-
Články
Reklama
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
ReklamaConcerted Activity of IgG1 Antibodies and IL-4/IL-25-Dependent Effector Cells Trap Helminth Larvae in the Tissues following Vaccination with Defined Secreted Antigens, Providing Sterile Immunity to Challenge Infection
Despite the high prevalence of gastrointestinal helminth parasites in human and animal populations throughout the world, no vaccines are yet available and we lack understanding of how anti-parasite protective immunity may operate effectively. We have used a model system with a natural mouse nematode parasite, Heligmosomoides polygyrus, which establishes long-term chronic infection in laboratory mice through the secretion of immunosuppressive molecules. Immunization of mice with as few as 3 secreted proteins, collected from parasites in vitro, confers complete immunity to challenge infection. We show here that immunity requires specific IgG1 antibodies directed to the secreted products, acting together with innate myeloid cells that require activation through the canonical Type 2 cytokine receptor, IL-4Rα, as well as through a pathway not previously known to be involved in effector mechanisms, IL-25. These myeloid cells act to trap and envelop helminth larvae while in the submucosal tissues of the small intestine, massing in large numbers and preventing their maturation and exit into the gut lumen. Thus the combined effects of specific antibodies from the adaptive immune system, and Type 2 cytokine activation of the innate immune system, co-operate to ensure elimination of the helminth parasite.
Published in the journal: . PLoS Pathog 11(3): e32767. doi:10.1371/journal.ppat.1004676
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004676Summary
Despite the high prevalence of gastrointestinal helminth parasites in human and animal populations throughout the world, no vaccines are yet available and we lack understanding of how anti-parasite protective immunity may operate effectively. We have used a model system with a natural mouse nematode parasite, Heligmosomoides polygyrus, which establishes long-term chronic infection in laboratory mice through the secretion of immunosuppressive molecules. Immunization of mice with as few as 3 secreted proteins, collected from parasites in vitro, confers complete immunity to challenge infection. We show here that immunity requires specific IgG1 antibodies directed to the secreted products, acting together with innate myeloid cells that require activation through the canonical Type 2 cytokine receptor, IL-4Rα, as well as through a pathway not previously known to be involved in effector mechanisms, IL-25. These myeloid cells act to trap and envelop helminth larvae while in the submucosal tissues of the small intestine, massing in large numbers and preventing their maturation and exit into the gut lumen. Thus the combined effects of specific antibodies from the adaptive immune system, and Type 2 cytokine activation of the innate immune system, co-operate to ensure elimination of the helminth parasite.
Introduction
The immune system has evolved suites of defense mechanisms to protect against infectious pathogens of all types ranging from viral and bacterial micro-organisms to more complex eukaryotic fungi, protozoa and helminths. In contrast to our detailed knowledge of anti-microbial immune mechanisms, however, we have yet to develop a clear picture of how immunity acts to eliminate parasites such as gastrointestinal helminths which even today infect over 1 billion people across the world [1].
The need to understand how the immune system can successfully eliminate helminth parasites is accentuated by the lack of appropriate new tools for control and eradication of these organisms. Although experimental models of protection show immunity to secondary challenge following drug-abbreviated primary infection, drug-induced clearance of helminths does not prevent rapid re-infection in human populations, and resistance to anthelmintic drugs has already emerged in veterinary use [2]. While vaccination would offer longer-term protection from infection, there are no currently available human anthelminthic vaccines, and the mechanisms of protective immunity on which new vaccines would depend have not been defined [3,4].
Helminth infection is, under natural conditions, near-ubiquitous and the mammalian immune system will have evolved specific mechanisms of activation and regulation to optimally respond to their challenge. Hence it is also likely that studying pathways of immunity to helminths will uncover new facets and properties of the immune system not apparent under conditions of infection with micro-organisms, and not necessarily predictable from our current knowledge of Type 2 activation in anti-helminth immunity [5,6].
The quest for anti-helminth vaccines began with live, radiation-attenuated organisms, which were effective in veterinary settings but unsuitable for human use [7]. Despite the successful translation of one veterinary vaccine to a molecular subunit formulation [8], in all other cases individual purified protein and recombinant vaccines have had modest effects, reducing worm loads without inducing full sterilising immunity in vaccinated recipients. While human trials have been initiated with partially-protective anti-hookworm and schistosome vaccines [9], it would appear that greater insight into the cellular and mechanistic basis of anti-helminth immunity, alongside the identification of protective antigens, will be essential if we are to enhance the choice and efficacy of future vaccines for human use.
Many helminths, of both humans and animals, are known to be highly immunomodulatory, mediating their effects at least in part through their repertoire of soluble secreted proteins which target the host immune system [10]. This has been particularly well-demonstrated in the natural mouse helminth parasite, Heligmosomoides polygyrus, which successfully establishes long-term infections in many strains of laboratory mice [11,12]. Immunomodulatory properties characterized in the H. polygyrus Excretory/Secretory (HES) products mediate a series of immunosuppressive effects on dendritic cells [13], airway epithelial cells [14] and T cells [15,16], prolonging parasite survival in an immunologically hostile environment.
In this study, we reasoned that if parasite secretions were promoting infection, that their blockade should generate protective immunity in this model system. As we demonstrate below, by immunizing mice with secreted products in an immunogenic fashion, we were able to generate complete protection against challenge infection. Protective immunization elicited IgG1 antibodies reacting against homologues of human vaccine candidates, as well as to several new targets conserved in parasites of human and veterinary importance. However, whilst a strong antibody response was a prerequisite for protection it alone was insufficient, and IL-25 signalling was also required for the expulsion of adult worms. Using intra-vital imaging of the intestinal mucosa, we show that protection is associated with enhanced recruitment and extravasation of LysM+ myeloid cells. Thus we show below that the antigen-specific adaptive response drives and unleashes a cytokine-dependent innate effector response for parasite elimination.
Results
HES immunization generates early immunity against larval parasite challenge
We have previously reported that immunization with the secreted products of adult H. polygyrus (HES) confers potent immunity against challenge infection [17,18]. Using a standard alum adjuvant regimen (Fig. 1A), we first showed that while HES vaccination generates sterile immunity, as reflected by the complete absence of adult parasites at day 28 post-challenge, immunization with H. polygyrus somatic extract does not significantly reduce parasite numbers (Fig. 1B). Adult worm fecundity, as measured by fecal egg counts, is an earlier and more sensitive measure of immune attrition, by which HES vaccination is seen to elicit almost complete immunity by day 14, while the effects of somatic extract immunisation are considerably more modest (Fig. 1C).
Fig. 1. HES vaccination elicits sterile immunity to challenge and blocks parasite maturation. 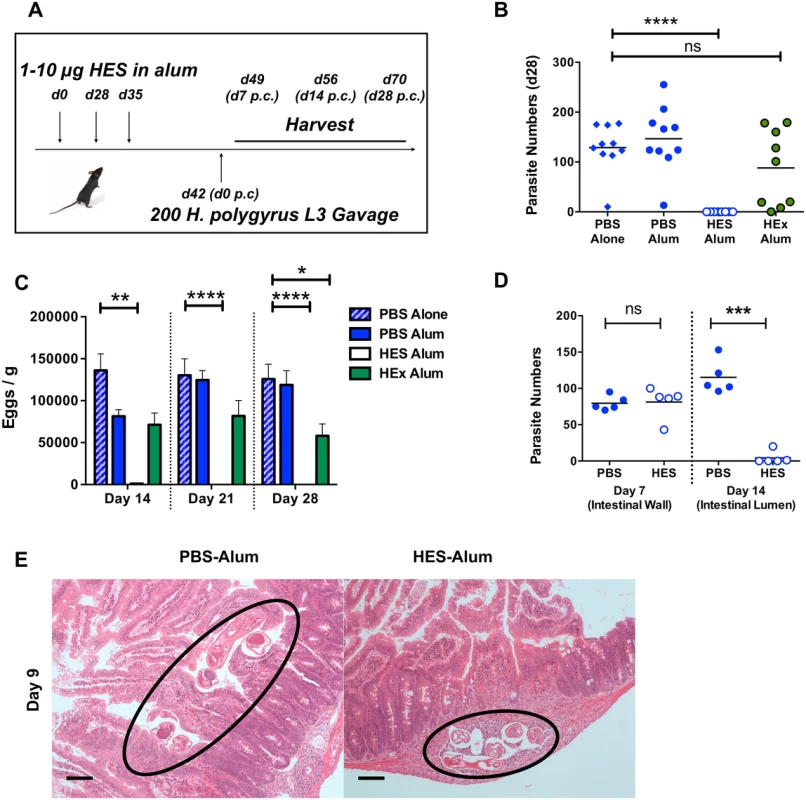
A. Schematic protocol for immunization. B. Parasite recoveries d 28 post-challenge following vaccination of C57BL/6 females with HES or somatic extract (HEx). Data shown are combined from two experiments each of 4–5 mice per group. Significance determined by ANOVA. C. Faecal egg counts (d 14, 21, 28 post-challenge) from (B). Significance determined by ANOVA. D. Parasite recoveries at d 7 (small intestinal wall) and 14 post-challenge (gut lumen). Representative of two independent experiments. Significance determined by t-test. E. H&E sections of duodenum in control and vaccinated animals at d9 post challenge; parasites in control animals are observed in gut lumen, but remain trapped in submucosa of vaccinated mice. Black circles indicate positions of parasite. Scale bar represents 100 μm. The low level of eggs present in the feces of vaccinated mice at day 14 post-challenge (Fig. 1C) indicated that immunity was acting early in infection. To confirm this we established that at day 7 post-challenge, similar numbers of fourth-stage (L4) larvae are found in the intestinal wall of vaccinated and control mice, but by day 14 the immune animals had eliminated almost every helminth from the lumen (Fig. 1D). Notably, while worms in control mice had migrated to the gut lumen by day 9, many of those in vaccinated mice remained trapped in the gut wall (Fig. 1E). Thus immunity induced by HES vaccination is directed against the immature parasite, with worm immobilisation and expulsion taking place between the first and second weeks of infection. In this respect, the molecular vaccine reproduces previous observations of immunity against immature stages in mice rendered immune by prior infection and drug-induced clearance of parasites [19,20].
The early host response was further characterized immunohistologically at the inflammatory foci surrounding larvae in the intestinal sub-mucosa (Fig. 2A). By day 7, these inflammatory foci have attracted large numbers of CD11b+ and Gr1+ myeloid cells, which localise more intensely around the parasite in vaccinated mice (Fig. 2B). To gain a greater understanding of the dynamic events that occur in vivo following parasite challenge, we used intra-vital two-photon microscopy to image cells and larval parasites from the serosal side of the intact intestine in live LysM-GFP mice, which have labeled neutrophils, monocytes and macrophages [21,22]. Early in infection, at day 3 post-challenge, GFP+ leukocytes showed decreased rolling velocity, and increased arrest and extravasation in non-vaccinated mice compared to naive controls; however, GFP+ cell arrest (Fig. 2C) and tissue infiltration was greatly enhanced in vaccinated recipients, with a >4-fold increase in tissue infiltrating cell numbers (Fig. 2D-F and S1 Movie A-C). GFP+ infiltrating cells accumulated around parasite larvae which are still viable at day 5, but particularly in the immune mice are constrained and partly immobilised by the cellular infiltrate (S1D and E Movie).
Fig. 2. HES vaccination promotes extensive myeloid cell extravasation and accumulation around site of larval invasion. 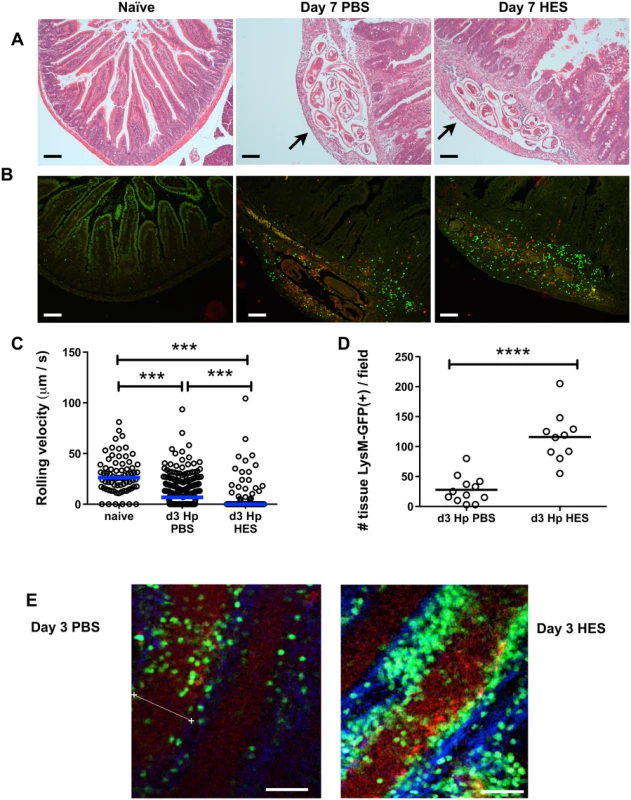
A. H&E staining of duodenal sections from naïve and d 7 post-challenge PBS control or HES-immunized C57BL/6 mice. Arrows indicate position of parasites. B. Immunohistology showing recruitment of CD11b+ (green) and Gr1+ (red) myeloid cells. Representative of 3–4 mice per group. Scale bar in A-B represents 100 μm. C. Rolling velocities of LysM-GFP+ cells along vessels imaged by two-photon microscopy of duodenum from the serosal aspect. Data accumulated from two mice per group, 3–7 vessels per mouse. Vessels were selected on basis of showing rolling behaviour. Significance determined by Kruskal-Wallis test. D. Tissue infiltration of LysM-GFP+ cells surrounding vessels in (C). Significance determined by t-test. E, F. Examples of two-photon microscopy from (D) showing tissue-infiltrating LysM-GFP+ cells in PBS- and HES-vaccinated mice. Scale bar represents 50μm. See also Supplemental Movie S1. Contrary to expectation, analysis of cell phenotypes in the intestinal tissue and draining mesenteric lymph nodes showed little difference between control and vaccinated animals, with similar levels of Th2 and Treg populations, although vaccinated mice have suppressed IFN-γ expression in the MLN (S1A-D Fig). In the lamina propria of infected mice, CD11b+Ly6G+ granulocytes, and both Ly6C+ and Ly6C– CD11b+F4/80int macrophages, showed expansion in all infected mice (S1E Fig), with a proportion of macrophages adopting an alternatively activated phenotype, characterised by expression of Ym1 and RELMα (S1F Fig). Notably, we observed higher levels of Ym1 transcript in total gut tissue from vaccinated mice by qPCR (S1F Fig).
Immunity requires IgG antibodies but not activating FcR or complement
Immunity to H. polygyrus generated by prior infection is known to be dependent on B cells, either or both through antibody and pro-Th2 cytokine production [19,23,24]. Vaccination induced marked phenotypic changes in MLN B cells with IgG1 class-switched CD19+ cells evident by day 7 post-challenge (Fig. 3A), alongside up-regulation of activation-associated CD23 (FcεRII), and reduced CD21/CD35 expression (Fig. 3B). Immunisation also evoked high titers of HES-specific IgG1 prior to challenge, as well as anti-HES IgA and E to a lesser degree (Fig. 3C). To assess whether B cells were required for immunity, we vaccinated wild-type C57BL/6 and congenic B cell-deficient μMT mice and found complete absence of immunity in the latter, as reflected in their higher worm and egg burdens (Fig. 3D, S2A Fig). To distinguish between humoral antibodies, and B cell-derived cytokines or signals, we also vaccinated MD4 mice with a fixed transgenic BCR for an unrelated protein, hen egg lysozyme (HEL). The inability of MD4 mice to develop protective immunity (S2B, C Fig) indicated that production of specific antibodies is critical for effective vaccination. Similar results were observed in CD40–/– animals which were unable to class switch to parasite-specific IgG1 (S2D, E Fig), and which (as μMT mice; Fig. 3D) showed significantly elevated parasite numbers in response to primary infection (PBS control groups).
Fig. 3. Immunity is dependent on cognate B cells and partially transferrable by antibody. 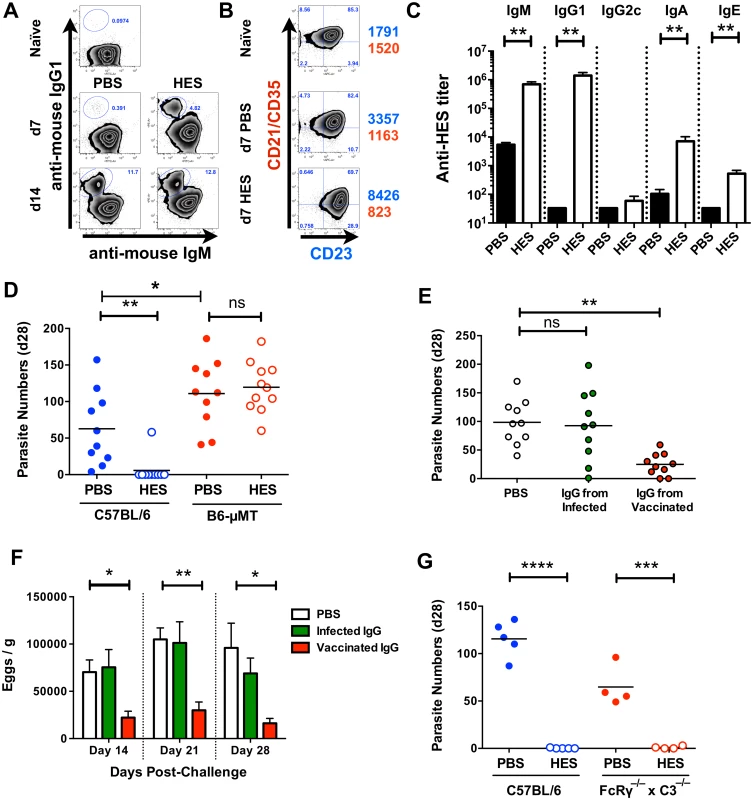
A. Gated CD19+ MLN cell expression of cell surface IgM and IgG1 in naïve and d 7 and d 14 post-challenge PBS and HES B6 mice. B. Gated CD19+ MLN cell expression of cell surface CD21/CD35 (CR2/CR1) and CD23 (FcεRII) in naïve and d 7 post-challenge PBS and HES B6 mice. Numbers represent geoMFI of CD23 (blue) and CD21/CD35 (red) expression. A-B representative of two independent experiments with 3–5 mice per group. C. Pre-challenge anti-HES IgM, IgG1, IgG2c, IgA and IgE titers in PBS and HES vaccinated B6 mice with 5 mice per group. D. Day 28 post-challenge worm burdens from C57BL/6 and μMT mice following HES immunization or PBS control. Data pooled from two experiments. Significance determined by ANOVA as indicated. E, F. Day 28 post-challenge worm and fecal egg burdens (d 14, 21, 28) in naïve C57BL/6 mice receiving IgG from vaccinated or primary infected mice, or PBS, as detailed in materials and methods. Data are pooled from two experiments, with significance determined by ANOVA Vs C57BL/6 PBS. G. Day 21 post-challenge adult worm burdens in C57BL/6 and FcRγ–/–x C3–/– mice following HES immunization or PBS control. Significance determined by t-test as indicated. See also S2 Fig. We next directly tested the protective potential of antibodies by passive transfer of purified IgG (predominantly IgG1, Fig. 3C) from the serum of HES-vaccinated animals into naïve recipients. Transfer resulted in significant reductions in adult worm (Fig. 3E), and egg (Fig. 3F) numbers, but not to the same extent as HES immunization. However, it was also noted that IgG transfer did not raise circulating anti-HES IgG1 titers in recipients to the same level as HES immunization (S2F Fig), and hence sterile immunity may require either extremely high antibody titers, or the activation of additional protective effector cell populations.
We next wished to see whether the protective antibody response was dependent on either activating FcR signalling and / or complement fixation. Vaccination of FcRγ–/–x C3–/– double transgenic mice resulted in undiminished immunity (Fig. 3G) showing that neither FcR signalling nor complement activation is required for protection. This suggests that HES immunisation generates protective IgG1 antibodies that function by binding to, and directly neutralising the function of, essential parasite excretory / secretory (ES) molecules.
Immunity requires IL-4 signaling, but is independent of eosinophils, mast cells, basophils and CCR2+ inflammatory monocytes
To investigate cellular effector components that may be required to complement the protective effects of IgG1 antibodies, we first confirmed that immunity is fully dependent upon IL-4R signalling. Thus, vaccinated IL-4Rα-/- mice fail to expel worms and actually incurred higher egg burdens than wild-type controls (Fig. 4A, S3A Fig), despite generating HES-specific IgG1 responses similar in titre to those seen in B6 primary infection (S3B Fig), and equivalent in specificity to immunised wild-type mice (S3C Fig).
Fig. 4. Vaccine-induced immunity requires IL-4R-mediated signaling but not eosinophils, mast cells, basophils or CCR2+ monocytes. 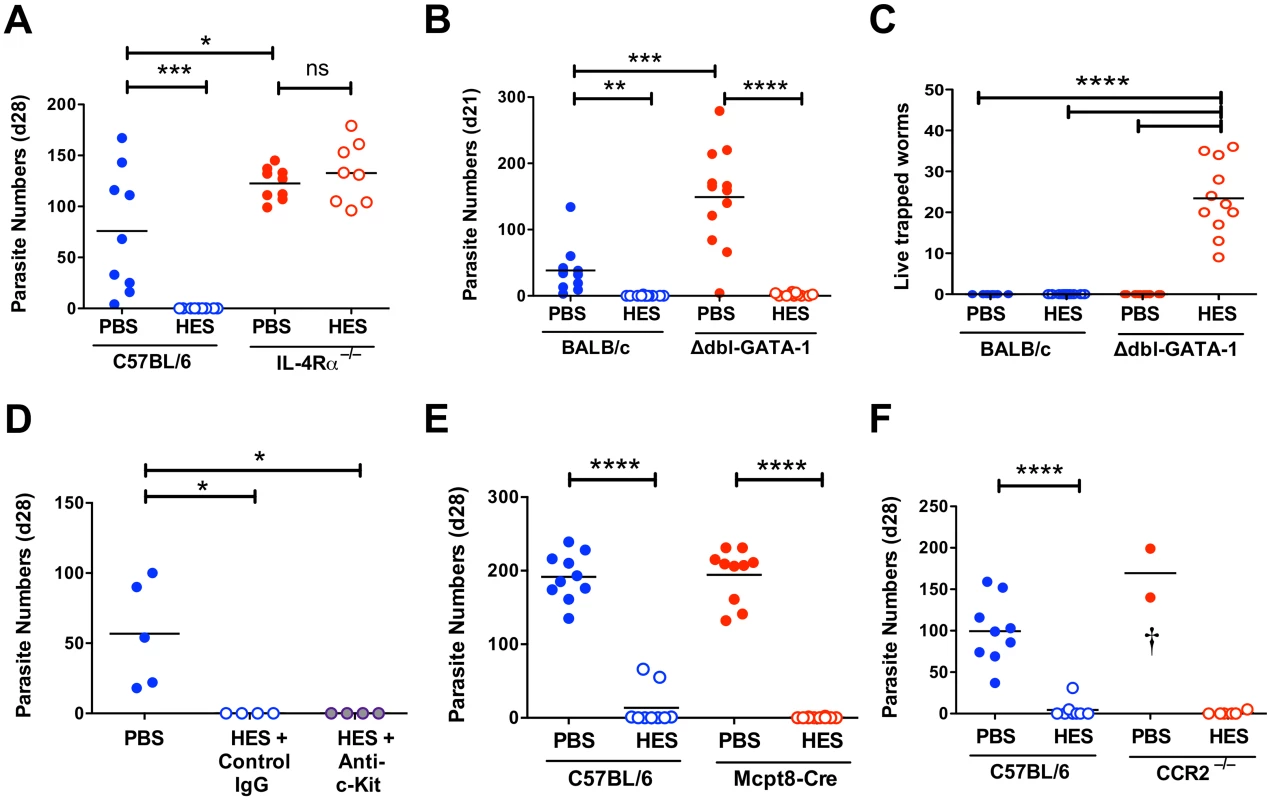
A-F. Day 21–28 worm counts in control and vaccinated (A) C57BL/6 and IL-4Rα–/– mice, (B-C) BALB/c and GATA-1 Δdbl mice, (D) ACK-2-treated and control C57BL/6 mice, (E) C57BL/6 4get/Mcpt8 cre and 4get controls and (F) CCR2–/– and C57BL/6 controls. n.b. PBS/alum immunized CCR2–/– mice died or were culled between 7–9 days post-challenge, with significant intestinal bleeding observed. Luminal worms in (A, B, D, E, and F), whereas counts in (C) represent live worms recovered from the intestinal wall at day 21 (i.e. presumed non-migratory parasites). Data in (A, B, C, E and F) pooled from 2 independent experiments. Significance in (A), (C) and (D) determined by ANOVA, in (B) by Kruskal-Wallis test, and (E-F) by unpaired t-test. See also S3 Fig. We then investigated type 2 effector populations using gene-targeting or antibody-depletion approaches as appropriate. Because of the marked expansion in eosinophil numbers in MLNs of vaccinated mice (S3D Fig), we assessed immunity in two models of eosinophil deficiency, C57BL/6 IL-5–/– mice and BALB/c Δdbl-GATA-1 mice (S3E-F Fig). Both eosinophil-deficient genotypes were fully protected from infection by HES vaccination (Fig. 4B and S3G Fig) and in both models anti-HES IgG1 titers were indistinguishable from wild-type mice (S3H, I Fig). Eosinophils have a role in killing parasites that are trapped in the intestinal wall of vaccinated mice, as a small but significant number of live worms (<40) were recovered from this site at day 21 post-challenge in vaccinated Δdbl-GATA-1 mice, but not BALB/c controls (Fig. 4C). Furthermore, PBS treated control Δdbl-GATA-1 mice had higher parasite burdens than their WT BALB/c counterparts, suggesting eosinophils have a role in the control of primary infection in this more resistant genotype (Fig. 4B).
Two other cells types thought to be important in Th2 mucosal immunity are mast cells and basophils. Mast cells gradually accumulate in the intestine of both control and vaccinated mice following challenge (S3J Fig). To focus on their potential role as effectors, rather than inducers, of type-2 immunity [25], we used the depleting anti-c-kit mAb (ACK-2) at the time of challenge. ACK treatment effectively depleted splenic mast cells, blocked serum mast cell protease mMCP-1 elevation and reduced intestinal levels (S3K-M Fig). Despite this, vaccine-induced immunity remained intact (Fig. 4D). The role of basophils was then assessed by immunization of basophil-deficient Mcpt8-Cre mice [26]. Again, HES vaccination rendered the mice fully resistant (Fig. 4E), indicating that immunity is intact in the absence of basophils as well as eosinophils and mast cells.
Similar genetic tools to selectively deplete other myeloid populations are less straightforward, and hence we employed clodronate depletion of phagocytes as previously described to impair immunity to secondary infection with H. polygyrus [27]. We found this treatment results in high haemorrhage-associated mortality (60–70%) in vaccinated and H. polygyrus-challenged mice, although the surviving mice remained immune. Because of the clear recruitment of Ly6C+ monocytes to the intestine following challenge (S1E Fig), and the requirement for IL-4R-dependent alternatively activated macrophages (AAMs) in resistance to secondary infection with H. polygyrus [27], we vaccinated and challenged CCR2-/- mice. Despite the severe reduction of circulating monocytes in these mice [28], they showed full immunity (Fig. 4F). As such, whilst Ly6C+ monocyte influx may represent part of the normal intestinal response to a variety of inflammatory insults [29–31] it is not required for successful anti-helminth immunity. Instead, we observed significant mortality in infected control (but not vaccinated) CCR2–/– mice, similar to that following clodronate treatment, revealing a key role for monocyte recruitment in either repairing parasite-induced damage as it migrates across the intestine, preventing bacterial translocation, or regulating the potentially pathogenic inflammatory response [31,32].
Protection requires IL-25 signaling, independent of antibodies
Recent studies have shown a key role for IL-25 (IL-17E) in the induction of protective anti-helminth immunity [33], and for IL-17A in promoting helminth-induced inflammation [34]. To test if either IL-25 or IL-17A was important for vaccine-induced immunity, we first immunised mice deficient in IL-17RA (the shared receptor subunit for both IL-17 and IL-25), and found identical anti-HES IgG1 titers compared to WT animals (Fig. 5A), indicating neither IL-17 nor IL-25 signalling is required for alum adjuvant-induced antibody production. Surprisingly, HES immunisation failed in these mice (Fig. 5B), with all mice containing worms at day 28 (range 1–214), many having worm burdens similar to non-vaccinated controls. Vaccination did impair egg production, indicating that a qualitatively lower level of functional immunity was induced in the absence of IL-17/25 signalling (Fig. 5C).
Fig. 5. Vaccine-induced immunity requires IL-25. 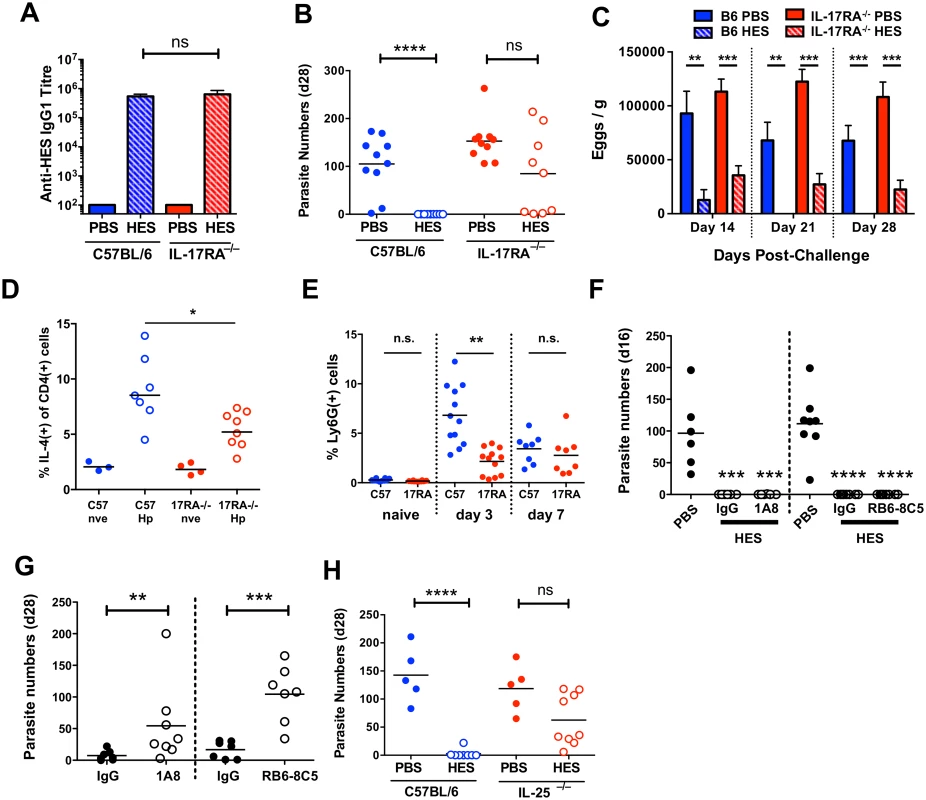
A. Anti-HES pre-challenge IgG1 titres in control and vaccinated C57BL/6 and IL-17RA-deficient mice. B, C. Day 28 worm counts and fecal egg burdens (d 14, 21 and 28) in control and vaccinated C57BL/6 and IL-17RA-deficient mice. D. Intracellular IL-4 production by naïve and d 7 post-challenge CD45+CD4+ lamina propria cells from C57BL/6 and IL-17RA–/– mice. Pooled from two experiments. E. Proportion of lamina propria CD45+CD11b+Ly6G+ in naïve, d 3 and d 7 challenged C57BL/6 and IL-17RA–/– mice. F. Adult worm burdens (d 16) in control and vaccinated C57BL/6 mice treated with anti-Ly6G (clone 1A8; left) or anti-Gr-1 (clone RB6-3C5; right) as detailed in materials and methods. G. Adult worm burdens (d 28) in primary infected BALB/c mice treated with anti-Ly6G, anti-Gr-1, or rat IgG control, as in (F). H. Day 28 worm counts in control and vaccinated C57BL/6 and IL-25-deficient mice. Significance in (A-E, G-H) determined by unpaired t-test or Mann-Whitney test, significance in (F) determined by ANOVA Vs PBS control. Data from A-G pooled from 2 independent experiments. See also S4 Fig. IL-25 expands lineage-negative innate lymphoid ILC2s that promote Th2 differentiation [33], but intestinal ILC IL-5 production was unimpaired in IL-17RA–/– mice (S4A Fig) and only a minor decrease in Th2 response was seen following challenge (Fig. 5D). It is important to note that in contrast to studies with the intestinal nematode Nippostrongylus brasiliensis [33,35,36] ILC2 expansion following H. polygyrus infection or challenge is relatively limited in susceptible wild-type C57BL/6 mice [12]. The muted induction of ILC2s by H. polygyrus implies that the parasite may have evolved effective means to suppress innate immune reactivity, such as that reported recently in the blockage of IL-33 release by its secreted products [14]. Instead, the major defect in IL-17RA-/- mice was impaired early recruitment of CD11b+Ly6G+ cells to the intestine (Fig. 5E). Cell sorting revealed these cells to have an irregular ring nucleus (S4B Fig), as observed in “type 2” neutrophils elicited by N. brasiliensis infection [37]. Because IL-17 family cytokines (including both IL-17 and IL-25) stimulate neutrophilia, we determined whether neutrophil depletion would abolish vaccine-induced immunity to H. polygyrus. Using antibodies that deplete either Ly6G+ cells (with 1A8 antibody) or all Gr-1+ cells (with RB6-8C5 antibody to both Ly6C and Ly6G), we established that immunity following vaccination was unaffected by the loss of neutrophils (Fig. 5F). However, as reported previously [38], resistance to primary infection (assayed in the more resistant BALB/c strain) is reduced by neutrophil depletion, showing that these cells can contribute to protection in the setting of partial immunity (Fig. 5G).
Because the IL-17RA–/– genotype does not distinguish between IL-17A and IL-25 signalling, we next immunised IL-25–/– mice and found them to have a similar deficiency in vaccine-induced protection, indicating that IL-25 is an important factor in expressing protective immunity in this model, independent of parasite-specific antibodies, (Fig. 5H), and that ablated IL-25 signalling can account for the phenotype of the IL-17RA–/– mouse, consistent with other data from our laboratory showing that antibody neutralisation of IL-17 does not influence susceptibility to H. polygyrus infection [39].
HES contains multiple protective components
To identify individual protective antigens within HES, we first established that immunity is elicited by heat-labile targets, rather than the abundant heat-stable glycans we recently identified [17]. Despite inducing similar titers of anti-HES IgG1 to native HES (S5A Fig), heat-denaturation markedly impaired the protective capacity of HES with all heat-treated HES immunised animals harboring between 1–90 adult parasites at day 28 (Fig. 6A). Nevertheless, heat-treated HES immunisation was still able to promote reductions in egg burdens (Fig. 6B). We then identified the dominant antigenic targets recognised by antibodies from vaccinated mice, using immunoprecipitation of native biotin-labeled HES, because key heat-sensitive epitopes are not detected by Western blot [17]. This analysis identified a restricted number of antigens, including known venom allergen-like (VAL) proteins, VAL-1, 2 and 3, and to a lesser extent VAL-4, as well as several additional spots (Fig. 6C).
Fig. 6. Identification of protective antigens following HES immunization. 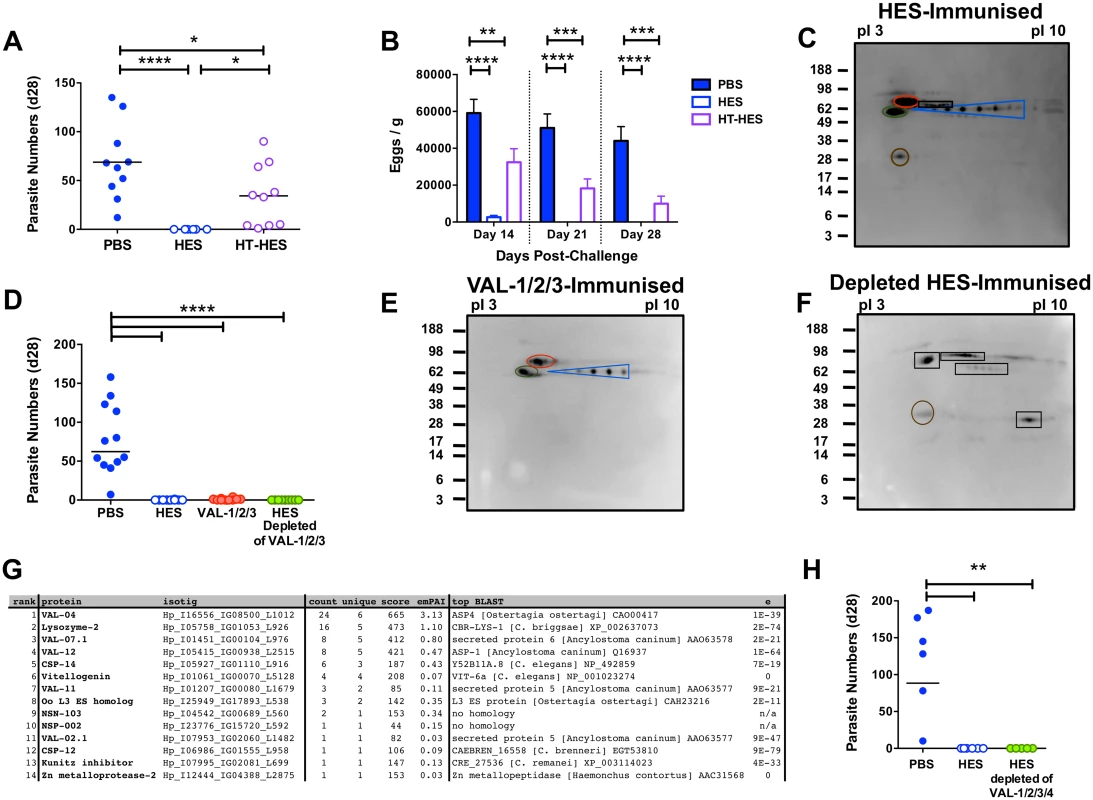
A-B. Day 28 post-challenge worm and fecal egg burdens (d 14, 21, 28) in C57BL/6 mice immunized with native or heat-treated HES. Data pooled from two experiments. Significance determined by ANOVA as indicated. C. Protective antibody targets revealed by immunoprecipitation of biotin-labeled HES by serum antibodies from immunized mice. Immunoprecipitated proteins were separated by pI (range 3–10) and molecular weights (indicated in kDa) and visualized with streptavidin HRP. Blue, red, green and brown circles represent VAL-1, 2, 3 and 4, respectively. Unknown antigens circled black. Sera pooled from 5 HES-vaccinated C57BL/6 mice pre-challenge and representative of two independent experiments. D. Day 28 post-challenge worm burdens from C57BL/6 mice immunized with a combination of VAL-1,-2 and -3, or with HES depleted of these 3 antigens. Data pooled from two experiments. Significance determined by ANOVA Vs PBS/alum. E-F. Immunoprecipitation of biotin-labeled HES antigens with vaccination sera as (C) from mice immunized with VAL-1/2/3 (E), or VAL-1/2/3 depleted HES (F). G. LC-MS/MS identification of antibody targets in mice immunized with VAL-1/2/3-depleted HES, performed on samples of unlabeled HES immunoprecipitated with serum antibodies from these mice. Proteins ranked according to spectral count (“count”). “Unique” represents number of unique peptide sequences in identification, “score” is Mascot score, and emPAI estimated abundance also shown. Highest scoring BLAST homolog with expect values indicated. H. Day 28 post-challenge worm burdens from C57BL/6 mice immunized with HES or HES depleted of VAL-1, -2, -3 and -4. See also S5 Fig. Next, we purified native VAL-1, 2 and 3 with specific monoclonal antibodies [17], providing also a fraction of HES devoid of these three dominant antigens (S5B-F Fig). Mice immunised with HES or with a cocktail of VAL-1, 2 and 3 generated IgG1 antibodies against these proteins, whereas mice given VAL-depleted HES failed to do so, while generating antibodies to other specificities (S5G-J Fig). Immunisation with the three major VAL immunogens induced a high, if not quite complete, level of protection against challenge (Fig. 6D, S5K Fig). Although 5/10 mice harbored up to 4 adult parasites, with eggs detected in 7/10 mice at day 28, this represented a highly significant reduction compared to levels in control animals. In contrast, HES depleted of the three major VAL proteins induced robust sterile immunity to challenge infection (Fig. 6D), with no eggs detected from day 21 (S5K Fig). The antibody targets in VAL-1/2/3 depleted HES (Fig. 6E, F) were then identified by mass spectrometry of antigens immunoprecipitated by the appropriate vaccinated mouse sera as four additional members of the VAL superfamily (particularly VAL-4) as well as a lysozyme, two novel proteins and several others with homologs in other parasitic and non-parasitic nematodes (Fig. 6G). We then further depleted HES of VAL-4 (S5L, M Fig), in addition to VAL-1, 2, and 3, and found it to again fully protect against challenge (Fig. 6H). These results show that the protective components in HES include VAL proteins, as well as additional non-VAL proteins, which are likely to be protective in combination.
Discussion
Together these studies show that immunization with secreted products from a gastrointestinal helminth induces long-lived sterile immunity against challenge infection through IgG1 antibodies acting in parallel with IL-4Rα - and IL-25-dependent effector cells. The ability of adult HES to drive immunity to immature stages is consistent with proteomic data demonstrating extensive antigen sharing between adult and larval secretomes, and the efficacy of ES from day 5 larvae in inducing full immunity [18]. Protective antibodies target both proteins of the VAL/ASP family that are known vaccine candidates [40], one of which induces natural IgE in exposed human populations [41], as well as non-VAL proteins with homologs in medically and veterinary important parasites. As antibodies induce protection in the absence of Fc receptor signalling, we suggest that they directly neutralise the function of parasite secretions.
Whilst antibodies reduce parasite fitness as reflected by egg production, they cannot alone lead to complete clearance of intestinal worms, which requires additional cytokine (IL-4Rα/IL-25)-stimulated cell population(s). We therefore hypothesise that protective antibodies neutralise parasite products that, in unvaccinated mice, are able to block the function of protective host innate cells. Such a model is consistent with greater myeloid cell recruitment to the intestine in vaccinated mice. Importantly, this suggests that successful vaccination against a mucosal pathogen may simply require the induction of sufficiently high titers of neutralising antibody to liberate host innate cells from parasite immunomodulation, allowing them to effect larval damage and killing.
While the ability of most HES-immunized mice to mount effective anti-larval immunity is striking, it is also notable that a small number of viable adult parasites survive to briefly produce eggs before they are also expelled. Two scenarios could explain this finding. First, parasites may be sufficiently damaged and stunted by immune attack in the tissues that their tenure in the luminal environment is short-lived. Secondly, there may be a distinct mechanism for expulsion of luminal parasites such as occurs to remove N. brasiliensis, whose tissue phase is within the lung. However, both tissue and luminal immunity appear to fully require activation through type 2 cytokines, and share many molecular and cellular pathways.
The dependence of helminth immunity on IL-4Rα signalling is a central paradigm established across many different systems [5,42] and suggests that indeed multiple Type 2 effector mechanisms are involved in elimination of H. polygyrus. However, we found that immunity in vaccinated mice was intact in the absence of various innate cell types reported to perform essential type-2 functions in other settings. For example, basophil-deficient Mcpt8Cre mice are more susceptible to primary infection with a related nematode, Nippostrongylus brasiliensis [26] and display impaired secondary immunity to H. polygyrus infection [43], yet are fully protected by HES vaccination. Likewise, mast cell-deficient Wsh mice are unable to clear primary H. polygyrus infection [25], but vaccine immunity is intact animals depleted of mast cells with ACK-2 antibody.
Eosinophils play intriguing roles in nematode infections, eliminating tissue-migrating larvae of some species [44–46], while in fact enhancing the survival of others [47]. In our system, the absence of eosinophils did incur higher primary levels of infection, but no deficit in immunity to challenge was observed other than greater viability of trapped larvae. Similarly, antibody depletion of neutrophils compromises resistance to primary infection but not vaccine-induced immunity to this parasite. So, whilst neutrophils may have some role in priming lung macrophages that promote early expulsion of N. brasiliensis following secondary challenge [37], they are not required for worm expulsion in the more chronic H. polygyrus model. An interesting observation is the expansion of CD11b+GR1+ subsets in the lamina propria, with phenotypes similar to myeloid-derived suppressor cells. However, they are unlikely to block immunity to the parasite as anti-Gr1 antibody compromises resistance to primary infection, and in the case of N. brasiliensis are able to promote expulsion of the luminal parasites [48,49].
Our finding that IL-25 is essential for vaccine-driven effector responses is somewhat surprising because it has been largely considered to be (alongside IL-33 and TSLP) a key inducer cytokine, acting at an early stage to drive ILC2s, mastocytosis and, in turn, Th2 immunity [50–55]. Whilst immunity to H. polygyrus is independent of TSLP [56] and IL-33 [57], IL-25 has a role in the partially protective response to primary infection [57]. Our data show that, whilst IL-25 is essential for the effector response that dislodges parasites from the intestine, it has little or no role in Th2 induction or the production of parasite-specific antibodies. This is most consistent with recent findings showing IL-25-deficiency does not to compromise the initiation of type 2 responsiveness following N. brasiliensis infection, but rather acts at a later stage to reduce immunity in both primary and challenge settings [58].
Interestingly, IL-25 is a key component in airway hypersensitivity, and antibody blockade of IL-25 in airway allergy was found to be most complete during the effector, rather than sensitization, phase [59]. Moreover, an IL-25-responsive myeloid cell was found to mediate allergic pathology in the lungs [60]. Although innate lymphoid cells, multipotent progenitor (mpp2) cells [61,62] and iNKT cells [63–65] are also responsive to IL-25, there are no data implicating any of these populations in the effector phase of anti-helminth immunity. Nevertheless, future studies will require lineage-specific deletions of the IL-25-specific receptor subunit (IL-17BR) to determine which populations are required to respond to this cytokine in the protective response to infection.
Resistance to secondary infection with H. polygyrus, following drug-induced clearance of primary parasites, is blocked by clodronate-loaded liposome depletion of phagocytes or pharmacological inhibition of Arginase-1, a key product of alternatively activated macrophages [27], while immunity to primary infection in genetically resistant mice is likewise compromised by clodronate administration [12]. In primary H. polygyrus infection, alternatively-activated macrophages adhere and immobilise tissue larvae [20], while transfer of macrophages (activated through IL-33) stimulates worm expulsion from chronically infected mice [66].
As has been noted elsewhere [67], the origin of intestinal macrophages during helminth-induced inflammation remains uncertain, and hence it is significant that immunity remains intact in CCR2-deficient mice. Monocyte egress from the bone marrow is impaired in these mice [28], and CCR2 ligation is also required for tissue entry, at least in Type 1 inflammatory settings [29]. Hence, immunity to H. polygyrus may be mediated by the coordinated recruitment of multiple (macrophage and non-macrophage) myeloid cell types utilising alternative receptors, but similarly driven through IL-4Rα and IL-25 to engage a similar effector gene program (including, for example, Arginase-1) to maximise immunity to the near-ubiquitous threat of gastrointestinal helminth parasites. Future work will be directed towards establishing if a suite of protective genes acting against helminth infection can be so defined, and if so the nature of their gene products and the range of cell phenotypes responsible for their production.
Materials and Methods
Mice, parasites, ES and extract preparation, immunization and antibody treatment
Mice were bred in house and kept in individually ventilated cages according to national guidelines. Transgenic strains were kindly provided as follows; C57BL/6 IL-17RA–/– mice [68] by Prof B. Ryffel, Orleans France; C57BL/6 μMT, MD4, and CD40-/- mice by Prof. D. Gray, Edinburgh, UK; C57BL/6 IL-4Rα–/–, IL-5–/–, and BALB/c GATA-1 Δ-dbl mice by Prof. J. Allen and Dr S. Babayan, Edinburgh, UK. C57BL/6 FcRγ–/–x C3–/–and CCR2–/– mice were maintained in Lausanne, Switzerland, C57BL/6 4get x Mcpt8Cre mice [26] in Erlangen, Germany, C57BL/6 IL-25–/– mice in the Malaghan Institute, New Zealand and C57BL/6 LysM-+/gfp in Washington University in St. Louis, USA. HES, heat-denatured HES and adult somatic extract (“HEx”) was produced from adult H. polygyrus bakeri (originally provided by Professor JM Behnke, University of Nottingham, UK) as described elsewhere [17,69,70]. Mice were immunized essentially as before [17] with 5 μg HES in alum adjuvant i.p., then boosted with 1 μg in alum on days 28 and 35, before challenge with 200 H. polygyrus L3 generally 1–2 weeks later. Fecal egg counts were determined on days 14, 21 and 28 post-challenge, and intestinal adult worms counted as indicated. For passive immunization, total serum IgG from day 28 post-infection control or HES immunized C57BL/6 mice was purified using a AKTA prime fast protein liquid chromatography (LC) with a HiTrap protein G HP column, dialysed into PBS, and then injected into naïve recipient C57BL/6 mice (1mg) on days -1, 0, 1, 3, 5, 7, 10, 13, 15, 17, 20, 22, 24 and 27 post-challenge with H. polygyrus. The ACK-2 hybridoma was provided by Prof. Richard Grencis, and purified as above. Anti-Gr-1 clone RB6-8C5 was produced in-house at Bioceros, Netherlands. Anti-Ly6G clone 1A8 was purchased from Bio-X-Cell. Antibody depletion regimes were as follows; ACK-2 (1 mg i.p.) on days -1, 0, 1, 3 and 5 post-challenge, 1A8 (500 μg i.p.) and RB6-8C5 (250μg i.p.) on days -1, 0, 1, 3, 5 and 7 post-challenge. Control mice received rat IgG purified from serum as above.
Ethics statement
All animal protocols adhered to the guidelines of the UK Home Office, complied with the Animals (Scientific Procedures) Act 1986, were approved by the University of Edinburgh Ethical Review Committee, and were performed under the authority of the UK Home Office Project Licence number 60/4105.
Cell isolation and FACS
Mesenteric lymph node and lamina propria cells were isolated for antigen-specific restimulation, surface and intracellular staining, or polyclonal stimulation for intracellular cytokine staining, as will be described elsewhere [12]. For FACS analysis, single cell suspensions were stained with live/dead aqua (Invitrogen) as per manufacturer’s instructions, washed into FACS buffer (PBS with 0.5% BSA and 0.05% sodium azide), blocked with 500 μg/ml rat IgG (sigma) for 10 min on ice, then surface stained with the following fluorochrome-conjugated antibodies: CD45.2 (clone 104), CD11b (M170), F4/80 (BM8), Ly6C (HK1.4), Ly6G (1A8), CD3 (17A2), CD19 (6D5), CD8α (53–6.7), Gr-1 (RB6-8C5), CD11c (N418), MHCII (M5/114.15.2), CD4 (RM4-5), anti-mouse IgM (RMM-1), anti-mouse IgG1 (RMG1-1), CD23 (B3B4), CD24 (M1/69), CD117 (2B8); all from Biolegend. CD49b (DX5) and siglecF PE (E50-2440) from BD Pharmingen, and CD103 (M290) from eBioscience. Intracellular antibodies were: IL-4 (11B11), IL-17A (TC11-18H10.1), IFNγ(XMG1.2) from Biolegend; IL-5 (TRFK5), IL-13 (ebio13A), Foxp3 (FJK-16) from eBioscience. Samples were acquired using an LSR II or Canto flow cytometer (BD Bioscience), and analysed with flowjo software (Tree Star). Lamina propria CD11b+Ly6G+ cells were sorted using a FACS aria (BD).
Cytokine and antibody ELISA
Serum antibody ELISA were performed as previously described [17] by coating with HES (1 μg/ml) or purified native VAL proteins (0.1 μg/ml), and detected using HRP-conjugated anti-mouse IgM, G1, G2a, A and E secondary antibodies (Southern Biotech). Controls included naïve mouse serum, day 28 primary infection sera and day 14 secondary infection sera. Gut homogenates were made in 1X cell lysis buffer (Cell Signaling) supplemented with PMSF protease inhibitor (Sigma) using a TissueLyzer (Qiagen). Serum and gut homogenate mouse mMCP-1 levels were determined with a commercially available ELISA kit (eBioscience).
Quantitative PCR
RNA was TRIzol (Invitrogen) extracted from duodenum (approx. 5 mm) according to manufacturer’s instructions. RNA (1–2 μg) was reverse transcribed with M-MLV reverse transcriptase (Promega), and cDNA transcript levels measured by quantitative PCR on a Roche Lightcycler 480 II with SYBR Green (Roche) using primers described previously [71].
Histology, immunohistochemistry, cytospins and two-photon microscopy
Transverse sections (5 μm) were cut from formalin-fixed paraffin-embedded duodenum and stained with either hemotoxylin and eosin or toluidine blue according to standard techniques. Pictures were taken using a DFC290 compound microscope and Application Suite software (both Leica). For antibody staining, paraffin sections were incubated with CD11b-FITC, Gr1-biotin/streptavidin APC. Intravital two-photon microscopy was carried out as previously described [22]. Briefly, mice were anesthetized with isofluorane, the peritoneal cavity opened, and duodenum (approximately 2–3 cm from the stomach) secured to a plastic coverslip with vetbond (3M). Non-targeted Q-dots (Qtracker 655nm, Invitrogen) were injected retro-orbitally to label the lumen of the blood vessels. Fluorescence was excited at 750 or 890 nm and images (~225 x 250 μm) were acquired from the serosal surface of intact intestine at 25 frames/second. Images were rendered and cells tracked using Imaris v7 (Bitplane, USA). Leukocyte recruitment was analysed as described in Kreisel et al [72].
2-D gel electrophoresis, western blotting, immunoprecipation and LC-MS
Identification of antibody targets in HES involved two separate approaches. First, for Fig. 6C, E and F, HES was biotin-labeled, immunoprecipitated with polyclonal sera from immunized mice, separated by 2-D gel electrophoresis and stained with HRP-conjugated Streptavidin as previously described [17]. Mice were immunized with specific VAL proteins purified from unlabeled HES by immunoprecipitation with Sepharose bead-conjugated mouse anti-VAL-1 (clone 5-S36), VAL-2 (clone 5-S2), VAL-3 mAb (clone 5-S1) and VAL-4 (clone 2–11). These mAb were generated from the spleens of secondary infected H. polygyrus mice and were conjugated as before [17]. In a second approach, shown in Fig. 6G to identify antigenic proteins by LC-MS, total serum Ig from uninfected C57BL/6 mice immunized with VAL-1, 2 and 3-depleted HES was isolated by ammonium sulfate precipitation and bead-conjugated as above. Immunoprecipitation of unlabeled total HES was carried out as above, and bound proteins were eluted and subjected to LC-MS/MS analysis as before [69]. Proteins were identified by comparison with a H. polygyrus transcriptomic assembly comprised of five different life-cycle stages (L3, day 3 larvae, day 5 larvae, adult and egg; Harcus et al, manuscript in preparation). MudPit scoring was used for LC-MS with a p<0.05 significance threshold, with single peptide hits more stringently filtered for expect values <0.01. False discovery rate for peptides at p<0.05 was 2.15%.
Statistical analysis
Statistical analyses were carried out as indicated with Prism 6 (Graphpad Software Inc.). Normally distributed two-way comparisons used unpaired t tests, and multiple comparisons used one-way ANOVA, followed by Tukey’s test. If normality was not achieved, Mann–Whitney (for two-way comparisons) and Kruskal–Wallis tests (for multiple comparisons, followed by Dunn’s test) were used. P values of <0.05 were considered significant. * p<0.05, ** p<0.01, *** p<0.001, **** p<0.0001.
Supporting Information
Zdroje
1. Hotez PJ, Brindley PJ, Bethony JM, King CH, Pearce EJ, et al. (2008) Helminth infections: the great neglected tropical diseases. J Clin Invest 118 : 1311–1321. doi: 10.1172/JCI34261 18382743
2. Sargison ND (2012) Pharmaceutical treatments of gastrointestinal nematode infections of sheep–future of anthelmintic drugs. Vet Parasitol 189 : 79–84. doi: 10.1016/j.vetpar.2012.03.035 22497871
3. Bethony JM, Cole RN, Guo X, Kamhawi S, Lightowlers MW, et al. (2011) Vaccines to combat the neglected tropical diseases. Immunol Rev 239 : 237–270. doi: 10.1111/j.1600-065X.2010.00976.x 21198676
4. Hewitson JP, Maizels RM (2014) Vaccination against helminth parasite infections. Expert Rev Vaccines 13 : 473–487. 24606541
5. Allen JE, Maizels RM (2011) Diversity and dialogue in immunity to helminths. Nat Rev Immunol 11 : 375–388. doi: 10.1038/nri2992 21610741
6. Grencis RK, Humphreys NE, Bancroft AJ (2014) Immunity to gastrointestinal nematodes: mechanisms and myths. Immunol Rev 260 : 183–205. doi: 10.1111/imr.12188 24942690
7. Maizels RM, Holland M, Falcone FH, Zang XX, Yazdanbakhsh M (1999) Vaccination against helminth parasites: the ultimate challenge for immunologists? Immunol Rev 171 : 125–148. 10582168
8. Assana E, Kyngdon CT, Gauci CG, Geerts S, Dorny P, et al. (2010) Elimination of Taenia solium transmission to pigs in a field trial of the TSOL18 vaccine in Cameroon. Int J Parasitol 40 : 515–519. doi: 10.1016/j.ijpara.2010.01.006 20138046
9. Loukas A, Gaze S, Mulvenna JP, Gasser RB, Brindley PJ, et al. (2011) Vaccinomics for the major blood feeding helminths of humans. OMICS 15 : 567–577. doi: 10.1089/omi.2010.0150 21679087
10. Hewitson JP, Grainger JR, Maizels RM (2009) Helminth immunoregulation: the role of parasite secreted proteins in modulating host immunity. Mol Biochem Parasitol 167 : 1–11.
11. Behnke JM, Menge DM, Noyes H (2009) Heligmosomoides bakeri: a model for exploring the biology and genetics of restance to chronic gastrointestinal nematode infections. Parasitology 136 : 1565–1580. doi: 10.1017/S0031182009006003 19450375
12. Filbey KJ, Grainger JR, Smith KA, Boon L, van Rooijen N, et al. (2014) Innate and adaptive type 2 immune cell responses in genetically controlled resistance to intestinal helminth infection. Immunology and Cell Biology 92 : 436–448. doi: 10.1038/icb.2013.109 24492801
13. Segura M, Su Z, Piccirillo C, Stevenson MM (2007) Impairment of dendritic cell function by excretory-secretory products: A potential mechanism for nematode-induced immunosuppression. Eur J Immunol 37 : 1887–1904. 17563917
14. McSorley HJ, Blair NF, Smith KA, McKenzie ANJ, Maizels RM (2014) Blockade of IL-33 release and suppression of type 2 innate lymphoid cell responses by helminth secreted products in airway allergy. Mucosal Immunol 7 : 1068–1078. doi: 10.1038/mi.2013.123 24496315
15. Telford G, Wheeler DJ, Appleby P, Bowen JG, Pritchard DI (1998) Heligmosomoides polygyrus immunomodulatory factor (IMF), targets T - lymphocytes. Parasite Immunol 20 : 601–611. 9990645
16. Grainger JR, Smith KA, Hewitson JP, McSorley HJ, Harcus Y, et al. (2010) Helminth secretions induce de novo T cell Foxp3 expression and regulatory function through the TGF-β pathway. J Exp Med 207 : 2331–2341. doi: 10.1084/jem.20101074 20876311
17. Hewitson JP, Filbey KJ, Grainger JR, Dowle AA, Pearson M, et al. (2011) Heligmosomoides polygyrus elicits a dominant nonprotective antibody response directed at restricted glycan and peptide epitopes. J Immunol 187 : 4764–4777. doi: 10.4049/jimmunol.1004140 21964031
18. Hewitson JP, Ivens AC, Harcus Y, Filbey KJ, McSorley HJ, et al. (2013) Secretion of protective antigens by tissue-stage nematode larvae revealed by proteomic analysis and vaccination-induced sterile immunity. PLOS Pathogens 9: e1003492. doi: 10.1371/journal.ppat.1003492 23966853
19. Liu Q, Kreider T, Bowdridge S, Liu Z, Song Y, et al. (2010) B cells have distinct roles in host protection against different nematode parasites. J Immunol 184 : 5213–5223. doi: 10.4049/jimmunol.0902879 20357259
20. Esser-von Bieren J, Mosconi I, Guiet R, Piersgilli A, Volpe B, et al. (2013) Antibodies trap tissue migrating helminth larvae and prevent tissue damage by driving IL-4Ralpha-independent alternative differentiation of macrophages. PLoS Pathog 9: e1003771. doi: 10.1371/journal.ppat.1003771 24244174
21. Faust N, Varas F, Kelly LM, Heck S, Graf T (2000) Insertion of enhanced green fluorescent protein into the lysozyme gene creates mice with green fluorescent granulocytes and macrophages. Blood 96 : 719–726. 10887140
22. McDole JR, Wheeler LW, McDonald KG, Wang B, Konjufca V, et al. (2012) Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature 483 : 345–349. doi: 10.1038/nature10863 22422267
23. McCoy KD, Stoel M, Stettler R, Merky P, Fink K, et al. (2008) Polyclonal and specific antibodies mediate protective immunity against enteric helminth infection. Cell Host Microbe 4 : 362–373. doi: 10.1016/j.chom.2008.08.014 18854240
24. Wojciechowski W, Harris DP, Sprague F, Mousseau B, Makris M, et al. (2009) Cytokine-producing effector B cells regulate type 2 immunity to H. polygyrus. Immunity 30 : 421–433. doi: 10.1016/j.immuni.2009.01.006 19249230
25. Hepworth MR, Daniłowicz-Luebert E, Rausch S, Metz M, Klotz C, et al. (2012) Mast cells orchestrate type 2 immunity to helminths through regulation of tissue-derived cytokines. Proc Natl Acad Sci U S A 109 : 6644–6649. doi: 10.1073/pnas.1112268109 22493240
26. Ohnmacht C, Schwartz C, Panzer M, Schiedewitz I, Naumann R, et al. (2010) Basophils orchestrate chronic allergic dermatitis and protective immunity against helminths. Immunity 33 : 364–374. doi: 10.1016/j.immuni.2010.08.011 20817571
27. Anthony RM, Urban JF Jr., Alem F, Hamed HA, Rozo CT, et al. (2006) Memory TH2 cells induce alternatively activated macrophages to mediate protection against nematode parasites. Nat Med 12 : 955–960. 16892038
28. Serbina NV, Pamer EG (2006) Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol 7 : 311–317. 16462739
29. Zigmond E, Varol C, Farache J, Elmaliah E, Satpathy AT, et al. (2012) Ly6C hi monocytes in the inflamed colon give rise to proinflammatory effector cells and migratory antigen-presenting cells. Immunity 37 : 1076–1090. doi: 10.1016/j.immuni.2012.08.026 23219392
30. Bain CC, Scott CL, Uronen-Hansson H, Gudjonsson S, Jansson O, et al. (2013) Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors. Mucosal Immunol 6 : 498–510. doi: 10.1038/mi.2012.89 22990622
31. Grainger JR, Wohlfert EA, Fuss IJ, Bouladoux N, Askenase MH, et al. (2013) Inflammatory monocytes regulate pathologic responses to commensals during acute gastrointestinal infection. Nat Med 19 : 713–721. doi: 10.1038/nm.3189 23708291
32. Kayama H, Ueda Y, Sawa Y, Jeon SG, Ma JS, et al. (2012) Intestinal CX3C chemokine receptor 1high (CX3CR1high) myeloid cells prevent T-cell-dependent colitis. Proc Natl Acad Sci U S A 109 : 5010–5015. doi: 10.1073/pnas.1114931109 22403066
33. Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, et al. (2010) Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature 464 : 1367–1370. doi: 10.1038/nature08900 20200518
34. Chen F, Liu Z, Wu W, Rozo C, Bowdridge S, et al. (2012) An essential role for TH2-type responses in limiting acute tissue damage during experimental helminth infection. Nat Med 18 : 260–266. doi: 10.1038/nm.2628 22245779
35. Price AE, Liang HE, Sullivan BM, Reinhardt RL, Eisley CJ, et al. (2010) Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc Natl Acad Sci U S A 107 : 11489–11494. doi: 10.1073/pnas.1003988107 20534524
36. Smith KA, Harcus Y, Garbi N, Hämmerling GJ, MacDonald AS, et al. (2012) Type 2 innate immunity in helminth infection is induced redundantly and acts autonomously following CD11c+ cell depletion. Infect Immun 80 : 3481–3489. doi: 10.1128/IAI.00436-12 22851746
37. Chen F, Wu W, Millman A, Craft JF, Chen E, et al. (2014) Neutrophils prime a long-lived effector macrophage phenotype that mediates accelerated helminth expulsion. Nat Immunol 15 : 938–946. doi: 10.1038/ni.2984 25173346
38. Pentilla IA, Ey PL, Lopez AF, Jenkin CR (1985) Suppression of early immunity to Nematospiroides dubius in mice by selective depletion of neutrophils with monoclonal antibody. Aust J Exp Biol Med Sci 63 (Pt 5): 531–543. 4091759
39. Smith KA, Maizels RM (2014) IL-6 controls susceptibility to helminth infection by impeding Th2 responsiveness and altering the Treg phenotype in vivo. Eur J Immunol 44 : 150–161. doi: 10.1002/eji.201343746 24185641
40. Cantacessi C, Campbell BE, Visser A, Geldhof P, Nolan MJ, et al. (2009) A portrait of the "SCP/TAPS" proteins of eukaryotes—Developing a framework for fundamental research and biotechnological outcomes. Biotechnol Adv 27 : 376–388. doi: 10.1016/j.biotechadv.2009.02.005 19239923
41. Diemert DJ, Pinto AG, Freire J, Jariwala A, Santiago H, et al. (2012) Generalized urticaria induced by the Na-ASP-2 hookworm vaccine: implications for the development of vaccines against helminths. J Allergy Clin Immunol 130 : 169–176 e166. doi: 10.1016/j.jaci.2012.04.027 22633322
42. Urban JF Jr., Noben-Trauth N, Donaldson DD, Madden KB, Morris SC, et al. (1998) IL-13, IL-4Rα and Stat6 are required for the expulsion of the gastrointestinal nematode parasite Nippostrongylus brasiliensis. Immunity 8 : 255–264. 9492006
43. Schwartz C, Turqueti-Neves A, Hartmann S, Yu P, Nimmerjahn F, et al. (2014) Basophil-mediated protection against gastrointestinal helminths requires IgE-induced cytokine secretion. Proc Natl Acad Sci U S A 111: E5169–E5177. doi: 10.1073/pnas.1412663111 25404305
44. Dent LA, Daly CM, Mayrhofer G, Zimmerman T, Hallett A, et al. (1999) Interleukin-5 transgenic mice show enhanced resistance to primary infections with Nippostrongylus brasiliensis but not primary infections with Toxocara canis. Infect Immun 67 : 989–993. 9916122
45. Martin C, Al-Qaoud KM, Ungeheuer MN, Paehle K, Vuong PN, et al. (2000) IL-5 is essential for vaccine-induced protection and for resolution of primary infection in murine filariasis. Medical microbiology and immunology 189 : 67–74. 11138639
46. Cadman ET, Thysse KA, Bearder S, Cheung AY, Johnston AC, et al. (2014) Eosinophils are important for protection, immunoregulation and pathology during infection with nematode microfilariae. PLoS Pathog 10: e1003988. doi: 10.1371/journal.ppat.1003988 24626328
47. Gebreselassie NG, Moorhead AR, Fabre V, Gagliardo LF, Lee NA, et al. (2012) Eosinophils preserve parasitic nematode larvae by regulating local immunity. J Immunol 188 : 417–425. doi: 10.4049/jimmunol.1101980 22131328
48. Saleem SJ, Martin RK, Morales JK, Sturgill JL, Gibb DR, et al. (2012) Cutting edge: mast cells critically augment myeloid-derived suppressor cell activity. J Immunol 189 : 511–515. doi: 10.4049/jimmunol.1200647 22706087
49. Martin RK, Saleem SJ, Folgosa L, Zellner HB, Damle SR, et al. (2014) Mast cell histamine promotes the immunoregulatory activity of myeloid-derived suppressor cells. J Leukoc Biol 96 : 151–159. doi: 10.1189/jlb.5A1213-644R 24610880
50. Fort MM, Cheung J, Yen D, Li J, Zurawski SM, et al. (2001) IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity 15 : 985–995. 11754819
51. Fallon PG, Ballantyne SJ, Mangan NE, Barlow JL, Dasvarma A, et al. (2006) Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J Exp Med 203 : 1105–1116. 16606668
52. Owyang AM, Zaph C, Wilson EH, Guild KJ, McClanahan T, et al. (2006) Interleukin 25 regulates type 2 cytokine-dependent immunity and limits chronic inflammation in the gastrointestinal tract. J Exp Med 203 : 843–849. 16606667
53. Angkasekwinai P, Park H, Wang Y-H, Wang Y-H, Chang SH, et al. (2007) Interleukin 25 promotes the initiation of proallergic type 2 responses. J Exp Med 204 : 1509–1517. 17562814
54. Zhao A, Urban JF Jr., Sun R, Stiltz J, Morimoto M, et al. (2010) Critical role of IL-25 in nematode infection-induced alterations in intestinal function. J Immunol 185 : 6921–6929. doi: 10.4049/jimmunol.1000450 20974983
55. Angkasekwinai P, Srimanote P, Wang YH, Pootong A, Sakolvaree Y, et al. (2013) Interleukin-25 (IL-25) promotes efficient protective immunity against Trichinella spiralis infection by enhancing the antigen-specific IL-9 response. Infect Immun 81 : 3731–3741. doi: 10.1128/IAI.00646-13 23897610
56. Massacand JC, Stettler RC, Meier R, Humphreys NE, Grencis RK, et al. (2009) Helminth products bypass the need for TSLP in Th2 immune responses by directly modulating dendritic cell function. Proc Natl Acad Sci U S A 106 : 13968–13973. doi: 10.1073/pnas.0906367106 19666528
57. Zaiss MM, Maslowski KM, Mosconi I, Guenat N, Marsland BJ, et al. (2013) IL-1β suppresses innate IL-25 and IL-33 production and maintains helminth chronicity. PLoS Pathog 9: e1003531. doi: 10.1371/journal.ppat.1003531 23935505
58. Mearns H, Forbes-Blom EE, Camberis M, Tang SC, Kyle R, et al. (2014) IL-25 exhibits disparate roles during Th2-cell differentiation versus effector function. Eur J Immunol 44 : 1976–1980. doi: 10.1002/eji.201344400 24737448
59. Ballantyne SJ, Barlow JL, Jolin HE, Nath P, Williams AS, et al. (2007) Blocking IL-25 prevents airway hyperresponsiveness in allergic asthma. J Allergy Clin Immunol 120 : 1324–1331. 17889290
60. Petersen BC, Budelsky AL, Baptist AP, Schaller MA, Lukacs NW (2012) Interleukin-25 induces type 2 cytokine production in a steroid-resistant interleukin-17RB+ myeloid population that exacerbates asthmatic pathology. Nat Med 18 : 751–758. doi: 10.1038/nm.2735 22543263
61. Saenz SA, Siracusa MC, Perrigoue JG, Spencer SP, Urban Jr JF, et al. (2010) IL25 elicits a multipotent progenitor cell population that promotes TH2 cytokine responses. Nature 464 : 1362–1366. doi: 10.1038/nature08901 20200520
62. Saenz SA, Siracusa MC, Monticelli LA, Ziegler CG, Kim BS, et al. (2013) IL-25 simultaneously elicits distinct populations of innate lymphoid cells and multipotent progenitor type 2 (MPPtype2) cells. J Exp Med 210 : 1823–1837. doi: 10.1084/jem.20122332 23960191
63. Stock P, Lombardi V, Kohlrautz V, Akbari O (2009) Induction of airway hyperreactivity by IL-25 is dependent on a subset of invariant NKT cells expressing IL-17RB. J Immunol 182 : 5116–5122. doi: 10.4049/jimmunol.0804213 19342692
64. Terashima A, Watarai H, Inoue S, Sekine E, Nakagawa R, et al. (2008) A novel subset of mouse NKT cells bearing the IL-17 receptor B responds to IL-25 and contributes to airway hyperreactivity. J Exp Med 205 : 2727–2733. doi: 10.1084/jem.20080698 19015310
65. Hams E, Locksley RM, McKenzie AN, Fallon PG (2013) Cutting edge: IL-25 elicits innate lymphoid type 2 and type II NKT cells that regulate obesity in mice. J Immunol 191 : 5349–5353. doi: 10.4049/jimmunol.1301176 24166975
66. Yang Z, Grinchuk V, Urban JF Jr., Bohl J, Sun R, et al. (2013) Macrophages as IL-25/IL-33-responsive cells play an important role in the induction of type 2 immunity. PLoS ONE 8: e59441. doi: 10.1371/journal.pone.0059441 23536877
67. Bain CC, Mowat AM (2014) Macrophages in intestinal homeostasis and inflammation. Immunol Rev 260 : 102–117. doi: 10.1111/imr.12192 24942685
68. Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, et al. (2001) Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med 194 : 519–527. 11514607
69. Hewitson JP, Harcus Y, Murray J, van Agtmaal M, Filbey KJ, et al. (2011) Proteomic analysis of secretory products from the model gastrointestinal nematode Heligmosomoides polygyrus reveals dominance of Venom Allergen-Like (VAL) proteins. J Proteomics 74 : 1573–1594. doi: 10.1016/j.jprot.2011.06.002 21722761
70. Johnston CJC, Robertson E, Harcus Y, Coakley G, Smyth DJ, et al. (2015) Cultivation of Heligmosomoides polygyrus: an immunomodulatory nematode parasite and its secreted products. Journal of Visualized Experiments In press.
71. McSorley HJ, O'Gorman MT, Blair N, Sutherland TE, Filbey KJ, et al. (2012) Suppression of type 2 immunity and allergic airway inflammation by secreted products of the helminth Heligmosomoides polygyrus. Eur J Immunol 42 : 2667–2682. doi: 10.1002/eji.201142161 22706967
72. Kreisel D, Nava RG, Li W, Zinselmeyer BH, Wang B, et al. (2010) In vivo two-photon imaging reveals monocyte-dependent neutrophil extravasation during pulmonary inflammation. Proc Natl Acad Sci U S A 107 : 18073–18078. doi: 10.1073/pnas.1008737107 20923880
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek A Phospholipase Is Involved in Disruption of the Liver Stage Parasitophorous Vacuole MembraneČlánek Host ESCRT Proteins Are Required for Bromovirus RNA Replication Compartment Assembly and FunctionČlánek Enhanced CD8 T Cell Responses through GITR-Mediated Costimulation Resolve Chronic Viral Infection
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 3- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- To Be or Not IIb: A Multi-Step Process for Epstein-Barr Virus Latency Establishment and Consequences for B Cell Tumorigenesis
- Is Antigenic Sin Always “Original?” Re-examining the Evidence Regarding Circulation of a Human H1 Influenza Virus Immediately Prior to the 1918 Spanish Flu
- The Great Escape: Pathogen Versus Host
- Coping with Stress and the Emergence of Multidrug Resistance in Fungi
- Catch Me If You Can: The Link between Autophagy and Viruses
- Bacterial Immune Evasion through Manipulation of Host Inhibitory Immune Signaling
- Evidence for Ubiquitin-Regulated Nuclear and Subnuclear Trafficking among Matrix Proteins
- BILBO1 Is a Scaffold Protein of the Flagellar Pocket Collar in the Pathogen
- Production of Anti-LPS IgM by B1a B Cells Depends on IL-1β and Is Protective against Lung Infection with LVS
- Virulence Regulation with Venus Flytrap Domains: Structure and Function of the Periplasmic Moiety of the Sensor-Kinase BvgS
- α-Hemolysin Counteracts the Anti-Virulence Innate Immune Response Triggered by the Rho GTPase Activating Toxin CNF1 during Bacteremia
- Induction of Interferon-Stimulated Genes by IRF3 Promotes Replication of
- Intracellular Growth Is Dependent on Tyrosine Catabolism in the Dimorphic Fungal Pathogen
- HCV Induces the Expression of Rubicon and UVRAG to Temporally Regulate the Maturation of Autophagosomes and Viral Replication
- Spatiotemporal Analysis of Hepatitis C Virus Infection
- Subgingival Microbial Communities in Leukocyte Adhesion Deficiency and Their Relationship with Local Immunopathology
- Interaction between the Type III Effector VopO and GEF-H1 Activates the RhoA-ROCK Pathway
- Attenuation of Tick-Borne Encephalitis Virus Using Large-Scale Random Codon Re-encoding
- Establishment of HSV1 Latency in Immunodeficient Mice Facilitates Efficient Reactivation
- XRN1 Stalling in the 5’ UTR of Hepatitis C Virus and Bovine Viral Diarrhea Virus Is Associated with Dysregulated Host mRNA Stability
- γδ T Cells Confer Protection against Murine Cytomegalovirus (MCMV)
- Rhadinovirus Host Entry by Co-operative Infection
- A Phospholipase Is Involved in Disruption of the Liver Stage Parasitophorous Vacuole Membrane
- Dermal Neutrophil, Macrophage and Dendritic Cell Responses to Transmitted by Fleas
- Elucidation of Sigma Factor-Associated Networks in Reveals a Modular Architecture with Limited and Function-Specific Crosstalk
- A Conserved NS3 Surface Patch Orchestrates NS2 Protease Stimulation, NS5A Hyperphosphorylation and HCV Genome Replication
- Host ESCRT Proteins Are Required for Bromovirus RNA Replication Compartment Assembly and Function
- Disruption of IL-21 Signaling Affects T Cell-B Cell Interactions and Abrogates Protective Humoral Immunity to Malaria
- Compartmentalized Replication of R5 T Cell-Tropic HIV-1 in the Central Nervous System Early in the Course of Infection
- Diminished Reovirus Capsid Stability Alters Disease Pathogenesis and Littermate Transmission
- Characterization of CD8 T Cell Differentiation following SIVΔnef Vaccination by Transcription Factor Expression Profiling
- Visualization of HIV-1 Interactions with Penile and Foreskin Epithelia: Clues for Female-to-Male HIV Transmission
- Sensing Cytosolic RpsL by Macrophages Induces Lysosomal Cell Death and Termination of Bacterial Infection
- PKCη/Rdx-driven Phosphorylation of PDK1: A Novel Mechanism Promoting Cancer Cell Survival and Permissiveness for Parvovirus-induced Lysis
- Metalloprotease NleC Suppresses Host NF-κB/Inflammatory Responses by Cleaving p65 and Interfering with the p65/RPS3 Interaction
- Immune Antibodies and Helminth Products Drive CXCR2-Dependent Macrophage-Myofibroblast Crosstalk to Promote Intestinal Repair
- Adenovirus Entry From the Apical Surface of Polarized Epithelia Is Facilitated by the Host Innate Immune Response
- The RNA Template Channel of the RNA-Dependent RNA Polymerase as a Target for Development of Antiviral Therapy of Multiple Genera within a Virus Family
- Neutrophils: Between Host Defence, Immune Modulation, and Tissue Injury
- CD169-Mediated Trafficking of HIV to Plasma Membrane Invaginations in Dendritic Cells Attenuates Efficacy of Anti-gp120 Broadly Neutralizing Antibodies
- Japanese Encephalitis Virus Nonstructural Protein NS5 Interacts with Mitochondrial Trifunctional Protein and Impairs Fatty Acid β-Oxidation
- Yip1A, a Novel Host Factor for the Activation of the IRE1 Pathway of the Unfolded Protein Response during Infection
- TRIM26 Negatively Regulates Interferon-β Production and Antiviral Response through Polyubiquitination and Degradation of Nuclear IRF3
- Parallel Epigenomic and Transcriptomic Responses to Viral Infection in Honey Bees ()
- A Crystal Structure of the Dengue Virus NS5 Protein Reveals a Novel Inter-domain Interface Essential for Protein Flexibility and Virus Replication
- Enhanced CD8 T Cell Responses through GITR-Mediated Costimulation Resolve Chronic Viral Infection
- Exome and Transcriptome Sequencing of Identifies a Locus That Confers Resistance to and Alters the Immune Response
- The Role of Misshapen NCK-related kinase (MINK), a Novel Ste20 Family Kinase, in the IRES-Mediated Protein Translation of Human Enterovirus 71
- Chitin Recognition via Chitotriosidase Promotes Pathologic Type-2 Helper T Cell Responses to Cryptococcal Infection
- Activates Both IL-1β and IL-1 Receptor Antagonist to Modulate Lung Inflammation during Pneumonic Plague
- Persistence of Transmitted HIV-1 Drug Resistance Mutations Associated with Fitness Costs and Viral Genetic Backgrounds
- An 18 kDa Scaffold Protein Is Critical for Biofilm Formation
- Early Virological and Immunological Events in Asymptomatic Epstein-Barr Virus Infection in African Children
- Human CD8 T-cells Recognizing Peptides from () Presented by HLA-E Have an Unorthodox Th2-like, Multifunctional, Inhibitory Phenotype and Represent a Novel Human T-cell Subset
- Decreased HIV-Specific T-Regulatory Responses Are Associated with Effective DC-Vaccine Induced Immunity
- RSV Vaccine-Enhanced Disease Is Orchestrated by the Combined Actions of Distinct CD4 T Cell Subsets
- Concerted Activity of IgG1 Antibodies and IL-4/IL-25-Dependent Effector Cells Trap Helminth Larvae in the Tissues following Vaccination with Defined Secreted Antigens, Providing Sterile Immunity to Challenge Infection
- Structure of the Low pH Conformation of Chandipura Virus G Reveals Important Features in the Evolution of the Vesiculovirus Glycoprotein
- PPM1A Regulates Antiviral Signaling by Antagonizing TBK1-Mediated STING Phosphorylation and Aggregation
- Lipidomic Analysis Links Mycobactin Synthase K to Iron Uptake and Virulence in .
- Roles and Programming of Arabidopsis ARGONAUTE Proteins during Infection
- Impact of Infection on Host Macrophage Nuclear Physiology and Nucleopore Complex Integrity
- The Impact of Host Diet on Titer in
- Antimicrobial-Induced DNA Damage and Genomic Instability in Microbial Pathogens
- Herpesviral G Protein-Coupled Receptors Activate NFAT to Induce Tumor Formation via Inhibiting the SERCA Calcium ATPase
- The Causes and Consequences of Changes in Virulence following Pathogen Host Shifts
- Small GTPase Rab21 Mediates Fibronectin Induced Actin Reorganization in : Implications in Pathogen Invasion
- Positive Role of Promyelocytic Leukemia Protein in Type I Interferon Response and Its Regulation by Human Cytomegalovirus
- NEDDylation Is Essential for Kaposi’s Sarcoma-Associated Herpesvirus Latency and Lytic Reactivation and Represents a Novel Anti-KSHV Target
- β-HPV 5 and 8 E6 Disrupt Homology Dependent Double Strand Break Repair by Attenuating BRCA1 and BRCA2 Expression and Foci Formation
- An O Antigen Capsule Modulates Bacterial Pathogenesis in
- Variable Processing and Cross-presentation of HIV by Dendritic Cells and Macrophages Shapes CTL Immunodominance and Immune Escape
- Probing the Metabolic Network in Bloodstream-Form Using Untargeted Metabolomics with Stable Isotope Labelled Glucose
- Adhesive Fiber Stratification in Uropathogenic Biofilms Unveils Oxygen-Mediated Control of Type 1 Pili
- Vaccinia Virus Protein Complex F12/E2 Interacts with Kinesin Light Chain Isoform 2 to Engage the Kinesin-1 Motor Complex
- Modulates Host Macrophage Mitochondrial Metabolism by Hijacking the SIRT1-AMPK Axis
- Human T-Cell Leukemia Virus Type 1 (HTLV-1) Tax Requires CADM1/TSLC1 for Inactivation of the NF-κB Inhibitor A20 and Constitutive NF-κB Signaling
- Suppression of RNAi by dsRNA-Degrading RNaseIII Enzymes of Viruses in Animals and Plants
- Spatiotemporal Regulation of a T4SS Substrate by the Metaeffector SidJ
- Antigenic Properties of the Human Immunodeficiency Virus Envelope Glycoprotein Gp120 on Virions Bound to Target Cells
- Dependence of Intracellular and Exosomal microRNAs on Viral Oncogene Expression in HPV-positive Tumor Cells
- Identification of a Peptide-Pheromone that Enhances Escape from Host Cell Vacuoles
- Impaired Systemic Tetrahydrobiopterin Bioavailability and Increased Dihydrobiopterin in Adult Falciparum Malaria: Association with Disease Severity, Impaired Microvascular Function and Increased Endothelial Activation
- Transgenic Expression of the Dicotyledonous Pattern Recognition Receptor EFR in Rice Leads to Ligand-Dependent Activation of Defense Responses
- Comprehensive Antigenic Map of a Cleaved Soluble HIV-1 Envelope Trimer
- Low Doses of Imatinib Induce Myelopoiesis and Enhance Host Anti-microbial Immunity
- Impaired Systemic Tetrahydrobiopterin Bioavailability and Increased Oxidized Biopterins in Pediatric Falciparum Malaria: Association with Disease Severity
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Bacterial Immune Evasion through Manipulation of Host Inhibitory Immune Signaling
- BILBO1 Is a Scaffold Protein of the Flagellar Pocket Collar in the Pathogen
- Antimicrobial-Induced DNA Damage and Genomic Instability in Microbial Pathogens
- Attenuation of Tick-Borne Encephalitis Virus Using Large-Scale Random Codon Re-encoding
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



