-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaNeutrophils: Between Host Defence, Immune Modulation, and Tissue Injury
Neutrophils, the most abundant human immune cells, are rapidly recruited to sites of infection, where they fulfill their life-saving antimicrobial functions. While traditionally regarded as short-lived phagocytes, recent findings on long-term survival, neutrophil extracellular trap (NET) formation, heterogeneity and plasticity, suppressive functions, and tissue injury have expanded our understanding of their diverse role in infection and inflammation. This review summarises our current understanding of neutrophils in host-pathogen interactions and disease involvement, illustrating the versatility and plasticity of the neutrophil, moving between host defence, immune modulation, and tissue damage.
Published in the journal: . PLoS Pathog 11(3): e32767. doi:10.1371/journal.ppat.1004651
Category: Review
doi: https://doi.org/10.1371/journal.ppat.1004651Summary
Neutrophils, the most abundant human immune cells, are rapidly recruited to sites of infection, where they fulfill their life-saving antimicrobial functions. While traditionally regarded as short-lived phagocytes, recent findings on long-term survival, neutrophil extracellular trap (NET) formation, heterogeneity and plasticity, suppressive functions, and tissue injury have expanded our understanding of their diverse role in infection and inflammation. This review summarises our current understanding of neutrophils in host-pathogen interactions and disease involvement, illustrating the versatility and plasticity of the neutrophil, moving between host defence, immune modulation, and tissue damage.
Neutrophil’s Life Cycle
Granulopoiesis
Neutrophils are the predominant immune cell population in human blood, where they patrol and protect us from pathogens, and diseases with neutropenia show that they are indispensable for controlling bacterial and fungal infections. Neutrophils develop in the bone marrow from haematopoietic stem cells in a process called “granulopoiesis” and mature neutrophils are characterised by their segmented nucleus and granules that are filled with >700 proteins [1]. Bone marrow neutrophil lineage cells can be divided into three compartments: (i) the stem cell pool composed of hematopoietic stem cells and pluripotent progenitors; (ii) the mitotic pool composed of proliferating, lineage-committed myeloblasts, promyelocytes, and myelocytes; and (iii) the post-mitotic pool composed of metamyelocytes, band cells, and mature neutrophils. Post-mitotic bone marrow neutrophils constitute 95% of the neutrophils in the body [2,3] and this reserve is easily mobilized and recruited rapidly to sites of infection.
Granulocyte colony-stimulating factor (G-CSF) is the predominant factor regulating the neutrophil’s life cycle by increasing cell proliferation, survival, differentiation, and trafficking/mobilization. Mice lacking G-CSF or its receptor have a profound, but not absolute, neutropenia in bone marrow and blood [2,4,5]. However, these mice can still produce mature neutrophils in steady state and increase their production and mobilization in “emergency” situations, indicating that other signals can provide partial or complete compensation [6]. Moreover, G-CSF induces proliferation and expression of anti-apoptotic proteins and regulates chemokine expression [7,8]. However, the precise mechanisms by which G-CSF signals regulate mitotic and post-mitotic neutrophils are not fully understood. Maintenance of neutrophil numbers is further regulated by phagocytosis of apoptotic neutrophils by macrophages, a process termed “efferocytosis.” Efferocytosis reduces the production of interleukin (IL)-23 and IL-17 and dampens G-CSF production [9]. G-CSF thus regulates the neutrophil life cycle at multiple levels and, consequently, has become an important therapeutic agent for neutropenic diseases, as discussed below. Recently, autophagy has been reported as a negative regulator of neutrophil development in the bone marrow [10].
Mobilization and trafficking
Chemokines orchestrate the balance between neutrophil release and retention. Bone marrow stromal cells produce C-X-C-motif chemokine ligand (CXCL) 12 that binds to C-X-C-motif chemokine receptor (CXCR) 4, leading to neutrophil retention, while release is mainly mediated by CXCR2 [11]. Pharmacologic CXCR2 inhibition in healthy humans, using ozone - or LPS-induced inflammation models [12–15], or in patients with severe asthma [16] or cystic fibrosis (CF) [17] showed that CXCR2 inhibition is safe and decreases neutrophilic inflammation in the airways.
Clearance of apoptotic neutrophils by macrophages, a mechanism involving liver X receptor (LXR), is essential for immune homeostasis and impaired clearance of apoptotic neutrophils has been linked to autoimmune disease [18–20]. Recent murine studies have extended this concept by highlighting the role of the bone marrow as a site of neutrophil clearance [21]. Intriguingly, homing of senescent neutrophils back to the bone marrow was found to regulate the circadian release of hematopoietic progenitors into the circulation [22]. However, the relevance of this circadian mechanism for neutrophil homeostasis in humans remains debatable. Another layer of complexity has been added by the concept of reverse neutrophil migration from peripheral organs back into the bloodstream. Reverse transmigration has been first observed in endothelial cells in vitro [23], and in mice in vivo [24], and has then been defined as a novel mechanism of inflammation resolution in zebrafish models [25]. Despite these fascinating mechanistic insights, their relevance for human diseases remains to be defined.
Released neutrophils are proposed to disseminate in the periphery into circulating and marginated neutrophil pools. The latter refers to neutrophils adherent to endothelial cells in the spleen, liver, bone-marrow, and the lung that can be recovered by exercise and adrenaline [26]. While a recent study highlights the importance of the pulmonary marginated pool in mice [27], its role in humans, at least under homeostatic conditions, remains questionable since the injection of non-primed autologous neutrophils did not lead to a significant retention in the pulmonary vasculature [28]. Further studies are required to shed more light on the marginated neutrophil pool in man and mice.
The traditional paradigm of neutrophils as short-lived immune cells has been challenged by in vivo-labelling studies, demonstrating a life span of 5.4 days for human neutrophils [29], ten times longer than previously estimated, and suggesting that neutrophils shape immune responses beyond rapid host–pathogen interactions. However, alternative explanations have been proposed [30,31] and there is no broad consensus yet regarding the life span of neutrophils in humans. Furthermore, in vivo-labelling studies are required to solve these controversies and to gain deeper insight into the neutrophil’s life span, particularly under infectious disease conditions.
Neutrophil serine proteases, serpins, and neutrophil survival
Amongst the broad armamentarium that neutrophils carry in their granules, neutrophil serine proteases directly kill microbes and inactivate bacterial toxins [32,33]. Excessive host tissue damage and inflammation driven by the uncontrolled activity of these proteases (namely elastase, proteinase-3, and cathepsin G) is counterbalanced by endogenous serine protease inhibitors (“serpins”) [34]. Besides protecting the body from free and harmful proteolytic activities, recent studies demonstrate that serpins have an unexpectedly broader role in regulating neutrophil survival. This novel function was first hinted at by mice lacking the intracellular inhibitor Serpinb1a, which showed reduced neutrophil survival and severe bone marrow neutropenia [35], rendering these mice susceptible to bacterial and viral lung infections [36,37]. Further studies showed that Serpinb1a is critical for maintaining neutrophil survival by blocking cell-intrinsic death mediated by cathepsin G and proteinase-3 [38,39].
Neutrophil–Pathogen Interactions
Recent findings have substantially expanded our view on the repertoire of antimicrobial effector functions of the neutrophil in host–pathogen interactions. Beyond phagocytosis, neutrophils employ neutrophil extracellular traps (NETs) [40] to capture microbes extracellularly and autophagy to digest them intracellularly [41,42]. Several factors decide which antibacterial effector functions are activated: (i) the presence of serum favors phagocytosis but inhibits the formation of NETs [43], (ii) transmigration triggers the release of contents from secretory vesicles and specific granules, and (iii) cell adherence lowers the threshold for integrin-dependent neutrophil activation. Importantly, intravital microscopy imaging approaches have expanded our understanding of neutrophil–pathogen interactions extensively. A recent study using this technology showed that phagocytosis and NETosis act in concert to combat Staphylococcus aureus in vivo, supporting a novel “neutrophil multitasking” concept in host–pathogen interactions [44]. Other in vivo studies demonstrated intracapillary neutrophil crawling in S. aureus infection [45] and dynamic and intravascular NET formation in bacterial [46] and viral infections [47]. Fig. 1 illustrates the diversity of neutrophil effector mechanisms in host–pathogen interactions.
Fig. 1. Neutrophil effector mechanisms. 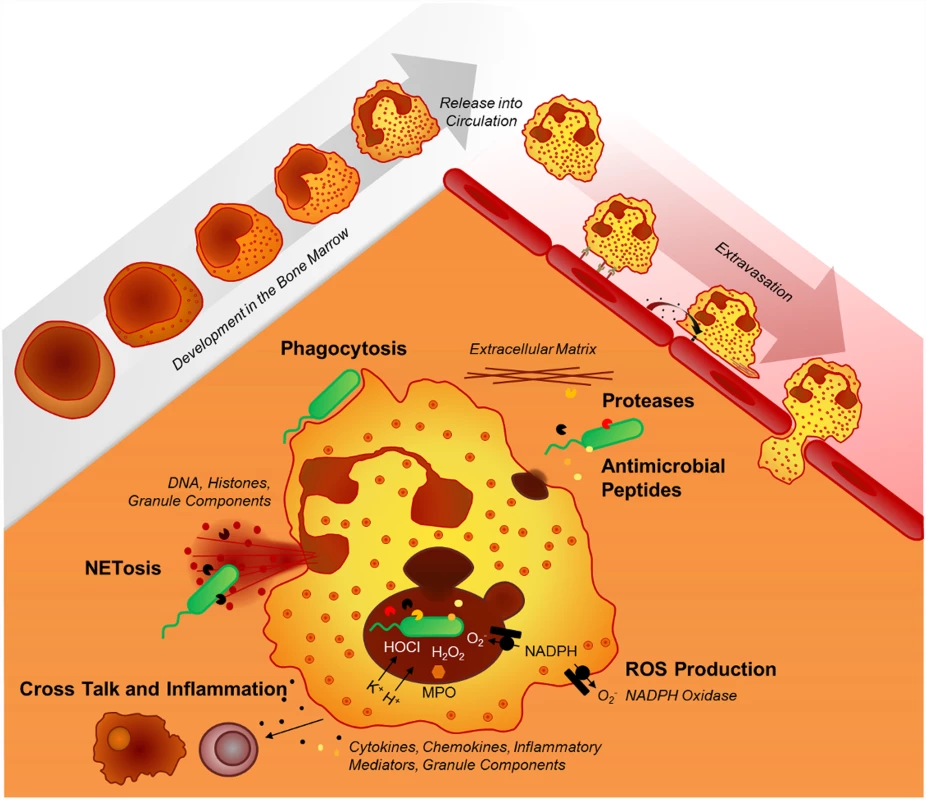
The mechanisms neutrophils employ to fight infections include phagocytosis, the release of various granule components into the extracellular space or into the phagosome (mainly proteases, oxidants, antimicrobial peptides), and the formation of neutrophil extracellular traps (NETs). In the paragraphs below, the key neutrophil effector functions are discussed.
Phagocytosis
Neutrophils are rapid and potent phagocytes. Upon ligation of opsonic receptors, such as Fcγ receptors, C-type lectins or complement receptors, the neutrophil membrane engulfs a particle or a microbe, a process that is mediated by a complex interplay of membrane lipids, intracellular signalling cascades, and cytoskeletal rearrangements. Subsequently, primary and secondary granules fuse with the phagosome and discharge their antimicrobial contents. Phagocytosis occurs within minutes and is enhanced by the complement system, IgG antibodies, and cell preactivation. Once specific granules fuse with the phagosome, the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase is assembled from the membrane-bound flavocytochrome b558, consisting of p91phox (phagocyte oxidase) and p22phox subunits, and the cytoplasmic components p47phox, p67phox, p40phox, and Rac. Potassium ion (K+) fluxes also occur in response to NADPH oxidase activation [48]. Reeves et al. hypothesised that K+, in turn, releases granule proteases that mediate bacterial killing, suggesting that the NADPH oxidase reaction mainly serves as an activator for proteases instead of being a microbicidal mechanism itself [49], a concept that has been challenged [50], most recently by a study showing that serine protease deficient human neutrophils show no impairment in killing bacteria [51]. The killing of S. aureus can be restored in NADPH oxidase-deficient neutrophils by adding liposomes loaded with glucose oxidase [52] or polyethylene glycol-conjugated D-amino acid oxidase [53], which produces H2O2. In these systems, neutrophils were still able to kill bacteria even when the NADPH oxidase was inactive. Moreover, inhibition of the NADPH oxidase does not impair killing of S. pneumoniae [54], and NADPH oxidase-deficient neutrophils from chronic granulomatous disease (CGD, see below) patients are still able to kill Escherichia coli, indicating additional microbicidal mechanisms independent of reactive oxygen species (ROS) production. A recent study further shows the involvement of the nucleotide-binding oligomerisation domain (NOD)-like receptor family, pyrin domain-containing protein (NLRP) 3 inflammasome in macrophage phagocytosis by demonstrating that activation of caspase-1 controls the phagosomal pH by regulating the NADPH oxidase (NOX) 2 to control phagosome function in gram-positive, but not gram-negative infections [55].
Neutrophil Extracellular Traps (NETs)
NET induction. The concept of NETosis was introduced in 2004 [40] as a novel mechanism for neutrophils to release extracellular DNA traps, composed of decondensed chromatin, histones, and granule proteins. This finding inspired a variety of studies employing different NETosis-inducing agents, including bacteria, fungi, protozoa, viruses, platelets, nitric oxide donors, and others. NET formation has been described as a novel form of cell death [56], involving the translocation of elastase from primary granules to the nucleus, where it cleaves specific histones and leads to chromatin decondensation [57]. Other studies support the notion that even viable neutrophils can form NETs, consisting of mitochondrial [58] or nuclear DNA, in a rapid and non-lytic manner [44]. Neutrophils treated with NADPH oxidase inhibitors or cells from CGD patients show impaired NET formation in response to phorbol-12-myristate-13-acetate (PMA) or S. aureus [56], and CGD gene therapy restored NET formation [59], suggesting that ROS are indispensable for NET formation. However, other studies suggested that ROS involvement depends on the stimulus [60–63]. In addition to ROS, several other pathways have been reported to be involved in NET formation, such as peptidyl arginine deiminase (PAD) 4 [64], the Raf-MEK (mitogen-activated kinase/ERK kinase)-ERK (extracellular signal-regulated kinase) pathway [65] and autophagy [66].
Microbial NETosis escape mechanisms. Pathogens have evolved a variety of mechanisms to escape from host defence. One of these mechanisms is the expression of DNase to degrade NETs. Streptococcal DNases render the bacteria resistant to NET-mediated killing [67–69]. Similar results have been obtained with other pathogens. However, the killing capacity of NETs was questioned by a study showing that pathogens entrapped in NETs survived and retained their ability to proliferate after being released from NETs [70], an issue requiring further investigations.
NETs in pathological conditions. Although NET generation has been described initially as an antimicrobial mechanism, recent data suggest that NETs could play a more important role in autoimmune and autoinflammatory pathologies, such as vasculitis [71], lung injury [72,73], atherosclerosis [74], rheumatoid arthritis [75,76], thrombosis [77–80], gout [81], and systemic lupus erythematosus (SLE) [43,82]. Several strategies have been proposed to interfere with NETs, particularly digestion of NET-DNA with DNase or targeting NET-associated proteins. DNase is successfully used to treat patients with CF, and its beneficial effect may be due to cleaving NETs [83,84]. Other approaches were so far mainly restricted to animal models and require clinical studies to assess their therapeutic usefulness.
Several aspects of NET formation remain enigmatic [85]. Despite a large number of studies demonstrating NET formation in vitro, our understanding of NET formation in vivo is limited. Most studies used PMA to induce NETs, while the major driver(s) of NET formation under (patho)physiological conditions remain poorly understood. A recent study suggests that particularly larger pathogens, such as fungal hyphae, induce NET formation [86]. Another open question is why some neutrophils form NETs and others do not. This heterogeneity remains enigmatic, but recent studies indicate that NET formation could be linked to olfactomedin 4 expression by a subset of neutrophils [87]. Moreover, the biological relevance of lytic/cell death-associated versus non-lytic/viable NET formation remains an open question [88]. While viable and rapid NET formation has been established in mice, human studies are scarce, but propose a role for this rapid NET formation mechanism in S. aureus infections [60]. Neutrophils have a relatively low content of mitochondrial DNA compared to nuclear DNA. Nevertheless, mitochondrial DNA is released following physiological stimuli and may represent a distinct form of NET formation [58]. While there is evidence for an involvement of elastase, PAD-4, and histone citrullination in NET formation, the precise cellular machinery leading to NET release is incompletely defined. For example, how elastase escapes from primary granules to reach the nucleus and what physical forces lead to propulsion of nucleic acids outside the cells remain largely elusive. How important are NETs in comparison to other neutrophil effector functions with regards to bacterial killing? A recent study in Papillon-Lefèvre syndrome, where neutrophils lack active serine proteases, such as elastase, showed that patient’s neutrophils failed to form NETs, but were fully competent to kill bacteria [51]. These data indicate that serine proteases are essential for NET formation, but that NET formation, in turn, could be dispensable for killing of certain bacteria. Further studies with more bacterial, fungal, and viral pathogens are required to solve these issues.
Antimicrobial peptides
Antimicrobial peptides play a major role at epithelial barriers and are secreted into various body fluids, such as sweat, alveolar lining fluid, milk, or intestinal mucus [89]. Most peptides in human neutrophil granules, like bactericidal/permeability-increasing protein, α-defensins, and cathelicidins, execute their microbicidal effects by disrupting bacterial membranes. Lactoferrin and lipocalin inhibit microbial iron uptake, and lysozyme cleaves cell wall peptidoglycans. Beyond their antimicrobial activities, cathelicidins (or their processed cleavage product LL-37) and α-defensins can also act as proinflammatory mediators and chemokine receptor agonists. Antimicrobial peptides, released by neutrophils, are also associated to NETs [85], facilitating a close interaction with NET-entangled pathogens.
Neutrophils and the Microbiome
The gut microbiome has attracted high interest in various fields of research and there is now compelling evidence to support the notion that mucosal commensals regulate granulopoiesis and neutrophil homeostasis at several levels. Prolonged antibiotic treatment leads to reduced neutrophil numbers and germ-free animals present a severe neutropenia, indicating a close and homeostatic link between microbiota and neutrophils [90–92]. While our understanding of cellular and molecular mechanisms remains incomplete, the regulation of neutrophil homeostasis by the microbiome seems to be mediated by structural features of bacteria that can activate innate pattern recognition receptors, leading to increased neutrophil production [93–95]. There is also emerging evidence that metabolites, such as short chain fatty acids, produced by gut commensals from dietary fibers, regulate neutrophil-mediated inflammation through the chemoattractant receptor G protein-coupled receptor (GPR) 43 expressed at high levels by neutrophils [96]. Dysbiosis (that is, altered microbiome composition) has been associated with increased incidence and severity of various chronic inflammatory diseases and cancer [97,98], yet the interaction between microbiota and neutrophils in these conditions remains to be established. Moreover, further studies are warranted to determine whether an altered microbiome induces changes in neutrophil phenotypes and functions and, vice versa, whether targeting neutrophils has an effect on the microbiome.
Neutrophil–Immune Cell Interactions
Neutrophils can interact with a variety of immune and non-immune cells at several levels, best studied for dendritic cells (DCs), macrophages, natural killer (NK) cells, and T cells [99,100]. Particularly, neutrophils and DCs frequently co-localize at sites of infection and DCs were found to capture neutrophils that have phagocytosed Leishmania and underwent apoptosis, a novel mechanism, which is how this pathogen undermines adaptive immune responses [101,102]. T helper 17 (Th17) cells orchestrate neutrophil recruitment by producing IL-17 and the neutrophil chemoattractant CXCL8. Neutrophils, in turn, recruit Th17 cells via release of the chemokines CCL20 and CCL2, resulting in a positive feedback loop between neutrophils and Th17 cells [103]. In addition, neutrophils interfere with T-cell proliferation as neutrophilic myeloid-derived suppressor cells (MDSCs). Neutrophils secrete the B-cell and plasma cell survival factors “B lymphocyte stimulator” (BLyS) [104] and “A proliferation-inducing ligand” (APRIL) [105]. In turn, a subset of splenic B cells produces GM-CSF [106] that provides differentiation signals for splenic neutrophils [107]. Human splenic neutrophils have been reported to activate marginal zone B-cell class switching and T-cell-independent antibody production, suggesting a distinct “B cell-helper neutrophil” phenotype [108]. However, these findings have recently been challenged by another study in which neutrophils with characteristics of the proposed “B cell-helper neutrophil” phenotype were not found in human spleens [109].
Murine and human studies indicate that distinct neutrophil phenotypes, initially identified as low-density neutrophils in cancer patients, inhibit T and NK cell proliferation. In analogy to monocyte subsets, those suppressive neutrophilic cells have been termed granulocytic/neutrophilic MDSCs [110]. The precise cellular mechanism(s) by which MDSCs suppress T-cell responses, however, have not been fully defined, but studies indicate that arginase, ROS, indoleamine 2,3-dioxygenase (IDO), Mac-1, PD-L1, and STAT3 might be involved [110–113]. Inter-species differences appear to exist, as mechanisms identified in mice do not seem to be always transferable to the human situation. Importantly, in systemic inflammation, a distinct subset of activated CD62Ldim neutrophils was found to suppress T cell proliferation mediated through a Mac-1-dependent mechanism [114].
Neutrophil Defects
Inherited neutrophil defects exemplify the importance of this cell type for various infectious and non-infectious conditions. These defects comprise severe congenital neutropenia (SCN), cyclic neutropenia, CGD, leukocyte adhesion deficiencies (LADs), myeloperoxidase (MPO) deficiency, warts, hypogammablogulinaemia, infections, myelokathexix (WHIM) syndrome, specific granule deficiency, defects in toll-like receptor (TLR)/interleukin 1 receptor-associated kinase (IRAK) 4/caspase activation and recruitment domain-containing protein (CARD) 9 signalling, and other less defined entities and are summarized in the chapters below.
Severe congenital neutropenia (SCN)
In SCN, neutrophilic differentiation is blocked at the promyelocyte/myelocyte stage, leading to isolated neutropenia with frequent severe bacterial and fungal infections and requiring lifelong treatment with G-CSF. Upon G-CSF treatment, SCN patients produce low numbers of neutrophils, sufficient to protect them from infections. The majority of SCN patients harbour autosomal dominant mutations in the neutrophil elastase gene (ELANE) [115]. Mechanistically, it has been proposed that intracellular accumulation of misfolded mutated neutrophil elastase activates the unfolded-protein response (UPR) and endoplasmatic reticulum (ER) stress [116,117] and promotes apoptosis [118]. Beyond ELANE, mutations in the haematopoietic cell-specific Lyn substrate (HCLS) 1-associated gene X1 (HAX1) were identified as a cause of neutropenia [119]. Further studies showed that the HAX1 interaction partner HCLS1 is activated by G-CSF, leading to the activation of the myeloid-specific transcription factor lymphoid enhancer binding factor (LEF)-1 [120,121]. A small subgroup of SCN patients have mutations in the transcriptional repressor oncoprotein growth factor independent (GFI) 1 [122], cytoskeletal regulator Wiskott-Aldrich Syndrome (WAS) protein [123], glucose-6 phosphatase catalytic subunit 3 (G6P3C) [124], endosomal adaptor protein p14 (also known as MAPBPIP) [125], or the endosomal trafficking vacuolar protein sorting 45 (VPS45) [126]. Depending on the type of the inherited gene mutation, SCN patients exhibit either isolated neutropenia (ELANE mutations) or additional syndromes such as lymphopenia (GFI1 gene mutations); monocytopenia (WAS-mutations); mental retardation/seizures (HAX1 mutations); prominent superficial venous network, atrial defects, and uropathy (G6PC3-associated disorders); and albinism and hyper-gammaglobulinemia (p14-mutated patients).
Chronic granulomatous disease (CGD)
CGD is caused by mutations in the genes encoding the subunits of the phagocyte NADPH oxidase complex, with an incidence around 1/200,000 [127]. CGD phagocytes cannot effectively produce superoxide (O2-) and fail to kill ingested pathogens, leading to severe infections, mainly by Staphylococcus and Aspergillus species [127,128]. CGD disease severity correlates inversely with the remaining neutrophil ROS production capacity [129]. The majority of CGD patients show mutations in gp91phox, which is the only CGD gene encoded on the X-chromosome [127–131]. X-linked CGD is characterised by an early onset and a high morbidity and mortality [128,129] and can also affect female carriers with an extreme X-chromosome inactivation pattern [132]. It has been proposed that the susceptibility of CGD patients to Aspergillus species might be due to a defect in ROS-dependent NET formation [59], an issue requiring further research. CGD patients exhibit a state of chronic immune activation, possibly due to diminished autophagy triggering excessive IL-1β release [133]. As a result, autoimmune conditions, such as SLE, discoid lupus erythematosus, and rheumatoid arthritis, are more prevalent among CGD patients [127,128,134]. Obstructive gastrointestinal and genitourinary granulomas in CGD are usually not caused by infections but by chronic inflammation [135] and often coincide with colonic inflammation reminiscent of Crohn’s disease [127,128,136]. This may be due to diminished intestinal epithelial defence as a result of the lack of ROS production by transmigrating CGD neutrophils [137].
Leukocyte adhesion deficiencies (LADs)
LADs are caused by several autosomal recessive genetic defects in molecules involved in the leukocyte adhesion cascade. The patients’ neutrophils cannot leave the circulation, leading to infection susceptibility [138]. In LAD-I, mutations in the gene encoding CD18, the integrin β2-chain, lead to the complete absence or decreased expression of the integrins αLβ2, αMβ2, αXβ2, and αDβ2 [139–143], rarely to normally expressed, but dysfunctional, β2-integrins [144]. Mutations in a Golgi guanosine diphosphate-fucose transporter (GFTP) protein, which is encoded by the SLC35C1 (solute carrier family 35 member C1) gene, lead to LAD-II [145,146], which was reclassified as the congenital disorder of glycosylation-IIc (CDG-IIc) [147]. Patients with LAD-II develop milder infections as in LAD-I, but have other abnormalities [147–149]. LAD-III, also known as LAD-I/variant, is caused by mutations in the gene FERMT3 (fermintin family homolog 3) encoding the β-integrin (β1, β2, and β3) adaptor protein kindlin-3. LAD-III patients also suffer from platelet defects and subsequent bleeding as well as osteopetrosis-like bone defects [150–154]. A combination of a LAD-like and CGD-like phenotype was described in two patients with mutations in Rac2 in which neutrophils showed defective chemotaxis due to impaired actin polymerisation and impaired NADPH oxidase activation [155,156]. An exciting, novel concept has recently emerged from studies of LAD-I patients in which defective neutrophil recruitment causes inflammatory periodontal bone loss. Surprisingly, the severe bone loss in these patients was not due to a lack of control of bacterial infections, but rather to the inability to control IL-17 production, which is normally reduced by phagocytosis of locally recruited neutrophils [157]. These findings suggest that neutrophils, the prototypic proinflammatory cells, have a profound anti-inflammatory function in steady state that is mediated by their effective efferocytosis. Thus, neutralizing IL-17 may be relevant for LAD-I and other diseases in which neutrophil recruitment to peripheral tissues is defective.
WHIM syndrome
In WHIM syndrome, CXCR4 gain-of-function mutations lead to neutropenia caused by increased retention of neutrophils in the bone marrow [158]. In addition to neutropenia, these patients often have lymphopenia and monocytopenia. The concept of CXCR4-dependent release of neutrophils from the bone marrow has recently been challenged by an intravital microscopy study showing that CXCR4 inhibition using plerixafor in mice triggers mobilization of neutrophils from the marginated pool of the lung and simultaneously prevents neutrophil homing to the bone marrow [27], highlighting the lung as a rich and easily mobilized source of neutrophils. The therapeutic relevance of these findings for neutropenic patients and other diseases remains to be defined in future studies.
Defects in innate immune receptor signalling
Neutrophils express various TLRs and TLR activation triggers antibacterial effector functions [159]. Mutations in myeloid differentiation primary response (MYD) 88 or IRAK4, central downstream mediators of TLR signalling, lead to recurrent, invasive bacterial infections in infancy and early childhood. This susceptibility decreases with age, so TLR-mediated signalling in neutrophils seems rather dispensable beyond adolescence. Neutrophils of these patients do not respond to TLR and IL-1R ligation, whereas ROS production is normal [160,161]. Acute-phase responses are delayed or even absent despite severe infections [162–166]. Mutations in CARD9, a protein essential for the sensing of fungi, lead to Candida infections [167], which was recently linked to impaired neutrophil killing [168]. Mutations in proteins regulating the activity of nuclear factor (NF)κB, namely NFκB essential modulator (NEMO) and inhibitor of NFκB kinase (IKK)α, lead to immunodeficiency in combination with other symptoms [166].
Specific Granule and MPO deficiency
Patients with specific-granule deficiency are extremely rare and suffer from recurrent bacterial infections due to a lack of secondary and tertiary granule proteins [169]. Specific-granule deficiency is caused by mutations in the transcription factor CCAAT/enhancer-binding protein ε (cEBPε), which is an important transcriptional regulator of granulopoiesis at the late stages of differentiation. Inherited MPO deficiency is frequent in humans, but MPO-deficient individuals are mostly asymptomatic, except for a small subset that suffers from diabetes [170].
Neutrophils in Acute Inflammation
Neutrophils are the first cells recruited to sites of infection and inflammation and, therefore, play a key role in several forms of acute inflammation, a topic that goes beyond the scope of this review. Here, we highlight several recent findings in this field and refer for further information to previous, more comprehensive reviews [171–174]. Neutrophil recruitment and activation are key events in acute inflammatory conditions, such as acute lung injury, and essential to provide a first cellular shield against bacterial and fungal pathogens. Neutrophil infiltration is not limited to infectious conditions and also occurs in sterile environments, mediated by a multistep migratory sequence, involving the inflammasome, chemokines, and formyl-peptide signals [175]. Physiologically, acute neutrophilic inflammation is followed by a resolution phase [176], important for tissue homeostasis. If these resolving mechanisms fail, neutrophils drive chronic inflammation, characterized by oxidant and protease release and leading to tissue injury. Consequently, a precise understanding of the local fine-tuning of neutrophil activation at sites of inflammation is mandatory. Full-blown neutrophil activation generally requires a two-step process, initiated by preactivation/priming through proinflammatory cytokines, such as tumor necrosis factor (TNF) α, IL-1β, IL-8, leukotriene (LT)B4 or GM-CSF, or bacterial products and by a later second-hit stimulus [177]. Priming is not a terminal event, as neutrophils can undergo cycles of priming and de-priming [178]. Importantly, the lung has been demonstrated recently to be a place for retention of primed neutrophils, a protective mechanism shown to be impaired in acute respiratory distress syndrome (ARDS) [28,179]. While various stimulants of neutrophil activity have been established, negative regulators are poorly defined. A recent study demonstrates that the sialic acid binding Ig-like lectin E (siglec-E) acts as an important negative regulator of neutrophil recruitment to the lung [180]. The NADPH oxidase, well known as the major source of ROS, has paradoxically also been shown to limit inflammation [134], yet the underlying mechanisms remained poorly defined. Davidson and coworkers now show that the NADPH oxidase dampened neutrophilic inflammation and was protective against acute lung injury by activating nuclear factor erythroid 2-related factor 2 (Nrf2), a transcriptional factor inducing antioxidative and cytoprotective pathways and suggesting Nrf2 as a novel anti-inflammatory therapeutic target [181]. Other recently described mechanisms in acute neutrophilic inflammation include the pulmonary endothelial glycocalyx [182] and myeloid-related protein-14 [183] in sepsis, midkine [184], hematopoietic progenitor kinase 1 (HPK1) [185], and chitinase-like proteins [186].
Neutrophils in Chronic Disease and Tissue Injury
While the role of neutrophils in acute inflammation is rather well established, their complexity in chronic diseases, tissue injury, and repair has just begun to evolve. Several chronic inflammatory disease conditions are characterized by a sustained influx of neutrophils, such as CF, chronic obstructive pulmonary disease, rheumatoid arthritis, nephritis, SLE, and cardiovascular diseases. Chemokines, lipid mediators, complement fragments, and tissue breakdown products trigger the migration of neutrophils into diseased tissues. Upon local activation, transmigrated neutrophils release their toxic ingredients, proteases and oxidants, which cause tissue injury and drive the production of chemokines that feed into the neutrophil recruitment loop. Neutrophil-derived serine and matrix metalloproteases (MMPs) cleave extracellular matrix (ECM; elastin and collagen) and impair host defence by degrading immune receptors and collectins. Neutrophils are a prominent source of MMPs, particularly MMP-9, thereby contributing to tissue damage and remodelling [187]. Consequently, neutrophil activities lead to cleavage of ECM into small fragments that, in turn, can stimulate the immune system and feed a positive feedback loop of tissue destruction and immune cell infiltration. Central to tissue injury and repair circuits is the tripeptide proline–glycine–proline (PGP), which is generated through the concerted action of several neutrophil-derived proteases, particularly MMP-8, MMP-9, and prolyl endopeptidase (PE). PGP can act as a chemokine mimetic and triggers neutrophil migration through CXCR1 and CXCR2 [188]. PGP is inactivated through a non-canonical functionality of the enzyme leukotriene A4 hydrolase (LTA4H) [189]. Persistence of PGP has been found in several neutrophilic diseases, including CF lung disease. Another neutrophil-derived protein involved in inflammation and tissue remodeling is high mobility group box protein-1 (HMGB1), a chromatin protein released from necrotic neutrophils and activating receptor for advanced glycation endproducts (RAGE) TLR and CXCR4 [190,191].
Neutrophil Heterogeneity and Plasticity
Heterogeneity of neutrophils is found at several levels: (i) nuclear appearance (band cells, mature and hypersegmented neutrophils); (ii) density (“low”-MDSCs versus “high” conventional density neutrophils [192]); (iii) NET-releasing versus non-NET-releasing neutrophils; and (iv) receptor expression profiles associated with distinct neutrophil subsets, which are summarized below:
Olfactomedin 4-expressing neutrophils, associated with a NET-releasing neutrophil subtype [87]
CD177 (NB1)-expressing neutrophils, associated with proteinase 3 and autoimmune diseases [193]
CD16brightCD62Ldim for suppressive neutrophils in systemic inflammation [114]
CD66b/CD33-expressing low-density neutrophils for neutrophilic MDSCs [192]
CXCR4+ for aged/senescent neutrophils [194]
CD63-expressing neutrophils in the airways of patients with CF [195]
CD49d-expressing neutrophils in atopic individuals [196]
VEGF-induced MMP-9 expressing “pro-angiogenic” neutrophils [187]
Neutrophils expressing a T-cell receptor-associated variable immunoreceptor complex [197]
ICAM-1/CD54-expressing neutrophils associated with systemic inflammation and reverse migration [23,198]
N1/N2 tumor-associated neutrophils (TANs) in murine tumor models (antitumorigenic, proinflammatory N1 neutrophils; protumorigenic, immunosuppressive N2 neutrophils) [199]
PMN-I (producing IL-12 and CCL3, inducing classically-activated macrophages) conferring protection against methicillin-resistant S. aureus (MRSA) infection and PMN-II (producing IL-10 and CCL2, inducing alternatively-activated macrophages) conferring susceptibility for MRSA infection [200]
The disease associations and functional characteristics of these described neutrophil phenotypes remain to be established. Could these markers be useful for subtyping different harmful versus beneficial neutrophil subsets at sites of chronic inflammation? Further studies in humans and mouse models are required to link phenotypic characteristics with functions.
Beyond heterogeneity, the potential of neutrophils to undergo phenotypic and/or functional plasticity has been addressed by some studies, demonstrating that neutrophils can transdifferentiate into: (i) DC-neutrophil hybrids upon GM-CSF stimulation with phenotypic and functional characteristics of both neutrophils and DCs [201,202]; (ii) DC-like cells in rheumatoid arthritis [203]; (iii) MHC-II-expressing antigen-presenting cells, induced by acidosis, cytokines, and growth factors and found in chronic inflammatory diseases [204,205]; and (iv) giant phagocytes, associated with autophagy [206]. Moreover, under inflammatory conditions, band-stage neutrophils were recently shown to transdifferentiate into monocytes [207]. Metabolic reprogramming, including the induction of anabolic pro-survival pathways, has been described in CF airway neutrophils, including up-regulation of the mammalian target of rapamycin (mTOR) pathway [208] and nutrient/glucose transporters [209]. Monocytic MDSCs have been shown to transdifferentiate into neutrophilic MDSCs [210]. While some of these neutrophil transdifferentiation phenotypes are found in chronic inflammatory and infective disease conditions [100,203,208], their overall disease relevance remains to be better defined.
Conclusions
Novel technologies, such as intravital microscopy and transgenic mice, have expanded our insights into neutrophil homeostasis and effector functions tremendously. Neutrophil steady-state homeostasis is not only regulated by growth factors, cytokines, and chemokines, but also involves microbial pattern recognition, linking hematopoiesis with microbiota. Neutrophil clearance seems to be even more complex, involving apoptosis/efferocytosis, reverse migration, and homing of senescent neutrophils back to the bone marrow, a process that, in turn, may regulate circadian neutrophil turnover. Besides their role as potent phagocytes, neutrophils expose their granule contents and DNA through NETosis, but its precise pathophysiological relevance in vivo remains to be defined. The spectrum of neutrophil plasticities has been further expanded by their role as immunomodulatory MDSCs that dampen T-cell activity. The clinical importance of neutrophils in host defence is underscored by the severity and complexity of infections in patients with inherited immunodeficiencies associated with a loss of neutrophil number or function. Faced with the emerging heterogeneity and plasticity of neutrophils, the challenge for the future remains how to target harmful, while promoting protective, neutrophil phenotypes. Based on these concepts, neutrophils in chronic inflammatory disease conditions are supposed to do more harm than good, rendering them promising therapeutic targets in chronic diseases. However, targeting neutrophils always bears the risk of increasing infection susceptibility, particularly towards bacterial and fungal pathogens. Small-molecule CXCR2 antagonists inhibit neutrophil chemotaxis and have been used in clinical studies in CF lung disease, with limited success [211]. Beyond these universal targeting approaches, inhibiting or depleting specific harmful neutrophil subsets, while preserving beneficial subtypes, would be preferential. However, this approach is limited by our current understanding of neutrophil heterogeneity. Which markers could be useful to identify harmful neutrophil phenotypes? Future studies using small molecules or antibodies targeting distinct neutrophil subsets will be required to address this question and to move forward towards a neutrophil subtype-selective pharmacologic treatment strategy.
Zdroje
1. Rørvig S, Østergaard O, Heegaard NHH, Borregaard N (2013) Proteome profiling of human neutrophil granule subsets, secretory vesicles, and cell membrane: correlation with transcriptome profiling of neutrophil precursors. Journal of Leukocyte Biology 94 : 711–721. doi: 10.1189/jlb.1212619 23650620
2. Semerad CL, Liu F, Gregory AD, Stumpf K, Link DC (2002) G-CSF is an essential regulator of neutrophil trafficking from the bone marrow to the blood. Immunity 17 : 413–423. 12387736
3. Summers C, Rankin SM, Condliffe AM, Singh N, Peters AM, et al. (2010) Neutrophil kinetics in health and disease. Trends Immunol 31 : 318–324. doi: 10.1016/j.it.2010.05.006 20620114
4. Lieschke GJ, Grail D, Hodgson G, Metcalf D, Stanley E, et al. (1994) Mice lacking granulocyte colony-stimulating factor have chronic neutropenia, granulocyte and macrophage progenitor cell deficiency, and impaired neutrophil mobilization. Blood 84 : 1737–1746. 7521686
5. Basu S, Hodgson G, Katz M, Dunn AR (2002) Evaluation of role of G-CSF in the production, survival, and release of neutrophils from bone marrow into circulation. Blood 100 : 854–861. 12130495
6. Hibbs ML, Quilici C, Kountouri N, Seymour JF, Armes JE, et al. (2007) Mice lacking three myeloid colony-stimulating factors (G-CSF, GM-CSF, and M-CSF) still produce macrophages and granulocytes and mount an inflammatory response in a sterile model of peritonitis. J Immunol 178 : 6435–6443. 17475873
7. Christopher MJ, Link DC (2007) Regulation of neutrophil homeostasis. Curr Opin Hematol 14 : 3–8. 17133093
8. Luo HR, Loison F (2008) Constitutive neutrophil apoptosis: mechanisms and regulation. Am J Hematol 83 : 288–295. 17924549
9. Stark MA, Huo Y, Burcin TL, Morris MA, Olson TS, et al. (2005) Phagocytosis of Apoptotic Neutrophils Regulates Granulopoiesis via IL-23 and IL-17. Immunity 22 : 285–294. 15780986
10. Rozman S, Yousefi S, Oberson K, Kaufmann T, Benarafa C, et al. (2014) The generation of neutrophils in the bone marrow is controlled by autophagy. Cell Death Differ.
11. Martin C, Burdon PC, Bridger G, Gutierrez-Ramos JC, Williams TJ, et al. (2003) Chemokines acting via CXCR2 and CXCR4 control the release of neutrophils from the bone marrow and their return following senescence. Immunity 19 : 583–593. 14563322
12. Holz O, Khalilieh S, Ludwig-Sengpiel A, Watz H, Stryszak P, et al. (2010) SCH527123, a novel CXCR2 antagonist, inhibits ozone-induced neutrophilia in healthy subjects. Eur Respir J 35 : 564–570. doi: 10.1183/09031936.00048509 19643947
13. Lazaar AL, Sweeney LE, MacDonald AJ, Alexis NE, Chen C, et al. (2011) SB-656933, a novel CXCR2 selective antagonist, inhibits ex vivo neutrophil activation and ozone-induced airway inflammation in humans. Br J Clin Pharmacol 72 : 282–293. doi: 10.1111/j.1365-2125.2011.03968.x 21426372
14. Virtala R, Ekman AK, Jansson L, Westin U, Cardell LO (2012) Airway inflammation evaluated in a human nasal lipopolysaccharide challenge model by investigating the effect of a CXCR2 inhibitor. Clin Exp Allergy 42 : 590–596. doi: 10.1111/j.1365-2222.2011.03921.x 22192144
15. Leaker BR, Barnes PJ, O'Connor B (2013) Inhibition of LPS-induced airway neutrophilic inflammation in healthy volunteers with an oral CXCR2 antagonist. Respir Res 14 : 137. doi: 10.1186/1465-9921-14-137 24341382
16. Nair P, Gaga M, Zervas E, Alagha K, Hargreave FE, et al. (2012) Safety and efficacy of a CXCR2 antagonist in patients with severe asthma and sputum neutrophils: a randomized, placebo-controlled clinical trial. Clin Exp Allergy 42 : 1097–1103. doi: 10.1111/j.1365-2222.2012.04014.x 22702508
17. Moss RB, Mistry SJ, Konstan MW, Pilewski JM, Kerem E, et al. (2013) Safety and early treatment effects of the CXCR2 antagonist SB-656933 in patients with cystic fibrosis. J Cyst Fibros 12 : 241–248. doi: 10.1016/j.jcf.2012.08.016 22995323
18. Vandivier RW, Henson PM, Douglas IS (2006) Burying the dead: the impact of failed apoptotic cell removal (efferocytosis) on chronic inflammatory lung disease. Chest 129 : 1673–1682. 16778289
19. A-Gonzalez N, Bensinger SJ, Hong C, Beceiro S, Bradley MN, et al. (2009) Apoptotic cells promote their own clearance and immune tolerance through activation of the nuclear receptor LXR. Immunity 31 : 245–258. doi: 10.1016/j.immuni.2009.06.018 19646905
20. Hong C, Kidani Y, A-Gonzalez N, Phung T, Ito A, et al. (2012) Coordinate regulation of neutrophil homeostasis by liver X receptors in mice. J Clin Invest 122 : 337–347. doi: 10.1172/JCI58393 22156197
21. Furze RC, Rankin SM (2008) The role of the bone marrow in neutrophil clearance under homeostatic conditions in the mouse. FASEB J 22 : 3111–3119. doi: 10.1096/fj.08-109876 18509199
22. Casanova-Acebes M, Pitaval C, Weiss LA, Nombela-Arrieta C, Chevre R, et al. (2013) Rhythmic modulation of the hematopoietic niche through neutrophil clearance. Cell 153 : 1025–1035. doi: 10.1016/j.cell.2013.04.040 23706740
23. Buckley CD, Ross EA, McGettrick HM, Osborne CE, Haworth O, et al. (2006) Identification of a phenotypically and functionally distinct population of long-lived neutrophils in a model of reverse endothelial migration. J Leukoc Biol 79 : 303–311. 16330528
24. Woodfin A, Voisin MB, Beyrau M, Colom B, Caille D, et al. (2011) The junctional adhesion molecule JAM-C regulates polarized transendothelial migration of neutrophils in vivo. Nat Immunol 12 : 761–769. doi: 10.1038/ni.2062 21706006
25. Shelef MA, Tauzin S, Huttenlocher A (2013) Neutrophil migration: moving from zebrafish models to human autoimmunity. Immunol Rev 256 : 269–281. doi: 10.1111/imr.12124 24117827
26. Athens JW, Haab OP, Raab SO, Mauer AM, Ashenbrucker H, et al. (1961) Leukokinetic studies. IV. The total blood, circulating and marginal granulocyte pools and the granulocyte turnover rate in normal subjects. J Clin Invest 40 : 989–995. 13684958
27. Devi S, Wang Y, Chew WK, Lima R, A-González N, et al. (2013) Neutrophil mobilization via plerixafor-mediated CXCR4 inhibition arises from lung demargination and blockade of neutrophil homing to the bone marrow. The Journal of Experimental Medicine 210 : 2321–2336. doi: 10.1084/jem.20130056 24081949
28. Summers C, Singh NR, White JF, Mackenzie IM, Johnston A, et al. (2014) Pulmonary retention of primed neutrophils: a novel protective host response, which is impaired in the acute respiratory distress syndrome. Thorax 69 : 623–629. doi: 10.1136/thoraxjnl-2013-204742 24706039
29. Pillay J, den Braber I, Vrisekoop N, Kwast LM, de Boer RJ, et al. (2010) In vivo labeling with 2H2O reveals a human neutrophil lifespan of 5.4 days. Blood 116 : 625–627. doi: 10.1182/blood-2010-01-259028 20410504
30. Tofts PS, Chevassut T, Cutajar M, Dowell NG, Peters AM (2011) Doubts concerning the recently reported human neutrophil lifespan of 5.4 days. Blood 117 : 6050–6052; author reply 6053–6054. doi: 10.1182/blood-2010-10-310532 21636720
31. Li KW, Turner SM, Emson CL, Hellerstein MK, Dale DC (2011) Deuterium and neutrophil kinetics. Blood 117 : 6052–6053; author reply 6053–6054. doi: 10.1182/blood-2010-12-322271 21636721
32. Pham CT (2006) Neutrophil serine proteases: specific regulators of inflammation. Nat Rev Immunol 6 : 541–550. 16799473
33. Borregaard N (2010) Neutrophils, from marrow to microbes. Immunity 33 : 657–670. doi: 10.1016/j.immuni.2010.11.011 21094463
34. Korkmaz B, Horwitz MS, Jenne DE, Gauthier F (2010) Neutrophil elastase, proteinase 3, and cathepsin G as therapeutic targets in human diseases. Pharmacol Rev 62 : 726–759. doi: 10.1124/pr.110.002733 21079042
35. Benarafa C, LeCuyer TE, Baumann M, Stolley JM, Cremona TP, et al. (2011) SerpinB1 protects the mature neutrophil reserve in the bone marrow. J Leukoc Biol 90 : 21–29. doi: 10.1189/jlb.0810461 21248149
36. Benarafa C, Priebe GP, Remold-O'Donnell E (2007) The neutrophil serine protease inhibitor serpinb1 preserves lung defense functions in Pseudomonas aeruginosa infection. J Exp Med 204 : 1901–1909. 17664292
37. Gong D, Farley K, White M, Hartshorn KL, Benarafa C, et al. (2011) Critical role of serpinB1 in regulating inflammatory responses in pulmonary influenza infection. J Infect Dis 204 : 592–600. doi: 10.1093/infdis/jir352 21791661
38. Baumann M, Pham CT, Benarafa C (2013) SerpinB1 is critical for neutrophil survival through cell-autonomous inhibition of cathepsin G. Blood 121 : 3900–3907, S3901–3906. doi: 10.1182/blood-2012-09-455022 23532733
39. Loison F, Zhu H, Karatepe K, Kasorn A, Liu P, et al. (2014) Proteinase 3-dependent caspase-3 cleavage modulates neutrophil death and inflammation. J Clin Invest 124 : 4445–4458. doi: 10.1172/JCI76246 25180606
40. Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, et al. (2004) Neutrophil extracellular traps kill bacteria. Science 303 : 1532–1535. 15001782
41. Mitroulis I, Kourtzelis I, Kambas K, Rafail S, Chrysanthopoulou A, et al. (2010) Regulation of the autophagic machinery in human neutrophils. Eur J Immunol 40 : 1461–1472. doi: 10.1002/eji.200940025 20162553
42. Mihalache CC, Simon HU (2012) Autophagy regulation in macrophages and neutrophils. Exp Cell Res 318 : 1187–1192. doi: 10.1016/j.yexcr.2011.12.021 22245582
43. Hakkim A, Furnrohr BG, Amann K, Laube B, Abed UA, et al. (2010) Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc Natl Acad Sci U S A 107 : 9813–9818. doi: 10.1073/pnas.0909927107 20439745
44. Yipp BG, Petri B, Salina D, Jenne CN, Scott BN, et al. (2012) Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nat Med 18 : 1386–1393. 22922410
45. Harding MG, Zhang K, Conly J, Kubes P (2014) Neutrophil Crawling in Capillaries; A Novel Immune Response to Staphylococcus aureus. PLoS Pathog 10: e1004379. doi: 10.1371/journal.ppat.1004379 25299673
46. McDonald B, Urrutia R, Yipp BG, Jenne CN, Kubes P (2012) Intravascular neutrophil extracellular traps capture bacteria from the bloodstream during sepsis. Cell Host Microbe 12 : 324–333. doi: 10.1016/j.chom.2012.06.011 22980329
47. Jenne CN, Wong CH, Zemp FJ, McDonald B, Rahman MM, et al. (2013) Neutrophils recruited to sites of infection protect from virus challenge by releasing neutrophil extracellular traps. Cell Host Microbe 13 : 169–180. doi: 10.1016/j.chom.2013.01.005 23414757
48. Rada BK, Geiszt M, Kaldi K, Timar C, Ligeti E (2004) Dual role of phagocytic NADPH oxidase in bacterial killing. Blood 104 : 2947–2953. 15251984
49. Reeves EP, Lu H, Jacobs HL, Messina CGM, Bolsover S, et al. (2002) Killing activity of neutrophils is mediated through activation of proteases by K+ flux. Nature 416 : 291–297. 11907569
50. Roos D, Winterbourn CC (2002) Immunology. Lethal weapons. Science 296 : 669–671. 11976433
51. Sørensen OE, Clemmensen SN, Dahl SL, Ostergaard O, Heegaard NH, et al. (2014) Papillon-Lefevre syndrome patient reveals species-dependent requirements for neutrophil defenses. J Clin Invest 124 : 4539–4548. doi: 10.1172/JCI76009 25244098
52. Gerber CE, Bruchelt G, Falk UB, Kimpfler A, Hauschild O, et al. (2001) Reconstitution of bactericidal activity in chronic granulomatous disease cells by glucose-oxidase-containing liposomes. Blood 98 : 3097–3105. 11698296
53. Nakamura H, Fang J, Mizukami T, Nunoi H, Maeda H (2012) PEGylated d-amino acid oxidase restores bactericidal activity of neutrophils in chronic granulomatous disease via hypochlorite. Experimental Biology and Medicine 237 : 703–708. doi: 10.1258/ebm.2012.011360 22715431
54. Standish AJ, Weiser JN (2009) Human neutrophils kill Streptococcus pneumoniae via serine proteases. J Immunol 183 : 2602–2609. doi: 10.4049/jimmunol.0900688 19620298
55. Sokolovska A, Becker CE, Ip WK, Rathinam VA, Brudner M, et al. (2013) Activation of caspase-1 by the NLRP3 inflammasome regulates the NADPH oxidase NOX2 to control phagosome function. Nat Immunol 14 : 543–553. doi: 10.1038/ni.2595 23644505
56. Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, et al. (2007) Novel cell death program leads to neutrophil extracellular traps. J Cell Biol 176 : 231–241. 17210947
57. Papayannopoulos V, Metzler KD, Hakkim A, Zychlinsky A (2010) Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J Cell Biol 191 : 677–691. doi: 10.1083/jcb.201006052 20974816
58. Yousefi S, Mihalache C, Kozlowski E, Schmid I, Simon HU (2009) Viable neutrophils release mitochondrial DNA to form neutrophil extracellular traps. Cell Death Differ 16 : 1438–1444. doi: 10.1038/cdd.2009.96 19609275
59. Bianchi M, Hakkim A, Brinkmann V, Siler U, Seger RA, et al. (2009) Restoration of NET formation by gene therapy in CGD controls aspergillosis. Blood 114 : 2619–2622. doi: 10.1182/blood-2009-05-221606 19541821
60. Pilsczek FH, Salina D, Poon KK, Fahey C, Yipp BG, et al. (2010) A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. J Immunol 185 : 7413–7425. doi: 10.4049/jimmunol.1000675 21098229
61. Parker H, Dragunow M, Hampton MB, Kettle AJ, Winterbourn CC (2012) Requirements for NADPH oxidase and myeloperoxidase in neutrophil extracellular trap formation differ depending on the stimulus. Journal of Leukocyte Biology 92 : 841–849. doi: 10.1189/jlb.1211601 22802447
62. Byrd AS, O'Brien XM, Johnson CM, Lavigne LM, Reichner JS (2013) An extracellular matrix-based mechanism of rapid neutrophil extracellular trap formation in response to Candida albicans. J Immunol 190 : 4136–4148. doi: 10.4049/jimmunol.1202671 23509360
63. Arai Y, Nishinaka Y, Arai T, Morita M, Mizugishi K, et al. (2014) Uric acid induces NADPH oxidase-independent neutrophil extracellular trap formation. Biochem Biophys Res Commun 443 : 556–561. doi: 10.1016/j.bbrc.2013.12.007 24326071
64. Wang Y, Li M, Stadler S, Correll S, Li P, et al. (2009) Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J Cell Biol 184 : 205–213. doi: 10.1083/jcb.200806072 19153223
65. Hakkim A, Fuchs TA, Martinez NE, Hess S, Prinz H, et al. (2011) Activation of the Raf-MEK-ERK pathway is required for neutrophil extracellular trap formation. Nat Chem Biol 7 : 75–77. doi: 10.1038/nchembio.496 21170021
66. Remijsen Q, Vanden Berghe T, Wirawan E, Asselbergh B, Parthoens E, et al. (2011) Neutrophil extracellular trap cell death requires both autophagy and superoxide generation. Cell Res 21 : 290–304. doi: 10.1038/cr.2010.150 21060338
67. Sumby P, Barbian KD, Gardner DJ, Whitney AR, Welty DM, et al. (2005) Extracellular deoxyribonuclease made by group A Streptococcus assists pathogenesis by enhancing evasion of the innate immune response. Proc Natl Acad Sci U S A 102 : 1679–1684. 15668390
68. Beiter K, Wartha F, Albiger B, Normark S, Zychlinsky A, et al. (2006) An endonuclease allows Streptococcus pneumoniae to escape from neutrophil extracellular traps. Curr Biol 16 : 401–407. 16488875
69. Walker MJ, Hollands A, Sanderson-Smith ML, Cole JN, Kirk JK, et al. (2007) DNase Sda1 provides selection pressure for a switch to invasive group A streptococcal infection. Nat Med 13 : 981–985. 17632528
70. Menegazzi R, Decleva E, Dri P (2012) Killing by neutrophil extracellular traps: fact or folklore? Blood 119 : 1214–1216. doi: 10.1182/blood-2011-07-364604 22210873
71. Kessenbrock K, Krumbholz M, Schonermarck U, Back W, Gross WL, et al. (2009) Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med 15 : 623–625. doi: 10.1038/nm.1959 19448636
72. Thomas GM, Carbo C, Curtis BR, Martinod K, Mazo IB, et al. (2012) Extracellular DNA traps are associated with the pathogenesis of TRALI in humans and mice. Blood 119 : 6335–6343. doi: 10.1182/blood-2012-01-405183 22596262
73. Caudrillier A, Kessenbrock K, Gilliss BM, Nguyen JX, Marques MB, et al. (2012) Platelets induce neutrophil extracellular traps in transfusion-related acute lung injury. J Clin Invest 122 : 2661–2671. doi: 10.1172/JCI61303 22684106
74. Doring Y, Manthey HD, Drechsler M, Lievens D, Megens RT, et al. (2012) Auto-antigenic protein-DNA complexes stimulate plasmacytoid dendritic cells to promote atherosclerosis. Circulation 125 : 1673–1683. doi: 10.1161/CIRCULATIONAHA.111.046755 22388324
75. Liu CL, Tangsombatvisit S, Rosenberg JM, Mandelbaum G, Gillespie EC, et al. (2012) Specific post-translational histone modifications of neutrophil extracellular traps as immunogens and potential targets of lupus autoantibodies. Arthritis Res Ther 14: R25. doi: 10.1186/ar3707 22300536
76. Khandpur R, Carmona-Rivera C, Vivekanandan-Giri A, Gizinski A, Yalavarthi S, et al. (2013) NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci Transl Med 5 : 178ra140.
77. Massberg S, Grahl L, von Bruehl ML, Manukyan D, Pfeiler S, et al. (2010) Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat Med 16 : 887–896. doi: 10.1038/nm.2184 20676107
78. Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, et al. (2010) Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci U S A 107 : 15880–15885. doi: 10.1073/pnas.1005743107 20798043
79. von Brühl ML, Stark K, Steinhart A, Chandraratne S, Konrad I, et al. (2012) Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J Exp Med 209 : 819–835. doi: 10.1084/jem.20112322 22451716
80. Martinod K, Demers M, Fuchs TA, Wong SL, Brill A, et al. (2013) Neutrophil histone modification by peptidylarginine deiminase 4 is critical for deep vein thrombosis in mice. Proc Natl Acad Sci U S A 110 : 8674–8679. doi: 10.1073/pnas.1301059110 23650392
81. Schauer C, Janko C, Munoz LE, Zhao Y, Kienhofer D, et al. (2014) Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nat Med 20 : 511–517. doi: 10.1038/nm.3547 24784231
82. Villanueva E, Yalavarthi S, Berthier CC, Hodgin JB, Khandpur R, et al. (2011) Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J Immunol 187 : 538–552. doi: 10.4049/jimmunol.1100450 21613614
83. Papayannopoulos V, Staab D, Zychlinsky A (2011) Neutrophil elastase enhances sputum solubilization in cystic fibrosis patients receiving DNase therapy. PLoS One 6: e28526. doi: 10.1371/journal.pone.0028526 22174830
84. Manzenreiter R, Kienberger F, Marcos V, Schilcher K, Krautgartner WD, et al. (2012) Ultrastructural characterization of cystic fibrosis sputum using atomic force and scanning electron microscopy. J Cyst Fibros 11 : 84–92. doi: 10.1016/j.jcf.2011.09.008 21996135
85. Yipp BG, Kubes P (2013) NETosis: how vital is it? Blood 122 : 2784–2794. doi: 10.1182/blood-2013-04-457671 24009232
86. Branzk N, Lubojemska A, Hardison SE, Wang Q, Gutierrez MG, et al. (2014) Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nat Immunol 15 : 1017–1025. doi: 10.1038/ni.2987 25217981
87. Welin A, Amirbeagi F, Christenson K, Bjorkman L, Bjornsdottir H, et al. (2013) The human neutrophil subsets defined by the presence or absence of OLFM4 both transmigrate into tissue in vivo and give rise to distinct NETs in vitro. PLoS One 8: e69575. doi: 10.1371/journal.pone.0069575 23922742
88. Peschel A, Hartl D (2012) Anuclear neutrophils keep hunting. Nat Med 18 : 1336–1338. doi: 10.1038/nm.2918 22961160
89. Peters BM, Shirtliff ME, Jabra-Rizk MA (2010) Antimicrobial peptides: primeval molecules or future drugs? PLoS Pathog 6: e1001067. doi: 10.1371/journal.ppat.1001067 21060861
90. Ohkubo T, Tsuda M, Tamura M, Yamamura M (1990) Impaired superoxide production in peripheral blood neutrophils of germ-free rats. Scand J Immunol 32 : 727–729. 1702900
91. Deshmukh HS, Liu Y, Menkiti OR, Mei J, Dai N, et al. (2014) The microbiota regulates neutrophil homeostasis and host resistance to Escherichia coli K1 sepsis in neonatal mice. Nat Med 20 : 524–530. doi: 10.1038/nm.3542 24747744
92. Kanther M, Tomkovich S, Xiaolun S, Grosser MR, Koo J, et al. (2014) Commensal microbiota stimulate systemic neutrophil migration through induction of serum amyloid A. Cell Microbiol 16 : 1053–1067. doi: 10.1111/cmi.12257 24373309
93. Clarke TB, Davis KM, Lysenko ES, Zhou AY, Yu Y, et al. (2010) Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat Med 16 : 228–231. doi: 10.1038/nm.2087 20081863
94. Bugl S, Wirths S, Radsak MP, Schild H, Stein P, et al. (2013) Steady-state neutrophil homeostasis is dependent on TLR4/TRIF signaling. Blood 121 : 723–733. doi: 10.1182/blood-2012-05-429589 23223360
95. Balmer ML, Schurch CM, Saito Y, Geuking MB, Li H, et al. (2014) Microbiota-Derived Compounds Drive Steady-State Granulopoiesis via MyD88/TICAM Signaling. J Immunol 193 : 5273–5283. doi: 10.4049/jimmunol.1400762 25305320
96. Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, et al. (2009) Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 461 : 1282–1286. doi: 10.1038/nature08530 19865172
97. Schwabe RF, Jobin C (2013) The microbiome and cancer. Nat Rev Cancer 13 : 800–812. doi: 10.1038/nrc3610 24132111
98. Gargano LM, Hughes JM (2014) Microbial origins of chronic diseases. Annu Rev Public Health 35 : 65–82. doi: 10.1146/annurev-publhealth-032013-182426 24365095
99. Mocsai A (2013) Diverse novel functions of neutrophils in immunity, inflammation, and beyond. J Exp Med 210 : 1283–1299. doi: 10.1084/jem.20122220 23825232
100. Scapini P, Cassatella MA (2014) Social networking of human neutrophils within the immune system. Blood 124 : 710–719. doi: 10.1182/blood-2014-03-453217 24923297
101. Peters NC, Kimblin N, Secundino N, Kamhawi S, Lawyer P, et al. (2009) Vector transmission of leishmania abrogates vaccine-induced protective immunity. PLoS Pathog 5: e1000484. doi: 10.1371/journal.ppat.1000484 19543375
102. Ribeiro-Gomes FL, Peters NC, Debrabant A, Sacks DL (2012) Efficient capture of infected neutrophils by dendritic cells in the skin inhibits the early anti-leishmania response. PLoS Pathog 8: e1002536. doi: 10.1371/journal.ppat.1002536 22359507
103. Pelletier M, Maggi L, Micheletti A, Lazzeri E, Tamassia N, et al. (2010) Evidence for a cross-talk between human neutrophils and Th17 cells. Blood 115 : 335–343. doi: 10.1182/blood-2009-04-216085 19890092
104. Scapini P, Carletto A, Nardelli B, Calzetti F, Roschke V, et al. (2005) Proinflammatory mediators elicit secretion of the intracellular B-lymphocyte stimulator pool (BLyS) that is stored in activated neutrophils: implications for inflammatory diseases. Blood 105 : 830–837. 15358625
105. Huard B, McKee T, Bosshard C, Durual S, Matthes T, et al. (2008) APRIL secreted by neutrophils binds to heparan sulfate proteoglycans to create plasma cell niches in human mucosa. J Clin Invest 118 : 2887–2895. doi: 10.1172/JCI33760 18618015
106. Rauch PJ, Chudnovskiy A, Robbins CS, Weber GF, Etzrodt M, et al. (2012) Innate response activator B cells protect against microbial sepsis. Science 335 : 597–601. doi: 10.1126/science.1215173 22245738
107. Cerutti A, Puga I, Magri G (2013) The B cell helper side of neutrophils. J Leukoc Biol 94 : 677–682. doi: 10.1189/jlb.1112596 23630389
108. Puga I, Cols M, Barra CM, He B, Cassis L, et al. (2012) B cell-helper neutrophils stimulate the diversification and production of immunoglobulin in the marginal zone of the spleen. Nat Immunol 13 : 170–180. doi: 10.1038/ni.2194 22197976
109. Nagelkerke SQ, aan de Kerk DJ, Jansen MH, van den Berg TK, Kuijpers TW (2014) Failure to detect functional neutrophil B helper cells in the human spleen. PLoS One 9: e88377. doi: 10.1371/journal.pone.0088377 24523887
110. Youn JI, Gabrilovich DI (2010) The biology of myeloid-derived suppressor cells: the blessing and the curse of morphological and functional heterogeneity. Eur J Immunol 40 : 2969–2975. doi: 10.1002/eji.201040895 21061430
111. Munder M, Mollinedo F, Calafat J, Canchado J, Gil-Lamaignere C, et al. (2005) Arginase I is constitutively expressed in human granulocytes and participates in fungicidal activity. Blood 105 : 2549–2556. 15546957
112. Kraaij MD, Savage ND, van der Kooij SW, Koekkoek K, Wang J, et al. (2010) Induction of regulatory T cells by macrophages is dependent on production of reactive oxygen species. Proc Natl Acad Sci U S A 107 : 17686–17691. doi: 10.1073/pnas.1012016107 20861446
113. Nagaraj S, Youn JI, Gabrilovich DI (2013) Reciprocal relationship between myeloid-derived suppressor cells and T cells. J Immunol 191 : 17–23. doi: 10.4049/jimmunol.1300654 23794702
114. Pillay J, Kamp VM, van Hoffen E, Visser T, Tak T, et al. (2012) A subset of neutrophils in human systemic inflammation inhibits T cell responses through Mac-1. J Clin Invest 122 : 327–336. doi: 10.1172/JCI57990 22156198
115. Dale DC, Person RE, Bolyard AA, Aprikyan AG, Bos C, et al. (2000) Mutations in the gene encoding neutrophil elastase in congenital and cyclic neutropenia. Blood 96 : 2317–2322. 11001877
116. Köllner I, Sodeik B, Schreek S, Heyn H, von Neuhoff N, et al. (2006) Mutations in neutrophil elastase causing congenital neutropenia lead to cytoplasmic protein accumulation and induction of the unfolded protein response. Blood 108 : 493–500. 16551967
117. Grenda DS, Murakami M, Ghatak J, Xia J, Boxer LA, et al. (2007) Mutations of the ELA2 gene found in patients with severe congenital neutropenia induce the unfolded protein response and cellular apoptosis. Blood 110 : 4179–4187. 17761833
118. Carlsson G, Aprikyan AA, Tehranchi R, Dale DC, Porwit A, et al. (2004) Kostmann syndrome: severe congenital neutropenia associated with defective expression of Bcl-2, constitutive mitochondrial release of cytochrome c, and excessive apoptosis of myeloid progenitor cells. Blood 103 : 3355–3361. 14764541
119. Klein C, Grudzien M, Appaswamy G, Germeshausen M, Sandrock I, et al. (2007) HAX1 deficiency causes autosomal recessive severe congenital neutropenia (Kostmann disease). Nat Genet 39 : 86–92. 17187068
120. Skokowa J, Cario G, Uenalan M, Schambach A, Germeshausen M, et al. (2006) LEF-1 is crucial for neutrophil granulocytopoiesis and its expression is severely reduced in congenital neutropenia. Nat Med 12 : 1191–1197. 17063141
121. Skokowa J, Klimiankou M, Klimenkova O, Lan D, Gupta K, et al. (2012) Interactions among HCLS1, HAX1 and LEF-1 proteins are essential for G-CSF-triggered granulopoiesis. Nat Med 18 : 1550–1559. doi: 10.1038/nm.2958 23001182
122. Person RE, Li FQ, Duan ZJ, Benson KF, Wechsler J, et al. (2003) Mutations in proto-oncogene GFI1 cause human neutropenia and target ELA2. Nature Genetics 34 : 308–312. 12778173
123. Devriendt K, Kim AS, Mathijs G, Frints SG, Schwartz M, et al. (2001) Constitutively activating mutation in WASP causes X-linked severe congenital neutropenia. Nat Genet 27 : 313–317. 11242115
124. Boztug K, Appaswamy G, Ashikov A, Schaffer AA, Salzer U, et al. (2009) A syndrome with congenital neutropenia and mutations in G6PC3. N Engl J Med 360 : 32–43. doi: 10.1056/NEJMoa0805051 19118303
125. Bohn G, Allroth A, Brandes G, Thiel J, Glocker E, et al. (2007) A novel human primary immunodeficiency syndrome caused by deficiency of the endosomal adaptor protein p14. Nat Med 13 : 38–45. 17195838
126. Vilboux T, Lev A, Malicdan MC, Simon AJ, Jarvinen P, et al. (2013) A congenital neutrophil defect syndrome associated with mutations in VPS45. N Engl J Med 369 : 54–65. doi: 10.1056/NEJMoa1301296 23738510
127. Winkelstein JA, Marino MC, Johnston RB Jr., Boyle J, Curnutte J, et al. (2000) Chronic granulomatous disease. Report on a national registry of 368 patients. Medicine (Baltimore) 79 : 155–169. 10844935
128. van den Berg JM, van Koppen E, Ahlin A, Belohradsky BH, Bernatowska E, et al. (2009) Chronic granulomatous disease: the European experience. PLoS One 4: e5234. doi: 10.1371/journal.pone.0005234 19381301
129. Köker MY, Camcioglu Y, van Leeuwen K, Kilic SS, Barlan I, et al. (2013) Clinical, functional, and genetic characterization of chronic granulomatous disease in 89 Turkish patients. J Allergy Clin Immunol.
130. Roos D, Kuhns DB, Maddalena A, Bustamante J, Kannengiesser C, et al. (2010) Hematologically important mutations: the autosomal recessive forms of chronic granulomatous disease (second update). Blood Cells Mol Dis 44 : 291–299. doi: 10.1016/j.bcmd.2010.01.009 20167518
131. Roos D, Kuhns DB, Maddalena A, Roesler J, Lopez JA, et al. (2010) Hematologically important mutations: X-linked chronic granulomatous disease (third update). Blood Cells Mol Dis 45 : 246–265. doi: 10.1016/j.bcmd.2010.07.012 20729109
132. Anderson-Cohen M, Holland SM, Kuhns DB, Fleisher TA, Ding L, et al. (2003) Severe phenotype of chronic granulomatous disease presenting in a female with a de novo mutation in gp91-phox and a non familial, extremely skewed X chromosome inactivation. Clin Immunol 109 : 308–317. 14697745
133. de Luca A, Smeekens SP, Casagrande A, Iannitti R, Conway KL, et al. (2014) IL-1 receptor blockade restores autophagy and reduces inflammation in chronic granulomatous disease in mice and in humans. Proc Natl Acad Sci U S A 111 : 3526–3531. doi: 10.1073/pnas.1322831111 24550444
134. Segal BH, Han W, Bushey JJ, Joo M, Bhatti Z, et al. (2010) NADPH oxidase limits innate immune responses in the lungs in mice. PLoS One 5: e9631. doi: 10.1371/journal.pone.0009631 20300512
135. Marciano BE, Rosenzweig SD, Kleiner DE, Anderson VL, Darnell DN, et al. (2004) Gastrointestinal involvement in chronic granulomatous disease. Pediatrics 114 : 462–468. 15286231
136. Jones LB, McGrogan P, Flood TJ, Gennery AR, Morton L, et al. (2008) Special article: chronic granulomatous disease in the United Kingdom and Ireland: a comprehensive national patient-based registry. Clin Exp Immunol 152 : 211–218. doi: 10.1111/j.1365-2249.2008.03644.x 18410635
137. Campbell EL, Bruyninckx WJ, Kelly CJ, Glover LE, McNamee EN, et al. (2014) Transmigrating neutrophils shape the mucosal microenvironment through localized oxygen depletion to influence resolution of inflammation. Immunity 40 : 66–77. doi: 10.1016/j.immuni.2013.11.020 24412613
138. Harris ES, Weyrich AS, Zimmerman GA (2013) Lessons from rare maladies: leukocyte adhesion deficiency syndromes. Curr Opin Hematol 20 : 16–25. doi: 10.1097/MOH.0b013e32835a0091 23207660
139. Sanchez-Madrid F, Nagy JA, Robbins E, Simon P, Springer TA (1983) A human leukocyte differentiation antigen family with distinct alpha-subunits and a common beta-subunit: the lymphocyte function-associated antigen (LFA-1), the C3bi complement receptor (OKM1/Mac-1), and the p150,95 molecule. J Exp Med 158 : 1785–1803. 6196430
140. Anderson DC, Schmalstieg FC, Arnaout MA, Kohl S, Tosi MF, et al. (1984) Abnormalities of polymorphonuclear leukocyte function associated with a heritable deficiency of high molecular weight surface glycoproteins (GP138): common relationship to diminished cell adherence. J Clin Invest 74 : 536–551. 6746906
141. Springer TA, Thompson WS, Miller LJ, Schmalstieg FC, Anderson DC (1984) Inherited deficiency of the Mac-1, LFA-1, p150,95 glycoprotein family and its molecular basis. J Exp Med 160 : 1901–1918. 6096477
142. Roos D, Meischl C, de Boer M, Simsek S, Weening RS, et al. (2002) Genetic analysis of patients with leukocyte adhesion deficiency: genomic sequencing reveals otherwise undetectable mutations. Exp Hematol 30 : 252–261. 11882363
143. van de Vijver E, Maddalena A, Sanal O, Holland SM, Uzel G, et al. (2012) Hematologically important mutations: leukocyte adhesion deficiency (first update). Blood Cells Mol Dis 48 : 53–61. doi: 10.1016/j.bcmd.2011.10.004 22134107
144. Mathew EC, Shaw JM, Bonilla FA, Law SK, Wright DA (2000) A novel point mutation in CD18 causing the expression of dysfunctional CD11/CD18 leucocyte integrins in a patient with leucocyte adhesion deficiency (LAD). Clin Exp Immunol 121 : 133–138. 10886250
145. Etzioni A, Frydman M, Pollack S, Avidor I, Phillips ML, et al. (1992) Brief report: recurrent severe infections caused by a novel leukocyte adhesion deficiency. N Engl J Med 327 : 1789–1792. 1279426
146. Lühn K, Wild MK, Eckhardt M, Gerardy-Schahn R, Vestweber D (2001) The gene defective in leukocyte adhesion deficiency II encodes a putative GDP-fucose transporter. Nat Genet 28 : 69–72. 11326279
147. Lubke T, Marquardt T, Etzioni A, Hartmann E, von Figura K, et al. (2001) Complementation cloning identifies CDG-IIc, a new type of congenital disorders of glycosylation, as a GDP-fucose transporter deficiency. Nat Genet 28 : 73–76. 11326280
148. Helmus Y, Denecke J, Yakubenia S, Robinson P, Luhn K, et al. (2006) Leukocyte adhesion deficiency II patients with a dual defect of the GDP-fucose transporter. Blood 107 : 3959–3966. 16455955
149. Etzioni A, Sturla L, Antonellis A, Green ED, Gershoni-Baruch R, et al. (2002) Leukocyte adhesion deficiency (LAD) type II/carbohydrate deficient glycoprotein (CDG) IIc founder effect and genotype/phenotype correlation. Am J Med Genet 110 : 131–135. 12116250
150. Kuijpers TW, Van Lier RA, Hamann D, de Boer M, Thung LY, et al. (1997) Leukocyte adhesion deficiency type 1 (LAD-1)/variant. A novel immunodeficiency syndrome characterized by dysfunctional beta2 integrins. J Clin Invest 100 : 1725–1733. 9312170
151. Kuijpers TW, van Bruggen R, Kamerbeek N, Tool AT, Hicsonmez G, et al. (2007) Natural history and early diagnosis of LAD-1/variant syndrome. Blood 109 : 3529–3537. 17185466
152. Kuijpers TW, van de Vijver E, Weterman MA, de Boer M, Tool AT, et al. (2009) LAD-1/variant syndrome is caused by mutations in FERMT3. Blood 113 : 4740–4746. doi: 10.1182/blood-2008-10-182154 19064721
153. Harris ES, Smith TL, Springett GM, Weyrich AS, Zimmerman GA (2012) Leukocyte adhesion deficiency-I variant syndrome (LAD-Iv, LAD-III): molecular characterization of the defect in an index family. Am J Hematol 87 : 311–313. doi: 10.1002/ajh.22253 22139635
154. van de Vijver E, De Cuyper IM, Gerrits AJ, Verhoeven AJ, Seeger K, et al. (2012) Defects in Glanzmann thrombasthenia and LAD-III (LAD-1/v) syndrome: the role of integrin beta1 and beta3 in platelet adhesion to collagen. Blood 119 : 583–586. doi: 10.1182/blood-2011-02-337188 22065596
155. Ambruso DR, Knall C, Abell AN, Panepinto J, Kurkchubasche A, et al. (2000) Human neutrophil immunodeficiency syndrome is associated with an inhibitory Rac2 mutation. Proc Natl Acad Sci U S A 97 : 4654–4659. 10758162
156. Accetta D, Syverson G, Bonacci B, Reddy S, Bengtson C, et al. (2011) Human phagocyte defect caused by a Rac2 mutation detected by means of neonatal screening for T-cell lymphopenia. Journal of Allergy and Clinical Immunology 127 : 535–538.e532. doi: 10.1016/j.jaci.2010.10.013 21167572
157. Moutsopoulos NM, Konkel J, Sarmadi M, Eskan MA, Wild T, et al. (2014) Defective neutrophil recruitment in leukocyte adhesion deficiency type I disease causes local IL-17-driven inflammatory bone loss. Sci Transl Med 6 : 229ra240.
158. Gulino AV, Moratto D, Sozzani S, Cavadini P, Otero K, et al. (2004) Altered leukocyte response to CXCL12 in patients with warts hypogammaglobulinemia, infections, myelokathexis (WHIM) syndrome. Blood 104 : 444–452. 15026312
159. Hayashi F, Means TK, Luster AD (2003) Toll-like receptors stimulate human neutrophil function. Blood 102 : 2660–2669. 12829592
160. Bouma G, Doffinger R, Patel SY, Peskett E, Sinclair JC, et al. (2009) Impaired neutrophil migration and phagocytosis in IRAK-4 deficiency. Br J Haematol 147 : 153–156. doi: 10.1111/j.1365-2141.2009.07838.x 19663824
161. van Bruggen R, Drewniak A, Tool AT, Jansen M, van Houdt M, et al. (2010) Toll-like receptor responses in IRAK-4-deficient neutrophils. J Innate Immun 2 : 280–287. doi: 10.1159/000268288 20375545
162. Picard C, Puel A, Bonnet M, Ku CL, Bustamante J, et al. (2003) Pyogenic bacterial infections in humans with IRAK-4 deficiency. Science 299 : 2076–2079. 12637671
163. Enders A, Pannicke U, Berner R, Henneke P, Radlinger K, et al. (2004) Two siblings with lethal pneumococcal meningitis in a family with a mutation in Interleukin-1 receptor-associated kinase 4. J Pediatr 145 : 698–700. 15520784
164. von Bernuth H, Picard C, Jin Z, Pankla R, Xiao H, et al. (2008) Pyogenic bacterial infections in humans with MyD88 deficiency. Science 321 : 691–696. doi: 10.1126/science.1158298 18669862
165. Picard C, von Bernuth H, Ghandil P, Chrabieh M, Levy O, et al. (2010) Clinical features and outcome of patients with IRAK-4 and MyD88 deficiency. Medicine (Baltimore) 89 : 403–425.
166. Picard C, Casanova JL, Puel A (2011) Infectious diseases in patients with IRAK-4, MyD88, NEMO, or IkappaBalpha deficiency. Clin Microbiol Rev 24 : 490–497. doi: 10.1128/CMR.00001-11 21734245
167. Glocker EO, Hennigs A, Nabavi M, Schaffer AA, Woellner C, et al. (2009) A homozygous CARD9 mutation in a family with susceptibility to fungal infections. N Engl J Med 361 : 1727–1735. doi: 10.1056/NEJMoa0810719 19864672
168. Drewniak A, Gazendam RP, Tool AT, van Houdt M, Jansen MH, et al. (2013) Invasive fungal infection and impaired neutrophil killing in human CARD9 deficiency. Blood 121 : 2385–2392. doi: 10.1182/blood-2012-08-450551 23335372
169. Gombart AF, Shiohara M, Kwok SH, Agematsu K, Komiyama A, et al. (2001) Neutrophil-specific granule deficiency: homozygous recessive inheritance of a frameshift mutation in the gene encoding transcription factor CCAAT/enhancer binding protein—epsilon. Blood 97 : 2561–2567. 11313242
170. Klebanoff SJ, Kettle AJ, Rosen H, Winterbourn CC, Nauseef WM (2013) Myeloperoxidase: a front-line defender against phagocytosed microorganisms. J Leukoc Biol 93 : 185–198. doi: 10.1189/jlb.0712349 23066164
171. Moraes TJ, Zurawska JH, Downey GP (2006) Neutrophil granule contents in the pathogenesis of lung injury. Curr Opin Hematol 13 : 21–27. 16319683
172. Grommes J, Soehnlein O (2011) Contribution of neutrophils to acute lung injury. Mol Med 17 : 293–307. doi: 10.2119/molmed.2010.00138 21046059
173. Kolaczkowska E, Kubes P (2013) Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol 13 : 159–175. doi: 10.1038/nri3399 23435331
174. Williams AE, Chambers RC (2014) The mercurial nature of neutrophils: still an enigma in ARDS? Am J Physiol Lung Cell Mol Physiol 306: L217–230. doi: 10.1152/ajplung.00311.2013 24318116
175. McDonald B, Pittman K, Menezes GB, Hirota SA, Slaba I, et al. (2010) Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science 330 : 362–366. doi: 10.1126/science.1195491 20947763
176. Serhan CN (2014) Pro-resolving lipid mediators are leads for resolution physiology. Nature 510 : 92–101. doi: 10.1038/nature13479 24899309
177. Condliffe AM, Kitchen E, Chilvers ER (1998) Neutrophil priming: pathophysiological consequences and underlying mechanisms. Clin Sci (Lond) 94 : 461–471. 9682667
178. Summers C, Chilvers ER, Peters AM (2014) Mathematical modeling supports the presence of neutrophil depriming in vivo. Physiol Rep 2: e00241. doi: 10.1002/phy2.241 24760504
179. Singh A, Zarember KA, Kuhns DB, Gallin JI (2009) Impaired priming and activation of the neutrophil NADPH oxidase in patients with IRAK4 or NEMO deficiency. J Immunol 182 : 6410–6417. doi: 10.4049/jimmunol.0802512 19414794
180. McMillan SJ, Sharma RS, McKenzie EJ, Richards HE, Zhang J, et al. (2013) Siglec-E is a negative regulator of acute pulmonary neutrophil inflammation and suppresses CD11b beta2-integrin-dependent signaling. Blood 121 : 2084–2094. doi: 10.1182/blood-2012-08-449983 23315163
181. Davidson BA, Vethanayagam RR, Grimm MJ, Mullan BA, Raghavendran K, et al. (2013) NADPH oxidase and Nrf2 regulate gastric aspiration-induced inflammation and acute lung injury. J Immunol 190 : 1714–1724. doi: 10.4049/jimmunol.1202410 23296708
182. Schmidt EP, Yang Y, Janssen WJ, Gandjeva A, Perez MJ, et al. (2012) The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nat Med 18 : 1217–1223. doi: 10.1038/nm.2843 22820644
183. Achouiti A, Vogl T, Urban CF, Rohm M, Hommes TJ, et al. (2012) Myeloid-related protein-14 contributes to protective immunity in gram-negative pneumonia derived sepsis. PLoS Pathog 8: e1002987. doi: 10.1371/journal.ppat.1002987 23133376
184. Weckbach LT, Gola A, Winkelmann M, Jakob SM, Groesser L, et al. (2014) The cytokine midkine supports neutrophil trafficking during acute inflammation by promoting adhesion via beta2 integrins (CD11/CD18). Blood 123 : 1887–1896. doi: 10.1182/blood-2013-06-510875 24458438
185. Jakob SM, Pick R, Brechtefeld D, Nussbaum C, Kiefer F, et al. (2013) Hematopoietic progenitor kinase 1 (HPK1) is required for LFA-1-mediated neutrophil recruitment during the acute inflammatory response. Blood 121 : 4184–4194. doi: 10.1182/blood-2012-08-451385 23460610
186. Sutherland TE, Logan N, Ruckerl D, Humbles AA, Allan SM, et al. (2014) Chitinase-like proteins promote IL-17-mediated neutrophilia in a tradeoff between nematode killing and host damage. Nat Immunol.
187. Christoffersson G, Vagesjo E, Vandooren J, Liden M, Massena S, et al. (2012) VEGF-A recruits a proangiogenic MMP-9-delivering neutrophil subset that induces angiogenesis in transplanted hypoxic tissue. Blood 120 : 4653–4662. doi: 10.1182/blood-2012-04-421040 22966168
188. Weathington NM, van Houwelingen AH, Noerager BD, Jackson PL, Kraneveld AD, et al. (2006) A novel peptide CXCR ligand derived from extracellular matrix degradation during airway inflammation. Nat Med 12 : 317–323. 16474398
189. Snelgrove RJ, Jackson PL, Hardison MT, Noerager BD, Kinloch A, et al. (2010) A critical role for LTA4H in limiting chronic pulmonary neutrophilic inflammation. Science 330 : 90–94. doi: 10.1126/science.1190594 20813919
190. Scaffidi P, Misteli T, Bianchi ME (2002) Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 418 : 191–195. 12110890
191. Schiraldi M, Raucci A, Munoz LM, Livoti E, Celona B, et al. (2012) HMGB1 promotes recruitment of inflammatory cells to damaged tissues by forming a complex with CXCL12 and signaling via CXCR4. J Exp Med 209 : 551–563. doi: 10.1084/jem.20111739 22370717
192. Brandau S, Dumitru CA, Lang S (2013) Protumor and antitumor functions of neutrophil granulocytes. Semin Immunopathol 35 : 163–176. doi: 10.1007/s00281-012-0344-6 23007469
193. Bauer S, Abdgawad M, Gunnarsson L, Segelmark M, Tapper H, et al. (2007) Proteinase 3 and CD177 are expressed on the plasma membrane of the same subset of neutrophils. J Leukoc Biol 81 : 458–464. 17077162
194. Hartl D, Krauss-Etschmann S, Koller B, Hordijk PL, Kuijpers TW, et al. (2008) Infiltrated neutrophils acquire novel chemokine receptor expression and chemokine responsiveness in chronic inflammatory lung diseases. J Immunol 181 : 8053–8067. 19017998
195. Tirouvanziam R, Gernez Y, Conrad CK, Moss RB, Schrijver I, et al. (2008) Profound functional and signaling changes in viable inflammatory neutrophils homing to cystic fibrosis airways. Proc Natl Acad Sci U S A 105 : 4335–4339. doi: 10.1073/pnas.0712386105 18334635
196. Sigua JA, Buelow B, Cheung DS, Buell E, Hunter D, et al. (2014) CD49d-expressing neutrophils differentiate atopic from nonatopic individuals. J Allergy Clin Immunol 133 : 901–904 e905. doi: 10.1016/j.jaci.2013.09.035 24360325
197. Puellmann K, Kaminski WE, Vogel M, Nebe CT, Schroeder J, et al. (2006) A variable immunoreceptor in a subpopulation of human neutrophils. Proc Natl Acad Sci U S A 103 : 14441–14446. 16983085
198. Beyrau M, Bodkin JV, Nourshargh S (2012) Neutrophil heterogeneity in health and disease: a revitalized avenue in inflammation and immunity. Open Biol 2 : 120134. doi: 10.1098/rsob.120134 23226600
199. Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, et al. (2009) Polarization of tumor-associated neutrophil phenotype by TGF-beta: "N1" versus "N2" TAN. Cancer Cell 16 : 183–194. doi: 10.1016/j.ccr.2009.06.017 19732719
200. Tsuda Y, Takahashi H, Kobayashi M, Hanafusa T, Herndon DN, et al. (2004) Three different neutrophil subsets exhibited in mice with different susceptibilities to infection by methicillin-resistant Staphylococcus aureus. Immunity 21 : 215–226. 15308102
201. Matsushima H, Geng S, Lu R, Okamoto T, Yao Y, et al. (2013) Neutrophil differentiation into a unique hybrid population exhibiting dual phenotype and functionality of neutrophils and dendritic cells. Blood 121 : 1677–1689. doi: 10.1182/blood-2012-07-445189 23305731
202. Geng S, Matsushima H, Okamoto T, Yao Y, Lu R, et al. (2013) Emergence, origin, and function of neutrophil-dendritic cell hybrids in experimentally induced inflammatory lesions in mice. Blood 121 : 1690–1700. doi: 10.1182/blood-2012-07-445197 23305733
203. Iking-Konert C, Ostendorf B, Sander O, Jost M, Wagner C, et al. (2005) Transdifferentiation of polymorphonuclear neutrophils to dendritic-like cells at the site of inflammation in rheumatoid arthritis: evidence for activation by T cells. Annals of the Rheumatic Diseases 64 : 1436–1442. 15778239
204. Radsak M, Iking-Konert C, Stegmaier S, Andrassy K, Hänsch GM (2000) Polymorphonuclear neutrophils as accessory cells for T-cell activation: major histocompatibility complex class II restricted antigen-dependent induction of T-cell proliferation. Immunology 101 : 521–530. 11122456
205. Pliyev BK, Sumarokov AB, Buriachkovskaia LI, Menshikov M (2011) Extracellular acidosis promotes neutrophil transdifferentiation to MHC class II-expressing cells. Cell Immunol 271 : 214–218. doi: 10.1016/j.cellimm.2011.08.020 21924707
206. Dyugovskaya L, Berger S, Polyakov A, Lavie L (2014) The development of giant phagocytes in long-term neutrophil cultures. J Leukoc Biol 96 : 511–521. doi: 10.1189/jlb.0813437 24577569
207. Köffel R, Meshcheryakova A, Warszawska J, Hennig A, Wagner K, et al. (2014) Monocytic cell differentiation from band-stage neutrophils under inflammatory conditions via MKK6 activation. Blood 124 : 2713–2724. doi: 10.1182/blood-2014-07-588178 25214442
208. Makam M, Diaz D, Laval J, Gernez Y, Conrad CK, et al. (2009) Activation of critical, host-induced, metabolic and stress pathways marks neutrophil entry into cystic fibrosis lungs. Proc Natl Acad Sci U S A 106 : 5779–5783. doi: 10.1073/pnas.0813410106 19293384
209. Laval J, Touhami J, Herzenberg LA, Conrad C, Taylor N, et al. (2013) Metabolic adaptation of neutrophils in cystic fibrosis airways involves distinct shifts in nutrient transporter expression. J Immunol 190 : 6043–6050. doi: 10.4049/jimmunol.1201755 23690474
210. Youn JI, Kumar V, Collazo M, Nefedova Y, Condamine T, et al. (2013) Epigenetic silencing of retinoblastoma gene regulates pathologic differentiation of myeloid cells in cancer. Nat Immunol 14 : 211–220. doi: 10.1038/ni.2526 23354483
211. Moss RB, Mistry SJ, Konstan MW, Pilewski JM, Kerem E, et al. (2013) Safety and early treatment effects of the CXCR2 antagonist SB-656933 in patients with cystic fibrosis. J Cyst Fibros 12 : 241–248. doi: 10.1016/j.jcf.2012.08.016 22995323
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek A Phospholipase Is Involved in Disruption of the Liver Stage Parasitophorous Vacuole MembraneČlánek Host ESCRT Proteins Are Required for Bromovirus RNA Replication Compartment Assembly and FunctionČlánek Enhanced CD8 T Cell Responses through GITR-Mediated Costimulation Resolve Chronic Viral Infection
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 3- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- To Be or Not IIb: A Multi-Step Process for Epstein-Barr Virus Latency Establishment and Consequences for B Cell Tumorigenesis
- Is Antigenic Sin Always “Original?” Re-examining the Evidence Regarding Circulation of a Human H1 Influenza Virus Immediately Prior to the 1918 Spanish Flu
- The Great Escape: Pathogen Versus Host
- Coping with Stress and the Emergence of Multidrug Resistance in Fungi
- Catch Me If You Can: The Link between Autophagy and Viruses
- Bacterial Immune Evasion through Manipulation of Host Inhibitory Immune Signaling
- Evidence for Ubiquitin-Regulated Nuclear and Subnuclear Trafficking among Matrix Proteins
- BILBO1 Is a Scaffold Protein of the Flagellar Pocket Collar in the Pathogen
- Production of Anti-LPS IgM by B1a B Cells Depends on IL-1β and Is Protective against Lung Infection with LVS
- Virulence Regulation with Venus Flytrap Domains: Structure and Function of the Periplasmic Moiety of the Sensor-Kinase BvgS
- α-Hemolysin Counteracts the Anti-Virulence Innate Immune Response Triggered by the Rho GTPase Activating Toxin CNF1 during Bacteremia
- Induction of Interferon-Stimulated Genes by IRF3 Promotes Replication of
- Intracellular Growth Is Dependent on Tyrosine Catabolism in the Dimorphic Fungal Pathogen
- HCV Induces the Expression of Rubicon and UVRAG to Temporally Regulate the Maturation of Autophagosomes and Viral Replication
- Spatiotemporal Analysis of Hepatitis C Virus Infection
- Subgingival Microbial Communities in Leukocyte Adhesion Deficiency and Their Relationship with Local Immunopathology
- Interaction between the Type III Effector VopO and GEF-H1 Activates the RhoA-ROCK Pathway
- Attenuation of Tick-Borne Encephalitis Virus Using Large-Scale Random Codon Re-encoding
- Establishment of HSV1 Latency in Immunodeficient Mice Facilitates Efficient Reactivation
- XRN1 Stalling in the 5’ UTR of Hepatitis C Virus and Bovine Viral Diarrhea Virus Is Associated with Dysregulated Host mRNA Stability
- γδ T Cells Confer Protection against Murine Cytomegalovirus (MCMV)
- Rhadinovirus Host Entry by Co-operative Infection
- A Phospholipase Is Involved in Disruption of the Liver Stage Parasitophorous Vacuole Membrane
- Dermal Neutrophil, Macrophage and Dendritic Cell Responses to Transmitted by Fleas
- Elucidation of Sigma Factor-Associated Networks in Reveals a Modular Architecture with Limited and Function-Specific Crosstalk
- A Conserved NS3 Surface Patch Orchestrates NS2 Protease Stimulation, NS5A Hyperphosphorylation and HCV Genome Replication
- Host ESCRT Proteins Are Required for Bromovirus RNA Replication Compartment Assembly and Function
- Disruption of IL-21 Signaling Affects T Cell-B Cell Interactions and Abrogates Protective Humoral Immunity to Malaria
- Compartmentalized Replication of R5 T Cell-Tropic HIV-1 in the Central Nervous System Early in the Course of Infection
- Diminished Reovirus Capsid Stability Alters Disease Pathogenesis and Littermate Transmission
- Characterization of CD8 T Cell Differentiation following SIVΔnef Vaccination by Transcription Factor Expression Profiling
- Visualization of HIV-1 Interactions with Penile and Foreskin Epithelia: Clues for Female-to-Male HIV Transmission
- Sensing Cytosolic RpsL by Macrophages Induces Lysosomal Cell Death and Termination of Bacterial Infection
- PKCη/Rdx-driven Phosphorylation of PDK1: A Novel Mechanism Promoting Cancer Cell Survival and Permissiveness for Parvovirus-induced Lysis
- Metalloprotease NleC Suppresses Host NF-κB/Inflammatory Responses by Cleaving p65 and Interfering with the p65/RPS3 Interaction
- Immune Antibodies and Helminth Products Drive CXCR2-Dependent Macrophage-Myofibroblast Crosstalk to Promote Intestinal Repair
- Adenovirus Entry From the Apical Surface of Polarized Epithelia Is Facilitated by the Host Innate Immune Response
- The RNA Template Channel of the RNA-Dependent RNA Polymerase as a Target for Development of Antiviral Therapy of Multiple Genera within a Virus Family
- Neutrophils: Between Host Defence, Immune Modulation, and Tissue Injury
- CD169-Mediated Trafficking of HIV to Plasma Membrane Invaginations in Dendritic Cells Attenuates Efficacy of Anti-gp120 Broadly Neutralizing Antibodies
- Japanese Encephalitis Virus Nonstructural Protein NS5 Interacts with Mitochondrial Trifunctional Protein and Impairs Fatty Acid β-Oxidation
- Yip1A, a Novel Host Factor for the Activation of the IRE1 Pathway of the Unfolded Protein Response during Infection
- TRIM26 Negatively Regulates Interferon-β Production and Antiviral Response through Polyubiquitination and Degradation of Nuclear IRF3
- Parallel Epigenomic and Transcriptomic Responses to Viral Infection in Honey Bees ()
- A Crystal Structure of the Dengue Virus NS5 Protein Reveals a Novel Inter-domain Interface Essential for Protein Flexibility and Virus Replication
- Enhanced CD8 T Cell Responses through GITR-Mediated Costimulation Resolve Chronic Viral Infection
- Exome and Transcriptome Sequencing of Identifies a Locus That Confers Resistance to and Alters the Immune Response
- The Role of Misshapen NCK-related kinase (MINK), a Novel Ste20 Family Kinase, in the IRES-Mediated Protein Translation of Human Enterovirus 71
- Chitin Recognition via Chitotriosidase Promotes Pathologic Type-2 Helper T Cell Responses to Cryptococcal Infection
- Activates Both IL-1β and IL-1 Receptor Antagonist to Modulate Lung Inflammation during Pneumonic Plague
- Persistence of Transmitted HIV-1 Drug Resistance Mutations Associated with Fitness Costs and Viral Genetic Backgrounds
- An 18 kDa Scaffold Protein Is Critical for Biofilm Formation
- Early Virological and Immunological Events in Asymptomatic Epstein-Barr Virus Infection in African Children
- Human CD8 T-cells Recognizing Peptides from () Presented by HLA-E Have an Unorthodox Th2-like, Multifunctional, Inhibitory Phenotype and Represent a Novel Human T-cell Subset
- Decreased HIV-Specific T-Regulatory Responses Are Associated with Effective DC-Vaccine Induced Immunity
- RSV Vaccine-Enhanced Disease Is Orchestrated by the Combined Actions of Distinct CD4 T Cell Subsets
- Concerted Activity of IgG1 Antibodies and IL-4/IL-25-Dependent Effector Cells Trap Helminth Larvae in the Tissues following Vaccination with Defined Secreted Antigens, Providing Sterile Immunity to Challenge Infection
- Structure of the Low pH Conformation of Chandipura Virus G Reveals Important Features in the Evolution of the Vesiculovirus Glycoprotein
- PPM1A Regulates Antiviral Signaling by Antagonizing TBK1-Mediated STING Phosphorylation and Aggregation
- Lipidomic Analysis Links Mycobactin Synthase K to Iron Uptake and Virulence in .
- Roles and Programming of Arabidopsis ARGONAUTE Proteins during Infection
- Impact of Infection on Host Macrophage Nuclear Physiology and Nucleopore Complex Integrity
- The Impact of Host Diet on Titer in
- Antimicrobial-Induced DNA Damage and Genomic Instability in Microbial Pathogens
- Herpesviral G Protein-Coupled Receptors Activate NFAT to Induce Tumor Formation via Inhibiting the SERCA Calcium ATPase
- The Causes and Consequences of Changes in Virulence following Pathogen Host Shifts
- Small GTPase Rab21 Mediates Fibronectin Induced Actin Reorganization in : Implications in Pathogen Invasion
- Positive Role of Promyelocytic Leukemia Protein in Type I Interferon Response and Its Regulation by Human Cytomegalovirus
- NEDDylation Is Essential for Kaposi’s Sarcoma-Associated Herpesvirus Latency and Lytic Reactivation and Represents a Novel Anti-KSHV Target
- β-HPV 5 and 8 E6 Disrupt Homology Dependent Double Strand Break Repair by Attenuating BRCA1 and BRCA2 Expression and Foci Formation
- An O Antigen Capsule Modulates Bacterial Pathogenesis in
- Variable Processing and Cross-presentation of HIV by Dendritic Cells and Macrophages Shapes CTL Immunodominance and Immune Escape
- Probing the Metabolic Network in Bloodstream-Form Using Untargeted Metabolomics with Stable Isotope Labelled Glucose
- Adhesive Fiber Stratification in Uropathogenic Biofilms Unveils Oxygen-Mediated Control of Type 1 Pili
- Vaccinia Virus Protein Complex F12/E2 Interacts with Kinesin Light Chain Isoform 2 to Engage the Kinesin-1 Motor Complex
- Modulates Host Macrophage Mitochondrial Metabolism by Hijacking the SIRT1-AMPK Axis
- Human T-Cell Leukemia Virus Type 1 (HTLV-1) Tax Requires CADM1/TSLC1 for Inactivation of the NF-κB Inhibitor A20 and Constitutive NF-κB Signaling
- Suppression of RNAi by dsRNA-Degrading RNaseIII Enzymes of Viruses in Animals and Plants
- Spatiotemporal Regulation of a T4SS Substrate by the Metaeffector SidJ
- Antigenic Properties of the Human Immunodeficiency Virus Envelope Glycoprotein Gp120 on Virions Bound to Target Cells
- Dependence of Intracellular and Exosomal microRNAs on Viral Oncogene Expression in HPV-positive Tumor Cells
- Identification of a Peptide-Pheromone that Enhances Escape from Host Cell Vacuoles
- Impaired Systemic Tetrahydrobiopterin Bioavailability and Increased Dihydrobiopterin in Adult Falciparum Malaria: Association with Disease Severity, Impaired Microvascular Function and Increased Endothelial Activation
- Transgenic Expression of the Dicotyledonous Pattern Recognition Receptor EFR in Rice Leads to Ligand-Dependent Activation of Defense Responses
- Comprehensive Antigenic Map of a Cleaved Soluble HIV-1 Envelope Trimer
- Low Doses of Imatinib Induce Myelopoiesis and Enhance Host Anti-microbial Immunity
- Impaired Systemic Tetrahydrobiopterin Bioavailability and Increased Oxidized Biopterins in Pediatric Falciparum Malaria: Association with Disease Severity
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Bacterial Immune Evasion through Manipulation of Host Inhibitory Immune Signaling
- BILBO1 Is a Scaffold Protein of the Flagellar Pocket Collar in the Pathogen
- Antimicrobial-Induced DNA Damage and Genomic Instability in Microbial Pathogens
- Attenuation of Tick-Borne Encephalitis Virus Using Large-Scale Random Codon Re-encoding
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



