-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaThe Great Escape: Pathogen Versus Host
article has not abstract
Published in the journal: . PLoS Pathog 11(3): e32767. doi:10.1371/journal.ppat.1004661
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1004661Summary
article has not abstract
Introduction
When bodily surface barriers have been breached, invading microorganisms are confronted by the innate immune system [1]. The first step in mounting a protective response is the rapid activation of an acute inflammatory response characterized by the migration and accumulation of immune cells at the site of invasion. As a primary defense against microbial infections, professional phagocytic cells such as macrophages will attempt to engulf and dispose of the invading microorganisms and their products. The recognition by effector cells of the innate immune system initiates signaling cascades, resulting in phagocytosis, secretion of microbicidal compounds and production of proinflammatory mediators. These early events culminate in the activation of adaptive immune responses; therefore, if launched early and effectively, innate immune responses limit the establishment of infectious foci and curb the severity of infections. However, it becomes more and more evident that microbial pathogens have developed very efficient strategies to circumvent and misguide host defenses, and therefore, their presence in the host results either in their elimination or in infection. Because of the critical role the innate immune system has in controlling microbial burden during the early stages of infection, the mechanisms employed by invading pathogens to thwart host immune defenses have attracted increasing interest. Here we synopsize some of the strategies exploited by two ubiquitous yet important human pathogens, the fungal species Candida albicans and the bacterial species Staphylococcus aureus [2,3]. In addition to possessing an array of virulence factors, these diverse species share many pathogenic characteristics, including the ability to form biofilms on host and abiotic surfaces, rapid development of antimicrobial resistance, and the ability to alter their transcriptome in response to stresses inflicted upon them by host immune cells. Importantly, although C. albicans and S. aureus are commensal species commonly colonizing various niches in the human host, they are the most frequent combination of organisms isolated from polymicrobial infections [4].
Staphylococcus aureus: A Resourceful Bacterial Species
Staphylococcus aureus is a precarious microbial species carried by about 30% of the population and has been implicated in a variety of diseases, ranging from minor skin infections to serious invasive diseases [2]. When the outer physical barriers of the body, comprising skin and mucous surfaces, have been breached by S. aureus, the organism is confronted by the host's immune system, both innate and acquired. S. aureus infection of the skin stimulates a strong inflammatory response, involving the migration of neutrophils and macrophages to the site of infection. However, multiple strategies have made S. aureus exceptionally successful in subverting its human host, thereby promoting its spread [5]. Among the numerous immune evasion mechanisms deployed by S. aureus is secretion of proteins that inhibit opsonization, complement activation and chemotaxis, or that lyse neutrophils and neutralize antimicrobial peptides [5]. Specifically, staphyloccocal Protein A, present on the surface of S. aureus, can bind to the Fc region of host immunoglobulins, compromising the phagocytic ability of innate immune cells. In addition, S. aureus can inhibit the complement pathway by inhibiting the formation of the C1qrs complex in the classical pathway via collagen adhesion (Cna) or by degrading C3b via clumpling factor A (ClfA). Further, S. aureus is known to overproduce a subset of immunomodulatory proteins known as the staphylococcal superantigen-like proteins (Ssls), and a family of phenol-soluble modulins (PSMs) have emerged as novel toxins causing lysis of red and white blood cells [6]. Additionally, a cysteine protease, Staphopain A, was recently identified as a chemokine receptor blocker inhibiting neutrophil migration [7]. Similarly, a metalloprotease, aureolysin, was also shown to be a potent inhibitor of phagocytosis and killing of bacteria by neutrophils [8]. Importantly, S. aureus is capable of surviving in phagosomes of phagocytic cells by expressing superoxide dismutase enzymes that remove O2- and release α-hemolysin to escape into the cytoplasm where it can remain viable in vacuolar compartments [9]. Later, through the expression of α-toxin, S. aureus can lyse the macrophage plasma membrane and escape into the surrounding environment [9]. Interestingly, findings from a recent study demonstrated that S. aureus is also capable of inducing immune cell death via secretion of a series of bacterial nucleases that degrade DNA released by neutrophils to trap immobilizing pathogens. Paradoxically, the degraded DNA components can ultimately activate caspase-3 in macrophages, thereby inducing apoptosis, which allows for staphylococcal persistence [10]. Not surprisingly, the ability of S. aureus to utilize such sophisticated systems has made it a model system for the study of novel virulence factors that compromise components of the innate immune system [5].
Candida albicans: An Evolved Fungal Species
Candida albicans is the most common and major invasive fungal pathogen of humans, causing diseases ranging from superficial mucosal to disseminated, systemic infections that are often life-threatening [3]. As part of the commensal flora, C. albicans asymptomatically inhabits the mucosal surfaces of most healthy individuals. However, as an opportunistic pathogen, when host defenses are weakened, such as in AIDS, C. albicans can proliferate, causing serious infections [11]. As a complex and highly evolved pathogen, C. albicans has acquired efficient strategies to avoid contact with immune cells, and these strategies are often mediated by masking of immunostimulatory surface molecules [12]. Following recognition, phagocytes initiate engulfment of C. albicans, ultimately leading to the formation of a specialized organelle: the phagolysosome [12]. The microbicidal microenvironment of this organelle is associated with a reduction in pH, the presence of hydrolytic enzymes and antimicrobial peptides, and the generation of toxic oxidative compounds. Nevertheless, C. albicans has developed strategies to survive within phagocytes by suppressing the generation of toxic compounds via a secreted mediator compound [13]. The dramatic transcriptional and translational reprogramming exhibited by C. albicans inside the macrophage is indicative of its rapid induction of survival strategies and adaptation to the harsh internal environment of the phagocyte, which causes severe nutrient limitation, oxidative stress and phagosomal acidifcation [14].
The interaction of C. albicans with phagocytes is highly dynamic. As a dimorphic fungal species, C. albicans has the ability to respond to environmental factors and switch morphologies accordingly, between yeast and hyphal forms, a property central to its pathogenesis [15]. Therefore, the conditions in which C. albicans is growing, such as available nutrients, temperature and pH, are important in selectively favoring the yeast or hyphal form. Thus, although some of the strategies C. albicans uses to survive attack from phagocytes are also employed by bacteria, the role of morphology in escaping from phagocytes is unique to C. albicans. While phagocytic cells are able to kill C. albicans, most of the ingested yeast cells survive. However, phagocytosis induces a switch in C. albicans morphology from the yeast to the hyphal form where elongating hyphae puncture through the macrophage membrane. This results in lysis and killing of macrophages, thereby allowing C. albicans to escape [16]. Although it is not quite clear what triggers morphogenesis within the phagocyte where the environment is acidic, recent studies demonstrated that in addition to carbon dioxide produced by macrophages, exposure to sublethal reactive oxygen species (ROS) concentrations in the phagocyte up-regulates C. albicans arginine biosynthesis, allowing C. albicans to neutralize the phagosome, inducing germination and hyphal morphogenesis [17]. Therefore, in addition to pathogenesis, the yeast-to-hyphae transition plays a pivotal role in facilitating C. albicans escape from phagocytes and dissemination. However, macrophage death by ingested C. albicans is not only the result of physical rupture by hyphae, but, similar to what was reported for S. aureus, a new model was recently described where C. albicans–induced macrophage lysis also occurs via pyroptosis, a proinflammatory host cell–programmed death pathway independent of hyphal formation [16,18].
C. albicans, S. aureus, and the Host
Polymicrobial infections caused by combinations of microorganisms are responsible for significant morbidity and mortality [19]. In these infections, the presence of one microorganism may predispose the host to colonization by others, and in additive polymicrobial infections, two or more nonpathogenic microorganisms together can cause disease [19]. C. albicans and S. aureus have been shown to co-adhere as they exist in mixed biofilms on host tissue and abiotic surfaces with S. aureus exhibiting high affinity to the C. albicans hyphae mediated by the hyphal-specific adhesin Als3p as a receptor [20]. Significantly, recent in vivo studies demonstrated a grave clinical implication to this interaction where using a mouse model, the co-colonization of C. albicans and S. aureus on oral tissue resulted in systemic staphylococcal infection despite the massive influx of host immune cells to site of infection [21]. Similar to the in vitro findings, in this model, Als3p was found to be crucial during the early stages of simultaneous co-colonization in the animals. In contrast, however, findings from a more recent study demonstrated that staphylococcal disease is not contingent upon Als3p once C. albicans colonization and infection are established [21,22]. Combined, these findings indicate that the process of C. albicans–S. aureus interaction is complex and multifactorial.
In addition to successful microbial persistence and enhanced virulence, infection by one pathogen can manipulate host immunity to the benefit of other pathogens. Using a mouse model of peritonitis, a recent study by Peters et al. [23] demonstrated that co-infection with C. albicans and S. aureus resulted in enhanced mortality, concomitant with significant increase in proinflammatory cytokines associated with an acute aggressive inflammatory response. Combined, the findings from these recent studies indicate that in addition to augmented pathogenicity, C. albicans and S. aureus co-infection modulate innate inflammatory events. Therefore, it is feasible to speculate that the interaction between these diverse species in a host may also involve immune co-evasion strategies to compromise the ability of immune cells against polymicrobial infections. These speculations were alluded to by co-phagocytosis studies using murine macrophages where S. aureus was seen co-escaping macrophages via its association with the invasive hyphae (Fig 1). Despite the important clinical implications, however, this aspect of fungal-bacterial interactions in the host is yet to be fully explored.
Fig. 1. False-colored scanning electron micrographs depicting the co-phagocytosis of C. albicans and S. aureus by murine macrophages. 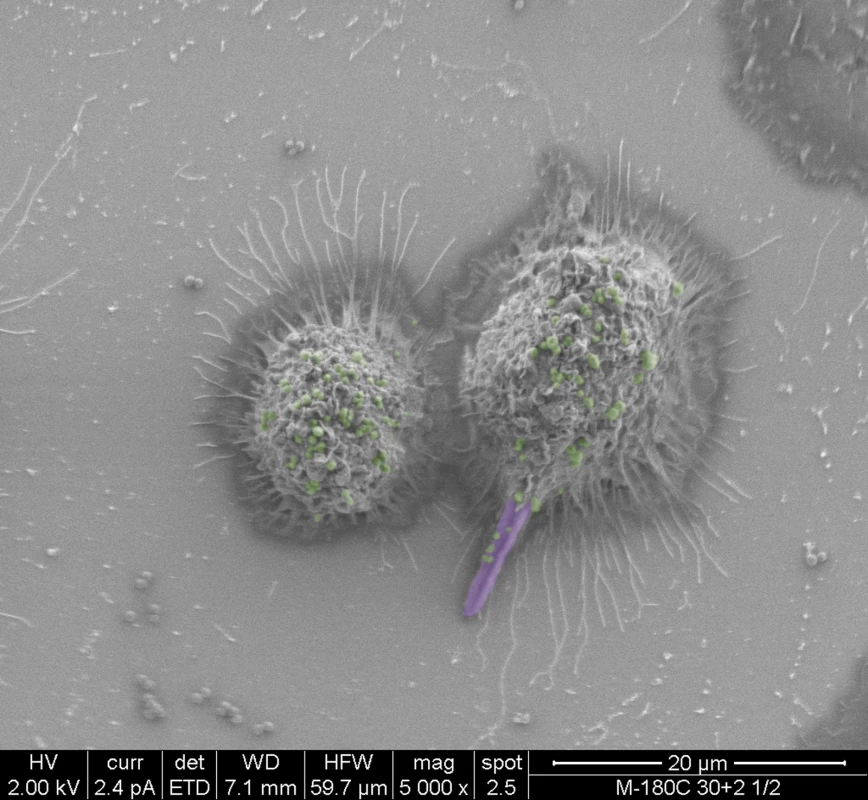
Within one hour of co-ingestion, C. albicans hyphae formed inside the macrophage pierce the cell membrane with S. aureus seen adhering to the protruding hyphae. Ultimately, the macrophage lyses, releasing intracellular S. aureus cells. C. albicans hyphae are purple; S. aureus cells are green. Future Perspectives
Ample information is available on the various strategies employed by microbial species to resist and survive host immune defenses. However, there are significant gaps in our understanding of the mechanisms that regulate host innate immunity during simultaneous infection with multiple microbial species. As modulatory immunotherapies continue to be developed, a fuller understanding of the complex circuitry directing pathogen response to host defences may allow us to develop more effective treatments in the context of complex polymicrobial infections. Therefore, future studies should be directed towards designing suitable animal models to explore the impact of interspecies interactions on host immunity, particularly when they involve ubiquitous commensal species with high pathogenic potential.
Zdroje
1. Akira S, Uematsu S, Takeuchi O (2006) Pathogen recognition and innate immunity. Cell Microbiol 124 : 783–801. 16497588
2. McGavin MJ, Heinrichs DE (2012) The staphylococci and staphylococcal pathogenesis. Front Cell Infect Microbiol 2 : 1–2. doi: 10.3389/fcimb.2012.00001 22919593
3. Calderone RA, ed. (2012) Candida and Candidiasis. Washington: ASM Press.
4. Klotz SA, Chasin BS, Powell B, Gaur NK, Lipke PN (2007) Polymicrobial bloodstream infections involving Candida species: analysis of patients and review of the literature. Diagn Microbiol Infect Dis 59 : 401–406. 17888612
5. Fedtke I, Gotz F, Peschel A (2004) Bacterial evasion of innate host defenses—the Staphylococcus aureus lesson. Int J Med Microbiol 294 : 189–194. 15493829
6. Peschel A, Otto M (2013) Phenol-soluble modulins and staphylococcal infection. Nat Rev Microbiol 11 : 667–673. doi: 10.1038/nrmicro3110 24018382
7. Laarman AJ, Mijnheer G, Mootz JM, van Rooijen WJ, Ruyken M, et al. (2012) Staphylococcus aureus Staphopain A inhibits CXCR2-dependent neutrophil activation and chemotaxis. EMBO J 31(17): 3607–19. doi: 10.1038/emboj.2012.212 22850671
8. Laarman AJ, Ruyken M, Malone CL, van Strijp JAG, Horswill AR, et al. (2011) Staphylococcus aureus Metalloprotease aureolysin cleaves complement C3 to mediate immune evasion. J Immunol 186(11): 6445–53. doi: 10.4049/jimmunol.1002948 21502375
9. Kubica M, Guzik K, Koziel J, Zarebski M, Richter W, et al. (2008) A potential new pathways for Staphylococcus aureus dissemination: the silent survival of S. aureus phagocytosed by human monocyte-derived macrophages. PLoS ONE 1 : 1–16.
10. Thammavongsa V, Missiakas DM, Schneewind O (2013) Staphylococcus aureus degrades neutrophil extracellular traps to promote immune cell death. Science 345 : 863–866.
11. Fidel PLJ (2011) Candida-Host Interactions in HIV Disease: Implications for oropharyngeal candidiasis. Adv Dent Res 23 : 45–49. doi: 10.1177/0022034511399284 21441480
12. Gow NAR, Netea MG, Munro CA, Ferwerda G, Bates B, et al. (2007) Immune recognition of Candida albicans β-glucan by dectin-1. J Infect Dis 196 : 1565–1571. 18008237
13. Collette JR, Zhou H, Lorenz MC (2014) Candida albicans suppresses nitric oxide generation from macrophages via a secreted molecule. PloS ONE. 9: e96203. doi: 10.1371/journal.pone.0096203 24755669
14. Lorenz MC, Bender JA, Fink GR (2004) Transcriptional response of Candida albicans upon internalization by macrophages. Eukaryot Cell 3 : 1076–1087 15470236
15. Saville SP, Lazzell AL, Monteagudo C, Lopez-Ribot JL (2003) Engineered control of cell morphology in vivo reveals distinct roles for yeast and filamentous forms of Candida albicans during infection. Eukaryot Cell 2 : 1053–1060. 14555488
16. Krysan DJ, Sutterwala FS, Wellington M (2014) Catching Fire: Candida albicans, macrophages, and pyroptosis. PLoS Path 10: e1004139.
17. Jiménez-López C, Collette JR, Brothers KM, Shepardson KM, Camer RA, et al. (2012) Candida albicans induces arginine biosynthetic genes in response to host-derived reactive oxygen species. Eukaryot Cell 12 : 91–100. doi: 10.1128/EC.00290-12 23143683
18. Uwamahoro N, Verma-Gaur J, Shen HH, Qu Y, Lewis R, et al. (2014) The pathogen Candida albicans hijacks pyroptosis for escape from macrophages. mBio. 5: e00003–14. doi: 10.1128/mBio.00003-14 24667705
19. Brogden KA, Guthmiller JM, Taylor CE (2005) Human polymicrobial infections. Lancet 365 : 253–255. 15652608
20. Peters BM, Ovchinnikova E, Schlecht LM, Hoyer LL, Busscher HJ, et al. (2012) Staphylococcus aureus adherence to Candida albicans hyphae is mediated by the hyphal adhesin Als3p. Microbiol 158 : 2975–86. doi: 10.1099/mic.0.062109-0 22918893
21. Schlecht LS, Peters BM, Krom B, Freiberg JA, Hänsch GM, et al. (2015) Systemic Staphylococcus aureus infection mediated by Candida albicans hyphal invasion of mucosal tissue. Microbiol 61 : 168–81.
22. Kong E, Kucharíková S, Van Dijck P, Peters BM, Shirtliff ME, et al. (2015) Clinical implications of oral candidiasis: host tissue damage and disseminated bacterial disease. Infect Immun 83(2). 604–613.
23. Peters BM, Noverr MC (2013) Candida albicans-Staphylococcus aureus polymicrobial peritonitis modulates host innate immunity. Infect Immun 81 : 2178–2189. doi: 10.1128/IAI.00265-13 23545303
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek A Phospholipase Is Involved in Disruption of the Liver Stage Parasitophorous Vacuole MembraneČlánek Host ESCRT Proteins Are Required for Bromovirus RNA Replication Compartment Assembly and FunctionČlánek Enhanced CD8 T Cell Responses through GITR-Mediated Costimulation Resolve Chronic Viral Infection
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 3- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- To Be or Not IIb: A Multi-Step Process for Epstein-Barr Virus Latency Establishment and Consequences for B Cell Tumorigenesis
- Is Antigenic Sin Always “Original?” Re-examining the Evidence Regarding Circulation of a Human H1 Influenza Virus Immediately Prior to the 1918 Spanish Flu
- The Great Escape: Pathogen Versus Host
- Coping with Stress and the Emergence of Multidrug Resistance in Fungi
- Catch Me If You Can: The Link between Autophagy and Viruses
- Bacterial Immune Evasion through Manipulation of Host Inhibitory Immune Signaling
- Evidence for Ubiquitin-Regulated Nuclear and Subnuclear Trafficking among Matrix Proteins
- BILBO1 Is a Scaffold Protein of the Flagellar Pocket Collar in the Pathogen
- Production of Anti-LPS IgM by B1a B Cells Depends on IL-1β and Is Protective against Lung Infection with LVS
- Virulence Regulation with Venus Flytrap Domains: Structure and Function of the Periplasmic Moiety of the Sensor-Kinase BvgS
- α-Hemolysin Counteracts the Anti-Virulence Innate Immune Response Triggered by the Rho GTPase Activating Toxin CNF1 during Bacteremia
- Induction of Interferon-Stimulated Genes by IRF3 Promotes Replication of
- Intracellular Growth Is Dependent on Tyrosine Catabolism in the Dimorphic Fungal Pathogen
- HCV Induces the Expression of Rubicon and UVRAG to Temporally Regulate the Maturation of Autophagosomes and Viral Replication
- Spatiotemporal Analysis of Hepatitis C Virus Infection
- Subgingival Microbial Communities in Leukocyte Adhesion Deficiency and Their Relationship with Local Immunopathology
- Interaction between the Type III Effector VopO and GEF-H1 Activates the RhoA-ROCK Pathway
- Attenuation of Tick-Borne Encephalitis Virus Using Large-Scale Random Codon Re-encoding
- Establishment of HSV1 Latency in Immunodeficient Mice Facilitates Efficient Reactivation
- XRN1 Stalling in the 5’ UTR of Hepatitis C Virus and Bovine Viral Diarrhea Virus Is Associated with Dysregulated Host mRNA Stability
- γδ T Cells Confer Protection against Murine Cytomegalovirus (MCMV)
- Rhadinovirus Host Entry by Co-operative Infection
- A Phospholipase Is Involved in Disruption of the Liver Stage Parasitophorous Vacuole Membrane
- Dermal Neutrophil, Macrophage and Dendritic Cell Responses to Transmitted by Fleas
- Elucidation of Sigma Factor-Associated Networks in Reveals a Modular Architecture with Limited and Function-Specific Crosstalk
- A Conserved NS3 Surface Patch Orchestrates NS2 Protease Stimulation, NS5A Hyperphosphorylation and HCV Genome Replication
- Host ESCRT Proteins Are Required for Bromovirus RNA Replication Compartment Assembly and Function
- Disruption of IL-21 Signaling Affects T Cell-B Cell Interactions and Abrogates Protective Humoral Immunity to Malaria
- Compartmentalized Replication of R5 T Cell-Tropic HIV-1 in the Central Nervous System Early in the Course of Infection
- Diminished Reovirus Capsid Stability Alters Disease Pathogenesis and Littermate Transmission
- Characterization of CD8 T Cell Differentiation following SIVΔnef Vaccination by Transcription Factor Expression Profiling
- Visualization of HIV-1 Interactions with Penile and Foreskin Epithelia: Clues for Female-to-Male HIV Transmission
- Sensing Cytosolic RpsL by Macrophages Induces Lysosomal Cell Death and Termination of Bacterial Infection
- PKCη/Rdx-driven Phosphorylation of PDK1: A Novel Mechanism Promoting Cancer Cell Survival and Permissiveness for Parvovirus-induced Lysis
- Metalloprotease NleC Suppresses Host NF-κB/Inflammatory Responses by Cleaving p65 and Interfering with the p65/RPS3 Interaction
- Immune Antibodies and Helminth Products Drive CXCR2-Dependent Macrophage-Myofibroblast Crosstalk to Promote Intestinal Repair
- Adenovirus Entry From the Apical Surface of Polarized Epithelia Is Facilitated by the Host Innate Immune Response
- The RNA Template Channel of the RNA-Dependent RNA Polymerase as a Target for Development of Antiviral Therapy of Multiple Genera within a Virus Family
- Neutrophils: Between Host Defence, Immune Modulation, and Tissue Injury
- CD169-Mediated Trafficking of HIV to Plasma Membrane Invaginations in Dendritic Cells Attenuates Efficacy of Anti-gp120 Broadly Neutralizing Antibodies
- Japanese Encephalitis Virus Nonstructural Protein NS5 Interacts with Mitochondrial Trifunctional Protein and Impairs Fatty Acid β-Oxidation
- Yip1A, a Novel Host Factor for the Activation of the IRE1 Pathway of the Unfolded Protein Response during Infection
- TRIM26 Negatively Regulates Interferon-β Production and Antiviral Response through Polyubiquitination and Degradation of Nuclear IRF3
- Parallel Epigenomic and Transcriptomic Responses to Viral Infection in Honey Bees ()
- A Crystal Structure of the Dengue Virus NS5 Protein Reveals a Novel Inter-domain Interface Essential for Protein Flexibility and Virus Replication
- Enhanced CD8 T Cell Responses through GITR-Mediated Costimulation Resolve Chronic Viral Infection
- Exome and Transcriptome Sequencing of Identifies a Locus That Confers Resistance to and Alters the Immune Response
- The Role of Misshapen NCK-related kinase (MINK), a Novel Ste20 Family Kinase, in the IRES-Mediated Protein Translation of Human Enterovirus 71
- Chitin Recognition via Chitotriosidase Promotes Pathologic Type-2 Helper T Cell Responses to Cryptococcal Infection
- Activates Both IL-1β and IL-1 Receptor Antagonist to Modulate Lung Inflammation during Pneumonic Plague
- Persistence of Transmitted HIV-1 Drug Resistance Mutations Associated with Fitness Costs and Viral Genetic Backgrounds
- An 18 kDa Scaffold Protein Is Critical for Biofilm Formation
- Early Virological and Immunological Events in Asymptomatic Epstein-Barr Virus Infection in African Children
- Human CD8 T-cells Recognizing Peptides from () Presented by HLA-E Have an Unorthodox Th2-like, Multifunctional, Inhibitory Phenotype and Represent a Novel Human T-cell Subset
- Decreased HIV-Specific T-Regulatory Responses Are Associated with Effective DC-Vaccine Induced Immunity
- RSV Vaccine-Enhanced Disease Is Orchestrated by the Combined Actions of Distinct CD4 T Cell Subsets
- Concerted Activity of IgG1 Antibodies and IL-4/IL-25-Dependent Effector Cells Trap Helminth Larvae in the Tissues following Vaccination with Defined Secreted Antigens, Providing Sterile Immunity to Challenge Infection
- Structure of the Low pH Conformation of Chandipura Virus G Reveals Important Features in the Evolution of the Vesiculovirus Glycoprotein
- PPM1A Regulates Antiviral Signaling by Antagonizing TBK1-Mediated STING Phosphorylation and Aggregation
- Lipidomic Analysis Links Mycobactin Synthase K to Iron Uptake and Virulence in .
- Roles and Programming of Arabidopsis ARGONAUTE Proteins during Infection
- Impact of Infection on Host Macrophage Nuclear Physiology and Nucleopore Complex Integrity
- The Impact of Host Diet on Titer in
- Antimicrobial-Induced DNA Damage and Genomic Instability in Microbial Pathogens
- Herpesviral G Protein-Coupled Receptors Activate NFAT to Induce Tumor Formation via Inhibiting the SERCA Calcium ATPase
- The Causes and Consequences of Changes in Virulence following Pathogen Host Shifts
- Small GTPase Rab21 Mediates Fibronectin Induced Actin Reorganization in : Implications in Pathogen Invasion
- Positive Role of Promyelocytic Leukemia Protein in Type I Interferon Response and Its Regulation by Human Cytomegalovirus
- NEDDylation Is Essential for Kaposi’s Sarcoma-Associated Herpesvirus Latency and Lytic Reactivation and Represents a Novel Anti-KSHV Target
- β-HPV 5 and 8 E6 Disrupt Homology Dependent Double Strand Break Repair by Attenuating BRCA1 and BRCA2 Expression and Foci Formation
- An O Antigen Capsule Modulates Bacterial Pathogenesis in
- Variable Processing and Cross-presentation of HIV by Dendritic Cells and Macrophages Shapes CTL Immunodominance and Immune Escape
- Probing the Metabolic Network in Bloodstream-Form Using Untargeted Metabolomics with Stable Isotope Labelled Glucose
- Adhesive Fiber Stratification in Uropathogenic Biofilms Unveils Oxygen-Mediated Control of Type 1 Pili
- Vaccinia Virus Protein Complex F12/E2 Interacts with Kinesin Light Chain Isoform 2 to Engage the Kinesin-1 Motor Complex
- Modulates Host Macrophage Mitochondrial Metabolism by Hijacking the SIRT1-AMPK Axis
- Human T-Cell Leukemia Virus Type 1 (HTLV-1) Tax Requires CADM1/TSLC1 for Inactivation of the NF-κB Inhibitor A20 and Constitutive NF-κB Signaling
- Suppression of RNAi by dsRNA-Degrading RNaseIII Enzymes of Viruses in Animals and Plants
- Spatiotemporal Regulation of a T4SS Substrate by the Metaeffector SidJ
- Antigenic Properties of the Human Immunodeficiency Virus Envelope Glycoprotein Gp120 on Virions Bound to Target Cells
- Dependence of Intracellular and Exosomal microRNAs on Viral Oncogene Expression in HPV-positive Tumor Cells
- Identification of a Peptide-Pheromone that Enhances Escape from Host Cell Vacuoles
- Impaired Systemic Tetrahydrobiopterin Bioavailability and Increased Dihydrobiopterin in Adult Falciparum Malaria: Association with Disease Severity, Impaired Microvascular Function and Increased Endothelial Activation
- Transgenic Expression of the Dicotyledonous Pattern Recognition Receptor EFR in Rice Leads to Ligand-Dependent Activation of Defense Responses
- Comprehensive Antigenic Map of a Cleaved Soluble HIV-1 Envelope Trimer
- Low Doses of Imatinib Induce Myelopoiesis and Enhance Host Anti-microbial Immunity
- Impaired Systemic Tetrahydrobiopterin Bioavailability and Increased Oxidized Biopterins in Pediatric Falciparum Malaria: Association with Disease Severity
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Bacterial Immune Evasion through Manipulation of Host Inhibitory Immune Signaling
- BILBO1 Is a Scaffold Protein of the Flagellar Pocket Collar in the Pathogen
- Antimicrobial-Induced DNA Damage and Genomic Instability in Microbial Pathogens
- Attenuation of Tick-Borne Encephalitis Virus Using Large-Scale Random Codon Re-encoding
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



