-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaAlzheimer’s disease: cost cuts call for novel drugs development and national strategy
Alzheimerova nemoc: význam nových léků a národní strategie pro snížení nákladů na léčbu
Duševní zdraví ovlivňuje kvalitu života velkého počtu osob a jejich rodinných příslušníků. V současné době činí celosvětově náklady na péči o lidi trpící demencemi více než 1 % hrubého domácího produktu (HDP). V budoucnu se předpokládá nárůst těchto výdajů zejména s ohledem na fakt, že populace vyspělých zemích stárnou a je prokázáno, že demence úzce souvisí se zvyšujícím se věkem. Je zřejmé, že vlády budou nuceny vyčlenit příslušné finanční, materiální a lidské zdroje na léčbu a péči o tyto pacienty. Cílem příspěvku je specifikovat současný stav v oblasti léčby a péče o pacienty trpící Alzheimerovou chorobou a analyzovat přímé a nepřímé náklady související s touto problematikou. Kromě toho je pozornost věnována problematice přístupu vlád a existence strategických plánů v oblasti Alzheimerovy choroby, které by řešily všechny související aspekty včetně investic do výzkumu vývoje léků. V současné době neexistuje léčba, která by uspokojivým způsobem zlepšovala stav těchto pacientů, a to zejména včasné fázi, stejně jako v České republice neexistuje žádný dobře fungující národní strategický plán.
Klíčová slova:
Alzheimerova choroba • náklady • léčba • strategický plán
Authors: Petra Marešová; Blanka Klímová; Kamil Kuča
Authors place of work: Faculty of Informatics and Management, Department of Economics ; University of Hradec Králové
Published in the journal: Čes. slov. Farm., 2015; 64, 25-30
Category: Původní práce
Summary
Mental health affects the quality of life for a large number of individuals and family members. Currently, globally costs for people with dementia amount to more than 1% of gross domestic product (GDP). In the future, the growth of expenditure is expected with regard to the fact that the population of developed countries is aging and the dementia is closely associated with increasing age. It is evident that governments have to allocate adequate financial, material and human resources to address a health problem on this scale. The purpose of this article is to explore the current state of treatment and care of patients suffering from Alzheimer’s disease (AD), analyze direct and indirect health care costs resulting from this disease. In addition, the authors of this article draw attention to the implementation of astrategic plan which would handle all the aspects of AD, including the research of drugs development since nowadays there are not still many drugs which would improve AD patients’ state, particularly in the early phases, as well as there does not exist any well-functioning national strategic plan in the Czech Republic which would bring a radical improvement in reducing the effects of AD.
Key words:
Alzheimer’s disease • costs • treatment • strategic planIntroduction
The aging population is the most characteristic feature of the demographic trend in the developed countries. This demographic trend seems to continue in future years1). By 2050 the demographic weight of Europe in the world will have diminished by more than two thirds. As for the population of the 27 member EU, today, at 492.3 million, it represents 7.4% of the world population (6,624 millions). Whilst the demographic importance of Europe may seem to be decreasing ineluctably, bearing in mind its low birth rate, one of the biggest challenges facing the Union today is not this decline in numbers but the aging of its population2). According to Eurostat’s latest set of population, projections were made till 2080. EU-28’s population is expected to increase to 520 million by 2080. As a result of the population movement between age groups, the EU-28’s old-age dependency ratio is projected to almost double from 27.5 % in 2013 to 51.0 % by 2080. The total age dependency ratio is projected to rise from 51.1 % in 2013 to 77.9 % by 20803). Figure 1 illustrates the development of population at the age of 80 and above at all continents by 2100.
Fig. 1. Population 80+ development in the years of 2013 to 2100 Source: own according to OECD database 
According to these data, the average economic burden on a patient increases with increasing age and the cost of treatment rises with medical progress. There is a synergy effect of ageing population and increasing costs of treatment, which might together cause an increased share of treatment costs around the year 20504). The most frequently mentioned diseases in old age include dementia.
The treatment of dementia is a significant economic problem. The International Association for Alzheimer’s disease (ADI) has computed that on a global level, 13 percent of people older than 60 years needed long-term care, which is 101 million people worldwide5). Treatment costs will rise in the future, not only because of the larger number of patients, but also due to the fact that more and more of them will be dependent on institutional care. Now mostly family members, who are doing it for free or with a small contribution, take care of people impaired by Alzheimer’s disease. More than two-thirds of persons suffering from Alzheimer’s disease stay at home, being taken care after by family and friends. Their illness has a significant impact on their families. As the disease advances, symptoms include confusion, irritability and aggression, mood swings, language breakdown, long-term memory loss, and the general withdrawal as senses decline. Gradually, bodily functions are lost6).The disease diminishes patients’ independence in Activities of Daily Living (ADL) and quality of life (QoL)7).
Unfortunately, nowadays, there are not still many drugs which would improve AD patients’ state as well as not a well-functioning national strategic plan which would bring a radical improvement in reducing the effects of AD8, 9). Therefore, the aim of this article is to explore the current state of treatment and care of patients suffering from Alzheimer’s disease (AD), analyze direct and indirect health care costs resulting from this disease and draw national government’s attention to the implementation of a strategic plan which would handle all the aspects of AD, including the research of drugs development.
Methods
For the purpose of this article a method of literature search of available sources describing direct and indirect costs of AD disease and a method of comparison of these costs are used to illustrate a necessity of further research and investment into this disease, particularly during its early stages.
The costs of Alzheimer’s disease
A major problem in the developed countries in future years will be the reimbursement of treating AD. The costs in this case involve the price of all goods and services that are invested to prevent, diagnose, treat and otherwise deal with dementia. Individuals, families and carers are influenced not only economically but also in terms of quality of life. Total costs consist of direct costs (containing hospital resources, medical services, drugs, social services, family payments to formal carers) and indirect costs (for instance, a loss of patient income and losses or restrictions even limitations for family members or careers). Finally, some literature defines intangible costs as those related to pain or deterioration of patient and caregivers’ quality of life.10)
Direct costs
Research studies specifying costs of AD treatment according to individual phases11–13). Table 1 below shows a comparison of treatment costs in three chosen EU countries which are selected on the basis of a similar system of health care financing and at the same time the studies which enable mutual comparison of costs in individual phases of AD. Attention is also paid to the currency of data, whose period is five years at maximum. Thus, the oldest study dates back to 2011.The costs are calculated in the same variable: Total monthly costs - mean (EUR).
Tab. 1. Direct costs of Alzheimer disease in chosen European countries according to the dementia stage 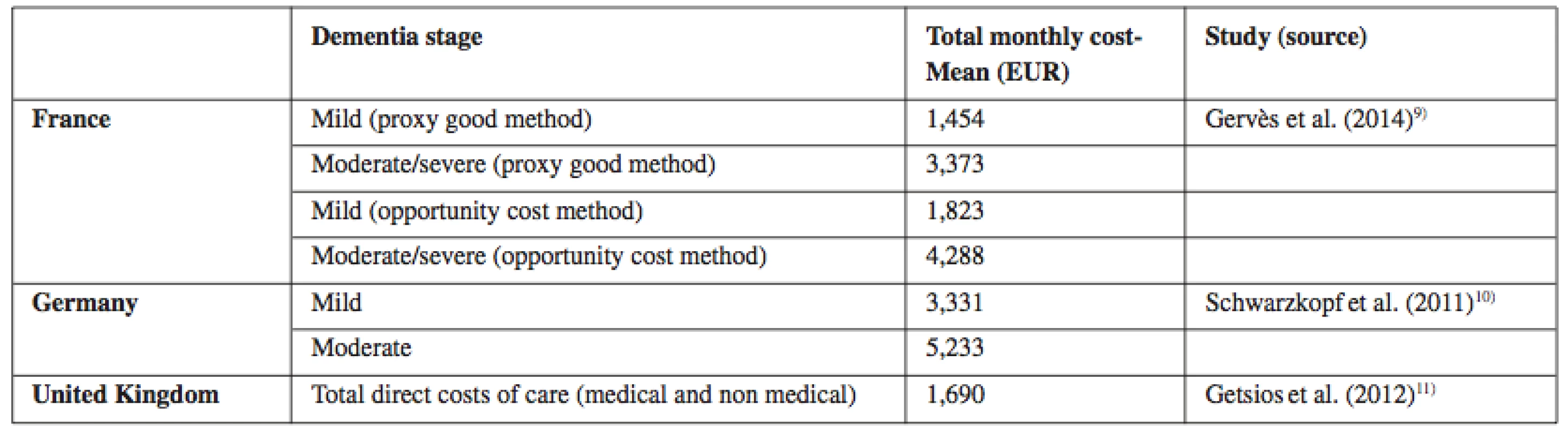
Source: own processing according to11–13) The British study by Getsios et al. (2012)11) estimates average assessment costs of 5,176 EUR per one diagnosed patient (2007 annual costs). In comparison with the scenario without the early diagnosis or pharmacologic treatment, the early diagnosis reduces health care costs by 4,545 EUR per patient and societal costs by 9,784 EUR. Savings are also significant with respect to the treatment without the early diagnosis, which makes 1,663EUR in health care costs, and 7,196 EUR in societal costs. In the probabilistic sensitivity analysis, the early diagnosis leads to savings or is highly cost-effective in the majority of cases. Similarly, in Germany this research was performed by Schwarzkopf et al. (2011)12). The generalized linear mixed model confirmed that costs of care rose with progressing dementia. The early diagnosis has significant up-front costs; on the other hand, it can save costs and brings health benefits compared with no treatment or treatment in the absence of the early diagnosis14).
At present there are altogether 81 compounds in all phases of AD, ranging from the preclinical phase to phase 4 which includes only four approved drugs15). Research studies16–20) compare costs between these four existing drugs and QALYs. A full table of all key clinical effectiveness estimates, modelled quality-adjusted life years (QALYs), modelled costs and incremental cost-effectiveness ratios (ICERs).
Table 2 below summarises the findings emphasising changes in evidence.
Tab. 2. Summary of cost-effectiveness for drugs for AD relative to BSC 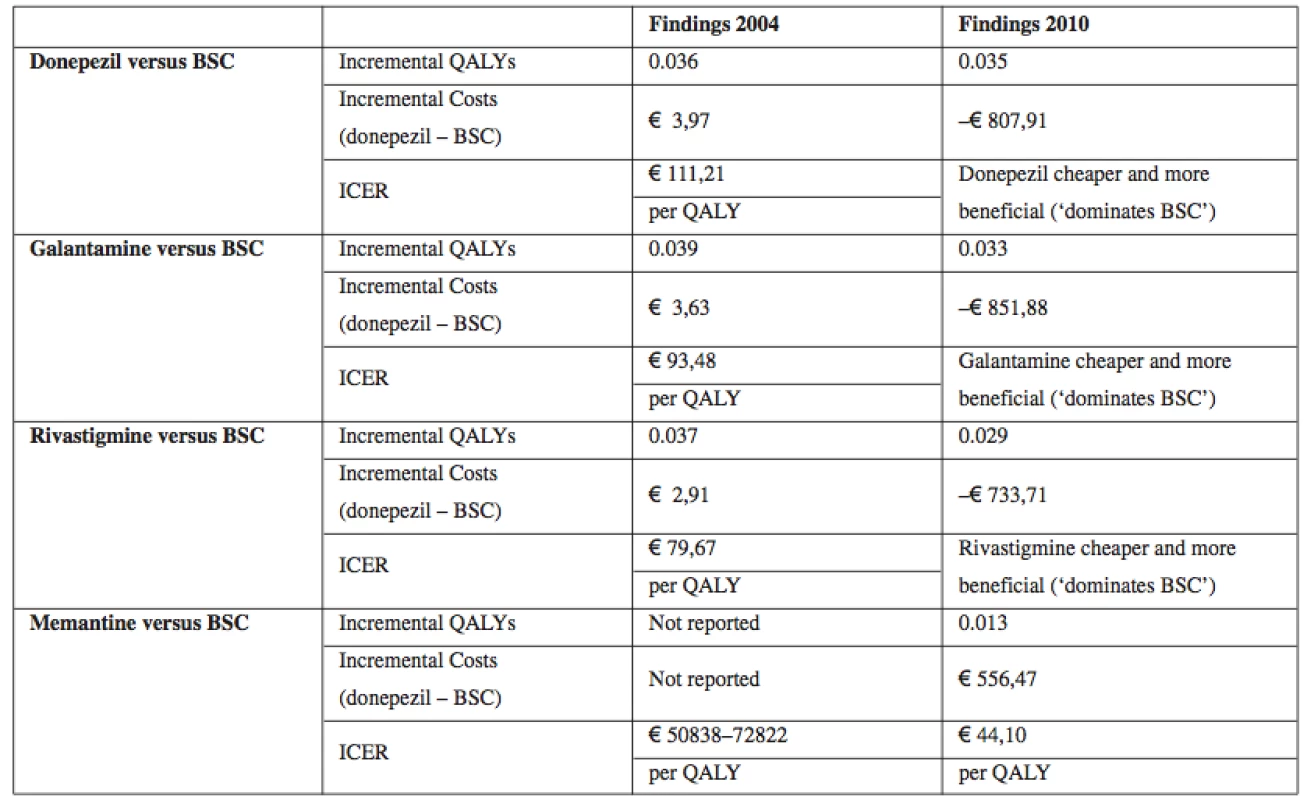
Source: own processing according to16) Table 2 indicates that there was the least additional information concerning the clinical effectiveness of donepezil between 2004 and 2010. The new review confirmed there were statistically significant benefits or trends towards benefit across all the main outcomes. Similarly with rivastigmine there was new evidence17–19), and the additional trials contributed across most of the outcomes. Again this led to quantified and much more precise estimates of effect on cognition, function and global impact, leading to much greater confidence about the beneficial effect of rivastigmine. Furthermore, this benefit occurred with the drug in the form of patches as well as tablets. With memantine there was also new evidence, with one new trial randomising 350 patients contributing across most outcomes20). An important general observation was that there was no new clinical effectiveness evidence on the impact on rates of, or time to institutionalisation. There were also changes in the evidence on cost effectiveness which were more marked than the changes in evidence on effectiveness. All drugs were more cost-effective compared with BSC than previously thought.
From the studies describing the costs in the individual phases of AD, effectiveness and impacts of the existing drugs, it is clear that investments into drugs development are highly important21). In case that drugs prolong the first phase of AD, there are considerable savings of costs, which leads to the reduction of health care system burden but also to the improvement of patients’ quality of life.
Indirect costs on caregivers
In addition, there are indirect costs on caregivers, particularly family members, whose caregiving burden increases with respect to the worsening of cognitive impairment of AD the patient22). Currently, there are about nine million family caregivers who assist their loved ones suffering from AD or a related dementia23). The role of these informal caregivers is particularly common in the Czech Republic where family is still the first informal caregiver in the health system. These informal caregivers are mostly middle aged people with a lot of other responsibilities. Sometimes, they even give up their jobs in order to help their loved ones. Furthermore, besides losing their regular income, they often lose their relationship, free time and eventually, they end up in social isolation. This consequently affects their health as well. They usually have sleep problems and behavioural disorders22). As research studies23–27). confirm the life of these caregivers is seriously modified by physical, emotional, financial and social overload.
Nowadays, there exist research studies, such as Van Durme, Macq, Jeanmart, & Gobert, (2011)28), which provide assessment scales that identify the above mentioned negative impacts of caregiving on the caregiver’s life and suggest timely interventions to reduce burden. These interventions might include different types of help. The most radical, on the one hand, for family caregivers, mainly spouses26), is institutionalization, but on the other hand, it results in great relief after some time. Daily centres, secondary caregivers coming to AD patients’ homes or psychological therapies via the Internet can be also a timely solution to reduce burden of family caregivers.
Discussion
Thus, each national government (see Table 3), including the Czech Republic, should develop its strategic plan on dealing with all aspects (economic, political, and social) of Alzheimer’s disease. These governments worldwide could follow the core principles for the advancement of the clinical delivery of care and scientific understanding of AD and related disorders which were set at the War Invitational Summit in the USA in 2012. These principles focus on the following needs29):
- integrate, but not duplicate services, resources, and research, including building on existing infrastructure where appropriate (e.g., resources available across different institutes such as the National Institutes of Health or Centres for Disease Control);
- shift resources and care delivery to the community and home, providing one stop shopping that incorporates both medical and social interventions along with research and training to improve the lives of people with AD and their families;
- increase awareness among the public, health care providers, and policy makers;
- deliver cost effective diagnostics, treatments and services;
- engage patients, family caregivers, and advocacy groups in the decision-making process;
- focus on affected persons and their families across the trajectory of AD disease; this requires a better understanding of the natural history of the disease and effects of multi-morbidity;
- focus on drug development and biomarker research towards the aim of reducing the incidence and progression of dementia; and
- ensure that dementia is a priority across all segments of societies and governments both nationally and internationally.
Tab. 3. An overview of countries with or without a functioning national strategic plan for AD 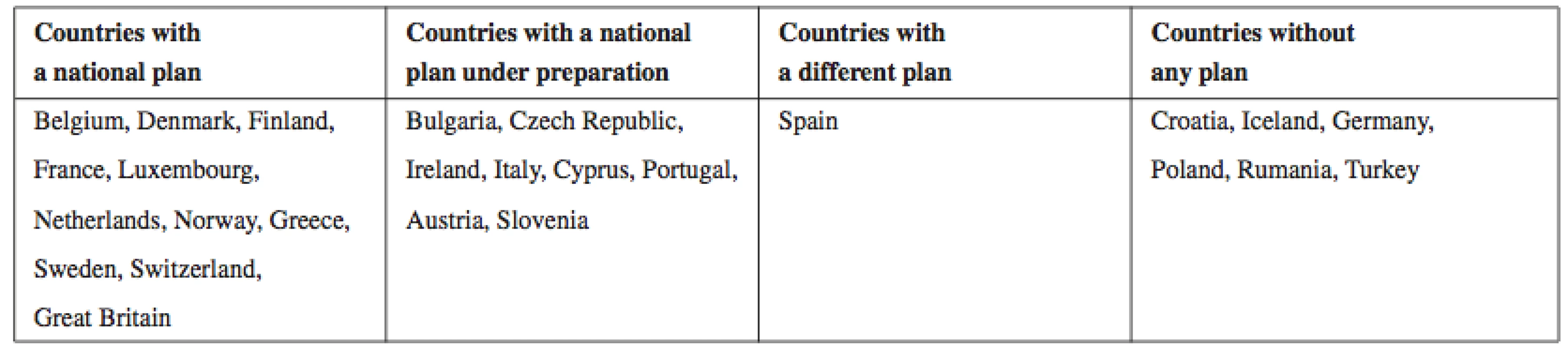
Source: own processing according to38) In order to meet the above described needs and to overcome certain barriers (e.g., underfunding, a lack of validated therapeutic targets and animal models, or a lack of technical infrastructure in areas such as bioinformatics and gene expression), co-investment by public and private sector stakeholders is needed. In addition, a clear consensus among the experts in industry and academia is a must30–32).
As Karran (2012)33) states at an example of UK, governments should establish a national dementia research strategy, with long-term, protected funding, and a lasting commitment to dementia research since there has been a crisis in the development of novel drugs for AD patients32). In fact, as Cummings, Morstorf and Zhong (2014) claim, no new treatments have been approved for AD since 200334).
Nowadays, as it has been already mentioned, there are only four core drugs which have been successfully clinically tested and publically used for AD treatment. Those are: the cholinesterase inhibitors (ChEI) donepezil, rivastigmine and galantamine for mild to moderate AD, and memantine (an NMDA receptor antagonist), and they can accommodate moderate to severe AD. In addition, all these four compounds have proved to considerably improve patients’ reported outcomes, that is, cognition, memory, communication and the ability to perform daily activities, which undoubtedly affect health-related quality of life (QoL). Therefore substantial improvements in drug discovery and clinical development methods are needed (cf. Schneider et al., 2014)35).
Furthermore, researchers claim there must be the collaborative efforts of the research, clinical, pharmaceutical, and regulatory communities, as well as policy makers, to identify barriers and solutions at each stage of drug development. There also exists share recognition of the complexity of the disease, the likelihood that multiple treatments are required at different stages of this disease and that more than one drug is needed. For example, several novel strategies for AD treatment are proposed – vaccination and immunization, use of modulators of secretases or use of statins (cf. Zemek et al., 2014; or Korabecny et al., 2014)36, 37).
Conclusion
The literature review and conducted comparisons show that the detection of AD patients in the initial stage of the outbreak of this disease is very desirable from an economic point of view because the costs of the day care in comparison with the institutionalization care are much lower7). Therefore, as it has been stated several times above, there is an urgent call for the development of novel drugs and a well-functioning national strategic plan which could significantly contribute to the cost cuts in the first phases of this disease, but which would also substantially reduce caregivers’ burden and contribute to the improvement of AD patients’ lives.
Acknowledgements
This research was supported by the project Excellence 2015 and internal research “Economic and managerial aspects of processes in biomedicine” (University of Hradec Kralove, Faculty of Informatics and Management).
Conflicts of interest: none.
Received 28 February 2015
Accepted 7 April 2015
Ing. Mgr. Petra Marešová, Ph.D. • B. Klímová
University of Hradec Králové
Faculty of Informatics and Management, Department of Economics
Rokitanskeho 62, 500 03 Hradec Králové, Czech Republic
e-mail: petra.maresova@uhk.cz
K. Kuča
University of Hradec Králové, Center for Basic and Applied Research
University Hospital Hradec Králové, Biomedical Research Center, Hradec Králové
Zdroje
1. MPSV. Preparing for aging. Portal of the Ministry of Labour and Social Affairs 2008; Available from http://www.mpsv.cz/cs/2856 (cited 2014 June 23).
2. European Commission. The impact of ageing on public expenditure: projections for the EU25 Member States on pensions, health care, long-term care, education and unemployment transfers. 2006. The report of the Economic Policy Committee and the Directorate General. 2006. http://www.worldbank.org/ en/news/feature/2013/01/17/the-eu-11-in-an-aging-europe (cited 2015 March 23).
3. Eurostat: Population structure and ageing. 2014. http://ec. europa.eu/eurostat/statistics-explained/index.php/Population_ structure_and_ageing (cited 2015 March 23).
4. Association of Independent Unions of the Czech Republic. Materials regular meeting of the 96th Plenary Session of 23rd February 2012. http://www.asocr.cz/cz/portal/dokumenty/tripartita/ materialy-na-radne-zasedani-96-plenarni-schuze-rhsd-cr-dne-23-unora-2012-526.htm. (cited 2014 June 23)
5. Ministry of Health of Czech Republic. Health 2020: Framework summary measures [online] 2013. file:///C:/Users/Maresova/ Downloads/WHO_Zdrav%C3%AD_2020_P%C5%99eklad_CZ.pdf (cited 2014 June 23)
6. Mega M. S., Cummings J. L., Fiorello T., Gornbein J. The spectrum of behavioral changes in Alzheimer’s disease. Neurology 1996; 46(1), 130–135.
7. Opara J. A. Activities of daily living and quality of life in Alzheimer disease. J. Med. Life 2012; 5(2), 162–167.
8. Maresova P., Mohelska H., Kuca K. Cooperation Policy of Rare Diseases in the European Union. 5th ICEEPSY International Conference on Education & Educational Psychology 2015; 171, 1302–1308.
9. Maresova P., Tomaskova H., Kuca K. The Use of Simulation Modelling in the Analysis of the Economic Aspects of Diseases in Old Age. EBES Conference Eurasia Business and Economics 2014; In press.
10. Castro D., Dillon C., Machnicki G., Allegri R. The economic cost of Alzheimer’s disease: family or public-health burden? Dement. Neuropsychol. 2010; 4, 262–267.
11. Gervès C., Chauvin P., Bellanger M. M., Meidani A. Evaluation of full costs of care for patients with Alzheimer’s disease in France: The predominant role of informal care. Health Policy 2014; 116, 114–122.
12. Schwarzkopf L., Menn P., Kunz S., Holle R., Lauterberg J., Marx P., Mehlig H., Wunder S., Leidl R., Donath C., Graessel E. Costs of care for dementia patients in community setting: An analysis for mild and moderate disease stage. Value in Health 2011; 14, 827–835.
13. Getsios D., Blume S., Khajak J. I., Maclaine G. and Hernández L. An economic evaluation of early assessment for Alzheimer’s disease in the united kingdom. Alzheimer’s 2012; 8, 22–30.
14. Wang G, Cheng Q, Zhang S, et al. Economic impact of dementia in developing countries: an evaluation of Alzheimer-type dementia in Shanghai, China. J. Alzheimer Dis. 2008; 15, 109–115.
15. Mangialasche F., et al. Alzheimer’s disease: clinical trials and drug development. Lancet Neurol. 2010; 9, 702–716.
16. Hyde Ch., Peters J., Bondi M., et al. Evolution of the evidence on the effectiveness and cost-effectiveness of acetylcholinesterase inhibitors and memantine for Alzheimer’s disease: systematic review and economic model. Age and Ageing 2013; 42, 14–20.
17. Feldman H. H., Lane R. Rivastigmine: a placebo controlled trial of twice daily and three times daily regimens in patients with Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 2007; 78, 1056–1063.
18. Mowla A., Mosavinasab M., Haghshenas H., Haghighi A. B. Does serotonin augmentation have any effect on cognition and activities of daily living in Alzheimer’s dementia? A double-blind, placebo-controlled clinical trial. J. Clin. Psychopharmacol. 2007; 27, 484–487.
19. Winblad B., Cummings J., Andreasen N., et al. A six-month double-blind, randomized, placebo-controlled study of a transdermal patch in Alzheimer’s disease: rivastigmine patch versus capsule. Int. J. Geriatr. Psychiatry 2007; 22, 456–467.
20. van Dyck C. H., Tariot P. N., Meyers B., Malca Resnick E. A 24-week randomized, controlled trial of memantine in patients with moderate-to-severe Alzheimer disease. Alzheimer Dis. Assoc. Disord. 2007; 21, 136–143.
21. Effectiveness and cost-effectiveness of acetylcholinesterase inhibitors. Downloaded from http://ageing.oxfordjournals.org/ by guest on November 17, 2014 patients with moderate-to-severe Alzheimer disease. Alzheimer Dis. Assoc. Disord. 2007; 21, 136–143.
22. Gaugler J., David R. L., Haley W. E., Mittelman M. Can counselling and support reduce Alzheimer’s caregivers’ burden and depressive symptoms during the transition to institutionalization? Results from the NYU caregiver intervention study. J. Am. Geriatr. Soc. 2008; 56(3), 421–428.
23. Sansoni J., Vellone E., Piras G. Anxiety and depression in community-dwelling, Italian Alzheimer’s disease caregivers. Int. J. Nurs. Pract. 2004; 10(2), 93–100.
24. Sansoni J., Anderson K. H., Varona L. M., Varela G. Caregivers of Alzheimer’s patients and factors influencing institutionalization of loved ones: some considerations on existing literature. Ann. Ig. 2013; 25, 235–246.
25. Blom M. M., Bosmans J. E., Cuijpers P., Zarit S. H., Pot A. M. Effectiveness and cost-effectiveness of an internet intervention for family caregivers of people with dementia: Design of a randomized controlled trial. BMC Psychiatry 2013; 13(17), 1–7.
26. Borghi A. C., de Castro V. C., Marcon S. S., Carreira L. Overload of families taking care of elderly people with Alzheimer’s disease: A comparative study. Rev. Latino-Am. Enfermagern 2013; 21(4), 876–883.
27. Diel L., Forster L. M. K., Kochhann R., Chaves M. L. F. Sociodemographic profile and level of burden of dementia patients’ caregivers who participate in a support group. Dement. Neuropsychol. 2010; 4(3), 232–237.
28. van Durme T., Macq J., Jeanmart C., Gobert M. Tools for measuring the impact of informal caregiving of the elderly: A literature review. Int. J. Nurs. Stud. 2011; 49(4), 490–504.
29. Nylor M., et al. Advancing Alzheimer’s diagnosis, treatment and care: Recommendations from the War Invitational Summit. Alzheimers Dement. 2012; 8(5), 445–452.
30. Ivinson A. J., Lane R., May P. C., Hosford D. A., Carrillo M. C., Siemers E. R. Partnership between academia and insudtry for drug discovery in Alzheimer’s disease. Alzheimer’s & Dementia 2008; 4, 80–88.
31. Socott, T. J., O’Connor, A. C., Link, A. N., & Beaulieu, T. J. Economic analysis of opportunities to accelerate Alzheimer’s disease research and development. Ann. Y. Y. Acad. Sci. 2014; 1313, 17–34.
32. Paul S. M., Mytelka D. S., Dunwiddie C. T., Persinger C. C., Munos B. H., Lindborg S. R., Schacht A. L. How to improve R & D productivity: The pharmaceutical industry’s grand challenge. Nat. Rev. Drug Discov. 2010; 9, 203–214.
33. Karran E. Alzheimer’s research U.K.: defeating dementia through research. Stem Cells Translational Medicine 2012; 1, 449–450.
34. Cummings J. L., Morstorf T., Zhong K. Alzeheimer’s disease drug-development pipeline: Few candidates, frequent failures. Alzeheimer’s Research & Therapy 2014; 6(37), 1–7.
35. Schneider L. S., et al. Clinical trials and late-stage drug development for Alzeheimer’s disease: An appraisal from 1984 to 2014. Journal of Internal Medicine 2014; 275, 251–283.
36. Zemek F., et al. Outcomes of Alzeheimer’s disease therapy with acetylcholinesterose inhibitors and memantine. Expert Opin. Drug Saf. 2014; 13(6), 759–776.
37. Korabecny J., et al. Pharmacotherapy of Alzeheimer’s disease: Current state and future perspectives. Frontiers in Drug Design and Discovery 2014; 6, 702–738.
38. Matl O., Holmerová I., Matlová M. Zpráva o stavu demence 2014. Downloaded from http://www.alzheimer.cz/alzheimerova-choroba-v-cr/ zverejnena-zprava-o-stavu-demence-2014/
Štítky
Farmacie Farmakologie
Článek vyšel v časopiseČeská a slovenská farmacie
Nejčtenější tento týden
2015 Číslo 1-2- Psilocybin je v Česku od 1. ledna 2026 schválený. Co to znamená v praxi?
- Ukažte mi, jak kašlete, a já vám řeknu, co vám je
- FDA varuje před selfmonitoringem cukru pomocí chytrých hodinek. Jak je to v Česku?
-
Všechny články tohoto čísla
- The effect of the size of a conical hopper aperture on the parameters of the flow equation of sorbitol and its size fractions
- Synthesis and anti-infective evaluation of 5-amino-N-phenylpyrazine-2-carboxamides
- Alzheimer’s disease: cost cuts call for novel drugs development and national strategy
- Effectiveness of phytotherapy in supportive treatment of type 2 diabetes mellitus Billberry (Vaccinium myrtillus)
- Use of selected OTC drugs: comparing Greece and the Czech Republic
- 5. postgradual and 3. postdoctoral conference Faculty of Pharmacy UK
- Jewish pharmacists in pharmacies of interwar Czechoslovakia and their lives during World War II
- Computational approach to search for novel antituberculostics
- Alkaloids from hydrastidis canadensis and their cholinesterase and prolyl oligopeptidase inhibitory
- K životnému jubileu pani doc. RNDr. Zuzany Vitkovej, PhD.
- Podpora aktivní účasti jednoho člena České farmaceutické společnosti na kongresu FIP v Düsseldorfu
- Výskum a vývoj liekových foriem v súčasnosti
- Zdravotnícke pomôcky. Legislatíva a regulácia.
- Alois Borovanský, farmaceutický chemik par excellence
- Jedová stopa.
- Antitrombotiká v klinickej praxi.
- Bioavailability and factors influencing its rate
- Česká a slovenská farmacie
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Bioavailability and factors influencing its rate
- Effectiveness of phytotherapy in supportive treatment of type 2 diabetes mellitus Billberry (Vaccinium myrtillus)
- Use of selected OTC drugs: comparing Greece and the Czech Republic
- Jedová stopa.
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



