-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaThe Bicoid Stability Factor Controls Polyadenylation and Expression of Specific Mitochondrial mRNAs in
The bicoid stability factor (BSF) of Drosophila melanogaster has been reported to be present in the cytoplasm, where it stabilizes the maternally contributed bicoid mRNA and binds mRNAs expressed from early zygotic genes. BSF may also have other roles, as it is ubiquitously expressed and essential for survival of adult flies. We have performed immunofluorescence and cell fractionation analyses and show here that BSF is mainly a mitochondrial protein. We studied two independent RNAi knockdown fly lines and report that reduced BSF protein levels lead to a severe respiratory deficiency and delayed development at the late larvae stage. Ubiquitous knockdown of BSF results in a severe reduction of the polyadenylation tail lengths of specific mitochondrial mRNAs, accompanied by an enrichment of unprocessed polycistronic RNA intermediates. Furthermore, we observed a significant reduction in mRNA steady state levels, despite increased de novo transcription. Surprisingly, mitochondrial de novo translation is increased and abnormal mitochondrial translation products are present in knockdown flies, suggesting that BSF also has a role in coordinating the mitochondrial translation in addition to its role in mRNA maturation and stability. We thus report a novel function of BSF in flies and demonstrate that it has an important intra-mitochondrial role, which is essential for maintaining mtDNA gene expression and oxidative phosphorylation.
Published in the journal: . PLoS Genet 7(10): e32767. doi:10.1371/journal.pgen.1002324
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002324Summary
The bicoid stability factor (BSF) of Drosophila melanogaster has been reported to be present in the cytoplasm, where it stabilizes the maternally contributed bicoid mRNA and binds mRNAs expressed from early zygotic genes. BSF may also have other roles, as it is ubiquitously expressed and essential for survival of adult flies. We have performed immunofluorescence and cell fractionation analyses and show here that BSF is mainly a mitochondrial protein. We studied two independent RNAi knockdown fly lines and report that reduced BSF protein levels lead to a severe respiratory deficiency and delayed development at the late larvae stage. Ubiquitous knockdown of BSF results in a severe reduction of the polyadenylation tail lengths of specific mitochondrial mRNAs, accompanied by an enrichment of unprocessed polycistronic RNA intermediates. Furthermore, we observed a significant reduction in mRNA steady state levels, despite increased de novo transcription. Surprisingly, mitochondrial de novo translation is increased and abnormal mitochondrial translation products are present in knockdown flies, suggesting that BSF also has a role in coordinating the mitochondrial translation in addition to its role in mRNA maturation and stability. We thus report a novel function of BSF in flies and demonstrate that it has an important intra-mitochondrial role, which is essential for maintaining mtDNA gene expression and oxidative phosphorylation.
Introduction
The maternal to zygotic transition, during which control of development shifts from maternally contributed mRNAs to genes expressed in the zygote, is of considerable interest. The maternally contributed bicoid mRNA, encoding a protein important for formation of anterior body patterning, is dependent on regulatory mechanisms controlling the cytoplasmic stability and localization of the mRNA in the zygote. The bicoid stability factor (BSF) is thought to be involved in this process as it binds the 3′UTR of the bicoid mRNA [1]. Mutation of the BSF binding site leads to reduced abundance of bicoid mRNA, whereas a P element insertion mutation that leads to a drastic reduction in BSF protein levels does not affect the abundance or distribution of endogenous bicoid mRNA [1]. In another study, BSF was reported to have a role in regulation of early zygotic genes by binding a short consensus sequence in the 5′UTR of genes expressed in the early zygote [2]. It was also reported that BSF is essential, as a P element insertion in the bsf open reading frame is lethal in the homozygous form [2]. However, homozygous bicoid mutant flies are viable and BSF therefore is likely to have an additional role besides regulating bicoid expression [2]. Characterization of global gene expression patterns in flies have shown that BSF is ubiquitously expressed in adults, further indicating that the role of BSF may not be limited to embryogenesis [3]. Studies of the subcellular localization of BSF have shown that it is present in cytoplasmic particles in oocytes and surrounding nurse cells [1] and in the cytoplasm and nucleus in early embryos [2]. Bioinformatics analyses suggest that BSF has some homology to the mammalian LRPPRC protein [1], [4], which belongs to the pentatricopeptide repeat class of proteins [5]. The LRPPRC protein has been reported to have roles in cytoplasmic RNA transport [6]–[8] and nuclear transcription [9], [10], but its main localization is in the mitochondrial matrix [4], [10], [11], where it has been suggested to stabilize mitochondrial transcripts [12].
The fly mtDNA is a small compact, circular molecule, encoding 13 essential polypeptides, which are components of the mitochondrial oxidative phosphorylation system, and RNA components of the mitochondrial translational system, which include 22 transfer RNAs and 2 ribosomal RNAs [13]. Mitochondrial transcription generates large polycistronic transcripts, which are processed by endonucleolytic cleavage to generate individual mRNAs [14], [15] These mRNAs are subsequently polyadenylated [16], in a process believed to take place in two steps. First, oligoadenylated transcripts are generated by addition of a short adenine tail to the 3‘ends of most mitochondrial mRNAs. The enzyme necessary for this oligoadenylation has not yet been identified. Second, the poly A polymerase enzyme will add up to <50 adenines to the oligoadenylated mRNAs to create the long polyadenylated tail [17].
The vast majority of the ∼103 mitochondrial proteins are encoded by nuclear genes, including the majority of the respiratory chain subunits, all proteins involved in replication and transcription of mtDNA and all proteins of the mitochondrial ribosome. The regulation of oxidative phosphorylation capacity is thus dependent on a crosstalk between two genomes and nuclear genes play a key role in this process as they regulate mtDNA expression at many different levels. The mitochondrial genomes of flies and mammals have the same gene content although there are substantial differences in gene order. The high level of conservation of mtDNA and important nuclear-encoded regulators of mtDNA expression suggests that key regulatory processes are similar in insects and mammals. We therefore hypothesized that BSF might have a role in fly mitochondria, in addition to its suggested regulatory roles in developmental processes. We report here that BSF is a bona fide mitochondrial protein involved in regulating mtDNA gene expression. Contrary to previous reports, our data demonstrate that BSF is involved in the maturation and polyadenylation of mitochondrial mRNAs and coordinates mitochondrial translation. We have thus identified an essential role for BSF in maintaining mtDNA gene expression and oxidative phosphorylation in flies.
Results
BSF is a mitochondrial protein and the fly homologue of LRPPRC
We previously identified two possible fly homologues for the mammalian LRPPRC protein [4]. Here we present additional phylogenetic analyses showing that BSF is the most closely related homologue to LRPPRC in flies (Figure 1A). Previous experiments have demonstrated a punctuated cytoplasmic localization of BSF in oocytes and a cytoplasmic and nuclear localization in early embryos [1], [2]. We further addressed the subcellular localization of BSF by transfecting Schneider (S2R+) and HeLa cells with a BSF-FLAG-GFP fusion construct. There was a perfect overlay between Mitotracker Deep Red and BSF-FLAG-GFP fluorescence with a co-localization rate of 91±1% in Schneider cells (N = 5) and 94±3% in HeLa cells (N = 7) (Figure 1B), thus indicating that BSF is localized to mitochondria. We also performed subcellular fractionation experiments of tissues from adult flies and found that BSF was present in the mitochondrial fraction (Figure 1C). Our results thus show that BSF is mainly localized to mitochondria, which is in good agreement with the known main localization of its mammalian homolog LRPPRC [4].
Fig. 1. Phylogenetic analysis and subcellular localization of BSF. 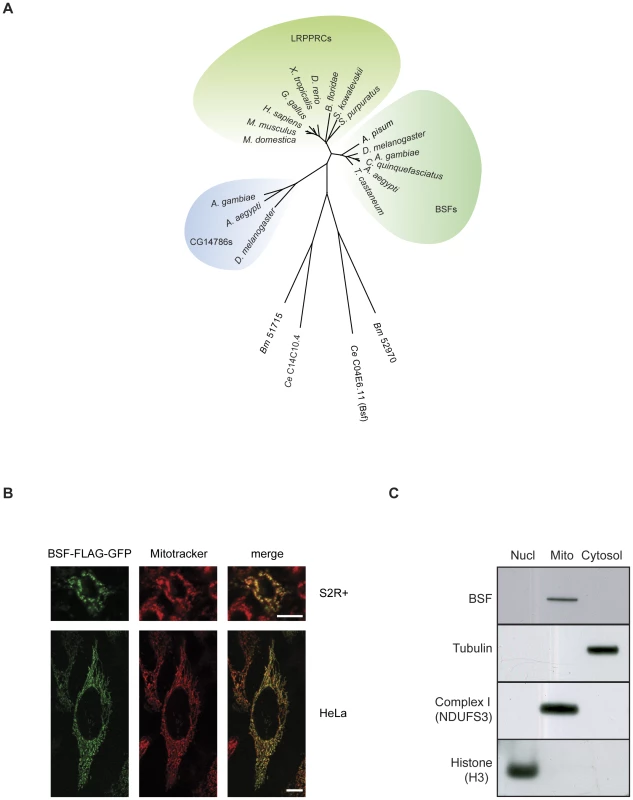
(A) Phylogenetic tree of the LRPPRC family of proteins. Phylogenetic analyses show two arthropod families related to LRPPRC, i.e. the Bicoid stability factor (BSF) family and the CG14786 family. The BSF family is orthologous to deuterostomian LRPPRC proteins. Dots indicate stable branches by non-parametric bootstrap analysis (found in at least 450 of 500 replicates). Orthologous sequences in nematodes are divergent, leading to long branches. Abbreviations and accession numbers are as follows: H. sapiens, Homo sapiens, NP_573566; M. musculus, Mus musculus, NP_082509; M. domesticus, Monodelphis domesticus, XP_001382190, G. gallus, Gallus gallus, XP_001234903; X. tropicalis, Xenopus tropicalis, NP_001039203; D. rerio, Danio rerio, NP_001136064; S. kowalevskii, Saccoglossus kowaleskii, XP_002734047; S. purpuratus, Strongylocentrotus purpuratus, XP_001200846; B. floridae, Branchiostoma floridae, XP_002613352; A. pisum, Acyrthosiphon pisum, BSF: XP_001944507; D. melanogaster, Drosophila melanogaster, BSF: NP_523596, CG14786: NP_569913; A. gambiae, Anopheles gambiae, BSF: XP_557938, CG14786: XP_321013; C. quinquefasciatus, Culex quinquefasciatus, BSF: XP_001846253; A. aegypti, Aedes aegypti, BSF: XP_001658446, CG14786: XP_001657384; T. castaneum, Tribolium castaneum, BSF: XP_975329. C. elegans, Caenorhabditis elegans, C04E6.11: NP_504540, C14C10.4: NP_506151; B. malayi, Brugia malayi, 52970: XP_001902062, 51715: XP_001901811. (B) S2R+ cells expressing a GFP-tagged BSF-FLAG fusion protein (BSF-FLAG-GFP), counterstained with Mitotracker Deep Red (upper panels), scale bar size 10 µm and HeLa cells expressing a GFP-tagged BSF-FLAG fusion protein (BSF-FLAG-GFP), counterstained with Mitotracker Deep Red (lower panels), scale bar size 10 µm. (C) Western blot analyses of nuclear, mitochondrial and cytoplasmic fractions to determine the subcellular localization of BSF. Antibodies against subunits NDUFS3 of mitochondrial complex I, tubulin and histone H3 were used to assess the purity of the fractions. Efficient RNAi induced knockdown of BSF in vivo
In order to analyze the in vivo function of BSF, we induced gene silencing by using the UAS-GAL4 system and two independent bsf RNAi-knock down fly lines, that target different, non-overlapping regions of the bsf transcript. In each case, the performed fly crosses generated the bsf knockdown lines wDahT;+;UAS-bsf-RNAi#1/daGAL4 or wDahT;UAS-bsf-RNAi#2/+;daGAL4/+ (w;;UAS-bsfRNAi#1/daGAL4 or w;UAS-bsfRNAi#2/+;daGAL4/+) and two control lines wDahT;+;daGAL4/+ (w;;daGAL4/+), and wDahT;+;UAS-bsfRNAi#1/+;+ or wDahT;UAS-bsfRNAi#2/+;+ (w;;UAS-bsfRNAi#1/+; or w;UAS-bsfRNAi#2/+), which all were analyzed in parallel in the experiments. Ubiquitous knockdown (KD), using the daughterless-GAL4 (daGAL4) driver line, resulted in an up to 80% down-regulation of bsf transcript levels both in third-instar larvae and in adult flies (Figure 2A). Western blot analyses revealed that the BSF protein was undetectable in third-instar KD larvae (Figure 2B) and KD flies (Figure S1A), demonstrating a highly efficient KD of BSF protein expression in both bsf-RNAi lines.
Fig. 2. Ubiquitous bsf KD negatively affects hatching rate, climbing ability, body weight, and life span of Drosophila. 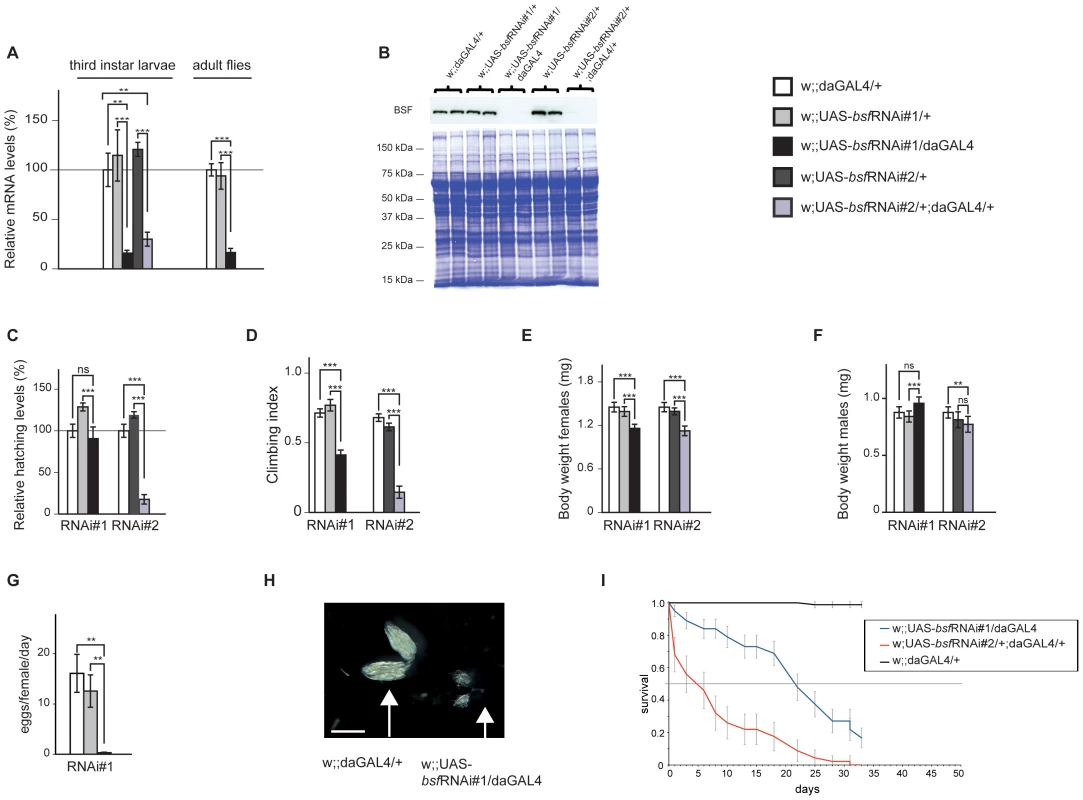
(A) QRT-PCR of BSF transcript levels in third-instar control (white and grey bars) and third-instar bsf KD (black and purple bars) larvae or six-day old flies (*p<0.05; **p<0.01; ***p<0.001, n = 4-5). (B) Western blot analyses were performed on mitochondrial protein preparations from third-instar larvae. Protein extracts, 10–20 µg, were separated by standard SDS-PAGE followed by Western blot analysis with antibodies against BSF. Coomassie staining of the membrane was used to assess loading. (C) The relative hatching rate in control lines (white and grey bars) and bsf KD lines (black bars). Hatching rates are shown relative to controls. ***p<0.001, Student-t-test. (D) bsf KD impairs climbing abilities of four-day old flies. ***p<0.001, Mann-Whitney U test. (E, F) Body weight of three-day-old female and male flies. ***p<0.001, Student-t–test. (G) Reduced fecundity upon ubiquitous bsf KD. **p<0.01, Student-t-test. (H) Ovaries from six-day old bsf KD females are significantly smaller (small arrow) than control ovaries (large arrow), scale bar size 1 mm. (I) bsf KD flies are short lived. Survival curves of bsf KD flies and control flies on the standard food. BSF deficiency affects climbing ability, fecundity, and life span in flies
Ubiquitous BSF KD caused a delay in pupal development, with the majority of pupae from line 1 hatching, and only 15% from line 2 managing to complete their eclosure (Figure 2C). KD flies had a significantly reduced climbing ability, suggesting muscle weakness (Figure 2D). Female flies from both KD lines weighed less (Figure 2E), whereas there was no consistent weight change in the males (Figure 2F). Continuous bsf KD male/female crossings resulted in no offspring, and KD flies laid significantly fewer eggs in comparison with controls (Figure 2G). Dissecting ovaries from adult bsf KD flies revealed a significant reduction in size in comparison with controls (45±21% v. 100±10%)(Figure 2H and Figure S1B), possibly contributing to the lower body weight of the female KD flies. Flies of both bsf KD lines had a drastically reduced life span compared with controls (Figure 2I), with most flies surviving less than 30 days. Together, these results identify BSF as an essential protein for fly survival.
BSF deficiency leads to a respiratory chain dysfunction and increased lactate levels
To investigate the biochemical consequence of reduced BSF expression levels we measured respiratory chain enzyme activities in isolated mitochondria from larval tissue. We found that all respiratory chain complexes containing mitochondrially-encoded subunits had reduced enzyme activities. Complex I was the most affected, but complex I+III, complex II+III and complex IV activities were also profoundly reduced in bsf KD flies (Figure 3A). Complex II, containing exclusively nuclear-encoded subunits, exhibited normal enzyme activity (Figure 3A), strongly supporting the notion that loss of BSF has specific effects on mtDNA gene expression. We also analyzed mitochondrial respiratory capacity by monitoring oxygen consumption in permeabilized tissues extracted from third-instar larvae or thoraces of flies. Maximal oxygen consumption levels were significantly reduced in mitochondria from third-instar bsf KD larvae and flies in the presence of substrates entering the respiratory chain at the level of complex I (CPI), but not with substrates (SUCC-G3P) that deliver electrons to complex II or glycerol-3-phosphate dehydrogenase, which both are upstream of complex III (Figure 3B). There was significant reduction of oxygen consumption with combined substrates (CPI-SUCC-G3P) in adult fly mitochondria (Figure 3B). We also observed a progression of the decrease in the uncoupled oxygen consumption with complex I substrates, when comparing third-instar larvae and flies (Figure 3B). In summary, the results from measurements of enzyme activities (Figure 3A) and oxygen consumption (Figure 3B) show that complex I is the most affected of the oxidative phosphorylation complexes, perhaps due to its high number of mtDNA-encoded subunits. As a result of the respiratory chain deficiency the [ATP]/[ADP] ratio in adult bsf KD flies was reduced to 44% of ratio in controls (Figure 3C). Furthermore, in adult bsf KD flies the [lactate]/[pyruvate] ratio was considerably increased (Figure 3D), most likely as a consequence of the compromised respiratory function, which leads to increased lactic acid fermentation in order to maintain the reduction-oxidation homeostasis.
Fig. 3. Biochemical measurements of respiratory chain function. 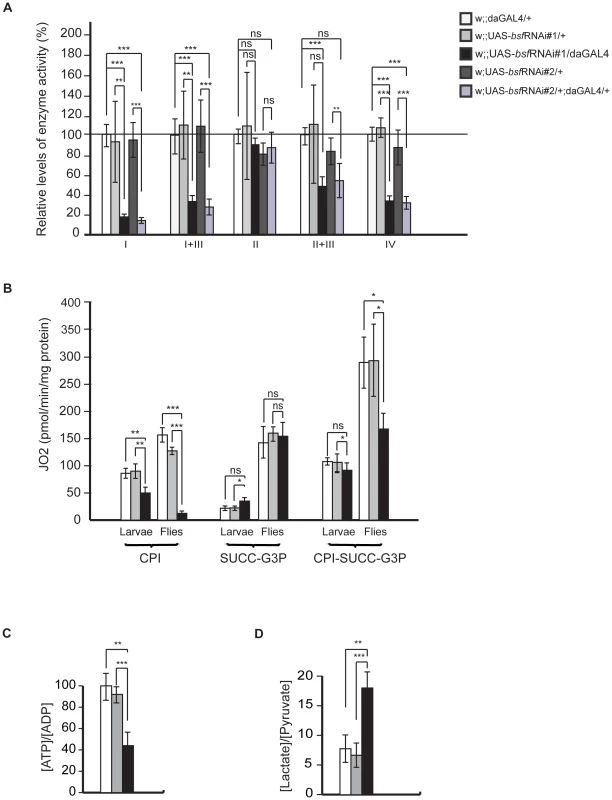
(A) Relative enzyme activities of respiratory chain enzyme complex I (NADH coenzyme Q reductase, NQR), complex I+III (NADH cytochrome c reductase, NCR), complex II (succinate dehydrogenase, SDH), complex II+III (succinate cytochrome c reductase, SCR) and complex IV (cytochrome c oxidase, COX) in third-instar control (white and grey bars) and bsf KD (black and purple bars) larvae are shown. Bars indicate mean ± SD (*p<0.05; **p<0.01; ***p<0.001; n = 6-7). (B) Oxygen consumption normalized to protein content in third-instar control (white and grey bars) and bsf KD (black bars) larvae and six-day old flies. Oxygen consumption was assessed in permeabilized larvae and thoraces from six-day old flies by using substrates entering at the level of complex I (CPI), complex II (SUCC), glycerol-3-phosphate dehydrogenase, (G3P) and complex I, II and glycerol-3-phosphate dehydrogenase (CPI-SUCC-G3P), (C) Relative ATP/ADP levels in six-day old bsf KD flies. (D) Lactate/pyruvate ratios in six-day old bsf KD flies. BSF affects steady-state levels of mitochondrial transcripts
We performed a detailed study on steady-state levels of mitochondrial transcripts in bsf KD lines by using both QRT-PCR and northern blot analyses. The levels of all analyzed mitochondrial mRNAs were reduced in third-instar bsf KD larvae (Figure 4A, 4C, 4D and Figure S1C) and flies (Figure 4B), with the exception of ND6, which was significantly reduced only in adult flies. QRT-PCR demonstrated slightly increased mtDNA levels in third-instar bsf KD larvae (Figure 4E), showing that the reduced mitochondrial mRNA levels cannot be explained by mtDNA depletion. In contrast, the mitochondrially encoded small ribosomal subunit rRNA (12S rRNA) was significantly increased, while the large ribosomal subunit rRNA (16S rRNA) was slightly decreased (Figure 4C and 4D). Additionally, there was a substantial increase in levels of all analyzed mitochondrial tRNAs in third-instar bsf KD larvae (Figure 4F and 4G). There was no clear correlation between the level of a particular tRNA and the location of its gene in fly mtDNA.
Fig. 4. Steady-state levels of mtDNA and mitochondrial transcripts. 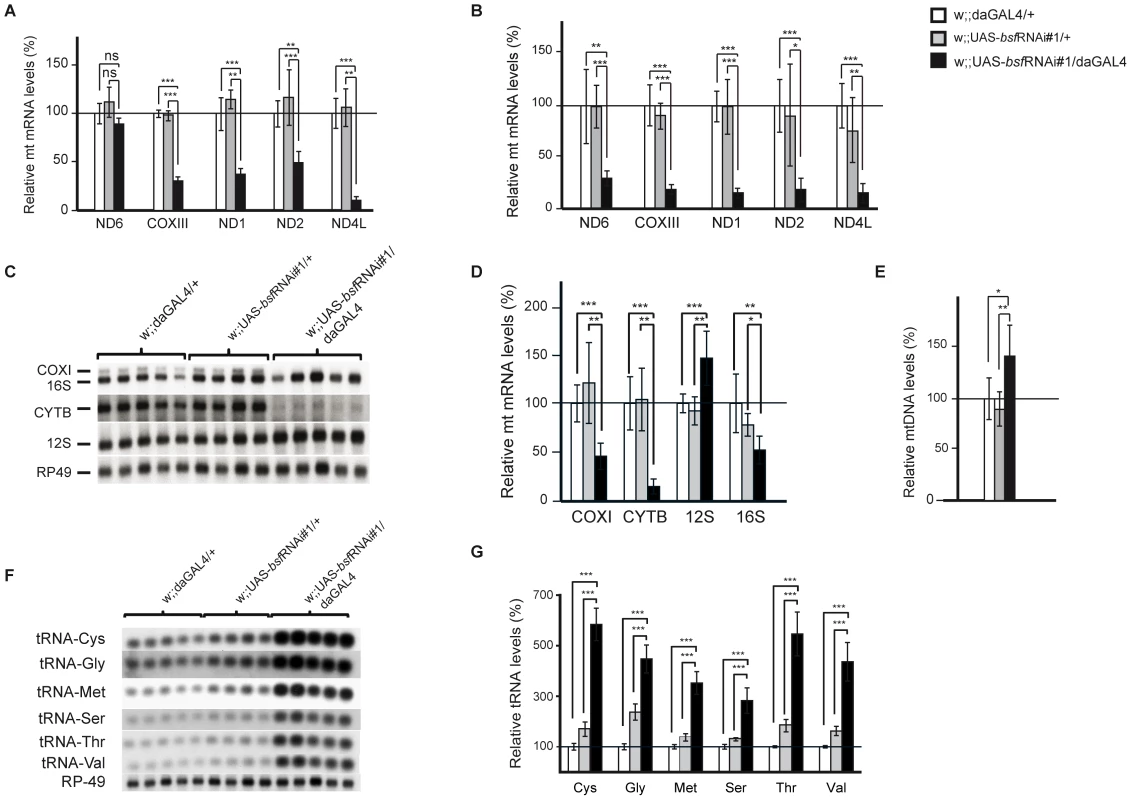
(A-B) QRT-PCR analysis of relative levels of mitochondrial mRNAs in comparison with the nuclear ribosomal protein 49 transcript in (A) third-instar larvae and (B) six-day old flies. Northern Blot analyses (C) and quantification (D) of mitochondrial transcripts normalized to nuclear ribosomal protein 49 transcript in third-instar larvae. (E) QRT-PCR analysis of mtDNA levels in third-instar larvae. Northern blot analyses (F) and quantification (G) of mitochondrial tRNA levels in third-instar larvae. (*p<0.05; **p<0.01; ***p<0.001. n = 5). Reduced BSF level leads to increased de novo mitochondrial transcription and aberrant translation
The presence of increased steady-state levels of tRNAs makes it unlikely that the reduction in levels of mRNAs are explained by reduced transcription as both types of mature transcripts are produced by processing of polycistronic precursor transcripts. We nevertheless assessed mitochondrial de novo transcription by performing in organello labeling experiments. Both third-instar bsf KD larvae (Figure 5A and Figure S2B) and bsf KD flies (Figure S2A) had a dramatic increase in de novo transcription, showing that the reduced mRNA levels must be explained by increased degradation. The increased mitochondrial de novo transcription and increased mtDNA copy number are likely parts of a compensatory mitochondrial biogenesis response induced by the respiratory chain deficiency, which, in turn, is caused by defective post-transcriptional regulation of mitochondrial mRNA stability in the absence of BSF. We further investigated whether the decrease in mRNA steady-state levels resulted in decreased mitochondrial translation by assessing de novo translation in isolated mitochondria. Surprisingly, third-instar bsf KD larvae demonstrated a selective increase in the synthesis of subunits of complex I (ND1-6 and ND4L) and complex IV (COXI-III), whereas the synthesis of subunits of complex III (Cyt b) and complex V (ATP6) remained unchanged (Figure 5B). We observed an exceptionally large increase in levels of the COXII subunit of complex IV (Figure 5B, asterisk). We also found an unidentified translation product migrating above the ND1 subunit of complex I (Figure 5B, arrow). Interestingly, both of these aberrant translation products were almost invisible after a 3-hour chase with cold methionine (Figure 5B, left panel), suggesting that they are subjected to an increased degradation shortly after synthesis.
Fig. 5. De novo mitochondrial transcription, de novo mitochondrial translation in bsf KD larvae, and polyadenylation profiles. 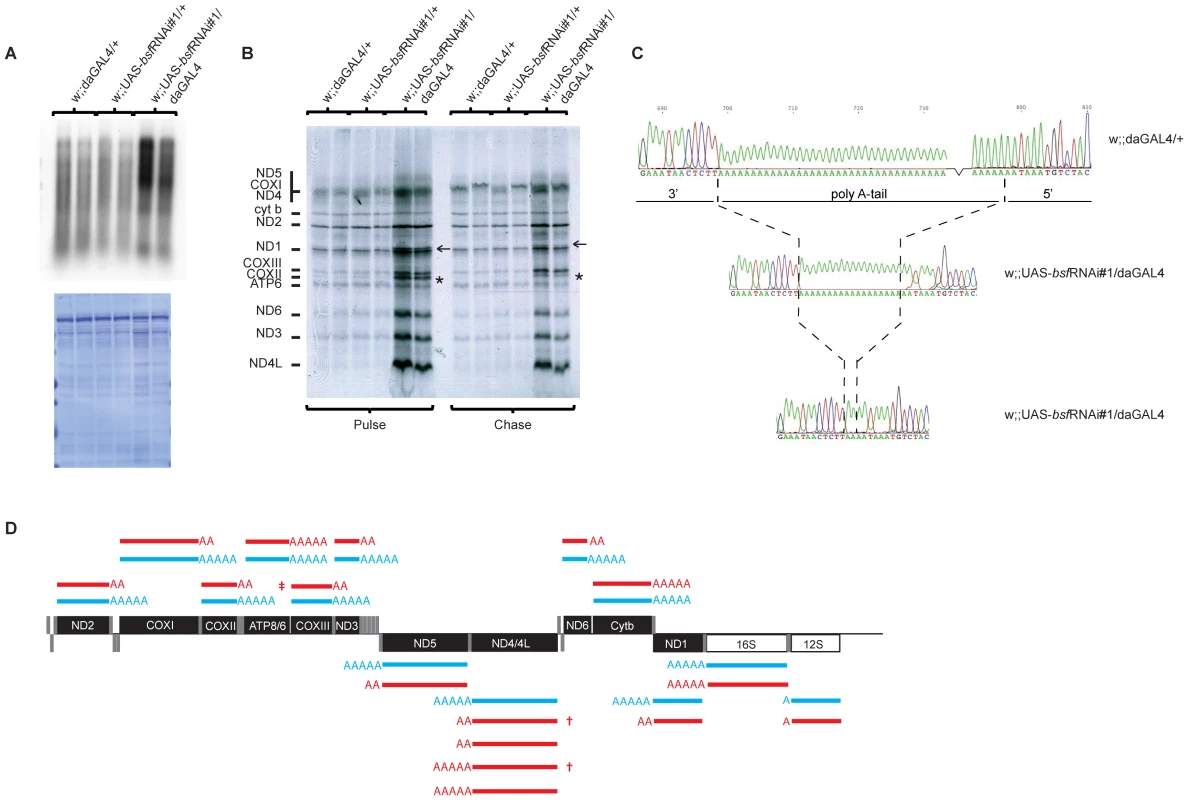
(A) De novo transcription of mtDNA as determined by α32P-UTP incorporation. Isolated mitochondria were incubated with α32P-UTP and labeled transcripts were separated on MOPS/formaldehyde agarose gels and normalized to the total mitochondrial protein content per µl of sample (lower panel). (B) Analysis of de novo mitochondrial translation as determined by S35-methionine incorporation, normalized to the total mitochondrial protein content per µl of sample (Figure 5A, lower panel) The asterisk indicates the COXII subunit. The arrow indicates an unidentified protein migration just above the ND1 subunit. (C) Examples of electropherograms showing the polyadenylation status of the COXII mRNA in mitochondria of control (w;;daGAL4/+) and bsf KD (w;;UAS-bsfRNAi/daGAL4) larvae. (D) A summary of the polyadenylation status of mRNAs in mitochondria of third-instar controls (w;;daGAL4/+) and bsf KD (w;;UAS-bsfRNAi/daGAL4) larvae. AAAA = 40-60 adenines, AA = <20 adenines. Polycistronic transcript of COXIII-ATP6/8 (‡), antisense tRNA-Threonine (†). BSF is required for polyadenylation of mitochondrial mRNAs
Decreased mitochondrial mRNA steady-state levels, in combination with increased de novo transcription and translation of specific mitochondrial polypeptides (ND1-6 and COX1-3) led us to investigate the nature of the mature mitochondrial mRNAs. We analyzed the 5′-and 3′-ends of mitochondrial transcripts, using RNA circularization, followed by reverse transcription and direct sequencing or cloning and subsequent sequencing (see Materials and Methods). The 5′ and 3′ ends of the 12S and 16S rRNA were identical in bsf KD and control larvae. However, we observed severely reduced poly A tail lengths of all mitochondrial mRNAs except Cytb and ATP6/8 (Figure 5C and 5D). Interestingly, those mRNAs with reduced poly A tail length encoded the polypeptides that showed increased levels of de novo synthesis. We further analyzed the 5′ and 3′ends of two polycistronic transcripts each containing an mRNA with a retained tRNA at its 3′ end (ND3 plus tRNA-Ala and COXII plus tRNA-Lys). In control samples these polycistronic transcripts were polyadenylated at their 3′-ends, showing that the polyadenylation process is not specific for fully processed mRNAs, but rather can occur at any free 3′end. In the bsf KD larvae, these polycistronic RNAs had severely reduced lengths of their polyA tail, thus suggesting that the lack of polyadenylation is a direct consequence of the loss of BSF and not a secondary effect of impaired translation. In the bsf KD larvae, we consistently failed to recover the mature COX III mRNA and only observed COXIII as part of a large polycistronic RNA. The 5′part of this RNA consisted of ATP6/8 and the 3‘part of COXIII. This RNA had undergone a correct processing at the 3′ end of COXIII but lacked polyadenylation. The lack of mature polyadenylated COXIII transcripts in the bsf KD larvae is thus likely explained by a combination of defective RNA processing and defective polyadenylation. Cloning and subsequent sequencing of the ND4/ND4L PCR product revealed several different RNA species in the bsf KD samples, indicating both polyadenylation and processing differences in comparison with control samples (Figure 5D). In summary, sequencing of RNA 5′ and 3′-ends in third-instar bsf KD larvae revealed a severe mRNA maturation defect with reduced poly A tail lengths and an enrichment of unprocessed polycistronic RNA intermediates containing COXIII and ND4/ND4L sequences.
The biochemical defect in bsf KD larvae is caused by reduced levels of assembled complex I, III, and IV
Despite the observed compensatory increase of de novo transcription and translation, third-instar bsf KD larvae show a severe respiratory chain dysfunction presumably causing delayed hatching and reduced lifespan. Sequencing of the mitochondrial mRNAs showed reduced length of poly A tails and processing defects, which could affect translational initiation or lead to the production of abnormal polypeptides. Subunits of complex I and IV were translated at increased rates, but a subset of the newly synthesized subunits were nevertheless preferentially degraded (Figure 5B), suggesting that they fail to assemble into mature complexes. We therefore assessed the levels of assembled respiratory chain enzyme complexes by using Blue-Native polyacrylamide gel electrophoresis (BN-PAGE). We observed reduced levels of assembled complex I, complex III, complex IV and supercomplexes in third-instar bsf KD larvae (Figure 6A and 6B) and flies (Figure S2C and S2D). The levels of assembled complex V (ATP synthase) were unaffected. This reduction in steady-state levels of assembled complexes was accompanied by reduced in-gel activity of complex I (Figure 6A, Figure S2C) and complex IV (Figure 6B, Figure S2D). The BN-PAGE and complex I in-gel activity analyses showed reduced steady-state levels of supercomplexes and the presence of a smaller, partially assembled, form of complex I in third-instar bsf KD larvae (Figure 6A, asterisk). Western blot analyses showed reduced levels of a nuclear encoded subunit of complex I (NDUFS3 subunit), indicating and supporting the notion that there is a severe reduction in steady-state levels of assembled complex I in third-instar bsf KD larvae (Figure 6C, Figure S2E) and flies (Figure 6D). There was no major reduction in steady-state levels of the nuclear-encoded complex V subunits (α-subunit of ATP synthase) in third-instar bsf KD larvae (Figure 6C, Figure S1B) and flies (Figure 6D), suggesting normal assembly. Together, these results show that third-instar bsf KD larvae and flies fail to assemble sufficient levels of complex I, III and IV as well as supercomplexes consisting of these complexes, which explains the observed profound reduction in oxidative phosphorylation capacity.
Fig. 6. Analyses of assembled respiratory chain enzyme complexes in bsf KD larvae. 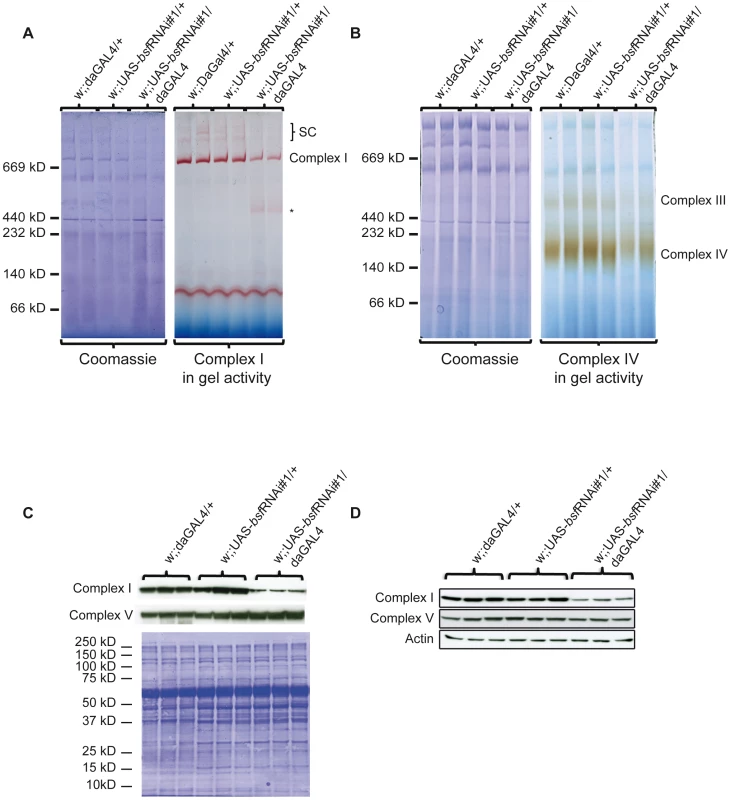
(A-B) BN-PAGE analysis of mitochondrial protein extracts from third-instar bsf KD and control larvae. (A) In-gel activity of complex I, (B) In-gel activity of complex IV. (C-D) Western blot analyses of levels of nuclear encoded subunit NDUFS3 (complex I) and the α-subunit of ATP synthase (complex V) in (C) third-instar bsf KD larvae and (D) one-day-old bsf KD flies. Discussion
BSF has previously been suggested to stabilize cytoplasmic mRNAs in oocytes and early zygotic cells during the first few hours of fly embryogenesis [1], [2]. However, the ubiquitous expression of the BSF RNA [3] and the punctuate cytoplasmic localization in flies has prompted us to re-investigate the function of BSF in the fly. Surprisingly, we were able to demonstrate that the BSF protein is mainly localized to mitochondria, where it controls polyadenylation of specific mitochondrial mRNAs. Further, BSF plays a key role in the regulation of mtDNA gene expression, by coordinating mitochondrial translation in flies.
GFP-tagged BSF localized to mitochondria in transfected tissue culture cells and cell fractionation experiments showed that BSF is mainly present in mitochondria of adult flies. Interestingly, BSF was not detectable in the cytoplasmic and nuclear fractions of adult flies. It is important to note that the mammalian homolog of BSF, LRPPRC, has also been reported to have roles in regulation of cytoplasmic mRNA transport [6]-[8] and in regulation of nuclear transcription [9], [10]. However, convincing evidence proposes that the main proportion of LRPPRC is localized to mitochondria [4], [10], [11]. In one study of tissue culture cells, endogenously expressed LRPPRC was not detectable in highly purified nuclei lacking mitochondrial contamination [4]. These findings do not exclude that there is a small fraction of BSF and LRPPRC localized in other subcellular compartments besides mitochondria. However, the proposed extramitochondrial functions of BSF and LRPPRC should be revisited in a new set of experiments focusing on avoiding mitochondrial contamination of isolated nuclei and cytoplasmic extracts.
Ubiquitous RNAi-induced reduction of endogenous bsf levels in flies causes mitochondrial dysfunction with decreased respiratory chain enzyme activities and reduced levels of assembled complex I, III and IV. This mitochondrial dysfunction leads to severe phenotypes, including delayed and incomplete eclosure, reduced fecundity, sterility and shortened life span.
Mammalian cell lines with reduced LRPPRC levels have reduced mitochondrial mRNA steady-state levels and reduced mitochondrial translation [12], [18]. Interestingly, downregulation of BSF protein levels in flies also leads to decreased steady-state levels of mitochondrial mRNAs but, in contrast with the results in mammals, translation is increased and aberrant translation products are generated. Some of the mitochondrial translation products that are synthesized at increased rates in flies are subject to increased degradation, suggesting that BSF might also act as a translational coordinator. Regulation of mitochondrial gene expression in response to different metabolic demands in animal cells is largely unknown, and most likely this regulation occurs at several different levels. The basic mtDNA transcription machinery has been defined [19], but it is unclear how it is regulated. Processing of mRNAs is required for correct translation [20], but the coupling between transcript processing and translation is poorly understood. Furthermore, mitochondria are in essence a prokaryotic system with no compartmentalization between transcription and translation, which makes it likely that both of these processes directly communicate.
Here we demonstrate that the loss of BSF results in incorrect processing of the polycistronic precursor transcripts and a failure to polyadenylate a subset of both processed and polycistronic transcripts. This failure to mature mitochondrial mRNAs, in turn, leads to the destabilization of mitochondrial mRNAs and reduced steady-state levels, despite a simultaneously increased de novo transcription. In animals all mitochondrial transcripts are polyadenylated as a part of becoming a mature mRNA ready for translation, except for ND6 of vertebrates, which in humans and fish has a long 3′UTR without any poly A tail [16], [21]. In the mammalian system it is believed that nearly all mitochondrial RNAs are oligoadenylated during transcription by a yet unknown enzyme, followed by the polyadenylation to approximately 50 adenines by the mitochondrial poly A polymerase (mtPAP) [22]. A number of mitochondrial transcripts require polyadenylation to generate functional stop codons, but otherwise the exact function of mRNA polyadenylation in mitochondria is unknown. While in bacteria and chloroplasts the poly A tail seems to promote transcript degradation, eukaryotes seem to polyadenylate nearly every fully processed cytosolic mRNA at the 3′ end, resulting in increased mRNA stability, increased translational efficiency, and promotion of transport of the processed mRNA from the nucleus to the cytoplasm [23]. A primary role of BSF in the maturation of mitochondrial mRNAs is supported by the recent observation that PPR proteins in trypanosomes affect polyadenylation of mitochondrial mRNAs. In this unicellular protozoon, the PPR proteins KPAF1 and KPAF2 associate with the mtPAP, stimulating mRNA polyadenylation and thereby coordinate stability and translation of mRNA [24]. Our results suggest that BSF is involved in the actual mRNA maturation process by controlling polyadenylation of specific transcripts.
The mammalian BSF homolog LRPPRC has been shown to be stabilized by a second RNA-binding protein called SLIRP, in a direct interaction [12]. A SLIRP homolog has also been suggested to exist in flies, raising the possibility that such an interaction with a second RNA binding protein is also required in fly mitochondria. Interestingly, bioinformatics analyses identified an additional LRPPRC homolog, besides BSF, in Drosphila melanogaster [4], suggesting that both homologs work together to control the polyadenylation and translation of different sets of mitochondrial mRNAs. This is supported by our observation that several mitochondrial transcripts, such as cytb and ATP6/8, are still polyadenylated in the bsf KD lines.
Loss of BSF also results in increased and aberrant translation, and reduced levels of assembled RC complex I, III and IV, suggesting that BSF coordinates mitochondrial translation and RC complex assembly. The coupling of transcription to translation is supported by studies of the yeast homologue of BSF and LRPPRC, PET309 [25]. This factor is implicated in activation of translation by binding to the 5′UTR of yeast mitochondrial mRNAs, thereby tethering mitochondrial polysomes to the inner mitochondrial membrane, which, in turn, is thought to ensure co-translational insertion of newly synthesized polypeptides into the assembling respiratory chain complexes [26], [27]. Mitochondrial mRNAs without these 5′-UTRs are translated at normal levels followed by rapid degradation, suggesting that targeting to the inner mitochondrial membrane is necessary for stability of the newly translated peptides [28]. It is important to point out that most mRNAs encoded by metazoan mtDNA lack 5′UTRs and targeting of translation to the inner membrane of animal mitochondria, if this occurs, must therefore involve, at least partly, different regulatory mechanisms.
Cytoplasmic mRNAs have several different types of destabilizing elements and it seems that translation is coupled to degradation of some classes of mRNAs [29]. If a similar mechanism exists in mitochondria, it would suggest that the increased de novo translation in BSF KD larvae results in increased degradation of the mitochondrial mRNAs. Some support for this proposed mechanism comes from characterization of a patient with a microdeletion between the genes for ATP6/8 and COXIII, which has been reported to cause incorrect processing of the corresponding mitochondrial mRNAs and severely reduced steady-state levels of the ATP6/8 transcript due to translationally induced deadenylation [30]. Additional support comes from the observation that human cells treated with the mitochondrial translation inhibitor thiamphenicol have increased steady-state levels of mitochondrial mRNAs [31]. However, decreased translation does not always increase mRNA steady-state levels as exemplified by the conditional mouse knockout for TFB1M, which abolishes mitochondrial translation, but does not affect the levels of most mitochondrial mRNAs despite activation of de novo transcription [32] .
In conclusion, we present here the unexpected result that the BSF protein mainly is localized to mitochondria, where it controls the polyadenylation of specific mitochondrial mRNAs. In addition, our results suggests that BSF has a novel role in coordinating mitochondrial translation as loss of BSF leads to increased and uncoordinated translation with increase synthesis of unstable translation products. BSF thus has an essential role in regulating mitochondrial function in the fly.
Materials and Methods
Drosophila stocks and maintenance
For in vivo KD studies two independent non-overlapping UAS-bsf RNAi lines were used. w;;UAS-bsfRNAi#1 (#10302R-I) was obtained from the National Institute of Genetics (Japan) and w;UAS-bsfRNAi#2 (#22839) was obtained from the Vienna Drosophila RNAi Center (VDRC). Ubiquitous bsf knock down was achieved by crossing UAS-bsf RNAi lines to a daughterless-GAL4 (da-GAL4) driver line. UAS-RNAi lines and daGAL4 driver lines were backcrossed for at least 6 generations into the white Dahomey background (wDahT). All fly stocks were free from the endosymbiontic bacterium Wolbachia. Flies were propagated and experiments were conducted at 25°C on a 12 h∶12 h light∶dark cycle at constant humidity on a standard sugar-agar-yeast medium.
Bioinformatics
Homologs of LRPPRC were collected using PSI-BLAST [33] against the RefSeq protein database at NCBI and aligned using ClustalX [34] with the BLOSUM matrix. Multiple sequence alignments were trimmed using the GBLOCKS server [35] with relaxed settings. The trimmed alignment was submitted to PhyML [36] using standard parameters and non-parametric bootstrap analysis with 500 replicates. The resulting tree was displayed using Dendroscope [37] and prepared for publication in Illustrator.
Colocalization studies
Full-length bsf cDNA was obtained from the Drosophila Genomics Resource Center (SD10676, AY058795). Two amino acid changing substitutions were identified in the bsf cDNA in comparison with the reference sequence (FBtr0081087). The corresponding mutations at nucleotide positions 415 and 710 were changed by site-directed mutagenesis of the cDNA, using the QuickChange II XL Site-Directed Mutagenesis Kit (Agilent Technologies). A cDNA encoding FLAG-tagged BSF was cloned into the plasmid pAcGFP1-N2 (Clontech, Mountain View, USA) to generate the vector pbsfFLAG-AcGFP1, which encodes a fusion protein consisting of BSF-FLAG with an in-frame addition of green fluorescent protein (GFP) to its carboxy-terminus (BSF-FLAG-GFP).
Schneider 2R+ and HeLa cells were transfected in microscopy dishes (µ-Dish, ibidi, Martinsried, Germany) with pbsfFLAG-AcGFP1 using the FuGENE HD Transfection Reagent (Roche Diagnostics, Mannheim, Germany). The mitochondrial counterstaining was achieved by 100 nm MitoTracker Deep Red FM (Invitrogen, Darmstadt, Germany). Live cell image acquisition was performed with a Leica TCS SP5-X confocal microscope (Leica Microsystems, Wetzlar, Germany).
The colocalization rate was determined by the software LAS AF (Leica Microsystems, Wetzlar, Germany) under the following conditions and calculations: threshold 30%, background 20%, colocalization rate [%] = colocalization area/area foreground, and area foreground = area image − area background.
Subcellular fractionation and Western blot analysis
All fractions were isolated from adult wDahT flies. Mitochondrial, cytoplasmic and nuclear fractions were isolated by differential centrifugation as previously described [38]. The used primary antibodies were: HISTONE H3 (Santa Cruz Biotechnology, dilution 1∶200), Complex I-subunit NDUFS3 (Mitoscience MS112, dilution 1∶1000), tubulin (Sigma, dilution 1∶1000) and polyclonal rat antisera raised against BSF (kindly provided by Professor MacDonald PM, Stanford University, dilution 1∶1000). Protein bands were visualized with ECL western blotting reagents (Bio-Rad).
Western blot analyses were performed using whole fly or mitochondrial protein extracts according to the Cell Signaling Technology protocol (CellSignaling). Additional primary antibodies used were: complex V (Mitoscience MS504, dilution 1∶5000), VDAC (Mitoscience MSAO3, dilution 1∶2000), actin (Sigma, dilution 1∶1000).
Hatching rate, lifespan, fecundity, and climbing assays of flies
For adult hatching rate measurements eggs were collected during a 3 hours time window and transferred to vials (80 eggs/vial) to ensure standard larval density. Hatching of adult flies was monitored in regular intervals. After hatching, virgin females and males were collected and mated for 2 days.
For lifespan analyses, 50–100 females per genotype were used at a density of 10 flies per vial. Flies were transferred to new vials every two to three days and dead flies were counted. For fecundity assays, 100 females were equally distributed in ten vials, transferred to new vials every day and the number of eggs was counted.
Climbing assays were conducted and performance index was calculated as described [39]. 100 three-day-old males per genotype were tested for their climbing ability.
DNA isolation and quantitative RT–PCR
DNA of third-instar larvae was extracted using the DNAeasy Kit (Qiagen). Mitochondrial DNA levels were determined by quantitative real-time PCR (QRT-PCR) on a 7900HT Real Time PCR system (Applied Biosystems), using SYBR green master mix (Invitrogen). Reactions were carried out in triplicates per sample in a final volume of 20 µl with 5 ng of DNA and 10 pmol of specific primers (primers are listed in Table S1).
RNA isolation, QRT-PCR, and Northern blot analysis
Total RNA from third-instar larvae or adult flies was extracted using the Totally RNA KIT (Ambion). Reverse transcription and QRT-PCR was performed using the High capacity RNA-to-cDNA kit (Applied Biosystems) and the Taqman 2x Universal PCR mastermix, No Amperase UNG (Applied Biosystems), respectively. Custom-made TaqMan probes against Drosophila mitochondrial transcripts were obtained from Applied Biosystems and are listed in Table S1.
For Northern blot analyses, RNA was fractionated on 1.2% agarose gels and blotted to Hybond-N+ membranes (Amersham Biosciences). Membranes were hybridized with 32P-labeled probes and afterwards exposed to PhosphoImager Screens and/or X-ray films. Labeling of mitochondrial double-stranded DNA probes and oligonucleotides was performed as described [40]. Primers and oligonucleotides used for Northern blot are listed in Table S1.
Biochemical evaluation of respiratory chain function
Isolation of mitochondria from third-instar larvae was performed as described [41] with modifications in buffer composition. Briefly, third-instar larvae were washed and gently homogenized in ice-cold MSB buffer (210 mM mannitol, 70 mM sucrose, 10 mM EDTA, 50 mM Tris, pH 7.5) using 15 ml Dounce homogenizers. Protein concentration was determined using Bradford assay and aliquots corresponding to 10 µg mitochondrial proteins were pelleted and resuspended in resuspension buffer (250 mM sucrose, 15 mM K2HPO4, 2 mM MgAc2, 0.5 mM EDTA and 0.5 g/L HSA, pH 7.2). Biochemical activities of respiratory chain complexes were determined as described [42].
Respiratory rates
Third-instar larvae (n = 10) or thoraces from adult flies (n = 5) were dissected in PBS and resuspended in 2 ml of respiratory buffer (120 mM sucrose, 50 mM KCl, 20 mM Tris-HCl, 4 mM KH2PO4, 2 mM MgCl2, 1 mM EGTA, 0.01% digitonin, pH 7.2). Oxygen consumption was measured at 25°C using an oxygraph chamber (OROBOROS). Complex I-dependent respiration was assessed by adding the substrates proline (10 mM), pyruvate (10 mM), malate (5 mM) and glutamate (5 mM). Succinate and glycerol-3-phosphate dehydrogenase activities were measured using 20 mM succinate (SUCC) and 15 mM glycerol-3-phosphate (G3P), respectively. Mitochondrial quality of each sample was assessed by measuring the respiratory control rate (RCR), using 1 mM ADP (state 3) or 1 mM ADP and 2.5 µg/ml oligomycin (pseudo state 4). Permeabilized control mitochondria consistently had RCR values between 4 and 7 with complex I substrates.
The respiration was uncoupled by the addition of 400 µM CCCP and the rotenone-sensitive flux was measured in the presence of 200 µM rotenone. Finally, the protein content was determined by the Bradford method (BioRad) in order to normalize the oxygen consumption flux to mitochondrial protein content.
ATP/ADP and lactate/pyruvate ratios
Flies (n = 10) were snap frozen directly in liquid nitrogen and kept at -80°C. Acidic extraction (PCA 7%) was performed, samples were centrifuged (16000 g, 10 min), the supernatant was neutralized with 2N KOH, 10 mM MOPS and metabolites were quantified. ADP and ATP levels were assessed as previously described [43], [44]. Briefly, ATP was quantified by using the ATPlite one step kit (PerkinElmer). For ADP levels, samples were incubated for 10 min at 37°C in 75 mM KCl, 8 mM MgSO4, 10 µg/ml pyruvate kinase and 2 mM phosphoenolpyruvate. Lactate and pyruvate concentrations were determined after 1 h incubation with horse radish peroxydase 5 U/ml, Amplex red 20 µM, 0.1 M phosphate, pH 7.2, supplemented with lactate oxidase or pyruvate oxidase, followed by fluorimetric analysis (ex:560 nm, em:590 nm), using an Infinite 200 Pro fluorimeter (Tecan).
In organello transcription and translation assays
For the preparation of mitochondria, third-instar larvae or flies were homogenized in ice-cold isolation buffer STE+BSA (250 mM sucrose, 5 mM Tris, 2 mM EGTA, 1% (w/v) BSA, pH 7.4) using a 15 ml Dounce homogenizer. Cellular debris were pelleted at 1000 g for 5 min and supernatants were transferred to new tubes. Mitochondria were washed two times and final mitochondrial pellets were resuspend in 1 ml STE buffer in the presence of 200 µg/ml emetine (Sigma) and 100 µg/ml cycloheximide (Sigma) to inhibit cytoplasmic translation. Protein concentrations were determined using the Bradford assay.
In organello transcription assays were performed as described [45] using 200 µg mitochondria/sample and a modified transcription buffer (25 mM sucrose, 75 mM sorbitol, 100 mM KCl, 10 mM K2HPO4, 50 µM EDTA, 5 mM MgCl2, 1 mM ADP, 10 mM glutamate, 2.5 mM malate, 10 mM Tris-HCl (pH 7.4) and 1% (w/v) BSA). In short, after labeling, mitochondrial RNA was isolated using Totally RNA kit (Ambion). Mitochondrial RNA was fractionated on 1.2% agarose gels and blotted to Hybond-N+ membranes (Amersham Biosciences).
In vitro assays to study mitochondrial de novo translation with [35S]-methionine were performed as described [46]. Equal amounts of total mitochondrial protein were loaded on 15% SDS-PAGE gels. Gels were fixed in isopropanol-acetic solution, stained with Coomassie, destained in ethanol-acetic acid solution and treated with Amplify Solution (GE Healthcare). Afterwards gels were dried and [35S]–methionine-labelled proteins were visualized by autoradiography. The mitochondrial translation profile was compared to previously published profiles in Schneider cell lines [47], additionally ND2 and ATP6 were identified by endopeptidase fingerprinting in the second dimension (data not shown) [48].
RNA circularization and RT–PCR
An RNA circularization protocol was modified from [49], [50]. Approximately 6 ng total mitochondrial RNA was circularized with 5 U T4 RNA ligase in 200 µl at 16°C for at least 16 h in manufacturer-supplied buffer (NEB). The circularized RNA was precipitated with an equal volume of isopropanol, incubated at -20°C for at least 4 h, and centrifuged for 20 min at top speed in a bench top centrifuge. The entire precipitate was used for complementary DNA synthesis with gene specific primers using GeneAmp RNA PCR kit (Applied Biosystems). PCR products were purified using ExoSAP-IT (Affymetrix) and sequenced. Selected PCR products were cloned into pCR-II (Invitrogen) and sequenced in order to confirm the results from direct sequencing. Primer sequences for RT-PCR and subsequent PCR are contained within Table S2.
Blue native-polyacrylamide gel electrophoresis (BN-PAGE) and in-gel histochemistry
For BN-PAGE, 75 µg of mitochondria were pelleted and lyzed in 50 µl ice-cold digitonin buffer (1% digitonin, 20 mM Tris pH 7.4, 0.1 mM EDTA, 50 mM NaCl, 10% glycerol, 1 mM PMSF). After 15 min of incubation on ice, unsolubilized material was removed by centrifugation at 4°C. The supernatant was mixed with 5 µl of 10 x loading dye (5% (w/v) Coomassie Brilliant Blue G-250, 100 mM Tris pH 7, 500 mM 6-aminocaproic acid) and loaded on 4–10% gradient BN-PAGE gels [51], [52]. In gel complex I activity was determined by incubating the BN-PAGE gels in 2 mM Tris-HCl pH 7.4, 0.1 mg/ml NADH (Roche) and 2.5 mg/ml iodonitrozolium (Sigma). In gel complex IV activity was determined by incubating the BN-PAGE gels in 50 ml of 0.05 mM phosphate buffer pH 7.4, 25 mg 3.3′-diamidobenzidine tetrahydrochloride (DAB), 50 mg cytochrome c, 3.75 g sucrose and 1 mg catalase. All stainings were carried out at room temperature.
Statistical analysis
Data were presented as mean ± SD. The Mann-Whitney test was used to analyze climbing index and the log-rank test was used to analyze lifespan. Unpaired t-test was used to analyze all other data statistically.
Supporting Information
Zdroje
1. ManceboRZhouXShillinglawWHenzelWMacdonaldPM 2001 BSF binds specifically to the bicoid mRNA 3′ untranslated region and contributes to stabilization of bicoid mRNA. Mol Cell Biol 21 3462 3471 doi:10.1128/MCB.21.10.3462-3471.2001
2. De RenzisSElementoOTavazoieSWieschausEF 2007 Unmasking activation of the zygotic genome using chromosomal deletions in the Drosophila embryo. PLoS Biol 5 e117 doi:10.1371/journal.pbio.0050117
3. ChintapalliVRWangJDowJAT 2007 Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat Genet 39 715 720 doi:10.1038/ng2049
4. SterkyFHRuzzenenteBGustafssonCMSamuelssonTLarssonN-G 2010 LRPPRC is a mitochondrial matrix protein that is conserved in metazoans. Biochemical and Biophysical Research Communications 398 759 764 doi:10.1016/j.bbrc.2010.07.019
5. LurinCAndrésCAubourgSBellaouiMBittonF 2004 Genome-wide analysis of Arabidopsis pentatricopeptide repeat proteins reveals their essential role in organelle biogenesis. Plant Cell 16 2089 2103 doi:10.1105/tpc.104.022236
6. MiliSShuHJZhaoYPiñol-RomaS 2001 Distinct RNP complexes of shuttling hnRNP proteins with pre-mRNA and mRNA: candidate intermediates in formation and export of mRNA. Mol Cell Biol 21 7307 7319 doi:10.1128/MCB.21.21.7307-7319.2001
7. TsuchiyaNFukudaHNakashimaKNagaoMSugimuraT 2004 LRP130, a single-stranded DNA/RNA-binding protein, localizes at the outer nuclear and endoplasmic reticulum membrane, and interacts with mRNA in vivo. Biochemical and Biophysical Research Communications 317 736 743 doi:10.1016/j.bbrc.2004.03.103
8. TopisirovicISiddiquiNLapointeVLTrostMThibaultP 2009 Molecular dissection of the eukaryotic initiation factor 4E (eIF4E) export-competent RNP. EMBO J 28 1087 1098 doi:10.1038/emboj.2009.53
9. LabialleSDayanGGayetLRigalDGambrelleJ 2004 New invMED1 element cis-activates human multidrug-related MDR1 and MVP genes, involving the LRP130 protein. Nucleic Acids Res 32 3864 3876 doi:10.1093/nar/gkh722
10. CooperMPQuLRohasLMLinJYangW 2006 Defects in energy homeostasis in Leigh syndrome French Canadian variant through PGC-1alpha/LRP130 complex. Genes Dev 20 2996 3009 doi:10.1101/gad.1483906
11. XuFMorinCMitchellGAckerleyCRobinsonBH 2004 The role of the LRPPRC (leucine-rich pentatricopeptide repeat cassette) gene in cytochrome oxidase assembly: mutation causes lowered levels of COX (cytochrome c oxidase) I and COX III mRNA. Biochem J 382 331 336 doi:10.1042/BJ20040469
12. SasarmanFBrunel-GuittonCAntonickaHWaiTShoubridgeEA 2010 LRPPRC and SLIRP interact in a ribonucleoprotein complex that regulates posttranscriptional gene expression in mitochondria. Mol Biol Cell 21 1315 1323 doi:10.1091/mbc.E10-01-0047
13. BooreJL 1999 Animal mitochondrial genomes. Nucleic Acids Res 27 1767 1780
14. MontoyaJOjalaDAttardiG 1981 Distinctive features of the 5′-terminal sequences of the human mitochondrial mRNAs. Nature 290 465 470
15. OjalaDMontoyaJAttardiG 1981 tRNA punctuation model of RNA processing in human mitochondria. Nature 290 470 474
16. TemperleyRJWydroMLightowlersRNChrzanowska-LightowlersZM 2010 Human mitochondrial mRNAs--like members of all families, similar but different. Biochim. Biophys. Acta 1797 1081 1085 doi:10.1016/j.bbabio.2010.02.036
17. TomeckiRDmochowskaAGewartowskiKDziembowskiAStepienPP 2004 Identification of a novel human nuclear-encoded mitochondrial poly(A) polymerase. Nucleic Acids Res 32 6001 6014 doi:10.1093/nar/gkh923
18. GohilVMNilssonRBelcher-TimmeCALuoBRootDE 2010 Mitochondrial and nuclear genomic responses to loss of LRPPRC expression. Journal of Biological Chemistry 285 13742 13747 doi:10.1074/jbc.M109.098400
19. FalkenbergMLarssonN-GGustafssonCM 2007 DNA replication and transcription in mammalian mitochondria. Annu Rev Biochem 76 679 699 doi:10.1146/annurev.biochem.76.060305.152028
20. GagliardiDStepienPPTemperleyRJLightowlersRNChrzanowska-LightowlersZMA 2004 Messenger RNA stability in mitochondria: different means to an end. Trends Genet 20 260 267 doi:10.1016/j.tig.2004.04.006
21. CoucheronDHNymarkMBreinesRKarlsenBOAndreassenM 2011 Characterization of mitochondrial mRNAs in codfish reveals unique features compared to mammals. Curr Genet 57 213 222 doi:10.1007/s00294-011-0338-2
22. BobrowiczAJLightowlersRNChrzanowska-LightowlersZ 2008 Polyadenylation and degradation of mRNA in mammalian mitochondria: a missing link? Biochem. Soc Trans 36 517 519 doi:10.1042/BST0360517
23. ColganDFManleyJL 1997 Mechanism and regulation of mRNA polyadenylation. Genes Dev 11 2755 2766
24. AphasizhevaIMaslovDWangXHuangLAphasizhevR 2011 Pentatricopeptide repeat proteins stimulate mRNA adenylation/uridylation to activate mitochondrial translation in trypanosomes. Mol Cell 42 106 117 doi:10.1016/j.molcel.2011.02.021
25. MoothaVKLepagePMillerKBunkenborgJReichM 2003 Identification of a gene causing human cytochrome c oxidase deficiency by integrative genomics. Proc Natl Acad Sci USA 100 605 610 doi:10.1073/pnas.242716699
26. MantheyGMMcEwenJE 1995 The product of the nuclear gene PET309 is required for translation of mature mRNA and stability or production of intron-containing RNAs derived from the mitochondrial COX1 locus of Saccharomyces cerevisiae. EMBO J 14 4031 4043
27. MantheyGMPrzybyla-ZawislakBDMcEwenJE 1998 The Saccharomyces cerevisiae Pet309 protein is embedded in the mitochondrial inner membrane. Eur J Biochem 255 156 161
28. SanchiricoMEFoxTDMasonTL 1998 Accumulation of mitochondrially synthesized Saccharomyces cerevisiae Cox2p and Cox3p depends on targeting information in untranslated portions of their mRNAs. EMBO J 17 5796 5804 doi:10.1093/emboj/17.19.5796
29. SachsAB 1993 Messenger RNA degradation in eukaryotes. Cell 74 413 421
30. TemperleyRJSenecaSHTonskaKBartnikEBindoffLA 2003 Investigation of a pathogenic mtDNA microdeletion reveals a translation-dependent deadenylation decay pathway in human mitochondria. Hum Mol Genet 12 2341 2348 doi:10.1093/hmg/ddg238
31. Chrzanowska-LightowlersZMPreissTLightowlersRN 1994 Inhibition of mitochondrial protein synthesis promotes increased stability of nuclear-encoded respiratory gene transcripts. J Biol Chem 269 27322 27328
32. MetodievMDLeskoNParkCBCamaraYShiY 2009 Methylation of 12S rRNA is necessary for in vivo stability of the small subunit of the mammalian mitochondrial ribosome. Cell Metab 9 386 397 doi:10.1016/j.cmet.2009.03.001
33. AltschulSFMaddenTLSchäfferAAZhangJZhangZ 1997 Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25 3389 3402
34. ChennaRSugawaraHKoikeTLopezRGibsonTJ 2003 Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res 31 3497 3500
35. TalaveraGCastresanaJ 2007 Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst Biol 56 564 577 doi:10.1080/10635150701472164
36. GuindonSDelsucFDufayardJ-FGascuelO 2009 Estimating maximum likelihood phylogenies with PhyML. Methods Mol Biol 537 113 137 doi:10.1007/978-1-59745-251-9_6
37. HusonDHRichterDCRauschCDezulianTFranzM 2007 Dendroscope: An interactive viewer for large phylogenetic trees. BMC Bioinformatics 8 460 doi:10.1186/1471-2105-8-460
38. CoxBEmiliA 2006 Tissue subcellular fractionation and protein extraction for use in mass-spectrometry-based proteomics. Nat Protoc 1 1872 1878 doi:10.1038/nprot.2006.273
39. GreeneJCWhitworthAJKuoIAndrewsLAFeanyMB 2003 Mitochondrial pathology and apoptotic muscle degeneration in Drosophila parkin mutants. Proc Natl Acad Sci USA 100 4078 4083 doi:10.1073/pnas.0737556100
40. FreyerCParkCBEkstrandMIShiYKhvorostovaJ 2010 Maintenance of respiratory chain function in mouse hearts with severely impaired mtDNA transcription. Nucleic Acids Res 38 6577 6588 doi:10.1093/nar/gkq527
41. Fernández-MorenoMAFarrCLKaguniLSGaresseR 2007 Drosophila melanogaster as a model system to study mitochondrial biology. Methods Mol Biol 372 33 49
42. WredenbergAWibomRWilhelmssonHGraffCWienerHH 2002 Increased mitochondrial mass in mitochondrial myopathy mice. Proc Natl Acad Sci USA 99 15066 15071 doi:10.1073/pnas.232591499
43. KimmichGARandlesJBrandJS 1975 Assay of picomole amounts of ATP, ADP, and AMP using the luciferase enzyme system. Anal Biochem 69 187 206
44. MourierADevinARigouletM 2010 Active proton leak in mitochondria: a new way to regulate substrate oxidation. Biochim Biophys Acta 1797 255 261 doi:10.1016/j.bbabio.2009.10.011
45. EnríquezJAPérez-MartosALópez-PérezMJMontoyaJ 1996 In organello RNA synthesis system from mammalian liver and brain. Meth Enzymol 264 50 57
46. CôtéCPoirierJBouletD 1989 Expression of the mammalian mitochondrial genome. Stability of mitochondrial translation products as a function of membrane potential. J Biol Chem 264 8487 8490
47. RobertiMBruniFLoguercio PolosaPManzariCGadaletaMN 2006 MTERF3, the most conserved member of the mTERF-family, is a modular factor involved in mitochondrial protein synthesis. Biochim Biophys Acta 1757 1199 1206 doi:10.1016/j.bbabio.2006.04.026
48. SasarmanFAntonickaHShoubridgeEA 2008 The A3243G tRNALeu(UUR) MELAS mutation causes amino acid misincorporation and a combined respiratory chain assembly defect partially suppressed by overexpression of EFTu and EFG2. Hum Mol Genet 17 3697 3707 doi:10.1093/hmg/ddn265
49. CouttetPFromont-RacineMSteelDPictetRGrangeT 1997 Messenger RNA deadenylylation precedes decapping in mammalian cells. Proc Natl Acad Sci USA 94 5628 5633
50. StewartJBBeckenbachAT 2009 Characterization of mature mitochondrial transcripts in Drosophila, and the implications for the tRNA punctuation model in arthropods. Gene 445 49 57 doi:10.1016/j.gene.2009.06.006
51. SchäggerHJagow vonG 1991 Blue native electrophoresis for isolation of membrane protein complexes in enzymatically active form. Anal Biochem 199 223 231
52. DekkerPJMüllerHRassowJPfannerN 1996 Characterization of the preprotein translocase of the outer mitochondrial membrane by blue native electrophoresis. Biol Chem 377 535 538
Štítky
Genetika Reprodukční medicína
Článek Macroautophagy Is Regulated by the UPR–Mediator CHOP and Accentuates the Phenotype of SBMA MiceČlánek Dynamic Replacement of Histone H3 Variants Reprograms Epigenetic Marks in Early Mouse EmbryosČlánek Mutations in a Guanylate Cyclase GCY-35/GCY-36 Modify Bardet-Biedl Syndrome–Associated Phenotypes in
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2011 Číslo 10
-
Všechny články tohoto čísla
- Transcriptional Robustness Complements Nonsense-Mediated Decay in Humans
- Identification, Replication, and Fine-Mapping of Loci Associated with Adult Height in Individuals of African Ancestry
- Genetic Determinants of Serum Testosterone Concentrations in Men
- A One Base Pair Deletion in the Canine Gene Causes Exon Skipping and Late-Onset Neuronal Ceroid Lipofuscinosis in the Tibetan Terrier
- Three Structure-Selective Endonucleases Are Essential in the Absence of BLM Helicase in
- Identification of Widespread Ultra-Edited Human RNAs
- Multiple Wnts Redundantly Control Polarity Orientation in Epithelial Stem Cells
- The Bicoid Stability Factor Controls Polyadenylation and Expression of Specific Mitochondrial mRNAs in
- Transcriptome-Wide Binding Sites for Components of the Non-Poly(A) Termination Pathway: Nrd1, Nab3, and Sen1
- Macroautophagy Is Regulated by the UPR–Mediator CHOP and Accentuates the Phenotype of SBMA Mice
- Genetic Rearrangements Can Modify Chromatin Features at Epialleles
- Novel Function of as a Gap Gene during Spider Segmentation
- A Genome-Wide Screen for Interactions Reveals a New Locus on 4p15 Modifying the Effect of Waist-to-Hip Ratio on Total Cholesterol
- Comparative Genomic Analysis of Human Fungal Pathogens Causing Paracoccidioidomycosis
- Genetic Diversity in Cytokines Associated with Immune Variation and Resistance to Multiple Pathogens in a Natural Rodent Population
- Mutator Suppression and Escape from Replication Error–Induced Extinction in Yeast
- Dynamic Replacement of Histone H3 Variants Reprograms Epigenetic Marks in Early Mouse Embryos
- A Barcode Screen for Epigenetic Regulators Reveals a Role for the NuB4/HAT-B Histone Acetyltransferase Complex in Histone Turnover
- HIF–VEGF Pathways Are Critical for Chronic Otitis Media in and Mouse Mutants
- A Conserved Developmental Patterning Network Produces Quantitatively Different Output in Multiple Species of Drosophila
- Role of Exonic Variation in Chemokine Receptor Genes on AIDS: Association with Pneumocystis Pneumonia
- Whole-Exome Sequencing Identifies Homozygous Mutations in a Spastic Ataxia-Neuropathy Syndrome Linked to Mitochondrial -AAA Proteases
- Von Hippel-Lindau () Inactivation in Sporadic Clear Cell Renal Cancer: Associations with Germline Polymorphisms and Etiologic Risk Factors
- A Systems Biology Approach Reveals the Role of a Novel Methyltransferase in Response to Chemical Stress and Lipid Homeostasis
- Identification of Genomic Regions Associated with Phenotypic Variation between Dog Breeds using Selection Mapping
- Global Mapping of Cell Type–Specific Open Chromatin by FAIRE-seq Reveals the Regulatory Role of the NFI Family in Adipocyte Differentiation
- Natural Selection Affects Multiple Aspects of Genetic Variation at Putatively Neutral Sites across the Human Genome
- MicroRNA Expression and Regulation in Human, Chimpanzee, and Macaque Brains
- An Adaptive Allelic Series Featuring Complex Gene Rearrangements
- Feed-Forward Microprocessing and Splicing Activities at a MicroRNA–Containing Intron
- Developmental Stability: A Major Role for in
- A Phenomics-Based Strategy Identifies Loci on , , and Associated with Metabolic Syndrome Phenotype Domains
- Association of , , , , and with Systemic Lupus Erythematosus
- Small RNAs Prevent Transcription-Coupled Loss of Histone H3 Lysine 9 Methylation in
- Successive Increases in the Resistance of to Viral Infection through a Transposon Insertion Followed by a Duplication
- Mutations in a Guanylate Cyclase GCY-35/GCY-36 Modify Bardet-Biedl Syndrome–Associated Phenotypes in
- The Glycobiome Reveals Mechanisms of Pentose and Hexose Co-Utilization in Bacteria
- Insights into Hox Protein Function from a Large Scale Combinatorial Analysis of Protein Domains
- Mutations Cause Seckel and Jawad Syndromes
- Zelda Binding in the Early Embryo Marks Regions Subsequently Activated at the Maternal-to-Zygotic Transition
- Temporal Coordination of Gene Networks by Zelda in the Early Embryo
- Genetic Interaction between MTMR2 and FIG4 Phospholipid Phosphatases Involved in Charcot-Marie-Tooth Neuropathies
- Oxr1 Is Essential for Protection against Oxidative Stress-Induced Neurodegeneration
- Transforming Growth Factor β Receptor Type 1 Is Essential for Female Reproductive Tract Integrity and Function
- Positional Cloning of a Type 2 Diabetes Quantitative Trait Locus; , a Negative Regulator of Insulin Secretion
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The Glycobiome Reveals Mechanisms of Pentose and Hexose Co-Utilization in Bacteria
- Global Mapping of Cell Type–Specific Open Chromatin by FAIRE-seq Reveals the Regulatory Role of the NFI Family in Adipocyte Differentiation
- Genetic Determinants of Serum Testosterone Concentrations in Men
- MicroRNA Expression and Regulation in Human, Chimpanzee, and Macaque Brains
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



