-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaIdentification and Functional Validation of the Novel Antimalarial
Resistance Locus in
The Plasmodium falciparum parasite's ability to adapt to
environmental pressures, such as the human immune system and antimalarial drugs,
makes malaria an enduring burden to public health. Understanding the genetic
basis of these adaptations is critical to intervening successfully against
malaria. To that end, we created a high-density genotyping array that assays
over 17,000 single nucleotide polymorphisms (∼1 SNP/kb), and applied it to
57 culture-adapted parasites from three continents. We characterized genome-wide
genetic diversity within and between populations and identified numerous loci
with signals of natural selection, suggesting their role in recent adaptation.
In addition, we performed a genome-wide association study (GWAS), searching for
loci correlated with resistance to thirteen antimalarials; we detected both
known and novel resistance loci, including a new halofantrine resistance locus,
PF10_0355. Through functional testing we demonstrated that
PF10_0355 overexpression decreases sensitivity to
halofantrine, mefloquine, and lumefantrine, but not to structurally unrelated
antimalarials, and that increased gene copy number mediates resistance. Our GWAS
and follow-on functional validation demonstrate the potential of genome-wide
studies to elucidate functionally important loci in the malaria parasite
genome.
Published in the journal: . PLoS Genet 7(4): e32767. doi:10.1371/journal.pgen.1001383
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1001383Summary
The Plasmodium falciparum parasite's ability to adapt to
environmental pressures, such as the human immune system and antimalarial drugs,
makes malaria an enduring burden to public health. Understanding the genetic
basis of these adaptations is critical to intervening successfully against
malaria. To that end, we created a high-density genotyping array that assays
over 17,000 single nucleotide polymorphisms (∼1 SNP/kb), and applied it to
57 culture-adapted parasites from three continents. We characterized genome-wide
genetic diversity within and between populations and identified numerous loci
with signals of natural selection, suggesting their role in recent adaptation.
In addition, we performed a genome-wide association study (GWAS), searching for
loci correlated with resistance to thirteen antimalarials; we detected both
known and novel resistance loci, including a new halofantrine resistance locus,
PF10_0355. Through functional testing we demonstrated that
PF10_0355 overexpression decreases sensitivity to
halofantrine, mefloquine, and lumefantrine, but not to structurally unrelated
antimalarials, and that increased gene copy number mediates resistance. Our GWAS
and follow-on functional validation demonstrate the potential of genome-wide
studies to elucidate functionally important loci in the malaria parasite
genome.Introduction
Plasmodium falciparum malaria is a major public health challenge that contributes significantly to global morbidity and mortality. Efforts to control and eliminate malaria combine antimalarial drugs, bed nets and indoor residual spraying, with vaccine development a longer-term goal. Genetic variation in the parasite population threatens to undermine these efforts, as the parasite evolves rapidly to evade host immune systems, drugs and vaccines. Studying genetic variation in parasite populations will expand our understanding of basic parasite biology and its ability to adapt, and will allow us to track parasites as they respond to intervention efforts. Such understanding is increasingly important as countries move towards reducing disease burden and the ultimate elimination of malaria.
Given the potential impact of rapid evolution of P. falciparum in response to control and eradication strategies, discovery and characterization of P. falciparum genetic diversity has accelerated in recent years. Since the first malaria genome was sequenced in 2002 [1], over 60,000 unique SNPs have been identified by concerted sequencing efforts [2]–[4], and several genomic tiling arrays [5]–[9] and low-density SNP arrays [10], [11] have been developed to query this genetic variation. Recently the first malaria GWAS was published [11], in which 189 drug-phenotyped parasites from Asia, Africa and the Americas were genotyped using a low-density array (3,257 SNPs); that study identified loci under positive selection and found several novel drug resistance candidates.
For our study, we adopted two strategies for identifying genes involved in the malaria parasite's adaptive response: searching for signals of recent or ongoing natural selection, and searching for loci associated with one important clinical adaptation—resistance to antimalarial drugs. To make these searches possible, we began by sequencing 9 geographically diverse strains of P. falciparum to identify novel variation, thereby doubling the number of publicly available SNPs to 111,536 (all accessible at plasmodb.org), and used these SNPs to develop a high-density genotyping array assaying 17,582 validated markers. After characterizing linkage disequilibrium and population structure in our samples, we used the arrays to search for evidence of both ongoing balancing selection and recent positive selection, and to carry out a GWAS that sought loci associated with resistance to thirteen antimalarial agents. We then followed up one of the novel loci associated with drug resistance in order to verify that variation there was biologically involved in modulating drug response.
Results
Genetic Diversity
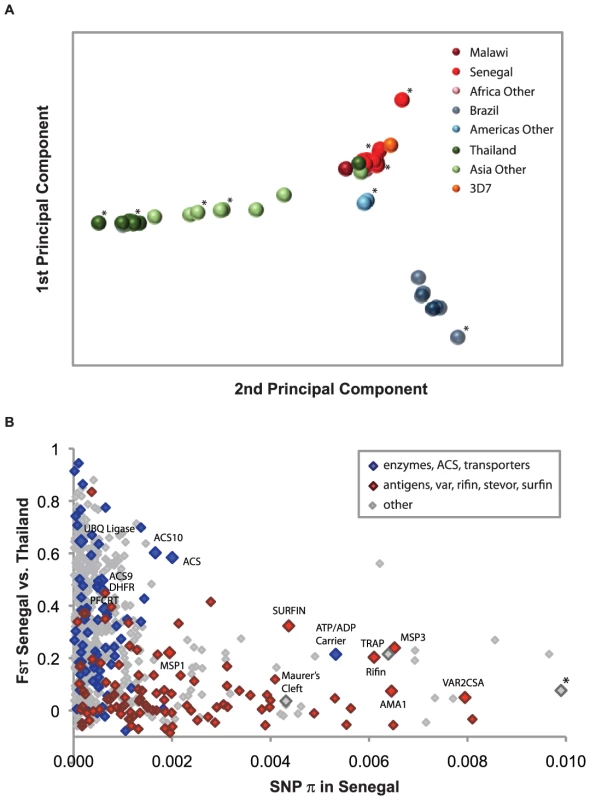
We identified global population structure among malaria parasites using principal components analysis (PCA) of 57 genotyped culture-adapted parasite samples (Figure 1A, Table S1, Figure S1). African, American and Asian samples form three distinct clusters, reflecting the likely independent introduction of P. falciparum from Africa into Asia and the Americas. There was little evidence for structure within Africa, suggesting high gene flow throughout the region (Figure S1). Asian and American parasites however both show substantial internal structure.
There are also dramatic differences in linkage disequilibrium (LD) between populations, with substantial LD extending less than 1 kb in Senegal, 10 kb in Thailand, and 100 kb in Brazil (Figure S2). These observations are consistent with previous findings, which showed that LD decays more rapidly in Africa, due either to founder effects in other continents [12] or to elevated outcrossing frequencies in Africa [12], [13], where higher transmission intensity leads to a greater likelihood of sexual outcrossing rather than selfing within the mid-gut of vector mosquitoes.
The short LD in malaria, driven by high levels of recombination, means that a high density of markers is required to identify candidate loci in association studies, since causal variants not on the array can seldom be tagged by neighboring alleles (Table S2). On the other hand, short LD can aid in fine-mapping candidate associations and greatly accelerates the search for causal genes. Short LD also aids in identifying genomic regions under recent positive selection with recombination-based methods, since the increased LD in selected regions should stand out against the short-LD background.
Detecting Signals of Natural Selection
We expect that many parasite proteins that interact with the host immune system will be under balancing selection, because they will be under selective pressure to maintain high levels of diversity. Indeed, previous studies have shown that regions of the P. falciparum genome that are highly polymorphic and appear to be under balancing selection encode antigens that are recognized by the human immune system [4]. We examined evidence for balancing selection in our data by searching for regions with high nucleotide diversity (as measured by SNP π) and low population divergence (as measured by FST) (Figure 1B). When we examined the loci lying in this region of the graph (Figure S3), we found a number of known antigens and vaccine candidates. Loci in the same region with unknown function are thus potential novel antigens that trigger human immune response to malaria, and may prove useful as biomarkers or as candidate vaccine molecules.
We carried out a similar search for loci under positive selection by identifying regions with both low nucleotide diversity within Senegal and Thailand and high population divergence between the two populations (Figure 1B). We observed throughout the genome that nucleotide diversity was lower for nonsynonymous SNPs than for intergenic SNPs (Figure S4), a characteristic result of widespread purifying selection. At the same time, nonsynonymous SNPs exhibited significantly greater divergence than intergenic SNPs in all pairwise population comparisons, suggesting the effect of positive selection in local P. falciparum populations. Nonsynonymous SNPs with low diversity within a population and high divergence between the two populations studied may represent polymorphisms responsible for adaptive evolution.
We also carried out a genome-wide scan for recent positive selection using the long-range haplotype (LRH) test [14], which identifies common variants that have recently spread to high prevalence using recombination as a clock. Approximately 15 genes were identified as having undergone recent positive selection by this approach, including known drug resistance loci (pfcrt and dhfr) as well as multiple members of the acyl-CoA synthetase (ACS) and ubiquitin protein ligase families (Figures S5 and S6); these latter genes also exhibit high divergence between Senegal and Thailand (Figure 1B), evidence for selection that is recent and population-specific. Taken as a group, the genes identified by the LRH test show a significant enrichment for higher than average population divergence (as measured by FST, Mann-Whitney U = 1583, P = 0.0071). All of these loci (Table S3, Dataset S1), which include genes in the folate metabolism, lipid biosynthesis and ubiquitin pathways, should be viewed as candidates for functional follow-up and further characterization.
Genome-Wide Associations with Drug Resistance
In order to directly assess the genetic basis for one important response to antimalarial intervention, we carried out a GWAS to identify loci associated with drug resistance in parasites. This same approach can potentially be applied to many other clinically relevant malaria phenotypes, e.g. host response, invasion, and gametocyte formation. Our first step was to measure drug resistance (IC50 values) to 13 antimalarial drugs (amodiaquine, artemether, artesunate, artemisinin, atovaquone, chloroquine, dihydroartemisinin, halofuginone, halofantrine, lumefantrine, mefloquine, piperaquine and quinine) in 50 culture-adapted parasites using a high-throughput assay (Tables S4 and S5, Text S1, Dataset S1).
We performed the genome-wide association analysis using two statistical tests: efficient mixed-model association (EMMA) and a haplotype likelihood ratio (HLR) test (Figures S7, S8, S9, S10, Methods). EMMA identifies quantitative trait associations in individuals with complex population structure and hidden relatedness; it has previously been shown to outperform both PCA-based and λGC-based correction approaches in highly inbred and structured mouse, maize, and Arabidopsis populations [15]. EMMA is particularly applicable for small and structured sample sets: one of its first applications was in a study of 24 diploid mouse strains [15], essentially the same sample size as in our study (50 haploid strains). The HLR test is a multi-marker test designed to detect the association of a single haplotype with a phenotype, and is particularly powerful when the associated haplotype experienced recent strong selection (and is therefore long) and occurs on a low-LD background [16]; it is therefore particularly appropriate for this study. We addressed the effect of population structure in the HLR test using population-specific permutation (Methods). When used together, these two complementary approaches provide a highly sensitive screen for association signals within the P. falciparum genome.
The well-characterized chloroquine resistance locus, pfcrt, served as a positive control for our GWAS methods (Figure 2A and 2C, Table S2), an important test given our small sample size and the limited LD present in P. falciparum. As expected, we found evidence for association with resistance to chloroquine using both tests, consistent with previous studies [11]; EMMA yielded evidence for association with genome-wide signficance, while the signal from the HLR test fell just short of genome-wide significance (Figure 2C).
Fig. 2. Genome-wide association study (GWAS) results. 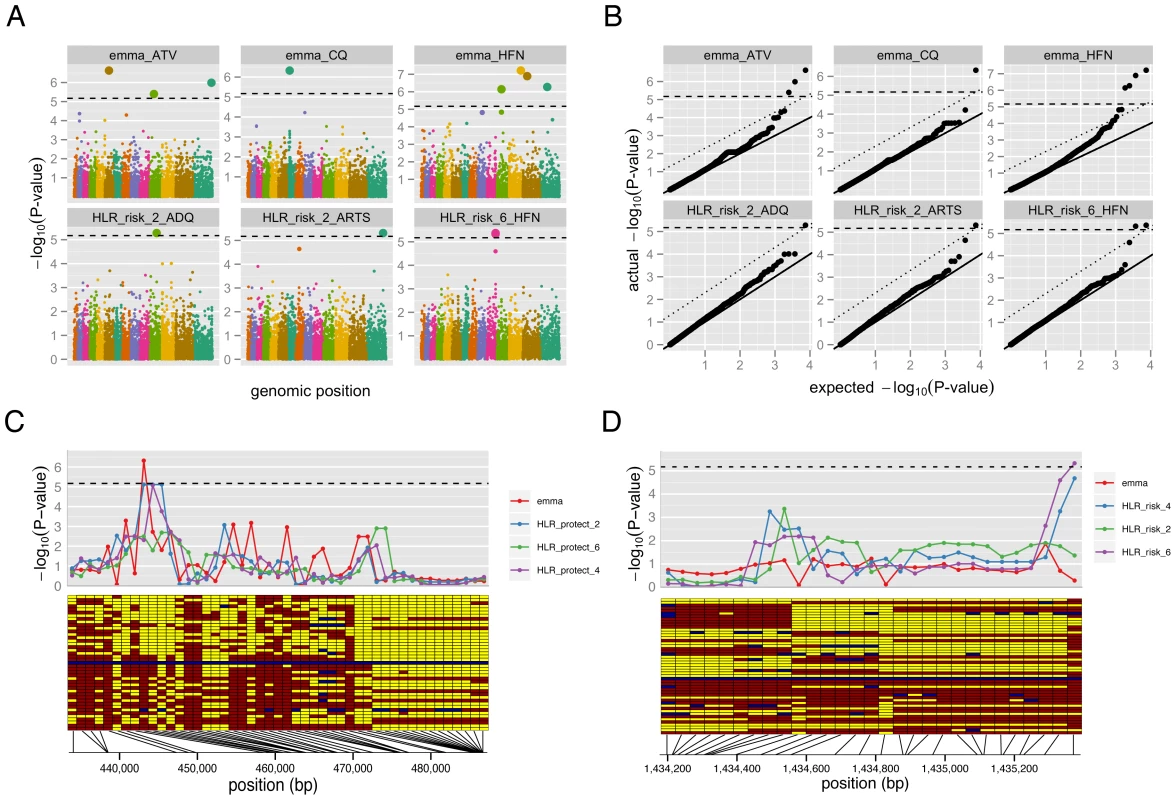
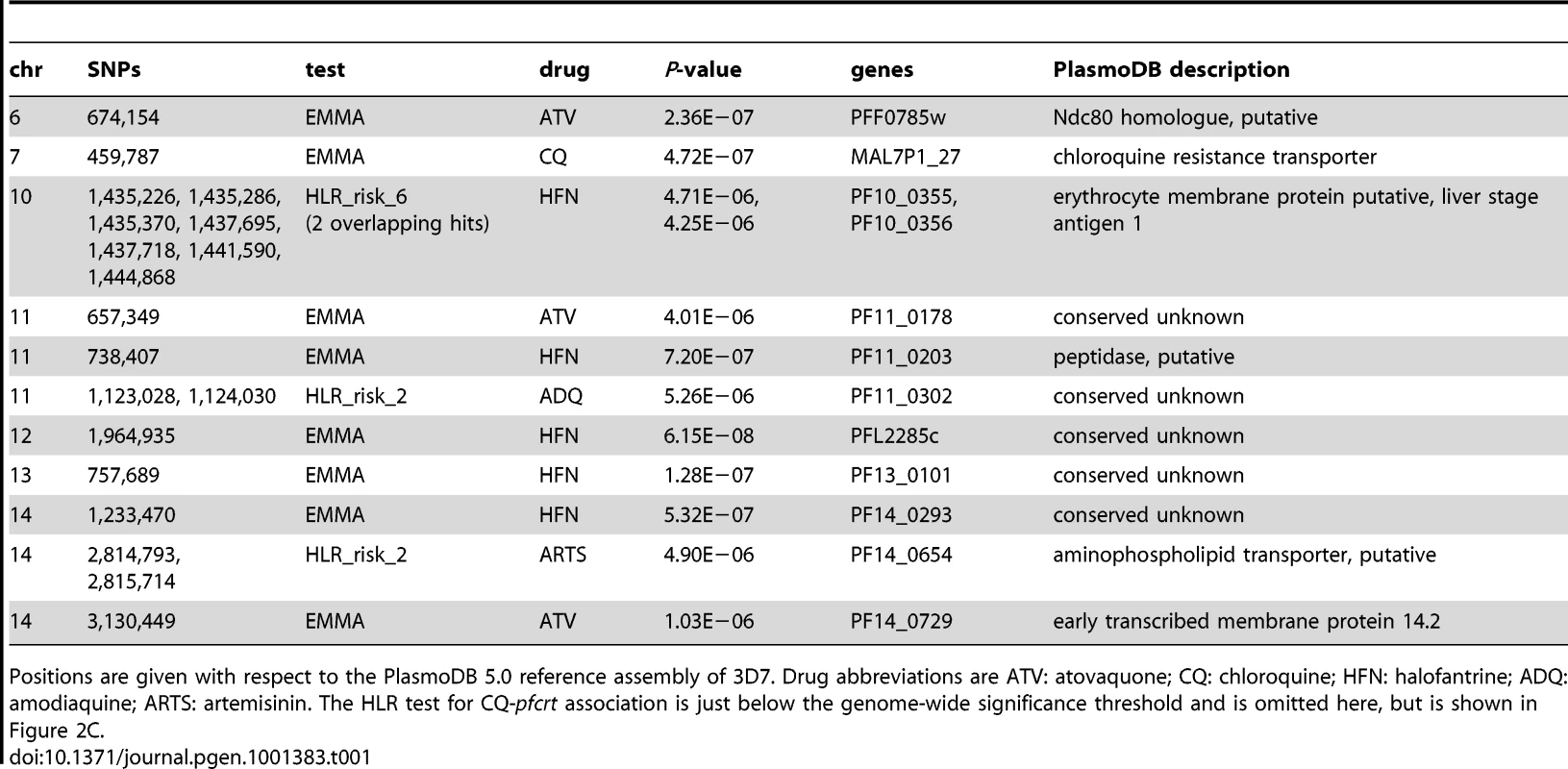
Applying the same tests to the other drug phenotypes, we detected numerous novel loci showing significant associations with drug resistance (Figure 2A and 2D, Table 1). Quantile-quantile plots for each test demonstrate that we were able to effectively control for population structure (Figure 2B). Despite our small sample size and the low LD in P. falciparum, in total eleven loci achieved genome-wide significance for association with resistance to five different drugs: amodiaquine, artemisinin, atovaquone, chloroquine and halofantrine. In most cases, the short extent of LD allowed localization to individual genes. Among the loci identified were various transporters and membrane proteins, as well as five conserved genes with unknown function (Table 1, Dataset S1).
Functional Validation of a Novel Resistance Candidate
Demonstrating that a signal of association actually reflects a causal molecular process requires functional testing and validation of the candidate locus, both because of concerns about power and reproducibility of genetic association tests, and because even a robust statistical correlation need not imply biological causation. To confirm the ability of GWAS to identify functionally relevant candidates, we investigated one of our association findings, PF10_0355, in greater depth. This gene contains multiple SNPs associated with halofantrine resistance (Figure 2D), and encodes a putative erythrocyte membrane protein (PlasmoDB.org) characterized by high genetic diversity.
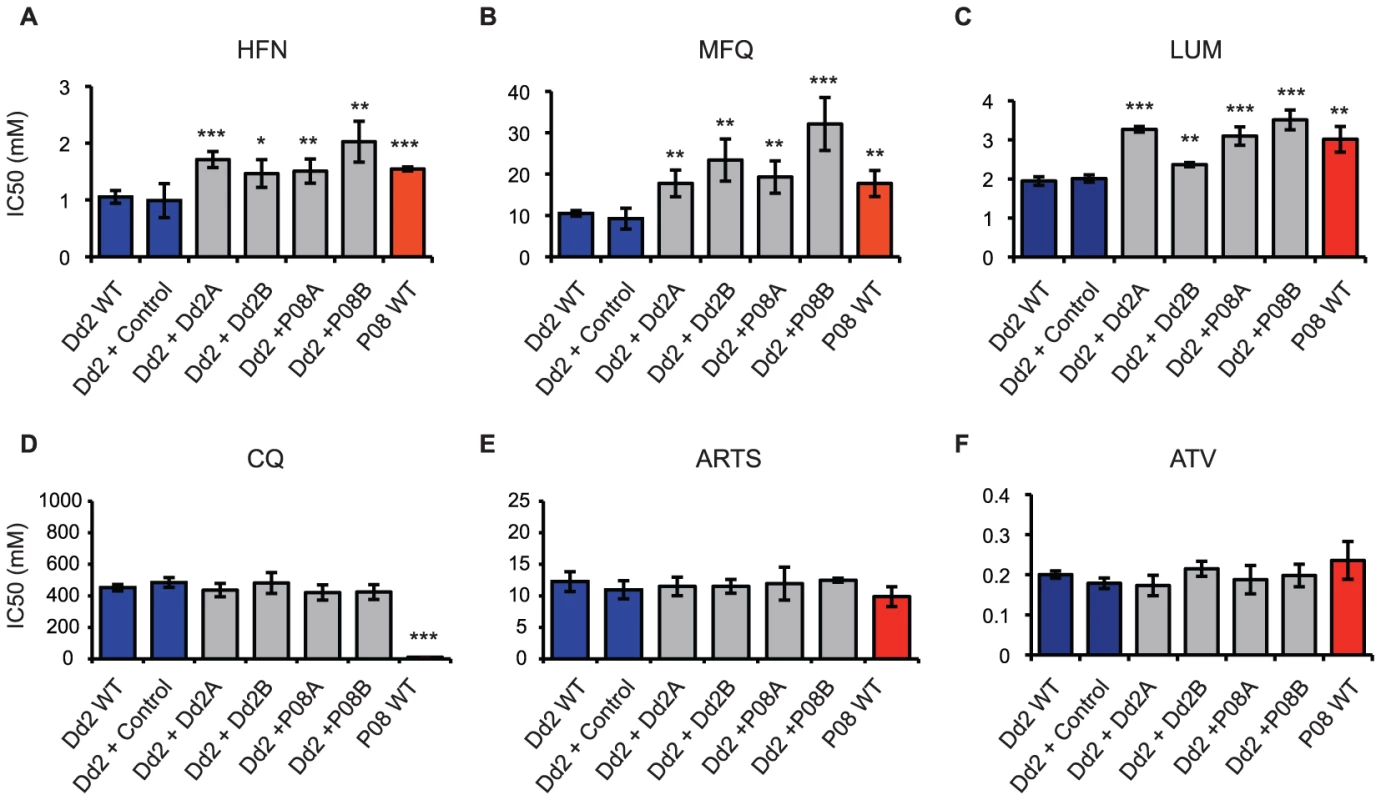
We set out to determine the role of PF10_0355 in halofantrine resistance by transfecting halofantrine-sensitive Dd2 parasites with episomal plasmids containing the PF10_0355 gene from a halofantrine-resistant parasite (SenP08.04), a technique that is used routinely for stable transgene expression [17]. Two independent transfectants overexpressing the PF10_0355 gene from SenP08.04 both showed reduced susceptibility to halofantrine when compared with the Dd2 parent or a transfection control (Figure 3A), suggesting that this gene is indeed involved in modulating parasite drug response.
Two independent transfectants overexpressing the endogenous PF10_0355 gene from halofantrine-sensitive Dd2 also showed reduced susceptibility to halofantrine (Figure 3A), however, pointing to a role of overexpression in the observed resistance. Because PF10_0355 is annotated as a putative erythrocyte membrane protein and belongs to the merozoite surface protein 3/6 family, we tested the hypothesis that the observed effect was the by-product of a growth or invasion-related process, rather than resistance due to a direct interaction with the antimalarial itself. To that end, we expanded our drug testing in the transfectant lines to include other antimalarials, some structurally related and some unrelated to halofantrine.
Overexpression of PF10_0355 from either the Dd2 or the SenP08.04 parent caused increased resistance to the structurally related antimalarials mefloquine and lumefantrine (Figure 3B and 3C), but had no effect on parasite susceptibility to the structurally unrelated antimalarials chloroquine, artemisinin or atovaquone (Figure 3D and 3E). Indeed, we found evidence of cross-resistance between halofantrine and both mefloquine and lumefantrine (Figure 4). We also observed cross-resistance between halofantrine and artemisinin, which is expected as cross-resistance between aminoquinolines and artemisinin compounds has been previously demonstrated [11], [18] and resistance to all these drugs has been shown to be mediated by changes in pfmdr1 copy number [19], [20]. Overexpression of PF10_0355, however, alters parasite susceptibility to the aminoquinolines but not to artemisinin, suggesting that this effect is specific for that set of structurally related compounds and distinct from the effect of pfmdr1, which seems to exert a global effect of resistance to unrelated compounds (i.e. both aminoquinolines and artemisinins). Using the Dd2 parasite line, which has amplified pfmdr1 copy number, as a background for PF10_0355 overexpression allowed us to distinguish between cross-resistance to a structurally related class of compounds (mediated by PF10_0355 overexpression) and pan-resistance to multiple classes of drugs.
Fig. 4. Correlations between antimalarial drugs tested. 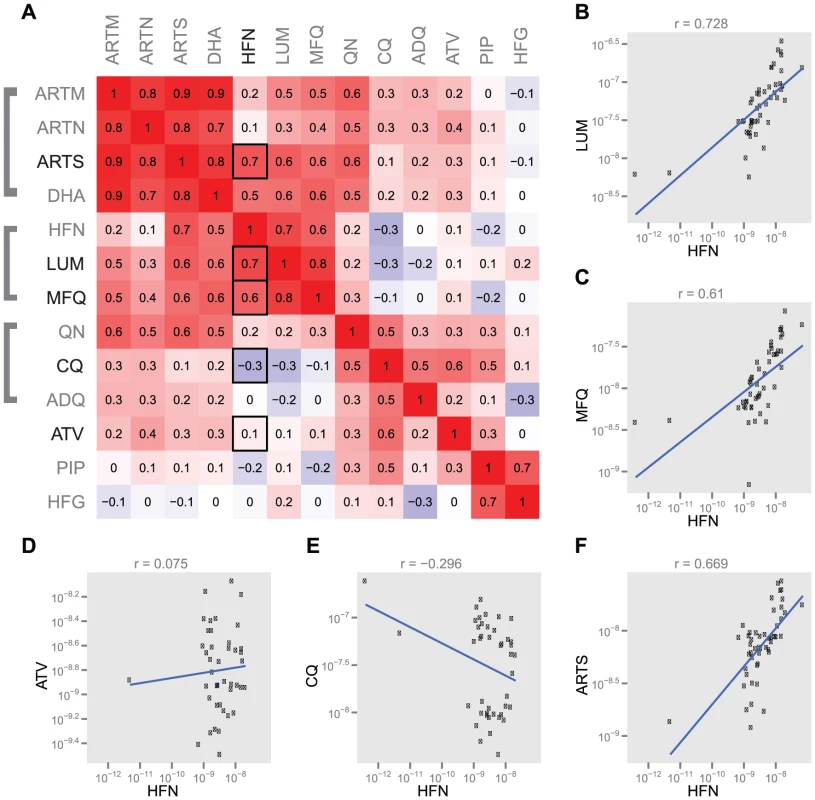
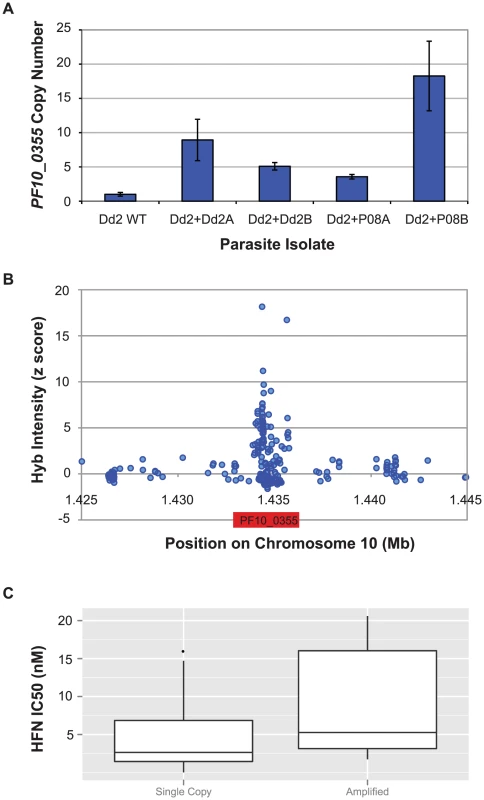
Given that overexpression of the PF10_0355 gene both from a halofantrine-resistant and from a sensitive parasite conferred resistance to halofantrine-related drugs, we investigated whether gene amplification might be driving the observed resistance, as it often does for antimalarial drugs [21]–[26]. We quantified PF10_0355 copy number in our transfectants and found that the transfectant with the highest IC50 for all three drugs (Dd2+P08B) also had the highest PF10_0355 copy number, as measured by quantitative PCR (qPCR) (Figure 5A). Furthermore, when we examined the PF10_0355 gene on our SNP array, we detected a substantial increase in hybridization intensity at the PF10_0355 locus compared to the genome average, suggesting that this gene is amplified in some parasites (Figure 5B). The amplified region appears only to contain the PF10_0355 gene itself and not surrounding loci. We observed a similar pattern at pfmdr1 on chromosome 5, where copy number variation is well established (Figure S11). Follow-up qPCR analysis of 38 parasite lines confirmed that parasites with amplified PF10_0355 have a greater mean halofantrine IC50. (Figure 5C, Table S6, Dataset S1). Copy number variation was further confirmed in a number of parasites by quantitative Southern blotting (Figure S12).
Discussion
In this study we used natural selection and genome-wide association methods to probe the genetic basis of adaptation in P. falciparum. These approaches are complementary: scanning for selected loci permits an unbiased search for unknown adaptive changes, but provides little information about the processes at work, while GWAS gives a focused look at one easily identified (and clinically critical) adaptive phenotype. Results from both approaches open up new avenues for study, as we seek to understand the biological significance of the findings.
The specifics of our strategy were designed to cope with two potential limitations in applying genome-wide population genetic approaches to malaria: small sample sizes, due to the difficulty in adapting parasites to culture and assessing drug and other phenotypes; and a lack of correlation (LD) between nearby variants in the parasite genome, which limits our ability to infer untyped SNPs from genotyped markers. The second limitation we addressed by developing a high-density genotyping array (based on new sequencing), to increase the fraction of genetic variation that we could directly interrogate, while the effect of the first was mitigated by the phenotype we targeted in our GWAS.
Drug resistance is a phenotype well-suited for GWAS because it is expected to be caused by common alleles of large effect at few genomic loci [27]. If this is the case, associations will be much easier to detect than in a typical human GWAS, in which the phenotype is caused by alleles at many loci that are either rare or of small effect. Additionally, the haploid nature of the intra-erythrocytic stage of P. falciparum further heightens GWAS power by eliminating the issue of allelic dominance. Finally, the increased LD caused by recent selection for drug resistance counteracts the loss of power that comes from short LD, small sample size, and the temporal and geographic stratification of the parasite population that we examined. Thus, despite the potential limitations, we were able to detect a known drug resistance locus (pfcrt), observed little P-value inflation in our GWAS data (Figures S8, S9, S10), and identified a number of genome-wide significant loci associated with drug resistance. Part of this success was likely due to specific tests we used to account for population structure.
Going beyond these statistical tests, we went on to functionally validate one of these loci, demonstrating that increased PF10_0355 copy number confer resistance to three structurally related antimalarial drugs. This demonstrates the feasibility of coupling GWAS and functional testing in the malaria parasite for identifying and validating novel drug resistance loci and illustrates the power of GWAS to find functionally important alleles.
Comparing our results to the recent GWAS described by Mu, et al. [11], which was also directed at finding drug-resistance loci, we see that, beyond the well-known pfcrt locus, there was no overlap between the associations identified by each study. Differing sets of drugs tested and analytical methods explain much of the disagreement. Of the eleven candidate associations in Table 1, one (that with pfcrt) was found by both studies, eight were associations with drugs not assayed in Mu, et al. (atovaquone and halofantrine), and two were found only with a haplotype-based test, an approach not used by Mu, et al. Our candidate locus at PF10_0355, in fact, would not have been detectable in the Mu study because it was identified only by the multi-marker HLR test, because it involved an association with halofantrine, and because the Mu, et al. genotyping array lacked markers within 4 kb of the gene (plasmoDB.org).
Different parasite populations and marker sets probably explain many of the dihydroartemisinin, mefloquine and quinine associations identified by Mu, et al. but not seen in our data set. The studies used different parasite population sets—theirs was weighted toward southeast Asian strains, and ours toward African strains—and selection pressures and selected alleles can both vary between populations. Our smaller sample size also means that we might lack power to identify some associations accessible to Mu, et al. These difficulties are reflected in human GWAS studies as well, where the ability to replicate associations using multiple tests and in different sample sets has also been challenging to achieve [28].
Ultimately, the disparities in loci identified point to the role of population analysis as a tool for candidate gene discovery and not as a definitive study. Even within each study, there is little overlap between the signals observed with different methods—our study detects only one gene (pfcrt) by both GWAS tests (EMMA and HLR), while Mu, et al. detected only two genes (unknowns, not pfcrt) by both of their GWAS tests (Eigensoft and PLINK). Even a well-designed GWAS serves only as a hypothesis-generating experiment, and it is vital to empirically validate candidate loci associated with a phenotype of interest. Especially given the small sample sizes and relatively sparse marker density used in both malaria GWAS studies to date, functional validation of candidates is necessary to address concerns about false positive results.
Our functional result, that increased PF10_0355 copy number confers decreased susceptibility to halofantrine, mefloquine and lumefantrine, raises additional questions for study. Further work will be needed to determine the precise contributions of copy number variation and gene mutation to the parasite's response to these drugs. The biological function of this gene's product is unknown, but previous work indicates putative localization to the parasite surface [29], as well as it being a potential target of host immunity and balancing selection [30]. While the protein itself does not appear to be a transporter, it is possible that it directly binds drug or perhaps couples with transport proteins to modulate drug susceptibility; interaction between membrane transporters and non-channel proteins has been demonstrated in cancer, plant and yeast systems [31]–[33]. Additional experiments are certainly required to determine the precise role of PF10_0355 in modulating parasite response to this class of compounds, including assessing its relevance to resistance in natural populations, but it is clear that alteration of this locus can mediate drug resistance in P. falciparum.
Although halofantrine, mefloquine and lumefantrine are not commonly used as primary interventions, widespread halofantrine use has recently been documented in West Africa. Notably, halofantrine was used to treat nearly 18 million patients between 1988 and 2005 [34], [35], and it remains in production and use today. Use of halofantrine, mefloquine or lumefantrine as monotherapy may further explain how mutations and copy number variation in the PF10_0355 gene were selected. Lumefantrine is also currently used as a partner drug in the artemisinin-based combination therapy (ACT) Coartem. The shorter half-life of artemether allows lumefantrine to be present as monotherapy, making it vulnerable to selection of drug resistant mutants. As genetic loci associated with drug responses are identified and validated, these provide new molecular biomarkers to evaluate drug use and response in malaria endemic settings. Thus, our findings have implications for defining molecular biomarkers for monitoring partner drug responses as intervention strategies, such as ACTs, are applied.
Beyond identifying a novel drug resistance locus, this study illustrates the general utility of a GWAS approach for the discovery of gene function in P. falciparum. Even with a small and geographically heterogeneous sample of parasites, we identified a number of new loci associated with drug response and validated one of them. Larger samples from a single population will have much greater power to detect additional loci, including those where multiple and low frequency alleles contribute to resistance. Future GWAS have the potential both to provide greater insights into basic parasite biology and to identify biomarkers for drug resistance and other clinically relevant phenotypes like acquired protection, pathogenesis, and placental malaria.
Future GWAS will be able to counteract the loss of power caused by low LD, either by focusing on parasite populations with reduced outcrossing rates, or by studying cases of very strong selective pressure. This issue will soon become moot, however, as the declining cost of whole-genome sequencing makes it practical to assay every nucleotide in the genome on a routine basis. Culture-adapted parasites are amenable to robust and reproducible phenotypic characterization, but their limitations—the potential for artifactual mutations during adaptation and for a biased selection of clones within a given infection—mean that genetic changes identified using them require both functional validation and demonstration that the changes are important during natural infection. As direct sequencing of clinical isolates with demonstrable clinical phenotypes such as ex vivo drug response or invasion properties becomes increasingly feasible, sequencing will enable us to directly identify genetic changes in the parasite associated with clinically relevant phenotypes. In the years ahead, genome analysis of P. falciparum has the potential to identify genetic loci associated with many phenotypes, enhance our understanding of the biology of this important human pathogen, and inform the development of diagnostic and surveillance tools for malaria eradication.
Methods
Parasites, Drug Testing, and DNA Isolation
Parasite samples and origins are detailed in Text S1 and Table S1. Parasites were maintained by standard methods [36] and were tested for their response to amodiaquine, artemether, artesunate, artemisinin, atovaquone, chloroquine, dihydroartemisinin, halofuginone, halofantrine, lumefantrine, mefloquine, piperaquine and quinine according to the methods outlined by Baniecki, et al. [37] (Table S4, Figure S13, Text S1). Follow-up drug testing was done by measuring uptake of 3H-hypoxanthine [38]. Nucleic acids were obtained from parasite cultures using Qiagen genomic-tips (Qiagen, USA). All DNA samples were evaluated by molecular barcode [39].
Array Genotyping
We sequenced nine geographically diverse parasite isolates to 1.25x coverage, nearly doubling the number of publicly available SNPs to 111,536 (Text S1). These parasites had been previously sequenced to 0.25x coverage [2] and the deeper sequencing allowed for more thorough SNP discovery. Using this combined marker set, we created a high-density Affymetrix-based SNP array for P. falciparum containing 74,656 markers. Arrays were hybridized to 57 independent parasite samples (Table S1), including 17 previously sequenced strains used as a validation set. Genotype calls were produced using the BRLMM-P algorithm [40]. Markers that did not demonstrate perfect concordance between sequence and array data for the 17 strains were removed (Text S1). The remaining 17,582 SNPs constituted the high-confidence marker set used throughout this study (median marker spacing 444 bp, mean spacing 1,316 bp). All genomic positions and translation consequences are listed with respect to the PlasmoDB 5.0 assembly and annotation. SNP genotype data are publicly available on plasmodb.org (release 6.0, July 2009) and dbSNP (Build B134, May 2011), accessible by searching for submission batches Pf_0002 (sequencing of nine isolates) and Pf_0003 (genotyping of 57 isolates) from submitter BROAD-GENOMEBIO. Genotype data is also available as Dataset S2.
Principal Component Analyses
Principal components analysis (PCA) was performed using the program SmartPCA [41]. All single-infection samples were used for the analysis in Figure 1. Samples that tightly clustered with the wrong continental population (A4, Malayan Camp and T2_C6) represented likely cases of contamination and were thus omitted from all other analyses.
Diversity/Divergence Analysis
We measured diversity using a statistic we term ‘SNP π,’ which quantifies the average number of pair-wise differences among samples from a given population at assayed SNPs. Population divergence was measured using FST, calculated using the method of Hudson, et al. [42]. Statistical evaluation of the significance of differences in SNP π and FST among populations was performed using a bootstrapping approach, where the SNP set was re-sampled with replacement and each statistic recomputed 1000 times.
Linkage Disequilibrium (LD) Analysis
The statistic r2 was calculated within each population for all pairs of SNPs sharing the same chromosome [43]; pairs were binned by distance and averaged within each bin. The level of LD between unlinked markers was estimated by calculating r2 between all pairs of SNPs on different chromosomes. To determine the bias caused by small sample size, the unlinked calculation was repeated, with the change that for each pair of SNPs, the genotype for one was taken from one strain while the genotype for the second was taken from another strain. This background value of r2 was calculated separately for the possible pairs of different strains and then averaged. Only single infections, as assessed by molecular barcode, were used.
Long Range Haplotype (LRH) Analysis
Because of the small number of samples, LRH results for individual continental populations had a high level of variance. Thus, we pooled together samples from Africa (n = 26) and Asia (n = 18, excluding India), as suggested by our PCA analysis. SNPs included in the analysis had a minor allele frequency of at least 0.05 and a call rate of at least 0.8; missing genotypes were imputed using PHASE. LRH analysis was performed using Sweep. Each SNP defined two core alleles, one base pair in length. We calculated relative extended haplotype homozygosity (REHH) for each core allele, to its left and right [44], yielding up to four REHH scores per SNP locus. We standardized the REHH scores as a function of core allele frequency, defined on a discrete grid from 0.05 to 0.95 with even spaces of 0.025. This yielded a normally-distributed set of Z-scores for which we calculated corresponding P-values and Q-values.
Genome-Wide Association Study (GWAS)
We performed a GWAS for drug resistance to thirteen antimalarials across 50 of our genotyped samples. 7,437 SNPs that had a minor allele count of five samples as well as an 80% call rate under every phenotype condition were used for GWAS. A Bonferroni significance threshold of –log10(P-value) >5.17 was used for all tests. See Text S1 for more details on GWAS methods.
The Efficient Mixed-Model Association (EMMA) test [15] models quantitative trait associations to a data set with complex population structure and hidden relatedness. It calculates a genotype similarity matrix instead of discrete categories and does not require a priori specification of populations. The resulting P-value distributions demonstrate little remaining effect from population structure (Figure S8) while retaining power to find a number of associations at genome-wide significance (Figure S8, Figure 2A, Table 1).
The Haplotype Likelihood Ratio (HLR) test [16] models the likelihood that a single, resistant haplotype rose to dominance while all other haplotypes proportionally decreased. PLINK [45] is used to produce sliding window haplotypes across the genome and calculate haplotype frequencies for input to the HLR test. We produced input for all 2-, 4 - and 6-marker windows. The LOD scores generated by the HLR test were converted to empirical pointwise P-values by performing approximately 370,000 permutations of the null model for each test condition, allowing us to calculate empirical P-values up to a significance of 10−5.6. We preserved population-specific phenotype frequencies by permuting only within each of three populations defined by our PCA analysis (Table S1). Resulting P-value distributions fit expectations well for the vast majority of test conditions (Figures S9, S10) and the test demonstrates power to detect a number of loci at genome-wide significance (Figure 2A, Table 1).
Copy Number Variation (CNV)
Copy number was assessed by evaluating the hybridization intensity at the PF10_0355 locus on the high-density SNP array (Text S1). Follow-up analyses were done by quantitative real-time PCR (qPCR) of the PF10_0355 locus using the Delta Delta Ct method [46]. PF10_0355 was compared to the reference locus PF07_0076 and 3D7 was used as a reference strain. A summary of PF10_0355 copy number for all parasite strains tested is provided in Table S6. Select resistant strains that were found to have multiple copies of PF10_0355 were further analyzed by quantitative Southern blotting and PF10_0355 copy number was compared to the dhps gene from the 3D7 strain [47].
PF10_0355 Overexpression
The full length ORF of PF10_0355 was amplified from either the Dd2 (HFN sensitive) or SenP08.04 (HFN resistant) parasite isolate and cloned into the pBIC009 plasmid under the expression of the Hsp86 promoter. Plasmid DNA was isolated, tranfected into the Dd2 parasite strain and stable transfectants were selected with 2.5 nM WR99210 [48]. Parasites from two independent experiments for each vector type (Dd2+Dd2 and Dd2+SenP08.04) were isolated and successful transfection was confirmed by plasmid rescue as well as episome-specific PCR and sequencing. Additionally, a vector control strain was made by transfecting Dd2 parasites with the pBIC009 plasmid containing the firefly luciferase gene (EC 1.13.12.7).
Supporting Information
Zdroje
1. Gardner
MJ
Hall
N
Fung
E
White
O
Berriman
M
2002
Genome sequence of the human malaria parasite Plasmodium
falciparum.
Nature
498
511
2. Volkman
SK
Sabeti
PC
DeCaprio
D
Neafsey
DE
Schaffner
SF
2007
A genome-wide map of diversity in Plasmodium
falciparum.
Nat Genet
39
113
119
3. Jeffares
DC
Pain
A
Berry
A
Cox
AV
Stalker
J
2007
Genome variation and evolution of the malaria parasite Plasmodium
falciparum.
Nat Genet
120
125
4. Mu
J
Awadalla
P
Duan
J
McGee
KM
Keebler
J
2007
Genome-wide variation and identification of vaccine targets in
the Plasmodium falciparum genome.
Nat Genet
126
130
5. Carret
CK
Horrocks
P
Konfortov
B
Winzeler
E
Qureshi
M
2005
Microarray-based comparative genomic analyses of the human
malaria parasite Plasmodium falciparum using Affymetrix
arrays.
Mol Biochem Parasitol
177
186
6. Kidgell
C
Volkman
SK
Daily
J
Borevitz
JO
Plouffe
D
2006
A systematic map of genetic variation in Plasmodium
falciparum.
PLoS Pathog
2
e57
doi:10.1371/journal.ppat.0020057
7. Jiang
H
Yi
M
Mu
J
Zhang
L
Ivens
A
2008
Detection of genome-wide polymorphisms in the AT-rich Plasmodium
falciparum genome using a high-density microarray.
BMC Genomics
398
8. Dharia
NV
Sidhu
AB
Cassera
MB
Westenberger
SJ
Bopp
SE
2009
Use of high-density tiling microarrays to identify mutations
globally and elucidate mechanisms of drug resistance in Plasmodium
falciparum.
Genome Bio
R21
9. Tan
JC
Patel
JJ
Tan
A
Blain
JC
Albert
TJ
2009
Optimizing comparative genomic hybridization probes for
genotyping and SNP detection in Plasmodium falciparum.
Genomics
93
543
550
10. Neafsey
DE
Schaffner
SF
Volkman
SK
Park
DJ
Montgomery
P
2008
Genome-wide SNP genotyping highlights the role of natural
selection in Plasmodium falciparum population divergence.
Genome Bio
R171
11. Mu
J
Myers
RA
Jiang
H
Liu
S
Ricklefs
S
2010
Plasmodium falciparum genome-wide scans for positive selection,
recombination hot spots and resistance to antimalarial
drugs.
Nat Genet
42
268
271
12. Anderson
TJ
Haubold
B
Williams
JT
Estrada-Franco
JG
Richardson
L
2000
Microsatellite markers reveal a spectrum of population structures
in the malaria parasite Plasmodium falciparum.
Mol Biol Evol
1467
1482
13. Mu
J
Awadalla
P
Duan
J
McGee
KM
Joy
DA
2005
Recombination hotspots and population structure in Plasmodium
falciparum.
PLoS Biol
3
e335
doi:10.1371/journal.pbio.0030335
14. Sabeti
PC
Reich
DE
Higgins
JM
Levine
HZ
Richter
DJ
2002
Detecting recent positive selection in the human genome from
haplotype structure.
Nature
419
832
837
15. Kang
HM
Zaitlen
NA
Wade
CM
Kirby
A
Heckerman
D
2008
Efficient control of population structure in model organism
association mapping.
Genetics
178
1709
1723
16. Lindblad-Toh
K
Wade
CM
Mikkelsen
TS
Karlsson
EK
Jaffe
DB
2005
Genome sequence, comparative analysis and haplotype structure of
the domestic dog.
Nature
438
803
819
17. Crabb
BS
Triglia
T
Waterkeyn
JG
Cowman
AF
1997
Stable transgene expression in Plasmodium
falciparum.
Mol Biochem Parasitol
90
131
144
18. Pradines
B
Hovette
P
Fusai
T
Atanda
HL
Baret
E
2006
Prevalence of in vitro resistance to eleven standard or new
antimalarial drugs among Plasmodium falciparum isolates from Pointe-Noire,
Republic of the Congo.
J Clin Microbiol
44
2404
2408
19. Sidhu
AB
Uhlemann
AC
Valderramos
SG
Valderramos
JC
Krishna
S
2006
Decreasing pfmdr1 copy number in plasmodium falciparum malaria
heightens susceptibility to mefloquine, lumefantrine, halofantrine, quinine,
and artemisinin.
J Infect Dis
194
528
535
20. Chavchich
M
Gerena
L
Peters
J
Chen
N
Cheng
Q
2010
Role of pfmdr1 amplification and expression in induction of
resistance to artemisinin derivatives in Plasmodium
falciparum.
Antimicrob Agents Chemother
54
2455
2464
21. Foote
SJ
Thompson
JK
Cowman
AF
Kemp
DJ
1989
Amplification of the multidrug resistance gene in some
chloroquine-resistant isolates of P. falciparum.
Cell
57
921
930
22. Wilson
CM
Serrano
AE
Wasley
A
Bogenschutz
MP
Shankar
AH
1989
Amplification of a gene related to mammalian mdr genes in
drug-resistant Plasmodium falciparum.
Science
244
1184
1186
23. Price
RN
Uhlemann
AC
Brockman
A
McGready
R
Ashley
E
2004
Mefloquine resistance in Plasmodium falciparum and increased
pfmdr1 gene copy number.
Lancet
364
438
447
24. Nair
S
Miller
B
Barends
M
Jaidee
A
Patel
J
2008
Adaptive copy number evolution in malaria
parasites.
PLoS Genet
4
e1000243
doi:10.1371/journal.pgen.1000243
25. Anderson
TJ
Patel
J
Ferdig
MT
2009
Gene copy number and malaria biology.
Trends Parasitol
25
336
343
26. Ribacke
U
Mok
BW
Wirta
V
Normark
J
Lundeberg
J
2007
Genome wide gene amplifications and deletions in Plasmodium
falciparum.
Mol Biochem Parasitol
33
44
27. Hayton
K
Su
XZ
2008
Drug resistance and genetic mapping in Plasmodium
falciparum.
Curr Genet
54
223
239
28. Lohmueller
KE
Pearce
CL
Pike
M
Lander
ES
Hirschhorn
JN
2003
Meta-analysis of genetic association studies supports a
contribution of common variants to susceptibility to common
disease.
Nat Genet
33
177
182
29. Singh
S
Soe
S
Weisman
S
Barnwell
JW
Perignon
JL
2009
A conserved multi-gene family induces cross-reactive antibodies
effective in defense against Plasmodium falciparum.
PLoS ONE
4
e5410
doi:10.1371/journal.pone.0005410
30. Ochola
LI
Tetteh
KK
Stewart
LB
Riitho
V
Marsh
K
2010
Allele frequency-based and polymorphism-versus-divergence indices
of balancing selection in a new filtered set of polymorphic genes in
Plasmodium falciparum.
Mol Biol Evol
31. Miletti-Gonzalez
KE
Chen
S
Muthukumaran
N
Saglimbeni
GN
Wu
X
2005
The CD44 receptor interacts with P-glycoprotein to promote cell
migration and invasion in cancer.
Cancer Res
65
6660
6667
32. Geisler
M
Girin
M
Brandt
S
Vincenzetti
V
Plaza
S
2004
Arabidopsis immunophilin-like TWD1 functionally interacts with
vacuolar ABC transporters.
Mol Biol Cell
15
3393
3405
33. Beese
SE
Negishi
T
Levin
DE
2009
Identification of positive regulators of the yeast fps1 glycerol
channel.
PLoS Genet
5
e1000738
doi:10.1371/journal.pgen.1000738
34. 2005
Halofantrine and fatal cardiac arrhythmia.
GSK
Global Clinical Safety and Pharmacovigilance
35. Bouchaud
O
Imbert
P
Touze
JE
Dodoo
AN
Danis
M
2009
Fatal cardiotoxicity related to halofantrine: a review based on a
worldwide safety data base.
Malar J
8
289
36. Trager
W
Jensen
JB
1976
Human malaria parasites in continuous culture.
Science
193
673
675
37. Baniecki
ML
Wirth
DF
Clardy
J
2007
High-throughput Plasmodium falciparum growth assay for malaria
drug discovery.
Antimicrob Agents Chemother
51
716
723
38. Webster
HK
Boudreau
EF
Pavanand
K
Yongvanitchit
K
Pang
LW
1985
Antimalarial drug susceptibility testing of Plasmodium falciparum
in Thailand using a microdilution radioisotope method.
Am J Trop Med Hyg
34
228
235
39. Daniels
R
Volkman
SK
Milner
DA
Mahesh
N
Neafsey
DE
2008
A general SNP-based molecular barcode for Plasmodium falciparum
identification and tracking.
Malar J
7
223
40. 2007
BRLMM-P: a Genotype Calling Method for the SNP 5.0 Array http://www.affymetrix.com/support/technical/whitepapers/brlmmp_whitepaper.pdf
41. Patterson
N
Price
AL
Reich
D
2006
Population structure and eigenanalysis.
PLoS Genet
2
e190
doi:10.1371/journal.pgen.0020190
42. Hudson
RR
Slatkin
M
Maddison
WP
1992
Estimation of levels of gene flow from DNA sequence
data.
Genetics
132
583
589
43. Hill
WG
Robertson
A
1968
Linkage Disequilibrium in Finite Populations.
Theoretical and Applied Genetics
38
226
231
44. Sabeti
PC
Reich
DE
Higgins
JM
Levine
HZ
Richter
DJ
2002
Detecting recent positive selection in the human genome from
haplotype structure.
Nature
832
837
45. Purcell
S
Neale
B
Todd-Brown
K
Thomas
L
Ferreira
MA
2007
PLINK: a tool set for whole-genome association and
population-based linkage analyses.
Am J Hum Genet
81
559
575
46. Ferreira
ID
Rosario
VE
Cravo
PV
2006
Real-time quantitative PCR with SYBR Green I detection for
estimating copy numbers of nine drug resistance candidate genes in
Plasmodium falciparum.
Malar J
5
1
47. Triglia
T
Duraisingh
MT
Good
RT
Cowman
AF
2005
Reticulocyte-binding protein homologue 1 is required for sialic
acid-dependent invasion into human erythrocytes by Plasmodium
falciparum.
Mol Microbiol
55
162
174
48. Fidock
DA
Wellems
TE
1997
Transformation with human dihydrofolate reductase renders malaria
parasites insensitive to WR99210 but does not affect the intrinsic activity
of proguanil.
Proc Natl Acad Sci U S A
94
10931
10936
Štítky
Genetika Reprodukční medicína
Článek Incorporating Biological Pathways via a Markov Random Field Model in Genome-Wide Association StudiesČlánek Survival Motor Neuron Protein Regulates Stem Cell Division, Proliferation, and Differentiation inČlánek Epigenetic Regulation of Cell Type–Specific Expression Patterns in the Human Mammary Epithelium
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2011 Číslo 4
-
Všechny články tohoto čísla
- Is Co-Expressed with Closely Adjacent Uncharacterised Genes Spanning a Breast Cancer Susceptibility Locus at 6q25.1
- The Liberation of Embryonic Stem Cells
- Genome-Wide Meta-Analysis Identifies Regions on 7p21 () and 15q24 () As Determinants of Habitual Caffeine Consumption
- A Sustained Dietary Change Increases Epigenetic Variation in Isogenic Mice
- The Exocyst Protein Sec10 Interacts with Polycystin-2 and Knockdown Causes PKD-Phenotypes
- Incorporating Biological Pathways via a Markov Random Field Model in Genome-Wide Association Studies
- Survival Motor Neuron Protein Regulates Stem Cell Division, Proliferation, and Differentiation in
- Identification and Functional Validation of the Novel Antimalarial Resistance Locus in
- Does Positive Selection Drive Transcription Factor Binding Site Turnover? A Test with Drosophila Cis-Regulatory Modules
- Protein Phosphatase 2A Controls Ethylene Biosynthesis by Differentially Regulating the Turnover of ACC Synthase Isoforms
- Ribosomal DNA Deletions Modulate Genome-Wide Gene Expression: “–Sensitive” Genes and Natural Variation
- Reciprocal Sign Epistasis between Frequently Experimentally Evolved Adaptive Mutations Causes a Rugged Fitness Landscape
- Variable Pathogenicity Determines Individual Lifespan in
- Evolution of Vertebrate Transient Receptor Potential Vanilloid 3 Channels: Opposite Temperature Sensitivity between Mammals and Western Clawed Frogs
- Towards Establishment of a Rice Stress Response Interactome
- Mouse Genome-Wide Association and Systems Genetics Identify As a Regulator of Bone Mineral Density and Osteoclastogenesis
- Quantitative Fitness Analysis Shows That NMD Proteins and Many Other Protein Complexes Suppress or Enhance Distinct Telomere Cap Defects
- Highly Precise and Developmentally Programmed Genome Assembly in Requires Ligase IV–Dependent End Joining
- PDP-1 Links the TGF-β and IIS Pathways to Regulate Longevity, Development, and Metabolism
- Genome-Wide Association Study Using Extreme Truncate Selection Identifies Novel Genes Affecting Bone Mineral Density and Fracture Risk
- Eight Common Genetic Variants Associated with Serum DHEAS Levels Suggest a Key Role in Ageing Mechanisms
- 14-3-3 Proteins Regulate Exonuclease 1–Dependent Processing of Stalled Replication Forks
- HDA6 Regulates Locus-Directed Heterochromatin Silencing in Cooperation with MET1
- Epigenetic Regulation of Cell Type–Specific Expression Patterns in the Human Mammary Epithelium
- Enhanced Statistical Tests for GWAS in Admixed Populations: Assessment using African Americans from CARe and a Breast Cancer Consortium
- Beyond Missing Heritability: Prediction of Complex Traits
- An Evolutionary Genomic Approach to Identify Genes Involved in Human Birth Timing
- Long-Lost Relative Claims Orphan Gene: in a Wasp
- PTG Depletion Removes Lafora Bodies and Rescues the Fatal Epilepsy of Lafora Disease
- Chromatin Organization in Sperm May Be the Major Functional Consequence of Base Composition Variation in the Human Genome
- GWAS of Follicular Lymphoma Reveals Allelic Heterogeneity at 6p21.32 and Suggests Shared Genetic Susceptibility with Diffuse Large B-cell Lymphoma
- Loss-of-Function Mutations in Cause Metachondromatosis, but Not Ollier Disease or Maffucci Syndrome
- DNA Damage, Somatic Aneuploidy, and Malignant Sarcoma Susceptibility in Muscular Dystrophies
- The Phylogenetic Origin of Coincided with the Origin of Maternally Provisioned Germ Plasm and Pole Cells at the Base of the Holometabola
- Genome Analysis Reveals Interplay between 5′UTR Introns and Nuclear mRNA Export for Secretory and Mitochondrial Genes
- Genome-Wide Association Analysis of Soluble ICAM-1 Concentration Reveals Novel Associations at the , , , and Loci
- The Complete Spectrum of Yeast Chromosome Instability Genes Identifies Candidate CIN Cancer Genes and Functional Roles for ASTRA Complex Components
- Dynamic Regulation of H3K27 Trimethylation during Differentiation
- Phosphorylation-Dependent Differential Regulation of Plant Growth, Cell Death, and Innate Immunity by the Regulatory Receptor-Like Kinase BAK1
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- PTG Depletion Removes Lafora Bodies and Rescues the Fatal Epilepsy of Lafora Disease
- Evolution of Vertebrate Transient Receptor Potential Vanilloid 3 Channels: Opposite Temperature Sensitivity between Mammals and Western Clawed Frogs
- Survival Motor Neuron Protein Regulates Stem Cell Division, Proliferation, and Differentiation in
- An Evolutionary Genomic Approach to Identify Genes Involved in Human Birth Timing
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání