-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaThe Seminal Fluid Protease “Seminase” Regulates Proteolytic and Post-Mating Reproductive Processes
Proteases and protease inhibitors have been identified in the ejaculates of animal taxa ranging from invertebrates to mammals and form a major protein class among Drosophila melanogaster seminal fluid proteins (SFPs). Other than a single protease cascade in mammals that regulates seminal clot liquefaction, no proteolytic cascades (i.e. pathways with at least two proteases acting in sequence) have been identified in seminal fluids. In Drosophila, SFPs are transferred to females during mating and, together with sperm, are necessary for the many post-mating responses elicited in females. Though several SFPs are proteolytically cleaved either during or after mating, virtually nothing is known about the proteases involved in these cleavage events or the physiological consequences of proteolytic activity in the seminal fluid on the female. Here, we present evidence that a protease cascade acts in the seminal fluid of Drosophila during and after mating. Using RNAi to knock down expression of the SFP CG10586, a predicted serine protease, we show that it acts upstream of the SFP CG11864, a predicted astacin protease, to process SFPs involved in ovulation and sperm entry into storage. We also show that knockdown of CG10586 leads to lower levels of egg laying, higher rates of sexual receptivity to subsequent males, and abnormal sperm usage patterns, processes that are independent of CG11864. The long-term phenotypes of females mated to CG10586 knockdown males are similar to those of females that fail to store sex peptide, an important elicitor of long-term post-mating responses, and indicate a role for CG10586 in regulating sex peptide. These results point to an important role for proteolysis among insect SFPs and suggest that protease cascades may be a mechanism for precise temporal regulation of multiple post-mating responses in females.
Published in the journal: . PLoS Genet 8(1): e32767. doi:10.1371/journal.pgen.1002435
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002435Summary
Proteases and protease inhibitors have been identified in the ejaculates of animal taxa ranging from invertebrates to mammals and form a major protein class among Drosophila melanogaster seminal fluid proteins (SFPs). Other than a single protease cascade in mammals that regulates seminal clot liquefaction, no proteolytic cascades (i.e. pathways with at least two proteases acting in sequence) have been identified in seminal fluids. In Drosophila, SFPs are transferred to females during mating and, together with sperm, are necessary for the many post-mating responses elicited in females. Though several SFPs are proteolytically cleaved either during or after mating, virtually nothing is known about the proteases involved in these cleavage events or the physiological consequences of proteolytic activity in the seminal fluid on the female. Here, we present evidence that a protease cascade acts in the seminal fluid of Drosophila during and after mating. Using RNAi to knock down expression of the SFP CG10586, a predicted serine protease, we show that it acts upstream of the SFP CG11864, a predicted astacin protease, to process SFPs involved in ovulation and sperm entry into storage. We also show that knockdown of CG10586 leads to lower levels of egg laying, higher rates of sexual receptivity to subsequent males, and abnormal sperm usage patterns, processes that are independent of CG11864. The long-term phenotypes of females mated to CG10586 knockdown males are similar to those of females that fail to store sex peptide, an important elicitor of long-term post-mating responses, and indicate a role for CG10586 in regulating sex peptide. These results point to an important role for proteolysis among insect SFPs and suggest that protease cascades may be a mechanism for precise temporal regulation of multiple post-mating responses in females.
Introduction
Proteolysis regulators are a component of the seminal fluid of many animal taxa, including insects and other invertebrates [1]–[7], fish [8]–[10], birds [11], [12], and mammals [13]–[18]. However, the mechanisms by which seminal proteases act, and most of the processes they affect, in mated females are poorly understood.
A mechanism by which proteases may effect physiological responses is through proteolytic cascades. Because most proteases are synthesized as inactive zymogens and require the removal of a short N-terminal sequence for activation [19], a protease cascade can be rapidly set in motion without new protein synthesis. For example, in mammals a seminal protease cascade activates the protease prostate specific antigen (PSA), in order to rapidly liquefy the seminal clot formed following ejaculation (reviewed in [15]). The action of PSA is regulated, in part, by the protease inhibitor PCI (reviewed in [20]), which controls the timing and extent of liquefaction. Seminal clots are an important feature of the post-mating response in many animals [13], [21], [22].
Given the prevalence of proteolysis regulators in seminal fluid, it seems likely that they are involved in other processes whose effects may extend past the first few minutes after mating. The study of seminal fluid protease functions would benefit greatly from a genetic approach. Drosophila melanogaster provides an excellent system in which to study the roles of seminal fluid proteolytic proteins. Analysis of Drosophila seminal fluid proteins (SFPs) capitalizes on a wide range of available genetic tools, physiological and behavioral assays, and both a well-annotated genome and seminal fluid proteome. In addition, though individual SFPs, including proteases [23], are not generally well-conserved between distant taxa [24], [25], the biochemical classes into which SFPs fall are conserved between insects and mammals [21], [26], suggesting that mechanisms of action are likely to be conserved as well.
Approximately 18% of the proteins in the Drosophila ejaculate have been identified as predicted proteases or protease inhibitors [2], [27]. Mass spectrometry-based estimates indicate that the abundance of individual proteolysis regulators varies, with some being the most abundant proteins in the ejaculate (e.g. Acp62F) and others being the least abundant (e.g. CG10587) [2]. Most SFP predicted proteolysis regulators are either serine proteases or serine protease inhibitors with unknown functions [2], [28], [29], though a few other protease classes have also been identified [26], [30]–[32]. Proteolysis regulators have been identified as expressed in male reproductive tract tissues of Tribolium [7] and directly in the ejaculates of honey bees [3] and mosquitoes [33]. In crickets, a predicted trypsin-like serine protease in the ejaculate is important for inducing egg laying in mated females [5]. In the nematode Caenorhabditis elegans, a trypsin SFP has recently been reported to function in activation of male sperm [10]. Though proteases are emerging as a common SFP class in animals, there have been no studies determining whether protease cascades (i.e. proteolytic pathways that require at least two proteases in sequence) are a common regulatory mechanism for seminal fluid-mediated post-mating traits.
In Drosophila, transfer of SFPs from male to female during mating induces physiological changes in mated females [reviewed in 6]. Two of these changes are increased egg production and reduced receptivity to remating. These changes occur in two phases: short-term and long-term, both of which are necessary for optimal fertility. The short-term response (STR) occurs within 24 hours of mating and is solely dependent on the receipt of SFPs [34], including the prohormone ovulin [35], CG33943 [36], the sperm storage protein Acp36DE [37], [38], and the action of free sex peptide (SP) that is not bound to sperm [39], [40].
Long-term persistence of post-mating changes (the long-term response, or LTR) requires SP and multiple other SFPs, and the presence of sperm in storage [40]. SP binds to sperm during mating. Cleavage by an unknown trypsin protease(s) is required to release the active portion of SP from sperm within the mated female [41]. SP is gradually cleaved from stored sperm during the approximately two weeks that they remain in storage. As long as SP is released into the female, she continues to lay eggs at a high rate and is more likely to reject courting males [41]. If SP cannot be released from sperm, the LTR does not occur [41].
Fertility defects arise if SP cannot bind to sperm in the mated female, or if it cannot be released from sperm. Sperm binding by SP requires the action of at least four other SFPs: the predicted serine protease CG9997, the Cysteine Rich Secretory Protein (CRISP) CG17575, and the gene duplicate pair lectins CG1652 and CG1656 [36], [42]. These four “LTR proteins”, together with SP, function in an interdependent network to bind SP to sperm as well as to localize each other to the seminal receptacle (SR), the major sperm storage organ of the female [42]. In this network, CG9997 is cleaved into a 36-kDa protein in the male ejaculatory duct/bulb, prior to transfer to the female and is required for the normal transfer of CG1652 and CG1656. CG17575 is required to localize CG1652 and CG1656 to sperm and the SR. This final step is then required for SP to bind sperm and accumulate in the SR. If any one of the four LTR proteins is absent, SP does not bind sperm. These SP-free sperm are still stored in normal numbers, but cannot be efficiently released from storage for fertilization past the first 24 hours after mating [42], because SP is also required for sperm release [43].
In addition to SP, two SFPs involved in post-mating traits are known to be cleaved following deposition into the female. The prohormone ovulin is initially cleaved at about 10 minutes after the start of mating (ASM) [44]. Ovulin is required for a maximal ovulation rate in the first 24 hours following mating [35], [45]. Processing occurs via three cleavage events from the N-terminus of ovulin that ultimately results in the production of one major cleavage product (approx. 25 kDa) and three minor products (each 5 kDa or smaller) [44]. Ectopic expression experiments have shown that both full-length ovulin as well as two C-terminal fragments, roughly corresponding to cleavage products of ovulin, are each able to independently induce ovulation in virgin females [46]. The glycoprotein Acp36DE is also cleaved within mated females [47], starting at approximately 20 minutes ASM, as detected by Western blot [47], [48]. Acp36DE is required for efficient sperm storage [37], [48]. This protein is responsible for the conformational changes of the uterus immediately following the start of copulation, which are thought to aid the movement of sperm into the storage organs [38], [49].
A previous study of 11 SFP proteases and protease inhibitors identified CG11864 as required for processing of both ovulin and Acp36DE [32]. Though all three proteins are produced in the male accessory glands, ovulin and Acp36DE are not cleaved until several minutes after their entry into the female reproductive tract. Therefore, three possibilities exist for the regulation of ovulin and Acp36DE cleavage. CG11864 may be activated during mating, a repressor of CG11864 activity may be removed during mating, or a combination of both may occur.
CG11864 is predicted to be a member of the astacin family of metalloproteases, based on sequence similarity [32]. Astacin family proteases, like many other proteases, require removal of an N-terminal pro-peptide for activation [50]. The activity of CG11864 thus may be regulated in a similar manner. CG11864 is produced in the male accessory glands as a 33-kDa protein and is cleaved to an approximately 30-kDa form [32]. This cleavage begins in the male reproductive tract, in the ejaculatory duct and/or bulb, while CG11864 is in transit to the female during mating [32]. The size of the cleaved form of CG11864 is consistent with removal of a predicted pro-peptide from the N-terminus. We hypothesize that cleavage of CG11864 is required for its activation. If this is the case, there should be factors produced by the male that regulate the activation of CG11864. However, the previous study involving 11 SFP proteolysis regulators did not suggest their requirement for the regulation of CG11864 [32]. A recent microarray analysis by Chintapalli et al. [51] and subsequent proteomic studies by Findlay et al. [2] identified additional serine proteases in the ejaculate. We, therefore, focused on these proteases to test for roles in the activation/regulation of seminal proteolysis.
Here, we used RNAi knockdown analysis to test five male-derived serine proteases for roles in ovulin cleavage and other reproductive events. We describe the first proteolytic cascade in fly seminal fluid that is regulated by a predicted trypsin-like serine protease, CG10586. We propose to rename this enzyme seminase (gene symbol: sems). Seminase is required for cleavage, and likely activation, of CG11864. Like CG11864, seminase is produced in the accessory glands and is cleaved in the male during copulation. We show that CG11864 is not able to undergo self-cleavage in the absence of seminase. In addition to regulating CG11864 and thus its downstream SFP substrates, we show that seminase is a member of the LTR network, a CG11864-independent pathway that results in SP binding to sperm.
Results
Seminase Is Required for Normal CG11864-Mediated Processing of Ovulin and Acp36DE
We tested five predicted protease SFPs for ovulin processing defects via Western blot to identify potential CG11864-interacting proteins. The tested SFPs were the predicted serine proteases ‘seminase’ (CG10586), CG10587, CG4815, CG12558, and CG32382 (sphinx2). Of these, only seminase, a predicted trypsin-type serine protease, was required for ovulin processing (Figure 1A). In addition to the results for seminase, we show for comparison the results for CG10587, which did not affect ovulin processing. The data for the other SFPs tested are not shown. Two independent insertion lines of the same RNAi construct were used to test the phenotype of seminase knockdown (see Materials and Methods); we obtained similar results with both lines. Western blotting confirmed that seminase is knocked down at least 98% by Tubulin-Gal4 driven expression of the RNAi construct in males of both lines (Figure S1A). Transcript levels of seminase were also confirmed to be knocked down by RT-PCR (Figure S1B).
Fig. 1. Processing of SFPs is defective in the absence of seminase. 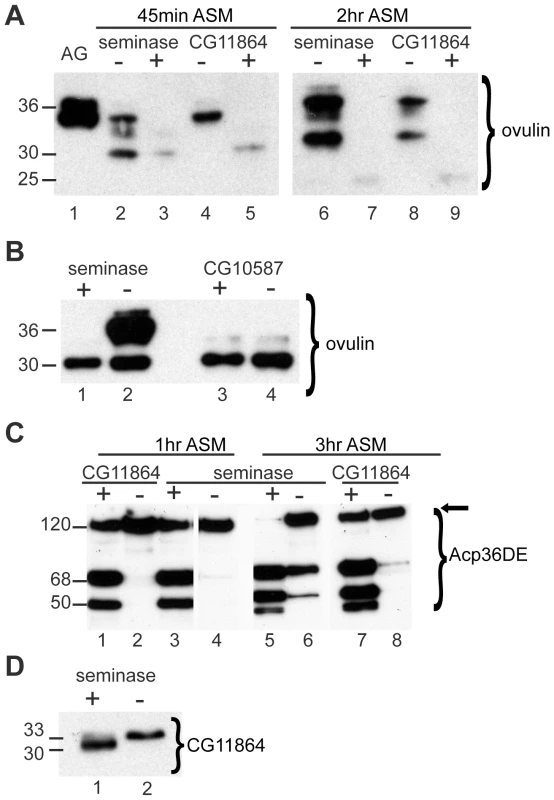
(A) Western blot probed with ovulin antibody. Lane 1: full-length ovulin in male accessory glands (AG). Lanes 2–9: female reproductive tracts (RT) dissected after mating to control (+) or RNAi (−) males for the gene given above the lanes. Females were dissected at 45 minutes after the start of mating (ASM) (lanes 2–5) or 2 hours ASM (lanes 6–9). All lanes are from the same gel with extraneous lanes removed for clarity. (B) Western blot probed with ovulin antibody. All lanes are female RT dissected at 30 minutes ASM. Lanes 1 and 2 are from females mated to seminase control (+) or RNAi (−) males. Lanes 3 and 4 are from females mated to CG10587 control (+) or RNAi (−) males. (C) Western blot probed with Acp36DE antibody. All lanes are from the same gel, with extraneous lanes cut out for clarity. Lanes 1–8 contain RT from females mated to males of the given genotype as in (A). Females were dissected at 1 hour ASM (lanes 2–5) or 3 hours ASM (lanes 6–9). Un-processed (full-length) Acp36DE runs at ∼122 kDa. (D) Western blot probed with CG11864 antibody. Female RTs dissected at 45 minutes ASM to seminase control (lane 1) or knockdown (lane 2) males. Numbers to the left of blots indicate approximate band size in kDa. Similar to the phenotype previously observed with CG11864 RNAi [32], some ovulin processing was observed in females mated to males knocked down for seminase, but ovulin was never processed fully in mates of seminase knockdown males, even at 2 hours ASM, the latest time at which ovulin can be reliably detected in female reproductive tracts when mated to controls (Figure 1A). Females mated to seminase knockdown males also failed to fully process Acp36DE at 1 hour ASM (Figure 1C), similar to CG11864 knockdown mates (Figure 1C). Even at 3 hours ASM, Acp36DE in females mated to seminase or CG11864 RNAi males had undergone only a small amount of processing relative to controls (Figure 1C).
Seminase Is Required for Putative CG11864 Pro-Peptide Cleavage
Females mated to seminase RNAi knockdown males received CG11864 protein, but it was of the full-length molecular weight (33-kDa); the cleaved form (30-kDa) was never observed (Figure 1D). However, females who mated to control males received both full-length and cleaved CG11864 (Figure 1D). Thus, seminase is required for the predicted pro-peptide cleavage of CG11864 during mating.
Seminase Is Specific to the Male Accessory Glands and Is Initially Cleaved in the Male during Transfer to the Female
Since many serine proteases are synthesized as zymogens (containing an N-terminal sequence that must be removed for activation), we tested whether seminase was also processed during or after mating. Seminase is detected as an apparent 29-kDa protein (predicted size: 28.2-kDa, excluding a predicted N-term secretion signal sequence) in the accessory glands, with no detectable expression in the testes or ejaculatory duct and bulb (Figure 2A). There was no evidence for seminase expression outside of the male accessory glands based on expression data in the FlyAtlas database [51] and our own RT-PCR (Figure S1B). We did not detect seminase protein in virgin females (Figure 2A).
Fig. 2. Seminase is produced in the accessory glands and is processed during and after mating. 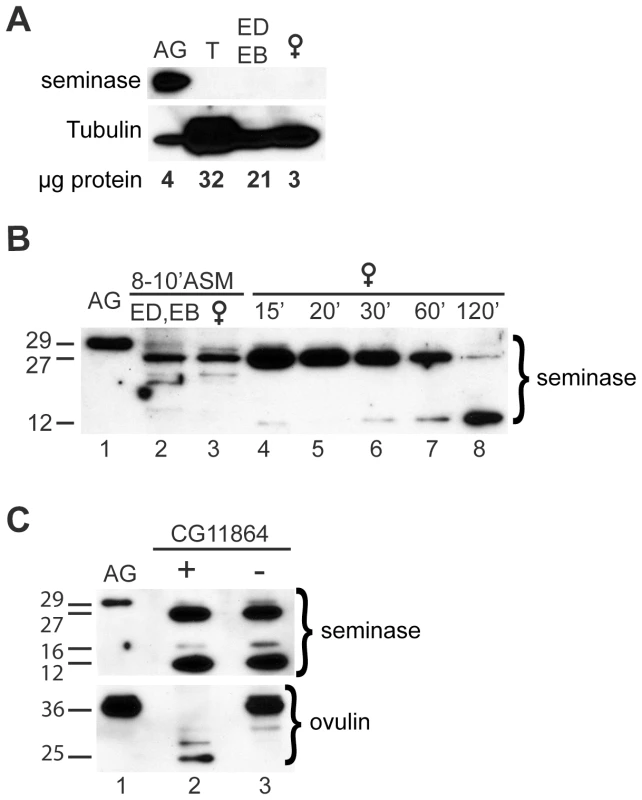
(A) Western blot probed with seminase antibody. Seminase in the male accessory glands (AG) has an apparent molecular weight of ∼29-kDa. No seminase was detected in testes (T), the ejaculatory duct and bulb (ED/EB), or in virgin female reproductive tracts. AG and T: tissue from 10 virgin males. ED/EB: tissue from 20 virgin males. Female: tissue from 4 virgin females. Tubulin is shown as a loading control. Total protein loaded is shown in micrograms as measured by BCA assay. (B) Western blot probed with seminase antibody. Lane 1: full-length seminase in AG. Lane 2: ED/EB dissected from 20 males at 8–10 minutes ASM. Lane 3: Reproductive tracts (RT) from 20 females dissected 8–10 minutes ASM. Lanes 4–8: Female RTs dissected at the times ASM indicated above the lanes. (C) Western blots probed for seminase (top) and ovulin (bottom). Lane 1: full length protein in AG. Lanes 2 and 3: RT from females mated to CG11864 control (+) or knockdown (−) males at 1 hour ASM. During mating, an additional, lower molecular weight band (approximately 27-kDa) of seminase appeared in the male ejaculatory duct and/or bulb (Figure 2B), consistent with removal of a 2.79-kDa pro-peptide (size prediction based on an NCBI conserved domain search at http://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi?INPUT_TYPE=live&SEQUENCE=NP_649270.1). Within the mated female, seminase was further cleaved, producing a ∼16-kDa product (visible in top panel of Figure 1C) and increasing the amount of the ∼12-kDa product (Figure 2B). We first detected the ∼12-kDa product in the female at around 15 minutes ASM. However, due to the difficulty in detecting the ∼16-kDa form, we could not determine at what time ASM it is first produced.
Given that some proteases can be cleaved by their own proteolytic substrates [for example, see 52], we tested whether knockdown of CG11864 affected processing of seminase after mating. Females mated to CG11864 knockdown males showed normal processing of seminase at 30 minutes ASM (Figure 2C, top panel). As expected [32], CG11864 knockdown does prevent ovulin cleavage (Figure 2C, bottom panel), indicating a unidirectional proteolytic pathway.
Seminase Is Also Required for CG11864-Independent Post-Mating Processes
The total number of eggs laid over 10 days was significantly lower in females mated to seminase knockdown males relative to their controls, and this was seen in both independent insertion lines (Line 1 Poisson regression: z = −29.56, p<0.0001, Figure 3A; Line 2 Poisson regression: z = −31.59, p<0.0001, Figure S1A). There was no difference in total number of eggs laid between females mated to CG4815 knockdown and control males (CG4815 Poisson regression: z = −0.79, p = 0.43, Figure 3A).
Fig. 3. Females mated to seminase knockdown males lay fewer eggs. 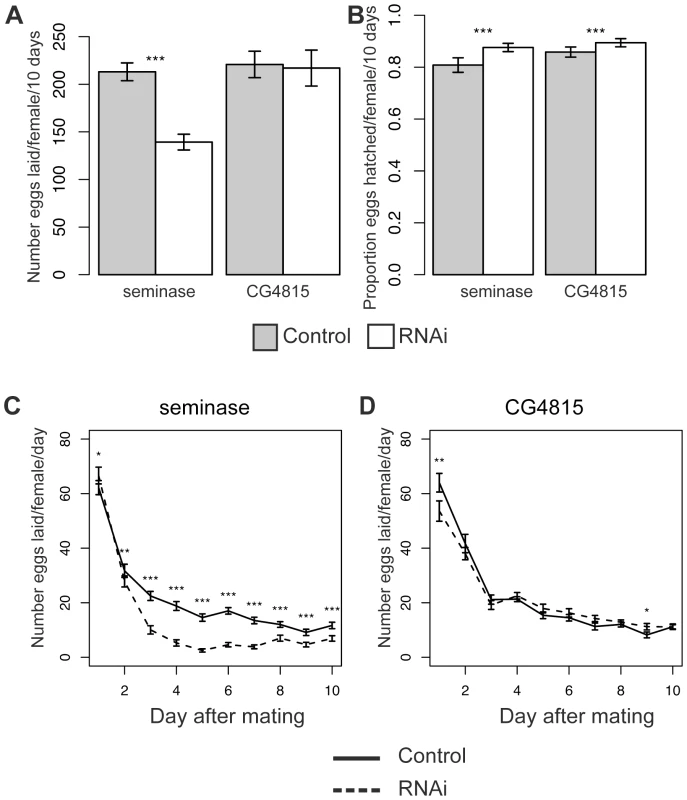
(A) The average number of eggs laid per female in a given treatment over 10 days. Seminase Line 1: Control N = 55, RNAi N = 59; CG4815: Control N = 20, RNAi N = 18. (B) Hatchability data for the corresponding experiments in (A). Hatchability is defined as the proportion of eggs that yielded adult progeny. CG4815 (a serine protease SFP) is included as a control for strain background. (A),(B) Asterisks indicate p<0.0001. (C) The data from (A) plotted as average number of eggs laid by females in each group on individual days of the experiment. (D) CG4815 egg laying data plotted as in (C). Asterisks indicate level of significance after Bonferroni correction (*p<0.05, **p<0.01, ***p<0.0001). Error bars indicate standard error of the raw data pooled over experiments. Eggs laid by seminase or CG4815 knockdown mates hatched in significantly larger proportions than eggs laid by control mates (Binomial regressions: Line 1: z = 10.1, p<0.001 and CG4815: z = 5.8, p<0.0001, Figure 3B; Line 2: z = 13.2, p<0.0001, Figure S1B), suggesting the egg laying defect was not accompanied by a hatchability defect (hatchability is defined as the proportion of eggs that produced adult progeny), but rather that there was a slight deleterious effect of the balancer control background on hatchability.
A repeated measures analysis of egg laying over time revealed a significant effect of male genotype on egg laying over time for both seminase lines and CG4815 (see Materials and Methods). To determine the days on which male genotype affected egg laying, data were analyzed separately for each individual day. The decrease in egg laying, relative to control, in mates of seminase knockdown males was only apparent after the first day following mating and persisted until at least 9 days post-mating (Figure 3C and Figure S1C). Females mated to seminase knockdown males laid slightly, though significantly, more eggs than females mated to control males on day 1, but only with seminase Line 1 males (Figure 3C). These results indicated that seminase only has a major role in egg laying after the first day post-mating.
Females mated to CG4815 knockdown males laid significantly fewer eggs than females mated to control males on day 1 and slightly, though significantly, more eggs than controls on day 9 (Figure 3D). Thus, CG4815 may have a short-term effect on egg laying, but no long-term effect similar to that observed with seminase knockdown mates. These results also indicate that the long-term egg laying effect of seminase is not an artifact of the VDRC strain background, which is shared by the CG4815 males.
A reduction in fecundity after the second day post-mating suggests seminase is a new member of the LTR pathway. Increased recovery of post-mating receptivity to courtship beginning after the first 24 hours post-mating is also associated with LTR defects. Therefore, we tested whether deficiency of seminase in the ejaculate also caused increased receptivity in females, relative to controls, after mating. Table 1 shows data for female receptivity at 24 hours, 2 days, and 4 days ASM to seminase knockdown or control males. Similar to previously described phenotypes of LTR SFPs [36], [42], [43], females mated to seminase knockdown males were significantly more likely to remate at 2 days and 4 days ASM than were controls. Females mated to males from seminase knockdown line 1 showed a smaller magnitude of difference in remating rate relative to their controls than did line 2. This is most likely due to a background effect in line 1 that is apparent in the control males, as the remating rate is similar in mates to knockdown males from both lines. Since the same RNAi construct is expressed in both lines, we assume that the higher remating rate for females mated to line 1 control males is due to the insertion locus of the transgene. There was no effect of CG4815 knockdown on receptivity (Table 1).
Tab. 1. Female receptivity to second mating. 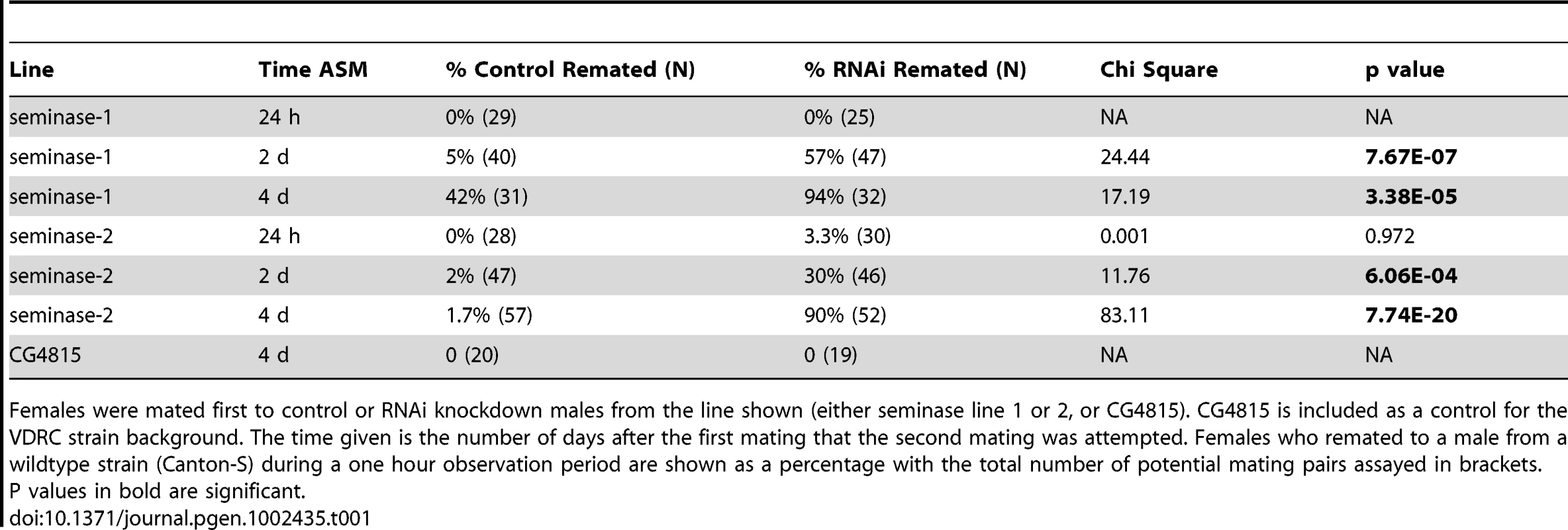
Females were mated first to control or RNAi knockdown males from the line shown (either seminase line 1 or 2, or CG4815). CG4815 is included as a control for the VDRC strain background. The time given is the number of days after the first mating that the second mating was attempted. Females who remated to a male from a wildtype strain (Canton-S) during a one hour observation period are shown as a percentage with the total number of potential mating pairs assayed in brackets. P values in bold are significant. Seminase Is Required for Sperm Release from Storage
In Drosophila females, sperm are stored in two types of storage organs: the seminal receptacle (SR) and the paired spermathecae. The bulk of the sperm are stored in the SR [53]. Because LTR SFPs (in concert with SP) affect the release of sperm from storage [36],[43], we tested whether mates of seminase knockdown males also showed a defect in sperm release. Mates to seminase knockdown males stored normal numbers of sperm (Figure 4 “2 h” bars), but significantly more sperm remained in storage at 10 days ASM in mates of seminase knockdown males than in control-mated females (Figure 4A). This effect was due to a failure to release sperm from the SR (Figure 4B), as sperm numbers in the spermathecae decreased at similar rates in females mated both to control and seminase knockdown males (Figure 4C). A slight, but significant, difference in sperm release was seen in the spermathecae at 4 days ASM, but this effect was in the opposite direction from that seen in the SR and was no longer apparent by 10 days ASM (Figure 4C). Similar effects were seen with seminase knockdown Line 2 (Figure S1D).
Fig. 4. Females mated to seminase knockdown males retain more sperm 4 and 10 days after mating. 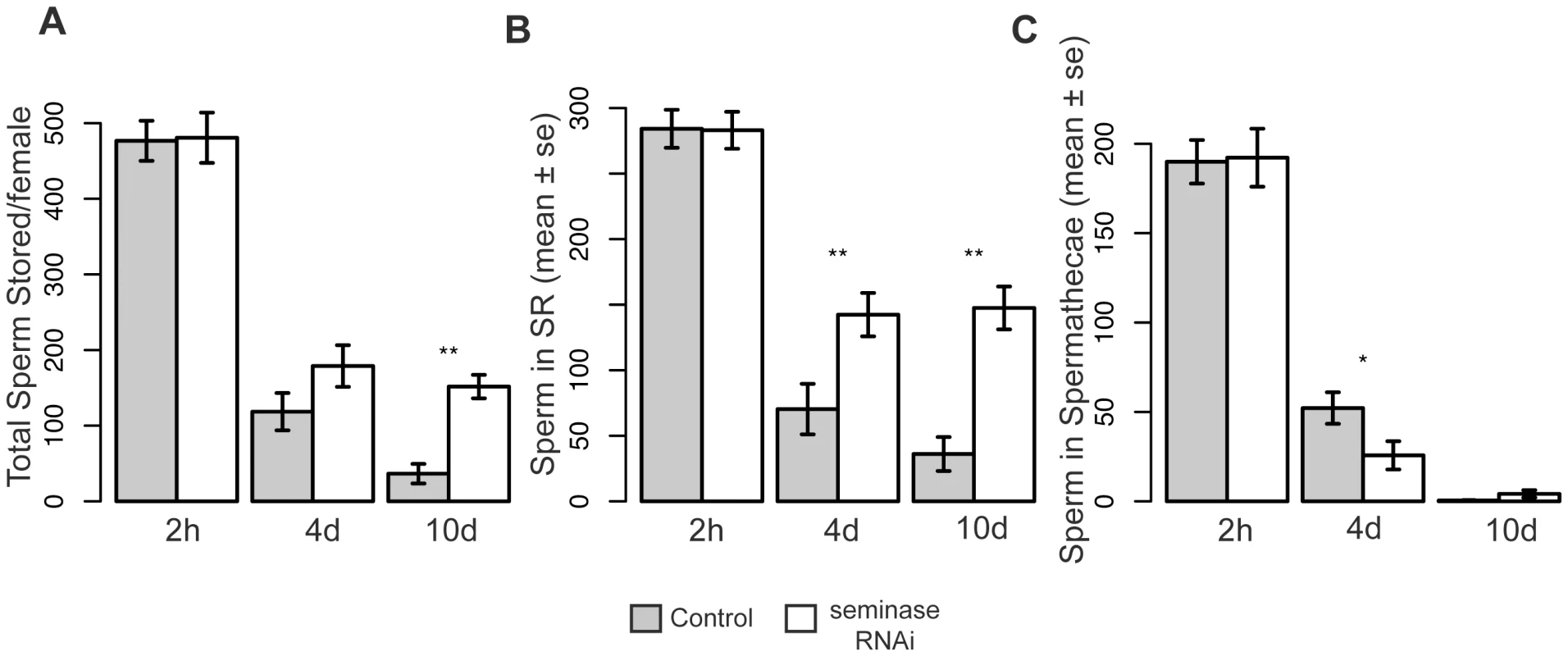
Sperm counts for seminase line 1 are shown for sperm stored at three timepoints after mating, as given on the x-axis. Line 2 yielded similar results. Asterisks indicate level of significance (*p<0.05; **p<0.01). (A) Total average number of sperm stored in both storage organs (2 h: t = −0.09, p = 0.93; 4 d: t = −1.6, p = 0.13; 10 d: t = −3.2, p<0.01). (B) Average number of sperm stored in the seminal receptacle only (2 h: t = −0.06, p = 0.96; 4 d: t = −2.8, p<0.01; 10 d: t = −3.2, p<0.01). (C) Average number of sperm stored in the paired spermathecae only; numbers are the sum of sperm stored in each spermatheca (2 h: t = −0.11, p = 0.91; 4 d: t = 2.2, p<0.05; 10 d: t = −1.1, p = 0.26). Samples sizes are, for bars from left to right: (A) 12, 14, 16, 10, 7, 15; (B) 17, 21, 18, 19, 7, 15; (C) 13, 14, 19, 10, 7, 15. Abbreviations: 2 h, 2 hours; 4 d, four days; 10 d, 10 days. Error bars indicate standard error. SP and Other LTR Proteins Fail to Localize to the Seminal Receptacle in the Absence of Seminase
The results above are consistent with seminase being a member of the LTR network. To determine the placement of seminase in this network, we tested whether knockdown of seminase affected the post-mating localization of SP and the three LTR proteins that localize to the SR: CG9997, CG1652, and CG1656. At 2 hours ASM, seminase was required for accumulation of SP, CG1652, and CG1656 in the SR (Figure 5A). CG9997 was not detected in the SR at 2 hours ASM, so we tested females at 1 hour ASM. Seminase was also required for accumulation of CG9997 in the SR at this time point (Figure 5A). However, seminase was not required for proper processing of CG9997 or transfer of any LTR SFPs to the female during mating (Figure 5A, RT lanes), including CG17575 (Figure 5B).
Fig. 5. LTR proteins fail to accumulate in the seminal receptacle in the absence of seminase. 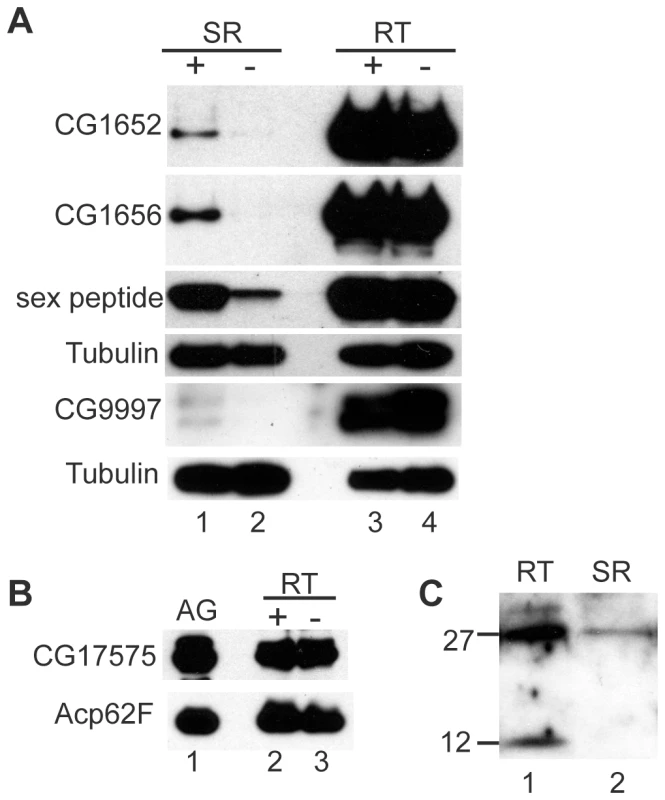
(A) Western blots probed for the proteins indicated to the left of blots. Lanes 1 and 2 contain 20 seminal receptacles (SR) dissected from females mated to control (+) or seminase knockdown (−) males at 2 hours ASM for SP, CG1656, and CG1652, and 1 hour ASM for CG9997. CG9997 was probed on a separate blot from CG1652, CG1656, and sex peptide. Lanes 4 and 5 contain 4 reproductive tracts (RT; not including ovaries or sperm storage organs, SSOs) from a subset of the females dissected in SR lanes. Tubulin is shown as a loading control for both gels. (B) Western blot probed for CG17575. Lane 1: male accessory glands (AG). Lanes 2 and 3 contain 4 RTs (excluding ovaries) from females mated to control (+) or seminase knockdown (−) males at 45 minutes ASM. Acp62F is shown as a loading control. (C) Western blot probed for seminase. Lane 1 contains female RT excluding ovaries and SSOs from wildtype mated females. Lane 2 contains 80 SR from wildtype mated females. A small amount of seminase also enters the SR (Figure 5C), suggesting that it could physically interact with other LTR proteins there. However, we were unable to determine whether other LTR proteins affected seminase localization to the SR due to the extremely low seminase signal within the SR. Multiple repetitions of the experiment failed to yield consistent results. As with previous efforts to detect LTR proteins in the spermathecae [42], we were not able to detect seminase in these organs (data not shown).
Discussion
An SFP, the Predicted Serine Protease Seminase, Regulates Processing of Other SFPs through a Proteolytic Cascade
To identify proteins that may interact with the predicted astacin-family protease CG11864 to process the SFPs ovulin and Acp36DE, we used RNAi to individually test five serine protease SFPs for ovulin processing defects. One of these proteins, the predicted trypsin-type serine protease ‘seminase’ (CG10586), is required for normal processing of ovulin as well as of the sperm storage protein Acp36DE. Because the phenotype of seminase knockdown was similar to that of CG11864 knockdown with respect to SFP processing, we hypothesized that both proteins might act in a single pathway. Additionally, because trypsin (serine) proteases are required for activational cleavage of some astacin-family proteases (of which CG11864 is one) [50], [54], we further hypothesized that seminase might act upstream of CG11864.
We therefore tested whether seminase regulates the cleavage, and thus activation, of CG11864. We found that seminase is required for the approximately 3-kDa mobility shift of CG11864 that is seen in the male reproductive tract very soon after mating begins, suggesting that seminase may activate CG11864 by cleaving its pro-peptide. The apparent processing of pro-CG11864 by seminase and the subsequent processing of downstream substrates is suggestive of a proteolytic cascade. No such proteolytic pathway has, to our knowledge, previously been identified in insect seminal fluid. With the identification of this pathway in Drosophila melanogaster, we have found a molecular model for dissecting proteolytic pathways involving SFPs that have consequences for fertility.
Proteolytic cascades, in their simplest form, typically have three steps [55]: 1) auto-activation of an initiator protease(s) present in low amounts and triggered by an external stimulus: 2) activation of a more abundant propagator protease(s) by the initiator protease; 3) activation of an executor protease(s) by the propagator, which will cleave the downstream substrates. In addition, the propagator may also cleave, and thereby continue to activate, the initiator. Altogether, this causes a rapid propagation of the initial external signal.
While protein abundance is not necessarily related to potency, it is intriguing that seminase (the putative initiator) is relatively scarce in the ejaculate of D. melanogaster, which is consistent with the above model. Abundance estimates are based on the normalized spectral abundance factor (NSAF) obtained by mass spectrometry on mated females [2]. NSAF is an approximate measure of the relative abundance of a protein in a complex sample. Seminase ranks at 130 out of 138 (NSAF = 1.34×10−4), with 1 being the most abundant and 138 the least [2]. CG11864 is similarly scarce (87/138; NSAF = 7.69×10×−4). This is in contrast to the much higher abundance of CG11864's substrates (ovulin: 20/138, NSAF = 1.2×10−2; Acp36DE: 19/138, NSAF = 1.21×10−2).
Also consistent with the protease cascade model, seminase is cleaved to a slightly smaller form during mating while still in the male reproductive tract. This may be an activational pro-peptide cleavage event, though this has not been directly tested. It is possible that seminase self-activates upon entering the ejaculatory duct, as is the case for many serine proteases [for example, among tissue kallikrein pathways; see 15]. Our data suggest that seminase acts as the initiator in the cascade, and CG11864 acts either as the propagator, the executor, or both.
After transfer, seminase itself undergoes additional processing in the female (after the initial pro-peptide cleavage in the male) that is not a result of CG11864 activity (Figure 2C). These cleavage products may be important for the function of seminase. On the other hand, they may simply be degradation products of seminase. However, both scenarios remain speculative.
We have shown that, in the absence of seminase, CG11864 is not cleaved to the predicted active form. However, the predicted pro-peptide cleavage site of CG11864, based on sequence threading to other astacin-family proteases [32], is not a trypsin site, as would be predicted if seminase were the only protease responsible for CG11864 activation. Interestingly, there are three trypsin cleavage sites present in the pro-peptide region of CG11864. It is possible that CG11864 is cleaved via a two-step mechanism (involving a trypsin and CG11864 itself), as is seen for the pro-peptide cleavage of Astacus astacus (crayfish) astacin, the prototype of the astacin family [54], [56]. Future studies using purified proteins in vitro will determine whether CG11864 is capable of self-cleavage and whether seminase acts to directly cleave CG11864.
Role of the Seminase/CG11864 Pathway
Despite a severe delay in ovulin processing, knockdown of neither seminase nor CG11864 results in an egg laying defect in the first 24 hours after mating [CG11864 data reported in 32]. This result is not surprising, however, given the rather small effect on egg laying seen with a complete knockout of ovulin [45]. Additionally, ectopic expression of full-length ovulin is sufficient to induce ovulation in virgin females [46], suggesting that the additional effect of ovulin processing may be too small to detect with the current assay. It is also possible that, while seminase was knocked down to very low levels, there may still be sufficient seminase present for its role in early egg laying.
We also do not observe a defect in sperm entry into storage following seminase knockdown, as seen with knockout of Acp36DE [37]. Instead, defects were seen in sperm release from storage at later timepoints, which is not a phenotype associated with Acp36DE knockout. Acp36DE processing may be important for other functions of this protein. For example, Acp36DE is a component of the mating plug [57], but its function in the mating plug is still unknown. Further research is required to determine the consequences of loss of proteolytic processing of both ovulin and Acp36DE.
Seminase Regulates Multiple Independent Post-Mating Processes
In contrast to CG11864, which seems specific to the STR, seminase has a second important activity: it is in the LTR pathway, which regulates the binding of sex peptide (SP) to sperm [36], [41]–[43] (See Figure 6 for overview). Similar to mates of SP null males, females mated to seminase knockdown males lay fewer eggs than controls over a 10 day period and also retain sperm in storage. The sperm retention phenotype is only apparent in the SR. Other LTR proteins are also known to affect sperm storage in the SR but not the spermathecae [36], though the reason for these differences is not understood. While the interaction between sperm release and egg laying is complex, over the long-term, egg laying and sperm release are independent of each other [58]. Sperm do not directly influence the release of eggs, though the presence of SP bound to sperm is required for both sperm release [43] and normal post-mating levels of egg laying [39], [40]. The failure of SP to accumulate in the SR indicates that seminase is likely required for SP to bind sperm.
Fig. 6. Seminase is required for two post-mating pathways. 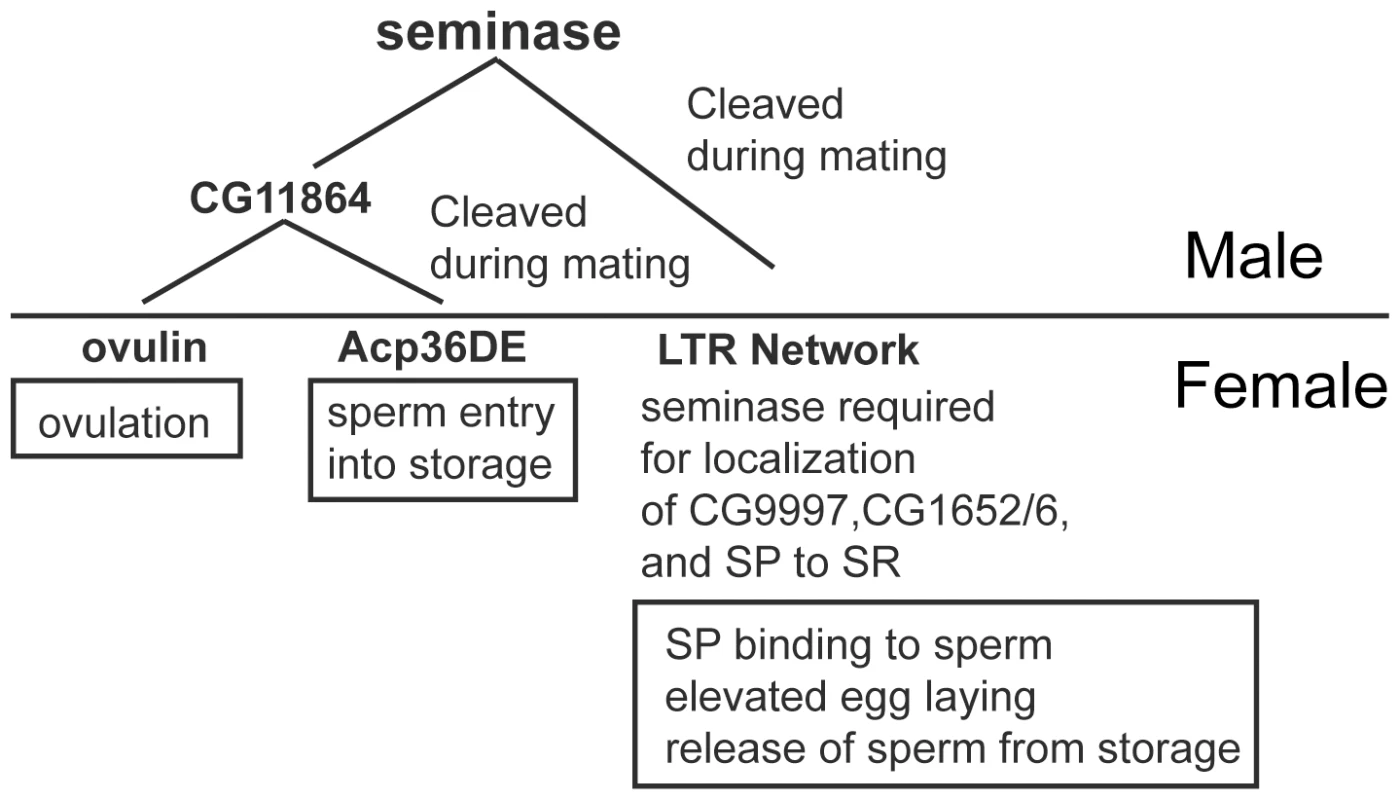
Seminase is cleaved during mating within the male reproductive tract where it is required for CG11864 cleavage. CG11864 then regulates processing of two SFPs, ovulin and Acp36DE (left branch). Seminase is also necessary for the long term response (LTR) pathway (right branch). Proteins/pathways downstream of seminase are involved in the processes shown in boxes, based on earlier knockout or knockdown studies. The consequences of proteolytic processing of ovulin and Acp36DE remain unknown. The requirement for seminase in two independent post-mating pathways suggests that its activation at mating may act as a regulatory “switch” that coordinates post-mating events in Drosophila. Identification of other seminase substrates, if they exist, will allow us to determine the extent of seminase's effects as a regulatory switch for post-mating events.
Evolutionary Implications of Seminase-Regulated Processes
In the context of evolution, SFPs represent a unique class of proteins in that they must, first and foremost, aid in successful fertilization, but are also tasked with representing the male's reproductive interests, sometimes in the face of opposing female interests [reviewed in 59]. This has the potential to set up a genetic conflict between the sexes and has been suggested to be one reason that SFPs in particular tend to be rapidly evolving [24], [25]. However, an SFP's evolutionary rate is also likely to be constrained by the need for the protein to maintain its interaction with other proteins in the seminal fluid, and/or with proteins expressed by the female. For example, seminase and CG11864 are processed first in the male, but must interact with the female environment to further process both seminase and the substrates of the proteolytic pathway. Seminase-regulated processes represent an opportunity to understand the evolution of SFP networks that contain a mixture of conserved proteins (e.g. the lectins CG1652 and CG1656 and the CRISP CG17575 [2]) and proteins under positive selection (e.g. ovulin [60] and the serine protease CG9997 [61]). Seminase itself shows no evidence of positive selection, either at the protein level [61] or at individual sites (personal communication, Geoff Findlay). However, seminase does have two very closely related SFP paralogs, CG11037 and CG10587, which show evidence for recent positive selection in the D. melanogaster lineage [61]. These three genes are clustered together in the genome of D. melanogaster and the other melanogaster subgroup species. CG10587 does not play a role in ovulin or Acp36DE processing (CG11037 has yet to be tested), suggesting that these genes arose from tandem duplications and later diverged in function, with seminase remaining as the more conserved of the paralogs.
Our data on seminase show that this member of a conserved protein class in the seminal fluid plays a vital role in reproductive success. We believe that future study of the seminase-regulated pathways in Drosophila will lead to new mechanistic and evolutionary insights related to proteolytic cascades and protein networks in seminal fluid.
Seminase-Dependent Processes: A Model for Pleiotropic SFPs
The regulation and mechanism of action of seminase constitutes a new in vivo model system for studying the regulation and physiological roles of pleiotropic SFPs. Pleiotropic effects of kallikrein-related proteases involved in the liquefaction of human semen have recently been reported [62], [63]. Further understanding of the various effects of seminal fluid proteolysis in post-mating processes may have important implications for human health (e.g. the role of PSA in cancer) and fertility. Our results indicate that genetic analysis in Drosophila will be an important complement to in vitro studies in mammalian systems for understanding the role of proteolytic processing in reproduction. Future studies of the regulatory mechanisms involved in the seminase/CG11864 proteolytic pathway may generate testable hypotheses for other SFP networks, including those in mammals.
Materials and Methods
Flies
Transgenic lines carrying RNAi constructs for CG10586 (‘seminase’) and CG4815 were purchased from the Vienna Drosophila Resource Center (VDRC, http://stockcenter.vdrc.at) GD RNAi library (P element library). VDRC lines used correspond to the following transformant ID numbers: 18795, 18796 (CG10586; same construct (ID 5539), different insertions), and 15410 (CG4815). CG10586-18795 is referred to here as “Line 1” and CG10586-18796 as “Line 2”. CG10586 VDRC lines are predicted to have an off-target, CG33306. However, we found no evidence for CG33306 knockdown in either line (Figure S2B, only Line 1 shown). All RNAi knockdown and control sibling flies were produced by crossing sympUAST-SFP or VDRC virgin females to ubiquitous driver males (Tubulin-Gal4/ TM3). UAS-RNAi / Tubulin-Gal4 (non-balancer) male progeny were knocked down for the SFP of interest and the sibling UAS-RNAi/TM3 (balancer) flies were used as controls that are wildtype for seminase expression.
Matings were carried out by placing single 3–6 day old virgin females of the Canton-S strain with a single 3–6 day old virgin male in a glass vial containing a moistened square of filter paper. Matings were observed and the time at which mating began was recorded. Mating pairs with unusually short matings (<15 minutes) were discarded. Mated females were flash frozen in liquid nitrogen at the appropriate time after the start of mating (ASM) for time points less than one hour ASM and stored at −80°C until dissection. All flies were reared in standard yeast-glucose media at room temperature (23±1°C) on a 12∶12 light/dark cycle.
Seminase Antibody Production
Generation of seminase fusion proteins and antibody purification was done following Ravi Ram et al. [64] and Cui et al. [65]. Briefly, we generated a 6×His fusion protein containing amino acids 101–200 from seminase-PA using the pDEST17 vector of the Gateway system (Invitrogen). Antibodies were generated in rabbits (Cocalico) as described previously for eight other Drosophila reproductive proteins, including CG11864 [64], and Wisp [65] except that rabbits were immunized with the 6×His-seminase fusion protein. Anti-seminase was affinity purified with a GST fusion protein of amino acids 101–200 of seminase, as described in the above references. Eluted antibodies were stored at −20°C in glycerol (1∶1), and used at a concentration of 1∶250 for Western blot analysis.
Sample Preparation and Western Blot Analysis
Sample preparation and Western blot analyses in Figure 1A and Figure 5A and 5B were carried out as in Ravi Ram and Wolfner [42]. Samples in other Westerns in this study were prepared similarly, except that they were separated using 5–15% gradient SDS/PAGE. Female reproductive tract samples (RT) are lower reproductive tract extracts (ovaries removed) from 4–6 mated females, unless otherwise noted.
A BCA (bicinchoninic acid) assay (Pierce BCA Protein Assay Kit, Thermo Scientific) was performed to determine the protein loading in Figure 1A. Samples identical to those used for the Western blot were prepared and protein concentration measured relative to a BSA standard, following the manufacturer's guidelines.
Fecundity/Fertility Assays
Number of eggs laid daily by mated females (fecundity) and number of progeny produced from those eggs (fertility) were quantified as in Ravi Ram and Wolfner [36]. Assays for the effect of seminase on fecundity and fertility were carried out three times for each independent insertion line, each time with 15–24 females measured for both knock down and control treatments. CG4815 knock down - and control-mated females were also measured for both fertility and fecundity, as a control for the VDRC background. Two assays were carried out with this line, each time with 7–12 females measured for each treatment. Hatchability was determined by dividing number of progeny by number of eggs (fertility/fecundity) [36]. Inspection of the data revealed non-significant variation in egg laying due to experimental block, so data were pooled across blocks. The effect of seminase or CG4815 knockdown on total 10-day egg laying was tested with a Poisson regression model (using the R function ‘glm()’. The statistical tests for hatchability were the same, except a Binomial regression was used.
A repeated measures analysis was performed to determine the effect of male genotype over time. This analysis was performed using a Poisson mixed-effects model with the R function ‘lmer()’ in the lme4 library. Two models were compared, a full model with day, genotype, and day-by-genotype interaction as fixed effects and female as the random effect, and a model with day as the only fixed effect. Comparison of the two models by ANOVA (R function ‘anova()’) revealed the full model was the better fit, indicating a significant effect of male genotype. To determine the statistical significance of male treatment on individual days post-mating, a Bonferroni correction for multiple tests was applied to the Poisson regressions. All plots were generated using the means and standard errors of the raw data pooled from all experiments. Statistical significance of the effect of male genotype on number of eggs laid is denoted by asterisks on the plots.
Receptivity Assays
Females who had previously mated with either control or seminase knockdown males were placed with a single wildtype male of the Canton-S strain for one hour either 24 hours, 2 days, or 4 days following the initial mating and the number of copulations beginning within one hour was recorded. Receptivity response to remating was tested for seminase as in Ravi Ram and Wolfner [36]. No fewer than 10 females were analyzed for control and experimental groups at 24 hours, 2 days, or 4 days after initial mating. Data were analyzed using a Chi-squared test (R function ‘chisq.test()’ with all parameters set to default).
Sperm Counts
Sperm counts were performed as in Avila, et al. [43] at 2 h ASM, 4 days, and 10 days ASM. Sample identity was coded to avoid bias and each slide was counted twice to assess counting precision (85%–100%). SR data used are the average of the two counts. Spermathecae data are the sum of the two averages (one for each spermatheca). For 4 and 10 day post-mating samples, individual female daily egg counts were also taken. Numbers of stored sperm at 4 and 10 days ASM were significantly negatively correlated with the number of eggs laid (Figure S2). Females that laid very few eggs on day 1 (less than 2 standard deviations below the mean) were removed from the dataset as they were likely unhealthy and may have had improper sperm storage. Data were analyzed using a two-tailed Student's t-test (R function ‘t.test()’).
Supporting Information
Zdroje
1. SirotLKPoulsonRLMcKennaMCGirnaryHWolfnerMF 2008 Identity and transfer of male reproductive gland proteins of the dengue vector mosquito, Aedes aegypti: potential tools for control of female feeding and reproduction. Insect Biochem Mol Biol 38 176 189
2. FindlayGDYiXMaccossMJSwansonWJ 2008 Proteomics reveals novel Drosophila seminal fluid proteins transferred at mating. PLoS Biol 6 e178 doi:10.1371/journal.pbio.0060178
3. BaerBHeazlewoodJLTaylorNLEubelHMillarAH 2009 The seminal fluid proteome of the honeybee Apis mellifera. Proteomics 9 2085 2097
4. KelleherESWattsTDLaFlammeBAHaynesPAMarkowTA 2009 Proteomic analysis of Drosophila mojavensis male accessory glands suggests novel classes of seminal fluid proteins. Insect Biochem Mol Biol 39 366 371
5. MarshallJLHuestisDLHiromasaYWheelerSOppertC 2009 Identification, RNAi knockdown, and functional analysis of an ejaculate protein that mediates a postmating, prezygotic phenotype in a cricket. PLoS ONE 4 e7537 doi:10.1371/journal.pone.0007537
6. AvilaFWSirotLKLaFlammeBARubinsteinCDWolfnerMF 2011 Insect seminal fluid proteins: identification and function. Annu Rev Entomol 56 21 40
7. SouthASirotLKLewisSM 2011 Identification of predicted seminal fluid proteins in Tribolium castaneum. Insect Mol Biol 20 447 456
8. WojtczakMDietrichGJCiereszkoA 2005 Transferrin and antiproteases are major proteins of common carp seminal plasma. Fish Shellfish Immunol 19 387 391
9. MommensMWojtczakMCiereszkoABabiakI 2008 Seminal plasma proteins of Atlantic halibut (Hippoglossus hippoglossus L.). Fish Physiol Biochem 34 349 355
10. SmithJRStanfieldGM 2011 TRY-5 Is a Sperm-Activating Protease in Caenorhabditis elegans Seminal Fluid. PLoS Genet 7 e1002375 doi:10.1371/journal.pgen.1002375
11. LessleyBABrownKI 1978 Purification and properties of a proteinase inhibitor from chicken seminal plasma. Biol Reprod 19 223 234
12. Kot3owskaMKowalskiRGlogowskiJJankowskiJCiereszkoA 2005 Gelatinases and serine proteinase inhibitors of seminal plasma and the reproductive tract of turkey (Meleagris gallopavo). Theriogenology 63 1667 1681
13. MurerVSpetzJFHengstUAltroggeLMde AgostiniA 2001 Male fertility defects in mice lacking the serine protease inhibitor protease nexin-1. Proc Natl Acad Sci USA 98 3029 3033
14. DacheuxJ-LGattiJLDacheuxF 2003 Contribution of epididymal secretory proteins for spermatozoa maturation. Microsc Res Tech 61 7 17
15. PampalakisGSotiropoulouG 2007 Tissue kallikrein proteolytic cascade pathways in normal physiology and cancer. Biochim Biophys Acta 1776 22 31
16. DeanMDClarkNLFindlayGDKarnRCYiX 2009 Proteomics and comparative genomic investigations reveal heterogeneity in evolutionary rate of male reproductive proteins in mice (Mus domesticus). Mol Biol Evol 26 1733 1743
17. ChenY-WLeeM-SLuchtAChouF-PHuangW 2010 TMPRSS2, a serine protease expressed in the prostate on the apical surface of luminal epithelial cells and released into semen in prostasomes, is misregulated in prostate cancer cells. Am J Pathol 176 2986 2996
18. DeanMDFindlayGDHoopmannMRWuCCMaccossMJ 2011 Identification of ejaculated proteins in the house mouse (Mus domesticus) via isotopic labeling. BMC Genomics 12 306
19. KhanARJamesMN 1998 Molecular mechanisms for the conversion of zymogens to active proteolytic enzymes. Protein Sci 7 815 836
20. SuzukiKKiseHNishiokaJHayashiT 2007 The interaction among protein C inhibitor, prostate-specific antigen, and the semenogelin system. Semin Thromb Hemost 33 46 52
21. PoianiA 2006 Complexity of seminal fluid: a review. Behavioral Ecology and Sociobiology 60 289 310
22. KnowlesLLMarkowTA 2001 Sexually antagonistic coevolution of a postmating-prezygotic reproductive character in desert Drosophila. Proc Natl Acad Sci USA 98 8692 8696
23. WongATurchinMCWolfnerMFAquadroC 2008 Evidence for positive selection on Drosophila melanogaster seminal fluid protease homologs. Mol Biol Evol 25 497 506
24. ClarkNLAagaardJESwansonWJ 2006 Evolution of reproductive proteins from animals and plants. Reproduction 131 11 22
25. HaertyWJagadeeshanSKulathinalRJWongAKristipatiRR 2007 Evolution in the fast lane: rapidly evolving sex-related genes in Drosophila. Genetics 177 1321 1335
26. MuellerJLRipollDRAquadroCWolfnerMF 2004 Comparative structural modeling and inference of conserved protein classes in Drosophila seminal fluid. Proc Natl Acad Sci USA 101 13542 13547
27. Ravi RamKWolfnerM 2007 Seminal influences: Drosophila Acps and the molecular interplay between males and females during reproduction. Integrative and Comparative Biology 47 427 445
28. MuellerJLKristipatiRRMcGrawLABloch QaziMCSiggiaED 2005 Cross-species comparison of Drosophila male accessory gland protein genes. Genetics 171 131 143
29. GubbDSanz-ParraABarcenaLTroxlerLFullaondoA 2010 Protease inhibitors and proteolytic signalling cascades in insects. Biochimie 92 1749 1759
30. LungOTramUFinnertyCMEipper-MainsMAKalbJM 2002 The Drosophila melanogaster seminal fluid protein Acp62F is a protease inhibitor that is toxic upon ectopic expression. Genetics 160 211 224
31. WalkerMJRylettCMKeenJNAudsleyNSajidM 2006 Proteomic identification of Drosophila melanogaster male accessory gland proteins, including a pro-cathepsin and a soluble gamma-glutamyl transpeptidase. Proteome science 4 9
32. Ravi RamKSirotLKWolfnerMF 2006 Predicted seminal astacin-like protease is required for processing of reproductive proteins in Drosophila melanogaster. Proc Natl Acad Sci USA 103 18674 18679
33. SirotLKHardstoneMCHelinskiMEHRibeiroJMCKimuraM 2011 Towards a semen proteome of the dengue vector mosquito: protein identification and potential functions. PLoS Negl Trop Dis 5 e989 doi:10.1371/journal.pntd.0000989
34. KalbJMDiBenedettoAJWolfnerMF 1993 Probing the function of Drosophila melanogaster accessory glands by directed cell ablation. Proc Natl Acad Sci USA 90 8093 8097
35. HerndonLAWolfnerMF 1995 A Drosophila seminal fluid protein, Acp26Aa, stimulates egg laying in females for 1 day after mating. Proc Natl Acad Sci USA 92 10114 10118
36. Ravi RamKWolfnerMF 2007 Sustained post-mating response in Drosophila melanogaster requires multiple seminal fluid proteins. PLoS Genet 3 e238 doi:10.1371/journal.pgen.0030238
37. Bloch QaziMCWolfnerMF 2003 An early role for the Drosophila melanogaster male seminal protein Acp36DE in female sperm storage. J Exp Biol 206 3521 3528
38. AvilaFWWolfnerMF 2009 Acp36DE is required for uterine conformational changes in mated Drosophila females. Proc Natl Acad Sci USA 106 15796 15800
39. ChapmanTBanghamJVintiGSeifriedBLungO 2003 The sex peptide of Drosophila melanogaster: female post-mating responses analyzed by using RNA interference. Proc Natl Acad Sci USA 100 9923 9928
40. LiuHKubliE 2003 Sex-peptide is the molecular basis of the sperm effect in Drosophila melanogaster. Proc Natl Acad Sci USA 100 9929 9933
41. PengJChenSBüsserSLiuHHoneggerT 2005 Gradual release of sperm bound sex-peptide controls female postmating behavior in Drosophila. Curr Biol 15 207 213
42. RamKRWolfnerMF 2009 A network of interactions among seminal proteins underlies the long-term postmating response in Drosophila. Proc Natl Acad Sci USA 106 15384 15389
43. AvilaFWRavi RamKBloch QaziMCWolfnerMF 2010 Sex peptide is required for the efficient release of stored sperm in mated Drosophila females. Genetics 186 595 600
44. ParkMWolfnerMF 1995 Male and female cooperate in the prohormone-like processing of a Drosophila melanogaster seminal fluid protein. Dev Biol 171 694 702
45. HeifetzYLungOFrongilloEAWolfnerMF 2000 The Drosophila seminal fluid protein Acp26Aa stimulates release of oocytes by the ovary. Curr Biol 10 99 102
46. HeifetzYVandenbergLNCohnHIWolfnerMF 2005 Two cleavage products of the Drosophila accessory gland protein ovulin can independently induce ovulation. Proc Natl Acad Sci USA 102 743 748
47. BertramMJNeubaumDMWolfnerMF 1996 Localization of the Drosophila male accessory gland protein Acp36DE in the mated female suggests a role in sperm storage. Insect Biochem Mol Biol 26 971 980
48. NeubaumDMWolfnerMF 1999 Mated Drosophila melanogaster females require a seminal fluid protein, Acp36DE, to store sperm efficiently. Genetics 153 845 857
49. AdamsEMWolfnerMF 2007 Seminal proteins but not sperm induce morphological changes in the Drosophila melanogaster female reproductive tract during sperm storage. J Insect Physiol 53 319 331
50. BondJSBeynonRJ 1995 The astacin family of metalloendopeptidases. Protein Sci 4 1247 1261
51. ChintapalliVRWangJDowJAT 2007 Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat Genet 39 715 720
52. BajajSPRapaportSIBrownSF 1981 Isolation and characterization of human factor VII. Activation of factor VII by factor Xa. J Biol Chem 256 253 259
53. GilbertD 1981 Ejaculate esterase-6 and initial sperm use by female Drosophila melanogaster. J Insect Physiol 27 641 650
54. YiallourosIKappelhoffRSchillingOWegmannFHelmsMW 2002 Activation mechanism of pro-astacin: role of the pro-peptide, tryptic and autoproteolytic cleavage and importance of precise amino-terminal processing. J Mol Biol 324 237 246
55. AmourABirdMChaudryLDeadmanJHayesD 2004 General considerations for proteolytic cascades. Biochem Soc Trans 32 15 16
56. GuevaraTYiallourosIKappelhoffRBissdorfSStöckerW 2010 Proenzyme structure and activation of astacin metallopeptidase. J Biol Chem 285 13958 13965
57. LungOWolfnerMF 2001 Identification and characterization of the major Drosophila melanogaster mating plug protein. Insect Biochem Mol Biol 31 543 551
58. Bloch QaziMCWolfnerMF 2006 Emergence of sperm from female storage sites has egg-influenced and egg-independent phases in Drosophila melanogaster. Biol Lett 2 128 130
59. SirotLKLaFlammeBASitnikJLRubinsteinCDAvilaFW 2009 Molecular social interactions: Drosophila melanogaster seminal fluid proteins as a case study. Adv Genet 68 23 56
60. AguadéM 1998 Different forces drive the evolution of the Acp26Aa and Acp26Ab accessory gland genes in the Drosophila melanogaster species complex. Genetics 150 1079 1089
61. WongATurchinMWolfnerMFAquadroCF 2011 Temporally variable selection on proteolysis related reproductive tract proteins in Drosophila. Mol Biol Evol [Published online ahead of print Sept. 22, 2011]
62. EmamiNDiamandisEP 2010 Potential role of multiple members of the kallikrein-related peptidase family of serine proteases in activating latent TGF beta 1 in semen. Biol Chem 391 85 95
63. MattssonJMValmuLLaakkonenPStenmanU-HKoistinenH 2008 Structural characterization and anti-angiogenic properties of prostate-specific antigen isoforms in seminal fluid. Prostate 68 945 954
64. Ravi RamKJiSWolfnerMF 2005 Fates and targets of male accessory gland proteins in mated female Drosophila melanogaster. Insect Biochem Mol Biol 35 1059 1071
65. CuiJSacktonKHornerVLKumarKEWolfnerMF 2008 Wispy, the Drosophila homolog of GLD-2, is required during oogenesis and egg activation. Genetics 178 2017 2029
Štítky
Genetika Reprodukční medicína
Článek USF-1 Is Critical for Maintaining Genome Integrity in Response to UV-Induced DNA PhotolesionsČlánek Checkpoints in a Yeast Differentiation Pathway Coordinate Signaling during Hyperosmotic StressČlánek Two-Component Elements Mediate Interactions between Cytokinin and Salicylic Acid in Plant Immunity
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 1
-
Všechny články tohoto čísla
- DNA Methylation and Gene Expression Changes in Monozygotic Twins Discordant for Psoriasis: Identification of Epigenetically Dysregulated Genes
- An siRNA Screen in Pancreatic Beta Cells Reveals a Role for in Insulin Production
- Parallel Mapping and Simultaneous Sequencing Reveals Deletions in and Associated with Discrete Inherited Disorders in a Domestic Dog Breed
- Cytoplasmic Polyadenylation Element Binding Protein Deficiency Stimulates PTEN and Stat3 mRNA Translation and Induces Hepatic Insulin Resistance
- Nucleolar Association and Transcriptional Inhibition through 5S rDNA in Mammals
- USF-1 Is Critical for Maintaining Genome Integrity in Response to UV-Induced DNA Photolesions
- Heterochromatin Formation Promotes Longevity and Represses Ribosomal RNA Synthesis
- Genetic Evidence for an Indispensable Role of Somatic Embryogenesis Receptor Kinases in Brassinosteroid Signaling
- Poly(ADP-Ribose) Polymerase 1 (PARP-1) Regulates Ribosomal Biogenesis in Nucleoli
- Genome Engineering in : A Feasible Approach to Address Biological Issues
- RIC-7 Promotes Neuropeptide Secretion
- Adaptation and Preadaptation of to Bile
- Checkpoints in a Yeast Differentiation Pathway Coordinate Signaling during Hyperosmotic Stress
- Progressive Polycomb Assembly on H3K27me3 Compartments Generates Polycomb Bodies with Developmentally Regulated Motion
- A High Density SNP Array for the Domestic Horse and Extant Perissodactyla: Utility for Association Mapping, Genetic Diversity, and Phylogeny Studies
- Two-Component Elements Mediate Interactions between Cytokinin and Salicylic Acid in Plant Immunity
- Cdc5-Dependent Asymmetric Localization of Bfa1 Fine-Tunes Timely Mitotic Exit
- A Genome-Wide Analysis of Promoter-Mediated Phenotypic Noise in
- Contribution of Intragenic DNA Methylation in Mouse Gametic DNA Methylomes to Establish Oocyte-Specific Heritable Marks
- Adaptive Evolution of the Lactose Utilization Network in Experimentally Evolved Populations of
- Microenvironmental Regulation by Fibrillin-1
- Unraveling the Regulatory Mechanisms Underlying Tissue-Dependent Genetic Variation of Gene Expression
- A Half-Century of Inspiration: An Interview with Hamilton Smith
- Reduced Lentivirus Susceptibility in Sheep with Mutations
- High-Density SNP Mapping of the HLA Region Identifies Multiple Independent Susceptibility Loci Associated with Selective IgA Deficiency
- Calpains Mediate Integrin Attachment Complex Maintenance of Adult Muscle in
- Genomic Ancestry of North Africans Supports Back-to-Africa Migrations
- Functional Specialization of the Plant miR396 Regulatory Network through Distinct MicroRNA–Target Interactions
- The Seminal Fluid Protease “Seminase” Regulates Proteolytic and Post-Mating Reproductive Processes
- Insulin Signaling Regulates Fatty Acid Catabolism at the Level of CoA Activation
- A Genome-Wide Association Study Identified as a Susceptibility Locus for Systemic Lupus Eyrthematosus in Japanese
- A Spontaneous Mutation of the Rat Gene Leads to Impaired Function of Regulatory T Cells Linked to Inflammatory Bowel Disease
- The Yeast Complex I Equivalent NADH Dehydrogenase Rescues Mutants
- A Flexible Bayesian Model for Studying Gene–Environment Interaction
- Sex Pheromone Evolution Is Associated with Differential Regulation of the Same Desaturase Gene in Two Genera of Leafroller Moths
- Genome-Wide Assessment of AU-Rich Elements by the ARE Algorithm
- Inference of Population Structure using Dense Haplotype Data
- A Gene Regulatory Network for Root Epidermis Cell Differentiation in Arabidopsis
- Sequencing of Pooled DNA Samples (Pool-Seq) Uncovers Complex Dynamics of Transposable Element Insertions in
- A Genome-Wide Association Scan on the Levels of Markers of Inflammation in Sardinians Reveals Associations That Underpin Its Complex Regulation
- Tempo and Mode in Evolution of Transcriptional Regulation
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Poly(ADP-Ribose) Polymerase 1 (PARP-1) Regulates Ribosomal Biogenesis in Nucleoli
- Microenvironmental Regulation by Fibrillin-1
- Parallel Mapping and Simultaneous Sequencing Reveals Deletions in and Associated with Discrete Inherited Disorders in a Domestic Dog Breed
- Two-Component Elements Mediate Interactions between Cytokinin and Salicylic Acid in Plant Immunity
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



