-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaSenescence in the Sbds-Deficient Murine Pancreas: Cell-Type Specific Consequences of Translation Insufficiency
Growth of all living things relies on protein synthesis. Failure of components of the complex protein synthesis machinery underlies a growing list of inherited and acquired multi—organ syndromes referred to as ribosomopathies. While ribosomes, the critical working components of the protein synthesis machinery, are required in all cell types to translate the genetic code, only certain organs manifest clinical symptoms in ribosomopathies, indicating specific cell-type features of protein synthesis control. Further, many of these diseases result in cancer despite an inherent deficit in growth. Here we report a range of consequences of protein synthesis insufficiency with loss of a broadly expressed ribosome factor, leading to growth impairment and cell cycle arrest at different stages. Apparent induction of p53-dependent cell death and arrest pathways included apoptosis in the fetal brain and senescence in the mature exocrine pancreas. The senescence, considered a tumour suppression mechanism, was accompanied by the expression of biomarkers associated with early stages of malignant transformation. These findings inform how cancer may initiate when growth is compromised and provide new insights into cell-type specific consequences of protein synthesis insufficiency.
Published in the journal: . PLoS Genet 11(6): e32767. doi:10.1371/journal.pgen.1005288
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005288Summary
Growth of all living things relies on protein synthesis. Failure of components of the complex protein synthesis machinery underlies a growing list of inherited and acquired multi—organ syndromes referred to as ribosomopathies. While ribosomes, the critical working components of the protein synthesis machinery, are required in all cell types to translate the genetic code, only certain organs manifest clinical symptoms in ribosomopathies, indicating specific cell-type features of protein synthesis control. Further, many of these diseases result in cancer despite an inherent deficit in growth. Here we report a range of consequences of protein synthesis insufficiency with loss of a broadly expressed ribosome factor, leading to growth impairment and cell cycle arrest at different stages. Apparent induction of p53-dependent cell death and arrest pathways included apoptosis in the fetal brain and senescence in the mature exocrine pancreas. The senescence, considered a tumour suppression mechanism, was accompanied by the expression of biomarkers associated with early stages of malignant transformation. These findings inform how cancer may initiate when growth is compromised and provide new insights into cell-type specific consequences of protein synthesis insufficiency.
Introduction
The protein translation machinery encompasses interrelated processes of ribosome biogenesis [1] as well as protein synthesis [2]. Mutations in genes that encode components of this machinery are implicated in a growing list of inherited and acquired disorders termed ribosomopathies. All aspects of cell growth require protein synthesis and deficiency in machinery biogenesis or function can be anticipated to have systemic effects with reduced growth caused by translation insufficiency. This was observed in the Drosophila minutes that were initially identified by diminutive size, and are now known to possess mutations in ribosome related genes [3]. Nevertheless, ribosomopathies present as clinical syndromes with select organ failure, often including the bone marrow [4,5]. The mechanisms dictating which organs are affected by any given ribosomopathy are unknown. Susceptibility to organ failure may reflect specific cell type expression levels or threshold requirements for translation [6]. Developmental requirements during organ expansion [6,7] and functional requirements during cued response to extrinsic signals may add other levels of complexity.
Most ribosomopathies are cancer predisposition syndromes. They can be associated with increased risk of hematological malignancies, and solid tumours have also been reported [4]. Numerous studies have linked defects in translational control and ribosome gene dosage to aberrant growth [8,9]. However, studies have primarily discussed cancer progression in the context of increased ribosome biogenesis and/or translation. What precipitates malignancies in a growth-disadvantaged context such as that of a ribosomopathy remains poorly understood.
A number of consequences have been noted with loss of the highly conserved ribosome-associated protein SBDS and its orthologs in various model systems with a common thread of deregulated protein synthesis. There are several lines of evidence indicating that SBDS functions in ribosome metabolism [10,11], specifically with eukaryotic initiation factor 6 (EIF6) and elongation factor Tu GTP binding domain containing 1 (EFTuD1) protein [12,13]. EIF6 is required for binding and maturation of the 60S ribosomal subunit [14,15] and has been shown to block ribosome subunit joining for formation of the 80S ribosome [16,17]; hence EIF6 is considered to limit translation initiation [18]. Gain of function mutations in the yeast ortholog of Eif6 rescued the severe slow-growth phenotype of SBDS-null yeast strains (sdo1Δ) and EFTuD1-null yeast strains (ria1Δ) [13,19]. The removal of EIF6 from the 60S ribosomal subunit was shown to require the GTPase activity of EFTuD1 [19]. Further, genetic and protein interactions between homologs of EFTuD1 and SBDS have been demonstrated [11,12,20,21]. The current working molecular model is that SBDS acts with EFTuD1 to promote EIF6 removal from the 60S ribosomal subunit [12,22,23].
Shwachman-Diamond syndrome (SDS) is a recessive ribosomopathy caused by biallelic loss-of-function mutations in SBDS [24]. SDS is a multisystem disorder presenting typically within the first year of life with failure to thrive, chronic infection and low blood counts [25]. Exocrine pancreatic dysfunction and blood lineage cytopenia (most often neutropenia) are defining features [26]. Other clinical findings include skeletal defects, decreased brain volume and cognitive impairment [27–30]. SDS is associated with high risk of hematological malignancies (up to 30%) [31]; more recently, early onset solid tumours have also been observed, notably including pancreatic carcinoma [32–34]. The exocrine pancreas has amongst the highest requirements for translation in the body as the site of reiterative digestive enzyme production [35]; SDS pancreatic dysfunction is characterized by severe digestive enzyme deficiency [36].
Studies in both patient-derived cell lines [21,37–39] and animal models of SDS [12,13,21,23,40,41] have demonstrated a role for SBDS in ribosome maturation and ribosome subunit joining. Furthermore, decreased global translation was demonstrated in mouse embryonic fibroblasts with disease-associated mutations of Sbds [41] and in human embryonic kidney 293 cell lines depleted for SBDS by siRNA [42]. It remains to be determined how SDS-related disruptions in translation manifest as acute dysfunctions in select organs.
Senescence is a permanent cell cycle arrest associated in vivo with tumour suppression and aging. In the context of tumours, senescence is considered to act as a rapid response to aberrant growth, particularly downstream of oncogene induction (e.g. RAS activation) [43]. Engagement of tumour suppressors including p53, CDKN2A (p16INK4A), pRB [43], TGFβ and CDKN2B (p15INK4B) [44,45], can initiate this permanent arrest of the cell cycle that is associated with quiescent cells that secrete inflammatory cytokines (senescence-associated secretory phenotype) and express senescence-associated β–galactosidase activity (SAβG) [43].
Here we sought to identify in vivo mechanisms underlying pancreas dysfunction, in comparison to other organs, in SDS. We used constitutive and targeted mouse models to establish the timing and type of organ responses to Sbds mutation. Specifically, we show the dependence of many responses on p53 and that SDS-related translation insufficiency induces a senescent cell cycle arrest through the induction of Tgfβ and p15Ink4b in the murine exocrine pancreas. Our study provides new insights into organ selectivity and tumorigenic potential in ribosomopathies.
Results
Loss of Sbds function results in growth and morphological defects
Mouse models with disease-associated missense (R126T) and null (–) alleles, SbdsR126T/R126T and SbdsR126T/–, displayed severe growth impairment and did not survive birth (S1 Table; [46]). Models demonstrated complete penetrance and consistent genotype-phenotype correlations, with more severe and earlier onset of disease phenotypes in the SbdsR126T/–embryos compared to SbdsR126T/R126T embryos (Fig 1). Heterozygous carriers of either the Sbds−or SbdsR126T alleles were indistinguishable from wildtype, consistent with a recessive mode of inheritance for SDS. Embryos were visibly smaller by two weeks gestation and at E18.5 were, on average, 38% (SbdsR126T/–) and 56% (SbdsR126T/R126T) of age-matched controls by mass (Figs 1A and S1A). Embryo length was also reduced (S1C Fig).
Fig. 1. Sbds mutants display ribosomopathy and SDS phenotypes. 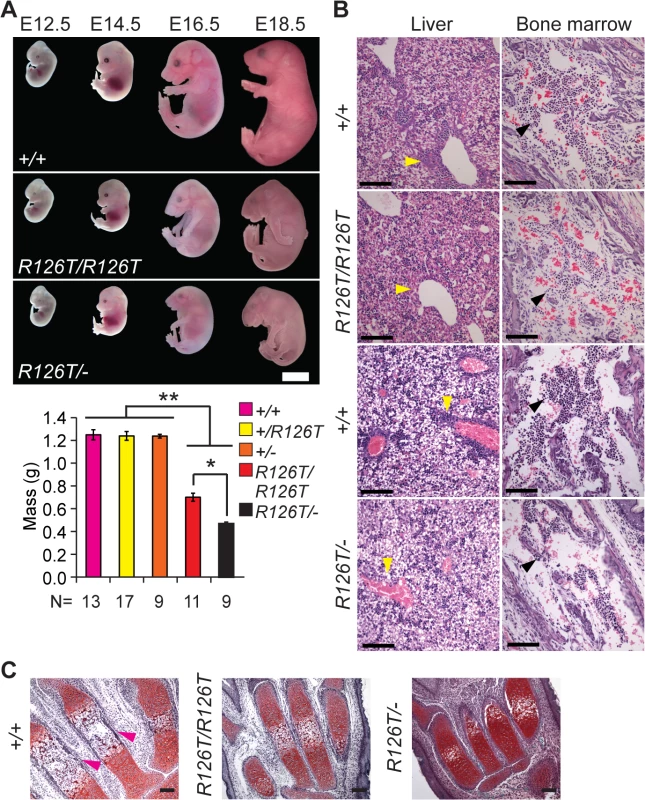
A, Embryos with biallelic mutations in Sbds have decreased mass compared with littermate controls, **P<4X10-6. SbdsR126T/–embryos are smaller than SbdsR126T/R126T embryos, *P = 1.9X10-4 (Wilcoxon Rank Sum Test; Kruskal-Wallis P = 3.0X10-8). Error bars represent ±SEM. Scale bar represents 5 mm (upper panel). B, Decreased granulocytes (dark purple, H&E, E18.5) in liver (cell cluster examples are indicated with yellow arrowheads) and bone marrow (black arrowheads) with loss of Sbds; N = 3 (SbdsR126T/R126T) and 4 (SbdsR126T/–). Scale bars represent 100 μm. C, Decreased bone ossification was observed in transverse metacarpal sections of mutants (corresponding regions of littermate controls that maintain red Safranin O staining in mutants are highlighted with magenta arrowheads, E18.5). Scale bars represent 100 μm. SDS mouse models recapitulated several features observed in human disease. Mutations in Sbds are associated with defects in hematopoiesis [47]. In the fetal period, the liver is the primary site of definitive hematopoiesis. In the SbdsR126T/R126T model near birth (E18.5), histopathology indicated decreased granulocytes in portal areas of the liver as well as pronounced bone marrow hypocellularity, with increased severity in the SbdsR126T/–model (Fig 1B).
SDS is also characterized by decreased ossification and delayed bone growth [27,48]. No gross skeletal defects were apparent in the constitutive models [41]; however, ossification was reduced in the metacarpals at late gestation (Fig 1C). In severe cases, asphyxiating thoracic dystrophy has been observed in SDS [49,50], presumably due in part to the skeletal dystrophy. Beyond this, lung pathology has not been specifically reported in SDS patients. We did observe a severe decrease in saccule expansion in the late fetal lung, despite presence of lung developmental stage biomarkers (S2A Fig).
A defining morphological feature of SDS is a small, fat-replaced pancreas [25,27,30]; we previously showed that pancreatic growth impairment, dysfunction and lipomatosis manifest only in the postnatal period [51].
Proliferation defects and cell death in absence of Sbds function in utero
Translation insufficiency impacts all tissues, and all ribosomopathies are associated with poor overall growth. To further investigate the observed decreases in granulocytes in the liver and hypoplasia of the bone marrow compartment we assessed the abundance of hematopoietic progenitors in the fetal liver of the SDS mouse models. Primary myocult cultures derived from E14.5 fetal livers revealed markedly decreased levels of all myeloid lineage progenitors in both the SbdsR126T/R126T and SbdsR126T/–models (S3 Fig). Decreased levels of granulocytes in the SbdsR126T/–model were also determined by flow cytometry of fetal liver cells (E16.5, S4A Fig). Unlike ribosome deficiency models with dominant inheritance [52–54], erythrocyte levels prior to birth (E18.5) were normal in both mouse models (S4B Fig), consistent with observations in SDS patients [31].
In contrast to other organs, Sbds mutations resulted in severe proliferation defects with pyknotic nuclei and apoptosis (detected by TUNEL staining) in the developing brain by E11.5 in both SbdsR126T/–and SbdsR126T/R126T models (Fig 2A). At E14.5, TUNEL staining was very prominent in the intermediate zone and bromodeoxyuridine labeling further indicated poor growth of neuronal progenitors in the ventricular zone of the developing cortices (Fig 2B). By E18.5, the brain showed multifocal lesions of necrotic neurons (S5 Fig). We did not observe an increase in TUNEL staining in other tissues at E18.5, including the liver and bone marrow, beyond what was observed in controls (S6 Fig).
Fig. 2. SDS brain is apoptotic. 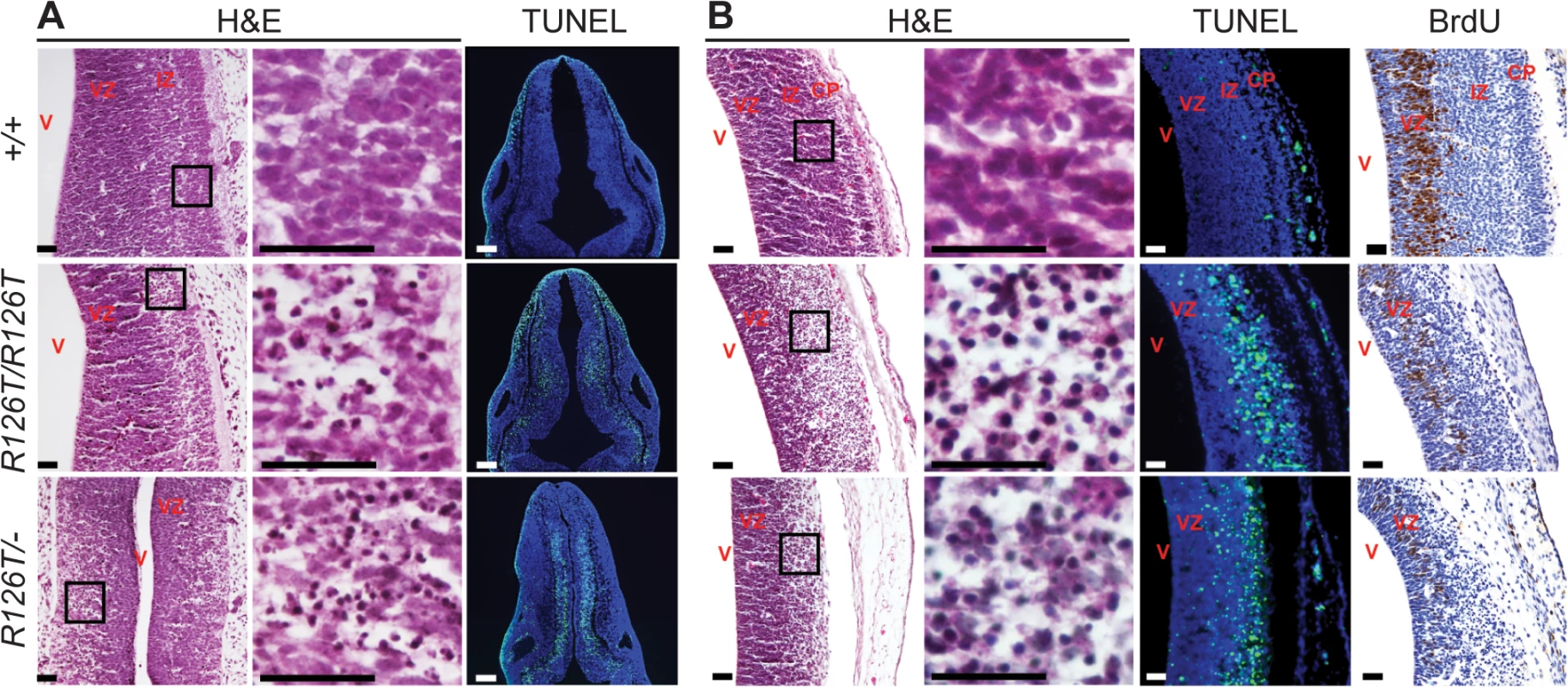
Histochemistry of transverse rhombencephalon (E11.5; A) and telencephalon (E14.5; B) brain sections indicate hypocellularity and neuronal cell death (green in TUNEL panels) in post-mitotic regions of SbdsR126T/R126T mice with earlier onset in the SbdsR126T/–mice (compare TUNEL panels in A and B). Bromodeoxyuridine labeling (brown in BrdU panels) highlighted reduced proliferation of neural progenitors. V, lateral ventricle; VZ, ventricular zone; IZ, intermediate zone; CP, cortical plate. Scale bars represent 25 μm. Loss of Sbds results in senescence in the postnatal pancreas
As mentioned above, the constitutive SDS models did not survive birth. Using a conditional knockout allele (CKO) in conjunction with a pancreas-specific Cre driver (Ptf1aCre) to circumvent lethality, we previously showed that biallelic loss-of-function mutations in Sbds result in a very small pancreas (53% of controls, relative to body mass [51]) with severe atrophy of the acinar component of the adult pancreas. This phenotype included a dramatic depletion of zymogen granules, the specialized vesicles that house digestive enzymes in pancreatic acinar cells. Furthermore, in contrast to the developing brain, poor pancreatic growth was not explained by apoptosis [51].
Given the loss of zymogen granules and acinar cell hypoplasia with a persistent absence of cell death markers, we considered that a senescent cell-cycle arrest might explain atrophy of the SDS pancreas. Several acinar cells of the SDS pancreas were positive for SAβG activity by 20 days of age, becoming more prominent by 30 days of age (Fig 3A). With this evidence of senescence, we next investigated the nature of this response by performing transcript analyses with reverse-transcriptase real-time quantitative PCR of a curated cellular senescence panel of genes. Pancreas samples from littermate control-mutant pairs were compared at two time points prior to the pronounced SAβG activity (Fig 3B, S2 Table). SDS pancreas transcripts showed a suite of changes that were, consistent with the literature, indicative of a senescent-associated cell cycle arrest and secretory program [55–57]. We then further investigated targets of the p53/p21Cip1 and Tgfβ/p15Ink4b networks with additional samples and time points (Fig 3C). We detected markedly increased expression of p15Ink4b (Cdkn2b) along with Tgfβ together with low Myc expression at 15 and 25 days of age (Fig 3C). Increased expression of p21Cip (Cdkn1a) occurred at the early time point of 15 days. Consistent with low Myc levels being permissive for p15Ink4b induction by Tgfβ [58], decreases in Myc transcript levels were noted already at one-week of age, preceding increases in Tgfβ and p15Ink4b (Fig 3C). An increase in p53 transcript expression (3.70 fold, relative to controls), a known mediator of the senescence response [43], also coincided with the onset of SAβG activity (Fig 3C).
Fig. 3. Senescence-associated markers in the SDS pancreas. 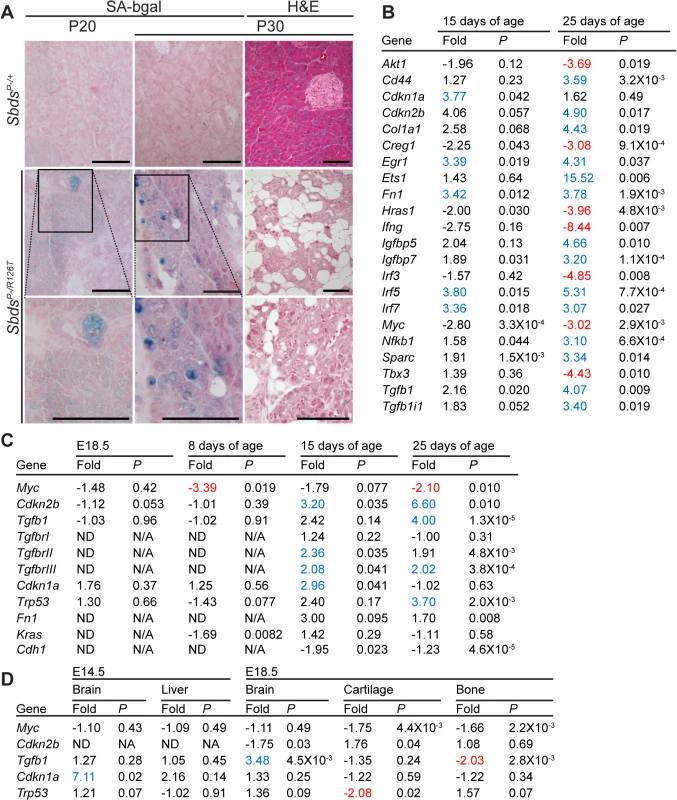
A, Senescence-associated β-galactosidase activity (SA-bgal, bright blue) was detected in acini of the SDS pancreas at 30 days of age (N = 5). SbdsP–/+ and SbdsP–/R126T are shorthand for SbdsCKO/+; Ptf1aCre/+ and SbdsCKO/R126T; Ptf1aCre/+, respectively [51]. Scale bars represent 100 μm. B, 84 cellular-senescence associated genes were assayed using the SABiosciences Cellular Senescence RT2 Profiler PCR Array (QIAGEN) with total RNA isolated from pancreata of mice at 15 and 25 days of age. Table lists transcripts that showed statistically significant changes relative to control genes at at least one of the two assayed time points (see also S2 Table). Fold change: SbdsP-/R126T/SbdsP-/+. Criteria for significance (as per supplier’s instructions): ≥3 fold difference with a P-value of <0.05, N = 3 at each time point. C and D, Quantitative transcript analysis. In C, fold change: SbdsP-/R126T/SbdsP-/+; N = 4 at each time point, except at E18.5 where N = 3. Criteria for significance: ≥2 fold change, P<0.05. E18.5 pancreas expression is relative to Tbp; P8-P25 expression is relative to Gapdh. In D, fold change: SbdsR126T/R126T/SbdsR126T/+; N = 4. Criteria for significance: ≥2 fold change, P<0.05. Brain and liver expression is relative to Actb; bone expression is relative to Tbp. All P-values calculated using unpaired, two-tailed T-tests. Red indicates down-regulation, blue indicates up-regulation. Abbreviations in B: Akt1: Thymoma viral proto-oncogene 1; Cd44: CD44 antigen; Cdkn1a: Cyclin-dependent kinase inhibitor 1A; Ckdn2b: Cyclin-dependent kinase inhibitor 2B; Col1a1: Collagen, type I, alpha 1; Creg1: Cellular repressor of E1A-stimulated genes 1; Egr1: Early growth response 1; Ets1: E26 avian leukemia oncogene 1, 5’ domain; Fn1: Fibronectin 1; Hras1: Harvey rat sarcoma virus oncogene 1; Ifng: Interferon gamma; Igfbp5: Insulin-like growth factor binding protein 5; Igfbp7: Insulin-like growth factor binding protein 7; Interferon regulatory factor 3; Irf5: Interferon regulatory factor 5; Irf7: Interferon regulatory factor 7; Myc: Myelocytomatosis oncogene; Nfkb1: Nuclear factor of kappa light polypeptide gene enhancer in B-cells 1, p105; Sparc: Secreted acidic cysteine rich glycoprotein (osteonectin; Tbx3: T-box-3; Tgfb1: Transforming growth factor, beta 1; Tgfb1i1: Transforming growth factor beta 1 induced transcript 1. Protein expression analyses of control-mutant littermate pairs from several litters paralleled the transcript changes in the SbdsP–/R126T pancreas with changes in Myc and Tgfβ signalling (Fig 4A). Steady-state protein levels of Myc and Tgfβ were consistently reduced and higher in mutants, respectively (Fig 4A). Tgfβ signalling is propagated by phosphorylation of the Smad proteins by Tgfβ receptors [59]. We noted less Smad3 phosphorylation, but more Smad2 phosphorylation in mutants than in controls (Fig 4A). We also observed increased transcript levels for Tgfβ receptors, TgfbrII and TgfbrIII, which can be upregulated during increased Tgfβ signalling [60] (Fig 3C).
Fig. 4. Tgfβ and p53 response in the SDS pancreas. 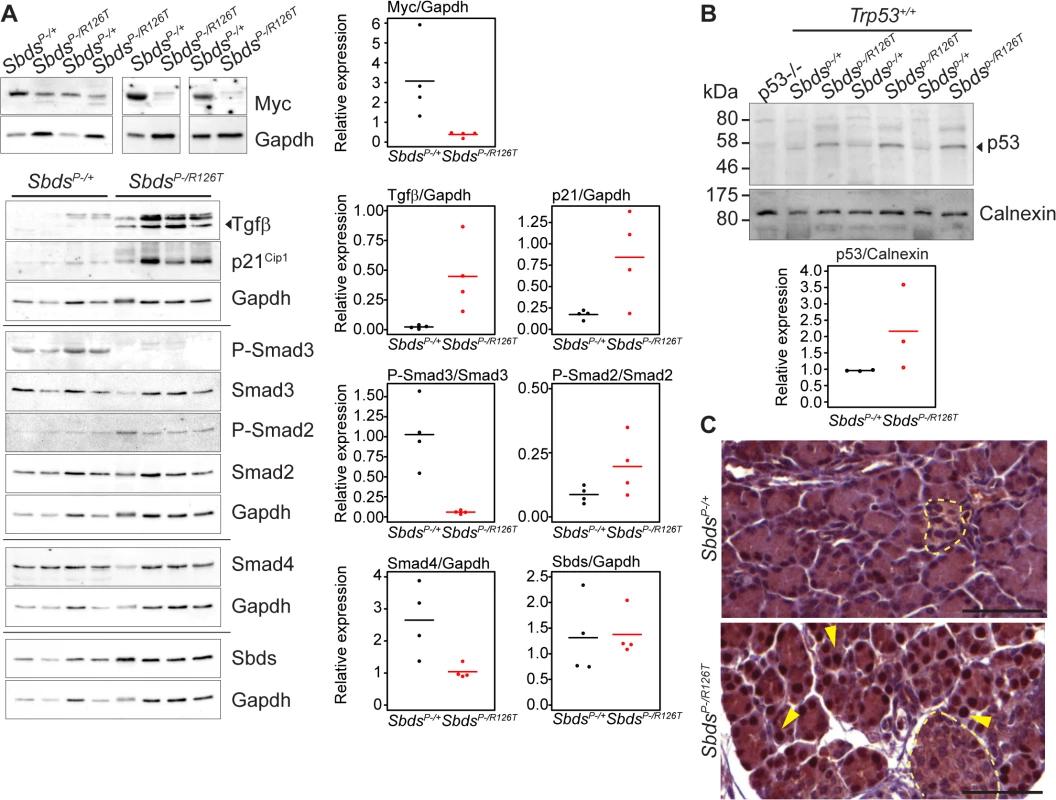
A, Steady state protein levels paralleled observed transcript changes with decreased Myc and increased p21Cip and Tgfβ expression, along with changes in Smad2 and Smad3 phosphorylation status in mutants. Representative immunoblots of lysates from four littermate pairs at 3 weeks of age are shown. Associated densitometry is shown in right graphs, with Myc, Tgfβ, and p21 relative to Gapdh expression, and phosphorylated-Smad2 and Smad3 relative to total Smad2 and Smad3, respectively. Horizontal lines in scatter plots indicate mean values. B, Representative immunoblot indicates stabilization of p53 protein in the SDS pancreas at 3 weeks of age with associated densitometry (expression relative to calnexin) below. Horizontal lines indicate mean values. C, Immunohistochemistry indicated p53 stabilization as early as 15 days of age (sections shown are of littermates). Yellow arrows highlight examples of positive nuclei. p53 staining was observed specifically in nuclei of acinar cells of the SDS pancreas model (islets denoted with pale yellow dashed outlines). Scale bars represent 50 μm. Expression of several factors implicated in the senescence-associated secretory phenotype [55,56,61] beyond Tgfβ, were also elevated. These included extracellular matrix proteins fibronectin (Fn1), osteonectin (Sparc) and collagen (Colla1) as well as innate immunity genes (e.g. Irf5, Irf7 and Nfkb1) and insulin growth factor binding proteins (Igfbp5 and Igfbp7) (Fig 3B; S2 Table), consistent with a senescence program.
Notably, indicators of replicative - and oxidative stress-induced senescence (e.g. Sod1 and Akt1, respectively [43]) were not elevated (S2 Table). Further, that expression of proto-oncogenes Akt1, Hras and Kras as well as Myc trended downwards or were reduced refuted an oncogene-induced senescence response (Fig 3B; S2 Table).
Tgfβ is a known driver of epithelial to mesenchymal transition [59] so we also considered that this process may be occurring in the SDS pancreas. We did observe indicators of dedifferentiation in the mature SDS pancreas (see below); however E-cadherin (Cdh1) transcript levels were not significantly reduced at young ages (Fig 3C).
To determine if the molecular signature of the pancreas senescence represented a common response to Sbds-ablation, we investigated whether cyclin inhibitors, Tgfβ, and Myc transcript level changes were evident in tissues of the constitutive SDS model (SbdsR126T/R126T). A marked increase in p21Cip transcript levels in the brain at E14.5 was observed when apoptosis was detected (Fig 3D). At this same early time point, Tgfβ expression was not altered in either mutant fetal brain or liver even though both organs manifested phenotypes (Fig 3D). Tgfβ expression was low in bone (Fig 3D) and unchanged in lung at E18.5 (S2B Fig). By E18.5, Tgfβ expression was elevated in the SDS mouse brain (Fig 3D), likely a late response to brain damage [62]. No changes in p15Ink4b or Myc expression levels were observed in fetal liver, lung, bone or cartilage tissues (Fig 3D; S2B Fig). These findings are consistent with Tgfβ/p15Ink4b-mediated senescence being a specific response of the pancreas to Sbds deficiency.
Growth impairment and senescence in the SDS pancreas are p53-dependent phenotypes
p53 is a known driver of senescent cell cycle arrest [57,63] and increased levels of p53 have been reported in SDS patients [64]. Moreover, studies of ribosomal gene haploinsufficiency have implicated p53 as a key factor in response to ribosome dysfunction [54,65]. We observed increased steady-state levels of p53 protein in the SDS mouse pancreas by immunoblotting (3 weeks of age, Fig 4B). Immunohistochemistry for p53 (15 days of age, prior to the detection of SAβG staining, Fig 4C) specifically highlighted nuclei of acinar cells, but not islet cells (Fig 4C). To determine if the senescence in the SDS pancreas is p53-dependent we bred the SDS pancreas model to a Trp53–/–mouse.
Complete genetic ablation of p53 alleviated the phenotypes of the SDS pancreas. SbdsP–/R126T;Trp53–/–animals demonstrated a notable improvement in pancreas mass as compared with SbdsP–/R126T;Trp53+/–animals (Fig 5A). Growth improvement was also evident at the histological level as acinar hypoplasia and fat infiltration did not occur in Sbds/Trp53 double mutants in direct contrast to single Sbds mutants (Fig 5B). The molecular signature associated with senescence in the SDS model pancreas was no longer detected; specifically Tgfβ, and p15Ink4b transcripts were not elevated and Myc transcript levels were not decreased (Fig 5C) at 25 days of age. Further, elevated SAβG activity was not detected at 32 days of age (S7 Fig).
Fig. 5. Atrophic SDS pancreas phenotype is p53-dependent. 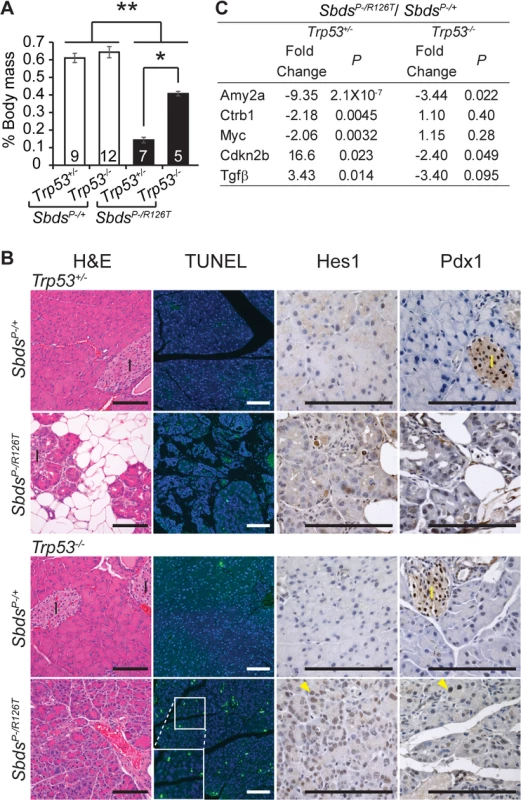
A, Improved mass of SDS pancreas with loss of p53 (SbdsP–/R126T; Trp53–/–; 30 days of age); *P = 0.0025, **P<4X10-5, Wilcoxon Rank Sum Test (Kruskal-Wallis Test P = 3.2X10-5). Inset numbers = N. Error bars represent ±SEM. B, Resolution of atrophy and hypocellularity as well as fat infiltration with loss of p53 in SDS pancreas at 60 days of age (H&E). However, multiple apoptotic acinar cells (TUNEL) per field of view and increased expression of dedifferentiation markers in acini (examples of Hes1 and Pdx1 positive cells, yellow arrowheads) highlighted dysplasia. I: islets. Scale bar represents 100 μm. C, Improvement in digestive enzyme expression and abrogation of senescence-related changes in Tgfβ/p15Ink4b and Myc expression in double mutants at 25 days of age. Fold changes correspond to the comparison of SbdsP–/R126T;Trp5+/–to SbdsP–/+;Trp53+/–or SbdsP–/R126T;Trp53–/–to SbdsP–/+;Trp53–/–transcript levels. P-values calculated using unpaired, two-sided T-tests. By one month of age, the architecture of the acinar epithelium in Sbds/Trp53 double deficient pancreata appeared disordered with many apoptotic cells evident by two months of age (Fig 5B). The morphology was consistent with early stages of acinar-ductal metaplasia. By 60 days of age, we had already noted that some acinar cells in the SDS model pancreas were positive for transcription factors Hes1 and Pdx1, both of which are associated with dedifferentiation (Fig 5B) [66]. With complete ablation of p53, staining of acinar cells with these dedifferentiation markers became widespread (Fig 5B). In contrast, we did not detect changes in islet structure, nor did islets contain apoptotic cells, consistent with our previous observation that mutations in Sbds specifically impact the acinar compartment of the pancreas [51].
p53–/–genetic background unmasks underlying translation-insufficiency of SbdsP–/R126T pancreas
The absence of p53 further revealed translation-insufficiency as a consequence of Sbds loss-of-function. A long established feature of ribosomal deficiency includes small cell size [7], a phenotype noted for the acinar cells of the Sbds/Trp53-double deficient pancreata. Quantification of micrographs of doubly deficient pancreas tissue revealed a nuclei count increase per acinar area compared to Trp53–/–controls (with Sbds), indicating more cells per area (and hence a decreased cell size; Fig 6A). Correspondingly, a smaller mean acinus diameter was also evident in the double mutant micrographs (Fig 6B).
Fig. 6. SDS pancreas senescence is downstream of p53-dependent changes in protein synthesis. 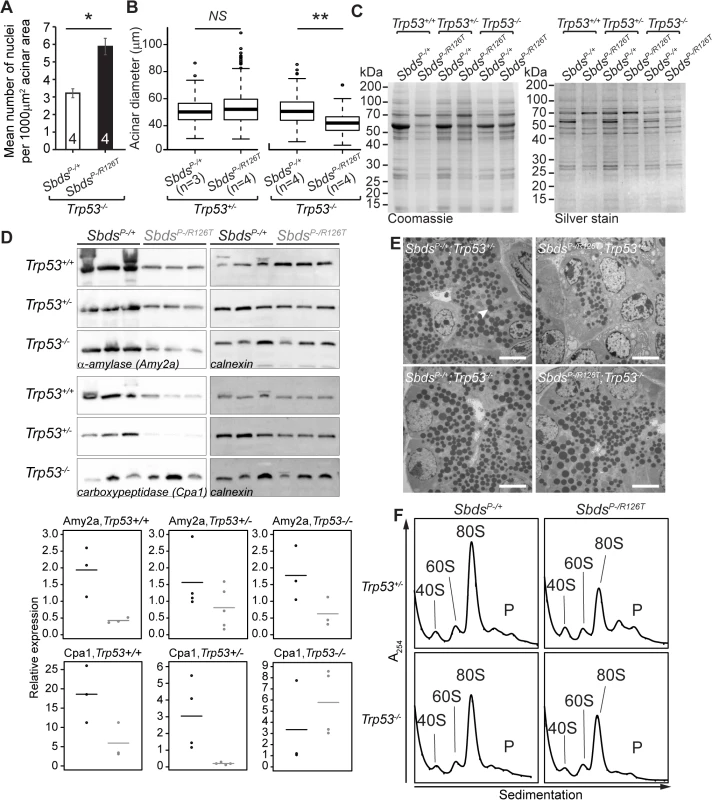
A, Increased nuclei per acinar area (*P = 0.029, Wilcoxon Rank Sum Test) and B, decreased mean acinus diameter (**P = 0.0073 and NS = not significant, P = 0.142; unpaired, two-sided T-test) in SbdsP–/R126T; Trp53–/–pancreas at 30 days of age. Error bars represent ±SEM; in boxplots, whiskers represent extreme values; circles, outliers. C, Coomassie and silver staining of pancreas lysate SDS-PAGE illustrated p53-dependent altered protein expression in the SDS pancreas (20 days of age, 6 μg total protein loaded). D, Immunoblotting confirmed that digestive enzymes were reduced in expression in the SDS pancreas, with expression of protease carboxypeptidase (Cpa1) increasing in a Trp53–/–genetic background (3 weeks of age, 25 μg total protein loaded). Representative blots are shown. Associated densitometry, with expression relative to Gapdh, is shown in lower panels, SbdsP–/+ black, SbdsP–/R126T grey, horizontal lines indicate mean values. E, Restoration of zymogen granules (example, white arrowhead) with loss of p53 was observed by one week of age (electron micrographs). Scale bar represents 5 μm. F, Representative polysome traces illustrate restoration of 80S peak in mutants to levels similar to those of controls with loss of p53 (20 days of age, 79 μg RNA loaded, N = 4 (Trp53+/–) and 3(Trp53–/–)). P: polysomes. Despite this indication of ribosomal deficiency, Sbds/Trp53 double mutants demonstrated a substantial rescue of digestive enzyme expression and zymogen granule abundance. In fact, SDS pancreas lysates showed qualitatively different protein expression patterns that became similar to controls when p53 was absent (Fig 6C). Although amylase expression remained low, increases in protease (carboxypeptidase) expression at three weeks (Fig 6D) as well as restoration of zymogen granules by one week (Fig 6E) in the absence of p53 supported improvement in exocrine function.
We previously suggested that loss of Sbds results in a moderate decrease in 80S monosome peak levels compared to littermate controls [51]. Corresponding increases in free ribosomal subunit levels were not apparent as would be expected if ribosome production was maintained, whereas quantification of the 80S monosome peak levels in the polysome profiles of mutant pancreata showed that the modest decrease normalized to that of controls with ablation of p53 (Figs 6F and S8).
p53 activation and tissue specific outcomes
In contrast to senescence and its molecular signature that included Tgfβ, p15Ink4b and Myc, p53-dependence was not specific to the pancreas as loss of p53 impacted many phenotypes of the SDS mouse model. Although the lethality and growth impairment with reduced mass in the constitutive SDS mouse embryo was not improved (S1 Table; S1 Fig), loss of p53 had a restorative effect on blood progenitor levels (Fig 7A; S3 Table) and led to reduced apoptosis in the early SDS mouse brain to non-detectable levels (Fig 7B). As in the pancreas, polysome profiles are perturbed in SbdsR126T/R126T fetal livers, however loss of p53 resulted in only modest effects, with 80S monosome levels remaining far short of control levels (Figs 7C and S8).
Fig. 7. Hematopoietic and neuronal SDS phenotypes are p53-dependent. 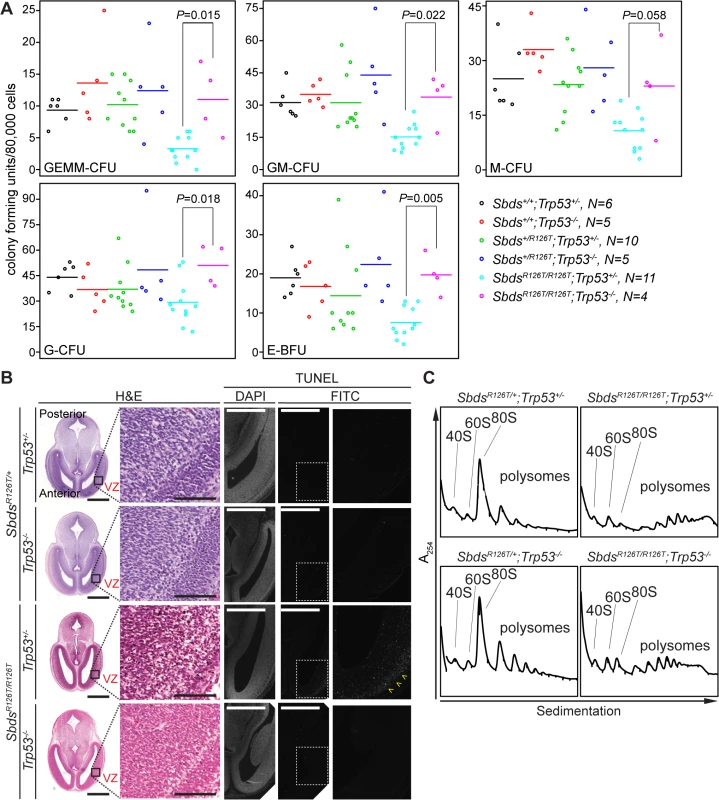
A, Restored hematopoietic progenitor colony forming units in constitutive SDS model with loss of p53 (E16.5 fetal liver; N = 5). CFU, colony forming unit; GEMM, granulocyte/erythroid/macrophage/ megakaryocyte; GM, granulocyte/macrophage; M, macrophage; G, granulocyte; BFU-E, burst forming unit-erythroid. P-values calculated using Wilcoxon Rank Sum Test comparing SDS models with (Trp53+/–) and without p53 (Trp53–/–). Horizontal lines indicate mean values. See S3 Table for complete statistics. B, Restored morphology and cell viability in the SDS mouse brain with loss of p53 (E14.5; VZ: ventricular zone). Scale bars for H&E sections represent 1000 μm (left panels) and 100 μm (right panels). Scale bars for TUNEL sections represent 50 μm. Yellow arrowheads highlight the zone of pronounced staining. C, Representative polysome traces illustrate that 80S peak levels remained low in mutant livers regardless of p53 status (E18.5, 100 μg RNA loaded). Discussion
Mutation of factors implicated in ribosome metabolism and translation lead to dramatic consequences for growth [3,8,9,67,68]. Our findings support the classification of SDS as a ribosomopathy [12,69]. Constitutive and targeted mouse models with SDS-associated Sbds alleles demonstrated severe growth impairment at both the organismal and organ levels. Mitogens were decreased in the SDS pancreas including low levels of the proto-oncogene Myc, a key regulator of exocrine pancreas expansion and acinar cell maintenance [70]. Moreover, arrest at the cell cycle level was evident with increased expression of cyclin inhibitors, senescence in the pancreas, and decreased BrdU-incorporation in the developing brain. With respect to direct evidence of a perturbation in ribosome metabolism, polysome analyses indicated a decrease in the proportion of 80S monosome levels in Sbds-ablated mutants relative to age-matched controls, with a notable difference in magnitude between the pancreas and liver. Although a subunit joining problem has been proposed previously in the context of Sbds mutations [12,21,37], our results are more consistent with an overall decrease in ribosome biogenesis, at least in the SDS pancreas. Finally, as observed in other ribosomopathies [54,65,71,72], we observed stabilization of p53 protein and increased Trp53 transcript levels in the SDS pancreas.
Constitutive ablation of Sbds in the mouse resulted in deficits in the hematopoietic and skeletal compartments, consistent with disease [25]. We previously demonstrated that targeted ablation of Sbds in the pancreas recapitulated all known SDS phenotypes of that organ [51]. Here we further identified a severe brain phenotype in constitutive models, with decreased proliferation in undifferentiated cells as well as pervasive cell death in differentiating neurons. These neural cell losses likely contribute to the perinatal lethality of the constitutive models. SDS is associated with cognitive impairment; imaging indicates reduced brain volume in patients [29,73] and approximately 20% of children with SDS meet criteria for intellectual disability [28] amongst other neurodevelopmental/behavioural concerns [27,28,74,75]. Neural cell death in the mouse occurred by p53-dependent apoptosis, consistent with neurological phenotypes observed in other ribosomopathy models [76–78].
Ribosome biogenesis and translational control have a significant impact on cell-cycle progression [79–81], therefore it is not surprising that cells with aberrant ribosome biogenesis and/or translation exhibit cell cycle arrest. However, while SDS-associated genotypes resulted in apoptosis in the fetal brain, senescence was observed in the postnatal pancreas. Given reports that Sbds loss may result in irregularities of the mitotic spindle [82,83], we considered that loss of Sbds could involve a DNA damage-induced senescence response. However, transcripts for DNA damage response factors Atm, Chek1 and Chek2 (S2 Table) were not upregulated. The senescence cell cycle arrest involved the p53/p21Cip1 and Tgfβ/p15Ink4b networks. Tgfβ is a key member of the senescence-associated secretory phenotype, with roles in both the establishment and maintenance of senescence [57]. Select activation of the Smad effector proteins to relay the Tgfβ signalling cascade is context specific [84]; our findings indicated Smad2 is involved in mediating Tgfβ senescence in the SDS pancreas.
Senescence is considered a hallmark of premalignant tumours [85]. A Tgfβ/p15Ink4b-mediated senescent response has been observed in the context of tumour suppression in hepatocellular carcinoma human cell lines [45] and in lymphomas [44]. Senescence can, over time, promote malignant transformation in neighbouring cells due to the chronic secretion of inflammatory cytokines that are part of the senescence-associated secretory phenotype [86]. Although we have not observed tumour formation up to 14 months in our mice, acinar cells did express markers of dedifferentiation and in light of recent reports of early onset, aggressive pancreatic cancers in SDS patients [32,34], we argue that senescent cells present in the SDS pancreas could contribute to malignant transformation.
That senescence and the underlying pathway involving Tgfβ were not invoked in other organs of the constitutive SDS model highlighted the diversity of outcomes with loss of Sbds. At the same time, both pancreatic senescence and neural apoptosis were abrogated with genetic depletion of p53, implicating p53 as a key mediator of the response to Sbds loss in these two tissues. In the pancreas, we detected stabilization of p53 expression, detectable by 15 days of age, specifically in nuclei of acinar cells. Moreover, we discovered that the characteristic pancreatic phenotypes in SDS are extensively p53-dependent, including organ morphology and the shutdown of the zymogen granule proteome which was evident at the transcription level.
A recent zebrafish model of SDS, generated via morpholino-mediated knockdown of homolog sbds (sbds-MO), demonstrated deficits in pancreatic progenitor proliferation that were phenocopied by ablation of ribosomal constituent proteins, highlighting hypersensitivity of the pancreas compartment to mutations in ribosome-associated genes [40,87].
The absence of p53 did not constitute the rescue of overall growth or perinatal survival of the SDS mouse model highlighting p53-dependent and p53-independent aspects of SDS pathology (as were reported for the zebrafish model [40]). Specifically, the apparent improvements in the Sbds/Trp53 pancreas double mutant phenotypes, were accompanied by a decrease in acinar cell size (a hallmark of translation insufficiency) supporting that protein synthesis remained compromised. Moreover, the profound 80S monosome loss in fetal liver cells (Fig 7) was not recovered with ablation of p53. We conclude that p53 is responding to Sbds deficiency by initiating cell cycle arrest (apoptosis or senescence) with some benefits. However, how the SDS-translation insufficiency triggers p53 activation, or how p53 activation achieves the apparent changes in phenotypes is not clear, perhaps through disturbed production or threshold shift of some critical checkpoint factor(s). With regard to the synthesis of specific proteins, we did note absence of recovery of amylase protein synthesis despite some resurgence of amylase transcript levels in the double mutant (Figs 5C and 6D).
In the pancreas, loss of Sbds is accompanied by cell cycle arrest and reduction of the zymogen granule transcriptome, leading to organ failure. Genetic ablation of Trp53 attenuated the response with rescued growth and increased staining for dedifferentiation markers. It remains to be determined what alternative network(s) may signal the apoptosis that was subsequently observed in absence of p53. Overall, our findings indicate a cellular imperative to shut down cells with disrupted ribosome metabolism, consistent with reports of protective cell shutdown in other ribosomopathy models [6,71].
Can the study of the Sbds-deficient models inform a key question of what dictates organ hypersensitivity to ribosome dysfunction? Robust and ubiquitous expression argues against Sbds expression levels being the limiting factor directly underlying the varied organ responses in SDS [24,46]. The responses of organs to the SDS-translation deficiency varied in both timing and molecular signature. However, despite these differences, many aspects of the brain, blood and pancreas pathologies are all downstream of p53. Our study suggests that the perceived organ paucity in ribosomopathies stems in part from a disparity in molecular responses to translation dysfunction, likely downstream of p53 activation. Such responses, with dependence on cell type, can thus result in vastly different tissue outcomes.
Materials and Methods
Mice
All animal experiments were carried out under the guidelines of the Canadian Council on Animal Care, with approval of procedures by The Animal Care Committee of the Toronto Centre for Phenogenomics, Toronto, AUP #0093. The generation of constitutive SDS and SDS pancreas mouse models was described elsewhere [46,51]. Heterozygous carriers of either the missense (R126T) or null (–) mutation were indistinguishable from wildtype littermates. All mouse lines were maintained on a C57BL/J6 background and no gender effects were observed. Excision of the floxed CKO allele was achieved by breeding with the Ptf1aCre mouse [88]. The p53 deficient strain B6.129S2-Trp53tm1Tyj/J (The Jackson Laboratory) was bred onto Sbds mutant lines for loss of p53 function studies. For embryonic staging, the morning of a vaginal plug was counted as embryonic day (E) 0.5. Mice were euthanized by decapitation, cervical dislocation or CO2 inhalation. Genotyping of DNA from tail samples was performed with the REDExtract-N-Amp Tissue PCR Kit (Sigma) using primers as previously described [51].
Polysome analysis
Flash frozen tissues were lysed in Polysome Buffer (100 mM KCl, 5 mM MgCl2, 10 mM Tris–HCl pH9.0, 1% Triton X–100 and 1% sodium deoxycholate in diethylpyrocarbonate—treated water) on ice using a polytron. Insoluble cell debris was pelleted by centrifugation at 2,500 X g for 15 min at 4°C. Cyclohexamide (0.1 mg/mL) and heparin (1 mg/mL) were added to the supernatant, and equal amounts of RNA (determined by A260 using a Nanodrop Spectrophotometer) were loaded onto a 10–50% sucrose gradient (100 mM KCl, 5 mM MgCl2, 10 mM Tris-HCl pH9.0). Sucrose gradients were subjected to ultracentrifugation (151,000 X g for 2 hours at 4°C) prior to fractionation using a density gradient fractionation system (Brandel). UV absorbance (A254) was recorded using PeakTrak software (Teledyne Isco). Area under the curve (AUC) was calculated using Adobe Photoshop CS5.1 as described [89]. Individual peak/compartment areas were expressed relative to the total AUC of the profile.
Histology and immunohistochemistry
For paraffin embedding, organs were dissected and fixed overnight in ice-cold 4% paraformaldehyde prior to processing into paraffin blocks. Sections with thickness of 5 μm were used. Safranin O (counterstained with Fast Green) staining was performed by the pathology core at the Toronto Centre for Phenogenomics. For immunohistochemistry, antigen retrieval was achieved by boiling in citrate buffer (10 mM sodium citrate, pH6.0), endogenous peroxidases were blocked with 6% H2O2, and non-specific epitopes were blocked with 5–10% goat serum. Antibodies used are given in S4 Table; antibody binding was visualized using diaminobenzidine reagent (Sigma). For senescence-associated β–galactosidase activity staining assays, fresh tissue was embedded and frozen in Tissue-Tek O.C.T. Compound (Sakura Finetek) as per supplier instructions. Frozen tissues were sectioned as 8 μm slices. Senescence staining was performed at pH5.5 as previously described [90]. Apoptosis was detected on paraffin sections by TUNEL assay either using the In Situ Cell Death Detection Kit (Roche) as per supplier’s instructions (fluorescein visualization) or by the pathology core at the Toronto Centre for Phenogenomics (diaminobenzidine visualization). 5–bromodeoxyuridine (50 μg/g, BD Biosciences) was injected in staged pregnant females 24 hours prior to embryo dissection at E14.5. 5–bromodeoxyuridine incorporation was detected using the BrdU In Situ Detection Kit (BD Biosciences). For cell size measurements, nuclei and acini from at least 3 non-overlapping micrographs taken at 40X magnification from 4 biological replicates were counted and measured.
Electron microscopy
One week old pancreata were dissected and fixed in 2% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH7.3). Fixed samples were processed and sectioned for electron microscopy by the joint Advanced Bioimaging Centre of The Hospital for Sick Children and Mount Sinai Hospital in Toronto.
Analysis of fetal liver cells
Single cell suspensions from E14.5 embryo livers were prepared by grinding and filtering tissue through a 40 μm cell strainer (BD Biosciences). Cell suspensions were stained with conjugated antibodies against cell surface antigens with a FACSCalibur system (BD Biosciences) as previously described [91]. Antibodies used were Gr-1, c-kit (FITC-conjugated), Mac-1, and Ter119 (BD Biosciences). Flow cytometry data were analyzed using FlowJo software (Tree Star, Inc.).
Colony forming assay for myeloid progenitor cells
Single cell suspensions from E16.5 embryo livers were prepared by grinding and filtering tissue through a 40 μm cell strainer (BD Biosciences). The number of cells per liver was determined by manual counting using a hemocytometer. Suspended cells (1X105 in 0.3 ml Dulbecco’s Modified Eagle Media) were mixed with 3 ml of methylcellulose media (Stem Cell Technologies) containing recombinant murine stem cell factor, recombinant murine IL-3, recombinant human IL-6 and recombinant human erythropoietin (Stem Cell Technologies), split into thirds and plated on three 35 mm tissue culture plates. Cells were incubated for 7 days at 37°C, 5% CO2 and ≥95% humidity. Colonies of each cell type were identified and counted using a light microscope according to supplier’s instructions. Counts for all three plates of each cell type were averaged and presented as counts per 80,000 cells plated. At least five embryos of each genotype were investigated.
Quantitative transcript analysis
Total RNA was isolated from RNAlater (QIAGEN) stabilized pancreas tissue (N = 3–4 for each genotype at each time point) or flash frozen tissues (brain, lung, liver, cartilage, bone; N = 4 for each genotype at each time point) using the RNeasy Mini Plus Kit (QIAGEN) according to manufacturer’s instructions with the addition of 5% β–mercaptoethanol in the homogenizing Buffer RLT Plus. For bone, cartilage and lung, homogenized tissues were first treated with Trizol (Life Technologies) before application to the RNeasy spin columns. Quality control and real-time quantitative PCR was performed as previously described [51]. Results are presented relative to the expression of the optimal control gene (four genes tested for each sample) for that tissue and time point as determined by GeNORM analysis [92]. A significant change was defined as a ≥2 fold difference with a P–value <0.05. Oligonucleotide primers are given in S5 Table. Expression levels of 84 cellular–senescence associated genes were assayed using the SABiosciences Cellular Senescence RT2 Profiler PCR Array (QIAGEN) with total RNA isolated from pancreata of mice at 15 and 25 days of age. A significant change was defined, as per supplier’s instructions, as a ≥3 fold difference with a P-value of <0.05. Selected gene results were confirmed by real-time quantitative PCR of independently prepared cDNA samples with distinct primer sets (with the exception of Cdkn2b where QIAGEN array primers were used).
Immunoblotting
Pancreas tissue (~30 mg) from 20 day old mice (prior to fat infiltration) was homogenized in RIPA buffer (150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 50 mM Tris-HCl, pH7.5) using a polytron over ice. Insoluble components were pelleted by centrifugation (17,000 X g at 4°C). Equal amounts of protein (determined by Lowry assay, BioRad) in 2X Laemmli buffer were separated by 12% SDS–PAGE and either stained with silver salts or Coomassie brilliant blue, or blotted using the Trans–Blot Turbo Transfer Pack with the Trans-Blot Turbo Transfer System (BioRad). Trans-Blot Turbo nitrocellulose membranes (BioRad) were blocked in 5% (w/v) powdered skim milk (5% (w/v) goat serum for Novacastra CM5 p53 antibody) prior to overnight incubation with primary antibodies followed by species appropriate horseradish peroxidase-conjugated secondary antibodies (S4 Table). Bound antibodies were visualized with Amersham ECL Prime Western Blotting Detection Reagent (GE Healthcare Life Sciences) on the ChemiDoc MP Imaging System using Image-Lab 4.1 Software (BioRad).
Statistical methods
All statistical tests were carried out using R statistical software (R Foundation, from http://www.r-project.org). For T-tests, Welch’s correction was used to adjust for non-constant variance. Wilcoxon Rank Sum Test and Kruskal-Wallis analysis of variance were used where data did not show normal distribution. Bonferroni adjusted critical values were used to declare significance, adjusting for the number of comparisons per analysis. Raw P-values are reported.
Supporting Information
Zdroje
1. Henras AK, Soudet J, Gerus M, Lebaron S, Caizergues-Ferrer M, et al. (2008) The post-transcriptional steps of eukaryotic ribosome biogenesis. Cell Mol Life Sci 65 : 2334–2359. doi: 10.1007/s00018-008-8027-0 18408888
2. Mathews M, Sonenberg N, Hershey JWB (2007) Translational control in biology and medicine. Cold Spring Harbor, N.Y.: Cold Spring Harbor Laboratory Press.
3. Marygold SJ, Roote J, Reuter G, Lambertsson A, Ashburner M, et al. (2007) The ribosomal protein genes and Minute loci of Drosophila melanogaster. Genome Biol 8: R216. 17927810
4. Narla A, Ebert BL (2010) Ribosomopathies: human disorders of ribosome dysfunction. Blood 115 : 3196–3205. doi: 10.1182/blood-2009-10-178129 20194897
5. Ruggero D, Shimamura A (2014) Marrow failure: a window into ribosome biology. Blood 124 : 2784–2792. doi: 10.1182/blood-2014-04-526301 25237201
6. Oliver ER, Saunders TL, Tarle SA, Glaser T (2004) Ribosomal protein L24 defect in belly spot and tail (Bst), a mouse Minute. Development 131 : 3907–3920. 15289434
7. Lambertsson A (1998) The minute genes in Drosophila and their molecular functions. Adv Genet 38 : 69–134. 9677706
8. Silvera D, Formenti SC, Schneider RJ (2010) Translational control in cancer. Nat Rev Cancer 10 : 254–266. doi: 10.1038/nrc2824 20332778
9. Montanaro L, Trere D, Derenzini M (2008) Nucleolus, ribosomes, and cancer. Am J Pathol 173 : 301–310. doi: 10.2353/ajpath.2008.070752 18583314
10. Boocock GR, Marit MR, Rommens JM (2006) Phylogeny, sequence conservation, and functional complementation of the SBDS protein family. Genomics 87 : 758–771. 16529906
11. Savchenko A, Krogan N, Cort JR, Evdokimova E, Lew JM, et al. (2005) The Shwachman-Bodian-Diamond syndrome protein family is involved in RNA metabolism. J Biol Chem 280 : 19213–19220. 15701634
12. Finch AJ, Hilcenko C, Basse N, Drynan LF, Goyenechea B, et al. (2011) Uncoupling of GTP hydrolysis from eIF6 release on the ribosome causes Shwachman-Diamond syndrome. Genes Dev 25 : 917–929. doi: 10.1101/gad.623011 21536732
13. Menne TF, Goyenechea B, Sanchez-Puig N, Wong CC, Tonkin LM, et al. (2007) The Shwachman-Bodian-Diamond syndrome protein mediates translational activation of ribosomes in yeast. Nat Genet 39 : 486–495. 17353896
14. Sanvito F, Piatti S, Villa A, Bossi M, Lucchini G, et al. (1999) The beta4 integrin interactor p27(BBP/eIF6) is an essential nuclear matrix protein involved in 60S ribosomal subunit assembly. J Cell Biol 144 : 823–837. 10085284
15. Si K, Maitra U (1999) The Saccharomyces cerevisiae homologue of mammalian translation initiation factor 6 does not function as a translation initiation factor. Mol Cell Biol 19 : 1416–1426. 9891075
16. Ceci M, Gaviraghi C, Gorrini C, Sala LA, Offenhauser N, et al. (2003) Release of eIF6 (p27BBP) from the 60S subunit allows 80S ribosome assembly. Nature 426 : 579–584. 14654845
17. Gartmann M, Blau M, Armache JP, Mielke T, Topf M, et al. (2010) Mechanism of eIF6-mediated inhibition of ribosomal subunit joining. J Biol Chem 285 : 14848–14851. doi: 10.1074/jbc.C109.096057 20356839
18. Gandin V, Miluzio A, Barbieri AM, Beugnet A, Kiyokawa H, et al. (2008) Eukaryotic initiation factor 6 is rate-limiting in translation, growth and transformation. Nature 455 : 684–688. doi: 10.1038/nature07267 18784653
19. Senger B, Lafontaine DL, Graindorge JS, Gadal O, Camasses A, et al. (2001) The nucle(ol)ar Tif6p and Efl1p are required for a late cytoplasmic step of ribosome synthesis. Mol Cell 8 : 1363–1373. 11779510
20. Krogan NJ, Cagney G, Yu H, Zhong G, Guo X, et al. (2006) Global landscape of protein complexes in the yeast Saccharomyces cerevisiae. Nature 440 : 637–643. 16554755
21. Wong CC, Traynor D, Basse N, Kay RR, Warren AJ (2011) Defective ribosome assembly in Shwachman-Diamond syndrome. Blood 118 : 4305–4312. doi: 10.1182/blood-2011-06-353938 21803848
22. Asano N, Atsuumi H, Nakamura A, Tanaka Y, Tanaka I, et al. (2014) Direct interaction between EFL1 and SBDS is mediated by an intrinsically disordered insertion domain. Biochem Biophys Res Commun 443 : 1251–1256. doi: 10.1016/j.bbrc.2013.12.143 24406167
23. Gijsbers A, Garcia-Marquez A, Luviano A, Sanchez-Puig N (2013) Guanine nucleotide exchange in the ribosomal GTPase EFL1 is modulated by the protein mutated in the Shwachman-Diamond syndrome. Biochem Biophys Res Commun 437 : 349–354. doi: 10.1016/j.bbrc.2013.06.077 23831625
24. Boocock GR, Morrison JA, Popovic M, Richards N, Ellis L, et al. (2003) Mutations in SBDS are associated with Shwachman-Diamond syndrome. Nat Genet 33 : 97–101. 12496757
25. Ginzberg H, Shin J, Ellis L, Morrison J, Ip W, et al. (1999) Shwachman syndrome: phenotypic manifestations of sibling sets and isolated cases in a large patient cohort are similar. J Pediatr 135 : 81–88. 10393609
26. Dror Y, Donadieu J, Koglmeier J, Dodge J, Toiviainen-Salo S, et al. (2011) Draft consensus guidelines for diagnosis and treatment of Shwachman-Diamond syndrome. Ann N Y Acad Sci 1242 : 40–55. doi: 10.1111/j.1749-6632.2011.06349.x 22191555
27. Aggett PJ, Cavanagh NP, Matthew DJ, Pincott JR, Sutcliffe J, et al. (1980) Shwachman's syndrome. A review of 21 cases. Arch Dis Child 55 : 331–347. 7436469
28. Kerr EN, Ellis L, Dupuis A, Rommens JM, Durie PR (2010) The behavioral phenotype of school-age children with shwachman diamond syndrome indicates neurocognitive dysfunction with loss of Shwachman-Bodian-Diamond syndrome gene function. J Pediatr 156 : 433–438. doi: 10.1016/j.jpeds.2009.09.026 19906387
29. Booij J, Reneman L, Alders M, Kuijpers TW (2013) Increase in central striatal dopamine transporters in patients with Shwachman-Diamond syndrome: additional evidence of a brain phenotype. Am J Med Genet A 161A: 102–107. doi: 10.1002/ajmg.a.35687 23239620
30. Toiviainen-Salo S, Raade M, Durie PR, Ip W, Marttinen E, et al. (2008) Magnetic resonance imaging findings of the pancreas in patients with Shwachman-Diamond syndrome and mutations in the SBDS gene. J Pediatr 152 : 434–436. doi: 10.1016/j.jpeds.2007.12.013 18280855
31. Donadieu J, Fenneteau O, Beaupain B, Beaufils S, Bellanger F, et al. (2012) Classification of and risk factors for hematologic complications in a French national cohort of 102 patients with Shwachman-Diamond syndrome. Haematologica 97 : 1312–1319. 22491737
32. Dhanraj S, Manji A, Pinto D, Scherer SW, Favre H, et al. (2013) Molecular characteristics of a pancreatic adenocarcinoma associated with Shwachman-Diamond syndrome. Pediatr Blood Cancer 60 : 754–760. doi: 10.1002/pbc.24453 23303473
33. Singh SA, Vlachos A, Morgenstern NJ, Ouansafi I, Ip W, et al. (2012) Breast cancer in a case of Shwachman Diamond syndrome. Pediatr Blood Cancer 59 : 945–946. doi: 10.1002/pbc.24052 22213587
34. Nakaya T, Kurata A, Hashimoto H, Nishimata S, Kashiwagi Y, et al. (2014) Young-age-onset pancreatoduodenal carcinoma in Shwachman-Diamond syndrome. Pathol Int 64 : 75–80. doi: 10.1111/pin.12133 24629175
35. Case RM (1978) Synthesis, intracellular transport and discharge of exportable proteins in the pancreatic acinar cell and other cells. Biol Rev Camb Philos Soc 53 : 211–354. 208670
36. Ip WF, Dupuis A, Ellis L, Beharry S, Morrison J, et al. (2002) Serum pancreatic enzymes define the pancreatic phenotype in patients with Shwachman-Diamond syndrome. J Pediatr 141 : 259–265. 12183724
37. Burwick N, Coats SA, Nakamura T, Shimamura A (2012) Impaired ribosomal subunit association in Shwachman-Diamond syndrome. Blood 120 : 5143–5152. doi: 10.1182/blood-2012-04-420166 23115272
38. Sezgin G, Henson AL, Nihrane A, Singh S, Wattenberg M, et al. (2013) Impaired growth, hematopoietic colony formation, and ribosome maturation in human cells depleted of Shwachman-Diamond syndrome protein SBDS. Pediatr Blood Cancer 60 : 281–286. doi: 10.1002/pbc.24300 22997148
39. Tulpule A, Kelley JM, Lensch MW, McPherson J, Park IH, et al. (2013) Pluripotent stem cell models of Shwachman-Diamond syndrome reveal a common mechanism for pancreatic and hematopoietic dysfunction. Cell Stem Cell 12 : 727–736. doi: 10.1016/j.stem.2013.04.002 23602541
40. Provost E, Wehner KA, Zhong X, Ashar F, Nguyen E, et al. (2012) Ribosomal biogenesis genes play an essential and p53-independent role in zebrafish pancreas development. Development 139 : 3232–3241. doi: 10.1242/dev.077107 22872088
41. Zhang S (2009) Elucidation of the function of SBDS and the pathobiology of Shwachman-Diamond syndrome with generation of mouse models. U of T Libraries: Univerisity of Toronto.
42. Ball HL, Zhang B, Riches JJ, Gandhi R, Li J, et al. (2009) Shwachman-Bodian Diamond syndrome is a multi-functional protein implicated in cellular stress responses. Hu Mol Genet 18 : 3684–3695. doi: 10.1093/hmg/ddp316 19602484
43. Campisi J, d'Adda di Fagagna F (2007) Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol 8 : 729–740. 17667954
44. Muller J, Samans B, van Riggelen J, Faga G, Peh KNR, et al. (2010) TGFbeta-dependent gene expression shows that senescence correlates with abortive differentiation along several lineages in Myc-induced lymphomas. Cell Cycle 9 : 4622–4626. 21127397
45. Senturk S, Mumcuoglu M, Gursoy-Yuzugullu O, Cingoz B, Akcali KC, et al. (2010) Transforming growth factor-beta induces senescence in hepatocellular carcinoma cells and inhibits tumor growth. Hepatology 52 : 966–974. doi: 10.1002/hep.23769 20583212
46. Zhang S, Shi M, Hui CC, Rommens JM (2006) Loss of the mouse ortholog of the shwachman-diamond syndrome gene (Sbds) results in early embryonic lethality. Mol Cell Biol 26 : 6656–6663. 16914746
47. Dror Y, Freedman MH (2002) Shwachman-diamond syndrome. Br J Haematol 118 : 701–713. 12181037
48. Makitie O, Ellis L, Durie PR, Morrison JA, Sochett EB, et al. (2004) Skeletal phenotype in patients with Shwachman-Diamond syndrome and mutations in SBDS. Clin Genet 65 : 101–112. 14984468
49. Keogh SJ, McKee S, Smithson SF, Grier D, Steward CG (2012) Shwachman-Diamond syndrome: a complex case demonstrating the potential for misdiagnosis as asphyxiating thoracic dystrophy (Jeune syndrome). BMC Pediatr 12 : 48. 22554078
50. Schaballie H, Renard M, Vermylen C, Scheers I, Revencu N, et al. (2013) Misdiagnosis as asphyxiating thoracic dystrophy and CMV-associated haemophagocytic lymphohistiocytosis in Shwachman-Diamond syndrome. Eur J Pediatr 172 : 613–622. doi: 10.1007/s00431-012-1908-0 23315050
51. Tourlakis ME, Zhong J, Gandhi R, Zhang S, Chen L, et al. (2012) Deficiency of Sbds in the mouse pancreas leads to features of Shwachman-Diamond syndrome, with loss of zymogen granules. Gastroenterology 143 : 481–492. doi: 10.1053/j.gastro.2012.04.012 22510201
52. Keel SB, Phelps S, Sabo KM, O'Leary MN, Kirn-Safran CB, et al. (2012) Establishing Rps6 hemizygous mice as a model for studying how ribosomal protein haploinsufficiency impairs erythropoiesis. Exp Hematol 40 : 290–294. doi: 10.1016/j.exphem.2011.12.003 22198155
53. McGowan KA, Mason PJ (2011) Animal models of Diamond Blackfan anemia. Semin Hematol 48 : 106–116. doi: 10.1053/j.seminhematol.2011.02.001 21435507
54. McGowan KA, Pang WW, Bhardwaj R, Perez MG, Pluvinage JV, et al. (2011) Reduced ribosomal protein gene dosage and p53 activation in low-risk myelodysplastic syndrome. Blood 118 : 3622–3633. doi: 10.1182/blood-2010-11-318584 21788341
55. Acosta JC, Banito A, Wuestefeld T, Georgilis A, Janich P, et al. (2013) A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat Cell Biol 15 : 978–990. doi: 10.1038/ncb2784 23770676
56. Coppe JP, Desprez PY, Krtolica A, Campisi J (2010) The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol 5 : 99–118. doi: 10.1146/annurev-pathol-121808-102144 20078217
57. Kuilman T, Michaloglou C, Mooi WJ, Peeper DS (2010) The essence of senescence. Genes Dev 24 : 2463–2479. doi: 10.1101/gad.1971610 21078816
58. Seoane J, Pouponnot C, Staller P, Schader M, Eilers M, et al. (2001) TGFbeta influences Myc, Miz-1 and Smad to control the CDK inhibitor p15INK4b. Nat Cell Biol 3 : 400–408. 11283614
59. Truty MJ, Urrutia R (2007) Basics of TGF-beta and pancreatic cancer. Pancreatology 7 : 423–435. 17898532
60. Massague J (1992) Receptors for the TGF-beta family. Cell 69 : 1067–1070. 1319842
61. Coppe JP, Patil CK, Rodier F, Krtolica A, Beausejour CM, et al. (2010) A human-like senescence-associated secretory phenotype is conserved in mouse cells dependent on physiological oxygen. PLoS One 5: e9188. doi: 10.1371/journal.pone.0009188 20169192
62. Doyle KP, Cekanaviciute E, Mamer LE, Buckwalter MS (2010) TGFbeta signaling in the brain increases with aging and signals to astrocytes and innate immune cells in the weeks after stroke. J Neuroinflammation 7 : 62. doi: 10.1186/1742-2094-7-62 20937129
63. Rodier F, Campisi J (2011) Four faces of cellular senescence. J Cell Biol 192 : 547–556. doi: 10.1083/jcb.201009094 21321098
64. Elghetany MT, Alter BP (2002) p53 protein overexpression in bone marrow biopsies of patients with Shwachman-Diamond syndrome has a prevalence similar to that of patients with refractory anemia. Arch Pathol Lab Med 126 : 452–455. 11900571
65. Bellodi C, Kopmar N, Ruggero D (2010) Deregulation of oncogene-induced senescence and p53 translational control in X-linked dyskeratosis congenita. EMBO J 29 : 1865–1876. doi: 10.1038/emboj.2010.83 20453831
66. Strobel O, Dor Y, Alsina J, Stirman A, Lauwers G, et al. (2007) In vivo lineage tracing defines the role of acinar-to-ductal transdifferentiation in inflammatory ductal metaplasia. Gastroenterology 133 : 1999–2009. 18054571
67. Ruggero D, Pandolfi PP (2003) Does the ribosome translate cancer? Nat Rev Cancer 3 : 179–192. 12612653
68. Scheper GC, van der Knaap MS, Proud CG (2007) Translation matters: protein synthesis defects in inherited disease. Nat Rev Genet 8 : 711–723. 17680008
69. Liu JM, Ellis SR (2006) Ribosomes and marrow failure: coincidental association or molecular paradigm? Blood 107 : 4583–4588. 16507776
70. Bonal C, Thorel F, Ait-Lounis A, Reith W, Trumpp A, et al. (2009) Pancreatic inactivation of c-Myc decreases acinar mass and transdifferentiates acinar cells into adipocytes in mice. Gastroenterology 136 : 309–319. doi: 10.1053/j.gastro.2008.10.015 19022256
71. Panic L, Tamarut S, Sticker-Jantscheff M, Barkic M, Solter D, et al. (2006) Ribosomal protein S6 gene haploinsufficiency is associated with activation of a p53-dependent checkpoint during gastrulation. Mol Cell Biol 26 : 8880–8891. 17000767
72. Barkic M, Crnomarkovic S, Grabusic K, Bogetic I, Panic L, et al. (2009) The p53 tumor suppressor causes congenital malformations in Rpl24-deficient mice and promotes their survival. Mol Cell Biol 29 : 2489–2504. doi: 10.1128/MCB.01588-08 19273598
73. Toiviainen-Salo S, Makitie O, Mannerkoski M, Hamalainen J, Valanne L, et al. (2008) Shwachman-Diamond syndrome is associated with structural brain alterations on MRI. Am J Med Genet A 146A: 1558–1564. doi: 10.1002/ajmg.a.32354 18478597
74. Cipolli M, D'Orazio C, Delmarco A, Marchesini C, Miano A, et al. (1999) Shwachman's syndrome: pathomorphosis and long-term outcome. J Pediatr Gastroenterol Nutr 29 : 265–272. 10467990
75. Kent A, Murphy GH, Milla P (1990) Psychological characteristics of children with Shwachman syndrome. Arch Dis Child 65 : 1349–1352. 1702966
76. Jones NC, Lynn ML, Gaudenz K, Sakai D, Aoto K, et al. (2008) Prevention of the neurocristopathy Treacher Collins syndrome through inhibition of p53 function. Nat Med 14 : 125–133. doi: 10.1038/nm1725 18246078
77. McGowan KA, Li JZ, Park CY, Beaudry V, Tabor HK, et al. (2008) Ribosomal mutations cause p53-mediated dark skin and pleiotropic effects. Nat Genet 40 : 963–970. doi: 10.1038/ng.188 18641651
78. Watkins-Chow DE, Cooke J, Pidsley R, Edwards A, Slotkin R, et al. (2013) Mutation of the diamond-blackfan anemia gene Rps7 in mouse results in morphological and neuroanatomical phenotypes. PLoS Genet 9: e1003094. doi: 10.1371/journal.pgen.1003094 23382688
79. Johnston GC, Pringle JR, Hartwell LH (1977) Coordination of growth with cell division in the yeast Saccharomyces cerevisiae. Exp Cell Res 105 : 79–98. 320023
80. Jorgensen P, Nishikawa JL, Breitkreutz BJ, Tyers M (2002) Systematic identification of pathways that couple cell growth and division in yeast. Science 297 : 395–400. 12089449
81. Maaloe O, Kjeldgaard NO (1966) Control of macromolecular synthesis. New York: W. A. Benjamin, Inc.
82. Austin KM, Gupta ML Jr., Coats SA, Tulpule A, Mostoslavsky G, et al. (2008) Mitotic spindle destabilization and genomic instability in Shwachman-Diamond syndrome. J Clin Invest 118 : 1511–1518. doi: 10.1172/JCI33764 18324336
83. Orelio C, Kuijpers TW (2009) Shwachman-Diamond syndrome neutrophils have altered chemoattractant-induced F-actin polymerization and polarization characteristics. Haematologica 94 : 409–413. doi: 10.3324/haematol.13733 19211642
84. Brown KA, Pietenpol JA, Moses HL (2007) A tale of two proteins: differential roles and regulation of Smad2 and Smad3 in TGF-beta signaling. J Cell Biochem 101 : 9–33. 17340614
85. Collado M, Gil J, Efeyan A, Guerra C, Schuhmacher AJ, et al. (2005) Tumour biology: senescence in premalignant tumours. Nature 436 : 642. 16079833
86. Campisi J (2011) Cellular senescence: putting the paradoxes in perspective. Curr Opin Genet Dev 21 : 107–112. doi: 10.1016/j.gde.2010.10.005 21093253
87. Provost E, Weier CA, Leach SD (2013) Multiple ribosomal proteins are expressed at high levels in developing zebrafish endoderm and are required for normal exocrine pancreas development. Zebrafish 10 : 161–169. doi: 10.1089/zeb.2013.0884 23697888
88. Kawaguchi Y, Cooper B, Gannon M, Ray M, MacDonald RJ, et al. (2002) The role of the transcriptional regulator Ptf1a in converting intestinal to pancreatic progenitors. Nat Genet 32 : 128–134. 12185368
89. Masek T, Valasek L, Pospisek M (2010) Polysome analysis and RNA purification from sucrose gradients. In: Nielsen H, editor: Humana Press. pp. 293–309.
90. Krizhanovsky V, Yon M, Dickins RA, Hearn S, Simon J, et al. (2008) Senescence of activated stellate cells limits liver fibrosis. Cell 134 : 657–667. doi: 10.1016/j.cell.2008.06.049 18724938
91. Tan JB, Visan I, Yuan JS, Guidos CJ (2005) Requirement for Notch1 signals at sequential early stages of intrathymic T cell development. Nat Immunol 6 : 671–679. 15951812
92. Hellemans J, Mortier G, De Paepe A, Speleman F, Vandesompele J (2007) qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol 8: R19. 17291332
Štítky
Genetika Reprodukční medicína
Článek Germline Mutations Confer Susceptibility to Acute Lymphoblastic Leukemia and ThrombocytopeniaČlánek Multiple In Vivo Biological Processes Are Mediated by Functionally Redundant Activities of andČlánek Temporal Expression Profiling Identifies Pathways Mediating Effect of Causal Variant on PhenotypeČlánek Simultaneous DNA and RNA Mapping of Somatic Mitochondrial Mutations across Diverse Human CancersČlánek A Legume Genetic Framework Controls Infection of Nodules by Symbiotic and Endophytic BacteriaČlánek The Eukaryotic-Like Ser/Thr Kinase PrkC Regulates the Essential WalRK Two-Component System inČlánek The Yeast GSK-3 Homologue Mck1 Is a Key Controller of Quiescence Entry and Chronological LifespanČlánek The Role of -Mediated Epigenetic Silencing in the Population Dynamics of Transposable Elements in
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 6
-
Všechny články tohoto čísla
- Expression of Concern: RNAi-Dependent and Independent Control of LINE1 Accumulation and Mobility in Mouse Embryonic Stem Cells
- Orphan Genes Find a Home: Interspecific Competition and Gene Network Evolution
- Minor Cause—Major Effect: A Novel Mode of Control of Bistable Gene Expression
- Germline Mutations Confer Susceptibility to Acute Lymphoblastic Leukemia and Thrombocytopenia
- Leveraging Identity-by-Descent for Accurate Genotype Inference in Family Sequencing Data
- Is Required for the Expression of Principal Recognition Molecules That Control Axon Targeting in the Retina
- Epigenetic Aging Signatures Are Coherently Modified in Cancer
- Silencing of DNase Colicin E8 Gene Expression by a Complex Nucleoprotein Assembly Ensures Timely Colicin Induction
- A Transposable Element within the Non-canonical Telomerase RNA of Modulates Telomerase in Response to DNA Damage
- The Orphan Gene Regulates Dauer Development and Intraspecific Competition in Nematodes by Copy Number Variation
- 9--13,14-Dihydroretinoic Acid Is an Endogenous Retinoid Acting as RXR Ligand in Mice
- The DnaA Protein Is Not the Limiting Factor for Initiation of Replication in
- FGFR3 Deficiency Causes Multiple Chondroma-like Lesions by Upregulating Hedgehog Signaling
- Multiple Changes of Gene Expression and Function Reveal Genomic and Phenotypic Complexity in SLE-like Disease
- Directed Evolution of RecA Variants with Enhanced Capacity for Conjugational Recombination
- The Regulatory T Cell Lineage Factor Foxp3 Regulates Gene Expression through Several Distinct Mechanisms Mostly Independent of Direct DNA Binding
- MreB-Dependent Inhibition of Cell Elongation during the Escape from Competence in
- DNA Damage Regulates Translation through β-TRCP Targeting of CReP
- Multiple In Vivo Biological Processes Are Mediated by Functionally Redundant Activities of and
- The Analysis of () Mutants Reveals Differences in the Fusigenic Potential among Telomeres
- The Causative Gene in Chanarian Dorfman Syndrome Regulates Lipid Droplet Homeostasis in .
- Temporal Expression Profiling Identifies Pathways Mediating Effect of Causal Variant on Phenotype
- The . Accessory Helicase PcrA Facilitates DNA Replication through Transcription Units
- AKTIP/Ft1, a New Shelterin-Interacting Factor Required for Telomere Maintenance
- Npvf: Hypothalamic Biomarker of Ambient Temperature Independent of Nutritional Status
- Transfer RNAs Mediate the Rapid Adaptation of to Oxidative Stress
- Connecting Circadian Genes to Neurodegenerative Pathways in Fruit Flies
- Response to “Ribosome Rescue and Translation Termination at Non-standard Stop Codons by ICT1 in Mammalian Mitochondria”
- Response to the Formal Letter of Z. Chrzanowska-Lightowlers and R. N. Lightowlers Regarding Our Article “Ribosome Rescue and Translation Termination at Non-Standard Stop Codons by ICT1 in Mammalian Mitochondria”
- Simultaneous DNA and RNA Mapping of Somatic Mitochondrial Mutations across Diverse Human Cancers
- Regulation of Insulin Receptor Trafficking by Bardet Biedl Syndrome Proteins
- Altered Levels of Mitochondrial DNA Are Associated with Female Age, Aneuploidy, and Provide an Independent Measure of Embryonic Implantation Potential
- Non-reciprocal Interspecies Hybridization Barriers in the Capsella Genus Are Established in the Endosperm
- Canine Spontaneous Head and Neck Squamous Cell Carcinomas Represent Their Human Counterparts at the Molecular Level
- Genetic Changes to a Transcriptional Silencer Element Confers Phenotypic Diversity within and between Species
- Functional Assessment of Disease-Associated Regulatory Variants Using a Versatile Dual Colour Transgenesis Strategy in Zebrafish
- Translational Upregulation of an Individual p21 Transcript Variant by GCN2 Regulates Cell Proliferation and Survival under Nutrient Stress
- Independent Neuronal Origin of Seizures and Behavioral Comorbidities in an Animal Model of a Severe Childhood Genetic Epileptic Encephalopathy
- The Human Blood Metabolome-Transcriptome Interface
- A Common Cancer Risk-Associated Allele in the Locus Encodes a Dominant Negative Inhibitor of Telomerase
- A Legume Genetic Framework Controls Infection of Nodules by Symbiotic and Endophytic Bacteria
- The Eukaryotic-Like Ser/Thr Kinase PrkC Regulates the Essential WalRK Two-Component System in
- The Yeast GSK-3 Homologue Mck1 Is a Key Controller of Quiescence Entry and Chronological Lifespan
- Dissection of a Complex Disease Susceptibility Region Using a Bayesian Stochastic Search Approach to Fine Mapping
- Exome Sequencing of Phenotypic Extremes Identifies and as Interacting Modifiers of Chronic Infection in Cystic Fibrosis
- The Role of -Mediated Epigenetic Silencing in the Population Dynamics of Transposable Elements in
- Ancestral Chromatin Configuration Constrains Chromatin Evolution on Differentiating Sex Chromosomes in
- Abnormal Activation of BMP Signaling Causes Myopathy in Null Mice
- Reproductive Mode and the Evolution of Genome Size and Structure in Nematodes
- Replication and Active Partition of Integrative and Conjugative Elements (ICEs) of the SXT/R391 Family: The Line between ICEs and Conjugative Plasmids Is Getting Thinner
- Motor and Sensory Deficits in the Mice Result from Mutation of the ESCRT Component HGS
- Senescence in the Sbds-Deficient Murine Pancreas: Cell-Type Specific Consequences of Translation Insufficiency
- Lipophorin Receptors Recruit the Lipoprotein LTP to the Plasma Membrane to Mediate Lipid Uptake
- Separable Crossover-Promoting and Crossover-Constraining Aspects of Zip1 Activity during Budding Yeast Meiosis
- Context-Dependent Functional Divergence of the Notch Ligands DLL1 and DLL4
- Multilayered Organization of Jasmonate Signalling in the Regulation of Root Growth
- Necrotic Cells Actively Attract Phagocytes through the Collaborative Action of Two Distinct PS-Exposure Mechanisms
- A Novel Feedback Loop That Controls Bimodal Expression of Genetic Competence
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Non-reciprocal Interspecies Hybridization Barriers in the Capsella Genus Are Established in the Endosperm
- Translational Upregulation of an Individual p21 Transcript Variant by GCN2 Regulates Cell Proliferation and Survival under Nutrient Stress
- Exome Sequencing of Phenotypic Extremes Identifies and as Interacting Modifiers of Chronic Infection in Cystic Fibrosis
- The Human Blood Metabolome-Transcriptome Interface
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



