-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaActive or Passive Exposure to Tobacco Smoking and Allergic Rhinitis, Allergic Dermatitis, and Food Allergy in Adults and Children: A Systematic Review and Meta-Analysis
Background:
Allergic rhinitis, allergic dermatitis, and food allergy are extremely common diseases, especially among children, and are frequently associated to each other and to asthma. Smoking is a potential risk factor for these conditions, but so far, results from individual studies have been conflicting. The objective of this study was to examine the evidence for an association between active smoking (AS) or passive exposure to secondhand smoke and allergic conditions.Methods and Findings:
We retrieved studies published in any language up to June 30th, 2013 by systematically searching Medline, Embase, the five regional bibliographic databases of the World Health Organization, and ISI-Proceedings databases, by manually examining the references of the original articles and reviews retrieved, and by establishing personal contact with clinical researchers. We included cohort, case-control, and cross-sectional studies reporting odds ratio (OR) or relative risk (RR) estimates and confidence intervals of smoking and allergic conditions, first among the general population and then among children.
We retrieved 97 studies on allergic rhinitis, 91 on allergic dermatitis, and eight on food allergy published in 139 different articles. When all studies were analyzed together (showing random effects model results and pooled ORs expressed as RR), allergic rhinitis was not associated with active smoking (pooled RR, 1.02 [95% CI 0.92–1.15]), but was associated with passive smoking (pooled RR 1.10 [95% CI 1.06–1.15]). Allergic dermatitis was associated with both active (pooled RR, 1.21 [95% CI 1.14–1.29]) and passive smoking (pooled RR, 1.07 [95% CI 1.03–1.12]). In children and adolescent, allergic rhinitis was associated with active (pooled RR, 1.40 (95% CI 1.24–1.59) and passive smoking (pooled RR, 1.09 [95% CI 1.04–1.14]). Allergic dermatitis was associated with active (pooled RR, 1.36 [95% CI 1.17–1.46]) and passive smoking (pooled RR, 1.06 [95% CI 1.01–1.11]). Food allergy was associated with SHS (1.43 [1.12–1.83]) when cohort studies only were examined, but not when all studies were combined.
The findings are limited by the potential for confounding and bias given that most of the individual studies used a cross-sectional design. Furthermore, the studies showed a high degree of heterogeneity and the exposure and outcome measures were assessed by self-report, which may increase the potential for misclassification.Conclusions:
We observed very modest associations between smoking and some allergic diseases among adults. Among children and adolescents, both active and passive exposure to SHS were associated with a modest increased risk for allergic diseases, and passive smoking was associated with an increased risk for food allergy. Additional studies with detailed measurement of exposure and better case definition are needed to further explore the role of smoking in allergic diseases.
Please see later in the article for the Editors' Summary
Published in the journal: . PLoS Med 11(3): e32767. doi:10.1371/journal.pmed.1001611
Category: Research Article
doi: https://doi.org/10.1371/journal.pmed.1001611Summary
Background:
Allergic rhinitis, allergic dermatitis, and food allergy are extremely common diseases, especially among children, and are frequently associated to each other and to asthma. Smoking is a potential risk factor for these conditions, but so far, results from individual studies have been conflicting. The objective of this study was to examine the evidence for an association between active smoking (AS) or passive exposure to secondhand smoke and allergic conditions.Methods and Findings:
We retrieved studies published in any language up to June 30th, 2013 by systematically searching Medline, Embase, the five regional bibliographic databases of the World Health Organization, and ISI-Proceedings databases, by manually examining the references of the original articles and reviews retrieved, and by establishing personal contact with clinical researchers. We included cohort, case-control, and cross-sectional studies reporting odds ratio (OR) or relative risk (RR) estimates and confidence intervals of smoking and allergic conditions, first among the general population and then among children.
We retrieved 97 studies on allergic rhinitis, 91 on allergic dermatitis, and eight on food allergy published in 139 different articles. When all studies were analyzed together (showing random effects model results and pooled ORs expressed as RR), allergic rhinitis was not associated with active smoking (pooled RR, 1.02 [95% CI 0.92–1.15]), but was associated with passive smoking (pooled RR 1.10 [95% CI 1.06–1.15]). Allergic dermatitis was associated with both active (pooled RR, 1.21 [95% CI 1.14–1.29]) and passive smoking (pooled RR, 1.07 [95% CI 1.03–1.12]). In children and adolescent, allergic rhinitis was associated with active (pooled RR, 1.40 (95% CI 1.24–1.59) and passive smoking (pooled RR, 1.09 [95% CI 1.04–1.14]). Allergic dermatitis was associated with active (pooled RR, 1.36 [95% CI 1.17–1.46]) and passive smoking (pooled RR, 1.06 [95% CI 1.01–1.11]). Food allergy was associated with SHS (1.43 [1.12–1.83]) when cohort studies only were examined, but not when all studies were combined.
The findings are limited by the potential for confounding and bias given that most of the individual studies used a cross-sectional design. Furthermore, the studies showed a high degree of heterogeneity and the exposure and outcome measures were assessed by self-report, which may increase the potential for misclassification.Conclusions:
We observed very modest associations between smoking and some allergic diseases among adults. Among children and adolescents, both active and passive exposure to SHS were associated with a modest increased risk for allergic diseases, and passive smoking was associated with an increased risk for food allergy. Additional studies with detailed measurement of exposure and better case definition are needed to further explore the role of smoking in allergic diseases.
Please see later in the article for the Editors' SummaryIntroduction
Allergic rhinitis, allergic dermatitis, and food allergy, in addition to asthma, are extremely common diseases worldwide. Indeed, allergic rhinitis affects 10% to 20% of the general population in Europe and the US [1],[2] and up to 40% of children [3]. The prevalence of allergy to any food varies between 3% and 35% [4], while that of allergic dermatitis reaches 20% in many countries [5]. These diseases have profound consequences on the patient's quality of life and imply a high cost both to the patient and insurance providers [6],[7]. Among infants, these costs reach more than US$4,000 per year per case of food allergy [8].
Recent studies have suggested that these diseases are but one unique set of immunoglobulin-E (IgE)-mediated allergic conditions, linked by the common thread of “atopic march” [9]. This concept postulates that those conditions are a continuous state that starts with dermatitis and food allergy and eventually progresses to asthma and allergic rhinitis. Indeed, these diseases often co-exist in the same patient and can predict the occurrence of each other [10].
Worldwide, the prevalence of allergic diseases has increased substantially in the last few decades [11],[12], which may have two explanations. On the one hand, increased clinician awareness, as well as patient and parental awareness, may have led to improved identification and increased case presentation to physicians [12]. On the other hand, it is possible that this increase is due to changing exposure to known and unknown risk factors [13], and among these factors, smoking may play a role. An increased risk of allergic diseases among individuals exposed to tobacco smoke is biologically plausible as smoking is known to facilitate sensitization to perennial indoor allergens, such as those caused by furry animals, as well as to some outdoor allergens such as pollen [14].
Increased risk of food allergy among infants exposed to tobacco smoke is also plausible. Food allergens are likely to be found in house dust. Swallowed foods are also inhaled or aspirated by infants, and thus, may cause sensitization that could be facilitated by exposure to tobacco smoke. The early and simultaneous exposure to tobacco smoke and food allergens may interfere with the normal development of immunologic tolerance and thus, facilitate sensitization to food [14].
Furthermore, smoking augments nasal responses to allergen in atopic subjects and increases IgE, immunoglobulin G4 (IgG4), and postallergen histamine levels in nasal lavage fluid [15],[16].
Allergic conditions are, in general, more prevalent in children. A potential effect of smoking would have a considerable impact on public health due to the frequency of exposure worldwide. Indeed, children and adolescents are exposed to secondhand smoke in a proportion that varies between 27.6% in Africa and 77.8% in Europe [17] and approximately 14% of all children were exposed to maternal smoking during pregnancy [18].
Several studies have assessed the association between smoking exposure and allergic diseases. In each of the allergic conditions, results were conflicting and alternated between the harmful effects of smoking [14],[19],[20] and protection [21]–[23], while some studies could not find evidence of any effect [24]–[26].
Except for a systematic review and meta-analysis examining the relationship between smoking and asthma in children [27], to our knowledge, there is no comprehensive meta-analysis that examines the evidence for a relationship between smoking and allergic conditions. We, therefore, summarized the scientific evidence and carried out a meta-analysis on exposure to active and passive smoking and the risk of allergic rhinitis, allergic dermatitis, and food allergy among adults and children/adolescents.
Methods
Data Sources and Searches
We searched databases from 1966 to June 30th, 2013, to identify all potentially eligible studies. For Medline, we applied the following algorithm both in medical subject heading and in free text words: (“SEASONAL ALLERGIC RHINITIS” OR “POLLEN ALLERG*” OR “POLLINOSIS” OR “POLLINOSES” OR “HAY FEVER” OR “RHINITIS, ALLERGIC, NONSEASONAL” OR “RHINITIS, ALLERGIC, PERENNIAL” OR “DERMATITIS, ATOPIC” OR ECZEMA OR “FOOD ALLERGIES” OR “HYPERSENSITIVITY, FOOD”) AND (SMOKING OR TOBACCO OR CIGARETT*). We used similar strategies to search Embase and the five regional bibliographic databases of the World Health Organization (AIM, LILACS, IMEMR, IMSEAR, WPRIM). We searched meeting abstracts using the ISI Proceedings database from its inception in 1990 to 2013. We also examined the references of every article retrieved and those of recent reviews of allergic rhinitis and smoking [16],[28]–[33] and established personal contact with clinical researchers to trace further publications or reports. We considered including any relevant article, independently of the language of publication.
Study Selection
Studies were included if: (1) they presented original data from cohort, case-control, or cross-sectional studies (ecologic studies were not included); (2) the outcome of interest was clearly defined as allergic rhinitis, allergic dermatitis, or food allergy; (3) one of the exposure factors was smoking, either by the subjects themselves or their relatives; (4) they provided estimates of odds ratio (OR), relative risk (RR), or prevalence odds ratio and their confidence intervals, or enough data to calculate them. If data on the same population were duplicated in more than one study, the most recent study was included in the analysis. When data for different types or levels of exposure were available in the same study, such as passive smoking, active smoking, or maternal smoking during pregnancy, we considered each type of exposure separately. We developed a standard data-recording form in which we recorded authors, year of publication, study location, sample size, outcome, outcome measurement details, effect estimator (OR, RR, other), effect estimate, 95% CIs, adjustment factors used, and study design including if the International Study of Asthma and Allergies in Childhood (ISAAC) methodology was followed. ISAAC is a large international epidemiologic study on risk factors of allergic diseases, the methods of which are widely used. When further clarification was necessary, we attempted to contact the authors. Abstracts were reviewed independently by two authors (BT and JS).
Quality Assessment
Study quality was assessed using a five-point binary scale specifically developed for this study. The scale is based on the Newcastle-Ottawa scale [34] with modifications in view of standard guidelines and our own judgment. The Newcastle-Ottawa scale is a scoring system that assesses every aspect of an observational epidemiologic study from a methodological point of view. For this meta-analysis, we tried to use those elements that were common to all epidemiologic designs and thus shortened the scale considerably. We used the following criteria labelled as “yes” or “no”: (1) whether assessment of the smoking habit included duration and/or quantity (yes) or not (no); (2) whether rhinitis diagnosis included clinical features and IgE or skin prick test (SPT) measurements (yes) or was based on clinical examination or questionnaire only (no), whether dermatitis diagnosis included clinically assessed diagnosis (yes) or was based on questionnaire information only (no), whether the diagnosis of food allergy was based on clinical diagnosis with SPT, IgE, or open-challenge test (yes) or was based on questionnaire information only (no); (3) whether results were adjusted for age, sex, and at least one other potential confounder (yes) or not (no); (4) whether participation exceeded 80% of the people initially approached (yes) or not (no); and, finally (5) whether the target population was clearly defined (yes) or, on the contrary, based on convenience sampling of subjects such as patients of a single consultation (no). Throughout this assessment, when the information on a specific item was not provided by the authors, we graded this item as “no.” We carried out a pooled analysis on those studies that fulfilled at least three criteria and compared with those that scored fewer than three. As a secondary analysis, we stratified our results on criterion 1 and present the pooled relative risks in Table S2.
Data extraction and quality scoring were performed independently by two reviewers (BT and JS) and the results were merged by consensus. The complete protocol and results for quality scoring are available in Table S1.
Data Synthesis and Analysis
We weighted the study-specific log odds ratios for case control and cross-sectional studies, and log relative risks for cohort studies by the inverse of their variance to compute a pooled relative risk and its 95% confidence interval. For each study, we used the estimate of the effect measure that was adjusted for the largest number of confounders. We present both fixed-effects and random effects pooled estimates but use the latter when heterogeneity was present. Odds ratios from case-control studies were assumed to be unbiased estimates of the relative risk [35].
We used a version adapted to small samples of the DerSimonian and Laird Q test to check for heterogeneity [36]. The null hypothesis of this test is the absence of heterogeneity. To quantify this heterogeneity we calculated the proportion of the total variance due to between-study variance (Ri statistic) [36]. Furthermore, we explored the origin of heterogeneity by restricting the analysis to subgroups of studies defined by study characteristics such as study design, type of exposure (active or passive smoking), and age of the participants (children/adolescents or adults).
To check whether the pooled estimates were significantly different between subgroups we carried out a meta-regression with the global effect as dependent variable and the subgroup variable as moderator.
We assessed publication bias, first visually, using funnel plots and then, more formally, using the test proposed by Egger and colleagues [37]. We also used the trim-and-fill method to correct for potential publication bias. All analyses were performed with the software HEpiMA version 2.1.3 [38] and STATA version 12 with its macros metabias, metareg, and metatrim.
The secondary analyses (children and adolescents/adults, ISAAC/other, cohort and case-control studies combined/cross-sectional studies, high quality/low quality) were planned a priori.
Results
We identified 196 studies, published in 139 different articles and carried out in 51 countries, on active or passive smoking and allergic diseases that met our inclusion criteria (Figure 1). The data from one study were obtained from the authors [39]. We found 97 studies on allergic rhinitis [19],[21],[24],[39]–[118], [91] on allergic dermatitis [19],[20],[22],[24]–[26],[44]–[46],[48],[53],[60]–[65],[67],[73],[75],[76],[78],[83]–[87],[91],[93],[96],[97],[99],[101]–[103],[105]–[107],[110],[111],[116]–[165], and eight on food allergies [14],[23],[26],[73],[126],[136],[166]–[168].
Fig. 1. Flow diagram for study selection. 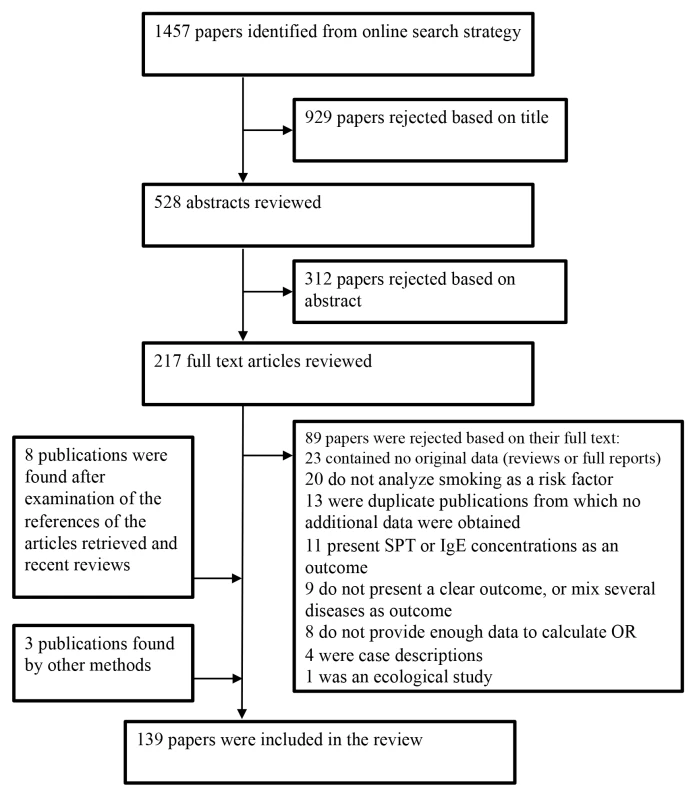
A large majority of the articles retrieved initially were excluded either because they did not provide any effect measure or the outcome was allergy at large. More specifically, of the studies that could have been relevant to our meta-analysis but were finally excluded, eight were discarded because they were an early version of cohort studies updated in subsequent publications [169]–[176]. Other studies published their results several times [175]–[181] in which case we chose to include the most complete report. Some studies were excluded because the outcome was not allergic rhinitis, dermatitis, or food allergy but rather SPT or IgE concentrations [182]–[192]. We also excluded nine studies that used either unspecific outcomes such as nasal symptoms [193],[194], or a mixture of allergic diseases as a single outcome [195]–[201]. Eight studies [138],[202]–[208] were excluded as they did not present any effect measure. Finally, one ecologic study was not considered further [209].
Globally, heterogeneity was substantial overall and similarly high after stratification by design, quality features (including adjustment for confounders), and study population. Given the substantial heterogeneity, we focused on the random effects analyses; however, the fixed effects analyses are presented for comparison and only discussed where they differ.
Allergic Rhinitis
Thirty-four studies on active smoking and 63 studies on passive smoking were available (Figures 2 and 3; Tables 1 and 2). The overwhelming majority of the studies assessed diagnosis through questionnaire and only seven studies used SPT or IgE measurements for the case definition [39],[42],[46],[52],[57],[101],[113]. The study by Wright and colleagues [42] measured SPT reactivity but used a definition of physician diagnosed allergic rhinitis that included both SPT-positive and SPT-negative children. More than half of the studies used ISAAC criteria for the definition of allergic rhinitis. Finally, 11 studies assessed maternal smoking during pregnancy [44],[45],[47]–[49],[60],[70],[81],[93],[99],[114].
Fig. 2. Study-specific and random effects pooled relative risks of active smoking and allergic rhinitis. 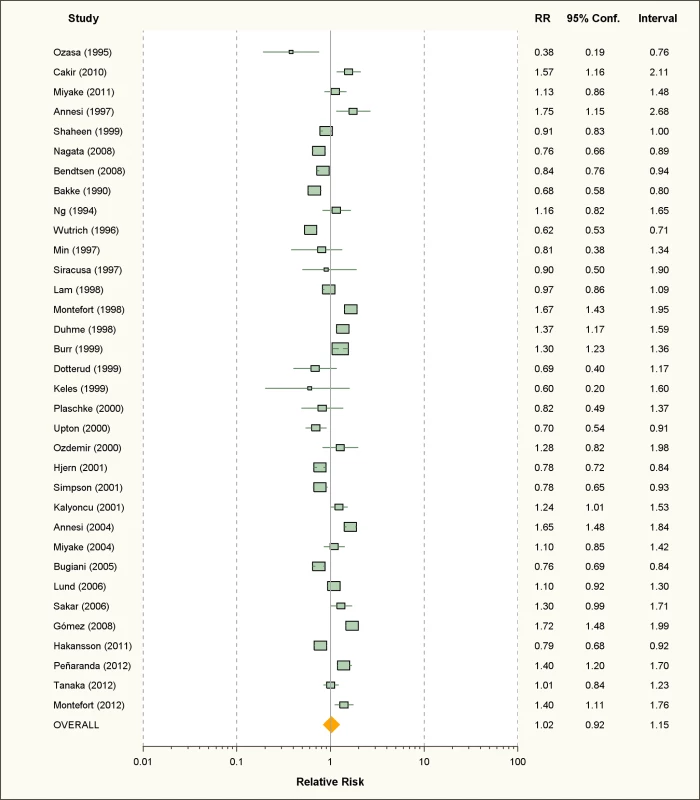
Fig. 3. Study-specific and random effects pooled relative risks of passive smoking and allergic rhinitis. 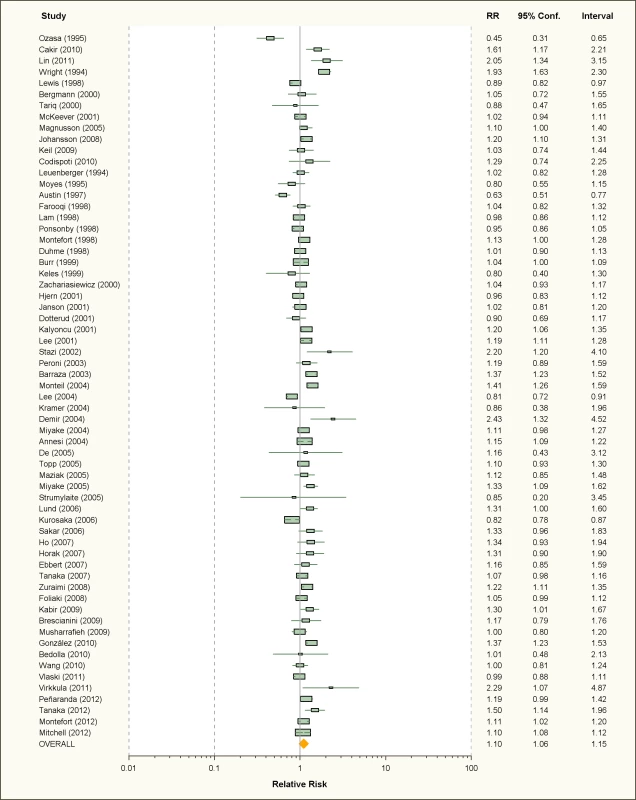
Tab. 1. Relative risks and 95% confidence intervals of allergic rhinitis by smoking exposure in case-control and cohort studies. 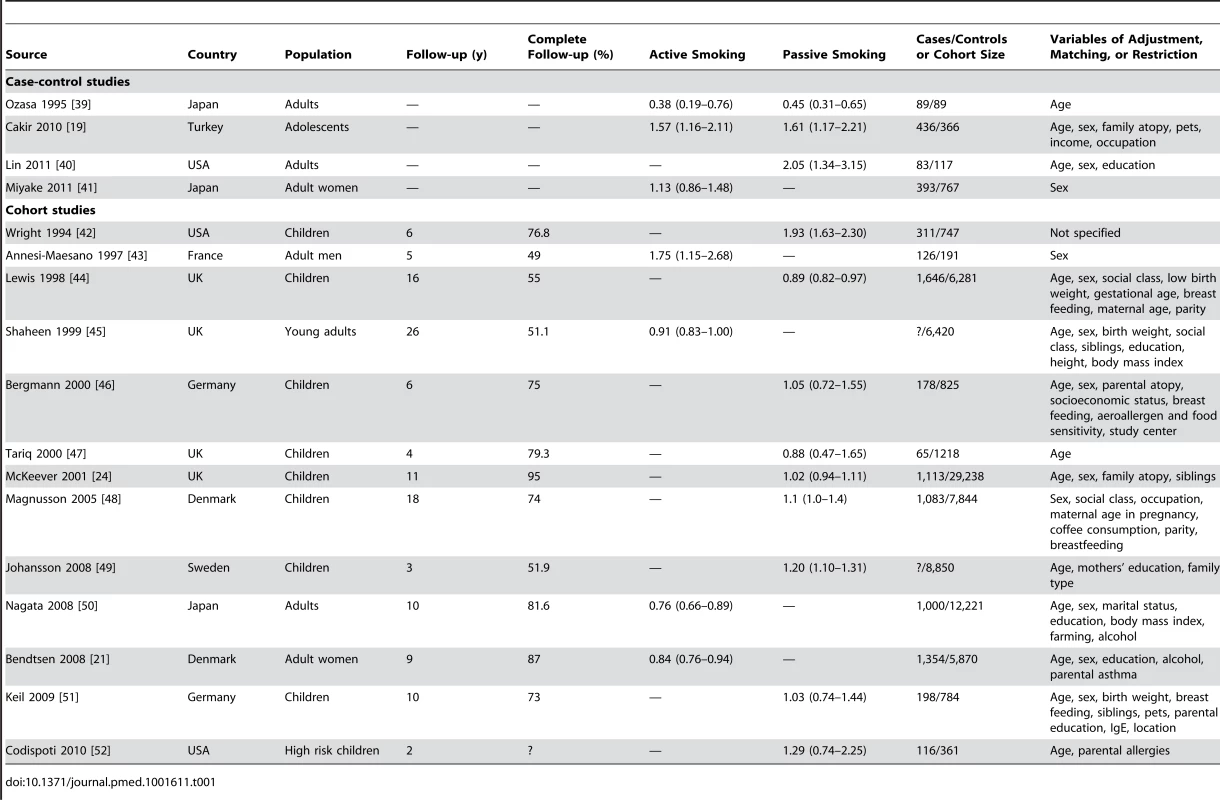
Tab. 2. Relative risks and 95% confidence intervals of allergic rhinitis by smoking exposure in cross-sectional studies. 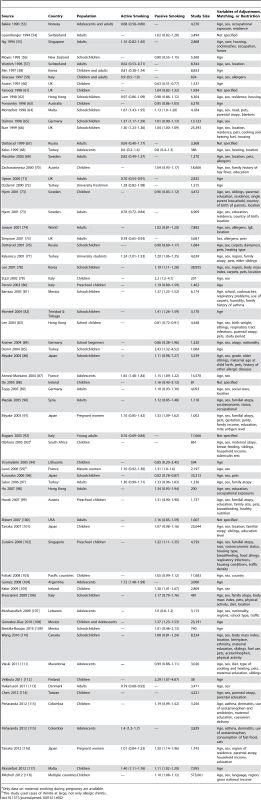
Only data on maternal smoking during pregnancy are available. Table 3 shows the results for associations between smoking and allergic rhinitis.
Tab. 3. Pooled relative risks and 95% confidence intervals of allergic rhinitis and smoking. 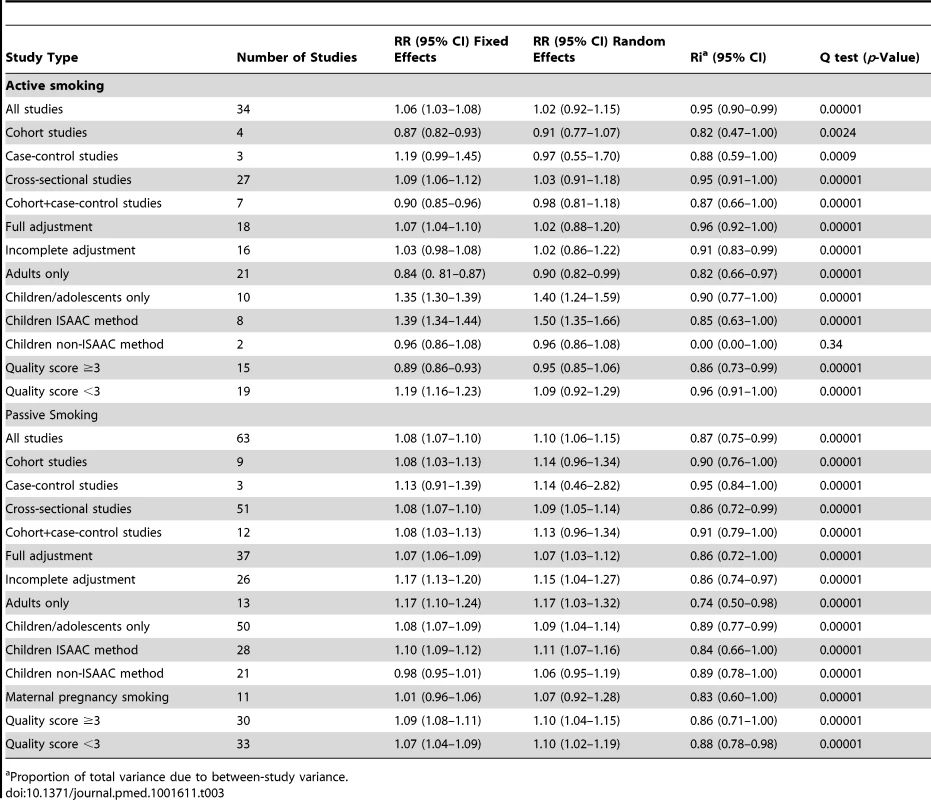
Proportion of total variance due to between-study variance. Active Smoking
Using random effects analysis, there was no significant association between active smoking and the risk of allergic rhinitis when all studies are considered (RR = 1.02; 95% CI 0.92–1.15). Using fixed effect analysis for all studies, there was a significant association between active smoking and risk of rhinitis (RR = 1.06, 95% CI 1.03–1.08); however, this may be explained by the considerable amount of heterogeneity due to differences in designs, case, and exposure definitions and adjustment for confounders. It is remarkable that, under the fixed effects model, the result of the cross-sectional subgroup (RR = 1.09; 95% CI 1.06–1.12) is statistically significant and opposed to the result of the cohort studies subgroup (RR = 0.87; 95% CI 0.82–0.93).
When restricting the analysis to the ten studies carried out on children and adolescents, active smoking was associated with an increased pooled relative risk of 1.40 (95% CI 1.24–1.59). In further sub-group analyses, the association was significant in the studies that used the standardized ISAAC protocol (RR = 1.50, 95% CI 1.35–1.66), but not those that used their own protocol (RR = 0.96, 95% CI 0.88–1.08). A reverse association between active smoking and allergic rhinitis was observed in adults only (RR = 0.90, 95% CI 0.82–0.99)
Passive Smoking
Using random effects analysis, there was a significant association passive smoking and allergic rhinitis (RR = 1.10, 95% CI 1.06–1.15). Similar findings were observed in subgroup analyses by adjustment for confounding variables (RR = 1.07; 95% CI 1.03–1.12 for full adjustment, RR = 1.15; 95% CI 1.04–1.27 for incomplete adjustment), quality scores (RR = 1.10; 95% CI 1.04–1.15 for high quality, RR = 1.10; 95% CI 1.02–1.19 for low quality), and for cross-sectional studies (RR = 1.09; 95% CI 1.05–1.14); however, there was no significant association between passive smoking and allergic rhinitis when restricting the analysis to cohort studies (RR = 1.14; 95% CI 0.96–1.34) or case-control studies (RR = 1.14; 95% CI 0.46–2.82).
In subgroup analyses based on age group, a significant association between passive smoking and allergic rhinitis was observed in adults only (RR = 1.17; 95% CI 1.03–1.32) and in children and adolescents (RR = 1.09; 95% CI 1.04–1.14). For maternal pregnancy smoking, there was no evidence for a a significant increase in the risk of allergic rhinitis in the offspring (RR = 1.07; 95% CI 0.92–1.28).
Publication Bias
The funnel plot of active smoking seems to be slightly skewed to the left, which indicates a potential lack of studies that favor a positive association of the disease with smoking (Figure 4). However, the Egger's test of asymmetry yielded a nonsignificant p-value of 0.27 and no hypothetical study was suggested as missing in the trim-and-fill procedure. The funnel plot for passive smoking (Figure 5) and the corresponding results of the Egger's test did not show any evidence of publication bias (p = 0.53), but two new studies were imputed in the trim-and-fill procedure yielding a modified pooled relative risk of 1.10 (95% CI 1.05–1.14).
Fig. 4. Funnel plot of relative risk versus standard error of relative risk: allergic rhinitis, active smoking. 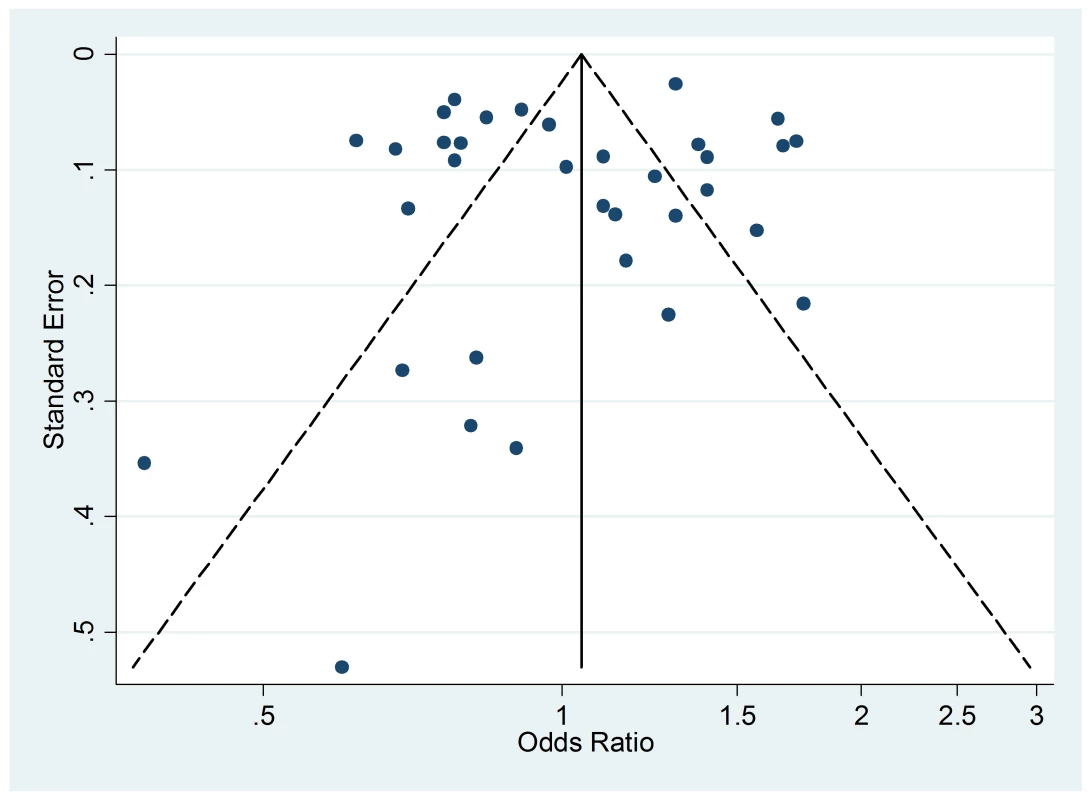
Fig. 5. Funnel plots of relative risk versus standard error of relative risk: allergic rhinitis, passive smoking. 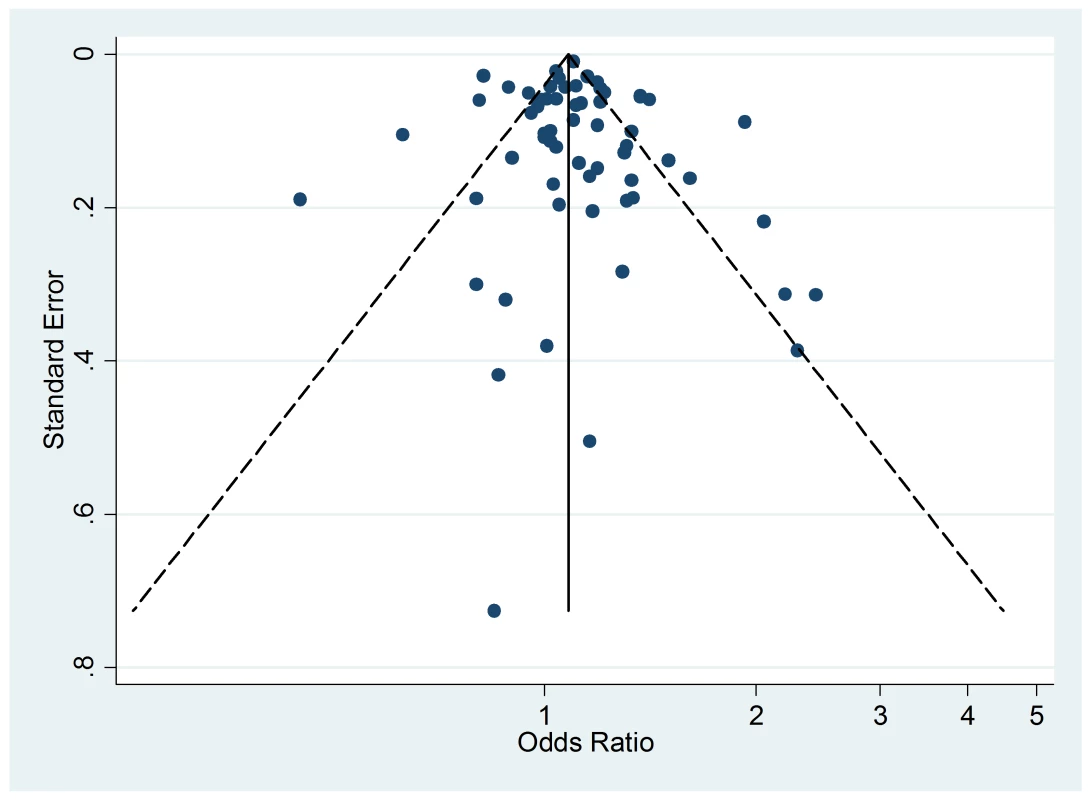
Allergic Dermatitis
We retrieved 33 studies on active smoking and 58 studies on passive smoking (Figures 6 and 7; Tables 4 and 5). About one-third of the studies used ISAAC criteria for case definition. Nineteen studies assessed maternal smoking during pregnancy [44],[45],[48],[83],[93],[99],[121],[127],[128],[130],[133]–[136],[140], [153], [158], [160], [164].
Fig. 6. Study-specific and random effects pooled relative risks of active smoking and allergic dermatitis. 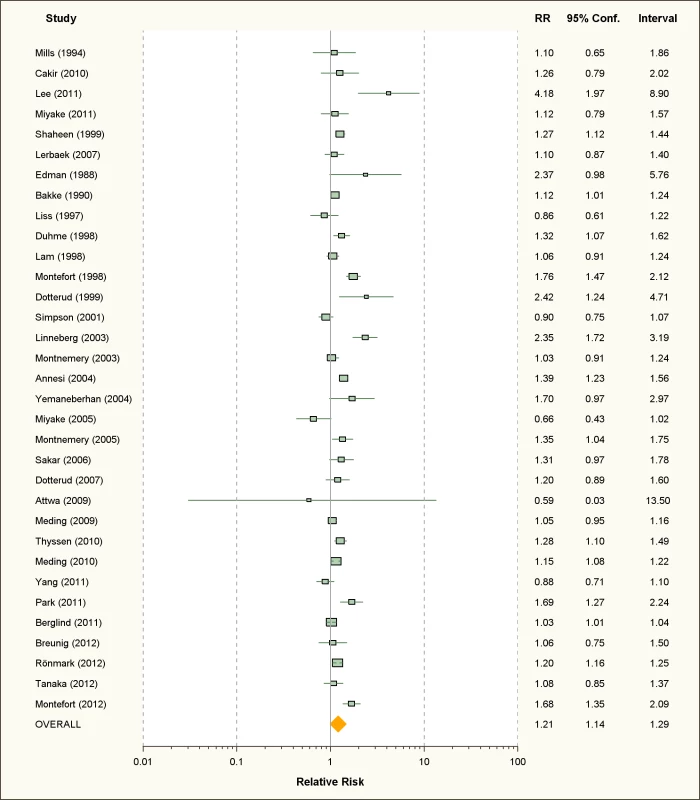
Fig. 7. Study-specific and random effects pooled relative risks of passive smoking and allergic dermatitis. 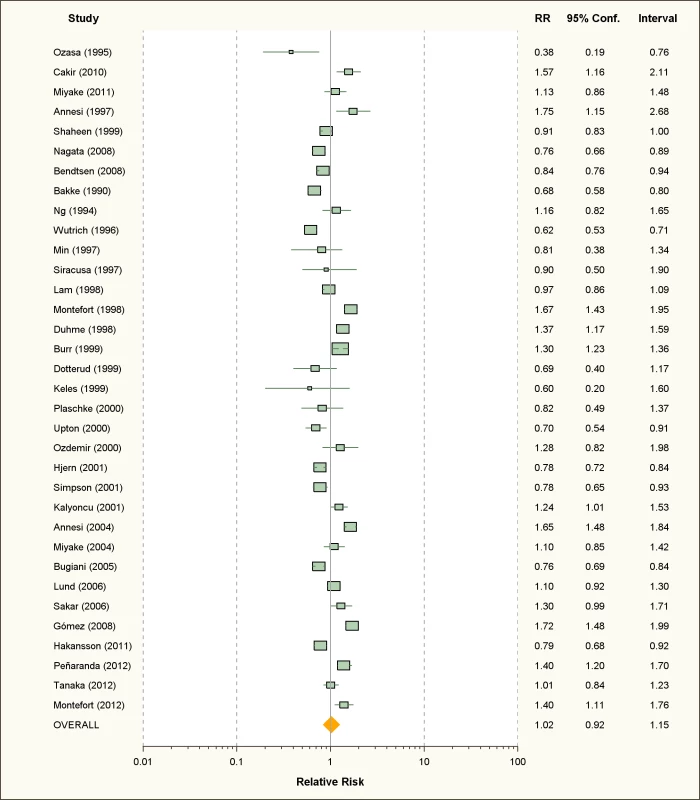
Tab. 4. Relative risks and 95% confidence intervals of dermatitis by smoking exposure in case-control and cohort studies. 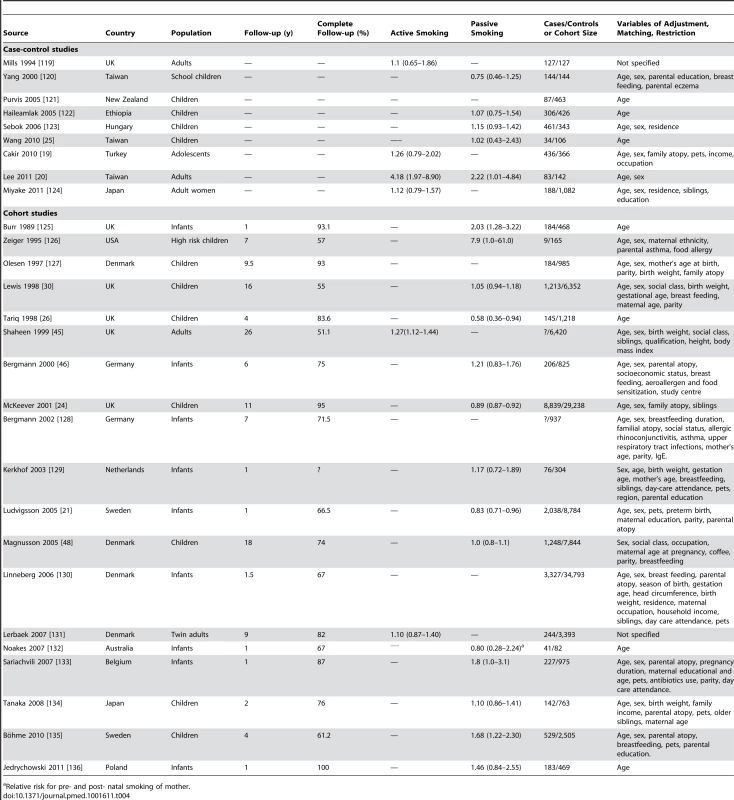
Relative risk for pre- and post- natal smoking of mother. Tab. 5. Relative risks and 95% confidence intervals of allergic dermatitis by smoking exposure in cross-sectional studies. 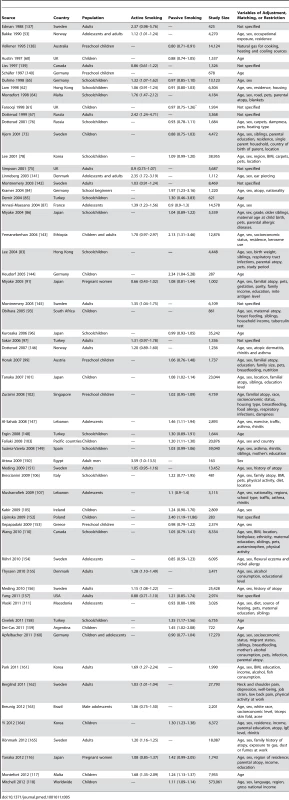
Tab. 6. Pooled relative risks and 95% confidence intervals of allergic dermatitis and smoking. 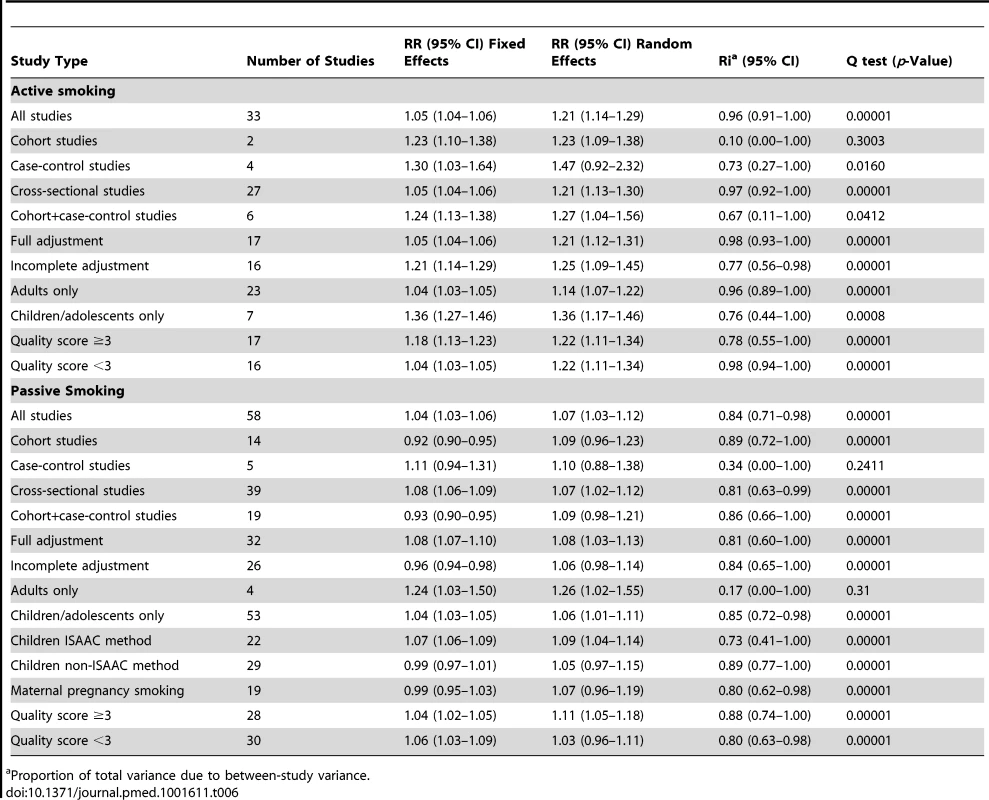
Proportion of total variance due to between-study variance. Active Smoking
Using random effects analysis, active smoking was significantly associated with an increased risk of allergic dermatitis overall (RR = 1.21; 95% CI 1.14–1.29) and in both adults (RR = 1.14; 95% CI 1.07–1.22) and in children and adolescents (RR = 1.36; 95% CI 1.17–1.46)
In sub-group analyses, the association between active smoking and allergic dermatitis was similar based on age, adjustment for confounding, quality scores, and for cohort studies and cross-sectional studies, although there was no significant association between active smoking and allergic dermatitis observed in the four case-control studies (RR = 1.47; 95% CI 0.92–2.32).
Passive Smoking
Using random effects analysis, passive smoking was associated with an increased risk of allergic dermatitis in the general population (RR = 1.07; 95% CI 1.03–1.12).
In sub-group analyses, the association between passive smoking and allergic dermatitis was significant when restricted to cross-sectional studies (RR = 1.07; 95% CI 1.02–1.12), but not for cohort (RR = 1.09; 95% CI 0.96–1.23) or case-control studies (RR = 1.10; 95% CI 0.88–1.38). A significant association between passive smoking and allergic dermatitis was observed for those studies with adjustment for confounding variables (RR = 1.08; 95% CI 1.03–1.13) and higher quality scores (RR = 1.11; 95% CI 1.05–1.18), but not those without adjustment (RR = 1.06; 95% CI 0.98–1.14) or low quality scores (RR = 1.03; 95% CI 0.96–1.11).
A significant association was observed in those studies including adults only (RR = 1.26; 95% CI 1.02–1.55) and in those including children and adolescents only (RR = 1.06; 95% CI 1.01–1.11). No significant association was observed between maternal smoking and allergic dermatitis (RR = 1.07; 95% CI 0.96–1.19).
Publication Bias
The Egger's test for asymmetry of the funnel plot of active smoking (Figure 8) yielded a p-value of 0.28 and no study was added in the trim-and-fill procedure. No asymmetry was detected for passive smoking (Figure 9) through the Egger's test (p = 0.33) but the trim-and-fill procedure suggested that ten potential studies were missing. The modified random effects pooled relative risk was 1.04 (95% CI 1.00–1.08).
Fig. 8. Funnel plots of relative risk versus standard error of relative risk: allergic dermatitis, active smoking. 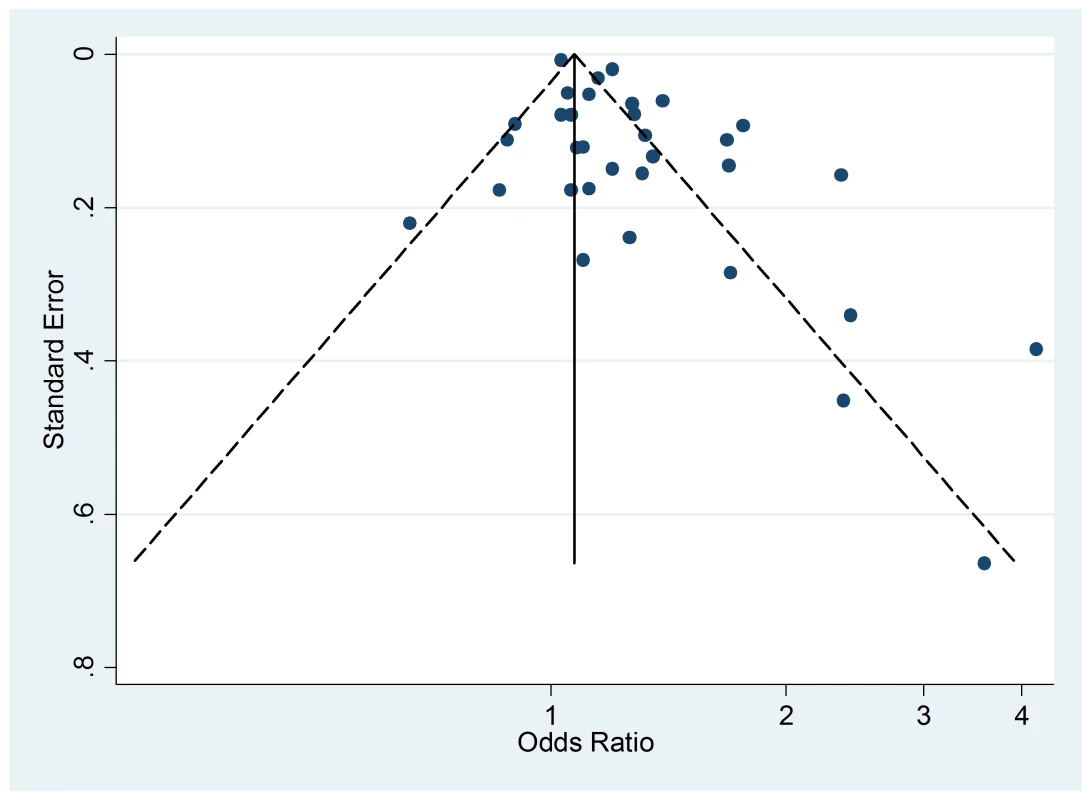
Fig. 9. Funnel plot of relative risk versus standard error of relative risk: allergic dermatitis, passive smoking. 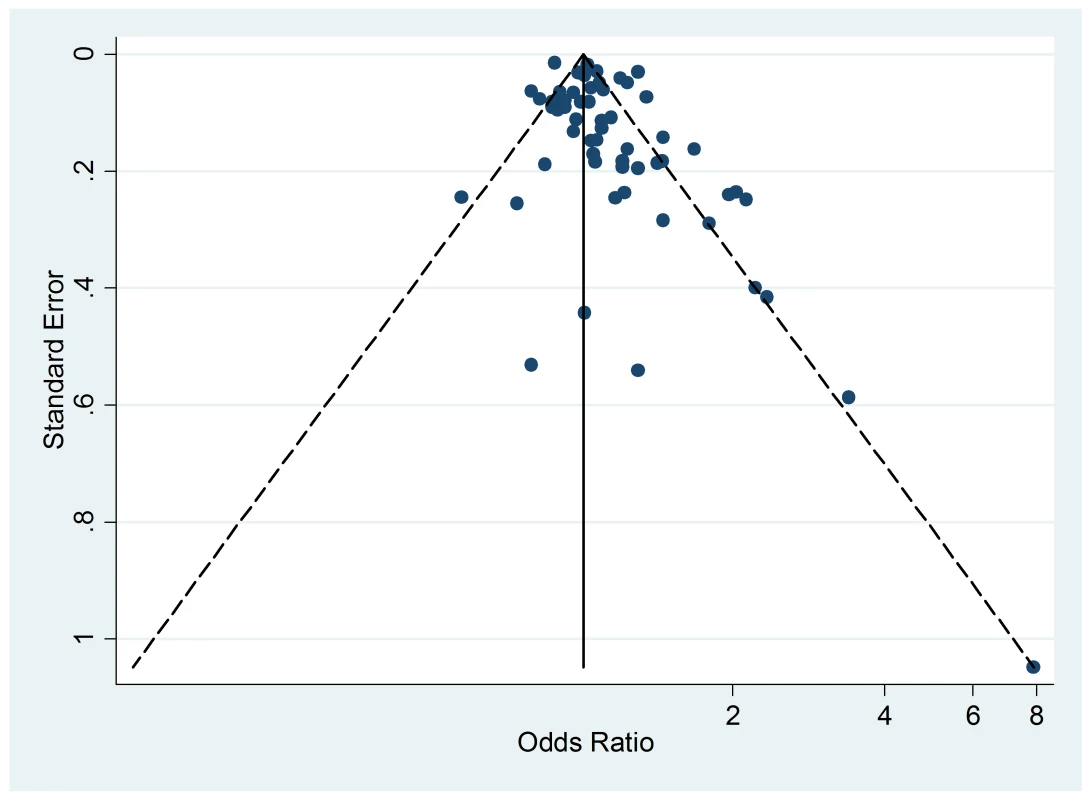
Food Allergies
We retrieved only one study for active smoking and six studies for passive smoking, while three studies assessed maternal smoking during pregnancy (Figure 10; Table 7). All were carried out in children or infants populations.
Fig. 10. Study-specific and random effects pooled relative risks of passive smoking and food allergies. 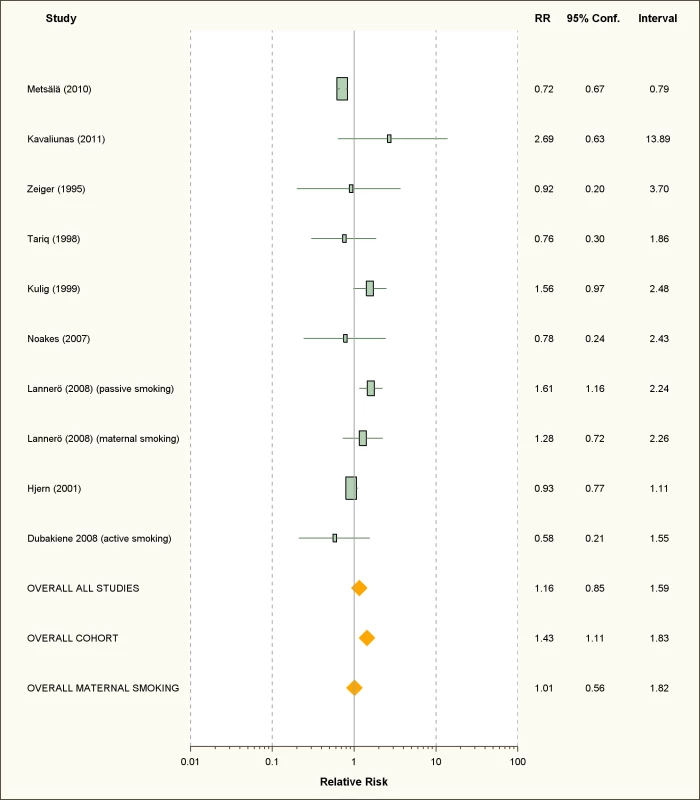
Tab. 7. Study-specific and 95% confidence intervals of food allergies and smoking. 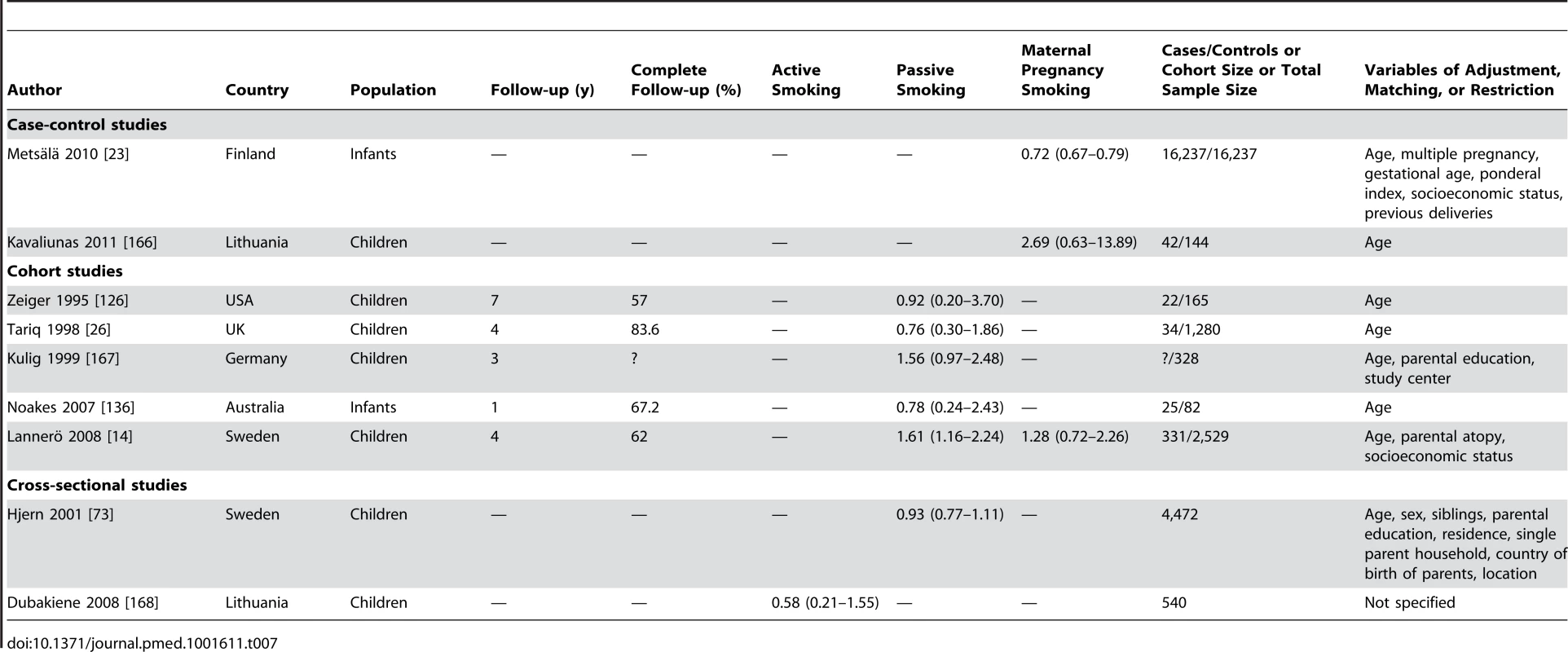
Active Smoking
The only available study on active smoking and food allergies did not show any significant association (RR = 0.58; 95% CI 0.21–1.55).
Passive Smoking
Using random effect analysis, including the six studies investigating exposure to secondhand smoke, showed that passive smoking was associated with a nonsignificant increase of the risk of food allergy (RR = 1.16; 95% CI 0.85–1.59). When the only cross-sectional study was excluded and the analysis was based on five cohort studies, passive smoking was significantly associated with an increased risk of food allergy (RR = 1.43; 95% CI 1.12–1.83) (Table 8). As with allergic rhinitis and allergic dermatitis, we could not detect any association with maternal smoking during pregnancy with food allergies (RR = 1.01; 95% CI 0.56–1.82) (Table 8).
Tab. 8. Pooled relative risks and 95% confidence intervals of food allergies and smoking. 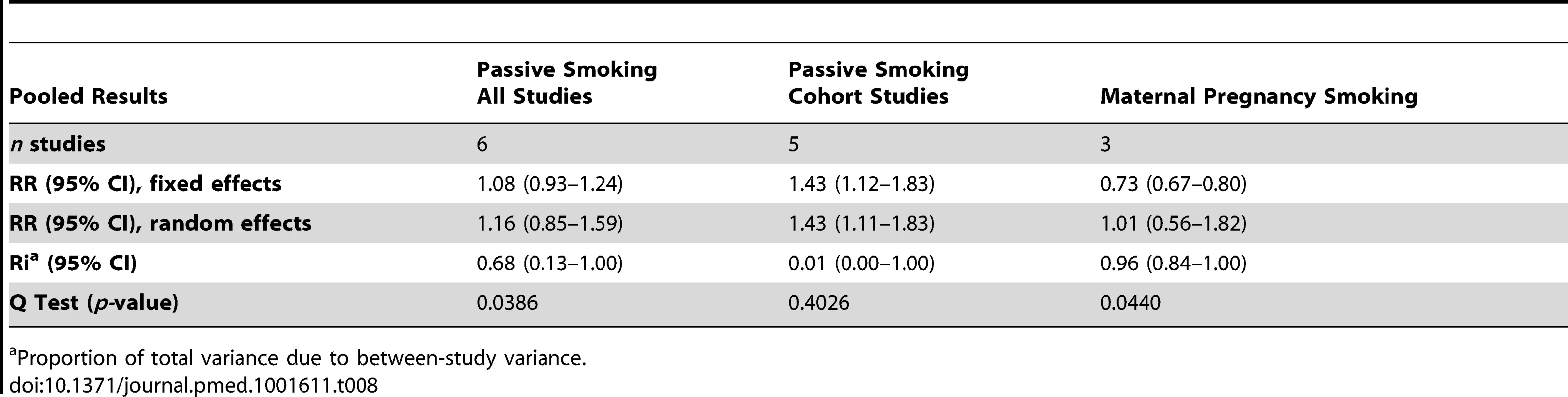
Proportion of total variance due to between-study variance. Publication Bias
The funnel plot (Figure 11), although not a valuable way to assess publication bias in this case due to the small sample size, did not provide evidence of asymmetry (p = 0.09).
Fig. 11. Funnel plot of relative risk versus standard error of relative risk: food allergy, passive smoking. 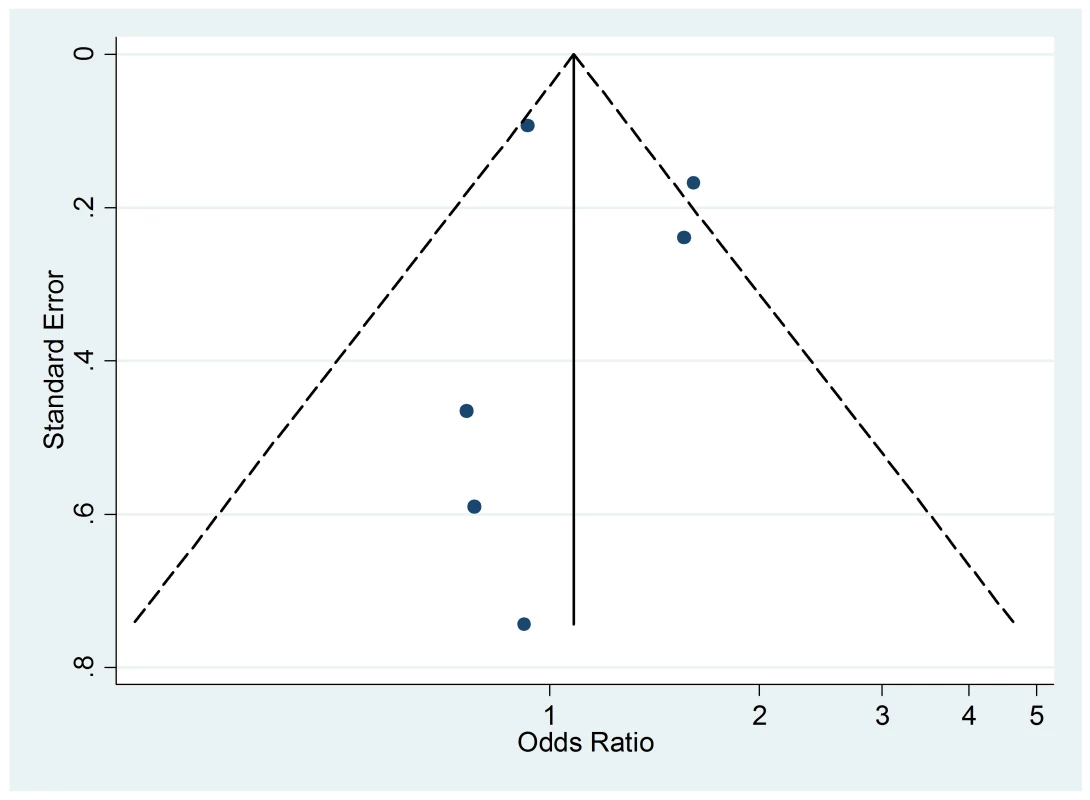
Meta-regression
The meta-regression with the pooled log relative risk as a dependent variable and the population variable as a moderator, introduced in the model as a dichotomous variable (adults/pediatric population), yielded the following results for the children and adolescents when compared to the adults: allergic rhinitis and active smoking: RR = 1.55, 95% CI 1.30–1.84; allergic rhinitis and passive smoking: RR = 0.93, 95% CI 0.81–1.06; allergic dermatitis and active smoking: RR = 1.18, 95% CI 1.01–1.39; and allergic dermatitis and passive smoking: RR = 0.83, 95% CI 0.65–1.06. These results suggest that the associations between allergic rhinitis and allergic dermatitis with active smoking are significantly greater among children and adolescents than among adults. Although these meta-regression RRs were not statistically significant at a 95% level for passive smoking, in Tables 3 and 6 we present the results of children and adolescent populations as a subgroup both for active and passive smoking.
Sub-group Analyses in Children and Adolescents
We calculated the random effects pooled relative risks for children cohort studies, then for children cohort studies and case-control studies combined. For cohort studies, passive smoking was not significantly associated with allergic rhinitis (RR = 1.14; 95% CI 0.96–1.34, nine studies), or allergic dermatitis (RR = 1.09; 95% CI 0.96–1.23, 14 studies), but was significantly associated with an increased risk of food allergy (RR = 1.43; 95% CI 1.11–0.83, five studies). For cohort and case-control studies combined, passive smoking was significantly associated with an increased risk for allergic rhinitis: RR = 1.17 (95% CI 1.00–1.38, ten studies), but not for allergic dermatitis: RR = 1.07 (95% CI 0.96–1.19, 18 studies).
Sensitivity Analysis
To further evaluate the possibility that the results obtained for children/adolescents were due to publication bias, we assumed that cross-sectional studies are the kind of studies that are most probably rejected by journals in case of null results and recalculated our pooled estimates under the following extreme assumptions: (1) published cross-sectional studies are only half of the studies of smoking and allergic rhinitis ever conducted among children, (2) all unpublished studies found an RR of 1, (3) the unpublished studies found the same prevalence of allergic diseases as the average of the published studies. Under these extreme assumptions, the random effects pooled estimates for active smoking still show a significant increase in risk: RR = 1.16 (95% CI 1.08–1.25) for allergic rhinitis and RR = 1.13 (95% CI 1.05–1.21) for allergic dermatitis.
Discussion
The results of our systematic review and meta-analysis suggest that active and passive smoking are associated with a modest increase in risk for some allergic diseases. In the overall population, active smoking was associated with a modest increase in the risk for allergic dermatitis but not allergic rhinitis, while passive smoking was associated with modest increases in the risks for both allergic dermatitis and allergic rhinitis. Among children and adolescents, we observed significant associations between both active and passive smoking and allergic rhinitis and allergic dermatitis, and passive smoking was associated with an increased risk for food allergy
In children and adolescents, while the observed increase in risk for allergic diseases associated with smoking was small, the findings are important given that to the prevalence of active and passive smoking in this population can be high. Worldwide, 14% of adolescents aged 13 to 15 are active smokers with some countries reaching a prevalence of 40%, and nearly 25% of the children who smoke have smoked their first cigarette before the age of 10 years [210]. Furthermore, in the US, more than one-third of children live with at least one adult smoker [211]. In other parts of the world, passive exposure to tobacco among children is even higher as nearly half of children were exposed to tobacco smoke at home [212]. On the basis of the figures above, in countries with high smoking prevalence we estimate that 14% of allergic rhinitis and 13% of allergic dermatitis are attributable to active smoking [213]. Eliminating active smoking in children and adolescents would then prevent one in every seven cases of allergic rhinitis and one in every eight cases of allergic dermatitis.
That age is an important effect modifier for the relation between tobacco exposure and risk of allergic diseases is biologically plausible. The US Surgeon General has suggested that the immaturity of the respiratory, nervous, and immune systems in children may make them vulnerable to health effects of smoking [214]. Furthermore, unlike adults, children have limited options for avoiding exposure to secondhand smoke and are unable to reduce the quantity of products inhaled [214].
Our finding that maternal exposure is not associated with the risk of allergic diseases in the offspring confirms the results from a previous meta-analysis that focused on the risk of allergic sensitization measured through skin prick positivity or IgE concentrations [30]. It is possible that the lack of observed association is due to the existence of bias given that parents of children at high risk of allergy may selectively avoid smoking during pregnancy.
The findings from our meta-analysis are subject to several limitations. The majority of studies were cross-sectional, a design that does not allow for causal inference and can overestimate relative risks given its reliance on prevalence ratios. When restricted to cohort studies our analyses showed that many of the results were no longer significant, especially for the subgroup analysis in children and adolescents. There is then some evidence that the findings may be impacted by study design.
Residual confounding (confounding remaining after adjustment) may explain some of our findings. For some of our analyses, we were unable to detect meaningful differences in the results between studies that had incomplete adjustment for confounders and those with more complete adjustment for confounders and our findings were broadly similar when restricting the analyses to studies with higher quality scores. However, there are likely to be other factors, such as genetic factors that were not controlled for and may play a role in the relationship between smoking and allergic diseases. Although publication bias cannot be ruled out, its magnitude is likely to be low as shown by the robustness of our sensitivity analysis.
Several studies assessed allergic diseases through self-report only, which can lead to misclassification of allergic and non-allergic conditions. Similarly, the findings are limited by measurement error in the smoking exposure given that a majority of studies assessed exposure to smoking in a qualitative fashion and often on a yes/no basis instead of using a quantitative assessment. Misclassification and measurement error in SHS assessment may result from a respondent's lack of knowledge about current or past exposure, biased recall, whether intentional or unintentional, and the difficulty in characterizing an exposure in complex indoor environments [215]. A standard set of items to identify passive smoking in distinct settings is needed [216]. If misclassification exists, it is probable that the outcome misclassification is not differential in regard to smoking and, similarly, measurement error in smoking assessment is not differential in regard to diagnosis. In this case, the results would be biased towards the null value, which means that the association with smoking observed in our meta-analysis is underestimated.
In our subgroup analyses, we were unable to identify any factors that accounted for study heterogeneity. Given the high heterogeneity estimates, we focused our interpretation on the random effects estimates. The random effects model gives increased weights to the effect of small studies, which may introduce bias in the estimation. It is worth noting that for some of the analyses, the fixed effects and random effects estimates differ substantially; this may be due to differences in case or exposure definition and in adjustment for potential confounders. AU: ok to delete>it appears that you have said this in the previous sentence.
Our subgroup analyses found stronger evidence for associations between smoking and allergic diseases in children and adolescents than adults. Furthermore, our meta-regression suggested that the association between active smoking and allergic disorders is larger in children and adolescents than in adults, which advocates for a transient effect through life. This finding is in accordance with the “atopic march” concept that suggests that the sequence of sensitization that starts in childhood may show a tendency to spontaneous remission later in life [217]. It is then plausible that sensitization to tobacco is mitigated by increasing age. Further research is needed to verify whether the association between smoking and risk of allergy in adults is similar for those who started smoking as an adult and those who started smoking during childhood or adolescents.
Future studies should minimize measurement error in the exposure and misclassification bias in the outcome. These studies should avoid cross-sectional designs, use extensive validated questionnaires in order to assess smoking in a quantitative fashion, and should be based on an optimal diagnosis of allergic diseases.
Supporting Information
Zdroje
1. OzdoganogluT, SonguM (2012) The burden of allergic rhinitis and asthma. Ther Adv Respir Dis 6 : 11–23.
2. BauchauV, DurhamSR (2004) Prevalence and rate of diagnosis of allergic rhinitis in Europe. Eur Respir J 24 : 758–764.
3. BergerWE (2004) Allergic rhinitis in children: diagnosis and management strategies. Paediatr Drugs 6 : 233–250.
4. RonaRJ, KeilT, SummersC, GislasonD, ZuidmeerL, et al. (2007) The prevalence of food allergy: a meta-analysis. J Allergy Clin Immunol 120 : 638–646.
5. DaVeigaSP (2012) Epidemiology of atopic dermatitis: a review. Allergy Asthma Proc 33 : 227–234.
6. MeltzerEO, BlaissMS, DereberyMJ, MahrTA, GordonBR, et al. (2009) Burden of allergic rhinitis: results from the Pediatric Allergies in America survey. J Allergy Clin Immunol 124: S43–S70.
7. NathanRA (2007) The burden of allergic rhinitis. Allergy Asthma Proc 28 : 3–9.
8. AlanneS, MaskunittyA, NermesM, LaitinenK, PekurinenM (2012) Costs of allergic diseases from birth to two years in Finland. Public Health 126 : 866–872.
9. SpergelJM (2010) From atopic dermatitis to asthma: the atopic march. Ann Allergy Asthma Immunol 105 : 99–106.
10. TanRA, CorrenJ (2011) The relationship of rhinitis and asthma, sinusitis, food allergy, and eczema. Immunol Allergy Clin North Am 31 : 481–491.
11. AsherMI, MontefortS, BjörksténB, LaiCK, StrachanDP, et al. (2006) Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet 368 : 733–743.
12. GhouriN, Hippisley-CoxJ, NewtonJ, SheikhA (2008) Trends in the epidemiology and prescribing of medication for allergic rhinitis in England. J R Soc Med 101 : 466–72.
13. MosgesR, KlimekL (2007) Today's allergic rhinitis patients are different: new factors that may play a role. Allergy 62 : 969–975.
14. LanneröE, WickmanM, van HageM, BergströmA, PershagenG, et al. (2008) Exposure to environmental tobacco smoke and sensitisation in children. Thorax 63 : 172–176.
15. Diaz-SanchezD, RumoldR, GongHJr (2006) Challenge with environmental tobacco smoke exacerbates allergic airway disease in human beings. J Allergy Clin Immunol 118 : 441–446.
16. PedenD, ReedCE (2010) Environmental and occupational allergies. J Allergy Clin Immunol 125(2 Suppl 2): S150–S160.
17. WarrenCW, JonesNR, PerugaA, ChauvinJ, BaptisteJP, et al. (2008) Global youth tobacco surveillance, 2000–2007. MMWR Surveill Summ 57 : 1–28.
18. MurinS, RafiiR, BilelloK (2011) Smoking and smoking cessation in pregnancy. Clin Chest Med 32 : 75–91.
19. CakirE, ErsuR, UyanZS, OktemS, VarolN, et al. (2010) The prevalence and risk factors of asthma and allergic diseases among working adolescents. Asian Pac J Allergy Immunol 28 : 122–129.
20. LeeCH, ChuangHY, HongCH, HuangSK, ChangYC, et al. (2011) Lifetime exposure to cigarette smoking and the development of adult-onset atopic dermatitis. Br J Dermatol 164 : 483–489.
21. BendtsenP, GrønbaekM, KjaerSK, MunkC, LinnebergA, et al. (2008) Alcohol consumption and the risk of self-reported perennial and seasonal allergic rhinitis in young adult women in a population-based cohort study. Clin Exp Allergy 38 : 1179–1185.
22. LudvigssonJF, MostromM, LudvigssonJ, DuchenK (2005) Exclusive breastfeeding and risk of atopic dermatitis in some 8300 infants. Pediatr Allergy Immunol 16 : 201–208.
23. MetsäläJ, LundqvistA, KailaM, GisslerM, KlaukkaT, et al. (2010) Maternal and perinatal characteristics and the risk of cow's milk allergy in infants up to 2 years of age: a case-control study nested in the Finnish population. Am J Epidemiol 171 : 1310–1316.
24. McKeeverTM, LewisSA, SmithC, CollinsJ, HeatlieH, et al. (2001) Siblings, multiple births, and the incidence of allergic disease: a birth cohort study using the West Midlands general practice research database. Thorax 56 : 758–762.
25. WangIJ, GuoYL, LinTJ, ChenPC, WuYN (2010) GSTM1, GSTP1, prenatal smoke exposure, and atopic dermatitis. Ann Allergy Asthma Immunol 105 : 124–129.
26. TariqSM, MatthewsSM, HakimEA, StevensM, ArshadSH, et al. (1998) The prevalence of and risk factors for atopy in early childhood: a whole population birth cohort study. J Allergy Clin Immunol 101 : 587–593.
27. BurkeH, Leonardi-BeeJ, HashimA, Pine-AbataH, ChenY, et al. (2012) Prenatal and passive smoke exposure and incidence of asthma and wheeze: systematic review and meta-analysis. Pediatrics 129 : 735–744.
28. Baena-CagnaniCE, GómezRM, Baena-CagnaniR, CanonicaGW (2009) Impact of environmental tobacco smoke and active tobacco smoking on the development and outcomes of asthma and rhinitis. Curr Opin Allergy Clin Immunol 9 : 136–140.
29. PattendenS, AntovaT, NeubergerM, NikiforovB, De SarioM, et al. (2006) Parental smoking and children's respiratory health: independent effects of prenatal and postnatal exposure. Tob Control 15 : 294–301.
30. StrachanDP, CookDG (1998) Health effects of passive smoking. 5. Parental smoking and allergic sensitisation in children. Thorax 53 : 117–123.
31. FreimanA, BirdG, MetelitsaAI, BarankinB, LauzonGJ (2004) Cutaneous effects of smoking. J Cutan Med Surg 8 : 415–423.
32. Just-SarobéM (2008) Smoking and the skin. Actas Dermosifiliogr 99 : 173–184.
33. National Institute for Public Health and the Environment (2010) Risk factors for food allergy. Bilthoven (The Netherlands): RIVM Report 340007001.
34. Wells G, Shea B, O'Connell D, Peterson J, Welch V, et al.. (2012) The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa Health Research Institute website. Available: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 16 August 2012.
35. Rothman KJ, Greenland S, Lash TL (2008) Measure of effect and measures of association. Modern epidemiology. 3rd edition. Philadelphia: Lippincott, Williams and Wilkins. P61.
36. TakkoucheB, Cadarso-SuarezC, SpiegelmanD (1999) Evaluation of old and new tests of heterogeneity in epidemiologic meta-analysis. Am J Epidemiol 150 : 206–215.
37. EggerM, Davey SmithG, SchneiderM, MinderC (1997) Bias in meta-analysis detected by a simple, graphical test. Br Med J 315 : 629–634.
38. Costa-BouzasJ, TakkoucheB, Cadarso-SuarezC, SpiegelmanD (2001) HEpiMA: software for the identification of heterogeneity in meta-analysis. Comput Methods Programs Biomed 64 : 101–107.
39. OzasaK, TakenakaH, TakagiN, AoikeA, KawaiK (1995) A case-control study of risk factors for Japanese cedar pollinosis. Jpn J Hyg 50 : 622–630.
40. LinSY, RehDD, ClippS, IraniL, Navas-AcienA (2011) Allergic rhinitis and secondhand tobacco smoke: a population-based study. Am J Rhinol Allergy 25: e66–e71.
41. MiyakeY, TanakaK, ArakawaM (2011) Case-control study of IL13 polymorphisms, smoking, and rhinoconjunctivitis in Japanese women: the Kyushu Okinawa Maternal and Child Health Study. BMC Med Genet 12 : 143.
42. WrightAL, HolbergCJ, HalonenM, MartinezFD, MorganW, et al. (1994) Epidemiology of physician-diagnosed allergic rhinitis in childhood. Pediatrics 94 : 895–901.
43. Annesi-MaesanoI, OryszczynM-P, NeukirchF, KauffmannF (1997) Relationship of upper airway disease to tobacco smoking and allergic markers: a cohort study of men followed up for 5 years. Int Arch Allergy Immunol 114 : 193–201.
44. LewisSA, BrittonJR (1998) Consistent effects of high socioeconomic status and low birth order, and the modifying effect of maternal smoking on the risk of allergic disease during childhood. Respir Med 92 : 1237–1244.
45. ShaheenSO, SterneJAC, MontgomerySM, AzimaH (1999) Birth weight, body mass index and asthma in young adults. Thorax 54 : 396–402.
46. BergmannRL, EdenharterG, BergmannKE, LauS, WahnU (2000) Socioeconomic status is a risk factor for allergy in parents but not in their children. Clin Exp Allergy 30 : 1740–1745.
47. TariqSM, HakimEA, MatthewsSM, ArshadSH (2000) Influence of smoking on asthmatic symptoms and allergen sensitisation in early childhood. Postgrad Med J 76 : 694–699.
48. MagnussonLL, OlesenAB, WennborgH, OlsenJ (2005) Wheezing, asthma, hayfever, and atopic eczema in childhood following exposure to tobacco smoke in fetal life. Clin Exp Allergy 35 : 1550–1556.
49. JohanssonA, LudvigssonJ, HermanssonG (2008) Adverse health effects related to tobacco smoke exposure in a cohort of three-year olds. Acta Pædiatr 97 : 354–357.
50. NagataC, NakamuraK, FujiiK, KawachiT, TakatsukaN, et al. (2008) Smoking and risk of cedar pollinosis in Japanese men and women. Int Arch Allergy Immunol 147 : 117–124.
51. KeilT, LauS, RollS, GruberC, NickelR, et al. (2009) Maternal smoking increases risk of allergic sensitization and wheezing only in children with allergic predisposition: longitudinal analysis from birth to 10 years. Allergy 64 : 445–451.
52. CodispotiCD, LevinL, LeMastersGK, RyanP, ReponenT, et al. (2010) Breast-feeding, aeroallergen sensitization, and environmental exposures during infancy are determinants of childhood allergic rinitis. J Allergy Clin Immunol 125 : 1054–1060.
53. BakkeP, GulsvikA, EideGE (1990) Hay fever, eczema and urticaria in southwest Norway. Lifetime prevalences and association with sex, age, smoking habits, occupational airborne exposures and respiratory symptoms. Allergy 45 : 515–522.
54. LeuenbergerP, SchwartzJ, Ackermann-LiebrichU, BlaserK, BologniniG, et al. (1994) Passive smoking exposure in adults and chronic respiratory symptoms (SAPALDIA Study) Swiss Study on Air Pollution and Lung Diseases in Adults, SAPALDIA Team. Am J Respir Crit Care Med 150 : 1222–1228.
55. NgTP, TanWC (1994) Epidemiology of allergic rhinitis and its associated risk factors in Singapore. Int J Epidemiol 23 : 553–558.
56. MoyesCD, WaldonJ, RamadasD, CraneJ, PearceN (1995) Respiratory symptoms and environmental factors in schoolchildren in the Bay of Plenty. N Z Med J 108 : 358–361.
57. WüthrichB, SchindlerC, MediciTC, ZellwegerJP, LeuenbergerP (1996) IgE levels, atopy markers and hay fever in relation to age, sex and smoking status in a normal adult Swiss population. SAPALDIA (Swiss Study on Air Pollution and Lung Diseases in Adults) Team. Int Arch Allergy Immunol 111 : 396–402.
58. MinY-G, JungH-W, KimHS, ParkSK, YooKY (1997) Prevalence and risk factors for perennial allergic rhinitis in Korea: results of a nationwide survey. Clin Otolaryngol 22 : 139–144.
59. SiracusaA, MarabiniA, SensiL, BacoccoliR, RipandelliA, et al. (1997) Prevalence of asthma and rhinitis in Perugia, Italy. Monaldi Arch Chest Dis 52 : 434–439.
60. AustinJB, RussellG (1997) Wheeze, cough, atopy, and indoor environment in the Scottish Highlands. Arch Dis Child 76 : 22–26.
61. FarooqiIS, HopkinJM (1998) Early childhood infection and atopic disorder. Thorax 53 : 927–32.
62. LamTH, ChungSF, BetsonCL, WongCM, HedleyAJ (1998) Respiratory symptoms due to active and passive smoking in junior secondary school students in Hong Kong. Int J Epidemiol 27 : 41–48.
63. PonsonbyAL, CouperD, DwyerT, CarmichaelA (1998) Cross sectional study of the relation between sibling number and asthma, hay fever, and eczema. Arch Dis Child 79 : 328–333.
64. MontefortS, LenickerHM, CarunaS, Agius MuscatH (1998) Asthma, rhinitis and eczema in Maltese 13–15 year-old schoolchildren – prevalence, severity and associated factors [ISAAC]. International Study of Asthma and Allergies in Childhood. Clin Exp Allergy 28 : 1089–1099.
65. DuhmeH, WeilandSK, RudolphP, WienkeA, KramerA, et al. (1998) Asthma and allergies among children in West and East Germany: a comparison between Münster and Greifswald using the ISAAC phase I protocol. International Study of Asthma and Allergies in Childhood. Eur Respir J 11 : 840–847.
66. BurrML, AndersonHR, AustinJB, HarkinsLS, KaurB, et al. (1999) Respiratory symptoms and home environment in children: a national survey. Thorax 54 : 27–32.
67. DotterudLK, FalkES (1999) Atopic disease among adults in northern Russia, an area with heavy air pollution. Acta Derm Venereol 79 : 448–450.
68. KeleşN, IlicaliC, DeğerK (1999) The effects of different levels of air pollution on atopy and symptoms of allergic rhinitis. Am J Rhinol 13 : 185–190.
69. PlaschkePP, JansonC, NorrmanE, BjörnssonE, EllbjärS, et al. (2000) Onset and remission of allergic rhinitis and asthma and the relationship with atopic sensitization and smoking. Am J Respir Crit Care Med 162 : 920–924.
70. ZacharasiewiczA, ZidekT, HaidingerG, WaldhorT, VutucC (2000) Symptoms suggestive of atopic rhinitis in children aged 6–9 years and the indoor environment. Allergy 55 : 945–950.
71. UptonMN, McConnachieA, McSharryC, HartCL, SmithGD, et al. (2000) Intergenerational 20 year trends in the prevalence of asthma and hay fever in adults: the Midspan family study surveys of parents and offspring. BMJ 321 : 88–92.
72. OzdemirN, UçgunI, MetintasS, KolsuzM, MetintasM (2000) The prevalence of asthma and allergy among university freshmen in Eskisehir, Turkey. Respir Med 94 : 536–541.
73. HjernA, HedbergA, HaglundB, RosénM (2001) Does tobacco smoke prevent atopic disorders? A study of two generations of Swedish residents. Clin Exp Allergy 31 : 908–914.
74. JansonC, ChinnS, JarvisD, ZockJP, TorénK, et al. (2001) Effect of passive smoking on respiratory symptoms, bronchial responsiveness, lung function, and total serum IgE in the European Community Respiratory Health Survey: a cross-sectional study. Lancet 358 : 2103–2109.
75. SimpsonBM, CustovicA, SimpsonA, HallamCL, WalshD, et al. (2001) NAC Manchester asthma and allergy study (NACMAAS): risk factors for asthma and allergic disorders in adults. Clin Exp Allergy 31 : 391–399.
76. DotterudLK, OdlandJO, FalkES (2001) Atopic diseases among schoolchildren in Nikel, Russia, an Arctic area with heavy air pollution. Acta Derm Venereol 81 : 198–201.
77. KalyoncuAF, DemirAU, OzcakarB, BozkurtB, ArtvinliM (2001) Asthma and allergy in Turkish university students: Two cross-sectional surveys 5 years apart. Allergol Immunopathol (Madr) 29 : 264–271.
78. LeeSI, ShinMH, LeeHB, LeeJS, SonBK, et al. (2001) Prevalences of symptoms of asthma and other allergic diseases in Korean children: a nationwide questionnaire survey. J Korean Med Sci 16 : 155–164.
79. StaziMA, SampognaF, MontaganoG, GrandolfoME, CouilliotMF, et al. (2002) Early life factors related to clinical manifestations of atopic disease but not to skin-prick test positivity in young children. Pediatr Allergy Immunol 13 : 105–112.
80. PeroniDG, PiacentiniGL, AlfonsiL, ZermanL, Di BlasiwP, et al. (2003) Rhinitis in pre-school children: prevalence, association with allergic diseases and risk factors. Clin Exp Allergy 33 : 1349–1354.
81. Barraza VillarrealA, Sanín AguirreLH, Téllez RojoMM, Lacasaña NavarroM, RomieuI (2003) Risk factors for asthma in school children from Ciudad Juárez, Chihuahua. J Asthma 40 : 413–423.
82. MonteilMA, JosephG, Chang KitC, WheelerG, AntoineRM (2004) Smoking at home is strongly associated with symptoms of asthma and rhinitis in children of primary school age in Trinidad and Tobago. Rev Panam Salud Publica 16 : 193–198.
83. LeeSL, WongW, LauYL (2004) Increasing prevalence of allergic rhinitis but not asthma among children in Hong Kong from 1995 to 2001 (Phase 3 International Study of Asthma and Allergies in Childhood). Pediatr Allergy Immunol 15 : 72–78.
84. KrämerU, LemmenCH, BehrendtH, LinkE, SchäferT, et al. (2004) The effect of environmental tobacco smoke on eczema and allergic sensitization in children. Br J Dermatol 150 : 111–118.
85. DemirAU, KarakayaG, BozkurtB, SekerelBE, KalyoncuAF (2004) Asthma and allergic diseases in schoolchildren: third cross-sectional survey in the same primary school in Ankara, Turkey. Pediatr Allergy Immunol 15 : 531–538.
86. MiyakeY, YuraA, IkiM (2004) Cross-sectional study of allergic disorders in relation to familial factors in Japanese adolescents. Acta Paediatr 93 : 380–385.
87. Annesi-MaesanoI, OryszczynMP, RaherisonC, KopferschmittC, PauliG, et al. (2004) Increased prevalence of asthma and allied diseases among active adolescent tobacco smokers after controlling for passive smoking exposure. A cause for concern? Clin Exp Allergy 34 : 1017–1023.
88. DeS, FentonJE, JonesAS, ClarkeRW (2005) Passive smoking, allergic rhinitis and nasal obstruction in children. J Laryngol Otol 119 : 955–957.
89. ToppR, ThefeldW, WichmannHE, HeinrichJ (2005) The effect of environmental tobacco smoke exposure on allergic sensitization and allergic rhinitis in adults. Indoor Air 15 : 222–227.
90. MaziakW, KennethD, WardKD, RastamS, MzayekF, et al. (2005) Extent of exposure to environmental tobacco smoke (ETS) and its dose-response relation to respiratory health among adults. Respir Res 6 : 13.
91. MiyakeY, MiyamotoS, OhyaY, SasakiS, MatsunagaI, et al. (2005) Association of active and passive smoking with allergic disorders in pregnant Japanese women: baseline data from the Osaka Maternal and Child Health Study. Ann Allergy Asthma Immunol 94 : 644–651.
92. BugianiM, CarossoA, MiglioreE, PiccioniP, CorsicoA, et al. (2005) ISAYA (ECRHS Italy) Study Group. Allergic rhinitis and asthma comorbidity in a survey of young adults in Italy. Allergy 60 : 165–70.
93. ObiharaCC, MaraisBJ, GieRP, PotterP, BatemanED, et al. (2005) The association of prolonged breastfeeding and allergic disease in poor urban children. Eur Respir J 25 : 970–977.
94. StrumylaiteL, KregzdyteR, VaitkaitieneE (2005) Pasyvus rukymas ir vaiku kvepavimo sutrikimai [Passive smoking and respiratory health of children]. Medicina (Kaunas) 41 : 348–354.
95. LundVJ, PreziosiP, HercbergS, HamoirM, DubreuilC, et al. (2006) Yearly incidence of rhinitis, nasal bleeding, and other nasal symptoms in mature women. Rhinology 44 : 26–31.
96. KurosakaF, NakataniY, TeradaT, TanakaA, IkeuchiH, et al. (2006) Current cat ownership may be associated with the lower prevalence of atopic dermatitis, allergic rhinitis, and Japanese cedar pollinosis in schoolchildren in Himeji, Japan. Pediatr Allergy Immunol 17 : 22–28.
97. SakarA, YorganciogluA, DincG, YukselH, CelikP, et al. (2006) The prevalence of asthma and allergic symptoms in Manisa, Turkey (A western city from a country bridging Asia and Europe). Asian Pac J Allergy Immunol 24 : 17–25.
98. HoSY, LamTH, ChungSF, LamTP (2007) Cross-sectional and prospective associations between passive smoking and respiratory symptoms at the workplace. Ann Epidemiol 17 : 126–131.
99. HorakE, MorassaB, UlmerbH (2007) Association between environmental tobacco smoke exposure and wheezing disorders in Austrian preschool children. Swiss Med Wkly 137 : 608–613.
100. EbbertJO, CroghanIT, SchroederDR, MurawskiJ, HurtRD (2007) Association between respiratory tract diseases and secondhand smoke exposure among never smoking flight attendants: a cross-sectional survey. Environ Health 6 : 28.
101. TanakaK, MiyakeY, ArakawaM, SasakiS, OhyaY (2007) Prevalence of asthma and wheeze in relation to passive smoking in Japanese children. Ann Epidemiol 17 : 1004–1010.
102. ZuraimiMS, ThamKW, ChewFT, OoiPL, DavidK (2008) Home exposures to environmental tobacco smoke and allergic symptoms among young children in Singapore. Int Arch Allergy Immunol 146 : 57–65.
103. FoliakiS, Annesi-MaesanoI, Tuuau-PotoiN, WaqatakirewaL, ChengS, et al. (2008) Risk factors for symptoms of childhood asthma, allergic rhinoconjunctivitis and eczema in the Pacific: an ISAAC Phase III study. Int J Tuberc Lung Dis 12 : 799–780.
104. GómezR, TeijeiroA, ZernottiM, CanonicaG, MimessiG, et al. (2008) Smoking is a risk factor for having rhinitis in adolescents. Allergy 63 : 419.
105. KabirZ, ManningPJ, HolohanJ, KeoganS, GoodmanPG, et al. (2009) Second-hand smoke exposure in cars and respiratory health effects in children. Eur Respir J 34 : 629–633.
106. BrescianiniS, BrunettoB, IacovacciP, D'IppolitoC, AlbertiG, et al. (2009) Prevalence of self-perceived allergic diseases and risk factors in Italian adolescents. Pediatr Allergy Immunol 20 : 578–84.
107. MusharrafiehU, Al-SahabB, ZaitounF, El-HajjMA, RamadanF, et al. (2009) Prevalence of asthma, allergic rhinitis and eczema among Lebanese adolescents. J Asthma 46 : 382–87.
108. González-DíazSN, Del Río-NavarroBE, Pietropaolo-CienfuegosDR, Escalante-DomínguezAJ, García-AlmarazRG, et al. (2010) Factors associated with allergic rhinitis in children and adolescents from northern Mexico: International Study of Asthma and Allergies in Childhood Phase IIIB. Allergy Asthma Proc 31 : 53–62.
109. Bedolla-BarajasM, Cuevas-RíosG, García-BarbozaE, Barrera-ZepedaAT, Morales-RomeroJ (2010) Prevalencia y factores asociados a la rinitis alérgica en escolares de Ciudad Guzmán, Mexico. [Prevalence and factors associated to allergic rhinitis among schoolchildren of Ciudad Guzmán, Mexico] Rev Invest Clin 62 : 244–251.
110. WangHY, PizzichiniMM, BeckerAB, DuncanJM, FergusonAC, et al. (2010) Disparate geographic prevalences of asthma, allergic rhinoconjunctivitis and atopic eczema among adolescents in five Canadian cities. Pediatr Allergy Immunol 21 : 867–877.
111. VlaskiE, StavricK, SeckovaL, KimovskaM, IsjanovskaR (2011) Do household tobacco smoking habits influence asthma, rhinitis and eczema among 13–14 year-old adolescents? Allergol Immunopathol 39 : 39–44.
112. VirkkulaP, LiukkonenK, SuomalainenAK, AronenET, KirjavainenT, et al. (2011) Parental smoking, nasal resistance and rhinitis in children. Acta Paediatr 100 : 1234–1238.
113. HåkanssonK, von BuchwaldC, ThomsenSF, ThyssenJP, BackerV, et al. (2011) Nonallergic rhinitis and its association with smoking and lower airway disease: a general population study. Am J Rhinol Allergy 25 : 25–29.
114. ChenBY, ChanCC, HanYY, WuHP, GuoYL (2012) The risk factors and quality of life in children with allergic rhinitis in relation to seasonal attack patterns. Paediatr Perinat Epidemiol 26 : 146–155.
115. PeñarandaA, AristizabalG, GarciaE, VasquezC, Rodriguez-MartinezCE, et al. (2012) Allergic rhinitis and associated factors in schoolchildren from Bogota, Colombia. Rhinology 50 : 122–128.
116. TanakaK, MiyakeY, ArakawaM (2012) Smoking and prevalence of allergic disorders in Japanese pregnant women: baseline data from the Kyushu Okinawa Maternal and Child Health Study. Environ Health 11 : 15.
117. MontefortS, EllulP, MontefortM, CaruanaS, GrechV, et al. (2012) The effect of cigarette smoking on allergic conditions in Maltese children (ISAAC). Pediatr Allergy Immunol 23 : 472–478.
118. MitchellEA, BeasleyR, KeilU, MontefortS, OdhiamboJ, et al. (2012) The association between tobacco and the risk of asthma, rhinoconjunctivitis and eczema in children and adolescents: analyses from Phase Three of the ISAAC programme. Thorax 67 : 941–949.
119. MillsCM, SrivastavaED, HarveyIM, SwiftGL, NewcombeRG, et al. (1994) Cigarette smoking is not a risk factor in atopic dermatitis. Int J Dermatol 33 : 33–34.
120. YangCY, ChengMF, HsiehYL (2000) Effects of indoor environmental factors on risk for atopic eczema in a subtropical area. J Toxicol Environ Health A 61 : 245–253.
121. PurvisDJ, ThompsonJM, ClarkPM, RobinsonE, BlackPN, et al. (2005) Risk factors for atopic dermatitis in New Zealand children at 3.5 years of age. Br J Dermatol 152 : 742–749.
122. HaileamlakA, DagoyeD, WilliamsH, VennAJ, HubbardR, et al. (2005) Early life risk factors for atopic dermatitis in Ethiopian children. J Allergy Clin Immunol 115 : 370–376.
123. SebõkB, SchneiderI, HarangiF (2006) Primary Care Paediatricians in Baranya County. Familiar and environmental factors influencing atopic dermatitis in the childhood. J Eur Acad Dermatol Venereol 20 : 418–422.
124. MiyakeY, TanakaK, ArakawaM (2011) IL13 genetic polymorphisms, smoking, and eczema in women: a case-control study in Japan. BMC Med Genet 12 : 142.
125. BurrML, MiskellyFG, ButlandBK, MerrettTG, Vaughan-WilliamsE (1989) Environmental factors and symptoms in infants at high risk of allergy. J Epidemiol Community Health 43 : 125–132.
126. ZeigerRS, HellerS (1995) The development and prediction of atopy in high-risk children: follow-up at age seven years in a prospective randomized study of combined maternal and infant food allergen avoidance. J Allergy Clin Immunol 95 : 1179–1190.
127. OlesenAB, EllingsenAR, OlesenH, JuulS, Thestrup-PedersenK (1997) Atopic dermatitis and birth factors: historical follow up by record linkage. BMJ 314 : 1003–1008.
128. BergmannRL, DiepgenTL, KussO, BergmannKE, KujatJ, et al. (2002) Breastfeeding duration is a risk factor for atopic eczema. Clin Exp Allergy 32 : 205–209.
129. KerkhofM, KoopmanLP, van StrienRT, WijgaA, SmitHA, et al. (2003) Risk factors for atopic dermatitis in infants at high risk of allergy: the PIAMA study. Clin Exp Allergy 33 : 1336–1341.
130. LinnebergA, SimonsenJB, PetersenJ, StensballeLG, BennCS (2006) Differential effects of risk factors on infant wheeze and atopic dermatitis emphasize a different etiology. J Allergy Clin Immunol 117 : 184–189.
131. LerbaekA, KyvikKO, RavnH, MennéT, AgnerT (2007) Incidence of hand eczema in a population-based twin cohort: genetic and environmental risk factors. Br J Dermatol 157 : 552–557.
132. NoakesP, TaylorA, HaleJ, BrecklerL, RichmondP, et al. (2007) The effects of maternal smoking on early mucosal immunity and sensitization at 12 months of age. Pediatr Allergy Immunol 18 : 118–127.
133. SariachviliM, DrosteJ, DomS, WieringaM, VellingaA, et al. (2007) Is breast feeding a risk factor for eczema during the first year of life? Pediatr Allergy Immunol 18 : 410–417.
134. TanakaK, MiyakeY, SasakiS, OhyaY, HirotaY, et al. (2008) Maternal smoking and environmental tobacco smoke exposure and the risk of allergic diseases in Japanese infants: the Osaka Maternal and Child Health Study. J Asthma 45 : 833–838.
135. BöhmeM, KullI, BergströmA, WickmanM, NordvallL, et al. (2010) Parental smoking increases the risk for eczema with sensitization in 4-year-old children. J Allergy Clin Immunol 125 : 941–943.
136. JedrychowskiW, PereraF, MaugeriU, Mrozek-BudzynD, MillerRL, et al. (2011) Effects of prenatal and perinatal exposure to fine air pollutants and maternal fish consumption on the occurrence of infantile eczema. Int Arch Allergy Immunol 155 : 275–281.
137. EdmanB (1988) Palmar eczema: a pathogenetic role for acetylsalicylic acid, contraceptives and smoking? Acta Derm Venereol 68 : 402–407.
138. VolkmerRE, RuffinRE, WiggNR, DaviesN (1995) The prevalence of respiratory symptoms in South Australian preschool children. II. Factors associated with indoor air quality. J Paediatr Child Health 31 : 116–120.
139. LissGM, SussmanGL, DealK, BrownS, CividinoM, et al. (1997) Latex allergy: epidemiological study of 1351 hospital workers. Occup Environ Med 54 : 335–342.
140. SchäferT, DirschedlP, KunzB, RingJ, UberlaK (1997) Maternal smoking during pregnancy and lactation increases the risk for atopic eczema in the offspring. J Am Acad Dermatol 36 : 550–556.
141. LinnebergA, NielsenNH, MennéT, MadsenF, JørgensenT (2003) Smoking might be a risk factor for contact allergy. J Allergy Clin Immunol 111 : 980–984.
142. MontnemeryP, NihlénU, Göran LöfdahlC, NybergP, SvenssonA (2003) Prevalence of self-reported eczema in relation to living environment, socio-economic status and respiratory symptoms assessed in a questionnaire study. BMC Dermatol 3 : 4.
143. YemaneberhanH, FlohrC, LewisSA, BekeleZ, ParryE, et al. (2004) Prevalence and associated factors of atopic dermatitis symptoms in rural and urban Ethiopia. Clin Exp Allergy 34 : 779–785.
144. HeudorfU, SchümannM, AngererJ, ExnerM (2005) Dermal and bronchial symptoms in children: are they caused by PAH containing parquet glue or by passive smoking? Int Arch Occup Environ Health 78 : 655–662.
145. MontnemeryP, NihlénU, LöfdahlCG, NybergP, SvenssonA (2005) Prevalence of hand eczema in an adult Swedish population and the relationship to risk occupation and smoking. Acta Derm Venereol 85 : 429–432.
146. DotterudLK, Smith-SivertsenT (2007) Allergic contact sensitization in the general adult population: a population-based study from Northern Norway. Contact Dermatitis 56 : 10–15.
147. Al-SahabB, AtouiM, MusharrafiehU, ZaitounF, RamadanF, et al. (2008) Epidemiology of eczema among Lebanese adolescents. Int J Public Health 53 : 260–267.
148. ErginS, OzşahinA, ErdoğanBS, AktanS, ZencirM (2008) Epidemiology of atopic dermatitis in primary schoolchildren in Turkey. Pediatr Dermatol 25 : 399–401.
149. Suárez-VarelaM, García-MarcosL, KoganMD, Llopis GonzálezA, Martínez GimenoA, et al. (2008) Parents' smoking habit and prevalence of atopic eczema in 6–7 and 13–14 year-old schoolchildren in Spain. ISAAC phase III. Allergol Immunopathol (Madr) 36 : 336–442.
150. AttwaE, el-LaithyN (2009) Contact dermatitis in car repair workers. J Eur Acad Dermatol Venereol 23 : 138–145.
151. MedingB, AlderlingM, AlbinM, BrismanJ, WrangsjöK (2009) Does tobacco smoking influence the occurrence of hand eczema? Br J Dermatol 160 : 514–518.
152. LipińskaKI, ElgalalA, KunaP (2009) Epidemiologia atopowego zapalenia skory w populacji ogolnej mieszkancow wojewodztwa lodzkiego [Epidemiology of atopic dermatitis in general population of Lodz province's citizens]. Pneumonol Alergol Pol 77 : 145–151.
153. XepapadakiP, ManiosY, LiarigkovinosT, GrammatikakiE, DouladirisN, et al. (2009) Association of passive exposure of pregnant women to environmental tobacco smoke with asthma symptoms in children. Pediatr Allergy Immunol 20 : 423–429.
154. RöhrlK, StenbergB (2010) Lifestyle factors and hand eczema in a Swedish adolescent population. Contact Dermatitis 62 : 170–176.
155. ThyssenJP, LinnebergA, MennéT, NielsenNH, JohansenJD (2010) The effect of tobacco smoking and alcohol consumption on the prevalence of self-reported hand eczema: a cross-sectional population-based study. Br J Dermatol 162 : 619–626.
156. MedingB, AlderlingM, WrangsjöK (2010) Tobacco smoking and hand eczema: a population-based study. Br J Dermatol 163 : 752–756.
157. YangYW, ChenYH, HuangYH (2011) Cigarette smoking may modify the risk of depression in eczema among adults: a preliminary study using NHANES 2005–2006. J Eur Acad Dermatol Venereol 25 : 1048–1053.
158. CivelekE, SahinerUM, YükselH, BozAB, OrhanF, et al. (2011) Prevalence, burden, and risk factors of atopic eczema in schoolchildren aged 10–11 years: a national multicenter study. J Investig Allergol Clin Immunol 21 : 270–277.
159. Dei-CasP, AcuñaMK, Dei-CasI (2011) Atopic dermatitis in children: a comparative survey among 2 age groups. Rev Chil Pediatr 82 : 410–418.
160. ApfelbacherCJ, DiepgenTL, SchmittJ (2011) Determinants of eczema: population-based cross-sectional study in Germany. Allergy 66 : 206–213.
161. ParkH, KimK (2011) Association of blood mercury concentrations with atopic dermatitis in adults: a population-based study in Korea. Environ Res 111 : 573–578.
162. BerglindIA, AlderlingM, MedingB (2011) Life-style factors and hand eczema. Br J Dermatol 165 : 568–575.
163. BreunigJA, de AlmeidaHLJr, DuquiaRP, SouzaPR, StaubHL (2012) Scalp seborrheic dermatitis: prevalence and associated factors in male adolescents. Int J Dermatol 51 : 46–49.
164. YiO, KwonHJ, KimH, HaM, HongSJ, et al. (2012) Effect of environmental tobacco smoke on atopic dermatitis among children in Korea. Environ Res 113 : 40–45.
165. RönmarkEP, EkerljungL, LötvallJ, WennergrenG, RönmarkE, et al. (2012) Eczema among adults: prevalence, risk factors and relation to airway diseases. Results from a large-scale population survey in Sweden. Br J Dermatol 166 : 1301–1308.
166. Kavaliūnas A (2011) Padidėjusio jautrumo maisto produktams ir alergijos maistui paplitimas tarp Vilniaus miesto gyventoju˛ [The prevalence of adverse reactions to food and food allergy among Vilnius city (Lithuania) inhabitants] [dissertation]. Vilnius: Institute of Public Health, Vilnius University.
167. KuligM, LuckW, LauS, NiggemannB, BergmannR, et al. (1999) Effect of pre - and postnatal tobacco smoke exposure on specific sensitization to food and inhalant allergens during the first 3 years of life. Multicenter Allergy Study Group, Germany. Allergy 54 : 220–228.
168. DubakienėR, ŠurkienėG, StukasR, Pirmaitytė-VileskoJ, KavaliūnasA (2008) Food allergies among 5th–9th grade schoolchildren in Vilnius (Lithuania). Ekologija 54 : 1–4.
169. TaylorB, WadsworthJ, GoldingJ, ButlerN (1983) Breast feeding, eczema, asthma, and hayfever. J Epidemiol Community Health 37 : 95–99.
170. ButlandBK, StrachanDP, LewisS, BynnerJ, ButlerN, et al. (1997) Investigation into the increase in hay fever and eczema at age 16 observed between the 1958 and 1970 British birth cohorts. BMJ 315 : 717–721.
171. ArshadSH, StevensM, HideDW (1993) The effect of genetic and environmental factors on the prevalence of allergic disorders at the age of two years. Clin Exp Allergy 23 : 504–511.
172. BiaginiJM, LeMastersGK, RyanPH, LevinL, ReponenT, et al. (2006) Environmental risk factors of rhinitis in early infancy. Pediatr Allergy Immunol 17 : 278–284.
173. RehDD, LinSY, ClippSL, IraniL, AlbergAJ, et al. (2009) Secondhand tobacco smoke exposure and chronic rhinosinusitis: a population-based case-control study. Am J Rhinol Allergy 23 : 562–567.
174. MerrettTG, BurrML, ButlandBK, MerrettJ, MiskellyFG, et al. (1988) Infant feeding and allergy: 12-month prospective study of 500 babies born into allergic families. Ann Allergy 61 : 13–20.
175. Dei-CasI, Dei-CasP, AcuñaK (2009) Atopic dermatitis and risk factors in poor children from Great Buenos Aires, Argentina. Clin Exp Dermatol 34 : 299–303.
176. WangIJ, HsiehWS, WuKY, GuoYL, HwangYH, et al. (2008) Effect of gestational smoke exposure on atopic dermatitis in the offspring. Pediatr Allergy Immunol 19 : 580–586.
177. DotterudLK, OdlandJØ, FalkES (2004) Atopic dermatitis and respiratory symptoms in Russian and northern Norwegian school children: a comparison study in two arctic areas and the impact of environmental factors. J Eur Acad Dermatol Venereol 18 : 131–136.
178. DotterudLK, OdlandJO, FalkES (2000) Atopic diseases among adults in the two geographically related arctic areas Nikel, Russia and Sør-Varanger, Norway: possible effects of indoor and outdoor air pollution. J Eur Acad Dermatol Venereol 14 : 107–111.
179. KuligM, LuckW, WahnU (1999) The association between pre - and postnatal tobacco smoke exposure and allergic sensitization during early childhood. Multicentre Allergy Study Group, Germany. Hum Exp Toxicol 18 : 241–244.
180. MiyakeY, OhyaY, TanakaK, YokoyamaT, SasakiS, et al. (2007) Home environment and suspected atopic eczema in Japanese infants: the Osaka Maternal and Child Health Study. Pediatr Allergy Immunol 18 : 425–432.
181. ThyssenJP, JohansenJD, MennéT, NielsenNH, LinnebergA (2010) Effect of tobacco smoking and alcohol consumption on the prevalence of nickel sensitization and contact sensitization. Acta Derm Venereol 90 : 27–33.
182. BarbeeRA, HalonenM, KaltenbornWT, BurrowsB (1991) A longitudinal study of respiratory symptoms in a community population sample. Correlations with smoking, allergen skin-test reactivity, and serum IgE. Chest 99 : 20–26.
183. ThomsenSF, UlrikCS, PorsbjergC, BackerV (2006) Early life exposures and risk of atopy among Danish children. Allergy Asthma Proc 27 : 110–114.
184. LarssonML, MagnusonA, MontgomerySM (2005) Parental smoking and allergic sensitization in offspring defined by skin prick testing. Pediatr Allergy Immunol 16 : 449–452.
185. LiptayS, BauerCP, GrüblA, FranzR, EmmrichP (1991) Atopieentwicklung in der fruhen Kindheit–Pradisponierende Faktoren [Development of atopic disease in early childhood–predisposing factors]. Monatsschr Kinderheilkd 139 : 130–135.
186. WittigHJ, McLaughlinET, LeiferKL, BelloitJD (1978) Risk factors for the development of allergic disease: analysis of 2,190 patient records. Ann Allergy 41 : 84–88.
187. LinnebergA, NielsenNH, MadsenF, FrølundL, DirksenA, et al. (2001) Smoking and the development of allergic sensitization to aeroallergens in adults: a prospective population-based study. The Copenhagen Allergy Study. Allergy 56 : 328–332.
188. ZetterströmO, OstermanK, MachadoL, JohanssonSG (1981) Another smoking hazard: raised serum IgE concentration and increased risk of occupational allergy. BMJ 283 : 1215–1217.
189. BråbäckL, KjellmanNI, SandinA, BjörksténB (2001) Atopy among schoolchildren in northern and southern Sweden in relation to pet ownership and early life events. Pediatr Allergy Immunol 12 : 4–10.
190. RaherisonC, Pénard-MorandC, MoreauD, CaillaudD, CharpinD, et al. (2008) Smoking exposure and allergic sensitization in children according to maternal allergies. Ann Allergy Asthma Immunol 100 : 351–357.
191. BakosN, SchöllI, SzalaiK, KundiM, UntersmayrE, et al. (2006) Risk assessment in elderly for sensitization to food and respiratory allergens. Immunol Lett 107 : 15–21.
192. Harris-RobertsJ, RobinsonE, WaterhouseJC, BillingsCG, ProctorAR, et al. (2009) Sensitization to wheat flour and enzymes and associated respiratory symptoms in British bakers. Am J Ind Med 52 : 133–140.
193. TsunodaK, OhtaY, ShinogamiM, SodaY (1995) Does passive smoking affect the incidence of nasal allergies? Am J Public Health 85 : 1019–1020.
194. JeebhayMF, RobinsTG, MillerME, BatemanE, SmutsM, et al. (2008) Occupational allergy and asthma among salt water fish processing workers. Am J Ind Med 51 : 899–910.
195. AngioniAM, FanciulliG, CorchiatC (1989) Frequency of and risk factors for allergy in primary school children: results of a population Survey. Paediatr Perinat Epidemiol 3 : 248–255.
196. FrankP, MorrisJ, HazellM, LinehanM, FrankT (2006) Smoking, respiratory symptoms and likely asthma in young people: evidence from postal questionnaire surveys in the Wythenshawe Community Asthma Project (WYCAP). BMC Pulm Med 6 : 10.
197. GuedesHTV, SouzaLSF (2009) Exposure to maternal smoking in the first year of life interferes in breast-feeding protective effect against the onset of respiratory allergy from birth to 5 yr. Pediatr Allergy Immunol 20 : 30–34.
198. StaikūnieneJ, SakalauskasR (2003) Ziedadulkiu sukelto alerginio rinito ir bronchu astmos imunologines savybes bei rizikos veiksniai [The immunological parameters and risk factors for pollen-induced allergic rhinitis and asthma]. Medicina (Kaunas) 39 : 244–253.
199. DubakienėR, VaicekauskaitėD, ŽidanavičiūtėJ, JoneliūnienėI, DrąsutienėG, et al. (2006) Human ecology studies: the role of environmental factors in pregnancy. Ekologija 4 : 18–21.
200. WoodsRK, AbramsonM, RavenJM, BaileyM, WeinerJM, et al. (1998) Reported food intolerance and respiratory symptoms in young adults. Eur Respir J 11 : 151–155.
201. HuangSW (2007) Follow-up of children with rhinitis and cough associated with milk allergy. Pediatr Allergy Immunol 18 : 81–85.
202. PegasPN, AlvesCA, ScottoMG, EvtyuginaMG, PioCA, et al. (2011) Factores de risco e prevalencia de asma e rinite em criancas em idade escolar em Lisboa [Risk factors and prevalence of asthma and rhinitis among schoolchildren in Lisbon]. Rev Port Pneumol 17 : 109–116.
203. GustafssonD, AnderssonK, FagerlundI, KjellmanNI (1996) Significance of indoor environment for the development of allergic symptoms in children followed up to 18 months of age. Allergy 51 : 789–795.
204. HagendorensMM, BridtsCH, LauwersK, van NuijsS, EboDG, et al. (2005) Perinatal risk factors for sensitization, atopic dermatitis and wheezing during the first year of life (PIPO study). Clin Exp Allergy 35 : 733–740.
205. LucasA, BrookeOG, ColeTJ, MorleyR, BamfordMF (1990) Food and drug reactions, wheezing, and eczema in preterm infants. Arch Dis Child 65 : 411–415.
206. VesseyMP, PainterR, PowellJ (2000) Skin disorders in relation to oral contraception and other factors, including age, social class, smoking and body mass index. Findings in a large cohort study. Br J Dermatol 143 : 815–820.
207. GirolomoniG, AbeniD, MasiniC, SeraF, AyalaF, et al. (2003) The epidemiology of atopic dermatitis in Italian schoolchildren. Allergy 58 : 420–425.
208. DubakieneR, RudzevicieneO, ButieneI, SezaiteI, PetronyteM, et al. (2012) Studies on early allergic sensitization in the Lithuanian birth cohort. Sci World J 2012 : 909524.
209. MitchellEA, StewartAW (2001) ISAAC Phase One Study Group (2001) International Study of Asthma and Allergy in Childhood. The ecological relationship of tobacco smoking to the prevalence of symptoms of asthma and other atopic diseases in children: the International Study of Asthma and Allergies in Childhood (ISAAC). Eur J Epidemiol 17 : 667–673.
210. The Global Youth Tobacco Survey Collaborative Group (2002) Tobacco use among youth: a cross country comparison. Tob Control 11 : 252–270.
211. KingK, MartynenkoM, BergmanMH, LiuYH, WinickoffJP, et al. (2009) Family composition and children's exposure to adult smokers in their homes. Pediatrics 123: e559–64.
212. Centers for Disease Control and Prevention (2007) Exposure to secondhand smoke among students aged 13–15 years-worldwide, 2000–2007. MMWR 56 : 497–500.
213. Rothman KJ (1986) Modern epidemiology. Boston: Little, Brown and Co. p.39.
214. US Department of Health and Human Services (2007) Children and secondhand smoke exposure. Excerpts from The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Atlanta: US Department of Health and Human Services, Centers for Disease Control and Prevention, Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health.
215. US Department of Health and Human Services (1986) The Health Consequences of Involuntary Smoking. A Report of the Surgeon General. Rockville (Maryland): US Department of Health and Human Services, Public Health Service, Centers for Disease Control, Center for Health Promotion and Education, Office on Smoking and Health.
216. Pérez-RíosM, SchiaffinoA, LópezMJ, NebotM, GalánI, et al. (2013) Questionnaire-based second-hand smoke assessment in adults. Eur J Public Health 23 : 763–767.
217. WahnU (2000) What drives the allergic march? Allergy 55 : 591–599.
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2014 Číslo 3- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Léčba bolesti u seniorů
- Benefity fixní kombinace tramadolu a paracetamolu v léčbě bolesti
- Flexofytol® – přírodní revoluce v boji proti osteoartróze kloubů
- Superoxidované roztoky v prevenci infekcí u dialyzovaných pacientů
-
Všechny články tohoto čísla
- How Can Journals Respond to Threats of Libel Litigation?
- and Water, Sanitation, and Hygiene: A Committed Relationship
- Representation and Misrepresentation of Scientific Evidence in Contemporary Tobacco Regulation: A Review of Tobacco Industry Submissions to the UK Government Consultation on Standardised Packaging
- Building Research Capacity in Africa: Equity and Global Health Collaborations
- Associations between Intimate Partner Violence and Health among Men Who Have Sex with Men: A Systematic Review and Meta-Analysis
- Active or Passive Exposure to Tobacco Smoking and Allergic Rhinitis, Allergic Dermatitis, and Food Allergy in Adults and Children: A Systematic Review and Meta-Analysis
- The Effectiveness of Community Action in Reducing Risky Alcohol Consumption and Harm: A Cluster Randomised Controlled Trial
- Assessing Causality in the Association between Child Adiposity and Physical Activity Levels: A Mendelian Randomization Analysis
- The Impact of Pyrethroid Resistance on the Efficacy of Insecticide-Treated Bed Nets against African Anopheline Mosquitoes: Systematic Review and Meta-Analysis
- Lung Function and Incidence of Chronic Obstructive Pulmonary Disease after Improved Cooking Fuels and Kitchen Ventilation: A 9-Year Prospective Cohort Study
- Early Experiences Implementing Pre-exposure Prophylaxis (PrEP) for HIV Prevention in San Francisco
- The Role of Viral Introductions in Sustaining Community-Based HIV Epidemics in Rural Uganda: Evidence from Spatial Clustering, Phylogenetics, and Egocentric Transmission Models
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- and Water, Sanitation, and Hygiene: A Committed Relationship
- Representation and Misrepresentation of Scientific Evidence in Contemporary Tobacco Regulation: A Review of Tobacco Industry Submissions to the UK Government Consultation on Standardised Packaging
- The Impact of Pyrethroid Resistance on the Efficacy of Insecticide-Treated Bed Nets against African Anopheline Mosquitoes: Systematic Review and Meta-Analysis
- The Role of Viral Introductions in Sustaining Community-Based HIV Epidemics in Rural Uganda: Evidence from Spatial Clustering, Phylogenetics, and Egocentric Transmission Models
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



