-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaEBV Latency Types Adopt Alternative Chromatin Conformations
Epstein-Barr Virus (EBV) can establish latent infections with distinct gene expression patterns referred to as latency types. These different latency types are epigenetically stable and correspond to different promoter utilization. Here we explore the three-dimensional conformations of the EBV genome in different latency types. We employed Chromosome Conformation Capture (3C) assay to investigate chromatin loop formation between the OriP enhancer and the promoters that determine type I (Qp) or type III (Cp) gene expression. We show that OriP is in close physical proximity to Qp in type I latency, and to Cp in type III latency. The cellular chromatin insulator and boundary factor CTCF was implicated in EBV chromatin loop formation. Combining 3C and ChIP assays we found that CTCF is physically associated with OriP-Qp loop formation in type I and OriP-Cp loop formation in type III latency. Mutations in the CTCF binding site located at Qp disrupt loop formation between Qp and OriP, and lead to the activation of Cp transcription. Mutation of the CTCF binding site at Cp, as well as siRNA depletion of CTCF eliminates both OriP-associated loops, indicating that CTCF plays an integral role in loop formation. These data indicate that epigenetically stable EBV latency types adopt distinct chromatin architectures that depend on CTCF and mediate alternative promoter targeting by the OriP enhancer.
Published in the journal: . PLoS Pathog 7(7): e32767. doi:10.1371/journal.ppat.1002180
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002180Summary
Epstein-Barr Virus (EBV) can establish latent infections with distinct gene expression patterns referred to as latency types. These different latency types are epigenetically stable and correspond to different promoter utilization. Here we explore the three-dimensional conformations of the EBV genome in different latency types. We employed Chromosome Conformation Capture (3C) assay to investigate chromatin loop formation between the OriP enhancer and the promoters that determine type I (Qp) or type III (Cp) gene expression. We show that OriP is in close physical proximity to Qp in type I latency, and to Cp in type III latency. The cellular chromatin insulator and boundary factor CTCF was implicated in EBV chromatin loop formation. Combining 3C and ChIP assays we found that CTCF is physically associated with OriP-Qp loop formation in type I and OriP-Cp loop formation in type III latency. Mutations in the CTCF binding site located at Qp disrupt loop formation between Qp and OriP, and lead to the activation of Cp transcription. Mutation of the CTCF binding site at Cp, as well as siRNA depletion of CTCF eliminates both OriP-associated loops, indicating that CTCF plays an integral role in loop formation. These data indicate that epigenetically stable EBV latency types adopt distinct chromatin architectures that depend on CTCF and mediate alternative promoter targeting by the OriP enhancer.
Introduction
Epstein-Barr virus (EBV) is a human gammaherpesvirus that is the etiological agent of infectious mononucleosis and is commonly associated with B-cell lymphomas, as well as epithelial malignancies [1], [2]. EBV latent infection is observed in endemic Burkitt's lymphoma (BL), the majority of Hodgkin's lymphomas (HL) and in lymphoproliferative diseases associated with immunosuppression [3]. During latency the EBV genome is maintained as a multicopy, chromatinized episome that can adopt one of four different gene expression patterns. These metastable gene expression patterns are generally referred to as latency types and are classified on the basis of EBNA and LMP protein expression [4]. Type 0 latency is defined as latency with no viral gene expression and is found in non-dividing B-cells [5]. In Type I latency, which is observed in BL and BL-derived cell lines, as well as in memory B-cells in a healthy host, EBNA1 is the only viral gene that is expressed [6], [7], [8], [9], [10]. In undifferentiated nasopharyngeal carcinoma (NPC), EBV-associated gastric carcinoma, HL, and T cell lymphomas EBV expresses LMP1 and LMP2 along with EBNA1 and this pattern is referred to as latency type II [11], [12], [13], [14], [15], [16], [17], [18], [19]. In immunoblastic lymphomas associated with immunosuppression and in vitro immortalized lymphoblastoid cell lines (LCLs) EBV is able to express all of the known latency associated genes, EBNA-1, -2, -3A, -3B, -3C, -LP, LMP1 and LMP2 [20]. These different patterns of gene expression are epigenetically stable, in that they are maintained over multiple cell divisions, and generally reflect developmental fate-associated properties of the host-cell.
Latency type gene expression patterns typically correspond to distinct primary transcripts that are initiated from different viral promoters [21], [22], [23], [24]. In type I latency EBNA1 is transcribed starting from Qp in the BamHI/Q region of the EBV genome, while in type III latency the polycistronic mRNA for EBNA proteins is initiated from Cp located in the BamHI C region of the EBV genome [22], [24], [25], [26]. Promoter selection is a critical step for establishing which gene expression program EBV adopts. Both viral and cellular transcription factors are known to regulate these alternative promoters [27], yet many questions still remain.
For example, EBNA1 binding sites at OriP serve as an essential enhancer of Cp in the establishment of type III latency, but it is not known how this enhancer physically targets Cp rather than Qp [28], [29], [30]. It is also known that EBNA1 binding to the Qp transcription initiation site inhibits Qp transcription in type III latency [31], but it is not known how this repression is reversed during the transition to type I latency. Furthermore, epigenetic modifications are known to play an important role in regulating promoter selection [32], [33]. In type I latency Cp is enriched for deacetylated histones and hypermethylated DNA, while Qp is associated with acetylated histones and elevated H3meK4 [34], [35], [36], [37]. In contrast, in type III latency H3meK4 levels are high at Cp along with histone acetylation and DNA hypomethylation while Qp lacks histone modifications associated with active transcription [35], [37]. How these DNA binding factors and chromatin modifications orchestrate the mutually exclusive alternative promoter selection of Cp or Qp remains an important unanswered question for understanding EBV latency control mechanisms.
Several recent findings have revealed that higher-ordered chromosome structures, including DNA loops, chromatin domains, and insulators, play an important role in gene expression programming [38], [39]. The use of new techniques such as Chromosome Conformation Capture (3C) and variations of this assay revealed a complex and diffuse network of long-distance interactions between DNA regions both intra - and inter-chromosomal [40], [41], [42], [43]. Many of these chromatin loops are dynamic and may reflect alternative genome packaging into the nucleus, while other interactions may reflect programmed long-distance interactions that play a crucial role in gene regulation. Identification of cellular factors that regulate loop-formation and long distance interactions support the importance of three-dimensional structures in regulating gene expression [44], [45].
The CCCTC-binding factor (CTCF) has been reported in several studies to be involved in promoting and regulating chromatin loop formation [46], [47], [48], [49], [50]. CTCF is a nuclear DNA binding protein that contains eleven zinc fingers and is well conserved among higher eukaryotes [51], [52], [53]. CTCF is involved in different functions including chromatin boundary formation, DNA loop formation, transcriptional activation and repression, and promoter-enhancer blocking activity [46], [47]. In EBV, CTCF binds at several key regulatory regions [35]. In particular, CTCF can bind between OriP and Cp to negatively regulate transcription initiating at Cp in type I latency [54]. In addition, CTCF can bind upstream of Qp, and this binding is crucial for maintaining transcription activation and protecting Qp from epigenetic silencing [37]. However, the role of CTCF in EBV chromatin conformation has not been addressed. Here, we use 3C and variations of 3C to probe the conformations of the EBV latency control regions in different latency types.
Results
3C analyses of EBV type I and type III genomes reveal distinct conformations of the OriP enhancer region
To determine if the differences in EBV latency type gene expression correlate with differences in three-dimensional organization of the EBV episome, we employed the 3C assay (Fig. 1). EBV positive B-cell lines with stable type I (Mutu I) or type III (Mutu-LCL) latency types were cross-linked with formaldehyde for 30 min and then processed for 3C after fragmentation with MseI restriction enzyme, which generates 342 fragments ranging from 4 bp to ∼4 kbp (Fig. 1A and B). Primers located in MseI fragments containing Cp (10894–11202) or Qp (49780–50493) were used as anchors for the PCR analysis of 3C interactions with several other regions of the EBV genome, including FR, DS, and the CTCF binding site at position 10.6 kb situated between DS and Cp [54]. A third anchor primer at position 35401–36017 was used as a control for 3C product specificity (Fig. 1B). As a positive control for primer efficiency we used a random ligation mixture of MseI digested EBV bacmid DNA in order to contain every possible combination of ligation products.
Fig. 1. OriP region forms alternative chromatin loops in type I and type III latency types. 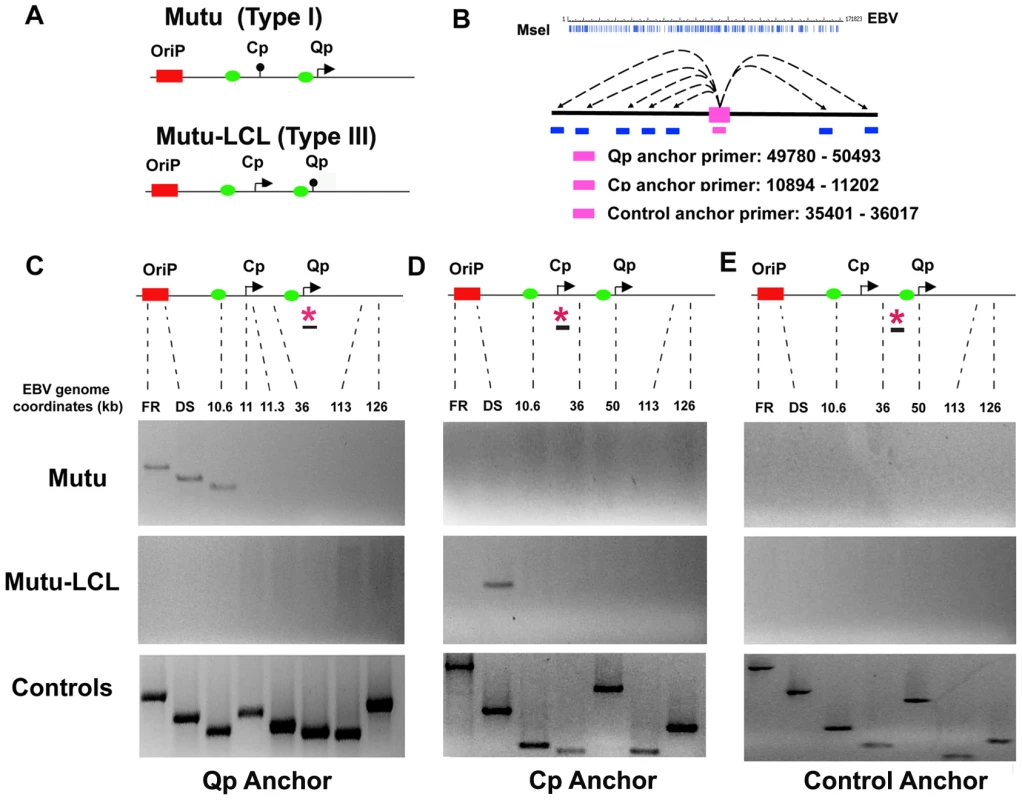
A) Schematic of type I and type III promoter usage and relative position of OriP, Cp, and Qp are indicated. Green boxes indicate known CTCF binding sites. B) Schematic of MseI restriction site map and the anchor primers used for Qp, Cp, and control. EBV genome coordinates for each anchor primer are indicated. C) 3C PCR analysis using the Qp anchor primers and several acceptor regions of EBV genome for FR, DS, 10.6, 11 11.3, 36, 113, and 126 kbp. Representative gel images for Mutu I (top), Mutu-LCL (middle) or control bacmid ligation mixtures (lower) are indicated. D) Representative gel images of 3C PCR analysis using the Cp anchor primer and several acceptor regions of the EBV genome as indicated. E) Representative gel images of 3C PCR analysis using the anchor primer within the fragment chosen as control and several regions of the EBV genome, as indicated. The Qp promoter is active in type I latency, and silenced in type III latency (Fig. 1A). When Qp anchor primers were used for 3C, we observed a strong ligation product between Qp and the OriP region in Mutu (Type I) (Fig. 1C, top panel). Interestingly, Qp interactions were identified for both DS (∼9000) and FR (∼7000) elements of OriP. Qp also interacted with the CTCF-binding site upstream the Cp promoter (∼10,600). Qp did not form detectable 3C ligation products with several other regions of the EBV genome, including regions at 11, 11.3, 36, 113 and 126 kb on the EBV genome. In contrast to type I latency, Qp did not form any 3C products in type III latency (Mutu-LCL), where Qp is transcriptionally silent (Fig. 1C, middle panel). These findings indicate that the 3C ligation products between Qp and OriP region correlate with transcription activity of Qp. All ligation products could be detected in control reactions with bacmid DNA (Fig. 1C, lower panel). These findings suggest that Qp forms specific DNA looping interactions with OriP and the CTCF binding site upstream of Cp in type I cells, but not in type III cells.
We next examined the three dimensional architecture of Cp in both type I and type III (Fig. 1C). In type I latency (Mutu I), where Cp is transcriptionally silent, the Cp anchor failed to form any detectable 3C products with acceptor primers (Fig. 1D, top panel). In type III latency (Mutu-LCL), where Cp is transcriptionally active, we found a predominant 3C interaction between Cp and the DS element of OriP (Fig. 1D, middle panel). All primer sets were capable of amplifying ligation products from control bacmid DNA (Fig. 1D, lower panel).
To verify that 3C interactions between Qp or Cp with OriP were specific and not due to a generic conformation of OriP in type I or type III, we used a primer at 35401 (a region equidistant from both Cp and Qp) as an anchor for the PCR analysis (Fig. 1E). The 35401 anchor primer was unable to form 3C interactions with OriP or any other region of the EBV genome assayed in type I (Fig. 1E, top panel) or type III (Fig. 1E, middle panel) cells. All primer sets were capable of generating PCR products from control ligation reactions with EBV bacmid DNA (Fig. 1E, lower panel). Furthermore, all major 3C products were gel purified, subjected to direct DNA sequencing, and validated for correct ligation products (data not shown). These finding indicate that the 3C products formed between Qp and OriP in type I, and Cp and OriP in type III cells, are sequence and cell-type specific interactions.
To validate the conventional PCR data and to better quantify the 3C interaction products, we analyzed the chromatin conformation by real time PCR using a different set of primer pairs (Fig. 2). When Qp anchor primers were assayed, interactions with OriP (FR and DS) and CTCF-Cp binding site (10.6) were observed in type I (Mutu), but not in type III (Mutu-LCL) (Fig. 2A). When Cp anchor primers were used, an interaction with DS was detected in type III (Mutu-LCL), but not in type I (Mutu) (Fig. 2B). The control anchor at 35.6kb failed to form any specific 3C products in type I or type III cells (Fig. 2C). In all cases, a strong interaction can be detected at regions immediately adjacent to the anchor primers, which is expected in quantitative 3C reactions, but does not indicate a specific chromatin conformation [55]. The specificity of the primer set was tested by agarose gel electrophoresis and dissociation curve analysis (Figures S1-4).
Fig. 2. Quantitative 3C analysis confirms alternative loop formation between type I and type III latency types. 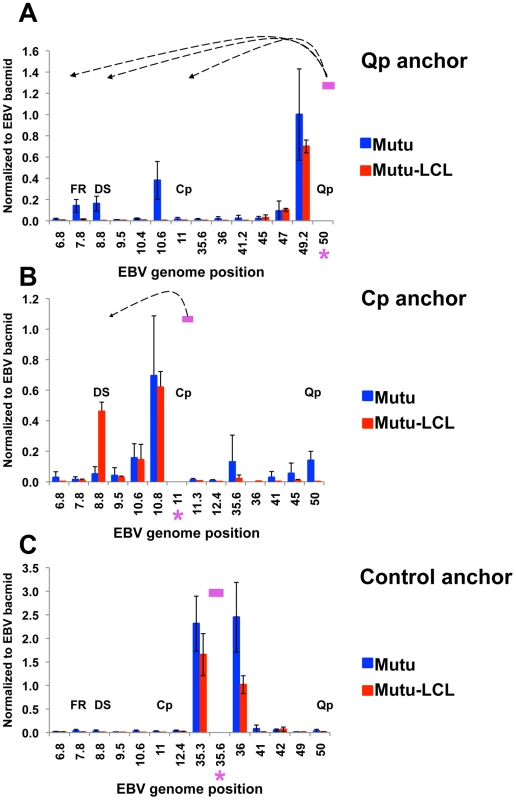
3C assay coupled with quantitative PCR was used to analyze chromatin architecture of the EBV genome in type I (Mutu) and type III (Mutu-LCL) latencies. EBV genome coordinates of all the Mse I fragments tested are indicated on the X-axis. A pink asterisk indicates fragment bearing the anchor primer. Loop formation between the anchor primer (pink box) and an Mse I fragment is indicated by dashed arrows. Data were analyzed using the ΔCt method, and Ct values generated by using 100 ng of EBV bacmid DNA Mse I digested and randomly ligated were used as the control. Data were obtained by three independent experiments and expressed as mean ± SE. Data generated by the anchor fragment self-ligation product were removed from analysis. (A) Quantitative 3C analysis using the anchor primer within the fragment containing Qp and several regions of the EBV genome. (B) Quantitative 3C analysis using the anchor primer within the fragment containing Cp and several regions of the EBV genome. (C) Quantitative 3C analysis using the anchor primer within the fragment chosen as control and several regions of the EBV genome. OriP binding factors EBNA1, ORC2 and TRF2 interact with active promoters for each latency type
To validate the presence of a chromatin loop between OriP and either Cp or Qp promoters in their respective latency types, we used a modified Chromatin Immunoprecipitation (ChIP) assay (Fig. 3). We reasoned that OriP binding factors should associate with either Cp or Qp if they were in close physical proximity due to a DNA loop between these different DNA loci. To measure these potentially indirect protein-DNA interactions, we increased the crosslink time as long as for 3C (30 min) in order to preserve any chromatin loop formation. Previous studies have demonstrated that cellular proteins TRF2 and ORC2, and the viral protein EBNA1 bind at the DS region of OriP in both type I and type III cells [56], [57]. We therefore assayed TRF2, ORC2, and EBNA1 for interactions with either Qp or Cp using this extended cross-linking ChIP assay. The DNA was analyzed by real time PCR using primers specific for different regions of the EBV genome. We found a significant enrichment of ORC2, TRF2 and EBNA1 at Cp in type III but not in type I (Fig. 3A), suggesting that the OriP region is in close proximity to the active (type III) Cp, in agreement with the 3C data. In contrast, ORC2 and TRF2 were enriched at Qp only in type I cells, where Qp is active (Fig. 3B). EBNA1 was able to bind at Qp both in type I and type III, consistent with the known sequence-specific binding of EBNA1 at Qp. It is worth noting that Qp is ∼40 kb from the OriP region and that these ChIP results are consistent with the chromatin loop observed in the 3C assay. As a positive control we tested the enrichment of all three proteins at the DS region of OriP (Fig. 3C). We found that EBNA1, ORC2, and TRF2 bound with high occupancy (>100 fold enrichment) at DS in type I and type III latencies. To rule out any non-specific binding caused by the increase in cross-linking time we evaluated the binding profile for all three proteins at OriLyt (Fig. 3D). We did not detect any significant enrichment for EBNA1, ORC2, or TRF2 with OriLyt in either latency type, indicating that the extended cross-linking time did not cause non-specific ChIP interactions.
Fig. 3. Interaction between OriP binding factors and promoters correlates with promoter activation. 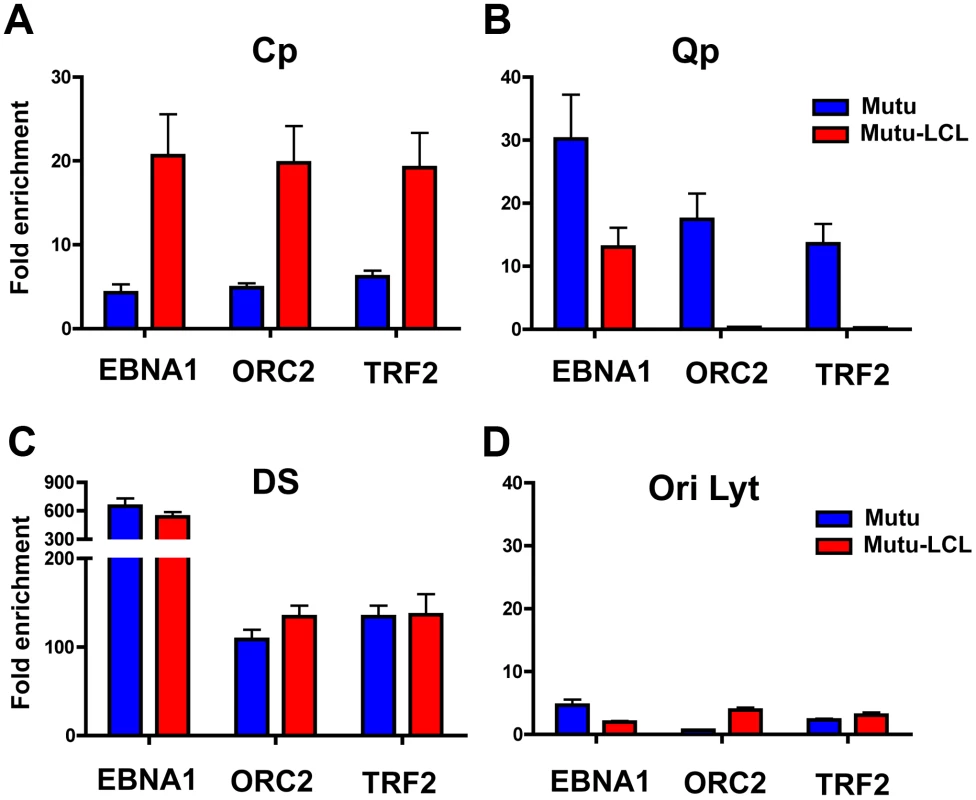
ChIP-qPCR analysis of EBNA1, ORC2, and TRF2 at target regions in type I (Mutu, blue) and type III (Mutu-LCL, red) type latency. Target regions were Cp (panel A), Qp (panel B), DS (panel C), and OriLyt (panel D), as indicated. ChIP-qPCR was performed in conditions that preserved chromatin loop formation. Each bar represents the mean ± SE of three independent experiments. Data are expressed as fold enrichment of specific factor binding relative to that of an IgG control. CTCF is involved in chromatin loop formation in EBV latency types
CTCF can bind upstream of both Cp and Qp, and influence their transcription activity [37], [54]. Since CTCF has been implicated in DNA looping and chromatin organization, we set out to determine if CTCF was involved in chromatin loop formation in EBV. To address this, we developed a new method, which we refer to as 3C-ChIP, that combines 3C and ChIP to address the role of a specific factor in mediating 3C linkages (Fig. 4A). Specifically, cells were subject to formaldehyde cross-linking for 30 min, followed by sonication and ChIP assay with CTCF-specific antibody. The ChIP DNA was then end-repaired by T4 DNA polymerase and ligated to its nearest cross-linked neighbor. Anchor regions for CTCF sites at Qp (49500) or Cp-CTCF (10350) were then used to design inverse PCR primers to amplify cross-linked DNA by circular inverse PCR. The inverse PCR-products were then analyzed by real time PCR using a 384-well array that covers the entire genome of EBV. When Cp-CTCF was used as the anchor in type I cells, we observed a peak at Cp and another one at Qp (Fig. 4B left panel). The peak at Cp-CTCF is mostly the self-ligated product generated during PCR amplification. The peak at Qp suggests that a chromatin loop exists between this region and the CTCF-binding site upstream of Cp. In type III (Mutu-LCL) cell lines, we detected the self-ligation product with Cp-CTCF, but also a strong peak at DS in the OriP region, consistent with the loop observed from 3C data in type III latency (Fig. 4B right panel). When Qp was used as the anchor, a large self-ligation peak appeared, as expected (Fig. 4C). In addition to the self-ligation, interactions between Qp and DS, and to a lesser extent with Cp, were revealed in type I cells (Mutu) (Fig. 4C, left panel). We also observed several minor peaks in the regions around ∼35500, ∼68000 and ∼138000 bp of the EBV genome. Interestingly, these regions all correspond to CTCF binding sites, which were mapped in a previous study [37]. In contrast, the Qp anchor region produced only self-ligation in type III cells (Mutu-LCL) (Fig. 4C right panel). None of the 3C-ChIP peaks were observed when the cross-linking step was eliminated from the protocol, indicating that these products are strictly dependent on CTCF interactions (Fig. S5). These data support the conclusion that DNA interactions between Qp and Cp are distinct in different latency types, and that these interactions may be mediated by CTCF.
Fig. 4. Chromosome Conformation Capture Chromatin Immunoprecipitation (3C-ChIP) analysis shows that CTCF is involved in chromatin looping in EBV. 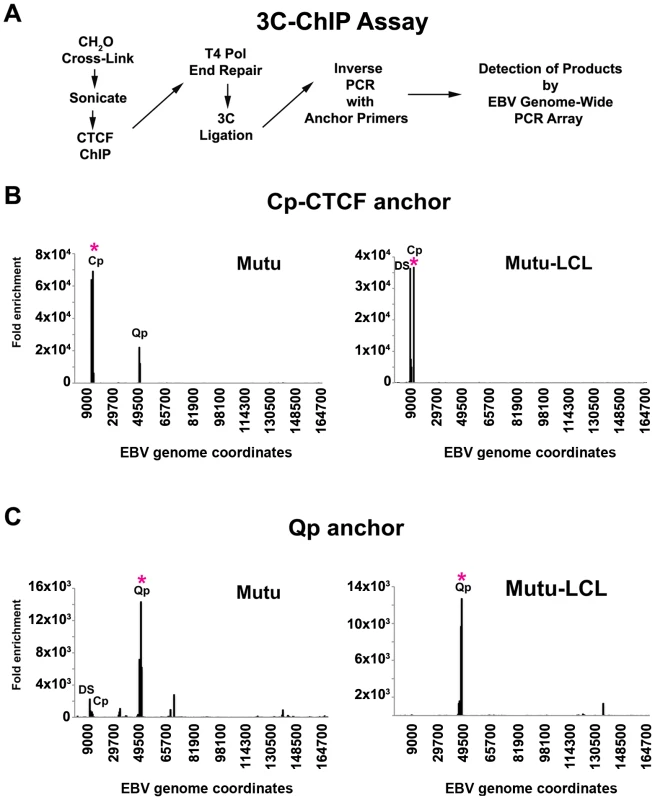
A) Schematic description of the 3C-ChIP assay used for analysis of CTCF mediated interactions with the EBV genome. B and C) 3C-ChIP assay using either Cp-CTCF anchor primers (panels B) or Qp anchor primers (panels C) with Mutu (left panel) or Mutu-LCL (right panel). Anchor primer regions are indicated by an asterisk. EBV genome coordinates of EBV 384-well genome array are indicated on the X-axis. Data are representative of two independent experiments and are expressed as fold enrichment of CTCF binding over that of an IgG control. Only data with a SD less then 10% were considered. The CTCF binding site at Qp is required for chromatin loop formation with OriP
To better study the role of CTCF in loop formation we employed EBV bacmids carrying mutations for the CTCF binding site at Qp and Cp. (Fig. 5A). We have previously shown that the mutations disrupting CTCF binding at either Qp or Cp alter viral transcription control and promoter selection in 293 cells [37], [54]. We therefore introduced the Wt, the ΔCTCF-Qp and the ΔCTCF-Cp bacmid in 293 cells and generated a stable cell line by selecting for hygromycin resistance and GFP expression. Consistent with a previous study, both ΔCTCF-Qp and ΔCTCF-Cp cells adopted a type III latency type expression profile with high levels of EBNA2 expression, Cp promoter activation, and Qp transcription repression, while Wt Bacmids adopted a type I latency expression pattern, which is typical of EBV in 293 cells [58] (Fig. 5B and S6). To examine the contribution of CTCF at Qp and Cp on EBV chromatin architecture, we compared Wt and ΔCTCF genomes using 3C assays (Fig. 5C and D). In Wt cells, we found that Qp is able to establish a strong interaction with OriP and CTCF-Cp (Fig. 5C, top panel), similar to the loop observed in Mutu I cell lines (Fig. 1). Interestingly, in ΔCTCF-Qp and ΔCTCF-Cp cells, loop formation was not detected between Qp and either OriP or CTCF-Cp binding site (Fig. 5C, middle and lower panels). When Cp was used as anchor primer, loop formation was detected between Cp and DS in ΔCTCF-Qp cells (Fig. 5D, middle panel), similar to the loop that was observed in type III Mutu LCL (Fig. 1). This interaction was not detected in Wt bacmid in 293 cells (Fig. 5D, top panel), consistent with Wt cells having a type I phenotype. Surprisingly, Cp was not able to form any loop in ΔCTCF-Cp cells (Fig. 5D, lower panel), suggesting that CTCF at Cp is necessary for the long-distance interaction with OriP. However, loss of CTCF binding had a net positive effect on Cp transcription, perhaps reflecting the ability of CTCF to insulate OriP enhancer activation at Cp. Control anchor primers (35401–36017) showed no significant loop formation in these assays, indicating that these 3C interactions are specific (Fig. S7).
Fig. 5. Mutation in the CTCF binding site at Qp and at Cp alters chromatin conformation and promoter activation. 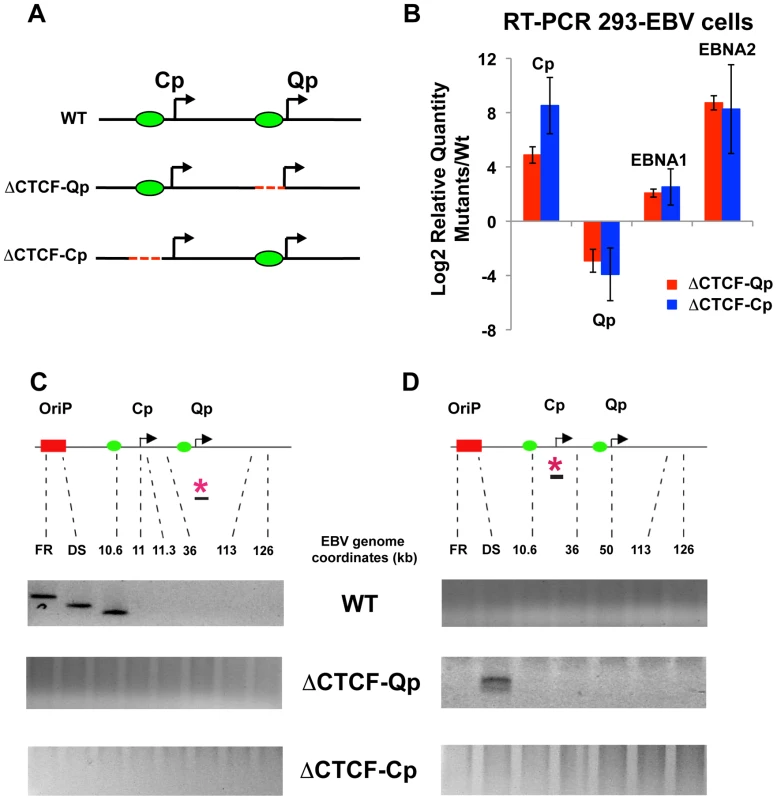
(A) Schematic representation of the EBV genome around the Cp and Qp promoters in the Wt and mutant bacmids. A red dashed line represents mutations that abolish CTCF binding. Green circles indicate known CTCF binding sites. (B) Analysis of mRNA expression for EBNA1 and EBNA2 gene and promoter utilization by RT-qPCR. Data are normalized to GFP protein and expressed as log22ΔΔC t. Each bar represents the mean ± SE of three independent experiments. (C) Representative gel images of 3C PCR analysis using the anchor primer within the fragment containing Qp promoter and several regions of EBV genome for Wt (top panel) or ΔCTCF-Qp (middle panel) or ΔCTCF-Cp (lower panel). (D) Representative gel imagine of 3C PCR analysis using the anchor primer within the fragment containing Cp promoter and several regions of the EBV genome for Wt (top panel) or ΔCTCF-Qp (middle panel) or ΔCTCF-Cp (lower panel). These 3C assays were repeated using different primer sets for more quantitative real-time PCR analysis of the 3C products (Fig. 6). Analysis of the Qp anchor interaction pattern confirmed the loop formation between OriP and the CTCF-Cp binding site in Wt EBV bacmid containing cell lines, but not in ΔCTCF-Qp and ΔCTCF-Cp bacmid genomes (Fig. 6A). Interestingly, in ΔCTCF-Qp cell lines a weak interaction was observed with the region at 41000 (Fig. 6A). Analysis of the Cp anchor region showed interactions between the Cp region and OriP in ΔCTCF-Qp but not in the Wt or ΔCTCF-Cp (Fig. 6B). The control anchor primer did not form any significant peaks at OriP or CTCF-Cp or Qp (Fig. 6C). These data suggest that CTCF binding at Qp and at Cp is important for directing chromatin looping between Qp and OriP, as well as restricting loop formation between Cp and OriP in 293 cell lines. Moreover, loop formation is correlated with promoter transcription activation at Qp.
Fig. 6. Quantitative 3C analysis confirms changes in chromatin architecture in ΔCTCF-Qp and ΔCTCF-Qp compared to Wt EBV bacmid. 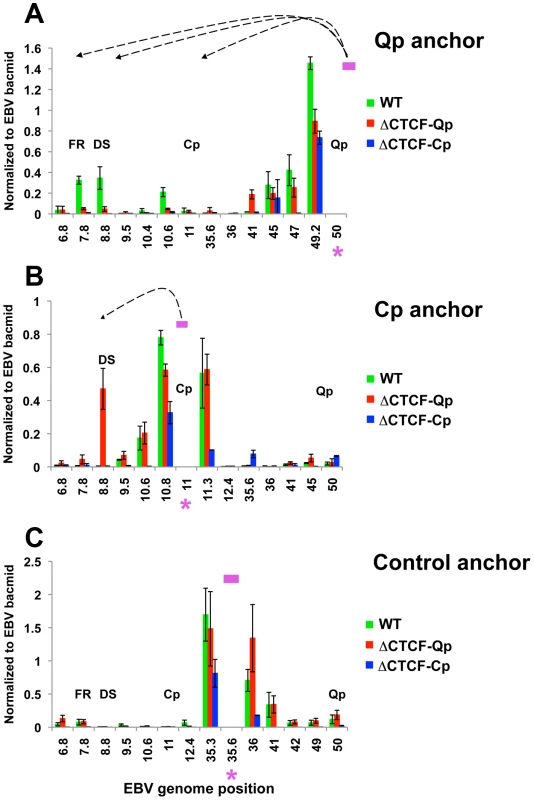
3C assay coupled with quantitative PCR was used to analyze EBV chromatin architecture in 293-EBV Wt bacmid, 293-EBV ΔCTCF-Qp and 293-EBV ΔCTCF-Cp stable cell lines. EBV genome coordinates of all the MseI fragments tested are indicated on the X-axis. A pink asterisk indicates the fragment bearing the anchor primer. Loop formation between the anchor primer (pink box) and a MseI fragment is indicated by dashed arrows. PCR amplification was analyzed using the ΔCt method, and Ct values generated by using 50 ng of EBV bacmid DNA Mse I digested and randomly ligated were used as the control. Data were obtained by three independent experiments and expressed as mean ± SE. Data generated by the anchor fragment self-ligation product were removed from analysis. (A) Quantitative 3C analysis using the anchor primer within the fragment containing Qp and several regions of the EBV genome. (B) Quantitative 3C analysis using the anchor primer within the fragment containing Cp and several regions of EBV genome. (C) Quantitative 3C analysis using the anchor primer within the fragment chosen as control and several regions of the EBV genome. OriP binding factors EBNA1, ORC2, and TRF2, interact with Wt-Qp but not ΔCTCF-Qp
We next investigated if OriP binding factors, EBNA1, ORC2 and TRF2, can interact with either Cp or Qp through loop formation in the EBV bacmids by using an extended cross-linking method for the ChIP assay (Fig. 7). We found that EBNA1, ORC2, and TRF2 were enriched at Cp in ΔCTCF genomes, but not in Wt cells (Fig. 7A), consistent with Cp activation in ΔCTCF cells. In contrast, EBNA1, ORC2, and TRF2 were enriched at Qp in Wt relative to ΔCTCF cells (Fig. 7B), consistent with Qp activation in Wt cells. EBNA1 binding at Qp was also reduced in ΔCTCF genomes, suggesting that CTCF can affect EBNA1 binding at Qp. We also evaluated the binding of EBNA1, ORC2, and TRF2 at DS (Fig. 7C). While EBNA1, ORC2, and TRF2 bound DS in Wt cells, binding was reduced in ΔCTCF-Qp cells, perhaps reflecting cooperative interactions between Qp and DS mediated by CTCF and chromatin looping. No significant binding of EBNA1, ORC2, or TRF2 was detected at the negative control OriLyt region (Fig. 7D). We also confirmed that CTCF binding at Qp was enriched in Wt and eliminated in ΔCTCF-Qp genomes, as expected (data not shown). These findings support the model that CTCF binding at Qp mediates interactions with OriP in type I latency.
Fig. 7. Absence of chromatin loop formation correlates with a loss of interaction between OriP binding factors ORC2 and TRF2 at Qp. 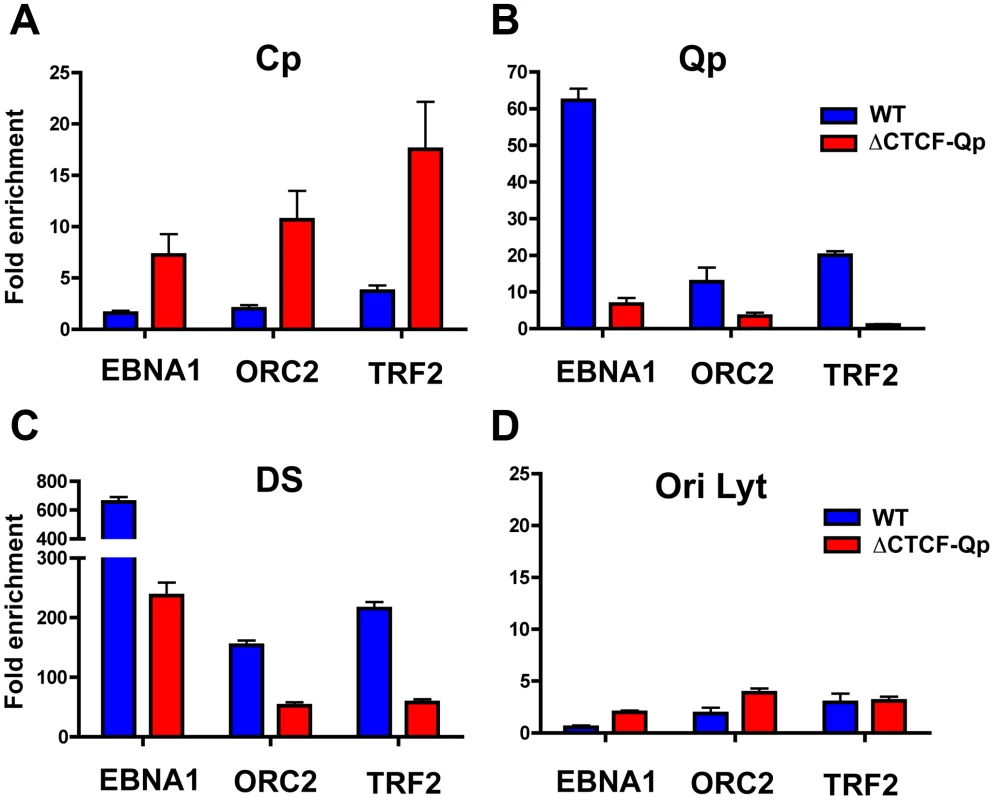
ChIP-qPCR analysis of EBNA1, ORC2 and TRF2 at target regions in Wt and ΔCTCF EBV-positive 293 stable cell lines. ChIP-qPCR was performed in conditions that preserved chromatin loop formation. Each bar represents the mean ± SE of three independent experiments. Data are expressed as fold enrichment of specific factor binding relative to that of an IgG control. Target regions used for PCR amplification were Cp (panel A), Qp (panel B), DS (panel C), and OriLyt (panel D). Depletion of CTCF affects the three-dimensional organization of the EBV genome
To determinate if CTCF levels in cells can influence chromatin loop formation 293-EBV Wt cell lines were transfected with siRNA against CTCF (siCTCF) or non-targeting control (siControl). We used 293-EBV positive cell lines because of their high transfection efficiency compared to human B cells (Fig. 8). siCTCF transfection completely depleted CTCF levels in 293-EBV Wt cells compared to siControl (Fig. 8A). By real time RT-PCR we investigated the effect of siCTCF transfection on promoter utilization and viral genome expression (Fig. 8B). We observed that CTCF depletion caused a moderate increase in Cp activation and a slight decrease in Qp transcription. However, neither EBNA1 nor EBNA2 mRNA levels were significantly affected by siCTCF. 3C analysis was then carried out to determine the effect of siCTCF on chromatin architecture. In 293-EBV Wt cells transfected with siControl we were able to observe chromatin loop formation between Qp and OriP (Fig. 8C, top panel). Interestingly, the chromatin loop between Qp and OriP was completely disrupted in siCTCF transfected cells (Fig. 8, lower panel). Consistent with previous observations, no chromatin loop was detected when Cp was used as the anchor primer in these EBV-293 cell lines (Fig. 8D). 3C assay coupled with real time PCR analysis was then employed to better quantify our analysis (Fig. 8E). Real time PCR confirmed that Qp-OriP interaction was significantly reduced in siCTCF but not in siControl transfected cells (Fig. 8E), left panel. No significant peaks were observed when the Cp region was used as anchor primer (Fig. 8E, right panel). The control anchor primer did not form any significant chromatin loops with OriP or Cp or Qp regions (Fig. S8). These data indicate that CTCF is critical for the establishment and maintenance of the OriP-Qp chromatin loop in EBV-transfected 293 cell lines.
Fig. 8. CTCF siRNA depletion disrupts chromatin architecture of EBV. 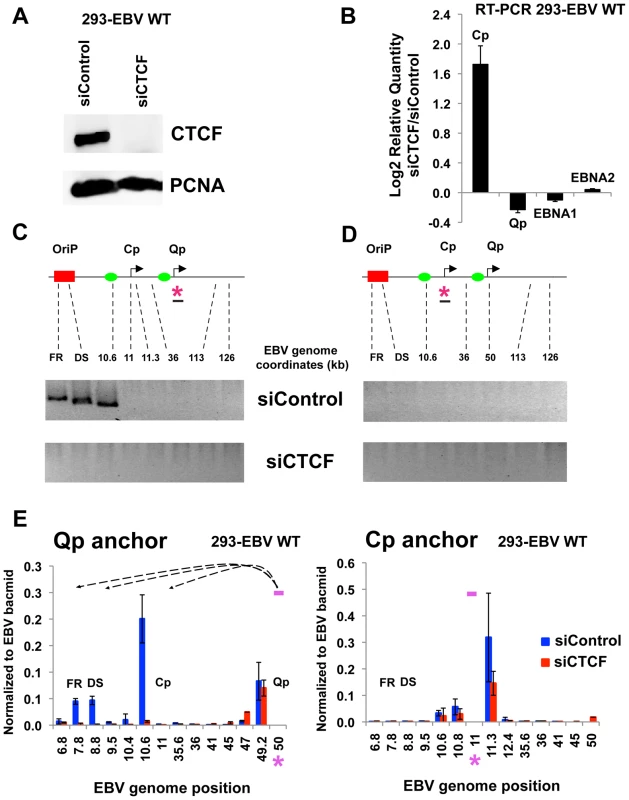
(A) 293-EBV Wt cell lines were transfected with siRNA against CTCF (siCTCF) or a non-targeting control (siControl). After 72 hrs cells were harvested and then analyzed by western blot with antibody to CTCF (top panel) or loading control PCNA (lower panel) to check depletion efficiency. (B) RT-qPCR analysis of promoter utilization and EBNA1 and EBNA2 gene expression after CTCF depletion. Data are normalized to GFP mRNA and expressed as −ΔΔCt values. Each bar represents the mean ± SE of three independent experiments. (C and D) 3C assay was used to analyze chromatin conformation after depletion of CTCF. Representative gel images of 3C PCR analysis using the anchor primer within the fragment containing Qp promoter (C) or Cp promoter (D) and several regions of the EBV genome for 293-EBV Wt cell line transfected with siControl or siCTCF siRNA. Amplified regions are indicated above each gel. (E) 3C assay coupled with quantitative PCR was used to analyze EBV chromatin architecture in 293-EBV Wt bacmid transfected with siControl or siCTCF siRNA, using the anchor primer within the fragment containing Qp promoter (left panel) or Cp promoter (right panel) and several regions of EBV genome. EBV genome coordinates of all the Mse I fragments tested are indicated on the X-axis. A pink asterisk indicates fragment bearing the anchor primer. Loop formation between anchor primer (pink box) and a Mse I fragment is indicated by dashed arrows. PCR amplification was normalized by using 50 ng of EBV bacmid DNA Mse I digested and randomly ligated. Data were obtained by three independent experiments and expressed as mean ± SE. Data generated by the anchor fragment self-ligation product were removed from analysis. Discussion
Chromatin structure is known to play an important role in gene expression by regulating the accessibility of the DNA to transcription factors and RNA polymerase II. Emerging evidence shows that not only the composition of the chromatin fiber, but also the three-dimensional structure of chromatin can influence gene transcription. We and others have shown that epigenetic features, including chromatin structure, play an important role in regulating EBV gene expression during latency [21], [34], [37], [54], [59]. We have also reported that the chromatin-organizing factor CTCF binds at Cp and Qp, and is important for maintaining the appropriate epigenetic pattern and transcription control at these promoters [37], [58]. Here, we explored the hypothesis that different latency types correspond to distinct chromatin conformations. Using 3C methodology, we found that different latency types form distinct chromatin-loops (Fig. 1 and 2). We identified two mutually exclusive loops that form between either OriP and Qp or OriP and Cp. These alternative loops correlate with promoter activity in their respective latency types. Genetic and siRNA depletion studies demonstrated that CTCF binding is essential for loop formation. We propose that chromosome conformation is an important epigenetic feature of latency type, and that CTCF plays a central role in generating EBV chromosome conformation (Fig. 9).
Fig. 9. Model of chromatin conformational control of EBV latency types. 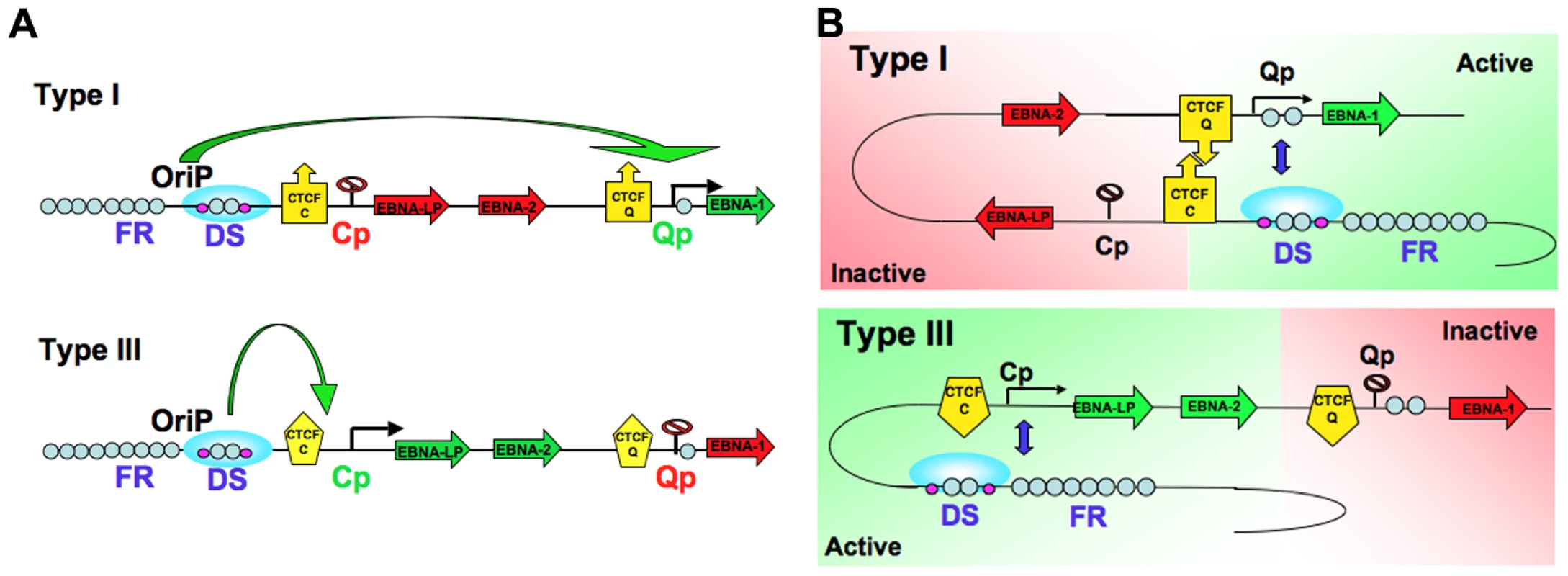
A) Linear model of EBV latency types depicting OriP enhancer interactions with Cp (type III) or Qp (type I). CTCF binding sites are shown as a yellow box with an arrow. B) Conformational model showing OriP recruiting Qp into an active loop and domain in type I cells (top panel), and the alternative conformation where OriP recruits Cp into a loop and active domain in type III cells (lower panel). CTCF has been implicated in chromatin loop formation in multiple species and chromosome contexts [46]. CTCF binds upstream of Qp and Cp in both type I and type III latency, although with some reduced occupancy at Cp in type III latency [37]. Biochemical methods used in this study suggest that CTCF is intimately involved in loop formation between OriP and either Cp or Qp. Using the 3C-ChIP assay, we show that the CTCF at Cp is in close physical proximity to Qp in type I and to DS in type III (Fig. 4B). Similarly, we found that CTCF bound at Qp was in close proximity to DS in type I, but not in type III (Fig. 4C). We also observed that Qp contacts several other regions of the EBV genome, suggesting that EBV genome architecture may be more complex than a single loop between OriP and either Cp or Qp. It is also likely that CTCF links multiple sites in a hub to create more intricate chromatin structures, as has been shown in other model systems [46], [48]. The use of the 3C-ChIP assay may help to elucidate some of these more complex interactions within the EBV chromosome.
Genetic methods were also used to address the role of CTCF in EBV chromatin conformation (Figs. 5–7). EBV bacmids carrying a mutation in the CTCF binding sites at either Qp or Cp revealed a strong correlation between loop formation and promoter activation. Previously, we showed that the ΔCTCF-Qp bacmids have a decrease in Qp utilization and a corresponding increase in EBNA2 gene expression [37]. Here, using 3C methods, we found that ΔCTCF-Qp bacmids fail to form a loop between OriP and Qp, while Wt bacmids form these loops (Fig. 5C and 6A). Moreover, ΔCTCF-Qp bacmids formed an alternative loop between OriP and Cp, suggesting that the failure to form a type I conformation (Qp-OriP) resulted in a type III conformation (Cp-CTCF) (Fig. 5D). The biological implications of this finding are not immediately clear, but suggest that the natural evolution of EBV towards a type I latency requires the ability to form a loop between OriP and Qp, which is mediated by CTCF binding sites. Interestingly, CTCF binding at Cp was also essential for Qp-OriP loop formation, since CTCF-ΔCp bacmids failed to form the OriP-Qp loop, as well as the OriP-Cp loop (Fig. 5D and 6B). This suggests that CTCF (Cp)-CTCF (Qp) interactions may be important for long-distance loop formation between OriP and Qp (Fig. 9). Consistent with this model, siRNA depletion of CTCF led to a complete loss of all measurable loop formation at either Qp or Cp (Fig. 8), suggesting that CTCF is generally required for chromosome conformations measured at these loci.
Other factors, in addition to CTCF, must also contribute to loop formation and promoter selection. Components of OriP are known to contribute to viral chromosome conformation during latency. EBNA1, TRF2, and ORC bind to OriP in both type I and III latency, and have been implicated in chromosome structure [57], [60]. In experiments with extended cross-linking, we showed that EBNA1, TRF2, and ORC are in close proximity to the active promoter, namely Cp in type III and Qp in type I (Fig. 3). EBNA1 is known to form homotypic interactions through its linking regions, and is an essential transcription activator of Cp during B-cell immortalization [28], [61]. Although not directly tested in this study, it seems likely that EBNA1 will be required for loop formation between OriP and Qp. In some 3C experiments, we observed more efficient linkage of Cp to the DS region relative to the FR region (Fig. 2B). This may be due to the additional components that bind to DS, like TRF2 and ORC, or it may be due to the proximity and orientation of DS to Cp. However, EBNA1 is known to promote looping between DS and FR in vitro [62], [63], suggesting that OriP elements are likely to interact with each other and function coordinately in vivo. In addition, the FR can function as an EBNA1-dependent transcriptional enhancer [28], [29], [30], [64], [65]. Since our 3C data can not readily dissect the relative contributions of FR and DS to loop formation, we suggest that OriP functions as a single structural element that forms stable chromatin loops with either Cp in type III or Qp in type I latency.
Chromatin loop structure is likely to be an important epigenetic regulatory component of EBV latency gene expression. How these different loops and conformations are selected remains an important unanswered question. We have shown the CTCF is one essential component required for the establishment and maintenance of DNA loops that form between the OriP enhancer and the promoters at Cp and Qp. EBNA1, and components of OriP, must also be important for these interactions. It is also likely that other epigenetic modifications, including histone tail modifications and DNA methylation, contribute to loop formation, chromatin conformation, and promoter selection. DNA methylation of Cp is known to play a central role in the transcriptional silencing of Cp in type I latency [21], [34], [36], [58], [66], [67]. Furthermore, DNA methylation is known to inhibit CTCF binding at some sites, and the Cp CTCF binding site may be sensitive to changes in DNA methylation. In addition to changes in CTCF DNA binding, variation in CTCF protein levels may also contribute to promoter selection and loop formation [54]. CTCF is also known to be subject to several post-translational modifications, which may also contribute to selective loop formation [68], [69], [70]. Although the precise molecular mechanism of loop formation and promoter selection is not yet elucidated, our findings provide strong evidence that EBV chromatin conformation is an important structural component of latency type determination and, more generally, the establishment of stable epigenetic fates.
Methods
Cells
293 cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum and antibiotic in a 5% CO2 incubator at 37°C. EBV positive Mutu I and Mutu-LCL were cultured in suspension in RPMI 1640 medium supplemented with 10% fetal bovine serum and antibiotics in a 5% CO2 incubator at 37°C. Mutu-LCL were established by primary infection of peripheral blood mononuclear cells (PBMC) with EBV virions generated from stimulated Mutu I cells. 293-EBV Wt, ΔCTCF-Qp, and ΔCTCF-Cp have been previously described [37], [54] and were cultured as adherent cells in DMEM supplemented with 10% fetal bovine serum and 100 µg/liter hygromycin B in 5% CO2 incubator at 37°C. 293-EBV Wt carry a bacmid containing the EBV genome and express a green fluorescent protein (GFP) and hygromycin B drug resistance gene. 293-EBV ΔCTCF-Qp cells carry a bacmid containing the EBV genome mutated at the CTCF binding site at 50082 and express a green fluorescent protein (GFP) and hygromycin B drug resistance gene [37]. 293-EBV ΔCTCF-Cp cells carry a bacmid containing the EBV genome deleted at the CTCF binding site at 10393–10590 and express a green fluorescent protein (GFP) and hygromycin B drug resistance gene [54].
Chromosome Conformation Capture (3C) assay
3C assay followed the protocol from Hagège et al. [71], with minor modifications. EBV-positive cells were filtered through a 70 µm to obtain a single cell preparation. 1×107 cells were then fixed in 1% formaldehyde for 30 min at room temperature. 30 min cross-linking was found to be optimal since 10 min cross-linking resulted in few detectable 3C products (data not shown). The reaction was quenched with 0.125 M glycine and cells were collected by centrifugation at 230 g at 4°C. The pellet was resuspended in 0.5 ml cold lysis buffer (10 mM Tris-HCl, pH 7.5; 10 mM NaCl; 5 mM MgCl2; 0.1 mM EGTA) with freshly added 1× complete protease inhibitors (Roche) and was lysed on ice for 10 min. The nuclei were collected by centrifugation at 500 g for 10 min at 4°C and were resuspended in 0.5 ml of 1.4× Mse I buffer (New England Biolabs), including 0.3% SDS and were incubated for 1 h at 37°C while shaking at 1200 rpm. 2% (final concentration) Triton X-100 was added to the nuclei and then the samples were incubated for 1 hr at 37°C while shaking. 500 U Mse I (50,000 U/ml New England Biolabs) was added to the nuclei and the samples were incubated at 37°C over night while shaking. 10 µl of the samples were collected before and after the Mse I reaction to evaluate digestion efficiency. The reaction was stopped by addition of 1.6% SDS (final concentration) and incubation at 65°C for 30 minutes while shaking at 1200 rpm. The sample was then diluted 10 fold with 1.3× ligation Buffer (Roche) with added 1% Triton X-100 and was incubated for 1 h at 37°C while shaking at 900 rpm. 100 U T4 DNA ligase (5 U/µl Roche) were added to the sample and the reaction was carried at 16°C for 4 hrs followed by 45 min at room temperature. 300 µg of Proteinase K were added to the sample and the reaction was carried at 65°C overnight. RNA was removed by adding 300 µg of RNAse and incubating the sample for 1 h at 37°C. DNA was purified by twice phenol-chloroform extraction and ethanol precipitation. Purified DNA was then analyzed by conventional or quantitative PCR. As control for ligation products EBV Wt bacmid was digested with 10 U of Mse I overnight and then incubated with 10 U T4 DNA-ligase at 16°C overnight. The DNA was extracted by phenol-chloroform and precipitated with ethanol. Purified DNA was then analyzed by conventional or quantitative PCR. For Real time PCR, the ΔCt method was applied for analyzing data, using EBV-bacmid Ct values as control. Ct values were normalized for each primer pair by setting the Ct value of 100 ng of EBV bacmid control random ligation matrix DNA at a value of 1. Primer sequences for conventional PCR are listed in Table S1. Primer sequences for quantitative PCR are listed in Table S2.
Chromatin Immunoprecipitation (ChIP) assay
ChIP assay followed the protocol provided by Upstate Biotechnology, Inc., with minor modifications as previously described [37]. Additional modifications are as follows. Cells were fixed in 1% formaldehyde for 30 minutes. DNAs were sonicated to between 200 - and 350-bp DNA fragments on a Diagenode Bioruptor according to the manufacturer's protocol, and real-time PCR was performed with SYBER green probe in an ABI Prism 7000 using 1/100 to 1/2,500 of the ChIP DNA according to the manufacturer's specified parameters. Chromatin was immunoprecipitated using polyclonal antibodies for CTCF (Millipore), ORC2 (Pharmigen), EBNA1 (which was raised against full-length EBNA1), and TRF2 ( which was raised against full-length TRF2). The primers for amplification were as follows: DS (atgtaaataaaaccgtgacagctcat and ttacccaacgggaagcatatg), and OriLyt (gcccgttgggtttcattaag and ccaaatctcgcggacctcta) and Qp (ggctcacgaagcgagac and acaggacctgcgttatagcc) and Cp (gccgtggaaaaaaatttatgg and cgccaacaaggttcaattttct).
Chromosome Conformation Capture Chromatin Immunoprecipitation (3C-ChIP) assay
EBV positive cells were fixed in 1% formaldehyde for 30 minutes at room temperature. The reaction was quenched by adding 0.125 M glycine (final concentration) and the cells were collected by centrifugation at 400 g for 5 min, washed twice with PBS and then 1×107 were resuspended in 1 ml cold lysis buffer (1% SDS; 10 mM EDTA; 50 mM Tris-HCl, pH 8) with 1× complete protease inhibitors (Roche) and lysed on ice for 10 min. DNA was sonicated to between 200 - and 350-bp DNA fragments on a Diagenode Bioruptor according to manufacturer's protocol. 100 µl sonicated cell lysate were then diluted 10 fold with immunoprecipitation (IP) buffer (0.01% SDS; 1.1% Triton X-100; 1.2 mM EDTA; 16.7 mM Tris-HCl, pH 8; 167 mM NaCl) and then pre-cleared by adding 50 µl 50% pre-blocked Protein A containing 25 µg of the heat-denaturated ssDNA and the samples were incubated for 30 min at 4°C with gentle rotation. The mixture was then centrifuged for 5 min at 750 g at 4°C and then the supernatant was carefully transferred into a new tube. Chromatin was precipitated by incubating the samples with 2.5 µg of antibody for CTCF (Millipore) or normal rabbit IgG (Santa Cruz) as negative control overnight at 4°C with rotation. Immune complexes were collected by incubation with 50 µl 50% slurry protein A sepharose beads (GE Healthcare) for 3 hrs at 4°C with rotation. Beads were collected by centrifugation at 800 g for 2 min and the washed once with 1 ml Low Salt buffer (0.1%SDS; 1% Triton X-100; 2 mM EDTA; 20 mM Tris-HCl, pH 8; 150 mM NaCl), then once with 1 ml High Salt buffer (0.1%SDS; 1% Triton X-100; 2 mM EDTA; 20 mM Tris-HCl, pH 8; 500 mM NaCl), then once with 1 ml LiCl buffer (250 mM LiCl; 1% NP-40; 1% Na-deoxycolate; 1 mM EDTA; 10 mM Tris-HCl, pH 8.0). Proteinase inhibitors cocktail (Sigma) was added to all the washing buffers before use. Before to eluting the immune complexes from beads we repaired the DNA ends generated by sonication in order to be ligated by T4 DNA ligation. The protein A beads were collected after the last wash by centrifugation at 800 g for 5 min and then resuspended into 100 µl kinase buffer [50 mM Tris-HCl, pH 7.5; 10 mM MgCl2; 1 mM DTT; 1 mM ATP; 35 µM dNTP; 6 U Klenow enzyme (Roche); 5 U T4 polynucleotide Kinase (Roche); 5 U T4 DNA polymerase (Roche)] and incubate for 90 minutes at 37°C with while shaking. The beads were centrifuged at 800 g for 3 minutes and then resuspended in 1 ml of 1× Ligation buffer (Roche) and 50 U T4 DNA ligase were added. The samples were then incubated at 16°C while shaking overnight. The beads were collected by centrifugation, washed twice with 1 ml TE and then resuspended in 150 µl Elution Buffer (1%SDS/TE) and incubated for 30 minutes at 65°C while shaking. The beads were centrifuged at 800 g for 5 minutes and the supernatant was carefully transferred into a new tube and incubated at 65°C overnight. Samples were then incubated with 45 µg Proteinase K for 3 hours at 50°C. After twice phenol/chloroform extraction and ethanol precipitation, purified DNA was amplified by nested PCR. For the first-round amplification we used High fidelity PlatinumTaq DNA Polymerase (Invitrogen) according to the manufacturer. The PCR conditions were the following: 94°C for 2 min followed by 35 cycles of 94°C for 30 s, 55°C for 30 s and 68°C for 1 min. For the second-round PCR 100 ng of first-round PCR DNA was used as template for Taq DNA polymerase (Roche). PCR conditions were the following: 94°C for 2 min followed by 30 cycles of 94°C for 30 s, 55°C for 30 s and 72°C for 1 min. Nested inverse primer sequences for both first and second round PCR are listed in Table S3. DNA generated by nested PCR was then analyzed by quantitative EBV-genome wide 384-well array.
RNA extraction and RT-PCR
RNA was extracted from 5×106 cells using the Qiagen RNA Extraction Kit according to the manufacturer's protocol (Qiagen). After extraction, the RNA was incubated with 2 U DNAse I at 37°C for 30 minutes, followed by the inactivation of the enzyme at 65°C for 10 minutes. The RNA was quantified and 2 µg of RNA was reverse transcribed using Super Script II Reverse Transcriptase from Invitrogen. 50 ng of cDNA was then analyzed by real time PCR, using the ΔCt method, using GFP gene as the internal control. Primer sequences are listed in Table S4.
siRNA mediated knockout of CTCF
293 cells containing EBV bacmid, at the density of 2×106 cells in 10 cm plate, were transfected with 50 pmol of short interfering RNA (siRNA) targeting CTCF (siD-020165-19) or non-targeting siRNA (si D-001810-01) (Dharmacon) as control, using DharmaFECT transfection reagent, according to manufacture's protocol. After 3 days post transfection cells were treated for 3C analysis as described above. Knockout efficiency was evaluated by western blot analysis.
Supporting Information
Zdroje
1. KieffE 2007 Epstein-Barr Virus and its replication.; FieldsBNKnipeDMHowleyPM Philadelphia Wolters Kluwer Health/Lippincott Williams & Wilkins 2 v. (xix, 3091, 3086 p.)
2. RickinsonABKieffE 2007 Epstein-Barr Virus.; FieldsBNKnipeDMHowleyPM Philadelphia Wolters Kluwer Health/Lippincott Williams & Wilkins 2 v. (xix, 3091, 3086 p.)
3. YoungLSRickinsonAB 2004 Epstein-Barr virus: 40 years on. Nat Rev Cancer 4 757 768
4. RoweMLearALCroom-CarterDDaviesAHRickinsonAB 1992 Three pathways of Epstein-Barr virus gene activation from EBNA1-positive latency in B lymphocytes. J Virol 66 122 131
5. MiyashitaEMYangBLamKMCrawfordDHThorley-LawsonDA 1995 A novel form of Epstein-Barr virus latency in normal B cells in vivo. Cell 80 593 601
6. BabcockGJDeckerLLVolkMThorley-LawsonDA 1998 EBV persistence in memory B cells in vivo. Immunity 9 395 404
7. BabcockGJHochbergDThorley-LawsonAD 2000 The expression pattern of Epstein-Barr virus latent genes in vivo is dependent upon the differentiation stage of the infected B cell. Immunity 13 497 506
8. KellyGBellARickinsonA 2002 Epstein-Barr virus-associated Burkitt lymphomagenesis selects for downregulation of the nuclear antigen EBNA2. Nat Med 8 1098 1104
9. QuLRoweDT 1992 Epstein-Barr virus latent gene expression in uncultured peripheral blood lymphocytes. J Virol 66 3715 3724
10. TierneyRJStevenNYoungLSRickinsonAB 1994 Epstein-Barr virus latency in blood mononuclear cells: analysis of viral gene transcription during primary infection and in the carrier state. J Virol 68 7374 7385
11. ShibataDWeissLM 1992 Epstein-Barr virus-associated gastric adenocarcinoma. Am J Pathol 140 769 774
12. Raab-TraubN 2002 Epstein-Barr virus in the pathogenesis of NPC. Semin Cancer Biol 12 431 441
13. FahraeusRFuHLErnbergIFinkeJRoweM 1988 Expression of Epstein-Barr virus-encoded proteins in nasopharyngeal carcinoma. Int J Cancer 42 329 338
14. GilliganKSatoHRajaduraiPBussonPYoungL 1990 Novel transcription from the Epstein-Barr virus terminal EcoRI fragment, DIJhet, in a nasopharyngeal carcinoma. J Virol 64 4948 4956
15. YoungLSDawsonCWClarkDRupaniHBussonP 1988 Epstein-Barr virus gene expression in nasopharyngeal carcinoma. J Gen Virol 69 Pt 5 1051 1065
16. ImaiSKoizumiSSugiuraMTokunagaMUemuraY 1994 Gastric carcinoma: monoclonal epithelial malignant cells expressing Epstein-Barr virus latent infection protein. Proc Natl Acad Sci U S A 91 9131 9135
17. NiedobitekGHansmannMLHerbstHYoungLSDienemannD 1991 Epstein-Barr virus and carcinomas: undifferentiated carcinomas but not squamous cell carcinomas of the nasopharynx are regularly associated with the virus. J Pathol 165 17 24
18. PallesenGHamilton-DutoitSJRoweMYoungLS 1991 Expression of Epstein-Barr virus latent gene products in tumour cells of Hodgkin's disease. Lancet 337 320 322
19. ChiangAKTaoQSrivastavaGHoFC 1996 Nasal NK - and T-cell lymphomas share the same type of Epstein-Barr virus latency as nasopharyngeal carcinoma and Hodgkin's disease. Int J Cancer 68 285 290
20. FarrellPJ 1995 Epstein-Barr virus immortalizing genes. Trends Microbiol 3 105 109
21. MinarovitsJ 2006 Epigenotypes of latent herpesvirus genomes. Curr Top Microbiol Immunol 310 61 80
22. SchaeferBCStromingerJLSpeckSH 1995 Redefining the Epstein-Barr virus-encoded nuclear antigen EBNA-1 gene promoter and transcription initiation site in group I Burkitt lymphoma cell lines. Proc Natl Acad Sci U S A 92 10565 10569
23. WoisetschlaegerMJinXWYandavaCNFurmanskiLAStromingerJL 1991 Role for the Epstein-Barr virus nuclear antigen 2 in viral promoter switching during initial stages of infection. Proc Natl Acad Sci U S A 88 3942 3946
24. WoisetschlaegerMYandavaCNFurmanskiLAStromingerJLSpeckSH 1990 Promoter switching in Epstein-Barr virus during the initial stages of infection of B lymphocytes. Proc Natl Acad Sci U S A 87 1725 1729
25. NonkweloCSkinnerJBellARickinsonASampleJ 1996 Transcription start sites downstream of the Epstein-Barr virus (EBV) Fp promoter in early-passage Burkitt lymphoma cells define a fourth promoter for expression of the EBV EBNA-1 protein. J Virol 70 623 627
26. BodescotMPerricaudetMFarrellPJ 1987 A promoter for the highly spliced EBNA family of RNAs of Epstein-Barr virus. J Virol 61 3424 3430
27. TierneyRNagraJHutchingsIShannon-LoweCAltmannM 2007 Epstein-Barr virus exploits BSAP/Pax5 to achieve the B-cell specificity of its growth-transforming program. J Virol 81 10092 10100
28. AltmannMPichDRuissRWangJSugdenB 2006 Transcriptional activation by EBV nuclear antigen 1 is essential for the expression of EBV's transforming genes. Proc Natl Acad Sci U S A 103 14188 14193
29. ReismanDSugdenB 1986 trans activation of an Epstein-Barr viral transcriptional enhancer by the Epstein-Barr viral nuclear antigen 1. Mol Cell Biol 5 3838 3846
30. PuglielliMTWoisetschlaegerMSpeckSH 1996 oriP is essential for EBNA gene promoter activity in Epstein-Barr virus-immortalized lymphoblastoid cell lines. J Virol 70 5758 5768
31. YoshiokaMCrumMMSampleJT 2008 Autorepression of Epstein-Barr virus nuclear antigen 1 expression by inhibition of pre-mRNA processing. J Virol 82 1679 1687
32. TakacsMSalamonDMyohanenSLiHSegesdiJ 2001 Epigenetics of latent Epstein-Barr virus genomes: high resolution methylation analysis of the bidirectional promoter region of latent membrane protein 1 and 2B genes. Biol Chem 382 699 705
33. TemperaILiebermanPM 2010 Chromatin organization of gammaherpesvirus latent genomes. Biochim Biophys Acta 1799 236 245
34. AmbinderRFRobertsonKDTaoQ 1999 DNA methylation and the Epstein-Barr virus. Semin Cancer Biol 9 369 375
35. DayLChauCMNebozhynMRennenkampAJShoweM 2007 Chromatin Profiling Of Epstein-Barr Virus Latency Control Region. J Virol 81 6389 401
36. RobertsonKDMannsASwinnenLJZongJCGulleyML 1996 CpG methylation of the major Epstein-Barr virus latency promoter in Burkitt's lymphoma and Hodgkin's disease. Blood 88 3129 3136
37. TemperaIWiedmerADheekolluJLiebermanPM 2010 CTCF prevents the epigenetic drift of EBV latency promoter Qp. PLoS Pathog 6 e1001048
38. GurudattaBVCorcesVG 2009 Chromatin insulators: lessons from the fly. Brief Funct Genomic Proteomic 8 276 282
39. BusheyAMDormanERCorcesVG 2008 Chromatin insulators: regulatory mechanisms and epigenetic inheritance. Mol Cell 32 1 9
40. LanctotCCheutinTCremerMCavalliGCremerT 2007 Dynamic genome architecture in the nuclear space: regulation of gene expression in three dimensions. Nat Rev Genet 8 104 115
41. DekkerJRippeKDekkerMKlecknerN 2002 Capturing chromosome conformation. Science 295 1306 1311
42. FraserPBickmoreW 2007 Nuclear organization of the genome and the potential for gene regulation. Nature 447 413 417
43. SextonTSchoberHFraserPGasserSM 2007 Gene regulation through nuclear organization. Nat Struct Mol Biol 14 1049 1055
44. KageyMHNewmanJJBilodeauSZhanYOrlandoDA 2010 Mediator and cohesin connect gene expression and chromatin architecture. Nature 467 430 5
45. LingJQLiTHuJFVuTHChenHL 2006 CTCF mediates interchromosomal colocalization between Igf2/H19 and Wsb1/Nf1. Science 312 269 272
46. PhillipsJECorcesVG 2009 CTCF: master weaver of the genome. Cell 137 1194 1211
47. OhlssonRRenkawitzRLobanenkovV 2001 CTCF is a uniquely versatile transcription regulator linked to epigenetics and disease. Trends Genet 17 520 527
48. OhlssonRLobanenkovVKlenovaE 2010 Does CTCF mediate between nuclear organization and gene expression? Bioessays 32 37 50
49. MajumderPBossJM 2010 CTCF controls expression and chromatin architecture of the human major histocompatibility complex class II locus. Mol Cell Biol 30 4211 4223
50. SplinterEHeathHKoorenJPalstraRJKlousP 2006 CTCF mediates long-range chromatin looping and local histone modification in the beta-globin locus. Genes Dev 20 2349 2354
51. FilippovaGNFagerlieSKlenovaEMMyersCDehnerY 1996 An exceptionally conserved transcriptional repressor, CTCF, employs different combinations of zinc fingers to bind diverged promoter sequences of avian and mammalian c-myc oncogenes. Mol Cell Biol 16 2802 2813
52. LobanenkovVVNicolasRHAdlerVVPatersonHKlenovaEM 1990 A novel sequence-specific DNA binding protein which interacts with three regularly spaced direct repeats of the CCCTC-motif in the 5′-flanking sequence of the chicken c-myc gene. Oncogene 5 1743 1753
53. KlenovaEMNicolasRHPatersonHFCarneAFHeathCM 1993 CTCF, a conserved nuclear factor required for optimal transcriptional activity of the chicken c-myc gene, is an 11-Zn-finger protein differentially expressed in multiple forms. Mol Cell Biol 13 7612 7624
54. ChauCMZhangXYMcMahonSBLiebermanPM 2006 Regulation of Epstein-Barr virus latency type by the chromatin boundary factor CTCF. J Virol 80 5723 5732
55. DekkerJ 2006 The three ‘C' s of chromosome conformation capture: controls, controls, controls. Nat Methods 3 17 21
56. DengZLezinaLChenCJShtivelbandSSoW 2002 Telomeric proteins regulate episomal maintenance of Epstein-Barr virus origin of plasmid replication. Mol Cell 9 493 503
57. DengZAtanasiuCBurgJSBroccoliDLiebermanPM 2003 Telomere repeat binding factors TRF1, TRF2, and hRAP1 modulate replication of Epstein-Barr virus OriP. J Virol 77 11992 12001
58. PaulsonEJFingerothJDYatesJLSpeckSH 2002 Methylation of the EBV genome and establishment of restricted latency in low-passage EBV-infected 293 epithelial cells. Virology 299 109 121
59. AlazardNGruffatHHiriartESergeantAManetE 2003 Differential hyperacetylation of histones H3 and H4 upon promoter-specific recruitment of EBNA2 in Epstein-Barr virus chromatin. J Virol 77 8166 8172
60. AtanasiuCDengZWiedmerANorseenJLiebermanPM 2006 ORC binding to TRF2 stimulates OriP replication. EMBO Rep 7 716 721
61. MackeyDMiddletonTSugdenB 1995 Multiple regions within EBNA1 can link DNAs. J Virol 69 6199 6208
62. MiddletonTSugdenB 1992 EBNA1 can link the enhancer element to the initiator element of the Epstein-Barr virus plasmid origin of DNA replication. J Virol 66 489 495
63. FrappierLO'DonnellM 1991 Overproduction, purification, and characterization of EBNA1, the origin binding protein of Epstein-Barr virus. J Biol Chem 266 7819 7826
64. GahnTASugdenB 1995 An EBNA-1-dependent enhancer acts from a distance of 10 kilobase pairs to increase expression of the Esptein-Barr virus LMP Gene. J Virol 69 2633 2636
65. WysokenskiDAYatesJL 1989 Multiple EBNA1-binding sites are required to form an EBNA1-dependent enhancer and to activate a minimal replicative origin within oriP of Epstein-Barr virus. J Virol 63 2657 2666
66. PaulsonEJSpeckSH 1999 Differential methylation of Epstein-Barr virus latency promoters facilitates viral persistence in healthy seropositive individuals. J Virol 73 9959 9968
67. ErnbergIFalkKMinarovitsJBussonPTurszT 1989 The role of methylation in the phenotype modulation of Epstein-Barr nuclear antigen 2 and latent membrane protein genes in cells latently infected with Epstein-Barr virus. J gen Virol 70 2989 3002
68. YuWGinjalaVPantVChernukhinIWhiteheadJ 2004 Poly(ADP-ribosyl)ation regulates CTCF-dependent chromatin insulation. Nat Genet 36 1105 1110
69. El-KadyAKlenovaE 2005 Regulation of the transcription factor, CTCF, by phosphorylation with protein kinase CK2. FEBS Lett 579 1424 1434
70. MacphersonMJBeattyLGZhouWDuMSadowskiPD 2008 The CTCF insulator protein is post-translationally modified by SUMO. Mol Cell Biol
71. HagegeHKlousPBraemCSplinterEDekkerJ 2007 Quantitative analysis of chromosome conformation capture assays (3C-qPCR). Nat Protoc 2 1722 1733
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek A Typhimurium-Typhi Genomic Chimera: A Model to Study Vi Polysaccharide Capsule Function In VivoČlánek SUMO Pathway Dependent Recruitment of Cellular Repressors to Herpes Simplex Virus Type 1 GenomesČlánek A Structural Model for Binding of the Serine-Rich Repeat Adhesin GspB to Host Carbohydrate ReceptorsČlánek Dynamic Evolution of Pathogenicity Revealed by Sequencing and Comparative Genomics of 19 IsolatesČlánek Widespread Endogenization of Genome Sequences of Non-Retroviral RNA Viruses into Plant GenomesČlánek The Cost of Virulence: Retarded Growth of Typhimurium Cells Expressing Type III Secretion System 1Článek A Role for the Chemokine RANTES in Regulating CD8 T Cell Responses during Chronic Viral Infection
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 7- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- What Do We Really Know about How CD4 T Cells Control ?
- “Persisters”: Survival at the Cellular Level
- E6 and E7 from Beta Hpv38 Cooperate with Ultraviolet Light in the Development of Actinic Keratosis-Like Lesions and Squamous Cell Carcinoma in Mice
- Selection of Resistant Bacteria at Very Low Antibiotic Concentrations
- The Extracytoplasmic Domain of the Ser/Thr Kinase PknB Binds Specific Muropeptides and Is Required for PknB Localization
- CD39/Adenosine Pathway Is Involved in AIDS Progression
- Hypoxia and a Fungal Alcohol Dehydrogenase Influence the Pathogenesis of Invasive Pulmonary Aspergillosis
- One Is Enough: Effective Population Size Is Dose-Dependent for a Plant RNA Virus
- Effects of Interferon-α/β on HBV Replication Determined by Viral Load
- A Typhimurium-Typhi Genomic Chimera: A Model to Study Vi Polysaccharide Capsule Function In Vivo
- Dual Chaperone Role of the C-Terminal Propeptide in Folding and Oligomerization of the Pore-Forming Toxin Aerolysin
- Rotavirus Stimulates Release of Serotonin (5-HT) from Human Enterochromaffin Cells and Activates Brain Structures Involved in Nausea and Vomiting
- Dissociation of Infectivity from Seeding Ability in Prions with Alternate Docking Mechanism
- The Impact of Recombination on dN/dS within Recently Emerged Bacterial Clones
- The Regulation of Sulfur Metabolism in
- Illumination of Parainfluenza Virus Infection and Transmission in Living Animals Reveals a Tissue-Specific Dichotomy
- A Permeable Cuticle Is Associated with the Release of Reactive Oxygen Species and Induction of Innate Immunity
- A Concerted Action of Hepatitis C Virus P7 and Nonstructural Protein 2 Regulates Core Localization at the Endoplasmic Reticulum and Virus Assembly
- SUMO Pathway Dependent Recruitment of Cellular Repressors to Herpes Simplex Virus Type 1 Genomes
- Re-localization of Cellular Protein SRp20 during Poliovirus Infection: Bridging a Viral IRES to the Host Cell Translation Apparatus
- Divergent Effects of Human Cytomegalovirus and Herpes Simplex Virus-1 on Cellular Metabolism
- A Structural Model for Binding of the Serine-Rich Repeat Adhesin GspB to Host Carbohydrate Receptors
- Transformation of Natural Genetic Variation into Genomes
- EBV Latency Types Adopt Alternative Chromatin Conformations
- Global mRNA Degradation during Lytic Gammaherpesvirus Infection Contributes to Establishment of Viral Latency
- Dynamic Evolution of Pathogenicity Revealed by Sequencing and Comparative Genomics of 19 Isolates
- Microbial Virulence as an Emergent Property: Consequences and Opportunities
- Widespread Endogenization of Genome Sequences of Non-Retroviral RNA Viruses into Plant Genomes
- Structural Basis of Chemokine Sequestration by CrmD, a Poxvirus-Encoded Tumor Necrosis Factor Receptor
- Cross-Species Transmission of a Novel Adenovirus Associated with a Fulminant Pneumonia Outbreak in a New World Monkey Colony
- An Interaction between KSHV ORF57 and UIF Provides mRNA-Adaptor Redundancy in Herpesvirus Intronless mRNA Export
- Elevated 17β-Estradiol Protects Females from Influenza A Virus Pathogenesis by Suppressing Inflammatory Responses
- The Role of IL-15 Deficiency in the Pathogenesis of Virus-Induced Asthma Exacerbations
- Fluorescence Lifetime Imaging Unravels Metabolism and Its Crosstalk with the Host Cell
- Programmed Death (PD)-1-Deficient Mice Are Extremely Sensitive to Murine Hepatitis Virus Strain-3 (MHV-3) Infection
- Hemoglobin Promotes Nasal Colonization
- Crystallography of a Lewis-Binding Norovirus, Elucidation of Strain-Specificity to the Polymorphic Human Histo-Blood Group Antigens
- The Cost of Virulence: Retarded Growth of Typhimurium Cells Expressing Type III Secretion System 1
- A Genome-Wide Approach to Discovery of Small RNAs Involved in Regulation of Virulence in
- Requires Glycerol for Maximum Fitness During The Tick Phase of the Enzootic Cycle
- C Metabolic Flux Analysis Identifies an Unusual Route for Pyruvate Dissimilation in Mycobacteria which Requires Isocitrate Lyase and Carbon Dioxide Fixation
- A Role for the Chemokine RANTES in Regulating CD8 T Cell Responses during Chronic Viral Infection
- Glycosaminoglycans and Sialylated Glycans Sequentially Facilitate Merkel Cell Polyomavirus Infectious Entry
- Regulation of Stomatal Tropism and Infection by Light in : Evidence for Coordinated Host/Pathogen Responses to Photoperiod?
- Multiple Translocation of the Effector Gene among Chromosomes of the Rice Blast Fungus and Related Species
- Comparative Genomics Yields Insights into Niche Adaptation of Plant Vascular Wilt Pathogens
- Unique Cell Adhesion and Invasion Properties of O:3, the Most Frequent Cause of Human Yersiniosis
- C-Terminal Region of EBNA-2 Determines the Superior Transforming Ability of Type 1 Epstein-Barr Virus by Enhanced Gene Regulation of LMP-1 and CXCR7
- Novel Chikungunya Vaccine Candidate with an IRES-Based Attenuation and Host Range Alteration Mechanism
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Requires Glycerol for Maximum Fitness During The Tick Phase of the Enzootic Cycle
- Comparative Genomics Yields Insights into Niche Adaptation of Plant Vascular Wilt Pathogens
- The Role of IL-15 Deficiency in the Pathogenesis of Virus-Induced Asthma Exacerbations
- “Persisters”: Survival at the Cellular Level
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



