-
Články
Reklama
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
ReklamaMultiple Translocation of the Effector Gene among Chromosomes of the Rice Blast Fungus and Related Species
Magnaporthe oryzae is the causal agent of rice blast disease, a devastating problem worldwide. This fungus has caused breakdown of resistance conferred by newly developed commercial cultivars. To address how the rice blast fungus adapts itself to new resistance genes so quickly, we examined chromosomal locations of AVR-Pita, a subtelomeric gene family corresponding to the Pita resistance gene, in various isolates of M. oryzae (including wheat and millet pathogens) and its related species. We found that AVR-Pita (AVR-Pita1 and AVR-Pita2) is highly variable in its genome location, occurring in chromosomes 1, 3, 4, 5, 6, 7, and supernumerary chromosomes, particularly in rice-infecting isolates. When expressed in M. oryzae, most of the AVR-Pita homologs could elicit Pita-mediated resistance, even those from non-rice isolates. AVR-Pita was flanked by a retrotransposon, which presumably contributed to its multiple translocation across the genome. On the other hand, family member AVR-Pita3, which lacks avirulence activity, was stably located on chromosome 7 in a vast majority of isolates. These results suggest that the diversification in genome location of AVR-Pita in the rice isolates is a consequence of recognition by Pita in rice. We propose a model that the multiple translocation of AVR-Pita may be associated with its frequent loss and recovery mediated by its transfer among individuals in asexual populations. This model implies that the high mobility of AVR-Pita is a key mechanism accounting for the rapid adaptation toward Pita. Dynamic adaptation of some fungal plant pathogens may be achieved by deletion and recovery of avirulence genes using a population as a unit of adaptation.
Published in the journal: . PLoS Pathog 7(7): e32767. doi:10.1371/journal.ppat.1002147
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002147Summary
Magnaporthe oryzae is the causal agent of rice blast disease, a devastating problem worldwide. This fungus has caused breakdown of resistance conferred by newly developed commercial cultivars. To address how the rice blast fungus adapts itself to new resistance genes so quickly, we examined chromosomal locations of AVR-Pita, a subtelomeric gene family corresponding to the Pita resistance gene, in various isolates of M. oryzae (including wheat and millet pathogens) and its related species. We found that AVR-Pita (AVR-Pita1 and AVR-Pita2) is highly variable in its genome location, occurring in chromosomes 1, 3, 4, 5, 6, 7, and supernumerary chromosomes, particularly in rice-infecting isolates. When expressed in M. oryzae, most of the AVR-Pita homologs could elicit Pita-mediated resistance, even those from non-rice isolates. AVR-Pita was flanked by a retrotransposon, which presumably contributed to its multiple translocation across the genome. On the other hand, family member AVR-Pita3, which lacks avirulence activity, was stably located on chromosome 7 in a vast majority of isolates. These results suggest that the diversification in genome location of AVR-Pita in the rice isolates is a consequence of recognition by Pita in rice. We propose a model that the multiple translocation of AVR-Pita may be associated with its frequent loss and recovery mediated by its transfer among individuals in asexual populations. This model implies that the high mobility of AVR-Pita is a key mechanism accounting for the rapid adaptation toward Pita. Dynamic adaptation of some fungal plant pathogens may be achieved by deletion and recovery of avirulence genes using a population as a unit of adaptation.
Introduction
Breeding for disease resistance is a cost-effective, labor-saving, and environmentally sound crop protection strategy. Cultivar resistance [1] conferred by major genes has been extensively used for breeding in many crop species. However, newly developed resistant cultivars have frequently been rendered ineffective within a few years after their release to farmer's fields [2], [3]. This breakdown of resistance has been caused by rapid adaptation of the pathogen, that is, evolution of new races that overcome the introduced resistance genes. The wide cultivation of a new cultivar with a major resistance gene and its breakdown by a new race has been called the boom-and-bust cycle [4]. The primary question is how plant pathogens adapt themselves to new resistance genes so quickly.
The rapid evolution of new races is attributed to rapid loss of function of avirulence effector genes that correspond to the resistance genes in a gene-for-gene manner [5]. Loss of the avirulence function can be due to point mutations, including repeat-induced point mutations [6], [7], insertions of transposable elements, or deletions of entire genes [8]. Extensive surveys of large natural populations of some fungal pathogens revealed that deletion of avirulence genes is a common mechanism of evolution towards virulence [7], [9], [10]. Then, the second question arises: how is a system involving continuous gene loss being sustained? Fungal populations often regain expelled avirulence genes after varieties containing the corresponding resistance genes are removed from the field [e.g. 11]. A simple explanation for the recovery is that isolates carrying the avirulence gene had survived as a minor race in the field during the prevalent cultivation of a resistant cultivar carrying its corresponding resistance gene. Then, when planting of the resistant cultivar came to a halt, their population increased again owing to the contribution of the avirulence gene to fitness. An alternative explanation is that isolates carrying the avirulence gene migrated from regions where the resistance gene had not been deployed. Sexual recombination would allow isolates that have undergone deletion of an avirulence gene to regain that gene, but pathogens such as the rice blast fungus are predominantly asexual in the field. It is of interest to understand if asexually-reproducing pathogen populations have a mechanism to regain deleted avirulence genes.
The genus Pyricularia is the causal agent of blast diseases of monocot species. This genus includes several morphological species such as P. higginsii, P. zingiberi, P. zizaniaecola, and the P. grisea/oryzae species complex [12]. The P. grisea/oryzae species complex is then composed of some cryptic species such as Pyricularia sp. (CE) pathogenic on Cenchrus spp. [12], P. grisea pathogenic on Digitaria spp. (including crabgrass) [13], and P. oryzae [13]. The most familiar species, P. oryzae, is the anamorph (asexual reproductive stage) of Magnaporthe oryzae [14], and is composed of several host-specific subgroups such as Oryza isolates pathogenic on Oryza spp. (including rice, O. sativa), Setaria isolates pathogenic on Setaria spp. (including foxtail millet, S. italica), Triticum isolates pathogenic on Triticum spp. (including common wheat, T. aestivum), Panicum isolates pathogenic on Panicum spp. (including common millet, P. miliaceum), etc. [13], [15]. Finally, Oryza isolates and Setaria isolates include various races which show different patterns of avirulence on cultivars of rice and foxtail millet, respectively [16]. Extensive population analyses of mating type distribution, sexual fertility, and genotypic diversity indicate that Oryza isolates responsible for rice blast disease are limited to asexual reproduction in most areas of the world [17]. The P. oryzae teleomorph can be produced on artificial media [18]–[20], but has not been found in natural fields.
Both subgroup - genus specificity (a type of traditional “host species specificity”) in P. oryzae and race - cultivar specificity in Oryza isolates are governed by gene-for-gene interactions [21]–[24]. Three avirulence genes have been cloned which are involved in host species specificity; PWL1 [25], PWL2 [26], and AVR1-CO39 [27]. Cloned avirulence effector genes involved in rice cultivar specificity are AVR-Pita [28], ACE1 [29], AvrPiz-t [30], AVR-Pia [31], [32], AVR-Pii [32], and AVR-Pik/km/kp [32]. As with other host-pathogen systems, deletion of P. oryzae avirulence genes is a common mechanism for the pathogen to overcome resistance genes deployed in the host. Indeed, cloning of AVR-Pita [28], PWL2 [26] and AVR-Pia [31] was facilitated by isolation of spontaneous virulent mutants with deletions of the avirulence genes. Similarly, association of avirulence phenotypes in field isolates with presence of candidate avirulence genes facilitated cloning of AVR-Pia, AVR-Pii, and AVR-Pik/km/kp [32]. Several studies confirmed that deletion of AVR-Pita is a common mechanism for overcoming resistance in the field, although point mutations and insertions of transposable elements also lead to virulence [28], [33]–[36].
AVR-Pita, which corresponds to the rice resistance gene Pita, is a subtelomeric gene (located adjacent to a telomere on chromosome 3), and it undergoes frequent spontaneous mutation in laboratory studies [28]. AVR-Pita encodes a protein with features typical of zinc metalloproteases, and the putative mature protease is predicted to bind directly to the cognate Pita protein [37] inside a plant cell to initiate hypersensitive resistance [38]. Recently, Khang et al. [39] showed that AVR-Pita is a member of a gene family, and they renamed it AVR-Pita1. Two additional family members named AVR-Pita2 and AVR-Pita3 shared 92% and 71% DNA sequence identity, respectively, to AVR-Pita1. AVR-Pita2 was functional as an avirulence gene, but AVR-Pita3 was not [39].
In this report we describe the process of evolution of the AVR-Pita family during the course of speciation and parasitic specialization of the genus Pyricularia based on analysis of morphological species, cryptic species, host-specific subgroups, and races of the rice blast pathogen. We focused on chromosomal locations of the AVR-Pita family for two reasons. First, Orbach et al. [28] suggested that the telomeric location of an avirulence gene may facilitate rapid adaptation of the blast fungus to its host. This strategy for avoiding the host recognition seemed similar to that adopted by animal parasites such as Trypanosoma brucei, a causal agent of African sleeping sickness [40]–[42], and Plasmodium falciparum, a causal agent of malaria. In P. falciparum, genes involved in antigenic variation (var, rif, etc) are concentrated in subtelomeric chromosomal regions [43]. Extraordinary diversity of var genes, produced by telomere-mediated ectopic recombination, makes it possible for the malaria parasite to evade attack by the host immune system [44]. This recombination can be associated with movement of var genes to new chromosome ends [44]. Second, based on differences in stability of the AVR-Pita gene among different fungal strains, Orbach et al. [28] hypothesized that the gene might reside at different chromosomal locations in different strains. Here, we demonstrate that AVR-Pita (AVR-Pita1 and AVR-Pita2) has been frequently translocated among chromosomes, particularly in Oryza isolates that have evolved in response to periodic deployment of Pita to control blast disease. We propose that “multiple translocation” of AVR-Pita may be associated with its frequent loss and recovery mediated by its transfer among individuals in asexual populations. This model implies that the high mobility of an avirulence gene is a key mechanism accounting for the rapid adaptation toward its corresponding resistance gene and that the system of continuous loss of an avirulence gene is sustained by its recovery from other individuals.
Results
AVR-Pita is unique to the Pyricularia grisea/oryzae species complex
Throughout this paper the anamorph (asexual) name “Pyricularia” will be used instead of the teleomorph (sexual) name “Magnaporthe” because fungal materials employed include several Pyricularia species whose sexual stages have not been recognized. To survey the distribution of AVR-Pita homologs in the genus Pyricularia, we chose 99 “core isolates” from our stock cultures that covered the entire variation of Pyricularia isolates we have collected. These isolates are listed in Table S1 with their code names, original hosts, place and year of isolation, etc. The code name (e.g., O-1G) is composed of its host genus (Oryza), a serial number within isolates from the same host genus (−1), and the country where it was collected (Guiana). The core isolates included seven “old” Oryza isolates (O-2J, O-3J, O-4J, O-5J, O-6J, O-7J, and O-8J) collected before 1960.
For Southern analysis, genomic DNAs from the core isolates were digested with AclI, BamHI, EcoRI, HindIII, and XhoI, and probed with APita766, a 766bp fragment (from O-5J) that spanned the middle of exon 1 through the end of the AVR-Pita ORF (see Materials and Methods, and Table S2). Under hybridization conditions used, APita766 hybridized to both AVR-Pita1 and AVR-Pita2, referred to collectively as AVR-Pita, but not to AVR-Pita3 [39]. Taking results with the five restriction enzymes together, copy numbers in individual isolates and RFLP types of these copies were determined. Blots of the AclI digests are shown in Figure 1 as examples. This restriction enzyme was the most useful for classifying and defining RFLP types because AVR-Pita had an AclI site just downstream from its stop codon. Oryza isolates of P. oryzae carried two copies of AVR-Pita on average (Figure 1A). Representative RFLP types were designated as J1, J2, J3, CH, and PO. J1 was the most common type, and was often accompanied by J2 or J3 in Japanese isolates and CH in Chinese isolates. PO was common among Indonesian isolates. A few isolates (O-28V, O-21IN) had no copies of AVR-Pita, indicating that AVR-Pita is dispensable for the survival of at least some Oryza isolates in natural fields.
Fig. 1. Southern blot analysis of AVR-Pita homologs in Pyricularia isolates. 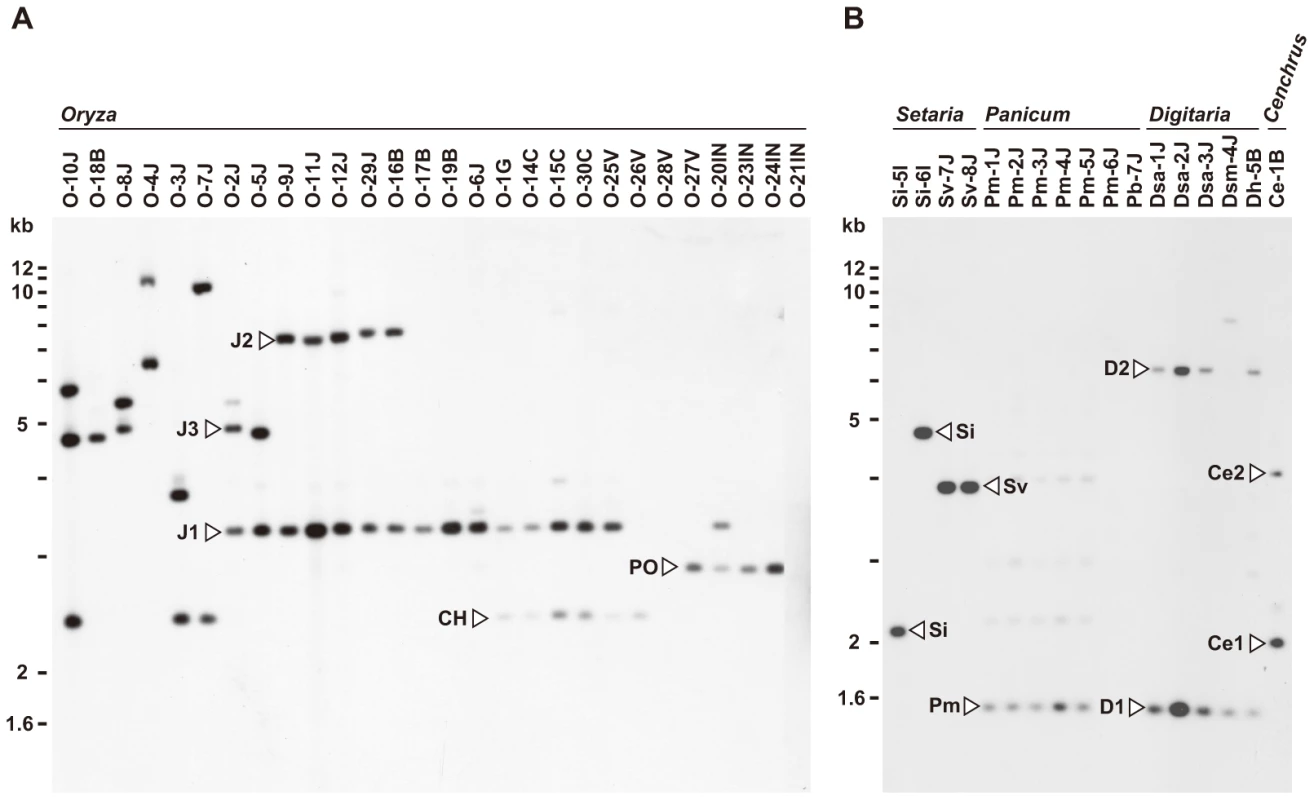
Genomic DNA was digested with AclI and hybridized with the 766-bp AVR-Pita fragment (APita766). (A) RFLP types (J1, J2, J3, CH, and PO) found in Oryza isolates. (B) RFLP types (Si, Sv, Pm, D1, D2, Ce1, and Ce2) found in Setaria, Panicum, Digitaria, and Cenchrus isolates. AVR-Pita or its homologs were also detected in other host-specific subgroups of P. oryzae (Setaria and Panicum isolates) and other cryptic species of the P. grisea/oryzae species complex (Digitaria and Cenchrus isolates) (Figure 1B). Hybridizing fragments in P. oryzae isolates from S. italica, S. viridis, and P. miliaceum were designated as Si, Sv, and Pm, respectively. The same symbol, Si, was assigned to the homologs in Si-5I and Si-6I despite their different-sized AclI fragments, because the same-sized fragments were found in EcoRI, HindIII, and XhoI digests. Homologous fragments in P. grisea (from Digitaria spp.) and Pyricularia sp. (CE) (from Cenchrus echinatus) were designated as D1/D2 and Ce1/Ce2, respectively.
When plotted on the phylogenic tree of Pyricularia spp. constructed by Hirata et al. [12], the AVR-Pita homologs were confined in the P. grisea/oryzae species complex, also known as the M. grisea species complex (Figure S1). This result suggests that AVR-Pita arose during the early stage of evolution of this species complex. It should be noted that P. oryzae isolates adapted for infection of finger millet (Eleusine spp.), ryegrass (Lolium spp.), and wheat (Triticum spp.) lack AVR-Pita homologs (Figure S1).
AVR-Pita homologs map to three locations on chromosome 4 in a cross between wild Oryza isolates
For simplification, hereafter AVR-Pita homologs will be represented by their RFLP types with codes for isolates they are derived from in parentheses. For example, J2(O-29J) represents the J2 type homolog from O-29J. As mentioned above, Oryza isolates carry two copies of AVR-Pita on average, and representative combinations are J1+J3, J1+J2, and J1+CH (Figure 1A). To map the AVR-Pita homologs and determine which confer avirulence, a linkage analysis was performed using an F1 population derived from a cross between O-29J and O-30C [45]. O-29J is avirulent on rice cultivar Yashiro-mochi (harboring Pita) and carries J1 and J2, while O-30C is virulent on Yashiro-mochi and carries J1 and CH (Figure 1A). For mapping J1, primers specific to J1(O-29J) (AVRPita-O23-3 and AVRPita-O29-5, Table S3) were designed using a single nucleotide polymorphism between J1(O-29J) and J1(O-30C). When segregation data for J1, J2, and CH were combined with those of molecular markers reported by Luo et al. [46], the three types mapped to different locations on a single linkage group (Figure 2A). Using data from Luo et al. [46] and information from the M. oryzae (70–15) genome database, this linkage group was deduced to be chromosome 4. Avirulence toward rice with the Pita resistance gene cosegregated perfectly with J2, but not with CH or J1 (Figure 2A). Therefore, J2 appeared to be a functional avirulence gene (confirmed by transformation assay as described later). Location of the functional AVR-Pita homolog J2(O-29J), ∼25.5 cM apart from a telomere of chromosome 4 (Figure 2A), was intriguing, because the functional AVR-Pita gene first cloned by Orbach et al. [28] adjoined a telomere of chromosome 3. This led us to a hypothesis that AVR-Pita might have frequently been translocated to different chromosomal locations.
Fig. 2. Linkage analyses of AVR-Pita homologs. 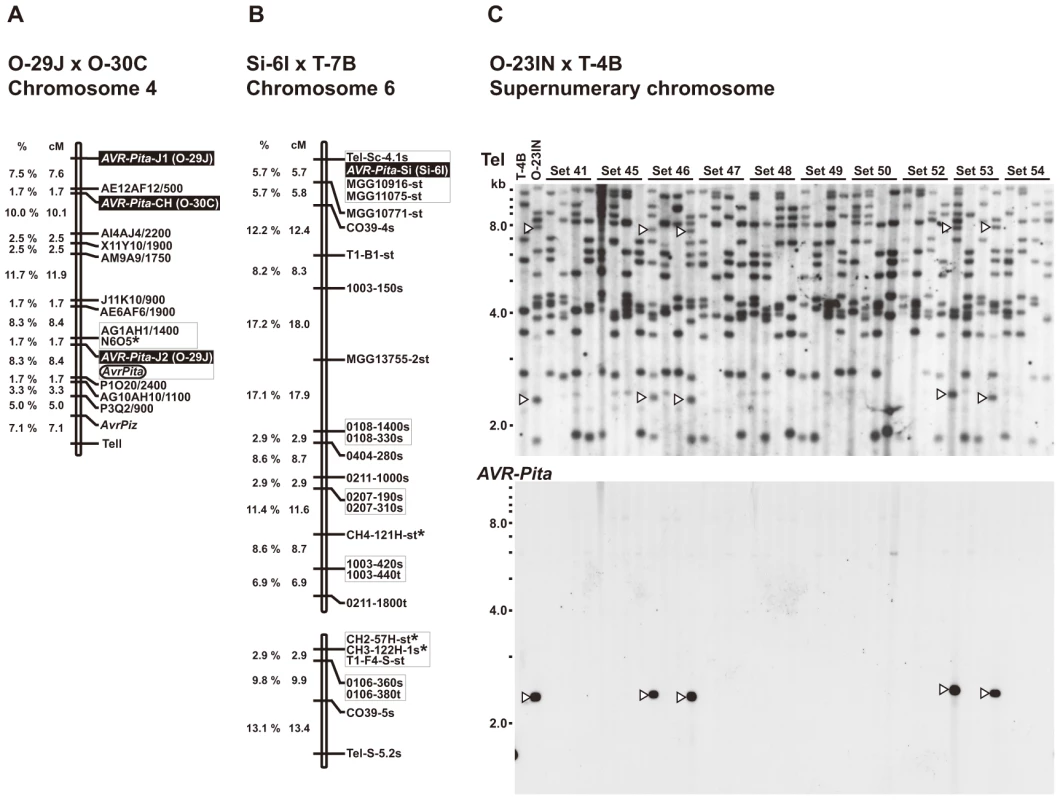
(A) A map of chromosome 4 constructed by using an F1 population derived from a cross between O-29J and O-30C. Segregation data of three AVR-Pita homologs (AVR-Pita-J1(O-29J), AVR-Pita-J2(O-29J), and AVR-Pita-CH(O-30C), enclosed in black rectangles) and the avirulence on cv. Yashiro-mochi (Pita carrier) determined by infection assay (AvrPita, enclosed in an open oval) were combined with those of genetic markers reported by Luo et al. [46]. A chromosome 4 – specific marker is indicated by an asterisk. “Tell” is a telomere signal produced by Southern hybridization with a telomere repeat oligonucleotide (TTAGGG)10. (B) A map of chromosome 6 constructed by using an F1 population derived from a cross between Si-6I and T-7B. An AVR-Pita homolog found in Si-6I is enclosed in a black rectangle. Chromosome 6 – specific markers [47] are indicated by asterisks. Markers prefixed with “Tel” are telomere signals produced by Southern hybridization with the telomere repeat probe. Letters at the end of the markers (s and t) represent parents they are derived from. For example, CH4-121H-st represents two cosegregating fragments, one from Si-6I (Setaria isolate) and one from T-7B (Triticum isolate). (C) Southern blot analysis of an F1 population derived from a cross between O-23IN and T-4B. Genomic DNAs representing each meiotic product from ten tetrads (Set41 through Set54) were digested with BamHI and hybridized with telomere repeat (upper panel) and AVR-Pita (lower panel) probes. Open arrowheads indicate restriction fragments inherited in a non-Mendelian manner. AVR-Pita homologs reside near telomeres on chromosome 6 (Setaria isolate) and a supernumerary chromosome (Oryza isolate)
A Setaria pathogen from India, Si-6I, contained an AVR-Pita homolog designated as the Si type (Figure 1B). To reveal its chromosomal location, Si-6I was crossed with T-7B (Triticum isolate lacking AVR-Pita), and an F1 population was produced which consisted of 33 cultures isolated randomly. A linkage map was constructed using various AFLP, RFLP, and telomere markers (Table S4). Si(Si-6I) cosegregated completely with a telomere marker that was located on a linkage group carrying chromosome 6 – specific markers reported by Nitta et al. [47] (Figure 2B). This result suggests that Si(Si-6I) resides in a subtelomeric region of chromosome 6.
An Oryza isolate from Indonesia, O-23IN, contained an AVR-Pita homolog of the PO type (Figure 1A). Segregation analysis was performed using 10 complete tetrads from a cross between O-23IN and T-4B (Triticum isolate lacking AVR-Pita) [22]. When BamHI-digested genomic DNA was hybridized with the telomere probe, most signals segregated in a 1∶1 ratio as expected (Figure 2C). However, two telomere fragments (7.8 kb and 2.4 kb) in O-23IN were inherited in a non-Mendelian manner (Figure 2C), as is characteristic of supernumerary chromosomes [48], [49]. Interestingly, the 2.4 kb telomere-containing fragment hybridized to APita766 (Figure 2C). Therefore, it appeared that PO(O-23IN) resided within 2.4 kb from a telomere of a supernumerary chromosome.
Chromosomes containing AVR-Pita homologs show extreme size variation in Oryzae isolates, but not in Digitaria isolates
To further survey variation in AVR-Pita-containing chromosomes in Pyricularia isolates, 66 isolates were chosen from the P. grisea/oryzae species complex, and their chromosomal DNAs were separated by contour-clamped homogeneous electric field (CHEF) gel electrophoresis (Figure 3). Electrophoretic karyotypes varied in Oryza isolates (Figure 3A, left) as reported previously [49], [50]. When the gel was blotted and probed with APita766, various sized chromosomes were detected (Figure 3B, left), suggesting that AVR-Pita homologs were located on different chromosomes in different field isolates. In contrast, hybridization with a chromosome 7 – specific marker (T1A11, Kobe University) showed that this smallest essential chromosome was approximately 3.5 Mb throughout the Oryza isolates (Figure 3C, left). It should be noted that the AVR-Pita-containing chromosomes in several isolates (including O-23IN mentioned above) are smaller than chromosome 7 (Figure 3A).
Fig. 3. CHEF-Southern analyses of chromosomal locations of AVR-Pita homologs in Pyricularia isolates. 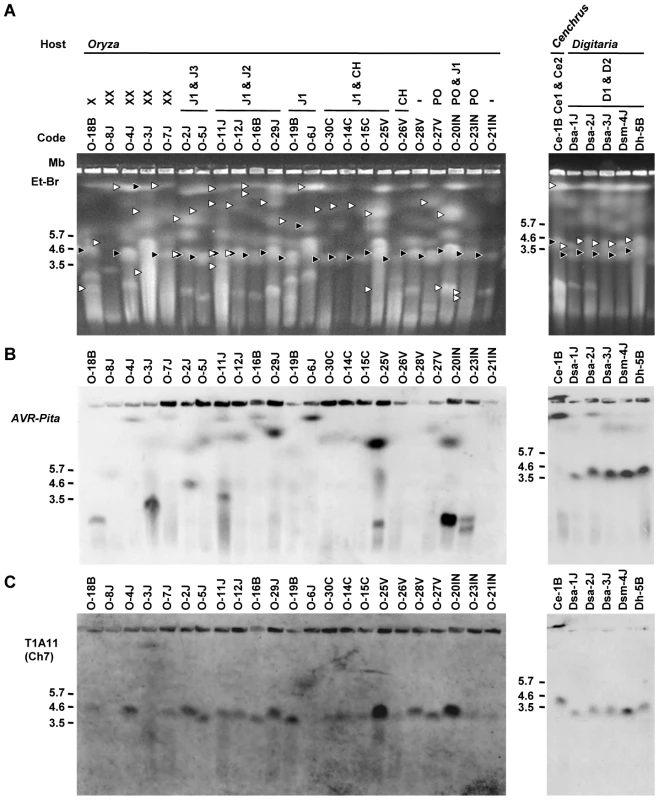
(A) Representative examples of chromosomal DNAs separated by contour-clamped homogeneous electric field (CHEF) gel electrophoresis. White arrowheads indicate chromosomal bands that hybridized to the AVR-Pita probe (APita766) in (B). Black arrowheads indicate chromosomal bands that hybridized to a chromosome 7 – specific cosmid marker, T1A11 (see Figure 4A), in (C). (B) Chromosomal bands carrying AVR-Pita homologs. The chromosomal DNAs in (A) were blotted and hybridized with the AVR-Pita probe (APita766). (C) The location of chromosome 7. The membrane in (B) was reprobed with the chromosome 7 – specific cosmid marker, T1A11. In contrast to Oryza isolates, Digitaria isolates (P. grisea) showed relatively little karyotype variation (Figure 3A, right). Sizes of AVR-Pita-containing chromosomes were almost uniform (Figure 3B, right), even between isolates from Japan and Brazil. These chromosomes (∼4 Mb) were a little larger than chromosome 7 (Figure 3A, B, C, right). Dsa-2J showed a weak signal on the top band, but this signal was not reproducibly detected under high-stringency conditions. From these results we concluded that the D1 and D2 homologs (Figure 1B) were both located on the ∼4 Mb chromosome in all the Digitaria isolates. A Cenchrus isolate (another cryptic species) had its AVR-Pita homologs on the largest chromosomal band (Figure 3A, B, right).
Chromosome-length polymorphisms, chromosome rearrangements, and AVR-Pita location in diverse field isolates
Determining which chromosomes contain AVR-Pita homologs in diverse field isolates, many of which are sexually infertile and contain chromosomal rearrangements [49], [50], required hybridization probes spanning each chromosome for use in CHEF analysis. To construct a system for accurate identification of chromosomes, we performed a comparative linkage and CHEF analysis. As a mapping population, we chose an F1 population between Si-1J (Setaria isolate collected in Japan) and T-4B (Triticum isolate collected in Brazil) that consisted of 78 random progenies [51], because this cross produced the most abundant polymorphic markers among crosses performed in our laboratories (at Kobe University and Saga University). The final map (Figure 4A) contained 160 markers, including 60 RFLP markers developed at Kobe University (KU markers), 24 chromosome-specific RFLP markers developed at University of Wisconsin-Madison (WU markers) [47], and 7 chromosome-specific SSR markers developed by Zheng et al. [52]. Based on this map, 48 RFLP markers were selected for comparative CHEF analysis (shown in colors, Figure 4A).
Fig. 4. Identification of chromosome specific markers spanning the P. oryzae chromosomes. 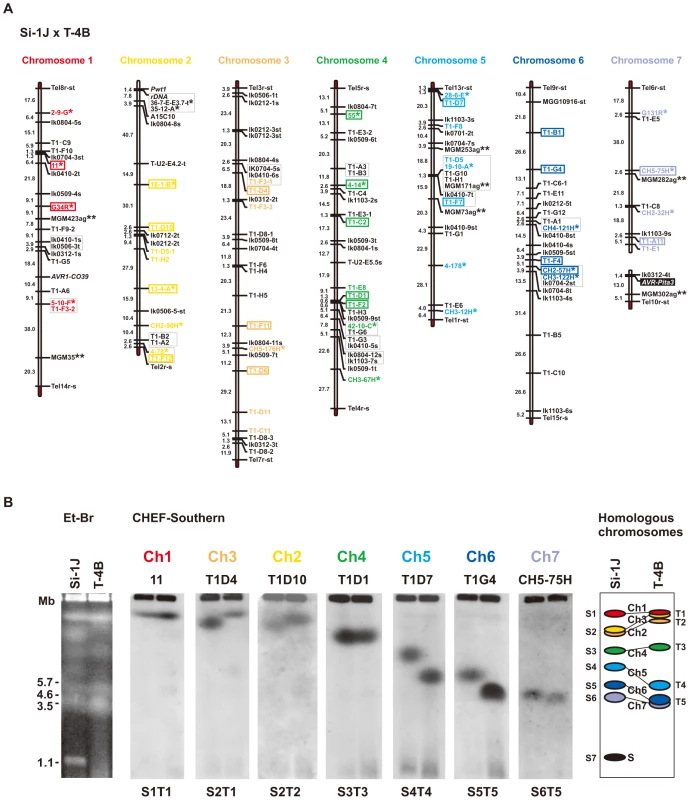
(A) A genetic map of a cross between P. oryzae isolates, Si-1J and T-4B. Asterisks indicate chromosome-specific markers reported by Nitta et al. [47]. Double asterisks indicate chromosome-specific SSR markers reported by Zheng et al. [52]. Markers prefixed with “Tel” are telomere signals produced by Southern hybridization with telomere repeats (TTAGGG)10. Letters at the end of the markers (s and t) represent parents they are derived from (s, from Si-1J; t, from T-4B). Markers in red, yellow, brown, green, light blue, blue, and violet, were used in the CHEF-Southern analysis (B) for identification of chromosomes 1, 2, 3, 4, 5, 6, and 7, respectively. Markers enclosed in rectangles were used for karyotype analysis in Figure 5A. (B) Identification of homologous chromosomes in the parental isolates (Si-1J and T-4B) on a CHEF gel. Chromosomal DNAs in the parental isolates were separated on a CHEF gel and stained with ethidium bromide (the left panel). Sizes of Schizosaccharomyces pombe chromosomes are indicated on the left of the gel photograph. The gel was then blotted and probed with the RFLP markers shown in the chromosome-specific colors in (A). Seven hybridization patterns (S1T1 through S6T5) are shown in the middle seven panels with marker names on the top as examples. Based on these Southern analyses, chromosome number(s) were assigned to each band (the right panel). The diagrammatic chromosomal bands are painted in the chromosome-specific colors used in (A). S (painted in black) indicates a supernumerary chromosome described previously [48]. When chromosomal DNAs of the parental isolates (Si-1J and T-4B) were run on a CHEF gel, chromosome-length polymorphisms were observed (Figure 4B, left panel). Chromosomal bands of Si-1J and T-4B were tentatively designated as S1 through S7 and T1 through T5, respectively, in the order of their sizes (Figure 4B, the right panel). When the gel was blotted and probed with the 48 markers, hybridization patterns were classified into seven types (S1T1 through S6T5) (Figure 4B, the middle seven panels). There was a perfect correspondence between the linkage groups (Figure 4A) and the hybridization patterns (Figure 4B). Chromosome number(s) were assigned to each band (right panel). Despite chromosome-length polymorphisms, the genomes of the Japanese Setaria and Brazilian Triticum isolates used for linkage analysis did not differ by major chromosome rearrangements such as translocations or duplications.
The membranes carrying chromosomal DNAs of the 66 isolates from the P. grisea/oryzae species complex were sequentially hybridized with 22 markers selected from the chromosome-specific markers (Figure 4A). The results are summarized in Figure 5A. Sometimes, one chromosomal band hybridized to markers that were assigned to different chromosomes. This was assumed to be caused by a translocation, a duplication, or co-migration of more than one chromosomal DNA. These three possibilities were differentiated as follows. In some isolates, markers assigned to a single chromosome in Figure 4 were split into two subgroups which hybridized to different chromosomal bands (e.g. O-4J, chromosome 6 in blue). This was considered to be caused by a chromosomal translocation. In some isolates each of markers assigned to a single chromosome hybridized to two chromosomal bands simultaneously (e.g. O-9J, chromosome 4 in green). This was considered to be caused by a chromosomal duplication. When there was no sign of translocation or duplication, the hybridization of a single band to multiple markers was considered to be attributable to the co-migration of more than one chromosomal DNA.
Fig. 5. Frequent AVR-Pita translocation occurred independently from major chromosomal translocations or duplications. 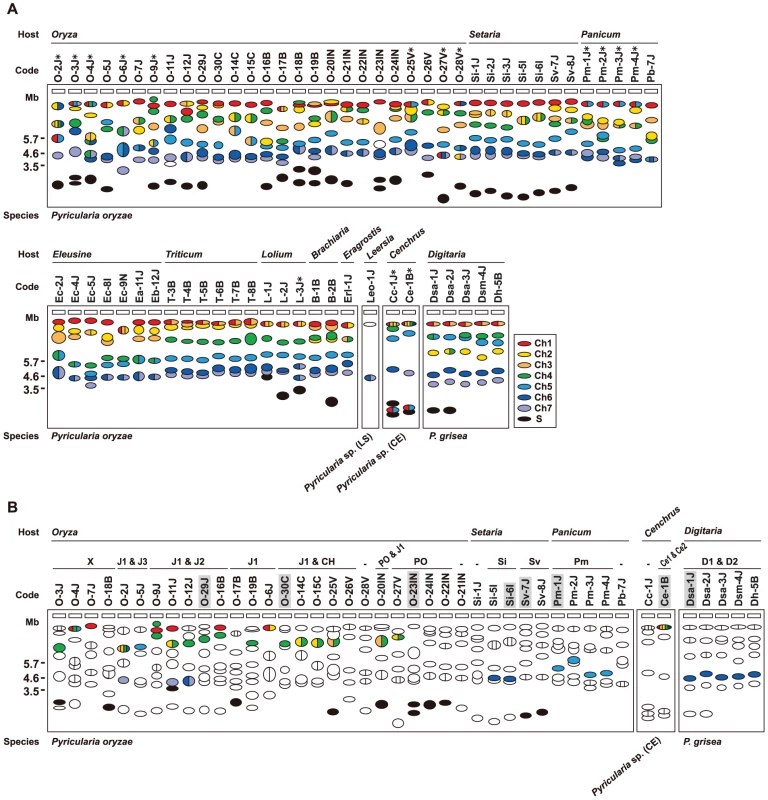
(A) A diagram of electrophoretic karyotypes of Pyricularia isolates revealed by Southern analyses. Blots of CHEF gels were hybridized with the markers enclosed in colored rectangles in Figure 4A. Chromosomal bands that hybridized exclusively to markers assigned to a single, same chromosome are painted in the color assigned to the chromosome in Figure 4. Chromosomal bands that hybridized to markers assigned to two or more, different chromosomes are divided with vertical lines and painted in the colors assigned to those chromosomes. Chromosomal bands that were smaller than the average size of chromosome 7 and did not hybridize to any chromosome-specific probes were considered to be supernumerary chromosomes and are painted in black. Asterisks indicate isolates which are deduced to have suffered from chromosomal rearrangements such as translocations or duplications. (B) A diagram of chromosomal locations of AVR-Pita homologs. Chromosomal bands that hybridized to the AVR-Pita probe (APita766) were painted with the chromosome-specific colors used in (A). The RFLP types defined in Figure 1 are shown above isolate codes. Hyphens indicate isolates carrying no AVR-Pita homologs that are detectable in the genomic Southern analysis. Shaded isolates are representatives chosen for further analyses of AVR-Pita flanks (see Figure 6). Isolates from Eleusine, Triticum, Lolium, Brachiaria, Eragrostis, and Leersia are omitted from this diagram because all isolates from these hosts are non-carriers of AVR-Pita homologs (see Figure S1). The AVR-Pita-containing chromosomes identified by CHEF-Southern analyses with the APita766 probe are summarized in Figure 5B. This analysis confirmed results from the linkage analysis (Figure 2A, B), which located AVR-Pita homologs on chromosomes 4 and 6 in O-29J/O-30C and Si-6I, respectively. AVR-Pita homologs in Digitaria isolates were stably located on chromosome 6, even though the isolates tested were from Japan and Brazil. Those in Panicum isolates were stably located on chromosome 5. By contrast, those in Oryza isolates were located on various chromosomes: for example, chromosome 1 in O-7J, chromosome 4 in O-19B, chromosome 5 in O-5J, chromosome 7 in O-2J, and a supernumerary chromosome in O-18B. Although this analysis detected chromosome rearrangements in some isolates (marked by asterisks in Figure 5A), there is no correlation between these chromosome rearrangements and AVR-Pita location.
Supernumerary chromosomes carrying AVR-Pita homologs were detected in significant numbers of P. oryzae isolates (Figure 5B). RFLP types of the homologs on supernumerary chromosomes varied, e.g. J1 in O-17B, PO in O-22IN, and Sv in Sv-7J, suggesting that multiple events produced these supernumerary chromosomes. Interestingly, O-23IN, which was suggested to have its AVR-Pita homolog on a supernumerary chromosome in the segregation analysis (Figure 2C), had two supernumerary chromosomes hybridizing to AVR-Pita (Figure 3B, 5B). The F1 cultures [22] used in the segregation analysis were produced in 1998. To check chromosomal constitutions at that time, O-23IN and O-20IN were retrieved from stock cultures produced in 1996, and subjected to CHEF-Southern analysis with the AVR-Pita probe. O-20IN from the old stock produced the same pattern of AVR-Pita signals as that shown in Figure 3B, whereas O-23IN from the old stock produced only one AVR-Pita signal (Figure S2). This analysis revealed that the AVR-Pita chromosome in the old O-23IN culture corresponded to the smaller AVR-Pita chromosome in the current culture (Figure 3B and Figure S2). These results suggest that the larger supernumerary chromosome in the current O-23IN culture was recently generated during subculture, possibly through chromosomal rearrangements such as duplication of the smaller one.
Association of the AVR-Pita homologs with the retrotransposons Inago1 and Inago2
To reveal mechanisms for the frequent changes of chromosomal locations, genomic DNA sequences around representative AVR-Pita homologs were examined using fosmid and plasmid clones containing the homologs (see Materials and Methods). An analysis of the J3 type was omitted because restriction analyses showed that J3 had an almost identical structure as J2. Most of the AVR-Pita homologs had a full length coding sequence (Figure S3). One exception was CH, which had a deletion of the 5′ half of the coding sequence (Figure S3). This homolog was apparently nonfunctional, and therefore, omitted from further analyses. Comparison of DNA sequences flanking the AVR-Pita coding sequence revealed that 30 bp after the stop codon and 441 bp before the start codon (yellow and orange regions in Figure S3) were conserved among P. oryzae isolates tested.
The AVR-Pita gene isolated from the strain 4224-7-8 closely adjoined a telomere [28]. A structure almost identical to this “authentic” AVR-Pita was found in the PO type carried by O-23IN, an isolate from Indonesia (Figure 6). The authentic AVR-Pita was, therefore, classified into the PO type and designated as PO(4224-7-8). The J1 and J2 types were accompanied by various transposable elements such as Inago1 (GenBank/EMBL accession no. AB334124), Inago2 (AB334125), Pyret (AB062507) [53], MGLR-3 (AF314096) [54], MGR583 (AF018033) [55], [56], Pot2 (Z33638) [57], Pot3 (U60989) [55], [58], and Occan (AB074754) [59]. The common feature shared by the three types (J1, J2, and PO) in Oryza isolates was that these AVR-Pita homologs were flanked by solo-LTRs of retrotransposons, Inago1 and Inago2 (Figure S3).
Fig. 6. Structures around AVR-Pita homologs in representative RFLP types. 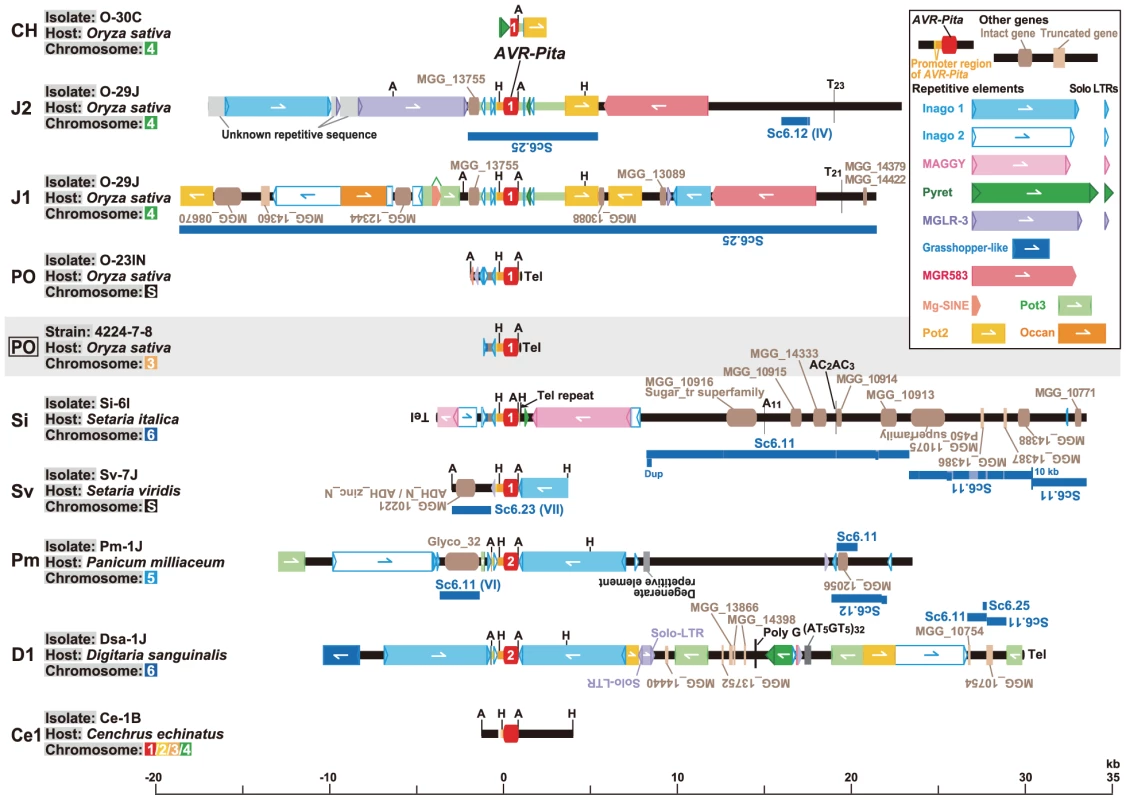
Structures of plasmid clones (in PO, Sv, and Ce1), whole fosmid clones (in J2, J1, Si, Pm, and D1), and a partial sequence of a fosmid clone (in CH) are shown with the structure of the authentic AVR-Pita reported by Orbach et al. [28] (PO enclosed in a rectangle). AVR-Pita homologs are depicted in red at the center. Those identified as AVR-Pita1 and AVR-Pita2 in sequence analysis (Figure 7A) were labeled with “1” and “2”, respectively. Rectangles with triangles at both ends in the same direction indicate LTR-retrotransposons. The triangles are direct, long terminal repeats (LTRs). Detached triangles indicate solo-LTRs. Rectangles with a triangle at one end indicate LINE- or SINE-like elements. Rectangles with oppositely directed small triangles at their ends indicate DNA transposons. Directions of those elements are indicated by arrows. Brown and light brown rectangles indicate intact and truncated genes, respectively, other than transposable elements. Directions of those genes are indicated by the orientation of gene names. Black ovals designated as ‘Tel’ represent telomere repeats. Dark blue lines indicate corresponding supercontigs in the M. oryzae (70–15) genome database ver.6 (http://www.broadinstitute.org/annotation/fungi/magnaporthe/). The scale is shown at the bottom of the figure. A, AclI sites; H, HindIII sites. We hypothesized that the solo-LTRs flanking AVR-Pita homologs in Oryza isolates are remnants of full-sized copies of Inago1 and Inago2 that had once flanked the ancestral gene. Analysis of homologs from non-rice isolates provided evidence to support this hypothesis. In Pm-1J belonging to the Panicum host-specific subgroup of P. oryzae, a full-sized copy of Inago1 was detected in the right flank of AVR-Pita (Figure 6). This Inago1 copy had a complete structure as a retrotransposon, that is, ORFs flanked by 5′-LTR and 3′-LTR, although its nucleotide sequence contained mutations leading to a truncation of the encoded protein. A similar structure was also detected in Sv-7J belonging to the Setaria host-specific subgroup. Furthermore, in Dsa-1J belonging to a different cryptic species (P. grisea), full-sized copies of Inago1 were detected in both flanks of the AVR-Pita homolog (the D1 type) (Figure 6). This fosmid clone from the Dsa-1J genomic library contained telomere repeats at one end, which was 28.8 kb downstream from the AVR-Pita homolog (Figure 6).
Considering the Cenchrus isolates, Ce-1B did not have Inago1 or its solo-LTRs in the flanks of the AVR-Pita homolog (the Ce1 type) (Figure 6). Interestingly, when genomic DNA of Ce-1B was hybridized with an Inago1 probe, >40 signals were detected (data not shown), indicating that its genome harbors many copies of Inago1. One of the features of retrotransposons is that they are not completely excised from genomic positions in which they reside. Therefore, we suggest that the flanks of Ce1(Ce-1B) are virgin regions which have not suffered from insertions of Inago1.
In contrast to the Sv type in the Setaria subgroup, the Si type showed a distinct structure lacking a full-sized copy of Inago1 sequences. Instead, Si(Si-6I) was accompanied by a full-sized copy of retrotransposon MAGGY (L35053) [60] in the right flank and its truncated copy in the left flank (Figure 6). In the linkage analysis (Figure 2B), Si(Si-6I) was suggested to reside in a subtelomeric region. Actually, this AVR-Pita homolog was located on a Si-6J fosmid clone containing telomere repeats (Figure 6). However, the telomere was located 3.8 kb upstream from the AVR-Pita homolog, not downstream as in the authentic AVR-Pita or the PO type.
The M. oryzae (70–15) genome database (http://www.broadinstitute.org/annotation/fungi/magnaporthe/) contains an AVR-Pita homolog with >99% homology to J1(O-29J). The structure around this homolog in the database was almost identical to that of the fosmid clone containing J1(O-29J); perfect synteny was observed from one end to the other of its insert (38.9 kb) (Figure 6). On the other hand, synteny with the fosmid clone containing J2(O-29) was confined to a 7.5 kb region around the AVR-Pita homolog (Figure 6). The AVR-Pita homolog in the database was, therefore, concluded to belong to the J1 type, and designated as J1(70–15).
Processes of rearrangements in the AVR-Pita-flanking regions
To reveal evolutionary relationships among the structures shown in Figure 6, 29 representative homologs were amplified from 24 isolates and sequenced. Their exon sequences were aligned with those of J1(70-15) and PO(4224-7-8) (Dataset S1) and used to construct a Bayesian tree. The resulting tree of a total of 31 homologs was rooted using AVR-Pita3 as an outgroup taxon. The RFLP types of these homologs were plotted on the tree with the diagrams showing structures in their flanks (Figure 7A). The AVR-Pita homologs were grouped into three clusters; Cl-X consisting of the RFLP types found only in P. oryzae, Cl-Y consisting of the P. grisea D1 and P. oryzae Pm types, and Cl-Z consisting of the Ce1 type. The homologs grouped into Cl-X and Cl-Y showed >98% homology to AVR-Pita1 and AVR-Pita2, respectively. This result indicates that J1, J2, J3, PO, Si, and Sv found in Oryza and Setaria isolates are AVR-Pita1 while Pm in Panicum isolates and D1 in Digitaria isolates are AVR-Pita2. The topology of the tree suggests that a key event in the evolution of AVR-Pita flanks was the insertion of full length copies of Inago1.
Fig. 7. Molecular evidence suggesting the course of evolution of the AVR-Pita family. 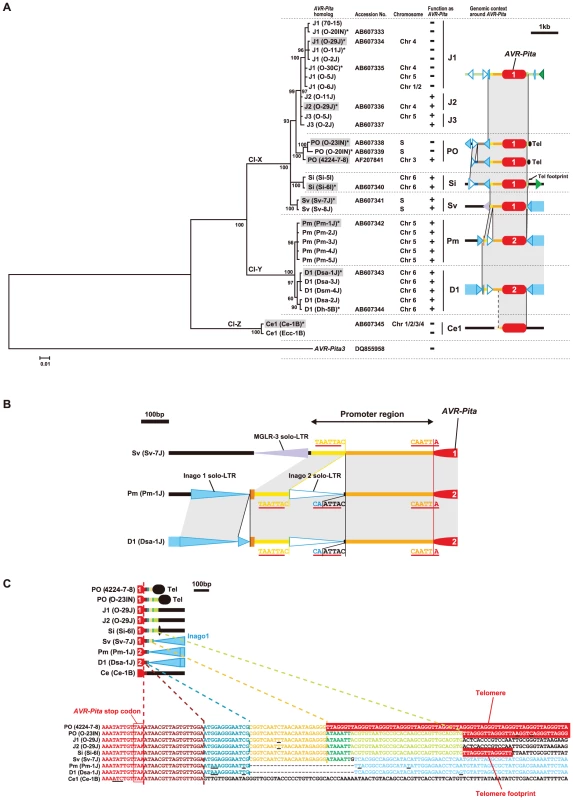
(A) A Bayesian tree constructed from exon sequences of AVR-Pita homologs. The number on each branch indicates posterior probability. The tree was rooted using AVR-Pita3 [39] as an outgroup taxon. Shaded are representative homologs whose flanks were analyzed in detail (see Figure 6). Asterisks indicate homologs used for the transformation assay (see Figure 8). The function of the homologs as an avirulence gene, which were deduced from the transformation assay and sequence analyses, is represented by + (functional) and – (nonfunctional) in the “function as AVR-Pita” column. Chromosomes carrying the homologs are shown in the “chromosome” column. “S” represents a supernumerary chromosome. The RFLP types of the homologs and structures of their flanks are depicted in the right column. See Figure 6 for legends of symbols. Cl-X, Cl-Y, and Cl-Z indicate three major clusters found in the present study. (B) Structure at the 5′ flanks of AVR-Pita homologs suggesting a horizontal transfer. (C) Structures at the 3′ flanks of AVR-Pita homologs suggesting stepwise stacking of blocks of DNA fragments. Each block is painted in a distinct color. Corresponding blocks in the diagrams (upper panel) and the nucleotide sequences (lower panel) are shown in the same color. Red boxes indicate telomere repeats. Underlines indicate nucleotide substitutions. It should be noted that Panicum isolates of P. oryzae (Figure S1) contained AVR-Pita2 characteristic of P. grisea (Digitaria isolates), instead of AVR-Pita1 characteristic of the other host species-specific subgroups in P. oryzae (Figure 7A). The Pm and D1 AVR-Pita2 genes shared a 99.7% identical coding sequence and a 97.4% identical promoter sequence including an inserted solo-LTR of Inago2 (Figure 7B) while the rDNA-ITS1, ITS2, actin genes, ß-tubulin genes, and calmodulin genes of the Panicum and Digitaria isolates shared 92.1%, 90.9%, 93.3%, 93.5%, and 84.7% identities in nucleotide sequences, respectively [12]. The AVR-Pita2 genes are located in non-homologous sequences on chromosomes 5 and 6 for the Pm and D1 types, respectively (Figure 6). These results suggest that the Pm type originated from a homolog that had been horizontally transferred from P. grisea to P. oryzae.
Additional clues suggesting evolutionary processes were found in structures in the 3′ flanks of the homologs. A comparison of nucleotide sequences of the 3′ flanks revealed that this region had been constructed through stepwise stacking of blocks (Figure 7C). The eight types other than Ce1 shared a 13 bp block shown in blue. Among the eight, the six types other than D1 and Pm shared a 22 bp block shown in yellow. Among the six, the five types other than PO(4224-7-8) shared a 7 bp block shown in green. Among the five, the four types other than Sv shared a 32 bp block shown in pale green. Intriguingly, short telomere repeats were found in the right flank of the pale green block in the Si type. The real telomere on this chromosome is located at 3.8 kb upstream from the AVR-Pita homolog as mentioned above (Figure 6, Figure S3). These results suggest that an ancestor of the Si type had a telomere at the position shown in Figure 7C (in the 3′ flank), but that the telomeric fragment containing the homolog was then excised and fused to the same or another telomere in the opposite direction.
Functional analysis of AVR-Pita homologs across the P. grisea/oryzae species complex
To assess avirulence activity of the AVR-Pita homologs, fosmid or plasmid clones (Table 1) carrying 13 representative homologs (indicated by asterisks in Figure 7A) were introduced into O-8J, an Oryza isolate virulent on rice cultivars Yashiro-mochi (Pita) and Shin2 (pita) (Figure 8A). Infection assays revealed that the fosmid clone with J2(O-29J) (shown in Figure 6) transformed O-8J to avirulence on Yashiro-mochi without a change in virulence on Shin2 (Figure 8A, Table 1). By contrast, the fosmid clone with J1(O-29) (shown in Figure 6) did not transform O-8J to avirulence on Yashiro-mochi. These results confirm the inference from the linkage analysis (Figure 2A) that the functional avirulence gene in O-29J is not J1, but J2. The J1 homologs were grouped into three variants at the level of nucleotide sequences of the gene (including the promoter and ORFs). These variants were amplified from genomic DNA of O-11J, O-20IN, and O-30C, and cloned into pBluescript II SK (+). None of these clones changed the virulence of O-8J on Yashiro-mochi (Table 1). Plasmid clones containing our PO homologs also did not have any effect on the virulence of O-8J. On the other hand, all plasmid clones containing Si, Sv, Pm, and D1 transformed O-8J to avirulence on Yashiro-mochi without a change in virulence on Shin2 (Figure 8A, Table 1). This result indicates that all these homologs encode proteins that are recognized by the rice resistance gene product Pita in spite of being derived from non-rice isolates. Only one type from the non-rice isolates, Ce1, failed to trigger Pita-mediated resistance.
Fig. 8. Functional analysis of AVR-Pita homologs with respect to avirulence activity. 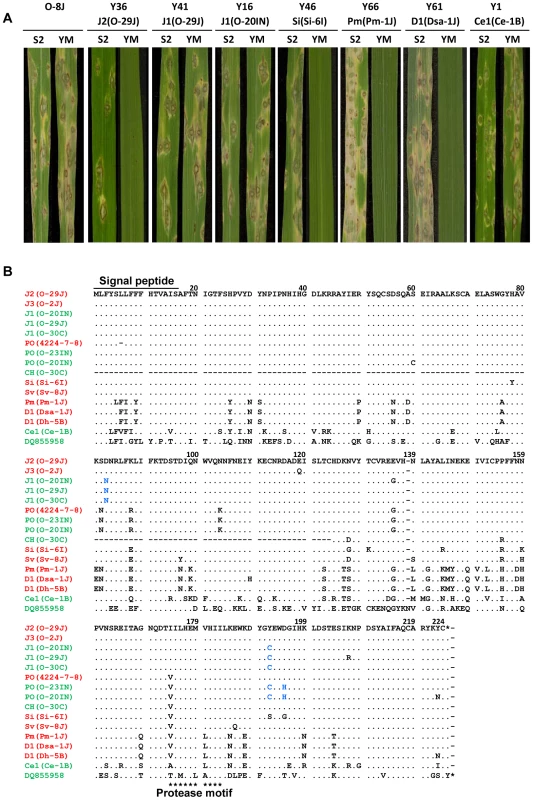
(A) Pathogenicity of Oryza isolate P-2b (O-8J) and its transformants expressing various AVR-Pita homologs (Y36 through Y1) on rice cultivars, Shin2 (S2) carrying pita and Yashiromochi (YM) carrying Pita, 7 days after inoculation. See Table 1 for details of the transgenes. (B) Alignment of amino acid sequences of the AVR-Pita homologs. DQ855958 is the accession number of AVR-Pita3 [39] used as an outgroup in Figure 7A. Functional (triggers Pita-mediated resistance) and non-functional (fails to trigger Pita-mediated resistance) homologs are shown in red and green, respectively. Amino acids shown in blue letters indicate substitutions shared by the three non-functional J1 homologs in comparison with the functional J2(O-29J) and those shared by the two non-functional PO homologs in comparison with the functional PO (4224-7-8). Tab. 1. Transformants of rice isolate P-2b (O-8J) with AVR-Pita homologs and their pathogenicity on rice cvs. Yashiromochi (YM) and Shin2 (S2). 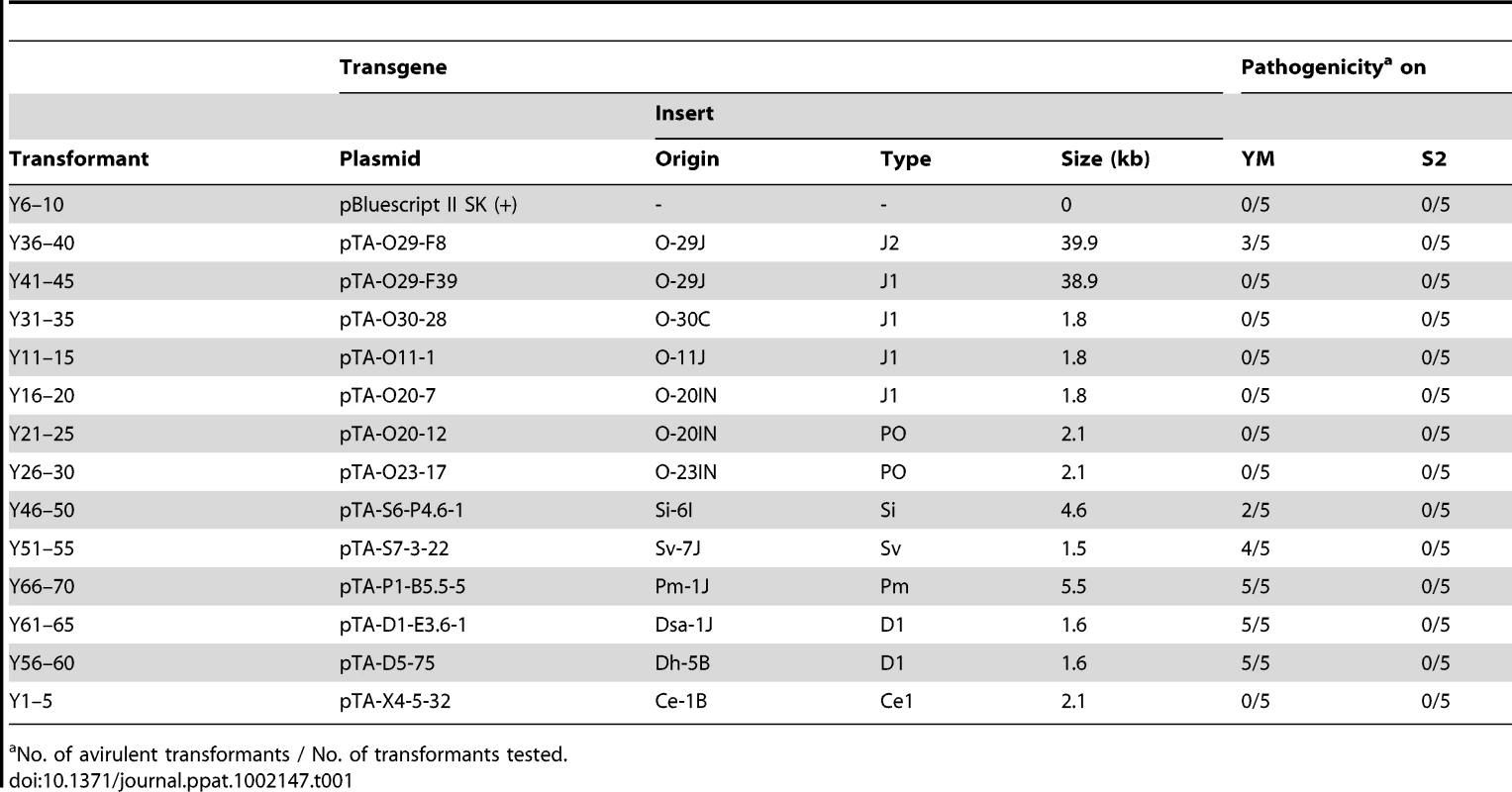
No. of avirulent transformants / No. of transformants tested. Avirulence function of the other homologs shown in Figure 7 was determined by sequence analyses or infection assay with wild isolates carrying them. For example, J1(70–15) from the database was considered to be non-functional (Figure 7A) because its nucleotide sequence (spanning the entire gene) was identical to that of J1(O-20IN), which was non-functional in the transformation assay (Figure 8A). Similarly, J3(O-5J), which shared a 100% identical nucleotide sequence with J2(O-29J), was considered to be functional as an avirulence gene. The five homologs belonging to the Pm type were all deduced to be functional (Figure 7A) because their nucleotide sequences were identical to that of Pm(Pm-1J). Taken together, all AVR-Pita homologs in non-rice isolates of P. oryzae and P. grisea were concluded to be functional as avirulence genes (Figure 7A).
Amino acid sequences of the AVR-Pita homologs were aligned and compared (Figure 8B). Within Oryza isolates, the homologs differed from one another by only a few amino acids. For example, J1(O-30C) differed from J2(O-29J) only by two amino acids, at the 83rd and 192nd positions. Nevertheless, the former was non-functional while the latter was functional. Similarly, PO(O-23IN) (non-functional) differed from PO(4224-7-8) (functional) only by two amino acids at the 192nd and 195th positions. When they were compared with homologs in other host-specific subgroups of P. oryzae or P. grisea, however, a high diversity of amino acid sequences was observed. For example, Si(Si-6I) and D1(Dsa-1J) differed from J2(O-29J) by 10 and 38 amino acids, respectively. Nevertheless, these three homologs were all functional. These results suggest that the AVR-Pita homologs are under different selection pressures within and outside the Oryza subgroup.
Nonfunctional AVR-Pita3 has been stably localized on chromosome 7
The AVR-Pita homologs described above did not include AVR-Pita3, a non-functional homolog reported by Khang et al. [39], because the APita766 probe did not hybridize to AVR-Pita3. To analyze AVR-Pita3, a fragment (958 bp) was amplified from genomic DNA of Ei-13I using AVR-Pita3-specific primers (Table S3). Direct sequencing of this fragment confirmed that it belonged to the AVR-Pita3 class. Southern hybridization with this AVR-Pita3-specific probe (APita3-958) indicated that AVR-Pita3 is widely distributed in P. oryzae, but absent in the other species (data not shown) as reported by Khang et al. [39].
Khang et al. [39] further reported that AVR-Pita3 was located on chromosome 7. APita3-958 produced the S6T5 pattern (Figure 4B) (which is specific to chromosome 7 markers) in Southern hybridization with the chromosomal DNAs of Si-1J and T-4B, indicating that the AVR-Pita3 homologs in these isolates are also located on chromosome 7. When BamHI digests of their genomic DNA were hybridized with APita3-958, 7.9 kb and 5.0 kb signals were detected in Si-1J and T-4B, respectively. In the mapping population derived from their cross, the two AVR-Pita3 signals (7.9 kb and 5.0 kb) cosegregated completely in repulsion and were mapped to a linkage group assigned to chromosome 7, apart from a telomere marker by 18.1 cM (Figure 4A).
To examine whether AVR-Pita3 has also frequently moved to different chromosomal locations, representative isolates were chosen from each host-specific subgroup of P. oryzae, and their chromosomal DNAs were again separated on a CHEF gel (Figure 9A). When the gel was blotted to a membrane and hybridized with APita3-958, similar sized fragments were identified for all isolates (Figure 9B). A comparison with the karyotype profiles on the other gel (Figure 5A) suggested that all of these signals corresponded to chromosome 7. To confirm it, the membrane was reprobed with chromosome 7 – specific markers (T1-A11 and CH5-75H). In all isolates, these chromosome 7 – specific markers hybridized to the chromosomal bands that hybridized to APita3-958 (Figure 9C). These results suggest that AVR-Pita3 has been stably located on chromosome 7 during the course of parasitic specialization into the host-adapted subgroups of P. oryzae.
Fig. 9. CHEF-Southern analyses of chromosomal locations of AVR-Pita3 in representative isolates of P. oryzae. 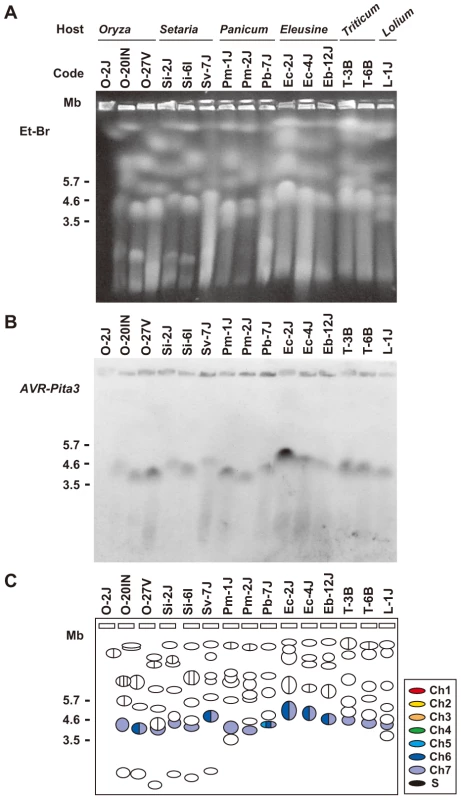
(A) Chromosomal DNAs separated in the contour-clamped homogeneous electric field (CHEF). The CHEF gel was stained with ethidium bromide. Note that the left five samples did not run straight. (B) Chromosomal bands carrying AVR-Pita3. The chromosomal DNAs in (A) were blotted and hybridized with the AVR-Pita3 probe (APita3-958). (C) Identification of the chromosomes hybridizing to the AVR-Pita3 probe. Chromosomes on the gel (A) were identified by reprobing the membrane (B) with chromosome – specific markers; e.g., T1-A11 and CH5-75H for chromosome 7 and T1-G4 for chromosome 6. Chromosomal bands that hybridized to the AVR-Pita3 probe in (B) were painted with the chromosome-specific colors used in Figure 5A. The membrane shown in Figure 9A contained chromosomes from three Oryza isolates. The membrane shown in Figure 3A (left) surveyed chromosomes from additional 20 Oryza isolates. Reprobing this membrane with APita3-958 produced the same pattern of chromosome 7-specific bands shown in Figure 3C. This suggests that AVR-Pita3 has been stably located on chromosome 7 during the course of race differentiation in the Oryza isolates.
Discussion
Multiple translocation of AVR-Pita homologs during parasitic specialization of the P. grisea/oryzae species complex
The most striking finding in the present study is that AVR-Pita is highly variable in its chromosomal location. In P. grisea, AVR-Pita2 is located within 30 kb of telomeric repeat sequence on chromosome 6 (Figure 6). AVR-Pita1 homologs in Setaria isolates (P. oryzae) reside at a different location near a chromosome 6 telomere (Si homologs) or on a supernumerary chromosome (Sv homologs) (Figure 6). The AVR-Pita chromosomal position was most variable in Oryza isolates, which were derived from asexual populations evolving in response to periodic deployment of the Pita resistance gene in rice (Figure 5B). In diverse Oryza isolates, AVR-Pita1 was absent or present in 1 or 2 copies residing on chromosomes 1, 3, 4, 5 or 7, or on different supernumerary chromosomes. Additionally, AVR-Pita1 types J1, J2 and CH mapped to three different locations on chromosome 4. This extraordinary variability in chromosomal position contrasts with the stable location of AVR-Pita3, a family member that lacks avirulence activity. AVR-Pita3 was stably localized on chromosome 7 in all isolates of all host-adapted forms of P. oryzae (Figure 9).
One simple explanation for the chromosomal position variation of AVR-Pita is that homologs have frequently been translocated to different chromosomal locations during the course of evolution of the P. grisea/oryzae species complex. An alternative explanation is that AVR-Pita originally occurred as a dispersed gene family in the ancestral strain, but family members were later lost in specific lineages (differential gene-loss). The large numbers of ancestral family members required (at least 12) for the gene-loss scenario make this hypothesis seem less likely, especially since contemporary isolates contain at most 3 copies of AVR-Pita. Additionally, the structures at the 3′ flanks of the AVR-Pita homologs indicate stepwise stacking of blocks of DNA fragments (Figure 7C), which is consistent with multiple steps of movement rather than with a process of differential gene-loss.
Aboukhaddour et al. [61] reported that genes for host-selective toxins (ToxA and ToxB) in Pyrenophora tritici-repentis were located on different sizes of chromosomes in different isolates. Unlike the case with AVR-Pita, however, most of these chromosomes were homologous. We shall call the frequent movement associated with AVR-Pita homologs “multiple translocation”, although the unit of movement in all cases seems to be a small DNA fragment (Figures 6 and 7C). It should be noted that the multiple translocation of AVR-Pita is not due to disintegration of chromosomal constitution. The mapping and CHEF analyses of the Si-1J x T-4B cross (Figure 4) suggested that, in spite of the chromosome-length polymorphisms between these two diverse parental isolates from the Setaria and Triticum host-adapted forms, the major, trunk regions of their chromosomes were syntenic. The relative chromosomal stability of the neutral chromosome-specific markers in our study resembles the stability of the nonrecognized AVR-Pita3 gene on chromosome 7 (Figure 9B, C).
AVR-Pita homologs are flanked by various transposable elements, suggesting that these elements have been involved in the multiple translocation events. Khang et al. [39] found fragments of retrotransposons, RETRO7-1 and RETRO6-1, in flanking regions of AVR-Pita homologs. The present study showed that these retrotransposons are portions of Inago1 (AB334124) and Inago2 (AB334125), respectively, and that full-sized copies are present in the flanking regions of some homologs (Pm and D1, Figure 6). All AVR-Pita homologs are accompanied by full-sized copies and/or solo-LTRs of Inago1, suggesting that this element has had a role in AVR-Pita movement (Figure 6). Based on this fact and phylogenetic relationships (Figure 7A), we suggest that the insertion of Inago1 into flanks of ancestral AVR-Pita homolog(s) was a key event that produced basic structure(s) associated with the AVR-Pita mobility. Retrotransposons and their solo-LTRs are known to mediate ectopic recombination through homologous recombination between preexisting elements located at non-allelic positions [62]. AVR-Pita may have been translocated through ectopic recombination mediated by Inago1 and its solo-LTR.
Why have AVR-Pita homologs undergone multiple translocation?
The extraordinary mobility of AVR-Pita in Oryza isolates compared to that in the other P. oryzae host-adapted forms suggests that the multiple translocation is associated with the long history of their arms race with rice varieties. In addition, the high mobility of AVR-Pita in contrast to AVR-Pita3, which lacks avirulence activity, suggests that the multiple translocation of AVR-Pita is associated with selection by Pita in rice. Then the first key question is why the recognition by Pita leads to the multiple translocation of AVR-Pita.
In laboratory studies, Orbach et al. [28] reported frequent spontaneous mutation of AVR-Pita to virulence on rice with Pita, and found that the majority of events were deletions of all or a portion of the gene. Similar mutations in AVR-Pita, including frequent deletions, are responsible for breakdown of Pita in the field [33], [36]. Takahashi et al. [35] recently reported that Oryza isolates collected in Japan carried two paralogs, AVR-Pita1JA and AVR-Pita1JB, which presumably correspond to J2/J3 and J1, respectively, and suggested that virulence on Pita has mainly evolved via deletion of AVR-Pita1JA. Nevertheless, the avirulence homolog is maintained in Oryza isolates from farmer's fields (Fig. 1). This leads to a second key question of how AVR-Pita is maintained in the current population of Oryza isolates. Without recovery of AVR-Pita, the system of continuous gene loss should not be sustainable.
We suggest that these two key questions could be simultaneously answered by assuming that the multiple translocation of AVR-Pita is associated with its frequent deletion and with recovery mediated by its transfer among individuals in asexual populations. It is well known that, after planting of resistant cultivars comes to a halt, the “unnecessary” virulence alleles at avirulence loci tend to disappear through “stabilizing selection” [63]. In other words, the frequency of avirulence genes in a population tends to increase again after the corresponding resistance genes are removed from the field. Recovery could result from restoration of minor races or migration of races from other regions. However, another possibility is that the avirulence genes may have actually been regained by the races that defeated the resistance genes. If DNA fragments carrying the avirulence genes are introduced into virulent isolates, they may insert into unstable chromosomal sites such as at telomeres. In this process of recovery, the avirulence genes will be recognized to have undergone multiple translocations.
Our data include evidence consistent with the transfer of genetic materials between individuals in the species complex. AVR-Pita1 was found in P. oryzae isolates while AVR-Pita2 was found in P. grisea isolates with one exception. The exception was the Pm type of AVR-Pita2 found in Panicum isolates of P. oryzae (Figure 7A). The nucleotide sequences of the AVR-Pita2 fragments in the Digitaria and Panicum isolates are more highly conserved than expected conserved genes such as the actin and ß-tubulin genes. It should also be noted that both types of AVR-Pita2 (Pm in P. oryzae and D1 in P. grisea) had an insertion of a solo-LTR of Inago2 into their 5′ flanks (Figure 7B). Retroelements are good markers for tracing evolutionary processes, and their insertion patterns can be treated as synapomorphic characters [64]. Although we cannot completely rule out the possibility that the Pm and D1 AVR-Pita2 genes have been highly conserved after introduction from a common ancestor, these data strongly suggest that AVR-Pita2 has been horizontally transferred beyond species. Therefore, there is no reason to exclude the possibility that AVR-Pita has been transferred among individuals within the same species (P. oryzae) and within the same host-specific subgroup (Oryza isolates).
If this scenario is correct, our results suggest that rare events in which AVR-Pita had been inserted into a new chromosomal location were selected for in the Oryza population. This implies that the avirulence allele of AVR-Pita confers a fitness advantage that is not provided by AVR-Pita3. So far, attempts to understand how AVR-Pita functions to promote disease in the absence of the Pita resistance gene have not succeeded. Defining this fitness role for the different AVR-Pita family members remains an important priority.
Potential role of supernumerary chromosomes in the multiple translocation of AVR-Pita homologs
An additional significant finding from this study is that AVR-Pita frequently appears on supernumerary chromosomes (Figure 5B), which were previously reported to be common in Oryza isolates reproducing asexually in the field [49]. Supernumerary chromosomes are extra chromosomes (B chromosomes) composed primarily of DNA not found in all representatives of the species [65]. In some fungal species, genes on such chromosomes play important roles in host-parasite interactions. In Nectria haematococca, a supernumerary chromosome carries genes encoding cytochrome P450 monooxygenases for phytoalexin detoxification and other genes contributing to pathogenicity [66], [67]. In Alternaria alternata, supernumerary chromosomes carry genes involved in the production of host-specific toxins [68]–[71].
Supernumerary chromosomes are structurally unstable [48], [66]. Here, we will point out three properties of supernumerary chromosomes that may have been associated with the dynamics of avirulence genes. First, supernumerary chromosomes are known to be spontaneously lost from the genome [70] because they are dispensable. This property of supernumerary chromosomes would confer a selective advantage upon isolates carrying avirulence genes on those chromosomes; such isolates could adapt quickly to cultivars carrying corresponding resistance genes by losing the supernumerary chromosomes. Second, supernumerary chromosomes are suggested to have contributed to gene expansion [72]. Some of the “expanded” genes appeared to have resulted from gene duplication events [72]. The present results with O-23IN suggest that supernumerary chromosomes may contribute to the gene duplication through duplication of themselves. Finally, supernumerary chromosomes or small chromosomes are suggested to have been horizontally transferred [68], [73], [74]. Supernumerary chromosomes in the P. grisea/oryzae species complex may have played significant roles in the loss, duplication, and multiple translocation of AVR-Pita.
How and where can AVR-Pita be transferred among isolates?
In laboratory studies, Oryza isolates are known to exchange DNA through parasexual recombination following hyphal anastomosis and transient diploid formation [75]. Zeigler et al. [17] suggested that parasexual DNA exchanges occur at a detectable frequency in the field. Furthermore, Oryza isolates are sometimes isolated from blast lesions on nonhost weeds, e.g., green bristlegrass (Setaria viridis) and crabgrass (Digitaria sanguinalis), around paddy fields (data not shown). They are considered to have colonized blast lesions produced by adapted isolates (Setaria isolates and Digitaria isolates) through opportunistic infection. Such lesions on weeds may provide opportunities for different isolates to exchange supernumerary chromosomes or DNA fragments carrying avirulence genes. Further studies are needed to test this hypothesis.
Does multiple translocation occur with other blast avirulence genes?
AVR-Pii, an avirulence gene corresponding to the Pii resistance gene of rice, appears to be subtelomeric like AVR-Pita, and is located on chromosome 7 [76]. AVR-Pia, an avirulence gene corresponding to the Pia resistance gene, also appears to be subtelomeric and is located on chromosome 7 in a cross [77]. However, in studies by Yasuda et al. [76], AVR-Pia was linked to three markers from chromosome 5 and one marker (40-12-G) from chromosome 7 that was presumed to reside in chromosome 5 in their isolates. AVR-Pik, an avirulence gene corresponding to the Pik resistance gene, was linked to DNA markers on chromosome 1 in some crosses [46], [78]. In Japanese isolate 84R-62B, however, this gene was located on a small 1.6Mb chromosome derived from fusion of a portion of chromosome 1 to a supernumerary chromosome [79]. These results suggest that AVR-Pia and AVR-Pik have undergone translocation, sometimes via supernumerary chromosomes. Our preliminary experiments also suggest that AVR-Pia, AVR-Pik, and AVR-Pii, have undergone multiple translocation. These data will be reported elsewhere.
Are the multiple translocations of these avirulence genes also mediated by loss/gain processes? Using Japanese isolates, Miki et al. [31] found a perfect correspondence between the presence or absence of AVR-Pia signals and avirulence or virulence on Pia, respectively. They also found that the homologs in avirulent isolates shared 100% identical nucleotide sequences. Similar results were obtained by Yoshida et al. [32]. These results suggest that the primary mechanism for Oryza isolates to overcome Pia is deletion of AVR-Pia from their genomes. AVR-Pik homologs (AVR-Pik/km/kp homologs) exhibited both presence/absence and nucleotide polymorphisms [32]. Yoshida et al. [32] found some isolates that were virulent on Pik in spite of carrying AVR-Pik homologs (A, B, C). These nonfunctional homologs were presumed to be paralogs rather than orthologous variants of the functional homolog (D) because several isolates contained both A and D homologs. This distribution of homologs resembles that of AVR-Pita. That is, most Oryza isolates carry AVR-Pita homologs, but they are composed of functional and non-functional paralogs (Figs. 1, 2), and deletion of the functional paralogs (J2 and J3) is primarily associated with the gain of virulence on Pita (Fig. 1 and unpublished data). Consequently, deletion of the functional AVR-Pik paralog seems to be a common mechanism for Oryza isolates to overcome Pik. AVR-Pii also appears to be deleted in virulent strains of the fungus [32]. Taken together, these results suggest that AVR-Pia, AVR-Pik, and AVR-Pii have undergone multiple translocations through the loss/gain processes. It is not clear why AVR-Pita and AVR-Pik have developed paralogs while AVR-Pia has not. It may be attributable to the structure in their flanking regions. Alternatively, the loss of AVR-Pita and AVR-Pik may pose higher fitness costs to isolates. If this is the case, the “nonfunctional” paralogs in terms of avirulence should retain their original function as effectors.
The involvement of the loss/gain processes in the arms race of P. oryzae against rice has also been suggested by Yoshida et al. [32] based on their observation that the majority of candidate effector loci in P. oryzae displayed low nucleotide diversity while frequently showing presence/absence polymorphisms. We assume that, in general, effector genes located on unstable chromosomal regions have a potential to be translocated sometimes within an individual and sometimes through loss/gain processes. M. oryzae may use the loss/gain system for adaptaion to resistance genes.
Jones and Dangl [80] noted that effector genes are often associated with transposable elements or telomeres and are commonly observed as presence/absence polymorphisms across bacterial and fungal pathogens. This observation led them to suggest that the simplest pathogen response to host recognition is to jettison the detected effector gene, provided the population's effector repertoire can cover the potential loss of fitness on susceptible hosts. It is of interest to examine whether the multiple translocation of avirulence genes may also be observed in other plant pathogens.
Comparison with telomeric genes in other microbes
Whatever the mechanism, multiple translocation has resulted in the dispersal of AVR-Pita to various sites (often in subtelomeric regions) of various chromosomes in the population of the P. grisea/oryzae species complex. Similar dispersal has been recognized in subtelomeric gene families of yeast that are involved in niche-specific processes [81]–[83]. Brown et al. [83] suggested that the evolvability of such subtelomeric gene families allows rapid adaptation to novel niches.
From the viewpoint of the importance of subtelomeric gene families in adaptation, we also notice a similarity to the immune evasion systems in animal pathogens. Several P. oryzae avirulence genes are mapped to terminal regions of chromosomes [46], [76], [77], [84]–[86], which are extraordinarily unstable in some strains [85]. One tempting hypothesis from this observation is that a telomere-based switching mechanism might underlie the pathogenic variability as in the animal pathogens [87]. However, Rehmeyer et al. [87] failed to identify the massively amplified families of surface protein genes or genes coding for secreted proteins in subtelomeric regions, which makes it unlikely that this fungus uses switching mechanisms like those in animal pathogens to evade the host defenses [85].
Here, we suggest that the strategy for host adaptation adopted by (fungal) plant pathogens is fundamentally different from that adopted by (protozoan) animal pathogens although they initially appear similar. First, antigenic genes are indispensable for the survival of the animal pathogens whereas individual avirulence genes are dispensable for the survival of the plant pathogens (as exemplified by O-28V and O-21IN in Figure 1). This makes it possible for the plant pathogens to survive after deletion of avirulence genes from their genomes, although the avirulence gene may provide an advantage in fitness in the field that favors regaining the gene after the resistance gene is removed [3]. Second, an individual of the animal pathogens carries a huge number of antigenic genes, e.g., ∼60 var genes in P. falciparum and more than 1000 VSG (variant surface glycoprotein) genes in T. brucei [88], whereas an isolate of the plant pathogens carry at most a few copies of individual avirulence genes. Third, antigenic genes are highly variable whereas avirulence genes themselves are relatively stable (Figure 8B). The high posterior probabilities on branches of the Bayesian tree (Figure 7A) suggest that the AVR-Pita homologs have not suffered from shuffling of gene fragments. Taken together, the interaction between animals and their protozoan pathogens appears to be a battle between individuals whereas the interaction between plants and their fungal pathogens seems to be a battle between populations. An animal host produces various antibodies in an individual. To evade the recognition by the antibodies, an animal pathogen carries a huge reservoir of antigenic genes in an individual. On the other hand, a plant individual carries at most a few resistance genes against a given pathogen. As a population, however, plants carry various resistance genes and, by using this variation, they survive pathogen attacks. Similarly, an isolate of plant pathogens carries at most a few copies of avirulence genes. As a population, however, a plant pathogen carries various avirulence genes at various chromosomal sites. In other words, a plant pathogen carries a reservoir of avirulence genes in its population. A Pyricularia population composed of various isolates, each of which carry avirulence genes at different sites on different chromosomes, may be an equivalent to an animal pathogen individual which carries antigenic genes at various sites of various chromosomes in its own nucleus. The dynamic adaptation of fungal plant pathogens may be primarily achieved by the deletion and recovery of avirulence genes using a population as a unit of adaptation. It appears to be the dispensability of individual avirulence genes that enables the fungal plant pathogens to adopt this strategy.
Materials and Methods
Pyricularia isolates and strains
Pyricularia isolates used in the present study are listed in Table S1. In addition to these field isolates, four F1 populations derived from crosses between them were employed for molecular mapping: (i) 60 random cultures derived from a cross, O-29J (84R-62B) x O-30C (Y93-245c-2) [46], (ii) 40 cultures (composed of ascospore cultures representing each meiotic product in ten tetrads) derived from a cross, O-23IN (PO12-7301-2) x T-4B (Br48) [22], (iii) 78 random cultures derived from a cross, Si-1J (GFSI1-7-2) x T-4B (Br48) [51], and (iv) 33 random cultures derived from a cross, Si-6I (IN77-20-1-1) x T-7B (Br116.5). In (i), (iii), and (iv), each culture was derived from a distinct ascus. The (iv) population was produced in the present study using methods described previously [89]. The eight ascospores in a single tetrad are derived by mitotic division of each product of meiosis. The (ii) population included only one representative of each of the four products of meiosis, which are easily differentiated based on cultural morphologies, mating types and other markers (Figure 2C). All cultures were maintained as described previously [12].
DNA extraction and Southern hybridization
Fungal genomic DNAs were extracted as described by Nakayashiki et al. [90], digested with restriction enzymes, electrophoresed on 0.7% agarose gels, and blotted to Hybond N+ membranes (GE healthcare, Buckinghamshire, U.K.). Southern hybridization of the genomic DNA digests was performed using the Alkphos Direct Labeling and Detection System (GE healthcare) for the telomere repeat probe (a synthetic oligonucleotide (TTAGGG)10) or the ECL Direct Nucleic Acid Labeling and Detection System (GE healthcare) for the other probes, according to the manufacturer's instructions. To detect AVR-Pita, a 766 bp fragment was amplified from O-5J using AVR-Pita-specific primers (AVRPita-ORF-F2 and AVRPita-ORF-R2) (Table S3), cloned into pBluescript II SK (+), and established as pP4-1 (Table S2). The insert was amplified from pP4-1 with the same primers and used as an AVR-Pita-specific probe, APita766. Sequence analysis revealed that the APita766 fragment was derived from J3(O-5J), the functional copy of O-5J. Homology of the APita766 fragment to AVR-Pita1, AVR-Pita2, and AVR-Pita3 [39] was >98%, ∼91%, and ∼70%, respectively, suggesting that APita766 hybridizes to AVR-Pita1 and AVR-Pita2, but not to AVR-Pita3, with the hybridization conditions used. To detect AVR-Pita3, a 958 bp fragment was amplified from Ei-13I using AVR-Pita3-specific primers (AVRPita-G22-2 and AVRPita-G22-3) (Table S3), and used as an AVR-Pita3-specific probe, APita3-958.
Genetic mapping
Molecular markers used in the genetic mapping are listed in Table S4. Clones for the WU markers were provided by S.A. Leong, University of Wisconsin-Madison, U.S.A. Nucleotide sequences of the WU markers were provided by M. Farman, University of Kentucky, U.S.A. Cosmid clones for the KU markers were selected from genomic libraries of Si-1J and T-4B which were constructed in the pMLF2 vector as described by Nakayashiki et al. [53]. SSR markers developed by Zheng et al. [52] were used as additional chromosome-specific markers. PCR products amplified from SSR loci were fractionated by electrophoresis through 6% denaturing polyacrylamide gels and stained using SILVER SEQUENCE DNA Staining Reagents (Promega, Madison, WI, USA). AFLP analysis was performed using AFLP Analysis System for Microorganisms (Invitrogen, Carlsbad, CA, USA) as described previously [51]. Segregation data were analyzed using MAPMAKER Macintosh V2.0 with the haploid program. Parameters for map construction were a minimum LOD of 3.0 and a maximum theta of 0.4. The Kosambi mapping function was employed to compute recombination distances in centimorgans (cM).
Electrophoretic karyotyping
Preparation of gel plugs, running conditions for CHEF gel electrophoresis, staining, blotting and hybridization procedures were described previously [48]. To prevent chromosomal degradation caused by Tris-radical reaction, 200 µM thiourea was added to TBE buffer as described by Römling and Tümmler [91].
Plasmid, cosmid, and fosmid clones
Cosmid, fosmid, and plasmid clones used for sequence analyses or complementation assays are listed in Table S2. A cosmid library of O-11J (2403-1) was constructed in the pMLF2 vector as described by Nakayashiki et al. [53]. Fosmid libraries of O-29J, O-30C, Si-1J, Si-6I, Pm-1J, and Dsa-1J were constructed with CopyControl Fosmid Library Production Kit (EPICENTRE, Madison, Wisconsin) by Takara Bio (Shiga, Japan). Total DNAs were sheared into approximately 40 kb fragments, blunt-ended, and ligated into the pCC1FOS vector. Clones containing AVR-Pita homologs were selected from these libraries by colony hybridization. Plasmid clones were constructed by inserting AVR-Pita-containing fragments into pBluescript II SK (+) as described in Table S2.
DNA sequencing
Cosmid, fosmid, and plasmid clones listed in Table S2 were sequenced using the ABI Prism Big Dye Terminator Cycle Sequencing Kit (Applied Biosystems, Foster City, CA) and the ABI 3100 Genetic Analyzer following the manufacturer's instructions. When PCR clones were used as templates, three or more clones from each ligation reaction were sequenced and compared to eliminate PCR errors. For Si(Si-5I) and Sv(Sv-8J) PCR amplicons were directly sequenced using the primers listed in Table S3. The resulting sequences were assembled using SeqManII (DNASTAR, Madison, WI), analyzed with Genetyx version 6.1.1 (GENETYX, Tokyo) and GeneQuest (DNASTAR, Madison, Wisconsin), and aligned with MEGA 4 [92].
Genetic complementation assay
A total of 13 clones containing AVR-Pita homologs were used for the transformation assay (Table 1 and Table S2). For J1(O-29J) and J2(O-29J), fosmid clones with these homologs selected from the O-29J fosmid library were used without subcloning as a negative and positive controls. For Si(Si-6I), Pm(Pm-1J), and D1(Dsa-1J), restriction fragments containing them were isolated from gels, ligated with pBluescript II SK(+), and transformed into E. coli. Colonies carrying the target homologs were detected by colony hybridization. For the other 8 homologs PCR cloning was performed. Each of the 8 homologs was amplified from genomic DNA so that the amplicon contained the conserved region, and cloned into pBluescript II SK (+). The 13 plasmid and fosmid clones were introduced into O-8J (P-2b) by co-transformation with pSH75 containing the hygromycin B phosphotransferase gene [93] as described by Tosa et al. [94]. Five transformants carrying full-length transgene(s) were chosen for each homolog through Southern hybridization, and employed for infection assay. Pathogenicity tests were performed as described previously [94].
Phylogenetic analysis
Two isolates (O-29J and O-30C) were added to the isolates used by Hirata et al. [12], and a phylogenic tree of Pyricularia isolates was constructed again as described previously [12]. The phylogenetic tree of AVR-Pita homologs was constructed using Bayesian inference in MrBayes v3.1.2 [95]. We chose the general time-reversible model and across-site rate variation for gamma distribution with a proportion of invariant sites for the analysis. Two independent Monte Carlo Markov Chain analyses for 500,000 generations were performed. The temperature for heating the chains was 0.2. We sampled once every 100 generations. After the 500,000 generations, average standard deviation of split frequency was 0.0076, which is acceptable for convergence between trees from the two parallel runs. After the first 1250 trees were discarded, a 50% majority rule consensus tree was constructed based on the remaining samples.
Accession numbers
Sequence data from this article can be found in the EMBL/GenBank data libraries under accession numbers: AB607333, J1(O-20IN); AB607334, J1(O-29J); AB607335, J1(O-30C); AB607336, J2(O-29J); AB607337, J3(O-2J); AB607338, PO(O-23IN); AB607339, PO(O-20IN); AB607340, Si(Si-6I); AB607341, Sv(Sv-7J); AB607342, Pm(Pm-1J); AB607343, D1(Dsa-1J); AB607344, D1(Dh-5B); AB607345, Ce1(Ce-1B).
Supporting Information
Zdroje
1. HeathMC 1981 A generalized concept of host-parasite specificity. Phytopathology 71 1121 1123
2. KiyosawaS 1982 Genetics and epidemiological modeling of breakdown of plant disease resistance. Ann Rev Phytopathol 20 93 117
3. LeachJECruzCMVBaiJLeungH 2001 Pathogen fitness penalty as a predictor of durability of disease resistance genes. Ann Rev Phytopathol 39 187 224
4. McDonaldBALindeC 2002 Pathogen population genetics, evolutionary potential, and durable resistance. Ann Rev Phytopathol 40 349 379
5. FlorHH 1956 The complementary genic systems in flax and flax rust. Adv Genet 8 29 54
6. FudalIRossSBrunHBesnardA-LErmelM 2009 Repeat-induced point mutation (RIP) as an alternative mechanism of evolution toward virulence in Leptosphaeria maculans. Mol Plant-Microbe Interact 22 932 941
7. Van de WouwAPCozijnsenAJHaneJKBrunnerPCMcDonaldBA 2010 Evolution of linked avirulence effectors in Leptosphaeria maculans is affected by genomic environment and exposure to resistance genes in host plants. PLoS Pathog 6 e1001180
8. De WitPJGMMehrabiRvan den BurgHAStergiopoulosI 2009 Fungal effector proteins: past, present and future. Mol Plant Pathol 10 735 747
9. GoutLKuhnMLVincenotLBernard-SamainSCattolicoL 2007 Genome structure impacts molecular evolution at the AvrLm1 avirulence locus of the plant pathogen Leptosphaeria maculans. Envion Microbiol 9 2978 2992
10. SchürchSLindeCCKnoggeWJacksonLFMcDonaldBA 2004 Molecular population genetic analysis differentiates two virulence mechanisms of the fungal avirulence gene NIP1. Mol Plant-Microbe Interact 17 1114 1125
11. KiyosawaSAndoIIshiguroKNemotoFHashimotoA 1991 Three examples of yearly change of frequencies of unnecessary virulence genes under field conditions where resistance genes for rice blast are not present. Bull Fukushima Pref Agri Exp Stn 30 23 36
12. HirataKKusabaMChumaIOsueJNakayashikiH 2007 Speciation in Pyricularia inferred from multilocus phylogenetic analysis. Mycol Res 111 799 808
13. KatoHYamamotoMYamaguchi-OzakiTKadouchiHIwamotoY 2000 Pathogenicity, mating ability and DNA restriction fragment length polymorphisms of Pyricularia populations isolated from Gramineae, Bambusideae and Zingiberaceae plants. J Gen Plant Pathol 66 30 47
14. CouchBCKohnLM 2002 A multilocus gene genealogy concordant with host preference indicates segregation of a new species, Magnaporthe oryzae, from M. grisea. Mycologia 94 683 693
15. TosaYHirataKTambaHNakagawaSChumaI 2004 Genetic constitution and pathogenicity of Lolium isolates of Magnaporthe oryzae in comparison with host species-specific pathotypes of the blast fungus. Phytopathology 94 454 462
16. KatoH 1983 Responses of Italian millet, oat, timothy, Italian ryegrass and perennial ryegrass to Pyricularia species isolated from cereals and grasses. Proc Kanto-Tosan Plant Prot Soc 30 22 23 (In Japanese)
17. ZeiglerRSScottRPLeungHBordeosAAKumarJ 1997 Evidence of parasexual exchange of DNA in the rice blast fungus challenges its exclusive clonality. Phytopathology 87 284 294
18. KatoHYamaguchiTNishiharaN 1976 The perfect state of Pyricularia oryzae Cav. in culture. Ann Phytopathol Soc Jpn 42 507 510
19. YaegashiHNishiharaN 1976 Production of the perfect stage in Pyricularia from cereals and grasses. Ann Phytopathol Soc Jpn 42 511 515
20. UeyamaATsudaM 1975 Formation of the perfect state in culture of Pyricularia sp. from some graminaceous plants (preliminary report). Trans Mycol Soc Jpn 16 420 422
21. TakabayashiNTosaYOhHSMayamaS 2002 A gene-for-gene relationship underlying the species-specific parasitism of Avena/Triticum isolates of Magnaporthe grisea on wheat cultivars. Phytopathology 92 1182 1188
22. TosaYTambaHTanakaKMayamaS 2006 Genetic analysis of host species specificity of Magnaporthe oryzae isolates from rice and wheat. Phytopathology 96 480 484
23. ValentBKhangCH 2010 Recent advances in rice blast effector research. Curr Opin Plant Biol 13 434 441
24. SilueDNotteghemJLTharreauD 1992 Evidence of a gene-for-gene relationship in the Oryza sativa – Magnaporthe grisea pathosystem. Phytopathology 82 577 580
25. KangSSweigardJAValentB 1995 The PWL host specificity gene family in the blast fungus Magnaporthe grisea. Mol Plant-Microbe Interact 8 939 948
26. SweigardJACarrollAMKangSFarrallLChumleyFG 1995 Identification, cloning, and characterization of PWL2, a gene for host species specificity in the rice blast fungus. Plant Cell 7 1221 1233
27. FarmanMLLeongSA 1998 Chromosome walking to the AVR1-CO39 avirulence gene of Magnaporthe grisea: Discrepancy between the physical and genetic maps. Genetics 150 1049 1058
28. OrbachMJFarrallLSweigardJAChumleyFGValentB 2000 A telomeric avirulence gene determines efficacy for the rice blast resistance gene Pi-ta. Plant Cell 12 2019 2032
29. BöhnertHUFudalIDiohWTharreauDNotteghemJ-L 2004 A putative polyketide synthase/peptide synthetase from Magnaporthe grisea signals pathogen attack to resistance rice. Plant Cell 16 2499 2513
30. LiWWangBWuJLuGHuY 2009 The Magnaporthe oryzae avirulence gene AvrPiz-t encodes a predicted secreted protein that triggers the immunity in rice mediated by the blast resistance gene Piz-t. Mol Plant-Microbe Interact 22 411 420
31. MikiSMatsuiKKitoHOtsukaKAshizawaT 2009 Molecular cloning and characterization of the AVR-Pia locus from a Japanese field isolate of Magnaporthe oryzae. Mol Plant Pathol 10 361 374
32. YoshidaKSaitohHFujisawaSKanzakiHMatsumuraH 2009 Association genetics reveals three novel avirulence genes from the rice blast fungal pathogen Magnaporthe oryzae. Plant Cell 21 1573 1591
33. DaiYJiaYCorrellJWangXWangY 2010 Diversification and evolution of the avirulence gene AVR-Pita1 in field isolates of Magnaporthe oryzae. Fun Genet Biol 37 973 980
34. KangSLebrunMHFarrallLValentB 2001 Gain of virulence caused by insertion of a Pot3 transposon in a Magnaporthe grisea avirulence gene. Mol Plant-Microbe Interact 14 671 674
35. TakahashiMAshizawaTHirayaeKMoriwakiJSoneT 2010 One of two major paralogs of AVR-Pita1 is functional in Japanese rice blast isolates. Phytopathology 100 612 618
36. ZhouEJiaYSinghPCorrellJCLeeFN 2007 Instability of the Magnaporthe oryzae avirulence gene AVR-Pita alters virulence. Fungal Genet Biol 44 1024 1034
37. BryanGTWuKSFarrallLJiaYHersheyHP 2000 A single amino acid difference distinguishes resistant and susceptible alleles of the rice blast resistance gene Pi-ta. Plant Cell 12 2033 2045
38. JiaYMcAdamsSABryanGTHersheyHPValentB 2000 Direct interaction of resistance gene and avirulence gene products confers rice blast resistance. EMBO J 19 4004 4014
39. KhangCHParkS-YLeeY-HValentBKangS 2008 Genome organization and evolution of the AVR-Pita avirulence gene family in the Magnaporthe grisea species complex. Mol Plant-Microbe Interact 21 658 670
40. BerrimanMGhedinEHertz-FowlerCBlandinGRenauldH 2005 The genome of the African trypanosome Trypanosoma brucei. Science 309 416 422
41. DreesenOLiBCrossGAM 2007 Telomere structure and function in trypanosomes: a proposal. Nat Rev Microbiol 5 70 75
42. TaylorJERudenkoG 2006 Switching trypanosome coats: what's in the wardrobe? Trends in Genet 22 614 620
43. GardnerMJHallNFungEWhiteOBerrimanM 2002 Genome sequence of the human malaria parasite Plasmodium falciparum. Nature 419 498 511
44. Freitas-JuniorLHBottiusEPirritLADeitschKWScheidigC 2000 Frequent ectopic recombination of virulence factor genes in telomeric chromosome clusters of P. falciparum. Nature 407 1018 1022
45. LuoCXHanamuraHSezakiHKusabaMYaegashiH 2002 Relationship between avirulence genes of the same family in rice blast fungus Magnaporthe grisea. J Gen Plant Pathol 68 300 306
46. LuoCXYinLFKoyanagiSFarmanMLKusabaM 2005 Genetic mapping and chromosomal assignment of Magnaporthe oryzae avirulence genes AvrPik, AvrPiz, and AvrPiz-t controlling cultivar specificity on rice. Phytopathology 95 640 647
47. NittaNFarmanMLLeongSA 1997 Genome organization of Magnaporthe grisea: integration of genetic maps, clustering of transposable elements and identification of genome duplications and rearrangements. Theor Appl Genet 95 20 32
48. ChumaITosaYTagaMNakayashikiHMayamaS 2003 Meiotic behavior of a supernumerary chromosome in Magnaporthe oryzae. Curr Genet 43 191 198
49. OrbachMJChumleyFGValentB 1996 Electrophoretic karyotypes of Magnaporthe grisea pathogens of diverse grasses. Mol Plant-Microbe Interact 9 261 271
50. TalbotNJSalchYPMaMHamerJE 1993 Karyotypic variation within clonal lineages of the rice blast fungus, Magnaporthe grisea. Appl Environ Microbiol 59 585 593
51. ChumaIZhanS-WAsanoSNgaNTTVyTTP 2010 PWT1, an avirulence gene of Magnaporthe oryzae tightly linked to the rDNA locus, is recognized by two staple crops, common wheat and barley. Phytopathology 100 436 443
52. ZhengYZhangGLinFWangZJinG 2008 Development of microsatellite markers and construction of genetic map in rice blast pathogen Magnaporthe grisea. Fungal Genet Biol 45 1340 1347
53. NakayashikiHMatsuoHChumaIIkedaKBetsuyakuS 2001 Pyret, a Ty3/gypsy retrotransposon in Magnaporthe grisea contains an extra domain between the nucleocapsid and protease domains. Nucleic Acids Res 29 4106 4113
54. KangS 2001 Organization and distribution pattern of MGLR-3, a novel retrotransposon in the rice blast fungus Magnaporthe grisea. Fungal Genet Biol 32 11 19
55. HamerJEFarrallLOrbachMJValentBChumleyFG 1989 Host species-specific conservation of a family of repeated DNA sequences in the genome of a fungal plant pathogen. Proc Natl Acad Sci USA 86 9981 9985
56. KachrooPAhujaMLeongSAChattooBB 1997 Organization and molecular analysis of repeated DNA sequences in the rice blast fungus Magnaporthe grisea. Curr Genet 31 361 369
57. KachrooPLeongSAChattooBB 1994 Pot2, an inverted repeat transposon from the rice blast fungus Magnaporthe grisea. Mol Gen Genet 245 339 348
58. FarmanMLTauraSLeongSA 1996 The Maganaporthe grisea DNA fingerprinting probe MGR586 contains the 3' end of an inverted repeat transposon. Mol Gen Genet 251 675 681
59. KitoHTakahashiYSatoJFukiyaSSoneT 2003 Occan, a novel transposon in the Fot1 family, is ubiquitously found in several Magnaporthe grisea isolates. Curr Genet 42 322 331
60. FarmanMLTosaYNittaNLeongSA 1996 MAGGY, a retrotransposon in the genome of the rice blast fungus Magnaporthe grisea. Mol Gen Genet 251 665 674
61. AboukhaddourRCloutierSBallanceGMLamariL 2009 Genome characterization of Pyrenophora tritici-repentis isolates reveals high plasticity and independent chromosomal location of ToxA and ToxB. Mol Plant Pathol 10 201 212
62. MieczkowskiPALemoineFJPetesTD 2006 Recombination between retrotransposons as a source of chromosome rearrangements in the yeast Saccharomyces cerevisiae. DNA repair 5 1010 1020
63. Van der PlankJE 1984 Disease Resistance in Plants. Academic Press, Inc 194
64. MurataSTakasakiNSaitohMOkadaN 1993 Determination of the phylogenetic relationships among Pacific salmonids by using short interspersed elements (SINEs) as temporal landmarks of evolution. Proc Natl Acad Sci USA 90 6995 6999
65. CovertSF 1998 Supernumerary chromosomes in filamentous fungi. Curr Genet 33 311 319
66. MiaoVPCovertSFVanEttenHD 1991 A fungal gene for antibiotic resistance on a dispensable (“B”) chromosome. Science 254 1773 1776
67. HanYLiuXBennyUKistlerCVanEttenHD 2001 Genes determining pathogenicity to pea are clustered on a supernumerary chromosome in the fungal plant pathogen Nectria haematococca. Plant J 25 305 314
68. AkagiYAkamatsuHOtaniHKodamaM 2009 Horizontal chromosome transfer, a mechanism for the evolution and differentiation of a plant-pathogenic fungus. Eukaryot Cell 8 1732 1738
69. HattaRItoKHosakiYTanakaTTanakaA 2002 A conditionally dispensable chromosome controls host-specific pathogenicity in the fungal plant pathogen Alternaria alternata. Genetics 161 59 70
70. JohnsonLJJohnsonRDAkamatsuHSalamiahAOtaniH 2001 Spontaneous loss of a conditionally dispensable chromosome from the Alternaria alternata apple pathotype leads to loss of toxin production and pathogenicity. Curr Genet 40 65 72
71. MasunakaAOhtaniKPeeverTLTimmerLWTsugeT 2005 An isolate of Alternaria alternata that is pathogenic to both tangerines and rough lemon and produces two host-selective toxins, ACT - and ACR-toxins. Phytopathology 95 241 247
72. ColemanJJRounsleySDRodriguez-CarresMKuoAWasmannCC 2009 The genome of Nectria haematococca: Contribution of supernumerary chromosomes to gene expansion. PLoS Genet 5 e1000618
73. HeCRusuAGPoplawskiAMIrwinJAGMannersJM 1998 Transfer of a supernumerary chromosome between vegetatively incompatible biotypes of the fungus Colletotrichum gloeosporioides. Genetics 150 1459 1466
74. MaLJvan der DoesHCBorkovichKAColemanJJDaboussiMJ 2010 Comparative genomics reveals mobile pathogenicity chromosomes in Fusarium. Nature 464 367 373
75. NoguchiMTYasudaNFujitaY 2006 Evidence of genetic exchange by parasexual recombination and genetic analysis of pathogenicity and mating type of parasexual recombinants in rice blast fungus, Magnaporthe oryzae. Phytopathology 96 746 750
76. YasudaNTsujimoto-NoguchiMFujitaY 2006 Partial mapping of avirulence genes AVR-Pii and AVR-Pia in the rice blast fungus Magnaporthe oryzae. Can J Plant Pathol 28 494 498
77. ChenQHWangYCLiANZhangZGZhengXB 2007 Molecular mapping of two cultivar-specific avirulence genes in the rice blast fungus Magnaporthe grisea. Mol Genet Genomics 277 139 148
78. FengSMaJLinFWangLPanQ 2007 Construction of an electronic physical map of Magnaporthe oryzae using genomic position-ready SSR markers. Chin Sci Bull 52 3346 3354
79. LuoCXYinLFOhtakaKKusabaM 2007 The 1.6 Mb chromosome carrying the avirulence gene AvrPik in Magnaporthe oryzae isolate 84R-62B is a chimera containing chromosome 1 sequences. Mycol Res 111 232 239
80. JonesJDGDanglJL 2006 The plant immune system. Nature 444 323 329
81. CarlsonMCelenzaJLEngFJ 1985 Evolution of the dispersed SUC gene family of Saccharomyces by rearrangements of chromosome telomeres. Mol Cell Biol 5 2894 2902
82. CharronMJReadEHautSRMichelsCA 1989 Molecular evolution of the telomere-associated MAL loci of Saccharomyces. Genetics 122 307 316
83. BrownCAMurrayAWVerstrepenKJ 2010 Rapid expansion and functional divergence of subtelomeric gene families in yeasts. Curr Biol 20 895 903
84. DiohWTharreauDNotteghemJLOrbachMJLebrunM-H 2000 Mapping of avirulence genes in the rice blast fungus, Magnaporthe grisea, with RFLP and RAPD markers. Mol Plant-Microbe Interact 13 217 227
85. FarmanML 2007 Telomeres in the rice blast fungus Magnaporthe oryzae: the world of the end as we know it. FEMS Microbiol Lett 273 125 132
86. ValentBChumleyFG 1994 Avirulence genes and mechanisms of genetic instability in the rice blast fungus. ZeiglerRSLeongSATengPS The Rice Blast Disease Cambridge International Rice Research Institute, Los Banos and Commonwealth Agricultural Bureaux 111 134 Chapter 7
87. RehmeyerCLiWKusabaMKimY-SBrownD 2006 Organization of chromosome ends in the rice blast fungus, Magnaporthe oryzae. Nucl Acid Res 34 4685 4701
88. VerstrepenKKFinkGR 2009 Genetic and epigenetic mechanisms underlying cell-surface variability in protozoa and fungi. Annu Rev Genet 43 1 24
89. MurakamiJTosaYKataokaTTomitaRKawasakiJ 2000 Analysis of host species specificity of Magnaporthe grisea toward wheat using a genetic cross between isolates from wheat and foxtail millet. Phytopathology 90 1060 1067
90. NakayashikiHKiyotomiKTosaYMayamaS 1999 Transposition of the retrotransposon MAGGY in heterologous species of filamentous fungi. Genetics 153 693 703
91. RömlingUTümmlerB 2000 Achieving 100% type ability of Pseudomonas aeruginosa by pulsed-field gel electrophoresis. J Clin Microbiol 38 464 465
92. TamuraKDudleyJNeiMKumarS 2007 MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) Software Version 4.0. Mol Biol Evol 24 1596 1599
93. KimuraNTsugeT 1993 Gene cluster involved in melanin biosynthesis of the filamentous fungus Alternaria alternata. J Bacteriol 175 4427 4435
94. TosaYOsueJEtoYOhHNakayashikiH 2005 Evolution of an avirulence gene, AVR1-CO39, concomitant with the evolution and differentiation of Magnaporthe oryzae. Mol Plant-Microbe Interact 18 1148 1160
95. RonquistFHülsenbeckJP 2003 MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19 1572 1574
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek A Typhimurium-Typhi Genomic Chimera: A Model to Study Vi Polysaccharide Capsule Function In VivoČlánek SUMO Pathway Dependent Recruitment of Cellular Repressors to Herpes Simplex Virus Type 1 GenomesČlánek A Structural Model for Binding of the Serine-Rich Repeat Adhesin GspB to Host Carbohydrate ReceptorsČlánek Dynamic Evolution of Pathogenicity Revealed by Sequencing and Comparative Genomics of 19 IsolatesČlánek Widespread Endogenization of Genome Sequences of Non-Retroviral RNA Viruses into Plant GenomesČlánek The Cost of Virulence: Retarded Growth of Typhimurium Cells Expressing Type III Secretion System 1Článek A Role for the Chemokine RANTES in Regulating CD8 T Cell Responses during Chronic Viral Infection
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 7- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- What Do We Really Know about How CD4 T Cells Control ?
- “Persisters”: Survival at the Cellular Level
- E6 and E7 from Beta Hpv38 Cooperate with Ultraviolet Light in the Development of Actinic Keratosis-Like Lesions and Squamous Cell Carcinoma in Mice
- Selection of Resistant Bacteria at Very Low Antibiotic Concentrations
- The Extracytoplasmic Domain of the Ser/Thr Kinase PknB Binds Specific Muropeptides and Is Required for PknB Localization
- CD39/Adenosine Pathway Is Involved in AIDS Progression
- Hypoxia and a Fungal Alcohol Dehydrogenase Influence the Pathogenesis of Invasive Pulmonary Aspergillosis
- One Is Enough: Effective Population Size Is Dose-Dependent for a Plant RNA Virus
- Effects of Interferon-α/β on HBV Replication Determined by Viral Load
- A Typhimurium-Typhi Genomic Chimera: A Model to Study Vi Polysaccharide Capsule Function In Vivo
- Dual Chaperone Role of the C-Terminal Propeptide in Folding and Oligomerization of the Pore-Forming Toxin Aerolysin
- Rotavirus Stimulates Release of Serotonin (5-HT) from Human Enterochromaffin Cells and Activates Brain Structures Involved in Nausea and Vomiting
- Dissociation of Infectivity from Seeding Ability in Prions with Alternate Docking Mechanism
- The Impact of Recombination on dN/dS within Recently Emerged Bacterial Clones
- The Regulation of Sulfur Metabolism in
- Illumination of Parainfluenza Virus Infection and Transmission in Living Animals Reveals a Tissue-Specific Dichotomy
- A Permeable Cuticle Is Associated with the Release of Reactive Oxygen Species and Induction of Innate Immunity
- A Concerted Action of Hepatitis C Virus P7 and Nonstructural Protein 2 Regulates Core Localization at the Endoplasmic Reticulum and Virus Assembly
- SUMO Pathway Dependent Recruitment of Cellular Repressors to Herpes Simplex Virus Type 1 Genomes
- Re-localization of Cellular Protein SRp20 during Poliovirus Infection: Bridging a Viral IRES to the Host Cell Translation Apparatus
- Divergent Effects of Human Cytomegalovirus and Herpes Simplex Virus-1 on Cellular Metabolism
- A Structural Model for Binding of the Serine-Rich Repeat Adhesin GspB to Host Carbohydrate Receptors
- Transformation of Natural Genetic Variation into Genomes
- EBV Latency Types Adopt Alternative Chromatin Conformations
- Global mRNA Degradation during Lytic Gammaherpesvirus Infection Contributes to Establishment of Viral Latency
- Dynamic Evolution of Pathogenicity Revealed by Sequencing and Comparative Genomics of 19 Isolates
- Microbial Virulence as an Emergent Property: Consequences and Opportunities
- Widespread Endogenization of Genome Sequences of Non-Retroviral RNA Viruses into Plant Genomes
- Structural Basis of Chemokine Sequestration by CrmD, a Poxvirus-Encoded Tumor Necrosis Factor Receptor
- Cross-Species Transmission of a Novel Adenovirus Associated with a Fulminant Pneumonia Outbreak in a New World Monkey Colony
- An Interaction between KSHV ORF57 and UIF Provides mRNA-Adaptor Redundancy in Herpesvirus Intronless mRNA Export
- Elevated 17β-Estradiol Protects Females from Influenza A Virus Pathogenesis by Suppressing Inflammatory Responses
- The Role of IL-15 Deficiency in the Pathogenesis of Virus-Induced Asthma Exacerbations
- Fluorescence Lifetime Imaging Unravels Metabolism and Its Crosstalk with the Host Cell
- Programmed Death (PD)-1-Deficient Mice Are Extremely Sensitive to Murine Hepatitis Virus Strain-3 (MHV-3) Infection
- Hemoglobin Promotes Nasal Colonization
- Crystallography of a Lewis-Binding Norovirus, Elucidation of Strain-Specificity to the Polymorphic Human Histo-Blood Group Antigens
- The Cost of Virulence: Retarded Growth of Typhimurium Cells Expressing Type III Secretion System 1
- A Genome-Wide Approach to Discovery of Small RNAs Involved in Regulation of Virulence in
- Requires Glycerol for Maximum Fitness During The Tick Phase of the Enzootic Cycle
- C Metabolic Flux Analysis Identifies an Unusual Route for Pyruvate Dissimilation in Mycobacteria which Requires Isocitrate Lyase and Carbon Dioxide Fixation
- A Role for the Chemokine RANTES in Regulating CD8 T Cell Responses during Chronic Viral Infection
- Glycosaminoglycans and Sialylated Glycans Sequentially Facilitate Merkel Cell Polyomavirus Infectious Entry
- Regulation of Stomatal Tropism and Infection by Light in : Evidence for Coordinated Host/Pathogen Responses to Photoperiod?
- Multiple Translocation of the Effector Gene among Chromosomes of the Rice Blast Fungus and Related Species
- Comparative Genomics Yields Insights into Niche Adaptation of Plant Vascular Wilt Pathogens
- Unique Cell Adhesion and Invasion Properties of O:3, the Most Frequent Cause of Human Yersiniosis
- C-Terminal Region of EBNA-2 Determines the Superior Transforming Ability of Type 1 Epstein-Barr Virus by Enhanced Gene Regulation of LMP-1 and CXCR7
- Novel Chikungunya Vaccine Candidate with an IRES-Based Attenuation and Host Range Alteration Mechanism
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Requires Glycerol for Maximum Fitness During The Tick Phase of the Enzootic Cycle
- Comparative Genomics Yields Insights into Niche Adaptation of Plant Vascular Wilt Pathogens
- The Role of IL-15 Deficiency in the Pathogenesis of Virus-Induced Asthma Exacerbations
- “Persisters”: Survival at the Cellular Level
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



