-
Články
Reklama
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
ReklamaIscR Is Essential for Type III Secretion and Virulence
Bacterial pathogens use regulators that sense environmental cues to enhance their fitness. Here, we identify a transcriptional regulator in the human gut pathogen, Yersinia pseudotuberculosis, which controls a specialized secretion system essential for bacterial growth in mammalian tissues. This regulator was shown in other bacterial species to alter its activity in response to changes in iron concentration and oxidative stress, but has never been studied in Yersinia. Importantly, Y. pseudotuberculosis experiences large changes in iron bioavailability upon transit from the gut to deeper tissues and iron is a critical component in Yersinia virulence, as individuals with iron overload disorders have enhanced susceptibility to systemic Yersinia infections. Our work places this iron-modulated transcriptional regulator within the regulatory network that controls virulence gene expression in Y. pseudotuberculosis, identifying it as a potential new target for antimicrobial agents.
Published in the journal: . PLoS Pathog 10(6): e32767. doi:10.1371/journal.ppat.1004194
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1004194Summary
Bacterial pathogens use regulators that sense environmental cues to enhance their fitness. Here, we identify a transcriptional regulator in the human gut pathogen, Yersinia pseudotuberculosis, which controls a specialized secretion system essential for bacterial growth in mammalian tissues. This regulator was shown in other bacterial species to alter its activity in response to changes in iron concentration and oxidative stress, but has never been studied in Yersinia. Importantly, Y. pseudotuberculosis experiences large changes in iron bioavailability upon transit from the gut to deeper tissues and iron is a critical component in Yersinia virulence, as individuals with iron overload disorders have enhanced susceptibility to systemic Yersinia infections. Our work places this iron-modulated transcriptional regulator within the regulatory network that controls virulence gene expression in Y. pseudotuberculosis, identifying it as a potential new target for antimicrobial agents.
Introduction
Type III secretion systems (T3SS) are important components in the progression of disease for a number of clinically relevant human pathogens, including those in the genera Shigella, Salmonella, Escherichia, Chlamydia, Vibrio, Pseudomonas, and Yersinia [1], [2]. The T3SS functions as an injectisome that delivers bacterial effector proteins directly into the host cell cytoplasm [2]. While the T3SS apparatus itself is structurally conserved, the repertoire of T3SS effector proteins used by each group of pathogens is distinct [2]. Thus, the effect of the T3SS on the host is unique to the needs of the pathogen [2]. While the T3SS is generally essential for a T3SS-expressing pathogen to cause disease, several aspects of the T3SS may be detrimental to bacterial growth [2]. For example, T3SS components are recognized by the host immune system [3], [4]. In addition, expression of the T3SS is energetically costly and, in some organisms, T3SS induction correlates with growth arrest [5]. Therefore, regulation is essential for proper T3SS function in order to ensure that it occurs only during host cell contact in the appropriate host tissue [2], [6].
Members of the genus Yersinia that utilize a T3SS are important human pathogens: Y. pestis, the causative agent of plague, and the enteropathogens Y. enterocolitica and Y. pseudotuberculosis. The Y. pseudotuberculosis Ysc T3SS is encoded on a 70-kb plasmid termed pYV [7]–[9] and is made up of approximately 25 known proteins comprising three main structures: the basal body, the needle apparatus, and the translocon [10], [11]. The basal body, which displays a high degree of similarity to the flagellar basal body, is made up of rings that span the inner and outer membranes and a rod that traverses the periplasmic space [12]. Basal body associated proteins include YscN, an ATPase that aids in the secretion and translocation of effector proteins [13]. The needle complex, which is thought to act as a molecular channel for effector protein translocation, is a straight hollow appendage approximately 60 nm in length and is made up of helical polymerized subunits of YscF [12]. The translocon is comprised of three proteins: YopD, YopB and LcrV, which are essential for pore formation in the target host membrane and proper translocation of effector proteins YopHEMOJTK to the host cytoplasm [12], [14]. Also encoded on pYV are chaperones important for efficient translocation of a subset of effector proteins [15]. Lastly, several transcriptional and post-transcriptional regulators of the T3SS are found on pYV, including the AraC-like transcriptional regulator LcrF. LcrF is responsible for expression of a number of T3SS structural genes and Yop effectors, specifically the virC and lcrGVH-yopBD operons as well as genes encoding effector Yops, the adhesin YadA, and the lipoprotein YlpA [16]-[22]. LcrF itself is thermoregulated at both the transcriptional and translational levels through the action of the histone-like protein YmoA and a cis-acting RNA thermosensor located on the lcrF transcript, respectively [23], [24]. This enables Yersinia to express T3SS genes at 37°C within the mammalian host, but not at lower temperatures [23], [24]. Importantly, proper LcrF-mediated control of T3SS expression is important for Y. pseudotuberculosis virulence [24].
IscR belongs to the Rrf2 family of winged helix-turn-helix transcription factors [25], [26] and has been studied extensively in E. coli, where its DNA-binding activity is dependent on coordination of an iron-sulfur [2Fe-2S] cluster through three conserved cysteines and a histidine [27]–[30]. E. coli IscR recognizes two distinct DNA motifs, type 1 and type 2, depending on the Fe-S status of the protein [31]. Holo-IscR coordinating an Fe-S cluster binds both type 1 and type 2 motifs, while clusterless apo-IscR recognizes only the type 2 DNA-binding motif [27], [32], [33]. As iron starvation, oxidative stress, and oxygen limitation affect the holo-IscR/apo-IscR ratio, these environmental cues are thought to have a direct effect on gene expression through IscR in E. coli [28]–[30]. For example, holo-IscR represses transcription of the housekeeping iscRSUA-hscBA-fdx Fe-S cluster biogenesis operon [32], [34], while either holo - or apo-IscR promotes transcription of the inducible sufABCDSE Fe-S cluster biogenesis operon [33], [35]. Both pathways function to insert Fe-S clusters onto proteins involved in a range of metabolic processes including electron transfer, substrate binding/activation, iron/sulfur storage, regulation, and enzyme activity [36]. In addition, E. coli IscR is also known to regulate transcription of other Fe-S cluster assembly genes such as erpA (yadR) as well as genes integral to oxidative stress resistance, biofilm formation, and anaerobic respiration [28]–[30], [34]. IscR is widely conserved among bacteria [25] and its regulatory activity is integral to the infectious process of the plant pathogen Erwinia chrysanthemi [37]. Furthermore, IscR plays an important role in the virulence of the human pathogens Pseudomonas aeruginosa through modulation of the catalase katA [38], Burkholderia mallei through resistance to reactive nitrogen species [39], and Vibrio vulnificus through induction of several virulence-associated pathways [39], [40]. While the iron-dependent transcriptional repressor Fur has been shown to control T3SS expression in Salmonella and Shigella [41], [42], IscR has never been linked to regulation of the T3SS in any organism and has not been studied in Yersinia.
In this study, we isolated two independent IscR transposon insertion mutants in a novel screen for Y. pseudotuberculosis genes important for T3SS function. We assessed the impact of iscR deletion on Y. pseudotuberculosis in vitro and in vivo growth, type III secretion, and global gene expression. We found IscR to be essential for full T3SS function and virulence in a mouse model of infection. In addition, we provide evidence that IscR control of the T3SS stems from direct transcriptional regulation of the T3SS master regulator LcrF.
Results
IscR is required for Y. pseudotuberculosis Ysc T3SS function
To identify regulators of the Y. pseudotuberculosis T3SS, we utilized a novel screen to isolate transposon mutants with defects in T3SS function. We previously showed that Y. pseudotuberculosis expressing a functional T3SS induces NFκB activation in HEK293T cells [43], enabling us to use host cell NFκB activation as a readout for T3SS function in Y. pseudotuberculosis transposon mutants. As some T3SS effector proteins inhibit NFκB signaling [44], we performed the screen using a Y. pseudotuberculosis transposon mutant library in a genetic background that lacked the known T3SS effector proteins YopHEMOJT (Δyop6; [43]). We identified several transposon mutants with defects in triggering activation of NFκB in HEK293T cells (L. Kwuan, N. Herrera, H. Ramirez, V. Auerbuch, data not shown), suggesting defective T3SS function. Among these were two strains with unique transposon insertions in YPTB2860 (Figure 1A), encoding a protein with 79% identity to the E. coli iron-sulfur cluster regulator IscR, part of the iscRSUA-hscBA-fdx operon involved in Fe-S cluster biogenesis (Figure 1B). Importantly, the helix-turn-helix DNA binding domain as well as the three cysteines and histidine known to coordinate an iron-sulfur (Fe-S) cluster in E. coli IscR are conserved in all three Yersinia species (Figure 1B). These data indicate that Yersinia IscR may coordinate an Fe-S cluster and, as in E. coli, may regulate gene transcription.
Fig. 1. Alignment of protein sequences shows a high level of conservation between E. coli and Yersinia IscR. 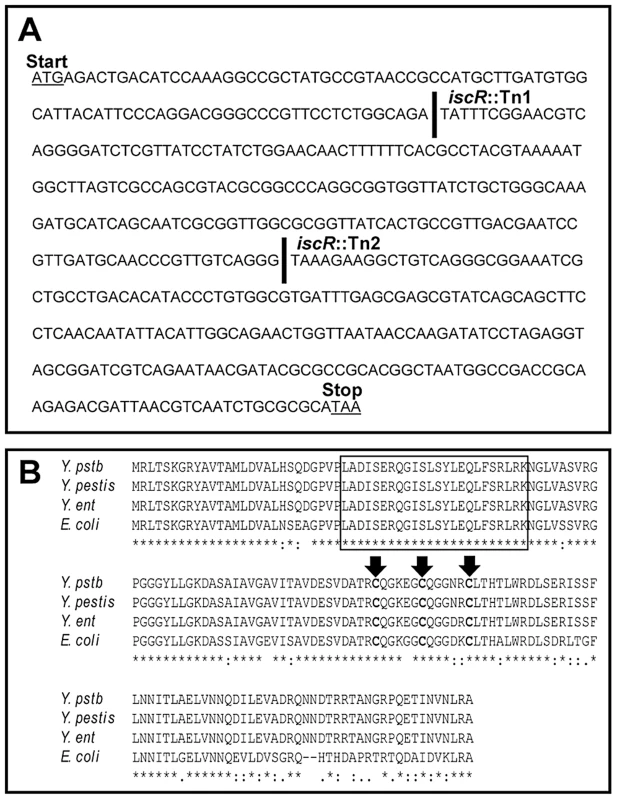
(A) The Y. pseudotuberculosis DNA sequence, which displays the unique insertions sites for the two transposon mutants generated from our genetic screen. A space in the DNA sequence and a solid black line indicate the site of insertion for either iscR::Tn1 or iscR::Tn2. (B) Multiple sequence alignment was performed on the IscR protein sequence from E. coli K12-MG1655 and each of the three human pathogenic Yersinia spp., Y. pseudotuberculosis IP 32953 (Y. pstb), Y. enterocolitica 8081 (Y. ent) and Y. pestis CO92 (Y. pestis) using ClustalW [86]. The N-terminal helix-turn-helix DNA-binding motif is indicated by a black box. The three conserved cysteine residues (C92, C98 and C104) responsible for coordinating an Fe-S cluster are in bold and identified by black arrows [33]. Asterisks indicate nucleotides that are conserved across all four species. To validate that loss of IscR in Y. pseudotuberculosis leads to T3SS defects, we isolated the two iscR transposon mutants (iscR::Tn1 and iscR::Tn2) from our library and again measured their ability to trigger NFκB activation in HEK293T cells compared to the Δyop6 parental strain and a ΔyscNU T3SS-null mutant [43]. In addition, we constructed an in-frame iscR deletion mutant in the Δyop6 genetic background (Δyop6/ΔiscR) and tested it in this assay. We found that disruption of iscR led to ∼2-fold less NFκB activation relative to the Δyop6 T3SS+ parental strain, although NFκB activation levels were still ∼5-fold higher than a strain with complete lack of T3SS function (ΔyscNU; Figure 2A), suggesting that loss of iscR leads to partial T3SS loss.
Fig. 2. IscR modulates Y. pseudotuberculosis T3SS function. 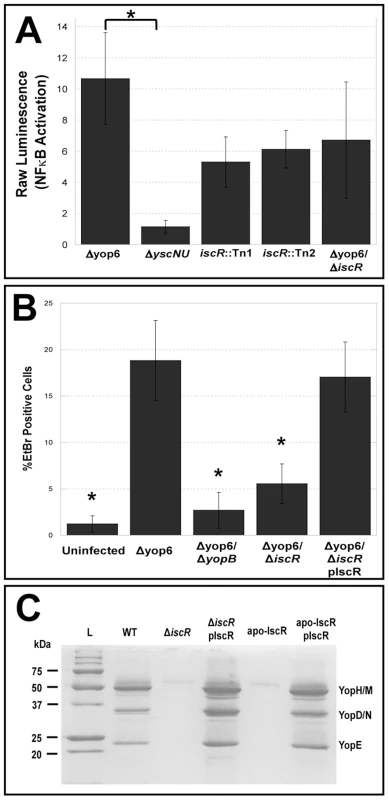
(A) HEK293T cells expressing an NFκB luciferase reporter gene were infected with either T3SS-positive Y. pseudotuberculosis lacking the six known effector proteins YopHEMOJT (Δyop6), two isogenic iscR transposon mutants (Δyop6/iscR::Tn1 and Δyop6/iscR::Tn2), the iscR deletion mutant (Δyop6/ΔiscR), or a T3SS null mutant (ΔyscNU). At 4 h post-inoculation, T3SS functionality was determined by assessing the ability of the mutants to trigger NFκB activation in host cells by measuring bioluminescence. Shown are the average raw luminescence values of the sample compared to uninfected ± standard error of the mean (SEM) from five independent experiments. *p≤0.05, as determined by one-way ANOVA followed by Bonferroni post hoc test where each indicated group was compared to the appropriate negative (ΔyscNU) and positive (Δyop6) controls. (B) To analyze T3SS-dependent pore formation in macrophages, C57Bl/6 immortalized BMDMs were infected with Δyop6, a T3SS-defective mutant lacking the translocator protein YopB (Δyop6/ΔyopB), the iscR deletion mutant (Δyop6/iscR), or the iscR complemented strain (Δyop6/iscR pIscR), or were left uninfected. At 2 h post-inoculation, pore formation was determined by assessing the number of cells that took up ethidium bromide (EtBr) compared to the total number of Hoechst-stained cells. Shown are the averages ± SEM from three independent experiments. *p≤0.05 relative to both Δyop6 and Δyop6/iscR pIscR, as determined by one-way ANOVA followed by Bonferroni post hoc test where each indicated group was compared to the appropriate negative (Δyop6/ΔyopB) and positive (Δyop6) controls. (C) Y. pseudotuberculosis IP2666 wild type (WT), iscR deletion (ΔiscR), iscR complemented (ΔiscR pIscR), apo-locked IscR (apo-IscR), and apo-IscR complemented (apo-IscR pIscR) strains were grown in 2xYT low calcium media at 37°C to induce type III secretion in the absence of host cells. Proteins in the bacterial culture supernatant were precipitated and visualized alongside a protein molecular weight marker (L) on a polyacrylamide gel using commassie blue. Sample loading was normalized for OD600 of each culture. Results are representative of three independent experiments. To verify further that deletion of iscR leads to alterations in T3SS function, we assessed the ability of the Δyop6/ΔiscR mutant to insert YopBD pores in target host cell membranes by measuring entry of ethidium bromide (EtBr) inside Y. pseudotuberculosis-infected bone marrow derived macrophages [45], [46]. Pore formation by the Δyop6/ΔiscR mutant was decreased by 7-fold (p<0.05) relative to the Δyop6 parental strain, which could be restored upon complementation with plasmid-encoded iscR (Figure 2B). To determine whether loss of iscR affects T3SS function in a wild type genetic background, we constructed an in-frame iscR deletion (ΔiscR) in the wild type Y. pseudotuberculosis IP2666 strain expressing six of the seven known T3SS effector proteins YopHEMOJK [47]. We then visualized the secretome of the ΔiscR mutant relative to wild type. Deletion of iscR led to a dramatic decrease in secretion of T3SS cargo relative to the wild type background, which can be restored upon complementation with plasmid-encoded iscR (Figure 2C). Importantly, this lack of type III secretion did not result from a defect in growth of the mutant, as the ΔiscR mutant actually grew better than wild type bacteria under T3SS-inducing conditions (Figure S1A). This is consistent with a T3SS defect in this strain, as wild type Yersinia display a characteristic growth arrest upon T3SS expression [5], [48], [49]. Collectively, these data demonstrate that Y. pseudotuberculosis IscR is required for proper T3SS function.
IscR is required for full virulence of Y. pseudotuberculosis
Based on the knowledge that the T3SS plays an important role in the virulence of human pathogenic Yersinia, we sought to investigate whether the diminished type III secretion observed in the Y. pseudotuberculosis ΔiscR strain would lead to a reduction in the infectious capacity of this mutant. Mice were orogastrically infected with 2×108 CFU of either the Y. pseudotuberculosis wild type or isogenic ΔiscR mutant strains. At 5 days post-inoculation, mice infected with the ΔiscR mutant displayed significantly decreased colonization of Peyer's patches and mesenteric lymph nodes (MLN) as well as diminished systemic colonization (Figure 3). Specifically, we noted 10 - and 130-fold reductions in CFU recovered from the Peyer's patches and MLNs, respectively, in mice infected with the ΔiscR mutant strain relative to wild type. Notably, we observed a 1000 - to 10,000-fold decrease in bacterial burden in the spleen and liver respectively. The diminished ability of the ΔiscR mutant strain to colonize deep tissue sites is underscored by the fact that bacteria were not detected in seven of the nine livers analyzed. These findings suggest that IscR is essential for Y. pseudotuberculosis virulence in an oral infection model.
Fig. 3. IscR is required for full virulence of Y. pseudotuberculosis. 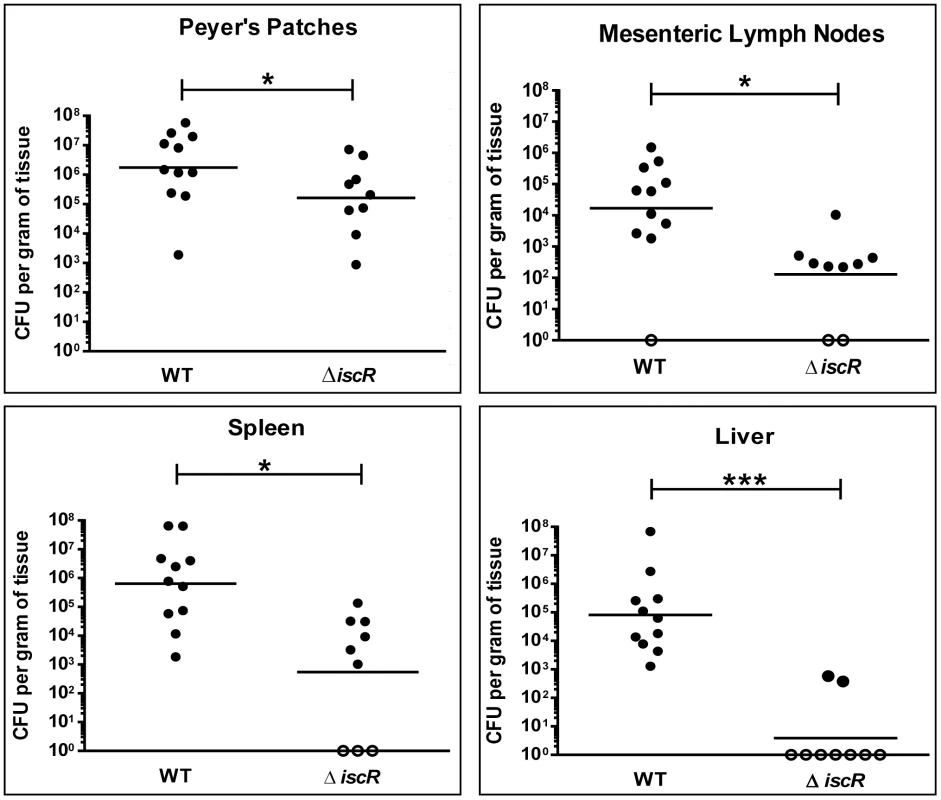
Mice were infected with 2×108 CFU of either WT Y. pseudotuberculosis or ΔiscR mutant via orogastric gavage. At 5 days post-inoculation, the Peyer's patches (PP), mesenteric lymph nodes (MLN), spleens and livers were collected, homogenized and CFU determined. Each symbol represents one animal. Unfilled symbols indicate that CFU were below the limit of detection. The data presented are from three independent experiments. *p<0.05, ***p<0.001 as determined by an unpaired Wilcoxon-Mann-Whitney rank sum test. Dashes represent the geometric mean. IscR deletion leads to global misregulation of gene expression in Y. pseudotuberculosis
To begin to understand the mechanistic contribution of IscR to Y. pseudotuberculosis pathogenesis, we performed high throughput transcriptome sequencing (RNAseq) analysis to determine the Y. pseudotuberculosis genes directly and indirectly controlled by IscR under iron replete, T3SS-inducing conditions. Total RNA was collected from wild type Y. pseudotuberculosis as well as the ΔiscR mutant strain grown in M9 at 37°C for 3 h, a point at which the ΔiscR and wild type strains display comparable growth rates (Figure S1A).
For the ΔiscR mutant relative to the wild type, a total of 226 genes demonstrated a statistically significant fold change of ≥2 (Table S1). Of these, 134 genes were up-regulated in the ΔiscR mutant relative to the wild type (Table 1 & Figure 4A), while 92 were down-regulated (Table 2 & Figure 4B). Genes found to be up-regulated in the ΔiscR mutant include key elements of Fe-S cluster biosynthesis, cellular detoxification, metabolism, and protein fate (Figure 4A). The most notable increases in transcription were observed for genes encoding Fe-S cluster biosynthesis proteins including those encoded in the isc operon, iscS (18.7-fold), iscU (21.7-fold) and iscA (13-fold) (Table 1 & Figure S2A). Additional genes encoding proteins involved in Fe-S cluster assembly and their respective fold increases include iscX/yfhJ (10.8), fdx (10.9), hscB (10), hscA (9.3), yadR/erpA (6.8), pepB (10.1) and nfuA (7.0). To validate these findings, we performed qRT-PCR analysis on the second gene encoded in the iscRSUA operon, iscS, as well as on the gene encoding the Fe-S biosynthesis protein ErpA. Transcription of iscS was increased by 30-fold, while erpA expression was increased 5-fold (p<0.05; Figure 5A). Bioinformatic analysis identified two IscR type 1 motifs upstream of the iscRSUA-hscBA-fdx operon (Figure S2B) as well as one site each located upstream of both erpA and nfuA (data not shown). Based on this data, we propose that Y. pseudotuberculosis IscR modulates Fe-S cluster biosynthesis expression in a manner akin to that of E. coli IscR.
Fig. 4. IscR impacts global gene expression in Y. pseudotuberculosis under iron replete conditions. 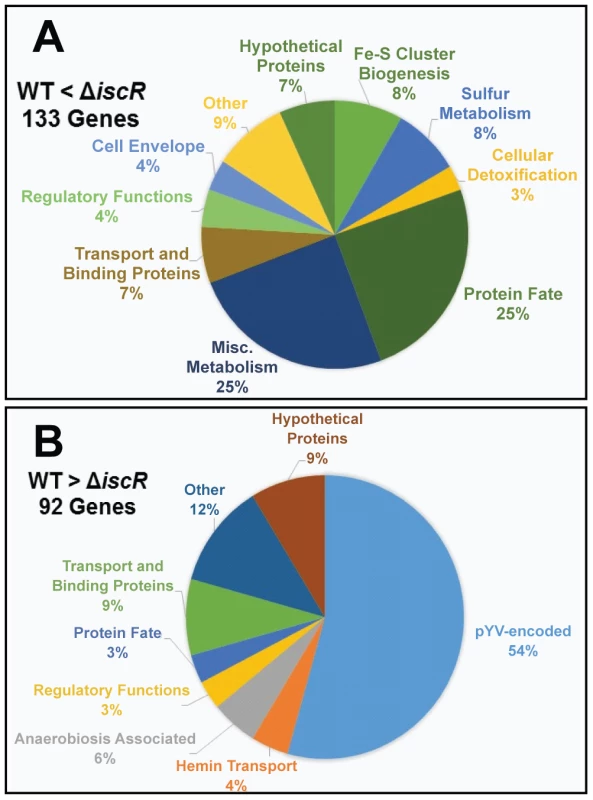
RNAseq analysis was performed on WT and ΔiscR Y. pseudotuberculosis after growth in M9 at 37°C for 3 h (T3SS-inducing conditions), at which point total RNA was collected and processed. The resulting libraries were sequenced using the HiSeq2500 Illumina sequencing platform for 50 bp single reads and analyzed via the CLC Genomics Workbench application (CLC bio). RPKM expression levels of 225 genes demonstrated a fold change of ≥2, and were deemed significant by Bayseq test with a corrected FDR post hoc test from three independent experiments (p≤0.05). Shown are the functional ontologies of the (A) 133 genes that are up-regulated in the ΔiscR mutant relative to the wild type and (B) 92 that are down-regulated. Fig. 5. Deletion of IscR leads to increased transcription of Fe-S cluster biogenesis genes and robust transcription of T3SS genes. 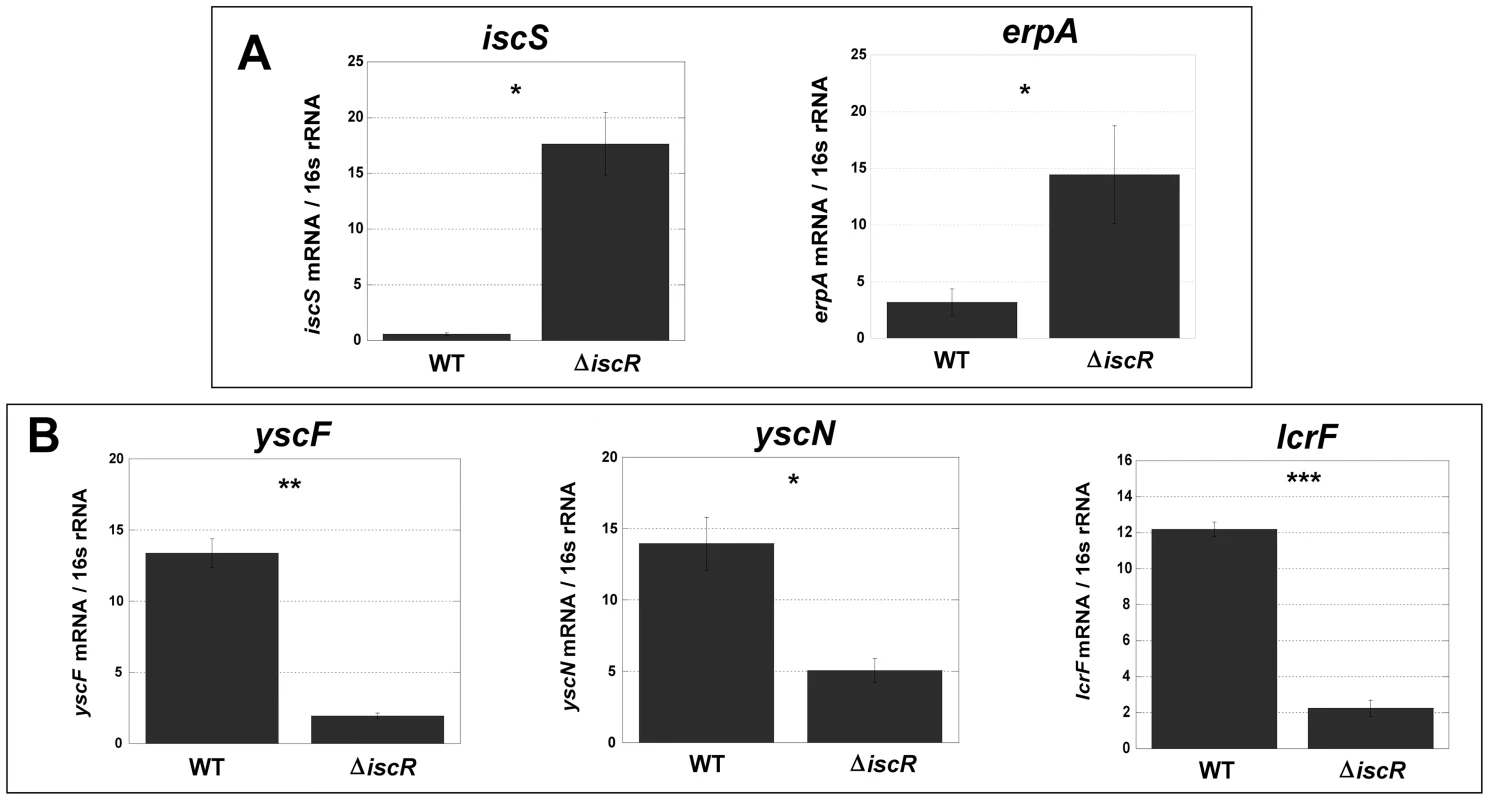
Quantitative real-time PCR analysis of WT and ΔiscR Y. pseudotuberculosis was performed (A) for the Fe-S cluster biogenesis genes, iscS and erpA and (B) for the T3SS genes, yscF, yscN and lcrF. Experiments were carried out from cultures grown in M9 at 37°C for 3 h. Shown are the averages ± SEM from three independent experiments. *p<0.05, **p<0.001, ***p<0.0001 as determined by a Student t test. Tab. 1. Genes repressed by IscR, identified by RNAseq analysis. 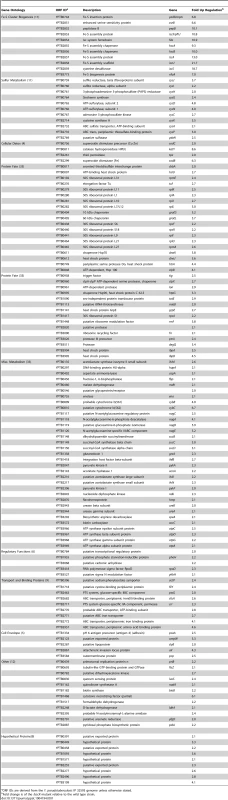
ORF IDs are derived from the Y. pseudotuberculosis IP 32593 genome unless otherwise stated. Tab. 2. Genes activated by IscR, identified by RNAseq analysis. 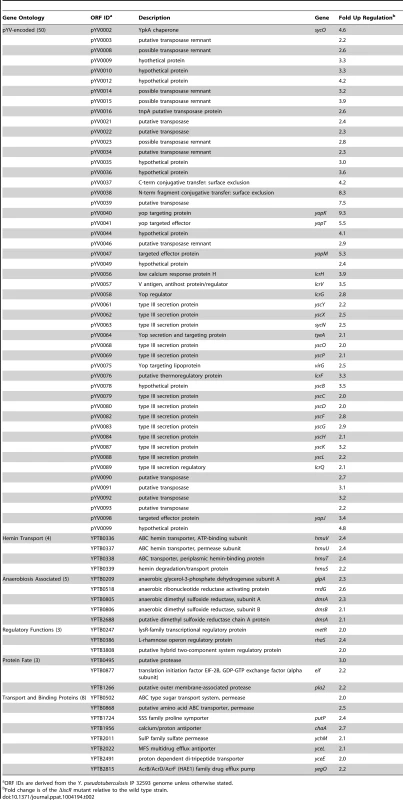
ORF IDs are derived from the Y. pseudotuberculosis IP 32593 genome unless otherwise stated. IscR is required for transcription of T3SS genes
In total, 92 genes were significantly down-regulated in the ΔiscR mutant relative to wild type Y. pseudotuberculosis (Table 2). These data demonstrate that the majority of pYV-encoded genes are decreased in the ΔiscR mutant relative to the wild type strain, including genes essential for proper T3SS expression and function. The virC and lcrGVH-yopBD operons as well as genes encoding the T3SS cargo YopHEMOJTK were the most affected upon deletion of iscR: the effector proteins YopJ (−3.4-fold), YopM (−5.3-fold) and YopT (−5.5-fold), the effector protein and translocation regulator YopK (−9.3-fold), as well as a number of genes encoding T3SS structural proteins. Genes encoding regulators that control T3SS expression and function were decreased in the mutant including lcrQ (−2.1-fold), lcrF (−3.3-fold), lcrG (−2.8-fold) and lcrH (−3.9-fold). To verify that T3SS gene expression was indeed decreased in the ΔiscR mutant, we measured the transcript levels of the genes encoding T3SS structural proteins YscN, YscF, and the T3SS transcriptional regulator LcrF via qRT-PCR. As detailed in Figure 5B, we observed fold decreases of 2.8-fold (p<0.05), 6.9-fold (p<0.001), and 5.4-fold (p<0.0001) for yscN, yscF, and lcrF, respectively. These data support our RNAseq analysis and confirm that IscR is required for robust transcription of Y. pseudotuberculosis T3SS genes.
In addition to T3SS genes, 25 other pYV-encoded genes were decreased in the mutant, but these are annotated as hypothetical proteins, transposases, and pseudogenes. Analysis of the relative abundance of pYV in the Y. pseudotuberculosis wild type and ΔiscR strains was performed in order to verify that the decreases in pYV-encoded genes were not a result of plasmid loss (Figure S3). The concentration of plasmid isolated from the wild type and ΔiscR mutant was comparable, suggesting that the decreased transcription of pYV-encoded genes, including those encoding the T3SS, are not a result of decreased stability of the pYV plasmid.
Y. pseudotuberculosis expressing only apo-locked IscR has a proton motive force defect and cannot secrete Yops
To assess the contribution of Fe-S cluster ligation to IscR control of the T3SS, we constructed an IscR mutant strain in which the three conserved cysteines were substituted with alanines (C92A, C98A, C104A; apo-locked IscR). Identical mutations in E. coli IscR render the protein unable to coordinate an iron-sulfur cluster, yet able to bind type 2 DNA binding motifs and to regulate target gene transcription [28]–[30]. We analyzed the secretome of the Y. pseudotuberculosis apo-locked IscR strain under T3SS-inducing conditions and found that the mutant was just as defective as the ΔiscR strain in Yop secretion (Figure 2C). This defect could be complemented with plasmid-encoded wild type IscR. As apo-locked IscR is insufficient to promote type III secretion, holo-IscR-mediated regulation of gene expression through one or more type 1 motifs may be specifically involved in regulating T3SS gene expression. Alternatively, forcing all IscR expression within the cell to the clusterless form, which leads to IscR overexpression, may lead to alterations of bacterial pathways that indirectly affect type III secretion.
Consistent with this latter explanation, the apo-locked IscR mutant exhibited decreased colony size on LB agar, slower growth in rich media (Figure S1), and decreased motility (Figure 6A). The flagellar basal body is a T3SS itself, indicating that the defect in the Ysc T3SS for this strain may be a result of gross abnormalities in secretion systems. Based on these findings, we set out to examine whether the apo-locked IscR mutant demonstrated alterations in membrane potential, as this has been shown to be important for both motility and Ysc T3S in Y. enterocolitica [50]. To this end, we examined bacterial membrane potential under T3SS inducing conditions. As demonstrated in Figure 6B, there is a notable decrease in membrane potential in the apo-locked IscR mutant relative to the wild type strain, which can be complemented upon addition of wild type iscR on a plasmid. Furthermore, the membrane potential of the ΔiscR mutant strain is comparable to that of the wild type. Collectively, these data suggest that the apo-locked IscR mutant has a proton motive force defect, leading to decreased type III secretion and motility. These findings highlight the importance for Yersinia to maintain appropriate levels of holo-IscR relative to apo-IscR in order maintain normal membrane potential.
Fig. 6. The apo-IscR mutant strain displays decreased motility and disruption of electrical potential. 
(A) Motility was analyzed by spotting 1 µl aliquots of either a nonmotile strain (Δyop6/flhDY.pestis), WT, ΔiscR, or apo-locked IscR Y. pseudotuberculosis onto motility agar plates. The diameters of the colonies were determined one day later and used to calculate percent motility relative to WT, which was set at 100%. Shown is the average percent motility ± SEM and is representative of three independent experiments. ***p≤0.0001 as determined by one-way ANOVA followed by Bonferroni post hoc test where each indicated group was compared to the appropriate negative (Δyop6/flhDY.pestis) and positive (WT) controls. (B) Proton motive force (PMF) was measured using JC-1 dye for Y. pseudotuberculosis IP2666 wild type (WT), iscR deletion mutant (ΔiscR), iscR complemented (ΔiscR pIscR), apo-IscR, and apo-IscR complemented (apo-IscR pIscR) strains grown in M9 at 37°C for 3 hours. The protonophore CCCP was added to a WT sample as a negative control (CCCP). Decreases in PMF were measured as a decrease in red (590 nm) fluorescent cells relative to green (530 nm). The data is presented as total fluorescence intensities at 590 (red) relative to 530 (green) ± SEM and is representative of three independent experiments. *p≤0.05, as determined by one-way ANOVA followed by Bonferroni post hoc test where each indicated group was compared to the appropriate negative (CCCP) and positive (WT) controls. IscR recognizes a type 2 motif upstream of the yscW-lcrF operon
To begin to understand the nature of the T3SS defect in the presence of only apo-IscR, we carried out RNAseq analysis on the Y. pseudotuberculosis apo-locked IscR mutant grown under T3SS-inducing conditions and compared the results with data from the wild type and ΔiscR strains. Curiously, the apo-locked IscR mutant displayed aberrant expression of genes involved in stress response, transport, cell envelope, as well as electron transport (data not shown). Of note, the Fe-S cluster biosynthesis proteins encoded in the iscRSUA-hscBA-fdx-iscX-pepB-sseB, yadR/erpA and nfuA operons are significantly increased in this background, similar to that of the ΔiscR mutant strain (Figure S2A), indicating that holo-IscR represses expression of these genes under the aerobic, iron-replete conditions used. In contrast, increases in the sufABCDS Fe-S cluster biogenesis operon were observed for the apo-locked IscR strain when compared to both the wild type and ΔiscR strains (Figure S4). As IscR is overexpressed by 30-fold (p<0.05) in the apo-locked iscR mutant compared to wild type (Figure S2A), we speculate that the suf operon is positively regulated by IscR in Yersinia as in E. coli. In contrast, the extensively studied E. coli IscR target, hyaABCDEF, is not encoded in the Y. pseudotuberculosis genome.
Importantly, our RNAseq analysis demonstrated that transcription of genes within the virA, virB, virC, yscW-lcrF, and lcrGVH-yopBD operons was restored in the apo-locked IscR mutant compared to the ΔiscR mutant (Figure 7 and Table S2). However, we observed a decrease in transcription of genes encoding the T3SS effector proteins YopH (−4.4-fold), YopM (−3.0-fold), YopK (−7.1-fold), and YopE (−2.1-fold) in the apo-locked IscR mutant compared to wild type. Transcription of yopE has been shown to be regulated by Yop secretion through a positive feedback loop [51], [52], suggesting that the defect in YopHEMK transcription observed in the apo-locked IscR mutant may be caused by the lack of Yop secretion we observed in this strain. Together, these data suggest that both holo - and apo-IscR can promote T3SS gene transcription, possibly through binding to one or more type 2 DNA motifs.
Fig. 7. Y. pseudotuberculosis lacking a functional IscR display decreased transcription of a number of pYV encoded genes. 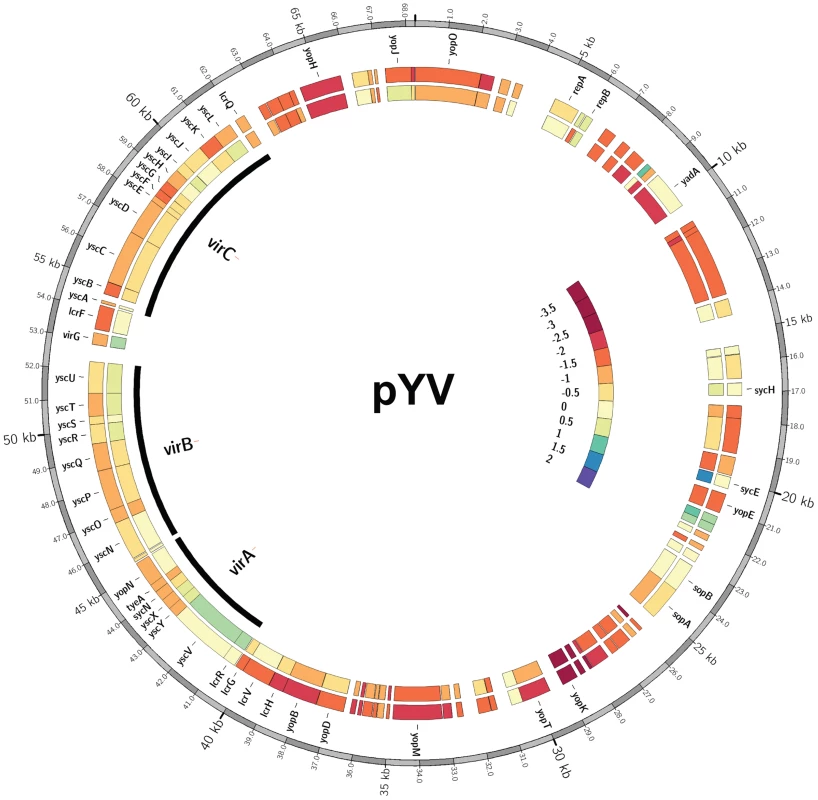
Middle and inner rings: heatmap [83] representations of log2-ratios (log2(RPKMmutant/RPKMwt) for each gene on the pYV plasmid for both the ΔiscR (middle ring) and apo-IscR (inner ring) mutants relative to wild type. Outer ring: pYV base coordinate position from Y. pseudotuberculosis IP32953. Known genes are identified and the virA, virB and virC operons highlighted by black arcs. On the interior right side is the color bar legend displaying log2-ratios from −3.5 to 2. Using this scale, orange/red colorations represent genes with decreased transcription in the mutant relative to the wild type strain and blue/green coloring represents increases in gene transcription for the mutant relative to the wild type. Tan/cream denotes no change. To determine whether IscR might directly regulate T3SS gene expression, we carried out bioinformatic analysis to search pYV for sequences resembling the E. coli IscR type 2 motif (xxWWWWCCxYAxxxxxxxTRxGGWWWWxx) [30], [31], [33], as the DNA-binding domain of Yersinia IscR is 100% identical to that of E. coli IscR (Figure 1A). We searched within the 150 nucleotides upstream of the 99 genes encoded on the pYV plasmid and obtained a ranked list of putative type 2 motifs (data not shown). Among these was a site located within the yscW-lcrF promoter region (Figure 8A) [24]. To test whether IscR bound specifically to this site, we performed equilibrium DNA competition assays utilizing purified E. coli IscR-C92A (apo-locked IscR) [33], with a fluorescently-labeled E. coli hya type 2 site previously identified by Nesbit et al. [33]. Purified E. coli IscR was utilized in this assay, as complementation of the Y. pseudotuberculosis ΔiscR mutant strain with IscR of E. coli encoded on a plasmid fully restored secretion of T3SS cargo (Figure 8B). Competitor DNA included unlabeled E. coli hya as a positive control, the identified site within the Yersinia yscW-lcrF promoter region, a mutated version of this sequence (mlcrF), where nucleotides previously demonstrated in E. coli to be important for type 2 motif binding were altered [33], as well as one of the Y. pseudotuberculosis isc type 1 motif sites we identified as a negative control (Figure S2B & Figure 8C). We found that unlabeled lcrF DNA competed as well as unlabeled hya DNA (IC50 27 nm and 61 nm, respectively), suggesting that IscR can indeed bind to the identified type 2 motif upstream of lcrF (Figure 8D). Furthermore, mutation of key nucleotides in the lcrF promoter sequence led to alleviation of competition and increased the IC50 to greater than 1000 nM, a level comparable to that of the isc negative control type 1 motif site (Figure 8D). These findings suggest that IscR may regulate transcription of the T3SS through a type 2 motif within the yscW-lcrF promoter region.
Fig. 8. IscR binds a novel motif 2 site within the lcrF promoter region. 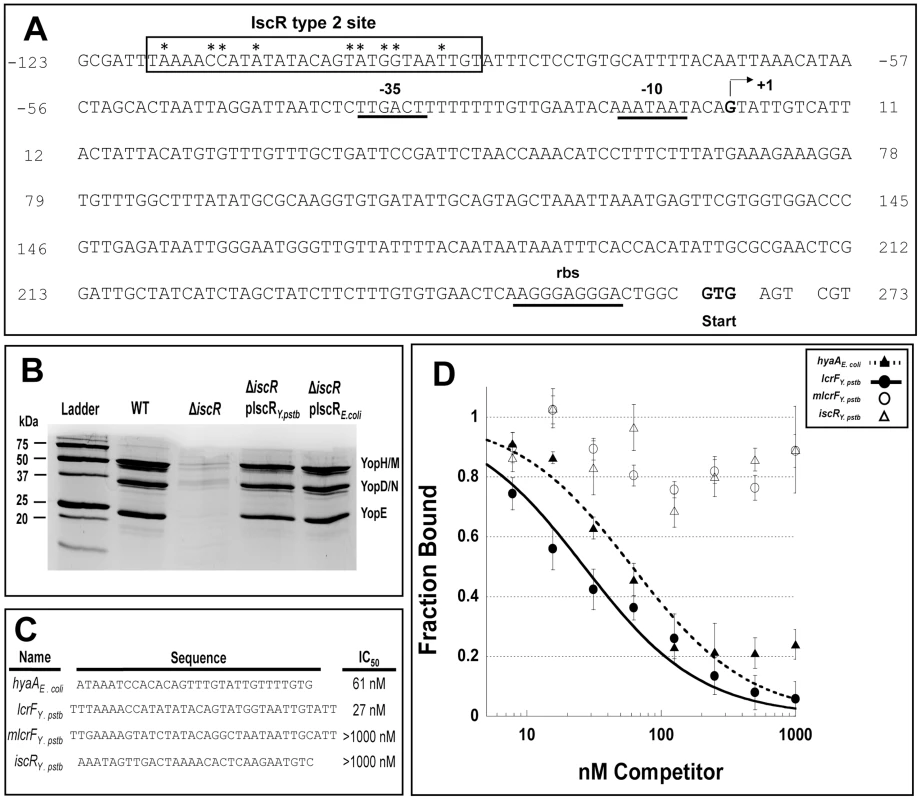
(A) Displayed is the promoter region of the yscW-lcrF operon including −35 and −10 regions, the transcriptional start site (+1) and the ribosome binding site (RBS) [24]. The IscR type 2 DNA-binding site is indicated by a black box. The nine bases previously found to be important for IscR binding are indicated by asterisks [35]. (B) Y. pseudotuberculosis IP2666 wild type (WT), iscR deletion (ΔiscR), ΔiscR complemented with Y. pseudotuberculosis iscR (ΔiscR pIscRY.pstb), and ΔiscR complemented with E. coli iscR (ΔiscR pIscRE.coli) strains were grown in 2xYT low calcium media at 37°C to induce type III secretion in the absence of host cells. Proteins in the bacterial culture supernatant were precipitated and visualized alongside a protein molecular weight marker (Ladder) on a polyacrylamide gel using commassie blue. Sample loading was normalized for OD600 of each culture. These results are representative of three independent experiments. (C) The competitor DNA sequences used for the competition assay and the resulting IC50 concentrations are displayed. Nucleotides in bold and underlined correspond to those that were changed in the mlcrF sequence and have been found to be important for IscR binding in E. coli [33]. (D) Competition assay utilizing 59 nM E. coli apo-locked IscR (IscR-C92A) and 5 nM TAMRA labeled hya DNA [33]. Assay were performed using a range of 8 to 1000 nM unlabeled competitor DNA, including the known E. coli hya site competitor (closed triangles), the in silico identified Y. pseudotuberculosis lcrF site competitor (closed circles), mutated lcrF (mlcrF) site competitor (open circles), and the negative control Y. pseudotuberculosis isc in silico identified motif I site competitor (open triangles). Shown are the averages ± SEM from three independent experiments. Discussion
In this study, we present the first characterization of the iron-sulfur cluster regulator, IscR, of Yersinia. Initially identified through a genetic screen for modulators of Ysc T3SS function, iscR-deficient Y. pseudotuberculosis had a dramatic defect in secretion of T3SS effector proteins and in targeting macrophages through their T3SS, yet displayed normal growth in broth culture and wild type flagellar motility. Bioinformatic and DNA binding analysis revealed an IscR binding site upstream of the operon encoding the T3SS master regulator LcrF, indicating that IscR controls expression of the Ysc T3SS. Collectively, these findings indicated that IscR is a central component of the Y. pseudotuberculosis T3SS regulatory cascade.
Both E. coli holo - and apo-IscR are active transcription factors with distinct DNA binding targets. Holo-IscR can bind both type 1 and 2 motifs whereas apo-IscR can only bind type 2 motifs. IscR of E. coli autoregulates the isc operon, iscRSUA-hscBA-fdx, through binding to type 1 motifs within the isc promoter region [34]. In addition, Giel et al. described increased transcription of the genes located immediately downstream of the isc operon, yfhJ-pepB-sseB, in an iscR mutant, suggesting a negative regulatory effect on these genes as well [30]. We observed derepression of the iscRSUA-hscBA-fdx operon and the yfhJ-pepB-sseB locus in the Y. pseudotuberculosis ΔiscR mutant as well as the mutant expressing apo-locked IscR. Furthermore, we identified two sites within the Y. pseudotuberculosis isc promoter that closely match the E. coli IscR motif I consensus sequence. These data indicate that the iscRSUA-hscBA-fdx operon, and possibly the yfhJ-pepB-sseB locus, are negatively regulated by holo-IscR in Yersinia as they are in E. coli (Figure 9A). IscR in E. coli is known to activate transcription of the sufABCDSE operon through binding to a type 2 motif [29]. Our analysis revealed that the Y. pseudotuberculosis apo-locked IscR mutant overexpresses the sufABCDS operon compared to the wild type and ΔiscR strains, which we predict results from the overexpression of IscR observed in the apo-locked mutant as found in E. coli [32], [33]. We identified a site within the Y. pseudotuberculosis suf promoter region that closely resembles an E. coli IscR type 2 motif (data not shown). Together, these data indicate that the suf operon is positively regulated by IscR in Yersinia as in E. coli. Thus, we propose that IscR of Y. pseudotuberculosis modulates transcription of both the isc and suf Fe-S cluster biosynthesis pathways via mechanisms established for its E. coli ortholog.
Fig. 9. Regulation of the isc and lcrF operons by IscR. 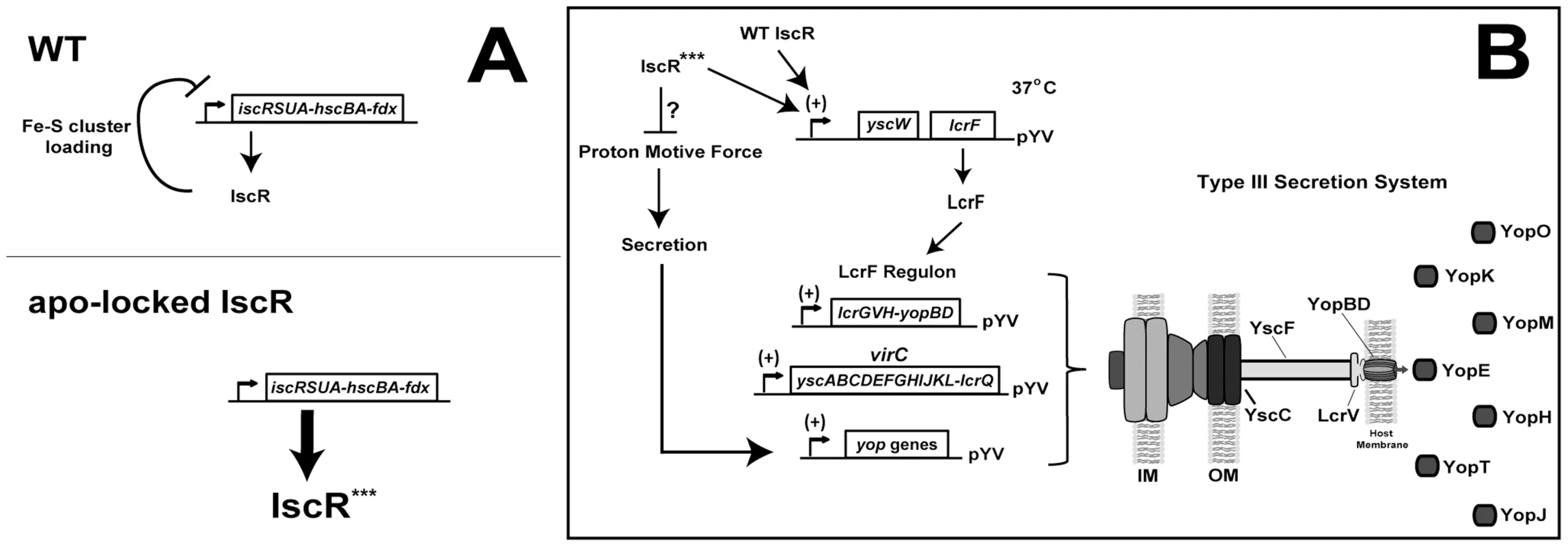
(A) Model of isc operon transcriptional control in the Y. pseudotuberculosis wild type and apo-locked IscR strains based on previous work on E. coli IscR [32], [34] and on data shown here. In wild type bacteria, the Isc Fe-S cluster biogenesis pathway loads a [2Fe-2S] cluster onto IscR (holo-IscR) [32], which recognizes a type 1 DNA-binding motif in the isc promoter to repress transcription in a negative feedback loop. Expression of the apo-locked IscR allele (***, IscR-C92A/C98A/C104A) results in loss of holo-IscR-mediated repression, thereby increasing transcription of the isc operon relative to wild type, resulting in a 30-fold increase in iscR. (B) Model depicting the mechanism by which IscR controls the Y. pseudotuberculosis Ysc T3SS. Holo- and apo-IscR are predicted to bind a newly identified type 2 DNA-binding site within the yscW-lcrF operon encoding the LcrF T3SS master regulator. Subsequently, LcrF expression leads to transcription of the LcrF regulon, which includes the lcrGVH-yopBD and virC operons and yop genes [17], [20], [22], [53], [54]. These genes encode the majority of T3SS structural, regulatory, and effector proteins. However, through an as yet undefined mechanism, overexpression of apo-locked IscR leads to a decrease in the proton motive force, which is required for type III secretion [50]. As Yop secretion positively regulates yop gene transcription [51], [52], the secretion defect of the apo-locked IscR mutant is predicted to lead to a decrease in effector yop transcription. In addition to control of Fe-S cluster biogenesis pathway expression, we present evidence that IscR controls expression and function of the Y. pseudotuberculosis T3SS. Bioinformatic analysis revealed a type 2 motif within the promoter of the T3SS master regulator LcrF that contained all nine bases previously found to be important for IscR binding (Figure 8A) [33]. Indeed, DNA binding assays demonstrated that IscR is able to specifically recognize this type 2 motif, suggesting that IscR may be acting directly to promote transcription of lcrF (Figure 9B). In support of this, we observed a marked decrease in transcription of numerous T3SS genes in the ΔiscR mutant strain. These include the gene that encodes LcrF, as well as a number of LcrF-regulated genes including the virC operon, yopK, yopT, yopM, yopH, yopJ, and lcrGVH-yopBD [17], [20], [22], [53], [54]. The lcrF type 2 motif is further upstream of the -10/-35 region previously identified by Böhme et al. [24] than other IscR binding sites that promote transcription [33], as we propose this site does. However, there may be an alternative −10/−35 region closer to the identified motif 2 site that might be used under specific growth conditions. Together, these data suggest that IscR is required for full expression of lcrF and LcrF-regulated genes through binding to a type 2 motif in the yscW-lcrF promoter (Figure 9B).
Based on these findings, an IscR mutant unable to coordinate an Fe-S cluster (apo-locked IscR) should lead to restoration of T3SS expression. Indeed, transcription of the yscW-lcrF and virC operons, as well as the majority of genes in the lcrGVH-yopBD operon, were no longer significantly decreased in the apo-locked IscR mutant compared to the ΔiscR strain. However, decreased transcription of yopE, yopK, yopM, and yopH as well as a severe defect in secretion of Yops was still observed. This could be explained by a deficiency in the apo-locked mutant's membrane potential, but not in the ΔiscR strain (Figure 9B). Wilharm et al., demonstrated that Y. enterocolitica motility and type III secretion requires the proton motive force [50]. Indeed, the apo-locked Y. pseudotuberculosis strain displayed a significant motility defect while the ΔiscR mutant was fully motile. Therefore, the type III secretion defect of the Y. pseudotuberculosis apo-locked IscR mutant can be explained by a deficiency in the proton motive force. Furthermore, the defect in YopHEMK transcription in the apo-locked IscR mutant may be explained by the fact that Yop secretion has a positive regulatory effect on Yop transcription [51], [52]. Together, these data suggest that apo-IscR can promote LcrF transcription, but that locking iscR is the apo form causes a proton motive force defect that prevents effector Yop transcription and secretion (Figure 9B).
It is unclear why locking IscR in the apo-locked form leads to a proton motive force defect. We observed ∼9-fold more suf transcript in the apo-locked IscR mutant compared to the ΔiscR strain that does not have a proton motive force defect, whereas the isc operon was expressed to the same degree in both mutants. Ezraty et al. recently showed that expression of the suf, but not the isc, operon in E. coli leads to a proton motive force defect, possibly as a result of impaired loading of Fe-S clusters into aerobic respiratory complexes [55]. Although the isc operon is expressed in the apo-locked Y. pseudotuberculosis mutant, perhaps overexpression of the suf pathway leads to misassembly of the Fe-S complexes of the electron transport chain that drive the proton motive force.
Both holo - and apo-IscR are predicted to bind to the type 2 motif within the yscW-lcrF promoter [33]. Based on previous data on E. coli IscR [28]–[30], [34], [56], low iron, aerobic growth, or high oxidative stress conditions are predicted to result in high expression of IscR through derepression of the isc operon, which in turn should increase T3SS gene expression. Likewise, high iron, anaerobic, or low oxidative stress conditions should lead to decreased IscR levels and therefore lower T3SS expression. Under normal aerobic culture conditions, we do not observe a change in wild type Y. pseudotuberculosis type III secretion when iron levels are altered (data not shown). However, in vivo, bacteria may be present in microaerophilic or anaerobic niches, where changes in iron bioavailability and reactive oxygen species production may impact iscR and T3SS gene expression. Upon ingestion by a host animal, Y. pseudotuberculosis enters the lumen of the intestine, which receives approximately 15 mg of iron per day [57], [58]. In the small intestine, Y. pseudotuberculosis can cross the gut barrier and enter the bloodstream and deeper tissues, which have very low iron bioavailability (∼10−24 M free serum iron) [59]–[61]. Sequestration of iron by iron carriers in mammalian tissues is an important host defense mechanism to prevent growth of bacterial pathogens, the majority of which require iron for growth [62]. The Ysc T3SS has been shown to be required for Y. pseudotuberculosis pathogenesis in these deep tissue sites that are low in iron bioavailability [44]. Perhaps Y. pseudotuberculosis uses IscR to sense iron, O2, and/or ROS concentration in order to optimally control T3SS expression in vivo.
Consistent with the severe T3SS expression defect displayed by the Y. pseudotuberculosis ΔiscR strain, this mutant was deficient in colonization of the Peyer's patches, spleen, and liver. Interestingly, the ΔiscR mutant was also defective in colonization of the mesenteric lymph nodes (MLN), yet T3SS mutants were previously shown to persist in the MLN and chromosomally-encoded factors were found to be important for Y. pseudotuberculosis survival in this tissue [24], [63], [64]. These results indicate that the virulence defect of the Y. pseudotuberculosis ΔiscR strain may not be due solely to misregulation of the T3SS, suggesting the existence of other IscR gene targets important for virulence. IscR of Pseudomonas aeruginosa has been shown to be important for full virulence through its ability to upregulate KatA, encoding a catalase that protects against oxidative stress [38], [65]–[67]. In Vibrio vulnificus, IscR upregulates two genes encoding the antioxidants peroxiredoxin and glutaredoxin 2, and is essential for survival during exposure to reactive oxygen species [40]. Interestingly, our analysis suggests that Y. pseudotuberculosis IscR plays an opposite regulatory role, as IscR negatively affects expression of the genes encoding cellular detoxification proteins KatY, Tpx, SodC and SodB. Furthermore, hydrogen peroxide sensitivity assays showed comparable levels of survival between the Y. pseudotuberculosis wild type and ΔiscR strains (Figure S5). This suggests that the virulence defect observed for the ΔiscR Y. pseudotuberculosis mutant is not due to increased susceptibility to oxidative stresses encountered during infection. Pathways other than the T3SS, such as the hmu hemin uptake system, were found to be misregulated in the Y. pseudotuberculosis ΔiscR strain (Table 2 & Figure 4B). While the hmu operon was shown to not affect Y. pestis virulence, it is possible that IscR control of the Y. pseudotuberculosis hmu pathway is important for virulence.
In summary, we present the first characterization for the iron-sulfur cluster regulator, IscR, of Yersinia. We reveal that IscR regulates genes involved in Fe-S cluster assembly in a manner akin to that of E. coli. Most notably, we demonstrate that mutation of IscR leads to decreased function of the Y. pseudotuberculosis T3SS and that this is due to a decrease in transcription of genes encoding structural, regulatory, and effector proteins. Furthermore, we present evidence showing that IscR is essential for the virulence of Y. pseudotuberculosis and that this attenuation is likely due, in part, to direct regulation of the T3SS by IscR. Collectively, this study argues for the important and novel role of IscR in the virulence of Y. pseudotuberculosis as well as regulation of the Ysc T3SS, and identifies IscR as a potential target for novel antimicrobial agents.
Materials and Methods
All animal use procedures were in strict accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the UCSC Institutional Animal Care and Use Committee.
Bacterial strains, plasmids and growth conditions
All strains used in this study are listed in Table 3. Y. pseudotuberculosis strains were grown in either 2xYT or M9 minimal media supplemented with casamino acids [68], referred to here as M9, at 26°C with shaking at 250 rpm, unless otherwise indicated. Where stated, Yop synthesis was induced via back-dilution of cultures into either M9 or low calcium media (2xYT plus 20 mM sodium oxalate and 20 mM MgCl2) to an OD600 of 0.2 and grown for 1.5 h at 26°C/shaking followed by 2 h at 37°C/shaking as previously described [69].
Tab. 3. <i>Y. pseudotuberculosis</i> strains used in this study. 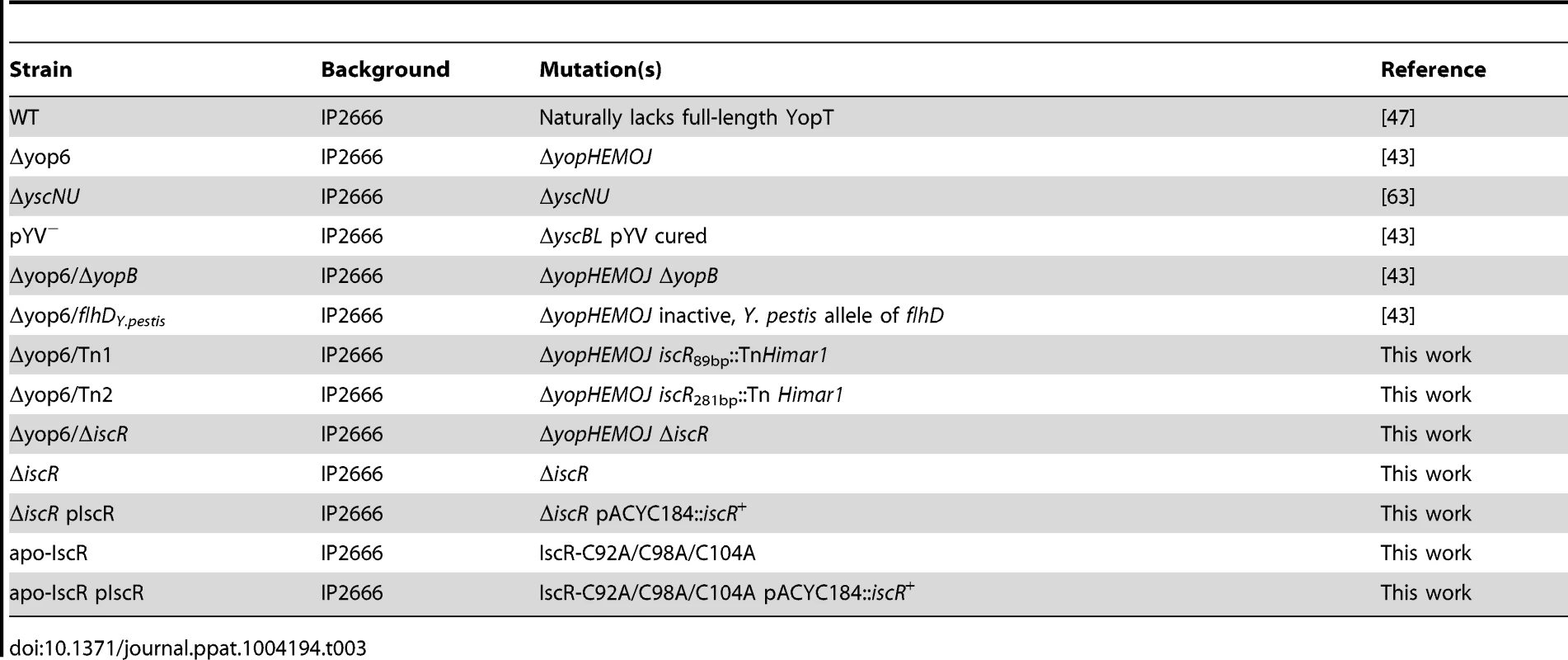
Construction of Y. pseudotuberculosis mutant strains
The iscR deletion mutant (ΔiscR) was generated via splicing by overlap extension PCR [70]. Primer pairs F5'iscR/R5'iscR and F3'iscR/R3'iscR (Table S3), designed using MacVector and Primer 3 software (http://fokker.wi.mit.edu/primer3/input.htm), were used to amplify ∼500 bp 5′ and 3′ of the iscR coding region, respectively. Amplified PCR fragments served as templates in an overlap extension PCR using the outside primers F5'iscR and R3'iscR.
The IscR-C92A/C98A/C104A mutant (apo-IscR) was generated via splicing by overlap extension PCR [70]. Primer pairs F5'apo-IscR/R5'apo-IscR and F3'apo-IscR/R3'apo-IscR (Table S3), designed using MacVector and Primer 3 software (http://fokker.wi.mit.edu/primer3/input.htm), were used to amplify ∼500 bp 5′ and 3′ within the iscR coding region, respectively. Amplified PCR fragments served as templates in an overlap extension PCR using the outside primers F5'apo-IscR and R3'apo-IscR. Nucleotide changes within the internal primers R5'apo-IscR and F3'apo-IscR allowed for amplification of iscR target containing sequences coding for alanine substitutions of the three conserved cysteines that coordinate an Fe-S cluster.
The resulting ∼1 kb fragments were cloned into the TOPO TA cloning vector (Invitrogen) and further subcloned into a BamHI - and NotI-digested pSR47s suicide plasmid (λpir-dependent replicon, kanamycinR (KanR), sacB gene conferring sucrose sensitivity) [71], [72]. Recombinant plasmids were transformed into E. coli S17-1 λpir competent cells and later introduced into Y. pseudotuberculosis IP2666 via conjugation. The resulting KanR, irgansanR (Yersinia selective antibiotic) integrants were grown in the absence of antibiotics and plated on sucrose-containing media to select for clones that had lost sacB (and by inference, the linked plasmid DNA). KanS, sucroseR, congo red-positive colonies were screened by PCR and subsequently sequenced to verify loss of the intended iscR coding region.
The iscR complement construct was generated by insertion of a fragment containing the iscR coding region as well as 530 bp of 5′ upstream sequence. This was PCR amplified using primer pair FiscRC and RiscRC, and cloned into the vector pACYC184 via BamHI/SalI restriction sites [73], [74]. Recombinant plasmids were transformed into E. coli S17-1 λpir competent cells and later introduced into Y. pseudotuberculosis IP2666 ΔiscR via a modified transformation method [75]. Briefly, recipient Yersinia strains were grown overnight in LB containing 2% glucose at 26°C. Cultures were centrifuged at 3,500 rpm for 3 min then washed with 750 µl of ice-cold sterile diH2O and repeated for a total of three washes. Washed pellets were resuspended in 100 µl of sterile diH2O, combined with 3 µl of plasmid and electroporated at EC2. Cells were allowed to recover in 1 mL SOC media for 1 h at 26°C followed by plating on LB containing carbenicillin to select for Yersinia bearing the plasmid of interest. Clones were confirmed by PCR analysis, using a combination of gene - and vector-specific primers, to construct both the ΔiscR complemented strain (ΔiscR pIscR) and the apo-IscR complemented strain (apo-IscR pIscR).
The nonmotile Δyop6/flhDCY.pestis mutant was generated by crossing in the Y. pestis flhDC gene into Y. pseudotuberculosis. Y. pestis flhD has a frameshift mutation, resulting in suppression of flagellin production [76]. The suicide plasmid pSB890 encoding a partial flhC gene and the full flhD gene from the Y. pestis KIM strain, generously provided by Dr. Brad Cookson, was conjugated into Y. pseudotuberculosis Δyop6 and nonmotile, recombinant mutants isolated as previously described [46].
Transposon screen generation and insertion site identification
Transposon mutagenesis was preformed similarly to Crimmins et al. [64]. Briefly, E. coli SM10λpir harboring pSC189, which encodes Himar1 [77], was mated with Y. pseudotuberculosis Δyop6. Mating culture was then pelleted, resuspended, spread out evenly among six 150 mm×15 mm petri plates containing LB supplemented with 2 µg mL−1 irgasan and 30 µg mL−1 Kan, and incubated for 3 days at room temperature. Colonies were patched onto LB supplemented with 100 µg mL−1 carbenicillin to ensure insertion of the transposon. Colony patches were used to grow 2xYT overnight cultures in 96-well plates, which were then frozen down to preserve the library. HEK293T cells were plated in 96-well white clear bottom plates (Corning) and transfected with a plasmid encoding a luciferase reporter gene fused to an NFκB-dependent promoter (Stratagene). Mutants from the transposon library were grown overnight in 96 well plates in M9 at 26°C and used to infect the transfected HEK293T cell monolayers. After 4 h incubation at 37°C, 100 µl of 1∶1 NeoLite:PBS solution was added to each well of the 96-well clear-bottom white plate (Corning), and luminescence was measured using a Victor3 plate reader (PerkinElmer). Each transposon mutant was assayed in duplicate. The positions of the transposons in the iscR::Tn1 and iscR::Tn2 mutants were determined by plasmid rescue, as previously described [78], except BamHI was used for digestion of genomic DNA.
NFκB activity assay
Validation of the transposon screen was performed through the use of an NFΚB activity assay, which is based on our previous work showing that Y. pseudotuberculosis induces NFκB activation in HEK293T cells dependent on expression of a functional T3SS [43]. Briefly, HEK293T cells were transfected with a plasmid encoding a luciferase reporter gene fused to an NFκB-dependent promoter (Stratagene). Bacterial strains were grown overnight in 2xYT and subcultured to an OD600 of 0.2 into low calcium media and grown at 26°C for 1.5 h followed by a shift to 37°C for an additional 1.5 h to induce the T3SS. Bacterial cultures were resuspended in prewarmed (37°C) DMEM and 200 µl aliquots were then used to infect the HEK293T cells containing the luciferase reporter plasmid at an MOI of 10. After 4 h incubation at 37°C, 100 µl of 1∶1 NeoLite:PBS solution was added to each well of the 96-well clear-bottom white plate (Corning), and luminescence was measured using a Victor3 plate reader (PerkinElmer). Data from three separate wells were averaged for each independent experiment.
Type III secretion assay
Visualization of T3SS cargo secreted in broth culture was performed as previously described [46]. Briefly, Y. pseudotuberculosis in M9 low calcium media (M9 plus 20 mM sodium oxalate and 20 mM MgCl2) was grown for 1.5 h at 26°C followed by growth at 37°C for 2 h. Cultures were normalized by OD600 and pelleted at 13,200 rpm for 10 min at room temperature. Supernatants were removed and proteins precipitated by addition of trichloroacetic acid (TCA) at a final concentration of 10%. Samples were incubated on ice for 20 min and pelleted at 13,200 rpm for 15 min at 4°C. Resulting pellets were washed twice with ice-cold 100% EtOH and subsequently resuspended in final sample buffer (FSB) containing 20% dithiothreitol (DTT). Samples were boiled for 5 min prior to running on a 12.5% SDS PAGE gel.
Ethidium bromide entry assay
Evaluation of pore formation was performed via the ethidium bromide (EtBr) entry assay as previously described [46]. Briefly, 2×104 immortalized C57Bl/6 BMDMs were plated in a 96 well clear bottom black plate (Corning) in 100 uL DMEM +10% FBS. Infection was performed in triplicate at an MOI of 25. Plates were centrifuged at 750×g at 4°C for 5 min to facilitate contact. Infections were carried out at 37°C with 5% CO2 for 2 h, at which point media was aspirated and replaced with 30 µL of PBS containing 25 µgmL−1 ethidium bromide (EtBr) and 12.3 µg mL−1 Hoechst dye. The cell monolayer was visualized using an ImageXpressMICRO automated microscope and MetaXpress analysis software (Molecular Devices). The percent of EtBr-positive cells was calculated by dividing the number of EtBr-stained cells by the number of Hoechst-stained cells. Data from three separate wells was averaged for each independent experiment.
Growth curves
Y. pseudotuberculosis strains were cultured overnight in 2xYT or M9 at 26°C and sub-cultured to an OD600 of 0.2 in 25 mL of either 2xYT or M9. Cultures were incubated at either 26°C or 37°C with shaking at 250 rpm and optical density measured at 600 nm every hour for 9 h.
Mouse infections
All animal use procedures were in strict accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the UC Santa Cruz Institutional Animal Care and Use Committee. Eleven to twelve-week-old 129S6/SvEvTac mice from our breeding facilities were used for oral infections as previously described [79]. Briefly, mice were orogastrically inoculated with 2×108 CFU in a 200 µl volume using a feeding needle. Mice were given food and water ad libitum and were euthanized at 5 days post-inoculation. Peyer's patches, mesenteric lymph nodes, spleens, and livers were isolated and homogenized for 30 s in PBS followed by serial dilution and plating on LB supplemented with 1 µg mL−1 irgasan for CFU determination.
RNAseq analysis
RNA was isolated from the IP2666 wild type and isogenic ΔiscR and apo-IscR strains grown for 3 h at 37°C in M9, using the RNeasy Mini Kit (Qiagen) as per the manufacturer's protocol. We chose M9 media for our RNASeq analysis because this condition enables expression of T3SS genes and secretion of T3SS cargo at 37°C [68]. Contaminating DNA was removed from the RNA samples using a DNA-free kit (Life Sciences). Samples were subjected to removal of contaminating rRNA via the Ribo-Zero Magnetic Kit for Gram-negative bacteria (Epicentre). The cDNA library was created using the NEBNext Ultra Directional RNA Library Prep Kit for Illumina (NEB). These studies were performed with three biological replicates per condition. Six indexed samples were sequenced per single lane using the HiSeq2500 Illumina sequencing platform for 50 bp single reads (UC Davis Genome Center) and subsequently analyzed and visualized via the CLC Genomics Workbench version 5.5.1 (CLC bio). Samples were normalized for both sequence depth and gene size by determining RPKM (Reads Per Kilo base per Million reads) and mapped to the Y. pseudotuberculosis genome (IP32953). Differentially regulated genes were identified as those displaying a fold change with an absolute value of 2 or greater. Statistical significance was determined by baySeq test with a corrected FDR post hoc test where p<0.05 was deemed significant [80].
Real-time PCR
Total RNA generated from our RNAseq analysis at a concentration of 2 µg was used to make cDNA as previously described [81]. SYBR Green PCR master mix (Applied Biosystems) was used for qPCR reactions according to the manufacturer's instructions and a 60°C annealing temperature. Primers used are listed in Table S4. Control primers were for the 16S rRNA as described previously [82]. Results were analyzed using the Bio-Rad CFX software.
Virulence plasmid map generation
Average RPKM values generated from RNAseq analysis for the wild type, ΔiscR and apo-IscR mutants were converted to log2-ratios (log2(RPKMmutant/RPKMwt) for each gene encoded on the virulence plasmid, pYV. These values were converted to a Circos heatmap [83] and plotted against the respective pYV base coordinate positions from Y. pseudotuberculosis IP32953.
Motif identification and search
Position specific scoring matrix (PSSM) was generated by the alignment of the known E. coli IscR type 2 motifs (Table S4) (Maverix Biomics, Inc) [31]. PSSM of type 2 was used to scan against the 150-nt upstream of 99 genes encoded on the Y. pseudotuberculosis pYV plasmid and obtained a ranked list of putative type 2 motifs.
DNA binding fluorescence anisotropy assays
Fluorescence anisotropy was measured similar to Nesbit et al., [33]. E. coli apo-IscR lacking the [2Fe-2S] cluster (IscR-C92A) was isolated anaerobically following the protocol described previously for wild type IscR [30]. Competition assays were performed using 5nM of 30-mer dsDNA of the known E. coli hyaA type 2 motif containing a 5′ TAMRA fluorophore (IDT) on the top strand and unlabeled competitor dsDNA concentrations ranged from 8 to 1000 nM (IDT, Table S4). DNA was annealed by heating equimolar concentrations of complementary DNA strands in annealing buffer (40 mM Tris (pH 7.9), 30 mM KCl) to 95°C for 5 min followed by slow cooling to room temperature over 2 hours. Annealed DNA was incubated with 90 nM apo-IscR in anisotropy buffer (40 mM Tris pH 7.9, 150 mM KCl, 100 ng ul−1 Salmon Sperm DNA) for 12 min at room temperature and anisotropy was measured using an EnVision 2103 Multilabel Reader (Perkin Elmer) with Wallac EnVision Manager software. Data is representative of experiments performed on three separate days.
Motility assay
Motility was analyzed by spotting 1 µl aliquots of either a nonmotile strain bearing an inactive, Y. pestis allele of flhD (Δyop6/flhDY.pestis), WT, ΔiscR, or apo-IscR strains onto motility agar plates (1% tryptone, 0.25% agar) from overnight cultures standardized to an OD600 of 2.5. Plates were incubated at room temperature for 1 day, at which point the diameters of the colonies were determined and used to calculate percent motility relative to WT, which was set at 100%.
Measurement of the membrane potential
The electrical potential was measured similar to the JC-1 red/green dye assay previously described for E. coli [84]. JC-1 is a membrane-permeable dye that emits green fluorescence (∼530 nm) upon excitation when the dye is in the monomeric form. Due to the membrane potential of the bacterial cell, JC-1 dye will form J aggregates which emit red fluorescence (∼590 nm). If the membrane potential decreases, there will be a decrease in J aggregate formation and subsequently a decrease in red fluorescence. As such, membrane potential can be displayed as a ratio of red/green fluorescence. Briefly, Y. pseudotuberculosis wild type and isogenic ΔiscR, ΔiscR complemented (ΔiscR pIscR), apo-IscR and apo-IscR complemented (apo-IscR pIscR) strains were grown overnight in M9 at 26°C. Strains were subcultured to an OD600 of 0.2 in M9 and grown at 37°C for 3 hours. A negative control containing a sample of wild type Y. pseudotuberculosis treated with 40 µM of the protonophore, CCCP (carbonyl cyanide m-chlorophenylhydrazone), during the last 30 min of growth was included in each experiment. After incubation at 37°C, 1 mL aliquots were harvested for each strain and pelleted at 4,500×g for 3 min followed by resuspension in 1 mL of permeabilisation buffer (10 mM Tris-HCl, pH 7.6, 1 mM EDTA and 10 mM glucose). Post resuspension, 2 µl of the membrane-permeable JC-1 dye (5 mg/mL) was added and the samples were incubated at room temperature for 30 min. Samples were then pelleted at 4,500×g for 3 min and resuspended in 500 µl of permeabilisation buffer. Slides were prepared by first coating with Poly-L-Lysine solution through addition of 100 µl aliquots of a 0.01% solution followed by a 5 min incubation at room temperature. Slides were washed a total of 3 times with sterile diH2O. Once dry, 10 µl of prepared sample was added to the slide and allowed to adhere for 5 min. Unattached bacteria were removed by washing with PBS and excess liquid removed via aspiration. A coverslip was applied and the cells were imaged using a LSM 5 PASCAL laser scanning microscope (Zeiss) fitted with a Plan-Apochromat 63x/1.4 Oil DIC objective and analyzed using the LSM 510 software (Zeiss). Quantification of image intensities was performed using ImageJ [85].
Supporting Information
Zdroje
1. TroisfontainesP, CornelisGR (2005) Type III secretion: more systems than you think. Physiology (Bethesda) 20 : 326–339.
2. CoburnB, SekirovI, FinlayBB (2007) Type III secretion systems and disease. Clin Microbiol Rev 20 : 535–549.
3. ZhangY, MenaP, RomanovG, LinJS, SmileyST, et al. (2012) A protective epitope in type III effector YopE is a major CD8 T cell antigen during primary infection with Yersinia pseudotuberculosis. Infect Immun 80 : 206–214.
4. BliskaJB, WangX, ViboudGI, BrodskyIE (2013) Modulation of innate immune responses by Yersinia type III secretion system translocators and effectors. Cell Microbiol 15 : 1622–1631.
5. BrubakerRR (1983) The Vwa+ virulence factor of yersiniae: the molecular basis of the attendant nutritional requirement for Ca++. Rev Infect Dis 5 Suppl 4S748–758.
6. HueckCJ (1998) Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol Mol Biol Rev 62 : 379–433.
7. GemskiP, LazereJR, CaseyT, WohlhieterJA (1980) Presence of a virulence-associated plasmid in Yersinia pseudotuberculosis. Infect Immun 28 : 1044–1047.
8. RuckdeschelK, RoggenkampA, LafontV, MangeatP, HeesemannJ, et al. (1997) Interaction of Yersinia enterocolitica with macrophages leads to macrophage cell death through apoptosis. Infect Immun 65 : 4813–4821.
9. LarocheY, van BouchauteM, CornelisG (1984) A restriction map of virulence plasmid pVYE439-80 from a serogroup 9 Yersinia enterocolitica strain. Plasmid 12 : 67–70.
10. CornelisGR (2006) The type III secretion injectisome. Nat Rev Microbiol 4 : 811–825.
11. YipCK, StrynadkaNC (2006) New structural insights into the bacterial type III secretion system. Trends Biochem Sci 31 : 223–230.
12. DewoodyRS, MerrittPM, MarketonMM (2013) Regulation of the Yersinia type III secretion system: traffic control. Front Cell Infect Microbiol 3 : 4.
13. BlaylockB, RiordanKE, MissiakasDM, SchneewindO (2006) Characterization of the Yersinia enterocolitica type III secretion ATPase YscN and its regulator, YscL. J Bacteriol 188 : 3525–3534.
14. MuellerCA, BrozP, CornelisGR (2008) The type III secretion system tip complex and translocon. Mol Microbiol 68 : 1085–1095.
15. CornelisGR, BolandA, BoydAP, GeuijenC, IriarteM, et al. (1998) The virulence plasmid of Yersinia, an antihost genome. Microbiol Mol Biol Rev 62 : 1315–1352.
16. Lambert de RouvroitC, SluitersC, CornelisGR (1992) Role of the transcriptional activator, VirF, and temperature in the expression of the pYV plasmid genes of Yersinia enterocolitica. Mol Microbiol 6 : 395–409.
17. MichielsT, VanooteghemJC, Lambert de RouvroitC, ChinaB, GustinA, et al. (1991) Analysis of virC, an operon involved in the secretion of Yop proteins by Yersinia enterocolitica. J Bacteriol 173 : 4994–5009.
18. BergmanT, HakanssonS, ForsbergA, NorlanderL, MacellaroA, et al. (1991) Analysis of the V antigen lcrGVH-yopBD operon of Yersinia pseudotuberculosis: evidence for a regulatory role of LcrH and LcrV. J Bacteriol 173 : 1607–1616.
19. HoeNP, MinionFC, GoguenJD (1992) Temperature sensing in Yersinia pestis: regulation of yopE transcription by lcrF. J Bacteriol 174 : 4275–4286.
20. CornelisG, SluitersC, de RouvroitCL, MichielsT (1989) Homology between virF, the transcriptional activator of the Yersinia virulence regulon, and AraC, the Escherichia coli arabinose operon regulator. J Bacteriol 171 : 254–262.
21. CornelisG, VanootegemJC, SluitersC (1987) Transcription of the yop regulon from Y. enterocolitica requires trans acting pYV and chromosomal genes. Microb Pathog 2 : 367–379.
22. ChinaB, MichielsT, CornelisGR (1990) The pYV plasmid of Yersinia encodes a lipoprotein, YlpA, related to TraT. Mol Microbiol 4 : 1585–1593.
23. HoeNP, GoguenJD (1993) Temperature sensing in Yersinia pestis: translation of the LcrF activator protein is thermally regulated. J Bacteriol 175 : 7901–7909.
24. BohmeK, SteinmannR, KortmannJ, SeekircherS, HerovenAK, et al. (2012) Concerted actions of a thermo-labile regulator and a unique intergenic RNA thermosensor control Yersinia virulence. PLoS Pathog 8: e1002518.
25. RodionovDA, GelfandMS, ToddJD, CursonAR, JohnstonAW (2006) Computational reconstruction of iron - and manganese-responsive transcriptional networks in alpha-proteobacteria. PLoS Comput Biol 2: e163.
26. ShepardW, SoutourinaO, CourtoisE, EnglandP, HaouzA, et al. (2011) Insights into the Rrf2 repressor family—the structure of CymR, the global cysteine regulator of Bacillus subtilis. FEBS J 278 : 2689–2701.
27. FleischhackerAS, StubnaA, HsuehKL, GuoY, TeterSJ, et al. (2012) Characterization of the [2Fe–2S] cluster of Escherichia coli transcription factor IscR. Biochemistry 51 : 4453–4462.
28. WuY, OuttenFW (2009) IscR controls iron-dependent biofilm formation in Escherichia coli by regulating type I fimbria expression. J Bacteriol 191 : 1248–1257.
29. YeoWS, LeeJH, LeeKC, RoeJH (2006) IscR acts as an activator in response to oxidative stress for the suf operon encoding Fe-S assembly proteins. Mol Microbiol 61 : 206–218.
30. GielJL, RodionovD, LiuM, BlattnerFR, KileyPJ (2006) IscR-dependent gene expression links iron-sulphur cluster assembly to the control of O2-regulated genes in Escherichia coli. Mol Microbiol 60 : 1058–1075.
31. RajagopalanS, TeterSJ, ZwartPH, BrennanRG, PhillipsKJ, et al. (2013) Studies of IscR reveal a unique mechanism for metal-dependent regulation of DNA binding specificity. Nat Struct Mol Biol 20 : 740–747.
32. GielJL, NesbitAD, MettertEL, FleischhackerAS, WantaBT, et al. (2013) Regulation of iron-sulphur cluster homeostasis through transcriptional control of the Isc pathway by [2Fe-2S]-IscR in Escherichia coli. Mol Microbiol 87 : 478–492.
33. NesbitAD, GielJL, RoseJC, KileyPJ (2009) Sequence-specific binding to a subset of IscR-regulated promoters does not require IscR Fe-S cluster ligation. J Mol Biol 387 : 28–41.
34. SchwartzCJ, GielJL, PatschkowskiT, LutherC, RuzickaFJ, et al. (2001) IscR, an Fe-S cluster-containing transcription factor, represses expression of Escherichia coli genes encoding Fe-S cluster assembly proteins. Proc Natl Acad Sci U S A 98 : 14895–14900.
35. LeeKC, YeoWS, RoeJH (2008) Oxidant-responsive induction of the suf operon, encoding a Fe-S assembly system, through Fur and IscR in Escherichia coli. J Bacteriol 190 : 8244–8247.
36. BeinertH, HolmRH, MunckE (1997) Iron-sulfur clusters: nature's modular, multipurpose structures. Science 277 : 653–659.
37. Rincon-EnriquezG, CreteP, BarrasF, PyB (2008) Biogenesis of Fe/S proteins and pathogenicity: IscR plays a key role in allowing Erwinia chrysanthemi to adapt to hostile conditions. Mol Microbiol 67 : 1257–1273.
38. KimSH, LeeBY, LauGW, ChoYH (2009) IscR modulates catalase A (KatA) activity, peroxide resistance and full virulence of Pseudomonas aeruginosa PA14. J Microbiol Biotechnol 19 : 1520–1526.
39. Jones-CarsonJ, LaughlinJ, HamadMA, StewartAL, VoskuilMI, et al. (2008) Inactivation of [Fe-S] metalloproteins mediates nitric oxide-dependent killing of Burkholderia mallei. PLoS One 3: e1976.
40. LimJG, ChoiSH (2014) IscR is a global regulator essential for pathogenesis of Vibrio vulnificus and induced by host cells. Infect Immun 82 : 569–578.
41. MurphyER, PayneSM (2007) RyhB, an iron-responsive small RNA molecule, regulates Shigella dysenteriae virulence. Infect Immun 75 : 3470–3477.
42. EllermeierJR, SlauchJM (2008) Fur regulates expression of the Salmonella pathogenicity island 1 type III secretion system through HilD. J Bacteriol 190 : 476–486.
43. AuerbuchV, GolenbockDT, IsbergRR (2009) Innate immune recognition of Yersinia pseudotuberculosis type III secretion. PLoS Pathog 5: e1000686.
44. ViboudGI, BliskaJB (2005) Yersinia outer proteins: role in modulation of host cell signaling responses and pathogenesis. Annu Rev Microbiol 59 : 69–89.
45. KirbyJE, VogelJP, AndrewsHL, IsbergRR (1998) Evidence for pore-forming ability by Legionella pneumophila. Mol Microbiol 27 : 323–336.
46. KwuanL, AdamsW, AuerbuchV (2013) Impact of host membrane pore formation by the Yersinia pseudotuberculosis type III secretion system on the macrophage innate immune response. Infect Immun 81 : 905–914.
47. BliskaJB, GuanKL, DixonJE, FalkowS (1991) Tyrosine phosphate hydrolysis of host proteins by an essential Yersinia virulence determinant. Proc Natl Acad Sci U S A 88 : 1187–1191.
48. SheridanJJ, LogueCM, McDowellDA, BlairIS, HegartyT (1998) A study of the growth kinetics of Yersinia enterocolitica serotype O:3 in pure and meat culture systems. J Appl Microbiol 85 : 293–301.
49. GoverdeRL, KustersJG, Huis in 't VeldJH (1994) Growth rate and physiology of Yersinia enterocolitica; influence of temperature and presence of the virulence plasmid. J Appl Bacteriol 77 : 96–104.
50. WilharmG, LehmannV, KraussK, LehnertB, RichterS, et al. (2004) Yersinia enterocolitica type III secretion depends on the proton motive force but not on the flagellar motor components MotA and MotB. Infect Immun 72 : 4004–4009.
51. PetterssonJ, NordfelthR, DubininaE, BergmanT, GustafssonM, et al. (1996) Modulation of virulence factor expression by pathogen target cell contact. Science 273 : 1231–1233.
52. KauppiAM, NordfelthR, UvellH, Wolf-WatzH, ElofssonM (2003) Targeting bacterial virulence: inhibitors of type III secretion in Yersinia. Chem Biol 10 : 241–249.
53. WattiauP, CornelisGR (1994) Identification of DNA sequences recognized by VirF, the transcriptional activator of the Yersinia yop regulon. J Bacteriol 176 : 3878–3884.
54. SkurnikM, ToivanenP (1992) LcrF is the temperature-regulated activator of the yadA gene of Yersinia enterocolitica and Yersinia pseudotuberculosis. J Bacteriol 174 : 2047–2051.
55. EzratyB, VergnesA, BanzhafM, DuvergerY, HuguenotA, et al. (2013) Fe-S cluster biosynthesis controls uptake of aminoglycosides in a ROS-less death pathway. Science 340 : 1583–1587.
56. ZhengM, WangX, TempletonLJ, SmulskiDR, LaRossaRA, et al. (2001) DNA microarray-mediated transcriptional profiling of the Escherichia coli response to hydrogen peroxide. J Bacteriol 183 : 4562–4570.
57. McCanceRA, WiddowsonEM (1938) The absorption and excretion of iron following oral and intravenous administration. J Physiol 94 : 148–154.
58. MiretS, SimpsonRJ, McKieAT (2003) Physiology and molecular biology of dietary iron absorption. Annu Rev Nutr 23 : 283–301.
59. MartinRB, SavoryJ, BrownS, BertholfRL, WillsMR (1987) Transferrin binding of Al3+ and Fe3+. Clin Chem 33 : 405–407.
60. AisenP, LeibmanA, ZweierJ (1978) Stoichiometric and site characteristics of the binding of iron to human transferrin. J Biol Chem 253 : 1930–1937.
61. KretchmarSA, ReyesZE, RaymondKN (1988) The spectroelectrochemical determination of the reduction potential of diferric serum transferrin. Biochim Biophys Acta 956 : 85–94.
62. SkaarEP (2010) The battle for iron between bacterial pathogens and their vertebrate hosts. PLoS Pathog 6: e1000949.
63. Balada-LlasatJM, MecsasJ (2006) Yersinia has a tropism for B and T cell zones of lymph nodes that is independent of the type III secretion system. PLoS Pathog 2: e86.
64. CrimminsGT, MohammadiS, GreenER, BergmanMA, IsbergRR, et al. (2012) Identification of MrtAB, an ABC transporter specifically required for Yersinia pseudotuberculosis to colonize the mesenteric lymph nodes. PLoS Pathog 8: e1002828.
65. JangS, ImlayJA (2007) Micromolar intracellular hydrogen peroxide disrupts metabolism by damaging iron-sulfur enzymes. J Biol Chem 282 : 929–937.
66. JangS, ImlayJA (2010) Hydrogen peroxide inactivates the Escherichia coli Isc iron-sulphur assembly system, and OxyR induces the Suf system to compensate. Mol Microbiol 78 : 1448–1467.
67. FlintDH, TuminelloJF, EmptageMH (1993) The inactivation of Fe-S cluster containing hydro-lyases by superoxide. J Biol Chem 268 : 22369–22376.
68. ChengLW, AndersonDM, SchneewindO (1997) Two independent type III secretion mechanisms for YopE in Yersinia enterocolitica. Mol Microbiol 24 : 757–765.
69. MecsasJ, RaupachB, FalkowS (1998) The Yersinia Yops inhibit invasion of Listeria, Shigella and Edwardsiella but not Salmonella into epithelial cells. Mol Microbiol 28 : 1269–1281.
70. WarrensAN, JonesMD, LechlerRI (1997) Splicing by overlap extension by PCR using asymmetric amplification: an improved technique for the generation of hybrid proteins of immunological interest. Gene 186 : 29–35.
71. AndrewsHL, VogelJP, IsbergRR (1998) Identification of linked Legionella pneumophila genes essential for intracellular growth and evasion of the endocytic pathway. Infect Immun 66 : 950–958.
72. MerriamJJ, MathurR, Maxfield-BoumilR, IsbergRR (1997) Analysis of the Legionella pneumophila fliI gene: intracellular growth of a defined mutant defective for flagellum biosynthesis. Infect Immun 65 : 2497–2501.
73. ChangAC, CohenSN (1978) Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol 134 : 1141–1156.
74. RoseRE (1988) The nucleotide sequence of pACYC184. Nucleic Acids Res 16 : 355.
75. WalkerKA, MillerVL (2004) Regulation of the Ysa type III secretion system of Yersinia enterocolitica by YsaE/SycB and YsrS/YsrR. J Bacteriol 186 : 4056–4066.
76. BergsbakenT, CooksonBT (2007) Macrophage activation redirects yersinia-infected host cell death from apoptosis to caspase-1-dependent pyroptosis. PLoS Pathog 3: e161.
77. ChiangSL, RubinEJ (2002) Construction of a mariner-based transposon for epitope-tagging and genomic targeting. Gene 296 : 179–185.
78. LawrenzMB, MillerVL (2007) Comparative analysis of the regulation of rovA from the pathogenic yersiniae. J Bacteriol 189 : 5963–5975.
79. AuerbuchV, IsbergRR (2007) Growth of Yersinia pseudotuberculosis in mice occurs independently of Toll-like receptor 2 expression and induction of interleukin-10. Infect Immun 75 : 3561–3570.
80. YaoJQ, YuF (2011) DEB: A web interface for RNA-seq digital gene expression analysis. Bioinformation 7 : 44–z45.
81. AuerbuchV, BrockstedtDG, Meyer-MorseN, O'RiordanM, PortnoyDA (2004) Mice lacking the type I interferon receptor are resistant to Listeria monocytogenes. J Exp Med 200 : 527–533.
82. ArafahS, RossoML, RehaumeL, HancockRE, SimonetM, et al. (2009) An iron-regulated LysR-type element mediates antimicrobial peptide resistance and virulence in Yersinia pseudotuberculosis. Microbiology 155 : 2168–2181.
83. KrzywinskiM, ScheinJ, BirolI, ConnorsJ, GascoyneR, et al. (2009) Circos: an information aesthetic for comparative genomics. Genome Res 19 : 1639–1645.
84. EnglC, BeekAT, BekkerM, de MattosJT, JovanovicG, et al. (2011) Dissipation of proton motive force is not sufficient to induce the phage shock protein response in Escherichia coli. Curr Microbiol 62 : 1374–1385.
85. SchneiderCA, RasbandWS, EliceiriKW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9 : 671–675.
86. LarkinMA, BlackshieldsG, BrownNP, ChennaR, McGettiganPA, et al. (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23 : 2947–2948.
87. SchianoCA, BellowsLE, LathemWW (2010) The small RNA chaperone Hfq is required for the virulence of Yersinia pseudotuberculosis. Infect Immun 78 : 2034–2044.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Recruitment of RED-SMU1 Complex by Influenza A Virus RNA Polymerase to Control Viral mRNA SplicingČlánek Systematic Phenotyping of a Large-Scale Deletion Collection Reveals Novel Antifungal Tolerance GenesČlánek The Contribution of Social Behaviour to the Transmission of Influenza A in a Human Population
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2014 Číslo 6- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Fungal Nail Infections (Onychomycosis): A Never-Ending Story?
- BdlA, DipA and Induced Dispersion Contribute to Acute Virulence and Chronic Persistence of
- Morphotype Transition and Sexual Reproduction Are Genetically Associated in a Ubiquitous Environmental Pathogen
- A Nucleic-Acid Hydrolyzing Single Chain Antibody Confers Resistance to DNA Virus Infection in HeLa Cells and C57BL/6 Mice
- HopW1 from Disrupts the Actin Cytoskeleton to Promote Virulence in Arabidopsis
- Ly6C Monocytes Become Alternatively Activated Macrophages in Schistosome Granulomas with Help from CD4+ Cells
- Recruitment of RED-SMU1 Complex by Influenza A Virus RNA Polymerase to Control Viral mRNA Splicing
- Contribution of Specific Residues of the β-Solenoid Fold to HET-s Prion Function, Amyloid Structure and Stability
- Antibody Responses to : Role in Pathogenesis and Diagnosis of Encephalitis?
- Discovery of a Novel Compound with Anti-Venezuelan Equine Encephalitis Virus Activity That Targets the Nonstructural Protein 2
- Activation of Focal Adhesion Kinase by Suppresses Autophagy via an Akt/mTOR Signaling Pathway and Promotes Bacterial Survival in Macrophages
- Crossing the Interspecies Barrier: Opening the Door to Zoonotic Pathogens
- Catching Fire: , Macrophages, and Pyroptosis
- IscR Is Essential for Type III Secretion and Virulence
- Selective Chemical Inhibition of Quorum Sensing in Promotes Host Defense with Minimal Impact on Resistance
- The Glycosylated Rv1860 Protein of Inhibits Dendritic Cell Mediated TH1 and TH17 Polarization of T Cells and Abrogates Protective Immunity Conferred by BCG
- A Genome-Wide Tethering Screen Reveals Novel Potential Post-Transcriptional Regulators in
- Structural Insights into SraP-Mediated Adhesion to Host Cells
- Human IGF1 Regulates Midgut Oxidative Stress and Epithelial Homeostasis to Balance Lifespan and resistance in
- Cycling Empirical Antibiotic Therapy in Hospitals: Meta-Analysis and Models
- Rab11 Regulates Trafficking of -sialidase to the Plasma Membrane through the Contractile Vacuole Complex of
- Mitogen and Stress Activated Kinases Act Co-operatively with CREB during the Induction of Human Cytomegalovirus Immediate-Early Gene Expression from Latency
- Profilin Promotes Recruitment of Ly6C CCR2 Inflammatory Monocytes That Can Confer Resistance to Bacterial Infection
- A Central Role for Carbon-Overflow Pathways in the Modulation of Bacterial Cell Death
- An Invertebrate Warburg Effect: A Shrimp Virus Achieves Successful Replication by Altering the Host Metabolome via the PI3K-Akt-mTOR Pathway
- The Highly Conserved Bacterial RNase YbeY Is Essential in , Playing a Critical Role in Virulence, Stress Regulation, and RNA Processing
- A Virulent Strain of Deformed Wing Virus (DWV) of Honeybees () Prevails after -Mediated, or , Transmission
- Systematic Phenotyping of a Large-Scale Deletion Collection Reveals Novel Antifungal Tolerance Genes
- Ubiquitin-Mediated Response to Microsporidia and Virus Infection in
- Preclinical Detection of Variant CJD and BSE Prions in Blood
- Toll-Like Receptor 8 Agonist and Bacteria Trigger Potent Activation of Innate Immune Cells in Human Liver
- Progressive Proximal-to-Distal Reduction in Expression of the Tight Junction Complex in Colonic Epithelium of Virally-Suppressed HIV+ Individuals
- The Triggering Receptor Expressed on Myeloid Cells 2 Inhibits Complement Component 1q Effector Mechanisms and Exerts Detrimental Effects during Pneumococcal Pneumonia
- Differential Activation of Acid Sphingomyelinase and Ceramide Release Determines Invasiveness of into Brain Endothelial Cells
- Forward Genetic Screening Identifies a Small Molecule That Blocks Growth by Inhibiting Both Host- and Parasite-Encoded Kinases
- Defining Immune Engagement Thresholds for Control of Virus-Driven Lymphoproliferation
- Growth Factor and Th2 Cytokine Signaling Pathways Converge at STAT6 to Promote Arginase Expression in Progressive Experimental Visceral Leishmaniasis
- Multimeric Assembly of Host-Pathogen Adhesion Complexes Involved in Apicomplexan Invasion
- Biogenesis of Influenza A Virus Hemagglutinin Cross-Protective Stem Epitopes
- Adequate Th2-Type Response Associates with Restricted Bacterial Growth in Latent Mycobacterial Infection of Zebrafish
- Protective Efficacy of Passive Immunization with Monoclonal Antibodies in Animal Models of H5N1 Highly Pathogenic Avian Influenza Virus Infection
- Fructose-Asparagine Is a Primary Nutrient during Growth of in the Inflamed Intestine
- The Calcium-Dependent Protein Kinase 3 of Influences Basal Calcium Levels and Functions beyond Egress as Revealed by Quantitative Phosphoproteome Analysis
- A Translocated Effector Required for Dissemination from Derma to Blood Safeguards Migratory Host Cells from Damage by Co-translocated Effectors
- Functional Characterization of a Novel Family of Acetylcholine-Gated Chloride Channels in
- Both α2,3- and α2,6-Linked Sialic Acids on O-Linked Glycoproteins Act as Functional Receptors for Porcine Sapovirus
- The Contribution of Social Behaviour to the Transmission of Influenza A in a Human Population
- MicroRNA-146a Provides Feedback Regulation of Lyme Arthritis but Not Carditis during Infection with
- Recombination in Enteroviruses Is a Biphasic Replicative Process Involving the Generation of Greater-than Genome Length ‘Imprecise’ Intermediates
- Cytoplasmic Viral RNA-Dependent RNA Polymerase Disrupts the Intracellular Splicing Machinery by Entering the Nucleus and Interfering with Prp8
- and Are Associated with Murine Susceptibility to Infection and Human Sepsis
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Fungal Nail Infections (Onychomycosis): A Never-Ending Story?
- Profilin Promotes Recruitment of Ly6C CCR2 Inflammatory Monocytes That Can Confer Resistance to Bacterial Infection
- Contribution of Specific Residues of the β-Solenoid Fold to HET-s Prion Function, Amyloid Structure and Stability
- IscR Is Essential for Type III Secretion and Virulence
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



