-
Články
Reklama
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
ReklamaManipulation of Behavioral Decline in with the Rag GTPase
Normal aging leads to an inexorable decline in motor performance, contributing to medical morbidity and decreased quality of life. While much has been discovered about genetic determinants of lifespan, less is known about modifiers of age-related behavioral decline and whether new gene targets may be found which extend vigorous activity, with or without extending lifespan. Using Caenorhabditis elegans, we have developed a model of declining neuromuscular function and conducted a screen for increased behavioral activity in aged animals. In this model, behavioral function suffers from profound reductions in locomotory frequency, but coordination is strikingly preserved until very old age. By screening for enhancers of locomotion at advanced ages we identified the ras-related Rag GTPase raga-1 as a novel modifier of behavioral aging. raga-1 loss of function mutants showed vigorous swimming late in life. Genetic manipulations revealed that a gain of function raga-1 curtailed behavioral vitality and shortened lifespan, while a dominant negative raga-1 lengthened lifespan. Dietary restriction results indicated that a raga-1 mutant is relatively protected from the life-shortening effects of highly concentrated food, while RNAi experiments suggested that raga-1 acts in the highly conserved target of rapamycin (TOR) pathway in C. elegans. Rag GTPases were recently shown to mediate nutrient-dependent activation of TOR. This is the first demonstration of their dramatic effects on behavior and aging. This work indicates that novel modulators of behavioral function can be identified in screens, with implications for future study of the clinical amelioration of age-related decline.
Published in the journal: . PLoS Genet 6(5): e32767. doi:10.1371/journal.pgen.1000972
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1000972Summary
Normal aging leads to an inexorable decline in motor performance, contributing to medical morbidity and decreased quality of life. While much has been discovered about genetic determinants of lifespan, less is known about modifiers of age-related behavioral decline and whether new gene targets may be found which extend vigorous activity, with or without extending lifespan. Using Caenorhabditis elegans, we have developed a model of declining neuromuscular function and conducted a screen for increased behavioral activity in aged animals. In this model, behavioral function suffers from profound reductions in locomotory frequency, but coordination is strikingly preserved until very old age. By screening for enhancers of locomotion at advanced ages we identified the ras-related Rag GTPase raga-1 as a novel modifier of behavioral aging. raga-1 loss of function mutants showed vigorous swimming late in life. Genetic manipulations revealed that a gain of function raga-1 curtailed behavioral vitality and shortened lifespan, while a dominant negative raga-1 lengthened lifespan. Dietary restriction results indicated that a raga-1 mutant is relatively protected from the life-shortening effects of highly concentrated food, while RNAi experiments suggested that raga-1 acts in the highly conserved target of rapamycin (TOR) pathway in C. elegans. Rag GTPases were recently shown to mediate nutrient-dependent activation of TOR. This is the first demonstration of their dramatic effects on behavior and aging. This work indicates that novel modulators of behavioral function can be identified in screens, with implications for future study of the clinical amelioration of age-related decline.
Introduction
The decline of motor function with age can lead to personal decreases in quality of life and medical morbidity, and also poses challenges for society as a whole. Life expectancy has increased substantially and may be expected to increase even further as a result of ongoing research into the molecular underpinnings of aging [1]. It will be increasingly urgent to identify ways to slow declining neuromuscular function. While research with model organisms has identified a large number of genes influencing lifespan itself across diverse species [2]–[10], much less is known about modifiers of the age-related decline in behavioral function.
Like other animals, Caenorhabditis elegans exhibits behavioral decline over the lifespan [11], [12]–[14]. Previously, genes which increase lifespan when reduced in function (in particular eat-2, daf-2, and age-1) have also been shown to slow motor decline, using locomotory speed as an index [12], [13], [15], [16]. Here we ask whether novel genes could be identified which improve behavioral aging by using a screen for enhanced locomotion in old animals, directly targeting activity as an endpoint. From this screen, we have identified raga-1 as a novel genetic target that alters the rate of behavioral aging.
raga-1 is the sole worm orthologue of the evolutionarily conserved ras-related GTPase RagA. RagA proteins include S. cerevisiae Gtr1p [17], Drosophila RagA (dRagA, CG11968) [18] and mammalian RagA and RagB [19], [20]. raga-1 is a particularly intriguing aging modulator, as it was recently identified as an upstream, amino acid-sensitive activator of the target of rapamycin complex 1 (TORC1) pathway [18], [21]. This pathway has been strongly implicated in regulating both growth and aging properties in many organisms: reducing TORC1 pathway activity extends lifespan in organisms from yeast to flies, including C. elegans [22]–[27]. Mammalian lifespan also responds to decreased TORC1 activity: in a recent study, rapamycin-treated mice were found to live longer even with treatment in mid-life [28]. This suggests that the TORC1 pathway's role in modulating lifespan is conserved. Here we show that genetic manipulation of raga-1 in this pathway can have positive or negative effects on the behavioral vitality and lifespan of the whole organism. Our results with raga-1 suggest we have identified a “tunable” upstream modulator of TORC1 capable of altering behavioral aging and lifespan. Upstream of TORC1, raga-1 may be a potential target for new interventions into the process of behavioral aging.
In this work we have sought to develop a new model of C. elegans age-related motor decline by examining both the speed and coordination of swimming, using digital analysis to provide quantitative information about behavioral aging. We have conducted a novel screen based on the hypothesis that new aging-related genes could be identified which prolong behavioral vitality, with or without also extending lifespan. We have identified raga-1 as one such new modulator of behavioral aging in C. elegans.
Results
Development of a C. elegans swimming model of behavioral aging
Previous studies have described behavioral decline in aging C. elegans using direct observation of locomotion as the animals crawled on solid media [12]–[14] or swam in liquid [15]. These studies have provided useful baseline information on the aging of behavior, but have relied on observer determinations of velocity or qualitative assessment of locomotion to chart decline. More recently, digital analysis was used to show an age-dependent exponential velocity decline in nematode crawling on solid media [16]. Here we develop a new model of C. elegans locomotory aging with a study of worm swimming using video analysis tools [29]. Worm swimming offers an alternative to crawling as a neuromuscular activity to monitor [15], [30] with several useful features: immersing worms in liquid is sufficient to initiate swimming, which helps standardize conditions and enhances the feasibility of genetic screens; a large amount of data can be gathered quickly as swimming cycles are rapid; and there is increased freedom of movement compared with crawling on solid media, so abnormal movements, if present, may be easier to detect.
In worm swimming, waves of curvature propagate along the body from anterior to posterior. In series of still frames, these appear as oscillations from “C”-shaped to a nearly straight posture and then to a reverse “C”-shape and back again, completing one cycle or “swim stroke” (Figure 1A). These can be examined to identify changes in the timing and coordinated postures, or kinematics, of swimming. Previously we used video image analysis to characterize swimming of young adults [29]. Here we examined aging nematodes in a longitudinal study. In this analysis, movies were taken of individual animals at time points over the worm's lifespan. One cycle for a young adult on day 1 (sequential frames are displayed), and the same adult at day 16 (every seventh frame is displayed), are shown in Figure 1A, which illustrates the similarity in swimming postures over the lifespan. Stills were image processed to convert each worm into a 13-point spine along the midline. To help visualize and to quantitate these postures, each of the 11 angles formed by the sets of three consecutive points along the worm spine were converted to color (red or blue) and then used to build a curvature matrix by arraying the spine data associated with each frame together sequentially to form a time series (Figure 1B and 1C). In these curvature matrices, each forward swim cycle begins at the moment the head initiates a bend to the ventral side. Cycles are marked by vertical bars in Figure 1C.
Fig. 1. Longitudinal study of swimming over the lifespan of wild-type N2 worms. 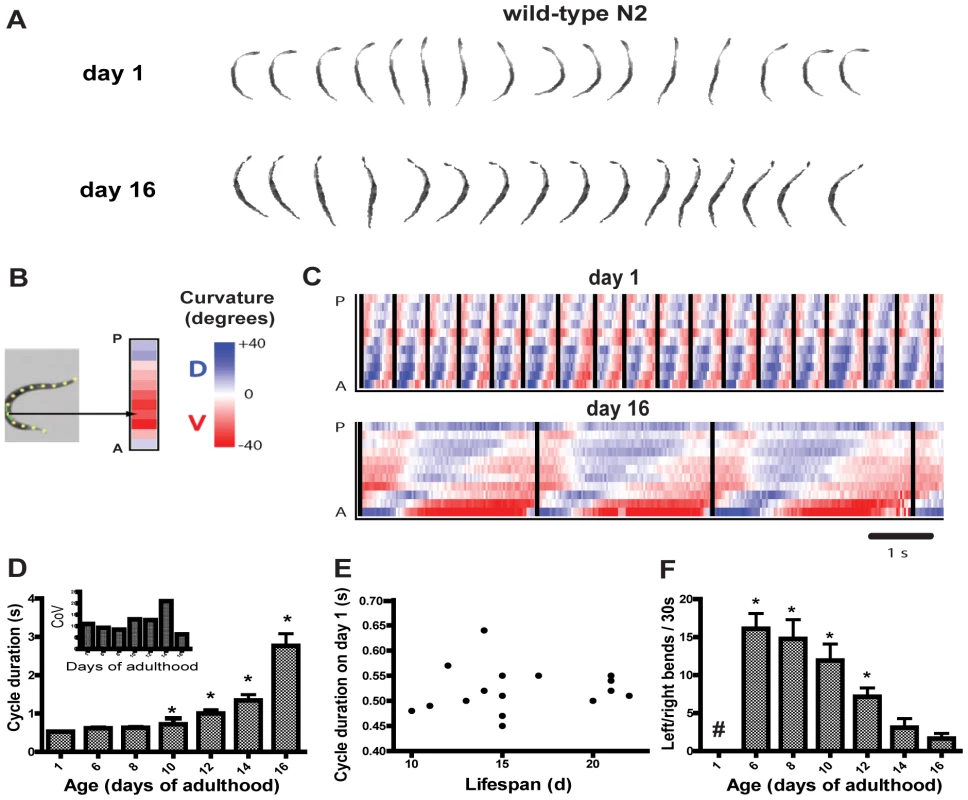
(A) Collage of swimming postures from one complete swim cycle. Every frame, or every seventh frame, is shown from a day 1 and day 16 animal respectively, from 30 frames per second movies. (B) Building of curvature matrix from a spine along the worm midline. Each angle along the worm is depicted using scaled blue or red coloring for curvature in the dorsal/ventral plane. The anterior is represented at the bottom of the matrix. (C) Representative curvature matrices from the same adult on day 1 of adulthood (top) and day 16 (bottom) show dramatic slowing of the swim cycle with relative preservation of waveform. (D) Mean (+/−S.D.) swim cycle duration in seconds (n = 18, 17, 16, 14, 12, 10, and 6 for days 1, 6, 8, 10, 12, 14, and 16 respectively). The increase is curvilinear, with rapid increases following a period of relative stability earlier in adulthood (*p<.05 versus day 1; Dunn's test). (Inset) Coefficient of variation for swim cycle duration on each day tested. Variability of cycle duration is fairly stable over the lifespan after correction for increases in the mean. (E) Plot of mean cycle duration on day 1 of adulthood versus lifespan for each individual in the cohort showing no correlation or predictive value of the cycle duration on day 1. Similar plots from later ages were not predictive of lifespan either (data not shown). Two worms' natural lifespan durations were not determined (i.e., were censored) due to accidental death. (F) Age-dependent incidence of left/right bends, out of the usual dorsal/ventral swimming plane (mean+/−S.E.M; *p<.05 versus day 1, Dunn's test; #, none were observed on day 1). For this experimental cohort, the mean lifespan was 16.0+/−0.9 days (mean+/−S.E.M.; n = 16 deaths observed). Data from a cohort of aging animals showed that swimming slowed profoundly over the lifespan (Figure 1D; Video S1, S2). The amount of slowing of the behavior is non-linear, with peak performance in youth, a substantial period of preserved function in “middle age” followed by a rapid functional decline (increase in cycle duration) in older worms, beginning around day 10–12 of adulthood (mean lifespan in this cohort was 16 days). These dynamics of changing performance contrast with crawling velocity which also slows with age, but very closely follows an exponential decay starting early in life [16]. In addition to an increase in cycle duration, the cycles become less uniform as animals age; cycle length is very strongly rhythmic and uniform on day 1, but later culminates in longer and more variable cycle durations in old age. (However, these increases in variability correspond fairly closely with the increased mean duration, as the coefficient of variation indicates; Figure 1D, inset). The predictive value of swim cycle duration was also examined, since biomarkers of aging are very useful [16], [31]. As previously shown for crawling velocity [16], the swimming cycle duration of young adults, for example on the first day of adulthood, was not predictive of the lifespan of the individual (Figure 1E; swim cycle durations on other days of life were also not predictive of lifespan, data not shown). Also, while the rate of decay of crawling velocity starts early in life and fits a single exponential curve which can be used to predict lifespan [16], we have not yet found early lifespan swimming performance parameters that are similarly predictive. These differing dynamics of decay suggest that the causes of swimming and crawling performance decline may differ to some extent.
In contrast, the coordination (or kinematics) of the behavior changed relatively little, as seen in movie stills (Figure 1A) and in curvature matrices (Figure 1C). As suggested by the similarity of the stills from the young and old animals' cycles, the postures adopted by the worm in swimming remained similar across the lifespan, but the rate of change between postures was greatly lengthened in older animals. This suggests that neuromuscular coordination persists well into old age in worms. However, though the swim cycles remained similar in form, we also noted an age-dependent increase in bends out of the usual dorsal/ventral swimming plane. In these bending movements, swimming cycles were interrupted by rolling or head-lifting movements to the left or right (up or down relative to the observer), then a return to normal swim cycle behavior (Video S3). These were noted extremely infrequently during our observation of young, wild-type animals on the first day of adulthood, but by day 6 of adulthood were readily seen, then decreased in number with age as worms moved more slowly (Figure 1F).
Identification of raga-1 as a modifier of behavioral aging
We hypothesized that novel genetic modifiers of the rate of behavioral aging could be found by directly screening for enhanced behavioral vitality in old age. Using the endpoint of increased activity in old age, we performed an RNAi pilot screen to identify such novel mediators of age-related behavioral decline. Early in the course of this pilot we found that raga-1 RNAi treatment improved locomotion at advanced ages when compared with empty vector control (unpublished observations). We were attracted to raga-1 as a target given its recent implication in mediating a link between nutrient sensing and activation of TORC1 [18], [21], especially since TOR itself has a role in aging in C. elegans [23], [27], [32]–[35] and yeast and flies [22], [25], [36]. As an initial step, we confirmed qualitatively that a deletion mutant, raga-1(ok386), showed similar alterations in age-related decline as the RNAi knockdown of raga-1 (data not shown).
We compared raga-1(ok386) swimming with wild-type over the lifespan. Both wild-type and raga-1(ok386) worms showed vigorous, rapid, and highly rhythmic swimming on day 1 of adulthood (Figure 2; Video S1, S4). These findings correspond well with previous studies of young animals and behavioral aging in worms [13], [15], [16]. The similar swimming form, short cycle duration, and low variability all suggest that raga-1 mutant worms developed healthy coordination for swimming and are, from the standpoint of locomotion, vigorous young adults much like wild-type worms. We then examined raga-1(ok386) worms later in life. As expected from the preliminary RNAi result with raga-1, over the lifespan wild-type and mutant swimming behavior diverged. At an advanced age, on average raga-1(ok386) mutant worms exhibited more rapid swimming than wild-type, as seen in comparing curvature matrices from an example individual of each genotype (Figure 2B; Video S2, S5). Despite differing swim rates, the waveforms of both appeared similar, suggesting that the underlying aging process of swimming is not affected greatly by the mutation. The divergence in behavioral aging corresponds to a shorter mean cycle duration in older mutant raga-1(ok386) worms (Figure 2A). However, ultimately raga-1(ok386) worms stop swimming and undergo a terminal phase of life with no coordinated locomotion as do wild-type worms. Finally, raga-1(ok386) worms also exhibited fewer of the age-dependent left/right, out of plane bends than wild-type worms as they swam (Figure S1). Overall, these results indicate that rates of behavioral decline are altered in raga-1(ok386).
Fig. 2. Comparison of swimming in aging raga-1(ok386), transgenic raga-1(GF), and wild-type animals. 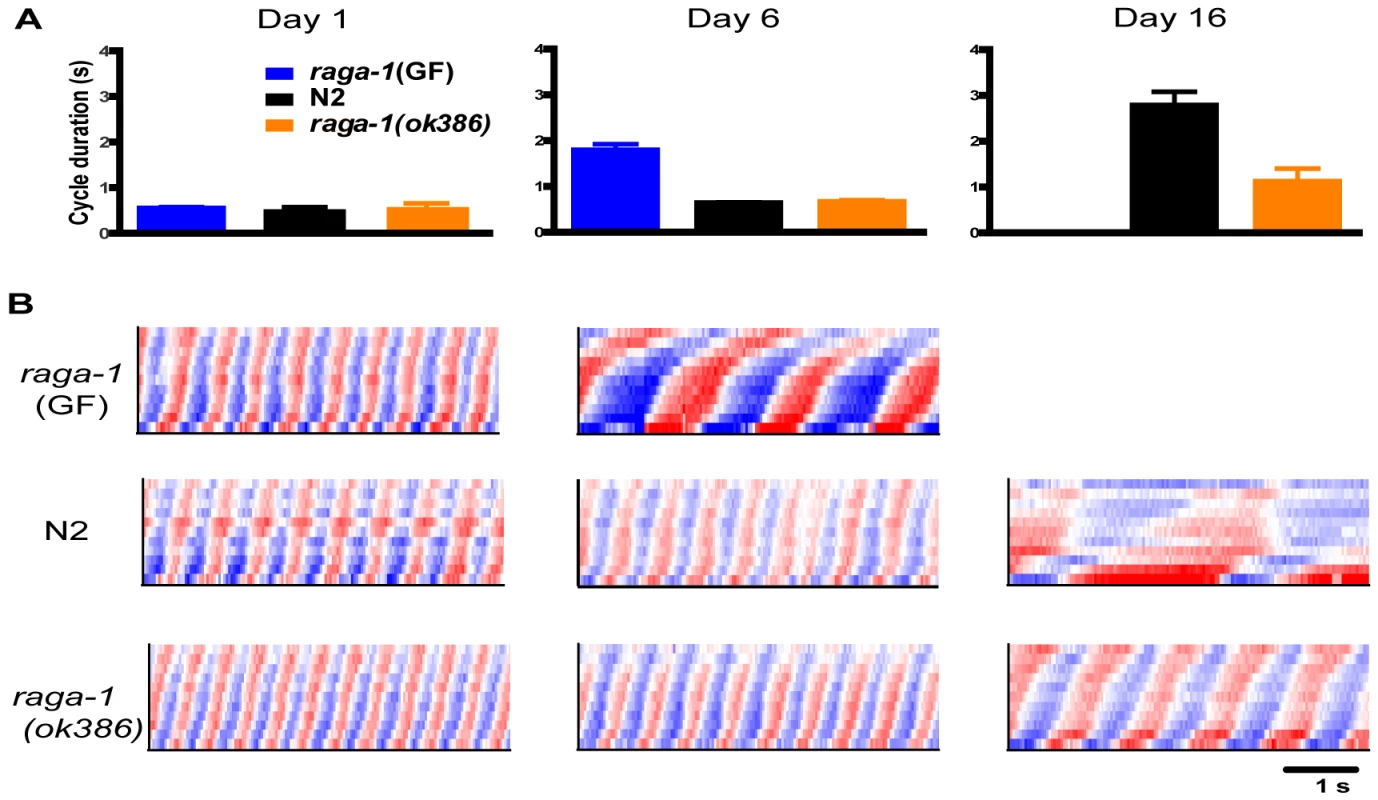
(A) Comparison of mean (+/−S.E.M.) cycle duration for raga-1(ok386), raga-1(GF) and wild-type N2. (Left) Mean cycle duration on day 1. Note the cycle lengths are similar for vigorous day 1 adults of all three genotypes. (Middle) Cycle durations on day 6 of adulthood; raga-1(GF) show substantial increases compared with N2 and raga-1(ok386) (*, p<.05, Dunn's test) (Right) Cycle durations on day 16, showing lengthening of wild-type cycles while raga-1 (ok386) still exhibits comparatively short cycles, though also increased from day 6. (B) Representative curvature matrices on day 1 (left column), day 6 (middle) and day 16 (right) for raga-1(GF) (top row), wild-type (middle) and raga-1(ok386) (bottom). As early as day 6, raga-1(GF) worms show slowing cycles; bends are also deeper than wild-type, suggesting qualitative changes in swimming occurred as well. By day 16, raga-1(ok386) worms swim substantially faster than wild-type (*, p<.05, Mann-Whitney U). Genetic alterations of raga-1 affect lifespan
Given that several important lifespan-influencing genes have been shown to slow the rate of behavioral aging [12], [13], [15], [16], we investigated whether overall lifespan was also affected by raga-1 mutations. raga-1 deletion mutant alleles (Figure S2) available from knock-out consortia were tested in survival analyses (Figure 3A; Table S1). raga-1(ok386) is predicted to preserve 48 N-terminal amino acids from RAGA-1, resulting in a deletion product comprised of the N-terminal 15% of the protein. raga-1(ok386) showed increased lifespan across multiple survival analyses (18.5+/−3.6% increase in lifespan; mean+/−S.E.M.; n = 7). A second raga-1 deletion allele, raga-1(ok701), which is predicted to preserve a much larger N-terminal fragment of 158 amino acids (plus 4 non-native amino acids coded by sequence 3′ to end of the deletion), or approximately 50% of the total protein length, produced a stronger lifespan extension (25.5+/−7.3%; n = 4), but also showed more severe pleiotropic phenotypes, for example reduced brood size (Figure S3; Table S2). A third deletion allele, raga-1(tm1862), which is predicted to lead to a fusion of 27 N-terminal RAGA-1 amino acids and 39 non-RAGA-1 amino acids after a frameshift introduced by the deletion, did not exhibit lifespan longer than wild-type (N2) (Table S1).
Fig. 3. Genetic alteration of raga-1 affects lifespan. 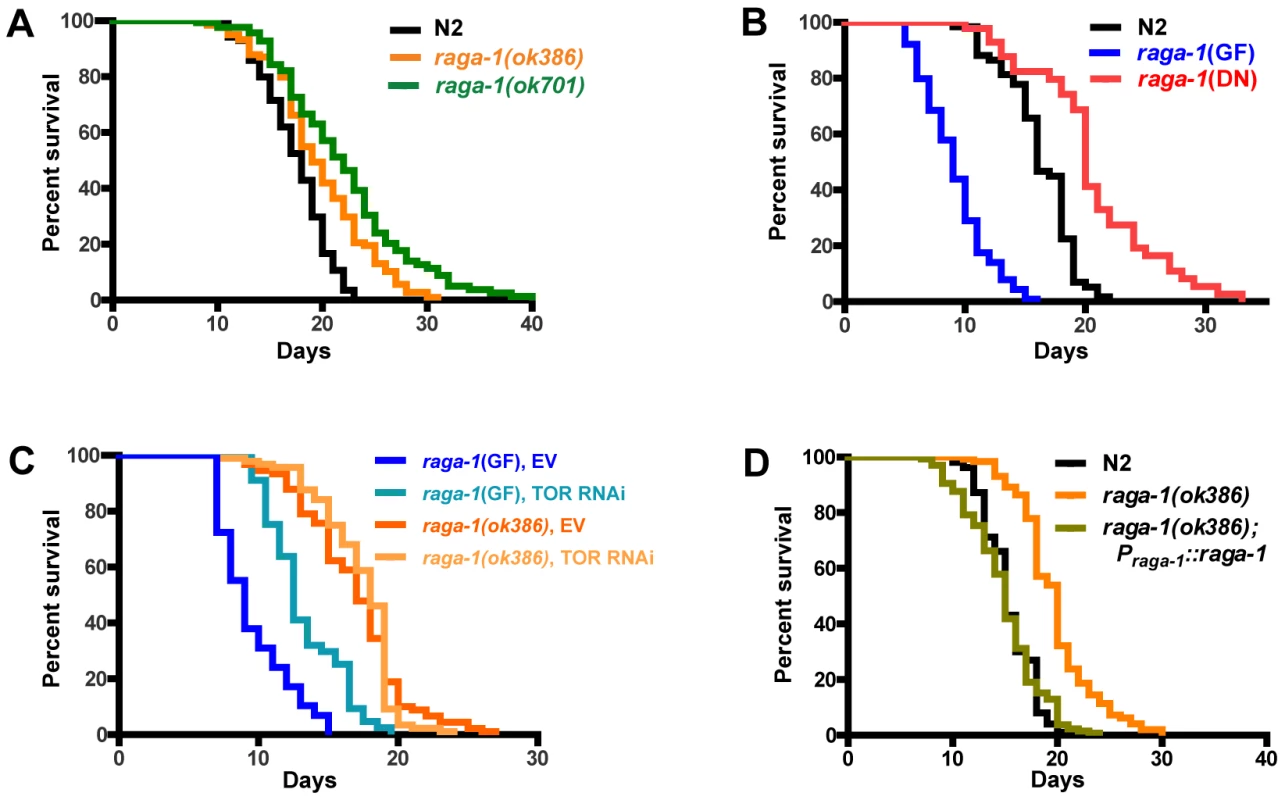
Lifespan assay results from representative experiments (see Table S1 for quantitative data and results of all experiments). (A) Comparison of raga-1(ok386) and raga-1(ok701) with wild-type N2 survival at 20°C. Strains harboring alleles ok386 and ok701 consistently showed increases in lifespan (N = 7 and 4 trials, respectively). (B) Transgenic expression of raga-1 dominant negative (DN) increased, while raga-1 gain of function (GF) decreased, lifespan versus wild-type (N = 5 and 4, respectively). (C) Effect of CeTOR (let-363) RNAi knockdown during adulthood on lifespan of raga-1(GF) and raga-1(ok386) at 25°C. See Table S3 for quantitative results of RNAi experiments. (D) Transgenic extrachromosomal expression of wild-type raga-1 under its native promoter rescues lifespan to wild-type duration in the raga-1(ok386) background. To further explore the effects of altering raga-1 function in worms, we designed genetic modifications of raga-1 based on studies of RagA function in mammalian cells and Drosophila [18], [21]. These studies made use of site-directed mutation of specific residues highly conserved among ras family proteins to produce dominant negative (DN) and gain of function (GF) forms of Rag proteins due to constitutive GDP - and GTP - binding, respectively. Previously published results indicated that an orthologous Rag GF was sufficient to induce activation through this pathway and resulted in increased TORC1 pathway activity [21]. In addition, transgenic expression of Rag GF and DN constructs in Drosophila cells resulted in increased and decreased size of the cells respectively, suggesting manipulation of downstream cellular TORC1 activity led to changes in cellular growth properties [18]. In order to examine these mutations' effects on the animal as a whole, we made corresponding DN (T18N in C. elegans raga-1) and GF (Q63L) mutations in worm raga-1 and expressed these transgenically in the wild-type N2 background using the raga-1operon promoter (Figure S2B).
raga-1(DN) expression closely phenocopied raga-1 deletion mutants ok386 and ok701, resulting in an increase in lifespan similar to raga-1(ok386) (17.7+/−3.5% (n = 5); Figure 3B). In addition, similar to these mutants, raga-1(DN) transgenic worms showed cold-dependent reductions in brood sizes at 14C (Figure S3, Table S2; this appeared to be due to cold-dependent increases in embryonic lethality, data not shown). This is reminiscent of the cold-sensitivity of orthologous yeast gtr1 mutants, and may suggest evolutionarily conserved functions which are disrupted at lower temperatures [17]. The DN phenotypic similarity to the deletion mutants suggests expression from the raga-1 operon promoter functionally approximates the native expression pattern.
Next we examined the effects of expressing a transgenic raga-1 gain of function mutation under the control of the raga-1 promoter. When expressed in a wild-type background, raga-1(GF) produced pronounced phenotypic changes, most notably a substantially decreased life span (Figure 3B). Reductions of lifespan can be difficult to interpret, in particular because worms may be generally sickened by genetic manipulations. However, the lack of general debility of raga-1(GF) is suggested by the absence of visible developmental anomalies and the relative preservation (though incomplete) of brood size (Figure S3, Table S2). In addition, expression of wild-type raga-1 under its own promoter in the raga-1(ok386) background rescued lifespan to N2 lengths (Figure 3), as well as partially rescued the increase in swimming cycle duration with aging (Figure S4). This suggests that changes in lifespan with different raga-1 transgenic versions were due to the specific properties of the transgenic version of RAGA-1 expressed, and that expression of raga-1 under this promoter sufficiently mimics native expression to rescue lifespan effects of the mutant. Possible explanations for the incomplete rescue of the swimming phenotype may be the relatively small sample size relative to lifespan experiments leading to higher variance in the result, or a higher sensitivity of this phenotype to the exact levels of RAGA-1 being expressed.
We then examined the effects of raga-1(GF) on behavioral aging. At young ages, these worms moved vigorously, also suggesting that these animals were not generally sick (Figure 2B; Video S6). However, swimming of raga-1(GF) changed rapidly with age, corresponding with its shortened lifespan (Figure 2B). Pooled data on swim cycle duration revealed an increase between day 1 and day 6 greater than that observed for wild-type or raga-1(ok386) (Figure 2A; Video S7). However, along with changes in swimming cycle duration, additional alterations in raga-1(GF) swimming were present and especially notable in day 6 worms, which bend more strongly than wild-type worms of any age (note the darker shades of red and blue indicating more pronounced postural curvature). Crawling on plates is also “loopy” on day 1 suggesting developmental changes in raga-1(GF) locomotion (data not shown). Thus, the effects of the GF are not necessarily restricted to modification of the aging process but also possibly affect development. The properties of the raga-1(GF) are likely to be a composite of accelerated aging and other changes due to the activity of the transgene during development. The results with raga-1(GF) worms are opposite to the effects of the dominant negative transgene on lifespan, suggesting that changing the activation of the TORC1 pathway by decreasing or increasing raga-1 activity can alter both lifespan and behavioral aging in either a positive or negative direction, respectively.
Since RagA activates the TORC1 pathway [18], [21], we tested the effects of CeTOR knockdown in different raga-1 backgrounds by TOR RNAi (Figure 3C, Table S3). While raga-1(GF) exhibited increased lifespan with TOR RNAi treatment compared to empty vector, raga-1(ok386) did not. This suggests that, as in other systems, raga-1 is acting through TOR to exert its effects on lifespan. Application of TOR RNAi earlier in the lifespan (feeding from larval stages onward) produced developmental alterations or arrest, as previously shown with both RNAi and with CeTOR mutations [33], [37]; presumptive loss of function CeTOR mutations are not viable as homozygotes (CeTOR is also known as lethal (let-) 363) [37]. Altering raga-1 activity may produce much less severe changes in phenotype than direct alteration of TOR itself (which also participates in a second complex, TORC2 [38], [39]) by producing milder changes in TORC1 activity than would be caused by direct manipulation of CeTOR itself.
We examined the effects of an rsks-1 (ribosomal S6 kinase) mutant on lifespan and swimming to investigate the effects of an additional genetic alteration in the TORC1 pathway. Ribosomal S6 kinase is a downstream mediator of TORC1 function in many systems [24], [40], [41], [42]. As previously shown [26], [35], [43], rsks-1mutation produced a moderate increase in lifespan compared to wild-type (Figure S7). In double mutants, raga-1(ok386) further increased rsks-1(ok1255) lifespan, suggesting that these genes may act at least partially in different pathways to exert lifespan effects. Thus, raga-1 likely acts through other TORC1 effectors, at least under these experimental conditions. We also examined the swimming behavior of this additional TORC1 pathway mutant (Figure S4). On day 1 of adulthood, rsks-1(ok1255) animals showed similar swim cycles and cycle durations to wild-type. Older day 16 rsks-1(ok1255) animals showed significantly less increase in swim cycle duration than wild-type, similar to raga-1(ok386). However, the swim cycles changed in form and were interrupted frequently by uncoordinated movements, for example twisting movements out of the plane of swimming and kinking or curling. Changes in swimming pattern can be seen in the color pattern of swim cycles reflecting alterations in the angles adopted by the animal during swimming; for example, in Figure S4, panel E the tail remains red due to a lack of movement, whereas in ordinary swim cycles the tail oscillates between red and blue reflecting movement to either side. Qualitatively, these animals appeared uncoordinated as they aged both when examined on plates or in liquid. This suggests that while genetic alterations interfering with the TORC1 pathway may share the property of improving swim cycle duration at advanced ages, they may also have pleiotropic effects on other aspects of locomotion. Although the mechanisms may be molecularly distinct, this combination of uncoordinated locomotion and long lifespan is reminiscent of daf-2(e1370) (a Class II daf-2 allele with pleiotropic phenotypes, including being uncoordinated (unc) [44]), and highlights the point that not all mutants with increased longevity will have improved locomotion in old age.
raga-1 animal size and expression pattern
Since TORC1, and Rag proteins more specifically, modulate growth properties, we examined adult animal size (except for the germ line, adults are post-mitotic in C. elegans but still increase in size with age, especially during early adulthood [45], [46]). On day one raga-1(ok386) adults were smaller than wild-type (Figure 4). This is reminiscent of expression of a dominant negative dRagA in Drosophila cells, which resulted in decreased size of the transgenic cells, and may be due to downregulation of TORC1 activity in a shift to “low growth” conditions [18]. The adult size increases in wild-type and raga-1(ok386) were parallel, suggesting that their rate of growth was similar in adulthood, and that the size differences as adults were due to changes during development. Surprisingly, raga-1(GF) expression also resulted in smaller animals (Figure 4). While raga-1(ok386) and wild-type animals continued to increase in size from day 1 to day 6 of adulthood, raga-1(GF) animals showed little increase in size over this period.
Fig. 4. Size of adult animals over the lifespan for wild-type N2, raga-1(ok386), and raga-1(GF). 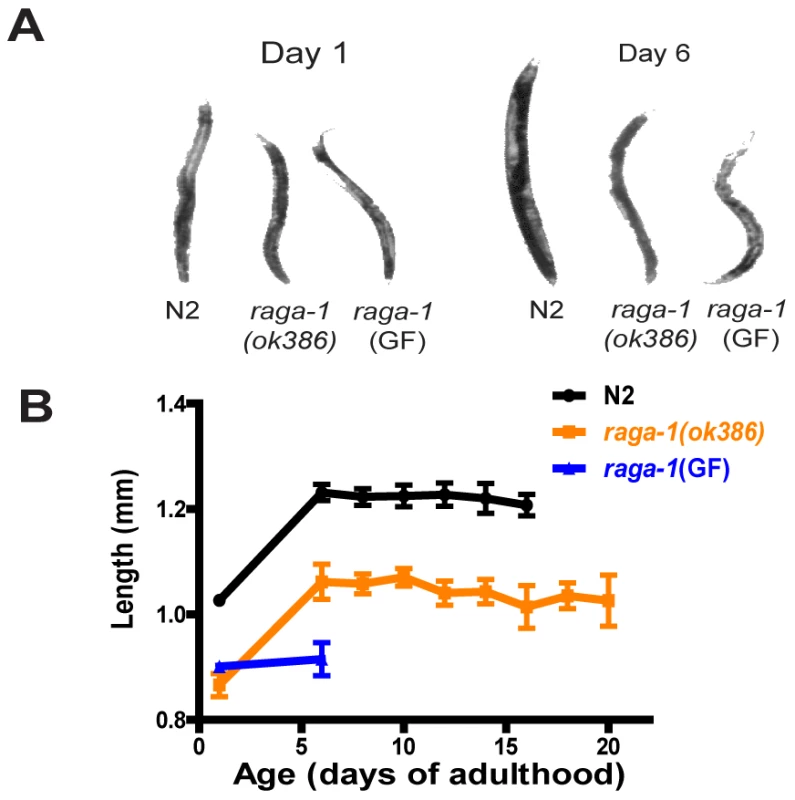
(A) Representative images of animals of each genotype to illustrate size differences. (B) Sizes of adult animals derived from measurement of length in video stills used for swimming analysis. Note differences in wild-type and raga-1(ok386) by day 1 of adulthood, suggesting different larval growth properties, which persist into adulthood even though animals continue to increase in size as adults. raga-1(GF) animals do not show any adult increase in size by day 6. To investigate the tissue basis of RAGA-1 action, we examined the expression pattern of the raga-1operon promoter driving mCherry or GFP in a wild-type background. Expression was widespread, perhaps ubiquitous, in larval animals (data not shown), similar to CeTOR [37]. In adults, expression was more restricted, and was most notable in the gut, unidentified head and tail neurons, and the distal tip cell of the somatic gonad (Figure S5). Examination of an additional independently generated line showed a similar pattern, but with higher adult levels of hypodermal expression (especially in the head), and in the entire somatic gonad including the spermatheca and gonadal sheath cells (data not shown). This restricted pattern of expression raises a question about the role of RAGA-1 in specific tissues in terms of behavior and aging in adulthood. To address this, we carried out experiments designed to test for tissue-specific phenocopy of the dominant negative or gain of function effects on lifespan. We generated transgenic lines using previously characterized promoters to drive expression of gain of function or dominant negative raga-1 in neurons, muscle, gut, or the distal tip cell of the somatic gonad. However, none of these lines showed substantial effects on lifespan (Table S4), which suggests larval, ubiquitous expression may be key to the RAGA-1 effect on lifespan. RNAi experiments also support this possibility, as raga-1 RNAi treatment in adulthood only did not extend lifespan, whereas RNAi treatment from hatching did prolong lifespan (Figure S6; Table S3). Alternatively, expression in the adult may also play a role but this may be dependent on tissues (or combinations of tissues) not tested here.
raga-1 genetically interacts with other aging genes
We combined raga-1(ok386) with other mutations known to affect lifespan (Figure S7; Table S5), focusing on those which mediate the complex relationship of metabolic and dietary status and lifespan. Results with daf-2/daf-16 insulin/insulin-like growth factor (IIS) pathway mutants suggest that raga-1 lifespan extension is dependent on daf-16, as daf-16; raga-1 double mutants show shortened lifespan similar to daf-16 alone. Upstream in the same pathway, results with daf-2(e1368) (a phenotypically less severe, Class I allele of daf-2 [44]) indicated that raga-1(ok386) lifespan was extended even further in combination with e1368. Examination of the stronger, Class II allele daf-2 (e1370) was more difficult to interpret because the strain appeared very debilitated with many morphological abnormalities accumulating over time, for example extrusion of the gut through the vulva. This may account for the biphasic appearance of the survival curve (Figure S7). However, maximum lifespan of raga-1 (ok386);daf-2(e1370) double mutants was similar to daf-2(e1370) alone. Taken together, the results suggest that raga-1 and the daf-2 pathways interact, and one possibility is that raga-1 may be upstream of this pathway such that daf-16 function is needed to at least partially carry out the beneficial effects of raga-1 mutation on lifespan extension. Previously it has been shown that the TOR pathway and daf-2 pathway interact in complex ways [23], [34], [35], and this data suggests these interactions may occur at many levels of the pathway.
We also examined the effects of two transcription factors which are crucial for dietary restriction-mediated longevity increases, skn-1 and pha-4 [34], [47], [48]. Under pha-4 RNAi treatment, raga-1(ok386) lifespan was only slightly longer than N2 (Figure S7), suggesting pha-4 does not strongly contribute to raga-1(ok386)'s extended lifespan, at least under these conditions. In contrast, a skn-1 mutation reversed the lifespan extension of raga-1(ok386) (Figure S7). This is consistent with previous results showing that skn-1 also interacts with the IIS pathway [48], and suggests that one effect of raga-1 mutation is to reduce signaling through the same output mechanisms as IIS, either acting upstream or in parallel with daf-2.
The requirement of skn-1 function, as well as the role the TORC1 pathway plays in nutrient sensing, suggested that the role of RAGA-1 in lifespan may vary with dietary conditions. Double mutants with eat-2 alleles ad1116 and ad465 indicated that raga-1(ok386) was not susceptible to the lifespan extension effects of dietary restriction using this genetic model, as the double mutants had similar lifespan extension to the respective single eat-2 mutants (Figure S8; Table S5). To look in more detail at this possibility, we tested the effects of dietary restriction directly by varying the concentration of bacterial food [49]. In these experiments, raga-1(ok386) responses to dietary restriction were strongly blunted in comparison to wild-type N2 (Figure 5; Table S6). The strongest effect of raga-1(ok386) on lifespan was at the highest concentration of bacterial feeding. This suggests RAGA-1 may have a role in mediating the shortening of lifespan under conditions of dietary repletion or excess, so that the raga-1(ok386) mutation confers partial protection from the negative effects of the high concentration feeding condition on lifespan.
Fig. 5. Effect of dietary restriction on raga-1(ok386) lifespan. 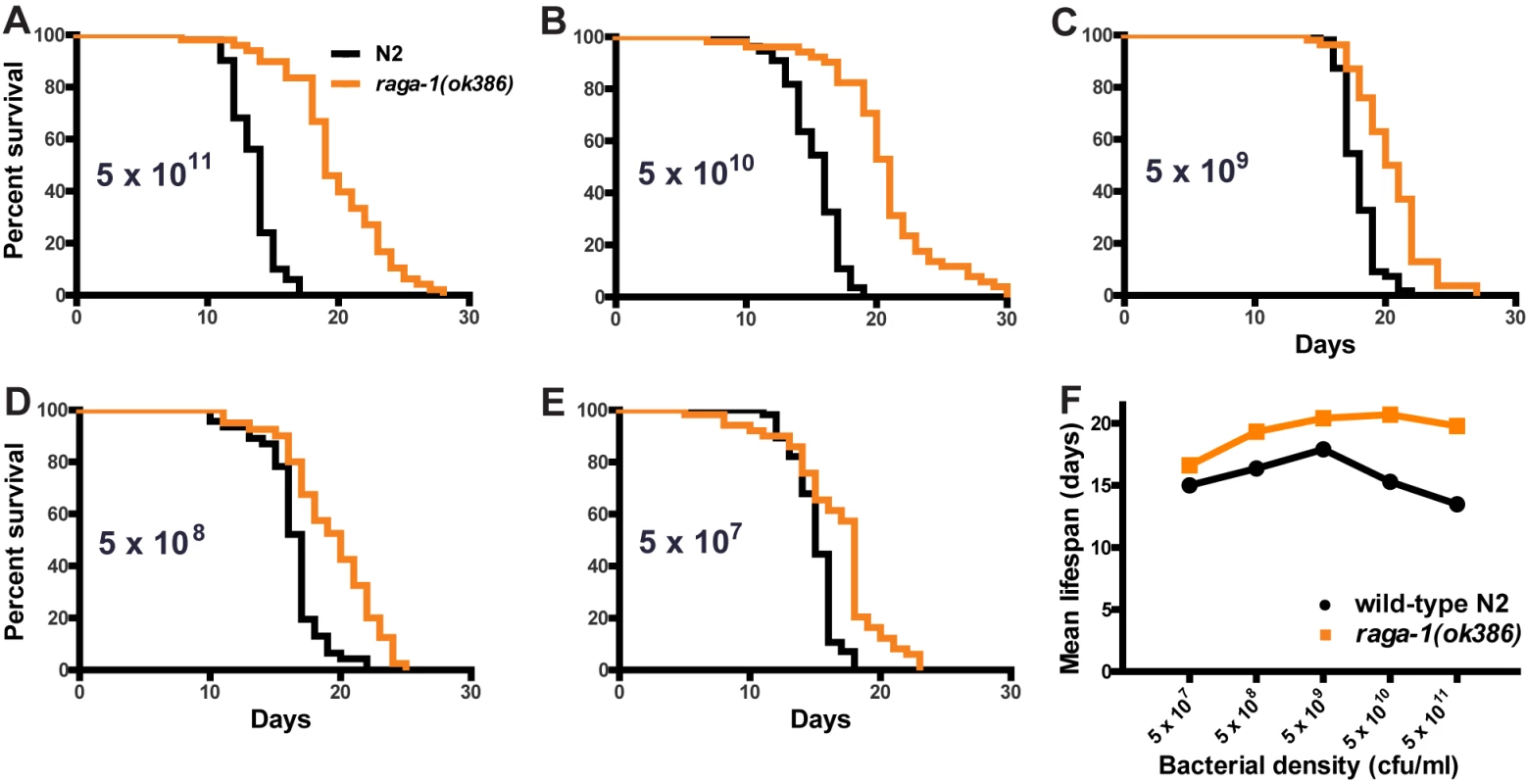
(A–E) Survival curves at 25°C under varying dietary conditions. The bacterial density of the food source is shown on each panel. The largest increase in lifespan seen for raga-1(ok386) versus N2 wild-type control is at the highest concentration of bacterial food. (F) Mean (+/−S.E.M.) lifespan plotted versus bacterial density. Wild-type N2 shows the expected parabolic response to dietary restriction, while raga-1(ok386) is much less sensitive. Discussion
In this study, age-dependent changes in swimming provided the basis for a screen to identify novel modulators of behavioral vitality in C. elegans. This screen yielded a new modulator of aging and behavior, raga-1. Of note, raga-1 has not previously been identified as an aging modulator, despite multiple genome-wide RNAi screens which have identified partially overlapping groups of genes which affect lifespan [50]–[55]. Identification of raga-1 in this screen may be due to our use of an RNAi hypersensitive strain [56], as N2 did not show lifespan extension with raga-1 RNAi treatment, while the hypersensitive strain did show a modest, statistically significant increased lifespan (Figure S6). In addition, raga-1 has not been identified as a downstream transcriptional target of the daf-2/daf-16 pathway [57], [58]. raga-1 does not appear to be strongly regulated by the elt transcriptional circuit which plays a role in longevity determination [59], and raga-1 transcription was not revealed as strongly regulated by age [60]. This suggests that new modulators of the aging process remain to be identified.
Our characterization of swimming in aged animals provides a model for quantitating behavioral decline and monitoring the effect of genetic manipulations on this process. Previous studies have shown that canonical aging-related genes influence the rate of locomotory decline. daf-2 pathway mutations that increase lifespan [61], [62] have been shown to improve behavioral aging; reduction of function mutants of daf-2 showed delayed age-related decreases in locomotion [15], while downstream in the same pathway an age-1 PI3 kinase mutant showed improved behavioral activity at advanced ages [13], [15]. daf-2 mutations also decreased the rate of behavioral aging as measured by computer-aided examination of crawling velocity [16]. The eat-2 mutant, a genetic model of dietary restriction [63], also modestly slowed crawling decline [16]. Here we have found remarkable preservation of coordination of swimming, as well as the profound slowing of locomotion noted previously. The cause of this slowing is unknown, but may be due to changes in muscle, especially since worm muscle shows severe decline with age, both in mass (sarcopenia) and structure, and this correlates well with qualitative assessments of crawling [14]. Additionally, as other physical properties of the worm age they may affect the mechanics of swimming, for example due to differences in cuticle stiffness. In contrast to structural changes, alterations in neuromuscular physiology might be expected to affect the coordination of swimming as well as the speed, and these may predominate during the terminal phase of decline in which there is no locomotion. This decrepit period immediately preceding death, approximately the last 15% of a worm's lifespan (unpublished observations), may correspond to the functional degradation of the nematode nervous system. In a key study of worm structural aging, however, neuronal structure was intact even in very old worms, though as noted in that study there may be functional changes in neurophysiology not readily seen at the structural level [14].
Our results identified raga-1 as a novel, “tunable” regulator of vitality and aging in the C. elegans TOR pathway [25], [64]. TORC1 is a critical integrator of metabolic information, integrating signals on nutrient sufficiency, growth factor activity, and energy status to influence growth rates via regulation of downstream targets [38]. C. elegans' TORC1 (comprised of CeTOR and associated proteins) [23], [37] has previously been shown to influence lifespan, as CeTOR mutation and RNAi knockdown lengthen life [23], [33]. In addition, increasing autophagy and decreasing protein synthesis, processes modulated by TORC1 inhibition, have also been shown to increase worm lifespan [26], [27], [35]. Recently, rheb-1, an additional modulator of TORC1, was found to mediate intermittent fasting-induced lifespan increases in worms [65]; unlike rheb-1, raga-1 has strong effects on behavior and lifespan under ordinary culture conditions. As these proteins interact functionally in mammalian systems [21], it will be of interest to examine their genetic interactions in C. elegans.
Rag proteins respond to amino acid levels and activate TORC1 when these levels are sufficient for growth [18], [21]. Speculatively, raga-1(GF), wild-type and raga-1(DN) animals may lie along of spectrum of behavioral vitality and lifespan phenotypes because of different levels of signaling through TORC1 (similarly, the daf-2 insulin/insulin like growth factor aging pathway may also undergo tuning [66]). In this model, raga-1(GF) animals are “hypermetabolic,” consuming resources at an excessive rate due to inappropriate positive signaling through TORC1. Although these animals are receiving normal levels of nutrition, there may be excessive protein synthesis, and depressed levels of autophagy, leading ultimately to a shortened lifespan. These results contrast with the extension of lifespan by inhibiting translation or enhancing autophagy in worms [26], [27], [35]. raga-1(DN) animals may mimic these conditions of functional starvation despite the presence of adequate nutrients, due to downregulated raga-1 signaling to TORC1. In this way, raga-1 provides a single point of entry for manipulating the activity of the TORC1 pathway to alter vitality and lifespan in intact animals.
The interaction between nutrients and raga-1 was also suggested by the effects of dietary restriction on lifespan. C. elegans have been used to model effects of dietary manipulations on lifespan extensively [47], [49], [63], [67]–[70]. Mutations in pha-4 and skn-1 ablate lifespan-extension by dietary restriction and result in shorter lifespan than wild-type when reduced in function. One model for the interaction of RAGA-1 and these mediators of dietary restriction is that, in raga-1(ok386), the animal is in a functionally starved state, extending lifespan, and this effect is dependent on mediators of dietary restriction.
In contrast, the effects of dietary restriction on raga-1(ok386) closely resemble the effects of hif-1(hypoxia inducible factor), a downstream target of the TOR pathway in C. elegans [49]. As in raga-1(ok386), the animals show the largest increase in lifespan under high levels of feeding (i.e., dietary repletion). These results suggest a model in which raga-1 and the TORC1 pathway, including hif-1, mediate the effects of dietary repletion, perhaps shortening lifespan when food is plentiful or excessive. Reduced RAGA-1 activity may protect animals from the deleterious effects of the highly concentrated diet.
TORC1 is already an important pharmacological target in medical practice, as rapamycin is clinically used in transplant rejection prevention and oncology. Recent results show that rapamycin treatment can extend the lifespan of mice, suggesting that the effects of manipulating this pathway are evolutionarily conserved [28]. It would be desirable, however, to avoid the immunosuppressant effects of rapamycin, which are its primary clinical use. Upstream regulators of TOR may be more appealing targets because of their more restricted functions. Our findings suggest raga-1 is a potentially useful target for upstream modulation of TOR activity, resulting in significant changes in worm locomotory function and lifespan, without as many severe effects as direct manipulation of TOR itself. raga-1 is an attractive target for further research on interventions into the TORC1 pathway.
Materials and Methods
C. elegans strains
C. elegans were cultured using standard techniques [71]. Strains used were: wild-type N2 (Bristol); VC222 raga-1(ok386) and VC533 raga-1(ok701) obtained from the C. elegans Knockout Consortium (University of Oklahoma) via the Caenorhabditis Genetics Center, University of Minnesota, supported by the NIH National Center for Research Resources (NCRR); and raga-1(tm1862), obtained from Prof. S. Mitani, National Bioresource Project, Tokyo Women's Medical University School of Medicine, Japan. Mutant strains were outcrossed to N2 4–6 times prior to phenotypic analysis. Additional strains used were: RB1206 rsks-1(ok1255), CF1041 daf-2(e1370), DR1572 daf-2(e1368), CF1037 daf-16(mu86), EU35 skn-1(zu169), DA1116 eat-2(ad1116), and DA465 eat-2(ad465).
Behavioral screen
A pilot screen yielding raga-1 was conducted based on swimming vigor at an advanced age. RNAi hypersensitive nre-1(hd20);lin-15b(hd126) animals (derived from strain VH624 after outcrossing once to our N2 stock) [56] were qualitatively screened for swimming activity at advanced ages under treatment with individual RNAi clones from the ORFeome library [72] (Open Biosystems). The pilot screen consisted of plating of 2342 clones from the library; 113 either failed to growth or became contaminated, and 258 clones were not screened due to a precluding phenotype (e.g., larval arrest). Of 1971 remaining clones behaviorally screened, 173 (8.8%) were selected using low stringency visual assessment of swimming vigor; of these, eighty considered qualitatively strongest were re-screened in quadruplicate. From these re-screened clones, raga-1 was the most reproducible across the four replicates and for this reason was selected for further analysis. Nine additional clones passed rescreening criteria in quadruplicate, but have not been further quantitatively characterized to verify the presence of enhanced swimming. Taking ten as the tentative total hits in this pilot screen, the yield was 0.5% (10/1971).
Locomotion assays
Swimming analysis was conducted as described previously [29]. Worms were placed singly into approximately 3 ml of assay buffer (NGM liquid, without cholesterol or agar) on a standard NGM plate. At each time point over the longitudinal assay, swimming was recorded digitally in a 30s, 30 frame per second movie using StreamPix (NorPix). Offline analysis with custom programming within ImagePro (Media Cybernetics) was used to obtain spines for worms with thirteen evenly spaced points along the worm midline. Spine tracking records were reviewed for quality control, and coordinates of these points were generated and subsequently analyzed using additional custom software written in IgorPro (WaveMetrics). Cycles were reviewed to ensure the accuracy of the cycle-finding algorithm; cycles with movements out of the dorsal/ventral plane, and cycles in which animals were moving backward (which were very rare under these conditions), were excluded from analysis of swim cycle parameters. Left-right bends were identified by their appearance by review of each movie. Individual worms were analyzed for swimming parameters, then average data from individuals was pooled to produce data in age-identical cohorts.
Transgenic worm construction
Transgenic worms bearing extra chromosomal arrays were generated with DNA at concentrations from 5–10 ng/ul using standard techniques. For tissue expression pattern and transgenic RAGA-1 mutants, expression was driven with a 4.2 kb sequence 5′ to the first gene in the predicted four-gene operon containing raga-1 (genomic features as predicted on wormbase, www.wormbase.org, Figure S2B) as a promoter to drive eGFP or mCherry. Transgenic constructs were coinjected with either Pofm-1::GFP or Pmyo-3::mCherry as a marker.
Mutant raga-1 constructs
Mutants were generated using overlap PCR methods [73] incorporating dominant negative T18N into the C. elegans coding sequence (orthologous to T21N in human RagA) or gain of function Q63L (orthologous to Q66L) single residue changes. Based on prior characterization, these mutations functionally produce Rag proteins with constitutive GDP or GTP binding respectively [18], [21]. Coding sequence including intended mutations was verified by sequencing. Alignments with predicted homology were generated using VectorNTI (Invitrogen).
CeTOR RNAi
Knockdown of CeTOR was done with a construct identical to that reported previously [37]. Adult worms were placed on RNAi plates with bacteria expressing control (empty vector) and allowed to lay eggs for several hours, then were removed. After growth at 20°C on control RNAi plates, L4 larvae were selected, and the following day were split onto control or TOR RNAi plates and placed at 25°C for lifespan assays.
Lifespan assays
Lifespan determination was performed using animals raised at 20°C, then carried out at the temperatures indicated (20°C for lifespan assays except RNAi and dietary restriction experiments which were carried out at 25°C). Adults were scored for viability (any movement in response to prodding with a platinum pick; lack of response defined death of the animal) throughout the lifespan until death. Worms were transferred regularly to fresh plates to escape progeny and to replenish food supply. Lifespan data were analyzed using the Mantel-Cox log rank test within GraphPad Prism (GraphPad Software). Worms that left the plate, died as a result of injury or prolapsed gonad (“exploded”) or internal hatching of progeny (“bagging”) were censored at that time point following standard procedures [54], [74].
Dietary restriction
Dietary restriction experiments were performed using the modified solid dietary restriction method as previously described [49]. Overnight cultures of OP50 E. coli were spun down to concentrate them and bacterial density was determined with a Petroff-Hausser counting chamber, then dilutions were made to the concentrations noted for each feeding condition. Modified NGM plates were used in dietary restriction lifespan assays, lacking peptone and containing carbenicillin (50 µg/ml) and, for the first week of the assay, FUdR (50 µg/ml) to inhibit reproduction.
Accession number
The wormbase (www.wormbase.org) gene identification number for raga-1 is WBGene00006414.
Supporting Information
Zdroje
1. VijgJ
CampisiJ
2008 Puzzles, promises and a cure for ageing. Nature 454 1065 1071
2. FinchCE
RuvkunG
2001 The genetics of aging. Annu Rev Genomics Hum Genet 2 435 462
3. GuarenteL
KenyonC
2000 Genetic pathways that regulate ageing in model organisms. Nature 408 255 262
4. PanowskiSH
DillinA
2009 Signals of youth: endocrine regulation of aging in Caenorhabditis elegans. Trends Endocrinol Metab 20 259 264
5. AntebiA
2007 Genetics of aging in Caenorhabditis elegans. PLoS Genet 3 e129 doi:10.1371/journal.pgen.0030129
6. FriedmanDB
JohnsonTE
1988 A mutation in the age-1 gene in Caenorhabditis elegans lengthens life and reduces hermaphrodite fertility. Genetics 118 75 86
7. GemsD
2000 An integrated theory of ageing in the nematode Caenorhabditis elegans. J Anat 197 Pt 4 521 528
8. KimSK
2007 Common aging pathways in worms, flies, mice and humans. J Exp Biol 210 1607 1612
9. RhodenizerD
MartinI
BhandariP
PletcherSD
GrotewielM
2008 Genetic and environmental factors impact age-related impairment of negative geotaxis in Drosophila by altering age-dependent climbing speed. Exp Gerontol 43 739 748
10. GrotewielMS
MartinI
BhandariP
Cook-WiensE
2005 Functional senescence in Drosophila melanogaster. Ageing Res Rev 4 372 397
11. HosonoR
SatoY
AizawaSI
MitsuiY
1980 Age-dependent changes in mobility and separation of the nematode Caenorhabditis elegans. Exp Gerontol 15 285 289
12. GlennCF
ChowDK
DavidL
CookeCA
GamiMS
2004 Behavioral deficits during early stages of aging in Caenorhabditis elegans result from locomotory deficits possibly linked to muscle frailty. J Gerontol A Biol Sci Med Sci 59 1251 1260
13. HuangC
XiongC
KornfeldK
2004 Measurements of age-related changes of physiological processes that predict lifespan of Caenorhabditis elegans. Proc Natl Acad Sci U S A 101 8084 8089
14. HerndonLA
SchmeissnerPJ
DudaronekJM
BrownPA
ListnerKM
2002 Stochastic and genetic factors influence tissue-specific decline in ageing C. elegans. Nature 419 808 814
15. DuhonSA
JohnsonTE
1995 Movement as an index of vitality: comparing wild type and the age-1 mutant of Caenorhabditis elegans. J Gerontol A Biol Sci Med Sci 50 B254 261
16. HsuAL
FengZ
HsiehMY
XuXZ
2008 Identification by machine vision of the rate of motor activity decline as a lifespan predictor in C. elegans. Neurobiol Aging
17. Bun-YaM
HarashimaS
OshimaY
1992 Putative GTP-binding protein, Gtr1, associated with the function of the Pho84 inorganic phosphate transporter in Saccharomyces cerevisiae. Mol Cell Biol 12 2958 2966
18. KimE
Goraksha-HicksP
LiL
NeufeldTP
GuanKL
2008 Regulation of TORC1 by Rag GTPases in nutrient response. Nat Cell Biol 10 935 945
19. HiroseE
NakashimaN
SekiguchiT
NishimotoT
1998 RagA is a functional homologue of S. cerevisiae Gtr1p involved in the Ran/Gsp1-GTPase pathway. J Cell Sci 111 ( Pt 1) 11 21
20. SchurmannA
BrauersA
MassmannS
BeckerW
JoostHG
1995 Cloning of a novel family of mammalian GTP-binding proteins (RagA, RagBs, RagB1) with remote similarity to the Ras-related GTPases. J Biol Chem 270 28982 28988
21. SancakY
PetersonTR
ShaulYD
LindquistRA
ThoreenCC
2008 The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science 320 1496 1501
22. KaeberleinM
PowersRW3rd
SteffenKK
WestmanEA
HuD
2005 Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science 310 1193 1196
23. JiaK
ChenD
RiddleDL
2004 The TOR pathway interacts with the insulin signaling pathway to regulate C. elegans larval development, metabolism and life span. Development 131 3897 3906
24. KapahiP
ZidBM
HarperT
KosloverD
SapinV
2004 Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr Biol 14 885 890
25. StanfelMN
ShamiehLS
KaeberleinM
KennedyBK
2009 The TOR pathway comes of age. Biochim Biophys Acta
26. PanKZ
PalterJE
RogersAN
OlsenA
ChenD
2007 Inhibition of mRNA translation extends lifespan in Caenorhabditis elegans. Aging Cell 6 111 119
27. HansenM
ChandraA
MiticLL
OnkenB
DriscollM
2008 A role for autophagy in the extension of lifespan by dietary restriction in C. elegans. PLoS Genet 4 e24 doi:10.1371/journal.pgen.0040024
28. HarrisonDE
StrongR
SharpZD
NelsonJF
AstleCM
2009 Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460 392 395
29. Pierce-ShimomuraJT
ChenBL
MunJJ
HoR
SarkisR
2008 Genetic analysis of crawling and swimming locomotory patterns in C. elegans. Proc Natl Acad Sci U S A 105 20982 20987
30. KortaJ
ClarkDA
GabelCV
MahadevanL
SamuelAD
2007 Mechanosensation and mechanical load modulate the locomotory gait of swimming C. elegans. J Exp Biol 210 2383 2389
31. GerstbreinB
StamatasG
KolliasN
DriscollM
2005 In vivo spectrofluorimetry reveals endogenous biomarkers that report healthspan and dietary restriction in Caenorhabditis elegans. Aging Cell 4 127 137
32. MeissnerB
BollM
DanielH
BaumeisterR
2004 Deletion of the intestinal peptide transporter affects insulin and TOR signaling in Caenorhabditis elegans. J Biol Chem 279 36739 36745
33. VellaiT
Takacs-VellaiK
ZhangY
KovacsAL
OroszL
2003 Genetics: influence of TOR kinase on lifespan in C. elegans. Nature 426 620
34. SheafferKL
UpdikeDL
MangoSE
2008 The Target of Rapamycin pathway antagonizes pha-4/FoxA to control development and aging. Curr Biol 18 1355 1364
35. HansenM
TaubertS
CrawfordD
LibinaN
LeeSJ
2007 Lifespan extension by conditions that inhibit translation in Caenorhabditis elegans. Aging Cell 6 95 110
36. KennedyBK
2008 The genetics of ageing: insight from genome-wide approaches in invertebrate model organisms. J Intern Med 263 142 152
37. LongX
SpycherC
HanZS
RoseAM
MullerF
2002 TOR deficiency in C. elegans causes developmental arrest and intestinal atrophy by inhibition of mRNA translation. Curr Biol 12 1448 1461
38. WullschlegerS
LoewithR
HallMN
2006 TOR signaling in growth and metabolism. Cell 124 471 484
39. SoukasAA
KaneEA
CarrCE
MeloJA
RuvkunG
2009 Rictor/TORC2 regulates fat metabolism, feeding, growth, and life span in Caenorhabditis elegans. Genes Dev 23 496 511
40. RuvinskyI
MeyuhasO
2006 Ribosomal protein S6 phosphorylation: from protein synthesis to cell size. Trends Biochem Sci 31 342 348
41. ThomasG
2002 The S6 kinase signaling pathway in the control of development and growth. Biol Res 35 305 313
42. SelmanC
TulletJM
WieserD
IrvineE
LingardSJ
2009 Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science 326 140 144
43. SyntichakiP
TroulinakiK
TavernarakisN
2007 eIF4E function in somatic cells modulates ageing in Caenorhabditis elegans. Nature 445 922 926
44. GemsD
SuttonAJ
SundermeyerML
AlbertPS
KingKV
1998 Two pleiotropic classes of daf-2 mutation affect larval arrest, adult behavior, reproduction and longevity in Caenorhabditis elegans. Genetics 150 129 155
45. CrollNA
SmithJM
ZuckermanBM
1977 The aging process of the nematode Caenorhabditis elegans in bacterial and axenic culture. Exp Aging Res 3 175 189
46. BolanowskiMA
RussellRL
JacobsonLA
1981 Quantitative measures of aging in the nematode Caenorhabditis elegans. I. Population and longitudinal studies of two behavioral parameters. Mech Ageing Dev 15 279 295
47. BishopNA
GuarenteL
2007 Two neurons mediate diet-restriction-induced longevity in C. elegans. Nature 447 545 549
48. TulletJM
HertweckM
AnJH
BakerJ
HwangJY
2008 Direct inhibition of the longevity-promoting factor SKN-1 by insulin-like signaling in C. elegans. Cell 132 1025 1038
49. ChenD
ThomasEL
KapahiP
2009 HIF-1 modulates dietary restriction-mediated lifespan extension via IRE-1 in Caenorhabditis elegans. PLoS Genet 5 e1000486 doi:10.1371/journal.pgen.1000486
50. HamiltonB
DongY
ShindoM
LiuW
OdellI
2005 A systematic RNAi screen for longevity genes in C. elegans. Genes Dev 19 1544 1555
51. LeeSS
LeeRY
FraserAG
KamathRS
AhringerJ
2003 A systematic RNAi screen identifies a critical role for mitochondria in C. elegans longevity. Nat Genet 33 40 48
52. CurranSP
RuvkunG
2007 Lifespan regulation by evolutionarily conserved genes essential for viability. PLoS Genet 3 e56 doi:10.1371/journal.pgen.0030056
53. SamuelsonAV
CarrCE
RuvkunG
2007 Gene activities that mediate increased life span of C. elegans insulin-like signaling mutants. Genes Dev 21 2976 2994
54. HansenM
HsuAL
DillinA
KenyonC
2005 New genes tied to endocrine, metabolic, and dietary regulation of lifespan from a Caenorhabditis elegans genomic RNAi screen. PLoS Genet 1 e17 doi:10.1371/journal.pgen.0010017
55. SmithED
KennedyBK
KaeberleinM
2007 Genome-wide identification of conserved longevity genes in yeast and worms. Mech Ageing Dev 128 106 111
56. SchmitzC
KingeP
HutterH
2007 Axon guidance genes identified in a large-scale RNAi screen using the RNAi-hypersensitive Caenorhabditis elegans strain nre-1(hd20) lin-15b(hd126). Proc Natl Acad Sci U S A 104 834 839
57. McElweeJJ
SchusterE
BlancE
ThomasJH
GemsD
2004 Shared transcriptional signature in Caenorhabditis elegans Dauer larvae and long-lived daf-2 mutants implicates detoxification system in longevity assurance. J Biol Chem 279 44533 44543
58. MurphyCT
McCarrollSA
BargmannCI
FraserA
KamathRS
2003 Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature 424 277 283
59. BudovskayaYV
WuK
SouthworthLK
JiangM
TedescoP
2008 An elt-3/elt-5/elt-6 GATA transcription circuit guides aging in C. elegans. Cell 134 291 303
60. LundJ
TedescoP
DukeK
WangJ
KimSK
2002 Transcriptional profile of aging in C. elegans. Curr Biol 12 1566 1573
61. KenyonC
ChangJ
GenschE
RudnerA
TabtiangR
1993 A C. elegans mutant that lives twice as long as wild type. Nature 366 461 464
62. KimuraKD
TissenbaumHA
LiuY
RuvkunG
1997 daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science 277 942 946
63. LakowskiB
HekimiS
1998 The genetics of caloric restriction in Caenorhabditis elegans. Proc Natl Acad Sci U S A 95 13091 13096
64. KapahiP
ZidB
2004 TOR pathway: linking nutrient sensing to life span. Sci Aging Knowledge Environ 2004 PE34
65. HonjohS
YamamotoT
UnoM
NishidaE
2009 Signalling through RHEB-1 mediates intermittent fasting-induced longevity in C. elegans. Nature 457 726 730
66. CohenE
BieschkeJ
PerciavalleRM
KellyJW
DillinA
2006 Opposing activities protect against age-onset proteotoxicity. Science 313 1604 1610
67. SteinkrausKA
SmithED
DavisC
CarrD
PendergrassWR
2008 Dietary restriction suppresses proteotoxicity and enhances longevity by an hsf-1-dependent mechanism in Caenorhabditis elegans. Aging Cell 7 394 404
68. SutphinGL
KaeberleinM
2008 Dietary restriction by bacterial deprivation increases life span in wild-derived nematodes. Exp Gerontol 43 130 135
69. KaeberleinTL
SmithED
TsuchiyaM
WeltonKL
ThomasJH
2006 Lifespan extension in Caenorhabditis elegans by complete removal of food. Aging Cell 5 487 494
70. PanowskiSH
WolffS
AguilaniuH
DurieuxJ
DillinA
2007 PHA-4/Foxa mediates diet-restriction-induced longevity of C. elegans. Nature 447 550 555
71. BrennerS
1974 The genetics of Caenorhabditis elegans. Genetics 77 71 94
72. RualJF
CeronJ
KorethJ
HaoT
NicotAS
2004 Toward improving Caenorhabditis elegans phenome mapping with an ORFeome-based RNAi library. Genome Res 14 2162 2168
73. HobertO
2002 PCR fusion-based approach to create reporter gene constructs for expression analysis in transgenic C. elegans. Biotechniques 32 728 730
74. MelendezA
HallDH
HansenM
2008 Monitoring the role of autophagy in C. elegans aging. Methods Enzymol 451 493 520
Štítky
Genetika Reprodukční medicína
Článek Epistasis of Transcriptomes Reveals Synergism between Transcriptional Activators Hnf1α and Hnf4αČlánek SMA-10/LRIG Is a Conserved Transmembrane Protein that Enhances Bone Morphogenetic Protein SignalingČlánek B1 SOX Coordinate Cell Specification with Patterning and Morphogenesis in the Early Zebrafish EmbryoČlánek Bulk Segregant Analysis by High-Throughput Sequencing Reveals a Novel Xylose Utilization Gene fromČlánek Abundant Quantitative Trait Loci Exist for DNA Methylation and Gene Expression in Human Brain
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2010 Číslo 5
-
Všechny články tohoto čísla
- Digital Quantification of Human Eye Color Highlights Genetic Association of Three New Loci
- Common Genetic Variants near the Brittle Cornea Syndrome Locus Influence the Blinding Disease Risk Factor Central Corneal Thickness
- Age- and Temperature-Dependent Somatic Mutation Accumulation in
- All About Mitochondrial Eve: An Interview with Rebecca Cann
- Aging and Chronic Sun Exposure Cause Distinct Epigenetic Changes in Human Skin
- Transposed Genes in Arabidopsis Are Often Associated with Flanking Repeats
- A Survey of Genomic Traces Reveals a Common Sequencing Error, RNA Editing, and DNA Editing
- Gene Transposition Causing Natural Variation for Growth in
- The Polyproline Site in Hinge 2 Influences the Functional Capacity of Truncated Dystrophins
- Epistasis of Transcriptomes Reveals Synergism between Transcriptional Activators Hnf1α and Hnf4α
- Integration of Light Signals by the Retinoblastoma Pathway in the Control of S Phase Entry in the Picophytoplanktonic Cell
- The Proprotein Convertase Encoded by () Is Required in Corpora Cardiaca Endocrine Cells Producing the Glucose Regulatory Hormone AKH
- Sgs1 and Exo1 Redundantly Inhibit Break-Induced Replication and Telomere Addition at Broken Chromosome Ends
- A MATE-Family Efflux Pump Rescues the 8-Oxoguanine-Repair-Deficient Mutator Phenotype and Protects Against HO Killing
- The Relationship among Gene Expression, the Evolution of Gene Dosage, and the Rate of Protein Evolution
- SMA-10/LRIG Is a Conserved Transmembrane Protein that Enhances Bone Morphogenetic Protein Signaling
- The Nuclear Receptor DHR3 Modulates dS6 Kinase–Dependent Growth in
- Involvement of Global Genome Repair, Transcription Coupled Repair, and Chromatin Remodeling in UV DNA Damage Response Changes during Development
- B1 SOX Coordinate Cell Specification with Patterning and Morphogenesis in the Early Zebrafish Embryo
- Linkage and Association Mapping of Flowering Time in Nature
- Bulk Segregant Analysis by High-Throughput Sequencing Reveals a Novel Xylose Utilization Gene from
- Abundant Quantitative Trait Loci Exist for DNA Methylation and Gene Expression in Human Brain
- Ablation of Whirlin Long Isoform Disrupts the USH2 Protein Complex and Causes Vision and Hearing Loss
- Characterization of Oxidative Guanine Damage and Repair in Mammalian Telomeres
- DNA Adenine Methylation Is Required to Replicate Both Chromosomes Once per Cell Cycle
- Genome-Wide Copy Number Variation in Epilepsy: Novel Susceptibility Loci in Idiopathic Generalized and Focal Epilepsies
- FACT Prevents the Accumulation of Free Histones Evicted from Transcribed Chromatin and a Subsequent Cell Cycle Delay in G1
- GC-Biased Evolution Near Human Accelerated Regions
- Liver and Adipose Expression Associated SNPs Are Enriched for Association to Type 2 Diabetes
- Myeloid Cell-Restricted Insulin Receptor Deficiency Protects Against Obesity-Induced Inflammation and Systemic Insulin Resistance
- The Mating Type Locus () and Sexual Reproduction of : Insights into the Evolution of Sex and Sex-Determining Chromosomal Regions in Fungi
- B-Cyclin/CDKs Regulate Mitotic Spindle Assembly by Phosphorylating Kinesins-5 in Budding Yeast
- Post-Replication Repair Suppresses Duplication-Mediated Genome Instability
- Genome Sequence of the Plant Growth Promoting Endophytic Bacterium sp. 638
- Transcription Factors Mat2 and Znf2 Operate Cellular Circuits Orchestrating Opposite- and Same-Sex Mating in
- The Use of Orthologous Sequences to Predict the Impact of Amino Acid Substitutions on Protein Function
- Mutation in Archain 1, a Subunit of COPI Coatomer Complex, Causes Diluted Coat Color and Purkinje Cell Degeneration
- Shelterin-Like Proteins and Yku Inhibit Nucleolytic Processing of Telomeres
- Affecting Function Causes a Dilated Heart in Adult
- Manipulation of Behavioral Decline in with the Rag GTPase
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Common Genetic Variants near the Brittle Cornea Syndrome Locus Influence the Blinding Disease Risk Factor Central Corneal Thickness
- All About Mitochondrial Eve: An Interview with Rebecca Cann
- The Relationship among Gene Expression, the Evolution of Gene Dosage, and the Rate of Protein Evolution
- SMA-10/LRIG Is a Conserved Transmembrane Protein that Enhances Bone Morphogenetic Protein Signaling
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



