-
Články
Reklama
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
ReklamaSMA-10/LRIG Is a Conserved Transmembrane Protein that Enhances Bone Morphogenetic Protein Signaling
Bone morphogenetic protein (BMP) pathways control an array of developmental and homeostatic events, and must themselves be exquisitely controlled. Here, we identify Caenorhabditis elegans SMA-10 as a positive extracellular regulator of BMP–like receptor signaling. SMA-10 acts genetically in a BMP–like (Sma/Mab) pathway between the ligand DBL-1 and its receptors SMA-6 and DAF-4. We cloned sma-10 and show that it has fifteen leucine-rich repeats and three immunoglobulin-like domains, hallmarks of an LRIG subfamily of transmembrane proteins. SMA-10 is required in the hypodermis, where the core Sma/Mab signaling components function. We demonstrate functional conservation of LRIGs by rescuing sma-10(lf) animals with the Drosophila ortholog lambik, showing that SMA-10 physically binds the DBL-1 receptors SMA-6 and DAF-4 and enhances signaling in vitro. This interaction is evolutionarily conserved, evidenced by LRIG1 binding to vertebrate receptors. We propose a new role for LRIG family members: the positive regulation of BMP signaling by binding both Type I and Type II receptors.
Published in the journal: . PLoS Genet 6(5): e32767. doi:10.1371/journal.pgen.1000963
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1000963Summary
Bone morphogenetic protein (BMP) pathways control an array of developmental and homeostatic events, and must themselves be exquisitely controlled. Here, we identify Caenorhabditis elegans SMA-10 as a positive extracellular regulator of BMP–like receptor signaling. SMA-10 acts genetically in a BMP–like (Sma/Mab) pathway between the ligand DBL-1 and its receptors SMA-6 and DAF-4. We cloned sma-10 and show that it has fifteen leucine-rich repeats and three immunoglobulin-like domains, hallmarks of an LRIG subfamily of transmembrane proteins. SMA-10 is required in the hypodermis, where the core Sma/Mab signaling components function. We demonstrate functional conservation of LRIGs by rescuing sma-10(lf) animals with the Drosophila ortholog lambik, showing that SMA-10 physically binds the DBL-1 receptors SMA-6 and DAF-4 and enhances signaling in vitro. This interaction is evolutionarily conserved, evidenced by LRIG1 binding to vertebrate receptors. We propose a new role for LRIG family members: the positive regulation of BMP signaling by binding both Type I and Type II receptors.
Introduction
Bone morphogenetic protein (BMP) receptor serine/threonine kinases (BMPRs) are pivotal signal transducers for the small, secreted BMP morphogens, members of the transforming growth factor β (TGF - β) superfamily (comprising subfamilies of TGF - βs, BMPs, activins, and others) [1], [2]. BMP dimers released from neighboring cells are received by these receptors, which leads to an intracellular cascade of transcriptional events. Depending on the specific pathway, cell type and milieu, these events result in a diverse array of cellular processes, from dorsal-ventral specification to cell cycle control and programmed cell death [3]. Understanding how growth factor pathways are regulated may lead not only to a better understanding of their normal physiological roles, but may also lead to potential treatments for a wide range of disorders and diseases [4], [5].
Secreted BMP dimers travel through the extracellular matrix to activate their receptors. Originally thought to be a process of simple diffusion, the movement of TGF-β superfamily members is now recognized to be highly regulated [6]. Many factors play a role in facilitating or preventing BMP ligand access to receptor. Post-translational processing and proteolysis of ligand, as well as seclusion of ligand by extracellular matrix (ECM) components like integrins and proteoglycans, for example, determine whether a ligand dimer can interact with its receptors [7]. Not only is the BMP's progress exquisitely controlled, but the receptors themselves are also subject to regulation [6], [8]. Inside the cell, receptor phosphorylation is inhibited by phosphatases, and SARA and Smurf proteins target receptors for polyubiquitination and degradation [8]. Outside the cell, the receptor complex can be inactivated by the decoy type I receptor BAMBI [9]. Coreceptors betaglycan/TGFβ receptor II (TGFβR3) and endoglin can bind certain BMPS and deliver them to receptors [10]–[14]. Endoglin also associates with select type I and type II receptors [13].
Pioneer studies in Caenorhabditis elegans and Drosophila melanogaster have identified components of the pathway and furthered understanding of BMP signaling [15], [16]. These studies have identified the conserved core of the signaling pathway, including the ligand, the type I and type II receptors, and the Smads. In C. elegans, a BMP-like pathway controls body size and male tail development (the Sma/Mab pathway). The receptors for the ligand DBL-1 are SMA-6 (type I) and DAF-4 (type II) [17], [18]. Receptor signals are transduced through the Smads SMA-2, SMA-3, and SMA-4 [19]. As in mammals, co-transcription factors that act with the Smads have been discovered, including SMA-9/Schnurri and RNT-1/RUNX [20], [21]. The body size phenotype of the Sma/Mab pathway is very sensitive to dose, indicating that the core pathway is tightly controlled [17], [18], [22], [23]. The only identified extracellular regulator of this pathway has been LON-2, a conserved heparan sulfate proteoglycan that binds DBL-1/BMP and attenuates pathway signaling [24].
To identify novel components of BMP signaling, we performed traditional forward genetic screens for mutations affecting the Sma/Mab signaling pathway ([25], unpublished results). From these genetic screens, we identified, cloned, and characterized sma-10. SMA-10 is a positive regulator of Sma/Mab signaling, and is absolutely and specifically required for body size regulation. It is a member of a cell surface localized family of proteins with leucine rich repeats and immunoglobulin-like domains (LRIG). The function of SMA-10 in BMP pathway signaling is conserved, as the Drosophila ortholog lambik rescues the body size defect of sma-10(lf) animals. Furthermore, SMA-10 promotes BMP signaling in mammalian cells. SMA-10 binds the pathway receptors SMA-6 and DAF-4 but not the BMP DBL-1, and a mammalian ortholog, LRIG1 (leucine-rich and immunoglobulin-like domains-1), also binds both type I and type II receptors. These studies identify a uniquely acting positive regulator of BMP signaling, SMA-10/LRIG, that directly interacts with type I and type II receptors from C. elegans to mammals.
Results
sma-10(lf) Alleles Were Identified in Screens for Small Body Size
The first small C. elegans mutants were identified in a large-scale screen for morphology and mobility mutants [26]. Their role in BMP signaling was elucidated when sma-2, sma-3 and sma-4 were characterized [19]. In an effort to identify additional genes that act in BMP signaling, we performed two genetic screens. From the first screen, in which body size mutant F2 animals were isolated from mutagenized N2/wild type P0 animals, two sma-10 alleles, wk26 and wk66, were identified [25]. From a lon-2(e678) suppressor screen, three additional alleles, wk88, wk89, and wk90, were identified and confirmed by complementation and sequencing.
sma-10 Mutants Display Body Size Defects But No Male Tail Abnormalities
The Sma/Mab pathway regulates both body size and male tail development. A reduction of dbl-1 pathway activity results in animals that are 55%–85% wild-type length [22],[23]. sma-10(lf) animals share the small body size defect, ranging from 79% to 88% the length of wild-type animals (Figure 1A and 1B, Table 1). The dbl-1 pathway also regulates the development and patterning of male tail structures [17]–[19], [22], [23], with mating spicules and sensory rays 5–7 being primarily affected. We therefore asked if sma-10 is also involved in patterning the male tail. Our studies revealed that all five alleles of sma-10, including a presumed null (wk88), have wild-type male tail rays and spicules (data not shown). These data suggest that SMA-10 is specifically involved in BMP signaling to control body size but not male tail development or patterning.
Fig. 1. sma-10 is required for normal body size. 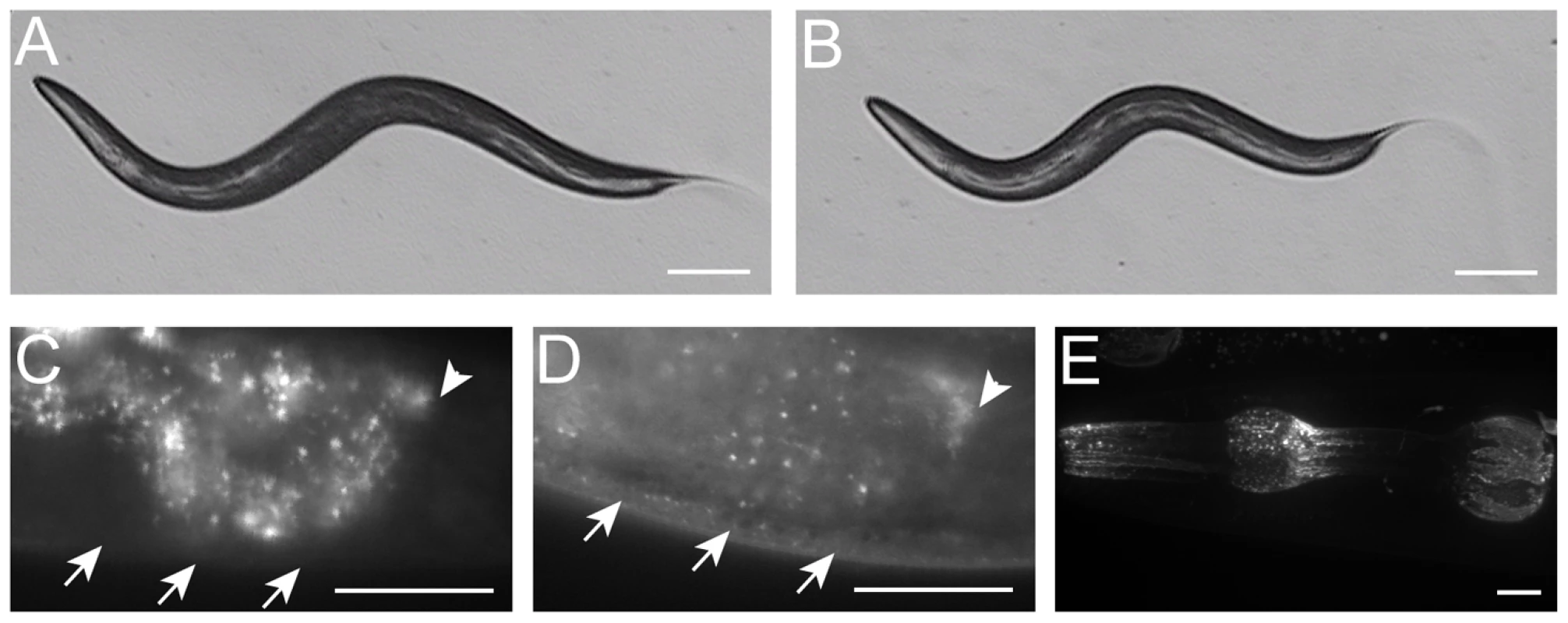
Microscope images of (A) a wild-type (N2) hermaphrodite and (B) a sma-10(wk66) hermaphrodite, both taken at 24 hours after the L4/adult molt (same magnification on a dissecting microscope, scale bar represents 0.1 mm). SMA-10:GFP is expressed in hypodermis (D) and pharynx muscle (E). No hypodermal fluorescence was detected in non-transgenic animals (C). Weak expression was detected in hypodermis (20-fold longer exposure of image in (C) than (D)). Arrows point to hypodermis in (C,D). Arrowheads point to the end of the intestine in (C,D). Intestinal autofluorescence is visible to the left of the arrowheads in (C,D). Strong expression of the SMA-10:GFP full-length fusion construct was observed in the pharynx muscle. Expression of the ttx-3::rfp marker in the AIY neuron is also visible in the upper right corner (E). Scale bars in (C–E) represent 0.01 mm. Anterior is to the left in all panels. Tab. 1. Quantitation of sma-10 mutant body size. 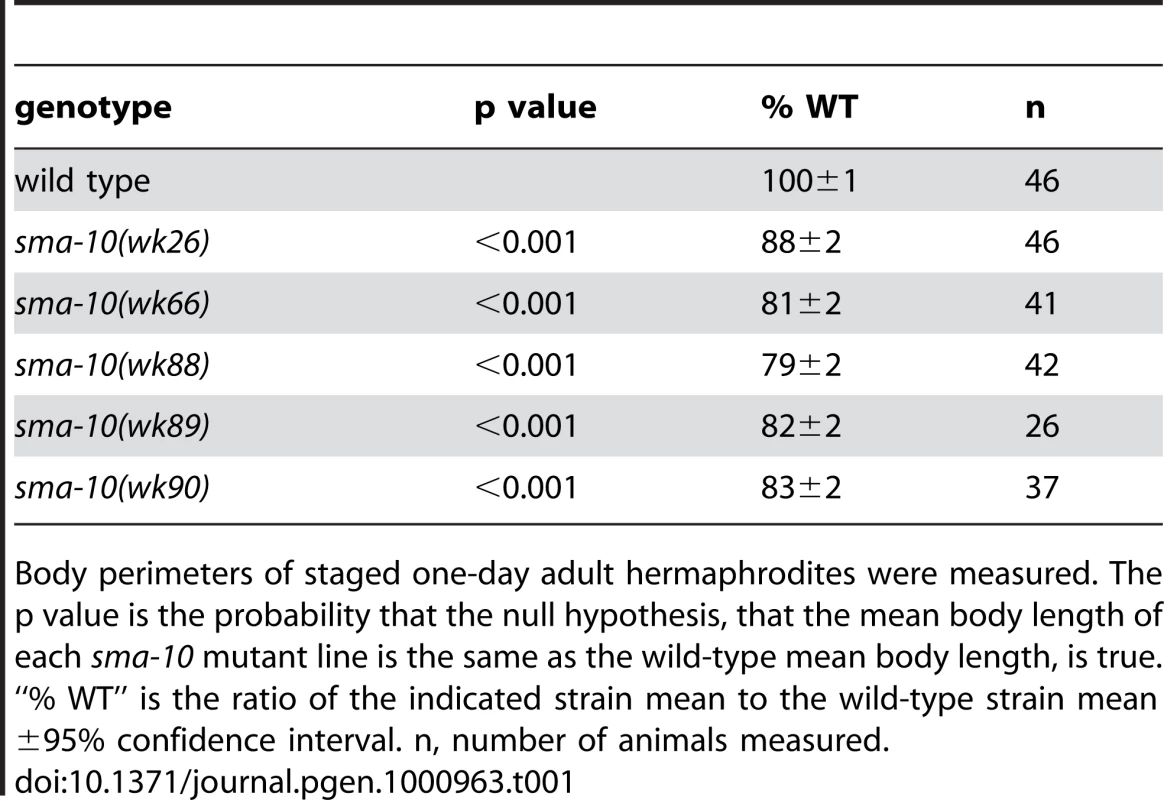
Body perimeters of staged one-day adult hermaphrodites were measured. The p value is the probability that the null hypothesis, that the mean body length of each sma-10 mutant line is the same as the wild-type mean body length, is true. “% WT” is the ratio of the indicated strain mean to the wild-type strain mean ±95% confidence interval. n, number of animals measured. SMA-10 Acts in the Sma/Mab Pathway
Because small body size is a hallmark phenotype of mutants in the Sma/Mab pathway, we asked if sma-10 genetically interacts with Sma/Mab pathway members. To determine the functional order of SMA-10 relative to Sma/Mab pathway members, we performed epistasis tests. We generated animals with mutations in sma-10 and DBL-1/BMP pathway genes that alone give opposite phenotypes (small and long, respectively), and asked what the terminal phenotype was for these strains (small or long). In a regulatory pathway, the terminal phenotype is a result of the loss of gene product with the most downstream effect bypassing the requirement for the second, upstream gene product. We created double mutant animals of sma-10 with lon-2, overexpressed dbl-1, or over expressed sma-6. We found that the small body size phenotype of sma-10(lf) was the terminal phenotype in double mutant animals with either lon-2 or over expressed dbl-1, but not for overexpressed sma-6 (Table 2). These results suggest that SMA-10 functions in the unique position between the receptor SMA-6 and the ligand DBL-1.
Tab. 2. sma-10 acts between ligand dbl-1 and receptor sma-6. 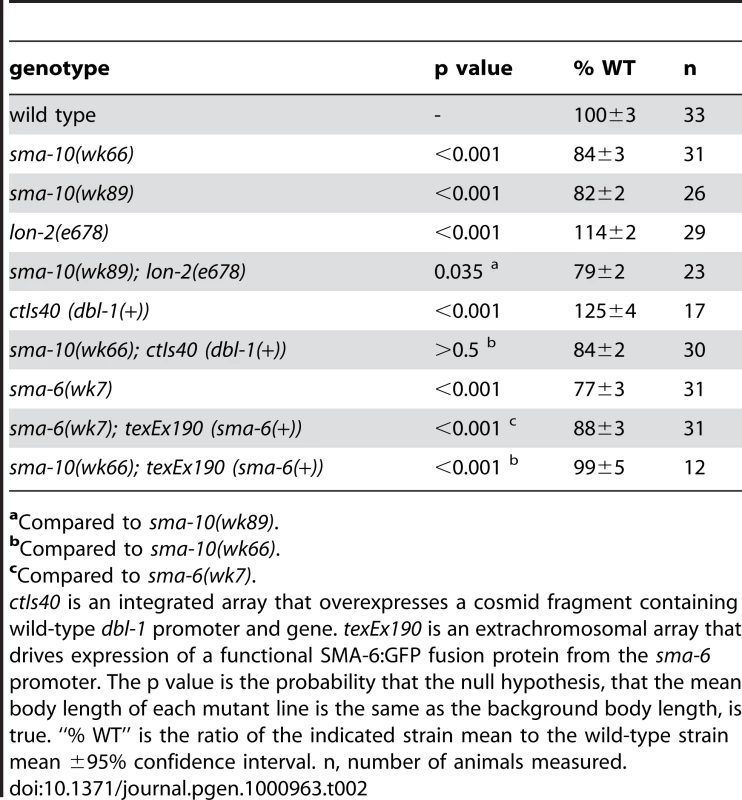
aCompared to sma-10(wk89). sma-10 Encodes a Protein with Leucine-Rich Repeats and Immunoglobulin-Like Domains
To understand the molecular nature of sma-10, we mapped and cloned the gene. sma-10 is located at LG IV: −26.82. Cosmid T21D12 conferred rescue of the sma-10(wk66) small phenotype, as does a sma-10 cDNA (Table S1). sma-10 encodes an 881 amino acid protein of the LRIG family (leucine rich repeats and immunoglobulin-like domains). SMA-10 is composed of an N-terminal signal sequence (amino acids 1–20), fifteen leucine-rich repeats (LRRs) flanked by an LRR N-terminal domain at amino acids 24–56 and an LRR C-terminal domain ending at amino acid 499, three immunoglobulin domains (spanning amino acids 503 to 802), a transmembrane domain (amino acids 839 to 861), and a short (19 amino acid) intracellular domain from amino acids 862 to 881 (Figure 2). The C-terminal tail is not conserved between C. elegans, Drosophila and mammals. This protein structure, which is largely extracellular and is transmembrane-bound, is consistent with the order of gene function, which places SMA-10 between the secreted DBL-1/BMP and its membrane-bound receptors.
Fig. 2. Structural comparison of SMA-10 to Drosophila Lambik and mammalian LRIGs. 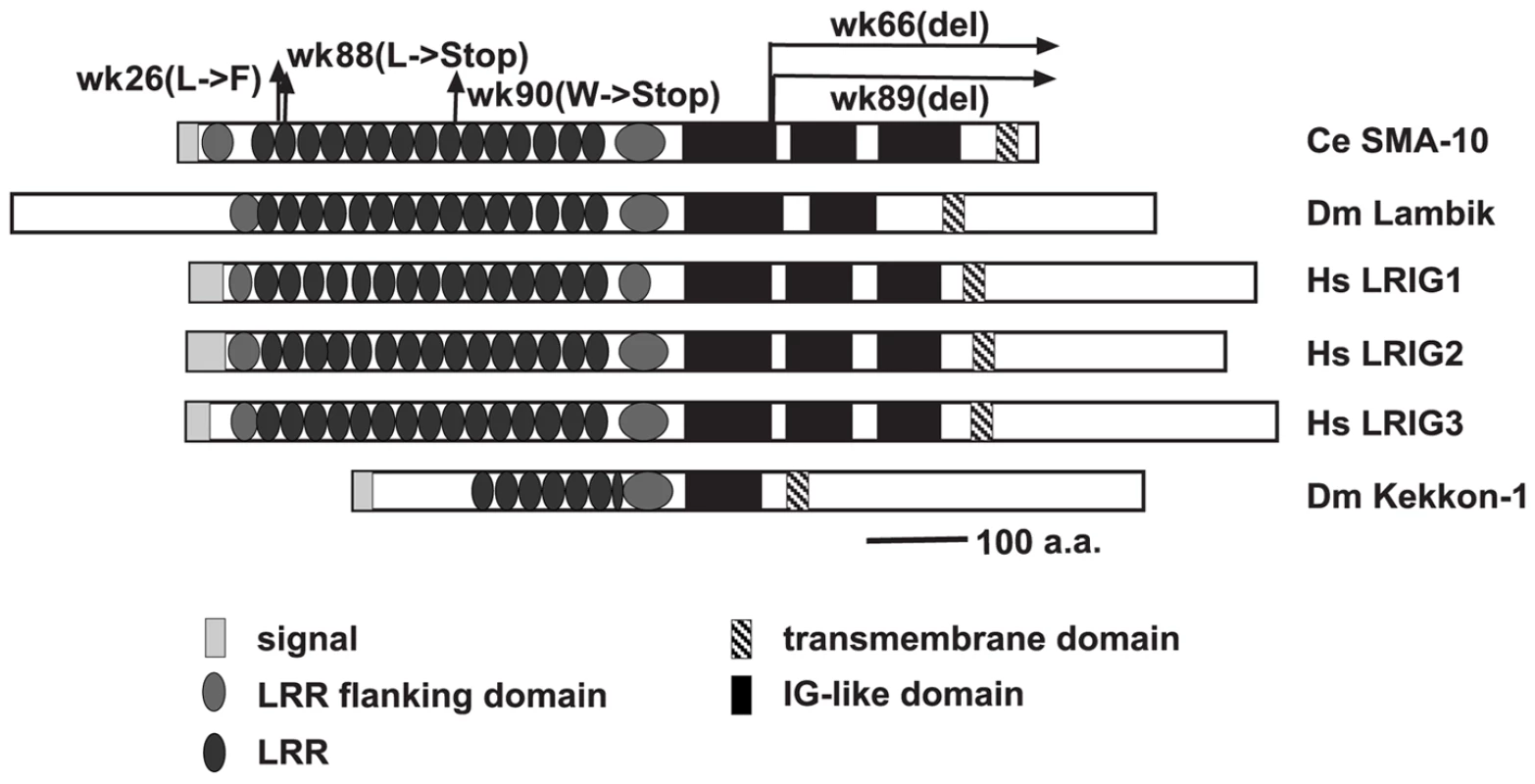
Schematic diagram of C. elegans SMA-10, Drosphila Lambik, human LRIG1, LRIG2, and LRIG3 and Drosophila Kekkon 1. Domains/motifs are represented as follows: N-terminal signal sequences, light gray rectangles; leucine rich repeat N- and C-terminal domains, medium gray ovals; leucine-rich repeats, dark gray ovals; immunoglobulin-like domains, black boxes; and transmembrane domains, striped rectangles. Arrows above the C. elegans protein sequence indicate mutations affecting the SMA-10 protein. Domains were placed according to SMART analyses [62], Guo et al (LRIGs) [27], and MacLaren et al (Kekkon 1) [63]. Sequencing DNA of mutant sma-10 strains confirmed lesions within T21D12.9 (Figure 2). sma-10(wk26) contains a T to C bp change at position 572, a substitution that changes a conserved leucine to a phenylalanine in the second LRR at amino acid 102. A deletion of 251 bps generates sma-10(wk66) (on cosmid T21D12 from base pairs 21092 to 21342, beginning in the gene at the end of exon 9). This transcript is predicted to result in a truncated protein with 609 a.a. of SMA-10 and 10 a.a. of novel sequence before terminating, deleting sequences after the first immunoglobulin-like domain. wk88 introduces a stop codon into amino acid position 112 in the LRR2, resulting in a severely truncated protein, and is a presumed null allele. wk89 is a 975 bp deletion from position 21301 to 22275 in T21D12, starting in intron 9 and ending in intron 11, deleting the protein after amino acid 611 and creating a frame shift and premature termination sequence, removing sequences after the first immunoglobulin-like domain. wk90 changes sequence encoding Trp 286 to a stop codon, deleting sequences after LRR9.
Multiple splice variants are predicted by cDNA sequencing (Wormbase.org). yk352c5, a full-length 2.6 kb cDNA of the longest splice variant T21D12.9a, driven by 1.2 kb of upstream sma-10 promoter sequence, is sufficient to confer rescue of sma-10(wk66) animals (Table S1). A function for the shorter splice variants (T21D12.9b, T21D12.9c.1, and T21D12.9c.2) is not known.
Database searches reveal orthologs in other metazoans. The hallmark of this family is the presence of fifteen leucine-rich repeats (LRRs) and three immunoglobulin-like repeats (Ig-like) followed by a transmembrane domain. In Drosophila, there is one SMA-10 ortholog, Lambik (www.flybase.org), and in vertebrates, there are three known sma-10 orthologs, LRIG1, LRIG2, and LRIG3 [27]. lambik corresponds to CG8434, but no phenotypic characterization has been published. A distantly related, distinct family is composed of the insect-specific Kekkon members, which have six LRRs and one Ig–like domain. One of these, Kekkon 1 (kek1), plays a role in inhibiting the epidermal growth factor receptor (EGFR) [28]. Another Kekkon, Kekkon5 (kek5), interacts genetically with a BMP signaling pathway [29].
sma-10 Is Required in the Hypodermis to Regulate Body Size
Although core Sma/Mab pathway components are expressed in many tissues, they are all required in the hypodermis for body size regulation. To determine if SMA-10 is also needed in these cells, we first asked where sma-10 is expressed. We created a functional translational fusion of SMA-10 with GFP at the C-terminus and expressed it using the sma-10 promoter. Expression of SMA-10:GFP was visible, though faint, in the hypodermis (Figure 1C and 1D), consistent with SMA-10's genetically identified role as a regulator of the DBL-1 signaling pathway, which functions in the hypodermis to regulate body size. Expression in the hypodermis of other DBL-1 pathway genes is also low [24], [30]–[33].
Bright fluorescent expression was also observed in the pharynx of animals from embryos to adults (Figure 1E). Pharyngeal expression was localized to the muscle segments pm1 (anterior cell of the procorpus), pm4 (metacarpus/anterior bulb), the anterior part of pm5 (isthmus), and pm7 (terminal bulb) (Figure 1E). Expression of SMA-10:GFP localized to both the cell surface and to puncta within the cells (Figure 1E). Expression of mRNA corresponding to the sma-10 locus has also been reported in intestine and renal gland cells in both larvae and adult animals [34]. Digestive tract expression (in the pharynx and intestine) of other Sma/Mab pathway members has been reported [18], [32], [33], [35], [36], and may reflect a proposed role for this pathway in an innate immune response [37], [38].
We then asked where SMA-10 is required for body size regulation. Because of its expression pattern and previous experiments showing that DBL-1/BMP pathway components are required in the hypodermis, we drove the expression of full-length genomic sma-10 from pharyngeal - or hypodermal-specific promoters in sma-10(wk66) animals and assayed for rescue of body size. We found that expression of sma-10(+) in hypodermis (using the rol-6 promoter), but not pharynx (using the myo-2 promoter), was sufficient to rescue animals to wild-type length (Table 3).
Tab. 3. Hypodermal sma-10 expression rescues the body size defect of sma-10(lf) animals. 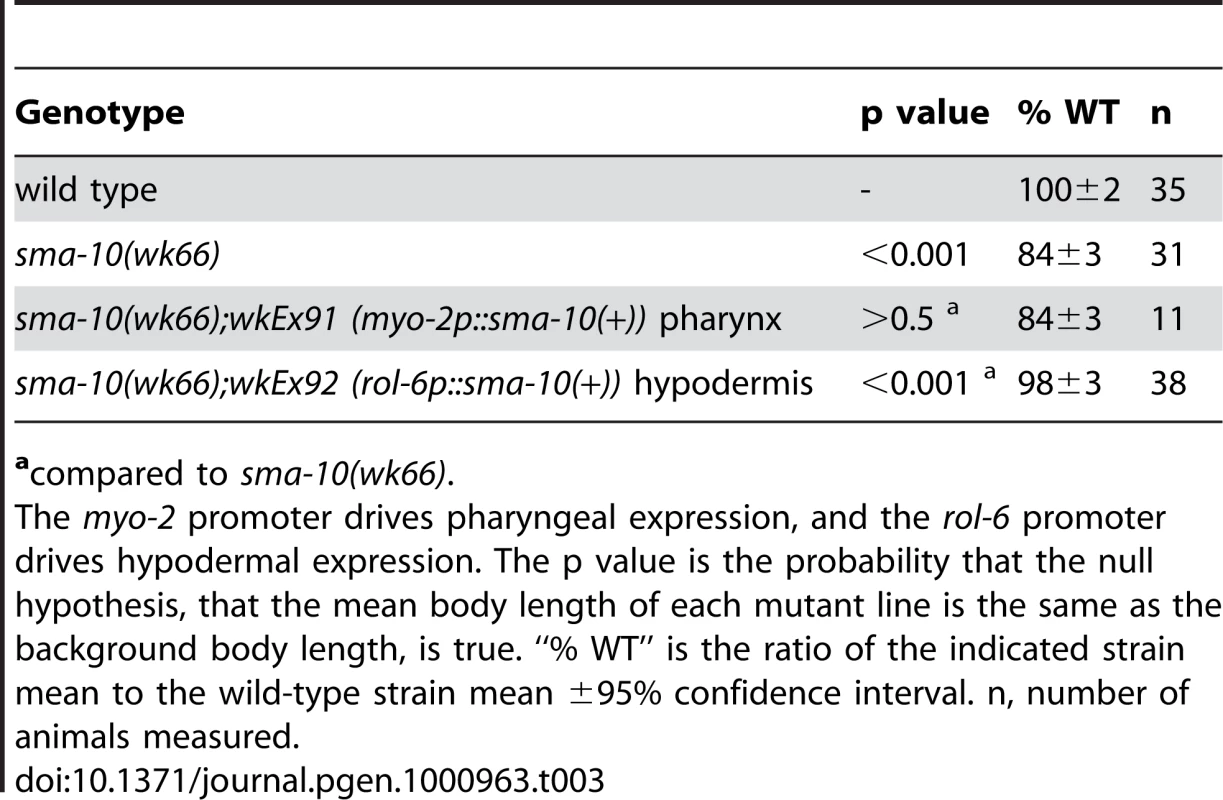
acompared to sma-10(wk66). Drosophila LRIG lambik Rescues sma-10(lf) Animals
Lambik is a Drosophila LRIG that is orthologous to vertebrate LRIGs and to SMA-10. Although the function of Lambik is currently unknown, we asked whether lambik could functionally substitute for sma-10 in C. elegans. We drove Drosophila lambik cDNA from the sma-10 promoter in sma-10(wk66) animals. Transgenic animals were rescued to the wild-type body size (Table 4), thereby showing functional conservation between divergent Drosophila and C. elegans LRIGS.
Tab. 4. Drosophila lambik rescues the body size defect of sma-10(lf) animals. 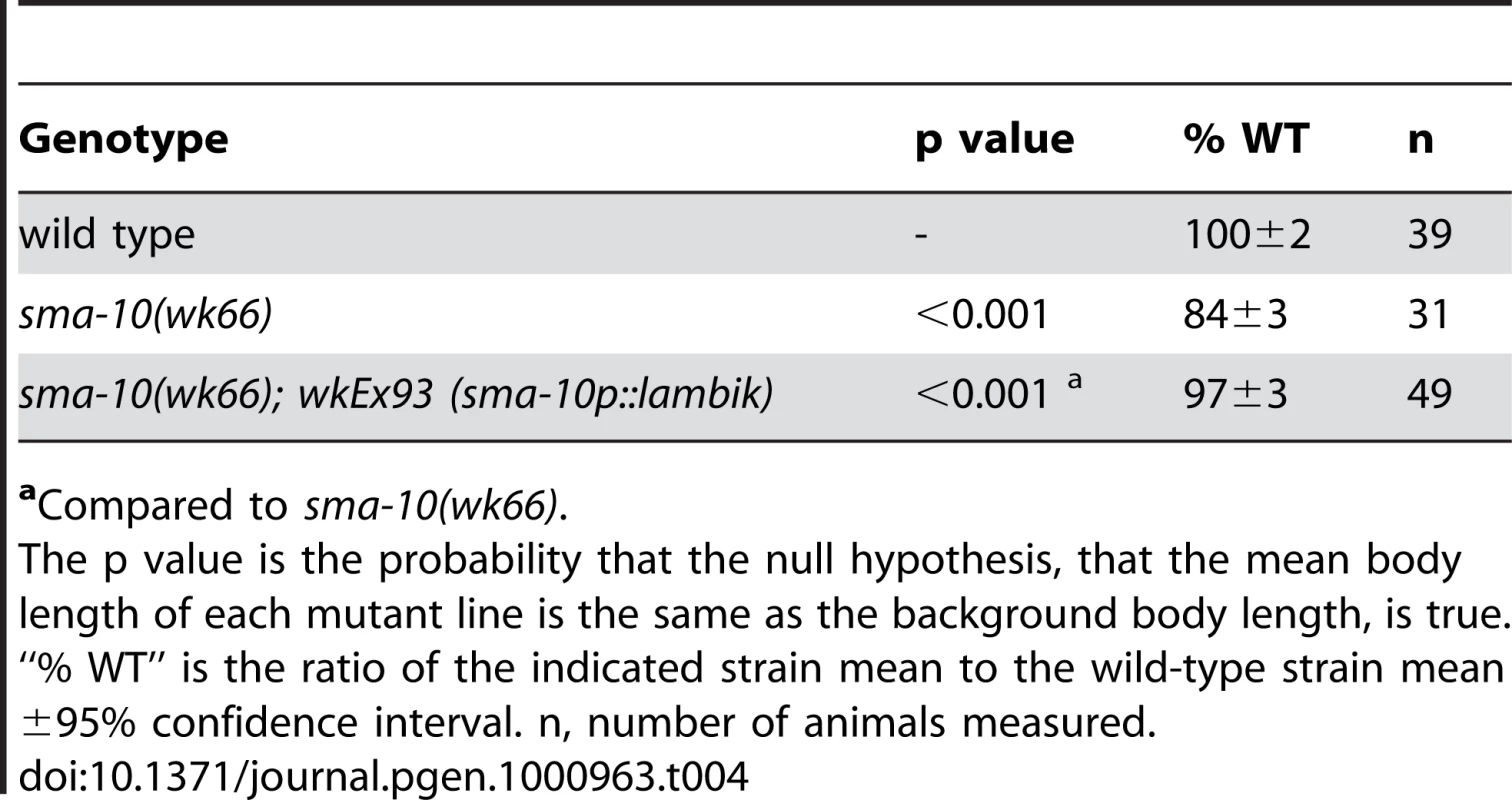
aCompared to sma-10(wk66). SMA-10 Promotes BMP Signaling in Mammalian Cells
Our genetic data demonstrate that SMA-10 acts within a nematode BMP-like pathway to promote signaling. To test whether this function is conserved, we asked if SMA-10 could regulate BMP signaling in mammalian cells. We used a standard reporter assay in a BMP-responsive human cell line (HepG2), the BMP-response element from the Smad7 gene driving luciferase [39]. BMP2 induced reporter activity significantly over the controls, whereas in the presence of SMA-10, the response of the promoter was increased 4.2-fold over BMP2 alone (Figure 3A). Thus, SMA-10 can directly promote mammalian BMP signaling.
Fig. 3. SMA-10 binds BMP receptors and enhances BMP signaling. 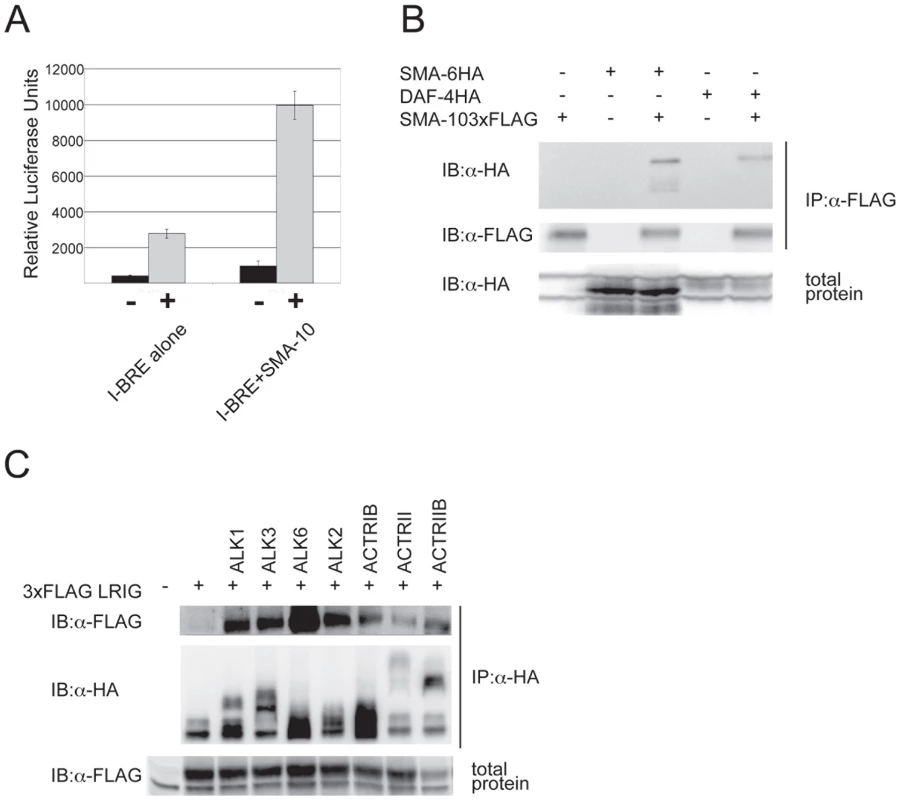
SMA-10 enhances BMP signaling in HepG2 cells (A). Cells were transfected with a BMP-responsive transcriptional reporter (I-BRE:luciferase) and either SMA-10 or a control vector. Cells were either left unstimulated (black bars) or were stimulated with 2.5 nM BMP2 (grey bars). The average of four transfected wells is shown plus and minus the standard deviation. SMA-10 binds SMA-6 and DAF-4 (B). 293T cells were co-transfected with FLAG-tagged SMA-10 and HA-tagged SMA-6 or DAF-4 receptors. Lysates were immunoprecipitated with anti-FLAG (SMA-10) and blotted for HA (SMA-6 or DAF-4). LRIG1 is able to interact with several mammalian BMP receptors (C). Cells were co-transfected with various HA-tagged BMP receptors and 3x FLAG-tagged LRIG1, then lysates were immunoprecipitated with anti-HA antibody and blotted with anti-FLAG to detect LRIG1. SMA-10 Binds BMP Receptors SMA-6 and DAF-4
The genetic and molecular data strongly support a model in which SMA-10 acts on the extracellular surface of the plasma membrane, and could act via physical interactions with ligand, receptors, or ligand and receptors. To examine these possibilities and to provide mechanistic insights into SMA-10's function, we asked whether SMA-10 directly binds to ligand or to the DBL-1 receptors, SMA-6 and DAF-4. For the first experiment, we employed affinity labeling and substituted BMP2 for DBL-1, since DBL-1 protein is not readily available and BMP2 has been shown to physically interact with other DBL-1 pathway members [17], [24]. SMA-10 was FLAG-tagged and BMP2 ligand was radio-iodinated with 125I. After anti-FLAG immunoprecipitation and blotting, the blot was exposed to film. However, we failed to detect any binding of BMP2 to SMA-10 (Figure S1). We next asked whether SMA-10 interacted with the receptors. FLAG-tagged SMA-10 and HA-tagged receptors were transfected into 293T cells. To isolate SMA-10 and associated proteins from cells, lysates were first immunoprecipitated with anti-FLAG antibody and blotted. The blot was then probed with anti-HA antibody to determine whether receptors were bound to SMA-10. Under these conditions, both SMA-6 and DAF-4 receptors co-immunoprecipitated with SMA-10 (Figure 3B). Thus, SMA-10 physically interacts with receptors and not with the ligand.
LRIG1 Binds Mammalian BMP Receptors
Given that SMA-10 binds the Sma/Mab pathway receptors, we tested whether this interaction was conserved in a mammalian system. Using the mammalian ortholog LRIG1 and mammalian BMP receptors, we assayed the ability of LRIG1 to bind the receptors. FLAG-tagged LRIG1 and HA-tagged versions of BMP receptors were transfected into mammalian cells. Lysates were immunoprecipitated with anti-FLAG antibody to pull down LRIG1, blotted, and probed with anti-HA antibody to determine whether LRIG1 binds any BMP receptors. We found that LRIG1 interacted strongly with type I receptor ALK6 and more weakly with the type I receptors ALK1, ALK2, ALK3, and ActRIB (Figure 3C). In addition, we detected weak interactions with type II receptors ActRII and ActRIIB (Figure 3C). Thus, LRIGs interact with BMP receptors and represent a new class of BMP receptor-associated proteins.
Discussion
Here we describe a conserved, novel regulator of BMP signaling, SMA-10, which is absolutely required for body size signaling in C. elegans. Loss of SMA-10 function results in a phenotype similar to a loss of any of the Sma/Mab pathway components. Molecular and genetic analyses place its function at the level of the cell membrane between the ligand and the receptors. Several lines of evidence support a model in which SMA-10 and its orthologs are conserved regulators of BMP signaling. The Drosophila homolog lambik rescues sma-10(lf) animals. Consistent with genetic evidence that SMA-10 is a positive effector of BMP signaling, SMA-10 stimulates a positive BMP-specific response in human cells. Furthermore, SMA-10 and LRIG1 physically interact with BMP type I and type II receptors. LRIGs have previously been shown to regulate EGF and Met receptor tyrosine kinase signaling [40], [41], and recently the insect-specific kekkon5 was shown to genetically interact with a BMP signaling pathway [29]. Our work reveals a new, conserved role for the LRIG subfamily in BMP receptor signaling.
SMA-10 Acts in BMP Signaling in C. elegans
We propose that SMA-10 acts at the hypodermal membrane surface, where the Sma/Mab receptors are located, to facilitate receptor signaling. Our genetic evidence shows that the SMA-10 protein acts between the secreted ligand and the transmembrane receptors (Table 2). The structure of SMA-10 indicates that it contains a secretion signal and a transmembrane region (Figure 2). Subcellular localization of a rescuing translational SMA-10:GFP fusion shows SMA-10 in hypodermal tissues and at cell membrane surfaces (Figure 1C and 1E). The requirement for sma-10 expression in the hypodermis to rescue the sma-10(lf) small body size further supports the model that SMA-10 acts at the hypodermal membrane to facilitate DBL-1 binding to its receptors (Table 3). We predict that the extracellular domain is responsible for this rescuing effect, as SMA-10's C-terminal intracellular tail is short (19 amino acids) and not conserved with Lambik, which has SMA-10 function, and SMA-10 is able to rescue when tagged with GFP at the C-terminus. We suggest that this intracellular sequence is not critical for transducing BMP signals.
Mutant animals for all previously characterized Sma/Mab core-signaling components have alterations in both body size and male tail defects. However, sma-10 only affects body size. This can be explained if sma-10 acts in a tissue-specific manner, namely the hypodermis, where DBL-1activated pathway signaling is required for body size control. Previous studies of targets downstream of this pathway (lon-1, mab-21, and mab-23) and one upstream regulator (LON-2) have shown that functions in the body size and male tail pathways are separable [22], [24], [30], [31], [42]. Given that sequencing reveals that one of the sma-10 alleles (wk88) is a presumed null, we favor the model that SMA-10 acts in a tissue-specific manner to enhance signal strength in the cells contributing to body size but not to the male tail. Consistent with this reasoning, differences in the requirement of signal strength between the tail and the hypodermis are seen with a hypomorphic allele of sma-6, where body size is reduced while tail morphology is normal [18]. It remains to be seen whether differential responses to Sma/Mab pathway dosage differences are sufficient to explain this tissue specificity or if there exists another male-specific factor that functions, like SMA-10 in the hypodermis, to positively regulate Sma/Mab signaling in the male tail. We note that all the sma-10 alleles result in small animals that are not as small as animals with loss-of-function mutations in previously identified core-signaling components. If tissue specificity in other organisms is a common feature, then this could explain why sma-10 orthologs were not previously identified as BMP signaling modulators. We note that mammals have three LRIGs, and mutating a single LRIG (LRIG1, a.k.a. LIG-1) results in a mild hyperplasia of epidermal cells [43]. This is a seemingly minor defect, but loss of LRIG1 function may be partially compensated by the other two functional LRIGs. Therefore, redundancy may also explain why LRIGs have eluded identification as regulators of BMP signaling in higher organisms.
SMA-10 Is a Conserved LRIG Family Member
The LRR and Ig-like domains exist singly in many other proteins, but only in the LRIG family do they exist in the same protein. The number of protein domains in the SMA-10/LRIG family, which excludes the insect Kekkon subfamily, is invariant. These domains have been shown to be involved with protein-protein interactions [44], [45]. In these studies, we show that SMA-10 and LRIG1 bind BMP type I and type II receptors, and our genetic evidence and studies based on overexpression in mammalian cells suggests that these proteins are positively required for BMP family signal transduction.
Kekkon 1 has been shown to be a negative regulator of the EGF pathway in Drosophila and acts by binding to the receptors [28]. Given the involvement of Kekkon 1 in EGF signaling, LRIG1 was tested for EGFR regulation in mammalian tissue culture [40]. LRIG1 was shown to also bind EGFRs and enhance their degradation [40]. Signaling of EGFs through EGFRs promotes cell proliferation. LRIG1 negatively regulates cell proliferation by down-regulating EGFR responsiveness by increasing activated receptor ubiquitination [28], [40]. LRIG1 binds the E3 ubiquitin ligase c-Cbl, bringing it into the EGFR complex. EGFR then phosphorylates c-Cbl, thereby activating it and promoting ubiquitination and degradation of both LRIG1 and EGFRs. LRIG1's amino acids 900 to 930 contain the binding site for c-Cbl [40], within its intracellular domain that is not shared by other LRIGs, SMA-10, or Lambik. An interaction of c-Cbl with LRIG2 or LRIG3 has not been demonstrated [46]. The ectodomain of LRIG1 alone inhibits EGFR signaling, but does so in a ubiquitin-independent fashion, showing that EGFR inhibition by LRIGs is not exclusively through ubiquitination [47]. EGFR signaling in C. elegans, mediated by a single EGFR (LET-23), directs several embryonic and larval cell fates and also ovulatory contractions in adult hermaphrodites [48]. We did not see any obvious defects associated with EGFR in sma-10(lf) animals that might suggest an interaction with EGFR. LRIG1 also binds to and inhibits signaling by hepatocyte growth factor receptor (Met), a tyrosine kinase that in many known cancers is mutated or misregulated to promote invasive growth, though its mechanism of inhibition is ubiquitin-independent [41]. C. elegans has no recognized Met receptor tyrosine kinase [49]. Another Kekkon, Kekkon5, affects BMP signaling, but the model, based on genetic and structure-function analyses, proposes that Kekkon5 regulates ligand distribution or activity rather than acts directly on receptors, as we show here for both C. elegans LRIG SMA-10 and mammalian LRIG1 [29].
Although their expression appears to be universal, human LRIGs are differentially expressed in various cancer cell types, being down regulated in many types studied, but being upregulated in others [46]. Various explanations have been proposed based on the current understanding of LRIG function [46]. Our research showing LRIG interaction with BMP receptors (BMPRs) leads us to propose a new model, where the cell-specific levels of BMP and EGF activity, which negatively and positively regulate cell growth, respectively, determine the cell's response to LRIG exposure.
The observation that SMA-10 localizes to intracellular puncta (Figure 1C) is reminiscent of endocytic vesicles, and suggests a model of action for SMA-10 and the Sma/Mab signaling pathway [50]. In other systems, there is evidence that TGF-β superfamily pathway signaling can be activated via receptor monoubiquitination and receptor complex endocytosis into early vesicles (reviewed in [51], [52]). SMA-10 may thus promote signaling by facilitating receptor internalization into early endosomes.
This work identifies a new, conserved component of BMP signaling, SMA-10/LRIG. Mammalian members play known roles in some receptor tyrosine kinase pathways, and this work identifies a new role for this family in BMP receptor serine/threonine kinase signaling. We have shown that two members of this family physically interact with both the type I and type II BMP receptors, and C. elegans SMA-10 enhances signaling in both the nematode and in mammalian cells.
Materials and Methods
Strains
Animals were maintained according to standard protocols [26]. All mutant strains used in this study were derived from the wild-type Bristol strain N2, and some mapping was accomplished using the wild-type Hawaii isolate CB4856. Alleles used include sma-10(wk26, wk66, wk88, wk89, wk90) [25], lon-2(e678), sma-6(wk7), ctIs40 [ZC421 (dbl-1(+)) + pTG96 (sur-5::gfp)] [23], bxIs16 [tph1::gfp + cat-2::yfp] [42]; and nIs128 [pkd-2::gfp] [53]. Arrays made for this study are wkEx47 (sma-10p::sma-10:gfp + pUC18 filler DNA, 50 ng/µl each), wkEx91 (myo-2p::sma-10(+)), wkEx92 (rol-6p::sma-10(+)), wkEx93 (sma-10p::lambik), texEx190 (sma-6p::sma-6(+):gfp), and texEx195 (sma-10p::sma-10(yk352c5).
Creation of transgenic arrays was performed by standard microinjection techniques [54]. Genomic sma-10 or Drosophila lambik cDNA was cloned into nematode expression vector pPD95.75 with the appropriate promoter sequence. wkEx47 was made by removing the stop codon of the sma-10 genomic sequence and fusing the gfp sequence in-frame at the 3′ end. Transgenic animals were generated by germline microinjection, using constructs at 50 ng/µl (HW480 sma-10p::lambik, CZ10.2 sma-10p::sma-10:gfp, and CZ9.1 sma-10(yk352c5)) or 0.5 ng/µl (HW469 rol-6p::sma-10 and HW477 myo-2p::sma-10) with the co-injection marker ttx-3p::rfp or ttx-3p::gfp at 50 ng/µl (with HW480 and CZ9.1) or 100 ng/µl (with HW469 and HW477). ttx-3p drives expression in AIY interneurons. One representative stable line for each transgene was measured.
Isolation of sma-10 Mutant Animals
Isolation of sma-10(wk26) and sma-10(wk66) was previously described [25]. In an effort to identify additional alleles of genes that act BMP signaling, lon-2(e678) hermaphrodites were mutagenized with 50 mM ethyl methanesulfonate (EMS) using standard procedures [26]. Mutagenized P0 animals were transferred to plates and allowed to segregate self-progeny. F1 animals were transferred to new plates to segregate progeny, which were then scored for a small phenotype in a quarter of the population. From about 9,000 mutagenized genomes screened, three additional alleles of sma-10 were isolated, wk88, wk89, and wk90. These alleles, as well as the two alleles isolated in the Sma screen [25], were outcrossed five times before further analyses were done.
Phenotypic Analyses
To measure body size, animals were picked at the L4 stage and photographed as young adults about 24 hours later. Images from individual animals were captured from a dissecting microscope using an Optronics MagnaFire CCD camera system and software (Optronics, Goleta, CA). Perimeters (Table 1) or lengths (Table 2, Table 3, Table 4) of animals were determined by using Image-Pro Plus measurement software (Media Cybernetics, Inc., Silver Spring, MD). The images for Figure 1C and 1D were captured using an Axiovert 200 M microscope (Carl Zeiss MicroImaging, Oberkochen, Germany) equipped with a digital CCD camera (C4742-95-12ER, Hamamatsu Photonics, Hamamatsu, Japan) and were deconvolved with AutoDeblur software (AutoQuant Imaging, Watervliet, NY). The Figure 1E image was obtained on an Olympus IX81 with a Carv Nipkow disk confocal unit (Atto Biosciences, Rockville, MD) and SensiCam QE camera (Cooke Corp., Auburn Hills, MI).
We performed statistical analyses on these measurements. Individual measurements from each strain were averaged. We determined the ratio and 95% confidence interval of the average measurement (mean) of each strain to the wild-type strain mean. To verify the significance of our findings, we tested the null hypothesis that the ratios of the compared means are the same. The ratios compared were the double mutant strain mean/wild type mean to the single sma/transgenic mutant strain mean/wild type strain mean. For populations measured on the same day, the denominator (the average of wild-type measurements) was the same and a Student's t-test was used to test the null hypothesis. For populations measured on different days, the Welch-Satterthwaite equation was used to calculate the effective degrees of freedom for this 2-tailed t-test for two ratios. We determined the value of t with the calculated degrees of freedom and compared the t-value to the Student's t table to construct the probability (p) of the null hypothesis.
Epistasis Analyses
sma-10(wk66); lon double mutant animals were constructed by crossing heterozygous sma-10(wk66) males with lon-2(e678) or lon-1(wk50) hermaphrodites, with animals overexpressing an integrated transgene with wild-type dbl-1 (ctIs40), or with animals overexpressing an extrachromosomal array encoding functional SMA-6 (texEx190) [15]. Wild-type F1 were isolated and small and long F2 animals were picked to individual plates. The F3 generation was then examined for the presence of long animals from a small F2 parent or small animals from a long F2 parent.
Positional Cloning of sma-10
sma-10(wk66) was previously mapped to chromosome IV by two-factor crosses [25]. We further refined its position by standard three-factor mapping and single nucleotide polymorphism mapping [26], [55]. We used microinjection and germline transformation rescue [54] to discover that YAC Y80C9 and cosmid T21D12 rescue the small phenotype of sma-10(wk66) animals. Each predicted gene on T21D12, with at least 1.5 kb of promoter sequence, was amplified by the polymerase chain reaction (PCR). The DNA product was purified and injected into sma-10(wk66) animals. Only T21D12.9 rescued the small phenotype. PCR confirmed altered or deleted sequences in all five sma-10 alleles.
Cell Culture
293T cells were cultured in Dulbecco's modified Eagle's medium containing high glucose and supplemented with 10% fetal bovine serum (FBS). HepG2 cells were cultured in minimal essential media supplemented with 1% non-essential amino acids and 10% FBS. Cells were transfected by calcium phosphate and lysed 48 hours after transfection in TNTE buffer containing 0.5% Triton-X-100 (150 mM NaCl, 50 mM Tris ph 7.4, and 1 mM EDTA) [56]. Immunoprecipitations were carried out using M2 anti-FLAG (Sigma) or 12CA5 anti-HA (made in-house) followed by incubation with Protein-G Sepharose beads (Amersham Biosciences, Uppsala, Sweden). Immunoprecipitates were then washed four times with lysis buffer containing 0.1% Triton-X-100. Proteins were separated by SDS-PAGE and immunoblotted with anti-HA (12CA5) or anti-FLAG (M2).
Signaling Assays
Transcriptional assays were carried out using a previously described BMP-responsive element from the mouse Smad7 gene (I-BRE) driving firefly luciferase [39]. Luciferase assays were carried out as described [39]. Renilla luciferase expressed from the CMV promoter was used as an internal control for transfection efficiency. Firefly luciferase values were normalized using Renilla luciferase values.
Constructs
1.2 kb promoter sequence 5′ of the sma-10 open reading frame driving expression of genomic sma-10 was sufficient to rescue body size defects in sma-10(lf) animals, and this same promoter region was fused to Drosophila lambik/CG8434 cDNA to address conservation of LRIG family function. Tissue specific expression was determined by driving expression of sma-10 genomic sequence with myo-2 (pharyngeal expression) or rol-6 (hypodermal expression) promoters [57]. We generated GFP-tagged SMA-10 by fusing the eGFP sequence to the 3′ terminus of wild-type sma-10 genomic sequence that lacked its termination codon.
The rescuing sma-6 construct was made by fusing the mCherry sequence in frame to the 3′ end of genomic sma-6 and expressing it using 1260 bp sma-6 promoter sequence.
3x FLAG SMA-10 was made by fusing the full-length sma-10 cDNA from the first Sal I site in frame to the 3x FLAG signal peptide containing vector p3xFLAG-CMV8 (Sigma). Similarly, we constructed the 3x FLAG-tagged LRIG-1 by subcloning the LRIG cDNA from an endogenous Sal I restriction site to the C-terminus into p3xFLAG-CMV8. This construct encodes a protein with the 3x FLAG tag between the N-terminal signal sequence and the first LRR of LRIG1. SMA-6 was C-terminally tagged with the HA1 epitope of influenza virus hemagglutinin (HA) and was cloned into the mammalian expression vector pCMV5 (T. Reguly and J. Wrana, unpublished work). DAF-4DC-HA is HA-tagged DAF-4 with a deletion of its C-terminal tail (T. Reguly and J. Wrana, unpublished work). ALKIHA, ALK3HA, ALK6HA, ALK2HA, ActRIBHA, ActRIIHA, ActRIIBHA have been previously described [58]–.
Supporting Information
Zdroje
1. DennlerS
GoumansMJ
ten DijkeP
2002 Transforming growth factor β signal transduction. J Leukoc Biol 71 731 740
2. ShiY
MassaguéJ
2003 Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell 113 685 700
3. MassaguéJ
BlainSW
LoRS
2000 TGFβ signaling in growth control, cancer, and heritable disorders. Cell 103 295 309
4. GordonKJ
BlobeGC
2008 Role of transforming growth factor-β superfamily signaling pathways in human disease. Biochim Biophys Acta 1782 197 228
5. MassagueJ
2008 TGFβ in Cancer. Cell 134 215 230
6. GumiennyTL
PadgettRW
2002 The other side of TGF-β superfamily signal regulation: thinking outside the cell. Trends Endocrinol Metab 13 295 299
7. UmulisD
O'ConnorMB
BlairSS
2009 The extracellular regulation of bone morphogenetic protein signaling. Development 136 3715 3728
8. ItohS
ten DijkeP
2007 Negative regulation of TGF-β receptor/Smad signal transduction. Cur Opin Cell Biol 19 176 184
9. OnichtchoukD
ChenYG
DoschR
GawantkaV
DeliusH
1999 Silencing of TGF-β signalling by the pseudoreceptor BAMBI. Nature 401 480 485
10. CheifetzS
WeatherbeeJA
TsangML
AndersonJK
MoleJE
1987 The transforming growth factor-β system, a complex pattern of cross-reactive ligands and receptors. Cell 48 409 415
11. MassaguéJ
LikeB
1985 Cellular receptors for type β transforming growth factor. Ligand binding and affinity labeling in human and rodent cell lines. J Biol Chem 260 2636 2645
12. CheifetzS
BellonT
CalesC
VeraS
BernabeuC
1992 Endoglin is a component of the transforming growth factor-β receptor system in human endothelial cells. J Biol Chem 267 19027 19030
13. BarbaraNP
WranaJL
LetarteM
1999 Endoglin is an accessory protein that interacts with the signaling receptor complex of multiple members of the transforming growth factor-β superfamily. J Biol Chem 274 584 594
14. KirkbrideKC
TownsendTA
BruinsmaMW
BarnettJV
BlobeGC
2008 Bone morphogenetic proteins signal through the transforming growth factor-β type III receptor. J Biol Chem 283 7628 7637
15. PattersonGI
PadgettRW
2000 TGFβ-related pathways. Roles in Caenorhabditis elegans development. Trends Genet 16 27 33
16. Savage-DunnC
2005 TGF-β signaling (September 9, 2005) In: Community TCeR, editor. WormBook. 10.1895/wormbook.1.22.1 ed: http://wormbook.org
17. EstevezM
AttisanoL
WranaJL
AlbertPS
MassaguéJ
1993 The daf-4 gene encodes a bone morphogenetic protein receptor controlling C. elegans dauer larva development. Nature 365 644 649
18. KrishnaS
MaduziaLL
PadgettRW
1999 Specificity of TGFβ signaling is conferred by distinct type I receptors and their associated SMAD proteins in Caenorhabditis elegans. Development 126 251 260
19. SavageC
DasP
FinelliAL
TownsendSR
SunCY
1996 Caenorhabditis elegans genes sma-2, sma-3, and sma-4 define a conserved family of transforming growth factor-β pathway components. Proc Natl Acad Sci U S A 93 790 794
20. JiYJ
NamS
JinYH
ChaEJ
LeeKS
2004 RNT-1, the C. elegans homologue of mammalian RUNX transcription factors, regulates body size and male tail development. Dev Biol 274 402 412
21. LiangJ
LintsR
FoehrML
TokarzR
YuL
2003 The Caenorhabditis elegans schnurri homolog sma-9 mediates stage - and cell type-specific responses to DBL-1 BMP-related signaling. Development 130 6453 6464
22. MoritaK
ChowKL
UenoN
1999 Regulation of body length and male tail ray pattern formation of Caenorhabditis elegans by a member of TGF-β family. Development 126 1337 1347
23. SuzukiY
YandellMD
RoyPJ
KrishnaS
Savage-DunnC
1999 A BMP homolog acts as a dose-dependent regulator of body size and male tail patterning in Caenorhabditis elegans. Development 126 241 250
24. GumiennyTL
MacNeilLT
WangH
de BonoM
WranaJL
2007 Glypican LON-2 is a conserved negative regulator of BMP-like signaling in Caenorhabditis elegans. Curr Biol 17 159 164
25. Savage-DunnC
MaduziaLL
ZimmermanCM
RobertsAF
CohenS
2003 Genetic screen for small body size mutants in C. elegans reveals many TGFβ pathway components. Genesis 35 239 247
26. BrennerS
1974 The genetics of Caenorhabditis elegans. Genetics 77 71 94
27. GuoD
HolmlundC
HenrikssonR
HedmanH
2004 The LRIG gene family has three vertebrate paralogs widely expressed in human and mouse tissues and a homolog in Ascidiacea. Genomics 84 157 165
28. GhiglioneC
CarrawayKL3rd
AmundadottirLT
BoswellRE
PerrimonN
1999 The transmembrane molecule Kekkon 1 acts in a feedback loop to negatively regulate the activity of the Drosophila EGF receptor during oogenesis. Cell 96 847 856
29. EvansTA
HaridasH
DuffyJB
2009 Kekkon5 is an extracellular regulator of BMP signaling. Dev Biol 326 36 46
30. MaduziaLL
GumiennyTL
ZimmermanCM
WangH
ShetgiriP
2002 lon-1 regulates Caenorhabditis elegans body size downstream of the dbl-1 TGFβ signaling pathway. Dev Biol 246 418 428
31. MoritaK
FlemmingAJ
SugiharaY
MochiiM
SuzukiY
2002 A Caenorhabditis elegans TGF-β, DBL-1, controls the expression of LON-1, a PR-related protein, that regulates polyploidization and body length. EMBO J 21 1063 1073
32. WangJ
TokarzR
Savage-DunnC
2002 The expression of TGFβ signal transducers in the hypodermis regulates body size in C. elegans. Development 129 4989 4998
33. YoshidaS
MoritaK
MochiiM
UenoN
2001 Hypodermal expression of Caenorhabditis elegans TGF-β type I receptor SMA-6 is essential for the growth and maintenance of body length. Dev Biol 240 32 45
34. McKaySJ
JohnsenR
KhattraJ
AsanoJ
BaillieDL
2003 Gene expression profiling of cells, tissues, and developmental stages of the nematode C. elegans. Cold Spring Harb Symp Quant Biol 68 159 169
35. PattersonGI
KoweekA
WongA
LiuY
RuvkunG
1997 The DAF-3 Smad protein antagonizes TGF-β-related receptor signaling in the Caenorhabditis elegans dauer pathway. Genes Dev 11 2679 2690
36. Reece-HoyesJS
ShinglesJ
DupuyD
GroveCA
WalhoutAJ
2007 Insight into transcription factor gene duplication from Caenorhabditis elegans promoterome-driven expression patterns. BMC Genomics 8 27
37. MalloGV
KurzCL
CouillaultC
PujolN
GranjeaudS
2002 Inducible antibacterial defense system in C. elegans. Curr Biol 12 1209 1214
38. ZugastiO
EwbankJJ
2009 Neuroimmune regulation of antimicrobial peptide expression by a noncanonical TGF-β signaling pathway in Caenorhabditis elegans epidermis. Nat Immunol 10 249 256
39. BenchabaneH
WranaJL
2003 GATA - and Smad1-dependent enhancers in the Smad7 gene differentially interpret bone morphogenetic protein concentrations. Mol Cell Biol 23 6646 6661
40. GurG
RubinC
KatzM
AmitI
CitriA
2004 LRIG1 restricts growth factor signaling by enhancing receptor ubiquitylation and degradation. EMBO J 23 3270 3281
41. ShattuckDL
MillerJK
LaederichM
FunesM
PetersenH
2007 LRIG1 is a novel negative regulator of the Met receptor and opposes Met and Her2 synergy. Mol Cell Biol 27 1934 1946
42. LintsR
JiaL
KimK
LiC
EmmonsSW
2004 Axial patterning of C. elegans male sensilla identities by selector genes. Dev Biol 269 137 151
43. SuzukiY
MiuraH
TanemuraA
KobayashiK
KondohG
2002 Targeted disruption of LIG-1 gene results in psoriasiform epidermal hyperplasia. FEBS Lett 521 67 71
44. BarclayAN
2003 Membrane proteins with immunoglobulin-like domains-a master superfamily of interaction molecules. Semin Immunol 15 215 223
45. KobeB
KajavaAV
2001 The leucine-rich repeat as a protein recognition motif. Curr Opin Struct Biol 11 725 732
46. HedmanH
HenrikssonR
2007 LRIG inhibitors of growth factor signalling—double-edged swords in human cancer? Eur J Cancer 43 676 682
47. Di GuglielmoGM
Le RoyC
GoodfellowAF
WranaJL
2003 Distinct endocytic pathways regulate TGF-β receptor signalling and turnover. Nat Cell Biol 5 410 421
48. MoghalN
SternbergPW
2003 The epidermal growth factor system in Caenorhabditis elegans. Exp Cell Res 284 150 159
49. RubinGM
YandellMD
WortmanJR
Gabor MiklosGL
NelsonCR
2000 Comparative genomics of the eukaryotes. Science 287 2204 2215
50. GrantB
HirshD
1999 Receptor-mediated endocytosis in the Caenorhabditis elegans oocyte. Mol Biol Cell 10 4311 4326
51. d'AzzoA
BongiovanniA
NastasiT
2005 E3 ubiquitin ligases as regulators of membrane protein trafficking and degradation. Traffic 6 429 441
52. Le RoyC
WranaJL
2005 Clathrin - and non-clathrin-mediated endocytic regulation of cell signalling. Nat Rev Mol Cell Biol 6 112 126
53. YuH
PrétôtRF
BürglinTR
SternbergPW
2003 Distinct roles of transcription factors EGL-46 and DAF-19 in specifying the functionality of a polycystin-expressing sensory neuron necessary for C. elegans male vulva location behavior. Development 130 5217 5227
54. MelloC
FireA
1995 DNA transformation. Methods Cell Biol 48 451 482
55. WicksSR
YehRT
GishWR
WaterstonRH
PlasterkRH
2001 Rapid gene mapping in Caenorhabditis elegans using a high density polymorphism map. Nat Genet 28 160 164
56. SambrookJ
RussellDW
1989 2344 Molecular Cloning: a Laboratory Manual: Cold Spring Harbor Press.
57. Jantsch-PlungerV
FireA
1994 Combinatorial structure of a body muscle-specific transcriptional enhancer in Caenorhabditis elegans. J Biol Chem 269 27021 27028
58. AttisanoL
CárcamoJ
VenturaF
WeisFM
MassaguéJ
1993 Identification of human activin and TGFβ type I receptors that form heteromeric kinase complexes with type II receptors. Cell 75 671 680
59. AttisanoL
WranaJL
MontalvoE
MassaguéJ
1996 Activation of signalling by the activin receptor complex. Mol Cell Biol 16 1066 1073
60. HoodlessPA
HaerryT
AbdollahS
StapletonM
O'ConnorMB
1996 MADR1, a MAD-related protein that functions in BMP2 signaling pathways. Cell 85 489 500
61. Macías-SilvaM
HoodlessPA
TangSJ
BuchwaldM
WranaJL
1998 Specific activation of Smad1 signaling pathways by the BMP7 type I receptor, ALK2. J Biol Chem 273 25628 25636
62. LetunicI
CopleyRR
PilsB
PinkertS
SchultzJ
2006 SMART 5: domains in the context of genomes and networks. Nucleic Acids Res 34 D257 D260
63. MacLarenCM
EvansTA
AlvaradoD
DuffyJB
2004 Comparative analysis of the Kekkon molecules, related members of the LIG superfamily. Dev Genes Evol 214 360 366
Štítky
Genetika Reprodukční medicína
Článek Epistasis of Transcriptomes Reveals Synergism between Transcriptional Activators Hnf1α and Hnf4αČlánek B1 SOX Coordinate Cell Specification with Patterning and Morphogenesis in the Early Zebrafish EmbryoČlánek Bulk Segregant Analysis by High-Throughput Sequencing Reveals a Novel Xylose Utilization Gene fromČlánek Abundant Quantitative Trait Loci Exist for DNA Methylation and Gene Expression in Human Brain
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2010 Číslo 5
-
Všechny články tohoto čísla
- Digital Quantification of Human Eye Color Highlights Genetic Association of Three New Loci
- Common Genetic Variants near the Brittle Cornea Syndrome Locus Influence the Blinding Disease Risk Factor Central Corneal Thickness
- Age- and Temperature-Dependent Somatic Mutation Accumulation in
- All About Mitochondrial Eve: An Interview with Rebecca Cann
- Aging and Chronic Sun Exposure Cause Distinct Epigenetic Changes in Human Skin
- Transposed Genes in Arabidopsis Are Often Associated with Flanking Repeats
- A Survey of Genomic Traces Reveals a Common Sequencing Error, RNA Editing, and DNA Editing
- Gene Transposition Causing Natural Variation for Growth in
- The Polyproline Site in Hinge 2 Influences the Functional Capacity of Truncated Dystrophins
- Epistasis of Transcriptomes Reveals Synergism between Transcriptional Activators Hnf1α and Hnf4α
- Integration of Light Signals by the Retinoblastoma Pathway in the Control of S Phase Entry in the Picophytoplanktonic Cell
- The Proprotein Convertase Encoded by () Is Required in Corpora Cardiaca Endocrine Cells Producing the Glucose Regulatory Hormone AKH
- Sgs1 and Exo1 Redundantly Inhibit Break-Induced Replication and Telomere Addition at Broken Chromosome Ends
- A MATE-Family Efflux Pump Rescues the 8-Oxoguanine-Repair-Deficient Mutator Phenotype and Protects Against HO Killing
- The Relationship among Gene Expression, the Evolution of Gene Dosage, and the Rate of Protein Evolution
- SMA-10/LRIG Is a Conserved Transmembrane Protein that Enhances Bone Morphogenetic Protein Signaling
- The Nuclear Receptor DHR3 Modulates dS6 Kinase–Dependent Growth in
- Involvement of Global Genome Repair, Transcription Coupled Repair, and Chromatin Remodeling in UV DNA Damage Response Changes during Development
- B1 SOX Coordinate Cell Specification with Patterning and Morphogenesis in the Early Zebrafish Embryo
- Linkage and Association Mapping of Flowering Time in Nature
- Bulk Segregant Analysis by High-Throughput Sequencing Reveals a Novel Xylose Utilization Gene from
- Abundant Quantitative Trait Loci Exist for DNA Methylation and Gene Expression in Human Brain
- Ablation of Whirlin Long Isoform Disrupts the USH2 Protein Complex and Causes Vision and Hearing Loss
- Characterization of Oxidative Guanine Damage and Repair in Mammalian Telomeres
- DNA Adenine Methylation Is Required to Replicate Both Chromosomes Once per Cell Cycle
- Genome-Wide Copy Number Variation in Epilepsy: Novel Susceptibility Loci in Idiopathic Generalized and Focal Epilepsies
- FACT Prevents the Accumulation of Free Histones Evicted from Transcribed Chromatin and a Subsequent Cell Cycle Delay in G1
- GC-Biased Evolution Near Human Accelerated Regions
- Liver and Adipose Expression Associated SNPs Are Enriched for Association to Type 2 Diabetes
- Myeloid Cell-Restricted Insulin Receptor Deficiency Protects Against Obesity-Induced Inflammation and Systemic Insulin Resistance
- The Mating Type Locus () and Sexual Reproduction of : Insights into the Evolution of Sex and Sex-Determining Chromosomal Regions in Fungi
- B-Cyclin/CDKs Regulate Mitotic Spindle Assembly by Phosphorylating Kinesins-5 in Budding Yeast
- Post-Replication Repair Suppresses Duplication-Mediated Genome Instability
- Genome Sequence of the Plant Growth Promoting Endophytic Bacterium sp. 638
- Transcription Factors Mat2 and Znf2 Operate Cellular Circuits Orchestrating Opposite- and Same-Sex Mating in
- The Use of Orthologous Sequences to Predict the Impact of Amino Acid Substitutions on Protein Function
- Mutation in Archain 1, a Subunit of COPI Coatomer Complex, Causes Diluted Coat Color and Purkinje Cell Degeneration
- Shelterin-Like Proteins and Yku Inhibit Nucleolytic Processing of Telomeres
- Affecting Function Causes a Dilated Heart in Adult
- Manipulation of Behavioral Decline in with the Rag GTPase
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Common Genetic Variants near the Brittle Cornea Syndrome Locus Influence the Blinding Disease Risk Factor Central Corneal Thickness
- All About Mitochondrial Eve: An Interview with Rebecca Cann
- The Relationship among Gene Expression, the Evolution of Gene Dosage, and the Rate of Protein Evolution
- SMA-10/LRIG Is a Conserved Transmembrane Protein that Enhances Bone Morphogenetic Protein Signaling
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



