-
Články
Reklama
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
ReklamaA Shared Role for RBF1 and dCAP-D3 in the Regulation of Transcription with Consequences for Innate Immunity
Previously, we discovered a conserved interaction between RB proteins and the Condensin II protein CAP-D3 that is important for ensuring uniform chromatin condensation during mitotic prophase. The Drosophila melanogaster homologs RBF1 and dCAP-D3 co-localize on non-dividing polytene chromatin, suggesting the existence of a shared, non-mitotic role for these two proteins. Here, we show that the absence of RBF1 and dCAP-D3 alters the expression of many of the same genes in larvae and adult flies. Strikingly, most of the genes affected by the loss of RBF1 and dCAP-D3 are not classic cell cycle genes but are developmentally regulated genes with tissue-specific functions and these genes tend to be located in gene clusters. Our data reveal that RBF1 and dCAP-D3 are needed in fat body cells to activate transcription of clusters of antimicrobial peptide (AMP) genes. AMPs are important for innate immunity, and loss of either dCAP-D3 or RBF1 regulation results in a decrease in the ability to clear bacteria. Interestingly, in the adult fat body, RBF1 and dCAP-D3 bind to regions flanking an AMP gene cluster both prior to and following bacterial infection. These results describe a novel, non-mitotic role for the RBF1 and dCAP-D3 proteins in activation of the Drosophila immune system and suggest dCAP-D3 has an important role at specific subsets of RBF1-dependent genes.
Published in the journal: . PLoS Genet 8(4): e32767. doi:10.1371/journal.pgen.1002618
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002618Summary
Previously, we discovered a conserved interaction between RB proteins and the Condensin II protein CAP-D3 that is important for ensuring uniform chromatin condensation during mitotic prophase. The Drosophila melanogaster homologs RBF1 and dCAP-D3 co-localize on non-dividing polytene chromatin, suggesting the existence of a shared, non-mitotic role for these two proteins. Here, we show that the absence of RBF1 and dCAP-D3 alters the expression of many of the same genes in larvae and adult flies. Strikingly, most of the genes affected by the loss of RBF1 and dCAP-D3 are not classic cell cycle genes but are developmentally regulated genes with tissue-specific functions and these genes tend to be located in gene clusters. Our data reveal that RBF1 and dCAP-D3 are needed in fat body cells to activate transcription of clusters of antimicrobial peptide (AMP) genes. AMPs are important for innate immunity, and loss of either dCAP-D3 or RBF1 regulation results in a decrease in the ability to clear bacteria. Interestingly, in the adult fat body, RBF1 and dCAP-D3 bind to regions flanking an AMP gene cluster both prior to and following bacterial infection. These results describe a novel, non-mitotic role for the RBF1 and dCAP-D3 proteins in activation of the Drosophila immune system and suggest dCAP-D3 has an important role at specific subsets of RBF1-dependent genes.
Introduction
The RB family proteins (pRB, p130 and p107 in humans; RBF1 and RBF2 in Drosophila) co-ordinate changes in gene expression. Understanding the types of programs that these proteins regulate is important because of the unequivocal link between the inactivation of RB proteins and human cancer. Mutation of the retinoblastoma tumor susceptibility gene (RB1) is the rate-limiting step in the genesis of retinoblastoma and over 90% of human tumors exhibit reduced pRB function [1], [2].
RB family members are best-known for their roles in the regulation of E2F-dependent transcription. E2F-controlled genes are needed for cell proliferation and RB proteins suppress the expression of these targets during G0 and G1 of the cell cycle [3]. In addition, RB proteins are also important for the regulation of genes that are not involved in cell cycle progression. For example, osteoblast differentiation is modulated by pRB through its interaction with Runx2 [4]; in muscle cells, pRB promotes the expression of muscle-specific differentiation markers, enabling these cells to irreversibly exit the cell cycle [5]–[7]; in Drosophila, RBF1 cooperates with the Hippo pathway to maintain photoreceptor differentiation, independent of dE2F1 activity [8]. Such E2F-independent functions may help to explain why the inactivation of RB proteins can have very different consequences in different cellular contexts. However, many of the E2F-independent activities of RB proteins are not well-understood. At present, it is unclear if pRB has different activities in different cell types, or whether there is a yet-to-be discovered, general process that allows RB proteins to activate or repress the expression of variable sets of genes in different cell types.
Recent studies have suggested that pRB family members may impact the organization of higher-order chromatin structures, in addition to their local effects on the promoters of individual genes [9]. Mutation of pRB causes defects in pericentric heterochromatin [10] and RBF1 is necessary for uniform chromatin condensation in proliferating tissues of Drosophila larvae [11]. Part of the explanation for these defects is that RBF1 and pRB promote the localization of the Condensin II complex protein, CAP-D3 to DNA both in Drosophila and human cells [11]. Depletion of pRB from human cells strongly reduces the level of CAP-D3 associated with centromeres during mitosis and causes centromere dysfunction [12].
Condensin complexes are necessary for the stable and uniform condensation of chromatin in early mitosis [13]–[16]. They are conserved from bacteria to humans with at least two types of Condensin complexes (Condensin I and II) present in higher eukaryotes. Both Condensin I and II complexes contain heterodimers of SMC4 and SMC2 proteins that form an ATPase which acts to constrain positive supercoils [17], [18]. Each type of Condensin also contains three specific non-SMC proteins that, upon phosphorylation, stabilize the complex and promote ATPase activity [14], [19], [20]. The kleisin CAPH and two HEAT repeat containing subunits, CAP-G and CAP-D2 are components of Condensin I, while the kleisin CAP-H2 and two HEAT repeat containing subunits, CAP-G2 and CAP-D3, are constituents of Condensin II.
Given the well-established functions of Condensins during mitosis, and of RBF1 in G1 regulation, the convergence of these two proteins was unexpected. Nevertheless, mutant alleles in the non-SMC components of Condensin II suppress RBF1-induced phenotypes, and immunostaining experiments revealed that RBF1 displays an extensive co-localization with dCAP-D3 (but not with dCAP-D2) on the polytene chromatin of Drosophila salivary glands [11]. This co-localization occurs in cells that will never divide, suggesting that Condensin II subunits and RBF1 co-operate in an unidentified process in non-mitotic cells. In various model organisms, the mutation of non-SMC Condensin subunits has been associated with changes in gene expression [21]–[24] raising the possibility that dCAP-D3 may affect some aspect of transcriptional regulation by RBF1. However, the types of RBF1-regulated genes that might be affected by dCAP-D3, the contexts in which this regulation becomes important, and the consequences of losing this regulation are all unknown.
Here we identify sets of genes that are dependent on both rbf1 and dCap-D3. The majority of genes that show altered expression in both rbf1 and dCap-D3 mutants (larvae or adults) are not genes involved in the cell cycle, DNA repair, proliferation, but are genes with cell type-specific functions and many are spaced within 10 kb of one another in “gene clusters”. To better understand this mode of regulation we have investigated the effects of RBF1 and dCAP-D3 on one of the most highly misregulated clusters which includes genes coding for antimicrobial peptides (AMPs). AMPs are produced in many organs, and one of the major sites of production is in the fat body. Following production in the fat body, AMPs are subsequently dumped into the hemolymph where they act to destroy pathogens [25]. RBF1 and dCAP-D3 are required for the transcriptional activation of many AMPs in the adult fly. Analysis of one such gene cluster shows that RBF1 and dCAP-D3 bind directly to this region and that they bind, in the fat body, to sites flanking the locus. RBF1 and dCAP-D3 are both necessary in the fat body for maximal and sustained induction of AMPs following bacterial infection, and RBF1 and dCAP-D3 deficient flies have an impaired ability to respond efficiently to bacterial infection. These results identify dCAP-D3 as an important transcriptional regulator in the fly. Together, the findings suggest that RBF1 and dCAP-D3 regulate the expression of clusters of genes in post-mitotic cells, and this regulation has important consequences for the health of the organism.
Results
RBF1 and dCAP-D3 regulate many of the same genes during the later stages of the D. melanogaster life cycle
Our previous data demonstrated that RBF1 co-localizes extensively with dCAP-D3 on polytene chromatin of non-dividing cells, leading us to hypothesize that the two proteins may co-operate to regulate transcription. To begin to test this idea, we first identified the stages of fly development where RBF1 and dCAP-D3 were most highly expressed. qRT-PCR using primers for dCap-D3 and rbf1 was performed on cDNA generated from various stages of the Drosophila life cycle (Figure 1A). The results demonstrate that both genes are transcribed at the highest levels in late third instar larval and adult stages. Concordantly, immunostaining for dCAP-D3 and RBF1 in cryosections of the abdomens of wild type flies confirmed that both proteins are highly expressed in the adult and that they are both present in the nuclei of many cells in normal adult tissues (Figure 1B).
Fig. 1. RBF1 and dCAP-D3 are highly expressed at later stages of development and co-localize in adult tissues. 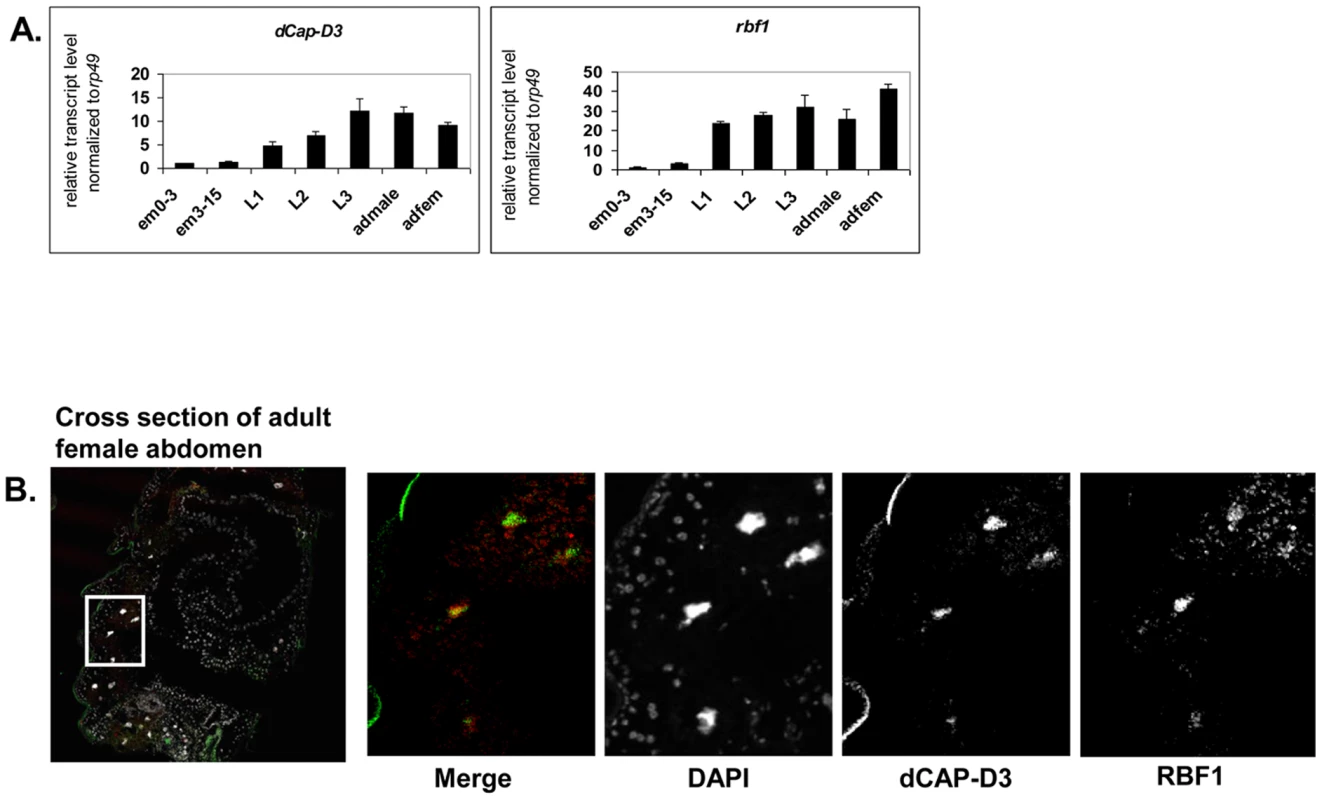
A) qRT-PCR for rbf1 transcript levels and dCap-D3 transcript levels in wild type Drosophila embryos aged for 0–3 hours (em0–3), embryos aged for 3–15 hours (em3–15), first instar larvae (L1), second instar larvae (L2), third instar larvae (L3), adult males (admale) or adult females (adfem) demonstrate high expression levels in the later life cycle stages. B) Immunostaining for RBF1 and dCAP-D3 in cryosections of adult female flies indicates co-localization in large nuclei of cells present underneath the cuticle. Images presented are a magnification of the area highlighted by the white box in the first image. Preliminary experiments showed that dCAP-D3 levels could influence the expression of very few of the previously identified RBF1-dependent transcripts. To gain a more complete understanding of the abundance and characteristics of RBF1/dCAP-D3 shared transcriptional targets, we carried out a microarray analysis of the entire Drosophila melanogaster genome and compared gene expression profiles of wild type, dCap-D3 and rbf1 mutant flies, at both the third instar larval and adult stages (Table S1). Since the null mutants are lethal, females expressing a transheterozygous combination of null and hypomorphic alleles were used for these experiments. The mutant flies used for microarray analysis expressed about 15% of wild type levels of each gene as judged by qRT-PCR and western blot (Figure S1). The microarray results revealed an extensive and highly significant overlap between RBF1 and dCAP-D3 regulated gene sets in both adults and larvae (Figure 2A and 2B). Shared target genes were evident in both upregulated and downregulated gene sets. Although some genes were mis-expressed in both larvae and adults, the majority of transcriptional changes were stage specific. The most highly significant p values for shared target gene sets were seen in upregulated larval genes (genes repressed by RBF1 and dCAP-D3 in the larvae, p≤6.34E-130) and downregulated adult genes (genes activated by RBF1 and dCAP-D3 in the adult, p≤9.88E-95) (Figure 2B). This suggests that RBF1 and dCAP-D3 may cooperate to repress specific programs during one stage of development and activate other programs in a later, more differentiated stage. Interestingly, at both stages, the genes dependent on both RBF1 and dCAP-D3 represented 15–17% of the total number of genes dependent on RBF1 in a given developmental stage and 47–55% of the total number of genes dependent on dCAP-D3 in a given developmental stage. Thus RBF1 appears to be important at close to half of the transcriptional targets of dCAP-D3.
Fig. 2. RBF1 and dCAP-D3 regulate many of the same transcripts in the fly. 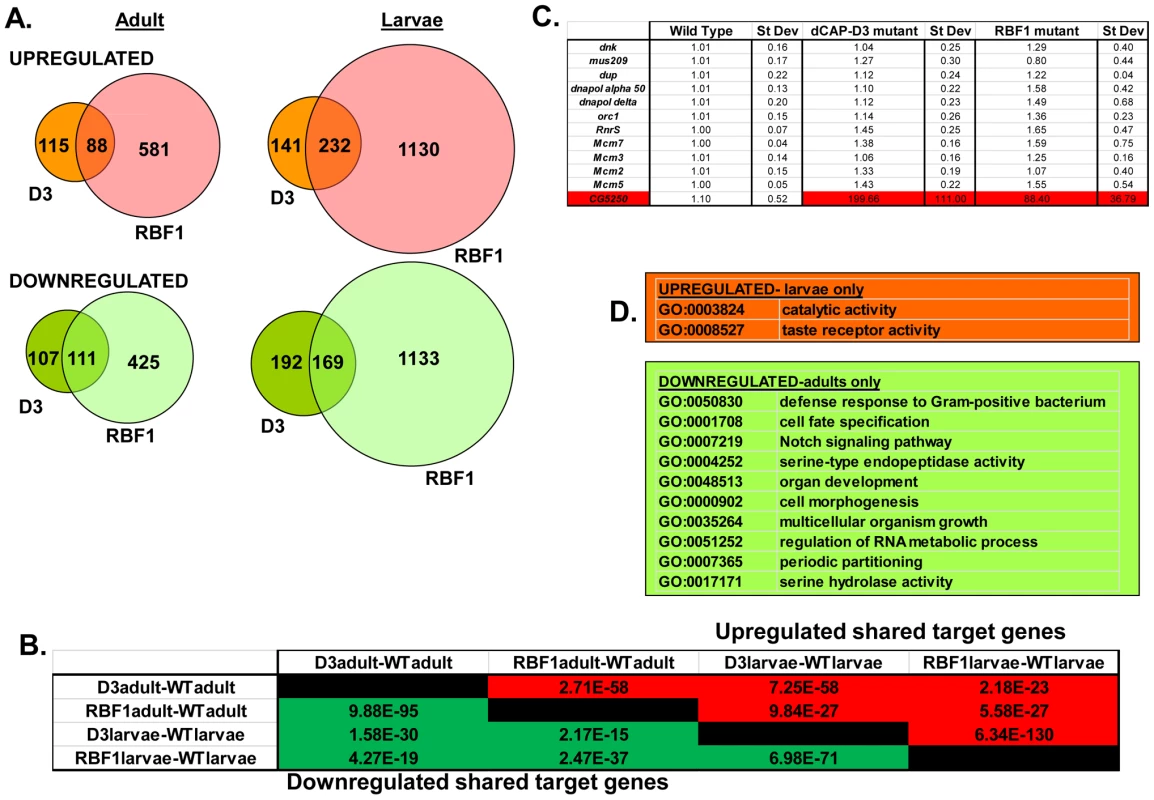
RNA was isolated from rbf1 mutant and dCap-D3 mutant female third instar larvae and adult flies. cDNA was hybridized to Nimblegen 385 k whole genome arrays. A) Venn diagrams show the numbers of RBF1, dCAP-D3 or RBF1/dCAP-D3 shared target genes which exhibited at least a 2 fold log change in expression with a p value of ≤0.15. Genes significantly upregulated in the mutant flies are shown in red while genes significantly downregulated are shown in green. B) P values for shared RBF1 and dCAP-D3 target genes indicate that RBF1 and dCAP-D3 regulate a significant number of the same genes in both adults and larvae. The numbers above the diagonal represent p-values for upregulated shared subsets and are colored red while the numbers below the diagonal represent p-values for downregulated shared genes and are colored green. C) qRT-PCR analyses of 12 E2F targets shows that the majority of RBF1/dCAP-D3 shared targets are not E2F targets. The one target that was significantly upregulated in dCAP-D3 and RBF1 mutant flies, CG5250, is highlighted in red. Results are the average of three independent experiments involving 10 female flies per genotype. D) Significant (p≤0.05) Gene Ontology (GO) groupings for shared target genes include defense response genes in the adult fly. The top box lists GO categories for upregulated shared genes in mutant larvae only, and the bottom box lists selected GO categories for downregulated shared genes in adults only. There were no significant GO groupings for upregulated shared target genes in adults or for downregulated shared target genes in the larvae. Characteristics of RBF1/dCAP-D3 shared target genes
We noticed that the lists of RBF1/dCAP-D3 shared target genes had two general properties. First, these genes are almost completely different from the lists of E2F-regulated genes that have been reported previously [26]. As expected, many of the targets that were upregulated in rbf1 mutant larvae could be categorized as E2F target genes involved in DNA repair, DNA replication and continuation of the cell cycle (comparison to microarray data from [26] and GO analyses of rbf1 mutant larvae - data not shown). However, few if any, of these cell cycle/proliferation related genes were altered in the dCap-D3 mutant flies (Figure 2C) suggesting that dCap-D3 regulates a different subset of RBF1 dependent targets. In fact, less than 6% of dCAP-D3/RBF1 shared target genes in larvae were found to be bound by dE2F1 in dE2F1 ChIP-chip experiments (Korenjak et. al., unpublished data). Unexpectedly, many of the known E2F target genes did not show a significant increase in expression in rbf1 mutant adults (Figure 2C). This may reflect cell-type specific differences in the requirement for RBF1. In support of this idea, qRT-PCR analysis of dissected tissues showed that few E2F-regulated genes were upregulated in ovaries of rbf1 mutants, but many did show a significant increase in the rest of the carcass (Figure S2). However, even in the tissues where these E2F-regulated proliferation genes did increase in expression levels in rbf1 mutant adults, these transcripts were not upregulated in tissues from dCap-D3 mutant flies (Figure S2). We infer that dCAP-D3 is not a key factor at most of the well-characterized E2F regulated genes in either larvae or adults. While unlikely, it is a formal possibility that the remaining amounts of dCAP-D3 protein present in the hypomorphic mutant flies might be sufficient for the regulation of E2F targets, but not for other target genes.
Second, we noted that genes that are similarly dependent on RBF1 and dCAP-D3 tend to be clustered on the genome and are often positioned within 10 kb of one another (Table 1). To determine whether this was an unusual feature, we compared the frequency of RBF1/dCAP-D3shared target genes positioned within 10 kb of one another to hundreds of simulations of randomly chosen Drosophila genes (Table 1). The results showed that genes exhibiting increased expression in rbf1 and dCap-D3 mutant adults (ie. genes that are apparently repressed both by RBF1 and dCAP-D3 are 25 times more likely to be clustered. Genes that were downregulated in rbf1 and dCap-D3 mutant adults (i.e. genes apparently activated by both RBF1 and dCAP-D3) are 15 times more likely to be clustered. Clustering of shared target genes was also seen in the larvae, although the fold difference was greatly diminished (5 fold) for the activated genes. Overall, the clustering effect was 3–7 fold more prevalent in dCAP-D3 regulated genes than in RBF-regulated genes. By way of comparison, RBF1/dCAP-D3 shared target genes in the larvae exhibited a much greater degree of clustering than the larval genes regulated by Hop or Nurf301, two other well-known chromatin remodeling proteins shown to regulate clusters of genes [27]. A list of the actual groupings of clustered genes is presented in Table S2.
Tab. 1. RBF1 and dCAP-D3 tend to regulate clusters of genes. 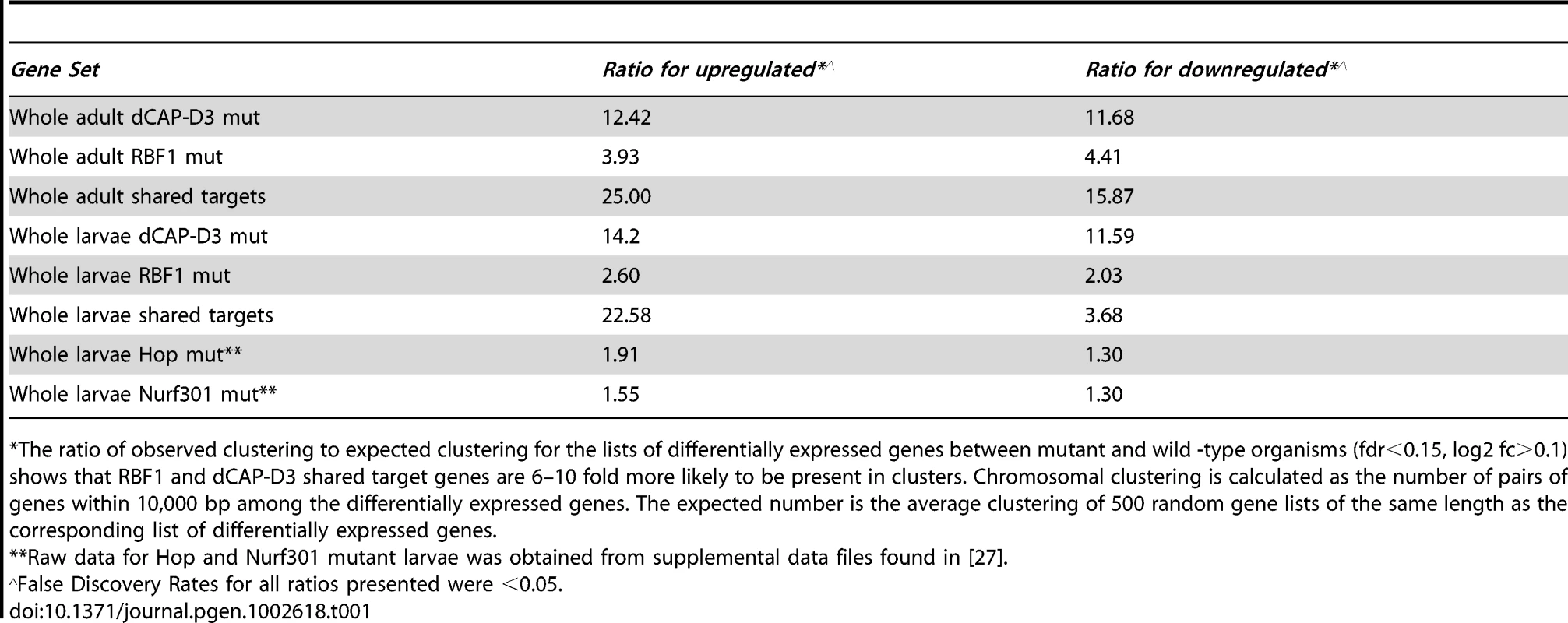
The ratio of observed clustering to expected clustering for the lists of differentially expressed genes between mutant and wild -type organisms (fdr<0.15, log2 fc>0.1) shows that RBF1 and dCAP-D3 shared target genes are 6–10 fold more likely to be present in clusters. Chromosomal clustering is calculated as the number of pairs of genes within 10,000 bp among the differentially expressed genes. The expected number is the average clustering of 500 random gene lists of the same length as the corresponding list of differentially expressed genes. Although proliferation-related genes were missing, gene ontology (GO) classification of the RBF1/dCAP-D3 shared target genes revealed many significant categories in the lists of up - and downregulated genes in the adult, and for shared, repressed target genes in the larvae (Figure 2D, complete list for downregulated adult genes in Table S3). One of the most interesting GO categories represented in the adults were the defense response genes (GO:0050830). The fly relies on an innate immune system to defend against invading pathogens. This immune system is comprised of three major mechanisms: 1) phagocytosis, 2) induction of coagulation and melanization, and 3) production of Antimicrobial Peptides or AMPs.
Phagocytosis is a conserved mechanism that is often the primary cellular defense used by many organisms to engulf and destroy pathogens. In Drosophila, circulating blood cells called hemocytes phagocytose bacteria, fungi, and parasitic wasp eggs [28]. RBF1 and dCAP-D3 mutant adult microarray data was analyzed for changes in levels of 19 different genes reported to be involved in phagocytosis in Drosophila (Table S4). Of these 19 genes, 2 genes demonstrated significant changes in transcript levels in adults. NimC1, a gene expressed in plasmatocytes which make up 95% of Drosophila hemocytes, has been shown to be necessary for phagocytosis of bacteria [29], and was significantly upregulated in RBF1 and dCAP-D3 mutant adults. Embryonic and larval hematopoiesis depends on a number of transcription factors including Gcm [30], [31]. Gcm transcripts were demonstrated to be downregulated in both RBF1 and dCAP-D3 adults (Table S4). While adult hemocytes do display phagocytic properties, they do not differentiate into specialized cells upon immune challenge [32], [33], and it is therefore unlikely that misregulation of gcm in adults would affect phagocyte numbers.
In response to septic injury, proteolytic cascades are triggered which lead to coagulation and melanization. Reactive oxygen species formed during these processes, as well as the actual deposition of melanin, are thought to be toxic to microorganisms [34]. After scanning the literature for genes involved in coagulation and melanization, and then analyzing RBF1 and dCAP-D3 mutant adult microarray data for changes in transcript levels of these genes, it was determined that only one of the reported genes, CG8193 was significantly increased in both RBF1 and dCAP-D3 mutant adults (Table S5). CG8193/PPO2 is thought to encode a phenol-oxidase constitutively expressed in crystal cells, a type of hemocyte cell involved in melanization [35]. However, overexpression of CG8193/PPO2 in cell lines or in flies does not induce constituitive melanization [36], nor did we see any evidence of melanotic lesions in RBF1 or dCAP-D3 mutant adults.
Several of the genes in the adult, downregulated GO category of “defense response to Gram positive bacteria” (Figure 2D and Table S3) fall into a family of proteins known as Antimicrobial Peptides or AMPs. In fact, two of these genes, AttA and AttB, represented some of the most highly deregulated targets in the mutant adults. Upon closer inspection of the microarray data, it was revealed that many other AMP genes were also deregulated in dCAP-D3 and/or RBF1 mutant adults, however their p-values were just below the confidence level. In addition, many of the AMP genes are present in clusters and located immediately next to one another in the genome (Figure 3A), making them an enticing group of genes for further study.
Fig. 3. RBF1 and dCAP-D3 activate basal transcript levels of genes coding for Antimicrobial Peptides (AMPs). 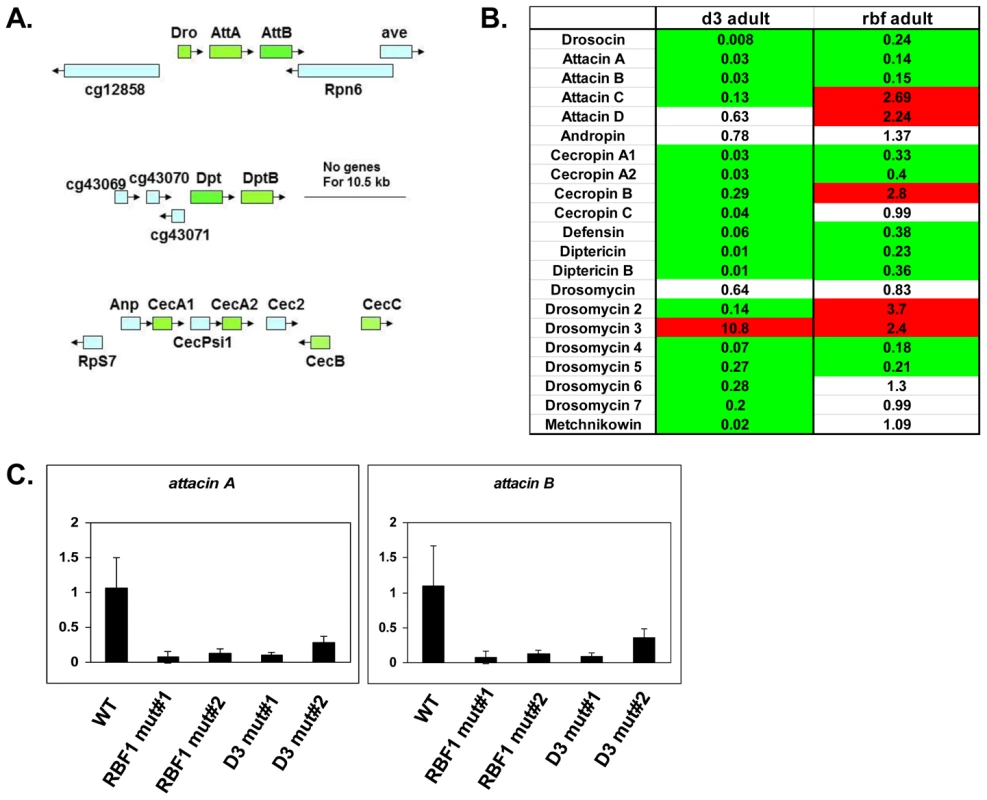
A) Graphic depictions of three separate AMP loci. The Attacin and Diptericin loci are located on Chromosome 2R at 51C1 and 55F8, respectively. The Cecropin locus is located on Chromosome 3R at 99E2. Genes within each locus are drawn in correct orientation to one another but are not drawn to scale. Genes colored in green are downregulated in dCAP-D3 mutant adult carcasses dissected of ovaries and most are also downregulated RBF1 mutants. Genes colored in blue remain unchanged in dCAP-D3 and RBF1 mutants. B) qRT-PCR analyses of transcript levels for 21 AMPs in female adult bodies (N = 10) with ovaries dissected shows that dCAP-D3 and RBF1 each regulate a much larger number of AMPs than originally indicated by the microarray results. Genes significantly upregulated in the mutants are highlighted in red and genes significantly downregulated in the mutants are highlighted in green. Transcript levels were normalized to tubulin 84B. All results had false discovery rates ≤0.05. C) qRT-PCR analysis of cDNA from wild type (WT/w1118), rbf1 mutant #1 (rbf1120a/rbf1Δ14), rbf1 mutant #2 (rbf1120a/rbf1120a), dCap-D3 mutant #1 (dCap-D3c07081/dCap-D3Δ25) and dCap-D3 mutant #2 (dCap-D3c07081/dCap-D3c07081) female adult whole flies confirms that two AMP target genes are regulated by both RBF1 and dCAP-D3, regardless of mutant genotype. Transcript levels were normalized to tubulin 84B mRNA levels. AMPs are shared targets of RBF1 and dCAP-D3 in the adult fat body
To confirm that the transcription of AMPs was indeed dependent on both RBF1 and dCAP-D3, qRT-PCR analysis was performed using cDNA generated from dCap-D3 or rbf1 transheterozygotes (using whole female mutant flies whose ovaries had been dissected) (Figure 3B). Results showed that 17 of the 21 AMPs tested were downregulated in the Cap-D3 mutants and 10 of those genes were similarly dependent on RBF1. qRT-PCR for AMPs performed on different allelic combinations of rbf1 and Cap-D3 mutants gave similar results (Figure 3C).
AMPs constitute one of the major defense mechanisms against bacterial and/or fungal infection in the fly [25], [37], [38]. They are produced in various adult tissues but one of the main organs responsible for their production is the fat body. Once produced in the fat body, AMPs are secreted into the hemolymph where they destroy or inhibit growth of pathogens [39].
We set out to test the hypothesis that RBF1 and dCAP-D3 regulate AMP genes in the adult fat body. First, we examined whether RBF1 and dCAP-D3 are expressed in this cell type. The yolk-GAL4 driver was used to express GFP in adult fat body cells, effectively marking this cell type in green. Combined immunostaining for RBF1 and dCAP-D3 localization in cryosections of adult wild type abdomens revealed a strong staining for both RBF1 and dCAP-D3 in the nuclei of adult fat body cells (Figure 4A, yellow arrows). yolk-GAL4 has been characterized to drive expression in Drosophila at approximately 2–5 days post eclosure [40], making it possible to drive expression of transgenes after the majority of fly development has occurred. The staining for RBF1 and dCAP-D3 in the adult abdomens was specific, as yolk-GAL4 driven expression of dsRNAs directed against RBF1 and dCAP-D3 specifically abrogated staining of their respective targets in fat body cells, without dramatically altering gross tissue morphology (Figure S3).
Fig. 4. Basal AMP transcript levels are activated by RBF1 and dCAP-D3 specifically in the fat body. 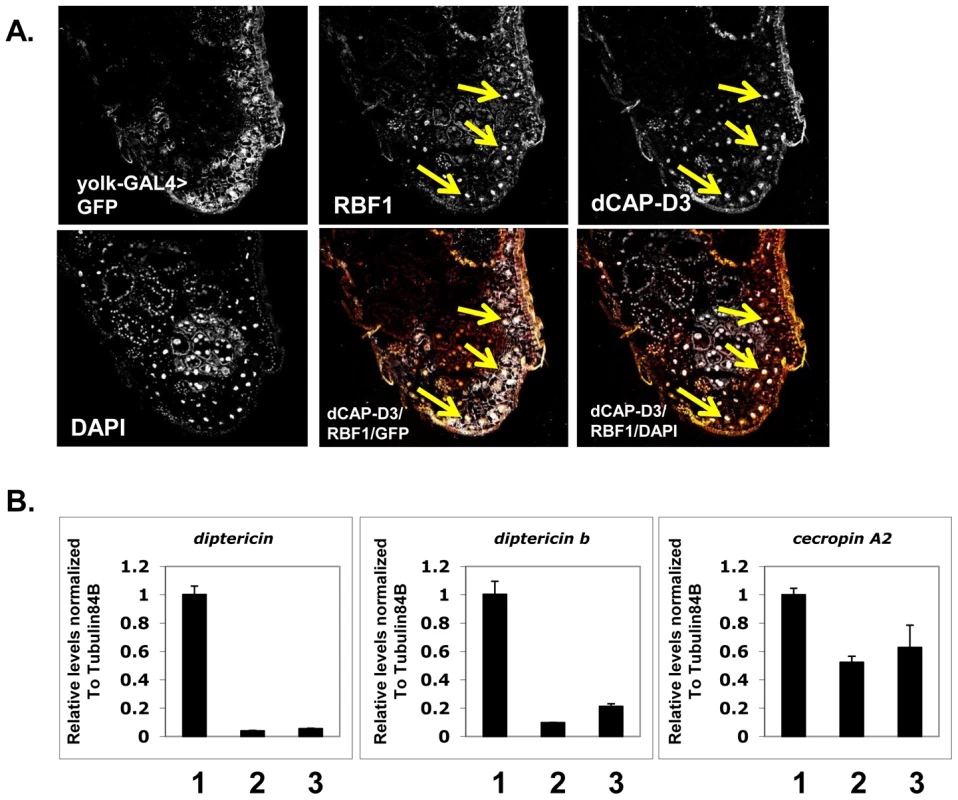
A) Immunofluorescence analysis of RBF1 and dCAP-D3 performed on cryosections of adult female flies expressing GFP under the control of the fat body specific yolk-GAL4 driver indicates that RBF1 and dCAP-D3 co-localize in the nuclei of fat body cells. Yellow arrows highlight fat body cells. B) qRT-PCR analysis of cDNA from 1) flies expressing driver alone (yolk-GAL4/+;+;+), 2) flies expressing rbf1 dsRNA (yolk-GAL4;+;UAS-rbf1 dsRNA) in the fat body cells and 3) flies expressing dCAP-D3 dsRNA (yolk-GAL4;+;UAS-dCAP-D3 dsRNA) in the fat body cells shows significant decreases in AMP levels. For each genotype, N = 10. Next, we measured the changes in expression of AMPs in animals where yolk-GAL4 driven expression of dsRNAs had reduced the expression of either RBF1 or dCAP-D3 in the fat body. qRT-PCR of cDNA from whole adult females showed a significant decrease in the expression of multiple AMP genes including diptericin, diptericin B and Cecropin A2 (Figure 4B) in the knockdown flies. Interestingly, the fold change in transcript levels for diptericin was comparable to the changes seen in dCap-D3 and rbf1 mutant animals. These results suggest that the yolk-GAL4-expressing cells are a primary site of constitutive diptericin expression in adult flies and that in these cells, RBF1 and dCAP-D3 are both needed to drive the basal expression levels of specific AMPs.
Regulation of an AMP cluster by RBF1 and dCAP-D3 is direct and dynamically changes over the course of bacterial infection
AMP genes can be regulated by multiple transcription factors [41]–[44]. We sought to determine, therefore, whether transcriptional regulation of these genes by RBF1 and dCAP-D3 was direct. For our ChIP analysis we focused on diptericin and diptericin B; two AMP genes that are situated within 1200 bp of one another (Figure 5B), that have well characterized promoters [45]–[47], and whose basal expression was dependent on both RBF1 and dCAP-D3 in the fat body (Figure 3B and Figure 4B). In addition, the basal transcript levels of at least one other gene in the region, CG43070, was found to be significantly activated by both RBF1 and dCAP-D3 (Figure S4).
Fig. 5. RBF1 and dCAP-D3 bind to an AMP locus in vivo. 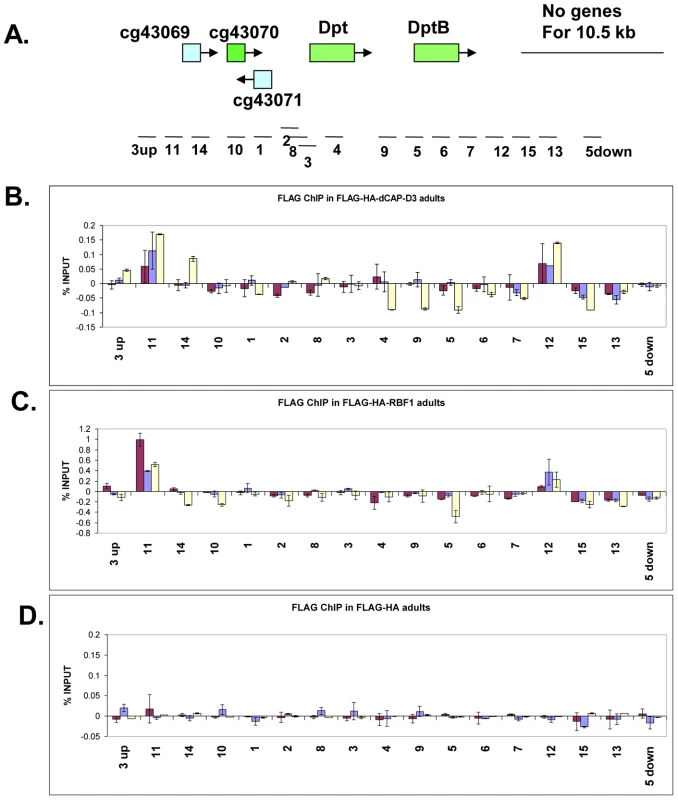
A) Graphic representation of the locus on which Chromatin Immunoprecipitation (ChIP) for RBF1 and dCAP-D3 in the adult fat body was performed. Genes highlighted in green are activated by both RBF1 and dCAP-D3 in the fat body. Positions of primer sets used are listed under the diagram of the locus. B) Chromatin immunoprecipitation for FLAG protein in female adult flies expressing FLAG-HA-dCAP-D3 in the fat body (yolk-GAL4/+; +; UAS-FLAG-HA-dCap-D3/+) demonstrates that the diptericin locus is a direct target of dCAP-D3. ChIP signal corresponding to FLAG-HA-dCAP-D3 binding in the absence of Staphylococcus aureus infection is colored in burgundy. ChIP signal corresponding to FLAG-HA-dCAP-D3 binding two and four hours after S. aureus infection is colored in blue and yellow, respectively. C) Chromatin immunoprecipitation for FLAG protein in female adult flies expressing FLAG-HA-RBF1 in the fat body (yolk-GAL4/+; +; UAS-FLAG-HA-rbf1/+) demonstrates that the locus is also a direct target of RBF1. Depiction of signal is as described in B. D) Chromatin immunoprecipitation for FLAG protein in female adult flies expressing FLAG-HA in the fat body (yolk-GAL4/+; +; UAS-FLAG-HA/+) demonstrates minimal non-specific binding of tag alone at the locus. Depiction of signal is as described in B. To study the binding of RBF1 and dCAP-D3 at the diptericin locus in vivo, transgenic fly lines were created which expressed N-terminally FLAG-HA tagged dCAP-D3 or N-terminally FLAG-HA tagged RBF1 under the control of the UAS promoter. These lines were then crossed to yolk-GAL4/FM7 lines to create progeny in which the tagged protein was specifically expressed in the adult fat body. ChIP using FLAG antibody in FLAG-HA-dCAP-D3 expressing flies demonstrated that dCAP-D3 binds to two separate regions located approximately 3 kb upstream and 900 bp downstream of the diptericin locus (Figure 5A and red bars in Figure 5B). Since diptericin is strongly induced in response to bacterial infection, we examined the effect of infection with S. aureus on the binding of dCAP-D3 to the diptericin locus. Strikingly, dCAP-D3 binding to the upstream site significantly increased after S. aureus infection (compare red bars to yellow bars, Figure 5B).
ChIP for FLAG in FLAG-HA-RBF1 expressing flies indicated that RBF1 binds to the identical upstream and downstream regions of the diptericin locus as dCAP-D3 (red bars in Figure 5C). This binding was detected both before and after infection with S. aureus (blue and yellow bars in Figure 5C), but unlike the results for dCAP-D3 binding, RBF1 binding was most significant prior to infection. ChIP for FLAG protein in flies expressing the FLAG-HA construct alone showed almost no signal at any of the primer sets used in these experiments (Figure 5D). Taken together, ChIP results show that 1) RBF1 and dCAP-D3 can bind directly to an AMP gene cluster at identical binding sites, 2) that the binding sites flank the diptericin and diptericin B genes, and 3) dCAP-D3 binding increases when gene expression is induced in response to bacterial infection.
For comparison, we also performed ChIP for dCAP-D3 on the CG5250 locus. CG5250 was the one previously identified direct target of RBF1 [26] that we found to be repressed by RBF1 and dCAP-D3 and to be consistently upregulated in all tissues of rbf1 and dCap-D3 mutant animals (Figures S2 and S5A). ChIP using FLAG antibody in FLAG-HA-dCAP-D3 expressing flies demonstrated a small amount of binding in the open reading frame of CG5250 (Figure S5B and S5C). This binding pattern obtained with the FLAG antibody closely resembled the ChIP signal found when a dCAP-D3 antibody was used to immunoprecipiate the endogenous dCAP-D3 protein expressed everywhere in the adult fly (Figure S5D).
The ability of RBF1 and dCAP-D3 to regulate basal levels of AMP transcription prompted the question of whether these proteins were also necessary for the regulation of AMP transcription in response to bacterial infection. cDNA was generated from female adult flies expressing dCAP-D3 or RBF1 dsRNAs specifically in the fat body, at various time-points post-infection with Staphylococcus aureus (Figure 6). dsRNAs have been used successfully in the past to decrease in vivo expression levels of proteins involved in innate immunity and to study their effects on responses to bacterial infection [48].
Fig. 6. Complete AMP induction following bacterial infection depends on dCAP-D3 and RBF1. 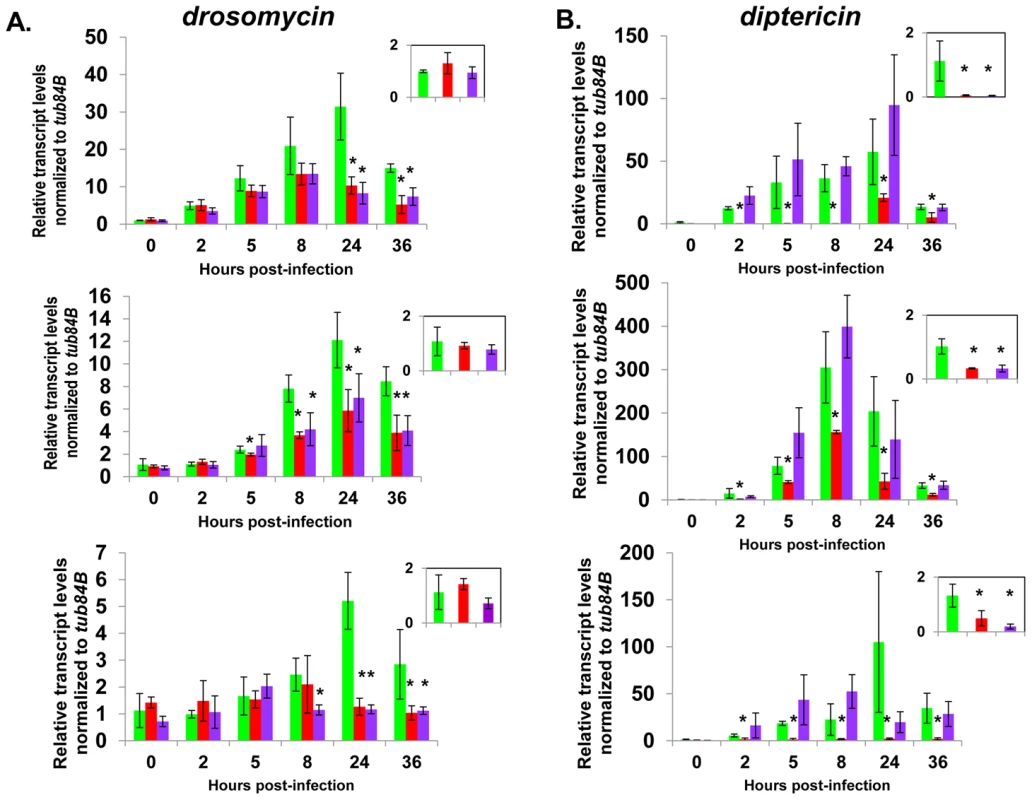
Adult female flies expressing RBF1 (purple) or dCAP-D3 (red) dsRNAs under the control of yolk-GAL4 were infected with the Gram positive bacteria, Staphylococcus aureus. A) qRT-PCR analyses for transcript levels of the Drosomycin AMP gene in these flies show that while flies expressing GFP dsRNAs under the control of yolk-GAL4 (green) undergo a large induction of AMPs at 8–24 hours post-infection, flies expressing dCAP-D3 or RBF1 dsRNA in the fat body fail to exhibit maximal, sustained induction. B) qRT-PCR analyses for transcript levels of the Diptericin AMP gene in these flies show that while flies expressing GFP or RBF1 dsRNAs under the control of yolk-GAL4 (green) undergo a large induction of AMPs at 8–24 hours post-infection, flies expressing dCAP-D3 dsRNA in the fat body fail to exhibit maximal, sustained induction. The inset boxes in the upper right corner of each graph are a larger representation of the 0 hour timepoint and depict basal transcription levels. Asterisks emphasize statistical significance (p≤0.05) as determined by a students paired t-test. Three independent experiments are shown and results for each experiment are the average of three sets of five infected adults per genotype, per timepoint. qRT-PCR for AMPs indicated two types of transcriptional defects in the RBF1 and dCAP-D3 deficient flies. In agreement with our earlier results, basal transcript levels of diptericin were reduced as a result of deficiency for either protein (Figure 6B, inset boxes). Following infection, diptericin transcripts remained very low in the dCAP-D3 deficient tissue and induction was minimal and severely delayed in comparison to GFP dsRNA expressing “wild type” control flies. RBF1 deficiency, however, allowed normal induction of diptericin transcripts. Drosomycin is an AMP gene downstream of the Toll pathway, and it is strongly induced following infection with Gram positive bacteria or fungi [49]. qRT-PCR for levels of Drosomycin revealed a much different defect in expression. Neither dCAP-D3 nor RBF1 deficiency in the fat body had any effect on basal levels of Drosomycin, a result consistent with our microarray data from whole flies. However, both dCAP-D3 and RBF1 deficiency caused significant decreases in the maximal expression levels of drosomycin at 24 hours post-infection (Figure 6A).
The biological response to bacterial infection in the fly requires dCAP-D3 and RBF1
Next, we tested whether the inefficient transcription of AMPs that results from decreased expression of RBF1 or dCAP-D3 has a significant effect on the ability of the fly to recover from exposure to pathogenic bacteria. Survival rates after infection with the Gram positive bacterium, Staphylococcus aureus (Figure 7A, Figure 8A) or with the Gram negative bacterium, Pseudomonas aeruginosa (Figure 7B, Figure 8B) were measured in five different genotypes: females expressing GFP dsRNAs under the control of the yolk-GAL4 driver (“wild-type controls”, yolk-GAL4 driving expression of dCAP-D3 dsRNA in the fat body, yolk-GAL4 driving expression of RBF1 dsRNA in the fat body, and positive control females which were either mutant for the Eater protein or expressing dsRNAs against the IMD protein. IMD is a major mediator of innate immune signaling in Drosophila [50]. Eater is a known phagocyctic receptor necessary for the response to infection with Gram positive bacteria [51]. We did not include data on flies expressing Eater dsRNAs under the control of yolk-GAL4, since Kocks et al [51] reported that Eater is not expressed in the fat body. In agreement with this, expression of Eater dsRNA in the fat body exhibited no changes in the ability of the fly to clear bacteria, while the Eater mutants described above showed a striking inability to phagocytize bacteria 5 hours following infection (data not shown and Figure 7). Following infection with S. aureus, both dCAP-D3 and RBF1 deficient flies were more susceptible to infection in comparison to flies expressing GFP dsRNAs (Figure 7A and Figure 8A). dCAP-D3 deficient flies were also more susceptible to infection with Gram negative bacteria, but this was not the case for RBF1 deficient flies, as their survival rates were not significantly decreased (Figure 7B and Figure 8B). These data demonstrate that acute knockdown of dCAP-D3 or RBF1 in the fat body of adult flies renders them more susceptible to bacterial infection, most likely due to inefficient transcription of AMP genes.
Fig. 7. dCAP-D3 is necessary for a proper immune response to bacterial infection. 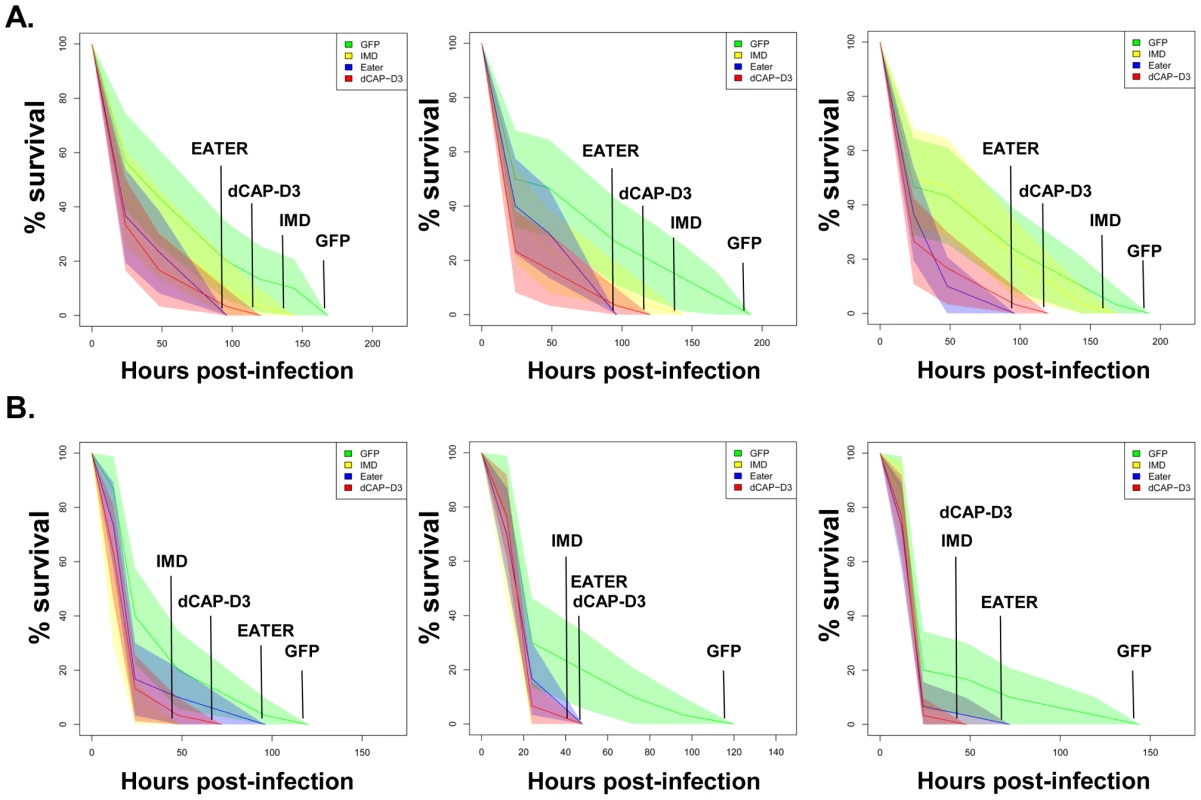
Adult female flies expressing dCAP-D3 (red) dsRNAs under the control of yolk-GAL4 were infected with the Gram positive bacterium, Staphylococcus aureus (A) or the Gram negative bacterium, Pseudomonas aeruginosa (B). Flies expressing GFP dsRNAs under the control of yolk-GAL4 (green) were used as “wild-type” controls. Eater mutants which are defective in phagocytosis (blue) or flies expressing IMD dsRNAs which are compromised in a major innate immune signaling pathway (yellow) were used as positive controls. Results demonstrate that flies expressing reduced levels of dCAP-D3 in the fat body cells are more susceptible to either type of infection than wild type controls. Three independent experiments are depicted with results of each experiment shown as the average of three sets of 10 infected adults per genotype. These experiments were also performed using a sterile needle dipped in PBS to rule out death as a result of wounding and survival curves matched those of yolk-GAL4 expressing flies (data not shown). Results are presented as cox regression models with statistical significance (p≤0.05) represented as shaded areas above and below the curves. Fig. 8. RBF1 is necessary for a proper immune response to Gram positive bacterial infection. 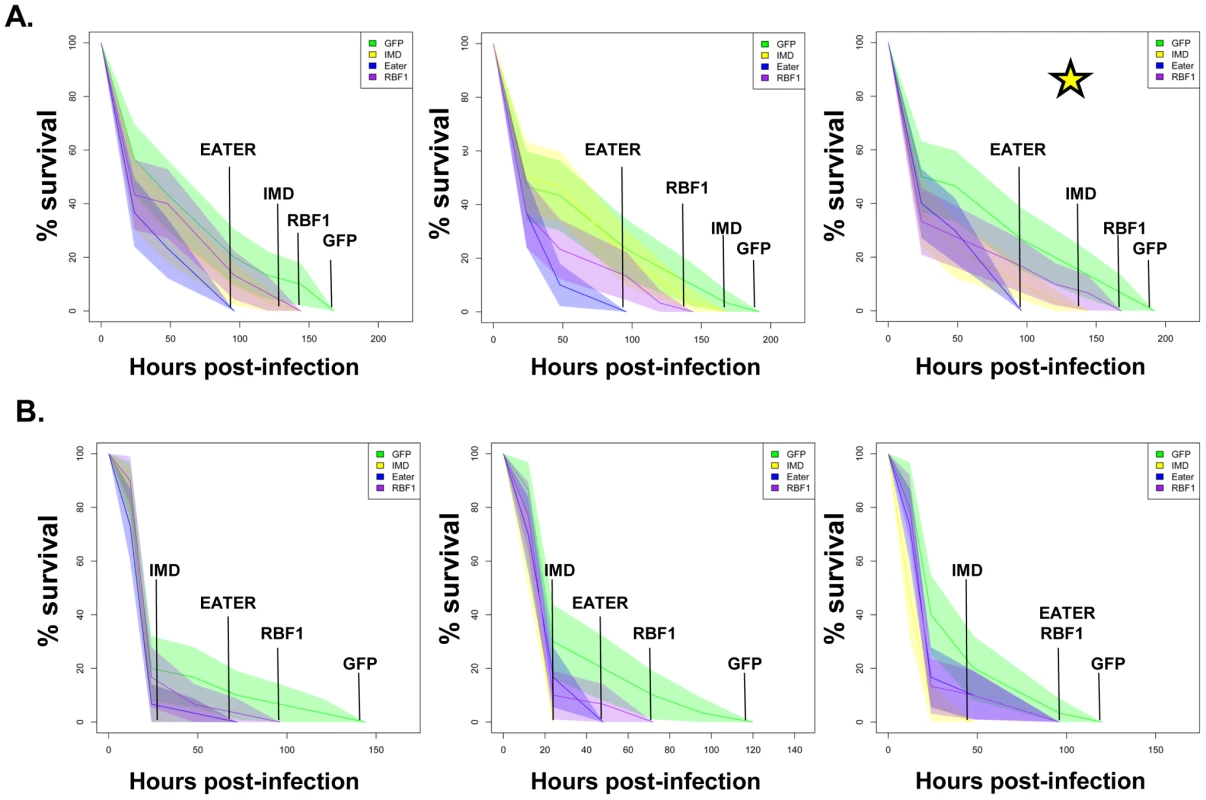
Adult female flies expressing RBF1 (purple) dsRNAs under the control of yolk-GAL4 were infected with the Gram positive bacterium, Staphylococcus aureus (A) or the Gram negative bacterium, Pseudomonas aeruginosa (B). Flies expressing GFP dsRNAs under the control of yolk-GAL4 (green) were used as “wild-type” controls. Eater mutants which are defective in phagocytosis (blue) or flies expressing IMD dsRNAs which are compromised in a major innate immune signaling pathway (yellow) were used as positive controls. Results demonstrate that flies expressing reduced levels of RBF1 in the fat body cells are more susceptible to infection with Gram positive bacteria (A) than wild type controls. Three independent experiments are depicted with results of each experiment shown as the average of three sets of 10 infected adults per genotype. Results are presented as cox regression models with statistical significance (p≤0.05) represented as shaded areas above and below the curves. In the third experiment in (A), which is highlighted by a star, the survival endpoint becomes significant when the confidence level is changed to 90% (p≤0.10) instead of 95% (p≤0.05). These experiments were also performed using a sterile needle dipped in PBS to rule out death as a result of wounding and survival curves matched those of yolk-GAL4 expressing flies (data not shown). Recently, a number of reports have identified genes whose mutation can reduce the ability of the fly to survive bacterial infection, without influencing the ability of the fly to clear bacteria [52]–[54]. These genes have been described as having affects not on the resistance mechanisms which exist in the fly, but on the tolerance mechanisms of the fly. Tolerance mechanisms limit the damage caused to the host by the infection, but do not actually limit the pathogen burden [55]. To determine whether loss of dCAP-D3 and/or RBF1 expression in the fat body did indeed result in diminished capacity of the fly to clear bacteria, we performed bacterial clearance assays and measured the number of bacteria present in the fly from 0–20 hours post-infection (Figure 9). Results showed that flies deficient for RBF1 or dCAP-D3 behave more like positive control flies deficient for IMD or Eater proteins, and exhibit significant increases in bacterial numbers at 15 hours post-infection with S. aureus. This suggests that RBF1 and dCAP-D3 most likely affect the resistance mechanisms (i.e. AMP transcription), and not the tolerance mechanisms of the fly.
Fig. 9. RBF1 and dCAP-D3 are necessary for the ability to clear bacteria in vivo. 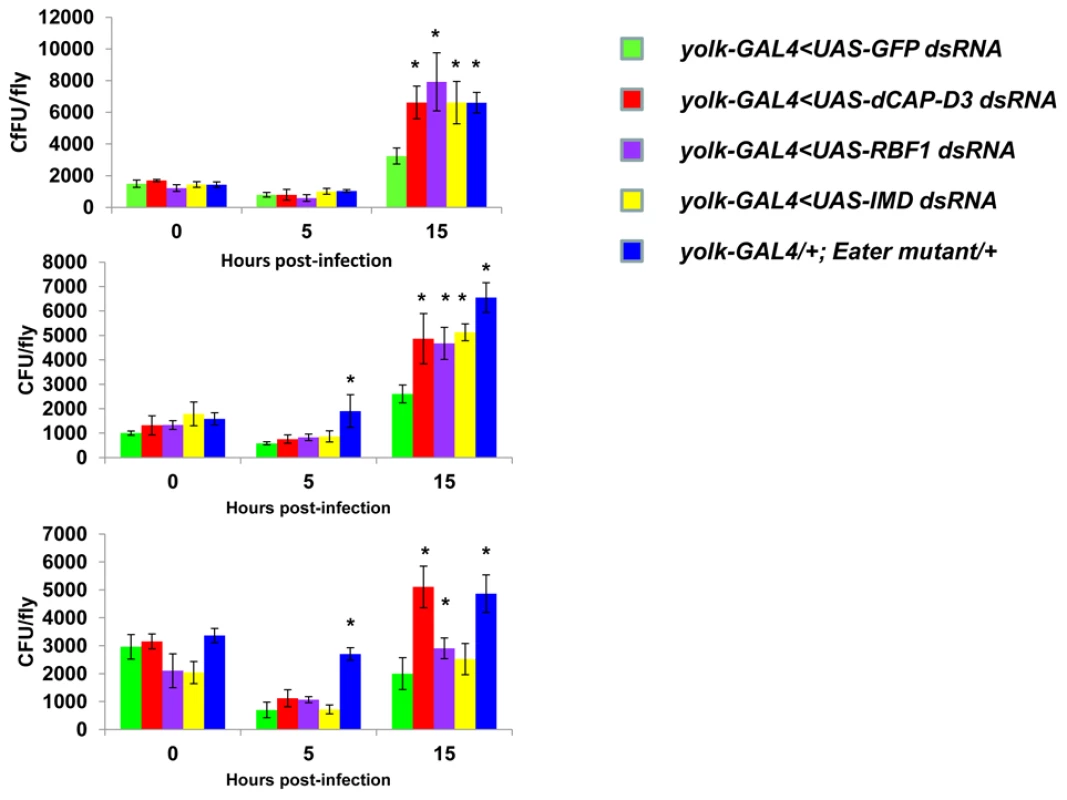
Adult female flies expressing RBF1 (purple) or dCAP-D3 (red) dsRNAs under the control of yolk-GAL4 were infected with the Gram positive bacterium, Staphylococcus aureus. Flies expressing GFP dsRNAs under the control of yolk-GAL4 (green) were used as “wild-type” controls. Eater mutants which are defective in phagocytosis (blue) or flies expressing IMD dsRNAs which are compromised in a major innate immune signaling pathway (yellow) were used as positive controls. Results demonstrate that at 15 hours following infection, flies expressing reduced levels of dCAP-D3 or RBF1 in the fat body cells exhibit increased numbers of bacteria in comparison to wild type controls. Three independent experiments are shown and results for each experiment are the average of three sets of three infected adults, per genotype, per timepoint. Asterisks emphasize statistical significance (p≤0.05) as determined by a students paired t-test. A second RBF family member, RBF2, regulates basal AMP transcription levels, but not induced levels, following infection
Since the observed defects in survival rates and AMP induction were not as severe for RBF1 deficient flies in comparison to dCAP-D3 deficient flies, we wondered whether the other Drosophila RBF member, RBF2, might compensate for loss of RBF1 activity. RBF2 has been shown to be upregulated upon depletion of RBF1, and co-regulates many genes with RBF1 as a part of the dREAM complex [26], [56], [57]. To address this question, we tested survival times and AMP induction in flies deficient for RBF2 in the fat body (yolk-GAL4<UAS-RBF2-dsRNA) or a combination of both RBF1 and RBF2 in the fat body (yolk-GAL4<UAS-RBF1 dsRNA, UAS-RBF2 dsRNA). The specific deficiencies in these flies were confirmed by qRT-PCR (Figure S6). qRT-PCR revealed that similar to the loss of RBF1 or dCAP-D3, loss of RBF2 or RBF1/RBF2 resulted in decreased basal transcript levels of diptericin but not drosomycin (Figure S7A and S7B, inset boxes). However, following infection with S. aureus, loss of RBF2 or RBF1/RBF2 did not cause decreased induction of either AMP transcript. In some cases, loss of both RBF1 and RBF2 actually resulted in an increase in diptericin transcription levels at 8 hours post infection. In response to infection with Gram positive bacteria (Figure S8A) or Gram negative bacteria (Figure S8B), RBF2 deficient or RBF1/RBF2 deficient flies did not exhibit any changes in survival rates that were significantly different from wild type control flies. These results demonstrate that RBF2 does regulate basal AMP transcript levels, but does not compensate for RBF1 in induction of AMP transcription in Drosophila following infection.
The shared regulation of innate immune gene clusters by RBF1 and dCAP-D3 may be conserved in human cells
AMPs are conserved in many metazoans and play a very important role in fighting pathogens in barrier epithelial cells at mucosal surfaces [58]. pRB and CAP-D3 have been previously shown to interact physically and functionally in human cells [11]. Remarkably, and perhaps unexpectedly, the regulation of AMP genes by RB and CAP-D3 proteins may also be conserved in human cells. To determine whether pRB and CAP-D3 could regulate genes in human cells and, more specifically, whether the co-regulation of AMPs was conserved, siRNAs were used to decrease pRB and CAP-D3 expression in human Retinal Pigment Epithelial (RPE-1) cells and in premonocytic U937 cells. (Figure S9A and data not shown). qRT-PCR analyses of the levels of five different AMPs revealed that two AMPs (DEFB-3 and DEFA-1) were expressed in RPE-1 cells and both genes were significantly downregulated following the depletion of either pRB or CAP-D3 (Figure S9B). Interestingly, these genes are also located in a very large gene cluster, the Defensin locus, encompassing over 20 different AMPs. These data raise the possibility that the regulation of AMPs by CAP-D3 and pRB, and the ability of these proteins to regulate gene clusters, are properties that may be conserved in human cells.
Discussion
In Drosophila, RB-family proteins are best known as transcriptional repressors of cell cycle and proliferation genes. Here we describe a different aspect of RB function and show that, together with the Condensin II protein dCAP-D3, RBF1 functions to regulate the expression of a large number of genes during Drosophila development. A surprising characteristic of RBF1/dCAP-D3 regulated genes is that they do not seem to be the classically repressed genes with functions in cell cycle progression, DNA damage and DNA replication. Instead, many RBF1/dCAP-D3-dependent genes are classified as being involved in cell-type specific functions and include genes that are involved in enzymatic cascades, organ development and cell fate commitment.
The idea that dCAP-D3 and RBF1 could cooperate to promote tissue development and differentiation is supported by the fact that both proteins are most highly expressed in the late stages of the fly life cycle, and accumulate at high levels in the nuclei of specific cell types in adult tissues. As an illustration of the cell-type specific nature of RBF1/dCAP-D3-regulation we show that dCAP-D3 and RBF1 are both required for the constituitive expression of a large set of AMP genes in fat body cells. The loss of this regulation compromises pathogen-induction of gene expression and has functional consequences for innate immunity. Interestingly, different sets of RBF1/dCAP-D3-dependent genes were evident in the gene expression profiles of mutant larvae and adults. Given this, and the fact that the gene ontology classification revealed multiple groups of genes, we suggest that the targets of RBF1/dCAP-D3-regulation do not represent a single transcriptional program, but diverse sets of cell-type specific programs that need to be activated (or repressed) in specific developmental contexts.
The changes in gene expression seen in the mutant flies suggest that RBF1 has a significant impact on the expression of nearly half of the dCAP-D3-dependent genes. This fraction is consistent with our previous data showing partial overlap between RBF1 and dCAP-D3 banding patterns on polytene chromatin, and the finding that chromatin-association by dCAP-D3 is reduced, but not eliminated, in rbf1 mutant animals and RBF1-depeleted cells. Although we have previously shown that RBF1 and dCAP-D3 physically associate with one another [11], and our current studies illustrate the fact that they each bind to similar sites at a direct target, the molecular events that mediate the co-operation between RBF1 and dCAP-D3 remain unknown.
These results represent the first published ChIP data for the CAP-D3 protein in any organism. Although we have only examined a small number of targets it is interesting to note that the dCAP-D3 binding patterns are different for activated and repressed genes (compare Figure 5 and Figure S5). More specifically, dCAP-D3 binds to an area within the open reading frame of a gene which it represses (Figure S5C and S5D). However, dCAP-D3 binds to regions which flank a cluster of genes that it activates (Figure 5). Whether or not this difference in binding is true for all dCAP-D3 regulated genes will require a more global analysis.
Human Condensin non-SMC subunits are capable of forming subcomplexes in vitro that are separate from the SMC protein - containing holocomplex [59], but currently, the extent to which dCAP-D3 relies on the other members of the Condensin II complex remains unclear. We note that fat body cells contain polytene chromatin. Condensin II subunits have been shown to play a role in the organization of polytene chromatin in Drosophila nurse cells [60]. Given that RB proteins physically interact with other members of the Condensin II complex [11], it is possible that RBF1 and the entire Condensin II complex, including dCAP-D3, may be especially important for the regulation of transcription on this type of chromatin template.
A potentially significant insight is that the genes that are deregulated in both rbf1 and dCap-D3 mutants tend to be present in clusters located within 10 kb of one another. This clustering effect seems to be a more general feature of regulation by dCAP-D3, which is enhanced by RBF1, since clustering was far more prevalent in the list of dCAP-D3 target genes than in the list of RBF1 target genes.
We chose to focus our studies on one of the most functionally related families of clustered target genes that were co-dependent on RBF1/dCAP-D3 for activation in the adult fly: the AMP family of genes. AMP loci represent 20% of the gene clusters regulated by RBF1 and dCAP-D3 in adults. ChIP analysis of one such region, a cluster of AMP genes at the diptericin locus, showed this locus to be directly regulated by RBF1 and dCAP-D3 in the fat body and revealed a pattern of RBF1 and dCAP-D3-binding that was very different from the binding sites typically mapped at E2F targets. Unlike the promoter-proximal binding sites typically mapped at E2F-regulated promoters, RBF1 and dCAP-D3 bound to two distant regions, one upstream of the promoter and one downstream of the diptericin B translation termination codon, a pattern that is suggestive of an insulator function. We hypothesize that RBF1 and dCAP-D3 act to keep the region surrounding AMP loci insulated from chromatin modifiers and accessible to transcription factors needed for basal levels of transcription. The modEncode database shows binding sites for multiple insulator proteins, as well as GATA factor binding sites, at these regions. GATA has been previously implicated in transcriptional regulation of AMPs in the fly [61], and future studies of dCAP-D3 binding partners in Drosophila fat body tissue may uncover other essential activators. Additionally, the chromatin regulating complex, Cohesin, which exhibits an almost identical structure to Condensin [62]–[64], has been shown to promote looping of chromatin and to bind proteins with insulator functions [65], [66]. Therefore, it remains a possibility that Condensin II, dCAP-D3 may actually possess insulator function, itself. We would like to propose that dCAP-D3 may be functioning as an insulator protein, both insulating regions of DNA containing clusters of genes from the spread of histone marks and possibly looping these regions away from the rest of the body of chromatin. This would serve to keep the region in a “poised state” available for transcription factor binding following exposure to stimuli that would induce activation. In the case of AMP genes, which are made constituitively in specific organs at low levels [37], [67], [68], dCAP-D3 would bind to regions flanking a cluster, and loop the cluster away from the body of chromatin. Upon systemic infection, these clusters would be more easily accessible to transcription factors like NF-κB. If dCAP-D3 is involved in looping of AMP clusters, then it may also regulate interchromosomal looping which could bring AMP clusters on different chromosomes closer together in 3D space, allowing for a faster and more coordinated activation of all AMPs.
AMP expression is essential for the ability of the fly to recover from bacterial infection. Experiments with bacterial pathogens show that RBF1 and dCAP-D3 are both necessary for induction and maintenance of the AMP gene, drosomycin following infection, but only dCAP-D3 is necessary for the induction of the diptericin AMP gene. Similarly, survival curves indicate, that while dCAP-D3 deficient flies die more quickly in response to both Gram positive and Gram negative bacterial infection, RBF1 deficient flies only die faster in response to Gram positive bacterial infection. The differences seen between RBF1 and dCAP-D3 deficient flies in diptericin induction cannot be attributed to functional compensation by the other Drosophila RB protein family member, RBF2, since results show that loss of RBF2 or both RBF2 and RBF1 do not decrease AMP levels following infection. Since results demonstrate that RBF1 binds most strongly to an AMP cluster prior to infection and regulates basal levels of almost all AMPs tested, we hypothesize that RBF1 (and possibly RBF2) may be more important for cooperating with dCAP-D3 to regulate basal levels of AMPs. Reports have shown that basal expression levels of various AMPs are regulated in a gene-, sex-, and tissue-specific manner, and it is thought that constituitive AMP expression may help to maintain a proper balance of microbial flora and/or help to prevent the onset of infections [37], [68], [69]. In support of this idea, one study in Drosophila which characterized loss of function mutants for a gene called caspar, showed that caspar mutants increased constituitive transcript levels of diptericin but not transcript levels following infection. This correlated with increased resistance to septic infection with Gram negative bacteria [70], proving that changes in basal levels of AMPs do have significant effects on the survival of infected flies. Additionally, disruption of Caudal expression, a protein which suppresses NF-κB mediated AMP expression following exposure to commensal bacteria, causes severe defects in the mutualistic interaction between gut and commensal bacteria [71]. It is therefore possible that RBF1 and dCAP-D3 may help to maintain the balance of microbial flora in specific organs of the adult fly and/or be involved in a surveillance-type mechanism to prevent the start of infection. RBF1 deficient flies also exhibit defects in Drosomycin induction following Gram positive bacterial infection. Mutation to Drosophila GNBP-1, an immune recognition protein required to activate the Toll pathway in response to infection with Gram positive bacteria has been show to result in decreased Drosomycin induction and decreased survival rates, without affecting expression of Diptericin [72], [73]. Therefore, it is possible that inefficient levels of Drosomycin, a major downstream effector of the Toll receptor pathway, combined with decreased basal transcription levels of a majority of the other AMPs, would cause RBF1 deficient flies to die faster following infection with Gram positive S. aureus but not Gram negative P. aeruginosa.
Some dCAP-D3 remains localized to DNA in RBF1 deficient flies [11] and it is also possible that other proteins may help to promote the localization of dCAP-D3 to AMP gene clusters following infection. Given that dCAP-D3 regulates many AMPs including some that do not also depend on RBF1 for activation, and given that dCAP-D3 binding to an AMP locus increases with time after infection whereas RBF1 binding is at its highest levels at the start of infection, it may not be too surprising that dCAP-D3 showed a more pronounced biological role in pathogen assays involving two different species of bacteria.
Remarkably, and perhaps unexpectedly, the levels of both RBF1 and dCAP-D3 impact the basal levels of human AMP transcripts, as well. This indicates that the mechanism of RBF1/dCAP-D3 regulation may not be unique to Drosophila. It is striking that many of the human AMP genes (namely, the defensins) are clustered together in a region that spans approximately 1 Mb of DNA. It seems telling that both the clustering of these genes, and a dependence on pRB and CAP-D3, is apparently conserved from flies to humans. The fact that dCAP-D3 and RBF1 dependent activation of Drosomycin was necessary for resistance to Gram positive bacterial infection in flies suggests the same could also be true for the human orthologs in human cells. Human AMPs expressed by epithelial cells, phagocytes and neutrophils are an important component of the human innate immune system. Human AMPs are often downregulated by various microbial pathogenicity mechanisms upon infection [58], [74]–[76]. They have also been reported to play roles in the suppression of various diseases and maladies including cancer and Inflammatory Bowel Disease [77]. We note that the chronic or acute loss of Rb expression from MEFs resulted in an unexplained decrease in the expression of a large number of genes that are involved in the innate immune system [78]. In humans, the bacterium, Shigella flexneri was recently shown to down regulate the host innate immune response by specifically binding to the LXCXE cleft of pRB, the same site that we had previously shown to be necessary for CAP-D3 binding [11], [79]. An improved understanding of how RB and CAP-D3 regulate AMPs in human cells may provide insight into how these proteins are able to regulate clusters of genes, and may also open up new avenues for therapeutic targeting of infection and disease. Further studies of in differentiated human cells may identify additional sets of genes that are regulated by pRB and CAP-D3.
Materials and Methods
Fly strains
W1118 flies were used as “wild type” controls for microarray experiments. Unless otherwise noted, the genotype of RBF mutants was a transheterozygous combination of rbf1Δ14/rbf1120a which was obtained by mating rbf1Δ14/FM7, GFP virgins to rbf1120a/FM7, GFP males at 18°C. Similarly, the genotype of CAP-D3 mutants was a transheterozygous combination of dCAP-D3Δ25/dCAP-D3c07081 which was obtained by mating dCAP-D3Δ25/CyO, GFP virgins to dCAP-D3c07081/CyO, GFP males at 23°C. yolk-GAL4/FM7c flies were a kind gift of M. Birnbaum and the timing of expression driven by yolk-GAL4 has been previously characterized in [40]. The RBF1, dCAP-D3, RBF2, and IMD dsRNA expressing strains were obtained from the VDRC and their transformant IDs were 10696, 29657, 100635, and 101834 respectively. UASt-FLAG-HA tagged strains were created by first amplifying the ORF from either the CAP-D3 RE18364 cDNA clone (DGRC) or the RBF1 LD02906 cDNA clone (DGRC) using Pfx polymerase (Invitrogen). The pENTR/D-TOPO Cloning Kit (Invitrogen) was used to clone the ORF into a Gateway entry vector as described in the manufacturer's protocol and at http://www.ciwemb.edu/labs/murphy/Gateway%20vectors.html. The LR Clonase kit (Invitrogen) was then used to recombine the ORF into the pUASt-FHW vector (DGRC) described in detail at the website mentioned above. pUASt-FLAG-HA-RBF1 and pUASt-FLAG-HA-dCAP-D3 vectors were then injected into embryos to create transgenic fly lines expressing the tagged proteins. Mutant flies used as positive controls in infection experiments included the Imd1 strain which was a generous gift from L. Stuart and the Eater mutant strain [51]. All flies were maintained at 25°C and placed in vials containing standard dextrose medium.
Cell culture and RNAi
hTERT-RPE-1 cells were grown in Dulbecco'sModified Essential Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin. RNAimax (Invitrogen) was used, according to manufacturer's protocol, to transfect non-targeting, RB, and CAP-D3 specific siRNAs (described in [12]) at final concentrations of 100 nM. Total RNA was harvested 48 hours post transfection and reverse transcribed into cDNA, as described below.
qRT–PCR
TRIzol (Invitrogen) was used to harvest total RNA from whole flies/specific tissues according to the manufacturer's protocol. After RNA was purified using the Qiagen RNAeasy kit, the Taqman Reverse Transcription kit (Applied Biosystems) was used to reverse transcribe 1.5 µg of RNA into cDNA. qRT-PCR was performed using the Roche Lightcycler 480 to amplify 15 µL reactions containing .5 µL of cDNA, .5 µL of a 10 µM primer mix and 7.5 µL of SYBR Green Master Mix (Roche). All qRT-PCR experiments were performed using three groups of 5 flies per genotype and three independent experiments were performed. Primer sequences are as follows: Rbf1qPCR F1-CTGCAGGGCTACGAGACGTAC, Rbf1qPCR R1 GTGTGCTGGTTCTTCGGCAGG, Rbf2qPCR R1-CTCCCAGTGCTTCTAGCACGC, Rbf2qPCR F1-CGTGAACGCCTTAGAGGTGCC, dCAP-D3 qPCR F3-CGTGCTGTTGCTTTACTTCGGCC, dCAP-D3 qPCR R3 - GGCGCATGATGAAGAGCATATCCTG, AttAqPCR F1-GTGGTCCAGTCACAACTGGCG, AttAqPCR R1 - CTTGGCATCCAGATTGTGTCTGCC, DroqPCR F1-CACCATCGTTTTCCTGCTGCTTGC, DroqPCR R1-GGTGATCCTCGATGGCCAGTG, AttBqPCR F1 - CTCAAAGCGGTCCAGTCACAACTG, AttBqPCR R1 - GAATAAATTGGCATGGGCCTCCTGC, Dro4qPCR F1 - GTTTGCTCTCCTCGCTGTGGTG, Dro4qPCR R1-GCCCAGCAAGGACCACTGAATC, Dro3qPCR F1 - GGCCAACACTGTTTTGGCACGTG, Dro3qPCR R1 - GTCCCTCCTCAATGCAGAGACG, Dro2qPCR F1 - GTTGTCCTGGCCGCCAATATGG, Dro2qPCR R1 - GGACTGCAGTGGCCACTGATATG, DptBqPCR F1 - GGACTGGCTTGTGCCTTCTCG, DptBqPCR R1 - CAGGGGCACATCAAAATTGGGAGC, DrsqPCR F1-GTACTTGTTCGCCCTCTTCGCTG, DrsqPCR R1 - CAGGTCTCGTTGTCCCAGACG, DptqPCR F1 - GCTTATCCGATGCCCGACGAC, DptqPCR R1-GTGACCCTGGACTGCAAAGCC, DefqPCR F1 - CAAACGCAGACGGCCTTGTCG, DefqPCR R1 - AAGCGAGCCACATGCGACCTAC, Dro5qPCR F1 - CAAGTTCCTGTACCTCTTCCTGGC, Dro5qPCR R1 - CAGGGTCCTCCGTATCTTCCAG, Dro6 qPCR F1-CTTCGCACCAGCATTGCAGCC, Dro6qPCR R1 - GAAGGTACAGACCTCCCTGTGC, Dro7qPCR F1 - GGCTGCAGTGTCCACTGGTTC, Dro7qPCR R1 - CACATGCCGACTGCCTTTCCG, MtkqPCR F1 - GATTTTTCTGGCCCTGCTGGGTG, MtkqPCR R1 - GGTTGGTTAGGATTGAAGGGCGAC, rp49qPCR F1 - TACAGGCCCAAGATCGTGAAG, rp49qPCR R1 - GACGCACTCTGTTGTCGATACC, CecCqPCR F1-CAATCGGAAGCCGGTTGGCTG, CecqPCR R1-GCGCAATTCCCAGTCCTTGAATGG, AndqPCR F1 - CATTTTGGCCATCAGCGTGGGTC, AndqPCR R1 - GGGCTTAGCAAAGCCAATTCCCAC, AttCqPCR F1 - GTACTTGGCTCCCTTGCGGTG, AttCqPCR R1 - CTTAGGTCCAATCGGGCATCGG, AttDqPCR F1 - CCAAGGGAGTTTATGGAGCGGTC, AttDqPCR R1 - GCTCTGGAAGAGATTGGCTTGGG, CecA1qPCR F1 - CAATCGGAAGCTGGGTGGCTG, CecA1qPCR R1 - GGCGGCTTGTTGAGCGATTCC, CecA2qPCR F1 - GGACAATCGGAAGCTGGTTGGC, CecA2qPCR R1 - GGCCTGTTGAGCGATTCCCAG, CecBqPCR F1 - GATTCCGAGGACCTGGATTGAGG, CecBqPCR R1 - GGCCATCAGCCTGGGAAACTC, tub84BqPCR F1 - GGCAAGGAGATCGTCGATCTGG, tub84BqPCR R1 - GACGCTCCATCAGCAGCGAG, hCAP-D3qPCR F1 - TCCGGAAGCAGGCCCTCCAG, hCAP-D3qPCR R1 - GGACCTGGCTGTCGTCCCCA, hRBqPCR F1 - AGCTGTGGGACAGGGTTGTGTC, hRBqPCR R1 - CAACCTCAAGAGCGCACGCC, eaterqPCR F1: CTCGTATCGGCTCAGATCTGCAC, eaterqPCR R1: CATCTGAGTGCGGAGCTCCTTAC, IMDqPCR F1 - CGAATCCACTGGAGCAACAGCTG, IMDqPCR R1 - GTTTCCACGCACTTGGGCGAG, hGAPDHqPCR F1 - AGCCTCCCGCTTCGCTCTCT, hGAPDHqPCR R1 - CCAGGCGCCCAATACGACCA, orc1qPCR F1 - CATCATCCTCAAACACGCGCTGC, orc1qPCR R1 - CCCTCGACGAGGCGTAAAAGC, cg5250qPCR F1 - GACATTGCCGGAGGTGAAGAGC, cg5250qPCR R1 - CTATTCGACTATGTGGTGGGCCTG, dupqPCR F1 - GGGTGGCGGTATTTTTGTGGGAG, dupqPCR R1 - CAACAGGAAACTCCGCGACGC, mus209qPCR F1 - CTTGTCGAAGCCATCGGAACGC, mus209qPCR R1 - GGGTCAAGCCACCATCCTGAAG, dnkqPCR F1 - CCGCCCCAACCAACAAGAAGC, dnkqPCR R1 - CCTCCAGCGTATTGTACATGCCC, RnrSqPCR F1 - GAAGAAGGCAAGCACGTGCGAG, RnrSqPCR R1 - CCAGTACCACGACATCTGGCAG, dnapoldeltaqPCR F1 - CCATCGCCCATTAGCAGAGTCTG, dnapoldeltaqPCR R1 - GGAACCTCCAATGGACATGCCAAG, mcm7qPCR F1 - CATTGAGCACCGCCTGATGATGG, mcm7qPCR R1 - GAGTGCGCCTTCTCTGTGGAC, mcm3qPCR F1 - CGAGGTGATGGAACAGGGTCG, mcm3qPCR R1 - GAAAGCAGCGAATCCTGCAGTCC, mcm2qPCR F1 - GAGATCCCGCAGGACTTGTTGC, mcm2qPCR R1 - CAAAAGACTCCTGTCGCAGCTGG, mcm5qPCR F1 - CTGGTCTCACGGCTTCGGTTATG, mcm5qPCR R1 - GCCACACGATCATCCTCTCGC, dnapolalpha50qPCR F1 - CCTTCTACCGTTGGCTATCGTATGG, dnapolalpha50qPCR R1 - CAGCTTGGGTATCAAAGCAGAGG, DEFA-1qPCR F1 - TGCCCTCTCTGGTCACCCTGC, DEFA-1qPCR R1 - GCCTGGAGTGGCTCAGCCTG, DEFB-3qPCR F1 - GCGTGGGGTGAAGCCTAGCA, DEFB-3qPCR R1 - AGCTGAGCACAGCACACCGG.
Generation of anti–dCAP-D3 antibody
The rabbit anti–dCAP-D3 YZ834 antibody was generated by Yenzyme Corporation. The antibody was purified using the BIO-RAD Affi-Gel 10 Gel according to manufacturer's protocol.
Immunofluorescence analysis of cryosections
Adult female flies were cryosectioned (10 µm) and stained as previously described [80]. Primary antibodies included RBF1 (DX2), dCAP-D3 (YZ384), and anti-GFP (Jackson Immunoresearch). Images were obtained using a Zeiss LSM510 Confocal microscope.
Preprocessing of array data
Nimblegen microarray data were pretreated according to the manufacturer's recommendation and replicate probes were averaged. Affymetrix microarray data was downloaded from array express as raw .CEL files and normalized by robust multi array averaging (RMA)[RMS] before further analysis [81]. The entire set of microarray data can be found in Table S1.
Hypothesis testing
Differentially expressed genes were identified using a linear model with a moderated T-test [82]. P values were corrected for multiple testing by calculating false discovery rates using the method of Benjamini and Hochberg [83]. Genes with a false discovery rate (FDR)<0.15 and a log2 fold change >0.1 were taken as significant. Gene ontology (GO) annotations were downloaded from FLYBASE [84], and gene ontology terms overrepresented on the lists of differentially expressed genes were identified using a hypergeometric test. P-values from the hypergeometric test were corrected for multiple testing using the same method as for the individual genes and GO-categories with FDR<0.05 were taken as significant.
Gene clustering analysis
Chromosomal positions of transcription start and stop sites for all genes on the chip were taken from FLYBASE. Genes were counted as clustered if they overlapped, or if the genes lay within 10 000 base pairs of each other. Overall chromosomal clustering for a list of genes was quantified as the number of genes that co-localize according to this criterion. Significance of co-localization was evaluated by comparing to lists of randomly selected genes from the same chip.
Infection of flies with pathogenic bacteria
S. aureus and P. aeruginosa bacteria were gifts from L. Stuart. S. aureus was grown in a shaking incubator at 37°C, in DIFCO Columbia broth (BD Biosciences) supplemented with 2% NaCl and P. aeruginosa was grown in a shaking incubator at 37°C in DIFCO Luria broth (BD Biosciences). Bacteria were inoculated in 10 mL cultures grown overnight. 10∧4 bacterial cells were then inoculated into a new 10 mL culture and this was grown to an OD600 nm of 0.5. These cultures were then centrifuged at 3000 rpm in a 1.5 mL eppendorf tube for 5 minutes at 4°C and subsequently washed twice with PBS. After a third centrifugation, PBS wash was removed from the pellet and 25 µL of new PBS was used to resuspend the pellet. Infections were performed as previously described [85]. Specifically, a .25 mm diameter straight stainless steel needle and pin vise (Ted Pella Inc, Redding, CA) were used to infect adult flies. The needle was dipped into the resuspended bacterial pellet and used to prick the thorax of a CO2-anesthetized adult fly in a region just underneath where the wing connects to the thorax. Flies were then separated from the needle using a brush and put into fresh vials containing standard dextrose medium with no more than 10 flies per vial.
Chromatin immunoprecipitation
40 flies per IP were used in all ChIP experiments. Flies were homogenized with a KONTES pellet pestle grinder (Kimble Chase) in 1 mL of buffer A (60 mM KCl, 15 mM NaCl, 4 mM MgCl2, 15 mM HEPES pH 7.6, .5% Triton X-100, .5 mM DTT, EDTA-free protease inhibitors cocktail (Roche)) containing 1.8% formaldehyde. Homogenized flies were incubated at RT for 15 minutes, at which point glycine was added to a concentration of 225 mM. 2–4 mLs of homogenized flies were transferred to 15 mL conical tubes and centrifuged at 4°C for 5 min at 4000 g. Supernatant was discarded and pellets were washed with 3 mL of buffer A. Tubes were centrifuged as described above, supernatant was discarded, and pellets were washed with 3 mL of buffer B (140 mM NaCl, 15 mM HEPES pH 7.6, 1 mM EDTA, .5 mM EGTA, 1% Triton, .5 mM DTT, .1% sodium deoxycholate, EDTA free protease inhibitors cocktail). Tubes were centrifuged as described above, supernatant was discarded, and 500 µL of buffer B+1% SDS per IP was added to each tube. Tubes were rotated at 4°C for 20 min. Samples were then sonicated using the Branson sonifier at a setting of 3, with 8 sonication intervals of 20 seconds interspersed by 10 second breaks. Tubes were centrifuged at 4°C for 5 min at 2000 RPM and 500 µL supernatant was used for each IP. 50 µL of Dynal Protein A beads (Invitrogen) per IP were prepared according to the manufacturer's recommendations. Beads were incubated with anti-FLAG M2 antibody (Sigma) or dCAP-D3 antibody (YZ384) for 2 hours at RT with rotation. Beads were washed according to manufacturer's protocol and added to the diluted chromatin samples which were then incubated at 4°C overnight, with rotation. Samples were centrifuged at 3000 RPM, 4°C for 1 min and washed three times with buffer B+.05% SDS and once with TE. Bound protein was eluted by adding 125 µL of Buffer C (1%SDS, .2% NaCl, TE) to the beads for 30 min at 65°C. Samples were again centrifuged and eluates were harvested and incubated for 4 hours at 65°C to reverse crosslinks. Samples were digested with Proteinase K and RNase A (Sigma), phenol-chloroform extracted, and ethanol precipitated. DNA pellets were dissolved in 105 µL of ddH2O and .5 µL was used per qRT-PCR reaction.
Survival curves
Flies were collected approximately 5–8 days after eclosure and were infected as described above. Following infection, each group of flies was placed in a new vial of food and monitored for the number of surviving flies at each timepoint. Three experiments were performed, with each experiment including 3 groups of 10 flies per genotype per timepoint. Survival statistics were calculated using a cox proportional hazard model, and hazard ratios with a two sided p-value less than 0.05 were taken as significant.
Bacterial clearance assays
Flies were anestitized by CO2 inhalation and infected as described above. Following infection, flies were dipped in 95% Ethanol, air dried, and placed into 1.5 mL Eppendorf tubes containing 500 µL of PBS. Flies were homogenized with a Kontes battery powered homogenizer and plastic pestle (USA scientific). The tubes were centrifuged for 2 min at 3000 rpm. Various dilutions were plated onto Columbia CNA with 5% Sheep's Blood Agar (Becton Dickinson and Company). This type of agar contains antibiotics to inhibit growth of organisms other than Staphylococcus aureus.
Supporting Information
Zdroje
1. HanahanDWeinbergRA 2000 The hallmarks of cancer. Cell 100 57 70
2. SherrCJMcCormickF 2002 The RB and p53 pathways in cancer. Cancer Cell 2 103 112
3. TrimarchiJMLeesJA 2002 Sibling rivalry in the E2F family. Nat Rev Mol Cell Biol 3 11 20
4. ThomasDMCartySAPiscopoDMLeeJSWangWF 2001 The retinoblastoma protein acts as a transcriptional coactivator required for osteogenic differentiation. Mol Cell 8 303 316
5. SchneiderJGuWZhuLMahdaviVNadal-GinardB 1994 Reversal of terminal differentiation mediated by p107 in Rb-/ - muscle cells 10.1126/science.8197461. Science 264 1467 1471
6. NovitchBMulliganGJacksTLassarA 1996 Skeletal muscle cells lacking the retinoblastoma protein display defects in muscle gene expression and accumulate in S and G2 phases of the cell cycle 10.1083/jcb.135.2.441. J Cell Biol 135 441 456
7. ZacksenhausEJiangZChungDMarthJDPhillipsRA 1996 pRb controls proliferation, differentiation, and death of skeletal muscle cells and other lineages during embryogenesis. Genes Dev 10 3051 3064
8. NicolayBNBayarmagnaiBMoonNSBenevolenskayaEVFrolovMV 2010 Combined inactivation of pRB and hippo pathways induces dedifferentiation in the Drosophila retina. PLoS Genet 6 e1000918 doi:10.1371/journal.pgen.1000918
9. LongworthMSDysonNJ 2010 pRb, a local chromatin organizer with global possibilities. Chromosoma 119 1 11
10. IsaacCEFrancisSMMartensALJulianLMSeifriedLA 2006 The retinoblastoma protein regulates pericentric heterochromatin. Mol Cell Biol 26 3659 3671
11. LongworthMSHerrAJiJYDysonNJ 2008 RBF1 promotes chromatin condensation through a conserved interaction with the Condensin II protein dCAP-D3. Genes Dev 22 1011 1024
12. ManningALLongworthMSDysonNJ 2010 Loss of pRB causes centromere dysfunction and chromosomal instability. Genes Dev 24 1364 1376
13. GerlichDHirotaTKochBPetersJMEllenbergJ 2006 Condensin I stabilizes chromosomes mechanically through a dynamic interaction in live cells. Curr Biol 16 333 344
14. OnoTLosadaAHiranoMMyersMPNeuwaldAF 2003 Differential contributions of condensin I and condensin II to mitotic chromosome architecture in vertebrate cells. Cell 115 109 121
15. SteffensenSCoelhoPACobbeNVassSCostaM 2001 A role for Drosophila SMC4 in the resolution of sister chromatids in mitosis. Curr Biol 11 295 307
16. HirotaTGerlichDKochBEllenbergJPetersJM 2004 Distinct functions of condensin I and II in mitotic chromosome assembly. J Cell Sci 117 6435 6445
17. Bazett-JonesDPKimuraKHiranoT 2002 Efficient supercoiling of DNA by a single condensin complex as revealed by electron spectroscopic imaging. Mol Cell 9 1183 1190
18. StrickTRKawaguchiTHiranoT 2004 Real-time detection of single-molecule DNA compaction by condensin I. Curr Biol 14 874 880
19. OnoTFangYSpectorDLHiranoT 2004 Spatial and temporal regulation of Condensins I and II in mitotic chromosome assembly in human cells. Mol Biol Cell 15 3296 3308
20. KimuraKCuvierOHiranoT 2001 Chromosome condensation by a human condensin complex in Xenopus egg extracts. J Biol Chem 276 5417 5420
21. GoslingKMMakaroffLETheodoratosAKimYHWhittleB 2007 A mutation in a chromosome condensin II subunit, kleisin beta, specifically disrupts T cell development. Proc Natl Acad Sci U S A 104 12445 12450
22. XuYLeungCGLeeDCKennedyBKCrispinoJD 2006 MTB, the murine homolog of condensin II subunit CAP-G2, represses transcription and promotes erythroid cell differentiation. Leukemia 20 1261 1269
23. CobbeNSavvidouEHeckMM 2006 Diverse mitotic and interphase functions of condensins in Drosophila. Genetics 172 991 1008
24. DejKJAhnCOrr-WeaverTL 2004 Mutations in the Drosophila condensin subunit dCAP-G: defining the role of condensin for chromosome condensation in mitosis and gene expression in interphase. Genetics 168 895 906
25. LemaitreBHoffmannJ 2007 The host defense of Drosophila melanogaster. Annu Rev Immunol 25 697 743
26. DimovaDKStevauxOFrolovMVDysonNJ 2003 Cell cycle-dependent and cell cycle-independent control of transcription by the Drosophila E2F/RB pathway. Genes Dev 17 2308 2320
27. KwonSYXiaoHGloverBPTjianRWuC 2008 The nucleosome remodeling factor (NURF) regulates genes involved in Drosophila innate immunity. Dev Biol 316 538 547
28. MeisterM 2004 Blood cells of Drosophila: cell lineages and role in host defence. Curr Opin Immunol 16 10 15
29. KuruczEMarkusRZsambokiJFolkl-MedzihradszkyKDarulaZ 2007 Nimrod, a putative phagocytosis receptor with EGF repeats in Drosophila plasmatocytes. Curr Biol 17 649 654
30. LebestkyTChangTHartensteinVBanerjeeU 2000 Specification of Drosophila hematopoietic lineage by conserved transcription factors. Science 288 146 149
31. AlfonsoTBJonesBW 2002 gcm2 promotes glial cell differentiation and is required with glial cells missing for macrophage development in Drosophila. Dev Biol 248 369 383
32. LanotRZacharyDHolderFMeisterM 2001 Postembryonic hematopoiesis in Drosophila. Dev Biol 230 243 257
33. Elrod-EricksonMMishraSSchneiderD 2000 Interactions between the cellular and humoral immune responses in Drosophila. Curr Biol 10 781 784
34. NappiAJOttavianiE 2000 Cytotoxicity and cytotoxic molecules in invertebrates. Bioessays 22 469 480
35. IrvingPUbedaJMDoucetDTroxlerLLagueuxM 2005 New insights into Drosophila larval haemocyte functions through genome-wide analysis. Cell Microbiol 7 335 350
36. WertheimBKraaijeveldARSchusterEBlancEHopkinsM 2005 Genome-wide gene expression in response to parasitoid attack in Drosophila. Genome Biol 6 R94
37. UvellHEngstromY 2007 A multilayered defense against infection: combinatorial control of insect immune genes. Trends Genet 23 342 349
38. BrennanCAAndersonKV 2004 Drosophila: the genetics of innate immune recognition and response. Annu Rev Immunol 22 457 483
39. ImlerJLBuletP 2005 Antimicrobial peptides in Drosophila: structures, activities and gene regulation. Chem Immunol Allergy 86 1 21
40. DiAngeloJRBirnbaumMJ 2009 Regulation of fat cell mass by insulin in Drosophila melanogaster. Mol Cell Biol 29 6341 6352
41. AggarwalKSilvermanN 2008 Positive and negative regulation of the Drosophila immune response. BMB Rep 41 267 277
42. FerrandonDImlerJLHetruCHoffmannJA 2007 The Drosophila systemic immune response: sensing and signalling during bacterial and fungal infections. Nat Rev Immunol 7 862 874
43. SengerKHarrisKLevineM 2006 GATA factors participate in tissue-specific immune responses in Drosophila larvae. Proc Natl Acad Sci U S A 103 15957 15962
44. TanjiTYunEYIpYT 2010 Heterodimers of NF-kappaB transcription factors DIF and Relish regulate antimicrobial peptide genes in Drosophila. Proc Natl Acad Sci U S A 107 14715 14720
45. MeisterMBraunAKapplerCReichhartJMHoffmannJA 1994 Insect immunity. A transgenic analysis in Drosophila defines several functional domains in the diptericin promoter. Embo J 13 5958 5966
46. GeorgelPMeisterMKapplerCLemaitreBReichhartJM 1993 Insect immunity: the diptericin promoter contains multiple functional regulatory sequences homologous to mammalian acute-phase response elements. Biochem Biophys Res Commun 197 508 517
47. KapplerCMeisterMLagueuxMGateffEHoffmannJA 1993 Insect immunity. Two 17 bp repeats nesting a kappa B-related sequence confer inducibility to the diptericin gene and bind a polypeptide in bacteria-challenged Drosophila. Embo J 12 1561 1568
48. ValanneSMyllymakiHKallioJSchmidMRKleinoA 2010 Genome-wide RNA interference in Drosophila cells identifies G protein-coupled receptor kinase 2 as a conserved regulator of NF-kappaB signaling. J Immunol 184 6188 6198
49. MengXKhanujaBSIpYT 1999 Toll receptor-mediated Drosophila immune response requires Dif, an NF-kappaB factor. Genes Dev 13 792 797
50. KanekoTSilvermanN 2005 Bacterial recognition and signalling by the Drosophila IMD pathway. Cell Microbiol 7 461 469
51. KocksCChoJHNehmeNUlvilaJPearsonAM 2005 Eater, a transmembrane protein mediating phagocytosis of bacterial pathogens in Drosophila. Cell 123 335 346
52. AyresJSFreitagNSchneiderDS 2008 Identification of Drosophila mutants altering defense of and endurance to Listeria monocytogenes infection. Genetics 178 1807 1815
53. AyresJSSchneiderDS 2008 A signaling protease required for melanization in Drosophila affects resistance and tolerance of infections. PLoS Biol 6 e305 doi:10.1371/journal.pbio.0060305
54. DionneMSPhamLNShirasu-HizaMSchneiderDS 2006 Akt and FOXO dysregulation contribute to infection-induced wasting in Drosophila. Curr Biol 16 1977 1985
55. SchneiderDSAyresJS 2008 Two ways to survive infection: what resistance and tolerance can teach us about treating infectious diseases. Nat Rev Immunol 8 889 895
56. KorenjakMTaylor-HardingBBinneUKSatterleeJSStevauxO 2004 Native E2F/RBF complexes contain Myb-interacting proteins and repress transcription of developmentally controlled E2F target genes. Cell 119 181 193
57. LewisPWBeallELFleischerTCGeorletteDLinkAJ 2004 Identification of a Drosophila Myb-E2F2/RBF transcriptional repressor complex. Genes Dev 18 2929 2940
58. DiamondGBeckloffNWeinbergAKisichKO 2009 The roles of antimicrobial peptides in innate host defense. Curr Pharm Des 15 2377 2392
59. KimuraKHiranoT 2000 Dual roles of the 11S regulatory subcomplex in condensin functions. Proc Natl Acad Sci U S A 97 11972 11977
60. HartlTASmithHFBoscoG 2008 Chromosome alignment and transvection are antagonized by condensin II. Science 322 1384 1387
61. PetersenUMKadalayilLRehornKPHoshizakiDKReuterR 1999 Serpent regulates Drosophila immunity genes in the larval fat body through an essential GATA motif. Embo J 18 4013 4022
62. HiranoT 2006 At the heart of the chromosome: SMC proteins in action. Nat Rev Mol Cell Biol 7 311 322
63. NasmythKHaeringCH 2005 The structure and function of SMC and kleisin complexes. Annu Rev Biochem 74 595 648
64. HiranoT 2005 SMC proteins and chromosome mechanics: from bacteria to humans. Philos Trans R Soc Lond B Biol Sci 360 507 514
65. NativioRWendtKSItoYHuddlestonJEUribe-LewisS 2009 Cohesin is required for higher-order chromatin conformation at the imprinted IGF2-H19 locus. PLoS Genet 5 e1000739 doi:10.1371/journal.pgen.1000739
66. WendtKSYoshidaKItohTBandoMKochB 2008 Cohesin mediates transcriptional insulation by CCCTC-binding factor. Nature 451 796 801
67. FerrandonDJungACCriquiMLemaitreBUttenweiler-JosephS 1998 A drosomycin-GFP reporter transgene reveals a local immune response in Drosophila that is not dependent on the Toll pathway. Embo J 17 1217 1227
68. RyuJHNamKBOhCTNamHJKimSH 2004 The homeobox gene Caudal regulates constitutive local expression of antimicrobial peptide genes in Drosophila epithelia. Mol Cell Biol 24 172 185
69. TzouPOhresserSFerrandonDCapovillaMReichhartJM 2000 Tissue-specific inducible expression of antimicrobial peptide genes in Drosophila surface epithelia. Immunity 13 737 748
70. KimMLeeJHLeeSYKimEChungJ 2006 Caspar, a suppressor of antibacterial immunity in Drosophila. Proc Natl Acad Sci U S A 103 16358 16363
71. RyuJHKimSHLeeHYBaiJYNamYD 2008 Innate immune homeostasis by the homeobox gene caudal and commensal-gut mutualism in Drosophila. Science 319 777 782
72. GobertVGottarMMatskevichAARutschmannSRoyetJ 2003 Dual activation of the Drosophila toll pathway by two pattern recognition receptors. Science 302 2126 2130
73. Pili-FlourySLeulierFTakahashiKSaigoKSamainE 2004 In vivo RNA interference analysis reveals an unexpected role for GNBP1 in the defense against Gram-positive bacterial infection in Drosophila adults. J Biol Chem 279 12848 12853
74. BergmanPJohanssonLAspVPlantLGudmundssonGH 2005 Neisseria gonorrhoeae downregulates expression of the human antimicrobial peptide LL-37. Cell Microbiol 7 1009 1017
75. ChakrabortyKGhoshSKoleyHMukhopadhyayAKRamamurthyT 2008 Bacterial exotoxins downregulate cathelicidin (hCAP-18/LL-37) and human beta-defensin 1 (HBD-1) expression in the intestinal epithelial cells. Cell Microbiol 10 2520 2537
76. SperandioBRegnaultBGuoJZhangZStanleySLJr 2008 Virulent Shigella flexneri subverts the host innate immune response through manipulation of antimicrobial peptide gene expression. J Exp Med 205 1121 1132
77. WehkampJSalzmanNHPorterENudingSWeichenthalM 2005 Reduced Paneth cell alpha-defensins in ileal Crohn's disease. Proc Natl Acad Sci U S A 102 18129 18134
78. MarkeyMPBergseidJBoscoEEStengelKXuH 2007 Loss of the retinoblastoma tumor suppressor: differential action on transcriptional programs related to cell cycle control and immune function. Oncogene 26 6307 6318
79. ZurawskiDVMumyKLFahertyCSMcCormickBAMaurelliAT 2009 Shigella flexneri type III secretion system effectors OspB and OspF target the nucleus to downregulate the host inflammatory response via interactions with retinoblastoma protein. Mol Microbiol 71 350 368
80. TsichritzisTGaentzschPCKosmidisSBrownAESkoulakisEM 2007 A Drosophila ortholog of the human cylindromatosis tumor suppressor gene regulates triglyceride content and antibacterial defense. Development 134 2605 2614
81. BolstadBMIrizarryRAAstrandMSpeedTP 2003 A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 19 185 193
82. SmythGK 2004 Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol 3 Article3
83. BenjaminiYHochbergY 1995 Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series 57 289 300
84. TweedieSAshburnerMFallsKLeylandPMcQuiltonP 2009 FlyBase: enhancing Drosophila Gene Ontology annotations. Nucleic Acids Res 37 D555 559
85. RomeoYLemaitreB 2008 Drosophila immunity: methods for monitoring the activity of Toll and Imd signaling pathways. Methods Mol Biol 415 379 394
Štítky
Genetika Reprodukční medicína
Článek A Genome-Wide Screen for Genetic Variants That Modify the Recruitment of REST to Its Target GenesČlánek Population Structure of Hispanics in the United States: The Multi-Ethnic Study of AtherosclerosisČlánek Differing Requirements for RAD51 and DMC1 in Meiotic Pairing of Centromeres and Chromosome Arms inČlánek Transcriptional Regulation of Rod Photoreceptor Homeostasis Revealed by NRL Targetome AnalysisČlánek Cell Contact–Dependent Outer Membrane Exchange in Myxobacteria: Genetic Determinants and MechanismČlánek Formation of Rigid, Non-Flight Forewings (Elytra) of a Beetle Requires Two Major Cuticular Proteins
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 4
-
Všechny články tohoto čísla
- Runs of Homozygosity Implicate Autozygosity as a Schizophrenia Risk Factor
- Modifier Genes and the Plasticity of Genetic Networks in Mice
- The DSIF Subunits Spt4 and Spt5 Have Distinct Roles at Various Phases of Immunoglobulin Class Switch Recombination
- A Genome-Wide Screen for Genetic Variants That Modify the Recruitment of REST to Its Target Genes
- Population Structure of Hispanics in the United States: The Multi-Ethnic Study of Atherosclerosis
- Deep Sequencing of Plant and Animal DNA Contained within Traditional Chinese Medicines Reveals Legality Issues and Health Safety Concerns
- Differing Requirements for RAD51 and DMC1 in Meiotic Pairing of Centromeres and Chromosome Arms in
- Insulin Signaling Mediates Sexual Attractiveness in
- Progressive Telomere Dysfunction Causes Cytokinesis Failure and Leads to the Accumulation of Polyploid Cells
- Long-Range Chromosome Organization in : A Site-Specific System Isolates the Ter Macrodomain
- Regulation of Budding Yeast Mating-Type Switching Donor Preference by the FHA Domain of Fkh1
- Polyglutamine Toxicity Is Controlled by Prion Composition and Gene Dosage in Yeast
- Patterns of Regulatory Variation in Diverse Human Populations
- Sequence-Specific Targeting of Dosage Compensation in Favors an Active Chromatin Context
- Whole-Exome Sequencing and Homozygosity Analysis Implicate Depolarization-Regulated Neuronal Genes in Autism
- Replication Fork Reversal after Replication–Transcription Collision
- Common Variants at 9p21 and 8q22 Are Associated with Increased Susceptibility to Optic Nerve Degeneration in Glaucoma
- Coordinate Regulation of Lipid Metabolism by Novel Nuclear Receptor Partnerships
- Epigenome-Wide Scans Identify Differentially Methylated Regions for Age and Age-Related Phenotypes in a Healthy Ageing Population
- A Coordinated Interdependent Protein Circuitry Stabilizes the Kinetochore Ensemble to Protect CENP-A in the Human Pathogenic Yeast
- Budding Yeast Dma Proteins Control Septin Dynamics and the Spindle Position Checkpoint by Promoting the Recruitment of the Elm1 Kinase to the Bud Neck
- , a Homolog of a Deaf-Blindness Gene, Regulates Circadian Output and Slowpoke Channels
- Transcriptional Regulation of Rod Photoreceptor Homeostasis Revealed by NRL Targetome Analysis
- Cell Contact–Dependent Outer Membrane Exchange in Myxobacteria: Genetic Determinants and Mechanism
- Defective Membrane Remodeling in Neuromuscular Diseases: Insights from Animal Models
- Formation of Rigid, Non-Flight Forewings (Elytra) of a Beetle Requires Two Major Cuticular Proteins
- SPE-44 Implements Sperm Cell Fate
- A Shared Role for RBF1 and dCAP-D3 in the Regulation of Transcription with Consequences for Innate Immunity
- A Companion Cell–Dominant and Developmentally Regulated H3K4 Demethylase Controls Flowering Time in via the Repression of Expression
- The HEN1 Ortholog, HENN-1, Methylates and Stabilizes Select Subclasses of Germline Small RNAs
- Improved Statistics for Genome-Wide Interaction Analysis
- The Probability of a Gene Tree Topology within a Phylogenetic Network with Applications to Hybridization Detection
- Context-Dependent Dual Role of SKI8 Homologs in mRNA Synthesis and Turnover
- Mu Insertions Are Repaired by the Double-Strand Break Repair Pathway of
- Competition between Replicative and Translesion Polymerases during Homologous Recombination Repair in Drosophila
- An Unbiased Assessment of the Role of Imprinted Genes in an Intergenerational Model of Developmental Programming
- Type 2 Diabetes Risk Alleles Demonstrate Extreme Directional Differentiation among Human Populations, Compared to Other Diseases
- Mutations in and Cause “Splashed White” and Other White Spotting Phenotypes in Horses
- Fine-Scale Mapping of Natural Variation in Fly Fecundity Identifies Neuronal Domain of Expression and Function of an Aquaporin
- Dynamics of Brassinosteroid Response Modulated by Negative Regulator LIC in Rice
- Genetic Inhibition of Solute-Linked Carrier 39 Family Transporter 1 Ameliorates Aβ Pathology in a Model of Alzheimer's Disease
- The Functions of Mediator in Support a Role in Shaping Species-Specific Gene Expression
- Patterns of Ancestry, Signatures of Natural Selection, and Genetic Association with Stature in Western African Pygmies
- Dissection of Pol II Trigger Loop Function and Pol II Activity–Dependent Control of Start Site Selection
- PIWI Associated siRNAs and piRNAs Specifically Require the HEN1 Ortholog
- Genome-Wide Patterns of Gene Expression in Nature
- Hypoxia Disruption of Vertebrate CNS Pathfinding through EphrinB2 Is Rescued by Magnesium
- A New Role for Translation Initiation Factor 2 in Maintaining Genome Integrity
- Sex Reversal in C57BL/6J XY Mice Caused by Increased Expression of Ovarian Genes and Insufficient Activation of the Testis Determining Pathway
- The Rac GTP Exchange Factor TIAM-1 Acts with CDC-42 and the Guidance Receptor UNC-40/DCC in Neuronal Protrusion and Axon Guidance
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- A Coordinated Interdependent Protein Circuitry Stabilizes the Kinetochore Ensemble to Protect CENP-A in the Human Pathogenic Yeast
- Coordinate Regulation of Lipid Metabolism by Novel Nuclear Receptor Partnerships
- Defective Membrane Remodeling in Neuromuscular Diseases: Insights from Animal Models
- Formation of Rigid, Non-Flight Forewings (Elytra) of a Beetle Requires Two Major Cuticular Proteins
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



