-
Články
Reklama
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
ReklamaFine-Scale Mapping of Natural Variation in Fly Fecundity Identifies Neuronal Domain of Expression and Function of an Aquaporin
To gain insight into the molecular genetic basis of standing variation in fitness related traits, we identify a novel factor that regulates the molecular and physiological basis of natural variation in female Drosophila melanogaster fecundity. Genetic variation in female fecundity in flies derived from a wild orchard population is heritable and largely independent of other measured life history traits. We map a portion of this variation to a single QTL and then use deficiency mapping to further refine this QTL to 5 candidate genes. Ubiquitous expression of RNAi against only one of these genes, an aquaporin encoded by Drip, reduces fecundity. Within our mapping population Drip mRNA level in the head, but not other tissues, is positively correlated with fecundity. We localize Drip expression to a small population of corazonin producing neurons located in the dorsolateral posterior compartments of the protocerebrum. Expression of Drip–RNAi using both the pan-neuronal ELAV-Gal4 and the Crz-Gal4 drivers reduces fecundity. Low-fecundity RILs have decreased Crz expression and increased expression of pale, the enzyme encoding the rate-limiting step in the production of dopamine, a modulator of insect life histories. Taken together these data suggest that natural variation in Drip expression in the corazonin producing neurons contributes to standing variation in fitness by altering the concentration of two neurohormones.
Published in the journal: . PLoS Genet 8(4): e32767. doi:10.1371/journal.pgen.1002631
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002631Summary
To gain insight into the molecular genetic basis of standing variation in fitness related traits, we identify a novel factor that regulates the molecular and physiological basis of natural variation in female Drosophila melanogaster fecundity. Genetic variation in female fecundity in flies derived from a wild orchard population is heritable and largely independent of other measured life history traits. We map a portion of this variation to a single QTL and then use deficiency mapping to further refine this QTL to 5 candidate genes. Ubiquitous expression of RNAi against only one of these genes, an aquaporin encoded by Drip, reduces fecundity. Within our mapping population Drip mRNA level in the head, but not other tissues, is positively correlated with fecundity. We localize Drip expression to a small population of corazonin producing neurons located in the dorsolateral posterior compartments of the protocerebrum. Expression of Drip–RNAi using both the pan-neuronal ELAV-Gal4 and the Crz-Gal4 drivers reduces fecundity. Low-fecundity RILs have decreased Crz expression and increased expression of pale, the enzyme encoding the rate-limiting step in the production of dopamine, a modulator of insect life histories. Taken together these data suggest that natural variation in Drip expression in the corazonin producing neurons contributes to standing variation in fitness by altering the concentration of two neurohormones.
Introduction
The life history of an organism—its reproductive schedule and lifespan—are fundamental characteristics intrinsically related to its evolutionary fitness. Due to the close relationship of life history with fitness, theory predicts life history traits should be subject to strong natural selection [1], [2]. However, the exact mode of action of natural selection on these traits is often complex and context dependent [3], [4]. Environmental heterogeneity, overdominance, frequency dependent selection or life history tradeoffs may thus maintain genetic variation in fitness traits [4]–[7]. Due to the complex behavior of natural selection on life history traits, there has been great interest to quantify their magnitude of genetic variation and to map natural alleles underlying this variation.
These efforts have mapped natural variation in life history traits to specific loci in a variety of organisms [8]. In Arabidopsis thaliana genetic variation in reproductive timing in response to vernalization has been mapped to polymorphisms in FLOWERING LOCUS C [9], [10] and FRIGIDA [11]. Genetic variation in population growth rate and dispersal in the Glanville fritillary butterfly, Melitaea cinxia, is associated with polymorphism in phosphoglucose isomerase and succinate dehydrogenase [12]. In the fruit fly, Drosophila melanogaster, genetic variation in reproductive diapause has been mapped to components of the insulin signaling [13], circadian clock [14], and neuronal development pathways [15]. For D. melanogaster in particular, extensive work has examined natural variation in age specific survival, a core life history component. Using traditional quantitative trait locus (QTL) mapping techniques, dozens of positional loci affecting lifespan have been identified [16]–[26]. Several of these QTL have been localized to specific genes including Dox-A2, tup, ms(2)35Ci, stc, Lim3, Ddc, and catsup; [18], [27]–[31]. A complimentary approach has described natural polymorphisms for lifespan in pro-longevity genes such as the G-coupled receptor methuselah [32] [33] that were previously identified through molecular techniques.
While considerable progress has been made uncovering the genetic basis for natural variation in lifespan, relatively little is known about natural variation in reproductive output. Natural genetic variation in only two genes has been associated with variation in fecundity (mth and InR; [33], [34]) although several studies have identified positional QTL for age specific female fecundity [35], male mating success [36] and ovariole number [37], [38] without resolving a molecular or specific genetic determinant. To advance this issue we identify novel genetic loci affecting fecundity in a natural population of D. melanogaster. We document segregating genetic variation in fecundity and identify a QTL underlying this variation. We localize the polymorphism in this QTL to a region containing 5 genes including the aquaporin, Drip. Drip expression in head tissue is variable in our mapping population as is two genes putatively downstream of Drip, Crz and pale. Drip has been previously described to function in malphigian tubules where it serves to regulate water balance [39] in an ecdysone dependent fashion [40]. Here we show that Drip is expressed in a small population of corazonin producing neurons in the fly brain and Drip expression in these neurons modulates fecundity. This work localizes a new polymorphic locus that effects a core life history trait and identifies a novel function and domain of expression for Drip.
Results/Discussion
To understand the genetic basis of phenotypic variation in female fecundity, we studied a set of 12 recombinant inbred lines (RILs) derived from an orchard population in Winters, CA. These RILs were chosen to maximize genetic variance in ovariole number and thorax length, two morphological determinants of fecundity [38]. RIL larvae were reared in the lab on diets of several yeast concentrations to identify nutrient dependent effects on female fecundity, egg-to-adult development time, ovariole number and thorax length. Significant genetic and genotype-by-environment variation is seen for each of these life history traits (Tables S1, S2, S3, S4). Measured as phenotypic variation among individuals, larger females have more ovarioles, develop quicker and lay more eggs, and standard and partial correlations between these traits are generally independent of larval rearing environment (Table S5, Figure S1A–S1F). We analyzed standard and partial correlations among line means to estimate the genetic contributions to these phenotypic patterns. Genetically, ovariole number and thorax length are positively correlated, and again independent of larval rearing environment (Table S5; cf [38]). On the other hand, total fecundity is only correlated with ovariole number when larvae are reared in the high larval yeast environment but this correlation does not appear when examining partial correlations (Table S5, Figure S1A–S1F). The discrepancy between phenotypic and genetic correlations for these traits suggests that the primary cause of the observed phenotypic correlations between fecundity and other measured life history traits derives from micro-environmental variation in food concentration within vials or asymmetric competition within rearing vials. Taken together these results suggest that genetic variation for fecundity is largely independent of other measured life history traits in this mapping population.
Given the genetic contribution to phenotypic variance in these traits within the population, we sought to identify chromosomal regions associated with these traits and their response to the environment. Multiple imputation QTL mapping [41] with ∼100 SNP markers spread along the three major D. melanogaster chromosomes [38] did not identify any significant QTL affecting ovariole number, thorax length or development time but did locate a single QTL affecting fecundity in chromosome 2R between bands 47D–E to 48E (Figure 1A) explaining ∼35% of the observed genetic variance. The effect of this QTL on fecundity is greater in flies reared in 0.6% yeast than flies reared in 0.2% yeast (Figure 1C). As expected from the small number of lines used for this analysis, we detect only a limited number of QTL; there is likely to be considerable unmapped genetic variation for these traits in our population and in the wild population from which these lines were derived.
Fig. 1. QTL and fine-scale mapping of female fecundity localizes to 5 positional candidate genes. 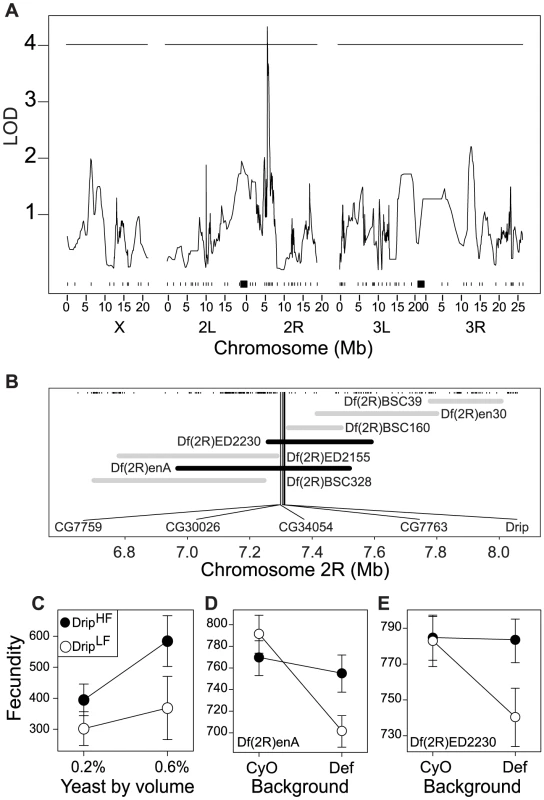
(A) QTL scan results for fecundity. The x-axis represents position along the three major D. melanogaster chromosomes in megabases (Mb). Tick marks represent location of polymorphic markers used for QTL mapping and black squares represent the approximate location of the centromeres. The y-axis represents the strength of association between a particular region and fecundity. The horizontal line represents the 95% permutation threshold. (B) Deficiency map of the QTL region on 2R. The x-axis represents position along chromosome 2R in megabases (Mb). Tick marks represent location of known genes and the horizontal bars represent the location (either molecularly defined or approximate) of deficiency break points. Grey bars represent deficiencies that complemented the RIL alleles, black bars represent deficiencies that failed to complement the RIL alleles. The five named genes are those genes identified by quantitative complementation as candidates genes affecting fecundity. (C) Estimated effect of the two RIL alleles at the QTL identified on chromosome 2R. Black and white circles represent high and low fecundity alleles, respectively. The x-axis represents larval rearing condition. The y-axis represents estimated fecundity. Error bars represent 95% CI calculated from amongst line variance. (D–E) Results from quantitative complementation tests with the two deficiencies that failed to complement the two alleles in the mapping population. The x-axis of each inset represents the tester chromosome (either “wild-type”- CyO, or deficiency – Def). Black vs. white circles represent the high and low fecundity RIL alleles, respectively. The y-axis of each inset represents estimated fecundity (see Materials and methods for more details). Error bars represent 95% CI based on non-parametric bootstrap resampling (5000 replicates), conditional on fly. The chromosome 2R genomic region affecting fecundity contains approximately 150 genes within the QTL bound by a 3-LOD interval. We used quantitative complementation with deficiencies to further identify the causal gene within this candidate region. Six independent RILs with the allele for relatively high fecundity and six RILs with the allele for relatively low fecundity were crossed to each of seven deficiencies that partially overlap across the QTL region (Figure 1B). From offspring we measured age specific fecundity to determine complementation. Deficiencies that do not complement the high/low fecundity alleles uncover a genomic subregion that contains a potentially causative locus for the variance in fecundity, assuming the two RIL alleles act in a semi-dominant manner and there is minimal epistasis. To statistically evaluate complementation with a likelihood-ratio test we tested the a priori contrast that fecundity among genotypes satisfied (A/balancer) = (B/balancer) = (A/deficiency)>(B/deficiency), where A is a second chromosome from high fecundity RIL allele, B is a second chromosome from the low fecundity RIL allele, balancer is the ‘wild-type’ balancer chromosome, and deficiency is particular aberration on the second chromosome. This contrast was only satisfied for two deficiencies: Df(2R)enA and Df(2R)ED2230 (Figure 1D–1E, Figure S2A–S2E, Table S6). Importantly these deficiencies overlap only in a small region, and thus imply that the causal locus is likely to be one of the five genes within this segment: CG7759, CG30026, CG34054, CG7763 and Drip (Figure 1B).
To determine which of these candidate genes could affect the observed variation in fecundity, we ubiquitously expressed RNAi from available UAS-lines for four of these five genes with the tubulin-Gal4 driver and measured fecundity. This experiment was not performed for CG34054 because the appropriate RNAi line did not exist. Each of these RNAi transgenes reduced mRNA of their targeted genes by 2.5 to 3.25 log2-fold (all p<0.05, Figure S3A). Expression of Drip-RNAi, but not of any other candidate, significantly reduced fecundity relative to the control at p<0.05 (Figure 2A, Table S7).
Fig. 2. Effects of overexpression of RNAi constructs against positional candidate genes on female fecundity. 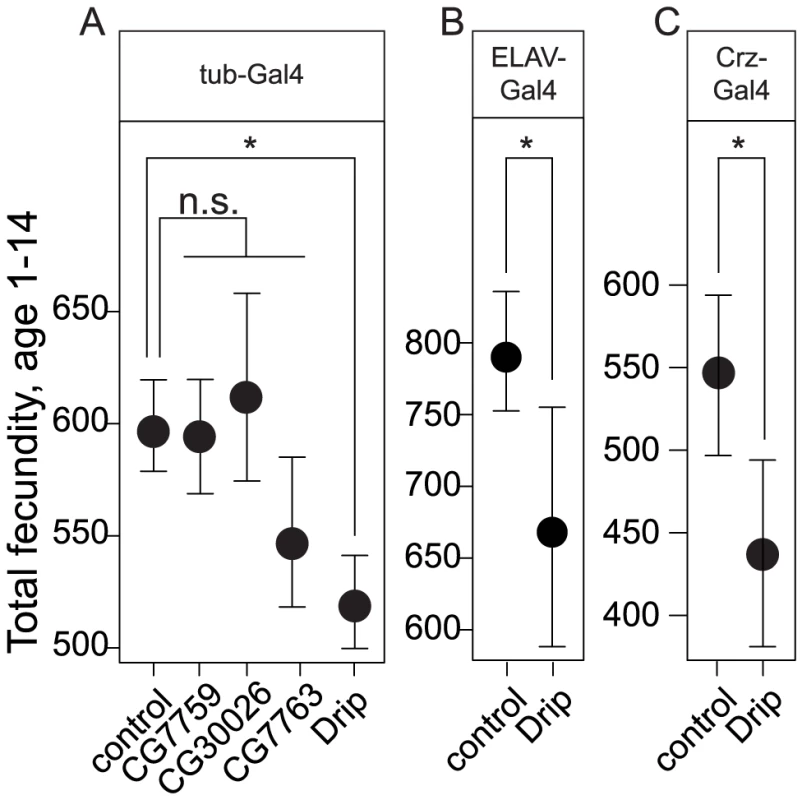
(A) Ubiquitous overexpression by the tub-Gal4 of RNAi constructs against driver for four of the five candidate genes. (B) Overexpression of Drip-RNAi with the pan-neuronal driver, Elav-Gal4, reduces female fecundity. (C) Overexpression of Drip-RNAi in corazonin producing neurons reduces female fecundity. Error bars represent 95% CI based on non-parametric bootstrap resampling (5000 replicates), conditional on fly. Asterisks represent significant difference from the control at p<0.05. Given that experimental repression of Drip reduces fecundity, we determined whether the endogenous expression of Drip differs amongst the high and low fecundity RIL alleles. Drip mRNA was measured from heads, ovaries plus lower reproductive tract (LRT) and carcasses (thorax, legs, wings, and the abdomen except the ovaries-LRT) from 3–5 day old mated females. Drip mRNA abundance did not differ among alleles in samples from the ovary-LRT or the carcass (ovary+LRT p = 0.3, carcass p = 0.28; Figure 3A). On the other hand, Drip mRNA was reduced ∼2.7 log2-fold in head tissue of RILs for the low fecundity allele relative to those of the high fecundity allele (p = 0.017, Figure 3A).
Fig. 3. Tissue-specific expression of Drip, Crz, and pale between the different RIL alleles. 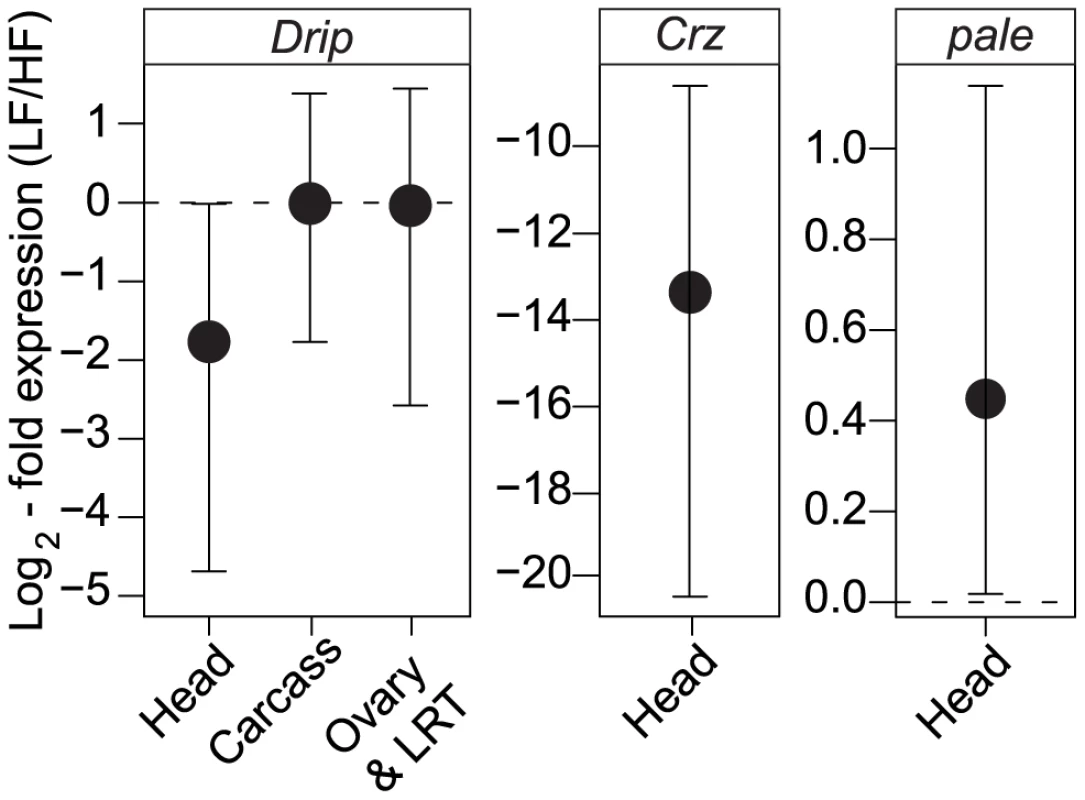
The y-axis represents fold change in Drip expression between low- and high-fecundity alleles, normalized to differences in Rpl32. Error bars represent 95% CI based on permutations; see text for details. The horizontal, dashed line represent the null hypothesis of no change in gene expression. To understand how variation in Drip might contribute to differences in fecundity we sequenced the Drip exons in the high and low fecundity RILs. Amongst the two alleles, we observed no non-synonymous SNPs and only two synonymous SNPs (Genbank accessions JN791442-3). Natural variation in fecundity caused by variation at the Drip locus is not likely to be caused by protein variation at Drip. As alternative explanations, nucleotide polymorphisms responsible for these expression differences might be caused by the synonymous SNPs if they affect mRNA stability or processing, or by polymorphisms in 3′ or 5′ UTRs or in intronic or 5′ enhancer regions. Based on the deficiency mapping, if the causative polymorphism resides in the 5′ enhancer region, it must occur within 6.5 Kb of the transcriptional start site.
To date, Drosophila Drip has been reported as expressed in the malphigian tubules [39]; a gut associated organ with kidney-like functions. In contrast, we observed Drip expression from the adult head. We used immunohistochemistry to determine which head tissues are responsible for this expression. Drip protein is stained in about 12 neurons located in the dorsolateral posterior compartments of the protocerebrum (Figure 4A–4B′). UAS-Drip-IR (Drip-RNAi) expressed with the pan neuronal driver ELAV-Gal4 effectively reduced Drip mRNA measured from adult heads by ∼1.75 log2-fold (p<0.001) and reduced fecundity by ∼20% (Figure 2B, Figure S3B, Table S7). Thus, neuronal expression of Drip is sufficient to affect fecundity.
Fig. 4. Drip is expressed in a neuropeptide corazonin (Crz) neurons located in protocerebrum of the brain. 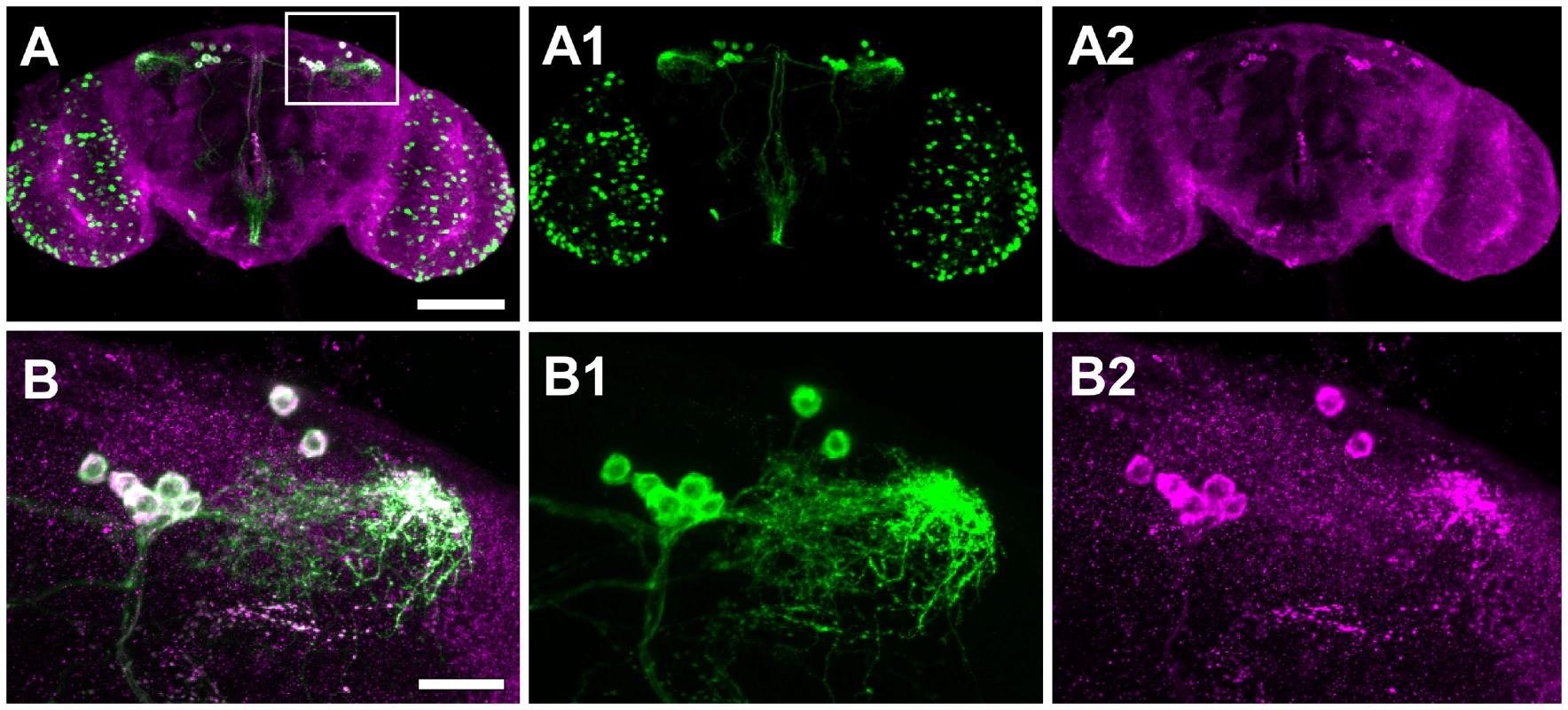
(A) Confocal sections of the brain of Crz-GL4/UAS-mCD8-EGFP female stained with anti-GFP (green, A1) and anti-Drip (magenta; A2). White indicates the overlap of these colors. Images are oriented with dorsal up. Scale bar, 100 µm. (B) Higher magnification views of the dorsolateral protocerebrum of the brain (inset box of A). Scale bar, 20 µm. To determine which dorsolateral posterior neurons express Drip, we screened neuropeptide-specific GAL4 lines for co-staining with anti-Drip antibody. Drip expression coincides with Crz-Gal4, which specifically marks neurons producing the neuropeptide corazonin ([42]; Figure 4A1–4B2). Drip-RNAi expressed in Crz neurons by Crz-Gal4 reduced both mRNA (p = 0.054, Figure S3C) and protein levels of Drip (Figure S4A–S4B′), and reduced fecundity by ∼20% (p = 8.5e-10, Figure 2C, Table S7).
Corazonin is a GnRH-like [43], stress response hormone [44] found in most insects [45]. In D. melanogaster, genetic ablation of corazonin producing neurons increases tolerance to starvation, osmotic and oxidative stress, decreases levels of trehalose [46], and increases triglyceride and dopamine [47] concentrations in the haemolymph. Accordingly, we tested if low and high fecundity RILs vary in Crz expression and if they vary in expression of the D. melanogaster homologue of tyrosine-hydroxylase, pale, the rate limiting step in the production of dopamine [48]. Low fecundity RILs show a 12 log2-fold decrease in Crz expression relative to high fecundity RILs (p<1e-6, Figure 3B) and a 0.5 log2 increase in pale expression (p = 0.01, Figure 3C). These results are consistent with the observation that exogenous application of dopamine in Drosophilid flies leads to a decrease in female fecundity [49].
Taken together, our data suggest that Drip mediates fecundity by altering corazonin and dopamine production. In drosophilids, genetic ablation of corazonin producing neurons increases stress resistance presumably by decreasing corazonin titer [44], [47]. Further, dopamine titer is positively correlated with stress resistance [47] and locomotor activity [50]. Here, we show that decreased Crz and increased pale expression are associated with a decrease in fecundity. Thus, these two neurohormones may contribute to the physiological basis for tradeoffs between reproduction and aspects of somatic maintenance contributing to increased stress resistance and activity. We hypothesize that Drip, an aquaporin involved in transport of small hydrophilic molecules such as water and glycerol across the plasma membrane [39], acts upstream of these neurohormones where it may function to signal hydration, nutritional status or osmolarity. If true, our model suggests that natural polymorphisms in Drip that affect female fecundity, a core life history trait, could be subject to balancing selection because they modulate the physiological basis for environmentally dependent life-history trade offs.
Materials and Methods
Fly stocks and husbandry
Initial QTL mapping was performed using twelve RILs (lines 69, 72, 94, 169, 218, 219, 252, 262, 285, 347, 369, 496). These lines are a subset of a larger population of RILs [38]. These RILs were made by crossing two isofemales lines derived from wild flies collected at the Wolfskill Orchard in Winters, CA (38°N, 121°W) in the summer of 2001. Any alleles segregating within this mapping population represent naturally segregating genetic variation. RILs were genotyped by oligoligation assay [51] at 102 SNPs along the X, 2nd and 3rd chromosomes and by PCR and Sanger sequencing at eleven additional genes near the QTL LOD peak in the RILs used in this study (see Text S1).
For phenotypic assays, RILs were reared at controlled density of 50 eggs/vial in media containing either 0.2% and 0.6% yeast by volume (YBV). Sugar, cornmeal, agar, and tegosept concentrations (11%, 8%, 5%, and 1% by volume, respectively) were kept constant. Rearing vials were kept at 25°C, 12L:12D at 40% relative humidity (RH). Each RIL was reared in four to five replicate vials for each of two replicate blocks. We also reared subsets of these RILs in 0.6% YBV under controlled densities for mRNA extraction. Extractions were made from three to five day old, mated females.
Quantitative complementation using deficiencies was performed by crossing RILs used for QTL mapping to one of seven deficiency stocks (Df(2R)BSC328, Df(2R)enA, Df(2R)ED2155, Df(2R)ED2230 [courtesy of H.A.J. Müller, [52]], Df(2R)BSC160, Df(2R)en30 and Df(2R)BSC39). Progeny of these crosses were reared at controlled density of 50 eggs/vial in media containing 0.6% YBV in four to five replicate vials.
RNAi over expression was performed using UAS-IR lines (CG7759, P{KK100412}; CG30026, P{KK107569}; Drip, P{KK107343}; CG7763, P{KK105972}) from the phiC31 insertion collection ([53]; courtesy VDRC, Vienna, Austria). As a control, we used y, w[1118]; P{attp, y[+], w[3′]} which contains the phiC31 landing site but no inverted repeat construct. These four UAS-IR lines and the control were crossed to tubulin-Gal4 (y1w*; P{w+mC, tub-Gal4}LL7/TM3, Sb−). UAS-IR against Drip along with the control were crossed to ELAV-Gal4 (w*, P{w+mW.hs = GawB}ElavC155) and Crz-Gal4 [[54]; (yw; P{w+mC, Crz-Gal4})]. Progeny of all crosses were reared in media containing 0.6% YBV as above.
Fecundity assays
Upon eclosion, virgin females were collected over ice and placed with two OreR males in vials containing media with 2.0% YBV (all other ingredients same as above) dyed green with food coloring (McCormick & Co, Inc.) and sprinkled with live yeast granules. Flies were kept at 25°C, 12L:12D and 40% RH during fecundity assays. Vials were changed daily for 14 days and stored at 4°C until the eggs were counted.
Ovariole number and thorax length
See Text S1.
Variance components, genetic and phenotypic correlation calculations of recombinant inbred lines
See Text S1.
Mapping
For QTL mapping, quantitative complementation and RNAi screens fecundity was analyzed as a function valued trait (e.g. [55]). We used a modified version of the triangular fecundity function [56] which we subsequently linearized (see SI) to facilitate efficient statistical analysis. The modified function takes the form , where , and where eggsx is the fecundity of an individual female at age x (agex), R is the set of random effects (e.g., vial effects) for a particular cross and ε is the normally distributed error. To calculate estimates of total fecundity (age 1–14) for a particular genotype we fit this function using the mixed effect model package lme4 [57] implemented in R 2.10 [58]. We then took the integral of the fitted function from days 1–14 and back transformed to produce estimates of total fecundity.
QTL mapping was performed with estimates of total fecundity for each RIL (see Text S1 for details) using multiple imputation [41] with 50 imputations per marker/pseudomarker and a pseudomarker step size of 3cM. We tested for association between genomic location and fecundity by fitting the full model, y = Mi+E+Mi:E+ε, and the reduced model, y = E+ε, where y is estimated fecundity, Mi is the effect of marker or pseudomarker i, E the effect of larval environment and Mi:E their interaction. The difference in LOD score between the full and reduced model represents the strength of association between a particular genomic region and variation in fecundity segregating amongst the RILs. Statistical significance of QTL was determined by permutation testing [59].
We tested for failure to complement in quantitative complementation tests by assessing the a priori contrast that A/Balancer = B/Balancer = A/Deficiency>B/Deficiency where A is the high fecundity RIL allele and B is the low fecundity RIL allele. We tested this contrast by fitting mixed effect model, using the R package lme4 [57], where A and B represent the fixed effect contrast matrices corresponding to [0,0,0,1] = [A/Bal, B/Bal, A/Def, B/Def] and R is the set of random effects that include RIL and rearing vial nested within RIL (see Text S1). In order to assess statistical significance of failure to complement we performed likelihood ratio tests between the above model and two reduced models that sequentially remove terms B and A. Two times the difference in log-likelihoods between the more complex and less complex model follows a χ2 distribution. Likelihood ratio tests for fixed effects such as these can be anticonservative when testing against a χ2 distribution with degrees of freedom equal to the difference number of parameters between the competing models (in this case, one; [60]). Therefore, we tested the likelihood ratio statistic against a χ2 distribution with two degrees of freedom which produces a conservative test. If the likelihood of models for a particular deficiency are significantly improved by including either A or B and if the parameter estimate for either A or B is below zero we regard that deficiency as failing to complement due to allelism.
We analyzed RNAi crosses in a similar fashion. For each Gal4 driver and UAS-RNAi cross, we fit the model where A and B are the contrast matrices [0,1] = [control, RNAi]. As above, we tested this model against two models that sequentially remove terms B and A and compared the likelihoods of these models. If the likelihoods of models for a particular Gal4-RNAi cross are significantly improved by including either A or B and if the parameter estimate for these contrast matrices is below zero, we conclude that the particular RNAi line reduces fecundity.
Quantitative PCR (qPCR)
mRNA transcript levels were measured with reverse-transcription qPCR. Flies were reared as above and frozen at −80°C until RNA extraction. To measure Drip expression in the head, ovary plus LRT and carcass in the RILs, flies were rinsed briefly in 95% ethanol, washed two times in phosphate buffered saline (PBS) and dissected in RNA Later (Qiagen). Tissue was stored overnight at 4°C in RNA Later prior to RNA extraction. RNA from the RNAi over expression experiments was extracted from whole flies for tub-Gal4 crosses and from heads in ELAV-Gal4 and Crz-Gal4 crosses in three biological replicates, each containing tissue from 10–15 female flies using TRIzol reagent (Invitrogen). Purity and quantity of RNA was determined spectrophotometrically (NanoDrop, ND-1000) and treated with DNase to remove residual DNA contamination (Ambion). Reverse transcription and quantification were performed using iScript One-Step RT-PCR kit with SYBR Green (Bio Rad) or SensiFAST SYBR One-Step Kit (Bioline) and measured on either a ABI prism 7300 Sequence Detection System (Applied Biosystems) or Eco (Illumina) qPCR machine. Gene expression for each biological replicate was measured three times (technical replicates). mRNA levels of Rpl32 were used to normalize mRNA levels of target genes. qPCR data were analyzed using the qpcR package [61] in R 2.10 [58]. Reported p values and confidence intervals were calculated from permutation tests. For more information on primers see Text S1.
Immunohistochemistry
3–5 day-old virgin females were dissected under PBS (pH 7.4). The brain tissues were fixed for 30 minutes at room temperature in 4% paraformaldehyde in PBS, and then were incubated in primary antibody for 48 hours at 4°C, and in secondary antibody for 24 hours at 4°C. Antibodies used were: rabbit anti-Drip ([62], 1∶500), mouse anti-GFP (1∶1000; Sigma G6539), Alexa 488 anti-rabbit (1∶1000, Invitrogen A11008), Alexa 488 anti-mouse (1∶1000, Invitrogen A11001), Alexa 568 anti-mouse (1∶1000, Invitrogen A11004). Images were acquired with a Zeiss LSM 700 and were processed in Image J [63].
Supporting Information
Zdroje
1. FisherRA 1958 The genetical theory of natural selection New York Dover Publications 291
2. RobertsonA 1968 The spectrum of genetic variation. LewontinRC Population Biology and Evolution Syracuse, N.Y., USA Syracuse University Press
3. LewontinRC 1974 The Genetic Basis of Evolutionary Change New York Columbia University Press
4. StearnsSC 1976 Life-history tactics: a review of the ideas. The Quarterly review of biology 51 3 47
5. LeveneH 1953 Genetic Equilibrium When More Than One Ecological Niche Is Available. American Naturalist 87 331 333
6. WrightS 1956 Modes of selection. The American Naturalist 90 5 24
7. HedrickPW 1972 Maintenance of genetic variation with a frequency-dependent selection model as compared to the overdominant model. Genetics 72 771 775
8. EllegrenHSheldonBC 2008 Genetic basis of fitness differences in natural populations. Nature 452 169 175
9. GazzaniSGendallARListerCDeanC 2003 Analysis of the molecular basis of flowering time variation in Arabidopsis accessions. Plant physiology 132 1107 1114
10. MichaelsSDHeYScortecciKCAmasinoRM 2003 Attenuation of FLOWERING LOCUS C activity as a mechanism for the evolution of summer-annual flowering behavior in Arabidopsis. Proceedings of the National Academy of Sciences of the United States of America 100 10102 10107
11. JohansonUWestJListerCMichaelsSAmasinoR 2000 Molecular analysis of FRIGIDA, a major determinant of natural variation in Arabidopsis flowering time. Science 290 344 347
12. WheatCWFescemyerHWKvistJTasEVeraJC 2011 Functional genomics of life history variation in a butterfly metapopulation. Molecular Ecology 20 1813 1828
13. WilliamsKDBustoMSusterMLSoAKBen-ShaharY 2006 Natural variation in Drosophila melanogaster diapause due to the insulin-regulated PI3-kinase. Proc Natl Acad Sci U S A 103 15911 15915
14. TauberEZordanMSandrelliFPegoraroMOsterwalderN 2007 Natural selection favors a newly derived timeless allele in Drosophila melanogaster. Science 316 1895 1898
15. SchmidtPSZhuCTDasJBataviaMYangL 2008 An amino acid polymorphism in the couch potato gene forms the basis for climatic adaptation in Drosophila melanogaster. Proc Natl Acad Sci U S A 105 16207 16211
16. NuzhdinSVPasyukovaEGDildaCLZengZBMackayTF 1997 Sex-specific quantitative trait loci affecting longevity in Drosophila melanogaster. Proc Natl Acad Sci U S A 94 9734 9739
17. LeipsJMackayTF 2000 Quantitative trait loci for life span in Drosophila melanogaster: interactions with genetic background and larval density. Genetics 155 1773 1788
18. PasyukovaEGVieiraCMackayTF 2000 Deficiency mapping of quantitative trait loci affecting longevity in Drosophila melanogaster. Genetics 156 1129 1146
19. VieiraCPasyukovaEGZengZBHackettJBLymanRF 2000 Genotype-environment interaction for quantitative trait loci affecting life span in Drosophila melanogaster. Genetics 154 213 227
20. CurtsingerJWKhazaeliAA 2002 Lifespan, QTLs, age-specificity, and pleiotropy in Drosophila. Mechanisms of ageing and development 123 81 93
21. LeipsJMackayTF 2002 The complex genetic architecture of Drosophila life span. Exp Aging Res 28 361 390
22. ValenzuelaRKForbesSNKeimPServicePM 2004 Quantitative trait loci affecting life span in replicated populations of Drosophila melanogaster. II. Response to selection. Genetics 168 313 324
23. ForbesSNValenzuelaRKKeimPServicePM 2004 Quantitative trait loci affecting life span in replicated populations of Drosophila melanogaster. I. Composite interval mapping. Genetics 168 301 311
24. NuzhdinSVKhazaeliAACurtsingerJW 2005 Survival analysis of life span quantitative trait loci in Drosophila melanogaster. Genetics 170 719 731
25. WilsonRHMorganTJMackayTF 2006 High-resolution mapping of quantitative trait loci affecting increased life span in Drosophila melanogaster. Genetics 173 1455 1463
26. LaiCQParnellLDLymanRFOrdovasJMMackayTF 2007 Candidate genes affecting Drosophila life span identified by integrating microarray gene expression analysis and QTL mapping. Mechanisms of ageing and development 128 237 249
27. CarboneMAJordanKWLymanRFHarbisonSTLeipsJ 2006 Phenotypic variation and natural selection at catsup, a pleiotropic quantitative trait gene in Drosophila. Curr Biol 16 912 919
28. De LucaMRoshinaNVGeiger-ThornsberryGLLymanRFPasyukovaEG 2003 Dopa decarboxylase (Ddc) affects variation in Drosophila longevity. Nat Genet 34 429 433
29. MackayTFCRoshinaNVLeipsJPasyukovaEG 2006 Complex genetic architecture of Drosophila longevity. MasaroEJAustadSN Handbook of the biology of Aging, Sixth Edition Burlington, MA Elsevier Academic Press 181 216
30. PasyukovaEGRoshinaNVMackayTF 2004 Shuttle craft: a candidate quantitative trait gene for Drosophila lifespan. Aging Cell 3 297 307
31. RybinaOYPasyukovaEG 2010 A naturally occurring polymorphism at Drosophila melanogaster Lim3 Locus, a homolog of human LHX3/4, affects Lim3 transcription and fly lifespan. PLoS ONE 5 e12621 doi:10.1371/journal.pone.0012621
32. FlattTSchmidtPS 2009 Integrating evolutionary and molecular genetics of aging. Biochim Biophys Acta 1790 951 962
33. PaabyABSchmidtPS 2008 Functional significance of allelic variation at methuselah, an aging gene in Drosophila. PLoS ONE 3 e1987 doi:10.1371/journal.pone.0001987
34. PaabyABlacketMJHoffmannAASchmidtPS 2010 Identification of a candidate adaptive polymorphism for Drosophila life history by parallel independent clines on two continents. Molecular Ecology 19 760 774
35. LeipsJGilliganPMackayTF 2006 Quantitative trait loci with age-specific effects on fecundity in Drosophila melanogaster. Genetics 172 1595 1605
36. HughesKALeipsJ 2006 Quantitative trait locus analysis of male mating success and sperm competition in Drosophila melanogaster. Evolution; international journal of organic evolution 60 1427 1434
37. WayneMLHackettJBDildaCLNuzhdinSVPasyukovaEG 2001 Quantitative trait locus mapping of fitness-related traits in Drosophila melanogaster. Genet Res 77 107 116
38. BerglandAOGenisselANuzhdinSVTatarM 2008 Quantitative trait loci affecting phenotypic plasticity and the allometric relationship of ovariole number and thorax length in Drosophila melanogaster. Genetics 180 567 582
39. KaufmannNMathaiJCHillWGDowJAZeidelML 2005 Developmental expression and biophysical characterization of a Drosophila melanogaster aquaporin. American journal of physiology Cell physiology 289 C397 407
40. GautamNKTapadiaMG 2010 Ecdysone signaling is required for proper organization and fluid secretion of stellate cells in the Malpighian tubules of Drosophila melanogaster. The International journal of developmental biology 54 635 642
41. SenSChurchillGA 2001 A statistical framework for quantitative trait mapping. Genetics 159 371 387
42. ChoiYJLeeGParkJH 2006 Programmed cell death mechanisms of identifiable peptidergic neurons in Drosophila melanogaster. Development 133 2223 2232
43. ParkYKimYJAdamsME 2002 Identification of G protein-coupled receptors for Drosophila PRXamide peptides, CCAP, corazonin, and AKH supports a theory of ligand-receptor coevolution. Proceedings of the National Academy of Sciences of the United States of America 99 11423 11428
44. VeenstraJA 2009 Does corazonin signal nutritional stress in insects? Insect biochemistry and molecular biology 39 755 762
45. BoerjanBVerleyenPHuybrechtsJSchoofsLDe LoofA 2010 In search for a common denominator for the diverse functions of arthropod corazonin: a role in the physiology of stress? General and comparative endocrinology 166 222 233
46. LeeGKimKMKikunoKWangZChoiYJ 2008 Developmental regulation and functions of the expression of the neuropeptide corazonin in Drosophila melanogaster. Cell and tissue research 331 659 673
47. ZhaoYBretzCAHawksworthSAHirshJJohnsonEC 2010 Corazonin neurons function in sexually dimorphic circuitry that shape behavioral responses to stress in Drosophila. PLoS ONE 5 e9141 doi:10.1371/journal.pone.0009141
48. NeckameyerWSQuinnWG 1989 Isolation and characterization of the gene for Drosophila tyrosine hydroxylase. Neuron 2 1167 1175
49. GruntenkoNEKarpovaEKAlekseevAAChentsovaNASaprykinaZV 2005 Effects of dopamine on juvenile hormone metabolism and fitness in Drosophila virilis. Journal of Insect Physiology 51 959 968
50. RiemenspergerTIsabelGCoulomHNeuserKSeugnetL 2011 Behavioral consequences of dopamine deficiency in the Drosophila central nervous system. Proceedings of the National Academy of Sciences of the United States of America 108 834 839
51. MacdonaldSJPastinenTGenisselACornforthTWLongAD 2005 A low-cost open-source SNP genotyping platform for association mapping applications. Genome biology 6 R105
52. GryzikTMullerHA 2004 FGF8-like1 and FGF8-like2 encode putative ligands of the FGF receptor Htl and are required for mesoderm migration in the Drosophila gastrula. Current biology: CB 14 659 667
53. DietzlGChenDSchnorrerFSuKCBarinovaY 2007 A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 448 151 156
54. ChoiYJLeeGHallJCParkJH 2005 Comparative analysis of Corazonin-encoding genes (Crz's) in Drosophila species and functional insights into Crz-expressing neurons. The Journal of comparative neurology 482 372 385
55. KingsolverJGGomulkiewiczRCarterPA 2001 Variation, selection and evolution of function-valued traits. Genetica 112–113 87 104
56. McMillanIFitz-EarleMRobsonDS 1970 Quantitative genetics of fertility. I. Lifetime egg production of Drosophila melanogaster–theoretical. Genetics 65 349 353
57. BatesDMaechlerM 2009 lme4: Linear mixed-effects models using S4 classes. R package version: 0.999375-32 ed
58. Team RCD 2009 R: A language and environment for statistical computing Vienna, Austria R Foundation for Statistical Computing
59. DoergeRWChurchillGA 1996 Permutation tests for multiple loci affecting a quantitative character. Genetics 142 285 294
60. PinheiroJCBatesD 2000 Mixed-effect models in S and S-PLUS New York, N.Y. Springer Verlag
61. RitzCSpiessAN 2008 qpcR: an R package for sigmoidal model selection in quantitative real-time polymerase chain reaction analysis. Bioinformatics 24 1549 1551
62. SpringJHRobichauxSRKaufmannNBrodskyJL 2007 Localization of a Drosophila DRIP-like aquaporin in the Malpighian tubules of the house cricket, Acheta domesticus. Comparative biochemistry and physiology Part A, Molecular & integrative physiology 148 92 100
63. AbramoffMDMagalhaePJRamSJ 2004 Image processing with ImageJ. Biophotonics International 11 36 42
Štítky
Genetika Reprodukční medicína
Článek A Genome-Wide Screen for Genetic Variants That Modify the Recruitment of REST to Its Target GenesČlánek Population Structure of Hispanics in the United States: The Multi-Ethnic Study of AtherosclerosisČlánek Differing Requirements for RAD51 and DMC1 in Meiotic Pairing of Centromeres and Chromosome Arms inČlánek Transcriptional Regulation of Rod Photoreceptor Homeostasis Revealed by NRL Targetome AnalysisČlánek Cell Contact–Dependent Outer Membrane Exchange in Myxobacteria: Genetic Determinants and MechanismČlánek Formation of Rigid, Non-Flight Forewings (Elytra) of a Beetle Requires Two Major Cuticular Proteins
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 4
-
Všechny články tohoto čísla
- Runs of Homozygosity Implicate Autozygosity as a Schizophrenia Risk Factor
- Modifier Genes and the Plasticity of Genetic Networks in Mice
- The DSIF Subunits Spt4 and Spt5 Have Distinct Roles at Various Phases of Immunoglobulin Class Switch Recombination
- A Genome-Wide Screen for Genetic Variants That Modify the Recruitment of REST to Its Target Genes
- Population Structure of Hispanics in the United States: The Multi-Ethnic Study of Atherosclerosis
- Deep Sequencing of Plant and Animal DNA Contained within Traditional Chinese Medicines Reveals Legality Issues and Health Safety Concerns
- Differing Requirements for RAD51 and DMC1 in Meiotic Pairing of Centromeres and Chromosome Arms in
- Insulin Signaling Mediates Sexual Attractiveness in
- Progressive Telomere Dysfunction Causes Cytokinesis Failure and Leads to the Accumulation of Polyploid Cells
- Long-Range Chromosome Organization in : A Site-Specific System Isolates the Ter Macrodomain
- Regulation of Budding Yeast Mating-Type Switching Donor Preference by the FHA Domain of Fkh1
- Polyglutamine Toxicity Is Controlled by Prion Composition and Gene Dosage in Yeast
- Patterns of Regulatory Variation in Diverse Human Populations
- Sequence-Specific Targeting of Dosage Compensation in Favors an Active Chromatin Context
- Whole-Exome Sequencing and Homozygosity Analysis Implicate Depolarization-Regulated Neuronal Genes in Autism
- Replication Fork Reversal after Replication–Transcription Collision
- Common Variants at 9p21 and 8q22 Are Associated with Increased Susceptibility to Optic Nerve Degeneration in Glaucoma
- Coordinate Regulation of Lipid Metabolism by Novel Nuclear Receptor Partnerships
- Epigenome-Wide Scans Identify Differentially Methylated Regions for Age and Age-Related Phenotypes in a Healthy Ageing Population
- A Coordinated Interdependent Protein Circuitry Stabilizes the Kinetochore Ensemble to Protect CENP-A in the Human Pathogenic Yeast
- Budding Yeast Dma Proteins Control Septin Dynamics and the Spindle Position Checkpoint by Promoting the Recruitment of the Elm1 Kinase to the Bud Neck
- , a Homolog of a Deaf-Blindness Gene, Regulates Circadian Output and Slowpoke Channels
- Transcriptional Regulation of Rod Photoreceptor Homeostasis Revealed by NRL Targetome Analysis
- Cell Contact–Dependent Outer Membrane Exchange in Myxobacteria: Genetic Determinants and Mechanism
- Defective Membrane Remodeling in Neuromuscular Diseases: Insights from Animal Models
- Formation of Rigid, Non-Flight Forewings (Elytra) of a Beetle Requires Two Major Cuticular Proteins
- SPE-44 Implements Sperm Cell Fate
- A Shared Role for RBF1 and dCAP-D3 in the Regulation of Transcription with Consequences for Innate Immunity
- A Companion Cell–Dominant and Developmentally Regulated H3K4 Demethylase Controls Flowering Time in via the Repression of Expression
- The HEN1 Ortholog, HENN-1, Methylates and Stabilizes Select Subclasses of Germline Small RNAs
- Improved Statistics for Genome-Wide Interaction Analysis
- The Probability of a Gene Tree Topology within a Phylogenetic Network with Applications to Hybridization Detection
- Context-Dependent Dual Role of SKI8 Homologs in mRNA Synthesis and Turnover
- Mu Insertions Are Repaired by the Double-Strand Break Repair Pathway of
- Competition between Replicative and Translesion Polymerases during Homologous Recombination Repair in Drosophila
- An Unbiased Assessment of the Role of Imprinted Genes in an Intergenerational Model of Developmental Programming
- Type 2 Diabetes Risk Alleles Demonstrate Extreme Directional Differentiation among Human Populations, Compared to Other Diseases
- Mutations in and Cause “Splashed White” and Other White Spotting Phenotypes in Horses
- Fine-Scale Mapping of Natural Variation in Fly Fecundity Identifies Neuronal Domain of Expression and Function of an Aquaporin
- Dynamics of Brassinosteroid Response Modulated by Negative Regulator LIC in Rice
- Genetic Inhibition of Solute-Linked Carrier 39 Family Transporter 1 Ameliorates Aβ Pathology in a Model of Alzheimer's Disease
- The Functions of Mediator in Support a Role in Shaping Species-Specific Gene Expression
- Patterns of Ancestry, Signatures of Natural Selection, and Genetic Association with Stature in Western African Pygmies
- Dissection of Pol II Trigger Loop Function and Pol II Activity–Dependent Control of Start Site Selection
- PIWI Associated siRNAs and piRNAs Specifically Require the HEN1 Ortholog
- Genome-Wide Patterns of Gene Expression in Nature
- Hypoxia Disruption of Vertebrate CNS Pathfinding through EphrinB2 Is Rescued by Magnesium
- A New Role for Translation Initiation Factor 2 in Maintaining Genome Integrity
- Sex Reversal in C57BL/6J XY Mice Caused by Increased Expression of Ovarian Genes and Insufficient Activation of the Testis Determining Pathway
- The Rac GTP Exchange Factor TIAM-1 Acts with CDC-42 and the Guidance Receptor UNC-40/DCC in Neuronal Protrusion and Axon Guidance
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- A Coordinated Interdependent Protein Circuitry Stabilizes the Kinetochore Ensemble to Protect CENP-A in the Human Pathogenic Yeast
- Coordinate Regulation of Lipid Metabolism by Novel Nuclear Receptor Partnerships
- Defective Membrane Remodeling in Neuromuscular Diseases: Insights from Animal Models
- Formation of Rigid, Non-Flight Forewings (Elytra) of a Beetle Requires Two Major Cuticular Proteins
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



