-
Články
Reklama
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
ReklamaClonality and Evolutionary History of Rhabdomyosarcoma
To decipher the dynamic mutational process and identify the causative genomic events in rhabdomyosarcoma (RMS), we developed a systematic method that incorporates multiple types of genomic information to estimate normal cell contamination, tumor clonality, and a timeline of somatic events that occurred prior to the tumor presentation. Our results demonstrate two distinct evolutionary paths resulting in PAX-fusion-negative-rhabdomyosarcoma (PFN-RMS) and PAX-fusion-positive-rhabdomyosarcoma (PFP-RMS): (1) In PFN-RMS, genomic loss of heterozygosity on chromosome 11p15.5 and non-synonymous mutations in RAS pathway and cell cycle genes (FGFR4, KRAS, NRAS, HRAS and CCDN1), as well as several genes not previously known to be drivers of RMS, including PKN1, CUL2, and TTK, occurs early in the evolutionary history of tumor; (2) In contrast, the PAX gene fusion event in PFP-RMS tumors is an early detectable event which consistently occurs prior to a whole genome duplication event. These findings provide new insights into the biology and molecular events that initiate RMS tumorigenesis and may help identify actionable drivers for targeted therapies.
Published in the journal: . PLoS Genet 11(3): e32767. doi:10.1371/journal.pgen.1005075
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005075Summary
To decipher the dynamic mutational process and identify the causative genomic events in rhabdomyosarcoma (RMS), we developed a systematic method that incorporates multiple types of genomic information to estimate normal cell contamination, tumor clonality, and a timeline of somatic events that occurred prior to the tumor presentation. Our results demonstrate two distinct evolutionary paths resulting in PAX-fusion-negative-rhabdomyosarcoma (PFN-RMS) and PAX-fusion-positive-rhabdomyosarcoma (PFP-RMS): (1) In PFN-RMS, genomic loss of heterozygosity on chromosome 11p15.5 and non-synonymous mutations in RAS pathway and cell cycle genes (FGFR4, KRAS, NRAS, HRAS and CCDN1), as well as several genes not previously known to be drivers of RMS, including PKN1, CUL2, and TTK, occurs early in the evolutionary history of tumor; (2) In contrast, the PAX gene fusion event in PFP-RMS tumors is an early detectable event which consistently occurs prior to a whole genome duplication event. These findings provide new insights into the biology and molecular events that initiate RMS tumorigenesis and may help identify actionable drivers for targeted therapies.
Introduction
Cancer development is driven by dynamic mutational processes and selective pressures which allow a tumor to adapt over time from the initiating oncogenic lesion towards clinical presentation [1–4]. High coverage, next-generation sequencing technologies have provided an unprecedented view of the mutational landscape of whole cancer genomes and demonstrated that cancer genomes have typically acquired thousands of somatic alterations by the time they are clinically detected [5–8]. Although a majority of these alterations do not have clear biological consequences, some alterations are recurrently found; implicating them as critical events in that tumor’s evolution. Subsequently, external factors such as the tumor microenvironment and therapy can confer selective advantages that allow successful clones to eventually supersede one another [1,2]. Two of the major findings of next generation sequencing studies are that biopsies taken at the time of tumor presentation already contain a significant amount of genetic heterogeneity [5,6] and importantly that often a rare subclone in the primary tumor subsequently becomes the founding clone of a metastatic or relapse tumor [5,6,9,10]. The remarkable accuracy and read coverage depth of whole genome sequencing technology now enables the inference of intra-tumor heterogeneity and cancer evolutionary history that is encrypted in its mutational profile [2,9,11–14]. The study of the evolutionary history of a cancer provides insight into which mutations are cancer-driving from the numerous passenger mutations and sheds light on the mechanism of tumorigenesis.
Rhabdomyosarcoma (RMS) is the most common soft-tissue sarcoma of childhood. Despite a growing understanding of the molecular mechanism underlying RMS, the disease continues to have significant mortality and morbidity especially when the tumor is metastatic or recurrent [15–17]. RMS tumors can be subdivided into two major subtypes: PAX fusion positive (PFP) and fusion negative (PFN), characterized by the presence or absence of a oncogenic fusion between the PAX3 or PAX7 and FOXO1 genes [18]. Fusion positive tumors tend to occur in adolescence and are associated with an adverse outcome. Fusion negative tumors typically occur at a younger age and have been associated with significant aneuploidy, loss of heterozygosity (LOH) at chromosome 11p15.5 [19] and mutations of NRAS, KRAS, HRAS [20], PIK3CA, CTNNB1 [21,22] and FGFR4 [22]. We have recently reported that PFP - and PFN-RMS have distinct landscapes of somatic genomic alterations that activate a common molecular pathway [23]. Another effort, using high coverage targeted re-sequencing of two relapsed fusion negative tumors, showed that indeed RMS relapse tumors are derived from a minor subclone discovered in the primary tumor [24].
In this study, we decipher for the first time, the evolutionary history of RMS using high coverage whole-genome sequencing of 44 primary tumors with their matched normal samples. We applied a framework of algorithms that enabled the prediction of the sequential order of mutational events and subclonality using chronological molecular information encoded in somatic mutations and allelic copy number (including copy number changes and allelic imbalance). The major inputs to our method include the variant allele fraction (VAF) of somatic mutations, the variant allele fraction of germline single nucleotide variants in tumor samples and somatic copy number status. We verified the accuracy of mutation detection and VAF estimation by deep sequencing on independent platforms. Our framework first estimates the rate of normal cell contamination and corrects its effect on VAF and copy number. Tumor subclones with different genomic profiles are then identified using the distribution of VAF of somatic mutations as well as the allelic copy number status. Finally, we estimate the temporal progression of the observed somatic events in each tumor genome based on the fact that somatic mutations that occur before and after aneuploidy events will have different values of VAF.
Through our analysis, we discovered that the initiating common lesions of PFN-RMS are the combination of loss of heterozygosity of chromosome 11p15.5 and point mutations in members of the RAS pathway in majority of cases. In a small number of PFN-RMS tumors where no obvious RAS pathway mutations were present, we identified alternative genes that were mutated early in the tumors progression, indicating their potential roles in oncogenesis. In PFP-RMS, we discover that a whole genome duplication event which results in tetraploidy consistently occurs in the middle or late stage of the development of these tumors and that the PAX3-FOXO1 fusion event occurs prior to the whole genome duplication event, making it probable that the fusion is an early event in the evolutionary history of these fusion-positive RMS. Finally, we find that in general RMS tumors are universally composed of a dominant clone, although each tumor contains subclonal populations with a unique mutational profile, which may provide selective advantage to a relapse or metastatic tumor. Because we sequenced only one tumor sample for each patient, a limitation of our study, despite a moderately high coverage of the whole genome sequencing (average 105X), is that we may miss some subclonal mutations and clones especially for subclonal mutations with a VAF<0.1. Nevertheless our findings allow us to propose a developmental model of how this devastating pediatric cancer initiates and evolves prior to presentation.
Results
Large-scale deep WGS analysis of RMS
We used data from whole-genome sequencing (WGS) of 44 primary RMS tumors (19 PAX - fusion-positive, and 25 PFN tumors) with paired blood samples (providing germline status) with an average of 105x coverage per genome base. The accuracy of somatic mutation detection has been estimated as 93% by experiments in which 604 non-silent mutations were verified by whole-exome sequencing and targeted sequencing on independent platforms [23]. To verify the observed VAF of somatic mutations, we re-sequenced the somatic mutations discovered in two RMS samples using multiplex PCR and deep sequencing (1997x coverage) with an orthologous platform. The verification rate of VAF was high by the targeted sequencing (90% accuracy respectively, S1 Fig.). In order to accurately estimate the timing of somatic alterations and dissect intra-tumor subclonality, we first estimated the portion of normal cells contaminating the tumor sample by surveying allelic copy number status and VAF of somatic mutations across the genome (S1 Text). The normal cell contamination rate was generally low in the 44 tumor samples, with a range of 0–33%, a median of 16% and a standard deviation of 9% (S1 Table). The effect of normal cell contamination was numerically corrected for in all subsequent analyses so that the VAF and copy number status are purely for tumor population. The efficacy of normal cell contamination correction is illustrated in S2–S4 Figs., where the observed non-integer allelic copy number was corrected to integers and the observed VAF distribution of somatic mutations was corrected to the expected distribution, e.g. VAF = 0.5 for heterozygous mutations on chromosomes without aneuploidy.
Subclonality analysis
Multiple studies have shown that subclones exist in individuals with cancer which may become a major clone at relapse or progression and this phenomenon has been reported in two fusion negative RMS tumors [24]. To identify those events which may provide resistance to therapy and allow recurrence, we searched for genomic alterations that specifically were confined to the subclonal population. Subclonal copy number alteration events and mutational events were identified using an in-silico method (for which the workflow is shown in S5 Fig.). Fifty point mutations were identified where the variant’s allele frequency was greater than 10% from the expected full clonal allele frequency (S2 Table). Included in this gene list were 7 COSMIC genes including ABL1, BUB1B, CDK12, ERBB2, IGF2, KDR, and SMARCA4. Gene ontology showed enrichment for genes involved in cell adhesion (GO:0007155 p = 0.002), negative regulation DNA repair (GO:0006281, p = 0.05) and cell migration (GO:0030335 p = 0.026). Across the population, several chromosomal events were recurrently found in the subclonal population, including gain of chromosomes, 5, 8, 11, 13, 14, 18 and 19 (S1 Table).
We illustrate our method in more detail using one PAX-fusion negative RMS tumor (RMS2110). Genome wide, this tumor had 3,889 somatic mutations including oncogenic mutation KRAS G13D and FGFR4 V550L [23]. Using the procedure described in the previous section, we estimated that this sample had 12% normal cell contamination (S1 Table). Copy number analysis showed that RMS2110 had large-segment allelic imbalance (including copy neutral LOH) and copy number alteration on eleven chromosomes (Fig. 1A).We employed the combination of somatic mutations, allelic imbalance and copy number alterations to dissect tumor clones that have unique genomic profiles. First, VAF of somatic mutations was determined to find subclonal mutations, based on the fact that a subclonal mutation (present only in a part of the tumor cells) usually has lower VAF than full-clonal mutations (present in all tumor cells) [2]. A typical scenario is on chromosomes without aneuploidy or allelic imbalance, where heterozygous full-clonal mutations have VAF equal to 0.5 while subclonal mutations have VAF<0.5. Analysis of RMS2110 showed that more than half of the chromosomes are without aneuploidy or allelic imbalance (Fig. 1A and 1B). On these chromosomes, a small portion of somatic mutations have observed VAF significantly lower than others indicating the presence of subclones (Fig. 1C, where there are two distinct VAF clusters, one is centered on VAF = 0.5 and the other is centered on VAF = 0.2; for individual chromosomes see Fig. 1D). Using a clustering algorithm with cluster-number-selection procedure (S1 Text) we can identify subclonal mutations as those with lower VAF. Given the depth of sequencing coverage, we estimated that we could detect subclonal mutations with VAF as low as 0.1 (S6 Fig.). Second, allelic copy number status is used to detect subclonal copy number alterations, based on the fact that subclones of different copy number will result in a non-integer allelic copy number for the whole tumor sample. The joint status of total copy number and lesser allele fraction (LAF—the ratio between the less allelic copy number and total copy number, estimated by germline single nucleotide variants, see Methods) reflects whether the allelic copy number is an integer—whether the observed allelic copy number (red dots) is on the expected position (blue crosses) in Fig. 1D. Therefore our approach predicts the subclonal copy number alterations and the fraction of tumor cells possessing these changes (S1 Text).
Fig. 1. Inferring evolutionary history for a typical rhabdomyosarcoma sample RMS2110. 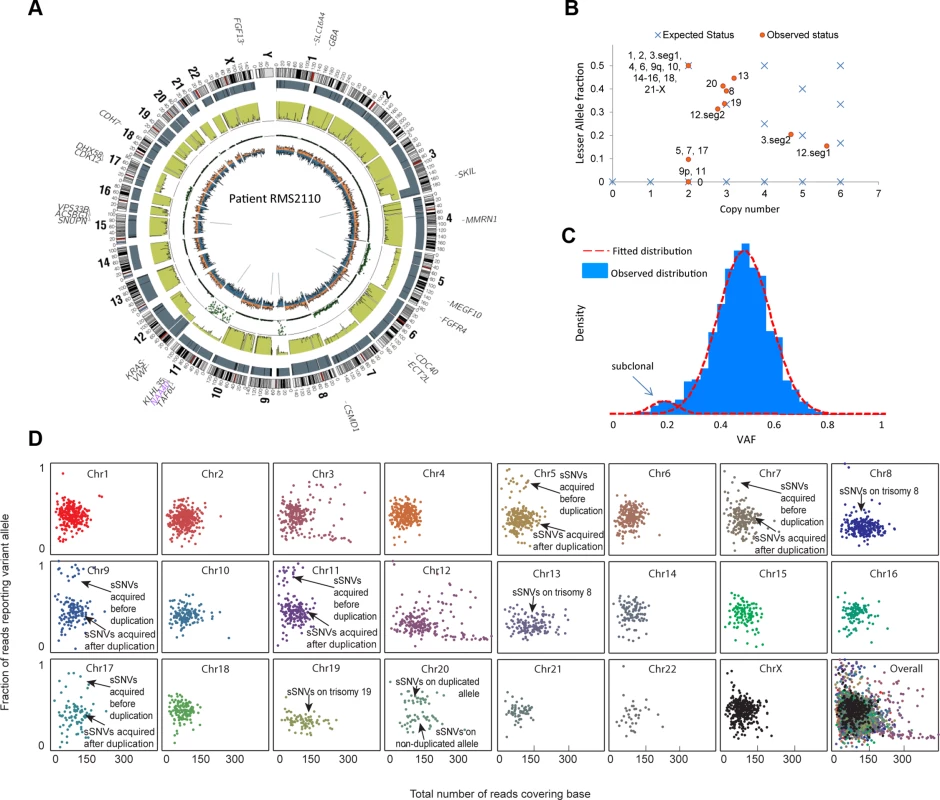
(a) A circos plot of tumor genome RMS2110 (before normal cell contamination correction). Gene symbols indicate genes with nonsynonymous mutations. Tracks from outermost to innermost depict chromosome banding, copy number (the height of bars denotes copy number), lesser allele fraction (the height of green bars represents the fraction of lesser allele at each genomic location, valued 0∼0.5), loss of heterogeneity status (each dot represents the probability of loss of heterozygosity for the adjacent segment), intensity of heterozygous (orange bar) and homozygous single nucleotide variants (blue bar), junctions or chromosomal rearrangement (grey lines). (b) Discrepancy between expected and observed status of allelic copy number suggests the existence of subclone(s) in tumor. Red dots denote chromosome segments, whereas blue crosses denote statuses of copy number and lesser allele fraction that leads to an integer allelic copy number. If there are tumor subclones with copy number status different from the major clone, the red dots will deviate from the blue crosses. (c) Observed VAF distribution of somatic mutations on chromosomes without aneuploidy. The normal-mixture like distribution suggest the existence of a minor subclone with VAF<0.5. (d) A scatter plot showing coverage (horizontal axis) and VAF (vertical axis) for somatic mutations (sSNV) on each chromosome. We thus predict that the subclonal somatic mutations made up only 5% of all the somatic mutations in RMS2110 tumor. Subclonal copy number alterations were detected on chromosomes 5, 7, 8, 12, 13, 17 and 20.
The method was then applied to all the 44 RMS tumors. Looking across all 44 genomes revealed that a dominant clonal lineage was present in each tumor sample (Fig. 2A). The dominant clone carried a large proportion (from 81–96%) of somatic mutations regardless of PAX-fusion status of the tumor. Meanwhile, a small percentage of the cells in each tumor did display evidence of subclonal changes (Fig. 2B). In addition, we observed more subclonal aneuploidy events in PFN tumors (more than half of the tumors have detectable subclones) than PFP tumors (Fig. 2C). The inferred subclonal aneuploidy and mutational events were listed in S1 and S2 Tables, respectively.
Fig. 2. Subclonality in in 44 rhabdomyosarcoma samples. 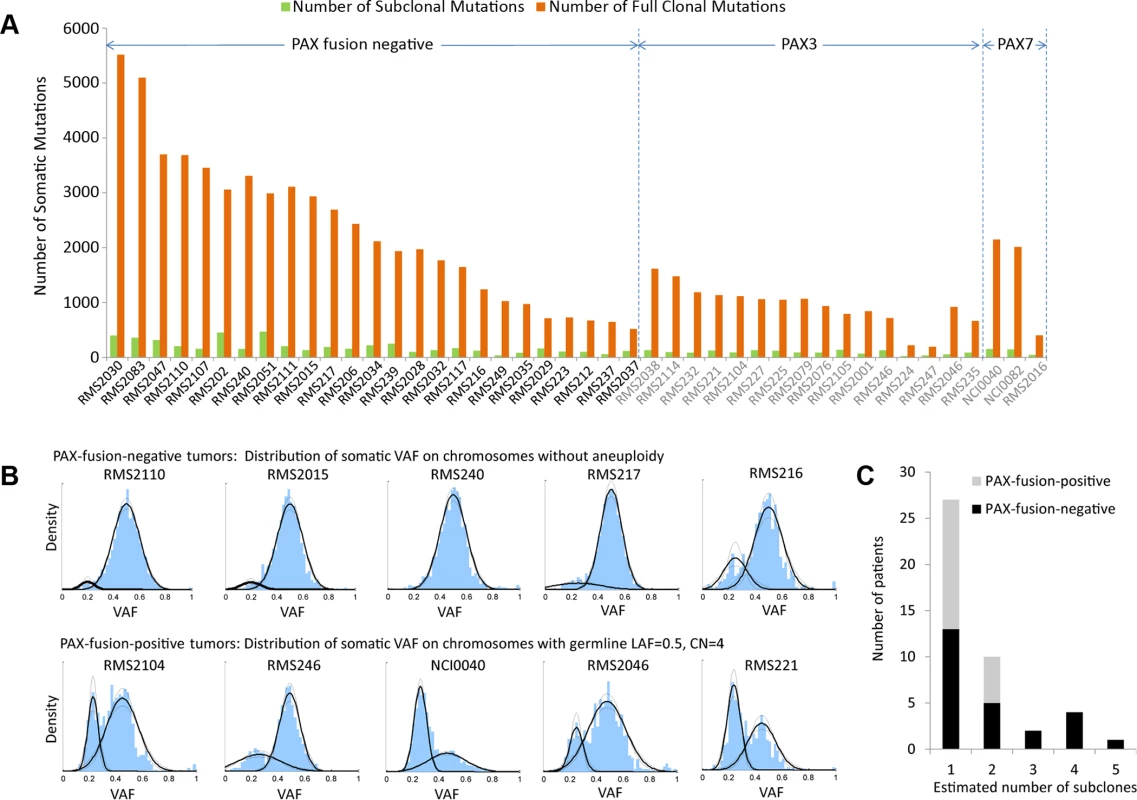
(a) Only a small number of somatic mutations are subclonal while the majority of them were present in all tumor cells. (b) The top panel shows the distribution of VAF of somatic mutations on chromosomes without aneuploidy for selected PFN-RMS samples, where a dominant clone is accompanied with a subclone(s). The lower panel shows the distribution of VAF of somatic mutations for selected PFP-RMS samples. Because these samples have undergone whole-genome duplication, the VAF of somatic mutations on tetraploid chromosomes is distributed around 0.5 and 0.25. (c) PFN samples have more detectable subclonal copy number alterations than PFP samples (p = 0.05, from Mann-Whitney test). It is possible that there are additional subclones that possess subclonal mutations or copy number alterations undetectable with our sequencing depth. Inference of the RMS cancer evolutionary history
The timing of genomic alteration events can be derived using chronological molecular information encoded in the somatic mutations and copy number alterations, because a specific order of genomic event will result in different VAF [2]. To explain the workflow of our method, we again take sample RMS2110 as an example.
The majority of this cancer genome was the expected diploid status with LAF = 0.5, however regions with aneuploidy or allelic imbalance provided the opportunity to identify the timing of genomic events, by comparing the allelic copy number status with the VAF distribution of somatic mutations (S7 Fig.). For example, chromosome 9p and 11 have 2 copies with LAF of 0 (Fig. 1B), which is likely the loss of one allele followed by a duplication of the remaining allele although we cannot formally exclude the possibility of the duplication of both alleles with subsequent loss of the 2 copies of one allele. This observation was confirmed by the fact that most germline single nucleotide variants on chromosome 9p and 11 had VAF near 1 (Fig. 3A). In this case, the somatic mutations occurring before the “LOH+duplication” event must be present on both copies, with an expected VAF of 1, whereas those occurring after the “LOH+duplication” event would be present on only one copy, with an expected VAF of 0.5. The data confirmed this prediction with the VAF displaying a bi-modal distribution with two peaks at 0.5 and 1, respectively (Fig. 3B). The ratio between the numbers of mutations in the two clusters reflects the fraction of “molecular time” it undergoes to accumulate mutations before and after the “LOH+duplication” event, assuming a constant accumulation rate [2]. We acknowledge that the somatic mutation accumulation rate varies among small genomic segments [5,25–27], but for chromosome-level segments used in this study, the average accumulation rates of somatic mutation were observed to be consistent with one another (r2 > 0.98, S8 Fig.). Therefore, the molecular timing inferred for different aneuploidy events were comparable among the segments within the same tumor sample.
Fig. 3. Inferring evolutionary history of a PFN sample, RMS2110. 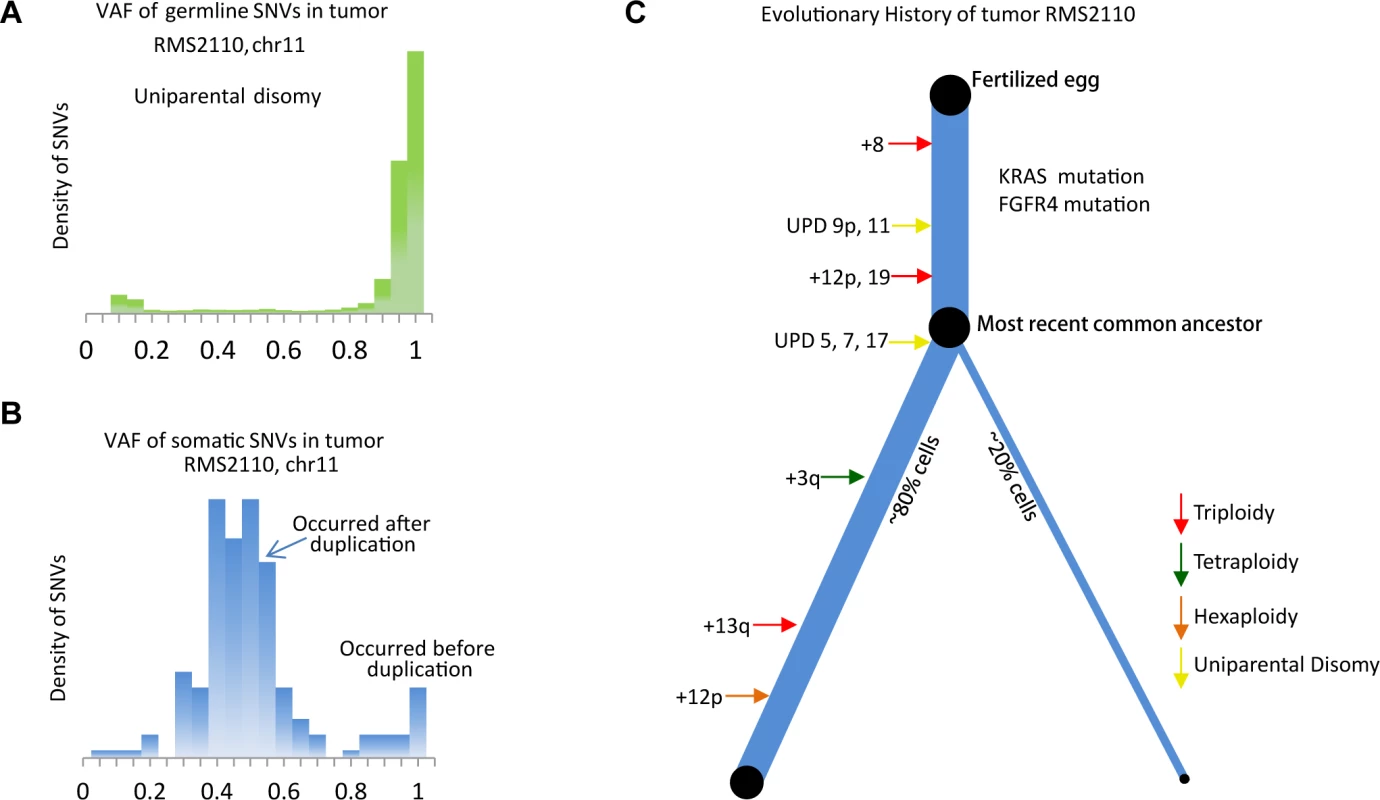
(a) The VAF of germline SNVs on chromosome 11 is distributed around 1, as a result of the uniparental disomy (LOH with duplication). (b) The VAF of somatic mutations is quite different from that of germline SNVs, suggesting majority of somatic mutations happen after the duplication event and thus are present in only one copy of chromosome 11. The earlier occurrence of the duplication of chromosome 11, the more somatic mutations with VAF = 0.5 are detected. S7 Fig. provides a more illustrative explanation. (c) Inferred evolutionary history represented as a phylogenetic tree. The thickness of the branches reflects the proportion of tumor cells in each lineage. The length of the branches reflects how much molecular time it undergoes with each lineage. This analysis was extended genome-wide and allowed the inference of the "phylogenetic tree" of tumor RMS2110 (Fig. 3C). Somatic mutations occurring before and after chromosome aneuploidy events had distinct VAF values, which allowed inference of the timing of two candidate driver mutations in this tumor, the nonsynonymous mutation of FGFR4 V550L and the nonsynonymous mutation of KRAS codon G12. The mutation of FGFR4 V550L has a VAF = 0.95, indicating that the mutation happened before the uniparental disomy event of chromosome 5, which is estimated to occur at 26% of molecular cancer lifetime (S1 Table). Therefore FGFR4 mutation should happen between 0 to 26% of molecular time. Similarly, the mutation of KRAS codon G12 has a VAF of 1, indicating that it occurred before the copy gain of chromosome 12p, timed at 21% of molecular cancer lifetime. Other early events included trisomy of chromosome 8 which happened at 8% of the molecular cancer lifetime and uniparental disomy of chromosomes 11 and 9p which occurred at 14% of the molecular cancer lifetime. Note that on both copies of chromosome 9p, there is a somatic focal deletion of CDKN2A (chr9 : 21981721-21952010), which is also verified by RNAseq (FPKM = 0).
Other aneuploidy events in this sample included: trisomy of chromosomes 12 and 19 which occurred at 21% of molecular cancer lifetime; uniparental disomy of chromosomes 5, 7 and 17 which happened at 26% of molecular cancer lifetime; tetrasomy of a segment of chromosome 3q which occurred around 50% of molecular cancer lifetime; trisomy of chromosome 13 which occurred at 77% of molecular cancer lifetime; hexasomy of 12p which happened at 85% of molecular cancer lifetime; and trisomy of chromosome 20 which occurred near the time of tumor presentation (Fig. 3C).
This method was applied to all the 44 tumor-normal sample pairs to build the evolutionary history of RMS (Fig. 4, S9 Fig., and S1 Table). In summary, our results showed three major findings. First, LOH of 11p15.5 was a consistent early founding event (in average occurred at 35% of molecular cancer lifetime) in PFN-RMS. In comparison, other highly recurrent aneuploidy events such as the copy gain of chromosome 8 and 2 were not consistent early occurring events (time ranges from 1%∼95% and 16%∼96%, respectively). Second, mutations in RAS pathway genes, including FGFR4, KRAS, NRAS and HRAS, were recurrent early events in PFN-RMS. In addition, mutations in other genes (PKN1, CCND1, CUL2, and TTK) occurred early suggesting their role in tumorigenesis. Third, PFP-RMS tumors in general had much fewer somatic alterations and few of them occurred early in the tumor’s molecular lifetime. Of note, a whole-genome duplication event consistently occurred at the middle or late point in the molecular lifetime of these tumors. The high recurrence suggests that this event might be crucial for the presentation of this cancer subtype. We will discuss these findings in more details in the following sections.
Fig. 4. Summary of recurrent somatic lesions found across the 44 tumors and the estimates of their occurrence time. 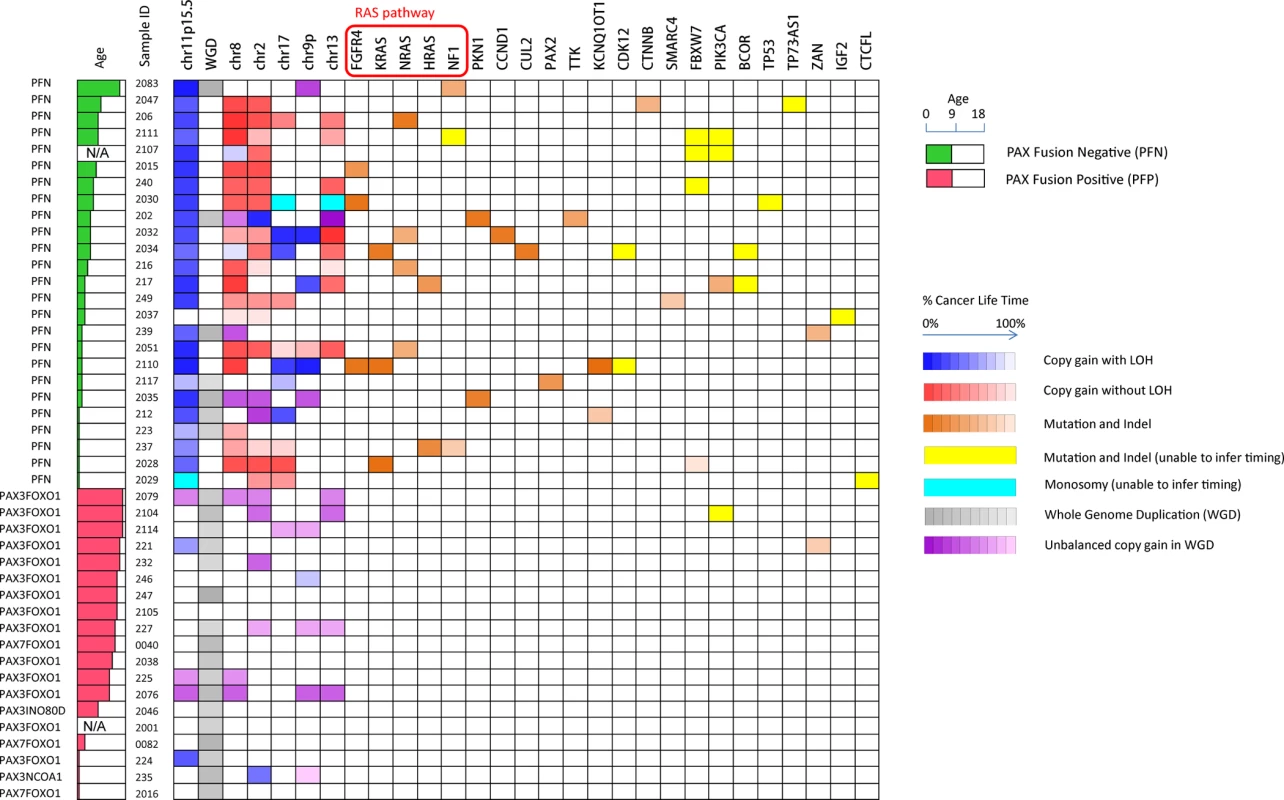
The heatmap denotes the occurrence of the lesions and their timing (the brightness of grids represents the percentage of cancer lifetime when the lesion occurred). The occurrence time of a mutation is represented as the median of its inferred time interval. Note that within a single chromosome, there may be multiple segments with different copy number status, and we only show the arm-level copy number alterations in this figure. 11p15.5 LOH with copy gain and the mutations in RAS pathway are consistently early events during the development of PFN rhabdomyosarcoma. Loss of Heterozygosity of 11p15.5 is a common founding event in PAX fusion negative RMS
The LOH of 11p15.5 is a critical event in fusion-negative tumors [19,28] and frequently results from uniparental disomy and trisomy. In our study, the LOH of 11p15.5 occurred in a total of 26 RMS tumors. As previously described, there was a distinct enrichment for the event in the PFN-RMS population (24/25 PFN samples). Of note, the remaining PFN sample had a small deletion event (3 bps) in the 3-prime non-coding region of IGF2 of undetermined significance. In contrast, only 2 out of 19 PFP-RMS samples had 11p15.5 LOH. The observed LOH usually was accompanied by a copy gain (23 out of 24 PFN samples with LOH on 11p15.5) of the remaining allele resulting in uniparental disomy (n = 8 in PFN and n = 1 in PFP), trisomy (n = 4 in PFN), tetrasomy (n = 8 in PFN and n = 1 in PFP), or pentasomy (n = 3 PFN) (S10 Fig.).
Applying our evolution-history-inference method across the RMS population revealed that the 11p15.5 LOH event universally occurred at an early time point in these tumors development (S11 Fig.). In these tumors, 11p15.5 had a LAF of 0 (S12 Fig. (A)-(C)), meaning that most germline single nucleotide variants present in the tumor, were homozygous. In contrast, the somatic mutations can be homozygous (before 11p15.5 copy gain) or not (after 11p15.5 copy gain). For instance, on chromosome 11 (uniparental disomy) of sample RMS2110, the majority of somatic mutations distributed around a VAF of 0.5 with a minority distributed around VAF of 1 (Fig. 3B). The cluster of somatic mutations discovered around a VAF of 0.5 indicated that these mutations occurred after chromosomal duplication. Comparison of the relative size of the mutations at VAF of 0.5 to those at VAF of 1 indicated that the duplication of chromosome 11 happened early in the cancer evolutionary history. On average, the “LOH+duplication” event of 11p15.5 occurred at 35% of molecular time of the 23 PFN-RMS tumors that had this event. The early occurrence of 11p15.5 “LOH+duplication” event was especially prominent in the patients diagnosed at a later age—the mean occurrence molecular time is 29% of lifetime and the variance is 10% in ≥3 year old patients (S1 Table).
In contrast to the high recurrence (92%) of 11p15.5 LOH in PFN tumors, 11p15.5 LOH was found in only 11% of our PFP tumors. Moreover, in contrast to the consistent early occurrence in PFN tumors, the LOH event did not consistently happen at early lifetime of PFP tumors—37% and 64% of lifetime for two PFP tumors, respectively.
Beyond 11p15.5, the LOH of chromosome 17 (6 out of 25 PFN-RMS) was identified as a recurrent alteration event, typically occurring at an early time point in the evolutionary history of RMS. The LOH region on chromosome 17 includes two small genomic regions that encompass the TP53 and NF1 locus, respectively (S10 Fig.). The observed lesions occurred at an average molecular timing of 38% of cancer evolutionary history. The LOH of 9p, was present in 3 out of 25 PFN-RMS and was found to occur at an average 41% of cancer evolutionary history. Additional accumulation of aneuploidy, such as gain of chromosome 2, 8 and 13, frequently followed the founding events and had no consistent timing pattern.
Whole genome duplication in PAX fusion positive tumors
Unlike PFN-RMS tumors which have significant chromosomal rearrangements, PFP tumors typically have an LAF at the expected 0.5 and a consistent copy number status across the genome. The VAF distribution of somatic mutations revealed that many samples have genome-wide tetraploidy as evidenced by a cluster of somatic mutations with VAF around 0.25 (Fig. 2B, PFP tumors). In total, we found similar VAF distributions in 17 out of 19 PFP tumors. While it is possible that there were two distinct subclones each occupying 50% of tumor cells, given that the cluster with VAF around 0.25 is consistently found across multiple tumors, tetraploidy is a much more likely assumption. Interestingly, when we analyzed these 17 PFP cancer genomes chromosome by chromosome, we found that the inferred tetraploidy occurred in one apparent event (S13 Fig.). This event typically occurred around the mid-point of the molecular lifetime of the tumor (62%±16%) (S1 Table).
In order to identify the sequential order of the PAX3-FOXO1 fusion event and the whole genome duplication event we interrogated the number of the sequencing reads across the PAX3-FOXO1 junction and found that the junction is duplicated. In contrary to PAX7-FOXO1, PAX3-FOXO1 is not known to be focally amplified [29], thus the observed duplication of PAX3-FOXO1 is likely due to the whole genome duplication. Our analysis demonstrated that the PAX3-FOXO1 fusion was consistently duplicated in the whole genome duplication and thus was likely to have occurred prior to the whole genome duplication event (S4B Fig., S3 Table).
Timing the evolutionary history of 44 RMS tumors demonstrates that RAS pathway mutations are critical events in the development of fusion negative RMS
In an effort to obtain a comprehensive picture of the evolutionary history of the RMS tumor genomes, we applied our method to estimate the timing of all the aneuploidy events and mutational events that occurred prior to each tumor’s presentation (Fig. 4). In addition to the recurrent and early 11p LOH event in fusion negative tumors, mutation of FGFR4, KRAS, NRAS and HRAS frequently occurred at an early time point in the cancers evolutionary history (S1 Table). Interestingly, many of the early-mutated genes belong to the RAS pathway (Fig. 4). In total 13/25 fusion negative samples carried the combination of early loss of LOH of 11p15.5 and the mutation of RAS, NF1 or FGFR4 which form the founding events in the evolutionary history of these tumors.
Timing and expression analysis of all the observed somatic mutations was used to discover potential driver mutations that occurred early during tumor development (S4 Table). These mutations included recurrent alteration in two PFN RMS of PKN1 (<30% and <42% of molecular lifetime) which encodes a kinase belonging to the protein kinase C superfamily, CCDN1 at <33% of molecular lifetime in RMS2032, CUL2 at <33% in RMS2034, PAX2 at <77% in RMS 2117, TTK at 17–78% in RMS202.
The E216K mutation of PKN1 inhibits terminal differentiation of C2C12 cells
Recurrent and early mutation of PKN1 in two of the evaluated tumors led us to hypothesize that these changes were involved in myogenic differentiation. To test our hypothesis and assess the potential of our method in identifying driver mutations, we conducted a cell line study described in this section. PKN1 is a member of the AGC-subfamily of serine/threonine kinases. The protein product of the PKN1 gene is composed of a C-terminal kinase domain with significant homology to that of the protein kinase C isoforms, but a unique auto-inhibitory N-terminus made up of 3 homologous stretches of anti-parallel coiled-coil folds (ACC1–3) known to bind to Rho-family GTPases in a nucleotide-dependent manner followed by a C2-like region, known to bind phospholipids and fatty acids. Rho-GTPase binding to the ACC regions causes a conformational change that allows PKN1 to be phosphorylated and activated by PDK1. Active PKN1 plays a role in diverse cellular processes such as regulation of the actin cytoskeleton, cell adhesion, vesicular transport and glucose metabolism [30]. PKN1 represses WNT/CTNNB1 signaling [31] and stimulates the ATF2 and MEF2A transcription factors via a signaling pathway that involves MAP2K3/MAP2K6 and MAPK12 [32]. The observed mutations in RMS202 (E216K) and RMS2035 (A298T) occurred prior to 30% and 42% of molecular lifetime of the tumors, respectively. Both mutations occur in the region of the third ACC domain and could potentially interfere with regulation of the kinase activity of PKN1. To test the functional consequences of the PKN1 E216K mutation, wild type and mutant PKN1 viral constructs were made and transduced into the mouse skeletal muscle precursor cell line C2C12. Using a differentiation assay described by [33], defects in terminal differentiation, reflected in expression of myosin heavy chain (MHC) were observed with the mutated version of PKN1, (Fig. 5A-C), suggesting that the mutant PKN1 prevented the C2C12 cell differentiation. C2C12 cells expressing wild type PKN1 could be induced to express MHC although cell fusion, as determined by the number of nuclei per MHC positive cell, was significantly inhibited. Since PKN1 is known to regulate the activity of several transcription factors known to play a role in myogenic differentiation, we performed expression analysis of the constructed cell lines to determined differentially expressed genes among the constructs. Interestingly, gene set enrichment analysis showed that YAP1 target genes were induced in myoblasts and skeletal muscle genes were repressed in myotubes (Fig. 5D) when the PKN1 mutation was present.
Fig. 5. PKN1 E216K mutation prevented muscle differentiation in C2C12 mouse myoblasts. 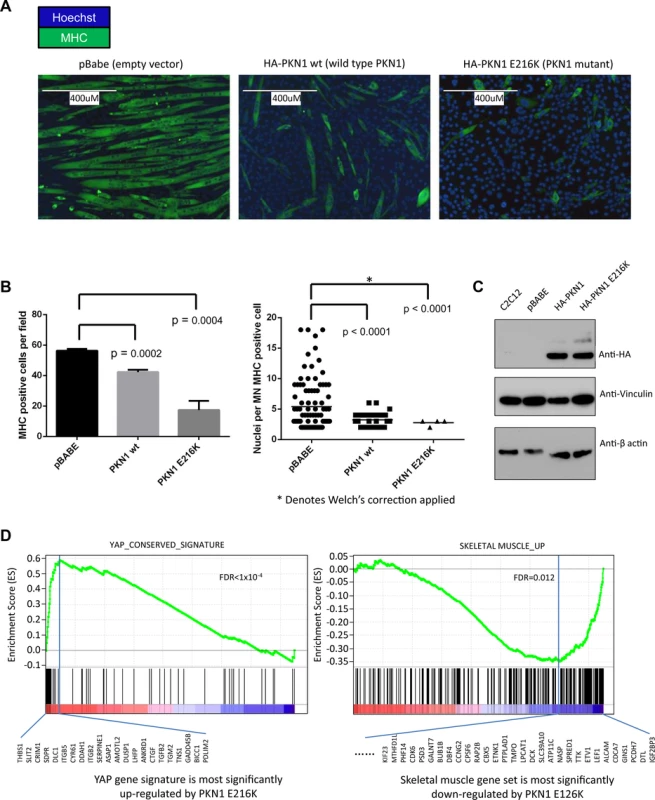
(a) C2C12 cells were transduced with viral constructs containing either empty vector (pBabe), wild type PKN1 (HA-PKN1 wt) or PKN1 E216 mutant (HA-PKN1 E216K). Immunofluorescence performed for myosin heavy chain showed a striking arrest of terminal muscle differentiation. The size bar represents 400um. (b) Quantitation of the observed phenotype showed a significant decrease myosin heavy chain positive cells when PKN1 E216K is expressed. (c) Immunoblot demonstrating expression of HA tagged wild type and mutant PKN1. (d) Gene set enrichment analysis (GSEA) of gene expression profiles demonstrated that PKN1 E216K mutant activated YAP1 target genes as well as cancer invasion-associated genes; while repressed muscle differentiation program in the myoblasts. Discussion
While a growing number of studies have used next generation sequencing to examine the evolutionary development of adult tumors [2,9,13], this study is the first application of whole-genome sequencing to the study of a pediatric solid tumor’s subclonality and evolutionary history. The implication from adult studies is that progression of a tumor cell follows a long course, with subsequent lineages of cells acquiring mutations until a particular alteration allows expansion of one clone; ultimately leading to clinical detection. Studies of multiple cancer types including melanoma [34] and colon [35] have shown that even potent oncogenic mutations may be relatively quiescent and thus remain in an undetected premalignant state until released by an additional genetic hit. The patients in our study had a median age at diagnosis of 6.5 years, indicating that the tumors in these children were developed over a relatively short time course, perhaps hinting at the relative potency of the observed somatic changes. Despite a shorter window of development, our study shows that the basic pattern of sequential mutation accumulation is maintained.
Given that similar analyses of adult tumors find the presence of a dominant clone [2], we anticipated that RMS tumors might have a similar finding. Our results show that a dominant clone accounts for more than 80% of cancer cells in a typical tumor sample and genome wide carries many hundreds or even thousands of accumulated point mutations. Even with our genome-wide sequencing and deep coverage, the subclones are frequently observed only at the limits of detection. Admittedly, our study may miss part of the subclonal mutations due to the limit of coverage and the complex nature of genomic subclonality, especially for subclonal mutations with a VAF<0.1. Nevertheless GO analysis of subclonal mutations showed enrichment for potential resistance and progression pathways including cell adhesion, DNA repair and cell migration. While our DNA extraction included relatively large tumor sections, it is also possible that further sampling of the tumor may yield additional subclonal populations as have been appreciated in the study of distant metastases [10]. Even with these constraints, we can conclude that one clone typically dominates these samples, and the majority of tumor cells share most of the detectable genomic alterations. In addition, our findings indicate that a significant proportion of the clonal heterogeneity in RMS is found in gain or loss of copy number, mirroring similar findings in a study of pediatric acute lymphoblastic leukemia [36]. Future efforts will be needed to determine if these alterations might play a role in defining a "cancer stem cell" which resists the selective pressures of the microenvironment and therapy to survive as a recurrence or metastatic lesion.
From the analyses described here, we can begin to understand the dynamics of RMS development. LOH of 11p15.5 has long been described as recurrent feature of several pediatric conditions including the overgrowth phenotype of Beckwith-Wiedemann Syndrome [37], Wilms tumor [38] and embryonal RMS. The proposed mechanism for oncogenesis of this lesion is loss of imprinting control over the IGF2 locus resulting in over-expression of this developmentally regulated growth factor. In this study, we find that not only is this lesion highly recurrent (>90%), it also appears to be the key early landmark in the evolution of fusion-negative tumors. The discovery of a somatic mutation of IGF2 within a fusion negative sample that does not harbor LOH of 11p15.5 (RMS2037) provides additional support to the role of dysregulation of IGF2 in PFN-RMS. In evolutionary terms, the presence of 11p15.5 LOH defines the “most recent common ancestor” when combined with a mutation in a gene in the RAS pathway (NRAS, KRAS, HRAS, FGFR4). While the progression we describe in this study indicates a possible common sequence of events (S1 Table), in some tumors it is equally as likely that the oncogenic mutation of a RAS pathway gene is the founding lesion. This corroborates the finding that patient’s with Costello Syndrome (HRAS germline mutation) [39], Noonan Syndrome (NRAS, KRAS, PTPN11 germline mutations) [40] and Neurofibromatosis (NF1 germline mutation) [41] all have increased risk of developing fusion-negative RMS. Interestingly, while RMS is certainly described in patients with Beckwith-Wiedemann Syndrome (germline uniparental disomy of 11p15.5), these patients appear to have a higher relative risk of developing Wilms’ tumor and hepatoblastoma than RMS [42]. Regardless of which lesion comes first, the combination of LOH of 11p15.5 with a RAS pathway mutation appears to set a clone on the course towards developing a fusion negative RMS tumor (Fig. 6A).
Fig. 6. Proposed evolutionary model of rhabdomyosarcoma. 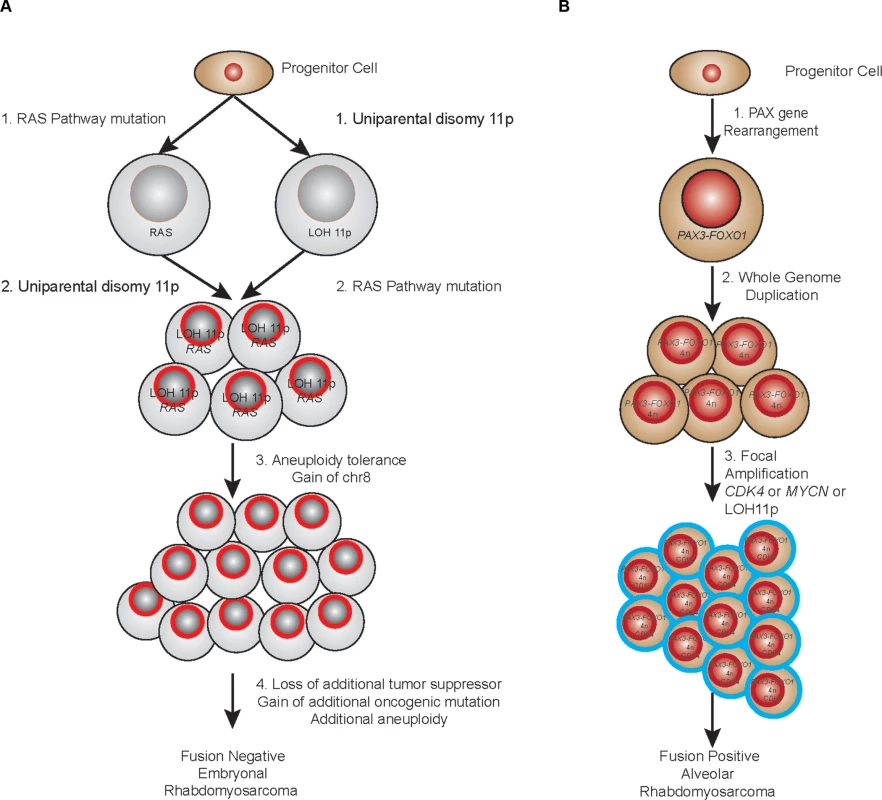
(a) PFN samples. (b) PFP samples. By examining other mutations that occur early in the molecular history of fusion negative tumors, especially those tumors that had no mutation in a candidate “RAS” pathway member, it is possible to nominate other potential founding lesions. In this analysis, mutation of PKN1 stands out as both recurrent (occurring in tumor RMS202 and RMS2035) and occurring early in the evolutionary history of these tumors (<30% and <42% respectively). PKN1 is a member of the protein kinase C family and has been implicated as a repressor of WNT/CTNNB1 signaling [43], a mediator of insulin signaling to the actin cytoskeleton [44] and an activator of MEF2A dependent transcription [32]. The observed mutations (E216K and A298T) occurred within the third ACC domain and may relieve N-terminal auto-inhibition of PKN1 kinase activity (S14 Fig.). Our functional assessments of the PKN1 mutation demonstrated that the observed mutation can inhibit terminal differentiation of skeletal muscle in a dominant-negative fashion. Gene expression analysis of PKN1 E216K expressing C2C12 cells grown in low serum media reveals that this differentiation block occurs as a result of repression of skeletal muscle specific genes and genes with MEF2A binding sites in the promoter. Expression of MEF2 proteins is induced by myogenin during normal skeletal muscle differentiation, and MEF2 factors, in complex with other transcriptional activators, are known to play a role of in myogenic differentiation [45]. One possible explanation for these results is that PKN1-dependent signaling leads to replacement of MEF2A at skeletal muscle specific genes with a transcriptional repressor. The identity of this transcriptional repressor is not yet known.
GSEA analysis of PKN1 E216K expressing C2C12 myoblasts reveals that the gene expression signature of these cells is enriched for a YAP gene signature. The YAP gene signature is composed of genes activated by YAP overexpression in human mammary cells, mouse liver tissues and immortalized mouse fibroblasts, and thus represents a list of evolutionary YAP target genes [46]. YAP1 has recently been identified as a potent ERMS oncogenic driver, and YAP1-TEAD complexes repress expression of genes important for myogenic differentiation, in part by impinging upon the binding of myogenic transcription factors including MEF2 [47]. Intriguingly, YAP1 is able to functionally compensate for oncogenic KRAS in colorectal and pancreatic cancer mouse models [48,49], which is consistent with the fact that RAS mutation and PKN1 mutation are mutually exclusive in the set of ERMS tumors presented here. The mechanism by which PKN1 leads to increased YAP1 activity is currently unknown.
With whole genome sequencing from a single biopsy, we were unable to reliably estimate the molecular timing of some of the recurrent genetic lesions observed in RMS. These events include mutations in PIK3CA, FBXW7, NF1, and BCOR, and the focal amplification of MDM2, CDK4, and MYCN. Moreover, somatic events such as the copy gain of chromosomes 2, 8 and 13 were estimated to occur in an inconsistent temporal pattern among RMS patients (occurring at multiple time points throughout the evolutionary history). Certainly, the recurrence of these lesions in multiple large sample sets speaks to their importance, however since they can occur either early or late in the cancers evolutionary history they do not represent the initiating lesion in the tumor but rather modifiers of the tumor as it grows towards presentation.
Early flow cytometry studies to evaluate DNA content [50] and cytogenetic analysis [51] in ARMS noted the frequent presence of tetraploidy. We find evidence that fusion positive tumors demonstrate a very high recurrence of a whole genome duplication event that results in a tetraploid genome. In general, the duplication event occurs around the midpoint of the tumor’s evolutionary history and prior to additional accumulation of aneuploidy (Fig. 6B). The mechanism of a cell moving from a diploid state to a tetraploid state can be due to a cytokinesis failure, a cell fusion event or mitotic slippage and it has long been speculated that tetraploidy is an intermediate in cancer progression [52]. This is born out in at least one premalignant state (Barrett’s esophagus), where tetraploidy represents an early intermediate as the cancer precursor develops towards esophageal adenocarcinoma [53]. Further experimental evidence of a tetraploidy intermediate state is demonstrated in TP53 null mouse mammary cells where tetraploid but not diploid cells promote tumorigenesis in nude mice [54]. Of note, in the mouse model of PFP ARMS both the increase in allelic copy number of the PAX3-FOXO1 fusion and the loss of TP53 or CDKN2A were found to be a critical component in increasing the penetrance of the phenotype [55]. Given the role of loss of TP53 in allowing a permissive environment for the tetraploid cell to escape cell cycle arrest, this is consistent with our findings. It is interesting to speculate that in our tumor series the whole genome duplication event is an attempt by the tumor to increase the allelic dose of the PAX fusion. Another intriguing possibility is that the whole genome duplication event alters the telomere length and activates telomerase; a critical cooperating genetic event in the temporal sequence that produces alveolar rhabdomyosarcoma from human myoblasts [56].
In conclusion, based on deep whole genome sequencing, we developed a systematic method to infer the evolutionary history and identify the causative lesions of this pediatric solid tumor. From our data, we provide a model for how these tumors develop. Our results demonstrate two distinct evolutionary paths resulting in a convergent phenotype of this soft tissue cancer: 1) genomic loss of heterozygosity on 11p15.5, nonsynonymous mutations on RAS pathway and cell cycle genes, including FGFR4, KRAS, NRAS, HRAS and CCDN1, as well as several other genes, including CUL2, TTK and PKN1, occur early in the evolution history of PFN-RMS; 2) recurrent whole genome duplication occurs in the middle or late stage of the tumors evolution in the PAX-fusion positive RMS tumors. PAX3-FOXO1 fusion occurs before the whole genome duplication event. Intriguingly, a recent report of the clonal evolution in two cases of relapsed fusion-negative RMS tumors after chemotherapy and radiation demonstrated that recurrent tumors are derived from the founding tumor’s minor clone [24]. Nevertheless given that survival following relapse is 30% [57], our findings that LOH of 11p15.5 and mutation of RAS genes form the “trunk” of the fusion negative RMS tumors has important therapeutic implications; at least in theory it is preferable for treatment to target mutations that are present in all of the tumor cells. In addition, while further biologic validation is required, our analysis identifies candidate lesions that may underpin the ability of the minor clone to survive therapy and proliferate during relapse or metastasis. Thus given clonal evolution and heterogeneity, our data suggests that future efforts to understand the emergence of resistant clones should include deep sequencing analysis on all patients at presentation and relapse. These efforts will add to the growing understanding of the biology of RMS and identify actionable genetic aberrations for targeted therapies.
Methods
Patient samples
All patient sample collection was approved by the institutional review board of the participating facility. All de-linked and de-identified patient sample information was collected under the approved institutional review board of the National Cancer Institute protocol 10-C-0086. Samples were assembled from collections at the Pediatric Oncology Branch of the National Cancer Institute, Children’s Oncology Group, and the Tumor Bank at The Children’s Hospital at Westmead (New South Wales, Australia). All tumors were collected at initial diagnosis and prior to any therapy with the exception of samples NCI0040 and NCI0080 which were collected at relapse. Samples were de-identified and histologic diagnosis and clinical information were compiled. Quality control genotyping for the whole genome samples was performed to ensure the match of tumor normal pairs.
Whole genome sequencing
Approximately 6 micrograms of DNA was sequenced using the paired end sequencing method of Complete Genomics. Data analysis was accomplished using CGA tools package v2.0 as well as a number of in house tools described in [23].
Targeted resequencing
A custom panel of oligonucleotides designed to incorporate somatic single nucleotide variants discovered in samples RMS2110 and RMS2107 was generated using Ion Ampliseq designer software. 150 base pair amplicon libraries were generated using multiplex PCR according to the Ion Torrent Ampliseq Library 2.0 kit. Individual samples were barcoded and the generated libraries were sequenced using a 318 chip on a Personal Genome Machine (Life Technologies). Compiled reads were mapped to Hg19 and the expected variants were analyzed for coverage and VAF. The targeted sequencing has a depth of 1997x. We used it to verify the VAF estimates of somatic mutations across whole genome of two samples. The result shows VAF estimation accuracy is as high as 90% (S1 Fig.).
SNP array
Illumina Omni 2.5M (97 paired plus 30 unmatched tumors) or 5M (10 paired samples) were performed according to the standard procedure from the manufacturer (Illumina, San Diego, CA) at the National Cancer, Cancer Genomics Research Laboratory. The data were previously reported in [23].
Whole transcriptome sequencing
PolyA selected RNA libraries were prepared for RNA sequencing on Illumina HiSeq2000. 100 bases long paired-end reads were assessed for quality and reads were mapped using CASAVA (Illumina, San Diego, CA). The generated fastq files were analyzed by TopHat2 [58] and Cufflinks [59] The data were previously reported in [23].
Somatic mutation calling
We used the somatic score from Complete Genomics (CG), a highly sensitive and specific somatic mutation-calling criteria [23,60,61], combined with principles previously used [25,62] to detect somatic signal nucleotide variants (SNVs). The somatic score is designed by systematically considering sequencing error, mapping error and read count and has been validated as an effective criterion by our previous studies as well as studies from other groups [23,61,63]. To ensure sensitivity and specificity of mutation calling, we set the somatic score cutoff as ≥0, with a set of additional filters for removing system artifacts and mapping errors, to select somatic mutations, based on the verification results from an independent sequencing platform [23]. By SOLiD whole exome sequencing (35x coverage) and Ion Torrent Ampliseq targeted sequencing (300x coverage) on 30 RMS tumor-normal sample pairs sequenced by CG, we verified that our filtering removes 99% of false positives while maintaining 80% of true positives [23] in CG’s comprehensive somatic mutation pool (called in a loose criterion to be as inclusive as possible) [23,60], which is premium given the less somatic mutations in pediatric tumors [3].
Copy number profiling
For the 44 matched tumor and normal sample pairs with whole genome sequencing, we used somatic copy number segmentation profile provided by Complete Genomics, with customized corrections. For a larger cohort, with 120 matched tumor and normal sample pairs measured by SNP array, we used the copy number profile provided by NEXUS copy number analysis.
In copy number profiling, an important quantity, lesser allele fraction (LAF), is estimated to represent the fraction of copies coming from each parental allele. Along with total copy number, LAF tells us the allelic copy number status. LAF is defined as the ratio between the copy number of the lesser allele (with fewer copies than the other allele) versus the total copy number. For example, a trisomic chromosome with allelic type “AAB” has LAF equal to 1/3. LAF is estimated based on germline heterozygous single nucleotide variants that are present in cancer genome. For each genomic segment of tumor sample, sites that have heterozygous single nucleotide variants in matched normal samples were selected and LAF was estimated by the ratio between the read count of lesser allele and the total read count for these sites.
Statistical analyses
In order to study the evolutionary history of RMS, we performed an integrated statistical analysis, using information including germline single-nucleotide variants, somatic mutations (single-nucleotide variants), somatic copy number alterations (CNA) and junctions (mapped breaking points), to estimate the normal cell contamination, intra-tumor heterogeneity (due to subclones) and the timing of somatic variants. The methods are detailed in the S1 Text.
Cell lines and retroviral constructs
Mouse myoblast cell line C2C12 was obtained as a generous gift from Dr. Marc Landanyi. The cells genotype was performed by the NCI Core Genotyping facility and the cell line was confirmed to be myocoplasm negative. All cell culture was performed in DMEM supplemented with 10% FBS. The differentiation assay was performed using 2% horse serum (Life Technologies) as previously described [33]. For retrovirus production, the pBabe vector system (Addgene) was used.
Retrovirus construction and transduction of C2C12 cells
Plasmids encoding human PKN1 cDNA was purchased from Addgene, and constructs were subcloned into pBABE containing a N-terminal HA tag. A cDNA encoding the PKN1 E216K mutant was generated using the GeneART Site-Directed Mutagenesis kit (Life Technologies) and was subcloned into pBabe. The generated mutation was confirmed by Sanger sequencing. Retroviruses were generated by contransfection of pBabe constructs with pCL-10A1 into 293T cells (American Type Culture Collection (ATCC), CRL-3216) subsequently used to infect the C2C12 cell line as previously described; infected cells were selected with 2 μg/ml puromycin (Life Technologies).
Immunofluorescence
Cells grown on Nunc chamber slides were fixed with 4% paraformaldehyde and permeabilized in PBS containing 0.5% Triton X-100 and blocked in Block-Aid (Life Technologies) for 1 h at room temperature. Cells were then incubated with MF20 monoclonal antibody (DSHB) against MHC (1 : 40 dilution;) overnight at 4 degrees. Secondary antibody Alexa Fluor 488-conjugated secondary antibody (1 : 200 dilution; Life Technologies) for 1 h at room temperature. Cells were mounted with ProLong Gold antifade reagent with DAPI (4′,6-diamidino-2-phenylindole; Life Technologies).
Immunoblotting
Cells were lysed in M-PER lysis buffer (Pierce Biotechnology). Lysates were denatured in 4× sample buffer at 70°C for 10 min, resolved on 4–12% NuPAGE gels (Life Technologies) and transferred onto PVDF (polyvinylidene fluoride) membranes. Membranes were blocked in 5% nonfat milk in TBST buffer (TBS with Tween-20) for 1 h at room temperature and probed with primary anti-HA antibody obtained from Covance (1 : 2,000 dilution). Bound antibodies were detected with peroxidase-labeled horse antibody to mouse IgG and visualized using enhanced chemiluminescence reagents (ThermoScientific).
Expression analysis
Total cellular RNA was isolated using the RNAeasy mini kit (Qiagen). Cellular RNA (250ng) was in vitro transcribed, fragmented, hybridized and applied to Affymetrix Mouse 430A arrays according to the standard operating procedure of the Laboratory of Molecular Technology core facility (http://atp.ncifcrf.gov/genetics-and-genomics/laboratory-of-molecular-technology) and the manufacturer’s instructions (Affymetrix, Santa Clara, CA). For gene set enrichment analysis (GSEA), the normalized gene expression data were z-scored and ranked according to absolute fold-change expression over the control. GSEA analysis (http://www.broadinstitute.org/gsea/index.jsp) was performed using default parameter settings.
Supporting Information
Zdroje
1. Yates LR, Campbell PJ (2012) Evolution of the cancer genome. Nat Rev Genet 13 : 795–806. doi: 10.1038/nrg3317 23044827
2. Nik-Zainal S, Van Loo P, Wedge DC, Alexandrov LB, Greenman CD, et al. (2012) The life history of 21 breast cancers. Cell 149 : 994–1007. doi: 10.1016/j.cell.2012.04.023 22608083
3. Tomasetti C, Vogelstein B, Parmigiani G (2013) Half or more of the somatic mutations in cancers of self-renewing tissues originate prior to tumor initiation. Proc Natl Acad Sci U S A 110 : 1999–2004. doi: 10.1073/pnas.1221068110 23345422
4. Stratton MR, Campbell PJ, Futreal PA (2009) The cancer genome. Nature 458 : 719–724. doi: 10.1038/nature07943 19360079
5. Chapman MA, Lawrence MS, Keats JJ, Cibulskis K, Sougnez C, et al. (2011) Initial genome sequencing and analysis of multiple myeloma. Nature 471 : 467–472. doi: 10.1038/nature09837 21430775
6. Ding L, Ellis MJ, Li S, Larson DE, Chen K, et al. (2010) Genome remodelling in a basal-like breast cancer metastasis and xenograft. Nature 464 : 999–1005. doi: 10.1038/nature08989 20393555
7. Ley TJ, Ding L, Walter MJ, McLellan MD, Lamprecht T, et al. (2010) DNMT3A mutations in acute myeloid leukemia. N Engl J Med 363 : 2424–2433. doi: 10.1056/NEJMoa1005143 21067377
8. Puente XS, Pinyol M, Quesada V, Conde L, Ordonez GR, et al. (2011) Whole-genome sequencing identifies recurrent mutations in chronic lymphocytic leukaemia. Nature 475 : 101–105. doi: 10.1038/nature10113 21642962
9. Ding L, Ley TJ, Larson DE, Miller CA, Koboldt DC, et al. (2012) Clonal evolution in relapsed acute myeloid leukaemia revealed by whole-genome sequencing. Nature 481 : 506–510. doi: 10.1038/nature10738 22237025
10. Yachida S, Jones S, Bozic I, Antal T, Leary R, et al. (2010) Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature 467 : 1114–1117. doi: 10.1038/nature09515 20981102
11. Landau DA, Carter SL, Stojanov P, McKenna A, Stevenson K, et al. (2013) Evolution and impact of subclonal mutations in chronic lymphocytic leukemia. Cell 152 : 714–726. doi: 10.1016/j.cell.2013.01.019 23415222
12. Shanafelt TD, Hanson C, Dewald GW, Witzig TE, LaPlant B, et al. (2008) Karyotype evolution on fluorescent in situ hybridization analysis is associated with short survival in patients with chronic lymphocytic leukemia and is related to CD49d expression. J Clin Oncol 26: e5–6. doi: 10.1200/JCO.2008.16.7874 18467710
13. Shah SP, Roth A, Goya R, Oloumi A, Ha G, et al. (2012) The clonal and mutational evolution spectrum of primary triple-negative breast cancers. Nature 486 : 395–399. doi: 10.1038/nature10933 22495314
14. Durinck S, Ho C, Wang NJ, Liao W, Jakkula LR, et al. (2011) Temporal Dissection of Tumorigenesis in Primary Cancers. Cancer Discovery 1 : 137–143. doi: 10.1158/2159-8290.CD-11-0028 21984974
15. Ognjanovic S, Linabery AM, Charbonneau B, Ross JA (2009) Trends in childhood rhabdomyosarcoma incidence and survival in the United States, 1975–2005. Cancer 115 : 4218–4226. doi: 10.1002/cncr.24465 19536876
16. Malempati S, Hawkins DS (2012) Rhabdomyosarcoma: review of the Children's Oncology Group (COG) Soft-Tissue Sarcoma Committee experience and rationale for current COG studies. Pediatr Blood Cancer 59 : 5–10. doi: 10.1002/pbc.24118 22378628
17. Breneman JC, Lyden E, Pappo AS, Link MP, Anderson JR, et al. (2003) Prognostic factors and clinical outcomes in children and adolescents with metastatic rhabdomyosarcoma—a report from the Intergroup Rhabdomyosarcoma Study IV. J Clin Oncol 21 : 78–84. 12506174
18. Linardic CM (2008) PAX3-FOXO1 fusion gene in rhabdomyosarcoma. Cancer Lett 270 : 10–18. doi: 10.1016/j.canlet.2008.03.035 18457914
19. Scrable H, Cavenee W, Ghavimi F, Lovell M, Morgan K, et al. (1989) A model for embryonal rhabdomyosarcoma tumorigenesis that involves genome imprinting. Proc Natl Acad Sci U S A 86 : 7480–7484. 2798419
20. Stratton MR, Fisher C, Gusterson BA, Cooper CS (1989) Detection of point mutations in N-ras and K-ras genes of human embryonal rhabdomyosarcomas using oligonucleotide probes and the polymerase chain reaction. Cancer Research 49 : 6324–6327. 2680062
21. Shukla N, Ameur N, Yilmaz I, Nafa K, Lau CY, et al. (2012) Oncogene mutation profiling of pediatric solid tumors reveals significant subsets of embryonal rhabdomyosarcoma and neuroblastoma with mutated genes in growth signaling pathways. Clin Cancer Res 18 : 748–757. doi: 10.1158/1078-0432.CCR-11-2056 22142829
22. Taylor JGt, Cheuk AT, Tsang PS, Chung JY, Song YK, et al. (2009) Identification of FGFR4-activating mutations in human rhabdomyosarcomas that promote metastasis in xenotransplanted models. J Clin Invest 119 : 3395–3407. doi: 10.1172/JCI39703 19809159
23. Shern JF, Chen L, Chmielecki J, Wei JS, Patidar R, et al. (2014) Comprehensive genomic analysis of rhabdomyosarcoma reveals a landscape of alterations affecting a common genetic axis in fusion-positive and fusion-negative tumors. Cancer Discov 4 : 216–231. doi: 10.1158/2159-8290.CD-13-0639 24436047
24. Chen X, Stewart E, Shelat AA, Qu C, Bahrami A, et al. (2013) Targeting oxidative stress in embryonal rhabdomyosarcoma. Cancer Cell 24 : 710–724. doi: 10.1016/j.ccr.2013.11.002 24332040
25. Cibulskis K, Lawrence MS, Carter SL, Sivachenko A, Jaffe D, et al. (2013) Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nat Biotechnol 31 : 213–219. doi: 10.1038/nbt.2514 23396013
26. Stamatoyannopoulos JA, Adzhubei I, Thurman RE, Kryukov GV, Mirkin SM, et al. (2009) Human mutation rate associated with DNA replication timing. Nature Genetics 41 : 393–395. doi: 10.1038/ng.363 19287383
27. Chen CL, Rappailles A, Duquenne L, Huvet M, Guilbaud G, et al. (2010) Impact of replication timing on non-CpG and CpG substitution rates in mammalian genomes. Genome Research 20 : 447–457. doi: 10.1101/gr.098947.109 20103589
28. Xia SJ, Pressey JG, Barr FG (2002) Molecular pathogenesis of rhabdomyosarcoma. Cancer Biol Ther 1 : 97–104. 12170781
29. Barr FG, Nauta LE, Davis RJ, Schafer BW, Nycum LM, et al. (1996) In vivo amplification of the PAX3-FKHR and PAX7-FKHR fusion genes in alveolar rhabdomyosarcoma. Human Molecular Genetics 5 : 15–21. 8789435
30. Mukai H (2003) The structure and function of PKN, a protein kinase having a catalytic domain homologous to that of PKC. Journal of Biochemistry 133 : 17–27. 12761194
31. James RG, Bosch KA, Kulikauskas RM, Yang PTT, Robin NC, et al. (2013) Protein Kinase PKN1 Represses Wnt/beta-Catenin Signaling in Human Melanoma Cells. Journal of Biological Chemistry 288 : 34658–34670. doi: 10.1074/jbc.M113.500314 24114839
32. Marinissen MT, Chiariello M, Gutkind JS (2001) Regulation of gene expression by the small GTPase Rho through the ERK6 (p38 gamma) MAP kinase pathway. Genes & Development 15 : 535–553.
33. Kohsaka S, Shukla N, Ameur N, Ito T, Ng CKY, et al. (2014) A recurrent neomorphic mutation in MYOD1 defines a clinically aggressive subset of embryonal rhabdomyosarcoma associated with PI3K-AKT pathway mutations. Nature Genetics 46 : 595–600. doi: 10.1038/ng.2969 24793135
34. Pollock PM, Harper UL, Hansen KS, Yudt LM, Stark M, et al. (2003) High frequency of BRAF mutations in nevi. Nature Genetics 33 : 19–20. 12447372
35. Chan TL, Zhao W, Leung SY, Yuen ST, Project CG (2003) BRAF and KRAS mutations in colorectal hyperplastic polyps and serrated adenomas. Cancer Research 63 : 4878–4881. 12941809
36. Mullighan CG, Phillips LA, Su X, Ma J, Miller CB, et al. (2008) Genomic analysis of the clonal origins of relapsed acute lymphoblastic leukemia. Science 322 : 1377–1380. doi: 10.1126/science.1164266 19039135
37. Henry I, Bonaitipellie C, Chehensse V, Beldjord C, Schwartz C, et al. (1991) Uniparental Paternal Disomy in a Genetic Cancer-Predisposing Syndrome. Nature 351 : 665–667. 1675767
38. KOUFOS A, HANSEN MF, LAMPKIN BC, WORKMAN ML, COPELAND NG, et al. (1984) Loss of alleles at loci on human chromosome 11 during genesis of Wilms' tumour. Nature 309 : 170–172. 6325936
39. Gripp KW (2005) Tumor predisposition in Costello syndrome. American Journal of Medical Genetics Part C-Seminars in Medical Genetics 137C: 72–77. 16010679
40. Kratz CP, Rapisuwon S, Reed H, Hasle H, Rosenberg PS (2011) Cancer in Noonan, Costello, Cardiofaciocutaneous and LEOPARD Syndromes. American Journal of Medical Genetics Part C-Seminars in Medical Genetics 157C: 83–89. doi: 10.1002/ajmg.c.30300 21500339
41. Sung L, Anderson JR, Arndt C, Raney B, Meyer WH, et al. (2004) Neurofibromatosis in children with rhabdomyosarcoma: A report from the intergroup Rhabdomyosarcoma Study IV. Journal of Pediatrics 144 : 666–668. 15127010
42. DeBaun MR, Tucker MA (1998) Risk of cancer during the first four years of life in children from The Beckwith-Wiedemann Syndrome Registry. Journal of Pediatrics 132 : 398–400. 9544889
43. James RG, Bosch KA, Kulikauskas RM, Yang PT, Robin NC, et al. (2013) Protein kinase PKN1 represses Wnt/beta-catenin signaling in human melanoma cells. J Biol Chem 288 : 34658–34670. doi: 10.1074/jbc.M113.500314 24114839
44. Dong LQ, Landa LR, Wick MJ, Zhu L, Mukai H, et al. (2000) Phosphorylation of protein kinase N by phosphoinositide-dependent protein kinase-1 mediates insulin signals to the actin cytoskeleton. Proc Natl Acad Sci U S A 97 : 5089–5094. 10792047
45. Potthoff MJ, Olson EN (2007) MEF2: a central regulator of diverse developmental programs. Development 134 : 4131–4140. 17959722
46. Cordenonsi M, Zanconato F, Azzolin L, Forcato M, Rosato A, et al. (2011) The Hippo Transducer TAZ Confers Cancer Stem Cell-Related Traits on Breast Cancer Cells. Cell 147 : 759–772. doi: 10.1016/j.cell.2011.09.048 22078877
47. Tremblay AM, Missiaglia E, Galli GG, Hettmer S, Urcia R, et al. (2014) The Hippo Transducer YAP1 Transforms Activated Satellite Cells and Is a Potent Effector of Embryonal Rhabdomyosarcoma Formation. Cancer Cell 26 : 273–287. doi: 10.1016/j.ccr.2014.05.029 25087979
48. Shao DD, Xue W, Krall EB, Bhutkar A, Piccioni F, et al. (2014) KRAS and YAP1 Converge to Regulate EMT and Tumor Survival. Cell 158 : 171–184. doi: 10.1016/j.cell.2014.06.004 24954536
49. Kapoor A, Yao WT, Ying HQ, Hua SJ, Liewen A, et al. (2014) Yap1 Activation Enables Bypass of Oncogenic Kras Addiction in Pancreatic Cancer. Cell 158 : 185–197. doi: 10.1016/j.cell.2014.06.003 24954535
50. De Zen L, Sommaggio A, d'Amore ES, Masiero L, di Montezemolo LC, et al. (1997) Clinical relevance of DNA ploidy and proliferative activity in childhood rhabdomyosarcoma: a retrospective analysis of patients enrolled onto the Italian Cooperative Rhabdomyosarcoma Study RMS88. Journal of Clinical Oncology 15 : 1198–1205. 9060564
51. Whang-Peng J, Knutsen T, Theil K, Horowitz ME, Triche T (1992) Cytogenetic studies in subgroups of rhabdomyosarcoma. Genes Chromosomes Cancer 5 : 299–310. 1283318
52. Ganem NJ, Storchova Z, Pellman D (2007) Tetraploidy, aneuploidy and cancer. Current Opinion in Genetics & Development 17 : 157–162.
53. Galipeau PC, Cowan DS, Sanchez CA, Barrett MT, Emond MJ, et al. (1996) 17P (p53) allelic losses, 4N (G(2)/tetraploid) populations, and progression to aneuploidy in Barrett's esophagus. Gastroenterology 110: A515–A515.
54. Fujiwara T, Bandi M, Nitta M, Ivanova EV, Bronson RT, et al. (2005) Cytokinesis failure generating tetraploids promotes tumorigenesis in p53-null cells. Nature 437 : 1043–1047. 16222300
55. Nishijo K, Chen QR, Zhang L, McCleish AT, Rodriguez A, et al. (2009) Credentialing a Preclinical Mouse Model of Alveolar Rhabdomyosarcoma. Cancer Research 69 : 2902–2911. doi: 10.1158/0008-5472.CAN-08-3723 19339268
56. Naini S, Etheridge KT, Adam SJ, Qualman SJ, Bentley RC, et al. (2008) Defining the Cooperative Genetic Changes That Temporally Drive Alveolar RhabdomyosarcomaU. Cancer Research 68 : 9583–9588. doi: 10.1158/0008-5472.CAN-07-6178 19047133
57. Pappo AS, Anderson JR, Crist WM, Wharam MD, Breitfeld PP, et al. (1999) Survival after relapse in children and adolescents with rhabdomyosarcoma: A report from the intergroup rhabdomyosarcoma study group. Journal of Clinical Oncology 17 : 3487–3493. 10550146
58. Trapnell C, Pachter L, Salzberg SL (2009) TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 25 : 1105–1111. doi: 10.1093/bioinformatics/btp120 19289445
59. Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, et al. (2010) Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol 28 : 511–515. doi: 10.1038/nbt.1621 20436464
60. Lam HY, Clark MJ, Chen R, Natsoulis G, O'Huallachain M, et al. (2012) Performance comparison of whole-genome sequencing platforms. Nat Biotechnol 30 : 78–82. doi: 10.1038/nbt.2065 22178993
61. Molenaar JJ, Koster J, Zwijnenburg DA, van Sluis P, Valentijn LJ, et al. (2012) Sequencing of neuroblastoma identifies chromothripsis and defects in neuritogenesis genes. Nature 483 : 589–593. doi: 10.1038/nature10910 22367537
62. Wei X, Walia V, Lin JC, Teer JK, Prickett TD, et al. (2011) Exome sequencing identifies GRIN2A as frequently mutated in melanoma. Nature Genetics 43 : 442–446. doi: 10.1038/ng.810 21499247
63. Turajlic S, Furney SJ, Lambros MB, Mitsopoulos C, Kozarewa I, et al. (2012) Whole genome sequencing of matched primary and metastatic acral melanomas. Genome Res 22 : 196–207. doi: 10.1101/gr.125591.111 22183965
Štítky
Genetika Reprodukční medicína
Článek NLRC5 Exclusively Transactivates MHC Class I and Related Genes through a Distinctive SXY ModuleČlánek Inhibition of Telomere Recombination by Inactivation of KEOPS Subunit Cgi121 Promotes Cell LongevityČlánek HOMER2, a Stereociliary Scaffolding Protein, Is Essential for Normal Hearing in Humans and MiceČlánek LRGUK-1 Is Required for Basal Body and Manchette Function during Spermatogenesis and Male FertilityČlánek The GATA Factor Regulates . Developmental Timing by Promoting Expression of the Family MicroRNAsČlánek Systems Biology of Tissue-Specific Response to Reveals Differentiated Apoptosis in the Tick VectorČlánek Phenotype Specific Analyses Reveal Distinct Regulatory Mechanism for Chronically Activated p53Článek The Nuclear Receptor DAF-12 Regulates Nutrient Metabolism and Reproductive Growth in NematodesČlánek The ATM Signaling Cascade Promotes Recombination-Dependent Pachytene Arrest in Mouse SpermatocytesČlánek The Small Protein MntS and Exporter MntP Optimize the Intracellular Concentration of Manganese
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 3
-
Všechny články tohoto čísla
- NLRC5 Exclusively Transactivates MHC Class I and Related Genes through a Distinctive SXY Module
- Licensing of Primordial Germ Cells for Gametogenesis Depends on Genital Ridge Signaling
- A Genomic Duplication is Associated with Ectopic Eomesodermin Expression in the Embryonic Chicken Comb and Two Duplex-comb Phenotypes
- Genome-wide Association Study and Meta-Analysis Identify as Genome-wide Significant Susceptibility Gene for Bladder Exstrophy
- Mutations of Human , Encoding the Mitochondrial Asparaginyl-tRNA Synthetase, Cause Nonsyndromic Deafness and Leigh Syndrome
- Exome Sequencing in an Admixed Isolated Population Indicates Variants Confer a Risk for Specific Language Impairment
- Genome-Wide Association Studies in Dogs and Humans Identify as a Risk Variant for Cleft Lip and Palate
- Rapid Evolution of Recombinant for Xylose Fermentation through Formation of Extra-chromosomal Circular DNA
- The Ribosome Biogenesis Factor Nol11 Is Required for Optimal rDNA Transcription and Craniofacial Development in
- Methyl Farnesoate Plays a Dual Role in Regulating Metamorphosis
- Maternal Co-ordinate Gene Regulation and Axis Polarity in the Scuttle Fly
- Maternal Filaggrin Mutations Increase the Risk of Atopic Dermatitis in Children: An Effect Independent of Mutation Inheritance
- Inhibition of Telomere Recombination by Inactivation of KEOPS Subunit Cgi121 Promotes Cell Longevity
- Clonality and Evolutionary History of Rhabdomyosarcoma
- HOMER2, a Stereociliary Scaffolding Protein, Is Essential for Normal Hearing in Humans and Mice
- Methylation-Sensitive Expression of a DNA Demethylase Gene Serves As an Epigenetic Rheostat
- BREVIPEDICELLUS Interacts with the SWI2/SNF2 Chromatin Remodeling ATPase BRAHMA to Regulate and Expression in Control of Inflorescence Architecture
- Seizures Are Regulated by Ubiquitin-specific Peptidase 9 X-linked (USP9X), a De-Ubiquitinase
- The Fun30 Chromatin Remodeler Fft3 Controls Nuclear Organization and Chromatin Structure of Insulators and Subtelomeres in Fission Yeast
- A Cascade of Iron-Containing Proteins Governs the Genetic Iron Starvation Response to Promote Iron Uptake and Inhibit Iron Storage in Fission Yeast
- Mutation in MRPS34 Compromises Protein Synthesis and Causes Mitochondrial Dysfunction
- LRGUK-1 Is Required for Basal Body and Manchette Function during Spermatogenesis and Male Fertility
- Cis-Regulatory Mechanisms for Robust Olfactory Sensory Neuron Class-restricted Odorant Receptor Gene Expression in
- Effects on Murine Behavior and Lifespan of Selectively Decreasing Expression of Mutant Huntingtin Allele by Supt4h Knockdown
- HDAC4-Myogenin Axis As an Important Marker of HD-Related Skeletal Muscle Atrophy
- A Conserved Domain in the Scc3 Subunit of Cohesin Mediates the Interaction with Both Mcd1 and the Cohesin Loader Complex
- Selective and Genetic Constraints on Pneumococcal Serotype Switching
- Bacterial Infection Drives the Expression Dynamics of microRNAs and Their isomiRs
- The GATA Factor Regulates . Developmental Timing by Promoting Expression of the Family MicroRNAs
- Accumulation of Glucosylceramide in the Absence of the Beta-Glucosidase GBA2 Alters Cytoskeletal Dynamics
- Reproductive Isolation of Hybrid Populations Driven by Genetic Incompatibilities
- The Contribution of Alu Elements to Mutagenic DNA Double-Strand Break Repair
- Systems Biology of Tissue-Specific Response to Reveals Differentiated Apoptosis in the Tick Vector
- Tfap2a Promotes Specification and Maturation of Neurons in the Inner Ear through Modulation of Bmp, Fgf and Notch Signaling
- The Lysine Acetyltransferase Activator Brpf1 Governs Dentate Gyrus Development through Neural Stem Cells and Progenitors
- PHABULOSA Controls the Quiescent Center-Independent Root Meristem Activities in
- DNA Polymerase ζ-Dependent Lesion Bypass in Is Accompanied by Error-Prone Copying of Long Stretches of Adjacent DNA
- Examining the Evolution of the Regulatory Circuit Controlling Secondary Metabolism and Development in the Fungal Genus
- Zinc Finger Independent Genome-Wide Binding of Sp2 Potentiates Recruitment of Histone-Fold Protein Nf-y Distinguishing It from Sp1 and Sp3
- GAGA Factor Maintains Nucleosome-Free Regions and Has a Role in RNA Polymerase II Recruitment to Promoters
- Neurospora Importin α Is Required for Normal Heterochromatic Formation and DNA Methylation
- Ccr4-Not Regulates RNA Polymerase I Transcription and Couples Nutrient Signaling to the Control of Ribosomal RNA Biogenesis
- Phenotype Specific Analyses Reveal Distinct Regulatory Mechanism for Chronically Activated p53
- A Systems-Level Interrogation Identifies Regulators of Blood Cell Number and Survival
- Morphological Mutations: Lessons from the Cockscomb
- Genetic Interaction Mapping Reveals a Role for the SWI/SNF Nucleosome Remodeler in Spliceosome Activation in Fission Yeast
- The Role of China in the Global Spread of the Current Cholera Pandemic
- The Nuclear Receptor DAF-12 Regulates Nutrient Metabolism and Reproductive Growth in Nematodes
- A Zinc Finger Motif-Containing Protein Is Essential for Chloroplast RNA Editing
- Resistance to Gray Leaf Spot of Maize: Genetic Architecture and Mechanisms Elucidated through Nested Association Mapping and Near-Isogenic Line Analysis
- Small Regulatory RNA-Induced Growth Rate Heterogeneity of
- Mitochondrial Dysfunction Reveals the Role of mRNA Poly(A) Tail Regulation in Oculopharyngeal Muscular Dystrophy Pathogenesis
- Complex Genomic Rearrangements at the Locus Include Triplication and Quadruplication
- Male-Biased Aganglionic Megacolon in the TashT Mouse Line Due to Perturbation of Silencer Elements in a Large Gene Desert of Chromosome 10
- Sex Ratio Meiotic Drive as a Plausible Evolutionary Mechanism for Hybrid Male Sterility
- Tertiary siRNAs Mediate Paramutation in .
- RECG Maintains Plastid and Mitochondrial Genome Stability by Suppressing Extensive Recombination between Short Dispersed Repeats
- Escape from X Inactivation Varies in Mouse Tissues
- Opposite Phenotypes of Muscle Strength and Locomotor Function in Mouse Models of Partial Trisomy and Monosomy 21 for the Proximal Region
- Glycosyl Phosphatidylinositol Anchor Biosynthesis Is Essential for Maintaining Epithelial Integrity during Embryogenesis
- Hyperdiverse Gene Cluster in Snail Host Conveys Resistance to Human Schistosome Parasites
- The Class Homeodomain Factors and Cooperate in . Embryonic Progenitor Cells to Regulate Robust Development
- Recombination between Homologous Chromosomes Induced by Unrepaired UV-Generated DNA Damage Requires Mus81p and Is Suppressed by Mms2p
- Synergistic Interactions between Orthologues of Genes Spanned by Human CNVs Support Multiple-Hit Models of Autism
- Gene Networks Underlying Convergent and Pleiotropic Phenotypes in a Large and Systematically-Phenotyped Cohort with Heterogeneous Developmental Disorders
- The ATM Signaling Cascade Promotes Recombination-Dependent Pachytene Arrest in Mouse Spermatocytes
- Combinatorial Control of Light Induced Chromatin Remodeling and Gene Activation in
- Linking Aβ42-Induced Hyperexcitability to Neurodegeneration, Learning and Motor Deficits, and a Shorter Lifespan in an Alzheimer’s Model
- The Complex Contributions of Genetics and Nutrition to Immunity in
- NatB Domain-Containing CRA-1 Antagonizes Hydrolase ACER-1 Linking Acetyl-CoA Metabolism to the Initiation of Recombination during . Meiosis
- Transcriptomic Profiling of Reveals Reprogramming of the Crp Regulon by Temperature and Uncovers Crp as a Master Regulator of Small RNAs
- Osteopetrorickets due to Snx10 Deficiency in Mice Results from Both Failed Osteoclast Activity and Loss of Gastric Acid-Dependent Calcium Absorption
- A Genomic Portrait of Haplotype Diversity and Signatures of Selection in Indigenous Southern African Populations
- Sequence Features and Transcriptional Stalling within Centromere DNA Promote Establishment of CENP-A Chromatin
- Inhibits Neuromuscular Junction Growth by Downregulating the BMP Receptor Thickveins
- Replicative DNA Polymerase δ but Not ε Proofreads Errors in and in
- Unsaturation of Very-Long-Chain Ceramides Protects Plant from Hypoxia-Induced Damages by Modulating Ethylene Signaling in
- The Small Protein MntS and Exporter MntP Optimize the Intracellular Concentration of Manganese
- A Meta-analysis of Gene Expression Signatures of Blood Pressure and Hypertension
- Pervasive Variation of Transcription Factor Orthologs Contributes to Regulatory Network Evolution
- Network Analyses Reveal Novel Aspects of ALS Pathogenesis
- A Role for the Budding Yeast Separase, Esp1, in Ty1 Element Retrotransposition
- Nab3 Facilitates the Function of the TRAMP Complex in RNA Processing via Recruitment of Rrp6 Independent of Nrd1
- A RecA Protein Surface Required for Activation of DNA Polymerase V
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Clonality and Evolutionary History of Rhabdomyosarcoma
- Morphological Mutations: Lessons from the Cockscomb
- Maternal Filaggrin Mutations Increase the Risk of Atopic Dermatitis in Children: An Effect Independent of Mutation Inheritance
- Transcriptomic Profiling of Reveals Reprogramming of the Crp Regulon by Temperature and Uncovers Crp as a Master Regulator of Small RNAs
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



