-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaMutation in MRPS34 Compromises Protein Synthesis and Causes Mitochondrial Dysfunction
Mitochondria make most of the energy required by eukaryotic cells and therefore they are essential for their normal function and survival. Mitochondrial function is regulated by both the mitochondrial and nuclear genome. Mutations in nuclear genes encoding mitochondrial proteins lead to mitochondrial dysfunction and consequently diminished energy production, a major symptom of metabolic and mitochondrial diseases. The molecular mechanisms that regulate mitochondrial gene expression and how dysfunction of these processes causes the pathologies observed in these diseases are not well understood. Messenger RNAs encoded by mitochondrial genomes are translated on mitochondrial ribosomes that have unique structure and protein composition. Mitochondrial ribosomes are a patchwork of core proteins that share homology with prokaryotic ribosomal proteins and mitochondria-specific proteins, which can be unique to different organisms. Mitochondria-specific ribosomal proteins have key roles in disease however their functions within mitochondria are not known. Here we show that a point mutation in a mammalian-specific ribosomal protein causes mitochondrial dysfunction, heart abnormalities and progressive liver disease. This mouse provides a valuable model to elucidate the pathogenic mechanisms and progression of metabolic diseases with age, while enabling a more thorough understanding of mitochondrial ribosomes and protein synthesis.
Published in the journal: . PLoS Genet 11(3): e32767. doi:10.1371/journal.pgen.1005089
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005089Summary
Mitochondria make most of the energy required by eukaryotic cells and therefore they are essential for their normal function and survival. Mitochondrial function is regulated by both the mitochondrial and nuclear genome. Mutations in nuclear genes encoding mitochondrial proteins lead to mitochondrial dysfunction and consequently diminished energy production, a major symptom of metabolic and mitochondrial diseases. The molecular mechanisms that regulate mitochondrial gene expression and how dysfunction of these processes causes the pathologies observed in these diseases are not well understood. Messenger RNAs encoded by mitochondrial genomes are translated on mitochondrial ribosomes that have unique structure and protein composition. Mitochondrial ribosomes are a patchwork of core proteins that share homology with prokaryotic ribosomal proteins and mitochondria-specific proteins, which can be unique to different organisms. Mitochondria-specific ribosomal proteins have key roles in disease however their functions within mitochondria are not known. Here we show that a point mutation in a mammalian-specific ribosomal protein causes mitochondrial dysfunction, heart abnormalities and progressive liver disease. This mouse provides a valuable model to elucidate the pathogenic mechanisms and progression of metabolic diseases with age, while enabling a more thorough understanding of mitochondrial ribosomes and protein synthesis.
Introduction
Mitochondria are composed of proteins encoded by the nuclear and mitochondrial genomes. Most of the mitochondrial proteins including the ribosomal proteins and translation factors that are responsible for the expression of the mitochondrial genome are synthesized on cytoplasmic ribosomes and imported into mitochondria post-translationally. In chordates, the mitochondrial genome encodes 22 tRNAs, 2 rRNAs and 11 mRNAs that are translated on mitochondrial ribosomes (mitoribosomes) into 13 polypeptides, all members of the oxidative phosphorylation complexes [1]. Mutations in mitochondrial genes or nuclear genes coding for mitochondrial proteins result in mitochondrial dysfunction and impaired energy production that cause mitochondrial diseases (reviewed in [2]). The most common cause of mitochondrial diseases are defects in the translational machinery (reviewed in [3]), however the mechanisms of mitochondrial protein synthesis are not well understood.
Mammalian mitoribosomes are 55S particles consisting of a 28S small subunit that includes the 12S rRNA and ~ 29 proteins and a 39S large subunit, which contains the 16S rRNA and ~ 48 proteins [4–7]. Mitoribosomes are distinct from their bacterial and cytoplasmic counterparts; they have reduced RNA content and an increased number of proteins [8]. The mitoribosome consists of proteins that share homology with bacterial ribosomes, some of which can have mitochondria-specific extensions, and additional, mitochondria-specific proteins that decorate the ribosomal surface, the mRNA entrance site and form some of the bridges linking the small and large subunits [6,7,9,10]. The increased ribosomal protein content does not entirely compensate for the loss of rRNA as many of the mitochondria-specific ribosomal proteins do not replace the missing RNA helices but instead have unique positions on the exterior of the mitochondrial ribosome [11,12]. Recent cryo-electron microscopy (cryo-EM) reconstructions indicate that the additional protein elements may fulfil roles necessitated by the unique features of the mitochondrial leaderless mRNAs, likely in their recognition, as well as facilitating the translation of the particularly hydrophobic proteins they encode and making the contacts between the small and large ribosomal subunits [6,7,9,10,12]. The mitoribosomes are located inside the matrix, the site of the transcriptome, and associate closely with the mitochondrial inner membrane through MRPL45 [7]. This positioning allows for co-translational insertion of the hydrophobic proteins, which are translated by the mitoribosome, into the inner membrane and their assembly into oxidative phosphorylation (OXPHOS) complexes [13].
There is very little known about the functions and roles of the mitochondria-specific ribosomal proteins, therefore characterizing their function within the ribosome and mitochondria will provide valuable insights into mitochondrial translation. The mitochondrial ribosomal protein of the small subunit 34 (MRPS34) has been identified as one of 15 mitochondria-specific proteins that are part of the small ribosomal subunit [14,15]. Although MRPS34 has been found localized to mitochondria and associated with the human homolog of the Drosophila discs large tumor suppressor protein (hDLG) [16], its role in mitochondria and protein synthesis has not been identified or characterized. Here we investigated the role of MRPS34 in mice carrying a homozygous mutation in the nuclear gene encoding this protein that causes a significant decrease of this protein. MRPS34 is required for protein synthesis of all 13 mitochondrially-encoded polypeptides and stability of the 12S rRNA and specific mRNAs. Dysfunction in the efficiency of mitochondrial protein synthesis leads to reduced mitochondrial oxygen consumption and respiratory complex activity in the mutant mice indicating that MRPS34 is essential for the stability of actively translating ribosomes. The Mrps34 mutation causes tissue-specific molecular and pathological changes that result in alterations in fractional shortening of the heart and pronounced liver steatosis that leads to fibrosis with age. Mitochondrial dysfunction caused by the Mrps34 mutation is likely caused by decreased levels of mitochondrial ribosomal subunits and translationally competent mitoribosomes.
Results
Mrps34mut/mut mice have reduced expression of the MRPS34 protein
A mouse line carrying an ENU-induced T203C point mutation in the Mrps34 gene that converts a leucine residue at position 68 to proline (Fig. 1A) was identified by whole exome sequencing [17]. Sanger sequencing shows that the Mrps34mut/mut mice are homozygous for the mutation that is absent in age and littermate matched control Mrps34wt/wt mice (Fig. 1B). The leucine residue at position 68 is conserved in vertebrates (Fig. 1A), and mutation to proline is predicted to disrupt the formation of an alpha helix. To determine whether the mutation caused changes in the abundance of the MRPS34 protein we carried out immunoblotting of mitochondrial lysates isolated from liver and heart of Mrps34 mut/mut and Mrps34wt/wt mice. The MRPS34 protein was reduced in the heart and liver of the mutant mice (Fig. 1C), indicating that the mutation causes instability of the protein. The MRPS34 protein is expressed in all examined tissues including brain, colon, heart, kidney, liver, thymus, pancreas, skin and testis and the Mrps34 mutation results in decreased levels of the protein in these tissues (S1 Fig). Mutations in nuclear genes encoding proteins that are part of the translational machinery have been shown to cause mitochondrial diseases with varying age of onset and diverse clinical pathologies that affect a range of different tissues [2,3]. Next we sought to determine the effects of the Mrps34 mutation, in young (6–8 week) and aged (28–30 week) mice, on mitochondrial function and the downstream effects on energy metabolism and disease pathology.
Fig. 1. A homozygous point mutation in the Mrps34 gene causes a decrease in the MRPS34 protein. 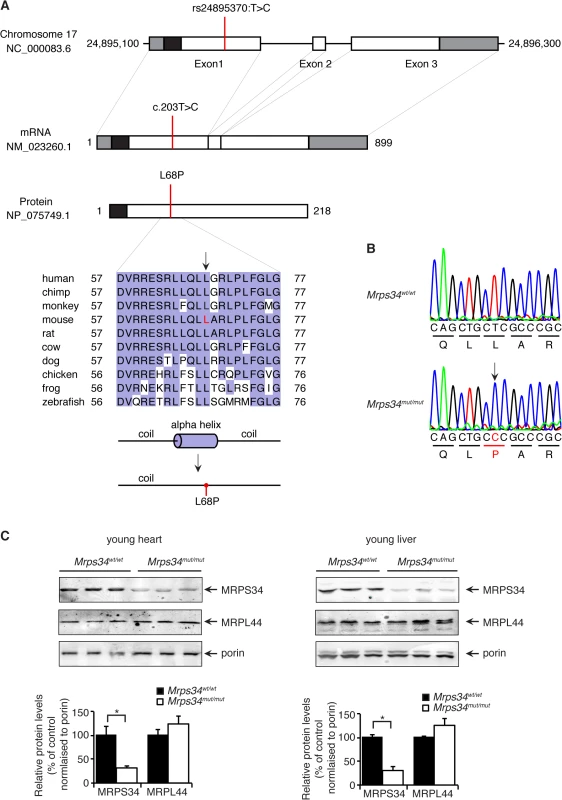
(A) Schematic showing the location of the mutation in the Mrps34 gene, mRNA and protein. The 5′- and 3′-untranslated regions are shown as grey boxes and the predicted mitochondrial targeting sequence is shown as a black box (predicted using MitoProtII, [40]). Conservation of the protein sequence surrounding the mutation is highlighted with residues identical to those in the mouse sequence boxed (the mutated leucine is shown in red in the mouse sequence). Sequences used were obtained from GenBank at NCBI (human, Homo sapiens, NP_076425.1; chimp, Pan troglodytes, XP_001160122.1; monkey, Macaca mulatta, NP_001244544.1; mouse, Mus musculus, NP_081622.1; rat, Rattus norvegicus, NP_001099241.1; cow, Bos taurus, NP_001192540.1; dog, Canis lupus familiaris, XP_003639160.1; chicken, Gallus gallus, NP_001264526.1; frog, Xenopus tropicalis, NP_001004910.2; zebrafish, Danio rerio, NP_001007377.2) and the alignment was produced using ClustalW2 [41]. The secondary structure of mouse MRPS34 was predicted using PSI-PRED [42] and the mutated region is shown. An alpha helical region is predicted to be lost when the L68P mutation is present. (B) The mutation was confirmed by Sanger sequencing of PCR amplicons from Mrps34mut/mut and littermate matched Mrps34wt/wt mice. (C) Mutation in the nuclear gene encoding the mitochondrial MRPS34 protein leads to its decreased abundance. MRPS34 and MRPL44 protein levels were investigated in mitochondria isolated from hearts and livers of Mrps34wt/wt and Mrps34mut/mut mice by immunoblotting. Porin was used as a loading control. The data are representative of results obtained from at least 8 mice from each strain. Data are means ± SEM of three separate experiments; *, p < 0.05 compared with control treatments by a 2-tailed paired Student’s t test. The Mrps34 mutation affects stability of the 12S rRNA and specific mitochondrial mRNAs
The mitochondrially-encoded 12S and 16S rRNAs form the scaffolds for the mitochondrial ribosomes that use 22 mitochondrial tRNAs to translate the 11 mRNAs [12]. Therefore we analyzed if the Mrps34 mutation affected the steady-state levels and stability of mitochondrial RNAs in heart and liver by northern blotting. The 12S rRNA levels were reduced in hearts and livers of the young Mrps34mut/mut mice, and the 16S rRNA level was unaffected (Fig. 2A), suggesting that the MRPS34 is required for the stability of the 12S rRNA. In addition, we observed that the levels of the mt-Nd5 mRNA were decreased in the livers, but not in the hearts of the young mutant mice (Fig. 2A), indicating that the Mrps34 mutation causes tissue specific effects on mitochondrial RNA metabolism. The 12S rRNA was also reduced significantly in the heart and livers of the mutant aged mice (Fig. 2B). Furthermore, the levels of specific mitochondrial mRNAs, mt-Co1, mt-Nd1 and mt-Nd5 were decreased in the livers of aged mice. However, in the hearts of aged Mrps34wt/wt and Mrps34mut/mut mice only mt-Nd5 was decreased but not mt-Co1 or mt-Nd1 suggesting that in the liver the stability of specific mRNAs is more severely affected by the Mrps34 mutation with age (Fig. 2A and 2B). The levels of mitochondrial tRNAs were unaffected in heart and liver mitochondria, suggesting that MRPS34 is necessary for the stability of 12S rRNA and for the stability of specific mRNAs.
Fig. 2. Decreased MRPS34 affects the stability of the 12S rRNA and specific mitochondrial mRNAs. 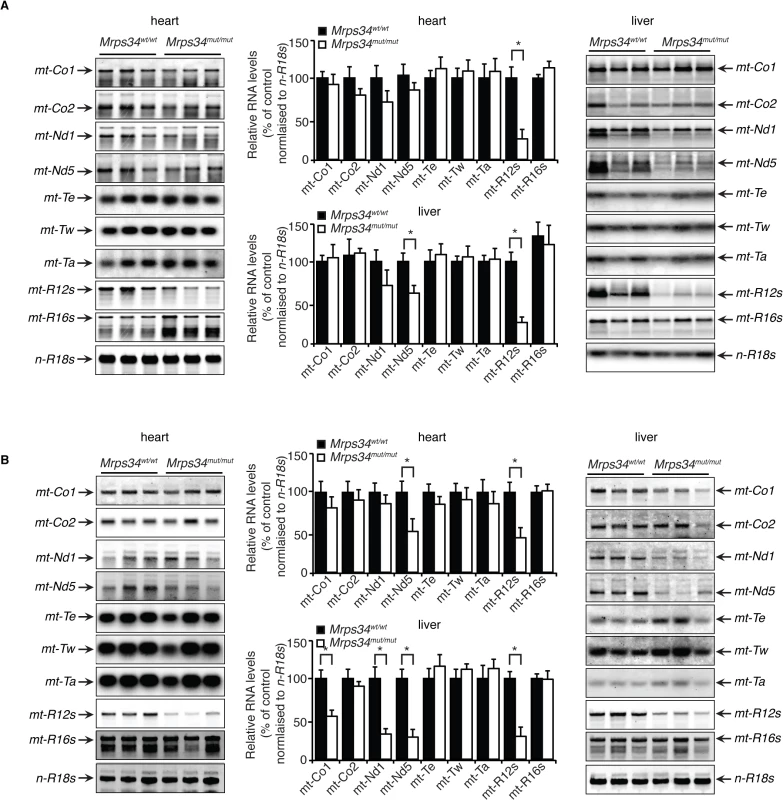
(A) The abundance of mature mitochondrial transcripts in mitochondria isolated from young Mrps34wt/wt and Mrps34mut/mut livers and hearts was analyzed by northern blotting. (B) The abundance of mature mitochondrial transcripts in aged liver and heart was analyzed by northern blotting. 18S rRNA was used as a loading control. The data are representative of results obtained from at least 8 mice from each strain. Data are means ± SEM of three separate experiments; *, p < 0.05 compared with control treatments by a 2-tailed paired Student’s t test. MRPS34 is required for mitochondrial translation
Since MRPS34 is a ribosomal protein we analyzed how decreased levels of this protein, as a result of the Mrps34 mutation, affect translation by measuring de novo protein synthesis of the 13 mitochondrially-encoded polypeptides in heart and liver mitochondria from Mrps34mut/mut and Mrps34wt/wt mice. The overall decrease of mitochondrial protein synthesis in the young and aged mutant mice compared to controls (S2 Fig) indicates that MRPS34 is required for mitochondrial protein synthesis. To investigate the effects of the mutation on the rate of protein synthesis between heart and liver mitochondria we measured mitochondrial translation over time. Interestingly we observed that the initial rate of translation in control liver mitochondria is faster compared to that of heart mitochondria (Figs. 3A, S2). In addition, we observed that the Mrps34 mutation causes a decrease in mitochondrial protein synthesis (Fig. 3A), however the initial rate in translation may account for the severity of the molecular changes found in the liver compared to heart mitochondria.
Fig. 3. Decreased MRPS34 levels cause a reduction in mitochondrial protein synthesis and steady state levels of mitochondrial proteins. 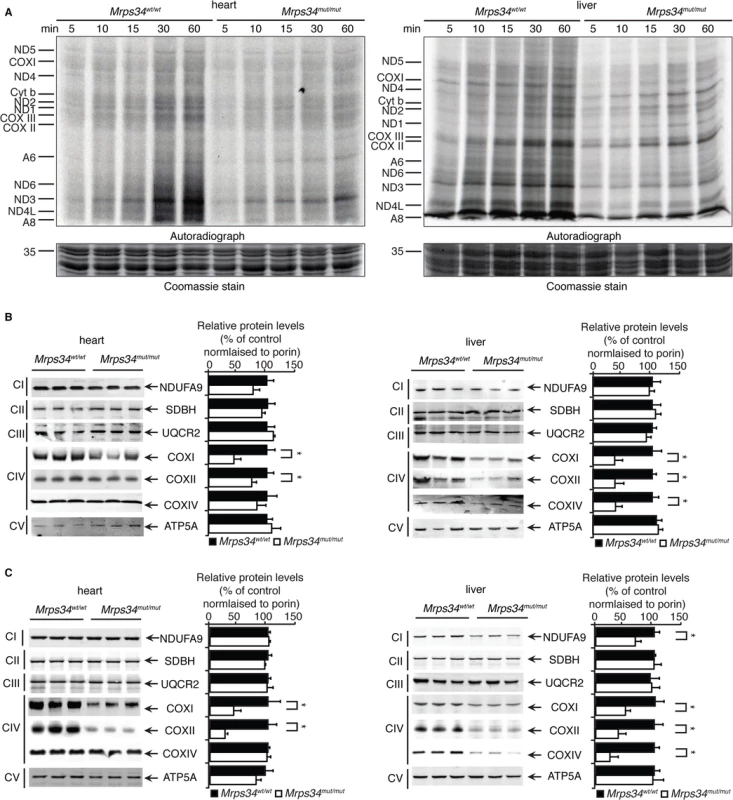
(A) Protein synthesis in heart and liver mitochondria isolated from Mrps34wt/wt and Mrps34mut/mut was measured by pulse incorporation of 35S-labelled methionine and cysteine over time. Equal amounts of mitochondrial lysates (50 μg) were separated by SDS–PAGE, Coomassie stained (bottom panel) and visualized by autoradiography (top panel). Mitochondrial lysates (20 μg) from Mrps34wt/wt and Mrps34mut/mut livers and hearts of young (B) and aged (C) mice were resolved by SDS-PAGE and immunoblotted using antibodies to assess changes in mitochondrial protein abundance. Porin was used as a loading control. Data are means ± SEM of four separate experiments; *, p < 0.05 compared with control treatments by a 2-tailed paired Student’s t test. Next we analyzed the effects of the Mrps34 mutation on the steady-state abundance of mitochondrial proteins by immunoblotting. The levels of the mitochondrially-encoded COXI and COXII were reduced in the livers and hearts of young Mrps34mut/mut mice compared to controls (Fig. 3C). In the aged mutant mice we observed a more pronounced decrease in COXI and COXII in both the heart and liver, suggesting that the effects of decreased MRPS34 levels on the steady state levels of these proteins are cumulative and the molecular defects have a late onset in the heart (Fig. 3D). In addition, we observed reduction in nuclear-encoded mitochondrial proteins, such as NDUFA9 and COXIV, possibly in a retrograde response to the decreased levels of mitochondrially-encoded proteins in the livers of aged Mrps34mut/mut mice (Fig. 3D).
We investigated the effects of Mrps34 mutation on the abundance of the mitochondrial respiratory complexes by blue native polyacrylamide gel electrophoresis (BN-PAGE). The reduction in the abundance of the respiratory complexes in hearts of Mrps34mut/mut mice is more apparent in the aged mice compared to the young (Fig. 4A and 4B). The reduction of the respiratory complexes was more significant in the livers of Mrps34mut/mut compared to Mrps34wt/wt mice (Fig. 4A and 4B) and this was confirmed by immunoblotting of each complex following BN-PAGE (Fig. 4C and 4D). Complexes I and IV were reduced in the hearts of both young and aged mice, whereas in the livers, Complexes I, III, IV and V were reduced (Fig. 4C and 4D). Taken together these findings provide evidence that MRPS34 is required for protein synthesis and that decreased mitochondrial translation as a result of the Mrps34 mutation can have varied effects on the abundance of mitochondrial proteins and respiratory complexes in different tissues.
Fig. 4. Reduced MRPS34 affects the abundance of mitochondrial respiratory complexes. 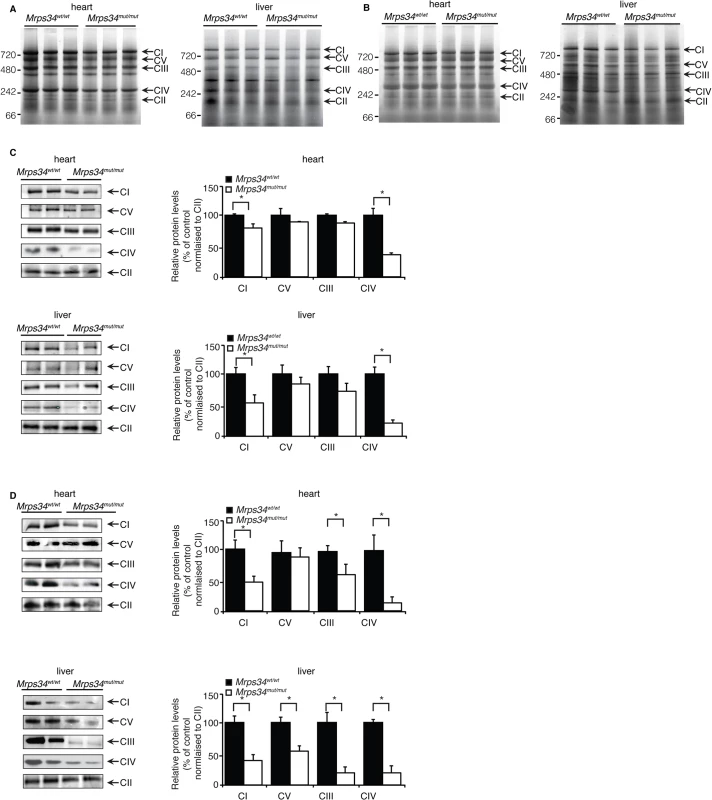
BN-PAGE was performed on 75 μg of mitochondria isolated from liver and heart of young (A) and aged (B) Mrps34 wt/wt and Mrps34 mut/mut mice. Mitochondria lysates (75 μg) isolated from liver and heart of Mrps34wt/wt and Mrps34mut/mut young mice (C) and aged mice (D) were analyzed by BN-PAGE and the gels used for immunoblotting. Specific antibodies representing proteins of each of the mitochondrial complexes were used to compare abundance of protein in the wild type and mutant mice. Data are means ± SEM of five separate experiments; *, p < 0.05 compared with control treatments by a 2-tailed paired Student’s t test. The mutation in the Mrps34 gene leads to decreased mitochondrial respiration and complex activity
Because we observed a decrease in the abundance of mitochondrial respiratory complexes we measured their enzyme activities in heart and liver mitochondria from young and aged Mrps34mut/mut and Mrps34wt/wt mice. The activities of the respiratory complexes were not significantly decreased in hearts of young Mrps34mut/mut mice (Fig. 5A), although there was a trend towards a decrease in in the activity of Complex IV, likely as a result of its decreased abundance (Fig. 4C). The activities of Complexes III and IV were significantly decreased in livers of the young mutant mice (Fig. 5B), consistent with the observed decrease in the levels of these complexes relative to those in control mice. In the hearts of aged Mrps34mut/mut mice only the activity of Complex IV was decreased (Fig. 5C), whereas the activities of both Complex III and IV were most significantly affected in the livers of these mice (Fig. 5D) as a result of reduction in the abundance of these complexes identified by immunoblotting (Fig. 4C and 4D). Measurements of oxygen consumption confirmed that mitochondrial respiratory function was affected more in the livers than hearts of young Mrps34mut/mut mice and this reduction was more dramatic with age in both tissues (Figs. 5E, 5F, and S3). Consistent with the observed molecular changes in response to the Mrps34 mutation, mitochondrial dysfunction was more pronounced in the liver compared to the heart of Mrps34mut/mut mice (Fig. 5E), suggesting that these two organs have different capacities to cope with changes in translational efficiency at different ages as observed when we measured mitochondrial translation (Fig. 3A).
Fig. 5. The Mrps34 mutation causes reduced oxygen consumption and respiratory complex activities in heart and liver mitochondria. 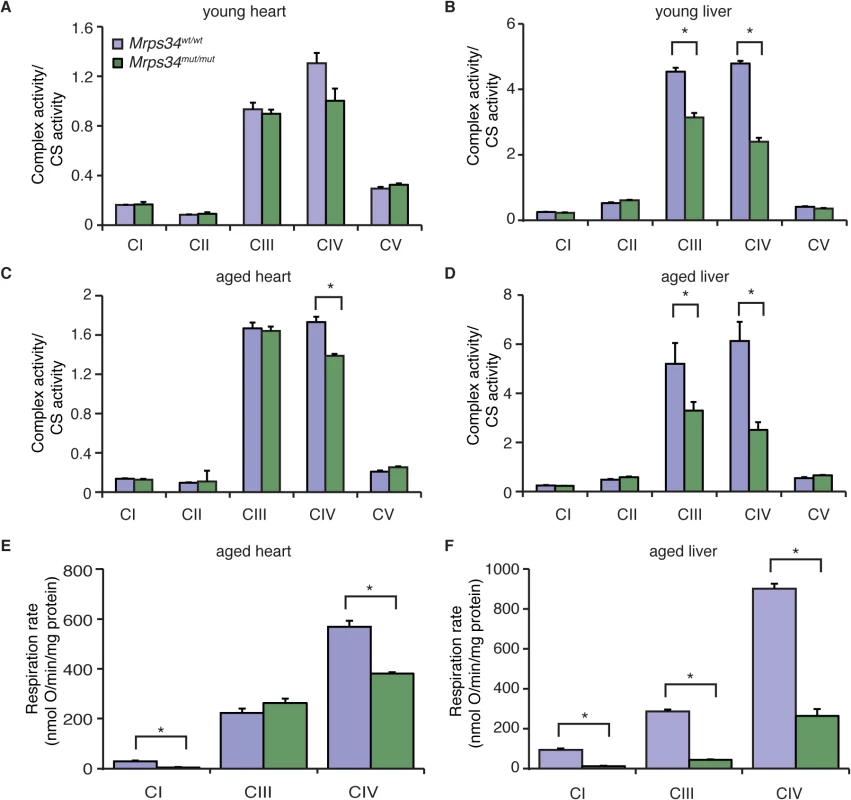
The activities of the five mitochondrial respiratory complexes were measured in mitochondria isolated from heart (A) and liver (B) of young Mrps34wt/wt and Mrps34mut/mut mice and aged mice (C and D), respectively. The respiratory complex activities were normalized relative to citrate synthase activity. Data are means ± SEM of three-four separate experiments; *, p < 0.05 compared with control treatments by a 2-tailed paired Student’s t test. State 3 and 4 respiration was measured in mitochondria isolated from hearts (E) and livers (F) of aged Mrps34wt/wt and Mrps34mut/mut mice using an OROBOROS oxygen electrode. Data are means ± SEM of three-four separate experiments; *, p < 0.05 compared with control treatments by a 2-tailed paired Student’s t test. Mrps34mut/mut mice develop heart hypertrophy and liver steatosis with age
Mutations in genes encoding mitochondrial ribosomal proteins have been shown to cause cardiomyopathy in patients that result in impaired mitochondrial protein synthesis and consequently reduction in the levels and activities of respiratory complexes [18,19]. The Mrps34mut/mut mice appeared similar to their Mrps34wt/wt mice littermates at birth and no developmental or fertility differences were observed compared to the control mice. However, with age the Mrps34mut/mut mice develop physiological changes that affect multiple tissues to varying extents. We observed slight reduction in vision in the Mrps34mut/mut compared to the Mrps34wt/wt mice, measured using optokinetic drum experiments, although the optic nerve was not affected (S4A–S4C, S4H Fig). The motor coordination, strength, and balance between the Mrps34wt/wt and Mrps34mut/mut mice was not significantly different (S4D–S4G Fig), although a slight difference at day 4 may suggest a potential motor learning deficit. We observed centralization of nuclei in the muscle of aged Mrps34mut/mut mice and decreased COX activity (S4I Fig). To investigate if reduced Complex IV activity, as a result of the Mrps34 mutation, affects heart function we carried out echocardiography on the Mrps34mut/mut and Mrps34wt/wt mice. We found that the hearts of mutant mice have increased fractional shortening, a thickening (hypertrophy) of the posterior wall and associated decreased oxygen consumption (Fig. 6A) that can be a common consequence of mitochondrial dysfunction. However, because we found greater mitochondrial dysfunction in the livers of Mrps34mut/mut mice we also investigated the effects of the mutation on the morphology and function of the livers in these mice compared to Mrps34wt/wt mice. Morphological examination revealed increased lipid accumulation in the livers of the young mutant mice and this was more pronounced in the aged mutant mice, suggesting that they have developed liver steatosis (Fig. 6B). Oil red O staining revealed extensive accumulation of lipid droplets in the livers of Mrps34mut/mut mice that was significantly exacerbated with age (Fig. 6C and 6D) and correlated with increased levels of alanine aminotransferase (ALT) in the serum of these mice (Fig. 6E and 6F), which is a marker of liver dysfunction, commonly associated with liver steatosis [20]. Extensive liver dysfunction can cause fibrosis, therefore we used Gomori’s trichome to stain the livers of Mrps34mut/mut and Mrps34wt/wt mice (Fig. 6B and 6C). In the young Mrps34mut/mut mice infiltration of collagen was found around portal tracts (Fig. 6B, arrows) compared to control mice, that was more pronounced in the aged Mrps34mut/mut mice, disrupting the morphology of the liver, encapsulating the tissue (arrow) and forming nodules that are markers of liver fibrosis (Fig. 6C). Our findings indicate that the Mrps34 mutation causes mitochondrial dysfunction that can affect multiple tissues, making these mice a model system to investigate how nuclear mutations can cause pathology with varying severity in different tissues.
Fig. 6. Mrps34mut/mut mice have hypertrophic hearts and increased lipid accumulation in their livers. 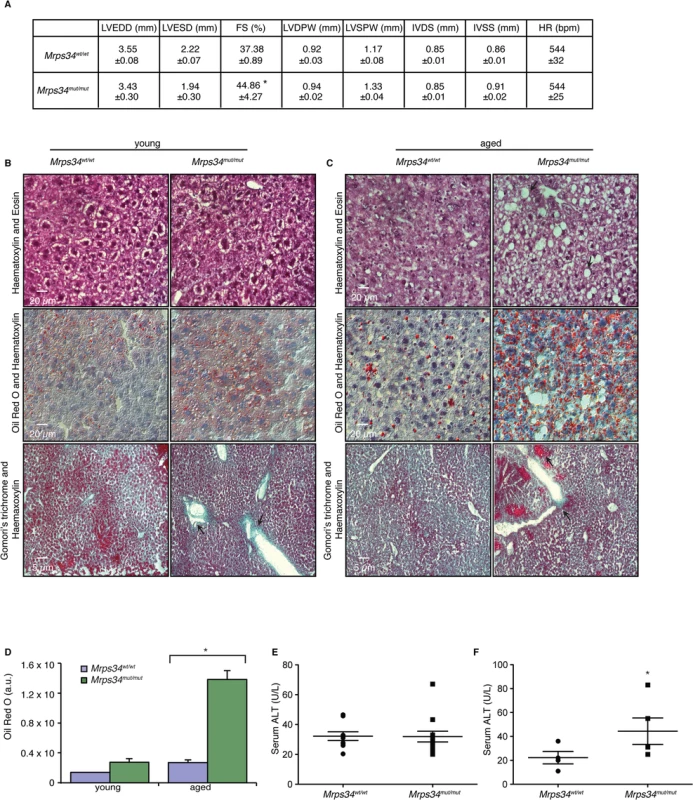
(A) Echocardiographic parameters of Mrps34wt/wt (n = 5) and Mrps34mut/mut (n = 5) mice. LVEDD, left ventricular end diastolic diameter; LVESD, left ventricular end systolic diameter; FS, fractional shortening; LVDPW, left ventricular posterior wall in diastole; LVSPW, left ventricular posterior wall in systole; IVDS, intraventricular septum in diastole; IVSS, intraventricular septum in systole. Values are means ± standard error. *p<0.05 compared with Mrps34wt/wt. Liver sections cut at 8–12 μm thickness were stained with Haematoxylin and Eosin, oil red O and Haematoxylin or Gomori trichrome from young (B) and aged (C) Mrps34wt/wt (n = 9) and Mrps34mut/mut (n = 9) mice and visualized at 40X magnification. (D) Quantitative measurement of oil red staining using Image J. Data are means ± SEM of four different mice; *, p < 0.05 compared with control treatments by a 2-tailed paired Student’s t test. Serum ALT levels in young (E) and aged (F) Mrps34wt/wt (n = 12) and Mrps34mut/mut (n = 12) mice. MRPS34 is required for the stability of the small ribosomal subunit and its association with the large subunit
To understand the role of MRPS34 within the mitochondrial ribosome we investigated the effects of the Mrps34 mutation on the levels of mitochondrial ribosomal proteins from the small and large subunit in Mrps34mut/mut compared to Mrps34wt/wt mice by immunoblotting. It has previously been shown that the loss of certain mitochondrial small ribosomal subunit proteins can disrupt the assembly of the small subunit and cause the loss or reduction of other small ribosomal subunit proteins [21], while loss of other small subunit proteins does not [22], potentially revealing their roles in ribosome assembly. We investigated the levels of MRPS16, MRPS25, and MRPS35 mitochondrial small ribosomal subunit proteins, and found that their levels closely paralleled those of MRPS34 (Fig. 7A), suggesting that the Mrps34 mutation causes destabilization of the small ribosomal subunit. This reduction in the small ribosomal subunit proteins is consistent with the reduced levels of the 12S rRNA (Fig. 2A and 2B). Although in the young mutant mice the levels of the large ribosomal subunit protein MRPL44 were slightly increased by the Mrps34 mutation (Fig. 1C), likely as a compensatory response to decreased small ribosomal subunit proteins (as previously observed in [23]), we observed that in the aged mutant mice the MRPL44 and MRPL23 proteins were also decreased compared to controls (Fig. 7A) both in heart and liver mitochondria. As ribosomal proteins are required in tightly regulated ratios, decrease in MRPS34 over time leads to reduction in other mitoribosomal proteins. Therefore we conclude that MRPS34 is required for the steady-state levels of small subunit ribosomal proteins, and thereby the stability of the small ribosomal subunit, which is necessary for decoding of mitochondrial mRNAs when coupled with the large subunit and consequently translation of the mitochondrially-encoded proteins.
Fig. 7. MRPS34 is required for the small ribosomal subunit and the stability of the mitoribosome. 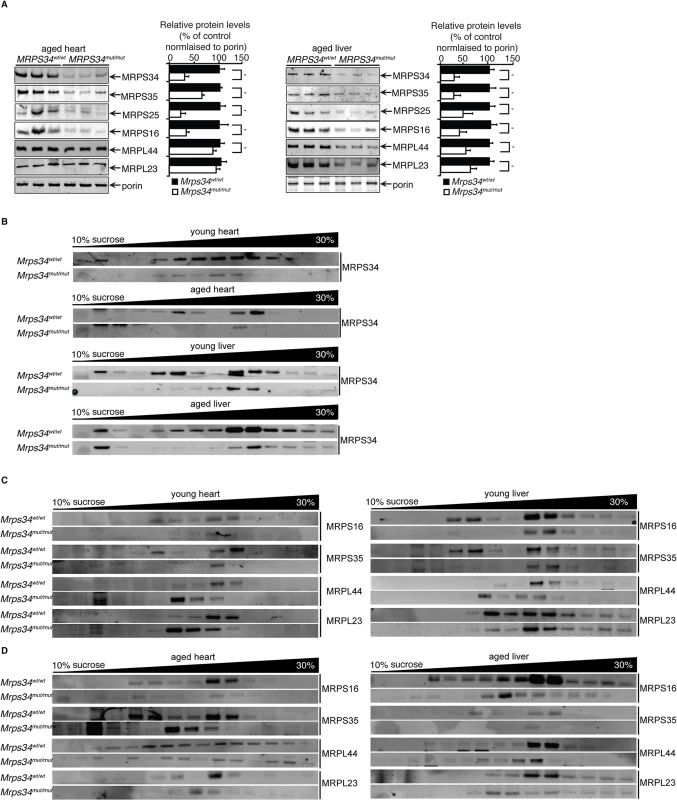
(A) Mitochondrial ribosomal protein levels in mitochondria (50 μg) isolated from liver and heart of Mrps34wt/wt compared to Mrps34mut/mut mice was determined by immunoblotting. (B) Distribution of the MRPS34 protein in 10–30% sucrose gradients of heart (0.8 mg) and liver (1.2 mg) mitochondria from young and aged Mrps34wt/wt and Mrps34mut/mut mice. Mitochondrial protein lysates from heart (0.8 mg) and liver (1.2 mg) of young (C) and aged (D) Mrps34wt/wt and Mrps34mut/mut were fractionated on 10–30% sucrose gradients. The distribution of ribosomal proteins was analyzed by immunoblotting against antibodies that are markers of the small and large ribosomal subunits. The data are typical of results from at least three independent biological experiments. Next we investigated if the residual levels of the mutant MRPS34 protein can interact with the mitochondrial ribosome using sucrose gradients followed by immunoblotting for this protein in heart and liver mitochondrial from the mutant and control mice (Fig. 7B). We observed that there are significantly reduced levels of the MRPS34 protein in both heart and liver mitochondria, however the mutant protein can associate with the small ribosomal subunit and constitute part of the actively translating mitoribosome.
To determine if MRPS34 is required for the stability of the mitoribosome we analyzed the mitochondrial ribosome profile of the large and small subunit, the monosome and polysome on sucrose gradients followed by immunoblotting of ribosomal marker proteins (Fig. 7C and 7D). In heart and liver mitochondria from young Mrps34mut/mut mice we observed a decrease in the presence of the small ribosomal proteins MRPS16 and MRPS35 and consequently decrease in the presence of actively translating mitochondrial ribosomes. Similarly, we observed a decrease in the actively translating mitochondrial ribosome in young heart and liver mitochondria when we immunoblotted for the large ribosomal subunit markers MRPL44 and MRPL23 and a small increase in the presence of the large subunit consistent with the slight increase in the steady state levels of these proteins (Fig. 1C), that are more apparent in heart mitochondria (Fig. 7C). The decreased levels of the small ribosomal subunit result in decreased formation of mitochondrial ribosomes.
In liver and heart mitochondria from aged mutant mice we observe decreased levels in both the small and the large ribosomal subunits and consequently reduced levels of the actively translating mitoribosomes (Fig. 7D). In the aged Mrps34mut/mut mice we found that both the ribosomal proteins from small and large mitochondrial subunits are redistributed compared to control mice and we observe reduced polysome formation in the mutant mice, indicating destabilization of mitoribosomes and polysomes with age (Fig. 7D), likely as a result of decreased MRPS34 levels (Fig. 7A and 7B). These results indicate that reduced abundance of the MRPS34 protein affects the rate of translation through destabilization of the small ribosomal subunit and the 12S rRNA that are required for mitoribosome formation.
Discussion
The mitochondrial ribosome is a unique molecular machine that has been honed through evolution to cope with the compaction of the mitochondrial genome [24]. The lack of substantial untranslated regions has given rise to leaderless mitochondrial mRNAs that are somehow recognized by the mitoribosome for translation. These ribosomes have acquired additional mitochondria-specific proteins that predominantly decorate the surface of the ribosome, giving clues that these proteins may play a role in specific recognition and recruitment of mRNAs to the mitoribosome, modulation of translation according to mitochondrial environmental changes, and the exclusive translation of hydrophobic membrane proteins [7,10]. The mitochondria-specific proteins are largely uncharacterized and understanding their roles in the mitoribosome would provide insight into the unique features of mitochondrial protein synthesis that are different from cytoplasmic and prokaryotic systems. The MRPS34 protein was identified as a constituent of the small ribosomal subunit [6,25], it shares little homology to other proteins and is conserved from humans to zebrafish. Here we show that a point mutation in the mouse Mrps34 gene, causing a leucine to proline change in a conserved alpha helix, results in a significant reduction of the MRPS34 protein in heart and liver mitochondria. Consequently, mitochondrial protein synthesis is impaired in the mice homozygous for the Mrps34 mutation leading to pathologies with varying severities in different tissues.
Decreased levels of MRPS34 affect the stability of mitochondrial RNAs and this is particularly pronounced in the liver. The 12S rRNA is specifically decreased in the hearts and livers of the mutant mice, although this reduction is more profound in the liver, particularly with age, indicating that MRPS34 is required for the stability of the 12S rRNA. Although the mutation causes significant reduction of MRPS34, the remaining protein is assembled within the small subunit of the mitoribosome. However the significant decrease in 12S rRNA levels as a result of the MRPS34 reduction suggests that this protein may be required early in the assembly of the small ribosomal subunit and is likely a 12S rRNA-binding protein. We found that reduction in MRPS34 levels affects the abundance of small ribosomal subunit proteins. In the young mice the proteins of the large ribosomal subunit were present in substantial amounts, suggesting that the large subunit was still fully assembled despite the significant loss of the small subunit. This is further corroborated by the reduction in abundance of the 12S rRNA, which is readily degraded unless incorporated into a ribosomal subunit [26], and the uninterrupted presence of the 16S rRNA. This finding suggests that there is no regulatory cross-talk monitoring the levels of the mitochondrial large and small subunit proteins, instead there seems to be a compensatory mechanism that increases the level of large ribosomal proteins in response to loss of small ribosomal proteins. However this may not be long lasting as with age persistent loss of the small ribosomal subunit also leads to reduction of the large ribosomal subunit proteins and destabilization of the actively translating ribosomes in the Mrps34mut/mut mice. In the mutant mice we observed re-distribution of mitochondrial ribosomal proteins suggesting that the assembly of mitochondrial ribosomes is compromised when there are insufficient levels of MRPS34. The differential distribution of the ribosomal subunits in the mutant compared to control mice suggests that there is accumulation of ribosome assembly intermediates due to the reduced MRPS34 levels. In the young mice this re-distribution is observed solely in the small ribosomal subunit proteins indicating that the small ribosomal subunit is dissociating while the large remains stable. This may result in the formation of inaccurately assembled ribosomes, which are easily dissociated into subunits or are unable to bind to docking sites on the inner mitochondrial membrane (IMM) affecting protein synthesis and OXPHOS biogenesis. As a result of this we find that over time, in the aged mice, the stability of the mitochondrial ribosome is affected causing a reduction in polysome formation. These changes may account for the more obvious mitochondrial dysfunction in the aged mutant mice. Furthermore, as the ribosomal proteins are required in a particular stoichiometry, loss of a ribosomal protein would destabilize this balance and would result in decreased assembly of de novo mitochondrial ribosomes. This is confirmed by our data where persistent decrease in MRPS34 in the aged mutant mice leads to reduction of actively translating ribosomes as a result of a decrease in small and large ribosomal subunit proteins.
We found decreased levels of specific mRNAs, including mt-Nd1, mt-Nd5 and mt-Co1 in the livers of mutant mice, while only the mt-Nd5 mRNA was reduced in the hearts of aged mutant mice. The levels of other mRNAs such as mt-Co2 and the tRNAs were unaffected in both heart and liver, and with age. Taken together these findings reveal that MRPS34 is necessary for the stability of specific mitochondrial mRNAs and may indicate that the active translation of certain mRNAs is linked to their stabilities. The different effects on the mitochondrial mRNAs between heart and liver reflect tissue-specific changes that have been found in mitochondrial disease patients previously [18,27,28], as well as recently in a mouse model of mitochondrial disease [29]. In the heart, mitochondrial RNAs account for at least 30% of the total RNA [30] reflecting the dependence of the heart on OXPHOS. It is likely that in the heart there is excess production of mitochondrial mRNAs relative to the required threshold for normal function, such that decreases in mRNA levels do not compromise mitochondrial function in the short term. This is not the case in the liver where the decrease in mitochondrial RNAs has more profound effects on energy metabolism. Furthermore we found that MRPS34 is more abundant in the heart compared to the liver of control mice, which may protect the heart from a more pronounced decline in function in the mutant mice. We find that the levels of the other mitoribosomal proteins in control mice is higher in the heart compared to the liver, likely due to the higher mitochondrial content in the heart. Finally we observe that the liver has a faster initial rate of mitochondrial translation that is compromised in the mutant mice and may contribute to the more pronounced defect in the liver compared to the heart. It could be that after the initial burst of translation in the liver the levels of mitoribosomes or their recycling become limiting so that subsequently translation plateaus, whereas in the heart the higher abundance of mitoribosomes contributes to the steady rate of translation. Unlike the heart, the liver is highly proliferative and it may require rapid bursts of mitochondrial translation for its normal function and during regeneration.
Mitochondrial diseases caused by mutations in nuclear genes encoding mitochondrial proteins can affect the function of many different tissues with varying severity (reviewed in [2,3]). Furthermore, nuclear mutations in mitochondrial proteins can result in remarkably heterogeneous defects with varying severity in different tissues that are poorly understood currently [2]. We observed similar varying defects in different tissues in the Mrps34mut/mut mice, which have a fractional shortening of their hearts, centralized nuclei in their muscles and increased lipid accumulation in their livers that causes liver steatosis. Hepatopathies have been identified along with a range of different symptoms in many mitochondrial disorders, although hepatopathy was the main consequence of a mutation in the tRNA 5-methylaminomethyl-2-thiouridylate methyltransferase (TRMU) gene that produces the enzyme responsible for 2-thiouridylation of the tRNAGlu, tRNAGln and tRNALys, causing a severe but reversible infantile hepatopathy [31–33]; the mutation in the Mrps34 gene is the first to show pronounced liver defects in mice providing the means to investigate the contribution of mitochondrial function to hepathopathy in the future. Many mutations in nuclear genes that encode protein components of the translational machinery result in compromised biogenesis of specific or all mitochondrial respiratory chain complexes and lead to decreased OXPHOS and some of these have been shown to cause accumulation of lipids in hepatocytes [3,34]. Although we observe overall decrease in mitochondrial protein synthesis as a result of the Mrps34 mutation, the greatest reduction is in the activity of Complex IV in both heart and liver, and this reduction is greater in the liver. Decrease in the mt-Co1 mRNA and consequently the COXI protein, that is necessary for the biogenesis of Complex IV [35] likely contributes to this pronounced decrease in its abundance and activity.
Our work has shown that MRPS34 plays a role in maintaining the stability of the small ribosomal subunit and the 12S rRNA, which are necessary formation of actively translating mitoribosomes. In addition, MRPS34 is required for the stability of specific mRNAs, indicating that the mitochondria-specific ribosomal proteins might have unique roles in mitochondrial RNA metabolism. Because the Mrps34 mutation in mice is not embryonic lethal it has provided a means to investigate how mitochondrial dysfunction can lead to disease in the whole body and the progression of the disease with age. Establishment of mouse models where mitochondrial dysfunction causes disease are particularly important for understanding the causes of human disease pathology, identification of drug targets and for the development of future treatments for these diseases.
Methods
Animals and housing
Male age - and litter-mate matched (6–8 weeks ‘young’ and 30 weeks ‘aged’) wild-type (Mrps34wt/wt) and homozygous (Mrps34mut/mut) ENU mutant mice on a C57BL/6J background were obtained from the Australian Phenomics Facility. The Mrps34 mice were bred onto a C57BL/6J background for 8–10 generations. Animals were singly housed in standard cages (45 cm × 29 cm × 12 cm) under a 12-h light/dark schedule (lights on 7 a.m. to 7 p.m.) in controlled environmental conditions of 22 + 2°C and 50 + 10% relative humidity. Normal chow diet (Rat & Mouse Chow, Speciality Foods, Glen Forrest, Western Australia) and water were provided ad libitum. The study was approved by the Animal Ethics Committee of the UWA (AEC 03/100/526) and performed in accordance with Principles of Laboratory Care (NHMRC Australian code for the care and use of animals for scientific purposes, 8th Edition 2013). Behavioral and motor tests are described in Supplemental Methods (S1 Text).
Tissue homogenate preparation
Tissues were homogenised in 100 μl of 100 mM Tris, 2 mM Na3VO4, 100 mM NaCl, 1% Triton X-100, 1 mM EDTA, 10% Glycerol, 1 mM EGTA, 0.1% SDS, 1 mM NaF, 0.5% deoxycholate, 20 mM Na4P2O7, pH 7.4 containing PhosSTOP Phosphatase Inhibitor Cocktail and EDTA-free Complete protease inhibitor cocktail and the supernatant was collected after centrifugation at 10,000 g.
Mitochondrial isolation
Mitochondria were isolated from homogenized hearts and livers and isolated by differential centrifugation as described previously [36] with some modifications. Livers were homogenized in buffer containing 250 mM sucrose, 5 mM Tris, 1mM EGTA, pH 7.4 with EDTA-free Complete protease inhibitor cocktail (Roche) and hearts were homogenized in 210 mM mannitol, 70 mM sucrose, 10 mM Tris, 0.1mM EDTA pH 7.4 containing EDTA-free Complete protease inhibitor cocktail.
Sucrose gradient subfractionation
Liver (1.2 mg of protein) and heart (0.8 mg of protein) mitochondria were lysed with 1% n-Dodecyl β-D-maltoside in 10mM Tris-HCl, pH 7.4, 260 mM sucrose, 100 mM KCl, 20 mM MgCl2 in the presence of RNasin and protease inhibitors for 30 min, the lysate centrifuged at 10,000 g for 45 min at 4°C, the clarified lysate was loaded on a continuous 10–30% sucrose gradient (in 10 mM Tris-HCl, pH 7.4, 100 mM KCl, 20 mM MgCl2 in the presence of RNasin and protease inhibitors) and centrifuged at 71,000 g in an Optima Beckman Coulter preparative ultracentrifuge as described before [6]. Fractions were collected and precipitated with 30% trichloroacetic acid, washed in acetone, and the entire fraction was resolved by SDS-PAGE. Protein markers of the mitochondrial ribosomal subunits were detected by immunoblotting as described below.
RNA isolation and northern blotting
RNA was isolated from heart and liver mitochondria using the miRNeasy Mini kit (Qiagen) incorporating an on-column RNase-free DNase digestion to remove all DNA. RNA (5 μg) was resolved on 1.2% agarose formaldehyde gels, then transferred to 0.45 μm Hybond-N+ nitrocellulose membrane (GE Lifesciences) and hybridized with biotinylated oligonucleotide probes specific to mouse mitochondrial tRNAs, mRNAs and rRNAs. The hybridizations were carried out overnight at 50°C in 5x SSC, 20 mM Na2HPO4, 7% SDS and 100 μg.ml-1 heparin, followed by washing. The signal was detected using streptavidin-linked infrared antibody (diluted 1 : 2,000 in 3x SSC, 5% SDS, 25 mM Na2HPO4, pH 7.5) using an Odyssey Infrared Imaging System (Li-Cor).
Immunoblotting
Specific proteins were detected using rabbit polyclonal antibodies against: MRPL44, MRPL23, MRPS16, MRPS35, MRPS25 (Proteintech, diluted 1 : 1000), MRPS34 (Sigma, diluted 1 : 1000) and mouse monoclonal antibodies against: porin, NDUFA9, Complex II, Complex III, COXI, COXII, COXIV and Complex V subunit (Abcam, diluted 1 : 1000), in Odyssey Blocking Buffer (Li-Cor). IR Dye 800CW Goat Anti-Rabbit IgG or IRDye 680LT Goat Anti-Mouse IgG (Li-Cor) secondary antibodies were used and the immunoblots were visualized using an Odyssey Infrared Imaging System (Li-Cor). Tissue specific immunoblotting analysis was performed on a Proteintech mouse tissue blot (Cat. No. M10005).
Mitochondrial protein synthesis
Mitochondrial de novo protein synthesis was analyzed using Expres35S Protein Labelling Mix [35S] (14 mCi, Perkin–Elmer) as described before [23].
Blue Native Page Electrophoresis
Liver and heart mitochondrial lysates were resolved by BN-PAGE to detect the respiratory complexes by Coomassie staining as described previously [8,37] or by immunoblotting as described above.
Complex enzyme assays
The enzyme activities of all five respiratory complexes and citrate synthase were measured in a 1 ml cuvette at 30°C using a Perkin Elmer lambda 35 dual beam spectrophotometer as described in [38].
Respiration
Mitochondrial respiration was evaluated as O2 consumption in isolated heart and liver mitochondria according to Kuznetsov et al. Mitochondria were supplemented with substrates for either complex I (10 mM glutamate/malate, Sigma), II (10 mM succinate, Sigma) or III (1 mM TMPD/1 mM ascorbate, Sigma). After addition of 1 mM adenosine diphosphate (ADP, Sigma) to the recording chamber, State 3 respiration activity was measured. ADP independent respiration activity (State 4) was monitored after addition of oligomycin (2 μg/ml, Sigma).
Echocardiography
Echocardiographic studies to measure left ventricular function were performed on mice under light methoxyflurane anesthesia with the use of an i13L probe on a Vivid 7 Dimension (GE Healthcare). Echocardiographic measurements were taken on M-mode in triplicate from each mouse and the quantitative measurements represent the average. M-mode recordings were made at a sweep speed of 200 mm/s. Measurements of left ventricular end diastolic diameter (LVEDD), left ventricular end systolic diameter (LVESD), fractional shortening (FS), left ventricular posterior wall in diastole (LVDPW), left ventricular posterior wall in systole (LVSPW), intraventricular septum in diastole (IVDS), and intraventricular septum in systole (IVSS) were made. Fractional shortening (FS) was calculated by the formula [(LVEDD-LVESD)/EDD] x 100.
Histochemistry
Fresh sections of the liver and muscle were frozen in Optimal Cutting Temperature (OCT) medium, sectioned and stained with Haematoxylin and Eosin, Gomori’s Trichrome and Haematoxylin, Oil Red O and Haematoxylin, COX or NADH stains. Images were acquired using a Nikon Ti Eclipse inverted microscope using a Nikon 40x objective and Oil Red O staining was quantified as described previously [39].
Supporting Information
Zdroje
1. Ojala D, Montoya J, Attardi G (1981) tRNA punctuation model of RNA processing in human mitochondria. Nature 290 : 470–474. 7219536
2. Vafai SB, Mootha VK (2012) Mitochondrial disorders as windows into an ancient organelle. Nature 491 : 374–383. doi: 10.1038/nature11707 23151580
3. Boczonadi V, Horvath R (2014) Mitochondria: Impaired mitochondrial translation in human disease. Int J Biochem Cell Biol 48 : 77–84. doi: 10.1016/j.biocel.2013.12.011 24412566
4. Sharma M, Koc E, Datta P, Booth T, Spremulli L, et al. (2003) Structure of the mammalian mitochondrial ribosome reveals an expanded functional role for its component proteins. Cell 115 : 97–108. 14532006
5. Suzuki T (2001) Proteomic Analysis of the Mammalian Mitochondrial Ribosome. Identification of protein components in the 28 S small subunit. J Biol Chem 276 : 33181–33195. doi: 10.1074/jbc.M103236200 11402041
6. Kaushal PS, Sharma MR, Booth TM, Haque EM, Tung C-S, et al. (2014) Cryo-EM structure of the small subunit of the mammalian mitochondrial ribosome. Proc Natl Acad Sci U S A. doi: 10.1073/pnas.1401657111
7. Greber BJ, Boehringer D, Leitner A, Bieri P, Voigts-Hoffmann F, et al. (2013) Architecture of the large subunit of the mammalian mitochondrial ribosome. Nature: 1–17. doi: 10.1038/nature12890
8. Sharma MRM, Booth TMT, Simpson LL, Maslov DAD, Agrawal RKR (2009) Structure of a mitochondrial ribosome with minimal RNA. Proc Natl Acad Sci U S A 106 : 9637–9642. doi: 10.1073/pnas.0901631106 19497863
9. Sharma M, Koc E, Datta P, Booth T, Spremulli L, et al. (2003) Structure of the mammalian mitochondrial ribosome reveals an expanded functional role for its component proteins. Cell 115 : 97–108. 14532006
10. Brown A, Amunts A, Bai X-C, Sugimoto Y, Edwards PC, et al. (2014) Structure of the large ribosomal subunit from human mitochondria. Science 346 : 718–722. doi: 10.1126/science.1258026 25278503
11. Suzuki T, Terasaki M, Takemoto-Hori C, Hanada T, Ueda T, et al. (2001) Structural compensation for the deficit of rRNA with proteins in the mammalian mitochondrial ribosome. Systematic analysis of protein components of the large ribosomal subunit from mammalian mitochondria. J Biol Chem 276 : 21724–21736. doi: 10.1074/jbc.M100432200 M100432200 [pii]. 11279069
12. Agrawal RK, Sharma MR (2012) Structural aspects of mitochondrial translational apparatus. Curr Opin Struct Biol 22 : 797–803. doi: 10.1016/j.sbi.2012.08.003 22959417
13. Ott MM, Prestele MM, Bauerschmitt HH, Funes SS, Bonnefoy NN, et al. (2006) Mba1, a membrane-associated ribosome receptor in mitochondria. EMBO J 25 : 1603–1610. doi: 10.1038/sj.emboj.7601070 16601683
14. Koc EC, Burkhart W, Blackburn K, Moseley A, Spremulli LL (2001) The small subunit of the mammalian mitochondrial ribosome. Identification of the full complement of ribosomal proteins present. J Biochem 276 : 19363–19374. Available: http://www.jbc.org/cgi/doi/10.1074/jbc.M100727200. 11279123
15. O'brien TW (2002) Evolution of a protein-rich mitochondrial ribosome: implications for human genetic disease. Gene 286 : 73–79. doi: 10.1016/S0378-1119(01)00808-3 11943462
16. Ogawa F, Adachi S, Kohu K, Shige K, Akiyama T (2003) Binding of the human homolog of the Drosophila discs large tumor suppressor protein to the mitochondrial ribosomal protein MRP-S34. Biochem Biophys Res Commun 300 : 789–792. doi: 10.1016/S0006-291X(02)02887-5 12507520
17. Andrews TD, Whittle B, Field MA, Balakishnan B, Zhang Y, et al. (2012) Massively parallel sequencing of the mouse exome to accurately identify rare, induced mutations: an immediate source for thousands of new mouse models. Open Biol 2 : 120061. doi: 10.1098/rsob.120061 22724066
18. Carroll CJ, Isohanni P, Poyhonen R, Euro L, Richter U, et al. (2013) Whole-exome sequencing identifies a mutation in the mitochondrial ribosome protein MRPL44 to underlie mitochondrial infantile cardiomyopathy. J Med Genet 50 : 151–159. doi: 10.1136/jmedgenet-2012-101375 23315540
19. Saada A, Shaag A, Arnon S, Dolfin T, Miller C, et al. (2007) Antenatal mitochondrial disease caused by mitochondrial ribosomal protein (MRPS22) mutation. J Med Genet 44 : 784–786. doi: 10.1136/jmg.2007.053116 17873122
20. Matthews VB, Allen TL, Risis S, Chan MHS, Henstridge DC, et al. (2010) Interleukin-6-deficient mice develop hepatic inflammation and systemic insulin resistance. Diabetologia 53 : 2431–2441. doi: 10.1007/s00125-010-1865-y 20697689
21. Emdadul Haque M, Grasso D, Miller C, Spremulli LL, Saada A (2008) The effect of mutated mitochondrial ribosomal proteins S16 and S22 on the assembly of the small and large ribosomal subunits in human mitochondria. Mitochondrion 8 : 254–261. doi: 10.1016/j.mito.2008.04.004 18539099
22. Davies SMK, Rackham O, Shearwood A-MJ, Hamilton KL, Narsai R, et al. (2009) Pentatricopeptide repeat domain protein 3 associates with the mitochondrial small ribosomal subunit and regulates translation. FEBS Letts 583 : 1853–1858. doi: 10.1016/j.febslet.2009.04.048 19427859
23. Ruzzenente B, Metodiev MD, Wredenberg A, Bratic A, Park CB, et al. (2011) LRPPRC is necessary for polyadenylation and coordination of translation of mitochondrial mRNAs. EMBO J 31 : 443–456. doi: 10.1038/emboj.2011.392 22045337
24. Small ID, Rackham O, Filipovska A (2013) Organelle transcriptomes: products of a deconstructed genome. Curr Opin Microbiol 16 : 652–658. doi: 10.1016/j.mib.2013.07.011 23932204
25. O'Brien TW (2000) Mammalian Mitochondrial Ribosomal Proteins (4). Amino acid sequencing, characterization, and identification of corresponding gene sequences. J Biol Chem 275 : 18153–18159. doi: 10.1074/jbc.M909762199 10751423
26. Dennis PP, Young RF (1975) Regulation of ribosomal protein synthesis in Escherichia coli B/r. J Bacteriol 121 : 994–999. 1090612
27. Antonicka H, Ostergaard E, Sasarman F, Weraarpachai W, Wibrand F, et al. (2010) Mutations in C12orf65 in patients with encephalomyopathy and a mitochondrial translation defect. Am J Hum Genet 87 : 115–122. S0002-9297(10)00307-1 [pii] doi: 10.1016/j.ajhg.2010.06.004 20598281
28. Janer A, Antonicka H, Lalonde E, Nishimura T, Sasarman F, et al. (2012) An RMND1 Mutation Causes Encephalopathy Associated with Multiple Oxidative Phosphorylation Complex Deficiencies and a Mitochondrial Translation Defect. Am J Hum Genet 91 : 737–743. doi: 10.1016/j.ajhg.2012.08.020 23022098
29. Dogan SA, Pujol C, Maiti P, Kukat A, Wang S, et al. (2014) Tissue-specific loss of DARS2 activates stress responses independently of respiratory chain deficiency in the heart. Cell Metab 19 : 458–469. doi: 10.1016/j.cmet.2014.02.004 24606902
30. Mercer TR, Neph S, Dinger ME, Crawford J, Smith MA, et al. (2011) The Human Mitochondrial Transcriptome. Cell 146 : 645–658. doi: 10.1016/j.cell.2011.06.051 21854988
31. Zeharia A, Fischel-Ghodsian N, Casas K, Bykhocskaya Y, Tamari H, et al. (2005) Mitochondrial myopathy, sideroblastic anemia, and lactic acidosis: an autosomal recessive syndrome in Persian Jews caused by a mutation in the PUS1 gene. J Child Neurol 20 : 449–452. 15971356
32. Schara U, Kleist-Retzow von J-C, Lainka E, Gerner P, Pyle A, et al. (2011) Acute liver failure with subsequent cirrhosis as the primary manifestation of TRMU mutations. J Inherit Metab Dis 34 : 197–201. doi: 10.1007/s10545-010-9250-z 21153446
33. Sasarman F, Antonicka H, Horvath R, Shoubridge EA (2011) The 2-thiouridylase function of the human MTU1 (TRMU) enzyme is dispensable for mitochondrial translation. Hum Mol Genet 20 : 4634–4643. doi: 10.1093/hmg/ddr397 21890497
34. Lee WS, Sokol RJ (2007) Mitochondrial hepatopathies: advances in genetics and pathogenesis. Hepatology 45 : 1555–1565. doi: 10.1002/hep.21710 17538929
35. Mick DU, Fox TD, Rehling P (2011) Inventory control: cytochrome c oxidase assembly regulates mitochondrial translation. Nat Rev Mol Cell Biol 12 : 14–20. doi: 10.1038/nrm3029 21179059
36. Rackham O, Davies SMK, Shearwood A-MJ, Hamilton KL, Whelan J, et al. (2009) Pentatricopeptide repeat domain protein 1 lowers the levels of mitochondrial leucine tRNAs in cells. Nucleic Acids Res 37 : 5859–5867. doi: 10.1093/nar/gkp627 19651879
37. Wittig I, Braun H-P, Schägger H (2006) Blue native PAGE. Nat Protoc 1 : 418–428. doi: 10.1038/nprot.2006.62 17406264
38. Davies SMS, Poljak AA, Duncan MWM, Smythe GAG, Murphy MPM (2001) Measurements of protein carbonyls, ortho - and meta-tyrosine and oxidative phosphorylation complex activity in mitochondria from young and old rats. Free Rad Biol Med 31 : 181–190. doi: 10.1016/S0891-5849(01)00576-7 11440830
39. Mehlem A, Hagberg CE, Muhl L, Eriksson U, Falkevall A (2013) Imaging of neutral lipids by oil red O for analyzing the metabolic status in health and disease. Nat Protoc 8 : 1149–1154. doi: 10.1038/nprot.2013.055 23702831
40. Claros M, Vincens P (1996) Computational method to predict mitochondrially imported proteins and their targeting sequences. Eur J Biochem 241 : 779–786. 8944766
41. McWilliam H, Li W, Uludag M, Squizzato S, Park YM, et al. (2013) Analysis Tool Web Services from the EMBL-EBI. Nucleic Acids Res 41: W597–W600. Available: http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=23671338&retmode=ref&cmd=prlinks. doi: 10.1093/nar/gkt376 23671338
42. Jones DT (1999) Protein secondary structure prediction based on position-specific scoring matrices. J Mol Biol 292 : 195–202. doi: 10.1006/jmbi.1999.3091 10493868
Štítky
Genetika Reprodukční medicína
Článek NLRC5 Exclusively Transactivates MHC Class I and Related Genes through a Distinctive SXY ModuleČlánek Inhibition of Telomere Recombination by Inactivation of KEOPS Subunit Cgi121 Promotes Cell LongevityČlánek HOMER2, a Stereociliary Scaffolding Protein, Is Essential for Normal Hearing in Humans and MiceČlánek LRGUK-1 Is Required for Basal Body and Manchette Function during Spermatogenesis and Male FertilityČlánek The GATA Factor Regulates . Developmental Timing by Promoting Expression of the Family MicroRNAsČlánek Systems Biology of Tissue-Specific Response to Reveals Differentiated Apoptosis in the Tick VectorČlánek Phenotype Specific Analyses Reveal Distinct Regulatory Mechanism for Chronically Activated p53Článek The Nuclear Receptor DAF-12 Regulates Nutrient Metabolism and Reproductive Growth in NematodesČlánek The ATM Signaling Cascade Promotes Recombination-Dependent Pachytene Arrest in Mouse SpermatocytesČlánek The Small Protein MntS and Exporter MntP Optimize the Intracellular Concentration of Manganese
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 3
-
Všechny články tohoto čísla
- NLRC5 Exclusively Transactivates MHC Class I and Related Genes through a Distinctive SXY Module
- Licensing of Primordial Germ Cells for Gametogenesis Depends on Genital Ridge Signaling
- A Genomic Duplication is Associated with Ectopic Eomesodermin Expression in the Embryonic Chicken Comb and Two Duplex-comb Phenotypes
- Genome-wide Association Study and Meta-Analysis Identify as Genome-wide Significant Susceptibility Gene for Bladder Exstrophy
- Mutations of Human , Encoding the Mitochondrial Asparaginyl-tRNA Synthetase, Cause Nonsyndromic Deafness and Leigh Syndrome
- Exome Sequencing in an Admixed Isolated Population Indicates Variants Confer a Risk for Specific Language Impairment
- Genome-Wide Association Studies in Dogs and Humans Identify as a Risk Variant for Cleft Lip and Palate
- Rapid Evolution of Recombinant for Xylose Fermentation through Formation of Extra-chromosomal Circular DNA
- The Ribosome Biogenesis Factor Nol11 Is Required for Optimal rDNA Transcription and Craniofacial Development in
- Methyl Farnesoate Plays a Dual Role in Regulating Metamorphosis
- Maternal Co-ordinate Gene Regulation and Axis Polarity in the Scuttle Fly
- Maternal Filaggrin Mutations Increase the Risk of Atopic Dermatitis in Children: An Effect Independent of Mutation Inheritance
- Inhibition of Telomere Recombination by Inactivation of KEOPS Subunit Cgi121 Promotes Cell Longevity
- Clonality and Evolutionary History of Rhabdomyosarcoma
- HOMER2, a Stereociliary Scaffolding Protein, Is Essential for Normal Hearing in Humans and Mice
- Methylation-Sensitive Expression of a DNA Demethylase Gene Serves As an Epigenetic Rheostat
- BREVIPEDICELLUS Interacts with the SWI2/SNF2 Chromatin Remodeling ATPase BRAHMA to Regulate and Expression in Control of Inflorescence Architecture
- Seizures Are Regulated by Ubiquitin-specific Peptidase 9 X-linked (USP9X), a De-Ubiquitinase
- The Fun30 Chromatin Remodeler Fft3 Controls Nuclear Organization and Chromatin Structure of Insulators and Subtelomeres in Fission Yeast
- A Cascade of Iron-Containing Proteins Governs the Genetic Iron Starvation Response to Promote Iron Uptake and Inhibit Iron Storage in Fission Yeast
- Mutation in MRPS34 Compromises Protein Synthesis and Causes Mitochondrial Dysfunction
- LRGUK-1 Is Required for Basal Body and Manchette Function during Spermatogenesis and Male Fertility
- Cis-Regulatory Mechanisms for Robust Olfactory Sensory Neuron Class-restricted Odorant Receptor Gene Expression in
- Effects on Murine Behavior and Lifespan of Selectively Decreasing Expression of Mutant Huntingtin Allele by Supt4h Knockdown
- HDAC4-Myogenin Axis As an Important Marker of HD-Related Skeletal Muscle Atrophy
- A Conserved Domain in the Scc3 Subunit of Cohesin Mediates the Interaction with Both Mcd1 and the Cohesin Loader Complex
- Selective and Genetic Constraints on Pneumococcal Serotype Switching
- Bacterial Infection Drives the Expression Dynamics of microRNAs and Their isomiRs
- The GATA Factor Regulates . Developmental Timing by Promoting Expression of the Family MicroRNAs
- Accumulation of Glucosylceramide in the Absence of the Beta-Glucosidase GBA2 Alters Cytoskeletal Dynamics
- Reproductive Isolation of Hybrid Populations Driven by Genetic Incompatibilities
- The Contribution of Alu Elements to Mutagenic DNA Double-Strand Break Repair
- Systems Biology of Tissue-Specific Response to Reveals Differentiated Apoptosis in the Tick Vector
- Tfap2a Promotes Specification and Maturation of Neurons in the Inner Ear through Modulation of Bmp, Fgf and Notch Signaling
- The Lysine Acetyltransferase Activator Brpf1 Governs Dentate Gyrus Development through Neural Stem Cells and Progenitors
- PHABULOSA Controls the Quiescent Center-Independent Root Meristem Activities in
- DNA Polymerase ζ-Dependent Lesion Bypass in Is Accompanied by Error-Prone Copying of Long Stretches of Adjacent DNA
- Examining the Evolution of the Regulatory Circuit Controlling Secondary Metabolism and Development in the Fungal Genus
- Zinc Finger Independent Genome-Wide Binding of Sp2 Potentiates Recruitment of Histone-Fold Protein Nf-y Distinguishing It from Sp1 and Sp3
- GAGA Factor Maintains Nucleosome-Free Regions and Has a Role in RNA Polymerase II Recruitment to Promoters
- Neurospora Importin α Is Required for Normal Heterochromatic Formation and DNA Methylation
- Ccr4-Not Regulates RNA Polymerase I Transcription and Couples Nutrient Signaling to the Control of Ribosomal RNA Biogenesis
- Phenotype Specific Analyses Reveal Distinct Regulatory Mechanism for Chronically Activated p53
- A Systems-Level Interrogation Identifies Regulators of Blood Cell Number and Survival
- Morphological Mutations: Lessons from the Cockscomb
- Genetic Interaction Mapping Reveals a Role for the SWI/SNF Nucleosome Remodeler in Spliceosome Activation in Fission Yeast
- The Role of China in the Global Spread of the Current Cholera Pandemic
- The Nuclear Receptor DAF-12 Regulates Nutrient Metabolism and Reproductive Growth in Nematodes
- A Zinc Finger Motif-Containing Protein Is Essential for Chloroplast RNA Editing
- Resistance to Gray Leaf Spot of Maize: Genetic Architecture and Mechanisms Elucidated through Nested Association Mapping and Near-Isogenic Line Analysis
- Small Regulatory RNA-Induced Growth Rate Heterogeneity of
- Mitochondrial Dysfunction Reveals the Role of mRNA Poly(A) Tail Regulation in Oculopharyngeal Muscular Dystrophy Pathogenesis
- Complex Genomic Rearrangements at the Locus Include Triplication and Quadruplication
- Male-Biased Aganglionic Megacolon in the TashT Mouse Line Due to Perturbation of Silencer Elements in a Large Gene Desert of Chromosome 10
- Sex Ratio Meiotic Drive as a Plausible Evolutionary Mechanism for Hybrid Male Sterility
- Tertiary siRNAs Mediate Paramutation in .
- RECG Maintains Plastid and Mitochondrial Genome Stability by Suppressing Extensive Recombination between Short Dispersed Repeats
- Escape from X Inactivation Varies in Mouse Tissues
- Opposite Phenotypes of Muscle Strength and Locomotor Function in Mouse Models of Partial Trisomy and Monosomy 21 for the Proximal Region
- Glycosyl Phosphatidylinositol Anchor Biosynthesis Is Essential for Maintaining Epithelial Integrity during Embryogenesis
- Hyperdiverse Gene Cluster in Snail Host Conveys Resistance to Human Schistosome Parasites
- The Class Homeodomain Factors and Cooperate in . Embryonic Progenitor Cells to Regulate Robust Development
- Recombination between Homologous Chromosomes Induced by Unrepaired UV-Generated DNA Damage Requires Mus81p and Is Suppressed by Mms2p
- Synergistic Interactions between Orthologues of Genes Spanned by Human CNVs Support Multiple-Hit Models of Autism
- Gene Networks Underlying Convergent and Pleiotropic Phenotypes in a Large and Systematically-Phenotyped Cohort with Heterogeneous Developmental Disorders
- The ATM Signaling Cascade Promotes Recombination-Dependent Pachytene Arrest in Mouse Spermatocytes
- Combinatorial Control of Light Induced Chromatin Remodeling and Gene Activation in
- Linking Aβ42-Induced Hyperexcitability to Neurodegeneration, Learning and Motor Deficits, and a Shorter Lifespan in an Alzheimer’s Model
- The Complex Contributions of Genetics and Nutrition to Immunity in
- NatB Domain-Containing CRA-1 Antagonizes Hydrolase ACER-1 Linking Acetyl-CoA Metabolism to the Initiation of Recombination during . Meiosis
- Transcriptomic Profiling of Reveals Reprogramming of the Crp Regulon by Temperature and Uncovers Crp as a Master Regulator of Small RNAs
- Osteopetrorickets due to Snx10 Deficiency in Mice Results from Both Failed Osteoclast Activity and Loss of Gastric Acid-Dependent Calcium Absorption
- A Genomic Portrait of Haplotype Diversity and Signatures of Selection in Indigenous Southern African Populations
- Sequence Features and Transcriptional Stalling within Centromere DNA Promote Establishment of CENP-A Chromatin
- Inhibits Neuromuscular Junction Growth by Downregulating the BMP Receptor Thickveins
- Replicative DNA Polymerase δ but Not ε Proofreads Errors in and in
- Unsaturation of Very-Long-Chain Ceramides Protects Plant from Hypoxia-Induced Damages by Modulating Ethylene Signaling in
- The Small Protein MntS and Exporter MntP Optimize the Intracellular Concentration of Manganese
- A Meta-analysis of Gene Expression Signatures of Blood Pressure and Hypertension
- Pervasive Variation of Transcription Factor Orthologs Contributes to Regulatory Network Evolution
- Network Analyses Reveal Novel Aspects of ALS Pathogenesis
- A Role for the Budding Yeast Separase, Esp1, in Ty1 Element Retrotransposition
- Nab3 Facilitates the Function of the TRAMP Complex in RNA Processing via Recruitment of Rrp6 Independent of Nrd1
- A RecA Protein Surface Required for Activation of DNA Polymerase V
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Clonality and Evolutionary History of Rhabdomyosarcoma
- Morphological Mutations: Lessons from the Cockscomb
- Maternal Filaggrin Mutations Increase the Risk of Atopic Dermatitis in Children: An Effect Independent of Mutation Inheritance
- Transcriptomic Profiling of Reveals Reprogramming of the Crp Regulon by Temperature and Uncovers Crp as a Master Regulator of Small RNAs
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



