-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaInformed Consent in the Genomics Era
article has not abstract
Published in the journal: . PLoS Med 5(9): e192. doi:10.1371/journal.pmed.0050192
Category: Essay
doi: https://doi.org/10.1371/journal.pmed.0050192Summary
article has not abstract
Since the Nuremberg trials, informed consent (IC) has been recognized as a basic ethical requirement for research involving human participants [1] (Table 1). Such consent encompasses two distinct elements: (1) researchers communicate detailed information about study procedures, outcomes, risks, and benefits for the participating individual or community, and (2) after understanding and careful consideration, the participants consent to take part under these conditions. However, the suitability of IC for genomic studies has been recently challenged [2,3]. Because the research protocol for such studies may evolve over time, the condition in IC of providing detailed information for a well-defined protocol is not easily satisfied.
Large amounts of data stored as electronic records allow multiple post-hoc analyses, which in many cases were not foreseen at the beginning of a study. The potential for analysis is constantly growing and recently has increased dramatically with the development of high-throughput sequencing and genotyping technologies. More than one million genetic variants of an individual may be determined within hours—and even the full genetic sequence within weeks [3]. Such technical advances expose participants to a new class of risk different from the physical harm usually considered in ethical reviews [4,5]. Release of genetic information could lead to uninsurability, unemployability, discrimination, and the breakdown of family relationships by unintentionally demonstrating missing or unknown relatedness. Moreover, participants usually do not get any direct benefit from the research. All of these concerns raise the question: are IC procedures still in accordance with the currently accepted ethical standards of autonomy, beneficence, and non-maleficence?
Summary Points
-
Genetic cohort studies storing biological materials hold great promise for medical research, but also present new problems that are profoundly different from the classical clinical trial for which informed consent was developed.
-
The classical risk/benefit analysis of physical harm doesn't take into account new threats to the individual such as uninsurability, unemployability, genetic discrimination, or disruption of family relationships.
-
Traditional informed consent may therefore no longer be appropriate when dealing with long-term studies using biological materials.
-
Informed consent should be seen as an ongoing process between researcher and participant, and not just as a once-and-for-all decision.
-
Research following the initial storage of samples needs to be likewise explained and may be announced using new communication methods.
There is pressure to harmonize different views, since a shared ethical and legal framework is still missing and approaches vary greatly [6–8]. A wide spectrum of opinions exists: Some researchers believe that available sample collections do not need any further consent, even for large-scale genotyping [9], while some institutional review boards recommend the destruction of samples immediately after testing (MW, personal observation).
Tab. 1. 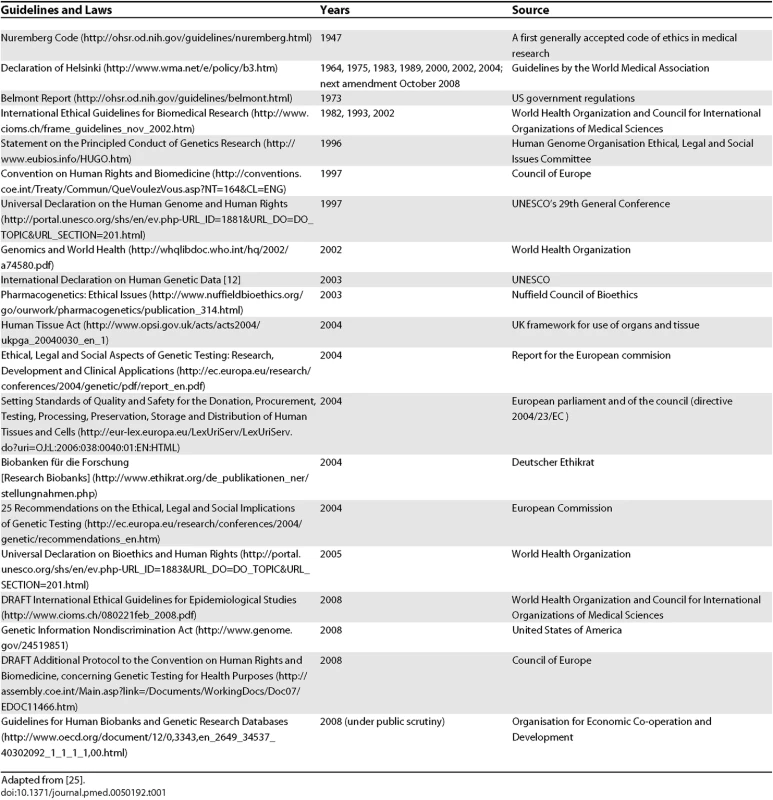
Adapted from [<em class="ref">25</em>]. Unfortunately, in current practice, the only moment when a person is really able to make a choice about participating in clinical research is when they sign the IC form. At this moment, the balance of power between overall research goals and individual interests should find equilibrium. As a study participant, however, one also has the right to know who owns the data, who guarantees proper handling, who will have further access to the data, and what security measures are in place. And all these concerns arise against a background in which the research questions themselves may rapidly change with the advancement of technical knowledge [10]. Any further genetic analysis may in fact severely compromise individual interests and autonomous choice, particularly if the individual is not fully aware of the very nature of the generated data and the implications of its use (or potential abuse). A stepwise informed consent should therefore be considered in accordance with the Council for International Organizations of Medical Sciences guidelines, one of the first to define IC not as a finite time step, but as an ongoing process [11] (Figure 1). Also, UNESCO's International Declaration on Human Genetic Data states that “clear, balanced, adequate and appropriate information shall be provided to the person whose prior, free, informed and express consent is sought” [12]. This goal seems to be largely in contrast with procedures in current genomic studies. Since this kind of detailed information is difficult to provide, a frequently favored approach is to ask for a broad consent [13].
Fig. 1. Informed Consent as a Process 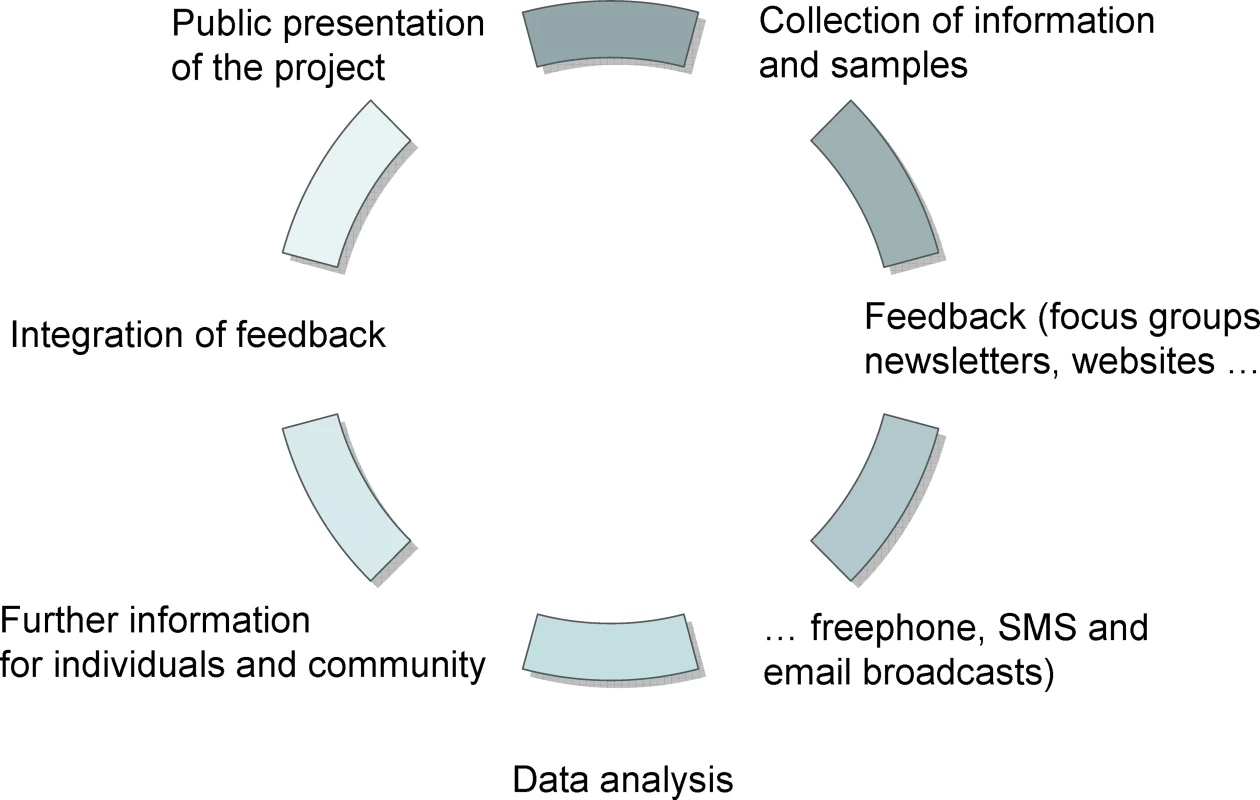
But a more detailed analysis of the rationale behind broad consent shows that “broad consent” is seldom if ever justified [14]. Although proponents of “broad consent” argue that individuals will maintain a right to withdraw from the study, this right seems to be more of a fig leaf than a true option. As the value of stored samples increases over time due to additional data being generated, a later withdrawal of consent is difficult; it would require not only removal of printed lists and questionnaires but also deletion of current computer files including backups as well as all samples and aliquots.
Box 1: An Implementation Plan
Carefully inform the community about the planned study
Collect feedback and take it into account for policy changes
Redesign informed consent forms so that consent may be initially given only for interview, physical examination, data, and sample storage
Explain terms without ambiguity (for instance, explain that anonymity may be limited even with anonymized samples)
Explain opt-in/opt-out procedures for further genetic testing
Establish conventional communication channels (face-to-face or group meetings, letters) but also use electronic communications (e-mail alerts, Web sites, RSS feeds, electronic voting, blogs, chat rooms, social platforms)
Offer freephone number, SMS broadcasts
Develop transparent annotation rules for individual genetic data
Respect the right to know and the right not to know Adjust protocols to local needs and individual wishes
Therefore the Council for International Organizations of Medical Sciences guidelines recommend broad consent be sought only under certain circumstances [11]. Such circumstances include (1) obtaining the approval of an ethical committee and (2) keeping data and samples anonymous. Anonymity, however, is in practice impossible to guarantee [15,16]. Genetic data are intrinsically self-identifying, hence their use in criminal forensic investigations [17]. Advances in computer science allow cross-matching of data not previously foreseen, as shown in the “Netflix” affair (http://www.nytimes.com/2007/06/04/technology/04netflix.htm). With every bit of additional data, anonymity is decreasing, in particular when genetic testing may be used to reconstruct ethnicity, sex, age, height, and other body characteristics such as eye and hair color [18]. Unfortunately, nobody can foresee the implications of these threats to genetic privacy. With the stability of DNA and its potential for use even with only trace amounts, genomics studies will have an almost infinite duration if not actively terminated. Moreover, any genetic variant determined in someone's DNA will have a good chance of also being present in that person's children (who may have never consented to the analysis of their genome in the first place). Hence there is a community dimension to IC for genomic studies, especially when combined with genealogical data.
There is substantial uncertainty in the development of genomics projects that may arise, which cannot be foreseen even by institutional review boards. Moreover, many researchers in the field currently see their institutional review board only as an “obstacle course” with an uncertain outcome [19]. With many issues now arising in the interplay between research-driven interests, new discoveries, and society, such as allocation of research resources, data ownership, and publication of results, the problem is not so much the doctrine of IC but its implementation [20]. Most if not all of the problems could certainly be addressed by a better researcher–participant relationship [21]. As Veatch notes: “In the past, research subjects have all too often been treated as passive “material” suitable for providing additional data points…[while] partners normally come together not because they share exactly the same interests or abilities, but because there is some mutuality of interests, some common point of intersection where each can help the other” [21]. A research partnership will demonstrate not only the potential benefits of such research, but also the current difficulties that need to be addressed. Reassuring research participants that anonymity will be preserved just to avoid a deeper discussion about the issues involved in genomics research may undermine the trust and collaborative spirit that is needed between science and society.
In current genomic studies, the relationship between researcher and participant may be different from the traditional physician–patient studies. Most of the research staff in current genomics projects will not have any direct contact with participants. In addition, these studies are usually not expected to give any direct benefit to the participants, as they are not seeking any new treatment. These two features of genomics research need to be reflected in the partnership between all actors involved in the research. Where possible, participants should be actively involved in information exchange and the decision-making process [3,18]. Several authors have already suggested an exploratory or participatory process prior to the implementation of a research project that should provide a better understanding of relevant issues through interest groups, consensus conferences, meetings, or surveys [10,16,20]. We propose a circular process of information exchange (Figure 1). Following a first phase of general information, a more detailed information exchange then takes place. IC in this context should be defined as an ongoing process instead of a once-and-for-all-time decision [22]. Understanding IC as a process will furthermore bring the “community dimension” back into focus. In addition to communicating the impact of the results together with the hope that these may bring forth improved diagnostics, personalized medicine, prevention, and health care, the circular IC process would also provide an opportunity to examine the difficulties and challenges raised by such research.
Kohane and colleagues have argued that the recent advances of information technology can further help in an ethical decision-making process [23]. At least in industrialized countries, such technology now allows the establishment of dynamic participant–researcher partnerships. In the past, contacting participants after a study was over would have been prohibitively expensive, but such contacts can now be easily managed by serialized SMS, e-mail, or regularly updated Web pages, with letters for those without Internet or mobile phone access (Box 1). Newsletters, public meetings, or local newspaper articles may also be considered as a channel to communicate research results. Participants may decide if they are willing to answer further questions, undergo additional tests, and give permission for their specimens to be included in a future series of genotyping, expression profiling, or proteomic analyses [24].
We are aware that implementing our suggested process of ongoing IC may require additional efforts, but we believe that our approach will lead to a better understanding and a new level of transparency in research. Reorienting IC in the genomics age as a circular process of communication involving researchers and participants as partners in an open dialogue is a great opportunity to build trust between science and society, while giving new force and meaning to the ethics of research.
Supporting Information
Zdroje
1. TaylorT
1993
The anatomy of the Nuremberg Trials
London
Bloomsbury Publishing
703
2. KnoppersB
2005
Consent revisited: Points to consider.
Health Law Rev
13
33
38
3. PetersenA
2007
Biobanks “engagements”: Engendering trust or
engineering consent.
Genomics Soc Policy
3
31
43
4. FuntowiczSR
1993
Science for the post-normal age.
Futures
25
735
755
5. KrimskyS
2004
Science in the private interest. Has the lure of profits corrupted biomedical
research
Lanham (MD)
Rowman & Littlefield
272
6. KnoppersB
2005
Biobanking: International norms.
J Law Med Ethics
33
7
14
7. KnoppersB
2004
Biobanks: Simplifying consent.
Nat Rev Genet
5
485
8. GibbonsSHelgasonHKayeJNomperAWendelL
2005
Lessons from European population genetic databases: Comparing the law in
Estonia, Iceland, Sweden and the United Kingdom.
Eur J Health Law
12
103
133
9. WichmannHGiegerCIlligT
2005
KORA-gen—Resource for population genetics, controls and a broad
spectrum of disease phenotypes.
Gesundheitswesen
67
Suppl 1
S26
S30
10. EdwardsJ
2002
Taking public understanding seriously.
New Genet Soc
21
315
325
11. Medical Research Council, Wellcome Trust
2006
CIOMS guidelines “Special ethical considerations for
epidemiological research”: Response from the Medical Research Council and the
Wellcome Trust.
Available: http://www.wellcome.ac.uk/stellent/groups/corporatesite/@policy_communications/documents/web_document/WTX035295.pdf.
Accessed 13 August 2008
12. United Nations Educational, Scientific and Cultural Organization
2003
International declaration on human genetic data.
Available: http://portal.unesco.org/shs/en/ev.php-URL_ID=1882&URL_DO=DO_TOPIC&URL_SECTION=201.html.
Accessed 13 August 2008
13. WinickoffD
2000
The biobanks law and decode genetics: Rhetoric equals cash in Iceland.
Genewatch
13
4
6
14. CaulfieldT
2007
Biobanks and blanket consent: The proper place of the public good and
public perception rationales.
Kings Coll Law J
18
209
226
15. ArnasonV
2004
Coding and consent: Moral challenges of the database project in Iceland.
Bioethics
181
27
49
16. TuttonR
2004
Genetic databases: Socio-ethical issues in the collection and use of DNA
London
Routledge
208
17. LinZAltmanRBOwenAB
2006
Confidentiality in genome research.
Science
313
441
442
18. LunshofJEChadwickRVorhausDBChurchGM
2008
From genetic privacy to open consent.
Nat Genet
9
406
411
19. LedfordH
2007
Human-subjects research: Trial and error.
Nature
448
530
532
20. LidzCWAppelbaumPS
1988
Two models of implementing informed consent.
Arch Intern Med
148
1385
1389
21. VeatchRM
1987
The patient as partner. A theory of human-experimentation ethics
Indiana University Press
256
22. WeillC
2003
Can consultation of both, experts and the public help developing public
policy.
Sci Public Policy
30
199
203
23. KohaneISMandlKDTaylorPLHolmIANigrinDJ
2007
Reestablishing the researcher-patient compact.
Science
316
836
837
24. CaulfieldTMcGuireALChoMBuchananJABurgessM
2008
Research ethics recommendations for whole-genome research: Consensus
statement.
PLoS Biol
6
e73
doi:10.1371/journal.pbio.0060073
25. ArnoldKvan der WaltJ
2007
Ethical issues in human genetic research: The global experience.
In:
WeinerMPGabrielSBStephensJC
editors
Genetic variation. A laboratory manual
Cold Spring Harbor (NY)
Cold Spring Harbour Laboratory Press
472
Štítky
Interní lékařství
Článek vyšel v časopisePLOS Medicine
Nejčtenější tento týden
2008 Číslo 9- Léčba bolesti u seniorů
- Benefity fixní kombinace tramadolu a paracetamolu v léčbě bolesti
- Berberin: přírodní hypolipidemikum se slibnými výsledky
- Flexofytol® – přírodní revoluce v boji proti osteoartróze kloubů
- Superoxidované roztoky v prevenci infekcí u dialyzovaných pacientů
-
Všechny články tohoto čísla
- Towards a Data Sharing Culture: Recommendations for Leadership from Academic Health Centers
- A 50-Year-Old Woman with Recurrent Generalised Seizures
- Tobacco Control Yields Clear Dividends for Health and Wealth
- Ethical and Practical Issues Associated with Aggregating Databases
- Publication of Clinical Trials Supporting Successful New Drug Applications: A Literature Analysis
- Informed Consent in the Genomics Era
- Birth Size and the Pathogenesis of Breast Cancer
- Animal Models of Inflammatory Bowel Disease at the Dawn of the New Genetics Era
- Making Sense of Non-Financial Competing Interests
- PLOS Medicine
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Informed Consent in the Genomics Era
- Publication of Clinical Trials Supporting Successful New Drug Applications: A Literature Analysis
- A 50-Year-Old Woman with Recurrent Generalised Seizures
- Ethical and Practical Issues Associated with Aggregating Databases
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



