-
Články
Reklama
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
ReklamaAntimicrobial Peptides: Primeval Molecules or Future Drugs?
article has not abstract
Published in the journal: . PLoS Pathog 6(10): e32767. doi:10.1371/journal.ppat.1001067
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1001067Summary
article has not abstract
Antimicrobial Peptides: Effector Substances of Innate Immunity
From the outside and within, we are constantly bombarded with a myriad of diverse microbial species. However, our bodies are equipped with an evolutionarily conserved innate immune defense system that allows us to thwart potential pathogens. Antimicrobial peptides (AMPs) are a unique and assorted group of molecules produced by living organisms of all types, considered to be part of the host innate immunity [1], [2]. These peptides demonstrate potent antimicrobial activity and are rapidly mobilized to neutralize a broad range of microbes, including viruses, bacteria, protozoa, and fungi [3]. More significantly, the ability of these natural molecules to kill multidrug-resistant microorganisms has gained them considerable attention and clinical interest [1]. With the growing microbial resistance to conventional antimicrobial agents, the need for unconventional therapeutic options has become urgent. This article provides an overview of AMPs, their biological functions, mechanism of action, and applicability as alternative therapeutic agents.
Structure and Classification
Antimicrobial peptides are small, positively charged, amphipathic molecules (which possess both hydrophobic and hydrophilic regions) of variable amino acid composition and length (six to 100 amino acids). Based on their secondary structure, AMPs are grouped into four major classes: β-sheet, α-helical, loop, and extended peptides (Figure 1) [1]. Currently, more than 800 natural AMPs with several different sequences have been isolated from a wide range of organisms (Antimicrobial Peptide Database, http://aps.unmc.edu/AP/main.php). In humans, the most prominent innate AMPs are the cathelicidins and defensins produced primarily by cells of the immune system and the histatins produced and secreted into the saliva by the parotid, mandibular, and submandibular salivary glands [4]–[6].
Fig. 1. Protein models representing the structural differences of the four classes of antimicrobial peptides. 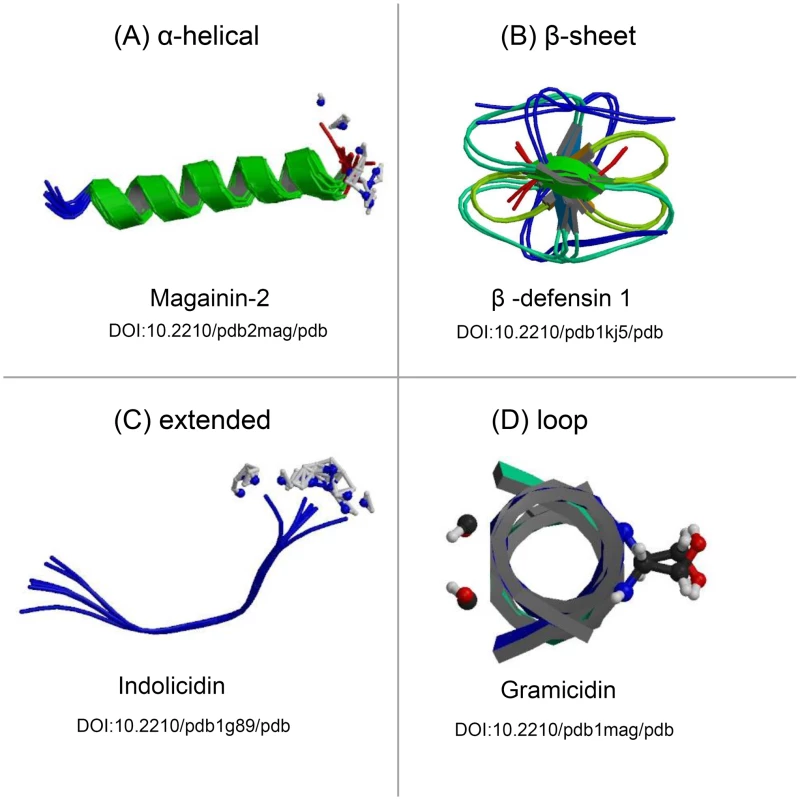
Antimicrobial peptides can be grouped into four major classes based on their secondary structures, including the (A) α-helical peptides, (B) peptides composed of a series of β-sheets, (C) peptides that adopt unconventional structures, such as extended helices, and (D) peptides that assemble into loops. All structures were obtained freely from the RCSB Protein Data Bank (PDB) (http://www.pdb.org/) and have been referenced according to their Digital Object Identifier (DOI) [23]. Additional information for each AMP may be obtained by consulting the RCSB PDB and cross-referencing the DOI. Mechanism of Antimicrobial Activity
Despite their vast diversity, most AMPs work directly against microbes through a mechanism involving membrane disruption and pore formation, allowing efflux of essential ions and nutrients. The molecular mechanism and pathway of membrane permeation may vary for different peptides depending on a number of parameters, such as the amino acid sequence, membrane lipid composition, and peptide concentration [3]. Although the mechanisms by which peptides associate with and permeabilize microbial cell membranes are not entirely clear, AMPs are proposed to bind to the cytoplasmic membrane, creating micelle-like aggregates, leading to a disruptive effect (Figure 2). However, a mounting body of evidence indicates the presence of additional or complementary mechanisms such as intracellular targeting of cytoplasmic components crucial to proper cellular physiology (Figure 2) [7], [8]. Thus, the initial interaction between the peptides and the microbial cell membrane would allow them to penetrate into the cell to bind intracellular molecules, resulting in the inhibition of cell wall biosynthesis and DNA, RNA, and protein synthesis. AMPs also possess anti-viral properties, inhibiting viral fusion and egress, thus preventing infection and viral spread via direct interactions with the membranous viral envelope and host cell surface molecules. These properties, combined with the broad range of activity and the short contact time required to induce killing, have led to the consideration of AMPs as excellent candidates for development as novel therapeutic agents. Therefore, insights into the mechanisms employed by AMPs will facilitate new approaches to discover and develop pharmacologic agents.
Fig. 2. The proposed diverse mechanistic modes of action for antimicrobial peptides in microbial cells. 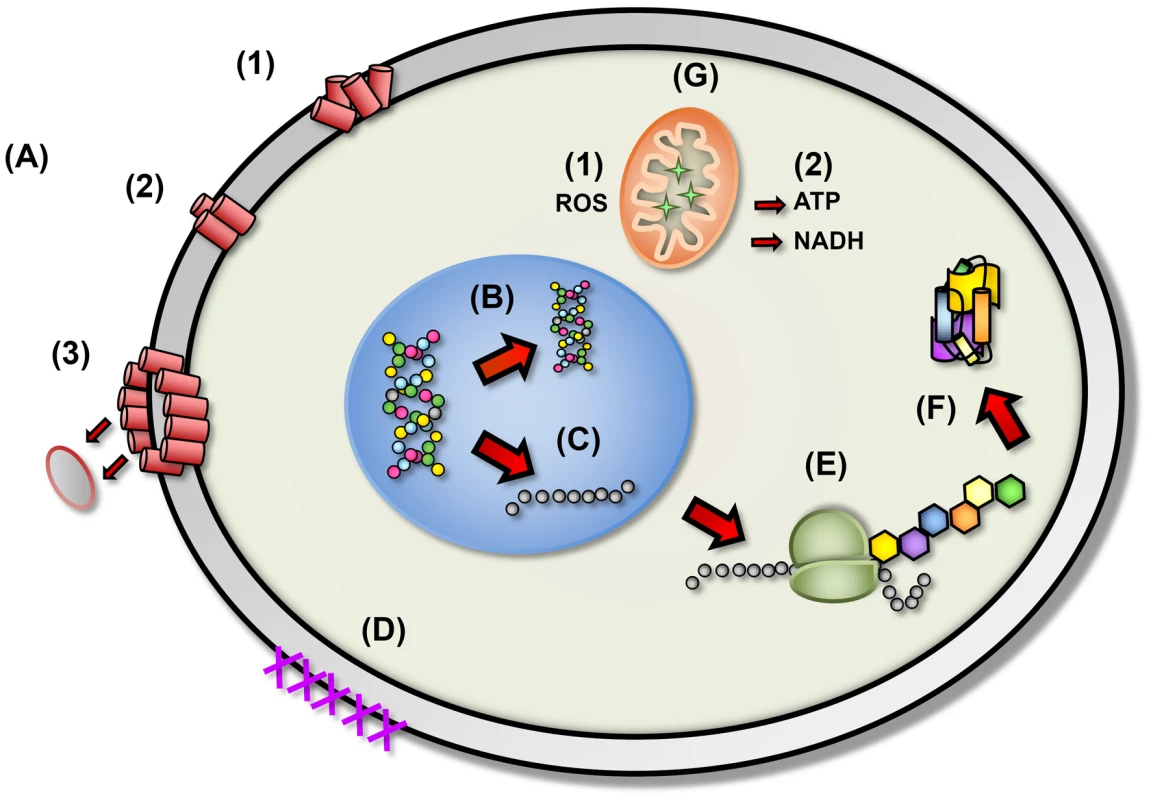
(A) Disruption of cell membrane integrity: (1) random insertion into the membrane, (2) alignment of hydrophobic sequences, and (3) removal of membrane sections and formation of pores. (B) Inhibition of DNA synthesis. (C) Blocking of RNA synthesis. (D) Inhibition of enzymes necessary for linking of cell wall structural proteins. (E) Inhibition of ribosomal function and protein synthesis. (F) Blocking of chaperone proteins necessary for proper folding of proteins. (G) Targeting of mitochondria: (1) inhibition of cellular respiration and induction of ROS formation and (2) disruption of mitochondrial cell membrane integrity and efflux of ATP and NADH. Therapeutic Potential
An essential requirement for any antimicrobial agent is that it has selective toxicity for the microbial target, which is an important feature of AMPs, as their preferential interaction with microbial cells makes them non-toxic to mammalian cells. Based on the significant distinctions between mammalian and microbial cells, several factors determine the selectivity of AMPs, such as membrane composition, transmembrane potential, polarization, and structural features [7]–[9]. AMPs have a number of potential advantages as future therapeutics; in addition to their broad spectrum antimicrobial activity and rapid killing of microbes, they neutralize endotoxin and are unaffected by classical antibiotic resistance mechanisms [7], [9], [10]. Significantly, given their proclivity to permeabilize target microbial membranes, the most promising potential application for AMPs is for enhancement of the potency of existing antimicrobials by facilitating access into the microbial cell, resulting in synergistic therapeutic effects [8], [11]. Moreover, unlike conventional antibiotics, which microbes readily circumvent, AMPs do not appear to induce antibiotic resistance, most likely due to the profound changes in membrane structure warranted to confer the microbial cell with resistance. Interestingly, although the fundamental biological role of AMPs is antimicrobial activity, recent studies have highlighted novel alternative functions for these molecules, including immunomodulatory activities, neutralization of endotoxins, wound healing, and anti-neoplastic properties [12]–[16]. Based on the broad and diverse biological functions of these endogenous peptides, AMPs currently are being widely used as blueprints for the development of innovative therapeutic agents that may be used as antimicrobials, modifiers of inflammation, or in cancer therapy.
Hurdles: Resistance and Immune Evasion
There are several barriers that might impede the development of AMPs as commercial therapeutic agents or restrict their applicability. Among the potential impediments are the high production costs and susceptibility to proteolytic degradation, although AMPs are amenable to extensive chemical modification, which may alleviate some of these obstacles [9]. However, with microbial drug resistance becoming a global public health problem, it has become imperative that new antimicrobials combat the increasing rise in resistance. The development of microbial resistance against AMPs is rare [10]. Nevertheless, microbial pathogens have the capabilities to coordinate countermeasures to circumvent antimicrobial peptide targeting and evade host immune defenses [8], [17], [18].
The newly identified mechanism of immune evasion employed by the pathogenic fungal species Candida albicans, the etiologic agent of oral candidiasis (thrush), serves as a good example of microbial strategies to thwart the deleterious effects of AMPs [5]. The salivary antimicrobial peptide histatin-5 exhibits potent anti-candidal properties and therefore is considered to be important in protecting the oral cavity against candidiasis. However, recently C. albicans was described to be capable of cleaving histatin-5 not only at specific amino acid residues required for successful intracellular uptake of histatin-5, but also at sites located within the antimicrobial fragment of histatin-5, resulting in deactivation of its anti-candidal potency [19]. This proteolytic ability was attributed to a family of proteolytic enzymes produced by C. albicans, the secreted aspartyl proteases, responsible for host tissue degradation and invasion by the fungus [20]. The outcome of this host–pathogen interaction in terms of clinical manifestation was demonstrated to be subject to the ratio of histatin-5 in the saliva to C. albicans cell density in the oral cavity [19]. Although C. albicans is innately sensitive to histatin-5, compensatory up-regulation of proteolytic enzymes would constitute a potential mechanism for the pathogen to evade host innate defenses [19], [20]. Similarly, the yeast Candida glabrata appears to be unaffected by concentrations of AMPs due to increased baseline expression of drug efflux pumps [21].
While acquired resistance to AMPs is less likely to occur as compared to the traditional antimicrobial therapies, some gram-negative bacteria have evolved to utilize various cellular enzymes to biochemically modify and reduce the net negative charge of their cell membranes. By making the net charge more positive, static repulsive forces antagonize insertion of positively charged AMPs into the bacterial membrane [22]. Several other strategies have been described that can result in decreased susceptibility of bacteria to AMPs, such as secretion of inactivating proteins or exportation via efflux pumps. Therefore, we cannot exclude the fact that resistance may evolve if microbial populations are consistently exposed to AMPs. Nevertheless, future research aimed at broadening our understanding of the mechanisms used by both host and microbe will undoubtedly lead to new therapeutic options for managing resistant microbial infections.
Conclusion
Presently, AMPs represent one of the most promising future strategies for combating infections and microbial drug resistance. This is evident by the increasing number of studies to which these peptides are subjected. As our need for new antimicrobials becomes more pressing, the question remains: can we develop novel drugs based on the design principles of primitive molecules?
Zdroje
1. GiulianiA
PirriG
NicolettoS
2007
Antimicrobial peptides: an overview of a promising class of therapeutics.
Cent Eur J Biol
2
1
33
2. ZasloffM
2002
Antimicrobial peptides of multicellular organisms.
Nature
415
389
395
3. ShaiY
2002
From innate immunity to de-novo designed antimicrobial peptides.
Curr Pharm Des
8
715
725
4. BalsR
WilsonJM
2003
Cathelicidins–a family of multifunctional antimicrobial peptides.
Cell Mol Life Sci
60
711
720
5. KavanaghK
DowdS
2004
Histatins: antimicrobial peptides with therapeutic potential.
J Pharm Pharmacol
56
285
289
6. KlotmanME
ChangTL
2006
Defensins in innate antiviral immunity.
Nat Rev Immunol
6
447
456
7. HarrisM
Mora-MontesHM
GowNA
CootePJ
2009
Loss of mannosylphosphate from Candida albicans cell wall proteins results in enhanced resistance to the inhibitory effect of a cationic antimicrobial peptide via reduced peptide binding to the cell surface.
Microbiology
155
1058
1070
8. YeamanMR
YountNY
2003
Mechanisms of antimicrobial peptide action and resistance.
Pharmacol Rev
55
27
55
9. BradshawJ
2003
Cationic antimicrobial peptides : issues for potential clinical use.
BioDrugs
17
233
240
10. PeschelA
SahlHG
2006
The co-evolution of host cationic antimicrobial peptides and microbial resistance.
Nat Rev Microbiol
4
529
536
11. TangYQ
YeamanMR
SelstedME
2002
Antimicrobial peptides from human platelets.
Infect Immun
70
6524
6533
12. BrownKL
HancockRE
2006
Cationic host defense (antimicrobial) peptides.
Curr Opin Immunol
18
24
30
13. DiamondG
BeckloffN
WeinbergA
KisichKO
2009
The roles of antimicrobial peptides in innate host defense.
Curr Pharm Des
15
2377
2392
14. MaderJS
HoskinDW
2006
Cationic antimicrobial peptides as novel cytotoxic agents for cancer treatment.
Expert Opin Investig Drugs
15
933
946
15. MookherjeeN
HancockRE
2007
Cationic host defence peptides: innate immune regulatory peptides as a novel approach for treating infections.
Cell Mol Life Sci
64
922
933
16. SteinstraesserL
KoehlerT
JacobsenF
DaigelerA
GoertzO
2008
Host defense peptides in wound healing.
Mol Med
14
528
537
17. MullardA
2008
Immune evasion: Overcoming defensins.
Nat Rev Micro
6
415
415
18. SperandioB
RegnaultB
GuoJ
ZhangZ
StanleySLJr
2008
Virulent Shigella flexneri subverts the host innate immune response through manipulation of antimicrobial peptide gene expression.
J Exp Med
205
1121
1132
19. MeillerTF
HubeB
SchildL
ShirtliffME
ScheperMA
2009
A novel immune evasion strategy of Candida albicans: proteolytic cleavage of a salivary antimicrobial peptide.
PLoS ONE
4
e5039
doi:10.1371/journal.pone.0005039
20. NaglikJR
ChallacombeSJ
HubeB
2003
Candida albicans secreted aspartyl proteinases in virulence and pathogenesis.
Microbiol Mol Biol Rev
67
400
428
21. HelmerhorstEJ
VenuleoC
SanglardD
OppenheimFG
2006
Roles of cellular respiration, CgCDR1, and CgCDR2 in Candida glabrata resistance to histatin 5.
Antimicrob Agents Chemother
50
1100
1103
22. RoyH
DareK
IbbaM
2009
Adaptation of the bacterial membrane to changing environments using aminoacylated phospholipids.
Mol Microbiol
71
547
550
23. BermanHM
WestbrookJ
FengZ
GillilandG
BhatTN
2000
The Protein Data Bank.
Nucleic Acids Res
28
235
242
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek The Combined Effect of Environmental and Host Factors on the Emergence of Viral RNA RecombinantsČlánek Functional Interchangeability of Late Domains, Late Domain Cofactors and Ubiquitin in Viral BuddingČlánek Parvovirus Minute Virus of Mice Induces a DNA Damage Response That Facilitates Viral ReplicationČlánek Phenolglycolipid-1 Expressed by Engineered BCG Modulates Early Interaction with Human Phagocytes
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 10- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Social Media and Microbiology Education
- Antimicrobial Peptides: Primeval Molecules or Future Drugs?
- Phylodynamics and Human-Mediated Dispersal of a Zoonotic Virus
- Retroviral RNA Dimerization and Packaging: The What, How, When, Where, and Why
- Distinct Clones of Caused the Black Death
- Strain-Specific Differences in the Genetic Control of Two Closely Related Mycobacteria
- The Combined Effect of Environmental and Host Factors on the Emergence of Viral RNA Recombinants
- MHC Class I Bound to an Immunodominant Epitope Demonstrates Unconventional Presentation to T Cell Receptors
- Stimulates Immune Gene Expression and Inhibits Development in
- Crystal Structure of DotD: Insights into the Relationship between Type IVB and Type II/III Secretion Systems
- Cytomegalovirus microRNAs Facilitate Persistent Virus Infection in Salivary Glands
- Strategies to Avoid Killing by Human Neutrophils
- Transforming Growth Factor-β: Activation by Neuraminidase and Role in Highly Pathogenic H5N1 Influenza Pathogenesis
- Autoimmunity in Arabidopsis Is Mediated by Epigenetic Regulation of an Immune Receptor
- Functional Interchangeability of Late Domains, Late Domain Cofactors and Ubiquitin in Viral Budding
- Dengue Virus Ensures Its Fusion in Late Endosomes Using Compartment-Specific Lipids
- Nucleocapsid Promotes Localization of HIV-1 Gag to Uropods That Participate in Virological Synapses between T Cells
- Host Genetics and HIV-1: The Final Phase?
- Variations in the Hemagglutinin of the 2009 H1N1 Pandemic Virus: Potential for Strains with Altered Virulence Phenotype?
- High-Resolution Functional Mapping of the Venezuelan Equine Encephalitis Virus Genome by Insertional Mutagenesis and Massively Parallel Sequencing
- Viral Replication Rate Regulates Clinical Outcome and CD8 T Cell Responses during Highly Pathogenic H5N1 Influenza Virus Infection in Mice
- Calcineurin Inhibition at the Clinical Phase of Prion Disease Reduces Neurodegeneration, Improves Behavioral Alterations and Increases Animal Survival
- Parvovirus Minute Virus of Mice Induces a DNA Damage Response That Facilitates Viral Replication
- -Induced Inactivation of the Macrophage Transcription Factor AP-1 Is Mediated by the Parasite Metalloprotease GP63
- Epstein Barr Virus-Encoded EBNA1 Interference with MHC Class I Antigen Presentation Reveals a Close Correlation between mRNA Translation Initiation and Antigen Presentation
- Fidelity Variants of RNA Dependent RNA Polymerases Uncover an Indirect, Mutagenic Activity of Amiloride Compounds
- The Spread of Tomato Yellow Leaf Curl Virus from the Middle East to the World
- Concerted Action of Two Formins in Gliding Motility and Host Cell Invasion by
- Requirements for Receptor Engagement during Infection by Adenovirus Complexed with Blood Coagulation Factor X
- Release of Intracellular Calcium Stores Facilitates Coxsackievirus Entry into Polarized Endothelial Cells
- Gene Annotation and Drug Target Discovery in with a Tagged Transposon Mutant Collection
- Nuclear Export and Import of Human Hepatitis B Virus Capsid Protein and Particles
- Retention and Loss of RNA Interference Pathways in Trypanosomatid Protozoans
- Identification and Genome-Wide Prediction of DNA Binding Specificities for the ApiAP2 Family of Regulators from the Malaria Parasite
- Controlling Cellular P-TEFb Activity by the HIV-1 Transcriptional Transactivator Tat
- Direct Visualization of Peptide/MHC Complexes at the Surface and in the Intracellular Compartments of Cells Infected by
- Phenolglycolipid-1 Expressed by Engineered BCG Modulates Early Interaction with Human Phagocytes
- In Vitro and In Vivo Studies Identify Important Features of Dengue Virus pr-E Protein Interactions
- Inhibition of Nipah Virus Infection In Vivo: Targeting an Early Stage of Paramyxovirus Fusion Activation during Viral Entry
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Retroviral RNA Dimerization and Packaging: The What, How, When, Where, and Why
- Viral Replication Rate Regulates Clinical Outcome and CD8 T Cell Responses during Highly Pathogenic H5N1 Influenza Virus Infection in Mice
- Antimicrobial Peptides: Primeval Molecules or Future Drugs?
- Crystal Structure of DotD: Insights into the Relationship between Type IVB and Type II/III Secretion Systems
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



