-
Články
Reklama
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
ReklamaRetroviral RNA Dimerization and Packaging: The What, How, When, Where, and Why
article has not abstract
Published in the journal: . PLoS Pathog 6(10): e32767. doi:10.1371/journal.ppat.1001007
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1001007Summary
article has not abstract
The Encapsidated Viral Genome: What Is Packaged?
Retroviral genomic RNAs (gRNAs) are packaged as dimers, joined near their 5′ ends in non-covalent linkages that withstand modest heat treatment but dissociate at ∼65°C. Determinants of gRNA dimerization and recruitment for packaging map to the same ∼100 to ∼300 base regions and are, for the most part, physically and genetically inseparable [1]. Synthetic RNAs containing these sequences dimerize in vitro. Because transplanting these sequences onto a cell mRNA confers selective packaging, and ablating them greatly reduces gRNA packaging, these sequences are known as Ψ (psi), for “packaging signal” (Figure 1A) [2].
Fig. 1. Retroviral RNA dimerization and packaging. 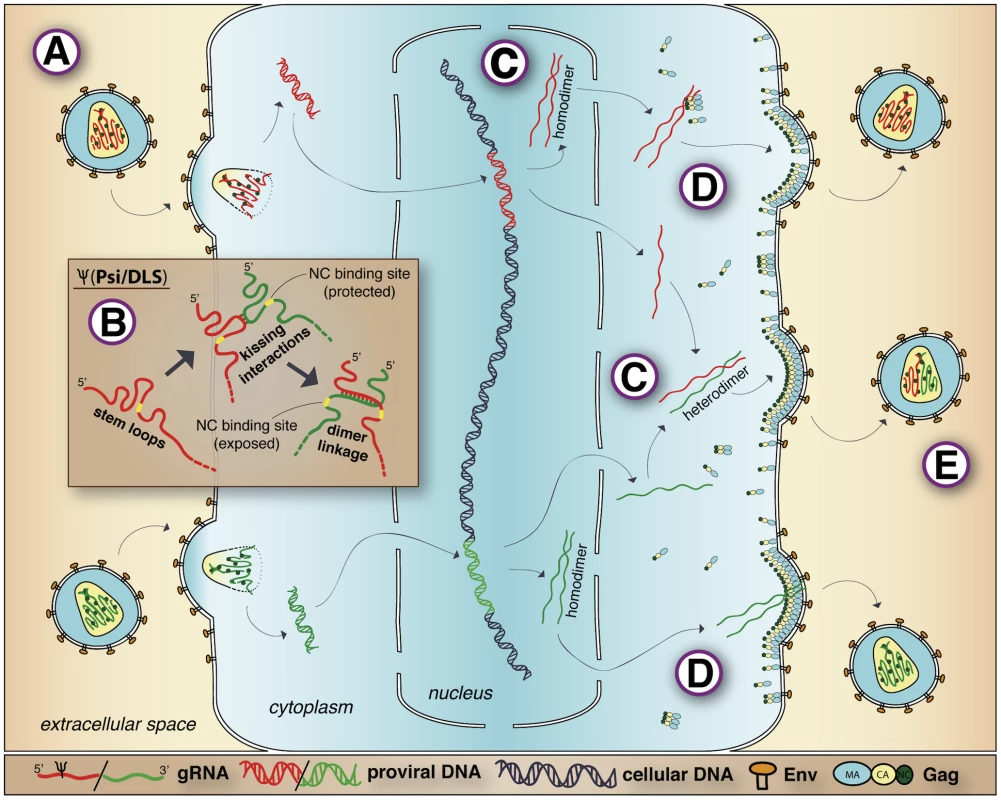
The description of retrovirus genomic RNA dimerization and packaging is based on a representative co-infected cell and depicts properties of C-type retroviruses such as HIV-1 and MLV. Note that this figure represents concepts schematically and is not intended to accurately represent structures or scale. (A) What is packaged: two genetically complete but nicked copies of plus sense gRNA (shown in red at top or green at bottom) are packaged within the capsid core and joined by a dimer linkage. The co-packaged gRNAs are condensed in the core and bound by NC (shown as green circles). (B) How gRNAs are recruited: initial gRNA dimerization occurs via kissing interactions between palindromic stem loops. Subsequent basepairing register-shifts that occur during dimer linkage maturation expose single-stranded NC binding motifs (indicated in yellow) that were previously basepaired and thus sequestered in gRNA monomers, allowing for Gag binding during recruitment. (C) When gRNAs associate in dimers: the point at which RNA dimerization partners first associate is different for HIV-1 and MLV. MLV gRNA dimers first associate near sites of transcription in the nucleus, which leads to disproportionately large amounts of one homodimer or the other (shown in red at top and green at bottom). HIV-1 gRNA dimers first associate in the cytoplasm, leading to a random assortment of homodimeric and heterodimeric gRNAs. (D) Where gRNAs join assembling virions: gRNAs may form subassemblies with Gag in the cytoplasm (shown at top) or may associate at the plasma membrane (shown at bottom) after active transport separate from all or most Gags. (E) Why two gRNAs are packaged: the packaging of two gRNAs may aid in packaging specificity as well as the promotion of genomic integrity. In addition, the packaging of two genetically distinct gRNAs (shown as a red/green heterodimer) promotes genetic recombination, which leads to genetic diversity in viral progeny. Within virions, gRNAs are coated with a basic viral protein called nucleocapsid (NC) at a density of about one NC per five to eight RNA bases [3]. This nucleoprotein complex resides within the mature virion core. Total gRNA length, were it in an A-helix, exceeds the core inner diameter by more than 30-fold [4]. Thus, encapsidated gRNA is highly condensed. The co-packaged gRNAs likely are not aligned along their lengths because they are identical and cannot basepair in register, but the nature of their compaction is unknown.
If Ψ is experimentally removed from gRNA but viral proteins are still expressed, morphologically normal virions can form, which are devoid of gRNA. These contain random samples of host mRNA [5]. Each Ψ+ or Ψ − virion also contains several copies of certain host RNAs such as 7SL, the RNA scaffold of signal recognition particles. Other than the primer tRNA, any roles of these host non-coding RNAs in retroviruses are unknown.
Usually, a virion's two gRNAs are identical. However, if a producer cell contains two distinct dimer-compatible proviruses, virions can contain gRNA heterdimers. Both gRNAs are genetically complete but not intact, as they appear to contain nicks and run as smears on denaturing gels. Accordingly, it has been speculated that retroviruses' dimeric genome organization may serve in part as defense against antiviral nucleases that would otherwise restrict replication [4]. RNA degradation during reverse transcription further limits provirus synthesis to one or fewer per virion [6]. Transmission of no more than one allele at each locus explains why, although they package two gRNAs, retroviruses are not truly diploid.
How Are gRNA Dimers Recruited?
gRNA packaging specificity results from high-affinity interactions between NC and the dimer linkage structure (DLS) that forms when two gRNAs join [1] (Figure 1B). The NC: Ψ/DLS interaction differs from RNA coating by NC, both in its specificity and because the interaction occurs while NC is a domain of the Gag polyprotein. Changes to the NC coding region lead to deficiencies in gRNA packaging, and NC dictates packaging in chimeras where one retrovirus's NC is exchanged for another's [7].
Secondary structure and mutant analyses show that the Ψ/DLS region contains a series of RNA stem-loops. Single-stranded loops on some of these hairpins contain palindromes, allowing one gRNA to basepair via “kissing” interactions with a second gRNA (Figure 1B). This initial dimer contact is followed by structural transitions that extend intermolecular basepairing to form the encapsidated dimer linkage [1].
Despite 25 years of research and impressive advances in understanding secondary structure, we still lack a three-dimensional understanding of any virus's Ψ/DLS and what, minimally, is necessary for packaging. Conflicting reports may reflect complications in teasing apart elements in a multifunctional genome region. The propensity of retroviruses to package suboptimal RNAs in the absence of gRNAs, differences among retroviruses, and the absence of uniform naming conventions also cloud the picture.
The structural basis of why gRNAs are packaged as dimers is best understood for murine leukemia virus (MLV) [1]. MLV NC binds unpaired UCUG motifs with high affinity. These are overrepresented in MLV's 5′ untranslated region, but are basepaired and inaccessible to NC in monomeric gRNA. However, once dimerized, secondary structure register shifts expose the high affinity binding sites. Thus, discrimination between monomeric and dimeric MLV gRNAs appears based on UCUG availability in one of two alternate gRNA folds.
Ψ's presence is not always sufficient to specify packaging. The packaging machinery must discriminate between complete gRNAs and subgenomic mRNAs. For HIV-1 and gammaretroviruses, this discrimination reflects Ψ's location downstream of the 5′ splice site and its removal from subgenomic mRNAs. However, for retroviruses like HIV-2, major packaging elements are present on both spliced and unspliced RNAs. Avian leukosis virus (ALV) spliced RNAs also retain Ψ. Interestingly, a heterologous RNA containing ALV Ψ, but not the natural ALV Ψ+ env mRNA, is packaged well [8], which implies env mRNAs contain negative packaging elements.
When Do the Two Co-Packaged gRNAs First Associate?
gRNAs are capped and polyadenylated unspliced RNA polymerase II transcripts, identical in sequence to gag mRNAs. All retroviruses that can mobilize virus gene-free retroviral vectors are by definition capable of at least some trans-packaging. However, whether an individual RNA can serve as both gag mRNA and gRNA varies among retroviruses.
All retroviruses must transport their unspliced RNAs to the cytoplasm, but retroviruses differ both in the RNA transport pathway used and in the replication step where mRNA and gRNA fates bifurcate [9]. Specifically, studies comparing gRNA and mRNA half-lives concluded both reside in a single, equilibrating pool for HIV-1, from which unspliced RNAs can be directed alternately into gRNA or gag mRNA fates well after transcription is completed [10]. In contrast, MLV gRNAs have shorter half-lives than mRNAs, and unspliced RNAs destined to serve gRNA roles appear to adopt this fate before exiting the nucleus [10], [11]. This suggests unspliced RNA fates are decided earlier for MLV than for HIV-1.
The timing of dimerization partner association is a slightly different question. By such criteria as their gel mobility, the gRNAs in immature virions can appear monomeric, and some have suggested monomeric gRNAs are the recruited species, with dimerization occurring later [12]. However, although means of recruiting gRNAs in a two-or-none fashion other than as immature dimers cannot be ruled out unambiguously, the preponderance of experimental evidence argues that initial gRNA dimer interactions occur prior to recruitment into assembling virions, with immature dimers matured upon protein processing. These observations include biases in gRNA co-packaging, the very poor packaging of gRNAs that are unable to dimerize, and the fact that even when gRNA is limiting and virions contain less than one gRNA on average, gRNAs are dimeric in those virions that contain them [13], [14].
The replication stage when dimerization partners first associate differs among retroviruses (Figure 1C). MLV gRNAs select dimerization partners at or near sites of transcription and proceed to assembly sites without releasing the partner they selected in the nucleus [15]. In contrast, HIV-1 gRNAs first associate for dimerization in the cytoplasm [16]. This trafficking difference has profound effects on virus genetics [4]. Retrovirus genomes are not segmented, but alleles re-assort at an exceptional rate due to high-frequency recombination, which results from template switching between dimerized gRNAs during reverse transcription. The timing of gRNA dimerization ensures that co-expressed HIV-1 gRNAs associate at random. In contrast, early self-associations of MLV gRNAs result in disproportionate co-packaging of identical RNAs, and template switching between identical gRNAs does not yield recombinants. As a result, genetic marker reassortment is about 10-fold lower for MLV than for HIV-1, even though these viruses' recombinogenic template switching rates are the same [4].
Where Does the Packaging of gRNAs into Virions Initiate?
On the gross morphologic level, orthoretroviruses adopt one of two assembly pathways. Beta - and deltaretroviruses form electron-dense particles in the cytoplasm, while assembly for “C-type” viruses like HIV-1, MLV, and ALV is first detectable on membranes. Whether Gag and gRNA first come together at the plasma membrane or associate earlier is incompletely resolved, but recent findings suggest that at least for HIV-1, initial interactions occur at the plasma membrane [17] (Figure 1D).
gRNA's intracellular routes to assembly sites differ among viruses and are not well understood. gRNA transport likely exploits host directional trafficking machinery [18]. For Rous sarcoma virus, a subset of viral Gag transits back into the nucleus to recruit gRNA en route to the plasma membrane [19].
Dimer linkage structures are recruited via high affinity interactions with perhaps a dozen NC domains [14], [17]. This is less than 1% of the total Gag precursors that form a virion. What prevents the other Gags from engaging additional dimer linkages? Because virions form readily without any gRNA, and also when provided with gRNA three times the normal length, a phage-like RNA “headful” mechanism seems unlikely [20]. Recent work with co-expressed gRNAs, each tethered to a different fluorescent protein, confirmed that HIV-1 essentially always packages precisely one gRNA dimer [21]. Is some sort of RNA quorum sensing triggered once one DLS is engaged? And if so, what substitutes in Ψ − particles? Interestingly, certain RNA binding-defective NC mutants phenocopy properties associated with budding defects, suggesting interactions with gRNA may help drive a late assembly step [22], [23]. However, no detectable change is observed in the time required for assembly, whether or not particles contain gRNA [17].
Why Do Retroviruses Package Dimeric gRNAs?
The fact that all other viruses encapsidate single copy genomes begs the question of why retroviruses co-package two gRNAs. One reason may be economy of scale: RNA dimerization allows formation of a unique structure—the dimer linkage—that distinguishes gRNAs from mRNAs. Monomeric HIV-1 RNAs are packaged when engineered with tandem dimer linkages, underscoring the importance of this RNA structure, and not gRNA counting per se, to packaging [24].
Co-packaging gRNAs allows retroviruses to generate intact proviruses despite pervasive gRNA nicking [4] (Figure 1E). Template switching during reverse transcription is probably why retroviruses maintain infectivity when their gRNAs are damaged by gamma rays, and why retroviruses are much less radiation-sensitive than RNA viruses like vescicular stomatitis virus [25]. Researchers previously thought retroviral recombination might be mutagenic, but these notions have been dispelled [4]. HIV-1 particles with two gRNAs generate full-length proviruses more efficiently than virions engineered to contain single gRNAs [26]. Thus, another advantage of gRNA dimers appears to be increased replication fidelity.
Co-packaging gRNAs promotes higher recombination frequencies for retroviruses than all other viruses, allowing rapid loss of deleterious alleles and re-assortment of genome segments. With approximately three to ten crossovers occurring during the synthesis of every provirus, recombination is perhaps 10-fold more frequent than reverse transcriptase base substitution rates, and is an evolutionary driving force for retroviruses such as HIV-1 that display high levels of replication and multi-strain infection [4].
These observations help seal the case for likely evolutionary advantages of dimeric genome packaging. The DLS in its immature form, which results only upon association of two gRNAs, likely provides the means for selective gRNA packaging. The need to generate an intact provirus provides a strong motive for packaging redundant genetic information. And because retroviruses encapsidate gRNA dimers, recombination can provide the opportunity for almost limitless combinatorial genetic sampling.
Zdroje
1. D'SouzaV
SummersMF
2005
How retroviruses select their genomes.
Nat Rev Microbiol
3
643
655
2. MannRS
MulliganRC
BaltimoreD
1983
Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus.
Cell
33
153
159
3. LevinJG
GuoJ
RouzinaI
Musier-ForsythK
2005
Nucleic acid chaperone activity of HIV-1 nucleocapsid protein: critical role in reverse transcription and molecular mechanism.
Prog Nucleic Acid Res Mol Biol
80
217
286
4. Onafuwa-NugaA
TelesnitskyA
2009
The remarkable frequency of human immunodeficiency virus type 1 genetic recombination.
Microbiol Mol Biol Rev
73
451
480
Table of Contents
5. RulliSJJr
HibbertCS
MirroJ
PedersonT
BiswalS
2007
Selective and nonselective packaging of cellular RNAs in retrovirus particles.
J Virol
81
6623
6631
6. ZhuangJ
MukherjeeS
RonY
DoughertyJP
2006
High rate of genetic recombination in murine leukemia virus: implications for influencing proviral ploidy.
J Virol
80
6706
6711
7. BerkowitzR
FisherJ
GoffSP
1996
RNA packaging.
Curr Top Microbiol Immunol
214
177
218
8. BanksJD
KealohaBO
LinialML
1999
An Mpsi-containing heterologous RNA, but not env mRNA, is efficiently packaged into avian retroviral particles.
J Virol
73
8926
8933
9. ButschM
Boris-LawrieK
2002
Destiny of unspliced retroviral RNA: Ribosome and/or virion?
Journal of Virology
76
3089
3094
10. DormanN
LeverA
2000
Comparison of viral genomic RNA sorting mechanisms in human immunodeficiency virus type 1 (HIV-1), HIV-2, and Moloney murine leukemia virus.
J Virol
74
11413
11417
11. LevinJG
RosenakMJ
1976
Synthesis of murine leukemia virus proteins associated with virions assembled in actinomycin D-treated cells: evidence for persistence of viral messenger RNA.
Proc Natl Acad Sci U S A
73
1154
1158
12. SongR
KafaieJ
YangL
LaughreaM
2007
HIV-1 viral RNA is selected in the form of monomers that dimerize in a three-step protease-dependent process; the DIS of stem-loop 1 initiates viral RNA dimerization.
J Mol Biol
371
1084
1098
13. HibbertCS
MirroJ
ReinA
2004
mRNA molecules containing murine leukemia virus packaging signals are encapsidated as dimers.
J Virol
78
10927
10938
14. MiyazakiY
GarciaEL
KingSR
IyallaK
LoeligerK
2010
An RNA structural switch regulates diploid genome packaging by Moloney murine leukemia virus.
J Mol Biol
396
141
152
15. KharytonchykSA
KireyevaAI
OsipovichAB
FominIK
2005
Evidence for preferential copackaging of Moloney murine leukemia virus genomic RNAs transcribed in the same chromosomal site.
Retrovirology
2
3
16. MooreMD
NikolaitchikOA
ChenJ
HammarskjoldML
RekoshD
2009
Probing the HIV-1 genomic RNA trafficking pathway and dimerization by genetic recombination and single virion analyses.
PLoS Pathog
5
e1000627
doi:10.1371/journal.ppat.1000627
17. JouvenetN
SimonSM
BieniaszPD
2009
Imaging the interaction of HIV-1 genomes and Gag during assembly of individual viral particles.
Proc Natl Acad Sci U S A
106
19114
19119
18. SwansonCM
MalimMH
2006
Retrovirus RNA trafficking: from chromatin to invasive genomes.
Traffic
7
1440
1450
19. Garbitt-HirstR
KenneySP
ParentLJ
2009
Genetic evidence for a connection between Rous sarcoma virus gag nuclear trafficking and genomic RNA packaging.
J Virol
83
6790
6797
20. ShinNH
Hartigan-O'ConnorD
PfeifferJK
TelesnitskyA
2000
Replication of lengthened Moloney murine leukemia virus genomes is impaired at multiple stages.
J Virol
74
2694
2702
21. ChenJ
NikolaitchikO
SinghJ
WrightA
BencsicsCE
2009
High efficiency of HIV-1 genomic RNA packaging and heterozygote formation revealed by single virion analysis.
Proc Natl Acad Sci U S A
106
13535
13540
22. LeeEG
LinialML
2006
Deletion of a Cys-His motif from the Alpharetrovirus nucleocapsid domain reveals late domain mutant-like budding defects.
Virology
347
226
233
23. ThomasJA
BoscheWJ
ShatzerTL
JohnsonDG
GorelickRJ
2008
Mutations in human immunodeficiency virus type 1 nucleocapsid protein zinc fingers cause premature reverse transcription.
J Virol
82
9318
9328
24. SakuragiJ-I
ShiodaT
PanganibanA
2001
Duplication of the primary encapsidation and dimer linkage region of human immunodeficiency virus type ! RNA results in the appearance of monomeric RNA in virions.
Journal of Virology
75
2557
2565
25. ToyoshimaK
NiwaO
YutsudoM
SugiyamaH
TaharaS
1980
Sensitivity to gamma-rays of avian-sarcoma and murine leukemia viruses.
Virology
105
508
515
26. KingSR
DuggalNK
NdongmoCB
PacutC
TelesnitskyA
2008
Pseudodiploid genome organization aids full-length human immunodeficiency virus type 1 DNA synthesis.
J Virol
82
2376
2384
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek The Combined Effect of Environmental and Host Factors on the Emergence of Viral RNA RecombinantsČlánek Functional Interchangeability of Late Domains, Late Domain Cofactors and Ubiquitin in Viral BuddingČlánek Parvovirus Minute Virus of Mice Induces a DNA Damage Response That Facilitates Viral ReplicationČlánek Phenolglycolipid-1 Expressed by Engineered BCG Modulates Early Interaction with Human Phagocytes
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 10- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Social Media and Microbiology Education
- Antimicrobial Peptides: Primeval Molecules or Future Drugs?
- Phylodynamics and Human-Mediated Dispersal of a Zoonotic Virus
- Retroviral RNA Dimerization and Packaging: The What, How, When, Where, and Why
- Distinct Clones of Caused the Black Death
- Strain-Specific Differences in the Genetic Control of Two Closely Related Mycobacteria
- The Combined Effect of Environmental and Host Factors on the Emergence of Viral RNA Recombinants
- MHC Class I Bound to an Immunodominant Epitope Demonstrates Unconventional Presentation to T Cell Receptors
- Stimulates Immune Gene Expression and Inhibits Development in
- Crystal Structure of DotD: Insights into the Relationship between Type IVB and Type II/III Secretion Systems
- Cytomegalovirus microRNAs Facilitate Persistent Virus Infection in Salivary Glands
- Strategies to Avoid Killing by Human Neutrophils
- Transforming Growth Factor-β: Activation by Neuraminidase and Role in Highly Pathogenic H5N1 Influenza Pathogenesis
- Autoimmunity in Arabidopsis Is Mediated by Epigenetic Regulation of an Immune Receptor
- Functional Interchangeability of Late Domains, Late Domain Cofactors and Ubiquitin in Viral Budding
- Dengue Virus Ensures Its Fusion in Late Endosomes Using Compartment-Specific Lipids
- Nucleocapsid Promotes Localization of HIV-1 Gag to Uropods That Participate in Virological Synapses between T Cells
- Host Genetics and HIV-1: The Final Phase?
- Variations in the Hemagglutinin of the 2009 H1N1 Pandemic Virus: Potential for Strains with Altered Virulence Phenotype?
- High-Resolution Functional Mapping of the Venezuelan Equine Encephalitis Virus Genome by Insertional Mutagenesis and Massively Parallel Sequencing
- Viral Replication Rate Regulates Clinical Outcome and CD8 T Cell Responses during Highly Pathogenic H5N1 Influenza Virus Infection in Mice
- Calcineurin Inhibition at the Clinical Phase of Prion Disease Reduces Neurodegeneration, Improves Behavioral Alterations and Increases Animal Survival
- Parvovirus Minute Virus of Mice Induces a DNA Damage Response That Facilitates Viral Replication
- -Induced Inactivation of the Macrophage Transcription Factor AP-1 Is Mediated by the Parasite Metalloprotease GP63
- Epstein Barr Virus-Encoded EBNA1 Interference with MHC Class I Antigen Presentation Reveals a Close Correlation between mRNA Translation Initiation and Antigen Presentation
- Fidelity Variants of RNA Dependent RNA Polymerases Uncover an Indirect, Mutagenic Activity of Amiloride Compounds
- The Spread of Tomato Yellow Leaf Curl Virus from the Middle East to the World
- Concerted Action of Two Formins in Gliding Motility and Host Cell Invasion by
- Requirements for Receptor Engagement during Infection by Adenovirus Complexed with Blood Coagulation Factor X
- Release of Intracellular Calcium Stores Facilitates Coxsackievirus Entry into Polarized Endothelial Cells
- Gene Annotation and Drug Target Discovery in with a Tagged Transposon Mutant Collection
- Nuclear Export and Import of Human Hepatitis B Virus Capsid Protein and Particles
- Retention and Loss of RNA Interference Pathways in Trypanosomatid Protozoans
- Identification and Genome-Wide Prediction of DNA Binding Specificities for the ApiAP2 Family of Regulators from the Malaria Parasite
- Controlling Cellular P-TEFb Activity by the HIV-1 Transcriptional Transactivator Tat
- Direct Visualization of Peptide/MHC Complexes at the Surface and in the Intracellular Compartments of Cells Infected by
- Phenolglycolipid-1 Expressed by Engineered BCG Modulates Early Interaction with Human Phagocytes
- In Vitro and In Vivo Studies Identify Important Features of Dengue Virus pr-E Protein Interactions
- Inhibition of Nipah Virus Infection In Vivo: Targeting an Early Stage of Paramyxovirus Fusion Activation during Viral Entry
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Retroviral RNA Dimerization and Packaging: The What, How, When, Where, and Why
- Viral Replication Rate Regulates Clinical Outcome and CD8 T Cell Responses during Highly Pathogenic H5N1 Influenza Virus Infection in Mice
- Antimicrobial Peptides: Primeval Molecules or Future Drugs?
- Crystal Structure of DotD: Insights into the Relationship between Type IVB and Type II/III Secretion Systems
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



