-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
Reklama“Everything You Always Wanted to Know about Sex (but Were Afraid to Ask)” in after Two Decades of Laboratory and Field Analyses
Leishmaniases remain a major public health problem today (350 million people at risk, 12 million infected, and 2 million new infections per year). Despite the considerable progress in cellular and molecular biology and in evolutionary genetics since 1990, the debate on the population structure and reproductive mode of Leishmania is far from being settled and therefore deserves further investigation. Two major hypotheses coexist: clonality versus sexuality. However, because of the lack of clear evidence (experimental or biological confirmation) of sexuality in Leishmania parasites, until today it has been suggested and even accepted that Leishmania species were mainly clonal with infrequent genetic recombination (see [1] for review). Two recent publications, one on Leishmania major (an in vitro experimental study) and one on Leishmania braziliensis (a population genetics analysis), once again have challenged the hypothesis of clonal reproduction. Indeed, the first study experimentally evidenced genetic recombination and proposed that Leishmania parasites are capable of having a sexual cycle consistent with meiotic processes inside the insect vector. The second investigation, based on population genetics studies, showed strong homozygosities, an observation that is incompatible with a predominantly clonal mode of reproduction at an ecological time scale (∼20–500 generations). These studies highlight the need to advance the knowledge of Leishmania biology. In this paper, we first review the reasons stimulating the continued debate and then detail the next essential steps to be taken to clarify the Leishmania reproduction model. Finally, we widen the discussion to other Trypanosomatidae and show that the progress in Leishmania biology can improve our knowledge of the evolutionary genetics of American and African trypanosomes.
Published in the journal: . PLoS Pathog 6(8): e32767. doi:10.1371/journal.ppat.1001004
Category: Review
doi: https://doi.org/10.1371/journal.ppat.1001004Summary
Leishmaniases remain a major public health problem today (350 million people at risk, 12 million infected, and 2 million new infections per year). Despite the considerable progress in cellular and molecular biology and in evolutionary genetics since 1990, the debate on the population structure and reproductive mode of Leishmania is far from being settled and therefore deserves further investigation. Two major hypotheses coexist: clonality versus sexuality. However, because of the lack of clear evidence (experimental or biological confirmation) of sexuality in Leishmania parasites, until today it has been suggested and even accepted that Leishmania species were mainly clonal with infrequent genetic recombination (see [1] for review). Two recent publications, one on Leishmania major (an in vitro experimental study) and one on Leishmania braziliensis (a population genetics analysis), once again have challenged the hypothesis of clonal reproduction. Indeed, the first study experimentally evidenced genetic recombination and proposed that Leishmania parasites are capable of having a sexual cycle consistent with meiotic processes inside the insect vector. The second investigation, based on population genetics studies, showed strong homozygosities, an observation that is incompatible with a predominantly clonal mode of reproduction at an ecological time scale (∼20–500 generations). These studies highlight the need to advance the knowledge of Leishmania biology. In this paper, we first review the reasons stimulating the continued debate and then detail the next essential steps to be taken to clarify the Leishmania reproduction model. Finally, we widen the discussion to other Trypanosomatidae and show that the progress in Leishmania biology can improve our knowledge of the evolutionary genetics of American and African trypanosomes.
Introduction
A major challenge with parasitic pathogens is determining disease epidemiologies and transmission cycles. In addition to the different biotic and abiotic factors involved in the infectious pattern, the pathogen's reproductive system is one of the basic biological processes that condition the microorganism's ecology and disease spread. It has been largely demonstrated that improved knowledge on the population structure and reproductive strategy of pathogens provides key information to gaining a better understanding of transmission patterns and indispensable information for diagnostic and epidemiological inquiries as well as drug and vaccine development [2]–[5]. Nevertheless, it is often difficult to obtain such information on these microorganisms because the experimental investigations are often difficult to conduct and sometimes even almost impossible to develop. For a majority of pathogens, including the Trypanosomatidae family, the reproductive strategy was mainly deduced from population genetics analysis. The main goal of this review is to detail the progress made in the knowledge of the Leishmania reproduction model over the past 20 years.
Protozoan parasites of the Leishmania genus are responsible for human leishmaniases, a serious public health problem. Indeed, leishmaniases are worldwide vector-borne diseases in humans and domestic animals with approximately 350 million persons at risk and 2,357,000 new cases per year [6]. These parasitoses occur on all continents except Antarctica. Leishmania parasites have a complex life cycle (Figure 1). They are present in extremely diverse ecosystems and are able to infect a wide variety of mammals. In humans, the majority of Leishmania infections lead to asymptomatic cases. However, when the disease is declared, it is expressed in a variety of more or less serious clinical forms: cutaneous, mucocutaneous, and visceral. For all these reasons, Leishmania provides a complex biological model from the ecological, genetics, and phylogenetic points of view [1]. Despite numerous studies since the 1990s and recent advances in the molecular genetics of these organisms, the Leishmania parasite's mode of reproduction is still under debate. Two major hypotheses are postulated: clonality versus sexuality. At this stage, it seems useful to review and clarify the arguments that fuel this debate and discuss the latest hypotheses. Finally, we will detail the next essential steps needed to advance our understanding of the reproduction mode of Leishmania and the usefulness of these approaches for other kinetoplastid parasites such as American and African trypanosomes.
Fig. 1. Schematic life cycle of Leishmania parasites. 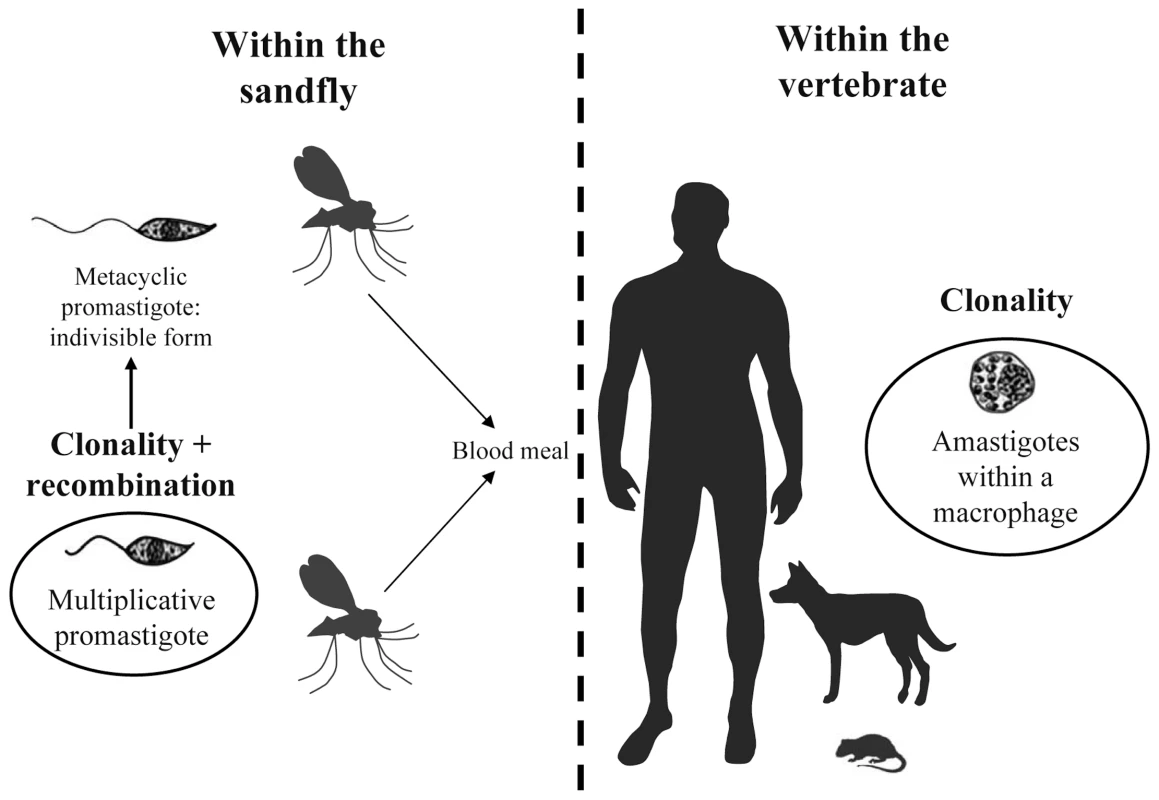
The life cycle starts when a parasitized female sandfly takes a blood meal from a vertebrate host (e.g., a human). As the sandfly feeds, infectious promastigote (metacyclic) forms of the parasite enter the vertebrate host. Within the vertebrate host, these forms are phagocytosed by macrophages where they differentiate into amastigote forms. The life cycle is completed when, during a blood meal, a female sandfly ingests infected macrophages. The parasites transform into multiplicative promastigotes inside the sandfly, and after migration into the sandfly's proboscis, promastigotes transform into metacyclic promastigotes (infectious form) and must be delivered to a new host for the life cycle to continue. The possible locations of clonality in the two hosts and of sexual events (recombination between two individuals) in the vector are indicated (figure adapted from http://www.dpd.cdc.gov/dpdx). Mode of Reproduction in the Leishmania Genus: The Debate
In the Leishmania genus, asexual reproduction was proposed long ago as the main mechanism of reproduction. Since 1990, Tibayrenc et al. have proposed the clonal theory for all or most Leishmania species [5], [7]–[9]. This theory was principally based on the concept that offspring are genetically identical to their parents. Consequently, several criteria have been proposed to test this theory (e.g., fixed heterozygosity, absence of recombinant genotypes, deviation from Hardy-Weinberg expectations, widespread identical genotypes, linkage disequilibrium, and a correlation between two independent sets of genetic markers (see glossary in Box 1)) [9]. Nevertheless, based on several observations of the interspecific hybrid profiles [10]–[13], the occurrence of infrequent recombination events was not excluded for Leishmania. At the same time, this clonal hypothesis has been challenged by other authors. Indeed, based on molecular karyotype analysis using the pulse field gel electrophoresis (PFGE) technique, Bastien, Blaineau, and Pagès argued for a predominance of automixis (corresponding in this case to the definition of autogamy; see Box 1) [14], [15]. In this study, for each chromosome analyzed, they observed a variation in the size of subtelomeric regions (which corresponds to different chromosome forms). Moreover, the results showed that, in each individual strain, only one chromosome form was observed and that, at the population level, various recombinations were observed across these different chromosome forms. To explain these results, authors proposed the existence of a predominantly autogamic sexual reproductive mode. Similarly, the existence of genetic recombination was brought back to the debate in 2007 by Kuhls et al. [16], who proposed the possible existence of selfing (producing an excess of homozygosity; see Box 1) on the basis of multilocus microsatellite typing performed on allopatric (see Box 1) Leishmania samples (91 strains collected in 18 different countries). However, because of the lack of clear evidence (experimental or biological confirmation) of sexuality in Leishmania, it was largely accepted that Leishmania species were mainly clonal with infrequent genetic events (see [1] for review).
Box 1. Glossary
-
Allopatric (antonym: sympatric): Species or populations occupying separate geographic areas.
-
Aneuploidy: The occurrence of one or more extra or missing chromosomes in the same genome.
-
Autogamy: A sexual reproductive mode where zygotes are produced by the fusion of two gametes that were produced by the same parental individual. This results in an increased homozygosity over time.
-
Automixis: A parthenogenetic reproductive mode in which the (unfertilized) eggs are diploid, although meiosis occurs during oocyte formation. The diploidy of the egg is either restored a posteriori or is due to a genome duplication of the female gamete mother cell just before meiosis (see [22] for a more detailed explanation).
-
Clonality: Reproduction with no sex. The descent is identical to the parental individual. Theoretically, excess heterozygotes and linkage disequilibrium are expected.
-
Endogamy: Recombination between related individuals, e.g., selfing (see below).
-
Inbreeding: A measure of the probability of identity by descent that tends to increase as a result of closed mating systems (selfing [see below], sib-mating) or limited population sizes.
-
Linkage disequilibrium: A characteristic expressing the nonrandom association between different loci (generally by pair). Many different factors (population structure, closed mating system, selection, etc.) can generate and maintain statistical association between loci.
-
Selfing: A sexual reproductive mode where an individual self-fertilizes its own ovules with its own spermatozoids. The resulting progenies tend to become highly homozygous at most genetic loci.
-
Wahlund effect: A phenomenon that occurs when a sample consists of individuals that were sampled from genetically differentiated subpopulations. This produces a loss of heterozygosity compared to expected heterozygosity under the assumption of the random union of gametes.
These previous studies did not always make use of suitable or sufficient molecular tools, sampling, or data interpretations to reach a definitive conclusion on the reproduction mode of Leishmania parasites. First, the genetic markers used (such as multilocus enzyme electrophoresis [MLEE], random amplified polymorphic DNA [RAPD], PFGE, and restriction fragment length polymorphism [RFLP]) have inherent limitations that make inferences on the population's genetic structure unreliable [17]. Indeed, these molecular markers have low resolution (MLEE or RFLP) or are dominant (RAPD) or multifactorial (reflecting global genomic organization in PFGE). Second, samplings were often too limited for robust population genetic interpretations because of small sample sizes and wide geographical and/or temporal distributions, which have important consequences on the structure of the data. Indeed, living organisms generally are not homogeneously distributed across their environment, and therefore most natural populations are subdivided into more or less small subpopulations. Natural populations are submitted to genetic drift and thus likely to display variable allele frequencies over time. This substructuring of populations has a great influence on the distribution of genetic information in terms of allelic frequencies. Theoretically, if allelic frequencies are not identical between subpopulations, an apparent loss of heterozygotes can be expected when considering the entire population compared to Hardy-Weinberg expectations; this is the Wahlund effect [18] (see Box 1). Third, clonality was mainly inferred from analyses of linkage disequilibria observed across loci. However, computer simulations showed that linkage disequilibria do not provide reliable measurements of the proportion of clonal or sexual reproduction in a population [19]. Furthermore, it was recently demonstrated that Wahlund effects (inappropriate sampling; see Box 1) in clonal populations can lead to highly biased perceptions of linkage disequilibrium [19]–[21].
Theoretical studies showed that strong heterozygote excesses are expected in diploid clones [22]–[26]. Judson and Normak reported that, for ancient diploid asexual lineages, the two alleles at one locus within an individual are expected to be highly divergent since they will accumulate different mutations independently [27], which results in substantial intralocus divergence. This phenomenon, called the Meselson effect (after the name of the leader of the laboratory in which this phenomenon was first described), has been empirically documented in bdelloid rotifers [23], [24]. Moreover, it is important to specify that even at an ecological time scale, clonal reproduction is expected to produce high heterozygosities. Thus, the homozygosity excesses recurrently observed in Leishmania population genetics studies contradict a dominant and ancient clonal reproductive mode.
More Sex in Leishmania?
Nowadays, with the development of elaborate experimental techniques and sophisticated statistical tools, our understanding of the evolutionary processes that govern the propagation of these parasites is continuously improving. Recently, two papers once again challenged the clonal hypothesis on the basis of empirical and experimental data. On one hand, a publication reporting a population genetics study on 125 human strains of Leishmania (Viannia) braziliensis based on 12 microsatellite loci revealed very high levels of homozygosity within samples [17]. The population structure studied at a finer scale by a Bayesian analysis demonstrated that a large part of this was explained by population substructuring (Wahlund effect). Nevertheless, there was still a substantial heterozygote deficit, which is theoretically incompatible with a predominant clonal mode of reproduction at ecological time scales, as explained above. Indeed, the high homozygosity obtained was more in agreement with the occurrence of mating between individuals from related strains (endogamy; see Box 1). We then proposed that L. (V.) braziliensis parasites could alternate between different modes of reproduction: clonality in both vertebrate host and insect vector and endogamy within the insect vector (as has been shown to occur for other kinetoplastid parasites, such as Trypanosoma brucei s.l. [28]), with occurrence of quite frequent recombination events between different (i.e., genetically divergent) individuals (see Figure 1) [29]. On the other hand, in parallel, the occurrence of mating in Leishmania parasites has been validated by a recent experimental study [30]. The authors provided evidence of genetic exchanges (i.e., recombination between individuals from two strains of the same species) within the vector host of Leishmania major. Using transgenic Leishmania strains resistant to different selective drugs, Akopyants et al. infected natural sandflies and then isolated parasites resistant to both drugs. They succeeded in producing hybrid progenies, characterized by full genomic complements from recombination between two related individuals, but with kinetoplast DNA maxicircles from only one parent. It should be noted that seven out of 18 hybrid progeny clones studied showed triploid DNA content, which could be explained by the capacity of Leishmania parasites to cope with aneuploidy (see Box 1). The authors proposed that recombination occurs only in the vector and that hybrid progeny are transmitted to the mammalian vertebrate host by sandfly bites. For the first time, this study directly and experimentally proved the existence of mating in L. major. These two studies combined challenge the assumption that the Leishmania parasites' mode of reproduction is overwhelmingly clonal.
What Will Be the Next Steps?
At this stage, it appears essential to extend population genetics approaches to different Leishmania species and various environments. Analysis of genetic variation at different hierarchical levels is often the only way to investigate natural population characteristics such as gene flow and reproductive strategies [31]. Moreover, given the observation of high substructuring of the population observed in the empirical study described above [17], working at finer geographic scales to detect and limit the substructuring (Wahlund effect) of such pathogens would be a desirable goal for future studies [17]. Thus, exploring the genetic patterns of these parasites (allelic frequencies, heterozygosity rates, or linkage disequilibrium) at microgeographic scales would be the next challenge. This will require samples taken in small geographic areas, within short time windows, and from different host species involved in the transmission cycle (i.e., symptomatic and asymptomatic hosts and different vector species). Very few studies fulfill these conditions, often because of problems arising in the field and/or limitations in molecular techniques (i.e., lack of sensitivity for characterizing parasites in asymptomatic hosts). Some as yet unpublished results from our team (S. Kako Ouraga, V. Rougeron, and A–L. Bañuls, unpublished data.) suggest that the demographic unit could reside in the individual host itself. We developed a pioneering experiment consisting of isolating each Leishmania cell by cell, providing a whole genome amplification on each cell, and then amplifying the microsatellite DNA of the parasite by a nested PCR (a technique developed by the team of F. Prugnolle for Plasmodium falciparum; personal communication, unpublished data). The preliminary tests were made on two different human strains of L. V. braziliensis, CH22B and CH25B, collected in Peru in 1994 from two different patients and known to be heterozygous at several loci [17]. Single-cell genotypic characterization revealed the actual coexistence of multiple genetic entities (i.e., genetically distinct Leishmania individuals) within each of these strains (CH22B and CH25B). This unexpected result strongly suggests that for these parasites the most nested subpopulation unit (the smallest demographic unit) might be individual hosts, a too frequently neglected scale for infectious agents, as stressed in recent papers [21], [32]. Thus, in future studies, it would be indispensable to attempt to analyze population genetic data from the most nested subpopulation. Ignoring these scales should invariably lead to more or less high-magnitude Wahlund effects, depending on the degree of isolation between these micro-subpopulations. We believe that this type of experiment should be generalized in order to improve our knowledge of Leishmania reproductive strategy.
Another step to define the evolutionary mechanisms involved in mating is to characterize the sexual events occurring within the vector in detail. Volf et al. suggested applying high-resolution imaging to identify when, where, and how these phenomena occur in the sandfly gut [33]. Moreover, they explained that stressful conditions applied in the sandfly gut can induce genetic exchanges between Leishmania parasites. This suggests that the environment could play a key role in the frequency of mating events in Leishmania, justifying the need to analyze the reproduction model in diverse environments and within various species (see above).
The progress in Leishmania biology and the development of experimental models will improve our knowledge of the evolutionary genetics of the Trypanosomatidae family. Empirical studies are in agreement with a purely clonal mode of reproduction for Trypanosoma brucei gambiense type 1 [34], while other studies have experimentally proved the possibility of recombination in the tsetse vector for other T. brucei subspecies [35]–[43]. Nevertheless, because Trypanosoma brucei brucei, T. brucei gambiense type 2, and Trypanosoma brucei rhodesiense are themselves extremely heterogeneous [35] and thus probably composed of different entities, the frequency of such events remains an unanswered question. These results show that different subspecies (or species given that trypanosomes' subspecific status is questionable) can display different reproductive strategies, suggesting how important it is to explore the reproduction model within a panel of different species before any definitive conclusion can be drawn for Leishmania. Regarding Trypanosoma cruzi, the genetic profiles observed in the literature (strong linkage disequilibrium and prevalent heterozygote deficits) [9], [44]–[47] may suggest evolutionary processes similar to those found in L. V. braziliensis. Moreover, Gaunt et al., by producing hybrid clones, showed that T. cruzi has an extant capacity for genetic exchange [46]. For all these reasons, research based on experimental techniques combined with sophisticated empirical approaches must be developed considering different species and various environments in order to provide a better understanding of the reproductive mode and the transmission cycle of these protozoan parasites.
Conclusion
In summary, we are still far from complete knowledge of the mechanisms that govern the multiplication, propagation, and diversity of Leishmania in particular and in kinetoplastids in general. It should be noted that we have not detailed the problem of aneuploidy here, which cannot be neglected since it has been largely demonstrated in previous investigations [14], [48] and recently confirmed experimentally [30]. Although no genetic data allow us to suspect a clear impact of aneuploidy for any of the microsatellite loci used so far in empirical approaches, this phenomenon should not be ignored in future studies. Additionally, although none of the published results on microsatellites suggests the existence of any gene conversion, the possibility of gene conversion has to be specifically studied in future population genetic studies. These parasites apparently have unsuspected resources to survive and propagate, and understanding why and how is of primary relevance for better knowledge of their epidemiology and evolutionary potential.
Zdroje
1. BañulsAL
HideM
PrugnolleF
2007 Leishmania and the leishmaniases: a parasite genetic update and advances in taxonomy, epidemiology and pathogenicity in humans. Adv Parasitol 64 1 109
2. MilgroomMG
1996 Recombination and the multilocus structure of fungal populations. Annu Rev Phytopathol 34 457 477
3. TaylorJW
GeiserDM
BurtA
KoufopanouV
1999 The evolutionary biology and population genetics underlying fungal strain typing. Clin Microbiol Rev 12 126 146
4. MacLeodA
TweedieA
WelburnSC
MaudlinI
TurnerCMR
TaitA
2000 Minisatellite marker analysis of Trypanosoma brucei: Reconciliation of clonal, panmictic, and epidemic population genetic structures. Proc Natl Acad Sci U S A 97 13442 13447
5. TibayrencM
AyalaFJ
2002 The clonal theory of parasitic protozoa: 12 years on. Trends Parasitol 18 405 410
6. WHO
2002 Leishmaniasis. Available: http://who.int/zoonoses/diseases/leishmaniasis/en. Accessed 26 July 2010
7. TibayrencM
1993 Clonality in Leishmania. Parasitol Today 9 58
8. VictoirK
DujardinJC
2002 How to succeed in parasitic life without sex? Asking Leishmania. Trends Parasitol 18 81 85
9. TibayrencM
KjellbergF
AyalaFJ
1990 A clonal theory of parasitic protozoa: the population structures of Entamoeba, Giardia, Leishmania, Naegleria, Plasmodium, Trichomonas, and Trypanosoma and their medical and taxonomical consequences. Proc Natl Acad Sci U S A 87 2414 2418
10. RavelC
CortesS
PratlongF
MorioF
DedetJP
CampinoL
2006 First report of genetic hybrids between two very divergent Leishmania species: Leishmania infantum and Leishmania major. Int J Parasitol 36 1383 1388
11. BañulsAL
GuerriniF
Le PontF
BarreraC
EspinelI
GuderianR
EcheverriaR
TibayrencM
1997 Evidence for hybridization by multilocus enzyme electrophoresis and random amplified polymorphic DNA between Leishmania braziliensis and Leishmania panamensis/guyanensis in Ecuador. J Eukaryot Microbiol 44 408 411
12. DujardinJC
BanulsAL
Llanos-CuentasA
AlvarezE
DeDonckerS
1995 Putative Leishmania hybrids in the Eastern Andean valley of Huanuco, Peru. Acta Trop 59 293 307
13. NolderD
RoncalN
DaviesCR
Llanos-CuentasA
MilesMA
2007 Multiple hybrid genotypes of Leishmania (Viannia) in a focus of mucocutaneous Leishmaniasis. Am J Trop Med Hyg 76 573 578
14. BastienP
BlaineauC
PagèsM
1992 Leishmania: sex, lies and karyotype. Parasitol Today 8 174 177
15. BlaineauC
BastienP
PagèsM
1992 Multiple forms of chromosome I, II and V in a restricted population of Leishmania infantum contrasting with monomorphism in individual strains suggest haploidy or automixy. Mol Biochem Parasitol 50 197 204
16. KuhlsK
KeilonatL
OchsenreitherS
SchaarM
SchweynochC
2007 Multilocus microsatellite typing (MLMT) reveals genetically isolated populations between and within the main endemic regions of visceral leishmaniasis. Microbes Infect 9 334 343
17. RougeronV
MeeûsTD
HideM
WaleckxE
BermudezH
2009 Extreme inbreeding in Leishmania braziliensis. Proc Natl Acad Sci U S A 106 10224 10229
18. WahlundS
1928 Zusammensetzung von populationen und korrelationsers-chinungen von standpunkt der vererbungslehre aus betrachtet. Hereditas 11 65 108
19. De MeeûsT
BallouxF
2004 Clonal reproduction and linkage disequilibrium in diploids: a simulation study. Infect Genet Evol 4 345 351
20. PrugnolleF
De MeeûsT
2008 The impact of clonality on parasite population genetic structure. Parasite 15 455 457
21. PrugnolleF
De MeeûsT
2010 Apparent high recombination rates in clonal parasitic organisms due to inappropriate sampling design. Heredity 104 135 140
22. De MeeûsT
PrugnolleF
AgnewP
2007 Asexual reproduction: genetics and evolutionary aspects. Cell Mol Life Sci 64 1355 1372
23. Mark WelchDB
MeselsonM
2000 Evidence for the evolution of bdelloid rotifers without sexual reproduction or genetic exchange. Science 288 1211 1215
24. Mark WelchDB
MeselsonMS
2001 Rates of nucleotide substitution in sexual and anciently asexual rotifers. Proc Natl Acad Sci U S A 98 6720 6724
25. PamiloP
1987 Heterozygosity in apomictic organisms. Hereditas 107 95 101
26. SuomalainenE
SauraA
LokkiJ
1976 Evolution of parthenogenetic insects. Evol Biol 9 209 257
27. JudsonOP
NormarkBB
1996 Ancient asexual scandals. Trends Ecol Evol 11 A41 A46
28. GibsonW
PeacockL
FerrisV
WilliamsK
BaileyM
2008 The use of yellow fluorescent hybrids to indicate mating in Trypanosoma brucei. Parasit Vectors 1 4
29. PeacockL
FerrisV
BaileyM
GibsonW
2009 Intraclonal mating occurs during tsetse transmission of Trypanosoma brucei. Parasit Vectors 2 43
30. AkopyantsNS
KimblinN
SecundinoN
PatrickR
PetersN
LawyerP
DobsonDE
BeverleySM
SacksDL
2009 Demonstration of genetic exchange during cyclical development of Leishmania in the sand fly vector. Science 324 265 268
31. NadlerSA
1995 Microevolution and the Genetic-Structure of Parasite Populations. J Parasitol 81 395 403
32. De MeeûsT
McCoyKD
PrugnolleF
ChevillonC
DurandP
2007 Population genetics and molecular epidemiology or how to “débusquer la bête”. Infect Genet Evol 7 308 332
33. VolfP
SadlovaJ
2009 Sex in Leishmania. Science 324 1644
34. KoffiM
SolanoP
BarnabéC
De MeeûsT
BuchetonB
2007 Genetic characterisation of Trypanosoma brucei ssp by microsatellite typing: new perspectives for the molecular epidemiology of human African trypanosomosis. Infect Genet Evol 7 675 684
35. GibsonW
1989 Analysis of genetic cross between Trypanosoma brucei rhodesiense and T. b. brucei. Parasitol Int 99 391 402
36. GibsonW
1995 The significance of genetic exchange in trypanosomes. Parasitol Today 11 465 468
37. MacLeodA
TweedieA
McLellanS
HopeM
TaylorS
2005 Allelic segregation and independent assortment in T. brucei crosses: proof that the genetic system is Mendelian and involves meiosis. Mol Biochem Parasitol 144 131 131
38. MacLeodA
TweedieA
McLellanS
TaylorS
CooperA
2005 Allelic segregation and independent assortment in T. brucei crosses: Proof that the genetic system is Mendelian and involves meiosis. Mol Biochem Parasitol 143 12 19
39. MacLeodA
TweedieA
McLellanS
TaylorS
HallN
2005 The genetic map and comparative analysis with the physical map of Trypanosoma brucei. Nucleic Acids Res 33 6688 6693
40. MacLeodA
TweedieA
McLellanS
TaylorS
HallN
2006 The genetic map and comparative analysis with the physical map of Trypanosoma brucei. Nucleic Acids Res 34 764 764
41. TaitA
MacLeodA
TweedieA
MasigaD
TurnerCMR
2007 Genetic exchange in Trypanosoma brucei: Evidence for mating prior to metacyclic stage development. Mol Biochem Parasitol 151 133 136
42. JenniL
MartiS
SchweizerJ
BetschartB
Le PageRW
1986 Hybrid formation between African trypanosomes during cyclical transmission. Nature 322 173 175
43. BarnabéC
BrisseS
TibayrencM
2000 Population structure and genetic typing of Trypanosoma cruzi, the agent of Chagas disease: a multilocus enzyme electrophoresis approach. Parasitology 120 513 526
44. MacedoAM
MachadoCR
OliveiraRP
PenaSD
2004 Trypanosoma cruzi: genetic structure of populations and relevance of genetic variability to the pathogenesis of chagas disease. Mem Inst Oswaldo Cruz 99 1 12
45. MachadoCA
AyalaFJ
2001 Nucleotide sequences provide evidence of genetic exchange among distantly related lineages of Trypanosoma cruzi. Proc Natl Acad Sci U S A 98 7396 7401
46. GauntMW
YeoM
FrameIA
StothardJR
CarrascoHJ
2003 Mechanism of genetic exchange in American trypanosomes. Nature 421 936 939
47. LlewellynMS
MilesMA
CarrascoHJ
LewisMD
YeoM
2009 Genome-scale multilocus microsatellite typing of Trypanosoma cruzi discrete typing unit I reveals phylogeographic structure and specific genotypes linked to human infection. PLoS Pathog 5 e1000410 doi:10.1371/journal.ppat.1000410
48. CruzAK
TitusR
BeverleySM
1993 Plasticity in chromosome number and testing of essential genes in Leishmania by targeting. Proc Natl Acad Sci U S A 90 1599 1603
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 8- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Dissecting the Genetic Architecture of Host–Pathogen Specificity
- The Battle for Iron between Bacterial Pathogens and Their Vertebrate Hosts
- Global Genotype-Phenotype Correlations in
- Burkholderia Type VI Secretion Systems Have Distinct Roles in Eukaryotic and Bacterial Cell Interactions
- Chitin Synthases from Are Involved in Tip Growth and Represent a Potential Target for Anti-Oomycete Drugs
- Distinct Merkel Cell Polyomavirus Molecular Features in Tumour and Non Tumour Specimens from Patients with Merkel Cell Carcinoma
- Biological and Structural Characterization of a Host-Adapting Amino Acid in Influenza Virus
- Functional Characterisation and Drug Target Validation of a Mitotic Kinesin-13 in
- CTCF Prevents the Epigenetic Drift of EBV Latency Promoter Qp
- The Human Fungal Pathogen Escapes Macrophages by a Phagosome Emptying Mechanism That Is Inhibited by Arp2/3 Complex-Mediated Actin Polymerisation
- Bim Nuclear Translocation and Inactivation by Viral Interferon Regulatory Factor
- Cyst Wall Protein 1 Is a Lectin That Binds to Curled Fibrils of the GalNAc Homopolymer
- Reciprocal Analysis of Infections of a Model Reveal Host-Pathogen Conflicts Mediated by Reactive Oxygen and imd-Regulated Innate Immune Response
- A Subset of Replication Proteins Enhances Origin Recognition and Lytic Replication by the Epstein-Barr Virus ZEBRA Protein
- Damaged Intestinal Epithelial Integrity Linked to Microbial Translocation in Pathogenic Simian Immunodeficiency Virus Infections
- Kaposin-B Enhances the PROX1 mRNA Stability during Lymphatic Reprogramming of Vascular Endothelial Cells by Kaposi's Sarcoma Herpes Virus
- Direct Interaction between Two Viral Proteins, the Nonstructural Protein 2C and the Capsid Protein VP3, Is Required for Enterovirus Morphogenesis
- A Novel CCR5 Mutation Common in Sooty Mangabeys Reveals SIVsmm Infection of CCR5-Null Natural Hosts and Efficient Alternative Coreceptor Use
- Micro RNAs of Epstein-Barr Virus Promote Cell Cycle Progression and Prevent Apoptosis of Primary Human B Cells
- Enterohemorrhagic Requires N-WASP for Efficient Type III Translocation but Not for EspF-Mediated Actin Pedestal Formation
- Host Imprints on Bacterial Genomes—Rapid, Divergent Evolution in Individual Patients
- UNC93B1 Mediates Host Resistance to Infection with
- The Transcription Factor Rbf1 Is the Master Regulator for -Mating Type Controlled Pathogenic Development in
- Protective Efficacy of Cross-Reactive CD8 T Cells Recognising Mutant Viral Epitopes Depends on Peptide-MHC-I Structural Interactions and T Cell Activation Threshold
- Bacteriophage Lysin Mediates the Binding of to Human Platelets through Interaction with Fibrinogen
- Insecticide Control of Vector-Borne Diseases: When Is Insecticide Resistance a Problem?
- Immune Modulation with Sulfasalazine Attenuates Immunopathogenesis but Enhances Macrophage-Mediated Fungal Clearance during Pneumonia
- PKC Signaling Regulates Drug Resistance of the Fungal Pathogen via Circuitry Comprised of Mkc1, Calcineurin, and Hsp90
- A Multi-Step Process of Viral Adaptation to a Mutagenic Nucleoside Analogue by Modulation of Transition Types Leads to Extinction-Escape
- “Everything You Always Wanted to Know about Sex (but Were Afraid to Ask)” in after Two Decades of Laboratory and Field Analyses
- Norovirus Gastroenteritis, Carbohydrate Receptors, and Animal Models
- Variations in TcdB Activity and the Hypervirulence of Emerging Strains of
- SWAN-1 Binds to EGL-9 and Regulates HIF-1-Mediated Resistance to the Bacterial Pathogen PAO1
- Conformational Adaptation of Asian Macaque TRIMCyp Directs Lineage Specific Antiviral Activity
- The Proteasome Active Site Threonine Is Essential for Persistence Yet Dispensable for Replication and Resistance to Nitric Oxide
- Characterization of Oseltamivir-Resistant 2009 H1N1 Pandemic Influenza A Viruses
- The Pneumococcal Serine-Rich Repeat Protein Is an Intra-Species Bacterial Adhesin That Promotes Bacterial Aggregation and in Biofilms
- Regulatory T Cell Suppressive Potency Dictates the Balance between Bacterial Proliferation and Clearance during Persistent Infection
- Structural Alterations in a Component of Cytochrome Oxidase and Molecular Evolution of Pathogenic in Humans
- A Limited Number of Antibody Specificities Mediate Broad and Potent Serum Neutralization in Selected HIV-1 Infected Individuals
- Spliced Leader Trapping Reveals Widespread Alternative Splicing Patterns in the Highly Dynamic Transcriptome of
- Contribution of Coagulases towards Disease and Protective Immunity
- Early Severe Inflammatory Responses to Uropathogenic Predispose to Chronic and Recurrent Urinary Tract Infection
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The Transcription Factor Rbf1 Is the Master Regulator for -Mating Type Controlled Pathogenic Development in
- PKC Signaling Regulates Drug Resistance of the Fungal Pathogen via Circuitry Comprised of Mkc1, Calcineurin, and Hsp90
- Contribution of Coagulases towards Disease and Protective Immunity
- Early Severe Inflammatory Responses to Uropathogenic Predispose to Chronic and Recurrent Urinary Tract Infection
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



