-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaCan Enhance Infection in Mosquitoes: Implications for Malaria Control?
article has not abstract
Published in the journal: . PLoS Pathog 10(9): e32767. doi:10.1371/journal.ppat.1004182
Category: Opinion
doi: https://doi.org/10.1371/journal.ppat.1004182Summary
article has not abstract
The symbiotic bacterium Wolbachia is an attractive agent for vector-borne pathogen control. It has long been studied for its ability to manipulate host reproduction and spread into arthropod populations [1]. These properties, coupled with the recently identified ability to inhibit diverse pathogens [2]–[6], open avenues for its use in controlling vector-borne disease. Numerous Wolbachia-based control strategies are being investigated (reviewed in [7]–[9]), with some studies having progressed to field trials [10], [11]. However, a worrying trend is emerging whereby Wolbachia infections have been demonstrated to enhance rather than suppress pathogens in some systems [12]–[18]. Plasmodium parasites, which are the causal agent of malaria, seem particularly prone to Wolbachia-mediated pathogen enhancement [13]–[16].
Wolbachia-based strategies have been proposed to control malaria [19]. Anopheles mosquitoes (the vectors of human malaria parasites) are not naturally infected by Wolbachia [20], [21], but artificial transfer of this bacterium between species can be accomplished in the laboratory (reviewed in [22]). Pathogen interference phenotypes appear to be most prominent when Wolbachia is transferred into a novel host [16], [23]. Given that Anopheles are for the most part naturally uninfected by Wolbachia (but see [24]), they can be considered an open niche for infection and a prime mosquito genus for Wolbachia-based control strategies. However, the main impediment for developing a control strategy is the difficulty in creating a stable artificial infection in Anopheles [19]. While examining Plasmodium interference in a stably infected host is the gold standard, a more convenient system is to transiently infect mosquitoes by intrathoracic microinjection. Using this system, the infection persists during the lifetime of the transinfected individual but is not transmitted to its offspring. Transient infection allows the rapid assessment of Wolbachia-host interactions without the need for generating stable artificial infections [5]. It is uncertain how representative transient infections are of stable inherited associations; however, similarities in tissues tropism and fitness costs incurred upon the host between stable and transiently infected Anopheles mosquitoes are evident [5], [14], [25]. Furthermore, both types of infection have been shown to inhibit the human malaria parasite Plasmodium falciparum [5], [25]. However, studies using transient infection models have found that Wolbachia can enhance certain Plasmodium species [13], [14].
The Plasmodium interference phenotype is therefore not universal, but context dependent. While P. falciparum is suppressed by the wAlbB strain of Wolbachia from Aedes albopictus [5], [25], transient infections have shown the opposite effect on rodent malaria parasites. Anopheles gambiae transiently infected with wAlbB exhibited enhanced P. berghei development at the oocyst stage [14]. Similarly, wAlbB increased the number of P. yoelii oocysts in An. stephensi, although the phenotype was modulated by temperature [13]. At a temperature optimal for parasite development, Wolbachia increased parasite intensity compared to uninfected controls, but at warmer temperatures, Wolbachia inhibited Plasmodium development [13].
While P. falciparum is a major parasite in sub-Saharan Africa, four other parasites also cause human malaria worldwide: P. malariae, P. ovale, P. knowlesi, and P. vivax (the etiological agent of the most prevalent form of relapsing malaria). To our knowledge, the effect of Wolbachia on these other human Plasmodium parasites is unknown. The question is relevant for two reasons. First, the precedent that a particular Wolbachia strain can inhibit one parasite yet enhance another has already been documented [5], [14], indicating that effects on parasites can be species-specific. Troublingly, P. malariae, P. ovale, P. knowlesi, and P. vivax are phylogenetically more closely related to rodent malaria parasites, which are enhanced by Wolbachia infections [13], [14], than they are to P. falciparum (Figure 1) [26], [27]. Second, many human Plasmodium parasites occur in sympatry and are transmitted by the same vectors. A case in point is P. falciparum and P. vivax, both of which occur in sympatry over large stretches of the Asian continent where they are both transmitted by An. stephensi [28], [29]. Any potential control strategy devised in regions where more than one parasite species occurs needs to thoroughly investigate the effect of Wolbachia on all parasite species transmitted by the vector, as well as other pathogens such as filarial worms or arboviruses (both as single infections and in the context of coinfections) to ensure that Wolbachia-infected mosquitoes do not inadvertently enhance transmission of secondary pathogens.
Fig. 1. Representative phylogenetic dendrogram of Plasmodium parasites, their vertebrate hosts, and the influence of Wolbachia infection on parasite development within the mosquito vector. 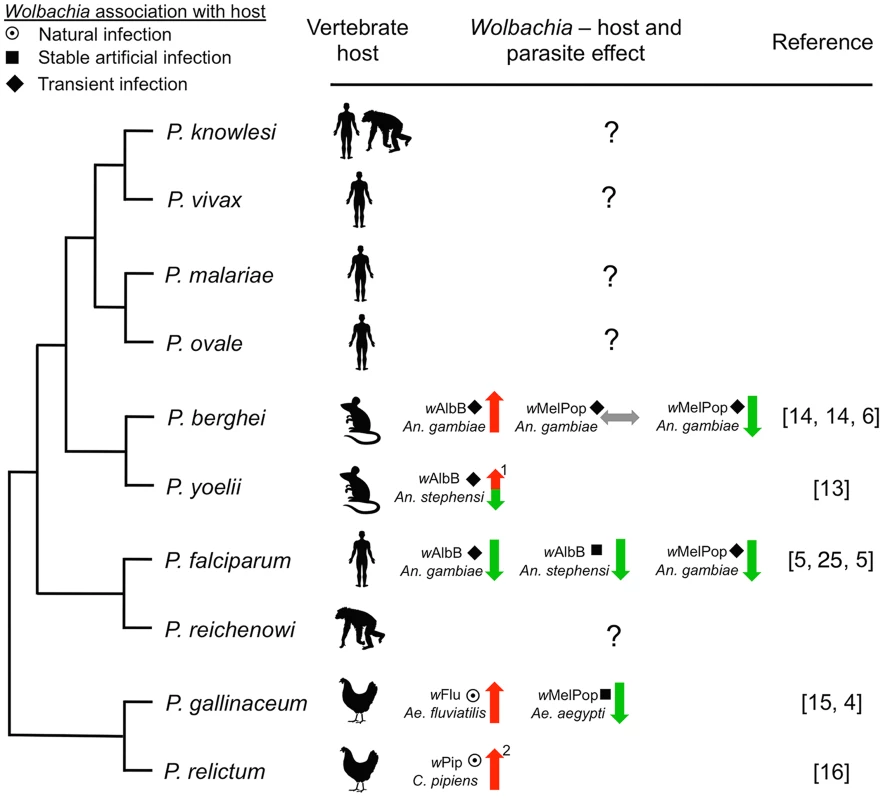
The protective effect of Wolbachia is variable and dependent on the Wolbachia strain and the insect host background, suggesting that complex tripartite interactions influence the effect on Plasmodium. The type of association between Wolbachia with the vector may also influence Plasmodium. Only one human malaria parasite (P. falciparum) has been assessed, while the effect of Wolbachia infection on the other four human parasites is unknown. Arrows indicate suppression (green), enhancement (red), or no effect (grey) of Plasmodium. The type of association within the host is depicted by symbols (target: natural infection, square: stable artificial infection, diamond: transient artificial infection). Numbers indicate: (1) the phenotype is temperature sensitive, (2) Wolbachia infection also increases insect life span [31], which has implications for pathogen transmission. Phylogeny was reconstructed based on work from Carlton et al. [26] and Martinsen et al. [27]. While difficult, forecasting the long-term evolutionary response in this tripartite relationship between Wolbachia, Plasmodium, and Anopheles is very important. Natural Wolbachia–mosquito associations in which the symbiont and the host have tightly coevolved exist and may provide powerful models for studying the long-term evolutionary effects of Wolbachia infections. The evidence currently available suggests that natural Wolbachia infections can also enhance malaria parasite development within the mosquito. Aedes fluviatilis naturally infected with the wFlu Wolbachia strain had a significantly higher number of P. gallinaceum oocysts compared to an Ae. fluviatilis line which had been cleared of the Wolbachia infection [15]. Ae. fluviatilis is not, however, a natural vector of P. gallinaceum, and it is well known that the outcome of experiments using such laboratory models can differ significantly from those of natural mosquito–Plasmodium combinations (e.g., Boete [30]). Recent studies carried out in Culex pipiens mosquitoes, which are naturally infected with the wPip Wolbachia strain and transmit the avian malaria parasite P. relictum, have also demonstrated Plasmodium enhancement. In this natural system, Wolbachia protects the mosquito host against the detrimental fitness effects incurred by Plasmodium infection [31] and increases the susceptibility of C. pipiens to P. relictum, with wPip-infected mosquitoes having a higher prevalence of Plasmodium sporozoites in the salivary glands [16]. These studies show that the Plasmodium-inhibiting properties of Wolbachia are far from universal; certain mosquito–Wolbachia–Plasmodium combinations and experimental conditions transform Wolbachia-infected mosquitoes into better vectors of malaria parasites. This is worrisome for the general implementation of Wolbachia-based control strategies.
Given that Wolbachia-based control strategies will use stable transinfected mosquitoes, the key question is whether stable and natural infections will behave in the same way. The stable transfer of Wolbachia into the host likely alters many aspects of host homeostasis, as evidenced by the novel phenotypes induced by infection [32]–[34], and as such, these associations likely differ from natural associations where Wolbachia and its host have coevolved. Another question is whether stable artificial infections will evolve over time. Theory and empirical studies show that these maternally transmitted bacteria will tend to evolve towards mutualistic associations with their host [35]–[38]. However, the evolutionary outcomes of pathogen interference or enhancement are harder to predict. A more complete mechanistic understanding of how Wolbachia infection modulates Plasmodium parasites is critical to address these important evolutionary questions and to evaluate if they are likely to occur in timescales relevant for disease control.
To date, two stable artificial Wolbachia transinfections have been assessed for their effect on Plasmodium. First, an Aedes aegypti line infected with wMelPop had inhibited P. gallinaceum infection [4]; Ae. aegypti is not, however, the natural vector of this parasite. Second, and more recently, the wAlbB strain was stably transferred into An. stephensi, one of the main vectors of human malaria in Asia [25]. This groundbreaking work demonstrated that stable artificial infections in epidemiologically relevant malaria vectors are feasible, and that P. falciparum can be inhibited by Wolbachia within its natural vector. If the severe fitness effects induced by Wolbachia in Anopheles can be overcome [25], then this approach is highly promising.
The work by Bian and colleagues [25] dramatically enhances the prospect for the use of Wolbachia in a malaria control strategy, but many questions still remain. What are the effects of Wolbachia on the other four species of Plasmodium parasites that infect humans? How relevant are transient infection models? Do some strains of Wolbachia enhance pathogens in a field context? What are the long-term evolutionary consequences of novel Wolbachia-host associations on Plasmodium development within the insect host? What are the mechanisms behind pathogen interference and enhancement of Wolbachia on Plasmodium parasites, and are the mechanisms of enhancement seen in rodent and avian model systems relevant to human malaria parasites? How influential are environmental variables on pathogen inhibition phenotypes? While many of these questions may be difficult to answer in the short term, assessing the relevance of transient infections would seem within the grasp of the scientific community. Although challenging, understanding the evolutionary consequences of novel Wolbachia associations on pathogen transmission and identifying the mechanisms behind Wolbachia modulation of Plasmodium is critical for developing effective control strategies and assessing their long-term feasibility. Insights from non-Anopheline systems where Wolbachia naturally infects the vector may be useful in this regard [16], [31], [39].
In conclusion, Wolbachia-based control of vector-borne pathogens is a promising novel strategy that has many advantages over other conventional and contemporary control methods. The development of a stable infection in Anopheles means the prospect of Wolbachia-based control of malaria can now be entertained [25], but many important questions need to be resolved before this idea can become a reality. While the concerns raised here focus on Plasmodium, these issues are relevant for Wolbachia control of any vector-borne pathogen [18]; we suggest that transinfected mosquitoes intended for release into nature should be assessed for inhibition (or lack thereof) of all relevant pathogens circulating in the system.
Zdroje
1. WerrenJH, BaldoL, ClarkME (2008) Wolbachia: master manipulators of invertebrate biology. Nat Rev Microbiol 6 : 741–751.
2. HedgesLM, BrownlieJC, O'NeillSL, JohnsonKN (2008) Wolbachia and virus protection in insects. Science 322 : 702.
3. KambrisZ, CookPE, PhucHK, SinkinsSP (2009) Immune activation by life-shortening Wolbachia and reduced filarial competence in mosquitoes. Science 326 : 134–136.
4. MoreiraLLA, Iturbe-OrmaetxeI, JefferyJA, LuG, PykeAAT, et al. (2009) A Wolbachia symbiont in Aedes aegypti limits infection with Dengue, Chikungunya, and Plasmodium. Cell 139 : 1268–1278.
5. HughesGL, KogaR, XueP, FukastuT, RasgonJL (2011) Wolbachia infections are virulent and inhibit the human malaria parasite Plasmodium falciparum in Anopheles gambiae. PLoS Pathog 7: e1002043.
6. KambrisZ, BlagboroughAM, PintoSB, BlagroveMSC, GodfrayHCJ, et al. (2010) Wolbachia stimulates immune gene expression and inhibits Plasmodium development in Anopheles gambiae. PLoS Pathog 6: e1001143.
7. McGrawEA, O'NeillSL (2013) Beyond insecticides: new thinking on an ancient problem. Nat Rev Microbiol 11 : 181–193.
8. BourtzisK, DobsonSL, XiZ, RasgonJL, CalvittiM, et al. (2014) Harnessing mosquito-Wolbachia symbiosis for vector and disease control. Acta Trop 132S: S150–S163.
9. Iturbe-OrmaetxeI, WalkerT, O' NeillSL (2011) Wolbachia and the biological control of mosquito-borne disease. EMBO Rep 12 : 508–518.
10. HoffmannAA, MontgomeryBL, PopoviciJ, Iturbe-OrmaetxeI, JohnsonPH, et al. (2011) Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature 476 : 454–457.
11. WalkerT, JohnsonPH, MoreiraLA, Iturbe-OrmaetxeI, FrentiuFD, et al. (2011) The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature 476 : 450–453.
12. GrahamRI, GrzywaczD, MushoboziWL, WilsonK (2012) Wolbachia in a major African crop pest increases susceptibility to viral disease rather than protects. Ecol Lett 15 : 993–1000.
13. MurdockCC, BlanfordS, HughesGL, RasgonJL, ThomasMB (2013) Temperature alters malaria transmission blocking by Wolbachia. Sci Rep 4 : 3932.
14. HughesGL, Vega-RodriguezJ, XueP, RasgonJL (2012) Wolbachia strain wAlbB enhances infection by the rodent malaria parasite Plasmodium berghei in Anopheles gambiae mosquitoes. Appl Environ Microbiol 78 : 1491–1495.
15. BatonLA, PacidônioEC, GonçalvesDDS, MoreiraLA (2013) wFlu: characterization and evaluation of a native Wolbachia from the mosquito Aedes fluviatilis as a potential vector control agent. PLoS ONE 8: e59619.
16. ZéléF, NicotA, BerthomieuA, WeillM, DuronO, et al. (2013) Wolbachia increases susceptibility to Plasmodium infection in a natural system. Proc Biol Sci 281 : 20132837.
17. HussainM, LuG, TorresS, EdmondsJH, KayBH, et al. (2013) Effect of Wolbachia on replication of West Nile virus in a mosquito cell line and adult mosquitoes. J Virol 87 : 851–858.
18. DodsonBL, HughesGL, PaulO, MatacchieroAC, KramerLD, et al. (2014) Wolbachia Enhances West Nile Virus (WNV) Infection in the Mosquito Culex tarsalis. PLoS Negl Trop Dis 8: e2965.
19. Walker T, Moreira LA (2011) Can Wolbachia be used to control malaria? Mem Inst Oswaldo Cruz 106 (Suppl. I): 212–217.
20. RicciI, CancriniG, GabrielliS, D'AmelioS, FaviG (2002) Searching for Wolbachia (Rickettsiales: Rickettsiaceae) in mosquitoes (Diptera: Culicidae): large polymerase chain reaction survey and new identifications. J Med Entomol 39 : 562–567.
21. RasgonJL, ScottTW (2004) An initial survey for Wolbachia (Rickettsiales: Rickettsiaceae) infections in selected California mosquitoes (Diptera: Culicidae). J Med Entomol 41 : 255–257.
22. HughesGL, RasgonJL (2014) Transinfection: a method to investigate Wolbachia-host interactions and control arthropod-borne disease. Insect Mol Biol 23 : 141–151.
23. BianG, XuY, LuP, XieY, XiZ (2010) The endosymbiotic bacterium Wolbachia induces resistance to Dengue virus in Aedes aegypti. PLoS Pathog 6: e1000833.
24. BaldiniF, SegataN, PomponJ, MarcenacP, Robert ShawW, et al. (2014) Evidence of natural Wolbachia infections in field populations of Anopheles gambiae.. Nat Commun 6 : 3985.
25. BianG, JoshiD, DongY, LuP, ZhouG, et al. (2013) Wolbachia invades Anopheles stephensi populations and induces refractoriness to Plasmodium infection. Science 340 : 748–751.
26. CarltonJM, EscalanteAA, NeafseyD, VolkmanSK (2008) Comparative evolutionary genomics of human malaria parasites. Trends Parasitol 24 : 545–550.
27. MartinsenES, PerkinsSL, SchallJJ (2008) A three-genome phylogeny of malaria parasites (Plasmodium and closely related genera): evolution of life-history traits and host switches. Mol Phylogenet Evol 47 : 261–273.
28. KorgaonkarNS, KumarA, YadavRS, KabadiD, DashAP (2012) Mosquito biting activity on humans & detection of Plasmodium falciparum infection in Anopheles stephensi in Goa, India. Indian J Med Res 135 : 120–126.
29. AdakT, SinghOP, DasMK, WattalS, NandaN (2005) Comparative susceptibility of three important malaria vectors Anopheles stephensi, Anopheles fluviatilis, and Anopheles sundaicus to Plasmodium vivax. J Parasitol 91 : 79–82.
30. BoëteC (2005) Malaria parasites in mosquitoes: laboratory models, evolutionary temptation and the real world. Trends Parasitol 21 : 445–447.
31. ZéléF, NicotA, DuronO, RiveroA (2012) Infection with Wolbachia protects mosquitoes against Plasmodium-induced mortality in a natural system. J Evol Biol 25 : 1243–1252.
32. ClancyDJ, HoffmannAA (1997) Behavior of Wolbachia endosymbionts from Drosophila simulans in Drosophila serrata, a novel host. Am Nat 149 : 975–988.
33. SuhE, MercerD, FuY, DobsonSL (2009) Pathogenicity of life-shortening Wolbachia in Aedes albopictus after transfer from Drosophila melanogaster. Appl Env Microbiol 75 : 7783–7788.
34. BouchonD, RigaudT, JuchaultP (1998) Evidence for widespread Wolbachia infection in isopod crustaceans: molecular identification and host feminization. Proc Biol Sci 265 : 1081–1090.
35. McGrawEA, MerrittDJ, DrollerJN, O'NeillSL (2002) Wolbachia density and virulence attenuation after transfer into a novel host. Proc Natl Acad Sci U S A 99 : 2918–2923.
36. WeeksAR, TurelliM, HarcombeWR, ReynoldsKT, HoffmannAA (2007) From parasite to mutualist: rapid evolution of Wolbachia in natural populations of Drosophila. PLoS Biol 5: E114.
37. TurelliM (1994) Evolution of incompatibility-inducing microbes and their hosts. Evolution 48 : 1500–1513.
38. CarringtonLB, HoffmannAA, WeeksAR (2010) Monitoring long-term evolutionary changes following Wolbachia introduction into a novel host: the Wolbachia popcorn infection in Drosophila simulans. Proc Biol Sci 277 : 2059–2068.
39. HughesGL, SamuelsSK, ShaikhK, RasgonJL, Vardo-ZalikAM (2014) Discrimination of the Plasmodium mexicanum vectors Lutzomyia stewarti and Lutzomyia vexator by a PCR-RFLP assay and Wolbachia infection. J Vector Ecol 39 : 224–227.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Out-of-Sequence Signal 3 as a Mechanism for Virus-Induced Immune Suppression of CD8 T Cell ResponsesČlánek RNF26 Temporally Regulates Virus-Triggered Type I Interferon Induction by Two Distinct MechanismsČlánek Mouse, but Not Human, ApoB-100 Lipoprotein Cholesterol Is a Potent Innate Inhibitor of Pneumolysin
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2014 Číslo 9- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Virus Control Goes Epigenetic
- The Role of Iron in Prion Disease and Other Neurodegenerative Diseases
- The Ins and Outs of Rust Haustoria
- Prion Strains and Amyloid Polymorphism Influence Phenotypic Variation
- Teaching Fido New ModiFICation Tricks
- Can Enhance Infection in Mosquitoes: Implications for Malaria Control?
- MIF Contributes to Associated Immunopathogenicity Development
- Persistence of Virus Reservoirs in ART-Treated SHIV-Infected Rhesus Macaques after Autologous Hematopoietic Stem Cell Transplant
- Bacillus Calmette-Guerin Infection in NADPH Oxidase Deficiency: Defective Mycobacterial Sequestration and Granuloma Formation
- EhCoactosin Stabilizes Actin Filaments in the Protist Parasite
- Molecular Insights Into the Evolutionary Pathway of O1 Atypical El Tor Variants
- LprG-Mediated Surface Expression of Lipoarabinomannan Is Essential for Virulence of
- Structural Correlates of Rotavirus Cell Entry
- Multivalent Adhesion Molecule 7 Clusters Act as Signaling Platform for Host Cellular GTPase Activation and Facilitate Epithelial Barrier Dysfunction
- The Effects of Vaccination and Immunity on Bacterial Infection Dynamics
- Myeloid Derived Hypoxia Inducible Factor 1-alpha Is Required for Protection against Pulmonary Infection
- Functional Characterisation of Germinant Receptors in and Presents Novel Insights into Spore Germination Systems
- Global Analysis of Neutrophil Responses to Reveals a Self-Propagating Inflammatory Program
- Host Cell Invasion by Apicomplexan Parasites: The Junction Conundrum
- Comparative Phenotypic Analysis of the Major Fungal Pathogens and
- Unravelling the Multiple Functions of the Architecturally Intricate β-galactosidase, BgaA
- Sialylation of Prion Protein Controls the Rate of Prion Amplification, the Cross-Species Barrier, the Ratio of PrP Glycoform and Prion Infectivity
- Symbionts Commonly Provide Broad Spectrum Resistance to Viruses in Insects: A Comparative Analysis of Strains
- Ontogeny of Recognition Specificity and Functionality for the Broadly Neutralizing Anti-HIV Antibody 4E10
- Identification and Characterisation of a Hyper-Variable Apoplastic Effector Gene Family of the Potato Cyst Nematodes
- Crimean-Congo Hemorrhagic Fever Virus Entry into Host Cells Occurs through the Multivesicular Body and Requires ESCRT Regulators
- Age-Dependent Enterocyte Invasion and Microcolony Formation by
- CD160-Associated CD8 T-Cell Functional Impairment Is Independent of PD-1 Expression
- Functional Fluorescent Protein Insertions in Herpes Simplex Virus gB Report on gB Conformation before and after Execution of Membrane Fusion
- The Tudor Domain Protein Spindlin1 Is Involved in Intrinsic Antiviral Defense against Incoming Hepatitis B Virus and Herpes Simplex Virus Type 1
- Transgenic Analysis of the MAP Kinase MPK10 Reveals an Auto-inhibitory Mechanism Crucial for Stage-Regulated Activity and Parasite Viability
- Evidence for a Transketolase-Mediated Metabolic Checkpoint Governing Biotrophic Growth in Rice Cells by the Blast Fungus
- Incomplete Deletion of IL-4Rα by LysM Reveals Distinct Subsets of M2 Macrophages Controlling Inflammation and Fibrosis in Chronic Schistosomiasis
- Identification and Functional Expression of a Glutamate- and Avermectin-Gated Chloride Channel from , a Southern Hemisphere Sea Louse Affecting Farmed Fish
- Out-of-Sequence Signal 3 as a Mechanism for Virus-Induced Immune Suppression of CD8 T Cell Responses
- Strong Epistatic Selection on the RNA Secondary Structure of HIV
- Hematopoietic but Not Endothelial Cell MyD88 Contributes to Host Defense during Gram-negative Pneumonia Derived Sepsis
- Delineation of Interfaces on Human Alpha-Defensins Critical for Human Adenovirus and Human Papillomavirus Inhibition
- Exploitation of Reporter Strains to Probe the Impact of Vaccination at Sites of Infection
- RNF26 Temporally Regulates Virus-Triggered Type I Interferon Induction by Two Distinct Mechanisms
- Helminth Infections Coincident with Active Pulmonary Tuberculosis Inhibit Mono- and Multifunctional CD4 and CD8 T Cell Responses in a Process Dependent on IL-10
- MHC Class II Restricted Innate-Like Double Negative T Cells Contribute to Optimal Primary and Secondary Immunity to
- Reactive Oxygen Species Regulate Caspase-11 Expression and Activation of the Non-canonical NLRP3 Inflammasome during Enteric Pathogen Infection
- Evolution of Plastic Transmission Strategies in Avian Malaria
- A New Human 3D-Liver Model Unravels the Role of Galectins in Liver Infection by the Parasite
- Translocates into the Myocardium and Forms Unique Microlesions That Disrupt Cardiac Function
- Mouse, but Not Human, ApoB-100 Lipoprotein Cholesterol Is a Potent Innate Inhibitor of Pneumolysin
- The Cofilin Phosphatase Slingshot Homolog 1 (SSH1) Links NOD1 Signaling to Actin Remodeling
- Kaposi's Sarcoma Herpesvirus MicroRNAs Induce Metabolic Transformation of Infected Cells
- Reorganization of the Endosomal System in -Infected Cells: The Ultrastructure of -Induced Tubular Compartments
- Distinct Dictation of Japanese Encephalitis Virus-Induced Neuroinflammation and Lethality via Triggering TLR3 and TLR4 Signal Pathways
- Exploitation of the Complement System by Oncogenic Kaposi's Sarcoma-Associated Herpesvirus for Cell Survival and Persistent Infection
- The Secreted Peptide PIP1 Amplifies Immunity through Receptor-Like Kinase 7
- Structural Insight into Host Recognition by Aggregative Adherence Fimbriae of Enteroaggregative
- The CD14CD16 Inflammatory Monocyte Subset Displays Increased Mitochondrial Activity and Effector Function During Acute Malaria
- Infection Induces Expression of a Mosquito Salivary Protein (Agaphelin) That Targets Neutrophil Function and Inhibits Thrombosis without Impairing Hemostasis
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The Secreted Peptide PIP1 Amplifies Immunity through Receptor-Like Kinase 7
- Symbionts Commonly Provide Broad Spectrum Resistance to Viruses in Insects: A Comparative Analysis of Strains
- MIF Contributes to Associated Immunopathogenicity Development
- The Ins and Outs of Rust Haustoria
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



