-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaSomatic Genetics Empowers the Mouse for Modeling and Interrogating Developmental and Disease Processes
With recent advances in genomic technologies, candidate human disease genes are being mapped at an accelerated pace. There is a clear need to move forward with genetic tools that can efficiently validate these mutations in vivo. Murine somatic mutagenesis is evolving to fulfill these needs with tools such as somatic transgenesis, humanized rodents, and forward genetics. By combining these resources one is not only able to model disease for in vivo verification, but also to screen for mutations and pathways integral to disease progression and therapeutic intervention. In this review, we briefly outline the current advances in somatic mutagenesis and discuss how these new tools, especially the piggyBac transposon system, can be applied to decipher human biology and disease.
Published in the journal: . PLoS Genet 7(7): e32767. doi:10.1371/journal.pgen.1002110
Category: Review
doi: https://doi.org/10.1371/journal.pgen.1002110Summary
With recent advances in genomic technologies, candidate human disease genes are being mapped at an accelerated pace. There is a clear need to move forward with genetic tools that can efficiently validate these mutations in vivo. Murine somatic mutagenesis is evolving to fulfill these needs with tools such as somatic transgenesis, humanized rodents, and forward genetics. By combining these resources one is not only able to model disease for in vivo verification, but also to screen for mutations and pathways integral to disease progression and therapeutic intervention. In this review, we briefly outline the current advances in somatic mutagenesis and discuss how these new tools, especially the piggyBac transposon system, can be applied to decipher human biology and disease.
Introduction
The recent revolution in high-throughput sequencing and genomic technologies has enabled geneticists to rapidly map disease susceptibility to genomic regions. As a result, there has been an explosion in the number of candidate genes identified for a multitude of human conditions [1]. We are now faced with the daunting task of verifying candidate disease genes, deciphering underlying mechanisms, and developing therapeutic strategies. The ability to genetically manipulate the mouse to study and model disease in vivo makes it an ideal tool to match the challenge. Current and future application of genetic tools such as somatic mosaicism, humanized rodents, and forward genetics will empower interrogation of mammalian biology and disease in the coming years. The ability to efficiently produce genetic mosaics facilitates gene analysis in somatic cells, which will reduce the time and cost that has been associated in producing germline models. Humanized rodents, which continue to evolve into better human models, can be combined with genetic mosaic tools to dissect mechanisms of human disease. Furthermore, the advent of forward genetic screening strategies like in vivo RNAi and insertional mutagenesis now allows investigators to identify novel players in mammalian disease and developmental processes. With these new tools in hand, investigators can use the mouse to rapidly identify key pathways in disease pathogenesis for targeted therapies.
It has become increasingly clear that somatic alterations, whether sequence changes or copy number variations, play a prominent role in human disease and physiology. An obvious example is cancer, where cells can be marked by hundreds of somatic mutations, many of which likely drive the progression of the disease [2], [3]. Interestingly, somatic mutations can also revert disease phenotypes, as illustrated recently in the case of ichthyosis with confetti, which has allowed for the identification of the causative mutation [4]. There are also established roles for somatic mutations and the accompanying mosaicism in shaping the defense repertoire of our immune system [5] and possibly creating neuronal diversity [6]–[8]. Clearly, genetic mosaicism is an important mechanism driving many developmental processes, but in an aberrant context can cause disease. The advent of genetic tools, which allow us to mirror somatic mosaicism by the introduction of mutations temporally and spatially in distinct populations of cells, provide a powerful means to study the cellular interplay that shapes disease and developmental processes (Table 1).
Tab. 1. Genetic Tools for Generating Mutant Clones and Somatic Mutagenesis in Mice. 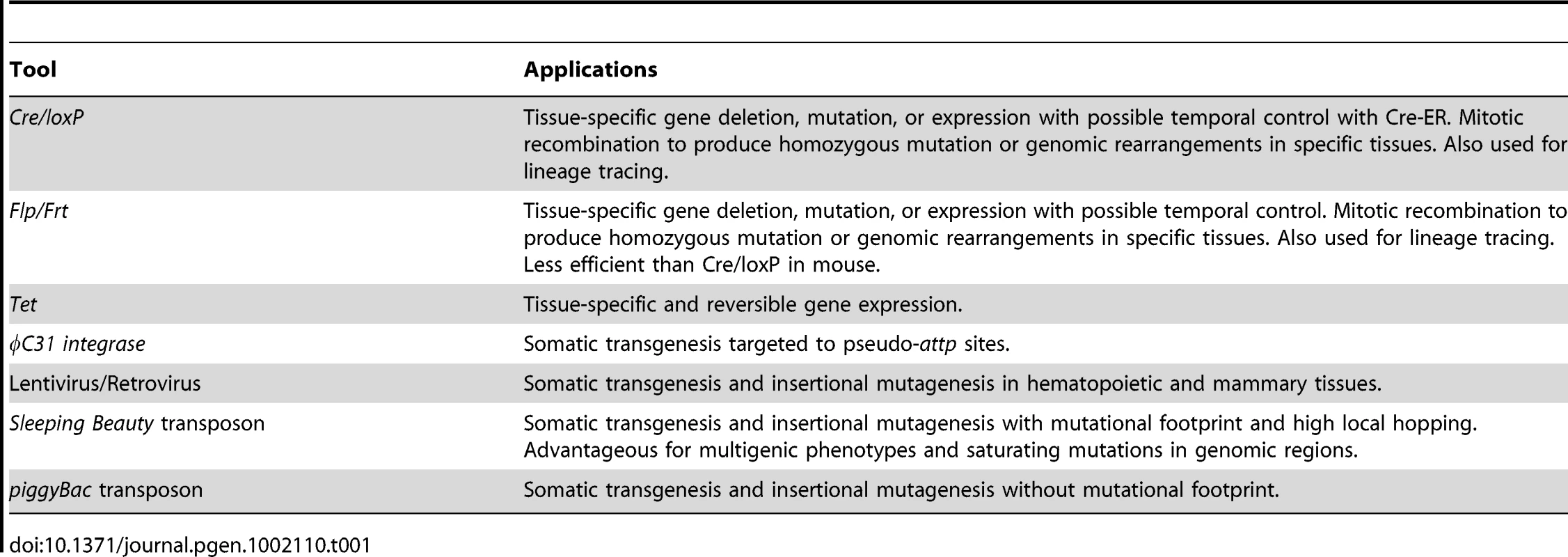
Rapid Dissection of Disease and Development in the Mouse
One of the major drawbacks of mouse genetics is the time it takes to generate germline transgenic or knockout lines and to combine multiple alleles into the same background. Performing experiments with somatic gene introduction has the potential to dramatically enhance the speed and broaden the scope of such experiments. Lentiviral - and transposon-mediated genetic manipulation will likely be widely used moving forward, due to the potential for genetically manipulating a variety of cell types and stably integrating transgenes. Although targeted mutations cannot be induced with these modalities, they can be introduced into lines harboring traditional germline-targeted alleles, thus broadening the utility of both systems.
Viral Vectors for Somatic Transgenesis
Integrating viral vectors like retrovirus or lentivirus have been used to create genetic mosaicism by overexpressing genes or knocking down mRNA with RNAi [9]–[12]. Specific tissues can be targeted by injecting the virus directly into the desired site, or transgenes can be used to specifically direct infection or expression to certain tissues. For example, the RCAS/TVA transgenic system allows for higher specificity of mutation through transgenic expression of the TVA receptor, which is needed for infection [13]. This elegant system has been used to study development as well as model cancer [14]–[17]. Alternatively, tissue-specific Cre lines can be used to direct expression of lentivirus transgenes, as recently utilized to model glioma [18]. The lentiviral approach has the added benefits of being able to infect non-dividing cells and carry larger DNA payloads [10], [19], [20]. Furthermore, extensive libraries of cDNA or shRNA constructs are commercially available in lentiviral backbones, which allows for rapid implementation and facilitates forward genetic screens.
Transposons and the φC31 Integrase for Somatic Transgenesis
The advent of in vivo transfection and electroporation to introduce plasmid DNA in mammalian tissues has allowed researchers to rapidly study gene function in the soma, as reviewed in [21]–[23]. Combining these gene delivery technologies with integrase or DNA cut-and-paste transposon systems allows for rapid and stable introduction of transgenes. In the case of the bacteriophage φC31 integrase system, plasmid DNA containing an attB site is transferred by the integrase into pseudo-attp sites in the mammalian genome [24]. The specific integration of plasmid DNA into a limited number of pseudo-attp sites by φC31 integrase is appealing for gene therapy approaches, as it reduces the chance of undesired phenotypes like cancer development due to insertional mutation [25]. For transposon-mediated gene transfer, the gene(s) are simply flanked in a plasmid by transposon end sequences and are introduced in trans with transposase to induce transposition into the target cell genome. Modified Sleeping Beauty (SB) and piggyBac (PB) transposon/transposase systems have been used to mediate stable integration and expression of transgenes in human cells and mice [26]–[32]. This strategy has been adapted to rapidly model brain tumors [33] and test gene therapy approaches in mice [28], [34]–[36]. Thus, the φC31 integrase and transposon tools can be rapidly constructed and implemented to efficiently integrate DNA into somatic cells.
The superior transposition efficiency and cargo capacity of the PB transposon make it an ideal tool for gene manipulation in the soma. The PB transposon system can transpose up to 10 kb of payload without a significant drop in transposition activity [26]. This large payload size allows for the combination of several cDNAs or RNAi hairpins to be combined into one construct. Such a strategy has been used to express multiple transcription factors in one PB transposon to reprogram differentiated fibroblasts into induced pluripotent stem (IPS) cells [37]. The unique ability of the PB transposon to excise from the genome without leaving a mutation also allows the transgene to be removed cleanly [37]. Furthermore, it is possible to transpose multiple transposons into the same cell, further enhancing the sophistication that can be incorporated into the genetic manipulation [38], . The PB system, like lentiviral constructs, will be useful in not only modeling disease, but also for dissecting molecular mechanisms (Figure 1). An immediate application would be to validate the increasing number of candidate cancer genes being identified by high-throughput sequencing and copy number analysis of human tumors [40]–[47]. In contrast, the traditional production of a murine transgenic or knockout allele for each one of these candidate genes in a similar effort would be extremely costly and time-consuming.
Fig. 1. Somatic phenotypes like cancer can be modeled and genetically dissected with transposon mutagenesis. 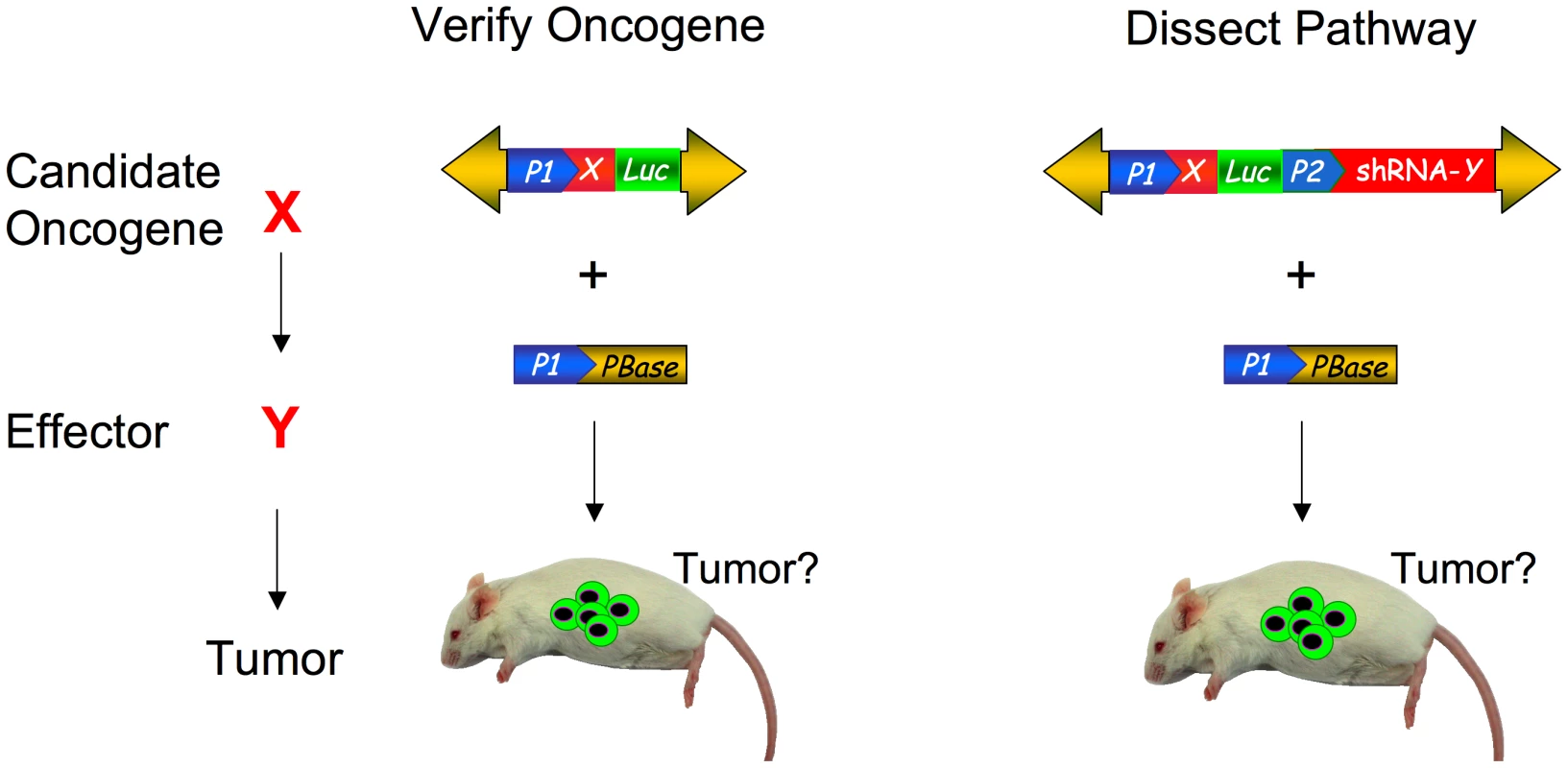
Potential oncogenic pathway to be interrogated with candidate oncogene X and effector Y in red (left). Depiction of PB transposon construct for verifying oncogene X (center). Yellow arrows detail transposon arms. Promoters are depicted by blue pointed boxes. Gene X is indicated by red box and luciferase marker is indicated by green box. To test if effector Y is involved in the oncogenic pathway, an shRNA cassette to knockdown gene Y is represented by the red box (right). The transposons are co-transfected or electroporated with PBase (lower yellow box) to stably integrate the transposon construct into the mouse cells. The green cells in the mouse indicate luciferase positive cells expressing the transposed construct, which are monitored for the tumor formation. Somatic Transgenesis of Human Tissues
Although mouse genetics and in vitro cell culture have been successful experimental surrogates for human disease, studying human cells in an in vivo environment is ideal. This can be accomplished by transplanting human cells into mice or rats to study developmental or disease processes. Such xenograft transplants have been widely used to model cancer as reviewed in [48]–[50]. However, a strategy involving the xenografting of normal human cells to develop chimeric rodents first, and then the induction of disease causing mutations would be much more attractive to study pathogenesis. Researchers are actively working to improve humanized models by increasing the engraftment and function of human cells into mouse tissues [51]–[53].
The ability to reprogram differentiated cells to embryonic stem cell–like IPS cells as first reported by the Yamanaka group [54] has opened up new avenues for personalized cellular therapies. These stem cells have the potential to serve as a source to replace damaged, diseased, or aged tissues. Investigators have performed proof-of-principle experiments in the mouse and rat to treat a variety of degenerative conditions by cellular transplantation of IPS or cells derived from IPS cells including: sickle cell anemia [55], spinal cord injury [56], hemophilia [57], diabetes [58], and Parkinson's disease [59]. Building upon the mouse experiments, IPS cells have been produced from normal and diseased human cells [60]–[62]. These disease-specific IPS cells could be used as donor cells to engraft for humanized mouse models.
Patient-derived disease-specific IPS cells are a perfect source of material to genetically dissect disease. Inducible transgenes can be introduced into these cells before engraftment into recipient mice such that the molecular mechanism of the disease could be studied in an in vivo environment. The PB transposon is ideally suited for such experiments. Inducible genes or shRNAs could be introduced to activate or repress cellular pathways to test the effect on disease progression. Rats would also be an attractive host for future models because of their well-studied pharmacological metabolism. Such experimental models will likely be ideal to identify therapeutic targets genetically and then to test drugs in preclinical trials.
Screening for Disease-Associated Phenotypes with Somatic Mutagens
Forward genetic screens, a phenotypic approach for screening mutants without a priori assumptions of the molecular nature of the affected genes, has been a powerful tool in lower organisms to map genes responsible for a variety of phenotypes. In mice, somatic forward genetic screens allow investigators to screen many genes in one animal by mosaic mutagenesis, thus saving time and money. To date, such somatic screens have been limited to the identification of genes involved in cancer, but the development of new genetic tools has now made screening for other phenotypes possible.
In Vivo RNAi Screens
Loss-of-function screens have been performed in vivo with viral RNAi libraries [63]–[65]. These screens involve isolating progenitor cells, transducing with an RNAi library, and transplanting the cells back into mice. The screens have successfully identified novel tumor suppressors in the development of hepatocellular carcinoma and leukemia. Unfortunately, not all tissues are currently amenable to such transplantation protocols, but future improvements to the efficiency of in vivo lentiviral transduction or transposon introduction may facilitate screens in other tissues. As a result, screening systems that do not rely on exogenous gene delivery are currently more broadly applicable for in vivo forward genetic screens.
Insertional Mutagenesis with Retrovirus
To date, the most widely used forward genetic strategy in the mouse has been insertional mutagenesis with replication-competent retroviruses. These retroviruses have been used as a mutagen to identify genes involved in cancer formation. The insertion of the retroviral provirus into the host genome can cause mutation by disrupting a gene sequence, by upregulating endogenous transcription from the viral LTR, or by producing hypomorphic alleles by inducing missplicing or alternative polyadenlation from the provirus. The provirus also serves as a tag to identify the insertion site and genes affected by PCR strategies. Thus, insertional mutagenesis with retrovirus mutates and tags genes, which allows rapid identification of causative mutations [66]. Indeed, many candidate genes identified in these screens have been confirmed to be bona fide cancer genes as reviewed in [67]. Modifier screens have also revealed cooperation between distinct oncogenic pathways [68], [69]. Although retroviral mutagenesis is a validated forward genetic tool, its broad utility is limited due to the fact that the tissue selectivity of the retroviral mutagen limits these screens to hematopoietic and mammary tissues.
Insertional Mutagenesis with Transposons
Transposon insertional mutagenesis (TIM) is a powerful tool for inducing and identifying mutations of interest and has been utilized with great effect in many organisms, from the bacterium to the fruit fly Drosophila melanogaster [70], [71]. The SB and PB DNA transposons have been developed for germline TIM in mice [26], [72]–[75]. TIM systems allow the induction and identification of mutations much like retroviral insertional mutagenesis, but with the ability to target any tissue where a promoter is available. The bipartite system consists of a transgenic line of non-autonomous mutagenic transposons and a line expressing the transposase, which promotes transposition [76]. Application of TIM in the mouse germline enables collections of mutant animals to be rapidly produced simply by breeding [26], [72]. In fact, a large-scale germline insertional mutagenesis with the PB transposon has already produced more than 5,000 mouse lines, each with a different gene mutated and a broad range of phenotypes (X. Wu and T. Xu, unpublished data; http://idm.fudan.edu.cn/PBmice). Furthermore, a Blm-deficient background can be used to increase the rate at which transposon insertions are converted from heterozygous to homozygous for rapid induction of recessive mutations [77]. The success of germline TIM in decoding gene and regulatory element function indicates that the strategy can be applied to the soma for rapid and efficient forward genetic screens.
Somatic forward genetic screens have the potency to interrogate thousands of genes for a wide range of phenotypes in a single animal. This has been illustrated by the successful application of TIM in somatic tissues for cancer gene discovery with SB and PB [78]–[83]. These screens have identified known human cancer genes and identified new players, thus confirming TIM as a viable technology for mapping mutations associated with somatic phenotypes. The induction of multiple tumors per mouse also reduced the number of animals required to identify candidate genes. By constructing latent transposase alleles with the Cre system, specific somatic tissues have also been directly targeted for screens [84]–[86]. With the proven success of the SB and PB systems in interrogating cancer development, it is likely that TIM can be applied to other developmental and disease processes.
The PB transposon has distinct advantages that make it an attractive tool for applying somatic mutagenesis for other phenotypes. First, PB has high transposition efficiency and can mobilize large DNA payloads [26], [31], [87], [88]. This allows for the creation of highly mutagenic transposons and therefore insertional screens to be performed with one or several copies of the transposon. Fewer transposons per cell is especially helpful in identifying causative mutations, because there will be less background or “bystander” insertions. The larger DNA payload also allows for the inclusion of fluorescent or bioluminescent markers for identifying mutated cells. Second, PB does not leave a footprint or mutation after excision like other transposons, so a direct correlation between insertion and the phenotype can be made [26], [89], [90]. However, a mutational footprint may be advantageous for inducing phenotypes that require multiple mutations as evidenced by efficient cancer induction with SB [78]–[82]. Third, the majority of PB insertions are genome wide compared to the extensive local hopping (transposon reinsertion close to original site) found with SB [26], [83]. Although local hopping is advantageous for saturating mutagenesis at certain genomic regions [91], it complicates the identification of causative mutations and contributes to the formation of genomic rearrangements [92]. Thus, the high efficiency of mutagenesis and ease of mapping causative mutations makes PB desirable for gene discovery in developmental and disease processes.
Theoretically, transposon mutagenesis can be performed in any tissue or cell type and applied to any phenotype. However, while tumors are readily identifiable, locating mutant clones is a prerequisite for screening and analyzing many other somatic phenotypes. Visibly marking mutant somatic clones has been employed in Drosophila, zebrafish, and mice and demonstrated to have tremendous utility in analyzing a variety of clonal behaviors in vivo [93]–[97]. Consequently, a somatic TIM system that incorporates the ability to track mutagenized cells would be ideal in somatic screens for phenotypes other than tumor formation. We have recently exploited PB's unique properties to develop a highly efficient, Cre-inducible TIM system and have demonstrated that tracking mutant clones with visible markers allows detection of altered cellular proliferation and infiltration, among other phenotypes induced by insertional mutation (S. Landrette, J. Cornett, T. Ni, M. Bosenberg, and T. Xu, unpublished data). Thus, TIM will likely be employed in future studies to identify novel players in mammalian disease and developmental pathways in vivo. Exciting areas for interrogation include immunology, neurobiology, and cancer metastasis.
Screening for Disease and Developmental Phenotypes in Humanized Mice
The next step would be to perform TIM screens in humanized mice such that disease phenotypes could be genetically interrogated in human cells in vivo. A TIM system consisting of multiple copies of a mutagenic transposon and an inducible transposase could be stably introduced into human IPS cells by transfection and selection. The cells would then be introduced into a humanized mouse and screened for disease or developmental phenotypes (Figure 2). Beyond inducing disease, it is also possible to screen for reversion of disease phenotypes, which will be particularly useful in identifying relevant targets for developing therapeutics. For example, IPS cells could be generated from a patient with a neurodegenerative disease. These cells could be introduced into a humanized mouse and mutagenized with a TIM system. Clones of neurons that survive in the mouse tissue and do not degenerate could be isolated and the transposon insertions mapped. The insertional mutations identified likely would reveal novel pathways involved in neurodegeneration that may be amenable to therapeutic targeting. Thus, somatic mutagenesis has the potential for unraveling the complexities of disease processes in human cells that are difficult to experimentally query in vitro or through human genome sequencing.
Fig. 2. Screening for phenotypes in humanized mice with patient-derived IPS cells. 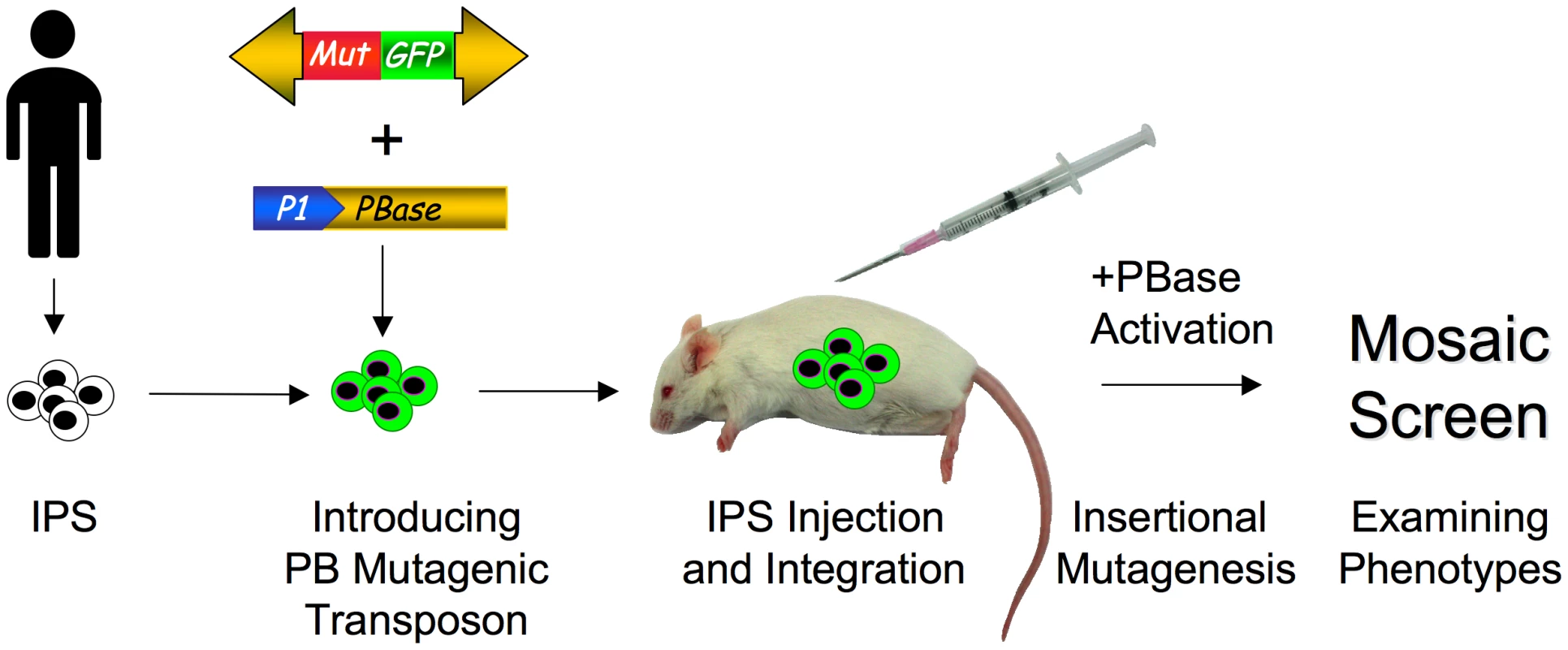
IPS cells are first created from a patient. A mutator transposon containing mutagenic elements (red box) and a GFP marker (green box) and an inducible PBase construct (utilizing the Cre-ER/lox or Tet system) is introduced into patient-derived IPS cells. Green cells indicate GFP expression from the stably integrated mutator transposon(s). The cells are then introduced into the mouse tissue by injection (syringe). Next, transposase activity is induced, which mobilizes the mutagenic transposon, resulting in insertional mutation. Finally, the mice are screened for the desired disease or developmental phenotype. Conclusion
In summary, recent technological advancements in mouse genetics have now provided opportunities to somatically interrogate the mouse and human genome that have previously only been possible in non-mammalian genetic model organisms. Great strides have been made in modeling somatic mosaicism, humanizing mice, and forward genetic screening. Moving forward, lentiviral and DNA transposon systems should be incredibly powerful in modeling and dissecting developmental and disease processes due to their ability to efficiently stably integrate large payloads of genetic sequence. Combining these genetic tools with humanized mice allows investigators to genetically manipulate human cells in vivo, which should push the boundaries of human biology. Furthermore, mammalian forward genetics is now at a point where novel causative mutations in cancer and beyond can be mapped. The advent of highly efficient TIM systems like PB allows genome-wide screens to be performed in small cohorts of mice. Thus, individual investigators now have the screening power to interrogate mammalian phenotypes in vivo. It is likely that screens will soon discover mutations that can revert disease phenotypes, which would accelerate the identification of new therapeutic avenues. The mouse continues to be the model of choice for in vivo verification and advances in somatic mutagenesis are evolving the mouse as an indispensable tool for gene discovery.
Zdroje
1. HeardETishkoffSToddJAVidalMWagnerGP Ten years of genetics and genomics: what have we achieved and where are we heading? Nat Rev Genet 11 723 733
2. HanahanDWeinbergRA Hallmarks of cancer: the next generation. Cell 144 646 674
3. VogelsteinBKinzlerKW 2004 Cancer genes and the pathways they control. Nat Med 10 789 799
4. ChoateKALuYZhouJChoiMEliasPM Mitotic Recombination in Patients with Ichthyosis Causes Reversion of Dominant Mutations in KRT10. Science
5. RajewskyK 1996 Clonal selection and learning in the antibody system. Nature 381 751 758
6. MuotriARChuVTMarchettoMCDengWMoranJV 2005 Somatic mosaicism in neuronal precursor cells mediated by L1 retrotransposition. Nature 435 903 910
7. CoufalNGGarcia-PerezJLPengGEYeoGWMuY 2009 L1 retrotransposition in human neural progenitor cells. Nature 460 1127 1131
8. SingerTMcConnellMJMarchettoMCCoufalNGGageFH LINE-1 retrotransposons: mediators of somatic variation in neuronal genomes? Trends Neurosci 33 345 354
9. NaldiniLBlomerUGageFHTronoDVermaIM 1996 Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proc Natl Acad Sci U S A 93 11382 11388
10. NaldiniLBlomerUGallayPOryDMulliganR 1996 In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 272 263 267
11. MillerADMillerDGGarciaJVLynchCM 1993 Use of retroviral vectors for gene transfer and expression. Methods Enzymol 217 581 599
12. TiscorniaGSingerOIkawaMVermaIM 2003 A general method for gene knockdown in mice by using lentiviral vectors expressing small interfering RNA. Proc Natl Acad Sci U S A 100 1844 1848
13. FederspielMJBatesPYoungJAVarmusHEHughesSH 1994 A system for tissue-specific gene targeting: transgenic mice susceptible to subgroup A avian leukosis virus-based retroviral vectors. Proc Natl Acad Sci U S A 91 11241 11245
14. DoetschFCailleILimDAGarcia-VerdugoJMAlvarez-BuyllaA 1999 Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 97 703 716
15. MurphyGJLeavittAD 1999 A model for studying megakaryocyte development and biology. Proc Natl Acad Sci U S A 96 3065 3070
16. HollandECCelestinoJDaiCSchaeferLSawayaRE 2000 Combined activation of Ras and Akt in neural progenitors induces glioblastoma formation in mice. Nat Genet 25 55 57
17. HollandECHivelyWPDePinhoRAVarmusHE 1998 A constitutively active epidermal growth factor receptor cooperates with disruption of G1 cell-cycle arrest pathways to induce glioma-like lesions in mice. Genes Dev 12 3675 3685
18. MarumotoTTashiroAFriedmann-MorvinskiDScadengMSodaY 2009 Development of a novel mouse glioma model using lentiviral vectors. Nat Med 15 110 116
19. OrsulicS 2002 An RCAS-TVA-based approach to designer mouse models. Mamm Genome 13 543 547
20. KumarMKellerBMakalouNSuttonRE 2001 Systematic determination of the packaging limit of lentiviral vectors. Hum Gene Ther 12 1893 1905
21. MirLMMollerPHAndreFGehlJ 2005 Electric pulse-mediated gene delivery to various animal tissues. Adv Genet 54 83 114
22. Barnabe-HeiderFMeletisKErikssonMBergmannOSabelstromH 2008 Genetic manipulation of adult mouse neurogenic niches by in vivo electroporation. Nat Methods 5 189 196
23. Tros de IlarduyaCSunYDuzgunesN Gene delivery by lipoplexes and polyplexes. Eur J Pharm Sci 40 159 170
24. KeravalaACalosMP 2008 Site-specific chromosomal integration mediated by phiC31 integrase. Methods Mol Biol 435 165 173
25. WoodardLEKeravalaAJungWEWapinskiOLYangQ Impact of hydrodynamic injection and phiC31 integrase on tumor latency in a mouse model of MYC-induced hepatocellular carcinoma. PLoS ONE 5 e11367 doi:10.1371/journal.pone.0011367
26. DingSWuXLiGHanMZhuangY 2005 Efficient transposition of the piggyBac (PB) transposon in mammalian cells and mice. Cell 122 473 483
27. IvicsZHackettPBPlasterkRHIzsvakZ 1997 Molecular reconstruction of Sleeping Beauty, a Tc1-like transposon from fish, and its transposition in human cells. Cell 91 501 510
28. YantSRMeuseLChiuWIvicsZIzsvakZ 2000 Somatic integration and long-term transgene expression in normal and haemophilic mice using a DNA transposon system. Nat Genet 25 35 41
29. CadinanosJBradleyA 2007 Generation of an inducible and optimized piggyBac transposon system. Nucleic Acids Res 35 e87
30. LuoGIvicsZIzsvakZBradleyA 1998 Chromosomal transposition of a Tc1/mariner-like element in mouse embryonic stem cells. Proc Natl Acad Sci U S A 95 10769 10773
31. WangWLinCLuDNingZCoxT 2008 Chromosomal transposition of PiggyBac in mouse embryonic stem cells. Proc Natl Acad Sci U S A 105 9290 9295
32. DupuyAJClarkKCarlsonCMFritzSDavidsonAE 2002 Mammalian germ-line transgenesis by transposition. Proc Natl Acad Sci U S A 99 4495 4499
33. WiesnerSMDeckerSALarsonJDEricsonKForsterC 2009 De novo induction of genetically engineered brain tumors in mice using plasmid DNA. Cancer Res 69 431 439
34. BellJBPodetz-PedersenKMAronovichELBelurLRMcIvorRS 2007 Preferential delivery of the Sleeping Beauty transposon system to livers of mice by hydrodynamic injection. Nat Protoc 2 3153 3165
35. SarideySKLiuLDohertyJEKajaAGalvanDL 2009 PiggyBac transposon-based inducible gene expression in vivo after somatic cell gene transfer. Mol Ther 17 2115 2120
36. NakanishiHHiguchiYKawakamiSYamashitaFHashidaM piggyBac transposon-mediated long-term gene expression in mice. Mol Ther 18 707 714
37. YusaKRadRTakedaJBradleyA 2009 Generation of transgene-free induced pluripotent mouse stem cells by the piggyBac transposon. Nat Methods 6 363 369
38. KahligKMSarideySKKajaADanielsMAGeorgeALJr Multiplexed transposon-mediated stable gene transfer in human cells. Proc Natl Acad Sci U S A 107 1343 1348
39. NakazawaYHuyeLEDottiGFosterAEVeraJF 2009 Optimization of the PiggyBac transposon system for the sustained genetic modification of human T lymphocytes. J Immunother 32 826 836
40. BeroukhimRMermelCHPorterDWeiGRaychaudhuriS The landscape of somatic copy-number alteration across human cancers. Nature 463 899 905
41. JonesSChenWDParmigianiGDiehlFBeerenwinkelN 2008 Comparative lesion sequencing provides insights into tumor evolution. Proc Natl Acad Sci U S A 105 4283 4288
42. JonesSZhangXParsonsDWLinJCLearyRJ 2008 Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science 321 1801 1806
43. LearyRJLinJCCumminsJBocaSWoodLD 2008 Integrated analysis of homozygous deletions, focal amplifications, and sequence alterations in breast and colorectal cancers. Proc Natl Acad Sci U S A 105 16224 16229
44. ParsonsDWJonesSZhangXLinJCLearyRJ 2008 An integrated genomic analysis of human glioblastoma multiforme. Science 321 1807 1812
45. SjoblomTJonesSWoodLDParsonsDWLinJ 2006 The consensus coding sequences of human breast and colorectal cancers. Science 314 268 274
46. WoodLDParsonsDWJonesSLinJSjoblomT 2007 The genomic landscapes of human breast and colorectal cancers. Science 318 1108 1113
47. MeyersonMGabrielSGetzG Advances in understanding cancer genomes through second-generation sequencing. Nat Rev Genet 11 685 696
48. SharplessNEDepinhoRA 2006 The mighty mouse: genetically engineered mouse models in cancer drug development. Nat Rev Drug Discov 5 741 754
49. FiebigHHBergerDPWinterhalterBRPlowmanJ 1990 In vitro and in vivo evaluation of US-NCI compounds in human tumor xenografts. Cancer Treat Rev 17 109 117
50. JohnsonJIDeckerSZaharevitzDRubinsteinLVVendittiJM 2001 Relationships between drug activity in NCI preclinical in vitro and in vivo models and early clinical trials. Br J Cancer 84 1424 1431
51. BrehmMAShultzLDGreinerDL Humanized mouse models to study human diseases. Curr Opin Endocrinol Diabetes Obes 17 120 125
52. LegrandNPlossABallingRBeckerPDBorsottiC 2009 Humanized mice for modeling human infectious disease: challenges, progress, and outlook. Cell Host Microbe 6 5 9
53. ShultzLDIshikawaFGreinerDL 2007 Humanized mice in translational biomedical research. Nat Rev Immunol 7 118 130
54. TakahashiKYamanakaS 2006 Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126 663 676
55. HannaJWernigMMarkoulakiSSunCWMeissnerA 2007 Treatment of sickle cell anemia mouse model with iPS cells generated from autologous skin. Science 318 1920 1923
56. TsujiOMiuraKOkadaYFujiyoshiKMukainoM Therapeutic potential of appropriately evaluated safe-induced pluripotent stem cells for spinal cord injury. Proc Natl Acad Sci U S A 107 12704 12709
57. XuDAlipioZFinkLMAdcockDMYangJ 2009 Phenotypic correction of murine hemophilia A using an iPS cell-based therapy. Proc Natl Acad Sci U S A 106 808 813
58. AlipioZLiaoWRoemerEJWanerMFinkLM Reversal of hyperglycemia in diabetic mouse models using induced-pluripotent stem (iPS)-derived pancreatic beta-like cells. Proc Natl Acad Sci U S A 107 13426 13431
59. WernigMZhaoJPPruszakJHedlundEFuD 2008 Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson's disease. Proc Natl Acad Sci U S A 105 5856 5861
60. YuJVodyanikMASmuga-OttoKAntosiewicz-BourgetJFraneJL 2007 Induced pluripotent stem cell lines derived from human somatic cells. Science 318 1917 1920
61. TakahashiKTanabeKOhnukiMNaritaMIchisakaT 2007 Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131 861 872
62. ParkIHAroraNHuoHMaheraliNAhfeldtT 2008 Disease-specific induced pluripotent stem cells. Cell 134 877 886
63. BricAMiethingCBialuchaCUScuoppoCZenderL 2009 Functional identification of tumor-suppressor genes through an in vivo RNA interference screen in a mouse lymphoma model. Cancer Cell 16 324 335
64. MeachamCEHoEEDubrovskyEGertlerFBHemannMT 2009 In vivo RNAi screening identifies regulators of actin dynamics as key determinants of lymphoma progression. Nat Genet 41 1133 1137
65. ZenderLXueWZuberJSemighiniCPKrasnitzA 2008 An oncogenomics-based in vivo RNAi screen identifies tumor suppressors in liver cancer. Cell 135 852 864
66. JonkersJBernsA 1996 Retroviral insertional mutagenesis as a strategy to identify cancer genes. Biochim Biophys Acta 1287 29 57
67. UrenAGKoolJBernsAvan LohuizenM 2005 Retroviral insertional mutagenesis: past, present and future. Oncogene 24 7656 7672
68. KoolJBernsA 2009 High-throughput insertional mutagenesis screens in mice to identify oncogenic networks. Nat Rev Cancer 9 389 399
69. MikkersHBernsA 2003 Retroviral insertional mutagenesis: tagging cancer pathways. Adv Cancer Res 88 53 99
70. CooleyLKelleyRSpradlingA 1988 Insertional mutagenesis of the Drosophila genome with single P elements. Science 239 1121 1128
71. KlecknerNRothJBotsteinD 1977 Genetic engineering in vivo using translocatable drug-resistance elements. New methods in bacterial genetics. J Mol Biol 116 125 159
72. WuSYingGWuQCapecchiMR 2007 Toward simpler and faster genome-wide mutagenesis in mice. Nat Genet 39 922 930
73. DupuyAJFritzSLargaespadaDA 2001 Transposition and gene disruption in the male germline of the mouse. Genesis 30 82 88
74. FischerSEWienholdsEPlasterkRH 2001 Regulated transposition of a fish transposon in the mouse germ line. Proc Natl Acad Sci U S A 98 6759 6764
75. HorieKKuroiwaAIkawaMOkabeMKondohG 2001 Efficient chromosomal transposition of a Tc1/mariner - like transposon Sleeping Beauty in mice. Proc Natl Acad Sci U S A 98 9191 9196
76. NguyenDXuT 2008 The expanding role of mouse genetics for understanding human biology and disease. Dis Model Mech 1 56 66
77. WangWBradleyAHuangY 2009 A piggyBac transposon-based genome-wide library of insertionally mutated Blm-deficient murine ES cells. Genome Res 19 667 673
78. BenderAMCollierLSRodriguezFJTieuCLarsonJD Sleeping beauty-mediated somatic mutagenesis implicates CSF1 in the formation of high-grade astrocytomas. Cancer Res 70 3557 3565
79. CollierLSAdamsDJHackettCSBendzickLEAkagiK 2009 Whole-body sleeping beauty mutagenesis can cause penetrant leukemia/lymphoma and rare high-grade glioma without associated embryonic lethality. Cancer Res 69 8429 8437
80. CollierLSCarlsonCMRavimohanSDupuyAJLargaespadaDA 2005 Cancer gene discovery in solid tumours using transposon-based somatic mutagenesis in the mouse. Nature 436 272 276
81. DupuyAJAkagiKLargaespadaDACopelandNGJenkinsNA 2005 Mammalian mutagenesis using a highly mobile somatic Sleeping Beauty transposon system. Nature 436 221 226
82. DupuyAJRogersLMKimJNannapaneniKStarrTK 2009 A modified sleeping beauty transposon system that can be used to model a wide variety of human cancers in mice. Cancer Res 69 8150 8156
83. RadRRadLWangWCadinanosJVassiliouG PiggyBac Transposon Mutagenesis: A Tool for Cancer Gene Discovery in Mice. Science
84. KengVWVillanuevaAChiangDYDupuyAJRyanBJ 2009 A conditional transposon-based insertional mutagenesis screen for genes associated with mouse hepatocellular carcinoma. Nat Biotechnol 27 264 274
85. StarrTKAllaeiRSilversteinKAStaggsRASarverAL 2009 A transposon-based genetic screen in mice identifies genes altered in colorectal cancer. Science 323 1747 1750
86. VassiliouGSCooperJLRadRLiJRiceS Mutant nucleophosmin and cooperating pathways drive leukemia initiation and progression in mice. Nat Genet
87. LiangQKongJStalkerJBradleyA 2009 Chromosomal mobilization and reintegration of Sleeping Beauty and PiggyBac transposons. Genesis 47 404 408
88. HuangXGuoHTammanaSJungYCMellgrenE Gene Transfer Efficiency and Genome-Wide Integration Profiling of Sleeping Beauty, Tol2, and PiggyBac Transposons in Human Primary T Cells. Mol Ther
89. ElickTABauserCAFraserMJ 1996 Excision of the piggyBac transposable element in vitro is a precise event that is enhanced by the expression of its encoded transposase. Genetica 98 33 41
90. LiuGAronovichELCuiZWhitleyCBHackettPB 2004 Excision of Sleeping Beauty transposons: parameters and applications to gene therapy. J Gene Med 6 574 583
91. KengVWYaeKHayakawaTMizunoSUnoY 2005 Region-specific saturation germline mutagenesis in mice using the Sleeping Beauty transposon system. Nat Methods 2 763 769
92. GeurtsAMCollierLSGeurtsJLOsethLLBellML 2006 Gene mutations and genomic rearrangements in the mouse as a result of transposon mobilization from chromosomal concatemers. PLoS Genet 2 e156 doi:10.1371/journal.pgen.0020156
93. LeeTLuoL 2001 Mosaic analysis with a repressible cell marker (MARCM) for Drosophila neural development. Trends Neurosci 24 251 254
94. PagliariniRAXuT 2003 A genetic screen in Drosophila for metastatic behavior. Science 302 1227 1231
95. XuTRubinGM 1993 Analysis of genetic mosaics in developing and adult Drosophila tissues. Development 117 1223 1237
96. WhiteRMSessaABurkeCBowmanTLeBlancJ 2008 Transparent adult zebrafish as a tool for in vivo transplantation analysis. Cell Stem Cell 2 183 189
97. ZongHEspinosaJSSuHHMuzumdarMDLuoL 2005 Mosaic analysis with double markers in mice. Cell 121 479 492
Štítky
Genetika Reprodukční medicína
Článek Pervasive Sign Epistasis between Conjugative Plasmids and Drug-Resistance Chromosomal MutationsČlánek Stress-Induced PARP Activation Mediates Recruitment of Mi-2 to Promote Heat Shock Gene ExpressionČlánek Histone Crosstalk Directed by H2B Ubiquitination Is Required for Chromatin Boundary IntegrityČlánek A Functional Variant at a Prostate Cancer Predisposition Locus at 8q24 Is Associated with ExpressionČlánek Replication and Explorations of High-Order Epistasis Using a Large Advanced Intercross Line PedigreeČlánek Expression of Tumor Suppressor in Spermatogonia Facilitates Meiotic Progression in Male Germ Cells
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2011 Číslo 7
-
Všechny články tohoto čísla
- Gene-Based Tests of Association
- The Demoiselle of X-Inactivation: 50 Years Old and As Trendy and Mesmerising As Ever
- Variants in and Underlie Natural Variation in Translation Termination Efficiency in
- SHH1, a Homeodomain Protein Required for DNA Methylation, As Well As RDR2, RDM4, and Chromatin Remodeling Factors, Associate with RNA Polymerase IV
- Genome-Wide Association Study Identifies as a Susceptibility Gene for Pediatric Asthma in Asian Populations
- Pervasive Sign Epistasis between Conjugative Plasmids and Drug-Resistance Chromosomal Mutations
- Genetic Anticipation Is Associated with Telomere Shortening in Hereditary Breast Cancer
- Identification of a Mutation Associated with Fatal Foal Immunodeficiency Syndrome in the Fell and Dales Pony
- Stress-Induced PARP Activation Mediates Recruitment of Mi-2 to Promote Heat Shock Gene Expression
- An Epigenetic Switch Involving Overlapping Fur and DNA Methylation Optimizes Expression of a Type VI Secretion Gene Cluster
- Recombination and Population Structure in
- A Rice Plastidial Nucleotide Sugar Epimerase Is Involved in Galactolipid Biosynthesis and Improves Photosynthetic Efficiency
- A Role for Phosphatidic Acid in the Formation of “Supersized” Lipid Droplets
- Colon Stem Cell and Crypt Dynamics Exposed by Cell Lineage Reconstruction
- Loss of the BMP Antagonist, SMOC-1, Causes Ophthalmo-Acromelic (Waardenburg Anophthalmia) Syndrome in Humans and Mice
- Interactions between Glucocorticoid Treatment and Cis-Regulatory Polymorphisms Contribute to Cellular Response Phenotypes
- Translation Reinitiation Relies on the Interaction between eIF3a/TIF32 and Progressively Folded -Acting mRNA Elements Preceding Short uORFs
- DAF-12 Regulates a Connected Network of Genes to Ensure Robust Developmental Decisions
- Adult Circadian Behavior in Requires Developmental Expression of , But Not
- Histone Crosstalk Directed by H2B Ubiquitination Is Required for Chromatin Boundary Integrity
- Proteins in the Nutrient-Sensing and DNA Damage Checkpoint Pathways Cooperate to Restrain Mitotic Progression following DNA Damage
- Complex Evolutionary Events at a Tandem Cluster of Genes Resulting in a Single-Locus Genetic Incompatibility
- () and Its Regulated Homeodomain Gene Mediate Abscisic Acid Response in
- A Functional Variant at a Prostate Cancer Predisposition Locus at 8q24 Is Associated with Expression
- LGI2 Truncation Causes a Remitting Focal Epilepsy in Dogs
- Adaptations to Endosymbiosis in a Cnidarian-Dinoflagellate Association: Differential Gene Expression and Specific Gene Duplications
- The Translation Initiation Factor eIF4E Regulates the Sex-Specific Expression of the Master Switch Gene in
- Somatic Genetics Empowers the Mouse for Modeling and Interrogating Developmental and Disease Processes
- Molecular Mechanisms Generating and Stabilizing Terminal 22q13 Deletions in 44 Subjects with Phelan/McDermid Syndrome
- Replication and Explorations of High-Order Epistasis Using a Large Advanced Intercross Line Pedigree
- Mechanisms of Chromosome Number Evolution in Yeast
- Regulatory Cross-Talk Links Chromosome II Replication and Segregation
- Ancestral Genes Can Control the Ability of Horizontally Acquired Loci to Confer New Traits
- Expression of Tumor Suppressor in Spermatogonia Facilitates Meiotic Progression in Male Germ Cells
- Rare and Common Regulatory Variation in Population-Scale Sequenced Human Genomes
- The Epistatic Relationship between BRCA2 and the Other RAD51 Mediators in Homologous Recombination
- Identification of Novel Genetic Markers Associated with Clinical Phenotypes of Systemic Sclerosis through a Genome-Wide Association Strategy
- NatF Contributes to an Evolutionary Shift in Protein N-Terminal Acetylation and Is Important for Normal Chromosome Segregation
- Araucan and Caupolican Integrate Intrinsic and Signalling Inputs for the Acquisition by Muscle Progenitors of the Lateral Transverse Fate
- Pathologic and Phenotypic Alterations in a Mouse Expressing a Connexin47 Missense Mutation That Causes Pelizaeus-Merzbacher–Like Disease in Humans
- Recombinant Inbred Line Genotypes Reveal Inter-Strain Incompatibility and the Evolution of Recombination
- Epistatic Relationships in the BRCA1-BRCA2 Pathway
- Genome-Wide Association Study Identifies Novel Restless Legs Syndrome Susceptibility Loci on 2p14 and 16q12.1
- Genetic Loci Associated with Plasma Phospholipid n-3 Fatty Acids: A Meta-Analysis of Genome-Wide Association Studies from the CHARGE Consortium
- Fine Mapping of Five Loci Associated with Low-Density Lipoprotein Cholesterol Detects Variants That Double the Explained Heritability
- CHD1 Remodels Chromatin and Influences Transient DNA Methylation at the Clock Gene
- Nonlinear Fitness Landscape of a Molecular Pathway
- Genome-Wide Scan Identifies , , and as Novel Risk Loci for Systemic Sclerosis
- Quantitative and Qualitative Stem Rust Resistance Factors in Barley Are Associated with Transcriptional Suppression of Defense Regulons
- A Systematic Screen for Tube Morphogenesis and Branching Genes in the Tracheal System
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Genome-Wide Association Study Identifies Novel Restless Legs Syndrome Susceptibility Loci on 2p14 and 16q12.1
- Loss of the BMP Antagonist, SMOC-1, Causes Ophthalmo-Acromelic (Waardenburg Anophthalmia) Syndrome in Humans and Mice
- Gene-Based Tests of Association
- Genome-Wide Association Study Identifies as a Susceptibility Gene for Pediatric Asthma in Asian Populations
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



