-
Články
Reklama
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
ReklamaSingle Nucleus Genome Sequencing Reveals High Similarity among Nuclei of an Endomycorrhizal Fungus
Nuclei of arbuscular endomycorrhizal fungi have been described as highly diverse due to their asexual nature and absence of a single cell stage with only one nucleus. This has raised fundamental questions concerning speciation, selection and transmission of the genetic make-up to next generations. Although this concept has become textbook knowledge, it is only based on studying a few loci, including 45S rDNA. To provide a more comprehensive insight into the genetic makeup of arbuscular endomycorrhizal fungi, we applied de novo genome sequencing of individual nuclei of Rhizophagus irregularis. This revealed a surprisingly low level of polymorphism between nuclei. In contrast, within a nucleus, the 45S rDNA repeat unit turned out to be highly diverged. This finding demystifies a long-lasting hypothesis on the complex genetic makeup of arbuscular endomycorrhizal fungi. Subsequent genome assembly resulted in the first draft reference genome sequence of an arbuscular endomycorrhizal fungus. Its length is 141 Mbps, representing over 27,000 protein-coding gene models. We used the genomic sequence to reinvestigate the phylogenetic relationships of Rhizophagus irregularis with other fungal phyla. This unambiguously demonstrated that Glomeromycota are more closely related to Mucoromycotina than to its postulated sister Dikarya.
Published in the journal: . PLoS Genet 10(1): e32767. doi:10.1371/journal.pgen.1004078
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004078Summary
Nuclei of arbuscular endomycorrhizal fungi have been described as highly diverse due to their asexual nature and absence of a single cell stage with only one nucleus. This has raised fundamental questions concerning speciation, selection and transmission of the genetic make-up to next generations. Although this concept has become textbook knowledge, it is only based on studying a few loci, including 45S rDNA. To provide a more comprehensive insight into the genetic makeup of arbuscular endomycorrhizal fungi, we applied de novo genome sequencing of individual nuclei of Rhizophagus irregularis. This revealed a surprisingly low level of polymorphism between nuclei. In contrast, within a nucleus, the 45S rDNA repeat unit turned out to be highly diverged. This finding demystifies a long-lasting hypothesis on the complex genetic makeup of arbuscular endomycorrhizal fungi. Subsequent genome assembly resulted in the first draft reference genome sequence of an arbuscular endomycorrhizal fungus. Its length is 141 Mbps, representing over 27,000 protein-coding gene models. We used the genomic sequence to reinvestigate the phylogenetic relationships of Rhizophagus irregularis with other fungal phyla. This unambiguously demonstrated that Glomeromycota are more closely related to Mucoromycotina than to its postulated sister Dikarya.
Introduction
The interaction of arbuscular endomycorrhizal (AM) fungi and land plants is a very successful symbiosis as it is ancient (∼450 million years), and maintained by the vast majority of plant species [1]. AM fungi are obligate biotrophs that infect roots and form highly branched structures (arbuscules) inside root cortical cells [1]. These arbuscules are connected to an extensive network of extraradical mycelium that facilitates uptake of nutrients from the soil, e.g. immobile phosphates.
AM hyphal networks form a continuous coenocytic compartment with numerous nuclei. AM fungi are considered to be ancient asexual organisms [2]–[4] and propagation occurs via spores that become filled with multiple nuclei that subsequently divide [5]. AM fungal individuals can be heterokaryotic, i.e. consist of genetically divergent nuclei, because single nucleus cellular stages never occur during the lifecycle, and because hyphae of different fungal individuals can fuse and exchange nuclei by anastomosis [6], [7]. Our knowledge of the genome structure of AM fungi is rudimentary. For instance, the degree to which a minimal gene set is present in a single nucleus, or is distributed over genetically distinct nuclei is unknown [2], [8]–[11]. Although there is evidence for genetic variability within single spores, the genomic organization of this variation remains elusive. Two competing hypotheses have been advocated. The genetic variation may be present in a single, possibly polyploid, nucleus [9], or it could be distributed over multiple nuclei in a single individual [8], [10]. However, in reality these hypotheses may represent extremes along a continuum of genetic variation among and within nuclei [2].
Extensive efforts to sequence the genome of the reference AM fungal species Rhizophagus irregularis DAOM197198 (previously known as Glomus intraradices [12], [13] have not been successful, possibly because of its heterokaryotic nature [14]. To address this issue and determine the extent to which nuclei are indeed markedly different, we conducted de novo genome sequencing of individual nuclei of an R. irregularis line isolated from the reference strain DAOM197198 (designated DAOM197198w). The resulting R. irregularis genome sequence revealed a surprisingly low level of polymorphism between nuclei.
Results and Discussion
Genome sequencing of individual nuclei reveals that R. irregularis is homokaryotic
Spores of a mycorrhized root culture of chicory (Cichorium intybus) were stained by 10 µM Sytox Green (Fig. 1A). Single nuclei were collected from a supernatant of crushed spores using a micromanipulator (Fig. 1B). Individual nuclei were immediately processed for whole genome amplification. To verify the quality of the amplified nuclear DNA ten randomly selected loci were PCR amplified, and also the extent of bacterial contamination was monitored. Four amplified single nucleus genomes were processed for sequencing, resulting in assembled genomes of 115, 90, 71 and 95 Mbps, respectively (Tables S1 and S2). The different sizes of the assemblies are likely reflecting variation in the whole genome amplification efficiencies among the four samples. First comparative analyses detected surprisingly few SNPs and indels across the four nuclei. This suggested that nuclei are markedly more similar than was expected. Therefore we decided to sequence also two DNA samples extracted from mycelium. The generated sequences of these DNA samples (designated DNA1 and DNA2) were assembled individually, resulting in genome assemblies of 116 and 117 Mbps, respectively (Table 1). Additionally, the six genome sequences were assembled together resulting in a reference genome for R. irregularis of 141 Mbps. A self-alignment of this reference genome revealed little redundancy ruling out the occurrence of (significant) artificial duplifications within the assembly (Fig. S1). By comparative genomic analysis, only 28,872 SNPs and 12,315 indels were detected across the six assemblies when compared to the reference genome (Fig. 1C, Table S2). Furthermore, a reference-independent comparison of the four single nuclei and the two mycelial samples also revealed a comparable low level of polymorphisms (Table S3). This indicates that more than 99.97% of the (aligned) genome sequence is identical between different nuclei. Furthermore, as the size of the assembled genome is in line with previous estimates of the DNA content of nuclei [15], we conclude that R. irregularis nuclei are haploid.
Fig. 1. Genome sequence of single R. irregularis DAOM197198w nuclei. 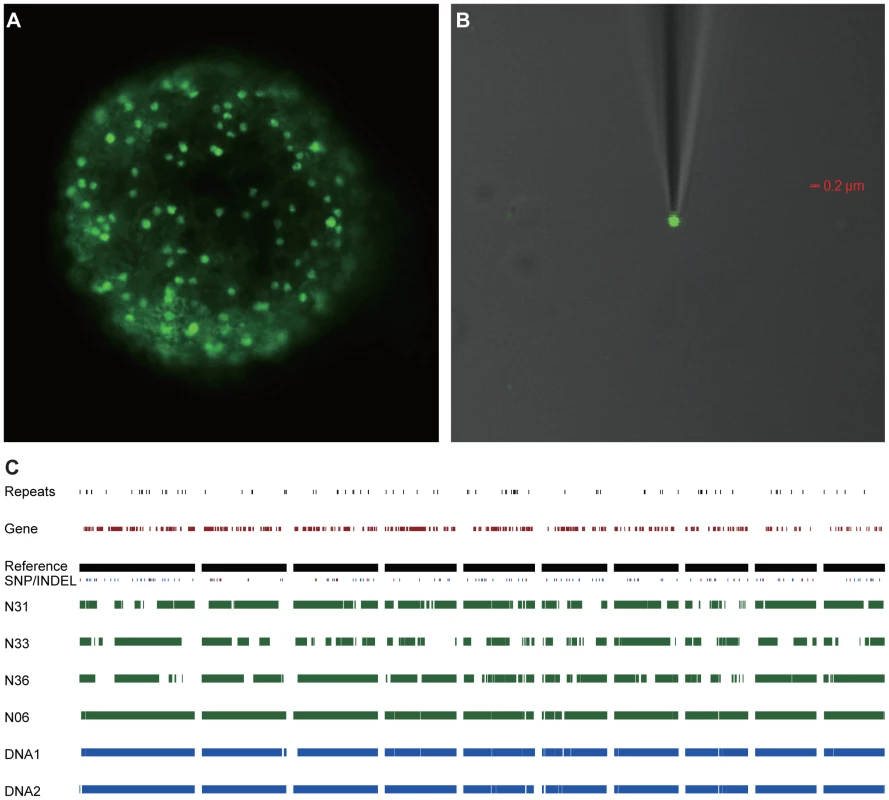
(A) Sytox Green stained spore containing numerous nuclei. (B) Single Sytox-stained nucleus trapped with a micropipette. (C) Level of homology between four individual nuclei (N6, N31, N33 and N36) and 2 mycelium DNA samples (DNA1 and DNA2). Presented are the 10 largest contigs of the reference genome (representing ∼1,278 kb). The occurrence of SNPs (marked in blue) and INDELs (marked in red), and gene distributions, in the different assemblies are indicated. Tab. 1. Characteristics of the seven genome assemblies from R. irregularis DAOM197198w. 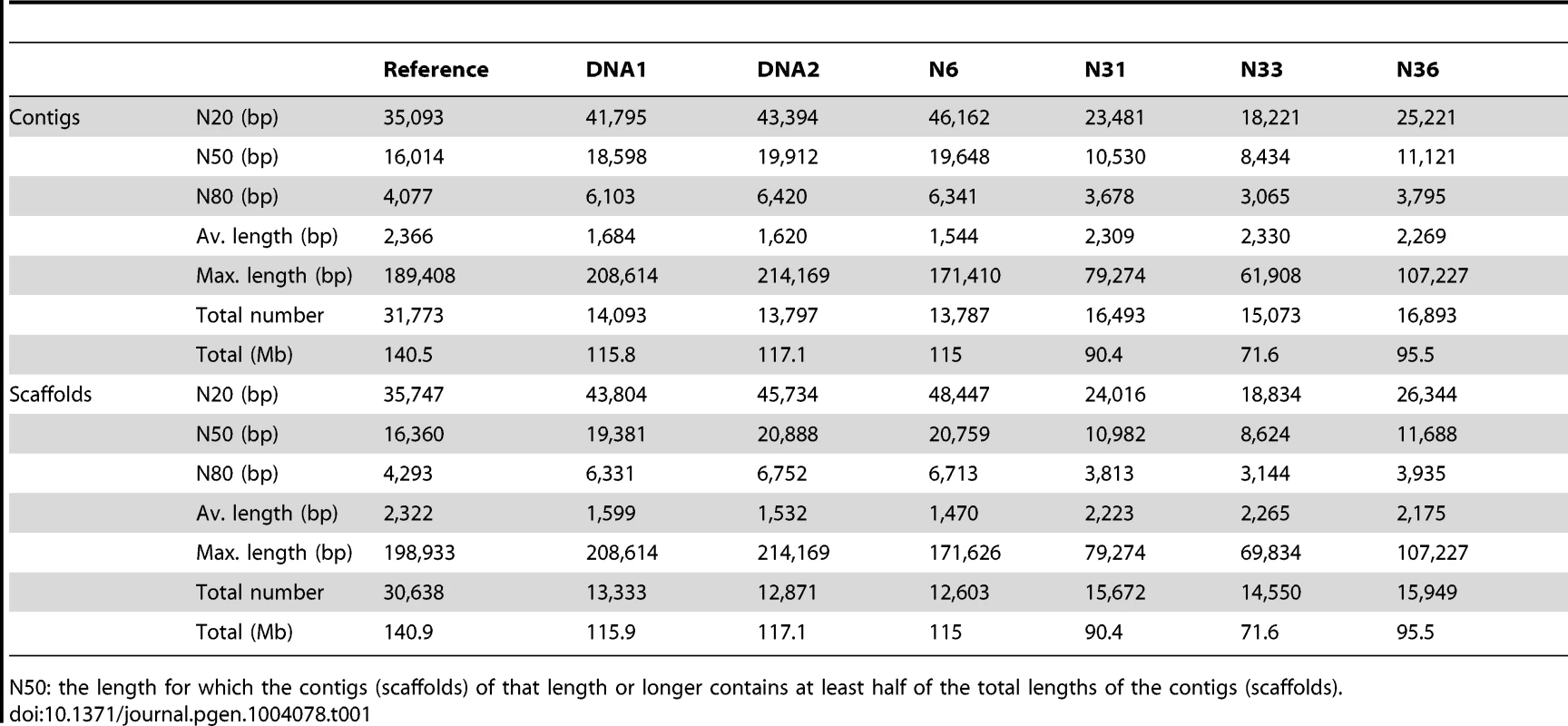
N50: the length for which the contigs (scaffolds) of that length or longer contains at least half of the total lengths of the contigs (scaffolds). Several loci have previously been used to determine genetic polymorphisms within AM individuals. These include Binding Protein (BIP), SSR marker Bg112, the internal transcribed spacers (ITS1 and ITS2) of the 45S rDNA locus in R. irregularis and POL1-Like Sequence (PLS) in Glomus etunicatum [8], [9], [16]. We compared these loci in the different genome assemblies. Only a single PLS homolog was identified in R. irregularis (RiPLS, RirG174000), whereas G. etunicatum has multiple copies that belong to two main types, of which the highly polymorphic PLS1 likely represents a pseudogene [9], [17]. No polymorphisms were found for RiPLS in the different assemblies (Fig. S2). For BIP three loci were identified and designated RiBIP1 (RirG196040), RiBIP2 (RirG160690) and RiBIP3 (RirG043980). Sequence and structure of these genes is highly conserved and homologous to a Rhizopus delemar 70 kD Heat shock protein (GenBank: EIE83965). RiBIP1, RiBIP2 and RiBIP3 are present also in nucleus 6 without allelic variation when compared to the DNA1 and DNA2 genome assemblies. This holds true also for the other three sequenced nuclei, though not all three BIP loci were covered in the genome assemblies, which can be attributed to incomplete amplification (Fig. S3). Next, we studied Bg112 for which three loci were identified. Again, no allelic variation was detected among the four nuclei (Fig. S4). The polymorphism of the ITS region of the multi-copy 45S rDNA locus was studied within each of the 4 nuclei. By mapping sequence reads to a reference R. irregularis ITS sequence (Genbank JF439109), many variants reported previously for strain DAOM197198 were identified within individual nuclei (Fig. 2) [8], [12]. This demonstrates that, in addition to reported intraspecific ITS variability within single R. irregularis spores [12], [18], the ITS region in the multi-repeat 45S rDNA locus is extremely variable even within individual nuclei, and that different nuclei can show quantitative variation in polymorphic ITS variants. In general, multi-repeat loci such as rDNA sequences are thought to be homogenized through concerted evolution [19], which presumably is most effective during meiosis [20], [21]. Therefore, the high level of heterogeneity among the copies within a single repeat seems to be consistent with ancient asexuality. However, also in several sexual fungal species varying levels of intra-individual polymorphism have been found [22], and R. irregularis may be an extreme case, although exact percentages cannot be deduced from the Illumina read data. Given the high level of ITS variability within single nuclei, we conclude that the 45rDNA ITS sequence is less suited for comparative studies of Glomeromycota. Based on the whole genome comparison of individual nuclei we conclude that the organization of the R. irregularis genome of the used reference culture DAOM197198w is basically homokaryotic. The high divergence observed among copies of the 45S rDNA repeat occurs within a single nucleus, indicating that this region is unsuited to claim that nuclei within a strain are highly divergent [8]. However, the presence of a low level of polymorphisms suggests that genetically, slightly divergent nuclei can arise and coexist in a single mycelium.
Fig. 2. Overview of polymorphisms in the R. irregularis 45S rDNA repeat unit in four individual nuclei. 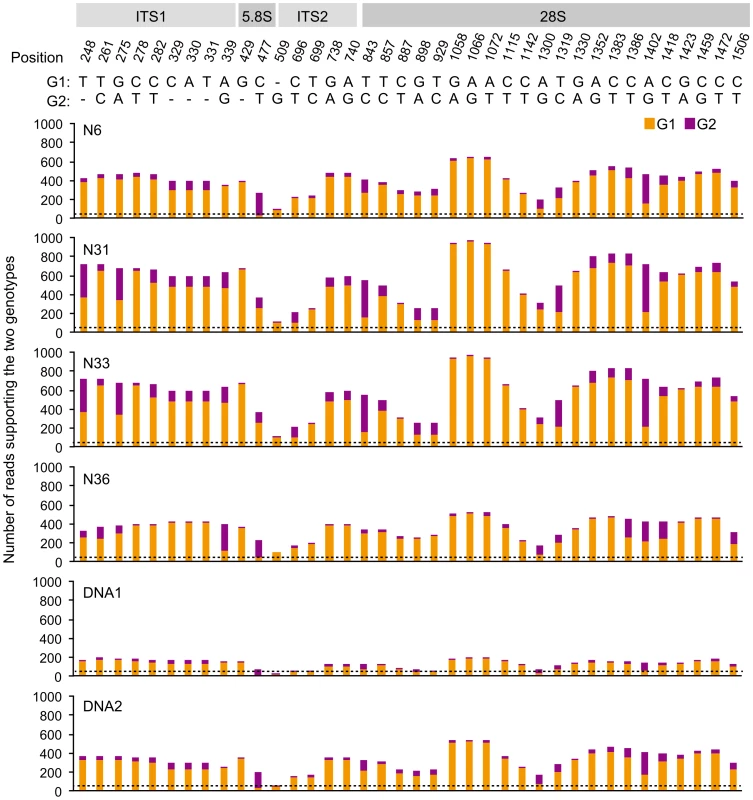
The top part indicates the various regions within the R. irregularis DAOM197198 45S rDNA reference sequence (Genbank JF439109). Position means the position of each polymorphic site on the reference. G1: genotype identical to reference; G2: polymorphic nucleotide. The six histograms show the numbers of sequenced reads supporting the two genotypes for N6, N31, N33, N36 and mycelium DNA samples DNA1 and DNA2. The dashed lines indicate the average sequencing depth for each sample. Genetic make-up of R. irregularis
The reference genome assembly of DAOM197198w covers about 97% of the current R. irregularis EST collection [23] indicating that it represents nearly the complete genic region of the genome. This is further supported by a survey of core eukaryotic genes (CEG), which shows that among the 248 CEG proteins 229 (92.3%) are included in the predicted protein-coding genes (Table S4). Genome annotation using EVidenceModeler resulted in 27,392 protein-coding gene models representing 30,003 putative transcripts. Of these models 11,145 are supported by at least one R. irregularis EST, whereas an additional 5,586 protein-coding gene models find support by homology to available protein sequences. Using an AHRD functional annotation pipeline we could assign putative functions to 14,073 protein-coding gene models (Table S5). To obtain insight into the R. irregularis gene repertoire a comparative approach using OrthoMCL was conducted on 10 species representing all five fungal phyla (Fig. 3). This resulted in 19,300 putative orthology groups (Table S6), of which 1,370 contained exclusively R. irregularis gene models that may represent genes unique for AM fungi (14,742 gene models in total). Of these 6,014 were functionally annotated (Table S7). A summary of the top ten Interpro domains is shown in Table S8. Interestingly, about 28% of these putative genes are predicted to encode proteins with a kinase domain, underling a striking overrepresentation of these signaling proteins in the R. irregularis genome. The second largest group (∼25%) that seems to be enriched especially in R. irregularis are BTB domain containing proteins (BTB-POZ (PF00651) and BTB-Kelch (BACK; PF07707)). Both findings are supported a recent transcriptome study [23].
Fig. 3. ML tree derived from the concatenation of 35 widespread, single-copy genes. 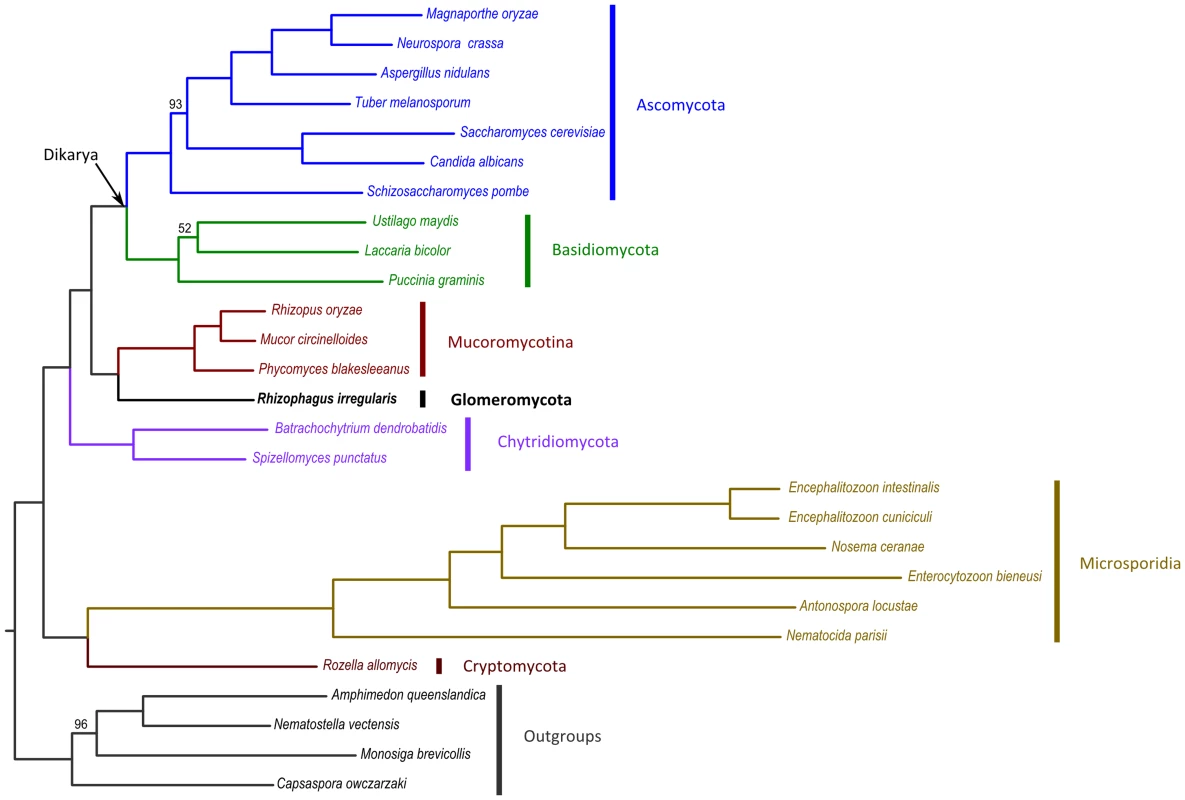
The amino acid alignment was trimmed as explained in the Materials and methods section to remove non-informative positions, resulting in 26,604 positions. The tree was estimated using the rtREV evolutionary model implemented in RAxML. Bootstrap analysis was performed based on 100 replicates, and the three nodes with support below 100 are indicated. Scale bar indicates average number of amino acid substitutions per site. We observed a high level of putative/predicted (retro-)transposable (TE) elements in the R. irregularis genome. In addition to well-known TE classes, representing 1.1% of the genome based on the Repbase [24] TE library (Table S9), potential novel TE repeats were identified, revealing that TE repeats represented ∼40% of the genome (Table S10). The presence of potential deleterious TE elements is difficult to reconcile with the ancient asexuality of Glomeromycota, as an uncontrolled accumulation of such elements would cause a deleterious load that leads to extinction [25], [26]. Therefore, the presence of such TE elements [27], together with the identification of meiotic recombination proteins [3] and signatures of recombination within populations [28]–[30], argues for the potential rare occurrence of so far unidentified sexual reproduction in R. irregularis [25], [26]. As an alternative, parasexual cycles where nuclei fuse and undergo recombination, together with observed exchange of nuclei through anastomoses, may explain both the spread of TE elements as well as restrain their intragenomic proliferation [2], [11].
Glomeromycota are related to Mucoromycotina
We noted that the gene repertoire of R. irregularis overlaps the most with the repertoire of sequenced Mucoromycotina species. Mucoromycotina have traditionally been classified as Zygomycota, which also have coenocytic hyphae, similarly as those in AM fungi. In general they are saprotrophic fungi, but some isolates can also act as opportunistic pathogens. A reconstruction of the early evolution of fungi largely based on the 45S rDNA locus suggested that the Zygomycota phylum is paraphyletic and that Glomeromycota are sister to the Dikarya phyla Ascomycota and Basidiomycota [31]. However, this has only limited statistical support, and analyses based on protein coding genes gave conflicting results [3], [32], [33]. As our data, together with that from others [12], [18] revealed that the 45S rDNA locus of R. irregularis is highly polymorphic we reinvestigated the phylogenetic relationships of R. irregularis within the fungi. To do so, we analysed a supermatrix of 35 highly conserved, putative single copy nuclear genes proposed by Capella-Gutiérez et al. [34], totaling a concatenated length of 26,604 aligned amino acids from 23 fungal species and 4 outgroups (Table S11). Phylogenetic analysis of this supermatrix using maximum-likelihood (ML) revealed that R. irregularis is related to Mucoromycotina rather than to the Dikarya phyla Basidiomycota and Ascomycota (Fig. 3). This phylogenetic placement of R. irregularis received maximal bootstrap support (100%; Fig. 3) and alternative placements resulted in significantly lower likelihoods (p< = 0.004; see Table S12). This finding is in concordance with gene repertoire reconstructions presented here, as well as phylogenetic studies based on genes encoding (meiotic) DNA repair proteins [3], [35], [36]. We note, however, that our taxonomic sampling includes Mucorales only. Additional lineages within Mucoromycotina (i.e. Mortierellales, Endogonales) and especially other currently unplaced subphyla traditionally classified as Zygomycota (e.g. Kickxellomycotina, Zoopagomycotina, Entomophthoromycotina) may better resolve the precise relationships of R. irregularis, as genome sequences for these members will become available in the future.
R. irregularis has a relatively small repertoire of effector-like proteins
In comparison to pathogenic fungi, AM fungi have an extremely broad host range. Pathogenic fungi suppress defence responses of their host by secreting effectors that interfere with this defence. This raises the question whether a particular repertoire of secreted putative effector proteins underlies the broad host range of AM fungi. From the deduced proteome of 30,003 putative proteins, we predicted the secretome to contain 299 proteins (1% of proteome) using stringent bioinformatics criteria, and 566 proteins (1.9% of proteome) using more relaxed criteria (Table S13). In relative sense, this is rather low compared with averages of other fungal secretomes such as plant pathogens (7.4%), animal pathogens (4.7%), and non-pathogens (5.3%) (Fig. 4). It is remarkable that AM fungi are able to colonize a broad range of plants despite the fact that it has a small secretome suggesting more research is needed on the effectors. The relative small secretome may have resulted from adaptation to a symbiotic lifestyle in which the secretome has been streamlined through the loss of unnecessary secreted protein genes. The proteins in the R. irregularis secretome identified with relaxed criteria were grouped into 254 tribes based on sequence similarity, annotated, and ranked based on potential effector features (Table S13). The top 100 tribes that are likely to contain effectors highlighted five protein tribes containing thirteen sequences with similarity to the known R. irregularis effector protein SP7 (Fig. 5) [37]. Alignment of these protein sequences identified conserved features also present in SP7 (Fig. S5), indicating that these proteins are good candidates to display effector functionality. To further analyze potential R. irregularis specific features, we compared the number of predicted secreted proteins of R. irregularis in each tribe with those of selected pathogenic and symbiotic fungi (Fig. S6). A survey of top 100 tribes, containing 16–134 members, revealed that R. irregularis was represented in only 26 tribes compared to for example 76 tribes and 64 tribes for the fungi Magnaporthe oryzae and Laccaria bicolor, respectively. This suggests that not only the secretome of R. irregularis is reduced, but also that it is missing some secreted proteins that are present in other fungi compared in this analysis. However, there is a 22-member tribe composed of R. irregularis proteins only (Tribe 62 based on the numbering of Fig. S6, equivalent to the largest R. irregularis Tribe 1 of Table S13). It is tempting to speculate that such effectors play important roles in the AM symbiosis.
Fig. 4. Comparison of secretomes of R. irregularis and other 43 fungi. 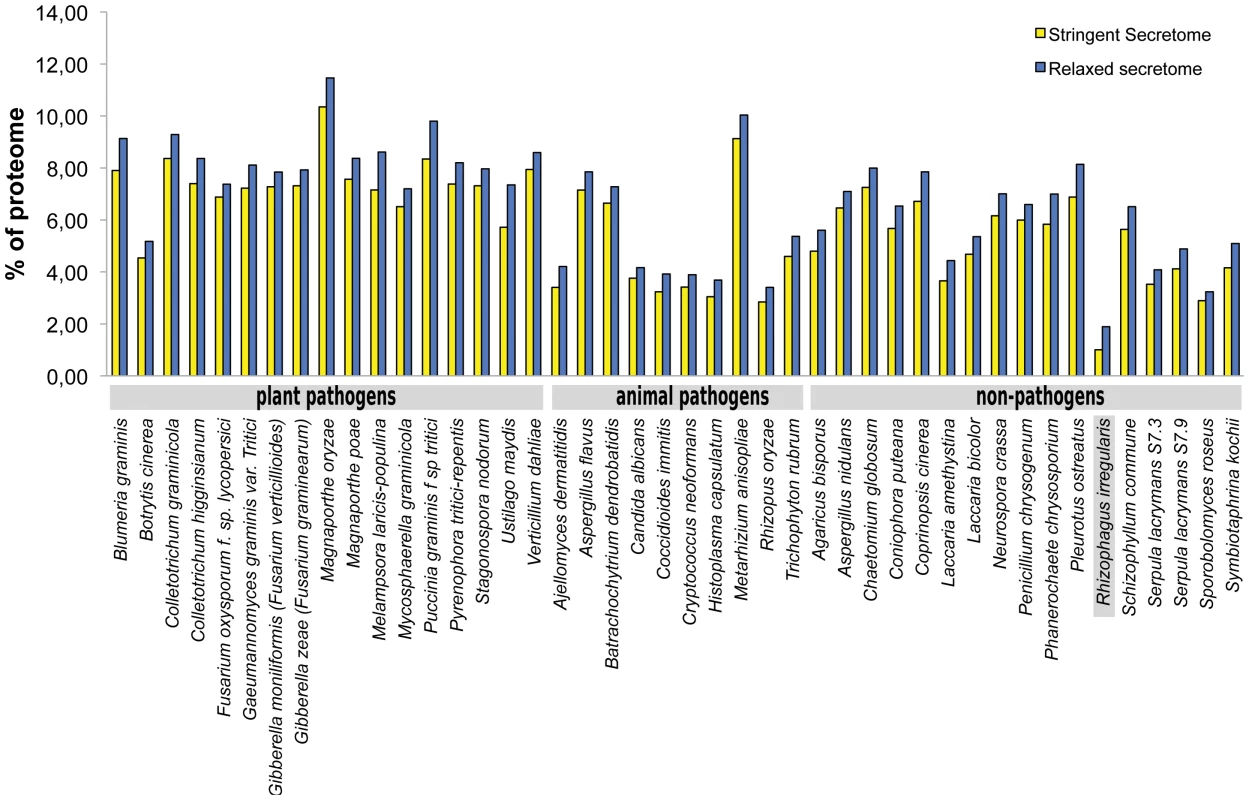
Percentage of predicted proteome representing putative effectors, using stringent (lacking transmembrane domains; yellow bars) or relaxed criteria (including proteins with predicted single transmembrane domain that overlapped with the signal peptide; blue bars). Fig. 5. Top 100 ranked protein tribes containing putative effector candidates. 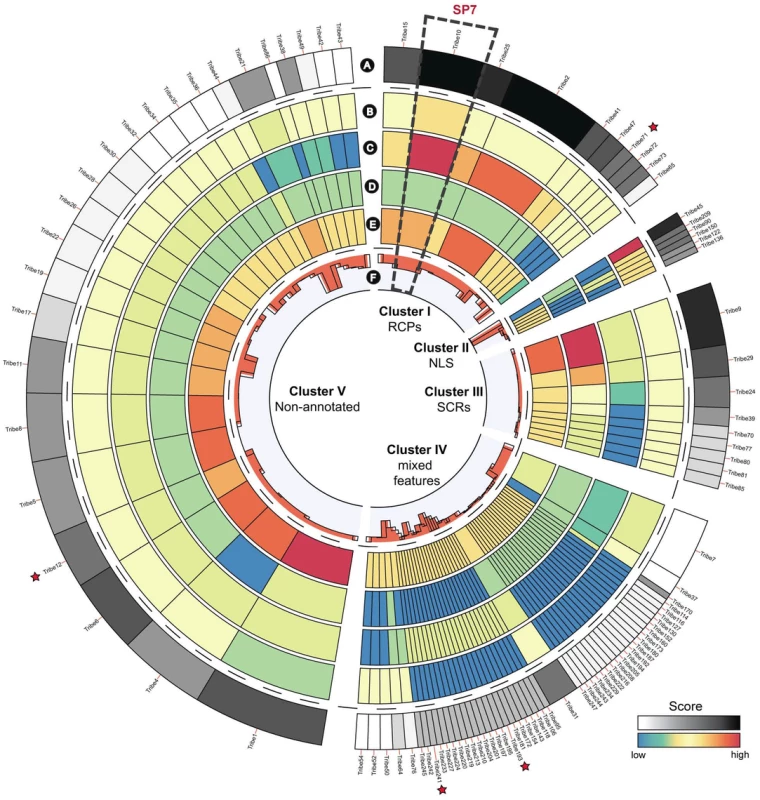
Clusters were determined using hierarchical clustering of the top 100 ranked tribes containing putative effector candidates. A. Rank associated with each tribe based on their content of effector features. B. Score for number of members containing a nuclear localization signals (NLS). C. Score for number of members classified as repeat containing (RCPs). D. Score reflecting number of members classified as small and cysteine rich (SCRs). E. Score for number of members not annotated by searches against swissprot. F. Average protein sequence length for tribe members (ranging from 55 to 856 amino acids). Stars indicate tribes that contain members with similarity to the characterised effector SP7. Among the putative effectors, a protein with a so-called Crinkler (CRN) domain was present (RirT087480; tribe 245). Secreted CRN domain effectors are abundantly present in oomycete plant pathogens of the Phytophthora genus [38], [39]. We searched the R. irregularis deduced proteome for proteins containing CRN domains using amino acid sequences of canonical CRN proteins from the potato blight pathogen Phytophthora infestans as query. This resulted in 42 sequences with positive scores for the so-called N-terminal LFLAK domain that is common to all CRN proteins (Table S14). Within this set, we also identified additional CRN domains (Fig. S7, Table S14). Among these 42 CRN-like proteins, only five have a putative signal peptide, similar as the canonical CRN proteins from P. infestans. Similar CRN domain effector-like proteins were identified in the Chytrid fungus Batrachochytrium dendrobatidis, but not yet in other sequenced fungal genomes. This led to speculations of horizontal acquisitions of these genes by this pathogenic fungus [40]. However, the occurrence of CRN genes in the R. irregularis genome makes a vertical descent equally well possible, and indicates that these proteins are encoded by an ancient eukaryotic gene family.
Conclusion
Genome sequencing of individual cells has previously been used for example to determine the genome of individual cancer cells [41]. However in these cases a reference genome was already available. Our study shows that it is possible to obtain a de novo genome sequence starting from a single haploid nucleus. This approach can be attractive for genomes of species with high heterozygocity that are notoriously difficult to assemble. We applied a single nucleus genome sequence approach on the AM fungus R. irregularis and provide solid evidence for the occurrence of homokaryosis in this strain. This demystifies the long lasting hypothesis that nuclei of a single Rhizophagus isolate are markedly different. The sequences of four nuclei, in combination with the reference genome sequence will provide the basis for future studies on AM fungi to address issues such as genetic selection, long-term persistence of asexuality, obligate endosymbiosis, adaptation to host plants and suppression of plant defense.
Materials and Methods
Isolation of nuclei, DNA extraction and whole genome amplification
A monoxenic culture of Agrobacterium rhizogenes (RiT-DNA) transformed chicory (Cichorium intybus) roots mycorrhized with the fungus R. irregularis DAOM197198 was obtained from Dr. Paola Bonfante and Dr. Andrea Genre (University of Torino) (originally obtained from GINCO (MUCL 43194)). This root culture was designated DAOM197198w and grown in a split-plate setup, where the fungus is allowed to grow into a compartment containing liquid M medium to allow easy collection of spores and extraradical mycelium [42].
Genomic R. irregularis DNA, used for meta-genome sequencing, was isolated from extraradical mycelium containing spores using the DNeasy Plant kit (Qiagen). Mycelium containing spores was washed 10× in sterile water. Spores were carefully teased out using forceps, washed by transferring through a series of (at least 5) sterile water droplets, and finally transferred to a small drop of 10 µM Sytox Green (Invitrogen) in Citifluor (Citifluor Ltd). To release the nuclei, spores were crushed using a teflon coated dounce and transferred to an eppendorf tube. The volume was adjusted to 25 µl with 10 µM Sytox Green. To remove cell debris, the crushed spore suspension was centrifuged for 1 min. at 4000 rpm.
Spore suspensions were loaded onto cover slips, from which individual nuclei were collected using a Narishige micromanipulator mounted to an inverted PASCAL Zeiss Confocal Laser Scanning microscope (excitation 480 nm; emission 505–530 nm). Individual isolated nuclei were transferred to a PCR tube containing 5 µl 1× ALB (200 mM KOH, 0.5 mM DTT) buffer, by breaking the tip of the glass microinjection needle containing the captured nucleus.
Whole genome amplification (WGA) was performed using the REPLI-g UltraFast midi-kit (Qiagen) according to the manufacturers instructions. Amplified DNA was diluted 100×. To verify the efficiency of the WGA a set of 10 selected amplicons was amplified using Premix Taq (Ex TaqVersion 2.0) polymerase (Takara Bio Inc). Amplicons could not be amplified from WGA-amplified control suspension lacking single nuclei. The extent of contamination of the WGA amplified DNA with bacterial DNA was checked by amplification of 16S rDNA amplicons. Primers for selected amplicons are listed in Table S15. From in total 40 WGA samples, 4 samples that allowed amplification of the selected R. irregularis amplicons and showed minimal bacterial contamination were selected for Illumina sequencing.
Illumina sequencing and assembly
Library construction and sequencing
The amplified DNAs were sheared into fragments of about 350 bp, using an ultrasonicator (Covaris), to construct a paired-end sequencing library for each sample according to the manufacturer's instructions (Illumina). All libraries were paired-end sequenced with a read length of 90 bp for each end on the Illumina Hiseq 2000. The duplicated reads, low-quality and adaptor sequences from each library were removed (Table S1).
Assembling for each sample
Paired-end reads from each sample were separately assembled by employing k-mer of optimized length (N31 : 59; N33 : 63; N36 : 59; N6 : 60; DNA1 : 63; DNA2 : 63) using SOAPdenovo2 [43]. Then, all paired-end reads were aligned to the assembled contigs. If two contigs were connected by more than 3 read pairs, they were constructed into a scaffold. Only the scaffolds with the length >100 bp were remained in the final assembly. In addition, the quality of each base was corrected by mapping the reads onto the assembly.
Assembling all reads from the six samples
A total of 21.5 Gb raw sequence data representing 150-fold coverage of R. irregularis genome were generated for the six samples. To reduce the sequencing errors to a large extent and to facilitate the assembly of the sequencing data from different samples, we also performed error correction using k-mer frequency spectrum. We used the MSR-CA assembler version 1.6 (ftp://ftp.genome.umd.edu/pub/MSR-CA/), which combines the advantage of de Bruijn graph and Overlap-Layout-Consensus assembly approaches, to generate the reference genome assembly. During the assembly, the program will compute the optimal k-mer size based on the read data and GC content (25–101 bp are supported). All contigs with the length of less than 200 bp were excluded in the final assembly.
Genome and gene functional annotation
Masking repeats
The genomic scaffolds were masked using RepeatMasker (http://www.repeatmasker.org, version 3.3.0) and the Repbase TE library [24] for identifying transposable elements across the genome. We found that the percentage of known transposable elements in the genome was about 1.1%.
Three software packages, PILER (version 1.0) [44], LTR_FINDER (version 1.05) [45], and RepeatScout (version 1.0.5) [46], were used to identify de novo repetitive elements in the reference genome, which was previously masked with Repbase TE library (version 20120418). Firstly, repetitive elements which belong to rRNA or satellites were filtered using BLASTN with parameters of E-value≤1e-10, identity ≥80%, coverage ≥50%, and match length ≥100 bp. Secondly, if comparison of two identified repeats met the criteria of E-value≤1e-10, identity ≥80%, coverage ≥80%, and match length ≥100 bp, then the shorter one was excluded. Through these two filtering steps, a non-redundant de novo transposable elements database was generated. Finally, RepeatMasker (version 3.3.0) was used to re-mask the reference genome with this de novo transposable elements' database, and we identified ∼40% transposable elements in the reference genome.
Identification of putative variants among a group of samples using Cortex pipeline
Cortex, designed for reference-free variant calling by de novo assembly of multiple samples, allows directly comparing samples without using a reference genome [47], [48]. We applied Cortex to data from (1) the four single nucleus samples, (2) the two mycelium samples, respectively. Thus, we could compare both results without a reference. We used the joint discovery workflow to directly compare all samples from the same group by using the Bubble Caller algorithm. In this workflow, we set the reference to be “Absent”, meaning that no reference was loaded into the graph and a fake reference is used to get the coordinates of variants. In addition, as suggested, we set k = 31(low k-mer for relatively low coverage at these sites) and k = 61(high k-mer for genome repeat content/genome complexity) to make different variants accessible.
Prediction of protein-coding genes
EVidenceModeler (EVM, version r03062010) [49], which is a nonstochastic weighted evidence evaluation system to produce consensus gene structure, was used to combine the alignments of proteins and transcripts to the genomic sequences, and various de novo predictions into a predicted gene set. A more detailed explanation as follows. Firstly, we processed evidence at the transcript level. Spaln (version 1.4.4) [50] mapped the fungal ESTs downloaded from NCBI (Sep 2012) onto our assembled genome and mapping by PASA (version rJAN_09_2011) [51] used R. irregularis ESTs [23]. These two processes/programs produced a dataset of putative intron-exon boundaries. Meanwhile, the alignment of ESTs to the reference genome by PASA also produced protein-coding gene models. Based on this set of gene models, we constructed a training set, which was used by de novo predictors, by selecting the genes with complete structures and at least 95% mapping rate for UniProt [52] proteins, and filtering out the redundant genes with more than 70% sequence identity by CD-HIT (version 4.1.1) [53]. Secondly, we focused on the evidence at the protein sequence level. The protein sequences from UniProt fungi (release 2012_09) [52] were mapped onto the genomic sequence using Spaln (version 1.4.4) [54] and TBLASTN [55]. The putative intron-exon boundaries were generated by Spaln. For TBLASTN mapping, we performed following procedures: (I) For each protein, joining all of the HSPs (1e-5) with the gap of 500 bp into a consecutive region; (II) selecting the region when the overlapping coverage of its HSPs with the protein is greater or equal to 80%; (III) extending 1000 bp at both ends of the region; (IV) applying GeneWise onto the region to identify the putative intron-exon boundaries of the predicted gene. Thirdly, we collected protein-coding evidence by de novo predictors. For this purpose, AUGUSTUS (version 2.4) [56], GeneID (version 1.4.4) [57], GeneMark-ES (version 2.3) [58], GlimmerHMM (version 3.0.1) [59] and SNAP (2006-07-28) [60] were used. Besides GeneMark-ES, all programs used the masked genomic sequences. August, GlilmmerHMM and Snap are supervised predictors with the training set generated by PASA abovementioned, while GeneID utilized the parameters of Schizosaccharomyces japonicus. Finally, all evidence for protein-coding genes collected by the methods abovementioned was combined into a consensus protein-coding gene models by EVM. In addition, based this set of gene models and the EST dataset, we also used PASA to polish the gene models by adding untranslated regions (UTRs), correcting gene models, and generating all possible alternatively spliced isoforms at the mRNA level.
Functional annotation
The putative biological functions of the protein-coding genes predicted were assigned by AHRD (developed by Schoof et al. https://github.com/groupschoof/AHRD), which integrates three types of evidence to describe gene functions using standard nomenclature. The three types of evidence are: (I) The best BLASTP alignments (E-value cutoff of 1e-4) of the SwissProt database (release 2011-03) [52] and yeast protein sequences downloaded from NCBI (2012-12-10); (II) The InterPro signatures determined by searching against the InterPro databases (v29.0) [61] with InterProScan (V4.7) [62]; (III) The GO terms assigned by BLAST2GO (version 2.5.0) [63] based on the gene ontologies (GO version 2012-11-03).
CEGMA (http://korflab.ucdavis.edu/datasets/cegma/) analysis was performed according to [64], to assess the completeness of the assembly.
Orthology assessment
OrthoMCL [65] was used to identify orthologous groups among the set of protein sequences extracted from the following eleven completely sequenced genomes: R. irregularis, Neurospora crassa, Tuber melanosporum, Saccharomyces cerevisiae, Laccaria bicolor, Ustilago maydis, Rhizopus oryzae, Phycomyces blakesleeanus, Batrachochytrium dendrobatidis, Magnaporthe grisea and Monosiga brevicollis [66]–[75]. Only the longest sequence of each protein-coding gene was chosen in the further analysis. The set contains 171,398 sequences. Three steps took as follows: (1) all-against-all comparison strategy was applied to the set of protein sequences by BLASTP with an E-value cutoff of 1e-5; (2) The distance matrix among all proteins was constructed by the OrthoMCL algorithm; (3) The orthologous groups were generated by MCL [76] (I = 1.5) algorithm based on the distance matrix. The software versions used in this process were: OrthoMCL version 2.02, MCL version mcl 10–201, and NCBI BLAST version 2.2.15.
Phylogenetic analyses
We reinvestigated the phylogenetic placement of R. irregularis within the fungi based on a set of 52 low-copy genes proposed by [34] with addition of orthologs from R. irregularis, Magnaporthe orzyzae, Tuber melanosporum, Ustilago maydis, and the Cryptomycete Rozella allomyces [77]. Amino acid sequences were aligned using MAFFT [78] and positions covering less than three species were trimmed. Seventeen gene alignments supported paralogy shared among different fungal lineages and were excluded from the analysis, leaving in a total number of 35 gene alignments that were concatenated into a supermatrix of 26,604 amino acids. Table S14 lists all included protein sequences. We then estimated a ML phylogenetic tree based on the supermatrix using RAxML 7.2.8 [79] applying the amino acid substitution model with the best fit on a maximum parsimony tree (rtREV; [80] with empirical frequencies and gamma-distributed rate heterogeneity (-m PROTGAMMARTREVF). Clade support was assessed using the rapid bootstrapping algorithm [81] with 100 alignment replicates.
To test alternative hypotheses of monophyly we imposed three alternative topological constraints on parallel RAxML analyses, with R. irregularis forming a clade with either Dikarya, Chytridiomycota, or Microsporidia and Cryptomycota. Branch lengths were optimized and all competing hypotheses were compared with an unconstrained analysis using the eight bootstrap probability tests implemented in CONSEL [82]; Table S12).
Effector mining
Identifying fungal secretomes
Proteomes of 43 fungi containing 17 plant pathogens, 10 animal pathogens and 16 non-pathogens were used to identify the secretomes of the fungi including R. irregularis. Therefore, we used the following approach; First, signal peptide containing proteins were predicted using SignalP V2.0 software [83] using the criteria of Torto et al. [84]. Second, the presence of transmembrane domains and mitochondrial signal peptides in these proteins was predicted using TMHMM V2.0c (http://www.cbs.dtu.dk/services/TMHMM/) and TargetP V1.1 [85] programs. Third, secretomes were established by removing the proteins that contain transmembrane domains and mitochondrial signals. For stringent prediction of secretome, proteins with one or more transmembrane domains were removed. For relaxed prediction of secretomes, proteins with single transmembrane domain that overlapped with the signal peptide were included in the secretome. Last, the secretome was assessed for the presence of endoplasmic reticulum (ER) retention signal by either searching for the canonical ER retention signal sequence “KDEL or HDE[LF]” [86] or by using the protein localization prediction program WoLF PSORT [87]. However, we would like to point out that in our experience these ER retention signals are not particularly robust for fungal proteomes.
Annotation and classification of candidate effectors
To identify and classify candidate effectors from R. irregularis, we implemented a modified version of the bioinformatics pipeline described in Saunders et al. [88]. Briefly, proteins in the secretome were annotated with (I) nuclear localization signal (PredictNLS, [89] and Prosite Scan with database release 20.91, [90], (II) cysteine content higher than 3% [88], [91], (III) repeat units (T-REK, [92], and (IV) BLASTP [93] hit against UniProtKB/Swiss-Prot protein database [94]. The proteins were then grouped into tribes based on sequence similarity of the mature proteins using Markov clustering [78]. To order and classify the secreted protein tribes, we used the aforementioned annotation criteria and associated the scores to each tribe based on their likelihood of containing potential effector proteins. Tribes were then ranked giving a higher weight to features that are distinctive to the only reported R. irregularis effector SP7 [37].
Identification of CNR-like proteins in R. irregularis
To identify CRN-like proteins in R. irregularis, we did BLASTP search with amino acid sequences of canonical CRN proteins from P. infestans against the R. irregularis proteome. We collected sequences that matched to CRN sequences with E-value less than 10−5 and searched for CRN motifs using a library of 36 CRN HMMs described in Haas et al [39]. 90 sequences were identified that had similarities to P. infestans CRN proteins from BLASTP search with E-value cutoff of 10−5. Among these, 42 sequences showed positive scores for LFLAK_domain HMM, which is common to all CRN proteins (Fig. S6, Table S14). Within this set, other CRN domains described in Haas et al. [39] were additionally identified, including DWL (18 proteins with positive score), DI (1), D2 (2), DBF (2), DC (1), DN5 (1), DN17 (10), DSV (1), DX8 (1), DX9 (1), DXS (2), and DXX (5) domains (Fig. S6, Table S14). SignalP2.0 was used to predict signal peptides, with HMM probability scores from 0.508 to 0.971, which are comparable to the canonical CRN proteins from P. infestans, which have scores of 0.541 to 0.984 [39]. CRN-domain containing proteins with scores less than 0.9 cutoff used for secretome prediction were omitted from the secretome. Trans-membrane domains were predicted by TM-HMM 2.0c program.
Accession numbers
The sequence data have been deposited into Genbank with accession number PRJNA230015. The R. irregularis reference genome and assemblies are also available at http://cmb.bnu.edu.cn/Rhizophagus_irregularis_v10/.
Supporting Information
Zdroje
1. ParniskeM (2008) Arbuscular mycorrhiza: the mother of plant root endosymbioses. Nat Rev Microbiol 6 : 763–775.
2. Bever JD, Kang H-J, Kaonongbua W, Wang M (2008). Genomic organization and mechanisms of inheritance in arbuscular mycorrhizal fungi: contrasting the evidence and Implications of current theories. In: Verma A, editor. Mycorrhiza, Springer Berlin Heidelberg, Berlin, Heidelberg, pp. 135–148.
3. HalaryS, MalikS-B, LildharL, SlamovitsCH, HijriM, et al. (2011) Conserved meiotic machinery in Glomus spp., a putatively ancient asexual fungal lineage. Genome Biol Evol 3 : 950–958.
4. RileyR, CorradiN (2013) Searching for clues of sexual reproduction in the genomes of arbuscular mycorrhizal fungi. Fungal Ecol 6 : 44–49.
5. MarleauJ, DalpéY, St-ArnaudM, HijriM (2011) Spore development and nuclear inheritance in arbuscular mycorrhizal fungi. BMC Evol Biol 11 : 51.
6. CrollD, GiovannettiM, KochAM, SbranaC, EhingerM, et al. (2008) Nonself vegetative fusion and genetic exchange in the arbuscular mycorrhizal fungus Glomus intraradices. New Phytol 181 : 924–937.
7. SbranaC, FortunaP, GiovannettiM (2011) Plugging into the network: belowground connections between germlings and extraradical mycelium of arbuscular mycorrhizal fungi. Mycologia 103 : 307–316.
8. KuhnG, HijriM, SandersIR (2001) Evidence for the evolution of multiple genomes in arbuscular mycorrhizal fungi. Nature 414 : 745–748.
9. PawlowskaTE, TaylorJW (2004) Organization of genetic variation in individuals of arbuscular mycorrhizal fungi. Nature 427 : 733–737.
10. HijriM, SandersIR (2005) Low gene copy number shows that arbuscular mycorrhizal fungi inherit genetically different nuclei. Nature 433 : 160–163.
11. SandersIR, CrollD (2010) Arbuscular Mycorrhiza: The challenge to understand the genetics of the fungal partner. Annu Rev Genet 44 : 271–292.
12. StockingerH, WalkerC, SchüsslerA (2009) “Glomus intraradices DAOM197198,” a model fungus in arbuscular mycorrhiza research, is not Glomus intraradices. New Phytol 183 : 1176–1187.
13. RedeckerD, SchüsslerA, StockingerH, StürmerSL, MortonJB, et al. (2013) An evidence based consensus for the classification of arbuscular mycorrhizal fungi (Glomeromycota). Mycorrhiza 23 : 515–531.
14. MartinF, Gianinazzi-PearsonV, HijriM, LammersP, RequenaN, et al. (2008) The long hard road to a completed Glomus intraradices genome. New Phytol 180 : 747–750.
15. SędzielewskaKA, FuchsJ, TemschEM, BaronianK, WatzkeR, et al. (2011) Estimation of the Glomus intraradices nuclear DNA content. New Phytol 192 : 794–797.
16. AngelardC, ColardA, Niculita-HirzelH, CrollD, SandersIR (2010) Segregation in a Mycorrhizal Fungus Alters Rice Growth and Symbiosis-Specific Gene Transcription. Curr Biol 20 : 1216–1221.
17. BoonE, ZimmermanE, LangBF, HijriM (2010) Intra-isolate genome variation in arbuscular mycorrhizal fungi persists in the transcriptome. J Evol Biol 23 : 1519–1527.
18. SchochCL, SeifertKA, HuhndorfS, RobertV, SpougeJL, et al. (2012) Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc Natl Acad Sci U S A 109 : 6241–6246.
19. EickbushTH, EickbushDG (2007) Finely orchestrated movements: evolution of the ribosomal RNA genes. Genetics 175 : 477–485.
20. CampbellCS, WojciechowskiMF, BaldwinBG, AliceLA, DonoghueMJ (1997) Persistent nuclear ribosomal DNA sequence polymorphism in the Amelanchier agamic complex (Rosaceae). Mol Biol Evol 14 : 81–90.
21. GandolfiA, BonilauriP, RossiV, MenozziP (2001) Intraindividual and intraspecies variability of ITS1 sequences in the ancient asexual Darwinula stevensoni (Crustacea: Ostracoda). Heredity 87 : 449–455.
22. NilssonRH, KristianssonE, RybergM, HallenbergN, LarssonKH (2008) Intraspecific ITS variability in the kingdom fungi as expressed in the international sequence databases and its implications for molecular species identification. Evol Bioinform Online 4 : 193–201.
23. TisserantE, KohlerA, Dozolme-SeddasP, BalestriniR, BenabdellahK, et al. (2012) The transcriptome of the arbuscular mycorrhizal fungus Glomus intraradices (DAOM 197198) reveals functional tradeoffs in an obligate symbiont. New Phytol 193 : 755–769.
24. JurkaJ, KapitonovVV, PavlicekA, KlonowskiP, KohanyO, et al. (2005) Repbase Update, a database of eukaryotic repetitive elements. Cytogenet Genome Res 110 : 462–467.
25. ArkhipovaIR (2005) Mobile genetic elements and sexual reproduction. Cytogenet Genome Res 110 : 372–382.
26. DolginES, CharlesworthB (2006) The fate of transposable elements in asexual populations. Genetics 174 : 817–827.
27. GollotteA, L'HaridonF, ChatagnierO, WettsteinG, ArnouldC, et al. (2006) Repetitive DNA sequences include retrotransposons in genomes of the Glomeromycota. Genetica 128 : 455–469.
28. VandenkoornhuyseP, LeyvalC, BonninI (2001) High genetic diversity in arbuscular mycorrhizal fungi: evidence for recombination events. Heredity 87 : 243–253.
29. CrollD, SandersIR (2009) Recombination in Glomus intraradices, a supposed ancient asexual arbuscular mycorrhizal fungus. BMC Evol Biol 9 : 13.
30. den BakkerHC, VankurenNW, MortonJB, PawlowskaTE (2010) Clonality and recombination in the life history of an asexual arbuscular mycorrhizal fungus. Mol Biol Evol 27 : 2474–2486.
31. JamesTY, KauffF, SchochCL, MathenyPB, HofstetterV, et al. (2006) Reconstructing the early evolution of Fungi using a six-gene phylogeny. Nature 443 : 818–822.
32. CorradiN, SandersIR (2006) Evolution of the P-type II ATPase gene family in the fungi and presence of structural genomic changes among isolates of Glomus intraradices. BMC Evol Biol 6 : 21.
33. LeeJ, YoungJPW (2009) The mitochondrial genome sequence of the arbuscular mycorrhizal fungus Glomus intraradices isolate 494 and implications for the phylogenetic placement of Glomus. New Phytol 183 : 200–211.
34. Capella-GutiérrezS, Marcet-HoubenM, GabaldónT (2012) Phylogenomics supports microsporidia as the earliest diverging clade of sequenced fungi. BMC Biol 10 : 47.
35. RedeckerD, RaabP (2006) Phylogeny of the glomeromycota (arbuscular mycorrhizal fungi): recent developments and new gene markers. Mycologia 98 : 885–895.
36. LiuYJ, HodsonMC, HallBD (2006) Loss of the flagellum happened only once in the fungal lineage: phylogenetic structure of kingdom Fungi inferred from RNA polymerase II subunit genes. BMC Evol Biol 6 : 74.
37. KloppholzS, KuhnH, RequenaN (2011) A secreted fungal effector of Glomus intraradices promotes symbiotic biotrophy. Curr Biol 21 : 1204–1209.
38. WinJ, KannegantiTD, Torto-AlaliboT, KamounS (2006) Computational and comparative analyses of 150 full-length cDNA sequences from the oomycete plant pathogen Phytophthora infestans. Fungal Genet Biol 43 : 20–33.
39. HaasBJ, KamounS, ZodyMC, JiangRH, HandsakerRE, et al. (2009) Genome sequence and analysis of the Irish potato famine pathogen Phytophthora infestans. Nature 461 : 393–398.
40. SunG, YangZ, KoschT, SummersK, HuangJ (2011) Evidence for acquisition of virulence effectors in pathogenic chytrids. BMC Evol Biol 11 : 195.
41. NavinN, KendallJ, TrogeJ, AndrewsP, RodgersL, et al. (2012) Tumour evolution inferred by single-cell sequencing. Nature 472 : 90–94.
42. Chabaud M, Harrison M, de Carvalho-Niebel F, Bécard G, Barker DG (2006) Inoculation and growth of Mycorrhizal fungi. In: Mathesius U, Journet EP, Sumner LW, editors. The Medicago truncatula handbook. ISBN 0-9754303-1-9 http://www.noble.org/MedicagoHandbook
43. LuoR, LiuB, XieY, LiZ, HuangW, et al. (2012) SOAPdenovo2: an empirically improved memory-efficient short-read de novo assembler. Gigascience 1 : 18.
44. EdgarRC, MyersEW (2005) PILER: identification and classification of genomic repeats. Bioinformatics 21: i152–i158.
45. XuZ, WangH (2007) LTR_FINDER: an efficient tool for the prediction of full-length LTR retrotransposons. Nucleic Acids Res 35: W265–W268.
46. PriceAL, JonesNC, PevznerPA (2005) De novo identification of repeat families in large genomes. Bioinformatics 21: I351–I358.
47. IqbalZ, CaccamoM, TurnerI, FlicekP, McVeanG (2012) De Novo Assembly and Genotyping of Variants Using Colored De Bruijn Graphs. Nature Genetics 44 : 226–232.
48. IqbalZ, TurnerI, McVeanG (2013) High-throughput microbial population genomics using the Cortex variation assembler. Bioinformatics 29 : 275–276.
49. HaasBJ, SalzbergSL, ZhuW, PerteaM, AllenJE, et al. (2008) Automated eukaryotic gene structure annotation using EVidenceModeler and the Program to Assemble Spliced Alignments. Genome Biol 9: R7.
50. GotohO (2008) A space-efficient and accurate method for mapping and aligning cDNA sequences onto genomic sequence. Nucleic Acids Res 36 : 2630–2638.
51. HaasBJ, DelcherAL, MountSM, WortmanJR, SmithRK, et al. (2003) Improving the Arabidopsis genome annotation using maximal transcript alignment assemblies. Nucleic acids Res 31 : 5654–5666.
52. BairochA, ApweilerR, WuCH, BarkerWC, BoeckmannB, et al. (2005) The Universal Protein Resource (UniProt). Nucleic. Acid. Res 33: D154–D159.
53. LiW, GodzikA (2006) Cd-hit: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics 22 : 1658–1659.
54. GotohO (2008) Direct mapping and alignment of protein sequences onto genomic sequence. Bioinformatics 24 : 2438–2444.
55. AltschulSF, GishW, MillerW, MyersEW, LipmanDJ (1990) Basic local alignment search tool. J Mol Biol 215 : 403–410.
56. StankeM, DiekhansM, BaertschR, HausslerD (2008) Using native and syntenically mapped cDNA alignments to improve de novo gene finding. Bioinformatics 24 : 637–644.
57. ParraG, BlancoE, GuigoR (2000) GeneID in Drosophila. Genome Res 10 : 511–515.
58. Ter-HovhannisyanV, LomsadzeA, ChernoffYO, BorodovskyM (2008) Gene prediction in novel fungal genomes using an ab initio algorithm with unsupervised training. Genome 18 : 1979–1990.
59. MajorosWH, PerteaM, SalzbergSL (2004) TigrScan and GlimmerHMM: two open source ab initio eukaryotic gene-finders. Bioinformatics 20 : 2878–2879.
60. KorfI (2004) Gene finding in novel genomes. BMC Bioinformatics 5 : 59.
61. ApweilerR, AttwoodTK, BairochA, BatemanA, BirneyE, et al. (2001) The InterPro database, an integrated documentation resource for protein families, domains and functional sites. Nucleic Acids Res 29 : 37–40.
62. QuevillonE, SilventoinenV, PillaiS, HarteN, MulderN, et al. (2005) InterProScan: protein domains identifier. Nucleic Acids Res 33: W116–W120.
63. ConesaA, GötzS, García-GómezJM, TerolJ, TalónM, et al. (2005) Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 21 : 3674–3676.
64. ParraG, GradnamK, KorfI (2007) CEGMA: a pipeline to accurately annotate core genes in eukaryotic genomes. Proc Natl Acad Sci USA 23 : 1061–1067.
65. LiL, StoeckertCJ, RoosDS (2003) OrthoMCL: Identification of ortholog groups for eukaryotic genomes. Genome Res 13 : 2178–2189.
66. MewesHW, AlbermannmK, BährM, FrishmanD (1997) Overview of the yeast genome. Nature 387: s7–s8.
67. GalaganJE, CalvoSE, BorkovichKA, SelkerEU, ReadND, et al. (2003) The genome sequence of the filamentous fungus Neurospora crassa. Nature 422 : 859–868.
68. DeanRA, TalbotNJ, EbboleDJ, FarmanML, Mitchell1TK, et al. (2005) The genome sequence of the rice blast fungus Magnaporthe grisea. Nature 434 : 980–986.
69. KämperJ, KahmannR, BölkerM, MaL-J, BrefortT, et al. (2006) Insights from the genome of the biotrophic fungal plant pathogen Ustilago maydis. Nature 444 : 97–101.
70. KingN, WestbrookMJ, YoungSL, KuoA, AbedinM, et al. (2008) The genome of the choanoflagellate Monosiga brevicollis and the origin of metazoans. Nature 451 : 783–788.
71. MartinF, AertsAD, AhrénD, BrunA, DanchinEGJ, et al. (2008) The genome of Laccaria bicolor provides insights into mycorrhizal symbiosis. Nature 452 : 88–92.
72. MaL-J, IbrahimAS, SkoryC, GrabherrMG, BurgerG, et al. (2009) Genomic analysis of the basal lineage fungus Rhizopus oryzae reveals a whole-genome duplication. PLoS Genet 5: e1000549.
73. JonesonS, StajichJE, ShiuS-H, RosenblumEB (2011) Genomic transition to pathogenicity in chytrid fungi. PLoS Pathog 7: e1002338.
74. MartinF, KohlerA, MuratC, BalestriniR, CoutinhoPM, et al. (2011) Périgord black truffle genome uncovers evolutionary origins and mechanisms of symbiosis. Nature 464 : 1033–1038.
75. TaguaVCG, MedinaHR, Martín-DomínguezR, EslavaAP, CorrochanoLM, et al. (2012) A gene for carotene cleavage required for pheromone biosynthesis and carotene regulation in the fungus Phycomyces blakesleeanu. Fungal Genet Biol 49 : 398–404.
76. EnrightAJ, Van DongenS, OuzounisCA (2002) An efficient algorithm for large-scale detection of protein families. Nucleic Acids Res 30 : 1575–1584.
77. JamesTY, PelinA, BonenL, AhrendtS, SainD, et al. (2013) Shared signatures of parasitism and phylogenomics unite cryptomycota and microsporidia. Curr Biol 23 : 1548–1553.
78. KatohK, TohH (2008) Recent developments in the MAFFT multiple sequence alignment program. Brief Bioinform 9 : 286–298.
79. StamatakisA (2006) RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22 : 2688–2690.
80. DimmicMW, RestJS, MindellDP, GoldsteinRA (2002) rtREV: an amino acid substitution matrix for inference of retrovirus and reverse transcriptase phylogeny. J Mol Evol 55 : 65–73.
81. StamatakisA, HooverP, RougemontJ (2008) A rapid bootstrap algorithm for the RAxML Web servers. Syst Biol 57 : 758–771.
82. ShimodairaH, HasegawaM (2001) CONSEL: for assessing the confidence of phylogenetic tree selection. Bioinformatics 17 : 1246–1247.
83. Nielsen H, Krogh A (1998) Prediction of signal peptides and signal anchors by a hidden Markov model. In: Proceedings of the International Conference on Intelligent Systems for Molecular Biology 6: : 122–130.
84. TortoTA, LiS, StyerA, HuitemaE, TestaA, et al. (2003) EST Mining and functional expression assays identify extracellular effector proteins from the plant pathogen Phytophthora. Genome Res 30 : 1575–1584.
85. EmanuelssonO, NielsenH, BrunakS (2000) Predicting subcellular localization of proteins based on their N-terminal amino acid sequence. J Mol Biol 300 : 1005–1016.
86. WrzeszczynskiKO, RostB (2004) Annotating proteins from endoplasmic reticulum and Golgi apparatus in eukaryotic proteomes. Cell Mol Life Sci 61 : 1341–1353.
87. HortonP, ParkKJ, ObayashiT, FujitaN, HaradaH, et al. (2007) WoLF PSORT: protein localization predictor. Nucleic Acids Res 35 (Web Server issue) W585–7.
88. SaundersDGO, WinJ, CanoLM, SzaboLJ, KamounS, et al. (2012) Using hierarchical clustering of secreted protein families to classify and rank candidate effectors of rust Fungi. PLoS ONE 7: e29847.
89. CokolM (2000) Finding nuclear localization signals. EMBO Rep 1 : 411–415.
90. SigristCJA, CeruttiL, HuloN, GattikerA, FalquetL, et al. (2002) PROSITE: A documented database using patterns and profiles as motif descriptors. Brief Bioinform 3 : 265–274.
91. StergiopoulosI, de WitPJ (2009) Fungal effector proteins. Annu Rev Phytopathol 47 : 233–263.
92. JordaJ, KajavaAV (2009) T-REKS: identification of Tandem REpeats in sequences with a K-meanS based algorithm. Bioinformatics 25 : 2632–2638.
93. AltschulS (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25 : 3389–3402.
94. MagraneM (2011) Uniprot Consortium (2011) UniProt Knowledgebase: a hub of integrated protein data. Database 2011: bar009.
Štítky
Genetika Reprodukční medicína
Článek Unwrapping BacteriaČlánek A Chaperone-Assisted Degradation Pathway Targets Kinetochore Proteins to Ensure Genome StabilityČlánek The Candidate Splicing Factor Sfswap Regulates Growth and Patterning of Inner Ear Sensory OrgansČlánek The SPF27 Homologue Num1 Connects Splicing and Kinesin 1-Dependent Cytoplasmic Trafficking inČlánek Down-Regulation of eIF4GII by miR-520c-3p Represses Diffuse Large B Cell Lymphoma DevelopmentČlánek Meta-Analysis Identifies Gene-by-Environment Interactions as Demonstrated in a Study of 4,965 Mice
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2014 Číslo 1
-
Všechny články tohoto čísla
- How Much Is That in Dog Years? The Advent of Canine Population Genomics
- The Sense and Sensibility of Strand Exchange in Recombination Homeostasis
- Unwrapping Bacteria
- DNA Methylation Changes Separate Allergic Patients from Healthy Controls and May Reflect Altered CD4 T-Cell Population Structure
- Evidence for Mito-Nuclear and Sex-Linked Reproductive Barriers between the Hybrid Italian Sparrow and Its Parent Species
- Translation Enhancing ACA Motifs and Their Silencing by a Bacterial Small Regulatory RNA
- Relationship Estimation from Whole-Genome Sequence Data
- Genetic Models of Apoptosis-Induced Proliferation Decipher Activation of JNK and Identify a Requirement of EGFR Signaling for Tissue Regenerative Responses in
- ComEA Is Essential for the Transfer of External DNA into the Periplasm in Naturally Transformable Cells
- Loss and Recovery of Genetic Diversity in Adapting Populations of HIV
- Bioelectric Signaling Regulates Size in Zebrafish Fins
- Defining NELF-E RNA Binding in HIV-1 and Promoter-Proximal Pause Regions
- Loss of Histone H3 Methylation at Lysine 4 Triggers Apoptosis in
- Cell-Cycle Dependent Expression of a Translocation-Mediated Fusion Oncogene Mediates Checkpoint Adaptation in Rhabdomyosarcoma
- How a Retrotransposon Exploits the Plant's Heat Stress Response for Its Activation
- A Nonsense Mutation in Encoding a Nondescript Transmembrane Protein Causes Idiopathic Male Subfertility in Cattle
- Deletion of a Conserved -Element in the Locus Highlights the Role of Acute Histone Acetylation in Modulating Inducible Gene Transcription
- Developmental Link between Sex and Nutrition; Regulates Sex-Specific Mandible Growth via Juvenile Hormone Signaling in Stag Beetles
- PP2A/B55 and Fcp1 Regulate Greatwall and Ensa Dephosphorylation during Mitotic Exit
- Differential Effects of Collagen Prolyl 3-Hydroxylation on Skeletal Tissues
- Comprehensive Functional Annotation of 77 Prostate Cancer Risk Loci
- Evolution of Chloroplast Transcript Processing in and Its Chromerid Algal Relatives
- A Chaperone-Assisted Degradation Pathway Targets Kinetochore Proteins to Ensure Genome Stability
- New MicroRNAs in —Birth, Death and Cycles of Adaptive Evolution
- A Genome-Wide Screen for Bacterial Envelope Biogenesis Mutants Identifies a Novel Factor Involved in Cell Wall Precursor Metabolism
- FGFR1-Frs2/3 Signalling Maintains Sensory Progenitors during Inner Ear Hair Cell Formation
- Regulation of Synaptic /Neuroligin Abundance by the /Nrf Stress Response Pathway Protects against Oxidative Stress
- Intrasubtype Reassortments Cause Adaptive Amino Acid Replacements in H3N2 Influenza Genes
- Molecular Specificity, Convergence and Constraint Shape Adaptive Evolution in Nutrient-Poor Environments
- WNT7B Promotes Bone Formation in part through mTORC1
- Natural Selection Reduced Diversity on Human Y Chromosomes
- In-Vivo Quantitative Proteomics Reveals a Key Contribution of Post-Transcriptional Mechanisms to the Circadian Regulation of Liver Metabolism
- The Candidate Splicing Factor Sfswap Regulates Growth and Patterning of Inner Ear Sensory Organs
- The Acid Phosphatase-Encoding Gene Contributes to Soybean Tolerance to Low-Phosphorus Stress
- p53 and TAp63 Promote Keratinocyte Proliferation and Differentiation in Breeding Tubercles of the Zebrafish
- Affects Plant Architecture by Regulating Local Auxin Biosynthesis
- The SET Domain Proteins SUVH2 and SUVH9 Are Required for Pol V Occupancy at RNA-Directed DNA Methylation Loci
- Down-Regulation of Rad51 Activity during Meiosis in Yeast Prevents Competition with Dmc1 for Repair of Double-Strand Breaks
- Multi-tissue Analysis of Co-expression Networks by Higher-Order Generalized Singular Value Decomposition Identifies Functionally Coherent Transcriptional Modules
- A Neurotoxic Glycerophosphocholine Impacts PtdIns-4, 5-Bisphosphate and TORC2 Signaling by Altering Ceramide Biosynthesis in Yeast
- Subtle Changes in Motif Positioning Cause Tissue-Specific Effects on Robustness of an Enhancer's Activity
- C/EBPα Is Required for Long-Term Self-Renewal and Lineage Priming of Hematopoietic Stem Cells and for the Maintenance of Epigenetic Configurations in Multipotent Progenitors
- The SPF27 Homologue Num1 Connects Splicing and Kinesin 1-Dependent Cytoplasmic Trafficking in
- Down-Regulation of eIF4GII by miR-520c-3p Represses Diffuse Large B Cell Lymphoma Development
- Genome Sequencing Highlights the Dynamic Early History of Dogs
- Re-sequencing Expands Our Understanding of the Phenotypic Impact of Variants at GWAS Loci
- Meta-Analysis Identifies Gene-by-Environment Interactions as Demonstrated in a Study of 4,965 Mice
- , a -Antisense Gene of , Encodes a Evolved Protein That Inhibits GSK3β Resulting in the Stabilization of MYCN in Human Neuroblastomas
- A Transcription Factor Is Wound-Induced at the Planarian Midline and Required for Anterior Pole Regeneration
- A Comprehensive tRNA Deletion Library Unravels the Genetic Architecture of the tRNA Pool
- A PNPase Dependent CRISPR System in
- Genomic Confirmation of Hybridisation and Recent Inbreeding in a Vector-Isolated Population
- Zinc Finger Transcription Factors Displaced SREBP Proteins as the Major Sterol Regulators during Saccharomycotina Evolution
- GATA6 Is a Crucial Regulator of Shh in the Limb Bud
- Tissue Specific Roles for the Ribosome Biogenesis Factor Wdr43 in Zebrafish Development
- A Cell Cycle and Nutritional Checkpoint Controlling Bacterial Surface Adhesion
- High Risk Population Isolate Reveals Low Frequency Variants Predisposing to Intracranial Aneurysms
- E3 Ubiquitin Ligase CHIP and NBR1-Mediated Selective Autophagy Protect Additively against Proteotoxicity in Plant Stress Responses
- Evolutionary Rate Covariation Identifies New Members of a Protein Network Required for Female Post-Mating Responses
- 3′ Untranslated Regions Mediate Transcriptional Interference between Convergent Genes Both Locally and Ectopically in
- Single Nucleus Genome Sequencing Reveals High Similarity among Nuclei of an Endomycorrhizal Fungus
- Metabolic QTL Analysis Links Chloroquine Resistance in to Impaired Hemoglobin Catabolism
- Notch Controls Cell Adhesion in the Drosophila Eye
- AL PHD-PRC1 Complexes Promote Seed Germination through H3K4me3-to-H3K27me3 Chromatin State Switch in Repression of Seed Developmental Genes
- Genomes Reveal Evolution of Microalgal Oleaginous Traits
- Large Inverted Duplications in the Human Genome Form via a Fold-Back Mechanism
- Variation in Genome-Wide Levels of Meiotic Recombination Is Established at the Onset of Prophase in Mammalian Males
- Age, Gender, and Cancer but Not Neurodegenerative and Cardiovascular Diseases Strongly Modulate Systemic Effect of the Apolipoprotein E4 Allele on Lifespan
- Lifespan Extension Conferred by Endoplasmic Reticulum Secretory Pathway Deficiency Requires Induction of the Unfolded Protein Response
- Is Non-Homologous End-Joining Really an Inherently Error-Prone Process?
- Vestigialization of an Allosteric Switch: Genetic and Structural Mechanisms for the Evolution of Constitutive Activity in a Steroid Hormone Receptor
- Functional Divergence and Evolutionary Turnover in Mammalian Phosphoproteomes
- A 660-Kb Deletion with Antagonistic Effects on Fertility and Milk Production Segregates at High Frequency in Nordic Red Cattle: Additional Evidence for the Common Occurrence of Balancing Selection in Livestock
- Comparative Evolutionary and Developmental Dynamics of the Cotton () Fiber Transcriptome
- The Transcription Factor BcLTF1 Regulates Virulence and Light Responses in the Necrotrophic Plant Pathogen
- Crossover Patterning by the Beam-Film Model: Analysis and Implications
- Single Cell Genomics: Advances and Future Perspectives
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- GATA6 Is a Crucial Regulator of Shh in the Limb Bud
- Large Inverted Duplications in the Human Genome Form via a Fold-Back Mechanism
- Differential Effects of Collagen Prolyl 3-Hydroxylation on Skeletal Tissues
- Affects Plant Architecture by Regulating Local Auxin Biosynthesis
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



