-
Články
Reklama
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
ReklamaHigh Risk Population Isolate Reveals Low Frequency Variants Predisposing to Intracranial Aneurysms
3% of the population develops saccular intracranial aneurysms (sIAs), a complex trait, with a sporadic and a familial form. Subarachnoid hemorrhage from sIA (sIA-SAH) is a devastating form of stroke. Certain rare genetic variants are enriched in the Finns, a population isolate with a small founder population and bottleneck events. As the sIA-SAH incidence in Finland is >2× increased, such variants may associate with sIA in the Finnish population. We tested 9.4 million variants for association in 760 Finnish sIA patients (enriched for familial sIA), and in 2,513 matched controls with case-control status and with the number of sIAs. The most promising loci (p<5E-6) were replicated in 858 Finnish sIA patients and 4,048 controls. The frequencies and effect sizes of the replicated variants were compared to a continental European population using 717 Dutch cases and 3,004 controls. We discovered four new high-risk loci with low frequency lead variants. Three were associated with the case-control status: 2q23.3 (MAF 2.1%, OR 1.89, p 1.42×10-9); 5q31.3 (MAF 2.7%, OR 1.66, p 3.17×10-8); 6q24.2 (MAF 2.6%, OR 1.87, p 1.87×10-11) and one with the number of sIAs: 7p22.1 (MAF 3.3%, RR 1.59, p 6.08×-9). Two of the associations (5q31.3, 6q24.2) replicated in the Dutch sample. The 7p22.1 locus was strongly differentiated; the lead variant was more frequent in Finland (4.6%) than in the Netherlands (0.3%). Additionally, we replicated a previously inconclusive locus on 2q33.1 in all samples tested (OR 1.27, p 1.87×10-12). The five loci explain 2.1% of the sIA heritability in Finland, and may relate to, but not explain, the increased incidence of sIA-SAH in Finland. This study illustrates the utility of population isolates, familial enrichment, dense genotype imputation and alternate phenotyping in search for variants associated with complex diseases.
Published in the journal: . PLoS Genet 10(1): e32767. doi:10.1371/journal.pgen.1004134
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004134Summary
3% of the population develops saccular intracranial aneurysms (sIAs), a complex trait, with a sporadic and a familial form. Subarachnoid hemorrhage from sIA (sIA-SAH) is a devastating form of stroke. Certain rare genetic variants are enriched in the Finns, a population isolate with a small founder population and bottleneck events. As the sIA-SAH incidence in Finland is >2× increased, such variants may associate with sIA in the Finnish population. We tested 9.4 million variants for association in 760 Finnish sIA patients (enriched for familial sIA), and in 2,513 matched controls with case-control status and with the number of sIAs. The most promising loci (p<5E-6) were replicated in 858 Finnish sIA patients and 4,048 controls. The frequencies and effect sizes of the replicated variants were compared to a continental European population using 717 Dutch cases and 3,004 controls. We discovered four new high-risk loci with low frequency lead variants. Three were associated with the case-control status: 2q23.3 (MAF 2.1%, OR 1.89, p 1.42×10-9); 5q31.3 (MAF 2.7%, OR 1.66, p 3.17×10-8); 6q24.2 (MAF 2.6%, OR 1.87, p 1.87×10-11) and one with the number of sIAs: 7p22.1 (MAF 3.3%, RR 1.59, p 6.08×-9). Two of the associations (5q31.3, 6q24.2) replicated in the Dutch sample. The 7p22.1 locus was strongly differentiated; the lead variant was more frequent in Finland (4.6%) than in the Netherlands (0.3%). Additionally, we replicated a previously inconclusive locus on 2q33.1 in all samples tested (OR 1.27, p 1.87×10-12). The five loci explain 2.1% of the sIA heritability in Finland, and may relate to, but not explain, the increased incidence of sIA-SAH in Finland. This study illustrates the utility of population isolates, familial enrichment, dense genotype imputation and alternate phenotyping in search for variants associated with complex diseases.
Introduction
About 3% of the population develops saccular intracranial aneurysms (sIAs) during life [1], [2]. Some 95% of subarachnoid hemorrhages are caused by ruptured sIA (sIA-SAH), a devastating form of stroke affecting individuals mainly in the sixth decade of life [3]. The annual incidence of SAH is 4–9 per 100 000 worldwide [4] but over twice as high in Finland and in Japan [5]. The sIA disease is a complex trait, the risk of which is affected by age, sex, smoking, hypertension, excess drinking [6], and in over 10% of the cases family history of sIA disease [7]–[9].
To date, genome wide association (GWA) studies have identified six definite and one probable loci with common variants associated to sIA: 4q31.23 (OR 1.22) [10], [11]; 8q11.23–q12.1 (OR 1.28); 9p21.3 (OR 1.31); 10q24.32 (OR 1.29); 12q22 (OR 1.16) [10]; 13q13.1 (OR 1.20); 18q11.2 (OR 1.22) [12] (Table S5). These seven loci were estimated to explain 6.1%, 4.4% and 4.1% of the four-fold sibling recurrence risk in Finland, Europe and Japan respectively [10]. In these previous GWA studies, results on 2q33.1 locus were inconsistent: the locus was significant in the first GWAS [13], not significant in the enlarged follow-up GWAS [12], and in the third GWAS the risk allele was reversed in the Japanese replication sample [10].
The population of Finland is one of the most thoroughly characterized genetic isolates. Due to the small size of the founder population, subsequent bottleneck effects and genetic drift, the Finnish population is enriched for rare and low frequency variants that are almost absent in other European populations and some variants rare elsewhere are increased in frequency [14]. This is best illustrated by the increased prevalence of 36 rare Mendelian, mostly recessive, disorders in Finland (www.findis.org); the so called Finnish disease heritage (FDH) [15]. We hypothesized that some of the enriched rare or low frequency variants could contribute to the increased sIA-SAH susceptibility in Finland.
In this GWA study we combined the power of 1000 Genomes imputation, the special benefits of a population isolate and enrichment of familial cases in the discovery cohort. Familial sIA patients more often carry multiple sIAs as compared to sporadic sIA patients, which may confer additional genetic burden to the sIA formation [8], [16], [17]. Therefore, in addition to the case vs. control analysis, we also analyzed the number of sIAs per individual as an intermediate phenotype. We conducted an association analysis in a discovery sample of 760 Finnish sIA cases and 2,513 matched controls followed by replication in an additional sample of 858 Finnish sIA cases and 4048 controls. The successfully replicated loci in Finland were further studied in a Dutch cohort of 717 sIA cases and 3004 controls to assess the extent to which the allele frequencies and risk effect sizes match between the isolate of Finland and a continental European population (Figure 1). In addition, we hypothesized that a previously inconclusive locus on 2q33.1 [10], [13], [18] is a true sIA risk locus at least in Finland and aimed to replicate the best discovery associations in the locus in this study in the Finnish and in the Dutch samples.
Fig. 1. Study design. 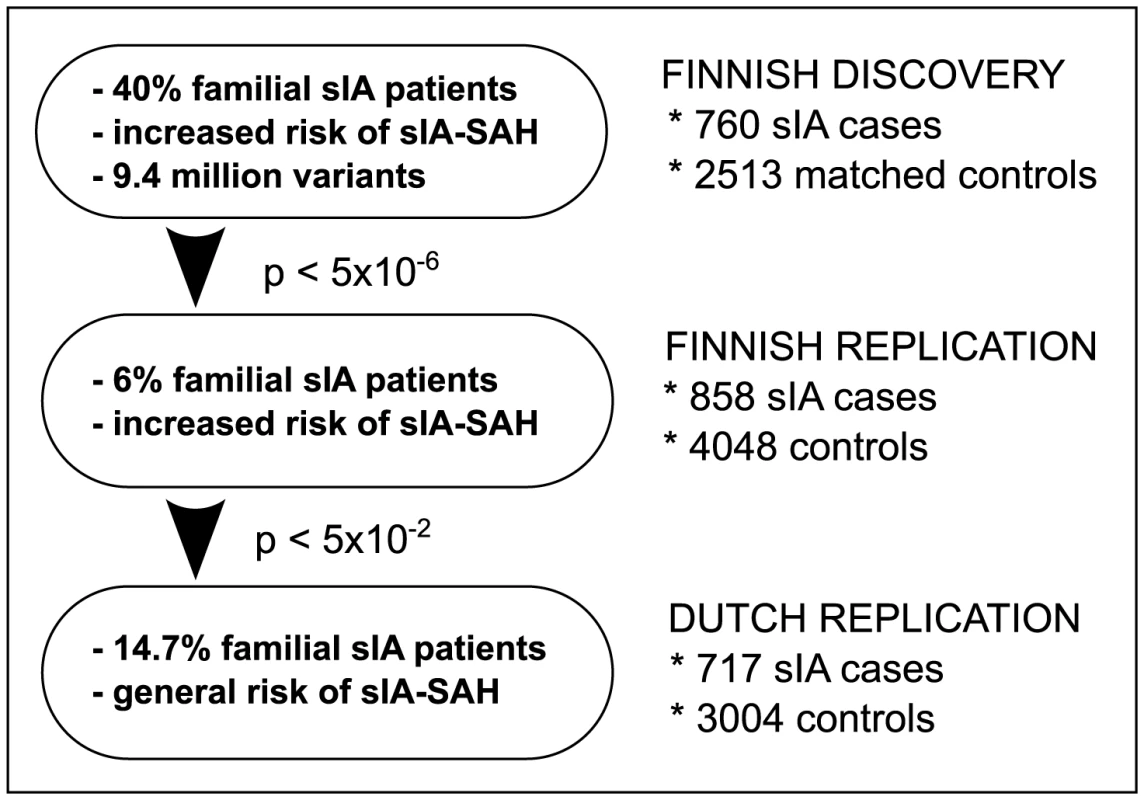
The Finnish discovery and replication cohorts represent a population with over two-fold increased risk of subarachnoid hemorrhage from ruptured saccular intracranial aneurysm (sIA-SAH). The Finnish discovery cohort was intentionally enriched with familial sIA patients, and 9.4M genotyped and imputed variants were studied. The loci with p<5E-6 were replicated in an independent and unselected Finnish sIA sample. The allele frequencies and effect sizes of the replicated variants in Finland were finally compared to continental European population using a Dutch sample. The sIA-SAH risk is not increased in the Netherlands (‘general risk’ in the figure). We successfully identified associations with low frequency variants in three novel loci in the case vs. control analysis and one in the aneurysm count analysis. Two of the case vs. control loci replicated also in the Dutch cohort with similar allele frequencies and comparable risk effect sizes. The variant in the aneurysm count locus demonstrated a strong bottleneck effect by being 15 times more frequent in the Finnish than in the Dutch controls. We also successfully replicated the previously inconclusive 2q33.1 locus.
Results
Case vs. control analysis in Finnish and Dutch samples
To increase the potential genetic load in the study sample, our discovery sample consisted of 760 cases from the isolated, high-risk Finnish population, purposefully enriched for familial sIA (40%) patients and 2513 genetically matched Finnish controls. The imputation of the 304,399 previously genotyped variants [12] against the 1000 Genomes Project reference panel (v3, March 2012 release) increased the number of common and low frequency variants available for the association analysis to 9,359,231. Quantile-quantile (QQ) plots of association p-values did not indicate substantial inflation (λ = 1.04) (Figure S1). The discovery association analysis revealed one locus at 12p11.1 driven by rs653464 at conventional genome-wide significance (p<5×10−8) and 14 other loci at p<5×10−6 (Table S1; Manhattan plot in Figure S3).
We chose 17 SNPs representing the 15 promising loci (p<5×10−6) above for replication in an independent sample of 858 Finnish sIA cases and 4,048 controls (Table 1). Four SNPs and one deletion were associated at p<0.05 with the sIA disease (Table S1), two of them in the previously reported sIA loci 9p21.3 (rs1333042; OR 1.3, p = 6.3×10−7) and 13q13.1 (rs113124623; OR 0.88, p = 0.01). The genome-wide significant 12p11.1 locus in the discovery sample did not replicate (p = 0.29).
Tab. 1. The Finnish and Dutch study samples used in the association analysis of saccular intracranial aneurysm (sIA) disease. 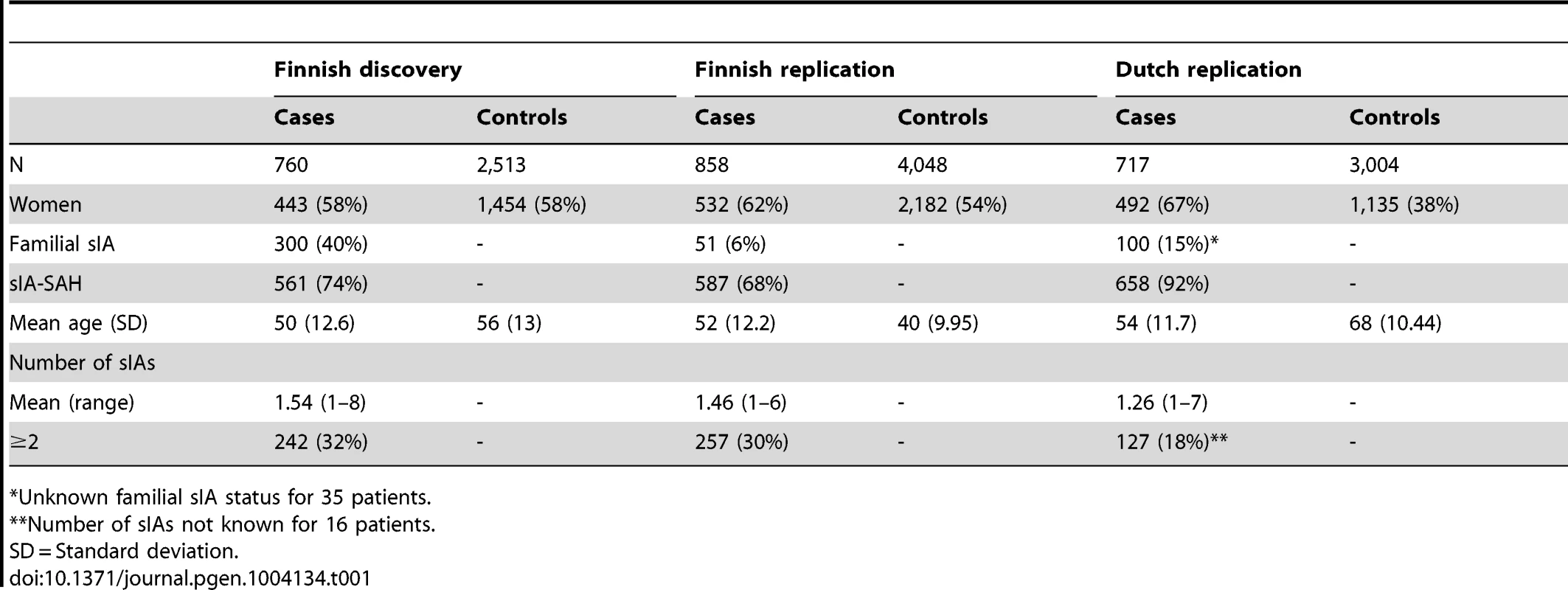
Unknown familial sIA status for 35 patients. In the meta-analysis of the two Finnish samples, four SNPs reached the commonly used level of genome-wide significance at p<5×10−8 (Table 2). Three were novel: 2q23.3 (rs74972714; OR 2.1, 95% CI 1.68–2.63, p = 7.4×10−11, control allele frequency or CAF 2.35%), 5q31.3 (rs113816216; OR 1.92, CI 1.53–2.40, p = 1.74×10−8, CAF 2.09%) and 6q24.2 (rs75018213; OR 1.97, CI 1.6–2.43, p = 2.25×10−10, CAF 2.53%). One was previously reported at 9p21.3 (rs1333042; OR 1.31, CI 1.21–1.42, p = 1.8×10−11, CAF 42.3%) (Table 2). We assessed the robustness of the associations controlling also for age and the effect sizes and p-values were almost identical (data not shown).
Tab. 2. Five loci with a genome-wide significant association to saccular intracranial aneurysm (sIA) disease in the Finnish and Dutch samples. 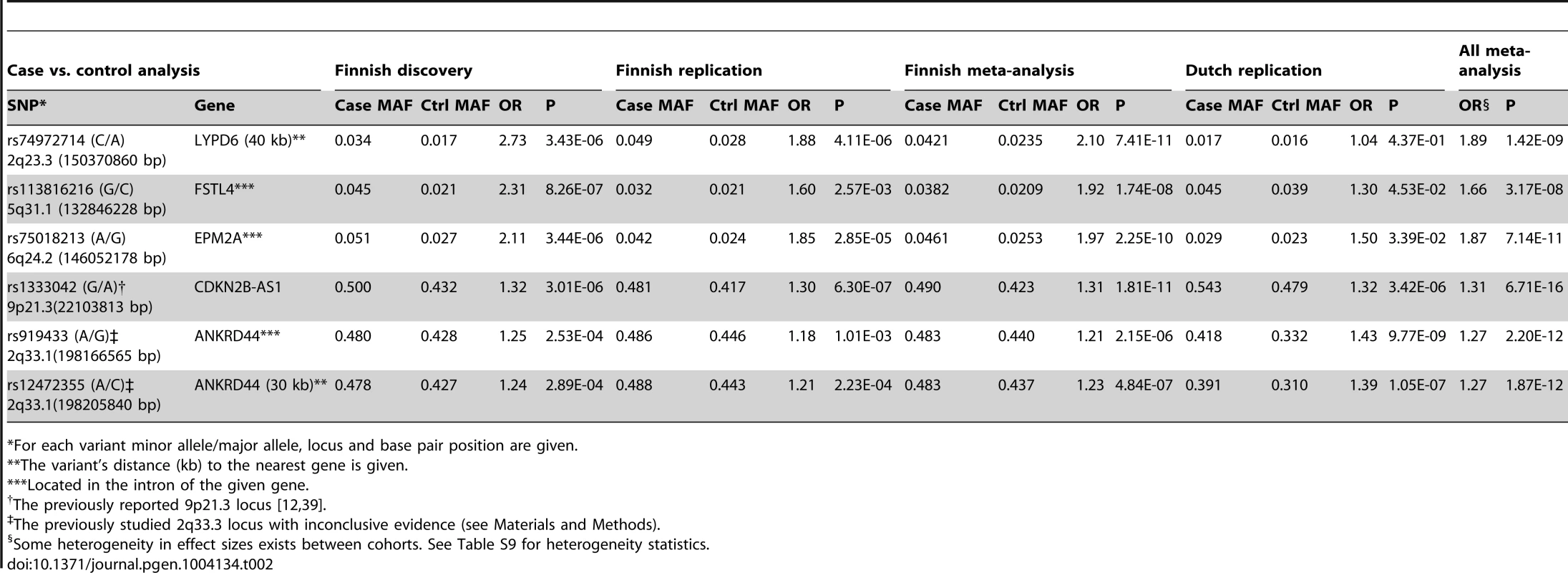
For each variant minor allele/major allele, locus and base pair position are given. To assess how the allele frequencies and effect sizes of variants identified in the Finnish population compare to other European populations, we studied those variants in a Dutch sample consisting of 717 sIA cases and 3,004 controls (Table 1). All three variants tagging the novel loci at 2q23.3, 5q31.3 and 6q24.2 had a similar low minor allele frequency (1.6–3.9%) in Finland and the Netherlands (Table 2). Two of them had similar effect sizes and were also replicated: 5q31.3 (rs113816216; OR 1.3, CI 0.98–1.75, p = 0.045, CAF 3.87%) and 6q24.2 (rs75018213; OR 1.5, CI 0.98–2.3 p = 0.034, CAF 2.3%). The previously reported 9p21.3 locus also replicated in the Dutch sample (rs1333042; OR 1.32, CI 1.17–1.49, p = 3.42×10−6, CAF 47.86%).
In the meta-analysis of the Finnish and Dutch samples, all three novel loci 2q23.3 (rs74972714; OR 1.89, p = 1.42×10−9), 5q31.3 (rs113816216; OR 1.66, p = 3.17×10−8) and 6q24.2 (rs75018213; 1.87, p = 7.1×10−11) were significantly associated to the sIA disease at genome-wide significance (Table 2; see Table S7 for imputation accuracy statistics). Some heterogeneity in effect sizes exists between samples (Table S9).
As the standard genome-wide significance 5×10−8 is estimated to correct for independent tests of common variants (MAF> = 5%) and we tested also a set of low-frequency variants, the common significance level may be too liberal. Based on Europeans of the 1000 Genomes project we estimated the significance level to be 3.82×10−8 (See Materials and Methods). All of the reported variants are below this level.
Association of variants to the number of sIAs
Some 20–30% of the sIA patients carry multiple sIAs, a phenomenon more commonly seen in familial sIA disease [8], [16], [17]. We hypothesized that an increased number of sIAs (≥2) in a given patient would reflect a higher underlying genetic load, motivating us to use aneurysm count as an intermediate phenotype to increase statistical power. The number of sIAs was used as a count data using the negative binomial regression analysis in the discovery sample of 760 Finnish sIA cases (1–8 sIAs per patient) and 2,513 controls. The QQ plot (Figure S2) and the genomic inflation factor (1.05) did not indicate substantial population stratification.
Nine loci had variants at p<5E-6 (Table S2; Manhattan plot in Figure S4). The most significant variant of each locus was selected for replication in the new Finnish sample of 858 sIA cases (1–6 sIAs per patient) and 4,048 controls. Two loci were replicated at p<0.05 : 7p22.1 (rs150927513; RR 1.39, p = 8.36×10−4, CAF 5.24%) and 16p13.3 (rs144159053; rate ratio (RR) 1.66, p = 4.4×10−3, CAF 1.27%) (Table S2). rs10802056 on 1p12 had a significant association p-value but the effect direction was different and thus was not considered as replicated. We assessed the robustness of the associations controlling also for age and the effect sizes and p-values were almost identical (data not shown).
In the meta-analysis of the Finnish samples, 7p22.1 was genome-wide significant (rs150927513; RR 1.6, CI 1.37–1.88, p = 4.92×10−9, CAF 4.61%);Table 3; See genotype to aneurysm count distribution in Table S3). The rate ratio (RR) estimate is the relative rate of aneurysm formation (i.e. change in expected number of aneurysms) per allele as compared to minor allele homozygotes.
Tab. 3. The locus with a genome-wide significant association to the number of saccular intracranial aneurysms (sIA) per individual in the Finnish samples. 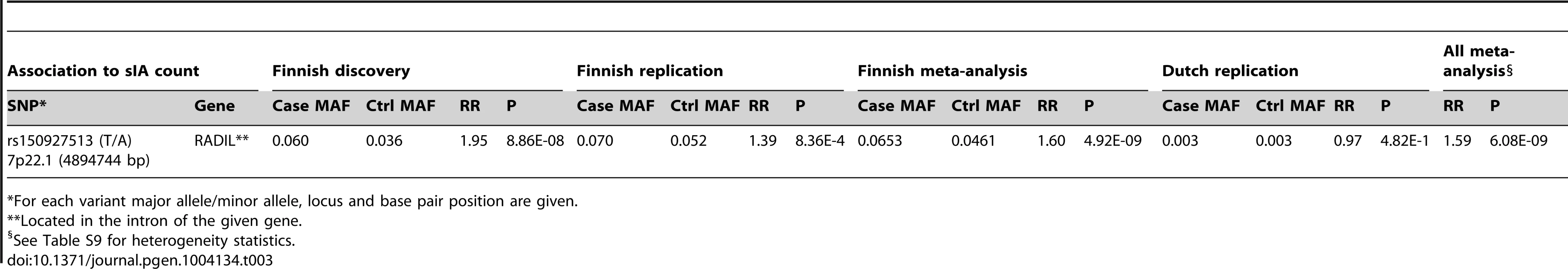
For each variant major allele/minor allele, locus and base pair position are given. To compare the allele frequency and effect size of rs150927513 identified in the Finnish population to those of continental European populations, we studied the variant also in the Dutch, but the imputation quality (Impute info 0.38) and estimated allele frequency (0.29%) were too low to obtain reliable estimates (RR 0.97; 95% CI 0.17–4.03, p = 0.97). We additionally checked the minor allele frequency of rs150927513 in 498 whole-genome sequenced Dutch individuals of GENOMEoftheNETHERLANDS-project (http://www.nlgenome.nl/). Only two individuals were heterozygous and the rest were major allele homozygotes (MAF 0.2%), which is in agreement with our imputation results of the Dutch sample.
Analysis of 2q33.1 locus
Previously published results on the 2q33.1 locus are inconsistent, being significant in the first GWAS [13], not significant in the enlarged follow-up GWAS [12], and uncertain in the third GWAS [10]. We aimed to study if the 2q33.1 would replicate in Finland, even though no variant in this region reached p<5E-6 in the discovery sample. We chose two of the most significant SNPs (in this study) at 2q33.1 for replication in the new Finnish replication sample, which was not used in the previous studies (rs12472355; OR 1.21, p = 2.23×10−4, CAF 44.3%, and rs919433; OR 1.18, p = 1.01×10−3, CAF 44.6%). They are in LD with the three previously investigated SNPs (rs787994, rs1429412, rs700651; LD r2 0.75–0.96). The variants rs12472355 (OR 1.23, CI 1.13–1.33, p = 4.84×10−7) and rs919433 (OR 1.21, CI 1.12–1.31, p = 2.15×10−6) did not reach genome-wide significance in the combined Finnish samples (Table 2). They were highly significant in the Dutch sample (rs12472355; OR, 1.39, CI 1.23–1.57, p = 1.05×10−7 and rs919433; OR 1.43, CI 1.26–1.61, p 9.77×10−9), and in the meta-analysis of all three samples they reached genome-wide significance (Table 2). The allele frequencies were notably higher in the Finnish samples (44% and 43.7%) than in the Dutch samples (33.2% and 31%).
Heritability estimate
We estimated the heritability explained by the reported variants. The four novel loci on 2q23.3, 5q31.3, 6q24.2 and 7p22.1 were estimated to explain 1.7% of the heritability in the combined Finnish samples. Adding the previously inconclusive 2q33.1 locus increases the heritability explained to 2.1%.
Genotype validation
For validating the imputation accuracy, we genotyped 87 individuals of the discovery sample using Sequenom genotyping. The concordance rates range from 96–99% except rs74972714 was slightly lower at 94% (Table S8). We did additional validation by Sanger sequencing 10 individuals per variant who were predicted to carry minor alleles. The imputation was near perfect in all other SNPs except rs75018213 had discrepancies between major allele homozygote and heterozygotes (Table S11). We further estimated by simulation, how likely it would be to get the observed OR for rs75018213 in the discovery sample just by change, given the imputation accuracy (See Text S1 for details). The probability of chance finding was very low (p: 0.0001) even if assuming that the minor allele would be over-imputed by 20% in cases (p: 0.004).
Some individuals were genotyped by both Sanger sequencing and Sequenom and the concordance between the two methods was perfect (Table S11). Finally, we estimated, in silico, the imputation efficiency of reported SNPs in Dutch population. 96 individuals of the Genome of the Netherlands project had both high coverage whole-genome sequencing (40×) data as well as GWA chip genotyping data available. We imputed the genotypes of reported SNPs using the same imputation methods, 1000 Genomes reference panel and set of SNPs in GWA chips as was done in the discovery and Dutch comparison analyses. The genotype concordance rates were excellent (Table S13). It is noteworthy that the imputation quality measure reported by the Impute2 program was higher in all of the SNPs in our Dutch replication cohort (Table S7) than in the in silico validation experiment. This indicates excellent imputation quality in the Dutch replication.
Fine mapping of the identified loci
We attempted to identify putative causative variants from whole exome sequencing data of 583 Finnish individuals. We focused on variants within 1 MB of the lead SNPs with high impact on protein product (i.e. variants affecting splice site, losing or gaining stop/start codon, altering reading frame) or non-synonymous coding SNPs. We additionally filtered variants if they were not in LD with the lead SNPs (r2<0.4, Europeans of 1000 Genomes if available). 254 variants were identified, most of which were rare. However 15 variants were enriched to low-frequency range (MAF>1%) (Table S12). The impact of these variants needs to be evaluated in follow-up studies.
Regulatory elements at identified loci
The UCSC Genome Browser and HaploReg version 2 [19] were used to search for ENCODE regulatory elements at the five genome-wide significant variants.
rs74972714 at 2q23.3 and rs150927513 at 7p22.1 reside within a DNAse hypersensitivity peak. The rs75018213 at 6q24.2 resides on an ENCODE GATA2 transcription factor binding site peak (Table S4).
Using genome-wide Chip-SEQ analysis, Ernst et al. constructed a predicted cell-type specific regulatory region map of nine chromatin marks in nine cell lines [20]. rs113816216 at 5q31.3 resides on a predicted erythroleukemia cell specific (K562) strong enhancer and rs75018213 at 6q24.2 on a predicted lymphoblastoid cell (GM12878) weak enhancer (Table S4).
We searched for putative transcription factor binding sites affected by the four variants, based on position weight matrices from Transfac, Jaspar and ENCODE (top 3 enriched motifs for each transcription factor, identified in transcription factor Chip-SEQ peaks [19]). rs74972714 at 2q23.3 affects putative binding sites for EBF1 (ENCODE), HDAC2 (ENCODE), RXRA:PPARG complex (Transfac), ZNF423 (Jaspar) and ZIC3 (Jaspar). rs113816216 at 5q31.3 affects the putative binding sites for RFX1 (Transfac), SREBP1 (ENCODE), STAT3 (Transfac) and IKZF3 (Transfac). rs150927513 at 7p22.1 affects putative binding sites of T (brachyury) (Transfac), CEBPB (Transfac) and P300 (ENCODE). rs75018213 at 6q24.2 is not directly on any putative transcription factor binding site. (Table S4).
At the 2q33.1 locus neither of the studied variants (rs919433, rs12472355) are on ENCODE DNAse hypersensitivity or transcription factor binding site peaks. However, rs919433 is on a predicted lymphoblastoid (GM12878) cell enhancer whereas rs12472355 is not directly on any regulatory region. rs919433 disrupts a putative transcription factor binding sites for RUNX2 (OSF2,Transfac) and the MYC:MAX complex (Transfac).
eQTL analysis
To study the potential effects of the variants in the five significant loci on the transcripts of nearby genes, we correlated the variants to expression levels of exons of nearby genes (expression quantitative trait locus (eQTL) analysis) obtained using RNA-sequencing in lymphoblasts of genotyped European individuals from the 1000 Genomes Project (Finnish, British, Toscani and CEPH populations, n = 373; www.geuvadis.org, [21]). Each variant was correlated to transcripts residing within 1 MB. There were 55 genes in 586 exons available for analysis (see Materials and Methods) and in total 748 tests were performed corresponding to Bonferroni corrected significance threshold of 8.7×10−5. Strongest association for each variant are reported below and all eQTL results in Table S6.
The most significant eQTL associations were observed at the 2q33.1 locus: rs12472355 associated significantly to the closest gene ANKRD44 (per allele fold change (FC) 0.94, p = 1.83×10−5) and also to HSPD1 (FC 0.94, p = 1.6×10−4), whereas rs919433 was associated to the same genes but in different order of significance; HSPD1 (FC 0.94, p = 3.8×10−5) and ANKRD44 (FC 0.95, p = 1.4×10−4). Among the novel low-frequency variants, only rs150927513 at 7p22.1 was significantly associated to TNRC18 (FC 1.23, p = 5.1×10−5). Nominal associations were observed for two other novel low frequency variants: rs113816216 at 5q31.3 to VDAC1 (FC 2.12, p 4.6E-4); rs74972714 at 2q23.3 to EPC2 (FC 0.75, p = 3.9×10−2). rs75018213 at 6q24.2 did not have any association even at nominal p<0.05 (Table S6).
We additionally investigated the eQTL landscape of identified loci by pairwise comparison of p-values from eQTL (MAF>0.05 p<0.001) and sIA analyses (Figure S5) and by plotting eQTL associations (p<0.001) in the implicated loci (Supplementary Figure S6 A–E). Only few loci show strong (p<1E-5) association in eQTL and at least nominal (p<0.05) association to sIA (Table S10). There does not seem to be stronger eQTL associations in LD with the lead SNPs. In the 2q33.1, where the lead SNPs were significantly associated to transcript levels, there seems to be a lot of regulatory potential in the same locus, even though not in direct LD with the lead variants (Figure S6 E).
Discussion
In this study, we used three approaches to improve the power to identify new loci associated to the sIA disease. First, we focused on the Finnish population isolate with increased risk for subarachnoid haemorrhage from ruptured sIAs (sIA-SAH) [5]. Second, we enriched the proportion of familial sIA patients in the discovery sample, thus possibly increasing the prevalence of risk alleles. Third, we increased genome-wide coverage through imputing ungenotyped variants based on 1000 Genomes Project data. The used 1000 Genomes Project imputation reference panel included 93 Finns, which made it well suited for discovery of enriched sIA associated variants in the Finnish population. Using this combination of strategies, we were able to identify three new loci associated with sIA disease, and one locus associated with the number of aneurysms. Additionally we replicated a locus where the evidence so far was inconclusive. Together these five loci account for 2.1% of the heritability in the Finnish samples. In comparison, the six previously identified SNPs explain 2.5% of the heritability in the discovery sample of the current study. Our results likely reflect the varying genetic background of complex traits, such as sIA, in different populations.
Four novel sIA loci
The lead SNPs in the four novel loci all have a low frequency (<5%) in the general population and could not have been identified without imputing the genotype data against the 1000 Genomes reference. One of the variants, rs150927513 at 7p22.1 that was associated with the number of sIAs, indicates a strong bottleneck effect, for it was 15 times more frequent in the controls of combined Finnish samples (4.6%) than in the Dutch sample (0.3%), and it is virtually non-existent in other populations (1000 Genomes). The three other loci had similar frequencies in Finland and other European populations (1000 Genomes). These four novel loci explain 1.7% of the heritability in the Finnish samples.
The four sIA loci had higher effect sizes (point estimates ranging from 1.59 to 1.88) than the lead SNPs identified by previous GWA studies. We cannot yet conclude whether relatively high ORs of low frequency risk alleles are a typical feature of sIA disease. Similar, and higher, odds ratios for low frequency and rare variants have been reported in isolates for other traits [22], [23]. It is likely that this first wave of low frequency and rare susceptibility variants represent “low hanging” fruits that do not allow general conclusions about the susceptibility landscape of sIA or other complex traits.
2q23.3 locus
The variant rs74972714 at 2q23.3 has a frequency of about 2% in European populations, including Finns. It was significantly associated to sIA in the Finnish samples but did not show a trend for being associated in the Dutch sample despite having a similar allele frequency. Further studies are required to find out whether this variant tags a risk allele specific to Finnish sIA patients. The variant is located 40 kb downstream of LYPD6 and 55 kb upstream of MMADHC (Figure 2 A). LYPD6 has recently been characterized as a member of the Ly-6 protein superfamily [24]. LYPD6 is ubiquitously expressed with highest levels in heart and brain. GPI-anchored Ly-6 proteins such as PLAUR function, e.g., in cellular adhesion [24]. LYPD6 overexpression can inhibit transcriptional activity of the AP1 transcription factor complex [24], a key inflammation mediator activated, e.g., in endothelial cells in atherogenic disturbed blood flow conditions, leading in turn to upregulation of pro-inflammatory molecules [25]. Similar transcriptional changes have been found in the ruptured human sIA wall [26]. MMADHC is an intracellular vitamin B12 trafficking gene. Mutations in this gene can cause methylmalonic aciduria or homocystinuria, or both [27].
Fig. 2. Regional association plots of the five identified saccular intracranial aneurysm (sIA) loci in the combined Finnish samples and the Dutch sample. 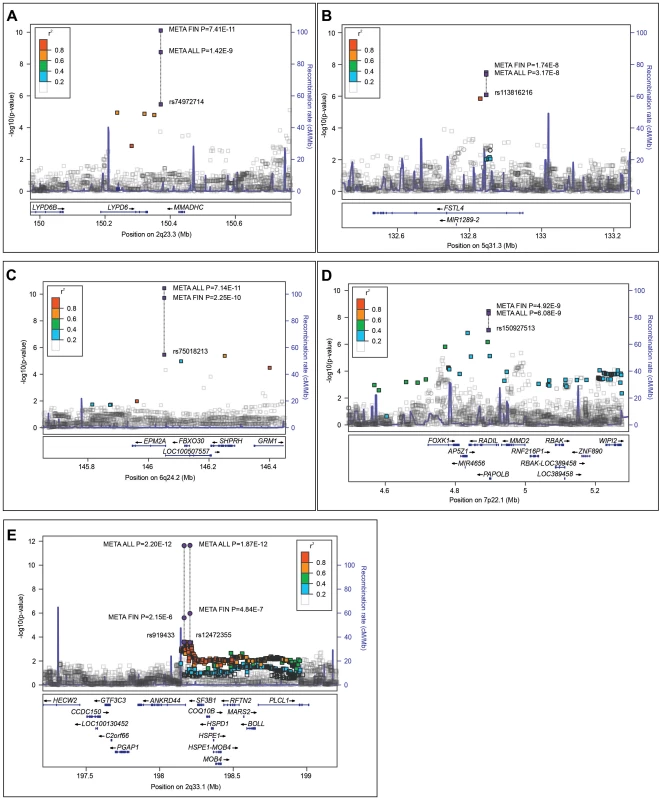
Association p-values (−log10 scale, y-axis) of variants are shown according to their chromosomal positions (x-axis). Blue lines indicate the genetic recombination rate (cM/Mb). Figures A–C present the loci identified in the case vs. control analysis at 2q23.3, 5q31.3, and 6q24.2, respectively. Figure D presents the 7p22.1 locus associated to the sIA count per patient. Figure E presents the 2q33.1 locus with inconclusive previous evidence. Purple rectangles indicate the most significant variant in a) the Finnish discovery sample and, along the dashed line, its p-values in b) the combined Finnish samples (META FIN) and in c) all samples (META ALL). Adjacent variants in linkage disequilibrium (r2; EUR populations, 1000 Genomes March 2012) to the index variant are shown in colours indicating their r2 levels (r2 box in each figure). 5q31.1 locus
The variant rs113816216 at 5q31.3 has a frequency of 1–3% in Finland and most other European populations, except in Spain (7%). It was significantly associated to the sIA disease in the Finnish samples and was also significant in the Dutch sample but had a somewhat lower OR there (Table 2). The meta-analysis of all combined samples exceeded the genome wide significance threshold. The variant is located in the intron of FSTL4 (Figure 2 B), a poorly characterized gene. FSTL1, a paralog of FSTL4, codes a protein inducing innate immunity as TLR4 agonist [28]. Increased tissue levels of FSTL1 were associated to the severity of heart failure [29] and to the coronary artery aneurysm formation in Kawasaki disease [30]. Variants in FSTL4 were modestly associated to human ischemic stroke [31], and a variant 70 kb from FSTL4 nominally to hypertension [32].
6q24.2 locus
The variant rs75018213 at 6q24.2 has similar frequencies (2%) in European populations, including Finns. It was significantly associated to the sIA disease in the Finnish samples and was also significant in the Dutch sample but had a somewhat lower OR there (Table 2). It is located in the intron of EPM2A. The LD spans over 300 kb downstream covering FBXO30, LOC100507557, SHPRH and GRM1 (Figure 2 C). In the ENCODE data, rs75018213 is located in a GATA2 transcription factor binding site RNA-seq peak. Homozygous deletions in the EPM2A gene result in progressive myoclonus epilepsy (PME) with Lafora bodies (OMIM 254780) [33]. No vascular anomalies have been reported in EPM2 deletion patients with a PME phenotype or their heterozygote parents. EPM2A encodes a phosphatase, which dephosphorylates glycogen, but it is likely that EPM2A has broader functions in regulation of glycogen biosynthesis, endoplasmic reticulum stress, autophagy, and possibly also cell cycle [34].
7p22.1 locus and the number of sIAs
The variant rs150927513 at 7p22.1 was significantly associated to sIA count per individual in the Finnish population (Table 1). Its frequency was 4.6% in the Finnish samples but only 0.3%, in the Dutch sample, in line with most European populations. This variant would therefore likely not have been identified if a sufficient number of Finnish individuals had not been included in the reference panel.
The variant is located in the intron of RADIL (Figure 2 D), a rap GTPase interactor, an essential effector of RAP1 in activation of integrins in cell-adhesive signalling by G protein-coupled receptors [35]. RADIL has also been shown to control, together with RAP1, neutrophil adhesion and chemotaxis [36]. Neutrophils seem to have a role in the formation and rupture of intracranial and abdominal aortic aneurysm [26], [37], [38]. The strongest eQTL association was to an exon of TNRC18 (FC 1.23, p = 5.1×10−5), a functionally uncharacterized gene.
As we analysed the number of sIAs as a count variable from 0–8, the inherent assumption was that the same variant would increase the risk of the first and the subsequent sIA formation. Thus, any variant associated to the number of sIAs will to some extent be associated in the case vs. control analysis. Indeed, in the analysis of combined Finnish cohorts rs150927513 was associated in the case-control analysis (OR 1.54, p = 6.5×10−7) and consistently also in the analysis of multiple vs. single sIA patients (OR 1.65, p = 8.4×10−4). The association of this variant, should be interpreted as reflecting the tendency of sIA formation, rather than considering multiple sIAs as a completely different dichotomous end point.
Previously identified 9p21.3 locus
The 9p21.3 locus has been robustly associated to the sIA disease [12] as well as to cardiovascular, metabolic and cancer traits [39], [40], and it has been extensively studied by others [41]. The allele frequency and effect size in the current study, although with a different lead SNP (r2 = 0.7 to previous lead SNP rs1333040), are in strong agreement with the previous study [12]. This locus is not therefore discussed further here.
2q33.1 locus with previously inconclusive evidence
Two common variants, rs12472355 and rs919433 at 2q33.1 were significantly associated to the sIA disease in the Finnish and Dutch samples (Table 2), rs919433 intronic and rs12472355 upstream 30 kb from ANKRD44 (Figure 2 E). The allele frequencies were somewhat higher in the Finnish samples (rs919433, 44%; rs12472355 43.7%) than in the Dutch samples (33.2%; 31%) or in the Japanese population according to 1000 Genomes Project (28.1%; 27.5%). In this locus, the risk allele was reversed in the Japanese cohort of the previous sIA GWA study [10]. ANKRD44 is likely a subunit of protein phosphatase 6 [42] that functions, e.g., in cell cycle control [43] and in inhibition of NF-κB activation [44]. NF-κB is a significant mediator in experimental sIA formation in rats, highly expressed in human sIA wall [45], and it was associated to human sIA wall rupture in transcriptomic profiling [26]. In eQTL analysis rs12472355 was significantly associated to ANKRD44 (FC 0.94, p = 1.83×10−5) and rs919433 to HSPD1 (FC 0.94, p = 3.8×10−5)
In conclusion, we identified four novel loci associated to sIA disease and confirmed one additional locus with previously inconclusive evidence, together explaining 2.1% of the sIA heritability in Finland. Our data illustrates the utility of high-risk population isolates, familial disease history, and dense genotype imputation in search for low-frequency variants associated to complex human diseases. The inclusion of Finnish individuals in the imputation reference panel and especially the highly differentiated variant in 7p22.1 would likely not have been identified
The identification of the four novel low frequency variants would likely have required much larger sample sizes in more mixed populations. Further studies of the identified five loci are needed to explain their functional mechanisms in the pathogenesis of sIA disease.
Materials and Methods
Ethics statement
For all of the Finnish and Dutch samples, the local ethics committees approved the study and all patients gave written informed consent.
Study samples
A. Finnish discovery sample
The initial discovery GWAS data consisted of previously Illumina genotyped 974 Finnish intracranial aneurysm patients and 740 controls [12]. The patients were collected from the registries of Neurosurgery, Kuopio University Hospital, and Neurosurgery, Helsinki University Hospital, solely serving their catchment populations in Eastern and Southern Finland, respectively. The sIAs were angiographically verified and the cases of subarachnoid hemorrhage from ruptured sIA (sIA-SAH) with computed tomography (CT). Patients with at least 1 first-degree relative carrying sIA disease were considered familial [8]. For the unruptured aneurysms we do not have the exact indications for these patients available. However in our aneurysm database in Neurosurgery of Kuopio University Hospital the indications for angiography of unruptured aneurysm patients were: 1) Incidental unruptured sIA (leading cause was headache) found in neuroimaging with non-related indications 383/467 = 83% 2) Incidential unruptured sIA found in neuroimaging screening of sIA family members 45/467 = 9.6% and 3) Symptomatic but unruptured sIA causing focal neurological symptoms 39/467 = 8.4%
The Helsinki Birth Cohort Study (HBCS) includes 8,760 individuals born in the Helsinki Central Hospital between 1934 and 1944 [46]. A subset of 1676 Illumina genotyped individuals were available for the present study. The Health 2000 Cohort (H2000) includes 2 402 Finns, and of those 2138 Illumina genotyped individuals were available for the present study [47], [48].
The discovery aneurysm cases, 740 population controls and Health 2000 controls have been used in the previous sIA GWA studies [10], [12].
The following 210 cases and 119 controls were removed from the discovery sample: fusiform aneurysm carriers (n = 5); duplicated cases (n = 9) and controls (n = 10); blind duplicate cases (n = 15) and controls (n = 5); genotyping rate <97% (29 cases, 31 controls); individuals with higher missingness from cryptically related pairs (Identity by descent (IBD)>0.1875, similarity halfway between 2nd and 3rd degree relatives: 69 cases, 55 controls); genetic distance to 5 nearest neighbours >4 standard deviations longer than the average distance (2 cases, 18 controls); patients not traceable from the database or with traumatic SAH (n = 81); polycystic kidney disease (n = 4).
The following SNPs were removed: missing genotypes >5%; minor allele frequency <1%; Hardy-Weinberg disequilibrium p-value in controls <1*10-6; symmetric SNPs (A/T, C/G); and SNPs not on all the genotyping platforms.
To minimize false positives, each sIA case was matched to three controls by gender and genetic distance from control individuals. First, a sliding window approach was used to thin the set of SNPs to be approximately independent of each other. A sliding window of 1500 SNPs was shifted by 150 SNPs at a time along chromosomes, and in each step SNPs were filtered if any pairwise r2 was >0.2, resulting in 79596 independent SNPs. Pairwise IBS distances of these SNPs were used in multidimensional scaling and four first dimensions were used in matching. Plink v. 1.07 [49] was used for thinning and MDS analysis. R package optmatch was used to pair each case to three controls. After 1∶3 matching, additionally all Eastern Finnish controls from the previous sIA study were included [12].
The final discovery sample consisted of 760 sIA cases and 2,513 controls (Table 1). After SNP filtering, there were 304,399 genotyped SNPs and 9,046,433 imputed SNPs and indels (see imputation paragraph for imputation QC) for the discovery sample.
B. Finnish replication sample
The replication sample consisted of 858 independent sIA patients from the registry of Neurosurgery, Kuopio University Hospital. There were 1,605 independent controls, 453 from Eastern Finland and 1152 from the FINRISK study, both genotyped using the Sequenom iPLEX technique. Additionally, 2,443 whole genome genotyped controls from The Cardiovascular Risk in Young Finns Study were acquired and replication SNPs were extracted after imputation (Table 1).
The Cardiovascular Risk in Young Finns Study is a follow-up study of cardiovascular risk factors from childhood to adulthood [50], [51]. The participants were randomly chosen from the Finnish Population Registry and recruited from five university cities in Finland. The baseline study launched in 1980 and included 3,596 individuals. Follow-ups have taken place at every three to six years with the last one in 2007 at 27 years of age.
The FINRISK cohort is a national survey on risk factors of chronic and non-communicable diseases in Finland [52]. The survey has been conducted every five years since 1972 in randomly selected, representative population samples from different parts of Finland.
C. Dutch replication sample
The Dutch sample consisted of previously GWAS genotyped 786 Dutch sIA cases (Yasuno 2010), and the 3,110 controls were recruited as part of the Nijmegen Biomedical Study (n = 1,832) and the Nijmegen Bladder Cancer Study (n = 1,278) [53], [54]. The relevant medical ethical committees approved all studies and all participants provided written informed consent.
The patients were admitted to the Utrecht University Medical Center between 1997 and 2007. The sIA-SAH cases were verified with CT scan and sIAs by angiography. Unruptured sIAs were identified by angiography in the absence of clinical or radiological signs of SAH [12]. Patients reporting at least 1 first-degree relative carrying sIA disease were considered familial.
The Nijmegen Biomedical Study is a population based cross-sectional study conducted by the Radboud University Nijmegen Medical Centre [53], [54]. Age and sex stratified, randomly selected adults (≥18 years) of Nijmegen (n = 22,452) received an invitation to fill out a postal questionnaire on lifestyle and medical history.
The following cases and controls were excluded: missingness ≥0.05 (n = 10); IBD≥0.2 (n = 102); heterozygosity >/<3 standard deviations from the mean (n = 46); and principal component analysis outliers (n = 43). The intersection of SNPs in different platforms was first extracted and symmetric SNPs were removed (A/T, C/G). SNPs prior to the imputation were filtered by the following QC criteria: genotype missingness >0.05; MAF<0.01; HWE p<0.001; differential missingness between cases and controls p<1E-5.
The final Dutch replication sample consisted of 717 cases and 3,004 controls (Table 1).
Replication strategy
From both of the analyses (the case vs. controls and the number of sIAs) the best independent SNPs were taken to replication if p<5E-6. Additional significant independent SNPs in a locus was tested by analyzing each SNP within 1 MB from the top SNP while adding the top SNP as a covariate. Additionally the most significant SNP in the current study in 2q33.1 region with uncertain evidence in previous sIA GWASs was taken to replication. Variant was considered replicated if it reached one-tailed significance of p<0.05 and was consistent in terms of risk allele. In all of the results, one-tailed p-values are given for the Finnish replication and in Dutch results.
Genotyping
Genomic DNA was extracted from peripheral blood and genotyped by Illumina arrays: the Finnish discovery sample and the Dutch replication cases by CNV370k DUO chip; the HBCS and YFS controls by Illumina Human670K customBeadChip; and the H2000 controls by Illumina Infinium HDHuman610-Quad BeadChip.
In the Finnish replication sample, DNA was genotyped using Sequenom MassARRAY system and iPLEX Gold assays (Sequenom Inc., San Diego, USA). The data was collected using the MassARRAY Compact System (Sequenom) and the genotypes were called using TyperAnalyzer software (Sequenom). Genotyping quality was examined by a detailed QC procedure consisting of success rate checks, duplicates, water controls and Hardy-Weinberg Equilibrium (HWE) testing. SNPs were filtered if genotype missingness >0.05 or if HWE p<0.001.
Imputation
For imputation of additional genotypes in the discovery sample, the Young Finns replication cohort and in the 2nd Dutch replication sample the genotypes were first pre-phased [55] using the Shape-IT [56] phasing software and the pre-phased haplotypes were subjected to imputation. The Impute version 2.2.2 software [57] with 1,000 Genomes Phase I integrated variant set release (v3) reference panel (05 Mar 2012 release downloaded from http://mathgen.stats.ox.ac.uk/impute/data_download_1000G_phase1_integrated.html) was used. Imputed genotypes were filtered if the Impute info measure was <0.5 or minor allele frequency <0.01 in the Finnish discovery sample.
eQTL analysis
We analyzed whether the identified genome-wide significant SNPs might affect gene expression by using the European samples of the Geuvadis RNA-sequencing data set, with mRNA sequencing data from LCLs of 373 samples from the FIN, CEU, GBR and TSI populations of 1000 Genomes project (for details, see [21]).
We did eQTL analysis for each of the associating variants and all the genes within a 1 MB window that were expressed in >50% of the individuals (Table eQTL). We used exon quantifications based on individual read counts per exon, after correction by the total number of mapped reads per sample and PEER normalization to remove technical variation. For each exon, we calculated linear regression between these expression values and genotype dosage of the associating variants in the 1000 Genomes data.
Regional association plots
Regional association plots were generated using LocusZoom with LD data from European populations of 1000 Genomes project (Hg19/March 2012) [58].
Search of regulatory elements at identified variants
The UCSC Genome Browser and HaploReg version 2 [19] were used to search for ENCODE regulatory element regions located at the five genome-wide significant variants. HaploReg database also annotates if SNP resides on a putative transcription-factor binding site (TFBS) according to Transfac or Jaspar TFBS profiles and also 10 most enriched TFBS profiles identified in ENCODE TF Chip-Seq peaks. We used all the Jaspar and Transfac annotations and three most enriched ENCODE based TFBS annotations for each TF.
Statistical analysis
GWA was performed against two complementary phenotypes: the case vs. control status and the number of sIAs.
Case vs. control analysis
SNPTEST v2.3.0 was used for the association analysis, assuming additive effect. Genotype uncertainty in the imputed SNPs was taken in to account by treating them as continuous expected genotype dosages. The gender was used as a covariate.
Aneurysm count analysis
The Vuong test [59] showed that the negative binomial model was a significantly better fit to the sIA count per individual when compared to the Poisson model. The zero-inflated negative binomial model was not significantly better either, so the simpler negative binomial model (glm.nb function in MASS R package) was used. When assessing the model fits, the gender was used as a predictor. Imputation uncertainty was taken in to account by treating the imputed SNPs as continuous expected genotype dosages, and the gender was used as a covariate.
Meta-analysis
The association evidence from the discovery and replication samples were combined by inverse variance-weighted fixed-effects meta-analysis, using Plink v.1.07 [49]. Heterogeneity statistic I2 and confidence intervals were calculated according to Higgins et al. [60] using metafor R package [61].
Genome-wide significance level estimation
As the standard genome-wide significance value of 5 * 10−8 is estimated to correct for independent tests when testing all common variants (MAF> = 5%). As we tested variants with MAF> = 1%, the standard genome-wide significance may be liberal. A simple Bonferroni correction would be much too string because of correlation between tested variants.
We estimated approximately independent number of variants by analysing chromosomes 1 and 7 of European individuals of the 1000 Genomes Project. We pruned the set of variants to be approximately independent (pairwise r2< = 0.6 within 250 kb of each other) using WDIST (https://www.cog-genomics.org/wdist/). This resulted in 308547 and 358834 independent variants out of 2215231 and 2553047 respectively. Taking the same proportion (14%) of SNPs from the 9 359 231 variants in the discovery is 1 303 594 variants which yields genome-wide significance of 3.82 * 10-8. We similarly estimated squared correlation r2 of the 528677 genotyped and imputed variants of all 3273 discovery samples in chromosome 7 using custom Python script. The proportion of approximately independent variants was 53 909 (10.2%), which is lower than in the full set of 1000 Genomes variants (threshold 5.2 * 10-8).
Heritability analysis
The fraction of additive genetic variance explained by the five identified loci was estimated using the liability threshold model [62]. The model assumes an additive effect at each locus, which shifts the mean of a normally distributed distribution of disease liability for each genotype. The combined genetic variance explained by the five SNPs (rs74972714, rs113816216, rs7501821, rs1509275133, rs12472355) in the five loci was assumed to be the sum of variances explained by each SNP. Risk allele frequencies in controls and OR's from combined Finnish samples was used and population prevalence of 3% of the sIA disease was assumed [1]. Heritability of the six previously identified lead SNPs (rs9298506, rs1333040, rs12413409, rs9315204, rs11661542, rs6841581) was estimated using the allele frequencies and effect sizes from the discovery cohort of the current study.
Supporting Information
Zdroje
1. VlakMH, AlgraA, BrandenburgR, RinkelGJ (2011) Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: a systematic review and meta-analysis. Lancet Neurol 10 : 626–636.
2. RonkainenA, MiettinenH, KarkolaK, PapinahoS, VanninenR, et al. (1998) Risk of harboring an unruptured intracranial aneurysm. Stroke 29 : 359–362.
3. Van GijnJ, KerrRS, RinkelGJE (2007) Subarachnoid haemorrhage. Lancet 369 : 306–318.
4. FeiginVL, LawesCMM, BennettDA, Barker-ColloSL, ParagV (2009) Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurol 8 : 355–369.
5. De RooijNK, LinnFHH, van der PlasJA, AlgraA, RinkelGJE (2007) Incidence of subarachnoid haemorrhage: a systematic review with emphasis on region, age, gender and time trends. J Neurol Neurosurg Psychiatry 78 : 1365–1372.
6. FeiginVL, RinkelGJE, LawesCMM, AlgraA, BennettDA, et al. (2005) Risk factors for subarachnoid hemorrhage: an updated systematic review of epidemiological studies. Stroke 36 : 2773–2780.
7. RonkainenA, HernesniemiJ, PuranenM, NiemitukiaL, VanninenR, et al. (1997) Familial intracranial aneurysms. Lancet 349 : 380–384.
8. HuttunenT, von und zu FraunbergM, FrösenJ, LeheckaM, TrompG, et al. (2010) Saccular intracranial aneurysm disease: distribution of site, size, and age suggests different etiologies for aneurysm formation and rupture in 316 familial and 1454 sporadic eastern Finnish patients. Neurosurgery 66 : 631–8 discussion 638.
9. RuigrokYM, BuskensE, RinkelGJ (2001) Attributable risk of common and rare determinants of subarachnoid hemorrhage. Stroke 32 : 1173–1175.
10. YasunoK, BakirciogluM, LowS-K, BilgüvarK, GaálE, et al. (2011) Common variant near the endothelin receptor type A (EDNRA) gene is associated with intracranial aneurysm risk. Proc Natl Acad Sci U S A
11. LowS-K, TakahashiA, ChaP-C, ZembutsuH, KamataniN, et al. (2012) Genome-wide association study for intracranial aneurysm in the Japanese population identifies three candidate susceptible loci and a functional genetic variant at EDNRA. Hum Mol Genet 21 : 2102–2110.
12. YasunoK, BilguvarK, BijlengaP, LowS-K, KrischekB, et al. (2010) Genome-wide association study of intracranial aneurysm identifies three new risk loci. Nat Genet 42 : 420–425.
13. BilguvarK, YasunoK, NiemeläM, RuigrokYM, von Und Zu FraunbergM, et al. (2008) Susceptibility loci for intracranial aneurysm in European and Japanese populations. Nat Genet 40 : 1472–1477.
14. The 1000 Genomes Project Consortium (2012) An integrated map of genetic variation from 1,092 human genomes. Nature 135 : 0–9.
15. PeltonenL, Jalankoa, VariloT (1999) Molecular genetics of the Finnish disease heritage. Hum Mol Genet 8 : 1913–1923.
16. RuigrokYM, RinkelGJE, AlgraA, RaaymakersTWM, Van GijnJ (2004) Characteristics of intracranial aneurysms in patients with familial subarachnoid hemorrhage. Neurology 62 : 891–894.
17. MackeyJ (2012) Unruptured intracranial aneurysms in the Familial Intracranial Aneurysm and International Study of Unruptured Intracranial Aneurysms cohorts: differences in multiplicity and location. J Neurosurg 117 : 192.
18. AkiyamaK, NaritaA, NakaokaH, CuiT, TakahashiT, et al. (2010) Genome-wide association study to identify genetic variants present in Japanese patients harboring intracranial aneurysms. J Hum Genet 55 : 656–661.
19. WardLD, KellisM (2012) HaploReg: a resource for exploring chromatin states, conservation, and regulatory motif alterations within sets of genetically linked variants. Nucleic Acids Res 40: D930–4.
20. ErnstJ, KheradpourP, MikkelsenTS, ShoreshN, LucasD, et al. (2011) Systematic analysis of chromatin state dynamics in nine human cell types. Nature 473 : 43–49.
21. LappalainenT, SammethM, FriedländerMR, 't Hoen P aC, MonlongJ, et al. (2013) Transcriptome and genome sequencing uncovers functional variation in humans. Nature 501 : 506–511.
22. SulemP, GudbjartssonDF, WaltersGB, HelgadottirHT, HelgasonA, et al. (2011) Identification of low-frequency variants associated with gout and serum uric acid levels. Nat Genet 43 : 1127–1130.
23. JonssonT, StefanssonH, SteinbergS, JonsdottirI, Jonsson PV, et al. (2013) Variant of TREM2 associated with the risk of Alzheimer's disease. N Engl J Med 368 : 107–116.
24. ZhangY, LangQ, LiJ, XieF, WanB, et al. (2010) Identification and characterization of human LYPD6, a new member of the Ly-6 superfamily. Mol Biol Rep 37 : 2055–2062.
25. NigroP, AbeJ-I, BerkBC (2011) Flow shear stress and atherosclerosis: a matter of site specificity. Antioxid Redox Signal 15 : 1405–1414.
26. KurkiMI, HäkkinenSK, FrösenJ, TulamoR, FraunbergM, et al. (2011) Upregulated signaling pathways in ruptured human saccular intracranial aneurysm wall: an emerging regulative role of Toll like receptor signaling and NF-κB, HIF1A and ETS transcription factors. Neurosurgery 68 : 1667–1676.
27. Lerner-ellisJP, RosenblattDS, NewboldRF, BaumgartnerMR, FowlerB (2008) Gene Identification for the cblD Defect of Vitamin B12 Metabolism. N Engl J Med 358 : 1454–1464.
28. MurakamiK, TanakaM, UsuiT, KawabataD, ShiomiA, et al. (2012) Follistatin-related protein/follistatin-like 1 evokes an innate immune response via CD14 and toll-like receptor 4. FEBS Lett 586 : 319–324.
29. Lara-PezziE, FelkinLE, BirksEJ, SarathchandraP, PanseKD, et al. (2008) Expression of follistatin-related genes is altered in heart failure. Endocrinology 149 : 5822–5827.
30. GorelikM, WilsonDC, CloonanYK, ShulmanST, HirschR (2012) Plasma follistatin-like protein 1 is elevated in Kawasaki disease and may predict coronary artery aneurysm formation. J Pediatr 161 : 116–119.
31. LukeMM, O'MearaES, RowlandCM, ShiffmanD, Bare La, et al. (2009) Gene variants associated with ischemic stroke: the cardiovascular health study. Stroke 40 : 363–368.
32. GuoY, TomlinsonB, ChuT, FangYJ, GuiH, et al. (2012) A genome-wide linkage and association scan reveals novel loci for hypertension and blood pressure traits. PLoS One 7: e31489.
33. MinassianBA, LeeJR, HerbrickJA, HuizengaJ, SoderS, et al. (1998) Mutations in a gene encoding a novel protein tyrosine phosphatase cause progressive myoclonus epilepsy. Nat Genet 20 : 171–174.
34. GentryMS, Romá-MateoC, SanzP (2013) Laforin, a protein with many faces: glucan phosphatase, adapter protein, et alii. FEBS J 280 : 525–537.
35. AhmedSM, DaulatAM, MeunierA, AngersS (2010) G protein betagamma subunits regulate cell adhesion through Rap1a and its effector Radil. J Biol Chem 285 : 6538–6551.
36. LiuL, AerbajinaiW, AhmedSM, RodgersGP, AngersS, et al. (2012) Radil controls neutrophil adhesion and motility through β2-integrin activation. Mol Biol Cell 23(24): 4751–65.
37. FrösenJ, TulamoR, PaetauA, LaaksamoE, KorjaM, et al. (2012) Saccular intracranial aneurysm: pathology and mechanisms. Acta Neuropathol 123(6): 773–86.
38. EliasonJL, HannawaKK, AilawadiG, SinhaI, FordJW, et al. (2005) Neutrophil depletion inhibits experimental abdominal aortic aneurysm formation. Circulation 112 : 232–240.
39. HelgadottirA, ThorleifssonG, MagnussonKP, GrétarsdottirS, SteinthorsdottirV, et al. (2008) The same sequence variant on 9p21 associates with myocardial infarction, abdominal aortic aneurysm and intracranial aneurysm. Nat Genet 40 : 217–224.
40. WellcomeT, CaseT, ConsortiumC (2007) Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature 447 : 661–678.
41. JohnsonAD, HwangS-J, VoormanA, MorrisonA, PelosoGM, et al. (2013) Resequencing and clinical associations of the 9p21.3 region: a comprehensive investigation in the framingham heart study. Circulation 127 : 799–810.
42. StefanssonB, OhamaT, DaughertyAE, BrautiganDL (2008) Protein phosphatase 6 regulatory subunits composed of ankyrin repeat domains. Biochemistry 47 : 1442–1451.
43. StefanssonB, BrautiganDL (2007) Protein phosphatase PP6 N terminal domain restricts G1 to S phase progression in human cancer cells. Cell Cycle 6 : 1386–1392.
44. StefanssonB, BrautiganDL (2006) Protein phosphatase 6 subunit with conserved Sit4-associated protein domain targets IkappaBepsilon. J Biol Chem 281 : 22624–22634.
45. TomohiroAoki, HiroharuKataoka, MunehisaShimamura, HironoriNakagami, KoujiWakayama, et al. (2007) NF-B Is a Key Mediator of Cerebral Aneurysm Formation. Circulation 116 : 2830.
46. BarkerDJP, OsmondC, ForsénTJ, KajantieE, ErikssonJG (2005) Trajectories of growth among children who have coronary events as adults. N Engl J Med 353 : 1802–1809.
47. Aromaa A, Koskinen S, editors (2004) HEALTH AND FUNCTIONAL CAPACITY IN FINLAND. Baseline Results of the Health 2000 Health Examination Survey. Publications of the National Public Health Institute.
48. THL - National Institute for Health and Welfare. (2000) Health (2000). Available: http://www.terveys2000.fi/indexe.html. Accessed 22 January 2013.
49. PurcellS, NealeB, ToddbrownK, ThomasL, FerreiraM, et al. (2007) PLINK: A Tool Set for Whole-Genome Association and Population-Based Linkage Analyses. Am J Hum Genet 81 : 559–575.
50. RaitakariOT, JuonalaM, RönnemaaT, Keltikangas-JärvinenL, RäsänenL, et al. (2008) Cohort profile: the cardiovascular risk in Young Finns Study. Int J Epidemiol 37 : 1220–1226.
51. The Cardiovascular Risk in Young Finns Study (2008). Available: http://vanha.med.utu.fi/cardio/youngfinnsstudy/index.html. Accessed 22 January 2013.
52. VartiainenE, LaatikainenT, PeltonenM, JuoleviA, MännistöS, et al. (2010) Thirty-five-year trends in cardiovascular risk factors in Finland. Int J Epidemiol 39 : 504–518.
53. WetzelsJFM, KiemeneyLA, SwinkelsDW, WillemsHL, den HeijerM (2007) Age - and gender-specific reference values of estimated GFR in Caucasians: the Nijmegen Biomedical Study. Kidney Int 72 : 632–637.
54. KiemeneyLA, ThorlaciusS, SulemP, GellerF, AbenKKH, et al. (2008) Sequence variant on 8q24 confers susceptibility to urinary bladder cancer. Nat Genet 40 : 1307–1312.
55. HowieB, FuchsbergerC, StephensM, MarchiniJ, AbecasisGR (2012) Fast and accurate genotype imputation in genome-wide association studies through pre-phasing. Nat Genet 44 : 955–959.
56. DelaneauO, MarchiniJ, ZaguryJ-F (2012) A linear complexity phasing method for thousands of genomes. Nat Methods 9 : 179–181.
57. HowieBN, DonnellyP, MarchiniJ (2009) A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet 5: e1000529.
58. PruimRJ, WelchRP, SannaS, TeslovichTM, ChinesPS, et al. (2010) LocusZoom: regional visualization of genome-wide association scan results. Bioinformatics 26 : 2336–2337.
59. VuongQH (1989) LIkelihood Ratio Tests for Model Selection and Non-Nested Hypotheses. Econometrica 57 : 307–333.
60. HigginsJPT, ThompsonSG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21 : 1539–1558.
61. ViechtbauerW (2010) Conducting Meta-Analyses in R with the metafor Package. J Stat Softw 36.
62. SoH-C, GuiAHS, ChernySS, ShamPC (2011) Evaluating the heritability explained by known susceptibility variants: a survey of ten complex diseases. Genet Epidemiol 35 : 310–317.
Štítky
Genetika Reprodukční medicína
Článek Unwrapping BacteriaČlánek A Chaperone-Assisted Degradation Pathway Targets Kinetochore Proteins to Ensure Genome StabilityČlánek The Candidate Splicing Factor Sfswap Regulates Growth and Patterning of Inner Ear Sensory OrgansČlánek The SPF27 Homologue Num1 Connects Splicing and Kinesin 1-Dependent Cytoplasmic Trafficking inČlánek Down-Regulation of eIF4GII by miR-520c-3p Represses Diffuse Large B Cell Lymphoma DevelopmentČlánek Meta-Analysis Identifies Gene-by-Environment Interactions as Demonstrated in a Study of 4,965 Mice
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2014 Číslo 1
-
Všechny články tohoto čísla
- How Much Is That in Dog Years? The Advent of Canine Population Genomics
- The Sense and Sensibility of Strand Exchange in Recombination Homeostasis
- Unwrapping Bacteria
- DNA Methylation Changes Separate Allergic Patients from Healthy Controls and May Reflect Altered CD4 T-Cell Population Structure
- Evidence for Mito-Nuclear and Sex-Linked Reproductive Barriers between the Hybrid Italian Sparrow and Its Parent Species
- Translation Enhancing ACA Motifs and Their Silencing by a Bacterial Small Regulatory RNA
- Relationship Estimation from Whole-Genome Sequence Data
- Genetic Models of Apoptosis-Induced Proliferation Decipher Activation of JNK and Identify a Requirement of EGFR Signaling for Tissue Regenerative Responses in
- ComEA Is Essential for the Transfer of External DNA into the Periplasm in Naturally Transformable Cells
- Loss and Recovery of Genetic Diversity in Adapting Populations of HIV
- Bioelectric Signaling Regulates Size in Zebrafish Fins
- Defining NELF-E RNA Binding in HIV-1 and Promoter-Proximal Pause Regions
- Loss of Histone H3 Methylation at Lysine 4 Triggers Apoptosis in
- Cell-Cycle Dependent Expression of a Translocation-Mediated Fusion Oncogene Mediates Checkpoint Adaptation in Rhabdomyosarcoma
- How a Retrotransposon Exploits the Plant's Heat Stress Response for Its Activation
- A Nonsense Mutation in Encoding a Nondescript Transmembrane Protein Causes Idiopathic Male Subfertility in Cattle
- Deletion of a Conserved -Element in the Locus Highlights the Role of Acute Histone Acetylation in Modulating Inducible Gene Transcription
- Developmental Link between Sex and Nutrition; Regulates Sex-Specific Mandible Growth via Juvenile Hormone Signaling in Stag Beetles
- PP2A/B55 and Fcp1 Regulate Greatwall and Ensa Dephosphorylation during Mitotic Exit
- Differential Effects of Collagen Prolyl 3-Hydroxylation on Skeletal Tissues
- Comprehensive Functional Annotation of 77 Prostate Cancer Risk Loci
- Evolution of Chloroplast Transcript Processing in and Its Chromerid Algal Relatives
- A Chaperone-Assisted Degradation Pathway Targets Kinetochore Proteins to Ensure Genome Stability
- New MicroRNAs in —Birth, Death and Cycles of Adaptive Evolution
- A Genome-Wide Screen for Bacterial Envelope Biogenesis Mutants Identifies a Novel Factor Involved in Cell Wall Precursor Metabolism
- FGFR1-Frs2/3 Signalling Maintains Sensory Progenitors during Inner Ear Hair Cell Formation
- Regulation of Synaptic /Neuroligin Abundance by the /Nrf Stress Response Pathway Protects against Oxidative Stress
- Intrasubtype Reassortments Cause Adaptive Amino Acid Replacements in H3N2 Influenza Genes
- Molecular Specificity, Convergence and Constraint Shape Adaptive Evolution in Nutrient-Poor Environments
- WNT7B Promotes Bone Formation in part through mTORC1
- Natural Selection Reduced Diversity on Human Y Chromosomes
- In-Vivo Quantitative Proteomics Reveals a Key Contribution of Post-Transcriptional Mechanisms to the Circadian Regulation of Liver Metabolism
- The Candidate Splicing Factor Sfswap Regulates Growth and Patterning of Inner Ear Sensory Organs
- The Acid Phosphatase-Encoding Gene Contributes to Soybean Tolerance to Low-Phosphorus Stress
- p53 and TAp63 Promote Keratinocyte Proliferation and Differentiation in Breeding Tubercles of the Zebrafish
- Affects Plant Architecture by Regulating Local Auxin Biosynthesis
- The SET Domain Proteins SUVH2 and SUVH9 Are Required for Pol V Occupancy at RNA-Directed DNA Methylation Loci
- Down-Regulation of Rad51 Activity during Meiosis in Yeast Prevents Competition with Dmc1 for Repair of Double-Strand Breaks
- Multi-tissue Analysis of Co-expression Networks by Higher-Order Generalized Singular Value Decomposition Identifies Functionally Coherent Transcriptional Modules
- A Neurotoxic Glycerophosphocholine Impacts PtdIns-4, 5-Bisphosphate and TORC2 Signaling by Altering Ceramide Biosynthesis in Yeast
- Subtle Changes in Motif Positioning Cause Tissue-Specific Effects on Robustness of an Enhancer's Activity
- C/EBPα Is Required for Long-Term Self-Renewal and Lineage Priming of Hematopoietic Stem Cells and for the Maintenance of Epigenetic Configurations in Multipotent Progenitors
- The SPF27 Homologue Num1 Connects Splicing and Kinesin 1-Dependent Cytoplasmic Trafficking in
- Down-Regulation of eIF4GII by miR-520c-3p Represses Diffuse Large B Cell Lymphoma Development
- Genome Sequencing Highlights the Dynamic Early History of Dogs
- Re-sequencing Expands Our Understanding of the Phenotypic Impact of Variants at GWAS Loci
- Meta-Analysis Identifies Gene-by-Environment Interactions as Demonstrated in a Study of 4,965 Mice
- , a -Antisense Gene of , Encodes a Evolved Protein That Inhibits GSK3β Resulting in the Stabilization of MYCN in Human Neuroblastomas
- A Transcription Factor Is Wound-Induced at the Planarian Midline and Required for Anterior Pole Regeneration
- A Comprehensive tRNA Deletion Library Unravels the Genetic Architecture of the tRNA Pool
- A PNPase Dependent CRISPR System in
- Genomic Confirmation of Hybridisation and Recent Inbreeding in a Vector-Isolated Population
- Zinc Finger Transcription Factors Displaced SREBP Proteins as the Major Sterol Regulators during Saccharomycotina Evolution
- GATA6 Is a Crucial Regulator of Shh in the Limb Bud
- Tissue Specific Roles for the Ribosome Biogenesis Factor Wdr43 in Zebrafish Development
- A Cell Cycle and Nutritional Checkpoint Controlling Bacterial Surface Adhesion
- High Risk Population Isolate Reveals Low Frequency Variants Predisposing to Intracranial Aneurysms
- E3 Ubiquitin Ligase CHIP and NBR1-Mediated Selective Autophagy Protect Additively against Proteotoxicity in Plant Stress Responses
- Evolutionary Rate Covariation Identifies New Members of a Protein Network Required for Female Post-Mating Responses
- 3′ Untranslated Regions Mediate Transcriptional Interference between Convergent Genes Both Locally and Ectopically in
- Single Nucleus Genome Sequencing Reveals High Similarity among Nuclei of an Endomycorrhizal Fungus
- Metabolic QTL Analysis Links Chloroquine Resistance in to Impaired Hemoglobin Catabolism
- Notch Controls Cell Adhesion in the Drosophila Eye
- AL PHD-PRC1 Complexes Promote Seed Germination through H3K4me3-to-H3K27me3 Chromatin State Switch in Repression of Seed Developmental Genes
- Genomes Reveal Evolution of Microalgal Oleaginous Traits
- Large Inverted Duplications in the Human Genome Form via a Fold-Back Mechanism
- Variation in Genome-Wide Levels of Meiotic Recombination Is Established at the Onset of Prophase in Mammalian Males
- Age, Gender, and Cancer but Not Neurodegenerative and Cardiovascular Diseases Strongly Modulate Systemic Effect of the Apolipoprotein E4 Allele on Lifespan
- Lifespan Extension Conferred by Endoplasmic Reticulum Secretory Pathway Deficiency Requires Induction of the Unfolded Protein Response
- Is Non-Homologous End-Joining Really an Inherently Error-Prone Process?
- Vestigialization of an Allosteric Switch: Genetic and Structural Mechanisms for the Evolution of Constitutive Activity in a Steroid Hormone Receptor
- Functional Divergence and Evolutionary Turnover in Mammalian Phosphoproteomes
- A 660-Kb Deletion with Antagonistic Effects on Fertility and Milk Production Segregates at High Frequency in Nordic Red Cattle: Additional Evidence for the Common Occurrence of Balancing Selection in Livestock
- Comparative Evolutionary and Developmental Dynamics of the Cotton () Fiber Transcriptome
- The Transcription Factor BcLTF1 Regulates Virulence and Light Responses in the Necrotrophic Plant Pathogen
- Crossover Patterning by the Beam-Film Model: Analysis and Implications
- Single Cell Genomics: Advances and Future Perspectives
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- GATA6 Is a Crucial Regulator of Shh in the Limb Bud
- Large Inverted Duplications in the Human Genome Form via a Fold-Back Mechanism
- Differential Effects of Collagen Prolyl 3-Hydroxylation on Skeletal Tissues
- Affects Plant Architecture by Regulating Local Auxin Biosynthesis
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



