-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaT Cell-Dependence of Lassa Fever Pathogenesis
Lassa virus (LASV), the causative agent of Lassa fever (LF), is endemic in West Africa, accounting for substantial morbidity and mortality. In spite of ongoing research efforts, LF pathogenesis and mechanisms of LASV immune control remain poorly understood. While normal laboratory mice are resistant to LASV, we report that mice expressing humanized instead of murine MHC class I (MHC-I) failed to control LASV infection and develop severe LF. Infection of MHC-I knockout mice confirmed a key role for MHC-I-restricted T cell responses in controlling LASV. Intriguingly we found that T cell depletion in LASV-infected HHD mice prevented disease, irrespective of high-level viremia. Widespread activation of monocyte/macrophage lineage cells, manifest through inducible NO synthase expression, and elevated IL-12p40 serum levels indicated a systemic inflammatory condition. The absence of extensive monocyte/macrophage activation in T cell-depleted mice suggested that T cell responses contribute to deleterious innate inflammatory reactions and LF pathogenesis. Our observations in mice indicate a dual role for T cells, not only protecting from LASV, but also enhancing LF pathogenesis. The possibility of T cell-driven enhancement and immunopathogenesis should be given consideration in future LF vaccine development.
Published in the journal: . PLoS Pathog 6(3): e32767. doi:10.1371/journal.ppat.1000836
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1000836Summary
Lassa virus (LASV), the causative agent of Lassa fever (LF), is endemic in West Africa, accounting for substantial morbidity and mortality. In spite of ongoing research efforts, LF pathogenesis and mechanisms of LASV immune control remain poorly understood. While normal laboratory mice are resistant to LASV, we report that mice expressing humanized instead of murine MHC class I (MHC-I) failed to control LASV infection and develop severe LF. Infection of MHC-I knockout mice confirmed a key role for MHC-I-restricted T cell responses in controlling LASV. Intriguingly we found that T cell depletion in LASV-infected HHD mice prevented disease, irrespective of high-level viremia. Widespread activation of monocyte/macrophage lineage cells, manifest through inducible NO synthase expression, and elevated IL-12p40 serum levels indicated a systemic inflammatory condition. The absence of extensive monocyte/macrophage activation in T cell-depleted mice suggested that T cell responses contribute to deleterious innate inflammatory reactions and LF pathogenesis. Our observations in mice indicate a dual role for T cells, not only protecting from LASV, but also enhancing LF pathogenesis. The possibility of T cell-driven enhancement and immunopathogenesis should be given consideration in future LF vaccine development.
Introduction
Lassa virus (LASV) is the causative agent of Lassa fever (LF) [1]. It accounts for an estimated number of 300′000 infections and several thousand deaths in endemic areas each year [2], while imported cases have been reported from around the globe [3]. The virus is listed category A by the Center for Disease Control and Prevention [4]. So far, LASV vaccines have remained unavailable for clinical use, and Ribavirin, the only available therapy, has shown limited efficacy [5]. The development of effective vaccination strategies would therefore benefit from further insight into the mechanisms of successful LASV immune control, as well as into the processes underlying LF development and pathogenesis.
It is generally agreed upon that the level of tissue damage observed at autopsy cannot by itself account for the severe nature of LF. Therefore, as with other viral hemorrhagic fevers [6],[7], a contribution of the host response to LF pathogenesis has long been a matter of debate. For instance, the manifestation of Dengue Hemorrhagic Fever (DHF) has long been accredited to pre-existing immunity [8],[9]. Apart from serotype cross-reactive antibodies [8],[9], memory T cells were recently identified as important players in the disease process [10], and susceptibility as well as resistance to DHF have been linked to particular MHC alleles [11],[12]. In addition, infected monocytes and macrophages play an important role in DHF by secreting inflammatory cytokines [13],[14].
Such contributions of the immune response to disease severity can represent a major hurdle in vaccine development [15]. For instance, formalin-inactivated vaccines to respiratory syncytial virus (RSV) and measles virus resulted in enhanced morbidity and mortality in response to natural infection [16],[17]. Animal models for RSV have since provided evidence that T cell subsets play an important role in disease enhancement [16],[18]. Interestingly, innate immune cells including eosinophils and polymorphonuclear granulocytes dominate the histological picture upon T cell-driven enhancement of RSV [18]. Similarly, inflammatory macrophage responses were found to be a common feature of viral hemorrhagic fevers [6]. In accordance with the “cytokine storm” hypothesis, macrophage-derived inflammatory cytokines [19],[20],[21],[22],[23] and nitric oxide (NO) [24],[25] are candidate mediators of capillary leakage and shock [26], and elevated levels of such mediators correlate with increased disease severity and worsened clinical outcome.
Still, LASV lacks a clear pathognomonic signature, and clinical manifestations of LF are largely unspecific, making it difficult to diagnose the infection accurately via clinical criteria alone [27]. In contrast to other hemorrhagic fevers, coagulation abnormalities and bleeding are largely absent in LF [27],[28], leading some to argue on pathological grounds that Lassa fever ought not be considered a hemorrhagic fever at all [6],[29]. More characteristic of severe LF cases are the vascular leakage with edema and effusions in the pleural and pericardial cavities [30],[31],[32]. At necropsy, liver and lung count among the organs most commonly affected during LF [29],[30],[31],[32],[33].
One of the few well-documented characteristics of primary LASV-directed immune response is that neutralizing antibody responses develop only weeks or months after the virus has been eliminated [28]. Also studies of vaccination-induced LASV immunity point toward a cell-mediated mechanism at the frontline of antiviral defense [34],[35]. Still, this notion remains to be addressed and verified directly, and the responsible T cell subtypes to be characterized. Further, a potential disease-enhancing effect of T cell responses in LF has not yet been given sufficient consideration.
Although normal laboratory mouse strains develop acute disease of the central nervous system when infected with LASV intracerebrally [1],[28],[36], they remain resistant to the systemic disease so characteristic of human LF, irrespective of the route used to infect them. Research on LF has therefore been limited to the use of guinea pigs and non-human primates [28], complicating mechanistic studies on immunity and pathogenesis. Here we report on a series of experiments triggered by accidental observations of serious disease in LASV-infected humanized mice (HHD mice [37], C57BL/6 background). HHD mice are genetically engineered to express a human/mouse-chimeric HLA-A2.1 molecule instead of the murine MHC class I gene products and are widely used to identify human HLA-A2.1-restricted peptide epitopes. Stimulated by these unexpected results, we were able to identify T cell-dependence of LASV control, but also of LF pathogenesis. These findings, combined with the propensity of LASV to target monocyte/macrophage lineage cells in vivo, followed by T cell-dependent activation of this cell population, provide a novel concept for virus-host relationship and pathogenesis of LASV. We anticipate that such understanding may aid rational refinement of both vaccine-mediated prevention and treatment of LASV infection.
Results
MHC class I-dependent control of LASV infection
We first compared viral replication in HHD mice and wild type C57BL/6 controls. C57BL/6 mice cleared LASV within about seven days after infection whereas HHD mice remained viremic for substantially longer periods of time (Fig. 1A and data not shown). A detailed analysis of the initial phase of infection documented a virtually immediate uptake of the inoculum into tissues (no virus in blood 2.5 hours after inoculation), followed by identical levels of viremia in wild type and HHD mice up to around day 4 (Fig. 1A). This demonstrates that viremia reflected viral replication in tissues rather than residual inoculum, and that the early phase of virus replication was identical in HHD and C57BL/6 mice. Clear differences in virus control became evident no earlier than seven days after infection (Fig. 1A). These differences in kinetics were compatible with differential adaptive immune control in HHD and C57BL/6 mice. Given that MHC class I (MHC-I) represents the only genetic difference between HHD and C57BL/6 mice, these findings suggested that H-2Db/H-2Kb-restricted T cell responses in C57BL/6 mice played an important role in virus control. Hence, we extended our study to further analyze the contribution of MHC-I - and MHC-II-restricted T cell responses to LASV control (Fig. 1B). MHC-II-deficient mice (lacking CD4+ T cells) efficiently resolved the infection whereas MHC-I-deficient animals (MHC-I-/-; targeted mutation of the β2-microglobulin gene; devoid of CD8+ T cells) developed persistent high-level viremia. This corroborated the key role of MHC-I-restricted T cell responses in LASV control and indicated further that MHC-II-restricted responses were of lesser importance. A time course analysis of viral titers in kidney, lung, liver and spleen of LASV-infected HHD, C57BL/6 and MHC-I-/- mice confirmed that viral replication was comparable on day 2 and day 4. By day 8, however, LASV in the organs of C57BL/6 mice approached the detection limit whereas comparably high titers of virus persisted in tissues of HHD and MHC-I-/- mice. These data provided additional independent support for the above conclusions on productive replication of LASV in mice and the key role of MHC-I-restricted T cells in its control.
Fig. 1. MHC-I but not MHC-II determines efficient LASV control. 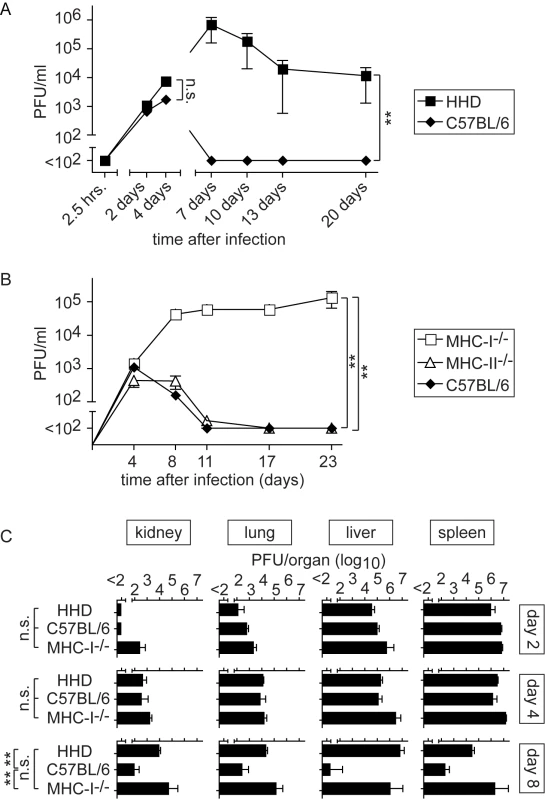
Mice of the indicated genotypes were infected with LASV. A, B: Blood samples were collected over time to determine viremia. A: The 2.5 hrs, 2 day and 4 day time points (left part of panel A) were determined in one experiment, and day 7, 10, 13 and 20 (right part of panel A) were determined in a second one. C: Mice were sacrificed on day 2, 4 and 8 after LASV infection to measure viral titers in organs. Axis breaks indicate the technical detection limit. Symbols and bars represent the mean±SEM of three to five mice per group. T cell-dependent disease in LASV-infected HHD mice
Between 7 and 12 days after LASV infection, HHD mice developed ruffled fur and reduced spontaneous activity. Some of them rapidly and unexpectedly deteriorated and progressed to a state of agony and death: Five out of twenty three mice (∼22%) infected in a total of five experiments succumbed to disease. In contrast but in accordance with the literature, all thirteen wild type C57BL/6 mice, serving as controls in three of these experiments, survived without clinical evidence of disease. Elevated serum aspartate aminotransferase activity (AST) represents the primary parameter for monitoring LF, and AST combined with viremia represent the best predictors for clinical outcome in humans [38]. In keeping with this manifestation of LF, serum AST activity remained mostly within normal ranges in wild type C57BL/6 controls but was significantly elevated in the serum of HHD mice, with a peak around day eight to twelve (Fig. 2A). To further investigate how T cell responses restricted to H-2Kb/H-2Db and to HLA-A2.1 influenced LASV control and disease, we crossed HHD mice to C57BL/6 mice. C57BL/6 x HHD F1 mice express H-2Kb, H-2Db and HLA-A2.1 molecules owing to hemizygosity at all relevant genetic loci. C57BL/6 x HHD F1 mice controlled LASV infection as efficiently as did C57BL/6 wild type mice, and their AST levels remained within normal ranges (Fig. 2B, C). This showed that H-2Kb/H-2Db-mediated virus control prevented disease even in the presence of the HLA-A2.1 molecule. At first it suggested also that persistent and high virus load was directly responsible for pathogenesis. Contrary to this notion, however, the experiments in MHC-I-deficient mice had not resulted in obvious disease despite persistent high-level viremia. This raised the possibility that primary T cell responses may contribute to LF in an immunopathological fashion, similar to the role of memory T cell responses in DHF [10],[11],[39]. To address this possibility, we infected HHD mice with LASV, and prior to infection depleted either CD8+ T cells or CD4+ T cells or both using monoclonal antibodies. MHC-I-/- mice are devoid of a CD8+ T cell compartment and were also included in the experiment. Unlike in untreated HHD mice and irrespective of comparably high levels of viremia in all groups (Fig. 2E), serum AST levels of CD8/CD4-double-depleted HHD mice remained in normal ranges (Fig. 2D). Also, depletion of only CD4+ or CD8+ T cells or genetic deficiency for MHC-I (affecting the CD8+ but not the CD4+ T cell compartment) afforded at least partial protection. In agreement with the results shown in Figs. 1B and 1C, these data suggested that T cells of HHD mice were unable to significantly influence viremia. Nevertheless CD8+ and CD4+ T cells played apparently an essential role in the pathogenesis of LF in HHD mice.
Fig. 2. MHC-I- and T cell-dependent AST elevation suggest T cell-dependent immunopathogenesis of LF. 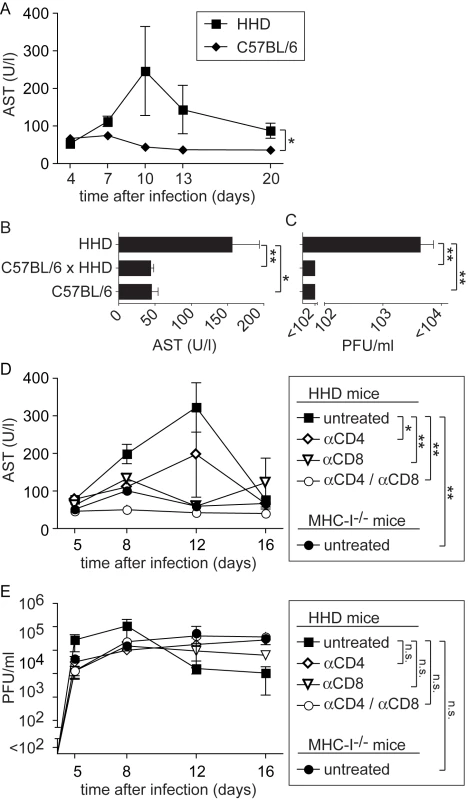
A: C57BL/6 and HHD mice were infected with LASV. AST activity was determined in serum samples collected over time. B, C: In a separate experiment comprising C57BL/6, C57BL/6 x HHD F1 and HHD mice, serum AST activity (B) and viremia (C) were measured on day 8 after LASV infection. D, E: HHD mice were either left untreated, were depleted of CD8+ T cells or of CD4+ T cells or were depleted of both populations. All groups of HHD mice and MHC-I-/- mice were subsequently infected with LASV. Serum AST activity (D) and viremia (E) was measured over time. Symbols and bars represent the mean±SEM of three to five mice per group and time point. Mopeia virus-induced T cell immunity protects HHD mice against Lassa fever
To further characterize the role of T cells in the HHD mouse model for LF, we analyzed whether such animals could be immunized against LASV. For this, we used Mopeia virus (MV), an apathogenic close relative of LASV. MV infection is known to elicit heterologous immunity against LASV in monkeys (analogous to vaccinia virus protecting against smallpox), and MV and recombinants thereof have therefore been postulated as LASV vaccines [40],[41]. MV infection of HHD mice did not result in detectable viremia (Fig. 3A) nor was AST elevation recorded at any time point (Fig. 3B). This pattern of susceptibility of HHD mice to LASV but not MV reflected the one reported in non-human primates [40]. Next we tested whether MV immunization could induce HLA-A2.1-restricted immunity against LF. A recent study has characterized HLA-A2.1-restricted T cell epitopes in the glycoprotein (GP) of LASV [42]. Here we found that MV infection of HHD mice elicited high frequencies of CD8+ T cells specific for the GP42-50 epitope of LASV and a somewhat weaker but clearly detectable response against the GP60-68 epitope (Fig. 3C). CD8+ T cells specific for a third known epitope in LASV-GP (GP441-449) were not induced to a detectable extent, owing to only partial sequence homology of MV and LASV. When subsequently challenged with LASV, MV immunization prevented viremia and serum AST elevation in HHD mice (Fig. 3D, E), and by day 14 after challenge the spleen, liver, lung and kidney of MV-immunized mice were free of detectable LASV (data not shown). MV and LASV are serologically distinct i.e. neutralizing antibodies elicited against one virus do not crossreact nor crossprotect against the other [43]. This suggested that T cell immunity protected MV-immunized HHD mice against subsequent LASV challenge (Fig. 3D, E), albeit primary T cell responses facilitated apparently the disease process in unvaccinated animals (Fig. 2D).
Fig. 3. Mopeia virus (MV) infection is apathogenic in HHD mice but elicits LASV-specific HLA-A2.1-restricted CD8+ T cells and protects against subsequent LASV challenge. 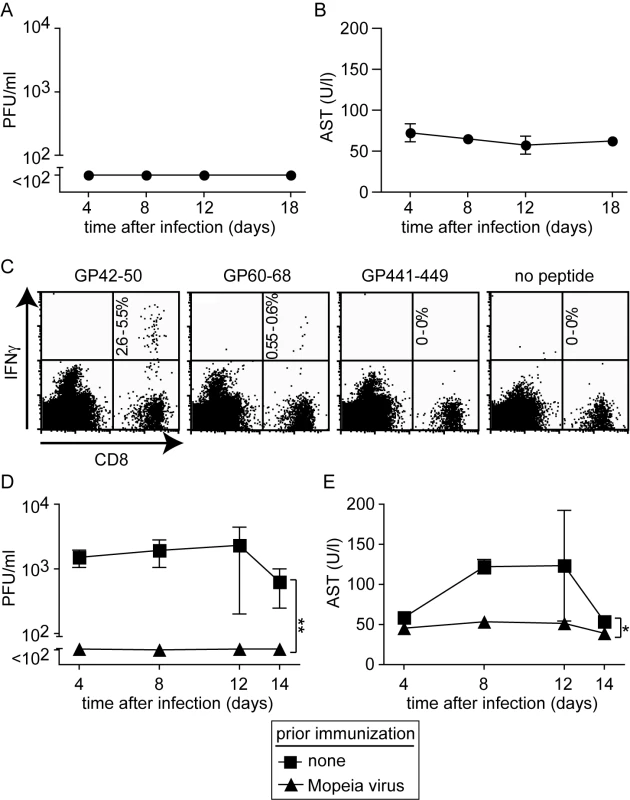
A, B: HHD mice were infected with MV. Serum and blood samples were collected at the indicated time points for measuring viremia (A) and AST (B), respectively. C: Epitope-specific CD8+ T cells in the spleen of MV-infected HHD mice were measured on day 8 (naive controls did not display detectable responses, data not shown). The percent range of IFNγ-producing CD8+ T cells is indicated in the upper right quadrant. D, E: HHD mice, either naive or infected with MV two weeks previously, were challenged with LASV. Viremia (D) and serum AST activity (E) were measured at the indicated time points. Symbols represent the mean ±SEM of four to five mice per group and time point. Histopathological alterations in diseased HHD mice are reminiscent of human Lassa fever and depend on T cells
Next we set out to characterize tissue alterations in LASV-infected HHD mice and to study their dependence on T cells. In all LASV-infected HHD mice analyzed, the lung showed severe pneumonitis with interlobular septal thickening and collapse of the alveolar lumen (Fig. 4A, B). In addition, macroscopic analysis at necropsy or in terminally diseased animals often revealed a substantial pleural effusion (up to about 0.5 ml in each hemithorax, not shown). Both observations matched those in human LF [30],[31]. In contrast to HHD mice, the lungs of C57BL/6 sacrificed at the same time point were only mildly affected or appeared normal (Fig. 4A, B). CD8/CD4-depletion prevented these alterations in HHD mice, and also MHC-I-/- mice exhibited considerably milder signs of peumonitis. Interestingly, HHD lungs contained dense infiltrations of rounded Iba-I+ monocyte/macrophage lineage cells (Fig. 4C), a finding that was less prominent or absent in C57BL/6 mice, T cell-depleted HHD mice or MHC-I-/- mice. Accumulation of T cells was also noted in HHD lungs (Fig. 4D) albeit to a lesser extent than for monocytes/macrophages (compare Fig. 4C). Moreover, similar infiltrations were also found in resistant C57BL/6 wild type mice and thus did not correlate with disease.
Fig. 4. T cell- and MHC-I-dependent alterations in the tissues of LASV-infected mice. 
HHD, C57BL/6, HHD mice depleted of CD8+ and CD4+ T cells, and MHC-I-/- mice were infected with LASV. Eight days later, lung (A-D), liver (E-G) and spleen (H-J) tissue was processed for histological analysis. H/E (A, B, E, H) or immunohistochemical staining of monocytes/macrophages (Iba-1; C, F, I) and T cells (CD3; D, G, J) are shown. Note the collapsed alveolar space, rounded and irregularly distributed monocytes/macrophages in liver and disruption of the splenic microarchitecture in LASV-infected HHD mice. These changes are less severe or largely restored in C57/BL/6 and CD8/CD4-depleted HHD mice, respectively, whereas MHC-I-/- mice display an intermediate picture. Magnification bars indicate 1000 µm (A), 100 µm (B), 50 µm (C, D, F), 30 µm E,G) and 200 µm (H-J), respectively. In the liver, nodules of mononuclear cells were found around the portal fields of all four groups of mice (Fig. 4E). Striking differences were, however, noted in the distribution, shape and orientation of hepatic monocyte/macrophage populations (including Kupffer cells, Fig. 4F). Like in uninfected mice, hepatic monocytes/macrophages of C57BL/6 and of T cell-depleted HHD mice formed predominantly a flat layer along liver sinusoids, oriented towards the central vein in a stellar pattern. In contrast, the architecture of this cell layer was disrupted in HHD mice (unless depleted of T cells) with the remaining cells enlarged, rounded up, disorganized and often accumulated in clusters, indicative of cellular activation and reminiscent of the vigorous hepatic macrophage response reported from human LF [29],[30],[31],[32],[33]. MHC-I-/- mice displayed an intermediate picture with only moderate monocyte/macrophage activation. Conversely, T cells were scattered at similarly moderate density throughout the liver parenchyma and in periportal inflammatory nodules of HHD and C57BL/6 mice (Fig. 4G and data not shown). The number of hepatic T cells did therefore not correlate with disease, similar to the findings in the lung.
Generalized immunosuppression is widely assumed to accentuate viral hemorrhagic fever [6]. A recent monkey study has tentatively attributed LASV immunosuppression to disorganization of the microarchitecture in secondary lymphoid organs [44]. Here we found that LASV infection of HHD mice resulted in disruption of the splenic white and red pulp compartments, whereas the spleens of C57BL/6, CD8/CD4-depleted HHD mice and MHC-I-/- mice were less affected (Fig. 4H). In correlation with these alterations, the marginal zone macrophage layer was lost in HHD mice but not in the other groups, and monocytes/macrophages were homogenously distributed throughout the splenic tissue of HHD mice (Fig. 4I). T cells were largely absent in CD8/CD4-depleted mice, as expected, and were also somewhat scarce in HHD mice (Fig. 4J), possibly indicating LASV-induced T cell depletion as reported from non-human primates [44].
Monocytes/macrophages represent a major target of LASV and produce IL-12p40 in a T cell-dependent fashion
The above morphological alterations had suggested T cell-dependent monocyte/macrophage activation in LASV-infected HHD mice. Classical activation of monocytes/macrophages e.g. by the T cell cytokine interferon gamma and cell-to-cell contact [45],[46],[47],[48] triggers the secretion of NO and inflammatory cytokines, and expression of the former is mediated by inducible NO synthase (iNOS) [45],[46],[47],[48],[49]. iNOS expression can therefore serve as a histological marker for inflammatory differentiation of monocytes/macrophages [50]. On day 8 after LASV infection we detected numerous iNOS-expressing monocyte/macrophage clusters in the liver parenchyma of HHD mice (Fig. 5A). Conversely, iNOS expression was not found in the liver of C57BL/6 mice, CD8/CD4-depleted HHD mice or MHC-I-/- mice infected with LASV. Furthermore, neither HHD nor C57BL/6 mice displayed hepatic iNOS expression or morphological evidence of monocyte/macrophage activation when assessed on day 2 and day 4 after infection (Fig. S1, analogous data in lung and spleen not shown), i.e. prior to the onset of the adaptive immune response.
Fig. 5. Monocytes/macrophages represent a major target of LASV and produce inflammatory mediators in a T cell-dependent fashion. 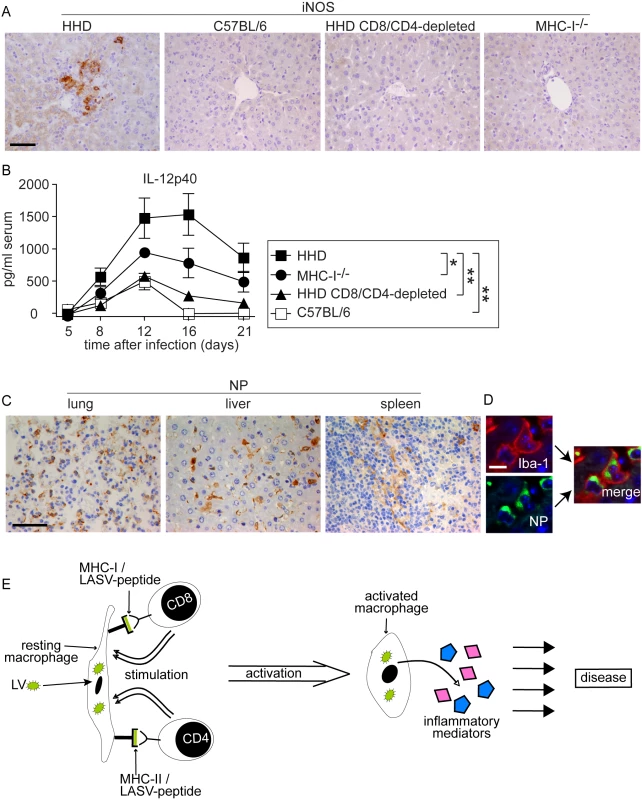
A, B: HHD, C57BL/6, HHD mice depleted of CD8+ and CD4+ T cells, and MHC-I-/- mice were infected with LASV. A: Eight days later, liver tissue was processed for histological analysis of iNOS expression. B. Serum samples were collected over time and IL-12p40 was determined by ELISA. C: Immunohistochemical staining of LASV-NP in lung, liver and spleen of HHD mice on day 8 after infection. D: Illustrative examples for cellular colocalization of the monocyte/macrophage marker Iba-1 (red) and LASV-NP (green) in the liver. Magnification bars indicate 50 µm (A, C) and 10 µm (D), respectively. E: Schematic describing the postulated mechanism how T cells may enhance LF pathogenesis. Synthesis of the inflammatory cytokine subunit IL-12p40 is restricted to macrophages, monocytes and dendritic cells, and its production is greatly enhanced by T cell stimulation [48],[51]. Within eight days after LASV infection, susceptible HHD mice displayed vastly elevated serum IL12-p40 levels (Fig. 5B). Resistant C57BL/6 mice showed comparably moderate IL-12p40 elevation. In agreement with the above results on iNOS, IL-12p40 secretion was strongly reduced by CD4/CD8-depletion in HHD mice, and MHC-I-/- mice exhibited an intermediate IL-12p40 response. Together, these findings suggested that T cells triggered an inflammatory differentiation of monocytes/macrophages with subsequent production of NO, IL-12p40 and likely other inflammatory mediators (see Discussion section). This process occurred, however, solely under conditions of unchecked LASV replication i.e. in HHD mice but not in C57BL/6 mice where the infection and thus the antigen were rapidly cleared.
We had noted high virus loads in lung, liver and spleen of HHD mice (Fig. 1C) where the above pathological changes were found. The cellular distribution of LASV in vivo remains unknown, albeit the virus has been shown to replicate productively in cultured primate macrophages and dendritic cells [21],[52],[53]. To better understand how viral replication might be associated with disease we assessed virus distribution in tissues by immunohistochemistry on day eight after infection (Fig. 5C). LASV nucleoprotein (NP) was readily detected in a distinct population of cells in lung, liver and spleen. Morphological criteria suggested that these cells were predominantly monocytes/macrophages. We therefore performed immunofluorescence double-stains for LASV nucleoprotein (LASV-NP) and the monocyte/macrophage marker Iba-I (Fig. 5D). By this method, 82±6.8% of LASV-NP+ cells in liver, 65±8.5% in the lung and 79±11% in the spleen (mean±SD of six mice) could be identified as monocytes/macrophages, suggesting that they served as a major target of LASV.
Discussion
The data in mice presented here suggest a dual role for T cells during LASV infection: T cells appear essential for rapid clearance of the virus, but if failing to do so they may play a key role in the ensuing disease process, too. Involvement of T cells in the pathogenesis of viral hemorrhagic fever has precedence in Dengue virus infection [10],[11],[39]. Unlike dengue hemorrhagic fever (DHF), where “original antigenic sin” of memory T cells appears to be involved, our findings suggest that T cell responses can have disease-enhancing effects during primary LASV infection. Importantly, we do not exclude roles for memory T cells in addition, but such aspects remain to be investigated (see below).
Our experiments modeled three prototypic scenarios of LASV-host balance, as defined by the parameters “T cells” (relative efficacy of antiviral T cell responses) and “Virus” (persistent virus load), thus delineating the extremes of a spectrum and resulting in the following outcomes:
-
T cells+ Virus−: Rapid and efficient virus control by T cells prevents disease (naive C57BL/6 and vaccinated HHD mice).
-
“T cells+ Virus+”: An antiviral T cell response of intermediate efficiency fails at eliminating the virus, but triggers severe disease (naive HHD mice).
-
“T cells− Virus+”: Complete absence of T cell defense allows LASV persistence, and results in only mild or absent disease (CD8/CD4-depleted HHD mice; partial protection in MHC-I-/- mice).
Several mechanisms can be envisaged by which T cell responses enhance LF [10],[11],[39] but additional studies will be needed to address them directly. The histological picture in HHD mice supports the current view [7] that direct T cell-mediated cytolysis [54] unlikely is the main mechanism responsible for the tissue damage in LF, such as hepatocyte death with subsequent AST release. Based on the available evidence we postulate that during persisting viremia, T cells continuously encounter LASV epitopes on infected monocytes/macrophages in MHC-I and MHC-II context (Fig. 5E). Additional interaction via co-stimulatory molecules, or stimulation via T cell cytokines may trigger infected monocytes/macrophages to differentiate and subsequently secrete inflammatory mediators of their own [45],[48],[49],[51]. Overstimulation of macrophages can result in severe hepatic and pulmonary damage besides mediating a shock syndrome [26],[55],[56], and such overstimulation provides a plausible mechanism for indirect T cell involvement in LF pathogenesis. LASV and related viruses are known to replicate in cultured macrophages without causing cellular activation or production of inflammatory cytokines [21],[52],[53]. Hence, classical T cell-driven monocyte/macrophage activation by IFNγ and direct cell-to-cell contact [45],[46],[47],[48] may augment inflammatory differentiation and cytokine release from LASV-infected monocytes/macrophages in vivo [19],[21],[22], similar to the ability of LPS to induce the activation of LASV-infected macrophages in culture [21],[52],[53]. T cell stimulation may thus facilitate a systemic inflammatory condition [57] as a potential pathogenetic correlate of the diverse and non-specific clinical manifestations of LF.
This view of the role of T cells in LF correlates well with our observations in scenario I, where one would predict that efficient virus elimination by C57BL/6 and immune HHD mice results in only short and transient antigen presentation on a limited number of infected monocytes/macrophages, and therewith lack of disease, as was indeed found. Similarly, the concept explains our findings in scenario III, where the absence of T cells to properly activate infected monocytes/macrophages in CD8/CD4-depleted HHD mice results in mild or absent disease.
In addition, at least two – seemingly paradoxical – observations in LASV-infected non-human primates would support our mechanistic postulate. The first observation is that high doses of LASV tend to be less lethal than low ones [28]. This phenomenon may be explained through the mechanisms of T cell “exhaustion” or “deletion” under high virus loads [58]. As such, a high initial virus inoculum may weaken the T cell response, thus attenuating disease through shifting conditions from scenario II towards scenario III.
The second paradoxical observation stems from a vaccination study, in which a recombinant vaccinia virus expressing the NP protein of LASV was used in monkeys. The vaccine turned out to protect only a minority of the animals and, intriguingly, those LASV-challenged monkeys not protected by the vaccine displayed a more acute form of disease than control monkeys that had not been vaccinated at all [34]. A likely explanation may be that, although the vaccination may not have protected all animals, the still accelerated (memory) T cell response of non-protected animals also accelerated their disease process. Support for such a scenario comes from infection of mice with another arenavirus, lymphocytic choriomeningitis virus (LCMV). LCMV-induced immunopathological disease of the central nervous system is T cell-dependent and, similar to the observation with LASV in monkeys, can be enhanced by prior vaccination with recombinant vaccinia viruses expressing LCMV antigens [59].
Last but not least, our study introduces a mouse model for LF, the lack of which has long hampered progress in this field of research. Only the general versatility of the mouse as a research model, including the availability of gene-targeted strains, makes mechanistic studies as presented here possible. Despite certain shortcomings as listed below, we think that the humanized mouse model could prove useful in further studies on LF pathogenesis, especially as the model lends itself well to the assessment of CD8+ T cell-based vaccines (compare Fig. 3). Although the ∼20% lethality we found in HHD mice using the Ba366 strain of LASV may contrast with the uniform lethality observed in LASV strain Josiah-infected strain 13 guinea pigs or monkeys [28], our lethality rates match those reported for Josiah-infected outbred Hartley guinea pigs [60], as well as inbred strain 13 and strain 2 guinea pigs inoculated with other LASV isolates [61]. Nevertheless, we cannot exclude the possibility that the HHD model fails to recreate certain aspects of human LF.
For the mechanistic analyses presented here, we used relatively high intravenous LASV doses of 106 PFU. The need for such doses to cause severe disease likely reflects the imperfect adaptation of LASV to mice (compare also the Methods section). However, preliminary experiments indicate that viremia and AST elevation can already be observed at lower doses, albeit with higher variability, and that LASV can replicate in HHD mice after subcutaneous administration (data not shown). We would therefore argue that viremia and AST elevation in HHD mice may represent useful surrogates [38] to assess vaccine efficacy prior to an eventual confirmation in non-human primates.
T cell densities in the spleen of LASV-infected HHD and C57BL/6 mice were comparable (day 8 : 3339±925 CD3+ cells/mm2 in HHD mice, 3826±2057 CD3+ cells/mm2 in C57BL/6 mice; mean±SD, n = 6; p = 0.61), arguing against preferential T cell depletion [44] as a potential reason for defective virus control in HHD mice. To characterize disease enhancing (HHD) vs. protective (C57BL/6) primary T cell responses against LASV, future studies will need to compare the magnitude, kinetics and effector-/cytokine-profile [62] of LASV epitope-specific CD4+ and CD8+ T cell responses. Similarly, serum IL-12p40 and iNOS expression have served as surrogates of the inflammatory monocyte/macrophage response in this study, but future work will have to determine its breadth in terms of cytokines, chemokines and inflammatory mediators such as NO, leukotrienes and prostaglandins, their production in tissues and systemic dissemination in blood. The experimental depletion and/or inhibition of monocyte/macrophage populations and of inflammatory mediators may provide additional insights into the cellular and molecular players in LF, indicating to which extent the “cytokine storm” hypothesis [57] can explain LF pathogenesis. With the availability of a mouse model for LASV such studies become possible, although the necessity for BSL-4 laboratory containment can represent a major practical hurdle.
Taken together, our results in mice suggest a two faced role of T cells in LASV infection, both in virus control and also in enhancing LF pathogenesis. This extends our understanding of LASV-host interactions and raises the possibility that heterogeneity in MHC-I and in overall T cell immunocompetence represents one explanation for the wide spectrum of clinical outcomes in a human population exposed to LASV [2]. Perhaps even more important, we think that beside beneficial also detrimental aspects of T cell-responses and -immunity [34],[59] should be given thorough consideration in future strategies for LF vaccine design.
Materials and Methods
Mice and animal experiments
C57BL/6, β2-microglobulin-deficient mice (MHC-I-/-) [63], MHC-II-/- [64] and HHD [37] mice were bred at the Institute for Laboratory Animal Sciences, University of Zurich, Switzerland. Experiments with Lassa virus were performed in the BSL-4 unit of the Bernhard Nocht Institute, Hamburg, Germany. Experiments with Mopeia virus were performed at the University of Geneva and at the University Hospital of Zurich, Switzerland. Permission for animal experiments was obtained from the authorities of the Freie und Hansestadt Hamburg, and from the Cantonal authorities of Geneva and Zurich, Switzerland, respectively. All experiments were performed in accordance with the Swiss and German law for animal protection, respectively.
Viruses, virus titration, infection and T cell depletion of mice
This model is based on the Ba366 [65] strain of LASV. Pilot experiments with a range of isolates representing the different endemic areas (Josiah, Sierra Leone; Lib90, Liberia; Ba366, Guinea; AV, Ivory coast/Burkina Faso; CSF, Nigeria) had indicated that Ba366 was the virus that most efficiently replicated in HHD mice. LASV and Mopeia virus (AN21366), were grown on BHK21 and Vero cells, respectively, and were administered to mice at a dose of 106 PFU i.v. unless stated differently. Virus stocks and viral infectivity in blood samples were determined in immunofocus assays as described [66]. CD8+ and/or CD4+ T cell populations were depleted by i.p. administration of monoclonal antibodies YTS169 (anti-CD8) and YTS191 (anti-CD4) on day -3 and day -1 of LASV infection as previously described [67]. The efficiency of depletion was verified by flow cytometry and was >99%.
Blood biochemistry and histology
Serum AST and ALT activities were determined by using commercially available colorimetric assay kits (Reflotron, Roche Diagnostics, Germany). Mouse tissues were fixed in 4% formalin and were embedded in paraffin. Sections were stained with hematoxilin/eosin (H/E) or processed for immunohistochemistry as follows: Upon inactivation of endogenous peroxidases (PBS/3% hydrogen peroxide) and blocking (PBS/10% FCS) sections were incubated with the primary antibodies rat anti-human CD3 (crossreactive with murine CD3 on mouse T cells; Serotec), rabbit anti-Iba-1 (monocytes/macrophages, Wako Pure Chemical Industries) or rat anti-Lassa nucleoprotein (see below). Bound primary antibodies were detected with biotinylated rat-specific (DakoCytomation) or rabbit-specific (Amersham) secondary antibody, followed by incubation with extraavidin peroxidase (Sigma Aldrich), and bound peroxidase was visualized by 3,3′-diaminobenzidine as chromogen (Sigma Aldrich). Haemalaun was used for counterstaining of nuclei. For fluorescence double labeling, primary antibodies were visualized using species specific Cy3 - or Cy2-conjugated secondary antibodies (all from Jackson ImmunoResearch Laboratories Inc.) with DAPI (Sigma-Aldrich) nuclear staining. To determine the percentage of monocytes/macrophages (Iba-1-positive cells) amongst LASV-infected cells, a total of 41 (liver) and 27 (lung) randomly captured 40x visual fields were analyzed.
Automated assessment of CD3+ T cell densities in spleen sections
Histological spleen sections stained with anti-CD3 antibody (T cells) and counterstained with Haemalaun (nuclei, see above) were scanned using the Dotslide System (Olympus GmBH) at a 200-fold magnification. For analysis, the images were automatically processed in a custom-programmed script of Cognition Network Language based on the Definiens Cognition Network Technology platform (Definiens Developer XD software). The Cognition Network Language is an object-based procedural computer language, designed for automated analysis of complex, context-dependent image analysis. In brief, the programmed script first discriminates spleen tissue and tissue-free surroundings by spectral difference detection. The surface of the resulting region of interest (spleen tissue, ROI) is calculated. Subsequently “CD3 positive cells” within the ROI are detected based on brown anti-CD3 staining and are counted, to calculate the number of CD3+ cells per mm2 of tissue.
ELISA
Serum IL-12p40 was determined using a sandwich ELISA kit (eBioscience) according to the manufacturer's instructions.
Generation of anti-LASV-NP serum
Recombinant NP of LASV strain BA366 was expressed in E. coli using the pET28 expression vector system (Novagen). Supernatants of pET28 constructs were purified using the Talon Metal Affinity Resin (Clontech) in a batch procedure. Urea (8 M) lysates were brought to nondenaturing conditions by increasingly substituting the buffer for sonication buffer during the resin-batch procedure. Proteins were eluted with 250 mM imidazole in sonication buffer on a gravity column (Bio-Rad). Rat antisera were raised against purified recombinant NP by s.c. immunization with recombinant NP emulsified in complete Freund's adjuvant containing 1 mg of Mycobacterium tuberculosis (H37RA; Difco Laboratories, Detroit, MI). Four weeks after the first immunization, animals were boosted with recombinant NP emulsified in incomplete Freund's adjuvant (Difco). Terminal bleedings were performed 4 weeks after the boost. The specificity of the anti-LASV-NP antiserum was verified by immunofluorescence tests on LASV-infected cells as well as on LASV-infected or non-infected tissues. Pre-immune serum from the rats used for immunization was included as a control in both settings.
Intracellular cytokine assay
Epitope-specific CD8+ T cells were enumerated by an intracellular cytokine assay for IFNγ as previously described [68]. In brief, 106 splenocytes were incubated in 200 µl of IMDM supplemented with 10% FCS and penicillin/streptomycin for 5 h at 37°C at a 10−6 M concentration of the LASV-GP-derived peptide epitope GP42-50 (GLVGLVTFL), GP60-68 (SLYKGVYEL), GP441-449 (YLISIFLHL) or with medium alone as a negative control. To enhance intracellular accumulation of IFN-γ, brefeldin A was added at a final concentration of 5 µg/ml for the last 3.5 hours of culture. Subsequently, the cells were washed with FACS buffer (PBS supplemented with 2% FCS, 0.01% NaN3 and 20 mM EDTA) and surface staining was performed with anti-CD8β-PE and anti-B220-PerCP antibody conjugates (both from BD Biosciences) for 30 min at 4°C. After washing twice with FACS buffer, the cells were fixed with 100 µl of 4% paraformaldehyde in PBS for 5 min at 4°C. Two milliliters of permeabilization buffer (FACS buffer supplemented with 0.1% w/v saponin, Sigma) were added and the cells were incubated for 10 min at 4°C. Subsequently, they were spun down and stained intracellularly with anti-mouse-IFNγ-APC (BD Biosciences) in permeabilisation buffer for 60 min at 4°C. After two washes with permeabilization buffer, the cells were resuspended in FACS buffer and were analyzed on a FacsCalibur (Becton Dickinson). FACS plots were gated on B220− lymphocytes.
Statistical analysis
Between group differences were analyzed by 1-way ANOVA and 2-way ANOVA for individual or multiple values of different groups, respectively, followed by LSD post tests. SPSS vs. 13 was used for analysis. P values <0.05 were considered statistically significant (indicated as * in figures). P<0.01 was considered highly significant (indicated as ** in figures). P>0.05 was considered as not significantly different (“n.s.”).
Supporting Information
Zdroje
1. BuckleySM
CasalsJ
DownsWG
1970 Isolation and antigenic characterization of Lassa virus. Nature 227 174
2. McCormickJB
WebbPA
KrebsJW
JohnsonKM
SmithES
1987 A prospective study of the epidemiology and ecology of Lassa fever. J Infect Dis 155 437 444
3. HaasWH
BreuerT
PfaffG
SchmitzH
KohlerP
2003 Imported Lassa fever in Germany: surveillance and management of contact persons. Clin Infect Dis 36 1254 1258
4. BorioL
InglesbyT
PetersCJ
SchmaljohnAL
HughesJM
2002 Hemorrhagic fever viruses as biological weapons: medical and public health management. Jama 287 2391 2405
5. CharrelRN
de LamballerieX
2003 Arenaviruses other than Lassa virus. Antiviral Res 57 89 100
6. GeisbertTW
JahrlingPB
2004 Exotic emerging viral diseases: progress and challenges. Nat Med 10 S110 121
7. GuntherS
LenzO
2004 Lassa virus. Crit Rev Clin Lab Sci 41 339 390
8. HalsteadSB
O'RourkeEJ
1977 Antibody-enhanced dengue virus infection in primate leukocytes. Nature 265 739 741
9. HalsteadSB
1979 In vivo enhancement of dengue virus infection in rhesus monkeys by passively transferred antibody. J Infect Dis 140 527 533
10. MongkolsapayaJ
DejnirattisaiW
XuXN
VasanawathanaS
TangthawornchaikulN
2003 Original antigenic sin and apopotosis in the pathogenesis of dengue hemorrhagic fever. Nat Med 9 921 927
11. LokeH
BethellDB
PhuongCX
DungM
SchneiderJ
2001 Strong HLA class I--restricted T cell responses in dengue hemorrhagic fever: a double-edged sword? J Infect Dis 184 1369 1373
12. LanNT
KikuchiM
HuongVT
Ha doQ
ThuyTT
2008 Protective and Enhancing HLA Alleles, HLA-DRB1*0901 and HLA-A*24, for Severe Forms of Dengue Virus Infection, Dengue Hemorrhagic Fever and Dengue Shock Syndrome. PLoS Negl Trop Dis 2 e304 doi:10.1371/journal.pntd.0000304
13. ChenYC
WangSY
2002 Activation of terminally differentiated human monocytes/macrophages by dengue virus: productive infection, hierarchical production of innate cytokines and chemokines, and the synergistic effect of lipopolysaccharide. J Virol 76 9877 9887
14. ChenST
LinYL
HuangMT
WuMF
ChengSC
2008 CLEC5A is critical for dengue-virus-induced lethal disease. Nature 453 672 676
15. HatchS
MathewA
RothmanA
2008 Dengue vaccine: opportunities and challenges. IDrugs 11 42 45
16. KimHW
CancholaJG
BrandtCD
PylesG
ChanockRM
1969 Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol 89 422 434
17. FulginitiVA
EllerJJ
DownieAW
KempeCH
1967 Altered reactivity to measles virus. Atypical measles in children previously immunized with inactivated measles virus vaccines. JAMA 202 1075 1080
18. OpenshawPJ
TregoningJS
2005 Immune responses and disease enhancement during respiratory syncytial virus infection. Clin Microbiol Rev 18 541 555
19. MahantyS
BauschDG
ThomasRL
GobaA
BahA
2001 Low levels of interleukin-8 and interferon-inducible protein-10 in serum are associated with fatal infections in acute Lassa fever. J Infect Dis 183 1713 1721
20. ter MeulenJ
SakhoM
KoulemouK
MagassoubaN
BahA
2004 Activation of the cytokine network and unfavorable outcome in patients with yellow fever. J Infect Dis 190 1821 1827
21. LukashevichIS
TikhonovI
RodasJD
ZapataJC
YangY
2003 Arenavirus-mediated liver pathology: acute lymphocytic choriomeningitis virus infection of rhesus macaques is characterized by high-level interleukin-6 expression and hepatocyte proliferation. J Virol 77 1727 1737
22. SchmitzH
KohlerB
LaueT
DrostenC
VeldkampPJ
2002 Monitoring of clinical and laboratory data in two cases of imported Lassa fever. Microbes Infect 4 43 50
23. GeisbertTW
HensleyLE
LarsenT
YoungHA
ReedDS
2003 Pathogenesis of Ebola hemorrhagic fever in cynomolgus macaques: evidence that dendritic cells are early and sustained targets of infection. Am J Pathol 163 2347 2370
24. LinderholmM
GroeneveldPH
TarnvikA
1996 Increased production of nitric oxide in patients with hemorrhagic fever with renal syndrome--relation to arterial hypotension and tumor necrosis factor. Infection 24 337 340
25. SanchezA
LukwiyaM
BauschD
MahantyS
SanchezAJ
2004 Analysis of human peripheral blood samples from fatal and nonfatal cases of Ebola (Sudan) hemorrhagic fever: cellular responses, virus load, and nitric oxide levels. J Virol 78 10370 10377
26. FeldmannH
BuganyH
MahnerF
KlenkHD
DrenckhahnD
1996 Filovirus-induced endothelial leakage triggered by infected monocytes/macrophages. J Virol 70 2208 2214
27. McCormickJB
KingIJ
WebbPA
JohnsonKM
O'SullivanR
1987 A case-control study of the clinical diagnosis and course of Lassa fever. J Infect Dis 155 445 455
28. PetersCJ
JahrlingPB
LiuCT
KenyonRH
McKeeKTJr
1987 Experimental studies of arenaviral hemorrhagic fevers. Curr Top Microbiol Immunol 134 5 68
29. WalkerDH
McCormickJB
JohnsonKM
WebbPA
Komba-KonoG
1982 Pathologic and virologic study of fatal Lassa fever in man. Am J Pathol 107 349 356
30. WinnWCJr
MonathTP
MurphyFA
WhitfieldSG
1975 Lassa virus hepatitis. Observations on a fatal case from the 1972 Sierra Leone epidemic. Arch Pathol 99 599 604
31. EdingtonGM
WhiteHA
1972 The pathology of Lassa fever. Trans R Soc Trop Med Hyg 66 381 389
32. FrameJD
BaldwinJMJr
GockeDJ
TroupJM
1970 Lassa fever, a new virus disease of man from West Africa. I. Clinical description and pathological findings. Am J Trop Med Hyg 19 670 676
33. McCormickJB
WalkerDH
KingIJ
WebbPA
ElliottLH
1986 Lassa virus hepatitis: a study of fatal Lassa fever in humans. Am J Trop Med Hyg 35 401 407
34. Fisher-HochSP
HutwagnerL
BrownB
McCormickJB
2000 Effective vaccine for lassa fever. J Virol 74 6777 6783
35. GeisbertTW
JonesS
FritzEA
ShurtleffAC
GeisbertJB
2005 Development of a new vaccine for the prevention of Lassa fever. PLoS Med 2 e183 doi:10.1371/journal.pmed.0020183
36. LukashevichIS
1985 Lassa virus lethality for inbred mice. Ann Soc Belg Med Trop 65 207 209
37. PascoloS
BervasN
UreJM
SmithAG
LemonnierFA
1997 HLA-A2.1-restricted education and cytolytic activity of CD8(+) T lymphocytes from beta2 microglobulin (beta2m) HLA-A2.1 monochain transgenic H-2Db beta2m double knockout mice. J Exp Med 185 2043 2051
38. JohnsonKM
McCormickJB
WebbPA
SmithES
ElliottLH
1987 Clinical virology of Lassa fever in hospitalized patients. J Infect Dis 155 456 464
39. FinkJ
GuF
VasudevanSG
2006 Role of T cells, cytokines and antibody in dengue fever and dengue haemorrhagic fever. Rev Med Virol 16 263 275
40. KileyMP
LangeJV
JohnsonKM
1979 Protection of rhesus monkeys from Lassa virus by immunisation with closely related Arenavirus. Lancet 2 738
41. LukashevichIS
PattersonJ
CarrionR
Moshkoffd
TicerA
2005 A live attenuated vaccine for Lassa fever made by reassortment of Lassa and Mopeia viruses. J Virol 79 13934 13942
42. BottenJ
AlexanderJ
PasquettoV
SidneyJ
BarrowmanP
2006 Identification of protective Lassa virus epitopes that are restricted by HLA-A2. J Virol 80 8351 8361
43. JahrlingPB
PetersCJ
1986 Serology and virulence diversity among Old-World arenaviruses, and the relevance to vaccine development. Med Microbiol Immunol (Berl) 175 165 167
44. CarrionRJr
BraskyK
MansfieldK
JohnsonC
GonzalesM
2007 Lassa virus infection in experimentally infected marmosets: liver pathology and immunophenotypic alterations in target tissues. J Virol 81 6482 6490
45. GordonS
2003 Alternative activation of macrophages. Nat Rev Immunol 3 23 35
46. StoutRD
SuttlesJ
XuJ
GrewalIS
FlavellRA
1996 Impaired T cell-mediated macrophage activation in CD40 ligand-deficient mice. J Immunol 156 8 11
47. TianL
NoelleRJ
LawrenceDA
1995 Activated T cells enhance nitric oxide production by murine splenic macrophages through gp39 and LFA-1. Eur J Immunol 25 306 309
48. ShuU
KiniwaM
WuCY
MaliszewskiC
VezzioN
1995 Activated T cells induce interleukin-12 production by monocytes via CD40-CD40 ligand interaction. Eur J Immunol 25 1125 1128
49. LowensteinCJ
PadalkoE
2004 iNOS (NOS2) at a glance. J Cell Sci 117 2865 2867
50. FongCH
BebienM
DidierlaurentA
NebauerR
HussellT
2008 An antiinflammatory role for IKKbeta through the inhibition of “classical” macrophage activation. J Exp Med 205 1269 1276
51. LangrishCL
McKenzieBS
WilsonNJ
de Waal MalefytR
KasteleinRA
2004 IL-12 and IL-23: master regulators of innate and adaptive immunity. Immunol Rev 202 96 105
52. BaizeS
KaplonJ
FaureC
PannetierD
Georges-CourbotMC
2004 Lassa virus infection of human dendritic cells and macrophages is productive but fails to activate cells. J Immunol 172 2861 2869
53. LukashevichIS
MaryankovaR
VladykoAS
NashkevichN
KoledaS
1999 Lassa and Mopeia virus replication in human monocytes/macrophages and in endothelial cells: different effects on IL-8 and TNF-alpha gene expression. J Med Virol 59 552 560
54. ZinkernagelRM
HaenselerE
LeistT
CernyA
HengartnerH
1986 T cell-mediated hepatitis in mice infected with lymphocytic choriomeningitis virus. Liver cell destruction by H-2 class I-restricted virus-specific cytotoxic T cells as a physiological correlate of the 51Cr-release assay? J Exp Med 164 1075 1092
55. SchumannJ
WolfD
PahlA
BruneK
PapadopoulosT
2000 Importance of Kupffer cells for T-cell-dependent liver injury in mice. Am J Pathol 157 1671 1683
56. SatoK
KadiiskaMB
GhioAJ
CorbettJ
FannYC
2002 In vivo lipid-derived free radical formation by NADPH oxidase in acute lung injury induced by lipopolysaccharide: a model for ARDS. FASEB J 16 1713 1720
57. ClarkIA
2007 The advent of the cytokine storm. Immunol Cell Biol 85 271 273
58. WherryEJ
BlattmanJN
Murali-KrishnaK
van der MostR
AhmedR
2003 Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol 77 4911 4927
59. OehenS
HengartnerH
ZinkernagelRM
1991 Vaccination for disease. Science 251 195 198
60. JahrlingPB
SmithS
HesseRA
RhoderickJB
1982 Pathogenesis of Lassa virus infection in guinea pigs. Infect Immun 37 771 778
61. JahrlingPB
FrameJD
SmithSB
MonsonMH
1985 Endemic Lassa fever in Liberia. III. Characterization of Lassa virus isolates. Trans R Soc Trop Med Hyg 79 374 379
62. AlmeidaJR
PriceDA
PapagnoL
ArkoubZA
SauceD
2007 Superior control of HIV-1 replication by CD8+ T cells is reflected by their avidity, polyfunctionality, and clonal turnover. J Exp Med 204 2473 2485
63. KollerBH
MarrackP
KapplerJW
SmithiesO
1990 Normal development of mice deficient in beta 2M, MHC class I proteins, and CD8+ T cells. Science 248 1227 1230
64. KontgenF
SussG
StewartC
SteinmetzM
BluethmannH
1993 Targeted disruption of the MHC class II Aa gene in C57BL/6 mice. Int Immunol 5 957 964
65. LecompteE
Fichet-CalvetE
DaffisS
KoulemouK
SyllaO
2006 Mastomys natalensis and Lassa fever, West Africa. Emerg Infect Dis 12 1971 1974
66. AsperM
SternsdorfT
HassM
DrostenC
RhodeA
2004 Inhibition of different Lassa virus strains by alpha and gamma interferons and comparison with a less pathogenic arenavirus. J Virol 78 3162 3169
67. PinschewerDD
PerezM
JeetendraE
BächiT
HorvathE
2004 Kinetics of protective antibodies are determined by the viral surface antigen. J Clin Invest 114 988 993
68. MerklerD
HorvathE
BruckW
ZinkernagelRM
de la TorreJC
2006 “Viral déjà vu” elicits organ-specific immune disease independent of reactivity to self. J Clin Invest 116 1254 1263
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2010 Číslo 3- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- All Mold Is Not Alike: The Importance of Intraspecific Diversity in Necrotrophic Plant Pathogens
- Tsetse EP Protein Protects the Fly Midgut from Trypanosome Establishment
- Perforin and IL-2 Upregulation Define Qualitative Differences among Highly Functional Virus-Specific Human CD8 T Cells
- N-Acetylglucosamine Induces White to Opaque Switching, a Mating Prerequisite in
- Origin and Evolution of Sulfadoxine Resistant
- Rapid Evolution of Pandemic Noroviruses of the GII.4 Lineage
- Natural Strain Variation and Antibody Neutralization of Dengue Serotype 3 Viruses
- Fine-Tuning Translation Kinetics Selection as the Driving Force of Codon Usage Bias in the Hepatitis A Virus Capsid
- Structural Basis of Cell Wall Cleavage by a Staphylococcal Autolysin
- Direct Visualization by Cryo-EM of the Mycobacterial Capsular Layer: A Labile Structure Containing ESX-1-Secreted Proteins
- Lipopolysaccharide Is Synthesized via a Novel Pathway with an Evolutionary Connection to Protein -Glycosylation
- MicroRNA Antagonism of the Picornaviral Life Cycle: Alternative Mechanisms of Interference
- Limited Trafficking of a Neurotropic Virus Through Inefficient Retrograde Axonal Transport and the Type I Interferon Response
- Direct Restriction of Virus Release and Incorporation of the Interferon-Induced Protein BST-2 into HIV-1 Particles
- RNAIII Binds to Two Distant Regions of mRNA to Arrest Translation and Promote mRNA Degradation
- Direct TLR2 Signaling Is Critical for NK Cell Activation and Function in Response to Vaccinia Viral Infection
- The Essentials of Protein Import in the Degenerate Mitochondrion of
- Dynamic Imaging of Experimental Induced Hepatic Granulomas Detects Kupffer Cell-Restricted Antigen Presentation to Antigen-Specific CD8 T Cells
- An Accessory to the ‘Trinity’: SR-As Are Essential Pathogen Sensors of Extracellular dsRNA, Mediating Entry and Leading to Subsequent Type I IFN Responses
- Innate Killing of by Macrophages of the Splenic Marginal Zone Requires IRF-7
- Exoerythrocytic Parasites Secrete a Cysteine Protease Inhibitor Involved in Sporozoite Invasion and Capable of Blocking Cell Death of Host Hepatocytes
- Inhibition of Macrophage Migration Inhibitory Factor Ameliorates Ocular -Induced Keratitis
- Membrane Damage Elicits an Immunomodulatory Program in
- Fatal Transmissible Amyloid Encephalopathy: A New Type of Prion Disease Associated with Lack of Prion Protein Membrane Anchoring
- Nucleophosmin Phosphorylation by v-Cyclin-CDK6 Controls KSHV Latency
- A Combination of Independent Transcriptional Regulators Shapes Bacterial Virulence Gene Expression during Infection
- Inhibition of Host Vacuolar H-ATPase Activity by a Effector
- Human Cytomegalovirus Protein pUL117 Targets the Mini-Chromosome Maintenance Complex and Suppresses Cellular DNA Synthesis
- Dispersion as an Important Step in the Biofilm Developmental Cycle
- Kaposi's Sarcoma-Associated Herpesvirus ORF57 Protein Binds and Protects a Nuclear Noncoding RNA from Cellular RNA Decay Pathways
- Differential Regulation of Effector- and Central-Memory Responses to Infection by IL-12 Revealed by Tracking of Tgd057-Specific CD8+ T Cells
- The Human Polyoma JC Virus Agnoprotein Acts as a Viroporin
- Expansion, Maintenance, and Memory in NK and T Cells during Viral Infections: Responding to Pressures for Defense and Regulation
- T Cell-Dependence of Lassa Fever Pathogenesis
- HIV and Mature Dendritic Cells: Trojan Exosomes Riding the Trojan Horse?
- Endocytosis of the Anthrax Toxin Is Mediated by Clathrin, Actin and Unconventional Adaptors
- A Capsid-Encoded PPxY-Motif Facilitates Adenovirus Entry
- Homeostatic Interplay between Bacterial Cell-Cell Signaling and Iron in Virulence
- Serological Profiling of a Protein Microarray Reveals Permanent Host-Pathogen Interplay and Stage-Specific Responses during Candidemia
- YfiBNR Mediates Cyclic di-GMP Dependent Small Colony Variant Formation and Persistence in
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Kaposi's Sarcoma-Associated Herpesvirus ORF57 Protein Binds and Protects a Nuclear Noncoding RNA from Cellular RNA Decay Pathways
- Endocytosis of the Anthrax Toxin Is Mediated by Clathrin, Actin and Unconventional Adaptors
- Perforin and IL-2 Upregulation Define Qualitative Differences among Highly Functional Virus-Specific Human CD8 T Cells
- Inhibition of Macrophage Migration Inhibitory Factor Ameliorates Ocular -Induced Keratitis
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



