-
Články
Reklama
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
ReklamaAerosols Transmit Prions to Immunocompetent and Immunodeficient Mice
Prions, the agents causing transmissible spongiform encephalopathies, colonize the brain of hosts after oral, parenteral, intralingual, or even transdermal uptake. However, prions are not generally considered to be airborne. Here we report that inbred and crossbred wild-type mice, as well as tga20 transgenic mice overexpressing PrPC, efficiently develop scrapie upon exposure to aerosolized prions. NSE-PrP transgenic mice, which express PrPC selectively in neurons, were also susceptible to airborne prions. Aerogenic infection occurred also in mice lacking B - and T-lymphocytes, NK-cells, follicular dendritic cells or complement components. Brains of diseased mice contained PrPSc and transmitted scrapie when inoculated into further mice. We conclude that aerogenic exposure to prions is very efficacious and can lead to direct invasion of neural pathways without an obligatory replicative phase in lymphoid organs. This previously unappreciated risk for airborne prion transmission may warrant re-thinking on prion biosafety guidelines in research and diagnostic laboratories.
Published in the journal: . PLoS Pathog 7(1): e32767. doi:10.1371/journal.ppat.1001257
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1001257Summary
Prions, the agents causing transmissible spongiform encephalopathies, colonize the brain of hosts after oral, parenteral, intralingual, or even transdermal uptake. However, prions are not generally considered to be airborne. Here we report that inbred and crossbred wild-type mice, as well as tga20 transgenic mice overexpressing PrPC, efficiently develop scrapie upon exposure to aerosolized prions. NSE-PrP transgenic mice, which express PrPC selectively in neurons, were also susceptible to airborne prions. Aerogenic infection occurred also in mice lacking B - and T-lymphocytes, NK-cells, follicular dendritic cells or complement components. Brains of diseased mice contained PrPSc and transmitted scrapie when inoculated into further mice. We conclude that aerogenic exposure to prions is very efficacious and can lead to direct invasion of neural pathways without an obligatory replicative phase in lymphoid organs. This previously unappreciated risk for airborne prion transmission may warrant re-thinking on prion biosafety guidelines in research and diagnostic laboratories.
Introduction
Transmissible spongiform encephalopathies (TSEs) are fatal neurodegenerative disorders that affect humans and various mammals including cattle, sheep, deer, and elk. TSEs are characterized by the conversion of the cellular prion protein (PrPC) into a misfolded isoform termed PrPSc [1]. PrPSc aggregation is associated with gliosis, spongiosis, and neurodegeneration [2] which invariably leads to death. Prion diseases have been long known to be transmissible [3], and prion transmission can occur after oral, corneal, intraperitoneal (i.p.), intravenous (i.v.), intranasal (i.n.), intramuscular (i.m.), intralingual, transdermal and intracerebral (i.c.) application, the most efficient being i.c. inoculation [4], [5], [6], [7], [8], [9], [10], [11], [12]. Several biological fluids and excreta (e.g. saliva, milk, urine, blood, placenta, feces) contain significant levels of prion infectivity [13], [14], [15], [16], [17], and horizontal transmission is believed to be critical for the natural spread of TSEs [18], [19], [20], [21], [22], [23]. Free-ranging animals may absorb infectious prion particles through feeding or drinking [24], [25], and tongue wounds may represent entry sites for prions [26].
PrPSc has also been found in the olfactory epithelium of sCJD patients [27], [28]. Prion colonization of the nasal epithelium occurs in various species and with various prion strains [11], [12], [29], [30], [31], [32], [33], [34], [35], [36], [37]. In the HY-TME prion model, intranasal application is 10–100 times more efficient than oral uptake [29] and, as in many other experimental paradigms [38], [39], [40], [41], [42], [43], [44], the lymphoreticular system (LRS) is the earliest site of PrPSc deposition. A publication demonstrated transmission of chronic wasting disease (CWD) in cervidized mice via aerosols and upon intranasal inoculation [45], yet two studies reported diametrically differing results on the role of the olfactory epithelium or the LRS in prion pathogenesis upon intranasal prion inoculation [11], [12], perhaps because of the different prion strains and animal models used. These controversies indicate that the mechanisms of intranasal and aerosolic prion infection are not fully understood. Furthermore, intranasal administration is physically very different from aerial prion transmission, as the airway penetration of prion-laden droplets may be radically different in these two modes of administration.
Here we tested the cellular and molecular characteristics of prion propagation after aerosol exposure and after intranasal instillation. We found both inoculation routes to be largely independent of the immune system, even though we used a strongly lymphotropic prion strain. Aerosols proved to be efficient vectors of prion transmission in mice, with transmissibility being mostly determined by the exposure period, the expression level of PrPC, and the prion titer.
Results
Prion transmission via aerosols
Prion aerosols were produced by a nebulizing device with brain homogenates at concentrations of 0.1–20% (henceforth always indicating weight/volume percentages) derived from terminally scrapie-sick or healthy mice, and immitted into an inhalation chamber. As per the manufacturer's specifications, aerosolized particles had a maximal diameter of <10 µm, and approximately 60% were <2.5 µm [46].
Groups of mice overexpressing PrPC (tga20; n = 4–7) were exposed to prion aerosols derived from infectious or healthy brain homogenates (henceforth IBH and HBH) at various concentrations (0.1, 2.5, 5, 10 and 20%) for 10 min (Fig. 1A, Table 1). All tga20 mice exposed to aerosols derived from IBH (concentration: ≥2.5%) succumbed to scrapie with an attack rate of 100%. The incubation time negatively correlated with the IBH concentration (2.5%: n = 4, 165±54 dpi; 5%: n = 4, 131±7 dpi; 10%: n = 5, 161±27 dpi; 20%: n = 6, 133±8 dpi; p = 0.062, standard linear regression on standard ANOVA; Fig. 1A and F, Table 1, Table S1A).
Fig. 1. Prion transmission through aerosols. 
(A) tga20 mice were exposed to aerosols generated from 0.1%, 2.5%, 5%, 10% or 20% prion-infected mouse brain homogenates (IBH) for 10 min. (B) Groups of tga20 and (C) CD1 mice were exposed for 1, 5 or 10 min to aerosols generated from a 20% IBH. Experiments were performed twice (different colors). (D) C57BL/6, (E) 129SvxC57BL/6, and Prnpo/o mice were exposed for 10 min to aerosols generated from 20% IBH. Kaplan-Meier curves describe the percentage of survival after particular time points post exposure to prion aerosols (y-axis represents percentage of living mice; x-axis demonstrates survival time in days post inoculation). Different colors and symbols describe the various experimental groups. (F) Jittered scatter plot of survival time against concentration of prion aerosols generated out of IBH with added linear regression fit (p = 0.0622). (G) Jittered scatter plot of survival time against exposure time for tga20 mice with added linear regression fit. The negative correlation between survival time and exposure time is significant (p<0.001***). (H) Consecutive paraffin sections of the right hippocampus of Prnpo/o, tga20, CD1 and C57BL/6 mice stained with HE (for spongiosis, gliosis, neuronal cell loss), SAF84 (PrPSc deposits), GFAP (astrogliosis) and Iba-1 (microglia). All animals had been exposed to aerosols generated from 20% IBH for 10 min. Scale bars: 100µm. Tab. 1. Survival times of different mouse strains exposed to prion aerosols for various periods. 
Upper panel: survival times of tga20 mice after 10-min exposure to aerosols generated from various concentrations of IBH. Lower panel: survival times of various mouse strains after exposure for 1, 5, or 10 min to infectious aerosols. Selected inoculations were repeated sequentially (asterisks) in order to estimate the reproducibility of these results. tga20 mice exposed to aerosolized 0.1% IBH did not develop clinical scrapie within the observational period (n = 4; experiment terminated after 300 dpi), yet displayed brain PrPSc indicative of subclinical prion infection (Fig. 1A and 2A). In contrast, control tga20 mice (n = 4) exposed to aerosolized HBH did not develop any recognizable disease even when kept for ≥300 dpi, and their brains did not exhibit any PrPSc in histoblots and Western blots (data not shown).
Fig. 2. PrPSc deposition in brains of mice infected with prion aerosols and profiling of NSE-PrP mice. 
(A) Western blot analysis of brain homogenates (10%) from terminal or subclinical tga20 mice exposed to aerosols from 20% or 0.1% IBH for 10 min. PK+ or −: with or without proteinase K digest; kDa: Kilo Dalton. (B–C): Western blot analyses of brain homogenates from tga20 (B) or CD1 (C) mice exposed to prion aerosols from 20% IBH. (D) Histoblot analysis of brains from mice exposed to prion aerosols. Brains of tga20 mice challenged with aerosolized 10% (middle panel) or 20% (right panel) IBH showed deposits of PrPSc in the cortex and mesencephalon. Because the brain of a Prnpo/o mouse showed no signal (left panel), we deduce that the signal in the middle and right panels represents local prion replication. (E) Histopathological lesion severity score analysis of 5 brain regions depicted as radar plots [51] (astrogliosis, spongiform change and PrPSc deposition) derived from tga20, CD1, C57BL/6 and 129SvxC57BL/6 mice exposed to prion aerosols. Numbers correspond to the following brain regions: (1) hippocampus, (2) cerebellum, (3) olfactory bulb, (4) frontal white matter, (5) temporal white matter. (F) Histopathological lesion severity score of 5 brain regions shown as radar blot (astrogliosis, spongiform change and PrPSc deposition) of i.c. prion inoculated tga20, CD1, C57BL/6 and 129SvxC57BL/6 mice. (1) hippocampus, (2) cerebellum, (3) olfactory bulb, (4) frontal white matter, (5) temporal white matter. (G) Survival curve and (H) lesion severity scores of NSE-PrP mice exposed to a 20% aerosolized IBH for 10 min. (I) Histological and immunohistochemical characterization of scrapie-affected hippocampi of NSE-PrP mice after exposure to aerosolized 20% IBH. Stain legend as in Fig. 1H. Scale bar: 100µm. In the above experiments, and in all experiments described in the remainder of this study, all PrP-expressing (tga20 and WT) mice diagnosed as terminally scrapie-sick were tested by Western blot analysis and by histology: all were invariably found to contain PrPSc in their brains (Fig. 2) and to display all typical histopathological features of scrapie including spongiosis, PrP deposition and astrogliosis (Fig. 1H).
Correlation of exposure time to prion aerosols and incubation period
We then sought to determine the minimal exposure time that would allow prion transmission via aerosols (Fig. 1B, Table 1). tga20 mice were exposed to aerosolized IBH (20%) for various durations (1, 5 or 10 min) in two independent experiments. Surprisingly, an exposure time of only 1 min was found to be sufficient to induce a 100% scrapie attack rate. Longer exposures to prion-containing aerosols strongly correlated with shortened incubation periods (Fig. 1B and G, Table 1, Table S1A and B).
In order to test the universality of the above results, we examined whether aerosols can transmit prions to various mouse strains (CD1, C57BL/6; 129SvxC57BL/6) expressing wild-type (wt) levels of PrPC. CD1 mice were exposed to aerosolized 20% IBH in two independent experiments (Fig. 1C, Table 1). After 5 or 10-min exposures, all CD1 mice succumbed to scrapie whereas shorter exposure (1 min) led to attack rates of 0–50% [1 min exposure (first experiment): scrapie in 0/3 mice; 1 min exposure (second experiment): 2/4 mice died of scrapie at 202±0 dpi; 5 min (first experiment): n = 4, attack rate 100%, 202±12 dpi; 5 min (second experiment): n = 3, attack rate 100%, 202±0 dpi; 10 min (first experiment): n = 4, attack rate 100%, 202±0 dpi; 10 min (second experiment): n = 4, attack rate 100%, 206±16 dpi]. In CD1 mice exposed to prion-containing aerosols for longer intervals, we detected a trend towards shortened incubation times which did not attain statistical significance (Table S1A and S1B).
We also investigated whether C57BL/6 or 129SvxC57BL/6 mice would succumb to scrapie upon exposure to prion aerosols (Fig. 1D and E, Table 1). A 10 min exposure time with a 20% IBH led to an attack rate of 100% (C57BL/6 : 10 min: n = 4; 185±11 dpi; 129SvxC57BL/6: n = 5; 10 min: 182±15 dpi). Control Prnpo/o mice (129SvxC57BL/6 background; n = 3) were resistant to aerosolized prions (20%, 10 min) as expected (Fig. 1E and H, Table 1).
Incubation time and attack rate depends on PrPC expression levels
When tga20 mice were challenged for 10 min, variations in the concentration of aerosolized IBH had a barely significant influence on survival times (p = 0.062; Fig. 1F), whereas variations in the duration of exposure of tga20 mice affected their life expectancy significantly (p<0.001; Fig. 1G). Furthermore tga20 mice, which express 6–9 fold more PrPC in the central nervous system (CNS) than wt mice [46], [47], [48], succumbed significantly earlier to scrapie upon prion aerosol exposure for 10 min (20%) (tga20 mice: 134±4 dpi; CD1 mice: 202±12 dpi, p<0.0001; C57BL/6 mice: 185±11 dpi, p = 0.003; 129SvxC57BL/6 mice: 182±15 dpi, p = 0.003; Fig. 1B–E, Fig. S1, Table S1A and S1C). Incubation time was prolonged and transmission was less efficient in CD1 mice than in tga20 mice after a 1 min exposure to prion aerosols (20%). The variability of incubation times between CD1 mice was low (1st vs. 2nd experiment with 5-min exposure: p = 0.62, 1st vs. 2nd experiment with 10-min exposure: p = 0.27; Fig. 1C, Table 1). This suggests that 1 min exposure of CD1 mice to prion aerosols (20%) suffices for uptake of ≤1LD50 infectious units. This finding underscores the importance of PrPC expression levels not only for the incubation time but also for susceptibility to infection and neuroinvasion upon exposure to aerosols. Histoblot analyses confirmed deposition of PrPSc in brains of tga20 mice exposed to prion aerosols derived from 10% or 20% IBH, whereas no PrPSc was found in brains of Prnpo/o mice exposed to prion aerosols (Fig. 2D).
We then performed a semiquantitative analysis of the histopathological lesions in the CNS. The following brain regions were evaluated according to a standardized severity score (astrogliosis, spongiform change and PrPSc deposition; [49]): hippocampus, cerebellum, olfactory bulb, frontal white matter, and temporal white matter. Scores were compared to those of mice inoculated i.c. with RML (Fig. 2E and F). Lesion profiles of terminally scrapie-sick mice (tga20, CD1, C57BL/6 and 129SvxC57BL/6) infected i.c. or through aerosols were similar irrespectively of genetic background or PrPC expression levels (Fig. 2E and F), with CD1 and 129SvxC57BL/6 hippocampi and cerebella displaying only mild histological and immunohistochemical features of scrapie regardless of the route of inoculation.
We attempted to trace PrPSc in the nasopharynx, the nasal cavity or various brain regions early after prion aerosol infection (1–6 hrs post exposure) and at various time points after intranasal inoculations (6, 12, 24, 72, 144 hrs, 140 dpi, and terminally) with various methods including Western blot, histoblot and protein misfolding analyses. However, none of these analyses detected PrPSc shortly after exposure to prion aerosols (6–72 hrs post prion aerosol exposure) whereas at 140 dpi or terminal stage PrPSc was detected by all of these methods (Fig. S2; data not shown).
PrPC expression on neurons allows prion neuroinvasion upon infection with prion aerosols
We then investigated whether PrPC expression in neurons would suffice to induce scrapie after exposure to prions through aerosols. NSE-PrP transgenic mice selectively express PrPC in neurons and if bred on a Prnpo/o background (Prnpo/o/NSE-PrP) display CNS-restricted PrP expression levels similar to wt mice [50].
Prnpo/o/NSE-PrP (henceforth referred to as NSE-PrP) mice were exposed to prion aerosols (20% homogenate; 10 min). All NSE-PrP mice succumbed to terminal scrapie (216±8 dpi; n = 4; Fig. 1E, 2G, Table 1), although incubation times were significantly longer than those of wt 129SvxC57BL/6 mice (180±15 dpi; n = 5; p = 0.004). Histology and immunohistochemistry confirmed scrapie in NSE-PrP brains (Fig. 2H and I). Histopathological lesion severity score analysis (see above) revealed a lesion profile roughly similar to that of control 129SvxC57BL/6 mice (Fig. 2E, H). More severe lesions were observed in NSE-PrP cerebella whereas olfactory bulbs were less affected.
Real time PCR analysis revealed 2–4 transgene copies per Prnp allele in Prnpo/o/NSE-PrP mice.
A detailed quantitative analysis of PrPC expression levels at various sites of the CNS was performed by comparing the signals obtained by blotting various amounts of protein from NSE-PrP, wt and tga20 tissues (Fig. S3). A value of 100 was arbitrarily assigned to expression of PrPC in wt tissues; olfactory epithelia of tga20 and NSE-PrP mice expressed ≥350 and ∼30, respectively (Fig. S3A). In olfactory bulbs, tga20 and NSE-PrP mice expressed ≥150 and 30, respectively (Fig. S3B). In brain hemispheres tga20 and NSE-PrP mice expressed >250 and >150, respectively (Fig. S3C). Therefore, NSE-PrP mice expressed somewhat more PrPC than wt mice in brain hemispheres, but somewhat less in olfactory bulbs and olfactory epithelia.
Aerosolic prion infection is independent of the immune system
In many paradigms of extracerebral prion infection, efficient neuroinvasion relies on the anatomical and physiological integrity of several immune system components [40], [42], [43], [44]. To determine whether this is true for aerosolic prion challenges, we exposed immunodeficient mouse strains to prion aerosols. This series of experiments included JH−/− mice, which selectively lack B-cells, and γCRag2−/− mice which are devoid of mature B-, T - and NK-cells (Fig. 3A). Upon exposure to prion aerosoIs (20% IBH; exposure time 10 min) both JH−/− and γCRag2−/− mice succumbed to scrapie with a 100% attack rate (JH−/−: n = 6, 181±21 dpi; γCRag2−/−: n = 11, 185±41 dpi, p = 0.65). The incubation times were not significantly different to those of C57BL/6 wt mice exposed to prion aerosols (JH−/− mice: p = 0.9; γCRag2−/− mice: p = 0.7).
Fig. 3. Prion transmission through aerosols in immunocompromised mice. 
Survival curves, lesion severity score analysis (radar plots), and representative histopathological micrographs of mice with genetically or pharmacologically impaired components of the immune system (JH−/−, γCRag2−/−A),129Sv mice treated with LTβR-Ig or with muIgG (B), and LTβR−/−, and CD40−/− mice (C). All mice were exposed for 10 min to aerosolized 20% IBH. Stain code: HE (spongiosis, gliosis, neuronal cell loss), SAF84 (PrPSc deposits), GFAP (astrogliosis) and Iba-1 (microglial activation) as in Fig. 1H. Scale bars: 100µm. Histological and immunohistochemical analyses confirmed scrapie in all clinically diagnosed mice. Lesion severity score analyses (Fig. 3A and 3E) showed that JH−/− and γCRag2−/− mice had lower profile scores in cerebella and higher scores in hippocampi and frontal white matter than C57BL/6 mice. Slightly higher scores in temporal white mater areas and the thalamus could be detected in JH−/− and γCRag2/−/− mice, whereas γCRag2−/− mice showed lower scores in olfactory bulbs. Consistently with several previous reports, γCRag2−/− mice (n = 4) did not succumb to scrapie after i.p. prion inoculation (100µl RML6 0.1% 6 log LD50) even when exposed to a prion titer that was twice higher than that used for intranasal inoculations (data not shown).
Depending on the exposure time and the IBH concentration, tga20 mice developed splenic PrPSc deposits. In contrast, none of the scrapie-sick JH−/−, LTβR−/− and γCRag2−/− mice displayed any splenic PrPSc on Western blots and/or histoblots (Fig. S4A–D) despite copious brain PrPSc.
Aerosol infection is independent of follicular dendritic cells
Follicular dendritic cells (FDCs) are essential for prion replication within secondary lymphoid organs and for neuroinvasion after i.p. or oral prion challenge [42], [44], [51]. Lymphotoxin beta receptor-Ig fusion protein (LTβR-Ig) treatment in C57BL/6 mice causes dedifferentiation of mature FDCs, resulting in reduced peripheral prion replication and neuroinvasion upon extraneural (e.g. intraperitoneal or oral) prion inoculation [52], [53]. We therefore investigated whether FDCs are required for prion replication after challenge with prion aerosols. C57BL/6 mice were treated with LTβR-Ig or nonspecific pooled murine IgG (muIgG) before and after prion challenge (−7, 0, and +7 days) (Fig. 3B). The effects of the LTβR-Ig treatment were monitored by Mfg-E8+/FDC-M1+ staining for networks of mature FDCs in lymphoid tissue. This analysis revealed a complete loss of Mfg-E8+/FDC-M1+ networks at the day of prion exposure and at 14 dpi (data not shown).
LTβR-Ig treatment and dedifferentiation of FDCs did not alter incubation times upon aerosol prion infection (LTβR-Ig: n = 3, attack rate 100%, 184±0 dpi; muIgG: n = 3, attack rate 100%, 184±0 dpi) (Fig. 3B, Table 2). The diagnosis of terminal scrapie was confirmed by histological and immunohistochemical analyses in all clinically affected mice (Fig. 3B; data not shown). Histopathological lesion severity scoring revealed that LTβR-Ig treated C57BL/6 mice displayed a higher score in all regions investigated than untreated C57BL/6 mice upon challenge with prion aerosols (20% IBH; 10 min) (Fig. 2E and 3B). We found slightly less severe scores in the olfactory bulbs of C57BL/6 mice treated with muIgG than in untreated C57BL/6 mice upon challenge with prion aerosols (Fig. 2E and 3B), and a slightly higher score in the temporal white matter (exposure to 20% aerosol for 10 min; Fig. 2E and 3B).
Tab. 2. Survival of mouse strains exposed to prion aerosols (upper panel) or intranasal administered prions (lower panel). 
Prion aerosol infection of mice lacking LTβR or CD40L
LTβR signaling is essential for proper development of secondary lymphoid organs and for maintenance of lymphoid microarchitecture, and was recently shown to play an important role in prion replication within ectopic lymphoid follicles and granulomas [40], [41], [44]. To investigate the role of this pathway in aerogenic prion infections, LTβR−/− mice were exposed to prion aerosols (20% IBH; 10 min exposure time). All LTβR−/− mice succumbed to scrapie (LTβR−/−: n = 4, 272±0 dpi) and displayed PrP deposits in their brains (Fig. 3C). Histological severity scoring of aerosol-exposed mice revealed higher scores in LTβR−/− hippocampi and lower scores in cerebellum, olfactory bulb, frontal and temporal white matter than in C57BL/6 controls (exposure: 20%; 10 min; Fig. 2E and 3C).
We then investigated the role of CD40 receptor in prion aerosol infection. CD40−/− mice fail to develop germinal centers and memory B-cell responses, yet CD40L−/− mice show unaltered incubation times upon i.p. prion challenge [54]. Similarly to the other immunocompromised mouse models investigated, CD40−/− mice developed terminal scrapie upon infection with prion aerosols with an attack rate of 100% (n = 3, 276±50 dpi). Lesion severity analyses of CD40−/− mice revealed a slightly higher score in the cerebellum and the temporal white matter than in C57BL/6 mice (Fig. 2E and 3C). Therefore, LTβR and CD40 signaling are dispensable for aerosolic prion infection.
Components of the complement system are dispensable for aerosolic prion infection
Certain components of the complement system (e.g. C3; C1qa) play an important role in early prion uptake, peripheral prion replication and neuroinvasion after peripheral prion challenge [43], [55], [56]. We have tested whether this is true also for exposure to prion aerosols. Mice lacking both complement components C3 and C4 (C3C4−/−) were exposed for 10 min to 20% aerosolized IBH. All C3C4−/− mice succumbed to scrapie (n = 3, 382±33 dpi; Fig. 4A). Histopathological evaluation of all scrapie affected mice revealed astrogliosis, spongiform changes and PrP-deposition in the CNS (Fig. 4A).
Fig. 4. Prion transmission through aerosols in complement-deficient and newborn mice. 
(A) C3C4−/− mice and (B) newborn tga20 and CD1 mice were exposed for 10 min to a 20% aerosolized IBH. Survival curves (right panels) as well as histological and immunohistochemical characterization of hippocampi indicate that all prion-exposed mice developed scrapie efficiently. Scale bars: 100µm. No protection of newborn mice against prion aerosols
The data reported above argued in favor of direct neuroinvasion via PrPC-expressing neurons upon aerosol administration. However, a possible alternative mechanism of transmission may be via the ocular route, namely via cornea, retina, and optic nerve [57], [58]. In order to test this possibility, newborn (<24 hours-old) tga20 and CD1 mice, whose eyelids were still closed, were exposed for 10 min to prion aerosols generated from a 20% IBH. All mice succumbed to scrapie and showed PrP deposits in brains (tga20 mice: n = 3, 173±23 dpi; CD1 mice: n = 3, 211±0 dpi) (Fig. 4B). Newborn tga20 mice succumbed to scrapie slightly later (p = 0.0043) than adult tga20 mice, whereas no differences were observed between newborn and adult CD1 mice exposed for 10 min to prion aerosols generated from a 20% IBH (p = 0.392).
The brains of all animals contained PK-resistant material, as evaluated by Western blot analysis (data not shown). In addition, untreated littermates or other sentinels which were reared or housed together with aerosol-treated mice immediately following exposure to aerosols showed neither signs of scrapie nor PrPSc in brains, even after 482 dpi. This suggests that prion transmission was the consequence of direct exposure of the CNS to prion aerosols rather than the result of transmission via other routes like ingestion from fur by grooming or exposure to prion-contaminated feces or urine.
Lack of PrPSc in secondary lymphoid organs of immunocompromised, scrapie-sick mice after infection with prion aerosols
We further investigated additional mice for the occurrence of PrPSc in secondary lymphoid organs upon exposure to prion aerosols. PK-resistant material was searched for in spleens, bronchial lymph nodes (bln) and mesenteric lymph nodes (mln) at terminal stage of disease. C57BL/6, 129SvxC57BL/6, muIgG treated C57BL/6 mice, newborn tga20 mice as well as newborn CD1 mice contained PrPSc in the LRS, whereas LTβR−/− mice and C57BL/6 mice treated with LTβR-Ig lacked PrPSc deposits in spleens (Fig. S4A–F; Table 3).
Tab. 3. PrPSc deposition in spleens of mice challenged with a range of aerosolized prion concentrations and exposure times. 
PrPSc was assessed on Western blots and histoblots of spleens of Prnpo/o, newborn CD1, adult CD1, 129SvxC57BL/6, newborn tga20, adult tga20, JH−/−, γCRag2−/−, C57BL/6 mice treated with LTβR-Ig or muIgG and LTβR−/− mice. +: PrPSc detectable in spleen; −: PrPSc undetectable; Nd: not determined; exp.: exposure time (minutes). The efficiency of intranasal prion inoculation depends on the level of PrPC expression
To dissect aerosol-mediated from non-aerosolic contributions to prion exposure, we directly applied a prion suspension (RML 6.0, 0.1%, 40µl, corresponding to 4×105 LD50 scrapie prions) to the nasal mucosa of various mouse lines (Fig. S5). Since mice breathe exclusively through their nostrils [59], [60] we reasoned that this procedure would simulate aerosolic transmission with sufficient faithfulness although the mechanisms of prion uptake could still differ between aerosolic and intranasal administration [11].
tga20 (n = 10), 129SvxC57BL/6 (n = 5), C57BL/6 (n = 8) and Prnpo/o mice (n = 8) were challenged intranasally with prions (Fig. S5). To test the possibility that the inoculation procedure itself might impact the life expectancy of mice, C57BL/6 mice (n = 4) were inoculated intranasally with healthy brain homogenate (HBH) for control (Fig. S5E). None of the animals that had been inoculated with HBH displayed a shortened life span, nor did they develop any clinical signs of disease - even when kept for ≥500 dpi. In contrast, after intranasal prion inoculation all C57BL/6, 129SvxC57BL/6 and tga20 mice succumbed to scrapie with an attack rate of 100% (Fig. S5A–C), whereas Prnpo/o mice were resistant to intranasal prions (Fig. S5D). After intranasal inoculation, tga20 mice (n = 10, 160±28 dpi) displayed a shorter incubation time (Fig. S5C) than 129SvxC57BL/6 (n = 5, 217±20 dpi) or C57BL/6 mice (n = 8, 266±33 dpi; Fig. S5A and S5B). Further, histological and immunohistochemical analyses for spongiosis, astrogliosis and PrP deposition pattern confirmed terminal scrapie (Fig. S5J). A histopathological lesion severity score analysis revealed similar lesion profiles as detected after exposure to prion aerosols (Fig. S5K). However, in the olfactory bulb of tga20 and 129SvxC57BL/6 mice the score was lower upon intranasal administration than in the aerosol paradigm (Fig. 2E).
Finally, we tested whether prion transmission via the intranasal route would be enabled by selective PrPC expression on neurons. For that, we inoculated NSE-PrP mice. All intranasally challenged NSE-PrP mice (n = 6, 291±86 dpi) succumbed to scrapie. The incubation time until terminal disease did not differ significantly from that of 129SvxC57BL/6 control mice (n = 5, 217±20 dpi; p = 0.0868).
Intranasal prion transmission in the absence of a functional immune system
Next, we sought to determine which components (if any) of the immune system are required for neuroinvasion upon intranasal infection with prions. To address this question, Rag1−/− and γCRag2−/− mice were intranasally inoculated with prions (inoculum RML 6.0, 0.1%, 40µl, equivalent to 4×105 LD50 scrapie prions). Remarkably, all intranasally prion-inoculated Rag1−/− (n = 9, 203±6 dpi) (Fig. 5A and H) and γCRag2−/− mice (n = 16, 243±24 dpi) (Fig. 5D and G) succumbed to scrapie, providing evidence for a LRS-independent mechanism of prion neuroinvasion upon intranasal administration. Incubation times in Rag1−/− were significantly different to those of intranasally challenged control mice (C57BL/6; attack rate 100%; n = 8, 266±33 dpi; p = 0.0009) whereas γCRag2−/− mice were not different from those of intranasally challenged control mice (Balb/c: attack rate 100%, n = 6, 209±48 dpi, p = 0.099) (Fig. 5B and Fig. 5D).
Fig. 5. Prion transmission by intranasal instillation. 
(A) Rag1−/− mice intranasally inoculated with RML6 0.1%, (B) C57BL/6 mice that have been intranasally inoculated with 3×105 LD50 prions. (C) Rag1−/− mice i.c. inoculated with 3×105 LD50, (D) γCRag2−/− mice intranasally inoculated with 4×105 LD50 or (E) Balb/c mice intranasally inoculated with 4×105 LD50 scrapie prions are shown. Survival curves (A–D) and respective Western blots (F–G) are indicative of efficient prion neuroinvasion. Brain homogenates were analyzed with (+) and without (−) previous proteinase K (PK) treatment as indicated. Brain homogenates derived from a terminally scrapie-sick and a healthy C57BL/6 mouse served as positive and negative controls (s: sick; h: healthy), respectively. Molecular weights (kDa) are indicated on the left side of the blots. (H and I) Histopathological lesion severity score described as radar blot (astrogliosis, spongiform change and PrPSc deposition) in 5 brain regions of both mouse lines exposed to prion aerosols. Numbers correspond to the following brain regions: (1) hippocampus, (2) cerebellum, (3) olfactory bulb, (4) frontal white matter, (5) temporal white matter. After intranasal prion administration, PrPSc was present in the CNS of Rag1−/− or γCRag2−/− mice. WB analysis corroborated terminal scrapie (Fig. 5G and H). Histopathological lesion severity scoring revealed a distinct lesion profile characterized by a high score in the temporal white matter and the thalamus in case of Rag1−/− mice. In case of γCRag2−/− mice the cerebellum, the olfactory bulb and the frontal white matter displayed lower scores (Fig. 5I and J). In contrast to the CNS spleens of the affected animals did not contain PK-resistant material in terminally sick Rag1−/− and γCRag2−/− mice (Fig. S6E).
For control, Rag1−/− as well as γCRag2−/− mice were intranasally inoculated with HBH to test the possibility that intranasal inoculation itself impacts their life expectancy. None of the mice inoculated with HBH died spontaneously or developed scrapie up to ≥300 dpi (n = 4 each; Fig. S6A, C–D). Further, Balb/c mice and C57BL/6 mice (n = 4 each) inoculated intranasally with HBH (Fig. S5E and S6D) did not develop any disease for ≥300 dpi.
As a positive control, Rag1−/− mice were i.c. inoculated with 3×105 LD50 scrapie prions. This led to terminal scrapie disease after approximately 130 days and an attack rate of 100% (n = 3, 131±8 dpi) (Fig. 5B and data not shown).
As additional negative controls, Rag1−/− and γCRag2−/− mice were i.p. inoculated with prions (100 µl RML 0.1%, 1×106 LD50). Although more infectious prions (approximately 2 fold more) were applied when compared to the intranasal route, i.p. prion inoculation did not suffice to induce scrapie in Rag1−/− and γCRag2−/− mice (attack rate: 0%, n = 4 for each group, experiment terminated after 400 dpi).
Relevance of the complement system for prion pathogenesis after intranasal challenge
The complement component C1qa is involved in facilitating the binding of PrPSc to complement receptors on FDCs [56]. Accordingly, C1qa−/− mice are resistant to prion infection upon low-dose peripheral inoculation. CD21−/− mice are devoid of the complement receptor 1, display a normal lymphoid microarchitecture and show a reduction in germinal center size. The incubation time in CD21−/− mice is greatly increased upon peripheral prion inoculation via the i.p. route [56].
To determine whether the complement system is involved in prion infection through aerosols, C1qa−/− and CD21−/− mice were intranasally inoculated with prions. C1qa−/− mice and CD21−/− mice succumbed to scrapie with an attack rate of 100% (C1qa−/− mice: n = 4, 288±26 dpi; CD21−/− mice: n = 10, 235±24 dpi) (Figs. 6A–C), with CD21−/− mice succumbing to scrapie slightly earlier when compared to C1qa−/− mice. However, survival times did not differ significantly from C57BL/6 control mice (n = 8, 266±33 dpi; C1qa−/− mice: p = 0.24; CD21−/− mice: p = 0.05) (Fig. S5A and S5B). Western blot analysis of one terminally scrapie-sick C1qa−/− mouse revealed one PrPSc positive spleen (1/4) (Fig. S6F). Two terminally scrapie-sick CD21−/− mice showed PK resistance in their spleens (2/10) (Fig. S6G). These results indicate that the complement components C1qa and CD21 are not essential for prion propagation upon intranasal application.
Fig. 6. Intranasal prion transmission in immnunodeficient mice. 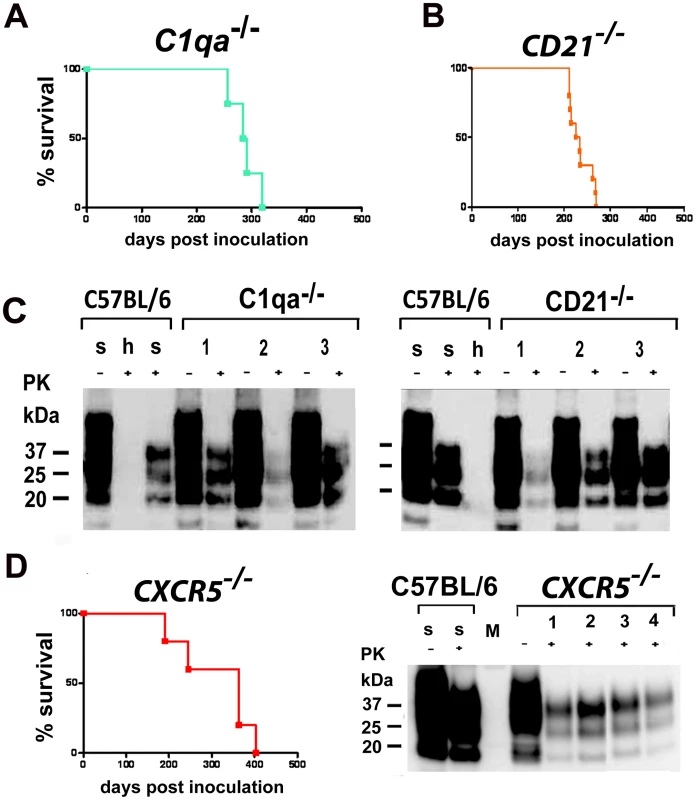
All mice were intranasally inoculated with 3×105 LD50 prions. (A) C1qa−/− mice intranasally inoculated and (B) CD21−/− mice intranasally inoculated are shown. Survival curves illustrate survival after intranasal prion challenge. Respective Western blots of C1qa−/− mice intranasally inoculated (C, left panel) and of CD21−/− mice intranasally inoculated (C, right panel) are shown. Survival curves of CXCR5−/− mice intranasally inoculated are shown (D). Respective Western blots of CXCR5−/− mice intranasally inoculated. Brain homogenates were analyzed with (+) and without (−) previous proteinase K (PK) treatment as indicated. Controls and legends are as in Fig. 5. CXCR5 deficiency does not shorten prion incubation time upon intranasal infection
CXCR5 controls the positioning of B-cells in lymphoid follicles, and the FDCs of CXCR5-deficient mice are in close proximity to nerve terminals, leading to a reduced incubation time after i.p. prion inoculation [39], [61]. Here we explored the impact of CXCR5 deficiency onto intranasal prion inoculation. CXCR5−/− mice exhibited attack rates of 100%, and incubation times did not differ significantly from those of C57BL/6 mice (n = 5, 313±91 dpi; p = 0.32) (Fig. 6D). 3 out of 5 terminally scrapie-sick CXCR5−/− mice revealed PK resistant material in their spleens (3/5), as detected by Western blot analysis (Fig. S6H).
Prion infection is independent of LTβR and TNFR1 signaling
Pharmacological inhibition of LTβR signaling strongly reduces peripheral prion replication and reduces or prevents prion neuroinvasion upon i.p. prion challenge [42], [44], [53]. To determine whether inhibition of LTβR signaling would affect prion transmission through the nasal cavity, we treated C57BL/6 mice with 100µg LTβR-Ig and for control with 100µg muIgG/mouse/week pre - and post-prion challenge (−7 days, 0 days, +7 days; 14 days). LTβR-Ig-treated mice were then inoculated intranasally with prions. 100% of the intranasally challenged mice died due to terminal scrapie (C57BL/6 mice treated with LTβR-Ig: n = 8, 476±200 dpi; Fig. 7A). MuIgG treated mice served as controls and showed an insignificantly shortened incubation time (attack rate: 100%, n = 9, 246±29 dpi) (Fig. 7B and C; C57BL/6 LTβR-Ig treated vs. muIgG treated mice: p = 0.014; C57BL/6 untreated vs. LTβR-Ig treated C57BL/6 mice: p = 0.021; C57BL/6 untreated vs. C57BL/6 muIgG treated mice: p = 0.22).
Fig. 7. Intranasal prion transmission is independent of lymphotoxin signaling. 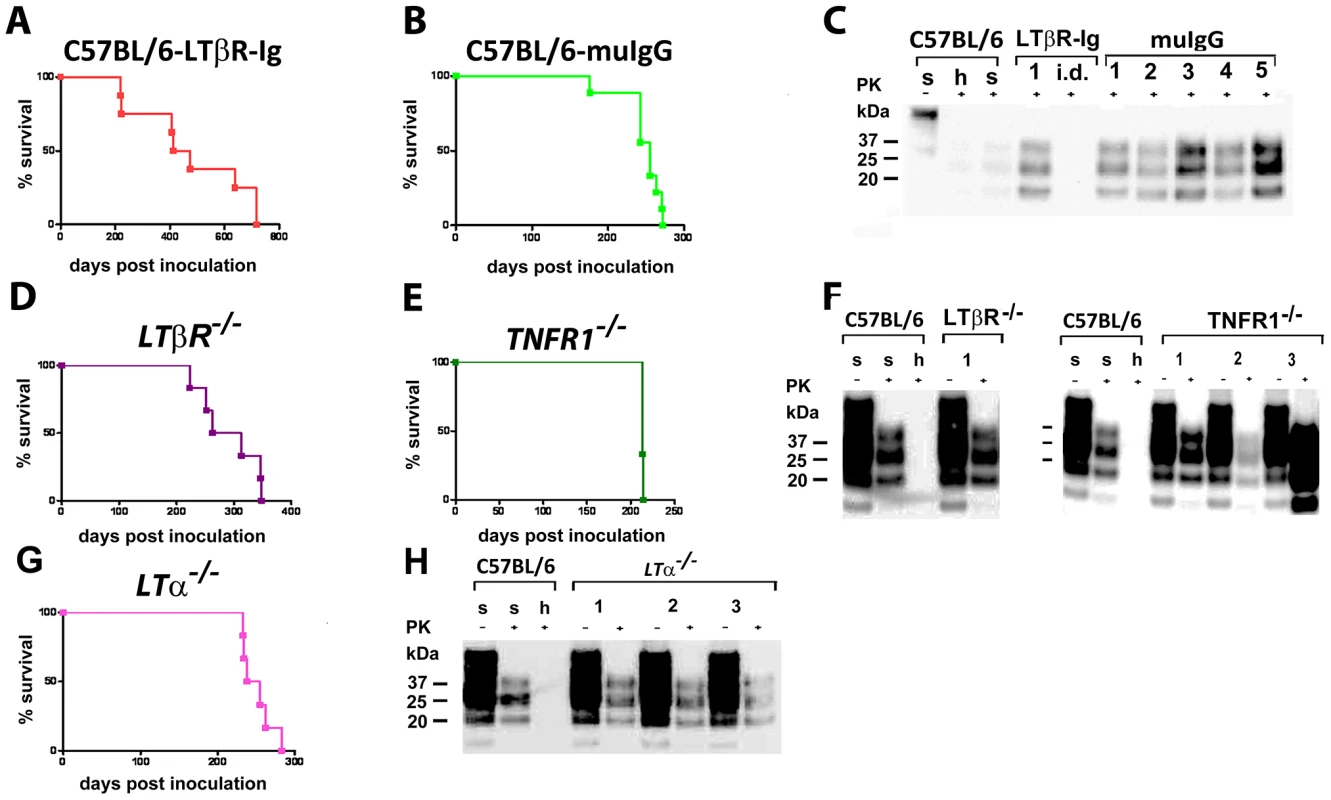
C57BL/6 mice treated with LTβR-Ig (A) or control muIgG (B), and mice lacking various components of the LT/TNF system (D–F, as indicated) were intranasally inoculated with 4×105 LD50 scrapie prions. Survival curves (A, B, D, E and G) and respective Western blots (C, F and H) indicate efficient prion infection and neuroinvasion. One animal that died early after intranasal inoculation (40 dpi) is reported as intercurrent death (i.d.) for reasons other than scrapie. Brain homogenates were analyzed with (+) and without (−) previous proteinase K (PK) treatment as indicated. Controls and legends used are as in Fig. 1H. We additionally challenged LTβR−/−, TNFR1−/− and LTα−/− mice intranasally with RML prions (Fig. 7D–H). Under these conditions all LTβR−/−, TNFR1−/− and LTα−/− mice developed terminal scrapie (LTβR−/− mice: n = 6, 291±52 dpi; TNFR1−/− mice: n = 3, 213±1 dpi; LTα−/− mice: n = 6, 251±20 dpi) (Fig. 7D–H). Terminal scrapie was confirmed by immunohistochemistry, histoblot and WB analysis (Fig. 7F and H, data not shown). Spleens of intranasally inoculated LTβR−/− and TNFR1−/− mice displayed no PK resistant material (LTβR−/− mice: 0/6; TNFR1−/− mice: 0/3). In LTα−/− mice 1 out of 6 spleens contained PrPSc, while splenic PrPSc deposits of PK-resistant material were abundantly found in terminally scrapie-sick tga20 mice (tga20 mice: 2/10)(Fig. S6I–L).
Discussion
Although aerial transmission is common for many bacteria and viruses, it has not been thoroughly investigated for prion aerosols [11], [12], [29], [30], [31], [32], [33], [34], [62] and prions are not generally considered to be airborne pathogens. Yet olfactory nerves have been discussed as a possible entry site for prions [11], and indeed contact-mediated prion exposure of nostrils can efficiently infect various species. We therefore set out to investigate the possible hazards of prion infection deriving from exposure to prion aerosols. Our results establish that aerosolized prion-containing brain homogenates that aerosols are efficacious prion vectors.
Incubation time and attack rate after exposure to prion aerosols depended primarily on the exposure time, the PrPC expression level of recipients and, to a lesser degree, the prion titer of the materials used to generate prion aerosols in a standardized inhalation chamber. The paramount role of the exposure time suggests that the rate of transepithelial ingress of prion through the airways may be limiting even when prions are offered in relatively low concentrations. Conversely, the total prion uptake capacity by the respiratory system was never rate-limiting, because the incubation time of scrapie decreased progressively with higher concentrations and longer exposure times, and because we were unable to establish a response plateau. The latter phenomenon may be explained by the large alveolar surface potentially available for prion uptake.
Since it occurred in wt mice of disparate genetic backgrounds (C57BL/6; CD1; 129SvxC57BL/6), aerosolic infection may represent a universal phenomenon untied to the genetic peculiarities of any specific mouse strain (Fig. S7 features a representative panel of histological features in CD1 mice). However, in CD1 mice the rapidity of progression to clinical disease did not correlate with the exposure time at a given concentration of IBH used for generating prion aerosols, suggesting the existence of genetic factors modulating the saturation of aerogenic prion intake.
The passage of infectivity from the peritoneum to the brain requires a non-hematopoietic conduit that expresses PrPC [63]. We therefore sought to determine whether such a conduit would be required for transfer of infectivity from the aerosols to the brains of recipients. Using NSE-PrP transgenic mice, we found that neuron-selective expression of PrPC sufficed to confer susceptibility of mice to prion infection by aerosols and intranasal application. Hence PrPC expression in non-neural tissues is not required for aerosolic or intranasal neuroinvasion.
Following peripheral exposure, many TSE agents accumulate and replicate in host lymphoid tissues, including spleen, lymph nodes, Peyers' patches, and tonsils [59], [64], [65], [66], [67], [68], [69] in B-cell and lymphotoxin-dependent process [70], [71]. After peripheral replication in the LRS, prions gain access to the CNS primarily via peripheral nerves [23]; the innervation of secondary lymphoid organs and the distance between FDCs and splenic nerve endings is rate-limiting step for neuroinvasion [38] [39].
In contrast to the above, aerosolic and intranasal exposure led to prion infection in the absence of B-, T-, NK-cells and mature FDCs. Although a trend towards a slight delay in incubation time was detected in certain immunodeficient mice (e.g. LTβR−/− and C3C4−/−) and after LTβR-Ig treatment, these differences were not statistically significant, and all other immunodeficient (JH−/−, Rag1−/− and γCRag2−/−) as well as complement-deficient (e.g. C3C4 and CD21) mice were susceptible to aerosolic and intranasal prion infection similarly to control mice. We conclude that transmission into the CNS upon aerosolic prion inoculation requires neither a functional adaptive immune system nor microanatomically intact germinal centers with mature FDCs. Further, the interference with LT signaling, be it by LTβR-Ig treatment or through ablation of the LTβR, indicates that the anatomical and functional intactness of lymphoid organs is dispensable for prion neuroinvasion, brain prion replication, and clinical scrapie.
Since genetic removal of the main cellular components of the LRS (e.g. by intercrosses with mice lacking T-, B-cells or NK-cells in, JH−/− or γCRag2−/− mice) as well as genetic (LTα−/−; LTβR−/−) or pharmacological (LTβR-Ig) depletion of follicular dendritic cells - the main cell responsible for prion replication in secondary lymphoid organs - did not change the course of disease upon infection with prion aerosols, we conclude that the above data demonstrate that the LRS is dispensable for prion infection through the aerogenic route. We therefore propose that airborne prions follow a pathway of direct prion neuroinvasion along olfactory neurons which extend to the surface of the olfactory epithelium. The infectibility of newborn mice supports this hypothesis, since these mice lacked a fully mature immune system at the time of prion exposure.
Our results contradict previous studies [12] claiming a role for the immune system in neuroinvasion upon intranasal prion infection, but are consistent with recent work [11] showing that prion neuroinvasion from the tongue and the nasal cavity can occur in the absence of a prion-infected LRS. Transmission of CWD to “cervidized” transgenic mice via aerosols and upon intranasal administration has also been shown [45].
Both LTβR−/− and LTα−/− mice lack Peyer's patches and lymph nodes as well as an intact NALT which may influence prion replication competence [11], [12], [29], [30], [31], [32], [33], [34], [63]. Furthermore, these mice display chronic interstitial pneumonia. Consistently with a role for LTβR-signaling in peripheral prion infection, these mice do not replicate intraperitoneally administered prions. On the other hand, TNFR1−/− mice lack Peyer's patches, show an aberrant splenic microarchitecture, an abnormal NALT, but have intact lymph nodes where prion replication can occur efficiently [72]. However, prion replication efficacy in spleen is almost completely abrogated [73] and TNFR1−/− mice die due to scrapie after a prolonged incubation time when peripherally challenged with prions.
In the present study, all LTα−/− mice succumbed to scrapie upon intranasal infection, whereas some LTα−/− mice acquired prion infection following nasal cavity exposure in a previous study [11]. The requirement for the LRS in intranasal prion infection may depend on the particular prion strain being tested and on the size of the administered inoculum. When present in sufficiently high titers, prions may be able to directly enter the nervous system via the nasal mucosa and olfactory nerve terminals (Fig. 8). However, at limiting doses, aerial prion infection may be potentiated by an LRS-dependent preamplification step (Fig. 8), e.g. in the bronchial lymph nodes (BLNs), the nose, the gut-associated lymphoid tissue (NALT; GALT), or the spleen. In this study, the particle size generated by the nebulizer ensured that the entire respiratory tract was flooded by the aerosol so that the prion-containing aerosolized brain homogenate would reach the alveolar surface of the lung. There, prions may also colonize airway-associated lymphoid tissues and gain access to the CNS (Fig. 8).
Fig. 8. Model of the possible pathways of aerogenic prion transmission. 
(left) Prion aerosols entering the nasal cavity (1) may directly migrate through the nasal epithelium towards olfactory nerve terminals (2). Subsequently, prions reach olfactory bulb neurons and colonize the limbic system and other regions of the brain (3). Prions may be taken up by the eyes from where they could be transported via the visual system (e.g. optic nerves) to the CNS. O: olfactory system; V: visual system. Alternatively (right) prions may be taken up by immune cells residing in (1) the nasal cavity, the lung, or (2) the gastrointestinal tract, from where they may be transferred to lymphoreticular system (LRS) components such as bronchial lymph nodes (BALT), nasal associated lymphoid tissue (NALT), gastrointestinal lymphoid tissue (GALT), mesenteric lymph nodes, or spleen for further amplification. Subsequently, prions traffic towards peripheral nerve terminals (PNS), from where they invade the central nervous system (CNS). SC: spinal cord. Arrows indicate possible migration directions of prions once they have invaded the spinal cord. Infection through conjunctival or corneal structures was not required, since newborn mice succumbed to scrapie with an incidence of 100% despite having closed eyelids. While newborn tga20, but not CD-1, mice experienced slightly prolonged incubation times when compared to adult (6–8 week-old) mice of the same genotype, the anatomical structures of the nasopharynx (e.g. olfactory epithelium and olfactory nerves) are not similarly developed at postnatal day one when compared to adulthood, potentially leading to a less efficient prion uptake upon aerosol exposure (e.g. via olfactory nerves). Although unlikely, it can not be excluded that infection through conjunctival or corneal structures might contribute to a more efficient prion infection upon aerosol exposure. Be as it may, all newborn mice of either genotypes succumbed to terminal scrapie upon aerosol prion infection despite their lack of fully developed lymphoid organs, thereby bolstering our conclusion that the immune system is dispensable for prion transmission through aerosols.
In summary, our results establish aerosols as a surprisingly efficient modality of prion transmission. This novel pathway of prion transmission is not only conceptually relevant for the field of prion research, but also highlights a hitherto unappreciated risk factor for laboratory personnel and personnel of the meat processing industry. In the light of these findings, it may be appropriate to revise current prion-related biosafety guidelines and health standards in diagnostic and scientific laboratories being potentially confronted with prion infected materials. While we did not investigate whether production of prion aerosols in nature suffices to cause horizontal prion transmission, the finding of prions in biological fluids such as saliva, urine and blood suggests that it may be worth testing this possibility in future studies.
Material and Methods
Ethics statement
Animals were maintained under specific pathogen-free conditions and experiments were approved and conform to the guidelines of the Swiss Animal Protection Law, Veterinary office, Canton Zurich. Mouse experiments were performed under licenses 40/2002 and 30/2005 according to the regulations of the Veterinary office of the Canton Zurich and in accordance with the regulations of the Veterinary office Tübingen.
Aerosols
Exposure of mice to aerosols was performed in inhalation chambers containing a nebulizer device (Art.No. 73-1963, Pari GmbH, Munich, Germany) run with a pressure of 1.5 bar generating 100% particles below 10 µm with 60% of the particles below 2.5 µm and 52% below 1.2 µm. Such particle sizes are considered to be able to reach upper and lower airways [74]. Prion infected material used throughout this study was RML6 strain obtained from the brains of diseased CD1 mice in its 6th passage (RML6). Mice were exposed to aerosolized prion infected brain homogenates for one, five or ten minutes.
Intracerebral prion inoculation of mice
tga20 mice serving as indicator mice were inoculated i.c. with brain tissue homogenate using 30 µl volumes (RML6 0.1%, 3×105 LD50 scrapie prions). The animals were checked on a daily basis and were sacrificed when showing defined neurological signs such as severe gait disorders.
Intranasal prion application in mice
Mice were anesthetized with Ketamine/Xylazin hydrochloride anaesthesia. 10 µl of RML6 (0.1%) were intranasally inoculated in each nostril and on the nasal epithelium by using a 10 µl pipette. The mice were held horizontally during inoculation process and for 1 minute following the inoculation. The whole procedure was repeated after a break of 20 minutes, reaching a final volume of 40 µl of RML6, 0.1% (4×105 LD50 scrapie prions).
Intraperitoneal prion application in mice
Mice were anesthetized with Ketamine/Xylazin hydrochloride anaesthesia. 100 µl of RML6 (0.1%) 1×106 LD50 scrapie prions were i.p. inoculated into Rag1−/− and γCRag2−/− mice.
Western blotting
Tissue homogenates were prepared in sterile 0.32 M sucrose using a Fast Prep FP120 (Savant, Holbrook, NY, USA) or a Precellys 24 (Bertin Technologies). For detection of PrPSc 15µl of a brain homogenate were digested with Proteinase K (30 µg/ml) and incubated for 30 min at 37°C. For detection of PrPC no digestion was performed. Proteins were separated by SDS-PAGE and transferred to a PVDF (Immobilon-P, Millipore, Bedford, Mass., USA) or nitrocellulose membrane (Schleicher & Söhne). Prion proteins were detected by enhanced chemiluminescence (Western blotting reagent, Santa Cruz Biotechnology, Heidelberg, Germany) or ECL (from PerbioScience, Lausanne, CH), using mouse monoclonal anti-PrP antibody POM-1 and horseradish peroxidase (HRP) conjugated goat anti-mouse IgG1 antibody (Zymed).
Histoblot analysis
Histoblots were performed as described previously [73]. Frozen brains that were cut into 12 µm-thick slices were mounted on nitrocellulose membranes. Total PrP, as well as PrPSc after digestion with 50 or 100 µg/ml proteinase K for 4 hrs at 37°C, were detected with the anti-prion POM1 antibody (1∶10000, NBT/BCIP, Roche Diagnostics).
Histological analysis
Formalin-fixed tissues were treated with concentrated formic acid for 60 min to inactivate prion infectivity. Paraffin sections (2µm) and frozen sections (5 or 10µm) of brains were stained with hematoxylin/eosin. Antibodies GFAP (1∶300; DAKO, Carpinteris, CA) for astrocytes were applied and visualized using standard methods. Iba-1 (1∶1000; Wako Chemicals GmbH, Germany) was used for highlighting activated microglial cells. Postfixation in formalin was performed for ∼8 hrs and tissues were embedded in paraffin. After deparaffinisation, for PrP staining sections were incubated for 6 min in 98% formic acid and washed in distilled water for 30 min. Sections were heated to 100°C in a steamer in citrate buffer (pH 6.0) for 3 min, and allowed to cool down to room temperature. Sections were incubated in Ventana buffer and stains were performed on a NEXEX immunohistochemistry robot (Ventana instruments, Switzerland) using an IVIEW DAB Detection Kit (Ventana). After incubation with protease 1 (Ventana) for 16 min, sections were incubated with anti-PrP SAF-84 (SPI bio, A03208, 1∶200) for 32 min. Sections were counterstained with hematoxylin.
Lesion profiling
We selected 5 anatomic brain regions from all investigated or at least 3 mice per experimental group. We evaluated spongiosis on a scale of 0–4 (not detectable, mild, moderate, severe and status spongiosus). Gliosis and PrP immunological reactivity was scored on a 0–3 scale (not detectable, mild, moderate, severe). A sum of the three scores resulted in the value obtained for the lesion profile for the individual animal. The ‘radar plots’ depict the scores for spongiform changes, gliosis and PrP deposition. Numbers correspond to the following brain regions: (1) hippocampus, (2) cerebellum, (3) olfactory bulb, (4) frontal white matter, (5) temporal white matter. Investigators blinded to animal identification performed histological analyses.
Misfolded Protein Assay (MPA)
Misfolded Protein Assay (MPA) was performed as described previously [75]. The assay, which was performed on a 96-well plate is divided into two parts: the PSR1 Capture and an ELISA. For the PSR1 Capture the set up of each reaction was as following: 3µL of PSR1 beads (buffer removed) and 100µl of 1× TBSTT were spiked with brain homogenate, incubated at 37°C for 1hr with shaking at 750rpm, the beads were washed on the plate washer (ELX405 Biotek) 8 times with residual 50 µl/well TBST. Then 75µl/well of denaturing buffer was added. This was incubated at RT for 10min with shaking at 750rpm. Subsequently 30µl/well of neutralizing buffer were added. An additional incubation at RT for 5min with shaking at 750rpm followed. The beads were pulled down with a magnet. The ELISA was performed as follows: 150µL/well of the sample was transferred to an ELISA plate which was coated with POM19. An incubation step at 37°C for 1hr with shaking at 300rpm followed. That was washed 6 times with wash buffer. POM2-AP conjugate had to be diluted to 0.01µg/mL in conjugate diluent. 150µL/well of diluted conjugate was added. Incubation at 37°C for 1hr without shaking followed. Washing 6 times with wash buffer was followed by preparation of enhanced substrate by adding 910µL of enhancer to 10mL of substrate (Lumiphos plus, Lumigen). 150µL/well of enhanced substrate was added. Incubation at 37°C for 30min was followed by reading by luminometer (Luminoskan Ascent) at default PMT, filter scale = 1.
Real-time RT-PCR for quantification of the transgene copy number in Prnpo/o/NSE-PrP mice
Real-time PCR was performed on purified genomic DNA from mouse tails on a 7900 HT Fast Real-Time PCR System (AB). Data were generated and analyzed using SDS 2.3 and RQ manager 1.2 software. The following primers were used: Forward primer annealing in mouse Prnp gene intron 1 : 5′ - GGT TTG ATG ATT TGC ATA TTA G - 3′. Reverse primer annealing in mouse Prnp gene exon 2 : 5′ - GGA AGG CAG AAT GCT TCA GC - 3′. The PCR product is approximately 200 bps in length. For control, the mouse Lymphotoxin alpha gene was analyzed. The following primers were used: Forward primer annealing to the Exon 1 of the mouse Lymphotoxin alpha gene: 5′ - CCT GGT GAC CCT GTT GTT GG - 3′. Reverse primer annealing to the mouse Lymphotoxin alpha gene Intron 1 : 5′ - GTG GGC AGA AGC ACA GCC - 3′. The PCR product is approximately 160 bps in lenght. Real time PCR analysis revealed 2–4 transgene copies per Prnp allele in Prnpo/o/NSE-PrP mice.
Statistical evaluation
Results are expressed as the mean+standard error of the mean (SEM) or standard deviation (SD) as indicated. Statistical significance between experimental groups was assessed using an unpaired two-sample Student's t-Test (Excel) and two-sample Welch t-Test for distributions with unequal variance (R). For survival analyses, Kaplan-Meier-survival curves were generated using SPSS or R software, statistical significance was assessed by performing log rank tests (R). Linear regression fits and analyses of variance (ANOVA) were conducted in R (www.r-project.org).
Supporting Information
Zdroje
1. PrusinerSB
1998 Prions. Proc Natl Acad Sci U S A 95 13363 13383
2. WillRG
1999 Prion related disorders. J R Coll Physicians Lond 33 311 315
3. CuilleJ
ChellePL
1939 Experimental transmission of trembling to the goat. C R Seances Acad Sci 208 1058 1160
4. WeissmannC
EnariM
KlohnPC
RossiD
FlechsigE
2002 Transmission of prions. J Infect Dis 186 Suppl 2 S157 165
5. HillAF
DesbruslaisM
JoinerS
SidleKC
GowlandI
1997 The same prion strain causes vCJD and BSE. Nature 389 448 450 526
6. MaignienT
LasmezasCI
BeringueV
DormontD
DeslysJP
1999 Pathogenesis of the oral route of infection of mice with scrapie and bovine spongiform encephalopathy agents. J Gen Virol 80 Pt 11 3035 3042
7. HeppnerFL
ChristAD
KleinMA
PrinzM
FriedM
2001 Transepithelial prion transport by M cells. Nat Med 7 976 977
8. HerzogC
SalesN
EtchegarayN
CharbonnierA
FreireS
2004 Tissue distribution of bovine spongiform encephalopathy agent in primates after intravenous or oral infection. Lancet 363 422 428
9. ZhangJ
ChenL
ZhangBY
HanJ
XiaoXL
2004 Comparison study on clinical and neuropathological characteristics of hamsters inoculated with scrapie strain 263K in different challenging pathways. Biomed Environ Sci 17 65 78
10. AguzziA
PolymenidouM
2004 Mammalian prion biology: one century of evolving concepts. Cell 116 313 327
11. BessenRA
MartinkaS
KellyJ
GonzalezD
2009 Role of the lymphoreticular system in prion neuroinvasion from the oral and nasal mucosa. J Virol 83 6435 6445
12. SbriccoliM
CardoneF
ValanzanoA
LuM
GrazianoS
2009 Neuroinvasion of the 263K scrapie strain after intranasal administration occurs through olfactory-unrelated pathways. Acta Neuropathol 117 175 184
13. MathiasonCK
PowersJG
DahmesSJ
OsbornDA
MillerKV
2006 Infectious prions in the saliva and blood of deer with chronic wasting disease. Science 314 133 136
14. SeegerH
HeikenwalderM
ZellerN
KranichJ
SchwarzP
2005 Coincident scrapie infection and nephritis lead to urinary prion excretion. Science 310 324 326
15. VascellariM
NonnoR
MutinelliF
BigolaroM
Di BariMA
2007 PrPSc in Salivary Glands of Scrapie-Affected Sheep. J Virol 81 4872 4876
16. TamguneyG
MillerMW
WolfeLL
SirochmanTM
GliddenDV
2009 Asymptomatic deer excrete infectious prions in faeces. Nature 461 529 532
17. LacrouxC
SimonS
BenestadSL
MailletS
MatheyJ
2008 Prions in milk from ewes incubating natural scrapie. PLoS Pathog 4 e1000238
18. DickinsonAG
StampJT
RenwickCC
1974 Maternal and lateral transmission of scrapie in sheep. J Comp Pathol 84 19 25
19. FosterJ
McKenzieC
ParnhamD
DrummondD
ChongA
2006 Lateral transmission of natural scrapie to scrapie-free New Zealand sheep placed in an endemically infected UK flock. Vet Rec 159 633 634
20. FosterJ
McKenzieC
ParnhamD
DrummondD
GoldmannW
2006 Derivation of a scrapie-free sheep flock from the progeny of a flock affected by scrapie. Vet Rec 159 42 45
21. MillerMW
WilliamsES
2003 Prion disease: horizontal prion transmission in mule deer. Nature 425 35 36
22. RyderS
DexterG
BellworthyS
TongueS
2004 Demonstration of lateral transmission of scrapie between sheep kept under natural conditions using lymphoid tissue biopsy. Res Vet Sci 76 211 217
23. AguzziA
HeikenwalderM
2006 Pathogenesis of prion diseases: current status and future outlook. Nat Rev Microbiol 4 765 775
24. HadlowWJ
KennedyRC
RaceRE
1982 Natural infection of Suffolk sheep with scrapie virus. J Infect Dis 146 657 664
25. MillerMW
WilliamsES
HobbsNT
WolfeLL
2004 Environmental sources of prion transmission in mule deer. Emerg Infect Dis 10 1003 1006
26. BartzJC
KincaidAE
BessenRA
2003 Rapid prion neuroinvasion following tongue infection. J Virol 77 583 591
27. ZanussoG
FerrariS
CardoneF
ZampieriP
GelatiM
2003 Detection of pathologic prion protein in the olfactory epithelium in sporadic Creutzfeldt-Jakob disease. N Engl J Med 348 711 719
28. TabatonM
MonacoS
CordoneMP
ColucciM
GiacconeG
2004 Prion deposition in olfactory biopsy of sporadic Creutzfeldt-Jakob disease. Ann Neurol 55 294 296
29. KincaidAE
BartzJC
2007 The nasal cavity is a route for prion infection in hamsters. J Virol 81 4482 4491
30. DeJoiaC
MoreauxB
O'ConnellK
BessenRA
2006 Prion infection of oral and nasal mucosa. J Virol 80 4546 4556
31. DotyRL
2008 The olfactory vector hypothesis of neurodegenerative disease: is it viable? Ann Neurol 63 7 15
32. CoronaC
PorcarioC
MartucciF
IuliniB
ManeaB
2009 Olfactory system involvement in natural scrapie disease. J Virol 83 3657 3667
33. HamirAN
KunkleRA
RichtJA
MillerJM
GreenleeJJ
2008 Experimental transmission of US scrapie agent by nasal, peritoneal, and conjunctival routes to genetically susceptible sheep. Vet Pathol 45 7 11
34. ParkCH
IshinakaM
TakadaA
KidaH
KimuraT
2002 The invasion routes of neurovirulent A/Hong Kong/483/97 (H5N1) influenza virus into the central nervous system after respiratory infection in mice. Arch Virol 147 1425 1436
35. ShawIC
1995 BSE and farmworkers [letter]. Lancet 346 1365
36. SawcerSJ
YuillGM
EsmondeTF
EstibeiroP
IronsideJW
1993 Creutzfeldt-Jakob disease in an individual occupationally exposed to BSE. Lancet 341 642
37. ReuberM
Al-DinAS
BaborieA
ChakrabartyA
2001 New variant Creutzfeldt-Jakob disease presenting with loss of taste and smell. J Neurol Neurosurg Psychiatry 71 412 413
38. GlatzelM
HeppnerFL
AlbersKM
AguzziA
2001 Sympathetic innervation of lymphoreticular organs is rate limiting for prion neuroinvasion. Neuron 31 25 34
39. PrinzM
HeikenwalderM
JuntT
SchwarzP
GlatzelM
2003 Positioning of follicular dendritic cells within the spleen controls prion neuroinvasion. Nature 425 957 962
40. HeikenwalderM
ZellerN
SeegerH
PrinzM
KlohnPC
2005 Chronic lymphocytic inflammation specifies the organ tropism of prions. Science 307 1107 1110
41. HeikenwalderM
KurrerMO
MargalithI
KranichJ
ZellerN
2008 Lymphotoxin-dependent prion replication in inflammatory stromal cells of granulomas. Immunity 29 998 1008
42. MabbottNA
YoungJ
McConnellI
BruceME
2003 Follicular dendritic cell dedifferentiation by treatment with an inhibitor of the lymphotoxin pathway dramatically reduces scrapie susceptibility. J Virol 77 6845 6854
43. MabbottNA
BruceME
BottoM
WalportMJ
PepysMB
2001 Temporary depletion of complement component C3 or genetic deficiency of C1q significantly delays onset of scrapie. Nat Med 7 485 487
44. MabbottNA
MackayF
MinnsF
BruceME
2000 Temporary inactivation of follicular dendritic cells delays neuroinvasion of scrapie. Nat Med 6 719 720
45. DenkersND
SeeligDM
TellingGC
HooverEA
2010 Aerosol and nasal transmission of chronic wasting disease in cervidized mice. J Gen Virol 91 1651 1658
46. FischerM
RülickeT
RaeberA
SailerA
MoserM
1996 Prion protein (PrP) with amino-proximal deletions restoring susceptibility of PrP knockout mice to scrapie. EMBO J 15 1255 1264
47. RadovanovicI
BraunN
GigerOT
MertzK
MieleG
2005 Truncated prion protein and Doppel are myelinotoxic in the absence of oligodendrocytic PrPC. J Neurosci 25 4879 4888
48. SigurdsonCJ
NilssonKP
HornemannS
HeikenwalderM
MancoG
2009 De novo generation of a transmissible spongiform encephalopathy by mouse transgenesis. Proc Natl Acad Sci U S A 106 304 309
49. SigurdsonCJ
NilssonKP
HornemannS
MancoG
PolymenidouM
2007 Prion strain discrimination using luminescent conjugated polymers. Nat Methods 4 1023 1030
50. RadovanovicI
BraunN
GigerOT
MertzK
MieleG
2005 Truncated Prion Protein and Doppel Are Myelinotoxic in the Absence of Oligodendrocytic PrPC. J Neurosci 25 4879 4888
51. MontrasioF
FriggR
GlatzelM
KleinMA
MackayF
2000 Impaired prion replication in spleens of mice lacking functional follicular dendritic cells. Science 288 1257 1259
52. MackayF
BrowningJL
1998 Turning off follicular dendritic cells. Nature 395 26 27
53. HuberC
ThielenC
SeegerH
SchwarzP
MontrasioF
2005 Lymphotoxin-beta receptor-dependent genes in lymph node and follicular dendritic cell transcriptomes. J Immunol 174 5526 5536
54. HeikenwalderM
FederauC
BoehmerL
SchwarzP
WagnerM
2007 Germinal center B cells are dispensable in prion transport and neuroinvasion. J Neuroimmunol 192 113 123
55. ZabelMD
HeikenwalderM
PrinzM
ArrighiI
SchwarzP
2007 Stromal complement receptor CD21/35 facilitates lymphoid prion colonization and pathogenesis. J Immunol 179 6144 6152
56. KleinMA
KaeserPS
SchwarzP
WeydH
XenariosI
2001 Complement facilitates early prion pathogenesis. Nat Med 7 488 492
57. WadsworthJDF
JoinerS
HillAF
CampbellTA
DesbruslaisM
LuthertPJ
CollingeJ
2001 Tissue distribution of protease resistant prion protein in variant CJD using a highly sensitive immuno-blotting assay. Lancet 358 171 180
58. SafarJ
WilleH
ItriV
GrothD
SerbanH
1998 Eight prion strains have PrP(Sc) molecules with different conformations. Nat Med 4 1157 1165
59. AgrawalA
SinghSK
SinghVP
MurphyE
ParikhI
2008 Partitioning of nasal and pulmonary resistance changes during noninvasive plethysmography in mice. J Appl Physiol 105 1975 1979
60. BatesJH
IrvinCG
2003 Measuring lung function in mice: the phenotyping uncertainty principle. J Appl Physiol 94 1297 1306
61. AguzziA
HeikenwalderM
MieleG
2004 Progress and problems in the biology, diagnostics, and therapeutics of prion diseases. J Clin Invest 114 153 160
62. DiringerH
1995 Proposed link between transmissible spongiform encephalopathies of man and animals. Lancet 346 1208 1210
63. BlättlerT
BrandnerS
RaeberAJ
KleinMA
VoigtländerT
1997 PrP-expressing tissue required for transfer of scrapie infectivity from spleen to brain. Nature 389 69 73
64. SigurdsonCJ
WilliamsES
MillerMW
SprakerTR
O'RourkeKI
1999 Oral transmission and early lymphoid tropism of chronic wasting disease PrPres in mule deer fawns (Odocoileus hemionus). J Gen Virol 80 2757 2764
65. HiltonDA
FathersE
EdwardsP
IronsideJW
ZajicekJ
1998 Prion immunoreactivity in appendix before clinical onset of variant Creutzfeldt-Jakob disease Lancet 352 703 704
66. MabbottNA
MacPhersonGG
2006 Prions and their lethal journey to the brain. Nat Rev Microbiol 4 201 211
67. HillAF
ZeidlerM
IronsideJ
CollingeJ
1997 Diagnosis of new variant Creutzfeldt-Jakob disease by tonsil biopsy. Lancet 349 99
68. MouldDL
DawsonAM
RennieJC
1970 Very early replication of scrapie in lymphocytic tissue. Nature 228 779 780
69. BeekesM
McBridePA
2000 Early accumulation of pathological PrP in the enteric nervous system and gut-associated lymphoid tissue of hamsters orally infected with scrapie. Neurosci Lett 278 181 184
70. KleinMA
FriggR
RaeberAJ
FlechsigE
HegyiI
1998 PrP expression in B lymphocytes is not required for prion neuroinvasion. Nat Med 4 1429 1433
71. KleinMA
FriggR
FlechsigE
RaeberAJ
KalinkeU
1997 A crucial role for B cells in neuroinvasive scrapie. Nature 390 687 690
72. PrinzM
MontrasioF
KleinMA
SchwarzP
PrillerJ
2002 Lymph nodal prion replication and neuroinvasion in mice devoid of follicular dendritic cells. Proc Natl Acad Sci U S A 99 919 924
73. TaraboulosA
JendroskaK
SerbanD
YangSL
DeArmondSJ
1992 Regional mapping of prion proteins in brain. Proc Natl Acad Sci U S A 89 7620 7624
74. RaabOG
YehHC
NewtonGJ
PhalenRF
VelasquezDJ
1975 Deposition of inhaled monodisperse aerosols in small rodents. Inhaled Part 4 Pt 1 3 21
75. LauAL
YamAY
MichelitschMM
WangX
GaoC
2007 Characterization of prion protein (PrP)-derived peptides that discriminate full-length PrPSc from PrPC. Proc Natl Acad Sci U S A 104 28 11551 6
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 1- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Salivary Gland NK Cells Are Phenotypically and Functionally Unique
- Genetic Epidemiology of Tuberculosis Susceptibility: Impact of Study Design
- Early Target Cells of Measles Virus after Aerosol Infection of Non-Human Primates
- Multiple Plant Surface Signals are Sensed by Different Mechanisms in the Rice Blast Fungus for Appressorium Formation
- Biofilm Development on by Is Facilitated by Quorum Sensing-Dependent Repression of Type III Secretion
- Distinct Patterns of IFITM-Mediated Restriction of Filoviruses, SARS Coronavirus, and Influenza A Virus
- Molecular Basis of Increased Serum Resistance among Pulmonary Isolates of Non-typeable
- A Helminth Immunomodulator Exploits Host Signaling Events to Regulate Cytokine Production in Macrophages
- HCMV Spread and Cell Tropism are Determined by Distinct Virus Populations
- Characteristics of the Earliest Cross-Neutralizing Antibody Response to HIV-1
- Induction of a Peptide with Activity against a Broad Spectrum of Pathogens in the Salivary Gland, following Infection with Dengue Virus
- Structural Basis for the Recognition of Cellular mRNA Export Factor REF by Herpes Viral Proteins HSV-1 ICP27 and HVS ORF57
- Identification and Characterization of the Host Protein DNAJC14 as a Broadly Active Flavivirus Replication Modulator
- Dual-Use Research and Technological Diffusion: Reconsidering the Bioterrorism Threat Spectrum
- Pathogenesis of the 1918 Pandemic Influenza Virus
- A Cardinal Role for Cathepsin D in Co-Ordinating the Host-Mediated Apoptosis of Macrophages and Killing of Pneumococci
- Critical Role of IRF-5 in the Development of T helper 1 responses to infection
- The Pel Polysaccharide Can Serve a Structural and Protective Role in the Biofilm Matrix of
- Selective C-Rel Activation via Malt1 Controls Anti-Fungal T-17 Immunity by Dectin-1 and Dectin-2
- Imaging Single Retrovirus Entry through Alternative Receptor Isoforms and Intermediates of Virus-Endosome Fusion
- Aerosols Transmit Prions to Immunocompetent and Immunodeficient Mice
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Dual-Use Research and Technological Diffusion: Reconsidering the Bioterrorism Threat Spectrum
- Pathogenesis of the 1918 Pandemic Influenza Virus
- Critical Role of IRF-5 in the Development of T helper 1 responses to infection
- A Cardinal Role for Cathepsin D in Co-Ordinating the Host-Mediated Apoptosis of Macrophages and Killing of Pneumococci
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



