-
Články
Reklama
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
ReklamaCritical Role of IRF-5 in the Development of T helper 1 responses to infection
The transcription factor Interferon Regulatory Factor 5 (IRF-5) has been shown to be involved in the induction of proinflammatory cytokines in response to viral infections and TLR activation and to play an essential role in the innate inflammatory response. In this study, we used the experimental model of visceral leishmaniasis to investigate the role of IRF-5 in the generation of Th1 responses and in the formation of Th1-type liver granulomas in Leishmania donovani infected mice. We show that TLR7-mediated activation of IRF-5 is essential for the development of Th1 responses to L. donovani in the spleen during chronic infection. We also demonstrate that IRF-5 deficiency leads to the incapacity to control L. donovani infection in the liver and to the formation of smaller granulomas. Granulomas in Irf5-/- mice are characterized by an increased IL-4 and IL-10 response and concomitant low iNOS expression. Collectively, these results identify IRF-5 as a critical molecular switch for the development of Th1 immune responses following L. donovani infections and reveal an indirect role of IRF-5 in the regulation of iNOS expression.
Published in the journal: . PLoS Pathog 7(1): e32767. doi:10.1371/journal.ppat.1001246
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1001246Summary
The transcription factor Interferon Regulatory Factor 5 (IRF-5) has been shown to be involved in the induction of proinflammatory cytokines in response to viral infections and TLR activation and to play an essential role in the innate inflammatory response. In this study, we used the experimental model of visceral leishmaniasis to investigate the role of IRF-5 in the generation of Th1 responses and in the formation of Th1-type liver granulomas in Leishmania donovani infected mice. We show that TLR7-mediated activation of IRF-5 is essential for the development of Th1 responses to L. donovani in the spleen during chronic infection. We also demonstrate that IRF-5 deficiency leads to the incapacity to control L. donovani infection in the liver and to the formation of smaller granulomas. Granulomas in Irf5-/- mice are characterized by an increased IL-4 and IL-10 response and concomitant low iNOS expression. Collectively, these results identify IRF-5 as a critical molecular switch for the development of Th1 immune responses following L. donovani infections and reveal an indirect role of IRF-5 in the regulation of iNOS expression.
Introduction
The protozoan parasite Leishmania donovani is the causative agent of visceral leishmaniasis (VL), a chronic life threatening disease if untreated. In the experimental model of VL, the two main target organs are the liver and the spleen [1]. While the spleen stays chronically infected, infection in the liver is self-resolving within 6-8 weeks due to the development of a Th1-dominated granulomatous response, which is characterized by high IFNγ production. This response is induced by IL-12 secreted by dendritic cells (DC) [2], [3], [4] and is crucial for parasite control and disease resolution in the liver, together with TNFα production and expression of inducible nitric oxide synthase (iNOS) by macrophages [1]. Studies using Myd88-/- mice have highlighted the importance of toll like receptors (TLRs) in the induction of IL-12 production by DC and the development of Th1 immune responses in Leishmania infection [5]. More recently, TLR9 has been shown to be required for IL-12 production by DC in a model of cutaneous leishmaniasis [6], [7] and also in Trypanosoma cruzi infected mice [8]. However, in contrast to T. cruzi infections, TLR9 deficiency in mice infected with L. major did not prevent the development of Th1 responses and only resulted in a transient disease exacerbation [6], [9]. As MyD88-/- mice are highly susceptible to Leishmania infection [5], this suggests that in addition to TLR9, other TLRs as well as IL-1 and IL-18 may also be involved in the generation of Th1 responses and in the induction of host protective immunity. Since Leishmania parasites reside in the phagolysosomes of the host cells, other endosomally localized TLRs, such as TLR 7 and 8 could be involved in the recognition of this pathogen [10], [11].
Interferon Regulatory Factor 5 (IRF-5) has been shown to be involved in the transcriptional activation of both Type I IFN genes and genes encoding key proinflammatory cytokines such as IL-12, TNFα and IL-6 [12], [13], [14], [15]. This transcription factor can be activated by TLR7 and TLR9 via the MyD88 signaling pathway and/or directly by viral infections and Type I interferon [16]. In vivo, IRF-5 has been shown to play a role in the innate antiviral immune response. Indeed, lack of IRF-5 expression in genetically modified Irf5-/- mice resulted in attenuation of Type I IFN, TNFα and IL-6 production in response to viral infection [13], [17], [18]. However, the antiviral effect of IRF-5 deficiency appeared to be cell type specific and mainly affected DCs and plasmacytoid DCs (pDCs), rather than macrophages [16], [17]. More recently, IRF-5 was also shown to cooperate with, among others, NOD2 and TBK1 in triggering expression of Type I interferon in response to Mycobacterium tuberculosis [19].
The aim of this study was to examine whether IRF-5 also plays a role in the regulation of the immune response to parasitic infections. Here we demonstrate that IRF-5 deficiency results in severe impairment in the development of Th1 immune responses following L. donovani infection. Moreover, Irf5-/- mice failed to develop typical Th1-type granulomas and to control infection in the liver, demonstrating a vital role for IRF-5 in the induction of the anti-parasitic response.
Results
IRF-5 is required for disease control in the liver
The transcription factor IRF-5 is an important downstream regulator of the TLR/MyD88 signaling pathway and is involved in the induction of several key proinflammatory cytokines [13], [16], [17]. As TLRs have been implicated in the recognition of Leishmania parasites [5], [6], [7], [20], [21], [22], [23], we first wanted to assess whether IRF-5 was at all involved in the generation of protective immunity against L. donovani. Hence, we infected wild type (WT) and Irf5-/- mice and monitored the course of infection at several time points. We observed similar parasite burdens for WT and Irf5-/- mice in the spleen (Fig. 1A). In contrast, the disease was exacerbated in the livers of Irf5-/- mice, with a parasite burden of approximately two times higher at d14 pi and almost 4-fold higher than in the WT controls by d28 pi (Fig. 1B). These data suggest that the requirements for IRF-5 in the immune response to L. donovani are strictly organ specific, at least until day 28p.i..
Fig. 1. Leishmania donovani infection in Irf5-/- mice. 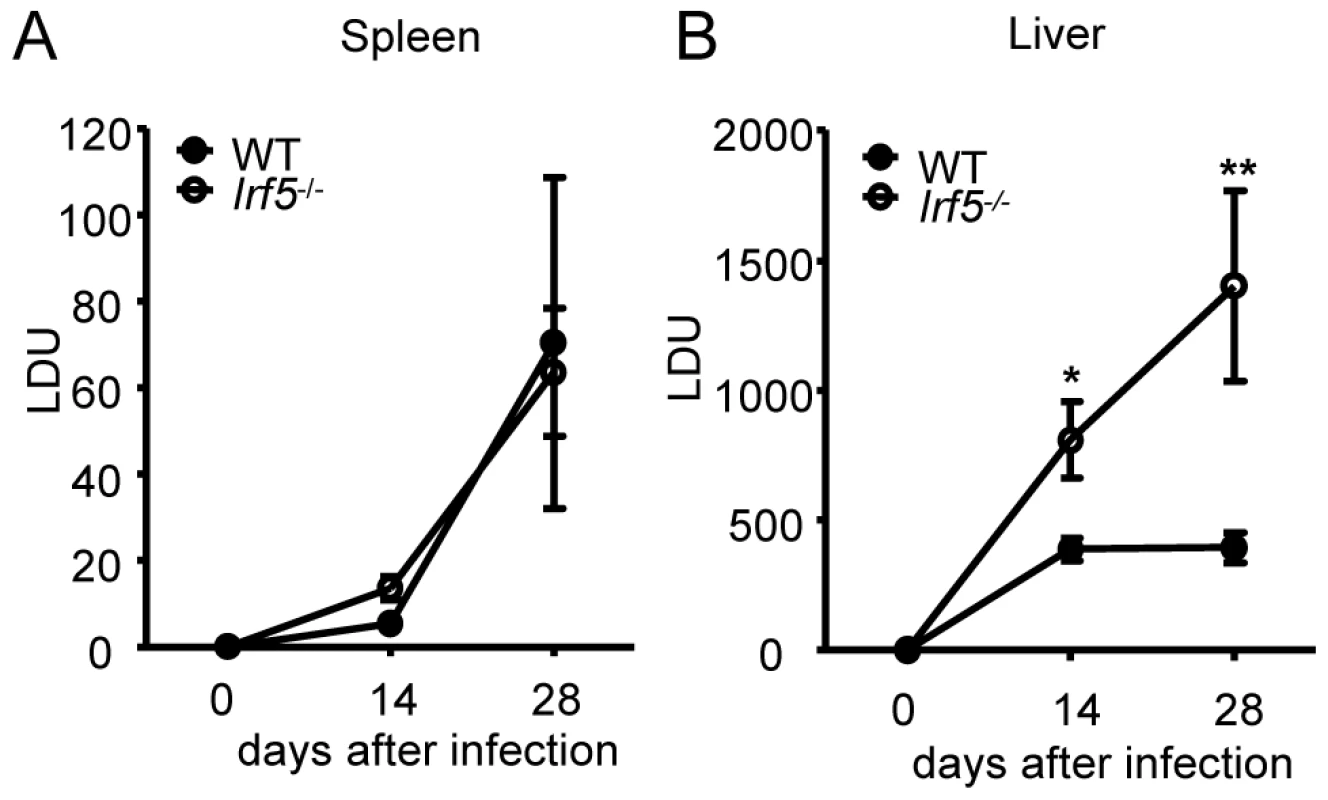
Parasite burdens in the spleen (A) and liver (B) were determined for infected WT and Irf5-/- mice at d14 and d28 pi as described. Data shown is the mean ± SEM and is representative of two independent experiments. * denotes p<0.05 and ** denotes p<0.01. IRF-5 deficiency results in defective Th1 responses
As IFNγ-producing CD4+ T helper cells are crucial for the control of L. donovani infection in the liver, we next investigated whether these responses were being generated in Irf5-/- mice. IFNγ–producing CD4+ T cells were already detected at d14 of infection in both the liver and spleen of WT mice, although still at low frequency. The frequency of IFNγ+ CD4+ T cells increased in the later stages of infection and by d28 pi, 39.1% of CD4+ T cells in the liver (Fig. 2A and B) and 7.6% in the spleen (Fig. 2C and D) of WT mice were producing IFNγ. In marked contrast, the generation of IFNγ-producing CD4+ T cells was significantly impaired in the livers of Irf5-/- mice (Fig. 2A), where only 13.2% of CD4+ T cells were IFNγ+ at d28 (a 60% reduction), and in the spleen (Fig. 2C), where only 2.8% of CD4+ T cells were found to be secreting IFNγ (a 60% reduction compared to WT controls). Interestingly, at day 14p.i., the frequency of IFNγ+ CD4+ T-cells in the liver and spleens of Irf5-/- mice was comparable, if not better than in WT mice (Fig. 2). This suggests that initially Th1 responses are being generated in the absence of IRF-5, however this transcription factor is essential for the maintenance and further expansion of Th1 responses.
Fig. 2. Irf5-/- mice have a defective Th1 response. 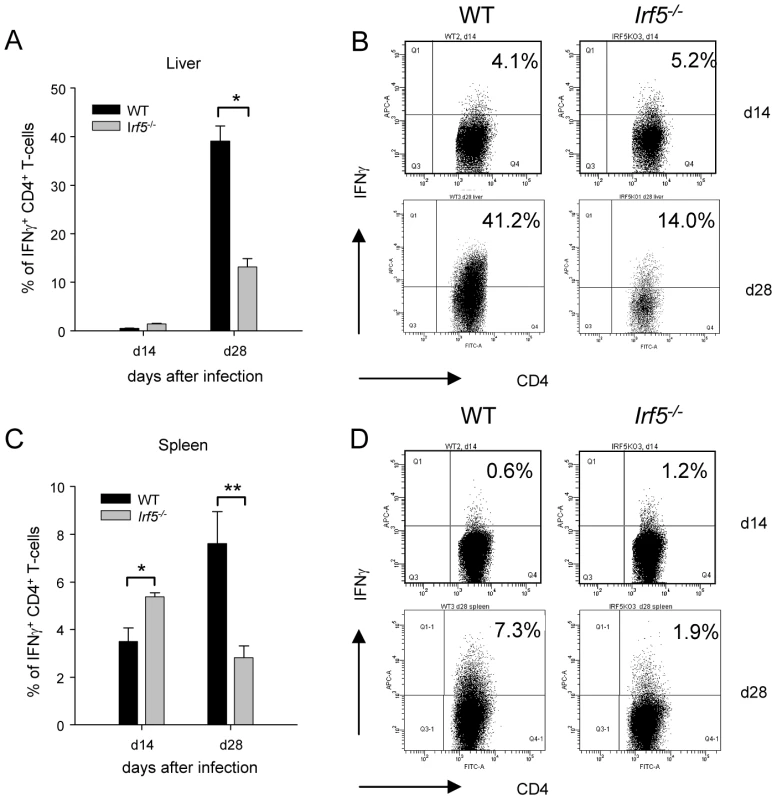
The percentage of CD4+ T cells producing IFNγ as a response to L. donovani infection in the liver (A) and the spleen (C) of WT and Irf5-/- mice was determined by intracellular flow cytometry. Representative scatter plots from WT and Irf5-/- mice infected with L. donovani showing IFNγ production by CD4+ T cells in the liver (B) and spleen (D) at different times p.i.. Data is shown as the mean ± SEM. Flow cytometry data is representative of two independent experiments. * denotes p<0.05 and ** denotes p<0.01. Irf5-/- mice fail to develop Th1-type granulomas
In order to determine whether the defective generation of Th1 responses detected in Irf5-/- mice had an impact on the Th1-type granuloma formation normally observed in the livers of L. donovani infected WT mice [1], we proceeded to analyze H&E stained sections of livers from WT and Irf5-/- mice. Despite an approximately 4-fold higher liver parasite burden in Irf5-/- mice (Fig. 1B), the number of granulomas/inflammatory foci observed in Irf5-/- mice at d28 p.i. was reduced approximately by 77% compared with WT mice (406.7±15.7 granuloma/100 microscopic fields in WT mice vs. 92±38 granuloma/100 microscopic fields in Irf5-/- mice). A closer histological analysis also revealed that the granulomas in Irf5-/- mice were smaller in size and had a different cellular composition, characterized by a marked infiltration of polymorphonucleated cells that were identified as neutrophils (Fig. 3A). Neutrophils were also observed at higher frequencies in the spleens of infected Irf5-/- mice compared to WT controls (Fig. S1 A and B). Increased neutrophil infiltration correlated with higher mRNA levels for CXCL1, a chemokine receptor known for its neutrophil chemoactractant activity, in the liver (day 14 p.i. only) and spleens of Irf5-/- compared to WT mice (Fig. 3 B and C). Of additional interest is the observation that the mononuclear cell infiltrates in the livers of Irf5-/- mice were significantly reduced at day 28 p.i. compared to WT mice (data not shown and Fig. 2B). Moreover, in Irf5-/- mice we could not observe any splenomegaly, which is a typical symptom of VL in WT mice (Fig. 3D). These results suggest that IRF-5 is an essential factor in the maintenance of the inflammatory responses generated during L. donovani infection. IL-23 has recently been shown to be involved in immune cell homing to infected target cells [24], hence we also assessed whether this cytokine was expressed at different levels in the livers of Irf5-/- mice. To our surprise, IL-23 p19 was slightly upregulated in Irf5-/- mice at day 14 p.i., but was induced at similar levels at later stages of infection in both WT and Irf5-/- mice (Fig. 3E).
Fig. 3. Irf5-/- mice do not develop Th1-type granulomas. 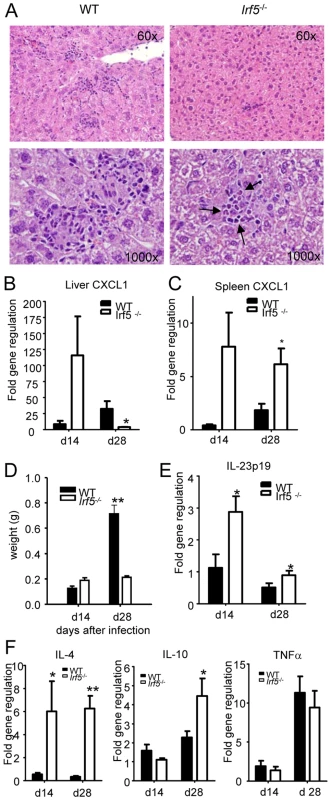
(A) Representative granulomas from H&E stained liver sections at d28 pi in WT and Irf5-/- mice. Pictures were taken at the indicated magnifications and arrows indicate the presence of neutrophils in the granulomas. (B, C) Real time PCR analysis of the livers (B) and spleen (C) measuring mRNA for CXCL1. (D) Spleen weights at d14 and d28 pi. The body weight of C57BL/6 WT and Irf5-/- mice was comparable. (E) Real time PCR analysis of the livers from infected mice measuring mRNA for IL-23 p19. (F) Real time PCR measurement of cytokine mRNA in the livers of infected mice indicates a skewing toward a Th2 environment. All data is presented as the mean ± SEM combined from two independent experiments. * denotes p<0.05 and ** denotes p<0.01. Since Irf5-/- granulomas were not the typical Th1-type granulomas which develop in infected WT mice and, unlike WT mice, Irf5-/- mice failed to eliminate the parasites, we next examined the cytokine environment in the livers of both groups of mice. IRF-5 deficiency resulted not only in a very weak IFNγ responses (Fig. 2B), but also in a significantly higher expression of IL-4 and IL-10 mRNA compared to WT mice (Fig. 3 F). Interestingly, the differences in cytokine mRNA levels were observed only at d28 pi but not during the first 2 weeks of infection. Surprisingly, however, we did not observe any significant differences in the relative levels of TNFα (Fig. 3E), IL-13, or IL-5 (data not shown) between the two groups. Moreover, Irf5-/- and WT mice showed comparable frequencies of IL-10 - and IL-17-producing CD4+ T cells at d28 pi (data not shown). Taken together, these data suggest that unlike in WT mice, L. donovani infection in Irf5-/- mice induces a very small inflammatory infiltration in the liver and results in the generation of an IL-4-dominated response, resulting in a failure to control parasite growth.
Attenuation of IL-12p35 expression in L. donovani infected Irf5-/- mice
It is well established that the production of IL-12 by DCs is crucial for the development of Th1 cells [25], although an IL-12-independent mechanism for the induction Th1 responses has also been described [26]. A study in Myd88-/- mice infected with L. major has highlighted the importance of TLRs, IL-1 and/or IL-18 in the induction of IL-12 and the generation of Th1 responses; MyD88 deficiency also resulted in complete abrogation of IFNγ production by CD4+ T cells and an inability to control infection [5]. More recently, TLR9 activation has been shown to play a crucial role in the induction of IL-12 secretion by DCs following T. cruzi and Leishmania infections [6], [7]. As the development of IFNγ-producing CD4+ T cell in L. donovani infected mice was shown to depend upon IL-12 production by conventional CD11chi DC (cDC), we examined whether IRF-5 deficient DC were able to produce IL-12 in vivo following L. donovani infection. In agreement with the literature [27], we detected a moderate increase in IL-12p35 mRNA levels in cDCs isolated from infected WT mice (Fig. 4). However, induction of IL-12p35 was not detected in IRF-5 deficient cDCs. In contrast, IL-12p40 mRNA expression was induced in cDCs of Irf5-/- at similar levels as in cDC of WT mice (data not shown). Since the p40 component of IL-12 is shared with IL-23, the upregulation of p40 may be caused by an increased expression of IL-23 (s. Fig. 3E).
Fig. 4. L. donovani fails to induce IL-12p35 expression in Irf5-/- mice. 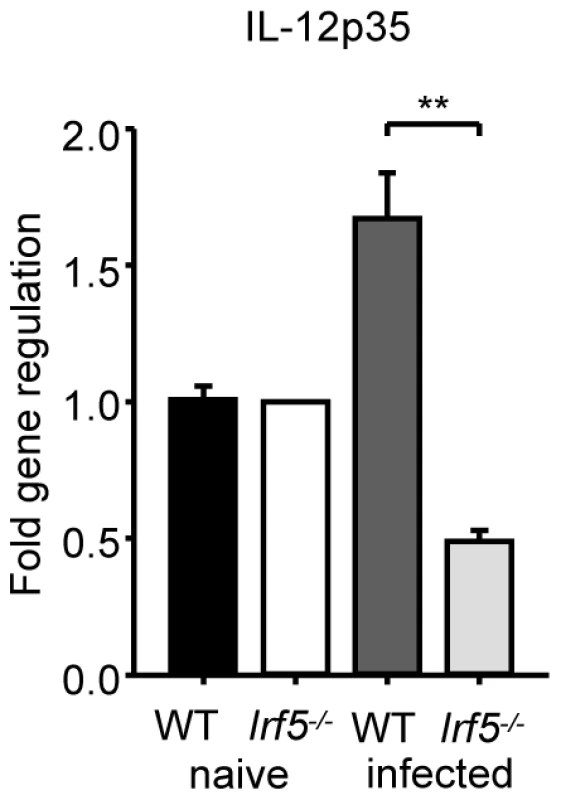
WT and Irf5-/- mice were infected with L. donovani amistigotes. At 5 h, splenic cDCs were purified. IL-12p35 mRNA was detected by real time PCR. Data is shown for n = 3 WT samples and n = 4 Irf5-/- samples.* denotes p<0.05 and ** denotes p<0.01. IRF-5 indirectly regulates iNOS expression
Inducible nitric oxide synthase (iNOS) is a key enzyme involved in the production of nitric oxide (NO), which has direct microbial toxicity and is also involved in the regulation of cytokine gene expression and cytokine responsiveness. NO is typically produced by classically activated macrophages upon triggering of IFN and TLR pathways that enhance the expression of iNOS [28], [29], [30]. In the absence of a strong IFNγ response and the presence of an IL-4 dominated immune response in infected Irf5-/- mice, we were curious as to whether iNOS (NOS2) was still able to be induced at all in Irf5-/- mice. To our surprise, we found no difference in the induction of iNOS mRNA in the livers of Irf5-/- and WT mice (Fig. 5A). As the absence of IRF-5 appeared to be leading to a Th2-like state in the liver, we were also interested in determining whether markers for the alternative activation of macrophages were being induced in mice deficient in IRF-5. Using real time PCR we only found a slight increase in the induction of Arg1 (Fig. 5B) and Fizz1 (Fig. 5C) in the livers of infected Irf5-/- mice compared to WT mice.
Fig. 5. IRF-5 deficiency results in defective iNOS production. 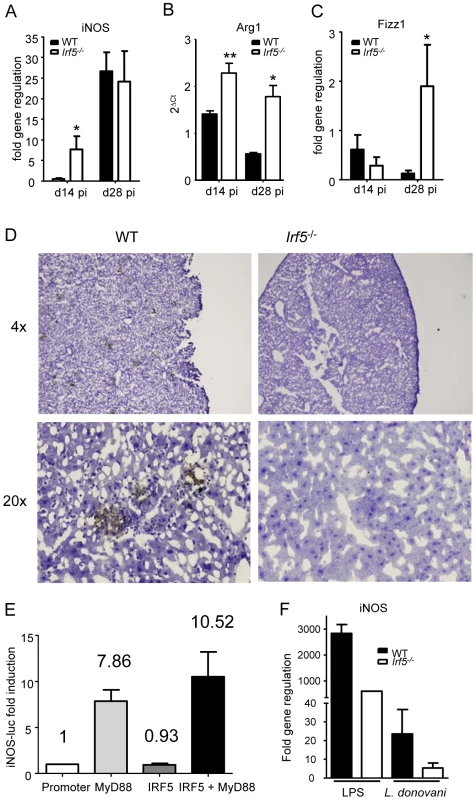
Real time PCR analysis of livers from infected mice measuring mRNA for iNOS (A), arginase1 (B) and Fizz1 (C). All data is presented as the mean ± SEM combined from two independent experiments, n = 6-9 mice per group. (D) iNOS protein in the liver was examined by immunohistochemistry. (E) Induction of the NOS2 promoter by MyD88 and IRF-5 was measured by luciferase assay. Data shown is fold induction of the promoter compared to promoter only controls and represents the mean± SEM for triplicate samples from 2 independent experiments. * denotes p<0.05 and ** denotes p<0.01. (F) Real time PCR analysis of iNOS mRNA in WT and Irf5-/- macrophages stimulated with LPS and/or L.donovani amastigotes. Data is shown for n = 3. Since iNOS mRNA levels were similar in Irf5-/- and WT mice, we next investigated whether this message was being translated into protein. iNOS protein was expressed in the livers of infected WT mice, mainly in granulomas (Fig. 5D). In contrast, it was not detected in infected Irf5-/- mice at any time point after infection (Fig. 5D).
To determine whether IRF-5 directly regulates transcription of iNOS, we used a luciferase reporter assay, where luciferase was under the control of the NOS2 promoter cotransfected with MyD88 and/or IRF-5 expression plasmids. As shown in Figure 5E and in agreement with the literature [28], [30], MyD88 expression induced a 7.86 fold increase in luciferase activity. In contrast, IRF-5 failed to induce luciferase activity, suggesting that overexpression of inactivated IRF-5 alone does not stimulate the transcriptional activity of the NOS2 promoter. Co - transfection with both MyD88 and IRF-5 expression plasmids resulted only in a slight increase in the transcriptional activity of the NOS2 promoter. Taken together, these results indicate that IRF-5 does not stimulate transcription of iNOS but may play a role to indirectly regulate the iNOS response to L. donovani at the posttranscriptional level.
We next infected WT and Irf5-/- bone marrow-derived macrophages in vitro with L. donovani and monitored the iNOS mRNA expression by real time PCR. In agreement with the results shown in Fig. 5E, IRF-5 deficiency did not affect the iNOS mRNA level (Fig. 5F) or the NO production (Fig. S2 A) following L. donovani infection, suggesting that IRF-5 does not directly induce transcription of iNOS. However, when we stimulated WT and Irf5-/- macrophages with LPS, we could see a marked decrease in both iNOS mRNA transcipts (Fig. 5F) and NO production (Fig. S2 A). This was possibly caused by a defective pro-inflammatory response in Irf5-/- macrophages (Fig. S2 B). This defect was not observed in Irf5-/- macrophages infected with L. donovani (Fig. S2 B), suggesting that the induction of pro-inflammatory cytokines in macrophages infected in vitro with L. donovani is IRF-5 independent. This data implies that the inflammatory signals downstream of TLR triggering largely contribute to the induction of iNOS. Thus it is possible that the defective pro-inflammatory response observed in L. donovani infected Irf5-/- mice (Fig. 2 and 3) is mainly responsible for the reduction in iNOS expression in these mice.
IRF-5 is upregulated in T-cells during L. donovani infection
Since IRF-5 seems to be involved in the maintenance of the inflammatory response to L. donovani and in the development of protective Th1-responses, we were interested in which cells IRF-5 expression is essential for displaying and/or inducing anti-leishmanial effector function. Thus, we analyzed the cell-specific expression pattern of IRF-5 mRNA in the liver and the spleen at different time points during infection (Fig. 6). Interestingly, in the spleen IRF-5 mRNA was only upregulated in the CD5+ population, corresponding to T-cells. The increase in IRF-5 mRNA levels was only detected at d28 p.i. (Fig. 6A). Splenic B-cells, macrophages, dendritic cells, and neutrophils did not show increased expression of IRF-5 mRNA at any time point analyzed (data not shown). In the liver, only the CD5 positive fraction (T-cells) had upregulated IRF-5 mRNA at d14 p.i. (Fig. 6B). However by d28 p.i., only few T-cells were present in the liver (Fig. 2 and 3) and at this time point T-cells did not express IRF-5 mRNA at levels greater than in naïve mice.
Fig. 6. IRF-5 is upregulated in T-cells during L. donovani infection. 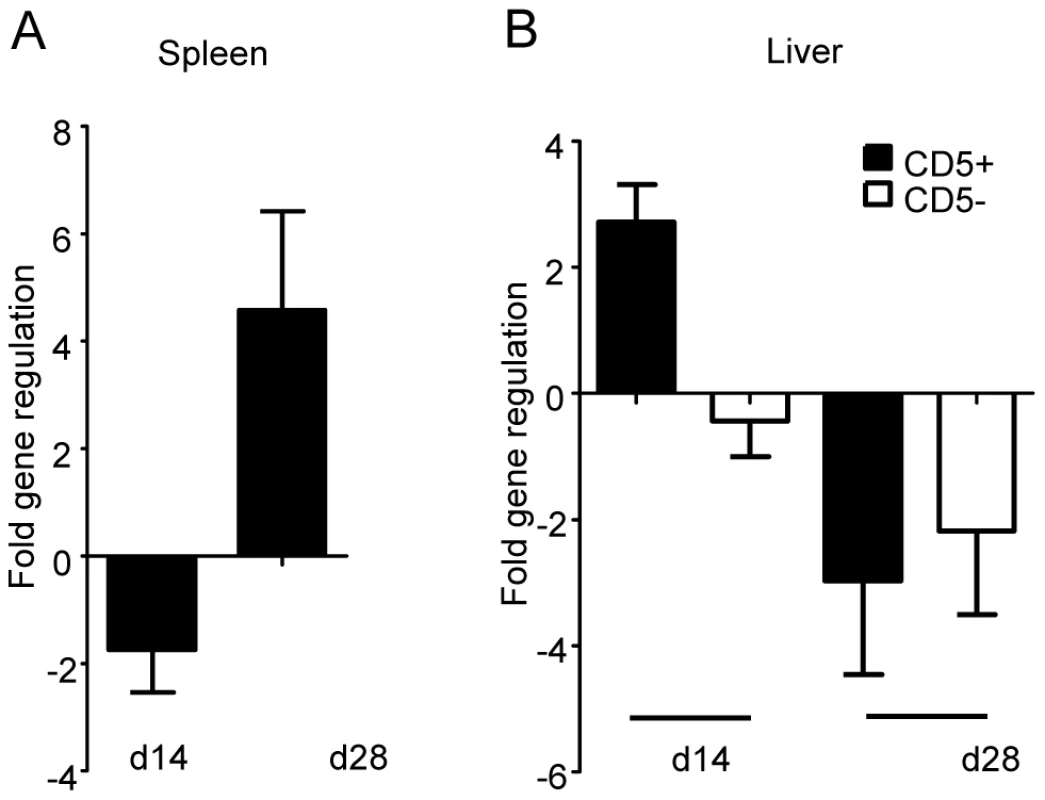
Real time PCR analysis of spleens (A) and livers (B) from infected WT mice measuring mRNA for IRF-5. All data is presented as the mean ± SEM combined from two independent experiments, n = 6 mice per group. TLR7 is essential for the development of host protective Th1 responses
We and others have previously shown that IRF-5 can be activated by TLR7 [16] and TLR9 [13] via the MyD88 signaling pathway. Indeed, it was recently shown that Leishmania infections in Tlr9-/- mice induced transiently deficient Th1 responses [7], [9], suggesting that the regulation of these responses in Leishmania infections might also be governed by other pathogen recognition pathways. Thus, we next investigated which TLR was involved in the activation of IRF-5 and consequently in the modulation of Th1 responses following L. donovani infection.
Hence we proceeded to assess whether TLR7 was involved in the recognition of L. donovani and in the generation of parasite-specific Th1 responses. To our surprise, L. donovani infection in Tlr7-/- mice resulted in a approximately 3-fold higher hepatic parasite burden at day 28pi compared to WT mice (Fig. 7A); as seen in Irf5-/- mice, the splenic parasite burden in Tlr7-/- mice was similar to the WT control group (data not shown). These results suggest that TLR7 plays a role in the recognition of L. donovani parasites and in the generation of protective immune responses against Leishmania.
Fig. 7. TLR7 activation is essential for the development of Th1 responses following L. donovani infection. 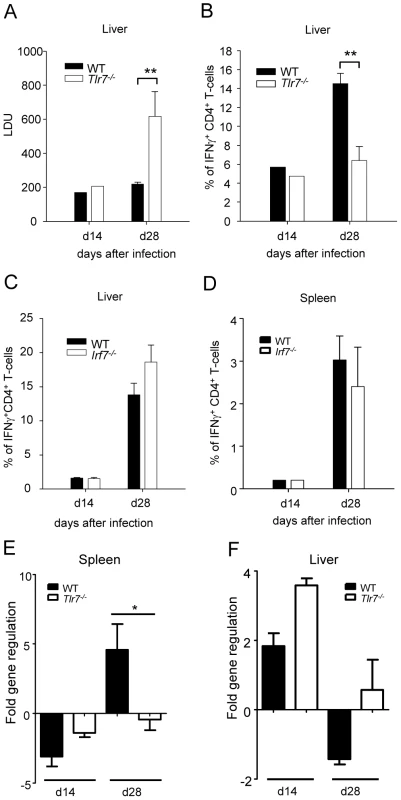
(A) The hepatic parasite burden was determined for infected WT and Tlr7-/- mice at d14 and d28 pi as described. (B) The percentage of CD4+ T cells producing IFNγ as a response to L. donovani infection in the liver of WT and Tlr7-/- mice was determined by intracellular flow cytometry. (C) Percentage of IFNγ+ CD4+ T-cells in the liver and (D) the spleen of WT and Irf7-/- mice. (E,F) Real time PCR analysis of spleens (E) and livers (F) from infected WT and Irf7-/- mice measuring mRNA for IRF5. Data is shown as the mean ± SEM. Data is representative of two independent experiments. We then determined the frequency of IFNγ+ CD4+ T-cells in infected Tlr7-/- mice and WT controls. At day 14 p.i the frequency of IFNγ producing CD4+ T-cells was similar in the liver of Tlr7-/- mice compared to the WT control group. However, at day 28, only 6.4% of CD4+ T-cells in the liver of Tlr7-/- mice was producing IFNγ compared to 14.5% in WT mice (Fig. 7B and S3). A similar reduction in IFNγ production was observed in the spleen (data not shown). Interestingly, the extent of the inflammatory cell infiltration in the livers of Tlr7-/- mice at day 28 p.i. was comparable to WT mice (data not shown). Taken together, these results suggest that the activation of TLR7 is crucial for the development of Th1 responses following L. donovani infection.
Since TLR7 not only signals through IRF5 but also through IRF7 [31] and IRF7 has recently been shown to regulate killing of intracellular Leishmania in marginal zone macrophages [32], we wanted to determine whether IRF7 contributed to the defect in Th1 responses we observed in Tlr7-/- mice. Hence, we infected Irf7-/- mice with L. donovani amastigotes and compared the development of Th1 responses in these mice to infected WT controls. The results revealed that in Irf7-/- mice, L. donovani induced IFNγ-producing CD4+ T-cells at frequencies comparable to infected WT mice (Fig. 7C and D).
Finally, we compared the cell-specific IRF-5 mRNA expression pattern in WT and Tlr7-/- mice at d14 and d28 p.i.. As previously shown (Fig. 6), only the CD5+ fraction (T-cells) had upregulated IRF-5 mRNA in the spleen of infected WT mice (Fig. 7E). The IRF-5 mRNA expression was not significantly higher in B-cells and non-B/non-T-cells from infected WT mice compared to the corresponding naïve WT cell populations (data not shown). Interestingly, we could not detect any upregulation of IRF-5 mRNA expression in splenic T-cells from infected Tlr7-/- mice at d28 p.i., suggesting that TLR7 is essential for the induction of IRF-5 in T-cells in the spleen at later stages of infection. In contrast, in the liver, IRF-5 mRNA expression was only upregulated in T-cells purified at day 14 p.i. from infected WT and Tlr7-/- mice (Fig. 7F). This suggests that increased IRF-5 expression in T-cells is independent of TLR7 during the early stages of infection in the liver. No IRF-5 mRNA could be detected at d28 p.i. in the liver of WT and Tlr7-/- mice (Fig. 7F).
Taken together, these data suggest that IRF-5 is a key molecular switch for the development of Th1 responses following L. donovani infection, and that TLR7-mediated IRF-5 activation plays a critical role during chronic infection.
Discussion
In the present study we have demonstrated that the TLR7-mediated activation of IRF-5 is required for the development of host-protective Th1 responses to L. donovani at later stages of infection. Moreover, IRF-5 deficiency resulted in an IL-4 dominated response, reduced iNOS expression, and failure to control parasite growth in the liver. To our knowledge, the role of IRF-5 in modulating adaptive T-cell responses is a novel and previously undescribed finding.
One of the key points in the induction of a protective immunity to Leishmania parasites is the generation of IFNγ-producing CD4+ T-cells. Although IL-12 production by DC is crucial for the development of Th1 cells [25], the mechanism leading to the generation of these responses remains elusive. A study in Myd88-/- mice infected with Leishmania has highlighted the importance of TLRs, IL-1 and/or IL-18 in the induction of IL-12 and the generation of Th1 responses. Indeed, MyD88 deficiency resulted in complete abrogation of IFNγ production by CD4+ T-cells and an inability to control infection [5]. More recently, TLR9 activation was shown to play a crucial role in the induction of IL-12 secretion by DCs following T. cruzi and/or Leishmania infections [6], [7]. However, unlike T. cruzi infections, attenuation of Th1 responses to Leishmania infection in Tlr9-/- mice was only transient [7], [9]. These studies suggest that the regulation of Th1 responses in Leishmania infections is not exclusively mediated by TLR9 and might be governed by other pathogen recognition pathways as well. Our study pinpoints IRF-5, a transcription factor in the MyD88-mediated TLR pathways, as an essential factor in the development of adaptive Th1 responses following Leishmania infection. Interestingly, while Th1 responses in Irf5-/- mice were severely impaired 4 weeks after infection, expression of IRF-5 was not required during the first 2 weeks of infection, since the frequency of IFNγ-producing CD4+ T-cells in Irf5-/- mice was comparable to WT mice. This observation suggests that IRF-5 is not essential for the early induction of Th1 responses but is crucial for their further development and expansion. Although IRF-5 has been shown to have a major impact on the innate immune response to viral infections [17], [18], its role in shaping the development of adaptive Th1 responses has not been previously demonstrated.
Defective Th1 responses were also observed in Tlr7-/- mice infected with L. donovani. IRF-5 and IRF-7 are both known to be activated by the MyD88-dependant TLR7 signaling pathway [16], [31], however IRF-7 deficiency did not result in defective Th1 responses to L. donovani. TLR7 has been shown to recognize single-stranded RNA [33], [34] and to play an essential role in the immunity to ssRNA virus [24], [35]. Recognition of a DNA virus, the murine cytomegalovirus, by TLR7 has also been reported [36]. To date there is no indication of TLR7 activation by parasites.
The analysis of cell-specific IRF-5 mRNA expression pattern revealed that the only cells that upregulated IRF-5 during L. donovani infection at d14 and 28 p.i. were T-cells. This does not exclude, though, that other cell types such as DCs and macrophages may express IRF5 during earlier stages of infection. Nevertheless, IRF-5 expression in T-cells differed between the liver and the spleen: in the liver IRF5 expression is upregulated during the first 2 weeks of infection in a TLR7-independent manner; in contrast, in the spleen the upregulation occurs 4 weeks into the infection and appears to be mediated by TLR7. Since the defect in the Th1 responses seems to mainly affect the hepatic infection, at least until d28 p.i., and IRF-5 only appears to be upregulated in T-cells in the spleen during chronic infection, it is tempting to speculate that effector Th1 cells generated in the spleen are required for controlling parasite growth in the liver. Future experiments involving splenectomized mice should be able to prove this hypothesis.
It is possible that the upregulation of IRF-5 in T-cells may be required depending on the subsets and activation status of the cells. A role for IRF-5 in T-cells is yet unknown. Whether IRF-5 expression by T-cells is directly mediated by TLR 7/9 triggering or indirectly induced by Type I IFN, produced by APC following TLR7/9 signaling, is still an open question. Recent studies have highlighted a role for MyD88 in T-cells [37], [38], supporting the possibility of a direct TLR7/9 –mediated IRF-5 induction. Mice with MyD88 deficient T-cells only develop defective Th1 responses in a Toxoplasma gondii model [37], suggesting that MyD88 signaling in T-cells is essential for Th1 cell development. Human T-cells purified from HIV [39] and from Hepatitis C [40] patients were also shown to express TLR7 and/or TLR9. Why TLR7/IRF-5 activation is only required at later stages of infection and what role it plays in various T-cell subsets are two more questions that remain yet to be answered. Future investigations will address these questions using T-cell-specific IRF-5 and TLR7 knockout mice.
Interestingly, the severe defect in Th1 responses seen in Irf5-/- mice affected the hepatic but did not seem to have any effect on the splenic parasite burden, at least until day 28 p.i.. This is in agreement with a study by Engwerda and colleagues who have demonstrated that IL-12 neutralization did exacerbate infection in the spleen during the first 4 weeks of infection, even though IFNγ production, which is mainly derived from CD4+ T-cells, was severely reduced [2]. Thus, in contrast to the liver, Th1 responses are not critical for controlling parasite growth in the spleen during the first 28 days of infection. These observations imply that requirements for protection against L. donovani in the liver and in the spleen are very distinct, and underline again the organ-specific nature of the immune response during VL.
IRF-5 deficiency also resulted in a dramatic decrease in the extent of the inflammatory cell infiltration in the liver at day 28 p.i.. Moreover, L. donovani failed to induce splenomegaly in Irf5-/- mice, which is characteristic for L. donovani infection in WT mice. Since TLR7 deficiency did not significantly impair the recruitment of inflammatory cells to the liver of L. donovani infected mice, we can assume that there is some redundancy between different TLRs recognizing different PAMPs, but commonly utilizing IRF-5 in the induction of the inflammatory response during VL. Such overlapping effects between TLR7 and TLR9 have been observed during murine cytomegalovirus infection [36]. However, TLR7 and TLR9 were recently shown to have distinct effects in a murine model of Lupus [41] and also during experimental West Nile Encephalitis, where Tlr7-/- mice, but not Tlr9-/- mice, showed an impaired CD45+ leukocyte and macrophage infiltration at the site of infection [24]. Failure of these cells to migrate to infected target organs was caused by a significant reduction in IL-23 responses [24]. In L. donovani infected Irf5-/- mice, the level of IL-23 p19 were slightly higher compared to WT mice, suggesting that the lack of lymphocyte infiltration in the liver was not caused by the decreased induction of IL-23, but by some other yet unidentified pathways. Furthermore, the frequency of IL-17 producing cells, which are typically induced by IL-23 [42], was comparable in both groups of mice. This indicates that IRF-5 deficiency only affected the development of Th1 responses, but not the generation of Th17 cells in L. donovani infected mice.
NO is an important leishmanicidal effector molecule. It has direct microbial toxicity and it is also involved in the regulation of cytokine gene expression and cytokine responsiveness. NO is typically produced by classically activated macrophages upon triggering of interferon and TLR pathways that enhance expression of iNOS [28], [29], [30]. Expression of iNOS in mice infected with L. donovani appears to be tissue-specific: this enzyme is induced in the liver, however only limited iNOS expression is observed in the spleen [43]. Despite defective IFNγ responses in Irf5-/- mice the level of iNOS mRNA expression was comparable to the WT control group. Since iNOS expression can also be triggered by TLR pathways, high levels of iNOS mRNA in Irf5-/- mice may most likely be due to the high parasite burden in the liver at d28pi. Interestingly though, iNOS protein was not detected in the livers of Irf5-/- mice. A possible explanation for the lack of iNOS protein in Irf5-/- mice is that iNOS may be competing with arginase 1. This enzyme is commonly linked to alternative pathway of macrophage activation [44]. However, arginase 1 can also be induced in classically activated macrophages and can reduce NO production through competition with iNOS for their common substrate arginine [45]. Arginase 1 was also shown in vitro to suppress translation and enzymatic activity of iNOS without affecting mRNA levels [46], [47]. Nevertheless, arginase 1 mRNA was only slightly increased in Irf5-/- mice compared to WT mice, an increase that is probably not sufficient to inhibit translation of iNOS protein. Another possible explanation is that the severe defect in pro-inflammatory and the concomitant increase in Th2 responses observed in Irf5-/- mice may be responsible for the lack of amplification of the iNOS expression. Further investigations are needed to clarify the role of IRF-5 in the molecular mechanisms involved in the regulation of iNOS production.
In conclusion, we have indentified IRF-5 as a critical component for the development of Th1 responses to Leishmania infection. Furthermore, IRF-5 is an essential factor for the maintenance of the inflammatory response and plays a role in the indirect regulation of iNOS expression during L. donovani infection. Further experiments have yet to determine the molecular mechanism by which IRF-5 affects the development of host protective immunity to L. donovani.
Materials and Methods
Mice and parasites
Ethics statement: All experiments were approved by and conducted in accordance with guidelines of the Animal Care and Use Committee of the Johns Hopkins University School of Medicine.
C57BL/6J mice were obtained from The National Cancer Institute (Frederick, MD, USA), and B6.129S7-Rag1tm1Mom/J from The Jackson Laboratory. All mice were housed in the Johns Hopkins University animal facilities (Baltimore, MD) under specific pathogen-free conditions and used at 6–8 weeks of age. Irf5-/- mice were a generous gift from Dr. T. Mak (University of Toronto, Canada) and were backcrossed to C57BL/6J for at least 10 generations. The SNP analysis confirmed that they are 100% C57BL/6. We have previously reported age related splenomegaly in Irf5-/- mice that was associated with changes in splenic architecture [48]. The background of these mice was only 92% C57BL/6. In this study we have used 6–8 weeks old Irf5-/- mice that have normal spleen size and splenic architecture. Tlr7-/- mice were a generous gift from Dr S. Akira (Osaka University, Japan). Tlr7-/-were backcrossed to C57BL/6J for 9 generations. Irf7-/- mice were a kind gift from Dr. T. Taniguchi (University of Tokyo, Japan). Irf7-/- were backcrossed to C57BL/6J for 8 generations. Leishmania donovani (strain LV9) parasites were maintained by serial passage in B6.129S7-Rag1tm1Mom/J mice, and amastigotes were isolated from the spleens of infected animals. Mice were infected by injecting 2×107 amastigotes intravenously via the lateral tail vein. Hepatic and splenic parasite burdens were determined by examining methanol-fixed, Giemsa stained tissue impression smears. Data are presented as Leishman Donovan Units (LDU).
Flow cytometry
Mice were euthanized at indicated time points. Mononuclear cells were purified from the liver as previously described [49]. Hepatic mononuclear cells and splenocytes were restimulated for 2 h at 37°C in the presence of bone marrow derived dendritic cells (BMDC) previously pulsed with paraformaldehyde fixed amastigotes. Brefeldin A was then added for a further 4 h. Cells were then stained with biotinylated anti-CD3 followed by PerCP-conjugated strepatvidin, FITC-conjugated anti-CD4, and APC-conjugated anti-IFNγ (all BD Bioscience). Flow cytometric analysis was performed with a LSRII flow cytometer (Becton Dickinson). 350,000 cells per sample were acquired and analyzed with the FACSDiva software.
Cell sorting
Spleens from infected and naive C57BL/6 mice were divided into 2 groups. Group A: B-cells were first enriched from splenic single cell suspension using anti-B220 beads (Miltenyi Biotec) following manufacturer instructions. B-cells were then sorted to >98% purity using FACSVantage (Becton Dickinson) based on their expression of CD19 and B220. The B220 negative fraction was then incubated with anti-CD5 beads for the enrichment of T-cells. T-cells were then sorted to >98% purity using FACSVantage based on their expression of CD4+, CD8+ and NK1.1-. Group B: splenocytes were first incubated with anti-CD11c beads and conventional splenic DCs were then sorted to >98% purity based on their expression of CD11c and MHCII. The CD11c negative fraction was then incubated with anti-CD11b beads. CD11b+ cells were sorted into different populations based on their expression of Gr1, MHCII and CD11b.
Livers from infected and naïve C57BL/6 mice and from infected Tlr7-/- mice were incubated with anti-CD5 beads in order to enrich T-cells. The purity of the T-cell preparation was >87%. Spleens from infected WT and Tlr7-/- mice were first incubated with anti-B220 beads; the B220 negative fraction was then incubated with anti-CD5 beads. >82% of the B220+ cells were B-cells; >94% of the CD5+ cells were T-cells.
Preparation and infection of bone marrow derived macrophages
Macrophages were differentiated from the bone marrow following red blood cell lysis. Cells were incubated with 30 ng/ml M-CSF (RnDSystems) for 5 days. Prior to use cells were counted and seeded at the 5×105 cells/well in a 24 well plate, rested in the absence of M-CSF for several hours and then treated with LPS (1 µg/ml) (Sigma-Aldrich) or L. donovani (MOI 10) for 1 h. Media was then changed to remove any extracellular parasites and cells incubated in fresh media for a further 18 h. At experimental end point supernatant was collected and cells washed in PBS and lysed for RNA extraction.
RNA extraction and real time PCR analysis
For the analysis of the IL-12p35/IL-12p40 mRNA induction, WT and Irf5-/- mice were infected with 2×107 L.donovani amastigotes. 5 h later, mice were euthanized and CD11c+ dendritic cells were isolated from splenic single cell suspensions using anti-CD11c beads (Miltenyi Biotec) following manufacturer instructions. The purity of the preparation was about 85%. RNA was extracted using Trizol (Invitrogen) as per manufacturer' instructions. For purified cell populations listed in the previous section RNA was extracted using the RNEasy Mini Kit (Qiagen). Reverse transcription was performed using the QuantiTect Reverse Transcription kit (Qiagen). SybrGreen was used to assay beta actin [17], IL-12p40 and IL-12p35. The following primers were used: IL-12p35 F - CCACCCTTGCCCTCCTAAAC and R-GGCAGCTCCCTCTTGTTGTG; IL-12p40 F - CTTGCAGATGAAGCCTTTGAAGA and R - GGAACGCACCTTTCTGGTTACA. For infected mice, livers and spleens were collected at indicated time points and whole tissue RNA extracted using Trizol, cDNA generated using the QuantiTect Reverse Transcription kit. Other than IL-12p35 and IL-12/23p40 all gene expression was analyzed using Taqman primers with a StepOnePlus cycler (Applied Biosystems).
NO quantiation
Nitric oxide was quantitated using the Greiss assay (Promega) according to manufacturer's instructions.
Immunohistochemistry
For frozen sections, livers from infected mice and uninfected controls were embedded in OCT (TissueTek), snap frozen and 5 µm sections cut. Sections were stained for iNOS/NOS2 using an anti-iNOS polyclonal antibody generated in rabbit (Chemicon/Millipore) and detected using anti-rabbit IgG conjugated to HRP and DAB substrate (both Vector Laboratories). Paraffin-embedded sections were prepared from liver fixed in neutral buffered formalin, cut to 5 µm and stained by H&E. All immunohistochemistry was analyzed by light microscopy and photographs taken at the indicated magnifications.
Luciferase assay
HEK293 cells were seeded at 1×105 cells/well in 96 well plates. Cells were transfected with the NOS2-luc reporter construct (50 ng) (Addgene) and Renilla luciferase (10 ng) with either MyD88 (50 ng) and/or murine IRF-5 (100 ng) expression plasmids. 24 hours after transfection cells were lysed and assayed for luciferase activity using the Promega Dual-luciferase reporter assay. Luciferase activity was normalized against Renilla. Data shown is the mean± SEM for triplicate samples from 2 independent experiments.
Statistical analysis
Results were analyzed using an unpaired Student t-test. P<0.05 was considered significant. Real time PCR results were analyzed using an unpaired Student t-test or the Mann-Whitney test. For bone marrow derived macrophages the non parametric t-test with Welch's correction was used. P<0.05 was considered significant. Experiments were repeated at least twice.
Supporting Information
Zdroje
1. KayePM
SvenssonM
AtoM
MaroofA
PolleyR
2004 The immunopathology of experimental visceral leishmaniasis. Immunol Rev 201 239 253
2. EngwerdaCR
MurphyML
CotterellSE
SmeltSC
KayePM
1998 Neutralization of IL-12 demonstrates the existence of discrete organ-specific phases in the control of Leishmania donovani. Eur J Immunol 28 669 680
3. GorakPM
EngwerdaCR
KayePM
1998 Dendritic cells, but not macrophages, produce IL-12 immediately following Leishmania donovani infection. Eur J Immunol 28 687 695
4. Scharton-KerstenT
AfonsoLC
WysockaM
TrinchieriG
ScottP
1995 IL-12 is required for natural killer cell activation and subsequent T helper 1 cell development in experimental leishmaniasis. J Immunol 154 5320 5330
5. MurailleE
De TrezC
BraitM
De BaetselierP
LeoO
2003 Genetically resistant mice lacking MyD88-adapter protein display a high susceptibility to Leishmania major infection associated with a polarized Th2 response. J Immunol 170 4237 4241
6. LieseJ
SchleicherU
BogdanC
2007 TLR9 signaling is essential for the innate NK cell response in murine cutaneous leishmaniasis. Eur J Immunol 37 3424 3434
7. SchleicherU
LieseJ
KnippertzI
KurzmannC
HesseA
2007 NK cell activation in visceral leishmaniasis requires TLR9, myeloid DCs, and IL-12, but is independent of plasmacytoid DCs. J Exp Med 204 893 906
8. BaficaA
SantiagoHC
GoldszmidR
RopertC
GazzinelliRT
2006 Cutting edge: TLR9 and TLR2 signaling together account for MyD88-dependent control of parasitemia in Trypanosoma cruzi infection. J Immunol 177 3515 3519
9. Abou FakherFH
RachinelN
KlimczakM
LouisJ
DoyenN
2009 TLR9-dependent activation of dendritic cells by DNA from Leishmania major favors Th1 cell development and the resolution of lesions. J Immunol 182 1386 1396
10. AkiraS
UematsuS
TakeuchiO
2006 Pathogen recognition and innate immunity. Cell 124 783 801
11. O'NeillLA
2006 How Toll-like receptors signal: what we know and what we don't know. Curr Opin Immunol 18 3 9
12. BarnesBJ
RichardsJ
ManclM
HanashS
BerettaL
2004 Global and distinct targets of IRF-5 and IRF-7 during innate response to viral infection. J Biol Chem 279 45194 45207
13. TakaokaA
YanaiH
KondoS
DuncanG
NegishiH
2005 Integral role of IRF-5 in the gene induction programme activated by Toll-like receptors. Nature 434 243 249
14. HondaK
TaniguchiT
2006 IRFs: master regulators of signalling by Toll-like receptors and cytosolic pattern-recognition receptors. Nat Rev Immunol 6 644 658
15. BarnesBJ
MoorePA
PithaPM
2001 Virus-specific activation of a novel interferon regulatory factor, IRF-5, results in the induction of distinct interferon alpha genes. J Biol Chem 276 23382 23390
16. SchoenemeyerA
BarnesBJ
ManclME
LatzE
GoutagnyN
2005 The interferon regulatory factor, IRF5, is a central mediator of toll-like receptor 7 signaling. J Biol Chem 280 17005 17012
17. PaunA
ReinertJT
JiangZ
MedinC
BalkhiMY
2008 Functional characterization of murine interferon regulatory factor 5 (IRF-5) and its role in the innate antiviral response. J Biol Chem 283 14295 14308
18. YanaiH
ChenHM
InuzukaT
KondoS
MakTW
2007 Role of IFN regulatory factor 5 transcription factor in antiviral immunity and tumor suppression. Proc Natl Acad Sci U S A 104 3402 3407
19. PandeyAK
YangY
JiangZ
FortuneSM
CoulombeF
2009 NOD2, RIP2 and IRF5 play a critical role in the type I interferon response to Mycobacterium tuberculosis. PLoS Pathog 5 e1000500
20. KropfP
FreudenbergMA
ModolellM
PriceHP
HerathS
2004 Toll-like receptor 4 contributes to efficient control of infection with the protozoan parasite Leishmania major. Infect Immun 72 1920 1928
21. de VeerMJ
CurtisJM
BaldwinTM
DiDonatoJA
SextonA
2003 MyD88 is essential for clearance of Leishmania major: possible role for lipophosphoglycan and Toll-like receptor 2 signaling. Eur J Immunol 33 2822 2831
22. LieseJ
SchleicherU
BogdanC
2008 The innate immune response against Leishmania parasites. Immunobiology 213 377 387
23. FlandinJF
ChanoF
DescoteauxA
2006 RNA interference reveals a role for TLR2 and TLR3 in the recognition of Leishmania donovani promastigotes by interferon-gamma-primed macrophages. Eur J Immunol 36 411 420
24. TownT
BaiF
WangT
KaplanAT
QianF
2009 Toll-like receptor 7 mitigates lethal West Nile encephalitis via interleukin 23-dependent immune cell infiltration and homing. Immunity 30 242 253
25. TrinchieriG
2003 Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol 3 133 146
26. SoaresH
WaechterH
GlaichenhausN
MougneauE
YagitaH
2007 A subset of dendritic cells induces CD4+ T cells to produce IFN-gamma by an IL-12-independent but CD70-dependent mechanism in vivo. J Exp Med 204 1095 1106
27. MaroofA
KayePM
2008 Temporal regulation of interleukin-12p70 (IL-12p70) and IL-12-related cytokines in splenic dendritic cell subsets during Leishmania donovani infection. Infect Immun 76 239 249
28. Thoma-UszynskiS
StengerS
TakeuchiO
OchoaMT
EngeleM
2001 Induction of direct antimicrobial activity through mammalian toll-like receptors. Science 291 1544 1547
29. BogdanC
2001 Nitric oxide and the immune response. Nat Immunol 2 907 916
30. BrightbillHD
LibratyDH
KrutzikSR
YangRB
BelisleJT
1999 Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science 285 732 736
31. KawaiT
SatoS
IshiiKJ
CobanC
HemmiH
2004 Interferon-alpha induction through Toll-like receptors involves a direct interaction of IRF7 with MyD88 and TRAF6. Nat Immunol 5 1061 1068
32. PhillipsR
SvenssonM
AzizN
MaroofA
BrownN
2010 Innate killing of Leishmania donovani by macrophages of the splenic marginal zone requires IRF-7. PLoS Pathog 6 e1000813
33. HeilF
HemmiH
HochreinH
AmpenbergerF
KirschningC
2004 Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science 303 1526 1529
34. DieboldSS
KaishoT
HemmiH
AkiraS
Reis e SousaC
2004 Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 303 1529 1531
35. LundJM
AlexopoulouL
SatoA
KarowM
AdamsNC
2004 Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc Natl Acad Sci U S A 101 5598 5603
36. ZucchiniN
BessouG
TraubS
RobbinsSH
UematsuS
2008 Cutting edge: Overlapping functions of TLR7 and TLR9 for innate defense against a herpesvirus infection. J Immunol 180 5799 5803
37. LaRosaDF
StumhoferJS
GelmanAE
RahmanAH
TaylorDK
2008 T cell expression of MyD88 is required for resistance to Toxoplasma gondii. Proc Natl Acad Sci U S A 105 3855 3860
38. ZhaoY
De TrezC
FlynnR
WareCF
CroftM
2009 The adaptor molecule MyD88 directly promotes CD8 T cell responses to vaccinia virus. J Immunol 182 6278 6286
39. SongY
ZhuangY
ZhaiS
HuangD
ZhangY
2009 Increased expression of TLR7 in CD8(+) T cells leads to TLR7-mediated activation and accessory cell-dependent IFN-gamma production in HIV type 1 infection. AIDS Res Hum Retroviruses 25 1287 1295
40. HammondT
LeeS
WatsonMW
FlexmanJP
ChengW
Toll-like receptor (TLR) expression on CD4+ and CD8+ T-cells in patients chronically infected with hepatitis C virus. Cell Immunol 264 150 155
41. ChristensenSR
ShupeJ
NickersonK
KashgarianM
FlavellRA
2006 Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity 25 417 428
42. AggarwalS
GhilardiN
XieMH
de SauvageFJ
GurneyAL
2003 Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem 278 1910 1914
43. MelbyPC
YangYZ
ChengJ
ZhaoW
1998 Regional differences in the cellular immune response to experimental cutaneous or visceral infection with Leishmania donovani. Infect Immun 66 18 27
44. GordonS
2003 Alternative activation of macrophages. Nat Rev Immunol 3 23 35
45. El KasmiKC
QuallsJE
PesceJT
SmithAM
ThompsonRW
2008 Toll-like receptor-induced arginase 1 in macrophages thwarts effective immunity against intracellular pathogens. Nat Immunol 9 1399 1406
46. BoucherJL
MoaliC
TenuJP
1999 Nitric oxide biosynthesis, nitric oxide synthase inhibitors and arginase competition for L-arginine utilization. Cell Mol Life Sci 55 1015 1028
47. El-GayarS
Thuring-NahlerH
PfeilschifterJ
RollinghoffM
BogdanC
2003 Translational control of inducible nitric oxide synthase by IL-13 and arginine availability in inflammatory macrophages. J Immunol 171 4561 4568
48. LienC
FangCM
HusoD
LivakF
LuR
2010 Critical role of IRF-5 in regulation of B-cell differentiation. Proc Natl Acad Sci U S A 107 4664 4668
49. StagerS
SmithDF
KayePM
2000 Immunization with a recombinant stage-regulated surface protein from Leishmania donovani induces protection against visceral leishmaniasis. J Immunol 165 7064 7071
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 1- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Salivary Gland NK Cells Are Phenotypically and Functionally Unique
- Genetic Epidemiology of Tuberculosis Susceptibility: Impact of Study Design
- Early Target Cells of Measles Virus after Aerosol Infection of Non-Human Primates
- Multiple Plant Surface Signals are Sensed by Different Mechanisms in the Rice Blast Fungus for Appressorium Formation
- Biofilm Development on by Is Facilitated by Quorum Sensing-Dependent Repression of Type III Secretion
- Distinct Patterns of IFITM-Mediated Restriction of Filoviruses, SARS Coronavirus, and Influenza A Virus
- Molecular Basis of Increased Serum Resistance among Pulmonary Isolates of Non-typeable
- A Helminth Immunomodulator Exploits Host Signaling Events to Regulate Cytokine Production in Macrophages
- HCMV Spread and Cell Tropism are Determined by Distinct Virus Populations
- Characteristics of the Earliest Cross-Neutralizing Antibody Response to HIV-1
- Induction of a Peptide with Activity against a Broad Spectrum of Pathogens in the Salivary Gland, following Infection with Dengue Virus
- Structural Basis for the Recognition of Cellular mRNA Export Factor REF by Herpes Viral Proteins HSV-1 ICP27 and HVS ORF57
- Identification and Characterization of the Host Protein DNAJC14 as a Broadly Active Flavivirus Replication Modulator
- Dual-Use Research and Technological Diffusion: Reconsidering the Bioterrorism Threat Spectrum
- Pathogenesis of the 1918 Pandemic Influenza Virus
- A Cardinal Role for Cathepsin D in Co-Ordinating the Host-Mediated Apoptosis of Macrophages and Killing of Pneumococci
- Critical Role of IRF-5 in the Development of T helper 1 responses to infection
- The Pel Polysaccharide Can Serve a Structural and Protective Role in the Biofilm Matrix of
- Selective C-Rel Activation via Malt1 Controls Anti-Fungal T-17 Immunity by Dectin-1 and Dectin-2
- Imaging Single Retrovirus Entry through Alternative Receptor Isoforms and Intermediates of Virus-Endosome Fusion
- Aerosols Transmit Prions to Immunocompetent and Immunodeficient Mice
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Dual-Use Research and Technological Diffusion: Reconsidering the Bioterrorism Threat Spectrum
- Pathogenesis of the 1918 Pandemic Influenza Virus
- Critical Role of IRF-5 in the Development of T helper 1 responses to infection
- A Cardinal Role for Cathepsin D in Co-Ordinating the Host-Mediated Apoptosis of Macrophages and Killing of Pneumococci
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



