-
Články
Reklama
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
ReklamaMechanisms of Trafficking to the Brain
During hematogenously disseminated disease, Candida albicans infects most organs, including the brain. We discovered that a C. albicans vps51Δ/Δ mutant had significantly increased tropism for the brain in the mouse model of disseminated disease. To investigate the mechanisms of this enhanced trafficking to the brain, we studied the interactions of wild-type C. albicans and the vps51Δ/Δ mutant with brain microvascular endothelial cells in vitro. These studies revealed that C. albicans invasion of brain endothelial cells is mediated by the fungal invasins, Als3 and Ssa1. Als3 binds to the gp96 heat shock protein, which is expressed on the surface of brain endothelial cells, but not human umbilical vein endothelial cells, whereas Ssa1 binds to a brain endothelial cell receptor other than gp96. The vps51Δ/Δ mutant has increased surface expression of Als3, which is a major cause of the increased capacity of this mutant to both invade brain endothelial cells in vitro and traffic to the brain in mice. Therefore, during disseminated disease, C. albicans traffics to and infects the brain by binding to gp96, a unique receptor that is expressed specifically on the surface of brain endothelial cells.
Published in the journal: . PLoS Pathog 7(10): e32767. doi:10.1371/journal.ppat.1002305
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002305Summary
During hematogenously disseminated disease, Candida albicans infects most organs, including the brain. We discovered that a C. albicans vps51Δ/Δ mutant had significantly increased tropism for the brain in the mouse model of disseminated disease. To investigate the mechanisms of this enhanced trafficking to the brain, we studied the interactions of wild-type C. albicans and the vps51Δ/Δ mutant with brain microvascular endothelial cells in vitro. These studies revealed that C. albicans invasion of brain endothelial cells is mediated by the fungal invasins, Als3 and Ssa1. Als3 binds to the gp96 heat shock protein, which is expressed on the surface of brain endothelial cells, but not human umbilical vein endothelial cells, whereas Ssa1 binds to a brain endothelial cell receptor other than gp96. The vps51Δ/Δ mutant has increased surface expression of Als3, which is a major cause of the increased capacity of this mutant to both invade brain endothelial cells in vitro and traffic to the brain in mice. Therefore, during disseminated disease, C. albicans traffics to and infects the brain by binding to gp96, a unique receptor that is expressed specifically on the surface of brain endothelial cells.
Introduction
Hematogenously disseminated candidiasis is a serious disease that remains associated with approximately 35% mortality, even with currently available treatment, and Candida albicans is the infecting organism in approximately 50% of patients [1], [2]. During this disease, C. albicans is carried by the bloodstream to virtually all organs of the body, including the brain. Although candidal infection of the brain may not be clinically evident in adults with disseminated candidiasis, it is frequently found at autopsy in patients who die of this disease [3]. Even more importantly, candidal brain infection, especially meningitis, is a significant problem in premature infants who have risk factors for disseminated candidiasis, even in the absence of detectable candidemia [4], [5].
To invade the brain parenchyma, blood-borne C. albicans cells must adhere to and traverse the endothelial cell lining of the blood vessels within the central nervous system. Brain endothelial cells are significantly different from those lining systemic blood vessels. For example, they have tight junctions that are not present in the endothelial cells in other vascular beds. Forming the blood brain barrier, brain endothelial cells restrict the diffusion of large or hydrophilic molecules into the central nervous system, while allowing the diffusion of small hydrophobic molecules [6]. More importantly, some microbial pathogens, such as Neisseria meningitidis, Streptococcus pneumonia, Escherichia coli K1, and Cryptococcus neoformans have an enhanced capacity to adhere to and invade human brain microvascular endothelial cells (HBMECs), which enables them to preferentially infect the central nervous system via the hematogenous route [7]–[11]. Thus, these pathogens can exploit the unique characteristics of HBMECs to specifically infect the brain.
Studies using human umbilical vein endothelial cells (HUVECs) as representative systemic endothelial cells have demonstrated that C. albicans adheres to, invades, and damages these cells in vitro [12], [13]. One mechanism by which C. albicans invades these cells is by stimulating its own endocytosis, which is induced when the C. albicans invasins, Als3 and Ssa1, bind to receptors such as N-cadherin and HER2 on the endothelial cell surface [14]–[17]. C. albicans yeast and hyphae can also invade HBMECs by inducing their own endocytosis [18], [19]. However, the mechanism by which this pathogen invades these endothelial cells and infects the brain is poorly understood.
Recently we discovered that C. albicans VPS51 is up-regulated by contact with HUVECs in vitro, and that a vps51/vps51 insertion mutant is defective in damaging these endothelial cells [20]. In Saccharomyces cerevisiae, Vps51 is known to bind to the Vps52/53/54 complex and is required for the retrograde transport of proteins from endosomes to the late Golgi [21], [22]. Although the function of Vps51 in C. albicans has not been studied in detail, the vps51/vps51 insertion mutant has a fragmented vacuole, similar to the corresponding S. cerevisiae mutant [20]–[22]. Thus, Vps51 likely plays a role in protein trafficking in C. albicans.
In the current study, we investigated how deletion of VPS51 affects the virulence of C. albicans during hematogenous infection. We found that the vps51Δ/Δ null mutant exhibits a preferential tropism for the brain. This tropism is mediated in part by the enhanced exposure of Als3 on the surface of the vps51Δ/Δ mutant, which binds to gp96 on the surface of HBMECs and mediates invasion of these endothelial cells. We further discovered that gp96 functions as a receptor for wild-type C. albicans on HBMECs, but not HUVECs, indicating that this organism invades the central nervous system by binding to a receptor that is expressed specifically on HBMECs.
Results
Deletion of VPS51 causes reduced mortality and decreased kidney and liver fungal burden during hematogenously disseminated candidiasis
To investigate the role of Vps51 in the virulence of C. albicans, we inoculated mice via the lateral tail vein with a wild-type strain, a vps51Δ/Δ mutant, and a vps51Δ/Δ+pVPS51 complemented strain and then monitored their survival over time. We found that all mice infected with the vps51Δ/Δ mutant survived for the entire 21-day observation period, whereas all mice infected with the wild-type strain died within 7 days after inoculation (Figure 1A). Complementing the vps51Δ/Δ mutant with an intact copy of VPS51 restored its virulence to wild-type levels, thus confirming that Vps51 is required for the maximal virulence of C. albicans. The greatly reduced virulence of the vps51Δ/Δ mutant was further verified by infecting mice with a 6-fold higher inoculum. As expected, mice infected with the wild-type strain at this higher inoculum died rapidly, with a median survival of only 3 days (Figure 1B). However, all mice infected with the vps51Δ/Δ mutant still survived. Therefore, Vps51 is necessary for the full virulence of C. albicans.
Fig. 1. A vps51Δ/Δ mutant has attenuated overall virulence, but increased brain tropism during hematogenously disseminated candidiasis. 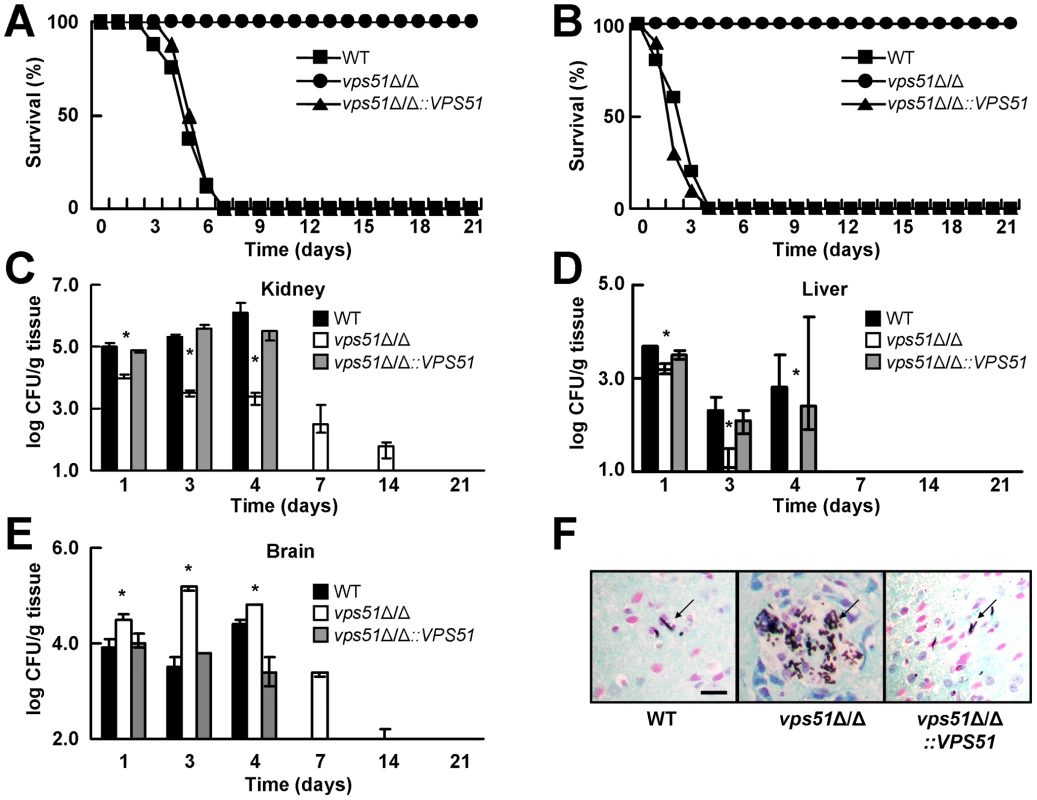
(A and B) Survival of mice after intravenous inoculation with 5×105 (A) or 3×106 (B) yeast phase cells of the indicated strains of C. albicans (n = 10 mice per strain). (C – E) Fungal burden of the kidneys (C), liver (D), and brain (E) of mice at the indicated times after inoculation with 5×105 cells of the various strains. Results from days 1, 4, 7, 14, and 21 are median ± interquartile range of a single experiment with 7 mice per strain at each time point. Data from day 3 are the combined results from two experiments, each with 6–7 mice per strain. Only mice infected with the vps51Δ/Δ mutant were analyzed at the 7, 14, and 21 day time points because all of the mice infected with the other C. albicans strains had died. At these later time points, the absence of a bar indicates that the fungal burden was below the limits of detection. *p<0.01 compared to the wild-type (WT) and vps51Δ/Δ+pVPS51 complemented strains by the Wilcoxon Rank Sum Test. (F). Histopathology of the hypothalamus of mice after 3 days of infection with the indicated strains. Sections were stained with Gomori methenamine silver. Scale bar 10 µm. Arrows indicate the organisms. The mouse model of hematogenous disseminated candidiasis mimics many aspects of this disease in humans, particularly the formation of microabscesses in most organs [23], [24]. We therefore investigated the effects of deleting VPS51 on organ fungal burden. During the first 4 days of infection, the kidneys and livers of mice infected with the vps51Δ/Δ mutant contained significantly fewer organisms than those of mice infected with either the wild-type or vps51Δ/Δ+pVPS51 complemented strain (Figure 1C and D). Furthermore, the kidney fungal burden of mice infected with the vps51Δ/Δ mutant progressively declined after the first day of infection. In contrast, the kidney fungal burden of mice infected with the wild-type and vps51Δ/Δ+pVPS51 complemented strains progressively increased for the first 4 days post-infection, after which these mice began to die. These results further demonstrate that the overall virulence of the vps51Δ/Δ mutant is decreased.
Absence of Vps51 or Vps53 results in increased brain fungal burden
A surprising result was that during the first 4 days of infection, the brain fungal burden of mice infected with the vps51Δ/Δ mutant was significantly greater than that of mice infected with either the wild-type or vps51Δ/Δ+pVPS51 complemented strain (Figure 1E). Indeed, after 3 days of infection, the brains of mice infected with the vps51Δ/Δ mutant contained a median of 50-fold more organisms than those of mice infected with the wild-type strain. Despite having a high brain fungal burden, the mice infected with the vps51Δ/Δ mutant did not appear to be sick and had no obvious signs of neurological disease. Moreover, beginning on the fourth day of infection, these mice progressively cleared the organisms from their central nervous system. These results suggest that while the overall virulence of the vps51Δ/Δ mutant is decreased, it has a distinct tropism for the brain.
We verified these quantitative culture results by performing histopathologic analysis of the brains of the infected mice. Foci containing multiple organisms were visible in the brains of mice infected with the vps51Δ/Δ mutant, especially in the hippocampus (Figure 1F). In sharp contrast, only rare organisms were visible in the brains of the mice infected with either the wild-type or vps51Δ/Δ+pVPS51 complemented strains, and these organisms were typically either solitary or in pairs.
To determine if another member of the Vps51/52/53/54 complex is required for maximal virulence and enhanced brain tropism, we constructed and analyzed a vps53Δ/Δ mutant. This strain also caused no mortality in mice following tail vein inoculation. (Figure 2A) Furthermore, it accumulated at significantly higher levels in the brain than did the wild-type and vps53Δ/Δ+pVPS53 complemented strains (Figure 2B). Collectively, these results demonstrate that the Vps51/52/53/54 complex plays a key role in virulence, and that C. albicans strains that lack components of this complex preferentially infect the brain.
Fig. 2. Deletion of VPS53 causes reduced mortality and increased brain fungal burden during hematogenously disseminated candidiasis. 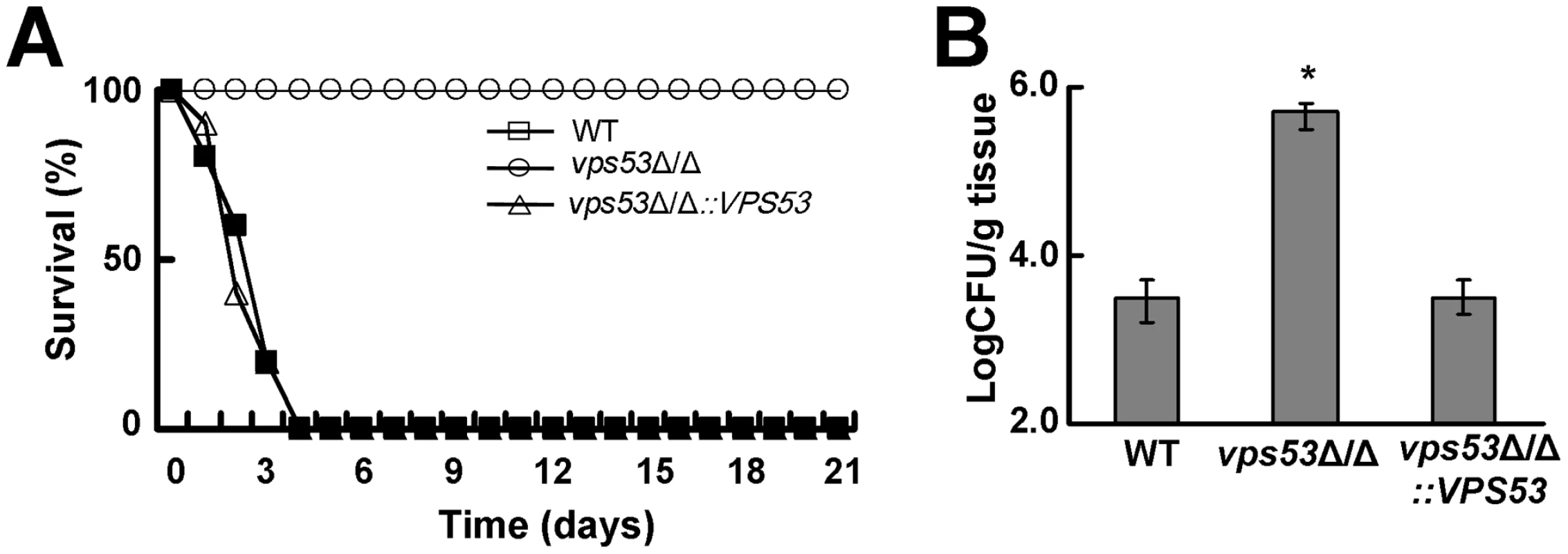
(A) Survival of mice after intravenous inoculation of with 3×106 yeast phase cells of the indicated strains. 10 mice were infected with each strain. (B) Increased brain fungal burden of mice 3 days after intravenous inoculation of 5×105 cells per strain. Results are the median ± interquartile ranges of 7 mice per strain. *p<0.01 compared to the wild-type and vps53Δ/Δ+pVPS53 complemented strains. The vps51Δ/Δ mutant has an increased capacity to adhere to and invade HBMECs
To cross the endothelial cell lining of the vasculature, C. albicans must first adhere to these endothelial cells and then invade through them. We hypothesized that the vps51Δ/Δ mutant had increased capacity to infect the brain because it preferentially adhered to and invaded the unique endothelial cells that line the blood vessels of central nervous system. To test this hypothesis, we compared the interactions of this mutant with HUVECs and HBMECs in vitro. We found that the adherence of the vps51Δ/Δ mutant to HUVECs was increased by only 22% compared to the wild-type strain (Figure 3A). However, the adherence of this mutant to HBMECs was increased by 95% (Figure 3B). There was an even greater difference in the capacity the vps51Δ/Δ mutant to induce its own endocytosis by HUVECs compared to HBMECs. The endocytosis of the vps51Δ/Δ mutant by HUVECs was 58% lower than that of the wild-type strain (Figure 3C). In contrast, the endocytosis of this mutant by HBMECs was 39% higher than the wild-type strain (Figure 3D). Complementing the vps51Δ/Δ mutant with an intact copy of VPS51 restored its interactions with both types of endothelial cells to wild-type levels. The increased capacity of the vps51Δ/Δ mutant to adhere to and invade HBMECs compared to HUVECs provides a likely explanation for the enhanced tropism of this mutant for the brain.
Fig. 3. The vps51Δ/Δ mutant interacts differently with human umbilical vein endothelial cells (HUVECs) versus human brain microvascular endothelial cells (HBMECs). 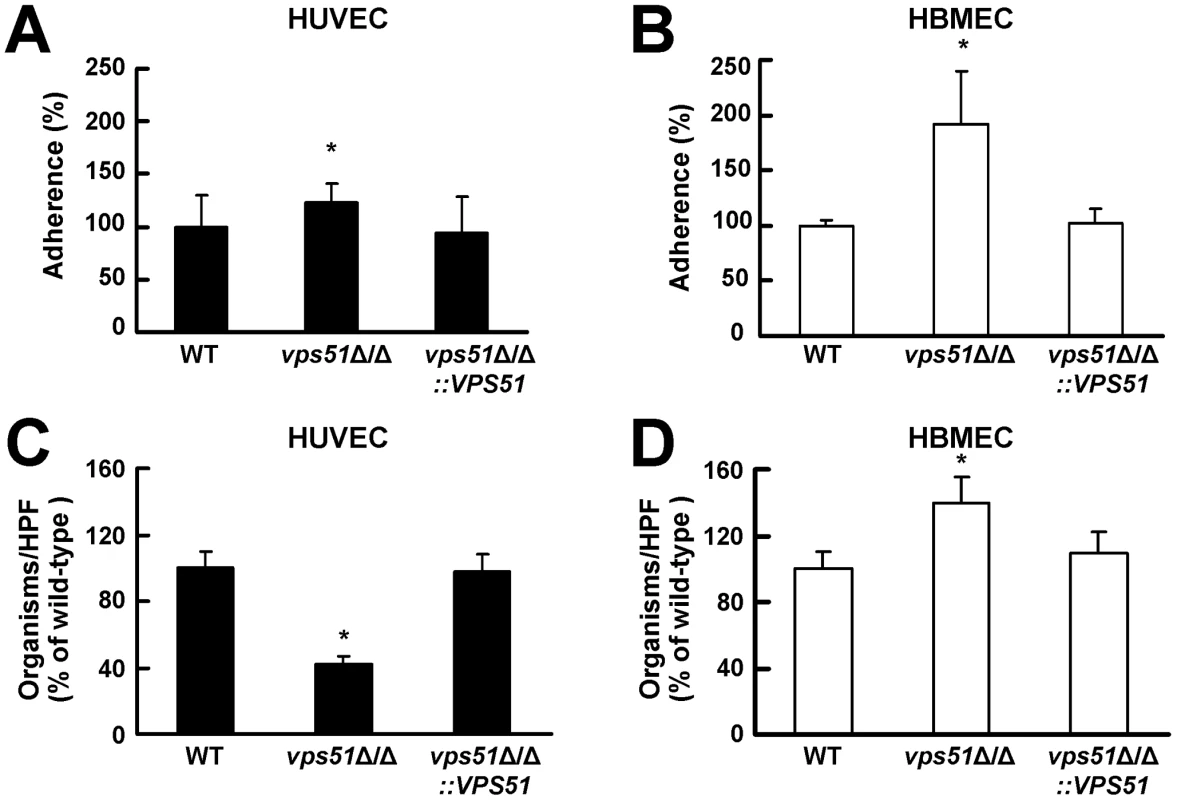
(A and B) Adherence of germ tubes of the indicated C. albicans strains to HUVECs (A) and HBMECs (B). (C and D) Endocytosis of hyphae of the indicated strains by HUVECs (C) and HBMECs (D). The results are expressed as a percentage of the wild-type strain and are the mean ± SD of 3 experiments, each performed in triplicate. The mean adherence of the wild-type strain to HUVECs and HBMECs was 47% and 28%, respectively. The mean number of wild-type cells endocytosed by HUVECs and HBMECs was 109 and 35 organisms per 10 high-powered fields (HPF), respectively. *p<0.01 compared to both the wild-type strain and the vps51Δ/Δ+pVPS51 complemented strain by Analysis of Variance. Gp96 mediates C. albicans invasion of HBMECs
Next, we sought to identify the HBMEC receptor for both wild-type C. albicans and the vps51Δ/Δ mutant. HBMECs are known to express high amounts of the heat shock protein, gp96 on their cell surface, whereas HUVECs do not [9]. Furthermore, gp96 functions as an HBMEC-specific receptor for E. coli K1 strains that cause neonatal meningitis [9]. We used multiple complementary approaches to evaluate whether gp96 expression is required for C. albicans to invade HBMECs. First, we tested the capacity of an anti-gp96 antibody to inhibit HBMEC endocytosis of C. albicans. This antibody reduced the endocytosis of wild-type C. albicans by 24% and the vps51Δ/Δ mutant by 48% (Figure 4A). Second, we determined the effects of siRNA-mediated knockdown of gp96 on endocytosis. HBMECs transfected with gp96 siRNA endocytosed 52% fewer wild-type C. albicans cells and 82% fewer vps51Δ/Δ cells than did HBMECs transfected with control siRNA (Figure 4B). Importantly, the effect of gp96 knockdown on endocytosis was specific for HBMECs because knockdown of gp96 in HUVECs had no effect on their capacity to endocytose C. albicans (Figure 4C). Also, knockdown of gp96 did not inhibit HBMECs endocytosis of transferrin (Figure 4D), demonstrating that reducing gp96 protein levels did not cause a global decrease in receptor-mediated endocytosis. Collectively, these results indicate that gp96 is required for maximal HBMEC endocytosis of both wild-type C. albicans and the vps51Δ/Δ mutant.
Fig. 4. C. albicans endocytosis by HBMECs is mediated by gp96. 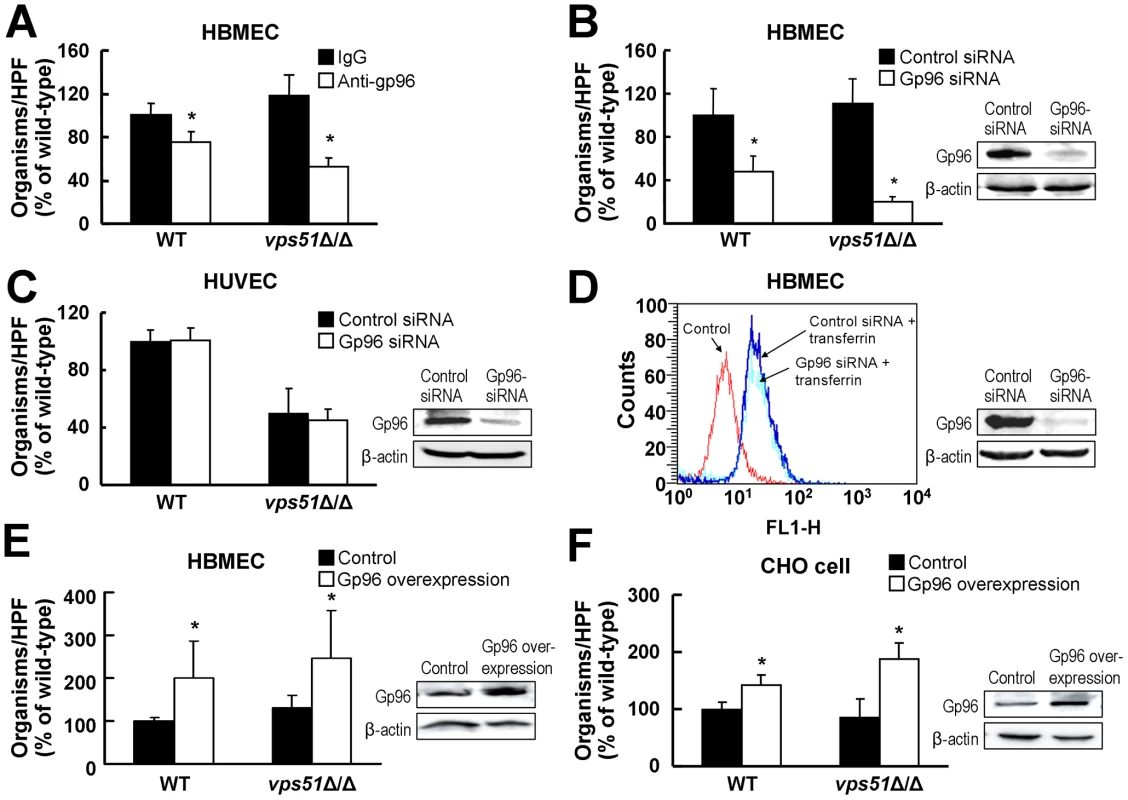
(A) Reduced HBMEC endocytosis of wild-type and vps51Δ/Δ mutant strains of C. albicans by an anti-gp96 antibody. (B and C) Knockdown of gp96 by siRNA inhibits the endocytosis of C. albicans by HBMECs (B), but not by HUVECs (C). (D) Gp96 knockdown has no effect on transferrin endocytosis by HBMECs. (E and F) Overexpression of human gp96 in HBMECs (E) and Chinese Hamster Ovary (CHO) cells (F) results in increased endocytosis of C. albicans. The results are expressed as a percentage of the wild-type strain and are the mean ± SD of 3 experiments, each performed in triplicate. The mean number of wild-type cells endocytosed by control HBMECs, HUVECs, and CHO cells was 41, 49, and 48 organisms per 10 HPF, respectively. *p<0.01 compared to control strains. Images to the right of the graphs are of representative immunoblots showing the effects of the interventions on total gp96 and β-actin protein levels in the cells. To further explore these findings, we investigated the effects of overexpressing gp96 on the endocytosis of C. albicans. We found that overexpression of gp96 in HBMECs enhanced the endocytosis of the wild-type strain and the vps51Δ/Δ mutant by 100% and 115%, respectively (Figure 4E). Similarly, heterologous expression of human gp96 in Chinese hamster ovary (CHO) cells resulted in a 42% increase in the endocytosis of wild-type C. albicans and a 102% increase in the endocytosis of the vps51Δ/Δ mutant compared to control CHO cells transfected with the empty vector (Figure 4F). Therefore, these combined results demonstrate that gp96 functions as an HBMEC receptor that mediates the endocytosis of both wild-type C. albicans and the vps51Δ/Δ mutant.
C. albicans Als3 and Ssa1 mediate HBMEC endocytosis in vitro
Our previous studies revealed that the C. albicans proteins Ssa1 and Als3 function as invasins that induce the endocytosis of this organism by HUVECs [14], [15]. To investigate the roles of these fungal proteins in HBMEC invasion, we analyzed ssa1Δ/Δ and als3Δ/Δ single mutants, as well as vps51Δ/Δ ssa1Δ/Δ and vps51Δ/Δ als3Δ/Δ double mutants. Approximately 30% fewer hyphae of the ssa1Δ/Δ single mutant were endocytosed by HBMECs as compared to wild-type parent strain and the ssa1Δ/Δ+pSSA1 complemented strain (Figure 5A). Similarly, the endocytosis of the vps51Δ/Δ ssa1Δ/Δ double mutant was significantly lower than the vps51Δ/Δ single mutant. Thus, Ssa1 is required for the maximal endocytosis of both wild-type and vps51Δ/Δ mutant strains of C. albicans by HBMECs in vitro.
Fig. 5. Deletion of SSA1 or ALS3 reduces HBMEC endocytosis of C. albicans. 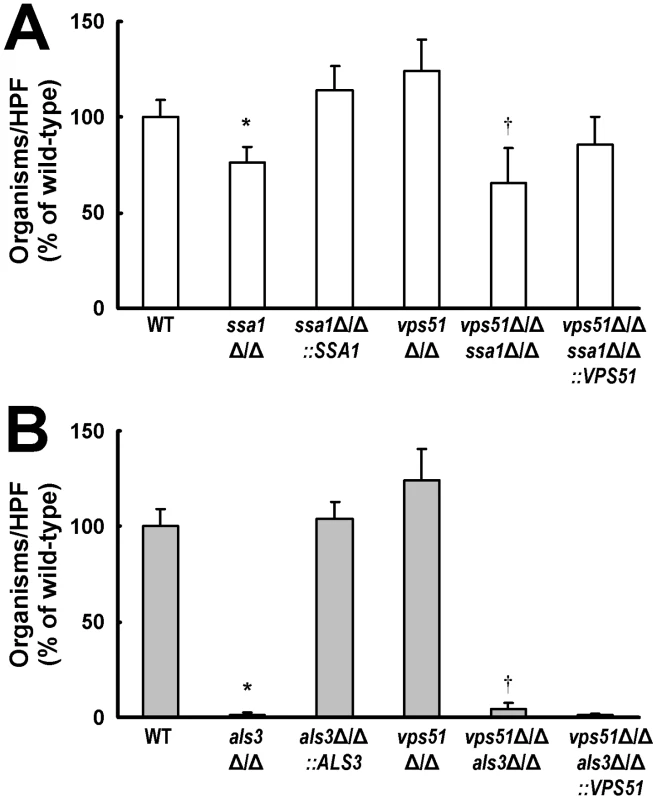
(A and B) Endocytosis of the indicated strains of C. albicans by HBMECs. The results are expressed as a percentage of the wild-type strain and are the mean ± SD of 3 experiments, each performed in triplicate. A mean 65 of wild-type cells per HPF was endocytosed by the HBMECs. *p<0.01 compared to the wild-type strain; †p<0.01 compared to the vps51Δ/Δ mutant. Als3 played a greater role than Ssa1 in stimulating the endocytosis of C. albicans by HBMECs in vitro. Both the als3Δ/Δ single mutant and the vps51Δ/Δ als3Δ/Δ double mutant were endocytosed extremely poorly by these endothelial cells (Figure 5B), indicating that Als3 is essential for the endocytosis of C. albicans by HBMECs in vitro.
To determine whether Ssa1 and Als3 mediate the endocytosis of C. albicans by directly interacting with endothelial cells, we used a heterologous expression strategy in which we expressed C. albicans SSA1 or ALS3 in the normally non-invasive yeast, Saccharomyces cerevisiae [25]. Expression of C. albicans SSA1 in S. cerevisiae resulted in a 300% increase in the endocytosis of this organism by HUVECs and a 43% increase in its endocytosis by HBMECs, as compared to the control strain of S. cerevisiae (Figure 6A and B). Moreover, expression of C. albicans ALS3 in S. cerevisiae resulted in a 2050% and 1880% increase in endocytosis by HUVECs and HBMECs, respectively (Figures 6C and D). Collectively, these data demonstrate that Ssa1 is a more potent inducer of fungal endocytosis by HUVECs than by HBMECs, whereas Als3 can induce endocytosis by HUVECs and HBMECs with similar efficacy. As HUVECs do not express gp96 on their surface [9], HUVEC endocytosis of S. cerevisiae expressing C. albicans SSA1 or ALS3 is mediated by receptors other than gp96, such as N-cadherin and HER2 [14]–[17].
Fig. 6. Both Als3 and Ssa1 induce HBMEC endocytosis, but Als3 has a greater effect than Ssa1. 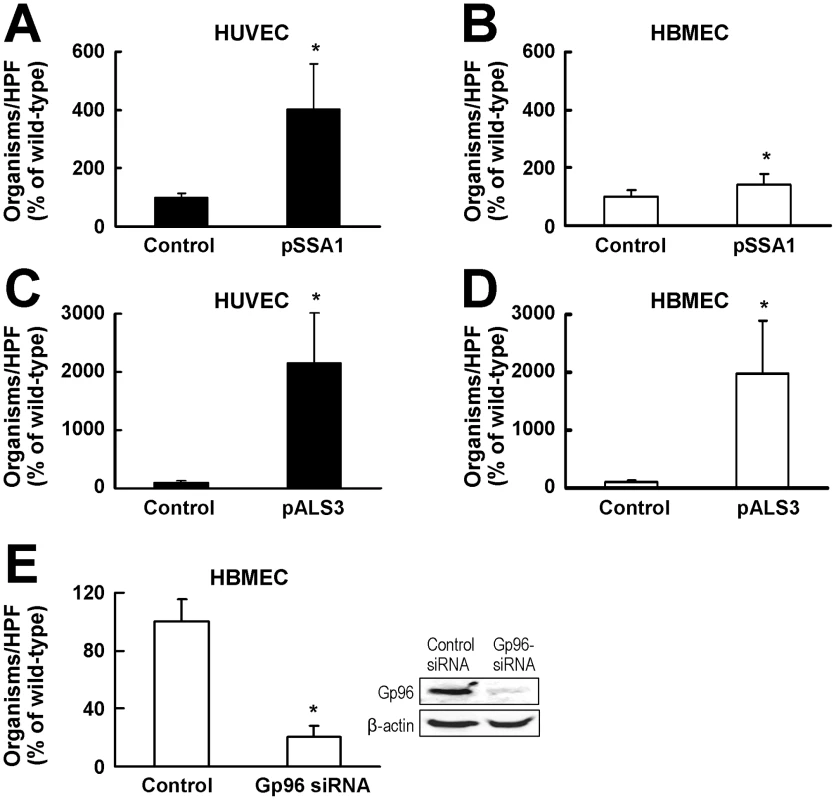
(A and B) Endocytosis by HUVECs (A) and HBMECs (B) of S. cerevisiae expressing C. albicans SSA1, or the backbone vector (control). (C and D) Endocytosis by HUVECs (C) and HBMECs (D) of S. cerevisiae expressing C. albicans Als3 or the backbone vector (control). (E) Effect of siRNA knockdown of gp96 on HBMEC endocytosis of S. cerevisiae expressing C. albicans ALS3. Image on the right is of a representative immunoblot of total HBMEC lysates probed for gp96 and β-actin. The endocytosis data are the mean ± SD of 3 experiments, each performed in triplicate. The results in (A–D) are expressed as a percentage of the control strain containing the backbone vector and the results in (E) are expressed as a percentage of the endocytosis of the ALS3 expressing strain by HBMEC transfected with control siRNA. The mean number of control cells endocytosed by the HUVECs and HBMECs in (A–D) was 3.2 and 1.2 organisms per 10 HPF, respectively. A mean of 49 cells of the Als3 expressing strain per HPF was endocytosed by HBMEC transfected with the control siRNA in (E). *p<0.01 compared to control strains. Als3 interacts with gp96 to induce HBMEC endocytosis
The above results suggested a model in which Als3 on the surface of C. albicans hyphae binds to gp96 on the surface of HBMECs and induces endocytosis. To test this model, we analyzed the effects of siRNA knockdown on the endocytosis of the S. cerevisiae strain that expressed C. albicans Als3. As predicted, knockdown of gp96 in HBMECs reduced the endocytosis of the Als3 expressing strain of S. cerevisiae by 79% compared to control HBMECs (Figure 6E).
We also tested the capacity of different C. albicans mutants and strains of S. cerevisiae to bind gp96 in HBMEC membrane protein extracts. As predicted by our endocytosis results, the vps51Δ/Δ mutant bound more gp96 than did the wild-type strain (Figure 7A). Also, the ssa1ΔΔ mutant bound slightly less gp96 than did the wild-type strain, and the als3Δ/Δ mutant bound very poorly to this protein. Finally, the strain of S. cerevisiae that expressed C. albicans Als3 bound to gp96, whereas the control strain of S. cerevisiae did not (Figure 7B), thus indicating that Als3 directly interacts with gp96.
Fig. 7. Effects of deletion of VPS51, SSA1, and ALS3 on binding to gp96 and fungal surface expression of Als3. 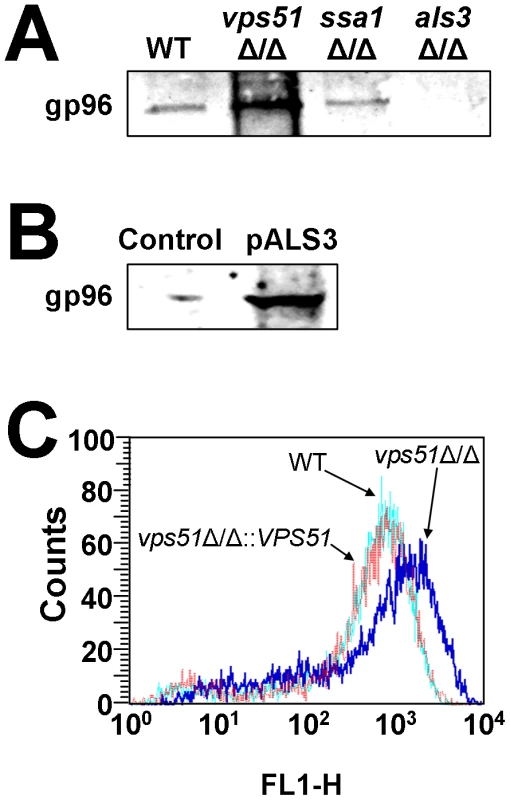
(A and B) Immunoblots of HBMEC membrane proteins that were bound by the indicated strains of C. albicans (A) or S. cerevisiae (B). Both blots were probed with an anti-gp96 antibody. (C) Flow cytometric analysis of Als3 exposure on the surface of hyphae of the wild-type, vps51Δ/Δ mutant, and vps51Δ/Δ+pVPS51 complemented strains. Next, we used flow cytometric analysis of C. albicans hyphae stained with either anti-HSP70 or anti-Als3 antibodies to quantify the levels of Ssa1 and Als3 that were exposed on the surface of the various strains. Although the vps51Δ/Δ mutant had normal Ssa1 surface exposure (data not shown), it had greater surface exposure of Als3 than did the wild-type and vps51Δ/Δ+pVPS51 complemented strains (Figure 7C). The greater surface exposure of Als3 by the vps51Δ/Δ mutant likely contributes its enhanced capacity to induced HBMEC endocytosis.
Ssa1 is important for brain invasion by wild-type C. albicans whereas Als3 is necessary for brain invasion by the vps51Δ/Δ mutant
Lastly, we investigated the roles of Ssa1 and Als3 in mediating brain invasion in vivo by both wild-type and vps51Δ/Δ mutant strains of C. albicans. Mice were inoculated with the various C. albicans strains via the tail vein and their brain fungal burden was determined 3 days later. Similar to our previous results [14], the brain fungal burden of mice infected with the ssa1Δ/Δ single mutant was significantly less than that of mice infected with either the wild-type strain or the ssa1Δ/Δ+pSSA1 complemented strain (Figure 8A). However, the brain fungal burden of mice infected with the vps51Δ/Δ ssa1Δ/Δ double mutant was only 1.7-fold lower than that of mice infected with the vps51Δ/Δ single mutant, a difference that did not achieve statistical significance (p = 0.053). Taken together, these results indicate that Ssa1 is necessary for wild-type C. albicans to cause maximal brain infection, but that it plays a relatively minor role in enhanced brain tropism of the vps51Δ/Δ mutant.
Fig. 8. Deletion of SSA1 and ALS3 have different effects on brain fungal burden. 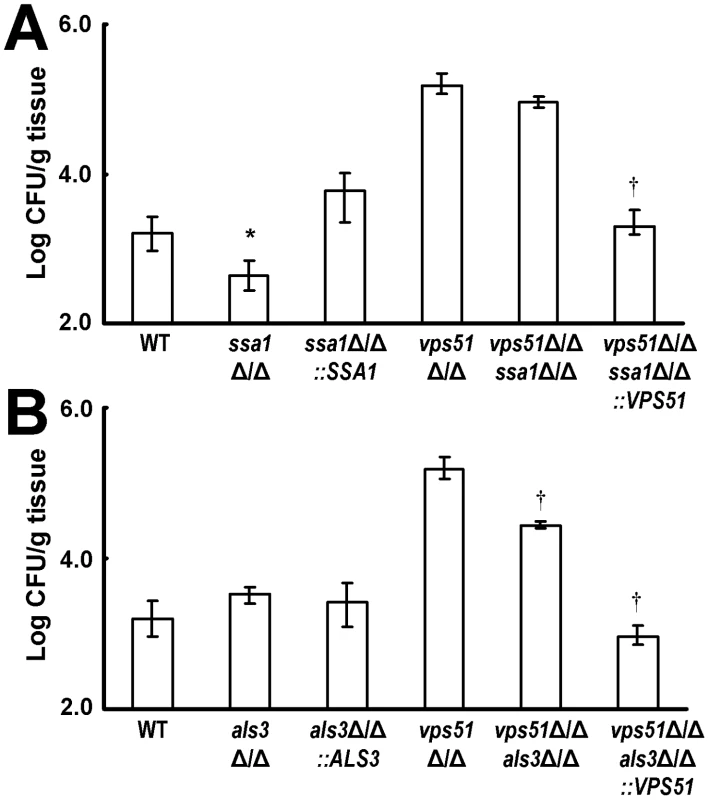
(A and B) Brain fungal burden of mice after 3 days of infection with the indicated strains of C. albicans. Results are median ± interquartile ranges of 7 mice per strain. *p<0.02 compared to mice infected with the wild-type strain; †p<0.001 compared to mice infected with the vps51Δ/Δ mutant. Different results were obtained with strains that lacked Als3. The brain fungal burden of mice infected with the als3Δ/Δ single mutant was similar to that of mice infected with the wild-type strain (Figure 8B). In contrast, mice infected with the vps51Δ/Δ als3Δ/Δ double mutant had 5.5-fold fewer organisms in their brain compared to mice infected with the vps51Δ/Δ single mutant. Therefore, although Als3 is dispensable for wild-type C. albicans to infect the brain, it is important for the vps51Δ/Δ mutant to achieve maximal brain fungal burden.
Discussion
In the mouse model of disseminated candidiasis, kidney fungal burden is directly correlated with mortality [23], [26]. Thus, many studies of this disease have used kidney fungal burden as the primary endpoint when analyzing either the virulence of mutant strains of C. albicans in mice or the susceptibility of mutant strains of mice to disseminated candidiasis [27]–[31]. However, during disseminated candidiasis in both mice and humans, C. albicans infects virtually all organs in the body. To do so, the blood-borne organisms must adhere to and invade the vascular beds of these organs. Importantly, there are significant differences among the endothelial cells that line the vasculature of the different organs, as well as the immunologic milieu of these organs [32], [33]. These differences provide a compelling rationale to investigate the capacity of C. albicans to traffic to and persist in organs other than the kidney. The brain is a particularly important target organ in neonates with hematogenously disseminated candidiasis [4], [5], and its blood vessels are lined with the unique endothelial cells that form the blood-brain barrier. Our studies with a vps51Δ/Δ mutant strain of C. albicans led us to discover that C. albicans traffics to the brain and invades cerebral blood vessels in part by binding to gp96 that is expressed on the surface of brain endothelial cells.
We had previously identified C. albicans VPS51 through a microarray study that was designed to discover genes that were up-regulated when the organism adhered to HUVECs [20]. In that study, we determined that a vps51/vps51 insertion mutant had reduced capacity to damage HUVECs and increased susceptibility to antimicrobial peptides [20]. These in vitro findings led us to predict that VPS51 would be required for the maximal virulence of C. albicans during disseminated disease. In the current study, we verified this prediction by determining that mice infected with a vps51Δ/Δ deletion mutant had no mortality and progressively cleared this strain from their kidneys and liver.
A unique and unexpected phenotype of the vps51Δ/Δ mutant was its marked propensity to infect the brain. In the few previous studies in which the brain fungal burden of mice infected with mutant strains of C. albicans was determined, the fungal burden in the brain generally paralleled the fungal burden in the kidney. For example, mice infected with ecm33Δ/Δ and hog1Δ/Δ mutants had improved survival and reduced fungal burden in both the kidney and the brain, as compared to mice infected with the wild-type strain [34], [35]. Thus, it was unusual to find that mice infected with the vps51Δ/Δ mutant had reduced kidney fungal burden, yet significantly increased brain fungal burden.
Our finding that the enhanced capacity of the vps51Δ/Δ mutant to adhere to and invade HBMECs, as compared to HUVECs, provides a likely explanation for its brain tropism. One difference between HBMECs and HUVECs is that the former cells express gp96 on their surface, whereas the latter cells do not [9]. Multiple lines of evidence indicate that gp96 functions as an HBMEC receptor for both wild-type C. albicans and the vps51Δ/Δ mutant. For example, an anti-gp96 antibody and siRNA knockdown of gp96 inhibited HBMEC endocytosis of C. albicans. Furthermore, overexpression of gp96 in HBMEC and the heterologous expression of human gp96 in CHO cells increased the endocytosis of C. albicans. Finally, wild-type C. albicans cells bound to gp96 in extracts of HBMEC membrane proteins, and the highly endocytosed vps51Δ/Δ mutant bound even more of this protein. Collectively, these data indicate that gp96 is an HBMEC receptor for C. albicans.
It was notable that in both the anti-gp96 antibody studies and the gp96 siRNA experiments, inhibition of gp96 function or expression had greater effect on the endocytosis of the vps51Δ/Δ mutant than the wild-type strain (78% reduction for the vps51Δ/Δ mutant vs. 38% reduction for the wild-type strain; p<0.0001). These results indicate that the vps51Δ/Δ mutant preferentially utilizes gp96 as a receptor to invade HBMECs. They further suggest that the enhanced brain tropism of the vps51Δ/Δ mutant is likely due to its increased binding to gp96 on the surface of brain endothelial cells.
Although these results strongly indicate that gp96 is important for HBMEC endocytosis of C. albicans, the findings that neither the anti-gp96 antibody nor siRNA knockdown of gp96 completely blocked the endocytosis of this organism suggest that it can invade HBMECs by additional mechanisms. Such mechanisms include the induction of endocytosis by binding to one or more receptors, such as N-cadherin that are independent of gp96 and active penetration, in which hyphae physically push their way into host cells by progressively elongating [16], [36].
Because gp96 also functions as a molecular chaperone [37], it is possible that it could be involved in the endocytosis of C. albicans by altering the expression or function of other proteins on the surface of HBMECs. Our data indicate that this possibility is remote because HBMEC endocytosis of C. albicans was inhibited by the anti-gp96 antibody, which is unlikely to affect the chaperone function of gp96. In addition, siRNA knockdown of gp96 inhibited the endocytosis of C. albicans by HBMECs, but not HUVECs, in which gp96 is located intracellularly. Moreover, gp96 knockdown did not affect transferrin uptake in HBMECs, a process that is mediated by the transferrin receptor. Thus, the role of gp96 in inducing the endocytosis of C. albicans is likely due to its function as a cell surface receptor rather than a chaperone.
Gp96 has been reported to be expressed on the surface of some epithelial cells where it functions as a receptor for Listeria monocytogenes, Neisseria gonorrhoeae and bovine adeno-associated virus [38]–[40]. In addition, gp96 on the surface of HBMEC is known to be bound by E. coli K1 OmpA [9]. This binding induces the endocytosis of E. coli by activating signal transducer and activator of transcription 3 (STAT3), which functions upstream of phosphatidylinositol-3 kinase and protein kinase C-α [41]–[43]. Whether the binding of C. albicans to gp96 activates a similar signaling pathway remains to be determined.
C. albicans possesses at least two invasin-like proteins, Ssa1 and Als3. Both of these proteins induce the endocytosis of C. albicans by HUVECs by binding to N-cadherin and other endothelial cell receptors [14], [15]. These two invasins may function cooperatively because the endocytosis defect of an ssa1Δ/Δ als3Δ/Δ double mutant is not greater than that of an als3Δ/Δ single mutant [14]. Our current studies with the C. albicans ssa1Δ/Δ and als3Δ/Δ mutants and strains of S. cerevisiae that overexpress C. albicans Ssa1 and Als3 demonstrate that both of these proteins can induce HBMEC endocytosis. The results of these in vitro experiments also indicate that Als3 is more important than Ssa1 in inducing HBMEC endocytosis, probably because it plays a greater role in binding to gp96.
Our mouse studies suggest that Ssa1 is required for the maximal trafficking of wild-type C. albicans to the brain because the brain fungal burden of mice infected with the ssa1Δ/Δ mutant was significantly less than that of mice infected with the wild-type strain. These results are similar to our previous data [14]. However, deletion of SSA1 in the vps51 mutant had only a minor effect on brain trafficking. It is probable that in the vps51Δ/Δ mutant, the effects of deleting SSA1 were masked by the increased surface expression of Als3.
A paradoxical finding was that although the endocytosis of the als3Δ/Δ mutant by HBMECs was severely impaired in vitro, this mutant had normal trafficking to the brain in mice. The normal virulence of an als3Δ/Δ mutant in the mouse model of disseminated candidiasis has recently been reported by others [44]. It is unclear why there is such a large discrepancy between the host cell interactions of the als3Δ/Δ mutant in vitro and its virulence in mice, especially because ALS3 is highly expressed in vivo [45], [46]. The most probable explanation for these paradoxical results is that other invasins, such as Ssa1 and perhaps other proteins, compensate for the absence of Als3. Because the in vitro experiments were performed using human endothelial cells and the virulence experiments were performed in mice, it is theoretically possible that differences between human and mouse gp96 may account for the differences between the in vitro and in vivo results. However, human and mouse gp96 are 97.5% identical at the amino acid level, making this possibility unlikely.
Importantly, our results indicate that Als3 does play a role in the enhanced brain tropism of the vps51Δ/Δ mutant because the brain fungal burden of mice infected with vps51Δ/Δ als3Δ/Δ double mutant was significantly lower than that of mice infected with the vps51Δ/Δ single mutant. Because protein trafficking is likely abnormal in the vps51Δ/Δ mutant, we speculate that this strain has reduced expression of compensatory proteins in response to deletion of ALS3. On the other hand, the vps51Δ/Δ als3Δ/Δ double mutant still had greater tropism for the brain compared to the wild-type strain. This result suggests that the overexpression of additional proteins, other than Als3, contributes to the brain tropism of the vps51Δ/Δ single mutant.
The combined results of these experiments support a model in which C. albicans invades the brain during hematogenously disseminated infection by binding to proteins that are specifically expressed on the surface of brain endothelial cells. One of these proteins is gp96, which is bound predominantly by C. albicans Als3 (Figure 9). At least one other brain endothelial cell protein functions as receptors for C. albicans Ssa1. As the endothelial cells of other vascular beds also express unique surface proteins, it is highly probable that blood-borne C. albicans utilizes different endothelial cell surface proteins to infect different organs. Identification of these organ-specific receptors for C. albicans may lead to novel approaches to block these receptors and thereby prevent hematogenous dissemination.
Fig. 9. Model of the receptor-ligand interactions that mediate the endocytosis of C. albicans by HBMECs. 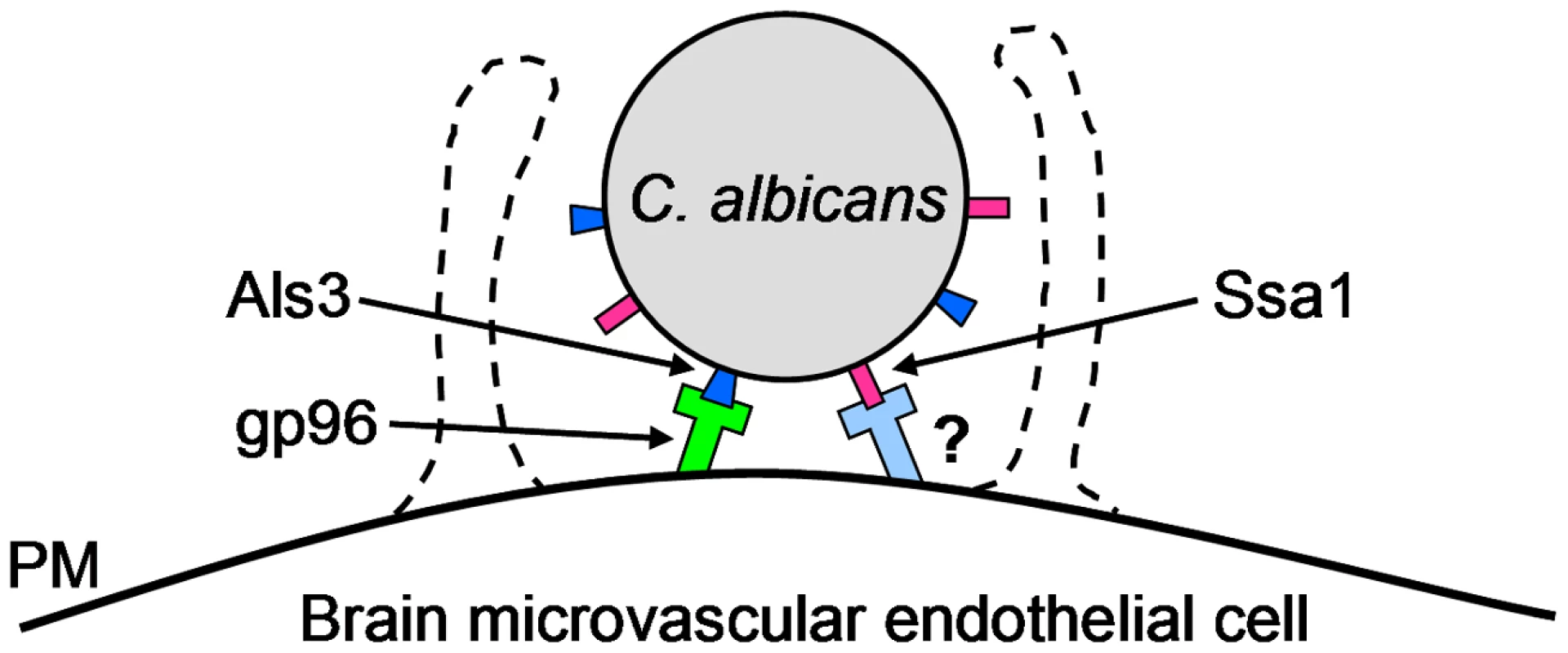
C. albicans Als3 binds to gp96 on the surface of HBMECs and induces endocytosis. C. albicans Ssa1 binds to an HBMEC surface protein other than gp96, which also induces endocytosis. PM, plasma membrane. Materials and Methods
Fungal strains and plasmids
The fungal strains used in this study are listed in Supplemental Table S1. All C. albicans mutant strains constructed for this study were derived from strain BWP17 [47]. Deletion of the entire protein coding regions of both alleles of VPS51 was accomplished by successive transformation with ARG4 and HIS1 deletion cassettes that were generated by PCR using the oligonucleotides vps51-f and vps51-r (The oligonucleotide sequences are listed in Supplemental Table S2) [47]. The resulting strain was subsequently transformed with pGEM-URA3 [47] to re-integrate URA3 at its native locus. The vps53Δ/Δ mutant was constructed similarly, using the oligonucleotides vps53-f and vps53-r. To construct the VPS51 complemented strain (vps51Δ/Δ+pVPS51), a 2.6 Kb fragment containing VPS51 was generated by high fidelity PCR with the primers vps51-rev-f and vps51-rev-r using genomic DNA from C. albicans SC5314 as the template. This PCR product was digested with NcoI, and then subcloned into pBSK-Ura, which had been linearized with NcoI. The resulting construct was linearized with NotI and PstI to direct integration at the URA3 locus of a Ura– vps51Δ/Δ mutant strain. The vps53Δ/Δ-complemented strain (vps53Δ/Δ+pVPS53) was generated similarly, except that primers vps53-rev-f and vps53-rev-r were used to PCR amplify a 3.3 Kb DNA fragment containing VPS53.
To delete the entire protein coding region of ALS3 in the vps51Δ/Δ mutant, deletion cassettes containing ALS3 flanking regions and the URA3 or NAT1 selection markers were amplified by PCR with primers als3-pgem-KO-f and als3-pgem-KO-r, using pGEM-URA3 [47] and pJK795 [48] as templates, respectively. These PCR products were then used to successively transform a Ura - ssa1Δ/Δ strain. The resulting als3Δ/Δ vps51Δ/Δ double mutant was plated on 5-fluoroorotic acid to select for a Ura - strain, which was then transformed with pGEM-URA3 as above. The als3Δ/Δ vps51Δ/Δ+pVPS51 complemented strain was generated the same way as was the vps51Δ/Δ+pVPS51 complemented strain. The ssa1Δ/Δ vps51Δ/Δ double mutant and its VPS51-complemented strain (ssa1Δ/Δ vps51Δ/Δ+pVPS51) were generated similarly to the als3Δ/Δ vps51Δ/Δ double mutant and its complemented strain, except that primers ssa1-pgem-f and ssa1-pgem-r were used to amplify the SSA1 deletion cassettes.
The construction of the S. cerevisiae strain that expressed C. albicans ALS3 under the control of the ADH1 promoter and its control strain containing the backbone vector was described previously [25]. To express C. albicans SSA1 in S. cerevisiae, a 2.0 kb fragment containing the SSA1 protein coding region was generated by PCR with primers ssa1-exp-bglii-f and ssa1-exp-xhoi-r using pRP10-SSA1ORF as template [49]. The resulting SSA1 fragment was cloned downstream of the GAL1 promoter of pYES2.1/V5-His-TOPO using the pYES2.1 TOPO TA Expression Kit (Invitrogen) following the manufacturer's instructions. The control strain of S. cerevisiae was transformed with the backbone vector alone. Expression of C. albicans SSA1 was induced by growth in SC minimal medium containing 2% galactose following the manufacturer's protocol.
Murine model of disseminated candidiasis
Male BALB/c mice weighing 18–20 g (Taconic Farms) were used for all animal experiments. For survival studies, 10 mice per strain were injected via the tail vein with either 5×105 or 3×106 yeast of the various C. albicans strains [50] and then monitored for survival three times daily. All inocula were confirmed by colony counting. In the organ fungal burden studies, the mice were inoculated with 5×105 yeast as above. At various time points, 7 mice per strain were sacrificed and the kidney, liver, and brain were harvested. These organs were weighed, homogenized and quantitatively cultured. For histopathological analysis, a portion of the excised tissue was fixed in zinc-buffered formalin followed by 70% ethanol. The tissue was then embedded in paraffin, after which thin sections were prepared and stained with Gomori methenamine silver. They were examined by light microscopy. All mouse experiments were approved by the Animal Care and Use Committee at the Los Angeles Biomedical Research Institute and carried out according to the National Institutes of Health (NIH) guidelines for the ethical treatment of animals.
Endothelial cells
HUVECs were harvested from umbilical cords with collagenase and grown in M-199 medium supplemented with 10% fetal bovine serum and 10% defined bovine calf serum (Gemini Bio-Products), and containing 2 mM L-glutamine with penicillin and streptomycin (Irvine Scientific) as previously described [51]. HBMECs were isolated from the capillaries in small fragments of the cerebral cortex, which were obtained by surgical resection from 4 - to 7-year-old children with seizure disorders at Children's Hospital Los Angeles. HBMECs were harvested from these capillaries and maintained in a mixture of M-199 and Ham's F-12 media (1∶1 v/v) supplemented with 10% fetal bovine serum, 1 mM sodium pyruvate, and 2 mM glutamine as described previously [52]. More than 98% of these cells were positive for Factor VIII-rag and carbonic anhydrase, and negative for GFAP by flow cytometry. In addition, 99% of the cells took up Dil-Ac-LDL by immunocytochemistry. CHO K-1 cells expressing human gp96 were generated and grown as outlined before [9]. All cell types were grown at 37°C in 5% CO2.
Knockdown of gp96 by siRNA
HBMECs or HUVECs were grown to 60% confluence in six-well plates, then transfected with either gp96 siRNA (Catalog number HSS110955; Invitrogen) or a random control siRNA using lipofactamine 2000 (Invitrogen), according to the manufacturer's instructions. Gp96 knockdown was verified by Western blotting of total endothelial cell lysates with an anti-gp96 monoclonal antibody (Santa Cruz Biotechnology).
Affinity purification of gp96 using intact organisms
HBMEC membrane proteins from host cells were isolated using octyl-glucopyranoside exactly as described previously [16]. Next, 2×108 hyphae of the various C. albicans strains or 8×108 yeast of the different S. cerevisiae strains were incubated on ice for 1 h with 250 µg of HBMEC membrane proteins in PBS with calcium and magnesium and containing 1.5% octyl-glucopyranoside and protease inhibitors. The unbound proteins were removed by extensive rinsing in the same buffer. Next, the proteins that had bound to the hyphae were eluted with 6M urea. The eluted proteins were separated by SDS-PAGE and detected by immunoblotting with the anti-gp96 antibody using enhanced chemiluminescence (Pierce).
Candidal adherence
The adherence of C. albicans to HUVECs and HBMECs grown in 6-well tissue culture plates was measured by a modification of our previously described method [25]. Briefly, germ tubes of the various strains were generated by a 1-h incubation in RPMI 1640 medium (Irvine Scientific) at 37°C. The germ tubes were enumerated with a hemacytometer and suspended in HBSS at 200 cells/ml. After rinsing the endothelial cell monolayers twice with HBSS, 1 ml of the germ tube suspension was added to each well. The cells were incubated for 30 min, after which the nonadherent organisms were aspirated and the endothelial cell monolayers were rinsed twice with HBSS in a standardized manner. Next, the wells were overlaid with YPD agar and the number of adherent organisms was determined by colony counting. The adherence results were expressed as a percentage of the initial inoculum, which was verified by quantitative culture. Each strain was tested in triplicate on three different days.
Candidal endocytosis
The number of organisms internalized by the endothelial cells was determined using our standard differential fluorescence assay [15], [16]. Briefly, endothelial cells on glass coverslips were infected with 105 yeast phase cells of each strain of C. albicans in RPMI 1640 medium. After incubation for 3 h, the cells were fixed with 3% paraformaldehyde. The noninternalized cells were stained with anti-C. albicans rabbit serum (Biodesign International) that had been conjugated with Alexa 568 (Invitrogen). Next, the endothelial cells were permeablized in 0.1% (vol/vol) Triton X-100 in PBS, after which both the internalized and the noninternalized organisms were stained with anti-C. albicans rabbit serum conjugated with Alexa 488 (Invitrogen). The coverslips were mounted inverted on a microscope slide and viewed under epifluorescence. The number of organisms endocytosed by the endothelial cells was determined by subtracting the number of noninternalized organisms (which fluoresced red) from the total number of organisms (which fluoresced green). At least 100 organisms were counted on each coverslip, and all experiments were performed in triplicate on at least three separate occasions.
Transferrin uptake
HBMECs were grown to 70% confluency in 6-well tissue culture plates and then incubated for 3 in serum-free medium to deplete endogenous transferrin. Next they were incubated for 45 min in serum-free medium containing AlexaFluor 555-labeled transferrin (Invitrogen; 10 µg/ml). The unincorporated transferrin was removed by rinsing, after which the cells were incubated for an additional 30 min. Any remaining surface bound transferrin was removed by rinsing the cells twice with ice-cold PBS containing Ca++ and Mg++ (PBS++) followed by two, 5 min incubations with ice-cold acid wash buffer (0.2 M acetic acid (pH 2.8) 0.5 M NaCl). Finally, the cells were washed three times with ice-cold PBS++, detached with Cell Dissociation Buffer (Invitrogen), and suspended in PBS++. Their transferrin content was determined by flow cytometry, analyzing at least 10,000 cells.
Flow cytometry
Flow cytometry was used to analyze the surface expression Als3p on hyphae of the various strains using a minor modification of our previously described method [14]. Briefly, hyphae of the different strains of C. albicans were fixed in 3% paraformaldehyde and blocked with 1% goat serum. The hyphae were then incubated with either a rabbit polyclonal antiserum raised against rAls3-N or purified rabbit IgG. After extensive rinsing, the cells were incubated with a goat anti-rabbit secondary antibody conjugated with Alexa 488. The fluorescent intensity of the hyphae was measured by flow cytometry. Fluorescence data for 10,000 cells of each strain were collected.
Statistical analyses
The capacity of the various strains of C. albicans and S. cerevisiae to adhere to, and be endocytosed to endothelial cells was compared using analyses of variance. Differences in the fungal burden of mice infected with these strains were analyzed using the Wilcoxon Rank Sum test. Differences in survival were analyzed using the Log-Rank test.
Ethics statement
The protocol for collecting umbilical cords for the harvesting of HUVECs used in these studies was approved by the Institutional Review Board of the Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center. This protocol was granted a waiver of consent because the donors remained anonymous. The protocol for using fragments of the cerebral cortex, obtained by surgical resection from 4 - to 7-year-old children with seizure disorders, for isolation of HBMECs was approved by the Institutional Review Board of Childrens Hospital Los Angeles. These fragments were obtained from anonymous donors in 1992-1993 and the HBMECs used in the current studies were isolated at that time and stored in liquid nitrogen. The use of HBMECs in our studies is exempted because the donors are unknown and there is no information linking the HBMECs with the donors. The mouse studies were carried out in accordance with the National Institutes of Health guidelines for the ethical treatment of animals. This protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of the Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center.
Supporting Information
Zdroje
1. WisplinghoffHBischoffTTallentSMSeifertHWenzelRP 2004 Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 39 309 317
2. HornDLNeofytosDAnaissieEJFishmanJASteinbachWJ 2009 Epidemiology and outcomes of candidemia in 2019 patients: data from the prospective antifungal therapy alliance registry. Clin Infect Dis 48 1695 1703
3. ParkerJCJrMcCloskeyJJLeeRS 1981 Human cerebral candidosis--a postmortem evaluation of 19 patients. Hum Pathol 12 23 28
4. BenjaminDKJrStollBJGantzMGWalshMCSanchezPJ 2010 Neonatal candidiasis: epidemiology, risk factors, and clinical judgment. Pediatrics 126 e865 873
5. FaixRGChapmanRL 2003 Central nervous system candidiasis in the high-risk neonate. Semin Perinatol 27 384 392
6. BroadwellRDBaker-CairnsBJFridenPMOliverCVillegasJC 1996 Transcytosis of protein through the mammalian cerebral epithelium and endothelium. III. Receptor-mediated transcytosis through the blood-brain barrier of blood-borne transferrin and antibody against the transferrin receptor. Exp Neurol 142 47 65
7. CoureuilMLecuyerHScottMGBoularanCEnslenH 2010 Meningococcus Hijacks a beta2-adrenoceptor/beta-Arrestin pathway to cross brain microvasculature endothelium. Cell 143 1149 1160
8. HuangSHWassCFuQPrasadaraoNVStinsM 1995 Escherichia coli invasion of brain microvascular endothelial cells in vitro and in vivo: molecular cloning and characterization of invasion gene ibe10. Infect Immun 63 4470 4475
9. PrasadaraoNVSrivastavaPKRudrabhatlaRSKimKSHuangSH 2003 Cloning and expression of the Escherichia coli K1 outer membrane protein A receptor, a gp96 homologue. Infect Immun 71 1680 1688
10. ChangYCStinsMFMcCafferyMJMillerGFPareDR 2004 Cryptococcal yeast cells invade the central nervous system via transcellular penetration of the blood-brain barrier. Infect Immun 72 4985 4995
11. OrihuelaCJMahdaviJThorntonJMannBWooldridgeKG 2009 Laminin receptor initiates bacterial contact with the blood brain barrier in experimental meningitis models. J Clin Invest 119 1638 1646
12. RotrosenDEdwardsJEJrGibsonTRMooreJCCohenAH 1985 Adherence of Candida to cultured vascular endothelial cells: mechanisms of attachment and endothelial cell penetration. J Infect Dis 152 1264 1274
13. FillerSGSwerdloffJNHobbsCLuckettPM 1995 Penetration and damage of endothelial cells by Candida albicans. Infect Immun 63 976 983
14. SunJNSolisNVPhanQTBajwaJSKashlevaH 2010 Host cell invasion and virulence mediated by Candida albicans Ssa1. PLoS Pathog 6 e1001181
15. PhanQTMyersCLFuYSheppardDCYeamanMR 2007 Als3 is a Candida albicans invasin that binds to cadherins and induces endocytosis by host cells. PLoS Biol 5 e64
16. PhanQTFrattiRAPrasadaraoNVEdwardsJEJrFillerSG 2005 N-cadherin mediates endocytosis of Candida albicans by endothelial cells. J Biol Chem 280 10455 10461
17. ZhuWBoontheungPLooJAFillerSG 2010 Roles of HER2 and EGFR signaling in host cell invasion by Candida albicans, [Abstract 180B]. p. 127. Abstr 10th ASM Conf Candida Candidiasis Miami, Florida, United States 22–26 March 2010
18. LossinskyASJongAFialaMMukhtarMButtleKF 2006 The histopathology of Candida albicans invasion in neonatal rat tissues and in the human blood-brain barrier in culture revealed by light, scanning, transmission and immunoelectron microscopy. Histol Histopathol 21 1029 1041
19. JongAYStinsMFHuangSHChenSHKimKS 2001 Traversal of Candida albicans across human blood-brain barrier in vitro. Infect Immun 69 4536 4544
20. ParkHLiuYSolisNSpotkovJHamakerJ 2009 Transcriptional responses of Candida albicans to epithelial and endothelial cells. Eukaryot Cell 8 1498 1510
21. ConibearECleckJNStevensTH 2003 Vps51p mediates the association of the GARP (Vps52/53/54) complex with the late Golgi t-SNARE Tlg1p. Mol Biol Cell 14 1610 1623
22. ReggioriFWangCWStromhaugPEShintaniTKlionskyDJ 2003 Vps51 is part of the yeast Vps fifty-three tethering complex essential for retrograde traffic from the early endosome and Cvt vesicle completion. J Biol Chem 278 5009 5020
23. MacCallumDMOddsFC 2005 Temporal events in the intravenous challenge model for experimental Candida albicans infections in female mice. Mycoses 48 151 161
24. CannomRRMFrenchSWJohnstonDEdwardsJEJrFillerSG 2002 Candida albicans stimulates local expression of leukocyte adhesion molecules and cytokines in vivo. J Infect Dis 186 389 396
25. SheppardDCYeamanMRWelchWHPhanQTFuY 2004 Functional and structural diversity in the Als protein family of Candida albicans. J Biol Chem 279 30840 30849
26. SpellbergBIbrahimASEdwardsJEJrFillerSG 2005 Mice with disseminated candidiasis die of progressive sepsis. J Infect Dis 192 336 343
27. LaFayetteSLCollinsCZaasAKSchellWABetancourt-QuirozM 2010 PKC signaling regulates drug resistance of the fungal pathogen Candida albicans via circuitry comprised of Mkc1, calcineurin, and Hsp90. PLoS Pathog 6 e1001069
28. NichollsSMacCallumDMKaffarnikFASelwayLPeckSC 2011 Activation of the heat shock transcription factor Hsf1 is essential for the full virulence of the fungal pathogen Candida albicans. Fungal Genet Biol 48 297 305
29. CorreiaALermannUTeixeiraLCercaFBotelhoS 2010 Limited role of secreted aspartyl proteinases Sap1 to Sap6 in Candida albicans virulence and host immune response in murine hematogenously disseminated candidiasis. Infect Immun 78 4839 4849
30. NeteaMGVan Der GraafCAVonkAGVerschuerenIVan Der MeerJW 2002 The role of toll-like receptor (TLR) 2 and TLR4 in the host defense against disseminated candidiasis. J Infect Dis 185 1483 1489
31. van SprielABSofiMGartlanKHvan der SchaafAVerschuerenI 2009 The tetraspanin protein CD37 regulates IgA responses and anti-fungal immunity. PLoS Pathog 5 e1000338
32. RochaSFAdamsRH 2009 Molecular differentiation and specialization of vascular beds. Angiogenesis 12 139 147
33. LionakisMSLimJKLeeCCMurphyPM 2011 Organ-specific innate immune responses in a mouse model of invasive candidiasis. J Innate Immun 3 180 199
34. Martinez-LopezRMonteolivaLDiez-OrejasRNombelaCGilC 2004 The GPI-anchored protein CaEcm33p is required for cell wall integrity, morphogenesis and virulence in Candida albicans. Microbiology 150 3341 3354
35. Alonso-MongeRNavarro-GarciaFMoleroGDiez-OrejasRGustinM 1999 Role of the mitogen-activated protein kinase Hog1p in morphogenesis and virulence of Candida albicans. J Bacteriol 181 3058 3068
36. DalleFWachtlerBCoralieLHollandGBannertN 2010 Cellular interactions of Candida albicans with human oral epithelial cells and enterocytes. Cell Microbiol 12 248 271
37. LiuBYangYQiuZStaronMHongF 2010 Folding of Toll-like receptors by the HSP90 paralogue gp96 requires a substrate-specific cochaperone. Nat Commun 1 79
38. CabanesDSousaSCebriaALecuitMGarcia-del PortilloF 2005 Gp96 is a receptor for a novel Listeria monocytogenes virulence factor, Vip, a surface protein. Embo J 24 2827 2838
39. RechnerCKuhleweinCMullerASchildHRudelT 2007 Host glycoprotein Gp96 and scavenger receptor SREC interact with PorB of disseminating Neisseria gonorrhoeae in an epithelial invasion pathway. Cell Host Microbe 2 393 403
40. Di PasqualeGKaludovNAgbandje-McKennaMChioriniJA 2010 BAAV transcytosis requires an interaction with beta-1-4 linked - glucosamine and gp96. PLoS One 5 e9336
41. MaruvadaRArgonYPrasadaraoNV 2008 Escherichia coli interaction with human brain microvascular endothelial cells induces signal transducer and activator of transcription 3 association with the C-terminal domain of Ec-gp96, the outer membrane protein A receptor for invasion. Cell Microbiol 10 2326 2338
42. SukumaranSKMcNamaraGPrasadaraoNV 2003 Escherichia coli K-1 interaction with human brain micro-vascular endothelial cells triggers phospholipase C-gamma1 activation downstream of phosphatidylinositol 3-kinase. J Biol Chem 278 45753 45762
43. SukumaranSKPrasadaraoNV 2002 Regulation of protein kinase C in Escherichia coli K1 invasion of human brain microvascular endothelial cells. J Biol Chem 277 12253 12262
44. ClearyIAReinhardSMMillerCLMurdochCThornhillMH 2011 The Candida albicans adhesin Als3p is dispensable for virulence in the mouse model of disseminated candidiasis. Microbiology 157 Pt 6 1806 15
45. ColemanDAOhSHZhaoXZhaoHHutchinsJT 2009 Monoclonal antibodies specific for Candida albicans Als3 that immunolabel fungal cells in vitro and in vivo and block adhesion to host surfaces. J Microbiol Methods 78 71 78
46. GreenCBZhaoXHoyerLL 2005 Use of green fluorescent protein and reverse transcription-PCR to monitor Candida albicans agglutinin-like sequence gene expression in a murine model of disseminated candidiasis. Infect Immun 73 1852 1855
47. WilsonRBDavisDMitchellAP 1999 Rapid hypothesis testing with Candida albicans through gene disruption with short homology regions. J Bacteriol 181 1868 1874
48. ShenJGuoWKohlerJR 2005 CaNAT1, a heterologous dominant selectable marker for transformation of Candida albicans and other pathogenic Candida species. Infect Immun 73 1239 1242
49. LiXSSunJNOkamoto-ShibayamaKEdgertonM 2006 Candida albicans cell wall Ssa proteins bind and facilitate import of salivary histatin 5 required for toxicity. J Biol Chem 281 22453 22463
50. SanchezAAJohnstonDAMyersCEdwardsJEJrMitchellAP 2004 Relationship between Candida albicans virulence during experimental hematogenously disseminated Infection and endothelial cell damage In vitro. Infect Immun 72 598 601
51. PhanQTBelangerPHFillerSG 2000 Role of hyphal formation in interactions of Candida albicans with endothelial cells. Infect Immun 68 3485 3490
52. StinsMFNemaniPVWassCKimKS 1999 Escherichia coli binding to and invasion of brain microvascular endothelial cells derived from humans and rats of different ages. Infect Immun 67 5522 5525
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Quorum Sensing in Fungi: Q&AČlánek Blood Feeding and Insulin-like Peptide 3 Stimulate Proliferation of Hemocytes in the MosquitoČlánek The DEAD-box RNA Helicase DDX6 is Required for Efficient Encapsidation of a Retroviral GenomeČlánek A Phenome-Based Functional Analysis of Transcription Factors in the Cereal Head Blight Fungus,Článek A Wide Extent of Inter-Strain Diversity in Virulent and Vaccine Strains of AlphaherpesvirusesČlánek The Anti-Sigma Factor TcdC Modulates Hypervirulence in an Epidemic BI/NAP1/027 Clinical Isolate ofČlánek Critical Roles for LIGHT and Its Receptors in Generating T Cell-Mediated Immunity during InfectionČlánek Frequent and Recent Human Acquisition of Simian Foamy Viruses Through Apes' Bites in Central Africa
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 10- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Quorum Sensing in Fungi: Q&A
- Discovery of an Ebolavirus-Like Filovirus in Europe
- Toll-like Receptor 7 Controls the Anti-Retroviral Germinal Center Response
- Tubule-Guided Cell-to-Cell Movement of a Plant Virus Requires Class XI Myosin Motors
- Herpesvirus Telomerase RNA (vTR) with a Mutated Template Sequence Abrogates Herpesvirus-Induced Lymphomagenesis
- Mitochondrial Peroxiredoxin Plays a Crucial Peroxidase-Unrelated Role during Infection: Insight into Its Novel Chaperone Activity
- Sustained CD8+ T Cell Memory Inflation after Infection with a Single-Cycle Cytomegalovirus
- Novel Mouse Xenograft Models Reveal a Critical Role of CD4 T Cells in the Proliferation of EBV-Infected T and NK Cells
- Toll-8/Tollo Negatively Regulates Antimicrobial Response in the Respiratory Epithelium
- Exhausted Cytotoxic Control of Epstein-Barr Virus in Human Lupus
- Structural and Functional Analysis of Laninamivir and its Octanoate Prodrug Reveals Group Specific Mechanisms for Influenza NA Inhibition
- Infection Drives IL-17-Mediated Neutrophilic Allergic Airways Disease
- Blood Feeding and Insulin-like Peptide 3 Stimulate Proliferation of Hemocytes in the Mosquito
- HIV-1 Replication in the Central Nervous System Occurs in Two Distinct Cell Types
- Deep Molecular Characterization of HIV-1 Dynamics under Suppressive HAART
- Fitness Landscape of Antibiotic Tolerance in Biofilms
- The DEAD-box RNA Helicase DDX6 is Required for Efficient Encapsidation of a Retroviral Genome
- Preventing Sepsis through the Inhibition of Its Agglutination in Blood
- A Phenome-Based Functional Analysis of Transcription Factors in the Cereal Head Blight Fungus,
- IFITM3 Inhibits Influenza A Virus Infection by Preventing Cytosolic Entry
- Targeting Cattle-Borne Zoonoses and Cattle Pathogens Using a Novel Trypanosomatid-Based Delivery System
- A Wide Extent of Inter-Strain Diversity in Virulent and Vaccine Strains of Alphaherpesviruses
- Coordinated Destruction of Cellular Messages in Translation Complexes by the Gammaherpesvirus Host Shutoff Factor and the Mammalian Exonuclease Xrn1
- Signal Transduction through CsrRS Confers an Invasive Phenotype in Group A
- Biochemical and Structural Insights into the Mechanisms of SARS Coronavirus RNA Ribose 2′-O-Methylation by nsp16/nsp10 Protein Complex
- Histone Deacetylase 8 Is Required for Centrosome Cohesion and Influenza A Virus Entry
- Severe Acute Respiratory Syndrome Coronavirus Envelope Protein Regulates Cell Stress Response and Apoptosis
- Co-opts the FGF2 Signaling Pathway to Enhance Infection
- IRAK-2 Regulates IL-1-Mediated Pathogenic Th17 Cell Development in Helminthic Infection
- Trafficking of Hepatitis C Virus Core Protein during Virus Particle Assembly
- The Anti-interferon Activity of Conserved Viral dUTPase ORF54 is Essential for an Effective MHV-68 Infection
- A Viral Nuclear Noncoding RNA Binds Re-localized Poly(A) Binding Protein and Is Required for Late KSHV Gene Expression
- Suppression of Methylation-Mediated Transcriptional Gene Silencing by βC1-SAHH Protein Interaction during Geminivirus-Betasatellite Infection
- ISG15 Is Critical in the Control of Chikungunya Virus Infection Independent of UbE1L Mediated Conjugation
- Non-Hematopoietic Cells in Lymph Nodes Drive Memory CD8 T Cell Inflation during Murine Cytomegalovirus Infection
- RNA Polymerase II Stalling Promotes Nucleosome Occlusion and pTEFb Recruitment to Drive Immortalization by Epstein-Barr Virus
- Noninfectious Retrovirus Particles Drive the / Dependent Neutralizing Antibody Response
- Endophytic Life Strategies Decoded by Genome and Transcriptome Analyses of the Mutualistic Root Symbiont
- An Integrated Approach to Elucidate the Intra-Viral and Viral-Cellular Protein Interaction Networks of a Gamma-Herpesvirus
- as an Animal Model for the Study of Biofilm Infections
- Homeostatic Proliferation Fails to Efficiently Reactivate HIV-1 Latently Infected Central Memory CD4+ T Cells
- The Anti-Sigma Factor TcdC Modulates Hypervirulence in an Epidemic BI/NAP1/027 Clinical Isolate of
- Enhances Protective and Detrimental HLA Class I-Mediated Immunity in Chronic Viral Infection
- The Mouse IAPE Endogenous Retrovirus Can Infect Cells through Any of the Five GPI-Anchored EphrinA Proteins
- The Urgent Need for Robust Coral Disease Diagnostics
- HacA-Independent Functions of the ER Stress Sensor IreA Synergize with the Canonical UPR to Influence Virulence Traits in
- A Novel Core Genome-Encoded Superantigen Contributes to Lethality of Community-Associated MRSA Necrotizing Pneumonia
- Critical Roles for LIGHT and Its Receptors in Generating T Cell-Mediated Immunity during Infection
- The SARS-Coronavirus-Host Interactome: Identification of Cyclophilins as Target for Pan-Coronavirus Inhibitors
- Frequent and Recent Human Acquisition of Simian Foamy Viruses Through Apes' Bites in Central Africa
- Mechanisms of Trafficking to the Brain
- Defining Emerging Roles for NF-κB in Antivirus Responses: Revisiting the Enhanceosome Paradigm
- The Role of Sialyl Glycan Recognition in Host Tissue Tropism of the Avian Parasite
- Evolutionarily Divergent, Unstable Filamentous Actin Is Essential for Gliding Motility in Apicomplexan Parasites
- The Herpes Simplex Virus-1 Transactivator Infected Cell Protein-4 Drives VEGF-A Dependent Neovascularization
- Distinct Single Amino Acid Replacements in the Control of Virulence Regulator Protein Differentially Impact Streptococcal Pathogenesis
- Soluble Rhesus Lymphocryptovirus gp350 Protects against Infection and Reduces Viral Loads in Animals that Become Infected with Virus after Challenge
- A Genetic Screen Reveals Arabidopsis Stomatal and/or Apoplastic Defenses against pv. DC3000
- Hepatitis C Virus Reveals a Novel Early Control in Acute Immune Response
- Fumarate Reductase Activity Maintains an Energized Membrane in Anaerobic
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Severe Acute Respiratory Syndrome Coronavirus Envelope Protein Regulates Cell Stress Response and Apoptosis
- The SARS-Coronavirus-Host Interactome: Identification of Cyclophilins as Target for Pan-Coronavirus Inhibitors
- Biochemical and Structural Insights into the Mechanisms of SARS Coronavirus RNA Ribose 2′-O-Methylation by nsp16/nsp10 Protein Complex
- Evolutionarily Divergent, Unstable Filamentous Actin Is Essential for Gliding Motility in Apicomplexan Parasites
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



