-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaExploring Host–Pathogen Interactions through Biological Control
article has not abstract
Published in the journal: . PLoS Pathog 11(6): e32767. doi:10.1371/journal.ppat.1004865
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1004865Summary
article has not abstract
Invasive pests impose a major burden on the agricultural industries and the natural environment, threatening some native species with extinction. Although chemical agents are often deployed to eliminate pests, unspecific toxicity makes their use undesirable. Biological agents are of interest as an alternative method to cull specific pest species with little residual environmental harm. The deliberate release of microbial pathogens, referred to as biological control (biocontrol), has, to date, largely involved viruses. Here, we summarise the past and proposed applications of the biocontrol of vertebrate pests. Despite understandable fears, vertebrate biocontrol has yet to be associated with adverse effects, and provides large-scale natural experiments by which to understand the intimate interactions between hosts and their pathogens.
A Short History of Biocontrol
Biocontrol, in which a biological agent is used to reduce the population size of a target pest organism, is widely used in agriculture to protect plants from arthropods and fungal infections [1]. Although biocontrol has, to date, played little role in human health, a recent and innovative application involves reducing burden of the vector-borne dengue virus (DENV) through Aedes aegypti mosquitoes artificially infected with Wolbachia bacteria [2]. While Wolbachia can strongly inhibit DENV in laboratory experiments, and can spread among wild mosquitoes, whether it will lead to a reduction in human DENV prevalence and incidence has yet to be determined [2,3].
The use of pathogens as biocontrol agents to control vertebrate populations has caused more concern, and the fear of inadvertent infection of other species, even humans, persists. In the 1950s, classical swine fever virus was released on small islands in California in an attempt to reduce the wild swine population, but the virus did not establish persistent transmission [4]. Indeed, to date, there have only been three successful viral biocontrols of vertebrates: the release of Feline panleukopenia virus (parvovirus) to eliminate cats on Marion Island and the well-documented cases of myxoma virus (MYXV) and rabbit haemorrhagic disease virus (RHDV) in Australia and New Zealand to control the feral rabbit population (Fig 1). In all three cases an immediate reduction in the size of the target population was observed and, critically, without widespread jumping to non-target hosts. A new biocontrol intervention is planned in Australia with the release of koi herpesvirus (KHV, or cyprinid herpesvirus-3) against the common carp (Cyprinus carpio), an invasive fish species that comprises up to 90% of fish biomass in the major Murray-Darling river system of southeast Australia and the cause of environmental degradation and loss of native fish biodiversity [5]. If KHV is released as planned, the future study of the carp-herpesvirus system may provide insights into the nature of host—pathogen coevolution that parallel Frank Fenner’s classic work on myxomatosis in Australia [6].
Fig. 1. Past and future viral biocontrols of vertebrate pest species. 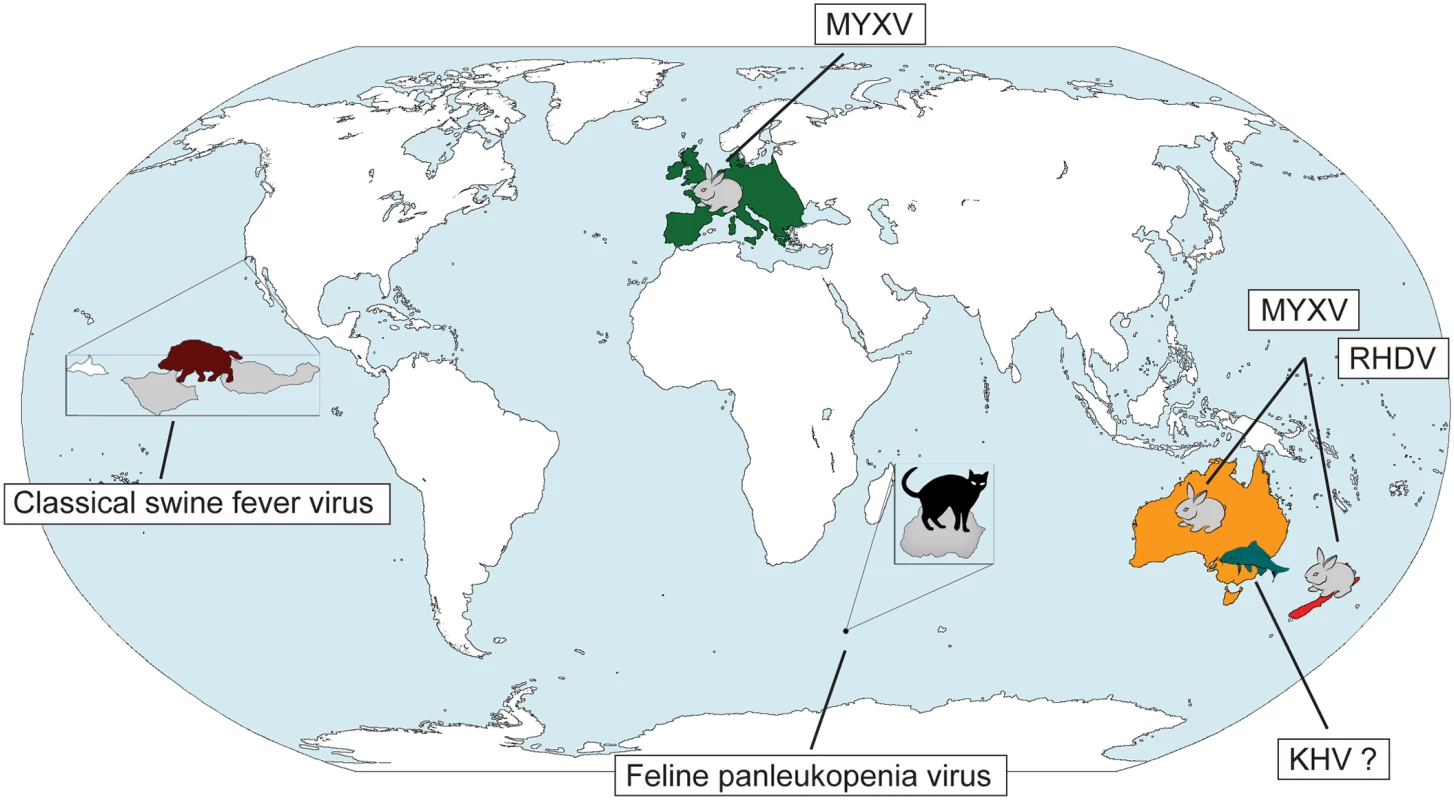
A limited number of viruses have been used to control invasive vertebrate pests on a global scale: (i) Feline panleukopenia virus against cats on Marion Island (magnified on the map in grey), (ii) MYXV against rabbits in Europe and Australia, and (iii) RHDV in Australia and New Zealand. Despite some initial success, classical swine fever virus proved to be an unsuccessful biocontrol against wild boar on the Channel Islands in California (the islands are magnified, with the two islands affected, Santa Cruz and Santa Rosa, shown in grey). Safety testing is currently underway for the possible release of KHV against carp in Australia. Abbreviations: KHV koi herpesvirus, MYXV myxoma virus, RHDV rabbit haemorrhagic disease virus. Parvovirus and the Cats of Marion Island
Marion Island is a small and remote island in the Southern Indian Ocean with a large seabird population. In 1949, cats were brought to the island to hunt mice in the island’s meteorological station. By 1975, the island had an estimated feral cat population of over 2,000, which posed a major threat to local avian species, with more than 45,000 birds predated each year. Feline parvovirus is a highly contagious (DNA) virus that is transmitted via body fluids or contaminated surfaces and resistant to temperature changes, making it ideal for a sub-Antarctic environment [7]. Following its release on Marion Island in 1977, the cat population declined to approximately 600 animals within five years, with the remaining cats successfully eradicated using traps and hunting. The island has been cat-free since 1991 [8].
Australia: The Evolving Story of Rabbits and Viruses
Australia’s unique fauna and flora are highly sensitive to imported species, and the deliberate or accidental introduction of feral animals has posed an enormous problem to local wildlife. Historically, the most important of these has been the European rabbit (Oryctolagus cuniculus). Following importation for hunting purposes in the 19th century, rabbits rapidly spread all across much of Australia, forming a “grey blanket” that caused major damage to vegetation [9]. Attempts to control the population, including large-scale rabbit-proof fences, failed [9], such that a new approach was clearly needed.
MYXV belongs to the family Poxviridae and is native to the Americas, causing only mild skin lesions in its natural lagomorph hosts (Sylvilagus spp.), but highly virulent in the European rabbit, resulting in myxomatosis and death within a few days [10]. In 1950, MYXV was successfully released as a biocontrol into wild rabbits in Australia, causing an immediate reduction in rabbit numbers. However, the rabbit population began to recover after a few years, reflecting a combination of evolving virus attenuation and rabbit resistance [11]. Fortuitously, a new candidate biocontrol agent—the calicivirus RHDV—was identified in a major outbreak in China in 1984, resulting in the death of 140 million rabbits in one year [12]. After its accidental spread to the Australian mainland, RHDV was officially approved as a biocontrol there in 1996, and the virus reduced the rabbit population to 5% of its former size within a few months [9]. In marked contrast to MYXV, RHDV has maintained its high virulence in rabbits, despite some resistance evolution.
Interestingly, MYXV was also released in New Zealand, but it failed to establish there, likely due to the lack of appropriate insect vectors [13]. The New Zealand government later refused the use of RHDV as a rabbit biocontrol. Nevertheless, in 1997, RHDV was illegally imported by farmers, spreading rapidly across the country and killing rabbits in a parallel manner to that observed in Australia, with a lethality rate of up to 84%. In both countries, the impact of RHDV varied among geographic regions, with lower death rates in wetter areas [9,14]. It is believed that this regional variation likely reflects cross-immunity to pre-existing rabbit caliciviruses in these localities in Australia [15].
Rabbit Biocontrol in Europe
Although a native species, the European rabbit is regarded as a pest in some parts of Europe. Hence, two years after the successful use of MYXV in Australia, the virus was privately released as a biocontrol in France. The impact was enormous. The virus spread rapidly through France and England, reducing the size of the rabbit population by more than 90% [9]. Thirty years later, the highly virulent RHDV naturally spread through European rabbit populations. However, the overall impact of RHDV on rabbit numbers in Europe was smaller than in Australia and New Zealand, again, perhaps reflecting cross-protection from related viruses present, as well as a longer time-scale of resistance evolution [12]. Importantly, the decline in the rabbit population caused by MYXV and RHDV also had a number of negative ecological consequences. For example, in Spain, the loss of rabbits reduced food availability for their natural predators, notably the Iberian lynx [12,13].
Insights into Virus Emergence and Evolution
The epizootics of MYXV and RHDV in Australia constitute a unique natural experiment in how viruses establish themselves in a novel and naïve host population, including the subsequent evolutionary “arms race” between hosts and pathogens and the evolution of virulence [15]. As such, they serve as a powerful analogy for natural emergence events, providing essential comparative data.
To be effective as a biocontrol, a virus needs to be species-specific, readily transmissible, and highly virulent. The breadth of host range of some viruses is so broad that they can be regarded as species “generalists,” with parvoviruses serving as an important example [16]. Indeed, the feline parvovirus used on Marion Island is a true host generalist, with a limited repertoire of mutations in the virus capsid protein enabling the infection of diverse carnivore (and perhaps other) species [17]. However, such generalists are likely rare, and most viruses have a more restricted host range, indicating that there must be important barriers to their establishment in novel species [18]. Because the host range of a pathogen is difficult to predict, and the barriers that prevent successful host jumps are largely obscure, rigorous species testing is needed before the use of any biocontrol. This is the case with both MYXV and RHDV as, to date, no inadvertent infections of species other than lagomorphs have been identified [15], although the genetic determinants of host range are unknown in both cases. Tests of species specificity are currently being performed with KHV in advance of its possible release, although Australia contains no native cyprinid fish [5].
Sustained high virulence (with high transmission intensity) is also critical to effective biocontrol. Ideally, the virus should spread quickly and kill rapidly, but retain the ability to readily transmit to new hosts. MYXV and RHDV are both highly virulent in European rabbits, killing within a few days. For reasons that are currently unclear, these viruses have experienced strikingly different trajectories of virulence evolution: the mean virulence of field strains of MYXV declined (although high virulence strains are still sampled), while there is no evidence that RHDV has attenuated [19]. Unfortunately, despite the remarkable economic and ecological benefits of both MYXV and RHDV, the rabbit population of Australia is recovering [20], which may exacerbate the decline and eventual extinction of Australia’s unique species diversity [21].
Outlook
If performed safely, biocontrol interventions are a potentially powerful way to control invasive pest species and have provided important insights into the patterns, dynamics and processes of virus—host coevolution. We await the release of koi herpersvirus with interest.
Zdroje
1. Moscardi F. Assessment of the application of baculoviruses for control of Lepidoptera. Annu Rev Entomol. 1999;44 : 257–289. 15012374
2. Hoffmann AA, Montgomery BL, Popovici J, Iturbe-Ormaetxe I, Johnson PH, Muzzi F, et al. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature. 2011;476 : 454–457. doi: 10.1038/nature10356 21866160
3. Frentiu FD, Zakir T, Walker T, Popovici J, Pyke AT, van den Hurk A, et al. Limited dengue virus replication in field-collected Aedes aegypti mosquitoes infected with Wolbachia. PLoS Negl Trop Dis. 2014;8: e2688. doi: 10.1371/journal.pntd.0002688 24587459
4. Saunders G, Cooke B, McColl K, Shine R, Peacock T. Modern approaches for the biological control of vertebrate pests: An Australian perspective. Biol Control. 2010;52 : 288–295.
5. McColl KA, Cooke BD, Sunarto A. Viral biocontrol of invasive vertebrates: Lessons from the past applied to cyprinid herpesvirus-3 and carp (Cyprinus carpio) control in Australia. Biol Control. 2014;72 : 109–117. doi: 10.1159/000360529 24993182
6. Fenner F. The Florey Lecture, 1983—Biological control, as exemplified by smallpox eradication and myxomatosis. Proc R Soc Lond B Biol Sci 1983;218 : 259–285. 6136042
7. van Rensburg PJJ, Skinner JD, Van Aarde RJ. Effects of feline panleucopaenia on the population characteristics of feral cats on marion island. J Appl Ecol. 1987;24 : 63–73.
8. Bester MN, Bloomer JP, van Aarde RJ, Erasmus BH, van Rensburg PJJ, Skinner JD, et al. A review of the successful eradication of feral cats from sub-Antarctic Marion Island, Southern Indian Ocean. S Afr J Wildl Res. 2002;32 : 65–73.
9. Cooke BD. Australia's war against rabbits: the story of rabbit haemorrhagic disease. Collingwood VIC 3066, Australia: CSIRO Publishing; 2014.
10. Ratcliffe FN, Myers K, Fennessy BV, Calaby JH. Myxomatosis in Australia; a step towards the biological control of the rabbit. Nature. 1952;170 : 7–11. 14957004
11. Fenner F, Fantini B. Biological control of vertebrate pests: the history of myxomatosis, an experiment in evolution. Wallingford, UK: CABI Publishing; 1999.
12. Abrantes J, van der Loo W, Le Pendu J, Esteves PJ. Rabbit haemorrhagic disease (RHD) and rabbit haemorrhagic disease virus (RHDV): a review. Vet Res. 2012;43 : 12. doi: 10.1186/1297-9716-43-12 22325049
13. Kerr PJ. Myxomatosis in Australia and Europe: a model for emerging infectious diseases. Antiviral Res. 2012;93 : 387–415. doi: 10.1016/j.antiviral.2012.01.009 22333483
14. Parkes JP, Norbury GL, Heyward RP, Sullivan G. Epidemiology of rabbit haemorrhagic disease (RHD) in the South Island, New Zealand, 1997–2001. Wildl Res. 2002;29 : 543–555.
15. Di Giallonardo F, Holmes EC. Viral biocontrol: grand experiments in disease emergence and evolution. Trends Microbiol. 2015;23 : 83–90. doi: 10.1016/j.tim.2014.10.004 25455418
16. Parrish CR, Holmes EC, Morens DM, Park EC, Burke DS, Calisher CH, et al. Cross-species virus transmission and the emergence of new epidemic diseases. Microbiol Mol Biol Rev. 2008;72 : 457–470. doi: 10.1128/MMBR.00004-08 18772285
17. Allison AB, Kohler DJ, Fox KA, Brown JD, Gerhold RW, Shearn-Bochsler VI, et al. Frequent cross-species transmission of parvoviruses among diverse carnivore hosts. J Virol. 2013;87 : 2342–2347. doi: 10.1128/JVI.02428-12 23221559
18. Holmes EC. Evolutionary history and phylogeography of human viruses. Annu Rev Microbiol. 2008;62 : 307–328. doi: 10.1146/annurev.micro.62.081307.162912 18785840
19. Elsworth P, Cooke BD, Kovaliski J, Sinclair R, Holmes EC, Strive T. Increased virulence of rabbit haemorrhagic disease virus associated with genetic resistance in wild Australian rabbits (Oryctolagus cuniculus). Virology. 2014;464–465 : 415–423.
20. Cox T, Strive T, Mutze G, West P, Saunders G. Benefits of rabbit biocontrol in Australia. Canberra, Australia: Invasive Animals Cooperative Research Centre; 2013.
21. Woinarski JCZ, Burbidge AA, Harrison PL. Ongoing unraveling of a continental fauna: decline and extinction of Australian mammals since European settlement. Proc Natl Acad Sci USA. 2015;
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Clearance of Pneumococcal Colonization in Infants Is Delayed through Altered Macrophage TraffickingČlánek An Model of Latency and Reactivation of Varicella Zoster Virus in Human Stem Cell-Derived NeuronsČlánek Protective mAbs and Cross-Reactive mAbs Raised by Immunization with Engineered Marburg Virus GPsČlánek Specific Cell Targeting Therapy Bypasses Drug Resistance Mechanisms in African TrypanosomiasisČlánek Peptidoglycan Branched Stem Peptides Contribute to Virulence by Inhibiting Pneumolysin ReleaseČlánek HIV Latency Is Established Directly and Early in Both Resting and Activated Primary CD4 T CellsČlánek Sequence-Specific Fidelity Alterations Associated with West Nile Virus Attenuation in Mosquitoes
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 6- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Introducing “Research Matters”
- Exploring Host–Pathogen Interactions through Biological Control
- Analysis of Bottlenecks in Experimental Models of Infection
- Expected and Unexpected Features of the Newly Discovered Bat Influenza A-like Viruses
- Clearance of Pneumococcal Colonization in Infants Is Delayed through Altered Macrophage Trafficking
- Recombinant Murine Gamma Herpesvirus 68 Carrying KSHV G Protein-Coupled Receptor Induces Angiogenic Lesions in Mice
- TRIM30α Is a Negative-Feedback Regulator of the Intracellular DNA and DNA Virus-Triggered Response by Targeting STING
- Targeting Human Transmission Biology for Malaria Elimination
- Two Cdc2 Kinase Genes with Distinct Functions in Vegetative and Infectious Hyphae in
- An Model of Latency and Reactivation of Varicella Zoster Virus in Human Stem Cell-Derived Neurons
- Protective mAbs and Cross-Reactive mAbs Raised by Immunization with Engineered Marburg Virus GPs
- Virulence Factors of Induce Both the Unfolded Protein and Integrated Stress Responses in Airway Epithelial Cells
- Peptide-MHC-I from Endogenous Antigen Outnumber Those from Exogenous Antigen, Irrespective of APC Phenotype or Activation
- Specific Cell Targeting Therapy Bypasses Drug Resistance Mechanisms in African Trypanosomiasis
- An Ultrasensitive Mechanism Regulates Influenza Virus-Induced Inflammation
- The Role of Human Transportation Networks in Mediating the Genetic Structure of Seasonal Influenza in the United States
- Host Delivery of Favorite Meals for Intracellular Pathogens
- Complement-Opsonized HIV-1 Overcomes Restriction in Dendritic Cells
- Inter-Seasonal Influenza is Characterized by Extended Virus Transmission and Persistence
- A Critical Role for CLSP2 in the Modulation of Antifungal Immune Response in Mosquitoes
- Twilight, a Novel Circadian-Regulated Gene, Integrates Phototropism with Nutrient and Redox Homeostasis during Fungal Development
- Surface-Associated Lipoproteins Link Virulence to Colitogenic Activity in IL-10-Deficient Mice Independent of Their Expression Levels
- Latent Membrane Protein LMP2A Impairs Recognition of EBV-Infected Cells by CD8+ T Cells
- Bank Vole Prion Protein As an Apparently Universal Substrate for RT-QuIC-Based Detection and Discrimination of Prion Strains
- Neuronal Subtype and Satellite Cell Tropism Are Determinants of Varicella-Zoster Virus Virulence in Human Dorsal Root Ganglia Xenografts
- Molecular Basis for the Selective Inhibition of Respiratory Syncytial Virus RNA Polymerase by 2'-Fluoro-4'-Chloromethyl-Cytidine Triphosphate
- Structure of the Virulence Factor, SidC Reveals a Unique PI(4)P-Specific Binding Domain Essential for Its Targeting to the Bacterial Phagosome
- Activated Brain Endothelial Cells Cross-Present Malaria Antigen
- Fungal Morphology, Iron Homeostasis, and Lipid Metabolism Regulated by a GATA Transcription Factor in
- Peptidoglycan Branched Stem Peptides Contribute to Virulence by Inhibiting Pneumolysin Release
- A Macrophage Subversion Factor Is Shared by Intracellular and Extracellular Pathogens
- A Novel AT-Rich DNA Recognition Mechanism for Bacterial Xenogeneic Silencer MvaT
- Reovirus FAST Proteins Drive Pore Formation and Syncytiogenesis Using a Novel Helix-Loop-Helix Fusion-Inducing Lipid Packing Sensor
- The Role of ExoS in Dissemination of during Pneumonia
- IRF-5-Mediated Inflammation Limits CD8 T Cell Expansion by Inducing HIF-1α and Impairing Dendritic Cell Functions during Infection
- Discordant Impact of HLA on Viral Replicative Capacity and Disease Progression in Pediatric and Adult HIV Infection
- Crystal Structure of USP7 Ubiquitin-like Domains with an ICP0 Peptide Reveals a Novel Mechanism Used by Viral and Cellular Proteins to Target USP7
- HIV Latency Is Established Directly and Early in Both Resting and Activated Primary CD4 T Cells
- HPV16 Down-Regulates the Insulin-Like Growth Factor Binding Protein 2 to Promote Epithelial Invasion in Organotypic Cultures
- The νSaα Specific Lipoprotein Like Cluster () of . USA300 Contributes to Immune Stimulation and Invasion in Human Cells
- RSV-Induced H3K4 Demethylase KDM5B Leads to Regulation of Dendritic Cell-Derived Innate Cytokines and Exacerbates Pathogenesis
- Leukocidin A/B (LukAB) Kills Human Monocytes via Host NLRP3 and ASC when Extracellular, but Not Intracellular
- Border Patrol Gone Awry: Lung NKT Cell Activation by Exacerbates Tularemia-Like Disease
- The Curious Road from Basic Pathogen Research to Clinical Translation
- From Cell and Organismal Biology to Drugs
- Adenovirus Tales: From the Cell Surface to the Nuclear Pore Complex
- A 21st Century Perspective of Poliovirus Replication
- Is Development of a Vaccine against Feasible?
- Waterborne Viruses: A Barrier to Safe Drinking Water
- Battling Phages: How Bacteria Defend against Viral Attack
- Archaea in and on the Human Body: Health Implications and Future Directions
- Degradation of Human PDZ-Proteins by Human Alphapapillomaviruses Represents an Evolutionary Adaptation to a Novel Cellular Niche
- Natural Variants of the KPC-2 Carbapenemase have Evolved Increased Catalytic Efficiency for Ceftazidime Hydrolysis at the Cost of Enzyme Stability
- Potent Cell-Intrinsic Immune Responses in Dendritic Cells Facilitate HIV-1-Specific T Cell Immunity in HIV-1 Elite Controllers
- The Mammalian Cell Cycle Regulates Parvovirus Nuclear Capsid Assembly
- Host Reticulocytes Provide Metabolic Reservoirs That Can Be Exploited by Malaria Parasites
- The Proteome of the Isolated Containing Vacuole Reveals a Complex Trafficking Platform Enriched for Retromer Components
- NK-, NKT- and CD8-Derived IFNγ Drives Myeloid Cell Activation and Erythrophagocytosis, Resulting in Trypanosomosis-Associated Acute Anemia
- Successes and Challenges on the Road to Cure Hepatitis C
- BRCA1 Regulates IFI16 Mediated Nuclear Innate Sensing of Herpes Viral DNA and Subsequent Induction of the Innate Inflammasome and Interferon-β Responses
- A Structural and Functional Comparison Between Infectious and Non-Infectious Autocatalytic Recombinant PrP Conformers
- Phosphorylation of the Peptidoglycan Synthase PonA1 Governs the Rate of Polar Elongation in Mycobacteria
- Human Immunodeficiency Virus Type 1 Nef Inhibits Autophagy through Transcription Factor EB Sequestration
- Sequence-Specific Fidelity Alterations Associated with West Nile Virus Attenuation in Mosquitoes
- EBV BART MicroRNAs Target Multiple Pro-apoptotic Cellular Genes to Promote Epithelial Cell Survival
- Single-Cell and Single-Cycle Analysis of HIV-1 Replication
- TRIM32 Senses and Restricts Influenza A Virus by Ubiquitination of PB1 Polymerase
- The Herpes Simplex Virus Protein pUL31 Escorts Nucleocapsids to Sites of Nuclear Egress, a Process Coordinated by Its N-Terminal Domain
- Host Transcriptional Response to Influenza and Other Acute Respiratory Viral Infections – A Prospective Cohort Study
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- HIV Latency Is Established Directly and Early in Both Resting and Activated Primary CD4 T Cells
- Battling Phages: How Bacteria Defend against Viral Attack
- A 21st Century Perspective of Poliovirus Replication
- Adenovirus Tales: From the Cell Surface to the Nuclear Pore Complex
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



