-
Články
Reklama
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
ReklamaWaterborne Viruses: A Barrier to Safe Drinking Water
article has not abstract
Published in the journal: . PLoS Pathog 11(6): e32767. doi:10.1371/journal.ppat.1004867
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1004867Summary
article has not abstract
What is the Global Status of Access to Safe, Pathogen-Free Drinking Water?
Nearly 25% of the global population (1.8 billion people in 2012) is consuming fecally-contaminated water [1]. This water can contain bacteria, protozoa, and viruses that can cause a variety of diseases in humans, most notably gastroenteritis. The impact on public health is staggering. Unsafe water, inadequate sanitation, and poor hygiene are responsible for about 90% of diarrheal deaths worldwide [2]. Not surprisingly, diarrhea is the second leading cause of death for children under the age of five globally (1.2 million deaths in 2012) [2]. In addition to the human cost, the World Bank estimates that lack of access to safe water and sanitation results in a global economic loss of US$260 billion annually [3].
The lack of access to improved water disproportionally affects those living in poverty in rural, developing regions; however, even populations living in countries with state-of-the-art water and waste treatment facilities are prone to waterborne disease outbreaks. For example, there were at least 33 outbreaks associated with drinking water reported in the United States of America during 2009–2010 [4]. Regardless of the socioeconomic status of a country, illnesses due to contaminated drinking water are considered significantly underreported because people do not seek medical attention for self-limiting infections and because of the current limitations on clinical detection of virus infections [4].
Are Waterborne Viruses a Particular Concern?
It is well known that bacteria are major causes of diarrhea transmitted through unsafe drinking water. What is less appreciated are viruses in these same drinking water sources and their impact on human health. Water-transmitted viral pathogens that are classified as having a moderate to high health significance by the World Health Organization (WHO) include adenovirus, astrovirus, hepatitis A and E viruses, rotavirus, norovirus and other caliciviruses, and enteroviruses, including coxsackieviruses and polioviruses [5]. Also, viruses that are excreted through urine like polyomaviruses [5] and cytomegalovirus [6] can potentially be spread through water. Other viruses, such as influenza and coronaviruses, have been suggested as organisms that can be transmitted through drinking water, but evidence is inconclusive [5].
Most of the above viruses are most commonly associated with gastroenteritis, which can cause diarrhea as well as other symptoms including abdominal cramping, vomiting, and fever. It should be noted that some of these same viruses could also cause more severe illnesses including encephalitis, meningitis, myocarditis (enteroviruses), cancer (polyomavirus), and hepatitis (hepatitis A and E viruses) [5]. Hepatitis E virus can also cause a mortality rate of up to 25% in pregnant women [5]. Viral infections are usually self-limiting in healthy individuals. They can cause greater morbidity in children under the age of five, the elderly, immunocompromised people, and pregnant women. Waterborne virus-based diseases may be higher in developing regions, where there is widespread malnutrition and large populations of HIV-positive people. Regardless, there are few broad spectrum anti-viral drugs to treat these diseases.
Certainly, there is good cause for controlling these waterborne viruses. Rotavirus, for example, is the leading cause of severe acute diarrhea in children under the age of five globally, resulting in over half a million deaths annually [7]. The worldwide use of new rotavirus vaccines may make the removal of this virus from water supplies less of an issue in the future. Similarly, better coverage with vaccinations against hepatitis A and poliovirus would also greatly decrease the health risks of these viruses in drinking water.
Waterborne viruses differ in terms of their genome content and capsid proteins, but these viruses share several properties that make them of particular concern regarding the risk of disease outbreak associated with drinking water contamination. Several of these viruses have extremely low infectious doses; the probability of infection from exposure to one rotavirus particle is 31% [8]. Viruses are shed in feces in very high numbers even asymptomatically. For example, up to 1011 norovirus particles can be present per gram of stool [9]. In addition, non-enveloped viruses can persist in water for long periods of time [10]. When considering these characteristics, inadequate disinfection of fecally contaminated drinking water could easily lead to outbreaks of viral gastroenteritis from ingestion. Notably, drinking water can also transmit viruses via inhalation (e.g., showering) or contact with skin and eyes (e.g., swimming) causing respiratory and ocular infections.
What is the State of the Art for Control of Viruses in Water?
Water treatment utilities routinely assay for the presence of fecal coliforms in water supplies, but they do not assay for the presence of infectious viruses because it is either impossible or not feasible to detect or propagate infectious virus particles in a cost-efficient and timely manner. Despite these barriers, the United States Environmental Protection Agency (USEPA) is evaluating adenovirus, caliciviruses, enteroviruses, and hepatitis A virus for potential regulatory action [11]. The current US regulations require the removal or inactivation of 99.99% of enteric viruses by approved treatment techniques, but specific virus families are not individually regulated. These approved treatment techniques are based on bench scale studies where a specific virus is exposed to a disinfectant at various environmental conditions until reaching 99.99% inactivation. By studying a range of enteric viruses, regulations are decided for each disinfectant based on an appropriate dose to adequately inactivate the most resistant enteric virus studied. Utilities must apply an appropriate disinfectant dose to meet enteric virus regulations.
Common water treatment techniques worldwide include physically removing pathogens through conventional treatment and inactivating pathogens by applying ultraviolet light or chemical oxidants such as chlorine, chloramines, ozone, and chlorine dioxide. Since viruses are so small, conventional treatment, including filtration, is ineffective at physically removing viruses. The application of disinfectants highly depends on water chemistry and local regulations. Free chlorine (i.e., sum of hypochlorous acid and hypochlorite ion formed by dissolution and hydrolysis of chlorine gas in water) is the most commonly used disinfectant worldwide and has been used to disinfect water since the early 1900s [12]. Most viruses are inactivated by this strong oxidant. However, free chlorine treatment may produce regulated toxic disinfection by-products (DBPs), and it is ineffective to control Cryptosporidium, a protozoan that is transmitted in water and causes diarrhea [13,14]. Thus, some drinking water utilities are moving towards using monochloramine (i.e., formed by mixing chlorine and ammonia with the latter in slight excess) to control the formation of regulated toxic DBPs, and either monochromatic (~254 nm) or polychromatic (200–300 nm) ultraviolet (UV) light for controlling both DBP formation and Cryptosporidium contamination. Unfortunately, these changes in disinfection practice come at a cost to virus control. For example, while adenovirus is susceptible to inactivation by free chlorine, it is highly resistant to inactivation by both monochloramine and UV light [15]. Chlorine dioxide and ozone, also strong oxidants, are both effective at controlling viruses, but they have operational challenges, such as the need for on-site generation, and the production of DBPs, including chlorite from chlorine dioxide and bromate from ozone [12].
Regardless of the disinfectant applied at a drinking water utility, as the treated water travels from the treatment plant to the tap, cross-contamination can occur throughout the miles of water distribution infrastructure due to cavitation and accidental depressurization; therefore, the use of secondary disinfectants in distribution systems is required. Unfortunately, the only two disinfectants capable of maintaining a residual in the distribution system are free chlorine and monochloramine. Although free chlorine is a stronger disinfectant with respect to pathogen inactivation, monochloramine provides a more stable residual in distribution systems, and so both are utilized.
What are the Barriers toward Disinfecting Viruses in Drinking Water?
There are several barriers that prevent viruses in drinking water from being detected. From a technological standpoint, as compared to detecting fecal coliforms, virus propagation requires the use of tissue culture, a system that requires increased time, labor, expertise, and expensive equipment [8]. Furthermore, several of these viruses cannot be grown easily (adenovirus serotypes 40 and 41) or at all (human norovirus, hepatitis A virus) in cell culture. Consequently, traditional viral growth assays (plaque assays) are either unavailable or too lengthy in time to be practical for water treatment facilities. For example, a 10-day incubation period is required to detect replicating adenoviruses via plaque assays. While ELISA or qPCR-based technologies can be used to rapidly detect viral proteins or genomes, respectively, they do not distinguish infectious versus non-infectious viral particles. Integrated cell culture-PCR (ICC-PCR) reduces time requirements of traditional plaque assays and allows for infectious viruses to replicate in host cells, but it still employs the use of cell culture that is impractical at water treatment utilities [8]. Although there have been advances in concentrating viruses from large volumes of water [16], there has yet to be a rapid way to detect viable viruses.
In addition to these detection technology limitations, there is not one “silver bullet” water treatment that will inactivate all virus types independently of water quality. For example, human adenovirus is nearly five times more resistant to monochromatic (254 nm) UV inactivation compared to other enteric viruses [17]. While bacteriophages are often used as models to study enteric eukaryotic viruses, no bacteriophage studied to date accurately represents enteric virus behavior for all disinfectants. The scientific community does not yet understand why viruses have different profiles of resistance to different disinfectants. Regardless, from a regulatory standpoint, a major barrier is that not one disinfection method is effective against all viruses that can be applied to all water quality conditions.
Waterborne viruses have a range of genome types (e.g., DNA, RNA, linear, segmented) and capsid protein structures that contribute to their resistance or susceptibility to specific disinfectants. Many studies have determined reaction rates of disinfectants with amino acids and nucleotides, and this information can be useful in analyzing the mechanism of virus inactivation. However, these data are not always predictive due to the complex nature of viral capsid structures and secondary reactions [18]. It was initially thought that UV treatment of viruses would damage the viral genome as the mechanism for disinfection, whereas chemical oxidants like free chlorine would damage viral capsid proteins as a means for disinfection. UV light is known to be more reactive with nucleotides than amino acids; however, UV light can also damage viral proteins [19], suggesting that UV light has multiple mechanisms to disinfect viruses. For example, UV irradiation inactivates bacteriophage MS2 by both site-specific backbone cleavage of the major capsid protein resulting in genome injection inhibition and by damaging the RNA genome leading to genomic replication inhibition [19]. Whether this is true for other viruses is unknown. Chemical oxidants like free chlorine typically have higher reaction rates with amino acids, but have been shown to damage both viral proteins and genomes [19–21]. Regardless, determining precisely how a disinfectant damages and neutralizes specific viruses is necessary to fully understand the mechanisms of virus inactivation.
What is the Future of Waterborne Virus Research?
How does the scientific community overcome the technological and knowledge barriers? First, we must increase our fundamental understanding of how individual viruses become inactivated by disinfectants on a molecular level. This will require detailed studies of the individual components of a viral particle as well as the virion as a whole (Fig 1). The state-of-the-art technique is to correlate virus disinfection with a block in the virus replication cycle as a means to pinpoint what alteration of the protein capsid and/or the viral genome by the disinfectant results in loss of infectivity [19,22]. Technologies including mass spectrometry will afford the opportunity to understand, on an amino acid level, how a disinfectant modifies viral capsid proteins, and how this may result in a non-infectious virus particle [18]. Labeling techniques can also be utilized to determine if viral capsid proteins have undergone specific modifications, such as using a biotin hydrazide that forms covalent bonds with carbonyl groups [23], one of many oxidative products formed on amino acids. The scientific field is now beginning to capitalize on the sensitivity of qPCR or RT-qPCR to detect specific regions of a viral genome that are damaged by a disinfectant. Disinfectant-induced genomic damage can block viral DNA/RNA replication in host cells or viral genome amplification in PCR reactions [24]. However, PCR cannot elucidate what type of genome modification is caused by a disinfectant (e.g., pyrimidine dimers, crosslinking to proteins, chlorine-carbon bonds) or if the host cell is able to repair the lesions [17,24]. A variety of techniques will need to be used and developed to determine on a molecular level how a disinfectant neutralizes a virus and what stage of the replication cycle is blocked. This will likely vary for different viruses and for each disinfectant.
Fig. 1. Future of waterborne virus research to provide safe drinking water globally. 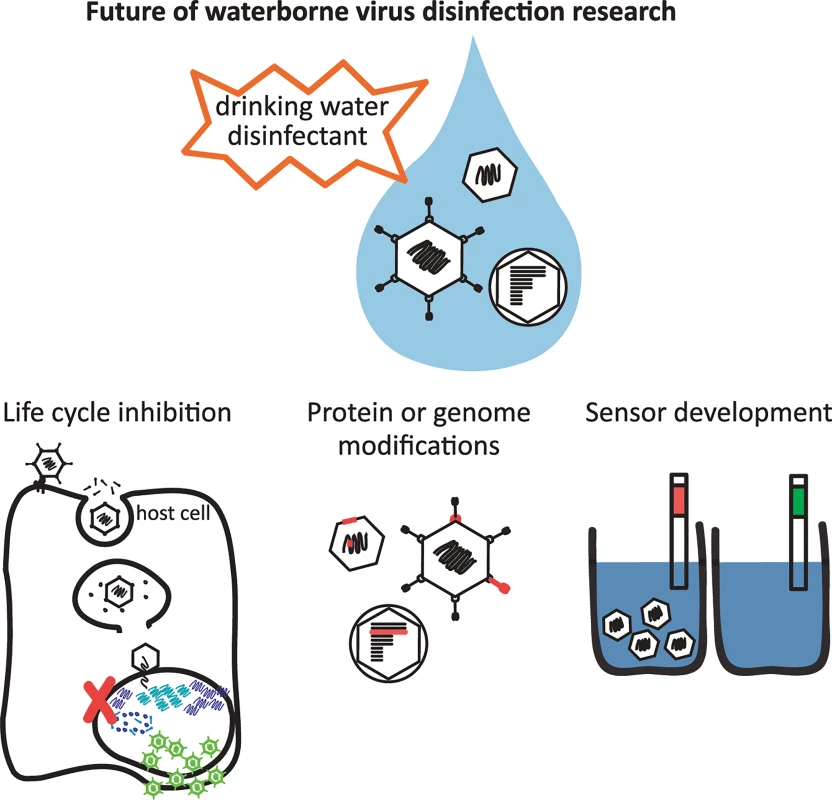
Gaining a better understanding of how viruses become inactivated by disinfectants requires detailed studies of many virus types and disinfectants to determine what stage of the virus replication cycle becomes blocked, and what modifications to the viral protein and/or genome lead to inactivation. The development of sensors to detect infectious viruses in drinking water will benefit from these studies and is also necessary to ensure safe drinking water. A grand challenge remains to design technology to rapidly distinguish infectious from damaged or portions of viral particles. Recently developed methods combine the antibody-based capture of viral capsids and subsequent qPCR amplification of genomes as a strategy to quantify structurally intact viruses in environmental samples [25]. This immunocapture-qPCR (IC-qPCR) technique is promising to detect infectious viral particles rather than fragments of viral nucleic acid and proteins using ELISA - or qPCR-based techniques alone. Another recent technique employs dyes (ethidium monoazide and propidium monoazide) that can penetrate damaged viral capsids and intercalate into viral nucleic acids inhibiting PCR amplification [26]. This method, like IC-qPCR, requires an intact viral capsid to detect an infectious virus. Unlike IC-qPCR, these dye-based assays rely on the mechanism of inactivation to be a leaky capsid structure. Results from these assays must be interpreted carefully because it is possible that a disinfectant modifies a capsid protein responsible for a key replication cycle event, like attachment, but does not affect the capsid structure or genome. Another new, promising technology to meet this grand challenge relies on the use of aptamers to selectively detect infectious virus particles. For this to occur, there is an in vitro selection from a large library of nucleic acids of aptamers that will selectively bind surface receptors of infectious viruses, but not those from non-infectious viral particles [27]. This technology has already been used in sensors to detect toxins and metals, and many aptamers have been selected for viral proteins and whole viruses for use in antiviral agents [28]. Indeed, an aptamer-based sensor can already distinguish between viable and heat-inactivated vaccinia virus, showing the promise of this technology for detecting other viruses [29]. Because aptamers are much more stable and cheaper than antibodies used in ELISA, such a technology would allow rapid detection of infectious viruses more cost-effectively and selectively.
Once we understand how disinfectants inactivate viruses, we can develop effective treatment protocols for water utilities and the next generations of sensors that rapidly detect and quantify infectious viruses in finished drinking water. Populations most vulnerable to unsafe drinking water live in rural and periurban areas of developing countries. Thus, scientists and engineers must design protocols and sensors to be cost-effective, rugged, and easy to use for these populations. Ultimately, the fundamental knowledge of how viruses become inactivated will ensure better control of viruses in drinking water, increasing access to safer drinking water globally.
Zdroje
1. WHO, UNICEF (2014) Progress on drinking water and sanitation: 2014 Update. Geneva, Switzerland: WHO Press.
2. UNICEF (2012) Pneumonia and diarrhoea: Tackling the deadliest diseases for the world's poorest children. New York, NY: Statistics and Monitoring Section - Division of Policy and Strategy.
3. WHO (2012) Global costs and benefits of drinking-water supply and sanitation interventions to reach the MDG target and universal coverage. Geneva, Switzerland: WHO Press, World Health Organization.
4. (2013) Center for Disase Control and Prevention. Morbidity and mortality weekly report. 62.
5. WHO (2011) Guidelines for drinking-water quality -4th ed. Geneva, Switzerland: WHO Press.
6. Cannon MJ, Hyde TB, Schmid DS (2011) Review of cytomegalovirus shedding in bodily fluids and relevance to congenital cytomegalovirus infection. Rev Med Virol 21 : 240–255. doi: 10.1002/rmv.695 21674676
7. WHO (2008) Global networks for surveillance of rotavirus gastroenteritis, 2001–2008. Weekly epidemiological record 47 : 421–428. 19024780
8. Fong TT, Lipp EK (2005) Enteric viruses of humans and animals in aquatic environments: health risks, detection, and potential water quality assessment tools. Microbiol Mol Biol Rev 69 : 357–371. 15944460
9. Hall AJ (2012) Noroviruses: the perfect human pathogens? J Infect Dis 205 : 1622–1624. doi: 10.1093/infdis/jis251 22573872
10. Reynolds KA, Mena KD, Gerba CP (2008) Risk of waterborne illness via drinking water in the United States. Rev Environ Contam Toxicol 192 : 117–158. 18020305
11. (2009) U.S. EPA. Drinking water contaminant candidate list 3: Final. Federal Register 74 : 51850–51862.
12. Crittenden JC (2005) MWH's water treatment: principles and design. Hoboken, N.J.: John Wiley and Sons.
13. (2006) U.S. EPA. 40 CFR Parts 9, 141, and 142. National primary drinking water regulations: stage 2 disinfectants and disinfection byproducts rule, final rule. Fed Regist 71 : 388–493.
14. (2006) U.S. EPA. 40 CFR Parts 9, 141, and 142. National primary drinking water regulations: Long term 2 enhanced surface water treatment rule. Federal Register 71 : 654–786.
15. Sirikanchana K, Shisler JL, Marinas BJ (2008) Effect of exposure to UV-C irradiation and monochloramine on adenovirus serotype 2 early protein expression and DNA replication. Appl Environ Microbiol 74 : 3774–3782. doi: 10.1128/AEM.02049-07 18424543
16. Ikner LA, Gerba CP, Bright KR (2012) Concentration and recovery of viruses from water: a comprehensive review. Food Environ Virol 4 : 41–67. doi: 10.1007/s12560-012-9080-2 23412811
17. Eischeid AC, Thurston JA, Linden KG (2011) UV Disinfection of Adenovirus: Present State of the Research and Future Directions. Critical Reviews in Environmental Science and Technology 41 : 1375–1396.
18. Wigginton KR, Kohn T (2012) Virus disinfection mechanisms: the role of virus composition, structure, and function. Curr Opin Virol 2 : 84–89. doi: 10.1016/j.coviro.2011.11.003 22440970
19. Wigginton KR, Pecson BM, Sigstam T, Bosshard F, Kohn T (2012) Virus inactivation mechanisms: impact of disinfectants on virus function and structural integrity. Environ Sci Technol 46 : 12069–12078. doi: 10.1021/es3029473 23098102
20. Sharma VK (2013) Halogenated species. Oxidation of amino acids, peptides, and proteins: Kinetics and mechanism. Hoboken, NJ: John Wiley & Sons, Inc. pp. 78–121.
21. Prutz WA (1996) Hypochlorous acid interactions with thiols, nucleotides, DNA, and other biological substrates. Arch Biochem Biophys 332 : 110–120. 8806715
22. Gall AM, Shisler JL, Marinas BJ (2015) Analysis of the Viral Replication Cycle of Adenovirus Serotype 2 after Inactivation by Free Chlorine. Environ Sci Technol.
23. Sano D, Pinto RM, Omura T, Bosch A (2010) Detection of oxidative damages on viral capsid protein for evaluating structural integrity and infectivity of human norovirus. Environ Sci Technol 44 : 808–812. doi: 10.1021/es9018964 20000802
24. Pecson BM, Ackermann M, Kohn T (2011) Framework for using quantitative PCR as a nonculture based method to estimate virus infectivity. Environ Sci Technol 45 : 2257–2263. doi: 10.1021/es103488e 21322644
25. Ogorzaly L, Bonot S, Moualij BE, Zorzi W, Cauchie HM (2013) Development of a quantitative immunocapture real-time PCR assay for detecting structurally intact adenoviral particles in water. J Virol Methods 194 : 235–241. doi: 10.1016/j.jviromet.2013.07.009 23850702
26. Leifels M, Jurzik L, Wilhelm M, Hamza IA (2015) Use of ethidium monoazide and propidium monoazide to determine viral infectivity upon inactivation by heat, UV - exposure and chlorine. Int J Hyg Environ Health.
27. Liu J, Cao Z, Lu Y (2009) Functional nucleic acid sensors. Chem Rev 109 : 1948–1998. doi: 10.1021/cr030183i 19301873
28. Gopinath SC (2007) Antiviral aptamers. Arch Virol 152 : 2137–2157. 17851732
29. Labib M, Zamay AS, Muharemagic D, Chechik AV, Bell JC, Berezovski M. (2012) Aptamer-based viability impedimetric sensor for viruses. Anal Chem 84 : 1813–1816. doi: 10.1021/ac203412m 22303883
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Clearance of Pneumococcal Colonization in Infants Is Delayed through Altered Macrophage TraffickingČlánek An Model of Latency and Reactivation of Varicella Zoster Virus in Human Stem Cell-Derived NeuronsČlánek Protective mAbs and Cross-Reactive mAbs Raised by Immunization with Engineered Marburg Virus GPsČlánek Specific Cell Targeting Therapy Bypasses Drug Resistance Mechanisms in African TrypanosomiasisČlánek Peptidoglycan Branched Stem Peptides Contribute to Virulence by Inhibiting Pneumolysin ReleaseČlánek HIV Latency Is Established Directly and Early in Both Resting and Activated Primary CD4 T CellsČlánek Sequence-Specific Fidelity Alterations Associated with West Nile Virus Attenuation in Mosquitoes
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2015 Číslo 6- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Introducing “Research Matters”
- Exploring Host–Pathogen Interactions through Biological Control
- Analysis of Bottlenecks in Experimental Models of Infection
- Expected and Unexpected Features of the Newly Discovered Bat Influenza A-like Viruses
- Clearance of Pneumococcal Colonization in Infants Is Delayed through Altered Macrophage Trafficking
- Recombinant Murine Gamma Herpesvirus 68 Carrying KSHV G Protein-Coupled Receptor Induces Angiogenic Lesions in Mice
- TRIM30α Is a Negative-Feedback Regulator of the Intracellular DNA and DNA Virus-Triggered Response by Targeting STING
- Targeting Human Transmission Biology for Malaria Elimination
- Two Cdc2 Kinase Genes with Distinct Functions in Vegetative and Infectious Hyphae in
- An Model of Latency and Reactivation of Varicella Zoster Virus in Human Stem Cell-Derived Neurons
- Protective mAbs and Cross-Reactive mAbs Raised by Immunization with Engineered Marburg Virus GPs
- Virulence Factors of Induce Both the Unfolded Protein and Integrated Stress Responses in Airway Epithelial Cells
- Peptide-MHC-I from Endogenous Antigen Outnumber Those from Exogenous Antigen, Irrespective of APC Phenotype or Activation
- Specific Cell Targeting Therapy Bypasses Drug Resistance Mechanisms in African Trypanosomiasis
- An Ultrasensitive Mechanism Regulates Influenza Virus-Induced Inflammation
- The Role of Human Transportation Networks in Mediating the Genetic Structure of Seasonal Influenza in the United States
- Host Delivery of Favorite Meals for Intracellular Pathogens
- Complement-Opsonized HIV-1 Overcomes Restriction in Dendritic Cells
- Inter-Seasonal Influenza is Characterized by Extended Virus Transmission and Persistence
- A Critical Role for CLSP2 in the Modulation of Antifungal Immune Response in Mosquitoes
- Twilight, a Novel Circadian-Regulated Gene, Integrates Phototropism with Nutrient and Redox Homeostasis during Fungal Development
- Surface-Associated Lipoproteins Link Virulence to Colitogenic Activity in IL-10-Deficient Mice Independent of Their Expression Levels
- Latent Membrane Protein LMP2A Impairs Recognition of EBV-Infected Cells by CD8+ T Cells
- Bank Vole Prion Protein As an Apparently Universal Substrate for RT-QuIC-Based Detection and Discrimination of Prion Strains
- Neuronal Subtype and Satellite Cell Tropism Are Determinants of Varicella-Zoster Virus Virulence in Human Dorsal Root Ganglia Xenografts
- Molecular Basis for the Selective Inhibition of Respiratory Syncytial Virus RNA Polymerase by 2'-Fluoro-4'-Chloromethyl-Cytidine Triphosphate
- Structure of the Virulence Factor, SidC Reveals a Unique PI(4)P-Specific Binding Domain Essential for Its Targeting to the Bacterial Phagosome
- Activated Brain Endothelial Cells Cross-Present Malaria Antigen
- Fungal Morphology, Iron Homeostasis, and Lipid Metabolism Regulated by a GATA Transcription Factor in
- Peptidoglycan Branched Stem Peptides Contribute to Virulence by Inhibiting Pneumolysin Release
- A Macrophage Subversion Factor Is Shared by Intracellular and Extracellular Pathogens
- A Novel AT-Rich DNA Recognition Mechanism for Bacterial Xenogeneic Silencer MvaT
- Reovirus FAST Proteins Drive Pore Formation and Syncytiogenesis Using a Novel Helix-Loop-Helix Fusion-Inducing Lipid Packing Sensor
- The Role of ExoS in Dissemination of during Pneumonia
- IRF-5-Mediated Inflammation Limits CD8 T Cell Expansion by Inducing HIF-1α and Impairing Dendritic Cell Functions during Infection
- Discordant Impact of HLA on Viral Replicative Capacity and Disease Progression in Pediatric and Adult HIV Infection
- Crystal Structure of USP7 Ubiquitin-like Domains with an ICP0 Peptide Reveals a Novel Mechanism Used by Viral and Cellular Proteins to Target USP7
- HIV Latency Is Established Directly and Early in Both Resting and Activated Primary CD4 T Cells
- HPV16 Down-Regulates the Insulin-Like Growth Factor Binding Protein 2 to Promote Epithelial Invasion in Organotypic Cultures
- The νSaα Specific Lipoprotein Like Cluster () of . USA300 Contributes to Immune Stimulation and Invasion in Human Cells
- RSV-Induced H3K4 Demethylase KDM5B Leads to Regulation of Dendritic Cell-Derived Innate Cytokines and Exacerbates Pathogenesis
- Leukocidin A/B (LukAB) Kills Human Monocytes via Host NLRP3 and ASC when Extracellular, but Not Intracellular
- Border Patrol Gone Awry: Lung NKT Cell Activation by Exacerbates Tularemia-Like Disease
- The Curious Road from Basic Pathogen Research to Clinical Translation
- From Cell and Organismal Biology to Drugs
- Adenovirus Tales: From the Cell Surface to the Nuclear Pore Complex
- A 21st Century Perspective of Poliovirus Replication
- Is Development of a Vaccine against Feasible?
- Waterborne Viruses: A Barrier to Safe Drinking Water
- Battling Phages: How Bacteria Defend against Viral Attack
- Archaea in and on the Human Body: Health Implications and Future Directions
- Degradation of Human PDZ-Proteins by Human Alphapapillomaviruses Represents an Evolutionary Adaptation to a Novel Cellular Niche
- Natural Variants of the KPC-2 Carbapenemase have Evolved Increased Catalytic Efficiency for Ceftazidime Hydrolysis at the Cost of Enzyme Stability
- Potent Cell-Intrinsic Immune Responses in Dendritic Cells Facilitate HIV-1-Specific T Cell Immunity in HIV-1 Elite Controllers
- The Mammalian Cell Cycle Regulates Parvovirus Nuclear Capsid Assembly
- Host Reticulocytes Provide Metabolic Reservoirs That Can Be Exploited by Malaria Parasites
- The Proteome of the Isolated Containing Vacuole Reveals a Complex Trafficking Platform Enriched for Retromer Components
- NK-, NKT- and CD8-Derived IFNγ Drives Myeloid Cell Activation and Erythrophagocytosis, Resulting in Trypanosomosis-Associated Acute Anemia
- Successes and Challenges on the Road to Cure Hepatitis C
- BRCA1 Regulates IFI16 Mediated Nuclear Innate Sensing of Herpes Viral DNA and Subsequent Induction of the Innate Inflammasome and Interferon-β Responses
- A Structural and Functional Comparison Between Infectious and Non-Infectious Autocatalytic Recombinant PrP Conformers
- Phosphorylation of the Peptidoglycan Synthase PonA1 Governs the Rate of Polar Elongation in Mycobacteria
- Human Immunodeficiency Virus Type 1 Nef Inhibits Autophagy through Transcription Factor EB Sequestration
- Sequence-Specific Fidelity Alterations Associated with West Nile Virus Attenuation in Mosquitoes
- EBV BART MicroRNAs Target Multiple Pro-apoptotic Cellular Genes to Promote Epithelial Cell Survival
- Single-Cell and Single-Cycle Analysis of HIV-1 Replication
- TRIM32 Senses and Restricts Influenza A Virus by Ubiquitination of PB1 Polymerase
- The Herpes Simplex Virus Protein pUL31 Escorts Nucleocapsids to Sites of Nuclear Egress, a Process Coordinated by Its N-Terminal Domain
- Host Transcriptional Response to Influenza and Other Acute Respiratory Viral Infections – A Prospective Cohort Study
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- HIV Latency Is Established Directly and Early in Both Resting and Activated Primary CD4 T Cells
- Battling Phages: How Bacteria Defend against Viral Attack
- A 21st Century Perspective of Poliovirus Replication
- Adenovirus Tales: From the Cell Surface to the Nuclear Pore Complex
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



