-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaTwo Frizzled Planar Cell Polarity Signals in the Wing Are Differentially Organized by the Fat/Dachsous Pathway
The regular array of distally pointing hairs on the mature Drosophila wing is evidence for the fine control of Planar Cell Polarity (PCP) during wing development. Normal wing PCP requires both the Frizzled (Fz) PCP pathway and the Fat/Dachsous (Ft/Ds) pathway, although the functional relationship between these pathways remains under debate. There is strong evidence that the Fz PCP pathway signals twice during wing development, and we have previously presented a Bidirectional-Biphasic Fz PCP signaling model which proposes that the Early and Late Fz PCP signals are in different directions and employ different isoforms of the Prickle protein. The goal of this study was to investigate the role of the Ft/Ds pathway in the context of our Fz PCP signaling model. Our results allow us to draw the following conclusions: (1) The Early Fz PCP signals are in opposing directions in the anterior and posterior wing and converge precisely at the site of the L3 wing vein. (2) Increased or decreased expression of Ft/Ds pathway genes can alter the direction of the Early Fz PCP signal without affecting the Late Fz PCP signal. (3) Lowfat, a Ft/Ds pathway regulator, is required for the normal orientation of the Early Fz PCP signal but not the Late Fz PCP signal. (4) At the time of the Early Fz PCP signal there are symmetric gradients of dachsous (ds) expression centered on the L3 wing vein, suggesting Ds activity gradients may orient the Fz signal. (5) Localized knockdown or over-expression of Ft/Ds pathway genes shows that boundaries/gradients of Ft/Ds pathway gene expression can redirect the Early Fz PCP signal specifically. (6) Altering the timing of ds knockdown during wing development can separate the role of the Ft/Ds pathway in wing morphogenesis from its role in Early Fz PCP signaling.
Published in the journal: . PLoS Genet 7(2): e32767. doi:10.1371/journal.pgen.1001305
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1001305Summary
The regular array of distally pointing hairs on the mature Drosophila wing is evidence for the fine control of Planar Cell Polarity (PCP) during wing development. Normal wing PCP requires both the Frizzled (Fz) PCP pathway and the Fat/Dachsous (Ft/Ds) pathway, although the functional relationship between these pathways remains under debate. There is strong evidence that the Fz PCP pathway signals twice during wing development, and we have previously presented a Bidirectional-Biphasic Fz PCP signaling model which proposes that the Early and Late Fz PCP signals are in different directions and employ different isoforms of the Prickle protein. The goal of this study was to investigate the role of the Ft/Ds pathway in the context of our Fz PCP signaling model. Our results allow us to draw the following conclusions: (1) The Early Fz PCP signals are in opposing directions in the anterior and posterior wing and converge precisely at the site of the L3 wing vein. (2) Increased or decreased expression of Ft/Ds pathway genes can alter the direction of the Early Fz PCP signal without affecting the Late Fz PCP signal. (3) Lowfat, a Ft/Ds pathway regulator, is required for the normal orientation of the Early Fz PCP signal but not the Late Fz PCP signal. (4) At the time of the Early Fz PCP signal there are symmetric gradients of dachsous (ds) expression centered on the L3 wing vein, suggesting Ds activity gradients may orient the Fz signal. (5) Localized knockdown or over-expression of Ft/Ds pathway genes shows that boundaries/gradients of Ft/Ds pathway gene expression can redirect the Early Fz PCP signal specifically. (6) Altering the timing of ds knockdown during wing development can separate the role of the Ft/Ds pathway in wing morphogenesis from its role in Early Fz PCP signaling.
Introduction
Planar Cell Polarity (PCP) describes the orientation of a cell within the plane of an epithelium. A primary model for studying the genetic control of PCP has been the organization of an array of cell hairs that point toward the distal tip of the Drosophila wing [1]. Two signaling pathways are known to control Drosophila wing PCP, the Frizzled (Fz) PCP pathway and the Fat/Dachsous (Ft/Ds) pathway [2], although the functional relationship between these two pathways remains subject to debate [3]. One model, the Tree-Amonlirdviman model, proposes a tiered structure in which long-range gradients of Ft/Ds signaling provide global polarity information that controls the direction of a local Fz PCP signal [4]. In the case of the wing, the proximal expression of Ds and distal expression of Four-jointed (Fj) are proposed to generate opposing activity gradients along the proximal-distal (P-D) wing axis that control the direction of the Fz PCP signal [5], [6]. In contrast, studies in the Drosophila abdomen have led to an alternative ‘Two Pathway’ model in which the Ft/Ds and Fz PCP pathways function independently to organize PCP [7]. The resolution of these distinct models is important since both Fz PCP and Ft/Ds pathways have also been shown to be critical for PCP in vertebrate development [8], [9] and are implicated in human disease [9]–[11].
In an earlier paper we showed that, in addition to organizing wing hair polarity, the Fz PCP pathway is required for the integrity and orientation of cuticle ridges that traverse the adult wing membrane [12]. However, although wing hairs have a common orientation across the wing, ridges are aligned with the anteroposterior (A-P) axis in the anterior wing and with the P-D axis in the posterior wing. Consequently, hair and ridge orientation are approximately orthogonal in the anterior wing, but are closely matched in the posterior wing. This presents the problem of how Fz PCP signaling can lead to these two distinct outcomes in anterior and posterior wing cells. Data from our work, and from other labs, has led us to propose a Bidirectional-Biphasic (Bid-Bip) model in which two distinct Fz PCP signaling events occur along different axes of the wing (Figure 1 and [12]). In the model, there is an Early Fz PCP signal aligned with the A-P axis that is approximately symmetric in the anterior and posterior wing. This is followed by a Late Fz PCP signal aligned with the P-D axis. For the model, the direction of Fz PCP signaling is defined as the hair polarity that would be specified by the signal.
Fig. 1. A Bidirectional-Biphasic (Bid-Bip) model for Fz PCP signaling in the Drosophila wing. 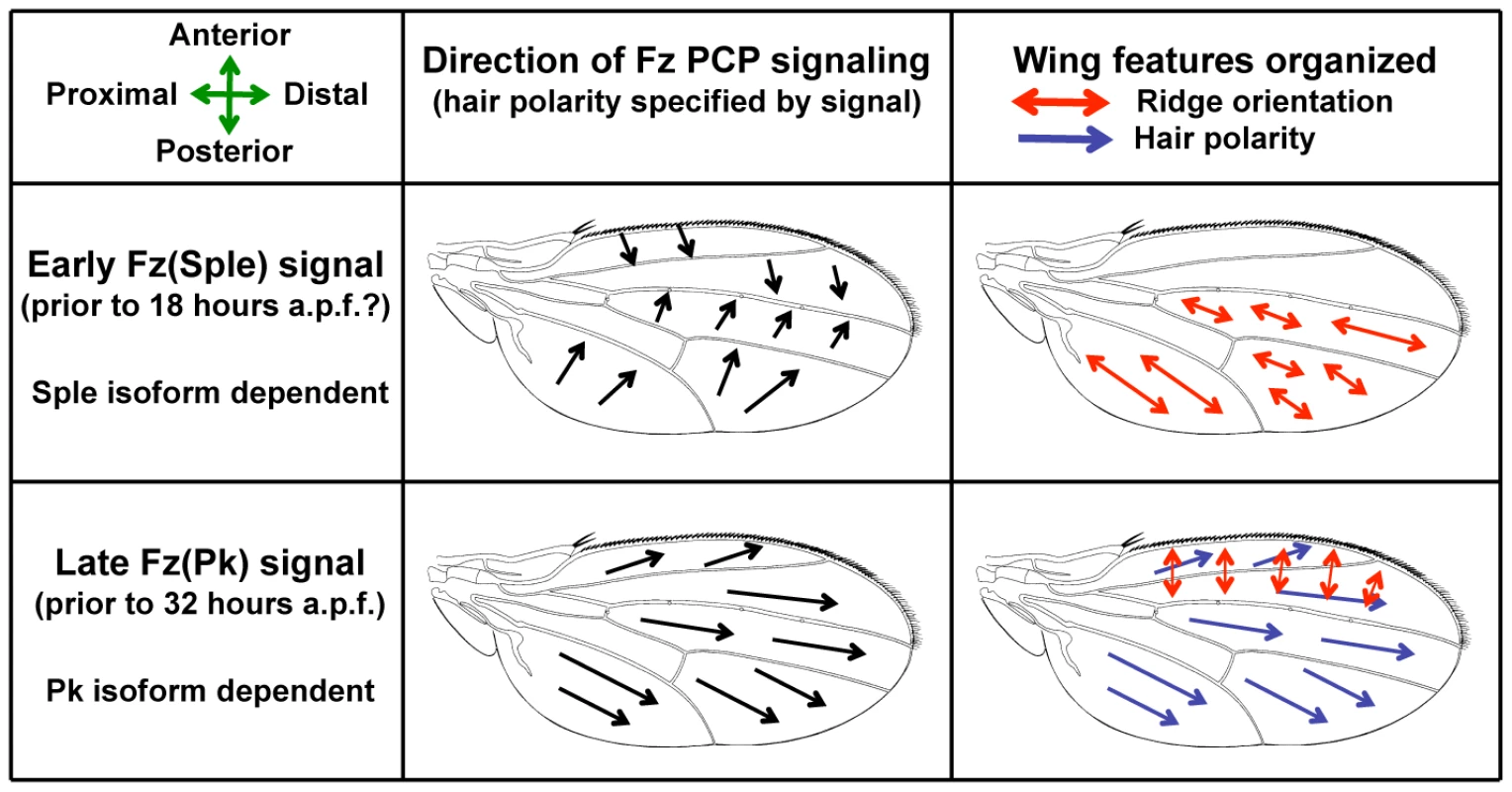
The model proposes two distinct Fz PCP signals that differ both in direction and in use of the Prickle protein isoforms, Pk and Sple. An Early Fz(Sple) signal along the A-P axis organizes posterior ridge orientation. A Late Fz(Pk) signal along the P-D axis organizes anterior ridge orientation and hair polarity. (a.p.f. = after pupal formation). The concept of two Fz PCP signaling events during wing development is not novel; the existence of a distinct Early Fz PCP signal around 18 hours after pupal formation (a.p.f.) has been well established by work in the Strutt lab [13], [14]. However, the notion that the Early Fz PCP signal is oriented along the A-P axis appears to be a novel feature of our Bid-Bip model [12]. Previous evidence for Fz PCP signaling along the A-P axis of the wing has come from the Adler lab. Adler showed that, in addition to the distal cell non-autonomy associated with fz mutant wing clones [15], fz clones in the anterior wing show cell non-autonomy posterior to the clone, whilst posterior fz clones show cell non-autonomy anterior to the clone [16]. Moreover, as well as the proximal cell non-autonomy associated with mutant clones of the Fz PCP pathway gene Van Gogh/strabismus (Vang/stbm) [17], anterior Vang/stbm clones show anterior cell non-autonomy and posterior Vang/stbm clones show posterior cell non-autonomy [16]. Adler's data argue for Fz PCP signaling along the A-P axis of the wing that has an opposite orientation in the anterior and posterior wing. This matches the description of our proposed Early Fz PCP signal (Figure 1).
Key to our Bid-Bip model is the notion that different features of the wing are organized by the two Fz PCP signaling events. The model proposes that posterior ridges are organized by the Early Fz PCP signal, while anterior ridges and wing hairs are organized by the Late Fz PCP signal (Figure 1). This is supported by our finding that early over-expression (e.g. 10 hours a.p.f.) of the Sple isoform of the PCP protein Prickle reorients hairs and ridges in both the anterior and posterior wing, whereas late Sple over-expression (e.g. 19 hours a.p.f.) reorients hairs and anterior ridges, but not posterior ridges [12]. This observation suggests that posterior ridges are specified earlier than anterior ridges. Consequently, the Bid-Bip model proposes that ridges and hairs are organized by the same Fz PCP signaling event in the anterior wing, but by different (and differently oriented) Fz PCP signaling events in the posterior wing. Thus, the model accounts for the differing relationships between ridge and hair orientation observed in the anterior and posterior wing. The model also implies that orthogonal hair and ridge orientation is the normal outcome of a single Fz PCP signaling event in the wing.
One further feature of our Bid-Bip model is the proposal that the Early and Late Fz PCP signals differ in the use of the Prickle protein isoforms Pk and Sple within the Fz PCP pathway. Pk and Sple share a C-terminus containing a PET domain and three LIM domains, but the 13 N-terminal amino acids in Pk are replaced by 349 N-terminal amino acids in Sple [18]. In the model, the Early Fz PCP signal employs the Sple isoform and the Late Fz PCP signal employs the Pk isoform. (For this reason, we will refer to the Early Fz PCP signal as Fz(Sple) and the Late Fz PCP signal as Fz(Pk) in this paper.) This agrees with previous work from Strutt that showed the Pk isoform is only required for Late Fz PCP signaling [13]. Consequently, a prediction of the Bid-Bip model is that loss of Pk isoform activity (i.e. a pkpk mutant) blocks Late Fz PCP signaling and so only the Early Fz PCP signal occurs. Consistent with this prediction, there is a regular, approximately orthogonal, relationship between hair and ridge orientation across the entire pkpk mutant wing suggesting that only a single Fz PCP signaling event (i.e. Fz(Sple)) has occurred [12]. Moreover, Adler has shown that in a pkpk mutant wing, the cell non-autonomy of fz− clones is primarily posterior to anterior clones and anterior to posterior clones [16], suggesting that Fz PCP signaling is principally along the A-P axis and in opposite directions in the anterior and posterior wing. This fits our model's proposal that only the Early Fz PCP signal is active in a pkpk mutant wing (Figure 1).
Our Bid-Bip Fz PCP signaling model differs significantly from previous models of PCP in the Drosophila wing. Therefore, it provides an alternative template for an evaluation of the role of the Ft/Ds pathway in wing PCP. The work presented in this paper addresses the relationship of the Ft/Ds and Fz PCP pathways in the context of our model and concludes that a primary role of the Ft/Ds pathway in wing PCP is to control the direction of the Early Fz(Sple) signal.
Results
A PCP discontinuity in the Drosophila wing
Membrane ridge orientation differs between the anterior and posterior of the wild-type Drosophila wing [12]. The boundary between these two regions lies in the vicinity of the L3 vein, but is not possible to pinpoint on wild-type wings, as ridge orientation is difficult to determine adjacent to wing veins. Homozygous rhove-1, vn1 wings lack wing veins L2-5 and display altered wing shape [19]. Using our Cuticle Refraction Microscopy (CRM) technique [12] in conjunction with conventional light microscopy, we find that rhove-1, vn1 wings retain wild-type hair polarity and ridge orientation (compare Figure 2A with Figure 3A). In the absence of veins on the rhove-1, vn1 wing, it becomes clear that the boundary between anterior A-P and posterior P-D ridge orientation can be mapped to a narrow region, about 2–3 cells wide, that forms an approximately straight line along the P-D axis of the wing (yellow shaded region in Figure 2A and 2B). Our ability to finely map this region implies an abrupt change in PCP on the wing and for this reason we refer to it as a ‘PCP Discontinuity’ (PCP-D). The absence of veins and unusual wing morphology of the rhove-1, vn1 wing make the location of the PCP-D difficult to pinpoint. To overcome this problem, we over-expressed Argos uniformly during dorsal wing development (MS1096-gal4; UAS-argos). The Argos protein is a negative regulator of EGF signaling and Argos over-expression in the dorsal wing antagonizes longitudinal vein development resulting in variable loss of dorsal longitudinal veins including L3 (Figure 2C and [20]). These wings reveal that the discontinuity in ridge orientation (i.e. the PCP-D) maps to the normal location of the L3 vein (Figure 2D).
Fig. 2. A PCP discontinuity (PCP-D) on the Drosophila wing. 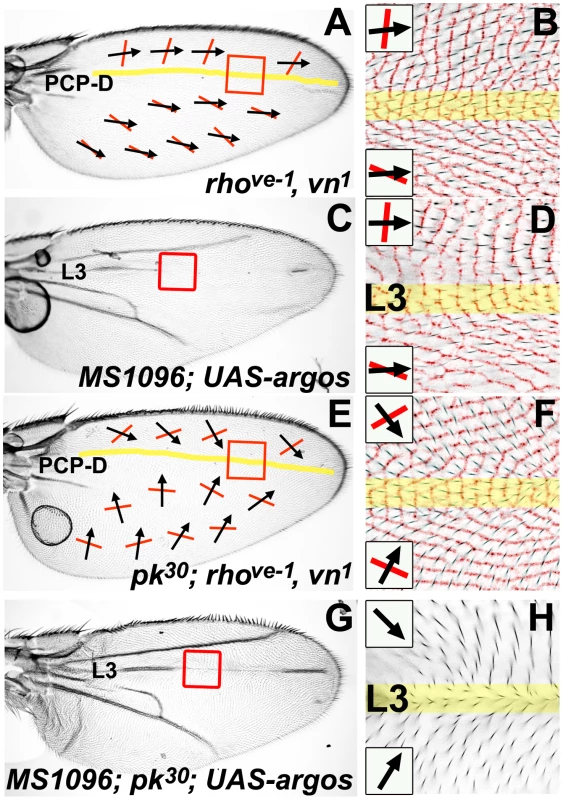
All micrographs are of the female dorsal wing surface. Black arrows indicate local hair polarity; red lines indicate local ridge orientation. Yellow shading represents the approximate location of a discontinuity in PCP (the PCP-D). Panels B, D, and F show light micrographs of hair polarity overlaid on an inverted and colorized (red) CRM image of ridge orientation in the same region. (A) rhove-1, vn1 homozygous wing. (B) Detail of rhove-1, vn1 homozygous wing (red-boxed region in (A)). (C) MS1096-gal4; UAS-argos wing. (D) Detail of MS1096-gal4; UAS-argos wing (red-boxed region in (C)). (E) pkpk30; rhove-1, vn1 homozygous wing. (F) Detail of pkpk30; rhove-1, vn1 wing (red-boxed region in (E)). (G) MS1096-gal4; pkpk30/pkpk30; UAS-argos wing. (H) Detail of MS1096-gal4; pkpk30/pkpk30; UAS-argos wing (red-boxed region in (G)) showing hair polarity. Fig. 3. Reduced Ft/Ds pathway gene activity alters posterior ridge orientation without affecting hair polarity. 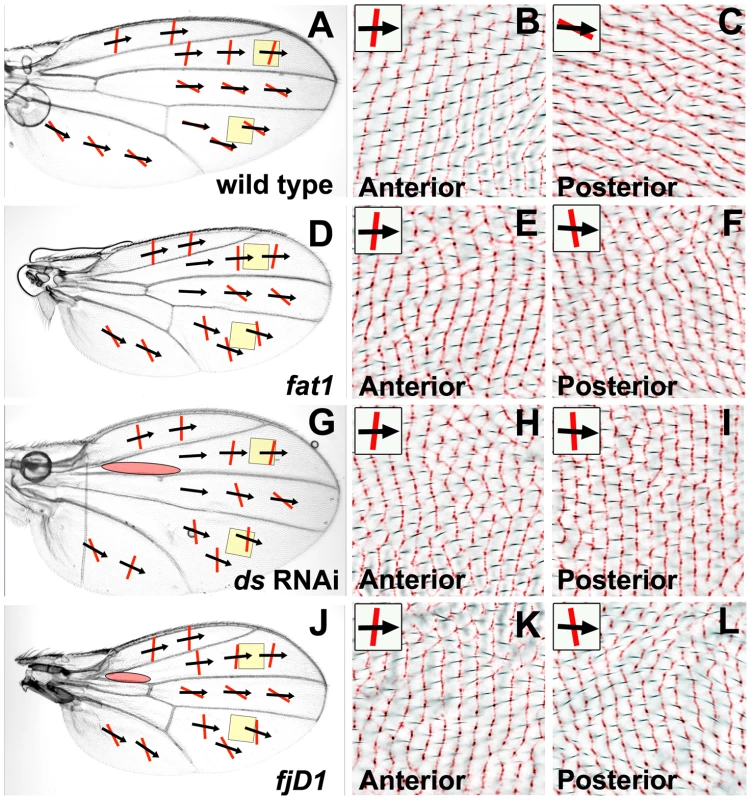
All micrographs are of the female dorsal wing surface. Black arrows indicate local hair polarity; red lines indicate local ridge orientation. Panels B, C, E, F, H, I, K and L show light micrographs of hair polarity overlaid on an inverted and colorized (red) CRM image of ridge orientation in the same region. (A) Wild-type (Canton S) wing. (B) Detail of anterior wild-type wing (anterior yellow shaded region in (A)). (C) Detail of posterior wild-type wing (posterior yellow shaded region in (A)). (D) ft1 homozygous wing. (E) Detail of anterior ft1 homozygous wing (anterior yellow shaded region in (D)). (F) Detail of posterior ft1 homozygous wing (posterior yellow shaded region in (D)). (G) MS109-Gal4; UAS-ds(IR) wing. (H) Detail of anterior MS109-Gal4; UAS-ds(IR) wing (anterior yellow shaded region in (G)). (I) Detail of posterior MS109-Gal4; UAS-ds(IR) wing (posterior yellow shaded region in (G)). (J) fjD1 homozygous wing. (K) Detail of anterior fjD1 homozygous wing (anterior yellow shaded region in (J)). (L) Detail of posterior fjD1 homozygous wing (posterior yellow shaded region in (J)). According to our Bid-Bip Fz PCP signaling model (Figure 1), loss of Pk isoform activity (i.e. a pkpk mutant) inactivates the Late Fz(Pk) signal, but not the Early Fz(Sple) signal [12]. Therefore, since only Early Fz(Sple) signaling is active on a pkpk mutant wing, the pkpk mutant hair polarity pattern should reflect the direction of the Fz(Sple) signal. At first glance, the intricate swirling hair patterns observed on a pkpk wing appear an improbable signaling output [12], [18], [21], [22]. However, since hair whorls, and other abrupt changes in hair polarity, on a pkpk wing are normally adjacent to wing veins, we hypothesized that an alternate hair pattern might appear in the absence of vein differentiation. To test this, we generated pkpk30; rhove-1, vn1 homozygous flies, which lack wing veins L2-5 and have no Pk isoform activity. We found that these wings lack most of the abrupt changes in hair polarity normally found on a pkpk mutant wing (see, for example, Figure S1). However, the approximately orthogonal relationship between hair polarity and ridge orientation, seen in both the anterior and posterior wing of a pkpk mutant wing [12], is maintained (Figure 2E and 2F). On a pkpk30; rhove-1, vn1 wing, anterior hairs consistently have a posterior component to their polarity and posterior hairs have an anterior component to their polarity. The boundary between anterior and posterior pointing hairs can be mapped to an approximately straight line, around 2–3 cells wide, along the P-D axis (yellow shaded region in Figure 2E and 2F). This position is also associated with a discontinuity in ridge orientation, which changes abruptly in this region (Figure 2F). To localize this PCP discontinuity, we over-expressed Argos in a pkpk mutant wing (MS1096-gal4; pkpk30/pkpk30; UAS-argos), to induce partial loss of dorsal longitudinal veins (Figure 2G). On these wings, it is clear that the discontinuity in hair and ridge orientation maps to the site of the L3 vein (Figure 2H).
In summary, we have identified a PCP discontinuity (PCP-D) in the Drosophila wing that maps to the site of the L3 wing vein (although physical differentiation of the L3 vein is not required for the formation of the PCP-D). In wild-type wings, the PCP-D represents a discontinuity in ridge orientation, but not hair polarity. However, in wings lacking Pk isoform activity, the PCP-D represents a discontinuity in both ridge orientation and hair polarity. According to our Bid-Bip Fz PCP signaling model (Figure 1), only Early Fz(Sple) signaling is active in a pkpk mutant wing, therefore we conclude that there is a discontinuity in Fz(Sple) signaling at the site of the L3 wing vein. We also note that, although hair polarity in a wild-type wing is not disrupted by the removal of wing veins, pkpk mutant hair polarity is significantly modified by wing vein removal (see Figure S1). This suggests that the output of the Early Fz(Sple) signal is significantly influenced by wing vein differentiation, whereas the Late Fz(Pk) signal is not. This observation is not consistent with a previous report which concluded that altered wing vein formation does not affect the pkpk mutant wing hair phenotype [21]. However, we note that the genes we have used to alter vein formation (rho, ve, argos) are all components of the EGF signaling pathway, whereas the genes used in the early work (knirpsri, cubitus interruptus and plexus) are not EGF components. This raises the possibility that it is altered EGF signaling that modifies the Early Fz(PCP) signal rather than the physical differentiation of wing veins.
Reduced activity of Ft/Ds pathway genes alters ridge orientation in the posterior wing without affecting hair polarity
The hypomorphic fat1 (ft1) mutant allele is homozygous viable and affects wing shape, but not hair polarity (Figure 3D). We mapped ridge orientation on a ft1 homozygous mutant wing using our CRM technique [12], and found that ridges in distal regions of the posterior wing show an A-P orientation, in contrast to the normal P-D orientation (compare Figure 3F with Figure 3C). In contrast, anterior ridges on the ft1 wing retain the normal A-P orientation (compare Figure 3E with Figure 3B). The abnormal wing morphology of viable dachsous (ds) mutants makes analysis of wing ridges by our CRM technique challenging, although we were able to confirm that the D region (between veins L4 and L5) of a dsUA071/ds05142 heterozygous wing, retains wild-type hair polarity, but has primarily A-P ridges (data not shown). However, we were largely able to overcome this problem by expressing gene-specific RNAi uniformly in the developing dorsal wing. Uniform expression of ds RNAi (VDRC transformant 36219GD [23]) in the dorsal wing (MS1096-gal4; UAS-ds(IR)) alters wing shape and disrupts crossveins (Figure 3G), but produces only localized hair polarity changes in the proximal wing (red shaded oval in Figure 3G). CRM analysis shows that uniform ds RNAi expression alters posterior ridges to a more A-P orientation (Figure 3I), but does not affect anterior ridges (Figure 3H). Uniform expression of ft RNAi (VDRC transformant 9396GD [23]) in the dorsal wing (MS1096-gal4; UAS-ft(IR)) results in a very similar wing phenotype to ds RNAi expression (data not shown). The control of PCP by the Ft/Ds pathway also requires the four-jointed gene [24], and we have mapped ridge orientation on wings homozygous for the amorphic fjD1 allele. Homozygous fjD1 wings have altered shape, but hair polarity is disrupted in only a small proximal region (red shaded oval in Figure 3J), the same region affected by uniform ft or ds knockdown. We found that posterior ridges on fjD1 homozygous wings also have a more A-P orientation than wild-type (Figure 3L), but anterior ridges are unchanged (Figure 3K).
The phenotypes generated using the VDRC ft and ds RNAi lines are unlikely to result from off-target RNAi activity as they phenocopy the established mutant phenotype of these genes. In addition, we were able to reproduce these phenotypes using independent ft (JF03245) and ds (JF02842) RNAi lines from the TRiP project (Transgenic RNAi Project, Harvard Medical School) in combination with the same Gal4 driver (data not shown). Curiously, uniform fj RNAi expression using either the VDRC (transformant 6774GD [23]) or TRiP (JF02843) stocks failed to give the characteristic fj mutant wing morphology and so these stocks were excluded from this study.
Our findings show that reduced activity of the Ft/Ds pathway genes ft, ds and fj alter ridge orientation in the distal posterior wing to a more A-P orientation, without affecting hair polarity in the same region or anterior ridge orientation. Since our Bid-Bip model proposes that posterior ridges are organized by the Early Fz(Sple) signal whereas anterior ridges and wing hairs are organized by the Late Fz(Pk) signal (see Figure 1), these results suggest that reduced activity of Ft/Ds pathway genes can alter the Early Fz(Sple) without affecting the Late Fz(Pk) signal. The fact that posterior ridges still form when Ft/Ds pathway activity is reduced suggests that the role of the Ft/Ds pathway is not to specify posterior ridges, but to direct ridge orientation, presumably by controlling the direction of the Early Fz(Sple) signal.
Reduced activity of Ft/Ds pathway genes modifies pkpk wing hair polarity to a more distal orientation
Our analysis of wing ridge phenotypes led us to conclude that reduced Ft/Ds pathway activity can affect the direction of the Early Fz(Sple) signal without altering the Late Fz(Pk) signal. Since the Late Fz(Pk) signal is inactivated in a pkpk mutant wing, the pkpk hair polarity phenotype should reflect the direction of the Early Fz(Sple) signal [12]. Consequently, if reducing Ft/Ds pathway activity affects the orientation of the Early Fz(Sple) signal, we predict that it should significantly modify the pkpk mutant wing hair phenotype. This turns out to be the case. For example, although a ft1 homozygous wing has wild-type hair polarity, the pkpk30 hair polarity phenotype is substantially modified in a ft1, pkpk30 double mutant wing (compare Figure 4A with 4D). Specifically, in comparison to a pkpk30 homozygote, ft1, pkpk30 hair polarity is more distal in both the anterior wing (compare Figure 4B with 4E) and in distal regions of the posterior wing (compare Figure 4C with 4F). We see a similar modification of the pkpk hair phenotype when driving uniform ft RNAi expression (VDRC transformant 9396GD) in a pkpk mutant wing (MS1096-gal4; pk30, UAS-ft(IR)/pk30), but with more extensive regions of distal hair polarity in the posterior wing and an anterior component to anterior hair polarity (data not shown). Driving uniform expression of ds RNAi (VDRC transformant 36219GD) in the dorsal wing of a pkpk mutant also modifies the pkpk hair phenotype to a more distal polarity in the anterior and distal posterior wing (Figure 4G, 4H and 4I). We also generated flies homozygous for both a pkpk allele and for an amorphic allele of lowfat (lft), a recently identified modulator of Ft/Ds signaling [25]. lftTG2 homozygous wings display altered wing morphology and aberrant posterior ridges, but wild-type hair polarity ([25] and data not shown). In lftTG2, pkpk homozygous wings, the pkpk hair phenotype is modified to a more distal polarity in the anterior and distal posterior wing (Figure 4J, 4K and 4L), in a similar manner to when ft or ds activity is reduced. Hair polarity on fjD1, pkpk30 homozygous wings is also more distal than the pkpk30 phenotype. However, this effect is less than observed for reduced ft, ds or lft activity and appears region specific. For example, hair polarity in the A region (anterior to the L2 vein) of a fjD1, pkpk30 wing is entirely distal, but in the B region (between the L2 and L3 vein) retains a significant posterior component and so is closer to the pkpk30 phenotype (data not shown).
Fig. 4. Reduced Ft/Ds pathway gene activity modifies the pkpk hair polarity phenotype. 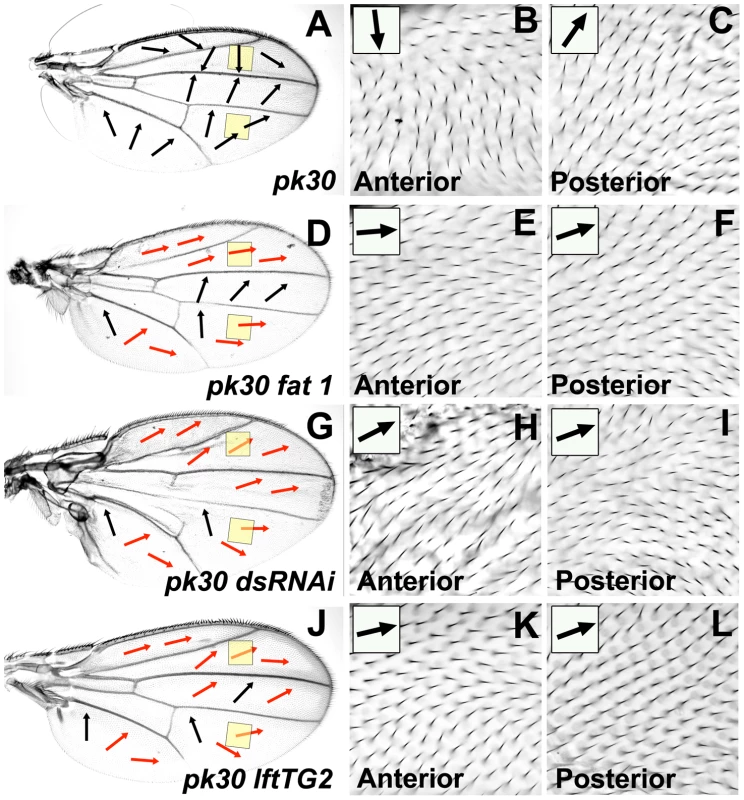
All micrographs are of the female dorsal wing surface. Arrows indicate local hair polarity, red arrows indicate where local hair polarity differs from that seen on a pkpk30 homozygous wing. (A) pkpk30 homozygous wing. (B) Detail of anterior pkpk30 homozygous wing (anterior yellow shaded region in (A)). (C) Detail of posterior pkpk30 homozygous wing (posterior yellow shaded region in (A)). (D) ft1, pkpk30 homozygous wing. (E) Detail of anterior ft1, pkpk30 homozygous wing (anterior yellow shaded region in (D)). (F) Detail of posterior ft1, pkpk30 homozygous wing (posterior yellow shaded region in (D)). (G) MS1096-gal4; UAS-ds(IR), pkpk30/pkpk30 wing. (H) Detail of anterior MS1096-gal4; UAS-ds(IR), pkpk30/pkpk30 wing (anterior yellow shaded region in (G)). (I) Detail of posterior MS1096-gal4; UAS-ds(IR), pkpk30/pkpk30 wing (posterior yellow shaded region in (G)). (J) lftTG2, pkpk30 homozygous wing. (K) Detail of anterior lftTG2, pkpk30 homozygous wing (anterior yellow shaded region in (J)). (I) Detail of posterior lftTG2, pkpk30 homozygous wing (posterior yellow shaded region in (J)). These findings show that reduced activity of the Ft/Ds pathway genes ft, ds, fj and lft modify the pkpk30 hair polarity phenotype to a more distal polarity in the anterior wing and distal regions of the posterior wing. This is despite the fact that hair polarity in these regions is not affected by the reduced activity of the same Ft/Ds pathway genes in a wild-type background (see Figure 3). In the context of our Bid-Bip model (Figure 1), this supports our proposal that reduced levels of Ft/Ds pathway activity can alter the direction of the Early Fz(Sple) signal without affecting the Late Fz(Pk) signal. Moreover, our results suggest that the role of Lft in wing PCP is entirely restricted to regulating the Early Fz(Sple) signal. In the posterior wing, reduced Ft/Ds pathway activity modifies the pkpk30 hair phenotype to a more distal polarity in the same regions in which reduced Ft/Ds pathway activity alters ridge orientation to a more A-P orientation (see Figure 3). Since we propose that a single Fz PCP signal specifies orthogonal hair and ridges, we would expect that a change in the Fz(Sple) signal direction that results in distal hair polarity should be associated with A-P ridges.
Uniform over-expression of Ft/Ds pathway genes modifies pkpk wing hair polarity to a more distal orientation
To complement the studies described above, we looked at the effect of over-expressing Ft/Ds pathway genes on the Early Fz(Sple) and Late Fz(Pk) signals. Uniform over-expression of ft (MS1096-gal4; UAS-ft) results in similar wing morphology to loss of ft activity (Figure 5B) and alters posterior ridges. ft over-expression alters hair polarity in the same proximal region of the wing affected by reduced Ft/Ds pathway gene activity (see Figure 3), but also generates variable hair polarity changes in more distal regions of the wing (red ovals in Figure 5B). Uniform over-expression of ds or fj results in a similar wing shape, posterior ridge and hair polarity phenotype to reduced activity of the same genes (Figure 5C and 5D). When ft, ds or fj are uniformly over-expressed in a pkpk mutant wing, the pkpk wing hair phenotype is modified to a more distal polarity in the anterior wing and in distal regions of the posterior wing (Figure 5F, 5G and 5H). These modifications of the pkpk hair phenotype are similar to those generated by reduced activity of the same Ft/Ds pathway genes (see Figure 4).
Fig. 5. Over-expression of Ft/Ds pathway genes modifies the pkpk hair polarity phenotype. 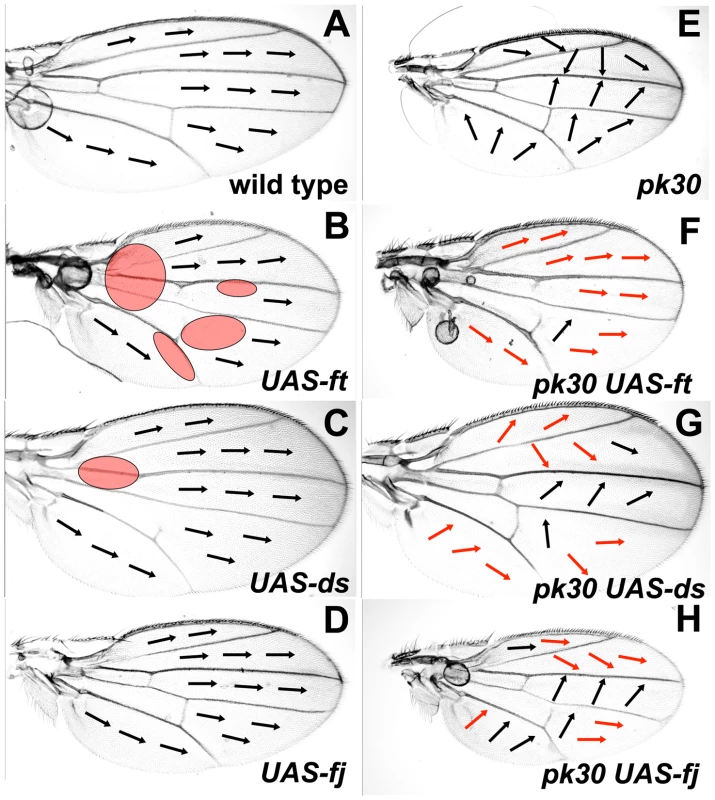
All micrographs are of the female dorsal wing surface. Arrows indicate local hair polarity. Red shaded ovals represent regions where hair polarity differs from wild-type. Red arrows indicate where local hair polarity differs from that seen on a pkpk30 homozygous wing. (A) Wild-type wing. (B) MS1096-gal4; UAS-ft wing. (C) MS1096-gal4; UAS-ds wing. (D) MS1096-gal4; UAS-fj wing. (E) pkpk30/pkpk30 wing. (F) MS1096-gal4; UAS-ft, pkpk30/pkpk30 wing. (G) MS1096-gal4; pkpk30/pkpk30; UAS-ds wing. (H) MS1096-gal4; UAS-fj, pkpk30/pkpk30 wing. These results show that uniform over-expression of ft, ds or fj modify the pkpk hair polarity phenotype in regions of the wing not affected by over-expression of these genes alone. In the context of our Bid-Bip model (Figure 1), this suggests that ft, ds and fj over-expression can alter the Early Fz(Sple) signal without affecting the Late Fz(Pk) signal. The results also imply that both over-expression, and reduced activity, of Ft/Ds pathway genes modify the direction of the Fz(Sple) signal to a more distal orientation.
ds is transiently expressed at the site of the L3 wing vein at the time of the Early Fz PCP signal
Conventionally, gradients of Ft/Ds activity, arising from localized expression of one or more Ft/Ds pathway genes, have been proposed to organize epithelial PCP [26]. In the wing, proximal Ds expression and distal Fj expression have been proposed to generate Ft/Ds activity gradients that organize hair polarity [6], [14], [27]. This proposal is supported by studies that show Ds expression is primarily in the proximal wing at 24–26 hours a.p.f., shortly before the Late Fz PCP signal [6], [27]. However, at 17 hours a.p.f., immediately before the Early Fz PCP signal [12], [14], Ds protein is present in a P-D stripe along the centre of the wing blade (see Figure 6H in [27]). We stained ds-lacZ wings at 18 hours a.p.f. and detected a corresponding stripe of beta-galactosidase activity that extends along the majority of the wing blade (Figure 6A). Beta-galactosidase activity reduces gradually both anterior and posterior to this stripe, suggesting symmetric gradients of ds expression along the A-P axis. To localize this ds expression, we stained for beta-galactosidase activity in an 18 hours a.p.f. ds-lacZ wing that also expressed Green Fluorescent Protein (GFP) under the control of the engrailed (en) promoter (en-gal4, UAS-gfp). The en promoter drives GFP expression throughout the posterior wing with a sharp anterior boundary 4–5 cells posterior to the L3 vein (Figure 6B). In ds-lacZ/en-gal4, UAS-gfp wings, the peak of beta-galactosidase activity (red arrowheads in Figure 6C and 6D) is located anterior to the anterior boundary of GFP expression (Figure 6D) implying that the peak of ds expression maps close to the site of the L3 vein.
Fig. 6. Symmetric ds expression gradients are centered on the L3 vein at 18 hours a.p.f. 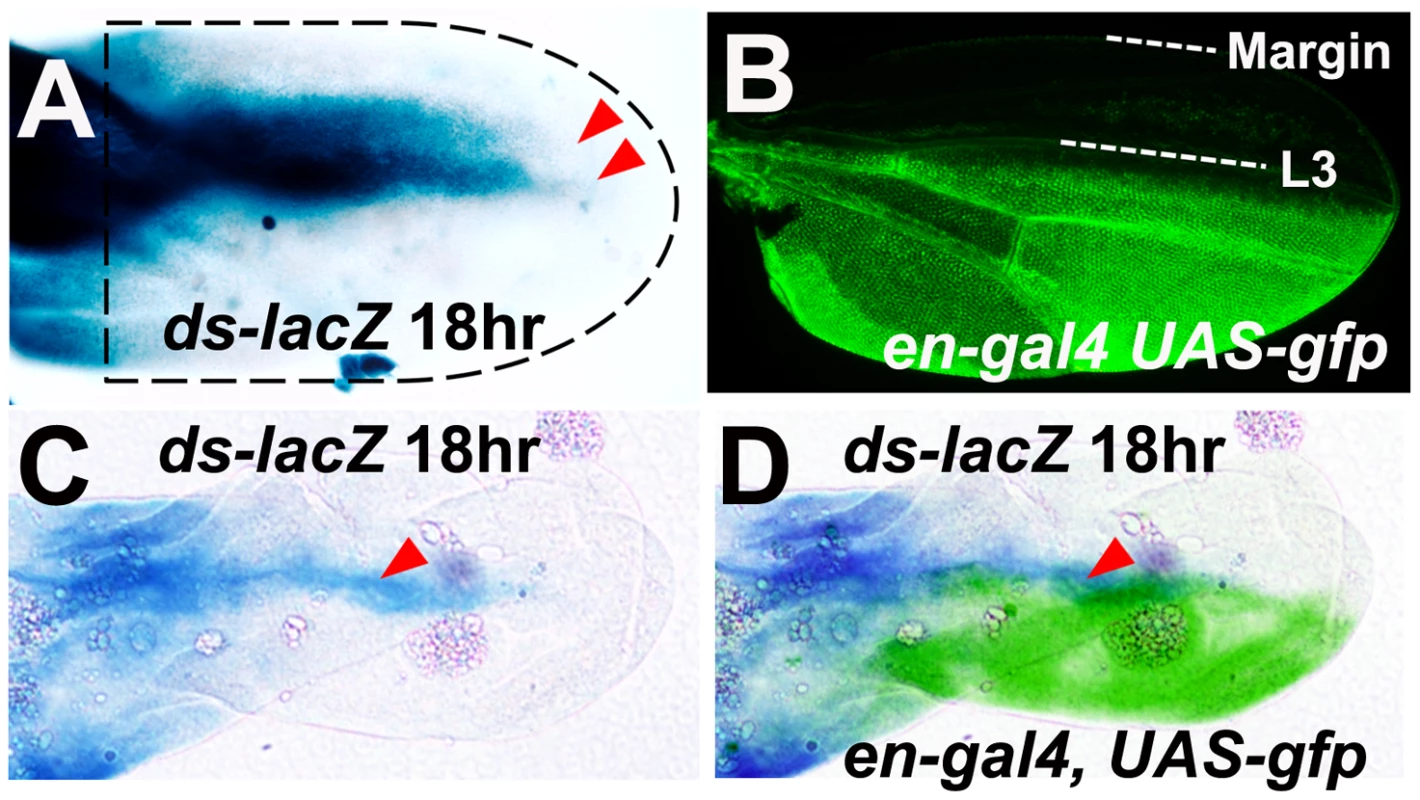
(A) An 18 hour ds-lacZ pupal wing stained for beta-galactosidase activity (blue). The dashed black line represents the approximate outline of the wing. Red arrows define the distal limit of beta-galactosidase activity on the dorsal and ventral epithelia. (B) Wing from a newly eclosed en-gal4, UAS-gfp fly showing GFP fluorescence (green) in the posterior wing. (C) An 18 hour ds-lacZ/en-gal4, UAS-gfp pupal wing stained for beta-galactosidase activity (blue). Note that this wing has been stained for a shorter period than the wing in (A) in order to identify just the highest levels of enzyme activity (indicated by red arrowhead). (D) Same image as (C) overlaid with a color-inverted and falsely colored (green) image of GFP staining in the same wing. There is no ds expression within the wing pouch of 3rd instar imaginal wing discs [28]–[30], and little ds expression within the pupal wing blade at 24–26 a.p.f. [6], [27]. Therefore, we conclude that ds is expressed transiently at the site of the L3 vein around 18 hours a.p.f., the time Strutt has defined for the Early Fz PCP signal [13], [14]. Since we propose that the Early Fz(Sple) signal converges at the site of the L3 vein and that ds is required for the normal orientation of the Fz(Sple) signal, this makes localized ds expression a strong candidate for an organizer of the Fz(Sple) signal. fj expression has previously been proposed to form an opposing gradient to ds in the wing, eye and abdomen [6], [27], [31]–[33]. However, although there is beta-galactosidase activity at the anterior and posterior wing margin of a fj-lacZ wing at 18 hours a.p.f., there is also expression at the distal margin and in distal intervein regions ([5] and data not shown). This pattern of fj expression does not suggest that there are simple opposing gradients of ds and fj expression in the anterior and posterior wing during the period of Early Fz PCP signaling.
Gradients/boundaries of Ft/Ds pathway gene expression reorient hair polarity on a pkpk mutant wing
If gradients of Ft/Ds pathway gene activity control the direction of the Early Fz(Sple) signal, we would expect that altering local levels of Ft/Ds pathway gene expression in the pupal wing should reorient the Fz(Sple) signal. We initially generated marked clones of ft, ds and fj knockdown or over-expression in a pkpk mutant wing to identify hair polarity changes that result from inducing novel gradients/boundaries of Ft/Ds signaling. However, interpreting the effects of clones of variable shape, size and position on the pkpk mutant hair phenotype proved unfeasible. To overcome this problem, we used the well-characterized sal-Gal4 driver to drive localized over-expression or knockdown of ft, ds and fj in both wild-type and pkpk mutant wings. The sal-Gal4 driver expresses Gal4 protein in the spalt expression pattern [34] (i.e. between the L2 vein and midway between the L4 and L5 veins (Figure 7A)), and has been used successfully to generate gradients of Ft/Ds pathway gene expression along the A-P wing axis [27]. Using the sal-Gal4 driver to knockdown ds or ft, or to over-express ds, ft or fj resulted in changes in wing morphology, but did not affect hair polarity outside the main sal-Gal4 expression domain (see Figure 7D, 7F, 7H, 7J and 7L). However, when the same experiments were done in a pkpk mutant wing, specific changes of hair polarity were observed outside of the main sal-Gal4 expression domain. For example, in the A region of the wing (anterior to the L2 vein) hair polarity on a pkpk mutant wing is posterior (see Figure 4 and [12], [18], [21]). However, hair polarity in the A region of a pkpk mutant wing becomes anterior when sal-Gal4 is used to drive ds knockdown or ft or fj over-expression (Figure 7E, 7K and 7M). In contrast, pkpk mutant wings in which sal-Gal4 drives ds over-expression or ft knockdown retain posterior hair polarity in the A region. In each case, hair polarity within the main sal-Gal4 expression domain resembles the modified pkpk phenotype seen when the same Ft/Ds pathway genes were knockdown or over-expressed uniformly in the wing (see Figure 4 and Figure 5), with the exception of fj over-expression which maintained the normal pkpk mutant phenotype within the sal-Gal4 expression domain. This last observation is curious, but may be due to the relative levels of expression driven by the MS1096-Gal4 and sal-Gal4 drivers.
Fig. 7. Gradients/boundaries of Ft/Ds pathway gene expression modify the pkpk hair polarity phenotype. 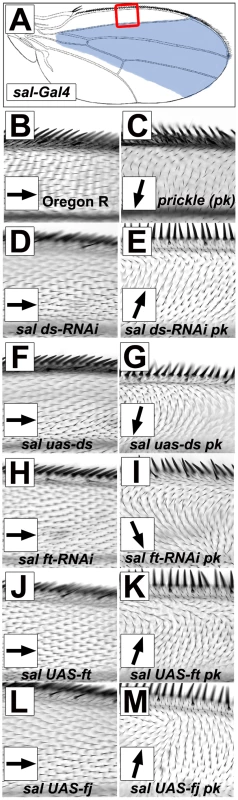
All micrographs show a detail of the A region of the female dorsal wing (red boxed region in (A)). Black arrows indicate local hair polarity. (A) Wing cartoon showing major expression domain of the sal-Gal4 driver (blue shading). (B) Wild-type (Oregon R). (C) pk30/pk30. (D) sal-Gal4/UAS-ds(IR). (E) pk30, UAS-ds(IR)/pk30, sal-Gal4. (F) sal-Gal4; UAS-ds. (G) pk30 sal-Gal4/pk30; UAS-ds. (H) sal-Gal4/UAS-ft(IR). (I) pk30, UAS-ft(IR)/pk30, sal-Gal4. (J) sal-Gal4/UAS-ft. (K) pk30, sal-Gal4/pk30, UAS-ft. (L) sal-Gal4/UAS-fj. (M) pk30, sal-Gal4/pk30, UAS-fj. We note that Ft/Ds pathway gene misexpression can affect hair polarity on a pkpk mutant wing ten or more cell diameters anterior to the main sal-Gal4 expression domain, suggesting a substantial degree of cell non-autonomy. We have found that driving RNAi knockdown of the cell-autonomous tricornered (trc) (VDRC transformant 107923KK [23]) or forked (f) (VDRC transformant 33200GD [23]) genes using the sal-Gal4 driver generates occasional cells carrying a mutant hair phenotype anterior to the L2 vein (data not shown). This raises the possibility there may be a gradient of sal-Gal4 expression extending several cell diameters anterior to the L2 vein that could generate corresponding gradients of Ft/Ds pathway gene activity. However, it is also possible that the boundary of Ft/Ds pathway gene expression generated using the sal-Gal4 driver may cause propagation of PCP changes outside of the expression domain, as has been observed in the Drosophila abdomen ([7] and see discussion) and in the control of cell proliferation by the Ft/Ds pathway [35].
In the context of our Bid-Bip model, these results suggest that generating gradients/boundaries of Ft/Ds pathway gene expression along the A-P wing axis can alter the direction of the Early Fz(Sple) signal, without affecting the Late Fz(Pk) signal. Specifically, we find that the Fz(Sple) signal is reoriented to point away from a region of reduced ds expression, but not from a region of ds over-expression. This is consistent with the observation that the Early Fz(Sple) signal normally points towards high levels of ds expression at the site of the L3 vein. The Early Fz(Sple) signal also points away from over-expressed ft or fj, which suggests that there are activity gradients of Ft and Fj that oppose the Ds expression gradient during the period of Early Fz(Sple) signaling.
To test if gradients/boundaries of Ft/Ds pathway gene expression can alter PCP in the absence of both the Pk and Sple protein isoforms, we used sal-Gal4 to drive ft or fj over-expression, and ft knockdown, in a pkpk-sple-14 homozygous mutant wing. These localized changes in ft or fj expression altered the morphology of the pkpk-sple-14 wing (compare Figure S2B with S2D, S2F and S2H), however, there were no significant changes in hair polarity at the boundaries of the sal-gal4 expression domain. For example, in the A region of a pkpk-sple-14 homozygous wing hair polarity is slightly more anterior than wild-type ([18], [22] and see Figure S2C), but is not altered when sal-gal4 is used to drive ft or fj over-expression, or ft knockdown (see Figure S2E, S2G and S2I). These results show that gradients/boundaries of Ft/Ds pathway gene expression, which can reorient the Fz(Sple) signal, do not alter PCP in the absence of Pk and Sple isoform activity.
The role of ds in wing morphogenesis is separable from its effect on the pkpk hair polarity phenotype
The Ft/Ds pathway controls wing morphogenesis by determining the orientation of cell divisions and clonal growth [36] and it has been proposed that altered wing hair polarity associated with loss of Ft/Ds pathway activity might also be a consequence of abnormal cell division [37]. Our data show that altered Ft/Ds pathway activity can change wing morphology without affecting hair polarity across most of the wing (see Figure 3). In the context of our Bid-Bip model, this suggests that the role of the Ft/Ds pathway in wing morphogenesis is largely separable from its role in organizing the Late Fz(Pk) signal. However, we find that changes in Ft/Ds activity that alter wing shape consistently modify the pkpk mutant hair phenotype. This suggests that we have been unable to separate the role of Ft/Ds in wing morphogenesis from its role in organizing the Early Fz(Sple) signal. To attempt to unlink these activities, we controlled the timing of ds RNAi expression during the development of a pkpk mutant wing. Constitutive expression of ds RNAi in the developing pkpk wing (using the MS1096-Gal4 driver) alters wing morphology and changes pkpk wing hair polarity to a more distal orientation (see Figure 4G, 4H and 4I). We controlled the timing of ds RNAi expression in MS1096-Gal4; UAS-ds(IR) wings by constitutive expression of Gal80ts, a temperature-sensitive Gal4 inhibitor, that binds and inactivates Gal4 at 18°C, but not at 30°C [38]. Consequently, animals of the genotype MS1096-Gal4/+; pk30, ds(IR)/pk30, tubP-GAL80ts can be cultured at 18°C (when Gal80ts is active and inhibits Gal4) and then shifted to 30°C at specific times a.p.f. to induce ds RNAi expression in the wing. When flies of this genotype were cultivated continuously at 18°C, they showed a typical pkpk mutant wing phenotype (Figure 8A and 8B), indicating that Gal80ts effectively inhibited Gal4 at this temperature. In contrast, when flies of this genotype were cultivated continuously at 30°C, they displayed wing morphology typical of reduced ds activity (Figure 8C), combined with more distal hair polarity than a pkpk mutant (Figure 8D). Flies shifted from 18°C to 30°C during pupal development showed close to wild-type wing morphology (e.g. Figure 8E, 8G and 8H), but a hair phenotype that was dependent upon timing of the temperature shift. Flies shifted before 30 hours a.p.f. displayed the more distal hair polarity typical of continuous ds knockdown (e.g. Figure 8F) and we still observed significant modification of the pkpk hair phenotype when pupae were shifted at 36 hours a.p.f.. However, pupae shifted after 40 hours a.p.f. displayed hair polarity phenotypes within the range of normal pkpk mutant wings.
Fig. 8. Reduced ds expression can modify pkpk hair polarity without affecting wing morphogenesis. 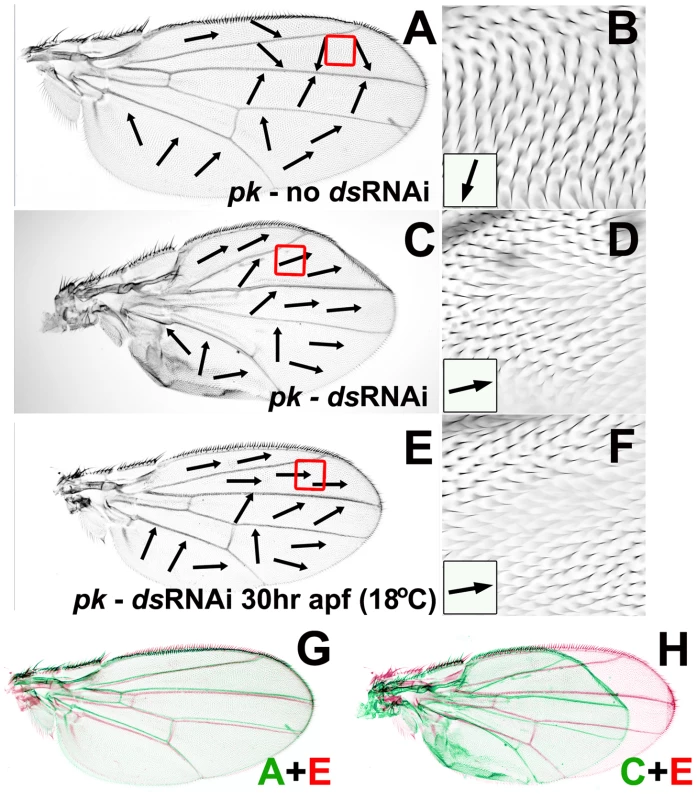
All micrographs show dorsal wing surface of MS1096-Gal4/+; pk30, UAS-ds(IR)/pk30, tubP-GAL80ts female flies. Black arrows indicate local hair polarity. (A) Wing from fly cultured at 18°C. (B) Enlargement of anterior region outlined by red box in (A). (C) Wing from fly cultured at 30°C. (D) Enlargement of anterior region outlined by red box in (C). (E) Wing from fly cultured at 18°C then shifted to 30°C at 30 hours a.p.f. (F) Enlargement of anterior region outlined by red box in (E). (G) Wing from (A) rescaled, colored green and overlaid on wing (E) colored red. (H) Wing from (C) rescaled, colored green and overlaid on wing (E) colored red. These results show that controlling the timing of ds knockdown during the development of pkpk pupal wings can generate wings that have close to wild-type morphology, but still have a modified pkpk wing hair phenotype. We conclude that the role of ds in wing morphology is largely separable from its role in the Early Fz(Sple) signal. In principle, ds knockdown should modify the pkpk wing hair phenotype prior to the Early Fz(Sple) signal, but should have no effect after the Early Fz(Sple) signal. We find that typical ds RNAi modification of the pkpk phenotype still occurs when pupae are shifted at 30 hours a.p.f. at 18°C (approximately equivalent to 13 hours a.p.f. at 25°C), and still see some modification of the pkpk phenotype when pupae are shifted at 36 hours a.p.f. at 18°C (approximately equivalent to 17–18 hours a.p.f. at 25°C), but not when pupae are shifted at 40 hours a.p.f. at 18°C (approximately equivalent to 19 hours a.p.f. at 25°C). These results are consistent with Strutt's proposal that the Early Fz PCP signal occurs at around 18 hours a.p.f. at 25°C [13], [14].
Discussion
A model for Ft/Ds control of the Early Fz(Sple) signal
The data presented in this report allow us to refine our Bid-Bip Fz PCP signaling model (Figure 1), particularly the nature of the proposed Early Fz(Sple) signal. We find that the Early Fz(Sple) signal is in opposing directions in the anterior and posterior wing and converges precisely at the site of the L3 vein. The site of the L3 vein, therefore, represents a discontinuity in Early Fz(Sple) signaling that we have called the PCP-D (see Figure 9). However, it is clear that physical differentiation of the L3 vein is not required for the formation of the PCP-D. The correspondence of the PCP-D with the site of the L3 vein is perhaps surprising as the compartment boundary (a barrier to clonal growth that runs a few cells anterior to the L4 vein) appears a more obvious boundary between the anterior and posterior wing. However, the L3 vein has been defined as a specific region of low Hedgehog signaling within the wing [39], suggesting this region has the molecular autonomy needed to function as a signaling centre. In addition, recently published work from the Eaton lab has also identified the L3 vein as the boundary between oppositely polarized cells in the anterior and posterior of early pupal wings [40].
Fig. 9. A model for PCP specification in the Drosophila wing. 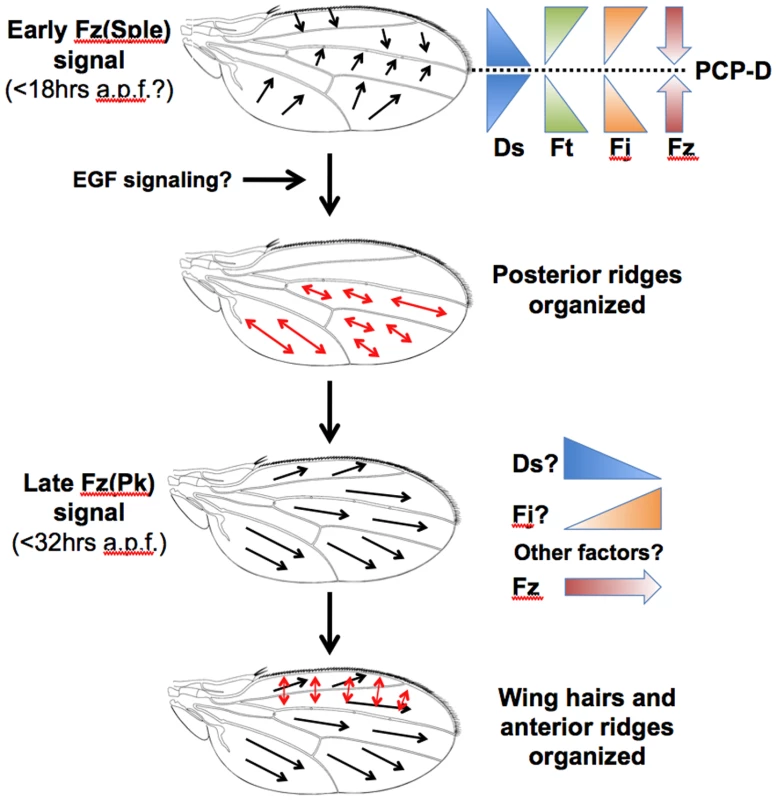
At 18 hours a.p.f., there are symmetric gradients of Ds expression that peak at the site of the L3 vein. The resulting Ds activity gradients are opposed by gradients of Ft and Fj activity. The Ft/Ds pathway organizes the direction of the Early Fz PCP signal, which points up the Ds activity gradient towards the site of the L3 vein. This Early Fz PCP signal employs the Sple isoform of the Prickle protein and determines the orientation of posterior wing ridges, which are specified orthogonal to the direction of Fz signaling. The outcome of the Early Fz PCP signal can be modified by the differentiation of wing veins, possibly due to EGF signaling. A second Fz signal occurs prior to wing hair initiation at 32 hours a.p.f. and employs the Pk isoform of the Prickle protein. The Late Fz PCP signal points distally and determines the orientation of hairs and also anterior ridges, which are specified orthogonal to the Fz activity gradient. The Late Fz PCP signal points down the contemporaneous Ds expression gradient and up a Fj expression gradient. However, it is likely that other factors are involved in controlling the direction of the Late Fz PCP signal. We find that both reduced activity and uniform over-expression of Ft/Ds pathway genes have similar effects on the direction of the Fz(Sple) signal, which becomes more distal in both the anterior wing and distal regions of the posterior wing. Significantly, the Eaton lab has recently shown that the subcellular localization of Vang/Stbm protein in the early (15 hours a.p.f.) pupal wing of a ds mutant is more distal than wild-type in both the anterior and distal posterior wing (see Figure 7C in [40]). Our results are consistent with the idea that the normal direction of the Fz(Sple) signal is controlled by gradients of Ft/Ds pathway activity that can be flattened through either reduced or uniform expression of individual pathway components. We have confirmed an observation made in the Blair lab [27] that ds is expressed transiently in a P-D stripe within the pupal wing blade at around the time of Early Fz PCP signaling (as defined by Strutt [13], [14]) and have localized the peak of Ds expression to the site of the L3 vein, the same location as the wing PCP-D. This implies that there are symmetric gradients of ds expression in the anterior and posterior wing and that the Early Fz(Sple) signal points up a ds expression gradient (Figure 9). This conclusion is supported by our finding that the Fz(Sple) signal reorients to point away from localized ds knockdown, but not from localized ds over-expression. The Early Fz(Sple) signal also points away from over-expressed ft or fj, which suggests that Ft or Fj activity has the opposite effect to Ds activity on direction of the Fz(Sple) signal (Figure 9). This is the same relationship between Ft, Ds and Fj activity that has been established in the Drosophila eye [41] and abdomen [31]. Recent molecular studies have shown that Fj, a golgi kinase, can phosphorylate cadherin domains within both Ft and Ds proteins [42], [43]. It has been proposed that this modification increases Ft activity, but decreases Ds activity.
We find that reducing ds expression (or increasing ft or fj expression) under the control of the sal-Gal4 driver redirects the Early Fz(Sple) signal for a significant distance (ten or more cell diameters) beyond the sal-Gal4 expression domain. In principle, reducing ds expression within the sal-Gal4 domain should generate a local reversal of the ds expression gradient at the boundary of sal-Gal4 expression (e.g. the L2 vein). This short reversed ds gradient should generate a correspondingly short region of reversed Fz(Sple) signal which should be visible (on a pkpk mutant wing) as a short region of reversed hair polarity adjacent to the L2 vein. Therefore, the propagation of reversed hair polarity significantly anterior to the L2 vein is surprising. However, a similar propagation of reversed polarity is seen adjacent to loss-of-function and over-expression clones of ds, ft or fj in the Drosophila abdomen [7], [31]. The model proposed for the propagation of altered polarity in the abdomen [7] may, therefore, also apply to the Early Fz(Sple) signal in the wing.
Since it has been established that wing hair polarity points down a gradient of Fz activity [16] and we propose that the direction of the Early Fz(Sple) signal (i.e. the hair polarity that would be specified by the signal) points up a Ds expression gradient, it appears that there are opposing gradients of Ds and Fz activity during Early Fz(Sple) signaling. This relationship between Ds and Fz gradients is consistent with that described in the Drosophila eye [32], although it is opposite to that previously proposed in the wing [6]. Our findings, therefore, may help resolve this discrepancy between the proposed relationships of Fz and Ds activity in the eye and wing that has been highlighted by Strutt, Mlodzik and others [2], [41], [44].
The role of Ft/Ds pathway in Late Fz(Pk) signaling
From this work, we conclude that for substantial regions of the wing (including most of the anterior wing and distal regions of the posterior wing), Ft/Ds pathway activity can be altered such that the Early Fz(Sple) signal is redirected, but the Late Fz(Pk) signal remains unaffected. For any specific experiment, this result might be explained by the specific properties of the mutant allele used or by the specific spatial or temporal activity of the Gal4 driver used to drive gene knockdown or over-expression. However, we have shown that numerous alleles, as well as both knockdown and over-expression, of Ft/Ds pathway genes, can redirect the Fz(Sple) signal in a similar way, without affecting the Fz(Pk) signal in the same region. This suggests that across most of the wing there is a different requirement for the Ft/Ds pathway in the Early Fz(Sple) and Late Fz(Pk) signals. Moreover, we have found that loss of the Ft/Ds pathway regulator Lft affects the Early Fz(Sple) signal, but not the Late Fz(Pk) signal. This suggests that the mechanism used by the Ft/Ds pathway to direct the Early Fz(Sple) signal differs from that used to organize the Late Fz(Pk) signal.
What, then, is the role of the Ft/Ds pathway in the Late Fz(Pk) signal? Since the Late Fz(Pk) signal organizes hair polarity (see Figure 1), characterizing the loss of Ft/Ds pathway activity on hair polarity should be informative. We have found that driving ft or ds RNAi uniformly in the wing results in altered wing morphology, but only localized proximal hair polarity changes. This might be due to incomplete gene knockdown, coupled with different requirements for Ft/Ds activity for Late Fz PCP signaling in different regions of the wing. However, it is suggestive that wings homozygous for a fj amorphic allele show only a localized hair polarity phenotype in this same proximal region, implying that Fj is only required for hair polarity in the proximal wing. These results raise the possibility the Ft/Ds pathway is normally only required for hair polarity in the proximal wing.
Since neither ft nor ds null flies are adult viable, previous studies have inferred the role of Ft and Ds in wing hair polarity from analyzing phenotypes of viable hypomorphic alleles, clones of amorphic alleles and localized over-expression [6], [14], [27], [45]. Some hypomorphic ds allele combinations display extensive wing hair polarity disruptions [27], [45], although the residual activity of these specific alleles has not been well characterized. Wing clones homozygous for amorphic ft or ds alleles can show hair phenotypes, although this is dependent upon the position and/or size of the clone [6], [14]. However, mutant clones generate ectopic Ft or Ds activity boundaries/gradients in the wing and it is known that localized mis-expression of Ft/Ds pathway genes can generate hair phenotypes in wing regions not affected by uniform over-expression [27]. Most telling, clones of fj affect hair polarity in regions of the wing that are not affected in amorphic fj wings [5]. These results clearly show that mis-regulated Ft/Ds activity can change wing hair polarity. However, they do not definitively establish a role for Ft/Ds pathway in the normal organization of hair polarity outside of the proximal wing. Therefore, it remains possible that Ft/Ds pathway activity is only required for hair polarity in the proximal wing, but mis-regulated Ft/Ds pathway activity can induce changes in hair polarity in other wing regions. This may restrict the normal role of the Ft/Ds pathway to organizing the Late Fz(Pk) signal in the proximal wing alone.
The logic of multiple Fz PCP signaling events during wing development
According to our Bid-Bip model, the two Fz PCP signaling events aligned with different axes of the developing wing allow membrane ridges to be organized in different directions in the anterior and posterior (see Figure 1). The ability of the insect wing to deform specifically is vital for insect flight and it has been proposed that wing membrane structure helps provide the appropriate wing rigidity and flexibility [46]. In the case of membrane ridges, the membrane should be flexible parallel to the ridges, but be resistant to folding perpendicular to the ridges. The A-P ridges in the anterior wing are perpendicular to longitudinal wing veins which suggests a rigid anterior wing structure, whereas the posterior ridges are almost parallel with longitudinal wing veins suggesting a more flexible posterior wing structure. This organization is typical for Dipteran wings which usually have a well-supported leading edge and a flexible trailing edge. Indeed, we have seen similar ridge organization in wings of other Drosophila species (data not shown). Therefore, the different orientation of ridges in the anterior and posterior wing may have a functional basis. The reason for the uniform distal hair polarity across the Drosophila wing is not well understood, but is conserved in a wide range of Dipteran species suggesting a functional constraint. Therefore, the two Fz PCP signals in different directions during Drosophila wing development may provide a mechanism that allows hairs and ridges to be organized appropriately using a single signaling pathway.
Are multiple Fz PCP signaling events active in other Drosophila tissues besides the developing wing? Intriguingly, the Prickle isoforms, Pk and Sple, play different roles in PCP in numerous Drosophila tissues, including the wing, eye, abdomen and leg [18], [21], [47]–[49]. This raises the possibility that there are multiple Fz PCP signals involving differential use of Pk and Sple isoforms in each of these tissues. However, the specific phenotypes associated with loss of either or both isoforms within the different tissues suggest that the details of our Bid-Bip model are unlikely to hold true for all tissues. How can multiple Fz PCP signals occur in different directions in the same developing tissue? One possibility is that changes in the molecular makeup of the Fz PCP pathway allow it to respond to different global signals within the tissue, or to respond in different ways to the same global signal. In the Drosophila wing, this might result from the differential use of the Pk and Sple isoforms. Alternatively, the individual Fz PCP signals may respond to different global signals present at different times during tissue development or to a single dynamic global cue. The significance of Prickle isoform switching and the possibility of dynamic global PCP signals are ongoing topics of interest in our lab.
Materials and Methods
Fly culture and stocks
Flies were cultured at 25°C on standard yeasted cornmeal media, unless stated otherwise. Fly mutations used in this study were: rhove-1, vn1 (J. de Celis), pk30 (D. Gubb), dsUA071 (P. Adler), lftTG2 (K. Irvine), UAS-ft, UAS-ds, UAS-fj (S.Blair), P{GD14350}v36219 (ds RNAi), P{GD881}v9396 (ft RNAi), P{GD430}v6774 (fj RNAi) (VDRC), TRiP.JF02842 (ds RNAi), TRiP.JF02843 (fj RNAi), TRiP.JF03245 (ft RNAi) (TRiP), UAS-argos, ft1, ds05142, fjD1, MS1096-Gal4, ds2D60B (ds-lacZ), fj9-11 (fj-lacZ), 459.2-Gal4 (sal-Gal4), en-Gal4, UAS-GFP.S65T(T2), tubP-Gal80ts(10) (Bloomington Stock Center).
Cuticle Refraction Microscopy (CRM)
We have described the CRM technique previously [12]. Briefly, adult wings were removed and laid gently on top of a thin layer of clear nail polish with the dorsal surface uppermost. The nail polish was allowed to dry, a cover slip placed on top and sealed with additional nail polish. Wings were viewed using an Olympus BX51 microscope (Olympus America Inc.) with the top lens of the condenser removed from the light path and the aperture diaphragm at its narrowest.
Detection of beta galactosidase activity in pupal wings
Prepupae of appropriate genotype were collected and aged for 18 hours at 25°C. Pupal wings were dissected, fixed with 4% formaldehyde (20 minutes) and beta-galactosidase activity assayed using X-gal by standard techniques.
Fluorescent microscopy of GFP-expressing adult wings
Newly unfolded wings were removed carefully from recently eclosed female flies and laid on a clean microscope slide. The wings were left in air, to prevent delaminated cells being washed out of the wing by mountant, and were either viewed directly or under a coverslip using appropriate spacers to prevent the coverslip contacting the wing surface.
Expression/knockdown of Ft/Ds pathway genes using the sal-Gal4 driver
Wings were mounted in GMM mountant. Discussion in the text refers to the hair polarity across the entire A region of the wing (between anterior wing margin and L2 vein), not just the region shown in Figure 7. All results described were consistent for the first 10 wings observed of each genotype, except where number of progeny was limiting specifically; sal-Gal4/UAS-ft(IR) (6/6 wings) and pk30 sal-Gal4/pk30; UAS-ds (6/6 wings).
Temporal control of ds knockdown in the pupal wing
Female flies of the genotype MS1096-Gal4/+; pk30, UAS-ds(IR)/pk30, tubP-GAL80ts were cultured at 18°C and isolated as white pre-pupae. The pre-pupae were incubated between 0 to 48 hours at 18°C before shifting to 30°C. A total of 140 adult wings were mounted in GMM and studied.
Supporting Information
Zdroje
1. EatonS
2003 Cell biology of planar polarity transmission in the Drosophila wing. Mech Dev 120 1257 1264
2. SeifertJR
MlodzikM
2007 Frizzled/PCP signalling: a conserved mechanism regulating cell polarity and directed motility. Nat Rev Genet 8 126 138
3. LawrencePA
StruhlG
CasalJ
2007 Planar cell polarity: one or two pathways? Nat Rev Genet 8 555 563
4. TreeDR
MaD
AxelrodJD
2002 A three-tiered mechanism for regulation of planar cell polarity. Semin Cell Dev Biol 13 217 224
5. ZeidlerMP
PerrimonN
StruttDI
2000 Multiple roles for four-jointed in planar polarity and limb patterning. Dev Biol 228 181 196
6. MaD
YangCH
McNeillH
SimonMA
AxelrodJD
2003 Fidelity in planar cell polarity signalling. Nature 421 543 547
7. CasalJ
LawrencePA
StruhlG
2006 Two separate molecular systems, Dachsous/Fat and Starry night/Frizzled, act independently to confer planar cell polarity. Development 133 4561 4572
8. JonesC
ChenP
2007 Planar cell polarity signaling in vertebrates. Bioessays 29 120 132
9. SaburiS
HesterI
FischerE
PontoglioM
EreminaV
2008 Loss of Fat4 disrupts PCP signaling and oriented cell division and leads to cystic kidney disease. Nat Genet 40 1010 1015
10. KibarZ
TorbanE
McDearmidJR
ReynoldsA
BerghoutJ
2007 Mutations in VANGL1 associated with neural-tube defects. N Engl J Med 356 1432 1437
11. BassukAG
WallaceRH
BuhrA
BullerAR
AfawiZ
2008 A homozygous mutation in human PRICKLE1 causes an autosomal-recessive progressive myoclonus epilepsy-ataxia syndrome. Am J Hum Genet 83 572 581
12. DoyleK
HoganJ
LesterM
CollierS
2008 The Frizzled Planar Cell Polarity signaling pathway controls Drosophila wing topography. Dev Biol 317 354 367
13. StruttD
StruttH
2007 Differential activities of the core planar polarity proteins during Drosophila wing patterning. Dev Biol 302 181 194
14. StruttH
StruttD
2002 Nonautonomous planar polarity patterning in Drosophila: dishevelled-independent functions of frizzled. Dev Cell 3 851 863
15. VinsonCR
AdlerPN
1987 Directional non-cell autonomy and the transmission of polarity information by the frizzled gene of Drosophila. Nature 329 549 551
16. AdlerPN
TaylorJ
CharltonJ
2000 The domineering non-autonomy of frizzled and van Gogh clones in the Drosophila wing is a consequence of a disruption in local signaling. Mech Dev 96 197 207
17. TaylorJ
AbramovaN
CharltonJ
AdlerPN
1998 Van Gogh: a new Drosophila tissue polarity gene. Genetics 150 199 210
18. GubbD
GreenC
HuenD
CoulsonD
JohnsonG
1999 The balance between isoforms of the prickle LIM domain protein is critical for planar polarity in Drosophila imaginal discs. Genes Dev 13 2315 2327
19. Garcia-BellidoA
de CelisJF
1992 Developmental genetics of the venation pattern of Drosophila. Annu Rev Genet 26 277 304
20. HowesR
WassermanJD
FreemanM
1998 In vivo analysis of Argos structure-function. Sequence requirements for inhibition of the Drosophila epidermal growth factor receptor. J Biol Chem 273 4275 4281
21. GubbD
Garcia-BellidoA
1982 A genetic analysis of the determination of cuticular polarity during development in Drosophila melanogaster. J Embryol Exp Morphol 68 37 57
22. LinYY
GubbD
2009 Molecular dissection of Drosophila Prickle isoforms distinguishes their essential and overlapping roles in planar cell polarity. Dev Biol 325 386 399
23. DietzlG
ChenD
SchnorrerF
SuKC
BarinovaY
2007 A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 448 151 156
24. BrayS
2000 Planar polarity: out of joint? Curr Biol 10 R155 158
25. MaoY
KucukB
IrvineKD
2009 Drosophila lowfat, a novel modulator of Fat signaling. Development 136 3223 3233
26. StruttH
StruttD
2005 Long-range coordination of planar polarity in Drosophila. Bioessays 27 1218 1227
27. MatakatsuH
BlairSS
2004 Interactions between Fat and Dachsous and the regulation of planar cell polarity in the Drosophila wing. Development 131 3785 3794
28. RodriguezI
2004 The dachsous gene, a member of the cadherin family, is required for Wg-dependent pattern formation in the Drosophila wing disc. Development 131 3195 3206
29. ClarkHF
BrentrupD
SchneitzK
BieberA
GoodmanC
1995 Dachsous encodes a member of the cadherin superfamily that controls imaginal disc morphogenesis in Drosophila. Genes Dev 9 1530 1542
30. ChoE
IrvineKD
2004 Action of fat, four-jointed, dachsous and dachs in distal-to-proximal wing signaling. Development 131 4489 4500
31. CasalJ
StruhlG
LawrencePA
2002 Developmental compartments and planar polarity in Drosophila. Curr Biol 12 1189 1198
32. SimonMA
2004 Planar cell polarity in the Drosophila eye is directed by graded Four-jointed and Dachsous expression. Development 131 6175 6184
33. YangCH
AxelrodJD
SimonMA
2002 Regulation of Frizzled by fat-like cadherins during planar polarity signaling in the Drosophila compound eye. Cell 108 675 688
34. ThomasU
JonssonF
SpeicherSA
KnustE
1995 Phenotypic and molecular characterization of SerD, a dominant allele of the Drosophila gene Serrate. Genetics 139 203 213
35. WilleckeM
HamaratogluF
Sansores-GarciaL
TaoC
HalderG
2008 Boundaries of Dachsous Cadherin activity modulate the Hippo signaling pathway to induce cell proliferation. Proc Natl Acad Sci U S A 105 14897 14902
36. Baena-LopezLA
BaonzaA
Garcia-BellidoA
2005 The orientation of cell divisions determines the shape of Drosophila organs. Curr Biol 15 1640 1644
37. StruttD
2005 Organ shape: controlling oriented cell division. Curr Biol 15 R758 759
38. McGuireSE
MaoZ
DavisRL
2004 Spatiotemporal gene expression targeting with the TARGET and gene-switch systems in Drosophila. Sci STKE 2004 pl6
39. HooperJE
2003 Smoothened translates Hedgehog levels into distinct responses. Development 130 3951 3963
40. AigouyB
FarhadifarR
StapleDB
SagnerA
RoperJC
2010 Cell flow reorients the axis of planar polarity in the wing epithelium of Drosophila. Cell 142 773 786
41. StruttH
StruttD
2002 Planar polarity: photoreceptors on a high fat diet. Curr Biol 12 R384 385
42. IshikawaHO
TakeuchiH
HaltiwangerRS
IrvineKD
2008 Four-jointed is a Golgi kinase that phosphorylates a subset of cadherin domains. Science 321 401 404
43. BrittleAL
RepisoA
CasalJ
LawrencePA
StruttD
2010 Four-jointed modulates growth and planar polarity by reducing the affinity of dachsous for fat. Curr Biol 20 803 810
44. WuJ
MlodzikM
2009 A quest for the mechanism regulating global planar cell polarity of tissues. Trends Cell Biol 19 295 305
45. AdlerPN
CharltonJ
LiuJ
1998 Mutations in the cadherin superfamily member gene dachsous cause a tissue polarity phenotype by altering frizzled signaling. Development 125 959 968
46. WoottonRJ
1992 Functional Morphology of Insect Wings. Annual Review of Entomology 37 113 140
47. HeldLIJr
1993 Segment-polarity mutations cause stripes of defects along a leg segment in Drosophila. Dev Biol 157 240 250
48. ChoiKW
MozerB
BenzerS
1996 Independent determination of symmetry and polarity in the Drosophila eye. Proc Natl Acad Sci U S A 93 5737 5741
49. GubbD
1993 Genes controlling cellular polarity in Drosophila. Dev Suppl 269 277
Štítky
Genetika Reprodukční medicína
Článek Break to Make a ConnectionČlánek A New Testing Strategy to Identify Rare Variants with Either Risk or Protective Effect on DiseaseČlánek The Architecture of Gene Regulatory Variation across Multiple Human Tissues: The MuTHER Study
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2011 Číslo 2
-
Všechny články tohoto čísla
- Break to Make a Connection
- A New Testing Strategy to Identify Rare Variants with Either Risk or Protective Effect on Disease
- Single-Tissue and Cross-Tissue Heritability of Gene Expression Via Identity-by-Descent in Related or Unrelated Individuals
- Pervasive Adaptive Protein Evolution Apparent in Diversity Patterns around Amino Acid Substitutions in
- The Architecture of Gene Regulatory Variation across Multiple Human Tissues: The MuTHER Study
- MiRNA Control of Vegetative Phase Change in Trees
- New Functions of Ctf18-RFC in Preserving Genome Stability outside Its Role in Sister Chromatid Cohesion
- Genome-Wide Association Studies of the PR Interval in African Americans
- Mapping of the Disease Locus and Identification of As a Candidate Gene in a Canine Model of Primary Open Angle Glaucoma
- Mapping a New Spontaneous Preterm Birth Susceptibility Gene, , Using Linkage, Haplotype Sharing, and Association Analysis
- A Population Genetic Approach to Mapping Neurological Disorder Genes Using Deep Resequencing
- and Genes Modulate the Switch between Attraction and Repulsion during Behavioral Phase Change in the Migratory Locust
- Targeted Sister Chromatid Cohesion by Sir2
- Correlated Evolution of Nearby Residues in Drosophilid Proteins
- Parallel Evolution of a Type IV Secretion System in Radiating Lineages of the Host-Restricted Bacterial Pathogen
- Lipophorin Receptors Mediate the Uptake of Neutral Lipids in Oocytes and Imaginal Disc Cells by an Endocytosis-Independent Mechanism
- Genome-Wide Association Study of Coronary Heart Disease and Its Risk Factors in 8,090 African Americans: The NHLBI CARe Project
- The Evolution of Host Specialization in the Vertebrate Gut Symbiont
- Genome-Wide Association of Familial Late-Onset Alzheimer's Disease Replicates and and Nominates in Interaction with
- Risk Alleles for Systemic Lupus Erythematosus in a Large Case-Control Collection and Associations with Clinical Subphenotypes
- Association between Common Variation at the Locus and Changes in Body Mass Index from Infancy to Late Childhood: The Complex Nature of Genetic Association through Growth and Development
- AID Induces Double-Strand Breaks at Immunoglobulin Switch Regions and Causing Chromosomal Translocations in Yeast THO Mutants
- A Study of CNVs As Trait-Associated Polymorphisms and As Expression Quantitative Trait Loci
- Whole-Genome Comparison Reveals Novel Genetic Elements That Characterize the Genome of Industrial Strains of
- Prevalence of Epistasis in the Evolution of Influenza A Surface Proteins
- Srf1 Is a Novel Regulator of Phospholipase D Activity and Is Essential to Buffer the Toxic Effects of C16:0 Platelet Activating Factor
- Two Frizzled Planar Cell Polarity Signals in the Wing Are Differentially Organized by the Fat/Dachsous Pathway
- Phosphoinositide Regulation of Integrin Trafficking Required for Muscle Attachment and Maintenance
- Pathogenic VCP/TER94 Alleles Are Dominant Actives and Contribute to Neurodegeneration by Altering Cellular ATP Level in a IBMPFD Model
- Meta-Analysis of Genome-Wide Association Studies in Celiac Disease and Rheumatoid Arthritis Identifies Fourteen Non-HLA Shared Loci
- A Genome-Wide Study of DNA Methylation Patterns and Gene Expression Levels in Multiple Human and Chimpanzee Tissues
- Nucleosomes Containing Methylated DNA Stabilize DNA Methyltransferases 3A/3B and Ensure Faithful Epigenetic Inheritance
- Mutations in Zebrafish Result in Adult-Onset Ocular Pathogenesis That Models Myopia and Other Risk Factors for Glaucoma
- [], the Prion Formed by the Chromatin Remodeling Factor Swi1, Is Highly Sensitive to Alterations in Hsp70 Chaperone System Activity
- Characterization of Transcriptome Remodeling during Cambium Formation Identifies and As Opposing Regulators of Secondary Growth
- The Cardiac Transcription Network Modulated by Gata4, Mef2a, Nkx2.5, Srf, Histone Modifications, and MicroRNAs
- Epistatic Interaction Maps Relative to Multiple Metabolic Phenotypes
- Quantitative Models of the Mechanisms That Control Genome-Wide Patterns of Transcription Factor Binding during Early Development
- Genome-Wide Transcript Profiling of Endosperm without Paternal Contribution Identifies Parent-of-Origin–Dependent Regulation of
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Meta-Analysis of Genome-Wide Association Studies in Celiac Disease and Rheumatoid Arthritis Identifies Fourteen Non-HLA Shared Loci
- MiRNA Control of Vegetative Phase Change in Trees
- Risk Alleles for Systemic Lupus Erythematosus in a Large Case-Control Collection and Associations with Clinical Subphenotypes
- The Cardiac Transcription Network Modulated by Gata4, Mef2a, Nkx2.5, Srf, Histone Modifications, and MicroRNAs
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



