-
Články
Reklama
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
ReklamaInflammasome-dependent Pyroptosis and IL-18 Protect against Lung Infection while IL-1β Is Deleterious
Burkholderia pseudomallei is a Gram-negative bacterium that infects macrophages and other cell types and causes melioidosis. The interaction of B. pseudomallei with the inflammasome and the role of pyroptosis, IL-1β, and IL-18 during melioidosis have not been investigated in detail. Here we show that the Nod-like receptors (NLR) NLRP3 and NLRC4 differentially regulate pyroptosis and production of IL-1β and IL-18 and are critical for inflammasome-mediated resistance to melioidosis. In vitro production of IL-1β by macrophages or dendritic cells infected with B. pseudomallei was dependent on NLRC4 and NLRP3 while pyroptosis required only NLRC4. Mice deficient in the inflammasome components ASC, caspase-1, NLRC4, and NLRP3, were dramatically more susceptible to lung infection with B. pseudomallei than WT mice. The heightened susceptibility of Nlrp3-/- mice was due to decreased production of IL-18 and IL-1β. In contrast, Nlrc4-/- mice produced IL-1β and IL-18 in higher amount than WT mice and their high susceptibility was due to decreased pyroptosis and consequently higher bacterial burdens. Analyses of IL-18-deficient mice revealed that IL-18 is essential for survival primarily because of its ability to induce IFNγ production. In contrast, studies using IL-1RI-deficient mice or WT mice treated with either IL-1β or IL-1 receptor agonist revealed that IL-1β has deleterious effects during melioidosis. The detrimental role of IL-1β appeared to be due, in part, to excessive recruitment of neutrophils to the lung. Because neutrophils do not express NLRC4 and therefore fail to undergo pyroptosis, they may be permissive to B. pseudomallei intracellular growth. Administration of neutrophil-recruitment inhibitors IL-1ra or the CXCR2 neutrophil chemokine receptor antagonist antileukinate protected Nlrc4-/- mice from lethal doses of B. pseudomallei and decreased systemic dissemination of bacteria. Thus, the NLRP3 and NLRC4 inflammasomes have non-redundant protective roles in melioidosis: NLRC4 regulates pyroptosis while NLRP3 regulates production of protective IL-18 and deleterious IL-1β.
Published in the journal: . PLoS Pathog 7(12): e32767. doi:10.1371/journal.ppat.1002452
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002452Summary
Burkholderia pseudomallei is a Gram-negative bacterium that infects macrophages and other cell types and causes melioidosis. The interaction of B. pseudomallei with the inflammasome and the role of pyroptosis, IL-1β, and IL-18 during melioidosis have not been investigated in detail. Here we show that the Nod-like receptors (NLR) NLRP3 and NLRC4 differentially regulate pyroptosis and production of IL-1β and IL-18 and are critical for inflammasome-mediated resistance to melioidosis. In vitro production of IL-1β by macrophages or dendritic cells infected with B. pseudomallei was dependent on NLRC4 and NLRP3 while pyroptosis required only NLRC4. Mice deficient in the inflammasome components ASC, caspase-1, NLRC4, and NLRP3, were dramatically more susceptible to lung infection with B. pseudomallei than WT mice. The heightened susceptibility of Nlrp3-/- mice was due to decreased production of IL-18 and IL-1β. In contrast, Nlrc4-/- mice produced IL-1β and IL-18 in higher amount than WT mice and their high susceptibility was due to decreased pyroptosis and consequently higher bacterial burdens. Analyses of IL-18-deficient mice revealed that IL-18 is essential for survival primarily because of its ability to induce IFNγ production. In contrast, studies using IL-1RI-deficient mice or WT mice treated with either IL-1β or IL-1 receptor agonist revealed that IL-1β has deleterious effects during melioidosis. The detrimental role of IL-1β appeared to be due, in part, to excessive recruitment of neutrophils to the lung. Because neutrophils do not express NLRC4 and therefore fail to undergo pyroptosis, they may be permissive to B. pseudomallei intracellular growth. Administration of neutrophil-recruitment inhibitors IL-1ra or the CXCR2 neutrophil chemokine receptor antagonist antileukinate protected Nlrc4-/- mice from lethal doses of B. pseudomallei and decreased systemic dissemination of bacteria. Thus, the NLRP3 and NLRC4 inflammasomes have non-redundant protective roles in melioidosis: NLRC4 regulates pyroptosis while NLRP3 regulates production of protective IL-18 and deleterious IL-1β.
Introduction
The ability to detect infection by pathogenic microbes and to restrict their growth are fundamental for the wellbeing of multicellular organisms. Pattern recognition receptors, including the Toll-like receptor (TLR) and the NLR, recognize microbial products and “danger signals” released by stressed cells and, in turn, activate signaling pathways that initiate the inflammatory response and regulate development of adaptive immunity. TLR are expressed on the cell surface or in endosomal compartments and their stimulation results in activation of the NF-κB, MAPK, and IRF signaling pathways culminating in transcriptional induction of a large number of genes. NLR, in contrast, are located in the cytoplasm, which they survey for evidence of danger or infection (reviewed in ref. [1]). Some NLR control activation of the inflammasome, a multiprotein complex that contains, in addition to a NLR, the adaptor molecule ASC and the protease caspase-1. Activation of caspase-1 in the context of the inflammasome is responsible for the proteolytic processing of the immature forms of IL-1β and IL-18, a modification required for the secretion and bio-activity of these proinflammatory cytokines. Activation of caspase-1 also triggers a form of cell death, known as pyroptosis, that effectively restricts intracellular bacterial growth [2], [3]. Production of IL-1β and IL-18 and induction of pyroptosis have been shown to be protective effector mechanisms against many infectious agents. NLRP3 and NLRC4 are the best characterized NLR molecules. NLRP3 controls caspase-1 activation in response to “danger signals”, several particles and crystals, and various bacteria, virus, and fungi. Although the logic that oversees the activation of the NLRP3 inflammasome is still elusive, it appears that disruption of cell membrane integrity may be a common event triggered by the NLRP3 activators. The NLRC4 inflammasome is responsive to a narrower spectrum of activators including cytoplasmically delivered bacterial flagellin and the basal rod constituent of various bacterial Type III secretion systems (T3SS). The T3SS apparatus is used by several bacteria, including Salmonella, Yersinia, Pseudomonas, Shigella, Legionella, and Burkholderia to inject virulence factors into the cytoplasm of target cells. Recent works demonstrated that the specificity of the mouse NLRC4 for flagellin or rod proteins is determined by its interaction with the NLR molecules NAIP5 or NAIP2, respectively [4], [5].
Burkholderia pseudomallei is a Gram-negative flagellate bacterium that causes melioidosis, a disease endemic to South-East Asia and other tropical regions [6], [7] and the most common cause of pneumonia-derived sepsis in Thailand. Because melioidosis carries a high fatality rate, B. pseudomallei is classified as category B potential bioterrorism agent by the Center for Disease Control and NIAID. B. pseudomallei infection can be contracted through ingestion, inhalation, or subcutaneous inoculation and leads to broad-spectrum disease forms including pneumonia, septicemia, and organ abscesses. Following infection of macrophages and other non-phagocytic cell types, B. pseudomallei is able to escape the phagosome and invade and replicate in the host cell cytoplasm, directly spreading from cell to cell using actin-tail propulsion. Macrophages and IFNγ have been shown to play a critical role in protection from melioidosis [8]–[10] and several B. pseudomallei virulence factors have been identified including the bacterial capsule [11], the lipopolysaccharide [12], and one of the three T3SS possessed by B. pseudomallei [13]. Analysis of mouse strains with different susceptibility to B. pseudomallei infection indicates that the early phases of the infection are crucial for survival [14], [15], emphasizing the necessity for better understanding of innate immune responses during melioidosis. With this goal in mind, using a murine model of melioidosis we have performed a detailed analysis of the role of the inflammasome components NLRP3, NLRC4, ASC, and caspase-1 and the effector mechanisms IL-1β, IL-18, and pyroptosis.
Results
NLRP3 and NLRC4 differentially regulate production of IL-1β and pyroptosis
To identify the pathway responsible for IL-1β and IL-18 secretion in response to infection with B. pseudomallei, bone marrow-derived macrophages (BMDM) or dendritic cells (BMDC) derived from WT mice or mice deficient in the inflammasome components ASC, NLRP3, NLRC4, or caspase-1 were infected in vitro with B. pseudomallei and secretion of IL-1β in culture supernatants was measured. As shown in figure 1A, secretion of IL-1β by Asc-/-, Nlrp3-/-, and Casp1-/- BMDM was markedly reduced compared to WT BMDM. Production of IL-1β during the first hours of the infection was also significantly reduced in Nlrc4-/- cells. However, later in the infection process (8 hours) Nlrc4-/- cells secreted IL-1β at levels considerably higher than WT cells. Secretion of IL-18 followed a similar pattern (data not shown). Immunoblotting of the supernatants confirmed processing of IL-1β and of caspase-1 to the mature 17 kDa and p20 forms, respectively (figure 1B). Interestingly, although caspase-1 was activated in Asc-/- cells, processing and secretion of IL-1β was not observed. NLRC4 possesses an amino-terminal CARD domain that can recruit and activate caspase-1 independently of ASC. It is unclear at present why activation of caspase-1 in Asc-/- cells is not sufficient to trigger secretion of mature IL-1β, a phenomenon previously reported by other groups [16]. The differences in IL-1β and IL-18 secretion were observed regardless of the number of bacteria used to infect cells (MOI 10, 50, or 100, data not shown) and were not due to differential induction of pro-IL-1β, which was present at comparable amounts in all the cell lysates. Thus, the NLRC4 and NLRP3 inflammasomes are both mediating release of IL-1β and IL-18 by myeloid cells infected with B. pseudomallei.
Fig. 1. NLRP3 and NLRC4 differentially regulate production of IL-1β and pyroptosis. 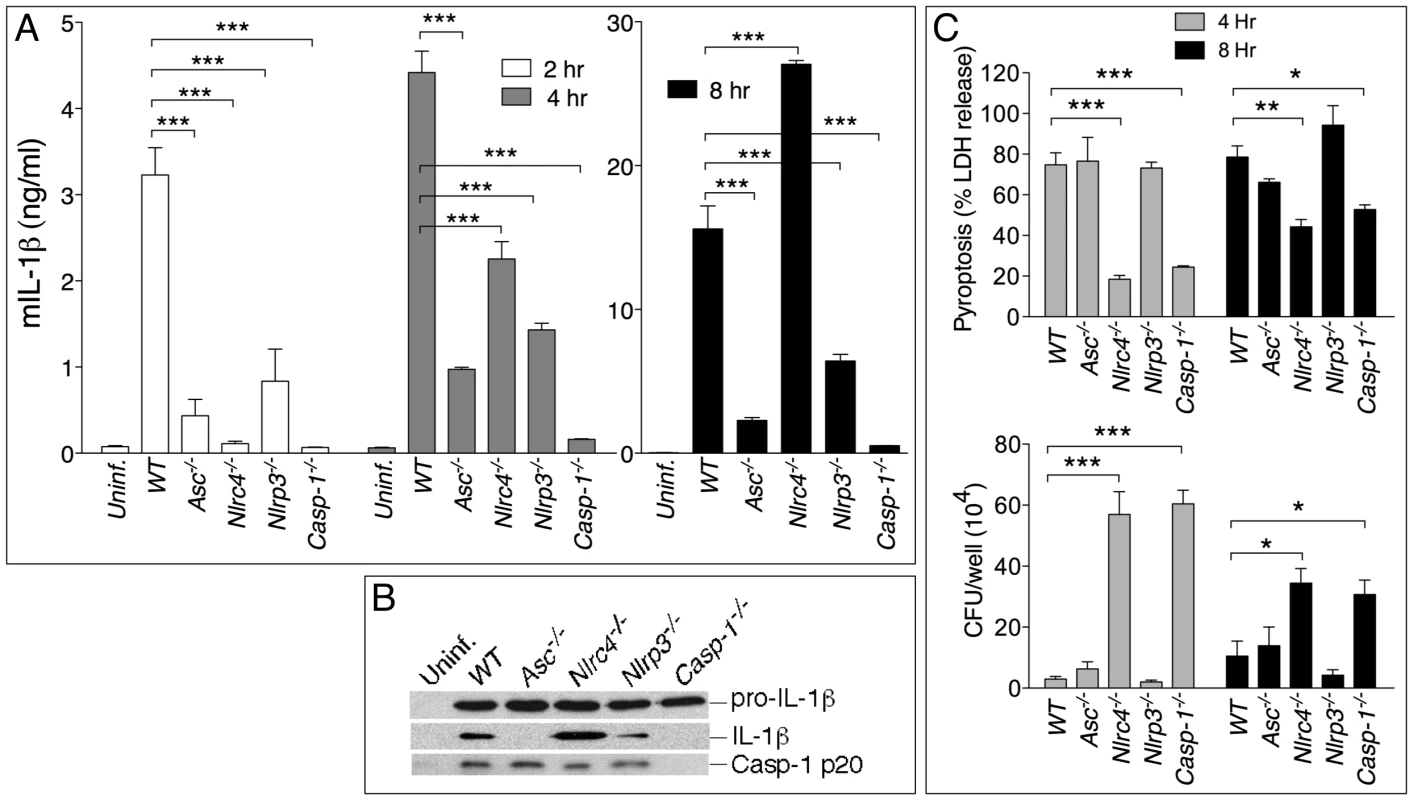
BMDM were infected with B. pseudomallei at MOI of 10. (A) Secretion of mature IL-1β was measured in conditioned supernatants of infected and uninfected cells at the indicated times. (B) Processing of IL-1β and caspase-1 was detected by immunoblot in 8 h conditioned supernatants from A. Pro-IL-1β was detected in cell lysate of the infected cells. (C) Induction of pyroptosis was measured as LDH release in conditioned supernatants of infected BMDM (MOI 10) (upper panel). Infected BMDM were lysed at the indicated time points after infection and intracellular bacteria growth was quantitated (lower panel). One experiment representative of three is shown. *p<0.05, **p<0.01, ***p<0.001 (1way ANOVA). Inflammasome-mediated induction of pyroptosis has been demonstrated to be a mechanism that restricts growth of certain intracellular bacteria [2], [3]. To measure induction of pyroptosis in cells infected with B. pseudomallei we used a kanamycin protection assay that allows only replication of intracellular bacteria whereas cells that undergo pyroptosis expose the bacteria to the microbicidal action of the antibiotic present in the medium. Induction of pyroptosis and intracellular bacterial replication were measured in WT or inflammasome-deficient BMDM infected with B. pseudomallei. As shown in figure 1C (upper graph), pyroptosis of infected cells (as measured by release of LDH in culture supernatants) was significantly reduced in Casp1-/- and Nlrc4-/- cells compared to WT and Nlrp3-/-. Importantly, induction of pyroptosis was not lost in Asc-/- cells. NLRC4-mediated pyroptosis induced by other bacteria is also reported to be ASC-independent [16]–[19]. Consistent with the role of pyroptosis as a mechanism to restrict intracellular bacteria growth, considerably less intracellular bacteria were recovered from WT, Nlrp3-/-, and Asc-/- cells than Casp1-/- or Nlrc4-/- cells at all time points (figure 1C, lower graph). Similar results regarding IL-1β processing and secretion and induction of pyroptosis were obtained using BMDC derived from the inflammasome–deficient mice (supplementary figure S1).
Taken together these results show that infection of macrophages and dendritic cells with B. pseudomallei leads to activation of the NLRC4 and NLRP3 inflammasomes. NLRC4 contributes to IL-1β during the early phase of the infection and induction of pyroptosis that restricts bacterial growth. NLRP3 does not control pyroptosis and primarily controls IL-1β secretion. It should be noted that the defective IL-1β production of Nlrc4-/- and Nlrp3-/- cells cannot be ascribed to the difference in induction of pyroptosis: thus Nlrp3-/- cells produce less cytokine than WT cells despite undergoing pyroptosis to the same extent as WT cells. Conversely, Nlrc4-/- cells, which are resistant to pyroptosis, still produce less cytokine than WT cells at the early time point. However, at later time points Nlrc4-/- cells produce considerably more IL-1β than WT cells. This is likely due to the fact that WT cells rapidly die after infection while Nlrc4-/- cells remain viable and continue to synthesize and secrete IL-1β.
Role of inflammasomes in murine melioidosis
The role of the inflammasome during in vivo B. pseudomallei infection was next analyzed using a mouse model of melioidosis (figure 2). WT mice or inflammasome-deficient mice were infected intranasally with B. pseudomallei (100 CFU) and their weight (not shown) and survival were monitored (figure 2A). All mice started to lose weight 2 days post-infection. Generally, mice that survived the infection started to recover weight 7 days post-infection. Casp1-/-, Nlrc4-/-, and Asc-/- mice were extremely susceptible to melioidosis compared to WT mice. Nlrp3-/- mice were also considerably more susceptible than WT mice but slightly more resistant than the other inflammasome deficient mice. Measurement of the bacterial burdens in lungs, spleens, and livers of infected mice 24 hours (data not shown) and 48 hours post-infection revealed that Nlrc4-/- and Casp1-/- mice carried considerably higher burdens in all three organs than WT mice (figure 2B). Surprisingly, the bacterial burden of Asc-/- and Nlrp3-/- mice was not significantly different from that of WT mice at the tested time points despite their higher mortality.
Fig. 2. Differential contributions of NLRP3 and NLRC4 to melioidosis. 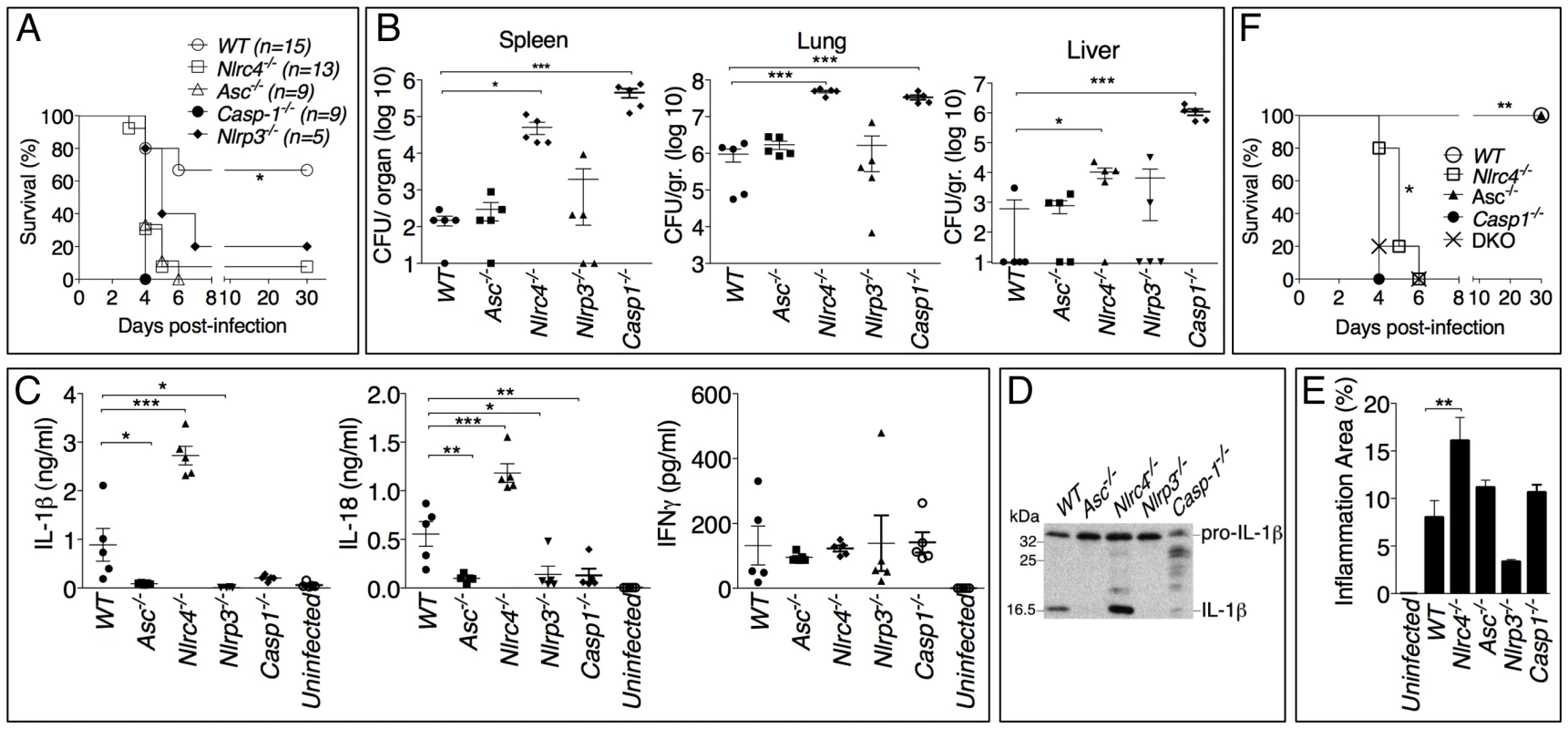
(A) Mice were intranasally infected with B. pseudomallei (100 CFU) and their survival was monitored. *p<0.05 (Kaplan-Meier), WT compared to other genotypes (B) Mice were sacrificed 48 hours post-infection and the bacterial burdens were measured in organ homogenates. (C) Cytokines were measured in BALF obtained 48 hours post-infection. (D) Processing of IL-1β was detected by immunoblot in BALF from C. (E) Lung sections were stained with H&E and the total area of the inflammatory nodules was measured and expressed as percentage of the total lung lobe area. *p<0.05, **p<0.01, ***p<0.001 (1way ANOVA). (F) Mice were intranasaly infected with B. pseudomallei (25 CFU) and their survival was monitored. **p<0.01 WT compared to other genotypes and *p<0.05 Casp-1-/- compared to Nlrc4-/- (Kaplan-Meier). Cytokine levels were measured in bronchio-alveolar lavage fluids (BALF) obtained from infected mice (figure 2C). Confirming the in vitro results, IL-1β and IL-18 levels were severely reduced in Asc-/-, Casp1-/- and Nlrp3-/- mice. In contrast, IL-1β and IL-18 were present in the lungs of Nlrc4-/- mice in amounts considerably higher than WT mice. Immunoblotting experiments confirmed that the IL-1β measured by ELISA was in fact the p17 mature form of IL-1β (figure 2D). Thus, although the in vitro experiments demonstrated that both the NLRP3 and the NLRC4 inflammasome contribute to IL-1β and IL-18 production in response to B. pseudomallei infection, it is the NLRP3 inflammasome that primarily mediates production of these cytokines in vivo. The levels of several other proinflammatory cytokines, including IL-1α (figure S2), were significantly elevated in Nlrc4-/- BALF. It is interesting to note that the levels of IL-18 in BALF of Asc-/- and Casp1-/- mice, although very low, were higher than uninfected mice suggesting the existence of inflammasome-independent mechanisms to produce IL-1β and IL-18, as it has been previously shown in models of highly neutrophilic inflammation [20]-[23].
Histological analysis of the infected lungs revealed extensive inflammatory cell infiltration in the lung parenchyma (data not shown). The area of the inflammatory nodules, relative to the total area of the lung lobe, was calculated for each given section and found to be significantly greater in Nlrc4-/- mice compared to WT mice (figure 2E). This result was consistent with the elevated levels of inflammatory cytokines and chemokines produced by Nlrc4-/- mice. Taken together these results suggest a scenario where failure of Nlrc4-/- infected macrophages to undergo pyroptosis results in higher bacterial burden and continued production of IL-1β and other factors that attract more inflammatory cells, perpetuating lung inflammation and promoting bacteria dissemination.
Thus, our results identified two distinct infammasome-mediated mechanisms that efficiently restrict B. pseudomallei growth and pathogenesis: production of the cytokines IL-1β and IL-18 and induction of pyroptosis. The high susceptibility of Nlrp3-/- and Asc-/- mice to meliodiosis is due to defective cytokine production while that of the Nlrc4-/- mice likely results from defective pyroptosis. Casp1-/- mice are impaired in both inflammasome effector mechanisms and, therefore, we predicted that they would be more vulnerable to B. pseudomallei than Asc-/- or Il1-r1-/--Il-18-/- double knock-out mice (DKO) (that are defective in cytokines but retain pyroptosis) or Nlrc4-/- mice (that retain IL-1β/IL-18 functionality but are deficient in pyroptosis). This prediction was found to be correct. As shown in figure 2F, when mice were infected with only 25 CFU (a non-lethal dose for WT mice) the mean time to death of Nlrc4-/- and Il-1r1-/--Il-18-/- DKO mice was slightly but significantly (p<0.05, Kaplan-Meier test) increased compared to Casp1-/- mice. Surprisingly, Asc-/- mice, which should be equivalent to DKO because of the absence of IL-1β or IL-18, survived the infection. This may be explained by the observation that IL-18, although drastically reduced, it is still detectable in Asc-/- mice at higher level than uninfected mice (figure 2C).
Role of IL-18 and IL-1β in murine melioidosis
We next analyzed the role of the inflammasome-dependent cytokines IL-1β and IL-18 during murine melioidosis. IL-18-deficient mice were extremely susceptible to B. pseudomallei infection even when infected with 25 CFU, a dose of bacteria that caused no mortality and only mild weight loss in WT mice (figure 3A). In contrast, Il-1r1-/- mice displayed increased resistance to B. pseudomallei infection compared to WT mice (figure 3A and see below). The survival of mice deficient in both IL-18 and IL-1RI (DKO) was indistinguishable from the Il-18-/- mice when the animals were infected with 100 CFU. However, in DKO mice infected with 25 CFU (figure 3A, right panel) the concomitant absence of IL-18 and IL-1RI provided a significant advantage over Il-18-/- mice (p<0.05) suggesting a detrimental role of IL-1RI-mediated signaling in melioidosis (see below).
Fig. 3. Differential contributions of IL-1 and IL-18 to melioidosis. 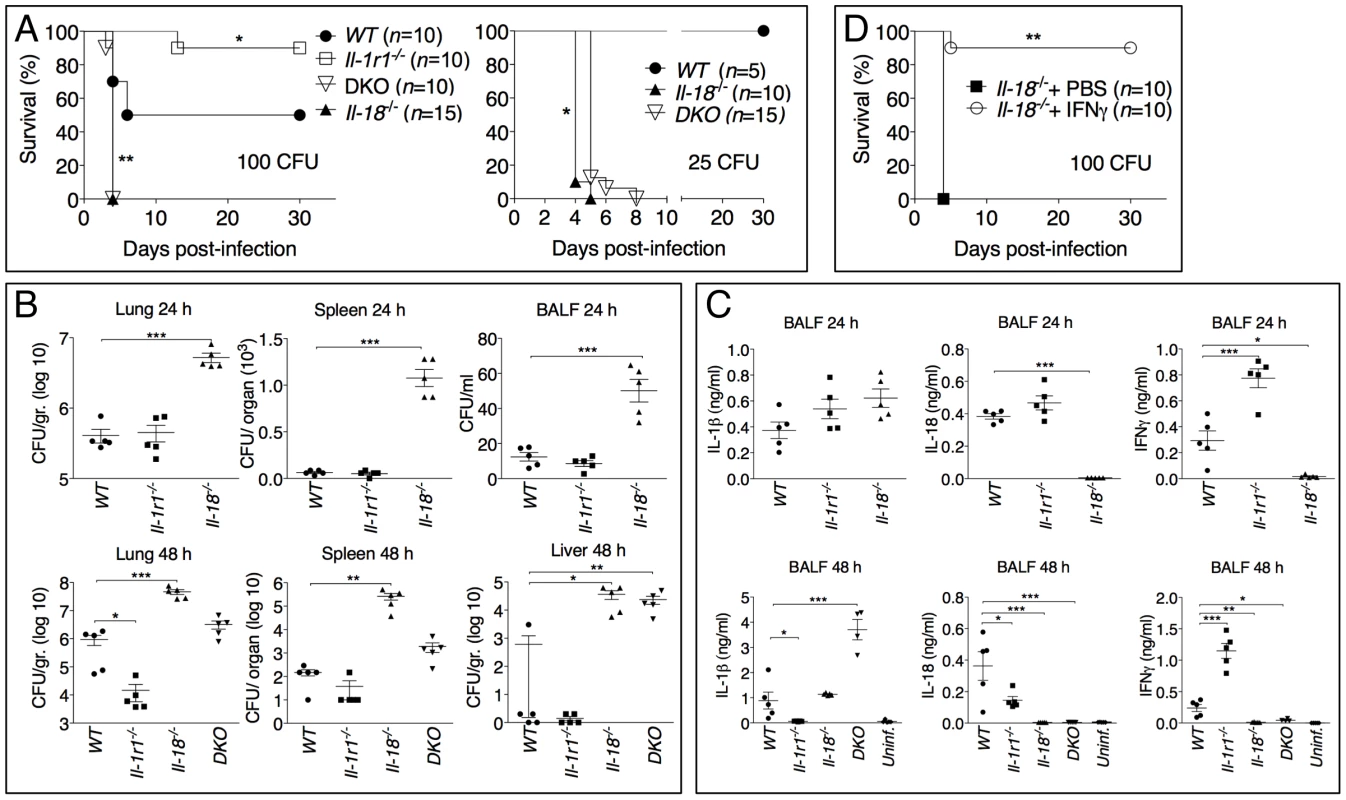
(A) Mice were intranasaly infected with B. pseudomallei (100 CFU, left, or 25 CFU, right) and their survival was monitored. *p<0.05, **p<0.01 (Kaplan-Meier), WT compared to other genotypes (left), Il-18-/- compared to DKO (right). (B, C) Mice infected with B. pseudomallei (100 CFU) were sacrificed 24 hours or 48 hours postinfection and the bacterial burdens in organ homogenates and BALF (B) or cytokines levels in BALF (C) were measured. *p<0.05, **p<0.01, ***p<0.001 (1way ANOVA). (D) Il-18-/- mice were intranasally infected with B. pseudomallei (100 CFU) and their survival monitored. Mice were administered daily injections of PBS or IFNγ (1 µg) for the first 8 days. **p<0.01 (Kaplan-Meier). Confirming the different susceptibilities of Il-18-/- and Il-1r1-/- mice to melioidosis, the bacterial burdens observed in the lungs, spleens, livers, and BALF of infected Il-18-/- mice were dramatically higher than that of WT mice even at early time points (24 hours post infection, figure 3B). In contrast, significantly lower amounts of bacteria were recovered 48 hours post infection from Il-1r1-/- mice compared to WT mice confirming their higher resistance.
Measurements of cytokines in the BALF obtained from mice at 24 and 48 hours post-infection (figure 3C) indicated that the levels of IFNγ were drastically reduced in Il-18-/- mice, a finding consistent with the established function of IL-18 as an IFNγ-inducing cytokine. Remarkably, IFNγ levels in Il-1r1-/- mice were greatly increased compared to WT mice. The levels of the neutrophil attractants Mip-2, KC, and IL-17 were also decreased in Il-1r1-/- mice and increased in Il-18-/- mice (figure S2). The number of inflammatory cells recovered from the BALF of infected Il-1r1-/- mice was significantly decreased compared to WT mice (see figure 4B, left panel). Histological analysis of the infected lungs revealed extensive inflammatory cell infiltration in the lung parenchyma of Il-18-/- mice (see figure 4C). The area of the inflammatory nodules, relative to the total area of the lung lobe, was calculated for each given section and found to be significantly greater in Il-18-/- mice compared to WT mice (figure 4D).
Fig. 4. Deleterious role of IL-1β in melioidosis. 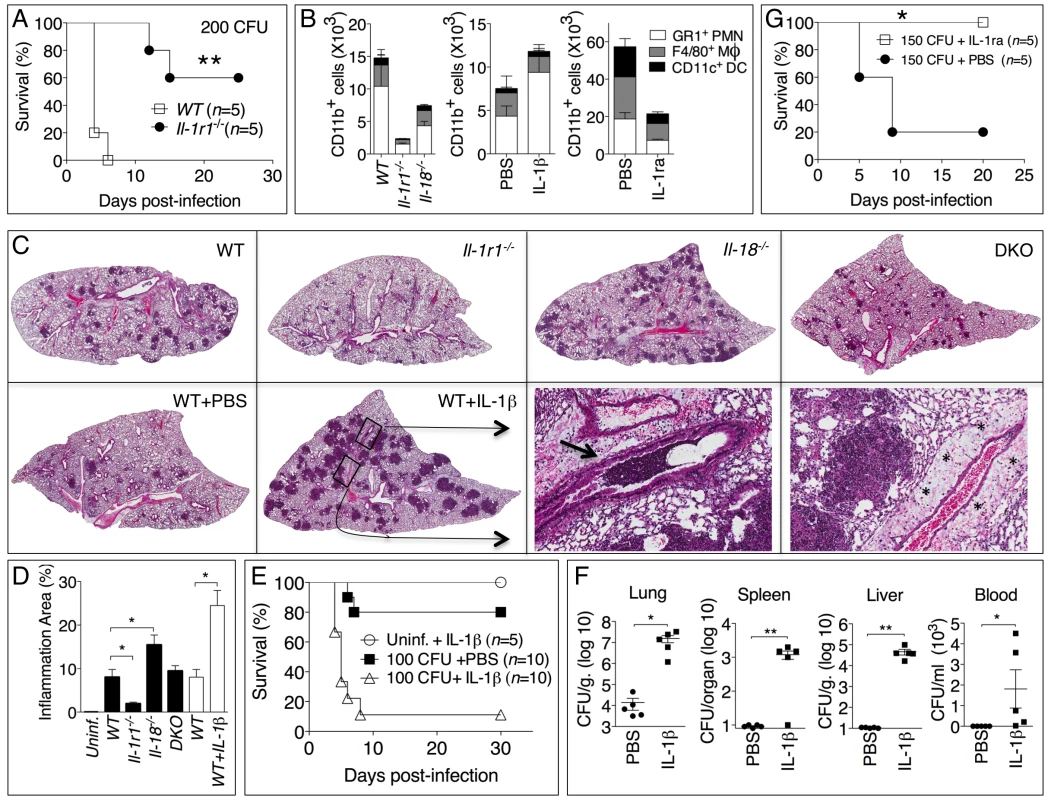
(A) Mice were infected intranasally with B. pseudomallei (200 CFU) and their survival was monitored. (B) Flow cytometric analysis for myeloid cell composition of BALF obtained from the indicated infected mouse strains at 24 h (left); infected WT mice injected with IL-1β at 48 h (center graph); or infected WT mice injected with PBS or IL-1ra at 72 h. *p<0.05, ***p<0.001 (1way ANOVA). (C) Histopathology of lungs of infected mice of indicated genotype (0.8X magnification) at 48 h post-infection (upper row) or WT mice injected with PBS or IL-1β at 72 hrs post-infection (lower row). Bottom right panel shows 10X magnification of the indicated insets from WT+IL-1β showing airways obstruction (arrow head) and perivascular edema (asterisks). One image representative of five animals/experimental group. (D) The total area of the inflammatory nodules of lung sections of C was measured and expressed as percentage of the total lung lobe area. *p<0.05 (t-test). (E) WT mice were infected intranasally with B. pseudomallei (100 CFU) and received daily i.p. injections of PBS or IL-1β (1 µg). One group of mice were treated with IL-1β but not infected. **p<0.01, ***p<0.001 (Kaplan-Meyer). (F) Mice infected and IL-1β-injected as in E were sacrificed 72 h post-infection and the bacterial burden was measured in organs and blood. *p<0.05, **p<0.01 (1way ANOVA). (G) WT mice were infected intranasally with B. pseudomallei (150 CFU) and were administered daily injections of PBS or IL-1ra. *p<0.05 (Kaplan-Meyer). Considering that IFNγ is known to play a protective role during several bacterial infections, including B. pseudomallei [8]–[10], these results suggested that the reduced resistance of Il-18-/- mice to B. pseudomallei infection may be due to lack of IFNγ induction. To test this hypothesis, a group of Il-18-/- mice infected with B. pseudomallei were given daily intraperitoneal injections of either recombinant IFNγ or PBS. As shown in figure 3D, exogenous IFNγ completely protected the mice suggesting that IL-18 exerts its protective action primarily through induction of IFNγ.
Deleterious role of IL-1β in melioidosis
The results of figure 3 showed that Il-1r1-/- mice were more resistant to lung infection with B. pseudomallei. This appeared even more evident when mice were infected with higher doses of B. pseudomallei that killed all WT mice but only a fraction of the Il-1r1-/- mice (figure 4A). Recruitment of neutrophils, macrophages, and dendritic cells into alveolar spaces was decreased in Il-1r1-/- mice compared to WT mice (figure 4B, left graph). Lower levels of the neutrophil enzyme myeloperoxidase (MPO) were detected in the BALF of Il-1r1-/- mice compared to WT (figure S3). The extent of lung inflammation, as measured by the number and size of inflammatory nodules, was also significantly decreased in Il-1r1-/- mice (figure 4C, and 4D).
To further test the hypothesis that IL-1R-mediated signaling has a deleterious role in this model of melioidosis, WT mice were infected with 100 CFU B. pseudomallei and were given daily intraperitoneal injections of IL-1β or PBS (figure 4E). All mice that received the cytokine succumbed to the infection compared to significantly higher survival of the control group. Injection of IL-1β in non-infected mice had no deleterious effect aside from a transient, negligible weight loss (not shown). The bacteria burdens in organs of IL-1β-treated mice 72 hours post infection were dramatically higher than the control group and bacteremia was detected in IL-1β-treated mice but not control mice (figure 4F). Higher number of neutrophils, macrophages, and dendritic cells were found in the BALF of IL-1β-treated mice (figure 4B, center graph). This correlated with increased level of MPO in BALF (figure S3). The increased inflammatory cell recruitment to the lungs of IL-1β-treated mice was likely due to the induction, by IL-1β, of neutrophil chemoattractans KC (CXCL1) and MIP-2 (CXCL-2), which in fact were detected at very high levels in the BALF of IL-1β-treated mice (figure S2). Histological analysis of lung sections of mice treated with IL-1β showed a dramatic increase in the number and size of the foci of infiltrating inflammatory cells (figure 4C, lower left panels) and evidence of perivascular edema and airway obstruction (figure 4C, lower right panels).
If IL-1β in fact has a detrimental effect during melioidosis, inhibition of its activity should lower morbidity and mortality of mice infected with B. pseudomallei. As shown in figure 4G, administration of the IL-1 receptor antagonist IL-1ra protected mice from infection with lethal doses of B. pseudomallei. Mice treated with IL-1ra had decreased recruitment of inflammatory cells to the alveolar spaces (figure 4B, right graph) lower level of MPO in BALF (figure S3), and less severe lung pathology (data not shown).
Neutrophils fail to restrict B. pseudomallei intracellular growth and are resistant to pyroptosis
Surprisingly, in our experiments lower numbers of neutrophils in Il-1r1-/- mice correlated with lower bacterial burdens while IL-1β administration resulted in increased neutrophil recruitment but also increased bacterial burdens and systemic dissemination. These results would be consistent with the notion that neutrophils are not very effective at containing B. pseudomallei infection and, in fact, may foster its spread. In support of this idea, human neutrophils infected with B. pseudomallei underwent pyroptosis at a much slower rate than infected monocytes (figure 5A). Concomitantly, intracellular bacteria growth increased with time in infected neutrophils but decreased in monocytes. Consistent with previously published results [3], neutrophils did not express NLRC4 mRNA (figure 5B) suggesting they may be resistant to pyroptosis. Similar results were obtained using neutrophils and CD11b+ monocytic cells isolated from mouse bone marrow (figure 5C). WT monocytes infected with B. pseudomallei underwent pyroptosis and failed to support bacteria replication whereas Nlrc4-/- cells were resistant to pyropotosis and supported B. pseudomallei intracellular replication. In contrast, both WT and Nlrc4-/- neutrophils did not undergo pyroptosis and supported B. pseudomallei intracellular replication to the same extent. These results suggest that the deleterious role of IL-1β during melioidosis may be due, in part, to excessive recruitment of neutrophils, a cell type that may be permissive for B. pseudomallei replication. We decided to test this hypothesis in Nlrc4-/- mice. As shown in figure 2E, infected Nlrc4-/- mice showed a significantly higher degree of lung inflammation. Consistent with higher neutrophil influx in the lung of Nlrc4-/- mice, the levels of the neutrophil enzyme MPO were significantly increased in their BALF compared to WT mice (figure 6A). To test the hypothesis that excessive neutrophil influx is deleterious during melioidosis, Nlrc4-/- mice were injected with IL-1ra or with antileukinate, a hexapeptide that acts as a CXCR2 neutrophil chemokine receptor antagonist. Both factors have been shown to inhibit neutrophil recruitment to inflammatory sites in different animal models including lung inflammation [24]–[26]. As shown in figure 6B, administration of IL-1ra or antileukinate protected Nlrc4-/- mice infected with low doses of B. pseudomallei. The number of inflammatory cells in the BALF of Nlrc4-/- mice treated with IL-1ra or antileukinate was reduced compared to mice who received PBS injection (figure 6C) and lower levels of MPO were detected in the BALF of injected mice (figure S3). Moreover, systemic spread of bacteria to spleen or liver was reduced by administration of either drug (figure 6D).
Fig. 5. Neutrophils do not undergo pyroptosis and fail to restrict B. pseudomallei replication. 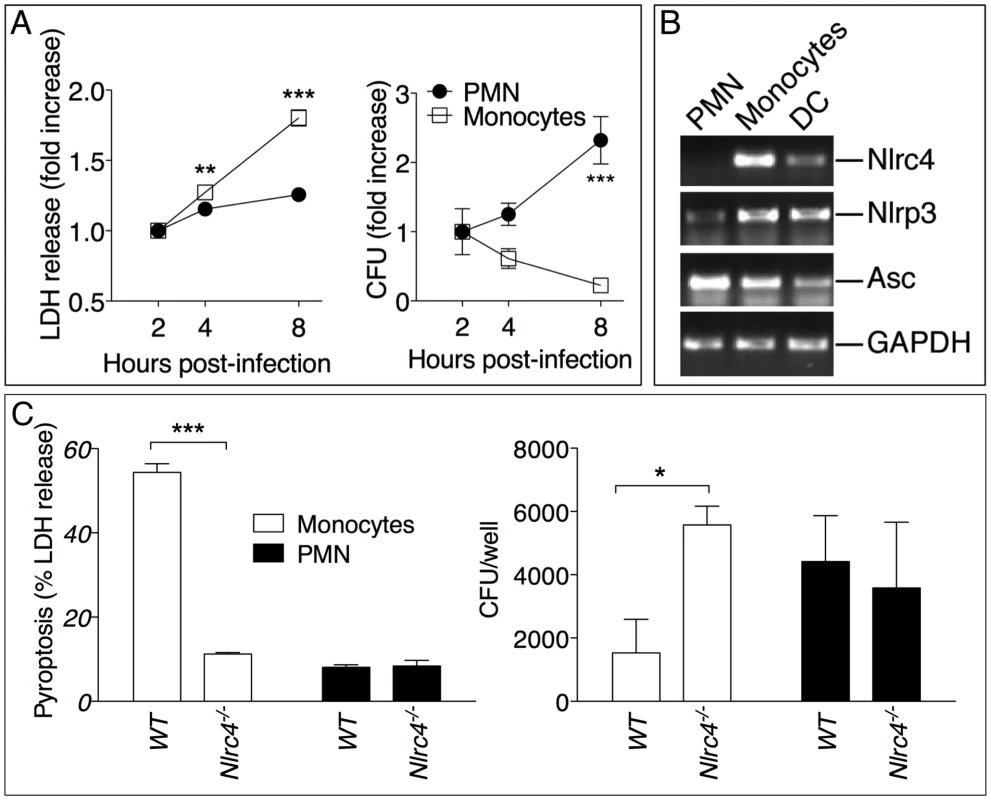
(A) Human neutrophils or monocytes were infected with B. pseudomallei (MOI 50) and pyroptosis and intracellular bacterial growth were measured at the indicated time points. One experiment representative of two is shown. Data are shown as the fold increase normalized to the 2 h values. (B) RT-PCR analysis of total RNA from the indicated cell types. (C) WT or Nlrc4-/- mouse neutrophils and monocytic cells were infected with B. pseudomallei (MOI 50) and pyroptosis and intracellular bacterial growth were measured 8 hours post infection. * p<0.05, **p<0.01, ***p<0.001 (2way ANOVA, post-test Bonferroni). Fig. 6. Inhibition of neutrophil recruitment to the lung protects Nlrc4-/- mice from melioidosis. 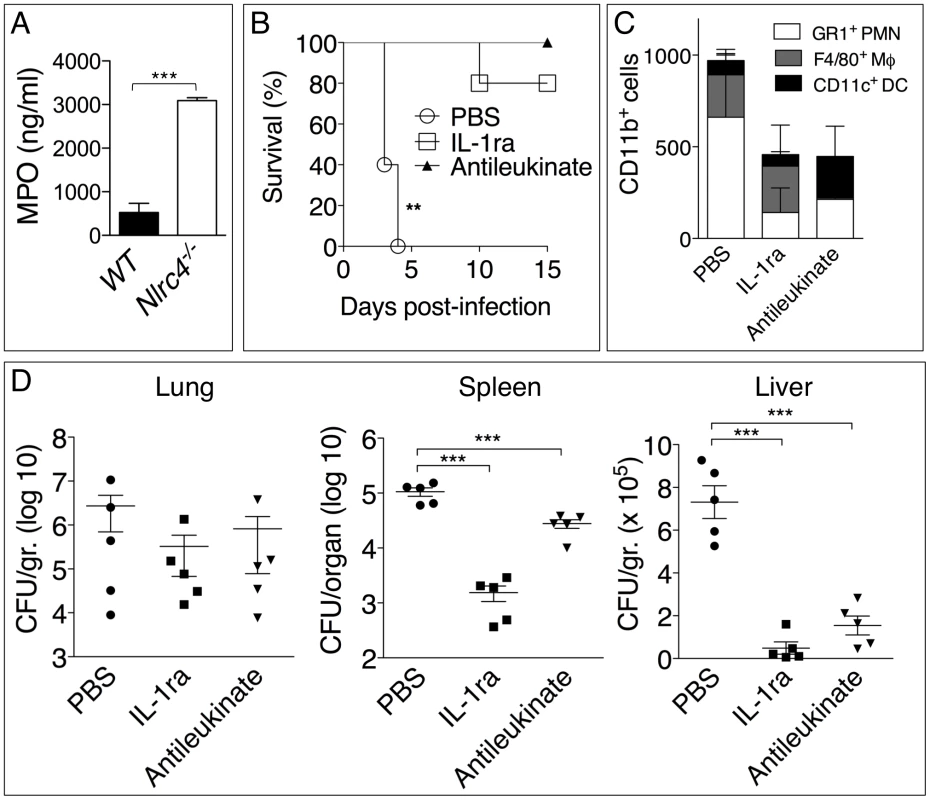
(A) Myeloperoxidase was measured in BALF of WT and Nlrc4-/- mice. (B) Nlrc4-/- mice were infected intranasally with B. pseudomallei (25 CFU) and were administered daily injections of PBS or IL-1ra (i.p.) or antileukinate (s.c.). **p<0.01, ***p<0.001 (Kaplan-Meyer). (C) Flow cytometric analysis for myeloid cell composition of BALF obtained from Nlrc4-/- mice infected and treated with IL-1ra or antileukinate. (D) Nlrc4-/- mice infected and treated with IL-1ra or antileukinate were sacrificed 48 h post-infection and the bacterial burden was measured in organs. *p<0.05, **p<0.01 (1way ANOVA). Discussion
The inflammatory response to infection consists of several protective effector mechanisms that must be activated and orchestrated in order to maximize microbicidal functions and stimulation of adaptive immunity while, at the same time, minimize damage to the host tissues. Alteration in this balance may result in excessive and non-resolving inflammation that leads to severe morbidity and mortality [27]. It is becoming clear that to be effective but non-pathogenic the inflammatory response must be tailored to each specific pathogen. Here we have analyzed the role of a very important inflammatory pathway during infection with the lung pathogen B. pseudomallei. Using a murine model of melioidosis we have determined the role of various components of the inflammasome and the downstream effector mechanisms (production of IL-1β, IL-18, and pyroptosis) and we report several novel discoveries that greatly increase our understanding of the pathogenesis of melioidosis (see model in figure 7).
Fig. 7. Inflammasomes-dependent protective and deleterious responses activated by B. pseudomallei. 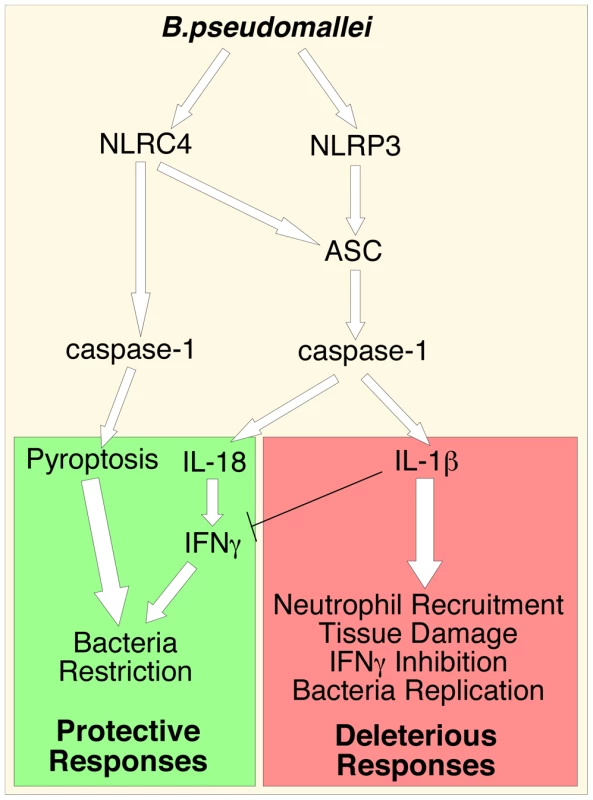
B. pseudomallei induces NLRC4-dependent pyroptosis that restricts intracellular bacterial growth. Activation of NLRP3-inflammasome leads to production of IL-18 and IL-1β. IL-18 is protective because of its induction of IFNγ. IL-1β deleterious role may be due to several reasons including excessive recruitment of neutrophils, which may support intracellular growth of B. pseudomallei, tissue damage, and inhibition of IFNγ production. First, we found that both NLRC4 and NLRP3 play non-redundant roles during detection of B. pseudomallei. Analysis of in vitro infected macrophages or dendritic cells allowed us to estimate the relative contribution of NLRC4 and NLRP3 to IL-1β production. Our findings indicated that production of IL-1β is primarily dependent on the NLRP3 inflammasome. During the early phase of the infection the NLRC4 inflammasome also significantly contributes to IL-1β production. We posit that this pattern likely reflects the fact that the NLRC4 inflammasome responds to T3SS deployment, which occurs early in the infection cycle, while activation of NLRP3 may require escape from the phagosome, which is a relatively slower event [28]. B. pseudomallei , including the strain used in our study, possesses at least three T3SS gene clusters, one of which is similar to the Salmonella SP-1 pathogenicity island and has been shown to be an important virulence factor required for escape from the phagosome, induction of IL-1β production, and pathogenicity [13], [28]. In addition to mediating host recognition of cytosol-delivered flagellin, NLRC4 also recognizes a structural motif found in the basal body rod components of the T3SS of various bacteria, including B. pseudomallei [29]. We have determined (data not shown) that transfection of B. pseudomallei flagellin protein into the cytoplasm of BMDC leads to NLRC4-dependent production of IL-1β. This result agrees with previously published evidence and indicates that B. pseudomallei (like some other bacteria) expresses multiple factors (e.g. flagellin, basal rods) that are recognized by the NLRC4 inflammasome. The mechanism responsible for NLRP3 activation by B. pseudomallei remains unclear.
In addition to controlling IL-1β and IL-18 production, NLRC4 also mediates pyroptosis, a form of cell death that is an effective mechanism to restrict growth and dissemination of intracellular bacteria [3]. Here we showed that B. pseudomallei-induced pyroptosis was caspase-1-dependent but ASC-independent, in agreement with works that showed ASC redundancy for pyroptosis induced by other bacteria [16]–[19]. However, the fact that production of IL-1β in response to B. pseudomallei infection is seriously compromised in Asc-/- cells indicates that this adaptor molecule plays a critical role in NLRC4-mediated cytokine production and suggests that NLRC4 can form two distinct inflammasomes: one that contains ASC and regulates IL-1β processing, and one devoid of ASC that activates caspase-1 and triggers pyroptosis, as recently proposed [30]. It has been recently shown [4], [5] that NAIP molecules determine the specificity of NLRC4 for its activators and, we would further speculate, for its down-stream effector mechanisms. Whether NLRC4 relies on other molecules to recognize B. pseudomallei remains to be ascertained. We tested the susceptibility to B. pseudomallei of C57BL/6J-Chr13A/J/NaJ mice, a consomic C57BL/6 strain that carries the A/J NAIP5 allele that renders them susceptible to Legionella infection [31]-[34], and found that they were indistinguishable from WT mice (data not shown).
Analysis of inflammasomes-deficient mice intranasaly infected with B. pseudomallei confirmed the importance of ASC, caspase-1, and both NLRP3 and NLRC4 inflammasomes for resistance to melioidosis. However, quite surprisingly, although production of IL-1β and IL-18 in vitro is mediated by both NLRP3 and NLRC4, in vivo it is exclusively dependent on the NLRP3-ASC-caspase-1 inflammasome (figure 2C). In contrast, Nlrc4-/- mice produce these cytokines in amounts that exceed even those detected in WT mice. Remarkably, despite the abundance of IL-1β and IL-18, Nlrc4-/- mice were dramatically more susceptible to melioidosis than WT mice, rapidly succumbed to the infection, and had very high organ's bacteria burden and worst neutrophilic lung inflammation. Thus, the critical role of NLRC4 during melioidosis is independent of IL-1β and IL-18 production. Rather, our results suggest that pyroptosis, which we show is defective in Nlrc4-/- cells, is a critical NLRC4 effector mechanism to fight B. pseudomallei and, in its absence, bacterial replication and IL-1β production proceeds unrestrained causing severe inflammation, morbidity and mortality. Moreover, our analysis indicates that pyroptosis and IL-18 are both required and contribute equally to resistance to melioidosis. Thus, deficiency of either is equally lethal while deficiency of both (Casp1-/- mice) further worsens the outcome. It is important to emphasize that our study is the first to demonstrate the importance of pyroptosis in the context of an infection with a clinically relevant human pathogen that has not been genetically manipulated, as opposed to the previous seminal work by Miao et al. [3] that elegantly employed genetically manipulated bacteria and mouse strains to identify pyroptosis as an effective innate immune defence mechanism against bacterial infections. Previous reports have demonstrated activation of both NLRP3 and NLRC4 inflammasomes in response to infection with Legionella pneumophila [16], Listeria monocytogenes [35], and Salmonella typhimurium [36]. However, in those infection models NLRP3 and NLRC4 appeared to play redundant roles while in our model we were able to assign distinct functions to each inflammasome.
A great number of publications have documented the role of IL-18 and IL-1β during infections with a variety of pathogens. Almost invariably, both cytokines were found to have a protective function. Remarkably, our results show that while Il-18-/- mice are profoundly vulnerable to melioidosis, as previously shown [37], Il-1r1-/- mice were unexpectedly more resistant than WT mice. The protective role of IL-18 during melioidosis appears to be related to its ability to induce IFNγ, as administration of exogenous IFNγ completely rescued the survival of Il-18-/- infected mice. IFNγ activates the microbicidal activity of macrophages and has been shown to be important for resistance against infection with many pathogens including B. pseudomallei [8]–[10]. It is interesting and surprising to see that Asc-/- and Nlrp3-/- mice, which are defective in IL-1β and IL-18 production, are more resistant to B. pseudomallei than mice lacking IL-18. It is worth noting that although IL-18 production is drastically reduced in Asc-/- and Nlrp3-/- mice, it is still detectable in these mice at higher level than uninfected mice. It is conceivable that this inflammasome-independent production of IL-18 may be sufficient to provide some level of protection to Asc-/- and Nlrp3-/- mice against infection with low B. pseudomallei CFU.
Our discovery that Il-1r1-/- mice were more resistant than WT to B. pseudomallei infection is quite surprising considering that this cytokine has been shown to be protective in several bacterial, viral, and fungal infection models [38]. Studies in humans have also shown that inhibition of the function of IL-1 using the IL-1R antagonist IL-1ra (Kineret) is associated with increased susceptibility to bacterial infection. Infected Il-1r1-/- mice had lower BALF levels of proinflammatory cytokines as well as a reduction of neutrophil influx into the lungs, bacterial burdens, and lung pathology. Consistent with a deleterious role of IL-1β in melioidosis, administration of recombinant IL-1β drastically increased mortality, inflammation, pathology, and bacteria burdens while administration of IL-1ra (Kineret) rescued the survival of WT mice infected with a lethal dose of B. pseudomallei.
The reason for the detrimental effect of IL-1β during melioidosis is unclear and it is likely that several factors determine this outcome. IL-1β is one of the most powerful proinflammatory cytokines, it affects virtually every organ, and several human pathologies are primarily driven by unrestrained IL-1β production. One possible mechanism to account for IL-1β's deleterious role in melioidosis may be related to its ability to inhibit IFNγ production through the induction of the cycloxigenase COX-2 and release of prostaglandin PGE2 [39], [40]. Our observation that the level of IFNγ, a protective factor against B. pseudomallei, was significantly higher in infected Il-1r1-/- than WT mice (figure 3C) supports this type of scenario in melioidosis. Interestingly IL-8, a potent neutrophil chemoattractant, was shown to enhance the intracellular growth and survival of B. cepacia in bronchial epithelial cell lines [41]. Whether IL-1β promotes B. pseudomallei intracellular replication is not known but our preliminary results indicated that induction of pyroptosis by B. pseudomallei was not affected by IL-1β.
IL-1β regulates neutrophil recruitment to inflammatory sites through multiple mechanisms including induction of KC, MIP-2, and IL-17, inflammatory mediators whose expression in our experiments correlated with the presence/absence of IL-1RI-mediated signaling. Excessive PMN recruitment is known to cause tissue damage leading to functional impairment of multiple organs, including the lungs [42], [43]. One of the most remarkable observations reported here is that the absence of IL-1 signaling was associated with reduced lung neutrophilic inflammation but also lower bacterial burdens in the lungs (figure 3B, 4B). Conversely, IL-1β administration resulted in increased neutrophil recruitment but also increased bacterial burdens and systemic dissemination. These results would be consistent with the idea that neutrophils are not very effective at containing B. pseudomallei infection and, in fact, may foster its spread despite their strong microbicidal activities. This notion is supported by our observation that human or mouse neutrophils infected with B. pseudomallei failed to undergo pyropotosis (figure 5), consistent with the finding that neutrophils do not express NLRC4 [3]. At the same time, intracellular B. pseudomallei replication proceeded unaffected in both WT and Nlrc4-/- neutrophils in agreement with a report that showed that B. pseudomallei is intrinsically resistant to killing by infected PMN [44]. In support for a deleterious role of neutrophils in melioidosis we found that inhibition of their recruitment by administration of IL-1ra or the CXCR2 neutrophil chemokine receptor antagonist antileukinate protected Nlrc4-/- mice from infection with low doses of B. pseudomallei and decreased systemic spread of bacteria (figure 6).
Taken together our results suggest the following scenario: failure of Nlrc4-/- infected macrophages to undergo pyroptosis results in higher bacteria burden and continued production of IL-1β and other factors that attract more inflammatory cells, including neutrophils, perpetuating excessive lung inflammation and promoting bacteria dissemination. It is tempting to speculate that IL-1β promotes B. pseudomallei growth possibly by increasing the local pool of infectable permissive cells, including PMN. Our conclusion that neutrophils are a permissive cell type for B. pseudomallei replication seems to contrast with a report [45] that indicated that depletion of neutrophils resulted in severe increase in mortality in a model of murine melioidosis. However, caution should be used in the interpretation of these types of experiments because systemic depletion of neutrophils devoids the host not only of their microbicidal function but also of the many immunomodulatory functions these cells exert [46]. Of note, mice deficient in osteopontin, a pleiotropic cytokine that is chemotactic for neutrophils, were shown to be more resistant to B. pseudomallei infection [47], supporting our conclusion that neutrophils have a detrimental role in melioidosis.
The notion that excessive inflammation may be detrimental in certain infection models is well accepted. For example, TLR-mediated signaling negatively affects the outcome of infections with West Nile Virus [48] or influenza virus [49]. The fact that IL-1β is deleterious in melioidosis but protective against other lung pathogens like Klebsiella, Francisella, Mycobacterium, Respiratory Syncytial Virus, and influenza virus likely reflects differences between the virulence strategy of B. pseudomallei and those other pathogens. The intensity, kinetics, and quality of the inflammatory response elicited by B. pseudomallei and its ability to suppress the induction of anti-inflammatory circuitries are phenomena that we are interested to investigate in detail. Despite an extensive literature search we could identify only a single report [26] where IL-1β was shown to be deleterious in bacterial infections. It was demonstrated that this cytokine had a negative effect on bacterial clearance in a model of pneumonia caused by Pseudomonas aeruginosa, an organism that shares features with Burkholderia, which was in fact previously classified in the Pseudomonas genus. Surprisingly, the same group also reported a deleterious role for IL-18 in this type of infection [50], a further indication that each pathogen displays unique virulence strategies. It has been shown that activation of the inflammasome exacerbates inflammation without restricting bacterial growth in a model of Mycobacterial infection [51]. That report did not examine the role of IL-1β but other work showed it is protective during tuberculosis [23].
This is the first report that has analyzed in detail the role of the inflammasome during melioidosis. Previous work has implicated caspase-1 [52] and IL-18 [37] in this infectious disease although the pathways that led to their activation were not investigated. Other species of Burkholderia have been used as model organisms to study aspects of inflammasome biology. Surprisingly, B. thailandensis, which is has been used as a model for melioidosis although it rarely causes disease in humans, was reported to cause similar disease in WT and IL-18 - IL-1β-double deficient mice [3] suggesting that species of Burkholderia other than B. pseudomallei may not be reliable models for melioidosis.
In summary, our work shows that NLRP3 and NLRC4 play non-redundant roles during B. pseudomallei infection by differentially regulating pyroptosis and production of IL-1β and IL-18; it demonstrates that pyroptosis is an efficient effector mechanism to restrict in vivo bacterial growth and dissemination; it identifies a deleterious role of IL-1β in melioidosis possibly due to excessive recruitment of neutrophils, a cell type that may be permissive to replication of B. pseudomallei; and, finally, it indicates that inhibition of IL-1RI-mediated signaling may be a beneficial therapeutical approach for the treatment of melioidosis.
Materials and Methods
Ethics statement
All the animal experiments described in the present study were conducted in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. All animal studies were conducted under a protocol approved by the University of Tennessee Health Science Center (UTHSC) Institutional Animal Care and Use Committee (IACUC, protocol #1854). All efforts were made to minimize suffering and ensure the highest ethical and humane standards. Research involving human blood is exempt from the human subjects regulations. Human neutrophils and monocytes were isolated from healthy donors Leukopacks obtained from Lifeblood Mid-South Regional Blood Center, Memphis TN. All leukopaks are obtained anonymously. The gender, race, and age of each donor are unknown to the investigators.
Mice
C57BL/6, Il-1r1-/-, Il-18-/-, C57BL/6J-Chr13A/J/NaJ mice were purchased from Jackson lab. Il-18-/--Il-1r1-/- double deficient mice (DKO) were obtained by crossing the parental single knock-out mice. Asc-/-, Nlrp3-/-, Nlrc4-/- (from Vishva Dixit, Genentec) and Casp1-/- (from Fayyaz Sutterwala) were bred in our facility. All mouse strains were on C57BL/6 genetic background and were bred under specific pathogen-free conditions. Age-(8–12 weeks old) and sex-matched animals were used in all experiments. Generally, experimental groups were composed of at least 5 mice. Animal and in vitro experiments involving B. pseudomallei were performed under biosafety level 3 conditions in accordance with standard operating procedures approved by the Regional Biocontainment Laboratory at UTHSC.
Bacteria, mice infection, and treatments
For all experiment the B. pseudomallei 1026b strain (a clinical virulent isolate) was used. Bacteria were grown in Luria broth (Difco) to mid-logarithmic phase, their titer was determined by plating serial dilutions on LB agar, and stocks were maintained frozen at −80°C in 20% glycerol. No loss in viability was observed over prolonged storage. For infections, frozen stocks were diluted in sterile PBS to the desired titer. Aliquots were plated on LB agar to confirm actual CFU. Mice were anesthetized with isoflurane using a Surgivet apparatus and 50 µl of bacteria suspension were applied to the nares. In some experiments, mice were injected i.p. daily with recombinant mouse IL-1β (1 µg) or IFNγ (1 µg). IL-1ra (Biovitrum) was administered by alternating s.c. and i.p. injections every 12 hours (60 mg/kg body weight). Antileukinate (American Peptide Company) was administered by s.c. injection (8 mg/kg body weight).
Generation of mouse BMDM and BMDC
Mouse macrophages or dendritic cells were generated by incubating bone marrow cells in RPMI 1640-10%FCS supplemented with either rmM-CSF or rmGM-CSF (20 ng/ml) for 8 days, respectively.
Isolation of mouse neutrophils
Neutrophils and monocytic cells were isolated from the bone marrow cells of WT or Nlrc4-/- mice using Miltenyi Ly6G microbeads. Flow-through cells, consisting mostly of monocytic cells, were further purified using Miltenyi CD11b microbeads.
Isolation of human neutrophils and monocytes
Human neutrophils and monocytes were isolated from healthy donors Leukopacks obtained from Lifeblood Mid-South Regional Blood Center, Memphis TN. Blood was mixed with Isolymph (CTL Scientific Supply Corp.) (5∶1 ratio) and RBC were allowed to sediment for 60 min at RT. The leukocytes-enriched supernatant was washed, resuspended in PBS, and stratified over Isolymph cushion and centrifuged at 1,350 rpm for 40 min. The cell pellet containing RBC and neutrophils was treated with 0.2% NaCl for 30 seconds to lyse RBC and immediatedly treated with an equal volume of 1.6% NaCl. The PBMC containing ring from the Isolymph centrifugation step was collected, washed, and monocytes were purified using CD14 microbeads (Miltenyi). The procedure routinely yield populations of purity greater than 95%.
Pyroptosis and intracellular bacteria growth (kanamycin protection assay)
Release of LDH in tissue culture media, a reflection of pyroptosis, was measured using the Roche Cytotox detection kit. BMDM, PMN, or monocytes (5×105 cells) were plated in 24 well plates. Bacteria at different MOI were added to the cell culture and the plates were centrifuged at 1500 rpm for 10 minutes to maximize and synchronize infection and incubated for 30 minutes at 37°C. Cells were washed with PBS to remove extracellular bacteria and medium containing kanamycin (200 µg/ml) was added to inhibit extracellular bacteria growth. Media were collected at 1, 2, 4, 8, 12 hours post infection for LDH measurement. Cells were lysed in PBS-2% saponin-15% BSA and serial dilutions of the lysates were plated on LB agar plates containing streptomycin (100 µg/ml) using the Eddy Jet Spiral Plater (Neutec). Bacterial colonies were counted 48 hours later using the Flash & Grow Automated Bacterial Colony Counter (Neutec).
Determination of bacteria growth in tissue culture and organs
Organs aseptically collected were weighted and homogenized in 1 ml PBS using 1 mm zirconium beads and the Mini16 bead beater. Serial dilutions were plated as described above.
Western blot
Conditioned supernatants were separated by 12% PAGE electrophoresis, transferred to PVDF membranes, and probed with rabbit anti-caspase-1 (Upstate Biotechnologies) or goat anti-mIL-1β (R&D Systems).
BALF collection and cytokines measurements
BALF were collected from euthanized mice by intratracheal injection and aspiration of 1 ml PBS. Cytokines levels in tissue culture conditioned supernatants and BALF were measured using the Milliplex mouse cytokine/chemokine panel (Millipore) and confirmed by ELISA using the following paired antibodies kits: mIL-1β and mIFNγ (eBioscience), mIL-18 (MBL Nagoya, Japan). MPO level in BALF were measured using the HyCult Biotech ELISA kit.
Flow cytometry
Cells obtained from BALF were counted and stained with CD45, CD11b, CD11c, F4/80, GR1 (Ly6G), and analyzed with a LSRII BD flow cytometer.
Histology and measurement of area of inflammatory foci
Formalin-fixed paraffin-embedded lung sections were stained with H&E and scanned using the Aperio Scanscope XT. The Aperio ImageScope software was used to quantitate the area of the inflammatory foci compared to the total lung lobe area. Results from lungs from 5 animals per group were combined.
RT-PCR
Total RNA was extracted using Trizol (Invitrogen) and 100 ng were amplified (27 cycles) using Superscript III One-step RT-PCR (Invitrogen) and primers specific for human Nlrc4, Nlrp3, Asc, and GAPDH (primers' sequence available upon request).
Statistical analysis
All data were expressed as mean ± S.E.M. Survival curves were compared using the log rank Kaplan-Meier test. 1way ANOVA and Tukey Post-test was used for analysis of the rest of data unless specified in the figure legends. Significance was set at p<0.05. Statistical analyses were performed using the GraphPad Prism 5.0.
Accession numbers
UniProtKB/Swiss-Prot ID: IL-1β, P10749; IL-1R1, P13504; IL-18, P70380; NLRP3, Q8R4B8; NLRC4, Q3UP24; ASC, Q9EPB4; Casp-1, P29452; NAIP5, Q8CGT2.
Supporting Information
Zdroje
1. DavisBKWenHTingJP 2011 The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu Rev Immunol 29 707 735
2. BrodskyIEMedzhitovR 2011 Pyroptosis: macrophage suicide exposes hidden invaders. Curr Biol 21 R72 75
3. MiaoEALeafIATreutingPMMaoDPDorsM 2010 Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat Immunol 11 1136 1142
4. KofoedEMVanceRE 2011 Innate immune recognition of bacterial ligands by NAIPs determines inflammasome specificity. Nature 477 592 595
5. ZhaoYYangJShiJGongYNLuQ 2011 The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature 477 596 600
6. ChengACCurrieBJ 2005 Melioidosis: epidemiology, pathophysiology, and management. Clin Microbiol Rev 18 383 416
7. WiersingaWJvan der PollTWhiteNJDayNPPeacockSJ 2006 Melioidosis: insights into the pathogenicity of Burkholderia pseudomallei. Nat Rev Microbiol 4 272 282
8. MiyagiKKawakamiKSaitoA 1997 Role of reactive nitrogen and oxygen intermediates in gamma interferon-stimulated murine macrophage bactericidal activity against Burkholderia pseudomallei. Infect Immun 65 4108 4113
9. SantanirandPHarleyVSDanceDARaynesJGDrasarBS 1997 Interferon-gamma mediates host resistance in a murine model of melioidosis. Biochem Soc Trans 25 287S
10. JonesALBeveridgeTJWoodsDE 1996 Intracellular survival of Burkholderia pseudomallei. Infect Immun 64 782 790
11. ReckseidlerSLDeShazerDSokolPAWoodsDE 2001 Detection of bacterial virulence genes by subtractive hybridization: identification of capsular polysaccharide of Burkholderia pseudomallei as a major virulence determinant. Infect Immun 69 34 44
12. DeShazerDBrettPJWoodsDE 1998 The type II O-antigenic polysaccharide moiety of Burkholderia pseudomallei lipopolysaccharide is required for serum resistance and virulence. Mol Microbiol 30 1081 1100
13. WarawaJWoodsDE 2005 Type III secretion system cluster 3 is required for maximal virulence of Burkholderia pseudomallei in a hamster infection model. FEMS Microbiol Lett 242 101 108
14. LiuBKooGCYapEHChuaKLGanYH 2002 Model of differential susceptibility to mucosal Burkholderia pseudomallei infection. Infect Immun 70 504 511
15. HoppeIBrennekeBRohdeMKreftAHausslerS 1999 Characterization of a murine model of melioidosis: comparison of different strains of mice. Infect Immun 67 2891 2900
16. CaseCLShinSRoyCR 2009 Asc and Ipaf Inflammasomes direct distinct pathways for caspase-1 activation in response to Legionella pneumophila. Infect Immun 77 1981 1991
17. MariathasanSNewtonKMonackDMVucicDFrenchDM 2004 Differential activation of the inflammasome by caspase-1 adaptors ASC and Ipaf. Nature 430 213 218
18. SuzukiTFranchiLTomaCAshidaHOgawaM 2007 Differential regulation of caspase-1 activation, pyroptosis, and autophagy via Ipaf and ASC in Shigella-infected macrophages. PLoS Pathog 3 e111
19. FranchiLStoolmanJKannegantiTDVermaARamphalR 2007 Critical role for Ipaf in Pseudomonas aeruginosa-induced caspase-1 activation. Eur J Immunol 37 3030 3039
20. FantuzziGKuGHardingMWLivingstonDJSipeJD 1997 Response to local inflammation of IL-1 beta-converting enzyme - deficient mice. J Immunol 158 1818 1824
21. CoeshottCOhnemusCPilyavskayaARossSWieczorekM 1999 Converting enzyme-independent release of tumor necrosis factor alpha and IL-1beta from a stimulated human monocytic cell line in the presence of activated neutrophils or purified proteinase 3. Proc Natl Acad Sci U S A 96 6261 6266
22. GumaMRonacherLLiu-BryanRTakaiSKarinM 2009 Caspase 1-independent activation of interleukin-1beta in neutrophil-predominant inflammation. Arthritis Rheum 60 3642 3650
23. Mayer-BarberKDBarberDLShenderovKWhiteSDWilsonMS 2010 Caspase-1 independent IL-1beta production is critical for host resistance to mycobacterium tuberculosis and does not require TLR signaling in vivo. J Immunol 184 3326 3330
24. MausUvon GroteKKuzielWAMackMMillerEJ 2002 The role of CC chemokine receptor 2 in alveolar monocyte and neutrophil immigration in intact mice. Am J Respir Crit Care Med 166 268 273
25. HayashiSYatsunamiJFukunoYKawashimaMMillerEJ 2002 Antileukinate, a hexapeptide inhibitor of CXC-chemokine receptor, suppresses bleomycin-induced acute lung injury in mice. Lung 180 339 348
26. SchultzMJRijneveldAWFlorquinSEdwardsCKDinarelloCA 2002 Role of interleukin-1 in the pulmonary immune response during Pseudomonas aeruginosa pneumonia. Am J Physiol Lung Cell Mol Physiol 282 L285 290
27. NathanCDingA 2010 Nonresolving inflammation. Cell 140 871 882
28. BurtnickMNBrettPJNairVWarawaJMWoodsDE 2008 Burkholderia pseudomallei type III secretion system mutants exhibit delayed vacuolar escape phenotypes in RAW 264.7 murine macrophages. Infect Immun 76 2991 3000
29. MiaoEAMaoDPYudkovskyNBonneauRLorangCG 2010 Innate immune detection of the type III secretion apparatus through the NLRC4 inflammasome. Proc Natl Acad Sci U S A 107 3076 3080
30. BrozPvon MoltkeJJonesJWVanceREMonackDM 2010 Differential requirement for Caspase-1 autoproteolysis in pathogen-induced cell death and cytokine processing. Cell Host Microbe 8 471 483
31. RenTZamboniDSRoyCRDietrichWFVanceRE 2006 Flagellin-deficient Legionella mutants evade caspase-1 - and Naip5-mediated macrophage immunity. PLoS Pathog 2 e18
32. MolofskyABByrneBGWhitfieldNNMadiganCAFuseET 2006 Cytosolic recognition of flagellin by mouse macrophages restricts Legionella pneumophila infection. J Exp Med 203 1093 1104
33. LightfieldKLPerssonJTrinidadNJBrubakerSWKofoedEM 2011 Differential requirements for NAIP5 in activation of the NLRC4 inflammasome. Infect Immun 79 1606 1614
34. ZamboniDSKobayashiKSKohlsdorfTOguraYLongEM 2006 The Birc1e cytosolic pattern-recognition receptor contributes to the detection and control of Legionella pneumophila infection. Nat Immunol 7 318 325
35. WarrenSEMaoDPRodriguezAEMiaoEAAderemA 2008 Multiple Nod-like receptors activate caspase 1 during Listeria monocytogenes infection. J Immunol 180 7558 7564
36. BrozPNewtonKLamkanfiMMariathasanSDixitVM 2010 Redundant roles for inflammasome receptors NLRP3 and NLRC4 in host defense against Salmonella. J Exp Med 207 1745 1755
37. WiersingaWJWielandCWvan der WindtGJde BoerAFlorquinS 2007 Endogenous interleukin-18 improves the early antimicrobial host response in severe melioidosis. Infect Immun 75 3739 3746
38. DinarelloCA 2009 Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol 27 519 550
39. LemosHPGrespanRVieiraSMCunhaTMVerriWAJr 2009 Prostaglandin mediates IL-23/IL-17-induced neutrophil migration in inflammation by inhibiting IL-12 and IFNgamma production. Proc Natl Acad Sci U S A 106 5954 5959
40. LeeJKKimSHLewisECAzamTReznikovLL 2004 Differences in signaling pathways by IL-1beta and IL-18. Proc Natl Acad Sci U S A 101 8815 8820
41. KazaSKMcCleanSCallaghanM 2008 IL-8 released from human lung epithelial cells induced by cystic fibrosis pathogens Burkholderia cepacia complex affects the growth and intracellular survival of bacteria. Int J Med Microbiol 301 26 33
42. AbrahamE 2003 Neutrophils and acute lung injury. Crit Care Med 31 S195 199
43. ZemansRLColganSPDowneyGP 2009 Transepithelial migration of neutrophils: mechanisms and implications for acute lung injury. Am J Respir Cell Mol Biol 40 519 535
44. ChanchamroenSKewcharoenwongCSusaengratWAtoMLertmemongkolchaiG 2009 Human polymorphonuclear neutrophil responses to Burkholderia pseudomallei in healthy and diabetic subjects. Infect Immun 77 456 463
45. EastonAHaqueAChuKLukaszewskiRBancroftGJ 2007 A critical role for neutrophils in resistance to experimental infection with Burkholderia pseudomallei. J Infect Dis 195 99 107
46. CassatellaMALocatiMMantovaniA 2009 Never underestimate the power of a neutrophil. Immunity 31 698 700
47. van der WindtGJWiersingaWJWielandCWTjiaICDayNP 2010 Osteopontin impairs host defense during established gram-negative sepsis caused by Burkholderia pseudomallei (melioidosis). PLoS Negl Trop Dis 4 pii e806
48. WangTTownTAlexopoulouLAndersonJFFikrigE 2004 Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis. Nat Med 10 1366 1373
49. Le GofficRBalloyVLagranderieMAlexopoulouLEscriouN 2006 Detrimental contribution of the Toll-like receptor (TLR)3 to influenza A virus-induced acute pneumonia. PLoS Pathog 2 e53
50. SchultzMJKnappSFlorquinSPaterJTakedaK 2003 Interleukin-18 impairs the pulmonary host response to Pseudomonas aeruginosa. Infect Immun 71 1630 1634
51. CarlssonFKimJDumitruCBarckKHCaranoRA 2010 Host-detrimental role of Esx-1-mediated inflammasome activation in mycobacterial infection. PLoS Pathog 6 e1000895
52. BreitbachKSunGWKohlerJEskeKWongprompitakP 2009 Caspase-1 mediates resistance in murine melioidosis. Infect Immun 77 1589 1595
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Genesis of Mammalian Prions: From Non-infectious Amyloid Fibrils to a Transmissible Prion DiseaseČlánek Role of Permissive Neuraminidase Mutations in Influenza A/Brisbane/59/2007-like (H1N1) VirusesČlánek Allelic Variation on Murine Chromosome 11 Modifies Host Inflammatory Responses and Resistance toČlánek Multifaceted Regulation of Translational Readthrough by RNA Replication Elements in a TombusvirusČlánek Latent KSHV Infection of Endothelial Cells Induces Integrin Beta3 to Activate Angiogenic PhenotypesČlánek Controlling Viral Immuno-Inflammatory Lesions by Modulating Aryl Hydrocarbon Receptor Signaling
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 12- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Inhibition of Apoptosis and NF-κB Activation by Vaccinia Protein N1 Occur via Distinct Binding Surfaces and Make Different Contributions to Virulence
- Genesis of Mammalian Prions: From Non-infectious Amyloid Fibrils to a Transmissible Prion Disease
- Kaposi's Sarcoma Herpesvirus microRNAs Target Caspase 3 and Regulate Apoptosis
- Nutritional Immunology: A Multi-Dimensional Approach
- Role of Permissive Neuraminidase Mutations in Influenza A/Brisbane/59/2007-like (H1N1) Viruses
- Vaccinomics and Personalized Vaccinology: Is Science Leading Us Toward a New Path of Directed Vaccine Development and Discovery?
- Symbiont Infections Induce Strong Cytoplasmic Incompatibility in the Tsetse Fly
- Allelic Variation on Murine Chromosome 11 Modifies Host Inflammatory Responses and Resistance to
- Computational and Biochemical Analysis of the Effector AvrBs2 and Its Role in the Modulation of Type Three Effector Delivery
- Granzyme B Inhibits Vaccinia Virus Production through Proteolytic Cleavage of Eukaryotic Initiation Factor 4 Gamma 3
- Association of Activating KIR Copy Number Variation of NK Cells with Containment of SIV Replication in Rhesus Monkeys
- Fungal Virulence and Development Is Regulated by Alternative Pre-mRNA 3′End Processing in
- versus the Host: Remodeling of the Bacterial Outer Membrane Is Required for Survival in the Gastric Mucosa
- Follicular Dendritic Cell-Specific Prion Protein (PrP) Expression Alone Is Sufficient to Sustain Prion Infection in the Spleen
- Autophagy Protein Atg3 is Essential for Maintaining Mitochondrial Integrity and for Normal Intracellular Development of Tachyzoites
- Longevity and Composition of Cellular Immune Responses Following Experimental Malaria Infection in Humans
- Sequential Adaptive Mutations Enhance Efficient Vector Switching by Chikungunya Virus and Its Epidemic Emergence
- Acquisition of Pneumococci Specific Effector and Regulatory Cd4 T Cells Localising within Human Upper Respiratory-Tract Mucosal Lymphoid Tissue
- The Meaning of Death: Evolution and Ecology of Apoptosis in Protozoan Parasites
- Deficiency of a Niemann-Pick, Type C1-related Protein in Is Associated with Multiple Lipidoses and Increased Pathogenicity
- Feeding Cells Induced by Phytoparasitic Nematodes Require γ-Tubulin Ring Complex for Microtubule Reorganization
- Eight RGS and RGS-like Proteins Orchestrate Growth, Differentiation, and Pathogenicity of
- Prion Uptake in the Gut: Identification of the First Uptake and Replication Sites
- Nef Decreases HIV-1 Sensitivity to Neutralizing Antibodies that Target the Membrane-proximal External Region of TMgp41
- Multifaceted Regulation of Translational Readthrough by RNA Replication Elements in a Tombusvirus
- A Temporal Role Of Type I Interferon Signaling in CD8 T Cell Maturation during Acute West Nile Virus Infection
- The Membrane Fusion Step of Vaccinia Virus Entry Is Cooperatively Mediated by Multiple Viral Proteins and Host Cell Components
- HIV-1 Capsid-Cyclophilin Interactions Determine Nuclear Import Pathway, Integration Targeting and Replication Efficiency
- Neonatal CD8 T-cell Hierarchy Is Distinct from Adults and Is Influenced by Intrinsic T cell Properties in Respiratory Syncytial Virus Infected Mice
- Two Novel Transcriptional Regulators Are Essential for Infection-related Morphogenesis and Pathogenicity of the Rice Blast Fungus
- Five Questions about Non-Mevalonate Isoprenoid Biosynthesis
- The Human Cytomegalovirus UL11 Protein Interacts with the Receptor Tyrosine Phosphatase CD45, Resulting in Functional Paralysis of T Cells
- Wall Teichoic Acids of Limit Recognition by the Drosophila Peptidoglycan Recognition Protein-SA to Promote Pathogenicity
- A Novel Role for the NLRC4 Inflammasome in Mucosal Defenses against the Fungal Pathogen
- Inflammasome-dependent Pyroptosis and IL-18 Protect against Lung Infection while IL-1β Is Deleterious
- CNS Recruitment of CD8+ T Lymphocytes Specific for a Peripheral Virus Infection Triggers Neuropathogenesis during Polymicrobial Challenge
- Latent KSHV Infection of Endothelial Cells Induces Integrin Beta3 to Activate Angiogenic Phenotypes
- A Receptor-based Switch that Regulates Anthrax Toxin Pore Formation
- Targeting of Heparin-Binding Hemagglutinin to Mitochondria in Macrophages
- Chikungunya Virus Neutralization Antigens and Direct Cell-to-Cell Transmission Are Revealed by Human Antibody-Escape Mutants
- Ce-Duox1/BLI-3 Generated Reactive Oxygen Species Trigger Protective SKN-1 Activity via p38 MAPK Signaling during Infection in
- Structural Elucidation and Functional Characterization of the Effector Protein ATR13
- Controlling Viral Immuno-Inflammatory Lesions by Modulating Aryl Hydrocarbon Receptor Signaling
- SAMHD1-Deficient CD14+ Cells from Individuals with Aicardi-Goutières Syndrome Are Highly Susceptible to HIV-1 Infection
- Acid Stability of the Hemagglutinin Protein Regulates H5N1 Influenza Virus Pathogenicity
- Cryo Electron Tomography of Herpes Simplex Virus during Axonal Transport and Secondary Envelopment in Primary Neurons
- A Novel Human Cytomegalovirus Locus Modulates Cell Type-Specific Outcomes of Infection
- Juxtamembrane Shedding of AMA1 Is Sequence Independent and Essential, and Helps Evade Invasion-Inhibitory Antibodies
- Pathogenesis and Host Response in Syrian Hamsters following Intranasal Infection with Andes Virus
- IRGM Is a Common Target of RNA Viruses that Subvert the Autophagy Network
- Epstein-Barr Virus Evades CD4 T Cell Responses in Lytic Cycle through BZLF1-mediated Downregulation of CD74 and the Cooperation of vBcl-2
- Quantitative Multicolor Super-Resolution Microscopy Reveals Tetherin HIV-1 Interaction
- Late Repression of NF-κB Activity by Invasive but Not Non-Invasive Meningococcal Isolates Is Required to Display Apoptosis of Epithelial Cells
- Polar Flagellar Biosynthesis and a Regulator of Flagellar Number Influence Spatial Parameters of Cell Division in
- Epstein-Barr Virus Nuclear Antigen 3C Stabilizes Gemin3 to Block p53-mediated Apoptosis
- The Enteropathogenic (EPEC) Tir Effector Inhibits NF-κB Activity by Targeting TNFα Receptor-Associated Factors
- Toward an Integrated Model of Capsule Regulation in
- A Systematic Screen to Discover and Analyze Apicoplast Proteins Identifies a Conserved and Essential Protein Import Factor
- A Host Small GTP-binding Protein ARL8 Plays Crucial Roles in Tobamovirus RNA Replication
- Comparative Pathobiology of Fungal Pathogens of Plants and Animals
- Synergistic Roles of Eukaryotic Translation Elongation Factors 1Bγ and 1A in Stimulation of Tombusvirus Minus-Strand Synthesis
- Engineered Immunity to Infection
- Inflammatory Monocytes and Neutrophils Are Licensed to Kill during Memory Responses
- Sialidases Affect the Host Cell Adherence and Epsilon Toxin-Induced Cytotoxicity of Type D Strain CN3718
- Eurasian-Origin Gene Segments Contribute to the Transmissibility, Aerosol Release, and Morphology of the 2009 Pandemic H1N1 Influenza Virus
- SARS Coronavirus nsp1 Protein Induces Template-Dependent Endonucleolytic Cleavage of mRNAs: Viral mRNAs Are Resistant to nsp1-Induced RNA Cleavage
- Identification and Characterization of a Novel Non-Structural Protein of Bluetongue Virus
- Functional Analysis of the Kinome of the Wheat Scab Fungus
- Norovirus Regulation of the Innate Immune Response and Apoptosis Occurs via the Product of the Alternative Open Reading Frame 4
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Controlling Viral Immuno-Inflammatory Lesions by Modulating Aryl Hydrocarbon Receptor Signaling
- Fungal Virulence and Development Is Regulated by Alternative Pre-mRNA 3′End Processing in
- Epstein-Barr Virus Nuclear Antigen 3C Stabilizes Gemin3 to Block p53-mediated Apoptosis
- Engineered Immunity to Infection
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



