-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaSgo1 Regulates Both Condensin and Ipl1/Aurora B to Promote Chromosome Biorientation
Accurate chromosome segregation is required for the equal distribution of genetic information to progeny. Failure to equally segregate chromosomes leads to aneuploidy, cell death or cancer. Proteins of the conserved shugoshin family contribute to accurate chromosome segregation in both meiosis and mitosis. The role of shugoshin in protection of centromeric cohesion during meiosis is well understood, but only little is known about shugoshin's function during mitosis. We show that Sgo1 mediates localization of the heterotrimeric phosphatase PP2A-Rts1 to the centromere and that this is in turn important for the efficient recruitment of condensin to the centromere. The failure to load centromeric condensin results in a defect during correction of improper microtubule-kinetochore attachments. Moreover, Sgo1 facilitates the maintenance of a centromeric pool of Aurora B/Ipl1, a conserved mitotic kinase essential for the correction of faulty microtubule-kinetochore attachments. Our results show that Sgo1 operates as a multifunctional hub that coordinates two centromeric functions essential for correct chromosome segregation.
Published in the journal: . PLoS Genet 10(6): e32767. doi:10.1371/journal.pgen.1004411
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004411Summary
Accurate chromosome segregation is required for the equal distribution of genetic information to progeny. Failure to equally segregate chromosomes leads to aneuploidy, cell death or cancer. Proteins of the conserved shugoshin family contribute to accurate chromosome segregation in both meiosis and mitosis. The role of shugoshin in protection of centromeric cohesion during meiosis is well understood, but only little is known about shugoshin's function during mitosis. We show that Sgo1 mediates localization of the heterotrimeric phosphatase PP2A-Rts1 to the centromere and that this is in turn important for the efficient recruitment of condensin to the centromere. The failure to load centromeric condensin results in a defect during correction of improper microtubule-kinetochore attachments. Moreover, Sgo1 facilitates the maintenance of a centromeric pool of Aurora B/Ipl1, a conserved mitotic kinase essential for the correction of faulty microtubule-kinetochore attachments. Our results show that Sgo1 operates as a multifunctional hub that coordinates two centromeric functions essential for correct chromosome segregation.
Introduction
Accurate chromosome segregation into daughter cells requires the formation of correct chromosome attachments to the mitotic spindle. Each of the sister kinetochores has to attach to microtubules (MTs) emanating from opposite spindle poles in order to achieve biorientation of sister chromatids. Correctly attached and bioriented kinetochores (KTs) generate tension caused by attached MTs, which is, in turn, opposed by sister chromatid cohesion [1]. Consequently, biorientation is achieved by stabilization of correct bipolar attachments that generate tension and continuous dissolution of incorrect, tensionless attachments [2], [3]. The spindle assembly checkpoint (SAC) recognizes KTs lacking attachments and halts the progression from metaphase to anaphase until all pairs of sister chromatids become bioriented. How exactly cells recognize erroneous tensionless attachments is not well understood. The chromosome passenger complex (CPC) consisting of the conserved kinase Aurora B/Ipl1, Incenp/Sli15, Survivin/Bir1, and Borealin/Nbl1 plays a crucial role in dissolving faulty connections by phosphorylating multiple substrates at the MT-KT interface, thereby creating unattached KTs leading, in turn, to SAC activation (reviewed in [1]). Other proteins, most prominently the conserved shugoshin/MEI-S322 family of proteins, have been proposed to facilitate the establishment of biorientation by promoting the correction of tensionless attachments [4]–[7]. Shugoshins recruit the heterotrimeric protein phosphatase PP2A-B56/Rts1 to the centromere via their conserved N-terminal coiled-coil domain to ensure the protection of cohesion during meiosis and in many organisms also in mitosis [8]–[11]. Besides this canonical function, shugoshin also affects Aurora B/Ipl1 kinase in mitosis by facilitating its centromeric localization in fission yeast [5], [12]. Moreover, Aurora B kinase activity in Xenopus egg extracts is impaired upon depletion of Sgo2 [13]. However, the exact function of shugoshin and PP2A in tension sensing and the establishment of biorientation remains unclear.
Kinetochores, large proteinaceous complexes assembled on centromeric DNA, are crucial interaction hubs for events necessary for accurate chromosome segregation. KTs are flexible and dynamic structures that become stretched when MTs attach and bipolarity is achieved [14], [15]. Furthermore, centromeric and pericentric chromatin creates a flexible spring-like filament that is responsive to the tension exerted by MT-mediated pulling forces of the spindle [16], [17]. This elasticity is ensured by the concerted activity of several multi-protein complexes including cohesin and condensin that bind and organize the pericentric chromatin into inter - and intramolecular loops (reviewed in [18]). Interestingly, mutants that are not able to maintain the functional organization of the pericentric region fail to create bioriented MT-KT attachments during mitosis [19]–[21]. Cohesin has been shown to control KT geometry [22] and condensin in C. elegans is required for centromere resolution [23]. Moreover, depletion of condensin I suppresses KT stretching in HeLa cells, thereby causing a SAC-mediated mitotic delay [15]. Taken together, cohesin and condensin contribute to the architecture and elastic properties of pericentric chromatin. However, whether the pericentric architecture modulates CPC activity or localization in response to lack of tension on sister KTs and how it affects the turnover of erroneous MT-KT attachments has not been clarified so far.
Here, we show that Sgo1 function is essential for the recruitment of condensin to the pericentric region. Moreover, Sgo1 localizes the PP2A subunit Rts1 to the same region, which facilitates the binding of condensin to centromeric chromatin. The failure to load functional condensin onto pericentric chromatin impairs the cellular response to tensionless attachments. Additionally, Sgo1 is required for the maintenance of the Ipl1 kinase on centromeres, which, in turn, allows for correction of erroneous MT-KT attachments. We propose that Sgo1 is a scaffold protein that via spatio-temporal modulation of kinase and phosphatase activity on KTs ensures a dual function - organization of the pericentric chromatin that mediates tension sensing and the maintenance of centromeric Ipl1 that facilitates correction of tensionless attachments.
Results
Sgo1-mediated recruitment of PP2A-Rts1 to the centromere is required for biorientation
Shugoshin proteins interact with PP2A-B56/Rts1 via their conserved N-terminal coiled-coil domains [8]–[11]. Earlier studies in higher eukaryotes and budding yeast have shown that substitution of a highly conserved asparagine residue within the N-terminus of Sgo1 abolishes the interaction with PP2A while retaining other functions of the protein [11]. We introduced this substitution (N51I) into budding yeast Sgo1 in order to study the role of the Sgo1-PP2A interaction during mitosis. As expected, the N51I mutation abrogated the interaction between the recombinantly purified N-terminus of Sgo1 and PP2A-Rts1 complexes purified from yeast mitotic lysates (Figure S1A), but did not affect the proper localization of the mutant protein to the centromeric region during mitosis (Figure 1A). PP2A is a ubiquitously expressed serine/threonine phosphatase with a broad substrate specificity [24]. It consists of a dimeric catalytic core that interacts with various regulatory subunits to promote diverse cellular functions. We found that the binding to Sgo1 is specific for PP2A complexes containing the Rts1 regulatory subunit, as we did not observe any specific interaction between Sgo1 and PP2A containing the regulatory subunit B55/Cdc55 (Figure S1B). Rts1 localizes diffusely to the cytoplasm and nucleus, but it becomes enriched specifically at the centromeric region in pre-anaphase cells (Figure 1B, [25]). Replacement of wild type SGO1 with the sgo1-N51I allele resulted in the reduction of the Rts1-GFP signal intensity between the spindle pole bodies (SPBs) (Figure 1B). Chromatin immunoprecipitation (ChIP) of Rts1-FLAG confirmed that, in comparison to a control locus, Rts1 is enriched at centromeres and this enrichment depends on the presence of Sgo1 in mitotic cells (Figure S1C), similarly as previously shown in budding yeast meiosis [26]. To further elucidate the interplay between Sgo1 and the two forms of PP2A, we analyzed genetic interactions among respective mutants. We found that the sgo1Δ rts1Δ double mutant shows a comparable growth defect as sgo1Δ alone and the sgo1-N51I rts1Δ mutant has a milder phenotype resembling rts1Δ. In contrast, we observed an additive growth defect of the sgo1Δ cdc55Δ double deletion that was only slightly improved by introduction of the sgo1-N51I allele (Figure S1D). Thus, protein interaction and localization analyses as well as genetic evidence suggest that Sgo1 interacts with PP2A-Rts1 (and not with PP2A-Cdc55) via its N-terminal coiled-coil domain.
Fig. 1. Sgo1-mediated PP2A recruitment to the centromere is essential for tension sensing. 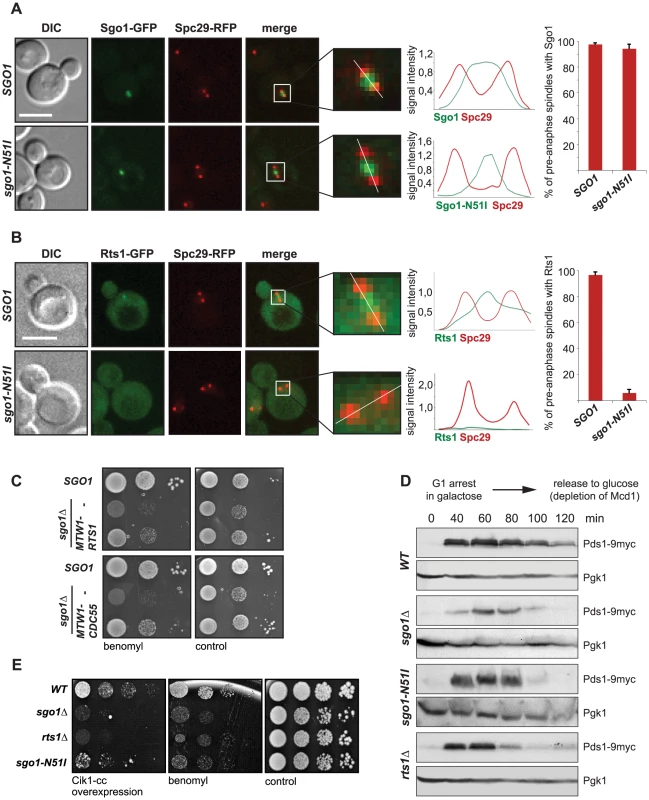
(A) Localization of the GFP-tagged Sgo1 and Sgo1-N51I mutant. Spindle pole bodies (SPBs) are visualized with Spc29-RFP. Only pre-anaphase spindles (SPB distance <2 µm, spindle located in the mother cell) were scored. Plots on the right: histogram of signal intensity across the white line in the insets and percentage of cells with localized GFP signal. Bar – 5 µm. (B) Localization of Rts1-GFP in wild type cells or in cells carrying the sgo1-N51I mutation. SPBs are visualized with Spc29-RFP. Only pre-anaphase spindles (SPB distance <2 µm, spindle located in the mother cell) were scored. Plots on the right: histogram of signal intensity across the white line in the insets and percentage of cells with localized GFP signal. Bar – 5 µm. (C) Sensitivity to the microtubule depolymerizing drug benomyl in cells lacking SGO1. Cell viability was scored upon artificial tethering of the PP2A regulatory subunit Rts1 and Cdc55, respectively, to the kinetochore. (D) Progression of the wild type, sgo1Δ, sgo1-N51I and rts1Δ mutants through cell cycle upon depletion of the cohesin subunit Mcd1 which leads to formation of tensionless kinetochores. (E) Sensitivity of the wild type, sgo1Δ, rts1Δ and sgo1-N51I mutants to microtubule poisons and to the overexpression of Cik1-cc which triggers the formation of syntelic attachments at high frequencies. Loss of Sgo1 or introduction of the sgo1-N51I mutation causes sensitivity to the microtubule-destabilizing drug benomyl, indicating that the recruitment of PP2A-Rts1 is crucial for Sgo1's functions in mitosis (Figure 1E, S2A, B). Importantly, this sensitivity was alleviated by artificially tethering Rts1 to the KT via fusion to the inner kinetochore protein Mtw1 (Figure 1C). Remarkably, artificial tethering of the second PP2A regulatory subunit Cdc55 partially rescued the benomyl sensitivity of sgo1Δ cells as well (Figure 1C), whereas the control Mtw1-GFP fusion protein did not affect the benomyl sensitivity of sgo1Δ cells (Figure S2C, D). Also, a fusion protein of mutant Sgo1-N51I and Cdc55 suppressed the sensitivity of sgo1Δ cells (Figure S2E). The observed rescuing effects cannot be explained by increased levels of PP2A regulatory subunits, because massive overexpression of Rts1 or Cdc55 by itself did not restore the growth of sgo1Δ cells on plates containing benomyl (Figure S2F, G). These observations suggest that the localization of PP2A complexes at the centromere is required for correct chromosome segregation in the presence of microtubule poisons.
By visualizing TetO arrays integrated 1 kb away from the centromere on chromosome 4 [27], we determined that loss of the centromeric Rts1 pool increases the frequency of segregation errors (Figure S3A). We found that 86% of wild type cells segregated chromosome 4 correctly into the daughter cells within 75 min after release from nocodazole arrest which elevates the rates of syntelic, tensionless MT-KT attachments (Figure S3A, B). In contrast, only about 50% of cells expressing the Sgo1-N51I mutant correctly segregated chromosome 4 after release from nocodazole-induced cell cycle arrest (Figure S3A, B). Similar results were obtained with cells lacking Rts1 (Figure S3A, B). The elevated frequency of chromosome missegregation in rts1Δ strains was rescued to the wild type level upon genetic complementation with a vector carrying RTS1 under the control of its endogenous promoter (Figure S3B). This finding supports the notion that Sgo1-dependent Rts1 localization to centromeres is important for the correction of erroneous MT-KT attachments.
The lack of Sgo1 or Rts1 leads to a failure to halt cell cycle progression upon loss of functional sister chromatid cohesion resulting in tensionless MT-KT attachments (Figure 1D, [4], [11]). In contrast, the SAC response in the presence of the microtubule depolymerizing drug nocodazole that creates unattached KTs is fully preserved (Figure S3C, [4]). To further elaborate the role of Sgo1/PP2A-Rts1 in the repair of tensionless MT-KT attachments, we took advantage of a recently developed genetic tool that promotes the formation of syntelic attachments at high frequencies by overexpression of the coiled-coil domain (Cik1-cc; amino acids 81-360) of the kinesin co-factor Cik1 [28]. Importantly, overexpression of Cik1-cc does not impair spindle geometry or localization of kinetochore proteins [28]. Galactose-induced overexpression of Cik1-cc is lethal in cells lacking Sgo1 (Figure 1E, [28]). The presence of Rts1 on centromeres also contributes to correct chromosome segregation, because cells lacking the Sgo1-Rts1 interaction or Rts1 alone cannot proliferate in conditions in which syntelic attachments are created at high frequencies (Figure 1E). Similarly, both rts1Δ and the sgo1-N51I mutant showed increased sensitivity to microtubule depolymerizing drugs (Figure 1E, S2A). Taken together, our results show that both Sgo1 and PP2A activities at the centromere are important for accurate chromosome segregation and play a critical role in inducing a cell cycle delay upon formation of tensionless, syntelic attachments. Yet, the finding that the sgo1Δ strain shows a stronger phenotype than rts1Δ and sgo1-N51I shows that Sgo1 performs both Rts1-dependent and Rts1-independent functions during chromosome segregation.
Lack of functional Sgo1 and Rts1 causes loss of condensin from centromeres
The response to tension across sister KTs is mediated by the structural integrity of pericentric chromatin that is maintained by cohesin and condensin complexes [16], [29], [30]. Therefore, we speculated that the defect observed in sgo1Δ and rts1Δ cells might result from an altered centromeric architecture. Since localization of cohesin and sister chromatid cohesion are not affected by deletion of SGO1 in budding yeast mitosis (Figure S4A, B, C, [31], [32]), we hypothesized that Sgo1 might affect the centromeric localization of condensin. Indeed, we found that whereas the non-SMC condensin subunit Ycg1-GFP was enriched between SPBs in mitotic wild type cells, cells lacking Sgo1 or Rts1 failed to enrich condensin (Figure 2A, B). In contrast, condensin localization was not impaired by deletion of the second PP2A regulatory subunit Cdc55 (Figure 2A, B). Similarly, the centromeric localization of another condensin subunit, Smc2-GFP, was severely decreased in the absence of Sgo1 or Rts1 (Figure S4D, E). Condensin is also highly enriched at rDNA repeats, which can be observed as a characteristic crescent-shaped sub-nuclear signal by imaging of GFP-labelled condensin subunits [33]. This localization was not affected in any of the analyzed mutants (Figure 2A, S4D). ChIP analysis confirmed that the lack of Sgo1 did not impair the enrichment of condensin on the rDNA locus, but the centromeric and pericentric pools of FLAG-tagged Smc2 were reduced in nocodazole-arrested cells lacking Sgo1 to 30% of the wild type level (Figure 2C). The reduced levels of Smc2 at centromeric regions were also observed in rts1Δ cells by ChIP-qPCR experiments, although to a lesser degree in comparison to sgo1Δ (58% of the wild type level; Figure 2D). Condensin co-localizes on the rDNA with Lrs4 and Csm1 and its recruitment to rDNA strictly depends on these proteins [34]. Whereas we observed Csm1-GFP to localize to rDNA throughout the cell cycle as previously reported [34], no enrichment was detected between SPBs in pre-anaphase cells (Figure S5A). Our results show that condensin is localized to the pericentric region via a Sgo1/PP2A-Rts1-dependent pathway and that this localization is independent of condensin's association with rDNA.
Fig. 2. Sgo1 and Rts1 are essential for the maintenance of condensin at centromeres. 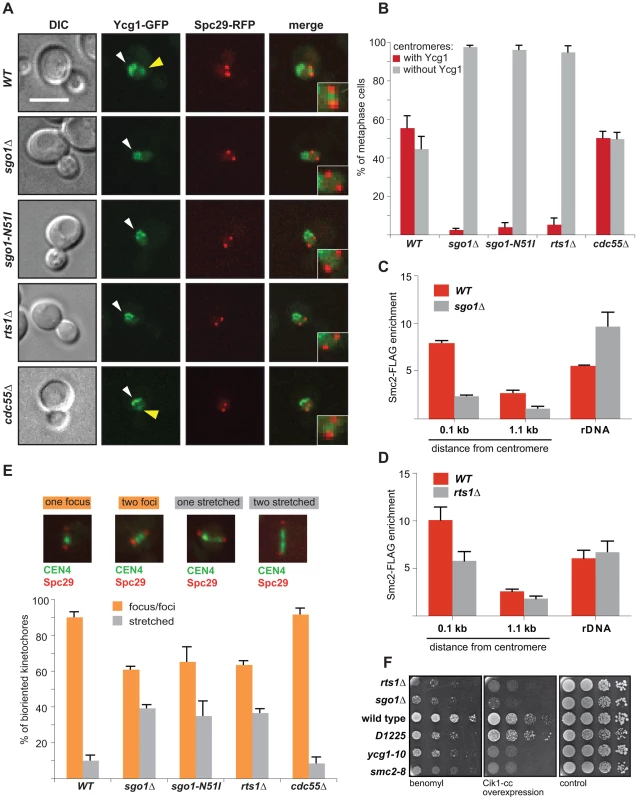
(A) Localization of the condensin subunit Ycg1-GFP in wild type cells and cells lacking Sgo1, centromeric Rts1 (rts1Δ and sgo1-N51I) or Cdc55. SPBs are visualized with Spc29-RFP. Bar – 5 µm. Yellow arrowhead: centromeric Ycg1-GFP signal; white arrowhead: Ycg1-GFP on rDNA. (B) Quantification of Ycg1-GFP localization. Only pre-anaphase spindles (SPB distance <2 µm, spindle located in the mother cell) were scored. Means with SD of three independent experiments are shown. At least 150 cells were scored in each experiment. (C) Enrichment of Smc2-FLAG on centromeric DNA (0.1 kb away from CEN1 and 1.1 kb away from CEN4) and on rDNA (NTS1-2) normalized to the levels of Smc2-FLAG bound to the arm of chromosome 10 in mitotic cells. Chromatin immunoprecipitation(ChIP)-qPCR experiments of Smc2-FLAG were performed using wild type and sgo1Δ cells arrested with nocodazole. Error bars represent the standard error of the mean. (D) Enrichment of Smc2-FLAG on centromeric DNA (0.1 kb away from CEN1 and 1.1 kb away from CEN4) and on rDNA (NTS1-2) normalized to the levels of Smc2-FLAG bound to the arm of chromosome 10 in mitotic cells. ChIP-qPCR experiments of Smc2-FLAG were performed using wild type and rts1Δ cells arrested with nocodazole. Error bars represent the standard error of the mean. (E) Stretching of centromeric DNA in wild type cells compared with cells that fail to localize Rts1 to the centromeric region (rts1Δ and sgo1-N51I) and with cells lacking Cdc55. CEN4 is visualized by TetR-GFP recruited to the TetO-repeats integrated 1 kb from the centromere. Only pre-anaphase spindles (SPB distance <2 µm, spindle located in the mother cell) were scored. Top – examples of scored categories. (F) Sensitivity of wild type cells and the indicated condensin mutants to microtubule poisons and to the overexpression of the Cik1-cc construct which triggers formation of syntelic attachments at high frequencies. Yeast strain D1225 expresses the non-posphorylatable mutants of the condensin subunits Ycg1, Brn1, and Ycs4 which impair anaphase-specific functions of condensin. Previously, it was shown that the lack of condensin reduces the ability to withstand the outward-directed forces of the mitotic spindle and leads to extensive centromeric stretching [16]. The finding that Sgo1 and Rts1 recruit condensin to the centromere predicts that the conformation of centromeres might be altered in sgo1Δ and rts1Δ cells. We therefore analyzed the compaction state of centromeric chromatin of chromosome 4. In wild type cells, most of the centromeres appear as a single spot that slightly separates during the process of biorientation (also called “kinetochore breathing”) and only 10% of cells contained stretched centromeres (Figure 2E, [16], [27]). In contrast, cells lacking SGO1 or RTS1 or carrying the sgo1-N51I allele contained up to 40% of stretched centromeres (Figure 2E). Since the absence of CDC55 did not affect centromeric stretching (Figure 2E), we conclude that the centromeric localization of condensin is facilitated by Sgo1 acting in collaboration with Rts1. Thus, the lack of Sgo1 or Rts1 affects centromeric conformation similarly as the lack of condensin, further strengthening our findings that Sgo1 and to a lesser degree also PP2A-Rts1 are needed for the maintenance of functional condensin on centromeric chromatin.
Since both sgo1Δ and rts1Δ cells are impaired in their response to lack of tension on sister KTs, we asked whether condensin mutations alone show a similar phenotype. The induction of syntelic attachments by Cik1-cc overexpression impaired the growth of cells carrying the temperature-sensitive condensin alleles smc2-8 or ycg1-10 even at a non-restrictive temperature, thus demonstrating that these mutants fail to recognize or repair syntelic attachments (Figure 2F). To test whether the pre-anaphase centromeric functions of condensin can be separated from its anaphase functions, we used the yeast strain D1225 that expresses non-posphorylatable mutants of the condensin subunits Ycg1, Brn1, and Ycs4, which interfere specifically with condensin's function in anaphase [35]. Importantly, the presence of syntelic attachments did not affect the proliferation of the D1225 mutant strain (Figure 2F). Additionally, deletion of the Lrs4 and Csm1 proteins that localize condensin to rDNA, but not to the centromere, did not impair the ability of cells to repair syntelic attachments induced by overexpression of Cik1-cc (Figure S5B). Thus, Sgo1/PP2A-Rts1-dependent recruitment of condensin to the pericentric region is essential for the pre-anaphase chromatin conformation and for the correction of faulty MT-KT attachments. This function of condensin can be clearly separated from its anaphase-specific function in chromosome compaction as well as from its function at repetitive rDNA sequences.
Condensin localizes to the centromere via its interaction with Sgo1 independently of PP2A-Rts1 phosphatase activity
To elucidate the mechanism of condensin localization to centromeres, we analyzed whether Sgo1 interacts with condensin in mitotic cells. To this end we precipitated Sgo1-TAP from yeast protein lysates. Indeed, we found that Sgo1 specifically pulls down the condensin subunit Smc2. This interaction is abolished in cells lacking Rts1, further strengthening the notion that Rts1 aids Sgo1 in condensin recruitment to the centromere (Figure 3A). The finding that Rts1 is important for the pulldown of Smc2 with Sgo1-TAP prompted us to ask whether the phosphatase activity of PP2A-Rts1 plays a role in the recruitment of condensin to centromeres. Therefore, we treated cells with okadaic acid (OKA), a potent inhibitor of the phosphatase activity of PP2A and analyzed its influence on condensin localization to centromeres in wild type cells. We found that the centromeric recruitment of the condensin subunit Ycg1-GFP is insensitive to treatment with OKA (Figure 3B, C). To exclude that this result is due to the low efficacy of the inhibitor in vivo, we analyzed the anaphase localization of Kin4, a well characterized target of PP2A-Rts1 [36], [37]. These experiments showed that the same concentration of OKA that did not affect Ycg1 localization efficiently delocalized Kin4 from the mother SPB within 30 min (Figure S6A, B). Similar observations were previously reported for Xenopus egg extracts and HeLa cells, where PP2A physically interacts with condensin II and recruits it to chromosomes independently of its phosphatase activity [38]. Taken together, our results suggest that Sgo1 and PP2A-Rts1 mediate condensin enrichment at the pericentric region. The recruitment of condensin does not depend on the phosphatase activity of PP2A, but rather relies on a physical interaction with Sgo1 supported by Rts1.
Fig. 3. Centromeric localization of condensin does not require PP2A's phosphatase activity. 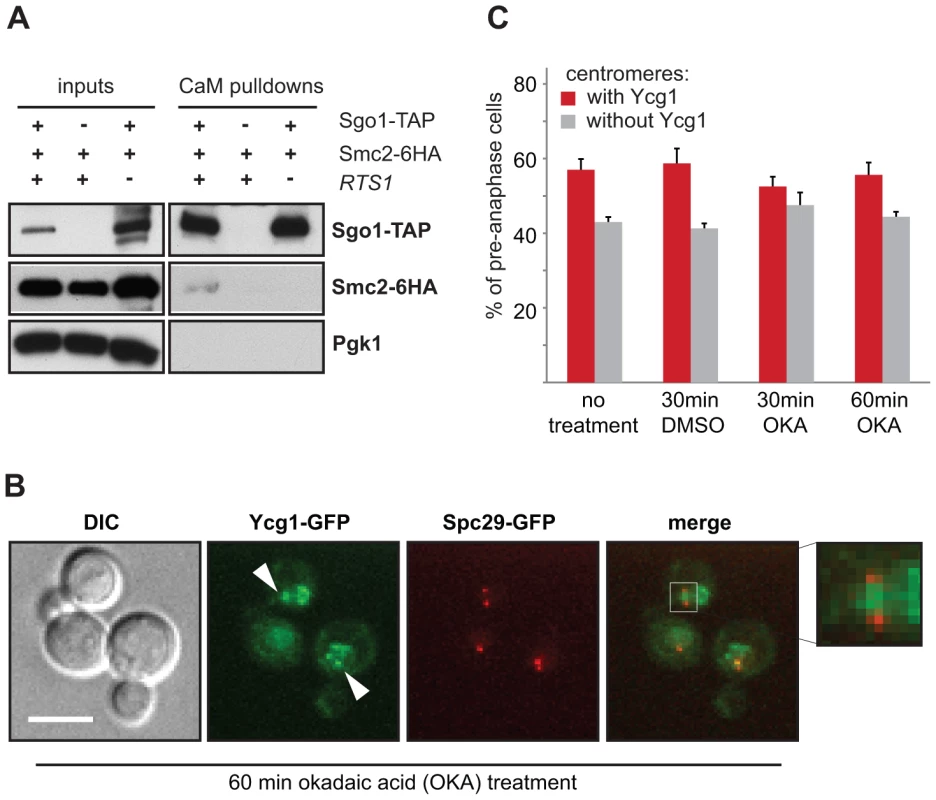
(A) Sgo1-TAP pulls down the condensin subunit Smc2-6HA. Co-immunoprecipitation of Smc2-6HA is dependent on the presence of Rts1. (B) Example of Ycg1-GFP localization after 60 min OKA treatment. SPBs are visualized with Spc29-RFP. Bar – 5 µm. (C) Quantification of subcellular localization of the condensin subunit Ycg1-GFP in the presence of OKA. Sgo1, PP2A and condensin affect correct Ipl1 localization
Recent high-throughput analysis revealed that condensin is required for the correct localization of the conserved kinase Aurora B/Ipl1 during metaphase and anaphase [39]. Since Sgo1 and PP2A-Rts1 are essential for condensin localization, we would expect a defective Aurora B/Ipl1 localization in sgo1Δ and rts1Δ mutants as well. Visualization of Ipl1-GFP revealed that the initial recruitment of the kinase to the KTs (before separation of the SPBs) was comparable in both wild type and sgo1Δ mutant cells (Figure S7A). In contrast, we observed a marked difference in Ipl1 localization at pre-anaphase spindles. Ipl1 localizes exclusively to centromeres and a diffuse nuclear signal was only rarely observed in wild type pre-anaphase cells (Figure 4A, B), which is in agreement with previous observations (e.g. [40] [41]). Careful analysis revealed that whereas cells with very short pre-anaphase spindles localize Ipl1-GFP between the SPBs, the cells with a diffuse nuclear localization of Ipl1-GFP had on average longer spindles (Figure S7B). In the absence of Sgo1, 57.8% of cells exhibited a diffuse nuclear signal of Ipl1-GFP, pointing to a defect in maintaining Ipl1 at the centromere (Figure 4A, B). Moreover, the correlation between spindle length and Ipl1-GFP localization was lost in sgo1Δ cells (Figure S7B). Ipl1 localization was also altered in cells lacking Rts1 or carrying the sgo1-N51I allele, although the phenotype was milder than in the cells lacking Sgo1 (25.0% and 31.2% of cells with diffuse Ipl1 signal, respectively; Figure 4A, B). No changes were observed in cells lacking Cdc55 (Figure 4A, B). ChIP analysis in cells arrested by nocodazole treatment showed that the levels of centromeric and pericentric Ipl1-FLAG were diminished in sgo1Δ cells to 52% of the wild type levels (Figure 4C). In contrast, the lack of RTS1 did not considerably impair Ipl1-FLAG recruitment to centromeres (Figure 4D). This indicates that Sgo1 might contribute to the maintenance of centromeric Ipl1 independently of Rts1 recruitment, or that, for technical reasons, the effect of PP2A-Rts1 is difficult to detect in nocodazole-arrested cells. Based on these observations we propose that Sgo1 is dispensable for the initial recruitment of Ipl1, but becomes essential for maintaining the centromeric localization of Ipl1 during the establishment of biorientation in budding yeast.
Fig. 4. Sgo1 and Rts1 are required for the maintenance of Ipl1 localization and activity at the centromere. 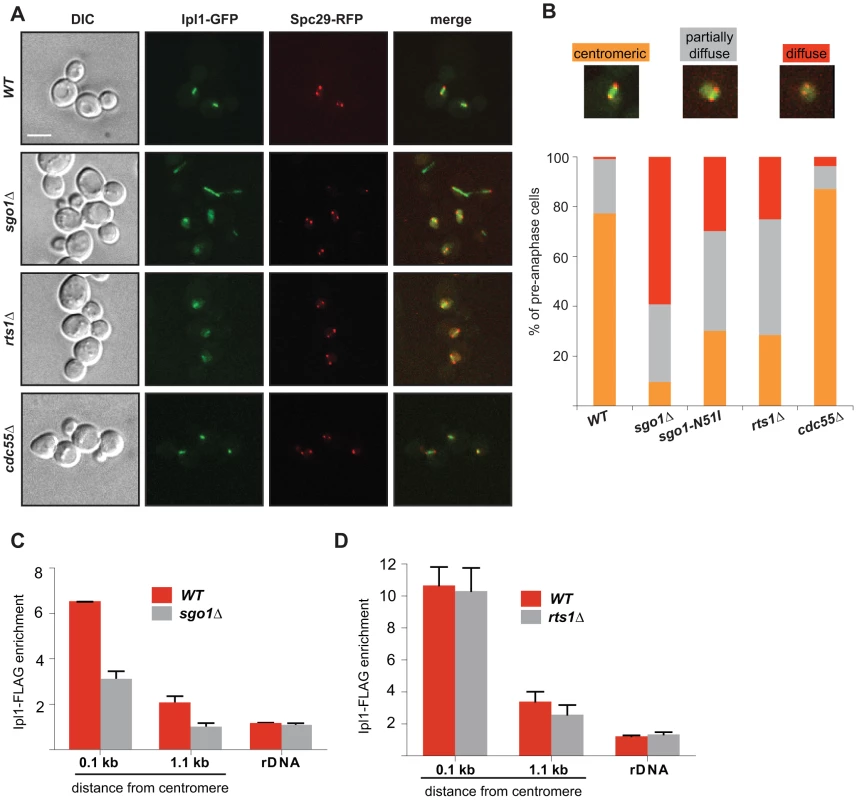
(A) Localization of Ipl1-GFP during mitosis in wild type cells and in cells lacking Sgo1, Rts1 or Cdc55. SPBs are visualized with Spc29-RFP. Bar – 5 µm. (B) Quantification of Ipl1-GFP localization on pre-anaphase spindles in wild type and sgo1Δ, sgo1-N51I, rts1Δ and cdc55Δ mutants (SPB distance <2 µm). Mean values of three independent experiments are shown. At least 150 cells were scored in each experiment. Top – examples of scored categories. (C) Enrichment of Ipl1-FLAG on centromeric DNA (0.1 kb away from CEN1 and 1.1 kb away from CEN4) and on rDNA (NTS1-2) normalized to the levels of Ipl1-FLAG bound to the arm of chromosome 10 in mitotic cells. ChIP-qPCR experiments of Ipl1-FLAG were performed using wild type and sgo1Δ cells arrested with nocodazole. Error bars represent the standard error of the mean. (D) Enrichment of Ipl1-FLAG on centromeric DNA (0.1 kb away from CEN1 and 1.1 kb away from CEN4) and on rDNA (NTS1-2) normalized to the levels of Ipl1-FLAG bound to the arm of chromosome 10 in mitotic cells. ChIP-qPCR experiments of Ipl1-FLAG were performed using wild type and rts1Δ cells arrested with nocodazole. Error bars represent the standard error of the mean. Next, we asked whether impairing the centromeric condensin pool leads to a more general defect in mitotic cells affecting the protein occupancy of the centromeric region, perhaps due to an altered DNA conformation. To test this possibility, we analysed strains carrying the temperature-sensitive condensin allele smc2-8. This mutation leads to a dramatic delocalization of Ycg1-GFP from centromeres at the non-permissive temperature (Figure S8A). However, we observed that Sgo1 and Rts1 localization was only marginally affected by the smc2-8 mutation (Figure S8B, C). Markedly, almost 40% of pre-anaphase spindles showed a diffuse Ipl1-GFP localization (Figure S8D). This suggests that condensin may contribute to the maintenance of Ipl1 on centromeres.
Both centromeric condensin and Ipl1 activity are required for the correction of syntelic attachments
The data presented above suggest a linear pathway where Sgo1, PP2A and condensin cooperate to ensure the maintenance of Ipl1 activity at the centromere. At the same time, we observed one marked difference: whereas condensin localization requires both Sgo1 and to a lesser degree also Rts1 function, the localization of Ipl1 depends only on Sgo1. To further explore the functional interactions, we exploited the finding that the CPC members Bir1 and Sli15 are high-copy-number suppressors of the benomyl sensitivity and ploidy-specific lethality of sgo1Δ mutants [41], [42]. We asked whether the overexpression of CPC subunits would restore the reduced levels of Ipl1 on the centromere in cells lacking centromeric Rts1. Indeed, Ipl1-GFP localization between the SPBs at pre-anaphase spindles was completely rescued by overexpression of Sli15 or Bir1 (Figure 5A, B). We next tested whether the restored localization of Ipl1 eliminates the defect in correction of tensionless MT-KT attachments in sgo1Δ and rts1Δ cells. As previously observed, overexpression of Sli15 partially rescued the growth of sgo1Δ in the presence of microtubule-depolymerizing drugs, but we found that the growth defect of rts1Δ cells was not improved (Figure 5C, [41]). We suggest that this difference reflects the different roles of Sgo1 and Rts1 in the maintenance of Ipl1 on centromeric DNA. Additionally, we found that the overexpression of Sli15 cannot rescue the exquisite sensitivity of sgo1Δ and rts1Δ mutants to syntelic attachments induced by Cik1-cc overexpression (Figure 5C). Thus, although the increased abundance of Sli15 or Bir1 increases the pool of Ipl1 localized between the SPBs, this is not sufficient to repair syntelic attachments when they are generated at high frequencies.
Fig. 5. Dual role of Sgo1 in localization of condensin and Ipl1. 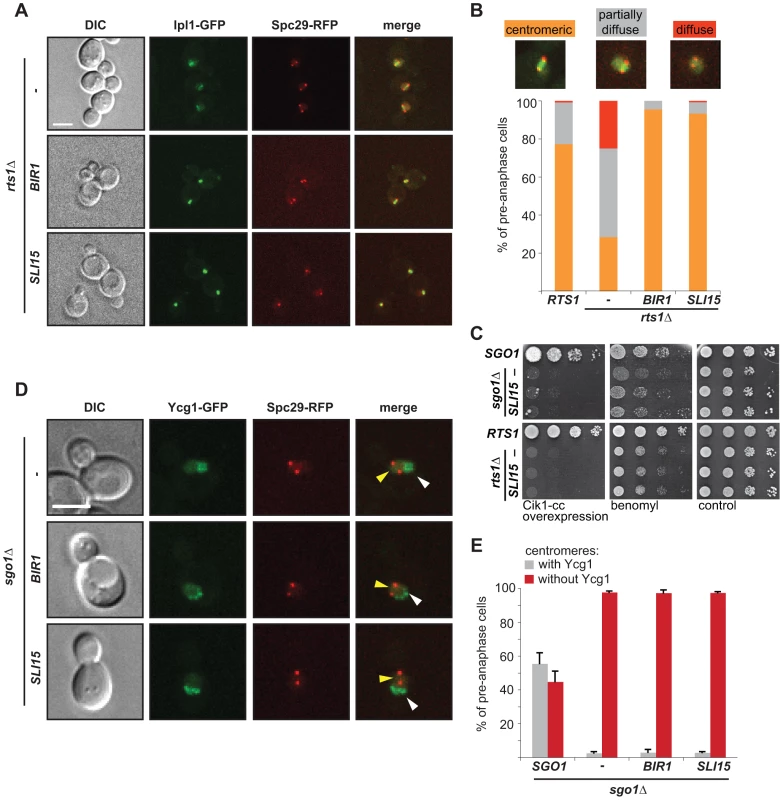
(A) Localization of Ipl1-GFP in rts1Δ cells that overexpress BIR1 or SLI15. SPBs are visualized with Spc29-RFP. Bar – 5 µm. (B) Quantification of Ipl1-GFP localization on pre-anaphase spindles. Means of three independent experiments are shown. At least 150 cells were scored in each experiment. (C) Growth of sgo1Δ and rts1Δ cells which overexpress BIR1 or SLI15 in the presence of microtubule poisons and under conditions leading to syntelic attachments. (D) Localization of the Ycg1-GFP signal in sgo1Δ cells which overexpress BIR1 or SLI15. SPBs are visualized with Spc29-RFP. Yellow arrowheads indicate lack of centromeric localization. White arrowheads indicate Ycg1-GFP localized to rDNA. Bar – 5 µm. (E) Quantification of Ycg1-GFP localization. Only pre-anaphase spindles were scored (SPB distance <2 µm). Means with SD of three independent experiments are shown. At least 150 cells were scored in each experiment. To determine why cells that localize Ipl1 properly, but lack centromeric Sgo1, cannot correct syntelic attachments, we analyzed the localization of the GFP-tagged condensin subunit Ycg1 in sgo1Δ cells overexpressing either Sli15 or Bir1. Importantly, we observed that the overexpression of CPC subunits did not restore centromeric condensin localization in sgo1Δ mutants (Figure 5D, E). From these results we conclude that the two functions of Sgo1 at the centromere – maintenance of the Ipl1 kinase at the MT-KT interface and providing flexibility to the pericentric region by condensin recruitment – are at least partially independent (Figure 6). Our findings illustrate that Sgo1 coordinates two essential functions at the centromere in order to facilitate chromosome biorientation during mitosis.
Fig. 6. Model of Sgo1 function during establishment of biorientation. 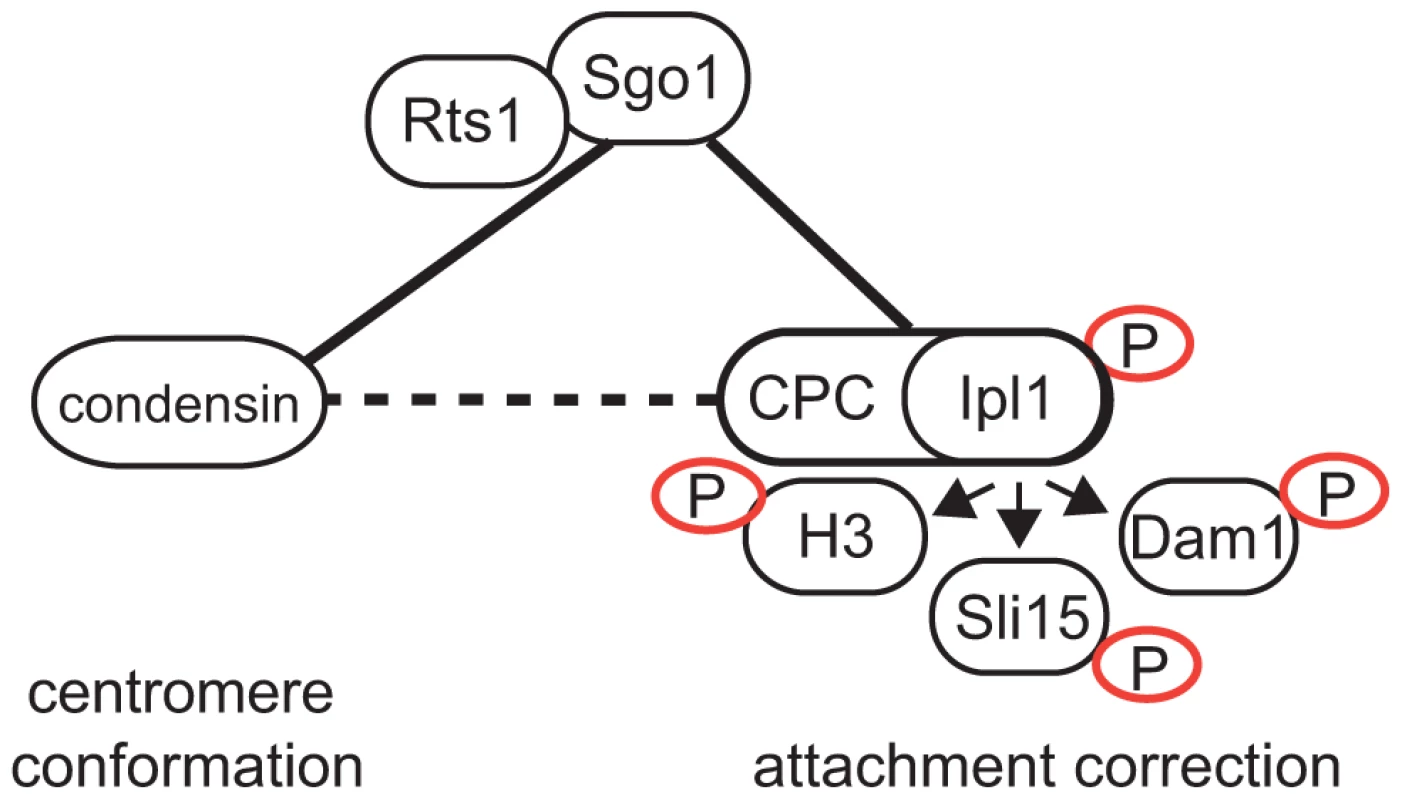
Connecting lines represent protein-protein interactions, dashed lines represent functional interactions. Full arrow depicts phosphorylation. Discussion
Here, we clarify the function of the Sgo1/PP2A-Rts1 interaction during the establishment of bioriented MT-KT attachments in budding yeast mitosis. We show that the Sgo1-dependent recruitment of PP2A-Rts1 is required for efficient localization of condensin to the centromere and that Sgo1 ensures the maintenance of centromeric Aurora B/Ipl1. Moreover, Sgo1 pulls down condensin in the presence of Rts1. Importantly, centromeric enrichment of both condensin and Aurora B/Ipl1 are essential for correct chromosome segregation. Our results are in agreement with the recent finding that shugoshin facilitates chromosome biorientation via condensin recruitment [43] and together suggest that Sgo1 serves as a hub protein that coordinates the molecular activities required for the biorientation of sister chromatids.
Budding yeast Sgo1, as all other members of the shugoshin protein family analyzed so far, interacts with the B56 regulatory subunit of protein phosphatase PP2A (Rts1 in budding yeast) via its N-terminal coiled-coil domain [8]–[11]. This interaction is crucial for shugoshin-mediated protection of centromeric cohesin from cleavage by separase during meiosis I and from phosphorylation-mediated removal during mitosis in vertebrates (reviewed in [44]). Yet, several lines of evidence suggest that in budding yeast, Sgo1 together with PP2A facilitates the establishment of biorientation, but by a mechanism independent of cohesin regulation. First, although lack of Sgo1 does not affect cohesion in budding yeast mitosis, the cells fail to recognize and correct improperly attached sister chromatids (Figure 1D, E, S4A, B, C, [4], [7], [41]). Moreover, cells carrying a mutation that impairs the Sgo1-Rts1 interaction fail to arrest in the presence of tensionless attachments induced by depletion of cohesin (Figure 1D, [11]). Additionally, cells lacking RTS1 or carrying mutations that impair the Sgo1-PP2A interaction show a marked sensitivity to microtubule depolymerizing drugs (Figure 1E, S2, [11]). We also observed that lack of Rts1 leads to defects in chromosome segregation (Figure S3A, B). Very recently, it was reported that Rts1 is not required for establishment of chromosome biorientation in cells arrested in metaphase by depletion of the anaphase regulator Cdc20, although a Sgo1 mutant that has lost the ability to interact with Rts1 showed a strong phenotype [43]. This result differs from our findings that Rts1 and the Sgo1-Rts1 interaction are required for correct chromosome segregation. Thus, future experiments should focus on dissecting the functions of Sgo1 and PP2A-Rts1 during the establishment of biorientation by identification of additional separation-of-function alleles.
The correct conformation of pericentric chromatin is essential for bioriented MT-KT attachments because it provides the rigidity and at the same time elasticity necessary for chromatin to withstand the opposing forces exerted by spindle MTs [17]. The inactivation of either cohesin or condensin results in the loss of tension across KTs and in defective turnover of syntelic attachments [30], [45]. We speculated that Sgo1 together with PP2A-Rts1 might affect the conformation of pericentric chromatin during metaphase, possibly by affecting loading of condensin onto this region. An increasing body of evidence supports this idea. First, condensin, similarly to cohesin, is a member of the SMC (structural maintenance of chromosomes) protein family and is, among others, required for the structure and organization of pericentric regions [18]. Second, condensin has been implicated in tension sensing and lack of condensin abolishes the conformational changes upon tension [16], [19], [20]. Finally, the ability of the inner KT to undergo conformational changes in response to altered tension is impaired in yeast cells lacking either Sgo1, or Bub1, a mitotic kinase essential for Sgo1 localization [21]. Here we demonstrate that Sgo1 and to a lesser degree also Rts1 are required for the localization of condensin specifically to centromeric and pericentric regions, but not to rDNA (Figure 2A, B, C, D, S4D, E). Importantly, cells with defective condensin cannot proliferate in the presence of microtubule poisons or when syntelic attachments are formed at high frequencies due to Cik1-cc overexpression and this condensin function can be separated from its anaphase functions in chromosome hypercondensation (Figure 2E). We hypothesized that PP2A might inhibit the phosphorylation of condensin to prevent its premature removal, similarly as was observed for cohesin molecules [44]. However, the localization of condensin is resistant to treatment with okadaic acid, a potent inhibitor of PP2A, suggesting that phosphatase activity is not required (Figure 3B, C). This is in agreement with studies in Xenopus egg extracts and HeLa cells, where recruitment of condensin II to chromosomes relies on the presence of PP2A independently of its phosphatase activity [38]. Yet, unlike yeast condensin, the localization of condensin II in higher eukaryotes is affected by the presence of okadaic acid; only the use of a catalytically inactive but correctly localized PP2A mutant revealed that chromosomal association of PP2A, but not its phosphatase activity, is essential for the targeting of condensin II to chromatin in Xenopus [38]. The difference might be explained by the fact that centromeric PP2A-Rts1 is recruited through an interaction with Sgo1, whereas Takomoto and colleagues analyzed the recruitment of condensin II to chromatin along entire chromosomes. Future research should clarify the nature of the interaction between Sgo1 and condensin as well as the role of Rts1 in condensin recruitment.
What is the function of condensin on centromeres? One possibility is that condensin maintains the conformation of centromeric regions, thereby facilitating the intrinsic bias of budding yeast KTs to biorient on the mitotic spindle [6]. This model would predict that cells lacking centromeric condensin should create monooriented attachments more often than wild type cells. However, cells lacking Sgo1 (and hence centromeric condensin) do not show any defect in the intrinsic bias of sister kinetochores to biorient [6]. Therefore, we suggest that the inability to localize centromeric condensin impairs error sensing and correction rather than contributing to the generation of more erroneous attachements.
We considered two possible roles for condensin in the correction of tensionless attachments. First, condensin might be required to facilitate the centromeric localization of Aurora B/Ipl1. Indeed, recently it has been shown that metaphase centromeres and KTs become deformed and Aurora B is mislocalized upon depletion of either condensin I or II in vertebrate cells [20]. Similarly, budding yeast lacking condensin fail to localize Ipl1 properly to the centromere during metaphase as well as to the spindle during anaphase [39]. This finding is in agreement with our observation that the absence of Sgo1 (and hence the absence of centromeric condensin) alters the localization of Ipl1 on centromeres during the establishment of biorientation. These results might imply a linear pathway where Sgo1 recruits PP2A, which, in turn, by recruiting condensin facilitates localization of Ipl1 to the centromere. Several important observations cannot be fully reconciled with this hypothesis. First, whereas full centromeric localization of condensin requires the presence of Rts1, localization of Ipl1 likely does not (compare Figure 2 and Figure 4). Second, although the overexpression of the CPC subunits Bir1 or Sli15 partially rescues the segregation defects and benomyl sensitivity of sgo1Δ cells and centromeric localization of Ipl1 in rts1Δ cells (Figure 5A, B, C, [41]), it does not affect the localization of condensin, nor the sensitivity of sgo1Δ and rts1Δ strains to Cik1-cc overexpression (Figure 5C, D, E). Additionally, deletion of SGO1 and mutations in condensin subunits are synthetically lethal, suggesting that they have non-overlapping functions [30]. Thus, although the functions of condensin and Ipl1 at the KT are closely linked, they are not arranged in a single linear pathway (Figure 6). We favor a model in which condensin is mainly required to establish the centromeric conformation that allows the generation of tension across bioriented KTs. Recent results suggest that condensin contributes to tension sensing by maintaining stiff, but flexible chromatin structure, which further supports our model [16].
In the wild type scenario, the majority of cells with very short spindles show an enrichment of Ipl1 between the SPBs, but with increased spindle length more cells with diffuse nuclear Ipl1 can be observed. One possible interpretation of this observation is that once tension is established, Ipl1 becomes more dynamic and is eventually released from the bioriented KTs. This is in agreement with the observation that Ipl1-GFP is often delocalized from centromeres in cells arrested in metaphase by Cdc20 depletion [40], [46]. The dynamics of Ipl1/Aurora B localization on centromeres is likely regulated by phosphorylation. A previous report demonstrated that shugoshin proteins from S. pombe and human cells interact with CPC subunits and this interaction depends on phosphorylation by Cdk1 [47]. Moreover, Ipl1 co-precipitates during mitosis with Sgo1 in budding yeast. Interestingly, this co-precipitation as well as centromeric Ipl1 localization was impaired in a strain carrying the mutant allele sgo1-3A which interferes with the Sgo1-Rts1 interaction [43]. Finally, Ipl1 is phosphorylated by the cyclin-dependent kinase Cdc28 upon anaphase onset; this phosphorylation prompts the binding of Ipl1 to Bim1, a microtubule plus-end tracking protein [46]. In the future, it should be determined whether Sgo1 affects the Cdc28-dependent phosphorylation of Aurora B/Ipl1 or other CPC subunits, thereby regulating their dynamics on the centromere during mitosis. Additionally, the role of protein phosphatases in this process remains to be characterized.
The centromeric localization and activity of Ipl1 in pre-anaphase cells is regulated by several factors. First, defective condensin impairs the localization of Ipl1 (but not vice versa [39]) and it has been shown that condensin subunits interact with Bir1, a member of the CPC [39]. Second, the overexpression of Bir1 or Sli15 localizes Ipl1 to the region between the SPBs in the absence of Rts1 (Figure 5A, B). Although we do not understand why Ipl1 localization is restored and whether under these conditions Ipl1 localizes exactly as in wild type cells, it has recently been postulated that Aurora B is tethered to the centromeric chromatin within the inner KT by Incenp/Sli15 [48]. The lack of Sgo1 also impairs the maintenance of Ipl1 localization during metaphase (Figure 4). This is in line with previous data that shugoshin proteins from human cells, fission and budding yeasts interact with the CPC, thereby contributing to the localization of Aurora B/Ipl1 at the pericentromere [43], [47]. Taken together, these results suggest the intriguing possibility that Sgo1 maintains centromeric localization of the CPC via a direct interaction (Figure 6). Lastly, the initial recruitment of Ipl1 to the centromere is independent of Sgo1 (Figure S7A, [41], [43]), indicating the existence of another recruiting mechanism, likely via the interaction of the CPC protein Bir1 with the KT protein Ndc10 [49], [50]. The fact that Aurora B/Ipl1 localization is regulated by several different mechanisms in both yeast and higher eukaryotes indicates that the CPC needs to be positioned correctly and in a timely manner in order to perform its functions. Yet, recent data suggest that even mislocalized Ipl1 can efficiently correct erroneous MT-KT attachments [51]. Further research should elucidate the exact spatio-temporal coordination of the molecular events during the establishment of biorientation. Our data show that Sgo1 is an important player during this event, serving as a hub modulating centromeric conformation and integrating phosphatase and kinase activities at the metaphase spindle.
Materials and Methods
Yeast strains and growth
All yeast strains used in this study are listed in Supporting Table S1 and derived from the genetic background of W303 (leu2-3, 112 trp1-1 can1-100 ura3-1 ade2-1 his3-11,15) or BY4741 (his3Δ1 leu2Δ0 met15Δ0 ura3Δ0). Gene deletions and epitope-tagging were performed using standard PCR-based methods. Cells were grown in either full (YP; 1% yeast extract, 2% Bacto-Peptone) or synthetic complete (SC; 1.34% yeast nitrogen base, 0.04% complete synthetic mix) medium supplemented with 2% glucose (YPD), 2% raffinose (YPR) or 2% galactose (YPG). Cells were arrested in G1 using 10 µM α-factor (Core Facility, Max Planck Institute of Biochemistry, Martinsried, Germany) or in mitosis using 30 µg/ml nocodazole (Santa Cruz Biotechnology). Mutants were grown at 25 °C to reduce chromosome missegregation. For viability assays cells were grown overnight in full medium, diluted to an OD600 of 0.3 and tenfold serial dilutions were spotted on YPD/YPG plates or on SC plates containing indicated concentrations of benomyl and nocodazole, respectively. The phosphatase inhibitor okadaic acid was used at concentrations of 10 or 20 µM as stated for the individual experiments.
Plasmid construction
All plasmids used in this study are listed in Supporting Table S2. Construction of plasmids was performed as described previously using standard cloning procedures. A fragment encoding amino acids 81–360 of Cik1 was cloned into the multiple cloning site (MCS) of pRS406 containing the inducible GAL1 promoter with a C-terminal TAP - or GFP-tag. SGO1 was cloned with its endogenous promoter into pRS405 with a C-terminal TAP-tag. Plasmids encoding the Mtw1-fusion proteins were constructed by cloning MTW1 with its endogenous promoter adjacent to the corresponding gene into the MCS of pRS405. Chromosomal integration of plasmids encoding Sgo1 - or Mtw1-fusion proteins was targeted into the endogenous locus using restriction enzymes cutting in the respective promoter region. Point mutations were introduced using the PCR-based Quick Change (Stratagene) site-directed mutagenesis approach.
Protein techniques
Protein extracts of S. cerevisiae were prepared either by glass bead lysis or alkaline lysis followed by TCA precipitation. Proteins were separated by SDS-PAGE, transferred to PVDF or nitrocellulose membranes and detected using antibodies according to standard protocols. Commercially available antibodies were used to detect individual proteins: myc (9E10, Santa Cruz Biotechnology), HA (Y-11, Santa Cruz Biotechnology), PAP (Sigma-Aldrich), Clb2 (y-180, Santa Cruz Biotechnology), Pgk1 (Invitrogen).
In vivo interaction analysis
Protein extracts from S. cerevisiae were prepared by glass bead lysis in a lysis buffer (10 mM Hepes, 200 mM KCl, 1 mM MgCl2, 1 mM DTT, 1 mM PMSF, 0.5% Triton X-100, supplemented with Roche Protease Inhibitor Mix) followed by centrifugation (100000 g, 45 min, 4°C). Sgo1-TAP was purified by incubation of approx. 3.5 mg protein extract with pre-washed calmodulin-coated beads (GE Healthcare). Beads were washed using lysis buffer containing increasing salt concentrations (up to 600 mM KCl) and finally eluted in the presence of 5 mM EGTA. Proteins were precipitated with TCA and co-purified proteins were analyzed by SDS-PAGE followed by immunoblotting.
PP2A – His-Sgo1-pulldown experiments
Heterotrimeric PP2A complexes were purified from mitotic lysates of S. cerevisiae via Rts1 - or Cdc55-TAP fusion proteins using calmodulin-coupled agarose as described above. Equal amounts of PP2A complexes were incubated with recombinant Ni-NTA agarose-bound His-Sgo1 fragments containing the conserved coiled-coil domain (amino acids 1–340) in a pulldown buffer (50 mM Tris, 300 mM NaCl, 10 mM imidazole, 1 mM PMSF supplemented with Roche protease inhibitor mix) for 2 hours. Proteins were eluted by boiling in SDS sample buffer and subjected to SDS-PAGE and immunoblotting to detect the bound PP2A subunits.
Fluorescence microscopy
Images of cells were obtained using a fully automated Zeiss inverted microscope (AxioObserver Z1) equipped with a MS-2000 stage (Applied Scientific Instrumentation, USA), a CSU-X1 spinning disk confocal head (Yokogawa, Herrsching), LaserStack Launch with selectable laser lines (Intelligent Imaging Innovations, USA) and an X-CITE Fluorescent Illumination System. Images were captured using a CoolSnap HQ camera (Roper Scientific, Canada) under the control of the Slidebook software (Intelligent Imaging Innovations, USA). All fluorescence signals were imaged with a 63x oil objective. A total of 10 z-stacks were collected and each optical section was 0.4 µm thick. Projected images were used for display. Only cells with spindles shorter than 2 µm and fully localized in the mother cell were scored to evaluate the localization in pre-anaphase. For Ipl1-GFP microscopy we defined three types of Ipl1-GFP localization: “centromeric” – fluorescence signal only in the area between the SPBs, “partially diffuse” – diffuse nuclear fluorescence signal and increased fluorescence intensity between the SPBs and “diffuse” – only diffuse nuclear fluorescence signal.
Chromatin-immunoprecipitation (ChIP)
Chromatin immunoprecipitation (ChIP) was performed as previously described [52]. In brief, 100 ml of exponentially growing yeast cultures were arrested with 20 µg/ml nocodazole for 3 h at room temperature and subsequently cross-linked with formaldehyde at a final concentration of 1%. The cross-linking reaction was stopped with glycine; the cells were harvested and lysed using Silica beads. Chromatin was sheared to 300–500 bp fragments by water-bath sonification (Bioruptor UCD-200, Diagenode). FLAG-tagged proteins were immunoprecipitated using the monoclonal ANTI-FLAG antibody coupled to superparamagnetic beads (Sigma-Aldrich, M8823). DNA was recovered by phenol/chloroform extraction followed by ethanol precipitation. Quantitative RT-PCR was performed using the Light Cycler LC480 system (Roche) to evaluate the enrichment of analyzed proteins. The ratio of DNA-IP to DNA-Input was calculated for centromeric/pericentromeric regions (0.1 kb away from CEN1, 1.1 kb away from CEN4 and 5 kb away from CEN12) as well as for the rDNA locus (NTS1-2). The relative enrichment was calculated by normalization to the IP/Input ratio for a control locus on the arm of chromosome 10. Three independent immunoprecipitation experiments from metaphase-arrested cells were performed for FLAG-tagged Smc2 and Ipl1; Rts1 and Mcd1 ChIP experiments were performed twice.
Supporting Information
Zdroje
1. LampsonMA, CheesemanIM (2011) Sensing centromere tension: Aurora B and the regulation of kinetochore function. Trends Cell Biol 21 : 133–140.
2. CheesemanIM, AndersonS, JwaM, GreenEM, KangJ, et al. (2002) Phospho-regulation of kinetochore-microtubule attachments by the Aurora kinase Ipl1p. Cell 111 : 163–172.
3. TanakaTU, RachidiN, JankeC, PereiraG, GalovaM, et al. (2002) Evidence that the Ipl1-Sli15 (Aurora kinase-INCENP) complex promotes chromosome bi-orientation by altering kinetochore-spindle pole connections. Cell 108 : 317–329.
4. IndjeianVB, SternBM, MurrayAW (2005) The centromeric protein Sgo1 is required to sense lack of tension on mitotic chromosomes. Science 307 : 130–133.
5. KawashimaSA, TsukaharaT, LangeggerM, HaufS, KitajimaTS, et al. (2007) Shugoshin enables tension-generating attachment of kinetochores by loading Aurora to centromeres. Genes & Development 21 : 420–435.
6. IndjeianVB, MurrayAW (2007) Budding yeast mitotic chromosomes have an intrinsic bias to biorient on the spindle. Curr Biol 17 : 1837–1846.
7. KiburzBM, AmonA, MarstonAL (2008) Shugoshin promotes sister kinetochore biorientation in Saccharomyces cerevisiae. Mol Biol Cell 19 : 1199–1209.
8. KitajimaTS, SakunoT, IshiguroK, IemuraS, NatsumeT, et al. (2006) Shugoshin collaborates with protein phosphatase 2A to protect cohesin. Nature 441 : 46–52.
9. RiedelCG, KatisVL, KatouY, MoriS, ItohT, et al. (2006) Protein phosphatase 2A protects centromeric sister chromatid cohesion during meiosis I. Nature 441 : 53–61.
10. TangZ, ShuH, QiW, MahmoodNA, MumbyMC, et al. (2006) PP2A is required for centromeric localization of Sgo1 and proper chromosome segregation. Dev Cell 10 : 575–585.
11. XuZ, CetinB, AngerM, ChoUS, HelmhartW, et al. (2009) Structure and function of the PP2A-shugoshin interaction. Mol Cell 35 : 426–441.
12. KawashimaS, NakabayashiY, MatsubaraK, SanoN, EnomotoT, et al. (2011) Global analysis of core histones reveals nucleosomal surfaces required for chromosome bi-orientation. EMBO J 30 : 3353–3367.
13. RiveraT, GhenoiuC, Rodriguez-CorsinoM, MochidaS, FunabikiH, et al. (2012) Xenopus Shugoshin 2 regulates the spindle assembly pathway mediated by the chromosomal passenger complex. EMBO J 31 : 1467–1479.
14. MarescaTJ, SalmonED (2009) Intrakinetochore stretch is associated with changes in kinetochore phosphorylation and spindle assembly checkpoint activity. J Cell Biol 184 : 373–381.
15. UchidaKS, TakagakiK, KumadaK, HirayamaY, NodaT, et al. (2009) Kinetochore stretching inactivates the spindle assembly checkpoint. J Cell Biol 184 : 383–390.
16. StephensAD, HaaseJ, VicciL, TaylorRM2nd, BloomK (2011) Cohesin, condensin, and the intramolecular centromere loop together generate the mitotic chromatin spring. J Cell Biol 193 : 1167–1180.
17. StephensAD, HaggertyRA, VasquezPA, VicciL, SniderCE, et al. (2013) Pericentric chromatin loops function as a nonlinear spring in mitotic force balance. J Cell Biol 200 : 757–772.
18. PoonBP, MekhailK (2011) Cohesin and related coiled-coil domain-containing complexes physically and functionally connect the dots across the genome. Cell Cycle 10 : 2669–2682.
19. RibeiroSA, GatlinJC, DongY, JoglekarA, CameronL, et al. (2009) Condensin regulates the stiffness of vertebrate centromeres. Mol Biol Cell 20 : 2371–2380.
20. SamoshkinA, ArnaoutovA, JansenLE, OuspenskiI, DyeL, et al. (2009) Human condensin function is essential for centromeric chromatin assembly and proper sister kinetochore orientation. PLoS One 4: e6831.
21. HaaseJ, StephensA, VerdaasdonkJ, YehE, BloomK (2012) Bub1 kinase and Sgo1 modulate pericentric chromatin in response to altered microtubule dynamics. Curr Biol 22 : 471–481.
22. SakunoT, WatanabeY (2009) Studies of meiosis disclose distinct roles of cohesion in the core centromere and pericentromeric regions. Chromosome Res 17 : 239–249.
23. MooreLL, StanvitchG, RothMB, RosenD (2005) HCP-4/CENP-C promotes the prophase timing of centromere resolution by enabling the centromere association of HCP-6 in Caenorhabditis elegans. Mol Cell Biol 25 : 2583–2592.
24. ShiY (2009) Serine/threonine phosphatases: mechanism through structure. Cell 139 : 468–484.
25. GentryMS, HallbergRL (2002) Localization of Saccharomyces cerevisiae protein phosphatase 2A subunits throughout mitotic cell cycle. Mol Biol Cell 13 : 3477–3492.
26. YuHG, KoshlandD (2007) The Aurora kinase Ipl1 maintains the centromeric localization of PP2A to protect cohesin during meiosis. J Cell Biol 176 : 911–918.
27. HeX, AsthanaS, SorgerPK (2000) Transient sister chromatid separation and elastic deformation of chromosomes during mitosis in budding yeast. Cell 101 : 763–775.
28. JinF, LiuH, LiP, YuHG, WangY (2012) Loss of function of the Cik1/Kar3 motor complex results in chromosomes with syntelic attachment that are sensed by the tension checkpoint. PLoS Genet 8: e1002492.
29. TanakaT, CosmaMP, WirthK, NasmythK (1999) Identification of cohesin association sites at centromeres and along chromosome arms. Cell 98 : 847–858.
30. Yong-GonzalezV, WangB-D, ButylinP, OuspenskiI, StrunnikovA (2007) Condensin function at centromere chromatin facilitates proper kinetochore tension and ensures correct mitotic segregation of sister chromatids. Genes to Cells 12 : 1075–1090.
31. KiburzBM, ReynoldsDB, MegeePC, MarstonAL, LeeBH, et al. (2005) The core centromere and Sgo1 establish a 50-kb cohesin-protected domain around centromeres during meiosis I. Genes Dev. 19 : 3017–3030.
32. KatisVL, GalovaM, RabitschKP, GreganJ, NasmythK (2004) Maintenance of Cohesin at Centromeres after Meiosis I in Budding Yeast Requires a Kinetochore-Associated Protein Related to MEI-S332. Current Biology 14 : 560–572.
33. FreemanL, Aragon-AlcaideL, StrunnikovA (2000) The Condensin Complex Governs Chromosome Condensation and Mitotic Transmission of Rdna. The Journal of Cell Biology 149 : 811–824.
34. JohzukaK, HoriuchiT (2009) The cis Element and Factors Required for Condensin Recruitment to Chromosomes. Molecular Cell 34 : 26–35.
35. St-PierreJ, DouziechM, BazileF, PascariuM, BonneilE, et al. (2009) Polo kinase regulates mitotic chromosome condensation by hyperactivation of condensin DNA supercoiling activity. Mol Cell 34 : 416–426.
36. ChanLY, AmonA (2009) The protein phosphatase 2A functions in the spindle position checkpoint by regulating the checkpoint kinase Kin4. Genes Dev 23 : 1639–1649.
37. D'AquinoKE, Monje-CasasF, PaulsonJ, ReiserV, CharlesGM, et al. (2005) The protein kinase Kin4 inhibits exit from mitosis in response to spindle position defects. Mol Cell 19 : 223–234.
38. TakemotoA, MaeshimaK, IkeharaT, YamaguchiK, MurayamaA, et al. (2009) The chromosomal association of condensin II is regulated by a noncatalytic function of PP2A. Nat Struct Mol Biol 16 : 1302–1308.
39. LiZ, VizeacoumarFJ, BahrS, LiJ, WarringerJ, et al. (2011) Systematic exploration of essential yeast gene function with temperature-sensitive mutants. Nat Biotechnol 29 : 361–367.
40. BuvelotS, TatsutaniSY, VermaakD, BigginsS (2003) The budding yeast Ipl1/Aurora protein kinase regulates mitotic spindle disassembly. J Cell Biol 160 : 329–339.
41. StorchovaZ, BeckerJS, TalarekN, KogelsbergerS, PellmanD (2011) Bub1, Sgo1, and Mps1 mediate a distinct pathway for chromosome biorientation in budding yeast. Mol Biol Cell 22 : 1473–1485.
42. StorchovaZ, BrenemanA, CandeJ, DunnJ, BurbankK, et al. (2006) Genome-wide genetic analysis of polyploidy in yeast. Nature 443 : 541–547.
43. VerzijlbergenKF, NerushevaOO, KellyD, KerrA, CliftD, et al. (2014) Shugoshin biases chromosomes for biorientation through condensin recruitment to the pericentromere. Elife 3: e01374.
44. Gutierrez-CaballeroC, CebolleroLR, PendasAM (2012) Shugoshins: from protectors of cohesion to versatile adaptors at the centromere. Trends Genet 28 : 351–360.
45. NgTM, WaplesWG, LavoieBD, BigginsS (2009) Pericentromeric sister chromatid cohesion promotes kinetochore biorientation. Mol Biol Cell 20 : 3818–3827.
46. ZimniakT, FitzV, ZhouH, LampertF, OpravilS, et al. (2012) Spatiotemporal regulation of Ipl1/Aurora activity by direct Cdk1 phosphorylation. Curr Biol 22 : 787–793.
47. TsukaharaT, TannoY, WatanabeY (2010) Phosphorylation of the CPC by Cdk1 promotes chromosome bi-orientation. Nature 467 : 719–723.
48. SantaguidaS, MusacchioA (2009) The life and miracles of kinetochores. EMBO J 28 : 2511–2531.
49. YoonHJ, CarbonJ (1999) Participation of Bir1p, a member of the inhibitor of apoptosis family, in yeast chromosome segregation events. Proc Natl Acad Sci U S A 96 : 13208–13213.
50. ChoUS, HarrisonSC (2012) Ndc10 is a platform for inner kinetochore assembly in budding yeast. Nat Struct Mol Biol 19 : 48–55.
51. CampbellCS, DesaiA (2013) Tension sensing by Aurora B kinase is independent of survivin-based centromere localization. Nature 497 : 118–121.
52. KalocsayM, HillerNJ, JentschS (2009) Chromosome-wide Rad51 spreading and SUMO-H2A.Z-dependent chromosome fixation in response to a persistent DNA double-strand break. Mol Cell 33 : 335–343.
Štítky
Genetika Reprodukční medicína
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2014 Číslo 6
-
Všechny články tohoto čísla
- Inflammation: Gone with Translation
- Recombination Accelerates Adaptation on a Large-Scale Empirical Fitness Landscape in HIV-1
- Caspase Inhibition in Select Olfactory Neurons Restores Innate Attraction Behavior in Aged
- Accurate, Model-Based Tuning of Synthetic Gene Expression Using Introns in
- A Novel Peptidoglycan Binding Protein Crucial for PBP1A-Mediated Cell Wall Biogenesis in
- Ancient DNA Analysis of 8000 B.C. Near Eastern Farmers Supports an Early Neolithic Pioneer Maritime Colonization of Mainland Europe through Cyprus and the Aegean Islands
- The Epidermal Growth Factor Receptor Critically Regulates Endometrial Function during Early Pregnancy
- Introgression from Domestic Goat Generated Variation at the Major Histocompatibility Complex of Alpine Ibex
- Netrins and Wnts Function Redundantly to Regulate Antero-Posterior and Dorso-Ventral Guidance in
- Coordination of Wing and Whole-Body Development at Developmental Milestones Ensures Robustness against Environmental and Physiological Perturbations
- Phenotypic Dissection of Bone Mineral Density Reveals Skeletal Site Specificity and Facilitates the Identification of Novel Loci in the Genetic Regulation of Bone Mass Attainment
- Deep Evolutionary Comparison of Gene Expression Identifies Parallel Recruitment of -Factors in Two Independent Origins of C Photosynthesis
- Loss of UCP2 Attenuates Mitochondrial Dysfunction without Altering ROS Production and Uncoupling Activity
- Translational Regulation of Specific mRNAs Controls Feedback Inhibition and Survival during Macrophage Activation
- Rosa26-GFP Direct Repeat (RaDR-GFP) Mice Reveal Tissue- and Age-Dependence of Homologous Recombination in Mammals
- Abnormal Type I Collagen Post-translational Modification and Crosslinking in a Cyclophilin B KO Mouse Model of Recessive Osteogenesis Imperfecta
- : Clonal Reinforcement Drives Evolution of a Simple Microbial Community
- Reviving the Dead: History and Reactivation of an Extinct L1
- Defective iA37 Modification of Mitochondrial and Cytosolic tRNAs Results from Pathogenic Mutations in TRIT1 and Its Substrate tRNA
- Early Back-to-Africa Migration into the Horn of Africa
- Aberrant Autolysosomal Regulation Is Linked to The Induction of Embryonic Senescence: Differential Roles of Beclin 1 and p53 in Vertebrate Spns1 Deficiency
- Microbial Succession in the Gut: Directional Trends of Taxonomic and Functional Change in a Birth Cohort of Spanish Infants
- Integrated Pathway-Based Approach Identifies Association between Genomic Regions at CTCF and CACNB2 and Schizophrenia
- Genetic Determinants of Long-Term Changes in Blood Lipid Concentrations: 10-Year Follow-Up of the GLACIER Study
- Palaeosymbiosis Revealed by Genomic Fossils of in a Strongyloidean Nematode
- Early Embryogenesis-Specific Expression of the Rice Transposon Enhances Amplification of the MITE
- PINK1-Mediated Phosphorylation of Parkin Boosts Parkin Activity in
- OsHUS1 Facilitates Accurate Meiotic Recombination in Rice
- Genetic Background Drives Transcriptional Variation in Human Induced Pluripotent Stem Cells
- Pervasive Divergence of Transcriptional Gene Regulation in Caenorhabditis Nematodes
- N-WASP Is Required for Structural Integrity of the Blood-Testis Barrier
- The Transcription Factor TFII-I Promotes DNA Translesion Synthesis and Genomic Stability
- An Operon of Three Transcriptional Regulators Controls Horizontal Gene Transfer of the Integrative and Conjugative Element ICE in B13
- Digital Genotyping of Macrosatellites and Multicopy Genes Reveals Novel Biological Functions Associated with Copy Number Variation of Large Tandem Repeats
- ATRA-Induced Cellular Differentiation and CD38 Expression Inhibits Acquisition of BCR-ABL Mutations for CML Acquired Resistance
- The EJC Binding and Dissociating Activity of PYM Is Regulated in
- JNK Controls the Onset of Mitosis in Planarian Stem Cells and Triggers Apoptotic Cell Death Required for Regeneration and Remodeling
- Mouse Y-Linked and Are Expressed during the Male-Specific Interphase between Meiosis I and Meiosis II and Promote the 2 Meiotic Division
- Rasa3 Controls Megakaryocyte Rap1 Activation, Integrin Signaling and Differentiation into Proplatelet
- Transcriptional Control of Steroid Biosynthesis Genes in the Prothoracic Gland by Ventral Veins Lacking and Knirps
- Souffle/Spastizin Controls Secretory Vesicle Maturation during Zebrafish Oogenesis
- The POU Factor Ventral Veins Lacking/Drifter Directs the Timing of Metamorphosis through Ecdysteroid and Juvenile Hormone Signaling
- The First Endogenous Herpesvirus, Identified in the Tarsier Genome, and Novel Sequences from Primate Rhadinoviruses and Lymphocryptoviruses
- Sequence of a Complete Chicken BG Haplotype Shows Dynamic Expansion and Contraction of Two Gene Lineages with Particular Expression Patterns
- Background Selection as Baseline for Nucleotide Variation across the Genome
- CPF-Associated Phosphatase Activity Opposes Condensin-Mediated Chromosome Condensation
- The Effects of Codon Context on Translation Speed
- Glycogen Synthase Kinase (GSK) 3β Phosphorylates and Protects Nuclear Myosin 1c from Proteasome-Mediated Degradation to Activate rDNA Transcription in Early G1 Cells
- Regulation of Gene Expression in Autoimmune Disease Loci and the Genetic Basis of Proliferation in CD4 Effector Memory T Cells
- Muscle Structure Influences Utrophin Expression in Mice
- BLMP-1/Blimp-1 Regulates the Spatiotemporal Cell Migration Pattern in
- Identification of Late Larval Stage Developmental Checkpoints in Regulated by Insulin/IGF and Steroid Hormone Signaling Pathways
- Transport of Magnesium by a Bacterial Nramp-Related Gene
- Sgo1 Regulates Both Condensin and Ipl1/Aurora B to Promote Chromosome Biorientation
- The HY5-PIF Regulatory Module Coordinates Light and Temperature Control of Photosynthetic Gene Transcription
- The Rim15-Endosulfine-PP2A Signalling Module Regulates Entry into Gametogenesis and Quiescence Distinct Mechanisms in Budding Yeast
- Regulation of Hfq by the RNA CrcZ in Carbon Catabolite Repression
- Loss of a Neural AMP-Activated Kinase Mimics the Effects of Elevated Serotonin on Fat, Movement, and Hormonal Secretions
- Positive Feedback of Expression Ensures Irreversible Meiotic Commitment in Budding Yeast
- Hecate/Grip2a Acts to Reorganize the Cytoskeleton in the Symmetry-Breaking Event of Embryonic Axis Induction
- Regulatory Mechanisms That Prevent Re-initiation of DNA Replication Can Be Locally Modulated at Origins by Nearby Sequence Elements
- Speciation and Introgression between and
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Early Back-to-Africa Migration into the Horn of Africa
- PINK1-Mediated Phosphorylation of Parkin Boosts Parkin Activity in
- Ancient DNA Analysis of 8000 B.C. Near Eastern Farmers Supports an Early Neolithic Pioneer Maritime Colonization of Mainland Europe through Cyprus and the Aegean Islands
- OsHUS1 Facilitates Accurate Meiotic Recombination in Rice
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



