-
Články
Reklama
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
ReklamaChk2 and p53 Are Haploinsufficient with Dependent and Independent Functions to Eliminate Cells after Telomere Loss
The mechanisms that cells use to monitor telomere integrity, and the array of responses that may be induced, are not fully defined. To date there have been no studies in animals describing the ability of cells to survive and contribute to adult organs following telomere loss. We developed assays to monitor the ability of somatic cells to proliferate and differentiate after telomere loss. Here we show that p53 and Chk2 limit the growth and differentiation of cells that lose a telomere. Furthermore, our results show that two copies of the genes encoding p53 and Chk2 are required for the cell to mount a rapid wildtype response to a missing telomere. Finally, our results show that, while Chk2 functions by activating the p53-dependent apoptotic cascade, Chk2 also functions independently of p53 to limit survival. In spite of these mechanisms to eliminate cells that have lost a telomere, we find that such cells can make a substantial contribution to differentiated adult tissues.
Published in the journal: . PLoS Genet 7(6): e32767. doi:10.1371/journal.pgen.1002103
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002103Summary
The mechanisms that cells use to monitor telomere integrity, and the array of responses that may be induced, are not fully defined. To date there have been no studies in animals describing the ability of cells to survive and contribute to adult organs following telomere loss. We developed assays to monitor the ability of somatic cells to proliferate and differentiate after telomere loss. Here we show that p53 and Chk2 limit the growth and differentiation of cells that lose a telomere. Furthermore, our results show that two copies of the genes encoding p53 and Chk2 are required for the cell to mount a rapid wildtype response to a missing telomere. Finally, our results show that, while Chk2 functions by activating the p53-dependent apoptotic cascade, Chk2 also functions independently of p53 to limit survival. In spite of these mechanisms to eliminate cells that have lost a telomere, we find that such cells can make a substantial contribution to differentiated adult tissues.
Introduction
In the 1930s, seminal work from Hermann Muller and Barbara McClintock showed that the normal termini of linear chromosomes can be distinguished from ends produced by chromosome breakage [1], [2]. Muller showed that normal ends did not participate in chromosome rearrangements induced by irradiation, and conversely, that broken ends created by ionizing radiation could not substitute for normal termini. McClintock demonstrated that broken chromosome ends undergo end-to-end fusion, leading to anaphase bridges during mitosis, followed by breakage which then led this process to repeat. This Breakage-Fusion-Bridge (BFB) cycle could continue for several rounds of mitosis. Evidence for telomere dysfunction and BFB cycles is seen in human tumors and may represent a precipitating early step in carcinogenesis [3]. However, the importance of telomere integrity to ongoing cellular viability is made clear by the discoveries that even cancer cells possess a mechanism for telomere maintenance, either by upregulation of telomerase or through the Alternative Lengthening of Telomeres pathway [4], [5]. If such maintenance mechanisms are lost, the cancer cells undergo apoptosis.
Previously, we showed that telomere loss in somatic cells of flies results in robust activation of caspase-3 mediated apoptosis [6]. This apoptosis is regulated by two p53-dependent pathways, with the majority mediated through loki (lok), which encodes the Drosophila ortholog of the Chk2 checkpoint kinase, and a much smaller fraction mediated through mei41 and grapes (grp), which encode the fly orthologs of mammalian DNA damage response proteins ATM and Rad3 related protein (ATR) and the Chk1 checkpoint kinase, respectively. When telomere loss is accompanied by the generation of aneuploidy, a p53-independent pathway to apoptosis is also activated, but is delayed by many hours [6]–[8]. However, despite the two-pronged robust apoptotic response, a karyotype analysis of neuroblasts demonstrated that a fraction of cells (up to 20%) are able to survive and divide repeatedly for up to 96 hours, until pupariation. Furthermore, a small subpopulation of these surviving cells had experienced repeated BFB cycles, showing that some cells can divide multiple times even though they carry a chromosome lacking a telomere [6].
In contrast, in both the male and female germlines there is clear evidence that chromosomes that have lost telomere can become healed by de novo telomere addition. This healing occurs efficiently in wildtype males [9], [10] or in females that carry the mu2 mutation [11]. These data suggest that different cell types have varying responses to the same genetic lesion, a missing telomere, and studies in model organisms will be pivotal to elucidate new targets for cancer therapy.
Although previous work has shown that some cells that have lost a telomere are able to differentiate [12], [13], the degree to which they participate in forming adult structures remains unclear, nor is it known whether escape from apoptosis is sufficient to allow a cell to fully differentiate after telomere loss. In the work reported here we quantitate the ability of cells to contribute to adult structures after telomere loss and we show that mutation of the DNA Damage Response (DDR) genes p53 and lok greatly enhances the survival and differentiation of such cells. Our results show that the genes encoding these proteins are haplo-insufficient. Furthermore, we find that Chk2 functions independently of p53 to limit cell survival.
Results
Bar and Telomere Loss assay
To determine the extent to which cells that have lost a telomere are capable of contributing to adult tissue we developed a highly sensitive assay called the Bar and Telomere Loss (BARTL) assay (Figure 1A). Cells that lose a single telomere normally suffer a high rate of apoptosis and, although some do survive and differentiate [12], [13], their ability to contribute to the adult is poorly defined. One drawback to the interpretation of those experiments is that telomere loss was accompanied by some degree of aneuploidy. We designed the BARTL assay so that, in somatic cells of the eye, a single telomere is lost from a dispensable Y chromosome and this coincides with loss of the dominant BarStone (BS) mutation. BS causes caspase-3-dependent cell death in the developing eye starting at least as early as second instar and continuing until only a small posterior segment of the eye imaginal disc remains, resulting in adults with bar-shaped eyes ([14], [15] and Figure S1). We reasoned that loss of BS could give cells a growth advantage and provide a favorable environment to assess their potential for growth and differentiation after telomere loss.
Fig. 1. Graphical depiction of assays and chromosomes employed in this work. 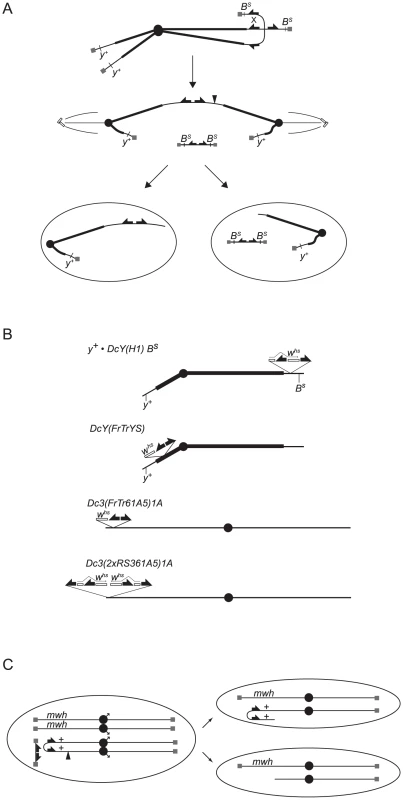
(A) Telomere loss in the BARTL assay: FLP mediates recombination between FRTs on sister chromatids to generate a dicentric chromosome and an acentric chromosome that contains both copies of BS. During anaphase the dicentric chromosome is pulled toward opposite poles and breaks delivering a chromosome with one broken end to each daughter cell. The acentric fragment does not segregate faithfully and is either lost or randomly segregates with one of the daughter cells. Cells that don't inherit the acentric fragment are B+. (B) The dicentric inducible chromosomes. FRTs are represented as filled half-arrows, the whs marker as an open rectangle, and centromeres as filled circles. The location of whs relative to FRTs on DcY(FrTrYS)4B1A is not known, and the representation here is arbitrary. (C) Telomere loss in the SMARTL assay: A chromosome 3 marked with the recessive multiple wing hairs (mwh) mutation is heterozygous with the dicentric inducible Dc3(FrTr61A5)1A that carries a mwh+ allele. FLP induces recombination distal to the mwh+ locus to generate a dicentric bridge. If the bridge breaks asymmetrically such that the break point is proximal to the mwh+ allele (at filled arrowhead) one daughter cell will be hemizygous for mwh. To induce telomere loss the FLP site-specific recombinase was used to mediate sister chromatid fusion by recombination between inverted FRTs on sister chromatids. This produces an acentric chromosome and a dicentric chromosome, which breaks in more than 90% of mitoses and delivers a chromosome with a non-telomeric end to each daughter cell (Figure 1A; [6]). BARTL uses the dicentric-inducible Y chromosome, DcY(H1), which carries inverted FRTs flanking the 3′ coding region of whs inserted proximal to BS (Figure 1B).
Cells that experience loss of a telomere in this assay have an advantage because they have lost BS, but are still subject to the telomere loss-induced DNA damage response that frequently results in apoptosis. To ascertain how effectively such cells would proliferate and differentiate in competition with unaltered BS cells, we measured the eyes of flies that carried DcY(H1) and an eyFLP transgene, which expresses FLP in the eye throughout development. Ten eyFLP lines were tested with DcY(H1): every combination produced eyes that, although rough and irregularly shaped, were significantly larger than BS (representative results shown in Figure 2). For further experiments we chose to use the P{eyFLP.N, ry+}2 line because the average eye size following telomere loss is ∼50% of wild type, permitting the identification of mutations that either limit or promote cell survival following telomere loss.
Fig. 2. The effect of telomere loss on cell survival in the eye using the BARTL assay. 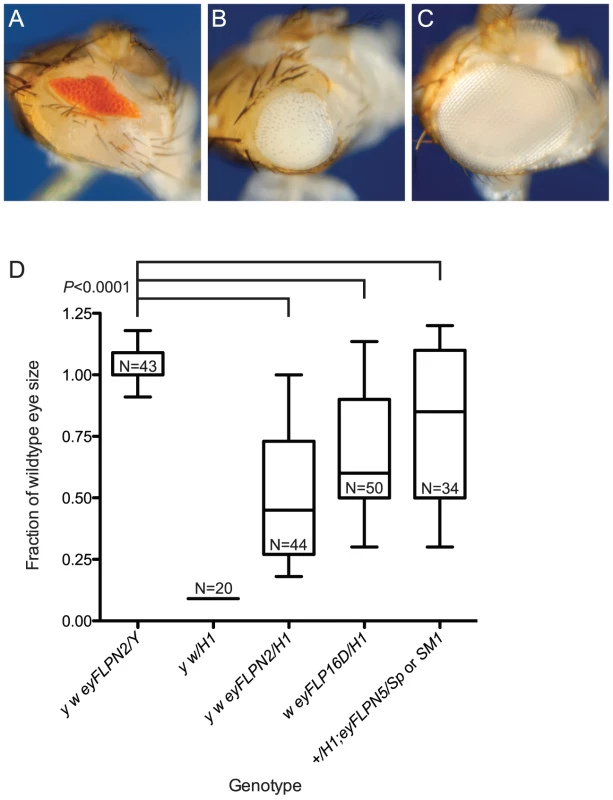
Representative eye phenotypes are pictured. (A) The BS phenotype of y w/DcY(H1) in the absence of FLP. (B) y w {eyFLP.N}2/DcY(H1) eyes that have experienced telomere and BS loss are larger than BS but smaller than wildtype eyes. (C) The y w {eyFLP.N}2/Y eyes used as controls are indistinguishable from wild type with respect to size and morphology. (D) The effect of three different eyFLPs combined with DcY(H1) are shown. Distribution of eye sizes are represented using box-and-whisker plots. Genotypes are indicated on the x-axis and eye size (area normalized to wildtype eye) is on the y-axis. The ends of the whiskers represent the 5th and 95th percentiles; the top and the bottom of the boxes represent the 25th and 75th percentiles of the range of eye sizes; the median (50th percentile) is represented by the horizontal line in the box. N is the number of eyes measured. Significance levels for critical comparisons are indicated. It is a formal possibility that the larger eye size seen following FLP induction results from loss of the entire Y chromosome, including BS. To test whether dicentric production precipitated chromosome loss we induced FLP expression by heat shock in flies carrying the heat-inducible 70FLP3F transgene and the DcY(H1) chromosome, then examined mitotic figures from larval neuroblasts 24 and 48 hours after FLP induction for loss of the Y chromosome. There was no increase in chromosome loss in flies that expressed FLP, compared to flies that did not (Table 1), confirming that the larger eye phenotypes seen in the BARTL assay were not the result of complete loss of the Y, but instead reflect extensive growth and differentiation of cells that lost a telomere.
Tab. 1. Assay for Y chromosome loss after dicentric induction. 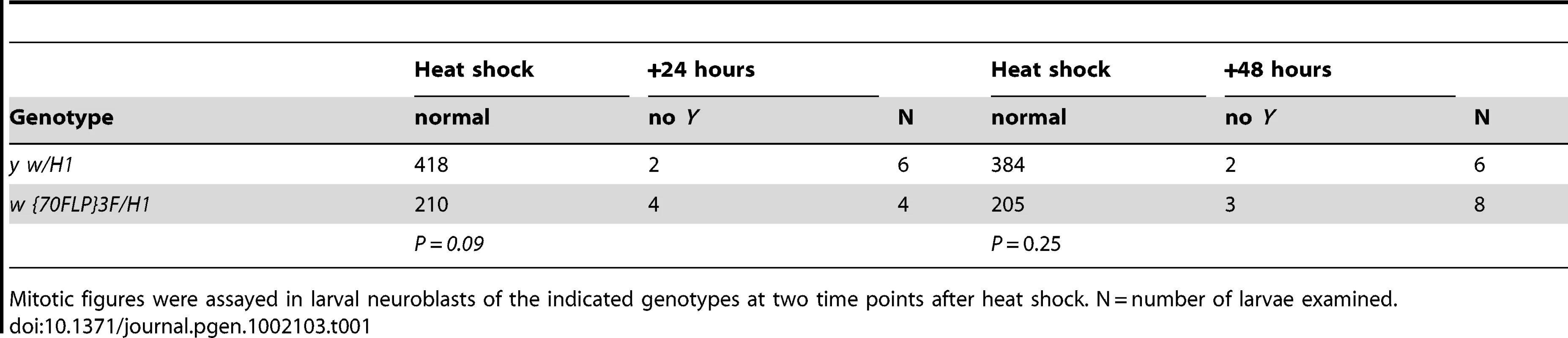
Mitotic figures were assayed in larval neuroblasts of the indicated genotypes at two time points after heat shock. N = number of larvae examined. Mutations in loki and p53 act semi-dominantly to increase cell survival after telomere loss
The DNA damage response, acting primarily through Chk2 and p53, mediates apoptosis in response to telomere loss [6]. To determine whether reducing or eliminating the apoptotic response would allow such cells to proliferate and differentiate we used the BARTL assay to examine the influence of mutations in these genes. The lokp6/+ heterozygous flies had eyes that were much larger than the lok+ control, and the lokp6/p6 homozygotes had eyes of nearly wildtype size and morphology (Figure 3A). When we tested two alleles of p53 (p535A-1-4 and p5311-1B-1) the heterozygotes had eyes that were also much larger than the p53+ control, with p53−/− homozygotes having eyes that were near wild type in size (Figure 4). As expected, the addition of a p53+ transgene reduced eye size significantly. We also tested a hemizygous deletion of 276 kb that removes p53, and found that it had a similar effect as the heterozygous p53 mutations. The lok and p53 mutations used in these studies had no effect on the BS phenotype in the absence of FLP expression and telomere loss (mean sizes ±1 SD, normalized to wildtype eye: y w/H1 = 0.090±2.8 e-17, N = 20; y w/H1; p535A-1-4 = 0.099±0.028, N = 10; y w/H1; lokp6 = 0.090±2.8 e-17, N = 24; P values are 0.6 and 0.9 respectively).
Fig. 3. The role of DNA damage checkpoint proteins in the elimination of cells that have lost a telomere. 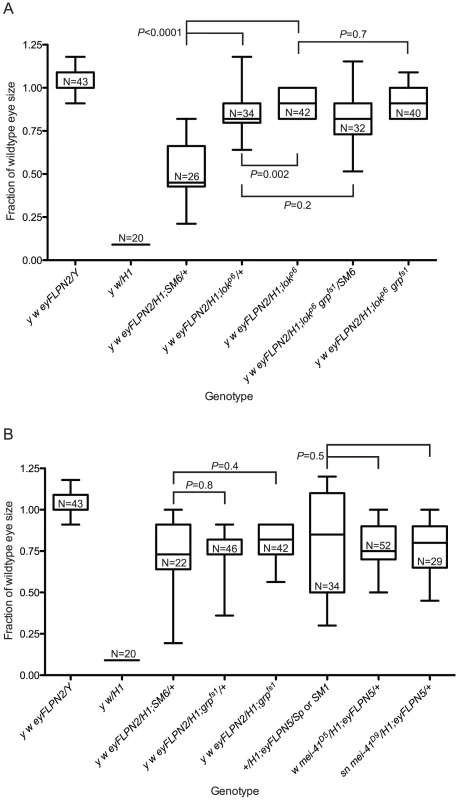
All sizes are presented after normalization to wild type. Significance levels for critical comparisons are indicated. The first two whisker plots in each graph are controls and are repeated here (from Figure 2) for ease of comparison. (A) The effects of lok mutations. (B) The effects of grp or mei-41 mutations. Fig. 4. The role of p53 in the elimination of cells that have lost a telomere. 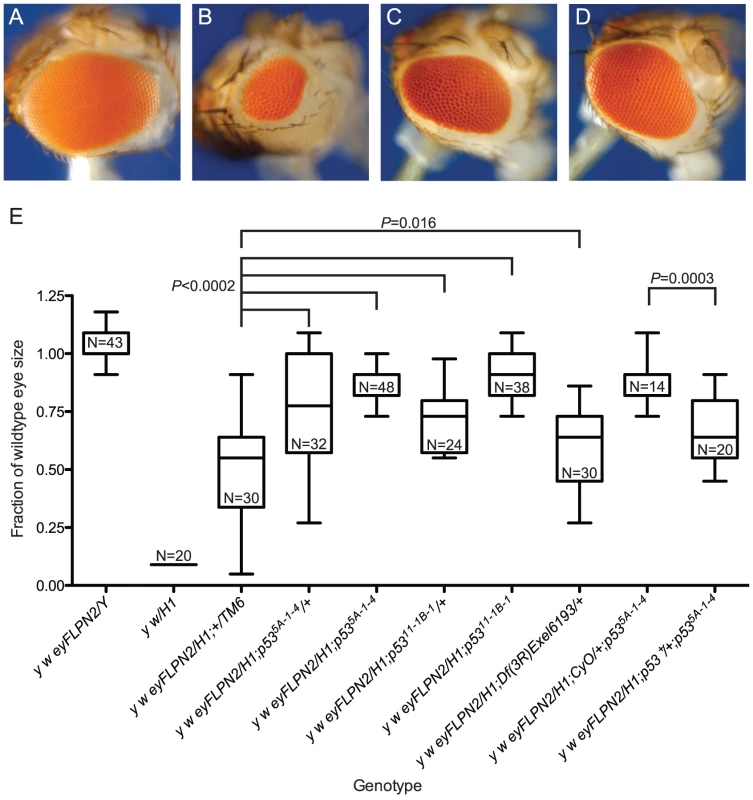
Representative eyes are pictured: (A) w {eyFLP}16D/Y; (B) w {eyFLP}16D/DcY(H1); (C) w {eyFLP}16D/H1; p535A-1-4/TM6, Ubx; (D) w {eyFLP}16D/DcY(H1); p535A-1-4. (E) BARTL results for various p53 mutant and wildtype combinations. The first two whisker plots are controls and are repeated here (from Figure 2) for ease of comparison. All sizes are presented after normalization to wild type. Significance levels for critical comparisons are indicated. To determine if our BARTL results were an anomaly of the extreme selection conferred by the context of surrounding BS cells, we induced telomere loss from an independently derived Y chromosome, DcY(FrTrYS)4B1A, that contains a P element with a white+ (whs) gene and inverted FRTs on the short arm of Y. Cells that lose whs must have experienced dicentric formation (and breakage), allowing positive identification of at least some of the cells that lose a telomere and survive to contribute to the adult eye. The eye shown in Figure 5B is typical of flies recovered in this experiment. The white sectors that predominate indicate that the majority of surviving cells have lost a telomere and the whs transgene.
Fig. 5. Cell survival after telomere loss from B+ Y or chromosome 3. 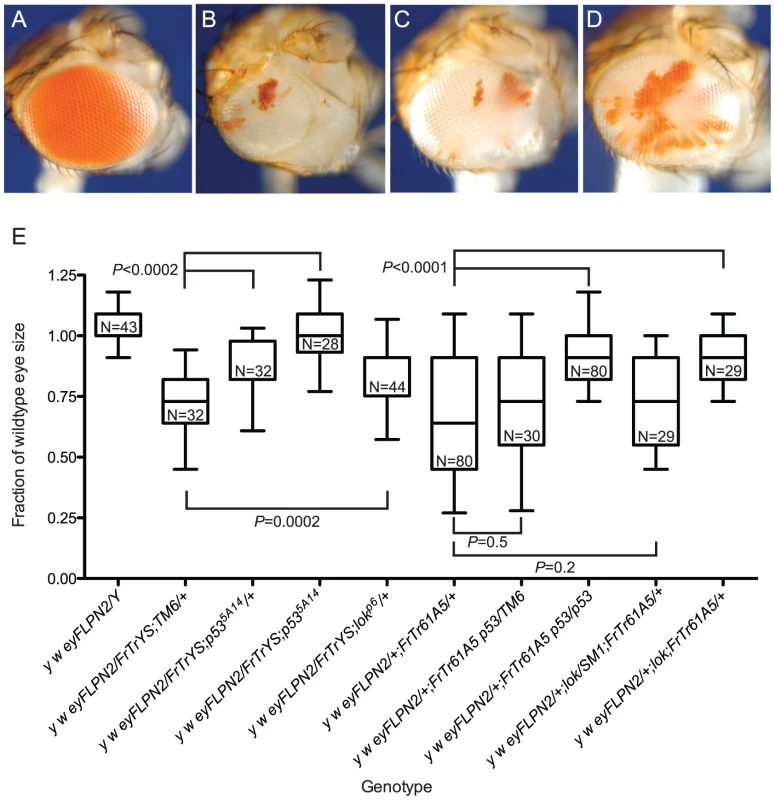
Representative eyes are pictured: (A) y w/DcY(FrTrYS)4B1A; (B) y w {eyFLP.N}2/DcY(FrTrYS)4B1A; (C) y w {eyFLP.N}2/DcY(FrTrYS)4B1A; p535A-1-4/+; (D) y w {eyFLP.N}2/FrTrY;p535A-1-4. White sectors in the eye positively mark cells that have lost a telomere. (E) Box-and-whisker plot showing DcY(FrTrYS)4B1A and Dc3(FrTr61A5) in p53 and lok mutant and wildtype backgrounds. The first whisker plot is a control and is repeated here (from Figure 2) for ease of comparison. All sizes are presented after normalization to wild type. Significance levels for critical comparisons are indicated. Since we do not know the orientation of the P element on this chromosome it is possible that some cells experiencing dicentric formation and breakage may retain whs. If, for instance, whs lies proximal to the FRTs, then the long chromosomes produced by asymmetrical breakage of a dicentric bridge will retain whs. However, since eyFLP expresses continuously through eye development and such cells retain inverted FRTs (Figure 1A) they may experience further rounds of dicentric formation, giving added opportunity to lose the whs gene. It is also possible that some cells may escape recombination entirely and retain whs. Notwithstanding this uncertainty about whether pigmented cells have lost a telomere (or not), our conclusion that most surviving cells experienced telomere loss remains valid since it is based on the predominant occurrence of cells that lack pigment.
In a wildtype background, flies with DcY(FrTrYS)4B1A and eyFLP had small and rough eyes that were on average 71% as large as normal eyes (Figure 5B and 5E). Although cells are capable of surviving telomere loss and contributing to the adult eye in the absence of BS selection, this observation that the eyes were smaller than wild type indicates that many of the cells that lost a telomere succumbed to apoptosis.
We then asked how lok and p53 mutants would alter the outcome in this context. We found that p53−/−, p53+/− and lok+/− had eyes that were significantly larger than the non-mutant controls (Figure 5C, 5D, 5E; lok−/− were not tested), demonstrating that the results from the BARTL assay are not an artifact imposed by the BS allele.
mei-41 and grp have no detectable effect on cell survival and differentiation after telomere loss
We previously showed that mutations of mei-41 and grp, the genes that encode the Drosophila orthologs of mammalian ATR and Chk1, have a detectable effect on reducing apoptosis after telomere loss only in a lok background [6]. Since the BARTL assay is sensitive enough to distinguish a heterozygous effect with p53 and lok, we also tested mei-41 and grp with this assay. BARTL flies homozygous for grp, or hemizygous for either of two alleles of mei-41, showed no significant change in eye size when compared to wildtype flies (Figure 3B). We further tested a role for grp by analyzing lok grp double mutants and found that the effect was not different from lok−/− single mutant flies (Figure 3A), confirming that these genes play a minor role in the elimination of cells that have lost a telomere.
Mutations in genes involved in telomere function
A number of genes that are essential for telomere protection have been identified. These include cav which encodes HOAP, Su(var)205 which encodes HP1a, tefu which codes for the ATM homolog, nbs, mre11, rad50 and hiphop [16]–[24]. The genes required for telomere protection are also required for cell viability, as loss of any one of these genes leads to global telomere dysfunction, multiple end-to-end fusions and ultimately to cell death. Use of the eyGAL4 transgene in conjunction with UAS-RNAi lines to knock down expression of cav, hiphop, or Su(var)205 in the eye, resulted in most flies dying as pharate adults with very small or no heads, indicating extensive cell death even in the absence of FLP-induced dicentric chromosome formation. The few that did survive had small rough eyes consistent with extensive cell death. Since RNAi-mediated knockdown of these genes strongly reduces cell viability, and homozygous mutants fail to develop past the early pupal stage, we were unable to assess their influence using the BARTL assay. However, we did test several genes as heterozygotes in the BARTL assay. We tested two components of the MRN complex, which consists of Mre11, Rad50 and Nbs, and is required for telomere protection and the DNA damage response [18], [24], [25]. BARTL flies heterozygous for nbs or rad50 mutations were not significantly different than controls (Figure 6). We also examined BARTL flies that were heterozygous for mutations of Su(var)205 or cav and saw no significant difference from controls (Figure 6).
Fig. 6. The role of genes required for telomere protection in cell survival after telomere loss. 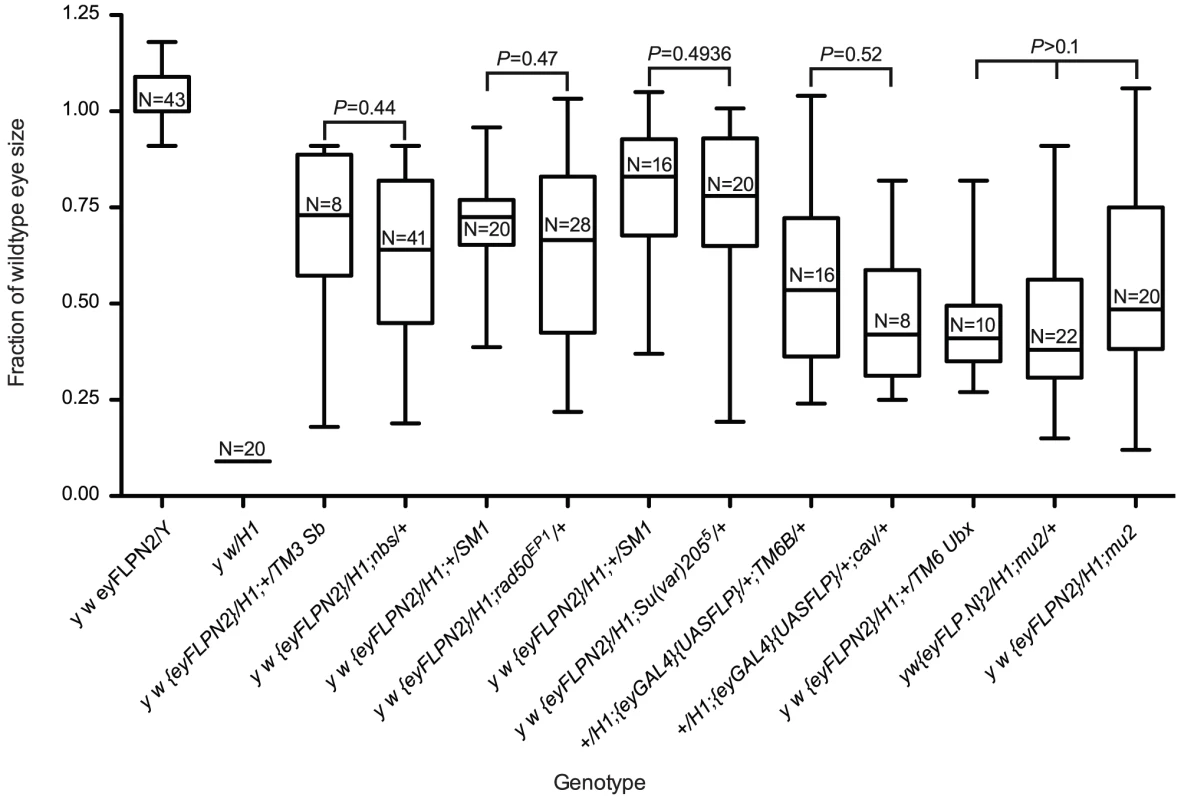
The first two whisker plots are controls and are repeated here (from Figure 2) for ease of comparison. All sizes are presented after normalization to wild type. Statistical comparisons were performed between genotypes produced from the same cross to minimize possible background effects (except a different cross was used to generate mu2 homozygotes). Significance levels for critical comparisons are indicated. Finally, we tested whether cell survival in the BARTL assay was affected by mu2 function. mu2 mutant females allow a high rate of recovery of broken-and-healed chromosomes through their germline, and examination of somatic cells suggests that Mu2 has a checkpoint function [26], [27]. If mu2 permits healing of broken chromosomes in the soma then we might expect to see increased survival of cells that have lost a telomere. We assayed two mu2 mutant genotypes: mu21/1 and an RNAi knock down construct, mu2P{GD12728}v28342, in the BARTL assay, and did not see a significant difference in eye size compared to controls (Figure 6 and data not shown). If de novo telomere addition does occur in the soma (of males at least), it does not appear to be controlled by mu2.
Autosomal telomere loss
We also investigated the effect of telomere loss on cell survival using an autosome instead of the Y. The Dc3(FrTr61A5)1A chromosome 3 has inverted FRTs inserted very near the tip of the left arm, with whs located just distal to these FRTs. Therefore whs will be located on the acentric chromosome produced by FLP-mediated recombination and is frequently lost after dicentric/acentric formation. Similar to the results with DcY(FrTr4B1A), flies carrying Dc3(FrTr61A5)1A and eyFLP had predominantly white eyes, indicating that the vast majority of cells that contribute to the eye have lost a telomere, and the eyes were rough and smaller than wild type indicating frequent cell death (Figure 5E). Also consistent with previous results, flies that were homozygous for mutations in p53 or lok had eyes that were significantly larger than p53+ or lok+ flies.
In contrast to results obtained with the BARTL assay or with DcY(FrTrYS)4B1A, eyes from p53+/− or lok+/− heterozygotes had eyes that were not significantly larger than p53+ or lok+ flies. We hypothesize that the semi-dominant effect of p53 and lok was not seen because in this case, where aneuploidy is produced, the p53-independent aneuploidy-triggered cell death pathway plays an additional role in elimination of many of the cells that have lost a telomere [6]–[8].
Survival of cells following telomere loss at different developmental time points
One limitation of the BARTL assay is that, because the eyeless promoter is used to drive FLP expression continuously, telomere loss may occur throughout development of the eye. We wished to assay the ability of cells that have lost a telomere to proliferate and differentiate for the full length of development, so we developed an assay, using Dc3(FrTr61A5)1A, that provided this capability. This system is similar to the often-used SMART (Somatic Mutation And Recombination Test [28]) assay, but since it is based on catalyzed telomere loss we call it SMARTL, for Somatic Multiplication After Recombinase-mediated Telomere Loss. Flies carry a normal chromosome 3 marked with the recessive multiple wing hairs (mwh) mutation, heterozygous with the Dc3(FrTr61A5)1A chromosome that carries mwh+ (Figure 1C). These flies also carry the heat-shock-inducible hsFLP1 transgene on the X so that FLP can be induced at any point during development by application of a heat pulse. When the chromosome 3 dicentric bridge that is produced breaks asymmetrically, such that the break point is proximal to the mwh+ allele, then one daughter cell will become hemizygous for mwh. Some of the cells that lose a telomere are then identifiable by their mwh phenotype, and are easily recognized in the adult wing. In our experiments we scored mwh clone number and clone size in at least 3 and up to 46 wings per time point, from flies heat-shocked at different times throughout development.
In wildtype flies that eclosed five days after heat shock (d.a.h.s), which corresponds to heat shock applied at approximately the time of pupariation, mwh cells in the wing were so frequent that it was not possible to distinguish separate clones. In wildtype flies collected six d.a.h.s, which translates to a pulse of FLP ∼24 hours before pupariation, the average number of mwh cells/wing was 6.11 (Figure 7A), suggesting that within 24 hours the majority of cells that lost a telomere were eliminated from the viable cell population, likely by apoptosis. However, the average number of mwh cells was still ∼11-fold greater than the number of mwh cells generated spontaneously. The number of mwh cells continued to be elevated in flies collected seven and eight d.a.h.s. (heat-shocked ∼48 and ∼72 hours before pupariation, respectively), at ∼2–4 times the spontaneous level. Taken together these data indicate that although most cells that experienced telomere loss were eliminated within 3–4 days of induction of telomere loss (8–9 d.a.h.s), some of these cells do survive for this period, and are capable of differentiation. Heat shocks given at even earlier developmental stages did not produce an increase in mwh cells compared to the no heat shock control, indicating that nearly all cells that lose a telomere are eliminated after 4–5 days of normal growth in a wildtype background.
Fig. 7. Cell survival after telomere loss at different time points in development. 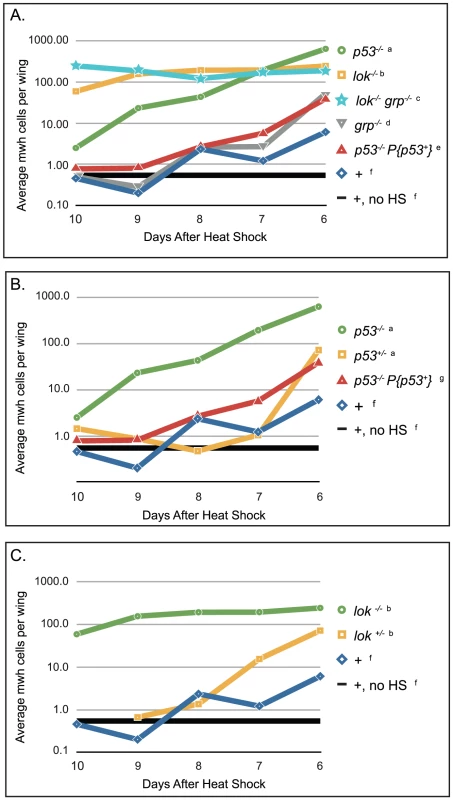
Wings were scored based on the number of days after heat shock that the flies eclosed (x-axis). The number and size of all mwh clones were scored and the product of those numbers is reported here, averaged over the number of wings scored (y-axis). Flies that were collected 6 days after heat shock experienced telomere loss late in development, as approximately third instar larvae, and flies that were collected 10 days after heat shock experience telomere loss early, as approximately embryos or hatching larvae. Genotypes of female progeny are listed, males were also counted. (A) Survival of mwh cells in wild type and homozygous mutant flies; (B) lok+/− heterozygous effect, and (C) p53+/− heterozygous effect. a. y w; Dc3(2xRS61A5)1A p535A-1-4 males X w1118 hsFLP1; mwh p535A-1-4 females, progeny scored were y w/w1118 hsFLP1; Dc3(2xRS61A5)1A p535A-1-4/mwh p535A-1-4. b. y w; lokp6/p6; Dc3(2xRS61A5)1A males X w1118 hsFLP1;lok+/p6; mwh females, progeny scored were y w/w1118 hsFLP1; lokp6/p6; Dc3(2xRS61A5)1A/mwh and y w/w1118 hsFLP1; lok+/p6; Dc3(2xRS61A5)1A/mwh, respectively. c. y w; lokp6/p6 grpfs1/fs1; Dc3(2xRS61A5)1A males X w1118 hsFLP1; lok+ grp+/lokp6 grpfs1; mwh females, progeny scored were y w/w1118 hsFLP1; lokp6/p6 grpfs1/fs1; Dc3(2xRS61A5)1A/mwh. d. y w; grpfs1/fs1; Dc3(2xRS61A5)1A males X w1118 hsFLP1; grp+/grpfs1; mwh females, progeny scored were y w/w1118 hsFLP1; grpfs1/fs1; Dc3(2xRS61A5)1A/mwh. e. y w; P{p53+, v+}3A; Dc3(2xRS61A5)1A p535A-1-4 males X w1118 hsFLP1; mwh p535A-1-4 females, progeny scored were y w/w1118 hsFLP1; P{p53+, v+}3A/+; Dc3(2xRS61A5)1A p535A-1-4/mwh p535A-1-4. f. y w; Dc3(2xRS61A5)1A males X w1118 hsFLP1; mwh females, progeny scored were y w/w1118 hsFLP1; Dc3(2xRS61A5)1A/mwh. g. y w; Dc3(2xRS61A5)1A males X w1118 hsFLP1; mwh p535A-1-4 females, progeny scored were y w/w1118 hsFLP1; Dc3(2xRS61A5)1A p53+/mwh p535A-1-4. Mutation of p53 or lok greatly improved the survival of cells that had lost a telomere at all time points tested (Figure 7A; all statisitical results shown in Table S1). However, the effects of these two mutations were significantly different from each other. When telomere loss was induced early in development (flies eclosing 8–10 d.a.h.s), cells that lost a telomere survived much better in lok−/− mutants (∼130× wild type) than in p53−/− mutants (∼5× wild type). When telomere loss was induced later in development the survival of cells that lost a telomere improved in p53−/−, but stayed about the same in lok−/−, so that with flies eclosing seven d.a.h.s, survival of such cells was similar in both genotypes (∼160× wild type), and with flies that eclosed six d.a.h.s survival was better in the p53−/− flies (∼100× wild type) than in lok−/− (∼40× wild type). The large number of mwh cells produced at early developmental stages in lok flies clearly depends on telomere loss, since without heat shock to induce FLP we observed only an average of 3.5 mwh cells per wing in 34 wings.
Both lok and p53 mutants exhibited haplo-insufficiency in these experiments (Figure 7B and 7C), as they did in the BARTL assay, but it was observed only in flies that differentiated within a day or two of the hsFLP induction. With early heat shocks, the heterozygotes were able to eliminate cells that lost a telomere as well as wild type. For p53, the addition of a wildtype transgene to the homozygous mutant produced results similar to the heterozygous mutant (Figure 7C).
As was the case in the BARTL assay, loss of Chk1 (grp−/−) did not produce a significant effect (Table S1). We also tested lokp6 grpfs1 double mutants and they were not consistently different from lokp6 single mutants (Figure 7A).
Discussion
One of the major roles of the complex of nucleic acids and proteins that form a telomere is to hide the chromosome terminus from machinery that mediates the DNA damage response [29], [30]. This response typically leads to the activation of p53, predominantly through phosphorylation by ATM and Chk2, major transducers of the DNA damage response [31], [32]. p53 is known to have a number of transcriptional targets, both in mammals and in flies [33]–[36]. The outcome of p53 activation ranges from cell cycle arrest and DNA repair to apoptosis, depending on the type and quantity of DNA lesions and the cellular context [37]–[41]. We previously showed that the primary cellular response to telomere loss in flies is rapid activation of apoptosis [6]. Nevertheless, by examining neuroblast karyotypes in otherwise wildtype flies we found that ∼20% of cells that lose a telomere survive and proliferate for at least 96 hours, even when they accumulate significant aneuploidy [6].
In the work reported here we investigated the capacity of cells that lose a telomere to survive through most of the life cycle of the developing fruit fly and differentiate into adult structures. The BARTL assay, based on the simultaneous loss of a telomere and the dominant BS mutation in the eye, is particularly useful because the phenotype can be readily scored in a semi-quantitative fashion, facilitating a rapid genetic screen. In this assay cells that have lost a telomere are conferred a selective advantage because the neighboring cells they must compete with are crippled by BS. Even so, the alternative tests we employed which do not confer a selective advantage, such as the SMARTL assay, confirmed the BARTL results.
We found that elimination of the apoptotic DNA damage response, either through mutation of lok, the gene encoding Chk2, or mutation of p53, greatly increased the ability of cells that had lost a telomere to proliferate and differentiate into adult tissues. It is striking that both lok and p53 mutants act semi-dominantly; in other words, the genes are haplo-insufficient for normal elimination of cells that have lost a telomere. This is highly reminiscent of Li-Fraumeni syndrome in humans, a cancer prone disorder that results from mutations in p53 [42], [43]. Human Chek2 mutants also confer a similar predisposition to tumors in multiple tissues [32], [42], and both p53 and Chek2 mutants are inherited as autosomal dominant diseases. Although the majority of p53 mutations that result in Li-Fraumeni syndrome are probably mis-sense, null alleles are also known. Moreover, many tumors arising in a mouse model of Li-Fraumeni do not show loss of heterozygosity for p53 [44]–[46], indicating that in mammals it is also likely that p53+ is haplo-insufficient. This high degree of conservation of function certainly makes Drosophila an appealing model for examining the role of p53 and other genes in the response to telomere loss.
In the SMARTL assay haplo-insufficiency of p53 and lok was only seen when telomere loss was induced during the last one to two days of larval development. When telomere loss was induced at earlier stages, in heterozygotes the mechanisms for eliminating such cells worked sufficiently so that there was no significant difference from wild type. Since most cancer cells show evidence of a period of early genomic instability that appears to stem from telomere dysfunction [47], a short lapse in the normal elimination of such cells may be sufficient to allow genomic reshuffling that can facilitate carcinogenesis, partially accounting for the increased incidence of cancer in humans that are heterozygous for p53 and Chek2 mutant alleles.
The results of the SMARTL experiment also indicate the existence of a Chk2-controlled pathway that functions independently of p53. When telomere loss was induced during the first few days of development, the lok−/− mutants allowed cells that had lost a telomere to proliferate and differentiate to a much greater extent than p53−/− mutants. This was not due to maternal deficiency of Chk2 because the female parents were lok+/− heterozygotes. Furthermore, the capability of p53−/− flies to eliminate cells that have lost a telomere early in development was not a result of maternal supply of p53 product, since the mothers in this experiment were homozygous for a p53 null allele. We previously showed that a p53-independent cell death pathway is triggered by aneuploidy [6]. Because the aneuploidy-triggered pathway is also independent of Chk2 [6], [8], that pathway cannot account for the difference. We conclude that loss of Chk2 in lok−/− mutants abrogates the apoptotic response that acts through p53 and also abolishes a second pathway to eliminate cells that have lost a telomere.
Chk2 has been shown to mediate several p53-independent processes [48], [49], including pathways that lead to cell cycle arrest [33], [50], [51] and apoptosis [52]. Because clones of cells that lost a telomere were both larger and more numerous in lok−/− mutants than in wild type or p53−/− (2), it appears that Chk2 may direct both cell cycle delay/arrest and apoptosis in response to telomere loss in addition to its role in activating p53. We propose that Chk2-mediated cell cycle delay or arrest could mimic the phenotype of a slow-growing Minute cell, and thus induce the Jnk pathway and apoptotic caspase cascade that eliminates poorly growing cells [7], [53] providing a route to cell death that does not require p53 (Figure 7).
One question that arises is why are cells that have lost a telomere in the SMARTL assay not eliminated by the aneuploidy-triggered pathway? It is likely that some cells are eliminated by this pathway. However, if aneuploidy-triggered death is a specific response to loss of Minute genes [7], then since mwh+ lies distal to all Minute genes [54] it is possible for dicentric breakage to produce a mwh cell that is not Minute. Such cells need not be subject to the aneuploidy-triggered death pathway and could proliferate extensively unless eliminated by other mechanisms.
Our results might be partially explained by an alternative hypothesis: that loss of Chk2 allows chromosomes that have lost a telomere access to a repair pathway which uses the homolog to restore the end of the chromosome. Break-induced replication is one such mechanism, and is similar to the ALT mechanism used for telomere maintenance in ∼15% of human cancers [55], [56]. Exchange with a normal chromosome is another possibility, analogous to telomere-sister chromatid exchange, except in this case the homolog would be used [57]. Although such mechanisms could operate in the SMARTL assay, where a genuine homolog is present, it is more difficult to imagine their employment in the experiments of the BARTL assay, where the flies carry no chromosome that is a DNA sequence homolog of the Y. So, even though it may occur, this is not likely to be the only mechanism by which cells can escape the apoptotic pathway.
The telomere-loss induced p53-dependent apoptotic response is also activated through a secondary pathway, albeit it to a much lesser extent, via the mei41 and grp gene products Atr and Chk1 [6]. However, we detected no significant effects of these mutants using either the BARTL or SMARTL assays. Taken together these data confirm that the contribution of this pathway to the elimination of cells that have lost a telomere is small.
The picture that emerges is of a complex multi-pronged response to telomere loss (Figure 8). Two pathways recognize damaged ends and invoke the DNA damage response to produce p53-mediated apoptosis. A third pathway is activated in the context of substantial aneuploidy. The p53-mediated and aneuploidy-mediated pathways converge in the activation of caspases. The Chk2 branch of the response to telomere loss bifurcates, leading to activation of the p53 apoptotic response and an alternative pathway that can also eliminate cells that have lost a telomere.
Fig. 8. A model of pathways for eliminating cells that have lost a telomere. 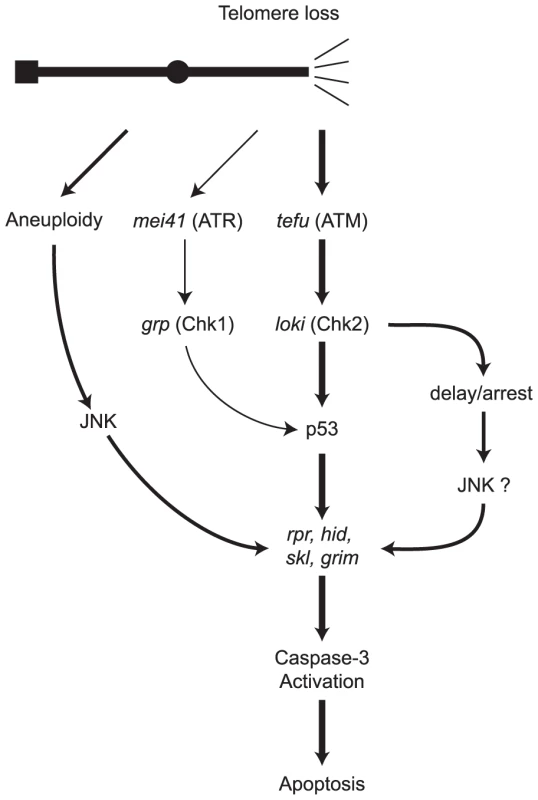
Following recognition of the missing telomere, ATM and ATR phosphorylate checkpoint kinases Chk2 and Chk1. Caspase-dependent cell death is mediated by signaling through p53, or alternatively, via the aneuploidy-triggered pathway. A second p53-independent cell death pathway is mediated through Chk2. Portions of this model are based on identified DNA damage response pathways in Drosophila and the role of ATM in mammalian cells [6]–[8], [31], [33], [34], [50], [68]–[72]. In our view, the most pressing issue is to fully identify and understand the mechanisms that allow a cell to escape the apoptotic responses to telomere loss, and then continue to divide. Such division may amplify and spread the existing genomic damage and, in humans, place cells in peril of becoming cancerous [3], [6], [47]. One possibility is that detection and response to this type of DNA damage is not 100% efficient. Certainly, our observation that p53 and lok are haplo-insufficient is consistent with the interpretation that the system is not overly sensitive to DNA damage. Cells could also escape telomere-loss-induced apoptosis by a process termed adaptation [58]–[60]. When yeast cells adapt to persistent DNA damage, the normal checkpoint is attenuated, but the DNA damage remains. The resumption of cell division leads to chromosome instability and chromosome loss [60], [61]. Cell division in the presence of DNA damage has been taken as de facto evidence of adaptation in mammalian cells [62]. By this definition, adaptation does occur in Drosophila. We previously showed that some cells could undergo several rounds of division after telomere loss. In these cells there was evidence of further chromosome rearrangement, indicating that they had unrepaired damage [6]. Thus, one may conclude that adaptation had occurred. Finally, the broken chromosome might become healed by construction of a new telomere on the broken end. Such a mechanism clearly exists for chromosomes that have lost a telomere in the germline [9]–[11]. Whether this process also occurs in the soma is still an unanswered question. However our experiments showed no effect of the mu2 mutant, which allows healing to occur on chromosomes broken in the female germline. If de novo telomere addition does occur in the soma, it is not controlled by mu2.
To fully understand the complex responses to telomere loss, it will be necessary to identify downstream mediators of the response and link them with specific upstream activators. The combination of powerful genetic and cytological tools in concert with multicellular development makes Drosophila an ideal system to examine the genetic regulation of the responses to telomere loss. The BARTL assay provides a facile method to screen for genes that are involved in this response. In conjunction with the SMARTL assay, the examination of cellular apoptosis, and the observation of karyotypes of surviving cells it should be possible to thoroughly characterize the roles of genes in long-term cell survival following telomere loss. The examination of germline effects, where chromosome healing is readily assayed, should help to distinguish the roles of such genes.
Materials and Methods
Fly stocks and immunohistochemistry
All flies were maintained and mated at 25°C on standard cornmeal food. Heat shocks were carried out in a circulating water bath at 38°C for 1 hour. The fly lines yd2 w1118 P{ey-FLPN}2, yd2 w1118; P{ey-FLPN}5, w P{ey4x-FLP.Exel}1, sn3 mei-41D9, mei-41D5, Df(3R)Exel6193, nbs, P{EP}rad50EP1, mu2, Su(var)2055, P{UAS-FLP1.D}JD1, and {GAL4-ey.H}4-8 were obtained from the Bloomington stock center. Several eyFLP lines, including eyFLP16D, were generated by mobilizing a P insertion, P{ey4x-FLP.Exel}1, located on the X and selecting lines with multiple insertions, by screening for stronger w+ expression, in order to generate lines with stronger eyFLP expression. The lokp6 stock was obtained from William Theurkauf; the grpfs1 lokp6 double mutant was obtained from Michael Brodsky; the grpfs1 mutant was obtained from William Sullivan; the cav mutant stock was obtained from Maurizio Gatti. Construction of the p53+ transgene was described previously [6]. Apoptosis in BS eye discs was visualized using the cleaved caspase-3 antibody (protocol adapted from [63]) from Cell Signaling Technologies (cat. no. 9661) and Alexa-Fluor 568 from Invitrogen (cat. no. A11036).
Dicentric-inducible chromosome construction
The DcY(H1) chromosome
The P element P{iw} carrying inverted FRTs [64] was transposed from an X, y w P{iw, whs}6, to a BsYy+ chromosome in males also carrying the Sb P{ry+t7.2 Δ2-3}99B transposase source [65]. From this cross, 720 individual Sb sons were collected and crossed to w1118 females; 121 produced at least one white+ Stubble+ Bar yellow+ male. We then tested for linkage of whs Y. Three independent whs Bs Y y+ chromosome derivatives were recovered from above screen, and are designated DcY(H1), DcY(H2) and DcY(H3).
The Dc3(2xRS61A5)1A chromosome
The CB-0127-03 fly line, which carries a P{RS3, whs} element inserted a 61A5, was acquired from the Drosdel collection [66]. Males carrying this insertion, which have a orange eye phenotype, and the Sb P{ry+t7.2 Δ2-3}99B transposase source were outcrossed to y1 w1118 virgins and the progeny were scored for darker eye pigmentation indicative two copies of whs resulting from duplication of the P{RS3, whs} element as a result of local transposition. Of the 47 independent two copy w+ lines 7 showed dicentric inducible phenotypes, i.e. rough eyes and notched wings [13], when crossed to a heat shock inducible FLP transgene, {70FLP, ry+}3F or P{ey4x-FLP.Exel}1 [9]. Two of the seven showed a median level of mwh clone generation sufficient for use in the SMARTL assay.
The Dc3(FrTr61A5)1A and DcY(FrTrYS)4B1A chromosomes were both made by crossing males carrying a whs and inverted FRT-bearing P-element, P{FrTr}, inserted on chromosome 2 to females carrying the transposase source. For DcY(FrTrYS)4B1A, male progeny were outcrossed to y w females and progeny were screened for w+ linkage to chromosomes other then 2. The insertion site on YS was deduced from DAPI-stained neuroblast analysis, where dicentric and acentric fragments were visualized two hours after FLP induction. For Dc3(FrTr61A5)1A we exchanged the P{FrTr, whs} on chromosome 2 with a remnant of the CB-0127-03 P-element at 61A5; for a full description see [9].
BARTL assay
Crosses were carried out using yd2 w1118 P{ey-FLPN}2 and DcY(H1) in mutant or wildtype backgrounds. P{ey-FLPN}5, which is located on chromosome 2, was used to evaluate the effect of multiple mei-41 alleles with DcY(H1). BARTL results were secondarily confirmed using multiple different insertions of P{ey4x-FLP.Exel}1 or the combination of P{UAS-FLP1.D}JD1 and P{GAL4-ey.H}4-8, as a FLP source.
To determine eye size, each eye was measured along the anterior-posterior axis and the dorsal-ventral axis using a digital filar micrometer and the two measurements were used to calculate the area of an ellipse. Statistical analysis used the Mann-Whitney test with Instat software for Macintosh. The area of the eyes was divided by the mean of P{eyFLPN}2/Y controls and size is presented as a fraction of wildtype size. Whisker plots were generated using Prism software for Macintosh. Eyes were photographed using a Nikon D200 digital camera and processed in Adobe Photoshop.
Mitotic cytology
Neuroblast figures were generated as described [67], stained with DAPI and visualized with a Zeiss Axioplan. A single brain was mounted per slide. Karyotypes were scored by scanning the entire brain and scoring every metaphase nucleus for the presence or absence of the Y chromosome.
Somatic telomere loss and recombination test
For the SMARTL assay flies were crossed and allowed to lay eggs for 5 days. The adults were then transferred to a new vial and the larvae were heat shocked for 1 hour at 38°C. Flies were immediately placed back at 25°C after heat shock and flies were collected every 24 hours for 10 days after heat shock. Wings were mounted on slides in isopropanol and mounting media, Cytoseal 60 Richard-Allan Scientific. Wing hairs were counted using a Zeiss Axioskop.
Supporting Information
Zdroje
1. MullerHJ 1940 An analysis of the process of structural change in chromosomes of Drosophila. J Genet 40 1 66
2. McClintockB 1939 The Behavior in Successive Nuclear Divisions of a Chromosome Broken at Meiosis. Proc Natl Acad Sci USA 25 405 416
3. ArtandiSEDePinhoRA 2010 Telomeres and telomerase in cancer. Carcinogenesis 31 9 18
4. HensonJDReddelRR 2010 Assaying and investigating Alternative Lengthening of Telomeres activity in human cells and cancers. FEBS Lett 584 3800 3811
5. ShayJWWrightWE 2010 Telomeres and telomerase in normal and cancer stem cells. FEBS Lett 584 3819 3825
6. TitenSWGolicKG 2008 Telomere loss provokes multiple pathways to apoptosis and produces genomic instability in Drosophila melanogaster. Genetics 180 1821 1832
7. McNameeLMBrodskyMH 2009 p53-independent apoptosis limits DNA damage-induced aneuploidy. Genetics 182 423 435
8. WichmannAJaklevicBSuTT 2006 Ionizing radiation induces caspase-dependent but Chk2 - and p53-independent cell death in Drosophila melanogaster. Proc Natl Acad Sci U S A 103 9952 9957
9. TitenSWGolicKG 2010 Healing of euchromatic chromosome breaks by efficient de novo telomere addition in Drosophila melanogaster. Genetics 184 309 312
10. AhmadKGolicKG 1998 The transmission of fragmented chromosomes in Drosophila melanogaster. Genetics 148 775 792
11. MasonJMChampionLEHookG 1997 Germ-line effects of a mutator, mu2, in Drosophila melanogaster. Genetics 146 1381 1397
12. AhmadKGolicKG 1999 Telomere loss in somatic cells of Drosophila causes cell cycle arrest and apoptosis. Genetics 151 1041 1051
13. GolicKG 1994 Local transposition of P elements in Drosophila melanogaster and recombination between duplicated elements using a site-specific recombinase. Genetics 137 551 563
14. MichinomaeMKajiS 1973 Cell death during the development of the Bar eye discs in Drosophila melanogaster. Japan J Genetics 48 307 310
15. FristromD 1969 Cellular degeneration in the production of some mutant phenotypes in Drosophila melanogaster. Mol Gen Genet 103 363 379
16. BiXWeiSCRongYS 2004 Telomere protection without a telomerase; the role of ATM and Mre11 in Drosophila telomere maintenance. Curr Biol 14 1348 1353
17. CenciGSiriacoGRaffaGDKellumRGattiM 2003 The Drosophila HOAP protein is required for telomere capping. Nature cell biology 5 82 84
18. CiapponiLCenciGGattiM 2006 The Drosophila Nbs protein functions in multiple pathways for the maintenance of genome stability. Genetics 173 1447 1454
19. FantiLGiovinazzoGBerlocoMPimpinelliS 1998 The heterochromatin protein 1 prevents telomere fusions in Drosophila. Molecular cell 2 527 538
20. GaoGWalserJCBeaucherMLMorcianoPWesolowskaN 2010 HipHop interacts with HOAP and HP1 to protect Drosophila telomeres in a sequence-independent manner. The EMBO journal 29 819 829
21. OikemusSRMcGinnisNQueiroz-MachadoJTukachinskyHTakadaS 2004 Drosophila atm/telomere fusion is required for telomeric localization of HP1 and telomere position effect. Genes Dev 18 1850 1861
22. SilvaETiongSPedersenMHomolaERoyouA 2004 ATM is required for telomere maintenance and chromosome stability during Drosophila development. Curr Biol 14 1341 1347
23. SongYHMireyGBetsonMHaberDASettlemanJ 2004 The Drosophila ATM ortholog, dATM, mediates the response to ionizing radiation and to spontaneous DNA damage during development. Curr Biol 14 1354 1359
24. CiapponiLCenciGDucauJFloresCJohnson-SchlitzD 2004 The Drosophila Mre11/Rad50 complex is required to prevent both telomeric fusion and chromosome breakage. Curr Biol 14 1360 1366
25. GorskiMMRomeijnRJEekenJCde JongAWvan VeenBL 2004 Disruption of Drosophila Rad50 causes pupal lethality, the accumulation of DNA double-strand breaks and the induction of apoptosis in third instar larvae. DNA Repair (Amst) 3 603 615
26. DronamrajuRMasonJM 2009 Recognition of double strand breaks by a mutator protein (MU2) in Drosophila melanogaster. PLoS Genet 5 e1000473 doi:10.1371/journal.pgen.1000473
27. MasonJMStrobelEGreenMM 1984 mu-2: mutator gene in Drosophila that potentiates the induction of terminal deficiencies. Proc Natl Acad Sci USA 81 6090 6094
28. GrafUWurglerFEKatzAJFreiHJuonH 1984 Somatic mutation and recombination test in Drosophila melanogaster. Environ Mutagen 6 153 188
29. PalmWde LangeT 2008 How shelterin protects mammalian telomeres. Annu Rev Genet 42 301 334
30. ChanSSChangS 2010 Defending the end zone: studying the players involved in protecting chromosome ends. FEBS Lett 584 3773 3778
31. KarlsederJBroccoliDDaiYHardySde LangeT 1999 p53 - and ATM-dependent apoptosis induced by telomeres lacking TRF2. Science 283 1321 1325
32. AntoniLSodhaNCollinsIGarrettMD 2007 CHK2 kinase: cancer susceptibility and cancer therapy - two sides of the same coin? Nat Rev Cancer 7 925 936
33. BrodskyMHWeinertBTTsangGRongYSMcGinnisNM 2004 Drosophila melanogaster MNK/Chk2 and p53 regulate multiple DNA repair and apoptotic pathways following DNA damage. Mol Cell Biol 24 1219 1231
34. AkdemirFChristichASogameNChapoJAbramsJM 2007 p53 directs focused genomic responses in Drosophila. Oncogene 26 5184 5193
35. HohJJinSParradoTEdingtonJLevineAJ 2002 The p53MH algorithm and its application in detecting p53-responsive genes. Proc Natl Acad Sci U S A 99 8467 8472
36. LevineAJ 1997 p53, the cellular gatekeeper for growth and division. Cell 88 323 331
37. ShilohY 2003 ATM and related protein kinases: safeguarding genome integrity. Nat Rev Cancer 3 155 168
38. VousdenKHPrivesC 2009 Blinded by the Light: The Growing Complexity of p53. Cell 137 413 431
39. Kenzelmann BrozDAttardiLD 2010 In vivo analysis of p53 tumor suppressor function using genetically engineered mouse models. Carcinogenesis 31 1311 1318
40. WellsBSYoshidaEJohnstonLA 2006 Compensatory proliferation in Drosophila imaginal discs requires Dronc-dependent p53 activity. Curr Biol 16 1606 1615
41. ShrivastavMDe HaroLPNickoloffJA 2008 Regulation of DNA double-strand break repair pathway choice. Cell Res 18 134 147
42. VarleyJM 2003 Germline TP53 mutations and Li-Fraumeni syndrome. Hum Mutat 21 313 320
43. IwakumaTLozanoGFloresER 2005 Li-Fraumeni syndrome: a p53 family affair. Cell Cycle 4 865 867
44. DonehowerLA 2009 Using mice to examine p53 functions in cancer, aging, and longevity. Cold Spring Harb Perspect Biol 1 a001081
45. VenkatachalamSShiYPJonesSNVogelHBradleyA 1998 Retention of wild-type p53 in tumors from p53 heterozygous mice: reduction of p53 dosage can promote cancer formation. EMBO J 17 4657 4667
46. DonehowerLA 1996 The p53-deficient mouse: a model for basic and applied cancer studies. Semin Cancer Biol 7 269 278
47. ArtandiSEDePinhoRA 2000 A critical role for telomeres in suppressing and facilitating carcinogenesis. Curr Opin Genet Dev 10 39 46
48. AbduUBrodskyMSchupbachT 2002 Activation of a meiotic checkpoint during Drosophila oogenesis regulates the translation of Gurken through Chk2/Mnk. Curr Biol 12 1645 1651
49. TakadaSKelkarATheurkaufWE 2003 Drosophila checkpoint kinase 2 couples centrosome function and spindle assembly to genomic integrity. Cell 113 87 99
50. XuJXinSDuW 2001 Drosophila Chk2 is required for DNA damage-mediated cell cycle arrest and apoptosis. FEBS Lett 508 394 398
51. MasrouhaNYangLHijalSLarochelleSSuterB 2003 The Drosophila chk2 gene loki is essential for embryonic DNA double-strand-break checkpoints induced in S phase or G2. Genetics 163 973 982
52. BakhratAPritchettTPeretzGMcCallKAbduU 2010 Drosophila Chk2 and p53 proteins induce stage-specific cell death independently during oogenesis. Apoptosis 15 1425 1434
53. MorenoE 2008 Is cell competition relevant to cancer? Nat Rev Cancer 8 141 147
54. MarygoldSJRooteJReuterGLambertssonAAshburnerM 2007 The ribosomal protein genes and Minute loci of Drosophila melanogaster. Genome Biol 8 R216
55. McEachernMJHaberJE 2006 Break-induced replication and recombinational telomere elongation in yeast. Annu Rev Biochem 75 111 135
56. HensonJDNeumannAAYeagerTRReddelRR 2002 Alternative lengthening of telomeres in mammalian cells. Oncogene 21 598 610
57. HagelstromRTBlagoevKBNiedernhoferLJGoodwinEHBaileySM 2010 Hyper telomere recombination accelerates replicative senescence and may promote premature aging. Proc Natl Acad Sci U S A 107 15768 15773
58. BartekJLukasJ 2007 DNA damage checkpoints: from initiation to recovery or adaptation. Curr Biol 19 238 245
59. ClemensonCMarsolier-KergoatMC 2009 DNA damage checkpoint inactivation: adaptation and recovery. DNA repair 8 1101 1109
60. SandellLLZakianVA 1993 Loss of a yeast telomere: arrest, recovery, and chromosome loss. Cell 75 729 739
61. GalgoczyDJToczyskiDP 2001 Checkpoint adaptation precedes spontaneous and damage-induced genomic instability in yeast. Mol Cell Biol 21 1710 1718
62. SyljuasenRGJensenSBartekJLukasJ 2006 Adaptation to the ionizing radiation-induced G2 checkpoint occurs in human cells and depends on checkpoint kinase 1 and Polo-like kinase 1 kinases. Cancer Res 66 10253 10257
63. LaurenconAPurdyASekelskyJHawleyRSSuTT 2003 Phenotypic analysis of separation-of-function alleles of MEI-41, Drosophila ATM/ATR. Genetics 164 589 601
64. AhmadKGolicKG 1996 Somatic reversion of chromosomal position effects in Drosophila melanogaster. Genetics 144 657 670
65. RobertsonHMPrestonCRPhillisRWJohnson-SchlitzDMBenzWK 1988 A stable genomic source of P element transposase in Drosophila melanogaster. Genetics 118 461 470
66. RyderEBlowsFAshburnerMBautista-LlacerRCoulsonD 2004 The DrosDel collection: a set of P-element insertions for generating custom chromosomal aberrations in Drosophila melanogaster. Genetics 167 797 813
67. GattiMBonaccorsiSPimpinelliS 1994 Looking at Drosophila mitotic chromosomes. Methods Cell Biol 44 371 391
68. SongYHMireyGBetsonMHaberDASettlemanJ 2004 The Drosophila ATM ortholog, dATM, mediates the response to ionizing radiation and to spontaneous DNA damage during development. Curr Biol 14 1354 1359
69. OllmannMYoungLMDi ComoCJKarimFBelvinM 2000 Drosophila p53 is a structural and functional homolog of the tumor suppressor p53. Cell 101 91 101
70. BrodskyMHNordstromWTsangGKwanERubinGM 2000 Drosophila p53 binds a damage response element at the reaper locus. Cell 101 103 113
71. PetersMDeLucaCHiraoAStambolicVPotterJ 2002 Chk2 regulates irradiation-induced, p53-mediated apoptosis in Drosophila. Proc Natl Acad Sci USA 99 11305 11310
72. SogameNKimMAbramsJM 2003 Drosophila p53 preserves genomic stability by regulating cell death. Proc Natl Acad Sci USA 100 4696 4701
Štítky
Genetika Reprodukční medicína
Článek Local Absence of Secondary Structure Permits Translation of mRNAs that Lack Ribosome-Binding SitesČlánek Independent Chromatin Binding of ARGONAUTE4 and SPT5L/KTF1 Mediates Transcriptional Gene SilencingČlánek Trade-Off between Bile Resistance and Nutritional Competence Drives Diversification in the Mouse GutČlánek FGF Signaling Regulates the Number of Posterior Taste Papillae by Controlling Progenitor Field SizeČlánek Mammalian BTBD12 (SLX4) Protects against Genomic Instability during Mammalian SpermatogenesisČlánek Identification of Nine Novel Loci Associated with White Blood Cell Subtypes in a Japanese PopulationČlánek Differential Effects of and Risk Variants on Association with Diabetic ESRD in African AmericansČlánek Dynamic Chromatin Localization of Sirt6 Shapes Stress- and Aging-Related Transcriptional Networks
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2011 Číslo 6
-
Všechny články tohoto čísla
- Local Absence of Secondary Structure Permits Translation of mRNAs that Lack Ribosome-Binding Sites
- Statistical Inference on the Mechanisms of Genome Evolution
- Revisiting Heterochromatin in Embryonic Stem Cells
- A Two-Stage Meta-Analysis Identifies Several New Loci for Parkinson's Disease
- Identification of a Sudden Cardiac Death Susceptibility Locus at 2q24.2 through Genome-Wide Association in European Ancestry Individuals
- Genomic Prevalence of Heterochromatic H3K9me2 and Transcription Do Not Discriminate Pluripotent from Terminally Differentiated Cells
- Epistasis between Beneficial Mutations and the Phenotype-to-Fitness Map for a ssDNA Virus
- Recurrent Chromosome 16p13.1 Duplications Are a Risk Factor for Aortic Dissections
- Telomere DNA Deficiency Is Associated with Development of Human Embryonic Aneuploidy
- Genome-Wide Association Study of White Blood Cell Count in 16,388 African Americans: the Continental Origins and Genetic Epidemiology Network (COGENT)
- Unexpected Role for DNA Polymerase I As a Source of Genetic Variability
- Transportin-SR Is Required for Proper Splicing of Genes and Plant Immunity
- How Chromatin Is Remodelled during DNA Repair of UV-Induced DNA Damage in
- Independent Chromatin Binding of ARGONAUTE4 and SPT5L/KTF1 Mediates Transcriptional Gene Silencing
- Two Evolutionary Histories in the Genome of Rice: the Roles of Domestication Genes
- Natural Allelic Variation Defines a Role for : Trichome Cell Fate Determination
- Multiple Common Susceptibility Variants near BMP Pathway Loci , , and Explain Part of the Missing Heritability of Colorectal Cancer
- Pathogenic Mechanism of the FIG4 Mutation Responsible for Charcot-Marie-Tooth Disease CMT4J
- A Functional Variant in Promoter Modulates Its Expression and Confers Disease Risk for Systemic Lupus Erythematosus
- Drift and Genome Complexity Revisited
- Chromosomal Macrodomains and Associated Proteins: Implications for DNA Organization and Replication in Gram Negative Bacteria
- Trade-Off between Bile Resistance and Nutritional Competence Drives Diversification in the Mouse Gut
- Pathways of Distinction Analysis: A New Technique for Multi–SNP Analysis of GWAS Data
- Web-Based Genome-Wide Association Study Identifies Two Novel Loci and a Substantial Genetic Component for Parkinson's Disease
- Chk2 and p53 Are Haploinsufficient with Dependent and Independent Functions to Eliminate Cells after Telomere Loss
- Exome Sequencing Identifies Mutations in High Myopia
- Distinct Functional Constraints Partition Sequence Conservation in a -Regulatory Element
- CorE from Is a Copper-Dependent RNA Polymerase Sigma Factor
- A Single Sex Pheromone Receptor Determines Chemical Response Specificity of Sexual Behavior in the Silkmoth
- FGF Signaling Regulates the Number of Posterior Taste Papillae by Controlling Progenitor Field Size
- Maps of Open Chromatin Guide the Functional Follow-Up of Genome-Wide Association Signals: Application to Hematological Traits
- Increased Susceptibility to Cortical Spreading Depression in the Mouse Model of Familial Hemiplegic Migraine Type 2
- Differential Gene Expression and Epiregulation of Alpha Zein Gene Copies in Maize Haplotypes
- Parallel Adaptive Divergence among Geographically Diverse Human Populations
- Genetic Analysis of Genome-Scale Recombination Rate Evolution in House Mice
- Mechanisms for the Evolution of a Derived Function in the Ancestral Glucocorticoid Receptor
- Mammalian BTBD12 (SLX4) Protects against Genomic Instability during Mammalian Spermatogenesis
- Interferon Regulatory Factor 8 Regulates Pathways for Antigen Presentation in Myeloid Cells and during Tuberculosis
- High-Resolution Analysis of Parent-of-Origin Allelic Expression in the Arabidopsis Endosperm
- Specific SKN-1/Nrf Stress Responses to Perturbations in Translation Elongation and Proteasome Activity
- Graded Nodal/Activin Signaling Titrates Conversion of Quantitative Phospho-Smad2 Levels into Qualitative Embryonic Stem Cell Fate Decisions
- Genome-Wide Analysis Reveals PADI4 Cooperates with Elk-1 to Activate Expression in Breast Cancer Cells
- Trait Variation in Yeast Is Defined by Population History
- Meiosis-Specific Loading of the Centromere-Specific Histone CENH3 in
- A Genome-Wide Survey of Imprinted Genes in Rice Seeds Reveals Imprinting Primarily Occurs in the Endosperm
- Multiple Regulatory Mechanisms to Inhibit Untimely Initiation of DNA Replication Are Important for Stable Genome Maintenance
- SIRT1 Promotes N-Myc Oncogenesis through a Positive Feedback Loop Involving the Effects of MKP3 and ERK on N-Myc Protein Stability
- Bacteriophage Crosstalk: Coordination of Prophage Induction by Trans-Acting Antirepressors
- Role of the Single-Stranded DNA–Binding Protein SsbB in Pneumococcal Transformation: Maintenance of a Reservoir for Genetic Plasticity
- Genomic Convergence among ERRα, PROX1, and BMAL1 in the Control of Metabolic Clock Outputs
- Genome-Wide Association of Bipolar Disorder Suggests an Enrichment of Replicable Associations in Regions near Genes
- Identification of Nine Novel Loci Associated with White Blood Cell Subtypes in a Japanese Population
- DNA Ligase III Promotes Alternative Nonhomologous End-Joining during Chromosomal Translocation Formation
- Differential Effects of and Risk Variants on Association with Diabetic ESRD in African Americans
- Finished Genome of the Fungal Wheat Pathogen Reveals Dispensome Structure, Chromosome Plasticity, and Stealth Pathogenesis
- Dynamic Chromatin Localization of Sirt6 Shapes Stress- and Aging-Related Transcriptional Networks
- Extracellular Matrix Dynamics in Hepatocarcinogenesis: a Comparative Proteomics Study of Transgenic and Null Mouse Models
- Integrating 5-Hydroxymethylcytosine into the Epigenomic Landscape of Human Embryonic Stem Cells
- Vive La Différence: An Interview with Catherine Dulac
- Multiple Loci Are Associated with White Blood Cell Phenotypes
- Nuclear Accumulation of Stress Response mRNAs Contributes to the Neurodegeneration Caused by Fragile X Premutation rCGG Repeats
- A New Mutation Affecting FRQ-Less Rhythms in the Circadian System of
- Cryptic Transcription Mediates Repression of Subtelomeric Metal Homeostasis Genes
- A New Isoform of the Histone Demethylase JMJD2A/KDM4A Is Required for Skeletal Muscle Differentiation
- Genetic Determinants of Lipid Traits in Diverse Populations from the Population Architecture using Genomics and Epidemiology (PAGE) Study
- A Genome-Wide RNAi Screen for Factors Involved in Neuronal Specification in
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Recurrent Chromosome 16p13.1 Duplications Are a Risk Factor for Aortic Dissections
- Statistical Inference on the Mechanisms of Genome Evolution
- Genome-Wide Association Study of White Blood Cell Count in 16,388 African Americans: the Continental Origins and Genetic Epidemiology Network (COGENT)
- Chromosomal Macrodomains and Associated Proteins: Implications for DNA Organization and Replication in Gram Negative Bacteria
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



