-
Články
Reklama
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
ReklamaA Novel Mutation in the Upstream Open Reading Frame of the Gene Causes a MEN4 Phenotype
The CDKN1B gene encodes the cyclin-dependent kinase inhibitor p27KIP1, an atypical tumor suppressor playing a key role in cell cycle regulation, cell proliferation, and differentiation. Impaired p27KIP1 expression and/or localization are often observed in tumor cells, further confirming its central role in regulating the cell cycle. Recently, germline mutations in CDKN1B have been associated with the inherited multiple endocrine neoplasia syndrome type 4, an autosomal dominant syndrome characterized by varying combinations of tumors affecting at least two endocrine organs. In this study we identified a 4-bp deletion in a highly conserved regulatory upstream ORF (uORF) in the 5′UTR of the CDKN1B gene in a patient with a pituitary adenoma and a well-differentiated pancreatic neoplasm. This deletion causes the shift of the uORF termination codon with the consequent lengthening of the uORF–encoded peptide and the drastic shortening of the intercistronic space. Our data on the immunohistochemical analysis of the patient's pancreatic lesion, functional studies based on dual-luciferase assays, site-directed mutagenesis, and on polysome profiling show a negative influence of this deletion on the translation reinitiation at the CDKN1B starting site, with a consequent reduction in p27KIP1 expression. Our findings demonstrate that, in addition to the previously described mechanisms leading to reduced p27KIP1 activity, such as degradation via the ubiquitin/proteasome pathway or non-covalent sequestration, p27KIP1 activity can also be modulated by an uORF and mutations affecting uORF could change p27KIP1 expression. This study adds the CDKN1B gene to the short list of genes for which mutations that either create, delete, or severely modify their regulatory uORFs have been associated with human diseases.
Published in the journal: . PLoS Genet 9(3): e32767. doi:10.1371/journal.pgen.1003350
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003350Summary
The CDKN1B gene encodes the cyclin-dependent kinase inhibitor p27KIP1, an atypical tumor suppressor playing a key role in cell cycle regulation, cell proliferation, and differentiation. Impaired p27KIP1 expression and/or localization are often observed in tumor cells, further confirming its central role in regulating the cell cycle. Recently, germline mutations in CDKN1B have been associated with the inherited multiple endocrine neoplasia syndrome type 4, an autosomal dominant syndrome characterized by varying combinations of tumors affecting at least two endocrine organs. In this study we identified a 4-bp deletion in a highly conserved regulatory upstream ORF (uORF) in the 5′UTR of the CDKN1B gene in a patient with a pituitary adenoma and a well-differentiated pancreatic neoplasm. This deletion causes the shift of the uORF termination codon with the consequent lengthening of the uORF–encoded peptide and the drastic shortening of the intercistronic space. Our data on the immunohistochemical analysis of the patient's pancreatic lesion, functional studies based on dual-luciferase assays, site-directed mutagenesis, and on polysome profiling show a negative influence of this deletion on the translation reinitiation at the CDKN1B starting site, with a consequent reduction in p27KIP1 expression. Our findings demonstrate that, in addition to the previously described mechanisms leading to reduced p27KIP1 activity, such as degradation via the ubiquitin/proteasome pathway or non-covalent sequestration, p27KIP1 activity can also be modulated by an uORF and mutations affecting uORF could change p27KIP1 expression. This study adds the CDKN1B gene to the short list of genes for which mutations that either create, delete, or severely modify their regulatory uORFs have been associated with human diseases.
Introduction
CDKN1B encodes the cyclin-dependent kinase (CDK) inhibitor, p27KIP1, which negatively regulates the Cdk2/cyclin E and Cdk2/cyclin A protein complexes, thereby preventing the progression from the G1 to the S phase of the cell cycle [1]. In G0 and early G1, p27KIP1 expression and stability are maximal. During the G1 phase gradual degradation of p27KIP1 is associated with an increased activity of Cdk2/cyclin E and Cdk2/cyclin A complexes to stimulate cell proliferation [2], [3]. Several mitogenic (i.e. MAPK, PI3K/AKT) and anti-proliferative (i.e. TGFβ/SMAD) signal transduction pathways regulate p27KIP1 expression and activity, making it a central integration point for cell-fate decision [4]. These pathways can regulate p27KIP1 at different levels, including transcription, translation, intracellular localization or ubiquitin-mediated proteasomal degradation [5].
p27KIP1 acts as an atypical tumor suppressor as it is rarely mutated in human cancers, but frequently underexpressed or mislocalized in human malignancies [4]. Although an augmented proteolysis was initially suggested as the major cause of p27KIP1 loss in human tumors [6], recent findings propose that reduced translation and/or transcription of CDKN1B also contributes to p27KIP1 deficiency [7]–[9].
Translation of CDKN1B may involve regulatory elements within its 5′UTR, including an internal ribosome entry site (IRES) and an upstream ORF (uORF) [10], [11]. The IRES supports p27KIP1 expression when cap-dependent translation is reduced, such as during quiescence or stress conditions [10], [12]. Reduced IRES-mediated translation, due to mutations in the pseudouridine synthase that alters the ribosome's ability to efficiently engage the CDKN1B IRES element, may contribute to the increased predisposition to cancer in X-linked congenital dyskeratosis [13].
Germline mutations in the CDKN1B gene have been recently associated with the development of a multiple endocrine neoplasia syndrome both in humans (MEN4, MIM 610755) and in rats (MENX) [14]. Multiple endocrine neoplasias, including type 1 (MEN1, MIM 131100) and type 2 variants, (MEN2, MIM 171400, MIM 162300), are a group of autosomal dominant syndromes characterized by varying combinations of tumors affecting at least two endocrine organs [15].
To date, seven CDKN1B germline mutations have been identified in MEN4 patients primarily associated with MEN1-related lesions, including parathyroid and pituitary tumors, but the presence of other malignancies such as renal angiomyolipoma, papillary thyroid carcinoma and pancreatic masses has also been reported [8], [9], [14], [16], [17]. Two further germline mutations have been more recently associated with sporadic hyperparathyroidism [18]. In MEN4, CDKN1B mutations either affect p27KIP1 cellular localization, protein stability or the binding with functional partners such as Cdk2 or Grb2 [8], [17]. Reduced transcription/translation efficiency due to mutations in elements regulating translation initiation (i.e., in the Kozak sequence, or forming a secondary stem loop structure within the CDKN1B 5′UTR), has also been described [8], [9].
Germline CDKN1B mutations are hence rare events in MEN1-like subjects (individuals with MEN1-related lesions, without MEN1 inactivating mutations), being identified in less than 3% of cases [8], [16] and a clear genotype-phenotype correlation has not been established to date.
In the present paper we analyzed the CDKN1B gene looking for point mutations and large rearrangements in order to determine the possible cause of multiple endocrine tumors in 25 consecutive sporadic and familial patients with typical MEN1-related symptoms. We identified a 4-bp deletion that modifies the regulatory uORF in the 5′UTR of the CDKN1B gene in a patient with tumors in the pituitary gland and the endocrine pancreas. Functional studies based on dual-luciferase assay and site-directed mutagenesis further support the deleterious influence of this deletion on translation reinitiation at the CDKN1B starting site, with a consequent reduction of p27KIP1 expression both in vitro and in vivo.
Results/Discussion
The regulatory uORF within the CDKN1B gene is mutated in an acromegalic patient affected by a well-differentiated pancreatic lesion
Among the 25 patients with MEN1-related symptoms, a 4-bp deletion (c.-456_-453delCCTT, NM_004064) within the 5′UTR of CDKN1B in a 62 year old female patient with acromegaly and a well-differentiated non-functioning pancreatic endocrine neoplasm has been identified. This sequence variant was not detected in either 600 chromosomes or in the dbSNP/1000 genomes databases.
The 5′UTR of the CDKN1B gene is highly structured, containing several translational regulatory elements. An IRES element sustains p27KIP1 translation under poor growth conditions [10], [12], while a G/C-rich hairpin domain contributes to cell-cycle dependent regulation of CDKN1B translation [11]. Downstream the G/C-rich domain and encompassing the c.-456_-453delCCTT, an uORF coding for a 29 amino acid-long peptide has been described that has been suggested to inhibit the in vitro synthesis of p27KIP1 and to enhance its cell cycle-dependent translation [11]. An extensive comparative analysis of DNA and protein sequences from multiple species (Figure 1) confirmed previous data of high evolutionary conservation among vertebrates of the uORF [11], and support the hypothesis of a functional role of this element [11].
Fig. 1. Comparison of the CDKN1B gene among species. 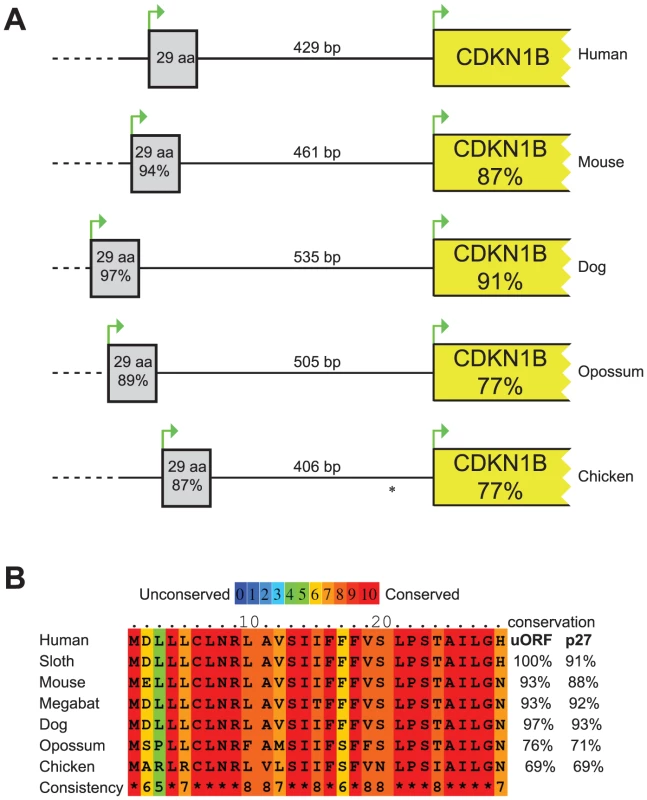
(A) Schematic representation of the CDKN1B gene in different species. The uORF has been reported as grey box while CDKN1B as a yellow one. Similarities at nucleotide level with human sequences have been reported within boxes. Intercistronic length is also shown. * In chicken CDKN1B 5′UTR a second uORF has been described. (B) uORF amino acid sequences in seven species. In all but one, conservation of the uORF encoded peptide is even higher than p27KIP1. The considered nucleotide and protein sequences are the following: Human, NM_004064.3, NP_04055.1; Mouse, NM_009875.4, NP_034005.2; Dog, HE804769, CCH35981; Opossum, HE804772, CCH35984; Chicken, NM_204256, NP_989587; Sloth, HE804770, CCH35982; Megabat, HE804771, CCH35983. Nucleotide and protein sequences alignments among species have been performed by LALIGN and PRALINE, respectively. In general, uORFs are small open reading frames located in the 5′UTR of genes that influence translation during ribosome scanning, thus modulating gene expression. A scanning ribosome encountering an uORF has multiple fates: it can i) translate the uORF; ii) scan through the sequence (leaky scanning) and reinitiate translation further downstream at a proximal or distal ATG; iii) induce ribosome stalling or premature dissociation at the uORF stop codon, thus reducing downstream-cistron translation [19] or down-regulating gene expression by promoting mRNA decay [20].
In our case the 4-bp deletion shifts the uORF termination codon, thus lengthening the uORF encoded peptide from 29 to 158 amino acids and shortening the intercistronic space from 429 to 38 bp, with a possible negative influence on translation reinitiation from the main ATG (Figure 2). Long uORFs and short intercistronic regions may indeed prevent the 40S ribosomal subunits from keeping and/or re-acquiring appropriate cofactors for translation resumption/reinitiation at the downstream ATG [21], [22].
Fig. 2. The c.-456_-453delCCTT affects protein translation by reducing reinitiation. 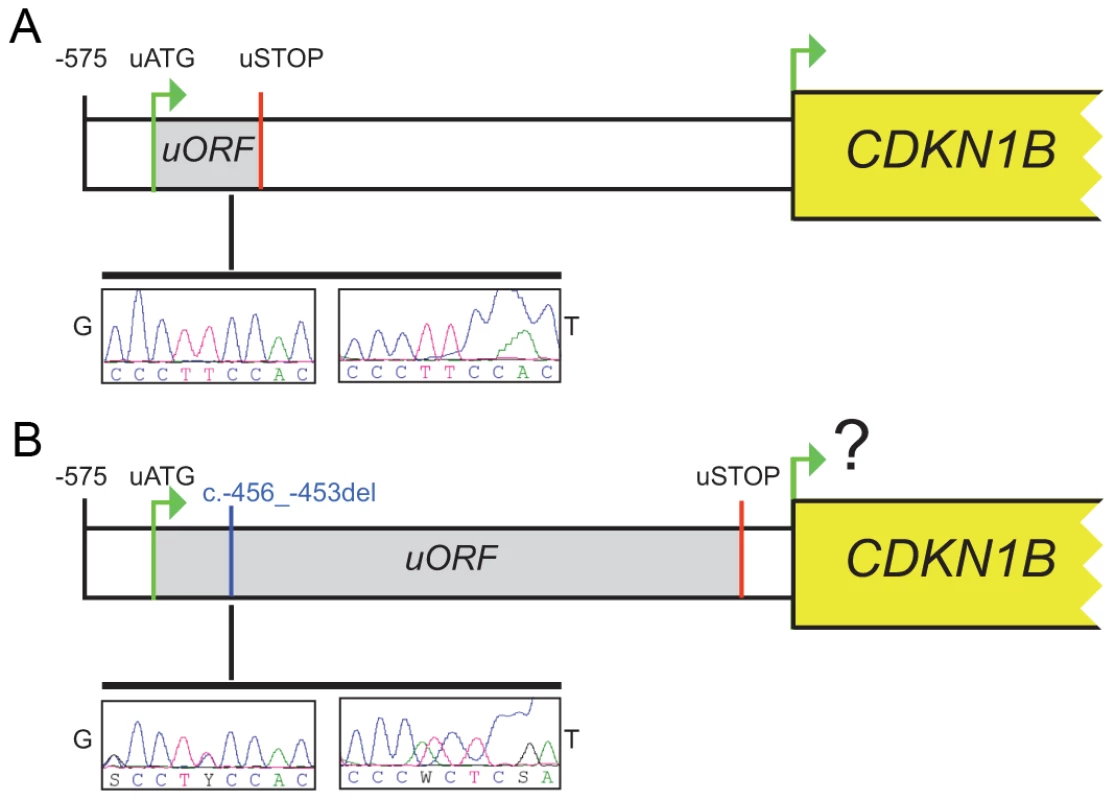
Schematic representation of the CDKN1B 5′UTR in the wild type (A) and in the c.-456_-453delCCTT carrier (B). The uORF coding sequence is shown as grey box. Electropherograms for germline (G) and tumor (T) DNA are shown. The c.-456_-453delCCTT affects p27KIP1 expression without altering neither the steady-state level of CDKN1B mRNA nor the promoter usage
To address the possibility that the 4-bp deletion affects transcription and/or mRNA stability, making a decreased translation rate due to reduced reinitiation efficiency biologically irrelevant, or alters the promoter usage pattern preventing transcription of the uORF-containing isoform [10], we measured the steady state levels of CDKN1B allelic mRNAs from whole blood by 5′RACE and allele-specific qPCR. As reported in Figure 3a, both wild type and mutated alleles were expressed in blood cells in almost equal amounts, suggesting that the identified deletion does not alter mRNA steady state levels, and therefore probably does not alter either transcription or mRNA stability. An apparently unique 5′UTR of >530 bp has been identified in the c.-456_-453delCCTT carrier and in healthy controls, supporting the concept that the transcription pattern is preserved in the mutated subject (Figure 3b).
Fig. 3. The c.-456_-453delCCTT does not alter the steady-state levels of CDKN1B allelic mRNA nor the promoter usage pattern. 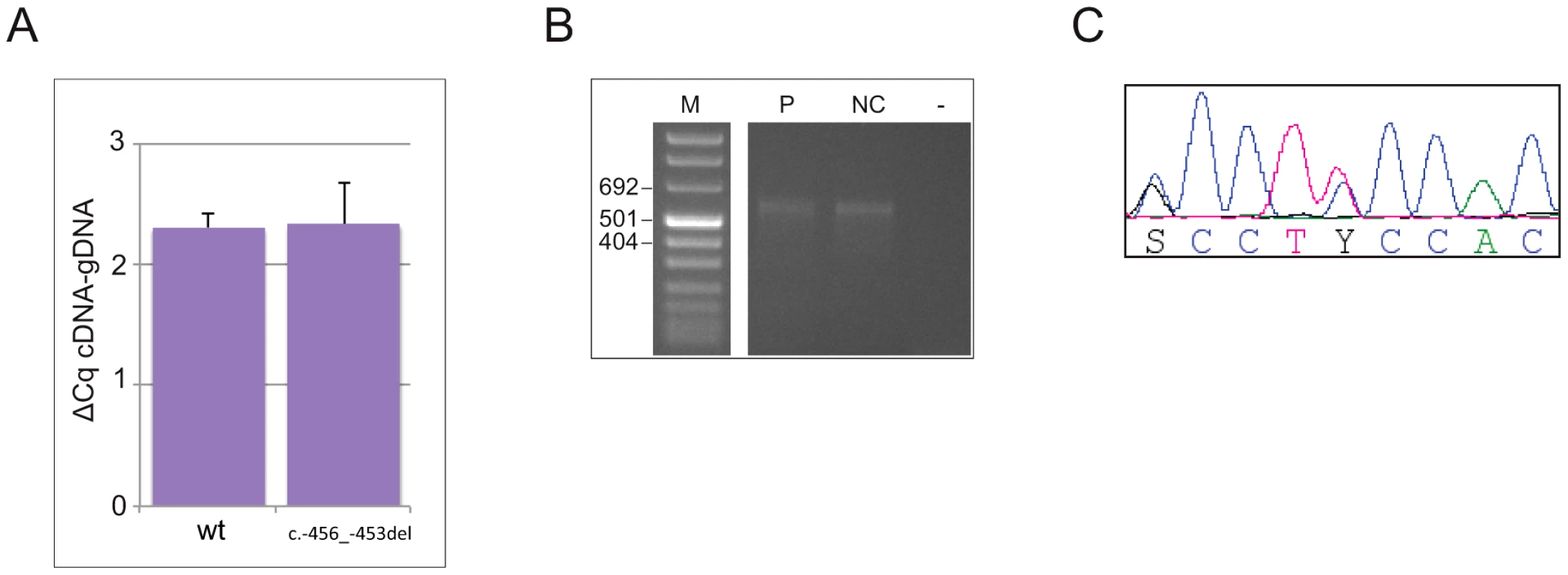
(A) The wild type and mutated alleles were present at similar level in blood-derived RNA. The amount of both alleles are expressed as differences between Cq values obtained for mRNA minus Cq for genomic DNA for removing the intrinsic variation between the two qPCR assays. (B) A single CDKN1B promoter is used in lymphocytes from both deletion carrier (P) and a normal control (NC). M, molecular marker; -, negative control. (C) Both wild type and mutated alleles are transcribed in pancreatic lesion. The pancreatic tumor of the mutated patient was then analyzed by immunohistochemistry for p27KIP1 expression and for the proliferation antigen Ki-67, and compared with similar tumors from CDKN1B-mutation negative subjects. Parallel differences in expression level and localization were found. We observed weak cytoplasmic staining in tumor cells and very strong nuclear staining in the interspersed normal endothelial cells in the MEN4 patient (Ki67<1%), while in contrast p27KIP1 nuclear staining was found in a high proportion of sporadic well-differentiated pancreatic tumors examined (Figure 4). The reduction in nuclear p27KIP1 and/or its cytoplasmic mislocalization has been reported in different cancers including breast, colon and prostate [4]. Loss of p27KIP1 may occur through different mechanisms, including augmented proteasome-mediated proteolysis and impaired translation [23]. On the other hand, the cytoplasmic mislocalization may be associated with imbalanced p27KIP1 phosphorylation due to the oncogenic activation of PI3K - and MEK-dependent kinases, mimicking protein loss [4]. Indeed, in the cytoplasm p27KIP1 is unable to exert its inhibitory activity on CDK even in the presence of anti-mitogenic stimuli.
Fig. 4. Expression of p27KIP1 and Ki-67 in pancreatic tumors and control tissue. 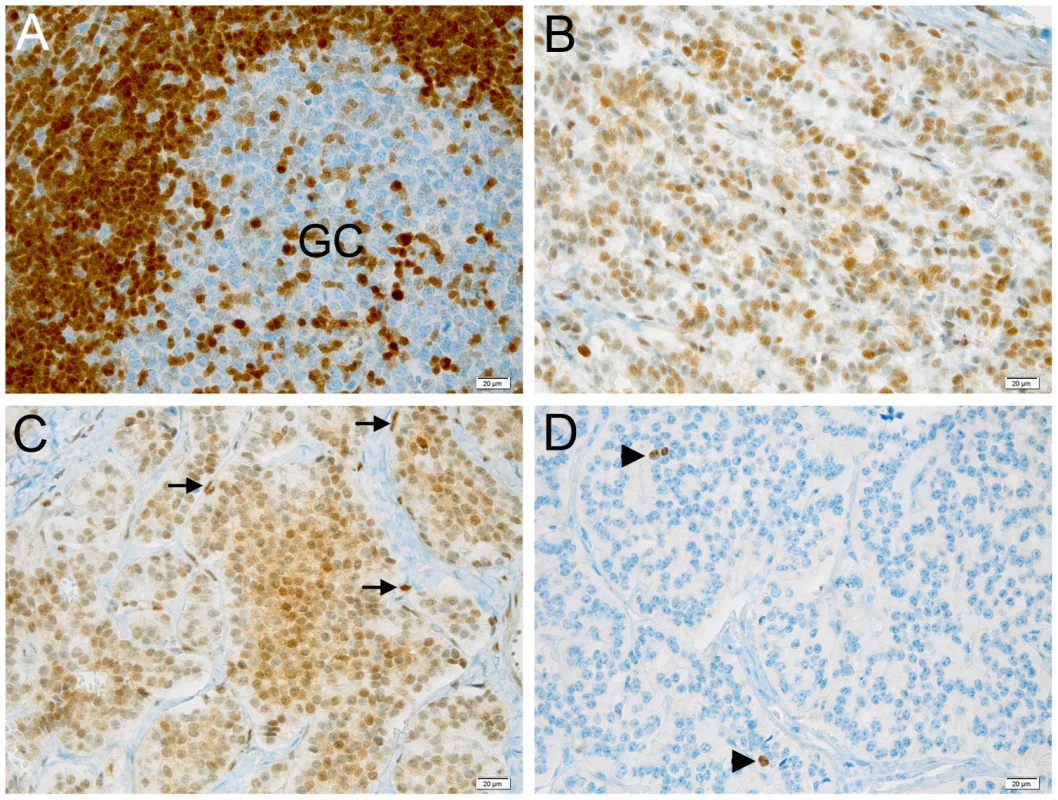
(A–C) Immunohistochemical staining with anti-p27KIP1 antibody. (D) Staining with anti-Ki67 antibody. (A) staining of a control human tonsil. Cells in the lymphatic nodule show the expected strong nuclear positivity for p27KIP1, while only few of the highly proliferating germinal center (GC) cells express p27KIP1. (B) Sporadic endocrine pancreatic tumor of an individual with no known germline mutations. The tumor cells show intermediate levels of nuclear immunoreactivity for p27KIP1. (C) Endocrine pancreatic tumor of the patient having the c.-456_-453delCCTT mutation. Tumor cells show low expression of p27KIP1 in the nucleus but also expression in the cytoplasm. Endothelial cells (arrows) show the typical strong nuclear positivity and serve as internal control to check for the adequacy of the staining. (D) Same tissue as in C stained for the proliferation marker Ki-67. Positive nuclei are indicated with arrowheads. The proliferation rate was estimated to be ca. 1%. Bar, 20 µM. Original magnification: A–D, 200×. On the same lesion loss of heterozygosity (LOH) analysis was then performed. No loss of the wild type allele was observed (Figure 2). Moreover, the biallelic expression of an uORF-containing transcript has been observed (Figure 3c), further confirming that p27KIP1 may act as a haploinsufficient tumor suppressor [24].
The c.-456_-453delCCTT affects p27KIP1 translation reinitiation by altering CDKN1B uORF length and the intercistronic distance in a cell cycle–independent manner
To identify possible additional uORF mutations, we extended the CDKN1B 5′UTR analysis to additional 41 patients with typical MEN1-like features previously reported negative for mutations in the CDKN1B coding sequence [17], [25]. A c.-469C>T substitution resulting in a silent change in the uORF was detected in a single patient but not in healthy controls (see above).
To determine whether the two identified substitutions negatively affect CDKN1B translation, the wild type and mutated 5′UTRs were cloned upstream of the firefly luciferase gene (Figure 5a). By transfecting lovastatin G1-synchronized or asynchronous HeLa and GH3 cells, we demonstrated that the c.-456_-453delCCTT, but not the c.-469C>T variant, significantly reduced luciferase activity in a cell cycle phase-independent manner (Figure 6a, 6b). When we analyzed the luciferase mRNA from the transfected cells by quantitative real-time RT-PCR, we demonstrated that the effects of the 4-bp deletion are largely due to reduction in translation rate rather than to changed steady-state mRNA levels (Figure 6c), in agreement with our observation on blood CDKN1B mRNA (Figure 3a) and with the trend observed in large-scale datasets [26].
Fig. 5. Constructs used in transfection experiments. 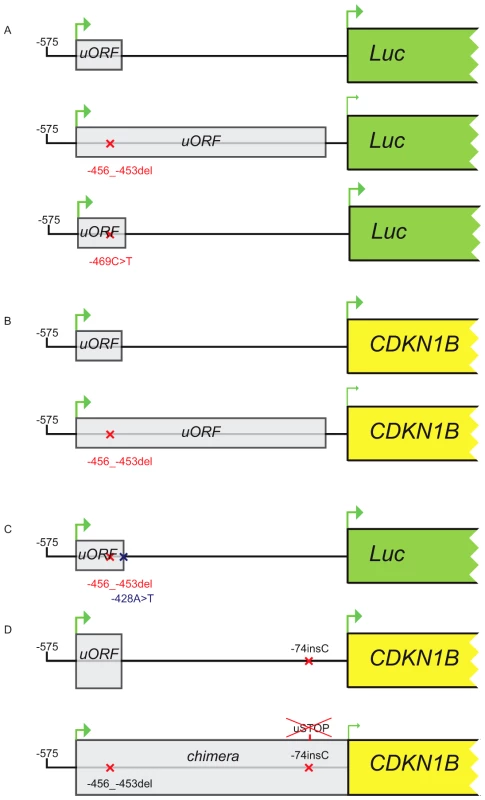
(A) Either the wild type, the c.-456_-453delCCTT or the c.-469C>T containing 5′UTRs were cloned upstream the firefly luciferase gene. (B) The wild type/c.-456_-453delCCTT 5′UTR were subcloned upstream the CDKN1B gene. (C) The introduction of the c.-428A>T substitution in the two former plasmids reported in panel A restores uORF length and intercistonic distance. (D) Constructs reported in panel B were mutated introducing a c.-74insC leading to the translation of a chimeric protein in the double mutant. Fig. 6. The c.-456_-453delCCTT affects protein translation by reducing reinitiation. 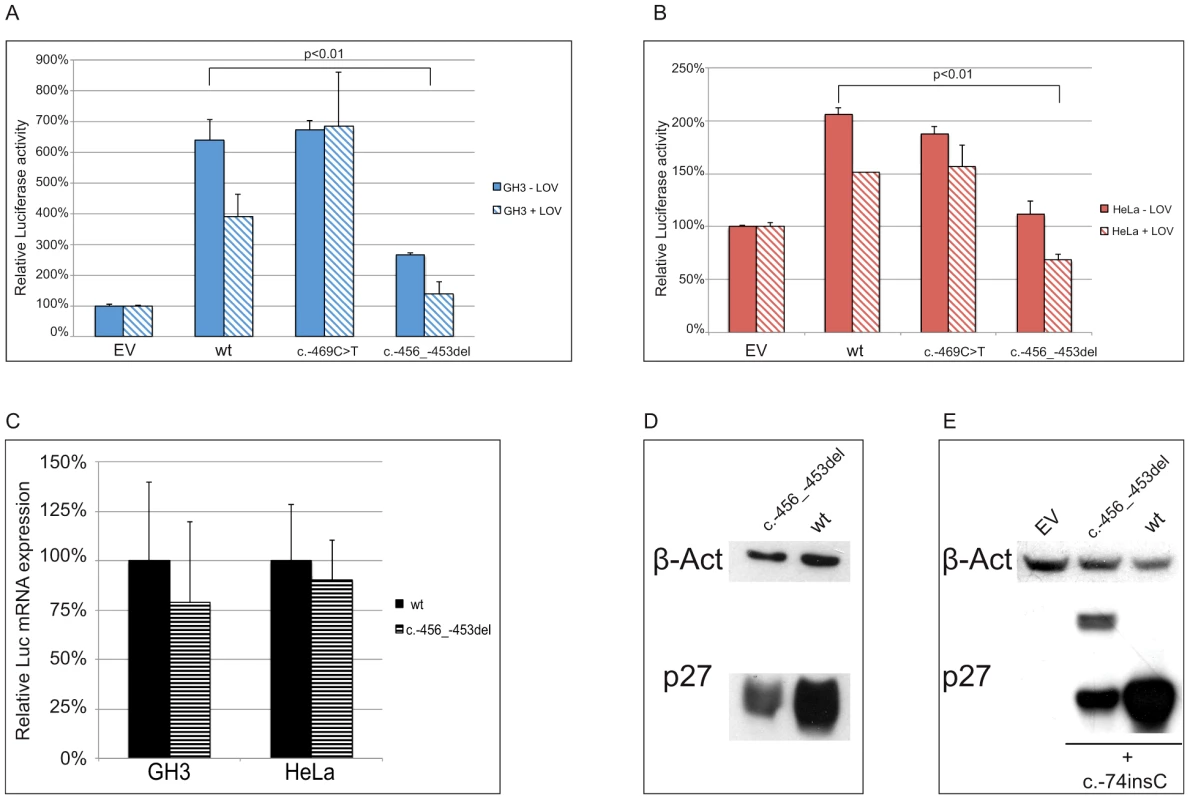
Relative luciferase activity for the wild type and the mutants (c.-456_-453delCCTT, c.-469C>T) 5′UTRs was measured in GH3 (A) and HeLa (B) cells. The effects of the 4 bp deletion on luciferase mRNA in HeLa and GH3 cells (C) and on p27KIP1 level in HEK293 cells (D) are shown. Panel E shows the presence of the chimeric product in HEK293 cells transfected with the double mutant construct (c.-456_-459delCCTT+c.-74insC), followed by substantial reduction of p27KIP1 expression, demonstrated by western blot. P-values were calculated using a two-tailed t-test. *p<0,01; EV, empty vector; wt, wild type; error bars, standard deviation. We then evaluated the effect of the 4 bp deletion on p27KIP1 translation by transfecting HEK293 cells with vectors with either the wild type or the mutated 5′UTRs cloned upstream the CDKN1B gene (Figure 5b). We confirmed a significant reduction in p27KIP1 protein levels as a consequence of the 5′UTR c.-456_-453delCCTT mutation (Figure 6d).
In a previous study on HeLa cells using an identical wild type construct, the CDKN1B 5′UTR induced luciferase expression only during G1 progression or in lovastatin-arrested cells [11]. Although we cannot exclude the presence of DNA variations on regulatory elements between the two cloned sequences, a possible biological variability between batches of cells from the same cell line seems the more plausible explanation. However, similar cell-cycle independent luciferase activation was observed under our experimental conditions in three additional cell lines, namely GH3 (Figure 6a), SH-SY5Y and HEK293 (Figure S1). Based on such observation we may therefore suggest the need for further studies for better clarifying the cell-cycle dependent translation of p27KIP1 regulated by the CDKN1B 5′UTR.
Site-directed mutagenesis (c.-428A>T) was then used to reintroduce a stop codon in the c.-456_-453delCCTT containing vector, thus restoring both uORF length and intercistronic distance (Figure 5c). After transfection, the uORF regulatory properties were almost completely rescued in the double mutant compared to the c.-428A>T construct (Figure 7a), further supporting the hypothesis that the 4 bp deletion affects translation reinitiation of the downstream CDKN1B ORF. In addition, the lack of complete recovery of the uORF modulatory activity, possibly due to differences in the C-terminus of the uORF-encoded peptide (Figure 7b), further confirms that the CDKN1B uORF belongs to the class of sequence-dependent uORFs that exert their inhibitory role by acting in cis to regulate components of the translation apparatus [19].
Fig. 7. Effect of the c.-428A>T restoring mutation. 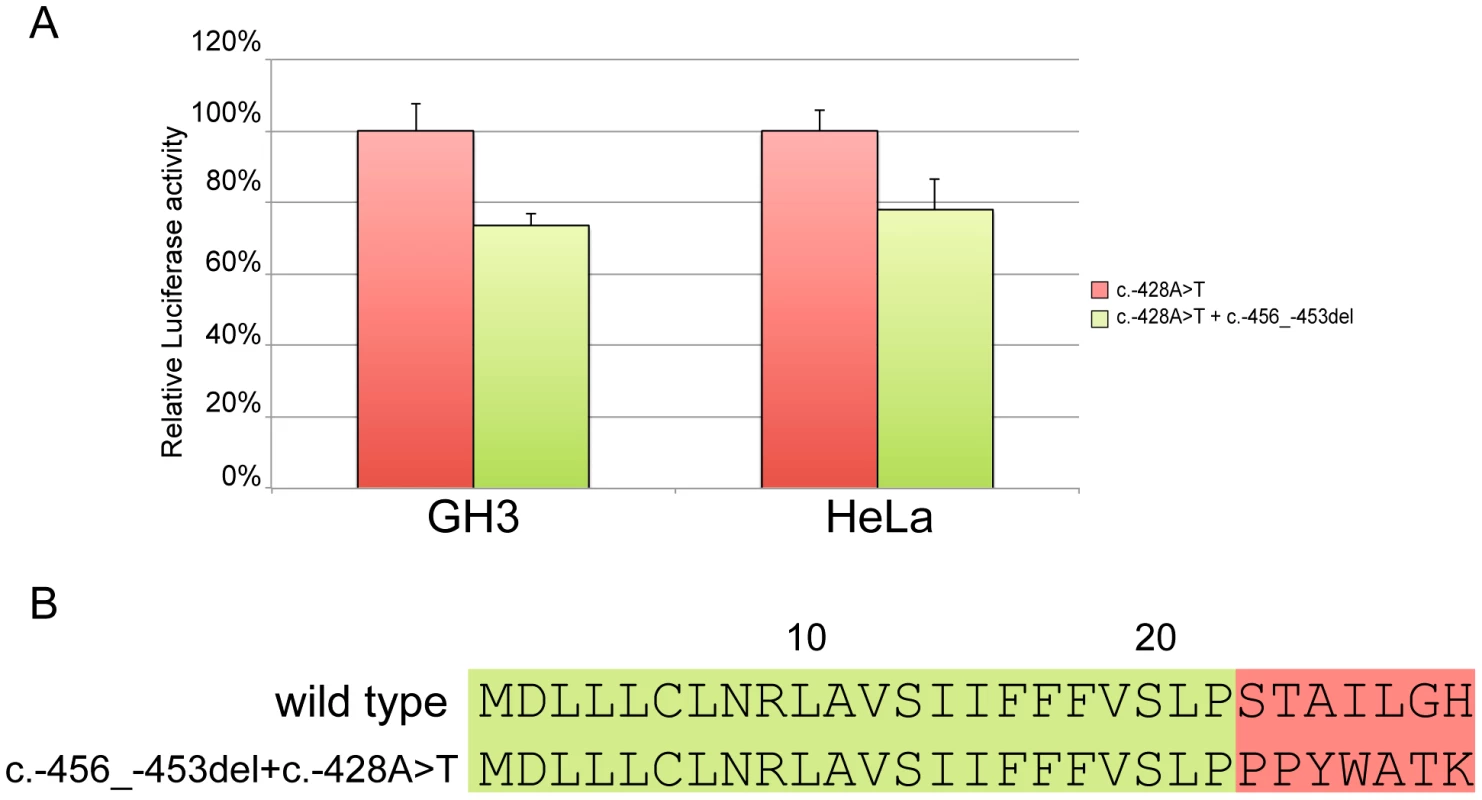
In both GH3 and HeLa cell lines c.-428A>T almost restores the modulation properties of the CDKN1B wild type uORF (A). Panel B shows the primary sequence differences in the wild type uORF compared with the -456_-453delCCTT+c.-428A>T. The CDKN1B uORF is efficiently translated
To evaluate the ability of the uORF to be translated, which represents the central point of our hypothesis on the possible deleterious effects of the c.-456_-453delCCTT change, the wild type or the mutated 5′UTRs were placed upstream of the CDKN1B open reading frame and the c.-74insC mutation was introduced by site-directed mutagenesis. This additional DNA variant leads to the in-frame fusion of the mutated uORF with the main gene (Figure 5d). As expected, the chimeric product was detected only in the c.-74insC+c.-456_-453delCCTT transfected HEK293 cells, and was again associated with a significant reduction of p27KIP1 expression (Figure 6e). To our knowledge, this is the first direct evidence of the translation of the CDKN1B uORF in a cellular system. However, it remains to be clarified if this peptide has additional biological functions other than repressing translation of the CDKN1B ORF as an effect of impaired reinitiation, as suggested for a subset of uORFs [27].
The c.-456_-453delCCTT transcript is less efficiently loaded onto polysomes than the wild-type one
To elucidate the molecular mechanism by which c.-456_-453delCCTT determines a decrease in p27KIP1 translational efficiency, we estimated the relative proportion of the two allelic mRNAs engaged in translation in the immortalized lymphoblastoid cells of the heterozygote patient. To this aim, the cell lysates were subjected to polysome fractionation through sucrose gradient ultracentrifugation [28] and we determined the level of each of the two allelic mRNAs for each fraction. Figure 8a reports the distribution of ribosomal RNA in the different fractions, showing a typical distribution with polysomes reproducibly spanning fractions 7–11. The distributions of the wild type and c.-456_-453delCCTT transcripts present an almost superimposable pattern, being for both about 90% of the total detectable mRNA localized in polysomes with a peak corresponding to fraction 9 (compare Figure 8b with Figure 8a). However, when the amounts of both alleles were expressed as differences between Cq values for mRNA and for genomic DNA for removing the intrinsic variation between the two qPCR assays, a clear preponderance on polysomes of the wild type CDKN1B mRNA could be observed (Figure 8c), which we can estimate to be of the order of about three times. Since the levels of the two allelic mRNAs in the cells are the same (Figure 3a), this implies that the c.-456_-453delCCTT mRNA suffers decreased average polysomal loading with respect to the wild type mRNA. Therefore, the two different CDKN1B mRNAs are differentially loaded in polysomes despite being present in the cells in the same relative amounts, and despite the fact that they share a distribution profile on polysomes of different molecular weights. The result is compatible with a decreased efficiency of translation reinitiation of the CDKN1B ORF due to the c.-456_-453delCCTT mutation.
Fig. 8. Polysome profiling of lymphoblastoid cells from the -456_-453delCCTT mutation carrier. 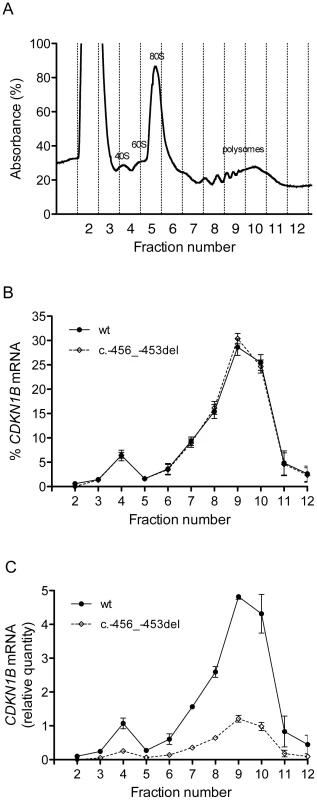
(A) Representative absorbance profile for RNA separated by velocity sedimentation through a 15–50% sucrose gradient. The positions of the 40S, 60S, 80S, and polysomal peaks are indicated. (B) The levels of CDKN1B mRNA (wt and c.-456_-453del) in each gradient fraction were measured by quantitative real-time PCR and plotted as a percentage of the total CDKN1B mRNA levels (wt or c.-456_-453del, respectively) in that sample. Data represent three independent experiments, with mean ± SD reported. (C) The levels of both alleles in each fraction were expressed as relative quantities calculated from differences in Cq values between mRNA and genomic DNA for removing the intrinsic variation between the two qPCR assays. Data represent the mean of three independent experiments ± SD. The role of uORF mutations in MEN4 and other human diseases
The data we presented here further confirm the role of CDKN1B germline mutations in predisposing to a MEN4 syndrome. Furthermore, they demonstrate that a reduced translation initiation rate of p27KIP1 due to the ineffective regulatory activity of its uORF may be associated with transformation. Based on our data, mutations in the CDKN1B-regulating uORF seem to be rare. However, previous studies on CDKN1B germline mutations in MEN1-like patients did not consider the uORF region [8], [9], [14], [16]–[18], [25], [29], and therefore the prevalence of this type of mutation remains to be established. Our results emphasize thus the need for the inclusion of the entire 5′UTR region of CDKN1B in molecular testing for MEN4.
Increasing evidence suggests that uORF-mediated translational control may represent an important mechanism in the regulation of gene expression. This is supported by the close relationship of mutations that introduce or disrupt uORFs and the pathophysiology of several human diseases, including cancer [30]. To date, only three well-known hereditary diseases have been associated with uORF-affecting mutations: i) thrombocythemia due to thrombopoietin mutation [31], ii) melanoma due to CDKN2A mutation [32] and iii) Marie Unna hypotrichosis due to mutations in the hairless gene [33]. Other diseases, such as breast cancer, Alzheimer's diseases, arrhythmogenic right ventricular cardiomyopathy have also been suggested to be associated to genes which have uORF-related control [34]–[36]. However, the pathogenic effects of deregulated uORF-mediated translation in these cases remain to be clarified [37].
Many important genes involved in controlling cell growth (i.e. receptors, oncogenes, growth factors) harbor uORF in their 5′UTR [38]. Some of these genes override the uORF-mediated translational repression and accumulate their protein product in cancer cells [39]. Translational derepression elements in the 3′UTR may counteract the inhibitory activity of uORFs on translation [39]; however, mutations inducing loss of uORF function in oncogenes might lead to a similar increase of translation rate and consequently to malignant transformation. Conversely, gain of function mutations in uORFs regulating tumor suppressor genes may reduce translation of protective proteins leading to tumor formation [32]. Similarly to a point mutation introducing a regulative uORF in the leader sequence of the tumor suppressor gene CDKN2A in hereditary melanoma [32], the 4-bp deletion in CDKN1B gene we describe here led to the reduced production of CDKN1B-encoded protein p27KIP1, probably due to a decreased translation reinitiation rate, which then results in predisposition to tumor development.
Conclusions
In conclusion, the CDKN1B mutation functionally characterized in this study represents a novel example of an uORF-affecting mutation. Our functional studies show the negative influence of this deletion on the translation reinitiation at the CDKN1B starting site thus providing novel insights into the role of uORFs in the pathogenesis of human diseases.
In addition to the classical mechanisms of degradation by the ubiquitin/proteasome pathway and by non-covalent cytoplasmic sequestration, our findings demonstrate that p27KIP1 activity can also be modulated by its uORF, and mutations affecting this sequence may lead to reduced expression of p27KIP1 protein.
Materials and Methods
Patients
The cohort of patients screened for mutations in the entire CDKN1B gene consisted of 25 consecutive patients with two or more typical MEN1-related symptoms (hyperparathyroidism, neuroendocrine tumors, pituitary adenoma). Patients were collected and diagnosed at the Division of Endocrinology (University/Hospital of Padova) and at the Familial Cancer Clinic and Oncoendocrinology (Veneto Institute of Oncology), Padova, Italy, following the recognized clinical practice guidelines [40]. All patients had negative mutational screening for MEN1, PRKAR1A and AIP genes. A second group of additional 41 patients with similar phenotype has been analyzed only for the uORF sequence since the rest of the gene has been analyzed and published previously without finding any pathogenic mutations [17], [25]. The study was conducted in accordance with the Helsinki declaration. Local ethical committees from each referring center approved the study, and all subjects gave written informed consent.
Mutational analysis for CDKN1B gene and LOH
The whole coding region, intron–exon boundaries, and 5′ - and 3′-UTRs of CDKN1B were amplified and directly sequenced as reported elsewhere [41]. All primer pairs used were designed by PRIMER3 (http://primer3.sourceforge.net/) and synthesized by IDT (Leuven, Belgium). Primers for point mutation analysis of the entire human CDKN1B gene were P0F, 5′-agcagtacccctccagcagt-3′; P0R, 5′-aaagcccgtccgagtctg-3′; P1F, 5′-ccaatggatctcctcctctg-3′; P1R, 5′-ggagccaaaagacacagacc-3′; P2F, 5′-ccatttgatcagcggagact-3′; P2R, 5′-gccctctaggggtttgtgat-3′; P3F, 5′-gagttaacccgggacttggag-3′; P3R, 5′-atacgccgaaaagcaagcta-3′; P4F, 5′-tgactatggggccaacttct-3′; P4R, 5′-tttgccagcaaccagtaaga-3′; P5F, 5′-ccccatcaagtatttccaagc-3′; P5R, 5′-cctcccttccccaaagttta-3′; P6F, 5′-tgcctctaaaagcgttggat-3′; P6R, 5′-tttttgccccaaactacctg-3′; P7F, 5′-gccctccccagtctctctta-3′; P7R, 5′-ggtttttccatacacaggcaat-3′; P8F, 5′-tctgtccatttatccacaggaa-3′; P8R 5′-tgccaggtcaaataccttgtt-3′.
Previously unreported nucleotide changes were screened in 300 healthy, anonymous, unrelated individuals by Tetra-primer ARMS-PCR [42] and searched in the dbSNP and 1000 genomes databases (http://www.ncbi.nlm.nih.gov/projects/SNP/; http://www.1000genomes.org/). The NHLBI Exome Sequencing Project - Exome Variant Server database (http://evs.gs.washington.edu/EVS) has been queried for the c.-469C>T. Primers for Tetra-primer ARMS-PCR were: hp27delOUTR, 5′-agccgctctccaaacctt-3′; hp27delOUTF, 5′-caatggatctcctcctctgttt-3′; hp27delINF, 5′-cttcttcgtcagcctcccac-3′; hp27-469INR, 5′-tggcggtggaagggaggctgacgcaa-3′; hp27-469INF, 5′-gactcgccgtgtcaatcattttcgtc-3′.
Gene dosage alteration was assessed by the quantitative multiplex PCR of short fluorescent fragments (QMPSFs) and by long-range PCR (LR-PCR) as previously described [41] using the following primers: CLIF, 5′-tggtcagagagtggcctttctc-3′; CLIR, 5′-tgccgagtagaggcatttagtca-3′; CLIIF, 5′-tgtctgtgacgccgttgtct-3′; CLIIR, 5′-aagggttttctagcacacataggaa-3′; 1IF, 5′-gccgcaaccaatggatctc-3′; 1IR, 5′-acgagccccctttttttagtg-3′; 1IIF, 5′-ctctgaggacacgcatttggt-3′; 1IIR, 5′-aaatcagaatacgccgaaaagc-3′; 2F, 5′-tttcccctgcgcttagattc-3′; 2R, 5′-ccaccgagctgtttacgtttg-3′; 3IF, 5′-ccccatcaagtatttccaagct-3′; 3IR, 5′-gttattgtgttgttgtttttcagtgctta-3′; 3IIF, 5′-aacttccatagctattcattgagtcaaa-3′; 3IIR, 5′ - tgagcgatgtggctcggct -3′.
Sequence based-LOH analysis was performed on the pancreatic lesion by direct analysis of the CDKN1B mutation.
Cell culture, transfection, and protein extraction
The human cervical carcinoma HeLa, the rodent p27KIP1-negative GH-secreting pituitary adenoma GH3, the human embryonic kidney HEK293 and the human neuroblastoma SH-SY5Y cell lines (American Type Culture Collection, Manassas, VA), were maintained at 37°C in a 5% CO2 in complete 10% FCS DMEM.
GH3 (1.5×105 cells/well), HeLa (1.0×105 cells/well), SH-SY5Y (1.30×105 cells/well) and HEK293 (2.5×105 cells/well) cells were plated 24 hours before transfection into 12-well plates. When necessary, 24 hours after seeding cells have been arrested in G1 phase by a 36-hour treatment with either 10 µM (GH3) or 20 µM (HeLa) lovastatin. In all cell lines but HEK293 (see below) transient transfection was performed by Superfect (Qiagen, Milan, Italy). 1.5 µg plasmid and a ratio µg DNA/µl Superfect of 1∶6 following manufacturer's protocol were used. The pRL-TK plasmid (Promega) encoding Renilla luciferase was cotransfected and used for normalization of transfection efficiency. After 3 hours, the medium was changed to DMEM with 2% FCS and incubated for further 24 hours. Cells were then harvested in passive lysis buffer (Promega) and the relative luciferase activity was measured using the Dual-Luciferase Assay System and a GloMax 20/20 luminometer (Promega) according to the manufacturer's instructions.
For expression experiments, HEK293 cells were seeded into 12-well plates, grew to 95% confluence and transfected with Lipofectamine 2000 (Invitrogen, Milan, Italy) following the manufacturer's protocol. Cells were harvested 24 hours post-transfection, lysed in RIPA Buffer supplemented with proteases inhibitors (MgCl2 10 mM, Pepstatin 1 µM, PMSF 1 mM, cOmplete 1X (Roche, Monza, Italy)). Samples were clarified by centrifugation at 13,000 rpm for 5 min at 4°C.
Western blotting
Concentrations of the HEK293 extracted proteins were determined using the Bio-Rad DC protein assay kit (Bio-Rad Italia, Milan, Italy) following the manufacturer's instructions. For each sample, 20 µg were resuspended in NuPAGE LDS sample buffer and NuPAGE sample reducing agent (Invitrogen), boiled for 10 min at 70°C and resolved by SDS-PAGE on 4–12% NuPAGE gels (Invitrogen) and Mes buffer (Invitrogen). Separated proteins were transferred onto nitrocellulose membrane by Trans-Blot Turbo transfer system (BioRad) that was blocked for 2 hours with 5% non-fat dry milk (BioRad). The membrane was incubated overnight at 4°C with anti-p27KIP1 monoclonal antibody (BD Bioscience Heidelberg, Germany) used at 1∶300. Expression was corrected for differences in protein loading by probing blots for 1 hour at RT with mouse anti-ß-actin antibody (clone AC-15 1∶5,000, Sigma-Aldrich, Milan, Italy). Blots were developed using Pierce ECL Substrate (Part No. 32106, Thermo Scientific, Rockford, IL USA) and exposed to CL-XPosure Film (Thermo Scientific).
RNA isolation from HEK293 cells, reverse transcription, and quantitative real-time PCR (qPCR)
For total RNA extraction, HEK293 cells were resuspended in TRIzol (Invitrogen) and processed according to the manufacturer's instructions. Plasmid DNA contamination was removed by DNase, treating total RNA twice with Turbo DNA free kit (Applied Biosystems, Milan, Italy). One µg of DNase-treated RNA was reverse-transcribed using M-MuLV Reverse Transcriptase RNase H - (F-572S, Finnzymes, Espoo, Finland). qPCR was done with Platinum SYBR Green qPCR SuperMix-UDG (Invitrogen) in an ABI PRISM 7900HT Sequence Detector (Applied Biosystems). A final concentration of 300 nM for both forward and reverse primers was used. Primers for qPCR were qLUCF, 5′-gcctgaagtctctgattaagt-3′; qLUCR, 5′-acacctgcgtcgaaga-3′; qrBActF, 5′-agattactgccctggctcct-3′; qrBActR, 5′-aacgcagctcagtaacagtccg -3′; qhGAPDHF, 5′ - ctctctgctcctcctgttcgac-3′; qhGAPDHR, 5′ - ctctctgctcctcctgttcgac-3′.
Threshold levels were set at the exponential phase of qPCR using Sequence Detection software, version 2.4 (Applied Biosystems). The amount of each target gene relative to the proper housekeeping gene (HK, rat β-actin or human GAPDH) was determined using a relative standard curve method and the results were expressed as a ratio of target gene/HK. A 38-cycle threshold was set, beyond which the gene was considered undetectable.
Mutation carrier's RNA, allele specific analysis, and rapid amplification of cDNA ends (5′RACE)
Total RNA from whole blood samples was obtained using Paxgene Blood RNA Kit (Qiagen) following manufacturer protocol, while RNA from paraffin-embedded pancreatic tumor tissue was extracted using a modified RNAzol method, as previously described [43]. RNA was reverse-transcribed as described above. Allele-specific analysis was evaluated by qPCR as described above using two different SYBR assays. A final concentration of 300 nM for both forward primers and 50 mM for the unique reverse primer was used (-456_-453del_wtF 5′ - cttcttcgtcagcctccctt-3′; -456_-453del_mutF 5′ - cttcttcgtcagcctcccac-3′; -456_-453del-R 5′-agccgctctccaaacctt-3′). Given the different efficiency that may characterize the two different assays, the value of each allele was referred to the genomic DNA expressed as ΔCq (Cq value obtained for mRNA minus Cq value for genomic DNA). The lack of a possible deletion/duplication of the corresponding genomic locus was proven by QMPSFs as described above.
5′RACE was performed using 5′RACE System 2.0 kit (Invitrogen) on whole blood derived total RNA following manufacturer's instructions. Briefly, first strand cDNA was synthesized from 2 µg of mRNA by SuperScriptII RNA polymerase reaction using the specific primers GSP1R (5′ - gttaactcttcgtggtcc -3′). After adding an oligo-dC tail to the cDNAs 3′-ends, a PCR reaction has been performed with GSP2R primer (5′-ttctcccgggtctgcacg-3′), coupled with an Abridged Anchor Primer (AAP). The resulting DNA fragments were eluted from agarose gel and analyzed by direct sequencing, as reported above.
Immunohistochemistry
Immunohistochemistry was performed on an automated immunostainer (Ventana Medical Systems, Frankfurt am Main, Germany), according to the manufacturer's protocols with minor modifications [44] using the monoclonal anti-p27KIP1 antibody cited above (1∶1,000). The monoclonal MIB5 antibody (1∶500, Dako, Hamburg, Germany) was used to detect the proliferation antigen Ki-67. Positive controls were used to confirm the adequacy of the staining.
Cloning and mutagenesis
PCR fragments were obtained by amplification of the mutation carrier with forward and reverse primers containing extra HindIII and NcoI sites, respectively (clonF, 5′ - catcataagcttccaccttaaggccgcgct -3′; clonR, 5′ - catcatccatggttctcccgggtctgcacg -3′). The PCR product was digested and inserted upstream the luciferase reporter gene into the pGL3 Control Vector (Promega). For expression studies the wild type and mutated 5′UTRs were subcloned into pcDNA3.1/p27HA (kind gift of Prof. Sylvain Meloche, Institute for Research in Immunology and Cancer, Université de Montréal, Canada). The c.-469C>T, c.-428A>T and c.-74insC modifications were introduced by QuikChange II XL kit (Stratagene, La Jolla, CA USA) following manufacturer's protocol.
Lymphoblastoid cell lines and polysomal RNA extraction
EBV-transformed lymphoblastoid cells were generated by infection of peripheral blood mononuclear cells from the c.-456_-453delCCTT mutation carrier with culture supernatant from the EBV-producing marmoset cell line B95.8 (American Type Culture Collection) and maintained in RPMI 1640 medium (Euroclone, Milano, Italy) supplemented with 10% FBS, 1 mM Na Pyruvate, 10 mM Hepes Buffer, 2 mM Ultraglutamine (Lonza BioWhittaker, Basel, Switzerland), 1% Antibiotic/antimycotic (Gibco, Invitrogen Corporation). Cyclosporin A (CsA, Sandoz Pharmaceuticals AG; Cham, Switzerland) was initially added to the cultures to inhibit T cell growth (final concentration, 0.7 µg/ml).
For polysomal RNA extraction lymphoblastoid cells (25×106) were incubated with 100 µg/ml cycloheximide for 4 minutes, washed once with phosphate buffer saline (PBS), resuspended in lysis buffer [10 mM NaCl, 10 mM MgCl2, 10 mM Tris–HCl, pH 7.5, 1% Triton X-100, 1% sodium deoxycholate, 100 µg/ml cycloheximide, 0.2 U/µl RNase inhibitor, 1 mM DTT] and transferred to a microcentrifuge tube. After 5 minutes incubation on ice, the extracts were centrifuged for 10 min at 12,000 g at 4°C. The supernatant was collected and stored at −80°C. The cytoplasmic lysates were fractionated by ultracentrifugation (Sorvall rotor, 100 min at 180,000 g) trough 15–50% linear sucrose gradient containing 30 mM Tris–HCl, pH 7.5, 100 mM NaCl, 10 mM MgCl2. Eleven fractions were collected monitoring the absorbance at 254 nm. The RNA in each fraction was isolated after proteinase K treatment, phenol–chloroform extraction and isopropanol precipitation. RNA was resuspended in 30 µl of water. For each fraction 1 µg RNA was reverse-transcribed and analyzed by qPCR using allele-specific assays as reported above.
Supporting Information
Zdroje
1. SherrCJ, RobertsJM (1999) CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev 13 : 1501–1512.
2. SherrCJ, RobertsJM (1995) Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev 9 : 1149–1163.
3. ReedSI, BaillyE, DulicV, HengstL, ResnitzkyD, et al. (1994) G1 control in mammalian cells. J Cell Sci Suppl 18 : 69–73.
4. ChuIM, HengstL, SlingerlandJM (2008) The Cdk inhibitor p27 in human cancer: prognostic potential and relevance to anticancer therapy. Nat Rev Cancer 8 : 253–267.
5. BorrielloA, CucciollaV, OlivaA, ZappiaV, Della RagioneF (2007) p27Kip1 metabolism: a fascinating labyrinth. Cell Cycle 6 : 1053–1061.
6. SlingerlandJ, PaganoM (2000) Regulation of the cdk inhibitor p27 and its deregulation in cancer. J Cell Physiol 183 : 10–17.
7. Le SageC, NagelR, AgamiR (2007) Diverse ways to control p27(Kip1) function: miRNAs come into play. Cell Cycle 6 : 2742–2749.
8. AgarwalSK, MateoCM, MarxSJ (2009) Rare germline mutations in cyclin-dependent kinase inhibitor genes in multiple endocrine neoplasia type 1 and related states. J Clin Endocrinol Metab 94 : 1826–1834.
9. MalangaD, De GisiS, RiccardiM, ScrimaM, De MarcoC, et al. (2012) Functional characterization of a rare germline mutation in the gene encoding the cyclin-dependent kinase inhibitor p27Kip1 (CDKN1B) in a Spanish patient with multiple endocrine neoplasia-like phenotype. Eur J Endocrinol 166 : 551–560.
10. KullmannM, GöpfertU, SieweB, HengstL (2002) ELAV/Hu proteins inhibit p27 translation via an IRES element in the p27 5′UTR. Genes Dev 16 : 3087–3099.
11. GöpfertU, KullmannM, HengstL (2003) Cell cycle-dependent translation of p27 involves a responsive element in its 5′-UTR that overlaps with a uORF. Hum Mol Genet 12 : 1767–1779.
12. MiskiminsWK, WangG, HawkinsonM, MiskiminsR (2001) Control of cyclin-dependent kinase inhibitor p27 expression by cap-independent translation. Mol Cell Biol 21 : 4960–4967.
13. YoonA, PengG, BrandenburgerY, ZolloO, XuW, et al. (2006) Impaired control of IRES-mediated translation in X-linked dyskeratosis congenita. Science 312 : 902–906.
14. PellegataNS, Quintanilla-MartinezL, SiggelkowH, SamsonE, BinkK, et al. (2006) Germ-line mutations in p27Kip1 cause a multiple endocrine neoplasia syndrome in rats and humans. Proc Natl Acad Sci USA 103 : 15558–15563.
15. FalchettiA, MariniF, LuziE, TonelliF, BrandiML (2008) Multiple endocrine neoplasms. Best Pract Res Clin Rheumatol 22 : 149–163.
16. GeorgitsiM, RaitilaA, KarhuA, van der LuijtRB, AalfsCM, et al. (2007) Germline CDKN1B/p27Kip1 mutation in multiple endocrine neoplasia. J Clin Endocrinol Metab 92 : 3321–3325.
17. MolatoreS, MarinoniI, LeeM, PulzE, AmbrosioMR, et al. (2010) A novel germline CDKN1B mutation causing multiple endocrine tumors: clinical, genetic and functional characterization. Hum Mutat 31: E1825–1835.
18. Costa-GudaJ, MarinoniI, MolatoreS, PellegataNS, ArnoldA (2011) Somatic mutation and germline sequence abnormalities in CDKN1B, encoding p27Kip1, in sporadic parathyroid adenomas. J Clin Endocrinol Metab 96: E701–706.
19. MorrisDR (2000) GeballeAP (2000) Upstream open reading frames as regulators of mRNA translation. Mol Cell Biol 20 : 8635–8642.
20. MatsuiM, YachieN, OkadaY, SaitoR, TomitaM (2007) Bioinformatic analysis of post-transcriptional regulation by uORF in human and mouse. FEBS Lett 581 : 4184–4188.
21. LuukkonenBG, TanW, SchwartzS (1995) Efficiency of reinitiation of translation on human immunodeficiency virus type 1 mRNAs is determined by the length of the upstream open reading frame and by intercistronic distance. J Virol 69 : 4086–4094.
22. KozakM (2001) Constraints on reinitiation of translation in mammals. Nucleic Acids Res 29 : 5226–5232.
23. LarreaMD, WanderSA, SlingerlandJM (2009) p27 as Jekyll and Hyde: regulation of cell cycle and cell motility. Cell Cycle 8 : 3455–3461.
24. FeroML, RandelE, GurleyKE, RobertsJM, KempCJ (1998) The murine gene p27Kip1 is haplo-insufficient for tumour suppression. Nature 396 : 177–180.
25. IgrejaS, ChahalHS, AkkerSA, GueorguievM, PopovicV, et al. (2009) Assessment of p27 (cyclin-dependent kinase inhibitor 1B) and aryl hydrocarbon receptor-interacting protein (AIP) genes in multiple endocrine neoplasia (MEN1) syndrome patients without any detectable MEN1 gene mutations. Clin Endocrinol (Oxf) 70 : 259–264.
26. CalvoSE, PagliariniDJ, MoothaVK (2009) Upstream open reading frames cause widespread reduction of protein expression and are polymorphic among humans. Proc Natl Acad Sci USA 106 : 7507–7512.
27. CroweML, WangXQ, RothnagelJA (2006) Evidence for conservation and selection of upstream open reading frames suggests probable encoding of bioactive peptides. BMC Genomics 26 : 7–16.
28. JohannesG, SarnowP (1998) Cap-independent polysomal association of natural mRNAs encoding c-myc, BiP, and eIF4G conferred by internal ribosome entry sites. RNA 4 : 1500–1513.
29. OzawaA, AgarwalSK, MateoCM, BurnsAL, RiceTS, et al. (2007) The parathyroid/pituitary variant of multiple endocrine neoplasia type 1 usually has causes other than p27Kip1 mutations. J Clin Endocrinol Metab 92 : 1948–1951.
30. ScheperGC, van der KnaapMS, ProudCG (2007) Translation matters: protein synthesis defects in inherited disease. Nat Rev Genet 8 : 711–723.
31. WiestnerA, SchlemperRJ, van der MaasAP, SkodaRC (1998) An activating splice donor mutation in the thrombopoietin gene causes hereditary thrombocythaemia. Nat Genet 18 : 49–52.
32. LiuL, DilworthD, GaoL, MonzonJ, SummersA, et al. (1999) Mutation of the CDKN2A 5′ UTR creates an aberrant initiation codon and predisposes to melanoma. Nat Genet 21 : 128–132.
33. WenY, LiuY, XuY, ZhaoY, HuaR, et al. (2009) Loss-of-function mutations of an inhibitory upstream ORF in the human hairless transcript cause Marie Unna hereditary hypotrichosis. Nat Genet 41 : 228–233.
34. BeffagnaG, OcchiG, NavaA, VitielloL, DitadiA, et al. (2005) Regulatory mutations in transforming growth factor-beta 3 gene cause arrhythmogenic right ventricular cardiomyopathy type 1. Cardiovasc Res 65 : 366–373.
35. ZhouW (2006) SongW (2006) Leaky scanning and reinitiation regulate BACE1 gene expression. Mol Cell Biol 26 : 3353–3364.
36. SpevakCC, ParkEH, GeballeAP, PelletierJ, SachsMS (2006) her-2 upstream open reading frame effects on the use of downstream initiation codons. Biochem Biophys Res Commun 350 : 834–841.
37. WethmarK, SminkJJ, LeutzA (2010) Upstream open reading frames: molecular switches in (patho)physiology. Bioessays 32 : 885–893.
38. KozakM (1991) An analysis of vertebrate mRNA sequences: intimations of translational control. J Cell Biol 115 : 887–903.
39. MehtaA, TrottaCR, PeltzSW (2006) Derepression of the Her-2 uORF is mediated by a novel post-transcriptional control mechanism in cancer cells. Genes Dev 20 : 939–953.
40. ThakkerRV, NeweyPJ, WallsGV, BilezikianJ, DralleH, et al. (2012) Clinical Practice Guidelines for Multiple Endocrine Neoplasia Type 1 (MEN1). J Clin Endocrinol Metab 97 : 2990–3011.
41. OcchiG, TrivellinG, CeccatoF, De LazzariP, GiorgiG, et al. (2010) Prevalence of AIP mutations in a large series of sporadic Italian acromegalic patients and evaluation of CDKN1B status in acromegalic patients with multiple endocrine neoplasia. Eur J Endocrinol 163 : 369–376.
42. YeS, DhillonS, KeX, CollinsAR, DayIN (2001) An efficient procedure for genotyping single nucleotide polymorphisms. Nucleic Acids Res 29: E88–8.
43. ChomczynskiP, SacchiN (2006) The single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction: twenty-something years on. Nat Protoc 1 : 581–585.
44. Quintanilla-MartinezL, KremerM, SpechtK, Calzada-WackJ, NathrathM, et al. (2003) Analysis of signal transducer and activator of transcription 3 (Stat 3) pathway in multiple myeloma: Stat 3 activation and cyclin D1 dysregulation are mutually exclusive events. Am J Pathol 162 : 1449–1461.
Štítky
Genetika Reprodukční medicína
Článek Ubiquitous Polygenicity of Human Complex Traits: Genome-Wide Analysis of 49 Traits in KoreansČlánek Alternative Splicing and Subfunctionalization Generates Functional Diversity in Fungal ProteomesČlánek RFX Transcription Factor DAF-19 Regulates 5-HT and Innate Immune Responses to Pathogenic Bacteria inČlánek Surveillance-Activated Defenses Block the ROS–Induced Mitochondrial Unfolded Protein ResponseČlánek Deficiency Reduces Adipose OXPHOS Capacity and Triggers Inflammation and Insulin Resistance in Mice
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 3
-
Všechny články tohoto čísla
- Power and Predictive Accuracy of Polygenic Risk Scores
- Rare Copy Number Variants Are a Common Cause of Short Stature
- Coordination of Flower Maturation by a Regulatory Circuit of Three MicroRNAs
- Ubiquitous Polygenicity of Human Complex Traits: Genome-Wide Analysis of 49 Traits in Koreans
- Genomic Evidence for Island Population Conversion Resolves Conflicting Theories of Polar Bear Evolution
- Mechanistic Insight into the Pathology of Polyalanine Expansion Disorders Revealed by a Mouse Model for X Linked Hypopituitarism
- Genome-Wide Association Study and Gene Expression Analysis Identifies as a Predictor of Response to Etanercept Therapy in Rheumatoid Arthritis
- Problem Solved: An Interview with Sir Edwin Southern
- Long Interspersed Element–1 (LINE-1): Passenger or Driver in Human Neoplasms?
- Mouse HFM1/Mer3 Is Required for Crossover Formation and Complete Synapsis of Homologous Chromosomes during Meiosis
- Alternative Splicing and Subfunctionalization Generates Functional Diversity in Fungal Proteomes
- A WRKY Transcription Factor Recruits the SYG1-Like Protein SHB1 to Activate Gene Expression and Seed Cavity Enlargement
- Microhomology-Mediated Mechanisms Underlie Non-Recurrent Disease-Causing Microdeletions of the Gene or Its Regulatory Domain
- Ancient Evolutionary Trade-Offs between Yeast Ploidy States
- Differential Evolutionary Fate of an Ancestral Primate Endogenous Retrovirus Envelope Gene, the EnvV , Captured for a Function in Placentation
- A Feed-Forward Loop Coupling Extracellular BMP Transport and Morphogenesis in Wing
- The Tomato Yellow Leaf Curl Virus Resistance Genes and Are Allelic and Code for DFDGD-Class RNA–Dependent RNA Polymerases
- The U-Box E3 Ubiquitin Ligase TUD1 Functions with a Heterotrimeric G α Subunit to Regulate Brassinosteroid-Mediated Growth in Rice
- Role of the DSC1 Channel in Regulating Neuronal Excitability in : Extending Nervous System Stability under Stress
- –Independent Phenotypic Switching in and a Dual Role for Wor1 in Regulating Switching and Filamentation
- Pax6 Regulates Gene Expression in the Vertebrate Lens through miR-204
- Blood-Informative Transcripts Define Nine Common Axes of Peripheral Blood Gene Expression
- Genetic Architecture of Skin and Eye Color in an African-European Admixed Population
- Fine Characterisation of a Recombination Hotspot at the Locus and Resolution of the Paradoxical Excess of Duplications over Deletions in the General Population
- Estrogen Mediated-Activation of miR-191/425 Cluster Modulates Tumorigenicity of Breast Cancer Cells Depending on Estrogen Receptor Status
- Complex Patterns of Genomic Admixture within Southern Africa
- Yap- and Cdc42-Dependent Nephrogenesis and Morphogenesis during Mouse Kidney Development
- Molecular Networks of Human Muscle Adaptation to Exercise and Age
- Alp/Enigma Family Proteins Cooperate in Z-Disc Formation and Myofibril Assembly
- Polycomb Group Gene Regulates Rice () Seed Development and Grain Filling via a Mechanism Distinct from
- RFX Transcription Factor DAF-19 Regulates 5-HT and Innate Immune Responses to Pathogenic Bacteria in
- Distinct Molecular Strategies for Hox-Mediated Limb Suppression in : From Cooperativity to Dispensability/Antagonism in TALE Partnership
- A Natural Polymorphism in rDNA Replication Origins Links Origin Activation with Calorie Restriction and Lifespan
- TDP2–Dependent Non-Homologous End-Joining Protects against Topoisomerase II–Induced DNA Breaks and Genome Instability in Cells and
- Recurrent Rearrangement during Adaptive Evolution in an Interspecific Yeast Hybrid Suggests a Model for Rapid Introgression
- Genome-Wide Association Study in Mutation Carriers Identifies Novel Loci Associated with Breast and Ovarian Cancer Risk
- Coincident Resection at Both Ends of Random, γ–Induced Double-Strand Breaks Requires MRX (MRN), Sae2 (Ctp1), and Mre11-Nuclease
- Identification of a -Specific Modifier Locus at 6p24 Related to Breast Cancer Risk
- A Novel Function for the Hox Gene in the Male Accessory Gland Regulates the Long-Term Female Post-Mating Response in
- Tdp2: A Means to Fixing the Ends
- A Novel Role for the RNA–Binding Protein FXR1P in Myoblasts Cell-Cycle Progression by Modulating mRNA Stability
- Association Mapping and the Genomic Consequences of Selection in Sunflower
- Histone Deacetylase 2 (HDAC2) Regulates Chromosome Segregation and Kinetochore Function via H4K16 Deacetylation during Oocyte Maturation in Mouse
- A Novel Mutation in the Upstream Open Reading Frame of the Gene Causes a MEN4 Phenotype
- Ataxin1L Is a Regulator of HSC Function Highlighting the Utility of Cross-Tissue Comparisons for Gene Discovery
- Human Spermatogenic Failure Purges Deleterious Mutation Load from the Autosomes and Both Sex Chromosomes, including the Gene
- A Conserved Upstream Motif Orchestrates Autonomous, Germline-Enriched Expression of piRNAs
- Statistical Analysis Reveals Co-Expression Patterns of Many Pairs of Genes in Yeast Are Jointly Regulated by Interacting Loci
- Matefin/SUN-1 Phosphorylation Is Part of a Surveillance Mechanism to Coordinate Chromosome Synapsis and Recombination with Meiotic Progression and Chromosome Movement
- A Role for the Malignant Brain Tumour (MBT) Domain Protein LIN-61 in DNA Double-Strand Break Repair by Homologous Recombination
- The Population and Evolutionary Dynamics of Phage and Bacteria with CRISPR–Mediated Immunity
- Long Noncoding RNA MALAT1 Controls Cell Cycle Progression by Regulating the Expression of Oncogenic Transcription Factor B-MYB
- Surveillance-Activated Defenses Block the ROS–Induced Mitochondrial Unfolded Protein Response
- DNA Topoisomerase III Localizes to Centromeres and Affects Centromeric CENP-A Levels in Fission Yeast
- Genome-Wide Control of RNA Polymerase II Activity by Cohesin
- Divergent Selection Drives Genetic Differentiation in an R2R3-MYB Transcription Factor That Contributes to Incipient Speciation in
- NODULE INCEPTION Directly Targets Subunit Genes to Regulate Essential Processes of Root Nodule Development in
- Spreading of a Prion Domain from Cell-to-Cell by Vesicular Transport in
- Deficiency in Origin Licensing Proteins Impairs Cilia Formation: Implications for the Aetiology of Meier-Gorlin Syndrome
- Deficiency Reduces Adipose OXPHOS Capacity and Triggers Inflammation and Insulin Resistance in Mice
- The Conserved SKN-1/Nrf2 Stress Response Pathway Regulates Synaptic Function in
- Functional Genomic Analysis of the Regulatory Network in
- Astakine 2—the Dark Knight Linking Melatonin to Circadian Regulation in Crustaceans
- CRL2 E3-Ligase Regulates Proliferation and Progression through Meiosis in the Germline
- Both the Caspase CSP-1 and a Caspase-Independent Pathway Promote Programmed Cell Death in Parallel to the Canonical Pathway for Apoptosis in
- PRMT4 Is a Novel Coactivator of c-Myb-Dependent Transcription in Haematopoietic Cell Lines
- A Copy Number Variant at the Locus Likely Confers Risk for Canine Squamous Cell Carcinoma of the Digit
- Evidence of Gene–Environment Interactions between Common Breast Cancer Susceptibility Loci and Established Environmental Risk Factors
- HIV Infection Disrupts the Sympatric Host–Pathogen Relationship in Human Tuberculosis
- Trans-Ethnic Fine-Mapping of Lipid Loci Identifies Population-Specific Signals and Allelic Heterogeneity That Increases the Trait Variance Explained
- A Gene Transfer Agent and a Dynamic Repertoire of Secretion Systems Hold the Keys to the Explosive Radiation of the Emerging Pathogen
- The Role of ATM in the Deficiency in Nonhomologous End-Joining near Telomeres in a Human Cancer Cell Line
- Dynamic Circadian Protein–Protein Interaction Networks Predict Temporal Organization of Cellular Functions
- Nuclear Myosin 1c Facilitates the Chromatin Modifications Required to Activate rRNA Gene Transcription and Cell Cycle Progression
- Robust Prediction of Expression Differences among Human Individuals Using Only Genotype Information
- A Single Cohesin Complex Performs Mitotic and Meiotic Functions in the Protist
- The Role of the Arabidopsis Exosome in siRNA–Independent Silencing of Heterochromatic Loci
- Elevated Expression of the Integrin-Associated Protein PINCH Suppresses the Defects of Muscle Hypercontraction Mutants
- Twist1 Controls a Cell-Specification Switch Governing Cell Fate Decisions within the Cardiac Neural Crest
- Genome-Wide Testing of Putative Functional Exonic Variants in Relationship with Breast and Prostate Cancer Risk in a Multiethnic Population
- Heteroduplex DNA Position Defines the Roles of the Sgs1, Srs2, and Mph1 Helicases in Promoting Distinct Recombination Outcomes
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Fine Characterisation of a Recombination Hotspot at the Locus and Resolution of the Paradoxical Excess of Duplications over Deletions in the General Population
- Molecular Networks of Human Muscle Adaptation to Exercise and Age
- Recurrent Rearrangement during Adaptive Evolution in an Interspecific Yeast Hybrid Suggests a Model for Rapid Introgression
- Genome-Wide Association Study and Gene Expression Analysis Identifies as a Predictor of Response to Etanercept Therapy in Rheumatoid Arthritis
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



