-
Články
Reklama
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
ReklamaHIV Infection Disrupts the Sympatric Host–Pathogen Relationship in Human Tuberculosis
The phylogeographic population structure of Mycobacterium tuberculosis suggests local adaptation to sympatric human populations. We hypothesized that HIV infection, which induces immunodeficiency, will alter the sympatric relationship between M. tuberculosis and its human host. To test this hypothesis, we performed a nine-year nation-wide molecular-epidemiological study of HIV–infected and HIV–negative patients with tuberculosis (TB) between 2000 and 2008 in Switzerland. We analyzed 518 TB patients of whom 112 (21.6%) were HIV–infected and 233 (45.0%) were born in Europe. We found that among European-born TB patients, recent transmission was more likely to occur in sympatric compared to allopatric host–pathogen combinations (adjusted odds ratio [OR] 7.5, 95% confidence interval [95% CI] 1.21–infinity, p = 0.03). HIV infection was significantly associated with TB caused by an allopatric (as opposed to sympatric) M. tuberculosis lineage (OR 7.0, 95% CI 2.5–19.1, p<0.0001). This association remained when adjusting for frequent travelling, contact with foreigners, age, sex, and country of birth (adjusted OR 5.6, 95% CI 1.5–20.8, p = 0.01). Moreover, it became stronger with greater immunosuppression as defined by CD4 T-cell depletion and was not the result of increased social mixing in HIV–infected patients. Our observation was replicated in a second independent panel of 440 M. tuberculosis strains collected during a population-based study in the Canton of Bern between 1991 and 2011. In summary, these findings support a model for TB in which the stable relationship between the human host and its locally adapted M. tuberculosis is disrupted by HIV infection.
Published in the journal: . PLoS Genet 9(3): e32767. doi:10.1371/journal.pgen.1003318
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003318Summary
The phylogeographic population structure of Mycobacterium tuberculosis suggests local adaptation to sympatric human populations. We hypothesized that HIV infection, which induces immunodeficiency, will alter the sympatric relationship between M. tuberculosis and its human host. To test this hypothesis, we performed a nine-year nation-wide molecular-epidemiological study of HIV–infected and HIV–negative patients with tuberculosis (TB) between 2000 and 2008 in Switzerland. We analyzed 518 TB patients of whom 112 (21.6%) were HIV–infected and 233 (45.0%) were born in Europe. We found that among European-born TB patients, recent transmission was more likely to occur in sympatric compared to allopatric host–pathogen combinations (adjusted odds ratio [OR] 7.5, 95% confidence interval [95% CI] 1.21–infinity, p = 0.03). HIV infection was significantly associated with TB caused by an allopatric (as opposed to sympatric) M. tuberculosis lineage (OR 7.0, 95% CI 2.5–19.1, p<0.0001). This association remained when adjusting for frequent travelling, contact with foreigners, age, sex, and country of birth (adjusted OR 5.6, 95% CI 1.5–20.8, p = 0.01). Moreover, it became stronger with greater immunosuppression as defined by CD4 T-cell depletion and was not the result of increased social mixing in HIV–infected patients. Our observation was replicated in a second independent panel of 440 M. tuberculosis strains collected during a population-based study in the Canton of Bern between 1991 and 2011. In summary, these findings support a model for TB in which the stable relationship between the human host and its locally adapted M. tuberculosis is disrupted by HIV infection.
Introduction
Host–pathogen co-evolution plays an important role in the biology of infectious diseases [1]. Coevolution between interacting host and pathogen species is difficult to demonstrate formally, but indirect evidence can be obtained by studying geographical patterns, which can indicate local adaptation of particular pathogen variants to geographically matched host variants [2]–[4]. Local adaptation is often studied using so-called reciprocal transplant experiments, in which the fitness of locally adapted (sympatric) pathogen variants is compared to the performance of allopatric pathogen variants [2]. Studies in several invertebrate systems have shown that sympatric pathogens (infection with a phylogeographically concordant strain) tend to outperform allopatric pathogens (infection with a phylogeographically discordant strain) in the corresponding host variants [1], [5]–[7].
Mycobacterium tuberculosis, the agent causing human tuberculosis (TB) is an obligate human pathogen, which has been affecting humankind for millennia [8]–[13]. Contrary to previous beliefs linking the origin of TB to animal domestication ∼10,000 years ago [14], more recent data suggest that M. tuberculosis evolved as a human pathogen in Africa, and might have co-existed with anatomically modern humans since their origins ∼200,000 years ago [8], [10], [12], [13], [15]. Analyses of multiple global strain collections have shown that M. tuberculosis exhibits a phylogeographic population structure consisting of six main phylogenetic lineages associated with different geographic regions and sympatric human populations [9], [11]–[13], [16]–[20]. Studies in San Francisco, London, and Montreal have shown that these sympatric host–pathogen associations persist in cosmopolitan settings, even under a presumed degree of host and pathogen intermingling [11], [18], [19]. Moreover, transmission of M. tuberculosis has been shown to occur more frequently in sympatric host–pathogen combinations compared to allopatric host–pathogen combinations [9]. Taken together, these observations are consistent with the notion that the different phylogeographic lineages of M. tuberculosis have adapted to specific sympatric human populations [21].
Based on the assumption that M. tuberculosis has been coevolving with humans, and that M. tuberculosis has locally adapted to sympatric human populations [9], we hypothesized that HIV co-infection will alter this relationship [22]. Specifically, we postulated that because HIV induces immune suppression in humans, and because variation in host immunity will likely play a role in local adaptation, M. tuberculosis strains will cause disease in HIV–infected patients, irrespective of their usual sympatric host–pathogen relationship. To test this hypothesis, we performed a population-based molecular-epidemiological study of HIV–infected and HIV–negative TB patients in Switzerland between 2000 and 2008, a country with a long history of immigration [23].
Results
Patient characteristics and phylogeographic distribution of M. tuberculosis lineages
A total of 518 patients were included in the study, of whom 112 (21.6%) were HIV–infected. Of these 518 patients, 233 (45.0%) were born in Europe (117 in Switzerland), 131 (25.3%) were born in sub-Saharan Africa, 48 (9.3%) in South-East Asia, 36 (7.0%) in the Indian subcontinent, and 24 (4.6%) in Central and South America. Similar to previous studies [9], [18], [19], we found an association between the patient's place of birth and the particular M. tuberculosis lineages (Figure 1). Lineage 4 (Euro-American lineage) was present in all regions, but particularly common in patients born in Europe and South America. Lineages 5 and 6 (West-African lineages also known as M. africanum [24]) were exclusively found in patients originating from West Africa, whereas Lineage 2 (which includes Beijing) and Lineage 1 were mainly seen in patients originating from the Western Pacific and East Asian regions. Patient characteristics are summarized in Table 1.
Fig. 1. Phylogeography of the six main Mycobacterium tuberculosis lineages. 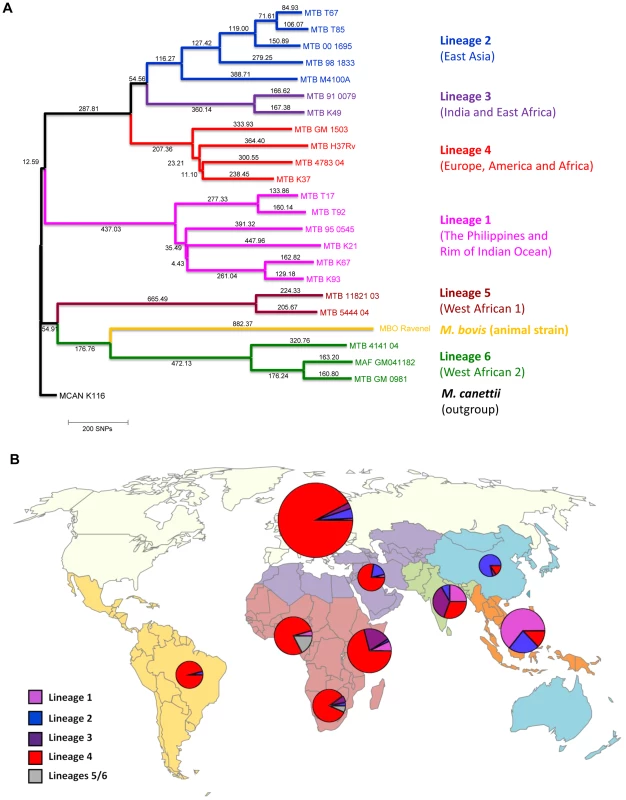
A: Phylogenetic tree of the main M. tuberculosis lineages described in our study based on the neighbor-joining phylogeny across 23 M. tuberculosis complex whole-genome sequences (from Ref. [72]). Numbers on branches refer to the corresponding number of single nucleotide polymorphisms inferred. B: Distribution of the main phylogenetic M. tuberculosis lineages among Swiss tuberculosis cases included in the study (n = 518), by geographic origin of the patients. In (A) Mycobacterium canettii was used as the outgroup. SNPs, single nucleotide polymorphisms. In (B) the sizes of the pie charts correspond to the number of patients included in the study: European region (233 patients), Middle-East/North Africa (27), Indian subcontinent (36), Western Pacific (19), Central/South America (24), South-East Asia (48), and Eastern (55), Western (46) and Southern region (30) of sub-Saharan Africa. Lineage 1: Indo-Oceanic lineage; Lineage 2: East-Asian lineage (includes Beijing strains); Lineage 3: Delhi/CAS; Lineage 4: Euro-American lineage; Lineages 5 and 6: West African lineages. Tab. 1. Patient characteristics of tuberculosis (TB) patients born in Europe, by presence of allopatric and sympatric Mycobacterium tuberculosis strains. 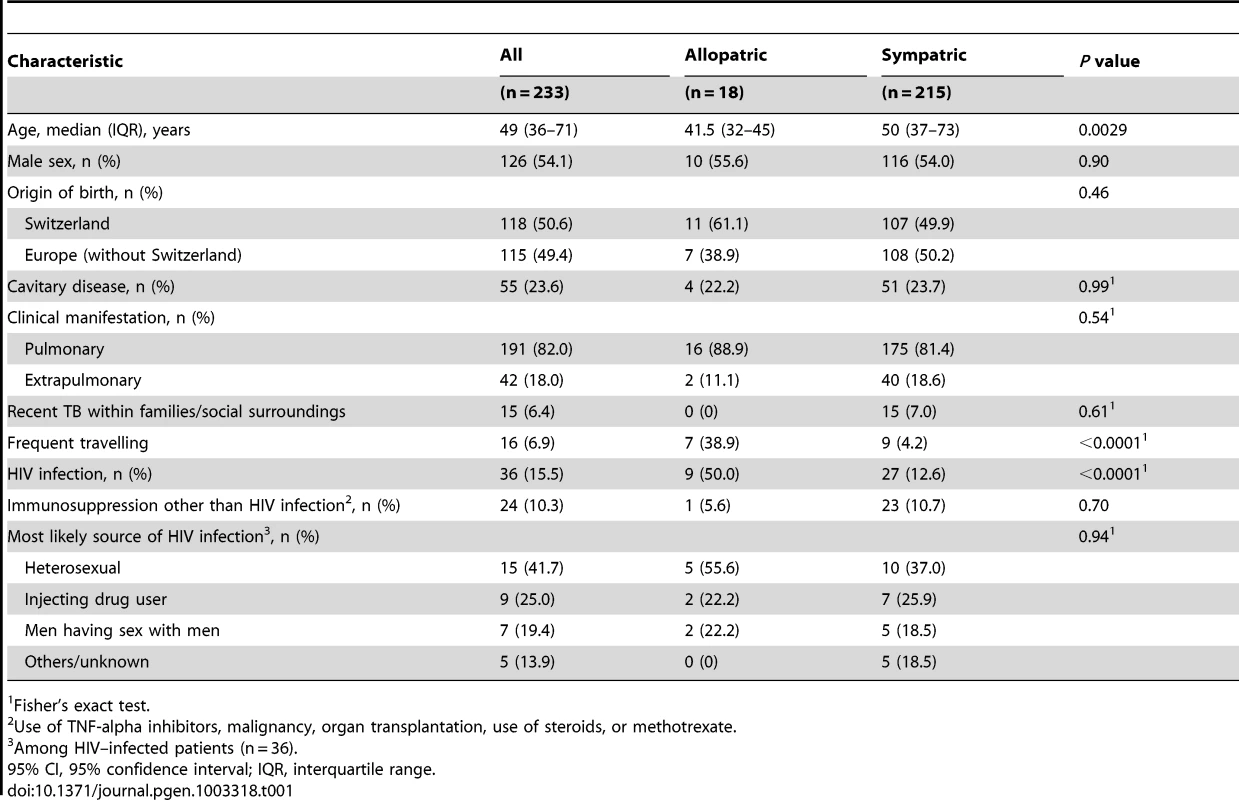
Fisher's exact test. Because in European-born patients the host–pathogen combinations defined as sympatric (i.e. Lineage 4/Euro-American lineage in European-born patients) or allopatric (i.e. all other lineages in European-born patients) have been well established [9], [18], [19], [25], we focused on this patient group (n = 233) for the remaining of our analyses.
M. tuberculosis transmission occurs primarily in sympatric host–pathogen combinations
M. tuberculosis transmission was more likely among patients in a sympatric host–pathogen relationship compared to patients in an allopatric host–pathogen relationship (adjusted odds ratio [OR] 7.5, 95% confidence interval [95% CI] 1.2-infinity, p = 0.03, Table 2). Of note, there was no molecular clustering among European-born TB patients infected with an allopatric M. tuberculosis strain. Moreover, we found that only the sympatric Lineage 4 (Euro-American lineage) was detected in European-born clusters as well as in mixed clusters (Table S1), suggesting that sympatric host–pathogen combinations in TB favor transmission.
Tab. 2. Recent transmission of Mycobacterium tuberculosis among tuberculosis (TB) cases born in the European region, according to sympatric and allopatric lineages. 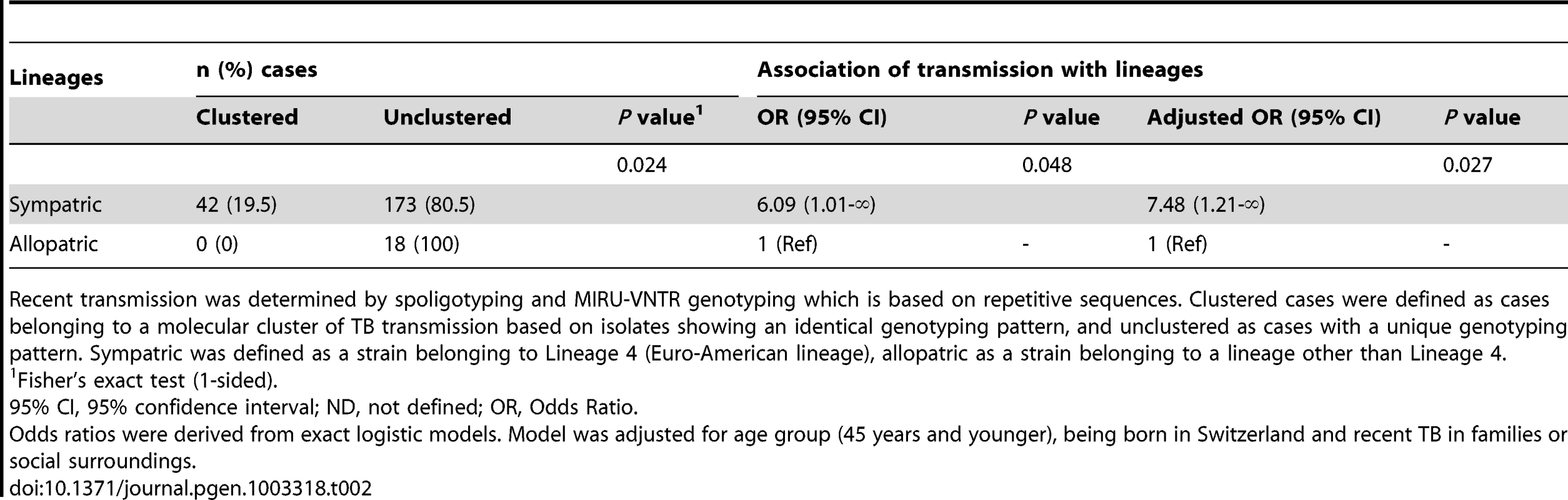
Recent transmission was determined by spoligotyping and MIRU-VNTR genotyping which is based on repetitive sequences. Clustered cases were defined as cases belonging to a molecular cluster of TB transmission based on isolates showing an identical genotyping pattern, and unclustered as cases with a unique genotyping pattern. Sympatric was defined as a strain belonging to Lineage 4 (Euro-American lineage), allopatric as a strain belonging to a lineage other than Lineage 4. Impact of HIV infection on the sympatric host–pathogen combination of M. tuberculosis among Europeans
Overall, we found that HIV infection was strongly associated with allopatric M. tuberculosis lineages among European-born TB patients (unadjusted OR 7.0, 95% CI 2.5–19.1, p<0.0001; Table 3). Among the allopatric lineages, we found that Lineages 1, 2 and 3 were more likely to be found in HIV–infected compared to HIV–negative patients when taking the sympatric Lineage 4 (Euro-American lineage) as the reference (Table S2). When investigating the ancestry of the nine HIV–infected patients with an allopatric M. tuberculosis strain, seven patients were confirmed to be of Swiss ancestry over the last three generations, and two patients had Swiss and Italian ancestors in the previous generations (Italian father in the previous generation, or emigrating from Italy in the previous generation).
Tab. 3. Unadjusted and adjusted associations between HIV infection and tuberculosis (TB) with an allopatric Mycobacterium tuberculosis strain among European patients (n = 233), in the context of other potential factors influencing the risk for an allopatric TB. 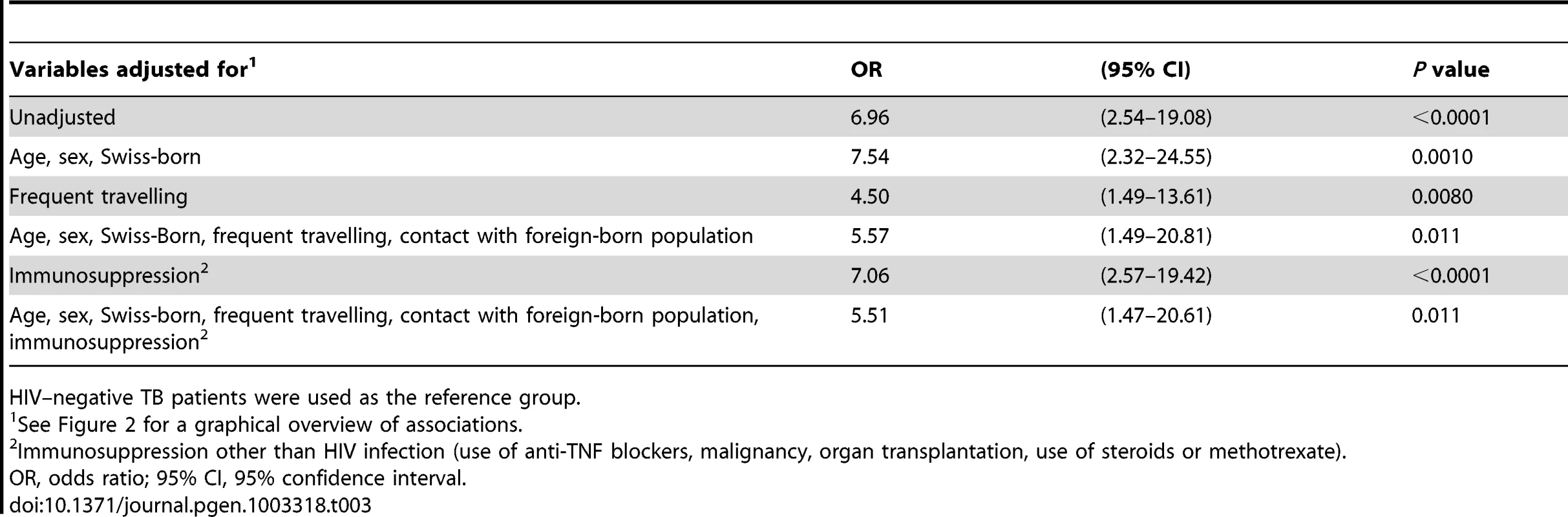
HIV–negative TB patients were used as the reference group. Several factors could contribute to the association between HIV infection and allopatric lineages. We found that patients with an allopatric M. tuberculosis lineage were younger (median age 41.5 versus 50 years), and had more often a history of frequent travelling (38.9% versus 4.2%, p<0.0001). Therefore, we developed a model (Figure 2) to take these and other putative confounding variables into account. These variables included age, sex, country of birth, frequent travelling, contact with the foreign-born population, and non–HIV associated immunosuppression. We considered “TB with an allopatric strain” as the outcome because disease is the only measurable outcome with a sympatric or allopatric M. tuberculosis strain (only diseased individuals can yield a positive mycobacterial culture). Our multivariate analyses revealed that the association between HIV infection and allopatry remained statistically significant after adjustment for all social and patient factors included in our model (OR 5.5, 95% CI 1.5–20.6, p = 0.01, Table 3). Age, sex, being Swiss-born, and non–HIV associated immunosuppression had only a minor effect on the association between HIV infection and TB with an allopatric strain (Table 3). In contrast, a history of repeated travelling to low-income countries had a stronger effect, decreasing the OR to 4.50 (95% CI 1.5–13.6, p = 0.008, Table 3) when adjusting for this variable.
Fig. 2. Graphical model showing direct and indirect potential effects of HIV infection on tuberculosis (TB) caused by an allopatric <i>Mycobacterium tuberculosis</i> strain, in the context of other potential factors influencing this association. 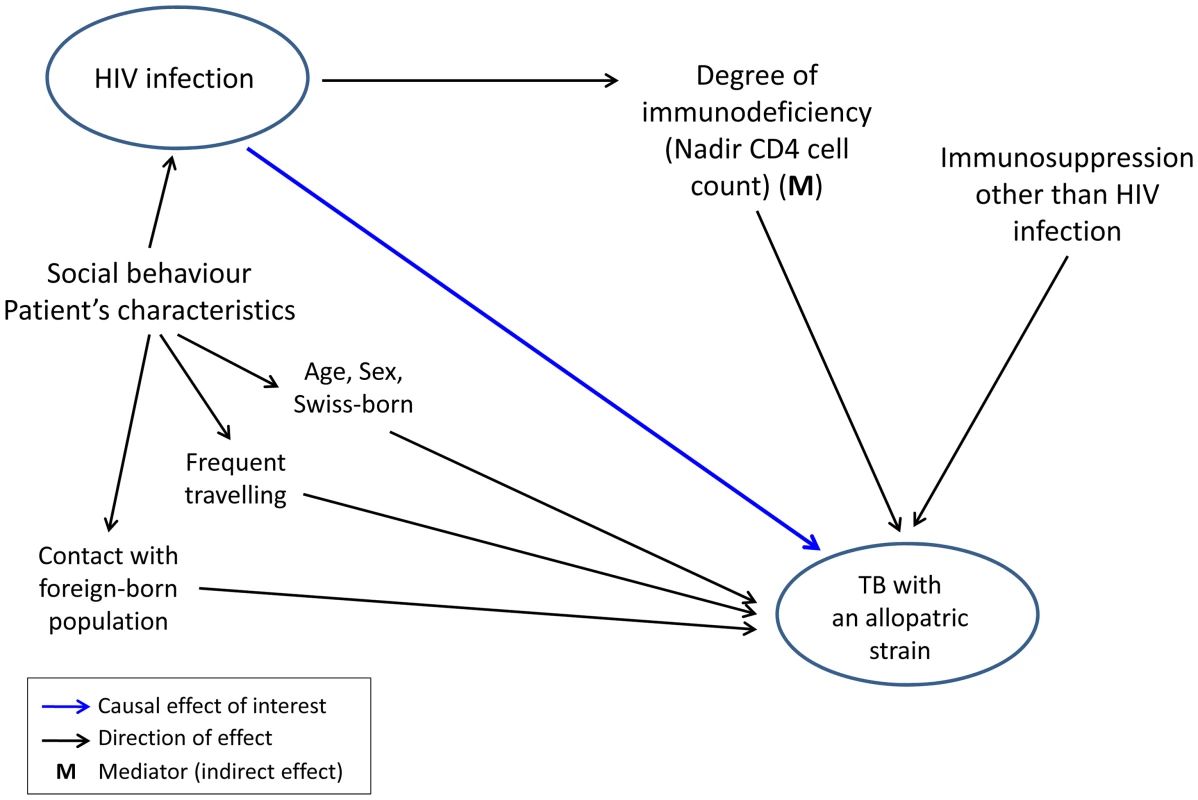
Impact of HIV infection on the sympatric host–pathogen association is a function of the degree of HIV–induced immunosuppression
We also tested if the degree of immunodeficiency as measured by the nadir CD4 T cell count (defined as the lowest CD4 T cell count ever measured in a patient) would have an impact on the association between host population and M. tuberculosis lineage. Among Europeans, the strength of the association between HIV infection and allopatric lineages increased with a decreasing nadir CD4 T cell count in a dose-dependent manner: from an OR of 4.6 (95% CI 0.9–24.7) in patients with a nadir CD4 T cell count of >200 cells/µL to an OR of 12.5 (95% CI 2.6–60.8) in patients with nadir CD4 T cell counts <50 cells/µL (test for trend p<0.0001; HIV–negative patients as reference). This trend remained statistically significant when adjusting for age, sex, being born in Switzerland, frequent travelling, contact with the foreign-born population, and non–HIV associated immunosuppression (Table 4).
Tab. 4. Association between the degree of immunodeficiency and tuberculosis with an allopatric Mycobacterium tuberculosis strain among European patients (n = 233). 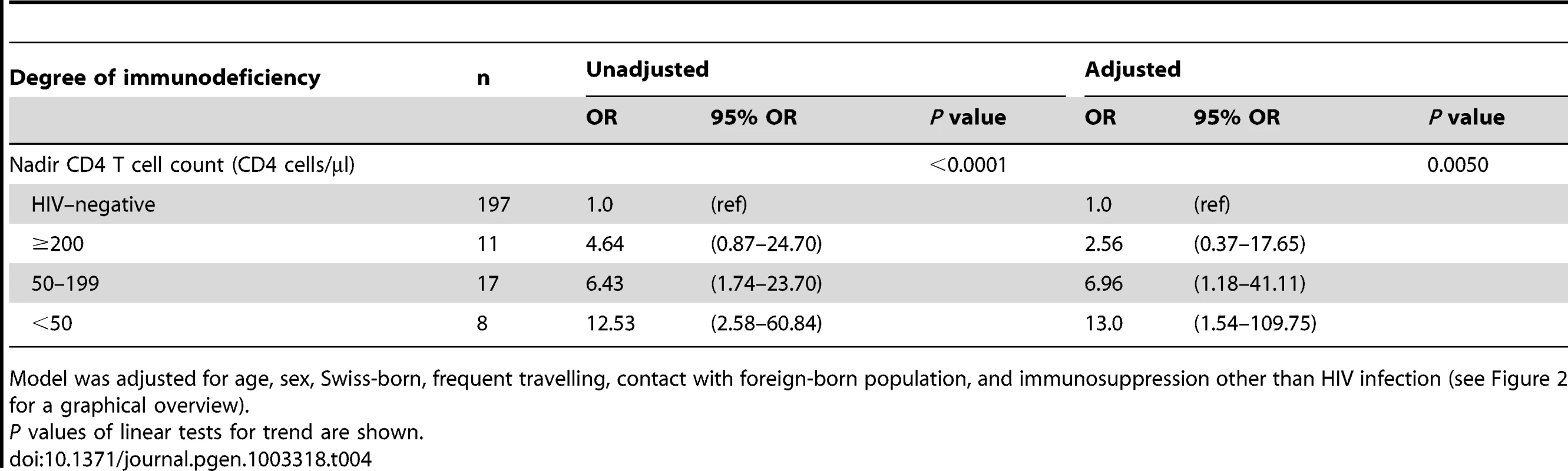
Model was adjusted for age, sex, Swiss-born, frequent travelling, contact with foreign-born population, and immunosuppression other than HIV infection (see Figure 2 for a graphical overview). Impact of social mixing on the sympatric host–pathogen association
Increased contact with foreigners originating from high TB burden countries, who have a higher risk of TB [26] and are more likely to have TB caused by an allopatric M. tuberculosis strain, could also lead to an allopatric host–pathogen relationship in European-born patients. However, the association between HIV and allopatry remained statistically significant when adjusting for this variable (Table 3). Furthermore, we examined molecular clusters defined by standard bacterial genotyping [27], [28], to test the hypothesis that HIV–infected patients were more frequently seen among ethnically mixed clusters where transmission occurred between non-European and European-born cases [29]. We found that the prevalence of HIV infection was similar among TB cases in mixed clusters (5 HIV–infected cases out of 26 cases, 19.2%) and among cases in clusters involving only European-born cases (4 out of 26 cases, 15.4%, see Table S1).
Sensitivity analyses
When restricting the main analysis (n = 233) to European-born patients without a history of frequent travelling, we found that the association between HIV infection and allopatric TB remained statistically significant (adjusted OR 6.96, 95% CI 1.25–38.88, p = 0.027). Furthermore, we explored associations of socio-demographic and clinical factors with TB with an allopatric M. tuberculosis strain in a model focusing on HIV–infected European patients only (Figure S1, Table S3): frequent travelling was confirmed to be an important factor, and patients with a low nadir CD4 T cell count tended to be associated with an allopatric TB although the associations did not reach statistical significance (Table S3). Finally, we obtained very similar results for the association between HIV infection and allopatric TB (Table S4), and for the association between the degree of immunodeficiency and allopatric TB (Table S5) when repeating analyses using a Bayesian approach [30], which is more robust when numbers are small.
Other supporting information
The birth origin of HIV–infected and non-infected patients is shown on a map in Figure S2. The main phylogenetic M. tuberculosis lineages stratified by place of birth and HIV status are presented in Table S6.
Replication in a second panel of M. tuberculosis strains
To replicate our main finding, we investigated a second panel of M. tuberculosis strains from an ongoing population-based TB study in the Canton of Bern, Switzerland, between 1991 and 2011. Of the 1,642 M. tuberculosis isolates analyzed, 1,350 (82.2%) belonged to Lineage 4 (Euro-American lineage), and 292 (17.8%) to non-Euro-American lineages (Lineages 1, 2, 3, 5 or 6). We compared all 40 European-born patients with an allopatric strain (non-Lineage 4) with 400 randomly selected European-born patients with a sympatric strain (Lineage 4). We found that the proportion of HIV infection was 4.5 (95% CI 1.6–11.9) times higher in patients with an allopatric strain compared to patients with a sympatric strain (12.5% versus 2.8%, p = 0.010, Table 5).
Tab. 5. HIV status in tuberculosis (TB) patients with an allopatric compared to patients with a sympatric Mycobacterium tuberculosis strain among European patients in a second panel. 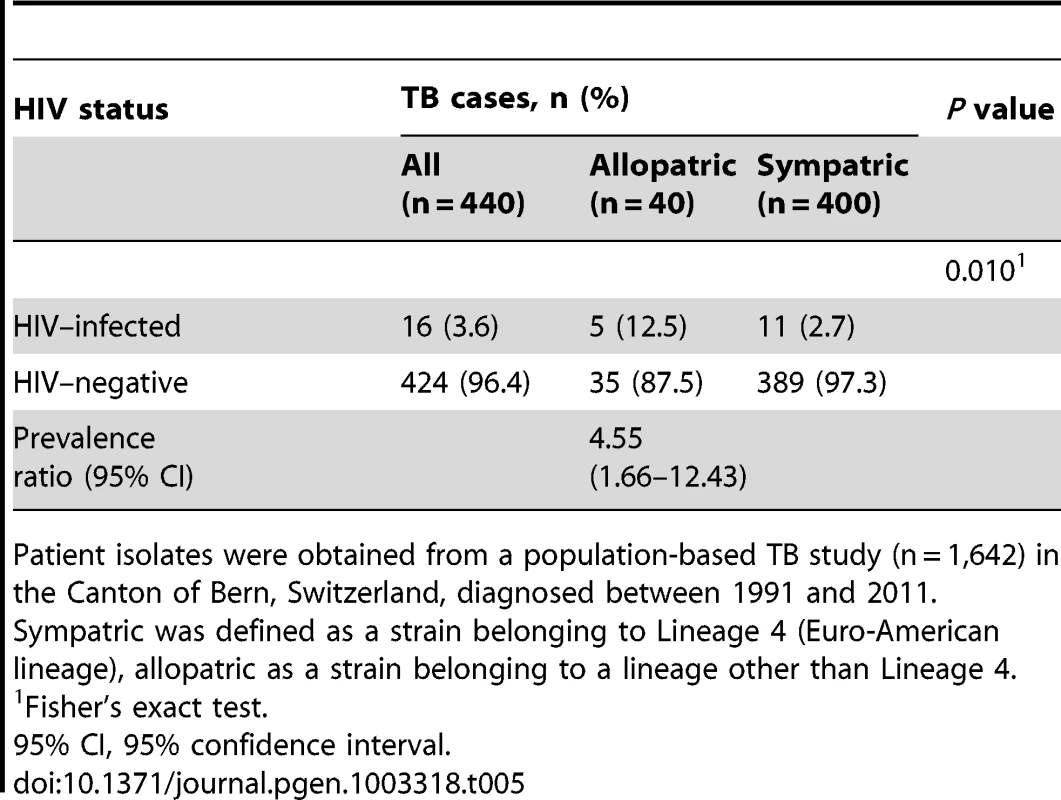
Patient isolates were obtained from a population-based TB study (n = 1,642) in the Canton of Bern, Switzerland, diagnosed between 1991 and 2011. Discussion
The phylogeographic distribution of M. tuberculosis lineages observed here suggests local adaptation to sympatric human populations. In contrast, we found that allopatric host–pathogen relationships in European-born TB patients were strongly associated with HIV co-infection. The association with HIV infection became stronger in a ‘dose-dependent’ manner in patients with a history of more pronounced immunodeficiency, and was not explained only by frequent travelling to high TB-incidence countries or increased social mixing with the foreign-born population. The association of M. tuberculosis lineages with sympatric patient populations reported here is in agreement with previous findings [9], [11]–[13], [16]–[19]. Similarly, our finding that recent TB transmission was more likely to occur in sympatric compared to allopatric host–pathogen combinations supports previous work [9]. Taken together, these data are consistent with local adaptation of M. tuberculosis to different human populations, which in turn can be viewed as indirect evidence for coevolution between M. tuberculosis and its human host [1]–[4], [9]–[13].
We found that TB allopatric host–pathogen combinations were strongly associated with HIV infection in a nation-wide study and a second panel of strains from one Canton of Switzerland. This supports the notion that M. tuberculosis lineages have evolved subtle differences in their interaction with different human immune systems. However, in the presence of HIV–induced immunodeficiency, any M. tuberculosis lineage seems to cause disease in a given human host. M. tuberculosis is an obligate human pathogen which lives in constant interaction with the host immune system [31]. Human populations, however, are known to differ genetically and immunologically [15]. The clinical disease reflects host-dependent immune-pathological processes [31]. In other words, while initially triggered by the pathogen, it is the host immune response which is ultimately responsible for the chronic inflammation and associated tissue destructions. These processes contribute to the successful transmission of M. tuberculosis [22], [32]. On the other hand, only 5–10% of the 2 billion individuals estimated to be latently infected with M. tuberculosis globally will develop active TB during their lifetime [33]–[35]. Hence most of the time, humans are able to control the infection. In our study, we chose culture-confirmed TB cases as the main endpoint which reflects successful transmission and progression from infection to active disease.
Our study on the association between allopatric TB and HIV was able to control for important cofactors [36], [37]. These cofactors included frequent travelling abroad and increased contact to foreign-born populations. A particularly important cofactor for allopatric TB was frequent travelling to high TB burden countries with potential exposure to foreign M. tuberculosis strains; HIV–infected individuals may be at a higher risk for travel-related infectious diseases [38]. However, the association between HIV infection and allopatric TB remained even when adjusting for these behavioral and other patient characteristics. A previous study reporting on allopatric TB and HIV was not able to control for these factors [9]. Furthermore, we found no evidence for increased social mixing among HIV–infected individuals, which argues against mere social factors leading to the association between allopatric TB and HIV.
A biological basis for this association is further supported by the striking dose-dependency we observed with increasing immunosuppression as defined by lower nadir CD4 T cell counts. Of note, this trend was also independent of other variables. Low nadir CD4 T cell counts are associated with incomplete immune recovery after starting combination antiretroviral therapy [39], [40] and impaired functional immune restoration despite normalization of CD4 T cells [41]. More generally, infection with HIV and M. tuberculosis interferes with the immune system in many ways [42], [43]. HIV infection disrupts the function of M. tuberculosis-infected macrophages [44], [45], but also seems to reduce the number and functionality of M. tuberculosis-specific T cells over time [46]. On the other hand, M. tuberculosis strains have been shown to induce variable immune responses [47]. Based on these observations, it is reasonable to hypothesize that HIV/TB co-infection might impact immune cell functions, intracellular signaling and immune regulation, perhaps leading to an immune response less capable of discriminating between M. tuberculosis variants.
Besides M. tuberculosis, several other human pathogenic bacteria exhibit phylogeographic population structures, possibly reflecting local adaptation to different human populations. These include Haemophilus influenzae [48], Streptococcus mutans [49], M. leprae [50] and Helicobacter pylori [51], [52]. Interestingly, like M. tuberculosis, all of these microbes are obligate human pathogens. In the case of H. pylori, functional studies have shown that strains associated with South America have adapted their adhesins to the human blood group O, which is particularly frequent in native populations of this region [53]. Similarly, a study of the bacterial genome evolution of an asymptomatic Escherichia coli bacteriuria strain showed adaptation at the genomic level in distinct human hosts [54]. No similar experimental work has yet been carried out in TB. However, several studies have reported associations between human genetic polymorphisms and particular M. tuberculosis lineages [55]–[59], indicating possible interaction between human and M. tuberculosis variation. Whether such variation in pathogen and host genetics can be attributed to co-evolution will be difficult to demonstrate conclusively, but the data presented here support this possibility.
The strength of our study was that we used a nation-wide sample to specifically look at the impact of HIV infection on the host–pathogen relationship in human TB. Yet, our study is limited by the relatively small sample size, and the difficulty to quantify the complex social context through which the host–pathogen relationship is influenced in human TB. In addition, we looked at European-born patients only, because sympatric and allopatric host–pathogen combinations are more easily defined for this patient population [9], [18], [19], [25]. Additional studies in large cosmopolitan cities of Asia and Africa would be required to test whether the association between allopatric TB and HIV holds true in these settings. Ultimately, detailed experimental work is needed to establish the biological basis of the host–pathogen association in human TB.
In conclusion, our data suggest that the phylogeographical host–pathogen relationship in TB influences transmission patterns. Among the studied European-born TB patients, we showed that HIV infection disrupts the sympatric host–pathogen relationship in human TB, and that this effect increased as a function of immunodeficiency. Various interactions between HIV and M. tuberculosis at the cellular level make an association biologically plausible [42], [43]. Further studies are needed to investigate the impact of HIV on the genetic population structure of M. tuberculosis with its consequences for transmission and clinical manifestations in high TB burden countries [36]. This will lead to a better understanding of biological factors that shape the current HIV/TB syndemic [60].
Methods
Study setting
The Swiss Molecular Epidemiology of Tuberculosis (SMET) study is a collaborative project between the Swiss HIV Cohort Study (SHCS), the National Center for Mycobacteria, diagnostic microbiology laboratories, departments of respiratory medicine and public health, and the Federal Office of Public Health (FOPH) [29], [61], [62]. The overarching aims were to examine the genetic population structure of M. tuberculosis and the associations between strain variation, patient origin, and clinical characteristics in HIV–infected and HIV–negative TB patients in Switzerland. Further information on the SMET project is available at www.tb-network.ch. All participating sites are listed in the Acknowledgements.
The SHCS is a prospective observational study of HIV–infected individuals followed up in HIV outpatient clinics in Switzerland [63]. All HIV–infected patients diagnosed with TB between 2000 and 2008 whose M. tuberculosis complex (MTBC) isolate was available were included in the SMET study [29]. Furthermore, we randomly selected 288 from the 4,221 culture-confirmed TB cases reported to the National TB Surveillance Registry during the same period (approximately three cases for one HIV–infected TB case within the SHCS). Finally, all reported drug-resistant TB cases were included. Two M. bovis isolates were excluded from this study as they are animal-adapted species within the MTBC and therefore represent a different host–pathogen relationship.
Clinical data collection and definitions
We obtained clinical data by standardized questionnaires sent to the treating physicians and extracted relevant data from the SHCS database. We collected socio-demographic data (age, sex, origin of birth, citizenship, legal status, immunosuppressive therapy, risk factors for TB such as recent TB within family or immediate social surroundings in the last two years), laboratory parameters (CD4 cell count and plasma HIV RNA in HIV–infected cases) and clinical information (site of disease, radiography findings). Chest radiography parameters were consolidation, cavitations, enlarged intrathoracic lymph nodes and pleural thickening. Any drug resistance was defined as any resistance to isoniazid, rifampicin or ethambutol as reported to the FOPH. Most TB cases in Switzerland are treated under the guidance of experienced infectious and respiratory disease specialists, and the clinical data were of high quality.
Geographic origin of patients was defined as the country of birth, and countries were grouped in seven geographic regions (see Figure 1) according to the current understanding of the phylogeography of M. tuberculosis [25]. Birth country was used as a proxy of the ancestry of the study population. Immunosuppression due to other causes than HIV infection was defined as use of TNF-alpha inhibitors, malignancy, solid organ transplantation, use of steroids or methotrexate. Nadir CD4 T cell count was defined as the lowest CD4 T cell count (cells/µL) ever measured in a patient. Nadir CD4 T cell count is a predictor of poor immune recovery after ART [39]. Travel history was extracted from the free text field “Risk factor for TB” and defined as repeated travelling of longer duration (>30 days) to low-income countries with a high TB burden and a relevant exposure to M. tuberculosis according to the physician's judgment. Belonging to a molecular cluster involving Swiss-born and foreign-born TB cases was used as a proxy for contact with the foreign-born population.
Molecular analyses
Mycobacterial isolates were cultured and DNA extracted according to standard laboratory procedures. We used spacer oligonucleotide typing (spoligotyping) and 24-loci mycobacterial interspersed repetitive units (MIRU-VNTR) which are based on repetitive DNA sequences as genotyping tools with high discriminatory power to identify recent TB transmission [29], [64]–[66]. Data were analyzed with the MIRU-VNTRplus online tool (http://www.miru-vntrplus.org). Molecular clusters were defined as a group of completely identical isolates in the spoligotyping and MIRU-VNTR pattern indicating a chain of TB transmission. In addition, we used single nucleotide polymorphisms (SNPs) as stable genetic markers to define the main phylogenetic M. tuberculosis lineages [67]. Lineages were determined by SNPs using multiplex real-time PCR with fluorescence-labeled probes (Taqman, Applied Biosystems, USA) adopted from previous studies [9], [12], [67], [68]. The SNP used to define Lineage 4 was originally described by Sreevatsan et al. [69] and shown to be specific to this lineage [9].
Graphical models
Graphical models were built using the principles of directed acyclic graphs [70]. Our model considered infection and disease as a combined outcome (“TB with an allopatric strain”). Our hypothesis that HIV infection causes TB with an allopatric strain is shown as a potentially causal direct effect, and risk factors potentially influencing this effect are shown in the hypothetical direction. Mediators represent variables that are caused by the independent variable and, in turn, have a direct effect on the outcome variable. We included age and sex in our model as risk factors for infection and disease [37]. We also considered contact with the foreign-born population who have a higher risk for TB compared to the native Swiss population [26] and who have a higher risk of exposure to “foreign” M. tuberculosis strains. Finally, we included frequent traveling to countries with a high TB burden, which increases exposure risk and thus potentially infection risk with “foreign” M. tuberculosis strains (Figure 2).
Statistical analyses
We used χ2 tests or Fisher's exact tests to assess differences between groups in binary variables, and the Wilcoxon rank sum test for continuous variables (Table 1, Table 2). Univariate and multivariate exact logistic regression models were fitted to estimate the association between transmission as defined by molecular clustering and patients with sympatric M. tuberculosis lineages (patients with allopatric lineages were used as the reference, Table 2). Results were presented as ORs unadjusted and adjusted for age group, being born in Switzerland and recent TB in families or social surroundings. To assess the association of HIV infection with allopatric TB, we fitted univariate and multivariate logistic models (Table 3), and presented ORs unadjusted and adjusted for age, sex, Swiss-born, frequent travelling, contact with foreign born populations, and/or immunosuppression. We used univariate and multivariate logistic models to estimate the association between the degree of immunodeficiency and allopatric TB (Table 4), and presented ORs unadjusted and adjusted for age, sex, Swiss-born, frequent travelling, contact with foreign-born populations, and immunosuppression other than HIV infection. Finally, we determined statistical significance of HIV prevalence in patients with allopatric M. tuberculosis lineages compared to patients with sympatric lineages using Fisher's exact tests (Table 5). All analyses were performed in Stata version 11.1 (Stata Corporation, College Station, TX, USA).
Sensitivity analyses
In sensitivity analyses, we excluded patients with a history of frequent travelling to remove its influence on the association between HIV infection and allopatric lineages. In addition, we repeated the analyses using fully probabilistic Bayesian methods using weakly informative prior distributions [71]. The CIs reported from these analyses are 95% credible intervals and correspond to tail probabilities of the coefficient's posterior distributions. Bayesian statistics are less sensitive to errors when calculating estimators and CIs in small datasets.
Second panel of M. tuberculosis strains
We obtained 1,642 M. tuberculosis isolates from all TB cases (n = 1,940, 84.6%) notified in the Canton of Bern, Switzerland, between 1991 and 2011. For all patient isolates, we determined the main phylogenetic M. tuberculosis lineages. Of these, we included all patient isolates belonging to a non-Euro-American lineage (Lineage 1, 2, 3, 5 or 6) from European-born TB patients (40 of a total of 292 isolates belonging to lineages other than Lineage 4). Furthermore, we randomly selected control strains belonging to the Euro-American lineage (Lineage 4) from European-born TB patients (400 of a total of 1,350 isolates belonging to Lineage 4). European ancestry was confirmed in HIV–infected patients with an allopatric M. tuberculosis strain. Finally, we determined the HIV status in these patients using the same procedures as in the main sample.
Ethics approval
The study was approved by the ethics committee of the Canton of Bern, Switzerland. Written informed consent was obtained from all patients enrolled in the SHCS. For patients outside the SHCS, written informed consent was obtained by the treating physicians. In some cases informed consent could not be obtained from the patient because he or she could not be located or was known to have died. For these cases we obtained permission from the Federal expert commission on confidentiality in medical research to use the data provided by the treating physician.
Supporting Information
Zdroje
1. WoolhouseME, WebsterJP, DomingoE, CharlesworthB, LevinBR (2002) Biological and biomedical implications of the co-evolution of pathogens and their hosts. Nat Genet 32 : 569–577.
2. GandonS, Van ZandtPA (1998) Local adaptation and host-parasite interactions. Trends Ecol Evol 13 : 214–216.
3. KaweckiTJ, EbertD (2004) Conceptual issues in local adaptation. Ecology Letters 7 : 1225–1241.
4. SchulteRD, MakusC, HasertB, MichielsNK, SchulenburgH (2011) Host-parasite local adaptation after experimental coevolution of Caenorhabditis elegans and its microparasite Bacillus thuringiensis. Proc Biol Sci 278 : 2832–2839.
5. AgnewP, KoellaC, MichalakisY (2000) Host life history responses to parasitism. Microbes Infect 2 : 891–896.
6. GandonS, AgnewP, MichalakisY (2002) Coevolution between parasite virulence and host life-history traits. Am Nat 160 : 374–388.
7. LivelyCM, DybdahlMF (2000) Parasite adaptation to locally common host genotypes. Nature 405 : 679–681.
8. BroschR, GordonSV, MarmiesseM, BrodinP, BuchrieserC, et al. (2002) A new evolutionary scenario for the Mycobacterium tuberculosis complex. Proc Natl Acad Sci U S A 99 : 3684–3689.
9. GagneuxS, DeRiemerK, VanT, Kato-MaedaM, de JongBC, et al. (2006) Variable host–pathogen compatibility in Mycobacterium tuberculosis. Proc Natl Acad Sci U S A 103 : 2869–2873.
10. GutierrezMC, BrisseS, BroschR, FabreM, OmaisB, et al. (2005) Ancient origin and gene mosaicism of the progenitor of Mycobacterium tuberculosis. PLoS Pathog 1: e5 doi:10.1371/journal.ppat.0010005.
11. HirshAE, TsolakiAG, DeRiemerK, FeldmanMW, SmallPM (2004) Stable association between strains of Mycobacterium tuberculosis and their human host populations. Proc Natl Acad Sci U S A 101 : 4871–4876.
12. HershbergR, LipatovM, SmallPM, ShefferH, NiemannS, et al. (2008) High functional diversity in Mycobacterium tuberculosis driven by genetic drift and human demography. PLoS Biol 6: e311 doi:10.1371/journal.pbio.0060311.
13. WirthT, HildebrandF, Allix-BeguecC, WolbelingF, KubicaT, et al. (2008) Origin, spread and demography of the Mycobacterium tuberculosis complex. PLoS Pathog 4: e1000160 doi:10.1371/journal.ppat.1000160.
14. Pearce-DuvetJM (2006) The origin of human pathogens: evaluating the role of agriculture and domestic animals in the evolution of human disease. Biol Rev Camb Philos Soc 81 : 369–382.
15. VinaMA, HollenbachJA, LykeKE, SzteinMB, MaiersM, et al. (2012) Tracking human migrations by the analysis of the distribution of HLA alleles, lineages and haplotypes in closed and open populations. Philos Trans R Soc Lond B Biol Sci 367 : 820–829.
16. BrudeyK, DriscollJR, RigoutsL, ProdingerWM, GoriA, et al. (2006) Mycobacterium tuberculosis complex genetic diversity: mining the fourth international spoligotyping database (SpolDB4) for classification, population genetics and epidemiology. BMC Microbiol 6 : 23.
17. FilliolI, MotiwalaAS, CavatoreM, QiW, HazbonMH, et al. (2006) Global phylogeny of Mycobacterium tuberculosis based on single nucleotide polymorphism (SNP) analysis: insights into tuberculosis evolution, phylogenetic accuracy of other DNA fingerprinting systems, and recommendations for a minimal standard SNP set. J Bacteriol 188 : 759–772.
18. BakerL, BrownT, MaidenMC, DrobniewskiF (2004) Silent nucleotide polymorphisms and a phylogeny for Mycobacterium tuberculosis. Emerg Infect Dis 10 : 1568–1577.
19. ReedMB, PichlerVK, McIntoshF, MattiaA, FallowA, et al. (2009) Major Mycobacterium tuberculosis lineages associate with patient country of origin. J Clin Microbiol 47 : 1119–1128.
20. GutackerMM, MathemaB, SoiniH, ShashkinaE, KreiswirthBN, et al. (2006) Single-nucleotide polymorphism-based population genetic analysis of Mycobacterium tuberculosis strains from 4 geographic sites. J Infect Dis 193 : 121–128.
21. GagneuxS (2012) Host–pathogen coevolution in human tuberculosis. Philos Trans R Soc Lond B Biol Sci 367 : 850–859.
22. BritesD, GagneuxS (2012) Old and new selective pressures on Mycobacterium tuberculosis. Infect Genet Evol 12 : 678–85.
23. Wicker HR, Fibbi R, Haug W (2004) “Ergebnisse des Nationalen Forschungsprogramms ‘Migration und interkulturelle Beziehungen’”. Seismo publishing, Zürich, Switzerland.
24. de JongBC, AntonioM, GagneuxS (2010) Mycobacterium africanum–review of an important cause of human tuberculosis in West Africa. PLoS Negl Trop Dis 4: e744 doi:10.1371/journal.pntd.0000744.
25. GagneuxS, SmallPM (2007) Global phylogeography of Mycobacterium tuberculosis and implications for tuberculosis product development. Lancet Infect Dis 7 : 328–337.
26. Federal Office of Public Health (2011) Tuberkulose in der Schweiz 2005–2009. [Erratum appears in Bull Bundesamt für Gesundheit (Switzerland) 2011;(no 13):277]. Bull BAG (no 10) 205–213.
27. BorgdorffMW, NagelkerkeN, Van SoolingenD, de HaasPE, VeenJ, et al. (1998) Analysis of tuberculosis transmission between nationalities in the Netherlands in the period 1993–1995 using DNA fingerprinting. Am J Epidemiol 147 : 187–195.
28. LillebaekT, AndersenÅB, BauerJ, DirksenA, GlismannS, et al. (2001) Risk of Mycobacterium tuberculosis transmission in a low-incidence country due to immigration from high-incidence areas. J Clin Microbiol 39 : 855–861.
29. FennerL, GagneuxS, HelblingP, BattegayM, RiederHL, et al. (2012) Mycobacterium tuberculosis transmission in a country with low tuberculosis incidence: role of immigration and HIV infection. J Clin Microbiol 50 : 388–395.
30. SpiegelhalterDJ, MylesJP, JonesDR, AbramsKR (1999) Methods in health service research. An introduction to bayesian methods in health technology assessment. BMJ 319 : 508–512.
31. LawnSD, ZumlaAI (2011) Tuberculosis. Lancet 378 : 57–72.
32. RodrigoT, CaylàJA, Garcia de OlallaP, Galdós-TangüisH, JansàJM, et al. (1997) Characteristics of tuberculosis patients who generate secondary cases. Int J Tuberc Lung Dis 1 : 352–357.
33. ComstockGW, LivesayVT, WoolpertSF (1974) The prognosis of a positive tuberculin reaction in childhood and adolescence. Am J Epidemiol 99 : 131–138.
34. VynnyckyE, FinePEM (2000) Life time risks, incubation period, and serial interval of tuberculosis. Am J Epidemiol 152 : 247–263.
35. HorsburghCRJr (2004) Priorities for the treatment of latent tuberculosis infection in the United States. N Engl J Med 350 : 2060–2067.
36. FennerL, EggerM, GagneuxS (2009) Annie Darwin's death, the evolution of tuberculosis and the need for systems epidemiology. Int J Epidemiol 38 : 1425–1428.
37. RiederHL (1999) Epidemiologic basis of tuberculosis control. International Union Against Tuberculosis and Lung Disease, Paris 1999.
38. FennerL, WeberR, SteffenR, SchlagenhaufP (2007) Imported infectious disease and purpose of travel, Switzerland. Emerg Infect Dis 13 : 217–222.
39. NegredoE, MassanellaM, PuigJ, Perez-AlvarezN, Gallego-EscuredoJM, et al. (2010) Nadir CD4 T cell count as predictor and high CD4 T cell intrinsic apoptosis as final mechanism of poor CD4 T cell recovery in virologically suppressed HIV–infected patients: clinical implications. Clin Infect Dis 50 : 1300–1308.
40. KelleyCF, KitchenCM, HuntPW, RodriguezB, HechtFM, et al. (2009) Incomplete peripheral CD4+ cell count restoration in HIV–infected patients receiving long-term antiretroviral treatment. Clin Infect Dis 48 : 787–794.
41. LangeCG, LedermanMM, MedvikK, AsaadR, WildM, et al. (2003) Nadir CD4+ T-cell count and numbers of CD28+ CD4+ T-cells predict functional responses to immunizations in chronic HIV–1 infection. AIDS 17 : 2015–2023.
42. DiedrichCR, FlynnJL (2011) HIV–1/Mycobacterium tuberculosis coinfection immunology: how does HIV–1 exacerbate tuberculosis? Infect Immun 79 : 1407–1417.
43. FalvoJV, RanjbarS, JasenoskyLD, GoldfeldAE (2011) Arc of a vicious circle: pathways activated by Mycobacterium tuberculosis that target the HIV–1 LTR. Am J Respir Cell Mol Biol 45 : 1116–24.
44. HoshinoY, NakataK, HoshinoS, HondaY, TseDB, et al. (2002) Maximal HIV–1 replication in alveolar macrophages during tuberculosis requires both lymphocyte contact and cytokines. J Exp Med 195 : 495–505.
45. PatelNR, ZhuJ, TachadoSD, ZhangJ, WanZ, et al. (2007) HIV impairs TNF-alpha mediated macrophage apoptotic response to Mycobacterium tuberculosis. J Immunol 179 : 6973–6980.
46. GeldmacherC, SchuetzA, NgwenyamaN, CasazzaJP, SangaE, et al. (2008) Early depletion of Mycobacterium tuberculosis-specific T helper 1 cell responses after HIV–1 infection. J Infect Dis 198 : 1590–1598.
47. PortevinD, GagneuxS, ComasI, YoungD (2011) Human macrophage responses to clinical isolates from the Mycobacterium tuberculosis complex discriminate between ancient and modern lineages. PLoS Pathog 7: e1001307 doi:10.1371/journal.ppat.1001307.
48. MusserJM, KrollJS, GranoffDM, MoxonER, BrodeurBR, et al. (1990) Global genetic structure and molecular epidemiology of encapsulated Haemophilus influenzae. Rev Infect Dis 12 : 75–111.
49. CaufieldPW (2009) Tracking human migration patterns through the oral bacterial flora. Clin Microbiol Infect 15 Suppl 1 : 37–39.
50. MonotM, HonoreN, GarnierT, ZidaneN, SherafiD, et al. (2009) Comparative genomic and phylogeographic analysis of Mycobacterium leprae. Nat Genet 41 : 1282–1289.
51. FalushD, WirthT, LinzB, PritchardJK, StephensM, et al. (2003) Traces of human migrations in Helicobacter pylori populations. Science 299 : 1582–1585.
52. LinzB, BallouxF, MoodleyY, ManicaA, LiuH, et al. (2007) An African origin for the intimate association between humans and Helicobacter pylori. Nature 445 : 915–918.
53. Aspholm-HurtigM, DailideG, LahmannM, KaliaA, IlverD, et al. (2004) Functional adaptation of BabA, the H. pylori ABO blood group antigen binding adhesin. Science 305 : 519–522.
54. ZdziarskiJ, BrzuszkiewiczE, WulltB, LiesegangH, BiranD, et al. (2010) Host imprints on bacterial genomes – rapid, divergent evolution in individual patients. PLoS Pathog 6: e1001078 doi:10.1371/journal.ppat.1001078.
55. CawsM, ThwaitesG, DunstanS, HawnTR, LanNT, et al. (2008) The influence of host and bacterial genotype on the development of disseminated disease with Mycobacterium tuberculosis. PLoS Pathog 4: e1000034 doi:10.1371/journal.ppat.1000034.
56. van CrevelR, ParwatiI, SahiratmadjaE, MarzukiS, OttenhoffTHM, et al. (2009) Infection with Mycobacterium tuberculosis Beijing genotype strains is associated with polymorphisms in SLC11A1/NRAMP1 in Indonesian patients with tuberculosis. J Infect Dis 200 : 1671–1674.
57. HerbF, ThyeT, NiemannS, BrowneEN, ChinbuahMA, et al. (2008) ALOX5 variants associated with susceptibility to human pulmonary tuberculosis. Hum Mol Genet 17 : 1052–1060.
58. IntemannCD, ThyeT, NiemannS, BrowneEN, AmanuaCM, et al. (2009) Autophagy gene variant IRGM −261T contributes to protection from tuberculosis caused by Mycobacterium tuberculosis but not by M. africanum strains. PLoS Pathog 5: e1000577 doi:10.1371/journal.ppat.1000577.
59. ThyeT, NiemannS, WalterK, HomolkaS, IntemannCD, et al. (2011) Variant G57E of mannose binding lectin associated with protection against tuberculosis caused by Mycobacterium africanum but not by M. tuberculosis. PLoS ONE 6: e20908 doi:10.1371/journal.pone.0020908.
60. KwanCK, ErnstJD (2011) HIV and tuberculosis: a deadly human syndemic. Clin Microbiol Rev 24 : 351–376.
61. FennerL, GagneuxS, JanssensJP, FehrJ, CavassiniM, et al. (2012) Tuberculosis in HIV–negative and HIV–infected patients in a low-incidence country: clinical characteristics and treatment outcomes. PLoS ONE 7: e34186 doi:10.1371/journal.pone.0034186.
62. FennerL, EggerM, BodmerT, AltpeterE, ZwahlenM, et al. (2012) Effect of mutation and genetic background on drug resistance in Mycobacterium tuberculosis. Antimicrob Agents Chemother 56 : 3047–3053.
63. Schoeni-AffolterF, LedergerberB, RickenbachM, RudinC, GunthardHF, et al. (2010) Cohort profile: the Swiss HIV Cohort study. Int J Epidemiol 39 : 1179–1189.
64. Allix-BeguecC, Fauville-DufauxM, SupplyP (2008) Three-year population-based evaluation of standardized mycobacterial interspersed repetitive-unit-variable-number tandem-repeat typing of Mycobacterium tuberculosis. J Clin Microbiol 46 : 1398–1406.
65. SupplyP, AllixC, LesjeanS, Cardoso-OelemannM, Rusch-GerdesS, et al. (2006) Proposal for standardization of optimized mycobacterial interspersed repetitive unit-variable-number tandem repeat typing of Mycobacterium tuberculosis. J Clin Microbiol 44 : 4498–4510.
66. OelemannMC, DielR, VatinV, HaasW, Rusch-GerdesS, et al. (2007) Assessment of an optimized mycobacterial interspersed repetitive - unit-variable-number tandem-repeat typing system combined with spoligotyping for population-based molecular epidemiology studies of tuberculosis. J Clin Microbiol 45 : 691–697.
67. StuckiD, MallaB, HostettlerS, HunaT, FeldmannJ, et al. (2012) Two new rapid SNP-typing methods for classifying Mycobacterium tuberculosis complex into the main phylogenetic lineages. PLoS ONE 7: e41253 doi:10.1371/journal.pone.0041253.
68. FennerL, MallaB, NinetB, DubuisO, StuckiD, et al. (2011) “Pseudo-Beijing”: Evidence for convergent evolution in the direct repeat region of Mycobacterium tuberculosis. PLoS ONE 6: e24737 doi:10.1371/journal.pone.0024737.
69. SreevatsanS, PanX, StockbauerKE, ConnellND, KreiswirthBN, et al. (1997) Restricted structural gene polymorphism in the Mycobacterium tuberculosis complex indicates evolutionarily recent global dissemination. Proc Natl Acad Sci U S A 94 : 9869–9874.
70. PearlJ (2010) An Introduction to Causal Inference. Int J Biostat 6: Article 7.
71. GelmanA, JakulinA, PittauMG, SuYS (2008) A weakly informative default prior distribution for logistic and other regression models. Ann Appl Stat 2 : 1360–1383.
72. BentleySD, ComasI, BryantJM, WalkerD, SmithNH, et al. (2012) The genome of Mycobacterium africanum West African 2 reveals a lineage-specific locus and genome erosion common to the M. tuberculosis complex. PLoS Negl Trop Dis 6: e1552 doi:10.1371/journal.pntd.0001552.
Štítky
Genetika Reprodukční medicína
Článek Ubiquitous Polygenicity of Human Complex Traits: Genome-Wide Analysis of 49 Traits in KoreansČlánek Alternative Splicing and Subfunctionalization Generates Functional Diversity in Fungal ProteomesČlánek RFX Transcription Factor DAF-19 Regulates 5-HT and Innate Immune Responses to Pathogenic Bacteria inČlánek Surveillance-Activated Defenses Block the ROS–Induced Mitochondrial Unfolded Protein ResponseČlánek Deficiency Reduces Adipose OXPHOS Capacity and Triggers Inflammation and Insulin Resistance in Mice
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 3
-
Všechny články tohoto čísla
- Power and Predictive Accuracy of Polygenic Risk Scores
- Rare Copy Number Variants Are a Common Cause of Short Stature
- Coordination of Flower Maturation by a Regulatory Circuit of Three MicroRNAs
- Ubiquitous Polygenicity of Human Complex Traits: Genome-Wide Analysis of 49 Traits in Koreans
- Genomic Evidence for Island Population Conversion Resolves Conflicting Theories of Polar Bear Evolution
- Mechanistic Insight into the Pathology of Polyalanine Expansion Disorders Revealed by a Mouse Model for X Linked Hypopituitarism
- Genome-Wide Association Study and Gene Expression Analysis Identifies as a Predictor of Response to Etanercept Therapy in Rheumatoid Arthritis
- Problem Solved: An Interview with Sir Edwin Southern
- Long Interspersed Element–1 (LINE-1): Passenger or Driver in Human Neoplasms?
- Mouse HFM1/Mer3 Is Required for Crossover Formation and Complete Synapsis of Homologous Chromosomes during Meiosis
- Alternative Splicing and Subfunctionalization Generates Functional Diversity in Fungal Proteomes
- A WRKY Transcription Factor Recruits the SYG1-Like Protein SHB1 to Activate Gene Expression and Seed Cavity Enlargement
- Microhomology-Mediated Mechanisms Underlie Non-Recurrent Disease-Causing Microdeletions of the Gene or Its Regulatory Domain
- Ancient Evolutionary Trade-Offs between Yeast Ploidy States
- Differential Evolutionary Fate of an Ancestral Primate Endogenous Retrovirus Envelope Gene, the EnvV , Captured for a Function in Placentation
- A Feed-Forward Loop Coupling Extracellular BMP Transport and Morphogenesis in Wing
- The Tomato Yellow Leaf Curl Virus Resistance Genes and Are Allelic and Code for DFDGD-Class RNA–Dependent RNA Polymerases
- The U-Box E3 Ubiquitin Ligase TUD1 Functions with a Heterotrimeric G α Subunit to Regulate Brassinosteroid-Mediated Growth in Rice
- Role of the DSC1 Channel in Regulating Neuronal Excitability in : Extending Nervous System Stability under Stress
- –Independent Phenotypic Switching in and a Dual Role for Wor1 in Regulating Switching and Filamentation
- Pax6 Regulates Gene Expression in the Vertebrate Lens through miR-204
- Blood-Informative Transcripts Define Nine Common Axes of Peripheral Blood Gene Expression
- Genetic Architecture of Skin and Eye Color in an African-European Admixed Population
- Fine Characterisation of a Recombination Hotspot at the Locus and Resolution of the Paradoxical Excess of Duplications over Deletions in the General Population
- Estrogen Mediated-Activation of miR-191/425 Cluster Modulates Tumorigenicity of Breast Cancer Cells Depending on Estrogen Receptor Status
- Complex Patterns of Genomic Admixture within Southern Africa
- Yap- and Cdc42-Dependent Nephrogenesis and Morphogenesis during Mouse Kidney Development
- Molecular Networks of Human Muscle Adaptation to Exercise and Age
- Alp/Enigma Family Proteins Cooperate in Z-Disc Formation and Myofibril Assembly
- Polycomb Group Gene Regulates Rice () Seed Development and Grain Filling via a Mechanism Distinct from
- RFX Transcription Factor DAF-19 Regulates 5-HT and Innate Immune Responses to Pathogenic Bacteria in
- Distinct Molecular Strategies for Hox-Mediated Limb Suppression in : From Cooperativity to Dispensability/Antagonism in TALE Partnership
- A Natural Polymorphism in rDNA Replication Origins Links Origin Activation with Calorie Restriction and Lifespan
- TDP2–Dependent Non-Homologous End-Joining Protects against Topoisomerase II–Induced DNA Breaks and Genome Instability in Cells and
- Recurrent Rearrangement during Adaptive Evolution in an Interspecific Yeast Hybrid Suggests a Model for Rapid Introgression
- Genome-Wide Association Study in Mutation Carriers Identifies Novel Loci Associated with Breast and Ovarian Cancer Risk
- Coincident Resection at Both Ends of Random, γ–Induced Double-Strand Breaks Requires MRX (MRN), Sae2 (Ctp1), and Mre11-Nuclease
- Identification of a -Specific Modifier Locus at 6p24 Related to Breast Cancer Risk
- A Novel Function for the Hox Gene in the Male Accessory Gland Regulates the Long-Term Female Post-Mating Response in
- Tdp2: A Means to Fixing the Ends
- A Novel Role for the RNA–Binding Protein FXR1P in Myoblasts Cell-Cycle Progression by Modulating mRNA Stability
- Association Mapping and the Genomic Consequences of Selection in Sunflower
- Histone Deacetylase 2 (HDAC2) Regulates Chromosome Segregation and Kinetochore Function via H4K16 Deacetylation during Oocyte Maturation in Mouse
- A Novel Mutation in the Upstream Open Reading Frame of the Gene Causes a MEN4 Phenotype
- Ataxin1L Is a Regulator of HSC Function Highlighting the Utility of Cross-Tissue Comparisons for Gene Discovery
- Human Spermatogenic Failure Purges Deleterious Mutation Load from the Autosomes and Both Sex Chromosomes, including the Gene
- A Conserved Upstream Motif Orchestrates Autonomous, Germline-Enriched Expression of piRNAs
- Statistical Analysis Reveals Co-Expression Patterns of Many Pairs of Genes in Yeast Are Jointly Regulated by Interacting Loci
- Matefin/SUN-1 Phosphorylation Is Part of a Surveillance Mechanism to Coordinate Chromosome Synapsis and Recombination with Meiotic Progression and Chromosome Movement
- A Role for the Malignant Brain Tumour (MBT) Domain Protein LIN-61 in DNA Double-Strand Break Repair by Homologous Recombination
- The Population and Evolutionary Dynamics of Phage and Bacteria with CRISPR–Mediated Immunity
- Long Noncoding RNA MALAT1 Controls Cell Cycle Progression by Regulating the Expression of Oncogenic Transcription Factor B-MYB
- Surveillance-Activated Defenses Block the ROS–Induced Mitochondrial Unfolded Protein Response
- DNA Topoisomerase III Localizes to Centromeres and Affects Centromeric CENP-A Levels in Fission Yeast
- Genome-Wide Control of RNA Polymerase II Activity by Cohesin
- Divergent Selection Drives Genetic Differentiation in an R2R3-MYB Transcription Factor That Contributes to Incipient Speciation in
- NODULE INCEPTION Directly Targets Subunit Genes to Regulate Essential Processes of Root Nodule Development in
- Spreading of a Prion Domain from Cell-to-Cell by Vesicular Transport in
- Deficiency in Origin Licensing Proteins Impairs Cilia Formation: Implications for the Aetiology of Meier-Gorlin Syndrome
- Deficiency Reduces Adipose OXPHOS Capacity and Triggers Inflammation and Insulin Resistance in Mice
- The Conserved SKN-1/Nrf2 Stress Response Pathway Regulates Synaptic Function in
- Functional Genomic Analysis of the Regulatory Network in
- Astakine 2—the Dark Knight Linking Melatonin to Circadian Regulation in Crustaceans
- CRL2 E3-Ligase Regulates Proliferation and Progression through Meiosis in the Germline
- Both the Caspase CSP-1 and a Caspase-Independent Pathway Promote Programmed Cell Death in Parallel to the Canonical Pathway for Apoptosis in
- PRMT4 Is a Novel Coactivator of c-Myb-Dependent Transcription in Haematopoietic Cell Lines
- A Copy Number Variant at the Locus Likely Confers Risk for Canine Squamous Cell Carcinoma of the Digit
- Evidence of Gene–Environment Interactions between Common Breast Cancer Susceptibility Loci and Established Environmental Risk Factors
- HIV Infection Disrupts the Sympatric Host–Pathogen Relationship in Human Tuberculosis
- Trans-Ethnic Fine-Mapping of Lipid Loci Identifies Population-Specific Signals and Allelic Heterogeneity That Increases the Trait Variance Explained
- A Gene Transfer Agent and a Dynamic Repertoire of Secretion Systems Hold the Keys to the Explosive Radiation of the Emerging Pathogen
- The Role of ATM in the Deficiency in Nonhomologous End-Joining near Telomeres in a Human Cancer Cell Line
- Dynamic Circadian Protein–Protein Interaction Networks Predict Temporal Organization of Cellular Functions
- Nuclear Myosin 1c Facilitates the Chromatin Modifications Required to Activate rRNA Gene Transcription and Cell Cycle Progression
- Robust Prediction of Expression Differences among Human Individuals Using Only Genotype Information
- A Single Cohesin Complex Performs Mitotic and Meiotic Functions in the Protist
- The Role of the Arabidopsis Exosome in siRNA–Independent Silencing of Heterochromatic Loci
- Elevated Expression of the Integrin-Associated Protein PINCH Suppresses the Defects of Muscle Hypercontraction Mutants
- Twist1 Controls a Cell-Specification Switch Governing Cell Fate Decisions within the Cardiac Neural Crest
- Genome-Wide Testing of Putative Functional Exonic Variants in Relationship with Breast and Prostate Cancer Risk in a Multiethnic Population
- Heteroduplex DNA Position Defines the Roles of the Sgs1, Srs2, and Mph1 Helicases in Promoting Distinct Recombination Outcomes
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Fine Characterisation of a Recombination Hotspot at the Locus and Resolution of the Paradoxical Excess of Duplications over Deletions in the General Population
- Molecular Networks of Human Muscle Adaptation to Exercise and Age
- Recurrent Rearrangement during Adaptive Evolution in an Interspecific Yeast Hybrid Suggests a Model for Rapid Introgression
- Genome-Wide Association Study and Gene Expression Analysis Identifies as a Predictor of Response to Etanercept Therapy in Rheumatoid Arthritis
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



