-
Články
Reklama
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
ReklamaMouse Genetics Suggests Cell-Context Dependency for Myc-Regulated Metabolic Enzymes during Tumorigenesis
c-Myc (hereafter called Myc) belongs to a family of transcription factors that regulates cell growth, cell proliferation, and differentiation. Myc initiates the transcription of a large cast of genes involved in cell growth by stimulating metabolism and protein synthesis. Some of these, like those involved in glycolysis, may be part of the Warburg effect, which is defined as increased glucose uptake and lactate production in the presence of adequate oxygen supply. In this study, we have taken a mouse-genetics approach to challenge the role of select Myc-regulated metabolic enzymes in tumorigenesis in vivo. By breeding λ-Myc transgenic mice, ApcMin mice, and p53 knockout mice with mouse models carrying inactivating alleles of Lactate dehydrogenase A (Ldha), 3-Phosphoglycerate dehydrogenase (Phgdh) and Serine hydroxymethyltransferase 1 (Shmt1), we obtained offspring that were monitored for tumor development. Very surprisingly, we found that these genes are dispensable for tumorigenesis in these genetic settings. However, experiments in fibroblasts and colon carcinoma cells expressing oncogenic Ras show that these cells are sensitive to Ldha knockdown. Our genetic models reveal cell context dependency and a remarkable ability of tumor cells to adapt to alterations in critical metabolic pathways. Thus, to achieve clinical success, it will be of importance to correctly stratify patients and to find synthetic lethal combinations of inhibitors targeting metabolic enzymes.
Published in the journal: . PLoS Genet 8(3): e32767. doi:10.1371/journal.pgen.1002573
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002573Summary
c-Myc (hereafter called Myc) belongs to a family of transcription factors that regulates cell growth, cell proliferation, and differentiation. Myc initiates the transcription of a large cast of genes involved in cell growth by stimulating metabolism and protein synthesis. Some of these, like those involved in glycolysis, may be part of the Warburg effect, which is defined as increased glucose uptake and lactate production in the presence of adequate oxygen supply. In this study, we have taken a mouse-genetics approach to challenge the role of select Myc-regulated metabolic enzymes in tumorigenesis in vivo. By breeding λ-Myc transgenic mice, ApcMin mice, and p53 knockout mice with mouse models carrying inactivating alleles of Lactate dehydrogenase A (Ldha), 3-Phosphoglycerate dehydrogenase (Phgdh) and Serine hydroxymethyltransferase 1 (Shmt1), we obtained offspring that were monitored for tumor development. Very surprisingly, we found that these genes are dispensable for tumorigenesis in these genetic settings. However, experiments in fibroblasts and colon carcinoma cells expressing oncogenic Ras show that these cells are sensitive to Ldha knockdown. Our genetic models reveal cell context dependency and a remarkable ability of tumor cells to adapt to alterations in critical metabolic pathways. Thus, to achieve clinical success, it will be of importance to correctly stratify patients and to find synthetic lethal combinations of inhibitors targeting metabolic enzymes.
Introduction
Activation of one of the three MYC oncogenes is frequently selected for during tumorigenesis. These genes encode the transcription factors c-Myc, N-Myc and L-Myc that regulate a large number of downstream target genes. Although most of the work on MYC oncogenes has involved their role in cell proliferation, it is becoming clear that they may be involved in most aspects of oncogenic transformation [1]. As such, unravelling the mechanisms by which Myc proteins activate genes, and which are the essential genes, is paramount as studies resolving these mechanisms may open up new avenues of targeted intervention against various cancers.
Some of Myc's earliest discovered transcriptional targets were genes encoding metabolic enzymes such as Ornithine decarboxylase [Odc] [2], [3], Lactate dehydrogenase A [Ldha] [4] and Carbamoyl-phosphate synthase/aspartate carbamoyltransferase/dihydroorotase [Cad] [5]. Later studies using expression profiling identified even more of these genes, indicating that Myc is a master regulator of cellular metabolism and cell growth [6], [7]. Interestingly, inhibition of polyamine biosynthetic enzymes Odc and Spermidine synthase have shown efficacy in chemoprevention of several cancers in experimental models [8]–[14] and in colon cancer patients [15]. Furthermore, Myc-regulated Ldha, Pyruvate kinase M2 and Glutaminase have also emerged as promising targets based on experimental models of human cancer [16]–[23], suggesting that targeting various metabolic pathways regulated by Myc may prove beneficial in cancer therapies of patients. To gain in vivo support for this notion we performed genetic ablation experiments in mice to determine the individual contribution to tumorigenesis of three different Myc-regulated metabolic enzymes.
Results
To identify critical Myc-regulated metabolic enzymes, we performed Illumina bead chip arrays on RNA isolated from 4–6 week old wildtype or precancerous, B cell lymphoma-prone λ-Myc transgenic mice, where the human MYC gene is under the control of the immunoglobulin (Ig) λ enhancer [24]. Interestingly, when we performed unsupervised Hierarchical clustering on 153 genes (Table S1) encoding metabolic enzymes involved in glycolysis, the Kreb's cycle, oxidative phosphorylation, serine synthesis and one-carbon metabolism, all expression profiles from Myc-transgenic B cells grouped together despite some intra-individual expression level differences that could be due to expression levels of MYC and developmental stage of the B-cell compartment (Figure 1A). Largely, these data are supportive of the Myc target gene database (http://www.myccancergene.org).
Fig. 1. Myc regulates the metabolic transcriptome. 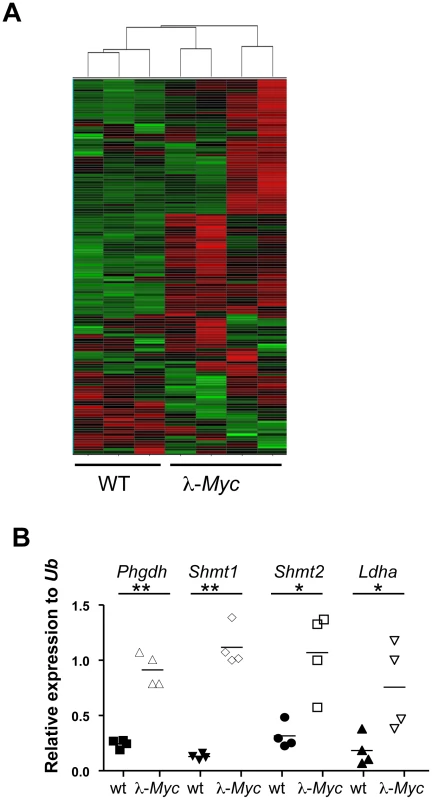
(A) Unsupervised hierarchical clustering of Illumina bead arrays made from RNA of splenic B cells from three wildtype and four precancerous λ-Myc transgenic mice. See Table S1 for genes used in the clustering. (B) qRT-PCR confirmation of 4 of the 20 most significantly, or most elevated expressed genes, in B220-sorted B cells from λ-Myc transgenic mice. *indicates p<0.05 and ** indicates p<0.01. Many tumor cells use aerobic glycolysis, producing lactate even in the presence of oxygen (the Warburg effect) [25]. Most of the glucose from the enhanced glucose uptake is however used to provide metabolites for anabolic processes, such as fat and nucleotide synthesis. Glycolysis and nucleotide metabolism are linked at several steps including the pentose phosphate shunt and via the phosphorylated pathway of serine synthesis (Figure S1). In the latter, 3-phosphoglycerate dehydrogenase (Phgdh) catalyzes the first step and serine hydroxymethyltransferases (Shmt1 and Shmt2) use the final product to produce folate metabolites that are critical for several metabolic pathways including methylation and thymidylate synthesis. Given that Myc regulates genes involved in many metabolic pathways we decided to focus on genes that were induced by Myc and for which there were genetic tools accessible at the start of this project. qRT-PCR analysis confirmed that the selected genes had elevated expression in Myc-transgenic B cells (Figure 1B).
Shmt1 and Shmt2 protein levels and their combined activity are elevated in B cells from λ-Myc transgenic mice (Figure S2A and S2B). So to analyze the role of Shmt1 in Myc-induced tumorigenesis, we obtained embryonic stem (ES) cells carrying a gene-trapped allele of Shmt1, which were injected into blastocysts to generate chimeric mice. The offspring from these mice generated wildtype, heterozygous or homozygous Shmt1 knockout mice at the expected Mendelian frequency (Figure S2C and S2D), corroborating a recent publication reporting that Shmt1 is dispensable for mouse development [26].
The homozygous Shmt1 mutant mice did not express any Shmt1 protein in the tissues analyzed (Figure S2E) making them suitable for the assessment of the role of this gene for Myc-induced tumorigenesis. To that end, we first back-crossed the Shmt1 knockout mouse for 10 generations to the C57BL/6 strain and then the interbred it with 3 different tumor models where Myc is either the direct cause of transformation (λ-Myc transgenic mice, Figure 2A [24]) or constitutes an important circuit (p53 knockout mice, Figure 2B [27] and ApcMin mice of intestinal tumorigenesis, Figure 2C [28]). Surprisingly, we did not observe any major negative impact of Shmt1 loss on tumor initiation and development in these models. The only significant effect was a clear acceleration of disease in the λ-Myc transgenic mice (Figure 2A), suggesting a B-cell specific event. Taken together these data argue against Shmt1 as a target for chemotherapy.
Fig. 2. Shmt1 loss accelerates lymphomagenesis in λ-Myc transgenic mice. 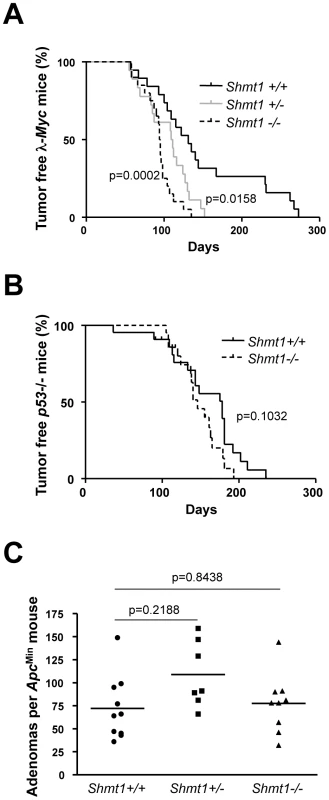
(A) Survival curve of λ-Myc mice generated from interbreedings between Shmt1 knockout and λ-Myc transgenic mice. λ-Myc; Shmt1+/+ n = 19, λ-Myc; Shmt1+/− n = 18, λ-Myc; Shmt1−/− n = 20. (B) Survival curve of p53 knockout mice of the indicated Shmt1 genotypes. p53−/−; Shmt1+/+ n = 22, p53−/−; Shmt1−/− n = 21. (C) Amount of adenomas in ApcMin mice with different Shmt1 genotypes. Serine and folate metabolites can also be made via pathways involving Shmt2 and Phgdh. However, to assess the role of Shmt2, we were forced to take a different approach, as Shmt2 gene-trap clones or knockout mice were not available when initiating this project. We hence infected Colon 26 cells, which carry an NMU-induced Kras mutation [29], with lentiviruses expressing shRNA directed against Shmt2 and Phgdh. Despite achieving potent knockdown levels, we did not observe any effect on viability or ability to form subcutaneous tumors when injected into syngenic Balb/c mice, as compared to cells infected with a control lentivirus (Figure S3A and S3B).
To further assess the effect of Phgdh loss in different tumor models, we obtained a Phgdh knockout mouse. Since Phgdh is essential for neurogenesis [30], Phgdh null embryos die at around embryonic day (E) 13.5, which prevented us from analyzing the effects of loss of Phgdh by conventional breeding to our tumor models. We therefore started out by assessing the impact of removal of just one allele of Phgdh on tumorigenesis in λ-Myc transgenic mice and in ApcMin mice. At variance with Odc, which is haploinsufficient for tumor progression in these models, Phgdh heterozygosity did not impact tumorigenesis in these tumor models (Figure 3A and 3B), despite the 50% reduction in Phgdh activity (Figure 3C). As an alternative approach, we crossed λ-Myc; Phgdh+/− mice with Phgdh+/− mice and isolated hematopoietic stem cells from E13.5 fetal livers. These cells were then used to reconstitute lethally irradiated syngenic recipients, creating lymphoma-prone mice with varying expression of Phgdh (Figure 3D). Even in this setting, Phgdh was dispensable for Myc-induced tumorigenesis (Figure 3E), suggesting that hematopoiesis and Myc-driven tumorigenesis can occur in the absence of Phgdh.
Fig. 3. Phgdh is dispensable for lymphomagenesis in λ-Myc transgenic mice. 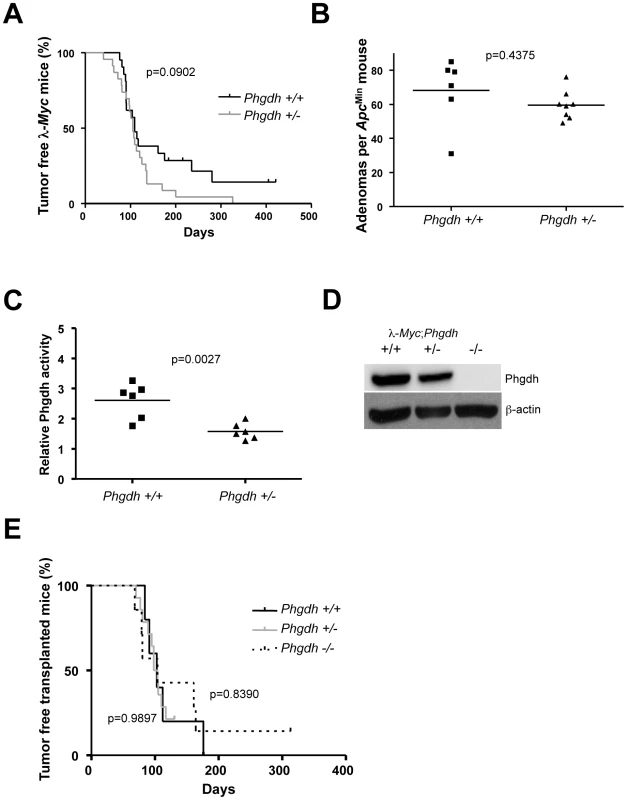
(A) Survival curve of λ-Myc mice generated from interbreedings between Phgdh+/− and λ-Myc transgenic mice. λ-Myc; Phgdh+/+ n = 21, λ-Myc; Phgdh+/− n = 23. (B) Amount of adenomas in ApcMin mice with different Phgdh genotypes. (C) Enzymatic activity of Phgdh analyzed in six λ-Myc; Phgdh+/+ and in six λ-Myc; Phgdh+/− lymphomas. (D) Western blot analysis confirming that Phgdh is absent in tumors arising in recipient mice from λ-Myc;Phgdh−/− embryos. (E) Survival curve of C57BL/6 mice transplanted with l-Myc transgenic E13.5 FLC of indicated Phgdh genotype. λ-Myc; Phgdh+/+ n = 5, λ-Myc; Phgdh+/− n = 14, λ-Myc; Phgdh−/− n = 7. Phgdh is linked to glycolysis and could potentially divert metabolites away from pyruvate usage in the TCA cycle in the mitochondrion. Pyruvate is also kept from entering the TCA cycle via the action of Ldha, encoded by another Myc-regulated gene [23]. Except for RNAi or antisense studies in established tumors, it is not known whether Ldha is needed for the actual transformation event in vivo. To assess this we used a mouse model carrying a procarbazine-induced homozygous germline mutation of Ldha [31]. The mutation has been mapped to an aspartate 223 to histidine exchange which results in a very strong phenotype in erythrocytes causing anemia that is counteracted by extra-medullary hematopoiesis with an associated splenomegaly (Figure S4A). We crossed Ldha mutant mice with λ-Myc mice to generate mice of all relevant genotypes. Some of these mice were sacrificed before they developed tumors to allow analysis of Ldh activity in splenic B cells. Other mice were aged and monitored for tumor development. As seen in Figure S4B, splenic B cells from λ-Myc mice exhibited an elevated level of Ldh activity - consistent with the expression analysis in Figure 1 – whereas the Ldha mutation severely diminished Ldh activity in B cells from both non-transgenic and Myc transgenic mice (Figure S4B). Unexpectedly, the Ldha mutation did not affect Myc-induced B-cell lymphomagenesis. In two independent survival curves generated at Umeå University and Helmholtz Center Munich the median survival time for λ-Myc;Ldhamut/mut was similar to that of λ-Myc;Ldhawt, with no statistical difference (Figure 4A for the Umeå-generated survival curve; Munich curve is shown in Figure S4C).
Fig. 4. Ldha is dispensable for Myc-induced lymphomagenesis but not for the development of Ras-induced fibrosarcomas. 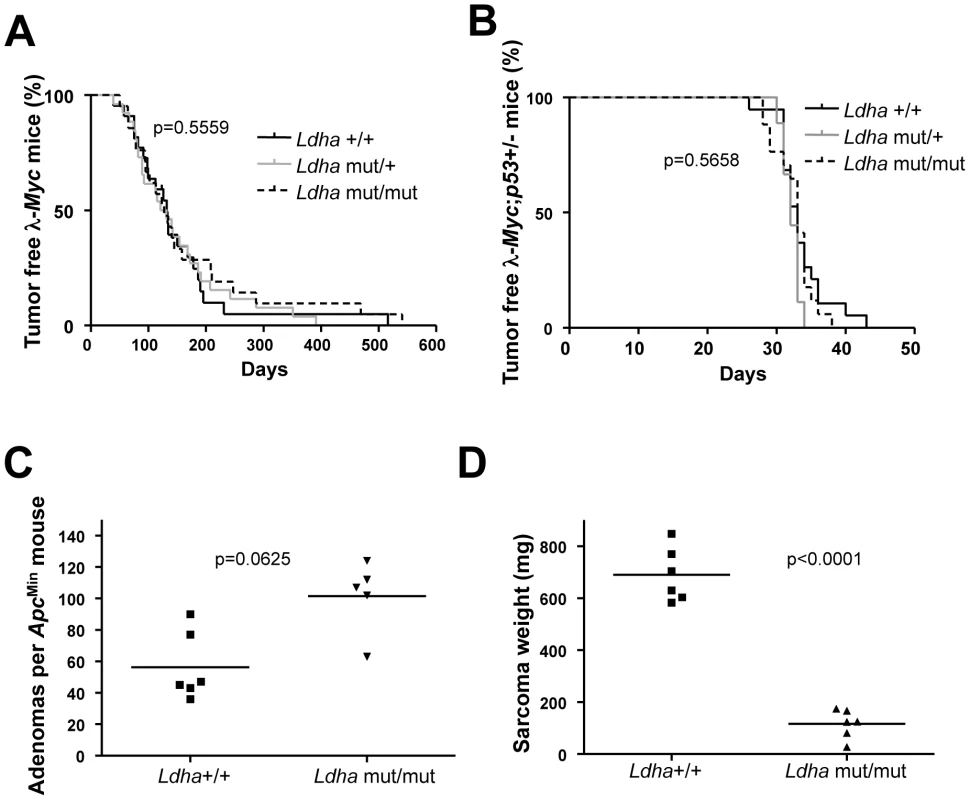
(A) Survival curve of λ-Myc mice generated from interbreedings between Ldhamut/mut and l-Myc transgenic mice. λ-Myc;Ldha+/+ n = 13, λ-Myc;Ldha+/mut n = 26, λ-Myc;Ldhamut/mut n = 21. The p-value is derived from comparing the survival of λ-Myc;Ldha+/+ to λ-Myc;Ldhamut/mut mice. (B) Survival curve of λ-Myc mice generated from interbreedings between Ldhamut/mut, p53 knockout and λ-Myc transgenic mice. λ-Myc;p53+/−;Ldha+/+ n = 19, λ-Myc;p53+/−;Ldha+/mut n = 9, λ-Myc;p53+/−;Ldhamut/mut n = 17. The p-value is derived from comparing the survival of λ-Myc;p53+/−;Ldha+/+ to λ-Myc; p53+/;Ldhamut/mut mice. (C) Amount of adenomas in ApcMin mice with different Ldha genotypes. (D) MEFs of different Ldha genotypes were infected with oncogenic Ras. After subcutaneous injection into syngenic C57BL/6 mice, tumor weights were monitored 16 days post-injection. Two mice were injected with cells per Ldha genotype and the experiment was repeated with three different MEF isolates per Ldha genotype. Our unexpected results suggest several possibilities: either Ldha is dispensable for Myc-induced lymphomagenesis; or a compensatory mechanism is occuring; or Ldha deficiency alters the route of transformation. Firstly, a compensation by another Ldh form is improbable since Ldh activity and Ldhb expression were very low or absent in tumors arising in λ-Myc;Ldhamut/mut mice (Figure S4D and S4E). Secondly, Myc-induced lymphomagenesis in the mouse is known to involve spontaneously arising, cooperating oncogenic mutations of tumor suppressors and other oncogenes to block the oncogenic stress response of Myc [32], [33]. Hence, overexpression of anti-apoptotic proteins such as Bcl-2 or genetic deletion of one tumor suppressor allele such as Arf or p53 dramatically accelerates lymphomagenesis [34]–[36]. To neutralize the genetic heterogeneity in the cooperating oncogenic lesion during lymphomagenesis we interbred the p53 knockout mouse with the Ldha mutant mouse and the λ-Myc mouse. λ-Myc;wildtype, λ-Myc;Ldhamut/wt or λ-Myc;Ldhamut/mut were made heterozygous for p53 by interbreeding. All mice developed disease at an accelerated rate as compared to λ-Myc mice (Figure 4B). The tumors that developed lost the wildtype p53 allele (data not shown). Moreover, the frequency of p53 mutation in the tumors that developed in the first cross (Figure 4A) was not different between Ldha genotypes (data not shown). We conclude that Myc-induced lymphomagenesis can occur normally in mice lacking fully functional Ldha.
Studies using antisense or RNAi have shown that Ldha is important for breast carcinoma, neuroblastoma, fumarase-deficient renal cell cancers, as well as fibroblast and B-cell tumor cells in vitro [17], [18], [20], [23], [37]. Although the specific combination of Myc overexpression with loss of p53 was previously unexplored, other explanations to the differences between our findings and those of others, like experimental methods, culture conditions, oxygen supply or oncogenic pathway, could be at play. To test if a dependency of Ldha could be revealed in settings where Myc is downstream rather than the primary oncogenic instigator, we interbred ApcMin mice and p53 knockout mice with the Ldha mutant mouse. As seen in Figure 4C, Ldha deficiency did not impact adenomagenesis in the ApcMin mice. However, we also created p53−/−;Ldhamut/mut or Ldhawt mouse embryo fibroblasts (MEFs) which were transduced with an oncogenic H-Ras (pBabe-HrasG12V-puro) retrovirus. Interestingly, whereas no growth defect could be observed in vitro, the Ldha mutant Ras-transformed p53 knockout MEFs generated significantly smaller tumors in vivo that appeared less vascularized (Figure 4D and Figure S5A). This effect was not a result of varying expression of oncogenic H-Ras in the different tumors (Figure S5B). Moreover, Colon 26 cells with an endogenous oncogenic Kras allele could not be propagated when transduced with lentiviruses expressing two different Ldha shRNAs despite the fact that these constructs were not lethal in NIH 3T3 cells (data not shown). To confirm the dependency on Ldha we also co-transfected a GFP expressing plasmid with the lentiviral expression constructs expressing Ldha shRNA into Colon 26. As seen in Figure S3C and S3D, we were able to knockdown expression of Ldha in the cells, which resulted in a progressive loss of cells from 48 h post-transfection.
To investigate the ability of Ldha-deficient fibroblasts to proliferate in a hypoxic environment, NIH 3T3 cells infected with Myc (pWLBlast-c-Myc) or Ras (pBabe-HrasG12V-hygro) retroviruses and control or Ldha shRNA lentiviruses were exposed to hypoxia. As expected, hypoxia resulted in the induction of Ldha and Pdk1, both downstream targets of Hif1α (Figure S5C). Interestingly, cells infected with the Ldha shRNA incorporated less 3H-thymidine than cells infected with a control lentivirus (Figure S5D). Thus, Ldha is required under defined conditions such as hypoxia and/or in cells with a deregulated Ras pathway. Therefore an Ldha dependency may not be manifested in a Myc-induced lymphomagenesis setting.
In agreement with this notion, the λ820 mouse B-cell lymphoma line established from λ-Myc mice [38] succumbed to apoptosis when exposed to hypoxia (Figure S6A and S6B), regardless of whether or not Ldha was knocked down (Figure S6C). In addition, Ldha knockdown did not impact lymphomagenesis in vivo (Figure S6D), although knockdown still left a substantial amount of Ldha transcript and activity in this highly Ldha-expressing cell line (Figure S6C and S6E). Nevertheless, given the sensitivity of λ-Myc lymphoma cells to hypoxia (Figure S6A and S6B), it is unlikely that tumorigenesis in this model contains a hypoxic component and thereby dependency on elevated Ldha activity. Indeed, immunohistochemistry showed that Ldha wildtype or mutant lymphomas from λ-Myc mice exhibited a remarkable sparse expression of angiogenic markers CD34 and SMA (Figure S7A). Despite this there were no signs of obvious necrotic areas, suggesting that nutrients and oxygen can diffuse in these non-solid tumors. The staining results were not due to non-functional antibodies as they readily detected the angiogenic markers in normal spleen and in lymphomas that had disseminated in spleens of λ-Myc mice (Figure S7B). It therefore appears as if lymphomas arising in lymph nodes of λ-Myc mice are neither angiogenic, hypoxic or dependent on Ldha activity.
Discussion
We are today beginning to appreciate the fact that oncogenes and tumor suppressor genes not only regulate cell proliferation, immortalization, apoptosis, metastasis and angiogenesis [39] but also cellular metabolism. The change in metabolism and the Warburg effect were for a long time believed to be self-evident and secondary to transformation. It is now known that the metabolic changes occur simultaneously and are governed by the same signal transduction pathways as those governing cell proliferation [40]. Since different tumor cells transform in response to variations of oncogenic mutations it is therefore likely that tumor cells can, or even have to, make different metabolic adaptations as well. Some of these adaptations may make the cells dependent on a certain enzyme, whereas others do not. Our study highlights the Ras oncogene as a potential pathway that requires Ldha, illustrated in Ras-transformed fibroblasts and Colon 26 cells, which carry an endogenous Kras mutation. Indeed, it has previously been shown that neu-transformed breast cancer cells are sensitive to Ldha inhibition by RNAi [37]. Since these cells have an activated Ras pathway [41], this may explain why knockdown of Ldha sensitizes these cells. The potential explanation why Ras-induced fibrosarcomas are sensitive to the Ldha mutation in vivo is that they are not inherently angiogenic, making them sensitive to metabolic perturbation before angiogenesis has occurred. The fact that Myc can stimulate angiogenesis independently of hypoxia-inducible factors [42]–[44] may account for the lack of impact on lymphomagenesis upon mutation of e.g. Ldha. Indeed, we show here that lymphomas from λ-Myc mice are very sensitive to hypoxia, most likely since they are Myc-driven and therefore rely on the TCA cycle and oxidative phosphorylation [45].
Folate biosynthesis has been linked to cancer both from studies on dietary supplements and by the identification of polymorphisms in genes encoding enzymes in folate biosynthesis like SHMT1, MTHFR and TS [46]. In addition, certain drugs like methotrexate target the folate biosynthetic pathway suggesting that this pathway is of critical importance for tumor cell survival. Interestingly, Shmt2 was first identified as a target in a screen for genes that can rescue the growth defect of Myc null rat fibroblasts [47]. In the same study Shmt1 was also shown to be a Myc transcriptional target gene but it was not further functionally characterized. Herein, we provide evidence that Shmt1 is dispensable for Myc-induced lymphomagenesis and that its deletion even accelerates tumorigenesis. The reason for this acceleration is unknown. It could involve effects on senescence or B-cell development as deletion of genes like Suv39h1 and E2f2 accelerates tumorigenesis by these mechanisms, respectively [48], [49]. However, our data corroborate other very recent studies. Using an Shmt1 knockout mouse [50], the Stover group showed that deletion of one allele of Shmt1 promotes adenomagenesis in the ApcMin mice when administered a special diet [26]. In our study the Shmt1 heterozygous ApcMin mice also have the largest mean amount of adenomas, albeit we did not investigate the impact of diet on this model. Interestingly, homozygous deletion of Shmt1 did not impact adenoma formation in ApcMin mice since there was a compensatory increase in thymidylate kinase (TK1) expression [47]: a salvage pathway for thymidine synthesis. We observed a stronger effect on acceleration of Myc-induced lymphomagenesis in homozygous mutant Shmt1 mice, suggesting that the salvage pathway is not completely penetrant. Taken together, we would argue that Shmt1 is a tumor-suppressing modifier in the context of B-cell lymphomas and colorectal adenomas.
One of the most important reasons for the systematic analysis of Myc target genes in tumorigenesis is the potential of identifying or validating future drug targets. Our lymphoma and adenoma data cast doubt on the utility of developing targeted interventions against Ldha, Phgdh and Shmt1. On the other hand, in these models, tumors arise in mice carrying germline mutations of both the oncogenic lesion and the genes encoding the metabolic enzymes. It is thus plausible that adaptations have occurred during development that would not have occurred in cells acutely exposed to an inhibitor. Nevertheless, our data suggest that tumor cells eventually will develop resistance to putative treatments directed against metabolic enzymes since tumor growth undoubtedly can occur in the absence of Shmt1, Phgdh or Ldha. Therefore, a correct stratification is needed to identify patients whose tumors would be sensitive to inhibitors that are under development, for instance against Ldh [6], [18]. Such stratification can be performed based on which oncogenic driver mutation the tumor has acquired. As shown here, oncogenic Ras or pathways utilizing this circuit could be a potential parameter. Moreover, two independent studies published while revising this manuscript suggest that PHGDH amplification could be another oncogenic driver mutation in breast cancer and melanoma, which would sensitize cells to inhibition of Phgdh [51], [52]. As shown here and in these two studies, Phgdh is important in some but not all contexts.
To date, very few inhibitors against Ldh have been identified and those known are either poorly bioavailable and/or have other targets. For instance, sodium oxamate is used in high millimolar concentrations but inhibits aspartate aminotransferase at concentrations where lactate production is not even affected when tumor cell growth is [53]. Gossypol, a natural compound from cottonseed first identified as a male contraceptive, also inhibits anti-apoptotic proteins of the Bcl-2 family making interpretation of anti-cancer activity difficult [54]. Even if improved inhibitors are developed the issue whether or not Ldh is a good target is unresolved. Our genetic study shows that cells carrying a defective Ldha are capable of forming tumors, albeit hindered by hypoxia. We and others also show that ablation of Ldha by RNAi can be detrimental for the cell. It is thus possible that either cell context determines sensitivity, or that the ablation of Ldha protein (RNAi) is more severe than inhibition of its activity (D223H mutation). This notion would lend support to the idea that Ldha may have other functions, potentially disconnected from its activity [55]. For instance, Ldha can be phosphorylated (Tyr238) and localized to the nucleus [56] and has recently been shown to exist in transcription complexes in ES cells [57]. Future studies should address if glycolysis-independent functions of Ldha, as suggested in transcription [58], [59], are the most important functions in some tumors. If so, focus on the development of new therapies should aim at blocking all activities of Ldha.
Materials and Methods
Ethics statement
All animal experiments were performed in accordance with the Regional Animal Ethic Committee Approval no. A6-08 or no. A18-08.
Mouse colonies
All transgenic mice in the study were on pure C57BL/6 background. The λ-Myc-mice and the Ldhmut/mut mice have been previously described [24], [31]. The p53 knockout mice and the ApcMin mice were from Jackson Labs, the C57BL/6 and Balb/c mice used as recipients were from Taconics, and the Phgdh knockout mice were from RIKEN BRC, Japan. Shmt1 knockout mice were generated by blastocyst injection of gene-trapped ES cells (clone AD0236, Sanger Institute Gene-trap Resources) at Umeå Transgene Core Facility. After confirmation of germline transmission, mice were backcrossed to C57BL/6 for at least ten generations. Illumina SNP genotyping confirmed that the mice were at least 96% C57BL/6 before starting interbreeding with λ-Myc transgenic mice, p53 knockout mice and ApcMin mice.
Tumor monitoring and analyses
All mice used in the study were monitored by group members and personnel at the animal facilities (Umeå Transgene Core Facility or Helmholtz Centre, Munich). When showing signs of disease, λ-Myc mice were sacrificed and lymphomas were collected for analyses. Dates of sacrifice were entered into GraphPad Prizm software for the generation of survival curves. Lymphomas were either snap frozen for RNA and protein analyses, or formalin-fixed and embedded in paraffin. Paraffin blocks were sectioned and processed by standard immunohistochemistry methodology using antibodies directed against smooth musle actin (SMA; Sigma) or CD34 (Abcam) at the Histocenter core facility (Göteborg, Sweden).
The ApcMin mice used for adenoma formation studies were sacrificed and analyzed between 120–140 days of age, or when showing signs of disease. The small intestine and colon were dissected out, washed with phosphate-buffered saline and cut length-wise at which point adenomas were counted and tissues were harvested for analyses. Adenomas were counted using dissection microscope as well as by eye by two independent observers. The adenomas were scored irrespective of size and numbers per mouse were entered into GraphPad Prizm software for generation of graphs.
Microarray analysis
The analysis of gene expression changes between magnetically sorted B cells from wildtype or λ-Myc transgenic mice was performed using the Illumina BeadChip system. For in vitro transcription amplification, 200 ng of RNA was used with the Illumina RNA Amplification Kit (Ambion). Amplified RNA (1.5 µg) was hybridized to the Sentrix MouseRef-8 Expression Beadchip (Illumina). The primary data were collected from the BeadChips using the manufacturer's BeadArray Reader and analyzed using the supplied scanner software. Data normalization was performed by cubic spline normalization using Illumina's Beadstudio v3 software. Clustering and visualization of genes encoding metabolic enzymes was done using the Spotfire software.
Cell culture
MEFs were generated by mechanical disruption and trypsin-digestion of E13.5 embryos from which the fetal liver and the head had been removed. The single-cell suspension was grown in DMEM supplemented with 10% fetal bovine serum (FBS), 50 µM β-mercaptoethanol, 1× glutamine, pyruvate, non-essential amino acids and antibiotics (Invitrogen). 293T cells and NIH 3T3 (from ATCC) were routinely maintained in DMEM supplemented with 10% FBS, glutamine and antibiotics. Colon 26 cells (from Cell Line Services) were cultured in RPMI supplemented with 10% FBS, glutamine and antibiotics. The λ820 cell line was established from λ-Myc transgenic mice and cultured as previously described [38].
Viral production and transductions
Retroviruses and lentiviruses were produced by calcium phosphate-mediated transfection of 293T cells. For retroviruses the following plasmids from Addgene were used: pBABE-HrasG12V-puro, pBABE-HrasG12V-hygro, MSCV-Myc-IRES-GFP, pWZL-Myc-blasticidine, pBABE-puro, pBABE-hygro together with pCL-Eco (encoding gag, pol and ecotropic envelope). Lentiviruses for RNAi were made using pLKO.1 puro vectors expressing shRNAs (Sigma Mission RNAi), together with packaging plasmids pCMV R8.2dvpr and pHCMV-EcoEnv (both from Addgene). Two or three different shRNAs were used per gene (Table S2) and they were compared to a control pLKO vector expressing a control shRNA with no known target in the mouse genome (non-target vector from Sigma). Thirty-six hours post-transfection, the media was harvested four times during an additional 36 h. The virus was filtered and either frozen down in aliquots or applied on target cells in the presence of 4–8 µg/ml polybrene. Following antibiotics selection for 48 h (or GFP analysis of FACS to confirm at least 90% positive cells) cells were expanded and used for experiments. The shRNA-containing viruses were always introduced into NIH 3T3 cells after the transduction with control, Myc or Ras retroviruses.
Subcutaneous tumor formation and analyses
MEFs used for sarcoma formation studies were infected with retroviruses encoding oncogenic Hras, whereas Colon 26 cells were infected with lentiviruses expressing shRNA against Shmt2, Phgdh and Ldha. For MEFs, 1×106 were injected subcutaneously into C57BL/6 recipients, whereas 5×105 Colon 26 cells were mixed with Matrigel (1∶1) and injected into Balb/c mice. When tumors appeared, the mice were sacrificed and tumors were weighed and material was harvested for analyses. For immunofluorescence, formalin-fixed tumors were embedded in paraffin and sectioned (8 µm) onto glass slides. Following deparaffinization and rehydration, slides were either stained with H&E or subjected to Hoechst and antibody staining using Cy3-conjugated control or anti-smooth muscle actin antibody (Sigma) according to standard methodology. Following mounting the sections they were analyzed in a fluorescence microscope.
Fetal liver transplants
Fetal livers of E13.5 embryos were dissected out of embryos from timed pregnancies between λ-Myc; Phgdh+/− males and Phgdh+/− females. Each individual liver was dissociated through a cell strainer and injected via the tail-vein into one lethally gamma-irradiated (9.25 Gy) C57BL/6 recipient. Tissue from each embryo was taken for genotyping and the mice positive for the λ-Myc transgene were followed for lymphoma development and treated as previously described in Mouse colonies and Tumor monitoring and analyses.
Protein and RNA expression
For protein expression analyses by Western blot, cells and tumors were lysed in an appropriate amount of lysis buffer on ice for 30 min. Following sonication, clearing by centrifugation and protein determination, an equal amount of protein per well was loaded on SDS-PAGE gels and separated by electrophoresis. The proteins were transferred to a nitrocellulose membrane, which was subsequently blocked with TBST containing 5% non-fat dried milk. The membranes were then blotted with primary and horseradish peroxidise-conjugated secondary antibodies dissolved in blocking solution. After washing with TBST, the bound proteins were visualized by enhanced chemoluminescence. The primary antibodies used were from BD Biosciences (H-Ras), Cell signalling (c-Myc and Pdk1), Sigma-Aldrich (Ldha, Ldhb, Shmt1, Shmt2 and β-actin) and Atlas Antibodies (Shmt1 and Phgdh).
RNA expression was measured by quantitative reverse transcriptase PCR (qRT-PCR). Briefly, RNA was prepared using the NucleoSpin RNA II kit (Macherey-Nagel). cDNA was prepared using the First strand synthesis kit (Fermentas) and the PCR was run using the KAPA mastermix (Biotools) on an iQ real-time PCR machine (Bio-Rad). Primer sequences can be found in Table S3.
Hypoxia experiment
NIH 3T3 cells expressing either HrasV12, c-Myc or both were used for parallel infections of lentiviruses encoding shRNAs against Ldha. The same amount of cells were seeded in 24-well format and subsequently infected. 72 hours post infection, each well was split into 3×96 well format in duplicate plates. One set of plates was placed in a hypoxic environment (using the Modular incubator chamber, Billups-Rothenberg Inc.) for 40 hours after which the hypoxic treatment was terminated and 3H-thymidine was added to all wells. After two hours, the plates were freeze-thawed and the cells harvested onto glass fibre filters. Microscint scintillation solution was administered to the dried filter, which were subsequently counted on a TopCount scintillation counter. λ820 cells were subjected to hypoxia in 24 well plates or 25 cm2 flasks (2×105 cells/ml) and were harvested for cell counting and apoptosis analyses or RNA analyses, respectively. For apoptosis analyses, cells were stained with Vindelövs reagent (10 mM Tris, 10 mM NaCl, 75 µM propidium iodine, 0.1% Igepal, and 700 units/liter RNase adjusted to pH 8.0) and then analyzed with a FACScalibur flow cytometer (BD Biosciences). Apoptosis was determined using DNA histograms and was based on the number of cells that carried less than diploid DNA content (sub-G1) in a logarithmic FL2 channel.
Enzyme activity assay
Total protein lysates were prepared as described above and the same amount of protein was assayed for LDH activity using the Cytotoxicity detection kit (Roche Applied Science). The reactions were read using the Tecan Infinite200 plate reader at 492 nm. Lysates were also used to assess Shmt activity and Phgdh in accordance with published methods [60], [61].
Supporting Information
Zdroje
1. EilersMEisenmanRN 2008 Myc's broad reach. Genes Dev 22 2755 2766
2. WagnerAJMeyersCLaiminsLAHayN 1993 c-Myc induces the expression and activity of ornithine decarboxylase. Cell Growth Differ 4 879 883
3. Bello-FernandezCPackhamGClevelandJL 1993 The ornithine decarboxylase gene is a transcriptional target of c-Myc. Proc Natl Acad Sci U S A 90 7804 7808
4. ShimHDoldeCLewisBCWuCSDangG 1997 c-Myc transactivation of LDH-A: implications for tumor metabolism and growth. Proc Natl Acad Sci U S A 94 6658 6663
5. MiltenbergerRJSukowKAFarnhamPJ 1995 An E-box-mediated increase in cad transcription at the G1/S-phase boundary is suppressed by inhibitory c-Myc mutants. Mol Cell Biol 15 2527 2535
6. DangCVLeAGaoP 2009 MYC-induced cancer cell energy metabolism and therapeutic opportunities. Clin Cancer Res 15 6479 6483
7. PatelJHLobodaAPShoweMKShoweLCMcMahonSB 2004 Analysis of genomic targets reveals complex functions of MYC. Nat Rev Cancer 4 562 568
8. RounbehlerRJLiWHallMAYangCFallahiM 2009 Targeting ornithine decarboxylase impairs development of MYCN-amplified neuroblastoma. Cancer Res 69 547 553
9. HogartyMDNorrisMDDavisKLiuXEvageliouNF 2008 ODC1 is a critical determinant of MYCN oncogenesis and a therapeutic target in neuroblastoma. Cancer Res 68 9735 9745
10. NilssonJAKellerUBaudinoTAYangCNortonS 2005 Targeting ornithine decarboxylase in Myc-induced lymphomagenesis prevents tumor formation. Cancer Cell 7 433 444
11. LiMRenSTilliMTFlawsJALubetR 2003 Chemoprevention of mammary carcinogenesis in a transgenic mouse model by alpha-difluoromethylornithine (DFMO) in the diet is associated with decreased cyclin D1 activity. Oncogene 22 2568 2572
12. GuptaSAhmadNMarengoSRMacLennanGTGreenbergNM 2000 Chemoprevention of prostate carcinogenesis by alpha-difluoromethylornithine in TRAMP mice. Cancer Res 60 5125 5133
13. JacobyRFColeCETutschKNewtonMAKelloffG 2000 Chemopreventive efficacy of combined piroxicam and difluoromethylornithine treatment of Apc mutant Min mouse adenomas, and selective toxicity against Apc mutant embryos. Cancer Res 60 1864 1870
14. MitsunagaSClapperMLitwinSWattsPBauerB 1997 Chemopreventive effect of difluoromethylornithine (DFMO) on mouse skin squamous cell carcinomas induced by benzo(a)pyrene. J Cell Biochem Suppl 28–29 81 89
15. MeyskensFLJrMcLarenCEPelotDFujikawa-BrooksSCarpenterPM 2008 Difluoromethylornithine plus sulindac for the prevention of sporadic colorectal adenomas: a randomized placebo-controlled, double-blind trial. Cancer Prev Res (Phila Pa) 1 32 38
16. WangJBEricksonJWFujiRRamachandranSGaoP 2010 Targeting mitochondrial glutaminase activity inhibits oncogenic transformation. Cancer Cell 18 207 219
17. QingGSkuliNMayesPAPawelBMartinezD 2010 Combinatorial regulation of neuroblastoma tumor progression by N-Myc and hypoxia inducible factor HIF-1alpha. Cancer Res 70 10351 10361
18. LeACooperCRGouwAMDinavahiRMaitraA 2010 Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proc Natl Acad Sci U S A 107 2037 2042
19. DavidCJChenMAssanahMCanollPManleyJL 2010 HnRNP proteins controlled by c-Myc deregulate pyruvate kinase mRNA splicing in cancer. Nature 463 364 368
20. XieHValeraVAMerinoMJAmatoAMSignorettiS 2009 LDH-A inhibition, a therapeutic strategy for treatment of hereditary leiomyomatosis and renal cell cancer. Mol Cancer Ther 8 626 635
21. GaoPTchernyshyovIChangTCLeeYSKitaK 2009 c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature
22. ChristofkHRVander HeidenMGHarrisMHRamanathanAGersztenRE 2008 The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature 452 230 233
23. ShimHChunYSLewisBCDangCV 1998 A unique glucose-dependent apoptotic pathway induced by c-Myc. Proc Natl Acad Sci U S A 95 1511 1516
24. KovalchukALQiCFTorreyTATaddesse-HeathLFeigenbaumL 2000 Burkitt lymphoma in the mouse. J Exp Med 192 1183 1190
25. WarburgO 1956 On the origin of cancer cells. Science 123 309 314
26. MacfarlaneAJPerryCAMcEnteeMFLinDMStoverPJ 2011 Shmt1 heterozygosity impairs folate-dependent thymidylate synthesis capacity and modifies risk of apcmin-mediated intestinal cancer risk. Cancer Res 71 2098 2107
27. FukasawaKWienerFVande WoudeGFMaiS 1997 Genomic instability and apoptosis are frequent in p53 deficient young mice. Oncogene 15 1295 1302
28. SansomOJMenielVSMuncanVPhesseTJWilkinsJA 2007 Myc deletion rescues Apc deficiency in the small intestine. Nature 446 676 679
29. WangYVan BecelaereKJiangPPrzybranowskiSOmerC 2005 A role for K-ras in conferring resistance to the MEK inhibitor, CI-1040. Neoplasia 7 336 347
30. YoshidaKFuruyaSOsukaSMitomaJShinodaY 2004 Targeted disruption of the mouse 3-phosphoglycerate dehydrogenase gene causes severe neurodevelopmental defects and results in embryonic lethality. J Biol Chem 279 3573 3577
31. PretschWMerkleSFavorJWernerT 1993 A mutation affecting the lactate dehydrogenase locus Ldh-1 in the mouse. II. Mechanism of the LDH-A deficiency associated with hemolytic anemia. Genetics 135 161 170
32. MurphyDJJunttilaMRPouyetLKarnezisAShchorsK 2008 Distinct thresholds govern Myc's biological output in vivo. Cancer Cell 14 447 457
33. ZindyFEischenCMRandleDHKamijoTClevelandJL 1998 Myc signaling via the ARF tumor suppressor regulates p53-dependent apoptosis and immortalization. Genes Dev 12 2424 2433
34. EischenCMWeberJDRousselMFSherrCJClevelandJL 1999 Disruption of the ARF-Mdm2-p53 tumor suppressor pathway in Myc-induced lymphomagenesis. Genes Dev 13 2658 2669
35. SchmittCAMcCurrachMEde StanchinaEWallace-BrodeurRRLoweSW 1999 INK4a/ARF mutations accelerate lymphomagenesis and promote chemoresistance by disabling p53. Genes Dev 13 2670 2677
36. StrasserAHarrisAWBathMLCoryS 1990 Novel primitive lymphoid tumours induced in transgenic mice by cooperation between myc and bcl-2. Nature 348 331 333
37. FantinVRSt-PierreJLederP 2006 Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell 9 425 434
38. HöglundANilssonLMForshellLPMacleanKHNilssonJA 2009 Myc sensitizes p53-deficient cancer cells to the DNA-damaging effects of the DNA methyltransferase inhibitor decitabine. Blood 113 4281 4288
39. HanahanDWeinbergRA 2000 The hallmarks of cancer. Cell 100 57 70
40. HsuPPSabatiniDM 2008 Cancer cell metabolism: Warburg and beyond. Cell 134 703 707
41. ReeseDMSlamonDJ 1997 HER-2/neu signal transduction in human breast and ovarian cancer. Stem Cells 15 1 8
42. BaudinoTAMcKayCPendeville-SamainHNilssonJAMacleanKH 2002 c-Myc is essential for vasculogenesis and angiogenesis during development and tumor progression. Genes Dev 16 2530 2543
43. DewsMHomayouniAYuDMurphyDSevignaniC 2006 Augmentation of tumor angiogenesis by a Myc-activated microRNA cluster. Nat Genet 38 1060 1065
44. PelengarisSLittlewoodTKhanMEliaGEvanG 1999 Reversible activation of c-Myc in skin: induction of a complex neoplastic phenotype by a single oncogenic lesion. Mol Cell 3 565 577
45. MorrishFNerettiNSedivyJMHockenberyDM 2008 The oncogene c-Myc coordinates regulation of metabolic networks to enable rapid cell cycle entry. Cell Cycle 7 1054 1066
46. UlrichCM 2005 Nutrigenetics in cancer research–folate metabolism and colorectal cancer. J Nutr 135 2698 2702
47. NikiforovMAChandrianiSO'ConnellBPetrenkoOKotenkoI 2002 A functional screen for Myc-responsive genes reveals serine hydroxymethyltransferase, a major source of the one-carbon unit for cell metabolism. Mol Cell Biol 22 5793 5800
48. ReimannMLeeSLoddenkemperCDorrJRTaborV 2010 Tumor stroma-derived TGF-beta limits myc-driven lymphomagenesis via Suv39h1-dependent senescence. Cancer Cell 17 262 272
49. RempelREMoriSGasparettoMGlozakMAAndrechekER 2009 A role for E2F activities in determining the fate of Myc-induced lymphomagenesis. PLoS Genet 5 e1000640 doi:10.1371/journal.pgen.1000640
50. MacFarlaneAJLiuXPerryCAFlodbyPAllenRH 2008 Cytoplasmic serine hydroxymethyltransferase regulates the metabolic partitioning of methylenetetrahydrofolate but is not essential in mice. J Biol Chem 283 25846 25853
51. LocasaleJWGrassianARMelmanTLyssiotisCAMattainiKR 2011 Phosphoglycerate dehydrogenase diverts glycolytic flux and contributes to oncogenesis. Nat Genet 43 869 874
52. PossematoRMarksKMShaulYDPacoldMEKimD 2011 Functional genomics reveal that the serine synthesis pathway is essential in breast cancer. Nature 476 346 350
53. ThornburgJMNelsonKKClemBFLaneANArumugamS 2008 Targeting aspartate aminotransferase in breast cancer. Breast Cancer Res 10 R84
54. KitadaSLeoneMSarethSZhaiDReedJC 2003 Discovery, characterization, and structure-activity relationships studies of proapoptotic polyphenols targeting B-cell lymphocyte/leukemia-2 proteins. J Med Chem 46 4259 4264
55. KimJWDangCV 2005 Multifaceted roles of glycolytic enzymes. Trends Biochem Sci 30 142 150
56. ZhongXHHowardBD 1990 Phosphotyrosine-containing lactate dehydrogenase is restricted to the nuclei of PC12 pheochromocytoma cells. Mol Cell Biol 10 770 776
57. KimJWooAJChuJSnowJWFujiwaraY 2010 A Myc network accounts for similarities between embryonic stem and cancer cell transcription programs. Cell 143 313 324
58. DaiRPYuFXGohSRChngHWTanYL 2008 Histone 2B (H2B) expression is confined to a proper NAD+/NADH redox status. J Biol Chem 283 26894 26901
59. ZhengLRoederRGLuoY 2003 S phase activation of the histone H2B promoter by OCA-S, a coactivator complex that contains GAPDH as a key component. Cell 114 255 266
60. AchouriYRiderMHSchaftingenEVRobbiM 1997 Cloning, sequencing and expression of rat liver 3-phosphoglycerate dehydrogenase. Biochem J 323 Pt 2 365 370
61. GellerAMKotbMY 1989 A binding assay for serine hydroxymethyltransferase. Anal Biochem 180 120 125
Štítky
Genetika Reprodukční medicína
Článek Physiological Notch Signaling Maintains Bone Homeostasis via RBPjk and Hey Upstream of NFATc1Článek Intronic -Regulatory Modules Mediate Tissue-Specific and Microbial Control of / TranscriptionČlánek Probing the Informational and Regulatory Plasticity of a Transcription Factor DNA–Binding DomainČlánek Repression of Germline RNAi Pathways in Somatic Cells by Retinoblastoma Pathway Chromatin ComplexesČlánek An Alu Element–Associated Hypermethylation Variant of the Gene Is Associated with Childhood ObesityČlánek Three Essential Ribonucleases—RNase Y, J1, and III—Control the Abundance of a Majority of mRNAsČlánek Genomic Tools for Evolution and Conservation in the Chimpanzee: Is a Genetically Distinct Population
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 3
-
Všechny články tohoto čísla
- Comprehensive Research Synopsis and Systematic Meta-Analyses in Parkinson's Disease Genetics: The PDGene Database
- Genomic Analysis of the Hydrocarbon-Producing, Cellulolytic, Endophytic Fungus
- Networks of Neuronal Genes Affected by Common and Rare Variants in Autism Spectrum Disorders
- Akirin Links Twist-Regulated Transcription with the Brahma Chromatin Remodeling Complex during Embryogenesis
- Too Much Cleavage of Cyclin E Promotes Breast Tumorigenesis
- Imprinted Genes … and the Number Is?
- Genetic Architecture of Highly Complex Chemical Resistance Traits across Four Yeast Strains
- Exploring the Complexity of the HIV-1 Fitness Landscape
- MNS1 Is Essential for Spermiogenesis and Motile Ciliary Functions in Mice
- A Fundamental Regulatory Mechanism Operating through OmpR and DNA Topology Controls Expression of Pathogenicity Islands SPI-1 and SPI-2
- Evidence for Positive Selection on a Number of MicroRNA Regulatory Interactions during Recent Human Evolution
- Variation in Modifies Risk of Neonatal Intestinal Obstruction in Cystic Fibrosis
- PIF4–Mediated Activation of Expression Integrates Temperature into the Auxin Pathway in Regulating Hypocotyl Growth
- Critical Evaluation of Imprinted Gene Expression by RNA–Seq: A New Perspective
- A Meta-Analysis and Genome-Wide Association Study of Platelet Count and Mean Platelet Volume in African Americans
- Mouse Genetics Suggests Cell-Context Dependency for Myc-Regulated Metabolic Enzymes during Tumorigenesis
- Transcriptional Control in Cardiac Progenitors: Tbx1 Interacts with the BAF Chromatin Remodeling Complex and Regulates
- Synthetic Lethality of Cohesins with PARPs and Replication Fork Mediators
- APOBEC3G-Induced Hypermutation of Human Immunodeficiency Virus Type-1 Is Typically a Discrete “All or Nothing” Phenomenon
- Interpreting Meta-Analyses of Genome-Wide Association Studies
- Error-Prone ZW Pairing and No Evidence for Meiotic Sex Chromosome Inactivation in the Chicken Germ Line
- -Dependent Chemosensory Functions Contribute to Courtship Behavior in
- Diverse Forms of Splicing Are Part of an Evolving Autoregulatory Circuit
- Phenotypic Plasticity of the Drosophila Transcriptome
- Physiological Notch Signaling Maintains Bone Homeostasis via RBPjk and Hey Upstream of NFATc1
- Precocious Metamorphosis in the Juvenile Hormone–Deficient Mutant of the Silkworm,
- Igf1r Signaling Is Indispensable for Preimplantation Development and Is Activated via a Novel Function of E-cadherin
- Accurate Prediction of Inducible Transcription Factor Binding Intensities In Vivo
- Mitochondrial Oxidative Stress Alters a Pathway in Strongly Resembling That of Bile Acid Biosynthesis and Secretion in Vertebrates
- Mammalian Neurogenesis Requires Treacle-Plk1 for Precise Control of Spindle Orientation, Mitotic Progression, and Maintenance of Neural Progenitor Cells
- Tcf7 Is an Important Regulator of the Switch of Self-Renewal and Differentiation in a Multipotential Hematopoietic Cell Line
- REST–Mediated Recruitment of Polycomb Repressor Complexes in Mammalian Cells
- Intronic -Regulatory Modules Mediate Tissue-Specific and Microbial Control of / Transcription
- Age-Dependent Brain Gene Expression and Copy Number Anomalies in Autism Suggest Distinct Pathological Processes at Young Versus Mature Ages
- A Genome-Wide Association Study Identifies Variants Underlying the Shade Avoidance Response
- -by- Regulatory Divergence Causes the Asymmetric Lethal Effects of an Ancestral Hybrid Incompatibility Gene
- Genome-Wide Association and Functional Follow-Up Reveals New Loci for Kidney Function
- A Natural System of Chromosome Transfer in
- Cell Size and the Initiation of DNA Replication in Bacteria
- Probing the Informational and Regulatory Plasticity of a Transcription Factor DNA–Binding Domain
- Repression of Germline RNAi Pathways in Somatic Cells by Retinoblastoma Pathway Chromatin Complexes
- Temporal Transcriptional Profiling of Somatic and Germ Cells Reveals Biased Lineage Priming of Sexual Fate in the Fetal Mouse Gonad
- Rapid Analysis of Genome Rearrangements by Multiplex Ligation–Dependent Probe Amplification
- Metabolic Profiling of a Mapping Population Exposes New Insights in the Regulation of Seed Metabolism and Seed, Fruit, and Plant Relations
- The Atypical Calpains: Evolutionary Analyses and Roles in Cellular Degeneration
- The Silkworm Coming of Age—Early
- Development of a Panel of Genome-Wide Ancestry Informative Markers to Study Admixture Throughout the Americas
- Balanced Codon Usage Optimizes Eukaryotic Translational Efficiency
- The Min System and Nucleoid Occlusion Are Not Required for Identifying the Division Site in but Ensure Its Efficient Utilization
- Neurobeachin, a Regulator of Synaptic Protein Targeting, Is Associated with Body Fat Mass and Feeding Behavior in Mice and Body-Mass Index in Humans
- Statistical Analysis of Readthrough Levels for Nonsense Mutations in Mammalian Cells Reveals a Major Determinant of Response to Gentamicin
- Gene Reactivation by 5-Aza-2′-Deoxycytidine–Induced Demethylation Requires SRCAP–Mediated H2A.Z Insertion to Establish Nucleosome Depleted Regions
- The miR-35-41 Family of MicroRNAs Regulates RNAi Sensitivity in
- Genetic Basis of Hidden Phenotypic Variation Revealed by Increased Translational Readthrough in Yeast
- An Alu Element–Associated Hypermethylation Variant of the Gene Is Associated with Childhood Obesity
- Modelling Human Regulatory Variation in Mouse: Finding the Function in Genome-Wide Association Studies and Whole-Genome Sequencing
- Novel Loci for Adiponectin Levels and Their Influence on Type 2 Diabetes and Metabolic Traits: A Multi-Ethnic Meta-Analysis of 45,891 Individuals
- Polycomb-Like 3 Promotes Polycomb Repressive Complex 2 Binding to CpG Islands and Embryonic Stem Cell Self-Renewal
- Insulin/IGF-1 and Hypoxia Signaling Act in Concert to Regulate Iron Homeostasis in
- EMF1 and PRC2 Cooperate to Repress Key Regulators of Arabidopsis Development
- Three Essential Ribonucleases—RNase Y, J1, and III—Control the Abundance of a Majority of mRNAs
- Contrasted Patterns of Molecular Evolution in Dominant and Recessive Self-Incompatibility Haplotypes in
- A Machine Learning Approach for Identifying Novel Cell Type–Specific Transcriptional Regulators of Myogenesis
- Genomic Tools for Evolution and Conservation in the Chimpanzee: Is a Genetically Distinct Population
- Nos2 Inactivation Promotes the Development of Medulloblastoma in Mice by Deregulation of Gap43–Dependent Granule Cell Precursor Migration
- Intracranial Aneurysm Risk Locus 5q23.2 Is Associated with Elevated Systolic Blood Pressure
- Heritability and Genetic Correlations Explained by Common SNPs for Metabolic Syndrome Traits
- A Genome-Wide Association Study of Nephrolithiasis in the Japanese Population Identifies Novel Susceptible Loci at 5q35.3, 7p14.3, and 13q14.1
- DNA Damage in Nijmegen Breakage Syndrome Cells Leads to PARP Hyperactivation and Increased Oxidative Stress
- DNA Resection at Chromosome Breaks Promotes Genome Stability by Constraining Non-Allelic Homologous Recombination
- Genetic Analysis of Floral Symmetry in Van Gogh's Sunflowers Reveals Independent Recruitment of Genes in the Asteraceae
- A Splice Site Variant in the Bovine Gene Compromises Growth and Regulation of the Inflammatory Response
- Promoter Nucleosome Organization Shapes the Evolution of Gene Expression
- The Nucleoside Diphosphate Kinase Gene Acts as Quantitative Trait Locus Promoting Non-Mendelian Inheritance
- The Ciliogenic Transcription Factor RFX3 Regulates Early Midline Distribution of Guidepost Neurons Required for Corpus Callosum Development
- Phosphorylation of the RNA–Binding Protein HOW by MAPK/ERK Enhances Its Dimerization and Activity
- A Genome-Wide Scan of Ashkenazi Jewish Crohn's Disease Suggests Novel Susceptibility Loci
- Parkinson's Disease–Associated Kinase PINK1 Regulates Miro Protein Level and Axonal Transport of Mitochondria
- LMW-E/CDK2 Deregulates Acinar Morphogenesis, Induces Tumorigenesis, and Associates with the Activated b-Raf-ERK1/2-mTOR Pathway in Breast Cancer Patients
- Mapping the Hsp90 Genetic Interaction Network in Reveals Environmental Contingency and Rewired Circuitry
- Autoregulation of the Noncoding RNA Gene
- The Human Pancreatic Islet Transcriptome: Expression of Candidate Genes for Type 1 Diabetes and the Impact of Pro-Inflammatory Cytokines
- Spo0A∼P Imposes a Temporal Gate for the Bimodal Expression of Competence in
- Antagonistic Regulation of Apoptosis and Differentiation by the Cut Transcription Factor Represents a Tumor-Suppressing Mechanism in
- A Downstream CpG Island Controls Transcript Initiation and Elongation and the Methylation State of the Imprinted Macro ncRNA Promoter
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- PIF4–Mediated Activation of Expression Integrates Temperature into the Auxin Pathway in Regulating Hypocotyl Growth
- Metabolic Profiling of a Mapping Population Exposes New Insights in the Regulation of Seed Metabolism and Seed, Fruit, and Plant Relations
- A Splice Site Variant in the Bovine Gene Compromises Growth and Regulation of the Inflammatory Response
- Comprehensive Research Synopsis and Systematic Meta-Analyses in Parkinson's Disease Genetics: The PDGene Database
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



