-
Články
Reklama
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
ReklamaPrecocious Metamorphosis in the Juvenile Hormone–Deficient Mutant of the Silkworm,
Insect molting and metamorphosis are intricately governed by two hormones, ecdysteroids and juvenile hormones (JHs). JHs prevent precocious metamorphosis and allow the larva to undergo multiple rounds of molting until it attains the proper size for metamorphosis. In the silkworm, Bombyx mori, several “moltinism” mutations have been identified that exhibit variations in the number of larval molts; however, none of them have been characterized molecularly. Here we report the identification and characterization of the gene responsible for the dimolting (mod) mutant that undergoes precocious metamorphosis with fewer larval–larval molts. We show that the mod mutation results in complete loss of JHs in the larval hemolymph and that the mutant phenotype can be rescued by topical application of a JH analog. We performed positional cloning of mod and found a null mutation in the cytochrome P450 gene CYP15C1 in the mod allele. We also demonstrated that CYP15C1 is specifically expressed in the corpus allatum, an endocrine organ that synthesizes and secretes JHs. Furthermore, a biochemical experiment showed that CYP15C1 epoxidizes farnesoic acid to JH acid in a highly stereospecific manner. Precocious metamorphosis of mod larvae was rescued when the wild-type allele of CYP15C1 was expressed in transgenic mod larvae using the GAL4/UAS system. Our data therefore reveal that CYP15C1 is the gene responsible for the mod mutation and is essential for JH biosynthesis. Remarkably, precocious larval–pupal transition in mod larvae does not occur in the first or second instar, suggesting that authentic epoxidized JHs are not essential in very young larvae of B. mori. Our identification of a JH–deficient mutant in this model insect will lead to a greater understanding of the molecular basis of the hormonal control of development and metamorphosis.
Published in the journal: . PLoS Genet 8(3): e32767. doi:10.1371/journal.pgen.1002486
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1002486Summary
Insect molting and metamorphosis are intricately governed by two hormones, ecdysteroids and juvenile hormones (JHs). JHs prevent precocious metamorphosis and allow the larva to undergo multiple rounds of molting until it attains the proper size for metamorphosis. In the silkworm, Bombyx mori, several “moltinism” mutations have been identified that exhibit variations in the number of larval molts; however, none of them have been characterized molecularly. Here we report the identification and characterization of the gene responsible for the dimolting (mod) mutant that undergoes precocious metamorphosis with fewer larval–larval molts. We show that the mod mutation results in complete loss of JHs in the larval hemolymph and that the mutant phenotype can be rescued by topical application of a JH analog. We performed positional cloning of mod and found a null mutation in the cytochrome P450 gene CYP15C1 in the mod allele. We also demonstrated that CYP15C1 is specifically expressed in the corpus allatum, an endocrine organ that synthesizes and secretes JHs. Furthermore, a biochemical experiment showed that CYP15C1 epoxidizes farnesoic acid to JH acid in a highly stereospecific manner. Precocious metamorphosis of mod larvae was rescued when the wild-type allele of CYP15C1 was expressed in transgenic mod larvae using the GAL4/UAS system. Our data therefore reveal that CYP15C1 is the gene responsible for the mod mutation and is essential for JH biosynthesis. Remarkably, precocious larval–pupal transition in mod larvae does not occur in the first or second instar, suggesting that authentic epoxidized JHs are not essential in very young larvae of B. mori. Our identification of a JH–deficient mutant in this model insect will lead to a greater understanding of the molecular basis of the hormonal control of development and metamorphosis.
Introduction
The number of larval instars in insects varies greatly across insect taxa, and can even vary at the intraspecific level [1], [2], [3]. In general, phylogenetically higher insects tend to have fewer larval instars (three to eight) compared to species from basal lineages, such as Ephemeroptera, Odonata and Plecoptera (more than ten) [1], [2], [3]. In many species, the number of larval instars is affected by genetic and environmental factors, such as temperature, nutritional conditions, photoperiod, humidity, injuries, and sex [1], [2]. The variation in the number of larval instars in the insect lifecycle is generally considered to be an adaptive response to diverse environmental conditions in order to ensure the attainment of a threshold-size for metamorphosis [1], [2], [3], [4].
The silkworm Bombyx mori, a classic model organism for endocrinology, has been reared by humans for thousands of years, and more than 1,000 strains are currently maintained [5], [6], [7]. Among these, several “moltinism” strains have been identified that exhibit variations in the number of larval instars [6], [7]. Silkworms typically have five larval instars, but the moltinism strains vary between three and seven [6], [7]. For example, precocious larval-pupal metamorphosis is observed in the mod (dimolting, chromosome 11–27.4 cM), rt (recessive trimolting, 7–9.0) and M3 (Moltinism, 6–24.1) strains, while extra larval molting is observed in the M5 (Moltinism, 6–24.1) strain [6], [7]. To date, however, none of these loci has been characterized at the molecular level. Given the availability of whole genome data and post-genomic tools in B. mori [8], [9], [10], these strains offer a valuable resource for elucidating the molecular mechanism that underlies plasticity in the number of larval instars.
Here we report the identification and characterization of the gene responsible for the mod mutation that causes precocious larval-pupal metamorphosis in the third or fourth instar [11]. Most mod larvae form larval-pupal intermediates, but some individuals can become miniature moths with normal fertility. Thus, the mod mutant strain can be maintained as homozygous stocks [6], [11], [12]. We demonstrate that the mod locus encodes CYP15C1, a cytochrome P450 involved in the biosynthesis of juvenile hormones (JHs), whose “status quo” action allows the progression of multiple larval-larval molting until the larva attains the required size for metamorphosis [13], [14], [15]. CYP15C1 is specifically expressed in the corpus allatum (CA), an endocrine organ that produces and secretes JHs. Enzymological analysis revealed that CYP15C1 converts farnesoic acid (FA) to JH acid (JHA) in a highly stereospecific manner. We further demonstrated that CYP15C1 plays an indispensable role in JH biosynthesis, and its molecular defect results in the loss of JHs in the hemolymph, thereby causing precocious metamorphosis in the mod strain. Remarkably, precocious larval-pupal transitions in mod larvae always occur after the larval third instar, but not in the first or second instar. Our data provide further evidence supporting the hypothesis that authentic (epoxidized) JHs are essential for the classic “status quo” molting in late larval stages (third and fourth instar), but not in early larval stages (first and second instar) of B. mori [16].
Results
The mod strain is a JH–deficient mutant
Larvae of standard B. mori strains undergo molting four times and thus have five larval instars; these larvae are conventionally termed “tetramolter” in silkworm genetics. The spontaneous mutant mod was identified in a standard strain [11] and mod larvae undergo precocious metamorphosis in the third (dimolter) or fourth instar (trimolter). First, we obtained a detailed developmental profile of larvae from two batches of the mod strain. All mod larvae underwent precocious metamorphosis in the fourth instar and no individuals reached the fifth instar (Figure 1A and 1B). We plotted the timing of the onset of spinning in the mod larvae (Figure 1C and 1D). Consistent with previous reports [11], [12], we found that spinning occurred at two clearly distinguishable timings: (1) from 56 to 80 h and (2) from 112 to 144 h after the third molt: these larvae were termed early - and late-maturing trimolters, respectively. This segregation in the timing of the onset of spinning was not observed in the standard strain p50T (Figure 1C) or other moltinism strains [11], and thus is a unique characteristic of the mod strain. Importantly, development in almost all early-maturing trimolters was arrested and they remained as larval-pupal intermediates (93.4%, 85/91 larvae); only 3 of the 91 larvae (3.3%) successfully survived to adulthood (Figure 1B). In contrast, the late-maturing trimolters did not show such severe developmental impairment and 88.5% (77/87) became miniature adults (Figure 1B). In the larval-pupal intermediates, we usually observed prothetelic phenotypes such as a mixed pupal cuticle on the exoskeleton of animals having overall a larval appearance (Figure 1A), suggesting that hormonal switching of molting and metamorphosis may be aberrant in the mod strain. Notably, despite their small body size, reproduction in mod moths seemed normal, and their eggs hatched without apparent abnormalities.
Fig. 1. Characterization of the mod mutant. 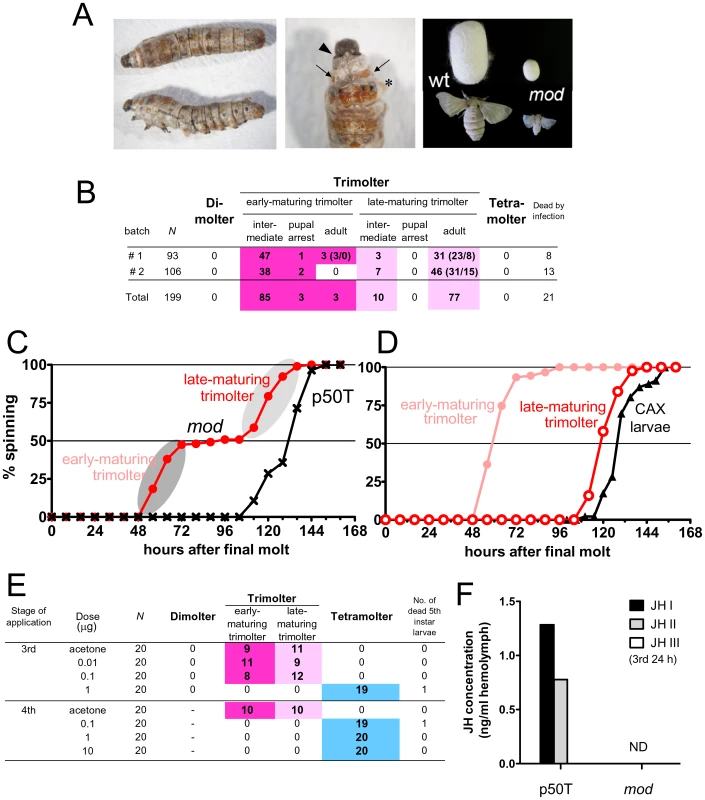
(A) Precocious metamorphosis observed in mod larvae. (left panel) Lateral and dorsal views and (middle panel) a magnified view of a larval-pupal intermediate. In intermediate animals, the new head capsule of the next instar (fifth) is formed (arrowhead). Beneath the old cuticles (asterisk), a new exoskeleton with larval eye spot markings (arrows) and brown-colored pupal cuticles are formed. (Right panel) Late-maturing trimolters form small cocoons and are able to develop into small but normal adults with normal fertility. (B) The developmental profiles of two batches of mod larvae (t011 strain). All of the larvae underwent precocious metamorphosis in the fourth instar, and no dimolters or tetramolters were observed. Larvae could be classified into two groups (early- and late-maturing trimolters) on the basis of the timing of onset of spinning. The numbers in parentheses indicate the sex of the moths (male/female). (C) Timing of the onset of spinning in mod (red, n = 178) and p50T (black, n = 28) strains after final larval molting. As highlighted by the grey ellipses, spinning was induced at two distinct timings in the mod strain, unlike the p50T strain. (D) Comparison of timings of the onset of spinning among early- and late-maturing trimolters of the mod strain and normal strain larvae that had been allatectomized (CAX) at the beginning of the fourth instar. Data on CAX larvae are from [17]; these larvae were reared at relatively low temperatures (23.0–25.5°C), which delays the timing of the onset of spinning to some extent. (E) Methoprene treatment of mod larvae. Selected doses of methoprene (0.01–10 µg/larva) were topically applied to newly molted third and fourth instar larvae (8–12 h after molting). As highlighted in blue, precocious pupation could be blocked by methoprene treatment. (F) Measurement of the JH titer in the hemolymph of third instar larvae of p50T and mod strains at 24 h after molting. Hemolymph was collected from ∼400 larvae using a microsyringe and the pooled sample was analyzed. JH in the hemolymph was converted to its corresponding methoxyhydrin derivatives and analyzed by GC-MS. JHs were not detected (ND) in the hemolymph of mod larvae. In the silkworm, premature metamorphosis can be induced by the loss of or low levels of JH signaling, which can occur due to the surgical removal of the CA [17] or to overexpression of the JH-degrading enzyme [16]. We therefore hypothesized that precocious metamorphosis in the mod strain was caused by the prevention of JH biosynthesis or signaling. To examine this hypothesis, we first determined whether the mod phenotype could be rescued by treatment with methoprene, a JH analogue. We topically applied several doses of methoprene to newly-molted third or fourth instar mod larvae and found that a fourth larval molting was induced by the treatment (Figure 1E). Fifth instar larvae that had undergone fourth larval ecdysis grew normally, began to spin after ∼6 days, and eventually metamorphosed to pupae and adults that were normal and fertile. This result suggests that JH reception and subsequent JH signaling is normal in the mod strain. Therefore, we next compared the JH titers in the hemolymph of third instar larvae of mod and p50T strains at 24 h after molting to the third instar. JHs were extracted from the hemolymph and their methoxyhydrin derivatives were analyzed by liquid chromatography-mass spectrometry (LC-MS). We detected JH I and JH II in the hemolymph of p50T, whereas the JH titer in the hemolymph of the mod strain was below the detectable level (Figure 1F). These results indicate that the mod strain is a JH-deficient mutant in which complete (or almost complete) loss of JH caused precocious metamorphosis.
Positional cloning of the mod locus
To identify the gene responsible for the mod locus, we performed positional cloning using backcross 1 progeny (BC1) obtained from crossing females of the mod strain (t011 strain, see http://www.shigen.nig.ac.jp/silkwormbase/index.jsp) with F1 heterozygote males of mod and p50T strains (see Figure S1). We mapped the mod locus within ∼400 kb region on the scaffold Bm_scaf16 (chromosome 11) [8] using 792 BC1 individuals. Twenty-five genes were predicted to be present within this region. Among them, we focused on BGIBMGA011708, a gene encodes a cytochrome P450 monooxygenase. Based on sequence homology and phylogenetic analysis (Figure 2B), the gene was designated as CYP15C1. We found that CYP15C1 shares high homology with the CYP15A1 of the cockroach Diploptera punctata, which is involved in JH biosynthesis in CA of the cockroach [18]. Given that the mod phenotype is a result of the loss of the JH titer (Figure 1F), we speculated that the mod phenotype is due to the loss of function of CYP15C1. To examine this possibility, we first determined the nucleotide sequence of the full-length CYP15C1 cDNA from p50T and mod strains. We identified a 68-bp deletion in the mod allele that introduces a premature stop codon in the coding region of CYP15C1 (Figure 2C–2E). This deletion seemed to produce a functionally null mutation in CYP15C1, since a heme-binding motif, which is essential for enzymatic activities in P450s [19], was eliminated in the mod allele (Figure 2D). This result indicates that CYP15C1 is a strong candidate for the mod locus. Therefore, we further characterized CYP15C1 and its gene product.
Fig. 2. Positional cloning of the mod locus. 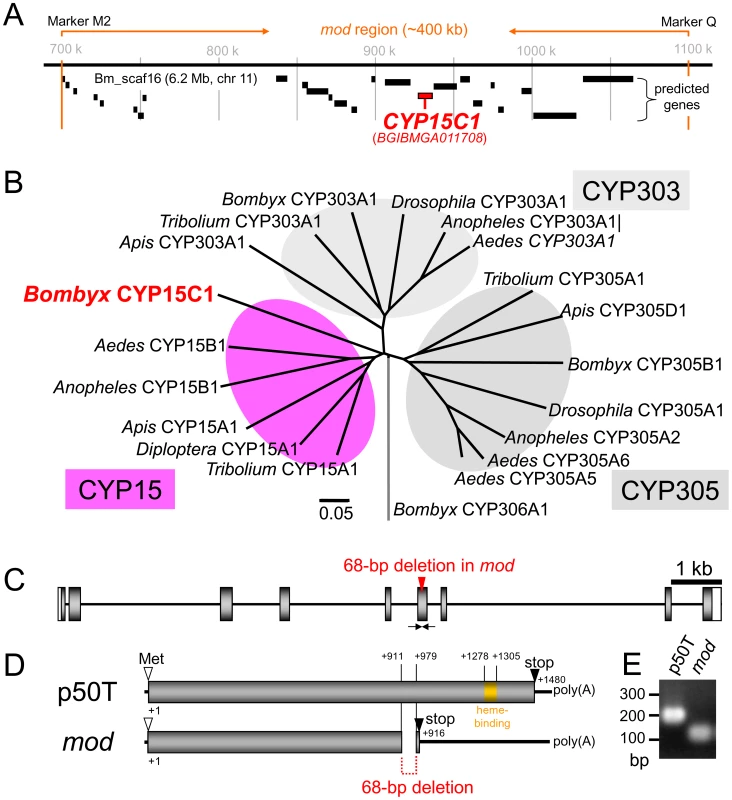
(A) Physical map showing the outcome of the linkage analysis using 792 BC1 individuals. The mod locus was narrowed to the genomic region flanked by the PCR markers M2 and Q, as indicated by the orange arrows. Putative genes predicted by the Gene model program [8], [9] are shown below the map, and CYP15C1 (BGIBMGA011708) is shown in red. For more details refer to Figure S1. (B) A phylogenetic tree showing the relationship of CYP15C1 and other related P450 genes. The rootless tree was constructed based on the entire amino acid sequence by the neighbor-joining method using the ClustalX program [55]. Sequences were retrieved from public databases, and the species names are abbreviated as follows: Aedes, A. aegypti; Anopheles, A. gambiae; Apis, A. mellifera; Bombyx, B. mori; Diploptera, D. punctata; Drosophila, D. melanogaster; and Tribolium, T. castaneum. The scale bar indicates the number of amino acid substitutions per site. Note that CYP15 was not found in D. melanogaster. (C) The genomic structure of CYP15C1 in the wild-type (p50T) strain. White box, grey box, and a black bar indicate untranslated, coding, and intronic regions, respectively. (D) Transcripts of CYP15C1 from p50T and mod strains. A 68-bp deletion was found in CYP15C1 of the mod strain, and this deletion introduced a premature stop codon as indicated in red. Heme-binding motifs of P450s [19] are indicated in orange. (E) Genomic PCR showing the presence of the 68-bp deletion in CYP15C1 from the mod strain. PCR primers (Table S1) that flank the deletion are shown by arrows in (C). Temporal and spatial expression of CYP15C1
The strict regulation of JH biosynthesis in CA is critical for the successful development and reproduction of insects [14], [15], [20]. We next examined the spatial expression pattern of CYP15C1 mRNA. We examined 12 tissues at four different developmental stages and found that CYP15C1 mRNA was highly specific to the corpus cardiacum (CC)-CA complex (Figure 3A). A whole mount in situ hybridization experiment in the brain (Br)-CC-CA complex (Figure 3B and Figure S2) showed that the signal for CYP15C1 was strictly limited to CA, where JH is synthesized, and could not be detected in the brain or CC. These results showed a close spatial correlation between CYP15C1 expression and JH biosynthesis.
Fig. 3. Temporal and spatial expression of CYP15C1. 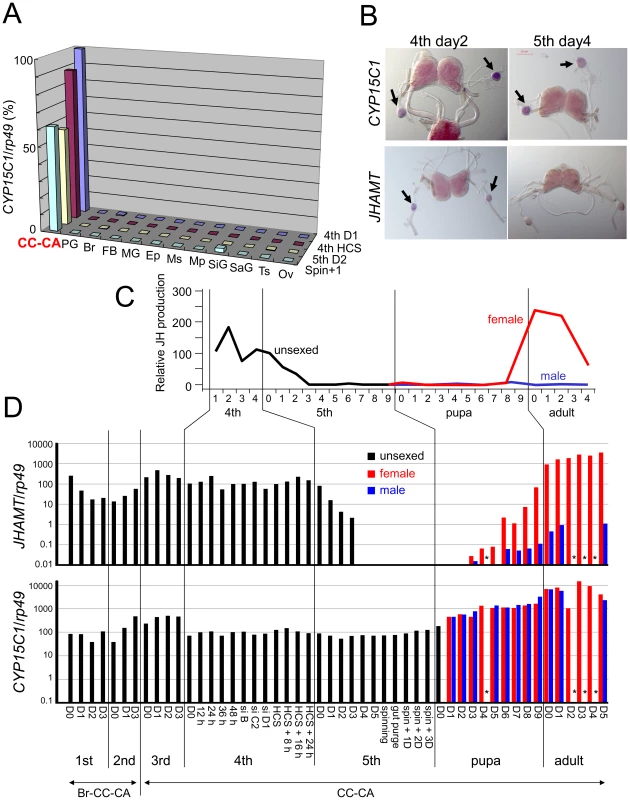
(A) qRT-PCR analysis of the spatial expression of CYP15C1 in the silkworm strain Kinshu×Showa. “CYP15C1/rp49” on the vertical axis indicates the level of CYP15C1 mRNA normalized to that of internal rp49 mRNA. RNAs were collected from larvae on day 1 of the fourth instar (4th D1), fourth instar larvae showing head capsule slippage (4th HCS), larvae on day 2 of the fifth instar (5th D2), and larvae on day 1 after the onset of spinning (Spin+1). CC-CA, corpus cardiacum-corpus allatum complex; PG, prothoracic gland; Br, brain; FB, fat body; MG, midgut; Ep, epidermis; Ms, muscle; Mp, Malpighian tubule; SiG, silk gland; SaG, salivary gland; Ts, testis; and Ov, ovary. (B) In situ mRNA hybridization of CYP15C1 and JHAMT. Br-CC-CA complexes on day 2 of the fourth instar and day 4 of the fifth instar were used for analysis. Signals of both genes were limited to CA as indicated by arrows, but JHAMT was not detected on day 4 of the fifth instar. The purple coloration in the brain is primarily due to ommochrome pigments and does not reflect gene expression. The result of control experiments using sense probes are shown in Figure S2. (C) Developmental changes in the rate of JH biosynthesis by B. mori CA in vitro. The data are based on Kinjoh et al. (2007). Black, red, and blue lines indicate CA from unsexed larvae, female and male animals, respectively. The activity in CA on day 1 of the fourth instar was set as 100. (D) Temporal expression patterns of JHAMT (upper) and CYP15C1 (lower) in the Br-CC-CA (first and second instar larvae) or CC-CA (third to fifth instar larvae, pupae, and adults) complex. Developmental stages are defined as h/days after certain developmental events [i.e., molting, head capsule slippage (HCS), spinning, or emergence] or by a spiracle index (si) [56]. Animals were unsexed during larval stages, while sexed during pupal and adult stages (female in red and male in blue). The expression profile of JHAMT after the second larval instar is based on published data (20). Expression levels measured on day 2 of the 4th larval instar are arbitrarily set at 100 (for actual transcript numbers per rp49) and are shown in a log scale. Asterisks indicate that data were not available. Next, we carried out a detailed analysis of the temporal expression pattern of CYP15C1 in the CC-CA complex and compared it to that of the gene for JHA methyltransferase (JHAMT), a key enzyme that acts in the final step of the JH biosynthetic pathway in CA [21]. CYP15C1 mRNA was constitutively expressed in CA from the first instar larval to adult stages (Figure 3D), even when JH is not synthesized (Figure 3C) [20]; no apparent differences in levels of CYP15C1 mRNA were observed between males and females during pupal and adult stages (Figure 3D). In contrast, the temporal expression pattern of JHAMT correlates well with the JH synthetic activity of CA (Figure 3D and Figure S2). JHAMT transcript completely disappeared by day 4 of the fifth instar when CA ceased production of JH (see Figure 3C). It reappeared from the mid-pupal stage and increased to a very high level in the female CA. This was consistent with the temporal profile of JH biosynthesis activity in CA as this occurs only in females during the pupal and adult stages [20]. Taken together, our results strongly indicate that CYP15C1 is involved in JH biosynthesis in CA, but does not appear to act as a rate-limiting factor for JH biosynthesis.
Enzymatic properties of CYP15C1
The cockroach CYP15A1, the ortholog of B. mori CYP15C1, catalyzes the epoxidation of (2E,6E)-methyl farnesoate (MF) to JH III [18]. Although biochemical studies predicted the presence of FA epoxidase in the CA of the lepidopteran insect Manduca sexta [22], [23], the corresponding gene has not been identified to date. Therefore we examined the enzymatic activity of B. mori CYP15C1 against two plausible substrates, FA and MF. First, we employed a transient expression system using Drosophila S2 cells. When S2 cells expressing CYP15C1 were incubated with medium containing FA, a major HPLC peak was generated that had the same retention time (15.1 min) as standard JH III acid (JHA III) (Figure 4A, middle). This peak did not appear when S2 cells expressing GFP were used (Figure 4A, bottom). The ESI-MS spectrum of this peak gave an [M-H]− at m/z 251, consistent with the C15H24O3 formula of JHA III, confirming that CYP15C1 catalyzes the conversion of FA to JHA III. The enzymatic properties of CYP15C1 was further examined in a stable Sf9 cell line (Sf9/BmCYP15C1) that constitutively expresses CYP15C1. When the Sf9/BmCYP15C1 cells were cultured in medium containing FA, significant levels of JHA III were detected; in contrast, JHA III production was difficult to detect when original Sf9 cells were used (Table S2, Exp.1). When Sf9/BmCYP15C1 cells were cultured in medium containing MF, JH III generation was detected at low levels. However, a similar level of JH III production was also detected in the original Sf9 cells when they were cultured in the same medium (Table S2, Exp.1). These results suggest that JH III production observed in Sf9/BmCYP15C1 was might be due to the presence of endogenous P450 epoxidases in Sf9 cells, which have been reported previously to have lower substrate specificity and stereospecificity [18], [24]. The addition of the JH esterase inhibitor 3-octylthio-1,1,1 - trifluropropan-2-one (OTFP) did not increase production of JHA III (Table S2, Exp. 2), indicating that the degradation of JH III by intrinsic JH esterases in the cells was negligible. Therefore, we were able to estimate the conversion ratio of FA and MF to JH III by CYP15C1. This showed that CYP15C1 exhibited at least 18-fold higher activity for FA than MF (Table S2, Exp. 1), a result that is consistent with previous biochemical studies on lepidopteran FA epoxidase in CA.
Fig. 4. Enzymatic properties of B. mori CYP15C1. 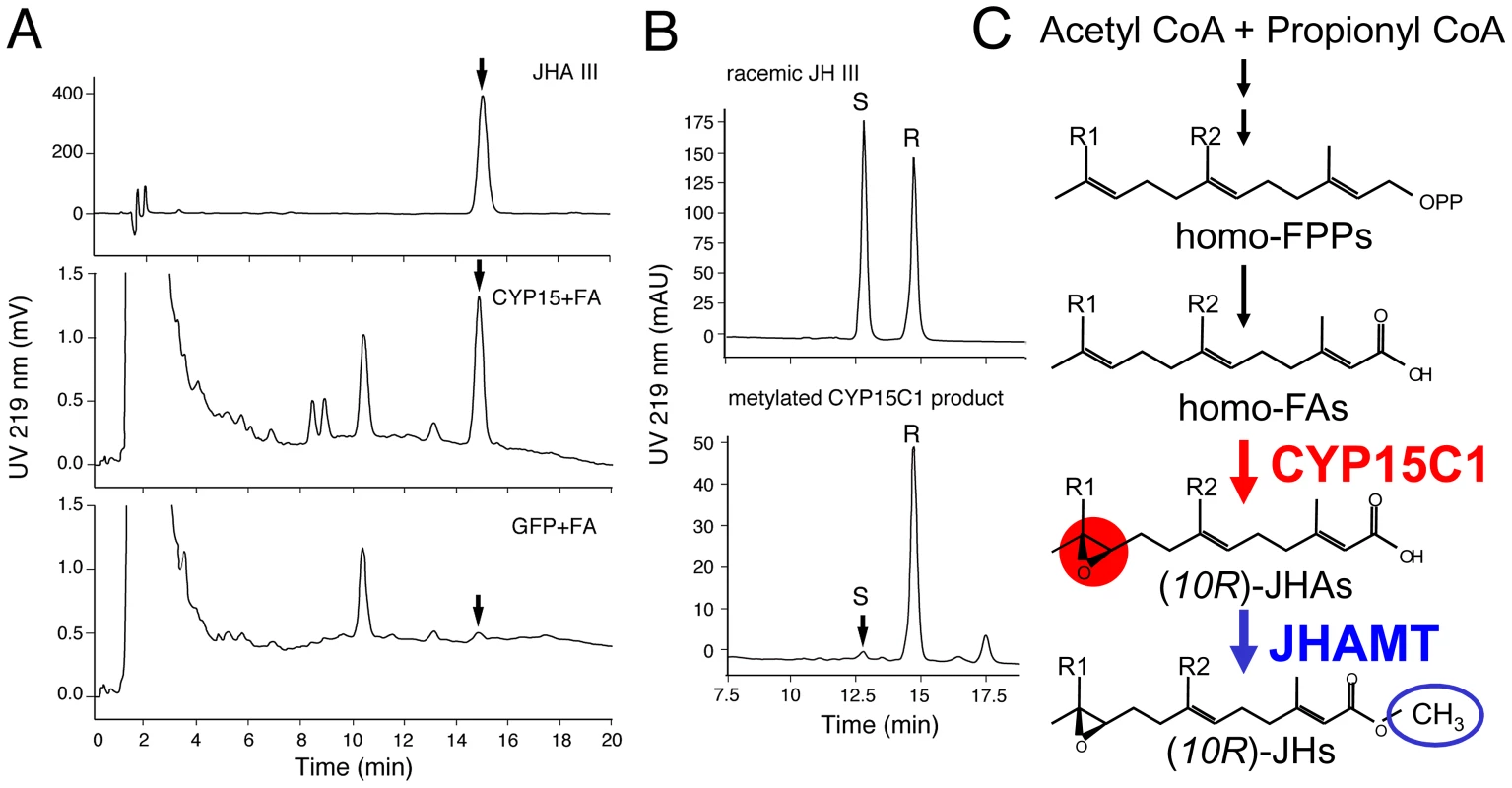
(A) Enzymatic activity against FA. Medium containing FA was incubated with Drosophila S2 cells transiently expressing CYP15C1 (middle) or GFP (bottom), and analyzed by HPLC. Standard JHA III (top). Arrows indicate peaks of JHA III. (B) Stereospecificity. JHA III generated from FA by Sf9 cells stably expressing CYP15C1 (Sf9/CYP15C1) was chemically methylated and analyzed by a Chiral-HPLC. R and S indicate peaks of (R)- and (S)-JH III enantiomers, respectively. The R∶S ratio of standard racemic JH III (top) was 50∶50, while that of CYP15C1-produced JH III (bottom) was 97∶3. (C) The late JH biosynthetic step in B. mori, in which major JHs in the hemolymph are JH I and II [28]. Ethyl-branched farnesyl diphosphates (homo-FPPs) are converted to homo-FAs, epoxidized to JHAs by the cytochrome P450 epoxidase CYP15C1 (this study), and then methylated by the JHA methyltransferase (JHAMT) [21]. JH I: R1 = R2 = C2H5, JH II: R1 = C2H5, R2 = CH3. To further examine the stereospecificity of CYP15C1, the JHA III generated by Sf9/CYP15C1 was chemically methylated and analyzed by a Chiral-HPLC. The methylated product had a major (R)-JH III and a minor (S)-JH III peak (R∶S = 97∶3) (Figure 4B). These results show that B. mori CYP15C1 encodes a functional P450 epoxidase that preferentially converts FA to JHA III rather than MF to JH III, and does so in a highly (R)-enantioselective manner (Figure 4C).
Transgenic rescue experiments using the GAL4/UAS system
To obtain direct evidence that CYP15C1 is responsible for the mod mutation, we performed transgenic rescue experiments using the GAL4/UAS system [25]. We generated transgenic silkworm lines carrying the UAS-CYP15C1 transgene with the eye-specific 3xP3-EGFP marker [26]. The UAS-CYP15C1 transgene was driven using a silkworm enhancer trap line ET14 in which GAL4 was strongly expressed in CA (Figure 5A), although weak expression was also detected in peripheral tissues including fat bodies and the midgut [9], [27]. As these lines were generated using the standard Shiro-C (w-1; +mod) strain, we changed the genetic background to w-1/w-1; mod/mod by crossing to the mod strain. The resultant w-1; mod; ET14/+ females were then crossed with w-1; mod; UAS-CYP15C1/+ males to determine whether the mod phenotype could be rescued by CYP15C1 overexpression. We used two independent UAS-CYP15C1 lines with ET-14 (Figure 5B). In both UAS-CYP15C1 lines, CYP15C1 overexpression efficiently prevented precocious metamorphosis and 97.1% of the larvae (34/35 in total) underwent the fourth larval molt to become fifth instar larvae (Figure 5B and 5C). Only one larva (1/35) became a late-maturing trimolter, but neither dimolters nor early-maturing trimolters appeared. This result was in contrast to what was observed in control larvae or larvae carrying either the GAL4 or UAS construct alone: approximately half of the larvae became dimolters and the remainder became trimolters, while no larvae became tetramolters. We also measured the JH titer in the hemolymph (Figure 5D). As expected, the JH titers in control, ET14, and UAS larvae were below the detectable limit. In contrast, we were able to detect JH I and JH II in the hemolymph of mod larvae carrying both ET14 and UAS-CYP15C1 constructs. Taken together, these results provide direct evidence that CYP15C1 is responsible for the mod mutation and is essential for JH biosynthesis.
Fig. 5. Transgenic rescue of mod. 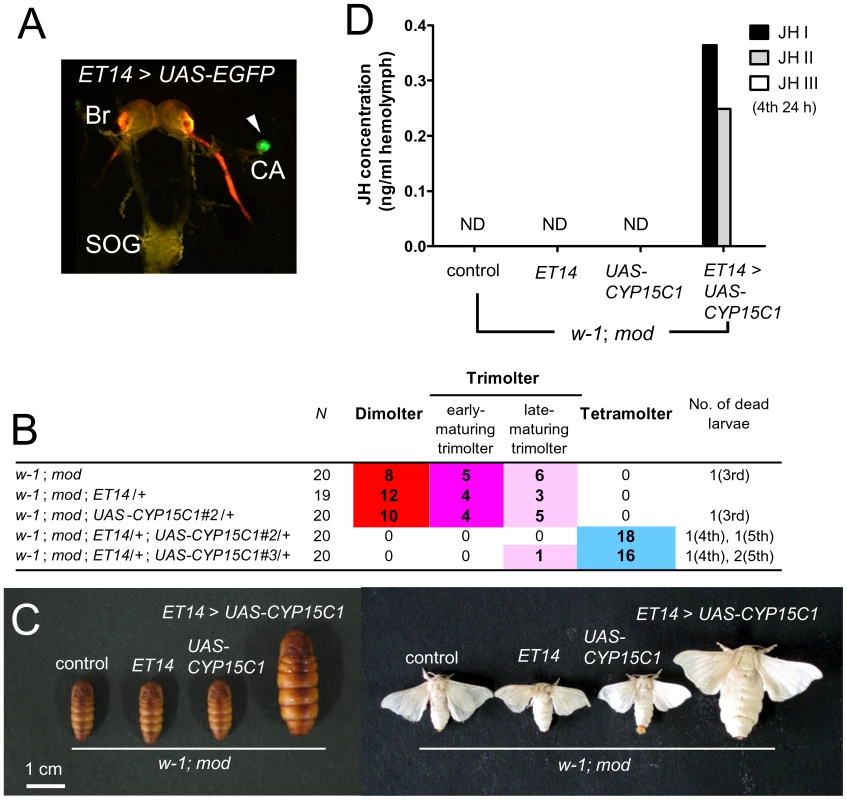
(A) Visualization of GAL4 expression in CA of the enhancer trap line ET14 carrying the UAS-GFP construct. GFP expression (green) is limited to CA (arrowhead). Red fluorescence in the optic nerve is due to DsRed2 expression driven by the 3xP3 promoter [26]. Br, brain; SOG, suboesophageal ganglion; and CA, corpus allatum. (B) Developmental profiles of binary GAL4/UAS transgenic lines. Male moths with a w-1; mod background and carrying UAS-CYP15C1 were crossed with w-1; mod female moths carrying ET14, and their progenies were analyzed. Tetramolters appeared in GAL4/UAS transgenic lines, but not in nonbinary lines. (C) Images of pupae and moths of GAL4/UAS transgenic lines. Larvae carrying both ET14 and UAS-CYP151 constructs entered the fifth larval instar and eventually formed larger adults. Control animals did not carry transgenic vectors. (D) Measurement of the JH titer in the hemolymph of GAL4/UAS transgenic lines on the w-1; mod background. Hemolymph was collected from fourth instar larvae at 24 h after molting and analyzed. JH was detected only in GAL4/UAS lines, but not in nonbinary lines. ND, not detected. Discussion
In this study, we identified and characterized the gene responsible for the mod locus that causes precocious larval-pupal metamorphosis in B. mori. The data we present here have two important implications. First, we provide direct genetic evidence for the significance of P450 epoxidase in the late step of the JH biosynthetic pathway, whose expression is essential for normal growth and metamorphosis. Second, we show that the mod strain is a JH-deficient mutant strain carrying a null allele of CYP15C1, in which developmental abnormalities are mostly limited to larval-pupal transitions and are not observed before the second larval molt.
Biochemical and physiological function of CYP15C1
JH III is the most common JH in many insect orders, although its ethyl-branched homologs (JH I and II) are the major JHs in the order Lepidoptera [22], [28]. Biochemical studies have shown that in the late steps of JH biosynthesis in many insect species, including cockroaches and locusts, FA is first methylated to MF and then epoxidized to JH III in CA [22]. However, the final two steps of JH biosynthesis are reversed in Lepidoptera: ethyl-branched homologs of FA (homo-FAs) are first epoxidized and the resultant JHAs (i.e., JHA I and II) are then methylated to the authentic JHs (i.e., JH I and II) [22]. This study showed that B. mori CYP15C1 epoxidizes FA to JHA III in a highly stereospecific manner. CYP15C1 might also epoxidize MF to JH III, but in a far less efficient manner (Table S2). Given that B. mori JHAMT can methylate both FA and JHAs with similar efficiencies [21], our data clearly demonstrate the major JH biosynthetic pathway in B. mori: homo-FAs are first epoxidized to JHAs by CYP15C1, and then methylated to JHs by JHAMT (Figure 4C and Figure 6A). Interestingly, D. punctata CYP15A1 does not convert FA to JHA III [18]. Thus, the difference in specificity of CYP15 to the substrates FA and MF may determine the order of the final steps of JH biosynthesis in insects.
Fig. 6. A model for JH biosynthetic pathway in the CA of wt and mod silkworms. 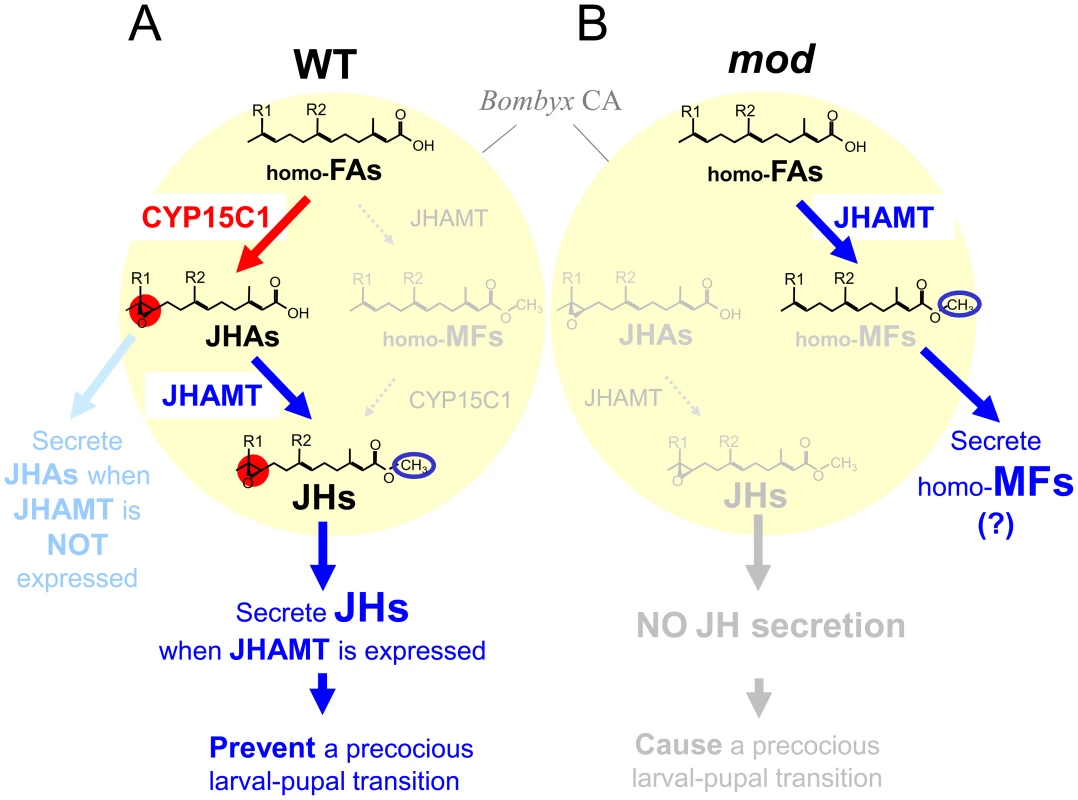
(A) In the B. mori CA, constitutive CYP15C1 expression allows the consistent conversion of homo-FAs to JHAs (predominantly JHA I and II in Lepidoptera). When JHAMT is expressed in CA, JHAs are further converted to JHs, and released from CA, thereby preventing precocious metamorphosis. When JHAMT expression is shut off (e.g., in the prepupal stage), JHAs are likely to be released from CA. (B) In CA of the mod strain, homo-FAs are not converted to JHAs because of the loss of CYP15C1, but instead, homo-FAs are converted to ethyl-branched homologs of MF (homo-MFs, i.e., unepoxidized JH I and II) by JHAMT. The loss of CYP15C1 does not allow the conversion of homo-MFs to the authentic JHs. Therefore, neither JHs is synthesized in nor released from CA of the mod strain, thereby causing precocious metamorphosis. The synthesized homo-MFs might be released from CA of the mod strain, similar to that of higher dipteran insects [57]. JH I: R1 = R2 = C2H5, JH II: R1 = C2H5, R2 = CH3. The expression of most early JH biosynthetic enzyme genes and JHAMT in B. mori is limited to the CA and shows dynamic developmental fluctuations [20], [21], [29]. In particular, the temporal expression profile of JHAMT correlates well with JH biosynthetic activity in B. mori [20], [21], [30], [31] and in the Eri silkworm Samia cynthia ricini [32], indicating that JHAMT is a key regulatory gene whose transcriptional control is critical for the regulation of JH biosynthesis in Lepidoptera. Here, we found that expression of CYP15C1 was also limited in CA but in a different pattern to other JH biosynthesis genes in that it was constitutively expressed from larval to adult stages. This result suggests that the transcriptional regulation of CYP15C1 is less important than JHAMT for the temporal regulation of JH production in B. mori. CA of the silkworm ceases JH biosynthesis by day 3 of the last (fifth) instar [20]; however, it is speculated that CA synthesizes and secretes JHAs during the following prepupal period. Our data indicate that this endocrine switch can be explained by constitutive CYP15C1 expression and the shut-off of JHAMT expression in CA (Figure 6A). During the larval-pupal transition, homo-FAs are constantly converted to JHA I and II by CYP15C1, and the resultant JHAs are secreted from the gland without further conversion because of the absence of JHAMT.
CYP15 P450 family members are found in both hemimetabolous and holometabolous insects [33]. In a similar manner as CYP15C1 expression in B. mori, CA-specific CYP15 expression has also been observed in two cockroach species, D. punctata and Blattella germanica [18], [34], in the locust Schistocerca gregaria [35], and in the mosquito Aedes aegypti [36], suggesting a conserved function in JH biosynthesis. However, the enzymatic properties of CYP15 products, with the exception of those of D. punctata [18] and B. mori (this study), have not been studied and the physiological role of CYP15s in the development of other insects remains unknown. By characterizing the CYP15C1-null mutant silkworm, we have demonstrated here that CYP15C1 plays an essential role in JH biosynthesis and for the maintenance of the proper timing of metamorphosis.
Accumulating data have suggested that CYP15 genes are evolutionarily diversified in terms of their gene regulation and nature. For example, unlike B. mori CYP15C1, A. aegypti CYP15 shows developmentally and dynamically regulated changes of expression, which appear to correlate well with the JH synthetic activity in the CA [36]. In addition, CYP15 is not present in the genome of D. melanogaster, but a P450 gene (Cyp6g2) is expressed in CA in a highly tissue-specific manner [37]. More extensive research on the transcriptional controls and enzymatic properties of JH epoxidases across a broader range of insect taxa will shed light on the roles of these enzymes.
Precocious pupation in mod larvae
Our results consistently indicate that the mod strain is a JH-deficient mutant that is unable to synthesize JHs in CA. One unique characteristic of the precocious pupation in the mod strain is the variation in the timing of the onset of spinning (Figure 1). The feeding period in early-maturing trimolters was unusually short (50 h after molting) compared with that observed in surgical allatectomy of newly molted fourth instar larvae. In the latter larvae, the feeding period was comparable in length to that of the late-maturing trimolters [e.g. ∼130 h [17]] and no timing segregation was observed [17]. In addition, most of the early-maturing trimolters displayed a larval-pupal intermediate phenotype and eventually died, unlike allatectomized larvae, most of which successfully developed into small but normal pupae [17]. One explanation for this phenomenon is that the early-maturing trimolters were destined to undergo larval molting to the fifth instar on day 2, while the late-maturing trimolters were destined for pupation after a prolonged fourth instar, similar to allatectomized larvae [17] (Figure 1D). Molting in early-maturing trimolters on day 2 usually resulted in the formation of larval-pupal intermediates. One possible explanation for this mixed phenotype is that metamorphosis in the mod strain is induced in the presence of homo-MFs (unepoxidized JH I and II), presumed products instead of epoxidized JH I and II in CA of the mod strain (see Figure 6B). MF is known as a crustacean JH and has recently been reported to have JH activity in D. melanogaster [38], [39]. Therefore, MF and its homologs might have JH-like activity but not able to fully substitute for authentic (epoxidized) JHs in the physiology of the silkworm. Alternatively, other P450 epoxidases in B. mori that have low substrate specificity and stereospecificity, like CYP9E1 [18] and CYP6A1 [24] in other insects, might substitute for the absence of CYP15C1 in peripheral tissues of mod larvae, and such locally-synthesized JHs may prevent precocious metamorphosis in the first and second instar larvae carrying the mod mutation. Further studies are needed to elucidate the mechanism for this unique characteristic of the mod strain.
We found that the precocious phenotype was more severe in the w-1; mod strain compared to that in t011, a genetic stock of the mod strain. We rarely observed dimolter larvae in the t011 stock (Figure 1B). However, in the original manuscript in 1956, it was reported that 28–92% of mod larvae became dimolters [11]. This difference might have developed as a consequence of unintended artificial selection during stock maintenance that favored broods producing trimolters in higher proportions, as it is difficult to obtain sufficient number of eggs using dimolter moths [11], [12]. Thus, we speculate that the present t011 stock may be genetically fixed to produce mostly trimolters, and that this attribute can be varied by outcrossing to other strains.
In the silkworm, premature metamorphosis can be induced by surgical removal of JH-producing CA (allatectomy) [17], by application of an imidazole-based insect growth regulator KK-42 [40] or an anti-juvenile hormone agent KF-13S [41], [42], or by continuous overexpression of the JH-degrading enzyme, JH esterase [16]. In any case, however, premature pupation is not induced in larvae younger than the third instar. In agreement with these studies, we did not observe precocious pupation in first or second instar mod larvae, nor did we observe apparent developmental abnormalities during these early instars. Therefore, our data support the hypothesis that there are two physiological phases in the life of silkworm larvae [16]: the JH-independent phase (first and second instar) in which JH does not have a morphogenetic function; and, the JH-dependent phase (third instar and thereafter) in which the morphostatic action of JH is required to prolong the larval stage until the attainment of the appropriate body size for metamorphosis. Given that most generally the minimum number of the larval instar in insects is three [1], [2], our data further imply that insect larvae need to experience at least one [e.g., L2 pupae in D. melanogaster [43]] or two (e.g., B. mori) larval-larval molts and/or require a certain length of time of postembryonic development in order to acquire competence for metamorphosis.
The silkworm is a classic model organism that has been used for pioneering studies in genetics, physiology, and biochemistry [5]. The availability of whole genome data [8], post-genomic tools [10], and unique mutant resources [6], together with the classic “status quo” responses to JHs in this insect [14], [15], [17], makes the silkworm well-suited for study of hormonal control of growth and development. Indeed, these advantages have greatly contributed to the identification of essential components in the biosynthesis of ecdysteroids, the insect molting hormones [44]. Moreover, recent success in targeted gene disruption using a zinc-finger nuclease [45] increases the utility of this model organism. We are hopeful that our present study will encourage further studies on other “moltinism” strains in the silkworm, and consequently pave the way for a greater understanding of physiological control, developmental plasticity, and evolutionary history of the number of larval molting in insects, which may reflect adaptive strategies of insects to diverse environmental conditions. It is also noteworthy that the late step of the JH biosynthetic pathway is insect-specific and is therefore a potential target for biorational insecticides [46].
Materials and Methods
Insects and cell lines
Silkworms were reared on an artificial diet or mulberry leaves at 25–27°C under standard conditions as described previously [47]. The silkworm strain t011 (mod/mod) was obtained from Kyushu University [6]. The Spodoptera frugiperda Sf9 and Drosophila melanogaster S2 cells were maintained as described previously [48]. To determine the developmental profile of mod, larvae from two batches of t011 were individually reared in plastic dishes, and their developmental stages were recorded at ∼8-h intervals.
Hormonal treatments
The JH analog, methoprene (a kind gift from S. Sakurai) was applied to newly molted third or fourth instar larvae (∼8–12 h after molting). Methoprene was diluted with acetone and the selected doses (0.01–10 µg/larva) were topically applied to the dorsum using a 10-µl Hamilton microsyringe. The same volume of acetone was applied as a control.
Positional cloning of the mod locus
Positional cloning of the mod locus was performed as described previously [49]. Codominant PCR markers and p50T-specific PCR markers were generated for each position of the scaffold Bm_scaf16 (chromosome 11) [9], and used for genetic analysis (Figure 2A and Figure S1). Homozygotes of the mod locus were collected from the BC1 population [t011×(p50×t011)] based on the phenotype of precocious pupation.
Cloning of CYP15C1
Total RNAs were collected from CA of day 0 fifth instar larvae of p50T and Kinshu×Showa strains and used for 5′ - and 3′-rapid amplification of cDNA ends (RACE) using the GeneRacer Kit (Invitrogen). PCR was performed using the primers listed in Table S1. The PCR products were subcloned and sequenced as described previously [47]. The obtained cDNA sequence was deposited in the GeneBank (accession number: AB124839).
Quantitative RT–PCR (qRT–PCR) analysis
qRT-PCR was performed essentially as described previously [21]. The primers used for the quantification of the CYP15C1 transcript are listed in Table S1.
In situ hybridization
In situ hybridization was performed essentially as described previously [50]. A CYP15C1 cDNA fragment (∼1.1 kb) was amplified by PCR listed in Table S1 and subcloned into a pDrive plasmid vector (QIAGEN).
Chemicals
(2E,6E)-farnesoic acid (FA) and (2E,6E)-methyl farnesoate (MF) were purchased from Echelon Research Laboratories (Salt Lake City) and racemic JH III from Sigma. JH III acid was prepared from the racemic JH III as described previously [21]. (R)-JH III was a kind gift from W.G. Goodman.
Enzyme assays of CYP15C1 in S2 cells
CYP15C1 overexpression in S2 cells was achieved using a GAL4/UAS system [51]. To generate a vector for expressing CYP15C1 under the control of the UAS promoter (UAS-CYP15C1-HA), a cDNA fragment coding the entire CYP15C1 ORF was ligated into the pUAST vector. UAS-GFP.RN3 [52] was used as a negative control. UAS-CYP15C1-HA or UAS-GFP.RN3 was transfected with the Actin5C-GAL4 construct (a gift from Yasushi Hiromi, National Institute of Genetics, Japan). Forty-eight hours after transfection of S2 cells in a 60-mm dish, the old medium was replaced with 2 ml of fresh medium. S2 cells were detached from the bottom of the dish by pipetting, and 1 ml of the cell suspension was transferred to a siliconized glass test tube. FA or MF (100 µM at final concentration) was then added to the tube. After incubation at 25°C for 16 h, 500 µl of medium was collected and mixed with 500 µl of acetonitrile. Samples were centrifuged for 10 min at 15,000 rpm, followed by incubation at 25°C for 10 min. After filtration using a 0.2 µm filter, 10–20 µl of each sample was subjected to HPLC analysis as described below.
Establishment of Sf9 cells stably expressing CYP15C1 and enzyme assay
A cDNA with the full ORF of CYP15C1 cDNA was subcloned into the pIZT/V5-His vector (Invitrogen). The plasmid was transfected into Sf9 cells with Cellfectin reagent (Invitrogen), then cells transiently expressing CYP15C1 were selected successively with Zeocin according to the manufacture's instruction and a cell line (Sf9/CYP15C1) stably expressing CYP15C1 was established. Sf9/CYP15C1 cells were placed in a glass tube (12×75 mm) coated with PEG20,000 and cultured in SF900-II SFM medium containing FA or MF (10 µg/ml) for either 2 or 6 h at 26°C. In some experiments, the specific JH esterase-specific inhibitor OTFP (6 µM) was added to the medium to prevent possible degradation of the generated JH III by intrinsic JH esterase present in the cells. After incubation, an equal volume of CH3CN was added to the medium, vortexed vigorously and centrifuged for 4,800 rpm for 10 min to remove cell debris. The supernatant was directly subjected to an HPLC analysis as described below for JH III acid or JH III, which were expected to be generated from FA and MF, respectively.
HPLC and ESI-MS analyses of JH III and JH III acid
JH III was analyzed by reversed-phase HPLC as described previously [21]. JH III acid was analyzed by reversed-phase HPLC (column, Shiseido ODS UG80, 150 mm×3.0 mm ID; solvent, CH3CN-20 mM CH3COONH4, pH 5.5, 35∶65, flow rate, 0.5 ml/min; detection, UV 219 nm). ESI-MS spectrum of JH III acid was obtained by TSQ system (Thermo Quest Finnigan, USA).
Analysis of the stereospecificity of JH III acid generated by CYP15C1
The stereospecificity of the epoxide group of JH III acid formed by CYP15C1 was analyzed as follows under semi-dark conditions. Sf9/CYP15 cells were cultured in medium containing 10 µg/ml FA for 48 hrs. An equal volume of CH3CN was added to the medium (2 ml), vortexed vigorously and centrifuged at 4,800 rpm for 10 min. One ml of 1 M CH3COONH, (pH 5.5) was added to the supernatant and extracted with 5 ml of CH2CH2; this step was performed 5 times. The extract was dehydrated with anhydrous Na2SO4 and evaporated to dryness in vacuo at 40°C, then the residue was dissolved in 200 µl of CH2Cl2, 50 µl of MeOH and 100 µl of TMS-diazomethane were then added and the solution was incubated at room temperature for 30 min. The reaction was dried with an N2 gas stream, the residue dissolved in 100 µl of hexane, and subjected to a normal-phase HPLC (column, Shiseido SG80, 250×4.6 mm ID; solvent, hexane-EtOH, 99∶1; flow rate, 0.5 ml/min; detection, UV 211 nm). The peak corresponding to JH III (r.t. = 9.8 min) was collected. The stereospecificity of the epoxide group of the JH III was analyzed by a chiral-HPLC (column, Chiralapack IA, 250×4.6 mm ID, DAICEL; solvent, hexane-EtOH, 99∶1; flow rate, 0.5 ml/min; detection UV 219 nm) as described previously [31].
Purification of JHs from hemolymphs and preparation for LC-MS analysis
Ten microliters of deuterium-substituted JH III (d3-JH III) [53] in toluene (67.1 pg/ml) was transferred to a clean glass tube to which 0.5 ml of methanol was added. The hemolymph sample (100 µl) was then added and mixed vigorously, and 1.5 ml of 2% NaCl was added to the JH sample. JH was extracted by partition with 0.5 ml hexane; this step was performed three times. The combined solvent containing JH (1.5 ml) was evaporated under a stream of nitrogen. One hundred microliters of methanol and 2 µl trifluoroacetic acid were added to the crude JH extract and mixture heated at 60°C for 30 min. After removal of the methanol, methoxyhydrin derivatives of JH (JH-MHs) were purified using a Pasture pipette packed with 1.0 g of aluminum oxide (activity grade III, ICN Ecochrom) prewashed with hexane. After loading the extract and washing with 2 ml of 30% ether in hexane, JH-MHs were eluted with 2 ml of 50% ethyl acetate in hexane and then dried under a stream of nitrogen. The residue was dissolved in 25 ml of 80% acetonitrile containing 5 µM sodium acetate.
Analytical condition for LC-MS
The HP1100MSD system (Agilent) was equipped with a 150×3 mm C18 reversed phase column (UG80, Shiseido) protected by a guard column with 70% acetonitrile containing 5 µM sodium acetate at a flow rate of 0.4 ml/min. For MS analysis, electrospray ionization in the positive mode was used under the conditions of drying gas temperature at 320°C with 10 l/min flow rate, ionization voltage of 70 V. Under these conditions, selected ion masses for each JH-MH were monitored as [M+Na]+, i.e., m/z 321, 324, 335, and 349 for JH III, d3-JH III, JH II, and JH I, respectively.
Transgenic rescue experiments
Overexpression of CYP15C1 was performed in transgenic silkworms using the GAL4/UAS system as described previously [25], [27], [54]. A coding sequence of CYP15C1 was introduced into a silkworm UAS vector carrying the marker gene 3xP3-EGFP. B. mori transformants were established using standard protocols [10]. To overexpress CYP15C1 on the mod/mod background, established UAS lines and an enhancer trap line ET14 [27] were crossed with the t011 strain, and the resultant F1 animals were sib mated to obtain the F2 generation. In the F2 generation, we collected animals showing premature pupation with white eyes (i.e., mod/mod; w-1/w-1) and confirmed the presence of the fluorescent marker gene using a fluorescent microscope (SZX12, Olympus). The established w-1; mod lines carrying UAS-CYP15C1 or ET14 were crossed, and their offspring were examined to determine whether precocious metamorphosis was blocked by CYP15C1 overexpression.
Supporting Information
Zdroje
1. SehnalF 1985 Growth and lifecycles. KerkutGAGilbertLI Comprehensive insect physiology, biochemistry and pharmacology Oxford Pergamon Press 1 81
2. EsperkTTammaruTNylinS 2007 Intraspecific variability in number of larval instars in insects. J Econ Entomol 100 627 645
3. NijhoutHF 1998 Insect hormones Princeton Princeton University Press
4. NijhoutHF 1981 Physiological control of molting in insects. Amer Zool 21 631 640
5. GoldsmithMRShimadaTAbeH 2004 The genetics and genomics of the silkworm, Bombyx mori. Annu Rev Entomol 50 71 100
6. BannoYFujiiHKawaguchiYYamamotoKNishikawaK 2005 A guide to the silkworm mutants 2005-gene name and gene Fukuoka, Japan Silkworm Genetics Division, Institute of Genetic Resorces, Kyushu University
7. TazimaY 1978 The silkworm: an important tool Tokyo Kodansha
8. The International Silkworm Genome Consortium 2008 The genome of a lepidopteran model insect, the silkworm Bombyx mori. Insect Biochem Mol Biol 38 1036 1045
9. ShimomuraMMinamiHSuetsuguYOhyanagiHSatohC 2009 KAIKObase: an integrated silkworm genome database and data mining tool. BMC Genomics 10 486
10. TamuraTThibertCRoyerCKandaTAbrahamE 2000 Germline transformation of the silkworm Bombyx mori L. using a piggyBac transposon-derived vector. Nat Biotechnol 18 81 84
11. OotaSWatanabeATokunagaH 1956 Genetical study on a spontaneous mutant, two molter, in the silkworm, Bombyx mori. J Insect Biotec Seric (In Japanese) 26 77 81
12. NinakiODoiraHChikushiH 1980 Genetical studies of the “dimolting” mutant in Bombyx mori. J Insect Biotec Seric 49 347 351
13. MirthCKRiddifordLM 2007 Size assessment and growth control: how adult size is determined in insects. Bioessays 29 344 355
14. TrumanJWRiddifordLM 2007 The morphostatic actions of juvenile hormone. Insect Biochem Mol Biol 37 761 770
15. RiddifordLM 1996 Juvenile hormone: the status of its “status quo” action. Arch Insect Biochem Physiol 32 271 286
16. TanATanakaHTamuraTShiotsukiT 2005 Precocious metamorphosis in transgenic silkworms overexpressing juvenile hormone esterase. Proc Natl Acad Sci U S A 102 11751 11756
17. FukudaS 1944 The hormonal mechanism of larval molting and metamorphosis in the silkworm. J Fac Sci Tokyo Univ Sect IV 6 477 532
18. HelvigCKoenerJFUnnithanGCFeyereisenR 2004 CYP15A1, the cytochrome P450 that catalyzes epoxidation of methyl farnesoate to juvenile hormone III in cockroach corpora allata. Proc Natl Acad Sci U S A 101 4024 4029
19. Werck-ReichhartDFeyereisenR 2000 Cytochromes P450: a success story. Genome Biol 1 REVIEWS3003
20. KinjohTKanekoYItoyamaKMitaKHirumaK 2007 Control of juvenile hormone biosynthesis in Bombyx mori: cloning of the enzymes in the mevalonate pathway and assessment of their developmental expression in the corpora allata. Insect Biochem Mol Biol 37 808 818
21. ShinodaTItoyamaK 2003 Juvenile hormone acid methyltransferase: a key regulatory enzyme for insect metamorphosis. Proc Natl Acad Sci U S A 100 11986 11991
22. SchooleyDABakerFC 1985 Juvenile hormone biosynthesis. KerkutGAGilbertLI Comprehensive insect physiology, biochemistry and pharmacology Oxford Pergamon Press 363 389
23. ReibsteinDLawJHBowlusSBKatzenellenbogenJA 1976 Enzymatic synthesis of juvenile hormone in Manduca sexta. GilbertLI The juvenile hormones New York Plenum Press 131 146
24. AndersenJFWaldingJKEvansPHBowersWSFeyereisenR 1997 Substrate specificity for the epoxidation of terpenoids and active site topology of house fly cytochrome P450 6A1. Chem Res Toxicol 10 156 164
25. ImamuraMNakaiJInoueSQuanGXKandaT 2003 Targeted gene expression using the GAL4/UAS system in the silkworm Bombyx mori. Genetics 165 1329 1340
26. HornCJaunichBWimmerEA 2000 Highly sensitive, fluorescent transformation marker for Drosophila transgenesis. Dev Genes Evol 210 623 629
27. UchinoKSezutsuHImamuraMKobayashiITatematsuK 2008 Construction of a piggyBac-based enhancer trap system for the analysis of gene function in silkworm Bombyx mori. Insect Biochem Mol Biol 38 1165 1173
28. KimuraMSakuraiSNakamachiTNarikiMNiimiS 1989 Qualitative and quantitative-analysis of juvenile-hormone in the larvae of the silkworm, Bombyx mori. Zool Sci 6 121 127
29. KanekoYShinodaTHirumaK 2011 Remodeling of the corpora cardiaca and the corpora allata during adult metamorphosis in Bombyx mori: identification of invisible corpora cardiaca by the expression of adipokinetic hormone. Appl Entomol Zool 46 87 93
30. MinakuchiCNamikiTYoshiyamaMShinodaT 2008 RNAi-mediated knockdown of juvenile hormone acid O-methyltransferase gene causes precocious metamorphosis in the red flour beetle Tribolium castaneum. FEBS J 275 2919 2931
31. NiwaRNiimiTHondaNYoshiyamaMItoyamaK 2008 Juvenile hormone acid O-methyltransferase in Drosophila melanogaster. Insect Biochem Mol Biol 38 714 720
32. ShengZTMaLCaoMXJiangRJLiS 2008 Juvenile hormone acid methyl transferase is a key regulatory enzyme for juvenile hormone synthesis in the Eri silkworm, Samia cynthia ricini. Arch Insect Biochem Physiol 69 143 154
33. NoriegaFGRibeiroJMKoenerJFValenzuelaJGHernandez-MartinezS 2006 Comparative genomics of insect juvenile hormone biosynthesis. Insect Biochem Mol Biol 36 366 374
34. MaestroJLPascualNTreiblmayrKLozanoJBellesX 2010 Juvenile hormone and allatostatins in the german cockroach embryo. Insect Biochem Mol Biol 660 665
35. MarchalEZhangJBadiscoLVerlindenHHultEF 2011 Final steps in juvenile hormone biosynthesis in the desert locust, Schistocerca gregaria. Insect Biochem Mol Biol 41 219 227
36. NouzovaMEdwardsMJMayoralJGNoriegaFG 2011 A coordinated expression of biosynthetic enzymes controls the flux of juvenile hormone precursors in the corpora allata of mosquitoes. Insect Biochem Mol Biol 41 660 669
37. ChungHSztalTPasrichaSSridharMBatterhamP 2009 Characterization of Drosophila melanogaster cytochrome P450 genes. Proc Natl Acad Sci U S A 106 5731 5736
38. LauferHBorstDBakerFCReuterCCTsaiLW 1987 Identification of a juvenile hormone-like compound in a crustacean. Science 235 202 205
39. HarshmanLGSongKDCasasJSchuurmansAKuwanoE 2010 Bioassays of compounds with potential juvenoid activity on Drosophila melanogaster: juvenile hormone III, bisepoxide juvenile hormone III and methyl farnesoates. J Insect Physiol 56 1465 1470
40. KuwanoETakeyaREtoM 1985 Synthesis and anti-juvenile hormone activity of 1-substituted-5-[(E)-2, 6-dimethyl-1, 5-heptadienyl]imidazoles. Agric Biol Chem 49 483 486
41. FurutaKAshibeKShirahashiHFujitaNYamashitaH 2007 Synthesis and anti-juvenile hormone activity of ethyl 4-(2-benzylalkyloxy)benzoates and their enantiomers. J Pestic Sci 32 99 105
42. KanekoYFurutaKKuwanoEHirumaK 2011 An anti-juvenile hormone agent, ethyl 4-(2-benzylhexyloxy)benzoate, inhibits juvenile hormone synthesis through the suppression of the transcription of juvenile hormone biosynthetic enzymes in the corpora allata in Bombyx mori. Insect Biochem Mol Biol 41 788 794
43. ZhouXZhouBTrumanJWRiddifordLM 2004 Overexpression of broad: a new insight into its role in the Drosophila prothoracic gland cells. J Exp Biol 207 1151 1161
44. NiwaRNamikiTItoKShimada-NiwaYKiuchiM 2010 Non-molting glossy/shroud encodes a short-chain dehydrogenase/reductase that functions in the ‘Black Box’ of the ecdysteroid biosynthesis pathway. Development 137 1991 1999
45. TakasuYKobayashiIBeumerKUchinoKSezutsuH 2010 Targeted mutagenesis in the silkworm Bombyx mori using zinc finger nuclease mRNA injection. Insect Biochem Mol Biol 759 765
46. MinakuchiCRiddifordLM 2006 Insect juvenile hormone action as a potential target of pest management. J Pestic Sci 31 77 84
47. DaimonTHamadaKMitaKOkanoKSuzukiMG 2003 A Bombyx mori gene, BmChi-h, encodes a protein homologous to bacterial and baculovirus chitinases. Insect Biochem Mol Biol 33 749 759
48. DaimonTKatsumaSIwanagaMKangWShimadaT 2005 The BmChi-h gene, a bacterial-type chitinase gene of Bombyx mori, encodes a functional exochitinase that plays a role in the chitin degradation during the molting process. Insect Biochem Mol Biol 35 1112 1123
49. DaimonTHirayamaCKanaiMRuikeYMengY 2010 The silkworm Green b locus encodes a quercetin 5-O-glucosyltransferase that produces green cocoons with UV-shielding properties. Proc Natl Acad Sci U S A 107 11471 11476
50. UedaHShinodaTHirumaK 2009 Spatial expression of the mevalonate enzymes involved in juvenile hormone biosynthesis in the corpora allata in Bombyx mori. J Insect Physiol 55 798 804
51. BrandAHPerrimonN 1993 Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118 401 415
52. NiwaRMatsudaTYoshiyamaTNamikiTMitaK 2004 CYP306A1, a cytochrome P450 enzyme, is essential for ecdysteroid biosynthesis in the prothoracic glands of Bombyx and Drosophila. J Biol Chem 279 35942 35949
53. IchikawaAOnoHFurutaKShiotsukiTShinodaT 2007 Enantioselective separation of racemic juvenile hormone III by normal-phase high-performance liquid chromatography and preparation of [(2)H(3)]juvenile hormone III as an internal standard for liquid chromatography-mass spectrometry quantification. J Chromatogr A 1161 252 260
54. SakudohTSezutsuHNakashimaTKobayashiIFujimotoH 2007 Carotenoid silk coloration is controlled by a carotenoid-binding protein, a product of the Yellow blood gene. Proc Natl Acad Sci U S A 104 8941 8946
55. ThompsonJDGibsonTJPlewniakFJeanmouginFHigginsDG 1997 The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25 4876 4882
56. KiguchiKAguiN 1981 Ecdysteroid levels and developmental events during larval moulting in the silkworm, Bombyx mori. J Insect Physiol 27 805 812
57. JonesDJonesG 2007 Farnesoid secretions of dipteran ring glands: what we do know and what we can know. Insect Biochem Mol Biol 37 771 798
Štítky
Genetika Reprodukční medicína
Článek Physiological Notch Signaling Maintains Bone Homeostasis via RBPjk and Hey Upstream of NFATc1Článek Intronic -Regulatory Modules Mediate Tissue-Specific and Microbial Control of / TranscriptionČlánek Probing the Informational and Regulatory Plasticity of a Transcription Factor DNA–Binding DomainČlánek Repression of Germline RNAi Pathways in Somatic Cells by Retinoblastoma Pathway Chromatin ComplexesČlánek An Alu Element–Associated Hypermethylation Variant of the Gene Is Associated with Childhood ObesityČlánek Three Essential Ribonucleases—RNase Y, J1, and III—Control the Abundance of a Majority of mRNAsČlánek Genomic Tools for Evolution and Conservation in the Chimpanzee: Is a Genetically Distinct Population
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 3
-
Všechny články tohoto čísla
- Comprehensive Research Synopsis and Systematic Meta-Analyses in Parkinson's Disease Genetics: The PDGene Database
- Genomic Analysis of the Hydrocarbon-Producing, Cellulolytic, Endophytic Fungus
- Networks of Neuronal Genes Affected by Common and Rare Variants in Autism Spectrum Disorders
- Akirin Links Twist-Regulated Transcription with the Brahma Chromatin Remodeling Complex during Embryogenesis
- Too Much Cleavage of Cyclin E Promotes Breast Tumorigenesis
- Imprinted Genes … and the Number Is?
- Genetic Architecture of Highly Complex Chemical Resistance Traits across Four Yeast Strains
- Exploring the Complexity of the HIV-1 Fitness Landscape
- MNS1 Is Essential for Spermiogenesis and Motile Ciliary Functions in Mice
- A Fundamental Regulatory Mechanism Operating through OmpR and DNA Topology Controls Expression of Pathogenicity Islands SPI-1 and SPI-2
- Evidence for Positive Selection on a Number of MicroRNA Regulatory Interactions during Recent Human Evolution
- Variation in Modifies Risk of Neonatal Intestinal Obstruction in Cystic Fibrosis
- PIF4–Mediated Activation of Expression Integrates Temperature into the Auxin Pathway in Regulating Hypocotyl Growth
- Critical Evaluation of Imprinted Gene Expression by RNA–Seq: A New Perspective
- A Meta-Analysis and Genome-Wide Association Study of Platelet Count and Mean Platelet Volume in African Americans
- Mouse Genetics Suggests Cell-Context Dependency for Myc-Regulated Metabolic Enzymes during Tumorigenesis
- Transcriptional Control in Cardiac Progenitors: Tbx1 Interacts with the BAF Chromatin Remodeling Complex and Regulates
- Synthetic Lethality of Cohesins with PARPs and Replication Fork Mediators
- APOBEC3G-Induced Hypermutation of Human Immunodeficiency Virus Type-1 Is Typically a Discrete “All or Nothing” Phenomenon
- Interpreting Meta-Analyses of Genome-Wide Association Studies
- Error-Prone ZW Pairing and No Evidence for Meiotic Sex Chromosome Inactivation in the Chicken Germ Line
- -Dependent Chemosensory Functions Contribute to Courtship Behavior in
- Diverse Forms of Splicing Are Part of an Evolving Autoregulatory Circuit
- Phenotypic Plasticity of the Drosophila Transcriptome
- Physiological Notch Signaling Maintains Bone Homeostasis via RBPjk and Hey Upstream of NFATc1
- Precocious Metamorphosis in the Juvenile Hormone–Deficient Mutant of the Silkworm,
- Igf1r Signaling Is Indispensable for Preimplantation Development and Is Activated via a Novel Function of E-cadherin
- Accurate Prediction of Inducible Transcription Factor Binding Intensities In Vivo
- Mitochondrial Oxidative Stress Alters a Pathway in Strongly Resembling That of Bile Acid Biosynthesis and Secretion in Vertebrates
- Mammalian Neurogenesis Requires Treacle-Plk1 for Precise Control of Spindle Orientation, Mitotic Progression, and Maintenance of Neural Progenitor Cells
- Tcf7 Is an Important Regulator of the Switch of Self-Renewal and Differentiation in a Multipotential Hematopoietic Cell Line
- REST–Mediated Recruitment of Polycomb Repressor Complexes in Mammalian Cells
- Intronic -Regulatory Modules Mediate Tissue-Specific and Microbial Control of / Transcription
- Age-Dependent Brain Gene Expression and Copy Number Anomalies in Autism Suggest Distinct Pathological Processes at Young Versus Mature Ages
- A Genome-Wide Association Study Identifies Variants Underlying the Shade Avoidance Response
- -by- Regulatory Divergence Causes the Asymmetric Lethal Effects of an Ancestral Hybrid Incompatibility Gene
- Genome-Wide Association and Functional Follow-Up Reveals New Loci for Kidney Function
- A Natural System of Chromosome Transfer in
- Cell Size and the Initiation of DNA Replication in Bacteria
- Probing the Informational and Regulatory Plasticity of a Transcription Factor DNA–Binding Domain
- Repression of Germline RNAi Pathways in Somatic Cells by Retinoblastoma Pathway Chromatin Complexes
- Temporal Transcriptional Profiling of Somatic and Germ Cells Reveals Biased Lineage Priming of Sexual Fate in the Fetal Mouse Gonad
- Rapid Analysis of Genome Rearrangements by Multiplex Ligation–Dependent Probe Amplification
- Metabolic Profiling of a Mapping Population Exposes New Insights in the Regulation of Seed Metabolism and Seed, Fruit, and Plant Relations
- The Atypical Calpains: Evolutionary Analyses and Roles in Cellular Degeneration
- The Silkworm Coming of Age—Early
- Development of a Panel of Genome-Wide Ancestry Informative Markers to Study Admixture Throughout the Americas
- Balanced Codon Usage Optimizes Eukaryotic Translational Efficiency
- The Min System and Nucleoid Occlusion Are Not Required for Identifying the Division Site in but Ensure Its Efficient Utilization
- Neurobeachin, a Regulator of Synaptic Protein Targeting, Is Associated with Body Fat Mass and Feeding Behavior in Mice and Body-Mass Index in Humans
- Statistical Analysis of Readthrough Levels for Nonsense Mutations in Mammalian Cells Reveals a Major Determinant of Response to Gentamicin
- Gene Reactivation by 5-Aza-2′-Deoxycytidine–Induced Demethylation Requires SRCAP–Mediated H2A.Z Insertion to Establish Nucleosome Depleted Regions
- The miR-35-41 Family of MicroRNAs Regulates RNAi Sensitivity in
- Genetic Basis of Hidden Phenotypic Variation Revealed by Increased Translational Readthrough in Yeast
- An Alu Element–Associated Hypermethylation Variant of the Gene Is Associated with Childhood Obesity
- Modelling Human Regulatory Variation in Mouse: Finding the Function in Genome-Wide Association Studies and Whole-Genome Sequencing
- Novel Loci for Adiponectin Levels and Their Influence on Type 2 Diabetes and Metabolic Traits: A Multi-Ethnic Meta-Analysis of 45,891 Individuals
- Polycomb-Like 3 Promotes Polycomb Repressive Complex 2 Binding to CpG Islands and Embryonic Stem Cell Self-Renewal
- Insulin/IGF-1 and Hypoxia Signaling Act in Concert to Regulate Iron Homeostasis in
- EMF1 and PRC2 Cooperate to Repress Key Regulators of Arabidopsis Development
- Three Essential Ribonucleases—RNase Y, J1, and III—Control the Abundance of a Majority of mRNAs
- Contrasted Patterns of Molecular Evolution in Dominant and Recessive Self-Incompatibility Haplotypes in
- A Machine Learning Approach for Identifying Novel Cell Type–Specific Transcriptional Regulators of Myogenesis
- Genomic Tools for Evolution and Conservation in the Chimpanzee: Is a Genetically Distinct Population
- Nos2 Inactivation Promotes the Development of Medulloblastoma in Mice by Deregulation of Gap43–Dependent Granule Cell Precursor Migration
- Intracranial Aneurysm Risk Locus 5q23.2 Is Associated with Elevated Systolic Blood Pressure
- Heritability and Genetic Correlations Explained by Common SNPs for Metabolic Syndrome Traits
- A Genome-Wide Association Study of Nephrolithiasis in the Japanese Population Identifies Novel Susceptible Loci at 5q35.3, 7p14.3, and 13q14.1
- DNA Damage in Nijmegen Breakage Syndrome Cells Leads to PARP Hyperactivation and Increased Oxidative Stress
- DNA Resection at Chromosome Breaks Promotes Genome Stability by Constraining Non-Allelic Homologous Recombination
- Genetic Analysis of Floral Symmetry in Van Gogh's Sunflowers Reveals Independent Recruitment of Genes in the Asteraceae
- A Splice Site Variant in the Bovine Gene Compromises Growth and Regulation of the Inflammatory Response
- Promoter Nucleosome Organization Shapes the Evolution of Gene Expression
- The Nucleoside Diphosphate Kinase Gene Acts as Quantitative Trait Locus Promoting Non-Mendelian Inheritance
- The Ciliogenic Transcription Factor RFX3 Regulates Early Midline Distribution of Guidepost Neurons Required for Corpus Callosum Development
- Phosphorylation of the RNA–Binding Protein HOW by MAPK/ERK Enhances Its Dimerization and Activity
- A Genome-Wide Scan of Ashkenazi Jewish Crohn's Disease Suggests Novel Susceptibility Loci
- Parkinson's Disease–Associated Kinase PINK1 Regulates Miro Protein Level and Axonal Transport of Mitochondria
- LMW-E/CDK2 Deregulates Acinar Morphogenesis, Induces Tumorigenesis, and Associates with the Activated b-Raf-ERK1/2-mTOR Pathway in Breast Cancer Patients
- Mapping the Hsp90 Genetic Interaction Network in Reveals Environmental Contingency and Rewired Circuitry
- Autoregulation of the Noncoding RNA Gene
- The Human Pancreatic Islet Transcriptome: Expression of Candidate Genes for Type 1 Diabetes and the Impact of Pro-Inflammatory Cytokines
- Spo0A∼P Imposes a Temporal Gate for the Bimodal Expression of Competence in
- Antagonistic Regulation of Apoptosis and Differentiation by the Cut Transcription Factor Represents a Tumor-Suppressing Mechanism in
- A Downstream CpG Island Controls Transcript Initiation and Elongation and the Methylation State of the Imprinted Macro ncRNA Promoter
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- PIF4–Mediated Activation of Expression Integrates Temperature into the Auxin Pathway in Regulating Hypocotyl Growth
- Metabolic Profiling of a Mapping Population Exposes New Insights in the Regulation of Seed Metabolism and Seed, Fruit, and Plant Relations
- A Splice Site Variant in the Bovine Gene Compromises Growth and Regulation of the Inflammatory Response
- Comprehensive Research Synopsis and Systematic Meta-Analyses in Parkinson's Disease Genetics: The PDGene Database
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



