-
Články
Reklama
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
ReklamaLoss of Catalytically Inactive Lipid Phosphatase Myotubularin-related Protein 12 Impairs Myotubularin Stability and Promotes Centronuclear Myopathy in Zebrafish
X-linked myotubular myopathy (XLMTM) is a congenital disorder caused by mutations of the myotubularin gene, MTM1. Myotubularin belongs to a large family of conserved lipid phosphatases that include both catalytically active and inactive myotubularin-related proteins (i.e., “MTMRs”). Biochemically, catalytically inactive MTMRs have been shown to form heteroligomers with active members within the myotubularin family through protein-protein interactions. However, the pathophysiological significance of catalytically inactive MTMRs remains unknown in muscle. By in vitro as well as in vivo studies, we have identified that catalytically inactive myotubularin-related protein 12 (MTMR12) binds to myotubularin in skeletal muscle. Knockdown of the mtmr12 gene in zebrafish resulted in skeletal muscle defects and impaired motor function. Analysis of mtmr12 morphant fish showed pathological changes with central nucleation, disorganized Triads, myofiber hypotrophy and whorled membrane structures similar to those seen in X-linked myotubular myopathy. Biochemical studies showed that deficiency of MTMR12 results in reduced levels of myotubularin protein in zebrafish and mammalian C2C12 cells. Loss of myotubularin also resulted in reduction of MTMR12 protein in C2C12 cells, mice and humans. Moreover, XLMTM mutations within the myotubularin interaction domain disrupted binding to MTMR12 in cell culture. Analysis of human XLMTM patient myotubes showed that mutations that disrupt the interaction between myotubularin and MTMR12 proteins result in reduction of both myotubularin and MTMR12. These studies strongly support the concept that interactions between myotubularin and MTMR12 are required for the stability of their functional protein complex in normal skeletal muscles. This work highlights an important physiological function of catalytically inactive phosphatases in the pathophysiology of myotubular myopathy and suggests a novel therapeutic approach through identification of drugs that could stabilize the myotubularin-MTMR12 complex and hence ameliorate this disorder.
Published in the journal: . PLoS Genet 9(6): e32767. doi:10.1371/journal.pgen.1003583
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003583Summary
X-linked myotubular myopathy (XLMTM) is a congenital disorder caused by mutations of the myotubularin gene, MTM1. Myotubularin belongs to a large family of conserved lipid phosphatases that include both catalytically active and inactive myotubularin-related proteins (i.e., “MTMRs”). Biochemically, catalytically inactive MTMRs have been shown to form heteroligomers with active members within the myotubularin family through protein-protein interactions. However, the pathophysiological significance of catalytically inactive MTMRs remains unknown in muscle. By in vitro as well as in vivo studies, we have identified that catalytically inactive myotubularin-related protein 12 (MTMR12) binds to myotubularin in skeletal muscle. Knockdown of the mtmr12 gene in zebrafish resulted in skeletal muscle defects and impaired motor function. Analysis of mtmr12 morphant fish showed pathological changes with central nucleation, disorganized Triads, myofiber hypotrophy and whorled membrane structures similar to those seen in X-linked myotubular myopathy. Biochemical studies showed that deficiency of MTMR12 results in reduced levels of myotubularin protein in zebrafish and mammalian C2C12 cells. Loss of myotubularin also resulted in reduction of MTMR12 protein in C2C12 cells, mice and humans. Moreover, XLMTM mutations within the myotubularin interaction domain disrupted binding to MTMR12 in cell culture. Analysis of human XLMTM patient myotubes showed that mutations that disrupt the interaction between myotubularin and MTMR12 proteins result in reduction of both myotubularin and MTMR12. These studies strongly support the concept that interactions between myotubularin and MTMR12 are required for the stability of their functional protein complex in normal skeletal muscles. This work highlights an important physiological function of catalytically inactive phosphatases in the pathophysiology of myotubular myopathy and suggests a novel therapeutic approach through identification of drugs that could stabilize the myotubularin-MTMR12 complex and hence ameliorate this disorder.
Introduction
X-linked myotubular myopathy (XLMTM) is a congenital disorder caused by mutations of the MTM1 gene that encodes myotubularin [1], [2]. Affected males are born with severe generalized hypotonia and weakness of skeletal muscles with respiratory insufficiency. In majority of cases the disease is fatal within the first months of life, but a proportion of affected males survive into their teens or beyond yet are non ambulant and require ventilatory support. Histopathologically, affected muscle fibers exhibit hypotrophy with a large number of centrally placed nuclei in a high proportion of myofibers. Thus, XLMTM is considered a subtype of centronuclear myopathy (CNM) [3]. MTM1 encodes a 3′-phosphoinositide (PtdIns3P) lipid phosphatase that catalyzes the dephosphorylation of phosphatidylinositol-3-phosphate (PtdIns3P) and phosphatidylinositol-3,5-bisphosphate PtdIns(3,5P)2 [4], [5], [6], [7]. Phosphoinositides (PIs) are critical for a variety of physiological processes, including cell proliferation, cell death, motility, cytoskeletal regulation, intracellular vesicle trafficking, autophagy and cell metabolism [8]. PtdIns3P and PtdIns(3,5)P2 are both important regulators of membrane trafficking [9]. Similar to humans, loss of myotubularin function in animal models also results in clinico-pathological symptoms similar to myotubular myopathy signifying evolutionary conservation of mechanisms involving myotubularin [10], [11], [12], [13].
Myotubularin is the prototypic member of one of the largest and most conserved protein lipid phosphatase subfamilies in eukaryotes, the myotubularin-related proteins (MTMRs) [14], . There are nine catalytically active members of the MTMR family, and six members whose catalytic sites have been inactivated through missense changes (designated myotubularin and MTMR1-14). While several active members are known to play crucial roles in physiology and human diseases presumably related to loss of their enzymatic activities, the cellular functions of catalytically inactive members are still poorly understood. Catalytically inactive MTMR family members are highly conserved and retained in vertebrate genome from teleosts to humans during evolution [16] suggesting that these inactive members have important roles in development and diseases independent of their enzymatic activities, as functionally redundant family members are often lost during evolution [17]. This is further evident by a recent study that showed that catalytically inactive form of myotubularin could ameliorate many of the structural abnormalities seen in Mtm1 knockout mice [18].
Myotubularins form homo - and heteroligomers with themselves and other members of the MTMR family, and the catalytically inactive MTMRs are thought to largely form oligomers with active family members [14], [19]. These interactions appear to regulate the sub-cellular localization or catalytic activity of the active members of the myotubularin family. In vitro, catalytically inactive MTMR5 and MTMR9 modulate the enzymatic activity of their interacting catalytically active partners, MTMR2, and MTMR6 and MTMR8, respectively [20], [21], [22]. Functional evidence for in vivo significance of these interactions comes from genetic studies in humans that show that mutations of either the catalytically active MTMR2 or its catalytically inactive binding partner MTMR13 result in similar forms of Charcot-Marie-Tooth disease [23], [24], [25], [26]. Similarly, mutations in either of the binding partners MTMR2 (catalytically active) or MTMR5 (catalytically inactive) lead to defective spermatogenesis in mice, suggesting the biological importance of these interactions [27], [28].
Myotubularin-related protein 12 (MTMR12), previously sometimes referred to as 3-phosphatase adaptor protein (3-PAP), is a catalytically inactive member of myotubularin family that interacts with MTM1 and MTMR2 to regulate their sub-cellular localization in in vitro studies of non-muscle cells [29], [30]. The in vivo significance of such interactions remains unknown, especially with reference to disease process in myotubular myopathy that primarily affects the skeletal muscles due to lack of myotubularin function [10]. Protein-protein interactions are critical components of almost every biological process and any disruption of these networks leads to pathological conditions resulting in related diseases [31], [32]. Therefore, a proper understanding of myotubular myopathy necessitates a comprehensive knowledge of the molecular mechanisms that govern such processes. We have employed zebrafish (Danio rerio) as a vertebrate animal model to understand the role of MTMR12 as a molecular regulator of myotubularin. Zebrafish are an excellent vertebrate model to study skeletal muscle development and disease due to high genomic synteny and similar clinico-pathological changes as seen in the human myopathies. Our studies revealed that deficiency of MTMR12 in zebrafish results in myopathy and impaired motor function similar to that caused by loss of myotubularin. Our data suggest MTMR12 affects muscle function primarily by regulating the stability of myotubularin in vivo. Studies in cells, mice and humans further implicate MTMR12 as an important player in the pathophysiology of XLMTM.
Results
MTMR12 interacts with myotubularin in vitro and in vivo in skeletal muscles
MTMR12 has been shown to interact with myotubularin and form oligomers in K562 and Cos7 cells [30]. To investigate if the binding between these proteins is a direct consequence of protein-protein interactions or is an indirect interaction mediated through another binding partner, GST-pull down assay was performed in vitro (Figure 1A). Human myotubularin full-length protein was expressed as a GST fusion protein in E. coli (MTM1-GST). Equivalent amounts MTM1-GST or control GST protein bound to glutathione beads were incubated with in vitro synthesized MTMR12 protein with a B10 tag (MTMR12-B10). MTM1-GST protein pulled down MTMR12-B10 protein whereas no interaction was observed with the GST alone, suggesting myotubularin and MTMR12 interact with each other by direct protein-protein interactions (Figure 1A). The interaction between myotubularin and MTMR12 was also examined in the Cos1 cell line. MTM1 and MTMR12-B10 were over-expressed in Cos1 cells and cell extracts were immunoprecipitated by an antibody against myotubularin (monoclonal, 1G1). This resulted in co-immunoprecipitation of MTMR12-B10 with myotubularin but not with control IgG or empty beads confirming that MTMR12 also interacts with myotubularin in the cellular context as reported earlier (Figure 1B) [30].
Fig. 1. Protein-protein interactions between myotubularin and MTMR12 proteins. 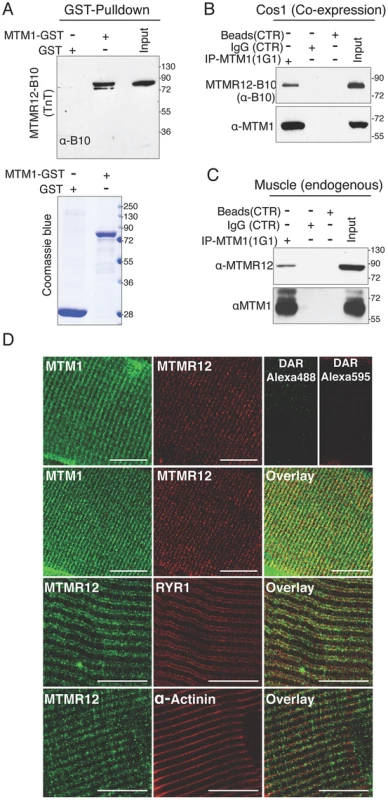
(A) GST pull down of MTM1-GST recombinant protein with MTMR12 showed a direct interaction between the two proteins (above). Coomassie blue stained gel (below) showing GST and MTM1-GST protein used in the pull down (B) Co-IP experiments from Cos-1 transfected cells with MTM1 and MTMR12-B10 constructs showing the interaction of the two proteins in the cellular context in-vivo (C) MTMR12 co-immunoprecipitates with myotubularin (using 1G1 monoclonal antibody) in mouse muscle lysates revealed with anti-MTMR12 polyclonal antibody (upper panel) and with the 2827 polyclonal anti-myotubularin (bottom panel). (D) Confocal microscopic immunofluorescence studies of longitudinal frozen sections of skeletal muscle from mouse tibialis anterior muscle with sarcomeric markers. Individual immunostaining with 2827 anti-myotubularin or anti-MTMR12 showed similar striated localization in skeletal muscle (top panel). Double-immunostaining with both proteins showed a co-localization of myotubularin and MTMR12 to Triads and partial co-localization with the ryanodine receptor, RyR1, a sarcoplasmic reticulum marker, but not with α-actinin, a Z-line marker. Scale bar = 10 µm. Mutations in MTM1 result in XLMTM, which is a congenital myopathy that primarily affects the skeletal muscles in human patients and animal models. Therefore, the interaction between myotubularin and MTMR12 was investigated in skeletal muscles. Co-immunoprecipitation was performed using protein extracts from murine skeletal muscle (tibialis anterior) using an anti-myotubularin monoclonal antibody (1G1) (Figure 1C). Antibody against myotubularin co-immunoprecipitated endogenous MTMR12 protein showing that myotubularin and MTMR12 interact in skeletal muscles. Control IgG or empty beads failed to immunoprecipitate MTMR12 suggesting the specificity of interactions between myotubularin and MTMR12. In vivo interaction between these proteins was also investigated by immuno-colocalization of myotubularin and MTMR12 proteins in murine skeletal muscles. Immunofluorescence showed a striated expression of endogenous MTMR12 in mouse tibialis anterior skeletal muscle similar to the pattern of myotubularin staining, and double immunolabeling revealed that these colocalize at the resolution of confocal microscopy (Figure 1D). In addition, partial co-localization of MTMR12 was observed with ryanodine receptors, a sarcoplasmic reticulum marker. The expression of MTMR12 was restricted to triads as no co-localization was observed with α-actinin at Z-lines (Figure 1D). Therefore, a combination of GST pull down, co-immunoprecipitation and co-localization studies support the hypothesis that myotubularin and MTMR12 interact with each other in skeletal muscle.
Mtmr12 knockdown results in skeletal muscle myopathy in zebrafish
To understand the function of MTMR12 in vivo, zebrafish was used as a vertebrate animal model. The zebrafish mtmr12 gene encodes a protein of 736 amino acids that is 69% similar and 52% identical with the human MTMR12 protein. The expression of mtmr12 was analyzed by whole mount in-situ hybridization and RT-PCR in developing zebrafish embryos. Whole mount in-situ hybridization showed that mtmr12 transcripts were expressed ubiquitously in developing eyes, brain, heart and skeletal muscles at 1 day post fertilization (dpf) (Figure 2A). This ubiquitous expression of mtmr12 was similar to the ubiquitous expression of mtm1 transcripts at 1 dpf. Similar to the zebrafish gene, human MTMR12 transcripts have also been found to express in all organs [33]. RT-PCR analysis further showed similar temporal expression of mtm1 and mtmr12 during zebrafish development (Figure 2A). RT-PCR revealed that mtmr12 and mtm1 were first detected as maternal transcripts at the 1 cell stage. Expression of these maternal transcripts decreased during gastrulation and zygotic expression appeared around 8 hours post fertilization (hpf). This zygotic expression persisted in all developmental stages tested (until 5 dpf). In murine muscle cells, Mtmr12 transcripts were detected during proliferation as well as differentiation stages in C2C12 cells (Figure 2B). The expression of Mtmr12 transcripts increased steadily during differentiation followed by a decrease in late differentiation. Comparison to Mtm1 expression revealed a similar expression pattern with a continuous increase with the progression of differentiation. Similar to the mRNA expression, MTMR12 protein expression was also highest during differentiation (Figure 2B). Similar expression of mtmr12 and mtm1 during zebrafish development and in muscle cell differentiation suggests that they may be involved in similar physiological processes.
Fig. 2. Expression patterns and morpholino-based knockdown of mtmr12 in developing zebrafish. 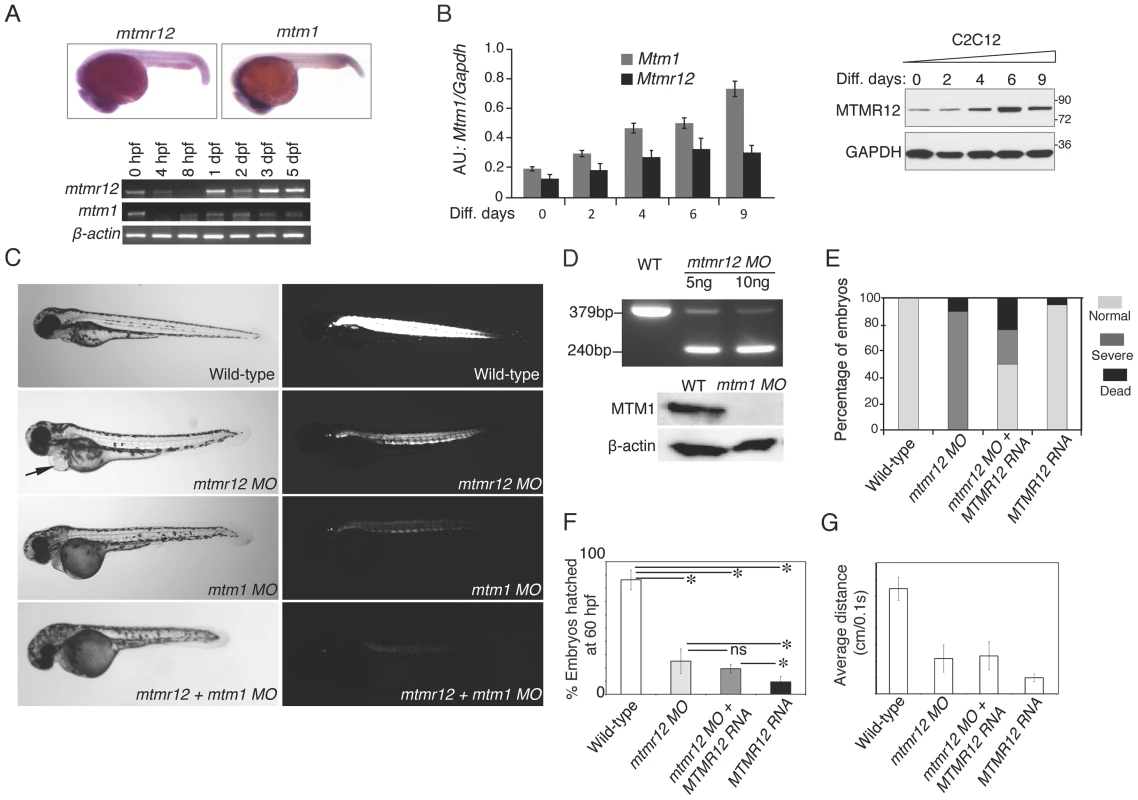
(A) Whole mount in-situ hybridization detected ubiquitous expression of mtmr12 and mtm1 transcripts in zebrafish embryos at 1 dpf (above). Below is RT-PCR analysis of mtm1 and mtmr12 expression during zebrafish development using RNA extracts from whole zebrafish embryos at indicated developmental timepoints. (B) Synergistic expression level of Mtm1 and Mtmr12 transcripts and protein at indicated time points of C2C12 differentiation (0–9 days) monitored by RT-quantitative PCR (corresponding histogram, *P≤0.05) and by western blot analysis (right panel). (C) Live embryos at 3 dpf injected with control, mtmr12, mtm1 or both mtmr12 and mtm1 morpholinos in normal (left) and polarized lights (right). mtmr12 morphant fish showed a dorsal curvature in skeletal muscle and reduced birefringence in polarized light similar to mtm1 morphant embryos. mtmr12 morphant fish also exhibited pericardial edema (arrow). mtmr12-mtm1 double knockdown fish exhibited smaller size and reduced birefringence relative to mtm1 or mtmr12 alone morphant fish. (D) mtmr12 mRNA levels in mtmr12 morphant zebrafish following injection of two different amounts of morpholino (indicated below, upper panel). In mtm1 morphant fish, no residual myotubularin was observed showing that mtm1 morpholinos are completely penetrant to the limits of detection for western blotting. (E) Over-expression of human MTMR12 mRNA rescued small body length and skeletal muscle abnormalities observed in mtmr12 morphant embryos. (F) Quantification of the chorion hatching at 60 hpf. The number of embryos was quantified in three independent clutches (number of embryos in each clutch = 90–120). (G) Quantification of touch evoke response at 3 dpf (n = 5–8 embryos were assayed in each morpholino group).*P≤0.01. To investigate in vivo functions of the mtmr12 gene in zebrafish, anti-sense morpholino technology was employed to achieve functional gene knockdown (Figure 2C–E). Morpholinos were designed to disrupt either the translation or splicing of mtmr12 transcripts. Knockdown using either a splice site-morpholino targeting the exon3-intron3 junction or the translational (ATG) morpholino resulted in similar phenotypes at relatively low morpholino concentrations (3.5–5.0 ng), suggesting the specificity of their action. Microinjection of 1cell embryos with splice site morpholinos resulted in mis-splicing and exclusion of exon 3 from the mature mRNA as detected by RT-PCR assay (Figure 2D). Mtmr12 knockdown fish (mtmr12 morphants) are smaller in size compared to controls and exhibited a dorsal curvature through the back and tail, instead of the normal flat dorsum, similar to mtm1 knockdown fish (Figure 2C). Axial skeletal muscles of zebrafish embryos were examined using a birefringence assay that involves examination of axial skeletal muscles of live zebrafish embryos using polarized filter microscopy. Skeletal muscles of mtmr12 morphant embryos showed a reduced birefringence in comparison to the control morpholino injected fish suggesting a defect in skeletal muscle organization. Several mtmr12 morphants also displayed pericardial edema. Similar phenotypes were obtained with both translational as well as spice-site morpholinos suggesting the specificity of morpholino knockdown. None of the commercially available antibodies showed reactivity to zebrafish MTMR12 protein, therefore, the rest of the studies were performed with the splice-site blocking morpholino against exon3-intron3 junction as mRNA knockdown could be assayed. To further validate the specificity of these phenotypes and to rule out any off-site targeting effect of morpholinos, mRNA rescues were performed. Human MTMR12 mRNA was co-injected with mtmr12 morpholino in zebrafish embryos. Over-expression of MTMR12 mRNA in mtmr12 morphant fish resulted in a rescue of phenotypes seen in mtmr12 morphant fish indicating the specificity of morpholino targeting (Figure 2E).
To understand if the interacting partners myotubularin and MTMR12 are involved in similar biological processes or have functions that are independent of each other, double knockdowns were performed. Double knockdown fish were smaller in size then either mtm1 or mtmr12 morphant fish. Polarized live microscopy of morphant fish also showed that birefringence of mtmr12-mtm1 double morphant zebrafish muscle was lower than in either mtm1 or mtmr12 morphant fish suggesting a severe muscle phenotype. The exacerbated phenotypes of double knockdown fish suggests that in addition to regulating similar biological processes these genes may be involved in different processes independent of each other.
MTMR12 deficiency results in impaired motor function in zebrafish
To understand the consequences of MTMR12 deficiency on motor function, behavioral analysis of zebrafish embryos was performed. During early development, zebrafish embryos hatch out of their chorions by regular contractions of their skeletal muscles. Typically, approximately 86±7.4% of control embryos hatch by 60 hpf. In contrast, only 25±9.3% of mtmr12 morpholino-injected embryos hatched by this time, consistent with a continued decrease in motor activity early in development (P<0.01, n = 100–170) (Figure 2F). Mtm1 morphant embryos displayed similar behavior as mtmr12 morphants (19.3±3.39, n = 100–150, P<0.001). Knockdown of both MTMR12 and myotubularin resulted in a significant decrease in hatching behavior in comparison to the mtmr12 alone morphant fish. (9.33%±4.02, P<0.001, n = 100–130).
Mtmr12 morphant fish were largely immotile and their touch evoked response was blunted; instead of rapidly swimming out of the field of view like control fish (6.44±0.712 cm/0.1 sec), they twitched and only moved several lengths when stimulated with a needle (2.16±(2.832 cm/0.1 sec), suggesting a significant degree of overall muscle weakness (Movie S1, Figure 2G). This decrease in touch-evoked response was similar to that of mtm1 morphant fish (2.32±0.855 cm/0.1 sec) (Movie S1, Figure 2G). The touch-evoked response was also evaluated in mtm1-mtmr12 double morphant fish. In comparison to either mtm1 or mtmr12 knockdown embryos, double morphant embryos showed a greater degree of reduction in touch-evoked escape response (0.98±0.230 cm/0.1 sec) (Movie S1, Figure 2G). The delayed chorion hatching and diminished touch-evoked escape behaviors showed that mtmr12 is required for normal motor function in zebrafish.
MTMR12 deficiency results in sarcomere disorganization with central nucleation in skeletal muscles
To study the effect of MTMR12 deficiency on skeletal muscle structure, ultrathin toluidine blue-stained longitudinal sections of control and mtmr12 knockdown fish were examined at 3 dpf. Histological examination of control muscle showed well-organized myofibers with elongated nuclei that were localized to the periphery of muscle fibers (Figure 3A). In mtmr12 knockdown fish, areas lacking sarcomeric organization were observed (Figure 3A, arrowhead). Moreover, occasional rounded, central nuclei were seen in skeletal muscles of the morphant fish but were absent in the control fish (Figure 3A, arrow). Remarkably, the sarcomeric disorganization with central nucleation was very similar to the histological changes observed in mtm1 knockdown fish (Figure 3A, arrow). The proportion of fibers with central nuclei was almost similar in mtmr12 morphant (54.5±7.1%) and mtm1 morphant (57.8±7.1%) fish and significantly higher than normal controls (2.2±1.1%). Mtm1-mtmr12 double knockdown fish exhibited severe muscle abnormalities with larger number of myofibers displaying sarcomeric disorganization and central nucleation (69.2±6.4%) than single morphants (Figure 3A). To evaluate if the sarcomeric defects observed in mtmr12 morphant fish are developmental or due to degenerative changes in muscle, skeletal muscle histology was evaluated at different time points during zebrafish development. A comparison of Hematoxylin and Eosin stained skeletal muscle sections at 2 dpf and 3 dpf showed an increase in sarcomeric disorganization at 3 dpf in mtmr12 morphant fish (Figure 3C). This suggests the sarcomeric defects seen in mtmr12 morphant fish are due to degenerative processes in disease state. The number of central nuclei per myofiber showed no significant change during development in mtmr12 morphants (Figure 3D). A comprehensive histological analysis of Hematoxylin and Eosin stained sections of wild-type and mtmr12 morphant showed no other histological abnormalities in other organs.
Fig. 3. Abnormal histology of MTMR12-deficient zebrafish. 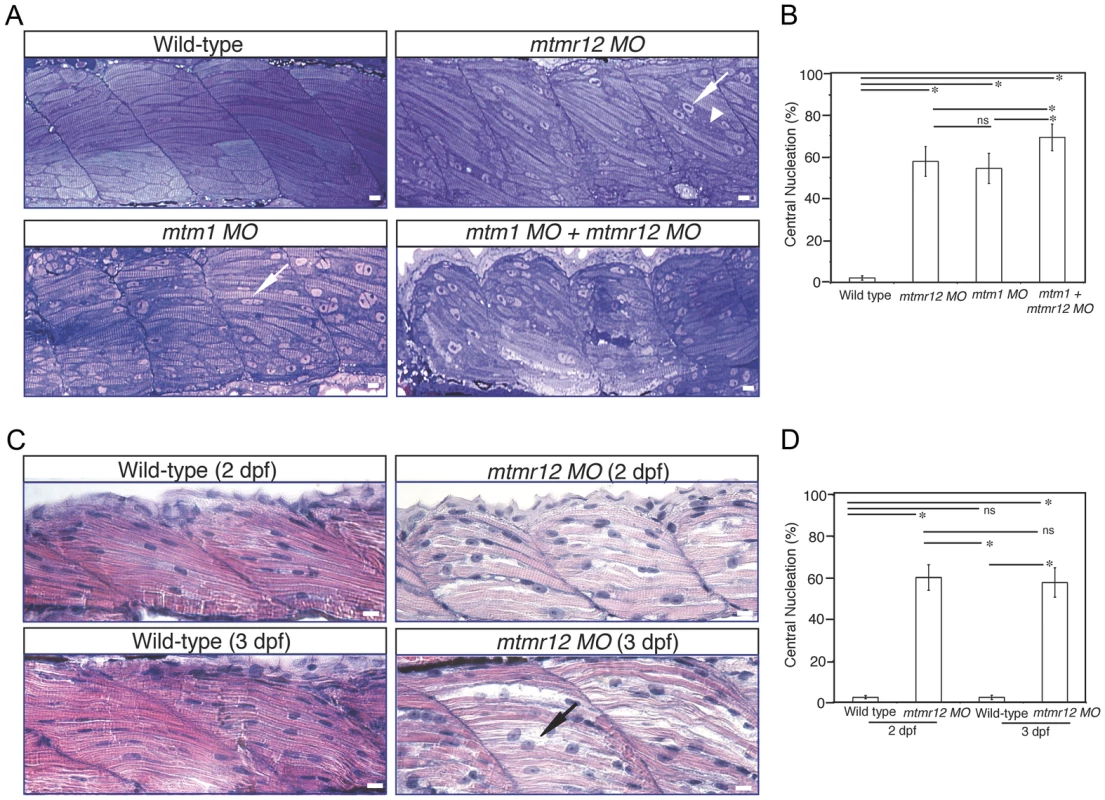
(A)Toluidine blue stained longitudinal sections of skeletal muscle in control and morphant fish at 3 dpf. In comparison to the control fish, mtmr12 morphants showed disorganized myofibers (arrowhead) with central nucleation (arrow), similar to histological changes observed in the skeletal muscle of mtm1 morphant fish (arrow). Knockdown of both mtm1 as well as mtmr12 results in severe muscle disorganization greater than seen in mtm1 or mtmr12 alone morphants. (B) Centrally nucleated myofibers were quantified. Serial sections from 3–4 different embryos were analyzed and the relative number of centrally nucleated fibers in the middle somites (10–13) were counted. (C) Hematoxylin and Eosin staining of mtmr12 morphant zebrafish at different time points. An increase in sarcomeric disorganization was observed at 3 dpf in comparison to 2 dpf in mtmr12 morphants (arrow) (D) Centrally nucleated myofibers were quantified. Serial sections from 6 different embryos were analyzed and the relative number of centrally nucleated fibers in the middle somites (10–13) were counted. Scale bar = 10 µm. To identify ultrastructural defects in sub-cellular compartments of skeletal muscle, transmission electron microscopy was performed at 3 dpf (Figure 4). Longitudinal views of the skeletal muscle in mtmr12 morphant fish revealed significant myofibrillar disarray in comparison to highly organized myofibrillar structures with peripheral elongated nuclei in control fish (Figure 4A, C). Notably, skeletal muscle of mtmr12 morphants showed an increase in absent or disorganized Triads in the myofibers in comparison to the control fish (Figure 4A, C). In addition, similar to the whorled membrane structures reported in myotubularin deficiency [13], mtmr12 fish also exhibited whorled membrane structures in skeletal muscle (Figure 4E). Mtm1 morphant fish displayed sarcomeric disorganization, with central nucleation and triad disorganization as previously reported for myotubularin deficiency in zebrafish, mouse and humans (Figure 4B). The ultrastructural defects in double knockdown fish were more severe than either mtm1 or mtmr12 knockdown fish (Figure 4D). Myofibers lacked the sarcomeric organization with absence of Z-lines in many myofibers. The numbers of disorganized triads also showed a small but significant increase in double knockdown fish (68.8±10.08%) compared to mtm1 (63.5±8.00%) or mtmr12 (57±12.12%) fish (p<0.005, n = 5 embryos, 15 myofibers in each embryo) (Figure 4G). Like in myotubularin and MTMR12 deficient muscles, whorled membrane structures were also observed in mtmr12-mtm1 double knockdown zebrafish (4F). These abnormal membrane structures are seen in several types of myopathies, however, their functional role in disease pathology is not known. In a previous study on double mtm1-mtmr14 knockdown, the severe phenotype was a result of an increase in autophagy in the absence of both proteins [34]. Ultrastructural examination of mtm1-mtmr12 double knockdown exhibited no increase in autophagic vacuoles suggesting different pathological mechanism in two different disease states.
Fig. 4. Ultrastructural abnormalities in MTMR12 deficiency. 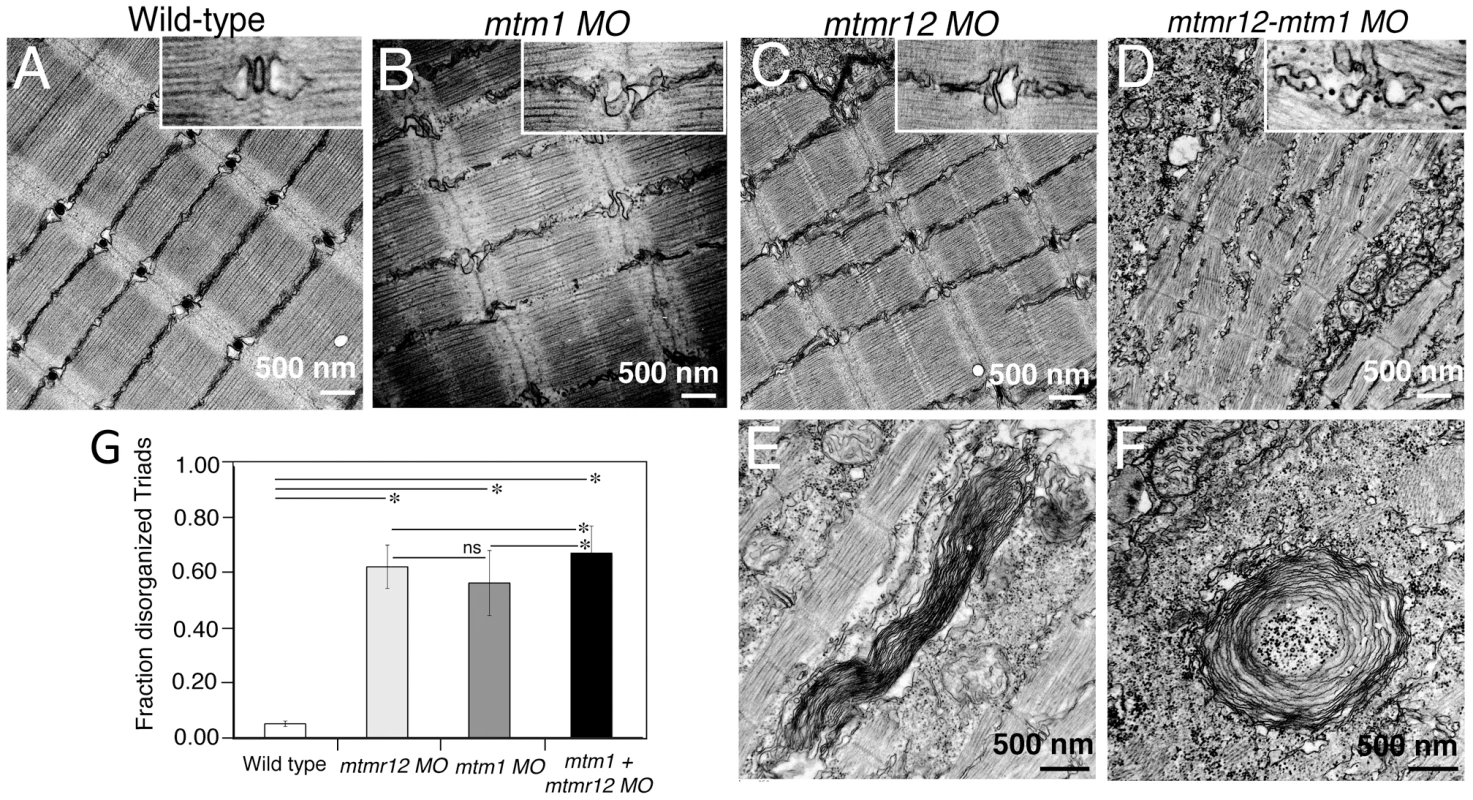
Transmission electron micrographs of skeletal muscles in control and morphant fish. In comparison to wild-type controls (A), mtmr12 morphant fish displayed abnormal triads (C, high magnification, inset). mtm1 morphant muscle also had absent or disorganized triads (B). mtmr12-mtm1 double knockdown morphants displayed exacerbated defects in muscle with many absent Z-lines in addition to disorganized triads (D inset shows high magnification view). Higher magnification examination showed whorled membranous structures in mtmr12 and mtmr12-mtm1 morphants (E–F). (G) Histograms represent quantification of disorganized triads of the single or double morphants. Total number of triads were counted in at least 15 myofibers within each embryo (n = 5 embryos). *P≤0.05, ns: statistically not significant. Loss of MTMR12 results in reduced stability of myotubularin protein in zebrafish
Catalytically inactive myotubularin family members have been shown to regulate the activity and/or sub-cellular localization of their catalytically active interacting partners. Previous co-transfection experiments in cell culture have suggested that MTMR12 is required to regulate the subcellular localization of myotubularin [30]. Therefore, to investigate if MTMR12 controls the subcellular localization of myotubularin in zebrafish skeletal muscle, immunofluorescence studies were performed. Immunostaining of skeletal muscle of control fish with antibody against myotubularin detected a striated expression pattern of myotubularin protein corresponding to the triad compartment (Figure 5). Strikingly, highly reduced levels of myotubularin labeling were seen in morphant muscle as compared to control skeletal muscle. However, the residual myotubularin protein showed similar localization as the control fish. These data suggest that loss of MTMR12 does not affect subcellular localization of myotubularin in skeletal muscle in vivo.
Fig. 5. Myotubularin and PtdIns3P alterations in mtmr12 morphants. 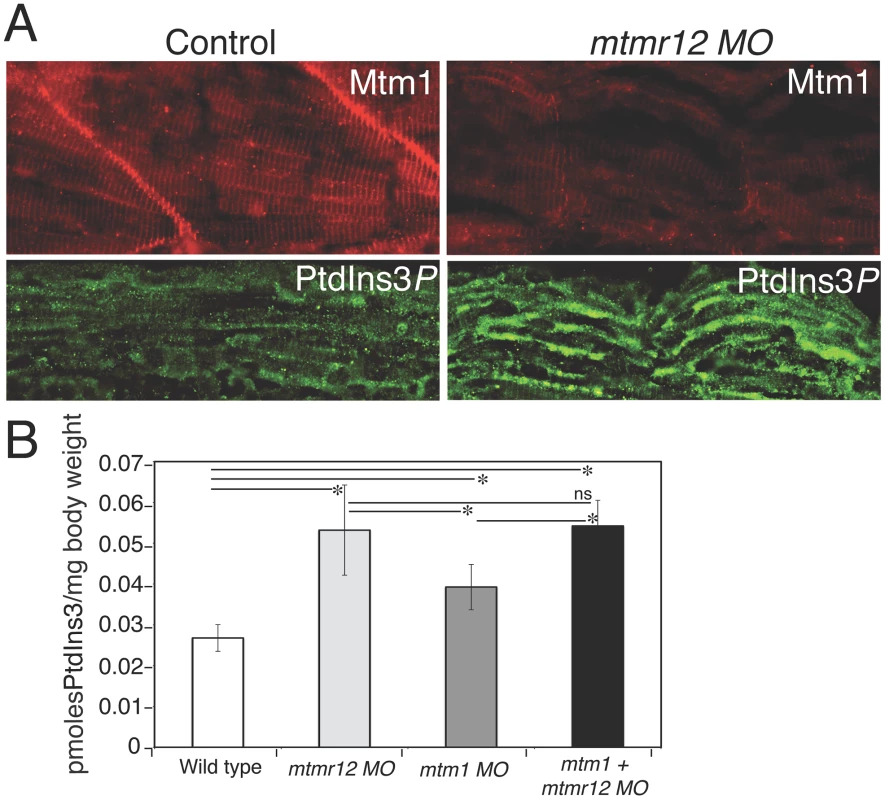
(A) Immunofluorescence of control and mtmr12 knockdown fish showed significantly decreased myotubularin staining in mtmr12 knockdown fish in images taken under identical conditions. Immunofluorescence detection of PtdIns3P showed apparent increases of this myotubularin substrate in mtmr12 morphant embryos as compared to controls. (B) PtdIns3P levels are increased in mtmr12, mtm1 and mtm1-mtmr12 morphant zebrafish, *P≤0.05. Total lipids were extracted from zebrafish at 3 dpf and PtdIns3P levels were measured using a lipid-protein overlay enzyme-linked immunosorbent assay. These observations suggest that MTMR12 is likely involved in regulating the stability of myotubularin. To address this point, we quantified myotubularin protein levels in mtmr12 morphant fish. Western blot analysis, performed in three independent groups of embryos injected with mtmr12 morpholino, revealed a ∼90% reduction in myotubularin levels in mtmr12 morphant fish in comparison to the controls (Figure 6A). This suggests that myotubularin-MTMR12 interactions result in stabilization of myotubularin in zebrafish skeletal muscle. To investigate whether MTMR12 regulates myotubularin levels in a mammalian system, Mtmr12 siRNA C2C12 cell lines were created. SiRNA-mediated knockdown of Mtmr12 in C2C12 myoblasts or differentiated myotubes resulted in decreased levels of myotubularin protein compared to scrambled control siRNAs (Figure 6B and 6C). MTMR12 deficient C2C12 myotubes also mimicked cellular changes previously seen in Mtm1 knockdown C2C12 cells [35]. Mtmr12 knockdown in C2C12 cells resulted in an increase in levels of the intermediate filament protein desmin which were associated with abnormal filament shape (arrow) in both myoblasts and myotubes (Figure 6C and 6D). However, no change in differentiation markers such as myogenin or myotube formation was observed in Mtmr12 knockdown cells (Figure 6C and 6E) suggesting that Mtmr12 deficiency does not affect the differentiation program in C2C12 cells.
Fig. 6. Loss of protein stability in the absence of myotubularin-MTMR12 interactions. 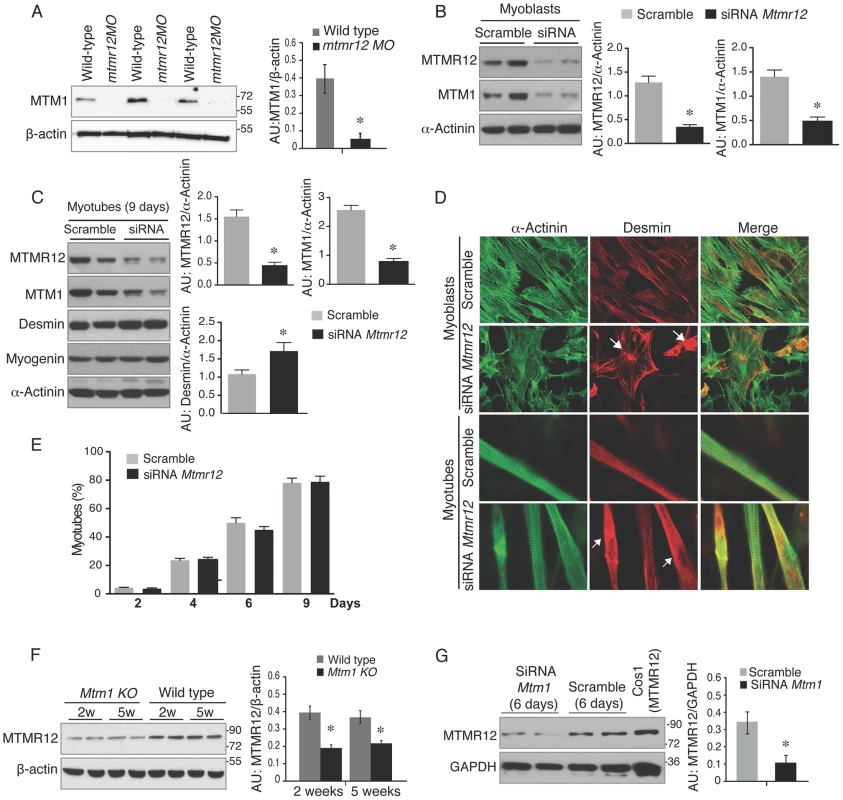
(A) Knockdown of mtmr12 resulted in a strong decrease of myotubularin protein in mtmr12 morphant zebrafish at 3 dpf by western blotting. Western blot analysis was done on three independently injected clutches (n in each clutch = 50–75). The histogram at right shows normalized amounts of myotubularin in control and mtmr12 morphant zerbrafish, *P≤0.01. (B) siRNA-mediated knockdown of Mtmr12 in C2C12 myoblasts leads to decreased protein levels of MTM1. Histograms showed western blot quantification of MTM1 and MTMR12 with reference to α-actinin as a loading control. Data represent mean of 3 independent experiments, * P<0.05. (C) Mtmr12 siRNA treated myoblasts were differentiated into myotubes and tested for protein expression of MTM1, MTMR12, desmin and myogenin. Mtmr12 knockdown in myotubes leads to decreased protein levels of MTM1 and increased amounts of the intermediate filament protein desmin, but do not affect myogenin levels. α-Actinin was used as the loading control (histograms). Data represent mean of 3 independent experiments, * P<0.05. (D) Labeling of α-actinin and desmin in C2C12 myoblast and myotubes treated with Mtmr12 siRNA or scramble control siRNA. Mtmr12 knockdown cells showed abnormal accumulation of desmin in both stages (arrow). (E) Quantification of myotubes at 2, 4, 6 and 9 days of differentiation showed no significant differences between siRNA-Mtmr12 cells and scramble siRNA. Data were obtained from 2 independent experiments (* P<0.05) and minimum of 100 cells per condition were counted. (F) Mtm1 knockout mice exhibited highly reduced levels of MTMR12 protein in skeletal muscle at pre-symptomatic (2 weeks) as well as symptomatic stages (5 weeks). The histogram on right shows normalized amounts of MTMR12 in control and Mtm1KO Mice, *P≤0.05. (G) siRNA-based Mtm1 knockdown in C2C12 cells led to no reduction in MTMR12 protein as seen by western blot analysis. β-actin/GAPDH were used as loading controls in western blots. The histogram at right shows normalized amounts of MTMR12 in control and Mtm1 knockdown cell line, *P≤0.05. To determine whether MTMR12 protein stability is regulated by myotubularin, MTMR12 levels were assessed in Mtm1 knockout mice and Mtm1 knockdown C2C12 cells (Figure 6F–G). In Mtm1 knockout mice, expression of MTMR12 was reduced in skeletal muscle in pre-symptomatic (2 weeks) as well as in symptomatic phases of disease progression (5 weeks) (Figure 6F). Similarly, in Mtm1 knockdown cell lines, a significant decrease in MTMR12 expression levels was also observed (Figure 6G). Combined, these data support the notion that the MTMR12-myotubularin interaction enhances stability of the complex in muscle cells in vitro and in skeletal muscle in vivo.
The only known and well-characterized biochemical functions of myotubularin related proteins is the dephosphrylation of phospholipids. Previous data have shown that Mtm1 mice and mtm1 zebrafish morphants display an increase in PtdIns3P levels (the substrate of myotubularin) [11], [13] To investigate if mtmr12 knockdown also affects PItdIns3P levels in skeletal muscle as seen in myotubular myopathy, PtdIns3P staining was performed on control and mtmr12 morphant skeletal muscle. Indirect immunofluorescence on MTMR12-deficient muscle showed an increase in PtdIns3P staining compared to control suggesting that the reduction of myotubularin levels is paralleled by an overall decrease in its enzymatic activity (Figure 5). To quantify the levels of PtdIns3P in the absence of either myotubularin or MTMR12 or both, a lipid-protein overlay PtdIns3P ELISA was performed on lipid extracts from 3 dpf zebrafish morphant fish. As shown previously [11], [13], absence of myotubularin resulted in an increase in PtdIns3P levels in mtm1 morphant embryos (Figure 5B). In the absence of MTMR12 or both myotubularin and MTMR12, a significant increase in PtdIns3P levels were seen over levels in both control and myotubularin morphant fish. This suggests that in addition to regulating myotubularin activity, MTMR12 may also be regulating the functions of other PtdIns3P phosphatases.
Over-expression of MTM1 improves skeletal muscle function in Mtmr12 morphants
Mtmr12 morphant fish displayed reduced levels of myotubularin protein and similar pathological changes to those seen in myotubularin deficiency, suggesting that the ability of MTMR12 to stabilize endogenous myotubularin levels in vivo may be a primary physiological role. These findings gave rise to the intriguing possibility that overexpression of myotubularin may be able to reverse the phenotypes associated with MTMR12 deficiency in zebrafish. To test this hypothesis, we overexpressed exogenous human MTM1 mRNA to investigate its ability to rescue the muscle phenotype in mtmr12 morphant fish and evaluated the resulting skeletal muscle phenotypes by birefringence assay and electron microscopy (Figure 7). Overexpression of MTM1 mRNA in mtmr12 morphant fish restored the birefringence to almost normal levels (Figure 7A). While the skeletal muscle birefringence was drastically improved, the length of zebrafish embryos was smaller (75±5.4% of control fish) suggesting MTMR12 may have additional functions independent of myotubularin. The ultra-structure of skeletal muscle was also significantly improved with a decrease in fraction of abnormal triads. This result strengthens the finding that an important role of MTMR12 is to provide stability to myotubularin protein and hence regulate its function. The mtm1-mtmr12 double knockdown fish exhibited severe muscle defects consistent with the idea that MTMR12 may have independent functional roles in addition to providing stability to myotubularin. This was investigated by overexpressing MTM1 RNA in mtm1-mtmr12 double knockdown fish. Overexpression of MTM1 resulted in a moderate improvement in birefringence as well as skeletal muscle pathology in double knockdowns as quantified by number of normal triads in skeletal muscle of rescued fish. Nevertheless, rescued fish were still smaller in size (0.64±0.029) than wild-type controls (1.00±0), consistent with the notion that MTMR12 may have functions independent of MTM1 in vivo (Figure 7 C and E).
Fig. 7. Rescue of mtmr12 morphant phenotypes by MTM1. 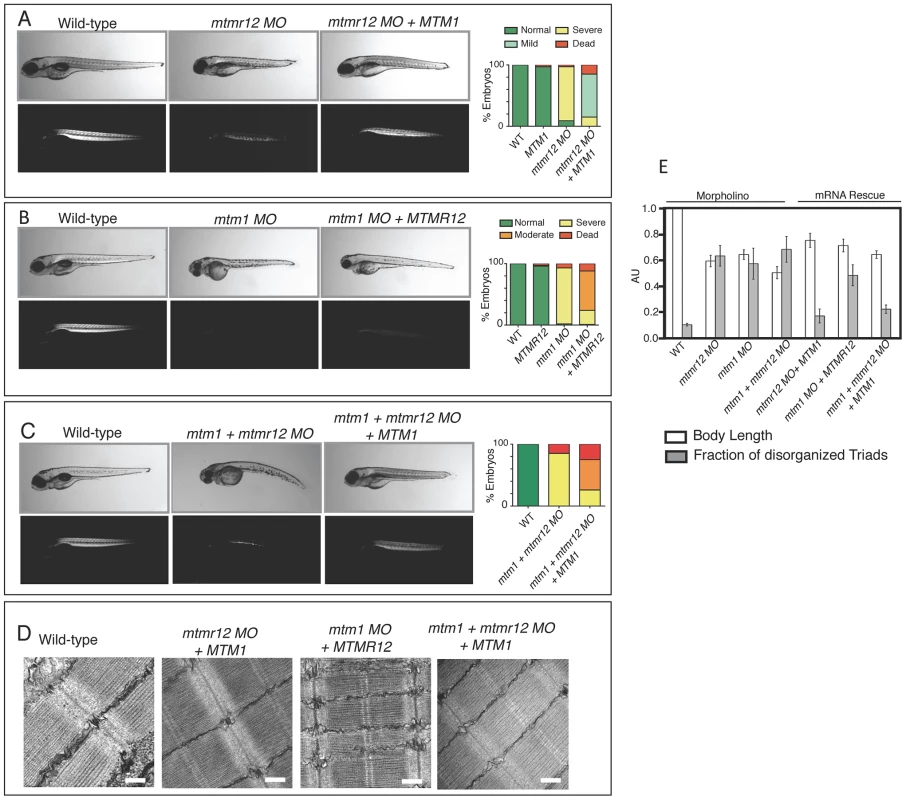
The ability of human MTM1 or MTMR12 transcripts to rescue abnormalities seen in morphant zebrafish was classified in to phenotypic index of five groups: Normal, mild, moderate, severe and dead, described in the table depending on body length, birefringence and ultrastructure of skeletal muscle. (A) Polarized light microscopy of 3 dpf live embryos showed that birefringence of mtmr12 morphant embryos increased significantly upon overexpression of human MTM1 mRNA. (B) Overexpression of human MTMR12 mRNA in mtm1 morphant fish resulted in a mild rescue of skeletal muscle defects as seen by birefringence of zebrafish embryos. (C) Overexpression of human MTM1 mRNA in mtm1-mtmr12 morphant fish resulted in a moderate rescue of skeletal muscle defects as seen by birefringence of zebrafish embryos. (D) Electron microscopy showed normal skeletal muscle structure of mtmr12 and mtm1-mtmr12 morphant fish rescued with MTM1 mRNA but displayed disorganized triads in mtm1 morphants that were rescued with MTMR12 mRNA. (E) Quantification of the body length and disorganized triads in morphant and rescued fish. Body length was measured in 10–15 embryos in each group. Total number of triads were counted in at least 15 myofibers within each embryo (n = 5 embryos). P≤0.05. To test the ability of MTMR12 to rescue the phenotypes associated with myotubularin deficiency in skeletal muscle, human MTMR12 RNA was overexpressed in mtm1 morphant fish. This resulted in partial rescue of the muscle phenotype in these fish as seen by an increase in birefringence and body length (71±4.9% of normal controls) of mtm1 morphant fish rescued with MTMR12 mRNA versus mtm1 morphant (64±3.68% of normal controls), however, without any significant reduction in disorganized triads (Figure 7B, C and E). These observations suggest that it is the missing catalytic activity or other gene-specific function of myotubularin that is primarily responsible for the pathology of XLMTM.
Myotubularin-MTMR12 interactions are perturbed in XLMTM
The significance of MTM1-MTMR12 interactions was investigated in the human neuromuscular disease XLMTM. In XLMTM, more than 200 mutations have been reported that are distributed on different domains of the myotubularin protein (http://www.dmd.nl/). In addition to nonsense mutations, a large number of missense mutations in various myotubularin domains have been shown to be pathogenic. To test, if disease causing mutations in myotubularin affect its interaction with MTMR12, interactions between mutant myotubularins and wild-type MTMR12 were examined. A series of human myotubularin proteins modeling human missense mutations in different domains were constructed (Figure 8A). Exogenous wild type or mutant myotubularins were co-expressed with MTMR12-GFP protein in Cos7 cells. Immunoprecipitation of protein extracts with a myotubularin specific antibody revealed that missense mutations in the GRAM or RID domains abolished the interaction of myotubularin with MTMR12 (Figure 8B). As over-expression in cell culture may not represent the physiological milieu, myotubes from XLMTM patients were also used to test if the pathogenic mutations on myotubularin protein affect myotubularin-MTMR12 interactions. Examination of XLMTM patient myotubes showed all mutations tested resulted in highly reduced levels of myotubularin compared to control samples. In addition, MTMR12 levels were also reduced in these patients suggesting an overall perturbation of myotubularin-MTMR12 complexes in XLMTM (Figure 8C).
Fig. 8. Myotubularin-MTMR12 interactions in XLMTM. 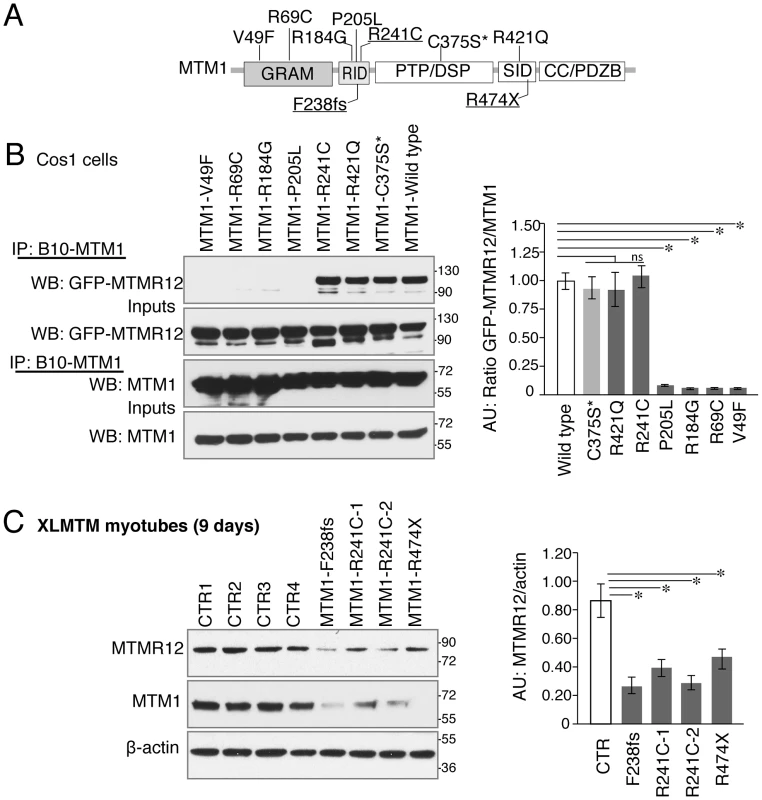
(A) Schematic diagram of different domains of myotubularin protein displaying representative pathogenic mutations found in XLMTM patients or an artificial inactivating mutation C375S* (GRAM, N terminal lipid or protein interacting domain; RID, putative membrane targeting motif; PTP/DSP, phosphatase domain; SID, protein-protein interacting domain; CC, coiled-coil domain; PDZB, PDZ binding site). (B) Wild-type or mutant MTM1-B10 fusion proteins with indicated missense mutations and wild-type MTMR12-GFP proteins were overexpressed in Cos1 cells. Immunoprecipitation of protein extracts with anti-B10 tag antibody showed that mutations on GRAM or RID domains disrupt the interactions between MTM1 and MTMR12. (C) Western blotting of XLMTM patient myotubes showed that mutants that decrease the stability of myotubularin protein also results in a reduction of MTMR12 levels. Histograms depict the western quantification for panels (B) and (C). Asterisks indicate statistically significant differences from measurements of wild type controls, P≤0.05. Discussion
Studies presented in this work were aimed at gaining insights into the molecular regulatory mechanism(s) of myotubularin function in vivo. Previous studies have shown that MTMR12 is an interacting partner of myotubularin in vitro [30], [33]. Here, we show that absence of catalytically inactive phosphatase MTMR12 protein resulted in skeletal muscle myopathy and pathological changes similar to XLMTM due to abrogation of protein-protein interactions between myotubularin and MTMR12 resulting in reduced stability and loss of myotubularin protein function.
The interaction between myotubularin and MTMR12 in skeletal muscle and co-localization at the triad certainly suggests that they might be functioning together in similar biological processes in muscle cells. This hypothesis is supported by knockdown studies on the mtmr12 gene in zebrafish that resulted in myopathic muscle in the affected fish similar to the myotubularin deficient zebrafish model [13]. To understand if these proteins function in similar biological processes or play roles in other processes independent of each other, mtm1-mtmr12 double knockdown zebrafish were created. The phenotype of double knockdown zebrafish was more severe than fish deficient in either myotubularin or MTMR12 alone. Further, inability of MTM1 to rescue all the defects observed in double knock-down fish suggests that these proteins may play additional functions that are independent of each other either by interacting with other proteins within or outside of the myotubularin family. Previous studies have shown that in addition to myotubularin, MTMR12 also interacts with another catalytically active member, MTMR2, by co-immunoprecipitation and yeast two hybrid interactions [29], [30]. Therefore, future studies on identification of protein complexes of myotubularin and MTMR12 proteins may help in identifying other pathways that are regulated by these proteins.
The presence of similar pathological changes in mtmr12 knockdown and XLMTM muscles, such as myofibrillar disarray, excessive central nucleation, triad disorganization and presence of whorled membranous structures, suggests that mtmr12 is a crucial regulator of disease pathology in XLMTM. Moreover, these pathological changes in zebrafish manifest early in zebrafish development (2–3 dpf), similar to pathological changes seen in human patients. As loss of mtmr12 resulted in clinical symptoms similar to those associated with centronuclear myopathies, MTMR12 represents an excellent candidate gene for patients with centronuclear myopathy but unknown genetic diagnosis. However, sequencing of 108 such cases failed to identify any pathogenic mutations in MTMR12, suggesting MTMR12 may account for a small subset of patients or is mutated in a clinically different disease, perhaps with a skeletal muscle component, but related also to other functions of MTMR12 (V.A. Gupta, unpublished data). In our cellular models of MTMR12 deficiency, highly reduced levels of MTM1 were observed. Therefore, any genetically unknown cases exhibiting low levels of myotubularin without any MTM1 mutations may also be good candidates for testing for MTMR12 mutations. Next generation sequencing technologies have been exhibited great promise in identifying rare gene variants and may yet identify MTMR12 mutations in the future. Regardless, these studies show a crucial role for MTMR12 function in XLMTM disease pathology.
Complex formation between some catalytically inactive and active partners in the myotubularin family has been shown to increase the activity of the catalytically active binding partner either by recruitment to specific membrane subdomains rich in lipid substrate or by increasing the allosteric activity. In the absence of MTMR12 an increase in PtdIns3P was observed suggesting a decrease in myotubularin and/or another partner's enzymatic activity. However, unlike other catalytically active-inactive pairs, MTMR12 primarily regulates the function of myotubularin protein by affecting protein levels instead of modulating the enzymatic activity [22], [36]. Interestingly, PtdIns3P levels were higher in mtmr12 morphant fish in comparison to mtm1 morphant fish, suggesting that MTMR12 may function in regulating the enzymatic activity/protein stability of other phospholipid phosphatases in cells. In the absence of MTMR12, highly reduced levels of myotubularin protein was observed suggesting that protein-protein interactions between these proteins are required for maintaining myotubularin stability. Similarly, decreased levels of MTMR12 were seen in myotubularin deficient cells and mice suggesting that stability of MTMR12 is also dependent on the interaction with myotubularin.
The abnormalities observed in MTMR12 deficient fish appear mainly due to loss of function of myotubularin as overexpression of MTM1 mRNA dramatically improved the skeletal muscle defects observed in mtmr12 morphant embryos while overexpression of catalytically inactive MTMR12 mildly improved muscle pathology in mtm1 morphant embryos. This suggests that enzymatic activity of myotubularin is required for its protein function, which was also seen, in a previous study where myotubularin deficiency could be rescued by catalytically active MTMR1 and MTMR2 [13]. Recent work also suggests that several but not all structural abnormalities observed in myotubularin deficiency can be rescued by over-expression of a catalytically inactive form of myotubularin [18]. As the catalytically inactive MTMR12 only partially but significantly rescues myotubularin function, it supports these recent findings [18] and suggests that MTMR proteins partially compensate for the lack of MTM1 through functions independent of their catalytic activity.
This implies that the inability of catalytically inactive MTMR12 to rescue myotubularin function is not only due to the lack of phosphatase activity but could also be due to other properties of myotubularin not compensated by all MTMRs. MTMR12 also interacts with another catalytically active member, MTMR2, by co-immunoprecipitation and yeast two hybrid [29], [30]. Further MTMR2 and MTMR13 also interact by direct protein-protein binding. Mutations in genes encoding MTMR2 and MTMR13 have both been associated with Charcot-Marie-Tooth (CMT) disease raising the possibility that MTMR12 deficiency may result in neurological defects such as those seen in CMT. Mtmr2 as well as Mtmr13 knockout mice exhibit a progressive neuropathy that becomes evident much later in the life span of these mice (∼6 months) [27], [37]. In comparison, no such neurological defects were seen in mtmr12 morphant fish, which could be due to early mortality of these embryos (between 3–5 dpf), before such changes become evident. Finally, it is worth noting that the ability of human MTM1 mRNA to rescue zebrafish mtm1 morphants reinforces the notion that these orthologues have been functionally conserved despite their considerable evolutionary distance, validating the relevance of the zebrafish model to studies of human XLMTM.
Studying the interaction of MTMR12 with myotubularins modeling various human mutations illuminates the role of myotubularin-MTMR12 interactions in XLMTM. Missense mutations in the N-terminal GRAM and RID domains resulted in abolishment of interaction between myotubularin and MTMR12. This was a surprising finding as previous studies have shown the myotubularin family members interact with each other through their SID or coiled-coil domains. It was also shown that an isoform of MTMR12 lacking the SID domain showed no interaction with myotubularin protein [30]. One reason for this discrepancy is that many of the previous studies have been performed using deletion constructs of different domains that may have a different effect on protein confirmation and thus its interactions than the missense changes we studied. Nonetheless a study of interactions between MTMR6 and KCa3.1 proteins has shown that along with the coiled-coil domain, the PH-GRAM domain is also required for protein-protein interactions [38]. Similarly, oligomerization of MTMR2-MTMR13 in a complex occurs independent of coiled-coil domains [39]. Analysis of XLMTM patient myotubes showed that many mutations of myotubularin that result in low levels of myotubularin protein also lead to decreases in MTMR12 levels. Many patients with these types of myotubularin mutations are described with severe phenotypes and a further reduction of MTMR12 may exacerbate the clinical severity as seen in myotubularin-MTMR12 double knockdown zebrafish.
Apart from the type III intermediate filament desmin [35], our study identifies a second interactor of myotubularin in skeletal muscle and underlines the concept that protein-interactions between myotubularin and MTMR12 are crucial for disease pathology in XLMTM. We propose a model whereby disruption of interactions between MTM1-MTMM12 results in destabilization of both partners in the complex, leading to centronuclear myopathy (Figure 9). As loss of MTMR12 results in reduction of myotubularin, we suspect that primary mutations of MTMR12 may result in centronuclear or related myopathies. As protein interactions play critical roles in almost all biological processes, efforts are currently focused on identifying drugs that can stabilize protein-protein interactions [40]. Stabilizing protein-protein interaction between myotubularin and MTMR12 may result in restoration of normal skeletal muscle function in XLMTM patients with certain missense mutations of myotubularin.
Fig. 9. MTM1-MTMR12 interactions in normal and disease states. 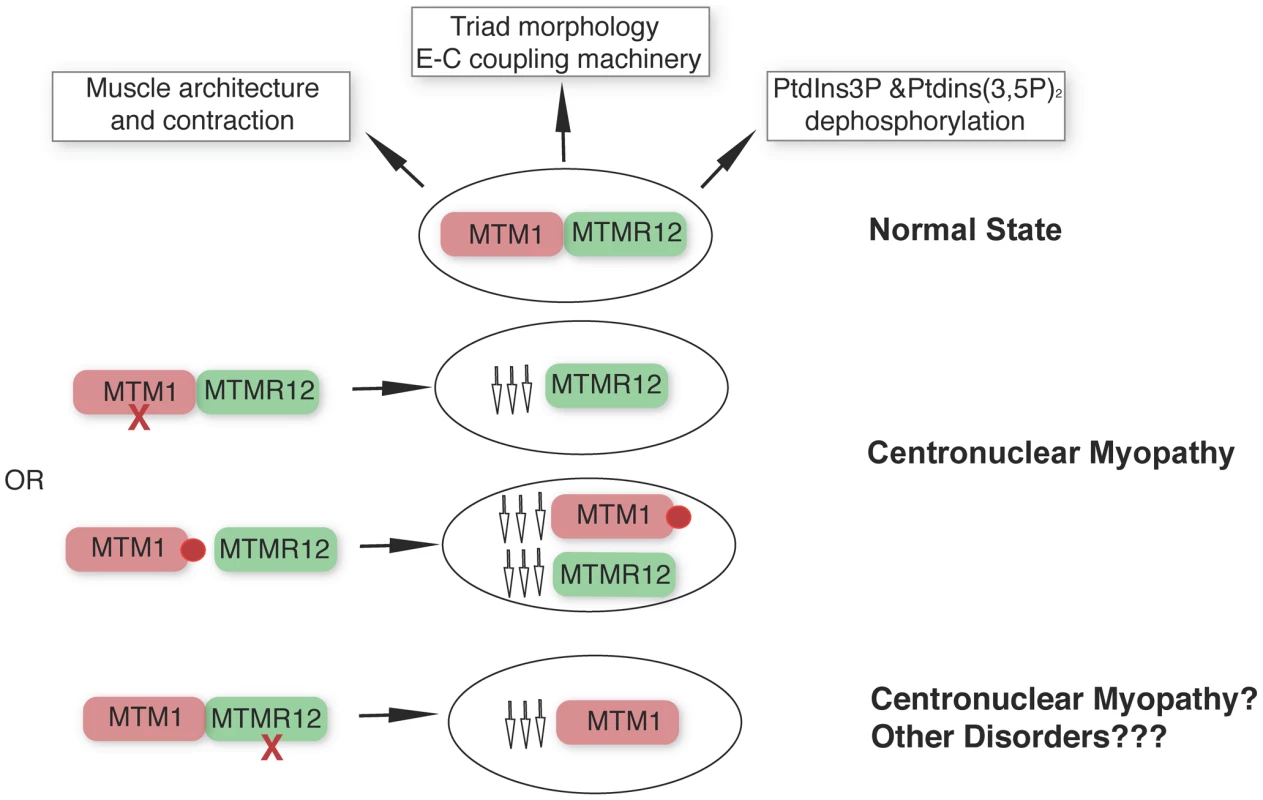
Under normal conditions, MTM1 and MTMR12 interact in skeletal muscle and regulate skeletal muscle architecture and function. Loss of function mutations of MTM1 (red cross) in skeletal muscle are associated with centronuclear myopathy and with a secondary reduction in MTMR12 levels. In centronuclear myopathy, disease causing missense mutations (red circle) that disrupt interactions between MTM1 and MTMR12 result in decreased stability of myotubularin causing myotubular myopathy associated with reduced levels of MTMR12. Loss of MTMR12 in zebrafish and mammalian cells, results in decreased levels of myotubularin resulting in pathological changes similar to centronuclear myopathy. Materials and Methods
Fish and embryo maintenance
Fish were bred and maintained as described previously [41]. Control embryos were obtained from the Oregon AB line and were staged by hours (hpf) or days (dpf) post fertilization at 28.5°C. All animal work was performed with approval from the Boston Children's Hospital Animal Care and Use Committee.
Ethics statement for mouse work
Animals were housed in a temperature-controlled room (19–22°C) with a 12 : 12 hr light/dark cycle. Mice were humanely killed by CO2 inhalation followed by cervical dislocation, according to national and European legislations on animal experimentation.
Whole mount in situ hybridization
Isoform-specific riboprobes were constructed from the 3′UTRs of mtm1 and mtmr12 using adult zebrafish RNA. Total RNA was extracted from adult zebrafish muscle tissue using Trizol (Invitrogen, Carlsbad, CA, USA). cDNAs were synthesized using superscript RT-PCR system (Invitrogen) and cloned in pZEM7Z(+) using XhoI and BamHI sites respectively. Sense or antisense digoxigenin-labeled riboprobes were synthesized by in vitro transcription using dig-labeling kits (Roche Applied Sciences, Indianapolis, IN, USA). Whole mount in situ hybridization was performed as described [42]. Imaging was performed using a Nikon SMZ1500 microscope with a Spot camera system.
Morpholino knockdown and mRNA rescue
Two splice site blocking morpholinos targeting different exon-intron boundaries, and a translational blocking morpholino, were designed to knockdown zebrafish mtm1 or mtmr12 transcripts (Genetools, Philomath, OR, USA). The morpholino sequences are mtm1 (translational): AGCCAGACCCTCGTCGAAAAGTCAT, mtmr12 (translational): CTCCTCCGCTCCCCAAACTCAACAT, mtmr12 (exon3-intron3): GCCCGGTCAACTGTCCTTACCATCT. Morpholino against human β-globin was used as a negative control for all injections. Morpholinos were dissolved in 1X Danieau buffer and 1–2 nl (1–10 ng) were injected into 1cell embryos.
For rescue experiments, full-length human MTM1 and MTMR12 cDNAs were cloned in to a PCSDest destination vector (a gift from Nathan Lawson) using Gateway technology (Invitrogen, Carlsbad, CA, USA). mRNA was synthesized in vitro using mMessage kits (Ambion, Austin, TX, USA). 50–200 pg of mRNA was injected into embryos at the 1 cell stage.
Immunofluorescence
Indirect immunofluorescence staining was performed on zebrafish frozen sections as described previously [43]. Primary antibodies used for zebrafish experiments were rabbit anti myotubularin HPA010008 (Sigma-Aldrich, St. Louis, MO, USA) and anti PtdIns3P, Z-P345b (Echelon Bioscience, Salt Lake City, UT, USA). For mouse muscle staining, tibialis anterior sections (8 µM) were labeled successively with the anti-MTM1 polyclonal antibody (2827) [35] and the anti-MTMR12 antibody (GTX119163, GeneTex Inc., Irvine, CA, USA). Briefly, after permeabilization and blocking, the anti-MTM1 antibody was applied (diluted at 1/500) on section for 2 h at RT. After washing steps, sections were incubated with the secondary antibody coupled to Alexa fluor 488 (Invitrogen) for 45 min followed by a second step of washing cycles and a blocking step. Then, the anti-MTMR12 antibody was applied on section for 2 h at RT and revealed by the incubation with a secondary antibody coupled to Alexa fluor 594 (Invitrogen). After final washing, sections were fixed, mounted and observed under confocal microscope (Leica SP2 MP confocal microscope). The monoclonal antibody against RyR1 (1C3) was a gift from Dr Isabelle Marty (Grenoble, France). The mouse anti α-actinin antibody (clone EA-53) was from Sigma (Sigma-Aldrich, St. Louis, MO, USA).
Western blotting
Zebrafish embryos were homogenized in a buffer containing Tris-Cl (20 mM, pH 7.6), NaCl (50 mM), EDTA (1 mM), NP-40 (0.1%) and complete protease inhibitor cocktail (Roche Applied Sciences). Western blotting was performed as described previously [43]. Primary antibodies used were mouse monoclonal anti-myotubularin 1G1 [19], rabbit polyclonal anti-myotubularin R2827 [44] and mouse anti-β-actin clone AC-15 (Sigma-Aldrich, A5441 at 1∶2000). Mouse monoclonal antibodies for Desmin and the sarcomeric α-actinin were purchased from Sigma-Aldrich (clone D33 and Clone EA-53, respectively). The mouse monoclonal antibody for myogenin was from R&D system (Clone 671037). Protein bands were quantified using Quantity One software (Biorad, Hercules, CA, USA).
Cell culture and siRNA
C2C12 cells were cultured in proliferation medium (Dulbecco medium supplemented with 20% FCS and 400 U/ml of Gentamycin) and differentiation was enhanced by decreasing the FCS in the media to 2% for 1 day and then accelerated by replacing the FCS by 5% Horse serum. Cells were kept in differentiation medium for 9 days with medium replacement every 2 days. For siRNA experiments the Accel Smart Pool siRNA (Thermo Scientific, Dharmacon) were used to knockdown Mtmr12 in C2C12. Myoblasts (at 30–40% of confluence) were washed with PBS and incubated with siRNA medium containing permeable Accel siRNA pool against Mtmr12 (CCAGCAGUAUAGAGGAAUA, GCGCUAUUUACGUUGGAUU, CCCGUGGGUUUAUAUAUUG, GGAUUAAGCUAUUAGACUG) or scrambled control siRNAs diluted to the appropriate concentration. After 72 hours, cells were washed with PBS and incubated with proliferation medium or differentiation medium and left for 9 days in order to obtain myotubes. Myotubes (containing min 2 nuclei) were counted blindly under the microscope (bright field) at different stages and minimums of 100 cells per well were counted.
GST pull down and co-immunoprecipitation (co-IP) assays
MTMR12 constructs were transferred to Gateway destination vectors for eukaryotic expression (pSG5 from Agilent Technologies (Santa Clara, CA, USA), with tag corresponding to the B10 epitope of estrogen receptor or GFP) and pSG5-MTM1-B10 and pcDNA3.1-MTM1 were used for co-immunoprecipitation assays in Cos1 cells. For GST-pull down experiment, MTM1 cDNA was inserted into the prokaryotic expression vector (pGex4T3, Invitrogen, Carlsbad, CA, USA). Myotubularin recombinant proteins were produced in the BL21-Rosetta 2 strain (Novagen, Billerica, MA, USA); GST fusion proteins were purified and coupled to glutathione sepharose beads as described before [35]. pSG5-MTMR12-B10 was translated in vitro according to the manufacture protocol (TNT coupled reticulocyte lysate System, Promega, Madison, WI, USA). Resulted translated protein was diluted in Co-IP buffer: (50 mM Tris-Cl pH 7.5, 100 mM NaCl, 5 mM EDTA, 5 mM EGTA, 1 mM DTT, 0,5% Triton X-100, 2 mM PMSF) supplemented with complete protease inhibitor tablet (Roche Applied Sciences, Indianapolis, IN, USA) and 1 mM Leupeptin and 1 mM pepstatin (Sigma, St. Louis, MO, USA). Homogenates were centrifuged at 14.000×g and pull down was performed as previously described [35]. GST coupled beads was used as negative control. For Co-IP Cos7 cells were transiently transfected with pcDNA3.1-MTM1 and pSG5-MTMR12-B10 constructs or with pSG5-MTM1-B10 and pSG5-MTMR12-GFP for 24 hours and homogenized in ice-cold lysis buffer (10 mM Tris-Cl, pH 7.6; 140 mM NaCl; 5 mM EDTA; 5 mM EGTA; 0.5% [v/v] Triton X-100; and 2 mM PMSF). Homogenates were incubated with mouse monoclonal anti-myotubularin 1G1 [19], or B10 epitope (Mab anti-B10, IGBMC, Illkirch, France) [35]. Interacting proteins were analysed by Western blot as mentioned before.
The panel of amino acid changes was engineered by PCR-based mutagenesis from the cDNA encoding the wild-type protein (MTM1) using PFU DNA polymerase (Agilent technologies). All constructs were verified by sequencing.
Co-IP experiments in muscle were performed from fresh murine tibialis anterior muscles that were dissected and homogenized with a dounce homogenizer in ice-cold co-IP buffer (50 mM Tris-Cl, pH 7.5; 100 mM NaCl; 5 mM EDTA; 5 mM EGTA; 1 mM DTT; 0.5% Triton X-100; and 2 mM PMSF) supplemented with 0.05% (w/v) SDS. Lysates were centrifuged at 14.000×g at 4°C and precleared with 50 µl of G-sepharose beads (GE Healthcare) and subsequently incubated with antibodies of interest for 12–24 hours at 4°C. Protein G-sepharose beads (50 ml) were then added for 4 hours at 4°C to capture the immune complexes. Beads were washed 4 times with co-IP buffer and 1 time with high stringency co-IP buffer (with 300 mM NaCl). For all experiments, two negative controls consisted of a sample lacking the primary antibody (Beads) and a sample incubated with IgG. Resulting immune-bound complexes were eluted in Laemmli buffer and analysed by SDS-PAGE and Western blotting.
PtdIns3P ELISA
PtdIns3P mass ELISA was performed on lipid extracts from whole zebrafish embryos from 3 independently injected clutches (number of embryos in each clutch = 50) following manufacturer's recommendations (Echelon Biosciences, Salt Lake City, UT, USA). Extracted lipids were resuspended in PBS-T with 3% protein stabilizer and then spotted on PtdIns3P Mass ELISA plate. Following ELISA, PtdIns3P levels were detected by measuring absorbance at 450 nm on a plate reader. Specific amounts were determined by comparison of values to a standard curve generated with simultaneous readings of known amounts of PtdIns3P.
RT-PCR
To detect mtmr12 knockdown in 3 dpf zebrafish embryos, RNA was prepared using RNeasy fibrous tissue mini kits (Qiagen, Valencia, CA, USA). cDNA was prepared using high capacity RNA-to-cDNA mastermix kits (Ambion, Austin, TX, USA). RT - PCR was performed using equal amounts of RNA from control and mtmr12 morphant zebrafish. To measure Mtmr12 and Mtm1 expression levels in C2C12 cells, total RNA was purified from C2C12 (from 0 to 9 days of differentiation) cells using Trizol reagent (Invitrogen) according to manufacturer's instructions. cDNAs were synthesized from 2 to 5 µg of total RNA using Superscript II reverse transcriptase (Invitrogen) and random hexamers. Quantitative PCR amplification of cDNAs was performed on Light-Cycler 480 and Light-Cycler 24 instruments (Roche Applied Sciences) using 58°C as melting temperature. Gapdh gene expression was used as control as expression of this gene varies little during C2C12 cell differentiation. Primers used were: Mtm1 (F: catgcgtcacttggaactgtgg, R : gcaattcctcgagcctcttt), Mtmr12 (F: tgtctgaggtacacaaaggag, R: agccttcattcacactcactg) and Gapdh (F: agctttccagaggggccatccaca, R : ccagtatgactccactcacggcaa).
Electron microscopy
Zebrafish embryos were fixed in formaldehyde-glutaraldehyde-picric acid in cacodylate buffer overnight at 4°C followed by osmication and uranyl acetate staining. Subsequently, embryos were dehydrated in a series of ethanol washes and finally embedded in TAAB epon (Marivac Ltd., Nova Scotia, Canada). 95 nm sections were cut with a Leica Ultracut microtome, picked up on 100 m Formvar coated Cu grids and stained with 0.2% Lead Citrate. Sections were viewed and imaged under a Philips Tecnai BioTwin Spirit Electron Microscope (Electron Microscopy Core, Harvard Medical School).
Quantification and statistical analysis
Centrally nucleated myofibers were quantified by analyzing serial sections from 6 different embryos. The relative number of centrally nucleated fibers in the middle somites (10–13) were counted. Total number of triads were counted in at least 15 myofibers within each embryo (n = 5 embryos). Body length was measured in 10–15 embryos in each group. Data were statistically analyzed by parametric Student t-test (two tailed) and were considered significant when P<0.05. All data analyses were performed using XLSTAT software.
Supporting Information
Zdroje
1. LaporteJ, HuLJ, KretzC, MandelJL, KioschisP, et al. (1996) A gene mutated in X-linked myotubular myopathy defines a new putative tyrosine phosphatase family conserved in yeast. Nat Genet 13 : 175–182.
2. Buj-BelloA, BiancalanaV, MoutouC, LaporteJ, MandelJL (1999) Identification of novel mutations in the MTM1 gene causing severe and mild forms of X-linked myotubular myopathy. Hum Mutat 14 : 320–325.
3. PiersonCR, TomczakK, AgrawalP, MoghadaszadehB, BeggsAH (2005) X-linked myotubular and centronuclear myopathies. J Neuropathol Exp Neurol 64 : 555–564.
4. BlondeauF, LaporteJ, BodinS, Superti-FurgaG, PayrastreB, et al. (2000) Myotubularin, a phosphatase deficient in myotubular myopathy, acts on phosphatidylinositol 3-kinase and phosphatidylinositol 3-phosphate pathway. Hum Mol Genet 9 : 2223–2229.
5. TaylorGS, MaehamaT, DixonJE (2000) Myotubularin, a protein tyrosine phosphatase mutated in myotubular myopathy, dephosphorylates the lipid second messenger, phosphatidylinositol 3-phosphate. Proc Natl Acad Sci U S A 97 : 8910–8915.
6. SchaletzkyJ, DoveSK, ShortB, LorenzoO, ClagueMJ, et al. (2003) Phosphatidylinositol-5-phosphate activation and conserved substrate specificity of the myotubularin phosphatidylinositol 3-phosphatases. Curr Biol 13 : 504–509.
7. CaldwellKK, LipsDL, BansalVS, MajerusPW (1991) Isolation and characterization of two 3-phosphatases that hydrolyze both phosphatidylinositol 3-phosphate and inositol 1,3-bisphosphate. The Journal of biological chemistry 266 : 18378–18386.
8. Di PaoloG, De CamilliP (2006) Phosphoinositides in cell regulation and membrane dynamics. Nature 443 : 651–657.
9. VicinanzaM, D'AngeloG, Di CampliA, De MatteisMA (2008) Phosphoinositides as regulators of membrane trafficking in health and disease. Cellular and molecular life sciences : CMLS 65 : 2833–2841.
10. Buj-BelloA, LaugelV, MessaddeqN, ZahreddineH, LaporteJ, et al. (2002) The lipid phosphatase myotubularin is essential for skeletal muscle maintenance but not for myogenesis in mice. Proc Natl Acad Sci U S A 99 : 15060–15065.
11. PiersonCR, Dulin-SmithAN, DurbanAN, MarshallML, MarshallJT, et al. (2011) Modeling the human MTM1 p.R69C mutation in murine Mtm1 results in exon 4 skipping and a less severe myotubular myopathy phenotype. Hum Mol Genet 21(4): 811–25.
12. BeggsAH, BohmJ, SneadE, KozlowskiM, MaurerM, et al. (2010) MTM1 mutation associated with X-linked myotubular myopathy in Labrador Retrievers. Proc Natl Acad Sci U S A 107 : 14697–14702.
13. DowlingJJ, VreedeAP, LowSE, GibbsEM, KuwadaJY, et al. (2009) Loss of myotubularin function results in T-tubule disorganization in zebrafish and human myotubular myopathy. PLoS Genet 5: e1000372.
14. HniaK, VaccariI, BolinoA, LaporteJ (2012) Myotubularin phosphoinositide phosphatases: cellular functions and disease pathophysiology. Trends Mol Med 18(6): 317–27.
15. WishartMJ, DixonJE (2002) PTEN and myotubularin phosphatases: from 3-phosphoinositide dephosphorylation to disease. Trends Cell Biol 12 : 579–585.
16. LecompteO, PochO, LaporteJ (2008) PtdIns5P regulation through evolution: roles in membrane trafficking? Trends in biochemical sciences 33 : 453–460.
17. WagnerA (1996) Genetic redundancy caused by gene duplications and its evolution in networks of transcriptional regulators. Biol Cybern 74 : 557–567.
18. AmoasiiL, BertazziDL, TronchereH, HniaK, ChicanneG, et al. (2012) Phosphatase-dead myotubularin ameliorates x-linked centronuclear myopathy phenotypes in mice. PLoS genetics 8: e1002965.
19. LaporteJ, BlondeauF, GansmullerA, LutzY, VoneschJL, et al. (2002) The PtdIns3P phosphatase myotubularin is a cytoplasmic protein that also localizes to Rac1-inducible plasma membrane ruffles. J Cell Sci 115 : 3105–3117.
20. KimSA, VacratsisPO, FiresteinR, ClearyML, DixonJE (2003) Regulation of myotubularin-related (MTMR)2 phosphatidylinositol phosphatase by MTMR5, a catalytically inactive phosphatase. Proc Natl Acad Sci U S A 100 : 4492–4497.
21. ZouJ, ChangSC, MarjanovicJ, MajerusPW (2009) MTMR9 increases MTMR6 enzyme activity, stability, and role in apoptosis. J Biol Chem 284 : 2064–2071.
22. ZouJ, ZhangC, MarjanovicJ, KisselevaMV, MajerusPW, et al. (2012) Myotubularin-related protein (MTMR) 9 determines the enzymatic activity, substrate specificity, and role in autophagy of MTMR8. Proc Natl Acad Sci U S A 109 : 9539–9544.
23. BolinoA, MugliaM, ConfortiFL, LeGuernE, SalihMA, et al. (2000) Charcot-Marie-Tooth type 4B is caused by mutations in the gene encoding myotubularin-related protein-2. Nat Genet 25 : 17–19.
24. AzzedineH, BolinoA, TaiebT, BiroukN, Di DucaM, et al. (2003) Mutations in MTMR13, a new pseudophosphatase homologue of MTMR2 and Sbf1, in two families with an autosomal recessive demyelinating form of Charcot-Marie-Tooth disease associated with early-onset glaucoma. Am J Hum Genet 72 : 1141–1153.
25. SenderekJ, BergmannC, WeberS, KetelsenUP, SchorleH, et al. (2003) Mutation of the SBF2 gene, encoding a novel member of the myotubularin family, in Charcot-Marie-Tooth neuropathy type 4B2/11p15. Hum Mol Genet 12 : 349–356.
26. NgAA, LoganAM, SchmidtEJ, RobinsonFL (2013) The CMT4B disease-causing phosphatases Mtmr2 and Mtmr13 localize to the Schwann cell cytoplasm and endomembrane compartments, where they depend upon each other to achieve wild-type levels of protein expression. Human molecular genetics 22 : 1493–1506.
27. BolinoA, BolisA, PrevitaliSC, DinaG, BussiniS, et al. (2004) Disruption of Mtmr2 produces CMT4B1-like neuropathy with myelin outfolding and impaired spermatogenesis. J Cell Biol 167 : 711–721.
28. FiresteinR, NagyPL, DalyM, HuieP, ContiM, et al. (2002) Male infertility, impaired spermatogenesis, and azoospermia in mice deficient for the pseudophosphatase Sbf1. J Clin Invest 109 : 1165–1172.
29. LorenzoO, UrbeS, ClagueMJ (2006) Systematic analysis of myotubularins: heteromeric interactions, subcellular localisation and endosome related functions. J Cell Sci 119 : 2953–2959.
30. NandurkarHH, LaytonM, LaporteJ, SelanC, CorcoranL, et al. (2003) Identification of myotubularin as the lipid phosphatase catalytic subunit associated with the 3-phosphatase adapter protein, 3-PAP. Proc Natl Acad Sci U S A 100 : 8660–8665.
31. OtiM, SnelB, HuynenMA, BrunnerHG (2006) Predicting disease genes using protein-protein interactions. J Med Genet 43 : 691–698.
32. MicheleDE, BarresiR, KanagawaM, SaitoF, CohnRD, et al. (2002) Post-translational disruption of dystroglycan-ligand interactions in congenital muscular dystrophies. Nature 418 : 417–422.
33. NandurkarHH, CaldwellKK, WhisstockJC, LaytonMJ, GaudetEA, et al. (2001) Characterization of an adapter subunit to a phosphatidylinositol (3)P 3-phosphatase: identification of a myotubularin-related protein lacking catalytic activity. Proc Natl Acad Sci U S A 98 : 9499–9504.
34. DowlingJJ, LowSE, BustaAS, FeldmanEL (2010) Zebrafish MTMR14 is required for excitation-contraction coupling, developmental motor function and the regulation of autophagy. Human molecular genetics 19 : 2668–2681.
35. HniaK, TronchereH, TomczakKK, AmoasiiL, SchultzP, et al. (2011) Myotubularin controls desmin intermediate filament architecture and mitochondrial dynamics in human and mouse skeletal muscle. J Clin Invest 121 : 70–85.
36. MochizukiY, MajerusPW (2003) Characterization of myotubularin-related protein 7 and its binding partner, myotubularin-related protein 9. Proc Natl Acad Sci U S A 100 : 9768–9773.
37. RobinsonFL, NiesmanIR, BeiswengerKK, DixonJE (2008) Loss of the inactive myotubularin-related phosphatase Mtmr13 leads to a Charcot-Marie-Tooth 4B2-like peripheral neuropathy in mice. Proc Natl Acad Sci U S A 105 : 4916–4921.
38. ChoudhuryP, SrivastavaS, LiZ, KoK, AlbaqumiM, et al. (2006) Specificity of the myotubularin family of phosphatidylinositol-3-phosphatase is determined by the PH/GRAM domain. J Biol Chem 281 : 31762–31769.
39. BergerP, BergerI, SchaffitzelC, TersarK, VolkmerB, et al. (2006) Multi-level regulation of myotubularin-related protein-2 phosphatase activity by myotubularin-related protein-13/set-binding factor-2. Hum Mol Genet 15 : 569–579.
40. LievensS, CaligiuriM, KleyN, TavernierJ (2012) The use of mammalian two-hybrid technologies for high-throughput drug screening. Methods 58(4): 335–42.
41. Westerfield M (2007) The Zebrafish Book. Eugene: University of Oregon Press.
42. ThisseC, ThisseB (2008) High-resolution in situ hybridization to whole-mount zebrafish embryos. Nature protocols 3 : 59–69.
43. GuptaV, KawaharaG, GundrySR, ChenAT, LencerWI, et al. (2011) The zebrafish dag1 mutant: a novel genetic model for dystroglycanopathies. Hum Mol Genet 20 : 1712–1725.
44. ToschV, VasliN, KretzC, NicotAS, GasnierC, et al. (2010) Novel molecular diagnostic approaches for X-linked centronuclear (myotubular) myopathy reveal intronic mutations. Neuromuscul Disord 20 : 375–381.
Štítky
Genetika Reprodukční medicína
Článek PARP-1 Regulates Metastatic Melanoma through Modulation of Vimentin-induced Malignant TransformationČlánek The Genome of : Evolution, Organization, and Expression of the Cyclosporin Biosynthetic Gene ClusterČlánek Distinctive Expansion of Potential Virulence Genes in the Genome of the Oomycete Fish PathogenČlánek USF1 and hSET1A Mediated Epigenetic Modifications Regulate Lineage Differentiation and TranscriptionČlánek Comprehensive High-Resolution Analysis of the Role of an Arabidopsis Gene Family in RNA EditingČlánek Extensive Intra-Kingdom Horizontal Gene Transfer Converging on a Fungal Fructose Transporter Gene
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2013 Číslo 6
-
Všechny články tohoto čísla
- BMS1 Is Mutated in Aplasia Cutis Congenita
- High Trans-ethnic Replicability of GWAS Results Implies Common Causal Variants
- How Cool Is That: An Interview with Caroline Dean
- Genetic Architecture of Vitamin B and Folate Levels Uncovered Applying Deeply Sequenced Large Datasets
- Juvenile Hormone and Insulin Regulate Trehalose Homeostasis in the Red Flour Beetle,
- Meiosis-Specific Stable Binding of Augmin to Acentrosomal Spindle Poles Promotes Biased Microtubule Assembly in Oocytes
- Environmental Dependence of Genetic Constraint
- H3.3-H4 Tetramer Splitting Events Feature Cell-Type Specific Enhancers
- Network Topologies and Convergent Aetiologies Arising from Deletions and Duplications Observed in Individuals with Autism
- Effectively Identifying eQTLs from Multiple Tissues by Combining Mixed Model and Meta-analytic Approaches
- Altered Splicing of the BIN1 Muscle-Specific Exon in Humans and Dogs with Highly Progressive Centronuclear Myopathy
- The NADPH Metabolic Network Regulates Human Cardiomyopathy and Reductive Stress in
- Negative Regulation of Notch Signaling by Xylose
- A Genome-Wide, Fine-Scale Map of Natural Pigmentation Variation in
- Transcriptome-Wide Mapping of 5-methylcytidine RNA Modifications in Bacteria, Archaea, and Yeast Reveals mC within Archaeal mRNAs
- Multiplexin Promotes Heart but Not Aorta Morphogenesis by Polarized Enhancement of Slit/Robo Activity at the Heart Lumen
- Latent Effects of Hsp90 Mutants Revealed at Reduced Expression Levels
- Impact of Natural Genetic Variation on Gene Expression Dynamics
- DeepSAGE Reveals Genetic Variants Associated with Alternative Polyadenylation and Expression of Coding and Non-coding Transcripts
- The Identification of -acting Factors That Regulate the Expression of via the Osteoarthritis Susceptibility SNP rs143383
- Pervasive Transcription of the Human Genome Produces Thousands of Previously Unidentified Long Intergenic Noncoding RNAs
- The RNA Export Factor, Nxt1, Is Required for Tissue Specific Transcriptional Regulation
- Inferring Demographic History from a Spectrum of Shared Haplotype Lengths
- Histone Acetyl Transferase 1 Is Essential for Mammalian Development, Genome Stability, and the Processing of Newly Synthesized Histones H3 and H4
- PARP-1 Regulates Metastatic Melanoma through Modulation of Vimentin-induced Malignant Transformation
- DNA Methylation Restricts Lineage-specific Functions of Transcription Factor Gata4 during Embryonic Stem Cell Differentiation
- The Genome of : Evolution, Organization, and Expression of the Cyclosporin Biosynthetic Gene Cluster
- Distinctive Expansion of Potential Virulence Genes in the Genome of the Oomycete Fish Pathogen
- Deregulation of the Protocadherin Gene Alters Muscle Shapes: Implications for the Pathogenesis of Facioscapulohumeral Dystrophy
- Evidence for Two Different Regulatory Mechanisms Linking Replication and Segregation of Chromosome II
- USF1 and hSET1A Mediated Epigenetic Modifications Regulate Lineage Differentiation and Transcription
- Methylation of Histone H3 on Lysine 79 Associates with a Group of Replication Origins and Helps Limit DNA Replication Once per Cell Cycle
- A Six Months Exercise Intervention Influences the Genome-wide DNA Methylation Pattern in Human Adipose Tissue
- The Gene Desert Mammary Carcinoma Susceptibility Locus Regulates Modifying Mammary Epithelial Cell Differentiation and Proliferation
- Hooked and Cooked: A Fish Killer Genome Exposed
- Distinct Neuroblastoma-associated Alterations of Impair Sympathetic Neuronal Differentiation in Zebrafish Models
- Mutations in Cause Autosomal Recessive Congenital Ichthyosis in Humans
- Integrated Transcriptomic and Epigenomic Analysis of Primary Human Lung Epithelial Cell Differentiation
- RSR-2, the Ortholog of Human Spliceosomal Component SRm300/SRRM2, Regulates Development by Influencing the Transcriptional Machinery
- Comparative Polygenic Analysis of Maximal Ethanol Accumulation Capacity and Tolerance to High Ethanol Levels of Cell Proliferation in Yeast
- SPO11-Independent DNA Repair Foci and Their Role in Meiotic Silencing
- Budding Yeast ATM/ATR Control Meiotic Double-Strand Break (DSB) Levels by Down-Regulating Rec114, an Essential Component of the DSB-machinery
- Comprehensive High-Resolution Analysis of the Role of an Arabidopsis Gene Family in RNA Editing
- Functional Analysis of Neuronal MicroRNAs in Dauer Formation by Combinational Genetics and Neuronal miRISC Immunoprecipitation
- DNA Ligase IV Supports Imprecise End Joining Independently of Its Catalytic Activity
- Extensive Intra-Kingdom Horizontal Gene Transfer Converging on a Fungal Fructose Transporter Gene
- Heritable Change Caused by Transient Transcription Errors
- From Many, One: Genetic Control of Prolificacy during Maize Domestication
- Neuronal Target Identification Requires AHA-1-Mediated Fine-Tuning of Wnt Signaling in
- Loss of Catalytically Inactive Lipid Phosphatase Myotubularin-related Protein 12 Impairs Myotubularin Stability and Promotes Centronuclear Myopathy in Zebrafish
- H-NS Can Facilitate Specific DNA-binding by RNA Polymerase in AT-rich Gene Regulatory Regions
- Prophage Dynamics and Contributions to Pathogenic Traits
- Global DNA Hypermethylation in Down Syndrome Placenta
- Fragile DNA Motifs Trigger Mutagenesis at Distant Chromosomal Loci in
- Disturbed Local Auxin Homeostasis Enhances Cellular Anisotropy and Reveals Alternative Wiring of Auxin-ethylene Crosstalk in Seminal Roots
- Causes and Consequences of Chromatin Variation between Inbred Mice
- Genome-scale Analysis of FNR Reveals Complex Features of Transcription Factor Binding
- Distinct and Atypical Intrinsic and Extrinsic Cell Death Pathways between Photoreceptor Cell Types upon Specific Ablation of in Cone Photoreceptors
- Sex-stratified Genome-wide Association Studies Including 270,000 Individuals Show Sexual Dimorphism in Genetic Loci for Anthropometric Traits
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- BMS1 Is Mutated in Aplasia Cutis Congenita
- Sex-stratified Genome-wide Association Studies Including 270,000 Individuals Show Sexual Dimorphism in Genetic Loci for Anthropometric Traits
- Distinctive Expansion of Potential Virulence Genes in the Genome of the Oomycete Fish Pathogen
- Distinct Neuroblastoma-associated Alterations of Impair Sympathetic Neuronal Differentiation in Zebrafish Models
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



