-
Články
Reklama
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
ReklamaVaginitis: When Opportunism Knocks, the Host Responds
article has not abstract
Published in the journal: . PLoS Pathog 10(4): e32767. doi:10.1371/journal.ppat.1003965
Category: Pearls
doi: https://doi.org/10.1371/journal.ppat.1003965Summary
article has not abstract
Introduction
Candida albicans, an opportunistic polymorphic fungus and resident of the normal vaginal microbiota, is the leading causative agent of vulvovaginal candidiasis (VVC) and presents major quality of life issues for women worldwide [1]. Candida vaginitis is characterized by itching, burning, pain, and redness of the vulva and vaginal mucosa and often accompanied by vaginal discharge. Predisposing factors for primary VVC include high-estrogen oral contraceptive use, hormone replacement therapy, antibiotic usage, and underlying diabetes mellitus. It is estimated that 75% of all women of childbearing age will be afflicted by VVC at least once in their lifetime [2]. Of these, approximately 5–8% (approximately 150 million worldwide) suffer from recurrent VVC (RVVC), resulting in idiopathic chronic episodes of vaginal irritation that require antifungal maintenance therapy (e.g., azoles) to partially control symptoms [1]. Although these treatments are typically effective at reducing organism burden and symptoms, the static function of azole activity and fungal recalcitrance to clearance are key factors resulting in recurrence [3]. It is proposed that RVVC and VVC both involve similar immunopathologies but that the triggers occur with greater sensitivity in individuals with RVVC. Continuously rising vaginitis-related healthcare costs are estimated at $1.8 billion annually in the United States alone [4]. These unsustainable costs further necessitate a comprehensive understanding of vaginitis and the host and fungal factors that contribute to its immunopathology.
The Role of Host Immunity in Candida Vaginitis: Historical and Contemporary Perspectives
Susceptibility to oral, chronic mucocutaneous, and gastrointestinal candidiasis has been clearly linked to deficiencies in cell-mediated immunity (CMI) [5]. Therefore, susceptibility to Candida vaginitis was also long believed to result from defects in the adaptive immune response. However, numerous clinical studies examining women with RVVC and the use of an experimental mouse model to evaluate roles for CMI or humoral immunity (HI) revealed no appreciable protection provided by local or systemic adaptive immune mechanisms [6], [7], [8]. In support of these findings, relatively high production of immunoregulatory factors (e.g., TGF-β, T-regs, and Υ/δ T-cells) in the vagina may partly explain the lack of functional local CMI [9], [10]. Despite a lack of supportive evidence for a role of adaptive immunity in vaginitis, the newly characterized Th17 axis of CMI, which links innate and adaptive immune responses, has been shown to be critical for local protection against oropharyngeal candidiasis (OPC) [11]. Accordingly, animal models were used to determine its potential role in mucosal immunity during vaginitis. However, discrepant findings amongst mouse models have led to contradictory conclusions: one study using a less stringent pharmacologic approach to Th17 blockade demonstrated a modest role for Th17 involvement [12], while a more rigorous approach using Th17 axis-knockout mice showed no such function [12], [13]. Thus, the role of Th17 responses during vaginitis remains unresolved and lacks any supportive clinical evidence. As for mucosal HI, some animal models have demonstrated modest antibody-mediated protection against vaginitis [14]. It is conceivable that protective human antibodies do exist but occur naturally at concentrations in vaginal secretions too low to sufficiently mediate protection.
While exhaustive efforts have found no major role for adaptive immunity in susceptibility to vaginitis, recent studies have identified the importance of innate immunity in regulating vaginitis symptomatology. A paramount study using women volunteers challenged with live C. albicans determined that vaginitis symptoms were associated with polymorphonuclear leukocyte (PMN) recruitment into the vagina and that organism burden alone was not predictive of disease [15]. Moreover, depletion of PMNs in mice did not result in increased fungal load but did decrease histological evidence of vaginal inflammation [16], [17]. Most recently, a family of calcium-binding proteins termed S100A8 and S100A9 “alarmins” have been implicated in the innate vaginal epithelial cell response to C. albicans (see Figure 1) [18]. Because these proteins have vigorous PMN chemotactic activity, it was hypothesized that epithelial expression of S100s may play a key role in controlling PMN migration into the vaginal lumen. However, while this was confirmed, studies using mice lacking expression of S100A8/9 proteins determined that these factors were sufficient but not necessary for driving the PMN response [19]. Collectively, these exciting new studies highlight the immunopathological response as a crucial element of vaginitis pathogenesis. Future clinical studies, however, are required to confirm the presence and function of alarmins during human infection.
Fig. 1. Working model of the immunopathogenesis of C. albicans vaginitis. 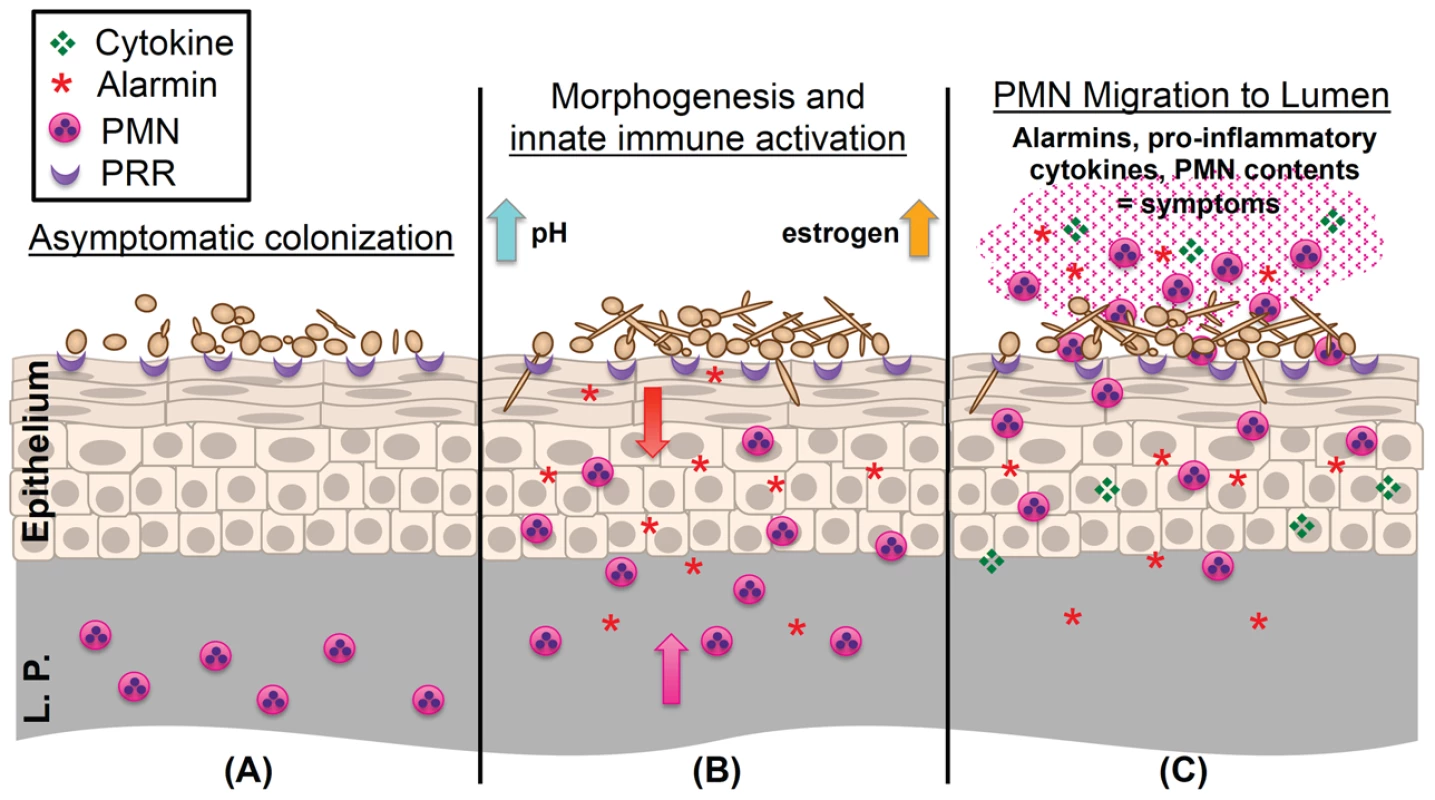
(A) Yeast forms of C. albicans asymptomatically colonize the vaginal epithelium despite the presence of numerous pattern recognition receptors (PRR) on the epithelial surface. (B) C. albicans begins to undergo the yeast-to-hypha switch under morphogenesis-inducing conditions (increases in estrogen, elevated vaginal pH, and microbiota disruption). Augmented recognition by PRRs, increased hyphal biomass, and expression of hypha-associated virulence factors elicits inflammatory signaling (S100A8/9 alarmins and proinflammatory cytokines) in the vaginal epithelium, resulting in initial migration of PMNs from the lamina propria (L.P.) to the vaginal lumen. (C) Failure to adequately reduce immunopathological triggers results in the continued expression of innate immune effectors by the vaginal epithelium. These initial signals, coupled with secondary amplification of immune effectors by recruited PMNs, contribute to symptomatic infection and characteristic immunopathology. As alluded to above, resultant findings from animal models must be translatable to the human host. One important point to consider is that laboratory rodents, unlike humans, do not naturally harbor C. albicans as commensal organisms. Although the estrogen-dependent mouse model of vaginitis closely mimics clinical infection, observed antifungal immune responses are considered primary and may be exaggerated as compared to human infection, in which repeated exposure, immunotolerance, or higher signaling thresholds to Candida may be encountered. Despite this shortcoming, the mouse model of vaginitis has been an indispensible tool for dissecting the immunological mechanisms associated with this highly complex disease.
Genetic Susceptibility to Fungal Infections: Applied Lessons to Vaginitis?
Several clinical studies have been designed to identify genetic polymorphisms in factors with putative importance for antifungal defense, such as the pathogen recognition receptors (PRRs) Dectin-1, Dectin-2, MINCLE (macrophage-inducible C-type lectin), TLR2/4 (Toll-like receptors 2 and 4), mannose binding lectin (MBL), mannose receptor (MR), and DC-SIGN/SIGNR1 (Dendritic Cell-Specific Intercellular adhesion molecule-3-Grabbing Non-integrin) [20]. Ligation of these receptors to their cognate ligands (typically C. albicans cell-wall components) induces proinflammatory signaling, initiating recruitment of both innate and adaptive immune cells to elicit potentially protective responses. Data acquired from associative genetic studies and their relevance to vaginitis pathogenesis is discussed below.
A homozygous polymorphism (Tyr238X stop codon) in the C-type lectin receptor (CLR) Dectin-1 (β-glucan recognition) confers susceptibility to chronic onychomycosis and RVVC but not OPC [21]. Studies conducted by Yano et al. surprisingly demonstrated lack of expression of Dectin-1 on murine vaginal epithelium and wild-type levels of neutrophil recruitment and fungal burden in Dectin-1-/ - mice during vaginal infection [19]. However, simultaneous blockade of TLR4 and SIGNR1 signaling reduced S100 alarmin expression in vaginal epithelial cells when challenged with C. albicans, suggesting an important function of these cell-surface receptors at the vaginal mucosa. Whereas the role of Dectin-1 during Candida vaginitis remains inconclusive, these other PRRs may serve more prominent roles. Signal transduction via CLRs converges on the common signaling adaptor CARD9. A homozygous point mutation (Q295X) abolishing CARD9 function has been associated with increased risk of fungal infection, including OPC and RVVC [22]. Unfortunately, lack of evidential expression data for Dectin-1 and CARD9 in the vaginal epithelium provokes further questions on their relevance in vaginitis pathogenesis. Functional defects in MBL and MR have also been suggested to mediate susceptibility to fungal infections. These host factors act by neutralizing or recognizing the carbohydrate mannan found ubiquitously on the surface of fungi. A series of clinical studies have elucidated an association between a polymorphism in the MBL-2 gene (mutant codon 54) and susceptibility to RVVC [23], [24]. While these findings are of significant clinical interest, protective roles for MBL and MR against Candida in the vagina remain unclear due to lack of supportive mechanistic studies.
Despite important new information gained from these studies, more in-depth molecular and immunological research is urgently required. While cell-surface receptors and signaling adaptors undoubtedly play a role during vaginal fungal infection, discrepancies between animal models and human clinical data further cloud the overall picture of vaginitis pathogenesis. Careful consideration must be taken to avoid overinterpretation of limited clinical datasets or mischaracterization of animal model data not relevant to the clinical scenario. Furthermore, the highly compartmentalized nature of immune responses to C. albicans at different anatomical sites warrants discretion in proposing overarching hypotheses without detailed experimentation.
Candida albicans Is a Robust Opportunistic Fungal Pathogen
Several properties of C. albicans have been proposed to play major roles in causing disease: morphogenesis, secreted factors, and biofilm formation. As a polymorphic fungus, C. albicans adopts two major morphological forms, the commensalistic ovoid yeast and the pathogenic filamentous hypha. The transition from yeast to hypha is under the control of a complex set of environmental sensors and has long been considered to be the major virulence factor of this pathogen [25]. In vitro studies utilizing Candida-infected, reconstituted human vaginal epithelium demonstrated a requirement for hyphae to uniquely induce innate immune signaling [26]. These findings were supported by challenging mice intravaginally with isogenic C. albicans strains, some of which were defective in key transcriptional regulators mediating the yeast-to-hypha switch. Despite similar colonization among strains, those defective in hypha formation displayed significantly reduced immunological markers of vaginitis symptomatology (including reduced PMNs and S100A8) [17]. Cumulatively, these studies confirm an important role for the morphogenetic response in vaginitis immunopathology. These points are illustrated in Figure 1 by yeast cells that asymptomatically colonize the vaginal epithelium despite being recognized by cell-surface PRRs. The yeast-to-hypha transition further engages additional PRRs, causes mechanical disruption of the epithelial surface, and augments expression of fungal virulence factors. Ultimately, these cellular insults result in damage-mediated responses leading to the recruitment of PMNs, culminating in vaginitis immunopathology.
Aside from the yeast-to-hypha switch, C. albicans also produces a number of secretory products that have been implicated in the pathogenesis of vaginitis. A family of homologous secreted aspartyl proteinases (SAPs) has demonstrated differential expression during vaginitis [27]. SAPs act by cleaving proteins on host epithelium, resulting in mucosal structural damage and enhanced fungal burden. Although not characterized in as much detail, candidal lipases have also been implicated in disease pathology [28].
Another major virulence property of C. albicans is its ability to form biofilms on both biotic and abiotic surfaces. Biofilms are complex, highly organized, three-dimensional structures formed by communities of microbes conferring properties of increased adhesion, recalcitrance to clearance by the host immune system, and enhanced antimicrobial resistance. It has been demonstrated that C. albicans forms biofilms on the vaginal mucosa of mice but biofilm formation is not required for colonization [29]. Furthermore, because morphogenesis and biofilm formation are intimately linked, it is unclear what role biofilms play in the context of immunity.
Vaginitis: A Multifactorial Disease
Other biologically relevant criteria are also critical in initiating disease pathology. The effects of estrogen on the vaginal mucosa appear to be crucial for vaginitis progression. Production of estrogen during the menstrual cycle causes the keratinized vaginal epithelium to cornify, thicken, and eventually slough. In fact, it is during these periods of high estrogenic activity that women are most susceptible to vaginitis [30]. This is further evidenced by the fact that prepubescent girls and postmenopausal women (low-estrogen producers) rarely develop vaginitis. However, postmenopausal women on hormone replacement therapy frequently become resusceptible [30]. Indeed, animal models of vaginitis almost always include administration of exogenous estrogen to maintain fungal burdens, emphasizing the importance of estrogen in disease pathology (Figure 1) [31]. Aside from physiological changes, estrogen also exhibits immunomodulatory effects, including decreased antimicrobial peptide expression, modulated PRR expression, reduced antigen presentation, decreased T cell priming, diminished mucosal antibody production, enhanced antigenic tolerance, and impairment of Th17 signaling [32], [33], [34]. Not only does estrogen have pleiotropic effects on the host, it also directly affects C. albicans itself. Estrogen enhances Candida adherence to vaginal epithelial cells, augments germ tube formation, and increases hyphal length [35], [36]. Furthermore, C. albicans encodes an estrogen binding protein (EBP), recently revealed to act as an oxidoreductase [37]. Inhibition of EBP by estrogen results in increased expression of the drug efflux pumps CDR1/2, antimicrobial resistance mechanisms employed by fungi against commonly used azoles [35]. Estrogen has also been shown to up-regulate expression of the fungal heat-shock protein Hsp90, conferring resistance to biological and chemical stressors [38]. Together, these estrogen-induced modifications of host and fungal responses may partly explain lack of protective immunity to vaginitis and recalcitrance to clearance.
The pH of the vaginal microenvironment and composition of the bacterial microbiota are also important for disease pathogenesis. The average adult human vaginal pH (4.5) is maintained at an acidic level to aid in the inhibition of microorganism overgrowth. A low vaginal pH inhibits the yeast-to-hypha switch in C. albicans [39]. Therefore, increases in pH levels are believed to promote vaginitis (Figure 1). Interestingly, mice maintain a neutral vaginal pH that favors hyphal formation, thus making them a robust model system for Candida vaginitis. The bacterial microbiota are also important in maintaining a healthy vaginal environment, as antibiotic administration is a major risk factor for developing vaginitis, presumably through disruption of the natural bacterial communities existing at the mucosal interface [40]. Decreased levels of ubiquitous acid-producing lactobacilli lead to increased vaginal pH levels and augmented colonization of potential pathogens, including C. albicans.
New Frontiers in Candida Vaginitis and Closing Remarks
A comprehensive understanding of the immune response to Candida vaginitis is far from complete. The fields of mucosal biology and immunology are rapidly expanding and may provide insights into this complex disease. Recent evidence suggests that danger–signal-mediated inflammasome signaling (i.e. processing, cleavage, and secretion of IL-1β) plays an important role in the innate response to various pathogens at mucosal surfaces, including C. albicans [17], [41], [42], [43]. However, more research is needed to address the role (if any) of inflammasome signaling during vaginitis and whether inflammasome activation may be linked to S100A8/9 production or PMN infiltration. In any case, novel immunomodulatory therapies may be viable options for curtailing vaginitis immunopathology, especially in RVVC.
Development of accurate diagnostics and preventive vaccines remains a priority. However, these developments do not come without significant challenges. An inexpensive, simple, and rapid diagnostic test that can simultaneously detect symptomatology (e.g., innate immune effectors) along with fungal presence is the ultimate goal in order to distinguish asymptomatic colonization from true infection. It is also imperative that such a diagnostic will discriminate between vaginitis of varying etiology (including those of fungal, bacterial, and protozoal origin) so that appropriate antimicrobial therapy may be administered without delay. Additionally, preliminary clinical trials of two antifungal vaccines (NDV-3, based on the recombinant candidal adhesin Als3, and PEV7, which utilizes SAP-2 as the immunogen) have provided promising results, efficaciously reducing fungal burden during intravaginal challenge with C. albicans [44], [45]. However, advanced clinical trials will be required to determine if these vaccines can reduce fungal burdens adequately to eliminate the triggers for vaginitis immunopathology in both VVC and RVVC populations.
Vaginitis is a complex disease, requiring a “perfect storm” scenario to initiate infection. Estrogen production, microbiota disruption, pH modification, fungal virulence factor expression, and exuberant innate immune activity must synchronize to culminate in symptomatic infection. Current research has elucidated a major paradigm shift in the philosophy of Candida vaginitis pathogenesis, highlighting the role of the host innate immune response in disease immunopathology. Moreover, another paradigm change is emerging that is shifting the focus on fungal burden as the sole outcome phenotype in the context of understanding virulence mechanisms and developing novel treatment strategies toward focusing on the immunopathological response instead. Accordingly, although much insight has been gained from extensive studies in this field, scientific endeavors aimed at preventive therapies, improved diagnostics, and pathogenesis will require careful attention to the immunopathogenic response to ultimately combat this highly significant, opportunistic fungal disease.
Zdroje
1. AchkarJM, FriesBC (2010) Candida infections of the genitourinary tract. Clin Microbiol Rev 23 : 253–273.
2. SobelJD (1997) Vaginitis. N Engl J Med 337 : 1896–1903.
3. SobelJD, ZervosM, ReedBD, HootonT, SoperD, et al. (2003) Fluconazole susceptibility of vaginal isolates obtained from women with complicated Candida vaginitis: clinical implications. Antimicrob Agents Chemother 47 : 34–38.
4. FoxmanB, BarlowR, D'ArcyH, GillespieB, SobelJD (2000) Candida vaginitis: self-reported incidence and associated costs. Sex Transm Dis 27 : 230–235.
5. AshmanRB (2008) Protective and pathologic immune responses against Candida albicans infection. Front Biosci 13 : 3334–3351.
6. FidelPLJr, LynchME, Redondo-LopezV, SobelJD, RobinsonR (1993) Systemic cell-mediated immune reactivity in women with recurrent vulvovaginal candidiasis. J Infect Dis 168 : 1458–1465.
7. FongIW, McClearyP, ReadS (1992) Cellular immunity of patients with recurrent or refractory vulvovaginal moniliasis. Am J Obstet Gynecol 166 : 887–890.
8. MendlingW, KoldovskyU (1996) Investigations by cell-mediated immunologic tests and therapeutic trials with thymopentin in vaginal mycoses. Infect Dis Obstet Gynecol 4 : 225–231.
9. TaylorBN, SaavedraM, FidelPLJr (2000) Local Th1/Th2 cytokine production during experimental vaginal candidiasis: potential importance of transforming growth factor-beta. Med Mycol 38 : 419–431.
10. WormleyFLJr, SteeleC, WozniakK, FujihashiK, McGheeJR, et al. (2001) Resistance of T-cell receptor delta-chain-deficient mice to experimental Candida albicans vaginitis. Infect Immun 69 : 7162–7164.
11. ContiHR, ShenF, NayyarN, StocumE, SunJN, et al. (2009) Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med 206 : 299–311.
12. PietrellaD, RachiniA, PinesM, PandeyN, MosciP, et al. (2011) Th17 cells and IL-17 in protective immunity to vaginal candidiasis. PLoS ONE 6: e22770.
13. YanoJ, KollsJK, HappelKI, WormleyF, WozniakKL, et al. (2012) The acute neutrophil response mediated by S100 alarmins during vaginal Candida infections is independent of the Th17-pathway. PLoS ONE 7: e46311.
14. de BernardisF, SantoniG, BoccaneraM, SpreghiniE, AdrianiD, et al. (2000) Local anticandidal immune responses in a rat model of vaginal infection by and protection against Candida albicans. Infect Immun 68 : 3297–3304.
15. FidelPLJr, BarousseM, EspinosaT, FicarraM, SturtevantJ, et al. (2004) An intravaginal live Candida challenge in humans leads to new hypotheses for the immunopathogenesis of vulvovaginal candidiasis. Infect Immun 72 : 2939–2946.
16. BlackCA, EyersFM, RussellA, DunkleyML, ClancyRL, et al. (1998) Acute neutropenia decreases inflammation associated with murine vaginal candidiasis but has no effect on the course of infection. Infect Immun 66 : 1273–1275.
17. PetersBM, PalmerGE, FidelPLJr, NoverrMC (2014) Fungal morphogenetic pathways are required for the hallmark inflammatory response during Candida vaginitis. Infect Immun 82 : 532–543.
18. YanoJ, LillyE, BarousseM, FidelPLJr (2010) Epithelial cell-derived S100 calcium-binding proteins as key mediators in the hallmark acute neutrophil response during Candida vaginitis. Infect Immun 78 : 5126–5137.
19. YanoJ, PalmerGE, EberleKE, PetersBM, VoglT, et al. (2014) Vaginal epithelial cell-derived S100 alarmins induced by C. albicans via pattern recognition receptor interactions is sufficient but not necessary for the acute neutrophil response during experimental vaginal candidiasis. Infect Immun 82 : 783–792.
20. NeteaMG, BrownGD, KullbergBJ, GowNA (2008) An integrated model of the recognition of Candida albicans by the innate immune system. Nat Rev Microbiol 6 : 67–78.
21. FerwerdaB, FerwerdaG, PlantingaTS, WillmentJA, van SprielAB, et al. (2009) Human dectin-1 deficiency and mucocutaneous fungal infections. N Engl J Med 361 : 1760–1767.
22. RosentulDC, PlantingaTS, OostingM, ScottWK, Velez EdwardsDR, et al. (2011) Genetic variation in the Dectin-1/CARD9 recognition pathway and susceptibility to candidemia. J Infect Dis 204 : 1138–1145.
23. BabulaO, LazdaneG, KroicaJ, LedgerWJ, WitkinSS (2003) Relation between recurrent vulvovaginal candidiasis, vaginal concentrations of mannose-binding lectin, and a mannose-binding lectin gene polymorphism in Latvian women. Clin Infect Dis 37 : 733–737.
24. DondersGG, BabulaO, BellenG, LinharesIM, WitkinSS (2008) Mannose-binding lectin gene polymorphism and resistance to therapy in women with recurrent vulvovaginal candidiasis. BJOG 115 : 1225–1231.
25. BiswasS, Van DijckP, DattaA (2007) Environmental sensing and signal transduction pathways regulating morphopathogenic determinants of Candida albicans. Microbiol Mol Biol Rev 71 : 348–376.
26. MoyesDL, MurcianoC, RunglallM, IslamA, ThavarajS, et al. (2011) Candida albicans yeast and hyphae are discriminated by MAPK signaling in vaginal epithelial cells. PLoS ONE 6: e26580.
27. TaylorBN, StaibP, BinderA, BiesemeierA, SehnalM, et al. (2005) Profile of Candida albicans-secreted aspartic proteinase elicited during vaginal infection. Infect Immun 73 : 1828–1835.
28. SchallerM, BorelliC, KortingHC, HubeB (2005) Hydrolytic enzymes as virulence factors of Candida albicans. Mycoses 48 : 365–377.
29. HarriottMM, LillyEA, RodriguezTE, FidelPLJr, NoverrMC (2010) Candida albicans forms biofilms on the vaginal mucosa. Microbiology 156 : 3635–36344.
30. Hong E, Dixit S, Fidel PL, Bradford J, Fischer G (2013) Vulvovaginal candidiasis as a chronic disease: diagnostic criteria and definition. J Low Genit Tract Dis.
31. HamadM, Abu-ElteenKH, GhalebM (2004) Estrogen-dependent induction of persistent vaginal candidosis in naive mice. Mycoses 47 : 304–309.
32. HickeyDK, FaheyJV, WiraCR (2013) Mouse estrous cycle regulation of vaginal versus uterine cytokines, chemokines, alpha-/beta-defensins and TLRs. Innate Immun 19 : 121–131.
33. RellosoM, Aragoneses-FenollL, LasarteS, BourgeoisC, RomeraG, et al. (2012) Estradiol impairs the Th17 immune response against Candida albicans. J Leukoc Biol 91 : 159–165.
34. WiraCR, FaheyJV, SentmanCL, PioliPA, ShenL (2005) Innate and adaptive immunity in female genital tract: cellular responses and interactions. Immunol Rev 206 : 306–335.
35. ChengG, YeaterKM, HoyerLL (2006) Cellular and molecular biology of Candida albicans estrogen response. Eukaryot Cell 5 : 180–191.
36. HollmerC, EssmannM, AultK, LarsenB (2006) Adherence and blocking of Candida albicans to cultured vaginal epithelial cells: treatments to decrease adherence. Infect Dis Obstet Gynecol 2006 : 98218.
37. MadaniND, MalloyPJ, Rodriguez-PomboP, KrishnanAV, FeldmanD (1994) Candida albicans estrogen-binding protein gene encodes an oxidoreductase that is inhibited by estradiol. Proc Natl Acad Sci U S A 91 : 922–926.
38. ZhangX, EssmannM, BurtET, LarsenB (2000) Estrogen effects on Candida albicans: a potential virulence-regulating mechanism. J Infect Dis 181 : 1441–1446.
39. VylkovaS, CarmanAJ, DanhofHA, ColletteJR, ZhouH, et al. (2011) The fungal pathogen Candida albicans autoinduces hyphal morphogenesis by raising extracellular pH. MBio 2: e00055–00011.
40. MaB, ForneyLJ, RavelJ (2012) Vaginal microbiome: rethinking health and disease. Annu Rev Microbiol 66 : 371–389.
41. BassoB, GimenezF, LopezC (2005) IL-1beta, IL-6 and IL-8 levels in gyneco-obstetric infections. Infect Dis Obstet Gynecol 13 : 207–211.
42. MatzingerP (2002) The danger model: a renewed sense of self. Science 296 : 301–305.
43. TomalkaJ, GanesanS, AzodiE, PatelK, MajmudarP, et al. (2011) A novel role for the NLRC4 inflammasome in mucosal defenses against the fungal pathogen Candida albicans. PLoS Pathog 7: e1002379.
44. De BernardisF, AmackerM, AranciaS, SandiniS, GremionC, et al. (2012) A virosomal vaccine against candidal vaginitis: immunogenicity, efficacy and safety profile in animal models. Vaccine 30 : 4490–4498.
45. IbrahimAS, LuoG, GebremariamT, LeeH, SchmidtCS, et al. (2013) NDV-3 protects mice from vulvovaginal candidiasis through T - and B-cell immune response. Vaccine 31 : 5549–5556.
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Affinity Proteomics Reveals Elevated Muscle Proteins in Plasma of Children with Cerebral MalariaČlánek The Transcriptional Activator LdtR from ‘ Liberibacter asiaticus’ Mediates Osmotic Stress ToleranceČlánek Complement-Related Proteins Control the Flavivirus Infection of by Inducing Antimicrobial PeptidesČlánek Fungal Chitin Dampens Inflammation through IL-10 Induction Mediated by NOD2 and TLR9 ActivationČlánek Parasite Fate and Involvement of Infected Cells in the Induction of CD4 and CD8 T Cell Responses to
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2014 Číslo 4- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- , , , Genetic Variability: Cryptic Biological Species or Clonal Near-Clades?
- Early Mortality Syndrome Outbreaks: A Microbial Management Issue in Shrimp Farming?
- Wormholes in Host Defense: How Helminths Manipulate Host Tissues to Survive and Reproduce
- Plastic Proteins and Monkey Blocks: How Lentiviruses Evolved to Replicate in the Presence of Primate Restriction Factors
- The 2010 Cholera Outbreak in Haiti: How Science Solved a Controversy
- Affinity Proteomics Reveals Elevated Muscle Proteins in Plasma of Children with Cerebral Malaria
- Noncanonical Role for the Host Vps4 AAA+ ATPase ESCRT Protein in the Formation of Replicase
- Efficient Parvovirus Replication Requires CRL4-Targeted Depletion of p21 to Prevent Its Inhibitory Interaction with PCNA
- Host-to-Pathogen Gene Transfer Facilitated Infection of Insects by a Pathogenic Fungus
- The Transcriptional Activator LdtR from ‘ Liberibacter asiaticus’ Mediates Osmotic Stress Tolerance
- Coxsackievirus B Exits the Host Cell in Shed Microvesicles Displaying Autophagosomal Markers
- TCR Affinity Associated with Functional Differences between Dominant and Subdominant SIV Epitope-Specific CD8 T Cells in Rhesus Monkeys
- Coxsackievirus-Induced miR-21 Disrupts Cardiomyocyte Interactions via the Downregulation of Intercalated Disk Components
- Ligands of MDA5 and RIG-I in Measles Virus-Infected Cells
- Kind Discrimination and Competitive Exclusion Mediated by Contact-Dependent Growth Inhibition Systems Shape Biofilm Community Structure
- Structural Differences Explain Diverse Functions of Actins
- HSCARG Negatively Regulates the Cellular Antiviral RIG-I Like Receptor Signaling Pathway by Inhibiting TRAF3 Ubiquitination Recruiting OTUB1
- Vaginitis: When Opportunism Knocks, the Host Responds
- Complement-Related Proteins Control the Flavivirus Infection of by Inducing Antimicrobial Peptides
- Fungal Chitin Dampens Inflammation through IL-10 Induction Mediated by NOD2 and TLR9 Activation
- Microbial Pathogens Trigger Host DNA Double-Strand Breaks Whose Abundance Is Reduced by Plant Defense Responses
- Alveolar Macrophages Are Essential for Protection from Respiratory Failure and Associated Morbidity following Influenza Virus Infection
- An Interaction between Glutathione and the Capsid Is Required for the Morphogenesis of C-Cluster Enteroviruses
- Concerted Spatio-Temporal Dynamics of Imported DNA and ComE DNA Uptake Protein during Gonococcal Transformation
- Potent Dengue Virus Neutralization by a Therapeutic Antibody with Low Monovalent Affinity Requires Bivalent Engagement
- Regulation of Human T-Lymphotropic Virus Type I Latency and Reactivation by HBZ and Rex
- Functionally Redundant RXLR Effectors from Act at Different Steps to Suppress Early flg22-Triggered Immunity
- The Pathogenic Mechanism of the Virulence Factor, Mycolactone, Depends on Blockade of Protein Translocation into the ER
- Role of Calmodulin-Calmodulin Kinase II, cAMP/Protein Kinase A and ERK 1/2 on -Induced Apoptosis of Head Kidney Macrophages
- An Overview of Respiratory Syncytial Virus
- First Experimental Model of Enhanced Dengue Disease Severity through Maternally Acquired Heterotypic Dengue Antibodies
- Binding of Glutathione to Enterovirus Capsids Is Essential for Virion Morphogenesis
- IFITM3 Restricts Influenza A Virus Entry by Blocking the Formation of Fusion Pores following Virus-Endosome Hemifusion
- Parasite Fate and Involvement of Infected Cells in the Induction of CD4 and CD8 T Cell Responses to
- Deficient IFN Signaling by Myeloid Cells Leads to MAVS-Dependent Virus-Induced Sepsis
- Pernicious Pathogens or Expedient Elements of Inheritance: The Significance of Yeast Prions
- The HMW1C-Like Glycosyltransferases—An Enzyme Family with a Sweet Tooth for Simple Sugars
- The Expanding Functions of Cellular Helicases: The Tombusvirus RNA Replication Enhancer Co-opts the Plant eIF4AIII-Like AtRH2 and the DDX5-Like AtRH5 DEAD-Box RNA Helicases to Promote Viral Asymmetric RNA Replication
- Mining Herbaria for Plant Pathogen Genomes: Back to the Future
- Inferring Influenza Infection Attack Rate from Seroprevalence Data
- A Human Lung Xenograft Mouse Model of Nipah Virus Infection
- Mast Cells Expedite Control of Pulmonary Murine Cytomegalovirus Infection by Enhancing the Recruitment of Protective CD8 T Cells to the Lungs
- Cytosolic Peroxidases Protect the Lysosome of Bloodstream African Trypanosomes from Iron-Mediated Membrane Damage
- Abortive T Follicular Helper Development Is Associated with a Defective Humoral Response in -Infected Macaques
- JC Polyomavirus Infection Is Strongly Controlled by Human Leucocyte Antigen Class II Variants
- Cationic Antimicrobial Peptides Promote Microbial Mutagenesis and Pathoadaptation in Chronic Infections
- Estimating the Fitness Advantage Conferred by Permissive Neuraminidase Mutations in Recent Oseltamivir-Resistant A(H1N1)pdm09 Influenza Viruses
- Progressive Accumulation of Activated ERK2 within Highly Stable ORF45-Containing Nuclear Complexes Promotes Lytic Gammaherpesvirus Infection
- Caspase-1-Like Regulation of the proPO-System and Role of ppA and Caspase-1-Like Cleaved Peptides from proPO in Innate Immunity
- Is Required for High Efficiency Viral Replication
- Modified Vaccinia Virus Ankara Triggers Type I IFN Production in Murine Conventional Dendritic Cells via a cGAS/STING-Mediated Cytosolic DNA-Sensing Pathway
- Evidence That Bank Vole PrP Is a Universal Acceptor for Prions
- Rapid Response to Selection, Competitive Release and Increased Transmission Potential of Artesunate-Selected Malaria Parasites
- Inactivation of Genes for Antigenic Variation in the Relapsing Fever Spirochete Reduces Infectivity in Mice and Transmission by Ticks
- Exposure-Dependent Control of Malaria-Induced Inflammation in Children
- A Neutralizing Anti-gH/gL Monoclonal Antibody Is Protective in the Guinea Pig Model of Congenital CMV Infection
- The Apical Complex Provides a Regulated Gateway for Secretion of Invasion Factors in
- A Highly Conserved Haplotype Directs Resistance to Toxoplasmosis and Its Associated Caspase-1 Dependent Killing of Parasite and Host Macrophage
- A Quantitative High-Resolution Genetic Profile Rapidly Identifies Sequence Determinants of Hepatitis C Viral Fitness and Drug Sensitivity
- Histone Deacetylase Inhibitor Romidepsin Induces HIV Expression in CD4 T Cells from Patients on Suppressive Antiretroviral Therapy at Concentrations Achieved by Clinical Dosing
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The 2010 Cholera Outbreak in Haiti: How Science Solved a Controversy
- , , , Genetic Variability: Cryptic Biological Species or Clonal Near-Clades?
- Efficient Parvovirus Replication Requires CRL4-Targeted Depletion of p21 to Prevent Its Inhibitory Interaction with PCNA
- An Overview of Respiratory Syncytial Virus
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



