-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaKeeping in Shape the Dogma of Mitochondrial DNA Maternal Inheritance
article has not abstract
Published in the journal: . PLoS Genet 11(5): e32767. doi:10.1371/journal.pgen.1005179
Category: Perspective
doi: https://doi.org/10.1371/journal.pgen.1005179Summary
article has not abstract
The Dogma: Uniparental (Maternal) Inheritance of mtDNA
It is textbook knowledge that the small multicopy mitochondrial genome (mtDNA) is maternally inherited in humans and mammals [1,2]. The uniparental mtDNA inheritance applies to most eukaryotic organisms, including animals exhibiting the doubly uniparental inheritance, such as the bivalve mollusks [3,4]. Occurrence of paternal mtDNA transmission has also been documented [5–7], and doubts on strict maternal inheritance in humans have been raised [8,9]. The best-documented case of paternal mtDNA inheritance was in a patient carrying a pathogenic mtDNA mutation [9], never replicated in following studies of patients with mitochondrial diseases due to various mtDNA defects [10–12].
The sperm mitochondria enter the oocyte during fertilization in mammals [13], but paternal mitochondria and mtDNA disappear at the initial cell divisions of the embryo in a stringently species-specific fashion [14]. In fact, the failure to efficiently eliminate paternal mtDNA from different species intercrosses [14,15] explains some of the cases of paternally inherited mtDNA [5]. Furthermore, recognition and targeted elimination of exogenous mtDNA entering the oocyte seems restricted to sperm mtDNA, not occurring with liver mtDNA, thus also displaying tissue specificity [16].
The way by which paternal mtDNA inheritance fails to occur in humans remains elusive, and it appears that several mechanisms have coevolved to avoid paternal mtDNA contribution to the embryo [17]. It has been observed that sperm mitochondria are ubiquitinated, suggestive of an “active elimination model” for paternal mtDNA [14], which may occur through different routes, such as proteosomal or lysosomal pathways [14,17]. Autophagy has been recently highlighted as the mechanism for paternal mtDNA elimination in Caenorhabditis elegans [18,19]. This was not observed in mice, for which elimination of mtDNA from prefertilization sperm and uneven persistence of paternal mtDNA in the embryo raised the possibility of a passive “dilution model” of disproportionate paternal versus maternal mtDNAs in mammals [20]. The consequent leakage of paternal mtDNA in the newborn may have remained “undetected” by the standard sequencing approaches.
The “Dilution Model” Tested in Humans
Taking advantage of deep sequencing techniques, Pyle and colleagues tackled the issue of detectability of diluted postfertilization paternal mtDNA in humans [21]. They first estimated a ratio of 1 : 15,860 for the amount of mtDNA in healthy human sperm and prefertilization oocytes, predicting an interval for the proportion of the paternal haplotypes at fertilization of 10–5 to 1.8 x 10–4. Then, these authors went on using extremely high-depth mtDNA resequencing, up to about 1.2 million-fold coverage, to screen trios where the father and the child had two or more variant differences within a <200 bp stretch of mtDNA, looking for paternal haplotypes at very low heteroplasmy in buccal-derived DNA. A long-template strategy was used to generate the amplicons for resequencing, minimizing the artifactual identification of mitochondrial pseudogene variants in the “nuclear mitochondrial DNA” (NUMTs). Four different trios suitable to such analysis were identified out of a pre-existing cohort, and the analysis revealed the occurrence of extremely rare variant haplotypes, which were not compatible with a paternal origin and were thus considered as “background noise.” Most importantly, this “noise” was observed also in the maternal samples and was consistent within trios, raising the possibility of very low level contamination occurring when the original samples were acquired. Overall, this “noise” was incorporated into the statistical analysis and did not change the study conclusions that there is no evidence for paternal mtDNA contribution in the child.
Is Buccal-Derived DNA Enough to Reject the “Dilution Model”?
This accurate study substantially rejects the hypothesis of a “dilution model” for paternal mtDNA transmission in humans [21]. The only debatable point remains the lack of a similar analysis in multiple tissues from the child. The confirmation of no paternal mtDNA haplotypes in multiple tissues, including postmitotic tissues, from the same individual would strengthen the current results, and there are a few reasons for this. According to Luo and colleagues [20], the skewed persistence of paternal mtDNA in only one of the 4-cell blastomers followed by subsequent uneven distribution to just a few cells at the morula stage of mouse embryos, would potentially lead to detectable paternal mtDNA only in some tissues of the newborn. Furthermore, age and tissue-dependent preferential shifts of one mtDNA haplotype over the other have been documented in heteroplasmic mice carrying a mixture of BALB and NZB mitochondrial genomes [22], potentially applying to the greatly disproportionate paternal versus maternal mtDNA ratio in the newborn tissues according to the “dilution model.”
Is Maternal Inheritance Selected to Avoid Heteroplasmy?
The key question of why uniparental (maternal) mtDNA inheritance has been evolutionarily successful remains to be convincingly answered. The quick answer that sperm mtDNA is damaged by oxidative stress, being thus of bad quality and unfit to contribute the mtDNA pool of the embryo, is unsatisfactory. Maternal mtDNA inheritance avoids the occurrence of heteroplasmy between potentially distant mtDNA haplotypes, if coinherited by biparental mtDNA inheritance. The possible conflict between different coexisting “normal” mtDNA haplotypes, which may slightly differ in terms of oxidative phosphorylation (OXPHOS) efficiency, has been shown to be maladaptive in heteroplasmic mice, leading to significant physiological, cognitive, and behavioral impairments as compared to the homoplasmic mice for each mtDNA haplotype [23]. As a consequence, a non-random segregation of the mtDNA haplotypes occurs during tissue aging and germline transmission, leading to the proposal that this may explain the advantage of uniparental inheritance of mtDNA [23].
Next: The “Active Elimination Model”
The study by Pyle and colleagues contributes to advance our understanding on how paternal mtDNA is not transmitted to newborns in humans [21]. The “active elimination model” takes over the “dilution model,” but besides ubiquitination of sperm mitochondria, we still do not know how their elimination is executed. Is ubiquitination targeting specific proteins? Prohibitin has been reported to be ubiquitinated in the sperm [24] and has also been proposed as the regulator of TFAM and mtDNA copy number [25]. In turn, reduction of TFAM and mtDNA copy number occurs during mammalian spermatogenesis [26]. Thus, a first step of a possibly multistep mechanism reduces sperm mtDNA to a minimal amount. Once the oocyte is fertilized, proteasomal and lysosomal pathways have been invoked for paternal mitochondria and mtDNA elimination. However, emerging aspects of mitochondrial quality control and dynamics [27] are poorly known in the contest of fertilized oocytes, which may turn out relevant to paternal mtDNA elimination. Finally, specific recognition and elimination of paternal mtDNA may occur at the molecular level. Endonuclease G has been implicated in reduction of sperm mtDNA copy number in Drosophila [28], but whether paternal mtDNA is directly targeted postfertilization remains unexplored.
Conclusions
The emerging picture is that of a multistep mechanism, with many different checkpoints composing a puzzle (Fig 1) needing more work to be completed to fully unwrap the dogma of mtDNA maternal inheritance.
Fig. 1. Schematic representation of the two models, “active elimination” and “dilution” of paternal mtDNA haplotypes, with multiple possible steps that ensure avoidance of paternal mtDNA inheritance. 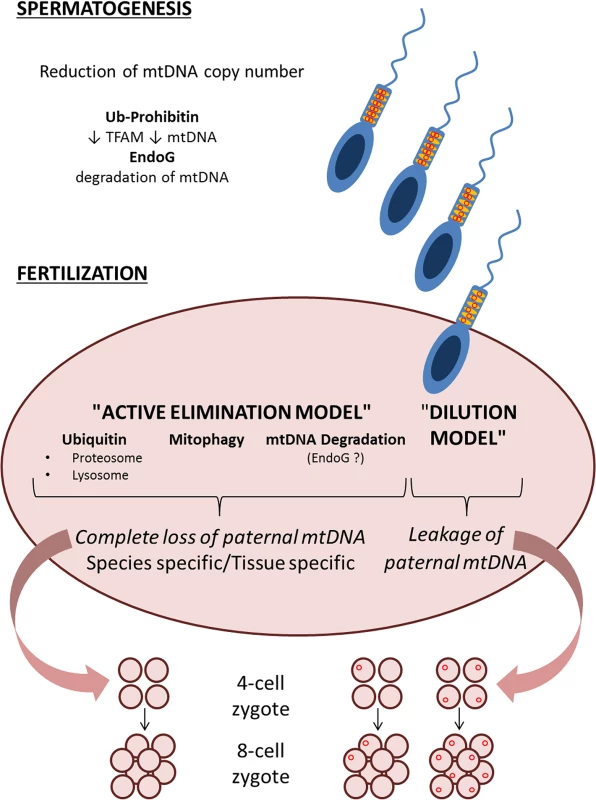
A first step for which there is evidence of reduction of mtDNA copy number is at the level of spermatogenesis and prefertilization sperm [20,24–26]. Postfertilization, according to the “dilution” model, the low levels of paternal mtDNA haplotypes may be evenly distributed among tissues, but the study by Pyle and colleagues finds no evidence of such a “dilution” [21]. Alternatively, if mtDNA haplotypes are unevenly distributed among the tissues of the newborn [20], or shift in an age and tissue-dependent fashion [22], there remains a possibility that paternal mtDNA is detectable only in certain tissues. The “active elimination” model, currently more supported by experimental evidence, may execute the paternal mtDNA elimination through multiple possible mechanisms, which are summarized in Fig 1. These include ubiquitination and active elimination of paternal mitochondria and mtDNA by proteasomal and lysosomal pathways [14], selective mitophagy of paternal mitochondria [18,19], or direct degradation of paternal mtDNA [28].
Zdroje
1. Hutchison CA 3rd, Newbold JE, Potter SS, Edgell MH. Maternal inheritance of mammalian mitochondrial DNA. Nature 1974; 251 : 536–8. 4423884
2. Giles RE, Blanc H, Cann HM, Wallace DC (1980) Maternal inheritance of human mitochondrial DNA. Proc Natl Acad Sci U S A 77 : 6715–6719. 6256757
3. Breton S, Beaupré HD, Stewart DT, Hoeh WR, Blier PU (2007) The unusual system of doubly uniparental inheritance of mtDNA: isn't one enough? Trends Genet 23 : 465–474. 17681397
4. Passamonti M, Ghiselli F (2009) Doubly uniparental inheritance: two mitochondrial genomes, one precious model for organelle DNA inheritance and evolution. DNA Cell Biol 28 : 79–89. doi: 10.1089/dna.2008.0807 19196051
5. Gyllensten U, Wharton D, Josefsson A, Wilson AC (1991) Paternal inheritance of mitochondrial DNA in mice. Nature 352 : 255–257. 1857422
6. Zhao X, Li N, Guo W, Hu X, Liu Z, et al. (2004) Further evidence for paternal inheritance of mitochondrial DNA in the sheep (Ovis aries). Heredity (Edinb) 93 : 399–403. 15266295
7. Dokianakis E, Ladoukakis ED (2014) Different degree of paternal mtDNA leakage between male and female progeny in interspecific Drosophila crosses. Ecol Evol 4 : 2633–2641. doi: 10.1002/ece3.1069 25077015
8. Awadalla P, Eyre-Walker A, Smith JM (1999) Linkage disequilibrium and recombination in hominid mitochondrial DNA. Science 286 : 2524–2525. 10617471
9. Schwartz M, Vissing J (2002) Paternal inheritance of mitochondrial DNA. N Engl J Med 347 : 576–580. 12192017
10. Taylor RW, McDonnell MT, Blakely EL, Chinnery PF, Taylor GA, et al. (2003) Genotypes from patients indicate no paternal mitochondrial DNA contribution. Ann Neurol 54 : 521–524. 14520666
11. Filosto M, Mancuso M, Vives-Bauza C, Vilà MR, Shanske S, et al. (2003) Lack of paternal inheritance of muscle mitochondrial DNA in sporadic mitochondrial myopathies. Ann Neurol 54 : 524–526. 14520667
12. Schwartz M, Vissing J (2004) No evidence for paternal inheritance of mtDNA in patients with sporadic mtDNA mutations. J Neurol Sci 218 : 99–101. 14759640
13. Ankel-Simons F, Cummins JM (1996) Misconceptions about mitochondria and mammalian fertilization: implications for theories on human evolution. Proc Natl Acad Sci U S A 93 : 13859–13863. 8943026
14. Sutovsky P, Moreno RD, Ramalho-Santos J, Dominko T, Simerly C, et al. (1999) Ubiquitin tag for sperm mitochondria. Nature 402 : 371–372. 10586873
15. Kaneda H. Hayashi J-I, Takahama S, Taya C, Fischer Lindahl K, Yonekawa H (1995) Elimination of paternal mitochondrial DNA in intraspecific crosses during early mouse embryogenesis. Proc Natl Acad Sci USA 92 : 4542–4546. 7753839
16. Shitara H, Kaneda H, Sato A, Inoue K, Ogura A, et al. (2000) Selective and continuous elimination of mitochondria microinjected into mouse eggs from spermatids, but not from liver cells, occurs throughout embryogenesis, Genetics 156 : 1277–1284. 11063701
17. Sato M, Sato K (2013) Maternal inheritance of mitochondrial DNA by diverse mechanisms to eliminate paternal mitochondrial DNA. Biochim Biophys Acta 1833 : 1979–1984. doi: 10.1016/j.bbamcr.2013.03.010 23524114
18. Sato M, Sato K (2011) Degradation of paternal mitochondria by fertilization triggered autophagy in C. elegans embryos. Science 334 : 1141–1144. doi: 10.1126/science.1210333 21998252
19. Al Rawi S, Louvet-Vallée S, Djeddi A, Sachse M, Culetto E, et al. (2011) Postfertilization autophagy of sperm organelles prevents paternal mitochondrial DNA transmission. Science 334 : 1144–1147. doi: 10.1126/science.1211878 22033522
20. Luo SM, Ge ZJ, Wang ZW, Jiang ZZ, Wang ZB, et al. (2013) Unique insights into maternal mitochondrial inheritance in mice. Proc Natl Acad Sci U S A 110 : 13038–13043. doi: 10.1073/pnas.1303231110 23878233
21. Pyle A, Hudson G, Wilson IJ, Coxhead J, Smertenko T, et al. (2015) Extreme-depth re-sequencing of mitochondrial DNA finds no evidence of paternal transmission in humans. Plos Genet 11: e1005040.
22. Jenuth JP, Peterson AC, Shoubridge EA (1997) Tissue-specific selection for different mtDNA genotypes in heteroplasmic mice. Nat Genet 16 : 93–95. 9140402
23. Sharpley MS, Marciniak C, Eckel-Mahan K, McManus M, Crimi M, et al. (2012) Heteroplasmy of mouse mtDNA is genetically unstable and results in altered behavior and cognition. Cell 151 : 333–343. doi: 10.1016/j.cell.2012.09.004 23063123
24. Thompson WE, Ramalho-Santos J, Sutovsky P (2003) Ubiquitination of prohibitin in mammalian sperm mitochondria: possible roles in the regulation of mitochondrial inheritance and sperm quality control. Biol Reprod 69 : 254–260. 12646488
25. Kasashima K, Sumitani M, Satoh M, Endo H (2008) Human prohibitin 1 maintains the organization and stability of the mitochondrial nucleoids. Exp Cell Res 314 : 988–996. doi: 10.1016/j.yexcr.2008.01.005 18258228
26. Rantanen A, Jansson M, Oldfors A, Larsson NG (2001) Downregulation of Tfam and mtDNA copy number during mammalian spermatogenesis. Mamm Genome 12 : 787–792. 11668394
27. Wakai T, Harada Y, Miyado K, Kono T (2014) Mitochondrial dynamics controlled by mitofusins define organelle positioning and movement during mouse oocyte maturation. Mol Hum Reprod 20 : 1090–1100. doi: 10.1093/molehr/gau064 25113836
28. DeLuca SZ, O'Farrell PH (2012) Barriers to male transmission of mitochondrial DNA in sperm development. Dev Cell 22 : 660–668. doi: 10.1016/j.devcel.2011.12.021 22421049
Štítky
Genetika Reprodukční medicína
Článek Minor Type IV Collagen α5 Chain Promotes Cancer Progression through Discoidin Domain Receptor-1Článek A Unique Virulence Gene Occupies a Principal Position in Immune Evasion by the Malaria ParasiteČlánek The Centrosomal Linker and Microtubules Provide Dual Levels of Spatial Coordination of CentrosomesČlánek Dissecting the Function and Assembly of Acentriolar Microtubule Organizing Centers in Cells In VivoČlánek MicroRNA-Dependent Transcriptional Silencing of Transposable Elements in Drosophila Follicle CellsČlánek PARP2 Is the Predominant Poly(ADP-Ribose) Polymerase in Arabidopsis DNA Damage and Immune ResponsesČlánek Cooperative Action of Cdk1/cyclin B and SIRT1 Is Required for Mitotic Repression of rRNA SynthesisČlánek The Developmental Intestinal Regulator ELT-2 Controls p38-Dependent Immune Responses in Adult .Článek Feeding and Fasting Signals Converge on the LKB1-SIK3 Pathway to Regulate Lipid Metabolism in
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 5
-
Všechny články tohoto čísla
- Genomic Heritability: What Is It?
- Triglyceride-Increasing Alleles Associated with Protection against Type-2 Diabetes
- Epistasis Is a Major Determinant of the Additive Genetic Variance in
- Genetic Regulation of Bone Metabolism in the Chicken: Similarities and Differences to Mammalian Systems
- Minor Type IV Collagen α5 Chain Promotes Cancer Progression through Discoidin Domain Receptor-1
- The Philosophical Approach: An Interview with Ford Doolittle
- Downregulation of the Host Gene by miR-92 Is Essential for Neuroblast Self-Renewal in
- A Unique Virulence Gene Occupies a Principal Position in Immune Evasion by the Malaria Parasite
- Y Fuse? Sex Chromosome Fusions in Fishes and Reptiles
- Regulation of Active DNA Demethylation by a Methyl-CpG-Binding Domain Protein in
- Overlapping Patterns of Rapid Evolution in the Nucleic Acid Sensors cGAS and OAS1 Suggest a Common Mechanism of Pathogen Antagonism and Escape
- Autoselection of Cytoplasmic Yeast Virus Like Elements Encoding Toxin/Antitoxin Systems Involves a Nuclear Barrier for Immunity Gene Expression
- Genetic Architecture of Abdominal Pigmentation in
- Whole Genome DNA Binding Analysis of the Bacterial Replication Initiator and Transcription Factor DnaA
- The Centrosomal Linker and Microtubules Provide Dual Levels of Spatial Coordination of Centrosomes
- Parp3 Negatively Regulates Immunoglobulin Class Switch Recombination
- Burden Analysis of Rare Microdeletions Suggests a Strong Impact of Neurodevelopmental Genes in Genetic Generalised Epilepsies
- Cell Cycle Control by the Master Regulator CtrA in
- Myopathic Lamin Mutations Cause Reductive Stress and Activate the Nrf2/Keap-1 Pathway
- Monoallelic Loss of the Imprinted Gene Promotes Tumor Formation in Irradiated Mice
- Phylum-Level Conservation of Regulatory Information in Nematodes despite Extensive Non-coding Sequence Divergence
- Clustering and Negative Feedback by Endocytosis in Planar Cell Polarity Signaling Is Modulated by Ubiquitinylation of Prickle
- Dissecting the Function and Assembly of Acentriolar Microtubule Organizing Centers in Cells In Vivo
- MicroRNA-Dependent Transcriptional Silencing of Transposable Elements in Drosophila Follicle Cells
- β-Catenin Signaling Biases Multipotent Lingual Epithelial Progenitors to Differentiate and Acquire Specific Taste Cell Fates
- A Simple Auxin Transcriptional Response System Regulates Multiple Morphogenetic Processes in the Liverwort
- Parallel Gene Expression Differences between Low and High Latitude Populations of and .
- The Nutrient-Responsive Hormone CCHamide-2 Controls Growth by Regulating Insulin-like Peptides in the Brain of
- Characterization of TCF21 Downstream Target Regions Identifies a Transcriptional Network Linking Multiple Independent Coronary Artery Disease Loci
- PARP2 Is the Predominant Poly(ADP-Ribose) Polymerase in Arabidopsis DNA Damage and Immune Responses
- Drosophila Spaghetti and Doubletime Link the Circadian Clock and Light to Caspases, Apoptosis and Tauopathy
- Coronary Artery Disease Associated Transcription Factor TCF21 Regulates Smooth Muscle Precursor Cells That Contribute to the Fibrous Cap
- Rescue of DNA-PK Signaling and T-Cell Differentiation by Targeted Genome Editing in a Deficient iPSC Disease Model
- Disruption of Transcriptional Coactivator Sub1 Leads to Genome-Wide Re-distribution of Clustered Mutations Induced by APOBEC in Active Yeast Genes
- Yeast Killer Elements Hold Their Hosts Hostage
- Keeping in Shape the Dogma of Mitochondrial DNA Maternal Inheritance
- Extreme-Depth Re-sequencing of Mitochondrial DNA Finds No Evidence of Paternal Transmission in Humans
- Trading Places—Switching Frataxin Function by a Single Amino Acid Substitution within the [Fe-S] Cluster Assembly Scaffold
- Natural Variation Identifies , a Universal Gene Required for Cell Proliferation and Growth at High Temperatures in
- Mutations in Gene Are Associated with Predisposition to Breast Cancer
- The Whole of a Scientific Career: An Interview with Oliver Smithies
- Cell Specific eQTL Analysis without Sorting Cells
- Cooperative Action of Cdk1/cyclin B and SIRT1 Is Required for Mitotic Repression of rRNA Synthesis
- Systemic Regulation of RAS/MAPK Signaling by the Serotonin Metabolite 5-HIAA
- Reprogramming LCLs to iPSCs Results in Recovery of Donor-Specific Gene Expression Signature
- The Developmental Intestinal Regulator ELT-2 Controls p38-Dependent Immune Responses in Adult .
- Genetic Mechanism of Human Neutrophil Antigen 2 Deficiency and Expression Variations
- Feeding and Fasting Signals Converge on the LKB1-SIK3 Pathway to Regulate Lipid Metabolism in
- Early Lineage Priming by Trisomy of Leads to Myeloproliferation in a Down Syndrome Model
- Turning into a Frataxin-Dependent Organism
- Accounting for Experimental Noise Reveals That mRNA Levels, Amplified by Post-Transcriptional Processes, Largely Determine Steady-State Protein Levels in Yeast
- Biological Significance of Photoreceptor Photocycle Length: VIVID Photocycle Governs the Dynamic VIVID-White Collar Complex Pool Mediating Photo-adaptation and Response to Changes in Light Intensity
- CTXφ Replication Depends on the Histone-Like HU Protein and the UvrD Helicase
- Disruption of miR-29 Leads to Aberrant Differentiation of Smooth Muscle Cells Selectively Associated with Distal Lung Vasculature
- Notch Is Required in Adult Sensory Neurons for Morphological and Functional Plasticity of the Olfactory Circuit
- Post-transcriptional Regulation of Keratinocyte Progenitor Cell Expansion, Differentiation and Hair Follicle Regression by
- PERK Limits Lifespan by Promoting Intestinal Stem Cell Proliferation in Response to ER Stress
- Casein Kinase 1 and Phosphorylation of Cohesin Subunit Rec11 (SA3) Promote Meiotic Recombination through Linear Element Formation
- Fibroblast Growth Factor 9 Regulation by MicroRNAs Controls Lung Development and Links Loss to the Pathogenesis of Pleuropulmonary Blastoma
- Auxin-Mediated Transcriptional System with a Minimal Set of Components Is Critical for Morphogenesis through the Life Cycle in
- The Broad-Spectrum Antiviral Protein ZAP Restricts Human Retrotransposition
- The 4E-BP Caf20p Mediates Both eIF4E-Dependent and Independent Repression of Translation
- Turning into a Frataxin-Independent Organism
- Promotion of Bone Morphogenetic Protein Signaling by Tetraspanins and Glycosphingolipids
- Essential Role of the ESX-5 Secretion System in Outer Membrane Permeability of Pathogenic Mycobacteria
- The Zinc-Finger Antiviral Protein ZAP Inhibits LINE and Alu Retrotransposition
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Drosophila Spaghetti and Doubletime Link the Circadian Clock and Light to Caspases, Apoptosis and Tauopathy
- Autoselection of Cytoplasmic Yeast Virus Like Elements Encoding Toxin/Antitoxin Systems Involves a Nuclear Barrier for Immunity Gene Expression
- Parp3 Negatively Regulates Immunoglobulin Class Switch Recombination
- PERK Limits Lifespan by Promoting Intestinal Stem Cell Proliferation in Response to ER Stress
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



