-
Články
Top novinky
Reklama- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Top novinky
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
Top novinky
ReklamaMutations in Gene Are Associated with Predisposition to Breast Cancer
In this study, we aimed to find novel breast cancer susceptibility genes by whole-exome sequencing in nine early-onset familial breast cancer patients without BRCA1/2 mutations. We found that two index cases carried a potentially deleterious mutation in the RECQL gene (RecQ helicase-like). We further screened the RECQL gene in an additional 439 unrelated familial breast cancer patients. In total, we found nine index cases carried a pathogenic mutation in the RECQL gene among the 448 BRCA-negative familial breast cancer patients. The RECQL is one of five RecQ helicase proteins (named RECQL, BLM, WRN, RECQL4 and RECQL5). RECQL is considered to be genome caretaker, and mutations in three of five RecQ genes, BLM, WRN and RECQ4 are associated with cancer predisposition and/or premature aging. Here, we are the first to report that mutations in the RECQL gene are associated with predisposition to breast cancer and this finding may have potential clinical implications and raise research questions about RECQL.
Published in the journal: . PLoS Genet 11(5): e32767. doi:10.1371/journal.pgen.1005228
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1005228Summary
In this study, we aimed to find novel breast cancer susceptibility genes by whole-exome sequencing in nine early-onset familial breast cancer patients without BRCA1/2 mutations. We found that two index cases carried a potentially deleterious mutation in the RECQL gene (RecQ helicase-like). We further screened the RECQL gene in an additional 439 unrelated familial breast cancer patients. In total, we found nine index cases carried a pathogenic mutation in the RECQL gene among the 448 BRCA-negative familial breast cancer patients. The RECQL is one of five RecQ helicase proteins (named RECQL, BLM, WRN, RECQL4 and RECQL5). RECQL is considered to be genome caretaker, and mutations in three of five RecQ genes, BLM, WRN and RECQ4 are associated with cancer predisposition and/or premature aging. Here, we are the first to report that mutations in the RECQL gene are associated with predisposition to breast cancer and this finding may have potential clinical implications and raise research questions about RECQL.
Introduction
Breast cancer is the most common malignancy disease in women world wide. Among these, approximately 10% of breast cancer patients have a family history of breast cancer (referred as familial breast cancer), but only 10–15% of familial breast cancer is owing to germline mutations in one of the two high penetrance breast cancer susceptibility genes—BRCA1 and BRCA2[1]. Additionally, mutations in the moderate breast cancer susceptibility genes, such as PALB2, ATM, CHEK2, BRIP1 and RAD51C, contribute to 5% of familial breast cancers[2–6]. Therefore, the genetic causes of approximately 70–80% familial breast cancer remain to be discovered.
Recently, next-generation sequencing assay provides a new platform to find cancer susceptibility genes. To find potential breast cancer susceptibility genes, we performed whole-exome sequencing in nine early-onset familial breast cancer patients who do not carry a germline mutation in the BRCA1/2 genes; all nine cases were diagnosed with breast cancer at or before the age of 35, the index case had at least one first-degree relative affected with breast cancer. Based on the data of whole-exome sequencing of the nine cases, we further screened the potential gene in an additional 439 unrelated familial breast cancer patients without BRCA1/2 mutations and performed in vitro functional analyses to evaluate whether the mutations disrupt the function of the potential gene. Finally, our results indicate that RECQL (RecQ helicase-like) is a novel breast cancer susceptibility gene.
Results
The detail information of the nine unrelated early-onset familial breast cancer patients who were subjected to whole-exome sequencing is presented in S1 Table. Approximately 46,000 variants were identified by whole-exome sequencing in each sample. We filtered the data for genes that harbored novel, heterozygous rare variants that were truncating mutations or splice-site variants. We further retained only genes that contain different variants shared in two or more cases (S2 Table).
As a result, there were three genes that fit these criteria and were validated by Sanger sequencing: TTLL2, VSIG2 and RECQL (S3 Table). Of these genes, RECQL is of great interest because it is involved in DNA repair process. The two mutations in the RECQL gene were found in two index cases through the whole-exome assay (S1A and S1B Fig), one index case carried a nonsense mutation in exon 4 of the RECQL gene (encoding L128X), leading to a premature stop codon; another index case carried a non-synonymous mutation (R539P) that was predicted to affect the function of RECQL.
To determine the germline mutations in the RECQL gene in an additional cohort of familial breast cancer patients, we screened the entire coding region of RECQL gene using Sanger sequencing assays in 439 familial breast cancer patients without BRCA1/2 mutations. In total, we found 15 germline variants in the RECQL gene in the 448 familial breast cancer patients (including the nine index cases for whole-exome sequencing) (Table 1 and S1 Fig). These variants contained three nonsense mutations (L128X, W172X and Q266X), two potential splice-site mutations (c.395-2 A>G and c.868-12_868-11del) and ten missense mutations (M1T, S63P, A195S, R215Q, N363S, R455C, M458K, R539P, H461R, and T562I) (Table 1). We further tested the germline mutations in the RECQL gene in 1,588 healthy controls, and three missense mutations (M1T, N363S and H461R) were found in the controls (Table 1). The three missense mutations (M1T, N363S and H461R) were present in both familial breast cancer patients and controls. Among these mutations, the mutation frequency of M1T (rs146924988) and N363S (rs138663409) was similar between familial breast cancer patients and controls. Thus, these two variants are more likely to be neutral (Table 1).
Tab. 1. Mutations in RECQL gene identified in the 448 BRCA1/2-negative familial breast cancer patients. 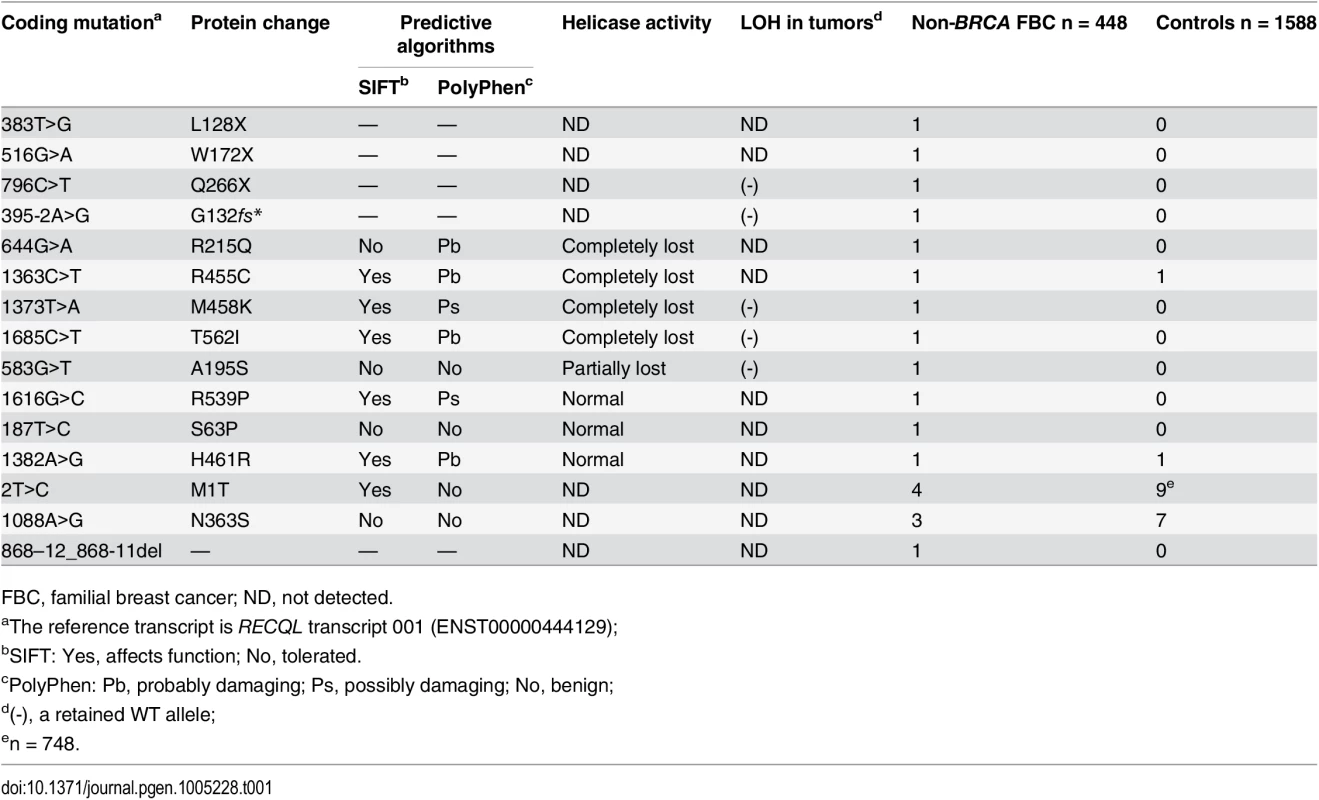
FBC, familial breast cancer; ND, not detected. The three nonsense mutations (L128X, W172X and Q266X) lead to premature protein termination, and therefore, we considered these to be clearly pathogenic. The two splice-site mutations were predicted to affect the splice site. RNA from peripheral blood samples was isolated from the index case who carried the 395-2A>G mutation. The RT-PCR pattern of the RECQL gene from the 395-2A>G mutation carrier revealed a reduced expression of the normal transcript and one new truncated product compared to the control. Further sequencing confirmed that the 395–2 A>G mutation resulted in exon 5 skipping in the abnormal RT-PCR product (Fig 1A). This product of the abnormal transcript disrupts the helicase domain of RECQL and leads to a premature stop signal (G132fs*); therefore, the 395-2A>G mutation is pathogenic. The pedigree of the index case who carried the RECQL 395-2A>G mutation is presented in Fig 1B. Both the index and the twin, who were negative for BRCA1/2 mutations, carried the mutation; they had the disease at the ages of 40 and 43, respectively. Additionally, at least ten individuals in this family had breast/ovarian cancer, lung cancer, cervical cancer, or peritoneal cancer (Fig 1B). Unfortunately, blood samples were only available for the twin; thus, it was not possible to perform the segregation analysis in this family. Another index carried the c.868-12_868-11del mutation; however, RT-PCR analysis revealed that this mutation did not affect the splicing of RECQL (S2 Fig). Thus, the c.868-12_868-11del mutation was neutral.
Fig. 1. Analysis of the splicing mutation 395–2 A>G. 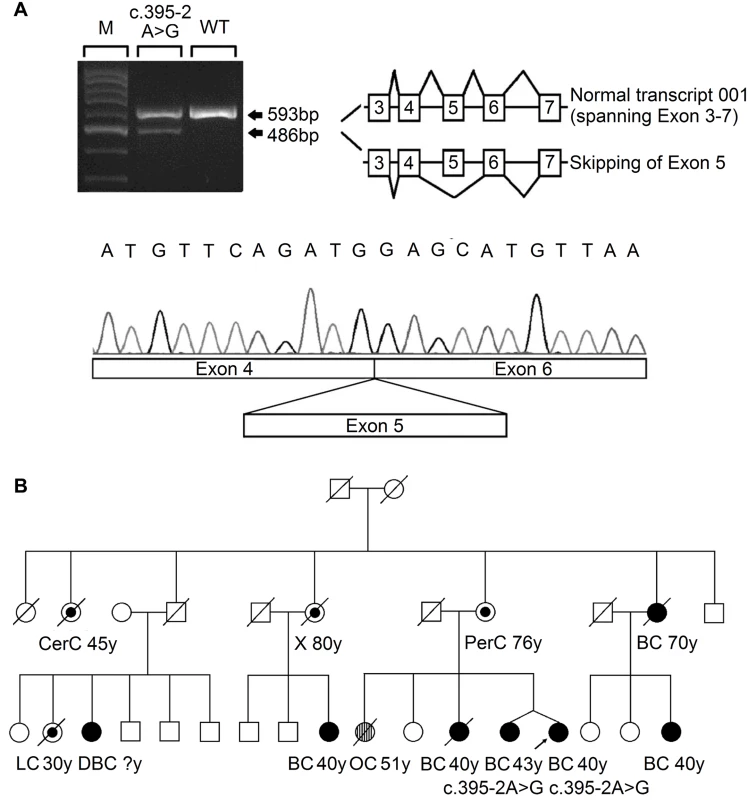
(A) RT-PCR analysis of RNA extracted from the blood sample of the affected index case, using primers located in exon 3 and exon 7. The PCR products showed a reduced normal transcript (ENST00000444129) and an additional aberrant splicing transcript skipping exon 5, as confirmed by Sanger sequencing. (B) The pedigree of the index case who carried the RECQL c.395-2 A>G mutation. Types of cancer and age in years (y) at first diagnosis are given underneath each symbol. Filled circles represent breast cancer (BC) and double breast cancer (DBC); streaked circles represent ovarian cancer (OC); centre circles represent other types of cancer (CerC, cervical cancer; LC, lung cancer; PerC, peritoneal cancer; X, uninformative cancer). To investigate whether the remaining eight missense mutations (S63P, A195S, R215Q, R455C, M458K, H461R, R539P, and T562I) influenced the efficacy of RECQL helicase activity, we first examined the structures of the RECQL protein (RCSB PDB, 2V1X and 2WWY) (S3 Fig). On the basis of the crystal structure, T562 is located in a β-hairpin, which is required for DNA unwinding [7,8]; A195 is involved in dimer interaction[8]; R215 is located near the ADP-binding pocket and is expected to weaken ATP hydrolysis[9]; the conserved residues R455 and M458 are located in the zinc binding subdomain, which is important for whole-protein stability[10](S3 Fig).
Next, we performed helicase activity assays using GST fusion proteins in vitro[11]. The K119A mutation served as a negative control that is reported to affect the helicase activity of RECQL[12]. By assessing the helicase ability to unwind forked DNA substrates, single-strand DNA was essentially undetectable with four mutations, R215Q, R455C, M458K, and T562I, suggesting that these four mutations completely disrupted the helicase activity (Fig 2A and 2B). Additionally, we found that the A195S mutant lost approximately 83.4% of the helicase activity compared to the wild-type of RECQL. The remaining three missense mutations (S63P, H461R and R539P) showed similarly effective helicase activities compared to the wild-type of RECQL (Fig 2A and 2B). Thus, the mutations of S63P, H461R and R539P were neutral. Taken together, five missense mutations (R215Q, R455C, M458K, T562I and A195S) were pathogenic.
Fig. 2. Functional analyses of the eight missense mutations in the RECQL gene. 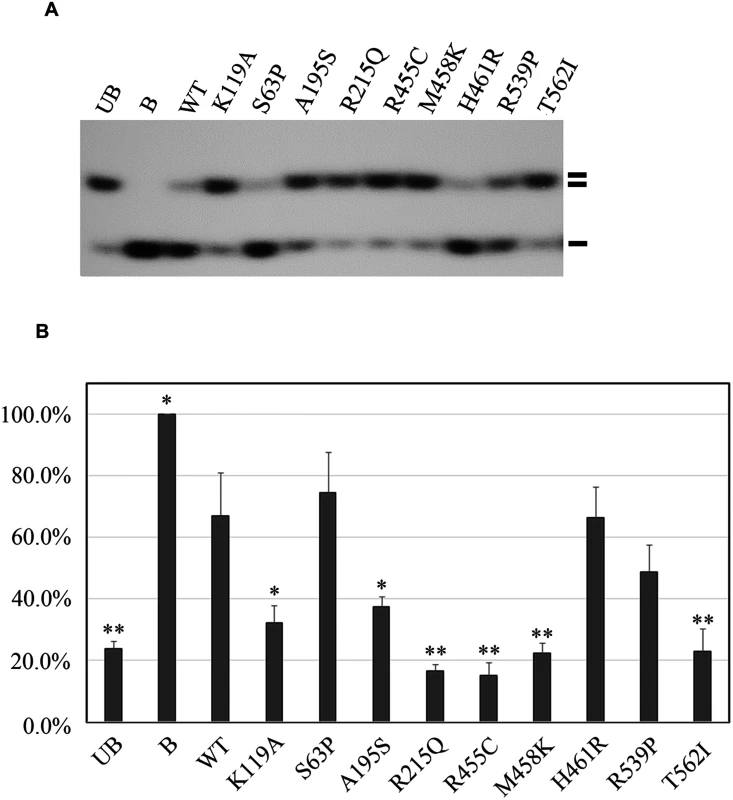
(A) helicase activity of the eight RECQL missense mutants. The helicase-defective K119A mutant was included as a negative control. B, boiled dsDNA; UB, unboiled dsDNA; WT, wild type; double lines, dsDNA; single lines, ssDNA. (B) Quantitative analysis of the helicase assay of RECQL mutants. Helicase data represent the mean of three independent experiments with the mean±S.D. indicated by error bars. The percent unwinding of mutants were compared to the WT level. The results of an unpaired, two-tailed t-test for the mutants are shown. *p < 0.05; **p <0.01. In total, nine germline mutations in the RECQL gene in the nine familial breast cancer patients were identified as pathogenic, including three nonsense mutations (L128X, W172X and Q266X), one splice-site mutation (395-2A>G) and five missense mutations (A195S, R215Q, R455C, M458K and T562) (Table 1 and Fig 3). The pedigrees of the nine families are present in Fig 1B and S4 Fig. The average number of the breast cancer cases in the nine families were 2.8 cases/per family, with a mean age of onset of 47.8 years. The overall frequency of the RECQL germline mutations in the 448 familial breast cancer patients without BRCA1/2mutations was 2.0% (9 of 448). One pathogenic mutation R455C was found in the 1,588 controls. The prevalence of the RECQL germline mutations was significantly higher in familial breast cancer patients than in the controls (9/448 vs. 1/1,588; the Fisher exact test, P = 9.14×10–6).
Fig. 3. Schematic representation of RECQL pathogenic mutations. 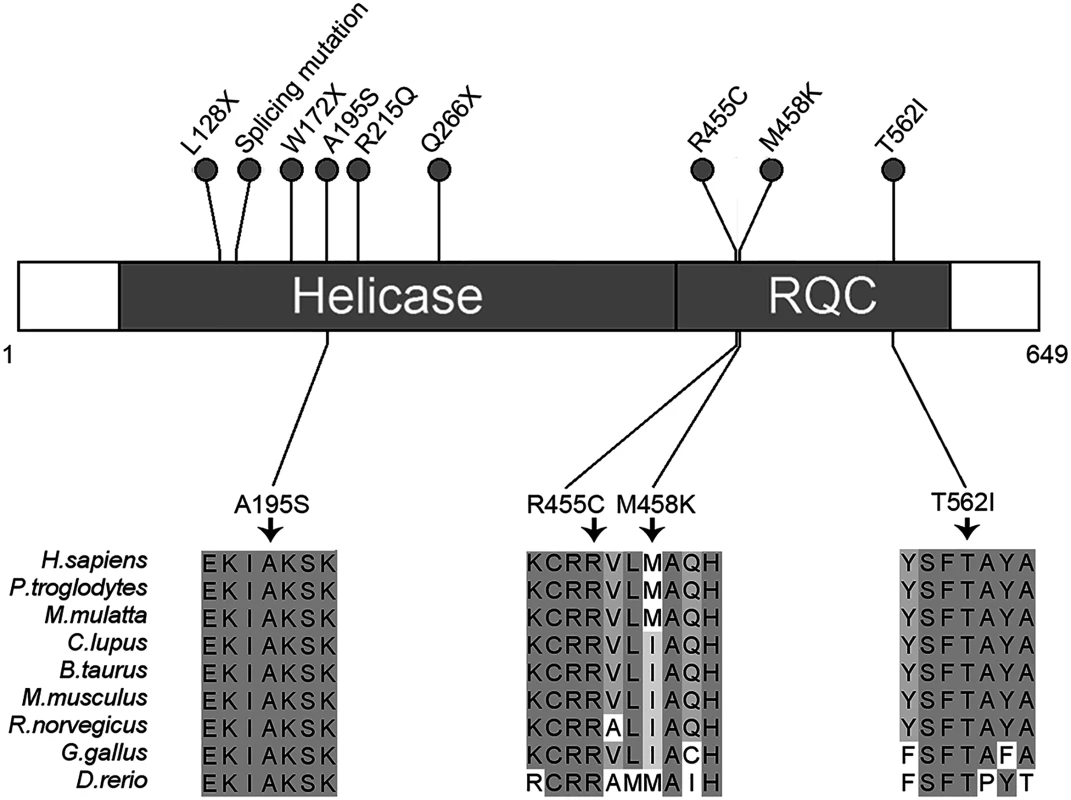
Two domains of RECQL are shown: The helicase domain (amino acids 63–418) and the RQC domain (amino acids 419–592). The nonsense, missense and splicing mutations are designated with a circle at the representative protein position. The splicing mutation is referred to as RECQL c.395-2A>G. The bottom part of the figure shows the four highly conserved mutations. Higher percentage identity at a given amino acid position is indicated. We then analyzed the clinical information of the nine cases with RECQL pathogenic mutations (S4 Table). The mean age at diagnosis of breast cancer in the nine cases with RECQL mutation was younger than in those without RECQL mutation (45.1vs.51.3 years; P = 0.12). The hormone receptor and HER2 status were available for all the nine cases: eight and five were positive for the estrogen and progesterone receptors (ER and PR), respectively. Seven cases had a HER2 negative tumor, and two had a positive tumor (S4 Table). These results indicated that RECQL-associated breast cancer was similar to those of BRCA2-associated breast cancer.
To test whether loss of heterozygosity (LOH) of RECQL occurred in the nine index cases who carried a pathogenic mutation, we performed the LOH assay in five index cases in which matched fresh tumor tissues and blood samples were available. As a result, no LOH was observed in the five cases (S5 Fig and Table 1).
Discussion
The RECQL gene is located on chromosome 12p12 and encodes a protein of 649 amino-acids. It contains two important domains, the helicase domain (residues 63–418) and the RecQ carboxy-terminal (RQC) domain (residues 419–592)[7]. These domains are highly conserved in the RecQ family and are essential for helicase activity.
Five RecQ helicase proteins (named RECQL, BLM, WRN, RECQL4 and RECQL5) in humans are highly conserved and are considered to be genome caretakers that suppress neoplastic transformation[13]. Although no hereditary disease has been linked with the RECQL gene to date, mutations in three of five RecQ genes, BLM, WRN and RECQ4, lead to Bloom, Werner, and Rothmund-Thomson syndromes, respectively, and are associated with cancer predisposition and/or premature aging. One recent study suggested that germline mutations in the BLM gene cause susceptibility to breast cancer, although the mutations are quite rare[14]. Another study indicated that RECQL5 polymorphisms are associated with an increased breast cancer risk in Chinese population[15].
Increasing evidence suggests that RECQL is involved in DNA double-strand break repair through the homologous recombination (HR) pathway[16]. RECQL-deficient cells or knockout mice exhibited chromosomal instability, sensitivity to ionizing radiation, and increased DNA damage, suggesting that RECQL plays an important role in the maintenance of genomic stability[17,18].
In this study, nine patients carried a pathogenic mutation in the RECQL gene. All of the pathogenic mutations are mapped to the above mentioned domains. The three nonsense mutations (L128X, W172X and Q266X) and one splice-site mutation (395–2 A>G) resulted in a premature truncated protein and lost the helicase activity; the five missense mutations (A195S, R215Q, R455C, M458K and T562I) were also confirmed to disrupt the function of helicase activity through a functional analysis. Therefore, mutations in the RECQL gene can lead to breast cancer tumorigenesis. In addition, no LOH was found in the RECQL mutation carriers, suggesting that RECQL-associated tumorigenesis may be through RECQL haploinsufficiency.
Here, we are the first to report that RECQL is a potential breast cancer susceptibility gene and that germline mutations in the RECQL gene are associated with predisposition to breast cancer. The 2.0% pathogenic mutation rate of the RECQL gene in familial breast cancer patients is remarkable and may be suitable for screening the mutations in BRCA1/2- negative breast cancer patients.
Materials and Methods
Study subjects
A total of 514 familial breast cancer patients were treated at the Breast Center, Peking University Cancer Hospital from 2003 to 2011. Among these, 448 index cases were negative for BRCA1/2 germline mutations. To maximize the chance of identifying a novel breast cancer susceptibility gene, 9 unrelated early-onset familial breast cancer patients were selected from the pool of 448 cases and were subjected to whole-exome sequencing (S1 Table). These nine cases were diagnosed with breast cancer at or before the age of 35 and had at least one first-degree relative affected with breast cancer. The remaining 439 unrelated familial breast cancer patients were used to screen the potential susceptibility gene. A total of 1,588 unrelated healthy women served as controls. Approximately 95% of familial breast cancer cases and controls are ethnic Chinese Han and reside in the northern region of China. The healthy controls were age-matched to cases. Genomic DNA was extracted from peripheral blood using standard protocols for all study subjects. This study was reviewed and approved by the Ethics Committee of Peking University Cancer Hospital (project No. 2011KT12). Informed written consent was obtained from all participants.
Whole-exome sequencing
Three micrograms of genomic DNA extracted from each blood sample was enriched for exonic regions using the SureSelect Biotinylated RNA Library (BAITS). The sequences of captured libraries were generated as 90-bp pair-end reads on an Illumina Hiseq2000. Exome capture and sequencing resulted in a minimum coverage of 10× for at least 91.3% of the capture target regions, and whole exomes were sequenced to an average mapped coverage of 108×.
Bioinformatics analysis and variant filtration
Sequencing reads were mapped to the reference GRCh37/hg19 human genome assembly using the Burrows-Wheeler Aligner(BWA)[19]. Further processing, including duplicate removal, local realignment and base quality recalibration, was performed using Picard and GATK. Single nucleotide variants (SNVs) and indels were detected by SOAPsnp[20]and SAMtools, respectively. Then, filters were applied to obtain variant results of higher confidence. We then used ANNOVAR[21]to perform annotation and classification. The variant collection was excluded from positions found in the dbSNP 132 and 1000 Genomes databases. Only genes that harbored heterozygous variants that were truncating mutations or splice-site variants were selected for further analyses. Candidate disease-causing variants were filtered for variants affecting the same gene in at least two samples using a straightforward criterion (S2 Table). SNV and indel data were analyzed separately.
RECQL mutation screening
After the filtering process, the variants in the RECQL gene and other potential genes found in the whole-exome sequencing assay were tested by Sanger sequencing using standard methods (S3 Table). We designed a set of 14 pairs of primers (S5 Table) to screen the entire coding regions of the RECQL gene in an additional 439 unrelated familial breast cancer patients and 1,588 healthy controls. The purified products were then sequenced on an ABI 3730 automated sequencer (Applied Biosystems). All mutations were confirmed in duplicate.
RT-PCR
Total RNA was isolated from the blood samples carrying the RECQL c.395-2A>G mutation. Then, 2.3 μg of total RNA was transcribed to cDNA by the Superscript II Reverse Transcriptase (Invitrogen) using a random primer. PCR was then carried out using the primer pair spanning exons 3–7 of RECQL transcript 001 (ENST00000444129). The PCR products were separated on a 2% agarose gel by electrophoresis. The DNA fragments were re-amplified with 30 cycles, visualized and directly sequenced after gel extraction. cDNA from the blood samples of a healthy volunteer were used as the control. The analysis of c.868-12_868-11del mutation was carried out using the primer pair spanning exons 6–11 (S5 Table).
Protein alignment and structural modeling
Multiple-sequence alignments were generated for homologous RECQL protein sequences using Clustal Omega. Jalview was used to visualize and format the alignment. Mutations in human RECQL were visualized using PyMOL on the crystal structure of the human RECQL protein (RCSB PDB, 2V1X) and the structure of this helicase in complex with a DNA substrate (RCSB PDB, 2WWY).
Cloning, protein purification, and helicase assay
cDNA encoding full-length RECQL was cloned into the pGEX-4T-1 plasmid to be expressed as a GST fusion protein. Eight missense mutations (S63P, A195S, R215Q, R455C, M458K, H461R, R539P and T562I) were introduced by site-directed mutagenesis and confirmed by sequencing. A helicase-defective mutant K119A was produced as well and served as a negative control. Recombinant proteins were purified from BL21(DE3)-RIL cells (Stratagene) using glutathione Sepharose columns (GE Healthcare) in a buffer containing 50mM Tris-HCl (pH 7.0), 250mM NaCl, 5mM β-mercaptoethanol and concentrated to 0.2 mg/ml in another buffer containing 50mM Tris-HCl (pH 7.0), 250mM NaCl, 5mMβ-mercaptoethanol and 20% glycerol. The proteins were assayed for helicase activity, detected by the displacement of a 32P-5’labeled 45mer oligonucleotide from a 44mer/45mer partial DNA duplex as previously described [12]. The unwinding of the substrate DNA was detected by autoradiography and quantified by Quantity One (Bio-Rad Laboratories, Inc.). The helicase data represent the mean of three independent experiments with the mean ± S.D. indicated by error bars.
Loss-of-heterozygosity (LOH)
Of the nine cases who carried a deleterious mutation in the RECQL gene, the fresh tumor tissues and blood samples were available for five cases, LOH analysis of the RECQL locus was carried out for these five cases. We specifically amplified the tumor DNA fragments using the same PCR conditions that we applied for germline DNA. Then, we directly sequenced them with an ABI 3730 automated sequencer (Applied Biosystems) and compared them to sequencing results of heterozygous germline DNA (S5 Fig).
Accession codes
The following GenBank reference sequences were used for variant annotation: RECQL, NM_002907, TTLL2, NM_031949 andVSIG2, NM_014312.
Web resources
1000 Genomes Project,http://browser.1000genomes.org/; dbSNP database, http://www.ncbi.nlm.nih.gov/projects/SNP/; Ensembl, http://www.ensembl.org/index.html; Picard, http://picard.sourceforge.net; GATK,http://www.broadinstitute.org/gatk/;SAMtools, http://samtools.sourceforge.net/;Clustal Omega, http://www.ebi.ac.uk/Tools/msa/clustalo/; Jalview, http://www.jalview.org/; PyMOL, http://www.pymol.org/; The Protein Data Bank, http://www.rcsb.org/pdb/.
Supporting Information
Zdroje
1. Narod SA (2002) Modifiers of risk of hereditary breast and ovarian cancer. Nat Rev Cancer 2 : 113–123. 12635174
2. Meijers-Heijboer H, van den Ouweland A, Klijn J, Wasielewski M, de Snoo A, et al. (2002) Low-penetrance susceptibility to breast cancer due to CHEK2(*)1100delC in noncarriers of BRCA1 or BRCA2 mutations. Nat Genet 31 : 55–59. 11967536
3. Renwick A, Thompson D, Seal S, Kelly P, Chagtai T, et al. (2006) ATM mutations that cause ataxia-telangiectasia are breast cancer susceptibility alleles. Nat Genet 38 : 873–875. 16832357
4. Seal S, Thompson D, Renwick A, Elliott A, Kelly P, et al. (2006) Truncating mutations in the Fanconi anemia J gene BRIP1 are low-penetrance breast cancer susceptibility alleles. Nat Genet 38 : 1239–1241. 17033622
5. Rahman N, Seal S, Thompson D, Kelly P, Renwick A, et al. (2007) PALB2, which encodes a BRCA2-interacting protein, is a breast cancer susceptibility gene. Nat Genet 39 : 165–167. 17200668
6. Meindl A, Hellebrand H, Wiek C, Erven V, Wappenschmidt B, et al. (2010) Germline mutations in breast and ovarian cancer pedigrees establish RAD51C as a human cancer susceptibility gene. Nat Genet 42 : 410–414. doi: 10.1038/ng.569 20400964
7. Pike AC, Shrestha B, Popuri V, Burgess-Brown N, Muzzolini L, et al. (2009) Structure of the human RECQ1 helicase reveals a putative strand-separation pin. Proc Natl Acad Sci U S A 106 : 1039–1044. doi: 10.1073/pnas.0806908106 19151156
8. Lucic B, Zhang Y, King O, Mendoza-Maldonado R, Berti M, et al. (2011) A prominent beta-hairpin structure in the winged-helix domain of RECQ1 is required for DNA unwinding and oligomer formation. Nucleic Acids Res 39 : 1703–1717. doi: 10.1093/nar/gkq1031 21059676
9. Mirzaei H, Schmidt KH (2012) Non-Bloom syndrome-associated partial and total loss-of-function variants of BLM helicase. Proc Natl Acad Sci U S A 109 : 19357–19362. doi: 10.1073/pnas.1210304109 23129629
10. Liu JL, Rigolet P, Dou SX, Wang PY, Xi XG (2004) The zinc finger motif of Escherichia coli RecQ is implicated in both DNA binding and protein folding. J Biol Chem 279 : 42794–42802. 15292213
11. Cui S, Arosio D, Doherty KM, Brosh RM Jr., Falaschi A, et al. (2004) Analysis of the unwinding activity of the dimeric RECQ1 helicase in the presence of human replication protein A. Nucleic Acids Res 32 : 2158–2170. 15096578
12. Doherty KM, Sharma S, Uzdilla LA, Wilson TM, Cui S, et al. (2005) RECQ1 helicase interacts with human mismatch repair factors that regulate genetic recombination. J Biol Chem 280 : 28085–28094. 15886194
13. Chu WK, Hickson ID (2009) RecQ helicases: multifunctional genome caretakers. Nat Rev Cancer 9 : 644–654. doi: 10.1038/nrc2682 19657341
14. Thompson ER, Doyle MA, Ryland GL, Rowley SM, Choong DY, et al. (2012) Exome sequencing identifies rare deleterious mutations in DNA repair genes FANCC and BLM as potential breast cancer susceptibility alleles. PLoS Genet 8: e1002894. doi: 10.1371/journal.pgen.1002894 23028338
15. He YJ, Qiao ZY, Gao B, Zhang XH, Wen YY (2014) Association between RECQL5 genetic polymorphisms and susceptibility to breast cancer. Tumour Biol 35 : 12201–12204. doi: 10.1007/s13277-014-2528-2 25394896
16. Wu Y, Brosh RM Jr. (2010) Distinct roles of RECQ1 in the maintenance of genomic stability. DNA Repair (Amst) 9 : 315–324. doi: 10.1016/j.dnarep.2009.12.010 20061189
17. Sharma S, Stumpo DJ, Balajee AS, Bock CB, Lansdorp PM, et al. (2007) RECQL, a member of the RecQ family of DNA helicases, suppresses chromosomal instability. Mol Cell Biol 27 : 1784–1794. 17158923
18. Sharma S, Brosh RM Jr. (2007) Human RECQ1 is a DNA damage responsive protein required for genotoxic stress resistance and suppression of sister chromatid exchanges. PLoS One 2: e1297. 18074021
19. Li H, Durbin R (2009) Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25 : 1754–1760. doi: 10.1093/bioinformatics/btp324 19451168
20. Li R, Li Y, Fang X, Yang H, Wang J, et al. (2009) SNP detection for massively parallel whole-genome resequencing. Genome Res 19 : 1124–1132. doi: 10.1101/gr.088013.108 19420381
21. Wang K, Li M, Hakonarson H (2010) ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res 38: e164. doi: 10.1093/nar/gkq603 20601685
Štítky
Genetika Reprodukční medicína
Článek Minor Type IV Collagen α5 Chain Promotes Cancer Progression through Discoidin Domain Receptor-1Článek A Unique Virulence Gene Occupies a Principal Position in Immune Evasion by the Malaria ParasiteČlánek The Centrosomal Linker and Microtubules Provide Dual Levels of Spatial Coordination of CentrosomesČlánek Dissecting the Function and Assembly of Acentriolar Microtubule Organizing Centers in Cells In VivoČlánek MicroRNA-Dependent Transcriptional Silencing of Transposable Elements in Drosophila Follicle CellsČlánek PARP2 Is the Predominant Poly(ADP-Ribose) Polymerase in Arabidopsis DNA Damage and Immune ResponsesČlánek Cooperative Action of Cdk1/cyclin B and SIRT1 Is Required for Mitotic Repression of rRNA SynthesisČlánek The Developmental Intestinal Regulator ELT-2 Controls p38-Dependent Immune Responses in Adult .Článek Feeding and Fasting Signals Converge on the LKB1-SIK3 Pathway to Regulate Lipid Metabolism in
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2015 Číslo 5
-
Všechny články tohoto čísla
- Genomic Heritability: What Is It?
- Triglyceride-Increasing Alleles Associated with Protection against Type-2 Diabetes
- Epistasis Is a Major Determinant of the Additive Genetic Variance in
- Genetic Regulation of Bone Metabolism in the Chicken: Similarities and Differences to Mammalian Systems
- Minor Type IV Collagen α5 Chain Promotes Cancer Progression through Discoidin Domain Receptor-1
- The Philosophical Approach: An Interview with Ford Doolittle
- Downregulation of the Host Gene by miR-92 Is Essential for Neuroblast Self-Renewal in
- A Unique Virulence Gene Occupies a Principal Position in Immune Evasion by the Malaria Parasite
- Y Fuse? Sex Chromosome Fusions in Fishes and Reptiles
- Regulation of Active DNA Demethylation by a Methyl-CpG-Binding Domain Protein in
- Overlapping Patterns of Rapid Evolution in the Nucleic Acid Sensors cGAS and OAS1 Suggest a Common Mechanism of Pathogen Antagonism and Escape
- Autoselection of Cytoplasmic Yeast Virus Like Elements Encoding Toxin/Antitoxin Systems Involves a Nuclear Barrier for Immunity Gene Expression
- Genetic Architecture of Abdominal Pigmentation in
- Whole Genome DNA Binding Analysis of the Bacterial Replication Initiator and Transcription Factor DnaA
- The Centrosomal Linker and Microtubules Provide Dual Levels of Spatial Coordination of Centrosomes
- Parp3 Negatively Regulates Immunoglobulin Class Switch Recombination
- Burden Analysis of Rare Microdeletions Suggests a Strong Impact of Neurodevelopmental Genes in Genetic Generalised Epilepsies
- Cell Cycle Control by the Master Regulator CtrA in
- Myopathic Lamin Mutations Cause Reductive Stress and Activate the Nrf2/Keap-1 Pathway
- Monoallelic Loss of the Imprinted Gene Promotes Tumor Formation in Irradiated Mice
- Phylum-Level Conservation of Regulatory Information in Nematodes despite Extensive Non-coding Sequence Divergence
- Clustering and Negative Feedback by Endocytosis in Planar Cell Polarity Signaling Is Modulated by Ubiquitinylation of Prickle
- Dissecting the Function and Assembly of Acentriolar Microtubule Organizing Centers in Cells In Vivo
- MicroRNA-Dependent Transcriptional Silencing of Transposable Elements in Drosophila Follicle Cells
- β-Catenin Signaling Biases Multipotent Lingual Epithelial Progenitors to Differentiate and Acquire Specific Taste Cell Fates
- A Simple Auxin Transcriptional Response System Regulates Multiple Morphogenetic Processes in the Liverwort
- Parallel Gene Expression Differences between Low and High Latitude Populations of and .
- The Nutrient-Responsive Hormone CCHamide-2 Controls Growth by Regulating Insulin-like Peptides in the Brain of
- Characterization of TCF21 Downstream Target Regions Identifies a Transcriptional Network Linking Multiple Independent Coronary Artery Disease Loci
- PARP2 Is the Predominant Poly(ADP-Ribose) Polymerase in Arabidopsis DNA Damage and Immune Responses
- Drosophila Spaghetti and Doubletime Link the Circadian Clock and Light to Caspases, Apoptosis and Tauopathy
- Coronary Artery Disease Associated Transcription Factor TCF21 Regulates Smooth Muscle Precursor Cells That Contribute to the Fibrous Cap
- Rescue of DNA-PK Signaling and T-Cell Differentiation by Targeted Genome Editing in a Deficient iPSC Disease Model
- Disruption of Transcriptional Coactivator Sub1 Leads to Genome-Wide Re-distribution of Clustered Mutations Induced by APOBEC in Active Yeast Genes
- Yeast Killer Elements Hold Their Hosts Hostage
- Keeping in Shape the Dogma of Mitochondrial DNA Maternal Inheritance
- Extreme-Depth Re-sequencing of Mitochondrial DNA Finds No Evidence of Paternal Transmission in Humans
- Trading Places—Switching Frataxin Function by a Single Amino Acid Substitution within the [Fe-S] Cluster Assembly Scaffold
- Natural Variation Identifies , a Universal Gene Required for Cell Proliferation and Growth at High Temperatures in
- Mutations in Gene Are Associated with Predisposition to Breast Cancer
- The Whole of a Scientific Career: An Interview with Oliver Smithies
- Cell Specific eQTL Analysis without Sorting Cells
- Cooperative Action of Cdk1/cyclin B and SIRT1 Is Required for Mitotic Repression of rRNA Synthesis
- Systemic Regulation of RAS/MAPK Signaling by the Serotonin Metabolite 5-HIAA
- Reprogramming LCLs to iPSCs Results in Recovery of Donor-Specific Gene Expression Signature
- The Developmental Intestinal Regulator ELT-2 Controls p38-Dependent Immune Responses in Adult .
- Genetic Mechanism of Human Neutrophil Antigen 2 Deficiency and Expression Variations
- Feeding and Fasting Signals Converge on the LKB1-SIK3 Pathway to Regulate Lipid Metabolism in
- Early Lineage Priming by Trisomy of Leads to Myeloproliferation in a Down Syndrome Model
- Turning into a Frataxin-Dependent Organism
- Accounting for Experimental Noise Reveals That mRNA Levels, Amplified by Post-Transcriptional Processes, Largely Determine Steady-State Protein Levels in Yeast
- Biological Significance of Photoreceptor Photocycle Length: VIVID Photocycle Governs the Dynamic VIVID-White Collar Complex Pool Mediating Photo-adaptation and Response to Changes in Light Intensity
- CTXφ Replication Depends on the Histone-Like HU Protein and the UvrD Helicase
- Disruption of miR-29 Leads to Aberrant Differentiation of Smooth Muscle Cells Selectively Associated with Distal Lung Vasculature
- Notch Is Required in Adult Sensory Neurons for Morphological and Functional Plasticity of the Olfactory Circuit
- Post-transcriptional Regulation of Keratinocyte Progenitor Cell Expansion, Differentiation and Hair Follicle Regression by
- PERK Limits Lifespan by Promoting Intestinal Stem Cell Proliferation in Response to ER Stress
- Casein Kinase 1 and Phosphorylation of Cohesin Subunit Rec11 (SA3) Promote Meiotic Recombination through Linear Element Formation
- Fibroblast Growth Factor 9 Regulation by MicroRNAs Controls Lung Development and Links Loss to the Pathogenesis of Pleuropulmonary Blastoma
- Auxin-Mediated Transcriptional System with a Minimal Set of Components Is Critical for Morphogenesis through the Life Cycle in
- The Broad-Spectrum Antiviral Protein ZAP Restricts Human Retrotransposition
- The 4E-BP Caf20p Mediates Both eIF4E-Dependent and Independent Repression of Translation
- Turning into a Frataxin-Independent Organism
- Promotion of Bone Morphogenetic Protein Signaling by Tetraspanins and Glycosphingolipids
- Essential Role of the ESX-5 Secretion System in Outer Membrane Permeability of Pathogenic Mycobacteria
- The Zinc-Finger Antiviral Protein ZAP Inhibits LINE and Alu Retrotransposition
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Drosophila Spaghetti and Doubletime Link the Circadian Clock and Light to Caspases, Apoptosis and Tauopathy
- Autoselection of Cytoplasmic Yeast Virus Like Elements Encoding Toxin/Antitoxin Systems Involves a Nuclear Barrier for Immunity Gene Expression
- Parp3 Negatively Regulates Immunoglobulin Class Switch Recombination
- PERK Limits Lifespan by Promoting Intestinal Stem Cell Proliferation in Response to ER Stress
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



