-
Články
Reklama
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
ReklamaRobust Antigen Specific Th17 T Cell Response to Group A Streptococcus Is Dependent on IL-6 and Intranasal Route of Infection
Group A streptococcus (GAS, Streptococcus pyogenes) is the cause of a variety of clinical conditions, ranging from pharyngitis to autoimmune disease. Peptide-major histocompatibility complex class II (pMHCII) tetramers have recently emerged as a highly sensitive means to quantify pMHCII-specific CD4+ helper T cells and evaluate their contribution to both protective immunity and autoimmune complications induced by specific bacterial pathogens. In lieu of identifying an immunodominant peptide expressed by GAS, a surrogate peptide (2W) was fused to the highly expressed M1 protein on the surface of GAS to allow in-depth analysis of the CD4+ helper T cell response in C57BL/6 mice that express the I-Ab MHCII molecule. Following intranasal inoculation with GAS-2W, antigen-experienced 2W:I-Ab-specific CD4+ T cells were identified in the nasal-associated lymphoid tissue (NALT) that produced IL-17A or IL-17A and IFN-γ if infection was recurrent. The dominant Th17 response was also dependent on the intranasal route of inoculation; intravenous or subcutaneous inoculations produced primarily IFN-γ+ 2W:I-Ab+ CD4+ T cells. The acquisition of IL-17A production by 2W:I-Ab-specific T cells and the capacity of mice to survive infection depended on the innate cytokine IL-6. IL-6-deficient mice that survived infection became long-term carriers despite the presence of abundant IFN-γ-producing 2W:I-Ab-specific CD4+ T cells. Our results suggest that an imbalance between IL-17 - and IFN-γ-producing CD4+ T cells could contribute to GAS carriage in humans.
Published in the journal: . PLoS Pathog 7(9): e32767. doi:10.1371/journal.ppat.1002252
Category: Research Article
doi: https://doi.org/10.1371/journal.ppat.1002252Summary
Group A streptococcus (GAS, Streptococcus pyogenes) is the cause of a variety of clinical conditions, ranging from pharyngitis to autoimmune disease. Peptide-major histocompatibility complex class II (pMHCII) tetramers have recently emerged as a highly sensitive means to quantify pMHCII-specific CD4+ helper T cells and evaluate their contribution to both protective immunity and autoimmune complications induced by specific bacterial pathogens. In lieu of identifying an immunodominant peptide expressed by GAS, a surrogate peptide (2W) was fused to the highly expressed M1 protein on the surface of GAS to allow in-depth analysis of the CD4+ helper T cell response in C57BL/6 mice that express the I-Ab MHCII molecule. Following intranasal inoculation with GAS-2W, antigen-experienced 2W:I-Ab-specific CD4+ T cells were identified in the nasal-associated lymphoid tissue (NALT) that produced IL-17A or IL-17A and IFN-γ if infection was recurrent. The dominant Th17 response was also dependent on the intranasal route of inoculation; intravenous or subcutaneous inoculations produced primarily IFN-γ+ 2W:I-Ab+ CD4+ T cells. The acquisition of IL-17A production by 2W:I-Ab-specific T cells and the capacity of mice to survive infection depended on the innate cytokine IL-6. IL-6-deficient mice that survived infection became long-term carriers despite the presence of abundant IFN-γ-producing 2W:I-Ab-specific CD4+ T cells. Our results suggest that an imbalance between IL-17 - and IFN-γ-producing CD4+ T cells could contribute to GAS carriage in humans.
Introduction
Group A streptococcus (GAS, Streptococcus pyogenes) is an important bacterial pathogen that causes many different clinical conditions ranging from pharyngitis, impetigo, toxic shock syndrome, necrotizing fasciitis to post infectious autoimmune sequelae like acute rheumatic fever and glomerulonephritis [1], [2]. GAS is an important cause of morbidity and mortality worldwide. Estimates are that 500,000 deaths occur each year due to severe GAS infections [3]. GAS produces a wide variety of virulence factors that play important roles in adhesion, immune evasion, dissemination, tissue destruction and toxicity [1], [4]. Remarkably, one third of those treated with antibiotics continue to shed streptococci and a significant number of these carriers have recurrent disease by the same strain [5], [6]. Carrier state is an important public health problem because it maintains the cycle of disease in a community. Tonsils are known to harbor and shed streptococci, even after intense antibiotic therapy. Osterlund et al found that 93% of tonsils excised from children retained intracellular GAS, and others reported isolation of GAS from excised tonsils, confirming that this secondary lymphoid tissue is an important reservoir [7].
The capacity of GAS to survive in lymphoid tissue is not understood. Although GAS induces a humoral immune response in most individuals, streptococci can still persist. GAS also induces a CD4+ T cell response [8]. CD4+ T cells express T cell antigen receptors (TCR) that recognize short peptides bound to MHCII molecules (pMHCII) expressed by host antigen-presenting cells. During primary infection, the rare naïve CD4+ T cells, which by chance express TCRs complementary to bacterial pMHCII complexes, proliferate and differentiate into Th1, Th2, or Th17 effector cells that produce cytokines such as IFN-γ, IL-4 or IL-17, respectively, which help to eliminate the pathogen. Which of these effector cell types will develop during infection is determined by cytokines produced by cells of the innate immune system. For example in mice, the differentiation of Th17 cells in vitro depends on IL-6 and Transforming Growth Factor-beta (TGF-β) [9]. We recently demonstrated that GAS infection of the NALT, the murine equivalent of the tonsils, generates Th17 cells, which contribute to immune protection [10]. Lu et al have shown that Th17 cells protect against other streptococcal infections as well [11].
Previous studies used adoptive transfer of monoclonal TCR transgenic T cells to study T cell responses to infection with antigen tagged GAS [8], [12]. A drawback of this approach is that it results in an abnormally large number of naïve precursors, which experience inefficient activation due to competition for limiting pMHCII [13]-[15]. Therefore, the nature of the CD4+ T cell response to GAS infection under physiological conditions is still unknown.
To avoid these limitations, we used a new cell enrichment method based on fluorochrome-labeled pMHCII tetramers and magnetic beads [16] to characterize the endogenous polyclonal CD4+ T cell response to GAS. This approach depended on the availability of a tetramer of the I-Ab MHCII molecule of the preferred C57BL/6 mouse strain bound to a peptide from the GAS proteome. Unfortunately, no such peptide has been identified to date. We therefore produced a recombinant GAS strain that expresses an immunogenic peptide (EAWGALANWAVDSA) called 2W [17] fused to the M1 protein on its surface. 2W:I-Ab tetramer staining and magnetic bead enrichment was used to characterize 2W:I-Ab+ CD4+ T cells from NALT and other lymphoid tissues after intranasal GAS-2W infection. Our results demonstrate that an intranasal infection is critical for mounting an effective IL-6-dependent pMHCII-specific Th17 response. A lack of this response led to a preponderance of Th1 cells and failure to control GAS infection. This work defines the Th17 response to GAS infection, and may shed light on the basis for the carrier state and autoimmune complications in humans.
Results
Generation of recombinant GAS strain that expresses the M1-2W fusion protein
An M1 GAS strain 90–226 was genetically engineered to express the 14 amino acid 2W peptide (EAWGALANWAVDSA) as a cell wall surface hybrid M1 fusion protein. The hybrid emm1.0::2W gene was constructed in plasmid pFW5 in E. coli and then introduced into the chromosomal emm1.0 gene by allelic replacement [18]. The corresponding chimeric protein is composed of the 14 amino acid 2W peptide inserted in frame after the first five amino acids of the mature M1 surface protein (Fig. 1A). The strain, designated 90–226 emm1.0::2W (GAS-2W), is genetically stable without spectinomycin selection due to replacement of the wild-type gene in the chromosome. The anti-phagocytic property of the M1-2W fusion protein was assessed to test whether insertion of the 2W peptide altered the function of the M protein. GAS-2W was as resistant to phagocytosis as the wild-type 90-226 in whole blood bactericidal assays (Fig. 1B). An M- variant 90-226 emm1.0::km (GAS-ΔM) [18] was included as a negative control and was susceptible to phagocytic killing as expected. Furthermore, the GAS-2W strain colonized mouse NALT as efficiently as the wild type (Fig. 1C).
Fig. 1. Construction of a Group A Streptococcus 90-226 strain that expresses the 2W (GAS-2W). 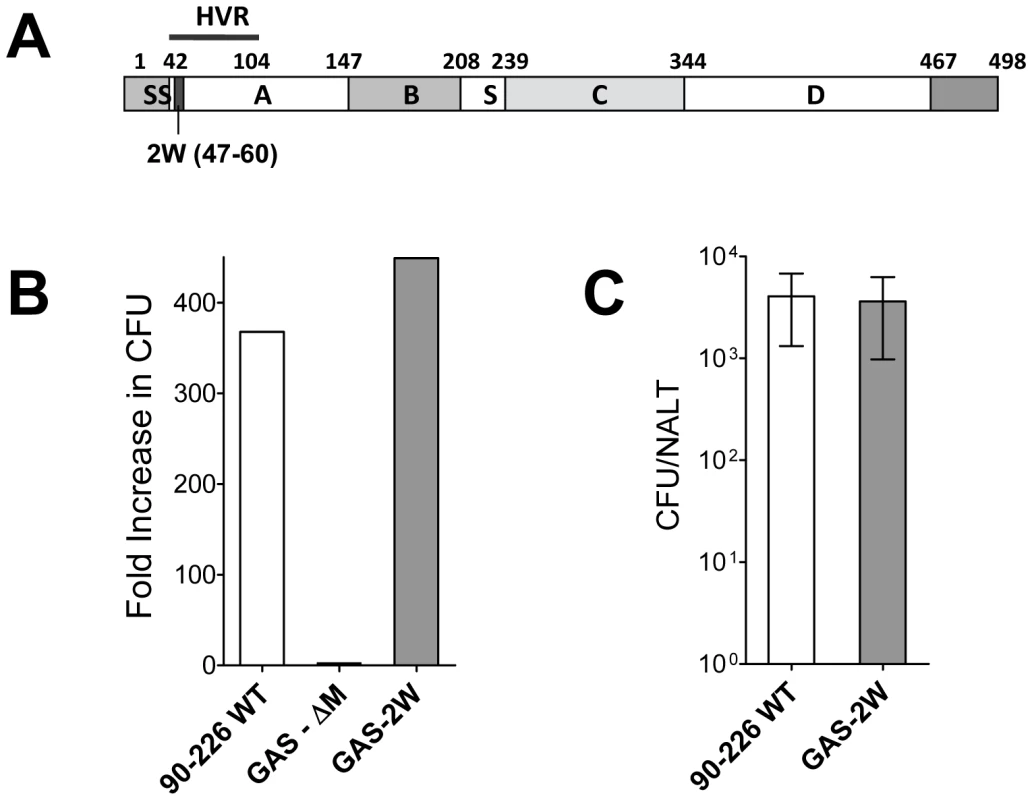
(A) Schematic diagram of emm1.0::2W fusion protein that is expressed on the surface of GAS-2W. SS-Signal sequence; A - A region; B - B repeats; S - spacer region; C – C repeats; D – D region; HVR – Hyper-variable region; Cell wall anchor (466-496) (B) Survival and growth of strain GAS-2W in phagocytic human blood. 90-226 WT is the parent of GAS-2W and the M protein negative mutant GAS-ΔM. Fold increase was calculated as the number of CFU after 3 h in rotated blood divided by the initial number of CFU. Representative result from one of two independent experiments is shown. (C) Colonization of mouse NALT by intranasally administered Streptococci. Viable counts of 90-226-WT or GAS-2W streptococci recovered from homogenized NALT 24h following inoculation with 2×108 CFU. The bars represent the mean ± SEM of four mice per group. Intranasal infection with GAS-2W induced a strong antigen-specific Th17 response in C57BL/6 mice
Spleen and lymph node cells from mice were pooled for quantification of 2W:I-Ab-specific CD4+ T cells by pMHCII-based cell enrichment at various times after infection with GAS-2W. Each C57BL/6 mouse had between 200 and 250 2W:I-Ab-specific CD4+ T cells before infection, and these cells expressed small amounts of CD44 (CD44Lo) on the surface as expected for naïve cells (Fig. 2A) [16], [19]. In mice that were inoculated intranasally with GAS-2W, this naïve 2W:I-Ab-specific CD4+ T cell population expanded to ∼105 cells in seven days (Fig. 2A). Most of these cells expressed large amounts of CD44 (CD44Hi), indicative of pMHCII-dependent activation. Expansion of 2W:I-Ab-specific CD4+ T cells was first detected three days after primary infection with GAS-2W and increased rapidly to a peak seven days after inoculation (Fig. 2B). 2W:I-Ab-specific CD4+ T cells then began to decline and reached 10% of the maximum level by day 20-post infection. Expansion of these CD4+ T cells was proportional to the dose of GAS-2W used for infection (Fig. 2C).
Fig. 2. 2W:I-Ab-specific naïve CD4+ T cells expanded in response to intranasal infection with GAS-2W. 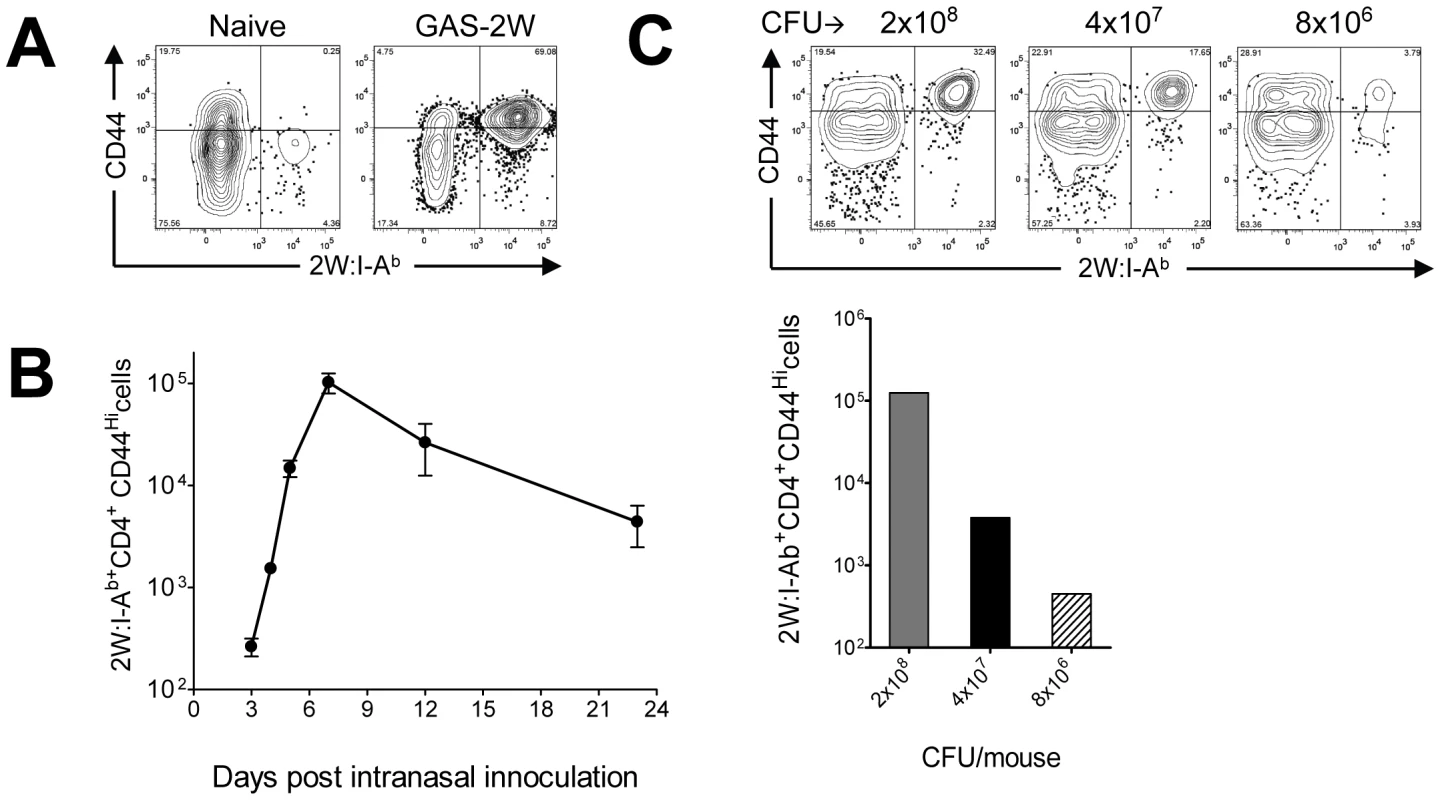
(A) B6 mice were infected with GAS-2W (intranasal, 2×108 CFU). Seven days later CD4+2W:I-Ab+ cells were enriched from cervical lymph nodes and spleens and analyzed by flow cytometry. One representative of at least ten independent experiments is shown. CD44 indicates the number of antigen experienced CD4+ T cells (B) Change in the total number of 2W:I-Ab-specific CD4+ T cells after a primary infection over time (n = 28). (C) Mean number of antigen specific CD4+2W:I-Ab+ T cells is dose dependent. B6 mice were inoculated intranasally with 2×108, 4×107 and 8×106 CFU/mouse, respectively. Seven days after infection CD4+2W:I-Ab+ cells were isolated from cervical lymph nodes and spleen, and analyzed. In vivo lymphokine production was measured by rechallenging mice with heat-killed GAS-2W (HK-GAS-2W) and then measuring intracellular lymphokines produced by 2W:I-Ab-specific T cells 3 hours later [20]. 2W:I-Ab+ cells in mice infected intranasally with live GAS-2W one week earlier produced IL-17A, but not IFN-γ after challenge with HK-GAS-2W (Fig. 3A). 2W:I-Ab+ cells in mice inoculated intranasally with HK-GAS-2W one week earlier also produced IL-17A after challenge with HK-GAS-2W (Fig. 3B). Therefore, exposure of NALT to either live or dead GAS induced bacterial pMHCII-specific Th17 cells. In order to compare the approximate percentage of 2W:I-Ab-specific Th17 cells to total GAS induced Th17 cells, B6 Mice were inoculated once intranasally with 2x108 CFU of GAS-2W. Ten days after the infection mice were restimulated in vivo by IV injection of heat killed GAS-2W. 2W:I-Ab+ CD4+ T cells from spleen were stained and enriched as described in the methods. Both bound (2W:I-Ab+ CD4+ T cells) and unbound (2W:I-Ab- CD4+ T cells or flow through) fractions were collected and analyzed for intracellular cytokines IL-17A and IFN-γ. Total numbers of CD4+2W:I-Ab+IL-17A+ and CD4+2W:I-Ab-IL-17A+ cells were calculated for the entire spleen. The approximate ratio of 2W:I-Ab+IL-17A+ cells to total 2W:I-Ab- IL-17A+ cells ranged from 1 : 7 to 1 : 12 (Fig S1).
Fig. 3. Primary intranasal GAS infection induces a Th17 response. 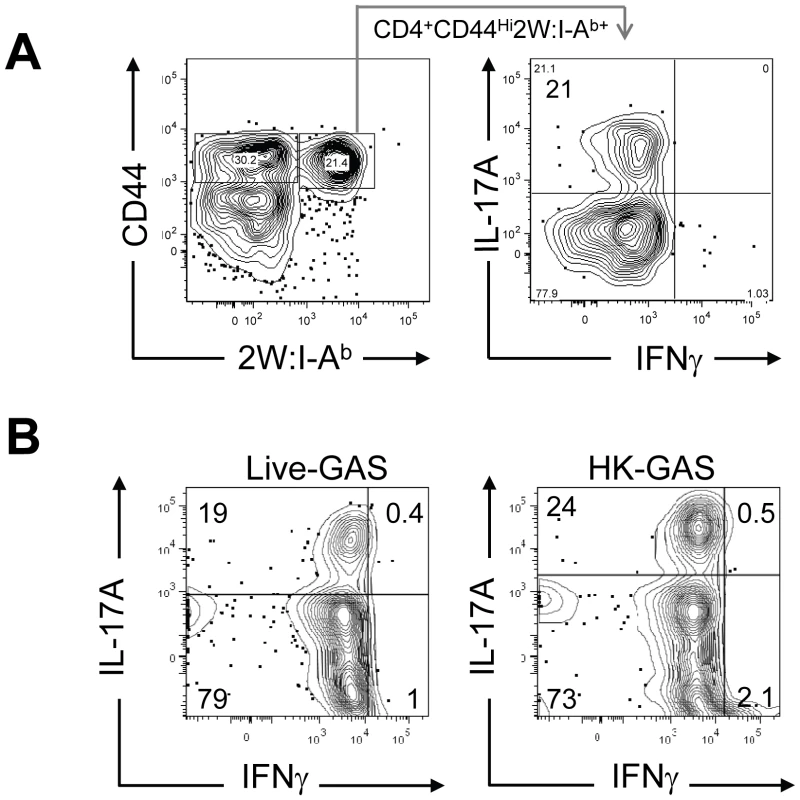
Mice were inoculated once intranasally with 2×108 CFU or equivalent numbers of heat-killed streptococci HK-GAS. (A) CD4+CD44Hi2W:I-Ab+ cells enriched from spleens of mice that were inoculated with live GAS-2W and restimulated after a week in vivo by IV injection of heat killed GAS-2W (HK-GAS-2W). (B) CD4+CD44Hi2W:I-Ab+ cells from mice that were inoculated intranasally with heat-killed GAS-2W and restimulated in vivo with HK-GAS-2W. One representative of three independent experiments is shown. Li et al reported that the Streptococcal superantigen, which contaminates commercial peptidoglycan preparations induced human lymphocytes to produce IL-17A [21]; therefore, experiments were performed to determine whether 2W:I-Ab-specific CD4+ T cell clonal expansion was pMHCII-specific. B6 mice were inoculated three times at weekly intervals with either 2×108 CFU of GAS-2W or wild type 2W- GAS (GAS-WT). Three days after the last infection, mice were euthanized and enriched CD4+2W:I-Ab+ T cells from spleen, cervical lymph nodes (CLN), and NALT were analyzed (Fig. 4.). Expansion of 2W:I-Ab-specific CD4+ T cells only occurred in mice inoculated with GAS-2W (Fig. 4A), and not in mice inoculated with GAS-WT that lacked the 2W epitope. The number of CD4+2W:I-Ab+ T cells in tissue from mice infected with GAS-WT was less than 200, similar to that of naïve mice. This indicates that the clonal expansion of 2W:I-Ab-specific CD4+ T cells is a pMHCII-specific response and not due to superantigens produced by this strain of GAS (Fig. 4A). The same samples were restimulated in vitro with pharmacologic TCR mimics, PMA and ionomycin, and stained for intracellular cytokines in order to evaluate their cytokine phenotype. Most of the 2W:I-Ab-specific CD4+ T cells from NALT, CLN and spleen produced IL-17A (Fig. 4B); greater than 75% of the 2W:I-Ab-specific CD4+ T cells from all three tissues had a Th17 phenotype (Fig. 4B). The response was more robust in NALT, which is a preferred target of intranasal GAS infection. In NALT pMHCII-specific IL-17A producers reached more than 90% of the total 2W:I-Ab-specific CD4+ T cells.
Fig. 4. Clonal expansion of 2W:I-Ab-specific CD4+ T cells and recall of IL-17A is antigen-specific and not due to Superantigens produced by GAS. 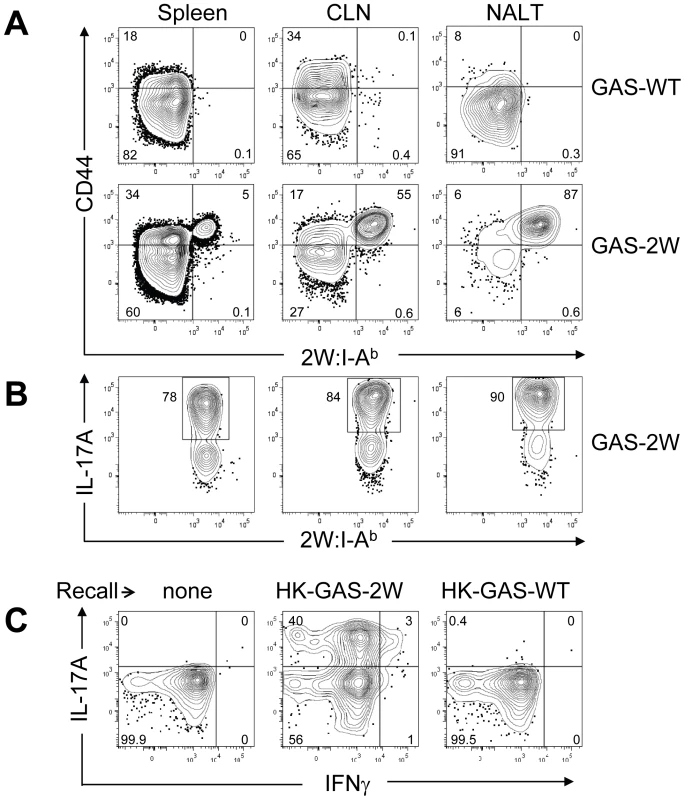
(A) Tetramer enriched CD4+ T cells from Spleen, CLN and NALT of mice after three inoculations at weekly intervals. Top panel is from wild type GAS (GAS-WT) infected B6 mice and the lower panel is from B6 mice inoculated with (GAS-2W). (B) Analysis of IL-17A expression by CD4+ CD44Hi 2W:I-Ab-specific T cells in NALT, CLN and Spleen from the lower panel of part A. (C) Analysis of the specificity of IL-17A activation in CD4+ 2W:I-Ab+ T cells originally primed by GAS-2W infection (twice). Primed mice were restimulated in vivo by IV injection of heat killed GAS-2W (HK-GAS-2W) or heat killed 2W negative GAS (HK-GAS-WT) 21 days after the last infection. CD4+CD44Hi2W:I-Ab+ cells were isolated as described in methods and analyzed for IL-17A and IFN-γ expression. Even though initial priming and expansion of CD4+2W:I-Ab+ T cells is independent of superantigen as shown above, it was possible that cytokine activation in T cells from infected mice was mediated by superantigens associated with heat-killed bacteria. To address specificity of the recall response, B6 mice that were inoculated twice with GAS-2W streptococci and then were restimulated in vivo by intravenous injection of either HK-GAS-2W or heat killed wild-type 2W-GAS (HK-GAS-WT) bacteria. CD4+CD44Hi2W:I-Ab+ cells from mice that were primed with GAS-2W had expanded significantly, produced IL-17A 21 days later following restimulation with HK-GAS-2W, but not with 2W- HK-GAS-WT bacteria (Fig. 4C). Thus both priming of CD4+ T cells and recall of cytokine expression by GAS is antigen-specific and independent of superantigens in this animal model.
Children often experience multiple episodes of GAS pharyngitis before reaching the age of 15. Therefore, we investigated whether repeated infection amplified, shifted or dampened the Th17 response. Following multiple infections, most of the CD4+CD44Hi2W:I-Ab+ T cells had elevated levels of intracellular IL-17A (Fig.4B). The response was more robust in NALT, the major infection site following intranasal GAS infection.
Route of inoculation influences the Th17 phenotype to GAS infection
Recently, Pepper et al showed that the intranasal route was important for the generation IL-17-producing 2W:I-Ab-specific T cells in response to a recombinant strain of Listeria monocytogenes that expressed the 2W peptide (LM-2W) [20]. Most GAS infections naturally involve oro-pharyngeal tissue and preferably colonize tonsils in humans and NALT in mice. Experiments were therefore performed to assess the impact of the intranasal route of inoculation of GAS on the CD4+ T cell response. B6 mice were inoculated with HK-GAS-2W intranasally, intravenously or subcutaneously. HK-GAS-2W bacteria were used to avoid spread of infection from the site of inoculation. 2W:I-Ab-specific T cells expanded in the spleens of these mice in response to all routes of inoculation. Intranasal infection with GAS-2W induced IL-17A-producing 2W:I-Ab-specific T cells; whereas intravenous and subcutaneous inoculations induced IFN-γ-producing cells (Fig. 5). The Th17/Th1 ratio was more than 40 times higher in cells from intranasally inoculated mice compared to that from mice inoculated intravenously or subcutaneously. Thus, the intranasal route of infection was critical for the generation of Th17 cells during GAS infection.
Fig. 5. Route of inoculation determines the Th17 phenotype to GAS infection. 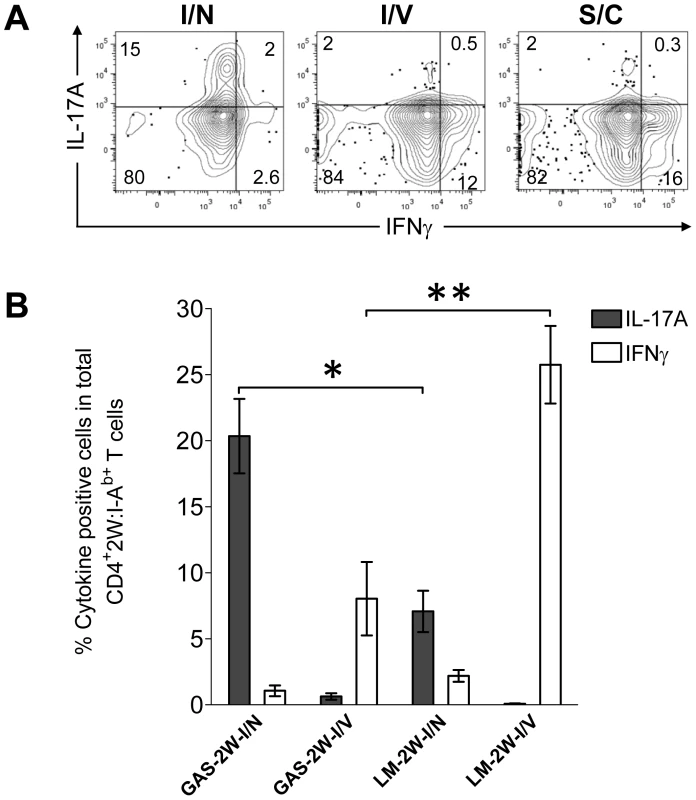
(A) B6 mice were inoculated intranasally (I/N), intravenously (I/V) or subcutaneously (S/C) with HK-GAS-2W. After one week mice were restimulated in vivo with HK-GAS-2W for 4 hours and then CD4+CD44Hi2W:I-Ab+ cells from spleen were stained for cytokines IL-17A and IFN-γ and analyzed by flow cytometry. (B) Comparison of the CD4+CD44Hi2W:I-Ab+ T cell response to GAS-2W or Listeria monocytogenes (LM-2W) intranasal and intravenous infections. Three weeks after a single intranasal or intravenous inoculation with bacteria, single cell suspensions from spleens were incubated with PMA and ionomycin for 4 hrs. CD4+CD44Hi2W:I-Ab+ cells were then enriched, stained and analyzed for IL-17A and IFN-γ expression by flow cytometry. (n = 7-12 mice per group). The bars represent the mean ± SEM. * = P<0.002, ** = P<0.001, Mann Whitney test. To compare the relative magnitude of Th17 induction by GAS and LM intranasal infections, mice were inoculated either intranasally or intravenously with either GAS-2W or LM-2W. Intranasally both GAS-2W and LM-2W induced primarily a Th17 response. Notably, however, a larger fraction of the 2W:I-Ab-specific T cell population produced IL-17A following intranasal GAS-2W infection than following intranasal LM-2W infection (Fig. 5B) [20] ; Furthermore, both GAS and LM primarily induced a Th1 response to intravenous inoculation but LM induced a significantly greater Th1 response than GAS (Fig. 5B). Therefore, the PAMPs expressed by or nature of GAS infections creates an environment more conducive to priming the Th17 phenotype than those of LM. Moreover, these data suggest that the 2W epitope does not influence the Th phenotype per se, since the same naïve 2W:I-Ab-specific CD4+ T cell population has expanded and differentiated into different phenotypes in response to alternative routes of infection.
IL-6-deficient mice fail to develop a Th17 response and to clear Group A Streptococci from NALT
Although IL-6 is known to be critical for some Th17 responses in mice, it is not known whether this is the case for streptococcal infection. We therefore compared the primary T cell response to GAS infection of B6 IL-6 knockout mice (IL-6 -/-) mice to age matched wild type B6 mice, inoculated intranasally with 2x108 cfu of GAS-2W. There was no significant difference in the colonization of both groups of mice 24 hrs after inoculation with this sublethal dose of bacteria. However, over the next 7 days nearly a third of the IL-6 -/- mice succumbed to infection (*P = 0.0473, Logrank Test) (Fig. 6). In contrast, wild-type B6 mice had no casualties and most had reduced their bacterial load in NALT. IL-6-/- mice that survived infection continued to harbor high bacterial counts for up to 60 days; whereas, all wild-type mice completely cleared the bacteria from NALT by day 7.
Fig. 6. Mice deficient in Th17 cells fail to clear primary GAS infections from NALT and become chronic carriers. 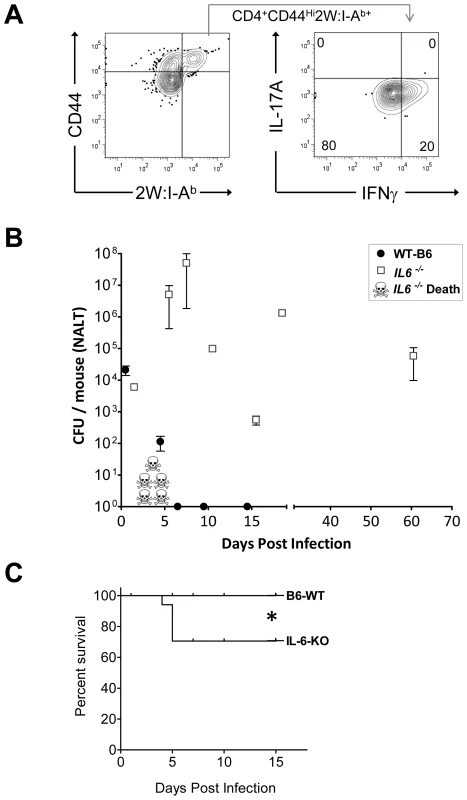
(A) Analysis of IL-17A and IFN-γ expression of CD4+CD44Hi2W:I-Ab+ T cells from IL-6 knockout mice (IL-6-/-) infected with GAS-2W. CD4+CD44Hi2W:I-Ab+ cells were enriched from spleens of IL-6-/- mice that were intranasally inoculated with HK-GAS-2W and restimulated in vivo after a week by IV injection of HK-GAS-2W. (B) Comparison of IL-6-/- mice (n = 17) to age matched wild type B6 mice (n = 14) for susceptibility to intranasal GAS infection. Mice were inoculated with 2×108 CFU/mouse intranasally. Mice were euthanized at different time points after inoculation and CFU associated with NALT were assessed. Data are presented as mean CFU ± SEM. (C) Kaplan-Meier survival curve of IL-6-/- and WT-B6 mice (*P<0.04, Logrank Test.) IL-6-/- mice that survived the first week of infection returned to health even though they retained significant numbers of GAS-2W organisms in NALT. T cells from both wild-type and IL-6-/- mice were restimulated in vivo after one week by intravenous injection of HK-GAS-2W. The 2W:I-Ab+ cells from IL-6-/- mice (Fig. 6A) had proliferated in response to infection with GAS-2W streptococci but failed to produce IL-17A. Instead, many of these cells produced IFN-γ (Fig. 6A). These results confirm that IL-6 is required to promote a Th17 response after GAS infection. Long-term persistence of GAS-2W in NALT from IL-6-/- mice is consistent with the possibility that clearance from NALT and perhaps human tonsils requires a vigorous Th17 adaptive response and that IFN-γ+ T cells lack potential to eliminate streptococci from lymphoid tissue.
Recurrent GAS infection shifts the antigen-specific population toward an IL-17A+ IFN-γ+ double positive phenotype in NALT
The Th17 phenotype is less stable than the Th1 phenotype [20], [22], [23]. For example, IL-17A+ T cells were observed to acquire the capacity to produce IFN-γ in vitro by varying the cytokine environment [24]. The regulatory program and precursor to these double positive T cells is unclear, as is the impact of infection on this phenotype. These considerations prompted experiments to test the influence of multiple intranasal infections on the Th17 population in NALT. Wild-type B6 mice usually clear GAS from NALT 5-7 days after an intranasal inoculation [25]. For recurrent infection, B6 mice were inoculated intranasally with 2×108 GAS-2W CFU at weekly intervals for 12 weeks. One week after the last inoculation cells were harvested from NALT, CLN and spleen, activated in vitro with PMA and ionomycin, and analyzed for intracellular cytokines IL-17A and IFN-γ. After repeated exposure to GAS-2W, IL-17A+ IFN-γ+ 2W:I-Ab-specific CD4+ cells preferentially accumulated in the NALT, whereas the primary phenotype of CD4+CD44Hi2W:I-Ab+ cells in spleen and CLN from the same mice retained a Th17 phenotype (Fig. 7A and 7B). More than 60% of CD4+CD44Hi2W:I-Ab+ cells were positive for both cytokines; whereas, fewer than 12% from spleens and cervical lymph nodes were double positive. The implications of this change will be discussed below.
Fig. 7. Recurrent GAS infection shifts the cytokine profile of CD4+ 2W:I-Ab+ T cells to IL-17A+, IFN-γ+ population in NALT. 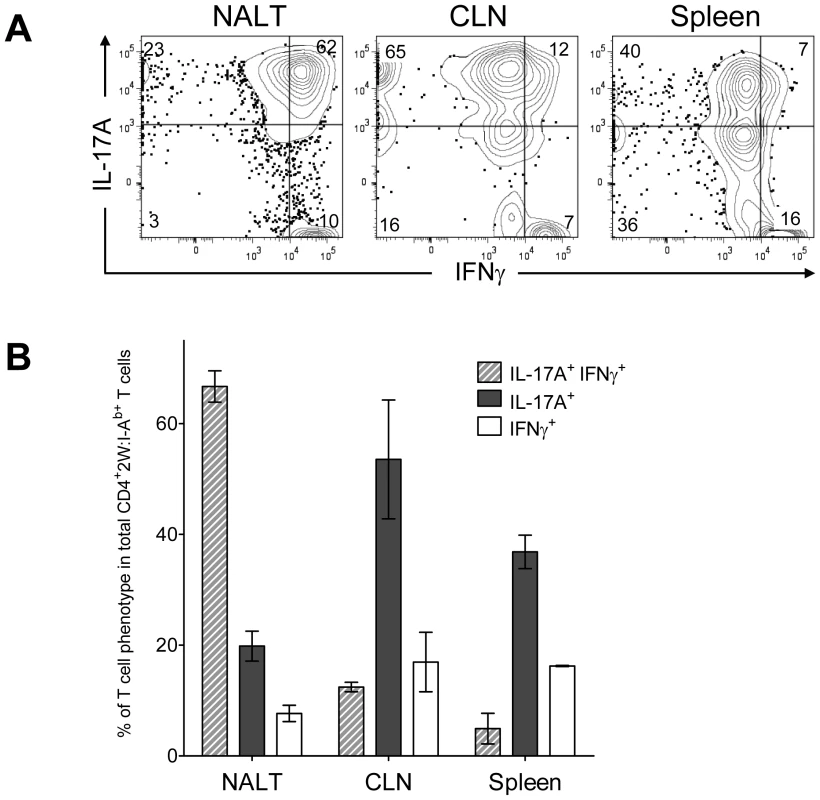
(A) B6 mice were inoculated intranasally with 2×108 CFU at weekly intervals for 12 weeks. CD4+CD44Hi2W:I-Ab+ T cells from Spleens, CLNs and NALT of infected mice were restimulated in vitro with PMA and ionomycin, stained and analyzed for IL-17A and IFN-γ and analyzed by flow cytometry. Representative result from one of two independent experiments is shown. (B) Pooled results of two independent experiments shown. (n = 4). Discussion
We recently discovered that GAS induces a polyclonal, TGF-β1 dependent Th17 response in intranasally infected mice. Moreover, adoptive transfer of CD4+ IL-17A+ T cells imparted partial protection to naïve mice against intranasal infection. That investigation raised several questions, which could be more definitively answered using pMHCII tetramers to quantify antigen specific T cells that recognize a streptococcal expressed antigen. In lieu of identifying immunodominant bacterial specific T cell epitopes, the surrogate 14 amino acid peptide 2W was fused to M1 protein and expressed on the streptococcal surface. The recombinant serotype M1 strain, GAS-2W, was constructed and M protein function confirmed by phagocytosis assays using human blood. Moreover, the GAS-2W strain colonized mouse NALT as efficiently as wild type 90-226 streptococci.
For unknown reasons, humans often fail to develop protective immunity after a single episode of pharyngitis or tonsillitis, and persistence of GAS in tonsils even after antibiotic intervention is common [7]. This problem could be explained by failure to develop a sufficient Th17 immune response. However, we found that a single intranasal infection with GAS-2W rapidly induced a robust 2W:I-Ab-specific Th17 population, which paralleled the clearance of bacteria in mice. The 2W:I-Ab-specific Th17 population represented ∼10% of the total GAS specific Th17 induced (Fig. S1). Superantigens are powerful mitogens that induce massive TCR V beta chain-specific clonal expansion of T cells. Group A streptococci, including the GAS-2W strain are known to produce many superantigens, which have been implicated in highly lethal streptococcal toxic shock and autoimmune sequelae [1], [26]. Such superantigens do not account for the expansion of 2W:I-Ab-specific CD4+ T cells following GAS-2W infection, despite the fact that some of these T cells express the relevant TCR V beta chains [19]. This conclusion may not reflect the situation during human infections because murine T cells are known to be relatively refractory to bacterial superantigens. Dominance of the Th17 response to GAS is impressive relative to that observed for other bacterial pathogens like Listeria. After a single infection 20-39% of the 2W-specific T cells produced IL-17A. By comparison, only about 5–12% of 2W:I-Ab-specific CD4+ cells produced IL-17A in response to primary LM-2W intranasal infection (Fig. 5, [20]). Furthermore, both GAS and LM primarily induced a Th1 response to intravenous inoculation and as expected LM induced a more robust Th1 response than GAS (Fig. 5B). Therefore, GAS might have other features that make for more efficient Th17 induction than LM. Furthermore, these results suggest that the 2W epitope per se does not influence the out come of the CD4+ T cell response. The more robust Th17 response could reflect the GAS tropism for NALT [8] and human tonsils [4], the potential for these bacteria to induce TGF-β1 production in these sites [10], expression of a unique pathogen-associated molecular patterns (PAMPs), or a combination of these factors. GAS was shown to induce TGF-β1 and IL-6 in NALT [10], but the specific PAMPs that lead to expression of these cytokines are unknown. The substantial hyaluronic acid capsule or its degradation products are known to induce TLR2 - and TLR4-mediated inflammation [27], [28], and TLR2 agonists are known to promote Th17 differentiation [29]. M1 protein is also a TLR2 agonist [30] and known to induce inflammatory cytokines, including IL-6 [31] and TGF-β1 [32], and is, therefore, another potential PAMP that could direct a Th17 response. The finding that intranasal inoculation, even with heat-killed GAS-2W cells induces an antigen-specific Th17 response, while other routes induce a Th1 response also has important implications for development and delivery of vaccines to protect against GAS and other mucosal pathogens. The pathogenic potential of IL-17A+ T cells and their short half-life questions the potential safety and utility of intranasal vaccines.
We previously observed that single cell suspensions of NALT, spleen, and cervical lymph nodes from intranasally infected mice secrete TGF-β1, IL-6 and IL-17 upon ex vivo restimulation [10]. Although IL-17 secretion was dependent on TGF-β1 receptor signals, dependence on IL-6 was not tested. As predicted [33], IL-6-/- mice failed to mount a Th17 response and instead developed IFN-γ+ T cells following intranasal infection. As reported by Diao et al, we found high mortality in IL-6-/- mice infected with GAS [34], perhaps as a consequence of exuberant TNF-α expression. Nearly a third of IL-6-/- mice, inoculated intranasally, developed lethal systemic infections; however, survivors fully recovered without subsequently developing systemic infections even though they retained large numbers of GAS in NALT (Fig. 6). This suggests that protection is compartmentalized, ie. opsonic antibody is required to clear bacteria from blood, but does not efficiently remove them from NALT or tonsils. On the other hand a robust Th17 cellular response would be required to eliminate streptococci from those lymphoid organs. Secretory antibody that baths mucosal surfaces may also protect against pharyngitis, but may not be able to reach bacteria sequestered within NALT. These findings are also consistent with studies of Th17 protection induced by intranasal infections of mice with encapsulated S. pneumoniae [11]. It is theoretically possible that local activation of antigen specific T cells could recruit phagocytes with potential to ingest other unrelated bacteria at that site, but recruitment of phagocytes to those infectious foci would still be antigen specific. In addition, IL-6-/- mice developed a robust antigen specific Th1 response (Fig. 6) but failed to clear the bacteria from NALT. This finding is consistent with the observation that IFN-γ-/- mice [35] cleared streptococci from NALT more rapidly than normal mice. We postulate that efficient elimination of GAS depends on IL-17A production by Streptococcal specific CD4+ T cells to attract neutrophils to infected NALT, a capacity that IFN-γ-producing T cells lack. Persistence of large numbers of GAS in NALT of IL-6-/- mice is reminiscent of the situation in human immune carriers who may also fail to develop an adequate Th17 response.
The Th17 response is known to contribute to immune protection against infections by several pathogens [10], [11], [36], [37], [38]; however, these T cells can also be pathogenic, as demonstrated in murine autoimmune models, such as experimental encephalomyelitis (EAE) [39], [40]. A potentially important discovery from our experiments is that repeated intranasal exposure to GAS-2W results in the accumulation of an IL-17A+ IFN-γ+ (double positive) 2W:I-Ab-specific CD4+ T cells in NALT. In contrast, 2W:I-Ab-specific CD4+ T cells in CLN and spleens from the same mice predominantly retained the Th17 phenotype. Preferential accumulation of double positive T cells in NALT could be explained by a unique homing potential, by more rapid expansion or a longer half-life of double positive cells relative to those only able to produce IL-17A. Whether the large population of antigen-specific IL-17+ IFN-γ+ double positive cells observed in NALT following recurrent GAS infection is associated with an autoimmune response is unknown: however, repeated intranasal infection of mice was reported to occasionally produce endocarditis and valvular lesions[41], suggesting a link to human autoimmune disease.
Methods
Ethics statement
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. All animal experiments were conducted under University of Minnesota Institutional Animal Care and Use Committee (IACUC) approved protocol number 0806A36362.
Bacterial strains and growth
Streptococci were grown in Todd-Hewitt broth supplemented with 2% neopeptone (THB-Neo) or on solid media containing Difco blood agar base and sheep blood at 37°C in 5% CO2. All growth media were purchased from Difco Laboratories, Detroit, MI. Strain 90–226 (serotype M1) was originally isolated from the blood of a septic patient [18].
Mice
Five to six weeks old female C57BL/6 mice were purchased from Taconic farms (Germantown, NY) and used at 7–8 weeks of age. IL-6-/- mice in C57BL/6 background were purchased from the Jackson Laboratory (Bar Harbor, ME) [42]. Mice were housed under specific pathogen-free conditions at RAR facilities of University of Minnesota. Mice inoculated with GAS were housed in biosafety level 2 facilities.
Generation of a recombinant GAS strain that expresses the 2W epitope in M protein
An M1 GAS strain 90–226 was genetically engineered to express the 14 amino acid 2W peptide (EAWGALANWAVDSA) on the bacterial surface as a fusion protein with streptococcal M protein (Fig. 1). The corresponding chimeric protein is composed of the 2W epitope inserted in-frame after the first five amino acids of the mature M1 surface protein. The hybrid emm1.0::2W gene was constructed using standard molecular biology techniques and then introduced into the chromosomal emm1.0 gene locus by allelic replacement. Briefly, the emm1.0 gene of strain 90–226 is contiguous with mga at the 5′ end and with the sic gene at the 3′ end, and has its own promoter. Plasmid pFW5 has two multiple cloning sites on either side of a spectinomycin resistance gene [8], [18]. The entire sic gene with its promoter was PCR amplified and inserted into the multiple cloning site downstream of the spectinomycin resistance gene in pFW5. A fragment containing the C-terminal half of mga through the entire emm1.0 gene was PCR amplified by a two-step method to insert the sequence of the 2W epitope into the emm1.0 gene. In the first step, DNA fragments, each coding for part of the 2W epitope sequence (overlapping) were amplified by PCR. In the second step these overlapping fragments were annealed, extended and further amplified to generate a 2.5-kb fragment, which was inserted into the multiple cloning site upstream of the spectinomycin resistance gene in pFW5. This plasmid was transformed into strain 90–226 emm1.0::km for gene replacement, and transformants were selected on spectinomycin (100 µg/ml). The spectinomycin-resistant transformants were then screened for kanamycin sensitivity. Positive clones (SpecRKanS) were screened for gene replacement and the entire region including the 2W insertion was amplified and sequence verified. The resulting strain was designated Streptococcus pyogenes 90-226 emm1.0::2W (GAS-2W). A whole blood phagocytosis assay was used to test whether GAS-2W streptococci are M+ [43].
Intranasal inoculation
C57BL/6 mice were anesthetized with isoflurane/oxygen mixture for 1 min and inoculated intranasally with GAS-2W by placing appropriate doses in a total volume of 15 µl PBS (7.5 µl/nostril). Fractionated droplets were placed with a pipette tip near the entrance of the nostril and the inoculum was involuntarily aspirated into the nasal cavity.
In vitro stimulation with PMA-ionomycin
NALT, Cervical lymph nodes (CLN) and Spleen from C57BL/6 mice were harvested by dissection, disrupted on a nylon screen in 1–2 ml of complete EHAA medium (cEHAA). Resulting single cell suspensions were filtered over a nylon screen and washed with cEHAA before invitro stimulation and staining. Freshly made single cell suspensions in cEHAA were stimulated in vitro with PMA and ionomycin for 4 hours. PMA and ionomycin were added at 50 ng/ml and 500 ng/ml final concentrations, respectively. Cells were incubated at 37C for 1 hour and Brefeldin A (BrA) was added to disrupt golgi and prevent cytokines from being secreted into the medium (1∶1000 of 10 mg/ml DMSO stock) and incubated for three additional hours before proceeding to tetramer enrichment.
Tetramer production
2W:I-Ab tetramers were generated as described in detail by Moon et al [16], [19]. Briefly, 2W:I-Ab was expressed in S2 cells. S2 cells were co-transfected with plasmids encoding the I-Ab alpha chain, the I-Ab beta chain and BirA gene. Transfected cells were selected, passaged in serum-free media, and cultures scaled up to one liter cultures. Expression was induced with copper sulfate and biotinylated 2W:I-Ab heterodimers were purified from supernatants with a Ni++ affinity column. Bound 2W:I-Ab molecules were eluted and purified. Tetramers were created by mixing biotinylated 2W:I-Ab molecules with Phycoerythrin (PE) or Allophycocyanin (APC)-conjugated streptavidin.
Peptide-MHC-II tetramer based magnetic bead enrichment
2W:I-Ab-specific CD4+ T cells were enriched as described previously [16], [19], [20]. Briefly mice were euthanized and NALT, cervical lymph nodes and Spleen were harvested separately. Single cell suspensions were prepared in 200 µl of Fc block (supernatant from 2.4G2 hybridoma cells grown in serum-free media + 2% mouse serum, 2% rat serum, 0.1% sodium azide) from each tissue. Phycoerythrin (PE) or Allophycocyanin (APC) conjugated tetramer was added at a final concentration of 10 nM and the cells were incubated at room temperature for 1 hr, followed by a wash in 15 ml of ice-cold FACS buffer (PBS + 2% fetal bovine serum, 0.1% sodium azide). The tetramer-stained cells were then resuspended in a volume of 200 µl of FACS buffer and mixed with 50 µl of anti-PE or anti-APC antibody conjugated magnetic microbeads (Miltenyi Biotech, Auburn, CA) and incubated on ice in dark for 30 min, followed by one wash with 10 ml of FACS buffer. The cells were resuspended in 3 ml of FACS buffer and passed over a magnetized LS column (Miltenyi Biotech). The column was washed with 3 ml of FACS buffer three times and then removed from the magnetic field. The bound cells were eluted by pushing 5 ml of FACS buffer through the column with a plunger. The resulting enriched fractions were resuspended in 0.1 ml of FACS buffer, and a small volume was removed for cell counting while the rest of the sample was stained with a cocktail of fluorochrome-labeled antibodies.
In vivo stimulation with heat-killed GAS-2W
GAS-2W was grown in THB-Neo media to O.D.600 of 0.5, washed once with PBS, pelleted and resuspended in appropriate volume of PBS and incubated at 60C for 30 minutes. Viability of bacteria was confirmed by plating out on blood agar plates. Heat-killed GAS-2W was stored in aliquots at -20C until use. To induce cytokine production by 2W:I-Ab-specific CD4+ T cells in vivo, 2 X 108 CFU heat-killed GAS-2W in 200 µl of PBS was inoculated intravenously through tail vein. Mice were sacrificed after 3–4 hrs and single cell suspensions of spleen were made in cEHAA medium supplemented with BrA.
Intracellular cytokine staining
Intracellular cytokine staining for IL-17A and IFN-γ was done using BD Cytofix/Cytoperm fixation-permeabilization solution and anti-IL-17A-PE and anti-IFN-γ-PE-Cy7 antibodies according to manufacturer's protocols. Briefly cells stimulated in vivo with heat-killed GAS-2W or in vitro with PMA-ionomycin were subjected to 2W-MHCII tetramer enrichment. Enriched cells were stained for surface markers, washed and fixed with 250 µl of Fixation/Permeabilization solution (BD Biosciences) per sample for 20 min on ice. All samples were washed with 1 ml of 1X Perm/Wash buffer (BD Biosciences) and anti IL-17A PE and anti IFN-γ-PE-Cy7 antibodies were added in 100 µl of 1X Perm/Wash buffer and incubated overnight at 4C in dark. The following day cells were washed with 1 ml of 1X Perm/Wash buffer and resuspended in 400 µl of FACS buffer and transferred to a micro tube within a FACS tube for analysis in LSR-II flow cytometer.
Flow cytometry and antibodies
A Becton Dickinson LSR-II flow cytometer was used to collect and analyze events that have the light scatter properties of lymphocytes and are CD4+ and 2W:I-Ab+. Pacific Blue-conjugated anti-B220, CD11b, F4/80 (Caltag), CD11c (eBioscience, San Diego, CA); Pacific Orange-conjugated CD8 (eBioscience); PerCP-conjugated CD4 (BD PharMingen); PE-Cy7-conjugated IFN-γ (eBioscience); PE conjugated IL-17A; APC-AF780 conjugated CD4 and AlexaFluor 700-conjugated CD44 (eBioscience); PE-conjugated CD4; FITC-conjugated CD4; PB-conjugated CD4; APC-conjugated CD4; AF700-conjugated CD4; PerCP-Cy5.5-conjugated CD4; APC-AF750-conjugated CD4. Antibodies were purchased from the indicated sources. FACS acquisition was performed using FACSDiva software and analysis was done using FlowJo (Tree Star, Ashland, OR).
Statistics
All values are expressed as mean ± SEM. Differences between groups were analyzed using Student's t-test by GraphPad Prism (Version 4.03 for Windows, GraphPad Software, San Diego, CA). Differences are considered significant at P<0.05.
Supporting Information
Zdroje
1. CunninghamMW 2008 Pathogenesis of group A streptococcal infections and their sequelae. Adv Exp Med Biol 609 29 42
2. GuilhermeLFaeKOshiroSEKalilJ 2005 Molecular pathogenesis of rheumatic fever and rheumatic heart disease. Expert Rev Mol Med 7 1 15
3. CarapetisJRSteerACMulhollandEKWeberM 2005 The global burden of group A streptococcal diseases. Lancet Infect Dis 5 685 694
4. TartAHWalkerMJMusserJM 2007 New understanding of the group A Streptococcus pathogenesis cycle. Trends Microbiol 15 318 325
5. QuinnRWFederspielCF 1973 The occurrence of hemolytic streptococci in school children in Nashville, Tennessee, 1961-1967. Am J Epidemiol 97 22 33
6. MartinJMGreenMBarbadoraKAWaldER 2004 Group A streptococci among school-aged children: clinical characteristics and the carrier state. Pediatrics 114 1212 1219
7. OsterlundAPopaRNikkilaTScheyniusAEngstrandL 1997 Intracellular reservoir of Streptococcus pyogenes in vivo: a possible explanation for recurrent pharyngotonsillitis. Laryngoscope 107 640 647
8. ParkHSCostalongaMReinhardtRLDombekPEJenkinsMK 2004 Primary induction of CD4 T cell responses in nasal associated lymphoid tissue during group A streptococcal infection. Eur J Immunol 34 2843 2853
9. BettelliECarrierYGaoWKornTStromTB 2006 Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441 235 238
10. WangBDileepanTBriscoeSHylandKAKangJ 2010 Induction of TGF-beta1 and TGF-beta1-dependent predominant Th17 differentiation by group A streptococcal infection. Proc Natl Acad Sci U S A 107 5937 5942
11. LuYJGrossJBogaertDFinnABagradeL 2008 Interleukin-17A mediates acquired immunity to pneumococcal colonization. PLoS Pathog 4 e1000159
12. CostalongaMClearyPPFischerLAZhaoZ 2009 Intranasal bacteria induce Th1 but not Treg or Th2. Mucosal Immunol 2 85 95
13. HatayeJMoonJJKhorutsAReillyCJenkinsMK 2006 Naive and memory CD4+ T cell survival controlled by clonal abundance. Science 312 114 116
14. CatronDMRuschLKHatayeJItanoAAJenkinsMK 2006 CD4+ T cells that enter the draining lymph nodes after antigen injection participate in the primary response and become central-memory cells. J Exp Med 203 1045 1054
15. BlairDALefrancoisL 2007 Increased competition for antigen during priming negatively impacts the generation of memory CD4 T cells. Proc Natl Acad Sci U S A 104 15045 15050
16. MoonJJChuHHHatayeJPaganAJPepperM 2009 Tracking epitope-specific T cells. Nat Protoc 4 565 581
17. ReesWBenderJTeagueTKKedlRMCrawfordF 1999 An inverse relationship between T cell receptor affinity and antigen dose during CD4(+) T cell responses in vivo and in vitro. Proc Natl Acad Sci U S A 96 9781 9786
18. DombekPECueDSedgewickJLamHRuschkowskiS 1999 High-frequency intracellular invasion of epithelial cells by serotype M1 group A streptococci: M1 protein-mediated invasion and cytoskeletal rearrangements. Mol Microbiol 31 859 870
19. MoonJJChuHHPepperMMcSorleySJJamesonSC 2007 Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity 27 203 213
20. PepperMLinehanJLPaganAJZellTDileepanT 2010 Different routes of bacterial infection induce long-lived TH1 memory cells and short-lived TH17 cells. Nat Immunol 11 83 89
21. LiHNoohMMKotbMReF 2008 Commercial peptidoglycan preparations are contaminated with superantigen-like activity that stimulates IL-17 production. J Leukoc Biol 83 409 418
22. AnnunziatoFRomagnaniS 2010 The transient nature of the Th17 phenotype. Eur J Immunol 40 3312 3316
23. PeckAMellinsED 2010 Plasticity of T-cell phenotype and function: the T helper type 17 example. Immunology 129 147 153
24. LeeYKTurnerHMaynardCLOliverJRChenD 2009 Late developmental plasticity in the T helper 17 lineage. Immunity 30 92 107
25. ParkHSFrancisKPYuJClearyPP 2003 Membranous cells in nasal-associated lymphoid tissue: a portal of entry for the respiratory mucosal pathogen group A streptococcus. J Immunol 171 2532 2537
26. SriskandanSFaulknerLHopkinsP 2007 Streptococcus pyogenes: Insight into the function of the streptococcal superantigens. Int J Biochem Cell Biol 39 12 19
27. JiangDLiangJFanJYuSChenS 2005 Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med 11 1173 1179
28. ScheibnerKALutzMABoodooSFentonMJPowellJD 2006 Hyaluronan fragments act as an endogenous danger signal by engaging TLR2. J Immunol 177 1272 1281
29. ReynoldsJMPappuBPPengJMartinezGJZhangY 2010 Toll-like receptor 2 signaling in CD4(+) T lymphocytes promotes T helper 17 responses and regulates the pathogenesis of autoimmune disease. Immunity 32 692 702
30. SigurdardottirTBjorckVHerwaldHMorgelinMRutardottirS 2010 M1 protein from streptococcus pyogenes induces nitric oxide-mediated vascular hyporesponsiveness to phenylephrine: involvement of toll-like receptor activation. Shock 34 98 104
31. PahlmanLIMorgelinMEckertJJohanssonLRussellW 2006 Streptococcal M protein: a multipotent and powerful inducer of inflammation. J Immunol 177 1221 1228
32. NishikawaYShibataROzonoYIchinoseHMiyazakiM 2000 Streptococcal M protein enhances TGF-beta production and increases surface IgA-positive B cells in vitro in IgA nephropathy. Nephrol Dial Transplant 15 772 777
33. KornTBettelliEOukkaMKuchrooVK 2009 IL-17 and Th17 Cells. Annu Rev Immunol 27 485 517
34. DiaoHKohanawaM 2005 Endogenous interleukin-6 plays a crucial protective role in streptococcal toxic shock syndrome via suppression of tumor necrosis factor alpha production. Infect Immun 73 3745 3748
35. HylandKABrennanROlmstedSBRojasEMurphyE 2009 The early interferon response of nasal-associated lymphoid tissue to Streptococcus pyogenes infection. FEMS Immunol Med Microbiol 55 422 431
36. LinYRitcheaSLogarASlightSMessmerM 2009 Interleukin-17 is required for T helper 1 cell immunity and host resistance to the intracellular pathogen Francisella tularensis. Immunity 31 799 810
37. ContiHRShenFNayyarNStocumESunJN 2009 Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med 206 299 311
38. IshigameHKakutaSNagaiTKadokiMNambuA 2009 Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity 30 108 119
39. AranamiTYamamuraT 2008 Th17 Cells and autoimmune encephalomyelitis (EAE/MS). Allergol Int 57 115 120
40. OukkaM 2008 Th17 cells in immunity and autoimmunity. Ann Rheum Dis 67 Suppl 3 iii26 29
41. StephenJKuruvillaAJohnsonJChandiSKrishnaswamiS 1998 Pharyngeal colonisation, ADNB response and development of cardiac lesions in mice following intranasal inoculation with group A beta haemolytic streptococcus M type 18. Indian J Exp Biol 36 292 297
42. KopfMBaumannHFreerGFreudenbergMLamersM 1994 Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature 368 339 342
43. HylandKAWangBClearyPP 2007 Protein F1 and Streptococcus pyogenes resistance to phagocytosis. Infect Immun 75 3188 3191
Štítky
Hygiena a epidemiologie Infekční lékařství Laboratoř
Článek Hostile Takeover by : Reorganization of Parasite and Host Cell Membranes during Liver Stage EgressČlánek A Trigger Enzyme in : Impact of the Glycerophosphodiesterase GlpQ on Virulence and Gene ExpressionČlánek An EGF-like Protein Forms a Complex with PfRh5 and Is Required for Invasion of Human Erythrocytes byČlánek Th2-polarised PrP-specific Transgenic T-cells Confer Partial Protection against Murine ScrapieČlánek Alterations in the Transcriptome during Infection with West Nile, Dengue and Yellow Fever Viruses
Článek vyšel v časopisePLOS Pathogens
Nejčtenější tento týden
2011 Číslo 9- Jak souvisí postcovidový syndrom s poškozením mozku?
- Měli bychom postcovidový syndrom léčit antidepresivy?
- Farmakovigilanční studie perorálních antivirotik indikovaných v léčbě COVID-19
- 10 bodů k očkování proti COVID-19: stanovisko České společnosti alergologie a klinické imunologie ČLS JEP
-
Všechny články tohoto čísla
- Unconventional Repertoire Profile Is Imprinted during Acute Chikungunya Infection for Natural Killer Cells Polarization toward Cytotoxicity
- Envelope Deglycosylation Enhances Antigenicity of HIV-1 gp41 Epitopes for Both Broad Neutralizing Antibodies and Their Unmutated Ancestor Antibodies
- Co-opts GBF1 and CERT to Acquire Host Sphingomyelin for Distinct Roles during Intracellular Development
- Nrf2, a PPARγ Alternative Pathway to Promote CD36 Expression on Inflammatory Macrophages: Implication for Malaria
- Robust Antigen Specific Th17 T Cell Response to Group A Streptococcus Is Dependent on IL-6 and Intranasal Route of Infection
- Targeting of a Chlamydial Protease Impedes Intracellular Bacterial Growth
- The Protease Cruzain Mediates Immune Evasion
- High-Resolution Phenotypic Profiling Defines Genes Essential for Mycobacterial Growth and Cholesterol Catabolism
- Plague and Climate: Scales Matter
- Exhausted CD8 T Cells Downregulate the IL-18 Receptor and Become Unresponsive to Inflammatory Cytokines and Bacterial Co-infections
- Maturation-Induced Cloaking of Neutralization Epitopes on HIV-1 Particles
- Murine Gamma-herpesvirus Immortalization of Fetal Liver-Derived B Cells Requires both the Viral Cyclin D Homolog and Latency-Associated Nuclear Antigen
- Rapid and Efficient Clearance of Blood-borne Virus by Liver Sinusoidal Endothelium
- Hostile Takeover by : Reorganization of Parasite and Host Cell Membranes during Liver Stage Egress
- A Trigger Enzyme in : Impact of the Glycerophosphodiesterase GlpQ on Virulence and Gene Expression
- Strain Specific Resistance to Murine Scrapie Associated with a Naturally Occurring Human Prion Protein Polymorphism at Residue 171
- Development of a Transformation System for : Restoration of Glycogen Biosynthesis by Acquisition of a Plasmid Shuttle Vector
- Monalysin, a Novel -Pore-Forming Toxin from the Pathogen Contributes to Host Intestinal Damage and Lethality
- Host Phylogeny Determines Viral Persistence and Replication in Novel Hosts
- BC2L-C Is a Super Lectin with Dual Specificity and Proinflammatory Activity
- Expression of the RAE-1 Family of Stimulatory NK-Cell Ligands Requires Activation of the PI3K Pathway during Viral Infection and Transformation
- Structure of the Vesicular Stomatitis Virus N-P Complex
- HSV Infection Induces Production of ROS, which Potentiate Signaling from Pattern Recognition Receptors: Role for S-glutathionylation of TRAF3 and 6
- The Human Papillomavirus E6 Oncogene Represses a Cell Adhesion Pathway and Disrupts Focal Adhesion through Degradation of TAp63β upon Transformation
- Analysis of Behavior and Trafficking of Dendritic Cells within the Brain during Toxoplasmic Encephalitis
- Exposure to the Viral By-Product dsRNA or Coxsackievirus B5 Triggers Pancreatic Beta Cell Apoptosis via a Bim / Mcl-1 Imbalance
- Multidrug Resistant 2009 A/H1N1 Influenza Clinical Isolate with a Neuraminidase I223R Mutation Retains Its Virulence and Transmissibility in Ferrets
- Structure of Herpes Simplex Virus Glycoprotein D Bound to the Human Receptor Nectin-1
- Step-Wise Loss of Bacterial Flagellar Torsion Confers Progressive Phagocytic Evasion
- Complex Recombination Patterns Arising during Geminivirus Coinfections Preserve and Demarcate Biologically Important Intra-Genome Interaction Networks
- An EGF-like Protein Forms a Complex with PfRh5 and Is Required for Invasion of Human Erythrocytes by
- Non-Lytic, Actin-Based Exit of Intracellular Parasites from Intestinal Cells
- The Fecal Viral Flora of Wild Rodents
- The General Transcriptional Repressor Tup1 Is Required for Dimorphism and Virulence in a Fungal Plant Pathogen
- Interferon Regulatory Factor-1 (IRF-1) Shapes Both Innate and CD8 T Cell Immune Responses against West Nile Virus Infection
- A Small Non-Coding RNA Facilitates Bacterial Invasion and Intracellular Replication by Modulating the Expression of Virulence Factors
- Evaluating the Sensitivity of to Biotin Deprivation Using Regulated Gene Expression
- The Motility of a Human Parasite, , Is Regulated by a Novel Lysine Methyltransferase
- Phosphodiesterase-4 Inhibition Alters Gene Expression and Improves Isoniazid – Mediated Clearance of in Rabbit Lungs
- Restoration of IFNγR Subunit Assembly, IFNγ Signaling and Parasite Clearance in Infected Macrophages: Role of Membrane Cholesterol
- Protease ROM1 Is Important for Proper Formation of the Parasitophorous Vacuole
- The Regulated Secretory Pathway in CD4 T cells Contributes to Human Immunodeficiency Virus Type-1 Cell-to-Cell Spread at the Virological Synapse
- Rerouting of Host Lipids by Bacteria: Are You CERTain You Need a Vesicle?
- Transmission Characteristics of the 2009 H1N1 Influenza Pandemic: Comparison of 8 Southern Hemisphere Countries
- Th2-polarised PrP-specific Transgenic T-cells Confer Partial Protection against Murine Scrapie
- Sequential Bottlenecks Drive Viral Evolution in Early Acute Hepatitis C Virus Infection
- Genomic Insights into the Origin of Parasitism in the Emerging Plant Pathogen
- Genomic and Proteomic Analyses of the Fungus Provide Insights into Nematode-Trap Formation
- Influenza Virus Ribonucleoprotein Complexes Gain Preferential Access to Cellular Export Machinery through Chromatin Targeting
- Alterations in the Transcriptome during Infection with West Nile, Dengue and Yellow Fever Viruses
- Protease-Sensitive Conformers in Broad Spectrum of Distinct PrP Structures in Sporadic Creutzfeldt-Jakob Disease Are Indicator of Progression Rate
- Vaccinia Virus Protein C6 Is a Virulence Factor that Binds TBK-1 Adaptor Proteins and Inhibits Activation of IRF3 and IRF7
- c-di-AMP Is a New Second Messenger in with a Role in Controlling Cell Size and Envelope Stress
- Structural and Functional Studies on the Interaction of GspC and GspD in the Type II Secretion System
- APOBEC3A Is a Specific Inhibitor of the Early Phases of HIV-1 Infection in Myeloid Cells
- Impairment of Immunoproteasome Function by β5i/LMP7 Subunit Deficiency Results in Severe Enterovirus Myocarditis
- HTLV-1 Propels Thymic Human T Cell Development in “Human Immune System” Rag2 gamma c Mice
- Tri6 Is a Global Transcription Regulator in the Phytopathogen
- Exploiting and Subverting Tor Signaling in the Pathogenesis of Fungi, Parasites, and Viruses
- The Next Opportunity in Anti-Malaria Drug Discovery: The Liver Stage
- Significant Effects of Antiretroviral Therapy on Global Gene Expression in Brain Tissues of Patients with HIV-1-Associated Neurocognitive Disorders
- Inhibition of Competence Development, Horizontal Gene Transfer and Virulence in by a Modified Competence Stimulating Peptide
- A Novel Metal Transporter Mediating Manganese Export (MntX) Regulates the Mn to Fe Intracellular Ratio and Virulence
- Rhoptry Kinase ROP16 Activates STAT3 and STAT6 Resulting in Cytokine Inhibition and Arginase-1-Dependent Growth Control
- Hsp90 Governs Dispersion and Drug Resistance of Fungal Biofilms
- Secretion of Genome-Free Hepatitis B Virus – Single Strand Blocking Model for Virion Morphogenesis of Para-retrovirus
- A Viral Ubiquitin Ligase Has Substrate Preferential SUMO Targeted Ubiquitin Ligase Activity that Counteracts Intrinsic Antiviral Defence
- Membrane Remodeling by the Double-Barrel Scaffolding Protein of Poxvirus
- A Diverse Population of Molecular Type VGIII in Southern Californian HIV/AIDS Patients
- Disruption of TLR3 Signaling Due to Cleavage of TRIF by the Hepatitis A Virus Protease-Polymerase Processing Intermediate, 3CD
- Quantitative Analyses Reveal Calcium-dependent Phosphorylation Sites and Identifies a Novel Component of the Invasion Motor Complex
- Discovery of the First Insect Nidovirus, a Missing Evolutionary Link in the Emergence of the Largest RNA Virus Genomes
- Old World Arenaviruses Enter the Host Cell via the Multivesicular Body and Depend on the Endosomal Sorting Complex Required for Transport
- Exploits a Unique Repertoire of Type IV Secretion System Components for Pilus Assembly at the Bacteria-Host Cell Interface
- Recurrent Signature Patterns in HIV-1 B Clade Envelope Glycoproteins Associated with either Early or Chronic Infections
- PLOS Pathogens
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- HTLV-1 Propels Thymic Human T Cell Development in “Human Immune System” Rag2 gamma c Mice
- Hostile Takeover by : Reorganization of Parasite and Host Cell Membranes during Liver Stage Egress
- Exploiting and Subverting Tor Signaling in the Pathogenesis of Fungi, Parasites, and Viruses
- A Viral Ubiquitin Ligase Has Substrate Preferential SUMO Targeted Ubiquitin Ligase Activity that Counteracts Intrinsic Antiviral Defence
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



