-
Články
Reklama
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
ReklamaTesticular Differentiation Occurs in Absence of R-spondin1 and Sox9 in Mouse Sex Reversals
In mammals, male sex determination is governed by SRY-dependent activation of Sox9, whereas female development involves R-spondin1 (RSPO1), an activator of the WNT/beta-catenin signaling pathway. Genetic analyses in mice have demonstrated Sry and Sox9 to be both required and sufficient to induce testicular development. These genes are therefore considered as master regulators of the male pathway. Indeed, female-to-male sex reversal in XX Rspo1 mutant mice correlates with Sox9 expression, suggesting that this transcription factor induces testicular differentiation in pathological conditions. Unexpectedly, here we show that testicular differentiation can occur in XX mutants lacking both Rspo1 and Sox9 (referred to as XX Rspo1KOSox9cKO), indicating that Sry and Sox9 are dispensable to induce female-to-male sex reversal. Molecular analyses show expression of both Sox8 and Sox10, suggesting that activation of Sox genes other than Sox9 can induce male differentiation in Rspo1KOSox9cKO mice. Moreover, since testis development occurs in XY Rspo1KOSox9cKO mice, our data show that Rspo1 is the main effector for male-to-female sex reversal in XY Sox9cKO mice. Thus, Rspo1 is an essential activator of ovarian development not only in normal situations, but also in sex reversal situations. Taken together these data demonstrate that both male and female sex differentiation is induced by distinct, active, genetic pathways. The dogma that considers female differentiation as a default pathway therefore needs to be definitively revised.
Published in the journal: . PLoS Genet 8(12): e32767. doi:10.1371/journal.pgen.1003170
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1003170Summary
In mammals, male sex determination is governed by SRY-dependent activation of Sox9, whereas female development involves R-spondin1 (RSPO1), an activator of the WNT/beta-catenin signaling pathway. Genetic analyses in mice have demonstrated Sry and Sox9 to be both required and sufficient to induce testicular development. These genes are therefore considered as master regulators of the male pathway. Indeed, female-to-male sex reversal in XX Rspo1 mutant mice correlates with Sox9 expression, suggesting that this transcription factor induces testicular differentiation in pathological conditions. Unexpectedly, here we show that testicular differentiation can occur in XX mutants lacking both Rspo1 and Sox9 (referred to as XX Rspo1KOSox9cKO), indicating that Sry and Sox9 are dispensable to induce female-to-male sex reversal. Molecular analyses show expression of both Sox8 and Sox10, suggesting that activation of Sox genes other than Sox9 can induce male differentiation in Rspo1KOSox9cKO mice. Moreover, since testis development occurs in XY Rspo1KOSox9cKO mice, our data show that Rspo1 is the main effector for male-to-female sex reversal in XY Sox9cKO mice. Thus, Rspo1 is an essential activator of ovarian development not only in normal situations, but also in sex reversal situations. Taken together these data demonstrate that both male and female sex differentiation is induced by distinct, active, genetic pathways. The dogma that considers female differentiation as a default pathway therefore needs to be definitively revised.
Introduction
Mammalian sex determination depends on the primary developmental decision of the gonad to differentiate as testis or ovary. The gonad develops as a bipotential organ with the capacity to respond to two different genetic stimuli: the activation of the SRY/SOX9 pathway that induces testicular development, or the expression of the R-spondin1 (RSPO1)/beta-catenin pathway that regulates ovarian differentiation [1]. Indeed in humans and mice, male sex determination is initiated by the expression of the Y-linked gene SRY [2], [3], [4]. Sry expression in turn activates the transcriptional regulator SOX9 [5]. Subsequently, SOX9 initiates Sertoli cell differentiation, the supporting cell of the testicular sex cords [6], [7]. Signaling pathways initiated in these cells contribute to the organization of the XY gonads [8], as well as to the differentiation of other testicular cell lineages such as the Leydig steroidogenic cells [9], [10] and the pro-spermatogonia [11], [12], ultimately leading to testis formation and, in turn, male development. In 46,XY patients, loss-of-function mutations in SRY and SOX9 promote male-to-female sex reversal [13], [14], whereas translocations of the SRY locus to another chromosome can yield 46,XX patients with female-to-male sex reversal [3]. Loss-of-function mutations [6], [7], [15], [16] and gain-of-function mutations [4], [17], [18] of Sry and Sox9 have been generated in mouse models, showing that Sry and Sox9 are necessary and sufficient to induce testis differentiation and the associated male development. As a consequence, these genes have been considered as the master inducers of testis differentiation and male development.
In the absence of SRY (XX individuals), up-regulation of RSPO1, an activator of the WNT/beta-catenin signaling pathway, promotes ovarian differentiation. Mutations in RSPO1 are responsible for skin disorders and female-to-male sex reversal in 46,XX patients [19]. Similarly, ablation of Rspo1 in mice yields female-to-male sex reversal and promotes Sox9 up-regulation correlated with differentiation of Sertoli cells and formation of testis cords at birth [20]. This gonadal dysgenesis yields development of an ovotestis, a gonad displaying both testicular and ovarian regions [20], [21]. Rspo1 expression in turn activates expression of Wnt4 [21], another activator of the WNT/beta-catenin signaling pathway involved in ovarian differentiation [22], [23]. When the canonical beta-catenin signaling pathway is activated in XY gonads, this induces male-to-female sex reversal indicating that this pathway acts on top of ovarian differentiation [23]. Indeed, activation of WNT/beta-catenin is required for expression of Foxl2 [24], a transcription factor involved in folliculogenesis [25], [26] and homeostasis of the ovary [27]. Thus Rspo1 appears to be the gene instructing the molecular network leading to ovarian development.
Since ablation of Rspo1 promotes SOX9 expression concomitantly with Sertoli cell differentiation [20], it was assumed that Sox9 is the sex reversal inducer in XX Rspo1KO mutants. We now show that i) testicular differentiation occurs in XX Rspo1KOSox9cKO mutants indicating that neither Sry nor Sox9 are required for female-to-male sex reversals; ii) testicular differentiation also occurs in XY Rspo1KOSox9cKO mutants indicating that Rspo1 is required for male-to-female sex reversal in XY Sox9cKO mutants.
Results/Discussion
Rspo1 is required for ovarian development in XY Sox9cKO mice
Sox9 is required for Sertoli cell differentiation, testis formation and male development. Indeed, deletion of Sox9 in XY Sox9fl/fl; Sf1:creTg/+, (referred to as XY Sox9cKO) triggers male-to-female sex reversal [16]. However the factor(s) inducing sex reversal in XY Sox9cKO remained to be identified. Given (i) the prominent role of RSPO1, an activator of beta-catenin signaling, in female sex determination [19], and (ii) the fact that ectopic activation of beta-catenin in XY gonads can induce male-to-female sex reversal [23], we hypothesized that Rspo1 expression induced male-to-female sex-reversal in XY Sox9cKO gonads. According to this scenario, neither testicular (which is Sox9-dependent) nor ovarian (which is Rspo1/beta-catenin-dependent) differentiation should occur in XY Sox9cKO gonads additionally lacking Rspo1. To test this hypothesis, we have generated and analyzed double loss-of-function mice (i.e. XY Rspo1−/−; Sox9fl/fl; Sf1:creTg/+, referred to as XY Rspo1KOSox9cKO).
Since previous results have shown that sex reversal can appear quite late during fetal development [20], [22], we first analyzed adult stages when the sexual development is likely to be completed. At P60 (postnatal day 60), the anogenital distance in XY Rspo1KOSox9cKO mice was equivalent to that of XX controls but the internal genitalia contained both male and female organs including oviducts, uterine horns and vaginal tissues, as well as epididymides, vasa deferensia, seminal vesicles and prostate (Figure S1). The XY Sox9cKO developed as ovaries (Figure 1b, 1g, 1l, 1q and Figure S1), as expected from a previous report [16]. Interestingly, XY Rspo1KOSox9cKO gonads developed as hypoplastic testes containing well-defined seminiferous tubules as evidenced by histological analysis (Figure 1c, 1h and Figure S1). We next examined whether the supporting cells forming the seminiferous tubules differentiated as granulosa cells, the ovarian supporting cells expressing FOXL2 [25], [28] or as Sertoli cells expressing DMRT1 [29]. In P21 gonads, immunostaining experiments showed that the supporting cells forming the seminiferous tubules in XY Rspo1KOSox9cKO gonads were DMRT1-expressing Sertoli cells (Figure 1r), even though SOX9 was clearly missing (Figure 1m). However, a few FOXL2-positive granulosa cells were found within the alignment of the Sertoli cells forming the seminiferous tubules (Figure 1m, r) and in a few XY Rspo1KOSox9cKO mice (3 out of 18), rare and abnormal follicles were observed (Figure S2A). The mixed genetic background of Rspo1KOSox9cKO mice is a likely factor causing the variation of this phenotype.
Fig. 1. Testicular differentiation in XY and XX Rspo1−/−; Sox9flox/flox; Sf1;creTg/+ (Rspo1KOSox9cKO) mice. 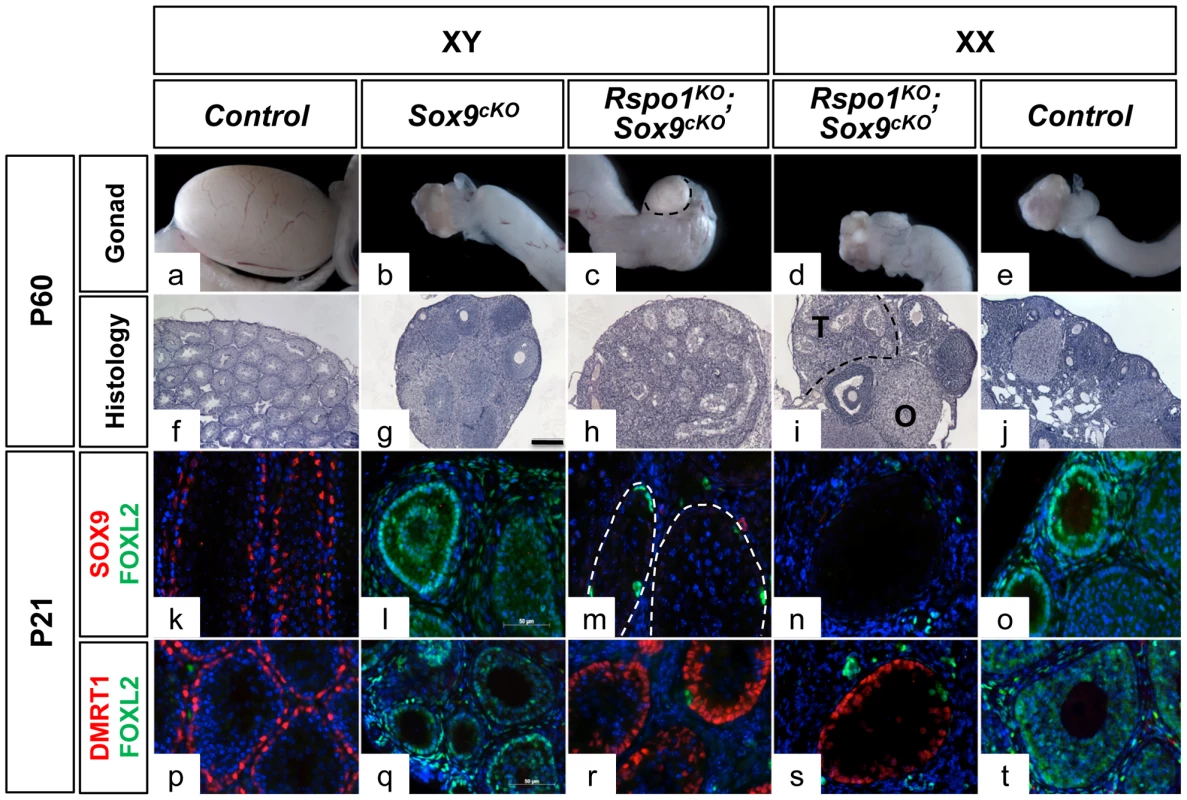
Macroscopic views of gonads of 2 month-old mice show hypoplasic testis and ovotestis development in XY (c) and XX (d) Rspo1KOSox9cKO mice, respectively. Seminiferous tubules are revealed by PAS histological analysis of XY (h) and XX (i) Rspo1KOSox9cKO gonadal sections. They are less abundant than in XY controls (f). XY Sox9cKO gonads (g) develop as ovaries (j). (T: testicular region, O: ovarian region, scale bar: 200 µm). Immunofluorescence of SOX9 (k–o) or DMRT1 (p–t) (a Sertoli cell marker, in red), FOXL2 (k–t) (a follicular cell marker, in green) and DAPI (a nuclear marker in blue) (scale bar, 50 µm). Deletion of Sox9 with Sf1:cre (Sox9cKO) eliminates SOX9 expression in Sertoli cells (l, m, n), and promotes male-to-female sex reversal in XY Sox9cKO gonads as highlighted by robust FOXL2 expression (l, q). However, Sox9 deletion no longer allows ovarian cells differentiation when Rspo1 is deleted in the XY (m, r) and XX (n, s) Rspo1KOSox9cKO mice. This is evidenced by the robust expression of DMRT1 in 3 week-old XY (s) and XX (r) mutant gonads and XY controls (p), and the low or absent expression of FOXL2 in these gonads (k, m, n, p, r, s). XY (a, f, k, p) and XX (e, j, o, t) Rspo1+/−; Sox9flox/flox controls, XY Sox9cKO gonads (b, g, l, q), XY (c, h, m, r) and XX (d, i, n, s) Sox9cKO Rspo1KO respectively. XX Rspo1KO and XX Sox9cKO Rspo1KO gonads appeared similar (see Figure S2B). Altogether this shows that a genetic pathway activated by RSPO1 is required for the male-to-female sex-reversal of XY Sox9cKO and indicates that Sertoli cell differentiation and seminiferous tubules formation can occur in the absence of SOX9.
Sertoli cell differentiation occurs without Sry and Sox9 in XX Rspo1KO gonads
Our study also allowed us to evaluate the effect of Sox9 removal in a female-to-male sex reversal context (i.e. in XX Rspo1KOSox9cKO). Given that homozygous mutations of Rspo1 promote Sertoli cell differentiation around birth, a process that is associated with Sox9 up-regulation in these cells [20], we hypothesized that Sox9 is the inducing factor of testicular differentiation in XX Rspo1KO mice. If Sox9 is indeed the main switch for female-to-male sex reversal in XX individuals, one expects an impaired differentiation of Sertoli cell and seminiferous tubules in the absence of both Rspo1 and Sox9 in XX Rspo1KOSox9cKO gonads. Unexpectedly, at P60, these XX double mutants displayed hermaphroditism of the reproductive tracts (Figure S1). Histological analysis revealed that XX Rspo1KOSox9cKO mice exhibited ovotestes with an extensive presence of sex cords (Figure 1d, 1i and in Figure S2B, f) as do XX Rspo1KOgonads (shown in Figure S2B, S2e and in previous analyses [20], [21]). Thus, the development of XX Rspo1KOSox9cKO mouse genitalia is indistinguishable from that of XX Rspo1KO mice indicating that the additional deletion of Sox9 in XX Rspo1KOSox9cKO gonads does not change the fate of XX Rspo1KO gonads. We next examined whether the supporting cells forming the sex cords differentiated as granulosa cells, the ovarian supporting cells expressing FOXL2 [25], [28], or as Sertoli cells expressing DMRT1 [29]. In three weeks old mice (P21), Sox9-depleted cells forming the seminiferous tubules generally lacked the follicular cell marker FOXL2 and instead expressed DMRT1 (Figure 1n, 1s). These data clearly indicate that Sertoli cell, seminiferous tubule and testis differentiation can occur in the absence of Sry and Sox9 in XX Rspo1KO gonads.
Steroidogenic cells are present in Rspo1KOSox9cKO embryonic gonads
Previous studies clearly show that the development of male genitalia depends on androgens secreted by the embryonic testis [30]. In XX Rspo1KO gonads, steroidogenic cells appear before Sertoli cell differentiation [20], [31] and this was also observed in Wnt4KO gonads [22], [32], Wnt4 being up-regulated upon Rspo1 expression in XX gonads [20], [21]. In addition, lack of Wnt4 expression was shown to allow ectopic migration of steroidogenic cells from the neighboring adrenals into gonads [32], [33] and subsequent androgen synthesis [34], which explains the development of male genitalia in these mutants. When investigating whether steroidogenic cells were present in XX and XY Rspo1KOSox9cKO gonads, we found that P450Scc, a gene encoding for a precursor involved in androgen synthesis was expressed at 14.5 dpc in XY controls, XY and XX Rspo1KOSox9cKO gonads and XX Rspo1KO gonads, but not in XX controls (Figure S3A). However, Cyp21, a marker for adrenal cells [35], was not strongly expressed in Rspo1KOSox9cKO gonads at 13.5 dpc (Figure S3A), suggesting that the steroidogenic cells in Rspo1KOSox9cKO gonads, did not come from the arenals or, alternatively, have undergone reprogramming as Leydig cells. Whatever the situation, it is likely that male hormones synthesized in the developing mutant gonads can contribute to stimulate epididymides, vasa deferentia and seminal vesicles development.
Delayed testicular formation in Rspo1KOSox9cKO mice
We next investigated the timing of testicular cord formation in XY and XX Rspo1KOSox9cKO gonads. In wild-type embryos, the earliest morphological sign of testis development occurs at 12.0–12.5 dpc when testis cord are formed [36]. Accordingly at 13.5 dpc, the testis cords were highlighted in XY controls by the prominent expression of SOX9 and AMH, two markers of Sertoli cells (Figure 2a). In contrast, Rspo1KOSox9cKO gonads did not show a clear testicular organization as they lack AMH at 13.5 dpc (Figure 2b, 2c, 2f, 2g). As AMH synthesis and secretion by Sertoli cells promotes the elimination of the female reproductive tract during embryogenesis [37], the absence of AMH in Rspo1KOSox9cKO gonads provides an explanation for the maintenance of the Mullerian derivatives (oviducts, uterine horns and vaginal tissues) in these mutant mice. In addition, Sertoli cell differentiation is delayed in gonads lacking both Sox9 and Rspo1, as indicated by the maintenance of SRY expression, in the XY Rspo1KOSox9cKO gonads at 13.5 dpc (Figure 2f), a stage at which SRY expression has already ceased for one day in the control situation [38], [39], [40]. Along these lines, the maintenance of SF1 expression in XX Rspo1KOSox9cKO gonads at 13.5 dpc (Figure 2j, 2k), a factor whose expression is normally down-regulated between 13.5 and 16.5 dpc in the ovary [41] (Figure 2l), also suggests that the XX Rspo1KOSox9cKO gonads are still undifferentiated or have differentiated as testis. However, with respect to the latter, the absence of AMH expression shows that no Sertoli cell differentiation has occurred (Figure 2c, 2g). Altogether these data indicate that the Rspo1KOSox9cKO gonads are still undifferentiated at 13.5 dpc.
Fig. 2. Non-differentiated XY and XX Rspo1KOSox9cKO gonads at 13.5 dpc. 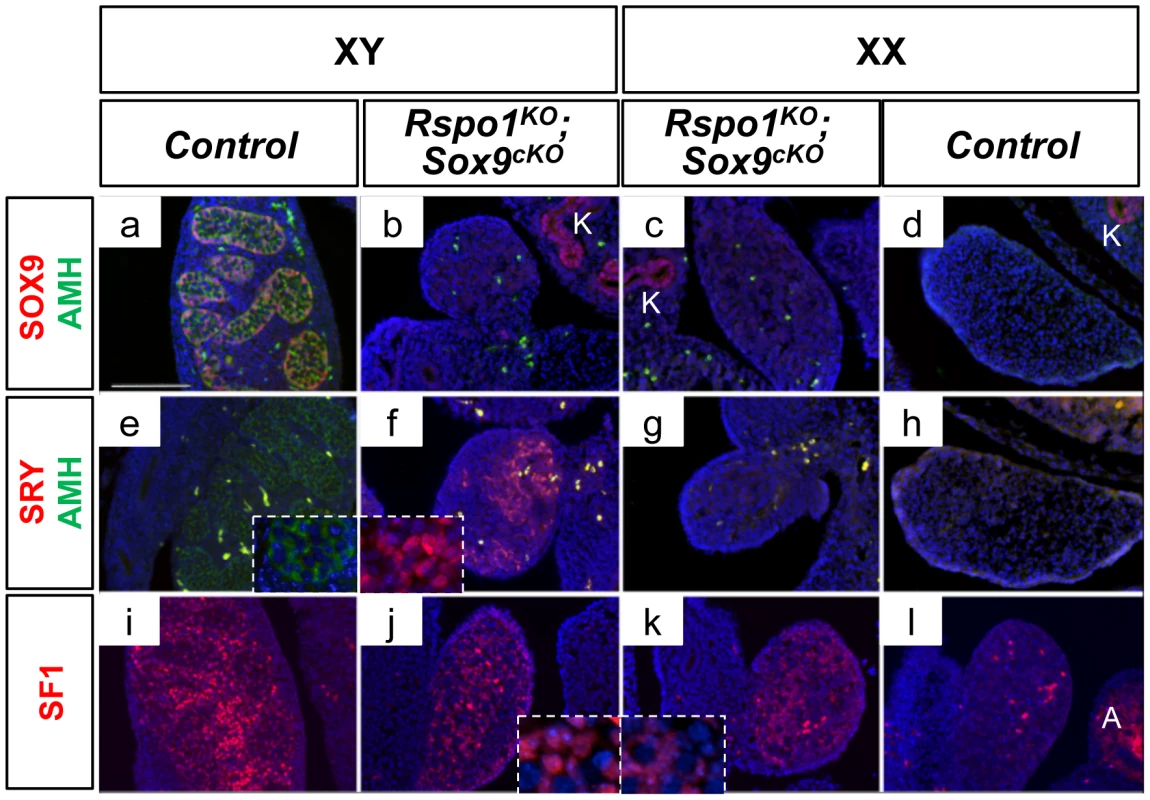
Immunofluorescence of SOX9 (Sertoli cell marker, in red) and AMH (Sertoli cell marker green) (a–d), AMH (Sertoli cell marker, in green) and SRY (pre-Sertoli and Sertoli cell marker in red) (e–h) and SF1 (undifferentiated supporting cell, Sertoli and Leydig cell marker) (i–l). Counterstain is DAPI (in blue). Lack of SOX9 and AMH expression in XY (b) and XX (c) Rspo1KOSox9cKO gonads shows that Sertoli cell differentiation did not occur at 13.5 dpc. Note that the kidneys (K) are positive for SOX9. This is accompagnied with the maintenance of SRY expression in the XY Rspo1KOSox9cKO gonads (f) whereas SRY expression has ceased in XY controls (e). SF1 expression is maintained in absence of Sertoli cells differentiation in XY and XX Rspo1KOSox9cKO gonads (j and k respectively) (scale bar: 100 µm). Note that SF1 is also expressed in steroidogenic cells of the adrenals (A). XY (a, e, i) and XX (d, h, l) Rspo1+/−; Sox9flox/flox controls, XY (b, f, j) and XX (c, g, k) Sox9cKO Rspo1KO respectively. The first signs of Sertoli cell differentiation appeared at 16.5 dpc in Rspo1KOSox9cKO gonads, with some rare DMRT1-positive cells in comparison to XY controls (Figure S3B). Then, few rudimentary testis cords were observed around 17.5 dpc (Figure S3B). At P0, Sertoli cells aligned to form sex cords as evidenced by the localization of DMRT1 positive-cells (Figure S4A c, d). Quantitative PCR experiments further confirmed that Dmrt1 expression was strongly expressed in XY Rspo1KOSox9cKO gonads and weakly in XX Rspo1KOSox9cKO gonads at P0, highlighting that more Sertoli cells were present in XY Rspo1KOSox9cKO gonads in comparison to XX Rspo1KOSox9cKO gonads (Figure S4C). In addition, some FOXL2-positive cells were also detected in Rspo1KOSox9cKO gonads (Figure S4A c, d). However, quantitative PCR experiments showed that Foxl2 expression was significantly reduced in comparison to XX control or XY Sox9cKO gonads (Figure S4B) as expected for a gonad developing as ovotestis or testis.
We then studied SDMG1 expression, a cytoplasmic marker of Sertoli cells and of granulosa cells when follicles form (Best et al. 2008). Using this marker, sex cords were evident at P0 (Figure 3c, 3d and Figure S5c, S5d) and, at puberty (P12), development of the seminiferous tubules appeared complete in Rspo1KOSox9cKO gonads (Figure 3h, 3i and Figure S5h, S5i). At puberty, androgen receptor (AR) immunostaining indicated that, in addition to Sertoli cells, peritubular myoid and Leydig cells were also present both in XY Rspo1KOSox9cKO testes (Figure 4i) and in testicular parts of the XX Rspo1KOSox9cKO ovotestes (data not shown). In addition, follicle development appeared at P12 in XX Rspo1KOSox9cKO ovotestes and XX control ovaries (Figure S2B d, f). Together our results indicate that seminiferous tubule development is delayed in the absence of Sox9 and Rspo1, thereby explaining the small size of the XY Rspo1KOSox9cKO testes (Figure 1c).
Fig. 3. Post-natal development of sex cords in XY and XX Rspo1KOSox9cKO mice. 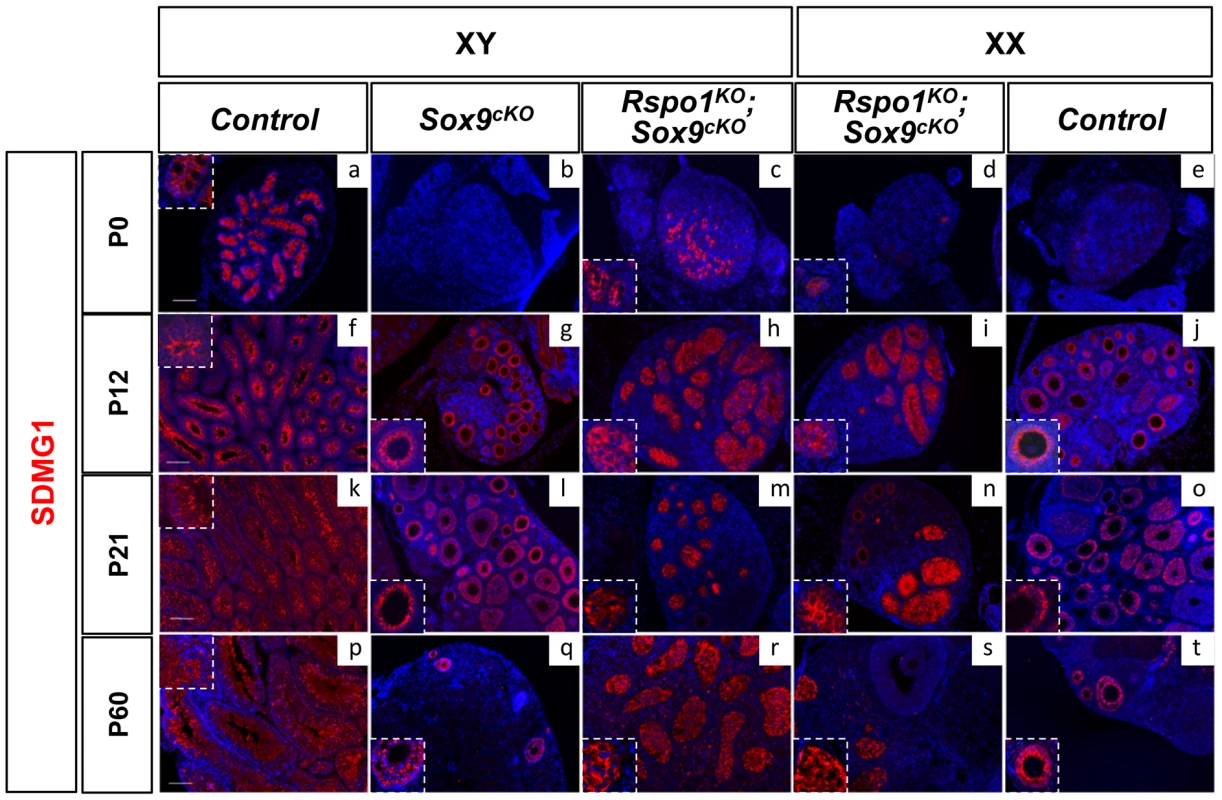
Immunofluorescence of SDMG1 (in red). Counterstain is DAPI (in blue). SDMG1 is expressed in Sertoli cells (XY controls a, f, k, p) and in follicular cells of growing ovaries as evidenced at P12 onwards (j, o, t). Sertoli cells are present and formed sex cords in both XY and XX Rspo1KOSox9cKO gonads, with more developing sex cords in XY Rspo1KOSox9cKO testis (c, h, m, r) in comparison to XX Rspo1KOSox9cKO ovotestis (d, i, n, s). At P12, the sex cords are fully developed in both XY (h) and XX (i) Rspo1KOSox9cKO mice. In XY Sox9cKO (b, g, l, q) and XX control (e, j, o, t) gonads, ovarian follicles express SDMG1 at P12, P21 and P60. At these stages, SDMG1 is also expressed in the follicles of the XX double mutant ovotestes (see n) and in XY double mutant follicles when they develop (scale bars: 100 µm). XY (a, f, k, p) and XX (e, j, o, t) Rspo1+/−; Sox9flox/flox controls, XY Sox9cKO gonads (b, g, l, q), XY (c, h, m, r) and XX (d, i, n, s) Rspo1KO Sox9cKO gonads respectively. Fig. 4. Sertoli cells support germ cell differentiation in XY Rspo1KOSox9cKO gonads. 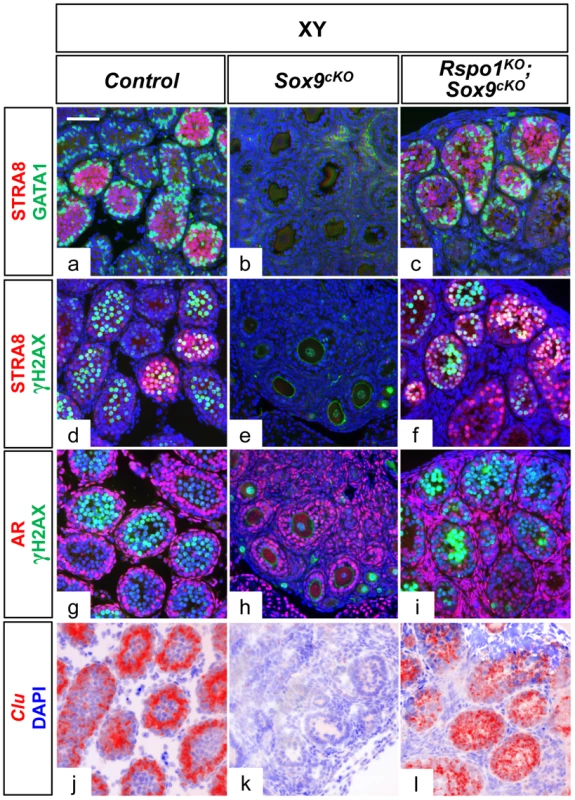
Immunofluorescence (a–i) of GATA1 (Sertoli cell marker, in green), AR (Androgen Receptor) (Sertoli, peritubular myoid and Leydig cell marker, in red), STRA8 (a premeiotic marker, in red), and γH2AX (a meiotic marker, in green) at P10. Counterstain is DAPI (in blue). In situ hybridization (j–l) using a probe for Clu transcripts, another marker for mature Sertoli cells, illustrated as computer–generated bright field superimpositions of the blue counterstain (DAPI) with the hybridization signal (red false color). GATA1, AR and Clu expression show that the Sertoli cells mature in XY controls (a, g, j) and XY Rspo1KOSox9cKO testes (c, i, l), and are able to support germ cell differentiation until meiosis initiation as revealed by STRA8 (a, c, d, f) and γH2AX (d, f, g, i) expression. Note that both Sertoli, peritubular myoid and Leydig cells of XY Sox9cKO mutant gonads normally expressed AR (h). (scale bars: 50 µm). XY (a, d, g, j) Rspo1+/−; Sox9flox/flox controls, XY Sox9cKO gonads (b, e, h, k) and XY Rspo1KOSox9cKO (c, f, i, l) gonads. SOX9-negative Sertoli cells can support germ cell differentiation until initiation of meiosis
We next investigated whether the Sertoli cells that differentiate in the Rspo1KOSox9cKO gonads can support germ cell differentiation. Since XX germ cells cannot survive in a testicular environment [42], [43], the analysis was only carried out in XY Rspo1KOSox9cKO gonads. In the normal fetal testis, following Sertoli cell differentiation, prospermatogonia become quiescent from 14.5 dpc and express the multipotency marker Oct4 until 17.5 dpc [44]. At that time, Cyp26b1, a protein involved in retinoic acid degradation, contributes to prevent germ cells from entering meiosis [45], [46]. As expected, the majority of prospermatogonia in XY Rspo1KOSox9cKO gonads expressed Oct4 at 14.5 dpc (Figure S6o). Nonetheless, some cells had already committed to meiosis (Figure S6k) and expressed the meiotic marker Stra8 [47], possibly because of the low level of Cyp26b1 expression in XY Rspo1KOSox9cKO gonads (Figure S6g). The reduced level of Cyp26b1 expression is however not sufficient to allow all germ cells to enter meiosis in XY Rspo1KOSox9cKO gonads.
At P10, GATA1, Androgen Receptors (AR) and Clusterin (Clu) were normally expressed in Sertoli cells of XY Rspo1KOSox9cKO gonads (Figure 4c, 4i, 4l), suggesting that these cells have acquired their identity and may be capable to support spermatogenesis. Accordingly, XY germ cells had committed to meiosis at P10, as assessed by immunodetection of the pre-meiotic and meiotic markers STRA8 and γH2AX, respectively (Figure 4c, 4f, 4i). However, later stages of spermatogenesis cannot however be analyzed, as hypoplasia of germ cells occurred within the seminiferous tubules of adult XY Rspo1KOSox9cKO gonads (Figure 1h and Figure S1m), most likely because of cryptorchidism.
Sox8 and Sox10 are expressed in the absence of Sox9
Interestingly, we found that AMH was expressed in Sertoli cells of both XX and XY Rspo1KOSox9cKO gonads at P12 (Figure 5A). Given that (i) Amh is a target-gene of SOX9 [48], [49], (ii) Amh expression can be induced by SOX8 [50] and SOX10 [51], and (iii) Sox10 ectopic up-regulation in XX gonads can induce testis differentiation [51], we hypothesized that a Sox factor distinct from Sox9 could have induced late AMH expression in Rspo1KOSox9cKO gonads and delayed testicular differentiation. In agreement with this possibility, expression of both Sox8 and Sox10 was activated in Rspo1KOSox9cKO mutants at P12 and P0 respectively (Figure 5B, 5C). Previous data have shown that Sox8 becomes crucial from 14.5 dpc onwards, for the maintenance of testis development [52], suggesting that Sertoli cell differentiation can be induced by Sox genes other than Sox9 during late embryogenesis. However, the function of these Sox genes during late development is likely not sufficient to replace the role of Sox9 in early Sertoli cells development, thus leading to the formation of an hypoplastic testis, as is the case in the XY Rspo1KOSox9cKO mice. To date, the only factors that have been shown to be able to induce Sertoli cell differentiation are Sox genes [51], [53], while other factors such as Dmrt1 are required after birth (P7) for the maintenance of Sertoli cell identity [54]. Further studies are required to address whether DMRT1 is able to allow Sertoli cell differentiation from undifferentiated supporting cells. Given that Sox9 expression is controlled by Wt1 when Sry expression has ceased [55], we can speculate that Wt1 might also be involved in Sox8 and Sox10 expression in these mutants. Furthermore, FGF9 or PGD2, two secreted factors synthesized in the undifferentiated gonads, [56], [57] can also contribute to Sertoli cell differentiation [58], [59], [60]. Whether Wt1, PGD2 or FGF9 signaling also regulate Sox8 and Sox10 remains to be investigated.
Fig. 5. AMH and SOX genes are expressed in XY and XX Rspo1KO Sox9cKO gonads. 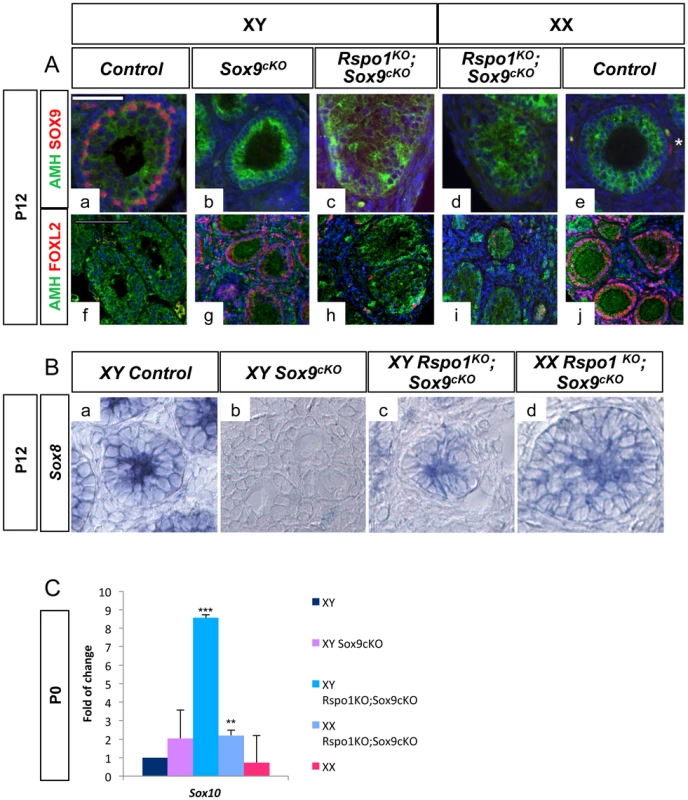
A- AMH expression in absence of SOX9. Immunofluorescence of SOX9 (in red) and AMH (in green). Counterstain is DAPI (in blue). SOX9 and AMH are synthetised in Sertoli cells of the testis (a, f). SOX9 is expressed in theca cells (white star in e) and AMH in follicular cells of the ovary at P12 (e, j). Deletion of Sox9 with Sf1:cre eliminates SOX9 expression in Sf1:cre positive cells of the gonads, which are Sertoli cells in XY (c) and XX (d) Rspo1KOSox9cKO gonads and theca cells of the ovarian region of XX Rspo1KOSox9cKO gonads (d) and XY Sox9cKO gonads (b). AMH expression is observed in Sertoli cells of the XY (c, h) and XX (d, i) Rspo1KOSox9cKO gonads even the absence of SOX9. (scale bar: 50 µm). Immunofluorescence of FOXL2 (in red) and AMH (in green). Counterstain is DAPI (in blue). Most of the AMH positive cells in the testicular cords of Rspo1KOSox9cKO gonads (h, i) are negative for FOXL2 indicating that they are not granulosa cells, some AMH/FOXL2 positive cells were observed outside of these cords indicating that they are granulosa cells (h, i). (scale bar: 100 µm). XY (a, f) and XX (e, j) Rspo1+/−;Sox9flox/flox controls, XY Sox9cKO gonads (b, g), XY (c, h) and XX (d, i) Rspo1KO Sox9cKO gonads respectively. B- Sox8 is expressed in XY and XX Rspo1KO Sox9cKO gonads. In situ hybridization of Sox8 transcripts. Sox8 is expressed in Sertoli cells at P5 in XY control (a), XY (c) and XX (d) Rspo1KOSox9cKO gonads, but not in XY Sox9cKO ovaries (b). (a) XY Rspo1+/−; Sox9flox/flox controls, (b) XY Sox9cKO gonads, XY (c) and XX (d) Rspo1KO Sox9cKO gonads respectively. C- Sox10 is expressed in XY and XX Rspo1KO Sox9cKO gonads. QPCR analysis shows that Sox10 is significantly up-regulated both in XY and XX Rspo1KOSox9cKO gonads, when compared to XY controls. The differences between XY controls and XY Sox9cKO are not significant. In addition, when XX and XY Rspo1KOSox9cKO gonads are compared at the same stage, XY gonads always appear more masculinized than XX gonads (Figure 1, Figure 3, Figure S1, Figure S3, Figure S5), because they contain more sex cords/seminiferous tubules. At a molecular level, the main difference between XX and XY Rspo1KOSox9cKO gonads is the expression of SRY in XY gonads (Figure 2). Indeed, SRY expression is maintained in XY Rspo1KOSox9cKO gonads at 13.5 dpc, while at this time point its expression has ceased in XY control gonads. This suggests that SRY participates in the masculinization of the XY Rspo1KOSox9cKO gonads by inducing the expression of genes other than Sox9 to promote sex cord formation.
In summary, here we have shown that (i) both SRY and SOX9 are dispensable for female-to-male sex reversal in Rspo1KO, (ii) RSPO1 signaling is required for male-to-female sex reversal in Sox9cKO, (iii) Sertoli cell differentiation and seminiferous tubule formation can occur in the absence of SOX9, possibly because of a functional redundancy with other SOX proteins such as SOX8 and SOX10. Indeed, ectopic expression of Sox10 in XX gonads has been shown to promote testicular differentiation [51]. Altogether these data show that SRY and SOX9 are not the only masculinizing factors, since other SOX proteins can induce female-to-male sex reversal in pathophysiological conditions (Figure 6). Following Sertoli cell differentiation, DMRT1 expression becomes required for the maintenance of the Sertoli cells and the testicular tissue [54]. Furthermore, our data suggest that the feminizing factors remaining in Rspo1KO mice can be overtaken by SOX proteins, when they are activated in XX gonads. Testicular differentiation in the absence of Rspo1 expression in XY Sox9cKO gonads was unexpected since female development is thought to be a default pathway [61], [62]. Our results imply that instead the female pathway needs to be activated. Therefore our genetic study suggests that mammalian sex determination is regulated by a finely tuned balance between two main factors [56], [63], which are the SOX genes on the one hand and the RSPO1/WNT/beta-catenin signaling pathway on the other hand.
Fig. 6. Opposing function of SOX and RSPO1 signaling in the fate of the gonad. 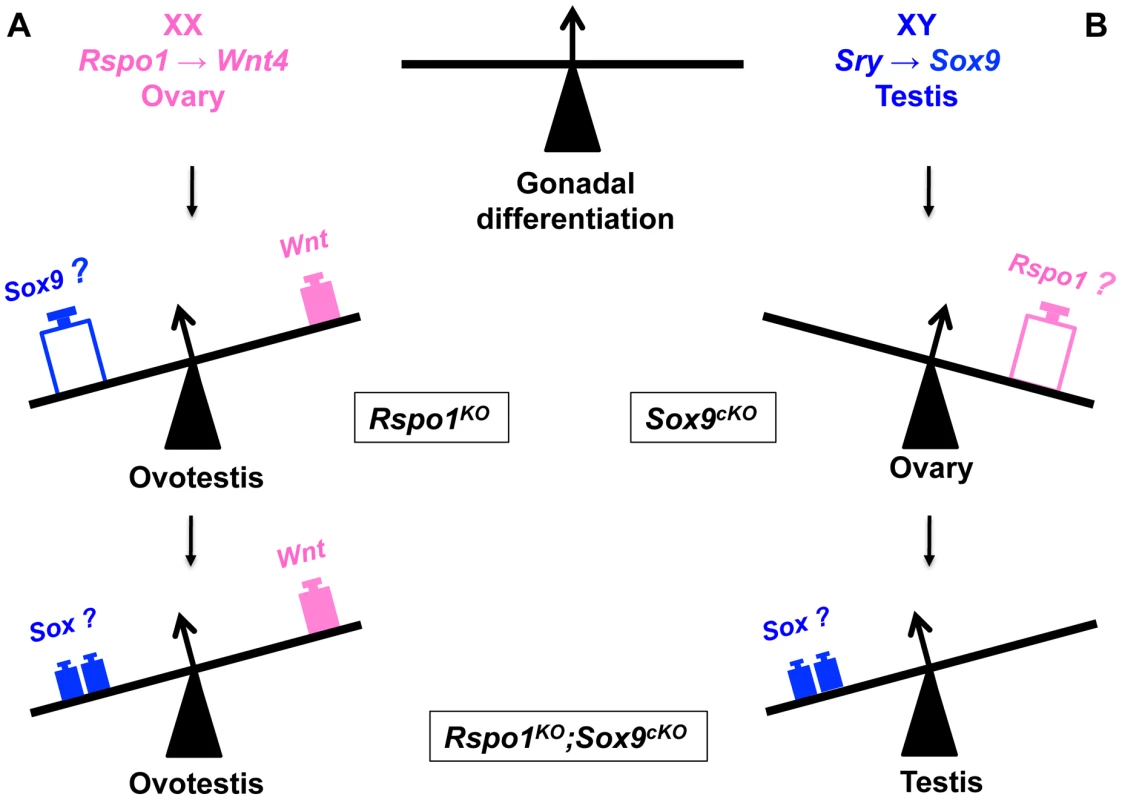
A- In XX gonads, RSPO1 activates WNT/beta-catenin signaling to promote ovarian differentiation. Ablation of Rspo1 results in partial sex reversal with ovotestis development, which coincides with Sox9 expression. However additional deletion of Sox9 in the XX Rspo1KO (i.e., Rspo1KOSox9cKO) still allows ovotestis formation, implying that Sry and Sox9 are not required for testicular differentiation in female-to-male sex reversal. B- In XY gonads, whereas Sox9 deletion triggers ovarian development, additional deletion of Rspo1 in XY Rspo1KOSox9cKO gonads restores testis development. This is associated with the expression of other SOX genes like SOX 8 and SOX10, other masculinising factors. Materials and Methods
Mouse strains and genotyping
The experiments here described were carried out in compliance with the relevant institutional and French animal welfare laws, guidelines and policies. They have been approved by the French ethics committee (Comité Institutionnel d'Ethique Pour l'Animal de Laboratoire; number NCE/2011-12). All mouse lines were kept on a mixed 129SV/C57BL6/J background. Rspo1−/−, Sox9flox/flox mice and Sf1:creTg/+ transgenic mice (a kind gift from Keith Parker) were described previously [64]. Rspo1−/− male were mated with Sox9flox/flox; Sf1:creTg/+ female [16] to obtain Rspo1+/−; Sox9flox/+; Sf1:creTg/+ females and Rspo1+/−; Sox9flox/+ males. Matings between these littermates allowed us to generate Rspo1−/−; Sox9fl/fl; Sf1:creTg/+ mice, referred to as Rspo1KO Sox9cKO mice, and controls. Gonad samples were collected from timed pups (day of birth = P0). Genotyping was performed as described [7], [20], [64] using DNA extracted from tail tip or ear biopsies of mice. The presence of the Y chromosome was determined as described previously [65]. Pax6 primer set (5′-GCAACAGGAAGGAGGGGGAGA-3′; 5′-CTTTCTCCAGAGCCTCAATCTG-3′) was included in each PCR reaction as an internal control.
Histological analysis
Urogenital organs were dissected, fixed in Bouin's solution overnight, and then embedded in paraffin. Microtome sections of 5 µm thickness were stained with periodic acid Schiff (PAS) or hematoxylin and eosin (H&E) according to standard procedures. Pictures were taken with an Axiocam mrm camera (Zeiss) and processed with Adobe Photoshop.
Immunological analyses
Gonad samples were fixed with 4% (w/v) paraformaldehyde overnight and then processed for paraffin embedding. Gonad samples for cryosections were successively fixed for 2 hours in 4% (w/v) paraformaldehyde, washed in cold phosphate-buffered saline (PBS), equilibrated in 10% (w/v) sucrose solution during 3 hours, then in 30% (w/v) sucrose solution overnight at 4°C, embedded in Cryomount (Histolab) and stored at −80°C. For paraffin-embedded and Cryomount-embedded samples, sections of 5 and 8 µm thickness were processed, respectively. The following dilutions of primary antibodies were used: AMH/MIS (C-20, sc-6886, Santa Cruz), 1∶200; AR (sc-816, SantaCruz), 1∶100; DMRT1 (kindly provided by David Zarkower), 1∶500; FOXL2 (ab5096, Abcam), 1∶250; γH2AX (U5-636, Upstate), 1∶500; GATA1 (sc-265, SantaCruz), 1∶50; SDMG1 (a kind gift from Ian Adams) 1∶2000; SF1 (kindly provided by Ken Morohashi) 1∶1500; SOX9 (HPA-001758, Sigma) 1∶250 and SRY [59], [66] 1∶100, STRA8 (ab49602, Abcam), 1∶100. Counterstain with 4′,6-diamidino-2-phenylindole (DAPI) was used to detect nuclei (in blue). Fluorescent studies were performed with a motorized Axio ImagerZ1 microscope (Zeiss) and pictures were taken with an Axiocam mrm camera (Zeiss) and processed with Axiovision LE.
In situ hybridization
Embryos were fixed with 4% paraformaldehyde (PFA) in 1×PBS at 4°C overnight. Further processing of embryos and in situ hybridization were carried out essentially as described [67]. Sox9 riboprobe was synthesized according to [68] and Sox8 to [69], P450scc, Stra8 and Oct4 riboprobes synthesis were carried out as described previously [20]. In situ hybridisation (ISH) with digoxigenin–labelled probes was performed as described [70], using 10 µm–thick cryosections. Each experiment was repeated on at least two gonads. Post–hybridization washes were done in 100 mM maleic acid pH7.5, 150 mM NaCl, 0.1% (v/v) tween–20 (MABT). To increase the sensitivity, 5% (v/v) polyvinyl alcohol (Sigma) was added to the staining solution [71]. Nuclei were counterstained with DAPI diluted in the mounting medium at 10 µg/ml (Vectashield, Vector laboratories). ISH signals corresponding to Clu-positive cells were converted into a red false color on the merged pictures. The plasmids containing Lgals1 (366 bp–long; exons 2–4; MGI:96777) or Clu (942 bp–long; exons 5–9; MGI:88423) cDNA fragments were linearized and used as a templates for the synthesis of the sense or antisense riboprobes.
Quantitative PCR analysis
Individual gonads were dissected in PBS from P0 animals (day of birth) and immediately frozen at −80°C. RNA was extracted using the RNeasy Qiagen kit, and reverse transcribed using the RNA RT–PCR kit (Stratagene). Primers and probes were designed at Roche Assay design center (https://www.rocheappliedscience.com/sis/rtpcr/upl/adc.jsp). Primers are 5′-TCCTCCTCAGACCGCTTTT-3′ and 5′-CCTGGTTCATCATCGCTAATC-3′ (probe 95) for Hprt1, and 5′-ATGTCAGATGGGAACCCAGA-3′ and 5′-GTCTTTGGGGTGGTTGGAG-3′ (probe 21) for Sox10, 5′-aagaagtgcagcctgattgc-3′ and 5′-ggtggctgatacccagttct-3′ (probe 40) for Dmrt1, and 5′-ggcgtcgtgaactcctaca-3′ and 5′-tgcagatgatgtgcgtgag-3′ (probe 51) for Foxl2. All real-time, quantitative, PCR assays (QPCR) were carried out using the LC-Faststart DNA Master kit Roche, according to the manufacturer's instructions. QPCR was performed on cDNA from one gonad and compared to a standard curve. QPCR were repeated at least twice. Relative expression levels of each sample were quantified in the same run, and normalized by measuring the amount of Hprt1 cDNA (which represents the total amount of gonadal cells).
Statistical analysis
For each genotype (n = 6 individuals), the fold-change was the mean normalized expression levels divided by the mean normalized expression levels of the XY samples considered as the reference. Graphs illustrate fold-changes +1 s.e.m. The results were analyzed using Graphpad for statistical significance that was assessed using one-way ANOVA followed by Tukey-Kramer post-test for selected pairs of genotypes. Asterisks indicate : * p<0.05, ** p<0.01 and *** p<0.001.
Supporting Information
Zdroje
1. DeFalcoT, CapelB (2009) Gonad morphogenesis in vertebrates: divergent means to a convergent end. Annu Rev Cell Dev Biol 25 : 457–482.
2. GubbayJ, CollignonJ, KoopmanP, CapelB, EconomouA, et al. (1990) A gene mapping to the sex-determining region of the mouse Y chromosome is a member of a novel family of embryonically expressed genes. Nature 346 : 245–250.
3. SinclairAH, BertaP, PalmerMS, HawkinsJR, GriffithsBL, et al. (1990) A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature 346 : 240–244.
4. KoopmanP, GubbayJ, VivianN, GoodfellowP, Lovell-BadgeR (1991) Male development of chromosomally female mice transgenic for Sry. Nature 351 : 117–121.
5. SekidoR, Lovell-BadgeR (2008) Sex determination involves synergistic action of SRY and SF1 on a specific Sox9 enhancer. Nature 453 : 930–934.
6. BarrionuevoF, Bagheri-FamS, KlattigJ, KistR, TaketoMM, et al. (2006) Homozygous inactivation of Sox9 causes complete XY sex reversal in mice. Biol Reprod 74 : 195–201.
7. ChaboissierMC, KobayashiA, VidalVI, LutzkendorfS, van de KantHJ, et al. (2004) Functional analysis of Sox8 and Sox9 during sex determination in the mouse. Development 131 : 1891–1901.
8. HiramatsuR, HarikaeK, TsunekawaN, KurohmaruM, MatsuoI, et al. (2010) FGF signaling directs a center-to-pole expansion of tubulogenesis in mouse testis differentiation. Development 137 : 303–312.
9. YaoHH, WhoriskeyW, CapelB (2002) Desert Hedgehog/Patched 1 signaling specifies fetal Leydig cell fate in testis organogenesis. Genes Dev 16 : 1433–1440.
10. DeFalcoT, TakahashiS, CapelB (2011) Two distinct origins for Leydig cell progenitors in the fetal testis. Dev Biol 352 : 14–26.
11. BarriosF, FilipponiD, PellegriniM, ParonettoMP, Di SienaS, et al. (2010) Opposing effects of retinoic acid and FGF9 on Nanos2 expression and meiotic entry of mouse germ cells. J Cell Sci 123 : 871–880.
12. BowlesJ, FengCW, SpillerC, DavidsonTL, JacksonA, et al. (2010) FGF9 suppresses meiosis and promotes male germ cell fate in mice. Dev Cell 19 : 440–449.
13. BertaP, HawkinsJR, SinclairAH, TaylorA, GriffithsBL, et al. (1990) Genetic evidence equating SRY and the testis-determining factor. Nature 348 : 448–450.
14. WagnerT, WirthJ, MeyerJ, ZabelB, HeldM, et al. (1994) Autosomal sex reversal and campomelic dysplasia are caused by mutations in and around the SRY-related gene SOX9. Cell 79 : 1111–1120.
15. Lovell-BadgeR, RobertsonE (1990) XY female mice resulting from a heritable mutation in the primary testis-determining gene, Tdy. Development 109 : 635–646.
16. LaveryR, LardenoisA, Ranc-JianmotamediF, PauperE, GregoireEP, et al. (2011) XY Sox9 embryonic loss-of-function mouse mutants show complete sex reversal and produce partially fertile XY oocytes. Dev Biol 354 : 111–122.
17. VidalVP, ChaboissierMC, de RooijDG, SchedlA (2001) Sox9 induces testis development in XX transgenic mice. Nat Genet 28 : 216–217.
18. BishopCE, WhitworthDJ, QinY, AgoulnikAI, AgoulnikIU, et al. (2000) A transgenic insertion upstream of sox9 is associated with dominant XX sex reversal in the mouse. Nat Genet 26 : 490–494.
19. ParmaP, RadiO, VidalV, ChaboissierMC, DellambraE, et al. (2006) R-spondin1 is essential in sex determination, skin differentiation and malignancy. Nat Genet 38 : 1304–1309.
20. ChassotAA, RancF, GregoireEP, Roepers-GajadienHL, TaketoMM, et al. (2008) Activation of beta-catenin signaling by Rspo1 controls differentiation of the mammalian ovary. Hum Mol Genet 17 : 1264–1277.
21. TomizukaK, HorikoshiK, KitadaR, SugawaraY, IbaY, et al. (2008) R-spondin1 plays an essential role in ovarian development through positively regulating Wnt-4 signaling. Hum Mol Genet 17 : 1278–1291.
22. VainioS, HeikkilaM, KispertA, ChinN, McMahonAP (1999) Female development in mammals is regulated by Wnt-4 signalling. Nature 397 : 405–409.
23. MaatoukDM, DiNapoliL, AlversA, ParkerKL, TaketoMM, et al. (2008) Stabilization of beta-catenin in XY gonads causes male-to-female sex-reversal. Hum Mol Genet 17 : 2949–2955.
24. ManuylovNL, SmagulovaFO, LeachL, TevosianSG (2008) Ovarian development in mice requires the GATA4-FOG2 transcription complex. Development 135 : 3731–3743.
25. SchmidtD, OvittCE, AnlagK, FehsenfeldS, GredstedL, et al. (2004) The murine winged-helix transcription factor Foxl2 is required for granulosa cell differentiation and ovary maintenance. Development 131 : 933–942.
26. OttolenghiC, OmariS, Garcia-OrtizJE, UdaM, CrisponiL, et al. (2005) Foxl2 is required for commitment to ovary differentiation. Hum Mol Genet 14 : 2053–2062.
27. UhlenhautNH, JakobS, AnlagK, EisenbergerT, SekidoR, et al. (2009) Somatic sex reprogramming of adult ovaries to testes by FOXL2 ablation. Cell 139 : 1130–1142.
28. UdaM, OttolenghiC, CrisponiL, GarciaJE, DeianaM, et al. (2004) Foxl2 disruption causes mouse ovarian failure by pervasive blockage of follicle development. Hum Mol Genet 13 : 1171–1181.
29. RaymondCS, MurphyMW, O'SullivanMG, BardwellVJ, ZarkowerD (2000) Dmrt1, a gene related to worm and fly sexual regulators, is required for mammalian testis differentiation. Genes Dev 14 : 2587–2595.
30. JosephA, YaoH, HintonBT (2009) Development and morphogenesis of the Wolffian/epididymal duct, more twists and turns. Dev Biol 325 : 6–14.
31. AugusteA, ChassotAA, GregoireEP, RenaultL, PannetierM, et al. (2011) Loss of R-spondin1 and Foxl2 amplifies female-to-male sex reversal in XX mice. Sex Dev 5 : 304–317.
32. Jeays-WardK, HoyleC, BrennanJ, DandonneauM, AlldusG, et al. (2003) Endothelial and steroidogenic cell migration are regulated by WNT4 in the developing mammalian gonad. Development 130 : 3663–3670.
33. Jeays-WardK, DandonneauM, SwainA (2004) Wnt4 is required for proper male as well as female sexual development. Dev Biol 276 : 431–440.
34. HeikkilaM, PrunskaiteR, NaillatF, ItarantaP, VuoristoJ, et al. (2005) The partial female to male sex reversal in Wnt-4-deficient females involves induced expression of testosterone biosynthetic genes and testosterone production, and depends on androgen action. Endocrinology 146 : 4016–4023.
35. ValP, Jeays-WardK, SwainA (2006) Identification of a novel population of adrenal-like cells in the mammalian testis. Dev Biol 299 : 250–256.
36. Nel-ThemaatL, GonzalezG, AkiyamaH, BehringerRR (2010) Illuminating testis morphogenesis in the mouse. J Androl 31 : 5–10.
37. BehringerRR, FinegoldMJ, CateRL (1994) Mullerian-inhibiting substance function during mammalian sexual development. Cell 79 : 415–425.
38. BullejosM, KoopmanP (2001) Spatially dynamic expression of Sry in mouse genital ridges. Dev Dyn 221 : 201–205.
39. HackerA, CapelB, GoodfellowP, Lovell-BadgeR (1995) Expression of Sry, the mouse sex determining gene. Development 121 : 1603–1614.
40. JeskeYW, BowlesJ, GreenfieldA, KoopmanP (1995) Expression of a linear Sry transcript in the mouse genital ridge. Nat Genet 10 : 480–482.
41. IkedaY, ShenWH, IngrahamHA, ParkerKL (1994) Developmental expression of mouse steroidogenic factor-1, an essential regulator of the steroid hydroxylases. Mol Endocrinol 8 : 654–662.
42. IsotaniA, NakanishiT, KobayashiS, LeeJ, ChumaS, et al. (2005) Genomic imprinting of XX spermatogonia and XX oocytes recovered from XX<–>XY chimeric testes. Proc Natl Acad Sci U S A 102 : 4039–4044.
43. ChassotAA, GregoireEP, LaveryR, TaketoMM, de RooijDG, et al. (2011) RSPO1/beta-catenin signaling pathway regulates oogonia differentiation and entry into meiosis in the mouse fetal ovary. PLoS ONE 6: e25641 doi:10.1371/journal.pone.0025641..
44. Maldonado-SaldiviaJ, van den BergenJ, KrouskosM, GilchristM, LeeC, et al. (2007) Dppa2 and Dppa4 are closely linked SAP motif genes restricted to pluripotent cells and the germ line. Stem Cells 25 : 19–28.
45. LiH, MacLeanG, CameronD, Clagett-DameM, PetkovichM (2009) Cyp26b1 expression in murine Sertoli cells is required to maintain male germ cells in an undifferentiated state during embryogenesis. PLoS ONE 4: e7501 doi:10.1371/journal.pone.0007501..
46. MacLeanG, LiH, MetzgerD, ChambonP, PetkovichM (2007) Apoptotic extinction of germ cells in testes of Cyp26b1 knockout mice. Endocrinology 148 : 4560–4567.
47. BaltusAE, MenkeDB, HuYC, GoodheartML, CarpenterAE, et al. (2006) In germ cells of mouse embryonic ovaries, the decision to enter meiosis precedes premeiotic DNA replication. Nat Genet 38 : 1430–1434.
48. ArangoNA, Lovell-BadgeR, BehringerRR (1999) Targeted mutagenesis of the endogenous mouse Mis gene promoter: in vivo definition of genetic pathways of vertebrate sexual development. Cell 99 : 409–419.
49. De Santa BarbaraP, BonneaudN, BoizetB, DesclozeauxM, MoniotB, et al. (1998) Direct interaction of SRY-related protein SOX9 and steroidogenic factor 1 regulates transcription of the human anti-Mullerian hormone gene. Mol Cell Biol 18 : 6653–6665.
50. SchepersG, WilsonM, WilhelmD, KoopmanP (2003) SOX8 is expressed during testis differentiation in mice and synergizes with SF1 to activate the Amh promoter in vitro. J Biol Chem 278 : 28101–28108.
51. PolancoJC, WilhelmD, DavidsonTL, KnightD, KoopmanP (2010) Sox10 gain-of-function causes XX sex reversal in mice: implications for human 22q-linked disorders of sex development. Hum Mol Genet 19 : 506–516.
52. BarrionuevoF, GeorgI, ScherthanH, LecureuilC, GuillouF, et al. (2009) Testis cord differentiation after the sex determination stage is independent of Sox9 but fails in the combined absence of Sox9 and Sox8. Dev Biol 327 : 301–312.
53. SuttonE, HughesJ, WhiteS, SekidoR, TanJ, et al. (2011) Identification of SOX3 as an XX male sex reversal gene in mice and humans. J Clin Invest 121 : 328–341.
54. MatsonCK, MurphyMW, SarverAL, GriswoldMD, BardwellVJ, et al. (2011) DMRT1 prevents female reprogramming in the postnatal mammalian testis. Nature 476 : 101–104.
55. GaoF, MaitiS, AlamN, ZhangZ, DengJM, et al. (2006) The Wilms tumor gene, Wt1, is required for Sox9 expression and maintenance of tubular architecture in the developing testis. Proc Natl Acad Sci U S A 103 : 11987–11992.
56. KimY, KobayashiA, SekidoR, DinapoliL, BrennanJ, et al. (2006) Fgf9 and wnt4 act as antagonistic signals to regulate Mammalian sex determination. PLoS Biol 4: e187 doi:10.1371/journal.pbio.0040187..
57. MoniotB, FarhatA, AritakeK, DeclosmenilF, NefS, et al. (2011) Hematopoietic prostaglandin D synthase (H-Pgds) is expressed in the early embryonic gonad and participates to the initial nuclear translocation of the SOX9 protein. Dev Dyn 240 : 2335–2343.
58. AdamsIR, McLarenA (2002) Sexually dimorphic development of mouse primordial germ cells: switching from oogenesis to spermatogenesis. Development 129 : 1155–1164.
59. WilhelmD, MartinsonF, BradfordS, WilsonMJ, CombesAN, et al. (2005) Sertoli cell differentiation is induced both cell-autonomously and through prostaglandin signaling during mammalian sex determination. Dev Biol 287 : 111–124.
60. KimY, BinghamN, SekidoR, ParkerKL, Lovell-BadgeR, et al. (2007) Fibroblast growth factor receptor 2 regulates proliferation and Sertoli differentiation during male sex determination. Proc Natl Acad Sci U S A 104 : 16558–16563.
61. JostA (1947) Recherche sur la différenciation sexuelle de l'embryon de lapin. Arch Anat Microsc Morph Exp 271–315.
62. McLarenA (1991) Development of the mammalian gonad: the fate of the supporting cell lineage. Bioessays 13 : 151–156.
63. MungerSC, AylorDL, SyedHA, MagwenePM, ThreadgillDW, et al. (2009) Elucidation of the transcription network governing mammalian sex determination by exploiting strain-specific susceptibility to sex reversal. Genes Dev 23 : 2521–2536.
64. BinghamNC, Verma-KurvariS, ParadaLF, ParkerKL (2006) Development of a steroidogenic factor 1/Cre transgenic mouse line. Genesis 44 : 419–424.
65. Hogan BL, Beddington RS, F C, E L (1994) Manipulating the Mouse Embryo. Cold Spring Harbor: Cold Spring Harbor Laboratory Press.
66. BradfordST, WilhelmD, KoopmanP (2007) Comparative analysis of anti-mouse SRY antibodies. Sex Dev 1 : 305–310.
67. Wilkinson DG (1992) Whole mount in situ hybridization of vertebrate embryos. Whole mount in situ hybridization of vertebrate embryos Oxford: Oxford University Press: 75–83.
68. Morais da SilvaS, HackerA, HarleyV, GoodfellowP, SwainA, et al. (1996) Sox9 expression during gonadal development implies a conserved role for the gene in testis differentiation in mammals and birds. Nat Genet 14 : 62–68.
69. SockE, SchmidtK, Hermanns-BorgmeyerI, BoslMR, WegnerM (2001) Idiopathic weight reduction in mice deficient in the high-mobility-group transcription factor Sox8. Mol Cell Biol 21 : 6951–6959.
70. VernetN, DennefeldC, Rochette-EglyC, Oulad-AbdelghaniM, ChambonP, et al. (2006) Retinoic acid metabolism and signaling pathways in the adult and developing mouse testis. Endocrinology 147 : 96–110.
71. DeBlockM, DebrouwerD (1993) RNA-RNA in situ hybridization using digoxigenin-labeled probes: the use of high-molecular-weight polyvinyl alcohol in the alkaline phosphatase indoxyl-nitroblue tetrazolium reaction. Anal Biochem 215 : 86–89.
72. JamesonSA, LinYT, CapelB (2012) Testis development requires the repression of Wnt4 by Fgf signaling. Dev Biol
73. HuntPA, WorthmanC, LevinsonH, StallingsJ, LeMaireR, et al. (1998) Germ cell loss in the XXY male mouse: altered X-chromosome dosage affects prenatal development. Mol Reprod Dev 49 : 101–111.
Štítky
Genetika Reprodukční medicína
Článek Defining the Pathways Underlying the Prolonged PR Interval in Atrioventricular Conduction DiseaseČlánek Translation in Giant Viruses: A Unique Mixture of Bacterial and Eukaryotic Termination SchemesČlánek Controls Gliogenesis by Regulating the Transient Expression of the Gcm/Glide Fate Determinant
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2012 Číslo 12
-
Všechny články tohoto čísla
- A Mystery Unraveled: Essentiality of RNase III in Is Caused by Resident Prophages
- Defining the Pathways Underlying the Prolonged PR Interval in Atrioventricular Conduction Disease
- Insertion/Deletion Polymorphisms in the Promoter Are a Risk Factor for Bladder Exstrophy Epispadias Complex
- Mi2β Is Required for γ-Globin Gene Silencing: Temporal Assembly of a GATA-1-FOG-1-Mi2 Repressor Complex in β-YAC Transgenic Mice
- Dissection of a Quantitative Trait Locus for PR Interval Duration Identifies as a Novel Modulator of Cardiac Conduction
- Dnmt3a Protects Active Chromosome Domains against Cancer-Associated Hypomethylation
- Construction of a Global Pain Systems Network Highlights Phospholipid Signaling as a Regulator of Heat Nociception
- Genes Contributing to Pain Sensitivity in the Normal Population: An Exome Sequencing Study
- Identification of , a Locus Controlling Dominant Resistance to Autoimmune Orchitis, as Kinesin Family Member 1C
- ATX1-Generated H3K4me3 Is Required for Efficient Elongation of Transcription, Not Initiation, at ATX1-Regulated Genes
- Dynamic and Differential Regulation of Stem Cell Factor FoxD3 in the Neural Crest Is Encrypted in the Genome
- Identification of Novel Type 2 Diabetes Candidate Genes Involved in the Crosstalk between the Mitochondrial and the Insulin Signaling Systems
- The Genetic Architecture of Adaptations to High Altitude in Ethiopia
- Population Genomics of the Endosymbiont in
- Translation in Giant Viruses: A Unique Mixture of Bacterial and Eukaryotic Termination Schemes
- Testicular Differentiation Occurs in Absence of R-spondin1 and Sox9 in Mouse Sex Reversals
- A Yeast GSK-3 Kinase Mck1 Promotes Cdc6 Degradation to Inhibit DNA Re-Replication
- Genetic Adaptation Associated with Genome-Doubling in Autotetraploid
- The Essential Function of RNase III Is to Silence Foreign Toxin Genes
- Long-Range Regulatory Polymorphisms Affecting a GABA Receptor Constitute a Quantitative Trait Locus (QTL) for Social Behavior in
- A New Isolation with Migration Model along Complete Genomes Infers Very Different Divergence Processes among Closely Related Great Ape Species
- Chromosome Fragile Sites in Harbor Matrix Attachment Regions That May Be Associated with Ancestral Chromosome Rearrangement Events
- Genome-Wide Association Study Implicates Testis-Sperm Specific as a Susceptibility Locus for Impaired Acrosome Reaction in Stallions
- A Mechanism of Gene Amplification Driven by Small DNA Fragments
- Base Damage within Single-Strand DNA Underlies Hypermutability Induced by a Ubiquitous Environmental Agent
- Integrative Analysis of a Cross-Loci Regulation Network Identifies as a Gene Regulating Insulin Secretion from Pancreatic Islets
- Reciprocal Signaling between the Ectoderm and a Mesendodermal Left-Right Organizer Directs Left-Right Determination in the Sea Urchin Embryo
- Population Genomics of Sub-Saharan : African Diversity and Non-African Admixture
- Genome-Wide Fine-Scale Recombination Rate Variation in
- Deciphering the Transcriptional-Regulatory Network of Flocculation in
- On Lung Function and Interactions Using Genome-Wide Data
- Genome-Wide Joint Meta-Analysis of SNP and SNP-by-Smoking Interaction Identifies Novel Loci for Pulmonary Function
- The Effective Population Size of Malaria Mosquitoes: Large Impact of Vector Control
- Recessive Mutations in Implicate β-III Spectrin in Both Cognitive and Motor Development
- An Excess of Gene Expression Divergence on the X Chromosome in Embryos: Implications for the Faster-X Hypothesis
- Reduced Life- and Healthspan in Mice Carrying a Mono-Allelic MVA Mutation
- Natural Variation at the MATE Transporter Locus Reveals Cross-Talk between Fe Homeostasis and Zn Tolerance in
- Histone Deacetylase HDA6 Is Functionally Associated with AS1 in Repression of Genes in
- A Framework for the Establishment of a Cnidarian Gene Regulatory Network for “Endomesoderm” Specification: The Inputs of ß-Catenin/TCF Signaling
- A Polycomb Group Protein Is Retained at Specific Sites on Chromatin in Mitosis
- Diapause Formation and Downregulation of Insulin-Like Signaling via DAF-16/FOXO Delays Axonal Degeneration and Neuronal Loss
- Genes That Act Downstream of Sensory Neurons to Influence Longevity, Dauer Formation, and Pathogen Responses in
- A Genome-Wide RNAi Screen Reveals MAP Kinase Phosphatases as Key ERK Pathway Regulators during Embryonic Stem Cell Differentiation
- Recurrent Targeted Genes of Hepatitis B Virus in the Liver Cancer Genomes Identified by a Next-Generation Sequencing–Based Approach
- Excessive Astrocyte-Derived Neurotrophin-3 Contributes to the Abnormal Neuronal Dendritic Development in a Mouse Model of Fragile X Syndrome
- Pre-Disposition and Epigenetics Govern Variation in Bacterial Survival upon Stress
- Controls Gliogenesis by Regulating the Transient Expression of the Gcm/Glide Fate Determinant
- Systems Genetic Analysis of Osteoblast-Lineage Cells
- Population Genomics of Inversion Polymorphisms in
- Spreading of Heterochromatin Is Limited to Specific Families of Maize Retrotransposons
- DNA Topoisomerases Maintain Promoters in a State Competent for Transcriptional Activation in
- A Histone Deacetylase Adjusts Transcription Kinetics at Coding Sequences during Morphogenesis
- Approaching the Functional Annotation of Fungal Virulence Factors Using Cross-Species Genetic Interaction Profiling
- Evidence for the Robustness of Protein Complexes to Inter-Species Hybridization
- Systematic Identification of Rhythmic Genes Reveals as a New Element in the Circadian Clockwork
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- Dnmt3a Protects Active Chromosome Domains against Cancer-Associated Hypomethylation
- Population Genomics of Sub-Saharan : African Diversity and Non-African Admixture
- Excessive Astrocyte-Derived Neurotrophin-3 Contributes to the Abnormal Neuronal Dendritic Development in a Mouse Model of Fragile X Syndrome
- Pre-Disposition and Epigenetics Govern Variation in Bacterial Survival upon Stress
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



