-
Články
Reklama
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
ReklamaControlling Meiotic Recombinational Repair – Specifying the Roles of ZMMs, Sgs1 and Mus81/Mms4 in Crossover Formation
A critical component of successful reproduction is ensuring that the correct number of chromosomes is distributed to the gametes (i.e. sperm, eggs). Incorrect numbers of chromosomes in our gametes can directly result in infertility, miscarriages and developmental disabilities such as Down syndrome. Gamete production involves meiosis, in which crossovers between parental chromosomes are required to promote proper chromosome segregation. However, other types of recombination can occur that are not productive towards appropriate chromosome segregation. In this study, we examine several genes that are thought to play important roles in crossover (CO) promotion. By interpreting the final recombination products using a sequencing based analysis of all four gametes of an individual meiosis in budding yeast, we can infer the roles of these genes in recombination. We find that one protein, Zip3, can direct biased cleavage of the dHJ intermediate but another protein, Msh4, in the same complex cannot. Moreover, we find that a minor resolvase, Mus81/Mms4 (Eme1) is crucial in limiting chromosome entanglements by suppressing multiple consecutive recombination events from initiating from a single double-strand break (DSB). We favor a model that Mms4 is needed to remove a 3′-flap such that second-end capture of the DSB can occur.
Published in the journal: . PLoS Genet 10(10): e32767. doi:10.1371/journal.pgen.1004690
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004690Summary
A critical component of successful reproduction is ensuring that the correct number of chromosomes is distributed to the gametes (i.e. sperm, eggs). Incorrect numbers of chromosomes in our gametes can directly result in infertility, miscarriages and developmental disabilities such as Down syndrome. Gamete production involves meiosis, in which crossovers between parental chromosomes are required to promote proper chromosome segregation. However, other types of recombination can occur that are not productive towards appropriate chromosome segregation. In this study, we examine several genes that are thought to play important roles in crossover (CO) promotion. By interpreting the final recombination products using a sequencing based analysis of all four gametes of an individual meiosis in budding yeast, we can infer the roles of these genes in recombination. We find that one protein, Zip3, can direct biased cleavage of the dHJ intermediate but another protein, Msh4, in the same complex cannot. Moreover, we find that a minor resolvase, Mus81/Mms4 (Eme1) is crucial in limiting chromosome entanglements by suppressing multiple consecutive recombination events from initiating from a single double-strand break (DSB). We favor a model that Mms4 is needed to remove a 3′-flap such that second-end capture of the DSB can occur.
Introduction
Homologous recombination during meiosis plays an integral role in ensuring that each gamete receives exactly one copy of each chromosome from its diploid parent. COs, representing reciprocal repair between homologs, become chiasmata – physical bridges between homologous chromosomes that are required for the proper alignment and subsequent segregation of the homologs during the first meiotic division. Perturbation of crossing over leads to missegregation of chromosomes resulting in infertility, developmental disabilities and miscarriages [1]. Given the adverse consequences stemming from problems in crossing over, there is a clear need to understand the underlying mechanisms by which COs are controlled, particularly how the cell balances the choice of partner for recombination: intersister (IS) vs. interhomolog (IH) and the choice in pathway: reciprocal exchange resulting in COs vs. nonreciprocal exchange resulting in NCOs.
Based on budding yeast studies [2]–[4], COs are thought to mainly arise from biased resolution of dHJ intermediates that can be observed physically as joint molecules (JM) using 2D gels [5]. This is not the case for NCOs. Although a minority of NCOs may arise through unbiased cutting of the JM [6] (Figure 1), the bulk of NCOs appears to form via synthesis-dependent strand annealing (SDSA) [7]–[9] or by topoisomerase-assisted dissolution [8]. NCO formation is temporally distinct from CO formation, since NCOs appear about 30 minutes earlier than JM resolution [2], [10]. The difference in the formation of COs and NCOs is further highlighted by the fact that NCO formation is independent of Cdc5, a polo-like kinase, whereas COs require Cdc5 activity for JM resolution [11]. Taken together these studies clearly point to distinct mechanisms and intermediates that exist in the formation of COs vs. NCOs during meiosis.
Fig. 1. Overview of meiotic recombination pathways. 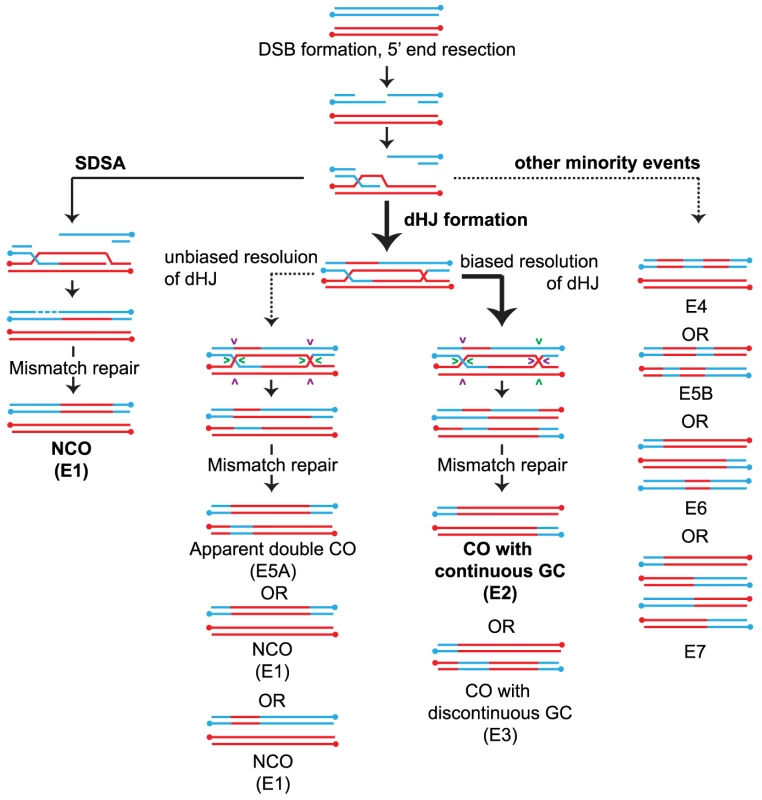
COs and NCOs normally form through two different pathways involving invasion of one of the resected 3′ ends of the DSB into a homolog followed by DNA repair (a) NCOs arise via SDSA where 3′ strand invasion is not accompanied by formation of a stable JM between the homologs. Disassembly of the SDSA intermediate allows reannealing and ligation to the other end of the DSB resulting in a NCO. (b) dHJ formation where the strand invasion is accompanied by stable JM formation. The majority of the dHJs are resolved into COs by biased cuts made to the two HJs followed by mismatch repair of the heteroduplexes. (Details about how heteroduplexes are resolved are shown in Figure S1). Pairs of cuts are indicated by similar colored arrowheads. A small fraction of the dHJs can resolve to form NCOs by unbiased cutting of the two HJs. (c) In addition, a minority of events likely results from multistrand invasions. Event classifications (E#) are described in the legend of Figure 2. Arrow weight indicates the degree of partitioning through the various pathways in WT. Recently, Sgs1, the yeast analog to the RecQ family helicase BLM has been identified as having a major role in directing recombinational repair in meiosis into either a NCO or JM fate [2], [12], [13]. Sgs1/BLM is a 3′-5′ helicase that is characterized as being part of an anti-CO complex or “dissolvasome” that can take apart DNA structures that stem from DNA replication or homologous recombination [14], [15]. In vitro, BLM can disrupt D-loops [16] and can displace a Rad51-coated single stranded DNA filament [17]. During meiosis, this type of displacement is postulated to lead to NCO formation by SDSA. The ability of Sgs1/BLM to branch migrate dHJs together followed by decatenation of TopoIIIA mediated single strand exchange (reviewed in [18]) is another mode by which meiotic NCOs can form, thereby diverting events away from a fate that would otherwise lead to COs. This ability of Sgs1/BLM to dismantle DNA structures has been taken to suggest that Sgs1 is also needed to disassemble aberrant recombination intermediates, an idea that is supported by the elevated levels of multichromatid recombination intermediates involving three or four chromatids in an sgs1 mutant [19].
In meiotic cells, Sgs1 exhibits an antagonistic relationship to synaptonemal complex proteins [19], [20] whose ability to promote crossing overs counters Sgs1 anti-CO activity [21]. The synaptonemal complex is a structure that resides between homologous chromosomes and is responsible for chromosome synapsis and CO promotion during meiotic prophase I. The synaptonemal complex proteins, collectively called ZMMs (Zip1/2/3/4(Spo22) Msh4/5 and Mer3), contribute in various ways to initiate synapsis and/or promote crossing over [22]. zmm mutants result in significant reductions in synapsis and crossing over. In ZMM deletion strains, removing Sgs1 and thus the ability to strand displace or promote dissolution of the D-loop restores crossing over to near wild-type levels [20]. ZMMs might protect JMs in at least two possible ways: 1) by providing an environment in which Sgs1 is unable to dismantle the JM intermediate and/or 2) by setting up a configuration in which biased cutting of the JM directs resolution solely towards the CO outcome.
Besides Sgs1, other recombination enzymes have been intensively investigated for their roles in meiotic CO formation. Recently, Mus81/Mms4 (Eme1), Slx1–Slx4, Yen1 and MutLγ-Exo1 have been shown to account for essentially all JM resolution in budding yeast, with the majority of meiotic JM resolution (∼49%) originating from the MutLγ-Exo1 pathway [13]. Although playing a major role in JM resolution in fission yeast [23], [24], Mus81/Mms4 (Eme1) is thought to have a reduced role in budding yeast [13]. Interestingly, both the sporulation efficiency (<12%) and spore viability (<51%) of a mms4 null mutant are more severely defective [25], [26] than the sporulation efficiency (73%) and spore viability (79%) of a MutLγ mutant such as mlh3 [27]. This might suggest that Mlh3 cannot resolve Mms4-dependent JMs or that there might be additional functions for Mus81/Mms4 (Eme1) beyond its JM resolution capabilities. Mus81-Mms4 (Eme1) has homology to XPF family endonucleases that are involved in nucleotide excision repair [28]. In vitro studies have shown that Mus81-Mms4 (Eme1) is efficient in cutting branched structures such as 3′ overhangs, forks, nicked HJs and D-loops [29]. It has been proposed that Mus81/Mms4 (Eme1) is needed to cleave 3′ flaps, created by overextension of DNA synthesis in the D-loop. This cleavage would promote ligation to the 5′ end permitting second end capture [25], [30]. In accordance, mms4 null mutants show larger gene conversion tracts [30]. However, based on biochemical characterizations of recombinant Mus81 and TEV-Mus81 cleavage activity [31], [32], this model fell into obscurity in lieu of the idea that Mus81/Eme1(Mms4) instead cleaves D-loops and half-junctions in order to resolve dHJs [33].
Recently, the development of high density oligonucleotide microarray mapping and high throughput sequencing of single nucleotide polymorphisms (SNPs) in meiotic tetrads has allowed detailed examination of the genome-wide distribution and composition of resolution patterns for both NCOs and COs in a method we now term RecSeq [6], [34]–[36]. In a yeast hybrid strain, a cross between YJM789 and S96, ∼60,000 SNPs can be genotyped to assess parental origin of DNA in progeny from the two strains [37]. Because each of the four products of a single meiosis can be isolated from a tetrad, NCOs and COs can be clearly distinguished by SNP analysis.
Here, we perform an extensive analysis of tetrads from sgs1, zip3, msh4, and mms4-md strains to further delineate the process by which COs and NCOs are formed. Through analysis of changes in the GC tract composition within COs and NCOs, we identify a recombination signature indicative of unbiased cleavage of dHJ intermediates for sgs1 and zip3, but not for msh4. In addition, detailed examination of conversion tracts in sgs1 and mms4-md mutants reveal distinct aberrant recombination events involving multiple chromatid invasions. In sgs1 mutants, these multiple invasions are generally multichromatid involving 3–4 chromatids; in mms4-md mutants the multiple invasions preferentially resolve into one or two chromatids. We suggest that Mms4 is needed to prepare the invading strand to properly dock with the second end of the double strand break (DSB) thus ensuring only a single round of invasion for both NCOs and COs. The loss of this alternative role for Mus81/Mms4, rather than removal of its function as a resolvase, could contribute to extreme loss of viability seen in gametes that lack Mus81/Mms4.
Results
Genome-Wide Analysis of Recombination in sgs1, zip3 and msh4 Mutants
It has been proposed that Sgs1 determines the outcome of NCOs and COs through an antagonistic relationship between itself and ZMM proteins [20]. To better understand this relationship between Sgs1 and the ZMM proteins, we turned to high throughput sequencing to ascertain the number, distribution and composition of COs and NCOs genome-wide in eleven sgs1, seven zip3, seven msh4, four zip3 sgs1 and five msh4 sgs1 tetrads that we compared to 52 wild-type tetrads. The wild-type tetrads combine six tetrads from sequencing [38] and 46 tetrads from high-density oligo microarrays [35] that we reanalyzed using our new classification scheme described below.
We first sorted recombination events into majority and minority categories (Figure 2) then subdivided events based on the number of chromatids involved. For wild type (WT), the majority of DSBs are repaired as either a NCO (Figure 2A, E1) or CO (Figure 2A, E2 and E3). Together these form the “majority” events since they are the dominant form of IH recombination events (92.4%) and the predicted outcomes of SDSA and biased dHJ resolution (Figure 1, Figure S1). Note that NCOs can also form from unbiased dHJ dissolution and unbiased dHJ resolution (Figure S1), though this is normally suppressed in wild-type strains. We named the remaining IH events that are seen less frequently “minority” events that include cases where two or more COs, NCOs or CO-NCOs are within 5 kb of each other (Figure 2B, E4–7). Most of these recombination events cannot be explained by simple resolution of a dHJ as shown in Figure 1 and therefore must arise from atypical resolution involving either multiple invasions or multiple nearby DSBs. Minority events make up 7.6% of all WT events (Figure 2B) and can potentially arise from a single DSB as demonstrated in Figure S2A. Of the total events, 4.4% are multichromatid (events on three or four chromatids) averaging 5.8 per meiosis (Figure 2B, E6–7). This finding is consistent with Oh et al. (2007) showing that probable intermediates of these events do occur in wild-type cells but in low abundance.
Fig. 2. Types of recombination signatures expected and observed by RecSeq. 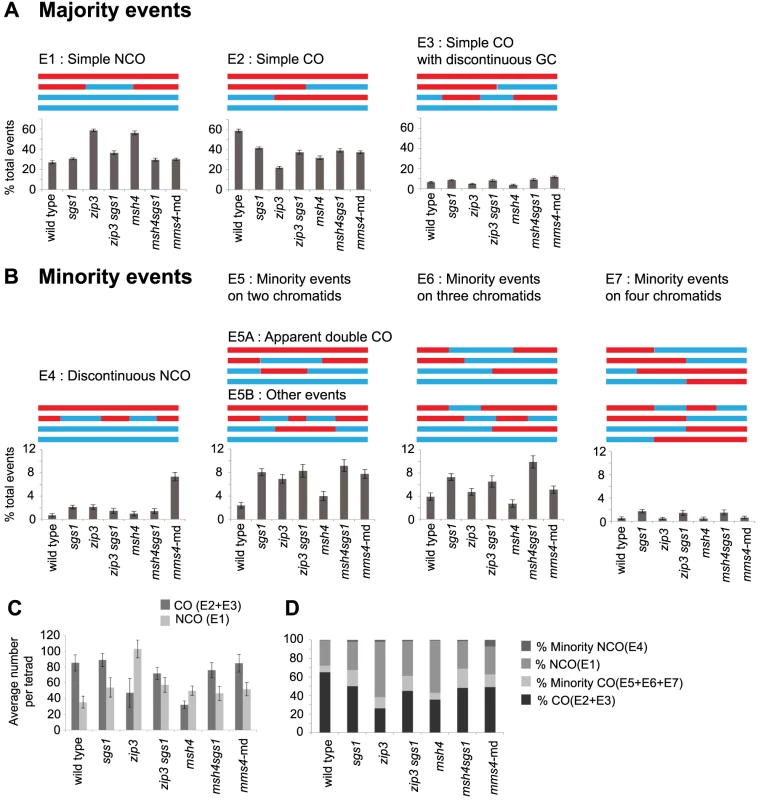
(A) The majority events are recombination signatures expected from normal SDSA and biased cleavage of JMs. Percentage of these events found for the various mutants are shown. Total events = E1–E7. (B) The minority events are recombination signatures that are found more rarely. Percentage of these events found for the various mutants are shown and can also be found in Table S1. WT has very few of these events. E1: Simple NCO is a 3∶1 tract on one chromatid and is not within 5 kb of another NCO or CO. E2: Simple CO is a CO with or without associated GC tracts and not within 5 kb of another CO or NCO. E3: Simple CO with discontinuous GC is a CO with or without associated GC tract with one or more GC within 5 kb and on one of the same chromatids as the CO chromatids. E4: Discontinuous NCO is two or more NCOs in a tandem fashion on one chromatid with regions of 2∶2 marker segregation separating them. E5 is an event where two or more COs or NCOs are within 5 kb of each other and involve only two chromatids. E5 is subdivided into E5A and E5B where E5A represents the signature of unbiased dHJ resolution and E5B represents all other E5 events. E6 is an event where two or more COs or NCOs are within 5 kb of each other and involve three chromatids. E7 is an event where two or more COs or NCOs are within 5 kb of each other and involve four chromatids. (Error bars – SE of proportions). T-test statistics comparing each mutant to another and WT (Table S2). (C) Average number of COs and NCOs per tetrad. Error bars, SD (D) Proportion of COs, NCOs and minority events out of total events. Note that some minority events, particularly E5A events can potentially arise through unbiased cutting of the dHJ (Figure 1, Figure S1). E5A events could also arise from two independent DSBs that get repaired as two NCOs. There are three possible configurations that could occur if two DSBs are very close to each other: (A) overlapping NCOs on a pair of homologous chromosomes, (B) non - overlapping NCOs on a pair of homologous chromosomes or (C) NCOs on sister chromatids. If these double NCOs arise from independent DSBs, we would expect each of these configurations to be equally likely. However, overlapping NCOs (E5A) form the majority of all E5 events (83 out of 167) (Figure S3) suggesting that these are more likely to arise from unbiased resolution of dHJ.
Distinct Signatures Indicative of Unbiased Resolution of dHJs Occur in sgs1 and zip3
In the presence of Sgs1 and ZMMs, JMs are thought to resolve predominantly by Exo1-MutLγ that cleaves the dHJ in a biased direction to direct resolution of the dHJs to form COs [39]. However in the absence of Sgs1, JMs become dependent on Mus81/Mms4, Slx1-Slx4 and Yen1 for resolution [13]. Dependence on these resolvases results in the loss of CO bias presumably because of unbiased cutting of the JM. Similarly, ZMMs are thought to play a role in ensuring biased resolution of the dHJ. But do all ZMMs contribute in the same way?
To determine whether unbiased cleavage is indeed occurring in the sgs1 and zmm mutants, we examined whether we could detect an increase in apparent double COs or E5A events per meiosis. E5A events are distinct recombination signatures predicted to arise if the cuts to the JMs are no longer biased (Figure 1, Figure S1). In agreement with the occurrence of unbiased cleavage in sgs1, we do observe an increase in number of E5A events per tetrad (5.7 sgs1 vs. 1.6 WT p = 0.0003, Table S1). The zip3 mutant also exhibits an increase in E5A events (3.6 zip3 vs. 1.6 WT p = 0.0122, Table S1). In contrast, msh4 shows a decrease in the number of E5A events (0.6 msh4 vs. 1.6 WT p = 0.0003) proportional to a decrease in CO levels. From these results, we infer that both Sgs1 and Zip3 normally prevent unbiased cutting of JMs, however Msh4 does not. In zip3, more NCOs arise either from SDSA and/or from unbiased dHJ cleavage likely contributing to the dramatic increase in NCOs in this mutant (Figure 2C). Correspondingly in msh4, the wild-type level of NCOs (Figure 2C) and the lack of unbiased signatures for this mutant are in accordance with NCOs arising predominantly from normal levels of SDSA.
Proportion of CO to NCO Levels Changes Dramatically in zmm Mutants but Not in sgs1
To investigate whether Sgs1 affects the CO-NCO decision via dictating the relative amounts of COs and NCOs, we examined the average CO and NCO levels in the sgs1 mutant. We find that overall levels of NCOs increase (t-test p value = <0.001) while CO levels of the majority class (E2,E3) remain unchanged (t-test p value = 0.21) (Figure 2C, Table S1). It follows that there is a small but significant decrease in the relative proportion of COs of the majority class (50%) to NCOs (31%) as compared to WT (z test of proportions p value = <0.001), for which 65% of the observed IH events are COs and 27% are NCOs (Figure 2D). In the case of the two null mutants of the ZMM genes ZIP3 and MSH4 a more dramatic change is observed. The relative proportion of COs to NCOs in both zip3 and msh4 deviate substantially from that of WT (zip3- 39% CO, 60.9% NCO; msh4 - 43% CO, 57% NCO, z test of proportions p value = <0.0001 for both) (Figure 2D). Removing Sgs1 in conjunction with eliminating either ZMM restores the proportion of COs and NCOs to near sgs1 levels [20]. Thus it seems that zmm mutants do affect the CO-NCO outcome through substantial changes to the CO/NCO ratio, but only when Sgs1 is present. Unlike the ZMMs, Sgs1's role in dictating the CO-NCO decision does not occur through major changes in the ratio of COs to NCOs, whether ZMMs are present or not.
Zip3-Dependent Population of Short NCOs Becomes Evident in the sgs1 Mutant
The distributions of NCO and CO-associated GCs (GCCOs) tract lengths are different for WT, both for the medians (Wilcoxon rank sum p<0.0001) [35] and for the shapes of the distribution (Kolmogorov-Smirnov (K-S), p<0.001) (Figure 3A). The distinctive distributions are not surprising given that COs and NCOs normally arise from separate intermediates. In sgs1, it was proposed that COs and NCOs arise from the same JM intermediate [10], [13] which led to our initial but incorrect prediction, that in this mutant, NCO and CO length distributions should closely overlap. Instead, we find that the two distributions do overlap for the most part except for one distinct difference. Notably, the NCO tract distribution is characterized by a novel population of short NCO tracts in the range of 0–500 bp in length (Figure 3B). These NCOs seem not to arise by a change in the restoration vs. conversion ratio since the we do not see a concomitant increase in the percentage of gene conversions associated with COs. Interestingly, the population of short NCOs seems dependent on Zip3, but not Msh4, since the sgs1 zip3 double mutant lacks this cohort of short NCOs, but in sgs1 msh4, they are still present (Figure 3C – first bin). Given that NCO timing parallels CO timing in an sgs1 mutant [2], [10], this suggests that the short NCOs may be a consequence of some NCOs forming from JMs or JM-like intermediates.
Fig. 3. Distribution of GCco and NCO tract lengths for sgs1 and zmm mutants. 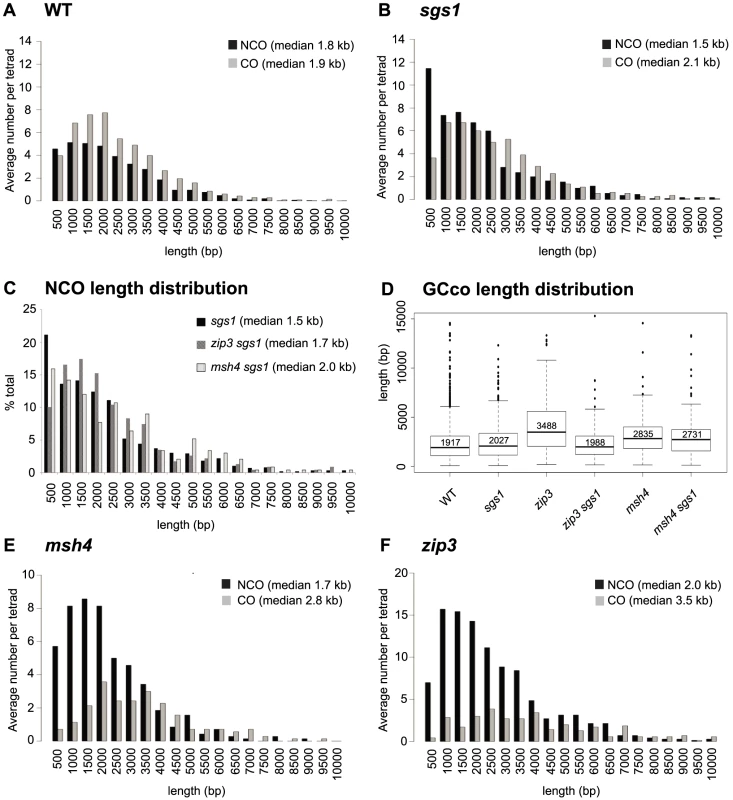
(A) Comparison of GCco vs. NCO tract lengths for WT (B) Comparison of GCco vs. NCO tract lengths for sgs1. (C) NCO length distributions for sgs1, msh4sgs1 and zip3sgs1 (D) Box plot of GCCO lengths. The dark line in each box represents median GCCO length. Each box outlines lower and upper quartile. The whiskers denote the boundaries of the interquartile range and the circles outside the whiskers represent outliers (outside 1.5 times the interquartile range). The value inside each box is the median GCCO length. (E) Comparison of GCCO vs. NCO tract lengths for msh4 (F) Comparison of GCCO vs. NCO tract lengths for zip3. zip3 but Not msh4 Exhibits Sgs1-Dependent Increases in GCCO Tract Lengths
Lack of ZMMs might be predicted to result in increased tract lengths of GCCOs since ZMMs may oppose Sgs1's postulated role in D-loop extension. Indeed we find that the zip3 mutant shows an increase in median GCco tract lengths as compared to WT (Figure 3D, 3F, Table S3 (p<0.001)). As expected, this increase is Sgs1-dependent, since GCCO tracts exhibit wild-type lengths in zip3 sgs1 (Figure 3D, Table S3 (p = 0.36)). Thus when Sgs1 is present, Zip3 is required to maintain wild-type lengths. This is consistent with the idea that Zip3 limits D-loop extension driven by Sgs1, by promoting efficient ligation at the second end of the DSB. Other evidence consistent with a role for Zip3 in promoting ligation is the increased frequency of minority events in zip3 (Figure 2B, Table S1), since multiple invasions would be a likely consequence of inefficiently ligated 3′ ends. Another possibility is that Zip3 limits extensive branch migration; however, extensive branch migration has yet to be shown in wild-type yeast. For msh4, we also find that median GCCO tract lengths are longer compared to WT, as was previously shown [35] (Figure 3D–3E Table S3 (p = <0.001)), though this increase was less than what was seen for zip3. Interestingly, the increase in tract length is independent of Sgs1 since in msh4 sgs1, the median GCCO tract size is equivalent to msh4 alone (Figure 3D, Table S3 (p = 0.23)). These results suggest that although Zip3 is needed to limit Sgs1 D-loop extension, Msh4 does not seem to be required. This is consistent with the observation that in a msh4 mutant, Zip3 still localizes normally [40] and potentially still functions to limit extension at the ligating end. In the discussion, we speculate how the GCCO tract lengths can increase in a msh4 mutant without being dependent on Sgs1.
zip3 but Not msh4 Exhibits an Increase in NCO Tract Lengths
We next examined NCO lengths. If NCOs predominantly occur via SDSA, we would not expect any change in NCO lengths in the zmm mutants since ZMM proteins are thought to be JM-specific. However, if NCOs are now being created through unbiased resolution of dHJs, we might expect a population of longer NCOs added to the normal population of NCOs occurring through SDSA. This is true for zip3. Here we see that NCO lengths increase by 184 bp, which is significantly longer than in WT (1960 bp zip3, 1778 WT, Wilcoxon p<0.0001, Table S1, Table S3). Although this only represents a ∼10% increase in median length, it is what might be expected given that the majority of NCOs likely still occur through SDSA. In contrast, in msh4, we observe no difference in the length of NCOs (1679 bp msh4, 1778 bp WT, Wilcoxon p = 0.19, Table S1, Table S3). Both the lack of increase in NCO conversion lengths and in E5As for this mutant suggests that NCOs do not arise through unbiased cutting in this msh4. Such findings further bolster the notion that Zip3 but not Msh4 influences biased cutting of the dHJ.
sgs1 Mutants Exhibit an Increased Number of Minority Events
The existence of multichromatid intermediates seen by physical analysis of recombination on 2D gels [19] predicts that an increase of such events should be observed in the final recombination products in sgs1 mutants. To examine whether such events are indeed resolved in viable spores, we further examined the minority events for sgs1 (Figure S2B). At the same time we wanted to analyze the mms4-md mutant since previous studies examining recombination intermediates suggest that Mus81/Mms4 (Eme1) collaborates with Sgs1 in resolving aberrant joint molecules [41], [42]. We find that minority events make up 19.2% of the total events for sgs1 (p<0.0001) and 20.9% for mms4-md (p<0.0001) compared to only 7.6% in WT (Figure 2B E4–E7). This increase is not a general feature of all meiotic recombination mutants since msh4 mutants show no significant change in the number of minority events (Figure 2B, E2, E4–E7). Further characterization of the minority events in sgs1 reveals a significant increase in E5As as compared to WT (Figure S2B) as well as higher levels of more complex events involving multiple combinations of COs, NCOs and GCs engaging 2, 3 and 4 chromatids (Figure 2B E5–E7, Figure S2C). Multichromatid events are also significantly higher in mms4-md than in WT, but they are fewer in comparison to sgs1.
Minority events can potentially arise from a single DSB or multiple DSBs (Figure S2A). In sgs1, we have more IH events than WT (Table S1); this either means that there are fewer intersister events and/or potentially more DSBs. Since Oh et al. (2007) showed that there is more intersister recombination in an sgs1 mutant, this raises the question whether minority events in sgs1 are in fact independent COs repaired from more than one DSB. Sister chromatid ratios allow us to distinguish between these possibilities. If the closely spaced COs with a minority event consist of more than one independent event, we would expect a 1∶2∶1 ratio for apparent double COs on two, three and four chromatids respectively since normally COs do not demonstrate chromatid interference [43]. In sgs1, the observed ratio is 6∶7∶1 suggesting that the apparent closely spaced double COs making up a minority event likely stem from a single event.
mms4-md Causes Discontinuities in NCO and GCCO Tracts
In mms4-md tetrads, we find that multichromatid events increase compared to WT but to a lesser extent than observed in sgs1 (Figure 2B: E5,E6,E7). Notably, mms4-md mutants preferentially exhibit prominent increases in events involving a discontinuous NCO (Figure 2B, E4) and in events in which COs exhibit discontinuous GCs (Figure 2A, E3). Therefore, although both sgs1 and mms4-md demonstrate a similar change in the number of minority events, clear differences exist in the resolution signatures between sgs1 and mms4-md.
The observation that there are more discontinuities that are part of the same event in mms4-md arose from the analysis of NCOs. Initially, we observed an unexpectedly high number of NCOs (∼118 per tetrad as compared to 39 per WT tetrad). Intriguingly, most of the NCOs appeared to be closely spaced (Figure 4A). Although previous studies merged events by imposing a cutoff for CO-CO and CO-NCO distances, no distance cutoffs for NCO-NCO distances were typically used to determine whether two closely spaced NCOs are part of the same event. By imposing the same 5 kb cutoff as we used for CO-CO and CO-NCO, the number of NCOs was reduced to an average of 51.9 per tetrad rather than the 118 identified initially (Figure 4B). Furthermore, the discontinuities observed in mms4-md now consist of many more tracts than observed for WT, with a range of 2–11 sequential tracts seen for mms4-md compared to the typical 2–3 tracts for WT.
Fig. 4. mms4-md results in an increase in discontinuous CO and NCO events. 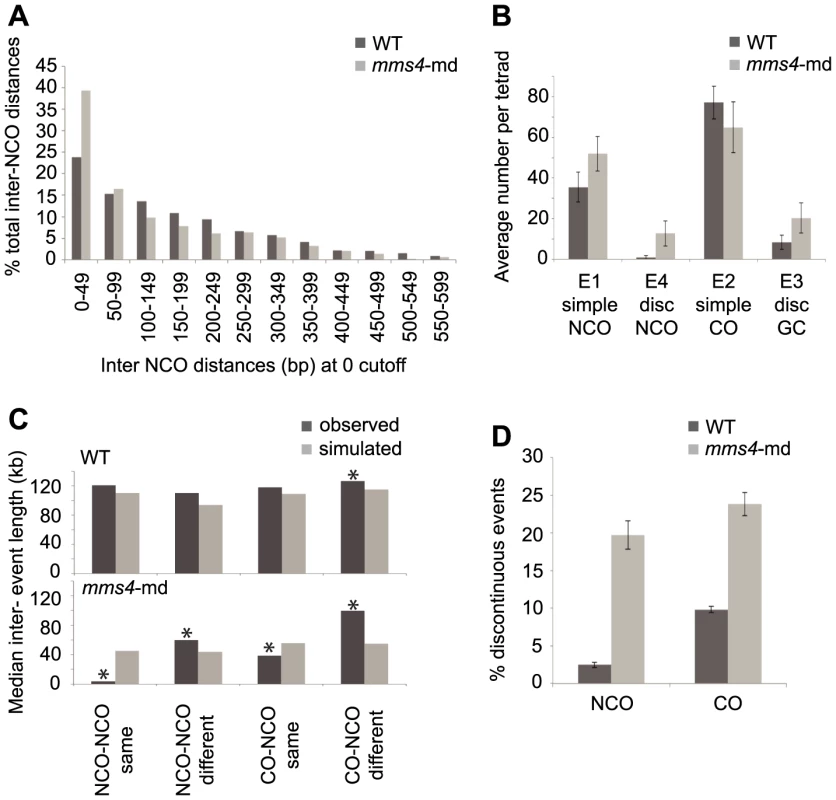
(A) Histogram of interNCO distances showing an increased frequency of closely spaced NCOs (1st bin) in mms4-md vs. WT. NCOs were identified with a 0 kb cutoff and then interNCO distances were calculated between each pair of adjacent NCOs. (B) Average number of simple NCO, discontinuous NCO, CO with GCco and CO with discGCco per tetrad plotted for WT and mms4-md. (Error bars – SD). (C) A random distribution of NCOs across the genome was simulated using the observed number of NCOs in WT and mms4-md tetrads respectively. CO positions were left unchanged. Distances were calculated between adjacent NCOs on same chromatid (NCO-NCO same), adjacent NCOs on different chromatids (NCO-NCO different), adjacent CO and NCO on same chromatid (CO-NCO same) and adjacent CO-NCO on different chromatids (CO-NCO different). Median distance for each category was plotted for WT and mms4-md. Asterisks indicate statistical significance (D) Percent of total NCOs and COs that show discontinuity in WT and mms4-md. (Error bars - SE of proportions). To rule out the possibility that the sheer number of NCOs when there is no cutoff would generate apparent discontinuities by random chance, we simulated over each tetrad's CO map a random distribution of NCOs using the experimentally determined NCO number from each tetrad. Calculating the distances between adjacent NCOs and between each CO and the adjacent NCO for the same and different chromatids, we then compared the simulated distances to the experimentally observed distances (Figure 4C). In WT, the observed distances between adjacent events for the majority of the cases do not differ greatly from the simulated values. Only for distances between CO-NCO on different chromatids do we see a small but significant difference between simulated and observed (Figure 4C). We thus can conclude for WT that the distribution of NCOs is generally, though not perfectly, consistent with a random dispersal of NCOs. This is in agreement with Mancera et al. (2008) who showed that NCOs and COs interfere with each other.
This is not the case for mms4-md. In mms4-md, all distances between observed and simulated distributions are different. Particularly striking is that distances between adjacent events on the same chromatid are significantly shorter than expected if NCOs were randomly distributed (Figure 4C). As a corollary, events on different chromatids are spaced farther apart than expected. Thus, in mms4-md, there is a strong chromatid bias for placement of adjacent NCOs such that they tend to be on the same chromatid. This argues that these closely spaced discontinuous NCOs are likely to arise from a single DSB. The same is also true for CO with discontinuous GC (discGCCOs) (Figure 4C). Surprisingly, a similar fraction of COs and NCOs show discontinuity in mms4-md (Figure 4D). On average we see 20.3 discGCCOs and 12.7 discontinuous NCOs per tetrad (Figure 4B, p value <0.05, p value<0.005, Table S1, Table S2) as compared to 8.4 and 0.9 in WT (Figure 4B, Table S1, Table S2). Since the absence of Mms4 affects NCO and GC formation to similar degrees, it suggests that Mms4 might act in a key mechanistic step common to the formation of both COs and NCOs.
mms4-md Does Not Disrupt the Mismatch Repair Pathway
Two possible models can explain the formation of discontinuous NCOs and GCs. One possibility is that without Mms4 each individual tract within the discontinuous event represents an IH invasion and repair cycle arising from multiple invasions (Figure 5A). The other possibility is that the mismatch repair pathway is disrupted by lack of Mms4 and the regions of heteroduplex DNA are not repaired completely giving rise to discontinuities (Figure 5B). In the disrupted mismatch repair model, NCO formation occurs normally, forming a heteroduplex. If the mismatch repair system is perturbed, this heteroduplex cannot be repaired completely. Partial mismatch repair within the heteroduplex DNA will create discontinuities.
Fig. 5. Multiple strand invasions can account for discontinuities seen in mms4-md. 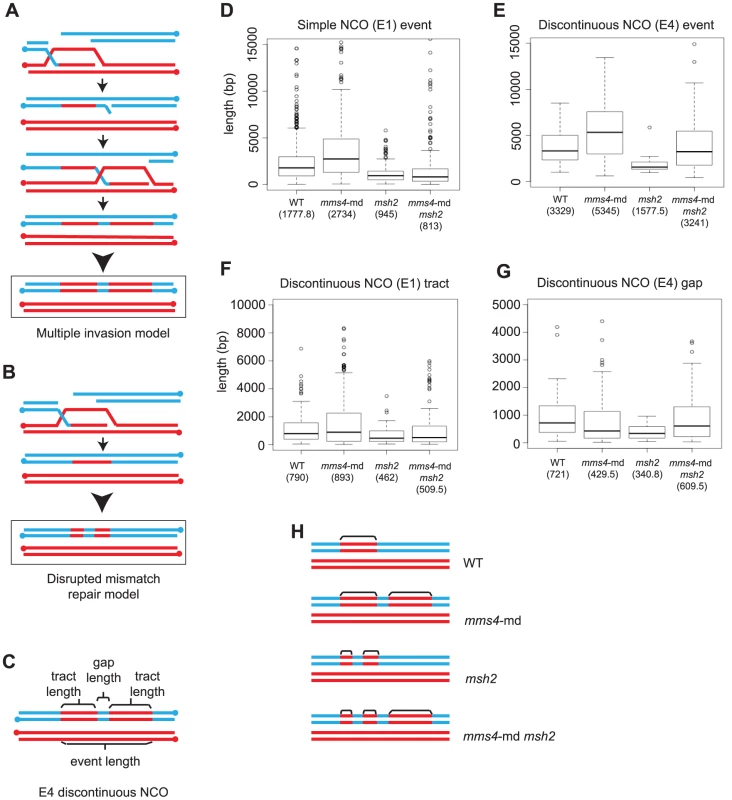
(A) Multiple strand invasions or (B) a defect in mismatch repair could potentially account for appearance of discontinuities in mms4-md. (C) Depiction of the various components of a discontinuous NCO event. (D) Box plot for tract lengths of simple NCOs (E1). The dark line in each box represents median length. Each box outlines lower and upper quartile. The whiskers denote minimum and maximum data and the circles outside the whiskers represent outliers (outside 1.5 times the inter quartile range). Values for the median length are given. (E) Box plot of event length for the entire discontinuous NCO. (F) Box plot of individual tract lengths within the discontinuous NCO. (G) Box plot of gap lengths between discontinuous NCOs. (H) Representation of observed discontinuous NCO events in mms4-md, msh2 and mms4-md msh2. Note that discontinuous events only make up 24% of all NCOs in a mms4-md mutant. Individual components of an entire discontinuous event can be measured (Figure 5C). The model in which mismatch repair is disrupted predicts discontinuous events in which the length of the entire event is similar to length of wild-type NCO and GCCO tracts but individual conversion tracts within these events are shorter. In contrast for the multiple invasion model, because each invasion is an independent D-loop, the entire discontinuous event would be much longer while the individual conversion tracts would be similar in length to wild-type simple NCOs. We find that lengths of simple NCOs in WT are significantly shorter than lengths of discontinuous events in mms4-md (median length of 5.4 kb, Fig. 5D, 5E, Table S1). Importantly, individual tract of a discontinuous event in mms4 are not different from the median tract length of a simple WT NCO (Figure 5D, 5F). Both of these observations are consistent with the view that in the absence of Mms4, discontinuous events arise through a multiple invasion pathway.
In mms4-md, Discontinuous Events Likely Arise through Multiple Invasions
Further evidence that the multiple invasion model may explain the discontinuous events in mms4-md comes from examining the discontinuities that arise when mismatch repair is compromised by the elimination of Msh2. Msh2 recognizes and repairs heteroduplex DNA during recombination. In the absence of Msh2, heteroduplex DNA remains unrepaired and after the first division following meiosis, a mixed population of genotypes results. However, other Msh2-independent repair mechanisms such as the short patch repair system still function [44]. Because this short patch repair is not as efficient as Msh2 mismatch repair, we expect the GC tracts to be repaired less efficiently thus creating shorter tract lengths and discontinuities. In this case, we expect discontinuous events in which 2∶2 regions within the GC tract would represent restored/repaired regions.
In absence of Msh2, 10% of all the NCOs show discontinuity. The median length of an entire discontinuous NCO event in msh2 is 1.6 kb, which is similar to the median length of a simple NCO in WT of 1.8 kb (p = 0.14) (Figure 5D, Figure 5E). The median length of conversion tracts within the discontinuous events is considerably shorter (0.5 kb) (Figure 5F) and the 2∶2 gap lengths between tracts are even shorter (0.3 kb) (Figure 5G) (Table S1). It is clear that the length distributions and discontinuity profiles of mms4-md and msh2 are distinct. mms4-md mutants show very long discontinuous tracts and each tract within the discontinuity is similar in length to a simple NCO, whereas in msh2, the entire length of a discontinuous event is similar in size to the length of a simple NCO and the lengths of tracts within are shorter. Further bolstering the argument that disrupted mismatch repair is not causing the discontinuities in mms4-md is the observation that in an msh2 mms4-md double mutant the resulting discontinuous events appear to be a convolution of the independent phenotypes of msh2 and mms4-md (Figure 5D–G). In Figure 5E, we see that in msh2 mms4-md, the entire discontinuous region is still long but the tracts making up the discontinuities are now short as in msh2 (Figure 5F). Figure 5H summarizes our findings for the various mutants. Note that discontinuous events are ∼20% of the total NCOs and do not correspond to 100% of the events. We thus conclude that compromised mismatch repair does not create the discontinuities seen in the mms4-md mutant and that multiple strand invasions occurring for both NCOs and COs are the likely reason for the increased tandem tracts.
Discussion
RecSeq Reveals Unbiased Cutting of the dHJ
In this study, we used RecSeq to examine the number, proportion and composition of final resolution signatures to better understand the relationship between Sgs1 and the ZMMs and their roles in regulating the CO-NCO decision. The recombination motifs we observed allowed us to attribute events arising from biased vs. unbiased cutting of the dHJ. Particularly, we have detected the appearance of apparent double COs (Type E5A) that are indicative of unbiased cleavage.
A Model Incorporating the Roles of Sgs1, Zip3 and Msh4 in CO and NCO Formation
Figures 6A–6C depicts models for WT, zip3 and sgs1 respectively and how they can perturb the CO-NCO relationship to result in the observed recombination signatures and the changed proportion of COs and NCOs. Based on the results from the zip3 mutant (Figure 6B), a key feature of the model for WT is that Zip3 is needed to ensure that biased resolution of the JM occurs. In zip3, E5A events increase substantially indicating that biased resolution has been perturbed or more likely completely lost given the 52% reduction of COs. In conjunction with unbiased cutting in zip3, an increase in the median length of both NCOs and GCco tracts is seen. Longer GCco tracts can arise if the two HJs making up the JM are farther apart increasing the heteroduplex length. If these HJs instead were cut in an unbiased manner, long NCOs would result thereby increasing the median NCO length as observed. Although Zip3 seems to play a role in directing biased cleavage, Zip3 also appears involved in stabilizing second end capture and promoting subsequent ligation to form the second HJ. This proposition stems from our observation that without Zip3, median GCco tracts are substantially longer. This extension is Sgs1-dependent since in zip3 sgs1 GCco tracts are WT in length. We postulate from these findings that Zip3 limits the ability of Sgs1 to extend the D-loop and/or limits Sgs1-dependent resection, thus promoting ligation of the second HJ.
Fig. 6. Model of the relationship between Sgs1, Zip3 and Msh4 in CO-NCO fate. 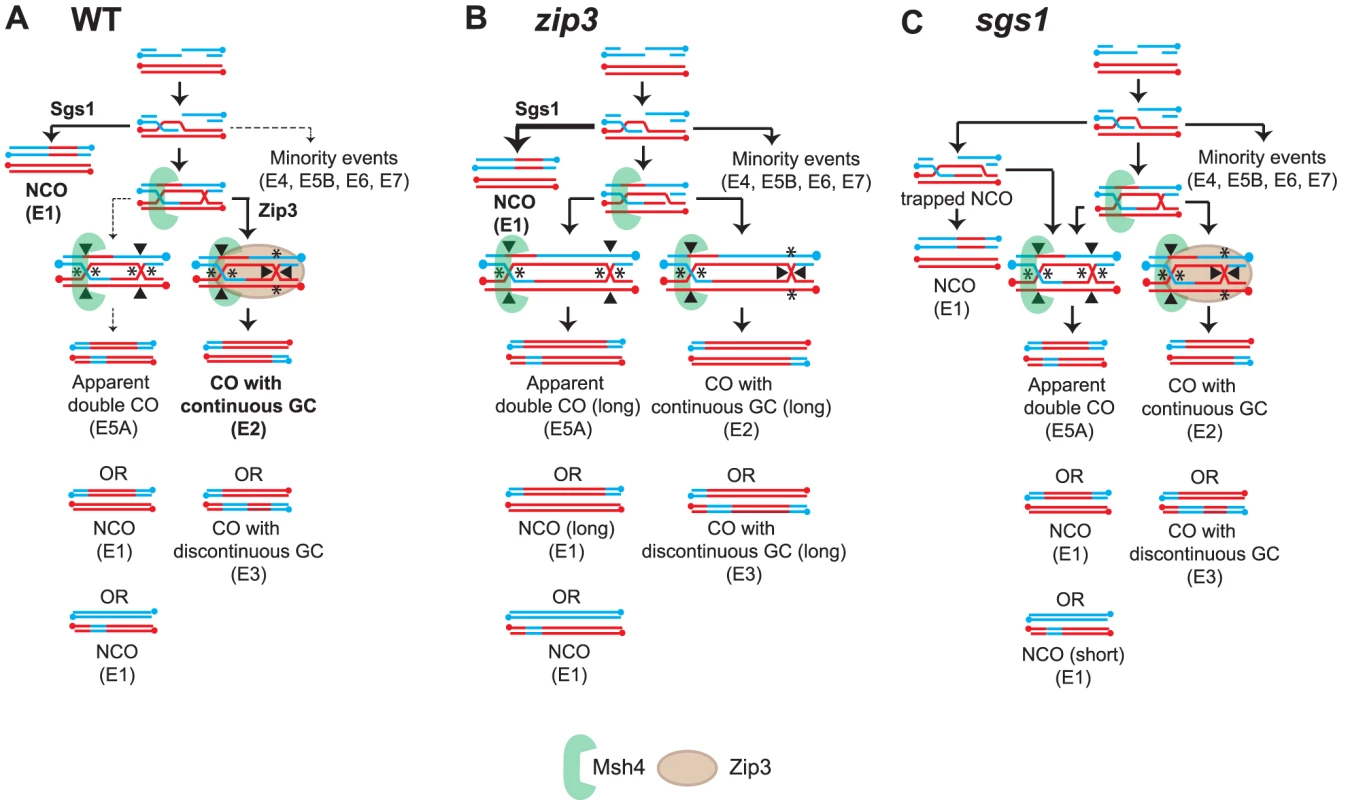
Models for how (A) WT, (B) zip3 and (C) sgs1 might produce the distribution of observed recombination types. Indicated in bold type are the main signatures observed. Arrow weight indicates the relative degree of partitioning through the pathway. Expected biased or unbiased cuts are indicated by asterisks or filled arrowheads. Another key feature in the model for wild-type CO-NCO balance is Sgs1's role in directing recombination both towards resolvase-independent NCO formation and towards ZMM-dependent CO formation as previously proposed by de Muyt et al. (2012). In sgs1 (Figure 6C), the appearance of E5A events suggests that unbiased cleavage of dHJs is occurring in this mutant. An important function of Sgs1 is to disassociate the extended invading strand from the D-loop to promote the formation of NCOs; we speculate that in sgs1 the lack of this ability will result in invasions originally destined to form NCOs becoming trapped in a JM-like intermediate (indistinguishable from JM on a 2D gel) that would require Cdc5 for release. In this trapped state, some strands will remain unligated but will presumably form NCOs upon Cdc5 induction by reannealing to the second end. In this case, a distinct population of small NCOs would form. We also speculate that a fraction of the trapped intermediate does become ligated, and is cut in an unbiased manner, giving rise to E5As. This model modifies a proposal by deMuyt et al. (2012) that suggests that in sgs1, NCOs mainly arise from JMs that are resolved by unbiased cutting. Our proposal of an unligated “trapped intermediate” was put forth in order to take into account the increase in the small NCO population (Figure S4). Unbiased cutting, although predicted to generate smaller NCOs, does not preferentially increase the frequency of small NCOs over NCOs of other sizes (Figure S1). Furthermore, zip3 sgs1 shows no increase in the population of short NCOs even though there is an increase in E5A events furthering bolstering the argument unbiased cutting alone cannot explain the increase in short NCOs in sgs1.
Msh4 Promotes Stability of the JM but Does Not Have a Role in Enforcing Biased Cutting
Unlike zip3 and sgs1, the msh4 mutant reveals no increase in E5As implying that biased designation is intact. In fact an in vitro study of hMSH4-hMSH5 [45] suggests that the role of Msh4-Msh5 is to recognize HJs and act as a meiosis-specific sliding clamp to stabilize dHJs. Our results showing that in msh4 1) GCco tracts are longer and 2) median GCco tract length remains long in msh4 sgs1 is in agreement with such a proposed role. Because extension of the D-loop is dependent on Sgs1, we envisage that the GCco length increase seen in msh4 is likely coming from the invading end of the DSB, which we speculate is not dependent on Sgs1 vs. the ligating end, which is dependent. If Msh4 normally stabilizes the invading end for JM formation, lack of this stabilization would cause the invading end to prematurely exit the D-loop and not get fully extended. Now this partially extended strand can invade again but this time the extended DNA is used as a primer for further extension, thus creating the potential for longer associated GCs. CO formation will be much more difficult to establish without sufficient JM stability thus accounting for the even lower amount of COs and spore viability than zip3. Note that NCO formation is not affected by loss of Msh4 since the number and lengths of NCOs are normal.
The Subpopulation of Small NCOs in sgs1 Is Potentially a Consequence of the Trapped NCO Intermediate
The trapped NCO intermediate postulated to form in sgs1 contributes to the appearance of the short NCOs we see in sgs1. Normally during SDSA the invading strand repairs using the homolog as a template (Figure S4). After annealing the remaining resected region repairs using the reannealed strand. However, in sgs1, when the invading strand cannot release and becomes trapped in an unligated JM-like intermediate, the non-invading end can now repair using the homolog just as it would if there were a CO-intermediate. The presence of this additional small heteroduplex can result in a short NCO. In sgs1 zip3, no short NCOs are observed suggesting that Zip3 is needed to stabilize the trapped intermediate. This notion is bolstered by the fact that in msh4 sgs1 in which Zip3 is present, a population of small NCOs is still discernable.
Changes in Total Interhomolog Events May Reflect Changes in DSBs, Homolog Bias or Conversion
Recently, Thacker et al. (2014) showed that strains lacking ZMMs exhibit greater than wild-type levels of DSBs [46]. For the zip3 mutant, this increase in DSBs is consistent with the increase in total interhomolog events (Table S1). However, in msh4, we find that total interhomolog events are decreased by 36%. One possibility is that more intersister repair than interhomolog repair occurs in msh4. However this seems unlikely since Oh et al. (2007) have shown that intersister repair decreases in msh5. We also cannot deduce if more NCOs were restored than converted. Although no decrease in the percentage of GCcos is observed (Table S1, E2% events with GC), any decrease would be obscured since GCco conversion tracts are longer in msh4 and thus increases detectability of GCcos. Therefore for this mutant, we cannot reliably deduce why total IH events decrease.
Mms4 and Sgs1 Limit Multiple-Strand Invasions through Different Mechanisms
As previously reported, both sgs1 and mms4-md showed an increase in the frequency of aberrant JMs as compared to WT [41]. However a difference was observed on how COs are now resolved in the different mutants [13]. In sgs1, a dependence on the Slx1-Slx4 resolvase was shown whereas in mms4-md, a role for the Yen1 resolvase was found. Our system for signature recognition also allowed us to detect important differences between the two mutants. In sgs1, multichromatid resolution patterns predominate whereas in mms4-md, there is a greater preponderance of multiple events on one or two chromatids. In Figure S5, we illustrate how mechanistically the multiple invasions might differ in the two mutants. In sgs1, multichromatid invasions are likely to arise from those events originally destined to be NCOs but trapped in JM-like intermediates. Because of the prolonged time in the trapped state, some of these will become ligated, forming JMs even without the presence of Zip3 and will resolve by unbiased cutting. The others are unligated. In this state, the 3′ end is still free to invade other chromatids increasing the probability of multichromatid events. On the other hand for mms4-md, multiple invasions arise when overextension of replication leads to a 3′ flap. Without the endonuclease activity of Mms4, ligation would be difficult thus permitting the 3′ end to invade again. This would manifest in both COs and NCOs. We suspect that the greater number of sequential invasions in mms4-md is the result of a combination of the low efficiency of ligation and the fact that Sgs1 is still around to divert the additional invasion into SDSA. Although both mutants will result in chromosome entanglements, the low efficiency of ligation might be a contributing factor why mms4Δ is more deleterious than sgs1Δ as evidenced by its poor spore viability.
Materials and Methods
Strain Construction
All yeast strains (see Text S1) were derived from a cross of haploid S96 and YJM789 parents. Most of the deletion strains were constructed by PCR mediated gene replacement using pFA6a-kanMX6 plasmid [47]. mms4-md strains were created by replacing the WT MMS4 promoter with mitosis specific CLB2 promoter using pFA6a-natMX4-pCLB2-3HA (pCA001) plasmid as a template. Haploid parents were mated for 8–12 hours before sporulation on 2% potassium-acetate plates at 30°C. Tetrads were dissected after 4–6 days. Colonies were streaked for single cells before growing up for DNA isolation.
Although 4-spore viable tetrads are used for the genomic analysis, the spore viability and sporulation frequencies of these strains are not at the level to trigger any major selection bias.
Actual bias has only been previously shown when the sporulation frequency was extremely poor (0.4%, 4-spore asci) as in the zip1 mutant [39]. In fact, except in the case of very poor sporulation, the genomic analysis agrees quite well with published reports of recombination frequencies in the same mutants obtained by physical analyses using 1-D, 2-D gels and classical genetics [43]. It is also important to note that although we can obtain an average resolution of ∼80 bp, this analysis cannot detect 1) NCOs that been fully restored rather than converted; 2) events that solely involve sister chromatids and 3) any events that lie under the resolution defined by the density of SNPs (e.g. in conserved regions lacking SNPs).
Sample Preparation
For most samples, genomic DNA was extracted using QIAGEN genomic tip 500/G from 100 mL overnight YPAD culture [48]. DNA library preparation was performed according to Illumina's protocol. Libraries from 4 or 16 spores were multiplexed using custom adapters (Text S1). Sequencing was performed at either Vincent J. Coates Genomics Sequencing Laboratory, UC Berkeley or at the Center for Advanced Technology, UCSF using Illumina's HiSeq sequencer. For four msh4 tetrads (3,4,5 6), two sgs1 tetrads (new1, new2) and msh4sgs1x2, sample preparation was performed using the NEXTflex DNA sequencing kit. For a list of barcodes used for multiplexing refer to Text S1.
Data Analysis
Fastq files generated by Illumina's Casava pipeline were the starting point for all data analyses. ReCombine software package was used to perform alignment to reference genomes, genotype the markers and designate CO and NCO locations for each tetrad [31]. In order to categorize the complex recombination patterns seen in mms4-md and sgs1 mutants, a custom analysis was performed using output data from ReCombine. To do so, the CrossOver program was run using a 0 kb range for close events instead of the default 5 kb. Next, COs and GCs within 5 kb of each other were grouped together as a single event. The categorization of events was performed based on the number of chromatids involved in the event and also the complexity of the event. Detailed segregation plots were generated using the plotSeg.R program [31]. Sequences will be available at SRA in BioProject #SRP028549 (WT) and #SRP041214 (mutants) and additional output from the analysis is deposited at Dryad Digital Depository (http://doi:10.5061/dryad.79hn1).
Statistical Analyses
The t-test of means was used to calculate statistical significance in the average number of events between mutants. To compare tract lengths, the non-parametric Wilcox test was used to compute statistical significance. Comparison between proportions was performed using the z test of proportions. A Kolmogorov-Smirnov test was used to calculate the statistical significance between different tract length distributions. P-values were not corrected for multiple comparisons.
Supporting Information
Zdroje
1. HassoldT, HuntP (2001) To err (meiotically) is human: the genesis of human aneuploidy. Nat Rev Genet 2 : 280–291.
2. AllersT, LichtenM (2001) Differential timing and control of noncrossover and crossover recombination during meiosis. Cell 106 : 47–57.
3. HunterN, KlecknerN (2001) The single-end invasion: an asymmetric intermediate at the double-strand break to double-holliday junction transition of meiotic recombination. Cell 106 : 59–70.
4. BörnerGV, KlecknerN, HunterN (2004) Crossover/noncrossover differentiation, synaptonemal complex formation, and regulatory surveillance at the leptotene/zygotene transition of meiosis. Cell 117 : 29–45.
5. SchwachaA, KlecknerN (1995) Identification of double Holliday junctions as intermediates in meiotic recombination. Cell 83 : 783–791.
6. MartiniE, BordeV, LegendreM, AudicS, RegnaultB, et al. (2011) Genome-wide analysis of heteroduplex DNA in mismatch repair-deficient yeast cells reveals novel properties of meiotic recombination pathways. PLoS Genet 7: e1002305.
7. PâquesF, HaberJE (1999) Multiple pathways of recombination induced by double-strand breaks in Saccharomyces cerevisiae. Microbiol Mol Biol Rev 63 : 349–404.
8. GilbertsonLA, StahlFW (1996) A Test of the Double-Strand Break Repair Model for Meiotic Recombination in Saccharomyces cerevisiae. Genetics 144 : 27–41.
9. McMahillMS, ShamCW, BishopDK (2007) Synthesis-dependent strand annealing in meiosis. PLoS Biol 5 : 2589–2601.
10. WuL, HicksonID (2003) The Bloom's syndrome helicase suppresses crossing over during homologous recombination. 426 : 870–874.
11. De MuytA, JessopL, KolarE, SourirajanA, ChenJ, et al. (2012) BLM helicase ortholog Sgs1 is a central regulator of meiotic recombination intermediate metabolism. Mol Cell 46 : 43–53.
12. SourirajanA, LichtenM (2008) Polo-like kinase Cdc5 drives exit from pachytene during budding yeast meiosis. Genes Dev 22 : 2627–2632.
13. ZakharyevichK, TangS, MaU, HunterN (2012) Delineation of joint molecule resolution pathways in meiosis identifies a crossover-specific resolvase. Cell 149 : 334–347.
14. CejkaP, PlankJL, BachratiCZ, HicksonID, KowalczykowskiSC (2010) Rmi1 stimulates decatenation of double Holliday junctions during dissolution by Sgs1-Top3. Nat Struct Mol Biol 17 : 1377–1382.
15. HicksonID, MankouriHW (2011) Processing of homologous recombination repair intermediates by the Sgs1-Top3-Rmi1 and Mus81-Mms4 complexes. Cell Cycle 10 : 3078–3085.
16. BachratiCZ, BortsRH, HicksonID (2006) Mobile D-loops are a preferred substrate for the Bloom's syndrome helicase. Nucleic Acids Res 34 : 2269–2279.
17. BugreevDV, YuX, EgelmanEH, MazinAV (2007) Novel pro - and anti-recombination activities of the Bloom's syndrome helicase. Genes Dev 21 : 3085–3094.
18. Larsen NB, Hickson ID (2013) DNA Helicases and DNA Motor Proteins. In: Spies M, editor. Advances in Experimental Medicine and Biology. New York, NY: Springer New York, Vol. 973. pp. 161–184.
19. OhSD, LaoJP, HwangPY, TaylorAF, SmithGR, et al. (2007) BLM ortholog, Sgs1, prevents aberrant crossing-over by suppressing formation of multichromatid joint molecules. Cell 130 : 259–272.
20. JessopL, RockmillB, RoederGS, LichtenM (2006) Meiotic chromosome synapsis-promoting proteins antagonize the anti-crossover activity of sgs1. PLoS Genet 2: e155.
21. RockmillB, FungJC, BrandaSS, RoederGS (2003) The Sgs1 helicase regulates chromosome synapsis and meiotic crossing over. Curr Biol 13 : 1954–1962.
22. LynnA, SoucekR, BörnerGV (2007) ZMM proteins during meiosis: crossover artists at work. Chromosome Res 15 : 591–605.
23. BoddyMN, GaillardPH, McDonaldWH, ShanahanP, YatesJR, et al. (2001) Mus81-Eme1 are essential components of a Holliday junction resolvase. Cell 107 : 537–548.
24. SmithGR, BoddyMN, ShanahanP, RussellP (2003) Fission yeast Mus81.Eme1 Holliday junction resolvase is required for meiotic crossing over but not for gene conversion. Genetics 165 : 2289–2293.
25. de los SantosT, LoidlJ, LarkinB, HollingsworthNM (2001) A role for MMS4 in the processing of recombination intermediates during meiosis in Saccharomyces cerevisiae. Genetics 159 : 1511–1525.
26. KaliramanV, MullenJR, FrickeWM, Bastin-ShanowerSA, BrillSJ (2001) Functional overlap between Sgs1-Top3 and the Mms4-Mus81 endonuclease. Genes Dev 15 : 2730–2740.
27. Sonntag BrownM, LimE, ChenC, NishantKT, AlaniE (2013) Genetic analysis of mlh3 mutations reveals interactions between crossover promoting factors during meiosis in baker's yeast. G3 (Bethesda) 3 : 9–22.
28. HaberJE, HeyerW (2001) The Fuss about Mus81 Minireview. 107 : 551–554.
29. SchwartzEK, HeyerW-D (2011) Processing of joint molecule intermediates by structure-selective endonucleases during homologous recombination in eukaryotes. Chromosoma 120 : 109–127.
30. de los SantosT, HunterN, LeeC, LarkinB, LoidlJ, et al. (2003) The Mus81/Mms4 Endonuclease Acts Independently of Double-Holliday Junction Resolution to Promote a Distinct Subset of Crossovers During Meiosis in Budding Yeast. 94 : 81–94.
31. GaillardP-HL, NoguchiE, ShanahanP, RussellP (2003) The endogenous Mus81-Eme1 complex resolves Holliday junctions by a nick and counternick mechanism. Mol Cell 12 : 747–759.
32. OsmanF, DixonJ, DoeCL, WhitbyMC (2003) Generating crossovers by resolution of nicked Holliday junctions: a role for Mus81-Eme1 in meiosis. Mol Cell 12 : 761–774.
33. HollingsworthNM, BrillSJ (2004) The Mus81 solution to resolution: generating meiotic crossovers without Holliday junctions. Genes Dev 18 : 117–125.
34. AndersonCM, ChenSY, DimonMT, OkeA, DeRisiJL, et al. (2011) ReCombine: a suite of programs for detection and analysis of meiotic recombination in whole-genome datasets. PLoS One 6: e25509.
35. ManceraE, BourgonR, BrozziA, HuberW, SteinmetzLM (2008) High-resolution mapping of meiotic crossovers and non-crossovers in yeast. Nature 454 : 479–485.
36. QiJ, WijeratneAJ, TomshoLP, HuY, SchusterSC, et al. (2009) Characterization of meiotic crossovers and gene conversion by whole-genome sequencing in Saccharomyces cerevisiae. BMC Genomics 10 : 475.
37. WinzelerEA, RichardsDR, ConwayAR, GoldsteinAL, KalmanS, et al. (1998) Direct allelic variation scanning of the yeast genome. Science (80-) 281 : 1194–1197.
38. LiuY, GainesWa, CallenderT, BusyginaV, OkeA, et al. (2014) Down-regulation of Rad51 activity during meiosis in yeast prevents competition with Dmc1 for repair of double-strand breaks. PLoS Genet 10: e1004005.
39. ZakharyevichK, MaY, TangS, HwangPY, BoiteuxS, et al. (2010) Temporally and biochemically distinct activities of Exo1 during meiosis: double-strand break resection and resolution of double Holliday junctions. Mol Cell 40 : 1001–1015.
40. ShinoharaM, OhSD, HunterN, ShinoharaA (2008) Crossover assurance and crossover interference are distinctly regulated by the ZMM proteins during yeast meiosis. Nat Genet 40 : 299–309.
41. OhSD, LaoJP, TaylorAF, SmithGR, HunterN (2008) RecQ helicase, Sgs1, and XPF family endonuclease, Mus81-Mms4, resolve aberrant joint molecules during meiotic recombination. Mol Cell 31 : 324–336.
42. JessopL, LichtenM (2008) Mus81/Mms4 endonuclease and Sgs1 helicase collaborate to ensure proper recombination intermediate metabolism during meiosis. Mol Cell 31 : 313–323.
43. ChenSY, TsubouchiT, RockmillB, SandlerJS, RichardsDR, et al. (2008) Global analysis of the meiotic crossover landscape. Dev Cell 15 : 401–415.
44. CoïcE, GluckL, FabreF (2000) Evidence for short-patch mismatch repair in Saccharomyces cerevisiae. EMBO J 19 : 3408–3417.
45. SnowdenT, AcharyaS, ButzC, BerardiniM, FishelR (2004) hMSH4-hMSH5 recognizes Holliday Junctions and forms a meiosis-specific sliding clamp that embraces homologous chromosomes. Mol Cell 15 : 437–451.
46. ThackerD, MohibullahN, ZhuX, KeeneyS (2014) Homologue engagement controls meiotic DNA break number and distribution. Nature 510 : 241–246.
47. LongtineMS, McKenzieAIII, DemariniDJ, ShahNG (1998) Additional Modules for Versatile and Economical PCR-based Gene Deletion and Modification in Saccharomyces cerevisiae. 961 : 953–961.
48. Chen SY, Fung JC (2011) Mapping of Crossover Sites Using DNA Microarrays. In: Tsubouchi H, editor. DNA Recombination. Methods in Molecular Biology. Totowa, NJ: Humana Press, Vol. 745. pp. 117–134.
Štítky
Genetika Reprodukční medicína
Článek Oligoasthenoteratozoospermia and Infertility in Mice Deficient for miR-34b/c and miR-449 LociČlánek The Kinesin AtPSS1 Promotes Synapsis and is Required for Proper Crossover Distribution in MeiosisČlánek Payoffs, Not Tradeoffs, in the Adaptation of a Virus to Ostensibly Conflicting Selective PressuresČlánek Examination of Prokaryotic Multipartite Genome Evolution through Experimental Genome ReductionČlánek BMP-FGF Signaling Axis Mediates Wnt-Induced Epidermal Stratification in Developing Mammalian SkinČlánek Role of STN1 and DNA Polymerase α in Telomere Stability and Genome-Wide Replication in ArabidopsisČlánek RNA-Processing Protein TDP-43 Regulates FOXO-Dependent Protein Quality Control in Stress ResponseČlánek Integrating Functional Data to Prioritize Causal Variants in Statistical Fine-Mapping StudiesČlánek Salt-Induced Stabilization of EIN3/EIL1 Confers Salinity Tolerance by Deterring ROS Accumulation inČlánek Ethylene-Induced Inhibition of Root Growth Requires Abscisic Acid Function in Rice ( L.) SeedlingsČlánek Metabolic Respiration Induces AMPK- and Ire1p-Dependent Activation of the p38-Type HOG MAPK PathwayČlánek Signature Gene Expression Reveals Novel Clues to the Molecular Mechanisms of Dimorphic Transition inČlánek A Mouse Model Uncovers LKB1 as an UVB-Induced DNA Damage Sensor Mediating CDKN1A (p21) DegradationČlánek Dominant Sequences of Human Major Histocompatibility Complex Conserved Extended Haplotypes from to
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2014 Číslo 10
-
Všechny články tohoto čísla
- An Deletion Is Highly Associated with a Juvenile-Onset Inherited Polyneuropathy in Leonberger and Saint Bernard Dogs
- Licensing of Yeast Centrosome Duplication Requires Phosphoregulation of Sfi1
- Oligoasthenoteratozoospermia and Infertility in Mice Deficient for miR-34b/c and miR-449 Loci
- Basement Membrane and Cell Integrity of Self-Tissues in Maintaining Immunological Tolerance
- The Kinesin AtPSS1 Promotes Synapsis and is Required for Proper Crossover Distribution in Meiosis
- Germline Mutations in Are Associated with Familial Gastric Cancer
- POT1a and Components of CST Engage Telomerase and Regulate Its Activity in
- Controlling Meiotic Recombinational Repair – Specifying the Roles of ZMMs, Sgs1 and Mus81/Mms4 in Crossover Formation
- Payoffs, Not Tradeoffs, in the Adaptation of a Virus to Ostensibly Conflicting Selective Pressures
- FHIT Suppresses Epithelial-Mesenchymal Transition (EMT) and Metastasis in Lung Cancer through Modulation of MicroRNAs
- Genome-Wide Mapping of Yeast RNA Polymerase II Termination
- Examination of Prokaryotic Multipartite Genome Evolution through Experimental Genome Reduction
- White Cells Facilitate Opposite- and Same-Sex Mating of Opaque Cells in
- BMP-FGF Signaling Axis Mediates Wnt-Induced Epidermal Stratification in Developing Mammalian Skin
- Genome-Wide Association Study of CSF Levels of 59 Alzheimer's Disease Candidate Proteins: Significant Associations with Proteins Involved in Amyloid Processing and Inflammation
- COE Loss-of-Function Analysis Reveals a Genetic Program Underlying Maintenance and Regeneration of the Nervous System in Planarians
- Fat-Dachsous Signaling Coordinates Cartilage Differentiation and Polarity during Craniofacial Development
- Identification of Genes Important for Cutaneous Function Revealed by a Large Scale Reverse Genetic Screen in the Mouse
- Sensors at Centrosomes Reveal Determinants of Local Separase Activity
- Genes Integrate and Hedgehog Pathways in the Second Heart Field for Cardiac Septation
- Systematic Dissection of Coding Exons at Single Nucleotide Resolution Supports an Additional Role in Cell-Specific Transcriptional Regulation
- Recovery from an Acute Infection in Requires the GATA Transcription Factor ELT-2
- HIPPO Pathway Members Restrict SOX2 to the Inner Cell Mass Where It Promotes ICM Fates in the Mouse Blastocyst
- Role of and in Development of Abdominal Epithelia Breaks Posterior Prevalence Rule
- The Formation of Endoderm-Derived Taste Sensory Organs Requires a -Dependent Expansion of Embryonic Taste Bud Progenitor Cells
- Role of STN1 and DNA Polymerase α in Telomere Stability and Genome-Wide Replication in Arabidopsis
- Keratin 76 Is Required for Tight Junction Function and Maintenance of the Skin Barrier
- Encodes the Catalytic Subunit of N Alpha-Acetyltransferase that Regulates Development, Metabolism and Adult Lifespan
- Disruption of SUMO-Specific Protease 2 Induces Mitochondria Mediated Neurodegeneration
- Caudal Regulates the Spatiotemporal Dynamics of Pair-Rule Waves in
- It's All in Your Mind: Determining Germ Cell Fate by Neuronal IRE-1 in
- A Conserved Role for Homologs in Protecting Dopaminergic Neurons from Oxidative Stress
- The Master Activator of IncA/C Conjugative Plasmids Stimulates Genomic Islands and Multidrug Resistance Dissemination
- An AGEF-1/Arf GTPase/AP-1 Ensemble Antagonizes LET-23 EGFR Basolateral Localization and Signaling during Vulva Induction
- The Proteomic Landscape of the Suprachiasmatic Nucleus Clock Reveals Large-Scale Coordination of Key Biological Processes
- RNA-Processing Protein TDP-43 Regulates FOXO-Dependent Protein Quality Control in Stress Response
- A Complex Genetic Switch Involving Overlapping Divergent Promoters and DNA Looping Regulates Expression of Conjugation Genes of a Gram-positive Plasmid
- ZTF-8 Interacts with the 9-1-1 Complex and Is Required for DNA Damage Response and Double-Strand Break Repair in the Germline
- Integrating Functional Data to Prioritize Causal Variants in Statistical Fine-Mapping Studies
- Tpz1-Ccq1 and Tpz1-Poz1 Interactions within Fission Yeast Shelterin Modulate Ccq1 Thr93 Phosphorylation and Telomerase Recruitment
- Salt-Induced Stabilization of EIN3/EIL1 Confers Salinity Tolerance by Deterring ROS Accumulation in
- Telomeric (s) in spp. Encode Mediator Subunits That Regulate Distinct Virulence Traits
- Ethylene-Induced Inhibition of Root Growth Requires Abscisic Acid Function in Rice ( L.) Seedlings
- Ancient Expansion of the Hox Cluster in Lepidoptera Generated Four Homeobox Genes Implicated in Extra-Embryonic Tissue Formation
- Mechanism of Suppression of Chromosomal Instability by DNA Polymerase POLQ
- A Mutation in the Mouse Gene Leads to Impaired Hedgehog Signaling
- Keeping mtDNA in Shape between Generations
- Targeted Exon Capture and Sequencing in Sporadic Amyotrophic Lateral Sclerosis
- TIF-IA-Dependent Regulation of Ribosome Synthesis in Muscle Is Required to Maintain Systemic Insulin Signaling and Larval Growth
- At Short Telomeres Tel1 Directs Early Replication and Phosphorylates Rif1
- Evidence of a Bacterial Receptor for Lysozyme: Binding of Lysozyme to the Anti-σ Factor RsiV Controls Activation of the ECF σ Factor σ
- Hsp40s Specify Functions of Hsp104 and Hsp90 Protein Chaperone Machines
- Feeding State, Insulin and NPR-1 Modulate Chemoreceptor Gene Expression via Integration of Sensory and Circuit Inputs
- Functional Interaction between Ribosomal Protein L6 and RbgA during Ribosome Assembly
- Multiple Regulatory Systems Coordinate DNA Replication with Cell Growth in
- Fast Evolution from Precast Bricks: Genomics of Young Freshwater Populations of Threespine Stickleback
- Mmp1 Processing of the PDF Neuropeptide Regulates Circadian Structural Plasticity of Pacemaker Neurons
- The Nuclear Immune Receptor Is Required for -Dependent Constitutive Defense Activation in
- Genetic Modifiers of Neurofibromatosis Type 1-Associated Café-au-Lait Macule Count Identified Using Multi-platform Analysis
- Juvenile Hormone-Receptor Complex Acts on and to Promote Polyploidy and Vitellogenesis in the Migratory Locust
- Uncovering Enhancer Functions Using the α-Globin Locus
- The Analysis of Mutant Alleles of Different Strength Reveals Multiple Functions of Topoisomerase 2 in Regulation of Chromosome Structure
- Metabolic Respiration Induces AMPK- and Ire1p-Dependent Activation of the p38-Type HOG MAPK Pathway
- The Specification and Global Reprogramming of Histone Epigenetic Marks during Gamete Formation and Early Embryo Development in
- The DAF-16 FOXO Transcription Factor Regulates to Modulate Stress Resistance in , Linking Insulin/IGF-1 Signaling to Protein N-Terminal Acetylation
- Genetic Influences on Translation in Yeast
- Analysis of Mutants Defective in the Cdk8 Module of Mediator Reveal Links between Metabolism and Biofilm Formation
- Ribosomal Readthrough at a Short UGA Stop Codon Context Triggers Dual Localization of Metabolic Enzymes in Fungi and Animals
- Gene Duplication Restores the Viability of Δ and Δ Mutants
- Selection on a Variant Associated with Improved Viral Clearance Drives Local, Adaptive Pseudogenization of Interferon Lambda 4 ()
- Break-Induced Replication Requires DNA Damage-Induced Phosphorylation of Pif1 and Leads to Telomere Lengthening
- Dynamic Partnership between TFIIH, PGC-1α and SIRT1 Is Impaired in Trichothiodystrophy
- Signature Gene Expression Reveals Novel Clues to the Molecular Mechanisms of Dimorphic Transition in
- Mutations in Moderate or Severe Intellectual Disability
- Multifaceted Genome Control by Set1 Dependent and Independent of H3K4 Methylation and the Set1C/COMPASS Complex
- A Role for Taiman in Insect Metamorphosis
- The Small RNA Rli27 Regulates a Cell Wall Protein inside Eukaryotic Cells by Targeting a Long 5′-UTR Variant
- MMS Exposure Promotes Increased MtDNA Mutagenesis in the Presence of Replication-Defective Disease-Associated DNA Polymerase γ Variants
- Coexistence and Within-Host Evolution of Diversified Lineages of Hypermutable in Long-term Cystic Fibrosis Infections
- Comprehensive Mapping of the Flagellar Regulatory Network
- Topoisomerase II Is Required for the Proper Separation of Heterochromatic Regions during Female Meiosis
- A Splice Mutation in the Gene Causes High Glycogen Content and Low Meat Quality in Pig Skeletal Muscle
- KDM5 Interacts with Foxo to Modulate Cellular Levels of Oxidative Stress
- H2B Mono-ubiquitylation Facilitates Fork Stalling and Recovery during Replication Stress by Coordinating Rad53 Activation and Chromatin Assembly
- Copy Number Variation in the Horse Genome
- Unifying Genetic Canalization, Genetic Constraint, and Genotype-by-Environment Interaction: QTL by Genomic Background by Environment Interaction of Flowering Time in
- Spinster Homolog 2 () Deficiency Causes Early Onset Progressive Hearing Loss
- Genome-Wide Discovery of Drug-Dependent Human Liver Regulatory Elements
- Developmentally-Regulated Excision of the SPβ Prophage Reconstitutes a Gene Required for Spore Envelope Maturation in
- Protein Phosphatase 4 Promotes Chromosome Pairing and Synapsis, and Contributes to Maintaining Crossover Competence with Increasing Age
- The bHLH-PAS Transcription Factor Dysfusion Regulates Tarsal Joint Formation in Response to Notch Activity during Leg Development
- A Mouse Model Uncovers LKB1 as an UVB-Induced DNA Damage Sensor Mediating CDKN1A (p21) Degradation
- Notch3 Interactome Analysis Identified WWP2 as a Negative Regulator of Notch3 Signaling in Ovarian Cancer
- An Integrated Cell Purification and Genomics Strategy Reveals Multiple Regulators of Pancreas Development
- Dominant Sequences of Human Major Histocompatibility Complex Conserved Extended Haplotypes from to
- The Vesicle Protein SAM-4 Regulates the Processivity of Synaptic Vesicle Transport
- A Gain-of-Function Mutation in Impeded Bone Development through Increasing Expression in DA2B Mice
- Nephronophthisis-Associated Regulates Cell Cycle Progression, Apoptosis and Epithelial-to-Mesenchymal Transition
- Beclin 1 Is Required for Neuron Viability and Regulates Endosome Pathways via the UVRAG-VPS34 Complex
- The Not5 Subunit of the Ccr4-Not Complex Connects Transcription and Translation
- Abnormal Dosage of Ultraconserved Elements Is Highly Disfavored in Healthy Cells but Not Cancer Cells
- Genome-Wide Distribution of RNA-DNA Hybrids Identifies RNase H Targets in tRNA Genes, Retrotransposons and Mitochondria
- The Chromosomal Association of the Smc5/6 Complex Depends on Cohesion and Predicts the Level of Sister Chromatid Entanglement
- Cell-Autonomous Progeroid Changes in Conditional Mouse Models for Repair Endonuclease XPG Deficiency
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The Master Activator of IncA/C Conjugative Plasmids Stimulates Genomic Islands and Multidrug Resistance Dissemination
- A Splice Mutation in the Gene Causes High Glycogen Content and Low Meat Quality in Pig Skeletal Muscle
- Keratin 76 Is Required for Tight Junction Function and Maintenance of the Skin Barrier
- A Role for Taiman in Insect Metamorphosis
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



