-
Články
Reklama
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
ReklamaThe Formation of Endoderm-Derived Taste Sensory Organs Requires a -Dependent Expansion of Embryonic Taste Bud Progenitor Cells
Gustatory perception is an evolutionary ancient sense, and the ability to discriminate toxic and digestible substances is vitally important for all organisms. In mammals, taste perception occurs in taste buds, groups of sensory cells that are housed in various types of taste papillae in the oral cavity. Little is known about the genetic and developmental programs that underlie the different architectures of these papillae. Using mouse models, we identified the transcription factor Pax9 as a major determinant for the development of endoderm-derived taste papillae, which develop in different locations in the back of the oral cavity. In these papillae, Pax9 regulates the expansion of the taste progenitor field, maintains the competence of these progenitors to interact with afferent nerve fibers of the glossopharyngeal nerve, and prevents their differentiation towards epidermal-like epithelial cells. In contrast, Pax9 is not required for the development of ectoderm-derived taste papillae that are distributed over the dorsum of the tongue. Our data reveal that mammals have evolved a specific developmental program to generate taste buds and associated papilla structures in different parts of the oral cavity.
Published in the journal: . PLoS Genet 10(10): e32767. doi:10.1371/journal.pgen.1004709
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004709Summary
Gustatory perception is an evolutionary ancient sense, and the ability to discriminate toxic and digestible substances is vitally important for all organisms. In mammals, taste perception occurs in taste buds, groups of sensory cells that are housed in various types of taste papillae in the oral cavity. Little is known about the genetic and developmental programs that underlie the different architectures of these papillae. Using mouse models, we identified the transcription factor Pax9 as a major determinant for the development of endoderm-derived taste papillae, which develop in different locations in the back of the oral cavity. In these papillae, Pax9 regulates the expansion of the taste progenitor field, maintains the competence of these progenitors to interact with afferent nerve fibers of the glossopharyngeal nerve, and prevents their differentiation towards epidermal-like epithelial cells. In contrast, Pax9 is not required for the development of ectoderm-derived taste papillae that are distributed over the dorsum of the tongue. Our data reveal that mammals have evolved a specific developmental program to generate taste buds and associated papilla structures in different parts of the oral cavity.
Introduction
Taste buds consist of a group of clustered sensory cells and have been identified in all vertebrates. In the mammalian tongue, taste buds develop in different types of taste papillae: in fungiform papillae (FUP) distributed over the anterior dorsum of the tongue, in circumvallate papillae (CVP) located medially at the back of the tongue, and in foliate papillae (FOP) located laterally at the back of the tongue (Figure 1A). In addition, taste buds form locally without associated papilla structures in the epithelium of the soft palate, throat, epiglottis and upper esophagus. Despite phyletic variations and different distribution patterns of taste papillae, taste buds in the dorsal tongue epithelium develop in all vertebrates, including amphibia, reptiles, birds and mammals. In contrast, larger taste papillae with higher morphological complexity such as the CVP and FOP evolved exclusively in the mammalian lineage [1], [2].
Fig. 1. Expression patterns of Pax9 in different taste papillae of the embryonic mouse tongue. 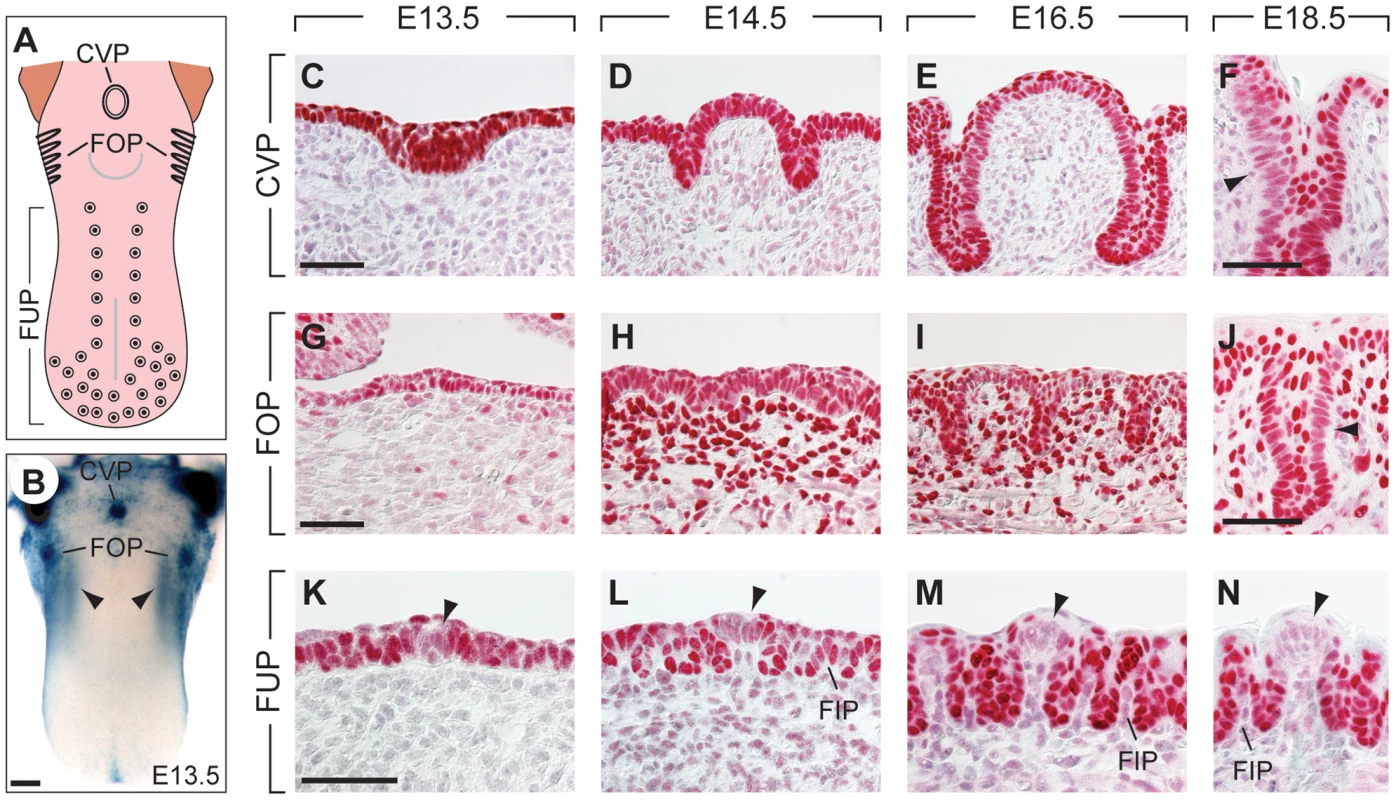
(A) Drawing showing the localization of the circumvallate papilla (CVP), foliate papillae (FOP), and fungiform papillae (FUP) in the mouse tongue. (B) Whole mount X-Gal staining of a Pax9+/LacZ mouse tongue at embryonic day 13.5 (E13.5). Note that expression is also seen in the mesenchyme adjacent to the developing FOP (arrowheads) and that the color reaction was stopped before epithelial staining began to obscure the mesenchymal expression domain. (C–N) Pax9 immunostaining of taste papillae during development on cross sections (C–F; K–N) and horizontal sections of the tongue (G–J). (C–F) Pax9 is expressed in the epithelium during CVP morphogenesis and is down-regulated in some regions of the trenches at E18.5 (arrowhead in F). (G–J) In addition to the epithelium, Pax9 is also expressed in the mesenchyme during FOP development, while reduced Pax9 levels were observed in the trenches at E18.5 (arrowhead in J). (K–N) In the anterior part of the tongue Pax9 is expressed in the FUP epithelium and in filiform papillae (FIP). Note that the expression is very weak or absent in the taste placodes (arrowheads). Scale bars: 200 µm in B; 50 µm in other panels. Embryonic induction and development of taste buds have been widely studied in amphibia and rodents (for a recent review, see [3]). These investigations concentrated mainly on the FUPs of mice and rats, which contain taste buds with taste pores that open directly into the oral cavity. FUP development starts around embryonic day 12.5 (E12.5) and involves the formation of an array of epithelial placodes in the anterior two thirds of the tongue. The early patterning of FUP development is regulated by complex signaling processes and involves interactions between the Wnt/β-catenin, Shh and Bmp pathways ([4]–[9]. In mice, each of approximately a total of 90 FUP contains a single taste bud, whereas in some mouse strains the single CVP may house more than 300 taste buds [10], which are located in epithelial trenches that begin to grow into the underlying mesenchyme at E14.5. In addition, small salivary glands (von Ebner's glands) develop together with the CVP and FOP [11] to facilitate gustatory sensation in taste buds located deep in the trenches. Thus, while taste buds of the FUP are formed by epithelial placodes that are established early in development, the placodes of the CVP and FOP undergo substantial morphological changes and intercalate a period of extensive epithelial growth to generate increased taste bud progenitor fields prior to the induction of taste bud cells.
Whereas the CVP and FOP of mammals house the vast majority of taste buds, our understanding of the genetic control of their morphogenesis is surprisingly fragmentary. A single trench was found to develop in the CVP of Tabby mice, which lack ectodysplasin A [12], [13]. A CVP placode is missing altogether in mice lacking a functional Fgf10 gene, which is expressed in the mesenchyme at the pre-placodal stage of CVP development [14]. A malformed CVP or reduction of CVP taste bud number has been described in mice lacking Dystonin, which show insufficient innervation caused by impaired development of the glossopharyngeal cranial nerve [15], as well as in mouse mutants that are compromised in attracting nerve endings due to missing expression of neurotrophins in the CVP epithelium [16]. A recent study revealed a role for Six1 and Six4 in CVP development, however, the morphological abnormalities may partly result from defects during cranial nerve formation, which are seen in Six1/Six4-deficient mice [17]. Thus there are considerable gaps in our knowledge about the developmental mechanisms that regulate the expansion of the early taste bud progenitor cell population in the CVP and FOP epithelium.
The paralogous genes Pax9 and Pax1 evolved from a single ancestral gene in the vertebrate lineage and form a subgroup within a total of nine members of the Pax gene family. Pax9 and Pax1 regulate different aspects of thymus, skeletal and craniofacial development [18]–[22]. Pax genes encode transcription factors and regulate the morphogenesis of a wide range of organs and are key factors for the development of mammalian sensory organs such as the eye (Pax6, Pax2), nose (Pax6) and ears (Pax2, Pax8) (for reviews, see [23], [24]). Here we show that Pax9, previously not implicated in the development of sensory organs, regulates essential steps during the development of endoderm-derived taste papillae.
Results
CVP and FOP development is arrested in Pax9-deficient mice
Epithelial expression of Pax9 in the developing oral apparatus of mice has been documented in the anterior foregut endoderm and its derivatives, as well as in the dorsal epithelium of the tongue [18], [25]. Whole mount X-Gal staining of a developing Pax9+/LacZ [21] mouse tongue at embryonic day 13.5 (E13.5) indicated that strong Pax9 expression is associated with the localization of placodes forming the CVP and FOP, respectively (Figure 1B). Immunostaining revealed Pax9 expression in the epithelium of placodes and trenches throughout the embryonic period of CVP and FOP development (Figure 1C–J). Pax9 was expressed normally in the region of the developing CVP of E13.5 and E14.5 mouse embryos lacking Fgf10 (Figure S1), a growth factor secreted by the posterior tongue mesenchyme and essential inducer of CVP development [14]. In contrast to the CVP, Pax9 is also expressed in the mesenchyme underlying the FOP epithelium (Figure 1G–J) in cells that are part of two discrete mesenchymal Pax9 expression domains at each side of the tongue (arrowheads in Figure 1B). The expression of Pax9 was down-regulated in some domains of the epithelial trenches at E18.5 (Figure 1F, J), a stage that precedes the early phase of taste bud induction in these papillae. Interestingly, while epithelial cells of the dorsal tongue were also stained, the central regions of placodes forming the FUP were negative for Pax9 at all stages of embryonic development (Figure 1K–N).
A histological analysis of serial sections of the three taste papilla types developing in the mouse tongue revealed that Pax9 is required for the formation of epithelial invaginations in both CVP and FOP. In homozygous Pax9LacZ/LacZ (for simplicity referred to as Pax9−/− hereafter) embryos, a CVP placode forms (Figure S2) but the epithelial trenches are growth retarded at E16.5 and E18.5 (Figure 2A–D). Similarly, invaginations of the FOP are missing and keratinocytes of the superficial layers are aberrantly enlarged in the mutant epithelium (Figure 2E–H). Moreover, the thickness of the mesenchymal cell layer was greatly reduced in the mutant FOP at E18.5. In contrast, the morphology of FUP appeared normal in Pax9−/− embryos (Fig. 2I–L).
Fig. 2. Arrest of CVP and FOP development in Pax9-deficient mouse embryos. 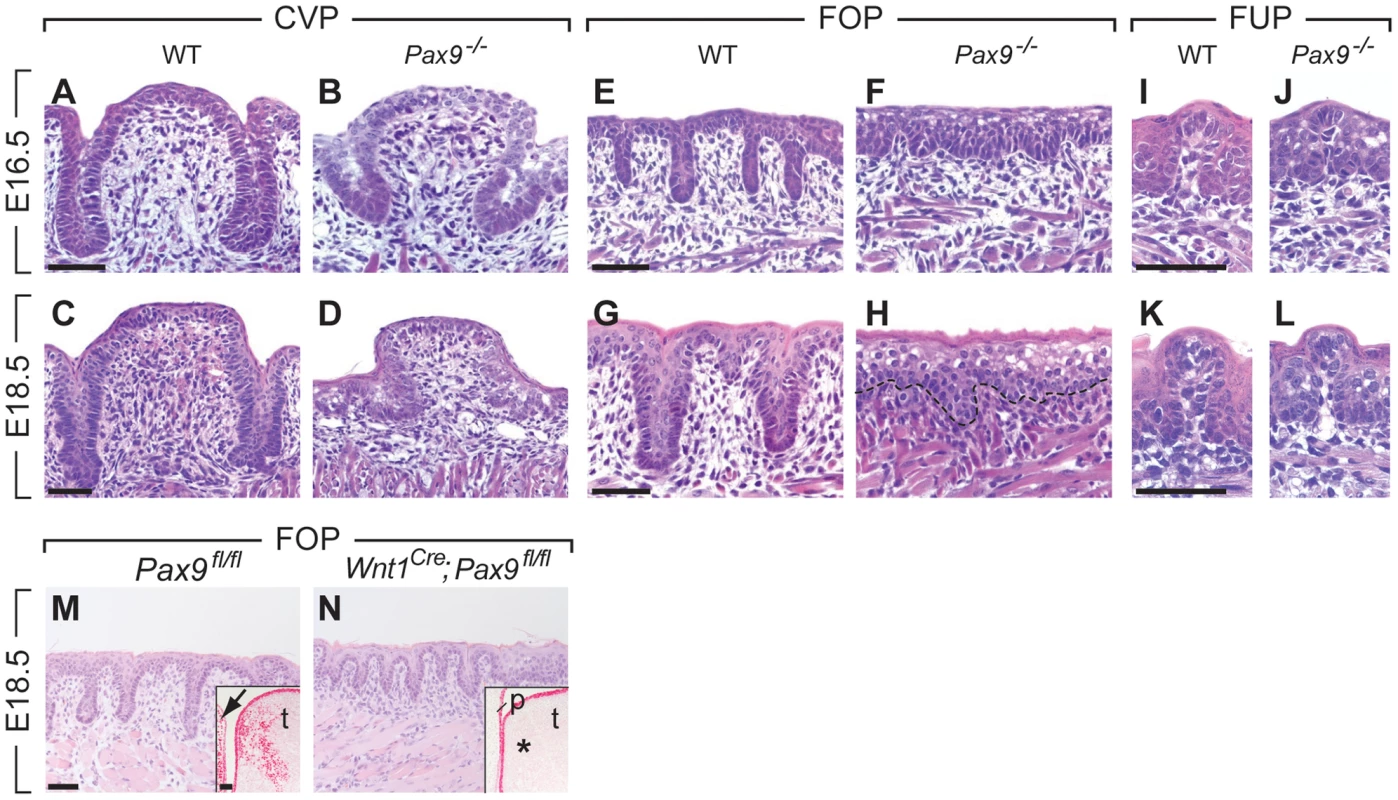
(A,C) In wild type (WT) embryos, the invaginating CVP epithelium forms deep trenches. (B,D) Rudimentary CVP trenches form in Pax9−/− embryos at E16.5 (B) but these trenches fail to invaginate (D). (E,G) A series of invaginations develop in the FOP of wild type embryos. (F,H) FOP trenches are absent in Pax9 mutants. (I–L) FUP development on the dorsal tongue. The FUP of wild type embryos (I,K) and Pax9−/− embryos (J,L) are morphologically indistinguishable. (M,N) FOP development in Pax9fl/fl embryos. (M) Without Cre expression, FOP development at E14.5 is normal and Pax9 expression is detectable in both epithelium and mesenchyme of the tongue (t), as well as in the adjacent lower jaw mesenchyme (arrow; inset shows a coronal section of the posterior region of the tongue). (N) Wnt1Cre-mediated inactivation of Pax9fl/fl did not disrupt the formation of epithelial invaginations. Note that Pax9-positive cells are not detectable in the tongue mesenchyme (asterisk in inset) or in the mesenchyme of the non-elevated secondary palate (p). Scale bars: 50 µm. To address the role of Pax9 in neural crest cell-derived mesenchymal cells located adjacent to the developing FOP (Figure 1G–J), we inactivated the Pax9 gene in these cells by crossing Pax9flox (Pax9fl) mice [26] with transgenic mice expressing Cre under the control of Wnt1 promoter (Wnt1Cre; [27]). While the Pax9fl/fl alleles were efficiently recombined in Wnt1Cre;Pax9fl/fl embryos, mesenchymal cells underlying the FOP were present and epithelial trenches formed in all (n = 5) mutant FOP of Wnt1Cre;Pax9fl/fl embryos (Figure 2M, N). These findings indicate that Pax9 function during FOP development is primarily required in epithelial cells.
Pax9 is dispensable in the developing and adult FUP
Postnatal Pax9 expression continues not only in the FUP epithelium but was also found in a few taste bud cells of the fully differentiated FUP (Figure 3A). Since FUP development is completed postnatally and since taste buds do not form prior to 2 days after birth we asked if Pax9 could be required at these later stages of FUP development. Because Pax9−/− embryos die at birth, we addressed this question by using transgenic mice expressing Cre under the control of Keratin 14 (K14Cre) promoter [28]. Previous studies showed that K14 is expressed in basal cells of the tongue epithelium and in FUP but not in actual taste bud cells. However, lineage tracing experiments identified K14-positive epithelial cells located directly adjacent to the taste bud as a niche of stem cells renewing taste bud cells in the adult mouse [29]. X-Gal staining of K14Cre;ROSAR26 embryos confirmed efficient Cre activity in the dorsal tongue epithelium from E13.5 onwards (Figure S3A,B) and Pax9 immunostaining revealed complete removal of Pax9 protein in both FUP and its associated taste buds in adult K14Cre;Pax9fl/fl mouse tongues (Figure 3B). Interestingly, the size and morphology of adult FUPs was not affected and FUP taste buds in these mutants appeared normal and formed taste pores (Figure 3C–F). In addition, the number of FUP visible on the dorsal aspect of K14Cre;Pax9fl/fl mouse tongues (30 per tongue, n = 5) did not differ significantly (p>0.79) from the number of FUPs of control mice (31 per tongue, n = 5).
Fig. 3. FUP maintenance and FUP taste bud renewal do not require Pax9 functions. 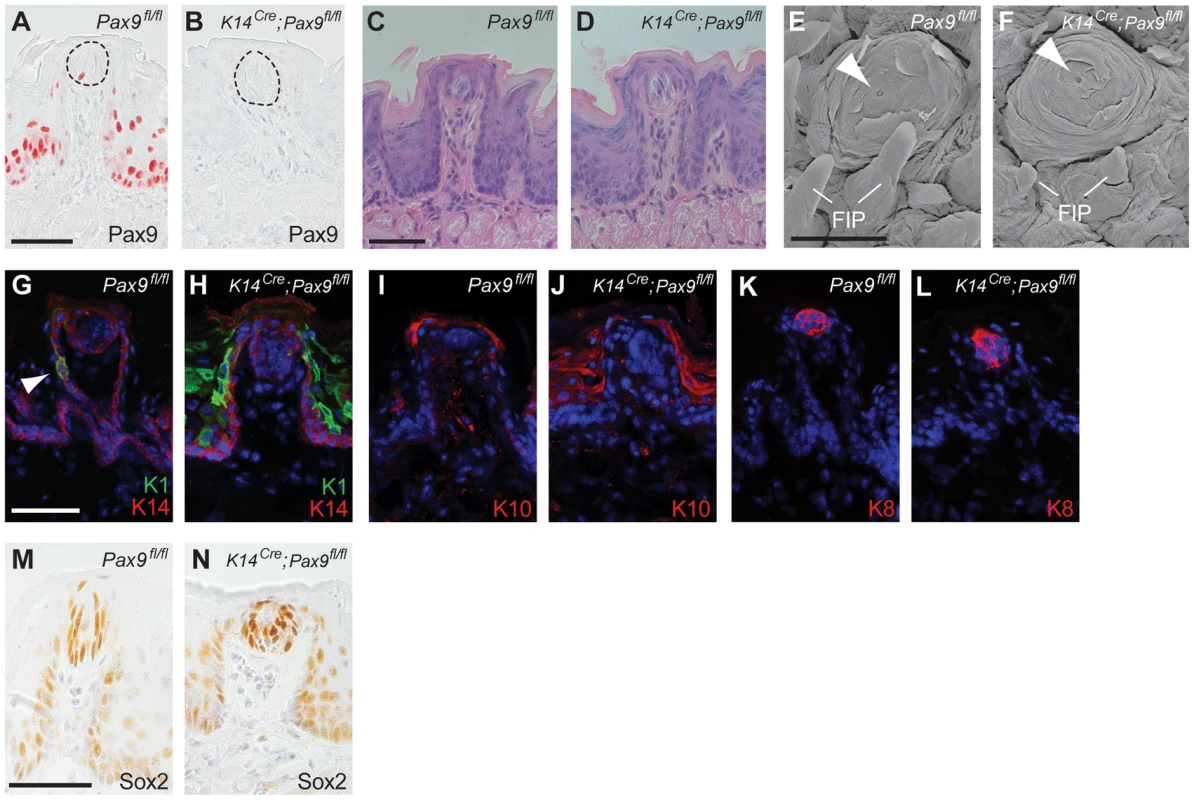
All analyses were carried out using 3–5 months old mice. (A,B) Pax9 immunostaining of FUPs. In Pax9fl/fl mice (A), Pax9 expression is detected in the FUP epithelium and in isolated taste bud cells (area of taste bud is indicated by dotted line). (B) No Pax9-positive cells are detectable in the FUP after K14Cre-mediated recombination of Pax9fl/fl. (C,D) Histological sections of FUP. Pax9fl/fl FUP (C) and K14Cre;Pax9fl/fl FUP (D) are morphologically indistinguishable. (E,F) Scanning electron microscopy images of FUP. The FUP of both Pax9fl/fl (E) and K14Cre;Pax9fl/fl (F) form taste pores (arrowhead), whereas the non-sensory FIP of the mutants (F) are hypoplastic. (G–L) Indirect immunofluorescent detection of keratins. Nuclei were stained with DAPI (blue). (G) In Pax9fl/fl mice, K14 is expressed in basal cells of the epithelium and K1 expression was seen in isolated epithelial cells of the FUP epithelium (arrowhead). (H) While K14 expression was not affected in the FUP of K14Cre;Pax9fl/fl mice, the number K1 expressing cells was strongly increased. (I,J) K10 expression is mainly restricted to the apical end of the FUP in Pax9fl/fl mice (I) whereas its expression is more extended in K14Cre;Pax9fl/fl mice (J). (K,L) K8 expression marks taste bud cells in both genotypes. (M,N) Immunohistochemical staining showing that Sox2 is expressed in mature taste buds of both Pax9fl/fl (M) and K14Cre;Pax9fl/fl (N) mice. Scale bars: 50 µm in A,C,G,M; 500 µm in E. To characterize the differentiation of the adult, Pax9-deficient FUP epithelium, we analyzed the expression of various keratin (K) proteins, which form intermediate filaments in cell type-specific combinations. We found that the keratin pair K1 and K10, which are normally expressed throughout the differentiated suprabasal layers of the epidermis, were strongly up-regulated in the K14Cre;Pax9fl/fl FUP epithelium, as well as in the interpapillary epithelium (Figure 3G–J). The expression of K6, which is often seen in hyperproliferative epidermal cells [30], was also up-regulated in the interpapillary epithelium, but not in the FUP epithelium itself (Figure S4A,B). In contrast, the expression of K14 and K5, which are normally found in basal cells of all stratified squamous epithelia, was not changed (Figure 3G,H; Figure S4C,D). Finally, we did not observe changes of the expression of K8, which marks taste bud cells in all taste papillae, as well as of Sox2, a marker of mature taste bud cells and critical regulator for the formation of taste sensory cell [31], in K14Cre;Pax9fl/fl mouse tongues (Figure 3K–N). Together, these results indicate that Pax9 is not functionally involved in the development of the mouse FUP. Furthermore, although the Pax9-deficient FUP epithelium shows alterations of keratin expression patterns, these changes are not associated with apparent defects of FUP maturation and FUP maintenance in the adult mouse. In contrast, Pax9 is required for the formation of filiform papillae (FIP), epithelial projections of the dorsal tongue epithelium that do not contain taste buds (Figure 3E,F; [25]).
Unlike the epithelium of the dorsal tongue, we did not detect full K14Cre activity in the CVP and FOP epithelium during embryonic development (Figure S3A,B). At perinatal stages, K14Cre activity expands to posterior regions of the tongue (Figure S3C) and while complete inactivation of Pax9 gradually manifests in the CVP and FOP of K14Cre;Pax9fl/fl mice, Pax9 deficiency was not associated with obvious morphological defects in these taste papillae (Figure S3D,E). In summary, the data indicate that Pax9 functions are not needed in adult taste papillae and that the requirement for Pax9 is restricted to the early steps of CVP and FOP morphogenesis.
Epithelial differentiation defects of the Pax9-deficient CVP are associated with the absence of proneural induction
While the FOP of Pax9−/− mutants does not form any epithelial trenches, the CVP exhibits rudimentary invaginations (Figure 2) and we thus chose the latter to characterize the cellular and molecular defects during embryonic CVP morphogenesis. SEM of the posterior tongue region showed that newborn Pax9−/− mice lack an accumulation of accessory papillae that normally surround the central domain of the CVP (Figure 4A,B). We also noted increased desquamation of the posterior tongue epithelium and diastase-controlled PAS staining revealed strongly increased levels of glycogen in the area in which the CVP trenches normally develop (Figure 4C,D). This differentiation defect is reminiscent of inappropriately increased deposition of glycogen regularly observed in the benign condition glycogenic acanthosis of the esophageal epithelium [32]. Moreover, a barrier assay revealed that only the central domain of the Pax9-deficient CVP was permeable to toluidine blue solution at E18.5, whereas the surrounding mutant tongue epithelium has prematurely established a full barrier (Figure 4E,F). Furthermore, the mutant CVP epithelium expresses high levels of Krt1 (Figure 4G,H), a keratin gene that is normally expressed in the mouse skin and not in the tongue [25] but was found to be up-regulated in oral dysplasia [33]. Together, these findings document the inappropriate differentiation of the Pax9-deficient CVP epithelium.
Fig. 4. Differentiation defects and lack of proneural induction in the Pax9-deficient CVP trench epithelium. 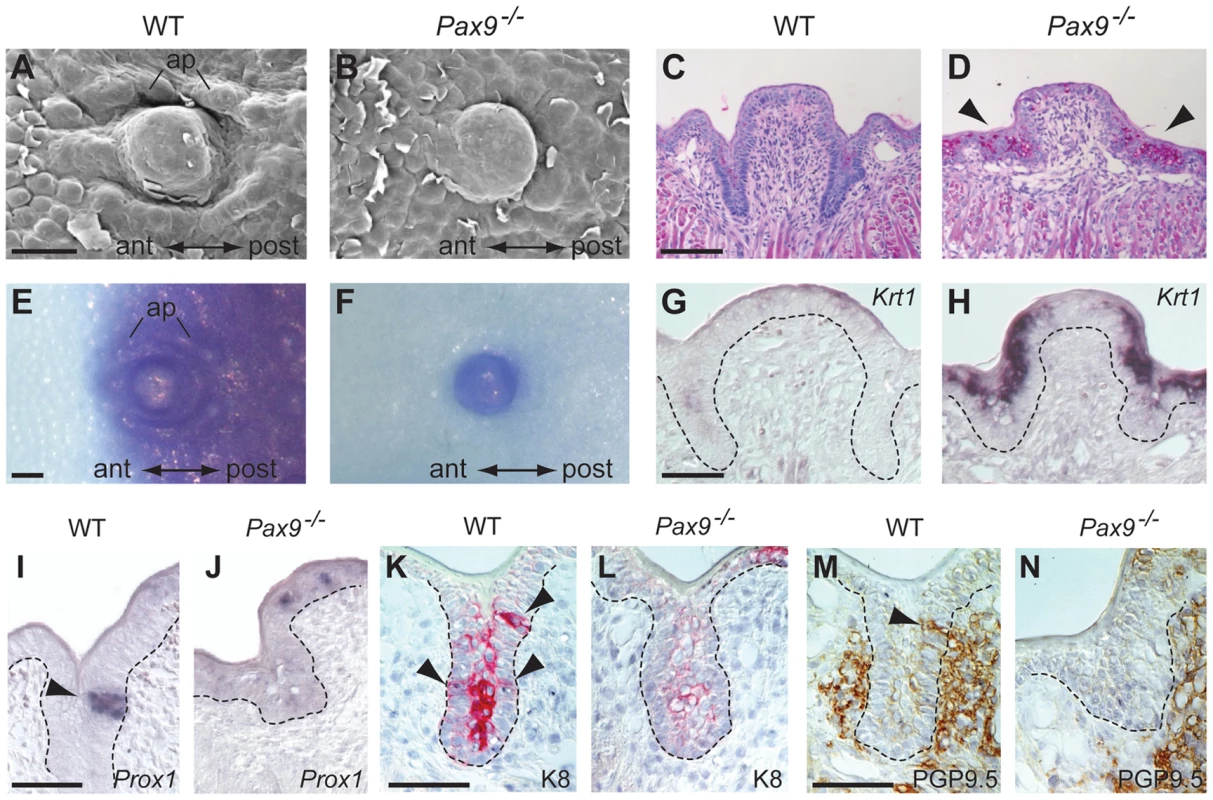
(A–N) Analyses of mouse embryos at E18.5. Anterior (ant) to posterior (post) orientation is indicated where appropriate. (A,B) SEM images of the CVP showing that both central dome and accessory papillae (ap) are well developed and separated by trenches in the wild type (A) but not in the Pax9−/− embryo (B). (C,D) PAS staining indicates intensively increased concentration of mucopolysaccharides in the mutant CVP trenches (arrowheads in (D). (E,F) Whole-mount barrier assay revealing that the CVP and the posterior tongue epithelium is permeable to toluidine blue in the wild type embryo (E), while a premature barrier has formed in the epithelium surrounding the mutant CVP (F). (G,H) Krt1 RNA in situ hybridization showing that Krt1 expression is strongly up-regulated in the Pax9−/− CVP (H). Dashed lines indicate the margin of the trench epithelium. (I,J) In situ hybridization of Prox1. Groups of epithelial trench cells express the proneural marker Prox1 in the wild type (I) but not in the Pax9−/− embryo (J). (K,L) Immunostaining of K8. Similar to Prox1, K8 is locally expressed in the wild type CVP (arrowheads in K). In contrast, only weak expression of K8 was detectable in the mutant CVP (L). (M,N) Immunostaining of PGP9.5. In the wild type CVP (M), nerve fibers contact the CVP trench epithelium (arrowhead; this section is directly adjacent to that shown in (K)), while nerve endings fail to invade the CVP trench epithelium of the Pax9−/− embryo (N). Scale bars: 100 µm in A,C,E; 50 µm in G,I,K,M. During mouse CVP development, taste buds become morphologically distinct from the surrounding trench epithelium two days after birth. Thus, to visualize epithelial domains that have started to initiate taste bud formation in the CVP at E18.5, we analyzed the expression of K8 and Prox1, which both mark taste bud primordia at this developmental stage [34], [35]. Both markers identified groups of cells in wild type epithelial trenches but not in the trenches of Pax9-deficient mice (Figure 4I–L). The same result was found using an Ascl1 (previously called Mash1) probe for in situ hybridization (Figure S5). In addition, K8 expression, normally found in loosely aligned cells in the middle of each trench (Figure 4K), was strongly reduced in the mutant CVP (Figure 4L). Interestingly, expression of Prox1 and K8 was also found in the apical domain of the CVP in both wild type (Figure S6A,B) and Pax9-deficient mice at E18.5 (Figure 4J,L; Figure S6C). We did not further investigate these structures, which are likely to represent immature taste buds that lack taste pores [36] and are known to disappear at early postnatal stages [37].
Afferent nerve fiber endings of the glossopharyngeal nerve make contact with the CVP epithelium from E14.5 onwards [38]. To analyze the pattern of CVP innervation, we stained for the neural marker PGP9.5 [39], which revealed a close contact of nerve fibers with the trench epithelium of the wild type CVP (Figure 4M). In contrast, although branches of the glossopharyngeal nerve were present at the Pax9-deficient CVP, we did not detect any penetration of the mutant trench epithelium by nerve endings (Figure 4N).
Disruption of the Shh signaling pathway in Pax9-deficient CVP and FOP
The Shh signaling pathway is active in taste papillae of the developing mouse tongue [40] and its inhibition was found to increase the number of FUP in the dorsal tongue epithelium [6], [7]. At the early stage of CVP development (E13.5), we found Shh expression in the epithelial placode in both control and Pax9-deficient embryos (Figure S2). At E14.5, in addition to the central, dome-like structure of the CVP, a ring of accessory papillae surrounding the center of the CVP was Shh-positive in controls, but not in Pax9−/− embryos (Figure 5A,B). Similar patterns were obtained with probes for the Shh pathway downstream genes Ptch1 and Gli1, in addition to a strong down-regulation of Gli1 expression in the center of the mutant CVP (Figure 5A,B). In the developing FOP, Shh expression was considerably weaker compared to that of the CVP but expression in an indistinctly delimited area was consistently identified on both sides in the posterior part of the wild type tongue (Figure 5C). In Pax9−/− mutants, Shh expression levels were below the detection threshold and only very weak expression of Ptch1 and Gli1 was found (Figure 5D). In contrast, consistent with unaffected FUP development, Shh was normally expressed in the dorsal tongue epithelium of Pax9−/− mutants (Figure 5E, F).
Fig. 5. Absence of Pax9 causes an endoderm-specific disruption of the Shh pathway in taste papillae. 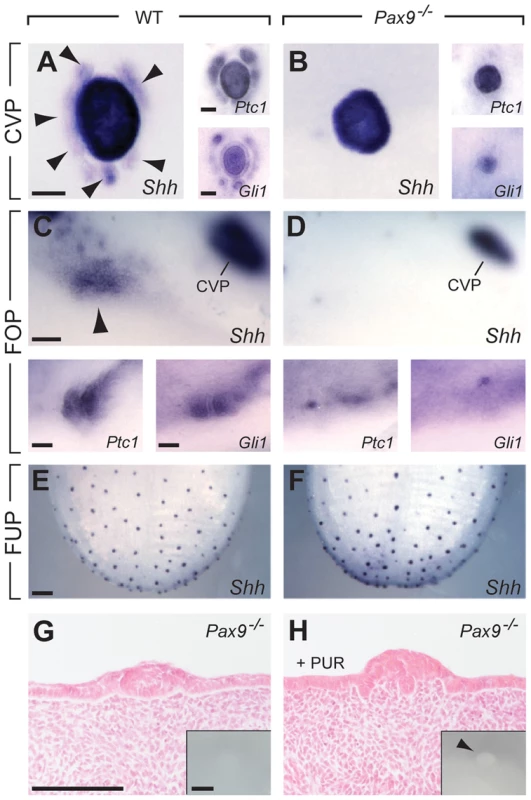
(A–F) Whole mount in situ hybridization of Shh, its receptor (Ptc1) and the downstream effector transcription factor (Gli1) at E14.5. (A,B) In the wild type CVP (A), Shh is expressed in the central dome as well as in a ring of accessory papillae (arrowheads). Ptc1 and Gli are expressed in a similar pattern. In the absence of Pax9, Shh, Ptc1 and Gli1 are only expressed in the central dome of the CVP (B). (C,D) In wild type embryos (C), patches of Shh, Ptc1 and Gli1 expression are detectable in the region of the developing FOP, whereas these expression patterns are missing (Shh) or are greatly reduced (Ptc1, Gli1) in Pax9−/− embryos (D). (E,F) Shh expression in FUP placodes is similar in wild type (E) and Pax9-deficient (F) embryos. (G, H) Histological sections of Pax9-deficient, cultured embryonic tongues. (G) In control medium the Pax9−/− CVP of cultured tongues is small and is not visible externally (inset). (H) In the presence of purmorphamine (PUR) the number of epithelial cells is increased in the dome of the CVP. Note the absence of trenches. Inset shows enlarged, protruded CVP dome (arrowhead) of the cultured tongue. Scale bars: 100 µm in A,C,G: 200 µm in E. Since the Shh pathway is an important modulator of epithelial morphogenesis during the development of various ectodermal appendages [41]–[43] we speculated that a reduction of Shh pathway activity in the developing CVP could be related to the impaired growth of the trenches in Pax9−/− embryos. To test this, we cultured mutant embryonic tongues in the presence of purmorphamine, a Shh signaling agonist that targets the Shh pathway effector protein Smoothened [44]. Under culture conditions used in this study, embryonic tongues dissected at E13.5 and cultured for 48 hours in control medium either formed a small CVP or an epithelial bud. In the presence of purmorphamine, the size of the mutant CVP (n = 4) was significantly increased but growth was primarily stimulated in the central, dome-like domain of the CVP (3 out of 4, Figure 5H). A similar response was observed in Pax9-deficient tongues cultured in the presence of a Shh protein-loaded bead placed next to the CVP. However, this result was only seen when the Shh protein-loaded bead was not displaced during culture (Figure S7A,B). In contrast, an enlarged CVP dome or enhanced trench formation was not observed after purmorphamine treatment of explants from wild type embryos (Figure S7C).
Pax1 is a critical target of Pax9 in the proliferating CVP trench epithelium
The incomplete ability of Shh pathway activation to restore epithelial growth of the Pax9-deficient CVP prompted us to search for additional developmental pathways that may be affected in the CVP of Pax9−/− mutants. To screen for early molecular defects, a genome-wide RNA expression analysis of wild type and Pax9-deficient CVP dissected at E14.5 was carried out. The array data suggested that two genes encoding the transcriptional regulators Sox9 and Pax1 might present early targets of Pax9 in the developing CVP. Immunostaining indeed confirmed that both transcription factors are strongly expressed at the tips of invaginating trenches of the normal CVP, but not in the CVP of Pax9−/− mutants (Figure 6A–D). Sox9 and Pax1 were shown to regulate epithelial cell proliferation in various developmental systems [45]–[47] and in agreement with these functions, counting of BrdU-positive cells at the tip of the growing trenches revealed a significant reduction of the number of proliferating cells in Pax9−/− mutants (Figure 6E–G).
Fig. 6. Pax1 and Sox9 are Pax9 targets in the proliferating compartment of the CVP trenches. 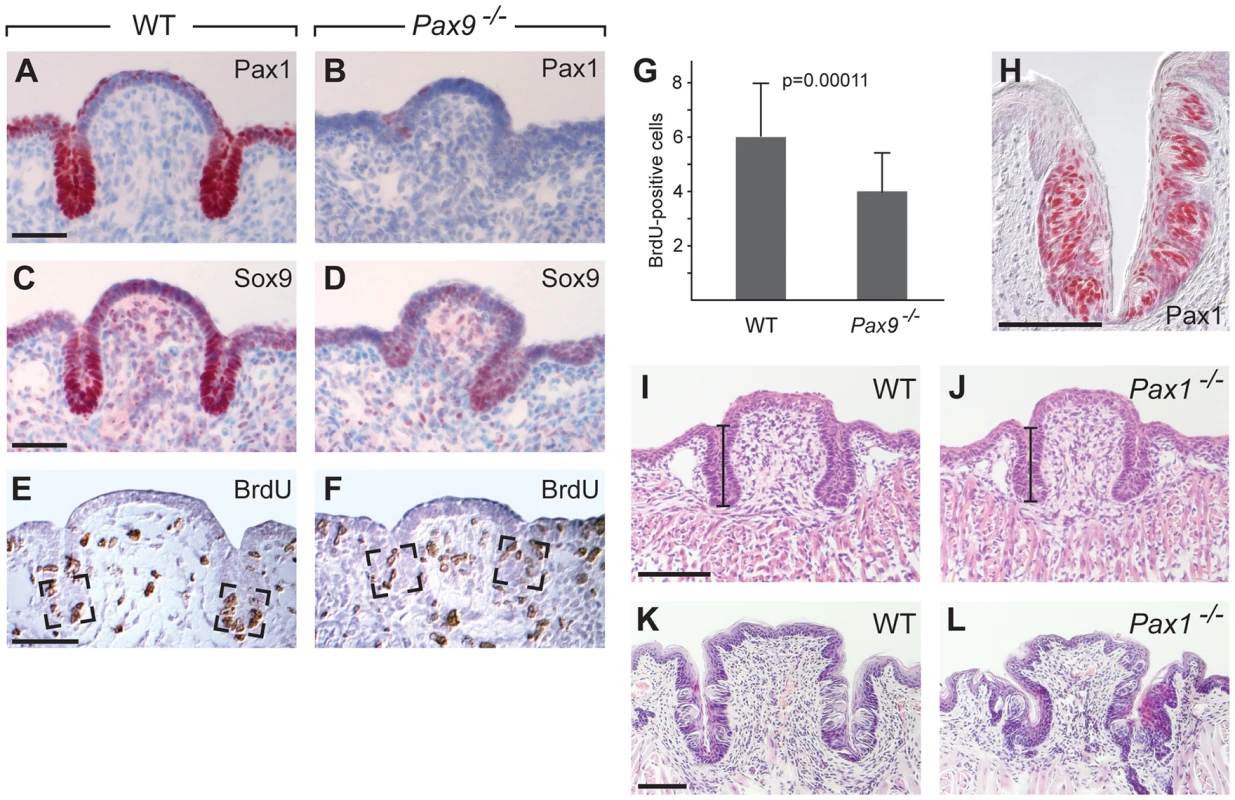
(A–F) Immunohistochemical staining on sections of the CVP at E15.5. (A,B) Pax1 is strongly expressed in the tips of epithelial trenches and in periderm cells covering the central dome of the wild type CVP (A), but not in the Pax9-deficient CVP (B). (C,D) Similarly, Sox9 expression is strongest in the epithelial trenches (C) and is barely detectable in the Pax9 mutant CVP (D). (E,F) BrdU-positive cells were counted in defined areas (boxed) of the CVP trenches from three wild type (n = 29 sections) and three Pax9 mutant CVPs (n = 28 sections). (G) The number of proliferating cells in the Pax9-deficient CVP is significantly reduced. Error bars illustrate standard deviation. (H) Pax1 immunostaining of one CVP trench in a 3 months old wild type mouse. (I,J) Morphology of the CVP at E18.5. The lengths of the CVP trenches (indicated by bars) were measured and shown to be reduced in the absence of Pax1 (for summary of measurements see Table S1). (K,L) Morphology of the CVP at postnatal day 16. In Pax1 mutants (n = 3) the trenches are growth-retarded and contain fewer taste buds. Scale bars: 50 µm in A,C,E; 100 µm in H,I,K. Pax1 and Pax9 are paralogous genes and while they have redundant functions during vertebral column development [22], the absence of Pax1 expression in the Pax9-deficient CVP rules out that Pax1 may compensate for the loss of Pax9 during early CVP development. Interestingly, Pax1 itself continues to be expressed and labels most taste bud cells in the wild type CVP and FOP of adult mice (Figure 6H; Figure S8A). In contrast, Pax1 is not expressed in the dorsal tongue epithelium during FUP development or in the FUP of adult mice (Figure S8B,C). Analysis of mouse mutants with a targeted deletion of Pax1 [48] showed that they develop shorter CVP trenches at E18.5 (111 µm in Pax1−/− mutants, 131 µm in control littermates, n = 8, p<0.01), while the width of the CVP was not significantly changed (Figure 6I,J; Table S1). Histological analysis of the CVP at postnatal day 16 revealed that the CVP of Pax1-deficient mice was noticeably smaller (n = 3; Figure 6K,L). Corresponding with this growth retardation, counting taste buds of a complete series of sections of one Pax1 mutant CVP indicated that the total number of taste buds was reduced by more than 50%. Thus Pax1 expression in the CVP trenches is required for epithelial growth and for the generation of the normal number of taste buds in the mouse CVP.
Taste placodes in the soft palate are missing in Pax9−/− mutants
The posterior part of the secondary palate forms the soft palate which, in contrast to the hard palate, is movable and not supported by bones. Moreover, the oral mucosa of the soft palate is part of the gustatory system and forms taste buds, however, these taste buds lack supporting papilla structures and are directly embedded in the epithelium (Figure 7D). During soft palate development, Pax9 expression was detected in the mesenchyme as well as in the epithelium prior to palatal shelf elevation (Figure 7A). Taste placodes of the soft palate begin to form as epithelial thickenings at E14.5 and express Shh [49]. Both taste placodes and soft palate epithelium are Pax9-positive, whereas Pax9 is not expressed in the epithelium of the hard palate, which lacks these placodes (Figure 7B,C). In newborn Pax9−/− mice, no clusters of taste bud progenitors were found in the soft palate epithelium (Figure 7D,E) and complete absence of Shh expression at E14.5 indicates that taste placode induction is not initiated in the soft palate of Pax9−/− mutants (Figure 7F,G).
Fig. 7. Pax9 is essential for taste placode formation in the soft palate. 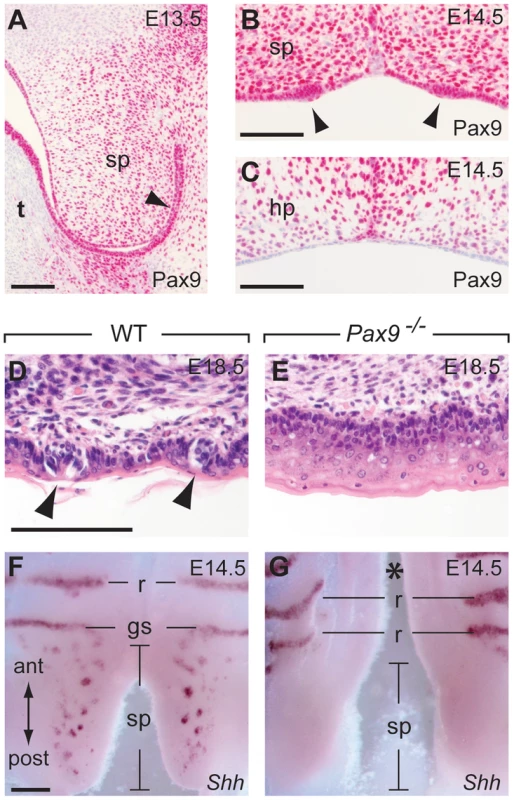
(A–C) Pax9 immunostaining of the secondary palate. (A) At E13.5, Pax9 expression is found in the mesenchyme as well as in those epithelial cells (arrowhead) of the soft palate (sp) facing the oral cavity after palatal shelf elevation. (B) At E14.5 the palatal shelves have elevated and Pax9 expression is seen in epithelial placodes (arrowheads) of the soft palate. (C) Pax9 is not expressed in the epithelium of the hard palate (hp). (D,E) Histological staining revealed taste bud precursors in wild type (D), but not in the Pax9-deficient (E) epithelium of the soft palate at E18.5. (F,G) Whole-mount Shh in situ hybridization at E14.5. In the wild type soft palate (F), Shh expression marks the taste placodes of the soft palate as well as the “Geschmacksstreifen” (gs). Note that palatal rugae (r) also express Shh at this stage. (G) Shh expression is not detectable in the soft palate of Pax9 mutants, which also form a cleft secondary palate (asterisk). Scale bars: 100 µm in A–D; 200 µm in F. Discussion
Taste perception at the back of the mammalian oral cavity serves as a critically important control mechanism to discriminate nutritious ingredients from substances that are potentially toxic to the organism. The formation of epithelial trenches that are rinsed by saliva produced in associated minor salivary glands enables a high concentration of functional taste buds to form in the narrow, posterior part of the tongue. The complex architecture of the CVP and FOP, and the close vicinity of numerous taste buds in these taste papillae predict the activities of developmental programs to differ from those regulating patterning and development of the FUP on the anterior dorsal tongue. Indeed, while loss of Fgf10 signaling in the mouse tongue mesenchyme results in the absence of the CVP, the spacing and size of FUP increased in these mutants [14]. In addition, similar to the differential expression of Pax1 shown in this work, expression of a Bmp4 reporter allele was detected in taste buds of the CVP but not in taste buds of FUP [50]. These fundamental differences may be attributed to the different embryonic origins of various taste papillae. Strong support for an entirely endoderm-derived origin of the CVP and FOP was recently provided by lineage tracing of Sox17-2AiCre/R26R mouse embryos [51],[52]. The study also indicated that the FUP on the dorsal tongue are exclusively derived from ectodermal cells.
During the development of the oral epithelium, expression of Pax9 is not restricted to endoderm-derived structures but is also seen in ectoderm-derived FUP as well as in non-sensory filiform papillae (this work; [25]). Our results clearly demonstrate that Pax9-deficiency does not affect patterning, development or maintenance of the mouse FUP. Although this result was unexpected, it reinforces the conclusion that endoderm-specific developmental pathways regulate the formation of the gustatory system in the posterior region of the oral cavity.
The early steps of CVP morphogenesis follow a sequence that is similar to that typically seen during the development of organs which form by epithelial-mesenchymal interactions. In analogy to the formation of, for example, a mammalian tooth or hair follicle, the CVP placode forms a bud-like epithelial structure that subsequently branches to form lateral invaginations. While the initial branching of the CVP bud is not affected in Pax9 mutant embryos, subsequent invagination of the epithelial trenches is blocked. Interestingly, a characteristic ring of accessory papillae normally surrounding the central dome of the CVP was not established in Pax9-deficient embryos. Whereas the developmental role of the accessory papillae has not been studied thus far, we found that they express Shh, suggesting that they may function as transient signaling centers and thereby contribute to CVP morphogenesis. The mitogenic effect of Shh has been documented in various epithelia [53]–[55] and we here found that activation of the Shh downstream pathway by purmorphamine increased the size of the Pax9-deficient CVP in embryonic tongue cultures. However, epithelial trench formation could not be rescued in these experiments, raising the possibility that precise timing and localization of Shh secretion by accessory papilla cells are required to restrict cell proliferation to the rudimentary trenches. Inhibition of the Shh pathway in rat embryonic tongue cultures was shown to increase the number of FUP [6]. While the external morphology of the CVP was not altered by Shh pathway inhibition, formation and growth of the epithelial trenches was not analyzed in these experiments. Recently, mouse reporter strains mapping the expression of the Shh pathway and its downstream genes in embryonic and adult FUP convincingly demonstrated an association between Shh expression and proliferation in neighboring epithelial cells [29], [56]. Thus, in vivo experiments using genetic tools suitable to inactivate or activate the Shh pathway in the CVP in an inducible manner should help to identify the specific roles of Shh for patterning and morphogenesis during CVP development.
Our analyses identified Pax9 as the first developmental regulator that is directly required for the expansion of taste progenitor cells in the developing mouse CVP. This progenitor field is normally established during a period of epithelial growth between E14.5 and E18.5 and our BrdU-labeling revealed a high proportion of cells that proliferate at the tip of the CVP trenches. Proliferation is significantly reduced in the invaginating epithelial CVP trench cells of Pax9−/− embryos, and this cellular defect is associated with a drastic down-regulation of Sox9, a known regulator of epithelial cell proliferation in other developing organs [45], [46], [57]. Beside this, Sox9 is necessary to establish the stem cell compartment in the hair follicle [58], raising the possibility that Sox9 could have a similar function in the CVP.
Pax1 and Pax9 exhibit similar expression patterns during embryonic development and function in a redundant manner during the formation of the vertebral column [18], [22]. Similarly, Pax1 and Pax9 both regulate aspects during the development of the thymus, which is derived from the foregut endoderm [20], [21]. Interestingly, while Pax1 is more critically required during vertebral column development, the role of Pax9 is more important in foregut-derived organs, to which the expression of the common Pax9/1 precursor is restricted in early chordates [59]. The moderate CVP phenotype of Pax1−/− mice identified in this work appears to support this conclusion. Together, these findings suggest that the mammalian Pax9 gene has retained the original function of the common Pax9/1 precursor gene in the foregut endoderm, while Pax1 has acquired a predominant role in the axial skeleton during vertebrate evolution.
Besides their functions in taste papilla formation, the expression of Pax9 and Pax1 in taste buds of adult mice suggests additional roles in the fully matured gustatory system. The absence of isolated, Pax9-positive cells in FUP taste buds after K14Cre-mediated recombination did not cause obvious morphological defects of the taste buds. However, as K14Cre is not active in actual taste bud cells, this finding supports the conclusion that stem cells from adjacent, non-sensory FUP cells contribute to the renewal of FUP taste buds [29]. While the roles of Pax9 and Pax1 in taste buds remain to be elucidated using appropriate genetic tools, it is tempting to speculate that they could be involved in the specification of sub-populations of mature taste bud cells.
Absent expression of K8 and Prox1 and lack of contact by nerve endings in the developing CVP trenches, as well as premature barrier formation indicates a highly defective differentiation program of the posterior tongue epithelium of Pax9-deficient embryos. In the mutants, the arrest of CVP morphogenesis is associated with ectopic expression of Krt1, a keratin gene known to be strongly up-regulated in dysplasia of the oral epithelium [33], as well as with increased levels of glycogen, a feature seen in the benign condition glycogenic acanthosis [32]. It therefore appears likely that premature and inappropriate terminal differentiation of the CVP epithelium accounts, at least in part, for the incompetence of the CVP trench cells to interact with nerve fiber endings and to generate taste bud progenitors.
Our data show that epithelial trench formation in the CVP and FOP is Pax9-dependent. A primary function for Pax9 in the expansion of taste progenitor fields in taste papillae with a higher degree of architectural complexity appears to be supported by the finding that taste papillae on the dorsal tongue, which lack epithelial trenches, develop normally in Pax9-deficient mice. However, although the soft palate epithelium does normally not form any recognizeable taste papilla structures, Pax9 is required for early Shh expression and for the induction of taste progenitor cells in this part of the oral cavity. Interestingly, lack of Shh expression in the taste placodes of the soft palate was also observed in mouse mutants lacking β-Catenin in the epithelium [60], raising the possibility that Pax9 might interact with Wnt-signalling. A complete secondary palate only evolved in the mammalian lineage, whereas the tongue is present in amphibia, reptiles, birds, and mammals [1]. While the molecular mechanisms regulated by Pax9 in the soft palate epithelium remain to be identified, it is conceivable that Pax9 may have acquired an additional, early role for taste placode formation in the soft palate epithelium at a later period during the evolution of tetrapods.
Materials and Methods
Ethics statement
All procedures were carried out under personal and project licenses issued by the Home Office, UK and were approved by the Local Ethics Committee.
Mouse husbandry and genotyping
Mice were housed as described previously [61]. Embryos were staged by taking mid-day on the day of vaginal plug detection as embryonic day 0.5 (E0.5). The following mouse lines were maintained on the indicated genetic background, intercrossed to produce relevant genotypes and PCR genotyped according to references: Pax9lacZ (C57BL/6; [21]), Pax9flox (C57BL/6 x 129S2/SvPas; [26]), Wnt1Cre (C57BL/6; [26]), K14Cre (FVB/N; [28]), Pax1 (C57BL/6; [48]), ROSA26R (C57BL/6; [62]).
Histology and immunohistochemistry
Mouse tissues were prepared, processed, paraffin-embedded, sectioned, stained with haematoxylin and eosin and photographically documented as described previously [61]. Diastase-controlled Periodic acid-Schiff (D-PAS) staining was performed as described [63]. CVP size was measured using AxioVision software v.4.3 (Carl Zeiss) and statistically analyzed by a two-tailed t-test (Excel software, Microsoft).
Pax9 immunohistochemical staining on paraffin sections was performed as described previously [64] with the following modifications. Antibodies were diluted in antibody diluent (Dako, S3022) and incubated in the following order: rat anti-Pax9 (1∶40), rabbit anti-rat IgG (Dako, Z0494; 1∶50), rat APAAP (Dako, D0488; 1∶50) with three TBS washes in between each step. The last two steps were repeated and alkaline phosphatase activity was visualized using Fast Red (Sigma) as a substrate. Other primary antibodies were detected using the Envision+ System-HRP kit (Dako, K4008 or K4010) according to the manufacturer's instructions. AEC (Dako, K4008) and DAB (Dako, K4010) substrates stain red and brown, respectively. Primary antibodies were used at the following dilutions: rabbit anti-PGP9.5 (7863-0504, AbD Serotec), 1∶200; rabbit anti-Sox2 (C70B1, Cell Signaling), 1∶100; rabbit anti-Sox9 (O9-1, [65]), 1∶1000; rat anti-Pax1 [66], 1∶40. Following incubation with rat anti-Pax1 antibody, HRP-conjugated rabbit anti-rat IgGs (Dako, P0450) were applied at 1∶200 dilution before using the rabbit-specific Envision+ detection system.
To visualize proliferating cells, BrdU labeling and detection was performed as described previously [67]. Serial sections from three wild type (29 sections) and three Pax9-deficient (28 sections) E15.5 CVPs dissected 90 minutes after BrdU injection were prepared and BrdU-positive cells counted in a defined area at the tip of epithelial trenches. Statistical significance was assessed using a two-tailed t-test.
For indirect immunofluorescence analysis, 5 µm cryosections were air-dried on Superfrost ultra plus slides (Thermo Scientific) for 2 hours at room temperature and then fixed for 10 minutes with pre-cooled acetone at −20°C. Immunofluorescence analysis was performed as previously described [68], using the following primary antibodies: rabbit anti-K1 (AF109, Covance), 1∶1000; mouse anti-K10 (DE-K10, Progen), 1∶160; rabbit anti-K5 (Covance), 1∶5000; guinea pig anti-K14 (GPCK14.2, Progen), 1∶50; mouse anti-K6 (Ks6.Ka12, Progen), 1∶10; rat anti-K8 (TROMA-I, Developmental Studies Hybridoma Bank), 1∶50. Nuclei were stained with DAPI (Invitrogen) and secondary antibodies were species-specific fluorochrome-conjugated goat antibodies: Cy3-conjugated anti-mouse and anti-guinea pig, both 1∶200; Alexa 594-conjugated anti-rat and Alexa 488-conjugated anti-rabbit, both 1∶400 (Molecular Probes). Microscopic analysis was performed using a Leica SP2 UV confocal microscope operated through LCS 2.61 software (Leica Microsystems).
Barrier assay, X-Gal staining and scanning electron microscopy (SEM)
Tongue barrier assays and whole-mount X-gal staining were performed as described previously [25], [21]. For SEM, tongues were fixed in 2% glutaraldehyde/PBS, dehydrated through a graded series of ethanol followed by carbon dioxide incubation in a Samdri 780 Critical Point Dryer. The specimens were then mounted on an aluminium stub with Acheson Silver Electrodag (Agar Scientific) and coated with gold using a Polaron SEM coating unit. Specimens were examined and photographed using a Stereoscan 240 scanning electron microscope. SEM images taken from flat-mounted tongues of 4 months old mice were also used to count the number of FUPs that were directly visible on the dorsal tongue surface.
RNA in situ hybridization
RNA in situ hybridization of whole embryonic specimens and of tissue sections using digoxygenin-labelled cRNA probes was performed as described previously [67]. cRNA probes were produced for Shh (0.6 kb; MGI:1327804), Ptch1 (2.2 kb; MGI:3833867), Gli1 (1.7 kb; MGI:12533), Prox1 (0.5 kb; [69]), Ascl1 (0.7 kb; [69]), and Krt1 (0.5 kb; [25]).
Embryonic tongue culture
Embryonic mandibles including tongues were dissected at E13.0 and cultured for two days as described previously [70], [4]. Before culture, the specimens were embedded in growth factor-reduced Matrigel (BD Biosciences, Cat. No. 305128) to prevent them from flattening during culture. To activate the Hh pathway, 4 µM purmorphamine (Calbiochem, Cat.No. 540220) was added to the culture medium. Alternatively, Affi-Gel Blue gel beads (Bio Rad, Cat.No. 153-7302) were soaked in recombinant mouse SHH protein (1.25 mg/mL in PBS; R&D Systems, Cat.No. 461-SH) or BSA for at least an hour and the beads were then placed onto the tongue epithelium close to the developing CVP.
Supporting Information
Zdroje
1. IwasakiS (2002) Evolution of the structure and function of the vertebrate tongue. J Anat 201 : 1–13.
2. NorthcuttRG (2004) Taste buds: development and evolution. Brain Behav Evol 64 : 198–206.
3. KapsimaliM, BarlowLA (2013) Developing a sense of taste. Semin Cell Dev Biol 24 : 200–209.
4. IwatsukiK, LiuHX, GrónderA, SingerMA, LaneTF, et al. (2007) Wnt signaling interacts with Shh to regulate taste papilla development. Proc Natl Acad Sci U S A 104 : 2253–2258.
5. LiuF, ThirumangalathuS, GallantNM, YangSH, Stoick-CooperCL, et al. (2007) Wnt-beta-catenin signaling initiates taste papilla development. Nat Genet 39 : 106–112.
6. MistrettaCM, LiuHX, GaffieldW, MacCallumDK (2003) Cyclopamine and jervine in embryonic rat tongue cultures demonstrate a role for Shh signaling in taste papilla development and patterning: fungiform papillae double in number and form in novel locations in dorsal lingual epithelium. Dev Biol 254 : 1–18.
7. HallJM, BellML, FingerTE (2003) Disruption of sonic hedgehog signaling alters growth and patterning of lingual taste papillae. Dev Biol 255 : 263–277.
8. ZhouY, LiuHX, MistrettaCM (2006) Bone morphogenetic proteins and noggin: inhibiting and inducing fungiform taste papilla development. Dev Biol 297 : 198–213.
9. BeitesCL, HollenbeckPL, KimJ, Lovell-BadgeR, LanderAD, et al. (2009) Follistatin modulates a BMP autoregulatory loop to control the size and patterning of sensory domains in the developing tongue. Development 136 : 2187–2197.
10. CongerAD, WellsMA (1969) Radiation and Aging Effect on Taste Structure and Function. Radiat Res 37 : 31–49.
11. LeeMJ, KimJY, LeeSI, SasakiH, LunnyDP, et al. (2006) Association of Shh and Ptc with keratin localization in the initiation of the formation of circumvallate papilla and von Ebner's gland. Cell Tissue Res 325 : 253–261.
12. GrünebergH (1971) The glandular aspects of the tabby syndrome in the mouse. J Embryol Exp Morphol 25 : 1–19.
13. WellsKL, MouC, HeadonDJ, TuckerAS (2010) Defects and rescue of the minor salivary glands in Eda pathway mutants. Dev Biol 349 : 137–146.
14. PetersenCI, JheonAH, MostowfiP, CharlesC, ChingS, et al. (2011) FGF signaling regulates the number of posterior taste papillae by controlling progenitor field size. PLoS Genet 7: e1002098.
15. IchikawaH, De RepentignyY, KotharyR, SugimotoT (2006) The survival of vagal and glossopharyngeal sensory neurons is dependent upon dystonin. Neuroscience 137 : 531–536.
16. NosratIV, AgermanK, MarinescuA, ErnforsP, NosratCA (2004) Lingual deficits in neurotrophin double knockout mice. J Neurocytol 33 : 607–615.
17. SuzukiY, IkedaK, KawakamiK (2011) Development of gustatory papillae in the absence of Six1 and Six4. J Anat 219 : 710–721.
18. NeubüserA, KosekiH, BallingR (1995) Characterization and developmental expression of Pax9, a paired-box-containing gene related to Pax1. Dev Biol 170 : 701–716.
19. WallinJ, WiltingJ, KosekiH, FritschR, ChristB, et al. (1994) The role of Pax-1 in axial skeleton development. Development 120 : 1109–1121.
20. WallinJ, EibelH, NeubüserA, WiltingJ, KosekiH, et al. (1996) Pax1 is expressed during development of the thymus epithelium and is required for normal T-cell maturation. Development 122 : 23–30.
21. PetersH, NeubüserA, KratochwilK, BallingR (1998) Pax9-deficient mice lack pharyngeal pouch derivatives and teeth and exhibit craniofacial and limb abnormalities. Genes Dev 12 : 2735–2747.
22. PetersH, WilmB, SakaiN, ImaiK, MaasR, et al. (1999) Pax1 and Pax9 synergistically regulate vertebral column development. Development 126 : 5399–5408.
23. KozmikZ (2005) Pax genes in eye development and evolution. Curr Opin Genet Dev 15 : 430–438.
24. BlakeJA, ZimanMR (2014) Pax genes: regulators of lineage specification and progenitor cell maintenance. Development 141 : 737–751.
25. JonkerL, KistR, AwA, WapplerI, PetersH (2004) Pax9 is required for filiform papilla development and suppresses skin-specific differentiation of the mammalian tongue epithelium. Mech Dev 121 : 1313–1322.
26. KistR, GreallyE, PetersH (2007) Derivation of a mouse model for conditional inactivation of Pax9. Genesis 45 : 460–464.
27. DanielianPS, MuccinoD, RowitchDH, MichaelSK, McMahonAP (1998) Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr Biol 8 : 1323–1326.
28. VasioukhinV, DegensteinL, WiseB, FuchsE (1999) The magical touch: genome targeting in epidermal stem cells induced by tamoxifen application to mouse skin. Proc Natl Acad Sci U S A 96 : 8551–8556.
29. OkuboT, ClarkC, HoganBL (2009) Cell lineage mapping of taste bud cells and keratinocytes in the mouse tongue and soft palate. Stem Cells 27 : 442–450.
30. MollR, FrankeWW, SchillerDL, GeigerB, KreplerR (1982) The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell 31 : 11–24.
31. OkuboT, PevnyLH, HoganBL (2006) Sox2 is required for development of taste bud sensory cells. Genes Dev 20 : 2654–2659.
32. SternZ, SharonP, LigumskyM, LevijM, RachmilewitzD (1980) Glycogenic acanthosis of the esophagus: a benign but confusing endoscopic lesion. Am J Gastroenterol 74 : 261–263.
33. BloorBK, TidmanN, LeighIM, OdellE, DoganB, et al. (2003) Expression of keratin K2e in cutaneous and oral lesions: association with keratinocyte activation, proliferation, and keratinization. Am J Pathol 162 : 963–975.
34. MbieneJP, RobertsJD (2003) Distribution of keratin 8-containing cell clusters in mouse embryonic tongue: evidence for a prepattern for taste bud development. J Comp Neurol 457 : 111–122.
35. MiuraH, KusakabeY, KatoH, Miura-OhnumaJ, TagamiM, et al. (2003) Co-expression pattern of Shh with Prox1 and that of Nkx2.2 with Mash1 in mouse taste bud. Gene Expr Patterns 3 : 427–430.
36. TakedaM, SuzukiY, ObaraN, NagaiY (1992) Neural cell adhesion molecule of taste buds. J Electron Microsc (Tokyo) 41 : 375–380.
37. StateFA, BowdenRE (1974) Innervation and cholinesterase activity of the developing taste buds in the circumvallate papilla of the mouse. J Anat 118 : 211–221.
38. AhPinP, EllisS, ArnottC, KaufmanMH (1998) Prenatal Development and Innervation of the Circumvallate Papilla in the Mouse. J Anat 162 : 33–42.
39. WakisakaS, MiyawakiY, YounSH, KatoJ, KurisuK (1996) Protein gene-product 9.5 in developing mouse circumvallate papilla: comparison with neuron-specific enolase and calcitonin gene-related peptide. Anat Embryol (Berl) 194 : 365–372.
40. HallJM, HooperJE, FingerTE (1999) Expression of sonic hedgehog, patched, and Gli1 in developing taste papillae of the mouse. J Comp Neurol 406 : 143–155.
41. St-JacquesB, DassuleHR, KaravanovaI, BotchkarevVA, LiJ, et al. (1998) Sonic hedgehog signaling is essential for hair development. Curr Biol 8 : 1058–1068.
42. DassuleHR, LewisP, BeiM, MaasR, McMahonAP (2000) Sonic hedgehog regulates growth and morphogenesis of the tooth. Development 127 : 4775–4785.
43. JaskollT, LeoT, WitcherD, OrmestadM, AstorgaJ, et al. (2004) Sonic hedgehog signaling plays an essential role during embryonic salivary gland epithelial branching morphogenesis. Dev Dyn 229 : 722–732.
44. SinhaS, ChenJK (2006) Purmorphamine activates the Hedgehog pathway by targeting Smoothened. Nat Chem Biol 2 : 29–30.
45. BastideP, DaridoC, PannequinJ, KistR, RobineS, et al. (2007) Sox9 regulates cell proliferation and is required for Paneth cell differentiation in the intestinal epithelium. J Cell Biol 178 : 635–648.
46. FantauzzoKA, KurbanM, LevyB, ChristianoAM (2012) Trps1 and its target gene Sox9 regulate epithelial proliferation in the developing hair follicle and are associated with hypertrichosis. PLoS Genet 8: e1003002.
47. SuD, EllisS, NapierA, LeeK, ManleyNR (2001) Hoxa3 and Pax1 regulate epithelial cell death and proliferation during thymus and parathyroid organogenesis. Dev Biol 236 : 316–329.
48. WilmB, DahlE, PetersH, BallingR, ImaiK (1998) Targeted disruption of Pax1 defines its null phenotype and proves haploinsufficiency. Proc Natl Acad Sci U S A 95 : 8692–8697.
49. NakayamaA, MiuraH, ShindoY, KusakabeY, TomonariH, et al. (2008) Expression of the basal cell markers of taste buds in the anterior tongue and soft palate of the mouse embryo. J Comp Neurol 509 : 211–224.
50. NguyenHM, BarlowLA (2010) Differential expression of a BMP4 reporter allele in anterior fungiform versus posterior circumvallate taste buds of mice. BMC Neurosci 11 : 129.
51. EngertS, LiaoWP, BurtscherI, LickertH (2009) Sox17-2A-iCre: a knock-in mouse line expressing Cre recombinase in endoderm and vascular endothelial cells. Genesis 47 : 603–610.
52. RothovaM, ThompsonH, LickertH, TuckerAS (2012) Lineage tracing of the endoderm during oral development. Dev Dyn 241 : 1183–1191.
53. CobourneMT, HardcastleZ, SharpePT (2001) Sonic hedgehog regulates epithelial proliferation and cell survival in the developing tooth germ. J Dent Res 80 : 1974–1979.
54. Gritli-LindeA, BeiM, MaasR, ZhangXM, LindeA, et al. (2002) Shh signaling within the dental epithelium is necessary for cell proliferation, growth and polarization. Development 129 : 5323–5337.
55. MillP, MoR, FuH, GrachtchoukM, KimPC, et al. (2003) Sonic hedgehog-dependent activation of Gli2 is essential for embryonic hair follicle development. Genes Dev 17 : 282–294.
56. LiuHX, ErmilovA, GrachtchoukM, LiL, GumucioDL, et al. (2013) Multiple Shh signaling centers participate in fungiform papilla and taste bud formation and maintenance. Dev Biol 382 : 82–97.
57. SeymourPA, FreudeKK, TranMN, MayesEE, JensenJ, et al. (2007) SOX9 is required for maintenance of the pancreatic progenitor cell pool. Proc Natl Acad Sci U S A 104 : 1865–1870.
58. VidalVP, ChaboissierMC, LützkendorfS, CotsarelisG, MillP, et al. (2005) Sox9 is essential for outer root sheath differentiation and the formation of the hair stem cell compartment. Curr Biol 15 : 1340–1351.
59. OgasawaraM, WadaH, PetersH, SatohN (1999) Developmental expression of Pax1/9 genes in urochordate and hemichordate gills: insight into function and evolution of the pharyngeal epithelium. Development 126 : 2539–2550.
60. LinC, FisherAV, YinY, MaruyamaT, VeithGM, et al. (2011) The inductive role of Wnt-β-Catenin signaling in the formation of oral apparatus. Dev Biol 356 : 40–50.
61. KistR, WatsonM, WangX, CairnsP, MilesC, et al. (2005) Reduction of Pax9 gene dosage in an allelic series of mouse mutants causes hypodontia and oligodontia. Hum Mol Genet 14 : 3605–3617.
62. SorianoP (1999) Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet 21 : 70–71.
63. Bancroft JD (2002) Chapter 10: Mucins. In: Bancroft JD, Gamble M, editors. Theory and Practice of Histological Techniques. 5 ed: Churchill Livingstone, Elsevier Science Limited. pp. 172–175.
64. GerberJK, RichterT, KremmerE, AdamskiJ, HöflerH, et al. (2002) Progressive loss of PAX9 expression correlates with increasing malignancy of dysplastic and cancerous epithelium of the human oesophagus. J Pathol 197 : 293–297.
65. StoltCC, LommesP, SockE, ChaboissierMC, SchedlA, et al. (2003) The Sox9 transcription factor determines glial fate choice in the developing spinal cord. Genes Dev 17 : 1677–1689.
66. SonnesenL, NoltingD, KjaerKW, KjaerI (2008) Association between the development of the body axis and the craniofacial skeleton studied by immunohistochemical analyses using collagen II, Pax9, Pax1, and Noggin antibodies. Spine 33 : 1622–1626.
67. NakatomiM, WangXP, KeyD, LundJJ, Turbe-DoanA, et al. (2010) Genetic interactions between Pax9 and Msx1 regulate lip development and several stages of tooth morphogenesis. Dev Biol 340 : 438–449.
68. ReicheltJ, BauerC, PorterR, LaneE, MaginV (1997) Out of balance: consequences of a partial keratin 10 knockout. J Cell Sci 110 : 2175–2186.
69. MiuraH, KusakabeY, KatoH, Miura-OhnumaJ, TagamiM, et al. (2003) Co-expression pattern of Shh with Prox1 and that of Nkx2.2 with Mash1 in mouse taste bud. Gene Expr Patterns 3 : 427–430.
70. MbieneJP, MaccallumDK, MistrettaCM (1997) Organ cultures of embryonic rat tongue support tongue and gustatory papilla morphogenesis in vitro without intact sensory ganglia. J Comp Neurol 377 : 324–340.
Štítky
Genetika Reprodukční medicína
Článek Oligoasthenoteratozoospermia and Infertility in Mice Deficient for miR-34b/c and miR-449 LociČlánek The Kinesin AtPSS1 Promotes Synapsis and is Required for Proper Crossover Distribution in MeiosisČlánek Payoffs, Not Tradeoffs, in the Adaptation of a Virus to Ostensibly Conflicting Selective PressuresČlánek Examination of Prokaryotic Multipartite Genome Evolution through Experimental Genome ReductionČlánek BMP-FGF Signaling Axis Mediates Wnt-Induced Epidermal Stratification in Developing Mammalian SkinČlánek Role of STN1 and DNA Polymerase α in Telomere Stability and Genome-Wide Replication in ArabidopsisČlánek RNA-Processing Protein TDP-43 Regulates FOXO-Dependent Protein Quality Control in Stress ResponseČlánek Integrating Functional Data to Prioritize Causal Variants in Statistical Fine-Mapping StudiesČlánek Salt-Induced Stabilization of EIN3/EIL1 Confers Salinity Tolerance by Deterring ROS Accumulation inČlánek Ethylene-Induced Inhibition of Root Growth Requires Abscisic Acid Function in Rice ( L.) SeedlingsČlánek Metabolic Respiration Induces AMPK- and Ire1p-Dependent Activation of the p38-Type HOG MAPK PathwayČlánek Signature Gene Expression Reveals Novel Clues to the Molecular Mechanisms of Dimorphic Transition inČlánek A Mouse Model Uncovers LKB1 as an UVB-Induced DNA Damage Sensor Mediating CDKN1A (p21) DegradationČlánek Dominant Sequences of Human Major Histocompatibility Complex Conserved Extended Haplotypes from to
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2014 Číslo 10
-
Všechny články tohoto čísla
- An Deletion Is Highly Associated with a Juvenile-Onset Inherited Polyneuropathy in Leonberger and Saint Bernard Dogs
- Licensing of Yeast Centrosome Duplication Requires Phosphoregulation of Sfi1
- Oligoasthenoteratozoospermia and Infertility in Mice Deficient for miR-34b/c and miR-449 Loci
- Basement Membrane and Cell Integrity of Self-Tissues in Maintaining Immunological Tolerance
- The Kinesin AtPSS1 Promotes Synapsis and is Required for Proper Crossover Distribution in Meiosis
- Germline Mutations in Are Associated with Familial Gastric Cancer
- POT1a and Components of CST Engage Telomerase and Regulate Its Activity in
- Controlling Meiotic Recombinational Repair – Specifying the Roles of ZMMs, Sgs1 and Mus81/Mms4 in Crossover Formation
- Payoffs, Not Tradeoffs, in the Adaptation of a Virus to Ostensibly Conflicting Selective Pressures
- FHIT Suppresses Epithelial-Mesenchymal Transition (EMT) and Metastasis in Lung Cancer through Modulation of MicroRNAs
- Genome-Wide Mapping of Yeast RNA Polymerase II Termination
- Examination of Prokaryotic Multipartite Genome Evolution through Experimental Genome Reduction
- White Cells Facilitate Opposite- and Same-Sex Mating of Opaque Cells in
- BMP-FGF Signaling Axis Mediates Wnt-Induced Epidermal Stratification in Developing Mammalian Skin
- Genome-Wide Association Study of CSF Levels of 59 Alzheimer's Disease Candidate Proteins: Significant Associations with Proteins Involved in Amyloid Processing and Inflammation
- COE Loss-of-Function Analysis Reveals a Genetic Program Underlying Maintenance and Regeneration of the Nervous System in Planarians
- Fat-Dachsous Signaling Coordinates Cartilage Differentiation and Polarity during Craniofacial Development
- Identification of Genes Important for Cutaneous Function Revealed by a Large Scale Reverse Genetic Screen in the Mouse
- Sensors at Centrosomes Reveal Determinants of Local Separase Activity
- Genes Integrate and Hedgehog Pathways in the Second Heart Field for Cardiac Septation
- Systematic Dissection of Coding Exons at Single Nucleotide Resolution Supports an Additional Role in Cell-Specific Transcriptional Regulation
- Recovery from an Acute Infection in Requires the GATA Transcription Factor ELT-2
- HIPPO Pathway Members Restrict SOX2 to the Inner Cell Mass Where It Promotes ICM Fates in the Mouse Blastocyst
- Role of and in Development of Abdominal Epithelia Breaks Posterior Prevalence Rule
- The Formation of Endoderm-Derived Taste Sensory Organs Requires a -Dependent Expansion of Embryonic Taste Bud Progenitor Cells
- Role of STN1 and DNA Polymerase α in Telomere Stability and Genome-Wide Replication in Arabidopsis
- Keratin 76 Is Required for Tight Junction Function and Maintenance of the Skin Barrier
- Encodes the Catalytic Subunit of N Alpha-Acetyltransferase that Regulates Development, Metabolism and Adult Lifespan
- Disruption of SUMO-Specific Protease 2 Induces Mitochondria Mediated Neurodegeneration
- Caudal Regulates the Spatiotemporal Dynamics of Pair-Rule Waves in
- It's All in Your Mind: Determining Germ Cell Fate by Neuronal IRE-1 in
- A Conserved Role for Homologs in Protecting Dopaminergic Neurons from Oxidative Stress
- The Master Activator of IncA/C Conjugative Plasmids Stimulates Genomic Islands and Multidrug Resistance Dissemination
- An AGEF-1/Arf GTPase/AP-1 Ensemble Antagonizes LET-23 EGFR Basolateral Localization and Signaling during Vulva Induction
- The Proteomic Landscape of the Suprachiasmatic Nucleus Clock Reveals Large-Scale Coordination of Key Biological Processes
- RNA-Processing Protein TDP-43 Regulates FOXO-Dependent Protein Quality Control in Stress Response
- A Complex Genetic Switch Involving Overlapping Divergent Promoters and DNA Looping Regulates Expression of Conjugation Genes of a Gram-positive Plasmid
- ZTF-8 Interacts with the 9-1-1 Complex and Is Required for DNA Damage Response and Double-Strand Break Repair in the Germline
- Integrating Functional Data to Prioritize Causal Variants in Statistical Fine-Mapping Studies
- Tpz1-Ccq1 and Tpz1-Poz1 Interactions within Fission Yeast Shelterin Modulate Ccq1 Thr93 Phosphorylation and Telomerase Recruitment
- Salt-Induced Stabilization of EIN3/EIL1 Confers Salinity Tolerance by Deterring ROS Accumulation in
- Telomeric (s) in spp. Encode Mediator Subunits That Regulate Distinct Virulence Traits
- Ethylene-Induced Inhibition of Root Growth Requires Abscisic Acid Function in Rice ( L.) Seedlings
- Ancient Expansion of the Hox Cluster in Lepidoptera Generated Four Homeobox Genes Implicated in Extra-Embryonic Tissue Formation
- Mechanism of Suppression of Chromosomal Instability by DNA Polymerase POLQ
- A Mutation in the Mouse Gene Leads to Impaired Hedgehog Signaling
- Keeping mtDNA in Shape between Generations
- Targeted Exon Capture and Sequencing in Sporadic Amyotrophic Lateral Sclerosis
- TIF-IA-Dependent Regulation of Ribosome Synthesis in Muscle Is Required to Maintain Systemic Insulin Signaling and Larval Growth
- At Short Telomeres Tel1 Directs Early Replication and Phosphorylates Rif1
- Evidence of a Bacterial Receptor for Lysozyme: Binding of Lysozyme to the Anti-σ Factor RsiV Controls Activation of the ECF σ Factor σ
- Hsp40s Specify Functions of Hsp104 and Hsp90 Protein Chaperone Machines
- Feeding State, Insulin and NPR-1 Modulate Chemoreceptor Gene Expression via Integration of Sensory and Circuit Inputs
- Functional Interaction between Ribosomal Protein L6 and RbgA during Ribosome Assembly
- Multiple Regulatory Systems Coordinate DNA Replication with Cell Growth in
- Fast Evolution from Precast Bricks: Genomics of Young Freshwater Populations of Threespine Stickleback
- Mmp1 Processing of the PDF Neuropeptide Regulates Circadian Structural Plasticity of Pacemaker Neurons
- The Nuclear Immune Receptor Is Required for -Dependent Constitutive Defense Activation in
- Genetic Modifiers of Neurofibromatosis Type 1-Associated Café-au-Lait Macule Count Identified Using Multi-platform Analysis
- Juvenile Hormone-Receptor Complex Acts on and to Promote Polyploidy and Vitellogenesis in the Migratory Locust
- Uncovering Enhancer Functions Using the α-Globin Locus
- The Analysis of Mutant Alleles of Different Strength Reveals Multiple Functions of Topoisomerase 2 in Regulation of Chromosome Structure
- Metabolic Respiration Induces AMPK- and Ire1p-Dependent Activation of the p38-Type HOG MAPK Pathway
- The Specification and Global Reprogramming of Histone Epigenetic Marks during Gamete Formation and Early Embryo Development in
- The DAF-16 FOXO Transcription Factor Regulates to Modulate Stress Resistance in , Linking Insulin/IGF-1 Signaling to Protein N-Terminal Acetylation
- Genetic Influences on Translation in Yeast
- Analysis of Mutants Defective in the Cdk8 Module of Mediator Reveal Links between Metabolism and Biofilm Formation
- Ribosomal Readthrough at a Short UGA Stop Codon Context Triggers Dual Localization of Metabolic Enzymes in Fungi and Animals
- Gene Duplication Restores the Viability of Δ and Δ Mutants
- Selection on a Variant Associated with Improved Viral Clearance Drives Local, Adaptive Pseudogenization of Interferon Lambda 4 ()
- Break-Induced Replication Requires DNA Damage-Induced Phosphorylation of Pif1 and Leads to Telomere Lengthening
- Dynamic Partnership between TFIIH, PGC-1α and SIRT1 Is Impaired in Trichothiodystrophy
- Signature Gene Expression Reveals Novel Clues to the Molecular Mechanisms of Dimorphic Transition in
- Mutations in Moderate or Severe Intellectual Disability
- Multifaceted Genome Control by Set1 Dependent and Independent of H3K4 Methylation and the Set1C/COMPASS Complex
- A Role for Taiman in Insect Metamorphosis
- The Small RNA Rli27 Regulates a Cell Wall Protein inside Eukaryotic Cells by Targeting a Long 5′-UTR Variant
- MMS Exposure Promotes Increased MtDNA Mutagenesis in the Presence of Replication-Defective Disease-Associated DNA Polymerase γ Variants
- Coexistence and Within-Host Evolution of Diversified Lineages of Hypermutable in Long-term Cystic Fibrosis Infections
- Comprehensive Mapping of the Flagellar Regulatory Network
- Topoisomerase II Is Required for the Proper Separation of Heterochromatic Regions during Female Meiosis
- A Splice Mutation in the Gene Causes High Glycogen Content and Low Meat Quality in Pig Skeletal Muscle
- KDM5 Interacts with Foxo to Modulate Cellular Levels of Oxidative Stress
- H2B Mono-ubiquitylation Facilitates Fork Stalling and Recovery during Replication Stress by Coordinating Rad53 Activation and Chromatin Assembly
- Copy Number Variation in the Horse Genome
- Unifying Genetic Canalization, Genetic Constraint, and Genotype-by-Environment Interaction: QTL by Genomic Background by Environment Interaction of Flowering Time in
- Spinster Homolog 2 () Deficiency Causes Early Onset Progressive Hearing Loss
- Genome-Wide Discovery of Drug-Dependent Human Liver Regulatory Elements
- Developmentally-Regulated Excision of the SPβ Prophage Reconstitutes a Gene Required for Spore Envelope Maturation in
- Protein Phosphatase 4 Promotes Chromosome Pairing and Synapsis, and Contributes to Maintaining Crossover Competence with Increasing Age
- The bHLH-PAS Transcription Factor Dysfusion Regulates Tarsal Joint Formation in Response to Notch Activity during Leg Development
- A Mouse Model Uncovers LKB1 as an UVB-Induced DNA Damage Sensor Mediating CDKN1A (p21) Degradation
- Notch3 Interactome Analysis Identified WWP2 as a Negative Regulator of Notch3 Signaling in Ovarian Cancer
- An Integrated Cell Purification and Genomics Strategy Reveals Multiple Regulators of Pancreas Development
- Dominant Sequences of Human Major Histocompatibility Complex Conserved Extended Haplotypes from to
- The Vesicle Protein SAM-4 Regulates the Processivity of Synaptic Vesicle Transport
- A Gain-of-Function Mutation in Impeded Bone Development through Increasing Expression in DA2B Mice
- Nephronophthisis-Associated Regulates Cell Cycle Progression, Apoptosis and Epithelial-to-Mesenchymal Transition
- Beclin 1 Is Required for Neuron Viability and Regulates Endosome Pathways via the UVRAG-VPS34 Complex
- The Not5 Subunit of the Ccr4-Not Complex Connects Transcription and Translation
- Abnormal Dosage of Ultraconserved Elements Is Highly Disfavored in Healthy Cells but Not Cancer Cells
- Genome-Wide Distribution of RNA-DNA Hybrids Identifies RNase H Targets in tRNA Genes, Retrotransposons and Mitochondria
- The Chromosomal Association of the Smc5/6 Complex Depends on Cohesion and Predicts the Level of Sister Chromatid Entanglement
- Cell-Autonomous Progeroid Changes in Conditional Mouse Models for Repair Endonuclease XPG Deficiency
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The Master Activator of IncA/C Conjugative Plasmids Stimulates Genomic Islands and Multidrug Resistance Dissemination
- A Splice Mutation in the Gene Causes High Glycogen Content and Low Meat Quality in Pig Skeletal Muscle
- Keratin 76 Is Required for Tight Junction Function and Maintenance of the Skin Barrier
- A Role for Taiman in Insect Metamorphosis
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



