-
Články
Reklama
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
ReklamaRecovery from an Acute Infection in Requires the GATA Transcription Factor ELT-2
Infections by bacterial pathogens often produce substantial tissue damage and alter metabolism in the host that, if left unchecked, could be detrimental to overall fitness. The cellular and systemic responses that resolve these alterations in the host are not well defined. Here, we examine transcriptional networks in an animal host that are modulated during the resolution phase of an intestinal infection treated with an antibiotic. Up-regulation of genes involved in detoxification and cellular homeostasis during the resolution phase is controlled by the conserved endodermal GATA transcription factor ELT-2. GATA transcription factors are known to be involved in the development, differentiation, and function of a diverse array of metazoan tissue types. Therefore, our results ascribe a new role to GATA transcription factors in recovery from an acute infection. Fully characterizing the host response during resolution of an infection will lead to a better understanding of human health concerns related to recurrent infections, wound healing, autoimmune diseases, and chronic inflammatory disorders.
Published in the journal: . PLoS Genet 10(10): e32767. doi:10.1371/journal.pgen.1004609
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004609Summary
Infections by bacterial pathogens often produce substantial tissue damage and alter metabolism in the host that, if left unchecked, could be detrimental to overall fitness. The cellular and systemic responses that resolve these alterations in the host are not well defined. Here, we examine transcriptional networks in an animal host that are modulated during the resolution phase of an intestinal infection treated with an antibiotic. Up-regulation of genes involved in detoxification and cellular homeostasis during the resolution phase is controlled by the conserved endodermal GATA transcription factor ELT-2. GATA transcription factors are known to be involved in the development, differentiation, and function of a diverse array of metazoan tissue types. Therefore, our results ascribe a new role to GATA transcription factors in recovery from an acute infection. Fully characterizing the host response during resolution of an infection will lead to a better understanding of human health concerns related to recurrent infections, wound healing, autoimmune diseases, and chronic inflammatory disorders.
Introduction
The course of human bacterial infections is controlled by a combination of immune responses, physiological changes, and, if necessary, antibiotic treatment. To recover from an infection and return to homeostasis, the host must activate mechanisms capable of controlling the damage caused by pathogen virulence factors, inflammation, and a potentially toxic antibiotic exposure. If these alterations in host physiology are not handled appropriately, the host risks entering a state of reduced fitness. This reduced fitness manifests in the form of recurrent infections, inappropriate wound healing, autoimmune diseases, and chronic inflammatory disorders. While the mechanisms involved in the recognition of microbial pathogens as such and the subsequent activation of the immune system have been extensively studied, the pathways involved in host recovery after an infection remain understudied.
To examine the biological changes that take place during the recovery phase of an acute bacterial infection, we decided to use the nematode Caenorhabditis elegans as a simple model host. Various human bacterial pathogens, including Pseudomonas aeruginosa, Salmonella enterica, Staphylococcus aureus, and Enterococcus faecalis, have been shown to colonize and kill C. elegans using conserved virulence mechanisms [1]–[4]. Moreover, C. elegans responds to infections using an inducible innate immune system that is controlled by several evolutionary conserved signaling cascades including the p38-MAPK (PMK-1), insulin-IGF (DAF-16), GATA (ELT-2), and TGF-B (SMA-6) pathways [5]–[8]. It is therefore likely that investigating C. elegans recovery from bacterial infection would shed light on host responses that reestablish homeostasis post-infection.
In this study, we established a C. elegans-S. enterica pathogenesis system as a model of acute infection by infecting nematodes with S. enterica and subsequently resolving the infection by treatment with the antibiotic Tetracycline. Using this acute infection model, we profiled gene expression changes in the host over the course of the infection and during the recovery phase of the infection. We found that during recovery, certain components of the host innate immune response were dampened, while mechanisms involved in xenobiotic detoxification, redox regulation, and cytoprotection were activated. A large number of the genes altered during recovery corresponded to intestinal genes regulated by ELT-2, which is a conserved GATA transcription factor that plays a key role in the control of intestinal functions in C. elegans. Further studies indicated that the recovery from acute S. enterica infection required ELT-2, indicating that ELT-2 controls not only induction of innate immune response genes but also genes that play a crucial role in the resolution of an infection.
Results
Use of a C. elegans-S. enterica pathogenesis system to model acute infections
Although host responses that limit microbial infection have been extensively studied, the mechanisms involved in the recovery phase of an infection are incompletely characterized at both the cellular and physiological level. We decided to use Caenorhabditis elegans as a simple model host for assessing biological changes during the recovery phase from an acute infection. C. elegans is propagated in the laboratory by feeding them E. coli strain OP50. E. coli is effectively disrupted by the C. elegans pharyngeal grinder and essentially no intact bacterial cells can be found in the intestinal lumen of young, immunocompetent animals. However, pathogenic bacteria such as Salmonella enterica are capable of killing C. elegans by infectious processes that correlate with the accumulation of bacteria in the intestine. As in mammalian hosts, a small inoculum of S. enterica is capable of establishing a persistent infection in C. elegans that does not require constant exposure to bacteria and cannot be prevented by transferring the infected animals to plates containing E. coli [2], [9].
To determine whether a long-lasting, chronic S. enterica infection could be easily reversed by antibiotic treatment to model a short, acute infection we used fer-1(b232ts) animals, which are fertilization defective at the restrictive temperature. This prevents losing track of the initially infected animals in the morass of progeny that would be otherwise generated following an acute infection. We first established that transferring S. enterica-infected animals to plates containing the bacteriostatic antibiotic Tetracycline and seeded with TetR E. coli was sufficient to significantly reduce bacterial burden (Figure S1). Subsequently, we decided to use 50 µg/ml Tetracycline treatment to reduce S. enterica burden to model an acute infection in C. elegans.
We monitored bacterial accumulation over the course of a 120 hour infection in synchronized larval stage 1 (L1) fer-1(b232ts) animals continuously grown on plates seeded with S. enterica-GFP or transferred to Tetracycline-containing plates seeded with TetR E. coli. Consistent with previous findings indicating that C. elegans larvae are highly resistant to pathogen-mediated killing and that death does not occur during the first several days of an S. enterica infection [9], [10], we observed that only 4.1% of the animals exposed to S. enterica-GFP starting at the L1 stage were colonized 72 hours later (Figure 1A). In contrast, at 96 and 120 hours post-exposure, 41.9% and 71.4% of the animals were colonized by S. enterica-GFP (Figure 1B–C). We found that transferring animals from S. enterica at 72 or 96 hours to Tetracycline-containing plates for 24 hours reduced bacterial burden (Figure 1B–C). Quantification of the number of live bacteria in animals that were infected with S. enterica-GFP for 72 hours and treated with Tetracycline for 24 hours showed a significant reduction of bacterial burden (Figure 1D), confirming that Tetracycline treatment can prevent S. enterica from persistently colonizing the C. elegans intestine and causing a chronic infection.
Fig. 1. Tetracycline treatment models S. enterica acute infection in C. elegans. 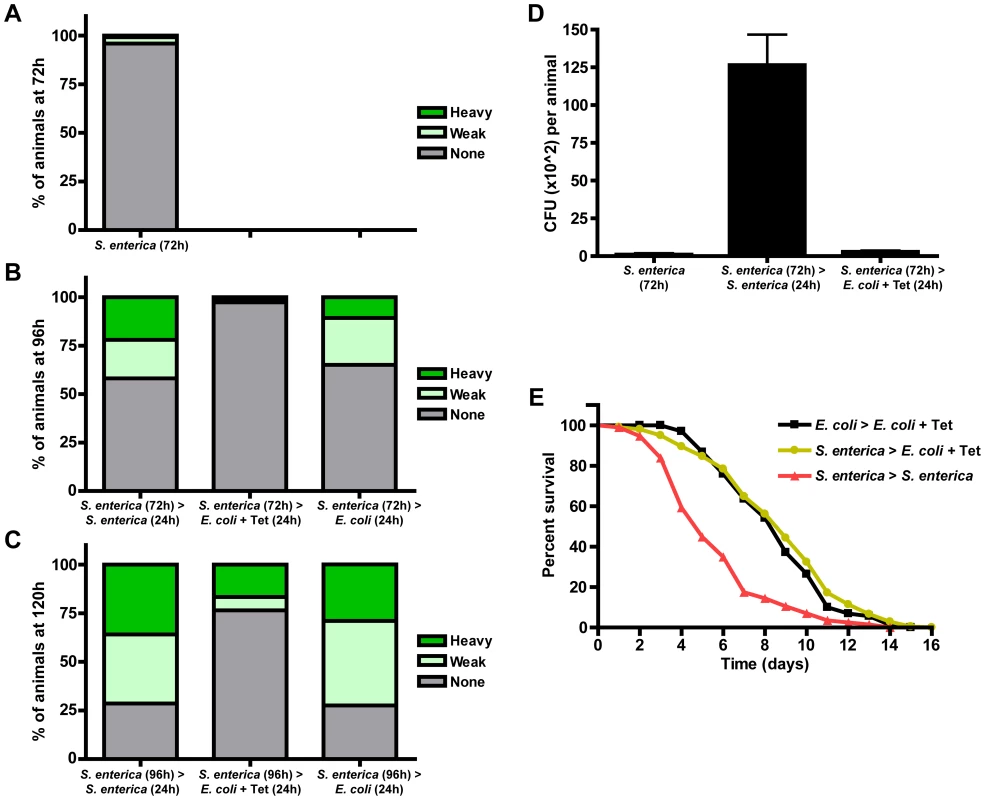
(A) fer-1(b232ts) L1 animals were exposed to S. enterica—GFP for 72 hours. (B) fer-1(b232ts) L1 animals were exposed to S. enterica—GFP for 72 hours and transferred to the indicated plates for 24 hours. (C) fer-1(b232ts) L1 animals were exposed to S. enterica—GFP for 96 hours and transferred to the indicated plates for 24 hours. At each time point, overall GFP intensity in the intestinal lumen was determined using an MZFLIII Leica stereomicroscope. Three levels of colonization were determined as heavy, weak, or none as described in Materials and Methods. N = 104–150 animals per condition. The graph represents the combined results of 2 independent experiments. (D) Quantification of colony forming units of fer-1(b232ts) L1 animals exposed to S. enterica—GFP for 72 hours, S. enterica—GFP for 96 hours, or S. enterica—GFP for 72 hours and then treated with Tetracycline for 24 hours. N = 10 animals per condition. The graph represents the combined results of 4 independent experiments. (E) fer-1(b232ts) L1 animals were exposed to E. coli or S. enterica—GFP for 72 hours and then transferred to E. coli plus Tetracycline or S. enterica—GFP. Animals were scored for survival 72 hour post initial exposure to S. enterica. N = 60 animals per condition. The graph represents the combined results of 5 independent experiments. Even though our results indicate that Tetracycline can prevent S. enterica from causing a persistent colonization of the C. elegans intestine, it was unclear whether acute pathogenic challenge would damage the animal and translate into an associated reduction in survival. As shown in Figure 1E, we found that the survival of animals infected with S. enterica and then treated with Tetracycline is significantly higher than that of animals continuously infected (Figure 1E; yellow vs. red lines). Also, survival of infected and then Tetracycline-treated animals is nearly equivalent to animals that were never infected (Figure 1E, yellow vs. black lines). Treatment with Tetracycline in the presence of killed bacteria only increased C. elegans mean lifespan from 14.2 to 14.9 days (Figure S2). Taken together, these studies show that an S. enterica infection can be resolved by treating the animals with Tetracycline and indicate that this type of treatment can be used to model an acute S. enterica infection that progresses towards chronicity if the animals were to remain untreated.
Recovery from an acute S. enterica infection results in the down-regulation of immune responses and up-regulation of cellular homeostatic mechanisms
To investigate cellular mechanisms potentially involved in recovery after an infection, we utilized Agilent C. elegans gene expression microarrays to identify changes in gene expression during infection and changes that take place after the infection is reversed by treatment with Tetracycline (Figure 2A, Tables S1 and S2). Initially, we focused our analysis on animals that were infected with S. enterica for 96 hours vs. animals that were infected for 72 hours and treated with Tetracycline for 24 hours to resolve the infection. At 96 hours, 99% of the animals were alive in both conditions (Figure 1E, Day 1). Overall, 243 genes, or approximately 1% of the C. elegans genome, were altered more than 2-fold (p<0.05) when comparing the 96-hour cohorts. Of these altered genes, 126 were down-regulated and 117 were up-regulated (Table S2).
Fig. 2. Whole genome expression analysis reveals down-regulated immune response genes and up-regulated detoxification genes during resolution of acute S. enterica infection. 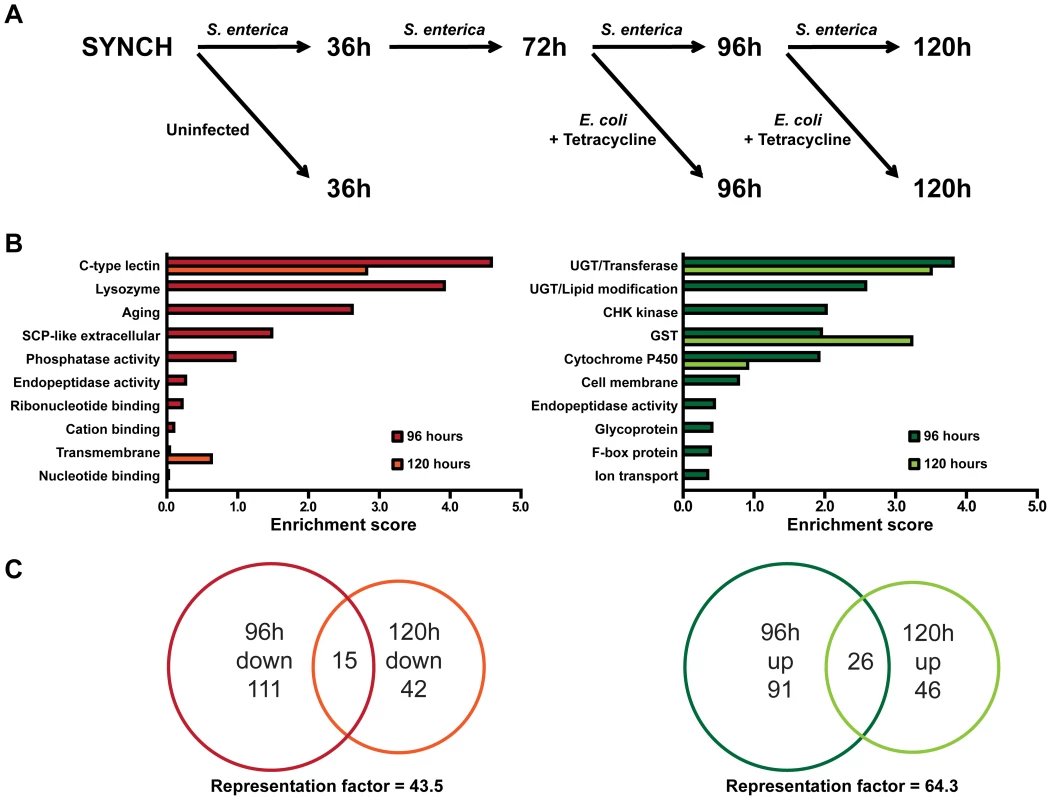
(A) Flowchart of animal cohorts collected for the microarray analysis. (B) Gene ontology analysis of genes regulated during recovery at the 96 and 120 hour time points using the DAVID Bioinformatics Database. Enrichment scores of the 96-hour and 120-hour down-regulated clusters are shown in the left panel. Enrichment scores of the 96-hour and 120-hour up-regulated clusters are shown in the right panel. (C) Venn diagrams showing the overlap of the 96-hour and 120-hour down-regulated genes, left, and overlap of the 96-hour and 120-hour up-regulated genes, right. Representation factors are 43.5 and 64.3, respectively. To identify related gene groups that are transcriptionally controlled by pathways potentially involved in the changes that take place after infection, we performed an unbiased gene enrichment analysis using the database for annotation, visualization and integrated discovery (DAVID, http://david.abcc.ncifcrf.gov/) [11]. The 10 gene ontology (GO) clusters with the highest DAVID enrichment score are shown in Figure 2B and Table S3. For the subset of down-regulated genes that respond to the resolution of the infection, the 2 top-scoring GO clusters, c-type lectins and lysozyme groupings, have previously been described as part of an inducible C. elegans immune response to a variety of pathogens [12]–[15]. For the subset of up-regulated genes that respond to the resolution of the infection, 4 of the top 10 highest scoring ontology clusters are associated with xenobiotic detoxification, redox regulation, or cytoprotection [16], [17]. These results indicate that the activation of the innate immune system of C. elegans by S. enterica infection is attenuated once the infection is resolved and that certain cellular homeostatic pathways are activated during recovery.
Since only 42% of the animals exposed to S. enterica for 96 hours exhibited visible bacterial colonization (Figure 1B), we decided to examine gene expression profiles of animals that were infected for 120 hours, which exhibited an even higher degree of bacterial colonization (Figure 1C). A comparison of gene expression profiles from animals that were infected with S. enterica for 120 hours vs. animals that were infected for 96 hours and treated with Tetracycline for 24 hours identified 57 and 72 genes that are down - or up-regulated greater than 2-fold (p<0.05), respectively (Table S2). Analysis of GO terms in these gene sets via DAVID gives a shorter but similar list of enriched gene clusters (Figure 2B and Table S3), confirming that, as the infection resolves, marker genes of immune activation are down-regulated while genes that correspond to cellular homeostatic pathways are up-regulated. Moreover, the significant overlap between 96 and 120 hour treatment gene sets indicates that the changes that take place after an infection is resolved are reproducible and that similar transcriptional profiles are elicited at different times (Figure 2C and Table S4). The smaller number of genes down - and up-regulated by the resolution of the infection at 120 hours compared to 96 hours could be a consequence of the higher heterogeneity of S. enterica colonization in the 120-hour population (comparison of Figures 1B and 1C). It is also possible that as the infection progresses, the animals suffer irreversible damage that makes them less responsive to antibiotic treatment.
To validate the results of the microarrays, we performed quantitative real-time PCR (qRT-PCR) on a subset of the 243 genes that change upon Tetracycline treatment of infected animals. This subset includes 11 up-regulated genes and 6 down-regulated genes that were either present in a high scoring GO cluster, were highly misregulated, or both. We performed qRT-PCR on RNA harvested from C. elegans that were subjected to the same conditions as in the microarray studies. As shown in Figure 3A and 3B, the changes in gene expression as assessed by qRT-PCR were comparable to those observed by microarray profiling. Further analysis indicated that 16 of the 17 genes had statistically significant expression changes during Tetracycline-mediated recovery from S. enterica infection (Figure 3A and 3B). Thus, the microarray data accurately reflects the majority of gene expression differences between treated and non-treated animals.
Fig. 3. Gene expression changes in infected animals treated with Tetracycline. 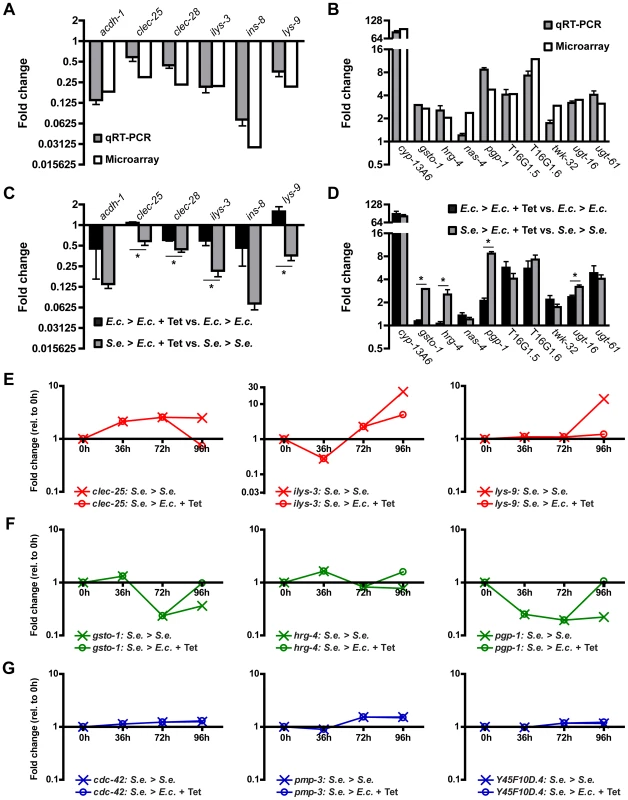
(A–B) Transcript levels of 6 selected down-regulated genes (A) and 10 selected up-regulated genes (B) from L1 animals grown on S. enterica for 72 hours and then treated with Tetracycline for 24 hours relative to L1 animals grown on S. enterica for 96 hours. Out of the 17 studied genes, only the 16 validated genes are shown. Gray bars represent fold change as determined using qRT-PCR. White bars represent fold change as determined using microarrays. (C–D) Transcript levels of 6 selected down-regulated genes (C) and 10 selected up-regulated genes (D) as determined using qRT-PCR. Black bars represent gene expression changes in L1 animals grown on E. coli for 72 hours and then treated with Tetracycline for 24 hours relative to L1 animals grown on E. coli for 96 hours. Gray bars represent gene expression changes in L1 animals grown on S. enterica for 72 hours and then treated with Tetracycline for 24 hours relative to animals grown on S. enterica for 96 hours. qRT-PCR studies were performed in triplicate. SEM is shown. Statistical significance is indicated (p<0.05: *). (E–G) Transcript levels of 3 selected down-regulated genes (E), 3 selected up-regulated genes (F), and 3 selected housekeeping genes (F) over the infection time course. The expression values of animals grown on S. enterica for 72 hours and then treated with Tetracycline for 24 hours are denoted with open circles. The expression values of animals grown on S. enterica for 96 hours are denoted with an X. Resolution of S. enterica infection by treatment with Tetracycline results in the down-regulation of genes that are markers of innate immunity and the up-regulation of genes that function in xenobiotic detoxification, redox regulation, and cytoprotection (Figure 2B). While the resolution of the infection may be responsible for altering the expression of these genes, it is also possible that Tetracycline is directly inducing these changes. To distinguish between these two possibilities, we compared changes in gene expression due to Tetracycline alone vs. changes in gene expression due to recovery from infection by treatment with Tetracycline. We found that expression of 8 out of 16 tested genes were significantly different (Figure 3C–D), highlighting the role of these 8 recovery genes in pathways that are altered during the resolution of the S. enterica infection. Considering that the genome wide microarray shows that 243 genes change their expression upon recovery at 96 hours, we estimate that approximately 122 genes are regulated by recovery from infection independently of Tetracycline while the remaining genes are regulated by the inclusion of Tetracycline alone. This suggests that Tetracycline may be directly inducing gene expression changes in the host that may help clear an infection independently of its antimicrobial activity.
To confirm the finding that a subset of genes is altered upon resolution of an S. enterica infection independent of Tetracycline, we performed equivalent experiments using the antibiotic Kanamycin. These studies indicate that Kanamycin alone did not alter the expression of 9 tested genes in uninfected animals (Figure S3A–B). Furthermore, 8 of the 9 alterations in gene expression seen in infected animals treated with Kanamycin are similar to those seen in infected animals treated with Tetracycline (Figure S3C–D).
To provide further insight into the behavior of genes altered during antibiotic-mediated recovery, we examined gene expression profiles over the course of the 96-hour infection (Table S1). We focused our analysis on qRT-PCR-confirmed genes that are known markers of immune activation and genes that correspond to cellular homeostatic pathways. The expression of innate immunity genes diminished significantly after the infection was resolved by Tetracycline treatment (Figure 3C and E). In contrast, genes involved in regulating cellular homeostasis were significantly up-regulated upon recovery from infection (Figure 3D and F). As a control, the expression of 3 select housekeeping genes remained relatively constant both during the course of the infection and during recovery (Figure 3G). Overall, these studies suggest that as the infection resolves, cellular homeostatic mechanisms are activated while elements of the immune response are attenuated.
The GATA transcription factor ELT-2 is required for the resolution of the S. enterica infection
Several of the GO clusters identified in the set of genes up-regulated during resolution of the infection correspond to genes whose products are involved in detoxification. We therefore hypothesized that the reduction of the pathogenic insult during the recovery phase of an infection may trigger processes involved in detoxification and clearance of immune effectors that, while necessary to combat pathogens, can have deleterious effects on the host. Recently, it was demonstrated that reactive oxygen species (ROS), a component of the C. elegans immune response to S. enterica and other pathogens [18]–[20], contributes to infectious pathogenicity (i.e., damage to the host). Thus, we decided to study gsto-1, which is an up-regulated gene that encodes an omega-class glutathione S-transferase that protects C. elegans from oxidative stress under non-infected conditions [21]. We found that survival of gsto-1(RNAi) animals infected with S. enterica and treated with Tetracycline was not significantly different from that of control animals (Figure S4).
The lack of a significant effect by gsto-1 RNAi could be attributed to incomplete RNAi or to functional redundancy among the multitude of detoxification genes that are up-regulated during recovery (Figure 2B and Table S3). The gsto-1 locus is transcriptionally regulated by the GATA transcription factor ELT-2 [21], leading us to consider a role for ELT-2 in controlling the expression of a set of genes required for resolution of an infection. Consequently, we applied several in silico approaches to determine whether ELT-2-regulated genes are present in the genes whose expression changes by the resolution of an infection. We compared the set of genes altered during recovery to previously identified ELT-2-regulated gene sets and to other control data sets. The ELT-2-regulated gene sets were among the 10 data sets with the strongest overlap with our recovery gene set (Figure 4A and Table S5). As ELT-2 regulates the expression of genes in the C. elegans intestine via trans-acting activity at TGATAA (extended GATA) cis-regulatory motifs [22], [23], we looked for the presence of TGATAA binding sites in the putative promoter regions of the down - and up - regulated genes. Approximately 63% of the 243 genes regulated by recovery contain at least 1 TGATAA site within the 1.5 kb sequence upstream of their transcriptional start site (Figure 4B). By comparison, only 54% of genes in 3 randomly selected gene sets (n = 243 each) have at least 1 TGATAA sequence in the equivalent 1.5 kb region (Figure 4B). Additionally, we observed that at least 1 TGATAA site is present in the putative promoter region of 7 of the 8 recovery genes verified by qRT-PCR (Table S6). While gsto-1 does not contain a TGATAA site in this 1.5 kb region, it does have a single site 3.8 kb upstream of the transcriptional start site. Moreover, it has been experimentally demonstrated that ELT-2 regulates the transcription of gsto-1 [21].
Fig. 4. ELT-2 regulates the expression of specific genes during recovery. 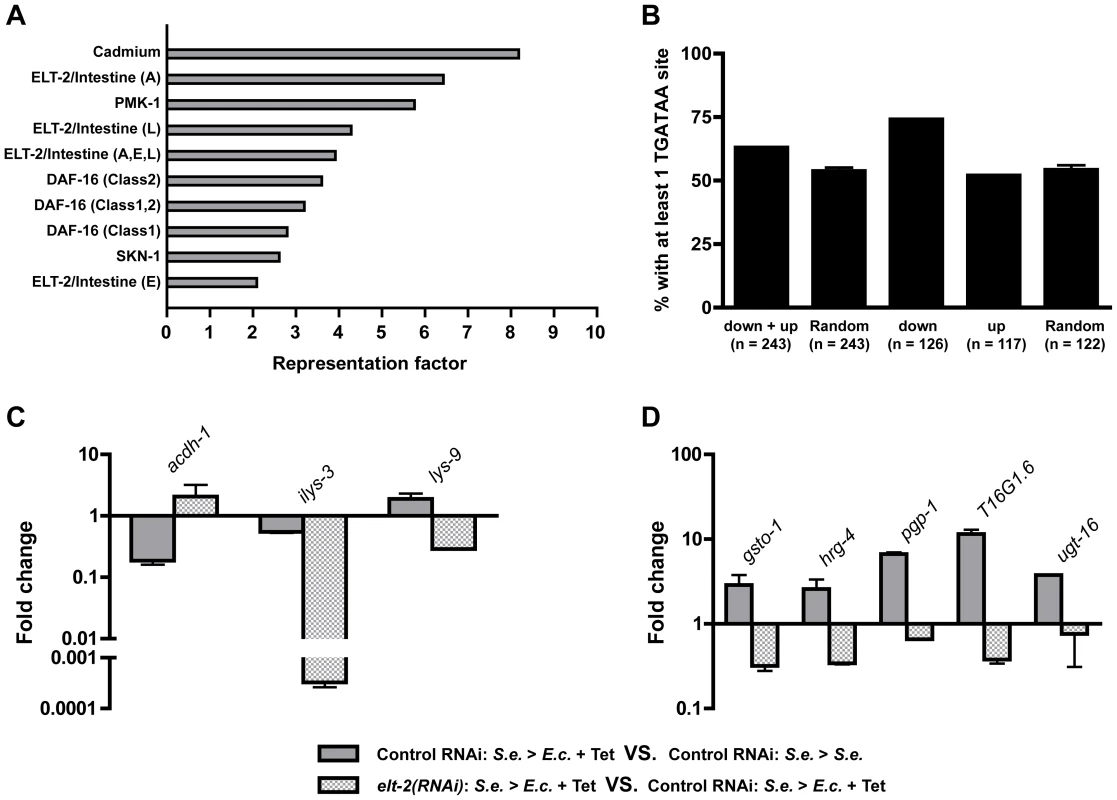
(A) Representation factors of previously studied data sets that overlap with genes altered during recovery at the 96-hour time point. (B) Percentage of down- and up-regulated recovery genes with at least one TGATAA site in the 1.5 kb sequence upstream of the transcriptional start site. Randomly selected gene sets are included as controls. (C–D) Transcript levels of 3 selected down-regulated genes (C) and 5 selected up-regulated genes (D) as determined using qRT-PCR. Gray bars represent gene expression changes in control fer-1(b232ts) young adult animals exposed to S. enterica—GFP for 36 hours and then treated with Tetracycline for 24 hours relative to control fer-1(b232ts) young adult animals grown on S. enterica—GFP for 60 hours. Checkered gray bars represent gene expression changes in fer-1(b232ts) elt-2(RNAi) young adult animals exposed to S. enterica for 36 hours and then treated with Tetracycline for 24 hours relative to control fer-1(b232ts) young adult animals exposed to S. enterica—GFP for 36 hours and then treated with Tetracycline for 24 hours. qRT-PCR studies were performed in duplicate. SEM is shown. Consistent with the post-developmental role of ELT-2 in the regulation of adult intestinal functions [5], [6], [24]–[26], another in silico approach showed that 17 out of 32 (53%) recovery genes with at least 1 TGATAA site and for which the data is available are expressed in the intestine (Table S7). Only 1 out of these 32 genes is expressed in the hypodermis where other GATA transcription factors function [27]. This analysis also showed that 4 out of 8 recovery genes verified by qRT-PCR are expressed in the intestine (Table S7). Taken together, our in silico analyses leads to the hypothesis that ELT-2 controls the expression of a subset of genes during the recovery phase of an infection.
To further substantiate a role for ELT-2 in the transcriptional regulation of genes during recovery, we studied the effect of elt-2 RNAi on the expression of recovery genes. As ELT-2 is essential for C. elegans larval development [22], RNAi was performed on late larval stage 4 (L4) animals. This approach has been used successfully to inhibit elt-2 expression for at least 6 days [28], [29]. As shown in Figure 4D, RNAi of elt-2 inhibited the expression of the 5 studied genes that are up-regulated during recovery from the infection by treatment with either Tetracycline (Figure 3D) or Kanamycin (Figure S3B). Inhibition of elt-2 by RNAi also further down-regulated the expression of ilys-3 and lys-9 (Figure 4C). However, RNAi of elt-2 does not result in the unselective down-regulation of recovery genes as acdh-1 is not down-regulated (Figure 4C). In addition, certain ELT-2-controlled immunity and structural genes [12], [28] are not significantly altered during recovery from S. enterica infection (Figure S5A–B). We further confirmed by qRT-PCR that transcript levels of clec-67, which is a known marker of immunity controlled by ELT-2 [12], are not altered upon recovery (Figure S5C). We conclude that expression of a specific intestinal gene program during resolution of an infection is dependent upon the action of the GATA transcription factor ELT-2.
To test whether ELT-2 is required for recovery after infection, we studied the survival of elt-2(RNAi) animals infected with S. enterica and treated with Tetracycline. RNAi inhibition of elt-2 starting at the L4 stage did not alter the survival of animals growing on live E. coli (Figure 5A; black lines), nor did it alter survival in the presence of Tetracycline (Figure S6). This data indicates that L4 elt-2(RNAi) animals are not sick merely due to disruptions in basal immunity or intestinal function. However, RNAi of elt-2 prevented the recovery of infected animals by treatment with Tetracycline (Figure 5B; yellow lines), highlighting the role of ELT-2 during the recovery phase of the infection. In agreement with previously published reports that ELT-2 regulates innate immunity [12], [28], RNAi of elt-2 did significantly reduce survival of animals continuously infected with S. enterica (Figure 5A; red lines). To address whether genes crucial for immunity are generally required for recovery, we studied pmk-1, which encodes a p38 mitogen-activated protein kinase that is a major regulator of innate immunity in C. elegans [14], [30], [31]. Even though RNAi of pmk-1 elicited sensitivity to S. enterica-mediated killing (Figure 5C; red lines), it did not prevent the recovery of infected animals by treatment with Tetracycline (Figure 5D). Taken together, these results indicate that ELT-2 is required for both early immune responses against pathogens and responses that are activated upon recovery from an infection by S. enterica.
Fig. 5. elt-2(RNAi) animals are unable to resolve an infection. 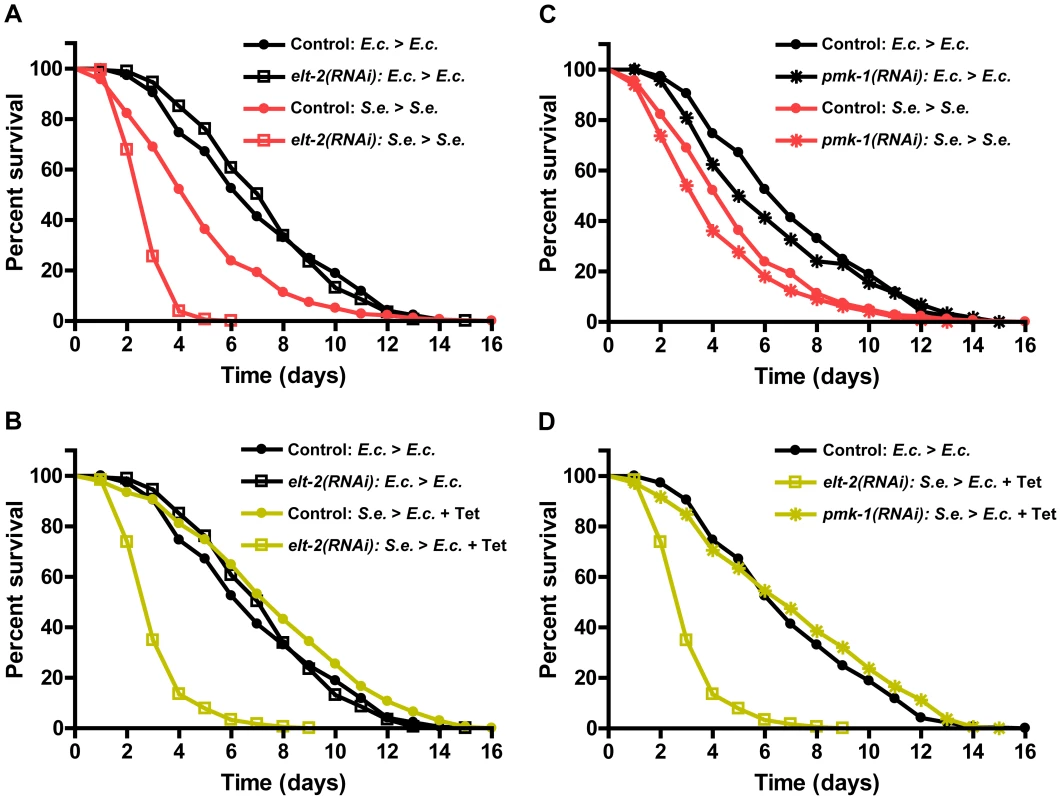
(A) Control fer-1(b232ts) or fer-1(b232ts) elt-2(RNAi) young adult animals were exposed to E. coli or S. enterica—GFP for 36 hours and then transferred to E. coli or S. enterica—GFP and scored for survival. (B) Control fer-1(b232ts) or fer-1(b232ts) elt-2(RNAi) animals were exposed to E. coli or S. enterica—GFP for 36 hours and then transferred to E. coli or E. coli plus Tetracycline and scored for survival. (C) Control fer-1(b232ts) or fer-1(b232ts) pmk-1(RNAi) young adult animals were exposed to E. coli or S. enterica—GFP for 36 hours and then transferred to E. coli or S. enterica—GFP and scored for survival. (D) Control fer-1(b232ts) or fer-1(b232ts) pmk-1(RNAi) animals were exposed to E. coli or S. enterica—GFP for 36 hours and then transferred to E. coli or E. coli plus Tetracycline and scored for survival. N = 60 animals per condition. The graphs represent the combined results of 3 independent experiments. Discussion
Using gene expression profiling, in silico analysis, and reverse genetic approaches, we have defined a novel post-developmental role for the GATA transcription factor ELT-2 during the resolution of an infection. ELT-2 was originally identified as a key regulator of C. elegans intestinal specification during development [22]. However, it is now becoming clear that ELT-2 has an extensive post-developmental role in the regulation of a plethora of adult intestinal functions. Under the control of ELT-2, the 20 cells of the adult intestine in C. elegans function in nutrient uptake, synthesis and storage of macromolecules, epithelial immunity, and host-microbial communication [5], [6], [24]–[26]. There are several additional GATA transcription factors encoded in the C. elegans genome, including ELT-4 and ELT-7, which regulate intestinal gene expression programs [32]. Further studies will be required to determine their possible contribution to the recovery process.
Owing to the multi-functional nature of the intestine and due to the fact that ELT-2 regulates nearly all intestinal genes [33], it is not surprising that half of the genes in the C. elegans genome have putative ELT-2 binding sites (Figure 4B). Thus, it is logical to conclude that the specification of different functional outputs that takes place in the C. elegans intestine during the complete course of an infection is controlled by additional co-factors that act together with ELT-2. Recent work has demonstrated that GATA transcription factors, including ELT-2, act coordinately with the insulin-IGF pathway transcriptional regulator DAF-16 in a cell-autonomous manner to regulate lifespan extension in C. elegans [34]. It is therefore plausible that DAF-16 acts with ELT-2 to co-regulate genes important for infection resolution. Indeed, we observed a significant enrichment of both ELT-2 - and DAF-16-controlled targets in our set of genes altered during recovery from infection (Figure 4A and Table S5). An emerging theme is that coordinated transcriptional activity of DAF-16 and ELT-2 would be necessary for the modulation of cytoprotective pathways that, in turn, are required for a majority of cellular stress response pathways [16].
Another candidate factor that may regulate damage response genes in conjunction with ELT-2 and/or DAF-16 is the Nrf1/SKN-1 transcription factor. Previous work has demonstrated that signaling cascades downstream of reactive oxygen species (ROS) induce a cytoprotective SKN-1 pathway [35], [36]. SKN-1 might regulate cytoprotective genes downstream of or in parallel to ELT-2 and/or DAF-16 to mediate resolution of an infection. Indeed, SKN-1-positively regulated targets are significantly enriched in the set of genes that are up-regulated during infection resolution (Table S5).
Mounting evidence indicates that ELT-2 activity is modulated under a variety of environmental conditions or physiological states. ELT-2-mediated immunity to a variety of pathogens is activated by currently unknown mechanisms. Interestingly, a paper by Lee and colleagues demonstrates that the intestinal pathogen B. pseudomallei can actively target and degrade ELT-2 to prevent host immune responses [29]. We failed to observe any alterations in ELT-2 protein localization or abundance caused by S. enterica infection or recovery. These pathogens, which kill C. elegans at a distinctly different rate, must utilize different mechanisms to overcome the host immune system.
Signals during the initial decline in infection may function to reprogram the transcriptional activity of ELT-2 from an innate immune program to a cytoprotective one. These unidentified signals may be bacterial - and/or host-derived. Specific bacterial-derived signals, such as those involved in biofilm formation or quorum sensing, may be the primary trigger for the ELT-2 switch [26]. These bacterial-derived signals might act directly on ELT-2 or they may transit through host-encoded genes. Alternatively, host-encoded regulators that normally function during development, such as the END-1/END-3 specification factors, might be re-activated during the resolution of infection to direct the transcription of detoxification genes by ELT-2. Interestingly, the END-1/END-3 system lies downstream of the oxidative stress (ROS) response protein SKN-1 in the development of the C. elegans intestine [24]. Alternatively, changes in ELT-2 activity may be controlled by local chromatin remodeling in a manner similar to the regulation of DAF-16 transcriptional activity [37].
In summary, our results identified a new, key role for ELT-2 during recovery from a bacterial infection. We revealed that during recovery from an infection, genes that are markers of innate immunity are down-regulated, while the expression of genes involved in xenobiotic detoxification, redox regulation, and cytoprotection is enhanced. Interestingly, a number of genes encoding antibacterial factors (ABFs) are up-regulated during the course of the S. enterica infection (Table S1). However, the expression of abf genes is not down-regulated once the infection is resolved. This could be due to a mechanism used by C. elegans to maintain high levels of abf genes throughout reproductive adulthood. It is also possible that ABFs have a high specificity for damaging prokaryotic cells, having little or no impact on host cells. Thus, there would be no immediate need to reverse their up-regulation once the infection is resolved, unlike the case of lysozyme-encoding genes, which could potentially damage host cells. The ELT-2 interaction with the aforementioned co-factors may dictate the specificity of the expression profile during the different phases of an infection. A number of microbial killing pathways and cellular homeostatic pathways are controlled by the nervous system in infected C. elegans [38]–[46]. An important question that remains to be evaluated is whether the nervous system also plays a role in the control of the mechanisms involved in recovery after infections have been cleared.
Materials and Methods
Nematode and bacterial strains
C. elegans strain HH142 fer-1(b232ts) was provided by the Caenorhabditis Genetics Center. C. elegans were maintained at 15°C on NGM—OP50 plates without antibiotics. The following bacterial strains were used for experiments: Escherichia coli strain OP50-1 [SmR] [47], E. coli—dsRed strain OP50 [AmpR, CbR] [47], E. coli strain HT115 [TetR] [48], E. coli strain HT115 pL4440 [AmpR, TetR] [48], E. coli strain DH5α pSMC21 [KanR] [49], Salmonella enterica enterica serovar Typhimurium strain 1344 [SmR] [50], S. enterica—GFP strain SM022 [SmR, KanR] [51]. Bacteria were grown overnight for 14 hours in 3 ml LB broth at 37°C.
Visualization of bacterial accumulation in the nematode intestine
fer-1(b232ts) animals were synchronized by treating gravid adults with sodium hydroxide and bleach. About 2,000 synchronized L1 animals were grown on full lawn S. enterica—GFP plates at 25°C for 36, 72, 96, or 120 hours. At designated transfer time points, animals were rinsed off S. enterica—GFP plates, washed with M9 (4 changes ×15 minutes), concentrated, and then transferred to plates with or without 50 µg/ml Tetracycline that were seeded with E. coli HT115 or S. enterica-GFP. At designated visualization time points, animals were picked to an NGM—OP50 plate for 1 hour before being picked to a new NGM—OP50 plate. Animals were then visualized at 20× using a Leica MZ FLIII fluorescence stereomicroscope. In heavily colonized animals (heavy) GFP fluorescence was visible in the presence of halogen white light set at 60%, while in weakly infected animals (weak) GFP fluorescence was only visible in the absence of white light. Animals where GFP fluorescence was not detected even in the absence of white light were scored as not infected (none).
Quantification of intestinal bacterial loads
For the quantification of colony forming units (CFUs), fer-1(b232ts) animals were synchronized by treating gravid adults with sodium hydroxide and bleach. About 2,000 synchronized L1 animals were grown on full lawn S. enterica—GFP plates at 25°C for 72 hours. At the designated transfer time points, animals were rinsed off S. enterica—GFP plates, washed with M9 (4 changes ×15 minutes), concentrated, and then transferred to S. enterica-GFP or E. coli plus 50 µg/ml Tetracycline plates. At designated CFU time points, animals were picked to 3 NGM—OP50 plates (20 minutes each) before being picked to a 1.5 ml eppendorf tube with 50 µl of PBS plus 0.1% Triton-X-100. A total of 10 animals per condition were mechanically disrupted using a mini-pestle. Serial dilutions of the lysates were spread onto LB/Kanamycin (50 µg/ml) plates to select for S. enterica—GFP cells and grown for 24 hours at 37°C.
Survival assays
Bacteria – E. coli HT115 or S. enterica were grown overnight for 14 hours in 3 ml LB broth at 37°C. A total of 50 µl (scoring) or 500 µl (exposure) of the resulting cultures were spread onto modified (0.35% peptone) NGM plates with or without 50 µg/ml Tetracycline and allowed to grow for 1–2 days at 25°C to produce a thick lawn. fer-1(b232ts) animals were synchronized by treating gravid adults with sodium hydroxide and bleach. Synchronized L1 animals were grown on full lawn S. enterica—GFP plates at 25°C for 72 hours before being transferred to the appropriate (treatment or not) plates. The assays were performed at 25°C. Animals were scored every day and were considered dead when they failed to respond to touch. Animals were transferred to fresh plates every other day for the entire length of the experiment. Survival was plotted using Kaplan-Meier survival curves and analyzed by the logrank test using GraphPad Prism (GraphPad Software, Inc., San Diego, CA). Survival curves resulting in p values of <0.05 were considered significantly different. A total of 60 animals per condition per experiment were used.
Longevity assays
E. coli was grown as described above. A 50 - µl drop of the bacteria was plated on a 3.5-cm plate of modified NGM agar containing 40 µg/ml fluoro-deoxyuridine with or without 50 µg/ml Tetracycline. A total of 100 animals per condition per experiment were used. The assays were performed at 25°C. Survival curves were analyzed as described above.
RNAi-coupled survival assays
gsto-1(RNAi). E. coli HT115(DE3) bacterial strains expressing double-stranded RNA [48] were grown for 9 hours in 5 ml LB broth containing Ampicillin (50 µg/ml) at 37°C. The resulting cultures were seeded onto NGM plates containing Carbenicillin (50 µg/ml) and isopropyl-1-thio-β-D-galactopyranoside (3 mM). dsRNA-expressing bacteria were allowed to grow for 2 days at 25°C to produce a thick lawn. fer-1(b232ts) L4 animals were placed on RNAi or vector control plates for 5 days at 15°C until F1 animals developed. fer-1(b232ts) F1 L4 animals were placed on a second RNAi or vector control plate and incubated for another 5 days at 15°C until adult F2 animals developed. Gravid F2 RNAi animals were picked to full lawn E. coli or S. enterica—GFP plates and allowed to lay eggs for 3 hours at 25°C to synchronize a third generation population. These third generation animals were kept on E. coli or S. enterica—GFP plates for 72 hours before being transferred to plates with or without 50 µg/ml Tetracycline that were seeded with E. coli or S. enterica-GFP. unc-22(RNAi) was used as positive control in all experiments to account for RNAi efficiency. The gsto-1 (mv_C29E4.7) RNAi vector was verified by DNA sequencing. A total of 60 animals per condition per experiment were scored for survival.
elt-2(RNAi) and pmk-1(RNAi). Production of RNAi plates was the same as described above. Gravid fer-1(b232ts) animals were allowed to lay eggs for 3 hours at 25°C on NGM-HT115 plates. Gravid animals were removed and the eggs/plates were incubated for 36 hours at 25°C. Synchronized L4 animals were then transferred to RNAi or vector control plates and incubated for an additional 36 hours at 25°C. Young adult RNAi or vector control animals were then transferred to and grown on full lawn E. coli or S. enterica—GFP plates for 36 hours at 25°C. Adult worms were then transferred to plates with or without 50 µg/ml Tetracycline that were seeded with E. coli or S. enterica-GFP. unc-22(RNAi) was used as positive control in all experiments to account for RNAi efficiency. The elt-2 (mv_AAC36130) and pmk-1 (sjj_B0218.3) RNAi vectors were verified by DNA sequencing. A total of 60 animals per condition per experiment were scored for survival.
RNA isolation for qRT-PCR
fer-1(b232ts). Animals were synchronized by treating gravid adults with sodium hydroxide and bleach. Synchronized L1 animals were grown on full lawn E. coli or S. enterica—GFP plates for 72 hours. At 72 hours, animals were rinsed off E. coli or S. enterica—GFP plates, washed with M9 (4 changes ×15 minutes), concentrated, and then transferred to E. coli, E. coli plus 50 µg/ml Tetracycline, E. coli plus 50 µg/ml Kanamycin, or S. enterica—GFP plates. At 24 hours post-transfer, animals were rinsed off plates, washed with M9 (4 changes ×15 minutes), and flash-frozen in Trizol (Life Technologies, Carlsbad, CA). Total RNA was extracted using the RNeasy Plus Universal Kit (Qiagen, Netherlands).
elt-2(RNAi); fer-1(b232ts). Animals were synchronized by treating gravid adults with sodium hydroxide and bleach. Synchronized L1 animals were grown on full lawn E. coli plates for 36 hours. At 36 hours, animals were rinsed off E. coli, washed with M9 (4 changes ×15 minutes), concentrated, and then placed on RNAi or vector control plates for 36 hours. At 72 hours, animals were rinsed off these plates washed with M9 (4 changes ×15 minutes), concentrated, and then placed on S. enterica—GFP plates for 36 hours. At 108 hours, animals were rinsed off S. enterica—GFP plates, washed with M9 (4 changes ×15 minutes), concentrated, and then transferred to E. coli, E. coli plus 50 µg/ml Tetracycline, or S. enterica—GFP plates for 24 hours. Animals were rinsed off plates, washed with M9 (4 changes ×15 minutes), and flash-frozen in Trizol (Life Technologies, Carlsbad, CA). Total RNA was extracted using the RNeasy Plus Universal Kit (Qiagen, Netherlands). All studies were performed at 25°C.
Quantitative Real-Time PCR (qRT-PCR)
Total RNA was obtained as described above. A total of 1 µg total RNA was oligo(dT) primed and reverse transcribed in a 50 µl volume using the SuperScript III First-Strand Synthesis System (Life Technologies, Carlsbad, CA). Reactions without the addition of reverse transcriptase (RT) were also performed and served as controls for contaminating genomic DNA in quantitative PCR experiments. Two µl of the resulting plus or minus RT reactions served as templates in quantitative PCR experiments using Power SYBR Green PCR Master Mix (Life Technologies, Carlsbad, CA) and the StepOnePlus Real-Time PCR System (Life Technologies, Carlsbad, CA). For each sample, 3 technical replicates were performed. Pan-actin-normalized Ct values were determined using the StepOnePlus Software (Life Technologies, Carlsbad, CA). Primer sequences are available upon request. When applicable a one or two variable t-test was performed.
RNA isolation for microarray analysis
fer-1(b232ts) animals were synchronized by treating gravid adults with sodium hydroxide and bleach. Synchronized L1 animals were grown on full lawn E. coli OP50 (uninfected) or full lawn S. enterica plates at 25°C for 36, 72, 96, or 120 hours. At designated transfer time points, animals were rinsed off S. enterica plates, washed with M9 (4 changes ×15 minutes), concentrated, and then transferred to S. enterica or E. coli plus 50 µg/ml Tetracycline plates. At designated harvesting time points, animals were rinsed off plates, washed with M9 (4 changes ×15 minutes), and flash-frozen in Trizol (Life Technologies, Carlsbad, CA). Total RNA was extracted using the RNeasy Plus Universal Kit (Qiagen, Netherlands).
Microarray analysis
Total RNA was assessed for quality with an Agilent 2100 Bioanalyzer G2939A (Agilent Technologies, Santa Clara, CA) and a Nanodrop 8000 spectrophotomer (Thermo, Wilmington, DE). 100 ng of total RNA was converted to 1.65 µg Cy-3-labeled, linearly amplified cRNA using the Low Input Quick Amp (LIQA) Labeling One-Color Microarray-Based Gene Expression Analysis Kit (Agilent Technologies, Santa Clara, CA). cRNA was fragmented and added to 44 K feature Agilent C. elegans Gene Expression Microarray V2 slides (Agilent Technologies, Santa Clara, CA). Hybridization was performed in the Agilent rotisserie Hybridization Oven for 17 hours. Arrays were subsequently washed and scanned with the Agilent B scanner according to standard Agilent protocols (Agilent Technologies, Santa Clara, CA). Scanned data was log2 transformed and quantile normalized using Partek Genomics Suite (St. Louis, MO). Analysis of variance (ANOVA) t tests and fold-change calculations were also performed using Partek Genomics Suite (St. Louis, MO). For each of the 5 time points, 2 biological replicates were assessed. The microarray data was deposited in the Gene Expression Omnibus database: GSE54212.
Bioinformatics
Gene lists were culled from the literature and passed through WormBase Converter [52] using the WS220 genome release as the output (references are noted in Table S5). A total of 20,834 WS220 genes are represented by 1 or more probes in the Agilent C. elegans V2 array (Agilent Technologies, Santa Clara, CA). Gene ontology analysis was performed using the DAVID Bioinformatics Database (david.abcc.ncifcrf.gov/). The most significant gene ontology term in each DAVID functional annotation cluster was set as the significance of the overall cluster. Statistical significance of the overlap between two gene sets was calculated using the following on-line program: nemates.org/MA/progs/overlap_stats.html. Representation Factor represents the number of overlapping genes divided by the expected number of overlapping genes drawn from 2 independent groups. A background gene list of 20,834 was used for the calculation. P values were calculated using the hypergeometric probability. 1.5 kb cis-regulatory sequences were identified using WormMart (wormbase.org). Expression patterns were determined using WormMine (wormbase.org). Detailed bioinformatics protocols are available upon request.
Supporting Information
Zdroje
1. Darby C (2005) Interactions with microbial pathogens. WormBook: 1–15.
2. TenorJL, McCormickBA, AusubelFM, AballayA (2004) Caenorhabditis elegans-based screen identifies Salmonella virulence factors required for conserved host-pathogen interactions. Curr Biol 14 : 1018–1024.
3. TanMW, RahmeLG, SternbergJA, TompkinsRG, AusubelFM (1999) Pseudomonas aeruginosa killing of Caenorhabditis elegans used to identify P. aeruginosa virulence factors. Proc Natl Acad Sci U S A 96 : 2408–2413.
4. GarsinDA, SifriCD, MylonakisE, QinX, SinghKV, et al. (2001) A simple model host for identifying Gram-positive virulence factors. Proc Natl Acad Sci U S A 98 : 10892–10897.
5. Pukkila-WorleyR, AusubelFM (2012) Immune defense mechanisms in the Caenorhabditis elegans intestinal epithelium. Curr Opin Immunol 24 : 3–9.
6. PartridgeFA, Gravato-NobreMJ, HodgkinJ (2010) Signal transduction pathways that function in both development and innate immunity. Dev Dyn 239 : 1330–1336.
7. IrazoquiJE, UrbachJM, AusubelFM (2010) Evolution of host innate defence: insights from Caenorhabditis elegans and primitive invertebrates. Nat Rev Immunol 10 : 47–58.
8. MeansTK, AballayA (2011) Models to study ancient host-pathogen interactions: lessons from Crete. EMBO reports 12 : 5–7.
9. AballayA, YorgeyP, AusubelFM (2000) Salmonella typhimurium proliferates and establishes a persistent infection in the intestine of Caenorhabditis elegans. Curr Biol 10 : 1539–1542.
10. LeroyM, MosserT, ManiereX, AlvarezDF, MaticI (2012) Pathogen-induced Caenorhabditis elegans developmental plasticity has a hormetic effect on the resistance to biotic and abiotic stresses. BMC evolutionary biology 12 : 187.
11. HuangDW, ShermanBT, LempickiRA (2009) Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nature protocols 4 : 44–57.
12. KerryS, TeKippeM, GaddisNC, AballayA (2006) GATA transcription factor required for immunity to bacterial and fungal pathogens. PLoS One 1: e77.
13. MalloGV, KurzCL, CouillaultC, PujolN, GranjeaudS, et al. (2002) Inducible antibacterial defense system in C. elegans. Curr Biol 12 : 1209–1214.
14. TroemelER, ChuSW, ReinkeV, LeeSS, AusubelFM, et al. (2006) p38 MAPK regulates expression of immune response genes and contributes to longevity in C. elegans. PLoS Genet 2: e183.
15. O'RourkeD, BabanD, DemidovaM, MottR, HodgkinJ (2006) Genomic clusters, putative pathogen recognition molecules, and antimicrobial genes are induced by infection of C. elegans with M. nematophilum. Genome Res 16 : 1005–1016.
16. ShoreDE, RuvkunG (2013) A cytoprotective perspective on longevity regulation. Trends Cell Biol 23 : 409–420.
17. XuC, LiCY, KongAN (2005) Induction of phase I, II and III drug metabolism/transport by xenobiotics. Archives of pharmacal research 28 : 249–268.
18. ChavezV, Mohri-ShiomiA, GarsinDA (2009) Ce-Duox1/BLI-3 generates reactive oxygen species as a protective innate immune mechanism in Caenorhabditis elegans. Infect Immun 77 : 4983–4989.
19. van der HoevenR, McCallumKC, GarsinDA (2012) Speculations on the activation of ROS generation in C. elegans innate immune signaling. Worm 1 : 160–163.
20. SemX, RhenM (2012) Pathogenicity of Salmonella enterica in Caenorhabditis elegans relies on disseminated oxidative stress in the infected host. PLoS One 7: e45417.
21. BurmeisterC, LuersenK, HeinickA, HusseinA, DomagalskiM, et al. (2008) Oxidative stress in Caenorhabditis elegans: protective effects of the Omega class glutathione transferase (GSTO-1). FASEB journal: official publication of the Federation of American Societies for Experimental Biology 22 : 343–354.
22. FukushigeT, HawkinsMG, McGheeJD (1998) The GATA-factor elt-2 is essential for formation of the Caenorhabditis elegans intestine. Dev Biol 198 : 286–302.
23. McGheeJD, FukushigeT, KrauseMW, MinnemaSE, GoszczynskiB, et al. (2009) ELT-2 is the predominant transcription factor controlling differentiation and function of the C. elegans intestine, from embryo to adult. Dev Biol 327 : 551–565.
24. McGheeJD (2013) The Caenorhabditis elegans intestine. Wiley interdisciplinary reviews Developmental biology 2 : 347–367.
25. WattsJL (2009) Fat synthesis and adiposity regulation in Caenorhabditis elegans. Trends in endocrinology and metabolism: TEM 20 : 58–65.
26. McFall-NgaiM, HadfieldMG, BoschTC, CareyHV, Domazet-LosoT, et al. (2013) Animals in a bacterial world, a new imperative for the life sciences. Proc Natl Acad Sci U S A 110 : 3229–3236.
27. ShaoJ, HeK, WangH, HoWS, RenX, et al. (2013) Collaborative regulation of development but independent control of metabolism by two epidermis-specific transcription factors in Caenorhabditis elegans. The Journal of biological chemistry 288 : 33411–33426.
28. ShapiraM, HamlinBJ, RongJ, ChenK, RonenM, et al. (2006) A conserved role for a GATA transcription factor in regulating epithelial innate immune responses. Proc Natl Acad Sci U S A 103 : 14086–14091.
29. LeeSH, WongRR, ChinCY, LimTY, EngSA, et al. (2013) Burkholderia pseudomallei suppresses Caenorhabditis elegans immunity by specific degradation of a GATA transcription factor. Proc Natl Acad Sci U S A 110 : 15067–15072.
30. KimDH, FeinbaumR, AlloingG, EmersonFE, GarsinDA, et al. (2002) A conserved p38 MAP kinase pathway in Caenorhabditis elegans innate immunity. Science 297 : 623–626.
31. AballayA, DrenkardE, HilbunLR, AusubelFM (2003) Caenorhabditis elegans innate immune response triggered by Salmonella enterica requires intact LPS and is mediated by a MAPK signaling pathway. Curr Biol 13 : 47–52.
32. McGhee JD (2007) The C. elegans intestine. WormBook: the online review of C elegans biology: 1–36.
33. McGheeJD, SleumerMC, BilenkyM, WongK, McKaySJ, et al. (2007) The ELT-2 GATA-factor and the global regulation of transcription in the C. elegans intestine. Dev Biol 302 : 627–645.
34. ZhangP, JudyM, LeeSJ, KenyonC (2013) Direct and indirect gene regulation by a life-extending FOXO protein in C. elegans: roles for GATA factors and lipid gene regulators. Cell Metab 17 : 85–100.
35. Glover-CutterKM, LinS, BlackwellTK (2013) Integration of the unfolded protein and oxidative stress responses through SKN-1/Nrf. PLoS Genet 9: e1003701.
36. HoevenR, McCallumKC, CruzMR, GarsinDA (2011) Ce-Duox1/BLI-3 generated reactive oxygen species trigger protective SKN-1 activity via p38 MAPK signaling during infection in C. elegans. PLoS Pathog 7: e1002453.
37. RiedelCG, DowenRH, LourencoGF, KirienkoNV, HeimbucherT, et al. (2013) DAF-16 employs the chromatin remodeller SWI/SNF to promote stress resistance and longevity. Nat Cell Biol 15 : 491–501.
38. StyerKL, SinghV, MacoskoE, SteeleSE, BargmannCI, et al. (2008) Innate immunity in Caenorhabditis elegans is regulated by neurons expressing NPR-1/GPCR. Science 322 : 460–464.
39. SunJ, SinghV, Kajino-SakamotoR, AballayA (2011) Neuronal GPCR controls innate immunity by regulating noncanonical unfolded protein response genes. Science 332 : 729–732.
40. ReddyKC, AndersenEC, KruglyakL, KimDH (2009) A polymorphism in npr-1 is a behavioral determinant of pathogen susceptibility in C. elegans. Science 323 : 382–384.
41. SunJ, LiuY, AballayA (2012) Organismal regulation of XBP-1-mediated unfolded protein response during development and immune activation. EMBO reports 13 : 855–860.
42. SinghV, AballayA (2012) Endoplasmic reticulum stress pathway required for immune homeostasis is neurally controlled by arrestin-1. The Journal of biological chemistry 287 : 33191–33197.
43. AnyanfulA, EasleyKA, BenianGM, KalmanD (2009) Conditioning protects C. elegans from lethal effects of enteropathogenic E. coli by activating genes that regulate lifespan and innate immunity. Cell Host Microbe 5 : 450–462.
44. KawliT, TanMW (2008) Neuroendocrine signals modulate the innate immunity of Caenorhabditis elegans through insulin signaling. Nat Immunol 9 : 1415–24.
45. EvansEA, KawliT, TanMW (2008) Pseudomonas aeruginosa suppresses host immunity by activating the DAF-2 insulin-like signaling pathway in Caenorhabditis elegans. PLoS Pathog 4: e1000175.
46. ZugastiO, EwbankJJ (2009) Neuroimmune regulation of antimicrobial peptide expression by a noncanonical TGF-beta signaling pathway in Caenorhabditis elegans epidermis. Nature immunology 10 : 249–256.
47. BrennerS (1974) The genetics of Caenorhabditis elegans. Genetics 77 : 71–94.
48. KamathRS, FraserAG, DongY, PoulinG, DurbinR, et al. (2003) Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature 421 : 231–237.
49. BurtonEA, PendergastAM, AballayA (2006) The Caenorhabditis elegans ABL-1 tyrosine kinase is required for Shigella flexneri pathogenesis. Appl Environ Microbiol 72 : 5043–5051.
50. HoisethSK, StockerBA (1981) Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature 291 : 238–239.
51. ValdiviaRH, FalkowS (1996) Bacterial genetics by flow cytometry: rapid isolation of Salmonella typhimurium acid-inducible promoters by differential fluorescence induction. Mol Microbiol 22 : 367–378.
52. EngelmannI, GriffonA, TichitL, Montanana-SanchisF, WangG, et al. (2011) A comprehensive analysis of gene expression changes provoked by bacterial and fungal infection in C. elegans. PLoS One 6: e19055.
Štítky
Genetika Reprodukční medicína
Článek Oligoasthenoteratozoospermia and Infertility in Mice Deficient for miR-34b/c and miR-449 LociČlánek The Kinesin AtPSS1 Promotes Synapsis and is Required for Proper Crossover Distribution in MeiosisČlánek Payoffs, Not Tradeoffs, in the Adaptation of a Virus to Ostensibly Conflicting Selective PressuresČlánek Examination of Prokaryotic Multipartite Genome Evolution through Experimental Genome ReductionČlánek BMP-FGF Signaling Axis Mediates Wnt-Induced Epidermal Stratification in Developing Mammalian SkinČlánek Role of STN1 and DNA Polymerase α in Telomere Stability and Genome-Wide Replication in ArabidopsisČlánek RNA-Processing Protein TDP-43 Regulates FOXO-Dependent Protein Quality Control in Stress ResponseČlánek Integrating Functional Data to Prioritize Causal Variants in Statistical Fine-Mapping StudiesČlánek Salt-Induced Stabilization of EIN3/EIL1 Confers Salinity Tolerance by Deterring ROS Accumulation inČlánek Ethylene-Induced Inhibition of Root Growth Requires Abscisic Acid Function in Rice ( L.) SeedlingsČlánek Metabolic Respiration Induces AMPK- and Ire1p-Dependent Activation of the p38-Type HOG MAPK PathwayČlánek Signature Gene Expression Reveals Novel Clues to the Molecular Mechanisms of Dimorphic Transition inČlánek A Mouse Model Uncovers LKB1 as an UVB-Induced DNA Damage Sensor Mediating CDKN1A (p21) DegradationČlánek Dominant Sequences of Human Major Histocompatibility Complex Conserved Extended Haplotypes from to
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2014 Číslo 10
-
Všechny články tohoto čísla
- An Deletion Is Highly Associated with a Juvenile-Onset Inherited Polyneuropathy in Leonberger and Saint Bernard Dogs
- Licensing of Yeast Centrosome Duplication Requires Phosphoregulation of Sfi1
- Oligoasthenoteratozoospermia and Infertility in Mice Deficient for miR-34b/c and miR-449 Loci
- Basement Membrane and Cell Integrity of Self-Tissues in Maintaining Immunological Tolerance
- The Kinesin AtPSS1 Promotes Synapsis and is Required for Proper Crossover Distribution in Meiosis
- Germline Mutations in Are Associated with Familial Gastric Cancer
- POT1a and Components of CST Engage Telomerase and Regulate Its Activity in
- Controlling Meiotic Recombinational Repair – Specifying the Roles of ZMMs, Sgs1 and Mus81/Mms4 in Crossover Formation
- Payoffs, Not Tradeoffs, in the Adaptation of a Virus to Ostensibly Conflicting Selective Pressures
- FHIT Suppresses Epithelial-Mesenchymal Transition (EMT) and Metastasis in Lung Cancer through Modulation of MicroRNAs
- Genome-Wide Mapping of Yeast RNA Polymerase II Termination
- Examination of Prokaryotic Multipartite Genome Evolution through Experimental Genome Reduction
- White Cells Facilitate Opposite- and Same-Sex Mating of Opaque Cells in
- BMP-FGF Signaling Axis Mediates Wnt-Induced Epidermal Stratification in Developing Mammalian Skin
- Genome-Wide Association Study of CSF Levels of 59 Alzheimer's Disease Candidate Proteins: Significant Associations with Proteins Involved in Amyloid Processing and Inflammation
- COE Loss-of-Function Analysis Reveals a Genetic Program Underlying Maintenance and Regeneration of the Nervous System in Planarians
- Fat-Dachsous Signaling Coordinates Cartilage Differentiation and Polarity during Craniofacial Development
- Identification of Genes Important for Cutaneous Function Revealed by a Large Scale Reverse Genetic Screen in the Mouse
- Sensors at Centrosomes Reveal Determinants of Local Separase Activity
- Genes Integrate and Hedgehog Pathways in the Second Heart Field for Cardiac Septation
- Systematic Dissection of Coding Exons at Single Nucleotide Resolution Supports an Additional Role in Cell-Specific Transcriptional Regulation
- Recovery from an Acute Infection in Requires the GATA Transcription Factor ELT-2
- HIPPO Pathway Members Restrict SOX2 to the Inner Cell Mass Where It Promotes ICM Fates in the Mouse Blastocyst
- Role of and in Development of Abdominal Epithelia Breaks Posterior Prevalence Rule
- The Formation of Endoderm-Derived Taste Sensory Organs Requires a -Dependent Expansion of Embryonic Taste Bud Progenitor Cells
- Role of STN1 and DNA Polymerase α in Telomere Stability and Genome-Wide Replication in Arabidopsis
- Keratin 76 Is Required for Tight Junction Function and Maintenance of the Skin Barrier
- Encodes the Catalytic Subunit of N Alpha-Acetyltransferase that Regulates Development, Metabolism and Adult Lifespan
- Disruption of SUMO-Specific Protease 2 Induces Mitochondria Mediated Neurodegeneration
- Caudal Regulates the Spatiotemporal Dynamics of Pair-Rule Waves in
- It's All in Your Mind: Determining Germ Cell Fate by Neuronal IRE-1 in
- A Conserved Role for Homologs in Protecting Dopaminergic Neurons from Oxidative Stress
- The Master Activator of IncA/C Conjugative Plasmids Stimulates Genomic Islands and Multidrug Resistance Dissemination
- An AGEF-1/Arf GTPase/AP-1 Ensemble Antagonizes LET-23 EGFR Basolateral Localization and Signaling during Vulva Induction
- The Proteomic Landscape of the Suprachiasmatic Nucleus Clock Reveals Large-Scale Coordination of Key Biological Processes
- RNA-Processing Protein TDP-43 Regulates FOXO-Dependent Protein Quality Control in Stress Response
- A Complex Genetic Switch Involving Overlapping Divergent Promoters and DNA Looping Regulates Expression of Conjugation Genes of a Gram-positive Plasmid
- ZTF-8 Interacts with the 9-1-1 Complex and Is Required for DNA Damage Response and Double-Strand Break Repair in the Germline
- Integrating Functional Data to Prioritize Causal Variants in Statistical Fine-Mapping Studies
- Tpz1-Ccq1 and Tpz1-Poz1 Interactions within Fission Yeast Shelterin Modulate Ccq1 Thr93 Phosphorylation and Telomerase Recruitment
- Salt-Induced Stabilization of EIN3/EIL1 Confers Salinity Tolerance by Deterring ROS Accumulation in
- Telomeric (s) in spp. Encode Mediator Subunits That Regulate Distinct Virulence Traits
- Ethylene-Induced Inhibition of Root Growth Requires Abscisic Acid Function in Rice ( L.) Seedlings
- Ancient Expansion of the Hox Cluster in Lepidoptera Generated Four Homeobox Genes Implicated in Extra-Embryonic Tissue Formation
- Mechanism of Suppression of Chromosomal Instability by DNA Polymerase POLQ
- A Mutation in the Mouse Gene Leads to Impaired Hedgehog Signaling
- Keeping mtDNA in Shape between Generations
- Targeted Exon Capture and Sequencing in Sporadic Amyotrophic Lateral Sclerosis
- TIF-IA-Dependent Regulation of Ribosome Synthesis in Muscle Is Required to Maintain Systemic Insulin Signaling and Larval Growth
- At Short Telomeres Tel1 Directs Early Replication and Phosphorylates Rif1
- Evidence of a Bacterial Receptor for Lysozyme: Binding of Lysozyme to the Anti-σ Factor RsiV Controls Activation of the ECF σ Factor σ
- Hsp40s Specify Functions of Hsp104 and Hsp90 Protein Chaperone Machines
- Feeding State, Insulin and NPR-1 Modulate Chemoreceptor Gene Expression via Integration of Sensory and Circuit Inputs
- Functional Interaction between Ribosomal Protein L6 and RbgA during Ribosome Assembly
- Multiple Regulatory Systems Coordinate DNA Replication with Cell Growth in
- Fast Evolution from Precast Bricks: Genomics of Young Freshwater Populations of Threespine Stickleback
- Mmp1 Processing of the PDF Neuropeptide Regulates Circadian Structural Plasticity of Pacemaker Neurons
- The Nuclear Immune Receptor Is Required for -Dependent Constitutive Defense Activation in
- Genetic Modifiers of Neurofibromatosis Type 1-Associated Café-au-Lait Macule Count Identified Using Multi-platform Analysis
- Juvenile Hormone-Receptor Complex Acts on and to Promote Polyploidy and Vitellogenesis in the Migratory Locust
- Uncovering Enhancer Functions Using the α-Globin Locus
- The Analysis of Mutant Alleles of Different Strength Reveals Multiple Functions of Topoisomerase 2 in Regulation of Chromosome Structure
- Metabolic Respiration Induces AMPK- and Ire1p-Dependent Activation of the p38-Type HOG MAPK Pathway
- The Specification and Global Reprogramming of Histone Epigenetic Marks during Gamete Formation and Early Embryo Development in
- The DAF-16 FOXO Transcription Factor Regulates to Modulate Stress Resistance in , Linking Insulin/IGF-1 Signaling to Protein N-Terminal Acetylation
- Genetic Influences on Translation in Yeast
- Analysis of Mutants Defective in the Cdk8 Module of Mediator Reveal Links between Metabolism and Biofilm Formation
- Ribosomal Readthrough at a Short UGA Stop Codon Context Triggers Dual Localization of Metabolic Enzymes in Fungi and Animals
- Gene Duplication Restores the Viability of Δ and Δ Mutants
- Selection on a Variant Associated with Improved Viral Clearance Drives Local, Adaptive Pseudogenization of Interferon Lambda 4 ()
- Break-Induced Replication Requires DNA Damage-Induced Phosphorylation of Pif1 and Leads to Telomere Lengthening
- Dynamic Partnership between TFIIH, PGC-1α and SIRT1 Is Impaired in Trichothiodystrophy
- Signature Gene Expression Reveals Novel Clues to the Molecular Mechanisms of Dimorphic Transition in
- Mutations in Moderate or Severe Intellectual Disability
- Multifaceted Genome Control by Set1 Dependent and Independent of H3K4 Methylation and the Set1C/COMPASS Complex
- A Role for Taiman in Insect Metamorphosis
- The Small RNA Rli27 Regulates a Cell Wall Protein inside Eukaryotic Cells by Targeting a Long 5′-UTR Variant
- MMS Exposure Promotes Increased MtDNA Mutagenesis in the Presence of Replication-Defective Disease-Associated DNA Polymerase γ Variants
- Coexistence and Within-Host Evolution of Diversified Lineages of Hypermutable in Long-term Cystic Fibrosis Infections
- Comprehensive Mapping of the Flagellar Regulatory Network
- Topoisomerase II Is Required for the Proper Separation of Heterochromatic Regions during Female Meiosis
- A Splice Mutation in the Gene Causes High Glycogen Content and Low Meat Quality in Pig Skeletal Muscle
- KDM5 Interacts with Foxo to Modulate Cellular Levels of Oxidative Stress
- H2B Mono-ubiquitylation Facilitates Fork Stalling and Recovery during Replication Stress by Coordinating Rad53 Activation and Chromatin Assembly
- Copy Number Variation in the Horse Genome
- Unifying Genetic Canalization, Genetic Constraint, and Genotype-by-Environment Interaction: QTL by Genomic Background by Environment Interaction of Flowering Time in
- Spinster Homolog 2 () Deficiency Causes Early Onset Progressive Hearing Loss
- Genome-Wide Discovery of Drug-Dependent Human Liver Regulatory Elements
- Developmentally-Regulated Excision of the SPβ Prophage Reconstitutes a Gene Required for Spore Envelope Maturation in
- Protein Phosphatase 4 Promotes Chromosome Pairing and Synapsis, and Contributes to Maintaining Crossover Competence with Increasing Age
- The bHLH-PAS Transcription Factor Dysfusion Regulates Tarsal Joint Formation in Response to Notch Activity during Leg Development
- A Mouse Model Uncovers LKB1 as an UVB-Induced DNA Damage Sensor Mediating CDKN1A (p21) Degradation
- Notch3 Interactome Analysis Identified WWP2 as a Negative Regulator of Notch3 Signaling in Ovarian Cancer
- An Integrated Cell Purification and Genomics Strategy Reveals Multiple Regulators of Pancreas Development
- Dominant Sequences of Human Major Histocompatibility Complex Conserved Extended Haplotypes from to
- The Vesicle Protein SAM-4 Regulates the Processivity of Synaptic Vesicle Transport
- A Gain-of-Function Mutation in Impeded Bone Development through Increasing Expression in DA2B Mice
- Nephronophthisis-Associated Regulates Cell Cycle Progression, Apoptosis and Epithelial-to-Mesenchymal Transition
- Beclin 1 Is Required for Neuron Viability and Regulates Endosome Pathways via the UVRAG-VPS34 Complex
- The Not5 Subunit of the Ccr4-Not Complex Connects Transcription and Translation
- Abnormal Dosage of Ultraconserved Elements Is Highly Disfavored in Healthy Cells but Not Cancer Cells
- Genome-Wide Distribution of RNA-DNA Hybrids Identifies RNase H Targets in tRNA Genes, Retrotransposons and Mitochondria
- The Chromosomal Association of the Smc5/6 Complex Depends on Cohesion and Predicts the Level of Sister Chromatid Entanglement
- Cell-Autonomous Progeroid Changes in Conditional Mouse Models for Repair Endonuclease XPG Deficiency
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The Master Activator of IncA/C Conjugative Plasmids Stimulates Genomic Islands and Multidrug Resistance Dissemination
- A Splice Mutation in the Gene Causes High Glycogen Content and Low Meat Quality in Pig Skeletal Muscle
- Keratin 76 Is Required for Tight Junction Function and Maintenance of the Skin Barrier
- A Role for Taiman in Insect Metamorphosis
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



