-
Články
Reklama
- Vzdělávání
- Časopisy
Top články
Nové číslo
- Témata
Reklama- Videa
- Podcasty
Nové podcasty
Reklama- Kariéra
Doporučené pozice
Reklama- Praxe
ReklamaLicensing of Yeast Centrosome Duplication Requires Phosphoregulation of Sfi1
Chromosomes are replicated during the cell cycle and distributed to two progeny cells on a machine called the spindle, such that each cell has one complete copy of the genome. The chromosomes are moved by attachment to the long microtubule polymers of the mitotic spindle, formed from a centrosome at each end of the spindle. Mechanisms that restrict DNA replication and centrosome duplication to once per cell cycle are critical, as defects in either event result in genetic instability and are associated with cancer. Cell cycle-dependent control of DNA replication has been extensively studied, but comparatively little is known about the regulation of centrosome duplication, particularly its restriction to once per cell cycle. Using genetics and cytology, including super-resolution imaging to detect reduplicated centrosomes, we show that cyclin-dependent kinase phosphorylation of Sfi1, a conserved component of centrosomes, prevents the occurrence of extra rounds of yeast centrosome duplication during the cell cycle. Additionally, we propose that dephosphorylation of Sfi1 by the phosphatase Cdc14 permits centrosome duplication for the next cell cycle. Our work is the first to provide a mechanism for how centrosome duplication, like DNA replication, occurs once during the cell cycle through cyclin-dependent kinase regulation.
Published in the journal: . PLoS Genet 10(10): e32767. doi:10.1371/journal.pgen.1004666
Category: Research Article
doi: https://doi.org/10.1371/journal.pgen.1004666Summary
Chromosomes are replicated during the cell cycle and distributed to two progeny cells on a machine called the spindle, such that each cell has one complete copy of the genome. The chromosomes are moved by attachment to the long microtubule polymers of the mitotic spindle, formed from a centrosome at each end of the spindle. Mechanisms that restrict DNA replication and centrosome duplication to once per cell cycle are critical, as defects in either event result in genetic instability and are associated with cancer. Cell cycle-dependent control of DNA replication has been extensively studied, but comparatively little is known about the regulation of centrosome duplication, particularly its restriction to once per cell cycle. Using genetics and cytology, including super-resolution imaging to detect reduplicated centrosomes, we show that cyclin-dependent kinase phosphorylation of Sfi1, a conserved component of centrosomes, prevents the occurrence of extra rounds of yeast centrosome duplication during the cell cycle. Additionally, we propose that dephosphorylation of Sfi1 by the phosphatase Cdc14 permits centrosome duplication for the next cell cycle. Our work is the first to provide a mechanism for how centrosome duplication, like DNA replication, occurs once during the cell cycle through cyclin-dependent kinase regulation.
Introduction
Centrosomes serve as the poles of the mitotic spindle in eukaryotic cells. Centrosome duplication only once per cell cycle is critical for bipolar spindle organization and proper chromosome segregation. Aberrant centrosome numbers are linked to chromosomal instability and are commonly observed in cancers [1]; thus, mechanisms that restrict duplication to a single event are essential. Cyclin-dependent kinase (Cdk), separase, and polo-like kinase 1 [2]–[5] are known regulators of centrosome duplication implicated in limiting this process to once per cell cycle. However, an understanding of the mechanisms through which they ensure that duplication is tightly coupled with other cell cycle events is still lacking. In budding yeast, deletion of all mitotic cyclins leads to reduplication of spindle pole bodies (SPBs, yeast centrosomes), indicating that mitotic cyclin/Cdk1 blocks SPB reduplication. Thus, a decrease in Cdk1 activity levels is needed to eliminate the block to SPB duplication, allowing SPBs to become licensed, or competent, for duplication [2]. However, even in budding yeast, in which mapping of Cdk1 substrates has been studied at a genome-wide level [6], no targets of Cdk1 that restrict licensing of SPB duplication have been identified.
In budding yeast, the SPB is embedded within the nuclear envelope. Its duplication begins during the G1 phase of the cell cycle when the half-bridge, a specialized region of the nuclear envelope on one side of the SPB, elongates and a density called the satellite forms at the cytoplasmic distal tip. The satellite develops into the new mature SPB, which is linked to the mother SPB via a complete bridge. After duplication is complete and the bridge is severed, the SPBs separate and move to opposite sides of the nucleus as they form the bipolar spindle [7]. All of the components of the SPB have been identified, and several are known to be Cdk1 substrates. In some cases, the role of Cdk1 phosphorylation is known to affect the SPB duplication cycle (Spc42, Sfi1) or spindle properties (γ-tubulin, Spc110) [8]–[14].
Sfi1 is a conserved component of centrosomes and SPBs and is required for SPB duplication in both budding and fission yeast [14]–[17]. Sfi1 is positioned on the cytoplasmic side of the half-bridge in an orientation-specific manner with the N terminus proximal and the C terminus distal to the SPB [18]. Based on this topology, Kilmartin and colleagues put forth a model in which Sfi1 is a central player in both SPB duplication and separation [18]. They proposed that SPB duplication initiates when Sfi1 molecules associate via C-terminal end-to-end interactions with Sfi1 molecules already present at the half-bridge, thus doubling the half-bridge length. A new SPB can then assemble at the free SPB-distal N-terminal domain created by this arrangement. After completion of SPB duplication, dissociation of the Sfi1 C termini at the bridge from each other would then allow SPB separation to occur. Based on this model, it has been proposed that Sfi1 may serve as a licensing factor for SPB duplication [18]–[20]. Interestingly, Sfi1 has been shown to be phosphorylated at numerous sites within C-terminal Cdk1 consensus motifs in vivo [11], [21] and in vitro by Cdk1 [14] and to be dephosphorylated by the protein phosphatase Cdc14 [14], [20], which is required for mitotic exit [22], [23]. This suggests that Cdk1 and Cdc14 phosphoregulation of Sfi1 contribute to licensing of SPB duplication whereby mitotic Cdk1 phosphorylation of Sfi1 blocks and Cdc14 dephosphorylation enables SPB duplication.
A recent study showed that cells with Sfi1 containing phosphomimetic Cdk1 sites arrest with a single SPB, supporting the idea that phosphorylation of Sfi1 inhibits the process of duplication [14]. However, no studies of Cdk1 targets have mimicked the reduplication phenotype revealed by depletion of mitotic Cdk1 activity [2]. In the current study, we show SPB reduplication in cells with reduced Cdk1 phosphorylation by mutating Sfi1 residues within Cdk1 sites or by activation of Cdc14 in a prolonged anaphase. Our results support the model that Sfi1 C terminus phosphorylation by Cdk1 is required to restrict licensing of SPB duplication and that dephosphorylation of the Sfi1 C terminus by Cdc14 licenses SPBs for duplication.
Results/Discussion
Cdk1 phosphorylation of Sfi1 is required for appropriate bipolar spindle assembly and chromosome segregation
Sfi1 contains five residues within full Cdk consensus motifs (S/T*-P-x-K/R), four at the C terminus known to be phosphorylated in vivo (T816, S855, S882, S892) [11], [21] and one immediately preceding the C terminus (S801; Fig. S1A). Using a recombinant fragment of Sfi1, we found that four of these residues (S801, T816, S855, S882) and S923 (an in vivo phosphorylation site within a minimal Cdk consensus motif (S/T*-P)) are phosphorylated by mitotic cyclin Clb2/Cdk1 in vitro (Fig. S1, B–G). In vitro Clb2/Cdk1 phosphorylation of three of these residues (T816, S855, S882) as well as S892 has recently been shown [14].
We created a nonphosphorylatable mutant allele collection with individual and combinations of these C-terminal residues converted to alanine. Alteration of S855 resulted in a temperature-sensitive growth defect at 37°C (Fig. 1A). Combinations of mutations resulted in varying phenotypes. We found that the alleles sfi1-C3A (T816A S882A S892A) and sfi1-C4A (T816A S855A S882A S892A) show growth defects at 24°C and little or no growth, respectively, at 37°C (Fig. 1A). Mutation of the minimal Cdk consensus site (S923A) in combination with sfi1-C3A or sfi1-C4A does not enhance the growth defect (Fig. 1A).
Fig. 1. sfi1-C4A displays impaired growth and spindle and chromosome segregation defects. 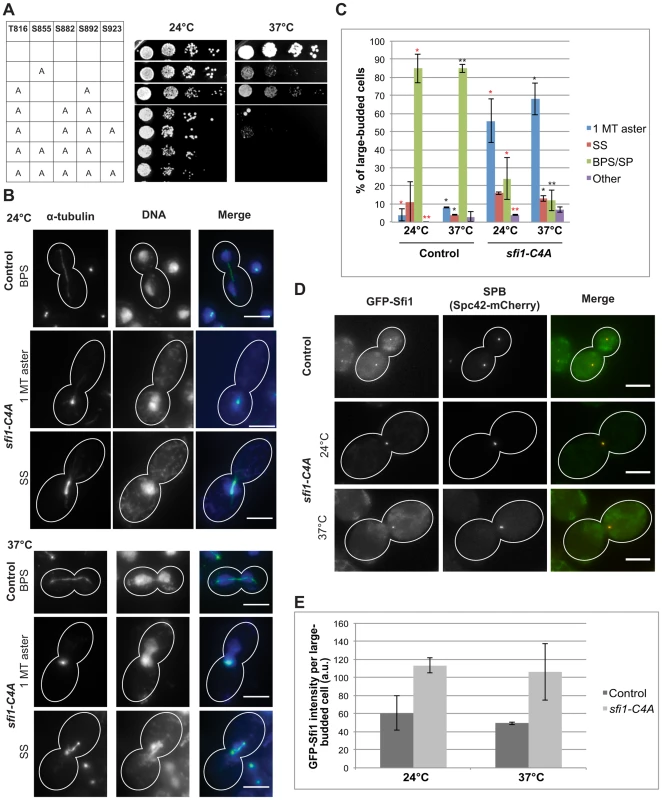
A. Dilution series of strains with mutations in SFI1 on YPD. B–C. Immunofluorescent staining (α-tubulin: green, DNA: blue) of fixed large-budded sfi1-C4A (JA249) or control (JA196) cells grown at 24°C or shifted to 37°C for 8.25 h in YPD. MT: microtubule, SS: short metaphase bipolar spindle, BPS/SP: bipolar spindle or separated poles. Bar: 5 µm. C. Quantification of B. Asterisks: statistically significant difference via Student's t test between sfi1-C4A and control for each phenotypic category at 24°C (red) and 37°C (black). **p<0.01. *: p<0.05. Significance only shown for comparisons between strains at each temperature. Error bars: SD. n≥192 cells per group from 2 experiments. D–E. GFP-sfi1-C4A pLEU-HIS-SPC42-mCherry (JA354) or GFP-SFI1 pLEU-HIS-SPC42-mCherry (JA311) grown in SC-Leu at 24°C or shifted to 37°C 9 h were fixed prior to localization (D) of Sfi1 (green) and Spc42 (red) and quantification of Sfi1 levels in arbitrary units (a.u.) (E). Error bars: SD. n≥62 cells per group from 2 experiments. Bar: 5 µm. The sfi1-C4A and sfi1-C3A mutant proteins fused to GFP localize to the SPB at levels similar or greater than that of GFP-Sfi1 (Fig. 1, D and E, and Fig. S2, C and D, respectively), indicating that the phenotypes observed are not caused by reduced levels of the mutant proteins but rather are likely due to loss of Cdk1 phosphorylation. In an asynchronous population of sfi1-C4A or sfi1-C3A, large-budded cells displayed multiple spindle morphology phenotypes (Fig. 1, B and C and Fig. S2, A and B, respectively). sfi1-C4A and sfi1-C3A displayed significant increases both in short spindles and in single microtubule asters with chromosome missegregation at 37°C compared to control. Defects were also observed at 24°C.
Cdk1 phosphorylation of Sfi1 is required for SPB separation and to block SPB reduplication
The phenotypes of sfi1-C4A in large-budded cells were examined using structured illumination microscopy (SIM; Fig. 2A). We used Spc42-GFP to label both the SPB and the assembly intermediate, the satellite [24]. SIM allowed us to resolve side-by-side SPBs and a satellite versus mother SPB into two Spc42-GFP foci. In contrast to control cells containing mainly two separated SPBs, we found that sfi1-C4A cells at 37°C commonly displayed one of the following three phenotypes: a single (Spc42-GFP) focus, two adjacent foci suggestive of side-by-side SPBs, or at least three foci (Fig. 2, A and B). Commonly, in sfi1-C4A large-budded cells that contained at least three GFP foci, two foci were located adjacent to one another, while a third GFP focus was located at a distance (13.71±6.92% at 24°C, 22.57±1.18% at 37°C; Fig. 2A). The observation of more than two SPBs suggests that an aberrant reduplication event has occurred.
Fig. 2. sfi1-C4A displays unseparated SPBs or bipolar spindles with enlarged or reduplicated SPBs. 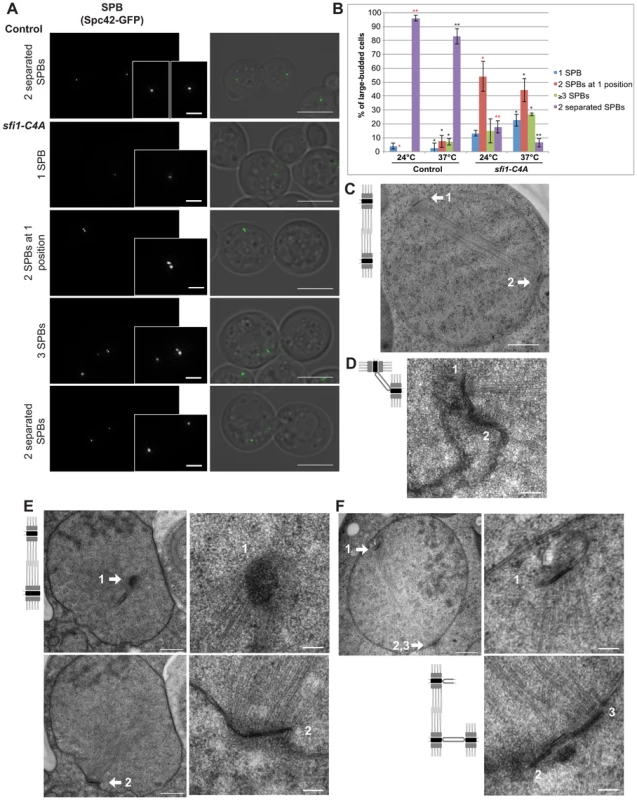
A. Asynchronous sfi1-C4A SPC42-GFP (JA302) and SFI1 SPC42-GFP (JA254) control cells grown at 24°C and shifted to 37°C for 9 h in YPD. Fixed large-budded cells were imaged by SIM. GFP on left (inset bar: 1 µm) and merge with transmitted image on right. Bar: 5 µm. B. Quantification of A. Asterisks: statistically significant difference via Student's t test between sfi1-C4A and control for each phenotypic category at 24°C (red) and 37°C (black). **p<0.01. *: p<0.05. Significance only shown for comparisons between strains at each temperature. Error bars: SD. n≥77 cells per group from 2 experiments. C. Asynchronous control cells (JA196) shifted to 37°C for 4 h in YPD and prepared for EM. Bipolar spindle with two SPBs (1, 2). Bar: 500 nm. D–F. Asynchronous sfi1-C4A (JA249) cells shifted to 37°C for 9 h in YPD and prepared for EM. Serial sections were examined by EM for 18 cells. D. Representative cell (n = 11) with two SPBs at one position. SPBs (1, 2) are at abnormal orientations to one another with a bridge. Bar: 100 nm. E–F. Multiple sections from the same cell are shown. Left panel(s): entire nucleus, 500 nm bar. Right panels: SPB(s), 100 nm bar. E. Representative cell (n = 2) containing a short bipolar spindle with SPB 1 at an orientation different from that of abnormally large SPB 2. F. Representative cell (n = 5) containing a short bipolar spindle with extra SPBs. SPB 1 is located at a region of the nuclear envelope opposite SPBs 2 and 3, which are connected via a bridge. Left panel shows the location of SPB 1, shown in the upper right panel at higher magnification, and SPBs 2 and 3. In the lower right panel, both SPBs 2 and 3 are clearly seen in a higher magnification view from a different serial section of the same cell. Average distance between adjacent SPBs: 108±10 nm. Using electron microscopy (EM), we further studied the phenotype of sfi1 mutant cells. Control large-budded cells display a bipolar spindle with two SPBs, one at either side of the nucleus (Fig. 2C). In sfi1-C4A large-budded cells, we observed either unseparated SPBs at the same position within the nuclear envelope (n = 11 of 18 cells), suggesting a defect in SPB separation (Fig. 2D), or short bipolar spindles with aberrant SPBs (n = 7 of 18 cells, Fig. 2, E and F). Some cells with short spindles have two SPBs, with one being enlarged (n = 2; Fig. 2E). However, the remaining cells (n = 5) contain three or four SPBs, in which two SPBs are adjacent, and the other SPB (or two adjacent SPBs when there are four total) is connected by the short spindle and lies on the other side of the nuclear envelope (Fig. 2F). Given that we typically see the presence of a bridge connecting two adjacent SPBs in these cells (n = 4 of 5 cells, Fig. 2F), and the distance between these SPBs is approximately that expected in wild type cells (Fig. 2F) [18], [25], we conclude that an aberrant reduplication event has occurred. A similar phenotype was also observed in sfi1-C3A (Fig. S3). This data is consistent with our SIM results and indicates that loss of Cdk1 phosphorylation of Sfi1 leads to SPB reduplication. Specifically, in cells with reduplicated SPBs, as seen previously in cells in which all mitotic cyclins are deleted [2], each extra SPB has duplicated using one of the original SPBs present in the spindle as a “template” during a single cell cycle. Thus, the nonphosphorylatable sfi1 mutations lead to premature licensing of SPB duplication during mitosis. Excitingly, this now points to Sfi1 as the first target of Cdk1 in blocking reduplication.
Since we propose that the aberrant SPB reduplication events observed in sfi1-C4A occur during mitosis within a single cell cycle, we would expect that mitotically-arrested sfi1-C4A cells would display reduplicated SPBs. Therefore, we arrested cells in mitosis by inducing overexpression of a nondegradable Pds1 (GAL1-pds1-mdb) [26] and then shifted to 37°C. The cells were initially arrested in G1, in which both SFI1 and sfi1-C4A GAL1-pds1-mdb cells commonly had two Spc42-GFP foci (>98%, see Fig. 3 legend), indicative of a mother SPB and satellite. As expected, SFI1 GAL1-pds1-mdb cells released from G1 and arrested in mitosis at 37°C displayed two separated (Spc42-GFP) foci, indicative of a bipolar spindle (86±2%; Fig. 3, A and B). In contrast, sfi1-C4A GAL1-pds1-mdb cells arrested in mitosis at 37°C only occasionally displayed two separated foci (15±1%). sfi1-C4A GAL1-pds1-mdb cells sometimes showed two foci at one position (30±3%), indicating that SPB duplication occurred, but not separation, but predominantly displayed at least three foci (41±3%; Fig. 3, A and B). A majority of sfi1-C4A cells with at least three Spc42-GFP foci contained at least two adjacent foci with additional SPB(s), indicative of reduplicated SPBs (36.8±1.4%; Fig. 3A). We confirmed the presence of reduplicated SPBs via EM (Fig. 3C). These findings support our conclusion that SPB reduplication within a single cell cycle is responsible for the presence of additional SPBs in cells lacking Cdk1 phosphorylation sites in Sfi1.
Fig. 3. Reduplicated SPBs upon mitotic arrest are enhanced in sfi1-C4A and are present upon Cdc14 activation. 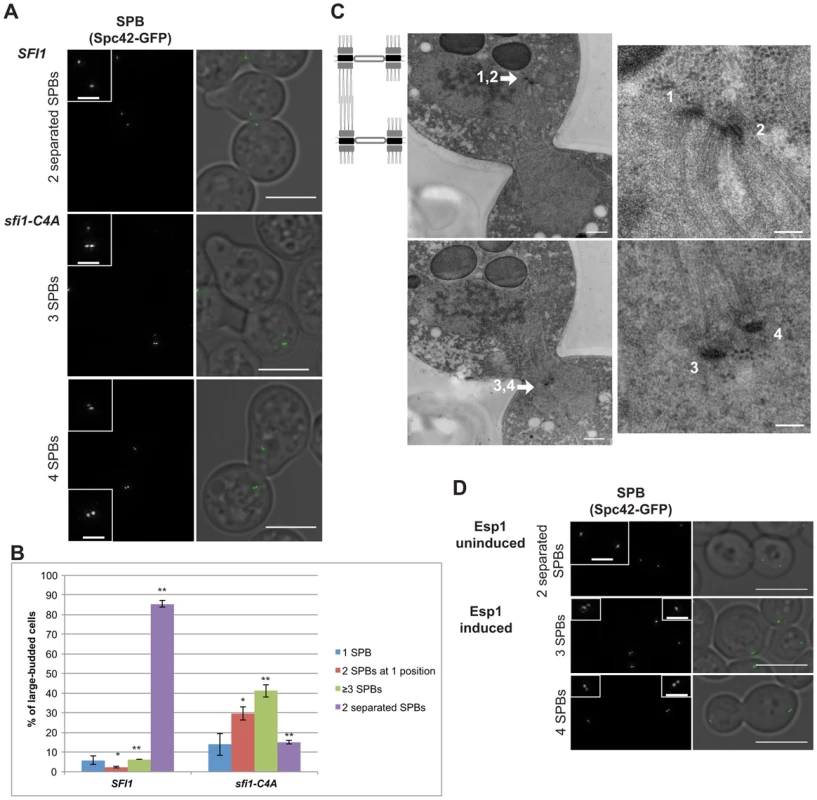
A–C. SFI1 (JA295) or sfi1-C4A (JA297) cells containing GAL1-pds1-mdb SPC42-GFP were grown to early-log phase at 24°C in YEP with 2% raffinose then arrested in G1 with α-factor (≤2 SPBs at G1 arrest: 98.1±0.4% for sfi1-C4A, 99.4±0.4% for SFI1; n≥219 per group from 2 experiments). G1-arrested cells were released into YEP with 2% galactose to arrest in mitosis and, once at mitotic arrest, were shifted to 37°C for 2.5 h. A. Fixed large-budded cells were imaged by SIM, with GFP on left (inset bar: 1 µm) and merge with transmitted image on right. Bar: 5 µm. Mitotically-arrested cells following 37°C shift. B. Quantification of A. Asterisks: statistically significant difference via Student's t test. **p<0.01. *: p<0.05. Error bars: SD. n≥185 cells per group from 2 experiments. C. A sfi1-C4A GAL1-pds1-mdb SPC42-GFP cell imaged by EM containing at least four SPBs (1–4) total. Left panels: entire nucleus, 500 nm bar. Right: SPBs, 100 nm bar. D. An asynchronous culture of MET-CDC20 cdh1Δ GALS-ESP1 SPC42-GFP (JA256) cells was grown to early-log phase in SC-Met with 3% raffinose. Methionine was added (2 mM final concentration) to arrest cells in metaphase and every 2 h for the experiment remainder. After metaphase arrest, Esp1 either was induced for 4 h using 3% galactose to release Cdc14 from the nucleolus or remained uninduced in raffinose. Fixed large-budded cells were imaged by SIM, with GFP on left (inset bar: 1 µm) and merge with transmitted image on right. Bar: 5 µm. n≥211 per group from 2 experiments. Upper panel: cells with no Esp1 induction with bipolar spindles (63±13%). Lower panels: cells with Esp1 induction with one or two reduplicated SPBs (3 or 4 Spc42-GFP foci total, respectively; 22±1%); p = 0.02 (Student's t test) for reduplicated SPBs versus without Esp1 induction (7±3%). Cdc14 is required to license SPB duplication
Cdk1 activity is counteracted by a phosphatase, Cdc14, which is activated during anaphase. Cdc14 is thought to remove Cdk1-dependent phosphates from a number of targets [22], [23] and has been shown to dephosphorylate Sfi1 [14], [20]. Several Cdk sites on Sfi1 are expected to be good Cdc14 target sites, as they are within Cdc14 consensus motifs (S-P-x-K/R; S801, S855, S882, S892) [14], [27], [28]. To determine whether Cdc14 acted on Sfi1 to control licensing of SPB duplication, we utilized an experimental strategy previously used by Cross and colleagues to examine another Cdc14-regulated dephosphorylation event in mitosis [20], [29]. Specifically, MET-CDC20 cdh1Δ GALS-ESP1 cells arrested in metaphase via repression of CDC20 were driven into anaphase by induction of separase (Esp1), which leads to Cdc14 release from the nucleolus and activation. Lack of both Cdc20 and Cdh1 prevents mitotic exit into G1 [20], [29], [30]. This engineered strain, both with and without Esp1 induction, includes a population of large-budded cells with three or more dispersed Spc42-GFP foci, which may arise from SPB missegregation events (17±7% and 25±8%, respectively; Fig. S4). However, notably, significantly more actual SPB reduplication events (two adjacent SPBs with additional SPBs in large-budded cells, 22±1%) were seen in cells in which Esp1 overexpression was induced and Cdc14 was activated than in cells in which Esp1 was not induced (7±3%; Fig. 3D), indicating that active Cdc14 promotes the presence of reduplicated SPBs.
A recent study showed that cdc14-2 cells forced into G1 were delayed in SPB duplication [14]. The authors conclude that Cdc14 promotes timely SPB duplication, which is consistent with our work demonstrating that Cdc14 is specifically involved in licensing of SPB duplication, as evidenced by the presence of reduplicated SPBs with prolonged activation of Cdc14. Schiebel and colleagues did not observe an increase in Sfi1 incorporation into the SPB, as measured by Sfi1 fluorescent signal intensity, with overexpression of Cdc14 in a metaphase arrest [14]. Using the protocol from the Cross lab [20], [29], we were able to arrest cells at a stage in the cell cycle that was permissive for Cdc14-driven reduplication, and we directly examined SPB assembly using SIM. While Cln/Cdk1 activity is required for SPB duplication in G1 [31], in mitotically-arrested cells in the absence of mitotic cyclin degradation [32]–[35], it appears that mitotic cyclins permit SPB duplication when Sfi1 phosphorylation is diminished.
Our findings support the model that Cdk1 phosphorylation of Sfi1 is required for SPB separation and a block to SPB reduplication (Fig. 4). Specifically, we propose that after SPB duplication is complete, Cdk1 phosphorylates at least four Sfi1 C-terminal residues, allowing for SPB separation and bipolar spindle formation during mitosis. These C-terminal residues remain phosphorylated during mitosis, restricting licensing of SPB duplication until downregulation of mitotic cyclin/Cdk1 at the end of mitosis. Dephosphorylation of Sfi1 by Cdc14 at the end of mitosis then licenses the SPBs for the next cycle of G1 SPB duplication.
Fig. 4. Model for licensing of yeast centrosome duplication via phosphoregulation of Sfi1. 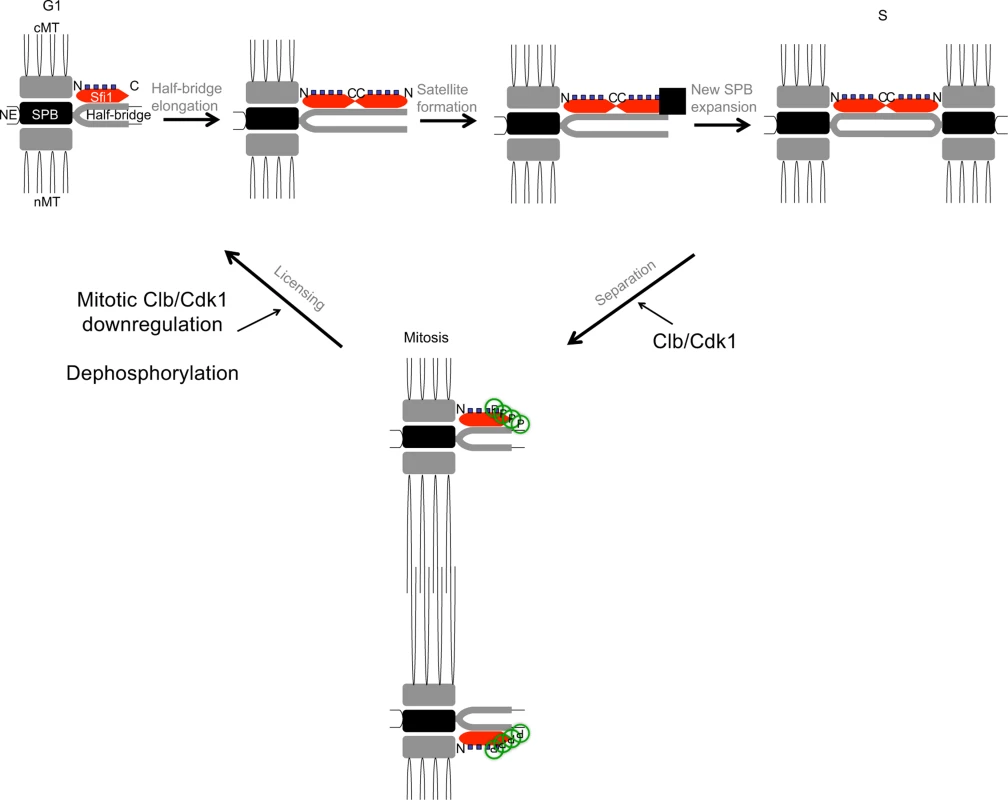
Cdk1 activity is required for SPB separation, in which Cdk1 phosphorylates the Sfi1 C terminus, ensuring SPB duplication does not begin until completion of mitosis and downregulation of mitotic cyclin/Cdk1. Dephosphorylation of Sfi1, likely by Cdc14, licenses SPB duplication to allow the next cycle of SPB duplication to begin at G1. cMT: cytoplasmic microtubules. nMT: nuclear microtubules. NE: nuclear envelope. ▪: satellite. Consistent with our findings, previous research has shown SPB separation requires mitotic cyclin/Cdk1 [2], [36], [37] and Cdk1 phosphorylation of the Sfi1 C terminus [14], [38]. In addition, Schiebel and colleagues showed that cells with Sfi1 containing six phosphomimetic Cdk1 sites arrest with a single SPB, indicating that Cdk1 phosphorylation of Sfi1 inhibits SPB duplication [14]. This result supports our conclusion that the block to SPB duplication by Cdk1 phosphorylation of Sfi1 must be removed to license SPB duplication. Importantly, we have reproduced the reduplication phenotype seen upon depletion of mitotic Cdk1 activity [2], either by nonphosphorylatable versions of Sfi1 or by inducing Cdc14 activity. Appropriate phosphoregulation of Sfi1 by Cdk1 and Cdc14 thus ensures SPB duplication occurs only once per cell cycle.
Future work examining whether the Sfi1 C termini directly interact with each other and/or interact with other components of the SPB and the impact of phosphorylation status on these interactions will be important in understanding the mechanism that initiates SPB duplication. Moreover, further studies will prove useful in determining a role for Cdks in licensing in metazoans. Given that human Sfi1 also contains multiple Cdk consensus motifs at the C terminus, this study could provide crucial insight into the licensing of centrosome duplication.
Materials and Methods
Yeast strains
The W303 strain background was used for all experiments (Table S1). Yeast transformation with pRS402 was used to create ADE2 strains where indicated in the strain list. Standard PCR mutagenesis, including use of the Quikchange II mutagenesis kit (Agilent), was used to create all mutations within the SFI1 sequence using a pRS305 vector base. Control and mutant strains (except JA256 and JA295) contain a silent mutation at Sfi1 residues E710 and L711 for a nucleotide change of A2130G T2131C in order to develop an additional restriction enzyme site within the Sfi1 sequence for plasmid manipulation purposes. Creation of single integrant mutants with the NATMX marker via PCR is as previously described [39]. This technique was used to create N-terminally tagged GFP-Sfi1 strains by transforming trp1::N-GFP-SFI1-TRP1 sfi1Δ::HIS5 (gift of John Kilmartin) [16]. Strains JA295 and JA297 were created by integrating pOC70 (gift of Orna Cohen-Fix) [26] at the LEU2 locus of heterozygous diploid strains, followed by dissection and selection of appropriate haploids. C-terminal tagging of SPC42 with yeGFP1 via PCR was performed as previously described [40], [41]. JA256 was created using YL165 (gift of Fred Cross) [29] via mating.
Five-fold dilution series with 5 µL of cells at an initial OD/mL of 0.1 in the left column were placed on YPD plates and grown at the indicated temperatures for 2 days. For temperature shift experiments of asynchronous cultures, cultures were shifted at early-log phase.
Cytological techniques
Imaging of live and fixed cells was performed at room temperature on an Eclipse Ti inverted microscope (Nikon, Tokyo, Japan) fitted with a CFI Plan Apo VC 60× H numerical aperture 1.4 objective (Nikon, Tokyo, Japan) and a CoolSNAP HQ2 charge-coupled device camera (Photometrics, Tuscon, AZ, USA). Metamorph imaging software (Molecular Devices, Sunnyvale, CA, USA) was used to collect images, and maximum projections are shown. The 1.5× intermediate magnification was used for fixed immunofluorescent images.
For immunofluorescence, cultures were fixed with 4% formaldehyde for 45 minutes, subjected to zymolyase and prepared on slides. YOL1/34 rat anti-tubulin antibody (1/150), FITC goat anti-rat secondary antibody (1/200), and Hoechst dye for DNA were used. For imaging of live GFP-expressing cells, cells were briefly centrifuged, washed, and imaged in 1× PBS. For brief fixation of GFP-expressing cells, cultures were fixed with 3.7% formaldehyde for 15 minutes, resuspended, and imaged in 1×PBS or KPO4/sorbitol.
SIM images were acquired at room temperature on an Applied Precision OMX Blaze microscope (Issaquah, WA, USA) equipped with a PCO Edge sCMOS camera (Kelheim, Germany). The objective used was an Olympus (Center Valley, PA, USA) 60× 1.42NA Plan Apo N oil objective. Image stacks were acquired at 125 nm intervals. SIM reconstruction was performed with the Applied Precision software package utilizing optical transfer functions measured with 100 nm green fluospheres in Prolong Gold on a coverslip surface (Life Technologies, Carlsbad, CA, USA) following the Applied Precision protocols. After reconstruction, SIM images were scaled 2 by 2 with bilinear interpolation through ImageJ software (National Institutes of Health, Bethesda, MD, USA) for future quantification.
Quantification of GFP intensities in live and fixed GFP-expressing cells was performed with Metamorph imaging software using maximum projections, in which the average pixel intensity was determined for the fluorescent focus and subtracted from the average pixel intensity for the immediate background border. Distances were measured using ImageJ software. GFP intensity (arbitrary units; a.u.) was averaged for each strain at each temperature, and the average of this value from two experiments for each condition was taken. A Student's t test was then performed on the average from both experiments.
Transmission electron microscopy
Log phase cells were high pressure frozen in a Wohlwend Compact 02 HPF and freeze-substituted in 2% osmium tetroxide, 0.1% uranyl acetate in acetone and embedded in Spurr's epoxy (JA188) or in Epon (JA196) or freeze-substituted in 0.25% glutaraldehyde, 0.1% uranyl acetate in acetone and embedded in Lowicryl HM20 (JA249, JA297) [42]. Imaging was conducted using a FEI Phillips CM100 electron microscope.
Distances in electron micrographs were measured using ImageJ software. Enlarged SPBs were classified as >213.28 nm diameter, which is >33.33% deviance from the predicted average diameter of 160 nm in a diploid [31].
Protein techniques and kinase reaction
Sfi1 residues 800 to 946 were amplified via PCR and cloned into the pKLD116 plasmid (gift of Ivan Rayment) immediately downstream of the rTEV site and in frame with the N-terminal 6×His and maltose binding protein tags. The resulting plasmid was transformed into C+ (DE3) competent E. coli cells (gift of Greg Odorizzi). IPTG induction was performed using 0.3 mM IPTG for 2.5 h at 23°C. The sample was flash frozen, treated with lysozyme, sonicated, and column purification using Talon metal affinity resin (Clontech Laboratories, Inc.) was performed using 200 mM imidazole for elution. An in vitro kinase reaction was performed using 1 µg of the Sfi1 C terminus fusion protein (6×His-MBP-rTEVsite-Sfi1C), 1 mM ATP, and Clb2 (purified from bacteria)/Cdc28 (purified from baculovirus co-infected with the Cdk activating kinase (CAK)) [8].
Mass spectrometry
The 20 µL kinase reaction was digested with 2 µg GluC (no denaturation nor reduction/alkylation) and incubated at room temperature for 1 h. 2 µL or 10% of the reaction was loaded directly onto a Waters nanoAcquity 75 µm×250 mm 1.7 µm BEH130 C18 column (no trapping nor desalting). Peptides were eluted with a gradient from 8% acetonitrile, 0.1% formic acid to 32% acetonitrile, 0.1% formic acid at a flow rate of 300 nL/min. Spectra were searched using Mascot v2.2 (Matrix Science) against a small custom database with the protein sequence of the recombinant Sfi1 fusion protein included. Phosphorylated peptides were identified by manual inspection of all MS/MS spectra with Mascot ions scores of at least 20. Multiple phosphorylated isoforms were identified as possible if the delta score was less than 4 (difference between the top two scoring phosphorylated positional isomers).
Supporting Information
Zdroje
1. GanemNJ, GodinhoSA, PellmanD (2009) A mechanism linking extra centrosomes to chromosomal instability. Nature 460 : 278–282 doi:10.1038/nature08136
2. HaaseSB, WineyM, ReedSI (2001) Multi-step control of spindle pole body duplication by cyclin-dependent kinase. Nat Cell Biol 3 : 38–42 doi:10.1038/35050543
3. VidwansSJ, WongML, O'FarrellPH (2003) Anomalous centriole configurations are detected in Drosophila wing disc cells upon Cdk1 inactivation. J Cell Sci 116 : 137–143.
4. TsouM-FB, StearnsT (2006) Mechanism limiting centrosome duplication to once per cell cycle. Nature 442 : 947–951 doi:10.1038/nature04985
5. TsouM-FB, WangW-J, GeorgeKA, UryuK, StearnsT, et al. (2009) Polo kinase and separase regulate the mitotic licensing of centriole duplication in human cells. Dev Cell 17 : 344–354 doi:10.1016/j.devcel.2009.07.015
6. UbersaxJA, WoodburyEL, QuangPN, ParazM, BlethrowJD, et al. (2003) Targets of the cyclin-dependent kinase Cdk1. Nature 425 : 859–864 doi:10.1038/nature02062
7. JaspersenSL, WineyM (2004) The budding yeast spindle pole body: structure, duplication, and function. Annu Rev Cell Dev Biol 20 : 1–28 doi:10.1146/annurev.cellbio.20.022003.114106
8. JaspersenSL, HuneycuttBJ, GiddingsTH, ResingKA, AhnNG, et al. (2004) Cdc28/Cdk1 regulates spindle pole body duplication through phosphorylation of Spc42 and Mps1. Dev Cell 7 : 263–274 doi:10.1016/j.devcel.2004.07.006
9. HuismanSM, SmeetsMFMA, SegalM (2007) Phosphorylation of Spc110p by Cdc28p-Clb5p kinase contributes to correct spindle morphogenesis in S. cerevisiae. J Cell Sci 120 : 435–446 doi:10.1242/jcs.03342
10. LinT, GombosL, NeunerA, SebastianD, OlsenJV, et al. (2011) Phosphorylation of the yeast γ-tubulin Tub4 regulates microtubule function. PloS One 6: e19700 doi:10.1371/journal.pone.0019700
11. KeckJM, JonesMH, WongCCL, BinkleyJ, ChenD, et al. (2011) A cell cycle phosphoproteome of the yeast centrosome. Science 332 : 1557–1561 doi:10.1126/science.1205193
12. LiangF, RichmondD, WangY (2013) Coordination of chromatid separation and spindle elongation by antagonistic activities of mitotic and S-phase CDKs. PLoS Genet 9: e1003319 doi:10.1371/journal.pgen.1003319
13. LinT-C, NeunerA, SchlosserYT, ScharfAN, WeberL, et al. (2014) Cell-cycle dependent phosphorylation of yeast pericentrin regulates γ-TuSC-mediated microtubule nucleation. eLife 3: e02208 doi:10.7554/eLife.02208
14. ElserafyM, SarićM, NeunerA, LinT-C, ZhangW, et al. (2014) Molecular Mechanisms that Restrict Yeast Centrosome Duplication to One Event per Cell Cycle. Curr Biol 24 : 1456–1466 doi:10.1016/j.cub.2014.05.032
15. MaP, WinderickxJ, NauwelaersD, DumortierF, De DonckerA, et al. (1999) Deletion of SFI1, a novel suppressor of partial Ras-cAMP pathway deficiency in the yeast Saccharomyces cerevisiae, causes G(2) arrest. Yeast 15 : 1097–1109.
16. KilmartinJV (2003) Sfi1p has conserved centrin-binding sites and an essential function in budding yeast spindle pole body duplication. J Cell Biol 162 : 1211–1221 doi:10.1083/jcb.200307064
17. LeeI-J, WangN, HuW, SchottK, BählerJ, et al. (2014) Regulation of spindle-pole body assembly and cytokinesis by the centrin-binding protein Sfi1 in fission yeast. Mol Biol Cell mbc.E13–11–0699 doi:10.1091/mbc.E13-11-0699
18. LiS, SandercockAM, ConduitP, RobinsonCV, WilliamsRL, et al. (2006) Structural role of Sfi1p-centrin filaments in budding yeast spindle pole body duplication. J Cell Biol 173 : 867–877 doi:10.1083/jcb.200603153
19. JonesMH, WineyM (2006) Centrosome duplication: is asymmetry the clue? Curr Biol CB 16: R808–810 doi:10.1016/j.cub.2006.08.041
20. BloomJ, CristeaIM, ProckoAL, LubkovV, ChaitBT, et al. (2011) Global analysis of Cdc14 phosphatase reveals diverse roles in mitotic processes. J Biol Chem 286 : 5434–5445 doi:10.1074/jbc.M110.205054
21. ChiA, HuttenhowerC, GeerLY, CoonJJ, SykaJEP, et al. (2007) Analysis of phosphorylation sites on proteins from Saccharomyces cerevisiae by electron transfer dissociation (ETD) mass spectrometry. Proc Natl Acad Sci U S A 104 : 2193–2198 doi:10.1073/pnas.0607084104
22. VisintinR, CraigK, HwangES, PrinzS, TyersM, et al. (1998) The phosphatase Cdc14 triggers mitotic exit by reversal of Cdk-dependent phosphorylation. Mol Cell 2 : 709–718.
23. StegmeierF, AmonA (2004) Closing mitosis: the functions of the Cdc14 phosphatase and its regulation. Annu Rev Genet 38 : 203–232 doi:10.1146/annurev.genet.38.072902.093051
24. AdamsIR, KilmartinJV (1999) Localization of core spindle pole body (SPB) components during SPB duplication in Saccharomyces cerevisiae. J Cell Biol 145 : 809–823.
25. WineyM, GoetschL, BaumP, ByersB (1991) MPS1 and MPS2: novel yeast genes defining distinct steps of spindle pole body duplication. J Cell Biol 114 : 745–754.
26. Cohen-FixO, PetersJM, KirschnerMW, KoshlandD (1996) Anaphase initiation in Saccharomyces cerevisiae is controlled by the APC-dependent degradation of the anaphase inhibitor Pds1p. Genes Dev 10 : 3081–3093.
27. BremmerSC, HallH, MartinezJS, EisslerCL, HinrichsenTH, et al. (2012) Cdc14 phosphatases preferentially dephosphorylate a subset of cyclin-dependent kinase (Cdk) sites containing phosphoserine. J Biol Chem 287 : 1662–1669 doi:10.1074/jbc.M111.281105
28. EisslerCL, MazónG, PowersBL, SavinovSN, SymingtonLS, et al. (2014) The Cdk/Cdc14 Module Controls Activation of the Yen1 Holliday Junction Resolvase to Promote Genome Stability. Mol Cell 54 : 80–93 doi:10.1016/j.molcel.2014.02.012
29. LuY, CrossF (2009) Mitotic exit in the absence of separase activity. Mol Biol Cell 20 : 1576–1591 doi:10.1091/mbc.E08-10-1042
30. SullivanM, UhlmannF (2003) A non-proteolytic function of separase links the onset of anaphase to mitotic exit. Nat Cell Biol 5 : 249–254 doi:10.1038/ncb940
31. ByersB, GoetschL (1974) Duplication of spindle plaques and integration of the yeast cell cycle. Cold Spring Harb Symp Quant Biol 38 : 123–131.
32. SchwabM, LutumAS, SeufertW (1997) Yeast Hct1 is a regulator of Clb2 cyclin proteolysis. Cell 90 : 683–693.
33. Cohen-FixO, KoshlandD (1999) Pds1p of budding yeast has dual roles: inhibition of anaphase initiation and regulation of mitotic exit. Genes Dev 13 : 1950–1959.
34. Tinker-KulbergRL, MorganDO (1999) Pds1 and Esp1 control both anaphase and mitotic exit in normal cells and after DNA damage. Genes Dev 13 : 1936–1949.
35. WäschR, CrossFR (2002) APC-dependent proteolysis of the mitotic cyclin Clb2 is essential for mitotic exit. Nature 418 : 556–562 doi:10.1038/nature00856
36. FitchI, DahmannC, SuranaU, AmonA, NasmythK, et al. (1992) Characterization of four B-type cyclin genes of the budding yeast Saccharomyces cerevisiae. Mol Biol Cell 3 : 805–818.
37. LimHH, GohPY, SuranaU (1996) Spindle pole body separation in Saccharomyces cerevisiae requires dephosphorylation of the tyrosine 19 residue of Cdc28. Mol Cell Biol 16 : 6385–6397.
38. AndersonVE, PruddenJ, ProchnikS, GiddingsTHJr, HardwickKG (2007) Novel sfi1 alleles uncover additional functions for Sfi1p in bipolar spindle assembly and function. Mol Biol Cell 18 : 2047–2056 doi:10.1091/mbc.E06-10-0918
39. TongAHY, BooneC (2006) Synthetic genetic array analysis in Saccharomyces cerevisiae. Methods Mol Biol 313 : 171–192.
40. LongtineMS, McKenzieA3rd, DemariniDJ, ShahNG, WachA, et al. (1998) Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14 : 953–961.
41. SheffMA, ThornKS (2004) Optimized cassettes for fluorescent protein tagging in Saccharomyces cerevisiae. Yeast 21 : 661–670 doi:10.1002/yea.1130
42. GiddingsTH, O'TooleET, MorphewM, MastronardeDN, McIntoshJR, et al. (2001) Using rapid freeze and freeze-substitution for the preparation of yeast cells for electron microscopy and three-dimensional analysis. Methods Cell Biol 67 : 27–42.
Štítky
Genetika Reprodukční medicína
Článek Oligoasthenoteratozoospermia and Infertility in Mice Deficient for miR-34b/c and miR-449 LociČlánek The Kinesin AtPSS1 Promotes Synapsis and is Required for Proper Crossover Distribution in MeiosisČlánek Payoffs, Not Tradeoffs, in the Adaptation of a Virus to Ostensibly Conflicting Selective PressuresČlánek Examination of Prokaryotic Multipartite Genome Evolution through Experimental Genome ReductionČlánek BMP-FGF Signaling Axis Mediates Wnt-Induced Epidermal Stratification in Developing Mammalian SkinČlánek Role of STN1 and DNA Polymerase α in Telomere Stability and Genome-Wide Replication in ArabidopsisČlánek RNA-Processing Protein TDP-43 Regulates FOXO-Dependent Protein Quality Control in Stress ResponseČlánek Integrating Functional Data to Prioritize Causal Variants in Statistical Fine-Mapping StudiesČlánek Salt-Induced Stabilization of EIN3/EIL1 Confers Salinity Tolerance by Deterring ROS Accumulation inČlánek Ethylene-Induced Inhibition of Root Growth Requires Abscisic Acid Function in Rice ( L.) SeedlingsČlánek Metabolic Respiration Induces AMPK- and Ire1p-Dependent Activation of the p38-Type HOG MAPK PathwayČlánek Signature Gene Expression Reveals Novel Clues to the Molecular Mechanisms of Dimorphic Transition inČlánek A Mouse Model Uncovers LKB1 as an UVB-Induced DNA Damage Sensor Mediating CDKN1A (p21) DegradationČlánek Dominant Sequences of Human Major Histocompatibility Complex Conserved Extended Haplotypes from to
Článek vyšel v časopisePLOS Genetics
Nejčtenější tento týden
2014 Číslo 10
-
Všechny články tohoto čísla
- An Deletion Is Highly Associated with a Juvenile-Onset Inherited Polyneuropathy in Leonberger and Saint Bernard Dogs
- Licensing of Yeast Centrosome Duplication Requires Phosphoregulation of Sfi1
- Oligoasthenoteratozoospermia and Infertility in Mice Deficient for miR-34b/c and miR-449 Loci
- Basement Membrane and Cell Integrity of Self-Tissues in Maintaining Immunological Tolerance
- The Kinesin AtPSS1 Promotes Synapsis and is Required for Proper Crossover Distribution in Meiosis
- Germline Mutations in Are Associated with Familial Gastric Cancer
- POT1a and Components of CST Engage Telomerase and Regulate Its Activity in
- Controlling Meiotic Recombinational Repair – Specifying the Roles of ZMMs, Sgs1 and Mus81/Mms4 in Crossover Formation
- Payoffs, Not Tradeoffs, in the Adaptation of a Virus to Ostensibly Conflicting Selective Pressures
- FHIT Suppresses Epithelial-Mesenchymal Transition (EMT) and Metastasis in Lung Cancer through Modulation of MicroRNAs
- Genome-Wide Mapping of Yeast RNA Polymerase II Termination
- Examination of Prokaryotic Multipartite Genome Evolution through Experimental Genome Reduction
- White Cells Facilitate Opposite- and Same-Sex Mating of Opaque Cells in
- BMP-FGF Signaling Axis Mediates Wnt-Induced Epidermal Stratification in Developing Mammalian Skin
- Genome-Wide Association Study of CSF Levels of 59 Alzheimer's Disease Candidate Proteins: Significant Associations with Proteins Involved in Amyloid Processing and Inflammation
- COE Loss-of-Function Analysis Reveals a Genetic Program Underlying Maintenance and Regeneration of the Nervous System in Planarians
- Fat-Dachsous Signaling Coordinates Cartilage Differentiation and Polarity during Craniofacial Development
- Identification of Genes Important for Cutaneous Function Revealed by a Large Scale Reverse Genetic Screen in the Mouse
- Sensors at Centrosomes Reveal Determinants of Local Separase Activity
- Genes Integrate and Hedgehog Pathways in the Second Heart Field for Cardiac Septation
- Systematic Dissection of Coding Exons at Single Nucleotide Resolution Supports an Additional Role in Cell-Specific Transcriptional Regulation
- Recovery from an Acute Infection in Requires the GATA Transcription Factor ELT-2
- HIPPO Pathway Members Restrict SOX2 to the Inner Cell Mass Where It Promotes ICM Fates in the Mouse Blastocyst
- Role of and in Development of Abdominal Epithelia Breaks Posterior Prevalence Rule
- The Formation of Endoderm-Derived Taste Sensory Organs Requires a -Dependent Expansion of Embryonic Taste Bud Progenitor Cells
- Role of STN1 and DNA Polymerase α in Telomere Stability and Genome-Wide Replication in Arabidopsis
- Keratin 76 Is Required for Tight Junction Function and Maintenance of the Skin Barrier
- Encodes the Catalytic Subunit of N Alpha-Acetyltransferase that Regulates Development, Metabolism and Adult Lifespan
- Disruption of SUMO-Specific Protease 2 Induces Mitochondria Mediated Neurodegeneration
- Caudal Regulates the Spatiotemporal Dynamics of Pair-Rule Waves in
- It's All in Your Mind: Determining Germ Cell Fate by Neuronal IRE-1 in
- A Conserved Role for Homologs in Protecting Dopaminergic Neurons from Oxidative Stress
- The Master Activator of IncA/C Conjugative Plasmids Stimulates Genomic Islands and Multidrug Resistance Dissemination
- An AGEF-1/Arf GTPase/AP-1 Ensemble Antagonizes LET-23 EGFR Basolateral Localization and Signaling during Vulva Induction
- The Proteomic Landscape of the Suprachiasmatic Nucleus Clock Reveals Large-Scale Coordination of Key Biological Processes
- RNA-Processing Protein TDP-43 Regulates FOXO-Dependent Protein Quality Control in Stress Response
- A Complex Genetic Switch Involving Overlapping Divergent Promoters and DNA Looping Regulates Expression of Conjugation Genes of a Gram-positive Plasmid
- ZTF-8 Interacts with the 9-1-1 Complex and Is Required for DNA Damage Response and Double-Strand Break Repair in the Germline
- Integrating Functional Data to Prioritize Causal Variants in Statistical Fine-Mapping Studies
- Tpz1-Ccq1 and Tpz1-Poz1 Interactions within Fission Yeast Shelterin Modulate Ccq1 Thr93 Phosphorylation and Telomerase Recruitment
- Salt-Induced Stabilization of EIN3/EIL1 Confers Salinity Tolerance by Deterring ROS Accumulation in
- Telomeric (s) in spp. Encode Mediator Subunits That Regulate Distinct Virulence Traits
- Ethylene-Induced Inhibition of Root Growth Requires Abscisic Acid Function in Rice ( L.) Seedlings
- Ancient Expansion of the Hox Cluster in Lepidoptera Generated Four Homeobox Genes Implicated in Extra-Embryonic Tissue Formation
- Mechanism of Suppression of Chromosomal Instability by DNA Polymerase POLQ
- A Mutation in the Mouse Gene Leads to Impaired Hedgehog Signaling
- Keeping mtDNA in Shape between Generations
- Targeted Exon Capture and Sequencing in Sporadic Amyotrophic Lateral Sclerosis
- TIF-IA-Dependent Regulation of Ribosome Synthesis in Muscle Is Required to Maintain Systemic Insulin Signaling and Larval Growth
- At Short Telomeres Tel1 Directs Early Replication and Phosphorylates Rif1
- Evidence of a Bacterial Receptor for Lysozyme: Binding of Lysozyme to the Anti-σ Factor RsiV Controls Activation of the ECF σ Factor σ
- Hsp40s Specify Functions of Hsp104 and Hsp90 Protein Chaperone Machines
- Feeding State, Insulin and NPR-1 Modulate Chemoreceptor Gene Expression via Integration of Sensory and Circuit Inputs
- Functional Interaction between Ribosomal Protein L6 and RbgA during Ribosome Assembly
- Multiple Regulatory Systems Coordinate DNA Replication with Cell Growth in
- Fast Evolution from Precast Bricks: Genomics of Young Freshwater Populations of Threespine Stickleback
- Mmp1 Processing of the PDF Neuropeptide Regulates Circadian Structural Plasticity of Pacemaker Neurons
- The Nuclear Immune Receptor Is Required for -Dependent Constitutive Defense Activation in
- Genetic Modifiers of Neurofibromatosis Type 1-Associated Café-au-Lait Macule Count Identified Using Multi-platform Analysis
- Juvenile Hormone-Receptor Complex Acts on and to Promote Polyploidy and Vitellogenesis in the Migratory Locust
- Uncovering Enhancer Functions Using the α-Globin Locus
- The Analysis of Mutant Alleles of Different Strength Reveals Multiple Functions of Topoisomerase 2 in Regulation of Chromosome Structure
- Metabolic Respiration Induces AMPK- and Ire1p-Dependent Activation of the p38-Type HOG MAPK Pathway
- The Specification and Global Reprogramming of Histone Epigenetic Marks during Gamete Formation and Early Embryo Development in
- The DAF-16 FOXO Transcription Factor Regulates to Modulate Stress Resistance in , Linking Insulin/IGF-1 Signaling to Protein N-Terminal Acetylation
- Genetic Influences on Translation in Yeast
- Analysis of Mutants Defective in the Cdk8 Module of Mediator Reveal Links between Metabolism and Biofilm Formation
- Ribosomal Readthrough at a Short UGA Stop Codon Context Triggers Dual Localization of Metabolic Enzymes in Fungi and Animals
- Gene Duplication Restores the Viability of Δ and Δ Mutants
- Selection on a Variant Associated with Improved Viral Clearance Drives Local, Adaptive Pseudogenization of Interferon Lambda 4 ()
- Break-Induced Replication Requires DNA Damage-Induced Phosphorylation of Pif1 and Leads to Telomere Lengthening
- Dynamic Partnership between TFIIH, PGC-1α and SIRT1 Is Impaired in Trichothiodystrophy
- Signature Gene Expression Reveals Novel Clues to the Molecular Mechanisms of Dimorphic Transition in
- Mutations in Moderate or Severe Intellectual Disability
- Multifaceted Genome Control by Set1 Dependent and Independent of H3K4 Methylation and the Set1C/COMPASS Complex
- A Role for Taiman in Insect Metamorphosis
- The Small RNA Rli27 Regulates a Cell Wall Protein inside Eukaryotic Cells by Targeting a Long 5′-UTR Variant
- MMS Exposure Promotes Increased MtDNA Mutagenesis in the Presence of Replication-Defective Disease-Associated DNA Polymerase γ Variants
- Coexistence and Within-Host Evolution of Diversified Lineages of Hypermutable in Long-term Cystic Fibrosis Infections
- Comprehensive Mapping of the Flagellar Regulatory Network
- Topoisomerase II Is Required for the Proper Separation of Heterochromatic Regions during Female Meiosis
- A Splice Mutation in the Gene Causes High Glycogen Content and Low Meat Quality in Pig Skeletal Muscle
- KDM5 Interacts with Foxo to Modulate Cellular Levels of Oxidative Stress
- H2B Mono-ubiquitylation Facilitates Fork Stalling and Recovery during Replication Stress by Coordinating Rad53 Activation and Chromatin Assembly
- Copy Number Variation in the Horse Genome
- Unifying Genetic Canalization, Genetic Constraint, and Genotype-by-Environment Interaction: QTL by Genomic Background by Environment Interaction of Flowering Time in
- Spinster Homolog 2 () Deficiency Causes Early Onset Progressive Hearing Loss
- Genome-Wide Discovery of Drug-Dependent Human Liver Regulatory Elements
- Developmentally-Regulated Excision of the SPβ Prophage Reconstitutes a Gene Required for Spore Envelope Maturation in
- Protein Phosphatase 4 Promotes Chromosome Pairing and Synapsis, and Contributes to Maintaining Crossover Competence with Increasing Age
- The bHLH-PAS Transcription Factor Dysfusion Regulates Tarsal Joint Formation in Response to Notch Activity during Leg Development
- A Mouse Model Uncovers LKB1 as an UVB-Induced DNA Damage Sensor Mediating CDKN1A (p21) Degradation
- Notch3 Interactome Analysis Identified WWP2 as a Negative Regulator of Notch3 Signaling in Ovarian Cancer
- An Integrated Cell Purification and Genomics Strategy Reveals Multiple Regulators of Pancreas Development
- Dominant Sequences of Human Major Histocompatibility Complex Conserved Extended Haplotypes from to
- The Vesicle Protein SAM-4 Regulates the Processivity of Synaptic Vesicle Transport
- A Gain-of-Function Mutation in Impeded Bone Development through Increasing Expression in DA2B Mice
- Nephronophthisis-Associated Regulates Cell Cycle Progression, Apoptosis and Epithelial-to-Mesenchymal Transition
- Beclin 1 Is Required for Neuron Viability and Regulates Endosome Pathways via the UVRAG-VPS34 Complex
- The Not5 Subunit of the Ccr4-Not Complex Connects Transcription and Translation
- Abnormal Dosage of Ultraconserved Elements Is Highly Disfavored in Healthy Cells but Not Cancer Cells
- Genome-Wide Distribution of RNA-DNA Hybrids Identifies RNase H Targets in tRNA Genes, Retrotransposons and Mitochondria
- The Chromosomal Association of the Smc5/6 Complex Depends on Cohesion and Predicts the Level of Sister Chromatid Entanglement
- Cell-Autonomous Progeroid Changes in Conditional Mouse Models for Repair Endonuclease XPG Deficiency
- PLOS Genetics
- Archiv čísel
- Aktuální číslo
- Informace o časopisu
Nejčtenější v tomto čísle- The Master Activator of IncA/C Conjugative Plasmids Stimulates Genomic Islands and Multidrug Resistance Dissemination
- A Splice Mutation in the Gene Causes High Glycogen Content and Low Meat Quality in Pig Skeletal Muscle
- Keratin 76 Is Required for Tight Junction Function and Maintenance of the Skin Barrier
- A Role for Taiman in Insect Metamorphosis
Kurzy
Zvyšte si kvalifikaci online z pohodlí domova
Současné možnosti léčby obezity
nový kurzAutoři: MUDr. Martin Hrubý
Všechny kurzyPřihlášení#ADS_BOTTOM_SCRIPTS#Zapomenuté hesloZadejte e-mailovou adresu, se kterou jste vytvářel(a) účet, budou Vám na ni zaslány informace k nastavení nového hesla.
- Vzdělávání



